Redefining Oncology Treatment with Next-Generation Radiopharmaceuticals

Why Radiotherapy, Why Perspective: Realizing the Untapped Value Across Oncology
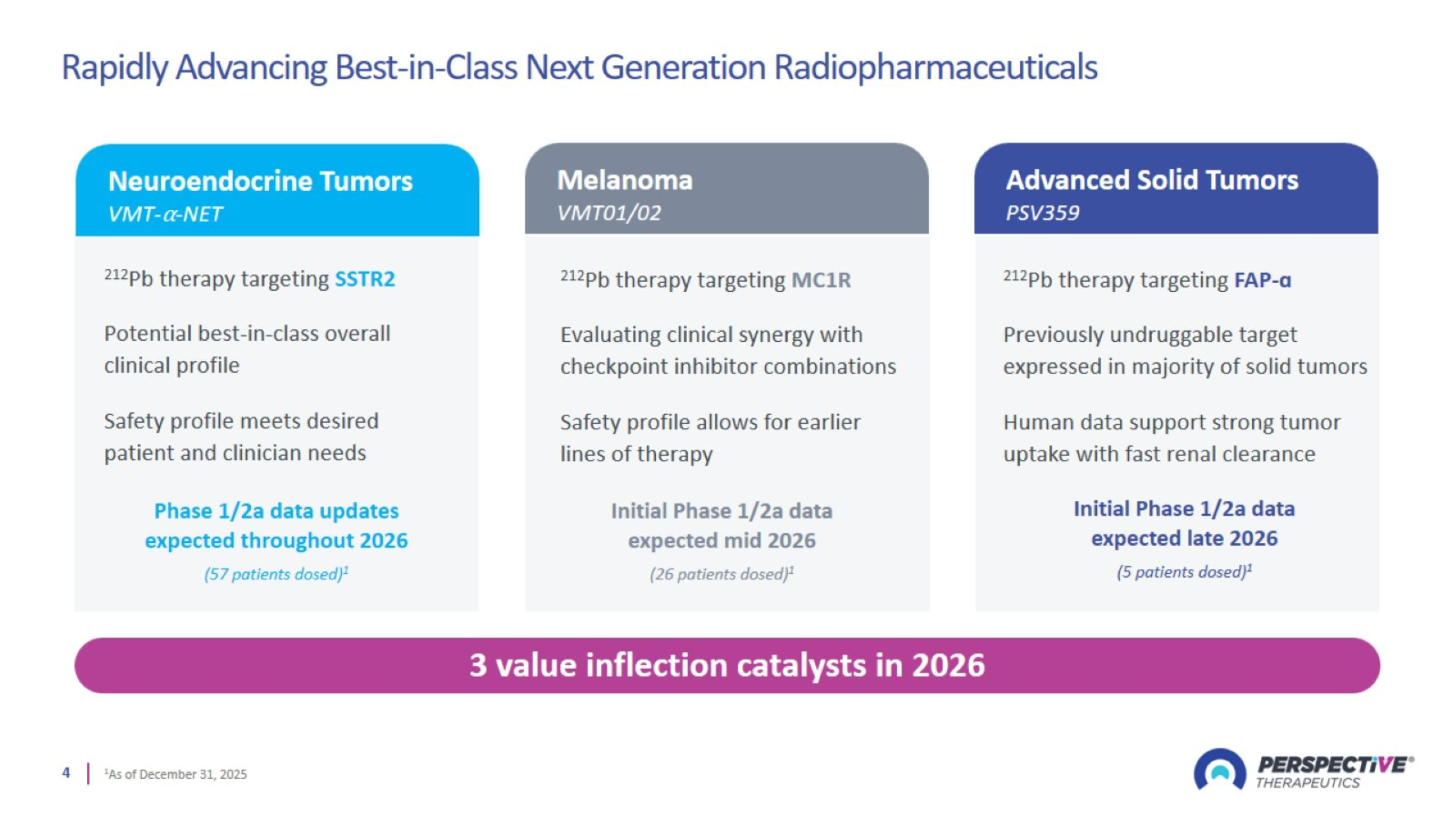
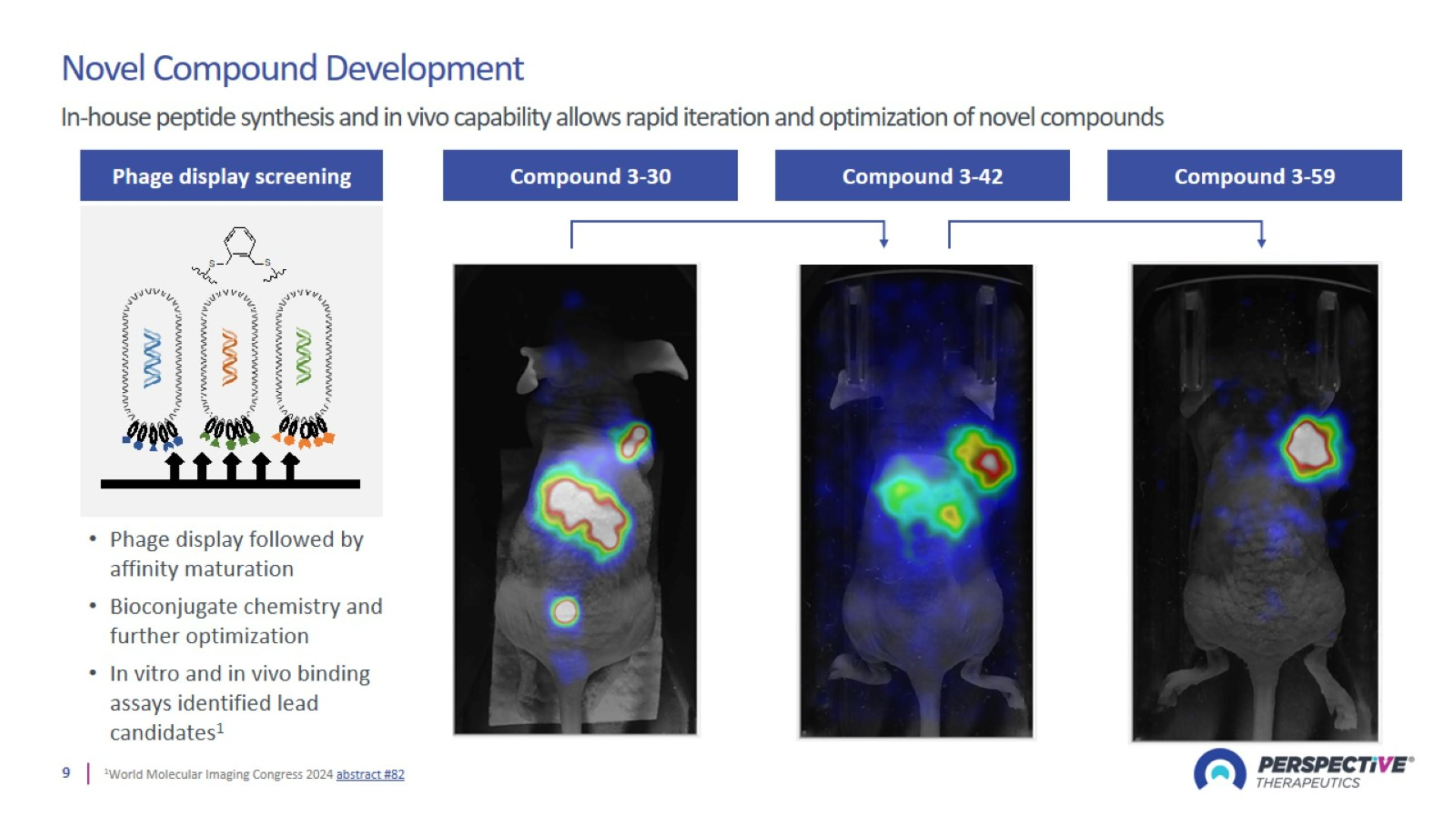
Rapidly Advancing Best-in-Class Next Generation Radiopharmaceuticals
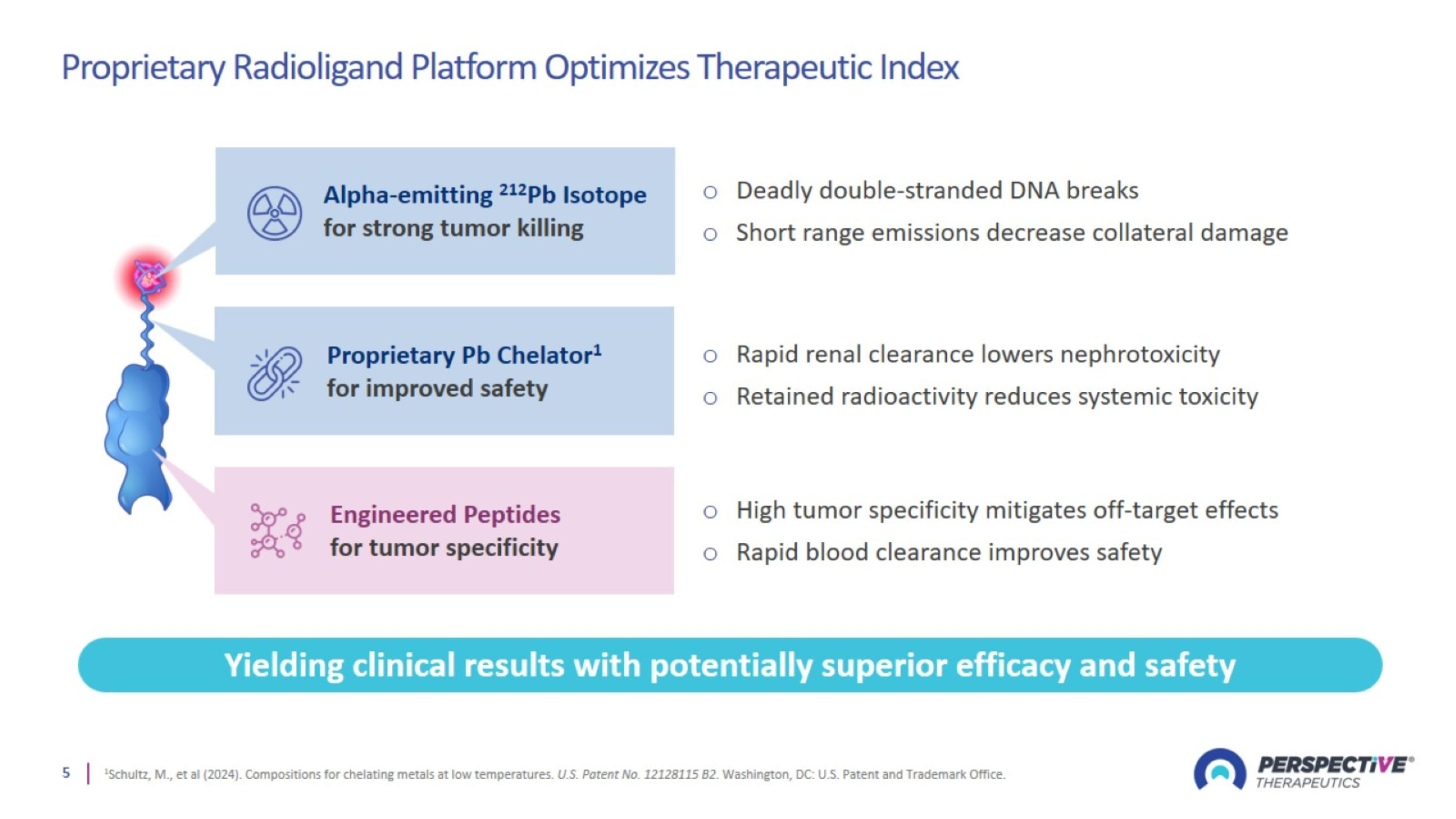
Proprietary Radioligand Platform Optimizes Therapeutic Index
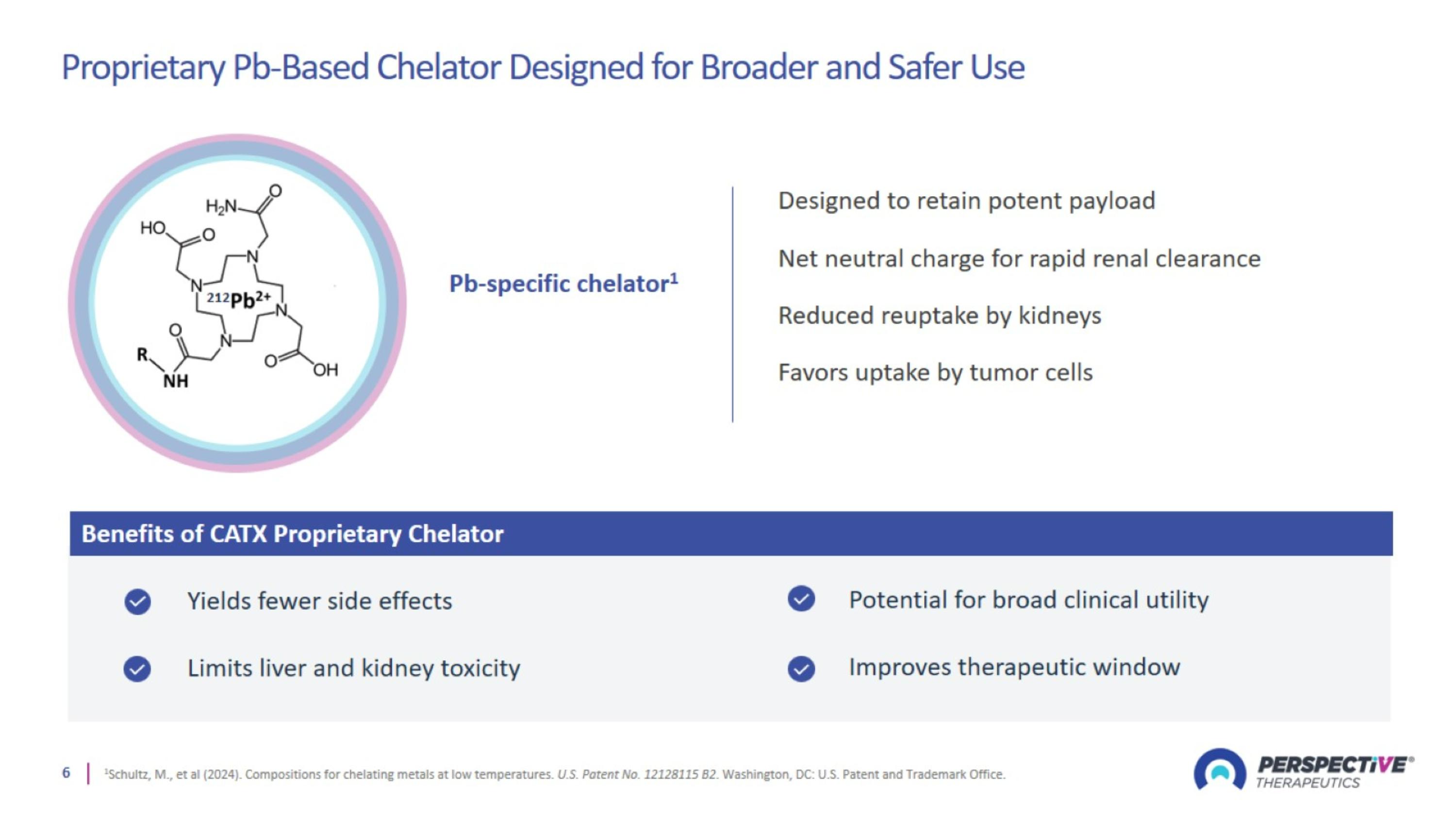
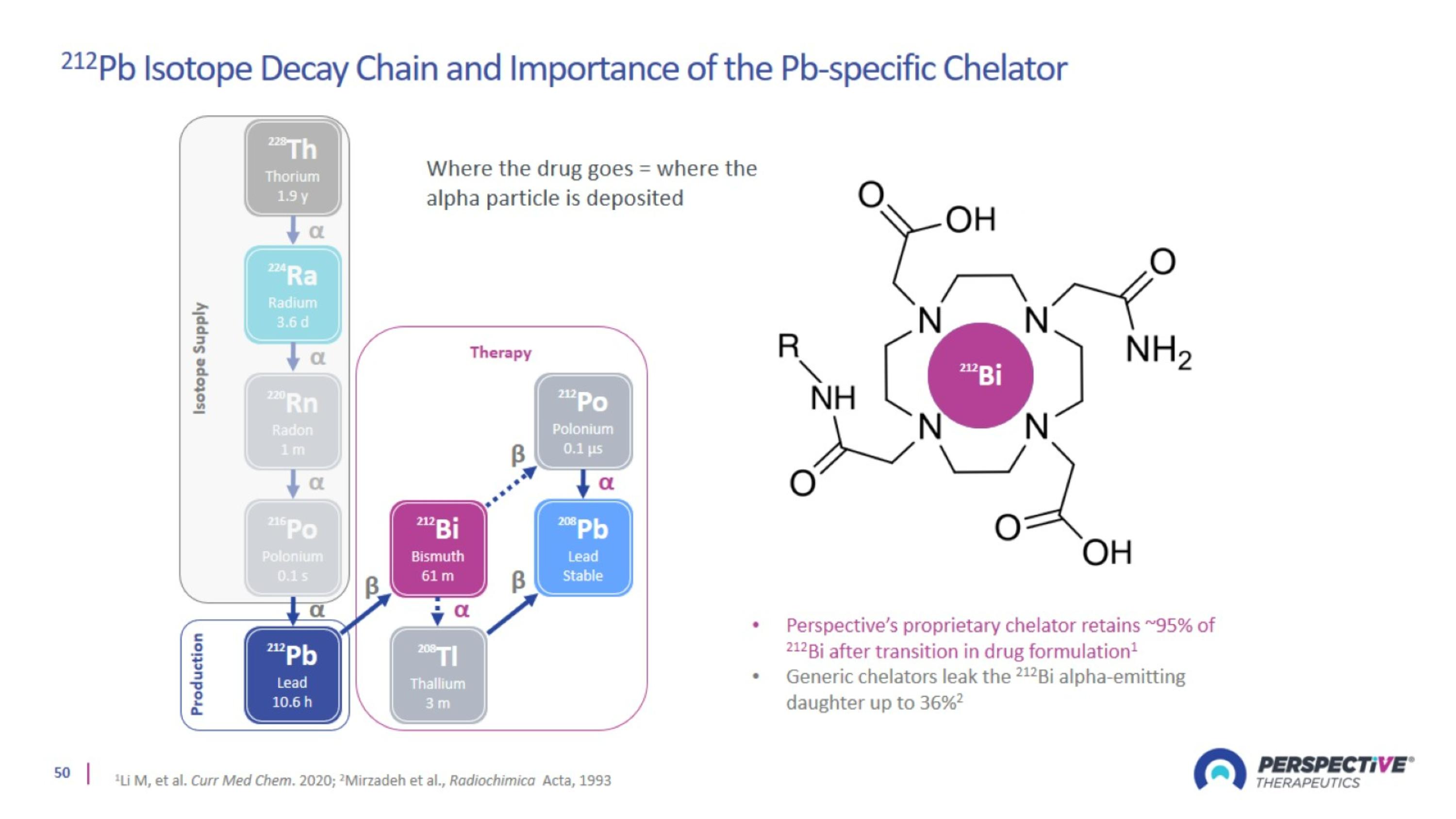
Proprietary Pb-Based Chelator Designed for Broader and Safer Use
