Please wait
0000014272DEF 14Afalseiso4217:USD00000142722024-01-012024-12-310000014272bmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272bmy:DrChrisBoernerMember2023-01-012023-12-3100000142722023-01-012023-12-3100000142722022-01-012022-12-3100000142722021-01-012021-12-3100000142722020-01-012020-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:PeoMember2022-01-012022-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:PeoMember2022-01-012022-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:PeoMember2022-01-012022-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:PeoMember2022-01-012022-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:PeoMember2021-01-012021-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:PeoMember2021-01-012021-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:PeoMember2021-01-012021-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:PeoMember2021-01-012021-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:PeoMember2020-01-012020-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:PeoMember2020-01-012020-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:PeoMember2020-01-012020-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:PeoMember2020-01-012020-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:PeoMember2024-01-012024-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:PeoMember2024-01-012024-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:PeoMember2024-01-012024-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:PeoMember2024-01-012024-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:AggtChngPnsnValInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:EqtyAwrdsInSummryCompstnTblForAplblYrMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:AggtPnsnAdjsSvcCstMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:PeoMemberbmy:DrGiovanniCaforioMember2023-01-012023-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:PeoMember2022-01-012022-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:PeoMember2022-01-012022-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:PeoMember2022-01-012022-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:PeoMember2022-01-012022-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:PeoMember2022-01-012022-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:PeoMember2022-01-012022-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:PeoMember2021-01-012021-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:PeoMember2021-01-012021-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:PeoMember2021-01-012021-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:PeoMember2021-01-012021-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:PeoMember2021-01-012021-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:PeoMember2021-01-012021-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:PeoMember2020-01-012020-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:PeoMember2020-01-012020-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:PeoMember2020-01-012020-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:PeoMember2020-01-012020-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:PeoMember2020-01-012020-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:PeoMember2020-01-012020-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:PeoMember2024-01-012024-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:PeoMember2024-01-012024-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:PeoMember2024-01-012024-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:PeoMember2024-01-012024-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:PeoMember2024-01-012024-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:PeoMember2024-01-012024-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:PeoMemberbmy:DrChrisBoernerMember2023-01-012023-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:NonPeoNeoMember2024-01-012024-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:NonPeoNeoMember2023-01-012023-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:NonPeoNeoMember2022-01-012022-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:NonPeoNeoMember2021-01-012021-12-310000014272ecd:YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMemberecd:NonPeoNeoMember2020-01-012020-12-310000014272ecd:DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMemberecd:NonPeoNeoMember2020-01-012020-12-31000001427212024-01-012024-12-31000001427232024-01-012024-12-31000001427252024-01-012024-12-31000001427272024-01-012024-12-31000001427222024-01-012024-12-31000001427242024-01-012024-12-31000001427262024-01-012024-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No.)
Filed by the Registrant ☒
Filed by a Party other than the Registrant ☐
Check the appropriate box:
☐ Preliminary Proxy Statement
☐ Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
☒ Definitive Proxy Statement
☐ Definitive Additional Materials
☐ Soliciting Material under §240.14a-12
Bristol-Myers Squibb Company
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
☒ No fee required.
☐ Fee paid previously with preliminary materials.
☐ Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11.
Table of Contents
| | | | | | | | | | | |
| | | |
| | |
| | | |
| Meeting Details Date: May 6, 2025 Time: 10:00 a.m. Eastern Time Location: Virtually | |
| | | |
| Your Vote is Important Regardless of the number of shares you own, your vote is important. If you do not attend the Annual Meeting to vote on the virtual meeting platform, your vote will not be counted unless a proxy representing your shares is presented at the meeting. To ensure that your shares will be voted at the meeting, please vote in one of these ways: | |
| | | |
| | Go to www.proxyvote.com and vote via the Internet; | |
| | | |
| | Call the toll-free telephone number (800) 690-6903 (this call is toll-free in the U.S.); or | |
| | | |
| | Mark, sign, date and promptly return the enclosed proxy card in the postage-paid envelope. | |
| If you do attend the Annual Meeting, you may revoke your proxy and vote your shares on the virtual meeting platform during the meeting. | |
| | | |
Notice of Annual Meeting of Shareholders
Notice is hereby given that the 2025 Annual Meeting of Shareholders (the “Annual Meeting” or “2025 Annual Meeting”) will be held virtually on May 6, 2025, at 10:00 a.m. Eastern Time for the following purposes as set forth in the accompanying Proxy Statement:
| | | | | |
| 1 | to elect to the Board of Directors the 11 persons nominated by the Board, each for a term of one year; |
| 2 | to conduct an advisory vote to approve the compensation of our Named Executive Officers; |
| 3 | to ratify the appointment of Deloitte & Touche LLP as the company’s independent registered public accounting firm for 2025; |
4-5 | to consider two shareholder proposals, if presented at the meeting; and |
| 6 | to transact such other business as may properly come before the meeting or any adjournments thereof. |
Holders of record of our common and preferred stock at the close of business on March 14, 2025, will be entitled to vote at the meeting.
The Annual Meeting will be held in a virtual-only meeting format. To be admitted to the Annual Meeting, you will need to visit www.virtualshareholdermeeting.com/BMY2025 and enter the 16-digit control number included on your Important Notice Regarding the Availability of Proxy Materials, on your proxy card, or on the instructions that accompanied your proxy materials. Guests may join the Annual Meeting in a listen-only mode, but they will not have the option to vote shares or ask questions during the virtual meeting. Once admitted, you may submit questions or vote during the Annual Meeting by following the instructions that will be available on the meeting website. You may log into the meeting platform beginning at 9:50 a.m. Eastern Time on May 6, 2025. To submit a question before the meeting, visit www.proxyvote.com with your 16-digit control number and select the “Submit a Question for Management” option. To submit a question during the meeting, visit www.virtualshareholdermeeting.com/BMY2025, enter your 16-digit control number and type your question into the “Ask a Question” field and click “Submit.” The company will provide direct and specific information to shareholder proponents on how they can present their shareholder proposals during the meeting.
By Order of the Board of Directors
Amy Fallone
Senior Vice President and Corporate Secretary
Dated: March 26, 2025
| | | | | |
Message from Christopher S. Boerner Board Chair and Chief Executive Officer | |
Dear BMS shareholders,
2024 was a productive year driven by strong execution for Bristol Myers Squibb (BMS). We made important progress, strengthened our financial foundation, and clearly articulated our strategy to reshape the company’s growth profile. Of course, we could not do any of this without our remarkable, global workforce and their continued commitment to delivering for patients. We are anchored in our mission, and I firmly believe we have the right talent and resources in place as we collectively write the next chapter in BMS’ history.
Last year the company saw 7% year-over-year revenue growth, with our Growth Portfolio now comprising 47% of annual total sales and our Legacy Portfolio generating significant cash flow to reinvest in future growth drivers. We closed and integrated several important transactions, received key regulatory approvals, executed well and advanced what I believe is the best pipeline we have ever had.
As we position the company to navigate the opportunities and challenges ahead, we are reshaping BMS to deliver sustained, top-tier growth and long-term shareholder returns. To do this, we are executing against three key objectives:
1.Focusing on transformational medicines where we have a competitive advantage;
2.Driving operational excellence throughout the organization; and
3.Strategically allocating capital for long-term growth and shareholder returns.
Focusing on Transformational Medicines
This past year, we re-established our presence in neuroscience with the U.S. approval of Cobenfy. This medicine epitomizes our focus on innovative treatments. It represents the first new mechanism of action in schizophrenia in decades and has the potential to expand into multiple additional indications.
We have extended our leadership positions across hematology, oncology and cardiovascular disease, while honing our efforts in immunology. In 2024, we received significant approvals and indication expansions in multiple major markets across key growth products, including Breyanzi, Camzyos and Reblozyl. We also extended the durability of our immuno-oncology portfolio with the U.S. approval of Opdivo Qvantig, the first and only subcutaneously administered PD-1 inhibitor, providing patients with a new option for faster administration.
Driving Operational Excellence
As our company evolves, our commitment to operational excellence will help drive efficiency, emphasize accountability and improve productivity. In 2024, we announced a new initiative to achieve $1.5 billion in cost savings by the end of 2025, and we realized the majority of these savings in 2024. We recently announced the expansion of this initiative to capture additional cost savings through 2027.
It is truly an exciting time for innovation at the company. Our collective focus on productivity and execution will continue to drive meaningful results. We are constantly pursuing bold science to deliver first-in-class or best-in class medicines as we define what is possible for patients—we are employing the responsible use of artificial intelligence (AI) to accelerate clinical trials in areas of significant unmet need. This includes pulmonary fibrosis, for which we are testing admilparant, an LPA1 antagonist, that has the potential to transform the current standard of care. We are also redefining anticoagulant therapy with the promise of FXIa inhibition in thrombotic diseases, and we are investing in CELMoDs, which can offer tailored treatment approaches in combinations across multiple myeloma and lymphoma.
Strategically Allocating Capital
Last year we strategically deployed our capital to (i) pursue sources of external innovation, (ii) return cash to shareholders, and (iii) strengthen our balance sheet. We closed the acquisitions of Karuna Therapeutics, bringing in Cobenfy, RayzeBio, adding radiopharmaceutical capabilities — one of the fastest-growing new modalities for treating patients with solid tumors, and Mirati Therapeutics, adding Krazati — to our portfolio.
The company announced a 5.3% dividend increase for 2025; the 15th consecutive year of a dividend increase and the 92nd consecutive year we have paid a dividend. We paid down $6 billion in debt consistent with our commitment to pay down approximately $10 billion of debt by 2026. These actions make clear our focus on maintaining our strong financial profile and prioritizing opportunities where we see the highest return for our patients and shareholders.
Our vision to transform patients’ lives through science extends to all patients. In 2024, we announced ASPIRE (Accessibility, Sustainability, Patient-centric, Impact, Responsibility and Equity), a strategy to advance access to our medicines and help patients in low- and middle-income countries (LMICs) gain access to potentially life-saving medicines. This is embedded in our overall commercial strategy.
We also received validation for our near-term and net-zero science-based targets from the Science Based Targets initiative (SBTi), highlighting our progress in reducing emissions across our operations and supply chain. Notably, our employee led initiative, Coast 2 Coast 4 Cancer, entered its 11th year and nearly 350 global colleagues representing 30 nations rode 6,200 miles cross-country to fund cancer research, further showing our mission in action.
As you can see, 2024 was a productive start to our broader transformation as a company. As we progress on this journey, we will be relentless in our pursuit to deliver innovative medicines to patients around the world. We have built the foundation and have a clear path forward.
Thank you for your continued investment in Bristol Myers Squibb and for your support of our mission. I am excited to see all that we will achieve together in 2025 and beyond.
Sincerely,
Christopher S. Boerner, Ph.D.
Board Chair and Chief Executive Officer
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| $48.3B annual revenues | | | 47% of revenues from Growth Portfolio | | | $6B of debt repaid as part of commitment to repay $10B by 2026 | | | 5.3% quarterly dividend increase for 2025 | | | 15 consecutive years of dividend increases | |
| | | | | | | | | | | | | | |
1 Bristol Myers Squibb | 2025 Proxy Statement
| | | | | |
Message from
Theodore R. Samuels Lead Independent Director | |
Dear fellow shareholders and stakeholders,
2024 was an eventful year for BMS and for the Board, which closely collaborated with management to strengthen the company’s governance profile and enhance the foundation for long-term, sustainable growth. We are excited to build on this progress as we write the next chapter in the company’s history.
When I first became Lead Independent Director, I said that maintaining our commitment to sound corporate governance was a top priority. This has not changed.
Since then, we have experienced a transition in leadership, and the relationship we have built with Chris in his capacity as CEO and Board Chair has been incredibly productive. I and my fellow independent directors are thankful for his and the management team’s continued partnership. Collectively, we value open communication and transparency, which fosters a constructive and continuous dialogue about how the company can continue delivering for patients, shareholders and all other stakeholders.
As directors, we play a significant role in supporting and overseeing BMS’s strategic direction. Each of our nine meetings with management last year offered opportunities to discuss the company’s execution against its strategy and the goal of becoming one of the sector’s fastest-growing companies by the end of the decade. We were consistently available to management and offered insights and guidance as the company approached and achieved key milestones, including its re-entry into neuroscience through the successful integration of Karuna Therapeutics and the U.S. launch of Cobenfy.
I’m confident that the Board is well positioned to help management navigate the challenges and opportunities ahead, especially as we continue identifying ways to enhance our effectiveness and expand our competencies to address oversight of existing and emerging risks, including drug pricing and access, artificial intelligence, and cybersecurity. Each year, as we complete an annual assessment of the Board, we are reminded it is critical that we continually evaluate our composition to ensure we maintain highly qualified directors with the right mix of skills to exercise proper oversight of management and the company’s strategy. In July 2024, we welcomed the newest member of our Board, Michael McMullen. Mike, the former president and CEO of Agilent Technologies, Inc., brings global management experience and a proven track record of business and cultural transformation that has already enhanced our contributions and the support we provide.
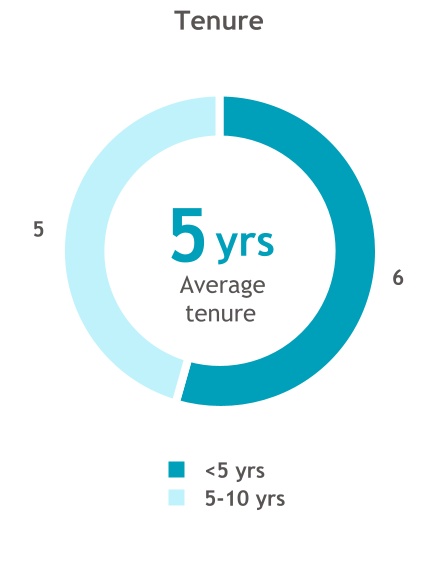
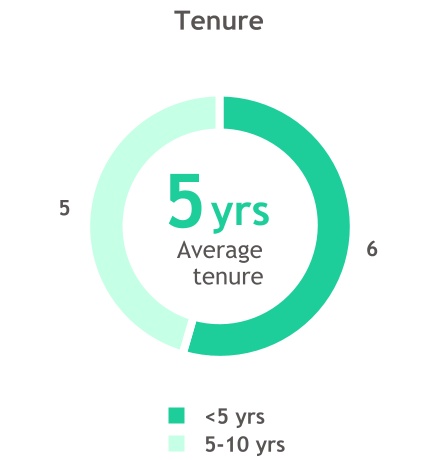
The Board we have today is strong and engaged, and I am tremendously excited about the impact we are making together. Our 11 director nominees bring a wide variety of experiences and expertise spanning corporate finance, commercialization, scientific research, risk management, practical medicine, academia, corporate leadership, and more. Six members were onboarded within the past five years, providing a steady refreshment of new talent and viewpoints.
Each year, we prioritize engaging with shareholders and answering their questions about the business and our areas of focus. In 2024, the company extended meeting invitations to approximately 50 of our top institutional shareholders, representing about 52% of our voting shares outstanding. We also used this opportunity to include our new CEO as well as the CFO and me in a few of the engagement meetings. These conversations are tremendously valuable, as they allow us to share recent milestones and key updates on the company’s progress. They also provide an opportunity to learn from our investors and better understand their perspectives on how BMS is performing and where we can improve.
Across our recent engagements, there was consistent interest in the company’s strategy, the relationship between management and the Board, and BMS’s plans to overcome potential near-term headwinds. The feedback we received was shared with the full Board and will help inform decisions we make throughout the coming year. We look forward to more of these discussions in 2025.
Beyond the governance, Board effectiveness and shareholder engagement efforts I’ve mentioned, we advanced our work to deliver value for patients and employees while furthering our environmental, social and governance (Sustainability and Social Impact) initiatives. Importantly, last year marked a watershed moment for BMS with the launch of our ASPIRE strategy, which is designed to enhance access to medicines in LMICs. The company also published its 2024 Climate Change Report, outlining a strategy for managing and mitigating climate risk.
Core to the company’s ongoing transformation are its people. We continue to prioritize a strong people strategy to ensure the best talent is in place to carry out our strategy. We are grateful to all BMS employees around the world who live our mission and deliver for patients day in and day out. The culture they have helped build, their tireless efforts, and the work of management and the Board to foster those efforts continue to be recognized, with Forbes naming the company to its 2024 list of the World’s Best Employers.
Thank you for your investment in our company and your support of our patient-centric mission. As you review the ballot items in this year’s Proxy Statement, please know that your vote matters. By taking the few minutes required to cast your vote, you are making your voice and opinions heard at the company’s Annual Meeting.
Theodore R. Samuels
Lead Independent Director
Chair, Committee on Directors and Corporate Governance
Bristol Myers Squibb | 2025 Proxy Statement 2
Bristol Myers Squibb
The Story
| | | | | | | | | | | |
| | | |
| At Bristol Myers Squibb, we are inspired by a single vision —transforming patients’ lives through science. | |
| | | |
| | | |
| | | |
| | Our vision is to transform patients’ lives through science. At Bristol Myers Squibb, we are in the business of breakthroughs — the kind that transform patients’ lives through life-saving, innovative medicines. Our talented workforce is relentlessly dedicated to our mission of discovering, developing and delivering innovative medicines that help patients prevail over serious diseases. Evolution is in our DNA, and we are reinventing to lead where we have a competitive advantage — because our success means success for patients in need. | |
| | | |
| | | |
| | We are a biopharmaceutical leader, powered by science. We combine the agility and mindset of a biotech with the reach and resources of an established pharmaceutical company to create a leading, global biopharmaceutical company. By extending our leadership positions across hematology, oncology and cardiovascular disease, honing our efforts in immunology and re-establishing our presence in neuroscience, we are focusing on innovations that drive meaningful change. Supported by a deep and innovative pipeline, we bring a human touch to every treatment we deliver. | |
| | | |
| | | |
| | We are committed to the health of people, society and the planet. Our passion for making an impact extends beyond the discovery, development and delivery of innovative medicines that help patients prevail over serious diseases. Through our Sustainability and Social Impact strategy, we seek to mobilize our capabilities and resources to positively impact the communities where we live and work, and those we serve around the world. Our Sustainability and Social Impact strategy and initiatives center on expanding the boundaries of science to address unmet patient needs and advancing equitable access to life-transforming medicines around the globe. This strategy is guided by our commitment to inclusion in all aspects of our business and to reducing our environmental impact. | |
| | | |
| | | |
| | We are people-focused, creating an environment for our employees to thrive. Among our highest priorities are the health, safety, professional development, and wellbeing of our employees. We prioritize our people by cultivating a high-performing and inclusive global workforce. We unite as ONE BMS, believing that the varied experiences and perspectives of all our employees help to bring out our best ideas, drive innovation and achieve transformative business results. We remain committed to providing a comprehensive rewards and wellbeing strategy to enable our workforce to deliver on our business strategy and transform patients' lives through science. | |
| | | |
| | | |
| | We put patients at the center of everything we do, defining what’s possible. Our focus on patients and their families motivates us to work smarter, faster and better. We commit to scientific excellence and invest in research and development (R&D) for our patients. Core to who we are is the belief that all patients who need our life-saving medicines should have access to them. We take a thoughtful approach to pricing our medicines and we support policies that help advance access. To that end, we are committed to working collaboratively with relevant stakeholders, including payers, physicians, advocates, patients and civil societies around the world. We are reimagining the patient experience by leveraging digital health to improve diagnosis, treatment and monitoring. We are motivated by the power of science. Together, we are reshaping the treatment of schizophrenia with the U.S. approval of Cobenfy — the first new mechanism of action in decades for the treatment of schizophrenia. The advancements of first- or best-in-class medicines Reblozyl, Camzyos and Breyanzi build on our legacy across disease areas in oncology, cardiovascular, and hematology. In immunology, we are focused on novel treatment approaches such as the use of cell therapy to reset the immune system. We are passionately pursuing the next wave of treatments and scientific advancements. This includes radiopharmaceutical therapeutics (RPTs), one of the fastest-growing new modalities for treating patients with solid tumors, which we’ve added to our manufacturing platform. We have a history of scientific excellence and transforming patient outcomes. This is driven by our global workforce, who work together to define what’s possible for the future of science and the patients we serve. | |
| | | |
3 Bristol Myers Squibb | 2025 Proxy Statement
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Reshaping BMS to achieve sustained top-tier growth, maximize long-term value and accelerate our ability to deliver breakthrough medicines to even more patients faster. 2024 was a productive year marked by strong execution and the advancement of the best pipeline in our history. Our Growth Portfolio delivered strong sales growth versus 2023, and we built momentum through important regulatory milestones, including the U.S. approval of Cobenfy and Opdivo Qvantig. To position the organization for continued success, we began reshaping the company by focusing on transformational medicines where we have a competitive advantage, driving operational excellence and strategically allocating capital. We serve millions of patients globally and our progress last year accelerated our ability to deliver more innovative medicines to more patients, strengthening our profile heading into 2025. | |
| | | | | | | |
| | | | | | | | | | |
| Highlights from the year include: üReceived 23 approvals from the FDA and other markets, including: •Cobenfy; launched the first-in-class transformational treatment for adults with schizophrenia in the U.S. in decades •Opdivo Qvantig; received U.S. approval for the subcutaneous formulation of nivolumab across most previously approved adult, solid tumor Opdivo indications •Reblozyl; expanded approval in the E.U. to include the first-line treatment of adult patients with transfusion-dependent anemia •Breyanzi; received U.S. approval to expand into follicular lymphoma and mantle cell lymphoma üStrong R&D and pipeline progress: •Advanced our promising pipeline with increased depth across therapeutic areas of focus to address high unmet needs •Took steps to expedite delivery through trial acceleration •Received nine investigational new drug/clinical trial authorization (IND/CTA)* approvals •Expanded our registrational pipeline, which includes over 15 assets •Began efforts to further increase and sustain R&D productivity and bring treatments to patients faster | | |
üLeveraged external innovation through business development and partnerships to address areas of high unmet need. •Closed and integrated the acquisitions of Mirati Therapeutics, RayzeBio, and Karuna Therapeutics. These transactions enhance our oncology portfolio, add an important radiopharmaceutical platform, and accelerate our re-entry into neuroscience. •Completed an exclusive license and collaboration agreement with SystImmune, adding another antibody-drug conjugate to our diverse pipeline. •Deepened our capabilities across our research platforms and core therapeutic areas through partnerships with Cellares and BioArctic. üAnnounced ASPIRE (Accessibility, Sustainability, Patient-centric, Impact, Responsibility and Equity) strategy to advance access to our innovative treatments and help patients in low- and middle-income countries (LMICs) gain access to potentially life-saving medicines and received validation for our near-term and net-zero science-based targets from the Science Based Targets initiative (SBTi), progressing our Sustainability and Social Impact initiatives. Throughout the year, we remained strategic in our approach to capital allocation. We harnessed our strong financial position to invest in both internal and external innovation. We remain committed to returning capital to shareholders and to the dividend, which we increased for the fifteenth consecutive year. We also strengthened our balance sheet, paying down $6 billion in debt in 2024. | |
| | | | | | |
| | | | | | |
23 Approvals from the FDA and other markets | 20 Submissions to the FDA and other markets | 9 IND/CTA approvals | | |
| | | | | | | | | | |
*An Investigational New Drug (IND) is a drug or biological product that has not been approved for general use by the FDA.
Bristol Myers Squibb | 2025 Proxy Statement 4
Who We Are
2025 Director Nominees
Our Board of Directors
Our Board of Directors (the “Board”) has nominated 11 current directors, Christopher Boerner, Ph.D., Theodore R. Samuels, Peter J. Arduini, Deepak L. Bhatt, M.D., M.P.H., M.B.A., Julia A. Haller, M.D., Manuel Hidalgo Medina, M.D., Ph.D., Michael R. McMullen, Paula A. Price, Derica W. Rice, Karen H. Vousden, Ph.D., and Phyllis R. Yale, to serve as directors of Bristol-Myers Squibb Company. All of our nominees are willing to serve as directors and have consented to being named in our proxy materials. When elected, these directors will hold office until the 2026 Annual Meeting or until their successors are duly elected.
We believe that tone is set at the top, so we begin by introducing you to who we are. We follow that with a description of how our directors are selected and elected, how we govern and are governed, how we are organized, how you can communicate with us and how we are paid. We ask in Item 1 for your voting support so we can continue our important work and build on our significant successes in 2025.
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| Board Nominees | | | | | | |
| Christopher S. Boerner, Ph.D. | Theodore R. Samuels | Peter J.
Arduini | Deepak L. Bhatt, M.D., M.P.H., M.B.A. | Julia A.
Haller, M.D. | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Manuel Hidalgo Medina, M.D., Ph.D. | Michael R. McMullen | Paula A.
Price | Derica W.
Rice | Karen H.
Vousden, Ph.D. | Phyllis R.
Yale | |
| | | | | | | |
All Director Nominees possess these qualities:
| | | | | | | | | | | |
| | | |
| | | |
| Leadership | Strategic Thinking | Sound Business Judgment | Integrity & Ethics |
| | | |
5 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Who We Are: 2025 Director Nominees |
Overview of 2025 Director Nominees Skills, Age & Tenure
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
| Chris S. Boerner, PhD (CEO) | Theodore R. Samuels (LID) | Peter J.
Arduini | Deepak L. Bhatt,
MD, MPH, MBA | Julia A.
Haller, MD | Manuel Hidalgo Medina, MD, PhD | Michael R. McMullen | Paula A.
Price | Derica W.
Rice | Karen H.
Vousden, PhD | Phyllis R.
Yale |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Director Since | 2023 | 2017 | 2016 | 2022 | 2019 | 2021 | 2024 | 2020 | 2020 | 2018 | 2019 |
| Compliance |
| Independent Director | | l | l | l | l | l | l | l | l | l | l |
| Designated Financial Expert | | l | | | | | l | l | l | | |
| Key Skills & Experience |
| Public Co. CEO | l | | l | | | | l | | | | |
| Public Co. CFO | | | | | | | | l | l | | |
| Healthcare | l | l | l | l | l | l | l | l | l | l | l |
| Science/Technology/Innovation | l | | l | l | l | l | l | | | l | |
| Financial | l | l | l | | l | | l | l | l | | l |
| Risk Management | l | l | l | | l | | l | l | l | | l |
| Sales & Marketing | l | l | l | | | | l | | | | l |
| International | l | l | l | | | l | l | l | l | l | |
Academia/
Non-Profit | | l | | l | l | l | | l | | l | l |
| Digital | l | | l | | | | l | l | l | | |
| Demographics |
Age (years)† | 54 | 70 | 60 | 57 | 70 | 57 | 64 | 63 | 60 | 67 | 67 |
Tenure (years)† | 2.0 | 8.2 | 9.1 | 2.9 | 5.5 | 3.9 | 0.8 | 4.7 | 4.7 | 7.4 | 5.5 |
† As of May 6, 2025 |
| | | | | | | | | | | |
Key Skills & Experience Definitions
Healthcare
Experience in relevant areas within the healthcare industry, including science, manufacturing, regulatory compliance, payer dynamics, and working with health care providers.
Science/Technology/Innovation
Relevant scientific expertise in the healthcare industry, and experience with technology and innovation, including the use of innovative technologies in the discovery, development and delivery of medicines.
Financial
Experience in corporate finance, and financial reporting and internal controls at a large organization.
Risk Management
Experience managing critical enterprise risks.
Sales & Marketing
Experience in commercialization, digital advertising, marketing and brand development.
International
Experience leading a complex global organization or understanding different regulatory and commercial requirements.
Public Company CEO/CFO
Experience serving as a CEO/CFO at a public or private company.
Academia/Non-Profit
Experience as professor, researcher or leader at a large university or non-profit organization.
Digital
Experience or expertise managing or overseeing information technology, including related to the use of digital technologies to facilitate business objectives, cybersecurity and data privacy.
Bristol Myers Squibb | 2025 Proxy Statement 6
| | |
|
| Who We Are: 2025 Director Nominees |
Overview of 2025 Director Nominees Background
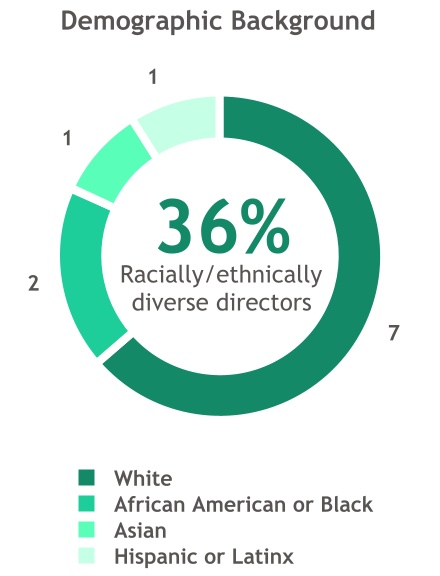
Our 11 Director nominees provide the Board with a comprehensive collection of varied backgrounds, industry experiences and personal characteristics.
| | | | | | | | | | | | | | | | | |
| | | | | |
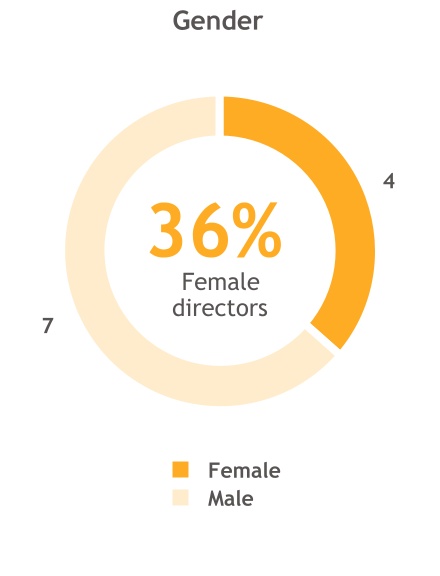
| Age distribution | | | Board refreshment | |
| 63 | | | 6 | |
| Average age of Directors (years) | | | New Directors over the past five years | |
| | | | | |
7 Bristol Myers Squibb | 2025 Proxy Statement
Item 1
Election of the Board of Directors
2025 Director Nominees
The following biographies of our director nominees reflect their Board Committee membership and Chair positions as of the date of this year’s Annual Meeting. Each of our Board members has experience and skills in the enumerated categories included in our skills matrix chart above, however, we have designated in the biographies only the top three to five skills to indicate that a director has particular strength in those areas.
Christopher S. Boerner, Ph.D.
Board Chair & Chief Executive Officer of the Company
Director Since: 2023
Age: 54
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •None | | | Other Public Company Boards •None | |
| | | | | |
| | | | | |
| Experience •Bristol Myers Squibb, Chief Executive Officer (November 2023-present) •Board Chair (April 2024-present); Board Member (2023-present) •Executive Vice President, Chief Operating Officer (April 2023-October 2023) •Executive Vice President, Chief Commercialization Officer (2018-2023) •Head of International Markets (2017-2018); Head of U.S. Commercial Markets (2015-2017) •Held leadership roles of increasing responsibility at Seattle Genetics, Inc. (2010-2015) •Served in marketing leadership roles at Genentech (2002-2010) •Worked at McKinsey & Company, earlier in his career, serving global pharmaceutical and biotechnology clients Key Skills and Experience •Public Company CEO/CFO •Healthcare •Sales & Marketing •Financial •Risk Management Other •Board of Directors of PhRMA (Pharmaceutical Research and Manufacturers of America) •Board member, Chair of the Nominating and Corporate Governance Committee of Achaogen, Inc. (2014-2015) | |
| | | | | |
Bristol Myers Squibb | 2025 Proxy Statement 8
| | |
|
| Item 1 – Election of the Board of Directors |
Theodore R. Samuels
Lead Independent Director & Chair of the Committee on Directors and Corporate Governance
Director Since: 2017
Age: 70
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Committee on Directors and Corporate Governance (Chair) •Audit Committee | | | Other Public Company Boards •Centene Corporation •Iron Mountain Incorporated Former Public Company Boards •Stamps.com •Perrigo Company plc | |
| | | | | |
| | | | | |
| Experience •President of the Capital Guardian Trust Company (2010-2016) •Board member, Capital Group (2005-2009); Capital Group Audit Committee; Capital Group Finance Committee (2013-2016); Chair of Capital International (North America) Proxy Committee; Capital Guardian Trust Company (North American) Management Committee member •Portfolio Manager at Capital Group (1990-2016 and analyst 1981-1990) Key Skills and Experience •Financial •Sales & Marketing •Risk Management •International Other •Director of BJC HealthCare •Trustee of Children’s Hospital Los Angeles Foundation; served as Director of Children’s Hospital Los Angeles (2004-2019; co-chair 2012-2015) •Director of the Edward Mallinckrodt, Jr. Foundation •Director of Research Corporation Technologies, Inc. •Trustee of the John Burroughs School, St. Louis (2018-2024) •Co-Chair of Tuft’s President Council (2016-2022) | |
| | | | | |
9 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Item 1 – Election of the Board of Directors |
Peter J. Arduini
Chair of the Compensation and Management Development Committee
Director Since: 2016
Age: 60
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Compensation and Management Development Committee (Chair) | | | Other Public Company Boards •GE Healthcare Former Public Company Boards •Integra LifeSciences Holdings Corporation | |
| | | | | |
| | | | | |
| Experience •President and Chief Executive Officer at GE Healthcare, a medical technology and digital solutions innovator (2022-present) •President and Chief Executive Officer at Integra LifeSciences Holdings Corporation (2012-2021); President and Chief Operating Officer (2010-2012) •Corporate Vice President and President of Medication Delivery, Baxter Healthcare (2005-2010) •Previously spent 15 years at General Electric Healthcare in a variety of management roles for domestic and global businesses, culminating in leading the global functional imaging business Key Skills and Experience •Public Company CEO/CFO •Healthcare •Sales & Marketing •Financial Other •Board of Directors of AdvaMed (the Advanced Medical Technology Association) •Board of Directors of the National Italian American Foundation •Board of Trustees of Susquehanna University (2016-2022) | |
| | | | | |
Bristol Myers Squibb | 2025 Proxy Statement 10
| | |
|
| Item 1 – Election of the Board of Directors |
Deepak L. Bhatt, M.D., M.P.H., M.B.A.
Director Since: 2022
Age: 57
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Science & Technology Committee •Compensation and Management Development Committee | | | Other Public Company Boards •None | |
| | | | | |
| | | | | |
| Experience •Director of Mount Sinai Fuster Heart Hospital and the Dr. Valentin Fuster Professor of Cardiovascular Medicine at the Icahn School of Medicine at Mount Sinai (2022-present) •Professor of Medicine at Harvard Medical School (2012-2022); Visiting Professor of Medicine at Harvard Medical School (2022-2024) •Executive Director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital (2013-2022) •Cardiologist at Dana Farber Cancer Institute (2009-2022) •Chief of Cardiology at VA Boston Heathcare (2008-2013) •Held a number of roles of increasing responsibility at the Cleveland Clinic in Cleveland, Ohio, in addition to being an attending physician, including Associate Director of the Cleveland Clinic Cardiovascular Coordinating Center, Director of the interventional cardiology fellowship, and Associate Director of the cardiovascular medicine fellowship (2001-2008) Key Skills and Experience •Science/Technology/Innovation •Healthcare •Academia/Non-Profit Other •Former Member of the Board of Trustees of the American College of Cardiology | |
| | | | | |
11 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Item 1 – Election of the Board of Directors |
Julia A. Haller, M.D.
Chair of the Science & Technology Committee
Director Since: 2019
Age: 70
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Science & Technology Committee (Chair) •Committee on Directors and Corporate Governance | | | Other Public Company Boards •Ophtea Limited •Outlook Therapeutics, Inc. Former Public Company Boards •Celgene Corporation •Eyenovia, Inc. | |
| | | | | |
| | | | | |
| Experience •Ophthalmologist-in-Chief of Wills Eye Hospital in Philadelphia, PA, where she holds the William Tasman, M.D. Endowed Chair (2007-present) •Professor and Chair of the Department of Ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University and Thomas Jefferson University Hospitals (present) •Member of the Johns Hopkins faculty, where she held the Katharine Graham Chair in Ophthalmology (until 2007) •Trained at the Wilmer Eye Institute at Johns Hopkins, where she served as the first female Chief Resident Key Skills and Experience •Academia/Non-Profit •Healthcare •Science/Technology/Innovation Other •Member of the National Academy of Medicine •Chair of the Board of Trustees for the College of Physicians of Philadelphia •Chair of the HEED Ophthalmic Foundation Board •President of the John Hopkins Medicine Alumni Society | |
| | | | | |
Bristol Myers Squibb | 2025 Proxy Statement 12
| | |
|
| Item 1 – Election of the Board of Directors |
Manuel Hidalgo Medina, M.D., Ph.D.
Director Since: 2021
Age: 57
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Committee on Directors and Corporate Governance •Science & Technology Committee | | | Other Public Company Boards •Guardant Health, Inc. | |
| | | | | |
| | | | | |
| Experience •Professor of Medicine and Chief of Division of Hematology and Medical Oncology at Weill Cornell Medical College (2019-present) •Attending Physician at New York-Presbyterian Hospital (2019-present) •Associate Director, Clinical Services of Meyer Cancer Center, Weill Cornell Medical College (2019-2024) •Deputy Associate Director, Clinical Sciences at Dana Farber/Harvard Cancer Center (2015-2019) •Chief of Division of Hematology, Oncology and Director at Rosenberg Clinical Cancer Center of Beth Israel Deaconess Medical Center (2015-2019) •Professor of Medicine at Harvard University (2015-2019) Key Skills and Experience •Science/Technology/Innovation •Healthcare •Academia/Non-Profit •International Other •Director of Methods of Special Conference Clinical Cancer Research Course of American Association for Cancer Research (2018-2024) •Steering Committee of Pancreatic Cancer Action Network (2016-present) •Director of American Association of Cancer Research (2024-2027) •Director of Guardant Health (2024-present) | |
| | | | | |
13 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Item 1 – Election of the Board of Directors |
Michael R. McMullen
Director Since: 2024
Age: 64
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Audit Committee •Compensation and Management Development Committee | | | Other Public Company Boards •KLA Corporation Former Public Company Boards •Agilent Technologies, Inc. •Coherent, Inc. | |
| | | | | |
| | | | | |
| Experience •Chief Executive Officer of Agilent Technologies, Inc. (2015-2024) •President and Chief Operating Officer of Agilent Technologies, Inc. (2014-2015) •Senior Vice President, Agilent and President, Chemical Analysis Group at Agilent (2009-2014) Key Skills and Experience •Public Company CEO/CFO •Science/Technology/Innovation •Healthcare •International •Digital Other •Board of Trustees, University of Delaware (2024-present) | |
| | | | | |
Bristol Myers Squibb | 2025 Proxy Statement 14
| | |
|
| Item 1 – Election of the Board of Directors |
Paula A. Price
Director Since: 2020
Age: 63
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Audit Committee •Committee on Directors and Corporate Governance | | | Other Public Company Boards •Accenture plc •Warner Brothers Discovery •Mondelez International, Inc. Former Public Company Boards •Davita, Inc. •Dollar General Corporation •Western Digital Corporation | |
| | | | | |
| | | | | |
| Experience •Executive Vice President and Chief Financial Officer at Macy’s, Inc. (2018-2020) •Senior Lecturer at Harvard Business School in the Accounting and Management Unit (2014-2018) •Executive Vice President and Chief Financial Officer of Ahold USA (2009-2014) •Senior Vice President, Controller and Chief Accounting Officer at CVS Caremark (2006-2009) Key Skills and Experience •Public Company CEO/CFO •Financial •Risk Management •Academia/Non-Profit •Digital Other •Director of Blue Cross Blue Shield of Massachusetts •Member of Advisory Board of Columbia University Mailman School of Public Health •Director of Mutual of America | |
| | | | | |
15 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Item 1 – Election of the Board of Directors |
Derica W. Rice
Chair of the Audit Committee
Director Since: 2020
Age: 60
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Audit Committee (Chair) •Compensation and Management Development Committee | | | Other Public Company Boards •Target Corporation •The Walt Disney Company •The Carlyle Group | |
| | | | | |
| | | | | |
| Experience •Executive Vice President of CVS Health, and President, Pharmacy Benefits Management Business of CVS Caremark (2018-2020) •Executive Vice President of Global Services (2010-2017) and Chief Financial Officer (2006-2017) of Eli Lilly and Company •Vice President and Controller (2003-2006) and various executive positions at Eli Lilly and Company (1990-2005) Key Skills and Experience •Public Company CEO/CFO •Financial •Healthcare •Risk Management •Digital Other •Director of Center for Leadership Development •Director of Tessera Therapeutics | |
| | | | | |
Bristol Myers Squibb | 2025 Proxy Statement 16
| | |
|
| Item 1 – Election of the Board of Directors |
Karen H. Vousden, Ph.D.
