
Obesity KOL Webinar ARO-INHBE and ARO-ALK7 Interim Phase 1/2a Clinical Data January 06, 2026

Introduction and Agenda Vince Anzalone, CFA Vice President, Finance and IR ARO-INHBE and ARO-ALK7 Interim Clinical Data Update January 2026

3 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including, without limitation, our developmental stage and limited operating history, our ability to successfully and timely develop products, entering into new collaborations and achieving existing projected milestones, rapid technological changes in our markets, demand for our future products, legislative, regulatory and competitive developments and general economic conditions. Our Annual Report on Form 10-K, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K, and other SEC filings discuss some of the important risk factors that may affect our ability to achieve the anticipated results, as well as our business, results of operations and financial condition. Readers are cautioned not to place undue reliance on these forward- looking statements. Additionally, Arrowhead disclaims any intent to update these forward- looking statements to reflect subsequent developments.

4 Agenda Topic Presenter Introduction Vince Anzalone, CFA The Future: What’s Next in Obesity Treatment to Address the Unmet Needs Carel Le Roux, M.D., Ph.D. University College Dublin, School of Medicine Therapeutic Rationale for Targeting the Activin E - ALK7 Axis James Hamilton, M.D., MBA Interim Results from Phase 1/2a Studies James Hamilton, M.D., MBA Key Takeaways Chris Anzalone, Ph.D. Q&A Panel

5 Obesity Key Opinion Leader Carel le Roux, M.D., Ph.D. Chair of Metabolic Medicine, University College Dublin, School of Medicine Professor Carel le Roux graduated from medical school in Pretoria South Africa, completed his specialist training in metabolic medicine and obtained his PhD from Imperial College London where he later took up a faculty position. He moved to University College Dublin for the Chair in Chemical Pathology and Metabolic Medicine. He also holds the position of Professor of Metabolic Medicine at Ulster University. He currently coordinates an Innovative Medicine Initiative project on obesity.

The Future: What’s Next in Obesity Treatment to Address the Unmet Needs Carel le Roux, M.D., Ph.D. University College Dublin, Ulster University, University of Pretoria Joseph Wright of Derby. The Alchemist Discovering Phosphorus. 1771. Derby Museum and Art Gallery

7 Conflicts of Interest • Consilient Health • Novo Nordisk • Herbalife • Johnson & Johnson • Covidien • Fractyl • GI Dynamics • Olympus • Lilly • Boehringer Ingelheim • Keyron • Astra Zeneca • Roche • Arrowhead • Amgen
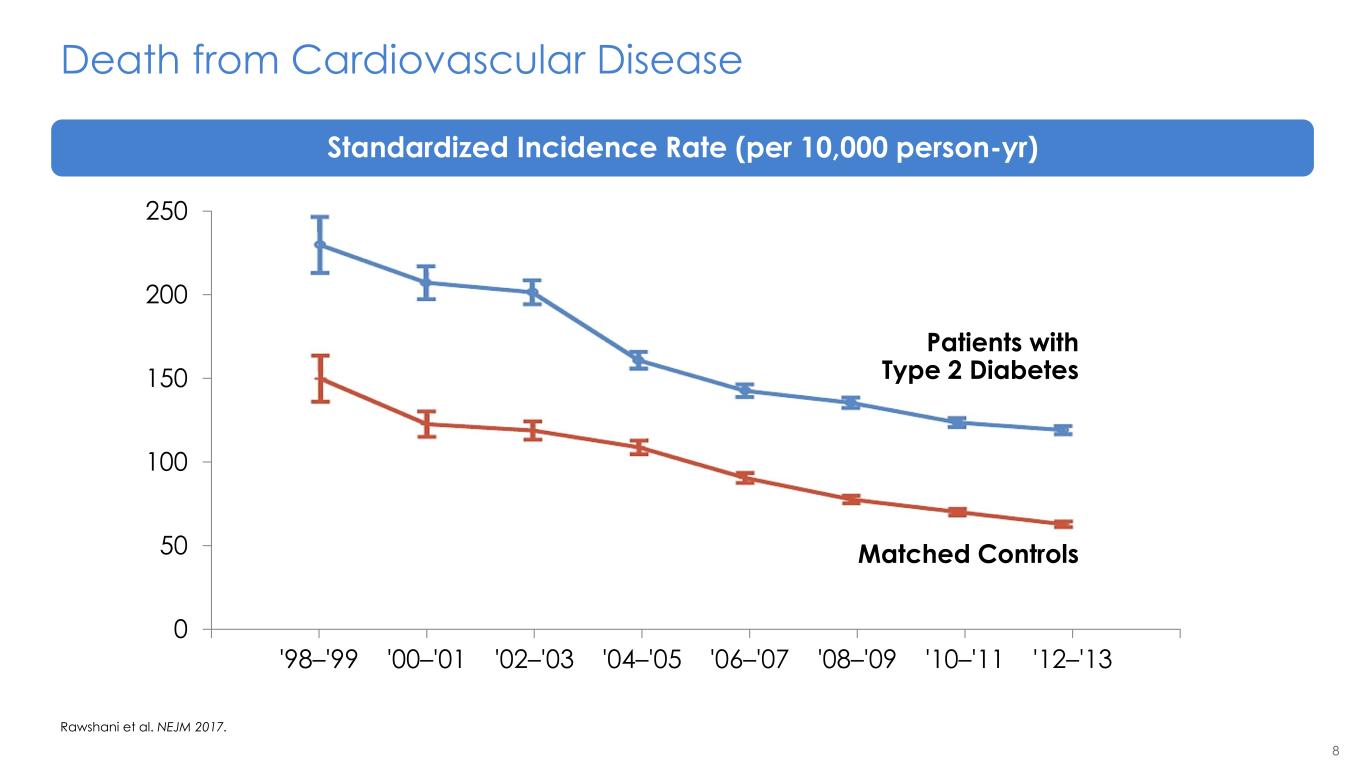
8 Death from Cardiovascular Disease Rawshani et al. NEJM 2017. 0 50 100 150 200 250 '98–'99 '00–'01 '02–'03 '04–'05 '06–'07 '08–'09 '10–'11 '12–'13 Patients with Type 2 Diabetes Matched Controls Standardized Incidence Rate (per 10,000 person-yr)

9 Visceral Fat Drives Metabolic Diseases
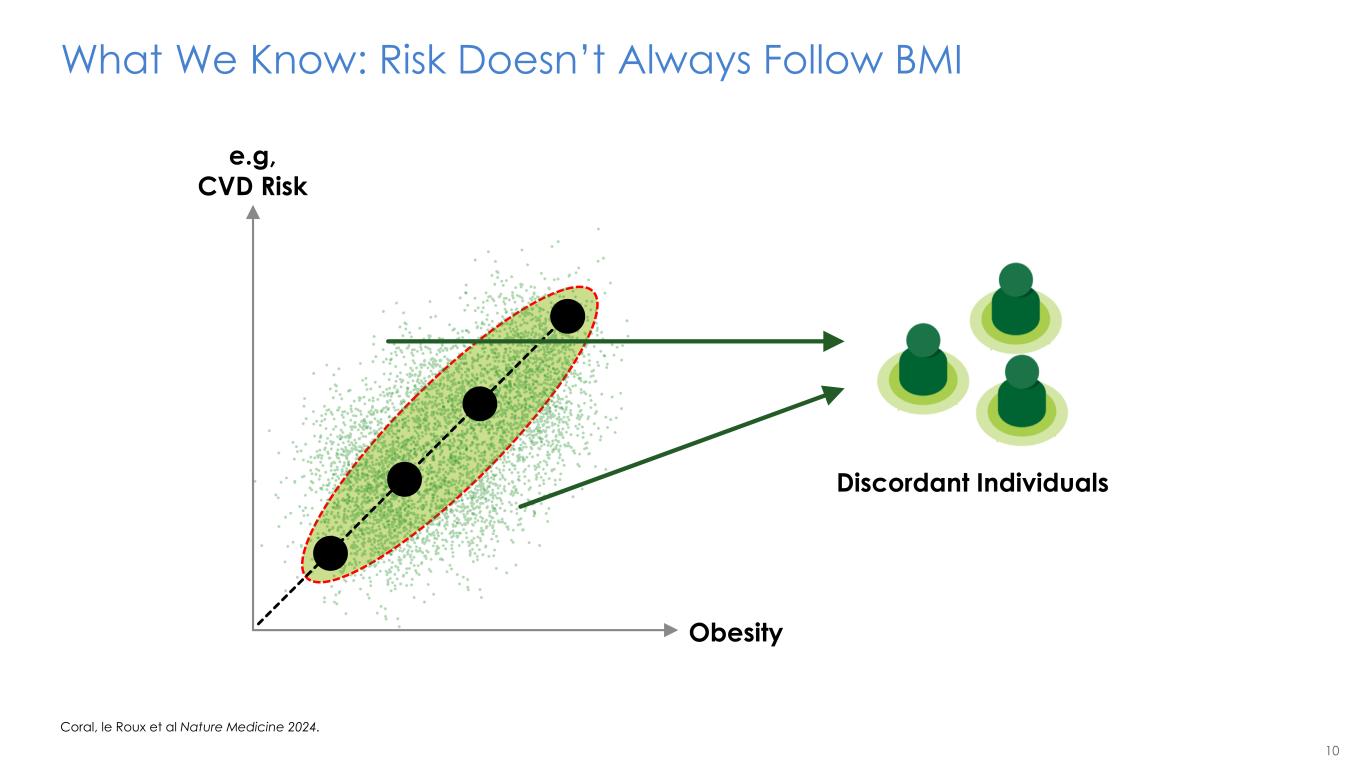
10 Obesity e.g, CVD Risk What We Know: Risk Doesn’t Always Follow BMI Coral, le Roux et al Nature Medicine 2024. Discordant Individuals
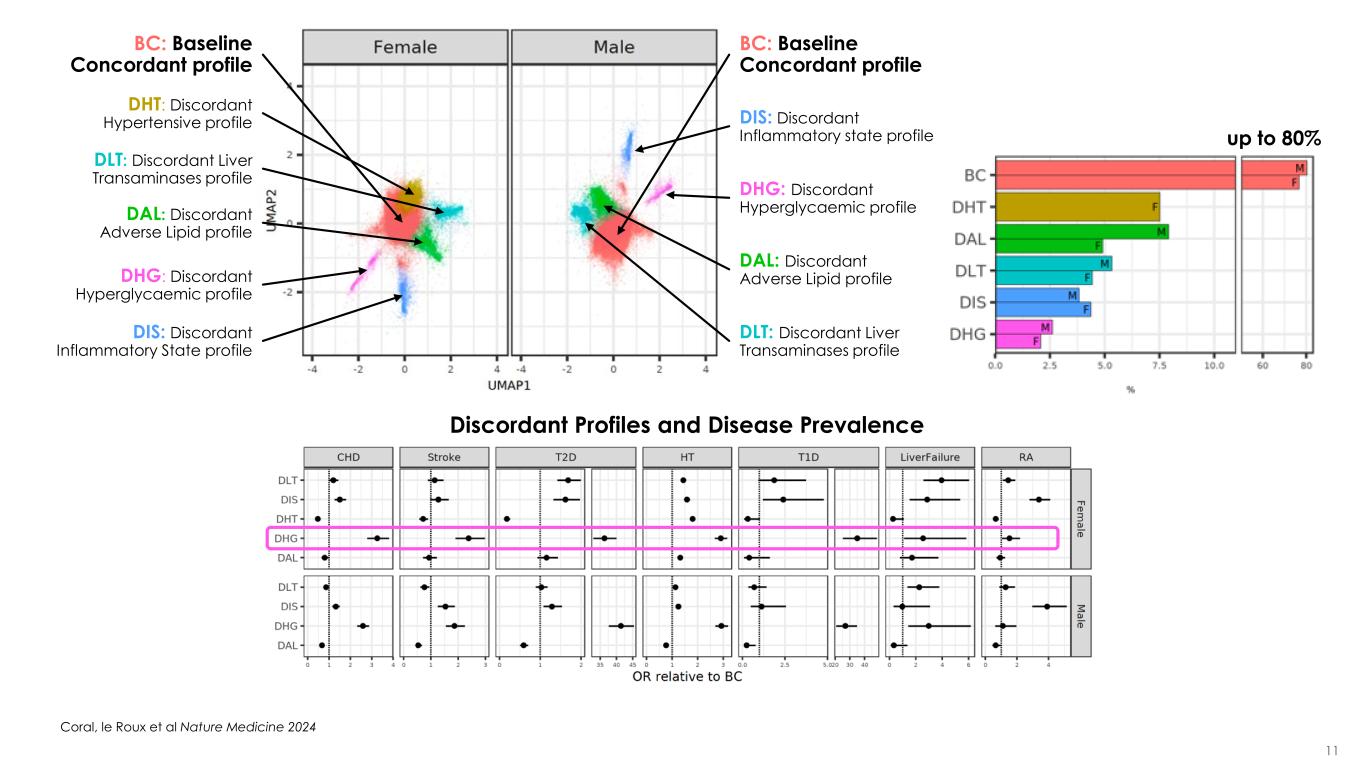
11 DIS: Discordant Inflammatory state profile DHG: Discordant Hyperglycaemic profile DAL: Discordant Adverse Lipid profile BC: Baseline Concordant profile DLT: Discordant Liver Transaminases profile DLT: Discordant Liver Transaminases profile BC: Baseline Concordant profile DAL: Discordant Adverse Lipid profile DHT: Discordant Hypertensive profile DHG: Discordant Hyperglycaemic profile DIS: Discordant Inflammatory State profile up to 80% Discordant Profiles and Disease Prevalence Coral, le Roux et al Nature Medicine 2024
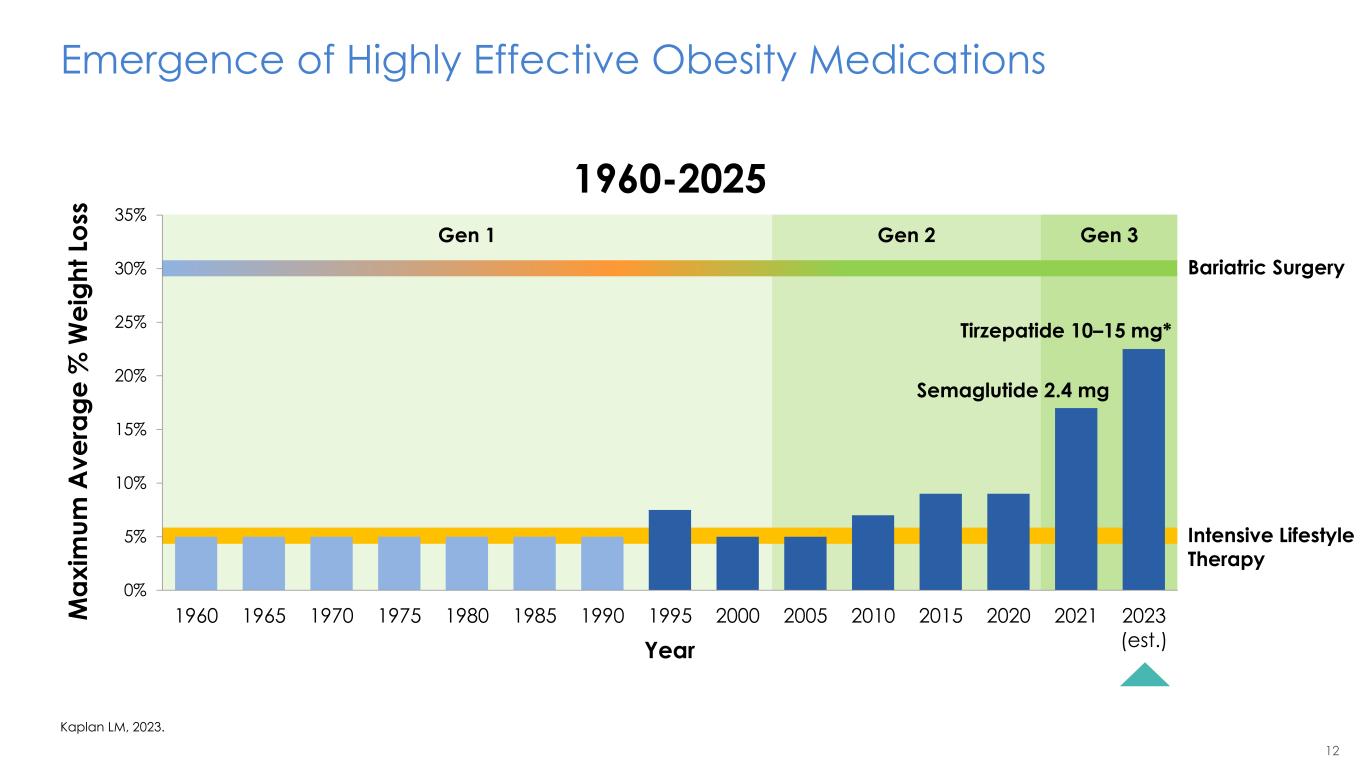
12 Gen 1 Gen 2 Gen 3 Intensive Lifestyle Therapy Bariatric Surgery Emergence of Highly Effective Obesity Medications Kaplan LM, 2023. 0% 5% 10% 15% 20% 25% 30% 35% 1960 1965 1970 1975 1980 1985 1990 1995 2000 2005 2010 2015 2020 2021 2023 (est.) Semaglutide 2.4 mg Tirzepatide 10–15 mg* Year M a x im u m A v e ra g e % W e ig h t Lo ss 1960-2025
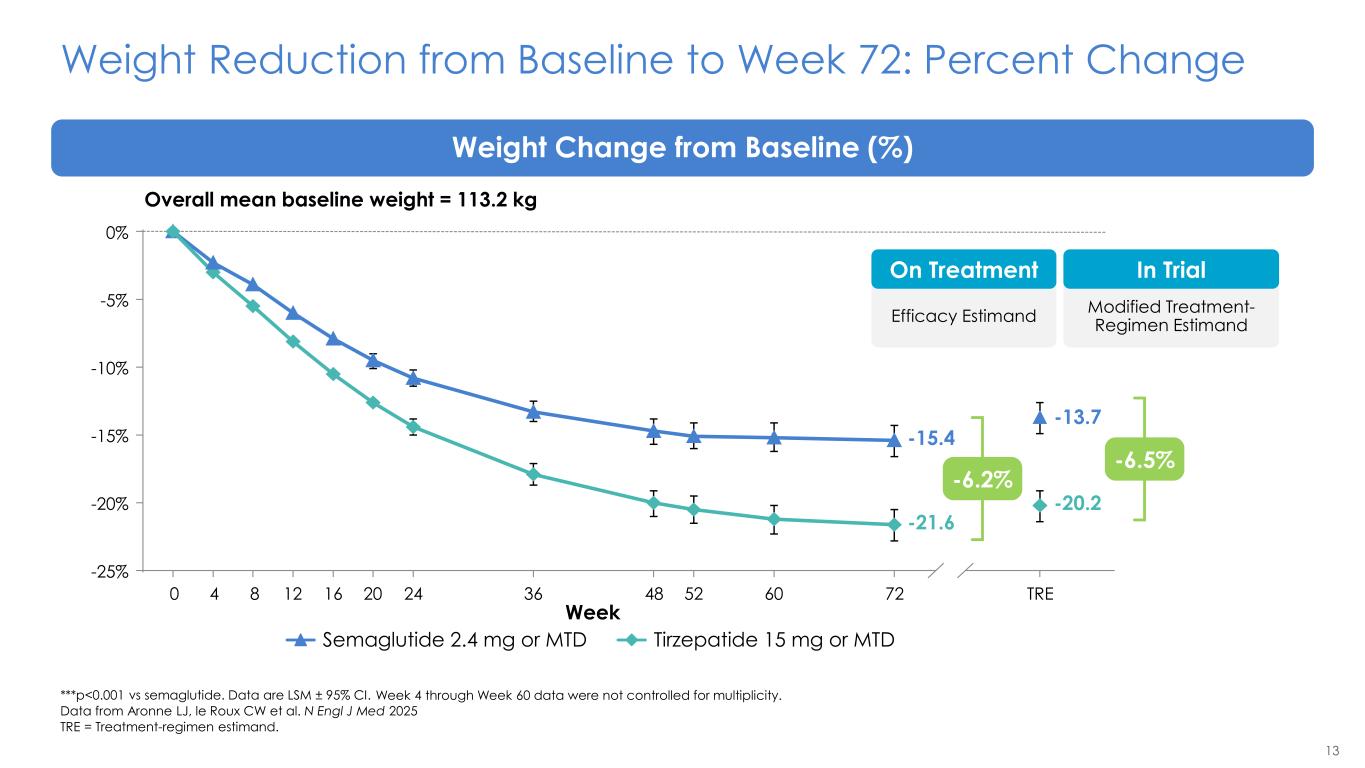
13 Weight Reduction from Baseline to Week 72: Percent Change ***p<0.001 vs semaglutide. Data are LSM ± 95% CI. Data from Aronne LJ, le Roux CW et al. N Engl J Med 2025 TRE = Treatment-regimen estimand. Weight Change from Baseline (%) Semaglutide 2.4 mg or MTD Tirzepatide 15 mg or MTD -25% -20% -15% -10% -5% 0% -20.2 -13.7 0 4 8 12 16 20 24 36 48 52 60 72 TRE -21.6 -15.4 Week Overall mean baseline weight = 113.2 kg Modified Treatment- Regimen Estimand In Trial Efficacy Estimand On Treatment -6.5% -6.2% Week 4 through Week 60 data were not controlled for multiplicity.
