

Redefining Oncology Treatment with Next-Generation Radiopharmaceuticals

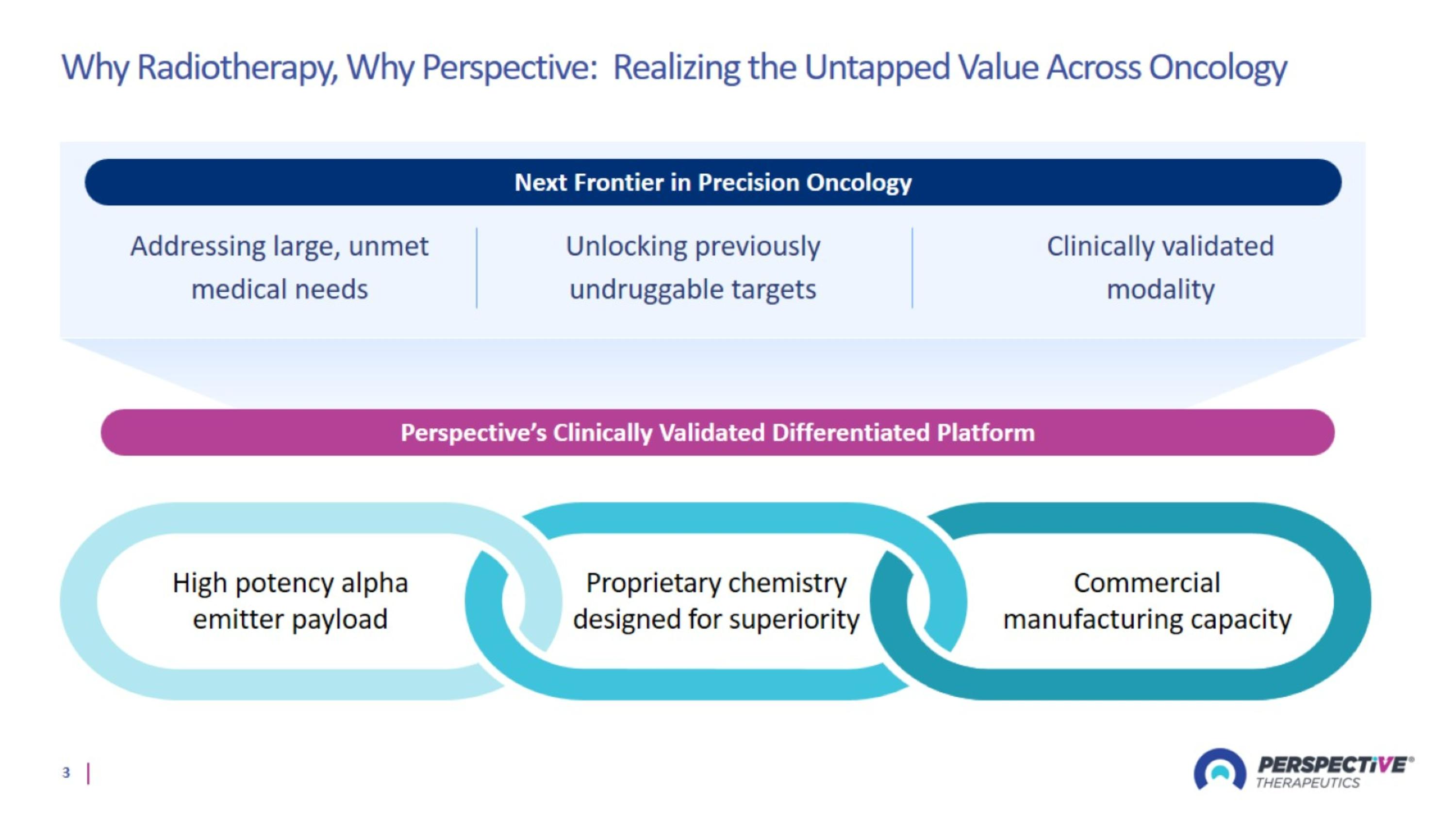
Why Radiotherapy, Why Perspective: Realizing the Untapped Value Across Oncology
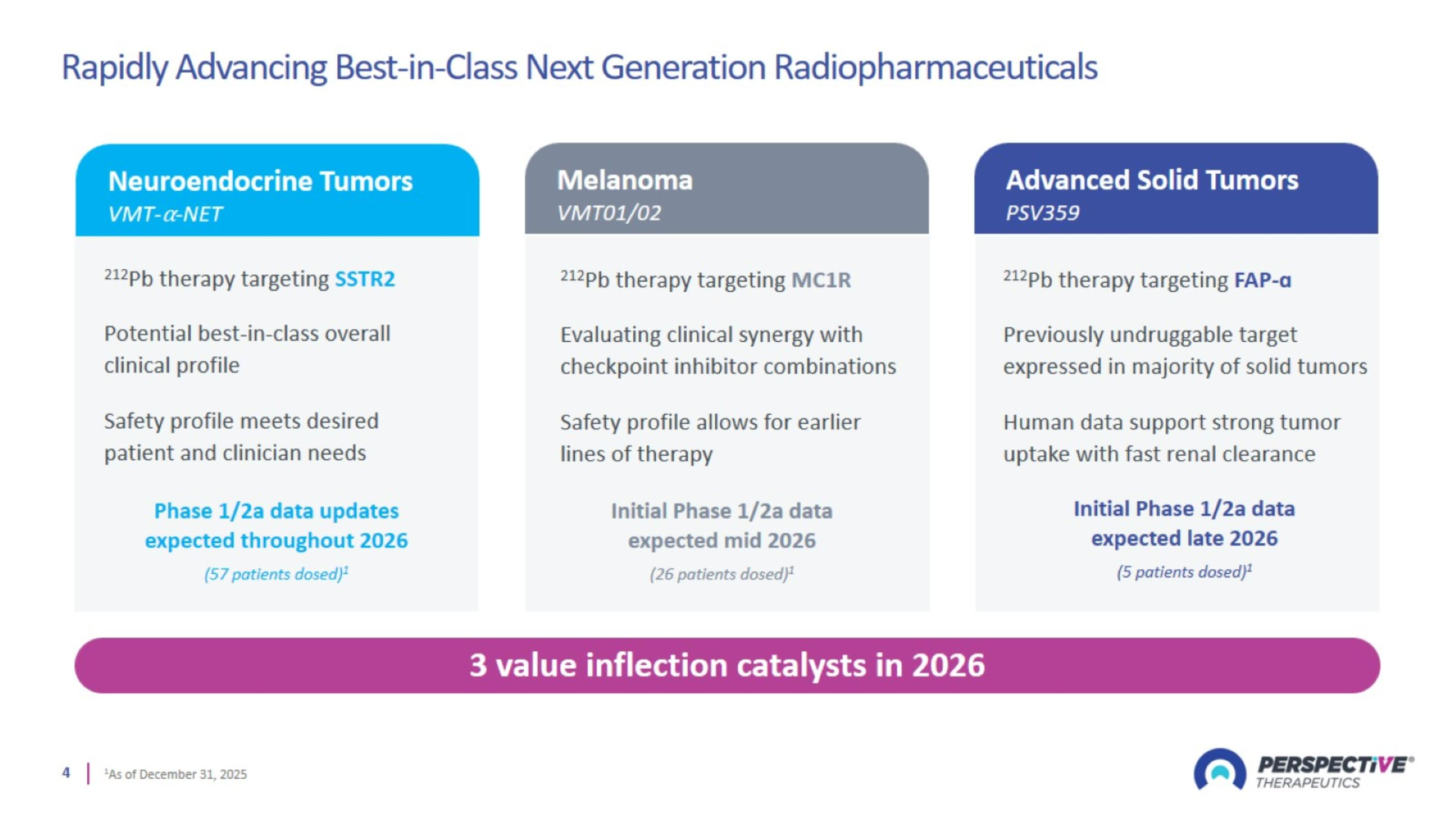
Rapidly Advancing Best-in-Class Next Generation Radiopharmaceuticals
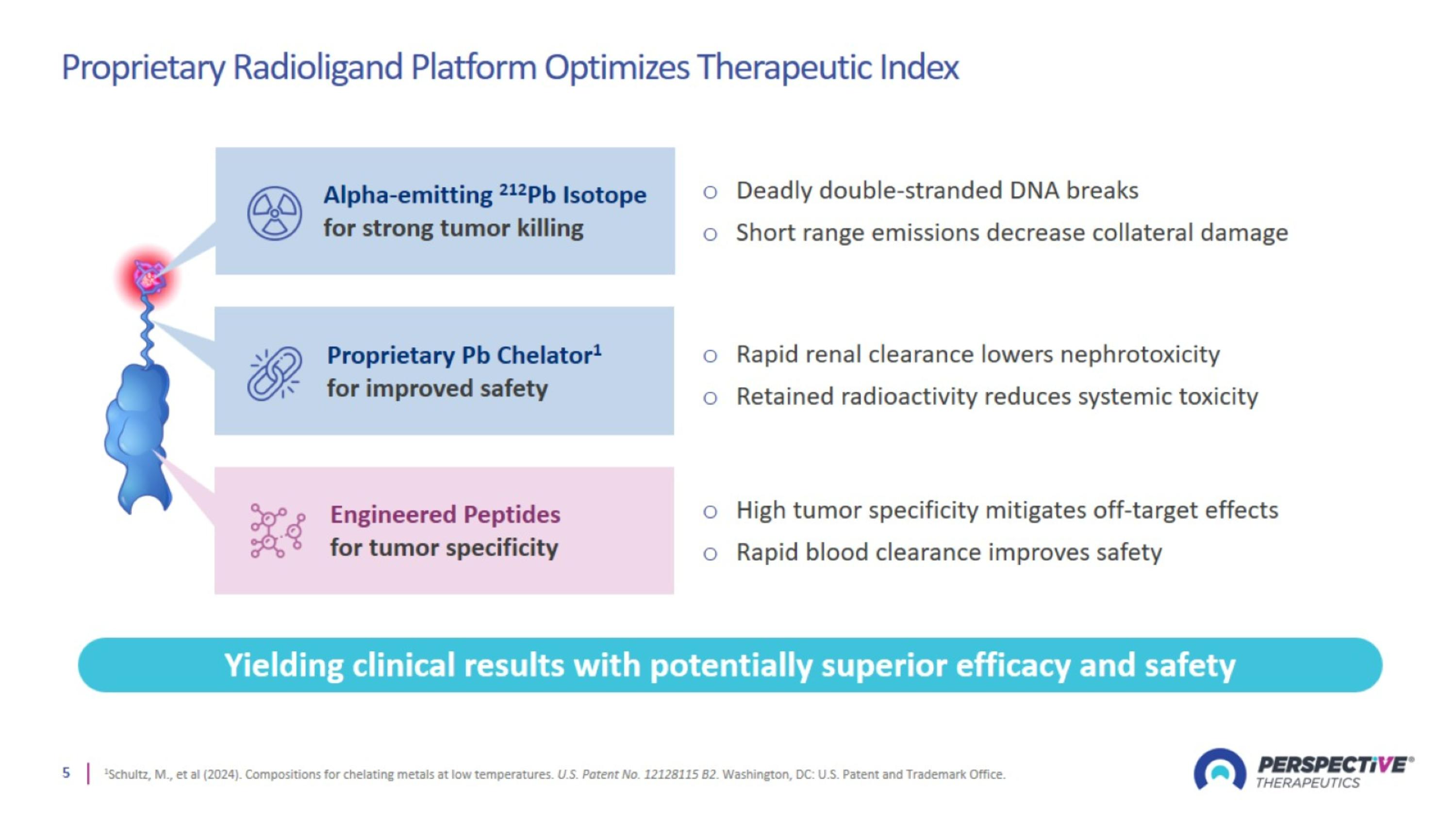
Proprietary Radioligand Platform Optimizes Therapeutic Index
