

Corporate Presentation

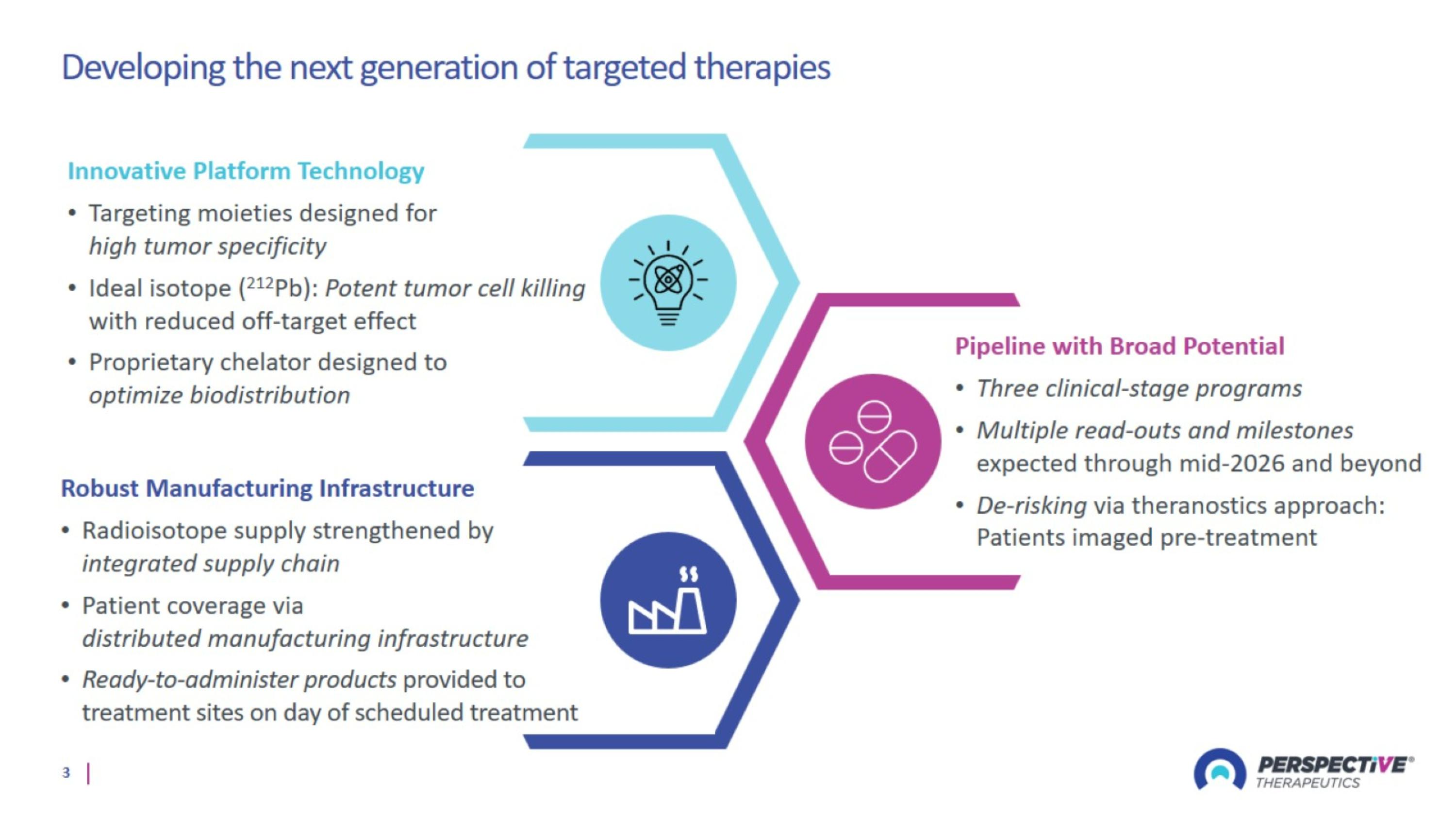
Developing the next generation of targeted therapies
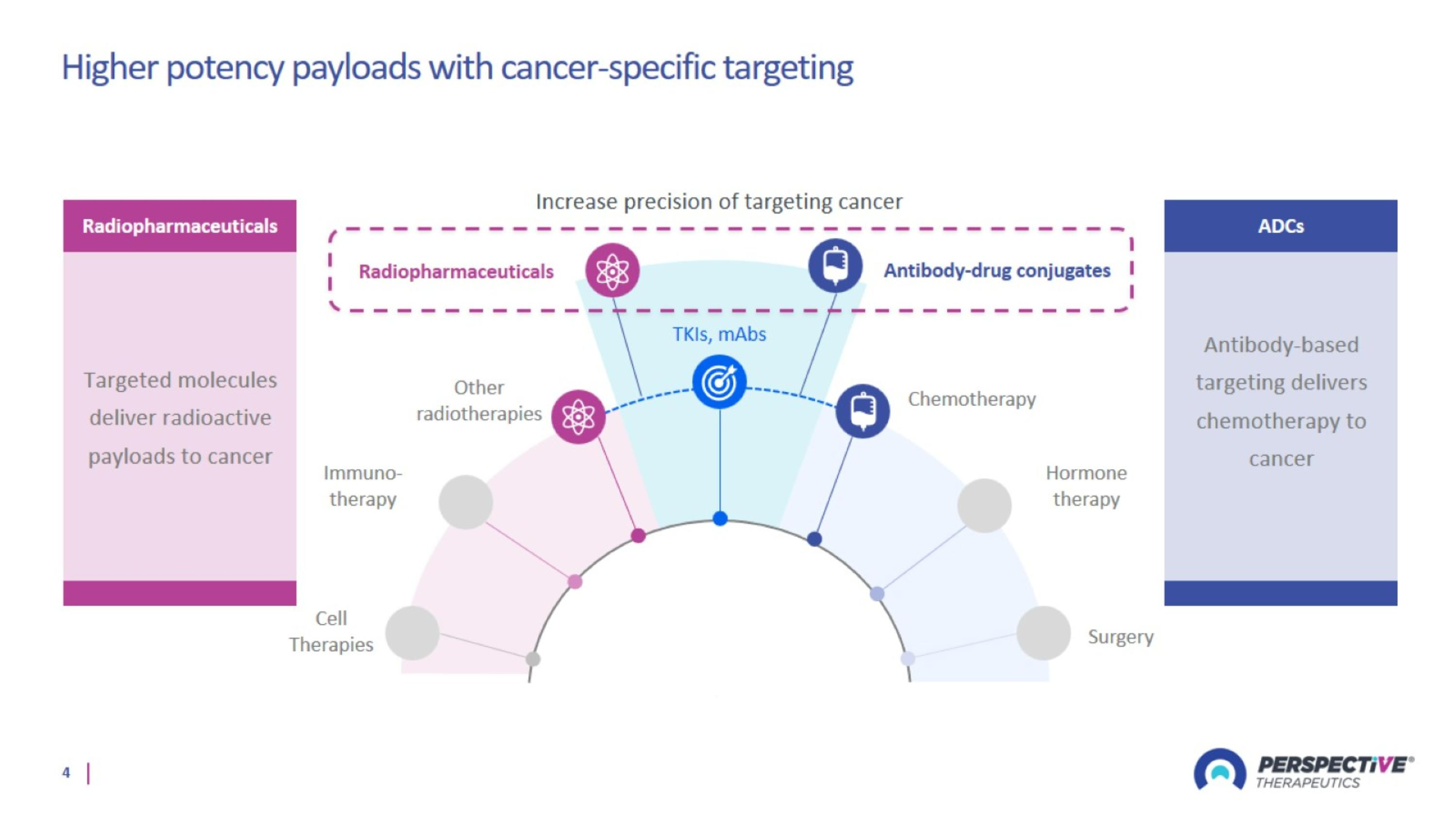
Higher potency payloads with cancer-specific targeting
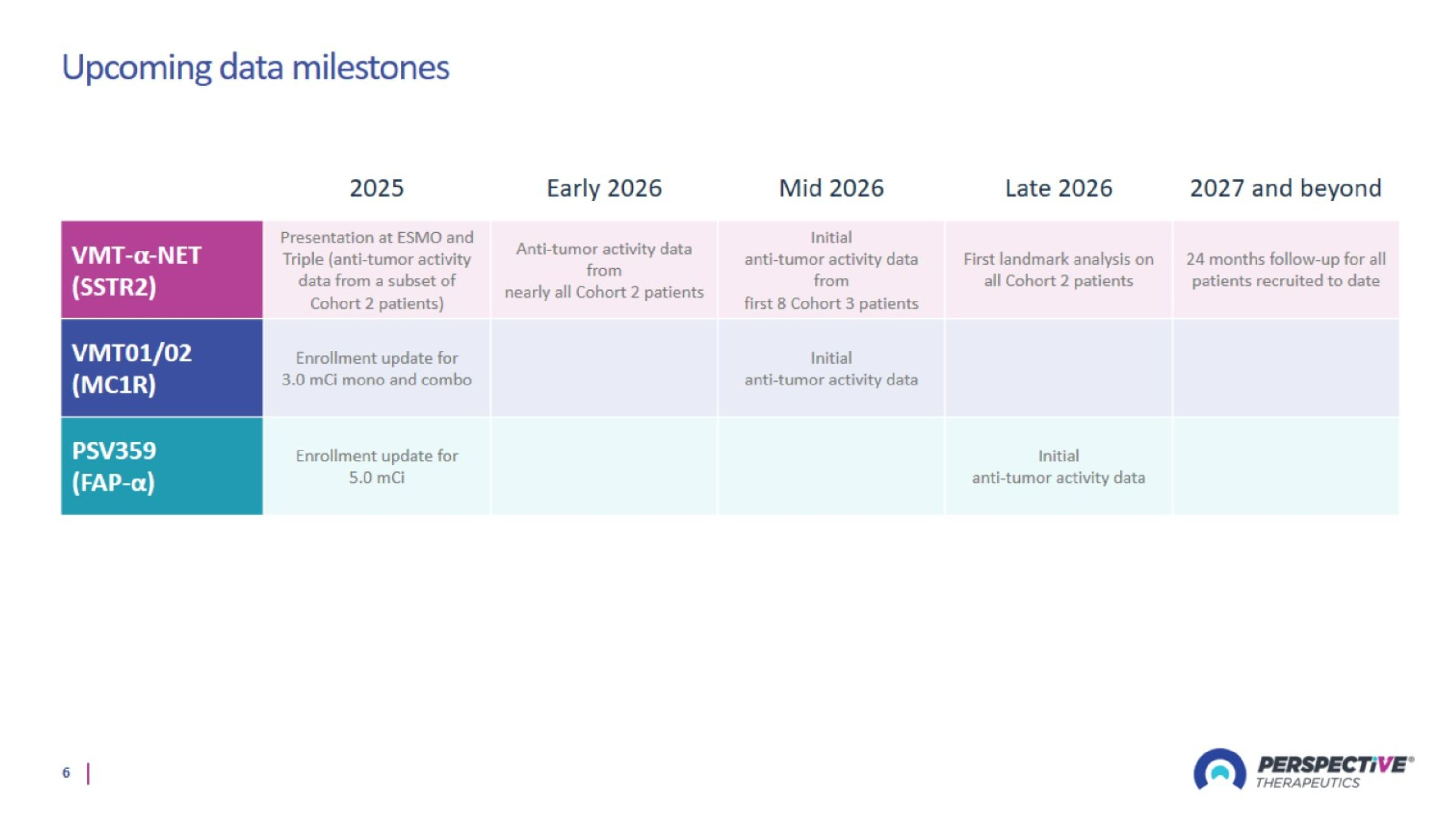
Upcoming data milestones
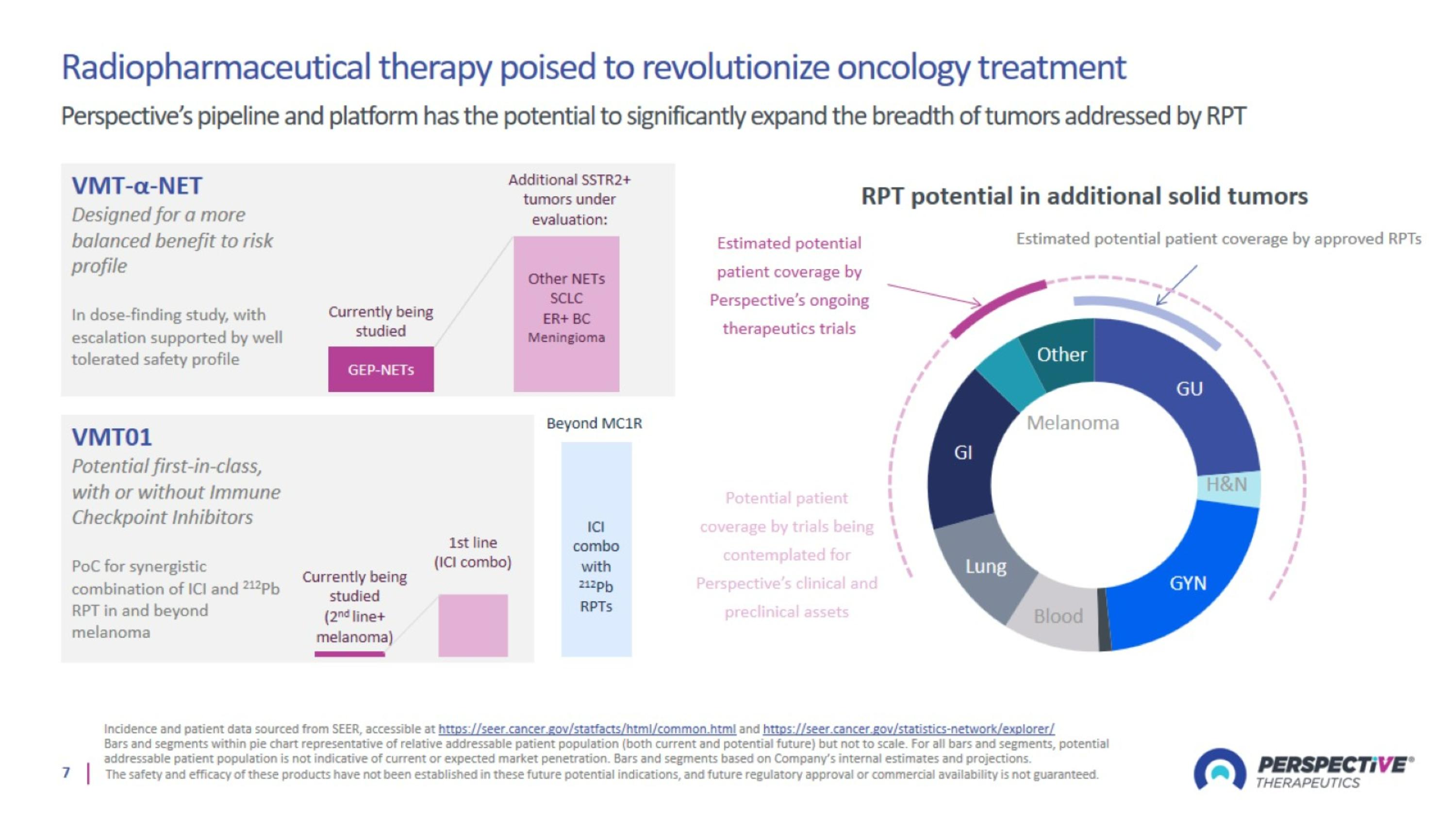
Radiopharmaceutical therapy poised to revolutionize oncology treatment Perspective’s pipeline and platform has the potential to significantly expand the breadth of tumors addressed by RPT
