

.2 Corporate Presentation NASDAQ: DCTH January 9, 2026

Forward-Looking Statement alternative indications; the impact of the COVID-19 pandemic or other pandemics on the The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward- completion of our clinical trials; the impact of the presentations at major medical looking statements made by the Company or on its behalf. This presentation contains conferences and future clinical results consistent with the data presented; uncertainties forward-looking statements, which are subject to certain risks and uncertainties that can relating to the timing and results of research and development projects; and uncertainties cause actual results to differ materially from those described. The words “anticipate,” regarding the Company’s ability to obtain financial and other resources for any research, “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” development, clinical trials and commercialization activities. These factors, and others, are “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended discussed from time to time in our filings with the Securities and Exchange Commission. to identify forward-looking statements, although not all forward-looking statements contain these identifying words. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these Factors that may cause such differences include, but are not limited to, uncertainties forward-looking statements to reflect events or circumstances after the date they are made. relating to: changes to the estimated preliminary results set forth herein as a result of The Company has not yet completed its financial close process for the fourth quarter and full audit adjustments and other developments that may arise between now the time the year 2025 and, as a result, actual results may vary from the estimated preliminary results set financial results for the fourth quarter and fiscal year ended December 31, 2025, are forth in this presentation. The estimated preliminary financial results have not been audited or finalized; the Company’s ability to successfully commercialize the HEPZATO KIT; reviewed by the Company’s independent registered public accounting firm. These estimates contributions to adjusted EBITDA; the Company's successful management of the should not be viewed as a substitute for the Company’s full, interim or annual audited HEPZATO KIT supply chain, including securing adequate supply of critical components financial statements. necessary to manufacture and assemble the HEPZATO KIT; successful FDA inspections of the facilities of Delcath and third-party suppliers/manufacturers; the Company's This presentation may include certain financial measures that were not prepared in successful implementation and management of the HEPZATO KIT Risk Evaluation and accordance with accounting principles generally accepted in the U.S. (GAAP). We believe that Mitigation Strategy; the potential of the HEPZATO KIT as a treatment for patients with the Non-GAAP financial measures provide additional insight into the ongoing economics of primary and metastatic disease in the liver; our ability to obtain reimbursement for our business. Non-GAAP financial measures are in addition to, not a substitute for, or superior commercialized product; the Company’s ability to successfully enter into any necessary to, measures of financial performance prepared in accordance with GAAP. purchase and sale agreements with users of the HEPZATO KIT; the timing and results of the Company’s clinical trials; our determination whether to continue a clinical trial program or to focus on other 2

Delcath Corporate Summary HEPZATO/CHEMOSAT Commercial Opportunity • 1Q 2024 HEPZATO (drug/device) US launch for mUM*, • Ultra orphan pricing with J-Code CHEMOSAT (device only) in EU • Focused call points • Included in NCCN Guidelines • US mUM TAM ~$500M • First and only FDA approved whole-liver directed therapy • 4Q 2025 Preliminary Total Revenue of $20.7M; Full Year Preliminary Total Revenue of $85.2M** Strong Financial Position** • Preliminary Cash and Investments as of 12/31/2025 = $91.0M • Q4 2025 Positive Operating Cash Significant upside beyond mUM • Repurchase of 629k shares for $6.0M in 2025 • HDS platform technology with utility across a broad set • No outstanding debt obligations of cancer types • Strong efficacy signals in multiple other tumor types • Unique interventional oncology asset Experienced Management Team • IND approved for mCRC (recruiting) and mBC trials • Expertise in commercializing high value, specialty products • CHOPIN data validates synergy with checkpoint inhibitors • TheraSphere (BSX) veterans * metastatic Uveal Melanoma (mUM) ** Q4 2025 and Full Year Financial Results are preliminary and unaudited 3

HIGH UNMET NEED: Primary/Metastatic Liver Cancers 4
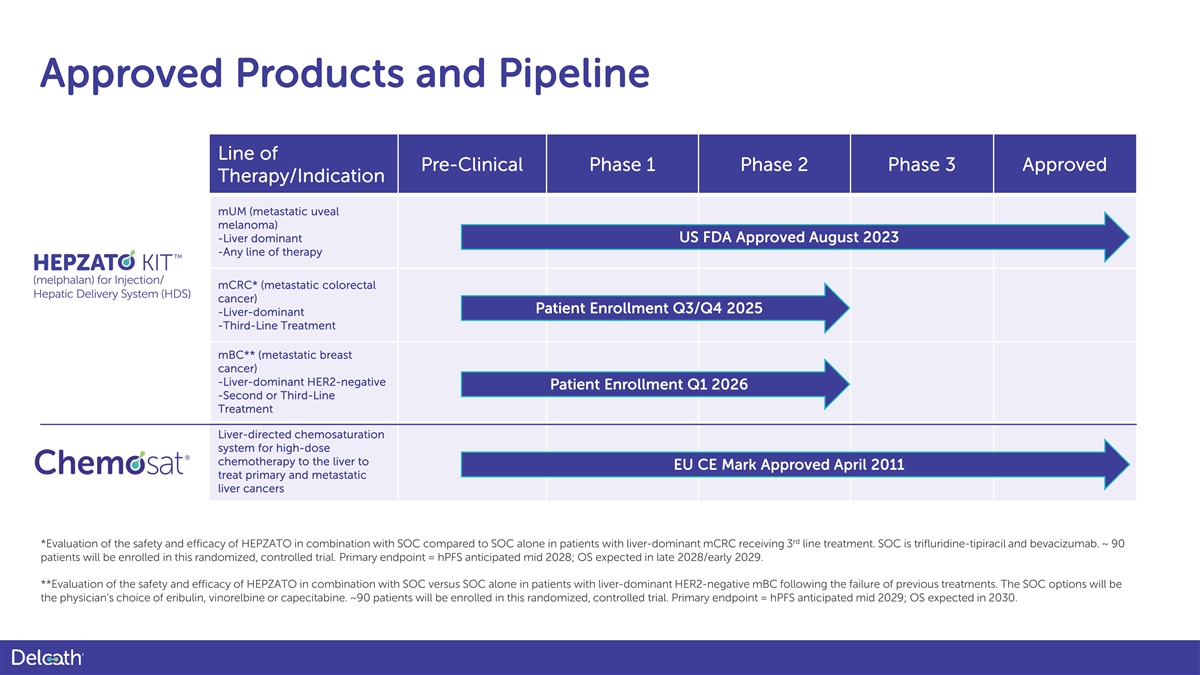
Approved Products and Pipeline Line of Pre-Clinical Phase 1 Phase 2 Phase 3 Approved Therapy/Indication mUM (metastatic uveal melanoma) -Liver dominant US FDA Approved August 2023 -Any line of therapy mCRC* (metastatic colorectal cancer) Patient Enrollment Q3/Q4 2025 -Liver-dominant -Third-Line Treatment mBC** (metastatic breast cancer) -Liver-dominant HER2-negative Patient Enrollment Q1 2026 -Second or Third-Line Treatment Liver-directed chemosaturation system for high-dose chemotherapy to the liver to EU CE Mark Approved April 2011 treat primary and metastatic liver cancers rd *Evaluation of the safety and efficacy of HEPZATO in combination with SOC compared to SOC alone in patients with liver-dominant mCRC receiving 3 line treatment. SOC is trifluridine-tipiracil and bevacizumab. ~ 90 patients will be enrolled in this randomized, controlled trial. Primary endpoint = hPFS anticipated mid 2028; OS expected in late 2028/early 2029. **Evaluation of the safety and efficacy of HEPZATO in combination with SOC versus SOC alone in patients with liver-dominant HER2-negative mBC following the failure of previous treatments. The SOC options will be the physician’s choice of eribulin, vinorelbine or capecitabine. ~90 patients will be enrolled in this randomized, controlled trial. Primary endpoint = hPFS anticipated mid 2029; OS expected in 2030.
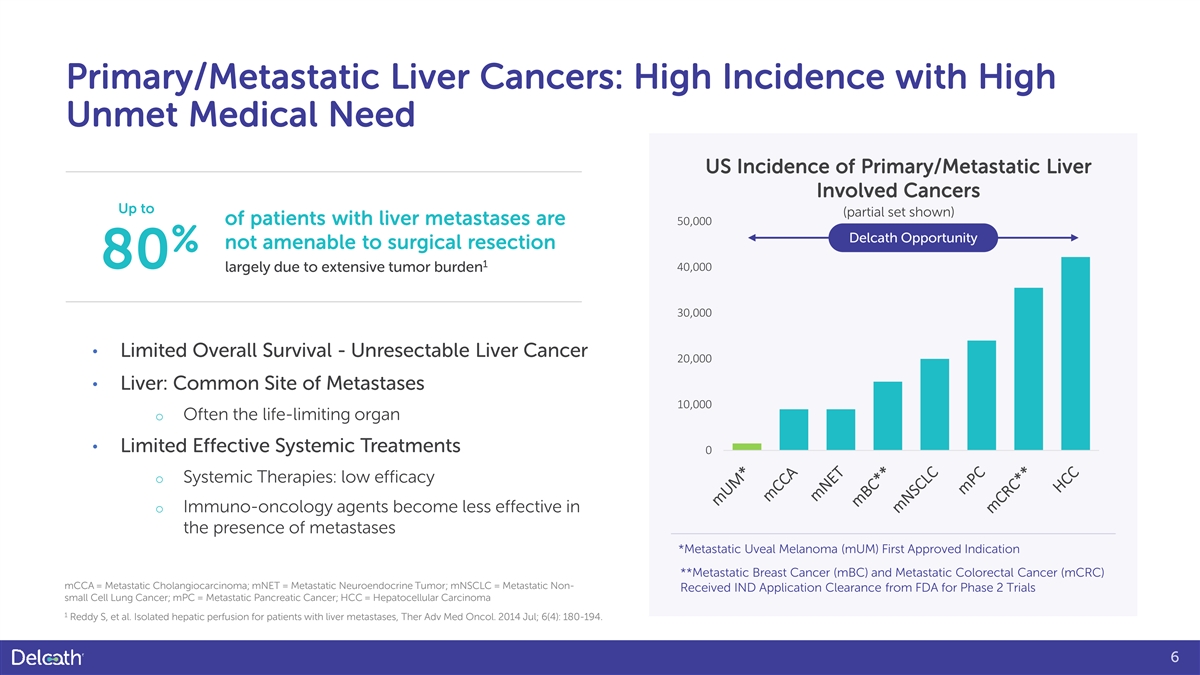
Primary/Metastatic Liver Cancers: High Incidence with High Unmet Medical Need US Incidence of Primary/Metastatic Liver Involved Cancers Up to (partial set shown) of patients with liver metastases are 50,000 Delcath Opportunity not amenable to surgical resection % 1 80 40,000 largely due to extensive tumor burden 30,000 • Limited Overall Survival - Unresectable Liver Cancer 20,000 • Liver: Common Site of Metastases 10,000 o Often the life-limiting organ • Limited Effective Systemic Treatments 0 o Systemic Therapies: low efficacy o Immuno-oncology agents become less effective in the presence of metastases *Metastatic Uveal Melanoma (mUM) First Approved Indication **Metastatic Breast Cancer (mBC) and Metastatic Colorectal Cancer (mCRC) mCCA = Metastatic Cholangiocarcinoma; mNET = Metastatic Neuroendocrine Tumor; mNSCLC = Metastatic Non- Received IND Application Clearance from FDA for Phase 2 Trials small Cell Lung Cancer; mPC = Metastatic Pancreatic Cancer; HCC = Hepatocellular Carcinoma 1 Reddy S, et al. Isolated hepatic perfusion for patients with liver metastases, Ther Adv Med Oncol. 2014 Jul; 6(4): 180-194. 6
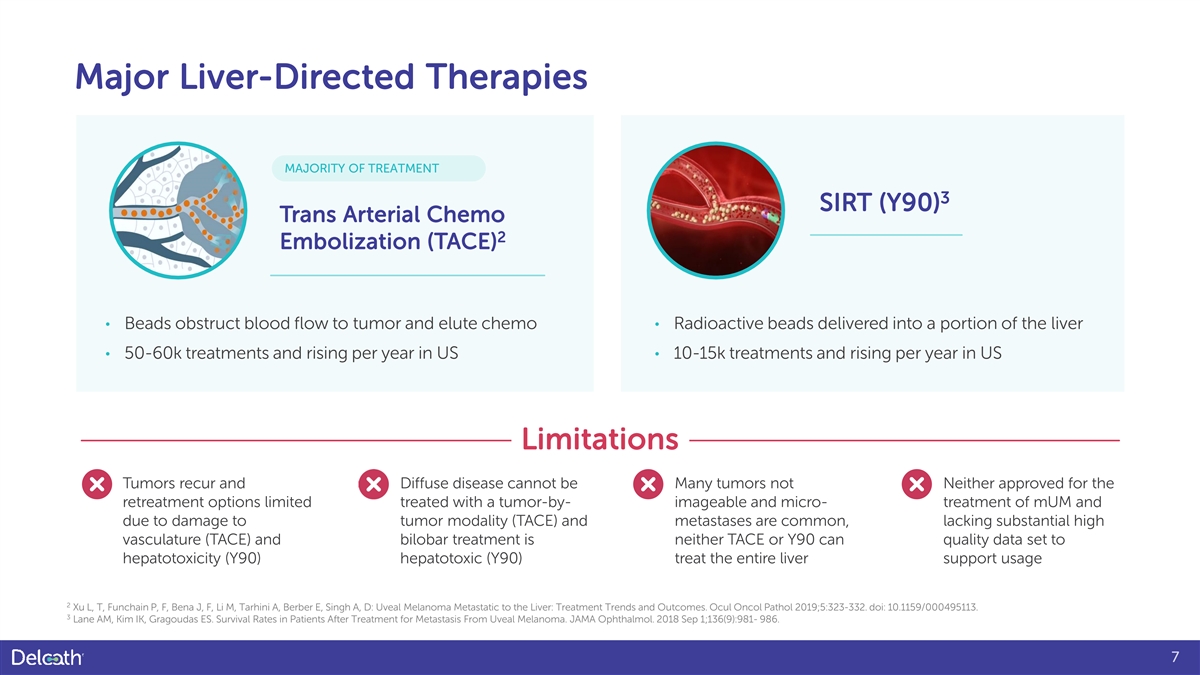
Major Liver-Directed Therapies MAJORITY OF TREATMENT 3 SIRT (Y90) Trans Arterial Chemo 2 Embolization (TACE) • Beads obstruct blood flow to tumor and elute chemo • Radioactive beads delivered into a portion of the liver • 50-60k treatments and rising per year in US • 10-15k treatments and rising per year in US Limitations Tumors recur and Diffuse disease cannot be Many tumors not Neither approved for the retreatment options limited treated with a tumor-by- imageable and micro- treatment of mUM and due to damage to tumor modality (TACE) and metastases are common, lacking substantial high vasculature (TACE) and bilobar treatment is neither TACE or Y90 can quality data set to hepatotoxicity (Y90) hepatotoxic (Y90) treat the entire liver support usage 2 Xu L, T, Funchain P, F, Bena J, F, Li M, Tarhini A, Berber E, Singh A, D: Uveal Melanoma Metastatic to the Liver: Treatment Trends and Outcomes. Ocul Oncol Pathol 2019;5:323-332. doi: 10.1159/000495113. 3 Lane AM, Kim IK, Gragoudas ES. Survival Rates in Patients After Treatment for Metastasis From Uveal Melanoma. JAMA Ophthalmol. 2018 Sep 1;136(9):981- 986. 7
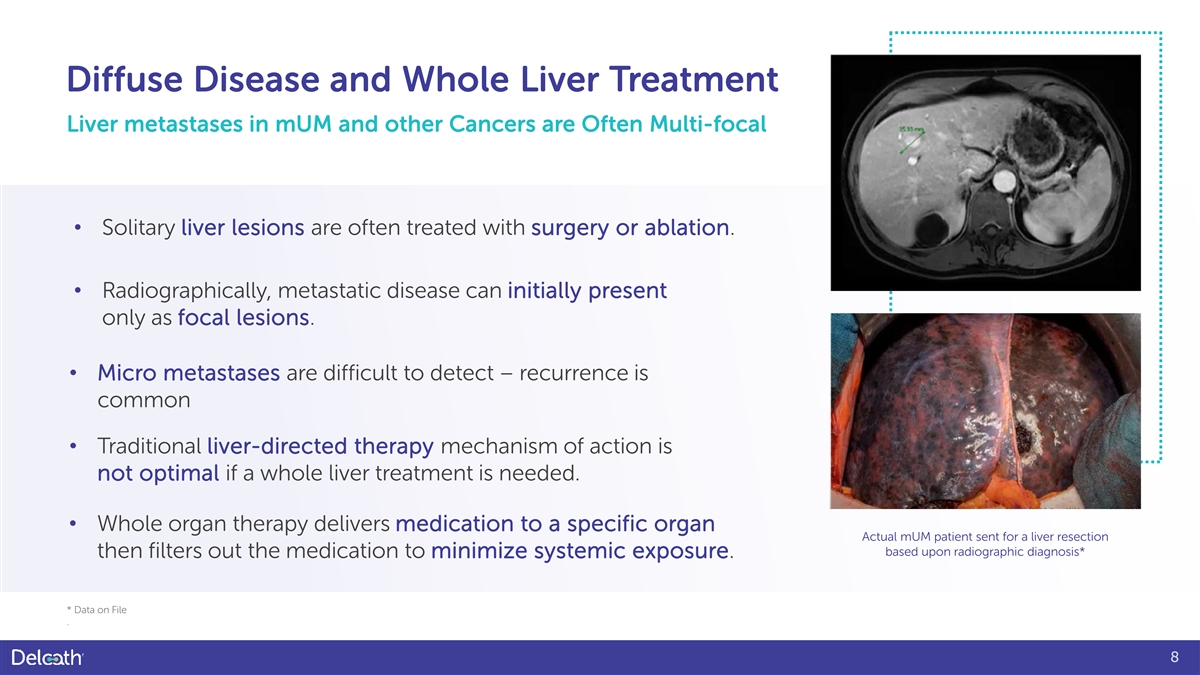
Diffuse Disease and Whole Liver Treatment Liver metastases in mUM and other Cancers are Often Multi-focal • Solitary liver lesions are often treated with surgery or ablation. • Radiographically, metastatic disease can initially present only as focal lesions. • Micro metastases are difficult to detect – recurrence is common • Traditional liver-directed therapy mechanism of action is not optimal if a whole liver treatment is needed. • Whole organ therapy delivers medication to a specific organ Actual mUM patient sent for a liver resection based upon radiographic diagnosis* then filters out the medication to minimize systemic exposure. * Data on File . 8

