
1 Bristol Myers Squibb: Built for Growth 44th Annual J.P. Morgan Healthcare
Conference Christopher Boerner, Ph.D., Board Chair & CEO January 12th, 2026

Forward Looking Statements 2 This presentation (as well as the oral statements
made with respect to the information contained in this presentation) contains statements about Bristol-Myers Squibb Company’s (the “Company”) future financial results, plans, business development strategy, anticipated clinical trials, results
and regulatory approvals that constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. All statements that are not statements of historical facts are, or may
be deemed to be, forward-looking statements. Actual results may differ materially from those expressed in, or implied by, these statements as a result of various factors, including, but not limited to: (i) new laws, government actions and
regulations, including with respect to pricing controls and market access and the imposition of new tariffs, trade restrictions and export regulations, including the potential for international reference pricing and most-favored nation drug
pricing for our products, (ii) our ability to obtain, protect and maintain market exclusivity rights and enforce patents and other intellectual property rights, (iii) our ability to achieve expected clinical, regulatory and contractual
milestones on expected timelines or at all, (iv) difficulties or delays in the development and commercialization of new products, (v) difficulties or delays in our clinical trials and the manufacturing, distribution and sale of our products,
(vi) adverse outcomes in legal or regulatory proceedings, (vii) risks relating to acquisitions, divestitures, alliances, joint ventures and other portfolio actions and (viii) political and financial instability, including changes in general
economic conditions. These and other important factors are discussed in the Company’s most recent annual report on Form 10-K and reports on Forms 10-Q and 8-K. These documents are available on the U.S. Securities and Exchange Commission’s