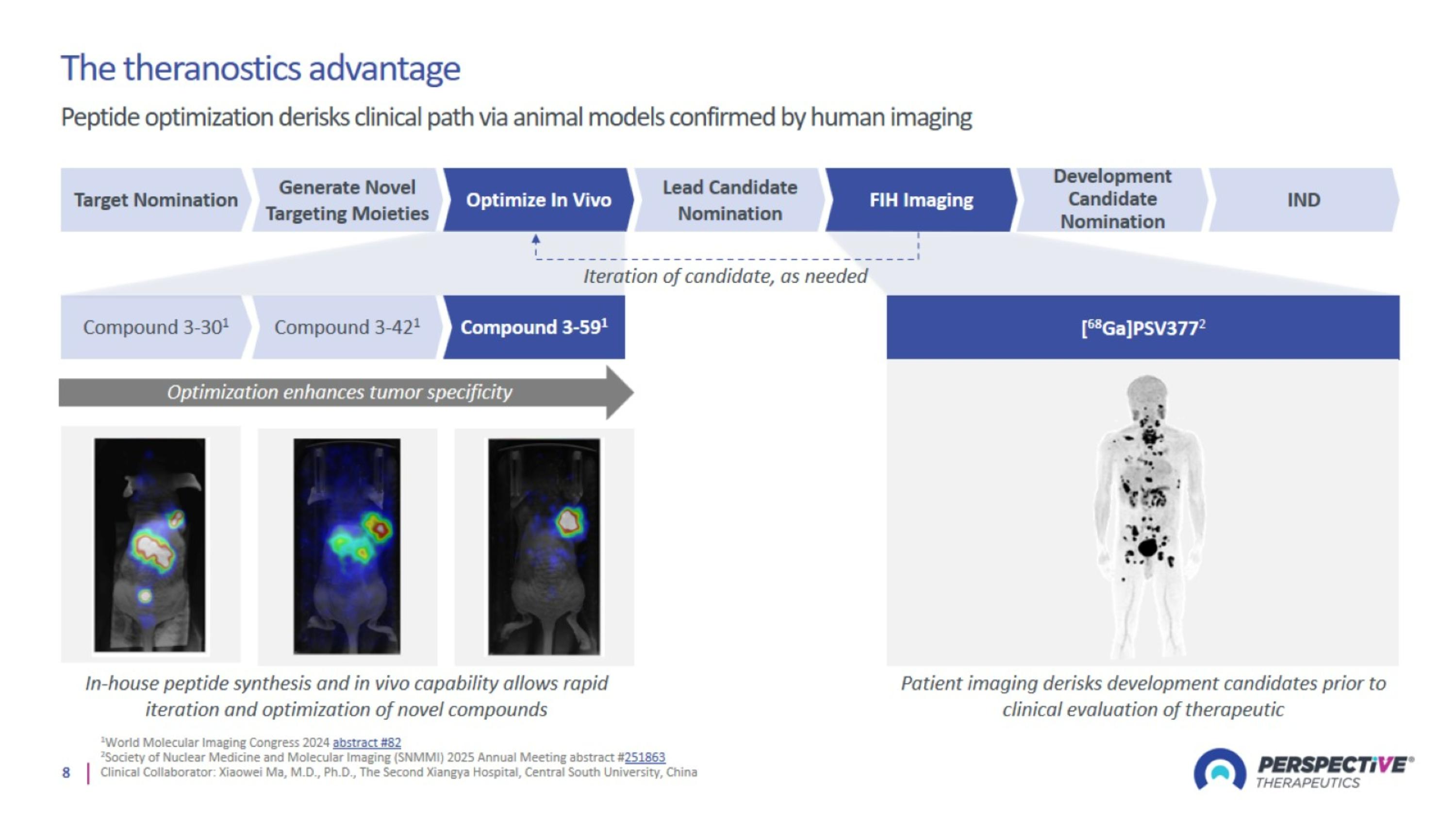

Perspective’s Innovative Platform
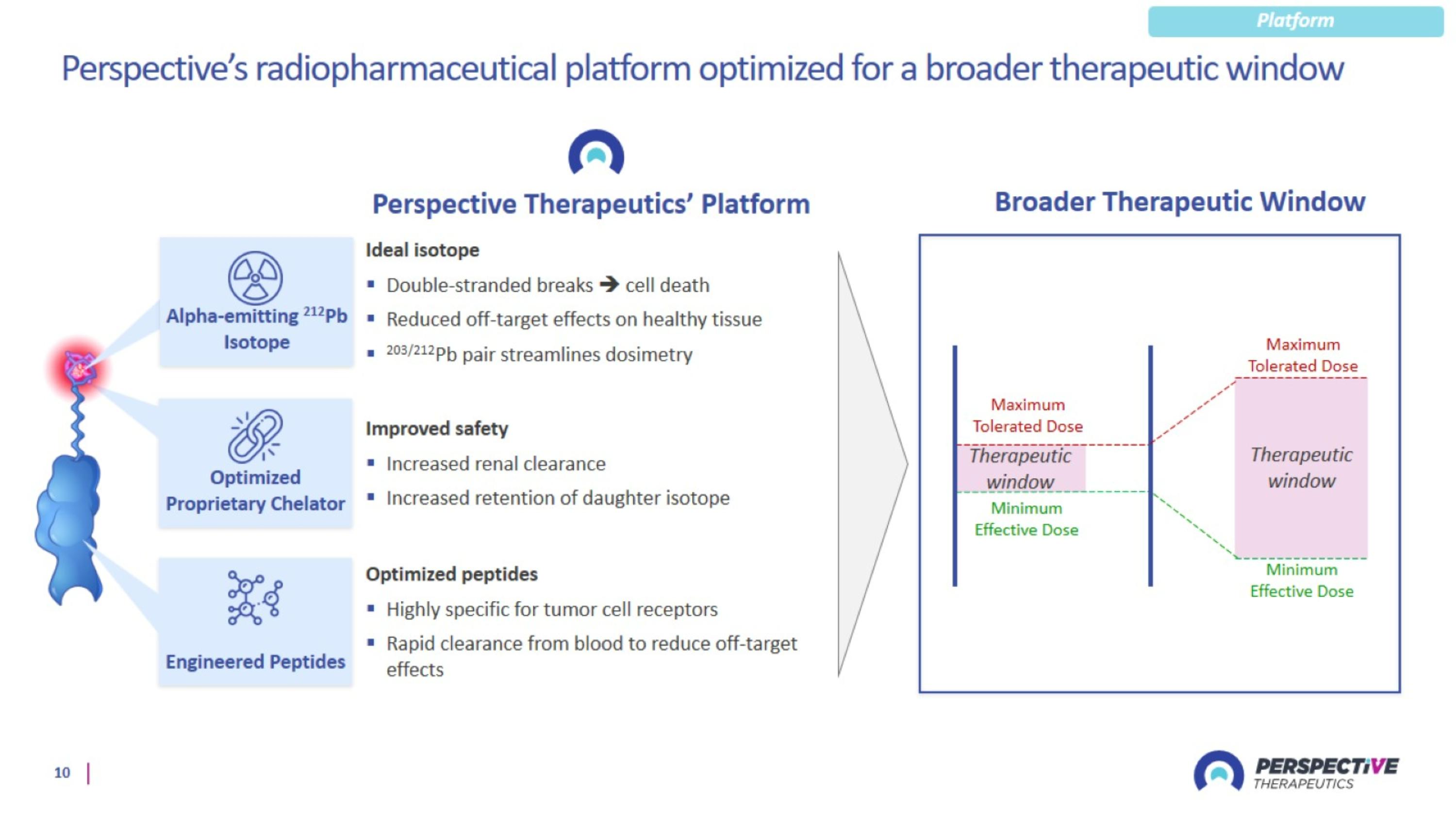
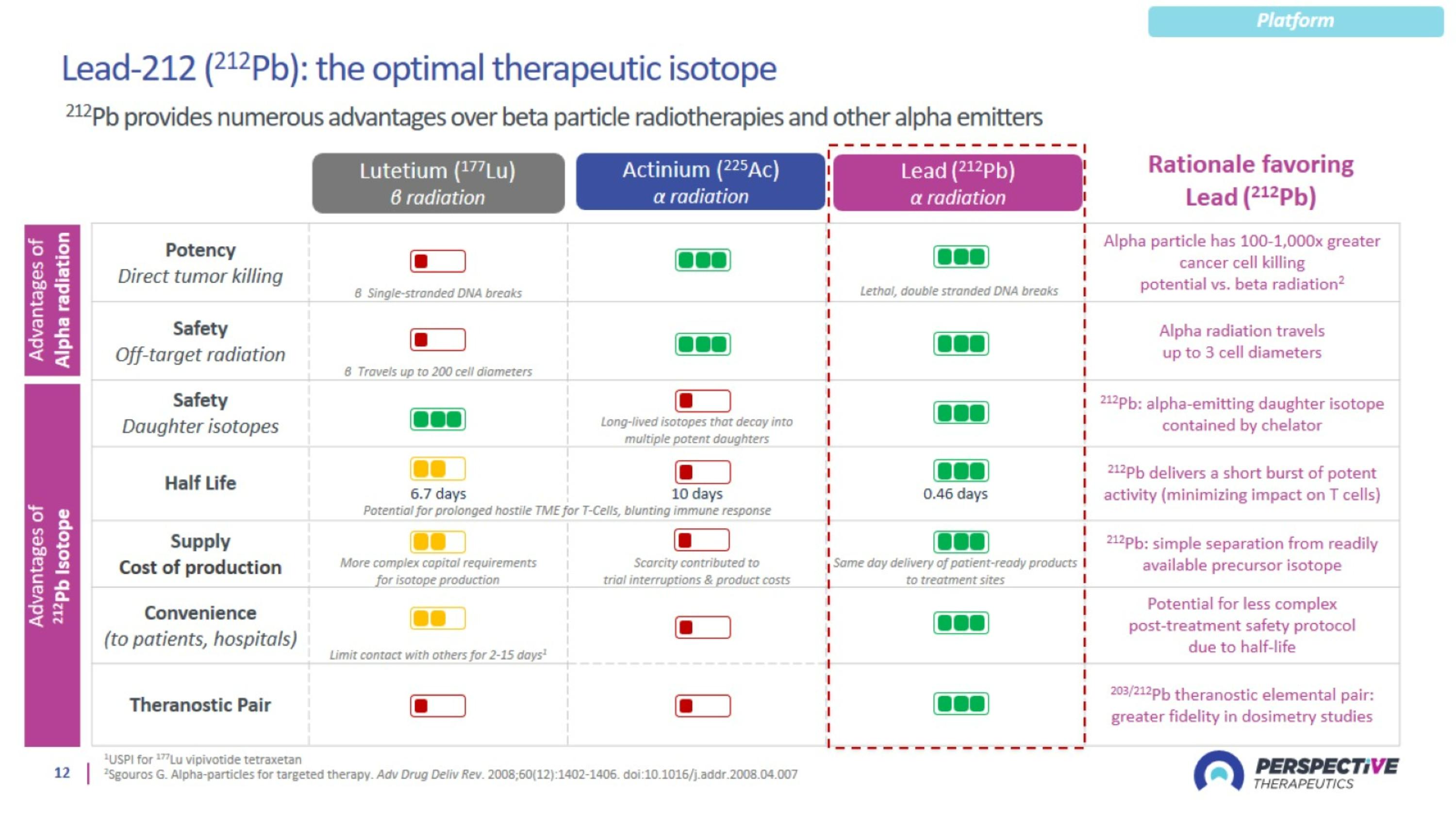
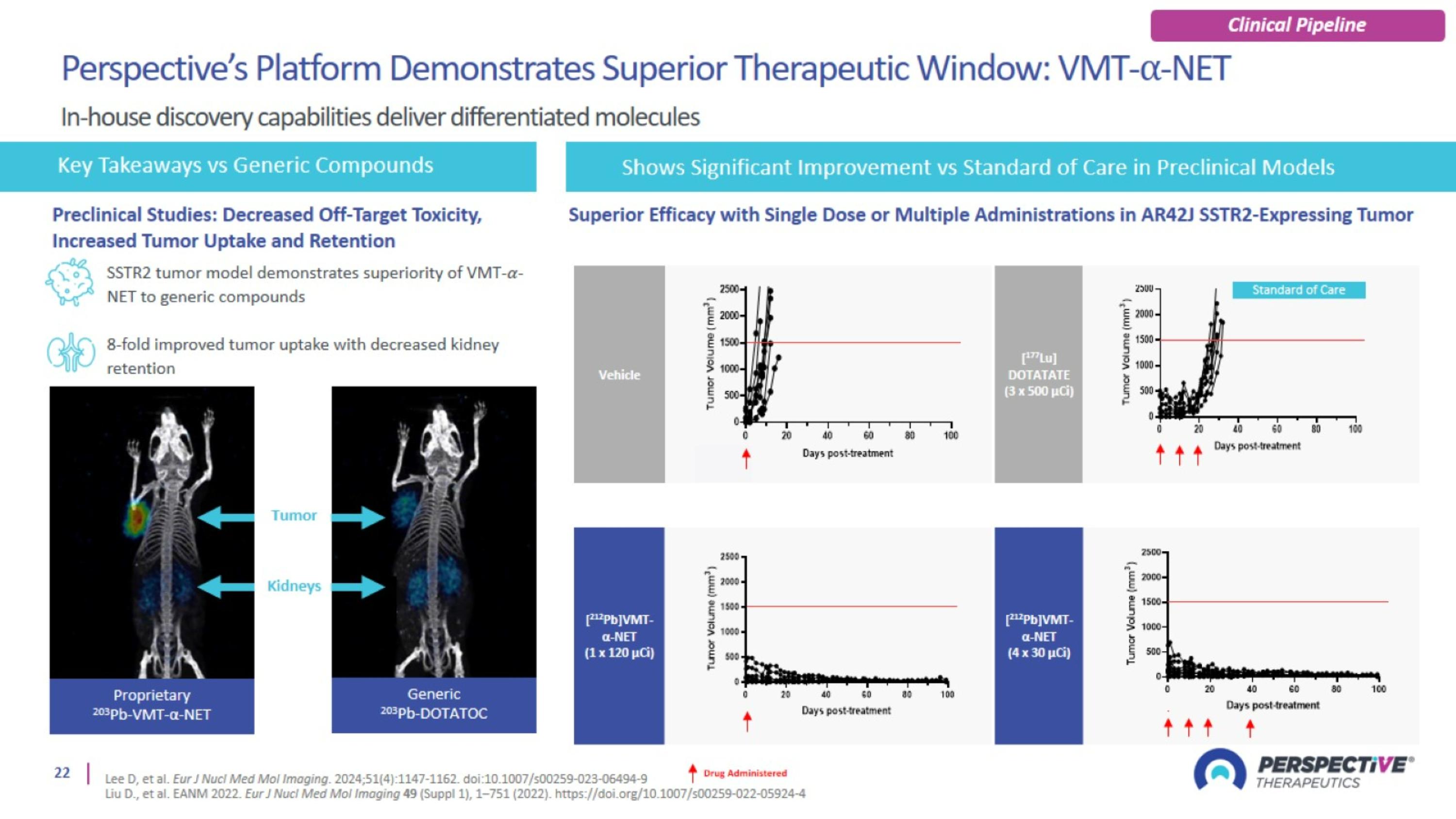
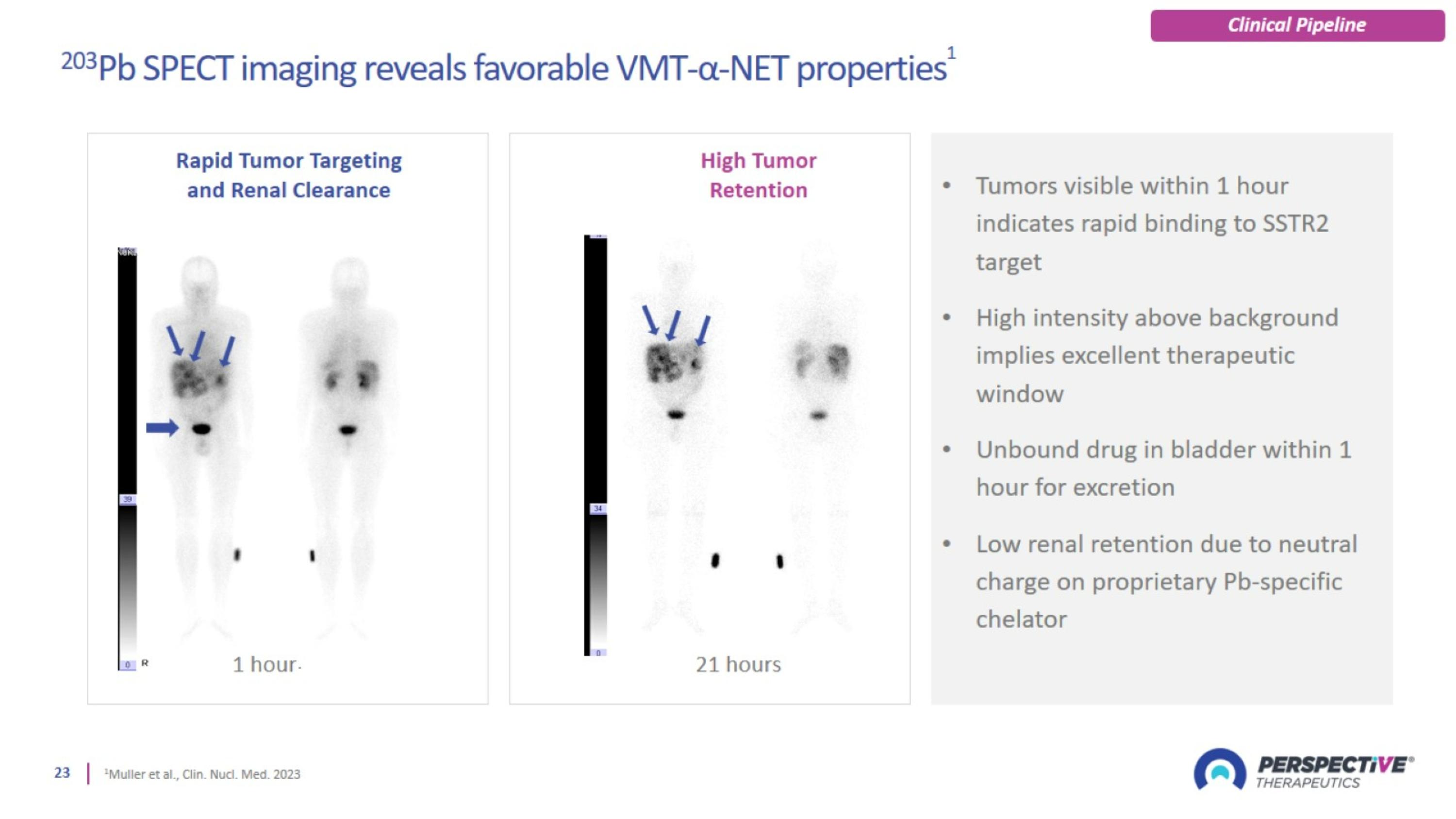
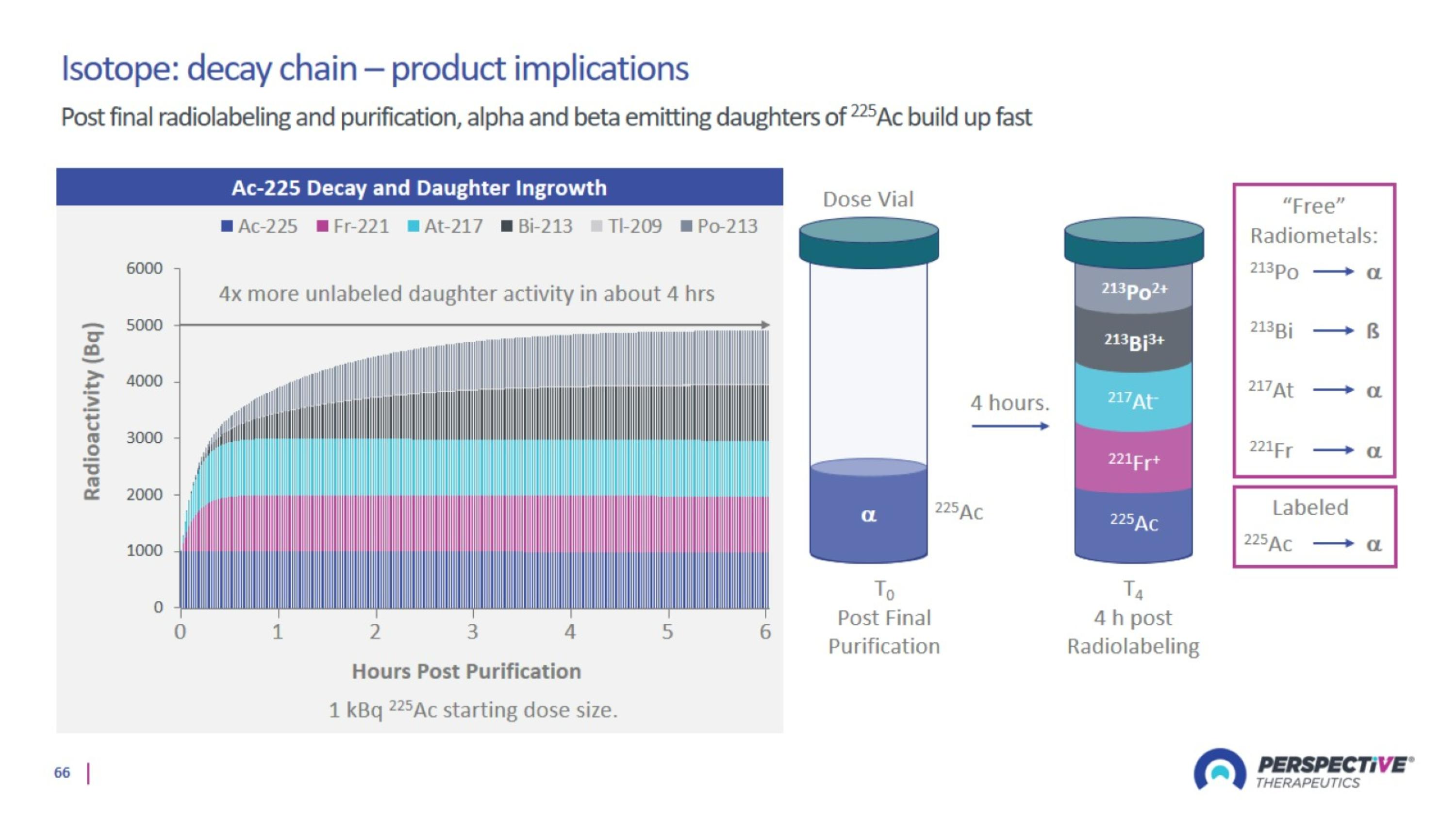
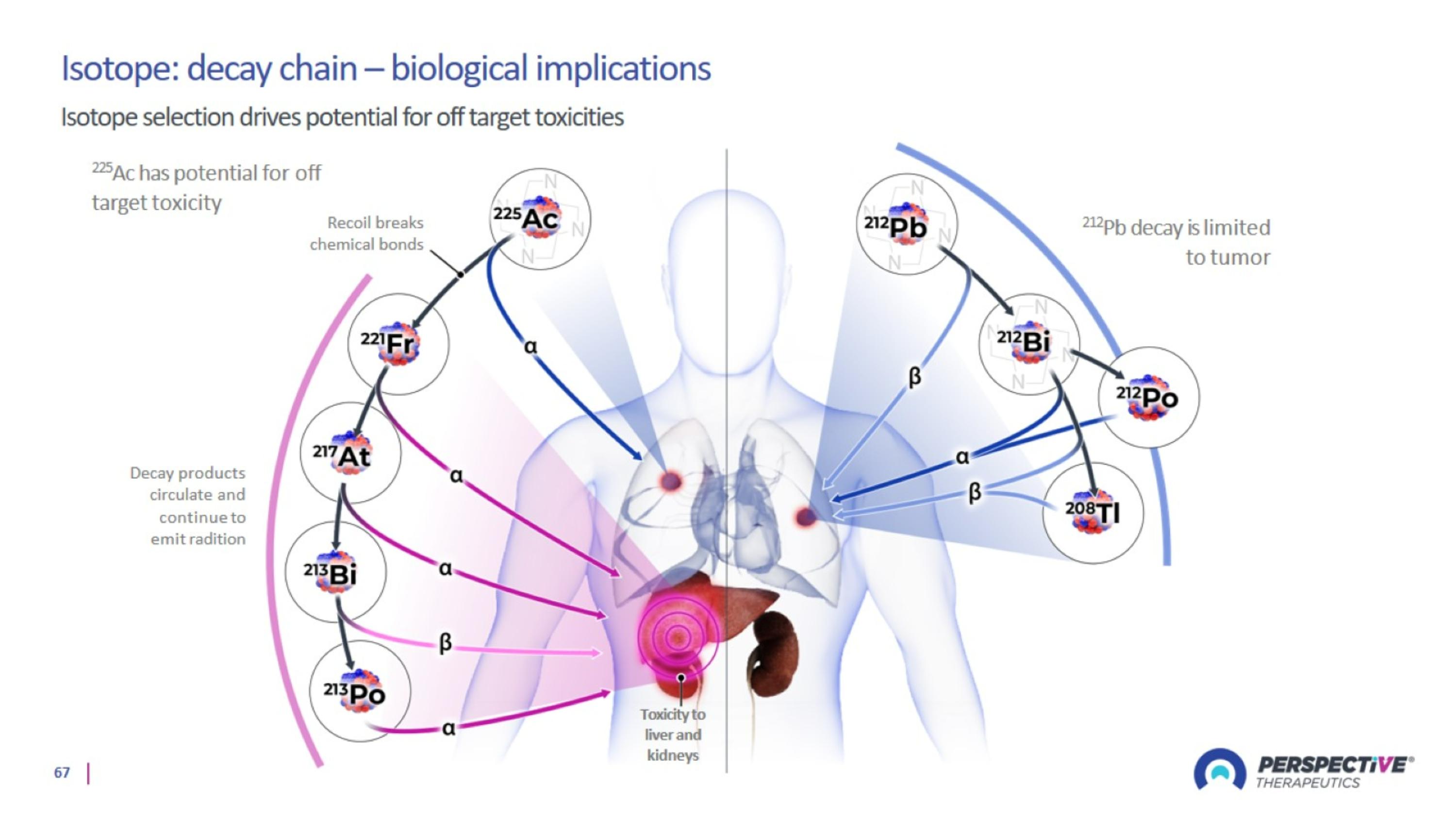
Perspective’s radiopharmaceutical platform optimized for a broader therapeutic window
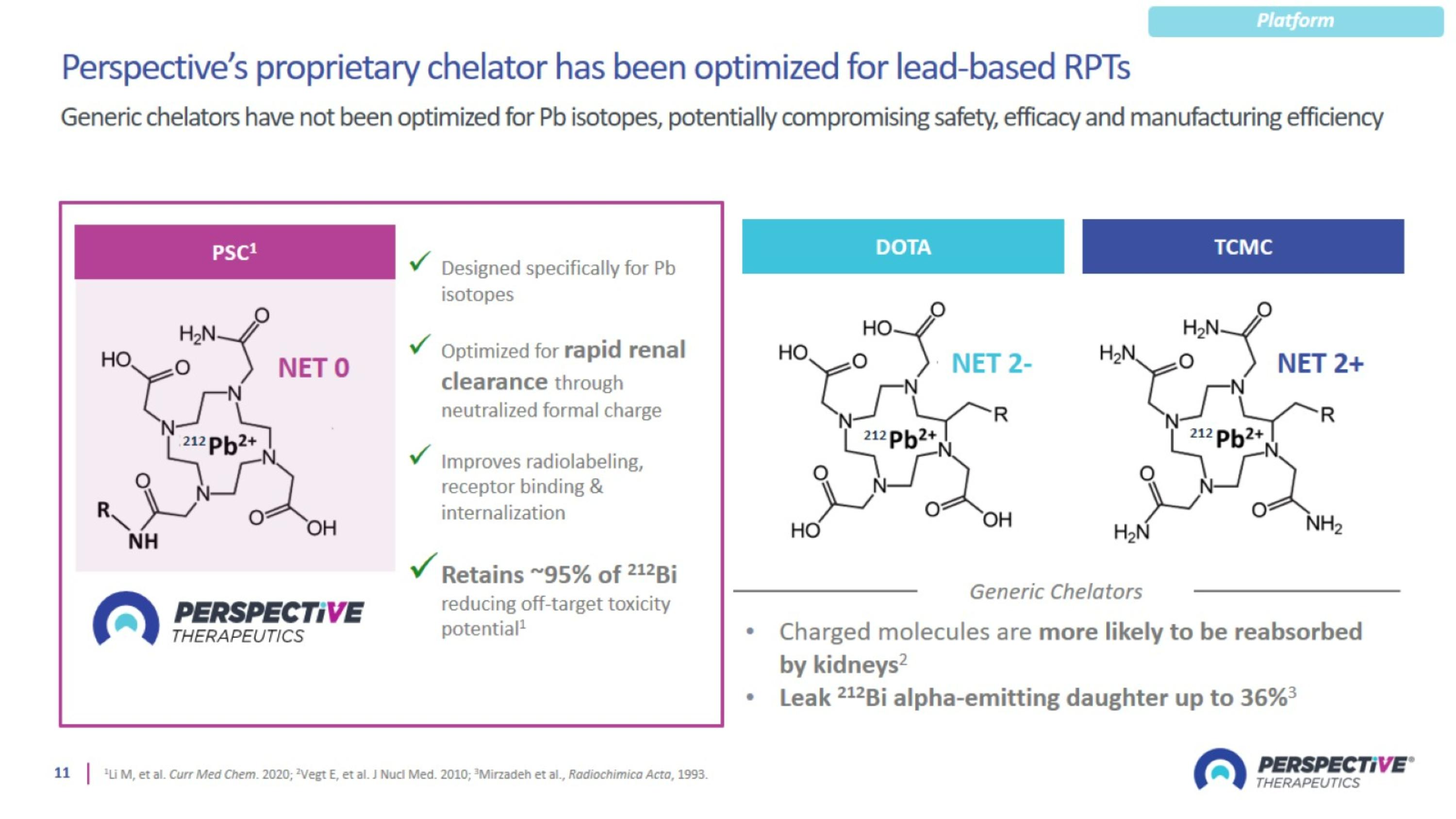
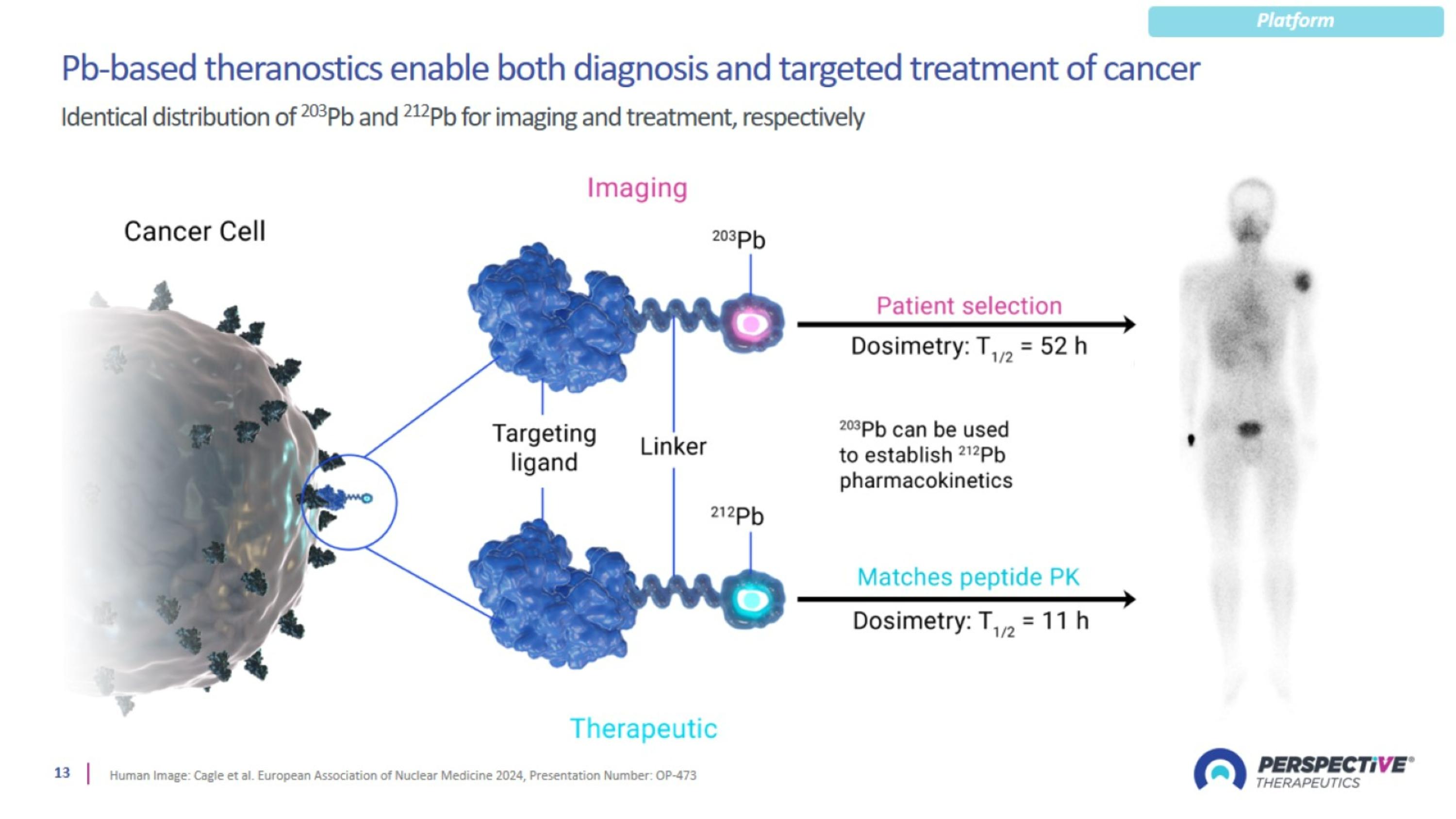