9
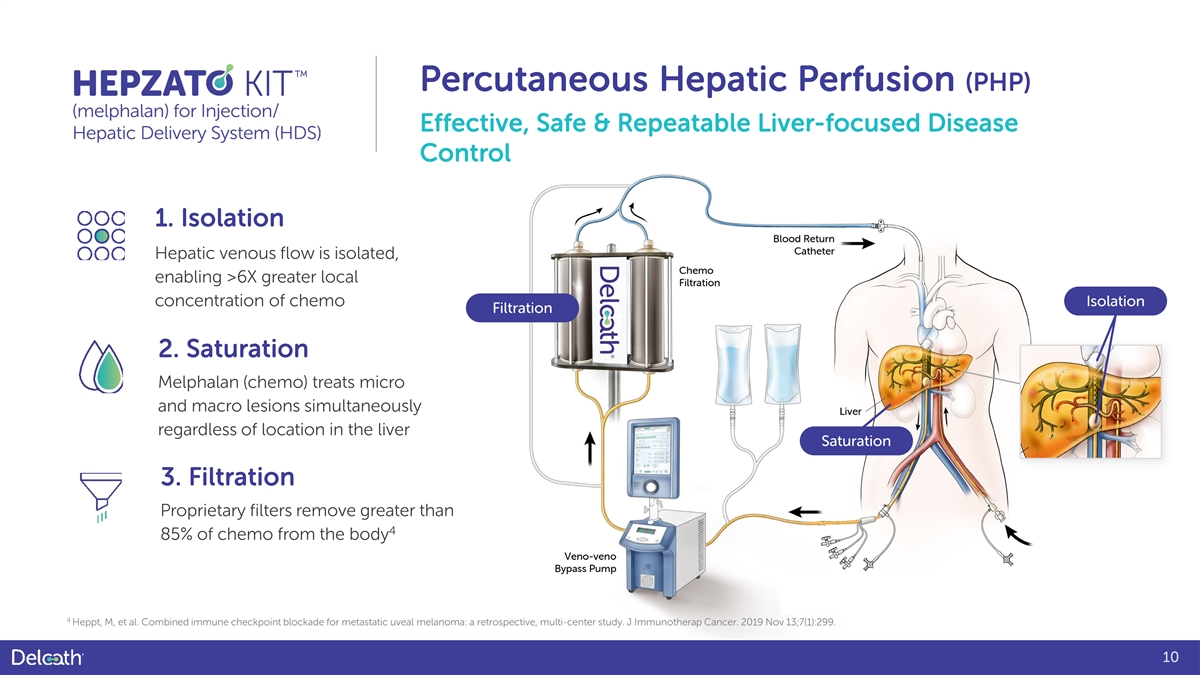
Percutaneous Hepatic Perfusion (PHP) Effective, Safe & Repeatable Liver-focused Disease Control 1. Isolation Hepatic venous flow is isolated, enabling >6X greater local concentration of chemo Isolation Filtration 2. Saturation Melphalan (chemo) treats micro and macro lesions simultaneously regardless of location in the liver Saturation 3. Filtration Proprietary filters remove greater than 4 85% of chemo from the body 4 Heppt, M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunotherap Cancer. 2019 Nov 13;7(1):299. 10
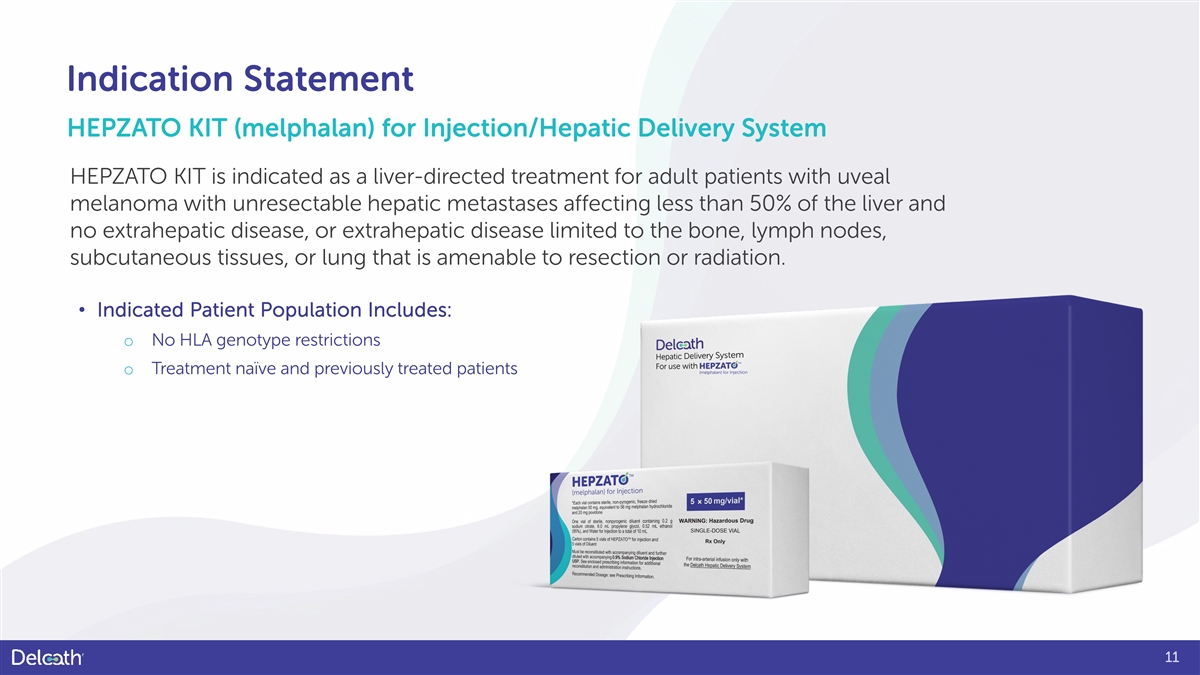
Indication Statement HEPZATO KIT (melphalan) for Injection/Hepatic Delivery System HEPZATO KIT is indicated as a liver-directed treatment for adult patients with uveal melanoma with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease, or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation. • Indicated Patient Population Includes: o No HLA genotype restrictions o Treatment naïve and previously treated patients 11
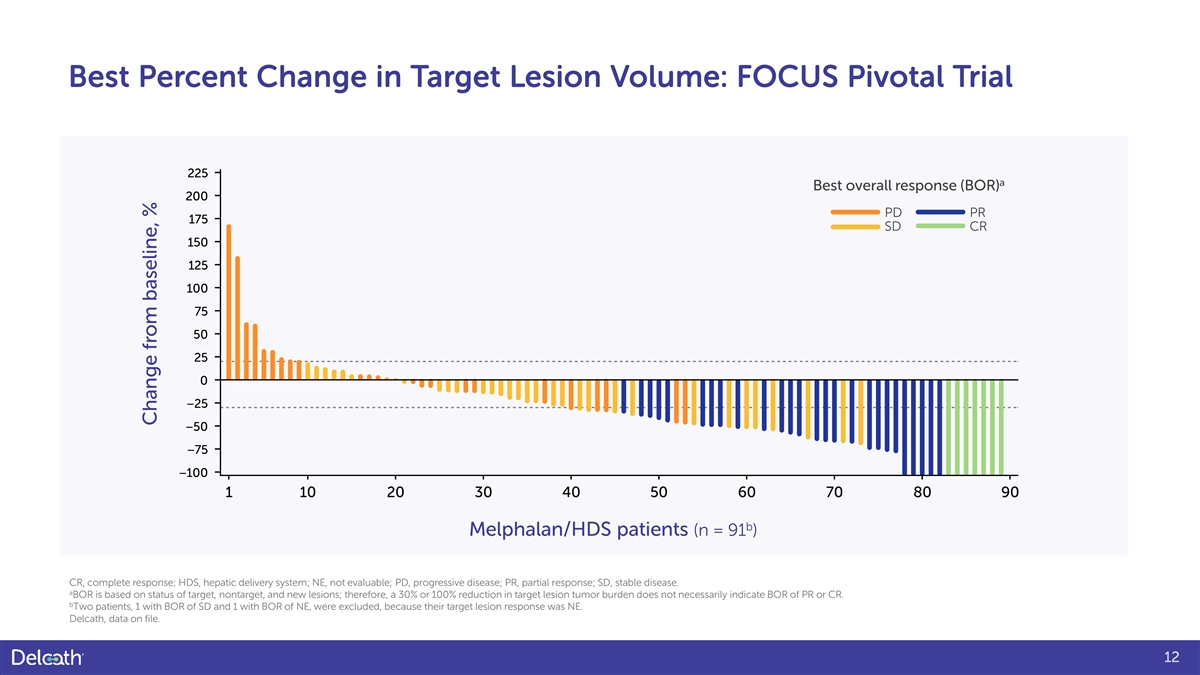
Best Percent Change in Target Lesion Volume: FOCUS Pivotal Trial 225 a Best overall response (BOR) 200 PD PR 175 SD CR 150 125 100 75 50 25 0 −25 −50 −75 −100 1 10 20 30 40 50 60 70 80 90 b Melphalan/HDS patients (n = 91 ) CR, complete response; HDS, hepatic delivery system; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease. a BOR is based on status of target, nontarget, and new lesions; therefore, a 30% or 100% reduction in target lesion tumor burden does not necessarily indicate BOR of PR or CR. b Two patients, 1 with BOR of SD and 1 with BOR of NE, were excluded, because their target lesion response was NE. Delcath, data on file. 12 Change from baseline, %