website, on the Company’s website or from Bristol-Myers Squibb Investor Relations. No forward-looking statements can be guaranteed. In addition, any forward-looking statements and clinical data included herein are presented only as of the
date hereof. Except as otherwise required by applicable law, the Company undertakes no obligation to publicly update any of the provided information, whether as a result of new information, future events, changed circumstances or otherwise.

Our goalis to build a company that is financially strong and delivers
industry-leading sustainable growth into the 2030s and beyond OPT 1
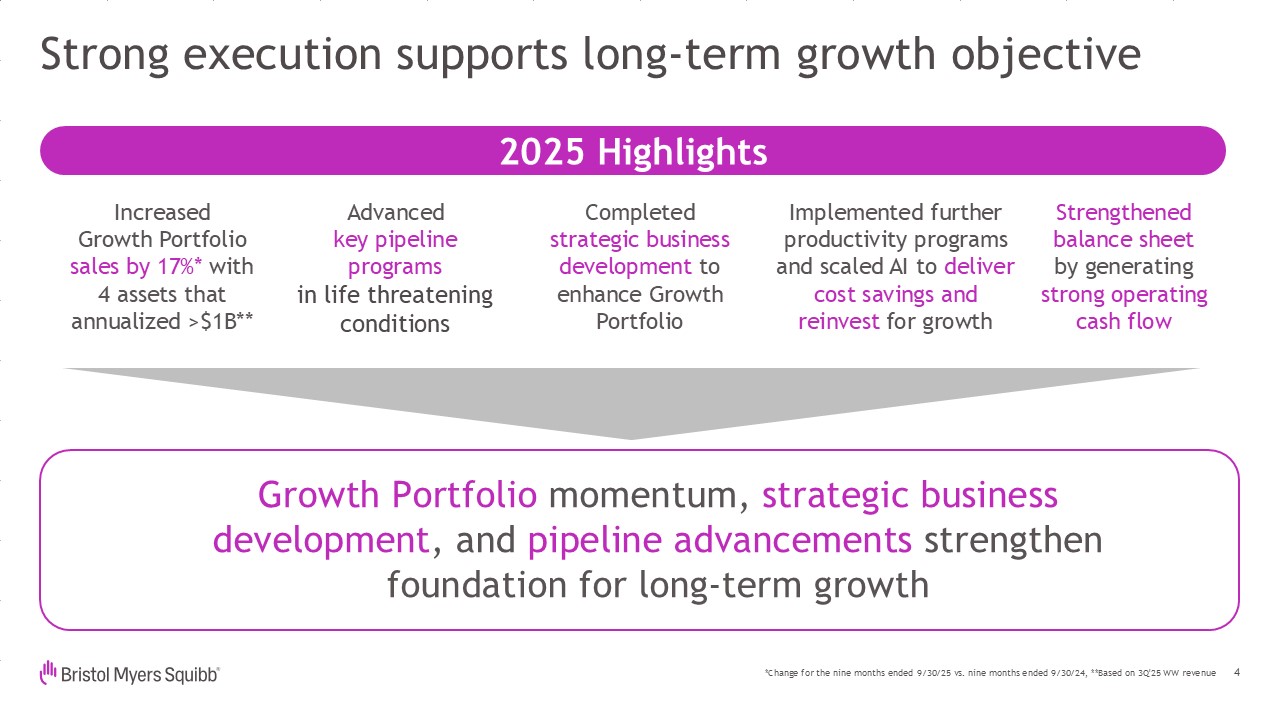
Strong execution supports long-term growth objective 4 IncreasedGrowth
Portfoliosales by 17%* with4 assets that annualized >$1B** Advancedkey pipeline programsin life threatening conditions Completedstrategic business development to enhance Growth Portfolio Implemented further productivity programs and
scaled AI to deliver cost savings and reinvest for growth Strengthened balance sheetby generating strong operating cash flow Growth Portfolio momentum, strategic businessdevelopment, and pipeline advancements strengthen foundation for
long-term growth 2025 Highlights *Change for the nine months ended 9/30/25 vs. nine months ended 9/30/24, **Based on 3Q'25 WW revenue
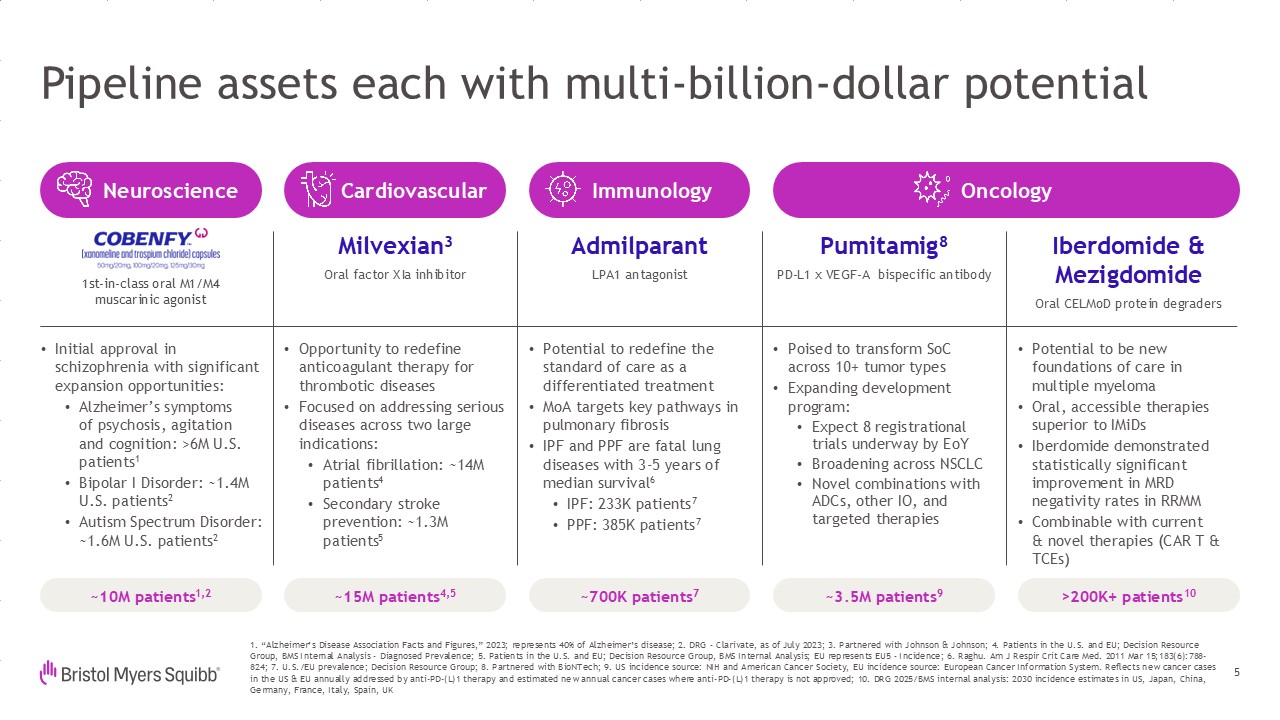
Pipeline assets each with multi-billion-dollar potential 5 Milvexian3 Oral
factor XIa inhibitor Admilparant LPA1 antagonist Pumitamig8 PD-L1 x VEGF-A bispecific antibody Iberdomide & Mezigdomide Oral CELMoD protein degraders 1. “Alzheimer’s Disease Association Facts and Figures,” 2023; represents 40%
of Alzheimer’s disease; 2. DRG – Clarivate, as of July 2023; 3. Partnered with Johnson & Johnson; 4. Patients in the U.S. and EU; Decision Resource Group, BMS Internal Analysis – Diagnosed Prevalence; 5. Patients in the U.S. and EU;
Decision Resource Group, BMS Internal Analysis; EU represents EU5 – Incidence; 6. Raghu. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788-824; 7. U.S./EU prevalence; Decision Resource Group; 8. Partnered with BioNTech; 9. US incidence