Perspective’s proprietary chelator has been optimized for lead-based RPTs
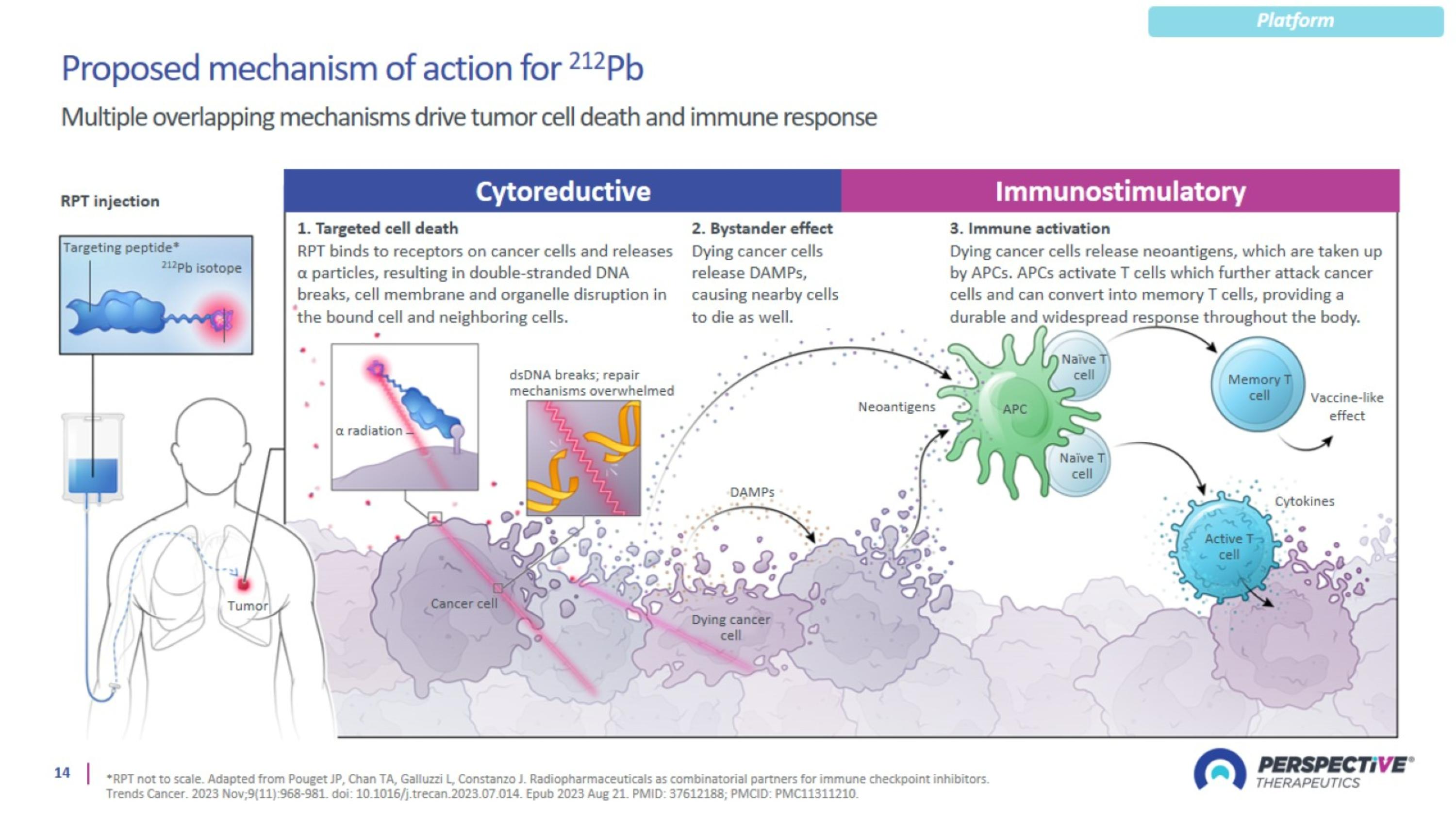
Proposed mechanism of action for 212Pb
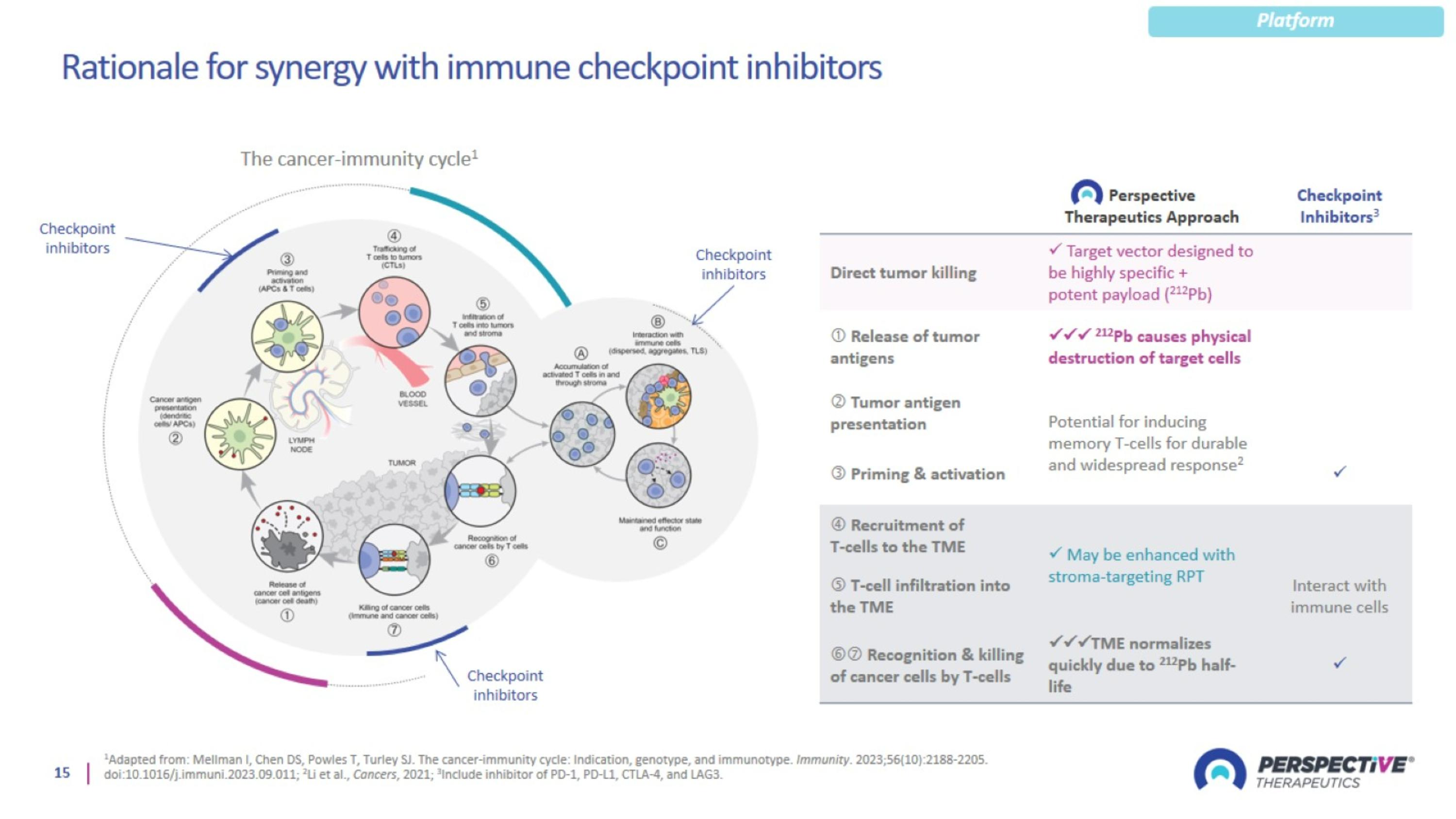
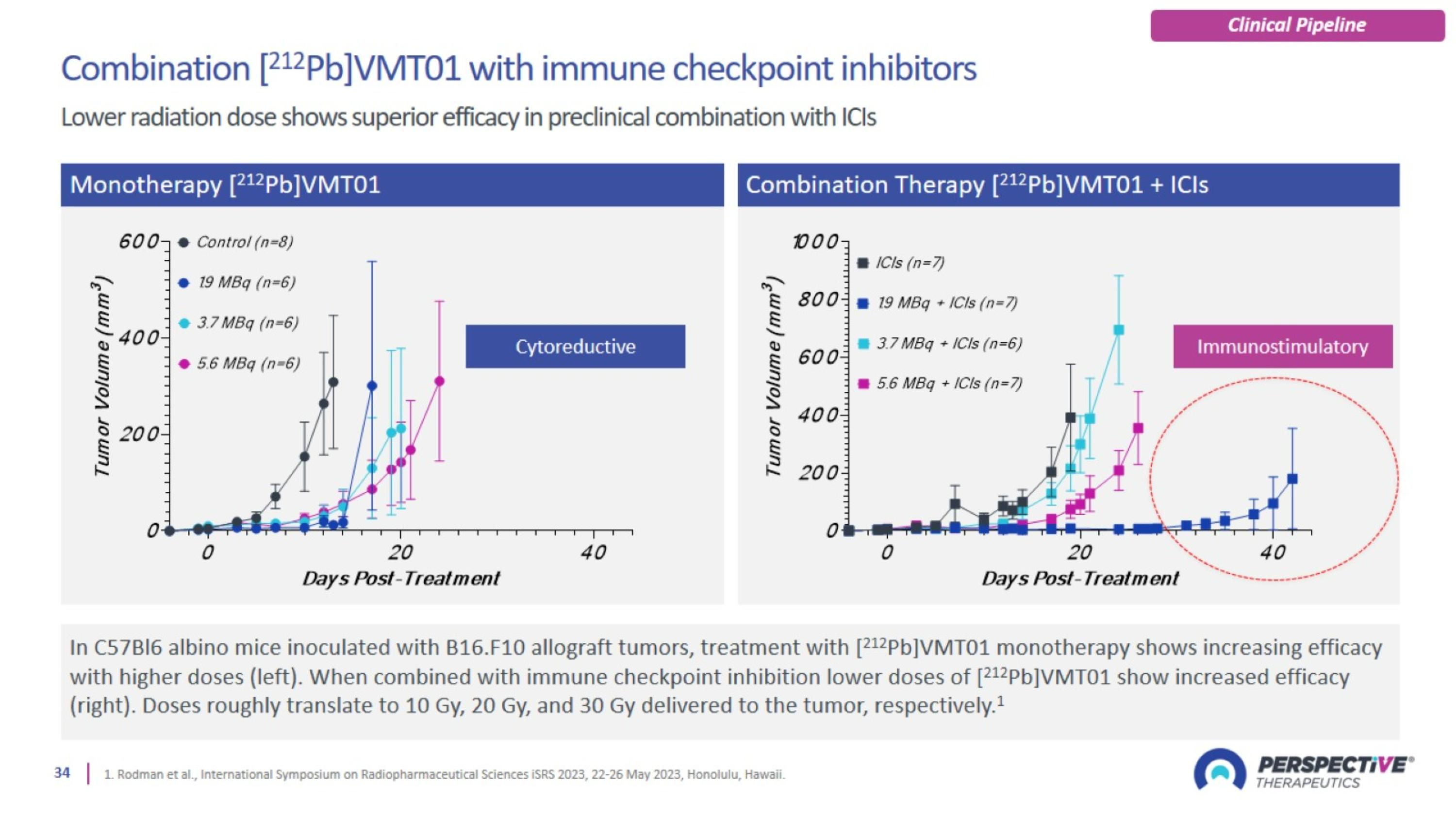
Rationale for synergy with immune checkpoint inhibitors

Supply Chain and Manufacturing Infrastructure
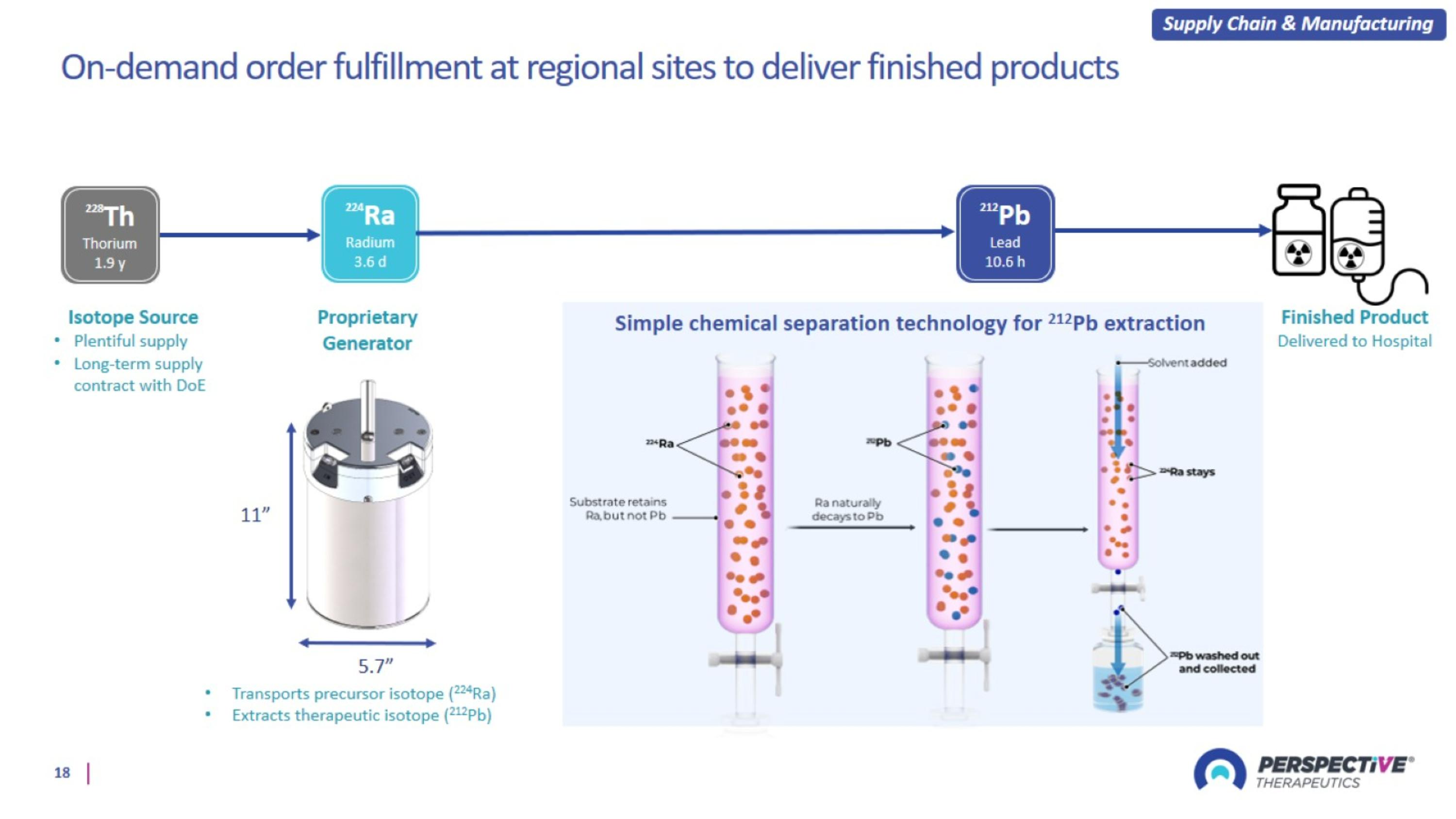
On-demand order fulfillment at regional sites to deliver finished products

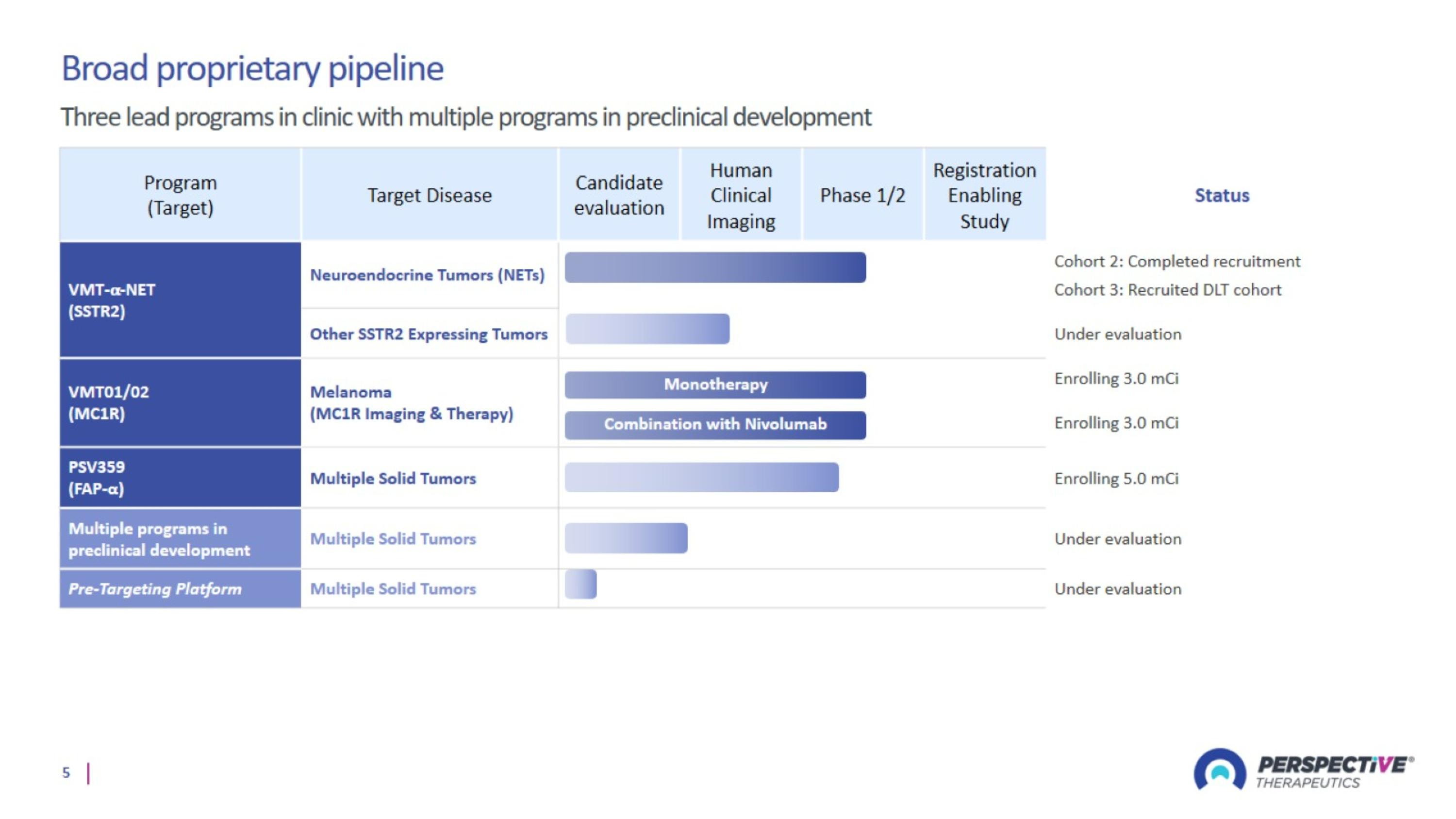