Director Since: 2018
Age: 67
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Compensation and Management Development Committee •Science & Technology Committee | | | Other Public Company Boards •None | |
| | | | | |
| | | | | |
| Experience •Principal Group Leader at the Francis Crick Institute in London (2017-present) •Chief Scientist of Cancer Research UK (2016-2022) •Director of the Cancer Research UK (CRUK) Beatson Institute in Glasgow (2002-2016) •Held leadership roles at the National Cancer Institute in Maryland (1995-2002) Key Skills and Experience •Academia/Non-Profit •Healthcare •Science/Technology/Innovation •International Other •Founder and Consultant of Faeth Therapeutics, Inc. •Member of the Science Advisory Boards of the Allison Institute (MD Anderson, Texas), Kovina Therapeutics, the Ludwig Institute for Cancer Research and Volastra Therapeutics •Fellow of the Royal Society •Foreign Member of the U.S. National Academy of Sciences •Former President of the British Association of Cancer Research | |
| | | | | |
17 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Item 1 – Election of the Board of Directors |
Phyllis R. Yale
Director Since: 2019
Age: 67
| | | | | | | | | | | | | | | | | |
| | | | | |
| Board Committees •Audit Committee •Science & Technology Committee | | | Other Public Company Boards •Davita, Inc. | |
| | | | | |
| | | | | |
| Experience •Advisory Partner, Bain & Company (2010-present); Partner (1987-2010) •Since 1982, has served in a number of leadership roles and has been a leader in building Bain’s healthcare practice Key Skills and Experience •Financial •Risk Management •Healthcare •Academia/Non-Profit Other •Director of Aledade, Inc. •Member of the Advisory Board of the Health Policy and Management Department at the Harvard Chan School of Public Health •Member of the Board of The Trustees of Reservations, a conservation and preservation organization •Former director of Blue Cross Blue Shield of Massachusetts •Former member of the Advisory Board of Harvard Business School Healthcare Initiative | |
| | | | | |
| | | | | | | | | | | | | | |
| | | | |
| | | We ask in Item 1 for your voting support so we can continue our important work and build on our significant successes in 2025. | |
| | | | |
Bristol Myers Squibb | 2025 Proxy Statement 18
How We Are Selected
and Elected
The BMS management team puts a great deal of thought into talent recruitment and retention, and as directors, we are similarly committed to identifying and attracting the best directors to serve the Company. In the subsections that follow, we describe our standards, policies and processes designed to achieve this goal.
Majority Vote Standard and Director Resignation Policy
A majority of the votes cast is required to elect directors. To be eligible for nomination to the Board, all director nominees must submit an irrevocable resignation, contingent on (A) that person not receiving a majority of the votes cast in an election that is not a contested election, and (B) acceptance of that resignation by the Board in accordance with policies and procedures adopted by the Board for such purpose. In the event an incumbent director fails to receive a majority of the votes cast in an election that is not a contested election, the Committee on Directors and Corporate Governance, without participation by any director tendering their resignation, will make a recommendation to the Board as to whether to accept or reject the resignation of such incumbent director, or whether other action should be taken. The Board, without participation by any director tendering their resignation, will act on the resignation, taking into account the committee’s recommendation at its next regularly scheduled meeting to be held within 60 days after the certification of the shareholder vote. BMS will publicly disclose the Board’s decision and, if such resignation is rejected, the reasons for that decision in a broadly disseminated press release that will also be furnished to the U.S. Securities and Exchange Commission (SEC) on Form 8-K within 90 days after the certification of the shareholder vote. The Committee on Directors and Corporate Governance, in making its recommendation, and the Board, in making its decision, each may consider any factors and other information that they consider appropriate and relevant. If any nominee is unable to serve, proxies will be voted in favor of the remainder of those nominated and may be voted for substitute nominees, unless our Board of Directors provides for a lesser number of directors.
Criteria for Board Membership
As specified in our Corporate Governance Guidelines, members of our Board should have broad experience in areas important to the operation and long-term success of BMS. These include areas such as business, science, medicine, finance/accounting, law, business strategy, crisis management, risk management, corporate governance, education or government. Board members should possess qualities reflecting integrity, independence, leadership, good business judgment, wisdom, an inquiring mind, vision, a proven record of accomplishment and an ability to work well with others.
We generally restrict the number of other public company boards that non-employee directors and a director serving as chief executive officer can serve on to four and two, respectively, including our Board, and all of our directors are in compliance with these restrictions.
Director Independence
Our Corporate Governance Guidelines provide that a substantial majority of Board members be independent from management. The Board observes all relevant criteria established by the SEC and the New York Stock Exchange (NYSE) and has adopted independence standards that meet the listing standards of the NYSE (See Exhibit A). The Board has determined that, except for Christopher Boerner, Ph.D., who is our Chief Executive Officer, each of our directors and each director nominee for election at this Annual Meeting is independent of BMS and its management.
| | | | | | | | |
| | |
| Independence | |
| 10 | |
| out of 11 director nominees are currently independent | |
| | |
19 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| How We Are Selected and Elected |
Process for Determining Independence
In accordance with our Corporate Governance Guidelines, the Board undertakes an annual review of director independence. Under our Corporate Governance Guidelines and the NYSE listing standards, a director is not independent unless the Board affirmatively determines that he or she does not have a direct or indirect material relationship with the Company. In February 2025, the Board considered all commercial and charitable relationships of our independent directors and director nominees, including the following relationship, which was deemed immaterial under our categorical standards:
•Mr. Arduini was appointed President and Chief Executive Officer of GE Healthcare in January 2022. BMS has a prior business relationship with GE Healthcare, pursuant to which we made ordinary course of business payments to GE Healthcare in 2024, including related to some early development and license agreements. All of the business dealings were entered into on terms no more favorable to GE Healthcare than terms that would be available to unaffiliated third parties under similar circumstances, and the payments made by BMS did not exceed the greater of $1 million or 2% of GE Healthcare’s consolidated gross revenues.
The Board determined that none of our independent directors had any relationships that would impair their independence under the NYSE’s independence standards or otherwise.
Director Succession Planning and Identification of Board Candidates
Regular Assessment of Our Board Composition
The Committee on Directors and Corporate Governance regularly assesses the appropriate size and composition of the Board. This assessment incorporates the results of the Board’s annual evaluation process, which are described more fully under “Annual Evaluation Process” beginning on page 22. The Committee also considers succession planning for its directors. Identification and Selection of Director Nominees
In connection with the Board’s ongoing director identification process, the Committee on Directors and Corporate Governance, in consultation with the Board Chair, conducts an initial evaluation of prospective nominees against the established Board membership criteria discussed above. The Committee also reviews the skills of the current directors and compares them to the particular skills of potential candidates. In particular, the Board is committed to identifying and evaluating all highly qualified candidates for Board positions, including candidates with varied backgrounds, industry experiences and personal characteristics. In addition, the Committee annually evaluates each current director’s prior service on and contributions to the Board, which includes consideration of each director’s attendance, job responsibilities, service on other public company boards, and leadership positions on such boards prior to recommending a director or nominee for election to our Board.
Bristol Myers Squibb | 2025 Proxy Statement 20
| | |
|
| How We Are Selected and Elected |
Candidates may come to the attention of the Committee on Directors and Corporate Governance through current Board members, third-party search firms, management, shareholders or others. Search firms together with management and directors develop a candidate profile that includes the relevant skills and experiences being sought at that time and incorporates the Board membership criteria. Prospective candidates are identified based on that profile. Additional information relevant to the qualifications of prospective nominees may be requested from third-party search firms, other directors, management or other sources. After this initial evaluation, prospective nominees may be interviewed by telephone or in person by the members of the Committee on Directors and Corporate Governance, the Board Chair, the Lead Independent Director and other directors, as applicable. After completing this evaluation and interview process, the Committee on Directors and Corporate Governance makes a recommendation to the full Board as to the persons who should be nominated by the Board, and the full Board determines the nominees after considering the recommendation and any additional information it may deem appropriate.
Following a robust process that began in 2023, Mr. McMullen was elected to join the Board, effective July 1, 2024. He was identified as a potential candidate for election to the Board by a third-party search firm retained by the Committee on Directors and Corporate Governance and was subsequently interviewed by members of the Board.
Shareholder Nominations for Director
In accordance with the Company’s Bylaws and other governing documents, the Board considers and evaluates qualified shareholder recommendations and nominations of candidates for election to the Board in substantially the same manner as other director nominees and will not impose unnecessary requirements for such candidates. Shareholder recommendations must be accompanied by disclosure, including written information about the recommended nominee’s business experience and background with consent in writing signed by the recommended nominee that he or she is willing to be considered as a nominee and, if nominated and elected, he or she will serve as a director. Shareholders should send their written recommendations of nominees accompanied by the required documents to: Bristol-Myers Squibb Company, Route 206 & Province Line Road, Princeton, NJ 08543, Attention: Corporate Secretary.
Proxy Access Shareholder Right
Following extensive engagement with our shareholders, the Board determined to adopt proxy access in 2016, permitting a shareholder or group of up to 20 shareholders, in each case, holding at least 3% of our outstanding shares of common stock for at least three years, to nominate a number of directors constituting the greater of two directors or 20% of the number of directors on the Board, as set forth in detail in our Bylaws. If you wish to propose any action pursuant to our proxy access bylaw provision, you must deliver a notice to BMS containing certain information set forth in our Bylaws, not less than 120 but not more than 150 days before the anniversary of the prior year’s filing of the proxy materials. For our 2026 Annual Meeting, we must receive this notice between October 27, 2025, and November 26, 2025. Shareholders should send their notices to our principal executive office: Bristol-Myers Squibb Company, Route 206 & Province Line Road, Princeton, NJ 08543, Attention: Corporate Secretary.
21 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| How We Are Selected and Elected |
Annual Evaluation Process
The Board recognizes the critical role Board and Committee evaluations play to ensure the effective functioning of the Board. We also believe in the importance of continuously improving the functioning of the Board and Board Committees. Under the leadership and guidance of our Lead Independent Director, the Committee on Directors and Corporate Governance annually reviews the Board evaluation process. For the last few years, the formal Board evaluation process has included a written questionnaire for the Board and the Board Committees. In addition to the Board and Board Committee questionnaires, for individual director assessments, the Lead Independent Director engages in one-on-one discussions with each director regarding, without limitation, governance, Board functioning and effectiveness, interaction with management, meetings and materials and performance. The Lead Independent Director conveys directors’ feedback on an ongoing basis to the Board Chair and has regular one-on-one discussions with the other members of the Board. The formal 2024 Board and Committee evaluation processes were as follows:
•Board: Directors completed an electronic questionnaire on an unattributed basis responding to questions about the Board and Committee structure and responsibilities, Board culture and dynamics, adequacy of information to the Board, Board skills and effectiveness, and Committee effectiveness. In addition, the Lead Independent Director completed one-on-one individual director assessments guided by predetermined questions covering the foregoing topics. The robust feedback and comments from the directors were anonymously compiled and then presented by the Board Chair and the Lead Independent Director to the full Board for discussion and action. The 2024 Board evaluation was completed in February 2025.
•Committees: Committee members completed an electronic questionnaire, which included questions approved by each Committee chair with topics covering each Committee’s composition, culture, and functioning as well as each Committee’s responsibilities and effectiveness. The results from the questionnaires were compiled and Committee chairs led discussions in executive sessions of their respective committees. Committee chairs then reported to the full Board the results of their respective committee’s evaluation and any follow-up actions. The 2024 Committee evaluations were completed in the beginning of 2025 and reported to the Board in February 2025.
Bristol Myers Squibb | 2025 Proxy Statement 22
How We Govern and Are Governed
Director Orientation and Continuing Education
Director education is an ongoing, year-round process, which begins when a director joins the Board. Upon joining the Board, new directors are provided with a comprehensive orientation program to learn about our company, including our business, strategy and governance. The orientation program is led by senior business and functional leaders from all areas of the company, where strategic priorities and key risks and opportunities are discussed. All of our directors periodically attend site visits to one or more of our locations. On an ongoing basis, our directors receive presentations on a variety of topics related to their work on the Board and within the biopharmaceutical industry, both from senior management and from experts outside of the company. We also encourage all of our directors to enroll, at our expense, in continuing education programs provided by third parties.
Active Board Oversight of Our Governance
Our business is managed under the direction of the Board pursuant to the Delaware General Corporation Law and our Bylaws. The Board has responsibility for establishing broad corporate policies and for the overall performance of our company. The Board keeps itself informed of company business through regular written reports and analyses from management, and regular discussions with the Chief Executive Officer and other company officers; by reviewing other materials provided by management and by external advisors; and by participating in Board and Board Committee meetings.
The Committee on Directors and Corporate Governance continually reviews corporate governance topics, trends, and is responsible for identifying and recommending the adoption of corporate governance initiatives. In addition, our Compensation and Management Development Committee regularly reviews our compensation policies and procedures and, when appropriate, recommends changes that strengthen our compensation practices.
The Board has adopted Corporate Governance Guidelines that govern its operation and that of its committees. The Board annually reviews the Corporate Governance Guidelines and, from time to time, revises them in response to changing regulatory requirements, evolving best practices and feedback from our shareholders and other stakeholders.
Board’s Role in Strategic Planning and Risk Oversight
The Board meets regularly to discuss our company’s strategic direction and the issues and opportunities facing our company in light of trends and developments in the biopharmaceutical industry and the broader business environment. The Board has been instrumental in determining our short-term and long-term company strategy.
The Board is dedicated to oversight of risk management and is responsible for risk oversight as part of its fiduciary duty of care to monitor business operations effectively. Specifically, the Board plays a critical role in the determination of the types and appropriate levels of risk undertaken by the company. Some of the key risks the Board is focused on relate to: (i) potential legislative or other regulatory actions impacting the pharmaceutical industry in the U.S. and internationally, including related to drug pricing and access; (ii) intellectual property protection and upcoming losses of exclusivity; (iii) competition; (iv) business continuity; (v) key environmental, social and governance risks, inclusive of human capital management, covering, among other things, compensation program design, and our Sustainability and Social Impact initiatives; and (vi) information technology, including cybersecurity, data privacy and artificial intelligence, among others.
23 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| How We Govern and Are Governed |
The Board administers its strategic planning and risk oversight function as a whole and through its Board Committees. The following are examples of how the Board Committees are involved in this process:
| | | | | | | | | | | | | | |
| | | | |
| Audit Committee | | Regularly reviews and discusses with management our policies and guidelines regarding risk assessment and risk management, including their effectiveness, our process for mitigating and monitoring enterprise risks, including those related to market/environment, strategic, financial, operational, legal, compliance, regulatory, cybersecurity and reputational risks. With respect to cybersecurity risk, the Audit Committee receives regular updates from management on matters related to cybersecurity and data privacy incidents. Our Chief Information Security Officer also provides updates on significant threats to our systems, risk mitigation strategies, program assessments, planned improvements, and status of information security initiatives. | |
| | | | |
| | | | |
| Compensation and Management Development Committee | | Annually evaluates our incentive compensation programs and determines whether incentive pay encourages excessive or inappropriate risk-taking. In particular, the Committee evaluates the components of our executive compensation program that work to minimize excessive or inappropriate risk-taking, including the use of different forms of long-term equity incentives, linking incentive payout opportunities to each executive’s demonstration and role modeling of our BMS Values, placing caps on our incentive award payout opportunities, requiring clawback/recoupment of incentive compensation when our policies are violated, following equity grant practices that limit potential for timing awards and having stock ownership and retention requirements. With such measures in place, we reduce the sensitivity to short-term performance and appropriately balance risk and reward in a manner that does not encourage our executives to expose the company to imprudent risk-taking. These elements work to ensure our compensation program is properly aligned with our strategy and the interests of our shareholders. | |
| | | | |
| | | | |
| Committee on Directors and Corporate Governance | | Focuses on risks associated with corporate governance, Board refreshment, Board succession planning and regularly considers and makes recommendations to the Board concerning the appropriate size, function and needs of the Board; determines the criteria for Board membership; provides oversight of our corporate governance affairs and reviews corporate governance practices and policies to manage related risks. Identifies and oversees monitoring and management of risks related to the company’s political activities; environmental, social and governance strategy and reporting and the impact on the company’s employees and shareholders. | |
| | | | |
| | | | |
| Science and Technology Committee | | Regularly reviews our pipeline and potential business development opportunities to evaluate our progress in achieving our near-term and long-term strategic R&D goals and objectives and assures that we make well-informed choices in the investment of our R&D resources, among other things. | |
| | | | |
Bristol Myers Squibb | 2025 Proxy Statement 24
| | |
|
| How We Govern and Are Governed |
The Board and Board Committees engage regularly with management on risks to the business, including the risks described above.
| | | | | | | | | | | | | | | | | |
| | | | | |
| Annual strategy deep-dive Specifically, each year, typically during the second and fourth quarters, the Board holds extensive meetings with senior management dedicated to discussing and reviewing: •our long-term operating plans, and •overall corporate strategy. As part of the meeting, our Chief Executive Officer leads: •a discussion of key risks to the plans, and •risk mitigation plans and activities. | | | Constant focus on strategy Throughout the year, the Board provides: •guidance to management on strategy, and •helps to refine operating plans to implement the strategy. In 2024, the Board met 9 times (7 regular and 2 special meetings) and held other information sessions to discuss the company’s execution of our strategy to renew our product pipeline with new life-changing medicines, including: •executing on key launches to enhance and diversify our portfolio, •progressing our pipeline, focusing on transformational medicines where we have a competitive advantage, •driving operational excellence throughout the organization, •succession planning and compensation matters, and •strategically allocating capital for long-term growth and shareholder returns, including entering into business development transactions that extend our scientific leadership and advance value creation. | |
| | | | | |
25 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| How We Govern and Are Governed |
Risk Assessment of Compensation Policies and Practices
The Compensation and Management Development Committee of the Board annually conducts a worldwide review of our material compensation policies and practices. Based on this review, the Committee concluded that our material compensation policies are appropriately balanced and do not incentivize imprudent risk taking and are therefore not likely to have a material adverse effect on the company. In fact, our global compensation policies and practices contain many design features that mitigate the likelihood of inducing excessive or inappropriate risk-taking behavior. These features include:
| | | | | | | | | | | |
| | | |
| ✓ | Balance of fixed and variable compensation, with variable compensation tied both to short-term objectives and the long-term value of our stock price | ✓ | Clawback and recoupment provisions and policies pertaining to annual incentive payouts and long‑term incentive awards |
| | | |
| | | |
| ✓ | Multiple metrics in our incentive programs that balance top‑line, bottom‑line and pipeline performance | ✓ | Share ownership and retention guidelines applicable to our senior executives |
| | | |
| | | |
| ✓ | Caps in our incentive program payout formulas | ✓ | Equity award policies that limit risk by having fixed annual grant dates |
| | | |
| | | |
| ✓ | Reasonable goals and objectives in our incentive programs | ✓ | Prohibition of speculative and hedging transactions by all employees and directors |
| | | |
| | | |
| ✓ | Compensation opportunity linked to each executive’s demonstration and role modeling of our BMS Values | ✓ | The participation by all employees worldwide—other than sales roles—in the same annual bonus plan generally applicable to our Named Executive Officers and that has been approved by the Compensation and Management Development Committee |
| | | |
| | | |
| ✓ | The Compensation and Management Development Committee’s ability to exercise discretion in determining incentive program payouts | ✓ | Mandatory training on our Principles of Integrity: BMS Standards of Business Conduct and Ethics (the Principles of Integrity) and other policies that educate our employees on appropriate behaviors and the consequences of taking inappropriate actions and where to escalate concerns anonymously |
| | | |
| | | |
| ✓ | Prohibition on timing the disclosure of material non-public information, or the granting of equity or equity-based grants, for the purpose of impacting the value of executive compensation | | |
| | | |
Meetings of the Board & Director Engagement
The Board meets on a regularly scheduled basis during the year to review significant developments affecting Bristol Myers Squibb and to act on matters requiring Board approval. It also holds special meetings when important matters require Board action between regularly scheduled meetings. Members of senior management regularly attend Board meetings to report on and discuss their areas of responsibility.
In 2024, the Board met 9 times (7 regular and 2 special meetings). The average aggregate attendance of directors at Board and Board Committee meetings was over 96%. No director attended fewer than 85% of the aggregate number of Board and Board Committee meetings during the period he or she served.
Bristol Myers Squibb | 2025 Proxy Statement 26
| | |
|
| How We Govern and Are Governed |
During these meetings, our independent directors met in executive sessions to discuss many topics, including advancing our strategy to renew our product pipeline with new life-changing medicines, executing on key launches that enhance and diversify our portfolio, progressing our pipeline with a focus on transformational medicines where we have a competitive advantage, driving operational excellence throughout the organization, succession planning and compensation matters, and strategically allocating capital for long-term growth and shareholder returns, including entering into important collaborations and exciting acquisition opportunities to extend our scientific leadership and advance value creation, among other things.
The Board and Board Committees also held information sessions throughout 2024, which supplemented the regularly scheduled Board and Board Committee meetings. These information sessions were especially important during 2024 and allowed the Board to provide effective oversight and support to our management team. For 2025, the Board and Board Committees will continue to supplement their regular meetings with information sessions as needed.
It is the expectation of the Board that each director has sufficient time to attend, prepare for and participate during Board and Board Committee meetings. The Committee on Directors and Corporate Governance periodically reviews the outside board service of our directors and has adopted internal procedures to address when a director’s outside public board service exceeds the limit included in the company’s Corporate Governance Guidelines.
As part of its annual review of director nominees for election, the Board reviews the attendance and other commitments, professional background and experience, as well as the valued contributions of individual directors, among other things. Mr. Rice and Ms. Price are each a current member of the audit committee of three other public company boards. The Board has determined that such simultaneous service does not impair the ability of Mr. Rice or Ms. Price to effectively serve on our Audit Committee, with Mr. Rice and Ms. Price recusing themselves from such determinations.
Annual Meeting of Shareholders
Directors are strongly encouraged, but not required, to attend the Annual Meeting of Shareholders. All of the 2025 nominees for director who were directors as of the 2024 Annual Meeting attended our 2024 Annual Meeting of Shareholders.
Codes of Conduct
The Principles of Integrity adopted by the Board set forth important company policies and procedures in conducting our business in a legal, ethical and responsible manner. These standards are applicable to all of our employees, including the Chief Executive Officer, the Chief Financial Officer and the Controller.
In addition, the Audit Committee has adopted the Code of Ethics for Senior Financial Officers that supplements the Principles of Integrity by providing more specific requirements and guidance on certain topics. The Code of Ethics for Senior Financial Officers applies to the Chief Executive Officer, the Chief Financial Officer, the Controller, the Treasurer and the heads of major operating units.
The Board has also adopted the Code of Business Conduct and Ethics for Directors that applies to all directors and sets forth guidance with respect to recognizing and handling ethical issues.
The Principles of Integrity, the Code of Ethics for Senior Financial Officers and the Code of Business Conduct and Ethics for Directors (collectively, the “Codes of Conduct”) are available on our website at https://www.bms.com/about-us/our-company/our-principles.html. We will post any substantive amendments to, or waivers from, applicable provisions of our Codes of Conduct on our website at https://www.bms.com/about-us/our-company/our-principles.html within two days following the date of such amendment or waiver.
Employees are required to report any conduct they believe in good faith to be an actual or apparent violation of our Codes of Conduct. In addition, as required under the Sarbanes-Oxley Act of 2002, the Audit Committee has established procedures to receive, retain and treat complaints received regarding accounting, internal accounting controls, or auditing matters and the confidential, anonymous submission by company employees of concerns regarding questionable accounting or auditing matters.
27 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| How We Govern and Are Governed |
BMS has a securities trading policy governing the purchase, sale, and other disposition of the company’s securities that applies to all directors, officers, employees, and certain other covered persons and their immediate family members. Additionally, BMS has a share repurchase program, authorized by the Board, allowing for repurchases of its shares, effected in the open market or through privately negotiated transactions in compliance with Rule 10b-18 under the Exchange Act, including through Rule 10b5-1 trading plans. We believe our securities trading policy and share repurchase program are reasonably designed to promote compliance with insider trading laws, rules, and regulations and NYSE listing standards. A copy of our securities trading policy was filed as Exhibit 19 to our Annual Report on Form 10-K for the year ended December 31, 2024.
Related Party Transactions
The Board has adopted a written policy and procedures for the review and approval of transactions involving the company and related parties, such as greater than 5% shareholders, directors, executive officers and their immediate family members. The policy and procedures cover any transaction or series of transactions (an “interested transaction”) in which the amount involved exceeds $120,000, the company is a participant, and a related party has a direct or indirect material interest (other than solely as a result of being a director or less than 10% beneficial owner of another entity). All interested transactions are subject to approval in accordance with the following policy and procedures:
•Management is responsible for determining whether a transaction is an interested transaction requiring review under this policy, in which case the transaction is disclosed to the Committee on Directors and Corporate Governance (the “Governance Committee”).
•The Governance Committee reviews the relevant facts and circumstances, including, among other things, whether the interested transaction is on terms no less favorable than terms generally available to an unaffiliated third-party under the same or ordinary circumstances and the related party’s interest in the transaction.
•No director participates in any discussion or approval of the interested transaction for which he or she is a related party, except that the director will provide all material information concerning the interested transaction to the Governance Committee.
•If an interested transaction is ongoing, the Governance Committee may establish guidelines for management to follow in its ongoing dealings with the related party and will review and assess such ongoing relationships on at least an annual basis.
•Certain types of interested transactions are deemed to be pre-approved by the Governance Committee, as applicable, even if the amount involved will exceed $120,000, including the employment of executive officers, director compensation, certain transactions with other companies or charitable contributions, transactions where all shareholders receive proportional benefits, transactions involving competitive bids, regulated transactions and certain banking-related services.
BlackRock, Inc. (“BlackRock”) and The Vanguard Group (“Vanguard”) are each considered a “Related Party” under our related party transaction policy because they each beneficially own more than 5% of our outstanding common stock. The Governance Committee approved the following related party transactions in accordance with our related party policy and procedures and Bylaws:
•Certain of our retirement plans use BlackRock and its affiliates to provide investment management services. In addition, we have certain investments in BlackRock-managed investment funds. In connection with these services, we paid BlackRock approximately $1.12 million in fees during 2024.
•Vanguard acts as an investment manager with respect to certain investment options under our savings and thrift plans. Participants in the plans pay Vanguard’s investment management fees if they invest in investment options managed by Vanguard; neither the plans themselves nor the company pays fees directly to Vanguard. In connection with these services, Vanguard received approximately $680,000 in fees during 2024.
Bristol Myers Squibb | 2025 Proxy Statement 28
| | |
|
| How We Govern and Are Governed |
The Governance Committee approved the above relationships on the basis that these entities’ ownership of our stock plays no role in the business relationship between us and them, and that the engagement of each entity was on terms no more favorable to them than terms that would be available to unaffiliated third parties under the same or similar circumstances.
Disclosure Regarding Political Activities
We provide semi-annual disclosure on our website at the link below of all political contributions to political committees, parties or candidates on both state and federal levels that are made by our employee political action committee, as well as annual disclosure of the portion of our dues or other payments made to trade associations to which we give $50,000 or more that can be attributed to lobbying expenditures.
Please see the company's website at: https://www.bms.com/about-us/sustainability/economic-responsibility/political-contributions.html under “Political Contributions.”
Sustainability and Social Impact
At BMS, our vision is to transform the lives of patients through science. We understand the future of our employees, our communities, our planet, and our business are inextricably linked. As a leading biopharma company, our passion for making an impact extends beyond the discovery, development and delivery of innovative medicines that help patients prevail over serious diseases. Through our environmental, social and governance (Sustainability and Social Impact) strategy, we seek to mobilize our capabilities and resources to positively impact the communities where we live, work, and serve around the world.
As we work to transform patients’ lives through science, we operate with effective governance, uncompromising quality and compliance, and the highest ethical standards to deliver our mission. These values have been central to who we are, what we do, and how we do it since our company was founded in the 1800s. We believe that driving long-term business value is at the heart of living our purpose, enabling us to be leaders and difference-makers for generations to come.
Being able to deliver scientific breakthroughs for patients starts with a strong governance profile, which includes direct oversight of Sustainability and Social Impact opportunities and risks, and relevant disclosure by our Committee on Directors and Corporate Governance. Oversight by this Committee strengthens our ability to operate with the highest levels of quality, integrity and ethics, a critical element of our Sustainability and Social Impact strategy.
Our Sustainability and Social Impact strategy is fully aligned with our corporate strategy and was established by, and is regularly updated, following a formal assessment of priority issues drawn from the Board of Directors, senior executives, employees, and external stakeholder groups such as patient advocacy organizations, shareholders, suppliers, multinational organizations, and academic institutions. As described in more detail below, our Sustainability and Social Impact strategy focuses on three core pillars: advancing patient health around the world, fostering a high-performing and inclusive global workforce to expand the boundaries of science, patient access and impact, and championing strong governance, engagement, accountability and transparency to support a culture of quality, integrity and ethics. Specifically, our strategy is centered on expanding the boundaries of science to address high unmet patient needs, advancing access for all patients to life-transforming medicines, while doing our part to reduce our environmental impact.
Advancing Patient Health Around the World
Patients, regardless of where they live, still encounter challenges to accessing medicines and healthcare services. We believe in long-term sustainable solutions to address health inequities globally, and we are allocating specific resources and developing new pathways and models to expand access to patients in low-, middle- and high-income countries.
As part of our ASPIRE (Accessibility, Sustainability, Patient-centric, Impact, Responsibility and Equity) strategy, BMS is developing initiatives to increase the affordability and availability of its medicines in low- and middle-income countries (LMICs). Our work to help ensure patients who need our potentially life-saving medicines can access them begins by ensuring that our marketed products are supported by access plans. Today we have defined access pathways for every country in which our medicines may be available to patients.
29 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| How We Govern and Are Governed |
With ASPIRE, we have identified a strategic opportunity to align our goal to expand access to our life-saving medicines with our international growth strategy. Among our strategic imperatives, we are focused on (i) expanding access to products with significant patient impact, lifecycle durability, and feasible risk management from our core brands or emerging market brands; (ii) tailoring access approaches to reflect the economic realities of LMICs and patients’ ability to pay; and (iii) maintaining sustainability for the different access pathways to ensure long-term viability.
By doing this, we are delivering on our commitment to reduce health inequities and continue to evolve our business to best meet the needs of our patients. Equitable access to innovative medicines benefits patients and society, while ensuring BMS continues to transform patients’ lives through science.
Fostering a High-Performing and Inclusive Global Workforce Expands the Boundaries of Science, Patient Access and Impact
We believe that a high-performing and engaged global workforce is essential to delivering the best possible outcomes for our patients. Our BMS Values—Integrity, Urgency, Accountability, Innovation, Passion, and Inclusion—are the foundation of our high-performing, patient-centric culture. Moreover, a workforce fueled by varied backgrounds, experiences, personal characteristics and viewpoints helps us expand our patient population, because it drives strategy designed to deliver greater access to varied clinical trial patients across geographic regions and address the changing demographics of the communities where we do business and the patients we serve. For further discussion, please see “People and Culture” beginning on page 49. We are committed to scientific excellence and investment in our R&D capabilities to provide more medicines to more patients faster. We leverage our expertise to accelerate drug discovery and development, and we entrust our talented researchers and innovators with the flexibility to drive research and development forward to meet unmet patient needs. With one of the most diversified portfolios and pipelines in the pharmaceutical industry, we are uniquely positioned to drive continued innovation and expand treatment options across therapeutic areas based on our differentiated research platforms which include cell therapy and targeted protein degradation.
We recognize the importance of enrolling clinical trial populations that are more reflective of broader patient populations and aligned with the epidemiology of the diseases we study. In doing so, we can better address barriers to health equity and deepen our clinicians’ understanding of the safety and efficacy of investigational medicines for all people. In 2024, approximately 43 percent of our clinical trials sites in the U.S. were located in diverse metro areas. The Bristol-Myers Squibb Foundation established a national program to train, engage, and support 250 early-stage investigator physicians and 250 medical students to help transform patient representation in the clinical trial landscape.
Championing Strong Governance, Engagement, Accountability and Transparency Supports a Culture of Quality, Integrity and Ethics at the Company
Our robust governance model includes oversight by the Board of Directors and the BMS Sustainability and Social Impact Council, a cross-functional management committee comprised of senior executives and subject matter experts from across the company, which reports to the Company’s Executive Leadership Team and Board of Directors.
•The work to advance all of our commitments is built on the foundation and legacy of ethical business conduct and acting with integrity in everything we do. Businesses are built on relationships and centered on trust, and the way we conduct ourselves reflects our company values. Good governance is vital to our success, and we aim to meet or exceed best practices, providing transparency and accountability to all of our stakeholders.
•We hold ourselves accountable to the highest patient safety and product quality standards. Our extensive quality and safety monitoring processes ensure that the integrity of our products and services meet or exceed expectations, as well as applicable laws and regulations. We are dedicated to achieving quality excellence through the relentless pursuit of continuous improvement.
Bristol Myers Squibb | 2025 Proxy Statement 30
| | |
|
| How We Govern and Are Governed |
As one of the world’s leading biopharma companies, we know we have a responsibility to help inform and influence public health and public policy to help strengthen healthcare systems around the world. Addressing health equity and access requires comprehensive strategies that encompass policy changes, healthcare system reforms and community engagement and education.
•We remain committed to working with policymakers, thought leaders, patient advocates and other stakeholders to shape a comprehensive system that provides accessible and affordable health care, while continuing to fuel innovation.
•At the same time, we support conscientious corporate citizenry and philanthropic efforts that make a positive difference in the world. Through charitable donations, corporate sponsorships, scholarship and fellowship support, independent patient and medical education, community engagement, and employee volunteerism, we are united in a commitment to invest our human and fiscal resources in local areas and to help build and maintain equitable healthcare systems.
We are acutely aware of our responsibility to minimize the impact of our operations on the environment to preserve the planet for future generations. Healthier environments support healthier people, which is why we seek out actionable solutions that minimize our environmental footprint and address the harmful effects of environmental degradation and climate change on public health.
•We have designed and implemented environmental goals that not only reflect our science-led, innovation-focused approach but ensure accountability to those we serve through strong governance and transparent reporting practices.
•In 2024, BMS received validation of both our near-term and our net-zero greenhouse gas emissions reduction targets from the Science Based Target initiative (SBTi). Our 2024 Climate Change Report (formerly the TCFD Report) highlights how we are continuing to understand and take action on our climate-related risks and opportunities.
•Importantly, our Sustainability and Social Impact strategy supports long-term value creation, and we are committed to adapting our business model to ensure we are best positioned to address the shared challenges that society faces, and governance issues—and to do so with a science-led, innovation-focused approach that is rooted in health equity.
To track our performance and enhance our transparency with our stakeholders, we regularly disclose our progress toward our Sustainability and Social Impact initiatives. Our reporting suite includes our annual Sustainability and Social Impact Report that is aligned with the Sustainability Accounting Standards Board (SASB), U.N. Sustainable Development Goals (SDGs), United Nations Global Compact Communication on Progress (COP), Carbon Disclosure Project (CDP) and the Task Force on Climate-Related Financial Disclosures (TCFD), as well as reporting to leading raters and rankers.
Among other highlights, in 2024, we continued to refine our governance structure by:
•enhancing transparency with greater voluntary reporting,
•building capabilities to align with the Corporate Sustainability Reporting Directive (CSRD), including completing a Double Materiality Assessment, and
•launching a new sustainability operating model to maximize impact.
For 2024, we again selected certain goals from our Sustainability and Social Impact commitments as a weighted metric for the measurement of company performance as part of our senior executives’ annual incentive program. We believe that creating joint executive accountability to achieve our Sustainability and Social Impact commitments is consistent with our mission as a leading purpose-driven biopharma company.
| | | | | | | | | | | |
| | | |
| | For more information please see our “Sustainability and Social Impact” Report on our website at https://www.bms.com/about-us/sustainability.html. | |
| |
| | | |
31 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| How We Govern and Are Governed |
Maintaining Transparency in Governance for our Drug Pricing & Patent Strategies
We take a thoughtful approach to pricing our products and exercising our legitimate intellectual property rights. We have internal processes and controls in place to ensure that decisions are thoroughly and appropriately vetted with the highest levels of management and are in line with our values and commitment prior to implementation. This process includes routine presentations to the Board on our drug pricing and patent strategies.
•The prices of our medicines reflect the value they deliver for patients, healthcare systems and society, as well as our investment in scientific innovation.
•We obtain intellectual property only by lawful and ethical means and enforce only those intellectual property rights we believe are valid. We place the highest priority on obtaining intellectual property for those innovations that provide the greatest medical benefit to patients.
| | | | | | | | | | | |
| | | |
| | For more information on our drug pricing and intellectual property transparency, please see “Position on Key Issues” on our company website at https://www.bms.com/about-us/responsibility/position-on-key-issues.html. | |
| | | |
Bristol Myers Squibb | 2025 Proxy Statement 32
How We Are Organized
Board Leadership Structure
Our governance documents provide the Board with flexibility to select the appropriate Board leadership structure for the company. They establish well-defined responsibilities with respect to the Board Chair and Lead Independent Director roles, including the requirement that the Board have a Lead Independent Director if the Board Chair is not an independent director. This information is set forth in more detail on our website at https://www.bms.com/about-us/our-company/governance.html.
The Board has dedicated significant consideration to our leadership structure, evaluating such structure annually. The Board’s most recent analysis of its leadership structure involved consideration of many factors, including the specific needs of the Board and the company, the strong role of our Lead Independent Director, our Corporate Governance Guidelines (including our governance practices that provide for independent oversight of management), the integration of acquired businesses into our company, the challenges specific to our company and the best interests of our shareholders.
Since joining the company in early 2015, Dr. Boerner has proven himself to be an exceptional leader and has been instrumental in shaping our strategy and our culture. His passion for science, his commitment to our workforce and his tireless focus on what matters most — our patients — make him uniquely suited for the role of Board Chair and Chief Executive Officer. Further, his deep knowledge of our strategy and pipeline, and proven execution across all geographies, make us confident that he is the right person to guide BMS through its next stage of growth and support our vision to be the world’s leading biopharma company that transforms patients’ lives through science.
After thoughtful and rigorous consideration, the Board determined that combining the Board Chair and Chief Executive Officer positions and electing Dr. Boerner as the Board Chair continues to be in the best interests of the company and our shareholders and is the best leadership for the company and its shareholders at this time. Specifically, our Board believes having Dr. Boerner serve in the combined role of Board Chair and Chief Executive Officer confers distinct advantages, including:
•having a Board Chair who can draw on detailed institutional knowledge of the company and industry experience from serving as Chief Executive Officer, providing the Board with focused leadership, particularly in discussions about the company’s strategy;
•a combined role ensures that the company presents its message and strategy to all stakeholders, including shareholders, employees and patients, with a unified voice; and
•the structure allows for efficient decision-making and focused accountability.
The Board recognizes the importance of appointing a strong Lead Independent Director to maintain a counterbalancing structure to ensure that the Board functions in an appropriately independent manner. The Lead Independent Director is selected annually by the independent directors. The independent directors of the Board have elected Mr. Samuels to serve in that position.