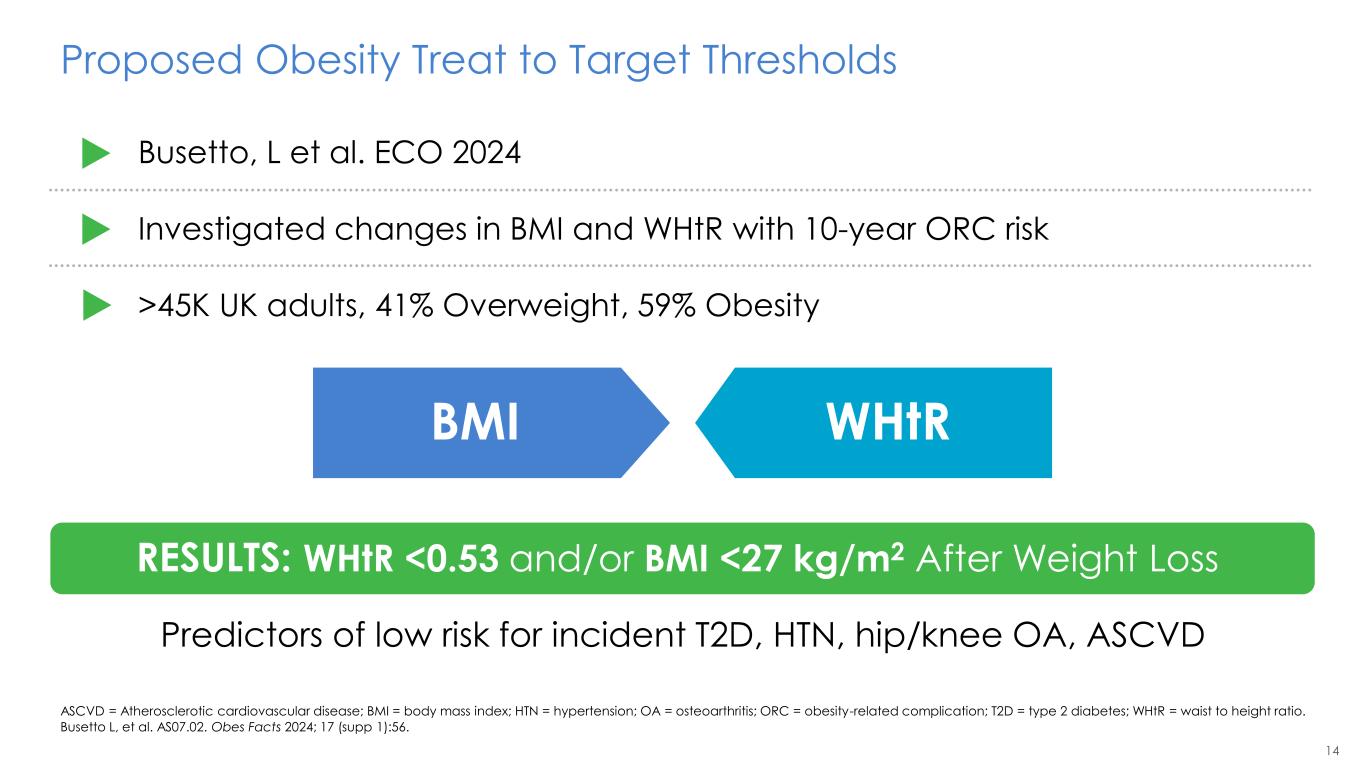
14 Proposed Obesity Treat to Target Thresholds ASCVD = Atherosclerotic cardiovascular disease; BMI = body mass index; HTN = hypertension; OA = osteoarthritis; ORC = obesity-related complication; T2D = type 2 diabetes; WHtR = waist to height ratio. Busetto L, et al. AS07.02. Obes Facts 2024; 17 (supp 1):56. Busetto, L et al. ECO 2024 Investigated changes in BMI and WHtR with 10-year ORC risk >45K UK adults, 41% Overweight, 59% Obesity RESULTS: WHtR <0.53 and/or BMI <27 kg/m2 After Weight Loss BMI WHtR Predictors of low risk for incident T2D, HTN, hip/knee OA, ASCVD
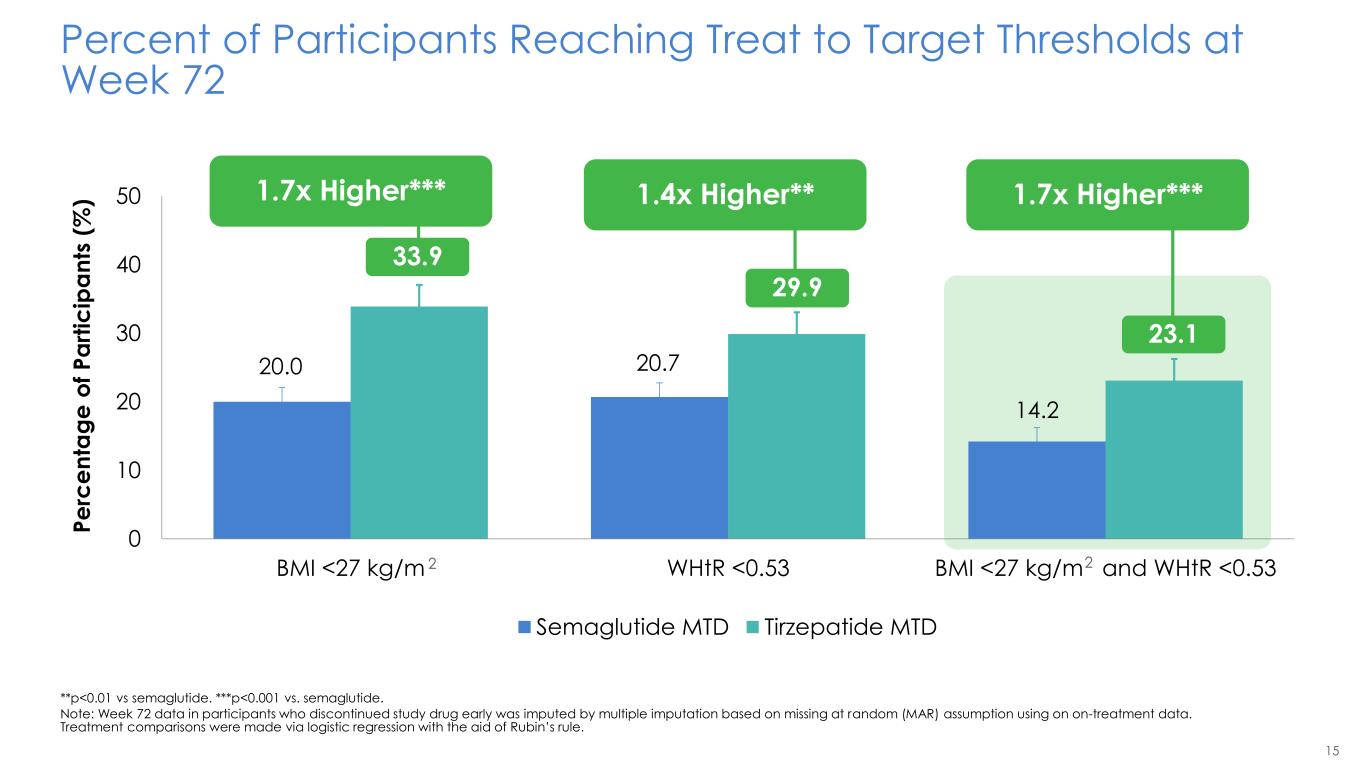
15 20.0 20.7 14.2 33.9 29.9 23.1 0 10 20 30 40 50 BMI <27 kg/m WHtR <0.53 BMI <27 kg/m and WHtR <0.53 Percent of Participants Reaching Treat to Target Thresholds at Week 72 **p<0.01 vs semaglutide. ***p<0.001 vs. semaglutide. Note: Week 72 data in participants who discontinued study drug early was imputed by multiple imputation based on missing at random (MAR) assumption using on on-treatment data. Treatment comparisons were made via logistic regression with the aid of Rubin’s rule. 2 2 P e rc e n ta g e o f P a rt ic ip a n ts ( % ) Semaglutide MTD Tirzepatide MTD 1.7x Higher*** 33.9 1.4x Higher** 29.9 1.7x Higher*** .
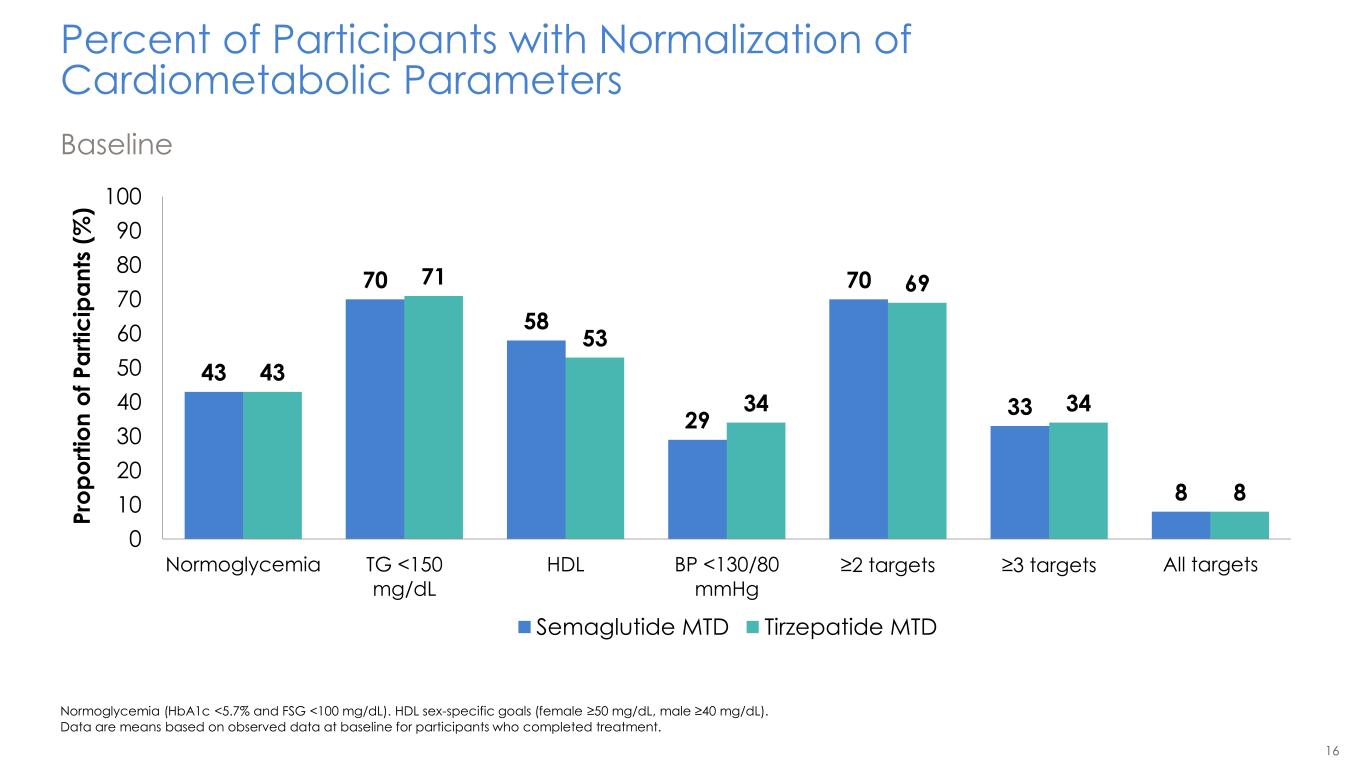
16 Percent of Participants with Normalization of Cardiometabolic Parameters Baseline Normoglycemia (HbA1c <5.7% and FSG <100 mg/dL). HDL sex-specific goals (female ≥50 mg/dL, male ≥40 mg/dL). Data are means based on observed data at baseline for participants who completed treatment. 43 70 58 29 70 33 8 43 71 53 34 69 34 8 0 10 20 30 40 50 60 70 80 90 100 Normoglycemia TG <150 mg/dL HDL BP <130/80 mmHg ≥2 targets ≥3 targets All targets P ro p o rt io n o f P a rt ic ip a n ts ( % ) Semaglutide MTD Tirzepatide MTD
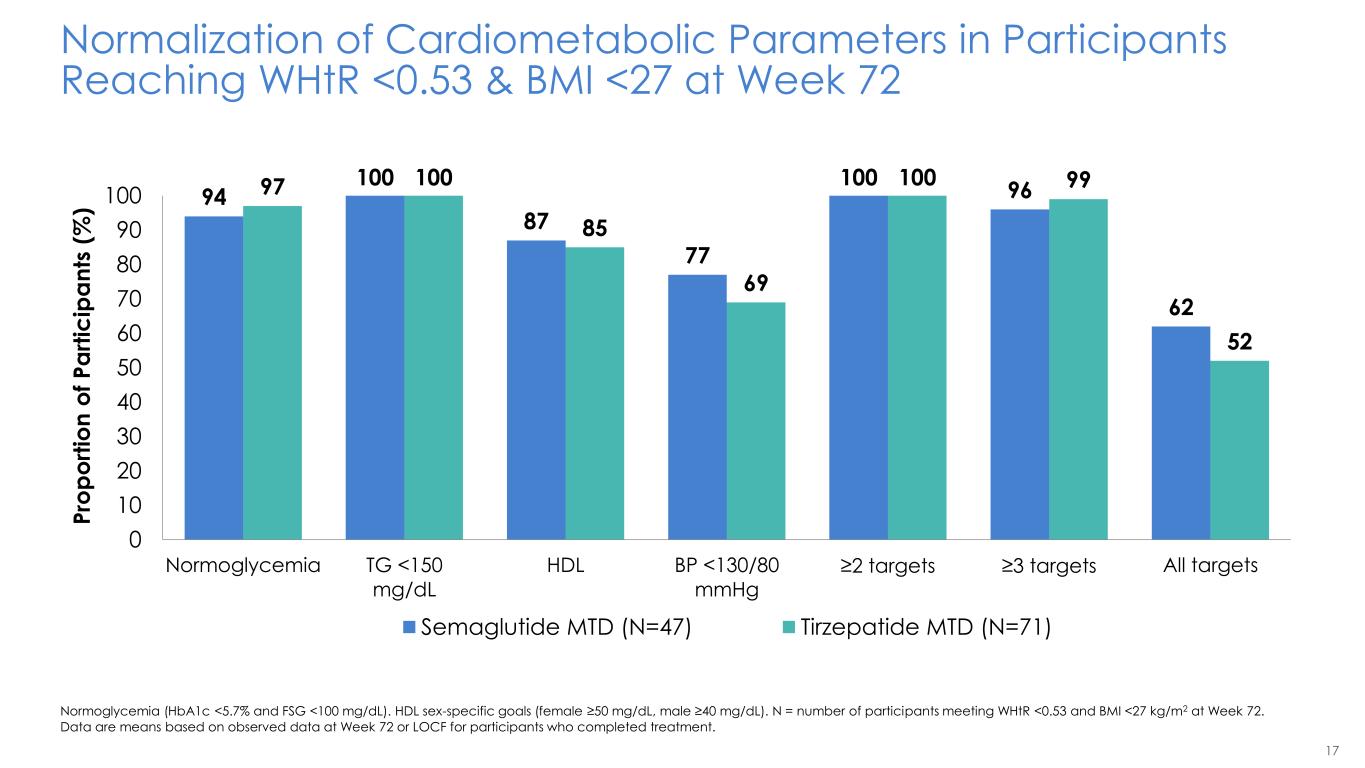
17 Normalization of Cardiometabolic Parameters in Participants Reaching WHtR <0.53 & BMI <27 at Week 72 Normoglycemia (HbA1c <5.7% and FSG <100 mg/dL). HDL sex-specific goals (female ≥50 mg/dL, male ≥40 mg/dL). N = number of participants meeting WHtR <0.53 and BMI <27 kg/m2 at Week 72. Data are means based on observed data at Week 72 or LOCF for participants who completed treatment. 94 100 87 77 100 96 62 97 100 85 69 100 99 52 0 10 20 30 40 50 60 70 80 90 100 Normoglycemia TG <150 mg/dL HDL BP <130/80 mmHg ≥2 targets ≥3 targets All targets P ro p o rt io n o f P a rt ic ip a n ts ( % ) Semaglutide MTD (N=47) Tirzepatide MTD (N=71)
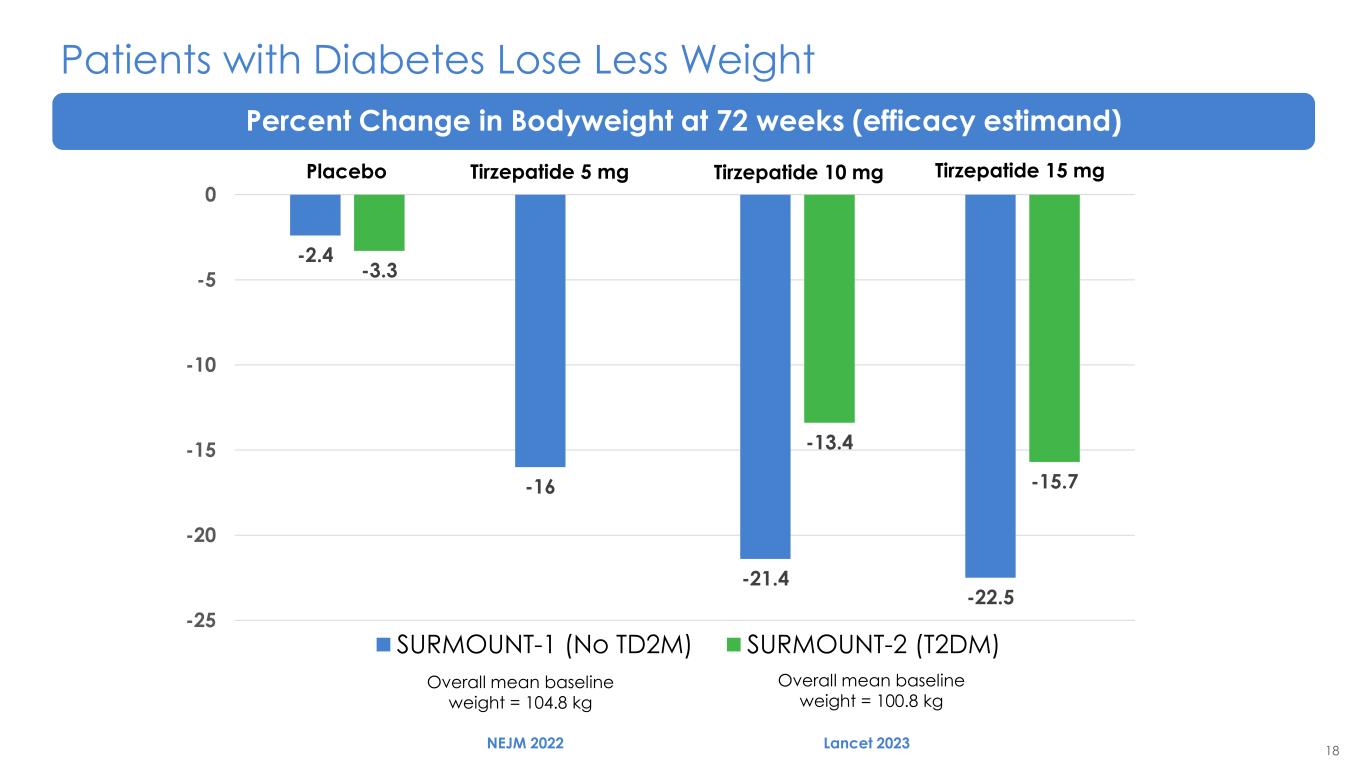
18 Patients with Diabetes Lose Less Weight Percent Change in Bodyweight at 72 weeks (efficacy estimand) Overall mean baseline weight = 104.8 kg Overall mean baseline weight = 100.8 kg -2.4 -16 -21.4 -22.5 -3.3 -13.4 -15.7 -25 -20 -15 -10 -5 0 SURMOUNT-1 (No TD2M) SURMOUNT-2 (T2DM) NEJM 2022 Lancet 2023 Placebo Tirzepatide 5 mg Tirzepatide 10 mg Tirzepatide 15 mg
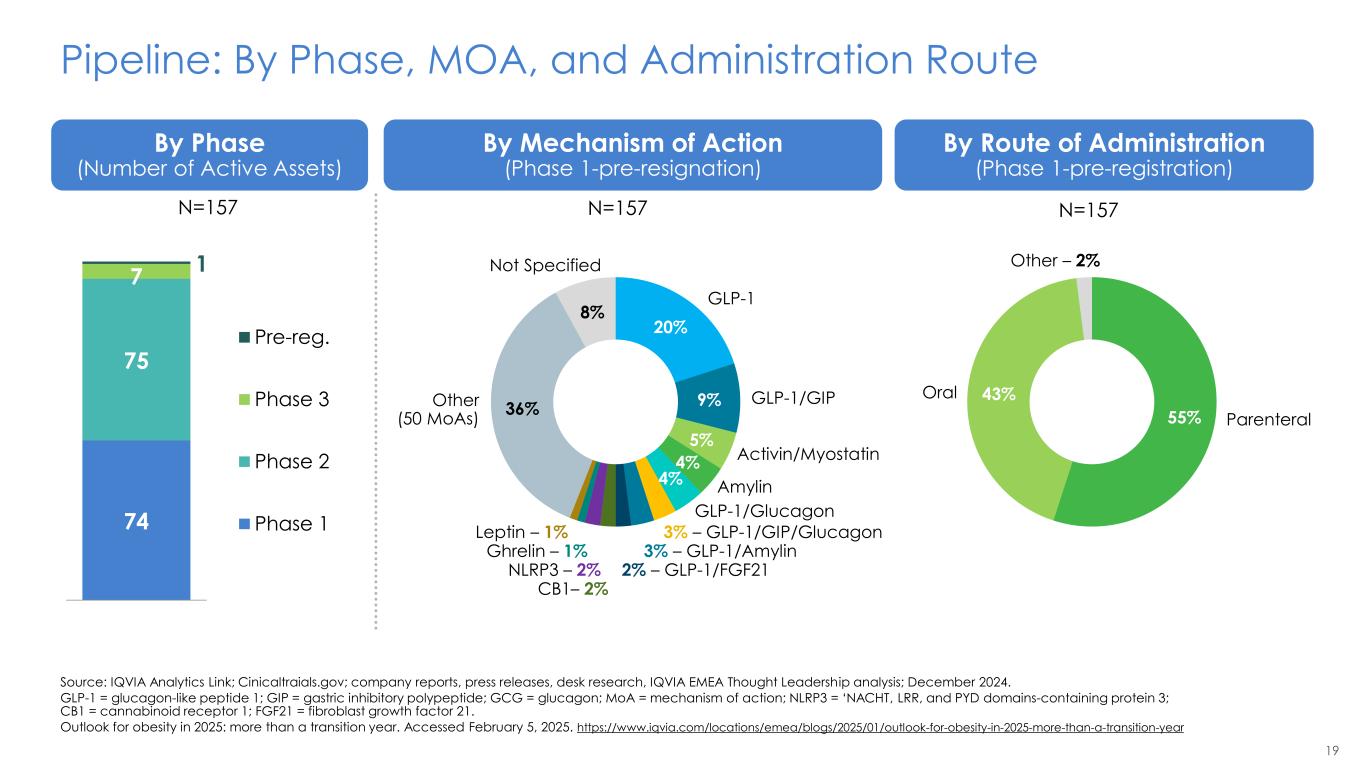