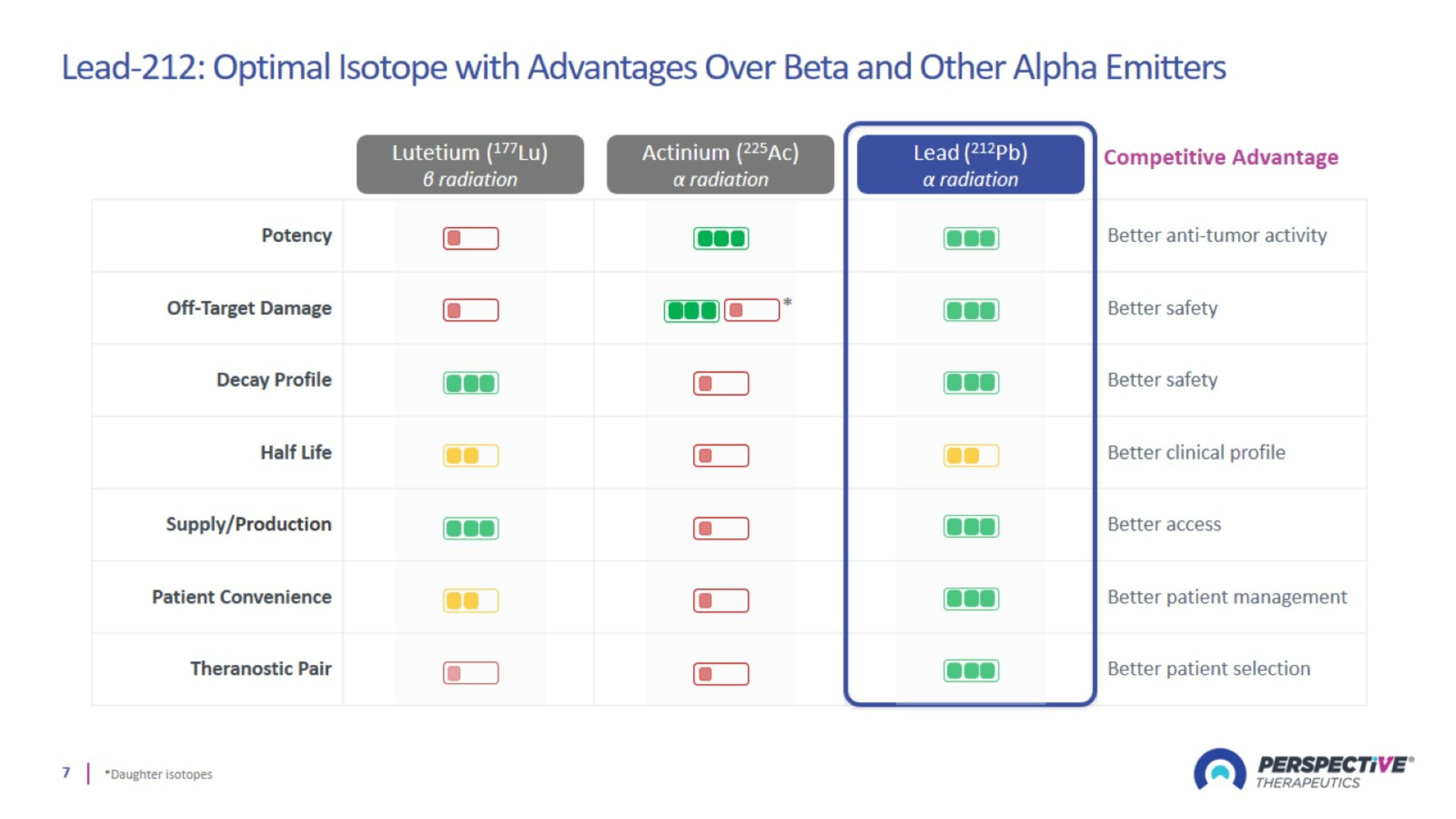
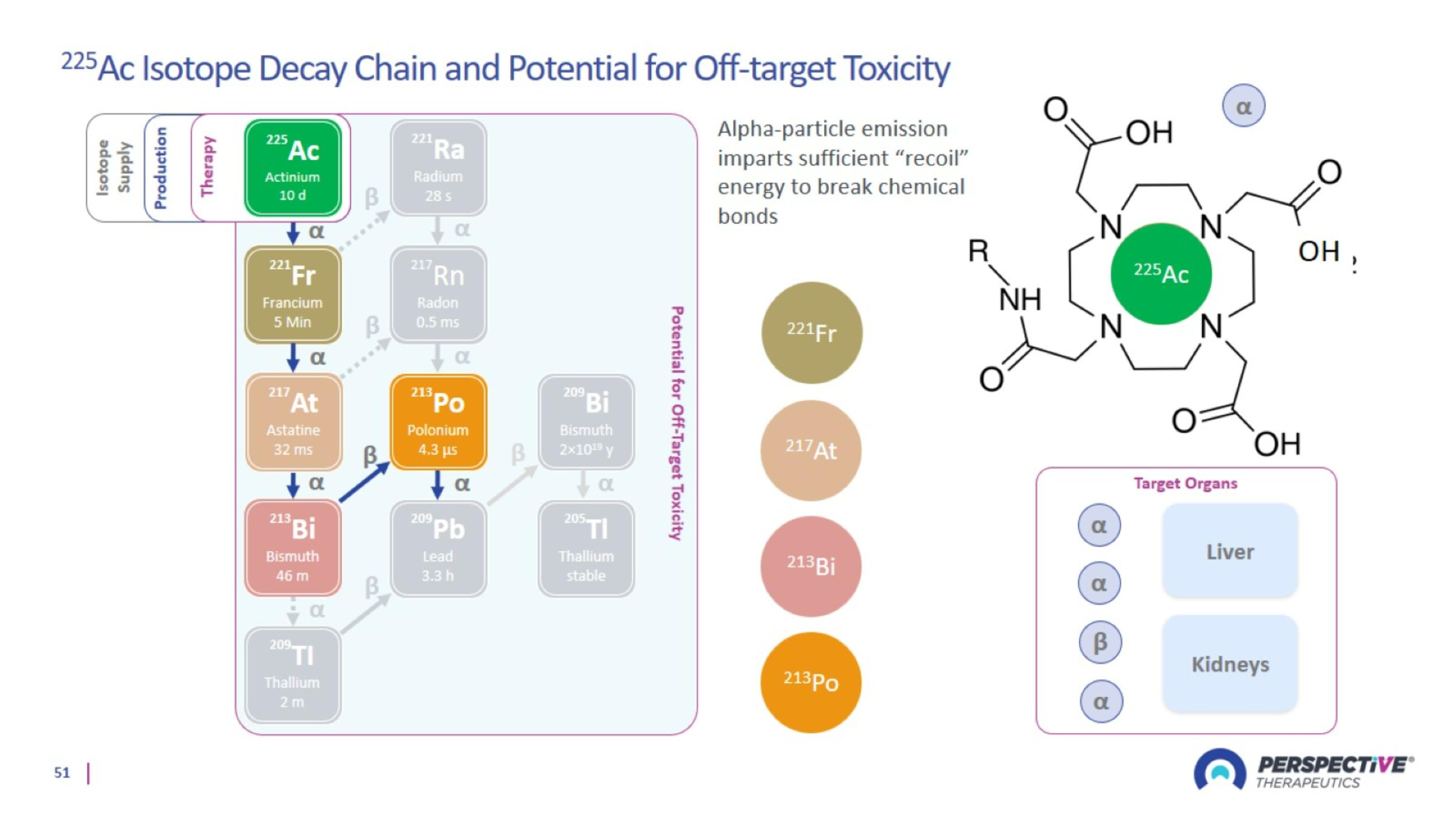
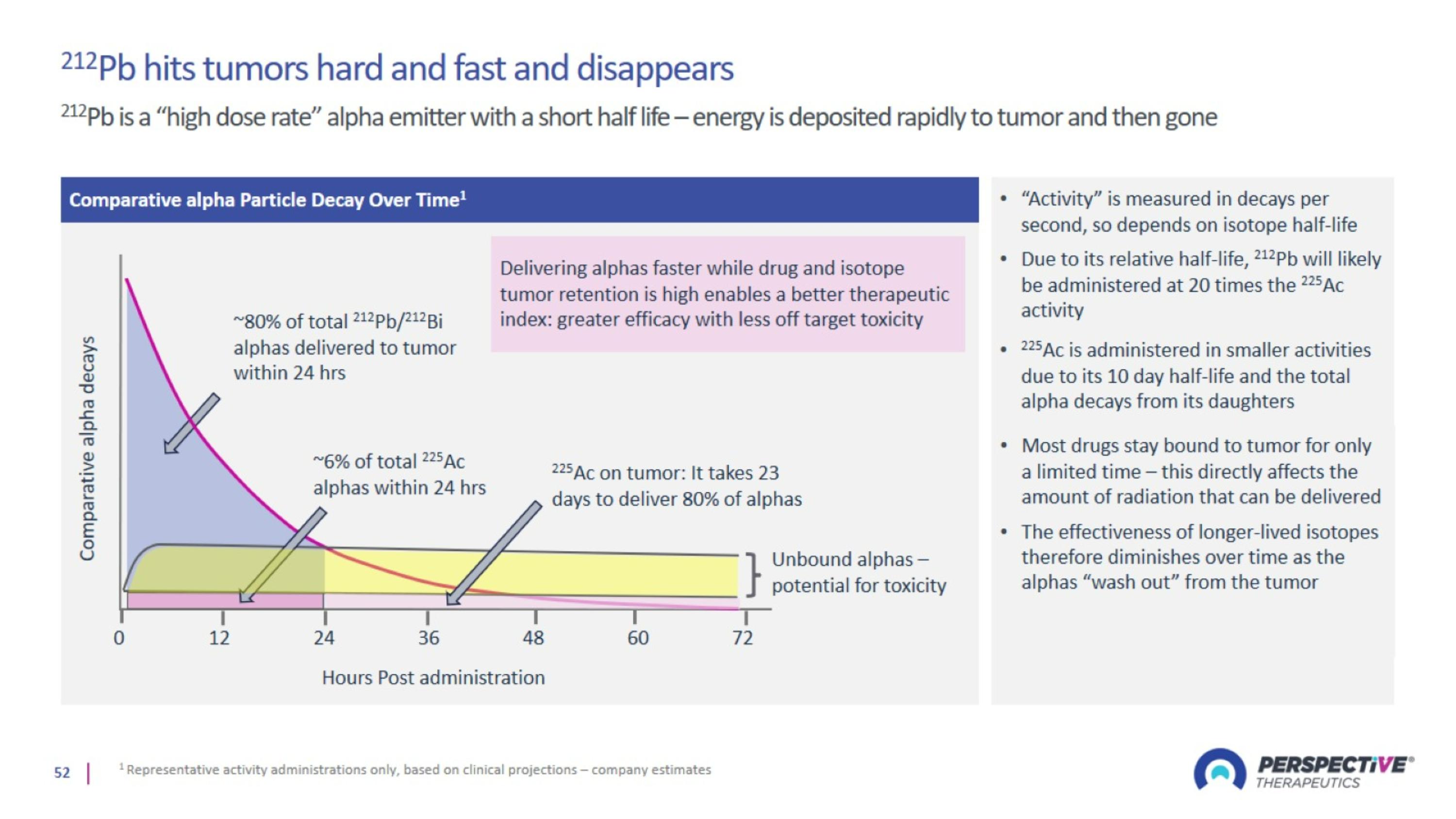
Lead-212: Optimal Isotope with Advantages Over Beta and Other Alpha Emitters
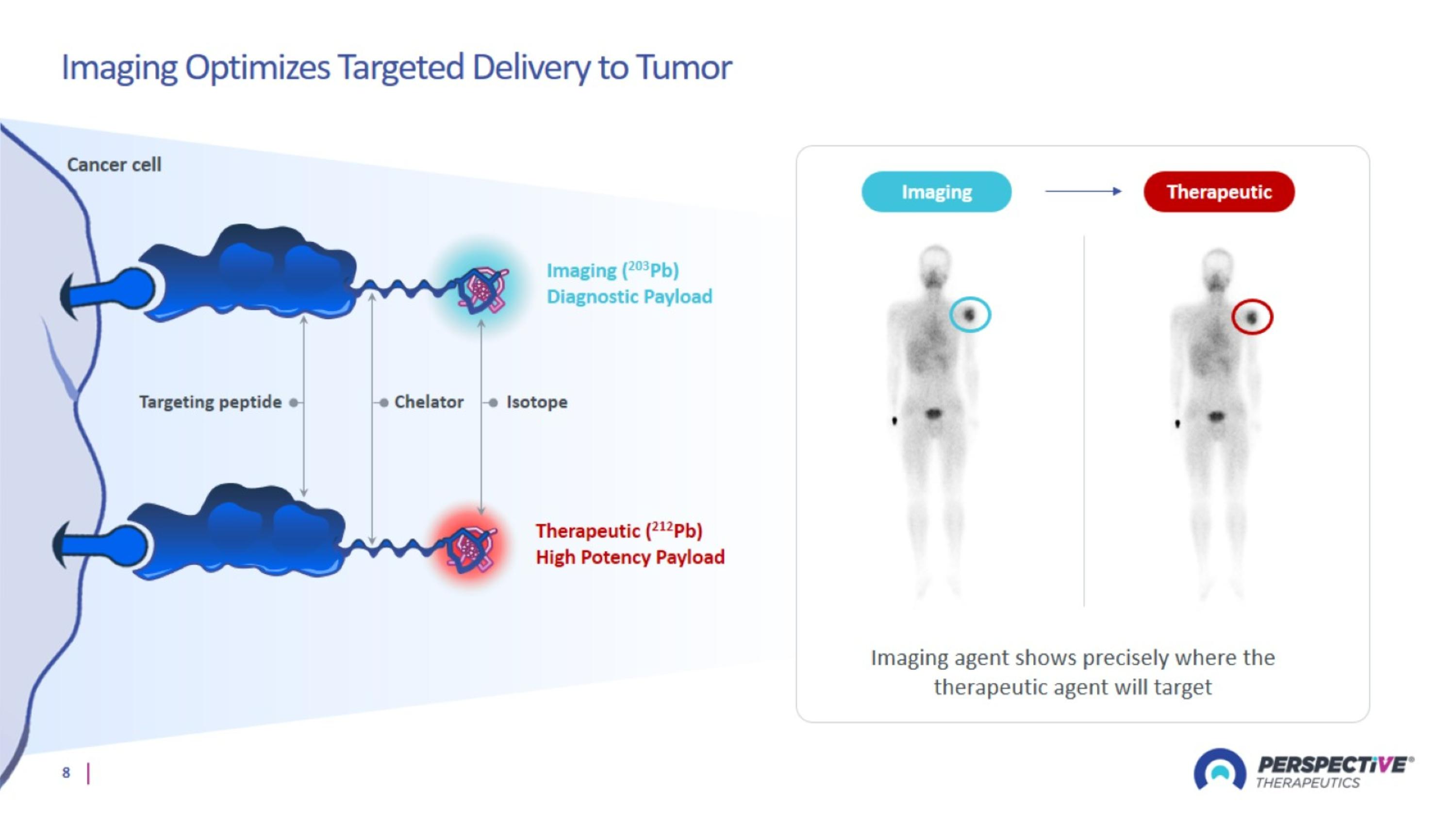
Imaging Optimizes Targeted Delivery to Tumor
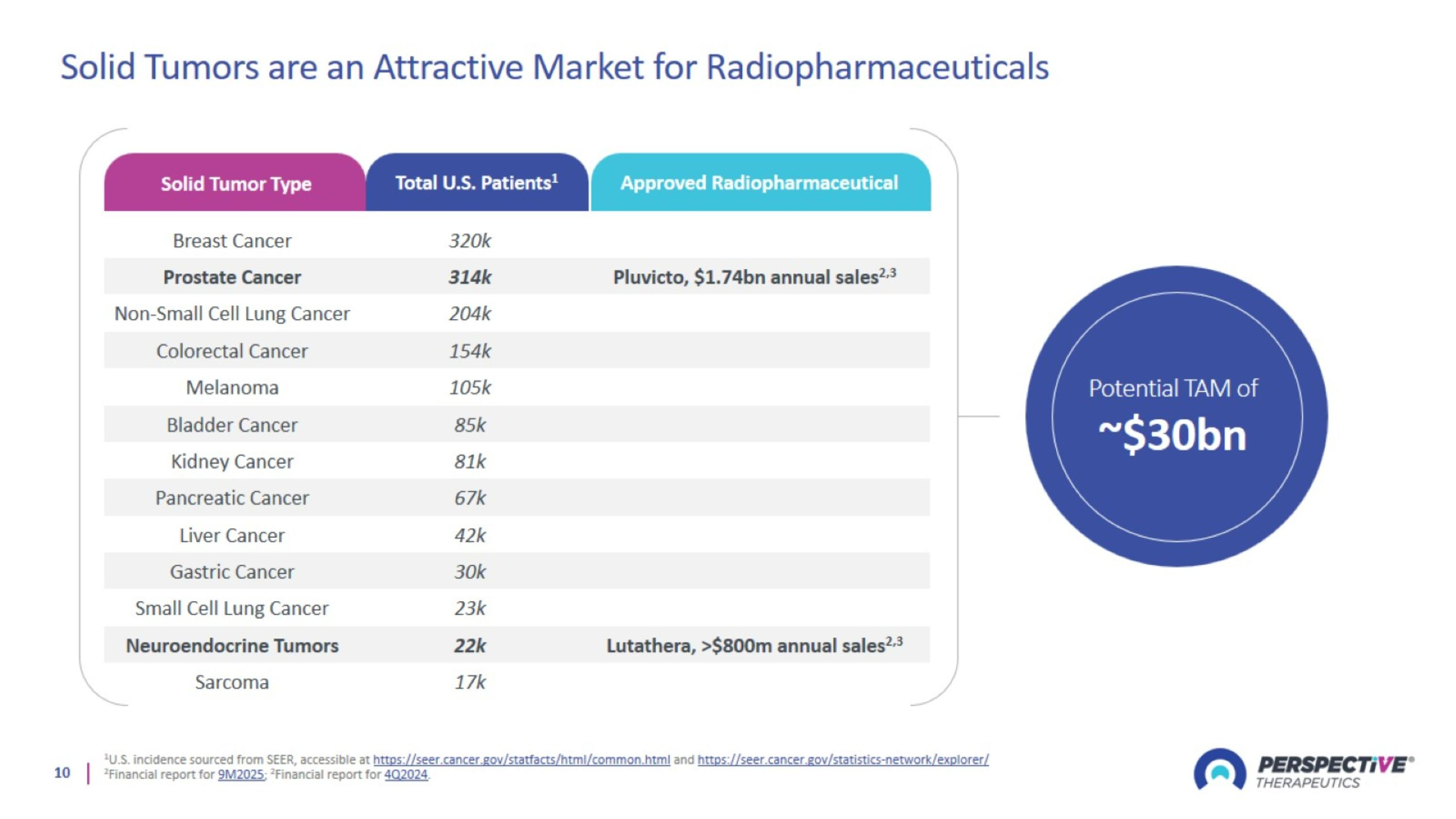
Solid Tumors are an Attractive Market for Radiopharmaceuticals
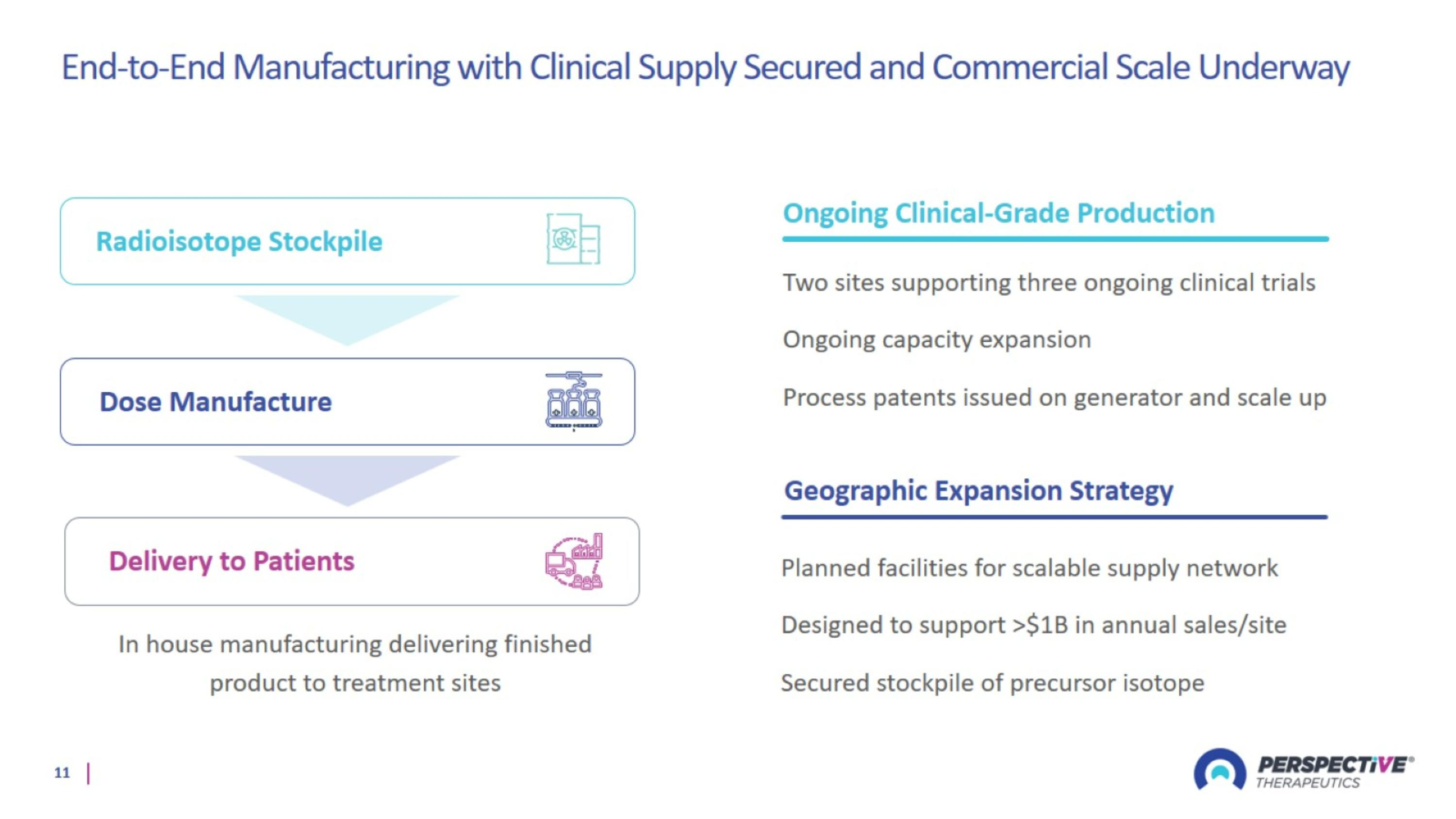
End-to-End Manufacturing with Clinical Supply Secured and Commercial Scale Underway
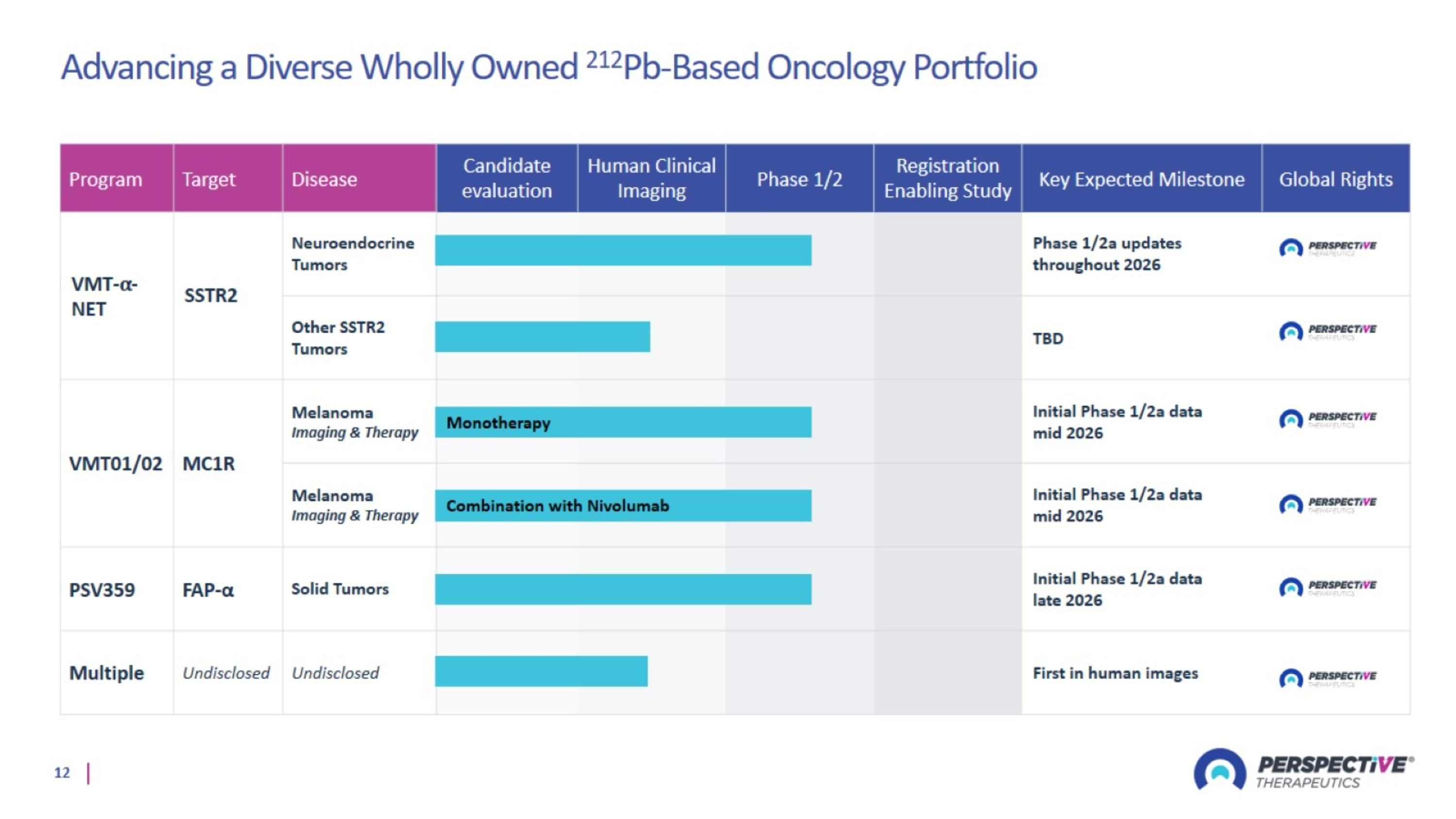