source: NIH and American Cancer Society, EU incidence source: European Cancer Information System. Reflects new cancer cases in the US & EU annually addressed by anti-PD-(L)1 therapy and estimated new annual cancer cases where anti-PD-(L)1
therapy is not approved; 10. DRG 2025/BMS internal analysis: 2030 incidence estimates in US, Japan, China, Germany, France, Italy, Spain, UK ~10M patients1,2 ~15M patients4,5 ~700K patients7 ~3.5M patients9 >200K+
patients10 Opportunity to redefine anticoagulant therapy for thrombotic diseases Focused on addressing serious diseases across two large indications: Atrial fibrillation: ~14M patients4 Secondary stroke prevention: ~1.3M
patients5 Potential to redefine the standard of care as a differentiated treatment MoA targets key pathways in pulmonary fibrosis IPF and PPF are fatal lung diseases with 3-5 years of median survival6 IPF: 233K patients7 PPF: 385K
patients7 Poised to transform SoC across 10+ tumor types Expanding development program: Expect 8 registrational trials underway by EoY Broadening across NSCLC Novel combinations with ADCs, other IO, and targeted therapies Potential to
be new foundations of care in multiple myeloma Oral, accessible therapies superior to IMiDs Iberdomide demonstrated statistically significant improvement in MRD negativity rates in RRMM Combinable with current& novel therapies (CAR T
& TCEs) Initial approval in schizophrenia with significant expansion opportunities: Alzheimer’s symptomsof psychosis, agitationand cognition: >6M U.S. patients1 Bipolar I Disorder: ~1.4M U.S. patients2 Autism Spectrum Disorder:
~1.6M U.S. patients2 Neuroscience Cardiovascular Immunology Oncology 1st-in-class oral M1/M4muscarinic agonist
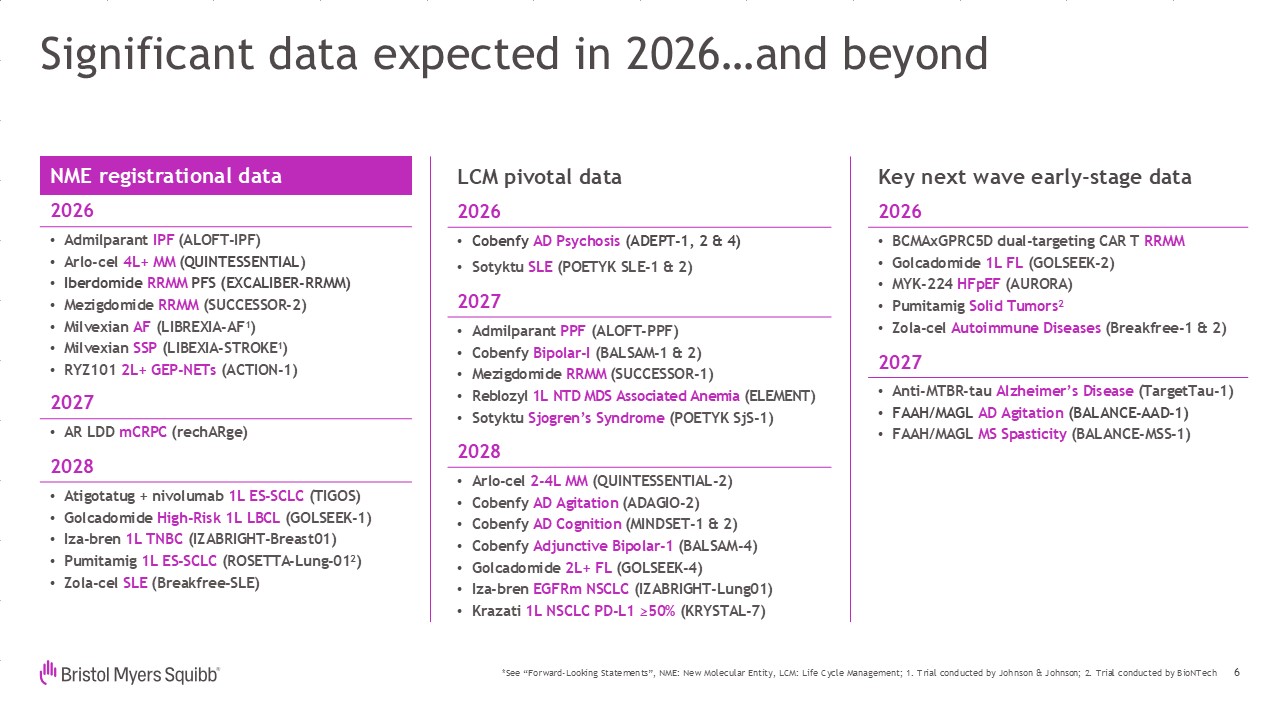
Significant data expected in 2026…and beyond NME registrational
data 2026 Admilparant IPF (ALOFT-IPF) Arlo-cel 4L+ MM (QUINTESSENTIAL) Iberdomide RRMM PFS (EXCALIBER-RRMM) Mezigdomide RRMM (SUCCESSOR-2) Milvexian AF (LIBREXIA-AF1) Milvexian SSP (LIBEXIA-STROKE1) RYZ101 2L+ GEP-NETs