Metastatic Uveal Melanoma (mUM) 13
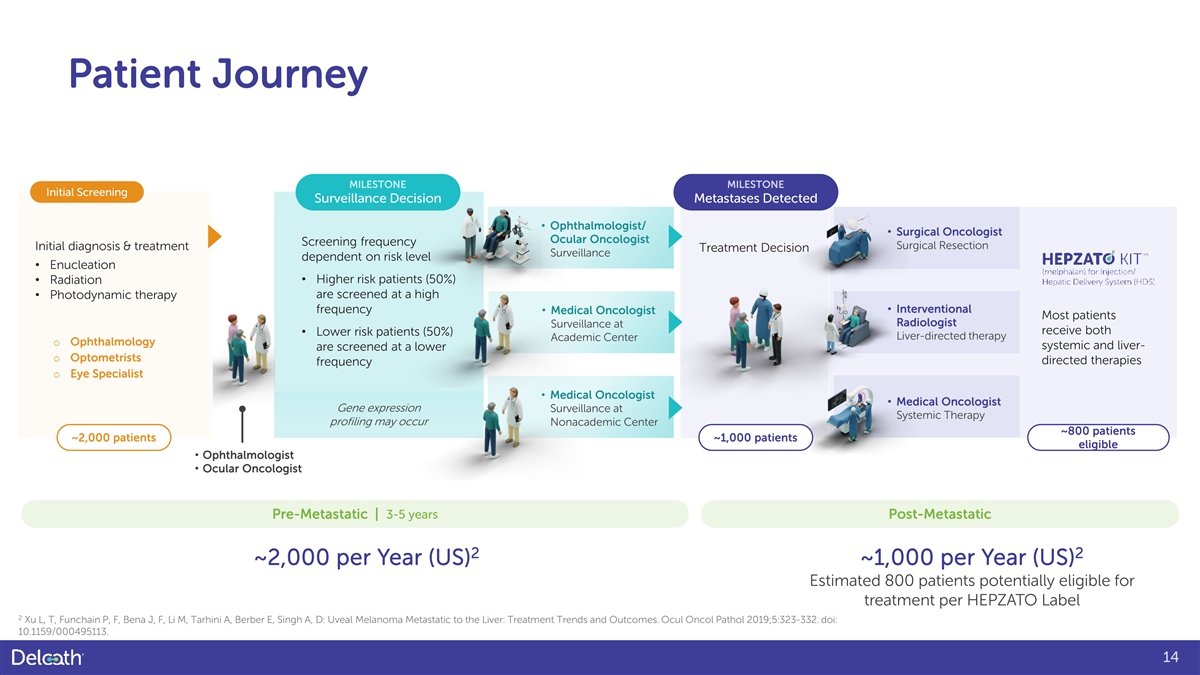
Patient Journey MILESTONE MILESTONE Initial Screening Surveillance Decision Metastases Detected • Ophthalmologist/ • Surgical Oncologist Ocular Oncologist Screening frequency Surgical Resection Initial diagnosis & treatment Treatment Decision Surveillance dependent on risk level • Enucleation • Radiation • Higher risk patients (50%) • Photodynamic therapy are screened at a high • Interventional frequency • Medical Oncologist Most patients Radiologist Surveillance at receive both • Lower risk patients (50%) Liver-directed therapy Academic Center o Ophthalmology systemic and liver- are screened at a lower o Optometrists directed therapies frequency o Eye Specialist • Medical Oncologist • Medical Oncologist Gene expression Surveillance at Systemic Therapy profiling may occur Nonacademic Center ~800 patients ~2,000 patients ~1,000 patients eligible • Ophthalmologist • Ocular Oncologist Pre-Metastatic | 3-5 years Post-Metastatic 2 2 ~2,000 per Year (US) ~1,000 per Year (US) Estimated 800 patients potentially eligible for treatment per HEPZATO Label 2 Xu L, T, Funchain P, F, Bena J, F, Li M, Tarhini A, Berber E, Singh A, D: Uveal Melanoma Metastatic to the Liver: Treatment Trends and Outcomes. Ocul Oncol Pathol 2019;5:323-332. doi: 10.1159/000495113. 14
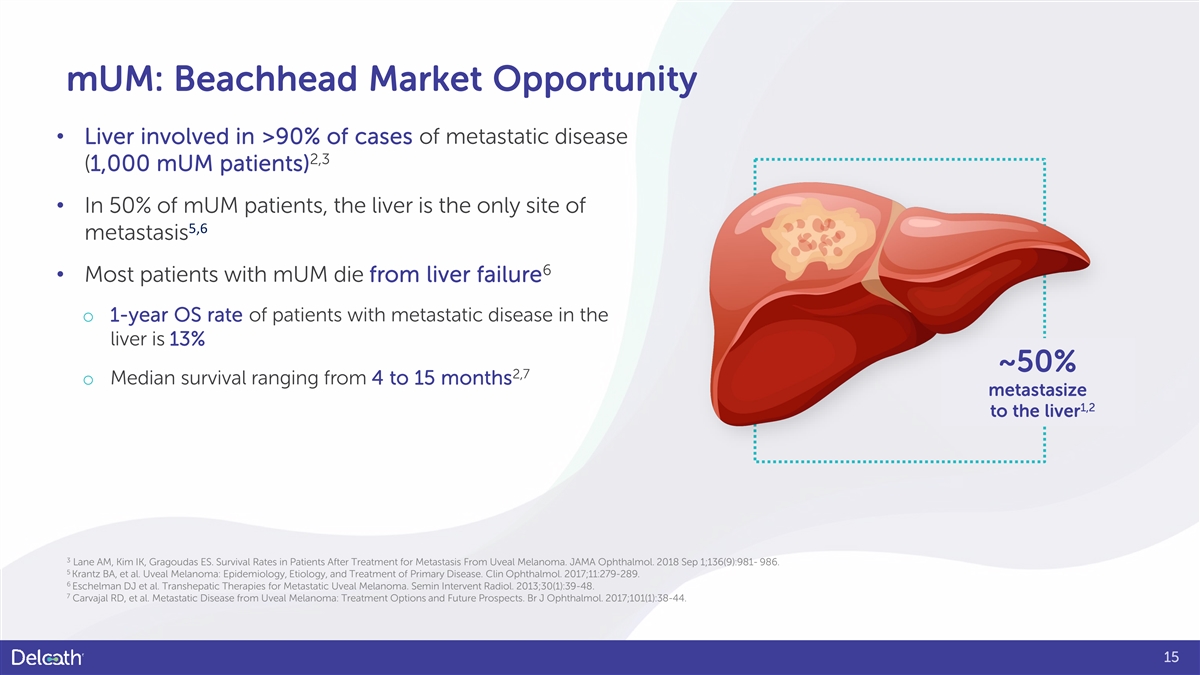
mUM: Beachhead Market Opportunity • Liver involved in >90% of cases of metastatic disease 2,3 (1,000 mUM patients) • In 50% of mUM patients, the liver is the only site of 5,6 metastasis 6 • Most patients with mUM die from liver failure o 1-year OS rate of patients with metastatic disease in the liver is 13% ~50% 2,7 o Median survival ranging from 4 to 15 months metastasize 1,2 to the liver 3 Lane AM, Kim IK, Gragoudas ES. Survival Rates in Patients After Treatment for Metastasis From Uveal Melanoma. JAMA Ophthalmol. 2018 Sep 1;136(9):981- 986. 5 Krantz BA, et al. Uveal Melanoma: Epidemiology, Etiology, and Treatment of Primary Disease. Clin Ophthalmol. 2017;11:279-289. 6 Eschelman DJ et al. Transhepatic Therapies for Metastatic Uveal Melanoma. Semin Intervent Radiol. 2013;30(1):39-48. 7 Carvajal RD, et al. Metastatic Disease from Uveal Melanoma: Treatment Options and Future Prospects. Br J Ophthalmol. 2017;101(1):38-44. 15