19 Pipeline: By Phase, MOA, and Administration Route Source: IQVIA Analytics Link; Cinicaltraials.gov; company reports, press releases, desk research, IQVIA EMEA Thought Leadership analysis; December 2024. GLP-1 = glucagon-like peptide 1; GIP = gastric inhibitory polypeptide; GCG = glucagon; MoA = mechanism of action; NLRP3 = ‘NACHT, LRR, and PYD domains-containing protein 3; CB1 = cannabinoid receptor 1; FGF21 = fibroblast growth factor 21. Outlook for obesity in 2025: more than a transition year. Accessed February 5, 2025. https://www.iqvia.com/locations/emea/blogs/2025/01/outlook-for-obesity-in-2025-more-than-a-transition-year By Phase (Number of Active Assets) By Mechanism of Action (Phase 1-pre-resignation) By Route of Administration (Phase 1-pre-registration) 74 75 7 1 Pre-reg. Phase 3 Phase 2 Phase 1 20% 9% 5% 4% 4% 36% 8% GLP-1 GLP-1/GIP Activin/Myostatin Amylin GLP-1/Glucagon 3% – GLP-1/GIP/Glucagon 3% – GLP-1/Amylin 2% – GLP-1/FGF21 CB1– 2% NLRP3 – 2% Ghrelin – 1% Leptin – 1% Not Specified Other (50 MoAs) 55% 43% Other – 2% Oral Parenteral N=157 N=157 N=157
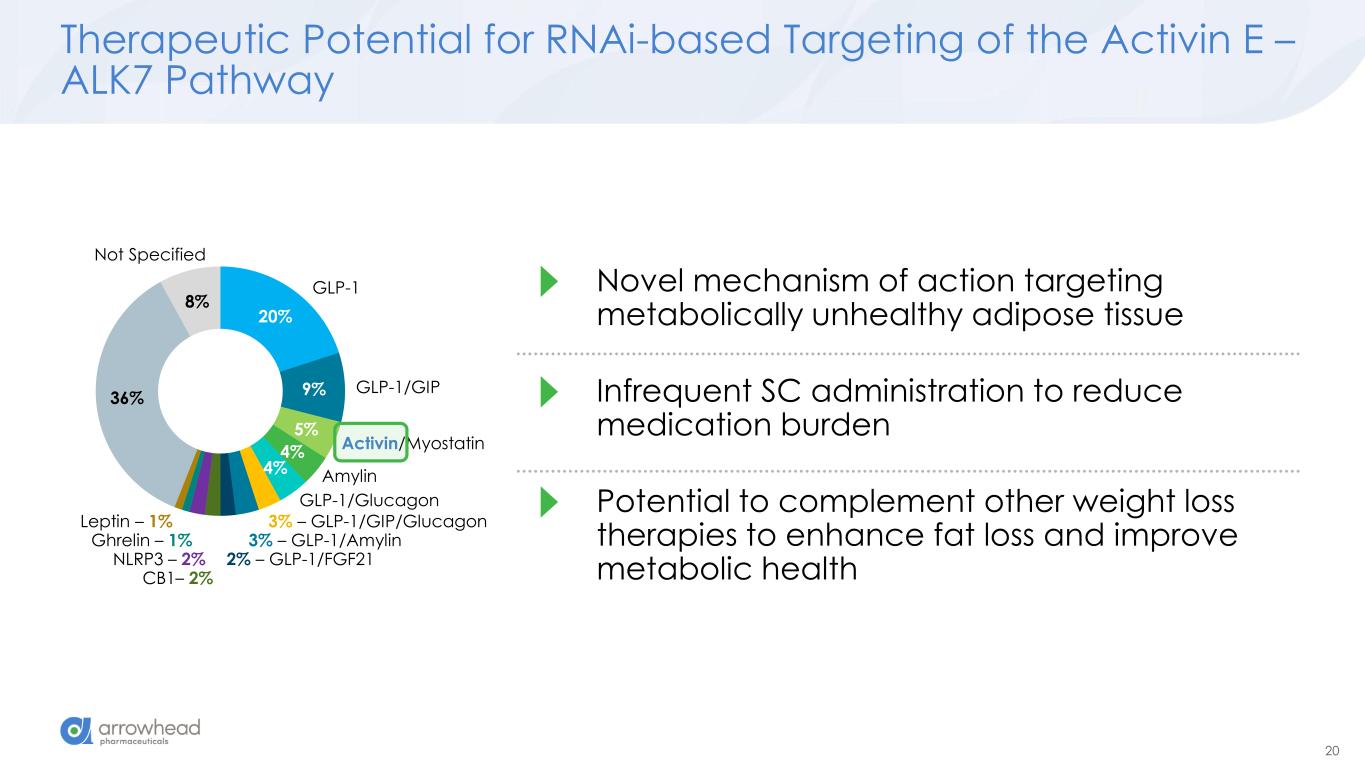
20 Therapeutic Potential for RNAi-based Targeting of the Activin E – ALK7 Pathway 20% 9% 5% 4% 4% 36% 8% GLP-1 GLP-1/GIP Activin/Myostatin Amylin GLP-1/Glucagon 3% – GLP-1/GIP/Glucagon 3% – GLP-1/Amylin 2% – GLP-1/FGF21 CB1– 2% NLRP3 – 2% Ghrelin – 1% Leptin – 1% Not Specified Novel mechanism of action targeting metabolically unhealthy adipose tissue Infrequent SC administration to reduce medication burden Potential to complement other weight loss therapies to enhance fat loss and improve metabolic health
21 Conclusions Recognising the different subtypes of obesity The Future of Obesity Care Will Include Reducing visceral fat to achieve better cardio-kidney- metabolic outcomes Combining therapies to achieve low cardiovascular risk state

Therapeutic Rationale for Targeting the Activin E – ALK7 Axis James Hamilton MD, MBA Chief Medical Officer and Head of R&D ARO-INHBE and ARO-ALK7 Interim Clinical Data Update
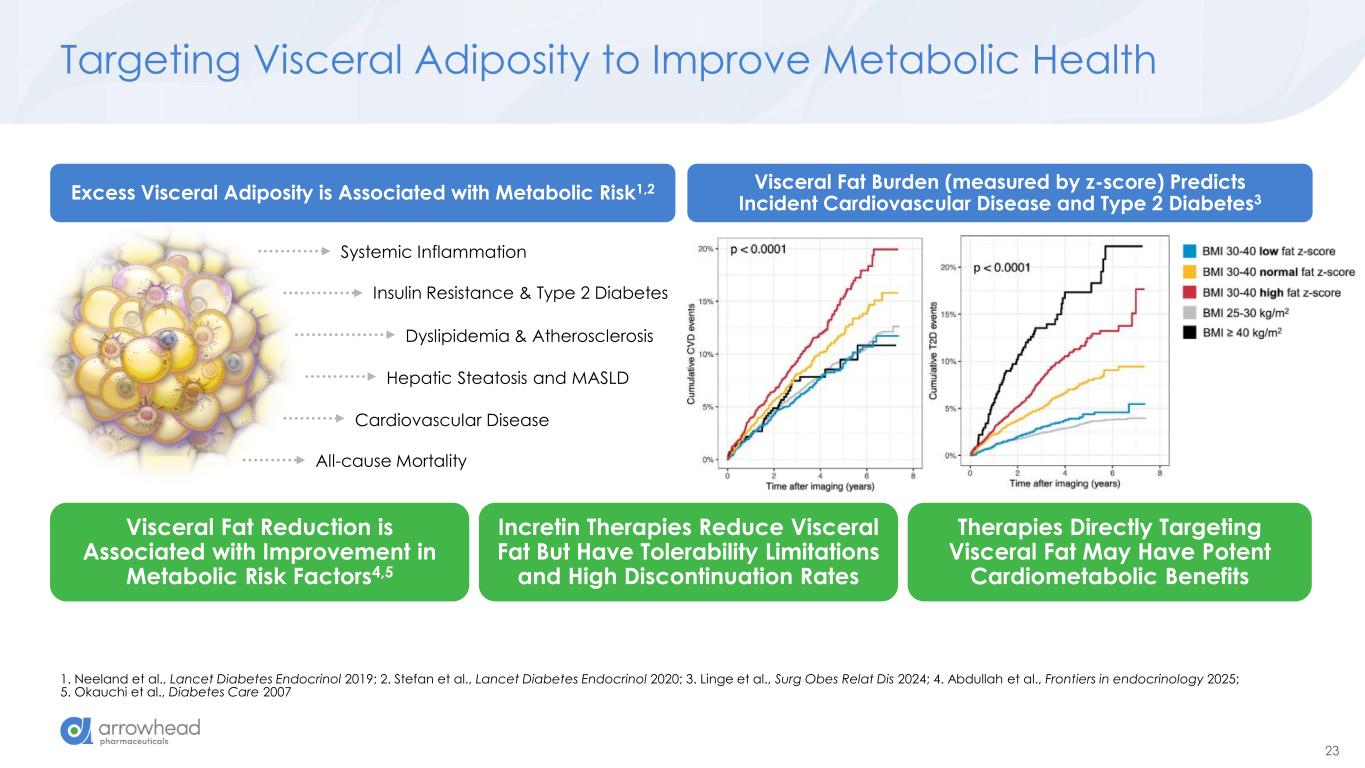
23 Targeting Visceral Adiposity to Improve Metabolic Health 1. Neeland et al., Lancet Diabetes Endocrinol 2019; 2. Stefan et al., Lancet Diabetes Endocrinol 2020; 3. Linge et al., Surg Obes Relat Dis 2024; 4. Abdullah et al., Frontiers in endocrinology 2025; 5. Okauchi et al., Diabetes Care 2007 Systemic Inflammation Insulin Resistance & Type 2 Diabetes Dyslipidemia & Atherosclerosis Cardiovascular Disease All-cause Mortality Hepatic Steatosis and MASLD Visceral Fat Reduction is Associated with Improvement in Metabolic Risk Factors4,5 Incretin Therapies Reduce Visceral Fat But Have Tolerability Limitations and High Discontinuation Rates Therapies Directly Targeting Visceral Fat May Have Potent Cardiometabolic Benefits Excess Visceral Adiposity is Associated with Metabolic Risk1,2 Visceral Fat Burden (measured by z-score) Predicts Incident Cardiovascular Disease and Type 2 Diabetes3
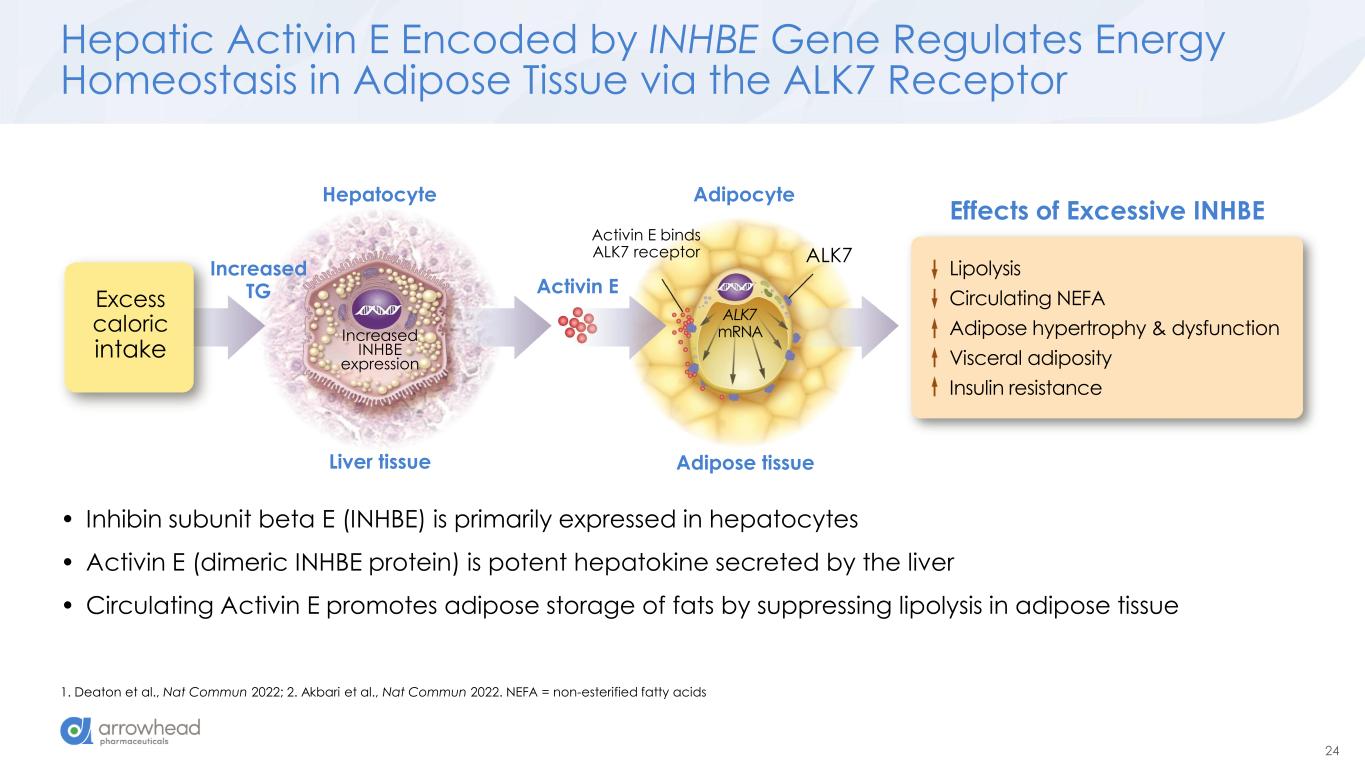
24 Hepatic Activin E Encoded by INHBE Gene Regulates Energy Homeostasis in Adipose Tissue via the ALK7 Receptor 1. Deaton et al., Nat Commun 2022; 2. Akbari et al., Nat Commun 2022. NEFA = non-esterified fatty acids Excess caloric intake Activin E Hepatocyte Liver tissue Adipose tissue Adipocyte Lipolysis Circulating NEFA Adipose hypertrophy & dysfunction Visceral adiposity Insulin resistance Increased INHBE expression Activin E binds ALK7 receptor ALK7 Effects of Excessive INHBE ALK7 mRNA Increased TG • Inhibin subunit beta E (INHBE) is primarily expressed in hepatocytes • Activin E (dimeric INHBE protein) is potent hepatokine secreted by the liver • Circulating Activin E promotes adipose storage of fats by suppressing lipolysis in adipose tissue
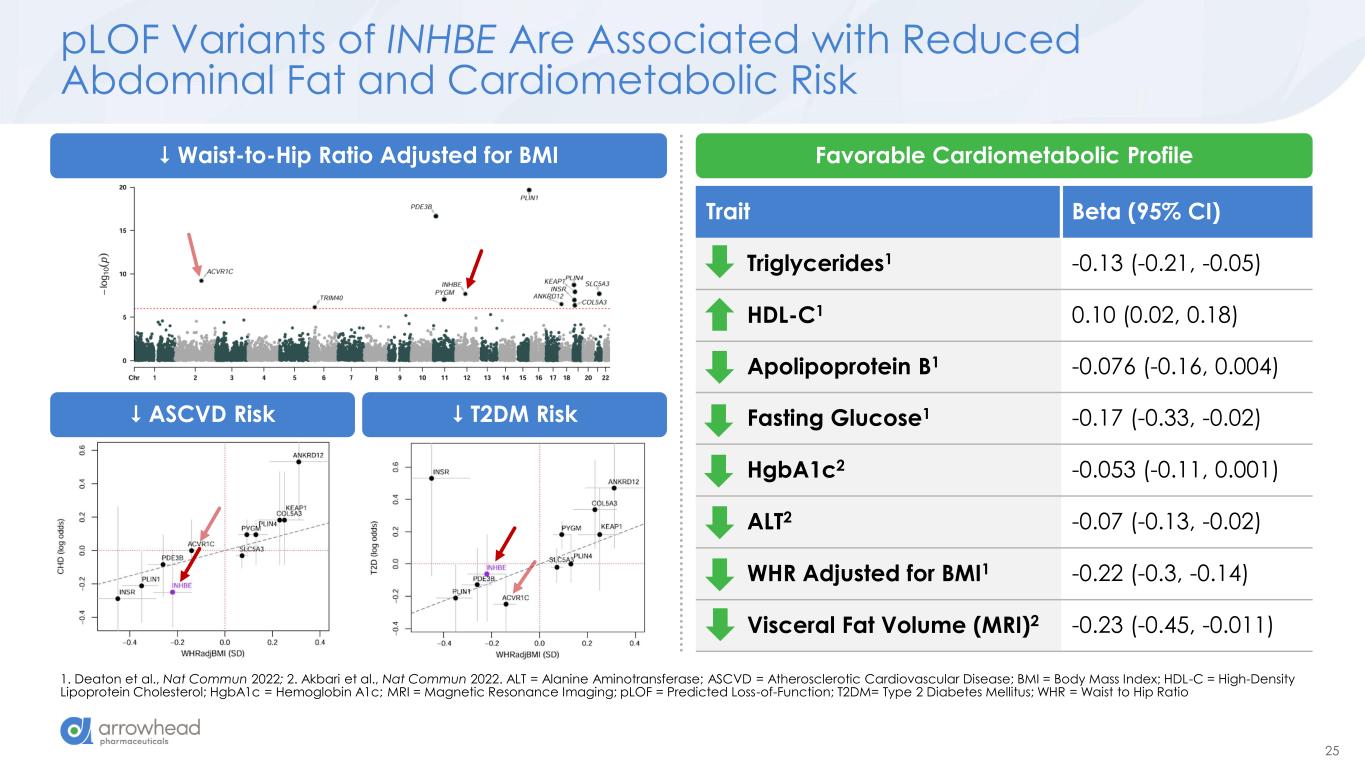