Advancing a Diverse Wholly Owned 212Pb-Based Oncology Portfolio
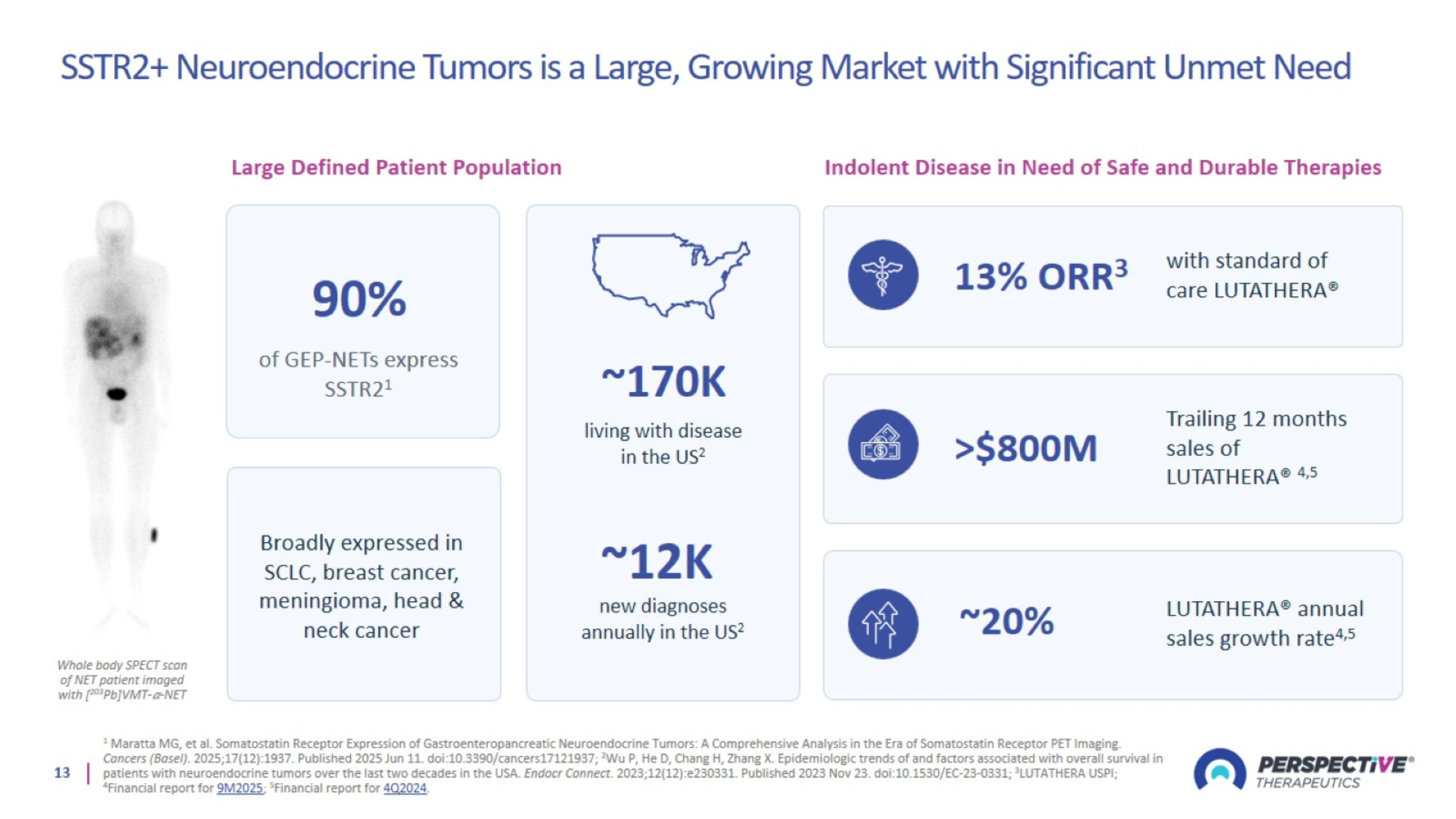
SSTR2+ Neuroendocrine Tumors is a Large, Growing Market with Significant Unmet Need
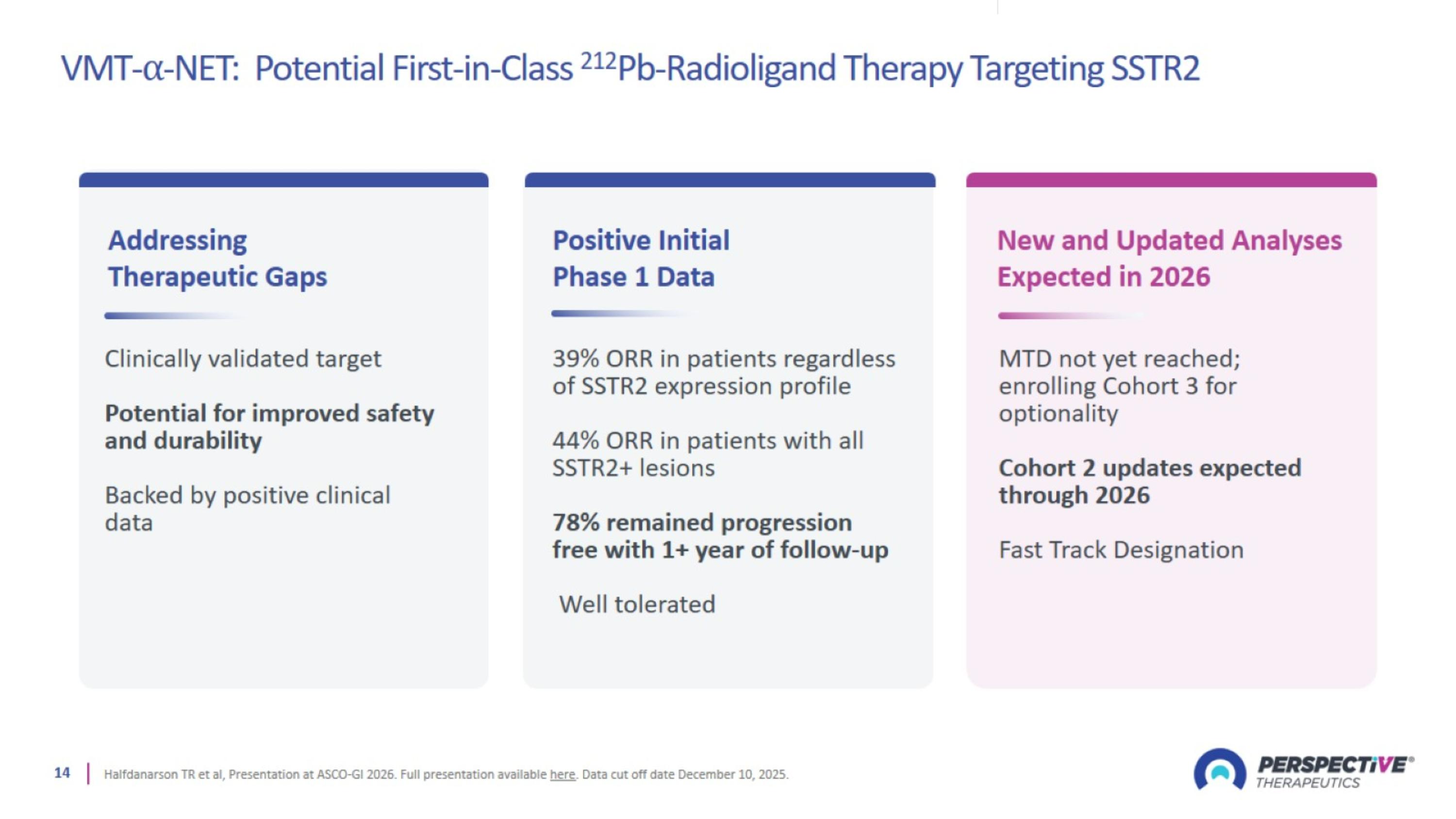
VMT-⍺-NET: Potential First-in-Class 212Pb-Radioligand Therapy Targeting SSTR2
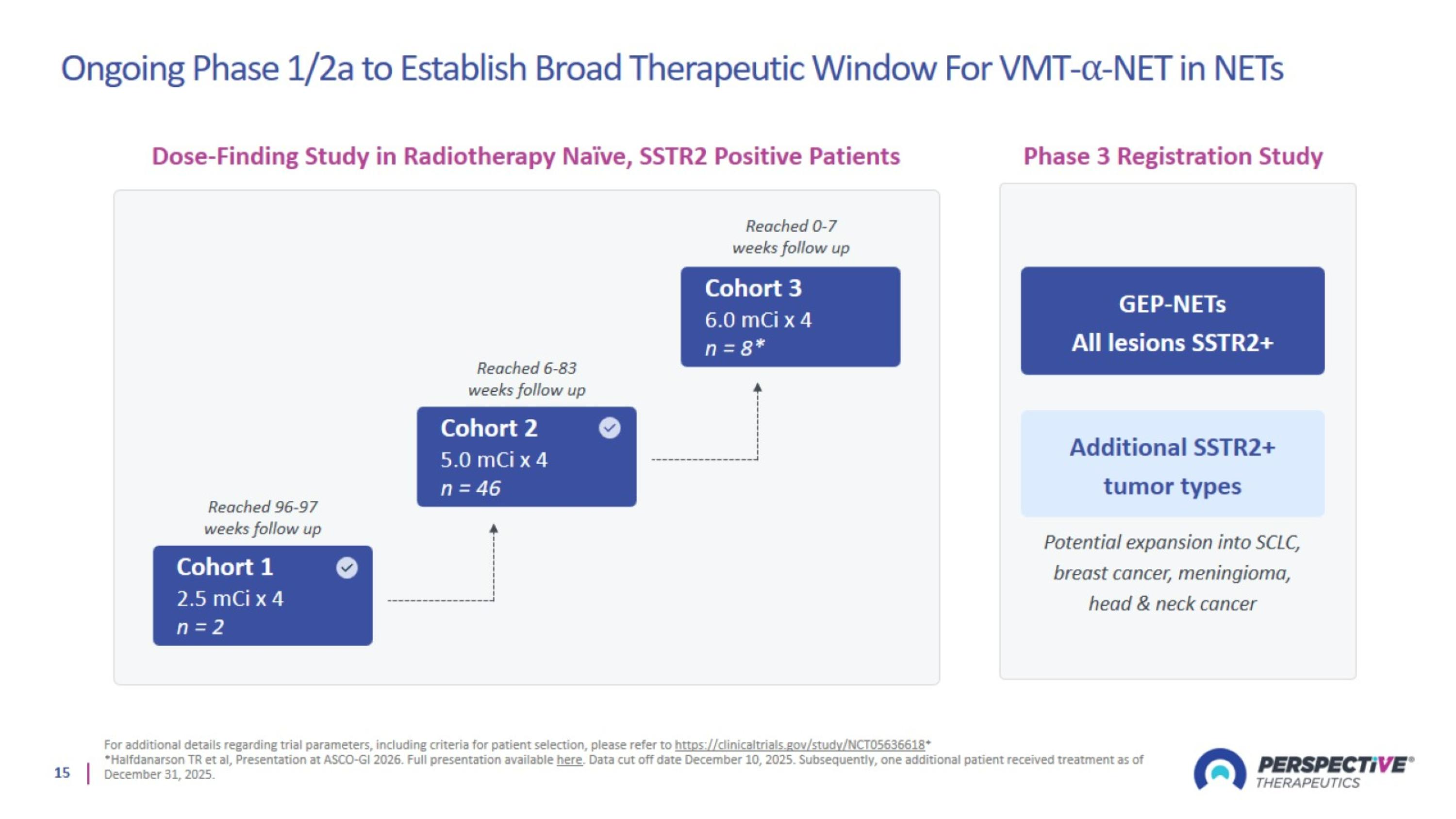
Ongoing Phase 1/2a to Establish Broad Therapeutic Window For VMT-⍺-NET in NETs
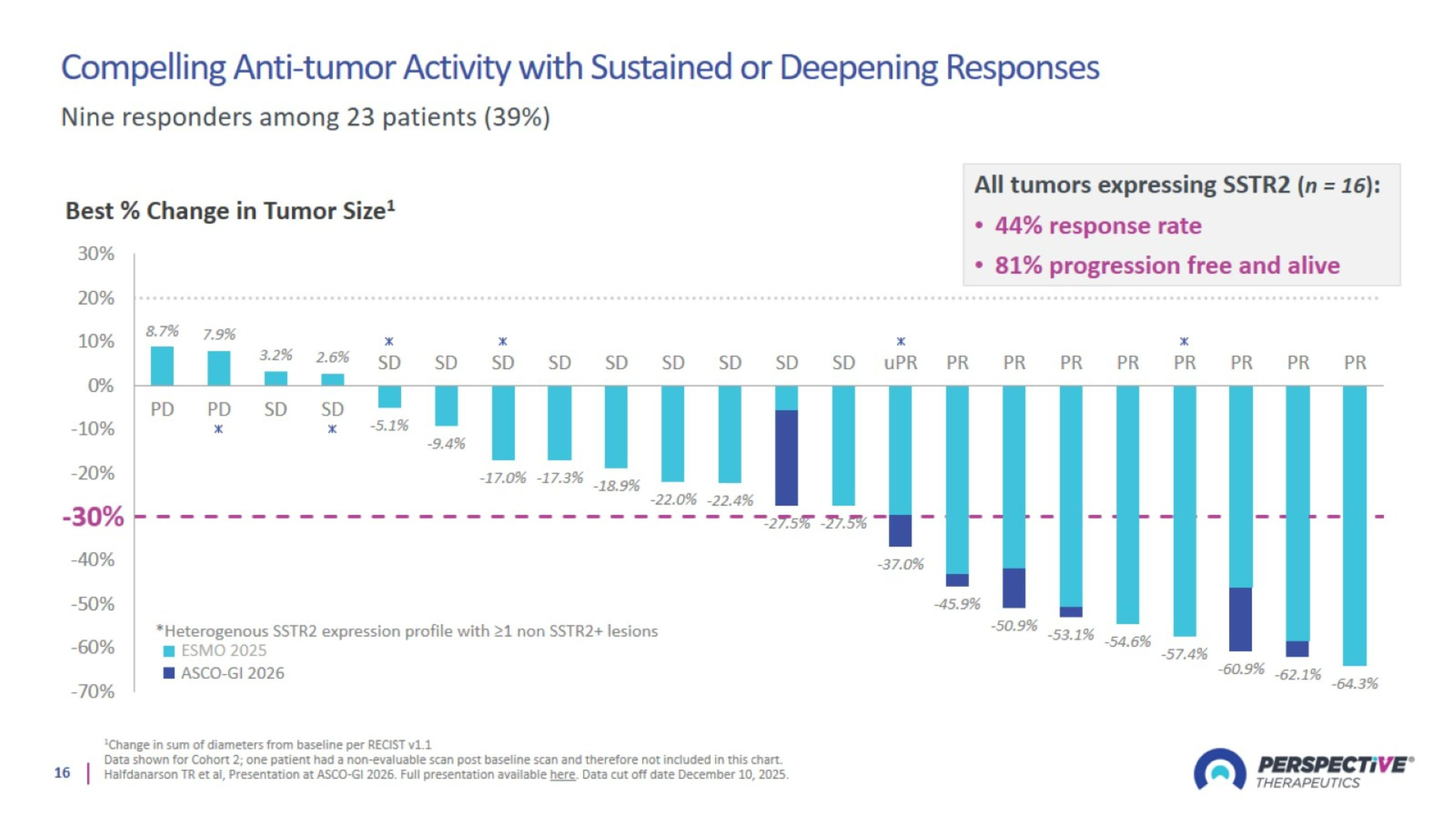
Compelling Anti-tumor Activity with Sustained or Deepening Responses