Competitive Landscape • 55% of patients have no approved systemic treatment option • Most patients treated with multiple lines of therapy Primary Systemic Competitors Primary LDT Competitors • Kimmtrak (tebentafusp) for HLA + (~45% of patients) • TACE (limited efficacy data, not suited for diffuse disease) • IPI/NIVO (in combination) for HLA – • SIRT (limited to two treatments, not suitable for multi-lobar disease) Competitive Positioning Competitive Positioning st st • Ideally all patients will receive a Liver Directed Therapy • 1 line for all that believe in LDT 1 line st nd (LDT) as either 1 or 2 line given most patients die of • Whole liver treatment vs. targeted treatment is necessary liver failure • PHP leaves options for additional LDT’s, Y90 and TACE • LDT and systemic therapies should be considered as do not complements in mUM 16

HEPZATO KIT: Commercialization 17

Active Commercial Centers As of 1/9/26 40 • Cleveland Clinic Main Campus - Cleveland, OH • Providence Saint John's Health Center - Santa Monica, CA • City of Hope - Duarte, CA • Regional One Health - Memphis, TN active centers targeted in 2026 • Duke Cancer Center - Durham, NC • Stanford Health Care - Stanford, CA • Emory University Hospital - Atlanta, GA • Thomas Jefferson University Hospital - Philadelphia, PA • HonorHealth Lincoln – Phoenix, AZ • UC San Diego Health - San Diego, CA • HonorHealth Scottsdale Shea - Scottsdale, AZ • UCLA Health - Santa Monica, CA • Massachusetts General Hospital - Boston, MA • UNC Health Medical Center - Chapel Hill, NC 32 • Mayo Clinic - Jacksonville, FL • University of Alabama - Birmingham, AL • Mayo Clinic - Scottsdale, AZ • University of Chicago Medical Center - Chicago, IL sites are accepting referrals • MD Anderson Cancer Center - Houston, TX • University of Iowa Medical Center, Iowa City, IA • Memorial Sloan Kettering Cancer Center - New York, NY • University of Miami Hospital - Miami, FL • Moffitt Cancer Center - Tampa, FL • University of Utah Hospital - Salt Lake City, UT • Northwestern Memorial Hospital – Chicago, IL • University of Kansas Cancer Center – Overland Park, KS • Ochsner Medical Center - New Orleans, LA • University of Virginia Medical Center - Charlottesville, VA 25 • Ohio State University - Columbus, OH • University of Wisconsin Hospital – Madison, WI active sites as of January 9, 2026 • Oregon Health and Sciences University - Portland, OR • UT Southwestern Medical Center - Dallas, TX View the HEPZATO KIT Healthcare Setting Locator + and sites now accepting referrals 20 sites in active conversation Click here 18

Delivering an Innovative Treatment with a Well-Trained Team Treatment with HEPZATO KIT involves training and a team approach. The team members below complete a preceptorship and proctorship as well as a risk evaluation and mitigation strategy (REMS) training. Interventional radiologist leads and performs the vascular interventional procedure Perfusionist establishes, monitors, and controls the extracorporeal pump and veno-venous bypass circuit Anesthesiologist manages sedation, analgesia, and respiratory and cardiovascular support All REMS materials are available at www.HEPZATOKITREMS.com or by calling the REMS Coordinating Center at 1-833-632-0457. 19
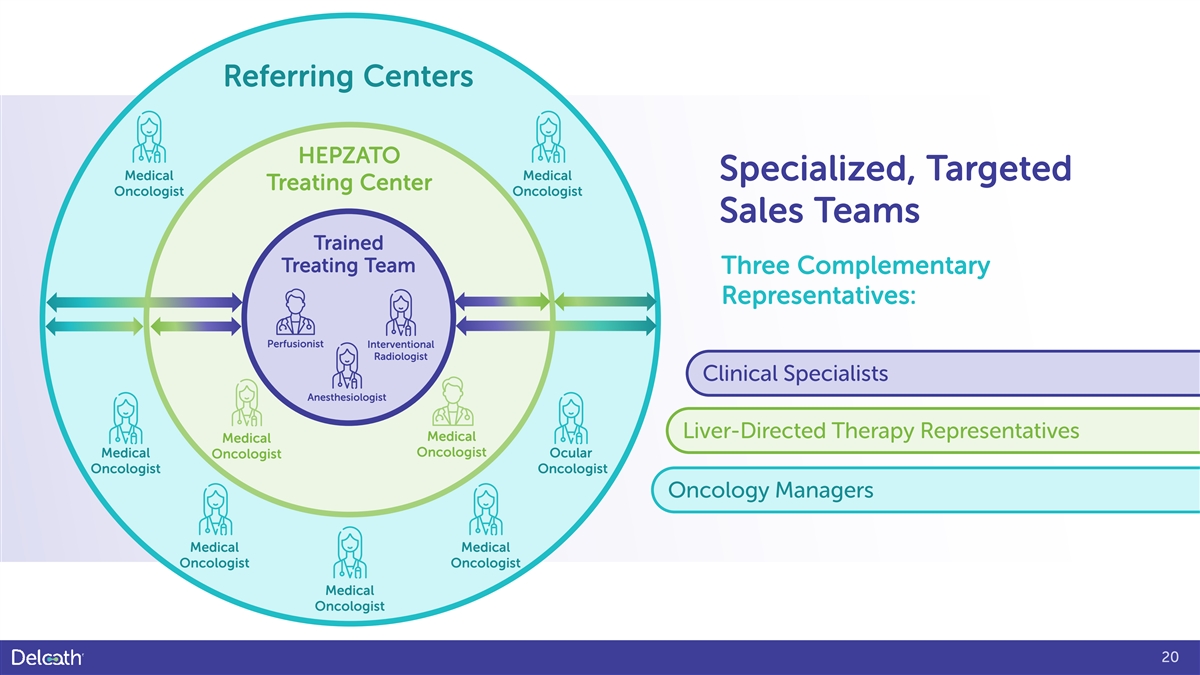
Referring Centers HEPZATO Medical Medical Specialized, Targeted Treating Center Oncologist Oncologist Sales Teams Trained Treating Team Three Complementary Representatives: Perfusionist Interventional Radiologist Clinical Specialists Anesthesiologist Liver-Directed Therapy Representatives Medical Medical Oncologist Medical Ocular Oncologist Oncologist Oncologist Oncology Managers Medical Medical Oncologist Oncologist Medical Oncologist 20

HEPZATO KIT: Reimbursement 21
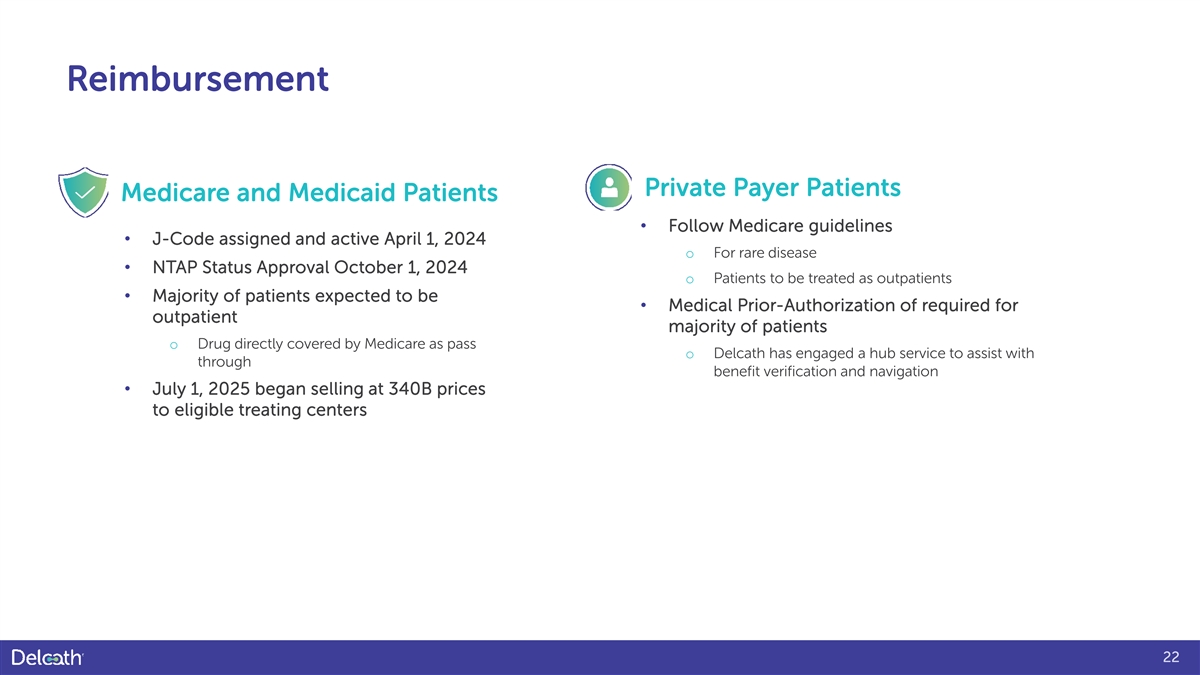
Reimbursement Private Payer Patients Medicare and Medicaid Patients • Follow Medicare guidelines • J-Code assigned and active April 1, 2024 o For rare disease • NTAP Status Approval October 1, 2024 o Patients to be treated as outpatients • Majority of patients expected to be • Medical Prior-Authorization of required for outpatient majority of patients o Drug directly covered by Medicare as pass o Delcath has engaged a hub service to assist with through benefit verification and navigation • July 1, 2025 began selling at 340B prices to eligible treating centers 22
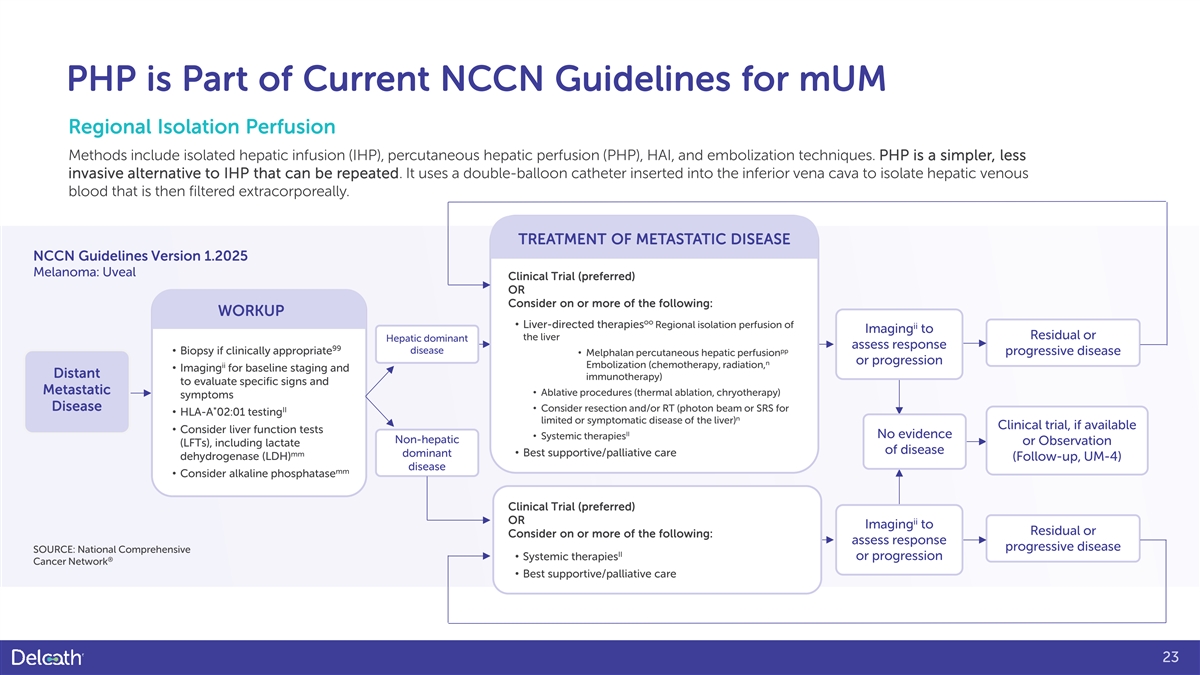
PHP is Part of Current NCCN Guidelines for mUM Regional Isolation Perfusion Methods include isolated hepatic infusion (IHP), percutaneous hepatic perfusion (PHP), HAI, and embolization techniques. PHP is a simpler, less invasive alternative to IHP that can be repeated. It uses a double-balloon catheter inserted into the inferior vena cava to isolate hepatic venous blood that is then filtered extracorporeally. TREATMENT OF METASTATIC DISEASE NCCN Guidelines Version 1.2025 Melanoma: Uveal Clinical Trial (preferred) OR Consider on or more of the following: WORKUP oo • Liver-directed therapies Regional isolation perfusion of ii Imaging to the liver Residual or Hepatic dominant assess response 99 disease pp • Biopsy if clinically appropriate progressive disease • Melphalan percutaneous hepatic perfusion n or progression ii Embolization (chemotherapy, radiation, • Imaging for baseline staging and Distant immunotherapy) to evaluate specific signs and Metastatic • Ablative procedures (thermal ablation, chryotherapy) symptoms Disease • Consider resection and/or RT (photon beam or SRS for * II • HLA-A 02:01 testing n limited or symptomatic disease of the liver) Clinical trial, if available • Consider liver function tests II No evidence • Systemic therapies Non-hepatic or Observation (LFTs), including lactate of disease mm • Best supportive/palliative care dominant dehydrogenase (LDH) (Follow-up, UM-4) disease mm • Consider alkaline phosphatase Clinical Trial (preferred) OR ii Imaging to Residual or Consider on or more of the following: assess response progressive disease SOURCE: National Comprehensive II • Systemic therapies or progression ® Cancer Network • Best supportive/palliative care 23
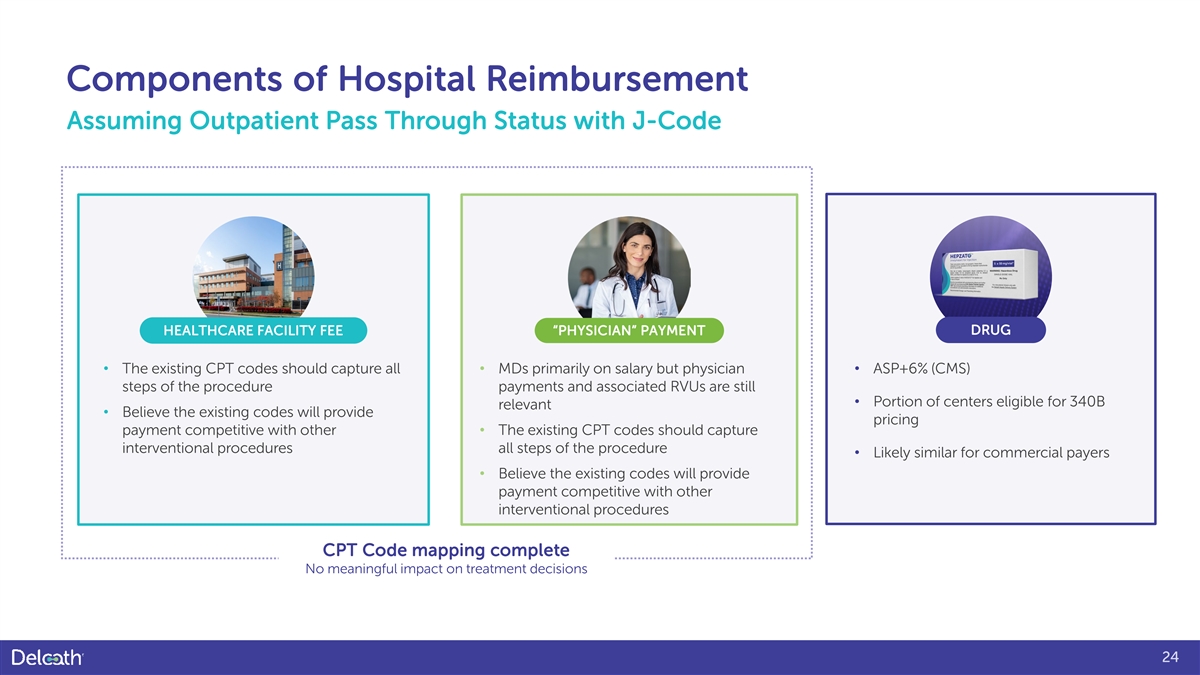
Components of Hospital Reimbursement Assuming Outpatient Pass Through Status with J-Code HEALTHCARE FACILITY FEE “PHYSICIAN” PAYMENT DRUG • The existing CPT codes should capture all • MDs primarily on salary but physician • ASP+6% (CMS) steps of the procedure payments and associated RVUs are still • Portion of centers eligible for 340B relevant • Believe the existing codes will provide pricing payment competitive with other • The existing CPT codes should capture interventional procedures all steps of the procedure • Likely similar for commercial payers • Believe the existing codes will provide payment competitive with other interventional procedures CPT Code mapping complete No meaningful impact on treatment decisions 24