25 Trait Beta (95% CI) Triglycerides1 -0.13 (-0.21, -0.05) HDL-C1 0.10 (0.02, 0.18) Apolipoprotein B1 -0.076 (-0.16, 0.004) Fasting Glucose1 -0.17 (-0.33, -0.02) HgbA1c2 -0.053 (-0.11, 0.001) ALT2 -0.07 (-0.13, -0.02) WHR Adjusted for BMI1 -0.22 (-0.3, -0.14) Visceral Fat Volume (MRI)2 -0.23 (-0.45, -0.011) pLOF Variants of INHBE Are Associated with Reduced Abdominal Fat and Cardiometabolic Risk 1. Deaton et al., Nat Commun 2022; 2. Akbari et al., Nat Commun 2022. ALT = Alanine Aminotransferase; ASCVD = Atherosclerotic Cardiovascular Disease; BMI = Body Mass Index; HDL-C = High-Density Lipoprotein Cholesterol; HgbA1c = Hemoglobin A1c; MRI = Magnetic Resonance Imaging; pLOF = Predicted Loss-of-Function; T2DM= Type 2 Diabetes Mellitus; WHR = Waist to Hip Ratio Waist-to-Hip Ratio Adjusted for BMI Favorable Cardiometabolic Profile ASCVD Risk T2DM Risk
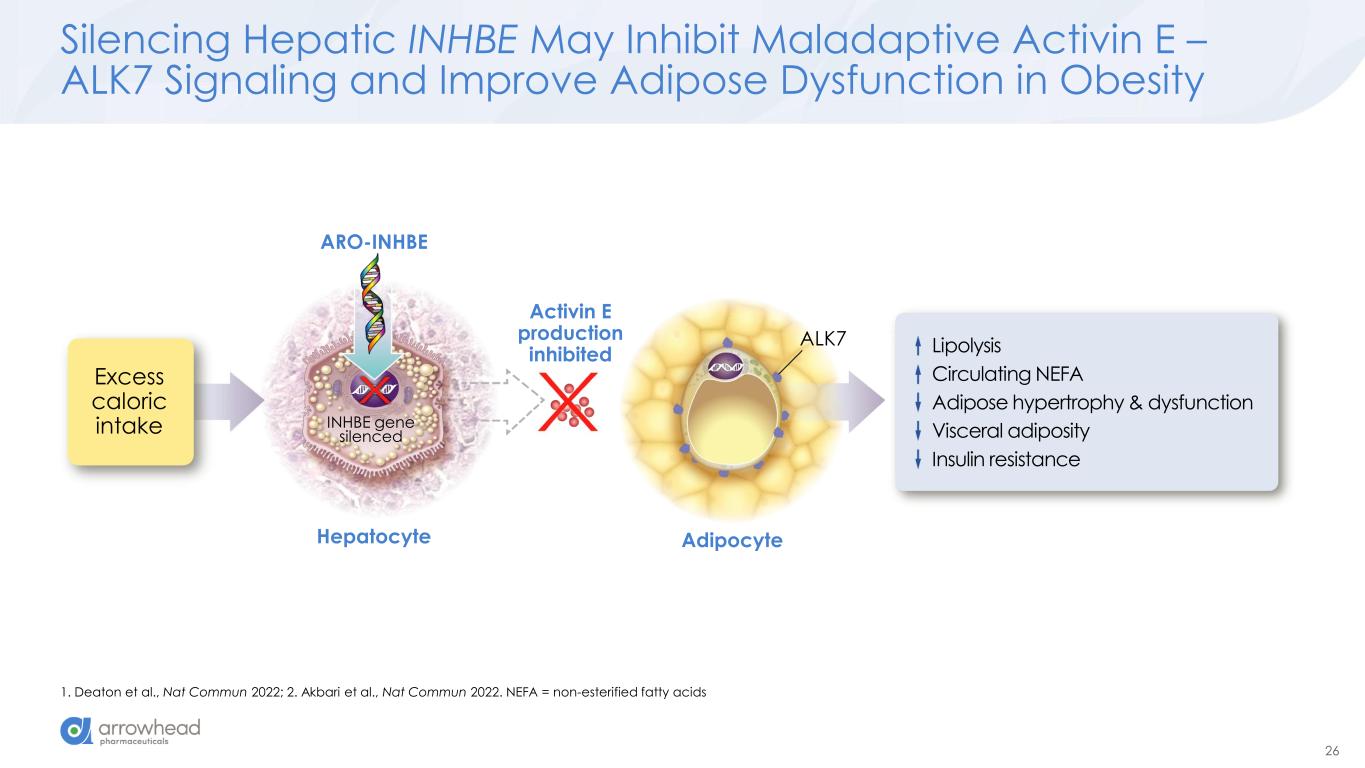
26 Silencing Hepatic INHBE May Inhibit Maladaptive Activin E – ALK7 Signaling and Improve Adipose Dysfunction in Obesity 1. Deaton et al., Nat Commun 2022; 2. Akbari et al., Nat Commun 2022. NEFA = non-esterified fatty acids Excess caloric intake INHBE gene silenced Activin E production inhibited Hepatocyte Adipocyte ALK7 ARO-INHBE Lipolysis Circulating NEFA Adipose hypertrophy & dysfunction Visceral adiposity Insulin resistance

Clinical Study Update from AROINHBE-1001 James Hamilton MD, MBA Chief Medical Officer and Head of R&D ARO-INHBE and ARO-ALK7 Interim Clinical Data Update
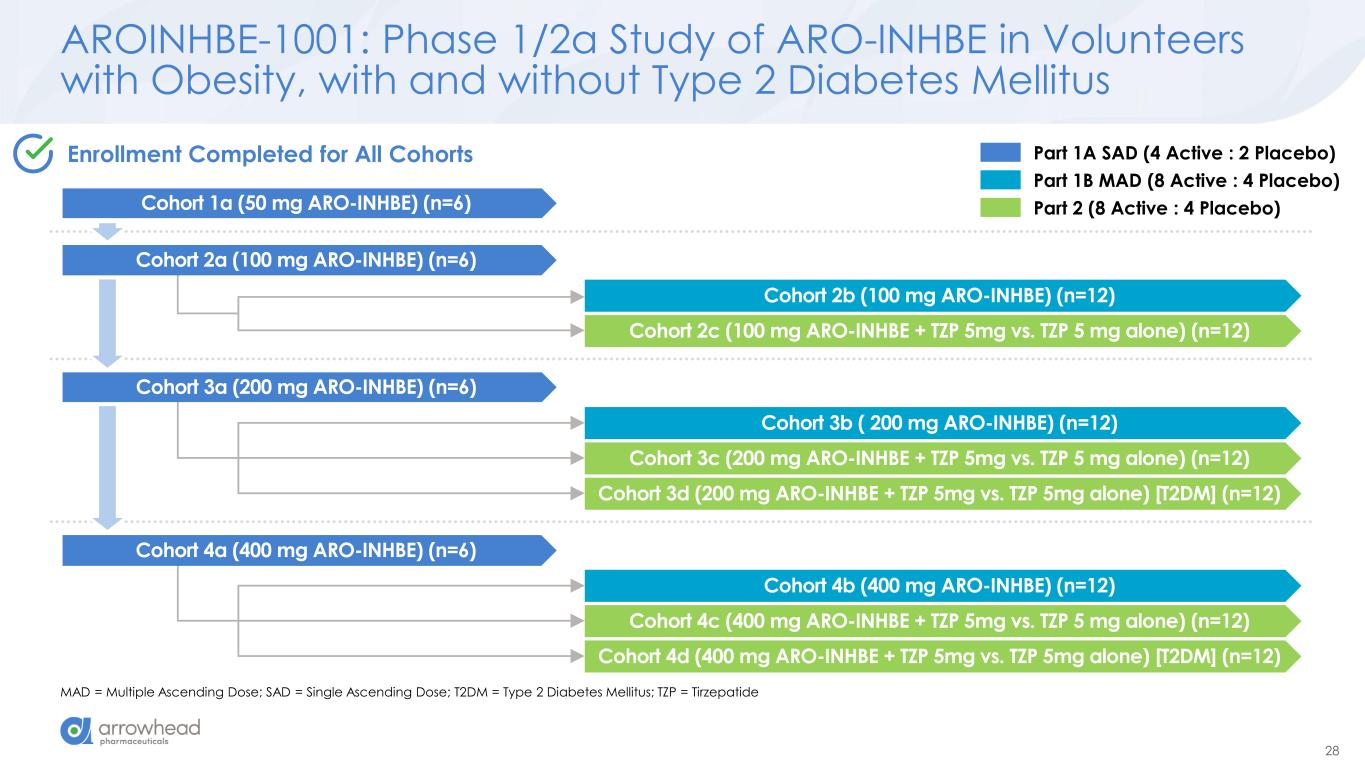
28 AROINHBE-1001: Phase 1/2a Study of ARO-INHBE in Volunteers with Obesity, with and without Type 2 Diabetes Mellitus MAD = Multiple Ascending Dose; SAD = Single Ascending Dose; T2DM = Type 2 Diabetes Mellitus; TZP = Tirzepatide Cohort 2b (100 mg ARO-INHBE) (n=12) Cohort 2c (100 mg ARO-INHBE + TZP 5mg vs. TZP 5 mg alone) (n=12) Cohort 3b ( 200 mg ARO-INHBE) (n=12) Cohort 3c (200 mg ARO-INHBE + TZP 5mg vs. TZP 5 mg alone) (n=12) Cohort 3d (200 mg ARO-INHBE + TZP 5mg vs. TZP 5mg alone) [T2DM] (n=12) Cohort 4b (400 mg ARO-INHBE) (n=12) Cohort 4c (400 mg ARO-INHBE + TZP 5mg vs. TZP 5 mg alone) (n=12) Cohort 4d (400 mg ARO-INHBE + TZP 5mg vs. TZP 5mg alone) [T2DM] (n=12) Part 1A SAD (4 Active : 2 Placebo) Part 1B MAD (8 Active : 4 Placebo) Part 2 (8 Active : 4 Placebo) Enrollment Completed for All Cohorts Cohort 1a (50 mg ARO-INHBE) (n=6) Cohort 2a (100 mg ARO-INHBE) (n=6) Cohort 3a (200 mg ARO-INHBE) (n=6) Cohort 4a (400 mg ARO-INHBE) (n=6)
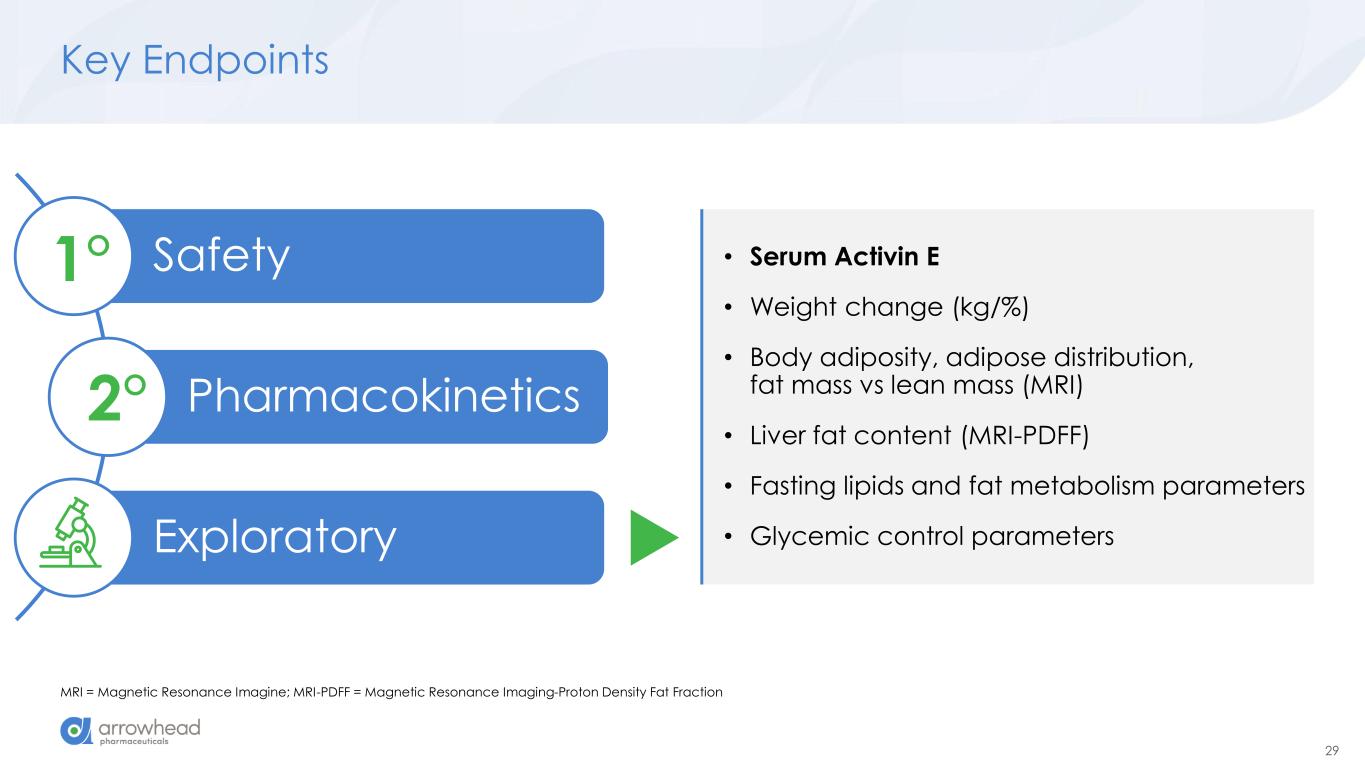
29 Key Endpoints MRI = Magnetic Resonance Imagine; MRI-PDFF = Magnetic Resonance Imaging-Proton Density Fat Fraction Safety Pharmacokinetics Exploratory 1° 2° • Serum Activin E • Weight change (kg/%) • Body adiposity, adipose distribution, fat mass vs lean mass (MRI) • Liver fat content (MRI-PDFF) • Fasting lipids and fat metabolism parameters • Glycemic control parameters
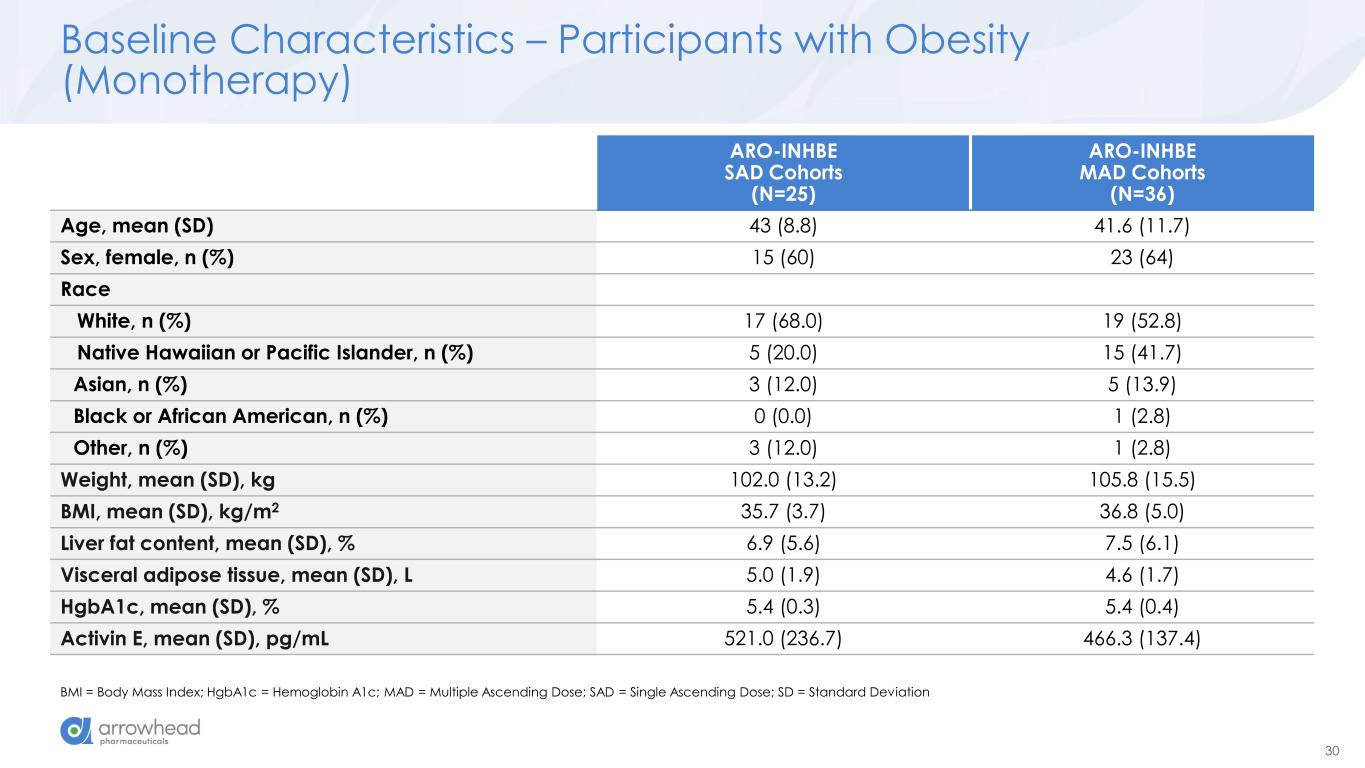
30 Baseline Characteristics – Participants with Obesity (Monotherapy) BMI = Body Mass Index; HgbA1c = Hemoglobin A1c; MAD = Multiple Ascending Dose; SAD = Single Ascending Dose; SD = Standard Deviation ARO-INHBE SAD Cohorts (N=25) ARO-INHBE MAD Cohorts (N=36) Age, mean (SD) 43 (8.8) 41.6 (11.7) Sex, female, n (%) 15 (60) 23 (64) Race White, n (%) 17 (68.0) 19 (52.8) Native Hawaiian or Pacific Islander, n (%) 5 (20.0) 15 (41.7) Asian, n (%) 3 (12.0) 5 (13.9) Black or African American, n (%) 0 (0.0) 1 (2.8) Other, n (%) 3 (12.0) 1 (2.8) Weight, mean (SD), kg 102.0 (13.2) 105.8 (15.5) BMI, mean (SD), kg/m2 35.7 (3.7) 36.8 (5.0) Liver fat content, mean (SD), % 6.9 (5.6) 7.5 (6.1) Visceral adipose tissue, mean (SD), L 5.0 (1.9) 4.6 (1.7) HgbA1c, mean (SD), % 5.4 (0.3) 5.4 (0.4) Activin E, mean (SD), pg/mL 521.0 (236.7) 466.3 (137.4)