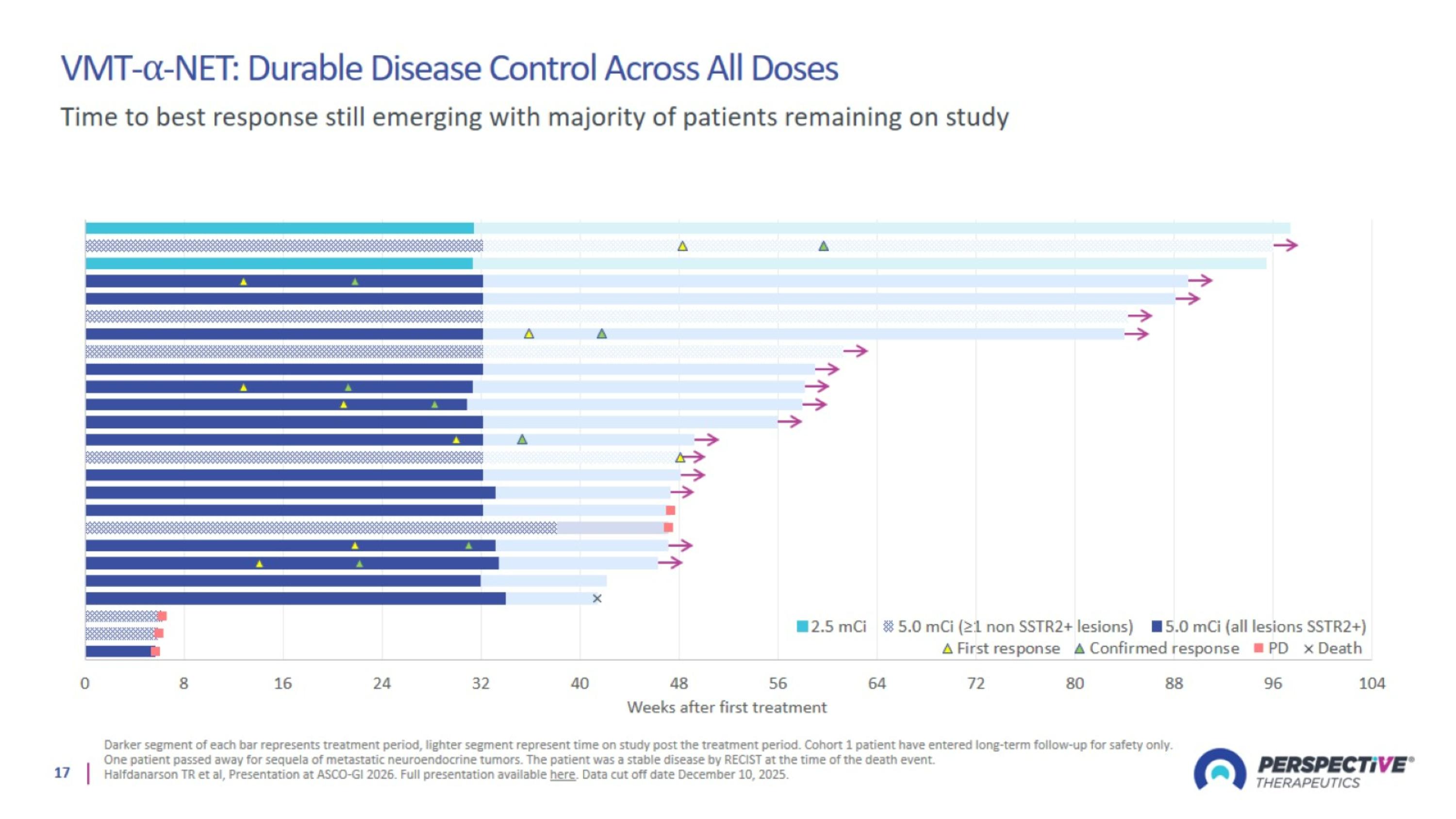
VMT-⍺-NET: Durable Disease Control Across All Doses
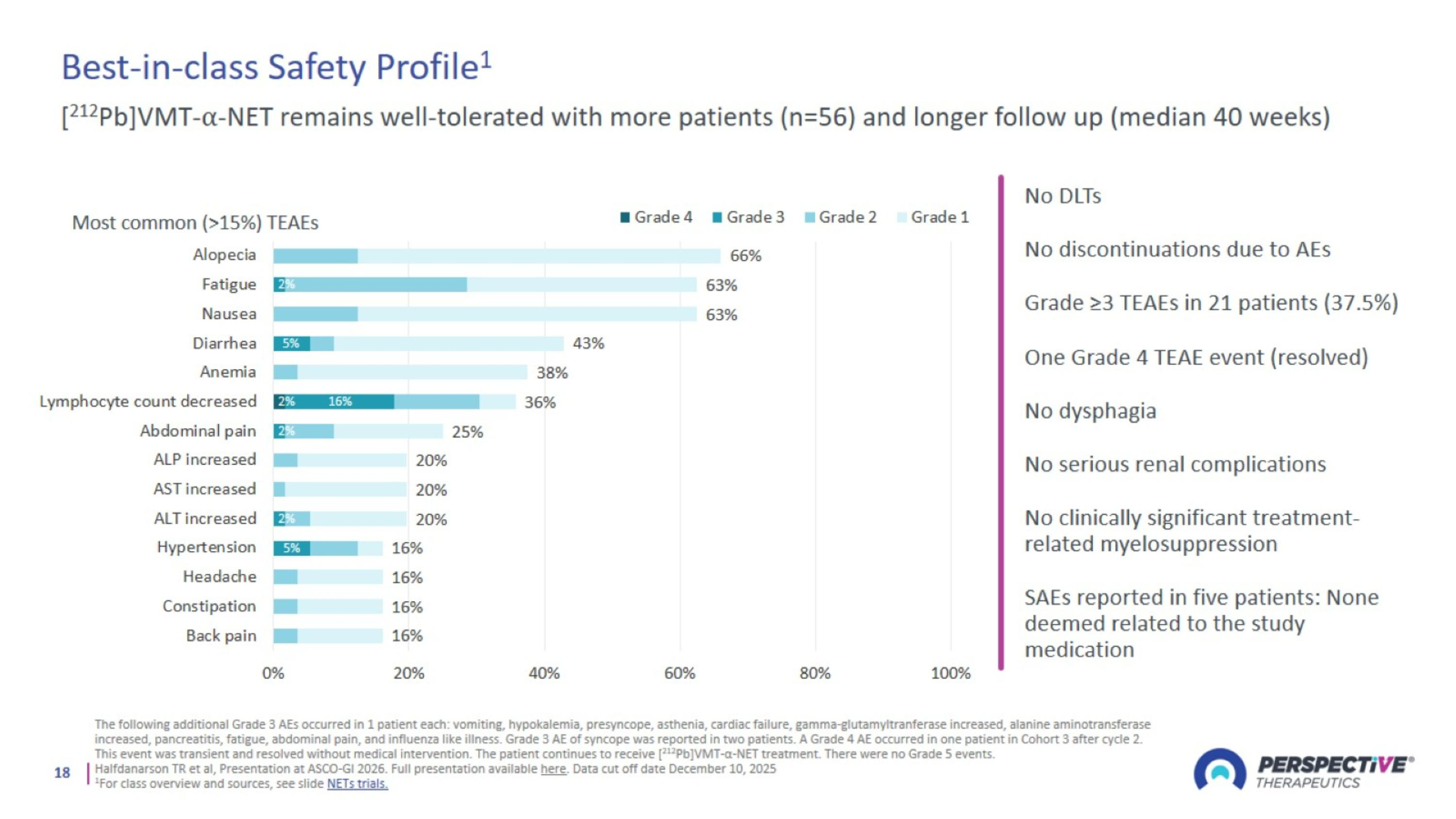
Best-in-class Safety Profile1
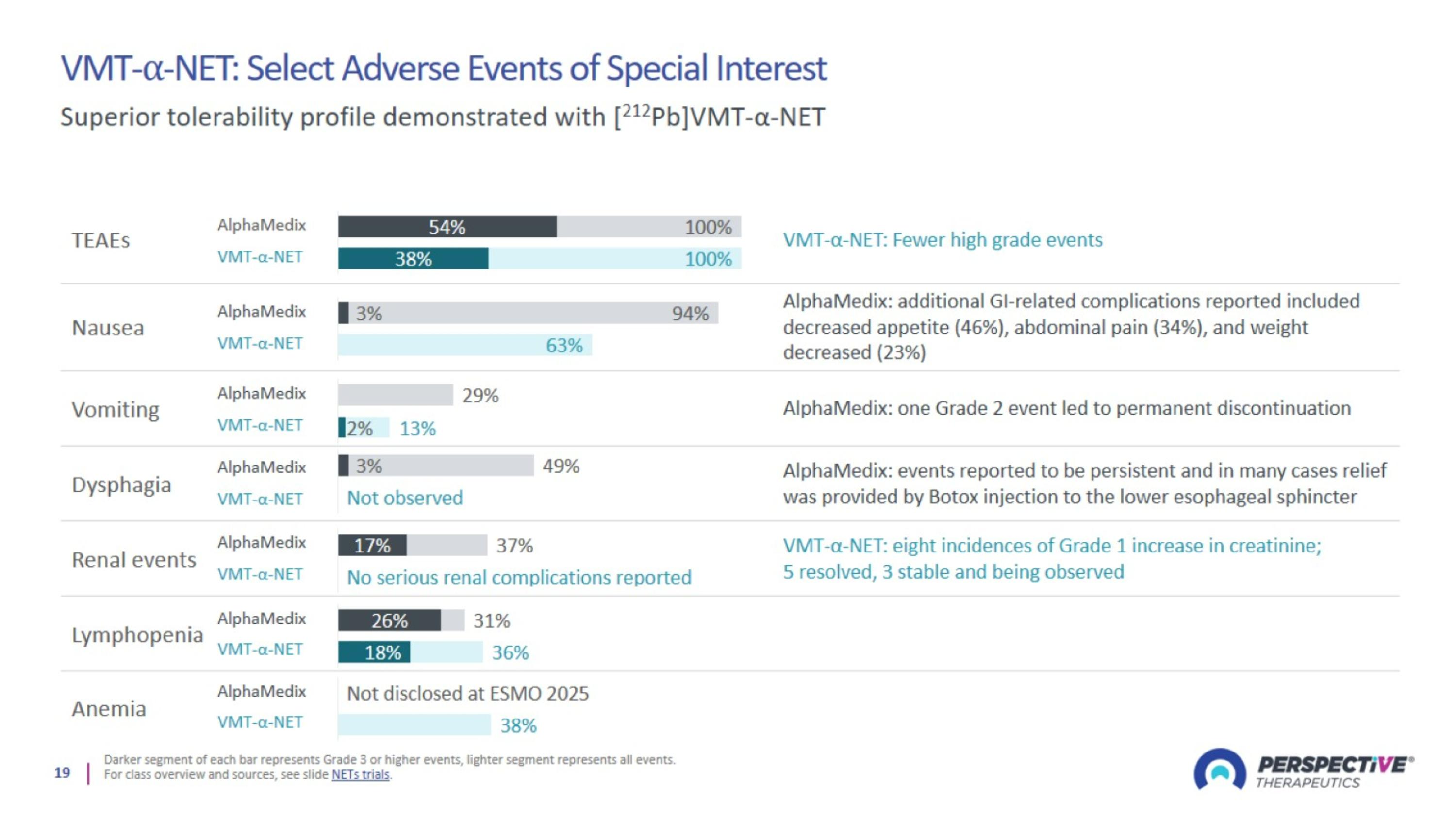
VMT-⍺-NET: Select Adverse Events of Special Interest
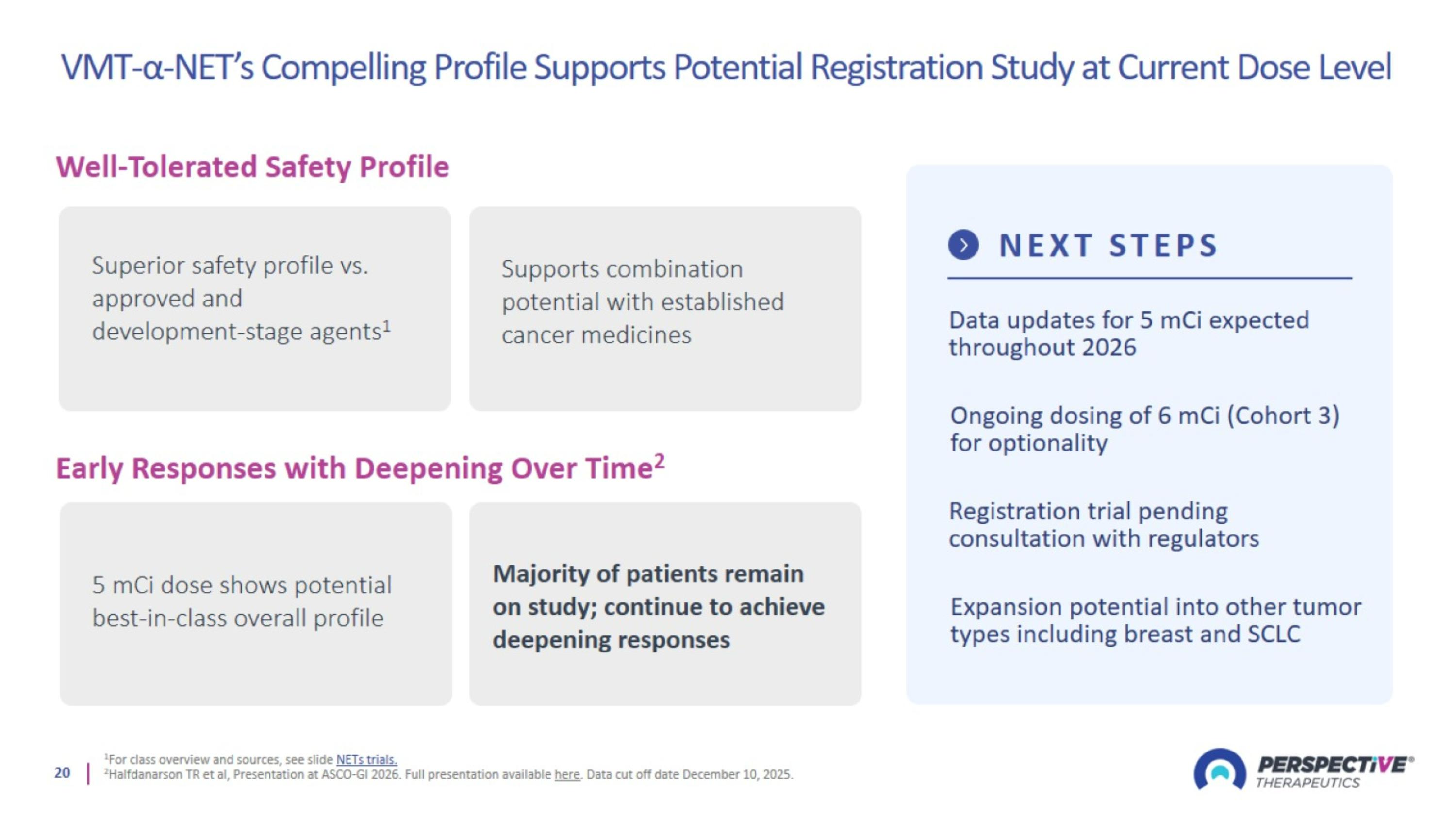
VMT-α-NET’s Compelling Profile Supports Potential Registration Study at Current Dose Level
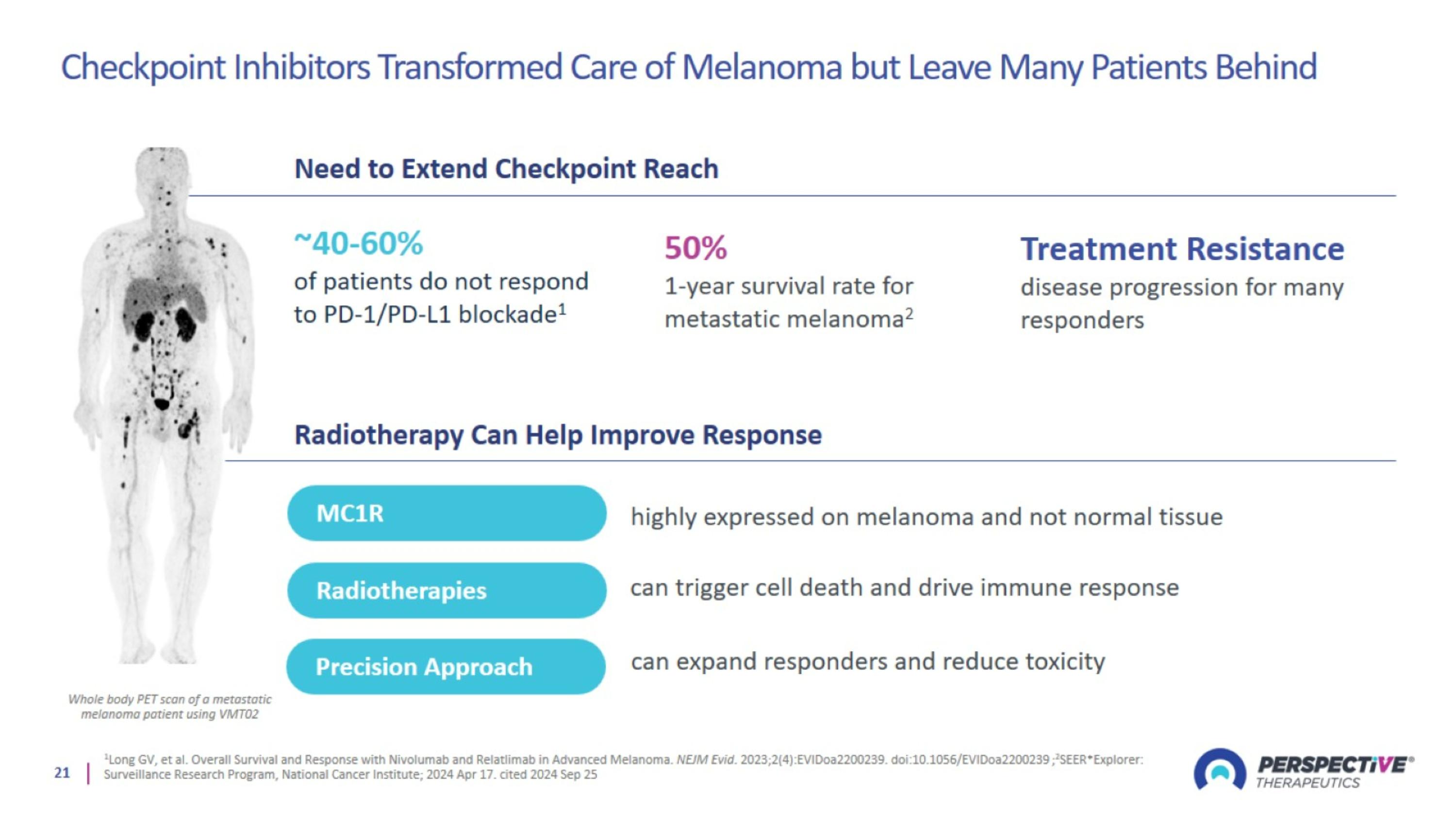
Checkpoint Inhibitors Transformed Care of Melanoma but Leave Many Patients Behind
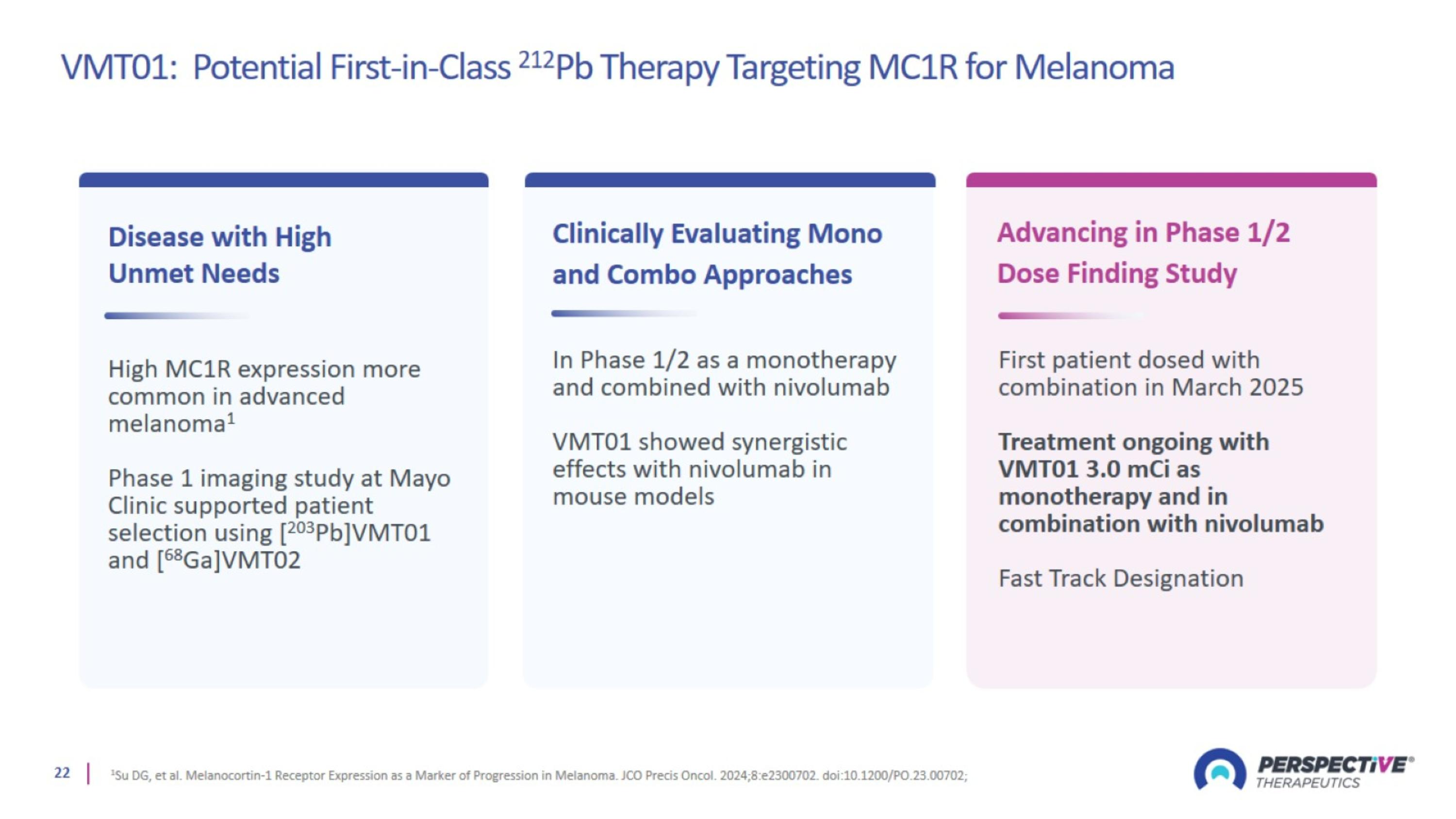