NEXT STEPS: Future Indications 25

Clinical Rationale for Broad Development Effort Melphalan has demonstrated clinical activity in multiple tumor types Promising ORR, DCR and PFS signals seen across multiple tumor types with CHEMOSAT in Europe and in earlier studies with IHP In many solid tumor patients, liver metastases are often life limiting HEPZATO is currently the only liver-directed treatment that can repeatedly treat the whole liver Potential for significant improvement in survival Converting unresectable liver metastases into resectable metastases and adjuvant usage to prevent recurrence Potential for sequential usage with Immune-Oncology (I/O) agents Liver metastases reduce I/O therapy efficacy due to the tumor microenvironment inducing immune tolerance, HEPZATO may reduce this effect 26
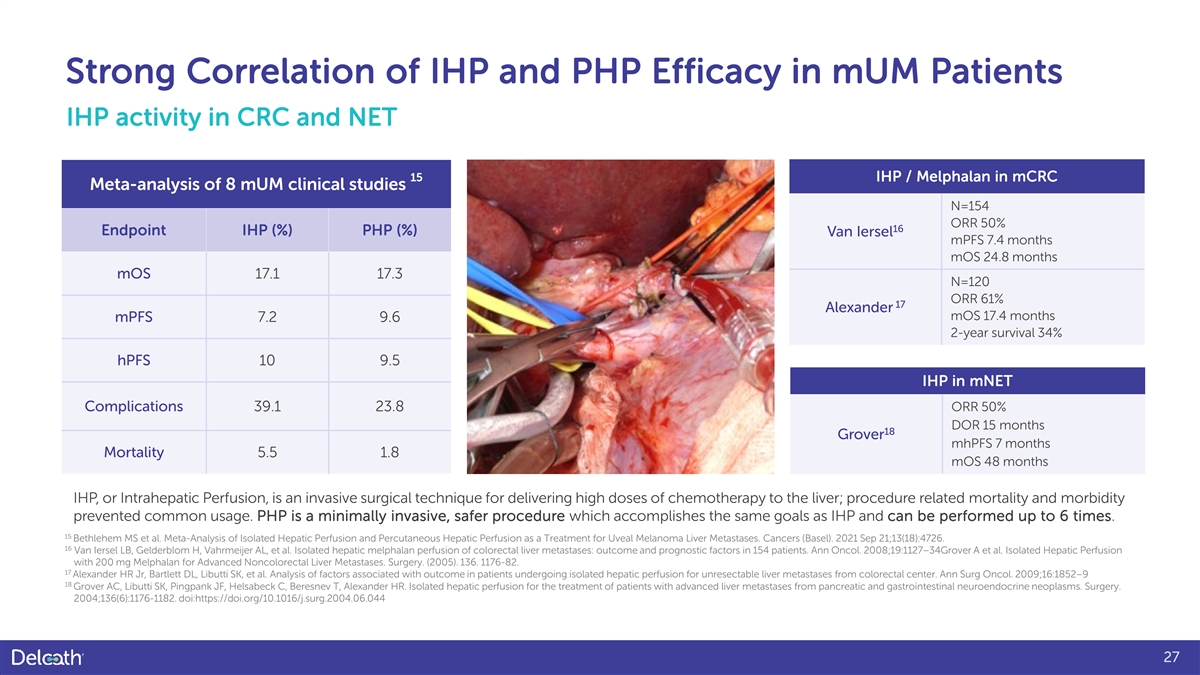
Strong Correlation of IHP and PHP Efficacy in mUM Patients IHP activity in CRC and NET 15 IHP / Melphalan in mCRC Meta-analysis of 8 mUM clinical studies N=154 ORR 50% 16 Endpoint IHP (%) PHP (%) Van Iersel mPFS 7.4 months mOS 24.8 months mOS 17.1 17.3 N=120 ORR 61% 17 Alexander mOS 17.4 months mPFS 7.2 9.6 2-year survival 34% hPFS 10 9.5 IHP in mNET Complications 39.1 23.8 ORR 50% DOR 15 months 18 Grover mhPFS 7 months Mortality 5.5 1.8 mOS 48 months IHP, or Intrahepatic Perfusion, is an invasive surgical technique for delivering high doses of chemotherapy to the liver; procedure related mortality and morbidity prevented common usage. PHP is a minimally invasive, safer procedure which accomplishes the same goals as IHP and can be performed up to 6 times. 15 Bethlehem MS et al. Meta-Analysis of Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver Metastases. Cancers (Basel). 2021 Sep 21;13(18):4726. 16 Van Iersel LB, Gelderblom H, Vahrmeijer AL, et al. Isolated hepatic melphalan perfusion of colorectal liver metastases: outcome and prognostic factors in 154 patients. Ann Oncol. 2008;19:1127–34Grover A et al. Isolated Hepatic Perfusion with 200 mg Melphalan for Advanced Noncolorectal Liver Metastases. Surgery. (2005). 136. 1176-82. 17 Alexander HR Jr, Bartlett DL, Libutti SK, et al. Analysis of factors associated with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal center. Ann Surg Oncol. 2009;16:1852–9 18 Grover AC, Libutti SK, Pingpank JF, Helsabeck C, Beresnev T, Alexander HR. Isolated hepatic perfusion for the treatment of patients with advanced liver metastases from pancreatic and gastrointestinal neuroendocrine neoplasms. Surgery. 2004;136(6):1176-1182. doi:https://doi.org/10.1016/j.surg.2004.06.044 27
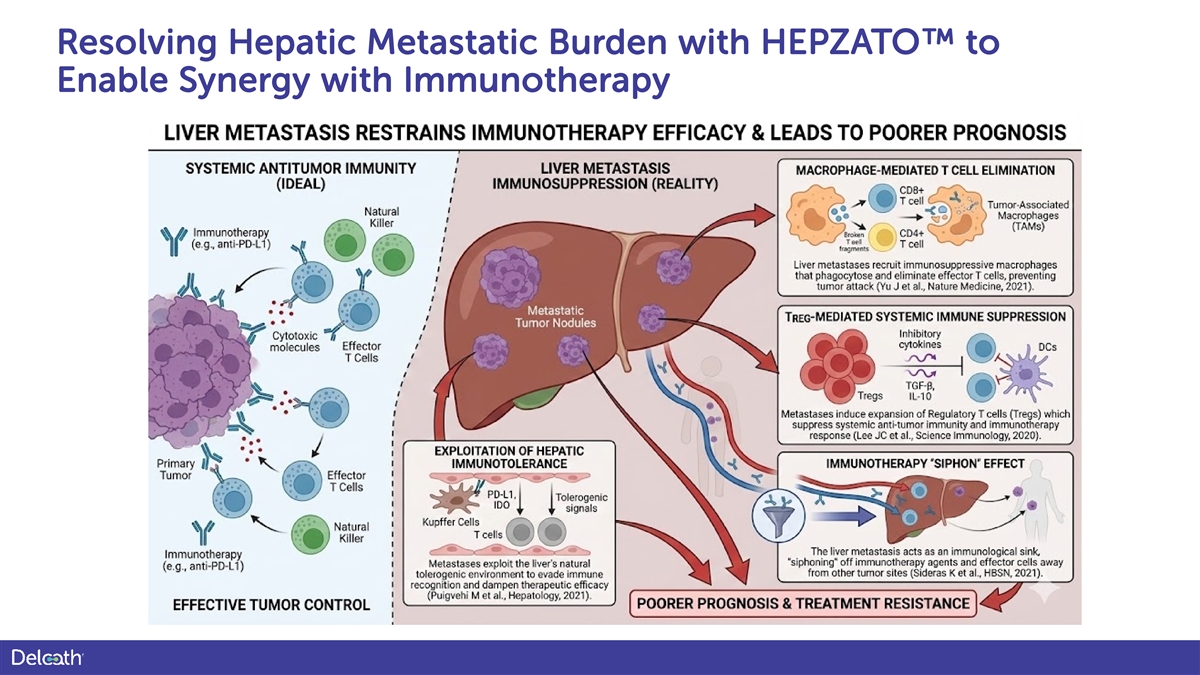