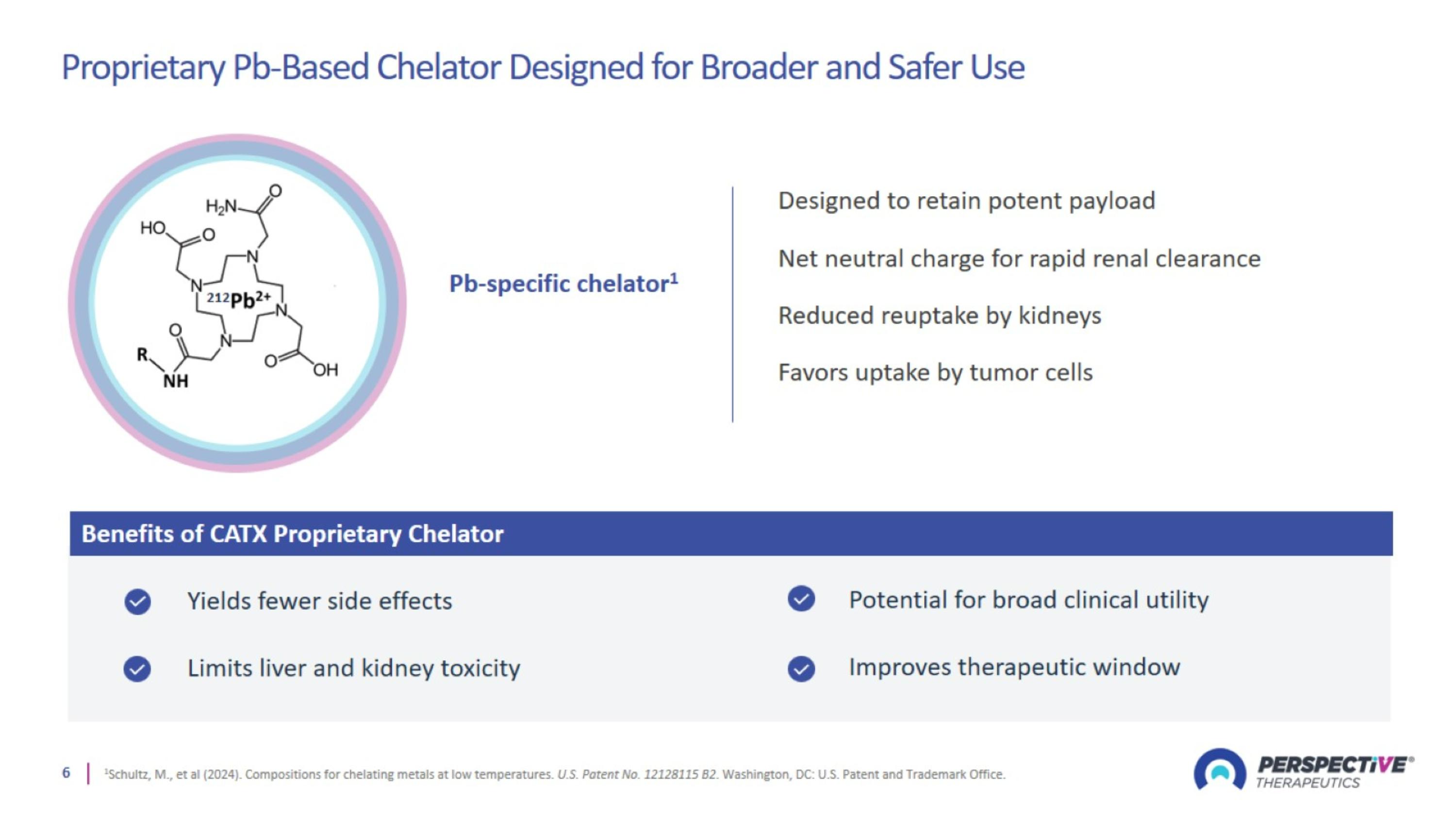
Proprietary Pb-Based Chelator Designed for Broader and Safer Use
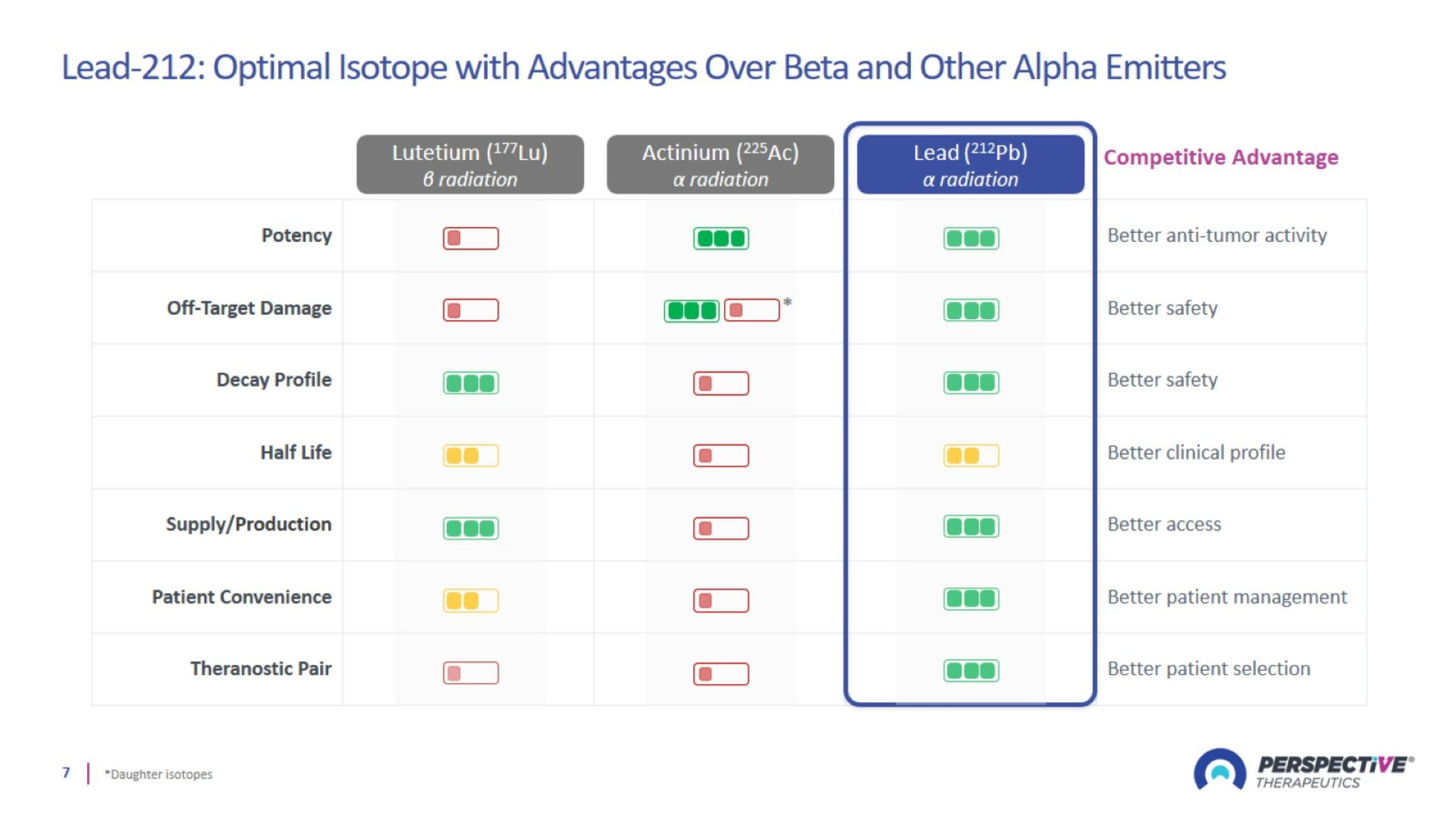
Lead-212: Optimal Isotope with Advantages Over Beta and Other Alpha Emitters
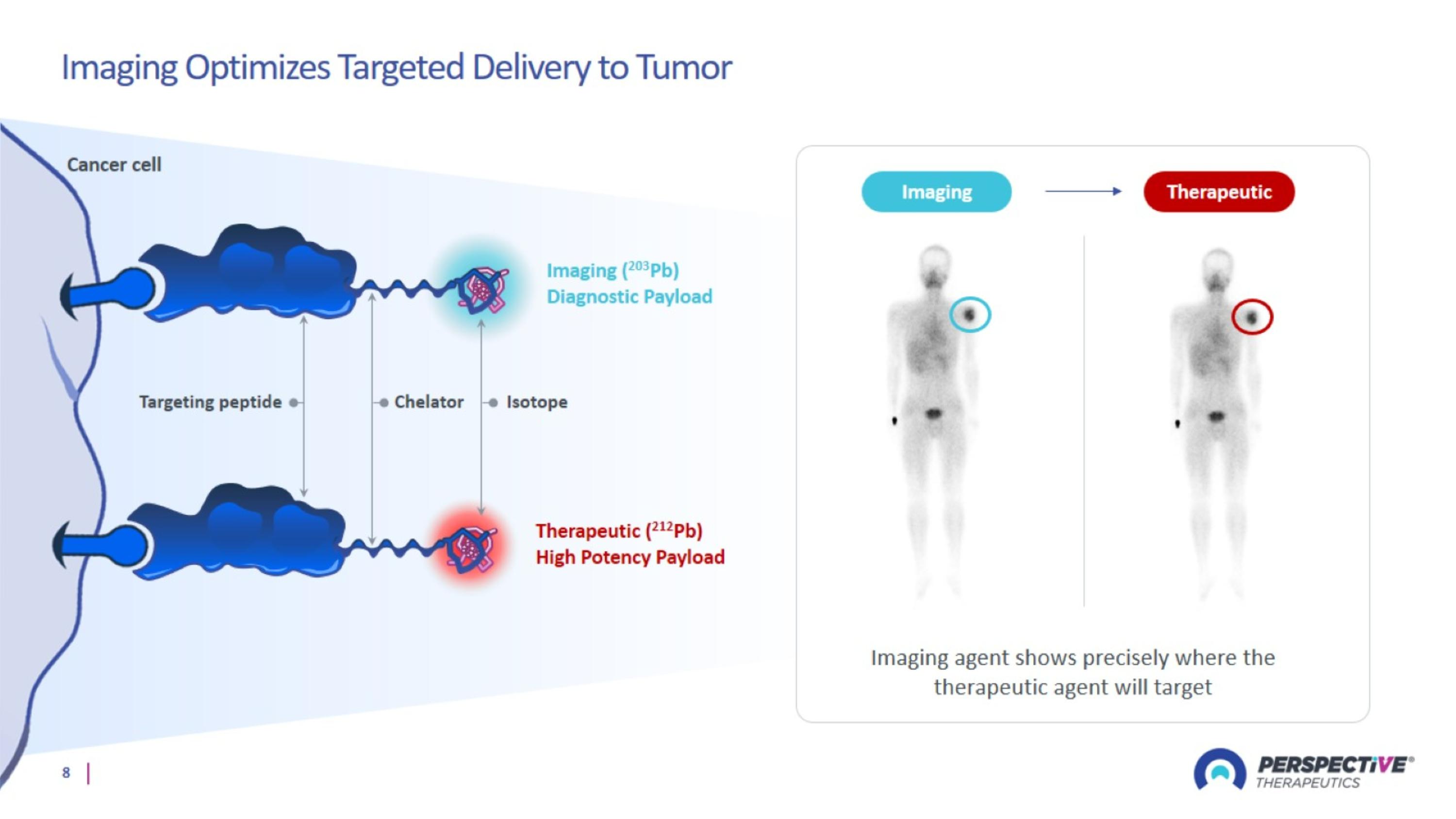
Imaging Optimizes Targeted Delivery to Tumor
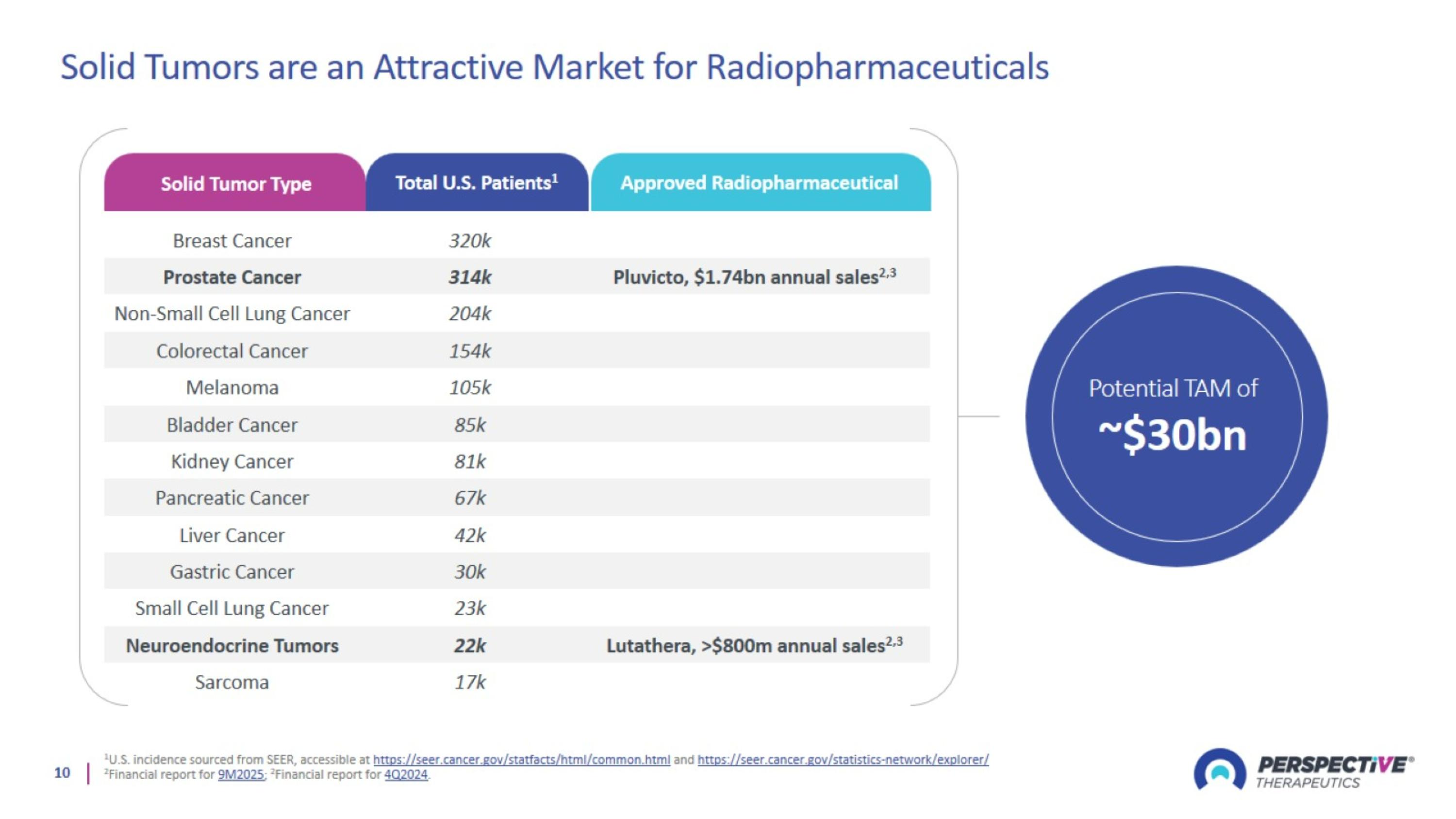