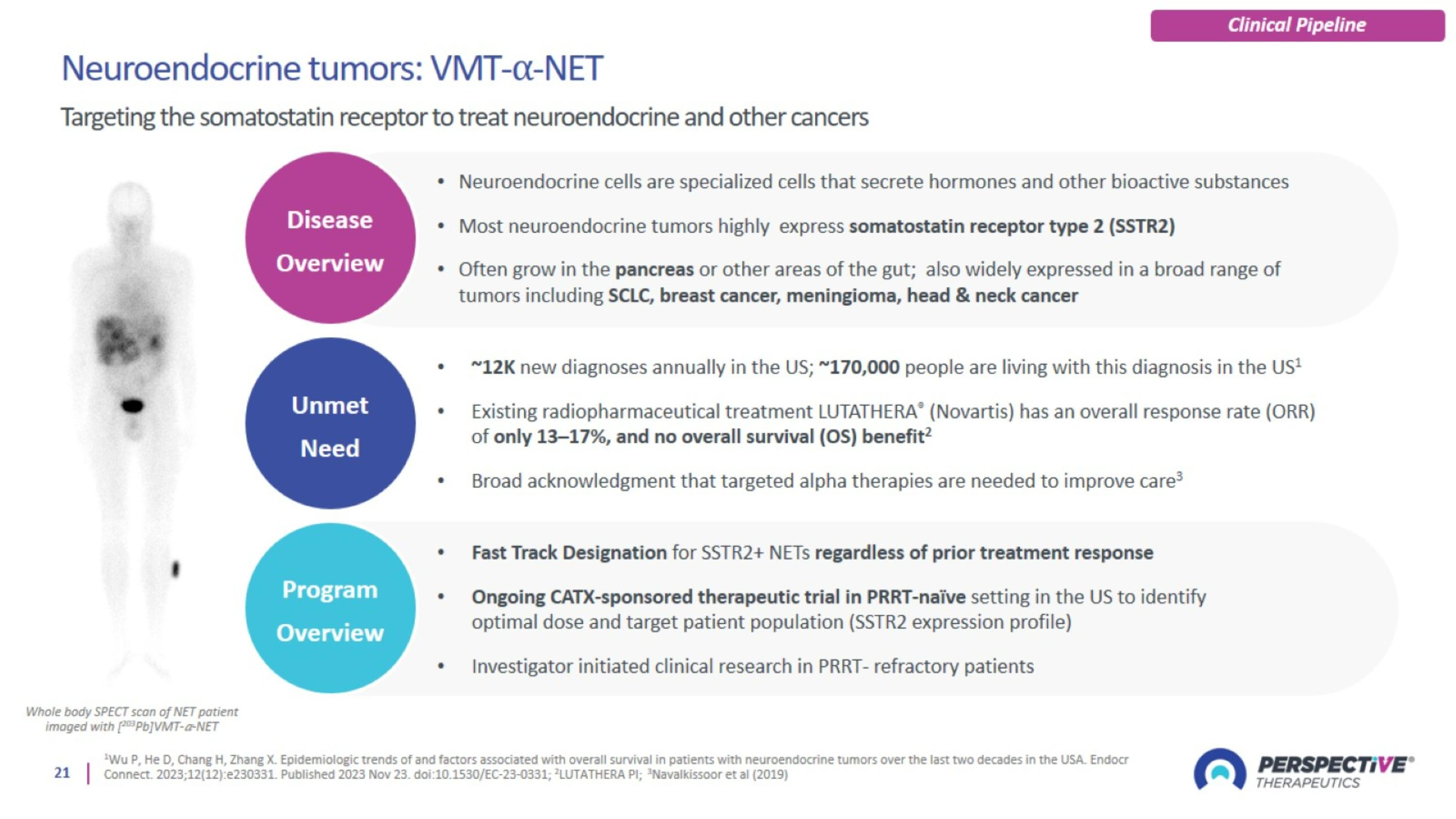
Neuroendocrine Tumors: VMT-⍺-NET
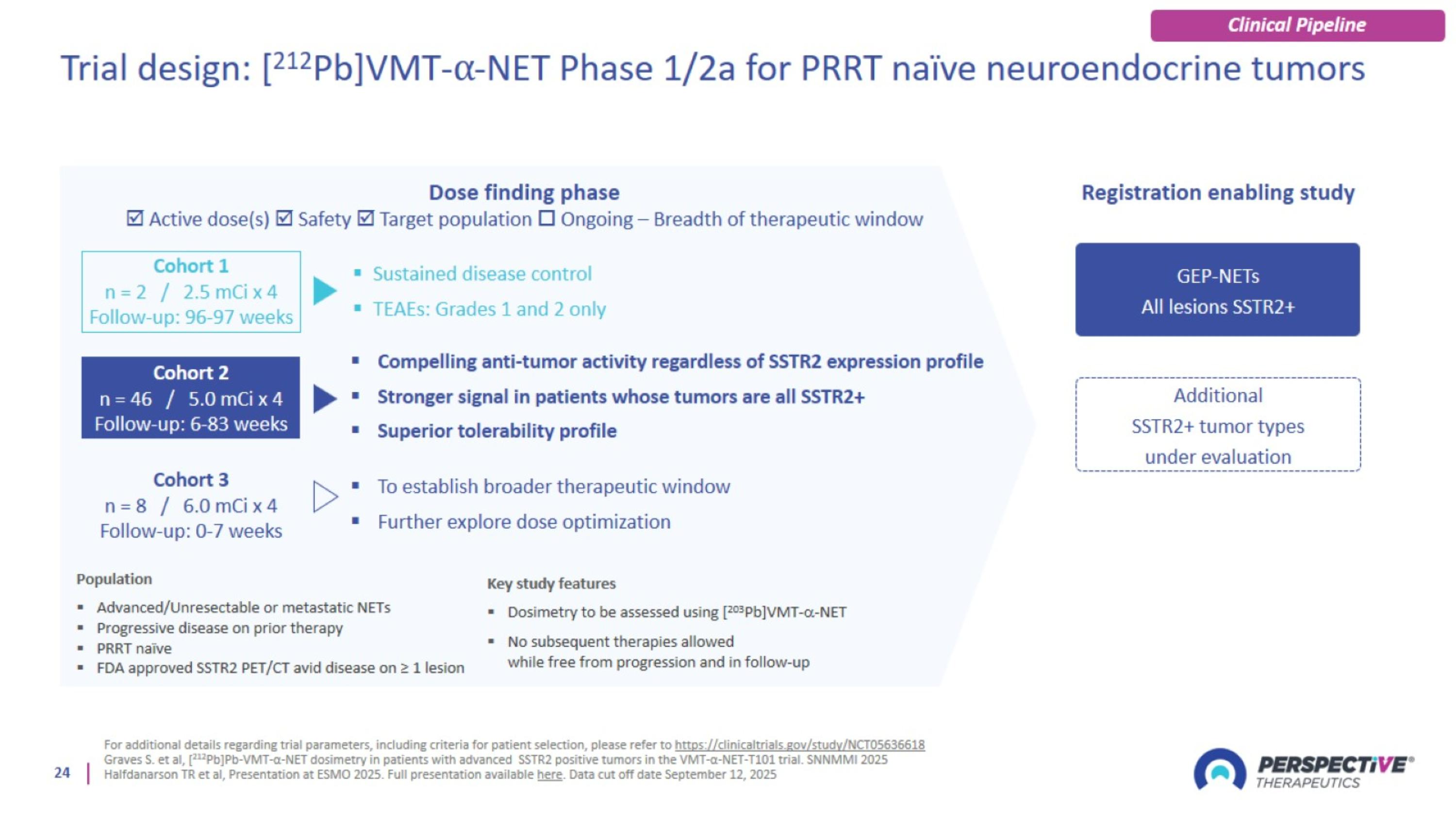
Trial design: [212Pb]VMT-⍺-NET Phase 1/2a for PRRT naïve neuroendocrine tumors
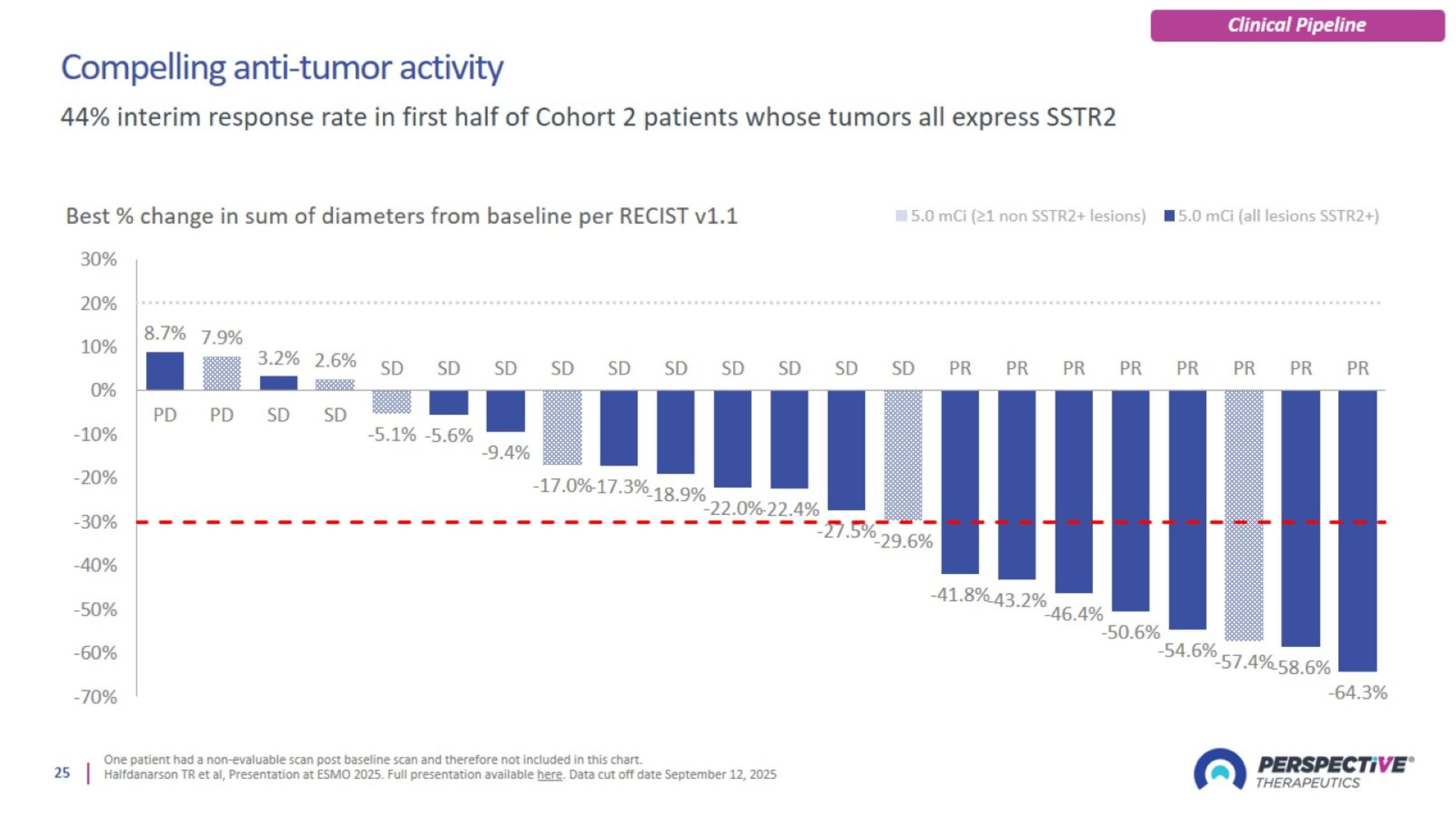
Compelling anti-tumor activity
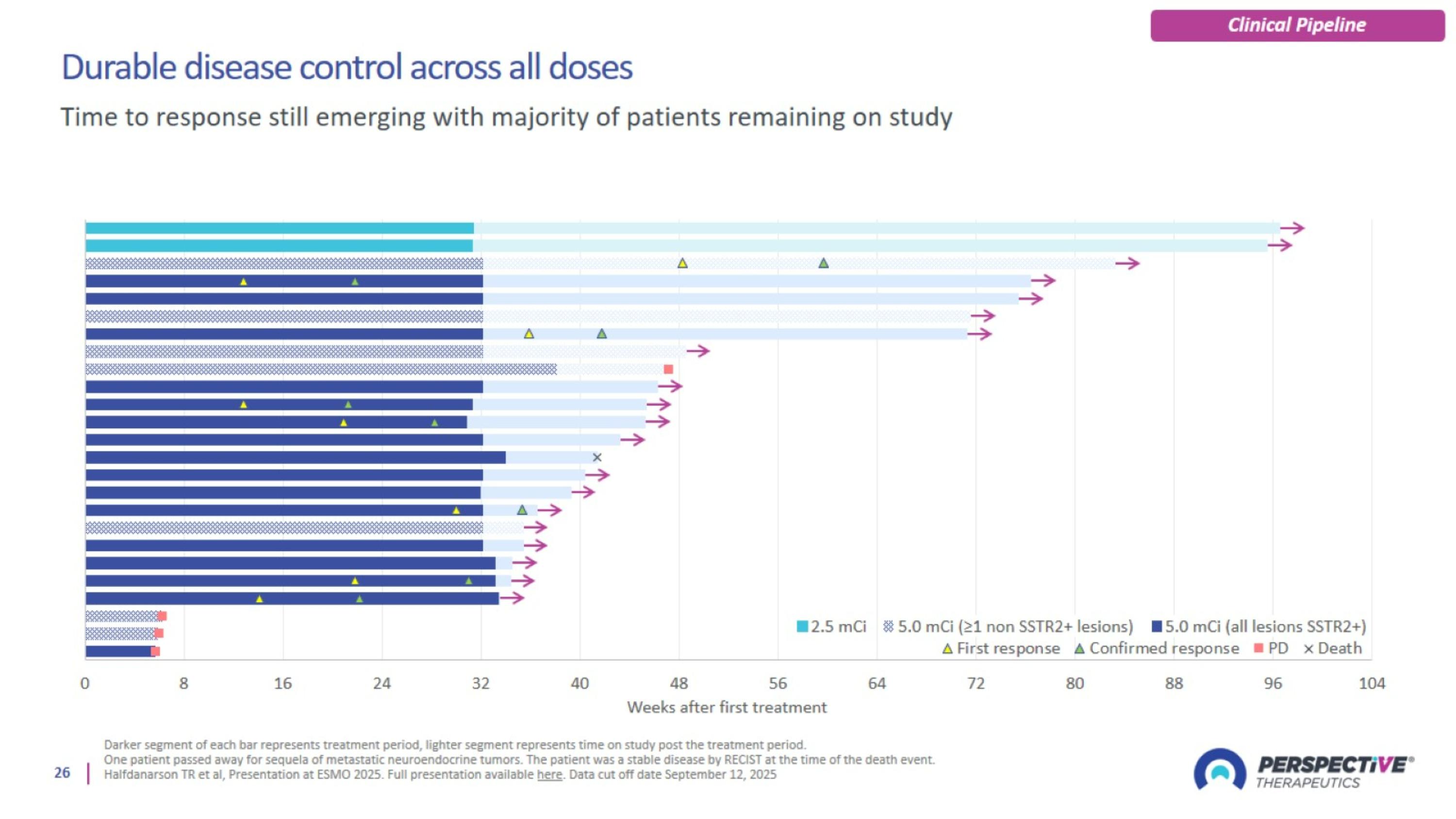
Durable disease control across all doses
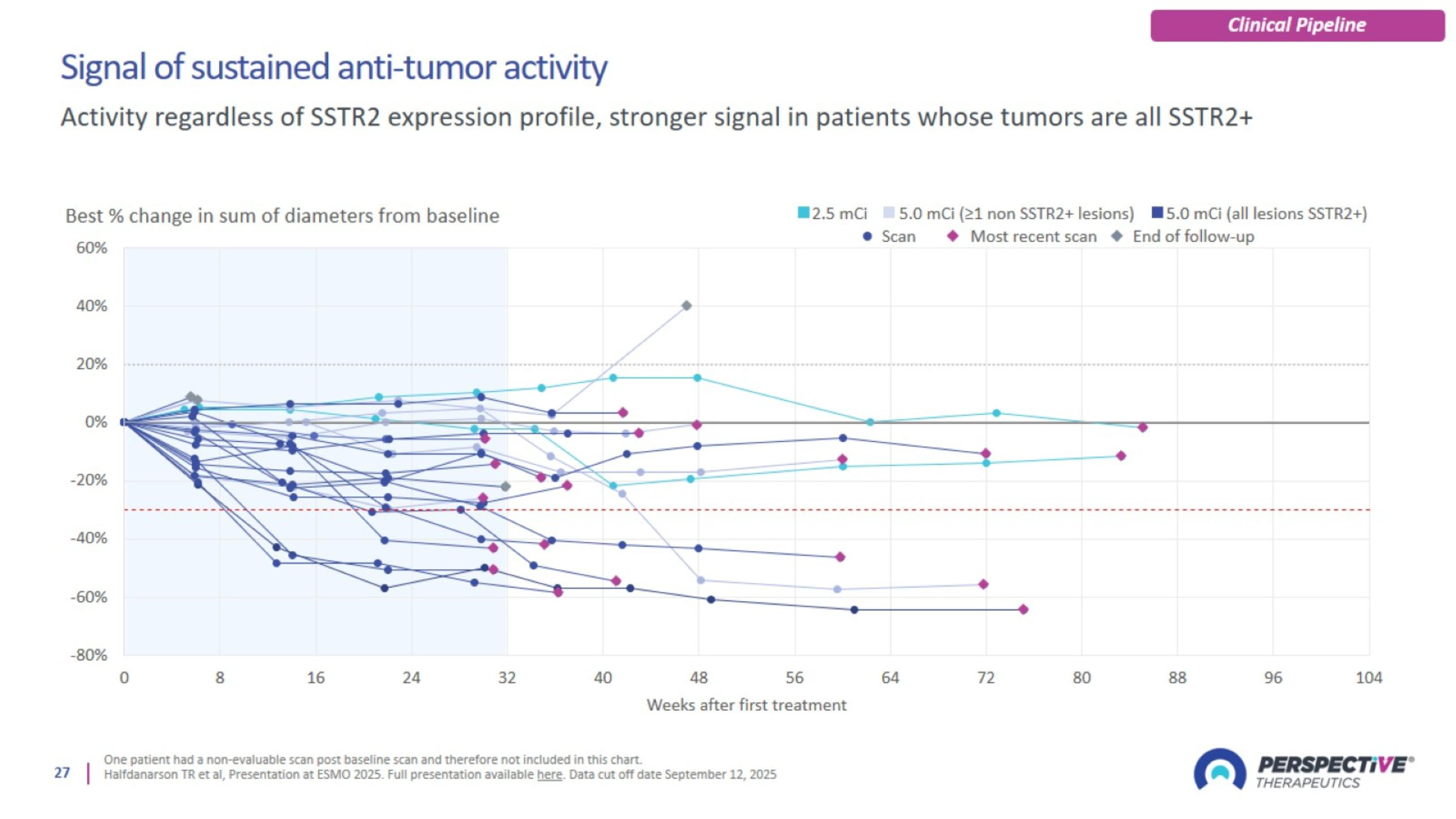
Signal of sustained anti-tumor activity
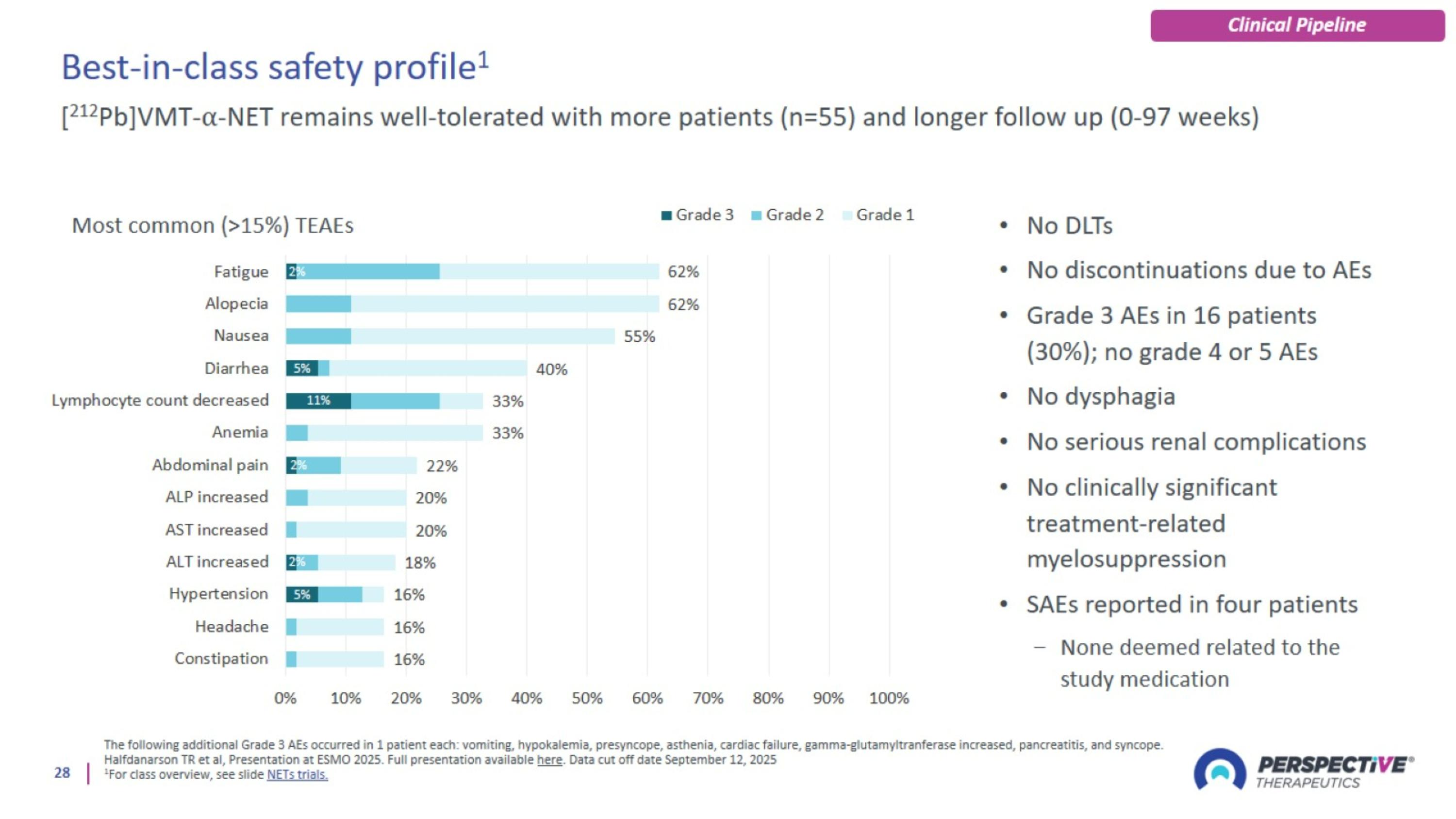
Best-in-class safety profile1