Resolving Hepatic Metastatic Burden with HEPZATO to Enable Synergy with Immunotherapy
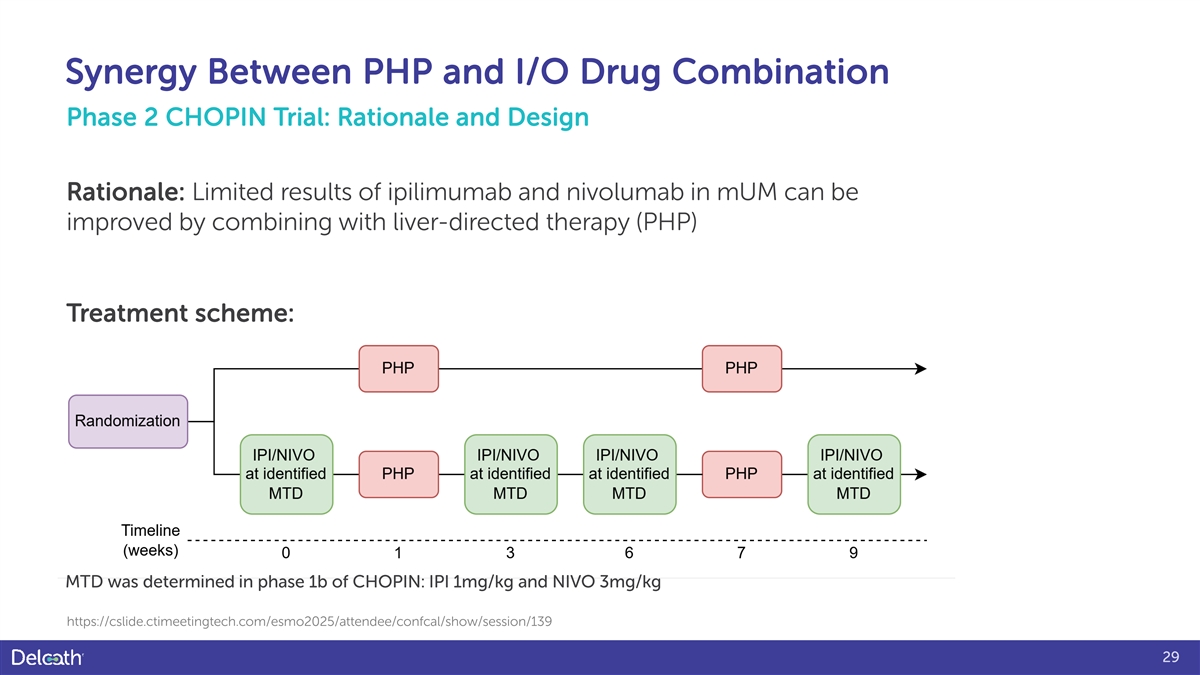
Synergy Between PHP and I/O Drug Combination Phase 2 CHOPIN Trial: Rationale and Design Rationale: Limited results of ipilimumab and nivolumab in mUM can be improved by combining with liver-directed therapy (PHP) Treatment scheme: MTD was determined in phase 1b of CHOPIN: IPI 1mg/kg and NIVO 3mg/kg https://cslide.ctimeetingtech.com/esmo2025/attendee/confcal/show/session/139 29
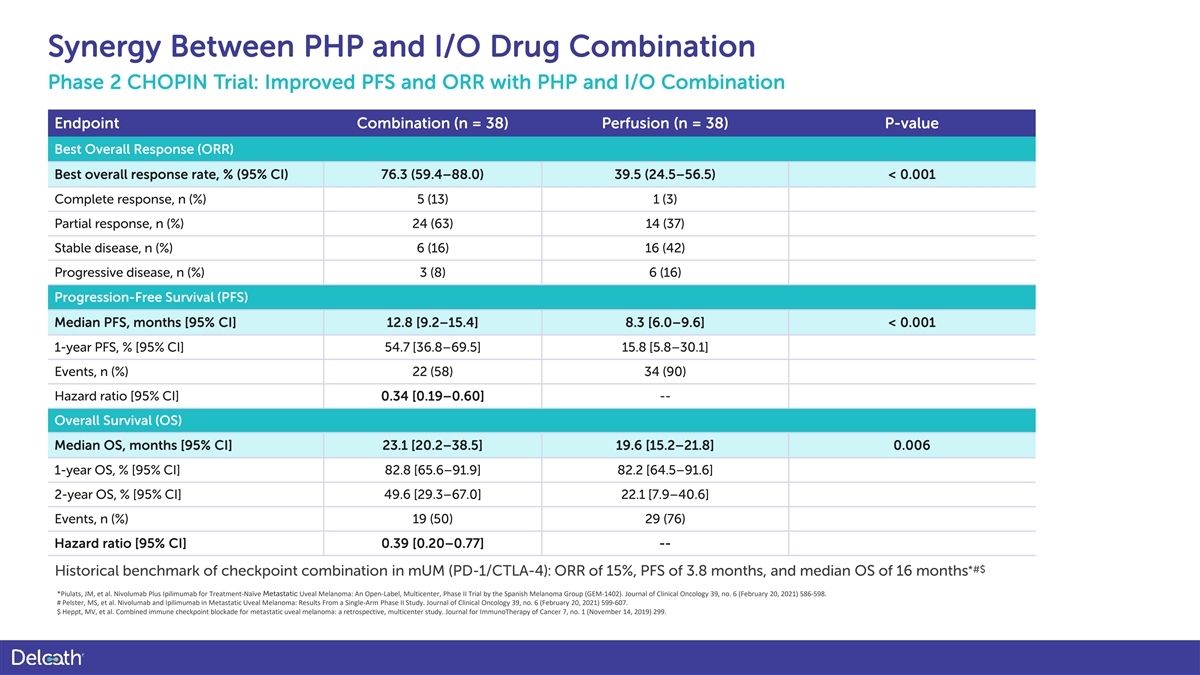
Synergy Between PHP and I/O Drug Combination Phase 2 CHOPIN Trial: Improved PFS and ORR with PHP and I/O Combination Endpoint Combination (n = 38) Perfusion (n = 38) P-value Best Overall Response (ORR) Best overall response rate, % (95% CI) 76.3 (59.4–88.0) 39.5 (24.5–56.5) < 0.001 Complete response, n (%) 5 (13) 1 (3) Partial response, n (%) 24 (63) 14 (37) Stable disease, n (%) 6 (16) 16 (42) Progressive disease, n (%) 3 (8) 6 (16) Progression-Free Survival (PFS) Median PFS, months [95% CI] 12.8 [9.2–15.4] 8.3 [6.0–9.6] < 0.001 1-year PFS, % [95% CI] 54.7 [36.8–69.5] 15.8 [5.8–30.1] Events, n (%) 22 (58) 34 (90) Hazard ratio [95% CI] 0.34 [0.19–0.60] -- Overall Survival (OS) Median OS, months [95% CI] 23.1 [20.2–38.5] 19.6 [15.2–21.8] 0.006 1-year OS, % [95% CI] 82.8 [65.6–91.9] 82.2 [64.5–91.6] 2-year OS, % [95% CI] 49.6 [29.3–67.0] 22.1 [7.9–40.6] Events, n (%) 19 (50) 29 (76) Hazard ratio [95% CI] 0.39 [0.20–0.77] -- *#$ Historical benchmark of checkpoint combination in mUM (PD-1/CTLA-4): ORR of 15%, PFS of 3.8 months, and median OS of 16 months *Piulats, JM, et al. Nivolumab Plus Ipilimumab for Treatment-Naïve Metastatic Uveal Melanoma: An Open-Label, Multicenter, Phase II Trial by the Spanish Melanoma Group (GEM-1402). Journal of Clinical Oncology 39, no. 6 (February 20, 2021) 586-598. # Pelster, MS, et al. Nivolumab and Ipilimumab in Metastatic Uveal Melanoma: Results From a Single-Arm Phase II Study. Journal of Clinical Oncology 39, no. 6 (February 20, 2021) 599-607. $ Heppt, MV, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multicenter study. Journal for ImmunoTherapy of Cancer 7, no. 1 (November 14, 2019) 299.
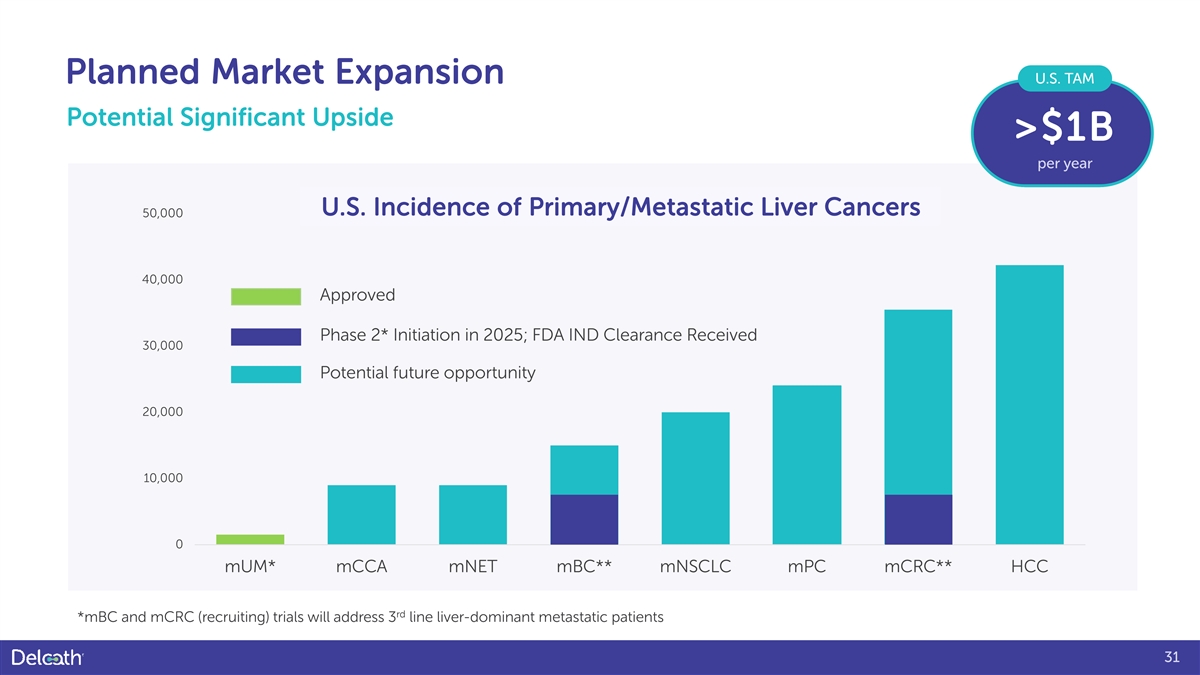
Planned Market Expansion U.S. TAM Potential Significant Upside >$1B per year U.S. Incidence of Primary/Metastatic Liver Cancers 50,000 40,000 Approved Phase 2* Initiation in 2025; FDA IND Clearance Received 30,000 Potential future opportunity 20,000 10,000 0 mUM* mCCA mNET mBC** mNSCLC mPC mCRC** HCC rd *mBC and mCRC (recruiting) trials will address 3 line liver-dominant metastatic patients 31