Solid Tumors are an Attractive Market for Radiopharmaceuticals
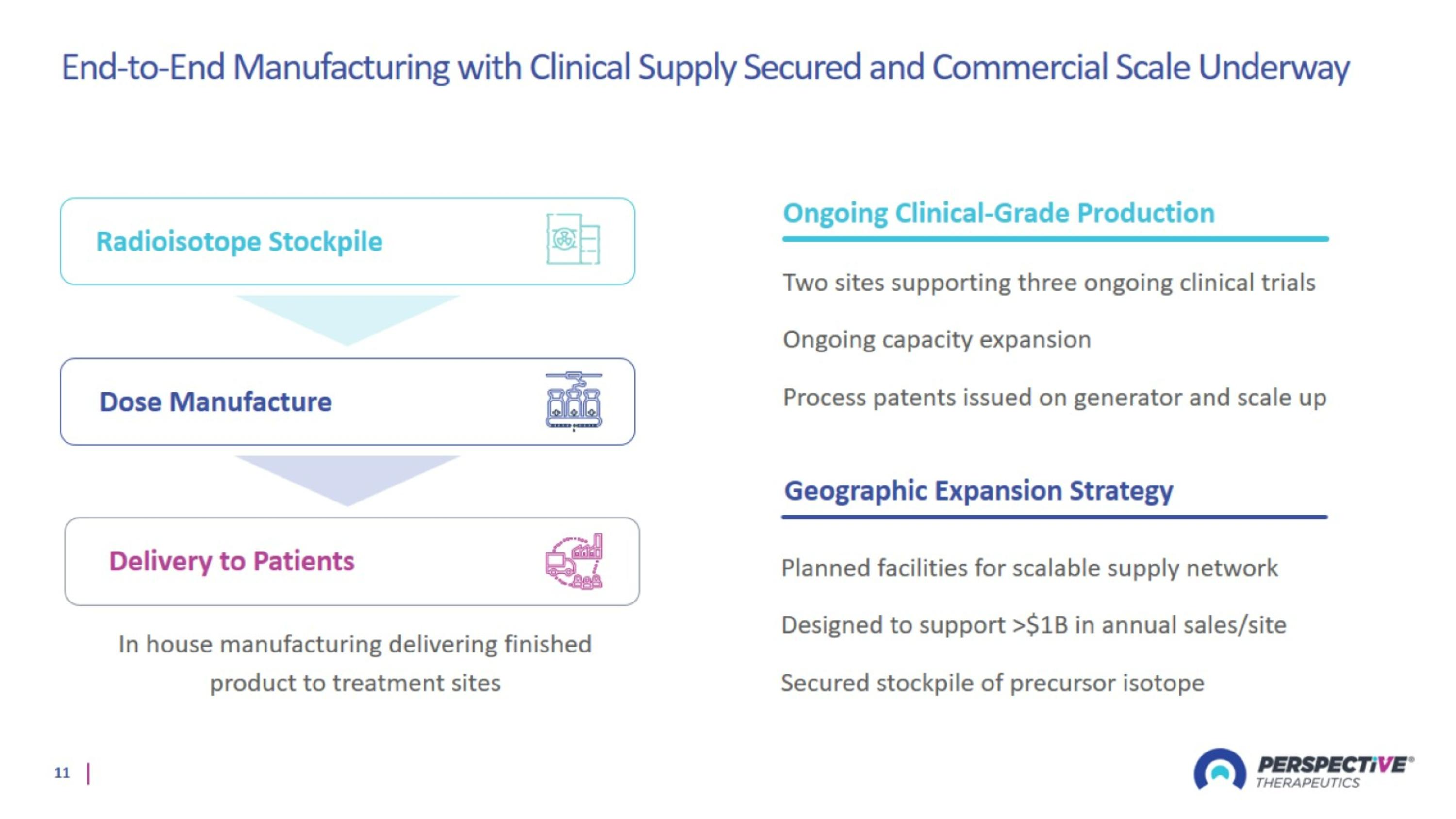
End-to-End Manufacturing with Clinical Supply Secured and Commercial Scale Underway
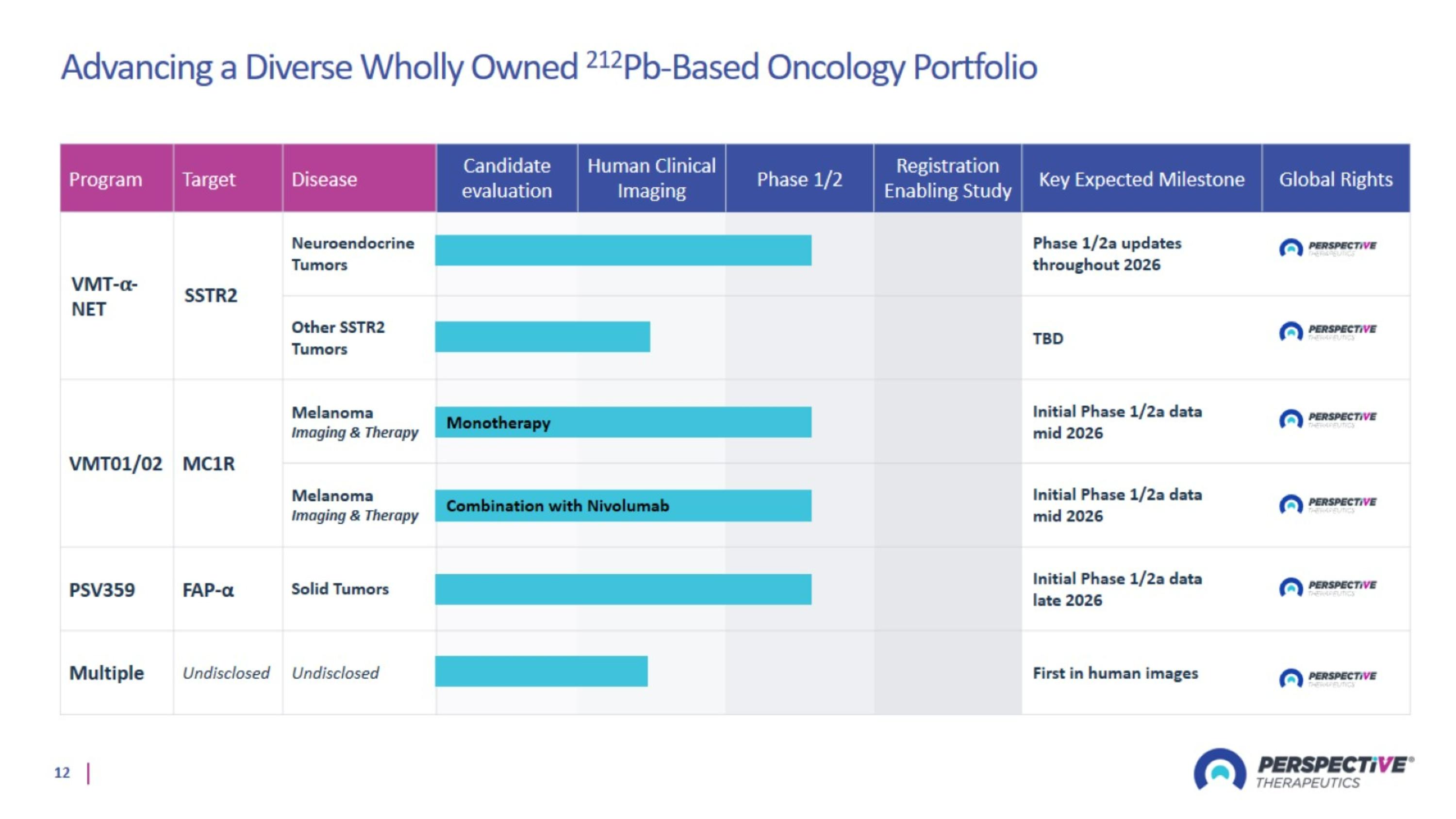
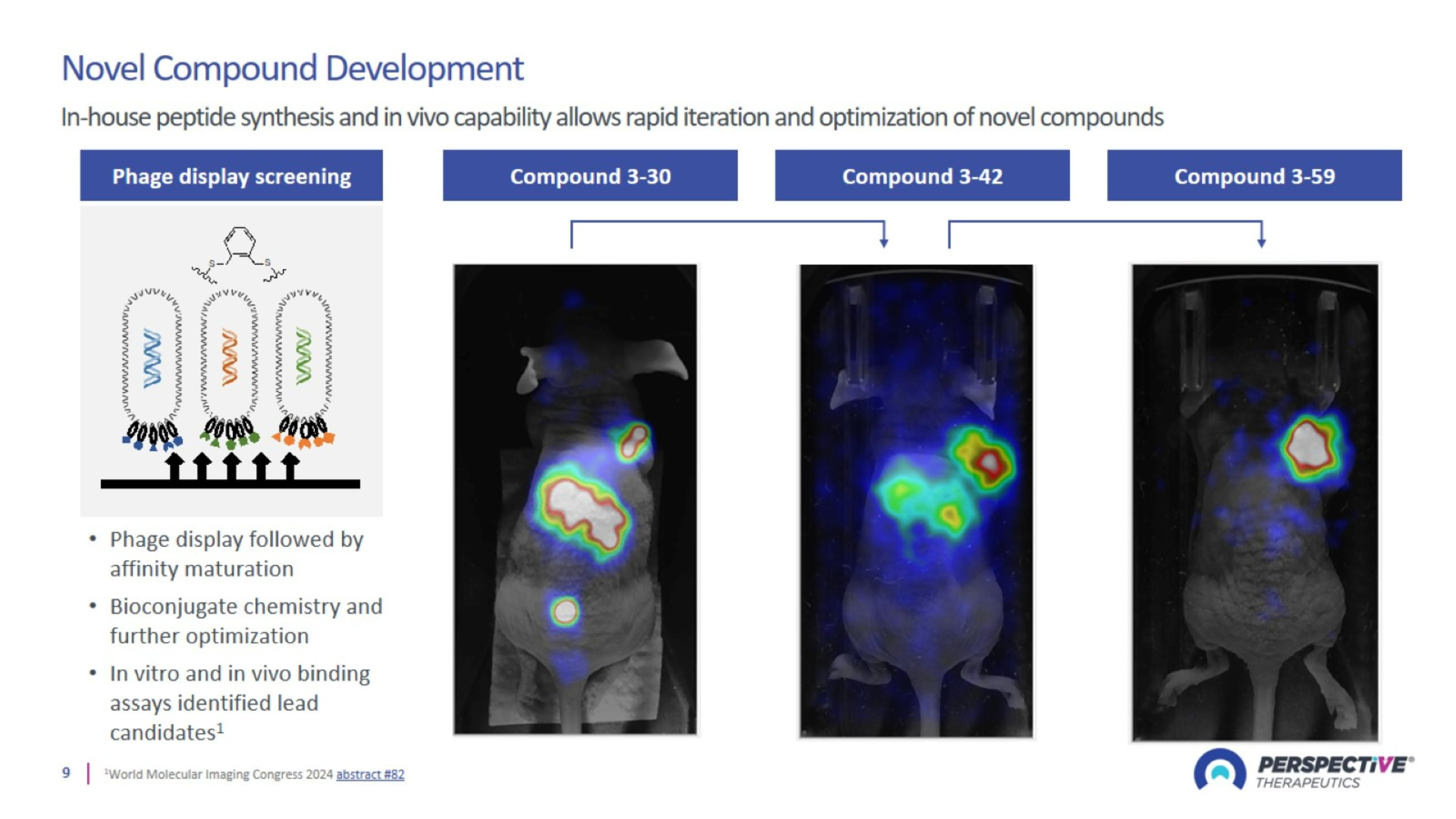
Advancing a Diverse Wholly Owned 212Pb-Based Oncology Portfolio
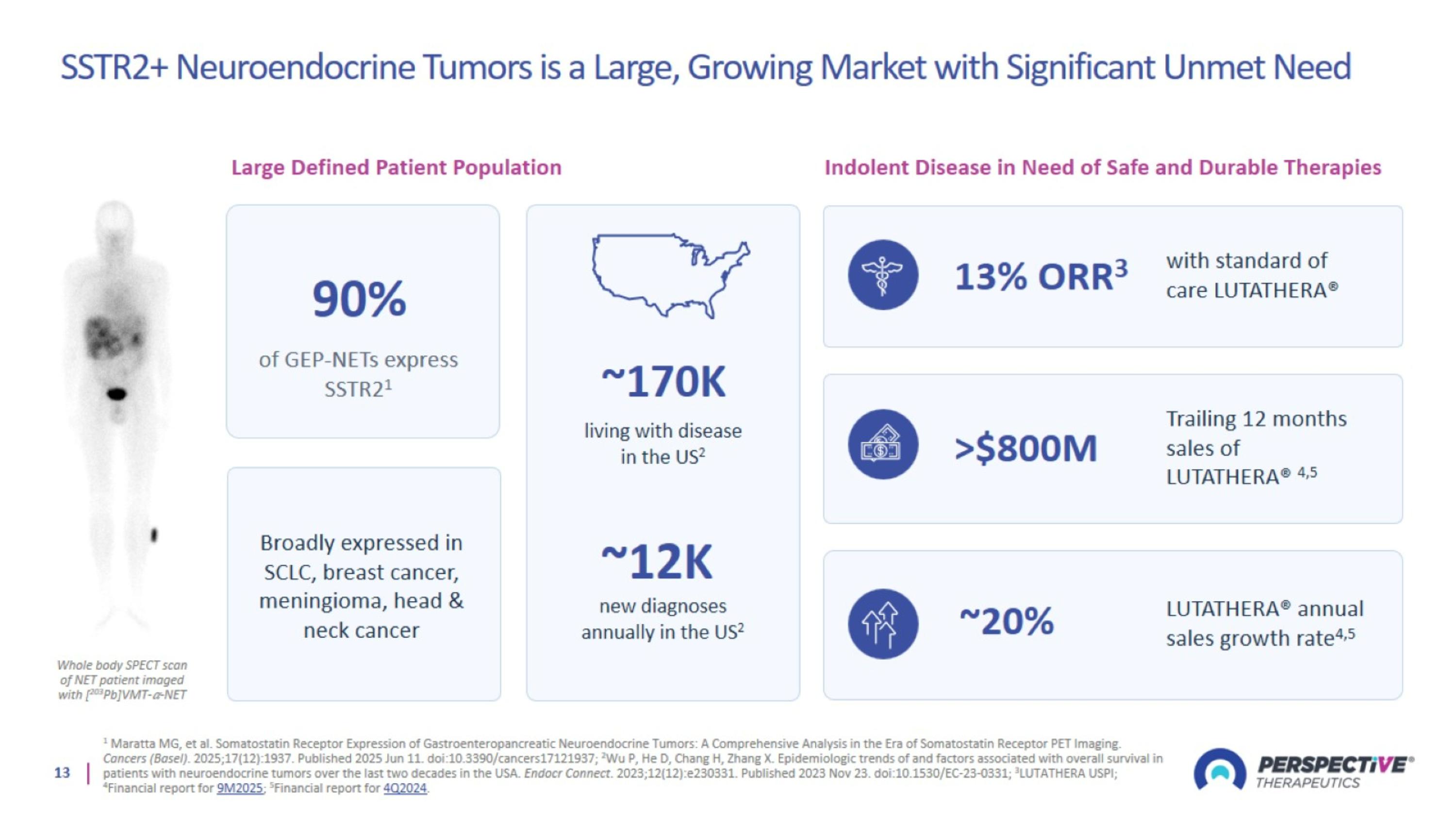