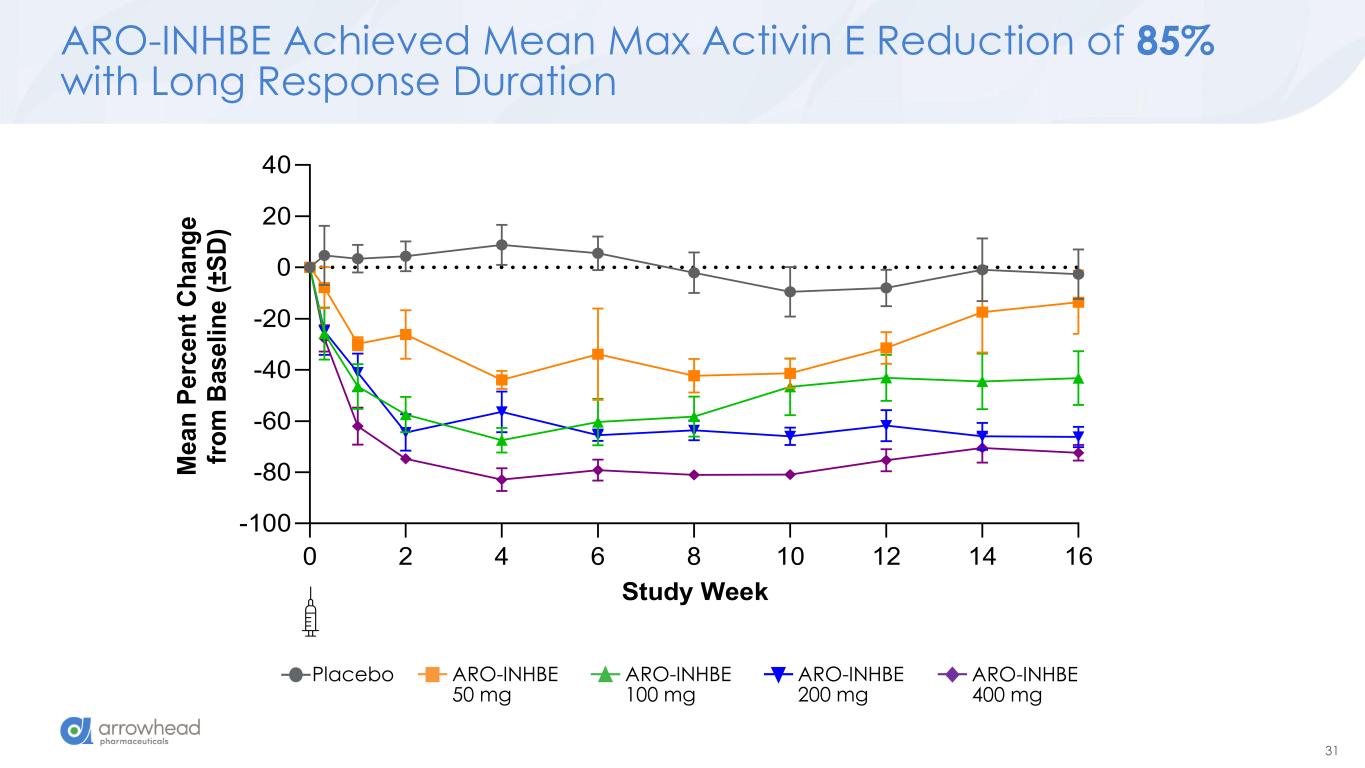
31 ARO-INHBE Achieved Mean Max Activin E Reduction of 85% with Long Response Duration 0 2 4 6 8 10 12 14 16 -100 -80 -60 -40 -20 0 20 40 Study Week M e a n P e rc e n t C h a n g e fr o m B a s e li n e ( ± S D ) Placebo ARO-INHBE 50 mg ARO-INHBE 100 mg ARO-INHBE 200 mg ARO-INHBE 400 mg
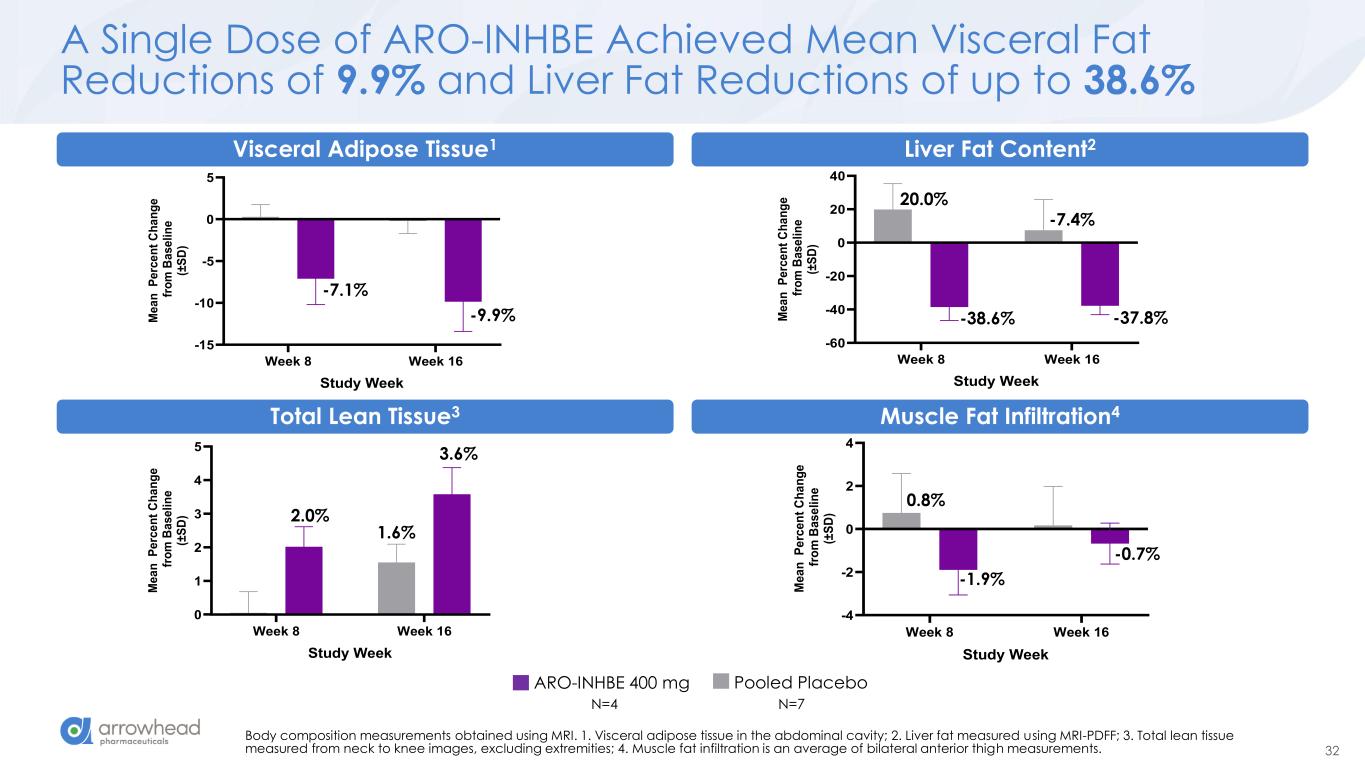
32 A Single Dose of ARO-INHBE Achieved Mean Visceral Fat Reductions of 9.9% and Liver Fat Reductions of up to 38.6% Body composition measurements obtained using MRI. 1. Visceral adipose tissue in the abdominal cavity; 2. Liver fat measured using MRI-PDFF; 3. Total lean tissue measured from neck to knee images, excluding extremities; 4. Muscle fat infiltration is an average of bilateral anterior thigh measurements. Week 8 Week 16 0 1 2 3 4 5 Total Lean Tissue (MRI) Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) 2.0% 3.6% 1.6% Week 8 Week 16 -4 -2 0 2 4 Muscle Fat Infiltration (MRI) Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) 0.8% -1.9% -0.7% Week 8 Week 16 -60 -40 -20 0 20 40 Liver Fat Content (MRI-PDFF) Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) 20.0% -38.6% -7.4% -37.8% Liver Fat Content2 Week 8 Week 16 -15 -10 -5 0 5 Visceral Adipose Tissue (MRI) Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) -7.1% -9.9% Vi ral Adipose Tissue1 Total Lean Tissue3 M s l Fat Infiltration4 ARO-INHBE 400 mg Pooled Placebo N=4 N=7
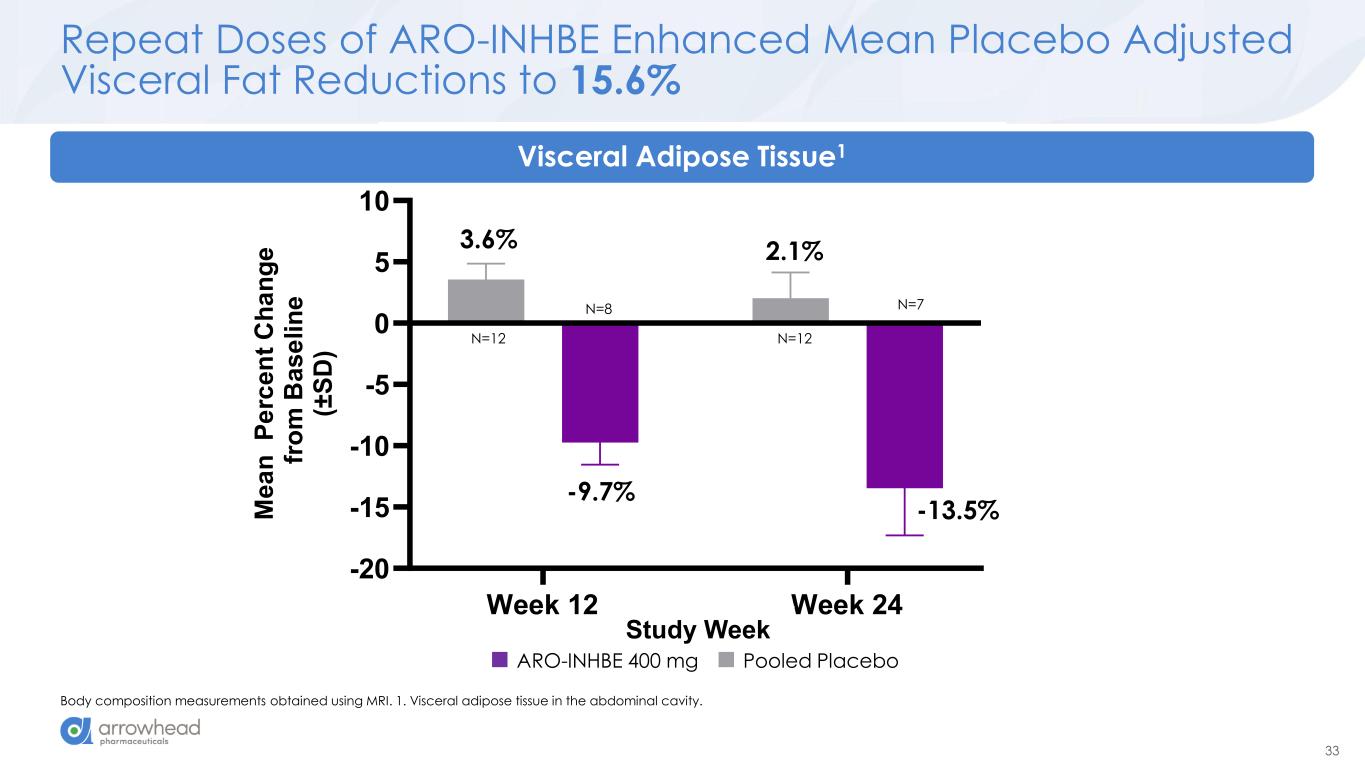
33 Week 12 Week 24 -20 -15 -10 -5 0 5 10 Visceral Adipose Tissue (MRI) Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) Repeat Doses of ARO-INHBE Enhanced Mean Placebo Adjusted Visceral Fat Reductions to 15.6% Body composition measurements obtained using MRI. 1. Visceral adipose tissue in the abdominal cavity. 3.6% -9.7% 2.1% -13.5% Visceral Adipose Tissue1 Study Week N=12 N=7N=8 N=12 ARO-INHBE 400 mg Pooled Placebo

Combination Therapy Update – Obesity with Type 2 Diabetes Mellitus
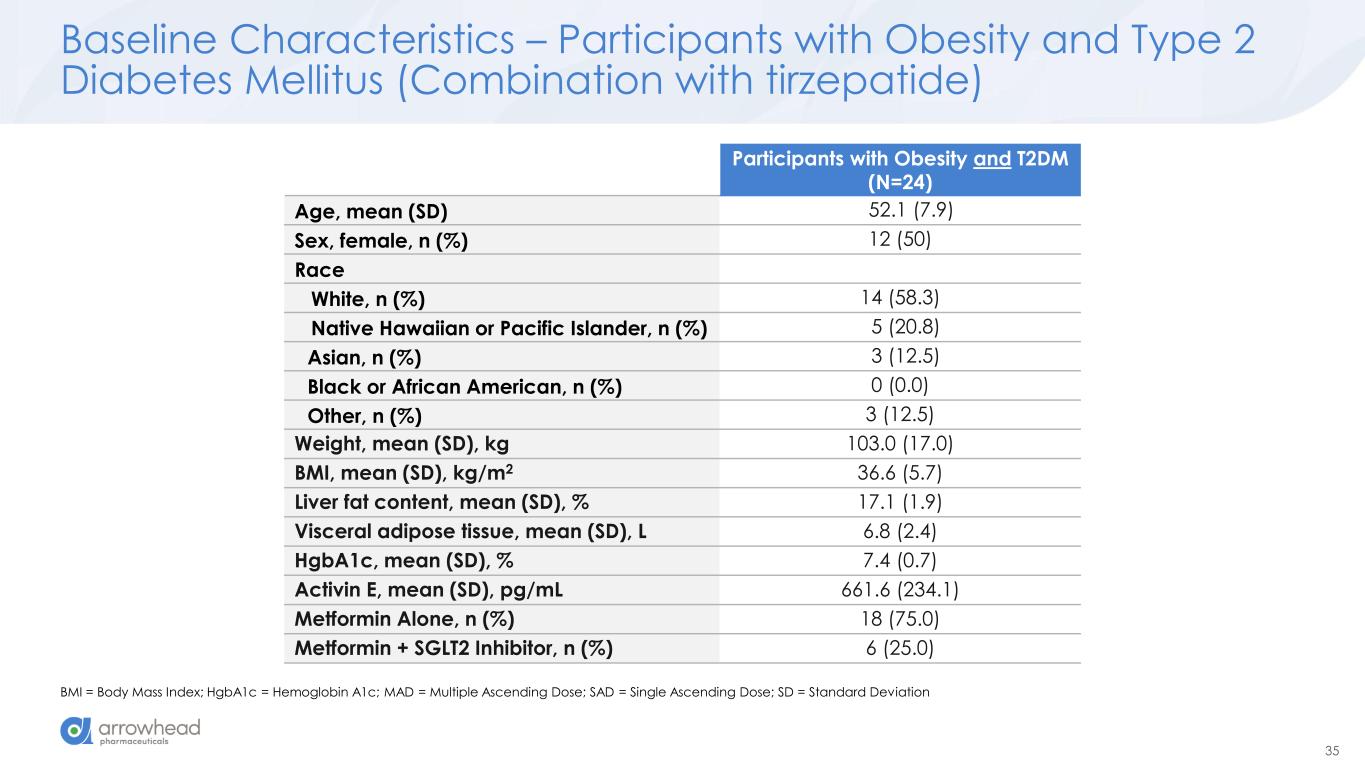
35 Baseline Characteristics – Participants with Obesity and Type 2 Diabetes Mellitus (Combination with tirzepatide) BMI = Body Mass Index; HgbA1c = Hemoglobin A1c; MAD = Multiple Ascending Dose; SAD = Single Ascending Dose; SD = Standard Deviation Participants with Obesity and T2DM (N=24) Age, mean (SD) 52.1 (7.9) Sex, female, n (%) 12 (50) Race White, n (%) 14 (58.3) Native Hawaiian or Pacific Islander, n (%) 5 (20.8) Asian, n (%) 3 (12.5) Black or African American, n (%) 0 (0.0) Other, n (%) 3 (12.5) Weight, mean (SD), kg 103.0 (17.0) BMI, mean (SD), kg/m2 36.6 (5.7) Liver fat content, mean (SD), % 17.1 (1.9) Visceral adipose tissue, mean (SD), L 6.8 (2.4) HgbA1c, mean (SD), % 7.4 (0.7) Activin E, mean (SD), pg/mL 661.6 (234.1) Metformin Alone, n (%) 18 (75.0) Metformin + SGLT2 Inhibitor, n (%) 6 (25.0)
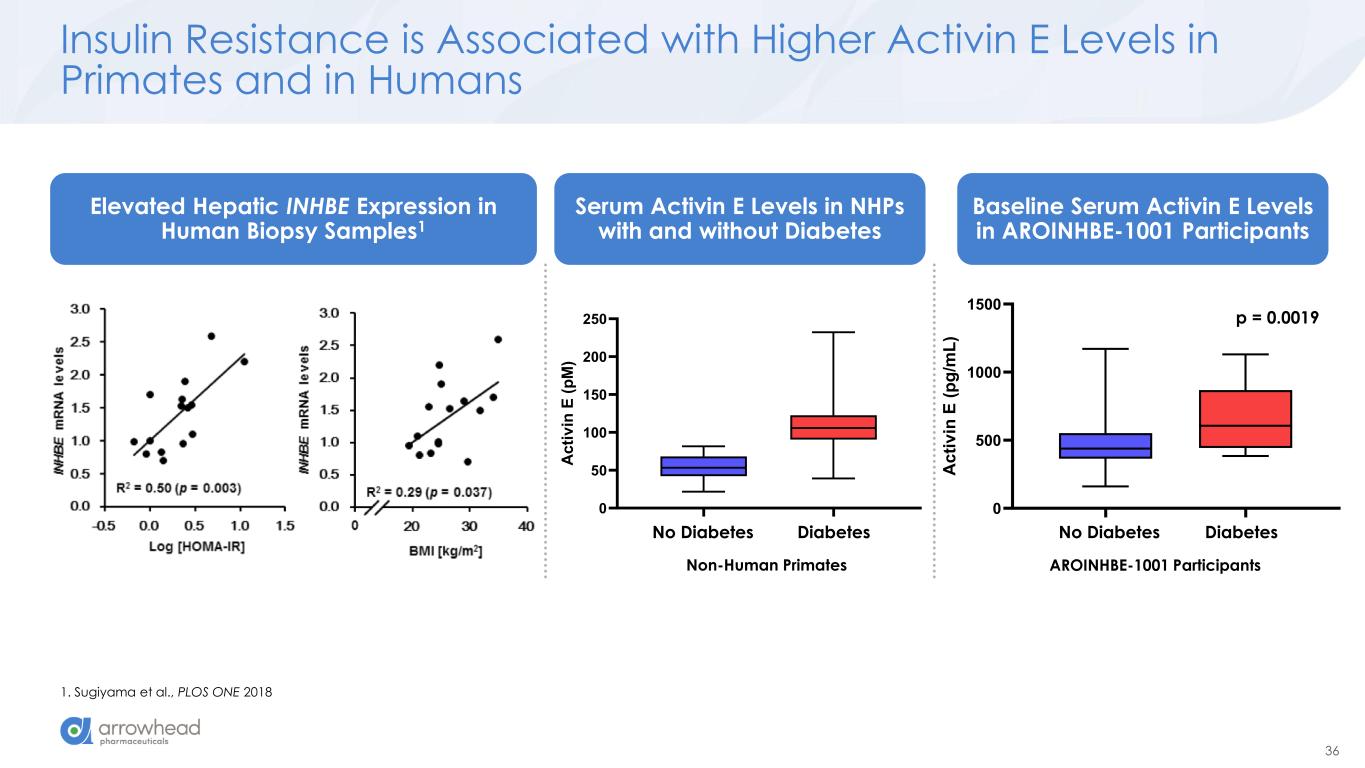