VMT01: Potential First-in-Class 212Pb Therapy Targeting MC1R for Melanoma
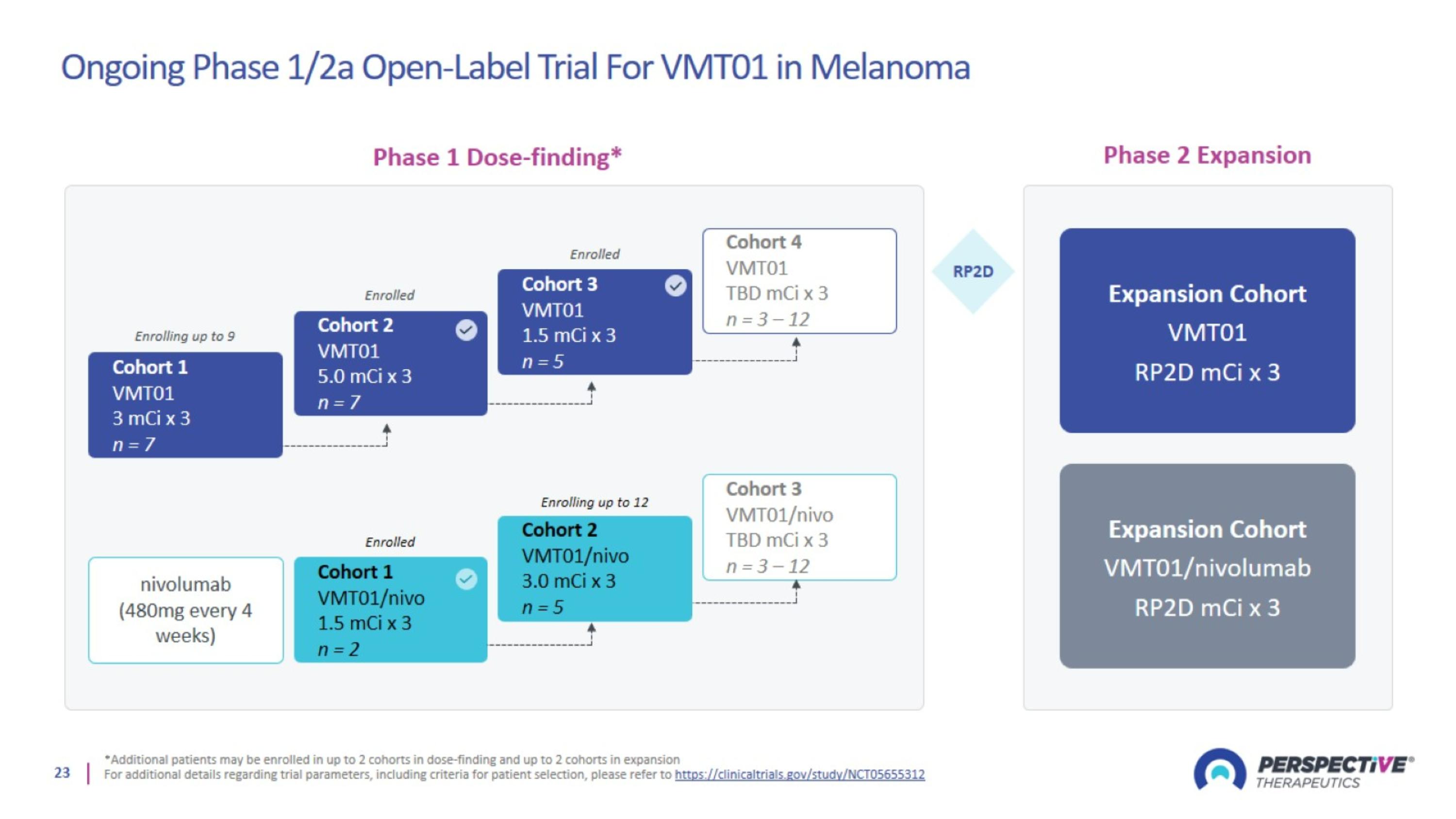
Ongoing Phase 1/2a Open-Label Trial For VMT01 in Melanoma
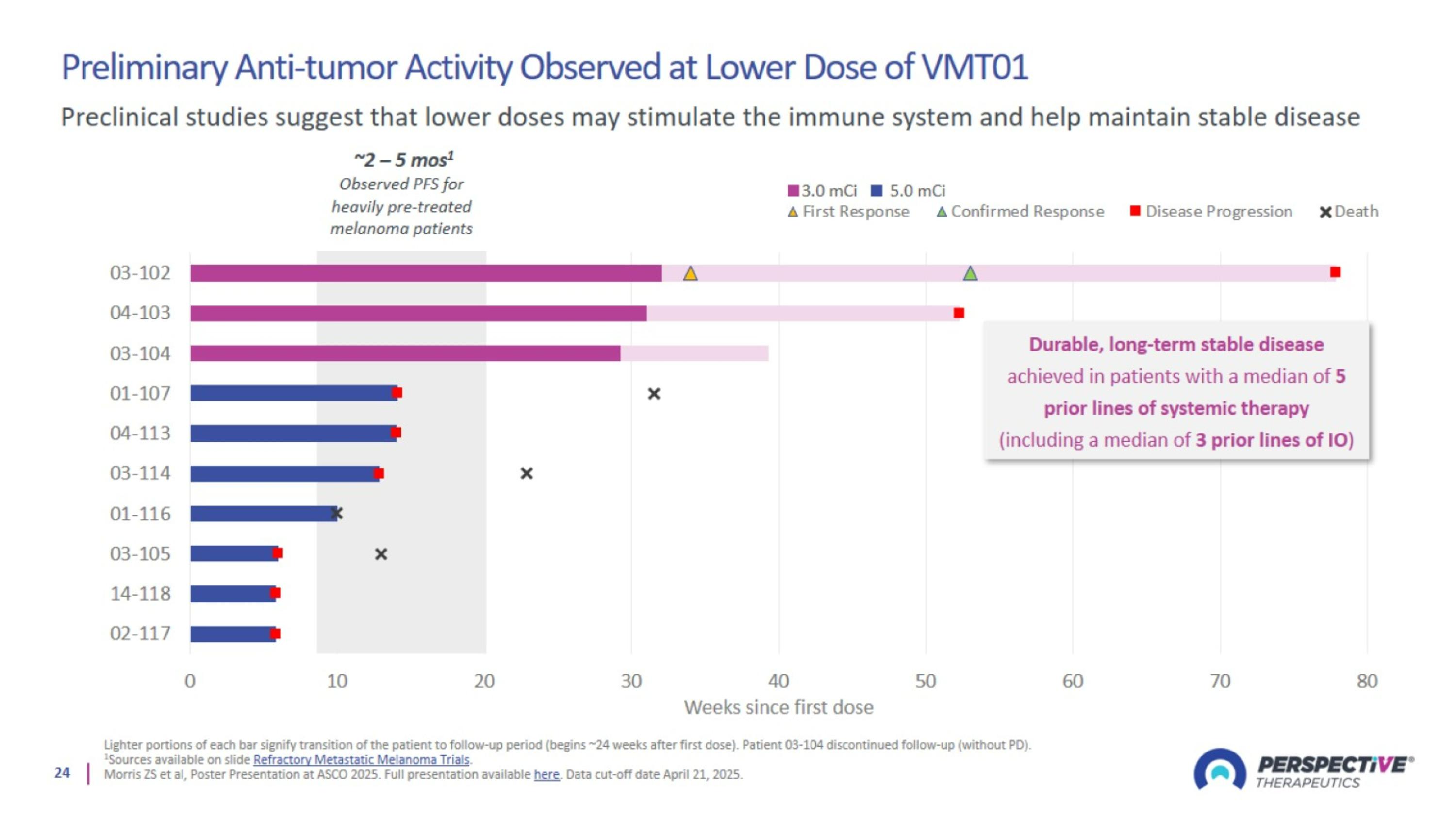
Preliminary Anti-tumor Activity Observed at Lower Dose of VMT01
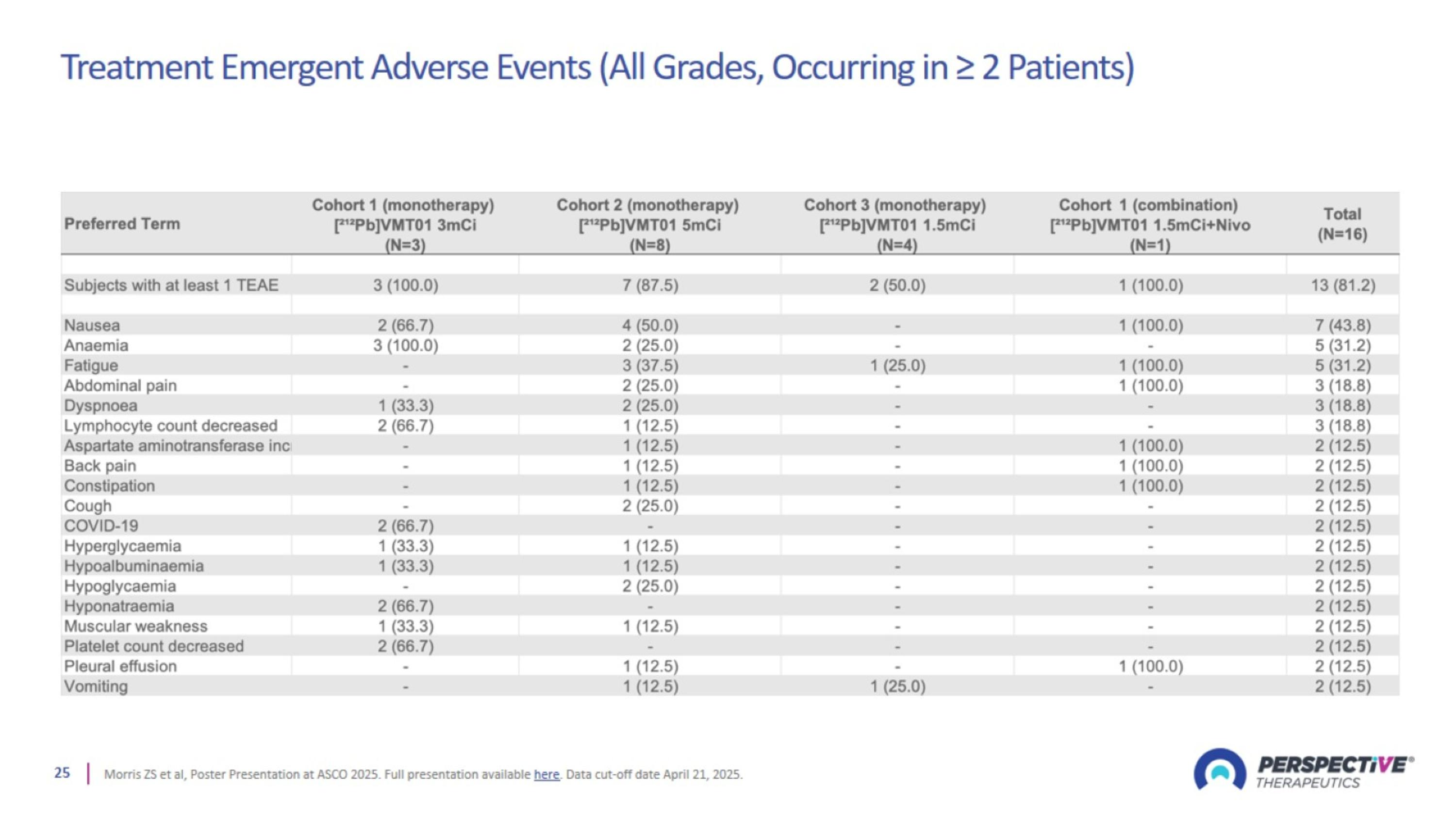
Treatment Emergent Adverse Events (All Grades, Occurring in ≥ 2 Patients)
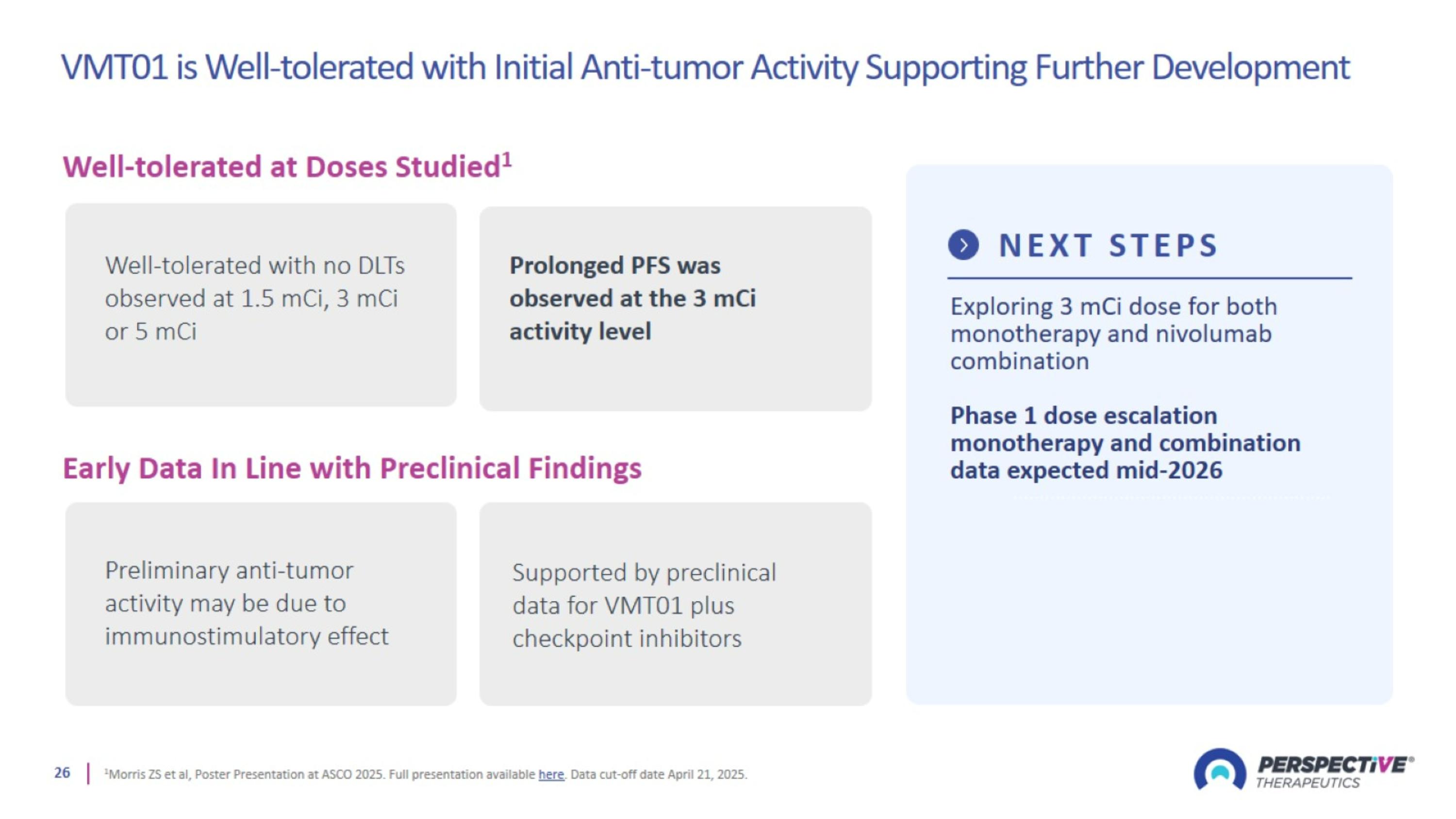
VMT01 is Well-tolerated with Initial Anti-tumor Activity Supporting Further Development
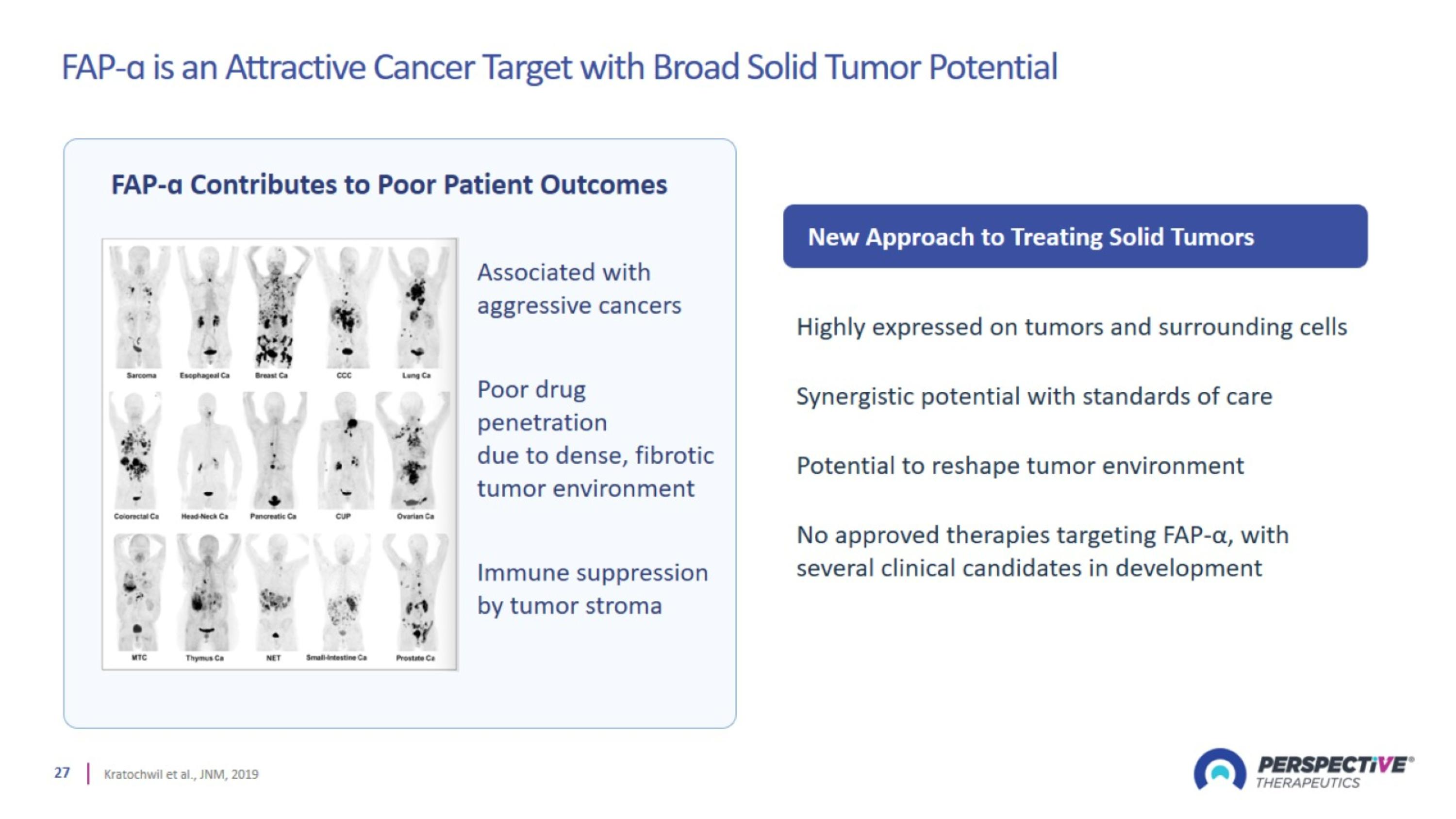