SSTR2+ Neuroendocrine Tumors is a Large, Growing Market with Significant Unmet Need
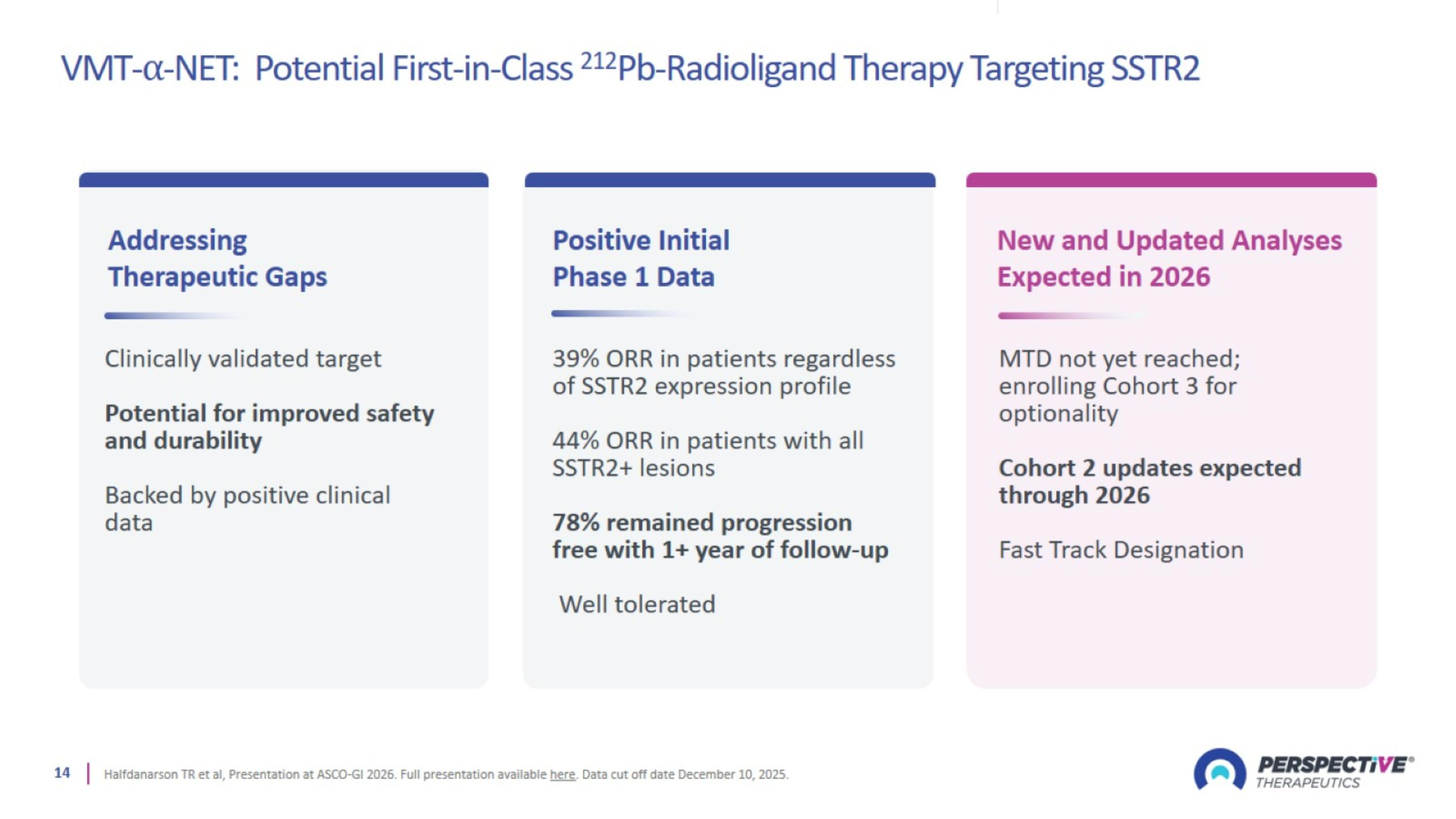
VMT-⍺-NET: Potential First-in-Class 212Pb-Radioligand Therapy Targeting SSTR2
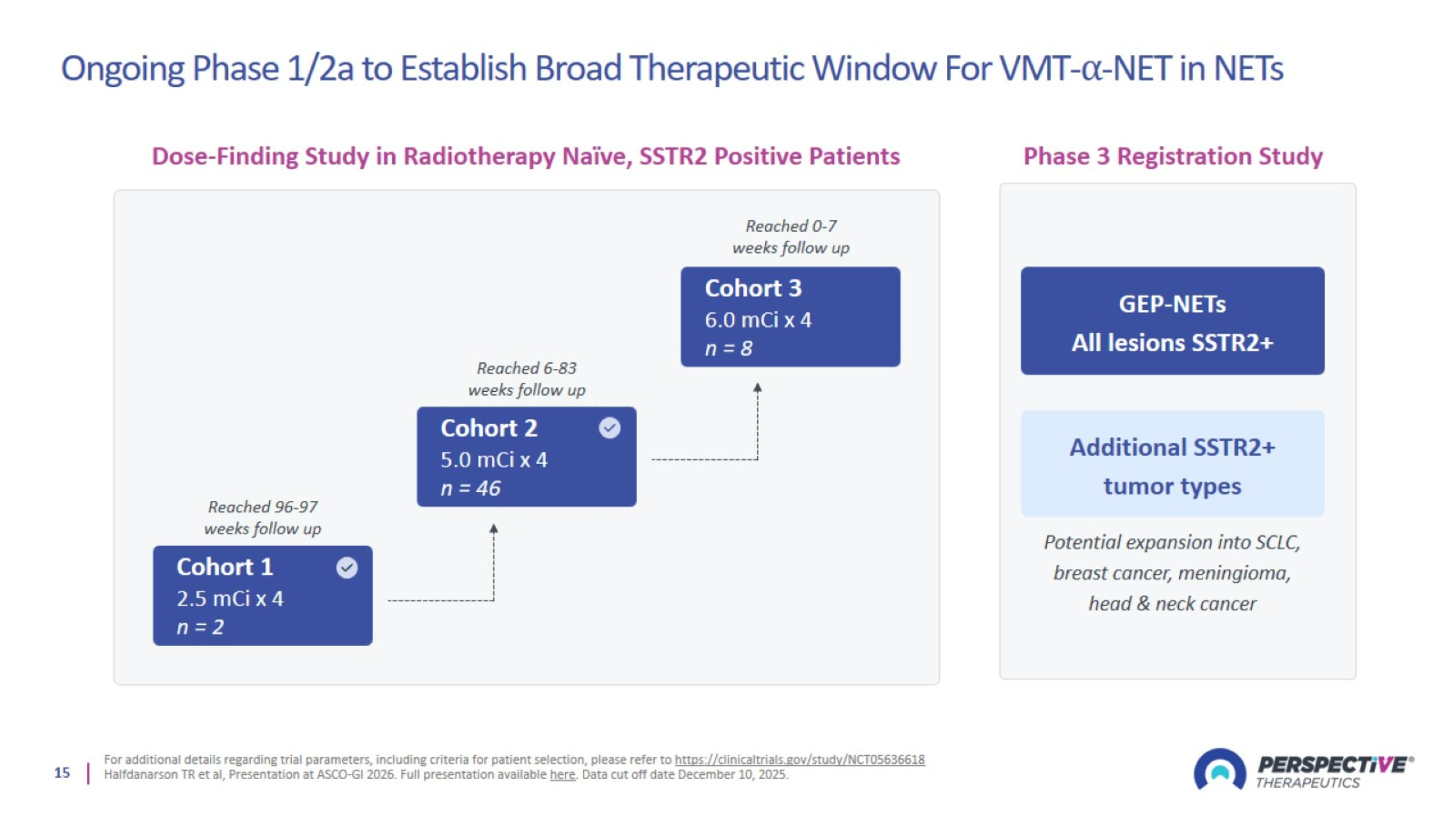
Ongoing Phase 1/2a to Establish Broad Therapeutic Window For VMT-⍺-NET in NETs
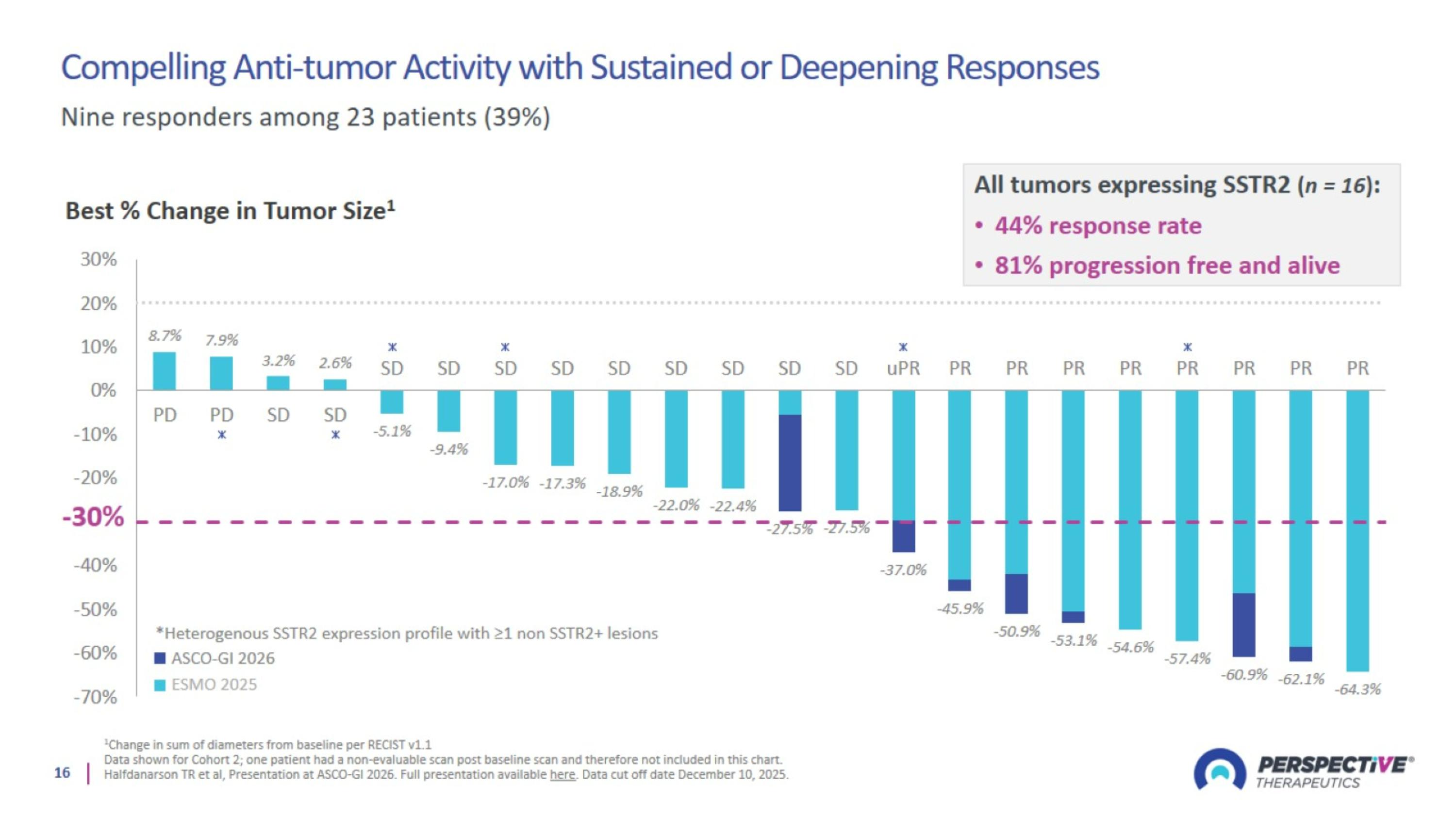
Compelling Anti-tumor Activity with Sustained or Deepening Responses