36 Non-Diabetic Diabetic 0 500 1000 1500 A c ti v in E ( p g /m L ) Insulin Resistance is Associated with Higher Activin E Levels in Primates and in Humans 1. Sugiyama et al., PLOS ONE 2018 Healthy Diabetic 0 50 100 150 200 250 A c ti v in E ( p M ) Non-Human Primates AROINHBE-1001 Participants DiabetesNo DiabetesDiabetesNo Diabetes Elevated Hepatic INHBE Expression in Human Biopsy Samples1 Serum Activin E Levels in NHPs with and without Diabetes Baseline Serum Activin E Levels in AROINHBE-1001 Participants p = 0.0019
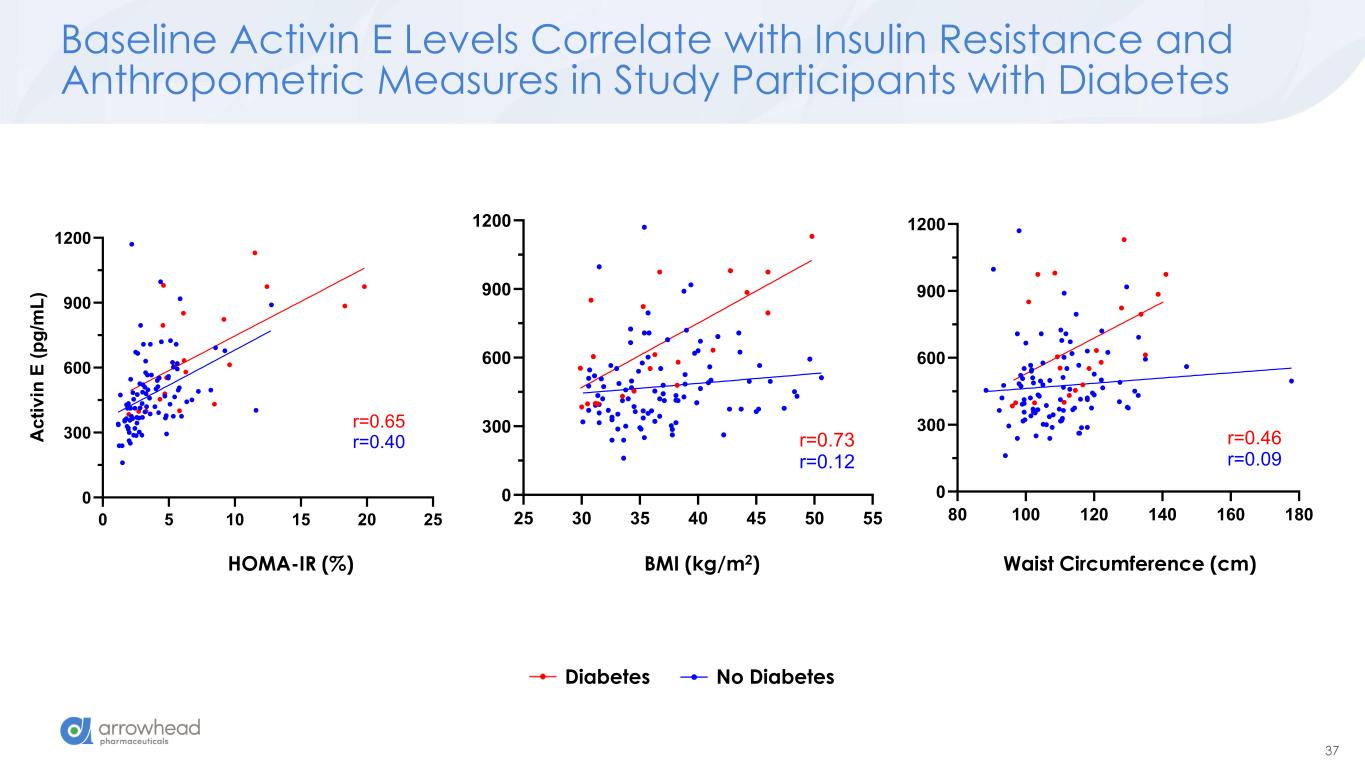
37 Baseline Activin E Levels Correlate with Insulin Resistance and Anthropometric Measures in Study Participants with Diabetes BMI (kg/m2)HOMA-IR (%) Waist Circumference (cm) Diabetes No Diabetes 0 5 10 15 20 25 0 300 600 900 1200 A c ti v in E ( p g /m L ) r=0.65 r=0.40 25 30 35 40 45 50 55 0 300 600 900 1200 r=0.73 r=0.12 80 100 120 140 160 180 0 300 600 900 1200 r=0.46 r=0.09
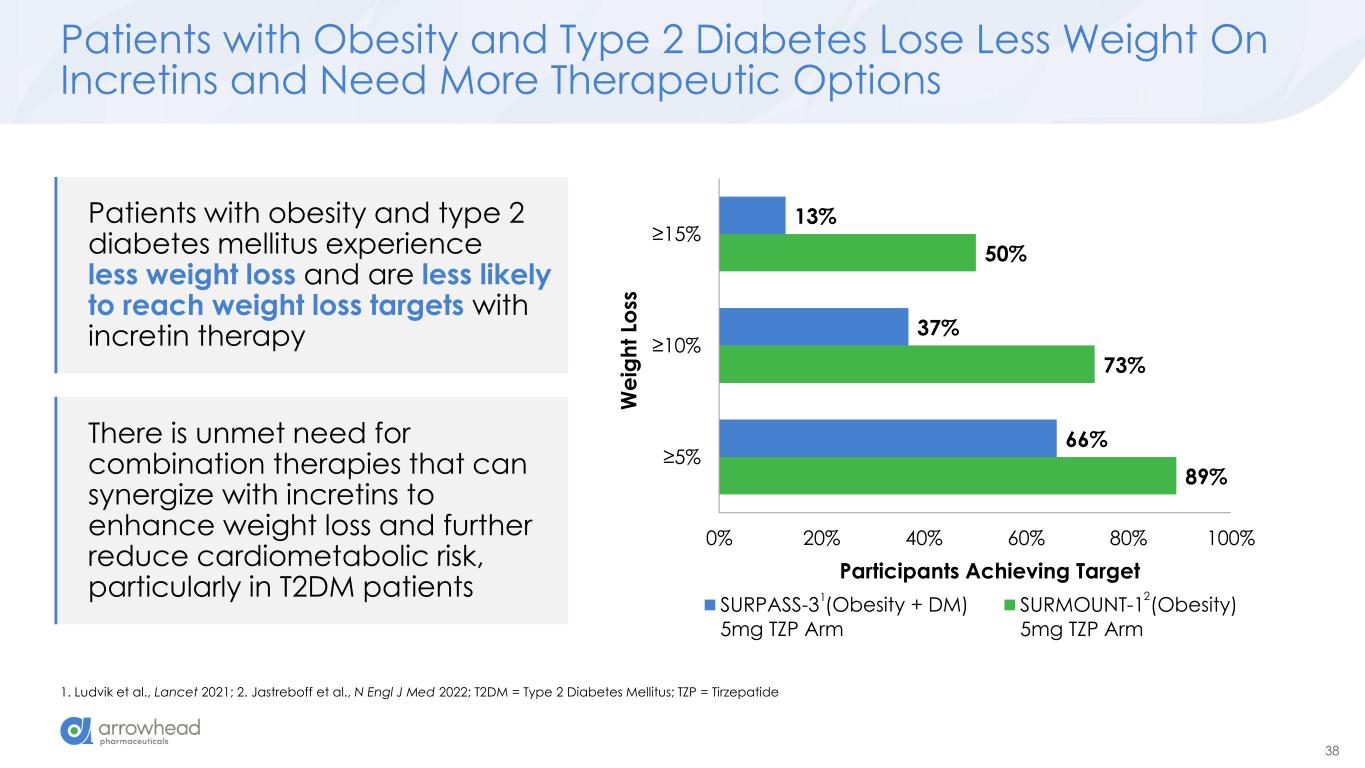
38 Patients with obesity and type 2 diabetes mellitus experience less weight loss and are less likely to reach weight loss targets with incretin therapy Patients with Obesity and Type 2 Diabetes Lose Less Weight On Incretins and Need More Therapeutic Options 1. Ludvik et al., Lancet 2021; 2. Jastreboff et al., N Engl J Med 2022; T2DM = Type 2 Diabetes Mellitus; TZP = Tirzepatide 89% 73% 50% 66% 37% 13% 0% 20% 40% 60% 80% 100% ≥5% ≥10% ≥15% Participants Achieving Target W e ig h t Lo ss SURPASS-3 (Obesity + DM) 5mg TZP Arm SURMOUNT-1 (Obesity) 5mg TZP Arm There is unmet need for combination therapies that can synergize with incretins to enhance weight loss and further reduce cardiometabolic risk, particularly in T2DM patients 1 2
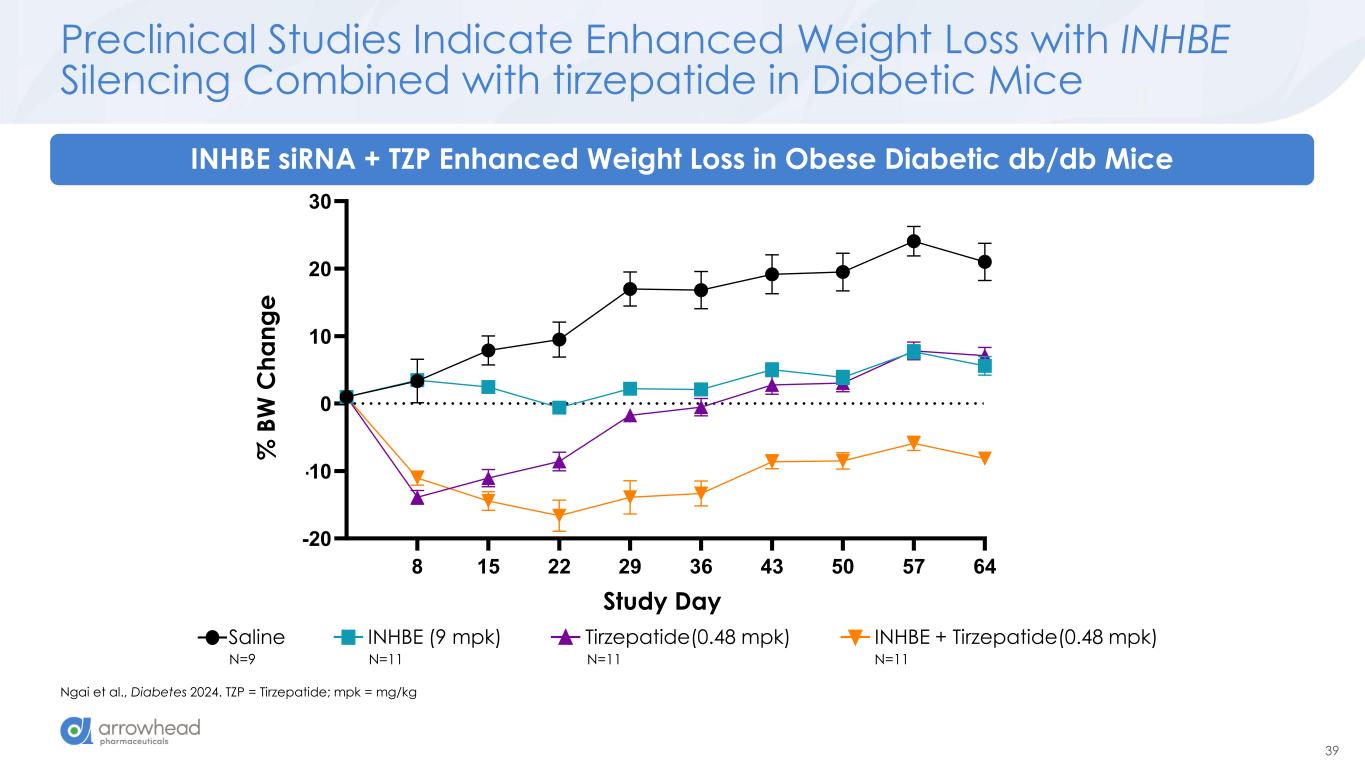
39 8 15 22 29 36 43 50 57 64 -20 -10 0 10 20 30 %BW change Study Day % B W c h a n g e Saline INHBE (9 mpk) Tirzepatide (0.48 mpk) INHBE + Tirzepatide (0.48 mpk) % B W C h a n g e Study Preclinical Studies Indicate Enhanced Weight Loss with INHBE Silencing Combined with tirzepatide in Diabetic Mice Ngai et al., Diabetes 2024. TZP = Tirzepatide; mpk = mg/kg INHBE siRNA + TZP Enhanced Weight Loss in Obese Diabetic db/db Mice Saline INHBE (9 mpk) Tirzepatide(0.48 mpk) INHBE + Tirzepatide(0.48 mpk) N=9 N=11 N=11 N=11
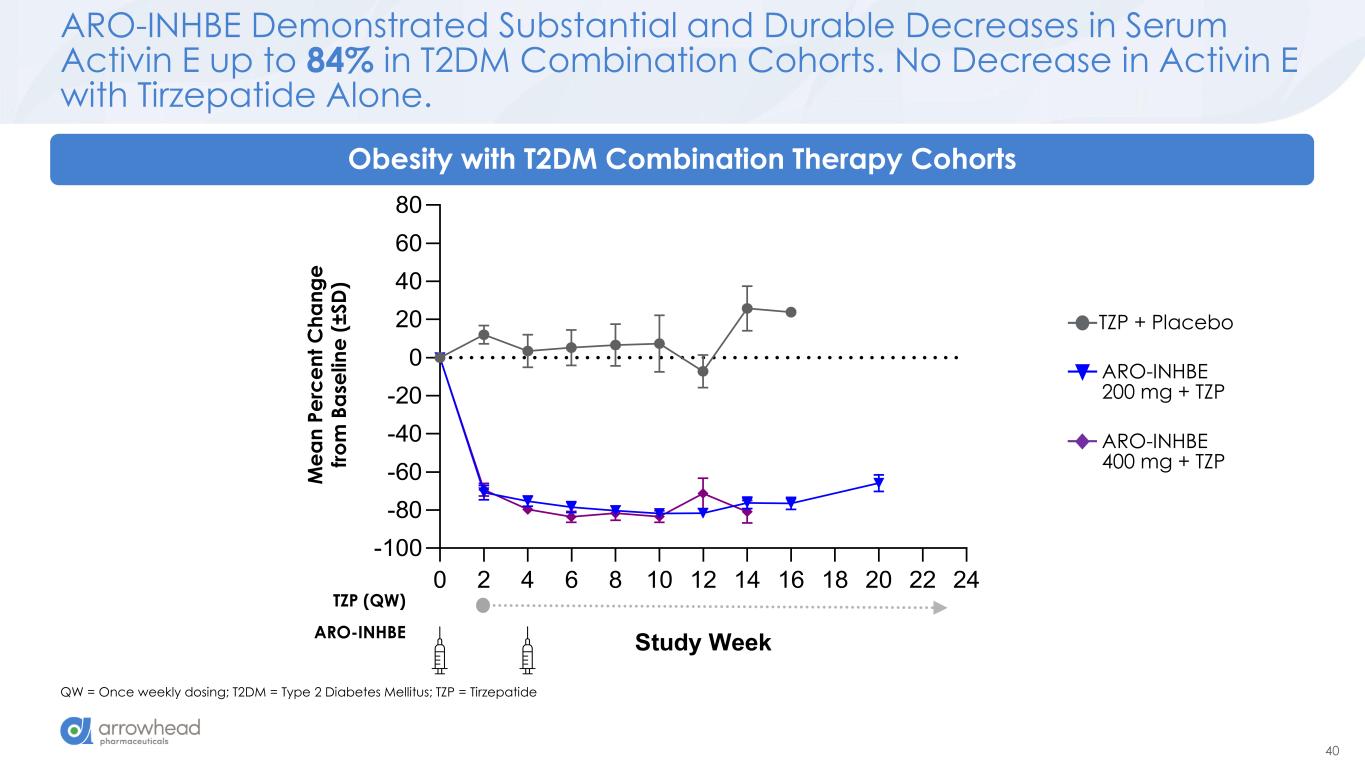
40 ARO-INHBE Demonstrated Substantial and Durable Decreases in Serum Activin E up to 84% in T2DM Combination Cohorts. No Decrease in Activin E with Tirzepatide Alone. QW = Once weekly dosing; T2DM = Type 2 Diabetes Mellitus; TZP = Tirzepatide Obesity with T2DM Combination Therapy Cohorts ARO-INHBE 400 mg + TZP TZP + Placebo ARO-INHBE 200 mg + TZP 0 2 4 6 8 10 12 14 16 18 20 22 24 -100 -80 -60 -40 -20 0 20 40 60 80 Study Week M e a n P e rc e n t C h a n g e fr o m B a s e li n e (± S D ) M e a n P e rc e n t C h a n g e fr o m B a se li n e ( ± S D ) ARO-INHBE TZP (QW)
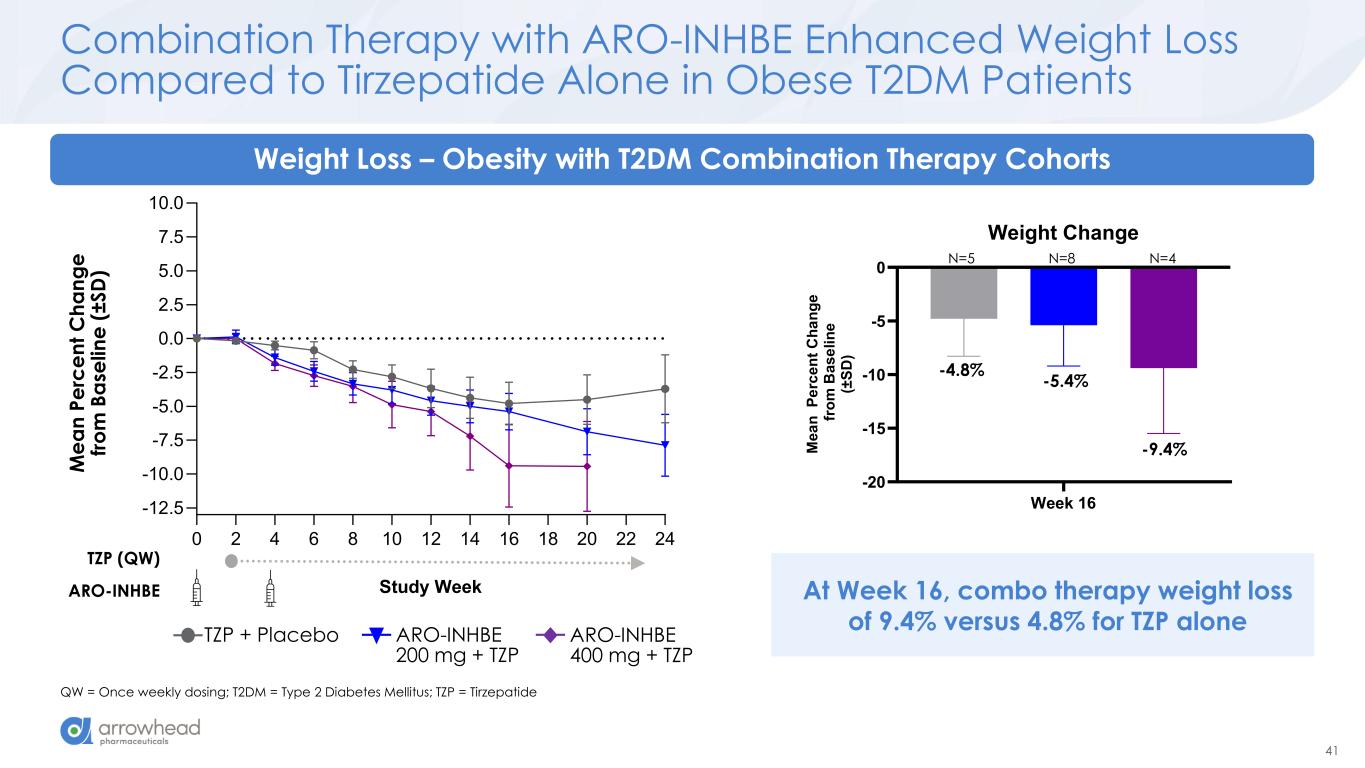
41 0 2 4 6 8 10 12 14 16 18 20 22 24 -12.5 -10.0 -7.5 -5.0 -2.5 0.0 2.5 5.0 7.5 10.0 Study Week M e a n P e rc e n t C h a n g e fr o m B a s e li n e (± S D ) Combination Therapy with ARO-INHBE Enhanced Weight Loss Compared to Tirzepatide Alone in Obese T2DM Patients QW = Once weekly dosing; T2DM = Type 2 Diabetes Mellitus; TZP = Tirzepatide ARO-INHBE TZP (QW) Weight Loss – Obesity with T2DM Combination Therapy Cohorts ARO-INHBE 400 mg + TZP TZP + Placebo ARO-INHBE 200 mg + TZP M e a n P e rc e n t C h a n g e fr o m B a se li n e ( ± S D ) At Week 16, combo therapy weight loss of 9.4% versus 4.8% for TZP alone Week 16 -20 -15 -10 -5 0 Weight Change M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) -4.8% -5.4% -9.4% N=5 N=8 N=4