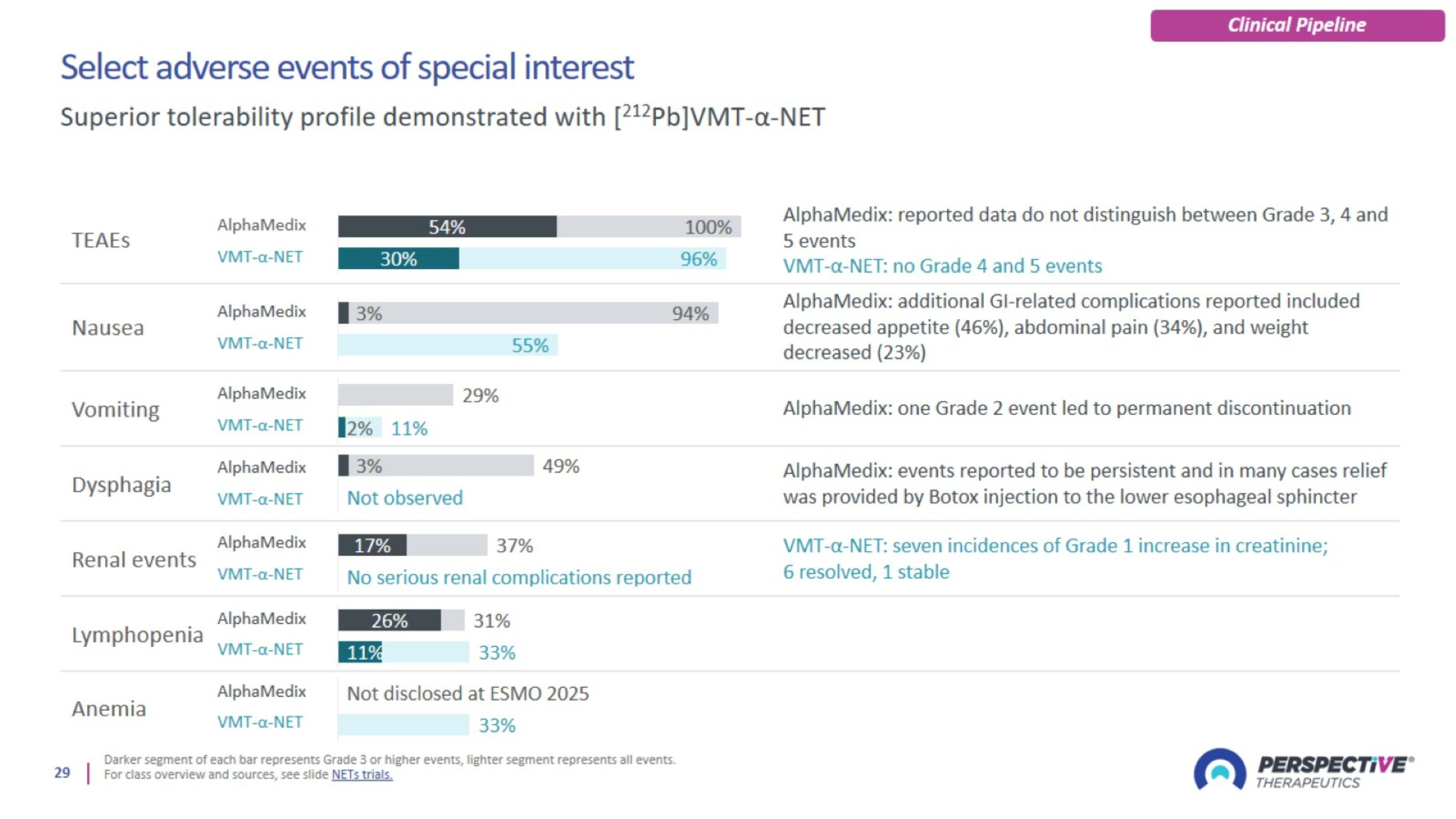
Select adverse events of special interest
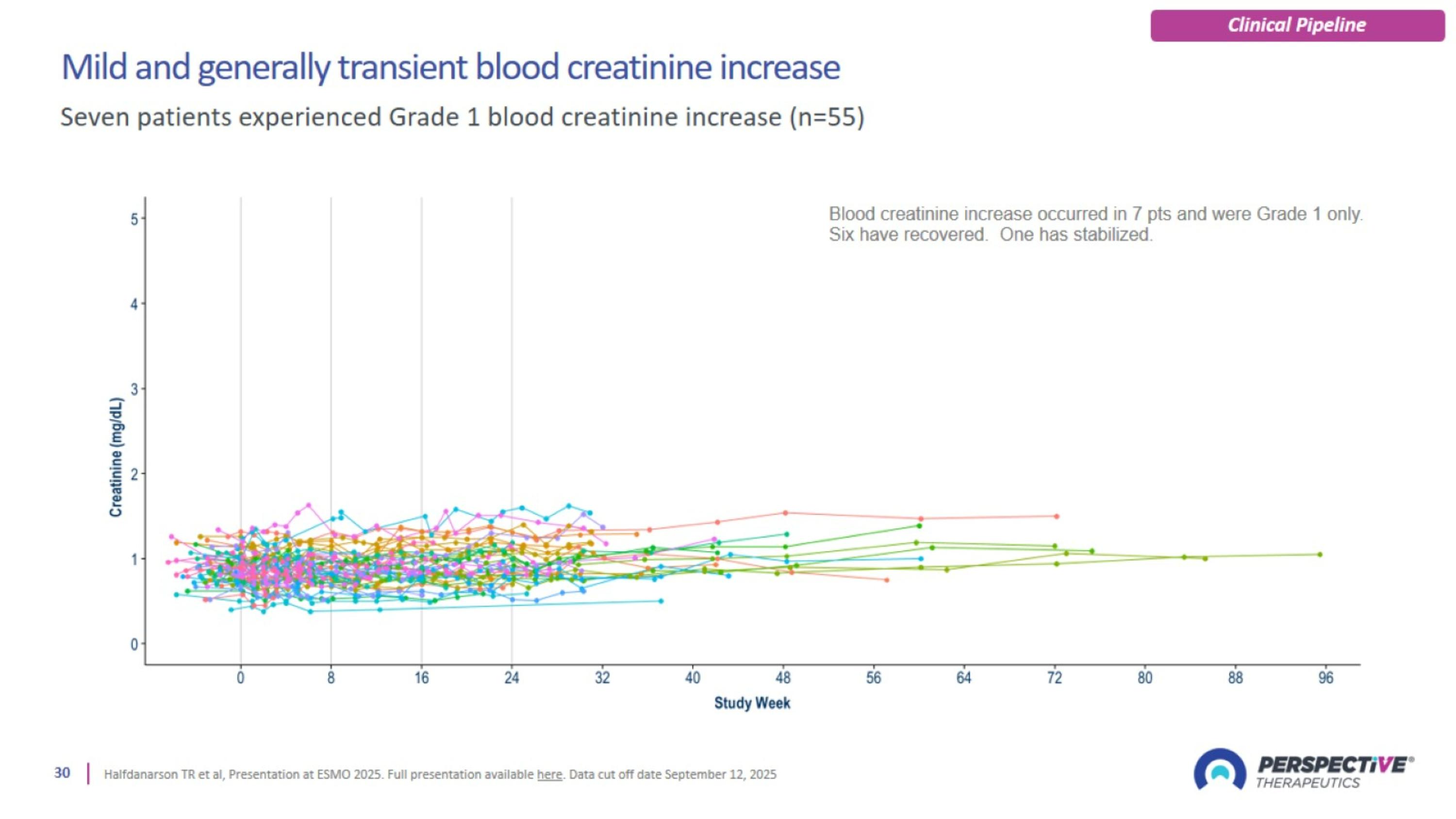
Mild and generally transient blood creatinine increase
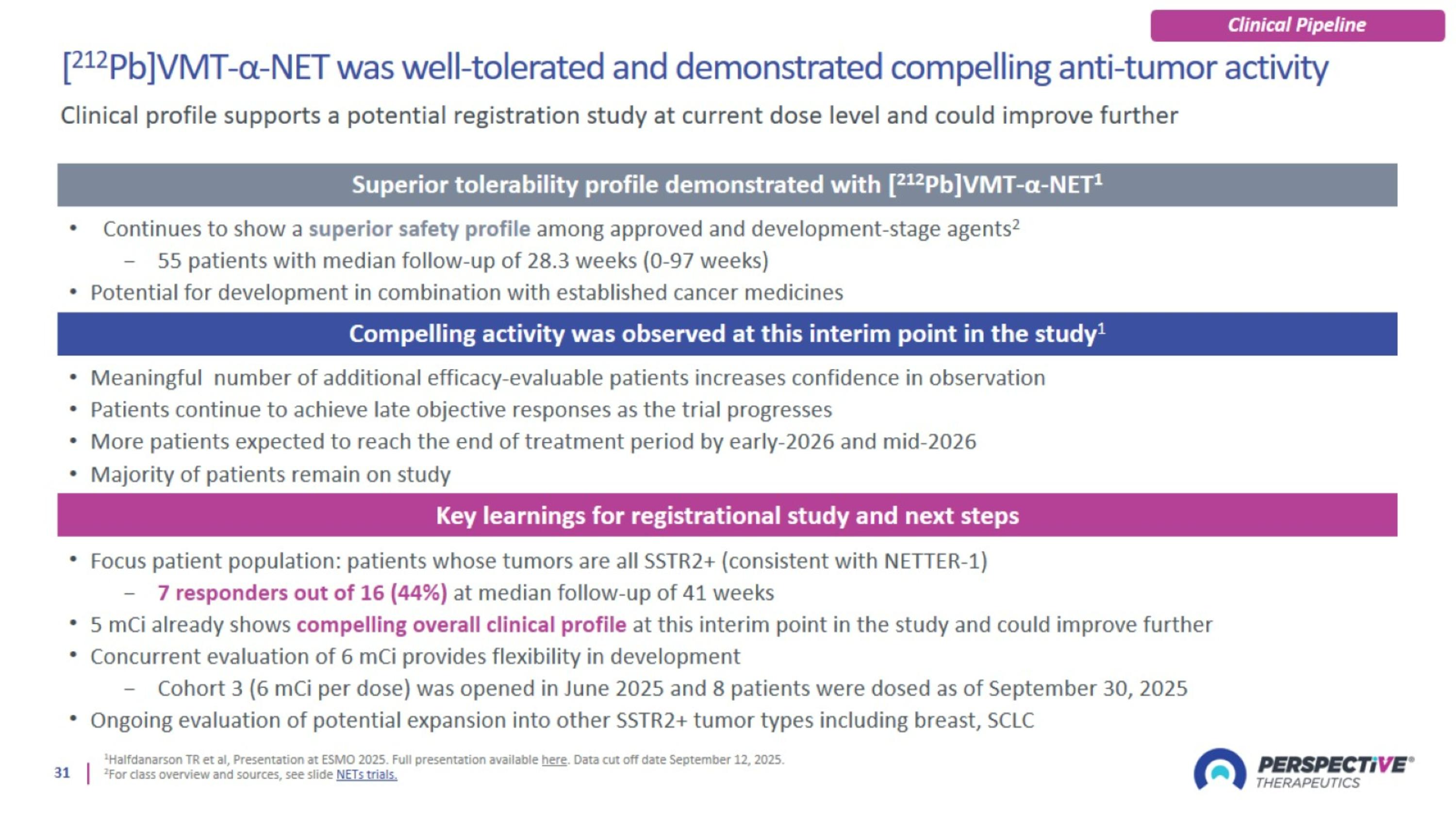
[212Pb]VMT-α-NET was well-tolerated and demonstrated compelling anti-tumor activity

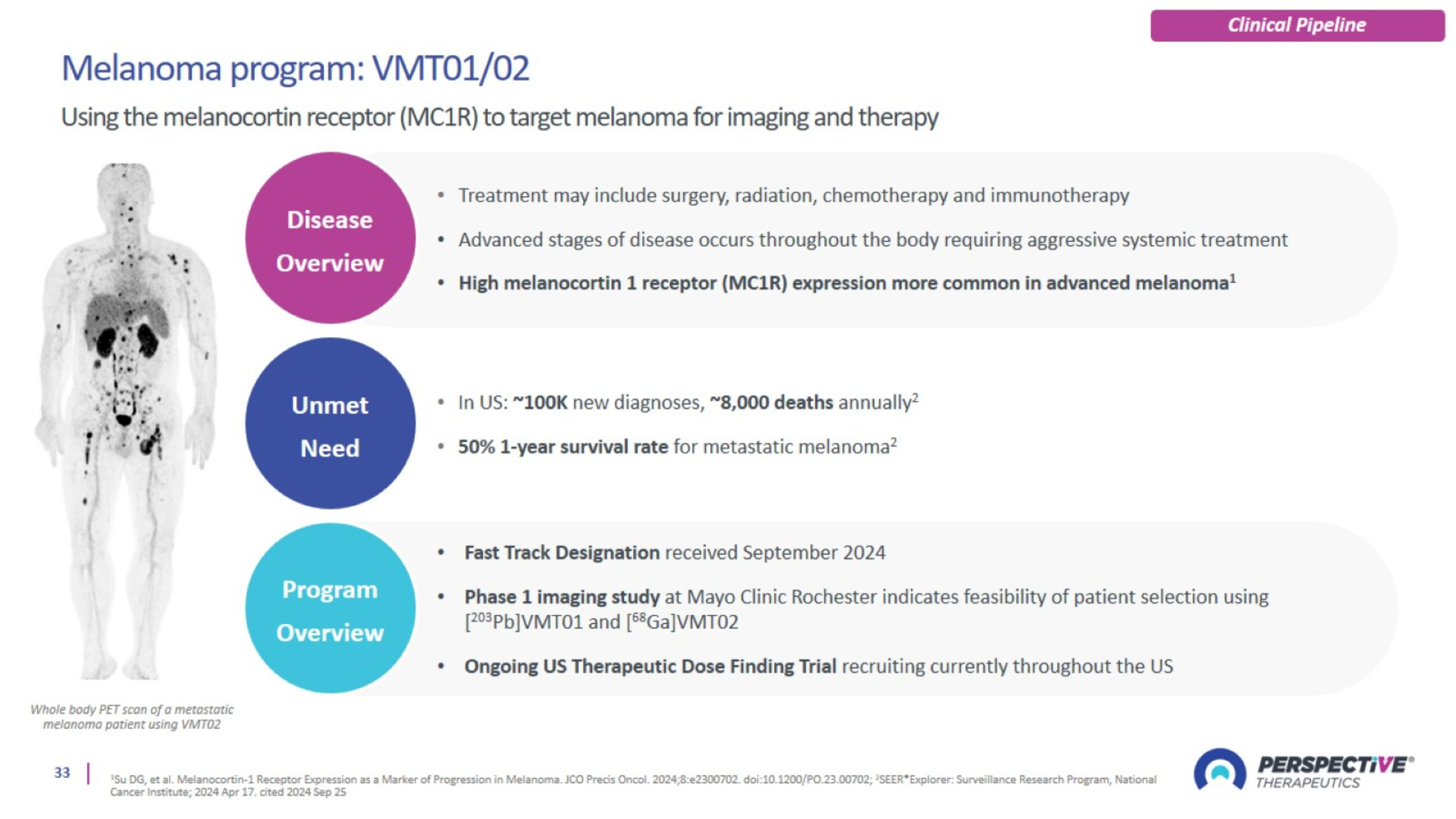
Melanoma Program: VMT01/02
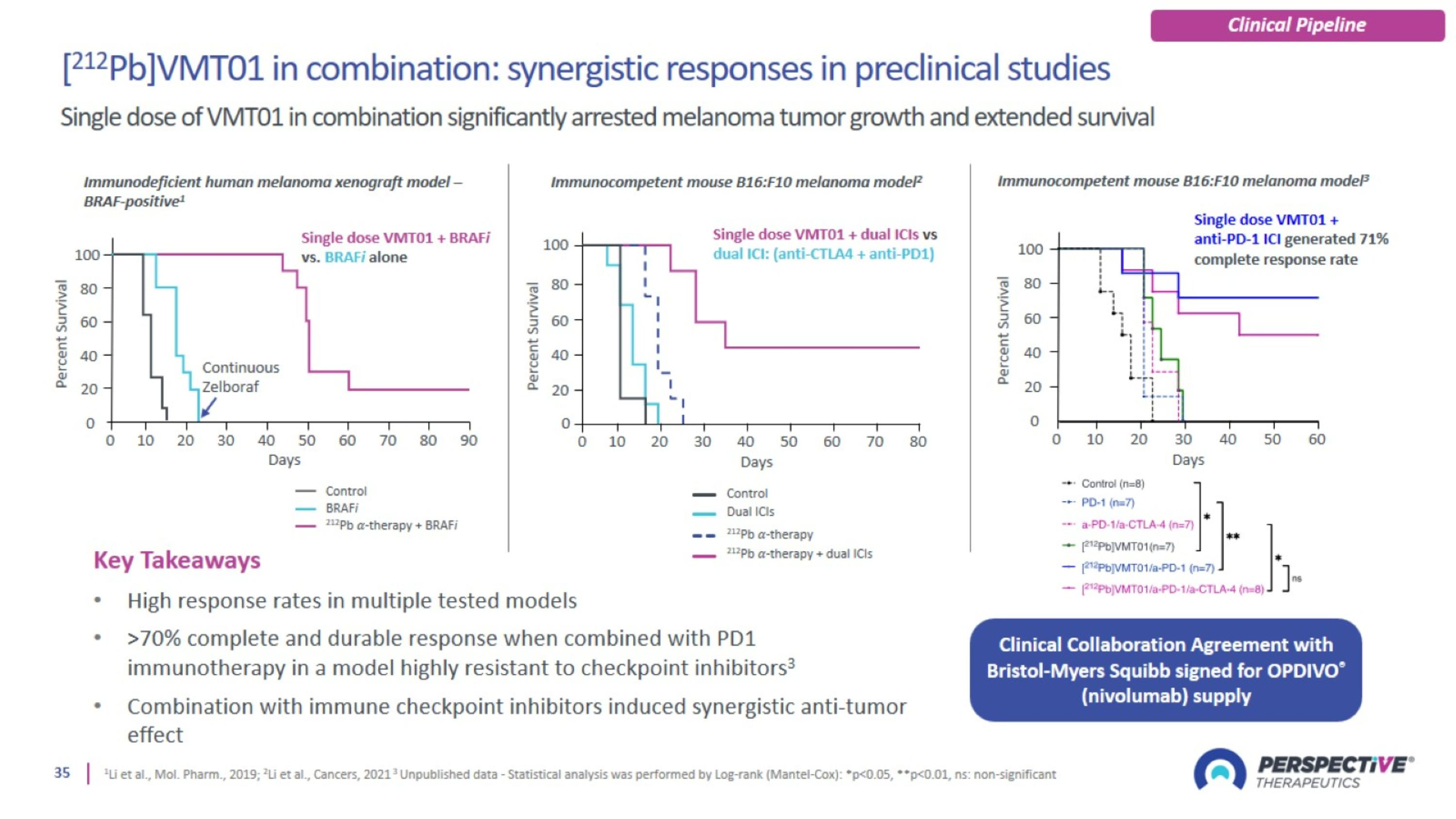
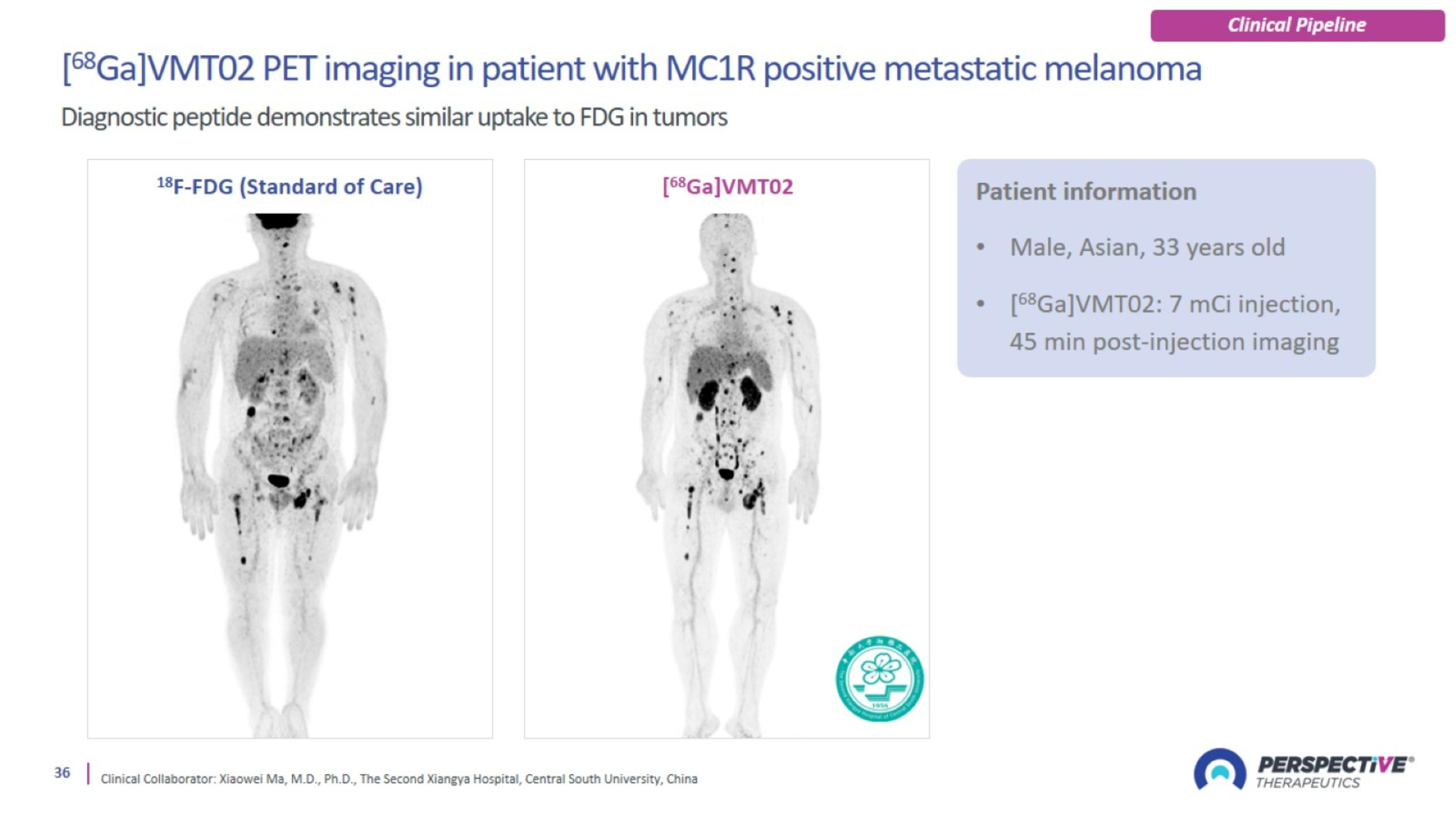
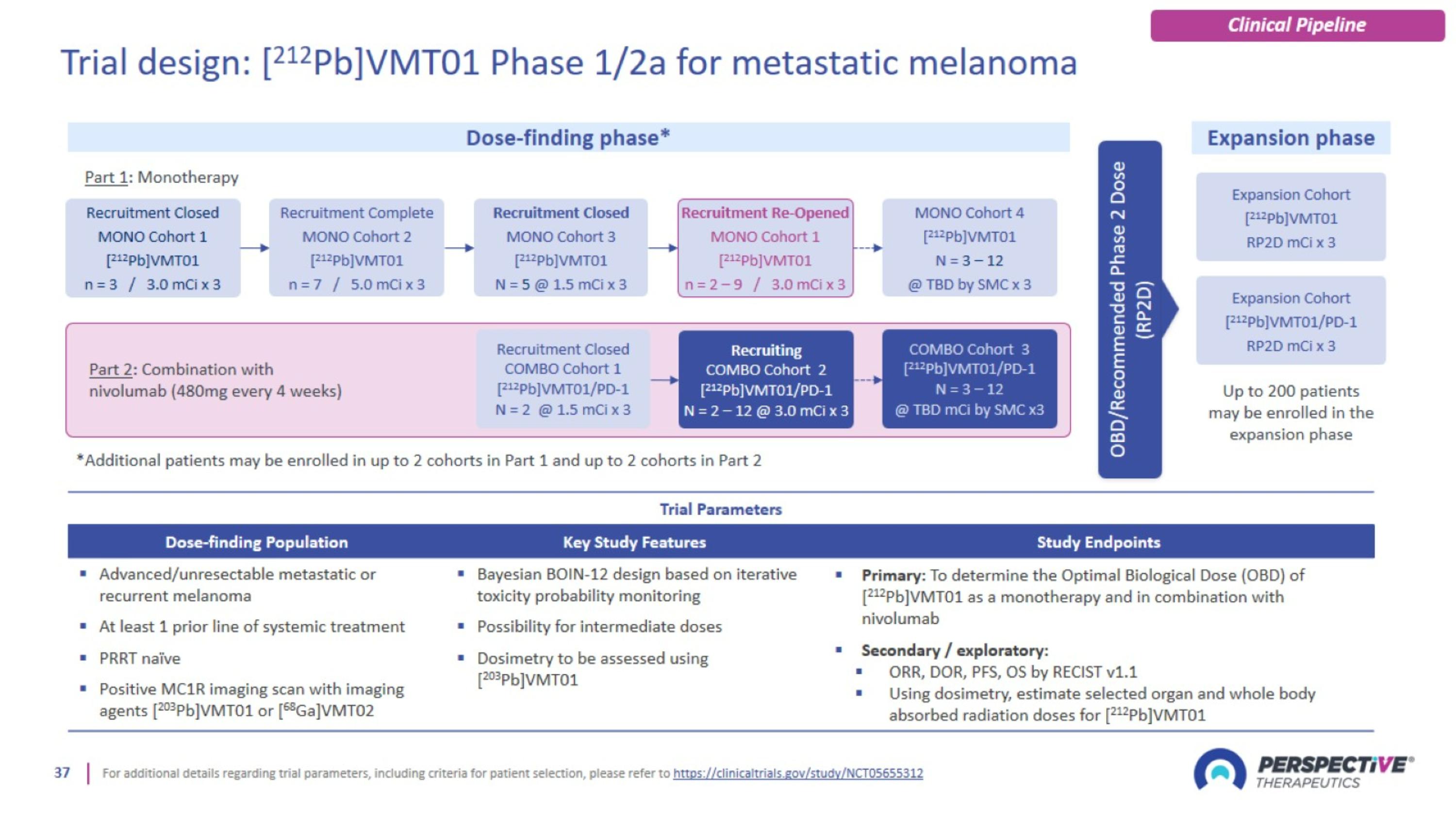