33 Bristol Myers Squibb | 2025 Proxy Statement
The Lead Independent Director’s responsibilities include, among others:
| | | | | | | | | | | |
| | | |
| ✓ | Serving as liaison between the independent directors and the Board Chair and Chief Executive Officer | ✓ | Approving the quality, quantity and timeliness of information sent to the Board |
| | | |
| | | |
| ✓ | Reviewing and approving meeting agendas and sufficiency of time | ✓ | Serving a key role in Board and Chief Executive Officer evaluations |
| | | |
| | | |
| ✓ | Calling meetings of the independent directors | ✓ | Responding directly to shareholder and stakeholder questions, as appropriate |
| | | |
| | | |
| ✓ | Presiding at all meetings of the independent directors and any Board meeting when the Board Chair and Chief Executive Officer is not present, including executive sessions of the independent directors | ✓ | Providing feedback from executive sessions of the independent directors to the Board Chair and Chief Executive Officer and other senior management |
| | | |
| | | |
| ✓ | Engaging with major shareholders, as appropriate | ✓ | Recommending advisors and consultants |
| | | |
The Board’s culture is open and promotes transparent dialogue and rigorous discussion. The Board deliberates on all major decisions with and without management present and effectively utilizes executive sessions under the leadership of the Lead Independent Director to drive board alignment.
The Board believes this structure provides an effective, high-functioning Board, as well as appropriate safeguards and oversight.
The Board will continue to evaluate its leadership structure in light of changing circumstances and will do so on at least an annual basis and make changes at such times as it deems appropriate.
| | | | | | | | | | | |
| | | |
| | Please see “Board’s Role in Strategic Planning and Risk Oversight” on page 23. | |
| | | |
Committees of Our Board
Our Bylaws specifically provide for an Audit Committee, Compensation and Management Development Committee, and Committee on Directors and Corporate Governance, all of which are composed entirely of independent directors. Our Bylaws also authorize the establishment of additional committees of the Board and, under this authorization, our Board established the Science and Technology Committee. The Board has appointed individuals from among its members to serve on these four standing committees and each committee operates under a written charter adopted by the Board, as amended from time to time. These charters are published on our website at https://www.bms.com/about-us/our-company/governance/board-committees-and-charters.html. Each of these Board Committees has the necessary resources and authority to discharge its responsibilities, including the authority to retain consultants or experts to advise the committees.
Bristol Myers Squibb | 2025 Proxy Statement 34
The table below indicates the current members and Chairs of our standing Board Committees and the number of meetings held in 2024:
| | | | | | | | | | | | | | | | | |
| Director | Audit Committee(1) | Committee on Directors and Corporate Governance | Compensation and Management Development Committee | Science and Technology Committee |
| | | | | |
| Peter J. Arduini(2) | | | C | |
| Deepak L. Bhatt, M.D., M.P.H., M.B.A. | | | l | l |
| Christopher S. Boerner, Ph.D.(3) | | | | |
| Julia A. Haller, M.D. | | l | | C |
| Manuel Hidalgo Medina, M.D., Ph.D. | | l | | l |
| Michael R. McMullen(4) | l | | | |
| Paula A. Price | l | l | | |
| Derica W. Rice | C | | l | |
| Theodore R. Samuels | l | C | | |
| Karen H. Vousden, Ph.D. | | | l | l |
| Phyllis R. Yale(5) | l | l | | |
| Number of 2024 Meetings | 8 regular meetings | 3 regular meetings | 6 regular meetings | 5 regular meetings |
“C” indicates Chair of the committee.
(1)Our Board has determined, in its judgment, that all members of the Audit Committee are financially literate and that all members of the Audit Committee meet additional, heightened independence criteria applicable to directors serving on audit committees under the New York Stock Exchange listing standards and applicable SEC rules. In addition, our Board has determined that Messrs. Rice, McMullen and Samuels and Ms. Price each qualify as an “audit committee financial expert” under the applicable SEC rule.
(2)Effective after the 2024 Annual Meeting, Mr. Arduini rotated from our Science & Technology Committee and became Chair of our Compensation and Management Development Committee.
(3)Dr. Boerner became Board Chair on April 1, 2024.
(4)Mr. McMullen joined the Board on July 1, 2024 and concurrently became a member of our Audit Committee. Effective May 6, 2025, Mr. McMullen will become a member of our Compensation and Management Development Committee.
(5)Effective May 6, 2025, Ms. Yale will rotate from the Committee on Directors and Corporate Governance and become a member of our Science & Technology Committee.
35 Bristol Myers Squibb | 2025 Proxy Statement
The following descriptions reflect each standing Board Committee’s membership and Chair effective as of May 6, 2025.
| | | | | | | | | | | | | | |
| | | | Key Responsibilities •Overseeing and monitoring the quality of our accounting and auditing practices, including, among others, reviewing and approving the internal audit charter, audit plan, audit budget and decisions regarding appointment and replacement of Chief Audit Officer •Appointing, compensating and providing oversight of the performance of our independent registered public accounting firm for the purpose of preparing or issuing audit reports and related work regarding our financial statements and the effectiveness of our internal control over financial reporting •Assisting the Board in fulfilling its responsibilities for general oversight of (i) compliance with legal and regulatory requirements, (ii) the performance of our internal audit function and (iii) the effectiveness of enterprise risk assessment and risk management policies and guidelines •Reviewing our disclosure controls and procedures, periodic filings with the SEC, earnings releases and earnings guidance •Producing the required Audit Committee Report for inclusion in our Proxy Statement •Overseeing the effectiveness of our compliance and ethics program •Overseeing our information security and data protection program, including by way of receiving regular updates from management and our Chief Information Security Officer |
| Audit Committee | | |
| | | |
| | | |
| Committee Chair Derica W. Rice Additional Members Michael R. McMullen Paula A. Price Theodore R. Samuels Phyllis R. Yale | | |
| | | | |
| | | | | | | | | | | | | | |
| | | | Key Responsibilities •Providing oversight of our corporate governance affairs and reviewing corporate governance practices and policies, including annually reviewing the Corporate Governance Guidelines and recommending any changes to the Board •Identifying individuals qualified to become Board members and recommending that our Board select the director nominees for the next annual meeting of shareholders •Reviewing and recommending annually to our Board the compensation of non-employee directors and overseeing and recommending any change in Board compensation •Considering questions of potential conflicts of interest involving directors and senior management and establishing, maintaining and overseeing related party transaction policies and procedures •Evaluating and making recommendations to our Board regarding the responsibilities of each Board committee, including its structure, operations and authority to delegate to subcommittees •Evaluating and making recommendations to the Board concerning director independence and defining specific categorical standards for director independence •Providing oversight of the company’s political activities as well as identifying and monitoring risks related to the Company’s political activities •Providing oversight of the company's environmental, social, governance strategy and reporting and the impact on the company’s workforce and shareholders •Overseeing the annual evaluation process of the Board and its Committees |
| Committee on Directors and Corporate Governance | | |
| | | |
| | | |
| Committee Chair Theodore R. Samuels Additional Members Julia A. Haller, M.D. Manuel Hidalgo Medina, M.D., Ph.D. Paula A. Price | | |
| | | | |
Bristol Myers Squibb | 2025 Proxy Statement 36
| | | | | | | | | | | | | | |
| | | | Key Responsibilities •Reviewing, approving and reporting to the Board on our major compensation and benefits plans, policies and programs •Reviewing corporate goals and objectives relevant to CEO compensation, evaluating the CEO’s performance in light of those goals and objectives and recommending for approval by at least three-fourths of the independent directors of our Board the CEO’s compensation based on this evaluation by the independent directors •Reviewing and evaluating the performance of senior management; and, in consultation with the CEO, approving the compensation of executive officers and certain senior management •Overseeing our senior management development programs, and performance assessment of our most senior executives and succession planning •Reviewing and discussing with management the Compensation Discussion and Analysis and related disclosures required for inclusion in our Proxy Statement, recommending to the Board whether the Compensation Discussion and Analysis should be included in our Proxy Statement, and producing the Compensation and Management Development Committee Report required for inclusion in our Proxy Statement •Establishing, overseeing, and administrating our compensation recoupment policies •Reviewing incentive compensation programs and determining whether incentive pay encourages inappropriate or excessive risk-taking throughout our business |
| Compensation and Management Development Committee | | |
| | | |
| | | |
| Committee Chair Peter J. Arduini Additional Members Deepak L. Bhatt, M.D., M.P.H., M.B.A Michael R. McMullen Derica W. Rice Karen H. Vousden, Ph.D. | | |
| | | | |
| | | | | | | | | | | | | | |
| | | | Key Responsibilities •Reviewing and advising our Board on the strategic direction of our research and development (R&D) programs, platforms and capabilities and our progress in achieving near-term and long-term R&D objectives •Reviewing and advising our Board on our internal and external investments in science and technology •Identifying, monitoring and discussing significant emerging trends and issues in science and technology and considering their potential impact on our company •Providing assistance to the Compensation and Management Development Committee in setting any pipeline performance metric under the company’s incentive compensation programs and reviewing the performance results |
| Science and Technology Committee | | |
| | | |
| | | |
| Committee Chair Julia A. Haller, M.D. Additional Members Deepak L. Bhatt, M.D., M.P.H., M.B.A. Manuel Hidalgo Medina, M.D., Ph.D. Karen H. Vousden, Ph.D. Phyllis R. Yale | | |
| | | | |
In addition, in 2024, the Board established an ad hoc Board Finance Committee to oversee and approve certain finance-related matters, including final terms of a notes issuance and other related financing transactions, among other things. The members of the Board Finance Committee were Christopher S. Boerner, Ph.D., Theodore R. Samuels, and Derica W. Rice. The Board Finance Committee acted by unanimous consent once during 2024.
37 Bristol Myers Squibb | 2025 Proxy Statement
How to Communicate With Us
We Value Input and Offer Many Means to Provide It.
We, members of the Board, know that we must actively seek information from a wide variety of sources—and not just from individuals and entities that work for us—to do our jobs optimally. We therefore create multiple means to hear from shareholders, employees at all levels, patients, medical professionals, policy experts and others to inform our work.
You can communicate with us via many of these means. You can provide us comments on your proxy card when you are voting.
You can attend our annual meeting and ask questions. You can accept our invitations to engage or ask us for a meeting when that is of value to you. You can participate in our various Investor Relations functions which we listen to both directly and indirectly. You can write to us via mail or use any of our reporting functions such as so-called Whistle Blower hotlines.
And, of course, we pay close attention to your voting and investment decisions as well.
Written Communication
The Board has created a process for anyone to communicate directly with the Board, any committee of the Board, the non-employee directors of the Board collectively or any individual director, including our Board Chair and Lead Independent Director. Any interested party wishing to contact the Board may do so in writing by sending a letter to Bristol-Myers Squibb Company, Route 206 & Province Line Road, Princeton, NJ 08543, Attention: Corporate Secretary.
Any matter relating to our financial statements, accounting practices or internal controls should be addressed to the Chair of the Audit Committee. All other matters should be addressed to the Chair of the Committee on Directors and Corporate Governance.
Our Corporate Secretary or her designee reviews all correspondence and forwards to the addressee all correspondence determined to be appropriate for delivery. Our Corporate Secretary periodically forwards to the Governance Committee a summary of all correspondence received. Directors may at any time review a log of the correspondence we receive that is addressed to members of the Board as well as copies of any such correspondence. Our process for handling communications to the Board has been approved by the independent directors.
Proactive Shareholder Engagement
We continued to place a high priority on proactive engagement with our shareholders in 2024, reaching out to approximately 50 of our top shareholders, representing about 52% of our voting shares outstanding. In 2024, management, including our CEO and CFO, as well as our Lead Independent Director, met with many of our shareholders and had a productive dialogue on a number of topics, including board composition and leadership, company strategy and execution, risk oversight, executive compensation, and execution against our Sustainability and Social Impact strategy.
The feedback received was generally positive and was shared with the entire Board and members of senior management.
We encourage our registered shareholders to use the space provided on the proxy card to let us know your thoughts about BMS or to bring a particular matter to our attention. If you hold your shares through an intermediary or received the proxy materials electronically, please feel free to write directly to us.
Bristol Myers Squibb | 2025 Proxy Statement 38
| | |
|
| How to Communicate With Us |
Responsiveness to Shareholder Feedback
Throughout the last few years, we have actively solicited feedback from shareholders on topical issues and offered additional insights on shareholder proposals that were included in our recent Proxy Statements. The results of these discussions are noted below:
| | | | | | | | | | | | | | | | | | | | | | | |
| Topic | | | Shareholder Feedback | | Company Response | |
| | | | | | | |
| | | | | | | |
| Sustainability and Social Impact Strategy and Reporting | | | Several shareholders inquired about our current Sustainability and Social Impact strategy, commitments and internal governance around Sustainability and Social Impact reporting.
| | Our Committee on Directors and Corporate Governance has direct oversight of our Sustainability and Social Impact strategy and reporting and ensures our ability to operate with the highest levels of quality, integrity and ethics. Our Sustainability and Social Impact strategy is fully aligned to our corporate strategy. Through active engagement with our shareholders and other key stakeholders, we evolved our Sustainability and Social Impact strategy over the last few years, and in 2024, we published our double materiality assessment results, our second Climate Change Report and our Task Force on Climate-Related Financial Disclosures (TCFD) report, and received validation of our science-based emissions reduction targets by the Science Based Targets Initiative (SBTi). Our Sustainability and Social Impact Report aligns with validated frameworks, such as the Global Reporting Initiative, the Sustainability Accounting Standards Board, the Biopharma Investor ESG Communications Guidance 4.0 and the TCFD. We will continue to update these reports annually. For further discussion, please see “Sustainability and Social Impact” beginning on page 29. | |
| | | | | | |
| | | | | | |
| | | A number of our shareholders requested we adopt a policy to publicly disclose our Consolidated EEO-1 Report yearly. | | We have been disclosing our latest EEO-1 report on our website since 2021. | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Access and Patent Exclusivity | | | In 2022, we received a shareholder proposal for the 2023 Annual Meeting from a group of shareholders asking the Board to establish and report on a process by which the impact of extended patent exclusivity on product access would be considered in deciding whether to apply for additional patent protection on new innovations after the main active ingredient/molecule patent(s) have been filed. | | We collaborated with the shareholders to include additional disclosure in our 2023 Proxy Statement to highlight the company’s existing robust access and intellectual property policies. Among other things, the disclosure notes that BMS obtains intellectual property only by lawful and ethical means, and enforces only those intellectual property rights we believe to be valid. We place the highest priority on obtaining intellectual property for those innovations that provide the greatest medical benefit to patients. This was responsive to the shareholders’ feedback and consistent with our shared desired outcome. This disclosure is included in this Proxy Statement beginning on page 32. | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Human Rights Policy | | | We received a shareholder proposal for the 2024 Annual Meeting requesting that the company adopt a more comprehensive human rights policy that, among other things, applies to its operations and those of its suppliers and references internationally recognized human rights standards. | | As highlighted in our Position Statement on Human Rights, we are a signatory to the U.N. Global Compact, and we strive to support and respect the protection of human rights and to avoid complicity in human rights abuses. The U.N. Global Compact requires the company to publish an annual Communication on Progress (“COP”) that outlines its progress toward implementing these principles. Our 2024 COP can be found on our website. We successfully negotiated a withdrawal of the proposal in exchange for updates to our Position Statement and a proposed action plan to enhance our human rights due diligence framework. | |
| | | | | | | |
39 Bristol Myers Squibb | 2025 Proxy Statement
How We Are Paid
Compensation of Directors
Director Compensation Program
We aim to provide a competitive compensation program to attract and retain high quality directors. The Committee on Directors and Corporate Governance (when used in this Compensation of Directors’ section, the “Committee”) annually reviews our non-employee directors’ compensation program, including a review of the director compensation programs at our executive compensation peer groups. For 2024 planning, we again engaged an outside consultant, Frederic W. Cook & Co., Inc. (“FWC”), to review market data and competitive information on director compensation. FWC recommended, and the Committee determined, that our executive compensation peer groups should be the primary source for determining director compensation.
Upon reviewing FWC’s analysis in December 2023, considering our core non-employee director compensation program had been unchanged since 2022 and was between the 25th percentile and median of our peer groups, among other reasons, the Committee determined to increase the annual equity award for service as a director for 2024 by $10,000 to $210,000. The Board also determined to increase the member annual cash retainer for service on the Board by $5,000 to $110,000. These changes place director compensation near the median of our peer groups. The Committee submitted its recommendations for director compensation to the full Board for approval, and the full Board approved the changes. Dr. Boerner does not receive any additional compensation for serving as a director.
The Committee believes the total 2024 compensation package for non-employee directors was reasonable, and appropriately aligned the interests of our directors with the interests of our shareholders by ensuring directors have an equity stake in BMS.
The Components of Our Non-Employee Director Compensation Program
In 2024, non-employee directors who served for the entirety of 2024 received:
| | | | | | | | | | | |
| Component | Value of Award | |
| | | |
| Annual Retainer | $110,000 | |
| Annual Equity Award | Deferred Share Units valued at $210,000 | |
| Lead Independent Director Annual Retainer | $50,000 | |
| Committee Chair Annual Retainer | $25,000 | |
| Committee Member (not Chair) Annual Retainer – Audit Committee, Compensation and Management Development Committee, Committee on Directors and Corporate Governance, and Science and Technology Committee | $15,000 | |
Annual Equity Award
On February 1, 2024, all non-employee directors serving on the Board at that time received an annual award of deferred share units valued at $210,000 under the company’s 1987 Deferred Compensation Plan for Non-Employee Directors, as amended (the “Plan”). These deferred share units are non-forfeitable at grant and are settleable solely in shares of BMS common stock upon retirement from the Board or at a future date previously specified by a director in accordance with the terms of the Plan. A new director on the Board who is eligible to participate in the Plan receives, on the date the director joins the Board, a pro-rata number of deferred share units based on the number of share units payable to participants as of the prior February 1.
Bristol Myers Squibb | 2025 Proxy Statement 40
Compensation of our Lead Independent Director
Our Lead Independent Director received an additional annual retainer of $50,000. Our Board has determined to award this retainer in light of the increased duties and responsibilities demanded by this role, which duties and responsibilities are described in further detail on page 34. Share Retention Requirements
All non-employee directors are required to acquire a minimum of shares and/or units of company stock valued at not less than five times their annual cash retainer within five years of joining the Board and to maintain this ownership level throughout their service as directors. We require at least 25% of the annual retainer to be deferred and credited to a deferred compensation account on behalf of each director, the value of which is determined by the value of our common stock, until a non-employee director meets our share retention requirement.
Deferral Program
A non-employee director may elect to defer payment of all or part of the cash compensation received as a director under the Plan. The election to defer is made in the year preceding the calendar year in which the compensation is earned. Deferred funds for compensation received in connection with service as a director in 2024 were credited to one or more of the following funds: a U.S. total bond index, a short-term fund, a total market index fund or a fund based on the return on our common stock. Deferred portions are payable in a lump sum or in a maximum of 10 annual installments. Payments under the Plan begin when a participant ceases to be a director or at a future date previously specified by the director.
Charitable Contribution Programs
Each director was eligible to participate in our company-wide matching gift programs in 2024. We matched a director’s contribution to qualified charitable and educational organizations up to $30,000. This benefit was also available to all company employees. In 2024, several of our directors participated in our matching gift programs as indicated in the Director Compensation Table below.
41 Bristol Myers Squibb | 2025 Proxy Statement
Director Compensation Table
The following table sets forth information regarding the compensation earned by our non-employee directors in 2024.
| | | | | | | | | | | | | | | | | | | | | | | |
| Name | Fees Earned or Paid in Cash(1) ($) | Stock Awards(2) ($) | Option Awards(3) ($) | All Other Compensation(4) ($) | Total
($) | |
| | | | | | | |
| P. J. Arduini | 136,790 | | 210,000 | | 0 | | 30,000 | | 376,790 | | |
| D. L. Bhatt, M.D., M.P.H., M.B.A. | 140,000 | | 210,000 | | 0 | | 0 | | 350,000 | | |
| J. A. Haller, M.D. | 150,000 | | 210,000 | | 0 | | 30,000 | | 390,000 | | |
| M. Hidalgo Medina, M.D., Ph.D. | 140,000 | | 210,000 | | 0 | | 0 | | 350,000 | | |
| M. R. McMullen | 62,500 | | 123,361 | | 0 | | 0 | | 185,861 | | |
| P. A. Price | 140,000 | | 210,000 | | 0 | | 30,000 | | 380,000 | | |
| D. W. Rice | 150,000 | | 210,000 | | 0 | | 30,000 | | 390,000 | | |
| T. R. Samuels | 200,000 | | 210,000 | | 0 | | 30,000 | | 440,000 | | |
| G. L. Storch (5) | 69,044 | | 210,000 | | 0 | | 10,000 | | 289,044 | | |
| K. H. Vousden, Ph.D. | 140,000 | | 210,000 | | 0 | | 0 | | 350,000 | | |
| P. R. Yale | 140,000 | | 210,000 | | 0 | | 30,000 | | 380,000 | | |
(1)Includes the annual retainer, committee chair retainers, committee membership retainers and Lead Independent Director retainer, as applicable. All or a portion of the cash compensation may be deferred until retirement, or a date specified by the director, at the election of the director. The directors listed in the below table deferred the following amounts in 2024, which amounts are included in the figures above. Mr. McMullen joined the Board effective July 1, 2024.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | Dollar Amount Deferred
($) | Percentage of Deferred Amount Allocated to U.S. Total Bond Index
(%) | Percentage of Deferred Amount Allocated to Short Term Fund
(%) | Percentage of Deferred Amount Allocated to Total Market Index Fund
(%) | Percentage of Company Deferred Amount Allocated to Deferred Share Units (%)* | Number of Company Deferred Share Units Acquired
(#) | |
| | | | | | | | |
| P. J. Arduini | 136,790 | | 0 | | 0 | | 0 | | 100 | | 2,418 | | |
| D. L. Bhatt, M.D., M.P.H., M.B.A. | 26,250 | | 0 | | 0 | | 0 | | 100 | | 464 | | |
| J. A. Haller, M.D. | 150,000 | | 20 | | 0 | | 80 | | 0 | | 2,652 | | |
| M. R. McMullen | 62,500 | | 0 | | 0 | | 0 | | 100 | | 1,105 | | |
| D. W. Rice | 150,000 | | 0 | | 0 | | 0 | | 100 | | 2,652 | | |
| T. R. Samuels | 200,000 | | 0 | | 0 | | 0 | | 100 | | 3,536 | | |
| G. L. Storch (5) | 69,044 | | 0 | | 0 | | 0 | | 100 | | 1,221 | | |
| P. R. Yale | 140,000 | | 0 | | 0 | | 0 | | 100 | | 2,475 | | |
*Per our share retention requirements, directors are required to defer at least 25% of their annual retainer until they meet our share retention requirement. Share retention requirements are evaluated at least quarterly and deferral amounts are appropriately adjusted from time to time to meet this requirement.
Bristol Myers Squibb | 2025 Proxy Statement 42
(2)Represents aggregate grant date fair value under FASB ASC Topic 718 of deferred share unit awards granted during 2024. On February 1, 2024, each of the non-employee directors then serving as a director received a grant of 4,314.773 deferred share units valued at $210,000 based on the fair market value of our common stock of $48.67 on the grant date. On July 1, 2024, in connection with his appointment to the Board, Mr. McMullen received a pro-rated grant of 2,986.941 deferred share units valued at $123,361 based on the fair market value of our common stock on the grant date of $41.3. The aggregate number of deferred share units held by each of these directors as of December 31, 2024, is set forth below. In some cases, these figures include deferred share units acquired through elective deferrals of cash compensation.
| | | | | | | | | | | |
| Name | # of Deferred Share Units | |
| | | |
| P. J. Arduini | 56,788 | | |
| D. L. Bhatt, M.D., M.P.H., M.B.A. | 10,814 | | |
| J. A. Haller, M.D. | 28,367 | | |
| M. Hidalgo Medina, M.D., Ph.D. | 14,127 | |
| M. R. McMullen | 4,221 | | |
| P. A. Price | 16,699 | | |
| D. W. Rice | 26,841 | | |
| T. R. Samuels | 52,749 | | |
| G. L. Storch (5) | 50,141 | | |
| K. H. Vousden, Ph.D. | 31,346 | | |
| P. R. Yale | 30,796 | | |
(3)There have been no stock options granted to directors since 2006 and except as noted below, no non-employee director had stock options outstanding as of December 31, 2024. On November 20, 2019, in connection with her appointment to the Board, effective upon the closing of the Celgene transaction, Dr. Haller’s stock options from Celgene were converted into BMS stock options. The aggregate number of shares of BMS common stock underlying stock options held by Dr. Haller as of December 31, 2024, is set forth below:
| | | | | | | | | | | |
| Name | # of Shares Underlying
Stock Options | |
| | | |
| J. A. Haller, M.D. | 83,469 | |
(4)Amounts include company matches of charitable contributions under our matching gift programs.
(5)Mr. Storch retired from the Board effective May 7, 2024.
43 Bristol Myers Squibb | 2025 Proxy Statement
| | | | | |
Message from
Peter J. Arduini Chair of the Compensation and Management Development Committee | |
This is the first time that I write to you on behalf of the Compensation and Management Development Committee (when used in this Compensation Discussion & Analysis (“CD&A”), the “Committee” or “CMDC”).
As you have seen throughout this Proxy Statement, last year marked the start of a transformational journey for BMS. Having the right individuals in place throughout the organization is critical to the company’s current and future success, particularly as we navigate changes to a complex and competitive industry and the loss of exclusivity on multiple products. In 2024, the Committee maintained a clear focus on our people, teams and culture and advanced its important work to ensure BMS can retain, motivate, reward, and attract the best possible talent to execute our strategy.
When we engage with management and review compensation and development programs, we do so through the lens of supporting the company’s continued transformation. This is why we thoughtfully design and deploy rewards and compensation structures that appropriately incentivize strong strategic execution. An executive’s compensation should be directly tied to the work he or she does to help the company carry out its strategy and create value for shareholders. We have structured our executive compensation programs with this in mind, and we’re proud of the shareholder support our recent pay proposals have generated.
BMS management in 2024 was guided by a commitment to near-term execution meant to build the foundation for long-term, sustainable growth and value creation. The company, under management’s direction, made meaningful strides in advancing and expanding the Growth Portfolio. It achieved several clinical and regulatory milestones, including the U.S. approval and launch of Cobenfy and the U.S. approval of Opdivo Qvantig. With a focus on long-term growth, management prioritized R&D productivity and high-value science while simultaneously pursuing operational excellence throughout the organization. The company strategically allocated capital — paying down debt and harnessing strong cash flow to invest in internal and external innovation and return cash to shareholders. Further, the company closed and integrated multiple acquisitions that added new assets and capabilities and enhanced its innovation engine.
Through these actions, the management team demonstrated its commitment to making BMS one of the sector’s fastest-growing companies by the end of the decade. The CMDC and the Board believe our current compensation programs are appropriately tailored to foster this commitment and drive continued strategic execution. Following regular review, the CMDC updated aspects of the 2024 compensation program, including the addition of the Growth Portfolio revenue metric to the annual incentive plan. Importantly, we also removed the individual performance component from this plan for senior executives, meaning payouts are now solely based on overall company performance. For the long-term incentive plan, Growth Portfolio revenue is also the revenue metric, and is aligned with the company’s long-term priorities of revenue renewal and R&D productivity and efficiency.
My predecessor, in his final letter as Chair, outlined the thorough, multi-year planning process that led to Chris’s selection as CEO, and the CMDC’s role in that process. Succession planning is a key part of our remit, and a responsibility that the Committee and the full Board take seriously. But, our focus on succession planning does not stop at the CEO or the broader management team. It’s about building and nurturing a top-notch talent pipeline across key positions and business units to guarantee there is never a break in our ability to execute against our strategy. This is particularly important following acquisitions, including the Karuna Therapeutics, RayzeBio, and Mirati transactions that closed in 2024, as new leaders and new talent enter the company. With this in mind, the Sustainability Scorecard component of our annual program included a metric to enhance the inclusive leadership capabilities of our executives through participation in learning experiences focused on inclusive talent practices and engagement. This supports a strong culture that encourages engagement and leads to greater employee productivity, satisfaction and retention.
Shareholder engagement continues to be a critical avenue for the Board to collect thoughts and suggestions on how company leaders are paid and incentivized, among other items. For example, we know through ongoing outreach that there is interest among investors in ensuring executive compensation stays aligned with company strategy and is appropriately balanced between fixed and variable compensation. It is something we are constantly evaluating, ensuring our compensation program maintains proper alignment with creating shareholder value. As the external environment shifts and new opportunities and challenges arise, we will continue seeking stakeholders’ perspectives on executive pay and make adjustments as needed.
In closing, I would like to share my appreciation for our employees and the work they do every day for our patients. On behalf of the Board and all BMS employees, thank you for your support of our compensation programs and company.
Peter J. Arduini
Chair, Compensation and Management Development Committee
Bristol Myers Squibb | 2025 Proxy Statement 44
Compensation Discussion
and Analysis
Business Overview
In 2024, Bristol Myers Squibb made meaningful strategic progress and strengthened our growth profile while navigating a dynamic operating environment and furthering scientific innovation. We enhanced and diversified our portfolio and progressed pipeline assets that will be critical to our success as we navigate upcoming challenges, including the loss of exclusivity on multiple products. We entered into important collaborations and completed and integrated key acquisitions to extend our scientific leadership and advance value creation. Our collective efforts across the organization helped build momentum as we create the foundation for long-term, sustainable growth and work to become one of the sector’s fastest growing companies by the end of the decade.
Critical to our performance in 2024 was our continued focus on growth, diversification and innovation — enabling us to bring more truly transformational medicines to patients faster. Working together, we furthered our strong financial position and achieved significant regulatory and clinical milestones, including the U.S. approval and launch of Cobenfy for patients with schizophrenia. Last year, the company received 23 approvals for initial and expanded indications in the U.S., E.U., JP and CN, including for Abecma, Augtyro, Breyanzi, Cobenfy, Krazati, Opdivo, and Reblozyl.
As an organization, our priorities are to (i) focus on transformational medicines where we have a competitive advantage, (ii) drive operational excellence, and (iii) strategically allocate capital for long-term growth and shareholder returns. Our mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases in the following core therapeutic areas: oncology and hematology with novel modalities in cell therapies, protein degraders, antibody-drug conjugates (ADCs) and radiopharmaceuticals; immunology with a focus on establishing new standards of care in pulmonology, rapidly advancing cell therapy into immunologic diseases and transformational programs to control inflammation, reset immune memory and promote homeostasis in dermatology, rheumatology and gastrointestinal disorders; cardiovascular diseases by leveraging deep expertise across thrombotic diseases, heart failures and cardiomyopathies; and neuroscience with a focus on developing new treatments in neuropsychiatry and neurodegeneration. We are working on accelerating our drug development and delivery of our innovative medicines to patients, enhancing our commercial operating model, as well as enhancing flexibility and reliability of our manufacturing network. We remain committed to strategic business development, maintaining a strong investment grade credit rating, the dividend and reducing debt.
Our focus on transformational medicines in areas where we have a competitive advantage continues to deliver results. We have launched 12 new medicines since 2019 and have established leadership positions across our oncology, hematology, and cardiovascular therapeutic areas. Within each of these businesses, we have leading in-line treatments and are rapidly progressing science to deliver more medicines to more patients faster. We are further enhancing productivity and efficiency within our research and development (R&D) programs—work that is helping us identify assets with an increased probability of success in areas of high unmet medical need. We have significant near-term launch opportunities and a rich pipeline, with platforms and technologies that provide opportunities for new approaches to the treatment of serious diseases. In addition, our financial strength and flexibility, as well as our differentiated business development strategy, remain crucial to the company’s ability to deliver long-term value.
45 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Looking ahead, we are writing the next chapter for BMS and have a clear path forward. We are expanding our registrational pipeline, which includes over 15 assets, while leveraging a very productive innovation engine with a robust early-stage pipeline of more than 30 assets that offer tremendous opportunities for delivering investigational new drugs every year.1 With strong cash flow from legacy brands to invest, we are well positioned for growth, and we are confident in our ability to deliver long-term sustainable value for shareholders. We are carrying significant momentum into 2025 as we pursue opportunities to further enhance efficiency, better execute, and drive growth, which we believe will maximize performance of our portfolio.
Executive Pay Program
We believe our pay programs, and particularly our executive incentive design, should reinforce our strategic priorities. For 2024, our executive pay programs remained focused on the company’s mission to discover, develop and deliver innovative medicines. While largely consistent with our 2023 program, we did make some changes to our 2024 incentive program to align to our priorities. In particular, (1) we changed our measure of revenue performance to Growth Portfolio Revenue, in both our annual and long-term incentive plans, to align to how we publicly report revenue performance during this period of revenue-renewal; (2) we changed our measure of profitability in our annual incentive plan from Earnings Per Share (EPS) to total company non-GAAP Operating Income; and (3) for our annual incentive plan, for our most senior executives, payouts were determined solely based on company performance without any modifications for individual performance.
The rationale for these changes is to incentivize our senior executives as one team – to deliver against our most important objectives. As noted above, we have identified three priority areas of execution to ensure our continued success as we navigate upcoming challenges, including the loss of exclusivity on multiple products. Growth Portfolio Revenue, which is included in both the annual and long-term incentive plans, is a measure of top line growth that creates a foundation for long-term sustainable growth and competitive superiority because it increases our focus on the strategic priority of revenue renewal from medicines where we have a competitive advantage. Non-GAAP Operating Income is a direct measure of profitability and ensures we are driving operational excellence and strategically allocating capital. And pipeline performance, as measured through annual goals for early research through late-stage development, ensures we will deliver on our near-term commercialization priorities while also having the pipeline and science to sustain our growth over the long-term. Together, these metrics help create the foundation for long-term, sustainable growth as we work to become one of the sector’s fastest growing companies by the end of the decade.
This Compensation Discussion and Analysis describes the actions taken by the Committee for our Named Executive Officers in 2024. In particular, the compensation program for 2024 was designed to provide the Committee with the tools and flexibility to appropriately incentivize, reward and retain our executives and align pay with company performance and our evolving strategic priorities.
1 An Investigational New Drug (IND) is a drug or biological drug that has not been approved for general use by the FDA.
Bristol Myers Squibb | 2025 Proxy Statement 46
| | |
|
| Compensation Discussion and Analysis |
2024 Named Executive Officers
This CD&A is intended to explain how our executive compensation program is designed and how it operates for our 2024 named executive officers, whom we refer to collectively as “Named Executive Officers” or “NEOs”. The table below lists our 2024 NEOs.2
| | | | | | | | | | | |
| Name | Principal Position | |
| | | |
| Christopher S. Boerner, Ph.D. | Board Chair and Chief Executive Officer | |
| David V. Elkins | EVP and Chief Financial Officer | |
| Samit Hirawat, M.D. | EVP and Chief Medical Officer, Head of Development | |
| Sandra Leung | EVP and General Counsel | |
| Karin Shanahan | EVP, Global Product Development & Supply | |
2024 Business Results
2024 was a year of strong growth for our growth and legacy portfolios. We had significant clinical and regulatory achievements and closed and integrated business development transactions that broadened our product portfolio and advanced our pipeline.
Key 2024 Financial Performance Highlights
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
| Total
Revenues of | | | GAAP diluted Loss per Share3 of | | | Non-GAAP diluted EPS of | | | A quarterly dividend increase of | |
| | | | | | | | | | | |
| $48.3B | | | $(4.41) | | | $1.15 | | | 5.3% | |
| an increase of 7% compared to 2023 | | | | | | a decrease of 85% compared to 20233 | | | for 2025, marking an increase for the 15th year in a row | |
| | | | | | | | | | | |
Completed & Integrated Key Business Development Transactions
•In 2024, we closed and integrated:
◦Mirati Therapeutics, strengthening and diversifying our oncology portfolio with the addition of Krazati (adagrasib), which is approved by the FDA as a second-line treatment for patients with KRASG12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC);
◦Karuna Therapeutics, bringing in Cobenfy for the treatment of schizophrenia in adults, re-establishing our presence in neuroscience; and
◦RayzeBio, further enhancing our oncology portfolio with the addition of an important radiopharmaceutical capability — one of the fastest-growing new modalities for treating patients with solid tumors.
•We also entered into partnerships to deepen our capabilities in neuroscience and autoimmune disease through agreements with Prothena Corporation, SystImmune, and Zenas BioPharma.
2 As previously disclosed, on February 14, 2025, Ms. Leung notified the company of her intention to retire from her position as Executive Vice President, General Counsel in 2025. Ms. Leung is expected to remain with the company for a transition period until her successor’s appointment in order to assist with the transition of her duties. In connection with her retirement, Ms. Leung will be entitled to retirement benefits in accordance with the company’s existing Senior Executive Severance Plan.
3 GAAP and non-GAAP diluted EPS include the net impact of Acquired IPRD charges and licensing income of ($6.39) in 2024 and ($0.28) in 2023. Acquired IPRD refers to certain in-process research and development (“Acquired IPRD”) charges resulting from upfront or contingent milestone payments in connection with asset acquisitions or licensing of third-party intellectual property rights.
47 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Delivered Strong Commercial Performance
In 2024, we delivered strong commercial execution, particularly for our growth portfolio despite challenges of increased competition and a dynamic regulatory and macro-economic environment.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
| Net sales for the Growth Portfolio was | | | Net sales for Legacy Portfolio was | |
| | | | | | | | | | | |
| $22.6B | | | $25.7B | |
| compared to $19.4 billion in the prior year, representing an increase of 17%, or 19% excluding foreign exchange. | | | compared to $25.6 billion in the prior year, remaining relatively flat, or increased 1% excluding foreign exchange. | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Growth Portfolio was largely driven by: | | | Legacy Portfolio was largely driven by: | |
| | | | | | | | | | | |
| Net sales of Opdivo of $9.3 billion; and | | | Net sales of Orencia of $3.7 billion. | | | Net sales of Eliquis of $13.3 billion; and | | | Net sales of Revlimid of $5.8 billion. | |
| | | | | | | | | | | |
Our overall business mix is beginning to transform as our Growth Portfolio becomes a bigger component, representing 47% of our net sales in 2024 compared to 43% in the prior year.
Achieved Positive Clinical and Regulatory Achievements
We leveraged our leading science and clinical development capabilities to achieve significant milestones, including achievement of all high value milestones for submissions and approvals:
•We launched Cobenfy, a combination M1/M4 muscarinic receptor agonist and muscarinic antagonist, for the treatment of schizophrenia in adults.
•We achieved significant advances in CAR-T cell therapy with approvals for: (i) Breyanzi in the U.S. and Japan for adults with relapsed or refractory follicular lymphoma (FL) and in the U.S. for adults with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) and mantle cell lymphoma (MCL); and (ii) Abecma in the U.S. and E.U. for triple-class exposed relapsed and refractory multiple myeloma (MM) after two or more prior lines of therapy.
•In hematology, Reblozyl received expanded approval to include the first-line treatment of adult patients with transfusion-dependent anemia due to very low, low and intermediate-risk myelodysplastic syndromes (MDS) in the E.U. and Japan.
•In oncology, we strengthened our portfolio with:
◦(i) (A) FDA approval of Opdivo Qvantig injection for subcutaneous use in most previously approved adult solid tumor Opdivo indications; (B) FDA approval of Opdivo for the treatment of adult patients with resectable NSCLC, in combination with platinum-doublet chemotherapy, followed by single-agent Opdivo as adjuvant treatment after surgery; (C) E.U. approval of Opdivo plus Yervoy for the first-line treatment of adult patients with microsatellite instability–high (MSI-High) or mismatch repair deficient unresectable or metastatic colorectal cancer (CRC); and (D) both in the U.S. and E.U., approval of Opdivo in combination with cisplatin and gemcitabine for first-line treatment of adult patients with unresectable or metastatic muscle-invasive urothelial carcinoma (MIUC);
◦(ii) accelerated approval in the U.S. of Krazati, in combination with cetuximab, for the treatment of adult patients with KRASG12C-mutated locally advanced or metastatic CRC;
◦(iii) approval in the U.S. of Augtyro for the treatment of patients with NTRK-positive locally advanced or metastatic solid tumors; and
◦(iv) approval in Japan of Augtyro for the treatment of patients with ROS1 fusion-positive, unresectable advanced or recurrent NSCLC.