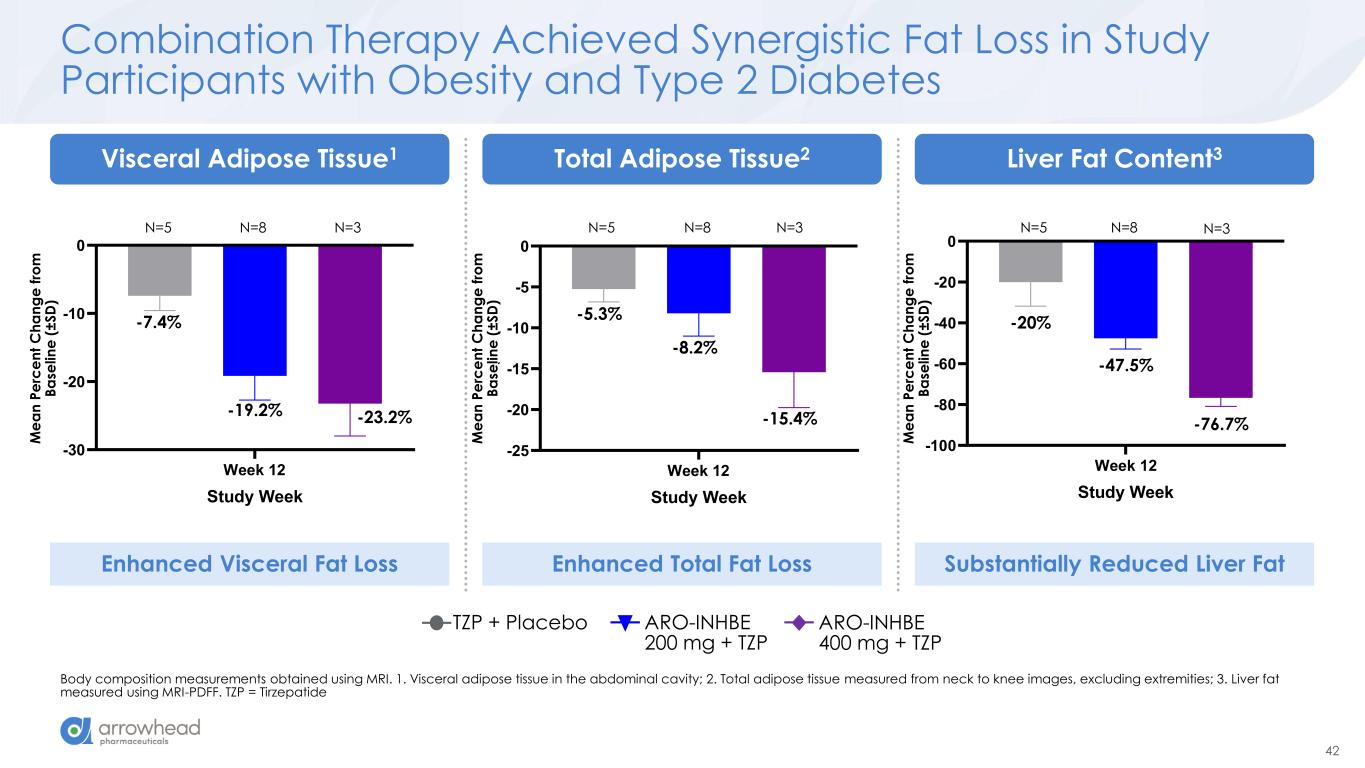
42 Week 12 -100 -80 -60 -40 -20 0 Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) Liver Fat Content (MRI-PDFF) Week 12 -25 -20 -15 -10 -5 0 Total Adipose Tissue Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) Week 12 -30 -20 -10 0 Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) Visceral Adipose Tissue Combination Therapy Achieved Synergistic Fat Loss in Study Participants with Obesity and Type 2 Diabetes Body composition measurements obtained using MRI. 1. Visceral adipose tissue in the abdominal cavity; 2. Total adipose tissue measured from neck to knee images, excluding extremities; 3. Liver fat measured using MRI-PDFF. TZP = Tirzepatide -7.4% Liver Fat Content3Total Adipose Tissue2Visceral Adipose Tissue1 ARO-INHBE 400 mg + TZP TZP + Placebo ARO-INHBE 200 mg + TZP M e a n P e rc e n t C h a n g e f ro m B a se li n e ( ± S D ) -19.2% -23.2% -5.3% -8.2% -15.4% -20% -47.5% -76.7% Enhanced Visceral Fat Loss Enhanced Total Fat Loss Substantially Reduced Liver Fat M e a n P e rc e n t C h a n g e f ro m B a se li n e ( ± S D ) M e a n P e rc e n t C h a n g e f ro m B a se li n e ( ± S D ) N=5 N=8 N=3 N=5 N=8 N=3 N=5 N=8 N=3
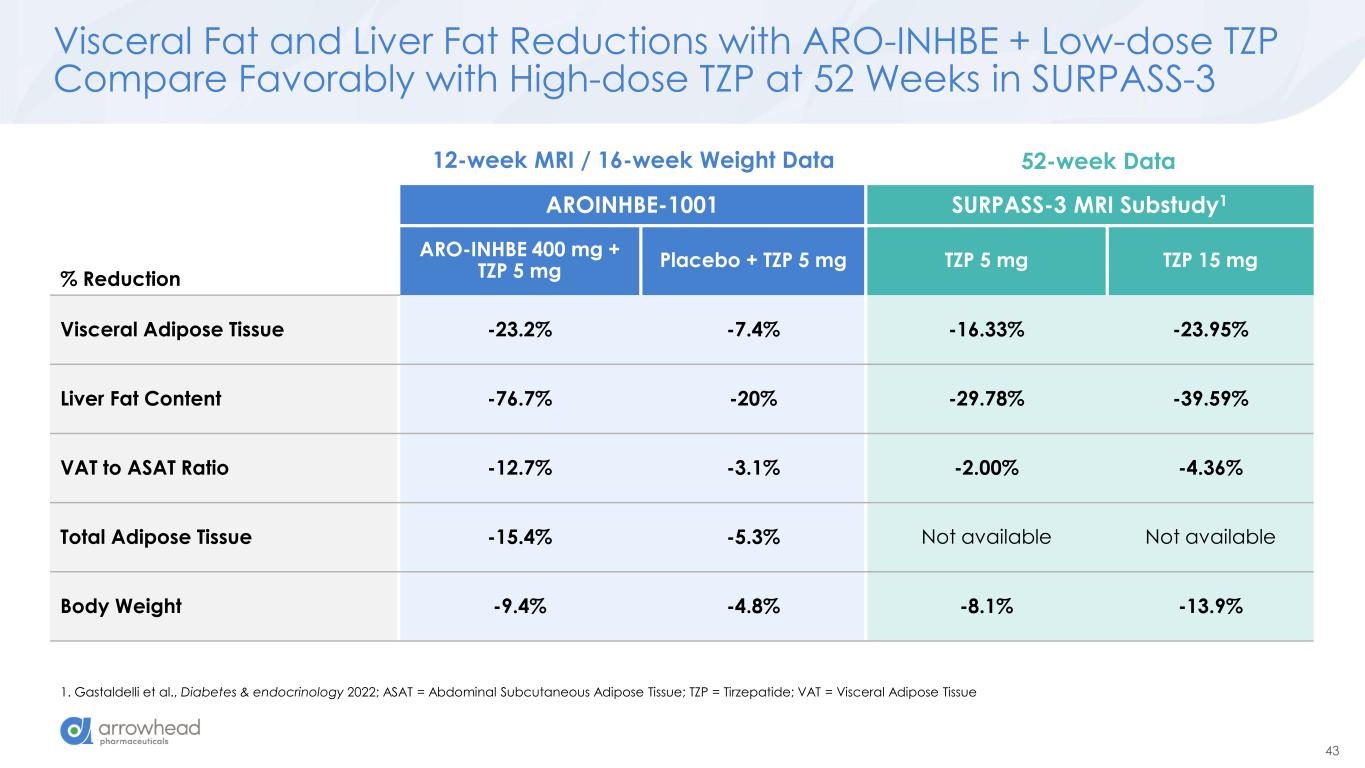
43 Visceral Fat and Liver Fat Reductions with ARO-INHBE + Low-dose TZP Compare Favorably with High-dose TZP at 52 Weeks in SURPASS-3 1. Gastaldelli et al., Diabetes & endocrinology 2022; ASAT = Abdominal Subcutaneous Adipose Tissue; TZP = Tirzepatide; VAT = Visceral Adipose Tissue % Reduction AROINHBE-1001 SURPASS-3 MRI Substudy1 ARO-INHBE 400 mg + TZP 5 mg Placebo + TZP 5 mg TZP 5 mg TZP 15 mg Visceral Adipose Tissue -23.2% -7.4% -16.33% -23.95% Liver Fat Content -76.7% -20% -29.78% -39.59% VAT to ASAT Ratio -12.7% -3.1% -2.00% -4.36% Total Adipose Tissue -15.4% -5.3% Not available Not available Body Weight -9.4% -4.8% -8.1% -13.9% 12-week MRI / 16-week Weight Data 52-week Data
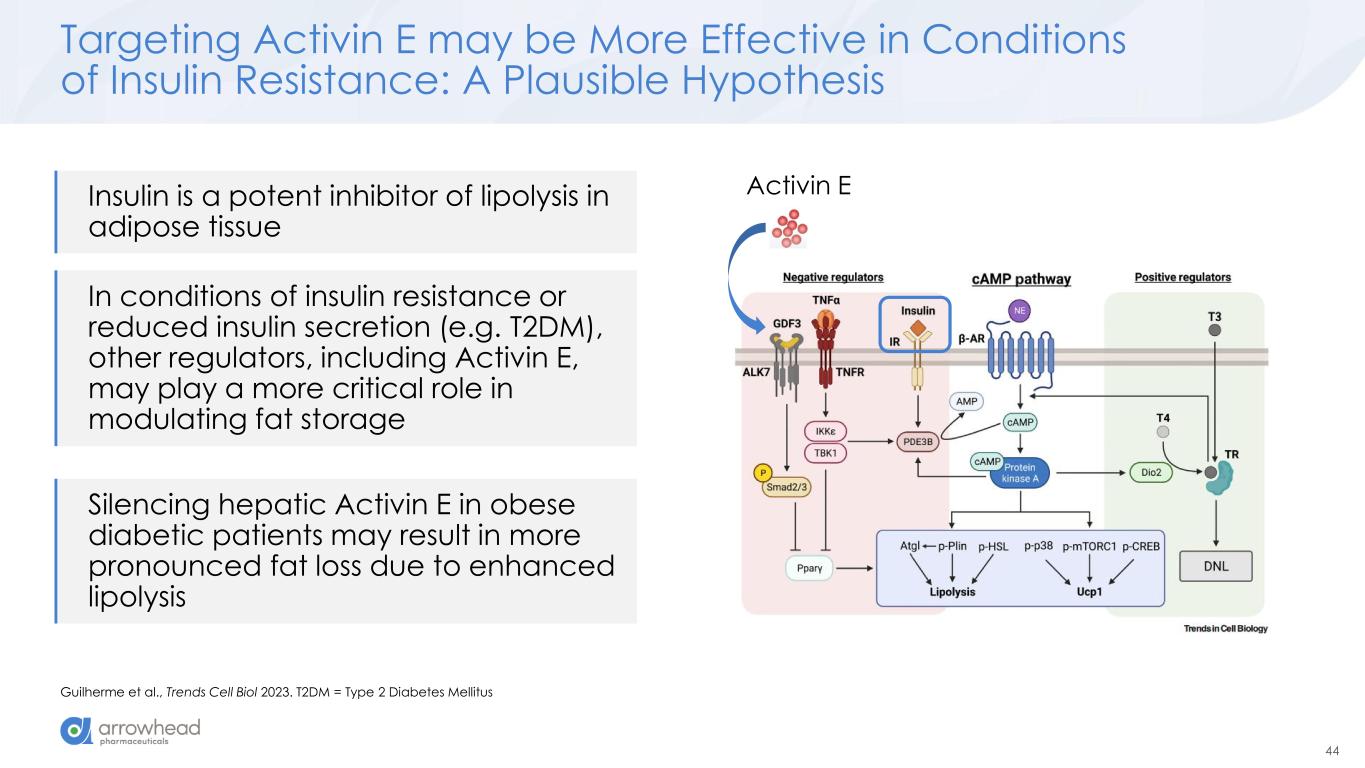
44 Targeting Activin E may be More Effective in Conditions of Insulin Resistance: A Plausible Hypothesis Guilherme et al., Trends Cell Biol 2023. T2DM = Type 2 Diabetes Mellitus Activin EInsulin is a potent inhibitor of lipolysis in adipose tissue In conditions of insulin resistance or reduced insulin secretion (e.g. T2DM), other regulators, including Activin E, may play a more critical role in modulating fat storage Silencing hepatic Activin E in obese diabetic patients may result in more pronounced fat loss due to enhanced lipolysis
45 ARO-INHBE Demonstrates a Favorable Safety Profile ARO-INHBE was well tolerated as monotherapy and in combination with tirzepatide in participants with obesity with and without type 2 diabetes. • Most TEAEs were mild in severity • No TEAEs led to study or study drug discontinuation Injection site reactions were generally mild and self-limited. Frequency of GI adverse events was similar in combination and tirzepatide monotherapy groups. One SAE of “limb abscess” was reported, managed with bedside drainage, and assessed as Unrelated to study treatment by both Sponsor and Site Investigator. No clinically significant adverse laboratory trends including in liver enzymes, glycemic indices, or lipid parameters were identified.