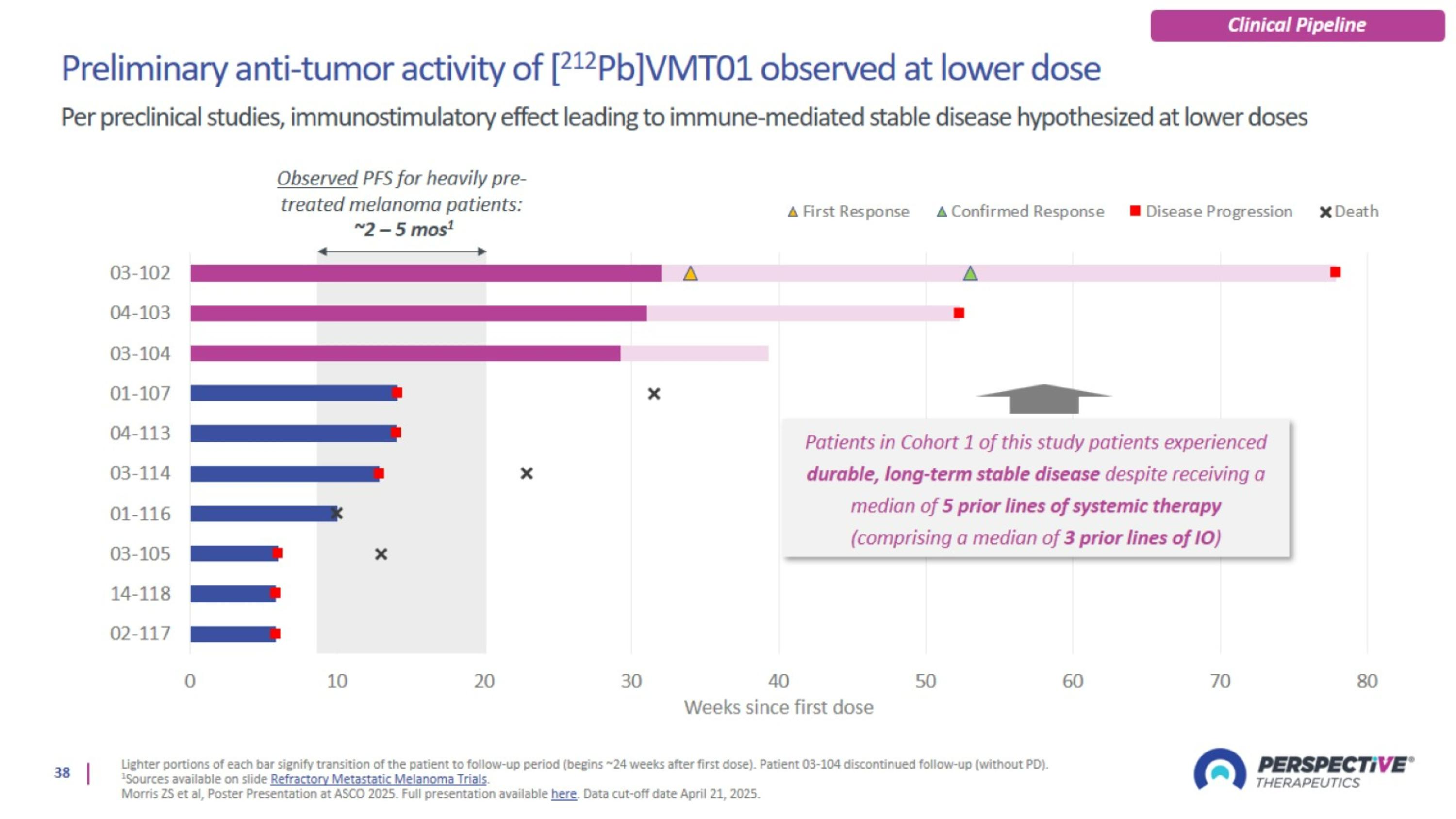
Trial design: [212Pb]VMT01 Phase 1/2a for metastatic melanoma
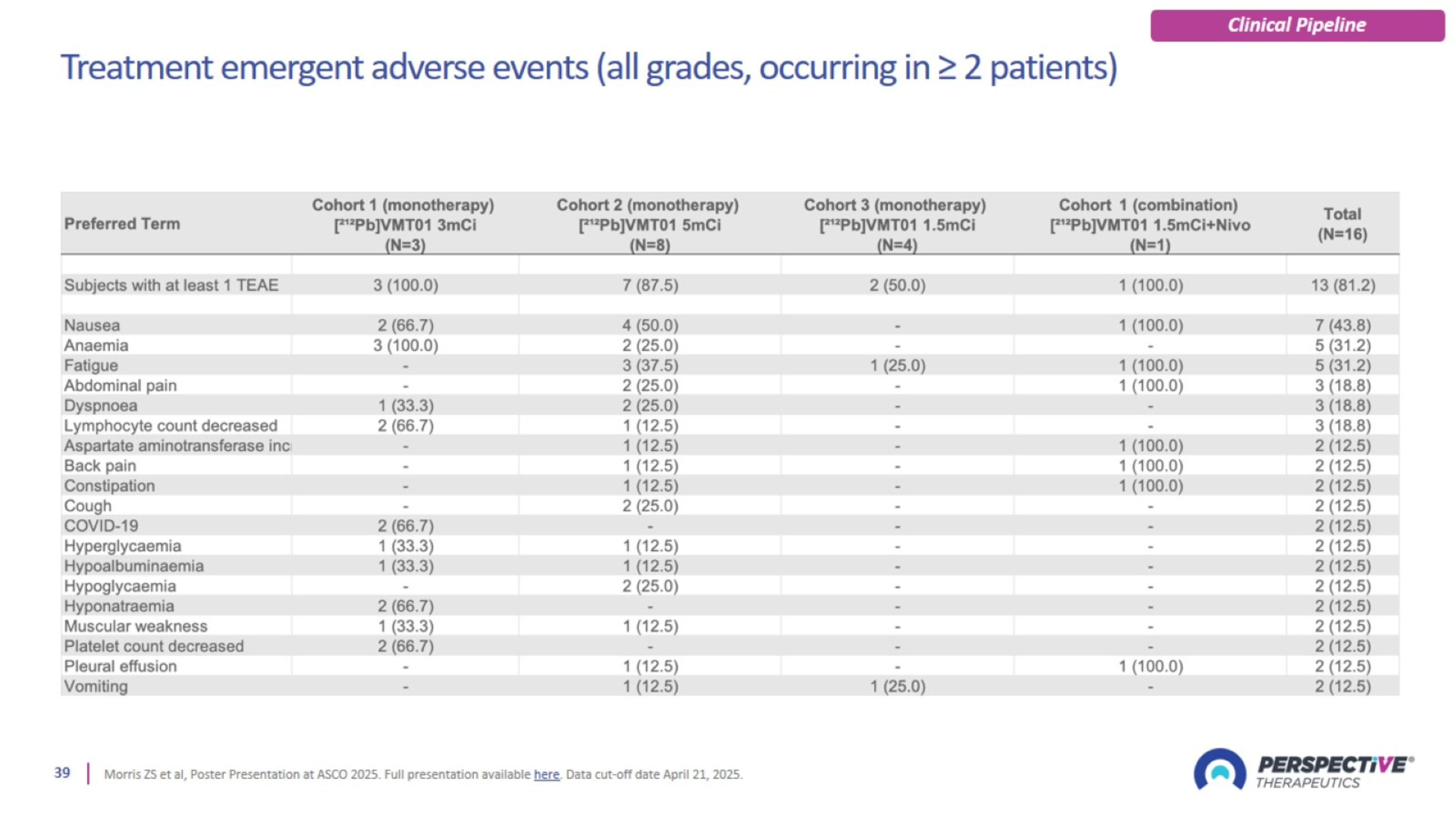
Treatment emergent adverse events (all grades, occurring in ≥ 2 patients)
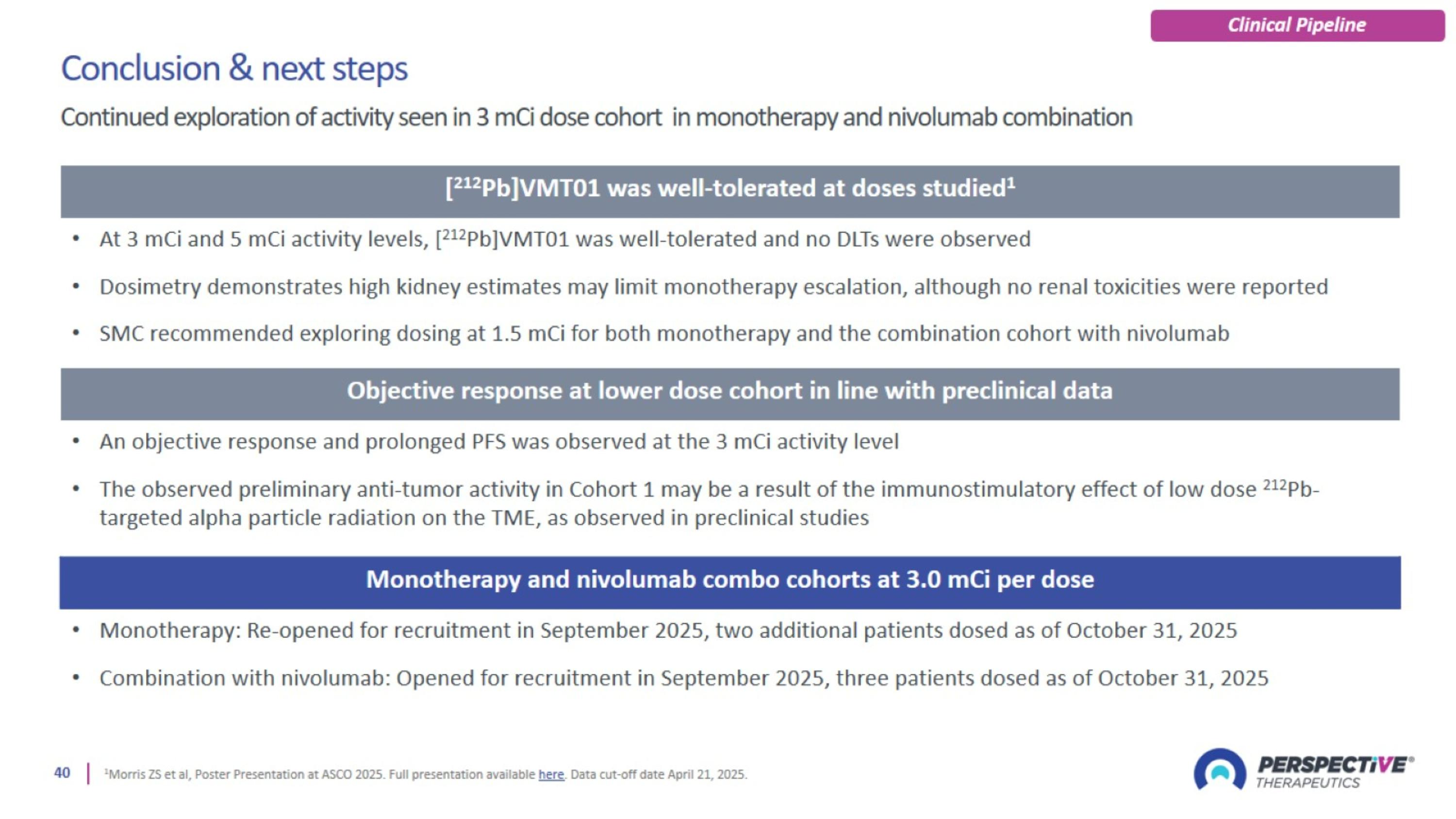

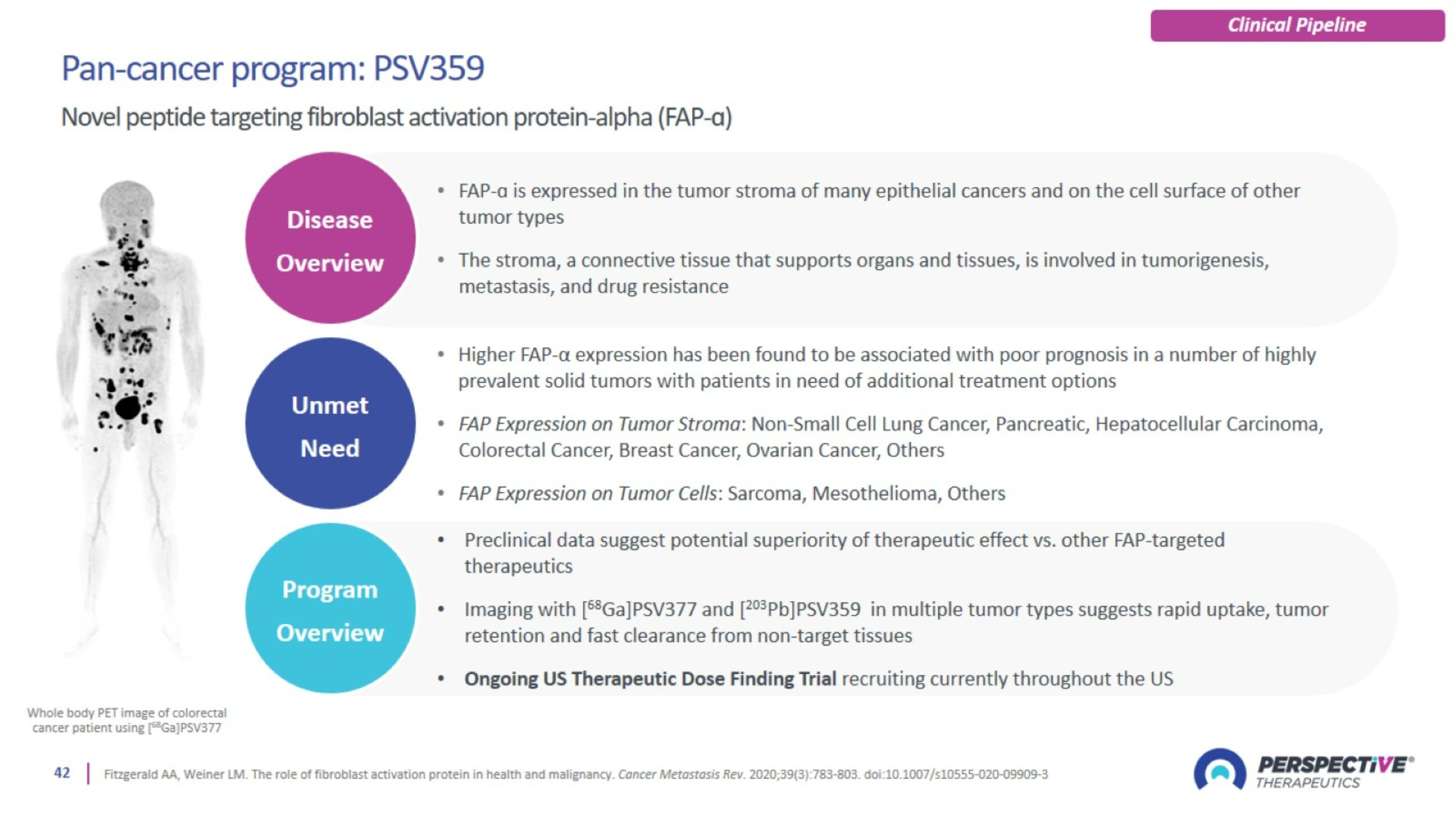
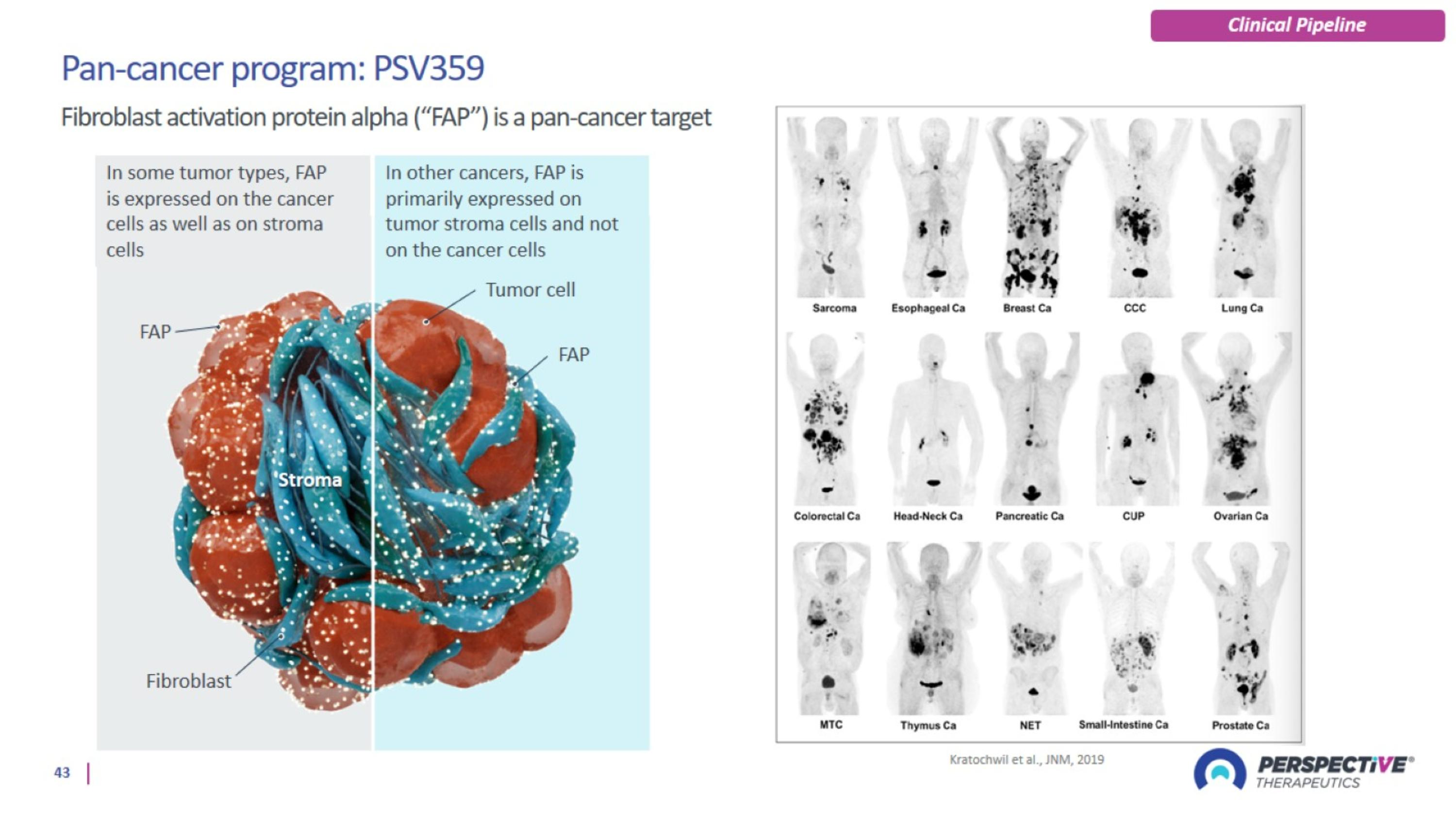
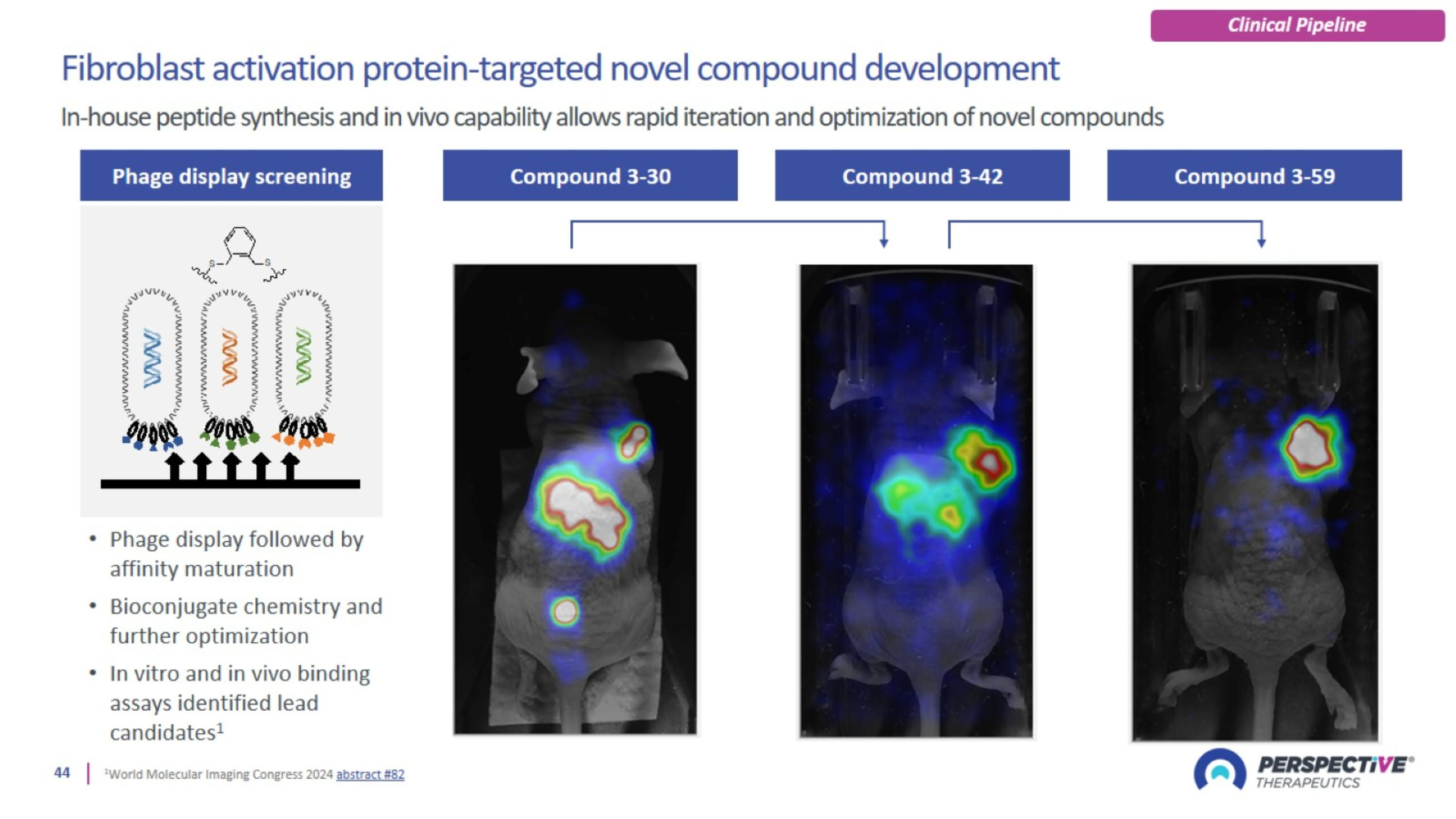
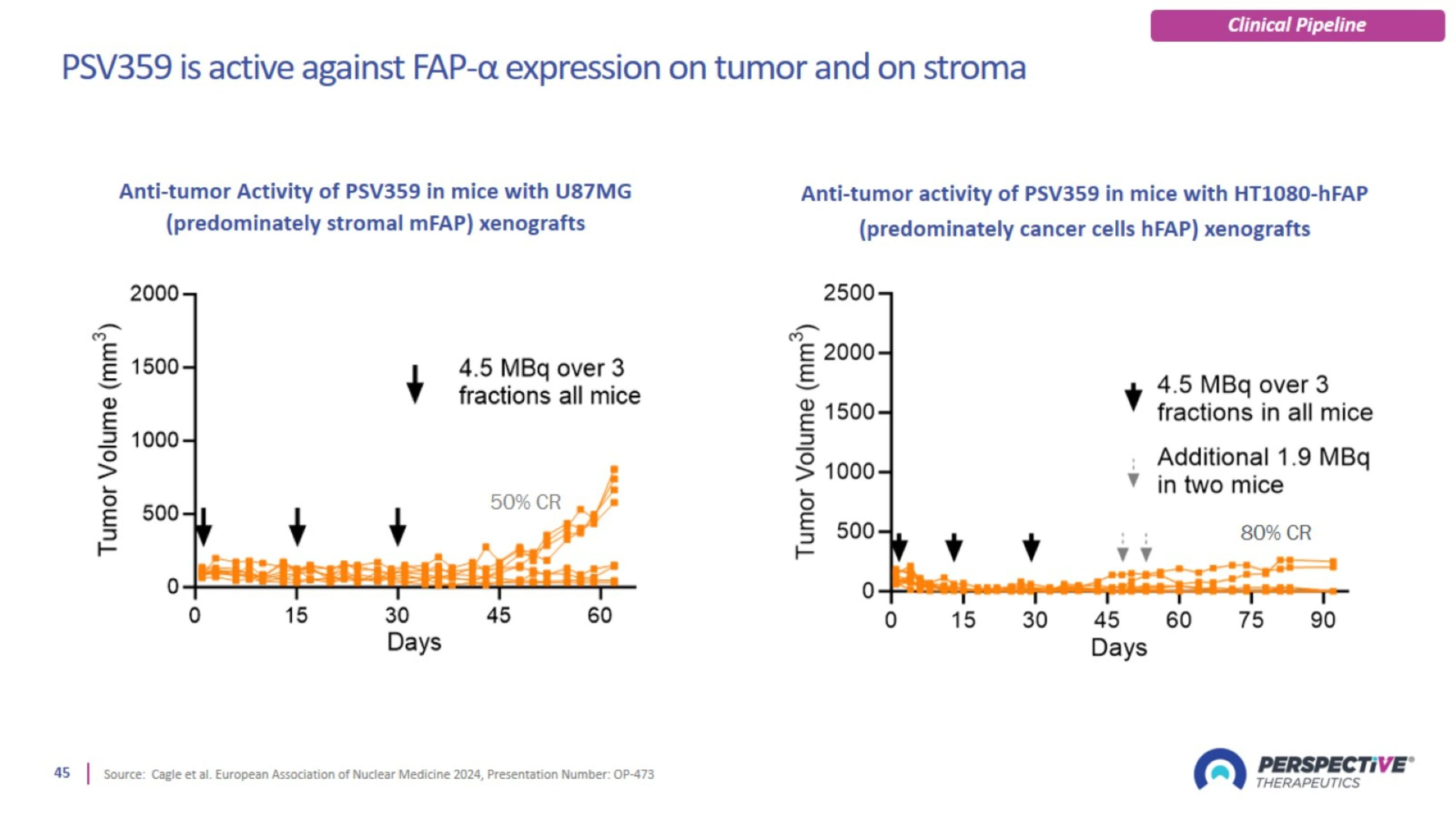
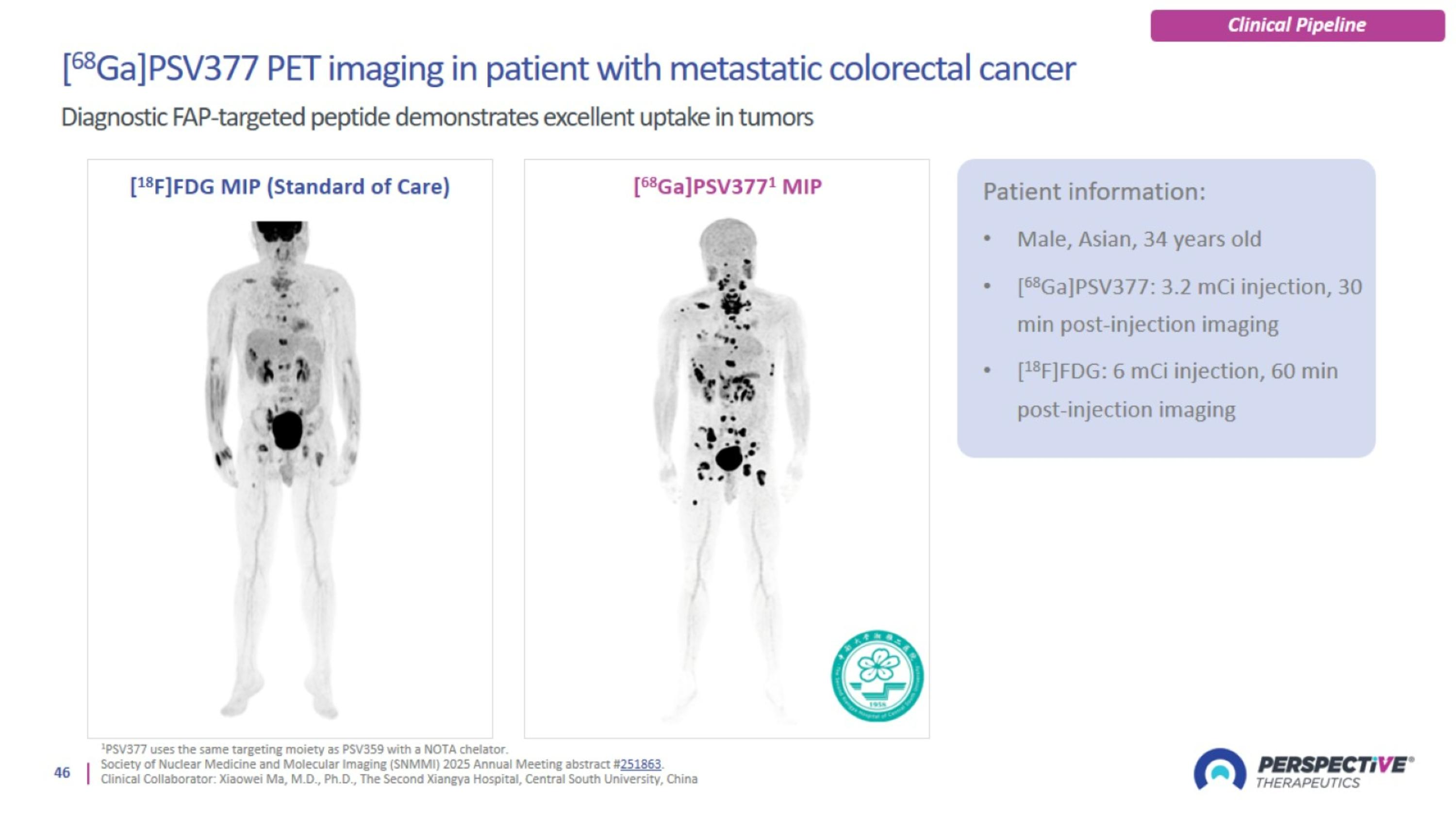
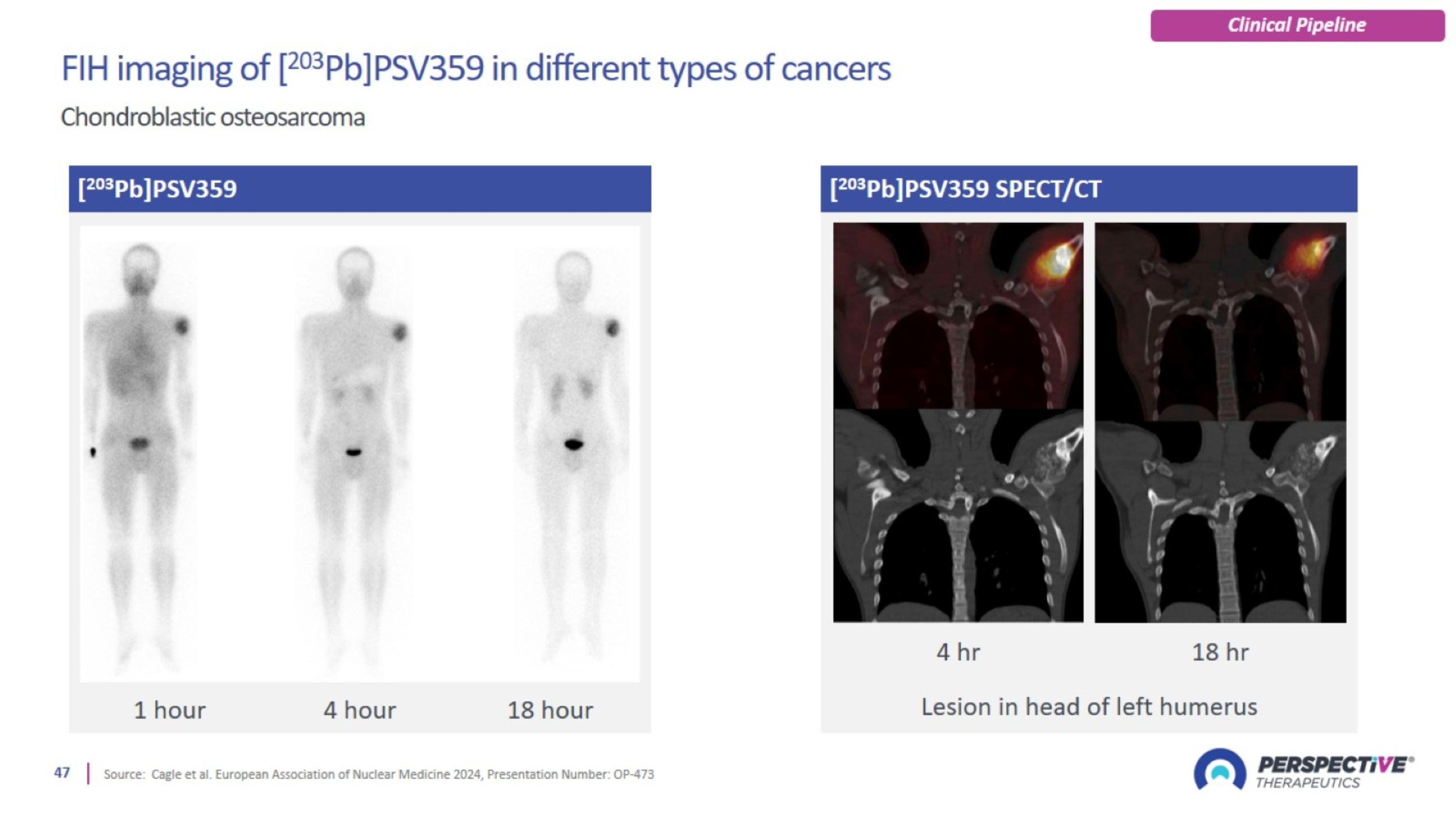