For a further discussion on our pipeline accomplishments, please see discussion under “2024 Annual Incentive Plan Program Outcomes” beginning on page 70. Bristol Myers Squibb | 2025 Proxy Statement 48
| | |
|
| Compensation Discussion and Analysis |
Delivering sustainable growth & innovation
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| $48.3B Total Revenues in 2024 | | | Strong Commercial Performance | |
| | | | | | | |
| | | $22.6B | | | $25.7B | |
| | | Growth Portfolio* net sales
compared to
$19.4B in 2023 | | | Legacy Portfolio** net sales
compared to
$25.6B in 2023 | |
| | | | | | | |
| | | ($4.41) | | | $1.15 | |
| | | GAAP diluted EPS† | | | Non-GAAP diluted EPS† (85%) versus 2023 | |
| | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| | Continued Pipeline Progress | |
| | | | | | |
| | 1 | | | 23 | |
| | New Molecular Entity approved in 2024 | | | Approvals in the U.S., E.U., JP & CN | |
| | | | | | |
| | | | 30+ | |
| | | | Early-stage assets | |
| | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| In First Quarter 2024, Completed Key Business Development Including: •Acquisitions of Mirati Therapeutics, RayzeBio and Karuna Therapeutics; and •Global Strategic Collaboration Agreement with SystImmune | | | Balanced Capital Allocation Strategy | |
| | | | | | | |
| | | $15.2B | | | 5.3% | |
| | | in cash flow from operating activities | | | quarterly dividend increase for 2025 | |
| | | | | | | |
| | | $6.0B | | | 15th | |
| | | debt repayment | | | consecutive year of annual increase | |
| | | | | | | | |
* Growth Portfolio includes Opdivo® (nivolumab), Orencia® (abatacept), Yervoy® (ipilimumab), Reblozyl® (luspatercept-aamt), Opdualag® (nivolumab and relatlimab-rmbw), Breyanzi® (lisocabtagene maraleucel), Camzyos® (mavacamten), Zeposia® (ozanimod), Abecma® (idecabtagene vicleucel), SotyktuTM (deucravacitinib), Krazati, AugtyroTM (repotrectinib), CobenfyTM (xanomeline and trospium chloride), and other growth products, including Onureg® (azacitidine), Inrebic® (fedratinib), Empliciti® (elotuzumab), and Nulojix® (belatacept), and royalty revenue products.
** Legacy Portfolio includes Eliquis® (apixaban), Revlimid® (lenalidomide), Pomalyst®/Imnovid® (pomalidomide), Sprycel® (dasatinib), Abraxane® (paclitaxel protein-bound particles for injectable suspension) (albumin-bound), and Mature and other products.
† GAAP and non-GAAP diluted EPS include the net impact of Acquired IPRD charges and licensing income of ($6.39) in 2024 and ($0.28) in 2023. A reconciliation of GAAP to non-GAAP measures can be found on our website at bms.com. See “Quarterly package of financial Information” available on bms.com/investors for information on the list of specified items excluded from non-GAAP EPS.
People and Culture
We believe that our employees are compassionate, purpose-driven professionals who embody our mission of discovering and delivering innovative medicines that help patients prevail over serious diseases. Together, their unyielding focus on patients defines our culture. They are the foundation of our success and our competitive advantage. They work together to bring our mission to life to help patients prevail over serious diseases.
49 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
People Strategy and Culture
Our People Strategy is designed to foster an inclusive and engaging work experience to attract, develop, and retain the most talented workforce that reflects the varied cultures, backgrounds, experiences, and perspectives of our patients and communities around the world. This is core to who we are and how we do business; it guides our decision-making and furthers our ability to deliver on our mission, execute our strategy and generate shareholder value. We strive to cultivate a culture that fosters collaboration and innovation, where everyone feels a sense of belonging and are valued for their unique perspectives.
Our innovation engine is focused on the most critical unmet medical needs and involves patients at every step of the R&D process to ensure our medicines can benefit all patient populations in a variety of contexts. Consequently, a workforce fueled by varied backgrounds, experiences, personal characteristics and viewpoints helps us expand our patient population, because it drives strategy designed to deliver greater access to varied clinical trial patients across geographic regions and address the changing demographics of the communities where we do business and the patients we serve.
The company is an equal opportunity employer and all employment decisions are merit-based. We prioritize investment in enterprise-wide, comprehensive and cohesive programs and policies that accelerate development and collaboration, which creates a competitive advantage in recruiting, developing and retaining our future workforce.
Career Growth and Development
BMS champions the learning and development of all of our people, our most important asset, and we aspire to create a ‘future ready’ workforce by developing the critical skills needed to tackle the organization’s most pressing strategic priorities. Our extensive library of resources, which includes on-demand, open-enrollment and nominations-based experiences, are available in multiple languages to our 30,000+ employees. In 2024, more than 6,000 employees participated in our professional, managerial, and leadership development programs.
Employee Engagement
Our workforce is focused on our patients and are asked to demonstrate our BMS values: Accountability, Inclusion, Innovation, Integrity, Passion and Urgency. By encouraging employees around the world to be their authentic selves at work, to ask questions, voice concerns, and think boldly, we create an energized environment of co-collaboration and co-design where bold ideas and solutions can lead to improved patient outcomes.
We conduct confidential surveys of our global workforce that measure employee sentiment and actively seek feedback on topics such as company culture and values, execution of our strategy, engagement, and individual development, among others. Survey results are reviewed by our executive officers and the Board, who analyze areas of progress or opportunity both at a company level as well as at a function level. We believe that our employee engagement initiatives and career growth and development opportunities help increase employee satisfaction and tenure. Given the criticality of an engaged and motivated workforce, select employee engagement goals are incorporated in our annual incentive program metrics for our most senior executives.
Compensation, Wellbeing, Employee Health and Safety
We provide highly competitive compensation, benefits, and work-life offerings that reflect a comprehensive rewards and wellbeing strategy to enable our workforce to deliver on our business strategy and transform patients' lives through science.
Compensation: Our compensation programs include market competitive base salaries, annual incentives that recognize and reward company performance (and outside of our senior executives, individual contributions), and long-term equity incentives that focus employees on long-term value creation. We also offer sales-based incentives, special allowances, and peer-to-peer individual recognition. With respect to senior executives, a substantial proportion of their pay is variable and at-risk based on our financial and operational results, and is delivered in the form of equity. This supports the alignment of our executive compensation plan with the creation of long-term value for our shareholders.
Bristol Myers Squibb | 2025 Proxy Statement 50
| | |
|
| Compensation Discussion and Analysis |
Wellbeing: We are committed to prioritizing the wellbeing of our workforce through Living Life Better, our strategy for encouraging the physical, emotional, work life, and financial wellbeing of our employees. For global consistency, we have established a framework with a set of standards concentrated on key areas: inclusive benefits, mental health, family care, and caregivers’ support, among others. Living Life Better is grounded in science and ensures that our employees have the support that best meets their individual needs at the right moment. Our signature Living Life Better programs include physical, emotional, work life, and financial programs that are summarized below.
| | | | | | | | | | | | | | | | | |
| | | | | |
| Compensation & Recognition | Wellbeing
& Protections | Time Away
from work | Managing Physical
& Mental Health | Balancing work
& Life | Caring for
Loved Ones |
| | | | | |
| | | | | |
| Global Programs |
| | | | | |
•Base Salary •Annual Incentives •Long-Term Incentives •Global Recognition Program | •Financial Savings & Wellbeing Resources •Financial & Income Protections •Business Travel Medical & Accidental •Life Insurance | •Bereavement Leave •Vacation Days •Holiday Time Off •Year-end Company Closure •Paid Time Off to Give Back | •Living Life Better Wellbeing Strategy •Employee Assistance Program (EAP) •Fitness Memberships •Health Care Benefits (vary by country) •Behavior Change Platform | •Tuition Reimbursement •Family Planning & Care •Mindfulness Training | •Parenting & Caregiving consultations |
| Specific to U.S. |
| | | | | |
| •401(k) & Non- Qualified Savings Plans •Health & Dependent Care Savings & Spending Accounts •Supplemental Personal Liability Protection •Employee & Dependent Life Insurance •Disability Coverage •Long Term Care - Life Insurance | •Military Leave •Military Family Support •Volunteer Time Off •Parental Leave •Bridge Back Parental Leave | •Medical & Pharmacy Benefits •Dental Benefits •Vision Benefits •Supplemental Health Insurance •Fertility/Infertility Benefits | •Commuter Benefits •Adoption/ Surrogacy/Doula Reimbursement | •Child, Elder Care & Pet Support |
Employee Health and Safety: We are committed to protecting our workforce, communities, and patients, thereby ensuring the continued supply of life-saving medicines. We have comprehensive policies that ensure all employees, contractors, and visitors to our sites can work or conduct their visit safely. We provide a comprehensive in-house occupational health service to ensure any work-related illness or disease is identified early so that worker health can be protected.
Employee share ownership
As noted above, we grant long-term incentives in the form of equity-based compensation, with vesting conditions, as part of our compensation strategy to create an ownership culture and better align employees’ interests with those of our shareholders. In 2024, the company granted a total of approximately 16,848,519 awards to all employees, including NEOs, which included (1) 13,607,953 Restricted Share Units (“RSU”), (2) 1,296,224 Market Share Units (“MSU”), and (3) 1,944,342 Performance Share Units (“PSU”). Of those awards, (i) our NEOs received (A) MSU and PSU grants totaling approximately 582,431 units, which are all 100% performance-based and at risk, and (B) Ms. Shanahan received an RSU grant of 18,843 units; and (ii) all other employees received a combination of MSU, PSU, and RSU grants totaling approximately 16,247,245. Additionally, non-employee directors received deferred share unit grants of approximately 62,953 in 2024.
51 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Shareholder Engagement
As noted earlier, in 2024, we reached out to approximately 50 of our top shareholders, representing about 52% of our total shares outstanding. As in previous years, we engaged on many important topics related to executive compensation and management development, including recent changes to the metrics in our short-term and long-term incentive programs to align with the evolution of our company strategy, the inclusion of the Sustainability Scorecard in our incentive programs, and the recent CEO succession. The feedback we received from shareholders was generally positive and supportive of our compensation program. Our 2024 say-on-pay proposal was approved by 94% of our shareholders, confirming continued support for our executive compensation program.
Additionally, we used the feedback from these engagement conversations as vital input into Committee discussions. The Committee remains committed to ongoing shareholder engagement and it will continue to actively consider shareholder feedback as it evaluates and optimize our executive compensation program in the future to drive execution of our strategy.
Executive Compensation Program Overview
Highlights of 2024 Compensation Program
2024 marked a critical period—we began writing the next chapter in our long history, and from now through the remainder of the decade, our long-term strategic priorities are to focus on transformational medicines where we have a competitive advantage, drive operational excellence, and strategically allocate capital for long-term growth and shareholder returns. The CMDC reviewed our program in light of our strategic priorities to ensure alignment.
For the 2024 annual incentive plan metrics, 35% was based on achieving Growth Portfolio Revenue, excluding foreign exchange impact, 30% was based on achieving non-GAAP Operating Income, together accounting for a total of 65% of the annual incentive plan. Additionally, 25% was based on our pipeline progression and, for senior executives, the remaining 10% was based on performance against our Sustainability Scorecard. The annual incentive plan was updated to remove individual performance from the calculation of our NEOs’ and senior executives’ payouts. This was an important change to reinforce the necessity for our senior leaders to work together as a team on the achievement of our most critical priorities, which are represented by our company performance goals.
Additionally, for the long-term incentive plan 2024 performance share unit awards, measured from 2024-2026, the revenue metric was also based on Growth Portfolio Revenue (40%) and, consistent with prior years, the earnings metric was based on non-GAAP Operating Margin (25%). The remaining 35%, consistent with prior years, was based on relative TSR.
Furthermore, the 2024 market shares units awards were updated such that performance will be measured using our Total Return (i.e., share price growth and the value of accumulated dividends over the performance period) over a three-year period from March 2024 to March 2027, with the entire award vesting at the end of the three-year period. The change to measure performance based on Total Return aligns to our investment profile, which provides returns to our shareholders through both share price growth and our dividend yield. By measuring performance over three years, with vesting occurring at the end of this period, we have aligned the performance period with our vesting period for our performance share units to create consistency in how we measure performance and returns, while also strengthening the retentive value of these awards.
We included non-GAAP Operating Income and Growth Portfolio Revenue metrics in our incentive plans because we believe these directly measure the execution of our strategic priorities. Total company non-GAAP Operating Income is a critical measure of profitability and ensures we are driving operational excellence and strategically allocating capital. Growth Portfolio Revenue is a measure of top line growth that creates a foundation for long-term sustainable growth and competitive superiority because it increases our focus on the strategic priority of revenue renewal from medicines where we have a competitive advantage.
Together, these changes further simplify the compensation program by providing greater clarity on program elements to our shareholders as well as our Named Executive Officers, and support execution of the company’s strategic priorities while driving sustainable long-term value.
Bristol Myers Squibb | 2025 Proxy Statement 52
| | |
|
| Compensation Discussion and Analysis |
2024 Design Supports Revenue Renewal and Execution of Our Core Strategy:
| | | | | | | | | | | | | | |
| | | | |
| Base Salary | | | •Allows us to attract and retain talent in a highly competitive labor market •Based on specialized qualifications, experience and role impact, pay levels of comparable positions within peer group and competitive market |
| | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| Annual Incentive (Paid in cash) | | | Non-GAAP Operating Income New for 2024 | Critical measure of annual profitability, aligning our employees with our shareholders | •No individual performance component. Payout based solely on the company performance |
| | | | | |
| | | | | |
| | | Growth Portfolio Revenue New for 2024 | •Foundation of long-term sustainable growth and competitive superiority. •Increases focus on strategic priority of revenue renewal. |
| | | | | |
| | | | | |
| | | Pipeline | Near-Term Value | Have evolved to: •Drive improved decision- making and operational rigor •Ensure alignment with company’s portfolio |
| | |
| | |
| | Long-Term Growth Potential |
| | |
| | |
| | Qualitative Overlay |
| | | | | |
| | | | | |
| | | Sustainability Scorecard | Aligned to our commitments on
sustainability and social impact |
| | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| Long-Term Incentive (Paid in shares) | | Performance Share Units | •Rewards the achievement of financial goals and further aligns executive compensation with the interests of our shareholders — Non-GAAP Operating Margin (25%), Growth Portfolio Revenue (40%) and relative Total Shareholder Return CAGR (35%), each measured over an applicable three-year performance period. | Market Share Units | •Rewards stock price appreciation, inclusive of the value of dividends accumulated during the performance period •Important component of attracting specialized talent •Rewards creation of incremental shareholder value •Promotes retention while maintaining pay-for-performance link |
| | | | | | |
53 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Executive Compensation Philosophy and Principles
At Bristol Myers Squibb, the cornerstone of our compensation philosophy and program structure is aligning pay to the achievement of both our short-term and long-term goals, engagement of our employees, the achievement of our mission and the delivery of value to our shareholders.
Each year, when evaluating company and senior management performance and making its pay decisions, the Committee considers our compensation philosophy and program structure, which underscores competitive compensation and pay for performance, with the goal of striking the appropriate balance among (i) directly aligning executives’ compensation with the fulfillment of our mission and the delivery of shareholder value, (ii) making a substantial portion of our executives’ compensation variable and at risk based on operational, financial, strategic and share price performance, and (iii) attracting, retaining and engaging executives who are capable of leading our business in a highly competitive, complex, and dynamic business environment. In addition, the Committee considers the information and recommendations it receives and may exercise discretion in modifying any recommended adjustments or awards to executives based on considerations it deems appropriate.
After reviewing our financial, operational, strategic, and share price performance, the Committee determined that the compensation of our executives under the program design continues to be appropriate.
| | | | | | | | |
| | |
| In 2024, the Committee reviewed how all the elements of our compensation program design worked together, focusing on the balance between short-term and long-term compensation and performance, top-line and bottom-line results, absolute and relative factors, and internal and market-based performance metrics. In evaluating 2024 performance, the Committee determined that the compensation of our executives appropriately reflects: •our financial and operational results; •the execution and advancement of the company’s long-term strategy in 2024; •the Committee’s holistic assessment of the performance of our executives in working together towards the achievement of our most critical company priorities; and •the execution of key regulatory milestones to renew our portfolio. We believe that the link between our compensation program design and the execution of our strategy will continue to create sustainable long-term value for shareholders. | |
| | |
Our Executive Compensation Philosophy Focuses on Two Core Elements:
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | Competitive Compensation | | | •We operate in a highly complex and competitive business environment that requires that we attract, retain and engage executives capable of leading our business. •By providing compensation that is competitive with our peer companies, we reduce the risk that our competitors can successfully recruit our executives. We are also able to maintain the highest ongoing levels of engagement of these talented executives to facilitate and sustain high performance. | |
| | | | | | |
| | | | | | |
| | | | | | |
| | Pay for Performance | | | •We structure our compensation program to closely align the interests of our executives with those of our shareholders. •We believe that an executive’s compensation should be directly tied to helping us achieve our mission and deliver value to our shareholders. Therefore, a substantial portion of our executives’ compensation is variable and at risk based on operational, financial, strategic and share price performance. | |
| | | | | | |
Bristol Myers Squibb | 2025 Proxy Statement 54
| | |
|
| Compensation Discussion and Analysis |
Based on this philosophy, our compensation program is designed with the following principles in mind:
•to pay our employees equitably based on the work they do, the capabilities and experience they possess, and the performance and values they demonstrate (including Accountability, Inclusion, Innovation, Integrity, Passion and Urgency);
•to motivate our executives and all our employees to deliver high performance with the highest integrity; and
•to implement best practices in compensation governance, including risk management and promotion of effective corporate policies.
The Role of Risk Assessment in Evaluating Performance Against Company Values
When determining the overall compensation payout opportunities for our most senior executives, the Committee considered each executive’s contributions to our company’s strategic achievements and financial and operational performance that support our long-term business strategy and shareholder value creation. The Committee also considered each executive’s demonstration and role modeling of the values of Accountability, Inclusion, Innovation, Integrity, Passion and Urgency defined as BMS Values (“BMS Values”).
The Committee evaluated our NEOs’ performance against clear and pre-defined objectives established at the beginning of the year and tied to the company’s key strategic objectives. We believe this structure appropriately incentivizes our executives to focus on our long-term business strategy, to achieve our mission to help patients prevail over serious diseases, and to attain sustained long-term value creation for our shareholders.
| | | | | | | | | | | |
| | | |
| BMS Values | |
| ü | Accountability | |
| ü | Inclusion | |
| ü | Innovation | |
| ü | Integrity | |
| ü | Passion | |
| ü | Urgency | |
| | | |
Also embedded in the determination of individual incentives is the ongoing assessment of enterprise risk, including reputational risk stemming from the dynamic external environment. This evaluation is one input into the determination of incentive payout opportunities. Given the direct link between BMS Values and our executive compensation program’s emphasis on sustainable long-term value, our goal is to minimize and appropriately reduce the possibility that our executive officers will make excessively or inappropriately risky decisions that could maximize short-term results at the expense of sustainable long-term value creation for our shareholders.
Benchmarking Analysis and Compensation Peer Groups
Benchmarking Approach
In general, our executive compensation program seeks to provide total direct compensation (base salary plus target annual incentive award, plus the grant date fair value of annual long-term equity incentive awards) at the median of our primary peer group and/or the Willis Towers Watson Pharmaceutical Human Resources Association Executive Compensation Survey (the “PHRA Survey”) peers (as defined below) when targeted levels of performance are achieved. In any given year, however, total direct compensation for a particular executive may be above or below the median of our primary peer group due to multiple factors. These factors include qualifications, experience, responsibilities, contributions, role criticality and/or potential as well as attracting and retaining talent within the highly competitive biopharmaceutical industry. Providing competitive pay when targeted levels of performance are achieved allows us to attract and retain the talent we need to continue driving performance, while enabling us to maintain a competitive cost base with respect to compensation expense.
55 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Benchmarking Process
The Committee’s independent compensation consultant annually conducts and shares with the Committee a review of compensation for our Named Executive Officers, including compensation information compiled from publicly filed disclosures of our primary and extended peer groups as well as the PHRA Survey. Pay levels of our primary and PHRA Survey peers, among other factors, are used as a reference point when determining individual pay decisions (i.e., base salary levels, target annual incentive levels and long-term equity incentive award size). For 2024, the independent compensation consultant was Farient Advisors LLC (“Farient”).
The market data, as applicable, is determined for our CEO and CFO and our other NEOs based on the prior year’s compensation and is reviewed by the Committee to inform compensation decisions made in March of each year as follows:
| | | | | | | | | | | | | | |
| | Data Source | Weighting | |
| | | | |
| CEO and CFO | Blended Peer Proxy and PHRA Survey Data | 50% Proxy Data and 50% Survey Data | |
| Other NEOs | PHRA Survey Data | 100% Survey Data | |
2024 Peer Groups
We regularly monitor the composition of our peer groups and make changes when appropriate. The Committee, with the support of Farient, reviewed our peer groups in 2024 and recommended two changes to the primary peer group to support the 2025 benchmarking process: (i) removal of Biogen and (ii) addition of Regeneron. Our peer groups in 2024 consisted of the following companies:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Company Name | BMS Peer Group | PHRA Survey Group |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | AbbVie Inc. | ü | ü |
| | | | | | | Amgen Inc. | ü | ü |
| | | Primary Peer Group | | | Biogen | ü | |
| | | | | Eli Lilly and Company | ü | ü |
Extended Peer Group1 | | | | Gilead Science | ü | ü |
| | | Johnson & Johnson | ü | ü |
| | | | | Merck & Co. | ü | ü |
| | | | | Pfizer Inc. | ü | ü |
| | | | | | | AstraZeneca PLC | ü | ü |
| | | | | | | GlaxoSmithKline PLC | ü | ü |
| | | | | | | Roche Holding AG | ü | ü |
| | | | | | | Novartis AG | ü | ü |
| | | | | | | Sanofi | ü | ü |
| | | | | | | Takeda Pharmaceutical Co. | | ü |
(1)Our extended peer group includes the primary peer group plus the five listed companies based outside the U.S.
Bristol Myers Squibb | 2025 Proxy Statement 56
| | |
|
| Compensation Discussion and Analysis |
Primary Peer Group and PHRA Survey Peers: The Committee believes the companies included in our 2024 primary peer and PHRA Survey peer groups are appropriate given the unique nature of the biopharmaceutical industry. The companies from these two groups represent our primary competitors for executive talent and operate in a similarly complex regulatory and research-driven environment.
In determining our peer groups, we believe emphasis should be placed on whether a company competes directly with us for the specialized talent necessary to further drive our success in creating the leading global biopharmaceutical company. We also consider company size in determining our peer groups. In particular, BMS’ revenue approximates the median 2024 revenue of our primary peer group. The median revenue of our primary and extended peer groups were $44.2 and $46.7 billion, respectively.
Extended Peer Group: We also selectively utilize an extended peer group, which comprises the eight companies in our primary peer group plus five companies based outside the U.S. This extended peer group as well as PHRA Survey data serves as an additional reference point for compensation practices, including an understanding of the competitive pay environment as it relates to the global nature of both our business and the competition for talent. Our extended peer group is also used to determine the relative Total Shareholder Return (“TSR”) component of our performance share unit and market share unit awards.
BMS Compensation Program Design Process
Compensation and Management Development Committee
The Committee is responsible for providing oversight of our executive compensation program for the Named Executive Officers as well as other members of senior management. The Committee is responsible for setting the compensation of the Chief Executive Officer and approving the compensation of all of the other Named Executive Officers and certain other members of senior management.
The Committee annually reviews and evaluates the executive compensation program with the intent to ensure that the program is aligned with our compensation philosophy and with our performance. The Committee may delegate any of its responsibilities to one or more duly formed and authorized subcommittees (which shall be comprised of at least two members of the Committee) or to management (with respect to matters affecting employees other than certain senior executive officers). During 2024, the Committee did delegate some of its responsibilities to management.
| | | | | | | | | | | |
| | | |
| | See page 36 for a discussion of the duties and responsibilities of the Committee in more detail. | |
| | | |
Role of the Independent Compensation Consultant
For 2024, the Committee retained Farient as its independent compensation consultant to provide executive compensation services to the Committee. Farient reported directly to the Committee, and the Committee directly oversaw the fees paid for services provided by Farient. The Committee instructed Farient to give advice to the Committee independent of management and to provide such advice for the benefit of our company and shareholders. Farient did not provide any consulting services to BMS beyond its role as compensation consultant to the Committee.
57 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
In 2024, Farient provided the following services:
•reviewed and advised on the composition of the peer group used for competitive benchmarking;
•participated in the review of our executive compensation program;
•provided an assessment of BMS senior executive pay levels and practices relative to peers and other competitive market data;
•provided an annual analysis of industry trends among the peers and best practices related to pay program design and other program elements;
•consulted on incentive plan design and compensation packages for senior executives;
•reviewed and advised on all materials provided to the Committee for discussion and approval; and
•attended all of the Committee’s regularly scheduled and special meetings in 2024 at the request of the Committee, and also met with the Committee chair without management present.
The Committee reviews the independence of its compensation consultant annually in accordance with its charter, applicable SEC rules and NYSE listing requirements. After review and consultation with Farient, the Committee determined that Farient was independent, and there was no conflict of interest resulting from retaining Farient during the year ended December 31, 2024.
Role of Company Management
The CEO makes recommendations to the Committee concerning the compensation of Named Executive Officers other than the CEO, as well as other members of senior management. In addition, the CEO, CFO and, in the case of our pipeline performance metric, both the EVP, Chief Research Officer and EVP, Chief Medical Officer, Head of Development, are involved in recommending for the Committee’s approval the performance goals for the annual and long-term incentive plans, as applicable. The Chief People Officer works closely with the Committee, its independent compensation consultant and management to (i) ensure that the Committee is provided with the appropriate information to make its decisions, (ii) propose recommendations for Committee consideration, and (iii) communicate those decisions to management for implementation.
Bristol Myers Squibb | 2025 Proxy Statement 58
| | |
|
| Compensation Discussion and Analysis |
2024 Compensation Program – Named Executive Officers
2024 Target Compensation Benchmarks
Dr. Boerner’s target compensation approximated the 35th percentile of Chief Executive Officers within our primary peer and PHRA Survey peer groups. The Committee believes Dr. Boerner's compensation package positions him appropriately among his peers when considering multiple factors, including his tenure as an executive officer and our CEO and provides opportunities for growth. On average, our other Named Executive Officers were generally around the 75th percentile of our primary peer and PHRA Survey peer groups, as applicable, with some variation by position.
The following charts provide an overview of the 2024 executive compensation components for the CEO and other NEOs, as originally granted and excluding one-time awards, and highlights the percentage of target compensation that is variable and at risk.
| | | | | | | | | | | | | | | | | |
| | | | | |
| CEO target compensation approximated the 35th percentile of our primary peer and PHRA Survey peer groups. | |
| | | | | |
| | | | | |
| 91% | | | 78% | |
| of target pay is performance-based and at risk | | | of target pay delivered in long-term equity incentives with three-year vesting | |
| | | | | |
| | |
| Average NEO Target Pay Mix (Excl. CEO) |
|
|
| | | | | | | | | | | | | | | | | |
| | | | | |
| Average NEO target compensation generally around the 75th percentile of our primary peer and PHRA Survey peer groups for the CFO and PHRA Survey peers only for other NEOs. | |
| | | | | |
| | | | | |
| 84% | | | 68% | |
| of average NEO target pay is performance-based and at risk | | | of average NEO target pay delivered in long-term equity incentives with three-year vesting | |
| | | | | |
59 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
This target pay mix supports the core elements of our executive compensation philosophy by emphasizing long-term, stock-based incentives while providing competitive annual cash components, thus aligning our executive compensation program with our business strategy.
The following sections discuss the primary components of our executive compensation program and provide detail on how specific pay decisions were made for each NEO in 2024.
Components of Our 2024 Compensation Program
| | | | | | | | | | | |
| Core Components of our 2024 Executive Compensation Program for NEOs: |
| | | |
| Components: | Purpose: | |
| | | |
| Base Salary | Fixed component of pay that is reflective of qualifications, experience and role impact; is aligned with comparable positions within our peer groups | |
| | | |
| | | |
| Annual Incentive Plan | Rewards annual company performance on key financial and strategic priorities | |
| | | |
| | | |
| Long-Term Incentive Program, comprising: •Performance Share Units •Market Share Units | Aligns executives’ interest with those of our shareholders and focuses executives on the execution of our long-term strategy; awards are 100% performance-based and at risk | |
| | | |
Base Salary
In 2024, in accordance with our company-wide salary review process, certain employees, including the Named Executive Officers, were eligible for a salary increase. The salaries of our executives are primarily based on the salary levels of comparable positions within our primary peer and PHRA Survey peer groups as well as the specialized qualifications, experience and criticality of the individual executive and/or his or her role. In addition to reviewing salaries annually based on the above criteria, there may be additional adjustments to salary from time to time to recognize, among other things, when an executive assumes significant increases in responsibility and/or is promoted. Effective April 1, 2024, Ms. Shanahan and Dr. Boerner received a salary increase of 16.7% and 3.3%, respectively. Other NEOs did not receive a salary increase.
Annual Incentive Plan
Our annual incentive plan is designed to reward performance that supports our business strategy of creating the leading biopharmaceutical company and our mission to help patients prevail over serious diseases. The annual incentive plan aligns with our business strategy and mission by sharpening management’s focus on key financial, pipeline and sustainability goals. Starting in 2024, the annual incentive plan was updated to remove the individual performance factor from the calculation of our NEO’s and senior executive payouts. This important change reinforces the necessity for our senior leaders to work together on the achievement of our most critical priorities, which are represented by our company performance goals. Namely, executives must advance our long-term strategic priorities to focus on transformational medicines where we have a competitive advantage, drive operational excellence, and strategically allocate capital for long-term growth and shareholder returns.
Each NEO’s target annual incentive is expressed as a percentage of base salary, which is set at a level to ensure competitive total direct compensation. Annual incentive awards for each NEO are determined by evaluating company performance (as measured by the Company Performance Factor). The maximum annual incentive opportunity for each NEO is 200% of target.
The Company Performance Factor can range from 0% to 200%, based on financial achievements, pipeline results and sustainability outcomes. The graphic below illustrates the calculation used to determine annual incentive awards.
Bristol Myers Squibb | 2025 Proxy Statement 60
| | |
|
| Compensation Discussion and Analysis |
Annual Incentive Award Calculation for Named Executive Officers
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
| Target Annual Bonus (As percentage of NEO base salary) | | X | | Company Performance Factor (Based on achievement of financial, pipeline and sustainability metrics) | | = | | Annual Bonus | |
| | | | | | | | | | |
The target annual incentive for each NEO is expressed as a percentage of the executive’s base salary. If mid-year salary and/or target bonus percentage adjustments are made, the target annual incentive award will include the pro-rated impact of the adjustments.
Performance Metrics Underlying the Company Performance Factor
Our 2024 annual incentive plan design has the following corporate-wide measures, which apply to all employees at the level of Senior Vice President and above (SVP+), including our Named Executive Officers.
| | | | | | | | | | | |
2024 Metric
and Weighting | | What It Is | Why It Is Important |
| | | |
Operating Income | | Non-GAAP Operating Income Total Revenue minus Cost of Goods Sold (COGS) minus Operating Expenses | A critical measure of annual profitability aligning our employees’ interests with those of our shareholders |
Growth Portfolio Revenue | | Growth Portfolio Revenue excludes certain key loss of exclusivity (LOE) brands such as Revlimid, Eliquis, Pomalyst, Sprycel and Abraxane. Revenues minus reserves for returns, discounts, rebates and other adjustments; excludes impact of foreign exchange •Near-Term Value •Submissions and approvals Long-Term Growth Potential •Qualitative Overlay | A measure of top line growth that creates a foundation of long-term sustainable growth and competitive superiority Increases focus on strategic priority of revenue renewal Have evolved to: •Drive improved decision-making and operational rigor •Ensure alignment with company’s portfolio |
Pipeline | |
Sustainability Scorecard | | •Environmental Reflects progress towards environmental commitments •Human Capital Management and Social Responsibility Reflects progress on patient access, enhancement of leadership capabilities and employee inclusive engagement trends | Aligns to BMS commitments on sustainability and social impact goals |
Financial and Pipeline Metric Target Setting Considerations
At the beginning of each year, the Committee undertakes an incentive target setting process to establish targets that it believes will motivate our executives appropriately to deliver the high performance that drives shareholder value creation in both the short- and long-term. When establishing targets and goals each year, the Committee considers budget, operational priorities, long-term strategic plans, historical performance, product pipeline and external factors, including external expectations, competitive developments, and the regulatory environment, among other things. Threshold, target, and maximum performance goals are evaluated independently and are set to provide appropriate awards across a wide but reasonable set of performance outcomes. When establishing our financial targets and our revenue target in particular, we consider expected product price increases.
61 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Financial targets are:
•Predefined;
•Target performance goals that are aligned with financial guidance and our operating plan;
•Tied to the key financial objectives of the company; and
•Aligned with industry benchmarks on speed of commercial launch and expected market adoption.
Pipeline performance targets are:
•Set in collaboration with the S&T Committee;
•Aligned with the company’s strategic plans, productivity initiatives, including a multi-year restructuring plan, and key value drivers;
•Aligned with industry benchmarks on typical clinical study duration and regulatory approval timelines;
•Separated into two performance categories, “Near-Term Value” and “Long-Term Growth Potential,” subject to a qualitative overlay; and
•Reflective of annual milestones that link short-term outcomes to long-term strategic R&D priorities (milestones for higher-value assets are emphasized in goal setting to provide a framework that assesses not only quantity, but also quality and impact of milestones).
The S&T Committee also identifies those highest-value assets and the integration of acquired assets, among other factors, the importance of which will inform the application of a qualitative overlay.
The Committee set incentive targets in the first quarter of 2024 in consideration of anticipated performance, in line with financial guidance provided to the market in early 2024 and in line with commercial, pipeline and sustainability expectations. Thereafter, we met or exceeded financial and operational goals in certain key areas, including revenues, non-GAAP earnings, regulatory and development milestones, important business development activities, and expense management, resulting in an update to financial guidance to the market for the year to reflect these changes.
Sustainability Scorecard Metric Target Setting Considerations
Similar to 2023, for the 2024 annual bonus plan, for our employees at the level of SVP+, including our NEOs, 10% of the company performance factor was based on a Sustainability Scorecard metric. This metric was included to incentivize our executives to timely achieve or progress important Sustainability and Social Impact milestones. We chose to include the Sustainability Scorecard in the annual bonus program as it incentivizes important progress toward various goals, in some cases, long-term commitments with varying completion dates, while also giving us the opportunity to annually re-align the metric goals to our strategic priorities and prior achievements. Below is a summary of the goals we selected for 2024, and we anticipate these goals to change each year.
In selecting the goals for inclusion in this metric, the CMDC included those across the broad pillars of our strategy. For each goal, the CMDC selected objective and measurable performance metrics linked to the Company’s strategy and long-term initiatives. In particular:
•environmental goals support our overall climate agenda and in particular the commitment to net zero emissions by 2050;
•progress on our patient access goals aligns to our external commitment to increase the number of low- and middle-income countries (LMICs) with access to our medicines;
•enhancement of leadership capabilities on inclusive talent practices across our Vice President and above population; and
•employee inclusive engagement score was set based on internal trend data covering employees’ observations of employee experience through pulse surveys administered three times a year that (i) measure progress, (ii) aid in the focus of our talent and workforce investment decisions, and (iii) address needed management interventions.
Bristol Myers Squibb | 2025 Proxy Statement 62
| | |
|
| Compensation Discussion and Analysis |
The selection and evaluation of these metrics were the product of a robust governance process. Namely, the goals and achievements were (i) established by the CMDC, (ii) reviewed by our Committee on Directors and Corporate Governance, which has primary oversight responsibility of our Sustainability and Social Impact strategy, and (iii) were finalized and approved by the CMDC.
2024 Annual Incentive Targets
| | | | | | | | | | | | | | | | | |
| Metric | 2024 Target (1) | Bonus Program Weight | |
| | |
| SVP+ (2) | All Others | |
| | | | | |
| Non-GAAP Operating Income ($M) | $17,500 | 30% | 30% | |
| Growth Portfolio Revenue (Ex-FX) ($M) | $22,500 | 35% | 35% | |
| Pipeline (1) | 3 | 25% | 25% | |
| Sustainability Scorecard (1) | 3 | 10% | — | |
| Individual Performance | — | — | 10% | |
| Total | | 100% | 100% | |
(1)Pipeline and Sustainability Scorecard performance metrics are determined on a scale of 1 to a maximum of 5.
(2)For our employees at the level of SVP+, including our NEOs, 10% of the company performance factor was based on a Sustainability Scorecard metric.
2024 Long-Term Incentive Program Grants
Long-Term Incentive Program
Our long-term incentive program employs only performance-based equity for our Named Executive Officers and is designed to promote creation of sustainable long-term value for shareholders by focusing on strong year-to-year financial and operational performance, and on the development and advancement of our pipeline over the long-term. Additionally, the design of our long-term equity incentive program promotes organizational alignment with our business strategy and serves as a retention lever, through vesting and payout over a three-year period.
The Committee’s Process for Granting Annual Long-Term Incentive Awards
Long-term incentive awards are typically approved each year on the date the Committee and full Board meet during the first week of March with a grant effective date of March 10. We believe that consistent timing of equity award grants is good corporate governance and reduces the risk of selecting a grant date with a preferential stock price.
For 2024, the Committee established annual equity award ranges for our Named Executive Officers, other than the CEO based on competitive market levels, among other factors. The CEO’s long-term equity incentive award level is assessed by the Committee and the Board annually. Once the grant value is established for each executive, 60% of the value is awarded as Performance Share Units (or “PSUs”) and 40% is awarded as Market Share Units (or “MSUs”).
In determining the size of the individual long-term equity incentive awards granted to our Named Executive Officers in March 2024, the Committee considered the annual equity award ranges, the executive’s performance in the prior year as well as ways to motivate our Named Executive Officers to focus on the company’s long-term performance. Given that each year’s awards have an overlapping performance period from the prior year, we believe these awards provide the right balance between short-term and long-term focus. Dr. Boerner’s long-term equity incentive award as CEO is determined annually by the Committee and the Board based on competitive benchmarks and his performance and contributions. Dr. Boerner’s March 2024 award took into account his strong performance during 2023 and a long-term equity incentive opportunity that was commensurate with his role as CEO and the competitive market pay for that position.
63 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
In 2024, we continued to offer two long-term award vehicles, each of which served a different purpose:
•Performance Share Unit Awards: rewards the achievement of key three-year end-to-end financial goals (non-GAAP Operating Margin and Growth Portfolio Revenue) and the value created for shareholders as measured by relative TSR CAGR over a three-year period ending in the first quarter of the applicable payout year.
•Market Share Unit Awards: rewards the creation of incremental shareholder value over a three-year period.
2024 Equity Incentive Program Summary
| | | | | | | | | | | | | | |
| | Performance Share Units (PSUs) | Market Share Units (MSUs) | |
| | | | |
| Proportion of Annual Grant | 60% | 40% | |
| Metrics & Weighting | •Non-GAAP Operating Margin: 25% •Growth Portfolio Revenue (Ex-FX): 40% •3-Year Relative TSR CAGR: 35% | Stock price appreciation or depreciation, inclusive of the value of dividends accumulated during the performance period, from the grant date (Total Return) | |
| Performance Period | •Financial Metrics: 3-year (2024-2026) •Relative TSR CAGR: 3-year (March 10, 2024 to February 28, 2027) | 3-year (March 10, 2024 to February 28, 2027)
| |
| Min / Max Payout (% of Target Units) | 0% / 200% | 0% / 225%* | |
| Vesting | 3‑Year Cliff (3rd anniversary of grant date) | 3-Year Cliff (3rd anniversary of grant date) | |
*The minimum Total Return achievement required to earn any shares from MSUs is 80% of the grant date stock price. Additionally, rTSR Floor feature provides a minimum level of payout if BMS stock price declines from the grant date but outperforms our peers based on the TSR percentile rank. The payout factor is the greater of Total Return and rTSR Floor.