FAP-ɑ is an Attractive Cancer Target with Broad Solid Tumor Potential
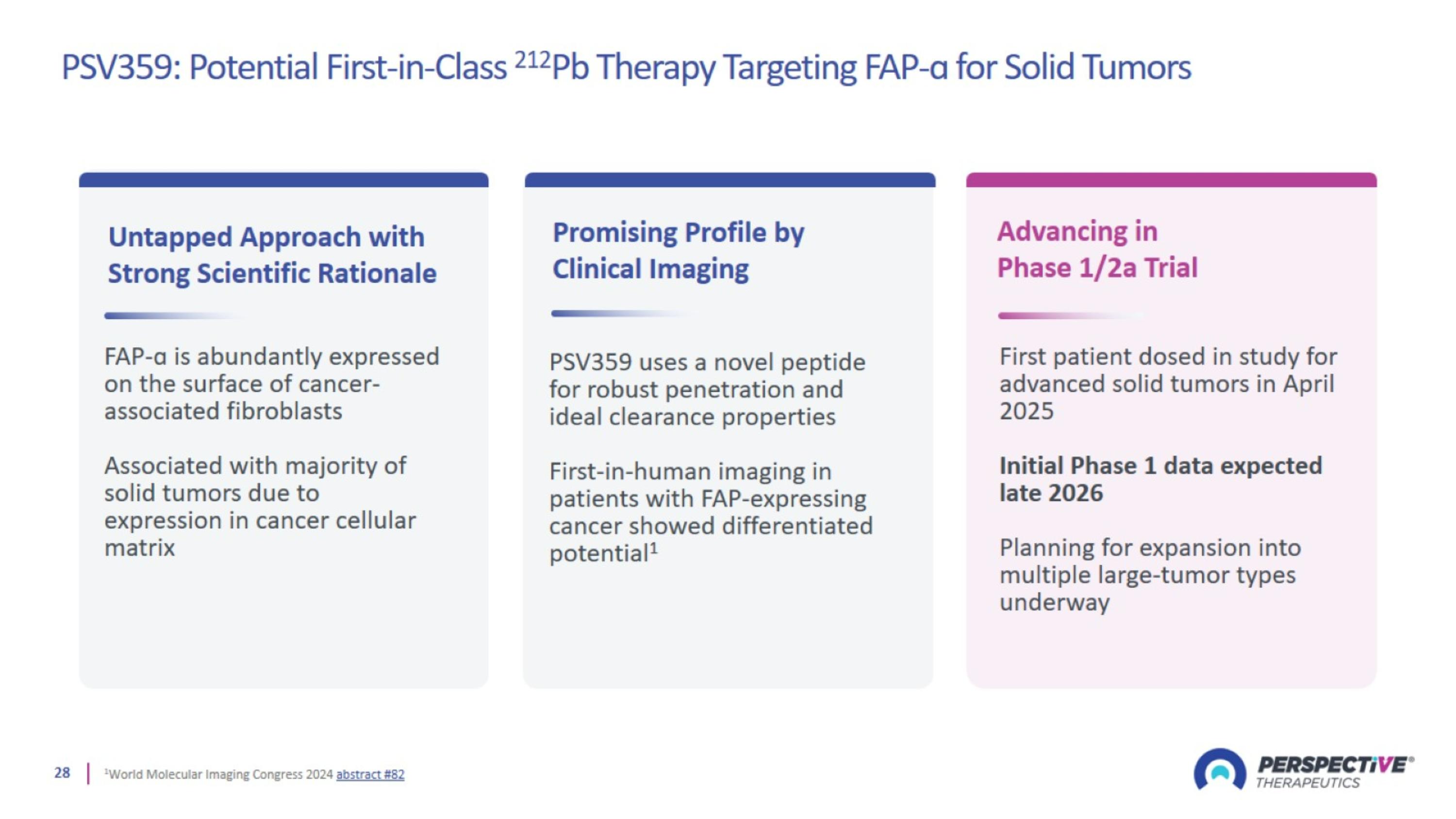
PSV359: Potential First-in-Class 212Pb Therapy Targeting FAP-ɑ for Solid Tumors
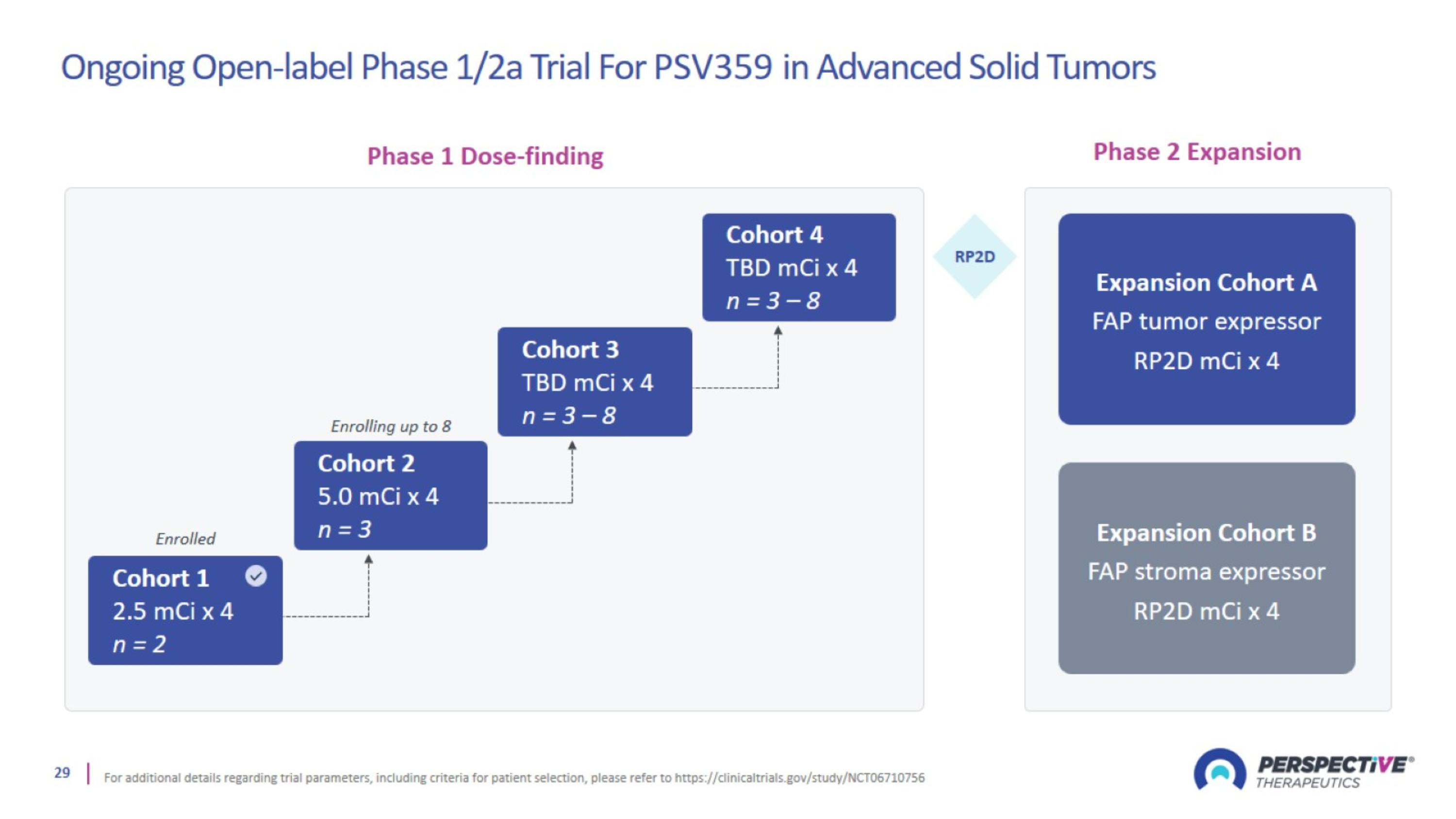
Ongoing Open-label Phase 1/2a Trial For PSV359 in Advanced Solid Tumors
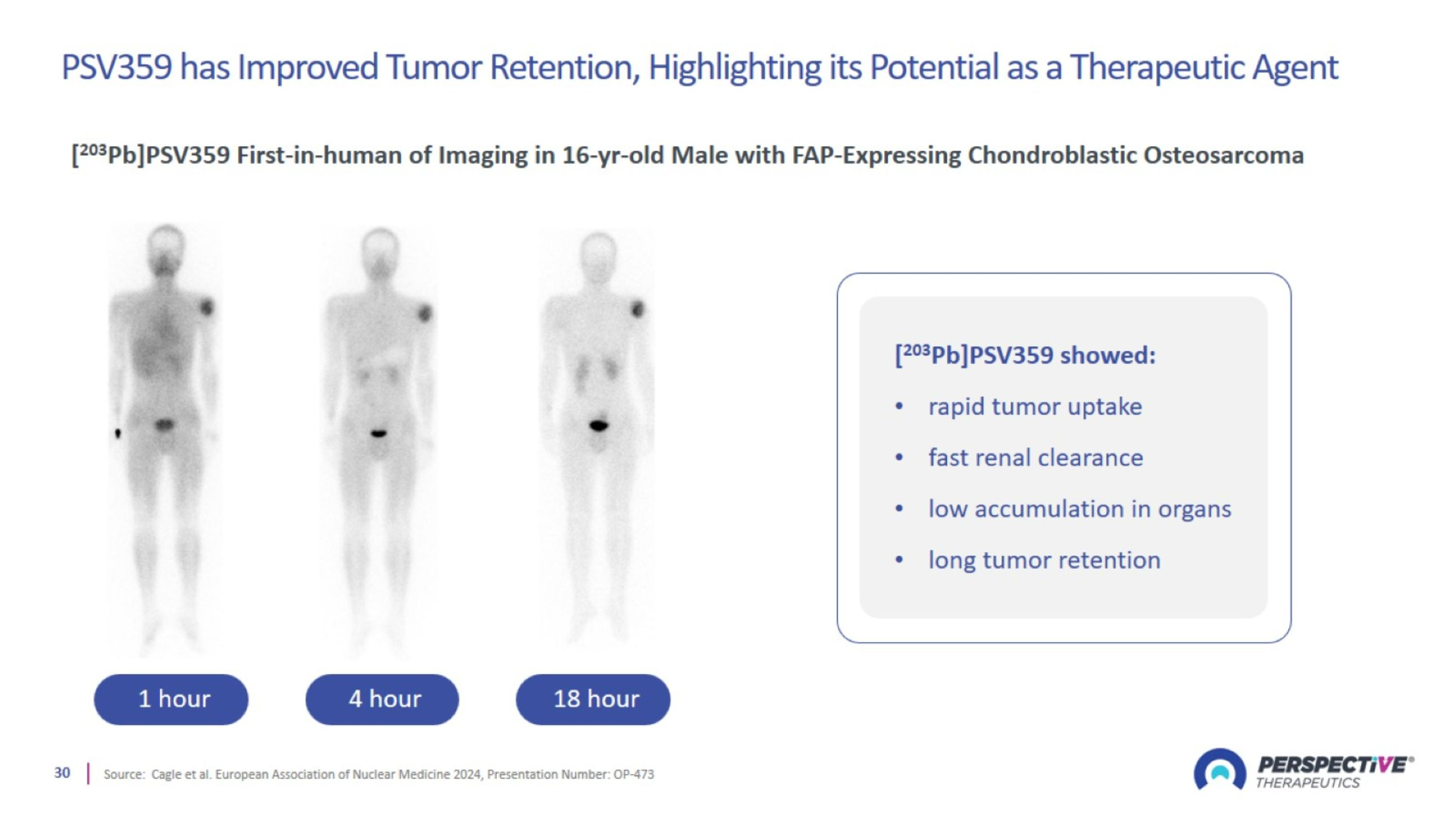
PSV359 has Improved Tumor Retention, Highlighting its Potential as a Therapeutic Agent
Strong IP Portfolio Covering All Aspects of Radiopharmaceutical Value Chain

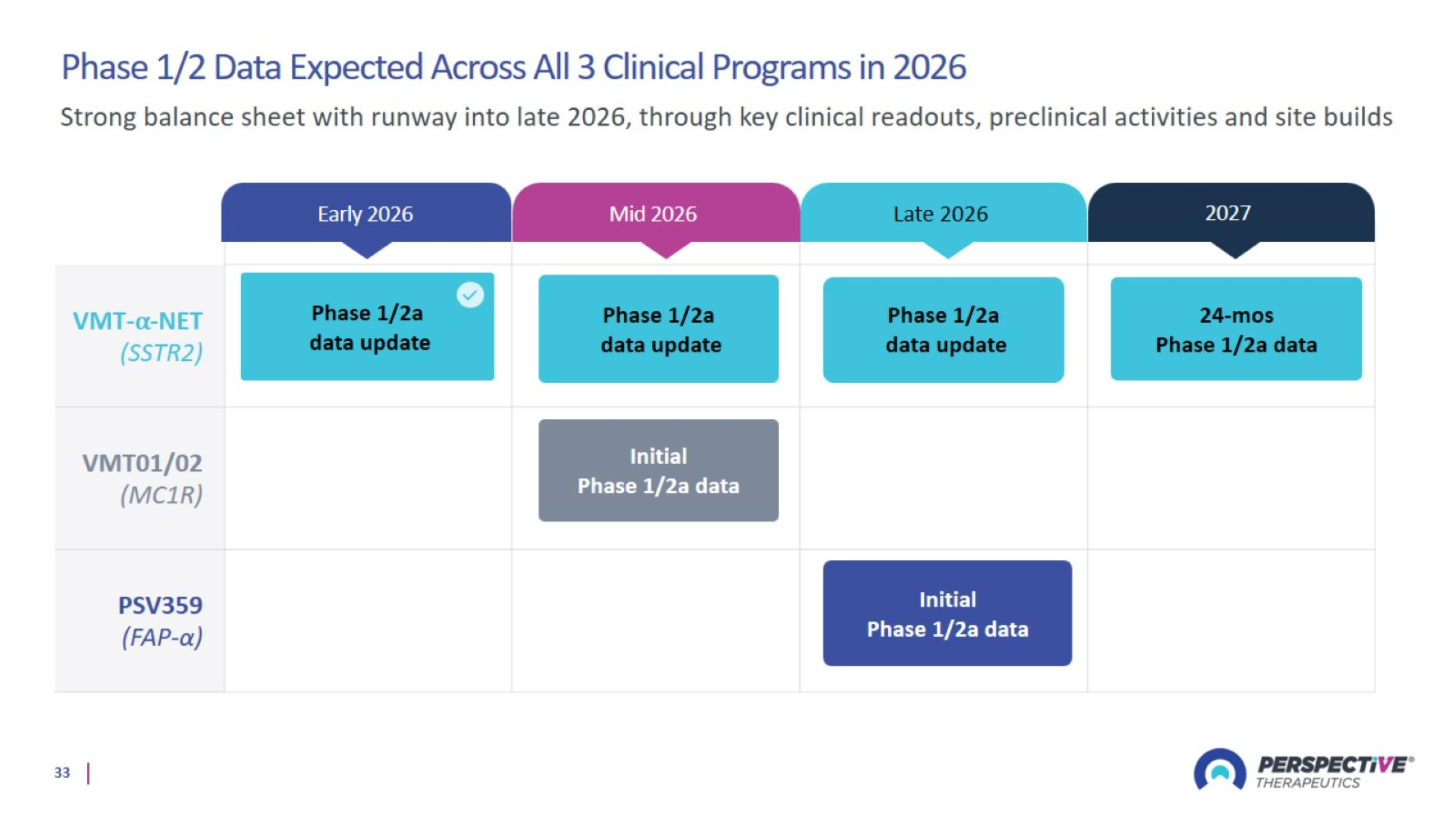