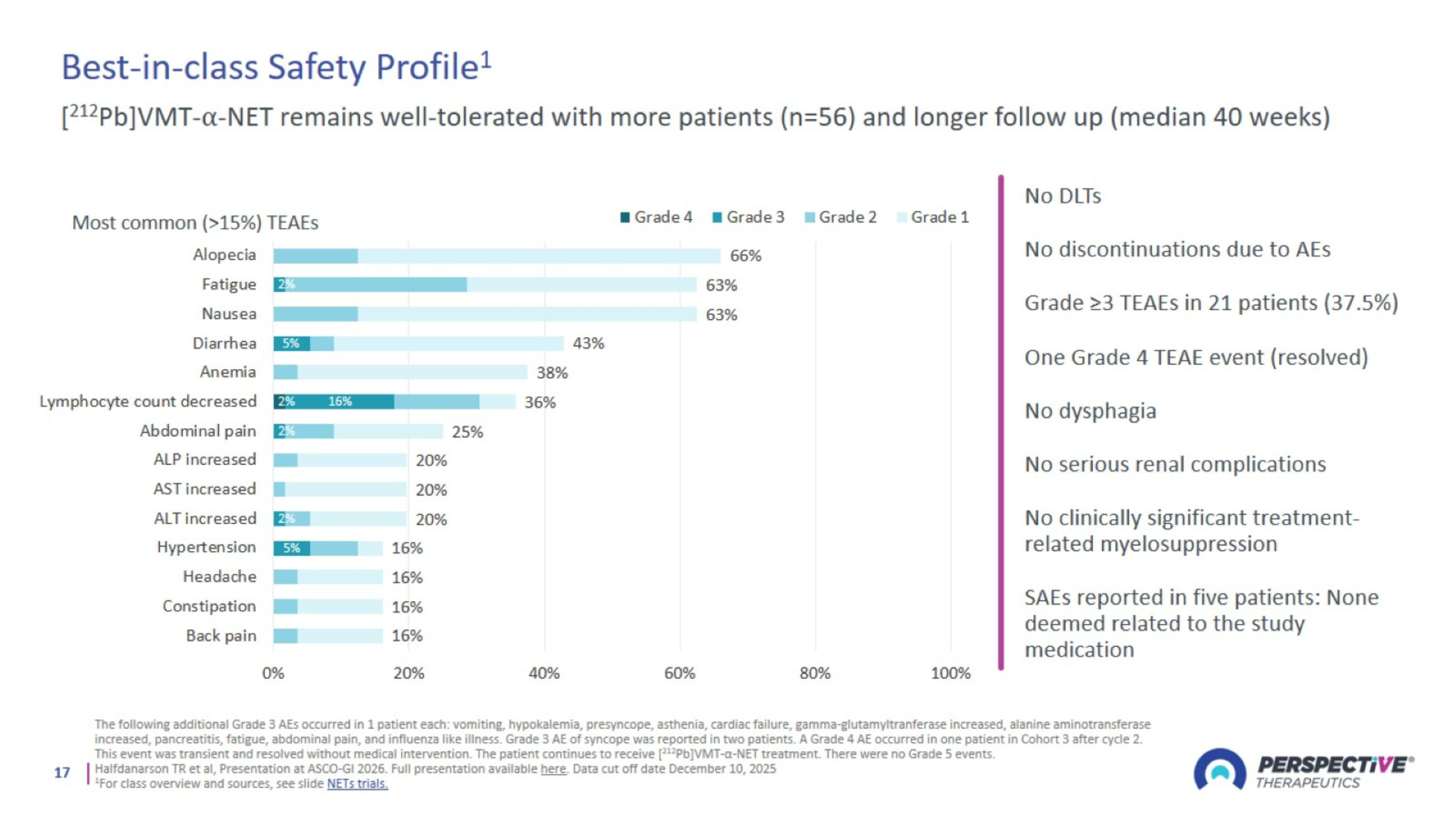
Best-in-class Safety Profile1
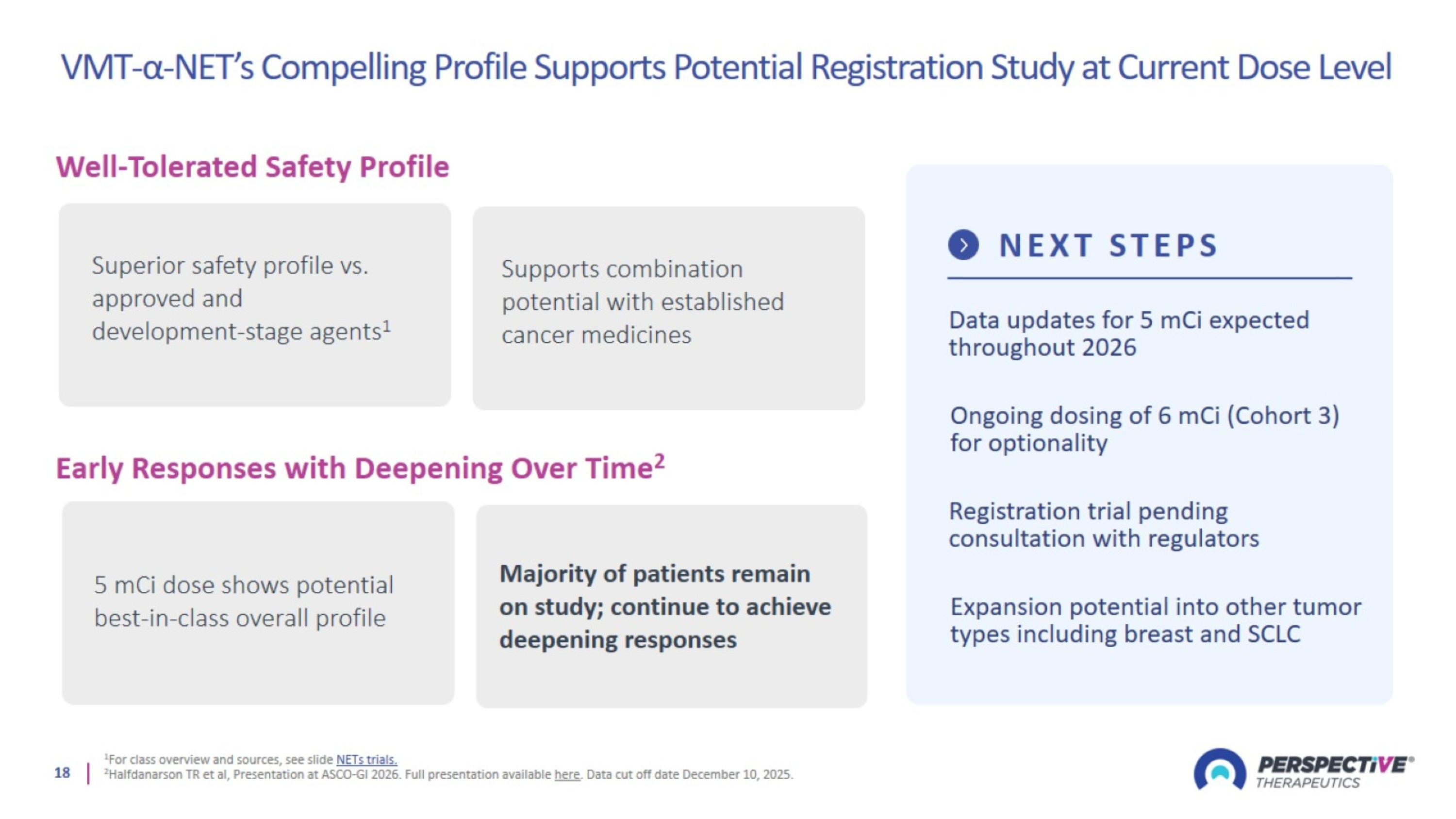
VMT-α-NET’s Compelling Profile Supports Potential Registration Study at Current Dose Level
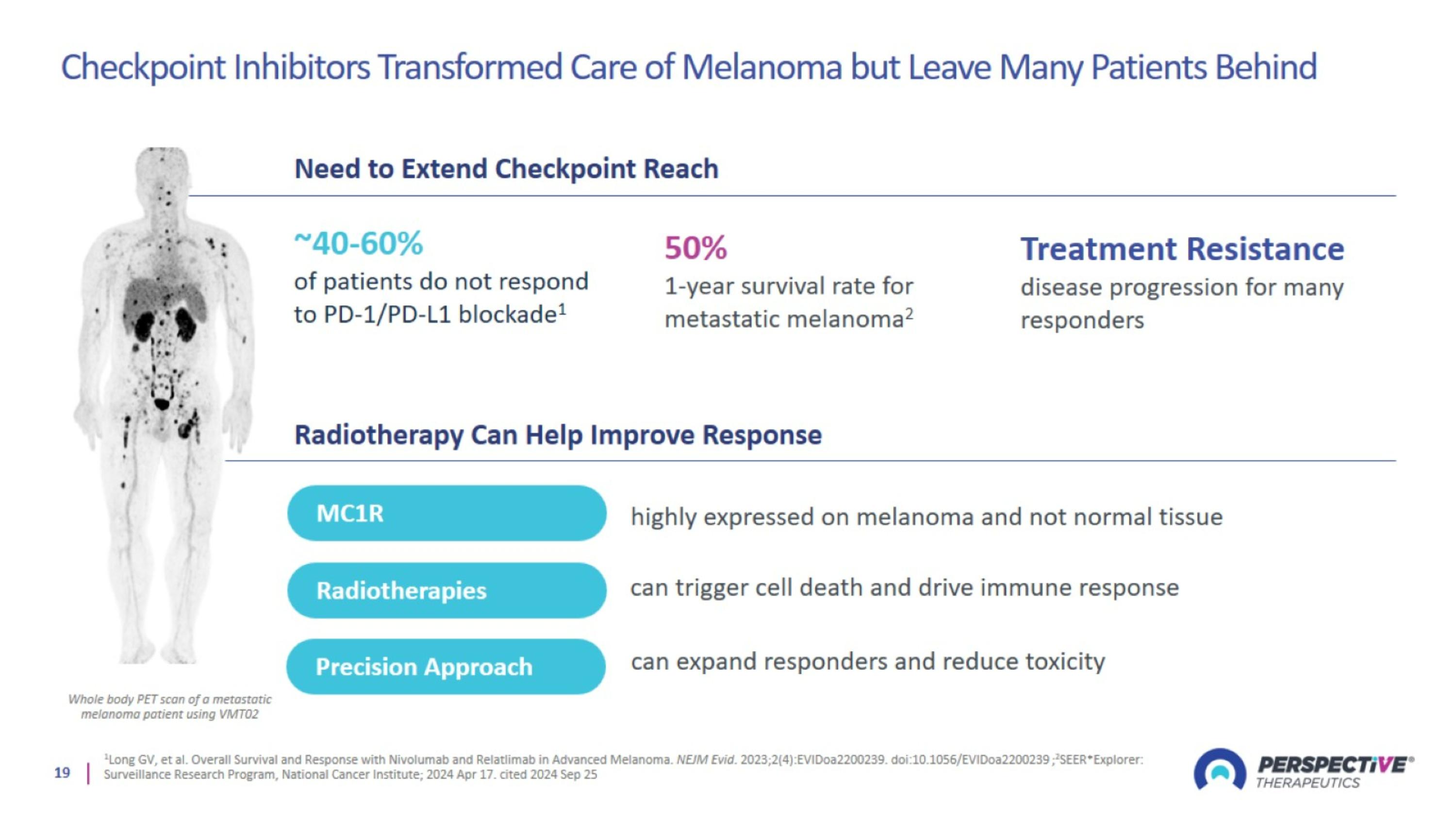
Checkpoint Inhibitors Transformed Care of Melanoma but Leave Many Patients Behind
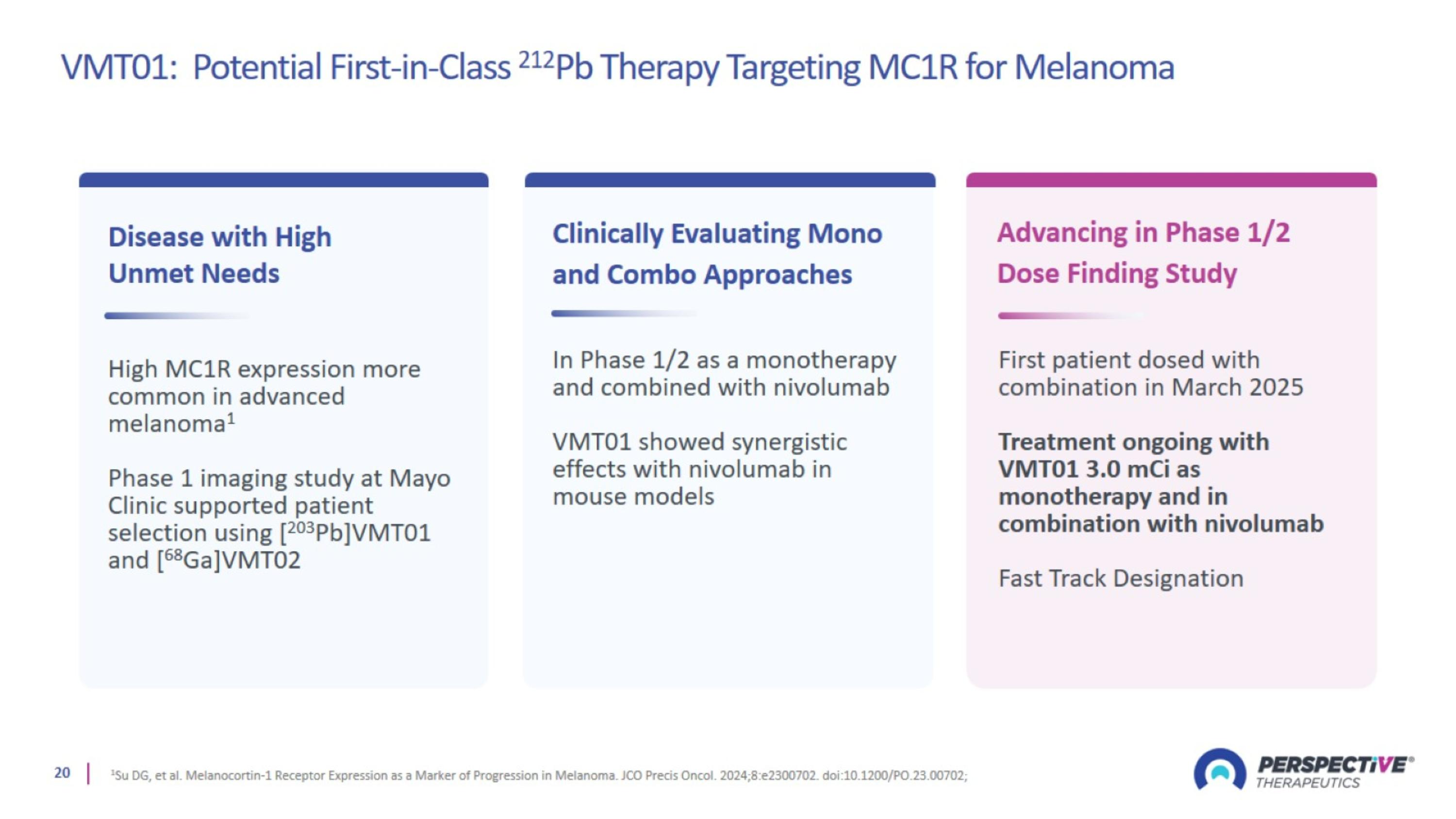