2024 Performance Share Unit Awards
The Performance Share Unit Awards made in 2024 are focused on motivating executives to deliver long-term top-line growth and improved margins, while also focusing on delivering shareholder value at a rate faster than our industry peers. For the 2024 PSU awards, the following metrics and goals were applied. Payouts are interpolated for performance between threshold, target and maximum.
2024-2026 PSU Payout Schedule
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2024-2026 Cumulative
Non-GAAP Operating Margin (25%) | | 2024-2026 Cumulative Growth Portfolio Revenue (Ex-FX) (40%) | | 3-Year
Relative TSR CAGR (35%) | |
| | | | | | | | | | |
| | Achievement
(%) | Payout
(%) | | Achievement
(%) | Payout
(%) | | TSR CAGR
Percentile | Payout
(%) | |
| | | | | | | | | | |
| Maximum | 105% | 200% | | 110% | 200% | | 5% Above 50 %ile | 200% | |
| Target | 100% | 100% | | 100% | 100% | | 50 %ile | 100% | |
| Threshold | 90% | 50% | | 90% | 50% | | 5% Below 50 %ile | 50% | |
| Below Threshold | <90% | 0 | | <90% | 0 | | More than 5% Below 50 %ile | 0 | |
For the non-GAAP Operating Margin and Growth Portfolio Revenue metrics, the targets were set using the Board-approved three-year operating plan. Our Board assessed the rigor of the targets and found that they were appropriate. In addition, our Board assessed the maximum and threshold achievement levels for each of these metrics and found that they were appropriate relative to the intended motivational effect of PSUs.
Bristol Myers Squibb | 2025 Proxy Statement 64
| | |
|
| Compensation Discussion and Analysis |
2024 Market Share Unit Awards
MSUs make up 40% of our executives’ target long-term equity incentives. For 2024, each grant of MSUs vests fully on the 3rd anniversary of the grant date, and the number of shares received by an executive upon vesting increases or decreases depending on the performance of our stock price plus the value of dividends accumulated during the three-year performance period versus the grant date (Total Return). Additionally, we introduced a relative total shareholder return feature to provide a minimum level of payout if our stock price declines from the grant date but outperforms our peers as described below (rTSR Floor). The final payout factor is the greater of Total Return and rTSR Floor.
2024-2026 MSU Payout Schedule
| | | | | | | | | | | | | | | | | | | | | | | |
| | 3-Year Total Return | | 3-Year rTSR Floor | |
| | | | | | | |
| | Total Return Achievement (%) | Payout
(%) | | rTSR Achievement (%ile) | Payout
(%) | |
| | | | | | | |
| Maximum | 225% | 225% | | Greater or Equal to 75 %ile | 100% | |
| Target | 100% | 100% | | Between 50 %ile and 74.99 %ile | 50% | |
| Threshold | 80% | 80% | | Below 50 %ile | 0% | |
| Below Threshold | <80% | 0% | | | | |
Total Return is a ratio of the 10-day average closing stock price on the measurement date, inclusive of the value of accumulated dividends, divided by the 10-day average closing stock price on the grant date (March 10). The measurement date is the February 28 immediately preceding the vesting date. The minimum Total Return performance that must be achieved to earn any payout is 80% of the grant date stock price. The maximum payout factor on Total Return is 225% of target. Payouts are interpolated for performance between threshold, target and maximum.
rTSR Floor performance is based on the TSR percentile rank among our peer companies such that a (i) TSR percentile rank below the 50th percentile yields a 0% payout, (ii) TSR percentile rank between the 50th and 74.99th percentiles yields a 50% payout, and (iii) TSR percentile rank at or above the 75th percentile yields a 100% payout. TSR Percentile Rank means the ranking of the company’s TSR (stock price appreciation or depreciation plus reinvestment of dividends) versus peer companies, expressed as a percentile ranking. There is no interpolation between these distinct points.
At vest, a payout factor is applied to the target number of MSUs to determine the total number of units vesting. If our Total Return increases during the performance period, both the number of units and value of shares that vest increases. If our Total Return declines during the performance period, both the number of units and value of shares that vest decreases. If both our Total Return performance is below 80% and rTSR Floor is below 50th percentile, then the award will be forfeited.
65 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Other Elements of 2024 Compensation
In addition to the components set forth above, our senior executives, including all of our NEOs, were entitled to participate in the following plans or arrangements in 2024:
| | | | | | | | |
| Other Elements of 2024 NEO Compensation | |
| Post-Employment Benefits •Change-in-Control Arrangements •Severance Plan •Non-Qualified Pension Plan (applicable only to Ms. Leung. The qualified Pension Plan was terminated on February 1, 2019) •Qualified and Non-Qualified Savings Plans | |
| Other Compensation | |
| •Perquisites: Financial Planning and Executive Physicals | |
Post-Employment Benefits
We offer certain plans which provide compensation and benefits to employees who have terminated their employment. These plans are periodically reviewed by the Committee to ensure that they are consistent with competitive market practice. The plans offered are intended to enhance our ability to attract and retain key talent.
Change-in-Control Arrangements
We have entered into change-in-control agreements with certain executives including the CEO and other NEOs. These agreements enable management to evaluate and support potential transactions that might be beneficial to shareholders even though the result would be a change-in-control of BMS. Additionally, the agreements provide for continuity of management in the event of a change-in-control. It is our policy that our agreements require a “double-trigger” before any payments are made to an executive. This means that payments are only made in the event of a change-in-control and subsequent involuntary termination or termination for good reason by the employee within either 36 months or 24 months after a change-in-control.
We do not gross up compensation on excess parachute payments for any of our executives, including our Named Executive Officers and it will continue to be our policy on a go-forward basis not to enter into any gross-up arrangements with any of our Named Executive Officers.
If a change-in-control occurs during the term of the agreement, the agreement will continue in effect for either 36 months or 24 months beyond the month in which such change-in-control occurs, as applicable. The value of this benefit for our Named Executive Officers is provided in the “Post-Termination Benefits” section beginning on page 89. Severance Plan
The Bristol Myers Squibb Senior Executive Severance Plan is intended to provide a competitive level of severance protection for certain senior executives (including our Named Executive Officers) to help us attract and retain key talent necessary to run our company.
| | | | | | | | | | | |
| | | |
| | The value of this benefit for our Named Executive Officers is shown in the “Post-Termination Benefits” section beginning on page 89. | |
| | | |
Bristol Myers Squibb | 2025 Proxy Statement 66
| | |
|
| Compensation Discussion and Analysis |
Benefit Equalization Plan—Retirement Plan
The Bristol-Myers Squibb Company Benefit Equalization Plan—Retirement Income Plan (BEP—Retirement Plan or U.S. BEP-RIP) is a non-qualified plan that provides income for employees after retirement in excess of the benefits that were payable under the Bristol-Myers Squibb Company U.S. Retirement Income Plan (Retirement Plan or US-RIP), a tax-qualified defined benefit plan that was terminated, effective February 1, 2019. As of December 31, 2009, BMS discontinued service accruals under the Retirement Plan and the BEP—Retirement Plan in the U.S for active plan participants and closed the plan to new entrants. Active plan participants of the Retirement Plan at year end 2009 were provided five additional years of pay growth in the Retirement Plan. Accordingly, 2014 was the last year of pay growth under the Retirement Plan and the BEP-Retirement Plan. Ms. Leung is the only 2024 NEO participant with a benefit under the BEP-Retirement Plan.
| | | | | | | | | | | |
| | | |
| | For a further discussion, please see “Benefit Equalization Plan–Retirement Plan” beginning on page 85. | |
| | | |
Savings Plans
Our savings plans allow employees to receive matching contributions from BMS to supplement their savings and retirement income. The Bristol-Myers Squibb Company Savings and Investment Program (Savings Plan or U.S. SIP) is a tax-qualified 401(k) plan, as defined under IRS regulations, and the Bristol-Myers Squibb Company Benefit Equalization Plan—Savings and Investment Program (BEP-Savings Plan or BEP-SIP) is a non-qualified deferred compensation plan that allows certain eligible employees to defer a portion of their total eligible cash compensation and to receive matching contribution credits from BMS in excess of contributions allowed under the Savings Plan.
The savings plans are designed to allow employees to accumulate savings for retirement on a tax-advantaged basis. The company matching contribution under the Savings Plan equals 100% of the first 6% of eligible compensation (generally, base salary and annual incentive) that an employee contributes to the Savings Plan for each pay period. If an employee is eligible and has timely elected to defer eligible compensation under the BEP-SIP after reaching an applicable IRS limit under the Savings Plan, the employee can receive a matching contribution credit under the BEP-SIP. The Savings Plan also provides for a discretionary employer non-elective contribution known as the annual additional company contribution that provides a percentage of eligible cash compensation based on a point system of an employee’s age plus service as follows: below 40 points, the percentage is 3%; between 40 and 59 points, is 4.5%; and at 60 points and above, is 6%. To the extent that an annual additional company contribution cannot be made to the Savings Plan due to IRS limits on tax-qualified plan contributions, the excess is credited to the employee under the BEP-SIP.
As of December 31, 2024, all NEOs had 60 or more points and therefore was eligible for a 6% additional annual contribution. The Summary Compensation Table reflects the value of annual additional company contributions to the Savings Plan and company contribution credits to the BEP-Savings Plan during 2024 in the “All Other Compensation” column. The Non-qualified Deferred Compensation Table provides more detail on amounts credited under the BEP Savings Plan.
Other Compensation
We offer very limited perquisites to our Named Executive Officers, including financial planning and counseling services and company-paid annual executive wellbeing checkups. We do not provide tax gross-ups for perquisites provided for the NEOs.
Financial Planning: Consistent with our focus on financial wellbeing, including counseling and financial workshops available to all employees, all of our senior executives, including our Named Executive Officers, are eligible for financial, estate and tax planning counseling paid by the company.
Executive Physicals: To assist our executives with proactively managing their health, and consistent with the value we place on wellbeing, we provide our SVP+ executives access to company-paid annual executive wellbeing checkups.
Fees are paid directly to the providers on the executive’s behalf and are fully taxable to the executive and not eligible for a tax gross-up.
67 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Additionally, for business purposes, the company owns fractional interests in two private aircraft arrangements. We generally do not allow personal use of any aircraft. However, in certain exigent circumstances, an aircraft may be used for personal travel. On very limited occasions, and subject to seat availability, family members may accompany our Named Executive Officers on an aircraft. When a family member accompanies an executive on business travel, this does not incur any incremental cost to the company. In general, incremental costs for personal use consist of the variable costs incurred by the company to operate the aircraft for such use. We did not reimburse any Named Executive Officer for any taxes paid on the taxable income for such personal use.
| | | | | | | | | | | |
| | | |
| | Please see “All Other Compensation” in the “Summary Compensation Table” beginning on page 80 for further discussion of all perquisites and other personal benefits provided to our Named Executive Officers. | |
| | | |
2024 Executive Performance Assessment and Compensation Payout Summary
The Committee noted the following with respect to each of our NEOs’ contributions in 2024. These contributions, as well as each executives’ demonstration of the BMS Values of Accountability, Inclusion, Innovation, Integrity, Passion and Urgency, are taken into account when determining target pay and equity grant values, making career and succession planning decisions and establishing development plans. In addition, the Committee has the discretion to adjust payouts negatively if an executive fails to meet critical performance objectives or does not demonstrate the BMS Values.
For Dr. Boerner, in the role of Chief Executive Officer, the Committee considered:
•performance against financial targets established at the beginning of the year, including total revenue, Growth Portfolio revenue, Non-GAAP EPS and Operating Margin, noting that the company exceeded these financial targets and delivered strong results for the Growth Portfolio and Legacy Portfolio, which supported meaningful progress towards strengthening our growth profile and establishing a younger and more diversified portfolio (together, the “2024 Financial Performance”);
•the exceptional clinical and regulatory performance, including successfully launching new medicines and indications, approval of new indications for late-stage assets, and positive clinical trials’ readouts, including: (i) approval and launch of Cobenfy, which represents the first new mechanism of action in schizophrenia in decades and the potential to expand into multiple additional indications, the twelfth new medicine since 2019; (ii) receiving 23 approvals from the FDA and other international regulatory authorities, including for Opdivo Qvantig for the subcutaneous formulation of nivolumab across most previously approved adult, solid tumor Opdivo indications, Reblozyl for expanded approval in the E.U. to include the first-line treatment of adult patients with transfusion-dependent anemia, Breyanzi for U.S. approval to expand into follicular lymphoma and mantle cell lymphoma, and Krazati in combination with cetuximab, for the treatment of adult patients with KRASG12C-mutated locally advanced or metastatic colorectal cancer; (iii) successfully executing 20 regulatory submissions, including: (A) U.S. and E.U. submissions for subcutaneous nivolumab for 2L RCC; (B) E.U. submission of Opdivo + Yervoy for 1L MSI-High CRC; and (C) E.U. submission of Opdivo for peri-adjuvant NSCLC, substantially achieving all high-value submission and approval goals; and (iv) achieving pivotal, positive clinical trial results, exceeding our 2024 long-term growth goals for Priority Study Execution, Proof of Concept Decisions, and achieving our goal for IND approvals, ensuring the long-term sustainability of our pipeline (together, the “2024 R&D Performance”);
•completion of the closing and integration of the Mirati and Karuna acquisitions, with Mirati strengthening and diversifying our oncology portfolio and Karuna re-establishing our presence in neuroscience with Cobenfy, and developing and implementing an effective operating model for RayzeBio to further enhance our oncology portfolio with the addition of an important radiopharmaceutical capability (together, the “2024 Business Development Transactions”);
Bristol Myers Squibb | 2025 Proxy Statement 68
| | |
|
| Compensation Discussion and Analysis |
•his exhibition of extraordinary leadership in shaping and executing a well-articulated vision and strategy for the company with a strong leadership team to support the company’s continued transformation, including through driving operational excellence and a high-performance inclusive culture; and
•the launch of ASPIRE strategy to advance access for patients in low- and middle- income countries to gain access to potentially life-saving medicines, and creation of a new health equity strategy focused on overcoming the geographic and socio-economic barriers to treatment in a sustainable way. The Committee also considered the progress made on our overall Sustainability and Social Impact priorities described in more detail beginning on page 72. Overall pipeline performance and key milestones are described in more detail on page 70. For Mr. Elkins, in the role of Chief Financial Officer, the Committee considered his: (i) contribution toward the company’s 2024 Financial Performance, noting strong performance for our initiative to achieve $1.5 billion in cost savings by the end of 2025, the majority of which were realized in 2024, and is primarily being reinvested to fund innovation and drive growth; (ii) leadership in forecasting key drivers of performance to maximize strategic allocation of resources, including driving a consistent, balanced approach to capital allocation focused on prioritizing investment for growth through business development, debt reduction, including the repayment of $6 billion in debt consistent with our commitment to pay down approximately $10 billion of debt by 2026, along with a commitment to the dividend and share repurchase; (iii) critical role supporting the 2024 Business Development Transactions, including through financial assessments, diligence, oversight of deal financing arrangements, integration planning, including related to organizational design, synergy capture and talent management; (iv) role in building and maintaining a strong financial foundation that provides flexibility to pursue strategic opportunities and return capital to shareholders; and (v) leadership evolving an integrated and effective finance organization that is poised to support execution of the company’s strategy.
For Dr. Hirawat, in the role of Chief Medical Officer, Head of Development, the Committee considered his: (i) leadership of the drug development organization which led to the exceptional 2024 R&D Performance, including the substantial achievement of all high value milestones for submissions and approvals and achievement of 23 additional priority studies; (ii) significant oversight of portfolio prioritization efforts, including substantial progress against key enterprise initiatives designed to support acceleration of our R&D engine, enhance productivity and efficiency to help identify assets with an increased probability of making it to market faster; and (iii) exceptional role in building and strengthening the drug development organization, including through creatively navigating regulatory challenges and patient needs, aligning team goals and accelerating clinical trial timelines by transforming ways of working and developing critical talent, which provide necessary support to enhance and diversify the company’s portfolio as we navigate a period of losses of exclusivity. Overall pipeline performance and key milestones are described in more detail on page 70. For Ms. Leung, in the role of General Counsel, the Committee considered her: (i) critical role in providing strategic support and advice to our Board and management; (ii) proactive role in managing key legal issues involving the Company, including our intellectual property and product pricing in a period of increased competition and a dynamic regulatory and macro-economic environment, and supporting key business development activities that advance value creation, build depth in scientific areas of focus and enhance our ability to drive growth; (iii) leadership in advancing our cohesive our overall environmental, social and governance strategy, including the validation of our near-term and net-zero science-based targets from the Science Based Target initiative (SBTi), and preparation for compliance with the Corporate Sustainability Reporting Directive (CSRD); and (iv) contributions and performance as a trusted and respected senior leader who provides valuable strategic advice and whose impact spans across all teams and functions.
For Ms. Shanahan, in the role of Global Product Development & Supply, the Committee considered her: (i) accountability for the quality and efficiency of our manufacturing process to deliver products, and optimizing our product supply organization, including through capacity building to support our evolving cell therapy franchise and critical product launches, including Cobenfy and Opdivo Qvantig (ii) leadership in our extensive product quality and safety monitoring processes to ensure the integrity of our products and services meet or exceed expectations, as well as applicable laws and regulations; and (iii) critical role in supporting the submission for validation of our near-term and net-zero science-based targets from the Science Based Target initiative (SBTi), which highlights our progress in reducing the emissions across our operations and supply chain.
69 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
2024 Annual Incentive Plan Program Outcomes
The payouts for the 2024 annual incentive plan were based on each NEO’s target bonus amount and the Company Performance Factor.
Company performance results for the year led to a Company Performance Factor of 140.29% for 2024. The calculation was based on the following performance against goals:
| | | | | | | | | | | | | | | | | | | | |
| Performance Measure | Target | Actual | % of Target | Resulting Payout Percentage | |
| | | | | | |
| Non-GAAP Operating Income ($M) | $17,500 | $18,577 | 106.2 | % | 138.45 | % | |
| Growth Portfolio Revenue (Ex-FX) ($M) | $22,500 | $22,799 | 101.3 | % | 100.00 | % | |
| Pipeline Score | 3 | 4.9 | 163.3 | % | 195.00 | % | |
| Sustainability Scorecard | 3 | 4.0 | 133.3 | % | 150.00 | % | |
| Total | | | 121.5 | % | 140.29 | % | |
Pipeline Performance
For the pipeline metric, the S&T Committee annually reviews performance in the near-term value and long-term growth potential categories and holistically assesses the quality of the results to determine a performance score using a scale of one to five, with three being target.
For 2024, we exceeded our target goal ranges for both submissions and approvals under near-term value, with all high value targets achieved. We advanced the new launch portfolio with approval of our 12th new medicine since 2019, Cobenfy, for the treatment of schizophrenia in adults. We achieved all high value milestones for submissions and approvals, including (i) (A) U.S. and E.U. submissions for subcutaneous nivolumab for 2L renal cell carcinoma (RCC); (B) U.S. and E.U. submission of Opdivo + Yervoy for 1L MSI-High CRC; and (C) E.U. submission of Opdivo for peri-adjuvant NSCLC; and (ii) (A) U.S. approval of Opdivo for peri-adjuvant NSCLC; (B) U.S. and E.U. approval of Opdivo for 1L MIUC cis-eligible; (C) E.U. and Japan approval of Reblozyl for 1L MDS; (D) U.S. approval of Breyanzi for 3L+ CLL; (E) U.S. and Japan approval of Breyanzi for relapsed or refractory FL; and (F) U.S. approval of Cobenfy for schizophrenia. We exceeded our 2024 long-term growth goals for Priority Study Execution, Proof of Concept Decisions, and achieved our goal for IND approvals.
We delivered exceptional results while also evolving our R&D organization, transforming ways of working and accelerating the portfolio despite increased competition and an evolving regulatory and macro-economic environment. The S&T Committee considered the specific milestones that were achieved and those that were not achieved and determined, based on a holistic review, to recommend a pipeline score of 4.9, which the Committee approved. In making its recommendation, the S&T Committee took into account: (i) the degree of difficulty of achievement; (ii) the substantial achievement of all high value milestones for submissions and approvals; (iii) the number of priority studies that exceeded their operating targets (17/24); (iv) the acceleration of the portfolio despite increased competition and an evolving regulatory and macro-economic environment; and (v) the impact the achievement of those goals has on the long- and short-term sustainability of our pipeline for our shareholders and our patients.
Bristol Myers Squibb | 2025 Proxy Statement 70
| | |
|
| Compensation Discussion and Analysis |
The following results were among the inputs considered in determining the pipeline score:
| | | | | | | | | | | | | | | | | | | | |
| Near-Term Value 43 Goals Achieved (Target 30-34) | | | Launches | | •Launched 12th New Molecular Entity (NME) in the US — Cobenfy (xanomeline and trospium chloride) for the treatment of schizophrenia in adults. |
| | | Approvals | | •Exceeded approvals goal with 23 achieved (target 15-17); achieved all 9 high-value approval goals. •Achieved approvals for key lifecycle management (LCM) opportunities: ◦Abecma for 3L+ multiple myeloma (US, EU) ◦Augtyro for ROS1-positive NSCLC (JP, CN); Augtyro for NTRK-positive solid tumors (US) ◦Breyanzi for 3L+ CLL (US); Breyanzi for relapsed or refractory FL (US, JP); Breyanzi for relapsed or refractory MCL (US) ◦Camzyos for obstructive hypertrophic cardiomyopathy (oHCM) (CN) ◦Eliquis for the Extension of Indication to include the pediatric treatment of venous thromboembolism (VTE) and prevention of recurrent VTE (EU) ◦Krazati for 3L+ CRC (US) ◦Opdivo for 1L MIUC cis-eligible (US, EU, JP); Opdivo for peri-adjuvant NSCLC (US); Opdivo + Yervoy for MSI-High CRC (EU, CN) ◦Reblozyl for 1L MDS (EU, JP) ◦Zeposia in moderate-to severe ulcerative colitis (JP) |
| | | Submissions | | •Exceeded submissions goal with 20 achieved (target 15-17); achieved all 5 high-value submission goals. |
| | | Topline Readouts | | •Achieved 4 positive topline readouts for key registrational programs: Opdivo + Yervoy in 1L MSI-High CRC (CheckMate -8HW), Opdivo + Yervoy in 1L hepatocellular carcinoma (CheckMate -9DW), cendakimab in eosinophilic esophagitis (CC-93538-EE-001), and Sotyktu in psoriatic arthritis. |
| | | Key Pipeline Advancement | | •Advanced key NME portfolio opportunities into registrational phase: ◦atigotatug + nivolumab in 1L extensive-stage small cell lung cancer (ES-SCLC) ◦arlocabtagene autoleucel (GPRC5D CAR T) in relapsed or refractory MM ◦AR LDD in metastatic castration-resistant prostate cancer (mCRPC) ◦golcadomide in 1L large B-cell lymphoma ◦nivolumab + relatlimab HD in 1L NSCLC |
| | | | | | | | | | | | | | | | | | | | |
| Long-Term Growth Potential 40 Goals Achieved (Target 25-31) | | | IND/CTA Approvals | | •Continued progressions of novel modalities into the clinic. •Achieved 9 Investigational New Drug/Clinical Trial Authorization (IND/CTA) Approvals (target 7-9), including two high-potential programs. |
| | | Priority Study Execution | | •Exceeded Priority Study Execution (PSE) goal, with 21 priority studies achieving or exceeding execution goals (target 14-16), with 17 of 21 exceeded (81%) with accelerations ranging from 2 weeks to 5 months. |
| | | Proof of Concept “Go/No-go” Decisions | | •Exceeded our Proof of Concept (PoC) ‘Go/No-go’ Decisions goal, with a total of 10 decisions (target 4-6), 6/10 are ‘Go’ decisions, including all 3 high-potential programs: atigotatug + nivolumab in 1L ES-SCLC; AR LDD in mCRPC; EGFR x HER3 Bispecific ADC in NSCLC; PRMT5 Inhibitor in solid tumors; CD19 NEX T in severe refractory systemic lupus erythematosus; MYK-224 in heart failure with preserved ejection fraction (HPpEF).1 |
(1)MYK-224 HFpEF ‘Go’ decision was based on MYK-224 oHCM Ph2 (MERCUTIO) and Camzyos HFpEF Ph2 (EMBARK) modeling/simulation
71 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Sustainability Scorecard Performance
For the Sustainability Scorecard metric, the Committee determines performance on a scale of 1-5 based on a quantitative assessment of achievement of the 2024 goals and a qualitative assessment of the relative impact of the goals. For 2024, based on the results we achieved, the CMDC determined that a score of 4 was appropriate for this metric.
| | | | | | | | | | | |
| Scorecard Element | 2024 Goals | Results |
| | | |
Environmental | Progress towards environmental commitments | •Science-based target initiative (SBTi) reviews and approves BMS’ SBTi submission which reflects near- and long-term milestones for achieving net zero greenhouse gas emissions by 2050 | Achieved |
Human Capital Management and Social Responsibility | Progress on patient access | •8 filings in LMIC countries in support of expanding access in order to meet long-term goal of increasing the number of patients in LMICs | Exceeded (11 filings) |
| Enhancement of leadership capabilities | •For leaders at the Vice President level and above, 75% will enhance their inclusive leadership capabilities through participation in learning experiences focused on inclusive talent practices and engagement | Exceeded (100% participation) |
| Employee Inclusive Engagement trends | •Average Inclusive Engagement score between 72 and 74 | Achieved (average 72) |
2024 Annual Incentive Award Payments
The actual annual incentive awards paid to our NEOs are shown in the table below and can also be found in the Summary Compensation Table under the Non-Equity Incentive Plan Compensation column:
| | | | | | | | | | | | | | |
| Executive | Target Incentive
Award
($) | Actual Payout: Applying Company Performance Factor(1) ($) | |
| | | | |
| Christopher S. Boerner, Ph.D. | 2,306,352 | | 3,235,581 | | |
| David V. Elkins | 1,115,000 | | 1,564,234 | | |
| Samit Hirawat, M.D. | 1,115,000 | | 1,564,234 | | |
| Sandra Leung | 1,150,000 | | 1,613,335 | | |
| Karin Shanahan | 911,434 | | 1,278,651 | | |
(1)Adjusted to reflect Company Performance Factor (financial, pipeline and sustainability metrics performance) earned at 140.29%.
As set forth in the table above, the Company Performance Factor of 140.29% was applied to each Named Executive Officer’s target incentive award.
Bristol Myers Squibb | 2025 Proxy Statement 72
| | |
|
| Compensation Discussion and Analysis |
2024 Long-Term Incentive Payout
Outcomes of the 2021-2024 PSUs
The 2021-2024 PSUs, granted on March 10, 2021, and having a three-year performance cycle were evaluated and certified in March 2024. The below table summarizes the outcome for each of the metrics included in the 2021 PSUs and the associated payout level in terms of percentage of target shares.
| | | | | | | | | | | | | | | | | | | | |
| Performance Metric | Target | Actual(2) | % of Target | Resulting Payout Percentage | |
| | | | | | |
| Cumulative 3-Year Total Revenues, Net of Foreign Exchange ($=MM)(1) | $ | 141,330 | | $ | 141,313 | | 100.00 | % | 99.98 | % | |
| Cumulative 3-Year Non-GAAP Operating Margin | 42.70 | % | 41.70 | % | 97.60 | % | 97.57 | % | |
| 3-Year Relative TSR (TSR Percentile Rank) | 50.0%ile | 17.1%ile | | 0 | % | |
| Total | | | | 65.19 | % | |
(1)Actual Total Revenues are restated to the 2021 Budget Rate.
(2)Includes adjustments for (i) SEC reporting change in 2022 to account for the net impact of IPRD & Licensing Income items that are no longer specified, (ii) the transfer of the Company’s commercial operations in Russia to a third-party distributor, (iii) the Turning Point acquisition, and (iv) timing of the generic competition for Eliquis in the UK and the Netherlands.
MSU Performance Results
The following table summarizes the payout factors relating to the outstanding MSU tranches that vested in 2024. Each award of MSUs granted prior to 2024 vests 25% on each of the first four anniversaries of the grant date, and the number of shares received by an executive upon vesting increases or decreases depending on the performance of our stock price during the one-, two-, three- and four-year performance periods. At vest, a factor is applied to the target number of MSUs to determine the total number of units vesting. The payout factor is a ratio of the 10-day average closing price on the measurement date divided by the 10-day average closing price on the grant date (March 10). The measurement date is the February 28 immediately preceding the vesting date. The minimum performance that must be achieved to earn any payout for awards granted in 2022 and 2023 is 80% and 60% for awards granted prior to 2022. The maximum payout factor is 225% for awards granted in 2022 and 2023 and 200% for awards granted prior to 2022. If our stock price performance is below 80% or 60%, respectively, then the portion of the award scheduled to vest will be forfeited.
| | | | | | | | | | | | | | | | | | | | |
| Grant Date | | Vesting Date | # of Years in
Performance Period | Payout Factor
(%) | |
| | | | | | |
| March 10, 2020 | | March 10, 2024 | 4 | 84.25 | % | |
| March 10, 2021 | | March 10, 2024 | 3 | 82.77 | % | |
| March 10, 2022 | | March 10, 2024 | 2 | 0.00 | % | |
| March 10, 2023 | | March 10, 2024 | 1 | 0.00 | % | |
| November 01, 2023 | (1) | November 01, 2024 | 1 | 98.13 | % | |
(1)Represents an MSU award granted to Dr. Boerner in connection with his appointment as CEO in November 2023.
Restricted Stock Units and Stock Options
Restricted stock units may be granted selectively to executives at other times of the year generally, as inducement grants as part of an offer in attracting candidates to BMS, for retaining employees, or for providing special recognition, such as when an employee assumes significant increases in responsibility. Among our Named Executive Officers, the Committee granted a one-time restricted stock unit award to Ms. Shanahan valued at $1.0 M on November 1, 2024. The award was provided to recognize her value to the company. The award will vest in three equal installments on the first three anniversaries of the grant date. This award is included in the “Stock Awards” column of the 2024 Summary Compensation Table on page 80. 73 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Executive Compensation Governance Practices
Best Practice Compensation Governance
We maintain a number of compensation governance best practices, which support our overarching compensation philosophy and are fully aligned with our compensation principles, as discussed in the following section. Our compensation practices also align with input we have received from shareholders.
| | | | | | | | | | | | | | | | | | | | |
| What We Do: | | | What We Don’t Do: | |
| | | | | | |
| | | | | | |
| ü | 100% performance-based annual and long-term incentives | | | X | No guaranteed incentives with our Named Executive Officers | |
| | | | | | |
| | | | | | |
| ü | Caps on the payouts under our annual and long-term incentive award programs | | | X | Prohibition on speculative and hedging transactions | |
| | | | | | |
| | | | | | |
| ü | Robust share ownership and share retention guidelines | | | X | Prohibition on pledging shares and holding them in a margin account | |
| | | | | | |
| | | | | | |
| ü | Neutralize share buyback impact on share-denominated compensation metrics | | | X | Proactively eliminate windfall gain potential | |
| | | | | | |
| | | | | | |
| ü | Robust recoupment and clawback policies | | | X | No employment contracts with our Named Executive Officers | |
| | | | | | |
| | | | | | |
| ü | Proactive shareholder engagement | | | X | Prohibition on re-pricing or backdating of equity awards | |
| | | | | | |
| | | | | | |
| ü | “Double trigger” change-in-control agreements | | | X | Minimal perquisites to our Named Executive Officers | |
| | | | | | |
Management Accountability & Compensation Recoupment
BMS employs a number of long-standing compensation best practices, which are designed to align pay to the achievement of both our short-term and long-term goals, engagement of our employees, the achievement of our mission, delivery of value to our shareholders and reinforcement of BMS Values.
In 2020, the company participated in an incentive deferral working group with members of Investors for Opioid and Pharmaceutical Accountability (“IOPA”). The participants included shareholders and corporate representatives from the pharmaceutical industry who worked to develop a set of principles that focus on incentive deferrals as one strategy to assist boards in recouping compensation in the event of misconduct.
We welcomed the opportunity to participate in the incentive deferral working group with other members of IOPA and provide greater insight into our existing compensation principles on this matter. The elements of our compensation plan that we discussed included our recoupment and clawback policy, share retention guidelines, long-term equity award performance periods and executive pre-clearance process for transactions involving company securities. We believe the many components of our plan provide the company with the ability to hold our executives accountable and recoup compensation in the event of misconduct. We are pleased that our long-standing practices meet the objectives outlined by shareholders within the working group.
Bristol Myers Squibb | 2025 Proxy Statement 74
| | |
|
| Compensation Discussion and Analysis |
The disclosures in the chart below, with additional detail following, highlight the levers the Committee could potentially use to hold executives accountable in the event they engage in any misconduct. This reflects and was responsive to the shareholders’ feedback and consistent with our shared desired outcome of greater transparency and disclosure.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Share Retention Requirements for Executives | | | |
Maximum flexibility of the Committee in ensuring that our pay programs are aligned with executives acting in the best interests of our shareholders | | | | | | |
| 1 | | | | |
| | | |
| | | | | |
| | | | Recoupment Policy |
| | | | | |
| 2 | | | | |
| | | |
| | | | | |
| | | Reduction or Elimination of Current Year Bonus & Equity Award | |
| | | | | |
| 3 | | | | |
| | | |
| | | | | |
| | | | Forfeiture Provisions |
| | | | | |
| 4 | | | | |
| | | |
| | | | | |
| | | Pre-Clearance Process for Section 16 Officers | |
| | | | | |
| 5 | | | | |
| | | |
| | | | | |
| | | | | | |
Share Ownership and Retention Policy
In order to preserve the link between the interests of our Named Executive Officers and those of our shareholders, certain senior executives, including our NEOs are expected to use the shares acquired upon the vesting of (i) Performance Share Unit awards, (ii) Market Share Unit awards and (iii) Restricted Stock Unit awards, if any, after satisfying the applicable taxes, to establish and maintain a significant level of direct ownership. This same expectation applies to shares acquired upon the exercise of any previously granted stock options. We continue to maintain long-standing share ownership expectations for our senior executives. Our current Named Executive Officers all comply with their ownership and retention requirements, as detailed in the following table:
| | | | | | | | | | | | | | | | | | | | |
| | Share Retention Policy—applied to all shares acquired, net of taxes | |
| | | | | | |
| Executive | Stock Ownership Guideline as a Multiple of Salary | Prior to Achieving Guideline | After Achieving Guideline1 | 2024 Compliance with Share Ownership and Retention Policy | |
| | | | | | |
| Christopher S. Boerner, Ph.D. | 6 x | 100% | 75% for 1 year | Yes | |
| David V. Elkins | 3 x | 100% | 75% for 1 year | Yes | |
| Samit Hirawat, M.D. | 3 x | 100% | 75% for 1 year | Yes | |
| Sandra Leung | 3 x | 100% | 75% for 1 year | Yes | |
| Karin Shanahan | 3 x | 100% | 75% for 1 year | Yes | |
(1)Our share retention policy requires executives to hold 75% of all newly acquired shares for 1 year after vesting even if they have met their share retention requirement. If they have not met their share retention requirement, they must hold 100% of the vested shares.
75 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
Clawback and Recoupment of Compensation Policies
Recoupment of Compensation for Compliance and Other Policy Violations
We maintain robust incentive compensation recoupment or “clawback” policies and practices for use in the event of certain compliance violations (the “Compliance Violations Recoupment Policy”), or other policy violations relating to our equity awards or financial statements (together, the “Other Policy Violations Recoupment Policies”). Our Compliance Violations Recoupment Policy and Other Policy Violations Recoupment Policies include clawback provisions relating to our short-term and long-term annual incentive programs, including related to stock options, restricted stock units, performance share units and market share units. The below table provides further details on these clawback provisions.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| If an executive or other employee, |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| engaged in misconduct | | engaged in misconduct or failed to appropriately supervise an employee who engaged in misconduct | | engaged in other misconduct |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| and the misconduct |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| caused or partially caused restatement of financial results | | was a material violation of a company policy relating to the research, development, manufacturing, sales or marketing of pharmaceutical products, which resulted in a significant negative impact on our results of operations or market capitalization | | was a violation of non-competition or non-solicitation agreements or caused detriments to company interest |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| then the Company can seek reimbursement/recoupment of: |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | 1 | | + | | 2 | | | + | | | 3 | | + | | 4 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Current/Relevant Period bonus(1) | Future bonus | | | Future award | Outstanding award(2) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Short-Term Annual Incentive | | | | | Long-Term Equity | |
(1)In 2005, the Board adopted a policy wherein the Board will seek reimbursement of annual incentive awards paid to an executive if such executive engaged in misconduct that caused or partially caused a restatement of financial results. The clawback amount will also include reasonable interest, where applicable.
(2)As a result of any such violation, (i) any portion of unvested and unsettled awards will be rescinded, (ii) vested but unsettled awards will be forfeited, and (iii) an employee must return shares received from awards that were vested and settled in the 12-months before the violation.
Our Compliance Violations Recoupment Policy also provides that, if legally permissible, the company will publicly disclose whenever a decision has been made to use such policy, so long as the underlying event has already been publicly disclosed with the SEC.
Bristol Myers Squibb | 2025 Proxy Statement 76
| | |
|
| Compensation Discussion and Analysis |
Recoupment of Compensation for Restatements
In addition to our existing clawback policies, effective December 2023, the Committee also approved a separate policy for the Recoupment of Compensation for Accounting Restatements (the “Restatement Recoupment Policy”), which is required pursuant to and consistent with, Rule 303A.14 of the NYSE and applies to current and certain former executive officers and requires the Board to recoup excess compensation paid to covered executive officers as a result of a financial statement restatement, regardless of any misconduct, fault or illegal activity on the part of the covered executive officer.
The full Compliance Violations Recoupment Policy and our separate Restatement Recoupment Policy may each be viewed on our website at www.bms.com.
Pre-clearance for Section 16 Officers
All members of the Board and all other Section 16 Officers, including our Named Executive Officers, must obtain pre-clearance from the Corporate Secretary’s office prior to making any sale, purchase, stock option exercise, gift, or other transaction in company securities. We work with our plan administrator to permanently restrict the account of all Section 16 Officers, effectively restricting any activity in their brokerage accounts related to our company securities. This permanent restriction and requirement for pre-clearance are mechanisms the company uses to administer our insider trading policy, the share ownership requirements for executives and our clawback policy. Together, these help to ensure that executives act in the best interest of BMS and our shareholders.
Forfeiture of Long-Term Equity Awards Upon Retirement or Termination
In general, in the event of retirement or a qualifying involuntary termination, upon signing a general release, employees are eligible to vest in a pro-rata portion of unvested RSUs, PSUs, and MSUs held at least one year from the grant date, subject to meeting applicable performance goals. However, executives who are found to have engaged in severe misconduct or in an activity, which may include a failure to take action, deemed detrimental to the interests of the company including, but not limited to acts involving dishonesty, violation of company policies, violation of safety rules, disorderly conduct, discriminatory harassment, unauthorized disclosure of confidential information, or the entry of a plea of nolo contendere to, or the conviction of, a crime, will be terminated and will forfeit any outstanding awards, as of the date such violation is discovered. As noted, these provisions help to ensure that executives act in the long-term best interest of BMS and our shareholders.
For further discussion on forfeiture provisions related to retirement, termination or death, please see the discussion under the header “Post-Termination Benefits” beginning on page 89. Equity Grant Policy
The Committee’s policy covering equity and equity-based grants for our Named Executive Officers is as follows:
Approval of Awards
•Awards granted to the CEO must be approved by the Committee and recommended by the Committee to, and approved by at least 75% of, the independent directors of our Board.
•The Committee approves awards to all other Named Executive Officers.
Grant Effective Date
Annual Awards
•Our regularly scheduled annual equity awards are approved on the date the Committee and full Board meet during the first week of March, with a grant effective date of March 10.
77 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Compensation Discussion and Analysis |
All Other Awards
•For awards granted to current employees at any other time during the year, the grant effective date is the first business day of the month following the approval date, except that if the approval date falls on the first business day of a given month, the grant effective date is the approval date.
•For awards granted to new hires, the grant effective date is the first business day of the month following the employee’s hire date, except that if the employee’s hire date falls on the first business day of a given month, the grant effective date is the employee’s hire date.