(ACTION-1) 2027 AR LDD mCRPC (rechARge) 2028 Atigotatug + nivolumab 1L ES-SCLC (TIGOS) Golcadomide High-Risk 1L LBCL (GOLSEEK-1) Iza-bren 1L TNBC (IZABRIGHT-Breast01) Pumitamig 1L ES-SCLC (ROSETTA-Lung-012) Zola-cel SLE
(Breakfree-SLE) 6 LCM pivotal data 2026 Cobenfy AD Psychosis (ADEPT-1, 2 & 4) Sotyktu SLE (POETYK SLE-1 & 2) 2027 Admilparant PPF (ALOFT-PPF) Cobenfy Bipolar-I (BALSAM-1 & 2) Mezigdomide RRMM (SUCCESSOR-1) Reblozyl 1L
NTD MDS Associated Anemia (ELEMENT) Sotyktu Sjogren’s Syndrome (POETYK SjS-1) 2028 Arlo-cel 2-4L MM (QUINTESSENTIAL-2) Cobenfy AD Agitation (ADAGIO-2) Cobenfy AD Cognition (MINDSET-1 & 2) Cobenfy Adjunctive Bipolar-1
(BALSAM-4) Golcadomide 2L+ FL (GOLSEEK-4) Iza-bren EGFRm NSCLC (IZABRIGHT-Lung01) Krazati 1L NSCLC PD-L1 ≥50% (KRYSTAL-7) Key next wave early-stage data 2026 BCMAxGPRC5D dual-targeting CAR T RRMM Golcadomide 1L FL (GOLSEEK-2) MYK-224
HFpEF (AURORA) Pumitamig Solid Tumors2 Zola-cel Autoimmune Diseases (Breakfree-1 & 2) 2027 Anti-MTBR-tau Alzheimer’s Disease (TargetTau-1) FAAH/MAGL AD Agitation (BALANCE-AAD-1) FAAH/MAGL MS Spasticity (BALANCE-MSS-1) *See
“Forward-Looking Statements”, NME: New Molecular Entity, LCM: Life Cycle Management; 1. Trial conducted by Johnson & Johnson; 2. Trial conducted by BioNTech

Drives wave of 10+ new product launch opportunities by 2030 Growth Portfolio
LCM NME / NME LCM *See “Forward-Looking Statements”; Not an exhaustive list of assets, programs, or indications; subject to positive registrational trials and regulatory approval; 1. Partnered with Zenas BioPharma; 2. Partnered with Johnson
& Johnson; 3. Partnered with SystImmune; 4. Partnered with BioNTech; 5. Reflects potential launches in the years during and beyond 2029 7 BCMA x GPRC5D CAR T MM Anti-CCR8 Solid
tumors RYZ801 HCC CD33-GSPT1 AML MYK-224 HFpEF Anti-MTBR-Tau Alzheimer’s Disease CD40xFAP Solid tumors PRMT5 NSCLC TRPC4/5 Mood/Anxiety Disorders AR-LDD mCRPC CEACAM5-Topo1 ADC Solid tumors PRMT5 PDAC WEE1 CELMoD Solid
tumors Arlo-cel RRMM eIF2B Activator Alzheimer’s Disease Pumitamig4 NSCLC Zola-cel IIM Atigotatug-Nivo SCLC FAAH/MAGL ADA/MSS Pumitamig4 SCLC Zola-cel MS BCL6
