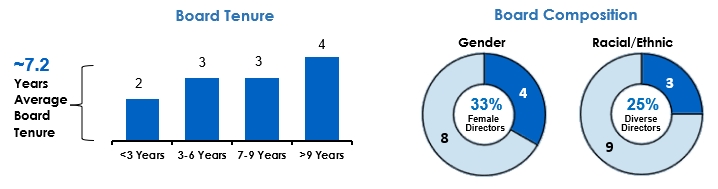
We are committed to corporate governance best practices overseen by our highly experienced and independent Board.
•We have a highly independent Board (11 of our 12 director nominees are independent) and only independent Board members serve on our key standing committees.(5)
•Our lead independent director, Robert A. Eckert, has substantial and specific duties and has been elected by our Board to serve as the lead independent director in 2025 subject to his re-election to the Board by stockholders.
•A director serving as our CEO should not serve on more than two outside public company boards and no director should serve on more than five public company boards. As part of its nominating process, the Governance and Nominating Committee conducts an annual review of director commitment levels and shares its findings with the Board. Currently, our CEO serves on one outside public company board and no director serves on more than two outside public company boards.
We have a long-standing practice of stockholder engagement and our Board has a history of responsiveness to stockholder feedback.
•We have a long-standing practice of stockholder engagement throughout the year and at our Annual Meeting. Consistent with prior years’ practices, since our 2024 annual meeting of stockholders, we have engaged in governance-focused outreach activities and discussions with stockholders comprising ~51% of our outstanding shares.
•In addition to our stockholders electing our Board annually by majority voting and having rights to act by special meeting, written consent, and proxy access, as well as our robust recoupment mechanisms, informed by engagement with our stockholders, we have expanded and enhanced a number of our disclosures, including those related to our: alignment of compensation program with strategic priorities; drug pricing practices; patents for our five top selling products; tax strategy; and oversight of political contributions, memberships in trade and industry associations, and lobbying.
We have implemented compensation best practices, including:
•A substantial majority of our Named Executive Officer (NEO) compensation is performance-based (including 80% of total target equity, of which 50% is in the form of three-year performance awards and 30% in the form of stock options).
•Our equity incentive plan provides that our equity awards are subject to a minimum vesting period of no less than one year for 95% of equity awards granted, with most equity grants vesting over four years to emphasize the long-term performance focus of our LTI equity award program and enhance retention.
•We have robust stock ownership and retention guidelines.
•We have a clawback policy providing for the mandatory recovery from our Section 16 officers, including our NEOs, of erroneously awarded incentive-based executive compensation including past cash bonuses and performance unit payouts granted, earned, or vested, wholly or in part, upon the attainment of any financial reporting measure that is the subject of a financial restatement.(6)
•We also have strong recoupment provisions for misconduct that permit a determination that cash incentive awards are not earned and to facilitate the forfeiture and cancellation of unvested or unexercised equity awards.(7)
Executive compensation is aligned with our business strategy and is performance-based.
•We pay for performance with a mix of incentives and targets (financial and operational) and pay outcomes reflect the achievements of our NEOs against our near- and long-term performance.
•76% of our CEO’s 2024 target direct compensation and 70% of our other NEOs’ target direct compensation was based solely on our Company’s performance.
•92% of our CEO’s 2024 target direct compensation and 83% of our other NEOs’ target direct compensation is “at-risk.”
•Our Compensation and Management Development Committee approves annual Company goals that are designed to focus our NEOs and all of our staff members on delivering our financial and operational objectives to drive annual performance, advance strategic priorities, and position us for longer-term success.