In no event will the grant effective date precede the approval date of a given award. We do not time the disclosure of material non-public information, or the granting of equity-based grants, for the purpose of impacting the value of executive compensation.
Grant Price
•The grant price of awards is a 10-day average closing price (i.e., an average of the closing price on the grant date plus the 9 prior trading days). For stock options that may be granted under special circumstances (none have been granted since 2009), the grant price will be the closing price on the date of grant.
Policy Prohibiting Hedging and Pledging
We consider it improper and inappropriate for our directors, officers, and other employees to engage in any transactions that hedge or offset, or are designed to hedge or offset, any decrease in the value of our securities. As such, our insider trading policy prohibits all employees, including directors and executive officers, from engaging in any speculative or hedging transactions or any other transactions that are designed to offset any decrease in the value of our securities. Our insider trading policy also prohibits all employees, including directors and executive officers, from holding our securities in a margin account or pledging our securities as collateral for a loan except in certain limited circumstances pre-approved by our Corporate Secretary when a person wishes to pledge our securities as collateral for a loan and clearly demonstrates the ability to repay the loan without selling such securities. None of our directors or executive officers has pledged shares of our stock as collateral for a loan or holds shares of our stock in a margin account.
Policy Prohibiting the Repricing of Stock Options Without Shareholder Consent
We have always maintained a consistent policy against the repricing of stock options. We believe this is a critical element in maintaining the integrity of the equity compensation program and ensuring alignment of senior executives’ interests with the interests of shareholders. Our Board has adopted a formal policy prohibiting the repricing of stock options. This policy may be viewed on our website at www.bms.com.
Policy Regarding Shareholder Approval of Severance
Our Board has approved a policy that requires shareholder approval of any future agreements that provide for cash severance payments in excess of 2.99 times the sum of an executive’s base salary plus annual incentive award. “Cash severance payments” exclude accrued incentive payments, the value of equity acceleration, benefits continuation or the increase in retirement benefits triggered by severance provisions or tax gross-up payments. This policy may be viewed on our website at www.bms.com.
Risk Assessment of Executive Compensation
The Committee annually reviews the compensation programs from a risk perspective. Based on that review of the executive compensation arrangements for our executives as detailed beginning on page 54, the Committee believes that our compensation program does not encourage executives to take excessive or inappropriate risks that could maximize short-term results at the expense of sustainable long-term value creation that may harm shareholder value. The Committee’s ongoing review of our business strategy and our extensive shareholder engagement efforts have allowed our executive compensation program to maintain close alignment with our strategic focus and the perspectives of our shareholders.
Bristol Myers Squibb | 2025 Proxy Statement 78
| | |
|
| Compensation Discussion and Analysis |
Our compensation program is intended to achieve this by striking an appropriate balance between short-term and long-term incentives, using a diversity of metrics to assess performance and payout under our incentive programs, placing caps on our incentive award payout opportunities, following equity grant practices that limit potential for timing awards and having stock ownership and retention requirements. For example, our current long-term equity incentive program (60% Performance Share Units and 40% Market Share Units) incorporates the company’s stock price into its performance measures and generally magnifies the impact of changes in our stock price as well as relative total shareholder return performance over the mid- and longer-term.
Also embedded in the Committee’s annual review is the ongoing assessment of enterprise risk, including reputational risks stemming from the dynamic external environment. In addition, we evaluate the performance of each of our executives based on a number of factors, including how they demonstrate our BMS Values in the execution of their day-to-day decisions. Those BMS Values include, among others, accountability. This evaluation is one input into the determination of incentive payout opportunities. Given the direct link between BMS Values and our executive compensation program’s emphasis on sustainable long-term value, we attempt to minimize and appropriately reduce the possibility that our executive officers will make excessively or inappropriately risky decisions that could maximize short-term results at the expense of sustainable long-term value creation for our shareholders.
Compensation and Management Development Committee Report
The Compensation and Management Development Committee of Bristol Myers Squibb has reviewed and discussed with management the “Compensation Discussion and Analysis” on pages 45 to 79 of this Proxy Statement as required under Item 402(b) of Regulation S-K. Based on its review and discussions with management, the Committee recommended to the full Board that the Compensation Discussion and Analysis be included in this Proxy Statement. Compensation and Management Development Committee
Peter J. Arduini, Chair
Deepak L. Bhatt, M.D., M.P.H., M.B.A.
Derica W. Rice
Karen H. Vousden, Ph.D.
Tax Implications of Executive Compensation Program
When setting executive compensation, we consider many factors, such as attracting and retaining executives and providing appropriate performance incentives. We also consider the after-tax cost to the company in establishing executive compensation programs, both individually and in the aggregate, but tax deductibility is not our sole consideration. Section 162(m) of the Internal Revenue Code generally disallows a federal income tax deduction to public companies for annual compensation over $1 million (per individual) paid to their chief executive officer, chief financial officer and the next three most highly compensated executive officers (as well as certain other officers who were covered employees in years after 2016). The 2017 Tax Act eliminated most of the exceptions from the $1 million deduction limit, except for certain arrangements in place as of November 2, 2017. As a result, most of the compensation payable to our Named Executive Officers in excess of $1 million per person in a year will not be fully deductible.
79 Bristol Myers Squibb | 2025 Proxy Statement
Executive
Compensation Tables
Summary Compensation Table
The following tables and notes present the compensation provided to Christopher S. Boerner, Ph.D., Board Chair and Chief Executive Officer, David V. Elkins, EVP and Chief Financial Officer and the three other most highly compensated Executive Officers.
Fiscal Years Ended December 31, 2024, 2023, and 2022
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and
Principal Position | Year | Salary(1) ($) | Bonus ($) | Stock Awards(2) ($) | Non-Equity Incentive Plan Compensation(3) ($) | Change in Pension Value and Non-Qualified Deferred Compensation Earnings(4) ($) | All Other Compensation(5) ($) | Total ($) | |
| | | | | | | | | | |
| Christopher S. Boerner, Ph.D. Board Chair and Chief Executive Officer | 2024 | 1,536,538 | | 0 | | 13,643,063 | | 3,235,581 | | 0 | | 372,436 | | 18,787,618 | | |
| 2023 | 1,256,921 | | 0 | | 5,326,178 | | 1,567,098 | | 0 | | 311,636 | | 8,461,833 | | |
| 2022 | 1,064,049 | | 0 | | 4,256,197 | | 1,274,626 | | 0 | | 285,348 | | 6,880,220 | | |
| David V. Elkins EVP and Chief Financial Officer | 2024 | 1,115,000 | | 0 | | 5,778,244 | | 1,564,234 | | 0 | | 250,093 | | 8,707,571 | | |
| 2023 | 1,106,176 | | 0 | | 4,882,143 | | 866,318 | | 0 | | 311,663 | | 7,166,300 | | |
| 2022 | 1,073,859 | | 0 | | 4,922,614 | | 1,397,863 | | 0 | | 252,954 | | 7,647,290 | | |
| Samit Hirawat, M.D. EVP, Chief Medical Officer, Head of Development | 2024 | 1,115,000 | | 0 | | 5,617,762 | | 1,564,234 | | 0 | | 289,018 | | 8,586,014 | | |
| 2023 | 1,103,781 | | 0 | | 3,616,394 | | 1,234,117 | | 0 | | 279,335 | | 6,233,627 | | |
| Sandra Leung EVP and General Counsel | 2024 | 1,150,000 | | 0 | | 3,959,204 | | 1,613,335 | | 0 | | 289,251 | | 7,011,790 | | |
| 2023 | 1,147,819 | | 0 | | 3,345,168 | | 1,123,091 | | 0 | | 307,403 | | 5,923,481 | | |
| 2022 | 1,133,074 | | 0 | | 3,441,053 | | 1,238,952 | | 0 | | 291,748 | | 6,104,827 | | |
| Karin Shanahan EVP, Global Product Development & Supply | 2024 | 1,009,615 | | 0 | | 4,145,908 | | 1,278,651 | | 0 | | 132,407 | | 6,566,581 | | |
(1)Reflects actual salary earned.
(2)Represents aggregate grant date fair value under FASB ASC Topic 718 of restricted stock unit ("RSU"), market share unit ("MSU") and performance share unit ("PSU") awards granted during a specified year. Further information regarding these awards, including the assumptions made in determining their values, is disclosed in the Grants of Plan-Based Awards Table in the Proxy Statements for the specified years. For PSU awards, the following represents the aggregate value based on the maximum number of shares that can be earned for the awards granted in the specified years.
Bristol Myers Squibb | 2025 Proxy Statement 80
| | |
|
| Executive Compensation Tables |
| | | | | | | | | | | | | | | | | |
| | Performance Share Units ($) | |
| | | | | |
| Name | 2022 | 2023 | 2024 | |
| | | | | |
| Christopher S. Boerner, Ph.D. | 4,252,783 | | 5,325,883 | | 12,371,347 | | |
| David V. Elkins | 4,918,660 | | 4,865,228 | | 5,239,599 | | |
| Samit Hirawat, M.D. | n.a. | 3,603,884 | | 5,094,094 | | |
| Sandra Leung | 3,438,220 | | 3,333,559 | | 3,590,153 | | |
| Karin Shanahan | n.a. | n.a. | 2,910,935 | | |
(3)Represents incentive award earned under our Company's annual incentive plan. For 2024, the payments were made on March 14, 2025. For 2023, the payments were made on March 6, 2024. For 2022, the payments were made on March 8, 2023.
(4)Includes increase in estimated value of accrued pension benefits under the BEP-Retirement Plan during the year. The Company does not pay above-market interest rates on deferred compensation. The present value of the accrued pension benefits for Ms. Leung, the only 2024 NEO participant in the BEP-Retirement Plan, decreased by $564,039 over the previous year due to updates to lump sum assumptions, increase in discount rate and one less year of payments.
(5)The amounts indicated for 2024 represent Company contributions to the Savings Plan and the BEP-SIP and value of perquisites. The details of the components of this column are provided in a table below, immediately following these footnotes. We generally do not allow personal use of any aircraft. On very limited occasions, and subject to seat availability, family members may accompany our Named Executive Officers on an aircraft. On occasion, a family member accompanied Dr. Boerner, at no incremental cost to the Company, when traveling on the Company's NetJets and HeliFlite accounts on business. Dr. Boerner paid the taxes on the imputed income as calculated using the Standard Industry Fare Level (SIFL) rate. We did not reimburse Dr. Boerner for the taxes he paid.
| | | | | | | | | | | | | | | | | | | | | | | |
| Name | Year | Financial Counseling and Tax Preparation(1) ($) | Executive Physical(2) ($) | Company Contributions to Savings Plans(3) ($) | Total All Other Compensation ($) | |
| | | | | | | |
| Christopher S. Boerner, Ph.D. | 2024 | 0 | | 0 | | 372,436 | | 372,436 | | |
| David V. Elkins | 2024 | 12,335 | | 0 | | 237,758 | | 250,093 | | |
| Samit Hirawat, M.D. | 2024 | 0 | | 7,124 | | 281,894 | | 289,018 | | |
| Sandra Leung | 2024 | 16,480 | | 0 | | 272,771 | | 289,251 | | |
| Karin Shanahan | 2024 | 12,343 | | 0 | | 120,064 | | 132,407 | | |
(1)Reflects bills paid for financial counseling and tax preparation services during 2024.
(2)Reflects bills paid for annual executive wellbeing checkups during 2024.
(3)Represent Company contributions to the Savings Plan and BEP-SIP.
81 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
Executive Compensation Tables |
Grants of Plan-Based Awards
2024 Fiscal Year
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | Award Type | Grant Date(1) | Approval Date | Estimated Future Payouts Under Non-Equity Incentive Plan Awards(2) | | Estimated Future Payouts Under Equity Incentive Plan Awards (shares) | | All Other Stock Awards: # of Shares of Stock of Units
($) | | Grant Date Fair Value of Stock and Option Awards
($) | |
| | | | | | | | | | |
| Threshold ($) | Target
($) | Maximum ($) | | Threshold (#) | Target (#) | Maximum (#) | | | |
| | | | | | | | | | | | | | | | |
| Christopher S. Boerner, Ph.D. | AIP | | | 115,318 | | 2,306,352 | | 4,612,705 | | | | | | | | | | |
| PSU | 03/10/24 | 03/01/24 | | | | | 18,503 | 148,026 | 296,052 | (3) | | | 7,857,220 | | (6) |
| MSU | 03/10/24 | 03/01/24 | | | | | 49,342 | 98,684 | 222,039 | (4) | | | 5,785,843 | | (7) |
| David V. Elkins | AIP | | | 55,750 | | 1,115,000 | | 2,230,000 | | | | | | | | | | |
| PSU | 03/10/24 | 03/01/24 | | | | | 7,837 | 62,693 | | 125,386 | (3) | | | 3,327,744 | | (6) |
| MSU | 03/10/24 | 03/01/24 | | | | | 20,898 | 41,796 | | 94,041 | (4) | | | 2,450,499 | | (7) |
| Samit Hirawat, M.D. | AIP | | | 55,750 | | 1,115,000 | | 2,230,000 | | | | | | | | | | |
| PSU | 03/10/24 | 03/01/24 | | | | | 7,619 | 60,952 | | 121,904 | (3) | | | 3,235,332 | | (6) |
| MSU | 03/10/24 | 03/01/24 | | | | | 20,318 | 40635 | 91,429 | (4) | | | 2,382,430 | | (7) |
| Sandra Leung | AIP | | | 57,500 | | 1,150,000 | | 2,300,000 | | | | | | | | | | |
| PSU | 03/10/24 | 03/01/24 | | | | | 5,370 | 42,957 | 85,914 | (3) | | | 2,280,158 | | (6) |
| MSU | 03/10/24 | 03/01/24 | | | | | 14,319 | 28,638 | 64,436 | (4) | | | 1,679,046 | | (7) |
| Karin Shanahan | AIP | | | 45,572 | | 911,434 | | 1,822,869 | | | | | | | | | | |
| PSU | 03/10/24 | 03/01/24 | | | | | 4,354 | 34,830 | 69,660 | (3) | | | 1,848,776 | | (6) |
| MSU | 03/10/24 | 03/01/24 | | | | | 11,610 | 23,220 | 52,245 | (4) | | | 1,361,389 | | (7) |
| RSU | 11/01/24 | 10/29/24 | | | | | | | | | 18,843 | (5) | 935,743 | | (8) |
(1)These equity awards were granted under our 2021 Stock Award and Incentive Plan.
(2)Target payouts under the 2024 annual incentive plan ("AIP") are based on a targeted percentage of annual base salary. The Committee reviews Company performance in determining the actual incentive award as reported in the Summary Compensation Table. The company performance for 2024 was based 35% on growth portfolio revenue, 30% on non-GAAP operating income, 25% on pipeline performance, and 10% on the Sustainability Scorecard metric. Maximum represents the maximum individual incentive award allowable under the 2024 AIP and for the Named Executive Officers, this is 200% of his or her target. For 2024, threshold payout for financial measures was 25% of target and for pipeline and Sustainability Scorecard was 50% of target. The threshold column above reflects the lowest possible combined payout of 5% of target based on the threshold payout on the least weighted metric.
(3)Reflects PSUs which cliff vest on the third anniversary of the grant date. Performance targets under these PSUs are based 40% on 3-year cumulative growth portfolio revenue (net of foreign exchange), 25% on 3-year cumulative non-GAAP operating margin, and 35% on 3-year relative TSR CAGR versus our peer group. Threshold payout for all three measures is 50% of target. The threshold column above reflects the lowest possible combined payout of 12.50% of target based on the threshold payout on the least weighted metric only. The maximum performance will result in a payout of 200% of target. PSUs do not accrue dividend equivalents.
Bristol Myers Squibb | 2025 Proxy Statement 82
| | |
|
| Executive Compensation Tables |
(4)Reflects MSUs which cliff vest on the third anniversary of the grant date. Each MSU converts into the number of shares of Common Stock determined by applying a payout factor to the target number of shares. The payout factor is the greater of the Total Return and relative TSR Floor. Total Return is a ratio of Common Stock price on the February 28 measurement date plus the nine prior trading days, inclusive of the value of accumulated dividends, divided by the average Common Stock price on the grant date (also a 10-day average). The minimum payout factor that must be achieved to earn a payout is 80% of the grant date stock price and the maximum payout factor is 225% of target. rTSR Floor performance is based on the Company’s TSR percentile rank among peer companies. Threshold payout is 50% of target. Maximum payout is 100% of target.
(5)Reflects RSUs which vest in equal annual installments on the first three anniversaries of the grant date.
(6)Fair value for the portion of these PSUs related to the relative TSR CAGR measure (35% weighting) is estimated as of the date of grant on March 10, 2024 using a Monte Carlo simulation. Estimated fair value of this portion was determined to be $64.55, which represents 120% of the grant date closing Common Stock price of $53.79. The assumptions used in this Monte Carlo simulation were as follows: volatility for BMY of 19.1%, the average for the peers of 23.1% and correlation co-efficient average of 29.8% based on three-year historical stock price data; assumed dividend yield of 4.46% based on the most recent annualized payment of $2.40 per share and the grant date stock price of $53.79; BMY’s starting TSR of 4.1% and the average for the peers of 0.4%, and a risk-free rate of 4.26%. Fair value for the remaining portion of these PSUs, related to Company financial measures (65% weighting), is calculated based on the grant date closing Common Stock price of $53.79 on March 8, 2024 and a probable outcome of a 100% payout, discounted for the lack of dividends. Estimated fair value of this portion was determined to be $46.91, which represents 87.2% of the grant date closing Common Stock price of $53.79. Therefore, the estimated grant date fair value for the whole PSU award equals $53.08, which represents 98.7% of the grant date closing Common Stock price of $53.79.
(7)Fair value of these MSUs is estimated as of the date of grant on March 10, 2024 using a Monte Carlo simulation. Estimated fair value was determined to be $58.63, which represents 109% of the grant date closing Common Stock price of $53.79 on March 8, 2024. The assumptions used in the Monte Carlo simulation were as follows: volatility for BMY of 19.1%, the average for the peers of 23.1%, correlation co-efficient average of 29.8% based on three-year historical stock price data; assumed dividend yield of 4.46% based on the most recent annualized payment of $2.40 per share and the grant date stock price of $53.79; BMY’s starting TSR and starting Total Return of 4.1%, the average starting TSR for the peers of 0.4%, and risk-free rate of 4.26%.
(8)The fair value of these RSUs is calculated based on the grant date closing Common Stock price of $54.32 on November 1, 2024, discounted for the lack of dividends. Estimated fair value was determined to be $49.66, which represents 91.4% of the grant date closing Common Stock price of $54.32.
Outstanding Equity Awards at Fiscal Year-End
2024 Fiscal Year
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | Grant Date/ Performance Award Period | Number of Shares or Units of Stock That Have Not Vested(1) (#) | Stock Awards | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Rights That Have Not Vested(2) ($) | |
| | | | | |
| Market Value of Shares or Units of Stock That Have Not Vested(1)(2) ($) | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Rights That Have Not Vested
(#) | | |
| | | | | | | | | |
| Christopher S. Boerner, Ph.D. | 1/1/2022-2/28/2025 | | | | 39,700 | (3) | 2,245,432 | | |
| 1/1/2023-2/28/2026 | | | | 58,186 | (4) | 3,291,000 | | |
| 1/1/2024-2/28/2027 | | | | 148,026 | (5) | 8,372,351 | | |
| 3/10/2021 | | | | 6,985 | (6) | 395,072 | | |
| 3/10/2022 | | | | 10,587 | (7) | 598,812 | | |
| 3/10/2023 | | | | 16,159 | (7) | 913,964 | | |
| 11/1/2023 | | | | 8,894 | (6) | 503,045 | | |
| 3/10/2024 | | | | 49,342 | (8) | 2,790,784 | | |
| David V. Elkins | 1/1/2022-2/28/2025 | | | | 45,916 | (3) | 2,597,009 | | |
| 1/1/2023-2/28/2026 | | | | 47,424 | (4) | 2,682,301 | | |
| 1/1/2024-2/28/2027 | | | | 62,693 | (5) | 3,545,916 | | |
| 3/10/2021 | | | | 8,045 | (6) | 455,025 | | |
| 3/10/2022 | | | | 12,245 | (7) | 692,566 | | |
| 3/10/2023 | | | | 18,970 | (7) | 1,072,921 | | |
| 3/10/2024 | | | | 20,898 | (8) | 1,181,991 | | |
83 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
Executive Compensation Tables |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | Grant Date/ Performance Award Period | Number of Shares or Units of Stock That Have Not Vested(1) (#) | Stock Awards | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Rights That Have Not Vested(2) ($) | |
| | | | | |
| Market Value of Shares or Units of Stock That Have Not Vested(1)(2) ($) | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Rights That Have Not Vested
(#) | | |
| | | | | | | | | |
| Samit Hirawat, M.D. | 1/1/2022-2/28/2025 | | | | 28,593 | (3) | 1,617,220 | | |
| 1/1/2023-2/28/2026 | | | | 35,129 | (4) | 1,986,896 | | |
| 1/1/2024-2/28/2027 | | | | 60,952 | (5) | 3,447,445 | | |
| 3/10/2021 | | | | 4,886 | (6) | 276,352 | | |
| 3/10/2022 | | | | 7,626 | (7) | 431,304 | | |
| 3/10/2023 | | | | 14,052 | (7) | 794,781 | | |
| 3/10/2024 | | | | 20,318 | (8) | 1,149,158 | | |
| Sandra Leung | 1/1/2022-2/28/2025 | | | | 32,096 | (3) | 1,815,350 | | |
| 1/1/2023-2/28/2026 | | | | 32,494 | (4) | 1,837,861 | | |
| 1/1/2024-2/28/2027 | | | | 42,957 | (5) | 2,429,648 | | |
| 3/10/2021 | | | | 5,858 | (6) | 331,328 | | |
| 3/10/2022 | | | | 8,560 | (7) | 484,154 | | |
| 3/10/2023 | | | | 12,998 | (7) | 735,190 | | |
| 3/10/2024 | | | | 14,319 | (8) | 809,883 | | |
| Karin Shanahan | 1/1/2022-2/28/2025 | | | | 14,683 | (3) | 830,470 | | |
| 1/1/2023-2/28/2026 | | | | 19,760 | (4) | 1,117,626 | | |
| 1/1/2024-2/28/2027 | | | | 34,830 | (5) | 1,969,985 | | |
| 3/10/2022 | | | | 3,916 | (7) | 221,489 | | |
| 3/10/2023 | | | | 7,904 | (7) | 447,050 | | |
| 3/10/2024 | | | | 11,610 | (8) | 656,662 | | |
| 4/1/2022 | 6,613 | 374031 | (9) | | | | |
| 11/1/2024 | 18,843 | 1,065,760 | | (10) | | | | |
(1)Represents RSUs outstanding as of December 31, 2024.
(2)Values are based on the closing Common Stock price on December 31, 2024 of $56.56.
(3)Represents target number of PSUs granted under the 2022-2024 award at target payout of 100%. The award vested on March 10, 2025.
(4)Represents target number of PSUs granted under the 2023-2025 award at target payout of 100%. These PSUs cliff vest on the third anniversary of the grant date.
(5)Represents target number of PSUs granted under the 2024-2026 award at target payout of 100%. These PSUs cliff vest on the third anniversary of the grant date.
(6)Represents MSUs at target payout of 100%. These MSUs vest in four equal installments of 25% on each of the first four anniversaries of the grant date, subject to a payout factor.
(7)Represents MSUs at threshold payout of 80%. These MSUs vest in four equal installments of 25% on each of the first four anniversaries of the grant date, subject to a payout factor.
(8)Represents MSUs at the lowest possible threshold payout of 50%. These MSUs cliff vest on the third anniversary of the grant date.
(9)These RSUs vest in four equal installments on each of the first, second, third, and fourth anniversaries of the grant date.
(10)These RSUs vest in three equal installments on each of the first, second, and third anniversaries of the grant date.
Bristol Myers Squibb | 2025 Proxy Statement 84
| | |
|
| Executive Compensation Tables |
Option Exercises and Stock Vested
2024 Fiscal Year
| | | | | | | | | | | | | | | | | |
| | Stock Awards(1) | |
| | | | | |
| Name | Number of Shares Acquired on Vesting
(#) | Value Realized On Vesting(2) ($) | | |
| | | | | |
| Christopher S. Boerner, Ph.D. | 0 | 0 | (3) | |
| 13,528 | 729,213 | | (4) | |
| 27,321 | 1,469,597 | | (5) | |
| David V. Elkins | 0 | 0 | (3) | |
| 13,419 | 721,808 | | (4) | |
| 31,459 | 1,692,180 | | (5) | |
| Samit Hirawat, M.D. | 0 | 0 | (3) | |
| 7,575 | 407,459 | | (4) | |
| 19,108 | 1,027,819 | | (5) | |
| Sandra Leung | 0 | 0 | (3) | |
| 9,724 | 523,054 | | (4) | |
| 22,907 | 1,232,168 | | (5) | |
| Karin Shanahan | 3,306 | 175,185 | | (3) | |
| 0 | 0 | (4) | |
| 0 | 0 | (5) | |
(1)There were no stock options exercised by any of our Named Executive Officers in 2024.
(2)The value realized for each RSU, MSU and PSU award was determined by multiplying the number of units that vested by the closing share price of our Common Stock on the respective vesting date.
(3)Reflects RSUs that vested during 2024.
(4)Reflects MSUs that vested during 2024.
(5)Reflects payouts of the vested 2021-2023 PSUs based on the closing Common Stock price of $53.79 on March 8, 2024.
Benefit Equalization Plan—Retirement Plan
The Bristol-Myers Squibb Company Benefit Equalization Plan—Retirement Income Plan (BEP—Retirement Plan or U.S. BEP-RIP) is a non-qualified plan that provides income for employees after retirement in excess of the benefits that were payable under the Bristol-Myers Squibb Company U.S. Retirement Income Plan (Retirement Plan or US-RIP), a tax-qualified defined benefit plan that was terminated effective February 1, 2019.
By way of background, as of December 31, 2009, BMS discontinued service accruals under the Retirement Plan and the BEP—Retirement Plan in the U.S for active plan participants and closed the plans to new entrants. Active plan participants of the Retirement Plan at year end 2009 were provided five additional years of pay growth in the pension plan. Accordingly, 2014 was the last year of pay growth under the Retirement Plan and the BEP-Retirement Plan. Ms. Leung is the only 2024 NEO with a benefit under the BEP-Retirement Plan.
85 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
Executive Compensation Tables |
Employees whose pay or benefits exceeded the IRS qualified plan limits of the Retirement Plan were eligible for the BEP—Retirement Plan. The key plan provisions of the terminated Retirement Plan and the BEP—Retirement Plan are as follows:
•The retirement benefit generally equals:
◦2% x Final Average Compensation x Years of Service through December 31, 2009, up to 40, minus
◦1/70th of the Primary Social Security Benefit x Years of Service through December 31,2009, up to 40.
•Final Average Compensation equals the average of the five consecutive years out of the last ten years, ending December 31, 2014, in which the employee’s compensation was the highest. Compensation equals the base salary rate plus the higher of annual incentive awards earned or paid during the year. In the BEP—Retirement Plan, there are no limits on compensation and benefits imposed under Section 401(a)(17) and Section 415(b) of the Internal Revenue Code.
•Normal retirement age is 65. Employees are eligible for early retirement at age 55 with 10 or more years of service.
•Employees eligible for early retirement may receive their pension without any reduction at age 60. The pension is generally reduced by 4% for each year that the retirement age precedes age 60. The same reduction factors also apply to employees who satisfy certain requirements relating to involuntary termination (known as “Rule of 70”).
•Employees are 100% vested after attaining five years of service.
•The BEP—Retirement Plan pension is paid as a cash lump sum or, if eligible and an election is made at least 12 months prior to retirement, the lump sum may be credited to the Bristol-Myers Squibb Company Benefit Equalization Plan—Savings and Investment Program. The BEP-Retirement Plan is subject to Section 409A of the Internal Revenue Code. Therefore, a distribution for an executive classified as a “Specified Employee” of the Company, as defined under Section 409A, is subject to a six-month delay following the executive’s separation from service in accordance with the Section 409A regulations.
Bristol Myers Squibb | 2025 Proxy Statement 86
| | |
|
| Executive Compensation Tables |
Present Value of Accumulated Pension Benefits
2024 Fiscal Year
| | | | | | | | | | | | | | | | | | | | |
| Name | Plan Name | # of Years of Credited Service(1) | Present Value of Accumulated Benefits(2) ($) | Payments During Last Fiscal Year
($) | |
| | | | | | |
| Christopher S. Boerner, Ph.D.(3) | Benefit Equalization Plan Retirement Plan | 0 | 0 | | 0 | | |
| David V. Elkins(3) | Benefit Equalization Plan Retirement Plan | 0 | 0 | | 0 | | |
| Samit Hirawat, M.D.(3) | Benefit Equalization Plan Retirement Plan | 0 | 0 | | 0 | | |
| Sandra Leung(4) | Benefit Equalization Plan Retirement Plan | 17.8 | 6,749,150 | | 0 | | |
| Karin Shanahan(3) | Benefit Equalization Plan Retirement Plan | 0 | 0 | | 0 | | |
(1)Reflects the years of credited service through December 31, 2009 at which time we discontinued service accruals under the BEP-Retirement Plan. The company terminated the US-RIP as of February 1, 2019.
(2)The present value of accumulated benefits was calculated based on the following assumptions which were used in the December 31, 2024 disclosure for the BEP-Retirement Plan:
•100% lump-sum utilization
•FTSE Pension Discount Curve rates as of the measurement date; and
•2025 IRS Applicable Mortality under IRC 417(e).
These assumptions are the same as those disclosed in conformity with generally accepted accounting principles. For active executives, payments are assumed to begin at age 60 for the BEP-Retirement Plan, the earliest age that employees are eligible for an unreduced pension, or current age if over age 60. The actual benefit received will vary based on age and interest rates at the time of retirement.
(3)Dr. Boerner, Mr. Elkins, Dr. Hirawat, and Ms. Shanahan are not participants in any of the company's defined benefit pension plans.
(4)Ms. Leung has met the age and service requirements for early retirement under the BEP—Retirement Plan.
Non-Qualified Deferred Compensation Plan
The Bristol-Myers Squibb Company Benefit Equalization Plan—Savings and Investment Program (BEP-Savings Plan or BEP—SIP) is a non-qualified deferred compensation plan that allows employees to defer a portion of their total eligible cash compensation and to receive company matching contribution credits in excess of contributions allowed under the Bristol-Myers Squibb Company Savings and Investment Program (Savings Plan or U.S. SIP). The Savings Plan is a tax-qualified plan, as defined under Sections 401(a) and 401(k) of the Internal Revenue Code. Employees who are eligible to participate in the Savings Plan, and whose compensation and/or total contributions exceed the IRS qualified plan limits, are eligible for the BEP—Savings Plan. The key provisions of the BEP—Savings Plan are as follows:
•Employee deferrals to the BEP—Savings Plan begin, in accordance with an employee’s timely-filed election, once the employee’s total eligible compensation paid for the year exceeds the limit under Section 401(a)(17) of the Internal Revenue Code, and/or total contributions to the Savings Plan exceed the limits under Section 415(c) of the Internal Revenue Code.
•Employees may defer no less than 2% and up to 75% of their eligible BEP compensation.
•The company matching contribution credit generally equals 100% of the employee’s contribution deferral credit on the first 6% of eligible BEP compensation that an employee elects to defer for each pay period.
87 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
Executive Compensation Tables |
•An additional, discretionary company contribution is applied to each individual account in the qualified Savings Plan annually, in an amount based on a point system of a participant’s age plus service, as follows: below 40 points—3% of total eligible cash compensation; between 40 and 59 points—4.5%; and at 60 points and above—6%. If the full amount of the annual additional company contribution calculated in accordance with the Savings Plan formula cannot be made to the qualified Savings Plan due to application of the Section 401(a)(17) limit on contributions and/or the Section 415(c) limit on contributions, the excess amount is credited to the employee’s account under the BEP-Savings Plan.
•The plan is unfunded. Benefits are paid from general assets of the Company.
•Employees may allocate their contributions among various notional investment options that provide different combinations of risk and return potential, and employees can generally elect to change their investment elections each business day.
•For amounts credited prior to January 2024, the employee’s full balance under the BEP—Savings Plan is paid following a separation from service, or, if eligible, an election can be made at least 12 months prior to a separation from service to defer payments until a later date that is no earlier than five (5) years following the date of separation from service. Effective for 2024 and later plan years, employees have the option each year of electing, for that year’s deferral, the form of payment of a termination distribution (lump sum payment or 2-15 year annual installments payable within 60 days after separation from service) and may also elect an optional in-service distribution of such amount (lump sum payment or 2-5 year annual installments payable at least 2 years after the deferral plan year) payable if the employee has not separated from service as of the designated in-service payment date. The BEP-Savings Plan is subject to Section 409A of the Internal Revenue Code. Therefore, in all cases, a distribution for an executive classified as a “Specified Employee” of the Company, as defined under Section 409A, is subject to a six-month delay following the executive’s separation from service in accordance with the Section 409A regulations.
Non-Qualified Deferred Compensation Plan
2024 Fiscal Year
| | | | | | | | | | | | | | | | | | | | | | | |
| Name | Executive Contributions in 2024(1) ($) | Registrant Contributions in 2024(2) ($) | Aggregate Earnings in 2024(3) ($) | Aggregate Withdrawals/ Distributions in 2024
($) | Aggregate Balance at December 31, 2024(2)(4) ($) | |
| | | | | | | |
| Christopher S. Boerner, Ph.D.(5) | 253,527 | | 331,036 | | 466,438 | | 0 | | 4,517,820 | | |
| David V. Elkins(5) | 114,542 | | 217,058 | | 385,551 | | 0 | | 2,142,075 | | |
| Samit Hirawat, M.D.(5) | 169,335 | | 258,583 | | 276,965 | | 0 | | 2,122,612 | | |
| Sandra Leung(5) | 134,262 | | 252,071 | | 1,670,177 | | 0 | | 15,134,447 | | |
| Karin Shanahan(5) | 0 | | 88,979 | | 9,148 | | 0 | | 203,147 | | |
(1)The contribution amounts in this column reflect the deferral of a portion of 2024 base salary and the 2023 annual incentive award that was paid in March 2024. The base salary deferral amount is included as 2023 Salary in the Summary Compensation Table. The 2023 annual incentive award deferral amount was also included as 2023 Non-Equity Incentive Plan Compensation in the 2023 Summary Compensation Table.
(2)The contribution amounts in this column are included as 2024 All Other Compensation in the Summary Compensation Table. Includes the additional annual registrant contributions earned in 2024 but credited to the BEP-SIP in February 2025.
(3)Aggregate earnings are not reflected in the 2024 Summary Compensation Table and were not reflected in prior years’ Summary Compensation Tables. The company does not pay above-market interest rates on non-qualified deferred compensation.
(4)Portions of the aggregate balances in this column reflect amounts reported in the Summary Compensation Tables in prior years as follows: Ms. Leung, $458,838 for 2021, $401,022 for 2022 and $390,019 for 2023; Dr. Boerner, $483,983 for 2021, $464,699 for 2022 and $506,356 for 2023; Mr. Elkins, $415,782 for 2021, $369,430 for 2022 and $432,867 for 2023; and Dr. Hirawat, $405,861 for 2023.
(5)Reflects 2024 activity and aggregate balances in the non-qualified BEP-Savings Plan.
Bristol Myers Squibb | 2025 Proxy Statement 88
| | |
|
| Executive Compensation Tables |
Post-Termination Benefits
Following is a description of payments and benefits available under different termination scenarios:
Voluntary Termination
The company does not offer any payments or benefits to salaried employees, including the Named Executive Officers, upon a voluntary termination, other than those that are vested at the time of termination, unless the applicable plan or award agreement provides otherwise.
Voluntary Termination for Good Reason
Under the Bristol-Myers Squibb Senior Executive Severance Plan, certain senior executives (including the Named Executive Officers) are eligible to receive severance payments and benefits if they voluntarily terminate their employment for “good reason,” where “good reason” is defined as:
•A material reduction in the executive’s weekly base salary;
•The material reduction in the executive’s grade level resulting in a material diminution of the executive’s authority, duties, or responsibilities; or
•The relocation of the executive’s job or office, so that it will be based at a location which is more than 50 miles farther (determined in accordance with the company’s relocation policy) from the executive’s primary residence than the executive’s work location immediately prior to the proposed change in their job or office.
A terminated executive who signs a general release will be eligible for the following:
•Severance payments in the amount of two times annual base salary for our senior most executives including the Named Executive Officers, and 1.5 times annual base salary for other senior executives;
•Continuation of subsidized health care coverage and company-paid basic life insurance (up to two times annual base pay), in each case, until the earlier of (i) 56 weeks from the executive’s termination date or (ii) the date the executive begins new employment; and
•Outplacement services.
Retirement and Death
The following benefits are generally available to all salaried employees including the Named Executive Officers:
Annual Incentive—Under the Annual Incentive Plan, employees are eligible for a pro-rata award based on the number of days worked in the performance period.
Restricted Stock Units—Employees are eligible to vest in a pro-rata portion of RSUs scheduled to vest next if such RSUs were held at least one year from the grant date; provided that if an employee turns 65 on or prior to their retirement or death, then any unvested RSUs held for at least one year will vest in full prior to their retirement or death.
Market Share Units—Employees are eligible to vest in a pro-rata portion of MSUs held at least one year from the grant date, subject to performance provisions; provided that if an employee turns 65 on or prior to their retirement or death, then any unvested MSUs held for at least one year will vest in full upon their retirement or death, subject to performance provisions.
Performance Share Units—Employees are eligible to vest in a pro-rata portion of unvested PSUs held at least one year from the grant date subject to performance provisions.
Defined Benefit Pension Excess Benefit Plan (U.S. BEP-RIP)—Accruals are no longer provided under the U.S. BEP-RIP. However, employees may be eligible for benefits previously accrued under that Plan.
89 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
Executive Compensation Tables |
Savings Plans—Employees are eligible for benefits accumulated under our Savings Plan and, if applicable, the BEP—Savings Plan (as well as a pro-rata annual contribution (if applicable) on eligible compensation paid in the year of separation from service or death).
Post-Retirement Medical and Life Insurance—Employees age 55 or older with 10 years of service or age 65 or over at the time of retirement are eligible for post-retirement medical and life insurance benefits provided that they were employed by a company participating in the Bristol-Myers Squibb Company Consolidated Health & Welfare Plan at the time that their employment ended. Employees retiring with less than 10 years of service are not eligible to receive a company subsidy for their post-retirement medical coverage.
Involuntary Termination Not for Cause
The following benefits are generally available to all salaried employees including the Named Executive Officers:
Annual Incentive—Under the Annual Incentive Plan, employees who are severance eligible and execute and do not revoke a separation agreement are eligible for a pro-rata award based on the number of days worked in the performance period if the termination occurs on or after September 30th of the plan year. Further, an employee who is severance eligible and whose age plus years of service equals or exceeds 70, and who has at least 10 years of service, upon signing and not revoking a separation agreement the employee is eligible for a pro-rata award based on the number of days worked in the performance period for a termination occurring at any point in the plan year.
Restricted Stock Units—Upon signing a general release, employees are eligible to vest in a pro-rata portion of RSUs held at least one year from the grant date; provided that if an employee turns 65 on or prior to their involuntary termination not for cause, then any unvested RSUs held for at least one year will vest in full upon their involuntary termination not for cause.
Market Share Units—Upon signing a general release, employees are eligible to vest in a pro-rata portion of unvested MSUs held at least one year from the grant date, subject to performance provisions; provided that if an employee turns 65 on or prior to their involuntary termination not for cause, then any unvested MSUs held for at least one year will vest in full upon their involuntary termination not for cause, subject to performance provisions.
Performance Share Units—Upon signing a general release, employees are eligible to vest in a pro-rata portion of unvested PSUs held at least one year from the grant date, subject to performance provisions.
Defined Benefit Pension Excess Benefit Plan—Employees may be eligible for benefits accrued under the BEP—Retirement Plan. If the employee’s age plus years of service equal or exceed 70 and the employee has at least 10 years of service, the employee is not eligible for early retirement, and the employee signs a general release, the retirement benefits are payable following termination of employment based upon enhanced adjustment factors similar to those applied to employees eligible for early retirement.