PSV359 is active against FAP-α expression on tumor and on stroma
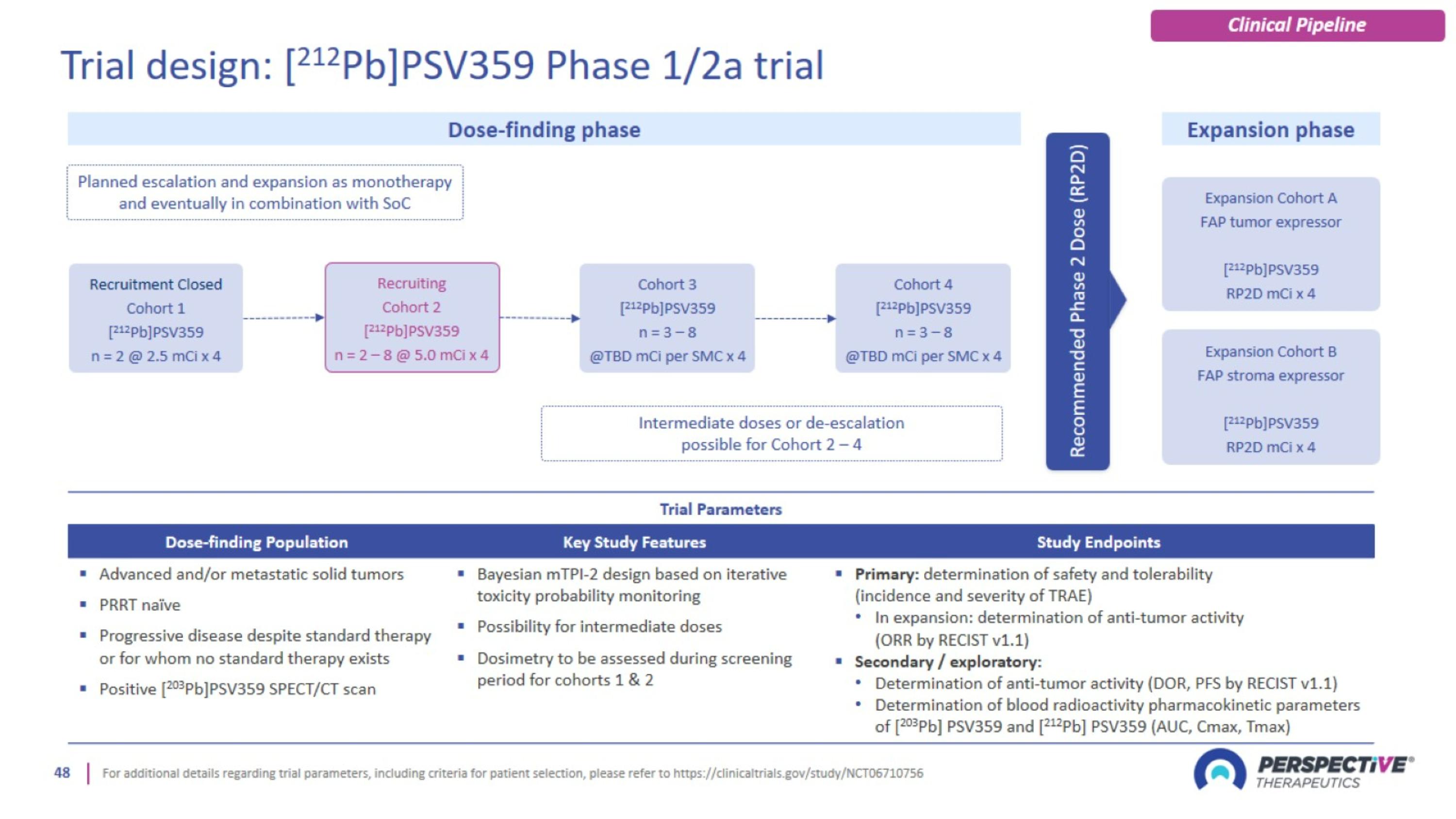
Trial design: [212Pb]PSV359 Phase 1/2a trial

General Corporate Information





Abbreviations
APPENDIX
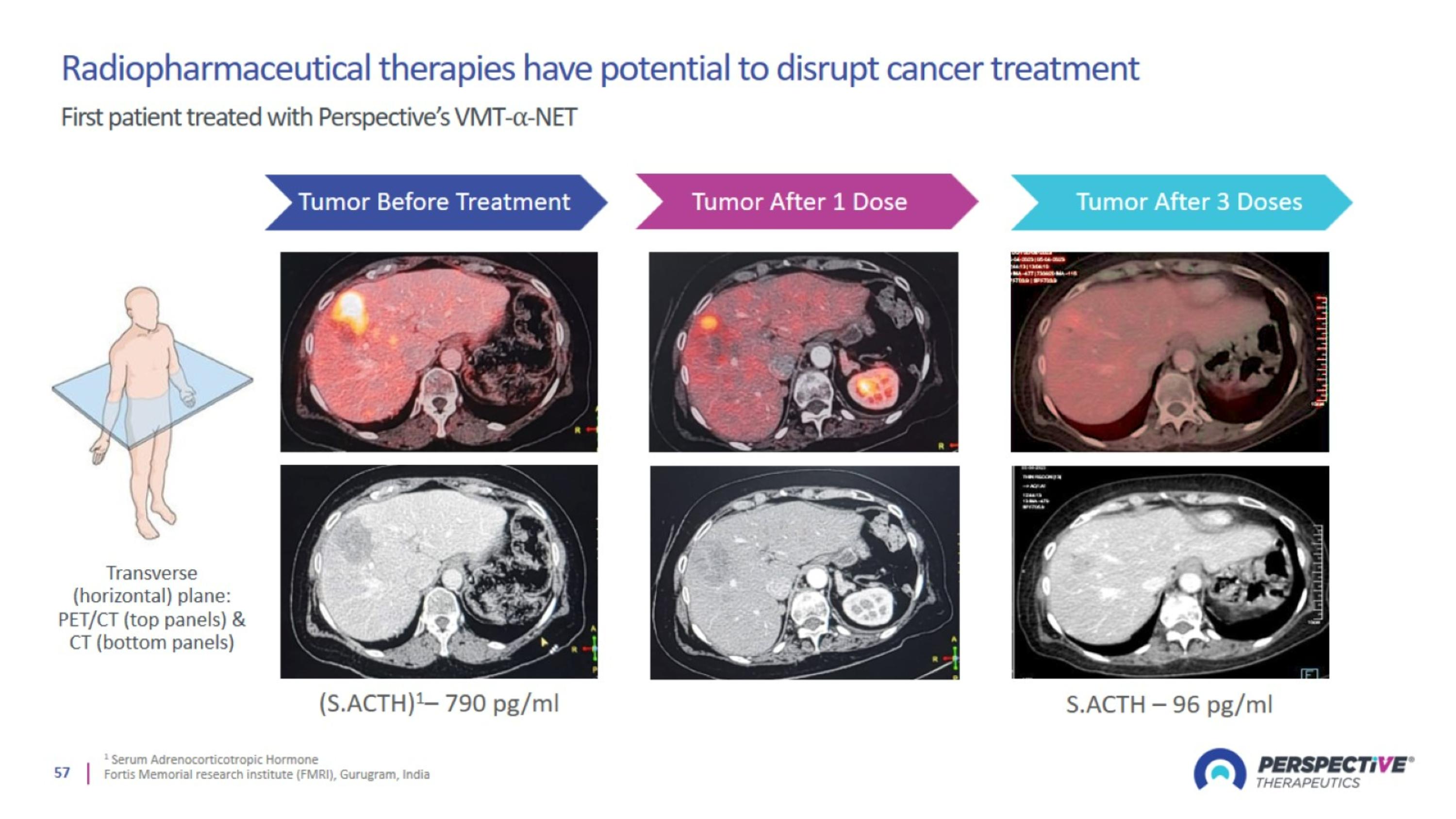
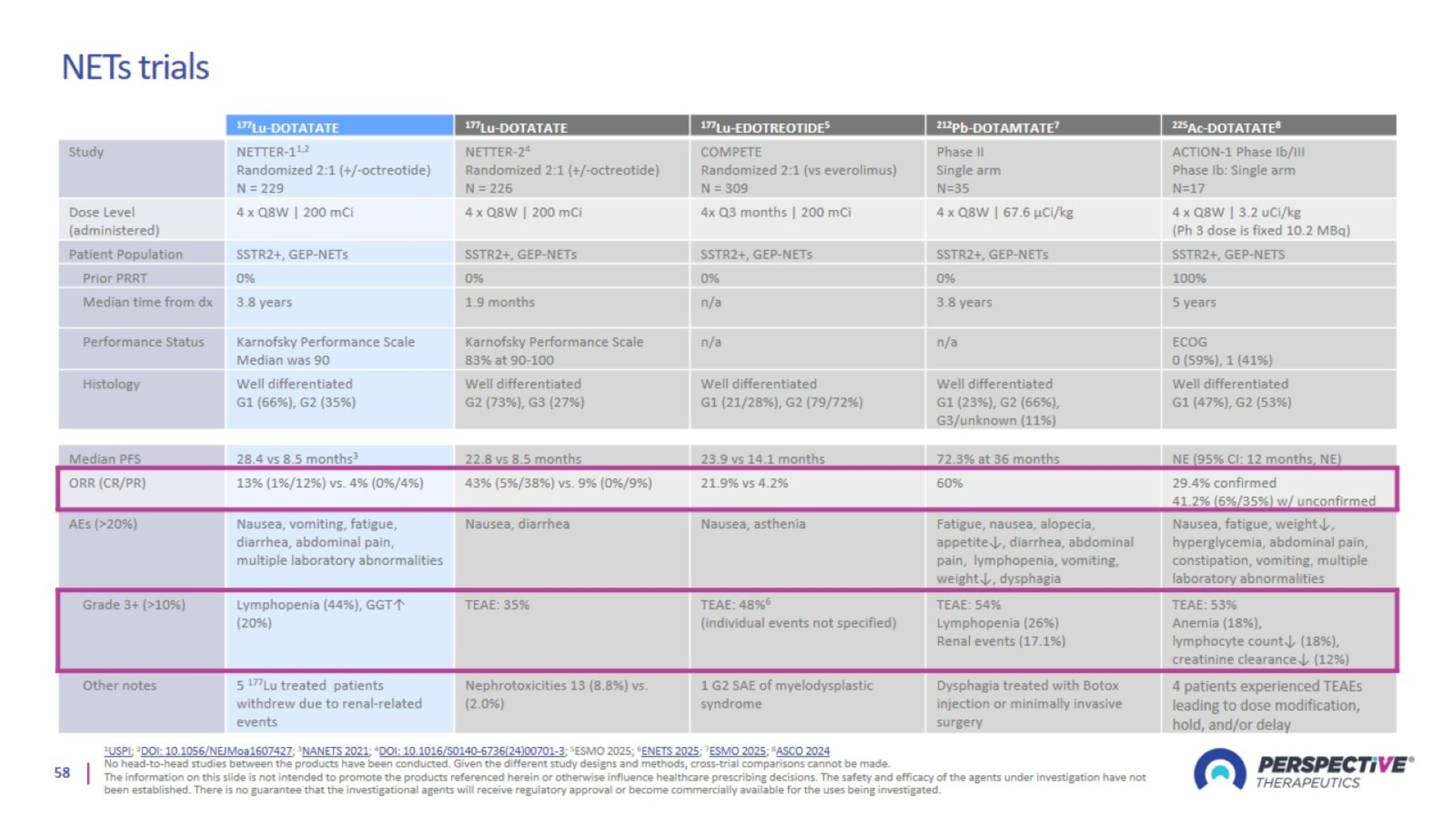
NETs trials
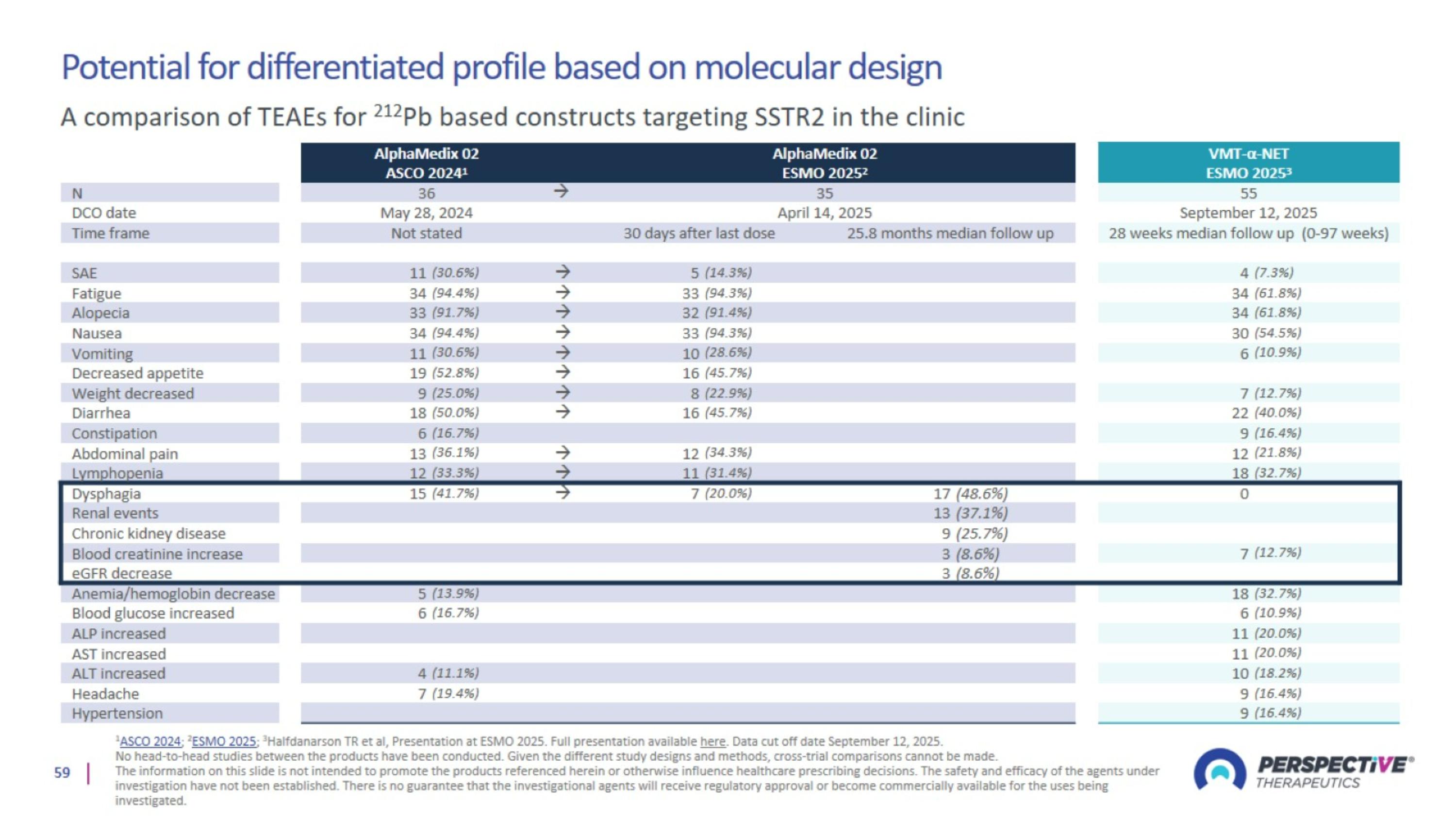
Potential for differentiated profile based on molecular design
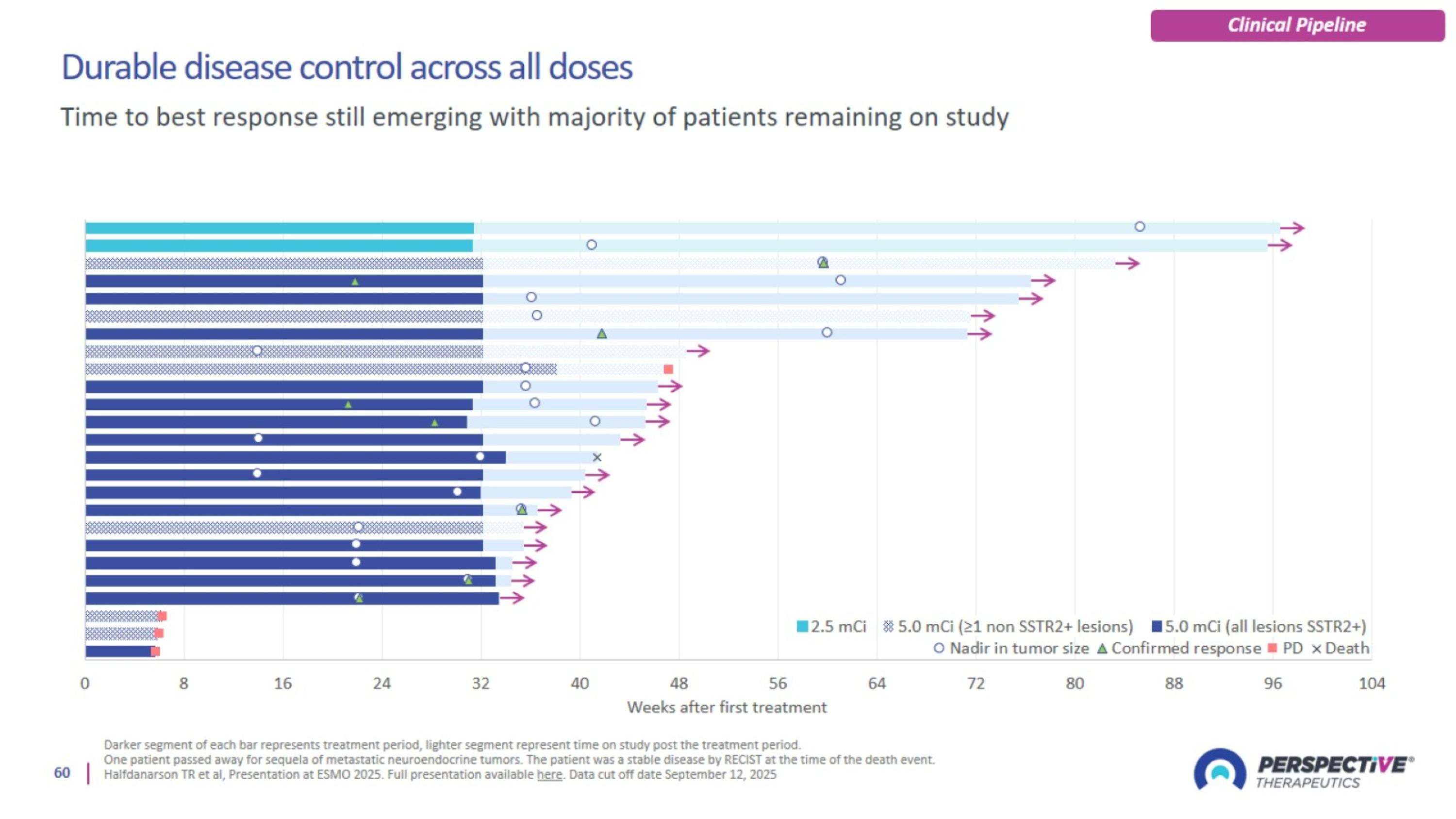
Durable disease control across all doses