Phase 1/2 Data Expected Across All 3 Clinical Programs in 2026


Abbreviations
APPENDIX

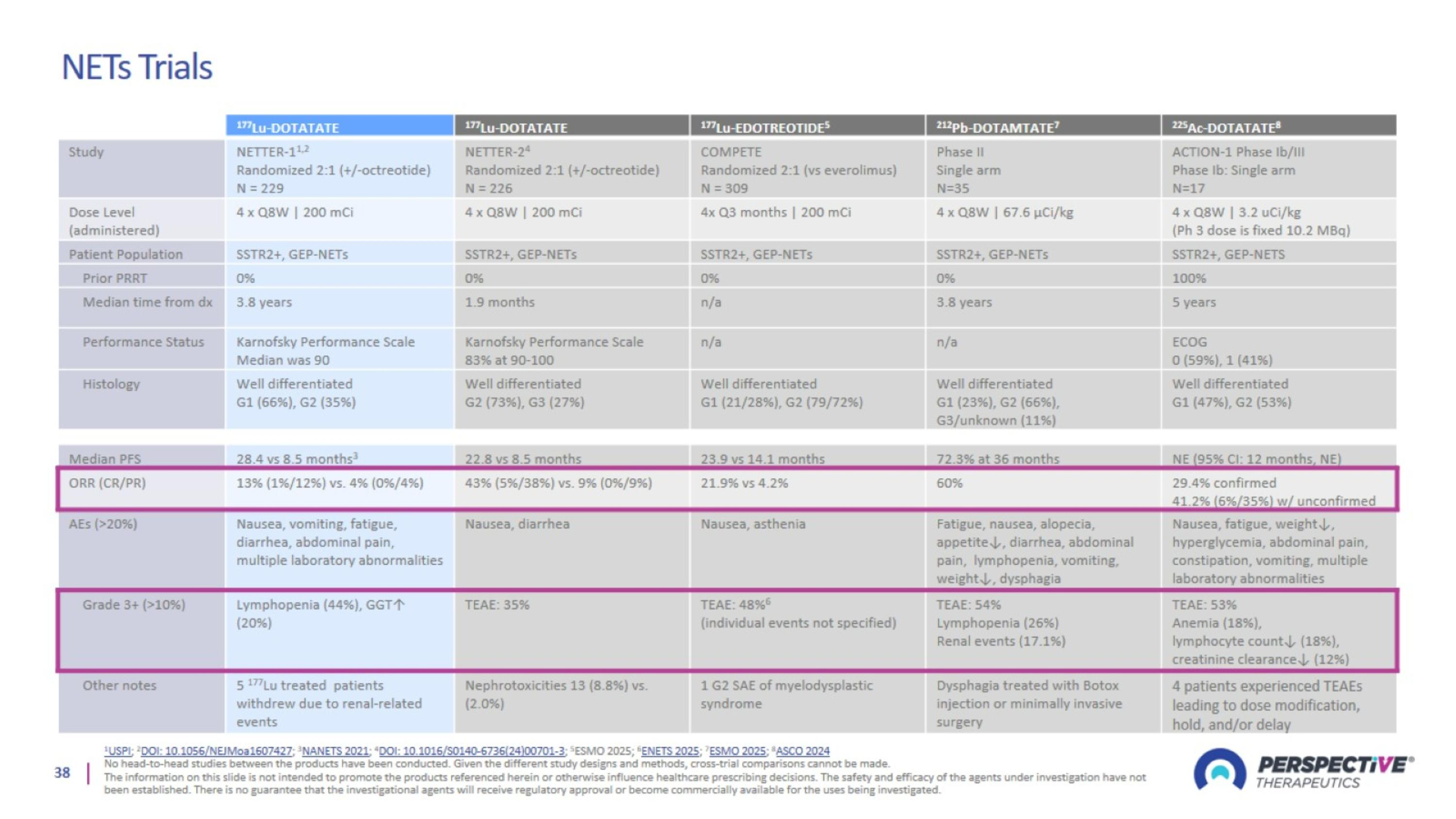
NETs Trials
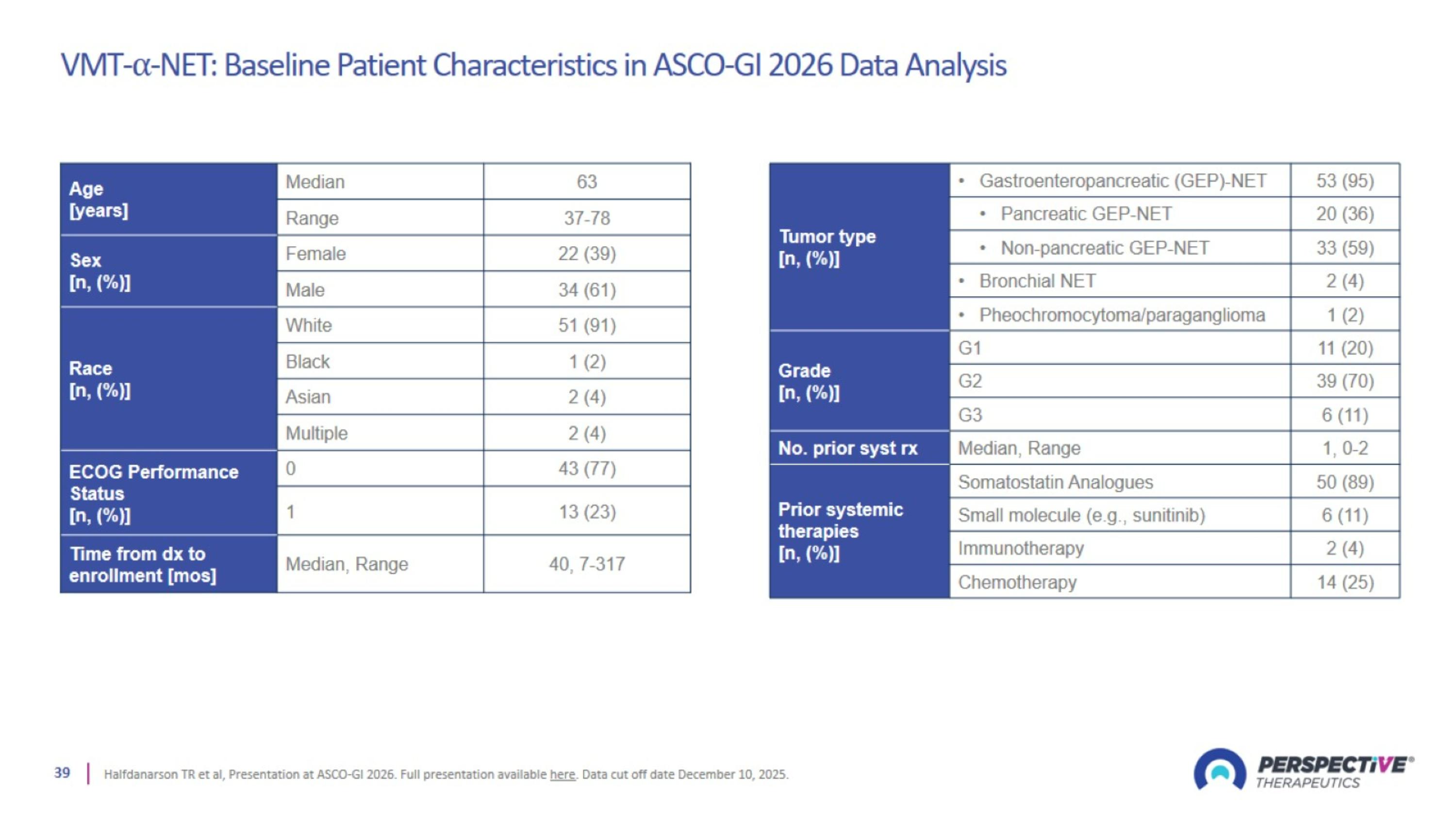
VMT-⍺-NET: Baseline Patient Characteristics in ASCO-GI 2026 Data Analysis
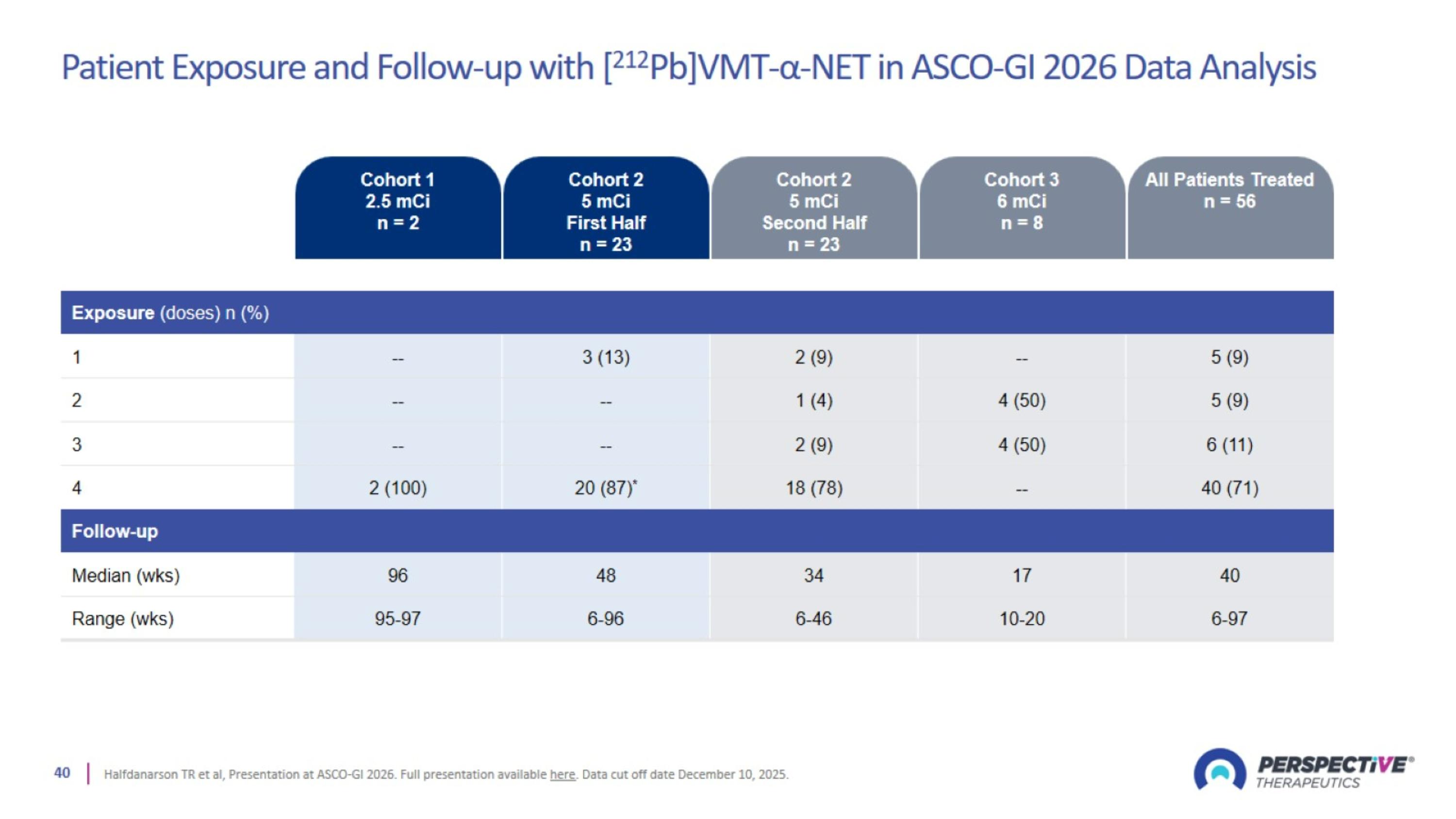
Patient Exposure and Follow-up with [212Pb]VMT-α-NET in ASCO-GI 2026 Data Analysis
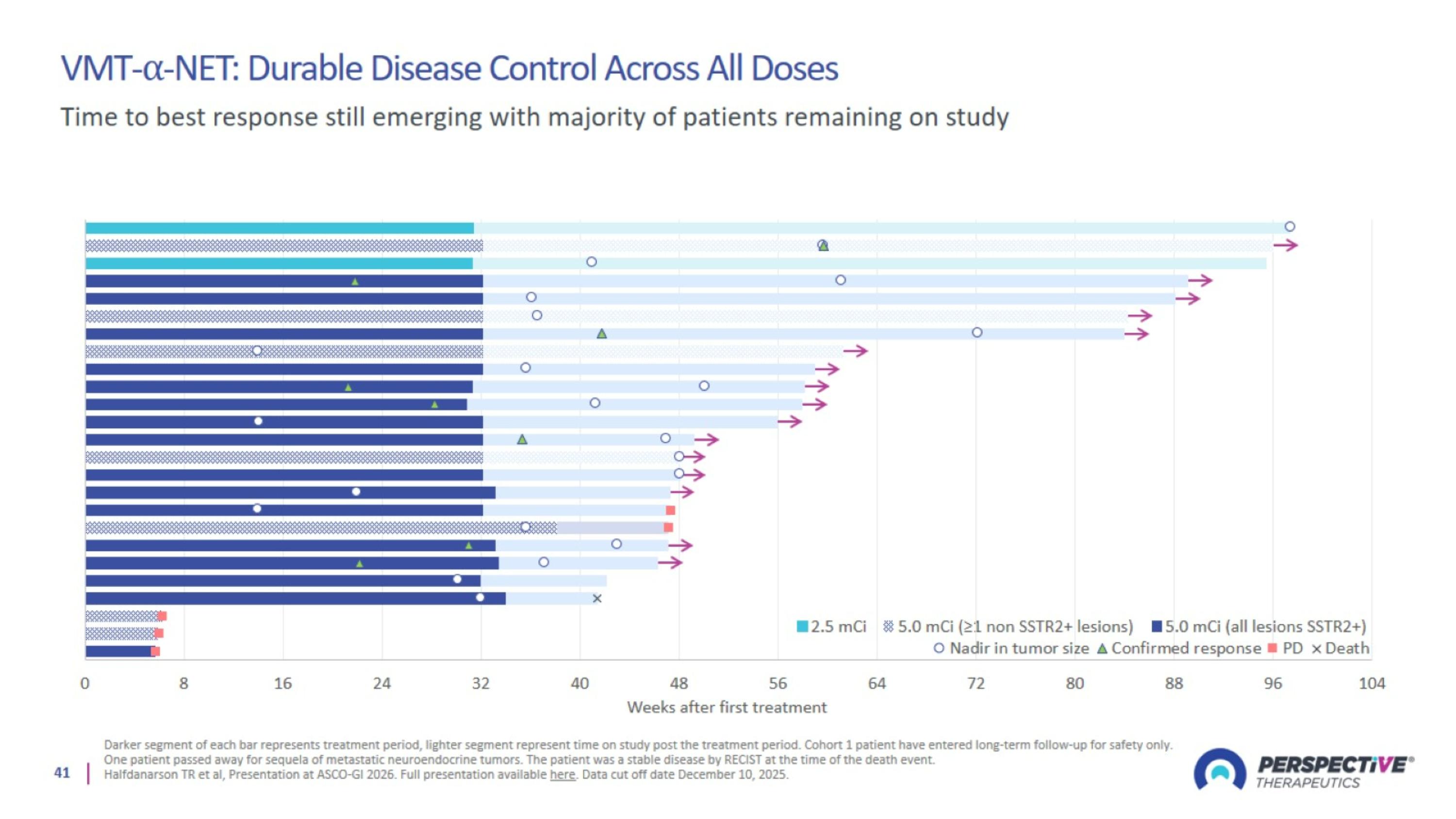
VMT-⍺-NET: Durable Disease Control Across All Doses
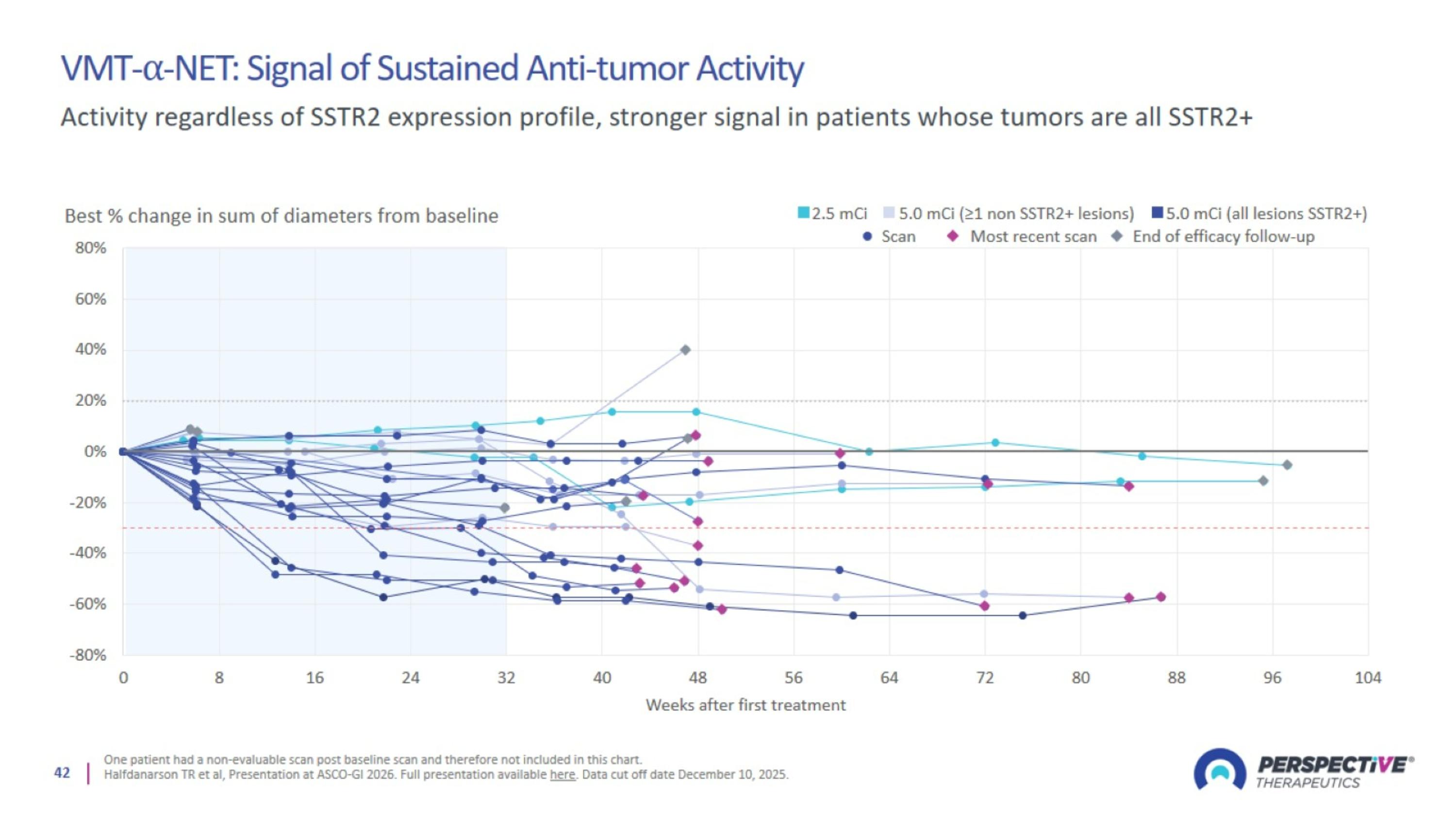
VMT-⍺-NET: Signal of Sustained Anti-tumor Activity