Savings Plans—Employees are eligible for benefits accumulated under our Savings Plan and, if applicable, the BEP—Savings Plan. Under the Savings Plan and the BEP-Savings Plan, if the employee is either (1) involuntarily terminated not for cause on or after September 30th and the employee is receiving severance and signs a general release or (2) the employee’s age plus years of service equal or exceed 70 and the employee has at least 10 years of service, the employee is not eligible for early retirement, and the employee is receiving severance and signs a general release, the employee is eligible for a pro-rata annual contribution (if applicable) based on eligible compensation paid in the year of separation from service.
Post Retirement Medical Insurance—If the employee’s age plus years of service equal or exceed 70 on the date of termination and the employee has at least 10 years of service, the employee is not eligible for early retirement, and the employee signs a general release, the employee is eligible for continued medical coverage beyond the severance and COBRA period, provided that they were employed by a company participating in the Bristol-Myers Squibb Consolidated Health & Welfare Plan at the time that their employment ended, and as long as no other group medical coverage is available, without company subsidy until age 55. At age 55, they become eligible for company-subsidized, post-retirement medical benefits. Company subsidy will be based on age & service on the date of termination.
Bristol Myers Squibb | 2025 Proxy Statement 90
| | |
|
| Executive Compensation Tables |
Senior Executive Severance Plan—Under the Bristol-Myers Squibb Senior Executive Severance Plan, certain senior executives (including the Named Executive Officers) are eligible to receive severance payments and benefits if they are involuntarily terminated not for “cause,” where “cause” is defined as:
•failure or refusal by the executive to substantially perform his or her duties (except where the failure results from incapacity due to disability); or
•severe misconduct or engaging in an activity, which may include a failure to take action, deemed detrimental to the interests of the company including, but not limited to, acts involving dishonesty, violation of company policies, violation of safety rules, disorderly conduct, discriminatory harassment, unauthorized disclosure of confidential information, or the entry of a plea of nolo contendere to, or the conviction of, a crime.
A terminated executive who signs a general release will be eligible for the following:
•Severance payments in the amount of two times annual base salary for our senior-most executives, including the Named Executive Officers, and 1.5 times annual base salary for other senior executives;
•Continuation of subsidized health care coverage and company-paid basic life insurance (up to two times annual base pay), in each case until the earlier of (i) 56 weeks from the executive’s termination date or (ii) the date the executive begins new employment; and
•Outplacement services.
Change in Control
As disclosed in the CD&A, the company has entered into change-in-control agreements with certain senior executives, including all of the Named Executive Officers. The agreements are automatically extended for one additional year on each January 1, unless not later than December 1 of the year before the renewal date, either the company or the executive gives prior notice of termination of the agreement or a change-in-control shall have occurred prior to January 1 of such year. Further, if a change-in-control occurs during the term of the agreement, the agreement will continue for 24 months after the change-in-control for all NEOs, except for Ms. Leung, which will continue for 36-months.
To trigger benefits, there must be both a change-in-control of the company and either (i) a subsequent involuntary termination without cause by the company or (ii) a good reason termination by the employee. Good reason is defined in the agreements and includes a substantial negative change in the nature and status of the executive’s responsibilities, certain changes in pay and benefits, and relocation of the executive’s principal place of employment beyond 50 miles. The executive has up to 120 days to assert a claim for payments under this provision. In general, for all of our Named Executive Officers, the change-in-control protection extends for 24 months following a change-in-control, except for Ms. Leung for whom it extends for 36 months.
“Change in Control” means the earliest to occur of any one of the following dates:
(i)The date any Person (as defined in Section 13(d)(3) of the Securities Exchange Act of 1934, as amended) shall have become the direct or indirect beneficial owner of thirty percent (30%) or more of the then outstanding common shares of the company;
(ii)The date of consummation of a merger or consolidation of the company with any other corporation other than (A) a merger or consolidation which would result in the voting securities of the company outstanding immediately prior thereto continuing to represent at least fifty one percent (51%) of the combined voting power of the voting securities of the company or the surviving entity outstanding immediately after such merger or consolidation, or (B) a merger or consolidation effected to implement a recapitalization of the company in which no Person acquires more than fifty percent (50%) of the combined voting power of the company’s then outstanding securities;
91 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
Executive Compensation Tables |
(iii)The date the stockholders of the company approve a plan of complete liquidation of the company or an agreement for the sale or disposition by the company of all or substantially all the company’s assets; or
(iv)The date there shall have been a change in the composition of the Board of Directors of the company within a two-year period such that a majority of the Board does not consist of directors who were serving at the beginning of such period together with directors whose initial nomination for election by the company’s stockholders or, if earlier, initial appointment to the Board, was approved by the vote of two-thirds of the directors then still in office who were in office at the beginning of the two-year period together with the directors who were previously so approved.
Each of our Named Executive Officers is eligible to receive, in general, the following benefits if he or she is terminated in connection with a change-in-control:
•A cash payment equal to two times the sum of the executive’s annual base salary and target annual incentive award for Dr. Boerner, Mr. Elkins, Dr. Hirawat, and Ms. Shanahan, and 2.99 times the sum of the executive’s annual base salary and target annual incentive award for Ms. Leung.
•Payout of annual incentive award on a pro-rata basis at target.
•Vesting of unvested stock options, if any, including options held less than one year.
•Vesting of unvested RSUs, if any, including units held less than one year.
•Vesting of unvested MSUs, subject to performance provisions, including units held less than one year.
•Vesting of all unvested PSUs at target, including units held less than one year.
•For Ms. Leung, 0.3 additional years of service and age for pension purposes under the BEP—Retirement Plan. For other NEOs, a payout of company matching contributions and annual additional company contributions equal to two additional years of service.
•Eligibility for retiree medical benefits after the continuation period for health and life insurance benefits (described in the next bullet) has ended,based on two years additional age and service for Mr. Elkins, Dr. Boerner, Dr. Hirawat and Ms. Shanahan and three years additional age and service for Ms. Leung.
•Continuation of life insurance and health benefits for two years for Dr. Boerner, Mr. Elkins, Dr. Hirawat and Ms. Shanahan and three years for Ms. Leung.
•Vesting of unvested match in the company’s savings plans.
•We no longer gross up compensation on excess parachute payments for any of our executives, including all of our Named Executive Officers.
•Payment of any reasonable legal fees incurred to enforce the agreement.
The following illustrates the potential payments and benefits under the company’s plans and programs to the Named Executive Officers upon a termination of employment assuming an effective date of December 31, 2024. To the extent payments and benefits are generally available to salaried employees on a nondiscriminatory basis, they are excluded from the table.
Bristol Myers Squibb | 2025 Proxy Statement 92
| | |
|
| Executive Compensation Tables |
Termination of Employment Obligations (Excluding Vested Benefits)
2024 Fiscal Year
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | Cash Severance(1) ($) | Restricted Stock Units (“RSUs”)(2)(5) ($) | Market Share Units (“MSUs”)(3)(5) ($) | Performance Share Units (“PSUs”)(4)(5) ($) | Pension Plans(6) ($) | Savings Plans(7) ($) | Health(8) ($) | Retiree Medical(9) ($) | Total ($) | |
| | | | | | | | | | | |
| Voluntary Termination for Good Reason | |
| Christopher S. Boerner, Ph.D. (10) | 3,100,000 | | 0 | | 0 | | 0 | | 0 | | 0 | | 42,952 | | 0 | | 3,142,952 | | |
| David V. Elkins (10) | 2,230,000 | | 0 | | 0 | | 0 | | 0 | | 0 | | 35,867 | | 0 | | 2,265,867 | | |
| Samit Hirawat, M.D.(10) | 2,230,000 | | 0 | | 0 | | 0 | | 0 | | 0 | | 39,590 | | 0 | | 2,269,590 | | |
| Sandra Leung(11) | 2,300,000 | | 0 | | 0 | | 0 | | 0 | | 0 | | 26,551 | | 0 | | 2,326,551 | | |
| Karin Shanahan (10) (11) | 2,100,000 | | 0 | | 0 | | 0 | | 0 | | 0 | | 33,617 | | 0 | | 2,133,617 | | |
| Involuntary Termination Not for Cause (Absent Change in Control) | |
| Christopher Boerner, Ph.D.(10) | 3,100,000 | | 0 | | 844,554 | | 4,100,657 | | 0 | | 0 | | 42,952 | | 0 | | 8,088,162 | | |
| David V. Elkins(10) | 2,230,000 | | 0 | | 945,514 | | 4,062,196 | | 0 | | 0 | | 35,867 | | 0 | | 7,273,576 | | |
| Samit Hirawat, M.D.(10) | 2,230,000 | | 0 | | 619,275 | | 2,721,271 | | 0 | | 0 | | 39,590 | | 0 | | 5,610,137 | | |
| Sandra Leung(11) | 2,300,000 | | 0 | | 0 | | 0 | | 0 | | 0 | | 26,551 | | 0 | | 2,326,551 | | |
| Karin Shanahan (10) (11) | 2,100,000 | | 0 | | 0 | | 0 | | 0 | | 0 | | 33,617 | | 0 | | 2,133,617 | | |
| Qualifying Termination Within 2 or 3 Years Following a Change in Control | |
| Christopher S. Boerner, Ph.D.(10) | 7,750,000 | | 0 | | 8,853,846 | | 13,908,783 | | 0 | | 927,762 | | 86,377 | | 65,126 | | 31,591,894 | | |
| David V. Elkins(10) | 4,460,000 | | 0 | | 4,968,853 | | 8,825,226 | | 0 | | 535,200 | | 72,449 | | 51,847 | | 18,913,575 | | |
| Samit Hirawat, M.D.(10) | 4,460,000 | | 0 | | 4,163,099 | | 7,051,561 | | 0 | | 535,200 | | 120,168 | | 47,442 | | 16,377,470 | | |
| Sandra Leung(12) | 6,877,000 | | 0 | | 2,766,180 | | 3,265,831 | | 3,669,555 | | 0 | | 7,987 | | 0 | | 16,586,553 | | |
| Karin Shanahan (10) (12) | 3,990,000 | | 1,298,910 | | 1,976,602 | | 2,461,717 | | 0 | | 474,772 | | 102,404 | | 0 | | 10,304,406 | | |
(1)For voluntary termination for good reason and involuntary termination not for cause, cash severance is equal to two times base annual salary. For change in control, cash severance is equal to two times the sum of annual base salary and target annual incentive award for Dr. Boerner, Mr. Elkins, Dr. Hirawat, and Ms. Shanahan, and 2.99 times for Ms. Leung.
(2)For involuntary termination not for cause, represents pro-rata portion of awards held at least one year. For change in control, represents all unvested units.
93 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
Executive Compensation Tables |
(3)For involuntary termination not for cause, represents pro-rata portion of awards held at least one year. For change in control, represents all unvested units. For awards granted prior to 2024, the payout factor applied is equal to the 10-day average closing price on December 31, 2024 divided by the 10-day average closing price on the grant date. For awards granted in 2024, the payout factor applied is equal to the 10-day average closing price on December 31, 2024 plus the value of accumulated dividends, divided by the 10-day average closing price on the grant date.
(4)For change in control, represents a full payout of the 2022-2024 and 2023-2025 and 2024-2026 PSU awards at target. For involuntary termination not for cause, represents a pro-rata payout of the 2022-2024 and 2023-2025 PSU awards at target. The 2024-2026 PSU award is forfeited because as of December 31, 2024 the award has not been held for at least one year since the grant date.
(5)Values as of December 31, 2024 based on the closing Common Stock price of $56.56 on that day.
(6)Reflects BEP - Retirement Plan. For Ms. Leung, change-in-control values include 0.3 additional years of credited service and age.
(7)Change in control values reflect Company matching contributions and annual additional company contributions under the company’s Savings Plan and employer credits under the BEP-SIP, in each case, equaling to two additional years of service.
(8)For voluntary termination for good reason and involuntary termination not for cause, reflects life insurance and health care benefit continuation through the severance period of 56 weeks. For change in control, represents continuation of life insurance and health care benefits for two years for Dr. Boerner, Mr. Elkins, Dr. Hirawat and Ms. Shanahan, and three years for Ms. Leung.
(9)Reflects cost to the Company for providing retiree medical benefits. For change in control, includes additional years of credited service and age.
(10)Dr. Boerner, Mr. Elkins, Dr. Hirawat and Ms. Shanahan are not participants in any of our pension plans.
(11)These Named Executive Officers are retirement-eligible under our stock plans and therefore are entitled to the following benefits, which are generally available to all retirement eligible participants in our stock plans:
•a pro-rata portion of RSUs held for one year from the grant date;
•a pro-rata portion of MSUs held for one year from the grant date, subject to performance provisions; and
•a pro-rata portion of PSUs held one year from the grant date, subject to performance provisions.
(12)These Named Executive Officers are retirement-eligible under our stock plans and therefore the number of units used to calculate the change-in-control value reflects:
•Restricted Stock Units - the difference between a pro-rata portion of RSUs held for one year from the grant date and all unvested RSUs including units held less than one year from the grant date;
•Market Share Units - the difference between a pro-rata portion of MSUs held for one year from the grant date and all unvested MSUs including units held less than one year from the grant date; and
•Performance Share Units - the difference between a pro-rata portion of PSUs held for one year from the grant date and all unvested units under the 2022-2024 and 2023-2025 PSUs and payout of the 2024-2026 PSU award at target.
Bristol Myers Squibb | 2025 Proxy Statement 94
Pay Ratio
To determine the ratio of the CEO’s annual total compensation to the median annual total compensation of all employees excluding the CEO, we identified the median employee as of October 1, 2023 using target total cash compensation (i.e., salary plus 2023 target incentive award). We believe this measure most reasonably reflects the typical annual compensation of our employee population and was consistently applied for all employees. We believe that there have not been any material changes in our employee population or compensation arrangements that would require us to change our methodology for 2024.
We calculated that the median employee’s 2024 total compensation, as determined in the same manner as “Total Compensation” in the 2024 Summary Compensation Table, was $160,144. Dr. Boerner had an annual total compensation equal to the amount included in the “Total Compensation” column of the 2024 Summary Compensation Table, which results in an annual total compensation for 2024 of $18,787,618. Based on this information, for 2024, the ratio of the annual total compensation of the CEO to the median of the annual total compensation of all other employees of the Company was 117 to 1.
95 Bristol Myers Squibb | 2025 Proxy Statement
Pay Versus Performance
The disclosure included in this section is prescribed by SEC rules and does not necessarily align with how the Company or the CMDC views the link between the Company’s performance and its NEOs’ pay. For a discussion of how the Company views its executive compensation structure, including alignment with Company performance, see the Compensation Discussion and Analysis (beginning on page 45). The CMDC did not consider the pay versus performance disclosure below in making its pay decisions for any of the years shown. The use of the term “compensation actually paid” (“CAP”) is required by the SEC’s rules. Neither CAP nor the total amount reported in the Summary Compensation Table (“SCT”) reflect the amount of compensation actually paid, earned or received during the applicable year. Per SEC rules, CAP was calculated by adjusting the SCT total values for the applicable year as described in the footnotes to the following table.
Tabular Disclosure of Pay Versus Performance
The following table sets forth information concerning the compensation actually paid to our CEO and other NEOs compared to Company performance for the years ended December 31, 2024, 2023, 2022, 2021 and 2020. The NEOs, including the CEO, represent the following individuals for years 2020 through 2022: Dr. Giovanni Caforio (CEO), Dr. Chris Boerner, Mr. David Elkins, Dr. Rupert Vessey and Ms. Sandra Leung. For 2023, it reflects the following individuals: Dr. Giovanni Caforio (former CEO), Dr. Chris Boerner (current CEO), Mr. David Elkins, Dr. Samit Hirawat, Ms. Sandra Leung, and Ms. Elizabeth Mily. For 2024, it reflects the following individuals: Dr. Chris Boerner (CEO), Mr. David Elkins, Dr. Samit Hirawat, Ms. Sandra Leung, and Ms. Karin Shanahan.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Value of Initial Fixed $100
Investment Based On | | | |
| | | | | | | | | | | |
| Year | Summary
Compensation
Table Total for
Former CEO
($) | Summary
Compensation
Table Total for
Current CEO
($) | Compensation Actually Paid to Former CEO(4) ($) | Compensation Actually Paid to Current CEO(4) ($) | Average
Summary
Compensation
Table Total
for
non-CEO NEOs
($) | Average Compensation Actually Paid to non-CEO NEOs(4) ($) | Total Shareholder Return(5) ($) | Peer Group Total Shareholder Return(5) ($) | Net Income (in Millions) (GAAP)(6) ($) | Total Revenues (in Millions)(7) ($) | |
| | | | | | | | | | | |
2024(1) | | 18,787,618 | | | 21,204,985 | | 7,717,989 | | 8,945,524 | | 105 | | 154 | | (8,948) | | 48,300 | | |
2023(2) | 19,661,434 | | 8,461,833 | | (6,907,611) | | 382,568 | | 5,917,907 | | (525,203) | | 91 | | 142 | | 8,025 | | 45,006 | | |
2022(3) | 20,053,032 | | | 35,057,807 | | | 6,996,472 | | 10,872,377 | | 123 | | 139 | | 6,327 | | 46,159 | | |
2021(3) | 19,784,806 | | | 16,505,622 | | | 7,140,446 | | 6,780,078 | | 103 | | 126 | | 6,994 | | 46,385 | | |
2020(3) | 20,150,902 | | | 17,644,676 | | | 7,050,986 | | 6,288,633 | | 100 | | 102 | | (9,015) | | 42,518 | | |
(1)The NEOs for 2024 are Dr. Chris Boerner, Mr. David Elkins, Dr. Samit Hirawat, Ms. Sandra Leung and Ms. Karin Shanahan.
(2)The NEOs for 2023 are Dr. Giovanni Caforio (Former CEO), Dr. Chris Boerner (Current CEO), Mr. David Elkins, Dr. Samit Hirawat, Ms. Sandra Leung and Ms. Elizabeth Mily.
(3)The NEOs for years 2020-2022 are Dr. Giovanni Caforio (CEO), Dr. Chris Boerner, Mr. David Elkins, Dr. Rupert Vessey and Ms. Sandra Leung.
(4)CAP reflects the total compensation reported in the 2024 SCT for the years ended December 31, 2024, 2023, 2022 and 2022 SCT for the years ended 2021 and 2020, in each case, adjusted to include or exclude the amounts shown in the tables below for the NEOs. To calculate CAP, the following amounts were deducted from and added to SCT total compensation, computed in accordance with Item 402(v) of Regulation S-K:
Bristol Myers Squibb | 2025 Proxy Statement 96
Former CEO SCT Total to CAP Reconciliation
| | | | | | | | | | | | | | | | | | | | | | | |
| Year | SCT Total for Former CEO
($) | Deduction of Change in Pension Values for Former CEO(a) ($) | Deduction of Stock Awards and Options Values for Former CEO(b) ($) | Addition of Pension Service Cost for Former CEO(c) ($) | Addition of Equity Values for Former CEO(d)* ($) | Compensation Actually Paid for Former CEO ($) | |
| | | | | | | |
| 2023 | 19,661,434 | | 0 | | (14,465,570) | | 0 | | (12,103,475) | | (6,907,611) | | |
| 2022 | 20,053,032 | | 0 | | (14,289,505) | | 0 | | 29,294,280 | | 35,057,807 | | |
| 2021 | 19,784,806 | | 0 | | (13,965,989) | | 0 | | 10,686,805 | | 16,505,622 | | |
| 2020 | 20,150,902 | | 0 | | (13,457,248) | | 0 | | 10,951,022 | | 17,644,676 | | |
Current CEO SCT Total to CAP Reconciliation
| | | | | | | | | | | | | | | | | | | | | | | |
| Year | SCT Total for Current CEO
($) | Deduction of Change in Pension Values for Current CEO(a) ($) | Deduction of Stock Awards and Options Values for Current CEO(b) ($) | Addition of Pension Service Cost for Current CEO(c) ($) | Addition of Equity Values for Current CEO(d)* ($) | Compensation Actually Paid for Current CEO
($) | |
| | | | | | | |
| 2024 | 18,787,618 | | 0 | | (13,643,063) | | 0 | | 16,060,430 | | 21,204,985 | | |
| 2023 | 8,461,833 | | 0 | | (5,326,178) | | 0 | | (2,753,087) | | 382,568 | | |
Average non-CEO NEOs SCT Total to CAP Reconciliation
| | | | | | | | | | | | | | | | | | | | | | | |
| Year | SCT Total
($) | Deduction of Change in Pension Values(a) ($) | Deduction of Stock Awards and Options Values(b) ($) | Addition of Pension Service Cost(c) ($) | Addition of Equity Values(d)* ($) | Compensation Actually Paid
($) | |
| | | | | | | |
| 2024 | 7,717,989 | | 0 | | (4,875,280) | | 0 | | 6,102,814 | | 8,945,524 | | |
| 2023 | 5,917,907 | | 0 | | (3,525,996) | | 0 | | (2,917,115) | | (525,203) | | |
| 2022 | 6,996,472 | | 0 | | (4,304,359) | | 0 | | 8,180,264 | | 10,872,377 | | |
| 2021 | 7,140,446 | | 0 | | (4,208,611) | | 0 | | 3,848,242 | | 6,780,078 | | |
| 2020 | 7,050,986 | | (221,035) | | (3,757,621) | | 0 | | 3,216,303 | | 6,288,633 | | |
(a)Represents change in pension value under the BEP-RIP reported in the SCT for each year shown. See the footnotes to the 2024 and 2022 SCTs for further detail regarding the amounts in this column.
(b)Represents the grant date fair value of equity-based awards granted each year and reported in the SCT for each year shown. See the footnotes to the 2024 and 2022 SCTs for further detail regarding the amounts in this column.
(c)Does not include any service cost for pension benefits under the U.S BEP-RIP as the plan was frozen in 2009 and, therefore, there is no service cost. The company terminated the US-RIP as of February 1, 2019.
(d)Reflects the value of equity calculated in accordance with the SEC methodology for determining the compensation actually paid for each year shown.
*The amounts in the Addition of Equity Values in the tables above are derived from the amounts set forth in the following table:
97 Bristol Myers Squibb | 2025 Proxy Statement
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year
($) | Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards
($) | Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year
($) | Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year
($) | Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year
($) | Value of Dividends or Other Earnings Paid on Stock or Option Awards Not Otherwise Included
($) | Total - Addition of Equity Values
($) | |
| | | | | | | | | |
| Former CEO | |
| 2023 | 4,874,459 | | (16,751,751) | | 0 | | (226,183) | | 0 | | 0 | | (12,103,475) | | |
| 2022 | 15,604,262 | | 7,721,151 | | 0 | | 5,968,867 | | 0 | | 0 | | 29,294,280 | | |
| 2021 | 13,701,811 | | (2,650,377) | | 0 | | (364,629) | | 0 | | 0 | | 10,686,805 | | |
| 2020 | 14,594,230 | | (1,605,636) | | 0 | | (2,037,572) | | 0 | | 0 | | 10,951,022 | | |
| Current CEO | |
| 2024 | 14,640,099 | | 1,437,395 | | 0 | | (17,064) | | 0 | | 0 | | 16,060,430 | | |
| 2023 | 2,266,251 | | (4,900,396) | | 0 | | (118,943) | | 0 | | 0 | | (2,753,087) | | |
| Average of Non-CEO NEOs | |
| 2024 | 5,246,975 | | 903,799 | | 0 | | (47,959) | | 0 | | 0 | | 6,102,814 | | |
| 2023 | 1,188,161 | | (4,033,124) | | 0 | | (72,151) | | 0 | | 0 | | (2,917,115) | | |
| 2022 | 4,700,394 | | 2,222,732 | | 0 | | 1,201,754 | | 0 | | 55,384 | | 8,180,264 | | |
| 2021 | 4,128,997 | | (478,162) | | 0 | | 25,413 | | 0 | | 171,994 | | 3,848,242 | | |
| 2020 | 4,205,108 | | (565,278) | | 0 | | (470,925) | | 0 | | 47,398 | | 3,216,303 | | |
(5)The Company TSR and the Peer Group TSR reflected in these columns for each of the applicable fiscal years is calculated based on a fixed investment of $100 at the applicable measurement point on the same cumulative basis as is used in Item 201(e) of Regulation S-K. The peer group used to determine the Company’s Peer Group TSR for the five fiscal years is the extended peer group that was used for purposes of disclosing our executive compensation benchmarking practices, as described in the section titled “Benchmarking Analysis and Compensation Peer Groups” on page 55 and also as disclosed in our Annual Report on Form 10-K pursuant to Item 201(e) of Regulation S-K for these years. The extended peer group is composed of AbbVie Inc., Amgen Inc., Biogen Inc., Eli Lilly and Company, Gilead Sciences Inc., Johnson & Johnson, Merck & Co., Pfizer Inc., AstraZeneca PLC, GlaxoSmithKline PLC, Roche Holding AG, Novartis AG, and Sanofi. (6)Net income is equivalent to “Net Earnings/(Loss) Attributable to BMS” as reported in the Company’s consolidated financial statements.
(7)SEC rules require us to designate a “Company-Selected Measure” that in our assessment represents the most important financial performance measure used by the Company to link the CAP of our NEOs, for the most recently completed fiscal year, to our performance. We have selected total revenues as this measure for fiscal year 2024.
Bristol Myers Squibb | 2025 Proxy Statement 98
Pay Versus Performance Comparative Disclosure
As described in more detail in the CD&A, a substantial portion of the Company’s executive compensation program is variable and at risk based on operational, financial, strategic and share price performance. While the Company utilizes several performance measures to align executive compensation with Company performance, not all of those Company measures are presented in the table above.
In accordance with Item 402(v) of Regulation S-K, the Company is providing the following descriptions of the relationships between the information presented in the table above.
CAP and Company TSR
CAP over the last five years is closely aligned with the Company’s TSR performance as presented in the chart below. This is because a significant portion of CAP is comprised of equity awards. As described in more detail in the section titled “2024 Target Compensation Benchmarks,” approximately 78% of the CEO’s 2024 target compensation and, on average, 68% of the non-CEO NEOs’ 2024 target compensation is comprised of equity awards delivered in MSUs and PSUs, tied to absolute and relative stock price performance in addition to financial performance.
The Company’s TSR over the 5-year period ending December 31, 2024 was 4.7%, while the Company’s peer group TSR was 50.6% for the same period of time.
CAP and Net Income
The Company’s net income and CAP have varied each year. The Company does not use net income as a performance metric in the annual and long-term incentive plans, and CAP is not meaningfully correlated with the company’s net income (GAAP).
99 Bristol Myers Squibb | 2025 Proxy Statement
CAP and Total Revenues
CAP is generally aligned with the Company’s total revenues over the five years. While the Company uses various financial and non-financial performance measures for the purpose of evaluating performance for the Company’s compensation programs, the Company has determined that total revenues is the Company’s most important financial performance measure (that is not otherwise required to be disclosed in the table) used to link NEO CAP to company performance for fiscal year 2024. The Company utilizes revenue as a performance metric for the Company’s annual incentive program and PSU awards granted to the NEOs in the long-term incentive program. As described in more detail in the section titled “Executive Compensation Program Overview” on page 52, 35% of annual incentives and 40% of the PSUs for 2024 were based on achieving revenue goals for our Growth Portfolio Revenue. Tabular Disclosure of Significant Financial and Non-Financial Performance Measures
The seven metrics listed below represent the most significant financial and non-financial performance measures as described in the “Annual Incentive Plan” and “Long-term Incentive Program” sections within our CD&A on pages 60 and 63, respectively. The measures in this table are not ranked. Please see the CD&A for a further description of these measures and how they are used in the Company’s executive compensation program. | | | | | | | | | | | | | | | | | |
| Significant Financial and Non-Financial Performance Measures |
| | | | | |
| Revenue | Sustainability Scorecard | Pipeline | Stock Price | |
| Operating Income | Operating Margin | Relative TSR | | |
Bristol Myers Squibb | 2025 Proxy Statement 100
Item 2
Advisory Vote to Approve the Compensation of Our Named Executive Officers
As required by Section 14A of the Securities Exchange Act of 1934, as amended, we are providing shareholders the opportunity to advise the Compensation and Management Development Committee and the Board of Directors regarding the compensation of our Named Executive Officers, as such compensation is described in the “Compensation Discussion and Analysis” section, the tabular disclosure regarding such compensation and the accompanying narrative disclosure, beginning on page 45, which we do annually. We strongly encourage you to read these sections for a detailed description of our executive compensation philosophy and programs, the compensation decisions the Committee has made under those programs, the factors considered in making those decisions, the changes approved to such programs and the feedback we received from our shareholder engagement. Accordingly, we are requesting your nonbinding vote on the following resolution: “RESOLVED, that the shareholders of Bristol-Myers Squibb Company approve, on an advisory basis, the compensation of the company’s Named Executive Officers, as described in the Compensation Discussion and Analysis section, the tabular disclosure regarding such compensation and the accompanying narrative disclosure set forth in the company’s 2025 Proxy Statement.”
Our executive compensation programs are designed to enable us to attract and retain talented executives capable of leading our business in the highly complex and competitive business environment in which we operate. We seek to accomplish this goal in a way that rewards performance and is aligned with our shareholders’ long-term interests. A significant portion of each executive’s pay depends on his or her individual performance against financial and operational objectives as well as a demonstration of key values necessary to our evolution as a leading biopharmaceutical company. In addition, a substantial portion of an executive’s compensation is in the form of equity awards that tie the executive’s compensation directly to creating shareholder value and achieving financial and operational results. We value input from our shareholders as expressed through their votes and other communications. As an advisory vote, this proposal is not binding on the company. However, consistent with our record of shareholder responsiveness, the Compensation and Management Development Committee will consider the outcome of the vote when making future executive compensation decisions.
| | | | | | | | | | | | | | |
| | | | |
| | | The Board of Directors unanimously recommends a vote “FOR” the approval, on an advisory basis, of the compensation of our Named Executive Officers. | |
| | | | |
101 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Item 2—Advisory Vote to Approve the Compensation of Our Named Executive Officers |
Equity Compensation Plan Information
The following table summarizes information concerning the company’s equity compensation plans and outstanding options, warrants and rights as of December 31, 2024:
| | | | | | | | | | | | | | | | | | | | | | | |
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights
(In millions) (a)
(#) | | Weighted-average exercise price of outstanding options, warrants and rights (b)
($) | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (In millions) (c)
(#) | |
| | | | | | | |
| Equity compensation plans approved by security holders | 37.8 | (1) | 59.02 | | (2) | 63.6 | (3) |
| Equity compensation plans not approved by security holders | 0 | | | | 0 | |
| Total | 37.8 | | 59.02 | | | 63.6 | |
(1)On December 31, 2024, there were a total of approximately 20.7 million shares subject to restricted stock units, approximately 1.9 million shares subject to market share units and approximately 3.7 million shares subject to performance share units. In the case of market share units and performance share units, which require performance conditions to be met for vesting, the number of awards reflected in the table assume achievement of target performance; under these awards, approximately 6.0 million additional shares would be issued if specified above-target performance levels were fully achieved in the applicable performance period.
(2)The weighted average exercise price of outstanding awards does not take into account the shares issuable upon settlement of outstanding restricted stock units, market share units or performance share units which have no exercise price. If the awards that have no exercise price were included in the calculation of weighted average exercise price of outstanding options, warrants and rights, the weighted average exercise price for all such outstanding awards would be $17.37.
(3)All available shares may be used for stock options and for equity awards that do not require payment of an exercise price, including restricted stock, restricted stock units, market share units, performance share units and similar full-value awards.
Bristol Myers Squibb | 2025 Proxy Statement 102
Item 3
Ratification of the Appointment of Independent Registered Public Accounting Firm
Our Board of Directors, upon the recommendation of its Audit Committee, has ratified the Audit Committee’s appointment of Deloitte & Touche LLP (“D&T”) as our independent registered public accounting firm for the year 2025. The Audit Committee and the Board believe that the continued retention of D&T to serve as our independent registered public accounting firm is in the best interests of the company and its shareholders. As a matter of good corporate governance, we are asking shareholders to ratify such appointment. In the event our shareholders fail to ratify the appointment, the Board of Directors and the Audit Committee will reconsider such appointment. It is understood that even if the appointment is ratified, the Audit Committee at its discretion, may direct the appointment of a new independent registered public accounting firm at any time during the year if the Audit Committee determines that such a change would be in the best interests of our company and our shareholders.
The Audit Committee is directly responsible for appointing, compensating and providing oversight of the performance of our independent registered public accounting firm for the purpose of issuing audit reports and related work regarding our financial statements and the effectiveness of our internal control over financial reporting. The Audit Committee is also responsible for approving the audit fees of our independent registered public accounting firm. In order to assure continuing auditor independence, the Audit Committee periodically considers whether there should be a rotation of the independent registered public accounting firm. Further, in conjunction with the mandated rotation of the audit firm’s lead engagement partner, every five years, the Audit Committee and its chairperson participate in the process for the selection of D&T’s new lead engagement partner.
Representatives from D&T will be present at the Annual Meeting to respond to appropriate questions and to make any additional statements they may choose.
| | | | | | | | | | | | | | |
| | | | |
| | | The Board of Directors unanimously recommends a vote “FOR” the ratification of the appointment of Deloitte & Touche LLP as Bristol-Myers Squibb Company’s independent registered public accounting firm for 2025. | |
| | | | |
103 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Item 3—Ratification of the Appointment of Independent Registered Public Accounting Firm |
Audit and Non-Audit Fees
The following table presents aggregate billed fees for professional audit services rendered by D&T for the fiscal years ended December 31, 2024, and 2023 for the audits of our annual financial statements and internal control over financial reporting, and fees billed for other services rendered by D&T during those periods.
| | | | | | | | | | | | | | |
| | 2024
($) | 2023
($) | |
| | | | |
| | (in millions) |
| | | | |
| Audit Fees | 19.01 | | 18.12 | | |
| Audit Related Fees | 0.46 | | 0.56 | | |
| Tax Fees | 8.93 | | 8.79 | | |
| All Other Fees | 0.01 | | 0.01 | | |
| Total | 28.40 | | 27.48 | | |
Audit fees for 2024 and 2023 were for professional services rendered for the audits of our consolidated financial statements, and of our internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act, statutory and subsidiary audits, timely reviews of quarterly financial statements, comfort letters, consents, and assistance with review of documents filed with the SEC.
Audit Related fees for 2024 and 2023 were primarily for agreed-upon procedures, special purpose financial statement audits and other audit-related services.
Tax fees were composed of both tax compliance and tax consulting fees as described below.
•Tax Compliance fees were incurred for services related to tax compliance, including the preparation of tax returns, claims for refund, assistance with tax audits and preparation of transfer pricing documentation studies. Such amounts were $7.48 million and $7.14 million in 2024 and 2023, respectively.
•Tax Consulting fees were incurred for tax planning (excluding planning related to transactions or proposals for which the sole purpose may be tax avoidance or for which tax treatment may not be supported by the Internal Revenue Code) and tax advice, including assistance with advice related to acquisitions, internal restructurings, legislative updates, and requests for rulings or technical advice from tax authorities. Such amounts were $1.45 million and $1.65 million in 2024 and 2023, respectively.
All Other fees for 2024 and 2023 were related to subscriptions to research databases.
Pre-Approval Policy for Services Provided by our Independent Registered Public Accounting Firm
The Audit Committee has established a policy to preapprove all audit and permissible non-audit services provided by our independent registered public accounting firm consistent with applicable SEC rules. Our independent registered public accounting firm is prohibited from providing tax consulting services relating to transactions or proposals in which the sole purpose may be tax avoidance or for which the tax treatment may not be supported by the Internal Revenue Code. Prior to the engagement of our independent registered public accounting firm for the next year’s audit, a schedule of the aggregate of services expected to be rendered during that year for each of the four categories of services described above is submitted to the Audit Committee for approval. Prior to engagement, the Audit Committee preapproves these services by category of service. The fees are budgeted by category of service and the Audit Committee receives periodic reports from our independent registered public accounting firm on actual fees versus the budget by category of service. During the year, circumstances may arise when it may become necessary to engage our independent registered public accounting firm for additional services not contemplated in the preapproval. In those instances, the Audit Committee requires specific preapproval before engaging our independent registered public accounting firm.
Bristol Myers Squibb | 2025 Proxy Statement 104
| | |
|
| Item 3—Ratification of the Appointment of Independent Registered Public Accounting Firm |
The Audit Committee may delegate pre-approval authority to one or more of its members. The member or members to whom such authority is delegated is required to report, for informational purposes, any pre-approval decisions to the Audit Committee at its next regularly scheduled meeting. During 2024, the Audit Committee did not delegate pre-approval authority to any of its members.
Audit Committee Report
As the Audit Committee of the Board of Directors, we are composed of independent directors as required by and in compliance with the listing standards of the NYSE and applicable SEC rules. We operate pursuant to a written charter adopted by the Board of Directors that is published on the company’s website.
Management has primary responsibility for the company’s financial reporting process, principles and internal controls as well as preparation of its consolidated financial statements. The independent registered public accounting firm is responsible for performing an audit in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”) to obtain reasonable assurance that Bristol Myers Squibb’s consolidated financial statements are free from material misstatement and expressing an opinion on the conformity of such financial statements with accounting principles generally accepted in the United States. We are responsible for overseeing and monitoring D&T’s auditing process on behalf of the Board of Directors.
As part of the oversight of the company’s financial statements, we review and discuss with both management and D&T all annual and quarterly financial statements prior to their issuance. Management advised us that each set of financial statements reviewed was prepared in accordance with accounting principles generally accepted in the United States. We have reviewed with management significant accounting and disclosure issues and reviewed with D&T matters required to be discussed pursuant to auditing standards adopted by the PCAOB and SEC requirements. Specifically, we reviewed and discussed with D&T the critical audit matters arising from the audit of our financial statements for fiscal year 2024.
In addition, we have received the written disclosures and the letter from D&T required by PCAOB Ethics and Independence Rule 3526, “Communication with Audit Committees Concerning Independence,” and have discussed with D&T their independence from Bristol Myers Squibb and its management. We have determined that D&T’s provision of non-audit services in 2024 was compatible with, and did not impair, its independence. We have also received written materials addressing D&T’s internal quality control procedures and other matters, as required by the New York Stock Exchange listing standards.
We have discussed with our internal auditors and D&T the overall scope and plans for their respective audits. We have met with the internal auditors and D&T, with and without management present, to discuss their evaluations of the company’s internal control over financial reporting, and the overall quality of the company’s financial reporting.
Based on the reviews and discussions described above, we recommended to the Board of Directors, and the Board has approved, that the audited consolidated financial statements for the year ended December 31, 2024 be included in Bristol-Myers Squibb Company’s Annual Report on Form 10-K for the year ended December 31, 2024 for filing with the Securities and Exchange Commission.
In addition, we have confirmed there have been no new circumstances or developments since our respective appointments to the Committee that would impair any of our member’s ability to act independently.
The Audit Committee
Derica W. Rice, Chair
Michael R. McMullen
Paula A. Price
Theodore R. Samuels
Phyllis R. Yale
105 Bristol Myers Squibb | 2025 Proxy Statement
Shareholder Proposals
The Company expects the following shareholder proposals (Items 4 and 5) to be presented at the 2025 Annual Meeting. The Board of Directors has recommended a vote against these proposals for the reasons set forth following each proposal. The stock holdings of each proponent will be provided upon request to the Corporate Secretary of Bristol-Myers Squibb Company.
Item 4—Shareholder Proposal on Corporate Financial Sustainability
The proponent of this resolution is the National Center for Public Policy Research, 2005 Massachusetts Avenue NW, Washington, DC 20036. This proposal has been included in this Proxy Statement pursuant to an agreement between the Company and the proponent.
Proposal 4 — Corporate Financial Sustainability
Corporate Financial Sustainability Report
Whereas: The Company’s policy positions, advocacy, partnerships and charitable giving on significant social policy and political matters should not alienate consumers, decrease sales, or diminish shareholder value.