VMT01: Potential First-in-Class 212Pb Therapy Targeting MC1R for Melanoma
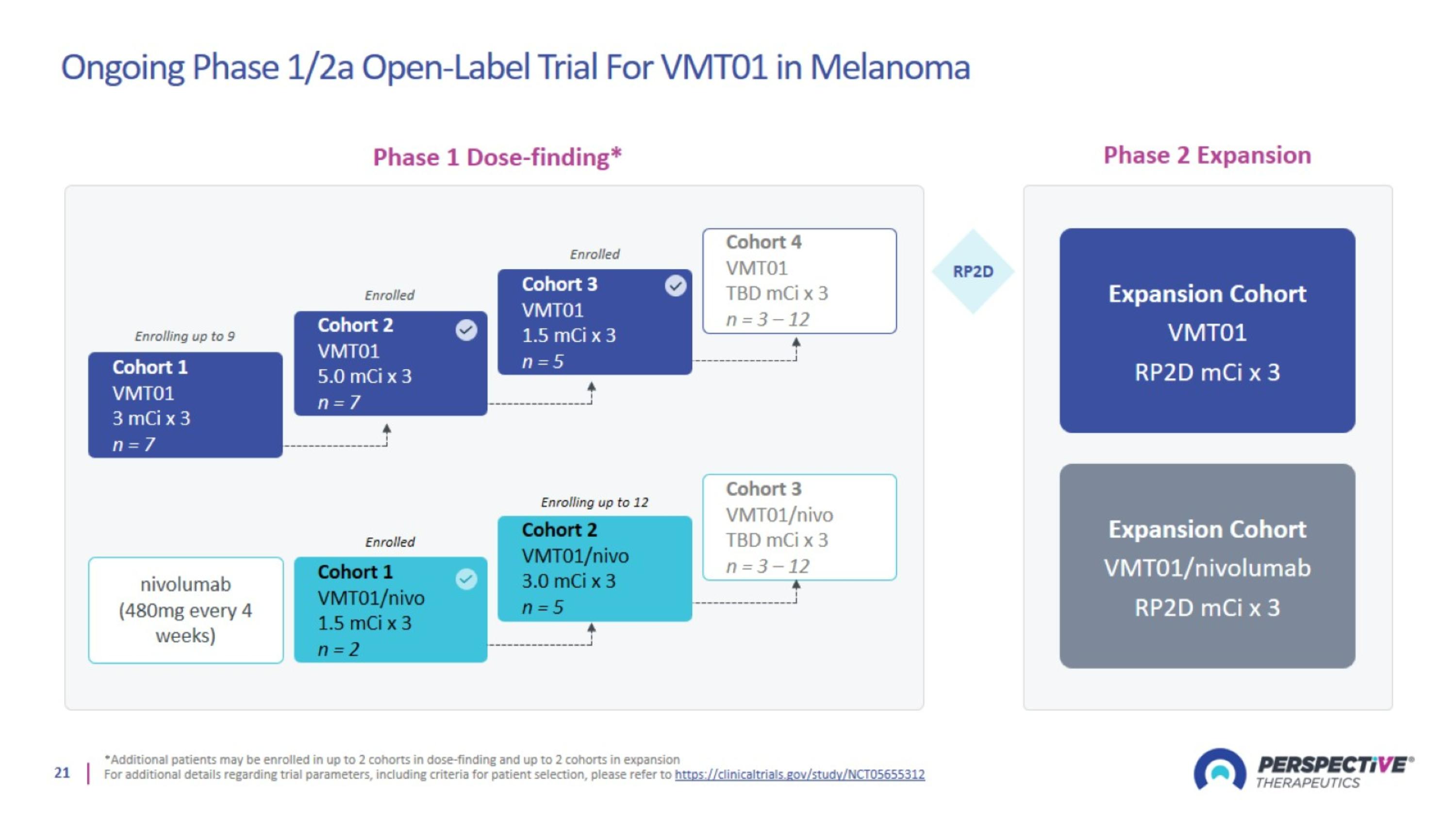
Ongoing Phase 1/2a Open-Label Trial For VMT01 in Melanoma
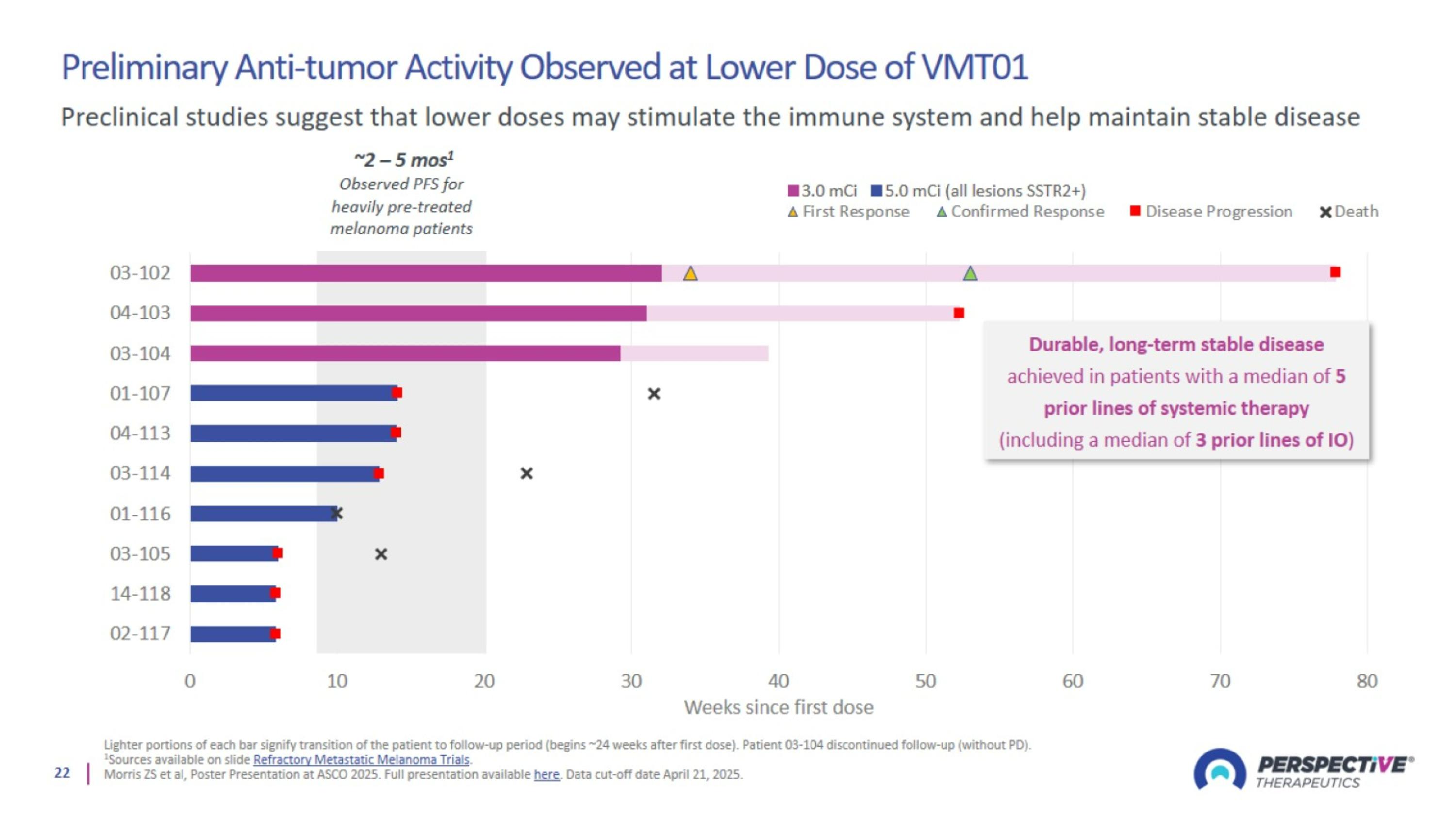
Preliminary Anti-tumor Activity Observed at Lower Dose of VMT01
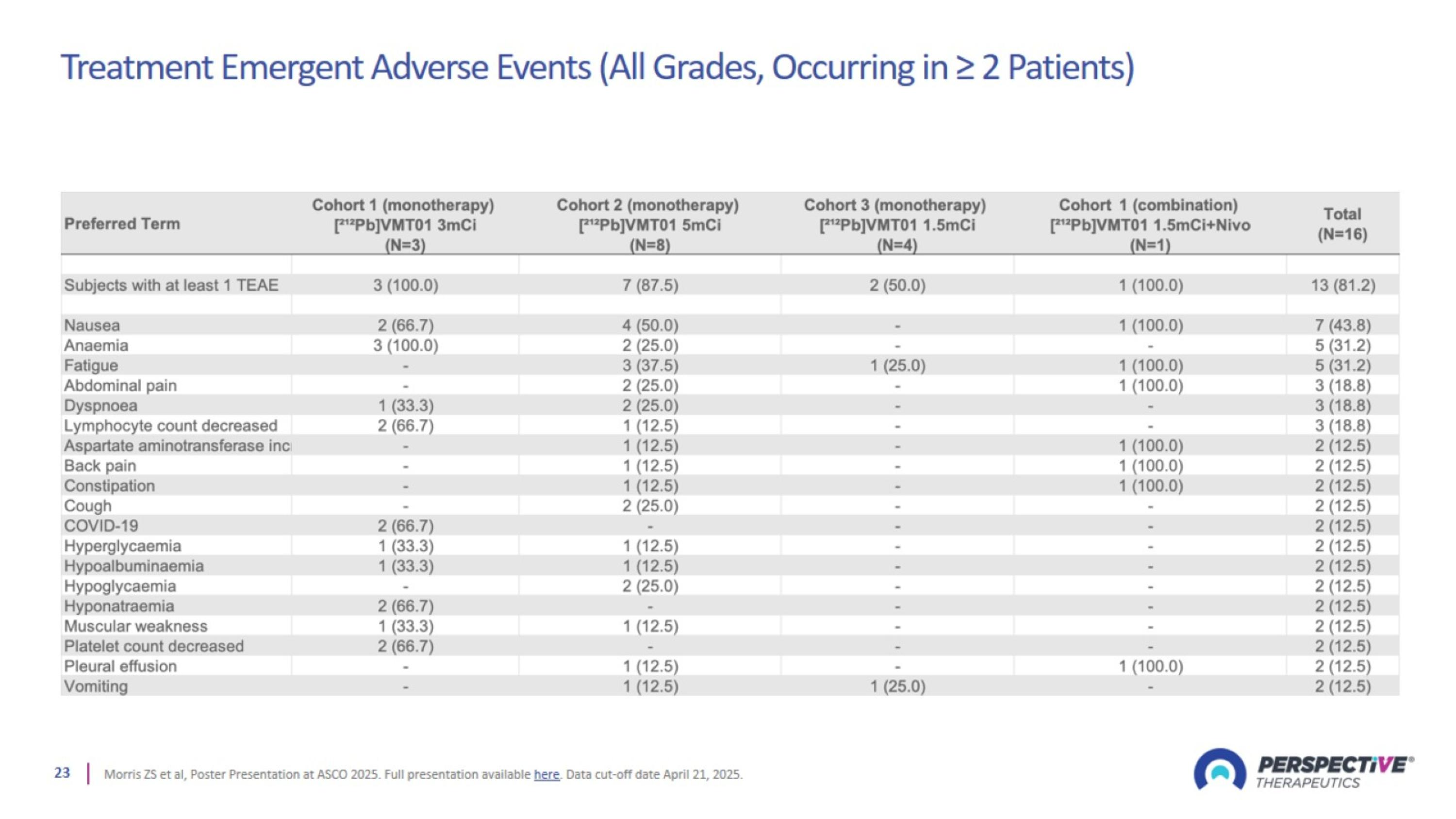
Treatment Emergent Adverse Events (All Grades, Occurring in ≥ 2 Patients)