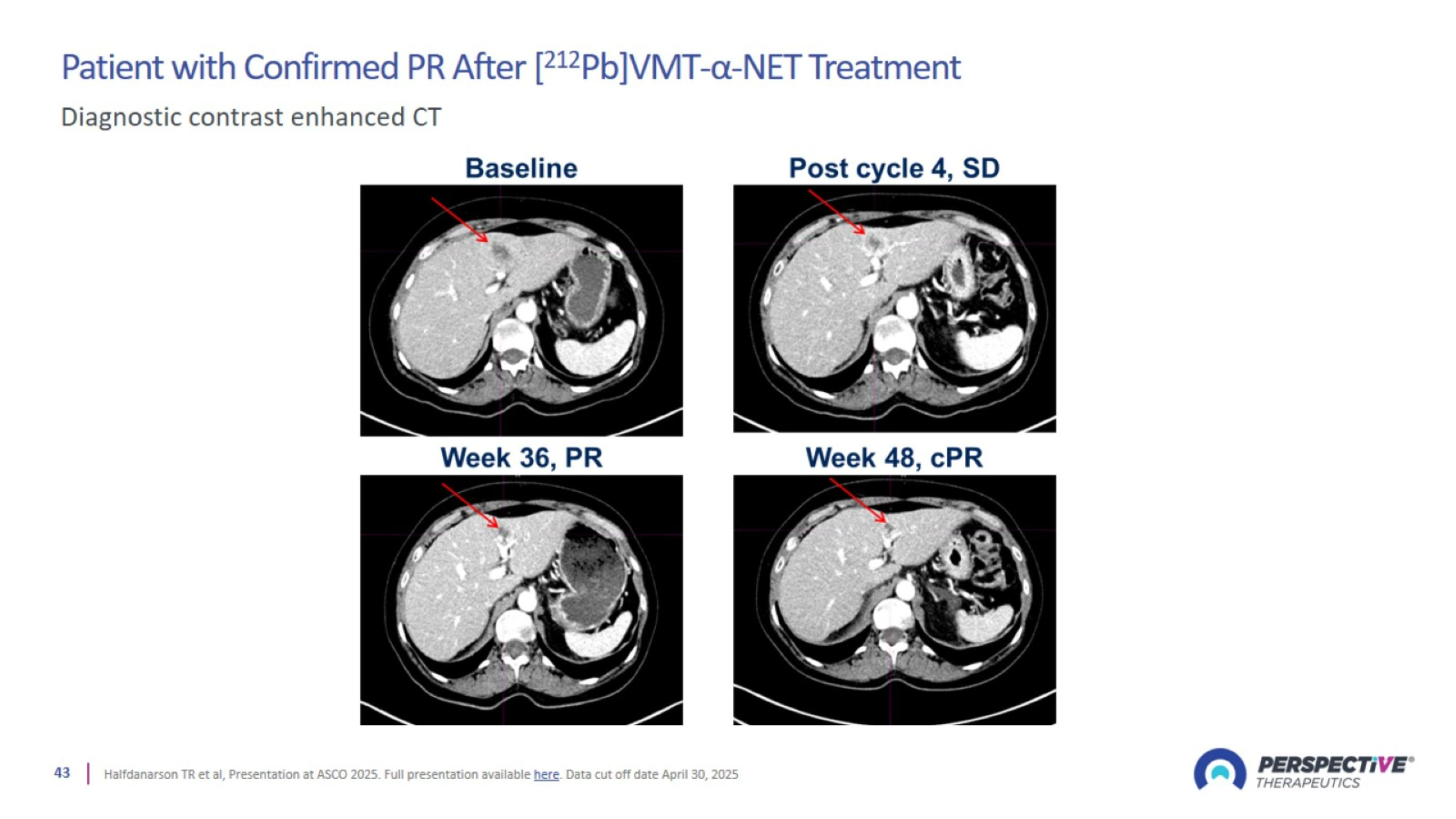
Patient with Confirmed PR After [212Pb]VMT-α-NET Treatment
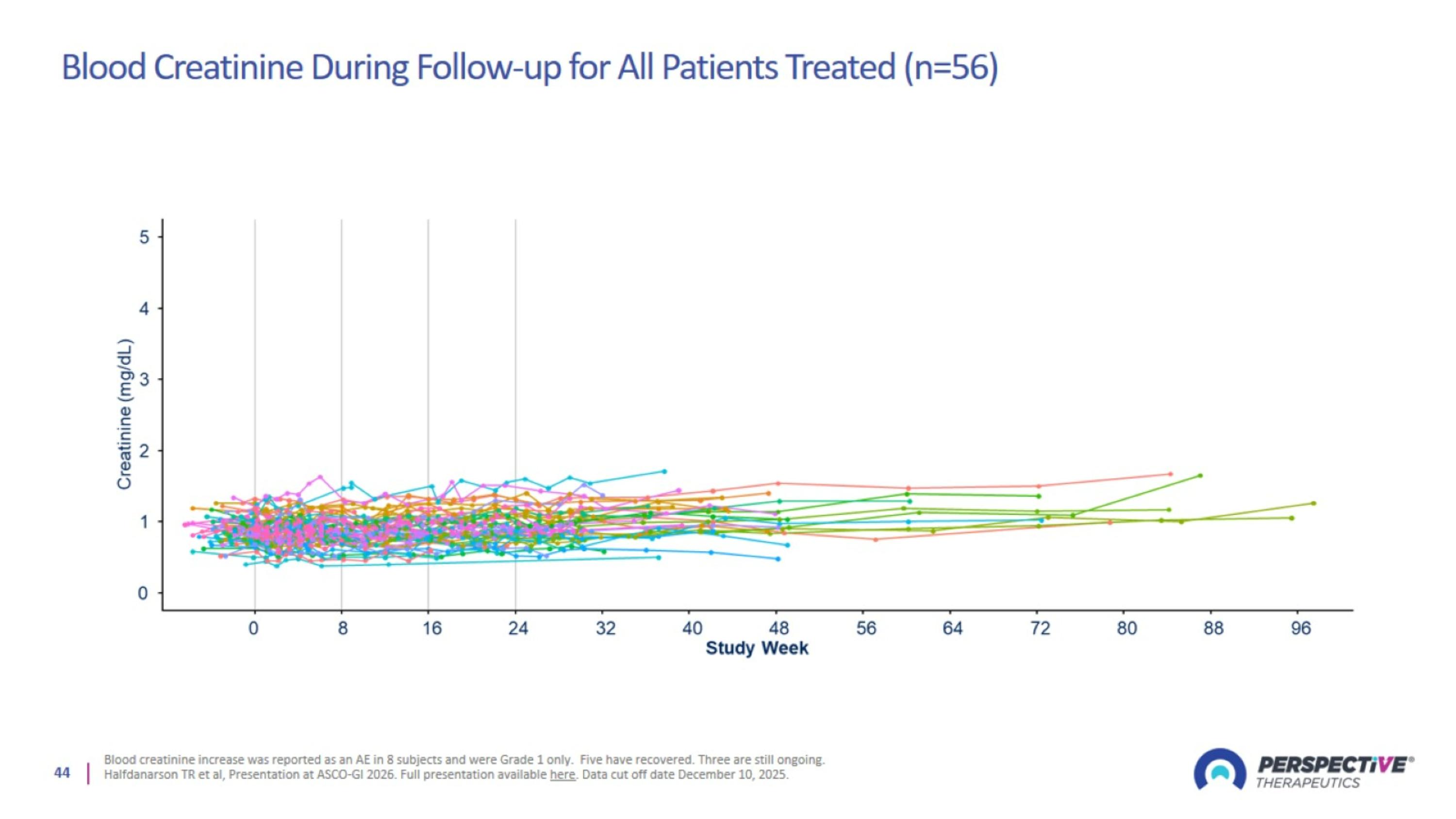
Blood Creatinine During Follow-up for All Patients Treated (n=56)
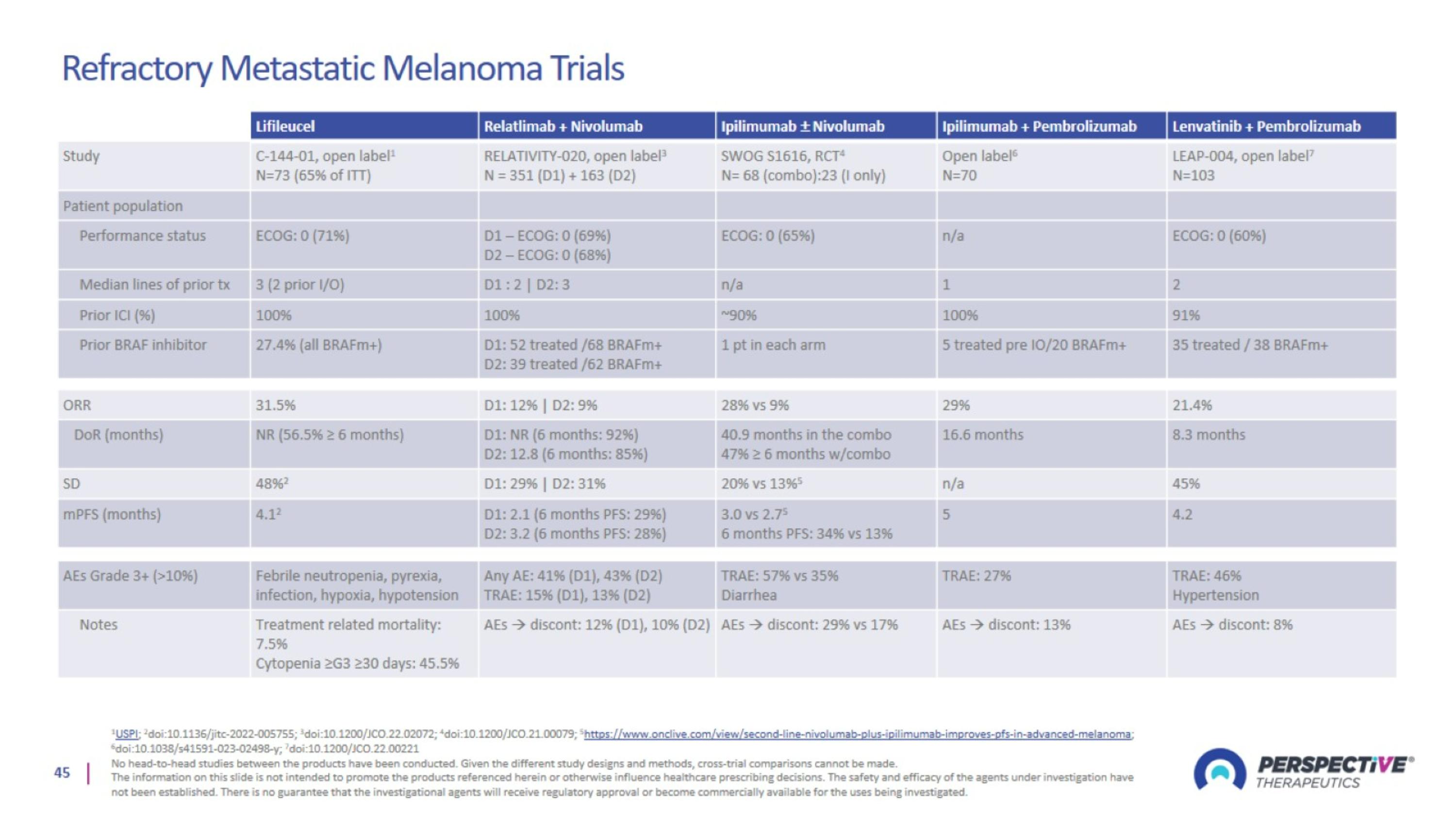
Refractory Metastatic Melanoma Trials
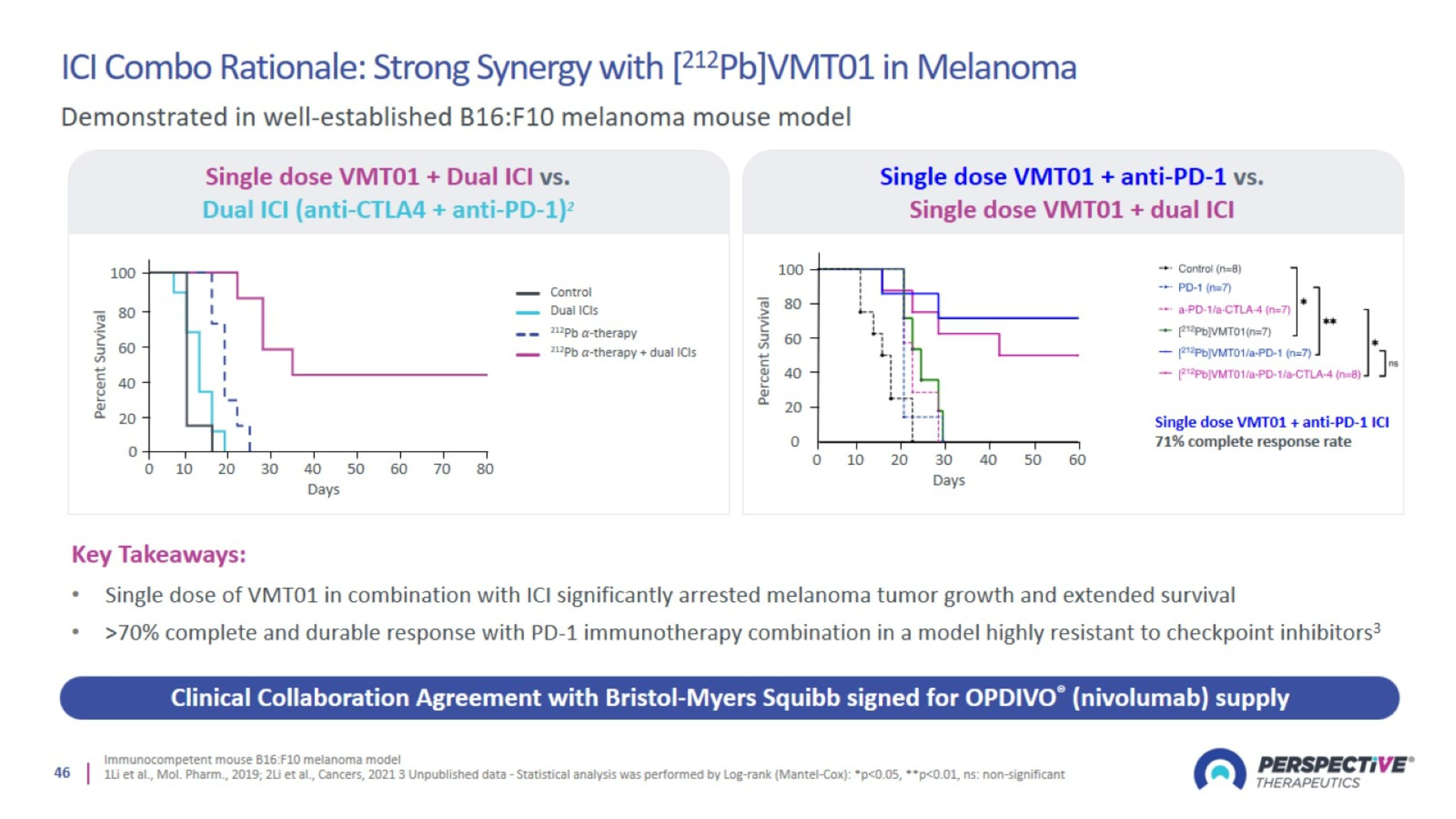
ICI Combo Rationale: Strong Synergy with [212Pb]VMT01 in Melanoma
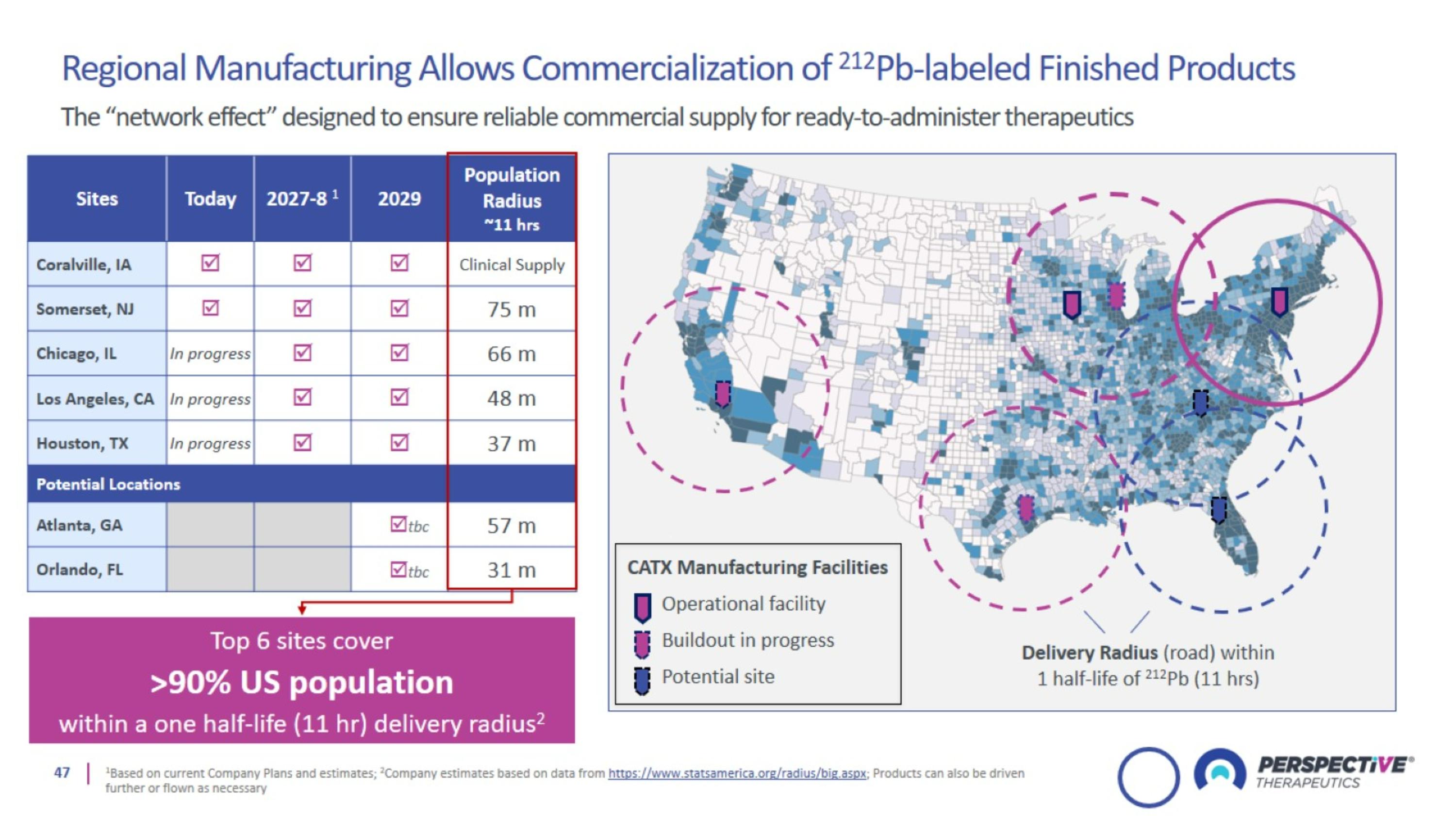

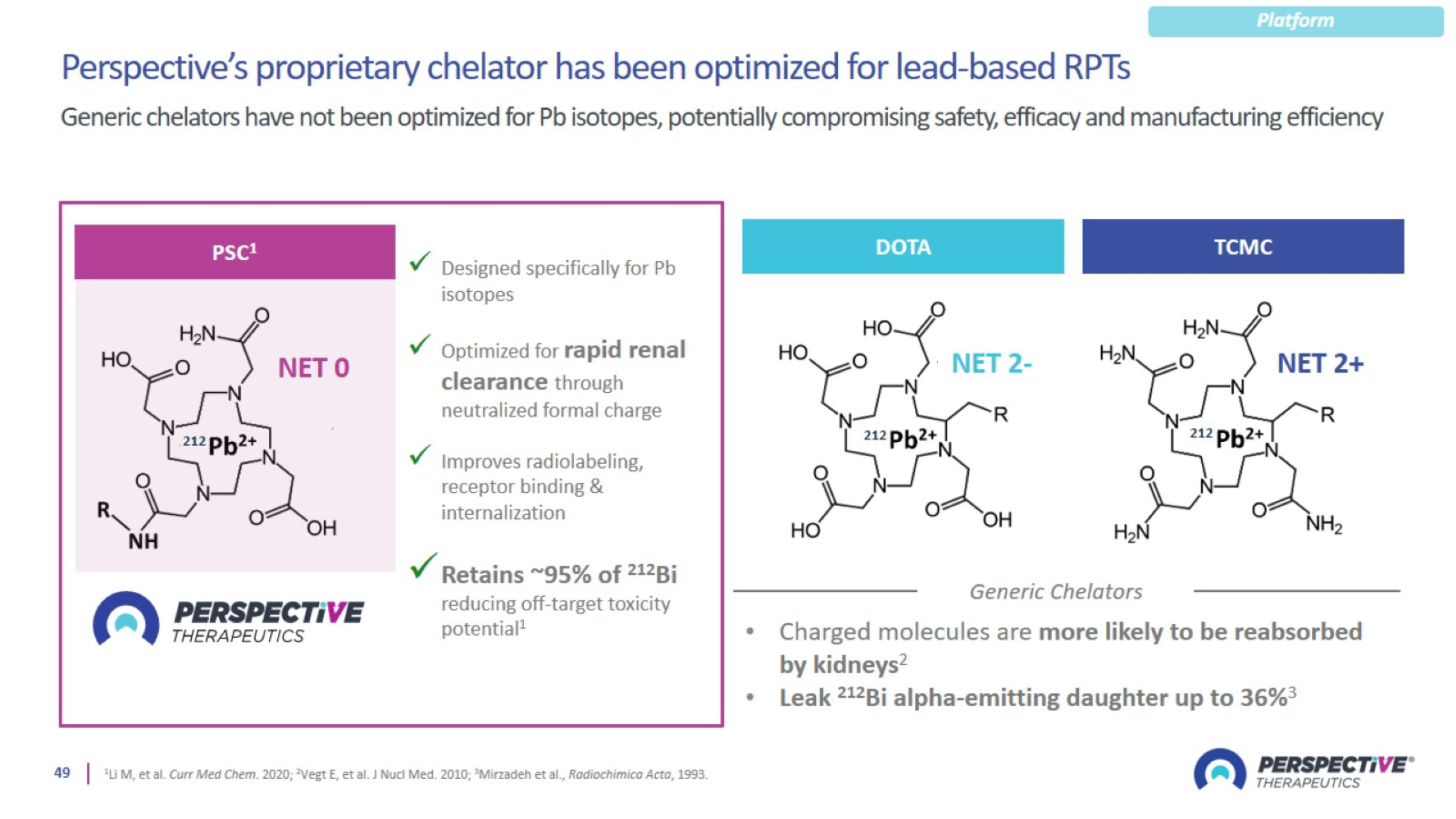
Perspective’s proprietary chelator has been optimized for lead-based RPTs