The Company also has a 100 percent rating on the Human Rights Campaign’s (HRC) “Corporate Equality Index.”1 Earning that score arguably requires spending shareholder assets to embrace highly partisan positions on hot-button issues, such as supporting legislation that eliminates religious liberties and discriminates against girls and women while opposing legislation to protect children from adult materials. In his 2021 book The Dictatorship of Woke Capital, Stephen Soukup describes HRC as “influencing businesses by employing a ‘soothsayer’s trick’” that boils down to increasing the radicalization of businesses by way of a strategy to “simply keep moving the goalposts.”2
The Company has been rated “high risk” by the 1792 Exchange on its “Spotlight Bias Report,”3 which is published to “shed light on corporate activism”4 and lists the following as potential concerns for BMS: (1) BMS “claims to protect its employees from viewpoint discrimination but fired multiple employees who refused to get Covid-19 vaccinations due to religious reasons.”5 (2) “BMS has pledged to vet vendors based on LGBTQ policies,”6 which may result in discriminating against religious vendors.
The Company has taken public and politically divisive positions over issues of significant social policy concern, including apparently supporting “the ill-named Equality Act” even though “scholars and legal experts [argue] that the law would eviscerate female sports and cancel federal religious freedom protections.”7
The Company is also listed as a Champion Tier Brand Partner by the Trevor Project,8 an organization that supports “gender affirming care,”9 which critics have argued translates into advocating for dangerous puberty blockers and genital mutilation for children.10 Trevor Project has also been accused of facilitating the hiding of gender confusion problems from parents.11
Supporting Statement: Recent events have made clear that company bottom-lines, and therefore value to shareholders, drop when companies take overtly political and divisive positions that alienate consumers. Following Bud Light’s embrace of partisanship and disparagement of its customer base, its revenue fell $395 million in North America when compared to the same time a year ago.12 This amounts to roughly 10 percent of its revenue in the months following its leap into contentious politics.13 Target Corporation’s market cap fell over $15 billion amid backlash for similar actions.14 And Disney stock fell 44 percent in 2022 – its worst performance in nearly 50 years – amid its decision to put extreme partisan agendas ahead of parents’ rights.15
Bristol Myers Squibb | 2025 Proxy Statement 106
Resolved: Shareholders request that the Board of Directors create a board committee on corporate financial sustainability to oversee and review the impact of the Company’s policy positions, advocacy, partnerships and charitable giving on social and political matters, and the effect of those actions on the Company’s financial sustainability. The Company should issue a public report on the committee’s findings by the end of 2025.
(1)https://corporate.kohls.com/news/archive-/2022/january/kohl-s-earns-top-score-in-human-rights-campaign-foundation-s-202
(2)Id.
(3)https://1792exchange.com/company/bristol-myers-squibb/
(4)https://1792exchange.com/spotlight-reports/
(5)https://1792exchange.com/company/bristol-myers-squibb/
(6)Id.
(7)https://nationalcenter.org/ncppr/2021/05/04/bristol-myers-squibb-slammed-for-efforts-to-destroy-womens-sports-and-religious-freedom/
(8)https://www.thetrevorproject.org/corporate-partners/
(9)https://www.thetrevorproject.org/research-briefs/gender-affirming-care-for-youth/
(10)https://aflegal.org/america-first-legal-demands-records-from-five-gender-clinics-in-georgia-iowa-ohio-utah-and-virginia-regarding-chemical-castration-and-genital-mutilation-known-as-gender-affirming-care/
(11)https://www.nationalreview.com/news/lgbtq-org-that-hosts-sexually-explicit-chatroom-racks-up-major-corporate-partnerships-millions-in-donations/
(12)https://www.cnn.com/2023/08/03/business/anheuser-busch-revenue-bud-light-intl-hnk/index.html;
(13)https://www.theguardian.com/business/2023/aug/03/bud-light-revenue-sales-anheuser-busch
(14)https://www.foxbusiness.com/media/target-market-cap-losses-hit-15-7-billion-share-near-52-week-low-amid-woke-backlash; https://nypost.com/2023/05/23/target-to-remove-some-lgbtq-merchandise-after-facing-customer-backlash/
(15)https://www.washingtonexaminer.com/policy/economy/disney-has-lost-50-billion-in-value-since-war-with-florida-began; https://www.hollywoodreporter.com/business/business-news/disney-stock-2022-1235289239/; https://markets.businessinsider.com/news/stocks/disney-stock-price-decline-bob-iger-pandemic-inflation-recession-streaming-2022-12; https://www.foxnews.com/media/disneys-decline-shows-woke-focus-alienating-fans-wsj-column
Board of Directors’ Position
The Board of Directors recommends a vote “AGAINST” the proposal for the following reasons.
The Board has carefully considered this proposal and believes the actions requested are not in the best interests of the Company and its shareholders.
Our existing corporate governance practices are designed to maximize shareholder value and enable the successful implementation of our strategy.
Effective corporate governance is essential for maximizing long-term value for the Company’s shareholders, and the Board’s existing corporate governance practices demonstrate its commitment to maintaining an effective structure to support the successful implementation of the Company’s strategy. The Board does not believe that establishment of the requested committee and development of the requested report are necessary to evaluate the Board’s oversight of the Company’s financial sustainability due to the existing allocation of responsibility of oversight among the Board and its committees in addition to the Company’s existing public disclosure and reports.
The proposal seeks to duplicate oversight responsibilities already undertaken by the Board or delegated to its committees.
Oversight of issues impacting the Company’s financial sustainability and risk is a responsibility of the Board and its current standing committees, and thus it would be redundant and inefficient to create a separate corporate financial sustainability committee, and the requested committee is not needed to oversee and review the Company’s policy positions, advocacy, partnerships or charitable giving on social and political matters and any resulting impacts or effects on the Company. The Board is dedicated to oversight of risk management and is responsible for risk oversight as part of its fiduciary duty to
107 Bristol Myers Squibb | 2025 Proxy Statement
monitor business operations effectively. In satisfaction of this responsibility, the Board and certain standing committees exercise extensive oversight of social and political matters, including the Company’s policy positions, advocacy, partnerships, and charitable giving, and the consideration of any impacts on the Company’s financial sustainability.
Specifically, among other things, the Audit Committee regularly reviews and discusses with management policies and guidelines regarding risk assessment and risk management, including their effectiveness, and the Board’s process for mitigating and monitoring enterprise risks, such as those related to strategic, financial, operational, legal, compliance, regulatory, and reputational risk, each of which directly relates to the Company’s financial sustainability. In addition, the Committee on Directors and Corporate Governance oversees the Company’s corporate governance affairs, reviews corporate governance practices and policies to manage related risks and identifies and oversees monitoring and management of risks related to the Company’s political activities and its Sustainability and Impact strategy, as well as any impact on the Company’s employees and shareholders.
These two committees report to the Board regarding their activities, findings, conclusions, and recommendations. In addition, the Board regularly discusses the Company’s positions on these matters and any corresponding impact on its stakeholders and the Company’s overall corporate financial sustainability and regularly reviews its committee structure and committee responsibilities to ensure that the Board has an appropriate committee structure focused on matters of financial, strategic and governance importance. The Board does not believe that establishing a separate committee to focus on corporate financial sustainability would be an effective or efficient use of the Company’s resources, as it would merely serve to duplicate work that the Board and its committees already perform. Accordingly, the proposal requests actions that are not necessary and are duplicative of existing Company practice.
BMS publishes annual reports on its corporate responsibility and sustainability efforts.
The Company’s existing public disclosures and reports detail our longstanding and unwavering positions on corporate financial sustainability, and the issuance of an additional report would result in unnecessary expense, detract management’s attention from operational matters and focus on the Company’s strategic priority.
On an annual basis, the Company publishes a report detailing some of its corporate responsibility activities and outlining its goals and progress toward meeting the evolving needs of patients and driving growth and value for the Company and its shareholders. As discussed above, the Board and its committees evaluate and review these activities and any resulting risks to the Company and its financial sustainability. Archived copies of recent reports are available on the Company’s website at https://www.bms.com/about-us/responsibility/transparency.html. The Company’s most recent Sustainability and Social Impact report discusses the Board’s and management’s collaborative approach to sound governance, the Company’s enterprise-wide sustainability strategy, and oversight and management of risks related to various topics affecting the sustainability of the company’s business.
In addition, the Company publishes numerous other public reports and disclosures that address the topics contemplated by this proposal. The Company’s Corporate Governance Guidelines and committee charters can be found at https://www.bms.com/about-us/our-company/governance.html, the Company’s Principles of Integrity, human rights disclosure and other pertinent information and reports can be found at https://www.bms.com/about-us/responsibility/transparency.html, and the Company’s policy and advocacy engagement and political contributions disclosure can be found at https://www.bms.com/about-us/responsibility/transparency/policy-and-advocacy-engagement-and-political-contributions.html. Each addresses topics regarding the Company’s evaluation of certain risks related to sustainability and its policy positions and partnerships. Of note, our Principles of Integrity are the building blocks of our Company policies, and they provide a common framework for how we interact with our colleagues, conduct business with our partners and suppliers, and serve our patients and the many communities in which we operate around the world. For these reasons, the Board believes that the report this proposal is requesting is neither necessary or in the best interests of the Company or its shareholders.
Bristol Myers Squibb | 2025 Proxy Statement 108
In sum, through its governance and Board oversight, the Company carefully considers how policy positions and actions impact long-term growth and shareholder value. Our Board, therefore, believes that forming the committee requested by the proposal would be unnecessary and duplicative as it would not provide any additional oversight or information and would not be an effective use of the Company’s time and resources or benefit our shareholders. Our Board also believes that the requested report would be unnecessary and duplicative as the information is already largely available and thus would not benefit shareholders.
| | | | | | | | | | | | | | |
| | | | |
| | | Accordingly, the Board of Directors unanimously recommends a vote “AGAINST” this proposal. | |
| | | | |
| | | | |
109 Bristol Myers Squibb | 2025 Proxy Statement
Item 5—Shareholder Proposal on a Request to Cease DEI Efforts
The proponent of this resolution is the National Center for Public Policy Research, 2005 Massachusetts Avenue NW, Washington, DC 20036.
Proposal 5 — Request to Cease DEI Efforts
Request to Cease DEI Efforts
Resolved:
Shareholders request that the Company consider abolishing its DEI program, policies, Department, and goals.
Supporting Statement:
Last year, the US Supreme Court ruled in SFFA v. Harvard that discriminating on the basis of race in college admissions violates the equal protection clause of the 14th Amendment.1 As a result, the legality of corporate Diversity, Equity and Inclusion (DEI) programs was called into question2 and thirteen Attorneys General warned that SFFA implicated corporate DEI programs.3
This year, those implications widened when the Supreme Court ruled in Muldrow v. City of St. Louis that Title VII of the Civil Rights Act protected against discriminatory job transfers.4 The ruling also lowered the bar for employees to successfully sue their employers for discrimination,5 and is therefore likely to lead to an increase in discrimination claims.
Since SFFA, a number of DEI-related lawsuits have been filed. Starbucks was successfully sued for discrimination by an employee for $25.6 million,6 and the risk of being sued for such discrimination is rising.7
Sensibly, many major companies have responded by rolling back their DEI commitments and laying off DEI departments due to the risks DEI creates of illegal discrimination.8 Alphabet and Meta cut DEI staff and DEI-related investments;9 and Microsoft and Zoom laid off their entire DEI teams.10 Since Muldrow, John Deere publicly halted DEI-related policies11 after Tractor Supply explicitly stated that it “eliminate[d] DEI roles and retire[d] our current DEI goals,”12 Lowe’s and Ford ended their participation in the Human Rights Campaign’s Corporate Equality (CEI),13 Harley Davidson ceased its DEI efforts,14 Jack Daniels ended both its DEI efforts and CEI participation,15 and Boeing got rid of its DEI department.16
DEI poses risks to companies and their shareholders, including the risk that adopting DEI policies that implement illegal discrimination will constitute a breach of fiduciary duties.
Despite these obvious risks, the SFFA and Muldrow decisions, and the wave of corporate DEI retreats, Bristol Myers Squibb still has a DEI program, which includes (1) race-based employee resource groups,17 (2) collaboration with the controversial Human Rights Campaign,18 (3) race-based workforce representation goals,19 (4) supplier diversity commitments,20 and (5) “affirmative action” policies.21
With over 30,000 employees,22 BMS likely has thousands of employees who are potentially victims of this type of discrimination. If even only a fraction of them file suit, and only some of those prove successful, the cost to BMS could reach billions of dollars.
(1)https://www.scotusblog.com/case-files/cases/students-for-fair-admissions-inc-v-president-fellows-of-harvard-college/
(2)https://freebeacon.com/democrats/starbucks-hired-eric-holder-to-conduct-a-civil-rights-audit-the-policies-he-blessed-got-the-coffee-maker-sued/
(3)https://ag.ks.gov/docs/default-source/documents/corporate-racial-discrimination-multistate-letter.pdf
(4)https://www.supremecourt.gov/opinions/23pdf/22-193_q86b.pdf
(5)https://www.skadden.com/insights/publications/2024/06/quarterly-insights/supreme-court-lowers-the-bar; https://www.dailysignal.com/2024/04/17/supreme-court-just-made-easier-sue-employers-dei-policies/
(6)https://www.foxbusiness.com/features/starbucks-manager-shannon-phillips-wins-25-million-lawsuit-fired-white-donte-robinson-rashon-nelson
Bristol Myers Squibb | 2025 Proxy Statement 110
(7)https://aflegal.org/america-first-legal-files-class-action-lawsuit-against-progressive-insurance-for-illegal-racial-discrimination/; https://aflegal.org/afl-files-federal-civil-rights-complaint-against-activision-for-illegal-racist-sexist-and-discriminatory-hiring-practices-and-sends-letter-to-activision-board-demanding-they-end-unlawful-dei-polici/; https://aflegal.org/america-first-legal-files-federal-civil-rights-complaint-against-kelloggs-warns-management-that-its-violating-fiduciary-duties/
(8)https://techcrunch.com/2024/07/29/dei-backlash-stay-up-to-date-on-the-latest-legal-and-corporate-challenges/
(9)https://www.cnbc.com/2023/12/22/google-meta-other-tech-giants-cut-dei-programs-in-2023.html
(10)https://www.businessinsider.com/microsoft-layoƯs-dei-leader-email-2024-7; https://www.bloomberg.com/news/articles/2024-02-06/zoom-dei-workers-fired-in-recent-round-of-job-cuts
(11)https://x.com/JohnDeere/status/181331897765084794411
(12)https://corporate.tractorsupply.com/newsroom/news-releases/news-releases-details/2024/Tractor-Supply-Company-Statement/default.aspx
(13)https://www.nbcnews.com/business/business-news/lowes-becomes-later-paring-back-dei-eƯorts-rcna168380; https://www.cbsnews.com/news/lowes-dei-harley-davidson-john-deere-tractor-supply/
(14)https://x.com/harleydavidson/status/182556413803223499414
(15)https://www.foxnews.com/lifestyle/jack-daniels-renounces-woke-agenda-latest-iconic-us-brand-bring-sanity-back-business
(16)https://www.bloomberg.com/news/articles/2024-10-31/boeing-dismantles-diversity-team-as-pressure-builds-on-new-ceo
(17)https://www.bms.com/about-us/global-diversity-and-inclusion/people-and-business-resource-groups.html
(18)https://www.hrc.org/resources/buyers-guide/bristol-myers-squibb-4; https://www.bms.com/about-us/working-at-bms.html
(19)https://news.bms.com/news/philanthropy/2023/Bristol-Myers-Squibb-Announces-Progress-Toward-Long-Term-Inclusion--Diversity-Goals-and-Health-Equity-Commitments/default.aspx; https://www.esg.bms.com/assets/bms-esg/us/documents/BMS-ESG-Report-2023.pdf
(20)https://www.bms.com/about-us/our-company/doing-business-with-us-as-a-supplier/supplier-diversity.html
(21)https://www.bms.com/about-us/global-diversity-and-inclusion/our-commitment-to-eeo/eeo-policy-statement.html; https://www.bms.com/about-us/global-diversity-and-inclusion/our-commitment-to-eeo.html
(22)https://www.macrotrends.net/stocks/charts/BMY/bristol-myers-squibb/number-of-employees
Board of Directors’ Position
The Board of Directors recommends a vote “AGAINST” the proposal for the following reasons.
The Board has carefully considered this proposal and believes the actions requested are not in the best interests of the Company and its shareholders.
Our commitment to inclusion, in compliance with applicable laws, furthers our strategic priorities and generates shareholder value.
We value inclusion and prioritize building an inclusive workforce in compliance with applicable non-discrimination laws, as do our shareholders. We believe our inclusion philosophy leads to greater financial and patient outcomes and generates shareholder value, because it helps drive our strategic goal to reach more patients with our transformative medicines. A workforce fueled by varied backgrounds, experiences, personal characteristics and viewpoints helps us expand our patient population, because it drives strategy designed to deliver greater access to varied clinical trial patients across geographic regions and address the changing demographics of the communities where we do business and the patients we serve.
Equal opportunity is a cornerstone of our culture. We prohibit workplace discrimination and retaliation and have a robust compliance program.
This proposal mischaracterizes the Company’s inclusion efforts by suggesting that such efforts are discriminatory. We believe the opposite is true. Our culture of inclusion enables us to create a respectful and welcoming work environment where all decisions are merit-based, and all employees are free to fully contribute and reach their maximum potential free from harassment and discrimination. Open communication is vital to our success. Employees are encouraged to speak up—to ask questions, raise concerns, and make suggestions about business practices, which enables our workforce to achieve their full potential and thereby better serve our patients.
111 Bristol Myers Squibb | 2025 Proxy Statement
Our employees, vendors, partners and independent contractors understand and share in the responsibility to comply with our Equal Employment Opportunity policies and legal requirements. The Company has been and will continue to be an equal opportunity employer. We seek to operate in compliance with applicable non-discrimination laws in all jurisdictions in which we operate, and we have developed practices and policies to address compliance risks while supporting our Principles of Integrity, which can be found at: https://www.bms.com/assets/bms/us/en-us/pdf/principles-of-integrity.pdf. We believe that implementation of this proposal would limit management’s ability to appropriately administer our compliance programs, and could call into question the very Company practices that ensure no employee is discriminated against.
As noted, we regularly evaluate our practices to ensure compliance with law. We believe that our current practices are legally appropriate, crucial to fulfillment of Company strategy and valued by shareholders—nothing in the proposal demonstrates otherwise. As such, we do not believe this proposal to be in the best interests of the Company or its shareholders.
| | | | | | | | | | | | | | |
| | | | |
| | | Accordingly, the Board of Directors unanimously recommends a vote “AGAINST” this proposal. | |
| | | | |
| | | | |
Bristol Myers Squibb | 2025 Proxy Statement 112
Voting Securities and Principal Holders
At the close of business on March 14, 2025, there were 2,034,763,196.509 shares of $0.10 par value common stock and 2,745 shares of $2.00 convertible preferred stock outstanding and entitled to vote.
Common Stock Ownership by Directors and Executive Officers
The following table sets forth, as of March 14, 2025 (except as otherwise noted), beneficial ownership of shares of our common stock by each director, each of our Named Executive Officers and all directors and executive officers as a group, in each case, as of such date. Shares are beneficially owned when an individual has voting and/or investment power over the shares or could obtain voting and/or investment power over the shares within 60 days. Voting power includes the power to direct the voting of the shares and investment power includes the power to direct the disposition of the shares. Unless otherwise noted, shares listed below are owned directly or indirectly with sole voting and investment power. None of our directors and executive officers, individually or as a group, beneficially owns greater than 1% of our outstanding shares of common or preferred stock.
| | | | | | | | | | | | | | | | | |
| Name | Total Common Shares Owned(1) (#) | Common Shares Underlying Options or Stock Units(2) (#) | Common Shares Underlying Deferred Share Units(3) (#) | |
| | | | | |
| P. J. Arduini | 60,938 | 0 | 60,938 | |
| D. L. Bhatt, M.D., M.P.H., M.B.A. | 14,488 | 0 | 14,488 | |
| C. Boerner, Ph.D. | 125,439 | 0 | 0 | |
| D. V. Elkins | 223,605 | 0 | 0 | |
| J. Haller, M.D. | 129,311 | 83,469 | 32,223 | |
| M. Hidalgo Medina, M.D., Ph.D. | 17,836 | 0 | 17,836 | |
| S. Hirawat, M.D. | 79,263 | 0 | 0 | |
| S. Leung | 465,374 | 0 | 0 | |
| M. R. McMullen | 7,827 | 0 | 7,827 | |
| P. A. Price | 20,434 | 0 | 20,434 | |
| D. W. Rice | 30,681 | 0 | 30,681 | |
| T. R. Samuels | 92,356 | 0 | 56,856 | |
| K. Shanahan | 15,086 | 0 | 0 | |
| K. H. Vousden, Ph.D. | 35,232 | 0 | 35,232 | |
| P. Yale | 34,677 | 0 | 34,677 | |
| All Directors and Executive Officers as a Group(4) | 1,453,003 | 83,469 | 311,193 | |
(1)Consists of direct and indirect ownership of shares, shares credited to the accounts of the executive officers under the Bristol-Myers Squibb Company Savings and Investment Program, stock options that are currently exercisable, restricted stock units that vest within 60 days, the target number of market share units that vest within 60 days and deferred share units.
113 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Voting Securities and Principal Holders |
(2)Consists of shares underlying stock options that are currently exercisable, restricted stock units that vest within 60 days, and the target number of market share units that vest within 60 days. None of these shares have any voting rights.
(3)Consists of deferred share units that are valued according to the market value and shareholder return on equivalent shares of common stock. Deferred share units have no voting rights.
(4)Includes 23 individuals.
Principal Holders of Voting Securities
The following table sets forth information regarding beneficial owners of more than 5% of the outstanding shares of our common stock. There are no beneficial owners of more than 5% of the outstanding shares of our preferred stock.
| | | | | | | | | | | | | | | | | |
| Name | Number of Shares Beneficially Owned
(#) | | Percent of Class
(%) | |
| | | | | |
| Vanguard Group Inc. 100 Vanguard Blvd Malvern, PA 19355 | 198,155,594 | (1) | 9.74 | % | (1) |
| BlackRock, Inc. 50 Hudson Yards New York, NY 10001 | 168,098,227 | (2) | 8.3 | % | (2) |
(1)This information is based on the Form 13G/A filed by The Vanguard Group with the SEC on February 13, 2024 reporting beneficial ownership as of December 31, 2023. The reporting person has sole voting power with respect to zero shares, shared voting power with respect to 2,641,512 shares, sole dispositive power with respect to 189,047,539 shares and shared dispositive power with respect to 9,108,055 shares.
(2)This information is based on the Schedule 13G/A filed by BlackRock, Inc. with the SEC on January 25, 2024 reporting beneficial ownership as of December 31, 2023. The reporting person has sole voting power with respect to 151,187,720 shares, shared voting power with respect to zero shares, sole dispositive power with respect to 168,098,227 shares and shared dispositive power with respect to zero shares.
Insider Trading Policy and Policy on Hedging and Pledging
Our insider trading policy prohibits all employees, including directors and executive officers, from engaging in any speculative or hedging transactions. Our insider trading policy also prohibits all employees, including directors and executive officers, from holding our securities in a margin account or pledging our securities as collateral for a loan except in certain limited circumstances pre-approved by our Corporate Secretary when a person wishes to pledge our securities as collateral for a loan and clearly demonstrates the ability to repay the loan without selling such securities. None of our directors or executive officers has pledged shares of our stock as collateral for a loan or holds shares of our stock in a margin account.
Bristol Myers Squibb | 2025 Proxy Statement 114
Other Matters
Advance Notice Procedures
As set forth in our Bylaws, if you wish to propose any action, including the nomination of directors, at next year’s annual meeting, you must deliver notice to BMS containing certain information set forth in our Bylaws, not less than 90 but not more than 120 days before the anniversary of the prior year’s annual meeting. For our 2026 Annual Meeting, we must receive this notice between January 6, 2026 and February 5, 2026. These requirements are separate and distinct from the SEC requirements that a shareholder must meet to have a shareholder proposal included in our proxy statement. For further information on how a shareholder may nominate a candidate to serve as a director, please see page 21. In addition to satisfying the foregoing advance notice requirements under our bylaws, to comply with the universal proxy rules under the Securities Exchange Act of 1934, as amended, shareholders who intend to solicit proxies in support of director nominees other than the company’s nominees must provide notice that sets forth the information required by Rule 14a-19 under the Securities Exchange Act of 1934, as amended, no later than March 6, 2026.
Our Bylaws are available on our website at https://www.bms.com/about-us/our-company/governance.html. In addition, a copy of the Bylaw provisions discussed above may be obtained by writing to us at our principal executive offices, Bristol- Myers Squibb Company, Route 206 & Province Line Road, Princeton, NJ 08543, Attention: Corporate Secretary.
2026 Shareholder Proposals
Shareholder proposals relating to our 2025 Annual Meeting of Shareholders must be received by us at our principal executive offices, Bristol-Myers Squibb Company, Route 206 & Province Line Road, Princeton, NJ 08543, Attention: Corporate Secretary, no later than November 26, 2025. Such proposals must comply with SEC regulations under Rule 14a-8 regarding the inclusion of shareholder proposals in company sponsored proxy materials. Shareholders are encouraged to contact the Office of the Corporate Secretary prior to submitting a shareholder proposal or any time they have a concern. At the direction of the Board of Directors, the Office of the Corporate Secretary acts as corporate governance liaison to shareholders.
Availability of Corporate Governance Documents
Our Corporate Governance Guidelines (including the standards of director independence), Principles of Integrity, Code of Ethics for Senior Financial Officers, Code of Business Conduct and Ethics for Directors, additional policies and guidelines, committee charters and links to Reports of Insider Transactions are available on our corporate governance webpage at https://www.bms.com/about-us/our-company/governance.html and are available to anyone who requests them by writing to: Corporate Secretary, Bristol-Myers Squibb Company, Route 206 & Province Line Road, Princeton, NJ 08543.
115 Bristol Myers Squibb | 2025 Proxy Statement
Frequently Asked Questions
Why Am I Receiving These Materials?
This Proxy Statement is being delivered to all shareholders of record as of the close of business on March 14, 2025, in connection with the solicitation of proxies on behalf of the Board of Directors for use at the Annual Meeting of Shareholders on May 6, 2025. We expect our proxy materials, including this Proxy Statement and the Annual Report, to be first made available to shareholders on or about March 26, 2025. Although the Annual Report and Proxy Statement are being delivered together, the Annual Report should not be deemed to be part of the Proxy Statement.
What Is “Householding” and How Does It Work?
“Householding” is a procedure we adopted whereby shareholders of record who have the same last name and address and who receive the proxy materials by mail will receive only one copy of the proxy materials unless we have received contrary instructions from one or more of the shareholders. This procedure reduces printing and mailing costs. If you wish to receive a separate copy of the proxy materials, now or in the future, at the same address, or if you are currently receiving multiple copies of the proxy materials at the same address and wish to receive a single copy, you may contact us by writing to Shareholder Services, Bristol-Myers Squibb Company, Route 206 & Province Line Road, Princeton, NJ 08543, Attention: Corporate Secretary or by calling us at (212) 546-3309. If you are a beneficial owner (your shares are held in the name of a bank, broker or other holder of record), the bank, broker or other holder of record may deliver only one copy of the Proxy Statement and Annual Report, or Notice of Internet Availability of Proxy Materials, to shareholders who have the same address unless the bank, broker or other holder of record has received contrary instructions from one or more of the shareholders. If you wish to receive a separate copy of the Proxy Statement and Annual Report, or Notice of Internet Availability of Proxy Materials, now or in the future, you may contact us at the address or phone number above and we will promptly deliver a separate copy. Beneficial owners sharing an address who are currently receiving multiple copies of the Proxy Statement and Annual Report, or Notice of Internet Availability of Proxy Materials, and wish to receive a single copy in the future, should contact their bank, broker or other holder of record to request that only a single copy be delivered to all shareholders at the shared address in the future.
Why Are You Holding a Virtual Annual Meeting?
Our goal for the Annual Meeting is to enable shareholders to participate in the meeting, while providing substantially the same access and possibilities for exchange with the Board and our senior management as an in-person meeting. We believe that this approach represents best practices for virtual shareholder meetings, including by providing a support line for technical assistance and addressing as many shareholder questions as time allows.
Who Can Attend the Virtual Annual Meeting?
Only shareholders of Bristol-Myers Squibb Company as of the record date, March 14, 2025, their authorized representatives and guests of Bristol-Myers Squibb Company may attend the Annual Meeting.
Only shareholders of record on the record date will be entitled to participate, vote their shares and ask questions during the virtual meeting via audio webcast. To be admitted to the virtual 2025 Annual Meeting, shareholders should visit www.virtualshareholdermeeting.com/BMY2025 and enter the 16-digit control number included on your Important Notice Regarding the Availability of Proxy Materials, on your proxy card, or on the instructions that accompanied your proxy materials.
We will have technicians ready to assist you with any technical difficulties you may have accessing the Annual Meeting. If you encounter any difficulties accessing the Annual Meeting or during the meeting time, there will be a 1-800 number and international number available on the website to assist you. Technical support will be available 15 minutes prior to the start of the meeting and through the conclusion of the meeting.
Bristol Myers Squibb | 2025 Proxy Statement 116
| | |
|
| Frequently Asked Questions |
How Do I Ask Questions in the Virtual Annual Meeting?
We are committed to ensuring that our shareholders have substantially the same opportunities to participate in the virtual Annual Meeting as they would at an in-person meeting.
To submit a question during the meeting, visit www.virtualshareholdermeeting.com/BMY2025, enter your 16-digit control number and type your question into the “Ask a Question” field and click “Submit.” If you would like to submit a question before the meeting, visit www.proxyvote.com with your 16-digit control number and select the “Submit a Question for Management” option. We encourage you to submit any question that is relevant to the business of the meeting. Questions pertinent to meeting matters will be answered during the meeting as time allows.
Who Is Entitled to Vote?
All holders of record of our $0.10 par value common stock and $2.00 convertible preferred stock at the close of business on March 14, 2025, will be entitled to vote at the 2025 Annual Meeting. Each share is entitled to one vote on each matter properly brought before the meeting.
How Do I Vote If I Am a Registered Shareholder?
Proxies are solicited to give all shareholders who are entitled to vote on the matters that come before the meeting the opportunity to do so whether or not they participate in the virtual meeting. If you are a registered holder, you can vote your shares by proxy in one of the following manners:
i)via Internet at www.proxyvote.com;
ii)by telephone at (800) 690-6903;
iii)via audio webcast during the virtual 2025 Annual Meeting; or
iv)by mail, if you received a paper copy of the proxy materials.
Choosing to vote via Internet or calling the toll-free number listed above will save us expense. In order to vote online or via telephone, have the voting form in hand and either call the number or go to the website and follow the instructions. If you vote via the Internet or by telephone, please do not return a signed proxy card.
If you wish to vote during the virtual Annual Meeting, you can vote your shares via audio webcast at www.virtualshareholdermeeting.com/BMY2025.
If you received a paper copy of the proxy materials and choose to vote by mail, specify how you want your shares voted on each proposal by marking the appropriate boxes on the proxy card enclosed with the Proxy Statement, date and sign it, and mail it in the postage-paid envelope.
How Do I Vote If I Am a Beneficial Shareholder?
If you are a beneficial shareholder, you have the right to direct your broker or nominee on how to vote the shares. You should complete a voting instruction card, which your broker or nominee is obligated to provide you. If you wish to vote at the virtual meeting, you must first obtain from the record holder a legal proxy issued in your name.
Under the rules of the New York Stock Exchange (“NYSE”), brokers that have not received voting instructions from their customers 10 days prior to the meeting date may vote their customers’ shares in the brokers’ discretion on the proposals regarding routine matters, which in most cases includes the ratification of the appointment of the independent registered public accounting firm.
Under NYSE rules, the election of directors, the advisory votes relating to executive compensation of our Named Executive Officers, and the approval of any shareholder proposals are considered “non-discretionary” items, which means that your broker cannot vote your shares on these proposals.
117 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Frequently Asked Questions |
What Items Will Be Voted Upon at the Virtual Annual Meeting?
At the Annual Meeting, we will consider and act on the following items of business:
i)the election to the Board of Directors of the 11 persons nominated by the Board, each for a term of one year;
ii)an advisory vote to approve the compensation of our Named Executive Officers;
iii)the ratification of the appointment of our independent registered public accounting firm; and
iv)two shareholder proposals, if presented at the meeting.
We do not know of any other matter that may be brought before the meeting. However, if other matters are properly presented for action, it is the intention of the named proxies to vote on them according to their best judgment.
What Are the Board of Directors’ Voting Recommendations?
For the reasons set forth in more detail in the Proxy Statement, our Board of Directors recommends a vote FOR the election of each director, FOR the advisory vote to approve the compensation of our Named Executive Officers, FOR the ratification of the appointment of Deloitte & Touche LLP as our independent registered public accounting firm for 2025, and AGAINST the shareholder proposals.
How Will My Shares Be Voted at the Virtual Annual Meeting?
Voting Options
| | | | | | | | | | | | | | | | | |
| Item | Proposal | Voting Options | Effect of Abstentions | Broker Discretionary Voting Allowed? | Effect of Broker Non-Votes |
| | | | | |
| 1 | Election of Directors | FOR, AGAINST or ABSTAIN (for each director nominee) | No effect—not counted as a vote cast | No | No effect |
| 2 | Advisory vote to approve the compensation of our Named Executive Officers | FOR, AGAINST or ABSTAIN | Treated as a vote AGAINST the proposal | No | No effect |
| 3 | Ratification of the appointment of an independent registered public accounting firm | FOR, AGAINST or ABSTAIN | Treated as a vote AGAINST the proposal | Yes | Not applicable |
| 4 | Shareholder Proposal on Corporate Financial Sustainability | FOR, AGAINST or ABSTAIN | Treated as a vote AGAINST the proposal | No | No effect |
| 5 | Shareholder Proposal on a Request to Cease DEI Efforts | FOR, AGAINST or ABSTAIN | Treated as a vote AGAINST the proposal | No | No effect |
How Many Votes Are Needed to Elect the Directors and to Approve Each of the Proposals?
Director Elections: A majority of votes cast with respect to each director’s election at the meeting is required to elect each director. A majority of the votes cast means that the number of votes cast “for” a director must exceed the number of votes cast “against” that director in order for the director to be elected. Abstentions will not be counted as votes cast for or against the director and broker non-votes will have no effect on this proposal.
Bristol Myers Squibb | 2025 Proxy Statement 118
| | |
|
| Frequently Asked Questions |
Advisory Vote to Approve Compensation of Our Named Executive Officers: The affirmative vote of a majority of our outstanding shares present in person or by proxy and entitled to vote on the matter is required for the approval of the advisory vote to approve the compensation of our Named Executive Officers. Because your vote is advisory, it will not be binding upon our Board of Directors. Abstentions will be counted as votes against this proposal and broker non-votes will have no effect on this proposal.
Ratification of Our Auditors: The affirmative vote of a majority of our outstanding shares present in person or by proxy and entitled to vote on the matter is required for the ratification of the appointment of our independent registered public accounting firm. Abstentions will be counted as votes against this proposal. As described above, a broker or other nominee may generally vote on routine matters such as this one, and therefore no broker non-votes are expected to exist in connection with this proposal.
Shareholder Proposals: The affirmative vote of a majority of our outstanding shares present in person or by proxy and entitled to vote on this matter is required for the approval of the shareholder proposals if presented at the meeting. Abstentions will be counted as votes against the proposals and broker non-votes will have no effect on the proposals.
How Are the Votes Counted?
In accordance with the laws of Delaware, our Amended and Restated Certificate of Incorporation and our Bylaws, for all matters being submitted to a vote of shareholders, only proxies and ballots that indicate votes “FOR,” “AGAINST” or “ABSTAIN” on the proposals, or that provide the designated proxies with the right to vote in their judgment and discretion on the proposals, are counted to determine the number of shares present and entitled to vote on a given matter. Broker non-votes are not counted as shares present and entitled to vote on a given matter but will be counted for purposes of determining quorum (whether enough votes are present to hold the Annual Meeting).
Can I Change My Vote After I Return the Proxy Card, or After Voting by Telephone or Electronically?
If you are a shareholder of record, you can revoke your proxy at any time before it is voted at the meeting by taking one of the following three actions:
i)by giving timely written notice of the revocation to the Corporate Secretary of Bristol-Myers Squibb Company;
ii)by casting a new vote by telephone or by the Internet prior to the deadline for voting; or
iii)by voting at the Annual Meeting.
If you are a beneficial owner of shares, you may submit new voting instructions by contacting your bank, broker or other holder of record. You may also vote at the Annual Meeting if you obtain a legal proxy.
All shares that have been properly voted and not revoked will be voted at the Annual Meeting.
How do I designate my proxy?
If you wish to give your proxy to someone other than the persons named as proxies in the enclosed form of proxy, you may do so by crossing out the names of all three persons named as proxies on the proxy card and inserting the name of another person. The signed card must be presented at the meeting by the person you have designated on the proxy card.
Who counts the votes?
An independent agent tabulates the proxies and the votes cast at the meeting. In addition, independent inspectors of election certify the results of the vote tabulation.
119 Bristol Myers Squibb | 2025 Proxy Statement
| | |
|
| Frequently Asked Questions |
Is My Vote Confidential?
Yes, any information that identifies a shareholder or the particular vote of a shareholder is kept confidential.
Who Will Pay for the Costs Involved in the Solicitation of Proxies?
We will pay all costs of preparing, assembling, printing and distributing the proxy materials as well as the solicitation of all proxies. We have retained Innisfree M&A Incorporated to assist in soliciting proxies for a fee of $30,000, plus reasonable out-of-pocket expenses. We may solicit proxies on behalf of the Board of Directors through the mail, in person, electronically, and by telecommunications. We will, upon request, reimburse brokerage firms and others for their reasonable expenses incurred for forwarding solicitation material to beneficial owners of stock.
Bristol Myers Squibb | 2025 Proxy Statement 120
EXHIBIT A
Categorical Standards of Independence
In determining director independence, the Board has adopted the following categorical standards to assist it in determining which relationships will be considered immaterial:
(a)an immediate family member of the director is or has been employed by the company, provided that such family member is not, and has not been for at least a period of three years, an executive officer of the company;
(b)more than three years has elapsed since i) the director was employed by the company, ii) an immediate family member of the director was employed by the company as an executive officer, or iii) an executive officer of the company was on the board of directors of a company that employed either the director or an immediate family member of the director as an executive officer;
(c)the director, or an immediate family member of the director, received, in any 12-month period within the last three years, $120,000 or less in direct compensation from the company (other than director’s fees or compensation that was deferred for prior service with the company);
(d)more than three years has elapsed since i) the director has been a partner with or employed by the company’s independent auditor or ii) an immediate family member personally worked on the company’s audit as a partner or employee of the company’s independent auditor;
(e)the director has an immediate family member who i) is an employee of, but not a partner of, the independent auditor and ii) does not personally work on the company’s audit;
(f)the director of the company, or an immediate family member of a director, is an executive officer or an employee of, or is otherwise affiliated with, another company that makes payment to, or receives payment from, the company for property or services in an amount which in any single fiscal year within the preceding three years, does not exceed the greater of $1 million or 2% of such other company’s consolidated gross revenues;
(g)the director of the company and/or an immediate family member of the director directly or indirectly owns, in the aggregate, 10% equity interest or less in another company that makes payment to, or receives payment from, the company for property or services;
(h)the director of the company, or an immediate family member of a director, is an executive officer, or employee of, or is otherwise affiliated with, a charitable organization or non-profit organization, and the company’s, or the Bristol-Myers Squibb Foundation’s discretionary charitable contributions to the organization, in the aggregate, in any single fiscal year within the preceding three years, do not exceed the greater of $1 million or 2% of that organization’s consolidated gross revenues; and
(i)an executive officer of the Company serves or served on the compensation committee of the board of directors of a company that, at the same time within the last three years, employs or employed either the director or an immediate family member of the director as an executive officer.
A-1 Bristol Myers Squibb | 2025 Proxy Statement