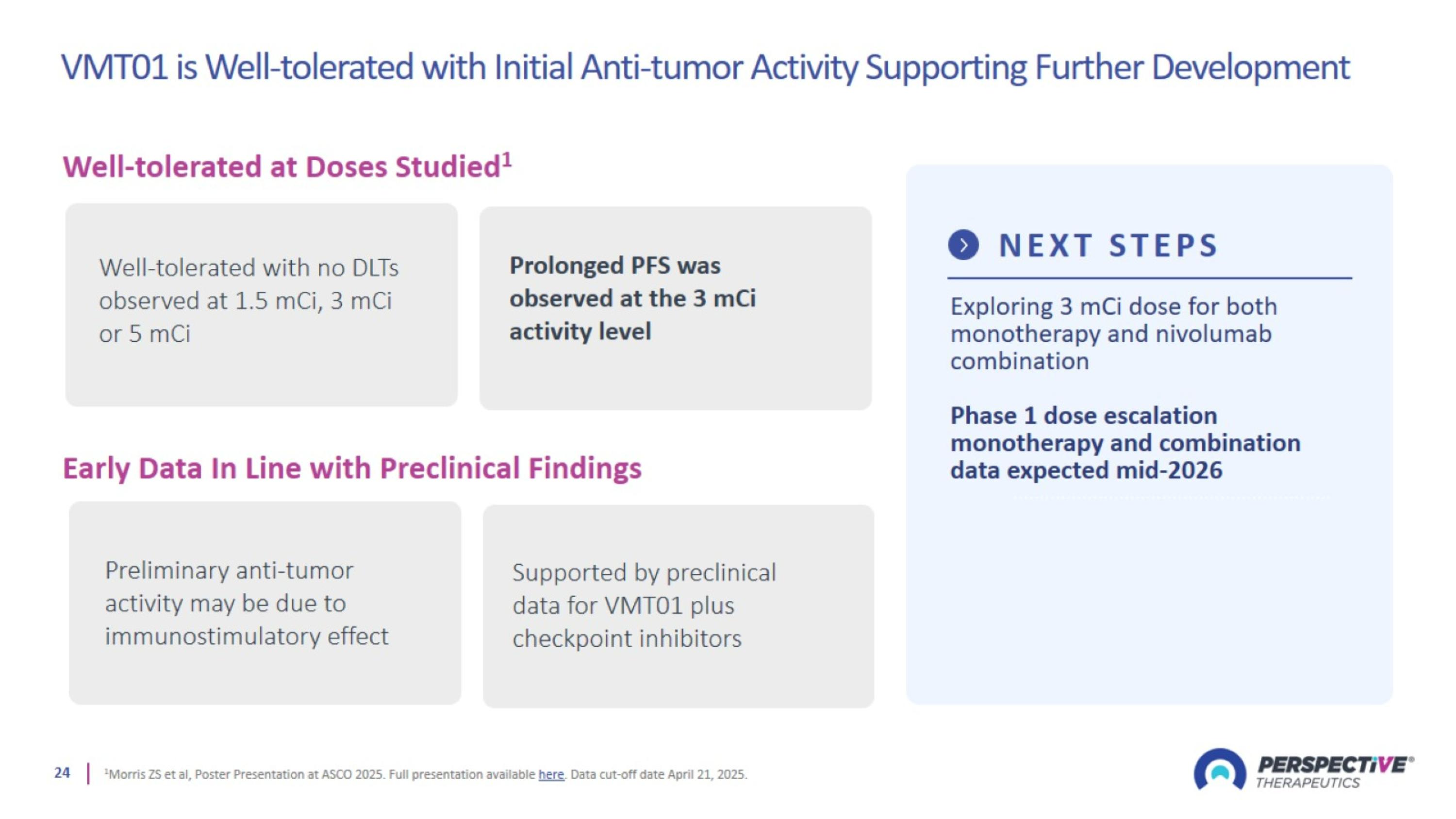
VMT01 is Well-tolerated with Initial Anti-tumor Activity Supporting Further Development
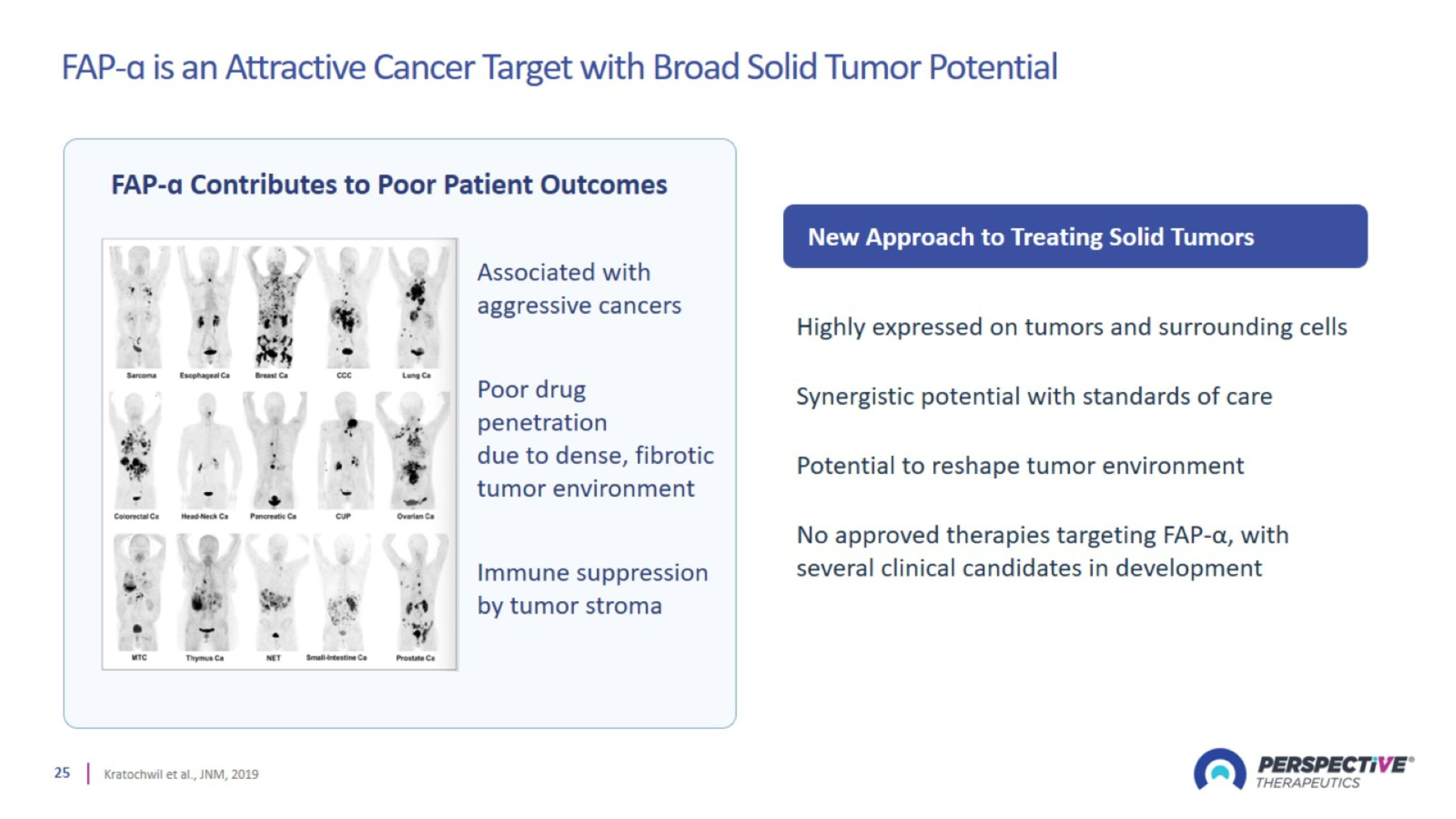
FAP-ɑ is an Attractive Cancer Target with Broad Solid Tumor Potential
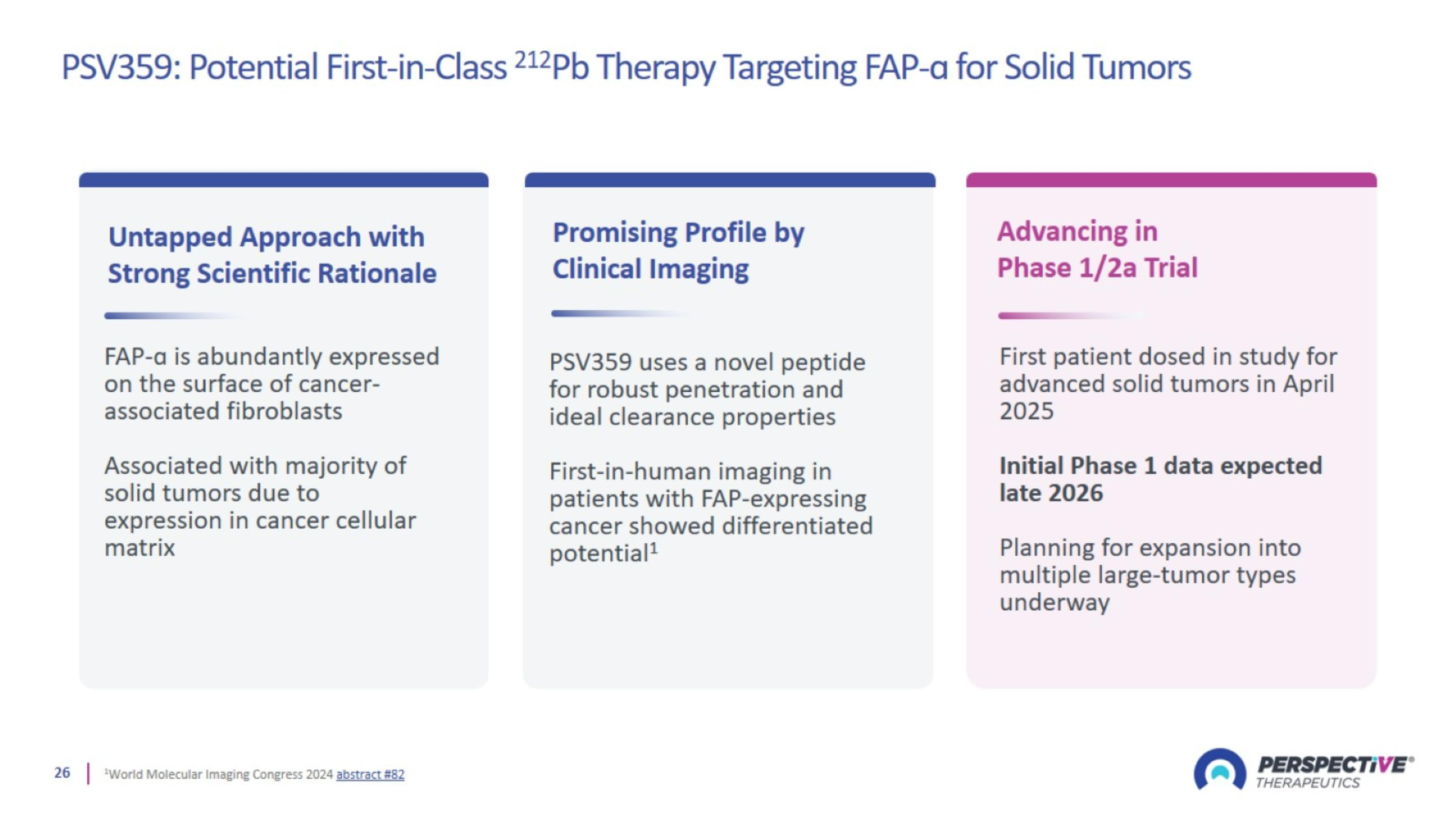
PSV359: Potential First-in-Class 212Pb Therapy Targeting FAP-ɑ for Solid Tumors
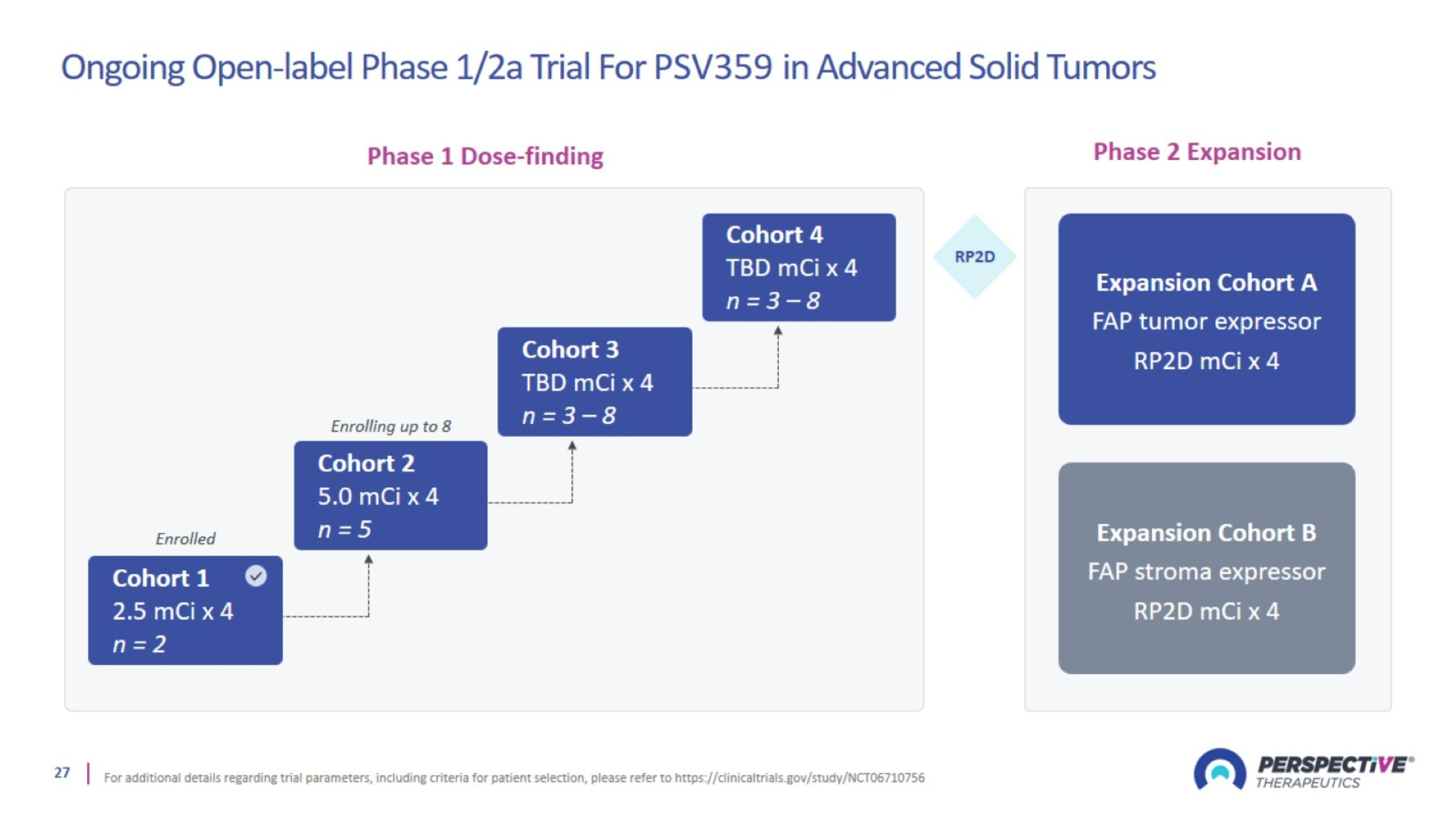