Financials 32
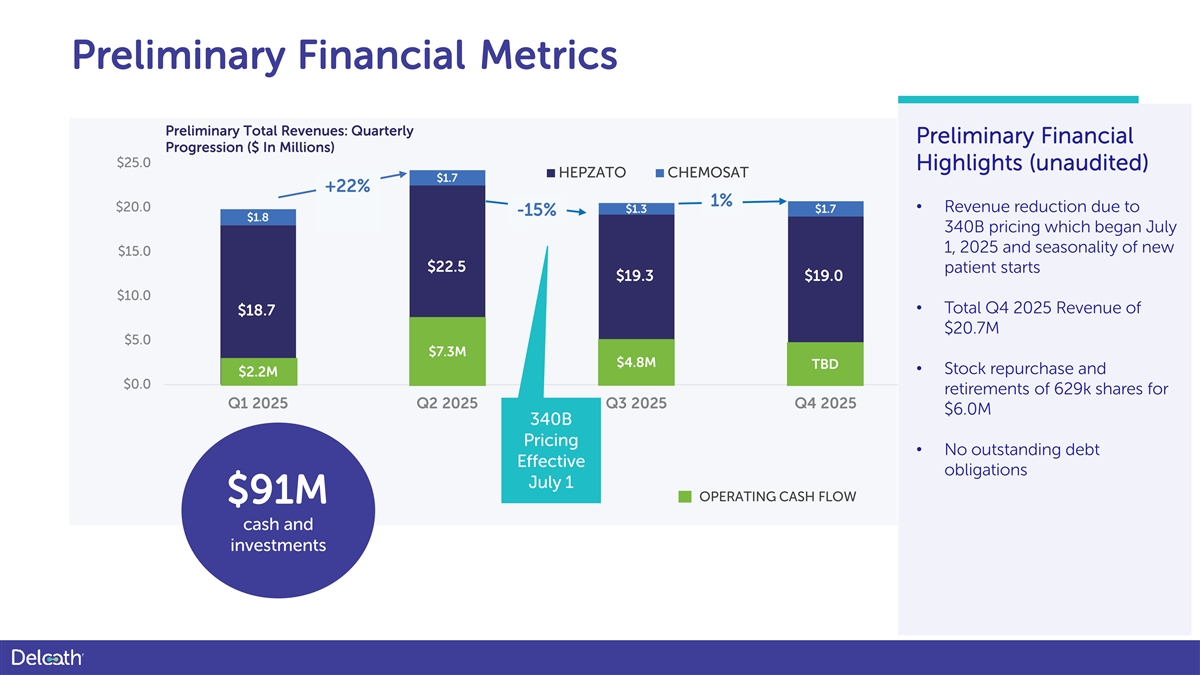
Preliminary Financial Metrics Preliminary Total Revenues: Quarterly Preliminary Financial Progression ($ In Millions) $25.0 Highlights (unaudited) HEPZATO CHEMOSAT $1.7 +22% 1% $20.0 $1.3 $1.7 • Revenue reduction due to -15% $1.8 340B pricing which began July 1, 2025 and seasonality of new $15.0 $22.5 patient starts $19.3 $19.0 $10.0 • Total Q4 2025 Revenue of $18.7 $18.0 $20.7M $5.0 $7.3M $4.8M TBD • Stock repurchase and $2.2M $0.0 retirements of 629k shares for Q1 2025 Q2 2025 Q3 2025 Q4 2025 $6.0M 340B Pricing • No outstanding debt Effective obligations July 1 OPERATING CASH FLOW $91M cash and investments
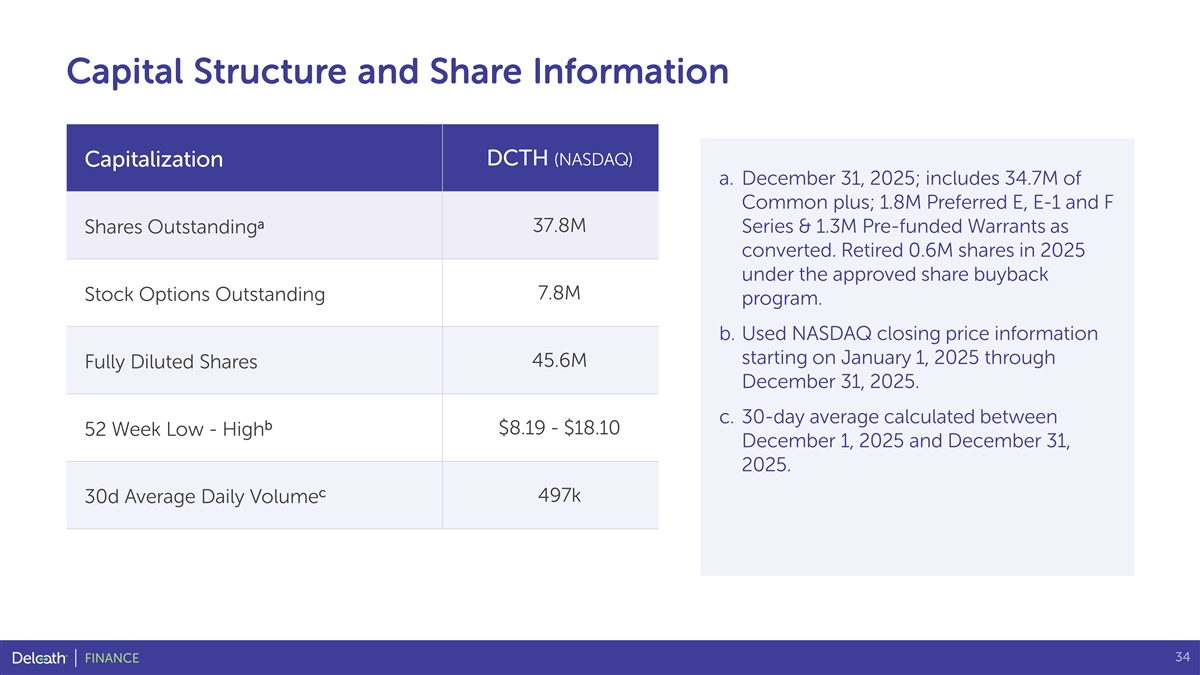
Capital Structure and Share Information DCTH (NASDAQ) Capitalization a. December 31, 2025; includes 34.7M of Common plus; 1.8M Preferred E, E-1 and F a 37.8M Series & 1.3M Pre-funded Warrants as Shares Outstanding converted. Retired 0.6M shares in 2025 under the approved share buyback 7.8M Stock Options Outstanding program. b. Used NASDAQ closing price information starting on January 1, 2025 through 45.6M Fully Diluted Shares December 31, 2025. c. 30-day average calculated between b $8.19 - $18.10 52 Week Low - High December 1, 2025 and December 31, 2025. c 497k 30d Average Daily Volume 34 FINANCE

Multi-Disciplinary, Experienced Leadership Team David Hoffman Gerard Michel Martha S. Rook, PhD GENERAL COUNSEL, CORP SECRETARY & CHIEF EXECUTIVE OFFICER CHIEF OPERATING OFFICER CHIEF COMPLIANCE OFFICER • 30+ yrs. pharma/medtech • 25+ yrs. molecular bio., process dev., manufacturing, supply chain and experience quality experience • 20+ yrs. advising biotech • C-suite roles at Vericel Corp, companies with a focus on the • Senior roles at insitro, Sigilon Biodel, & NPS commercialization of therapies Therapeutics, and MilliporeSigma • M.S. Microbiology, B.S. Biology & • Previously Associate General • Ph.D. Biochemistry from MIT, B.S. in Geology from the Univ. of Counsel and Chief Compliance chemistry from Texas A&M Rochester School of Medicine Officer at Vericel Corporation • Postdoctoral studies at Harvard • M.B.A. Simon School of Business & Medical School Leadership Kevin Muir Sandra Pennell Vojislav Vukovic, MD PhD GENERAL MANAGER, INTERVENTIONAL ONCOLOGY CHIEF FINANCIAL OFFICER CHIEF MEDICAL OFFICER • 20+ yrs. medtech/bioTx sales & • 20+ years' biotech financial marketing experience • Oncology dev. exec, global clinical oversight experience expertise • Senior leadership roles at BTG, • Manages global financial affairs, ClearFlow, Aragon Surgical, Kensey • Former CMO at Aileron, Taiho, Synta U.S. GAAP compliance Nash Corporation, and Kyphon • MD, Univ. of Sarajevo | MSc, PhD, • Led finance at Invivyd • Field Artillery officer, U.S. Army Univ. of Toronto • VP at Vericel Corp • B.S. in Management Systems • Published, AACR, ASCO, ASH, ESMO Engineering, U.S. Military Academy member • MSc, Accountancy, Univ. of Illinois at West Point John R. Sylvester, Chairman Elizabeth Czerepak, Director Dr. Gil Aharon, Ph.D., Director Board of Directors Bridget Martell, MA, MD, Director Steven Salamon, Director Gerard Michel, CEO 35