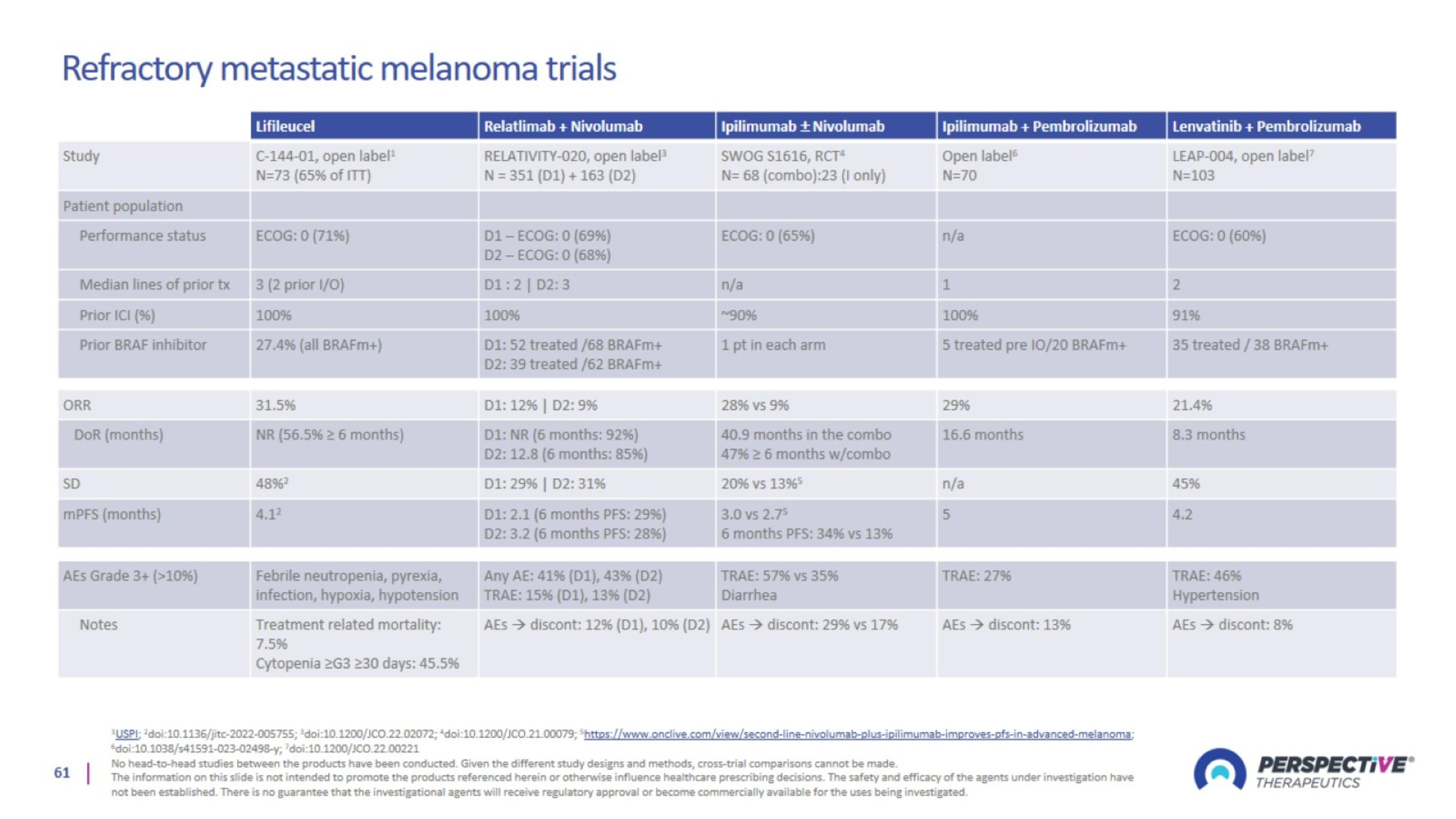
Refractory metastatic melanoma trials
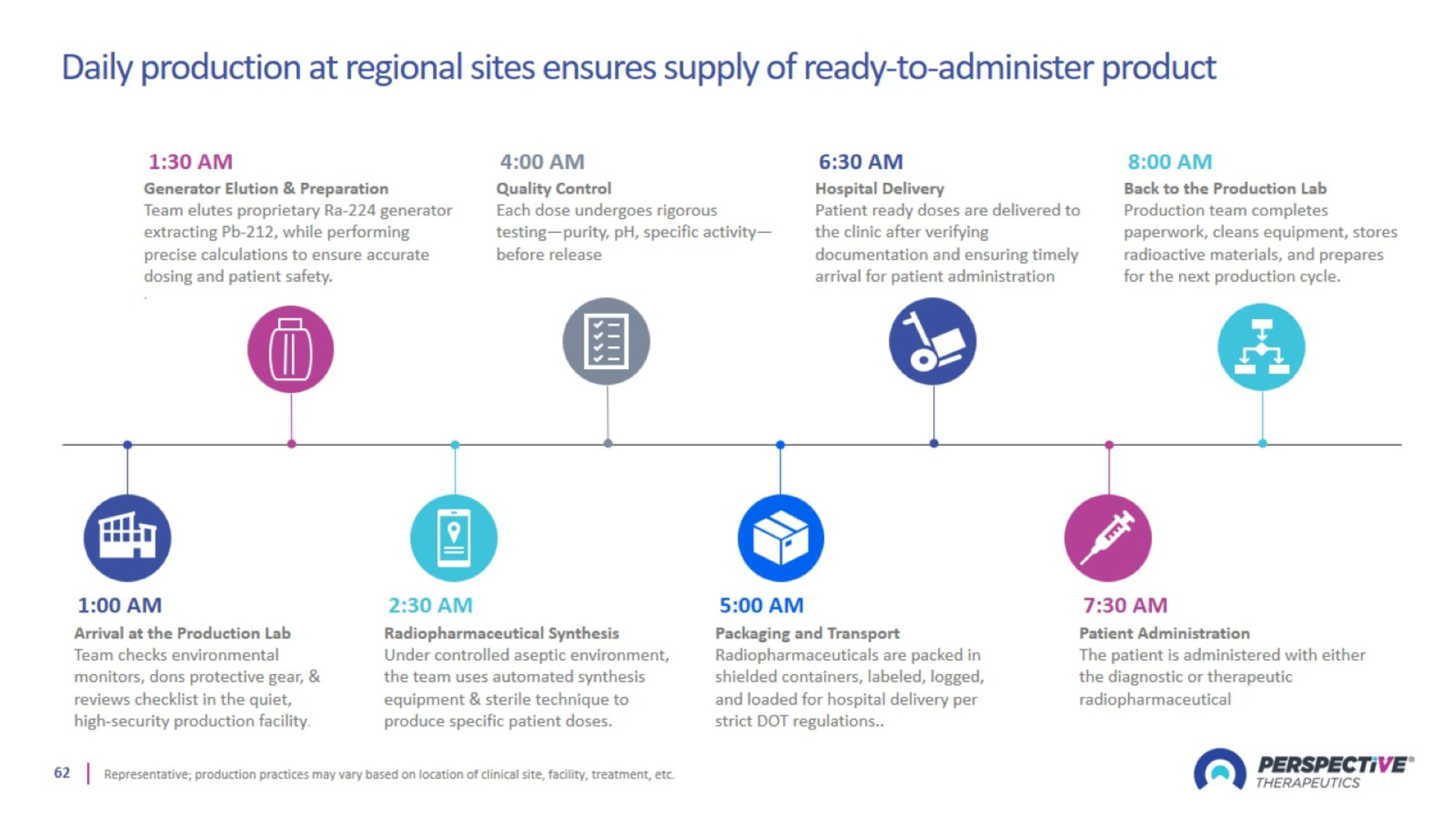
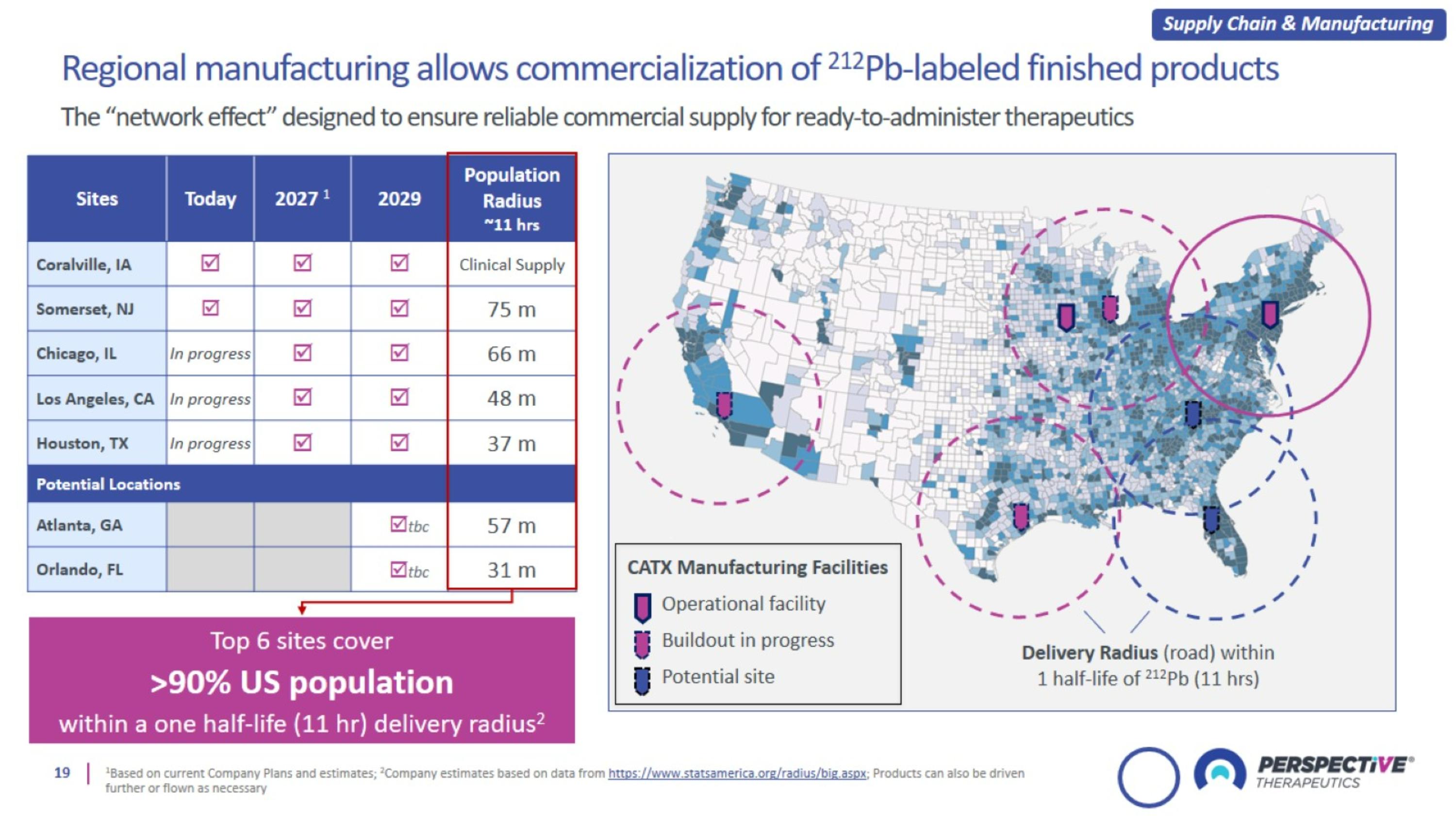
Daily production at regional sites ensures supply of ready-to-administer product
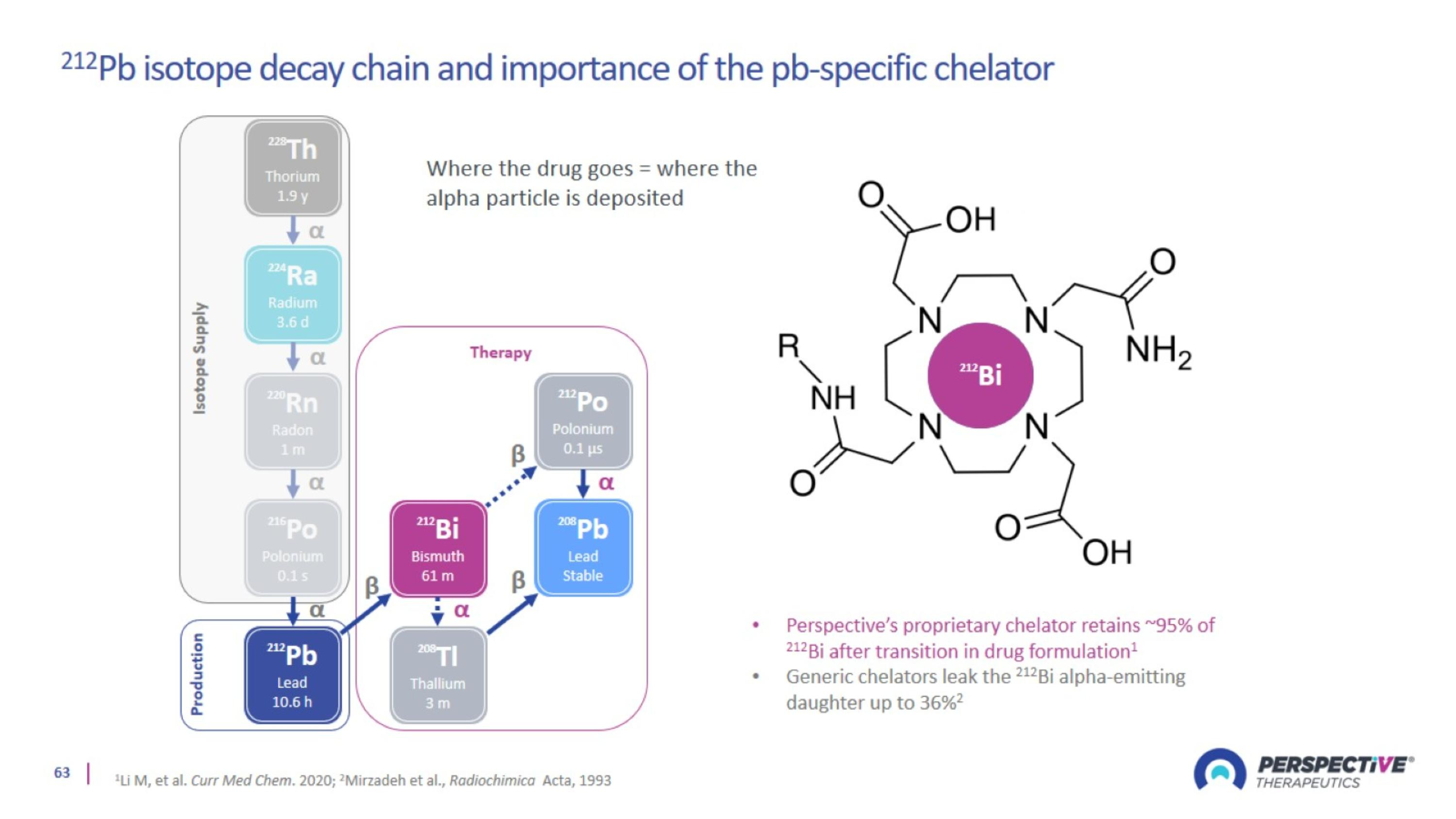
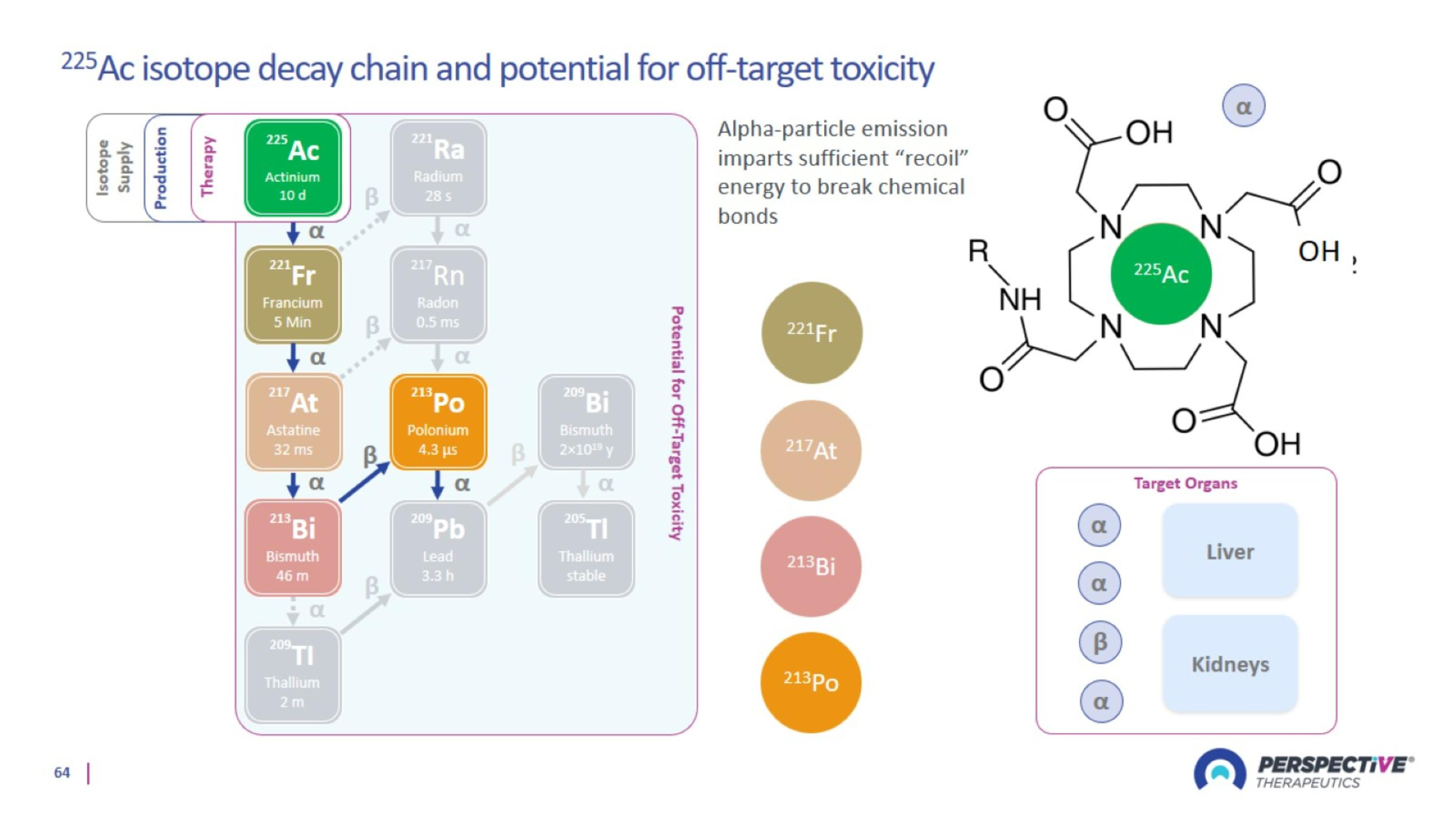
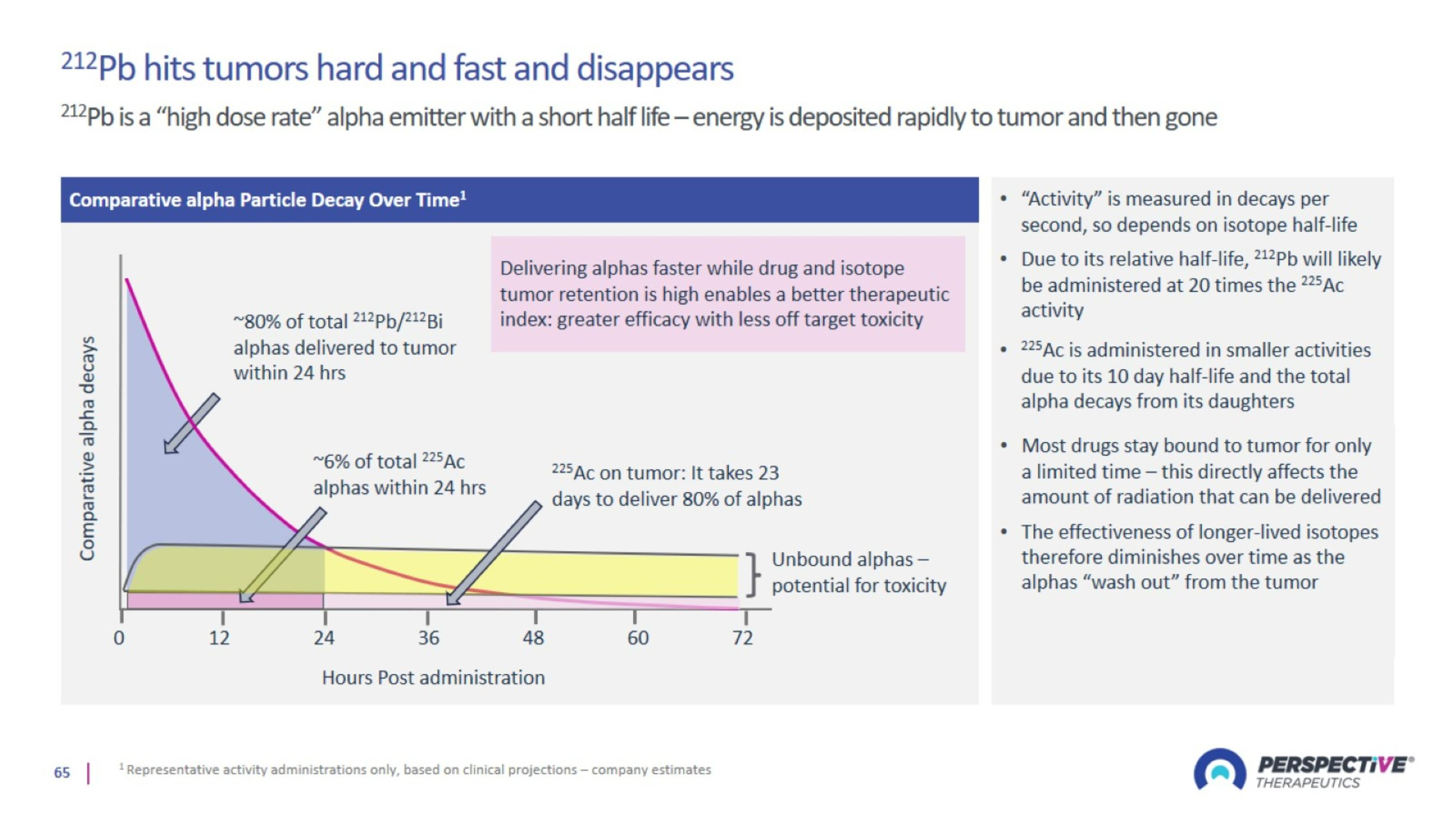
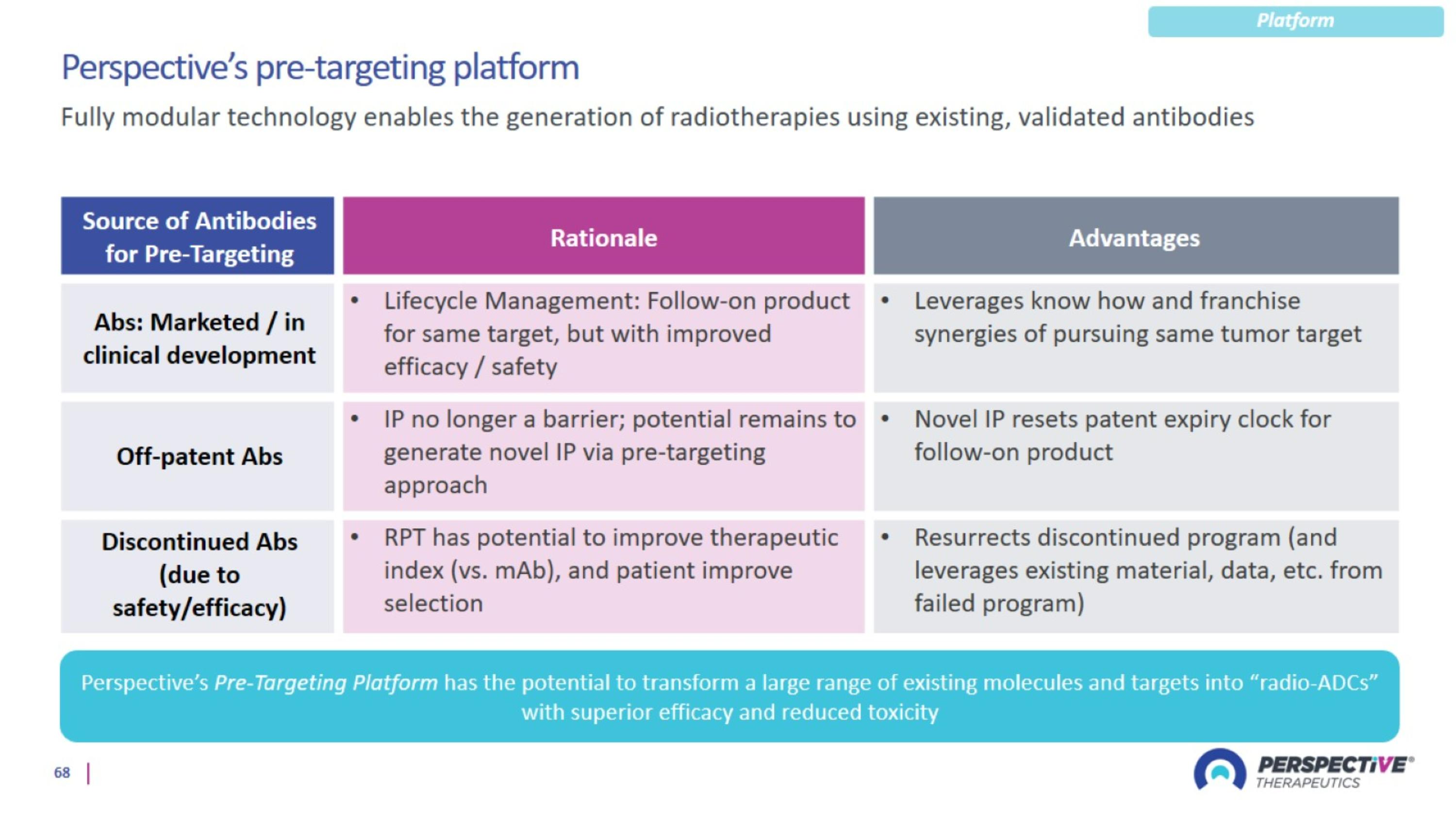
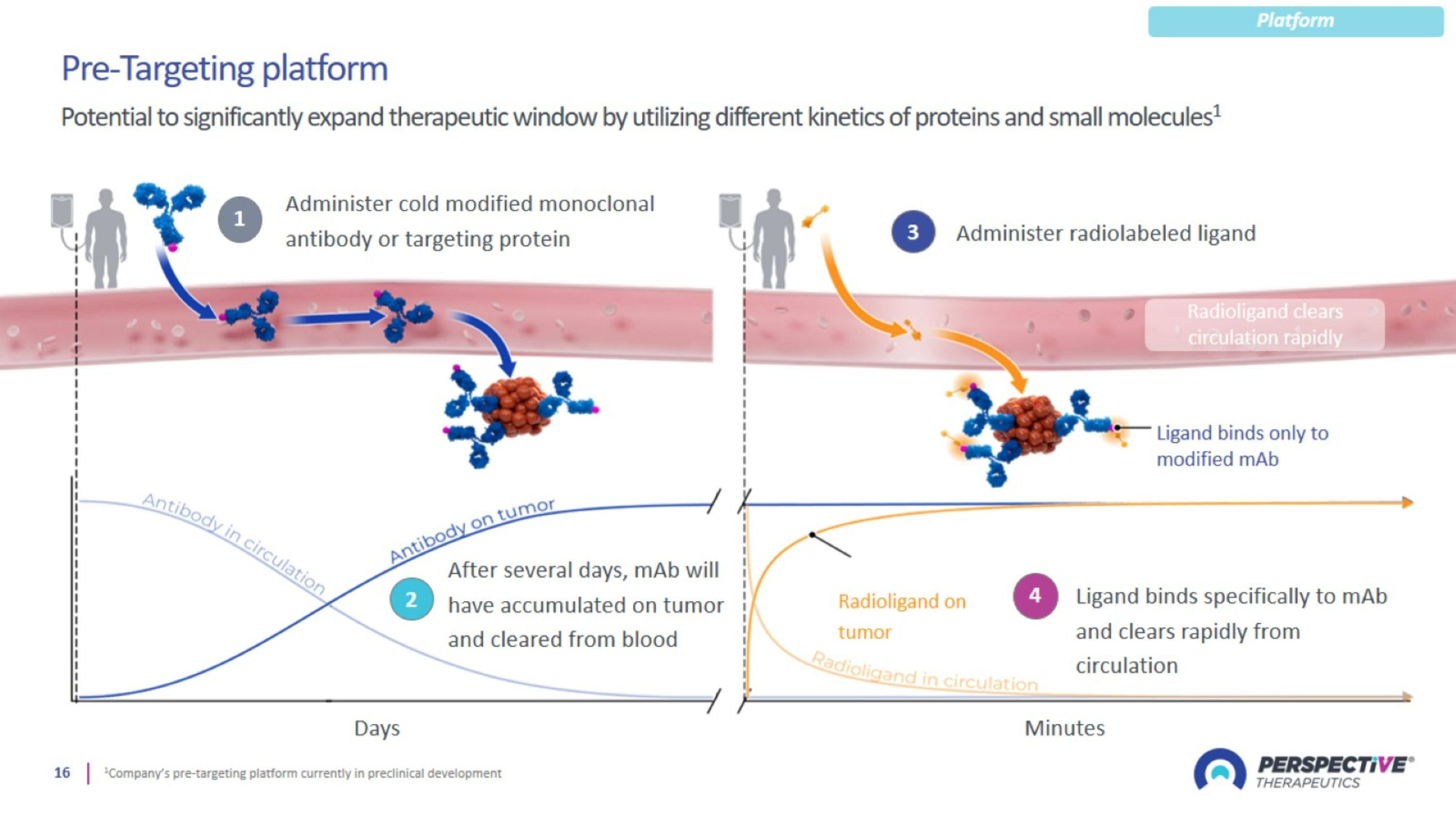
Perspective’s pre-targeting platform