LDD DLBCL Golcadomide FL Pumitamig4 Gastric Zola-cel Myasthenia Gravis Augtyro NSCLC ROS1+ TKI-naive Golcadomide DLBCL Pumitamig4 HCC Zola-cel SLE Cobenfy Autism irritability HbF
Inducer SCD Pumitamig4 H&N Zola-cel Systemic Sclerosis Cobenfy AD Cognition Iza-bren mUC Pumitamig4 MSS CRC Iberdomide MM maintenance Cobenfy AD Agitation Iza-bren3 NSCLC Pumitamig4 RCC RYZ101 SCLC Rela
HD+Nivo NSCLC Iza-bren TNBC Pumitamig4 TNBC RYZ101 BC Admilparant IPF Admilparant PPF Milvexian2 AF Milvexian2 SSP RYZ101 GEP-NETs Breyanzi FL 2L Cobenfy Bipolar I Disorder Krazati NSCLC 1L Reblozyl MDS 1L
NTD Sotyktu SLE Sotyktu SjS Arlo-cel MM 4L+ Iberdomide RRMM Mezigdomide RRMM Obexelimab1 IgG4-RD Cobenfy AD Psychosis Krazati CRC 2L Opdivo HCC Adj Opdivo+Chemo MIBC Adjuvant Opdivo+Chemo MIBC Neo-Adj Opdivo &
Qvantig H&N Sotyktu PsA Reblozyl MF 2028 2027 2026 2029++5
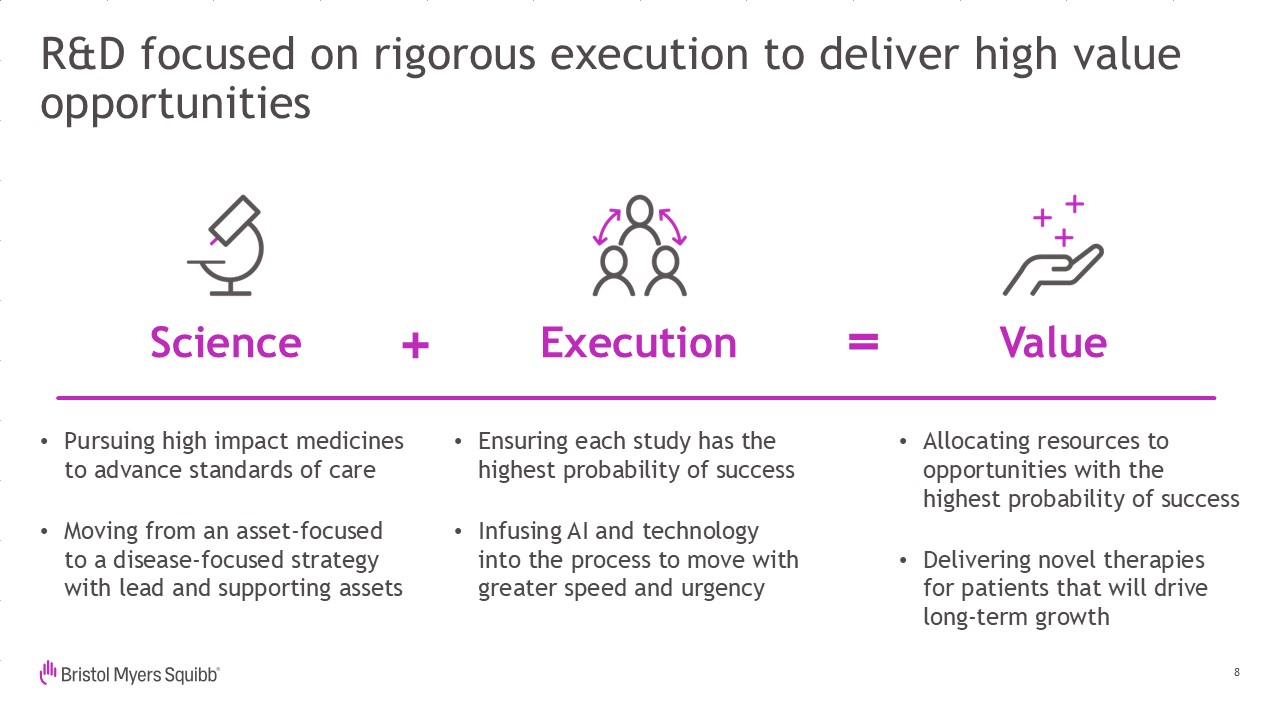
R&D focused on rigorous execution to deliver high value opportunities
Pursuing high impact medicines to advance standards of care Moving from an asset-focused to a disease-focused strategy with lead and supporting assets 8 Ensuring each study has the highest probability of success Infusing AI and
technologyinto the process to move with greater speed and urgency Allocating resources to opportunities with the highest probability of success Delivering novel therapiesfor patients that will drivelong-term
growth Science Execution Value +
Multiple paths for capital deployment to create value enabled by financial
strength and discipline 9 Investing inthe business Unlocking full potential of the Growth Portfolio Prioritizing highest opportunity assets and programs in R&D Deploying AI enterprise-wide Returning capitalto shareholders Solid
track record of returning capital to shareholders through ~$14.5Bin dividends and ~$8B in share repurchases over the past 3 years1 94 consecutive years of dividend payments2 Strategic business development 1. See “Forward-Looking
Statements”; 2. Latest dividend declared 12/10/2025 and payable 2/2/2026 on common stock of the company
Building our long-term growth profile 10 Focusing on transformational