Ongoing Open-label Phase 1/2a Trial For PSV359 in Advanced Solid Tumors
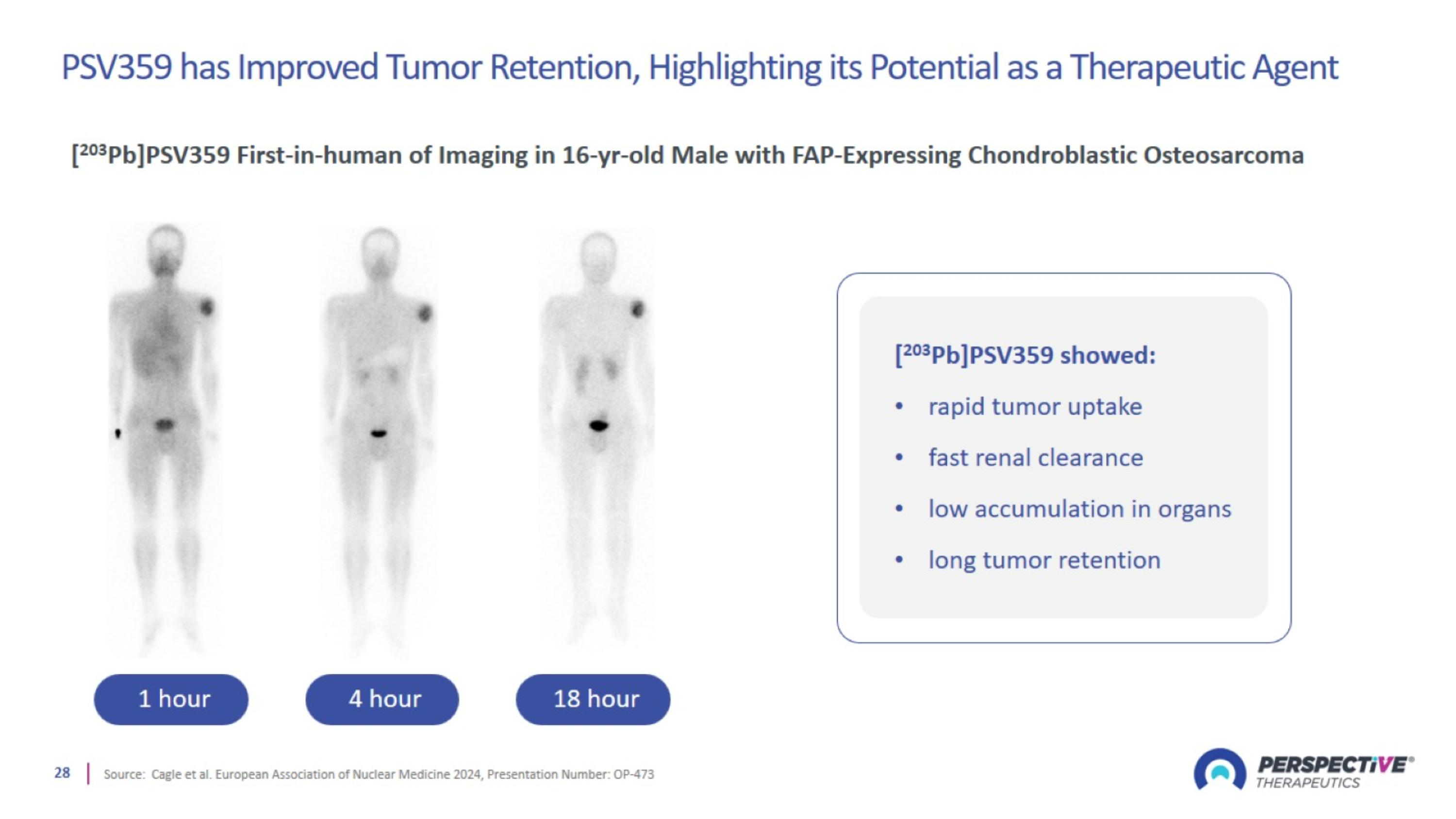
PSV359 has Improved Tumor Retention, Highlighting its Potential as a Therapeutic Agent
Strong IP Portfolio Covering All Aspects of Radiopharmaceutical Value Chain

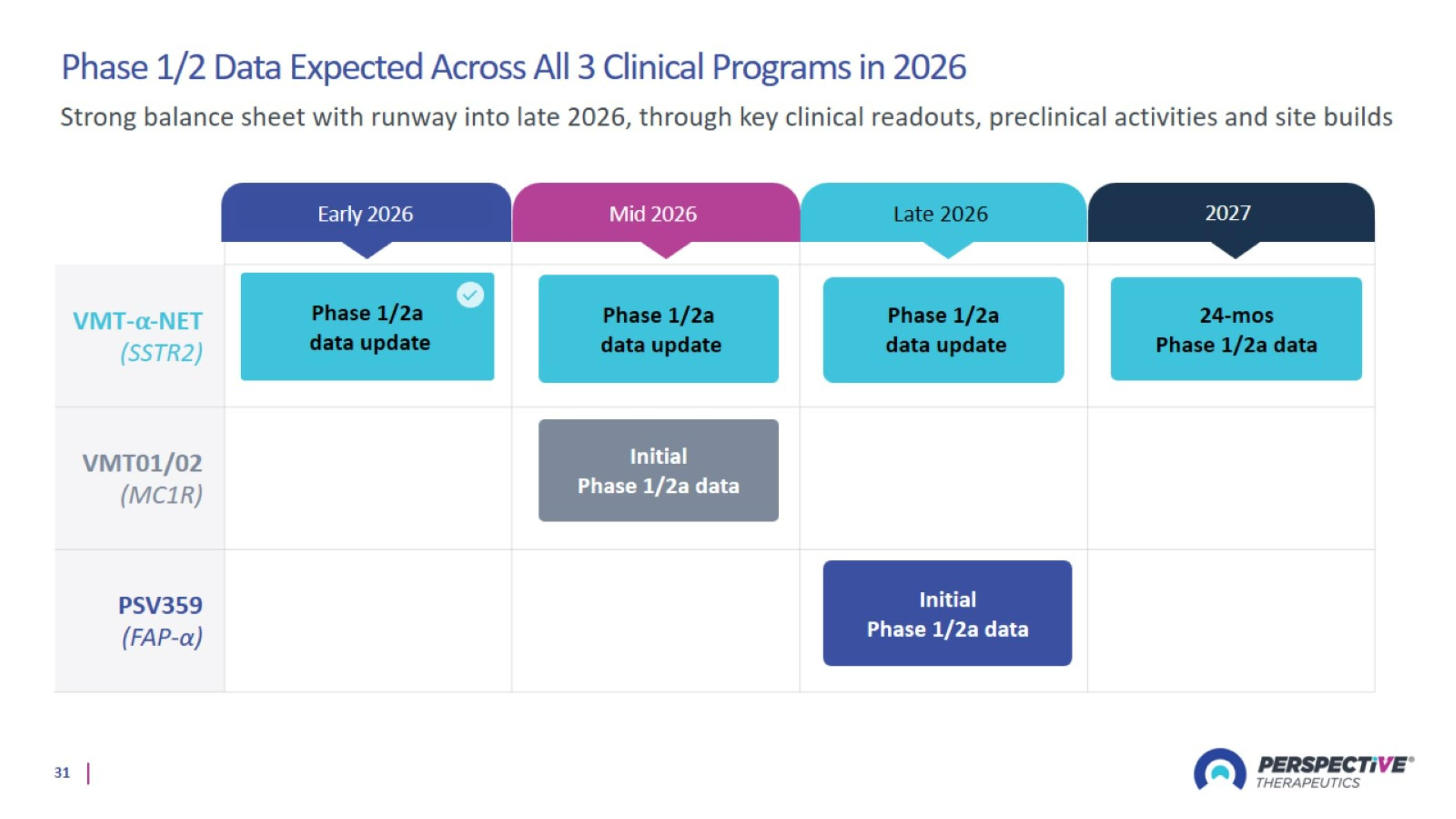
Phase 1/2 Data Expected Across All 3 Clinical Programs in 2026


Abbreviations