U.S. Registration Trial for the Treatment of Patients with mUM 36
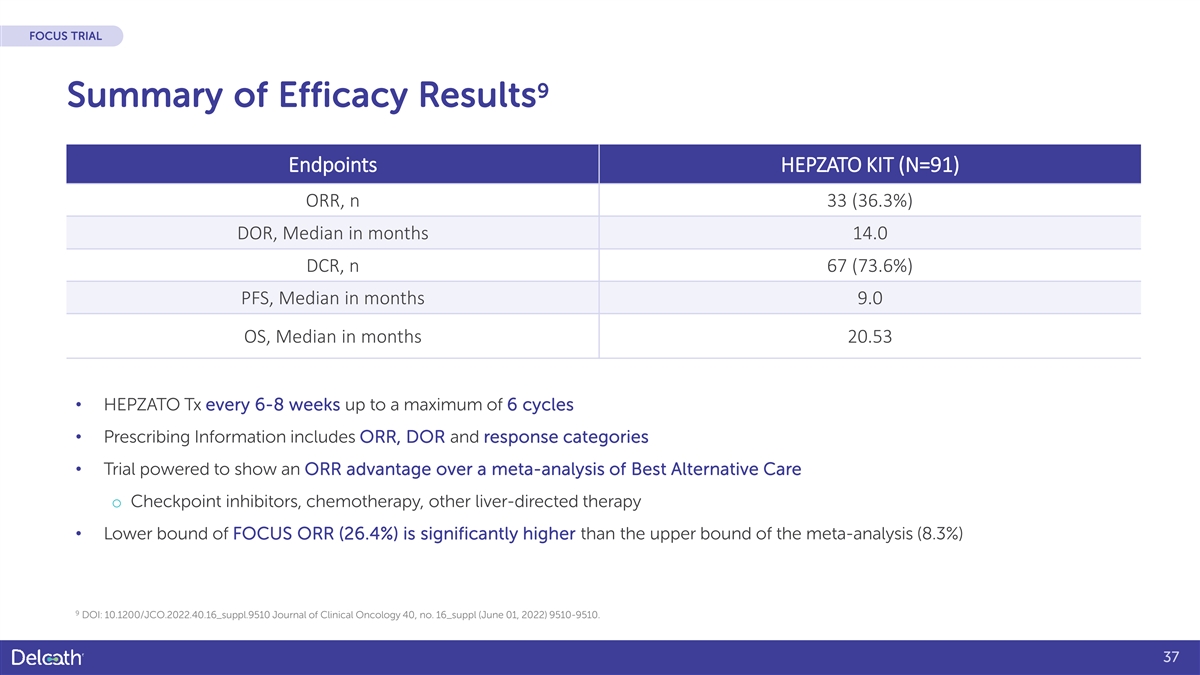
FOCUS TRIAL 9 Summary of Efficacy Results Endpoints HEPZATO KIT (N=91) ORR, n 33 (36.3%) DOR, Median in months 14.0 DCR, n 67 (73.6%) PFS, Median in months 9.0 OS, Median in months 20.53 • HEPZATO Tx every 6-8 weeks up to a maximum of 6 cycles • Prescribing Information includes ORR, DOR and response categories • Trial powered to show an ORR advantage over a meta-analysis of Best Alternative Care o Checkpoint inhibitors, chemotherapy, other liver-directed therapy • Lower bound of FOCUS ORR (26.4%) is significantly higher than the upper bound of the meta-analysis (8.3%) 9 DOI: 10.1200/JCO.2022.40.16_suppl.9510 Journal of Clinical Oncology 40, no. 16_suppl (June 01, 2022) 9510-9510. 37
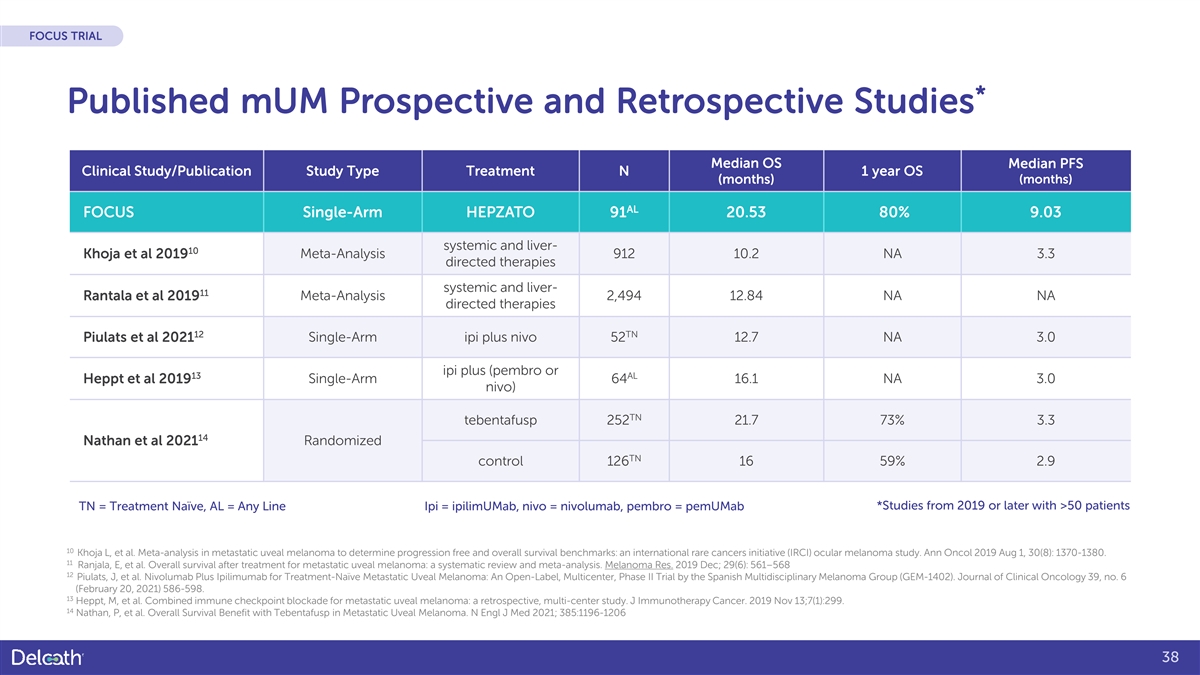
FOCUS TRIAL * Published mUM Prospective and Retrospective Studies Median OS Median PFS Clinical Study/Publication Study Type Treatment N 1 year OS (months) (months) AL FOCUS Single-Arm HEPZATO 91 20.53 80% 9.03 systemic and liver- 10 Khoja et al 2019 Meta-Analysis 912 10.2 NA 3.3 directed therapies systemic and liver- 11 Rantala et al 2019 Meta-Analysis 2,494 12.84 NA NA directed therapies 12 TN Piulats et al 2021 Single-Arm ipi plus nivo 52 12.7 NA 3.0 ipi plus (pembro or 13 AL Heppt et al 2019 Single-Arm 64 16.1 NA 3.0 nivo) TN tebentafusp 252 21.7 73% 3.3 14 Nathan et al 2021 Randomized TN control 126 16 59% 2.9 TN = Treatment Naïve, AL = Any Line Ipi = ipilimUMab, nivo = nivolumab, pembro = pemUMab *Studies from 2019 or later with >50 patients 10 Khoja L, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol 2019 Aug 1, 30(8): 1370-1380. 11 Ranjala, E, et al. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019 Dec; 29(6): 561–568 12 Piulats, J, et al. Nivolumab Plus Ipilimumab for Treatment-Naïve Metastatic Uveal Melanoma: An Open-Label, Multicenter, Phase II Trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). Journal of Clinical Oncology 39, no. 6 (February 20, 2021) 586-598. 13 Heppt, M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunotherapy Cancer. 2019 Nov 13;7(1):299. 14 Nathan, P, et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med 2021; 385:1196-1206 38
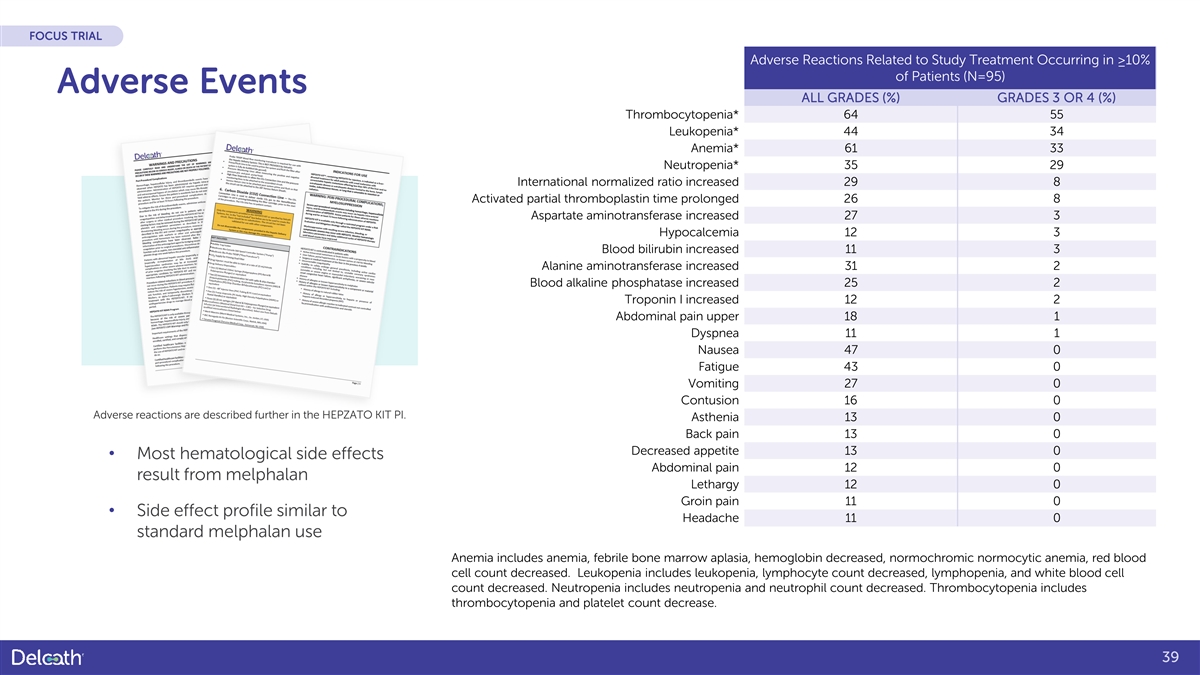
FOCUS TRIAL Adverse Reactions Related to Study Treatment Occurring in ≥10% of Patients (N=95) Adverse Events ALL GRADES (%) GRADES 3 OR 4 (%) Thrombocytopenia* 64 55 Leukopenia* 44 34 Anemia* 61 33 Neutropenia* 35 29 International normalized ratio increased 29 8 Activated partial thromboplastin time prolonged 26 8 Aspartate aminotransferase increased 27 3 Hypocalcemia 12 3 Blood bilirubin increased 11 3 Alanine aminotransferase increased 31 2 Blood alkaline phosphatase increased 25 2 Troponin I increased 12 2 Abdominal pain upper 18 1 Dyspnea 11 1 Nausea 47 0 Fatigue 43 0 Vomiting 27 0 Contusion 16 0 Adverse reactions are described further in the HEPZATO KIT PI. Asthenia 13 0 Back pain 13 0 Decreased appetite 13 0 • Most hematological side effects Abdominal pain 12 0 result from melphalan Lethargy 12 0 Groin pain 11 0 • Side effect profile similar to Headache 11 0 standard melphalan use Anemia includes anemia, febrile bone marrow aplasia, hemoglobin decreased, normochromic normocytic anemia, red blood cell count decreased. Leukopenia includes leukopenia, lymphocyte count decreased, lymphopenia, and white blood cell count decreased. Neutropenia includes neutropenia and neutrophil count decreased. Thrombocytopenia includes thrombocytopenia and platelet count decrease. 39

Thank You 40

References 1. Reddy S, et al. Isolated hepatic perfusion for patients with liver metastases, Ther Adv Med Oncol. 2014 Jul; 6(4): 180-194. 2. Xu L, T, Funchain P, F, Bena J, F, Li M, Tarhini A, Berber E, Singh A, D: Uveal Melanoma Metastatic to the Liver: Treatment Trends and Outcomes. Ocul Oncol Pathol 2019;5:323-332. doi: 10.1159/000495113. 3. Lane AM, Kim IK, Gragoudas ES. Survival Rates in Patients After Treatment for Metastasis From Uveal Melanoma. JAMA Ophthalmol. 2018 Sep 1;136(9):981- 986. 4. Heppt, M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunotherap Cancer. 2019 Nov 13;7(1):299. 5. Krantz BA, et al. Uveal Melanoma: Epidemiology, Etiology, and Treatment of Primary Disease. Clin Ophthalmol. 2017;11:279-289 6. Eschelman DJ et al. Transhepatic Therapies for Metastatic Uveal Melanoma. Semin Intervent Radiol. 2013;30(1):39-48. 7. Carvajal RD, et al. Metastatic Disease from Uveal Melanoma: Treatment Options and Future Prospects. Br J Ophthalmol. 2017;101(1):38-44. 8. Olofsson BR, et al. Isolated Hepatic Perfusion With Melphalan for Patients With Isolated Uveal Melanoma Liver Metastases: A Multicenter, Randomized, Open-Label, Phase III Trial (the SCANDIUM Trial). J Clin Oncol. 2023 Jun 1;41(16):3042-3050. 9. DOI: 10.1200/JCO.2022.40.16_suppl.9510 Journal of Clinical Oncology 40, no. 16_suppl (June 01, 2022) 9510-9510. 10. Khoja L, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol 2019 Aug 1, 30(8): 1370-1380. 11. Ranjala, E, et al. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019 Dec; 29(6): 561–568 12. Piulats, J, et al. Nivolumab Plus Ipilimumab for Treatment-Naïve Metastatic Uveal Melanoma: An Open-Label, Multicenter, Phase II Trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). Journal of Clinical Oncology 39, no. 6 (February 20, 2021) 586-598. 13. Heppt, M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunotherapy Cancer. 2019 Nov 13;7(1):299. 14. Nathan, P, et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med 2021; 385:1196-1206 15. Bethlehem MS et al. Meta-Analysis of Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver Metastases. Cancers (Basel). 2021 Sep 21;13(18):4726. 16. Van Iersel LB, Gelderblom H, Vahrmeijer AL, et al. Isolated hepatic melphalan perfusion of colorectal liver metastases: outcome and prognostic factors in 154 patients. Ann Oncol. 2008;19:1127–34Grover A et al. Isolated Hepatic Perfusion with 200 mg Melphalan for Advanced Noncolorectal Liver Metastases. Surgery. (2005). 136. 1176-82. 17. Alexander HR Jr, Bartlett DL, Libutti SK, et al. Analysis of factors associated with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal center. Ann Surg Oncol. 2009;16:1852–9. 18. Grover AC, Libutti SK, Pingpank JF, Helsabeck C, Beresnev T, Alexander HR. Isolated hepatic perfusion for the treatment of patients with advanced liver metastases from pancreatic and gastrointestinal neuroendocrine neoplasms. Surgery. 2004;136(6):1176-1182. doi:https://doi.org/10.1016/j.surg.2004.06.044 19. Tong TML et al. Combining Melphalan Percutaneous Hepatic Perfusion with Ipilimumab Plus Nivolumab in Advanced Uveal Melanoma: First Safety and Efficacy Data from the Phase Ib Part of the Chopin Trial. Cardiovasc Intervent Radiol. 2023 Mar;46(3):350-359. 41