medicines to treat life threatening diseases Focused on execution and building momentum in our Growth Portfolio Financial discipline and shareholder friendly capital allocation Strategic Priorities


Appendix

13 Clinical Development Portfolio — Phase I and II pumitamig 1L
Microsatellite Stable Colorectal Cancer 1L Gastric Cancer iza-bren ª 1L Triple-Negative Breast Cancer EGFR-mutated Post-TKI Non-Small Cell Lung Cancer Post-IO Metastatic Urothelial Cancer OPDIVO QVANTIG + YERVOY 1L Non-Small Cell Lung
Cancer PRMT5 Inhibitor 1L Non-Small Cell Lung Cancer ª 1L Pancreatic Ductal Adenocarcinoma 2L Non-Small Cell Lung Cancer arlo-cel ª 4L+ Multiple Myeloma golcadomide 1L Follicular Lymphoma REBLOZYL Alpha-Thalassemia MYK-224 ª
Heart Failure with Preserved Ejection Fraction CD19 NEX-T ª Systemic Lupus Erythematosus Anti-MTBR Tau ª Alzheimer’s Disease FAAH/MAGL Dual Inhibitor Alzheimer’s Disease Agitation ª Multiple Sclerosis Spasticity Anti-CCR8 ª Solid
Tumors BMS-986460^ ª Prostate Cancer BMS-986482+ ª Solid Tumors BMS-986488+ ª Solid Tumors BMS-986500+ ª Solid Tumors BMS-986506+ ª Solid Tumors BMS-986517 ª Solid Tumors BMS-986523 ª Solid Tumors CD40xFAP Bispecific ª Solid
Tumors CEACAM5-TOPO1 ADC ª Solid Tumors iza-bren 1L Non-Small Cell Lung Cancer* Metastatic Non-Small Cell Lung Cancer Solid Tumors* PRMT5 Inhibitor Solid Tumors pumitamig 1L Hepatocellular Carcinoma 1L Renal Cell Carcinoma 2L
Non-Small Cell Lung Cancer* RYZ101 Extensive-Stage Small Cell Lung Cancer HR+/HER2- Unresectable Metastatic Breast Cancer RYZ401 ª Solid Tumors RYZ801 ª Hepatocellular Carcinoma WEE1 CELMoD ª Solid Tumors BCL6
LDD Lymphoma CD33-GSPT1 ADC ª Acute Myeloid Leukemia Dual Targeting BCMAxGPRC5D CAR T ª RR Multiple Myeloma HbF Activating CELMoD ª Sickle Cell Disease BMS-986454 ª Rheumatoid Arthritis CD19 HD Allo CAR T ª Autoimmune
Diseases CD19 NEX-T Idiopathic Inflammatory Myopathies Rheumatoid Arthritis Systemic Sclerosis BMS-986495 ª Neurodegenerative Diseases* CD19 NEX-T Multiple Sclerosis Myasthenia Gravis eIF2B Activator ª Alzheimer’s Disease KarXT
Long-Acting Injectable ª Schizophrenia TRPC4/5 Inhibitor ª Mood and Anxiety Disorders Data as of Jan 12th, 2026 Hematology Immunology Oncology Neuroscience CV Phase I Phase II * Partner-run study NME leading
indication ^ CELMoD + LDD

14 Clinical Development Portfolio — Phase III Development Partnerships:
Anti-CCR8 + nivolumab, nivolumab + relatlimab HD, OPDIVO, YERVOY: Ono; BMS-986495: Prothena; COBENFY (KarXT): Zai Lab; pumitamig (BNT327/BMS-986545): BioNTech; iza-bren: SystImmune; milvexian: Johnson & Johnson; obexelimab: Zenas
BioPharma; REBLOZYL: Merck; rHuPH20: Halozyme Data as of Jan 12th, 2026 Phase III Registration US, EU, JP AR LDD ª Metastatic Castration-Resistant Prostate Cancer atigotatug + nivolumab ª 1L Extensive-Stage Small Cell Lung
Cancer KRAZATI 1L Non-Small Cell Lung Cancer 1L Non-Small Cell Lung Cancer PD-L1≥50% 2L Colorectal Cancer nivolumab + relatlimab HD ª 1L Non-Small Cell Lung Cancer PD-L1≥1% OPDIVO Adjuvant Hepatocellular Carcinoma Peri-adjuvant
Muscle-Invasive Urothelial Carcinoma pumitamig 1L Extensive-Stage Small Cell Lung Cancer* 1L Non-Small Cell Lung Cancer* 1L Triple-Negative Breast Cancer* RYZ101 ª 2L+ SSTR2+ Gastroenteropancreatic Neuroendocrine Tumors SC nivolumab +
relatlimab + rHuPH20 ª 1L Melanoma arlo-cel 2-4L Multiple Myeloma golcadomide 2L+ Follicular Lymphoma ª High Risk 1L Large B-cell Lymphoma iberdomide ª 2L+ Multiple Myeloma Post-ASCT Maintenance Newly Diagnosed Multiple
Myeloma mezigdomide ª 2L+ Multiple Myeloma Kd 2L+ Multiple Myeloma Vd REBLOZYL 1L NTD Myelodysplastic Syndrome Associated Anemia 1L TD Myelofibrosis Associated Anemia milvexian Atrial Fibrillation* Secondary Stroke
Prevention* admilparant ª Idiopathic Pulmonary Fibrosis LPA1 Antagonist Progressive Pulmonary Fibrosis obexelimab ª IgG4-Related Disease SOTYKTU Sjögren's Syndrome Systemic Lupus Erythematosus COBENFY Adjunctive Bipolar-I
Mania Agitation in Alzheimer’s Disease Alzheimer’s Disease Cognition Bipolar-I Mania Pediatric Autism Irritability Psychosis in Alzheimer’s Disease OPDIVO 1L Classical Hodgkin Lymphoma (US) BREYANZI R/R Marginal Zone Lymphoma
(JP) SOTYKTU Psoriatic Arthritis (US, EU, JP) Hematology Immunology Oncology Neuroscience CV * Partner-run study NME leading indication