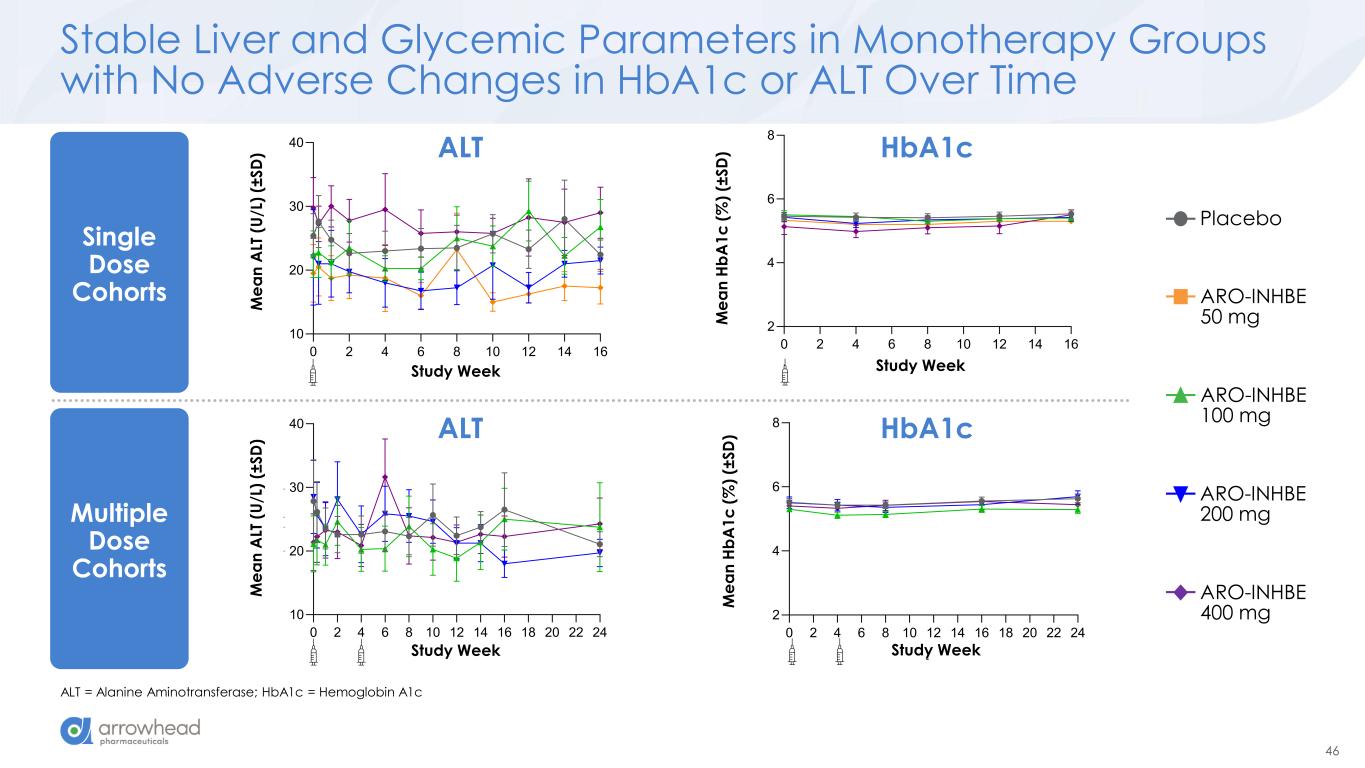
46 0 2 4 6 8 10 12 14 16 18 20 22 24 2 4 6 8 Study Week M e a n H b A 1 c ( % ) (± S D ) Stud ek 0 2 4 6 8 10 12 14 16 0 10 20 30 40 Study Week M e a n A L T (U /L ) (± S D ) 0 2 4 6 8 10 12 14 16 0 10 20 30 40 Study Week M e a n A L T (U /L ) (± S D ) Stable Liver and Glycemic Parameters in Monotherapy Groups with No Adverse Changes in HbA1c or ALT Over Time ALT = Alanine Aminotransferase; HbA1c = Hemoglobin A1c ALT HbA1c ALT HbA1c Single Dose Cohorts Placebo ARO-INHBE 50 mg ARO-INHBE 100 mg ARO-INHBE 200 mg ARO-INHBE 400 mg 0 2 4 6 8 10 12 14 16 10 20 30 40 Study Week M e a n A L T (U /L ) (± S D ) M e a n A LT ( U /L ) (± S D ) Study k 0 2 4 6 8 10 12 14 16 0 10 20 30 40 Study Week M e a n A L T (U /L ) (± S D ) 0 2 4 6 8 10 12 14 16 2 4 6 8 Study Week M e a n H b A 1 c ( % ) (± S D ) Study We 0 2 4 6 8 10 12 14 16 0 10 20 30 40 Study Week M e a n A L T (U /L ) (± S D ) 0 2 4 6 8 10 12 14 16 18 20 22 24 10 20 30 40 Study Week M e a n A L T (U /L ) (± S D ) Study k M e a n A LT ( U /L ) (± S D ) 0 2 4 6 8 10 12 14 16 0 1 20 30 40 Study Week M e a n A L T (U /L ) (± S D ) 0 2 4 6 8 10 12 14 16 0 10 20 30 40 Study Week M e a n A L T (U /L ) (± S D ) Multiple Dose Cohorts
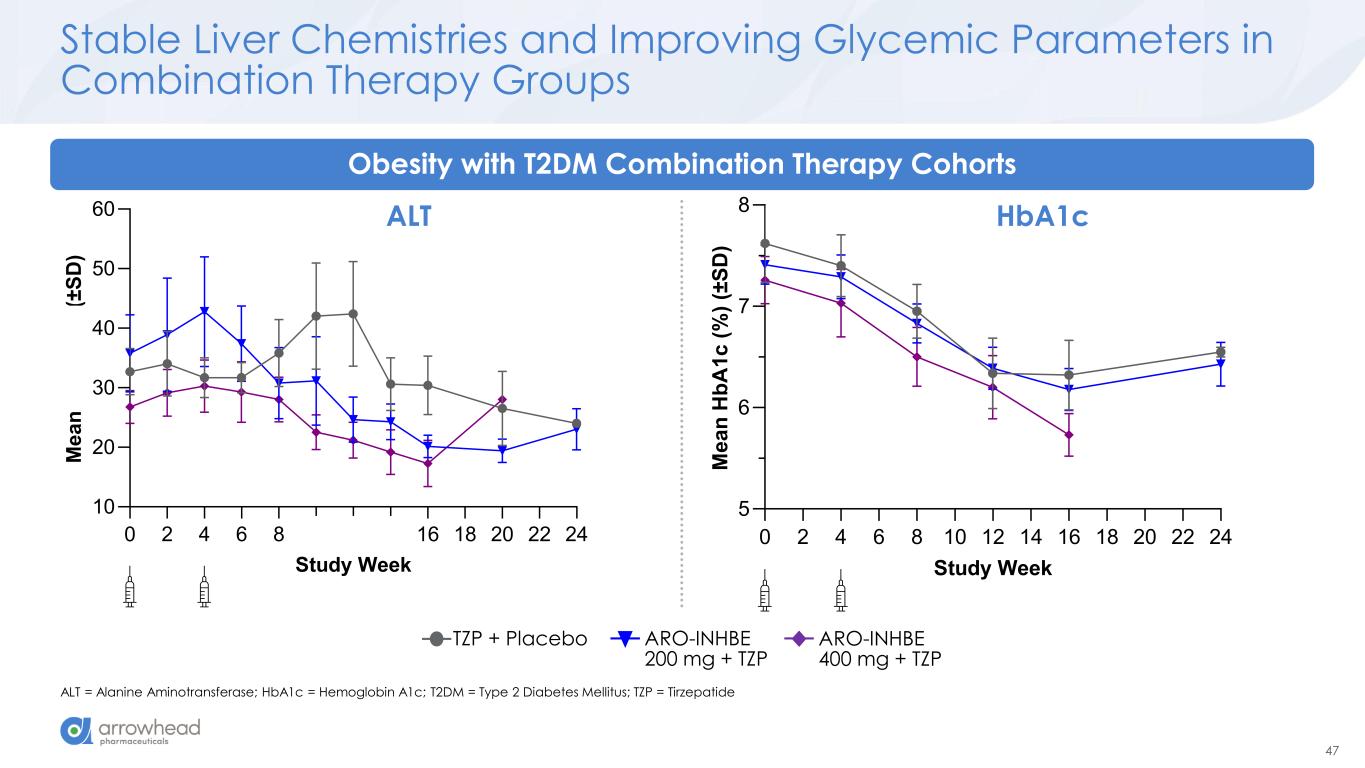
47 0 2 4 6 8 10 12 14 16 18 20 22 24 10 20 30 40 50 60 Study Week M e a n A L T ( U /L ) (± S D ) 0 2 4 6 8 10 12 14 16 18 20 22 24 5 6 7 8 Study Week M e a n H b A 1 c ( % ) (± S D ) Stable Liver Chemistries and Improving Glycemic Parameters in Combination Therapy Groups ALT = Alanine Aminotransferase; HbA1c = Hemoglobin A1c; T2DM = Type 2 Diabetes Mellitus; TZP = Tirzepatide Obesity with T2DM Combination Therapy Cohorts HbA1cALT ARO-INHBE 400 mg + TZP TZP + Placebo ARO-INHBE 200 mg + TZP

48 Summary and Next Steps HbA1c = Hemoglobin A1c ARO-INHBE was safe, well-tolerated, and achieved robust and sustained reductions in serum Activin E levels with no adverse changes in transaminases or HbA1c Monotherapy with ARO-INHBE achieved meaningful reductions in visceral and liver fat by MRI Combination therapy with tirzepatide in patients with obesity and type 2 diabetes enhanced fat mass loss and weight loss compared to tirzepatide alone, representing a unique opportunity for targeting metabolic health in a population that achieves less weight loss with GLP-1/GIP monotherapy Phase 2 study planning in progress

Clinical Study Update from AROALK7-1001 James Hamilton MD, MBA Chief Medical Officer and Head of R&D ARO-INHBE and ARO-ALK7 Interim Clinical Data Update
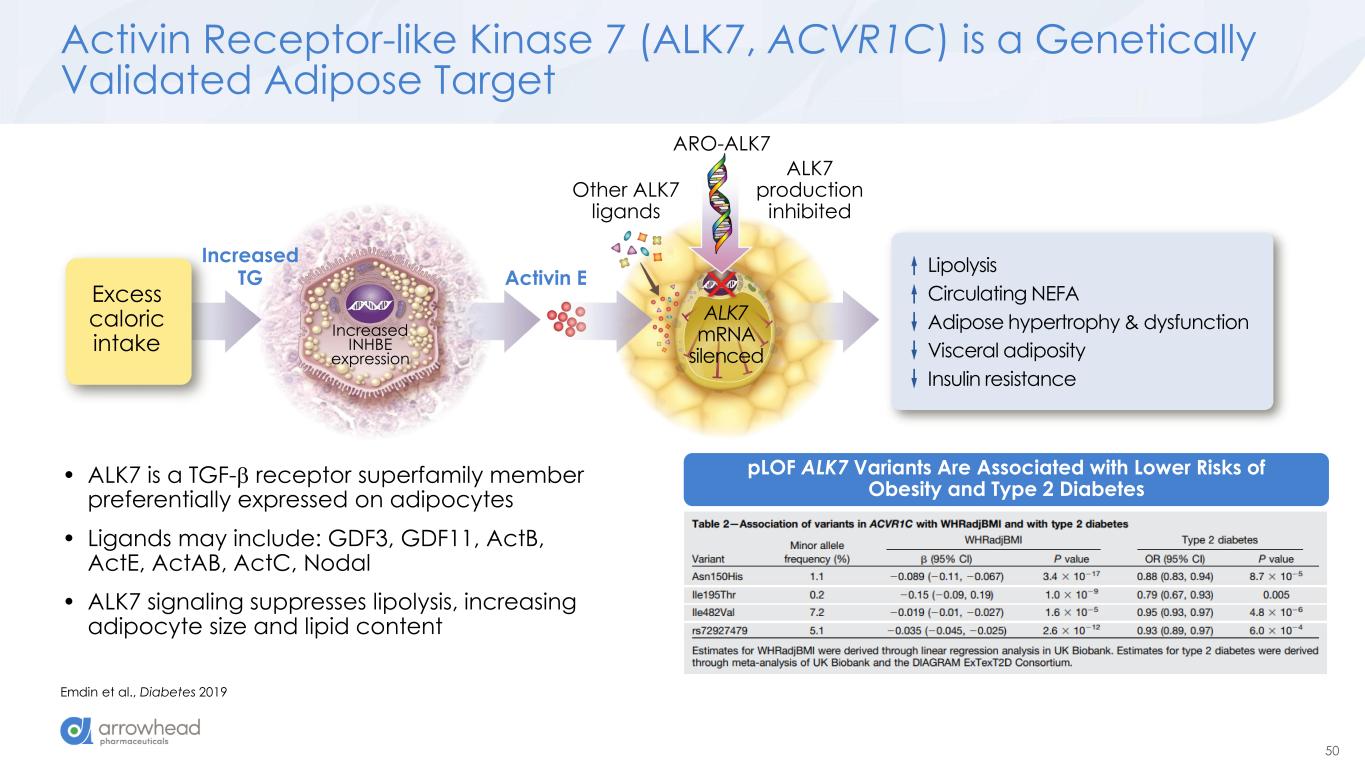
50 ARO-ALK7 Excess caloric intake Increased INHBE expression Activin E ALK7 production inhibited Lipolysis Circulating NEFA Adipose hypertrophy & dysfunction Visceral adiposity Insulin resistance Other ALK7 ligands ALK7 mRNA silenced Increased TG Activin Receptor-like Kinase 7 (ALK7, ACVR1C) is a Genetically Validated Adipose Target Emdin et al., Diabetes 2019 pLOF ALK7 Variants Are Associated with Lower Risks of Obesity and Type 2 Diabetes • ALK7 is a TGF- receptor superfamily member preferentially expressed on adipocytes • Ligands may include: GDF3, GDF11, ActB, ActE, ActAB, ActC, Nodal • ALK7 signaling suppresses lipolysis, increasing adipocyte size and lipid content
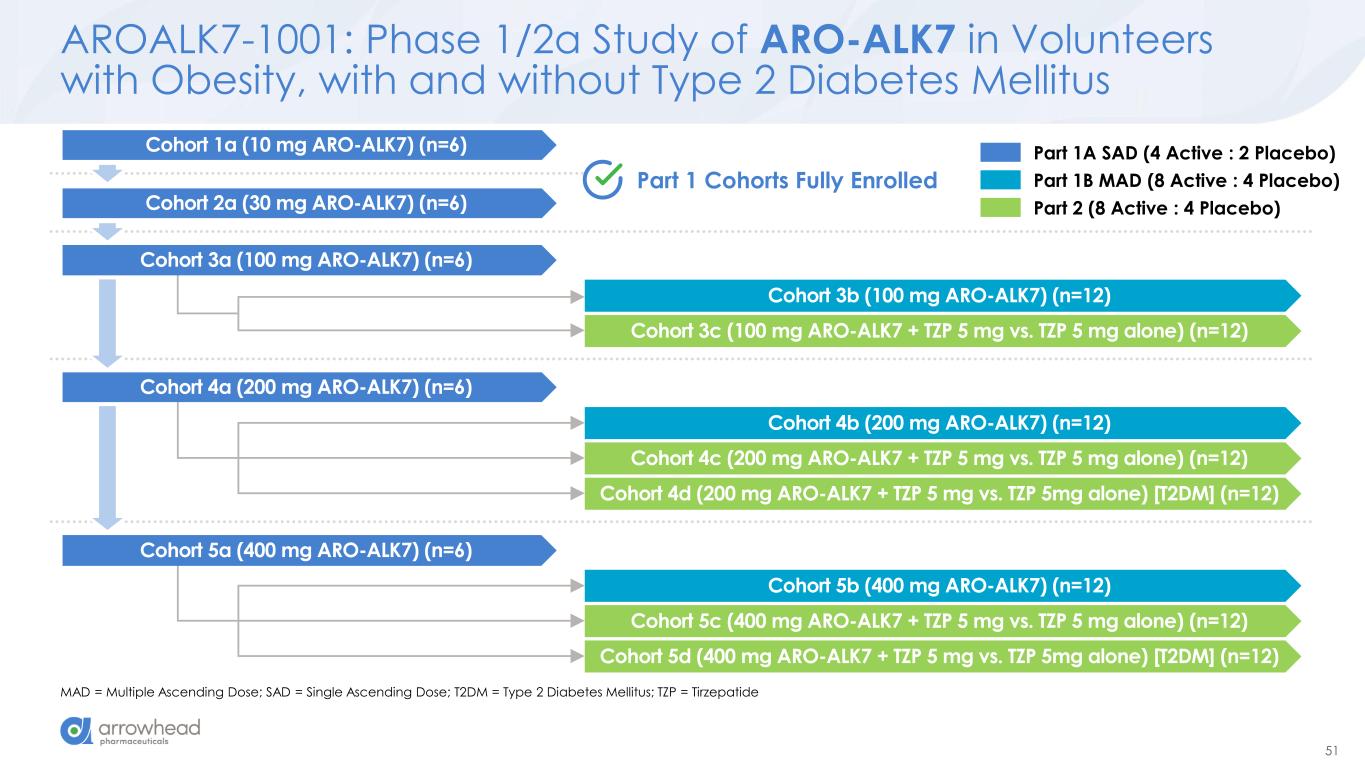
51 AROALK7-1001: Phase 1/2a Study of ARO-ALK7 in Volunteers with Obesity, with and without Type 2 Diabetes Mellitus MAD = Multiple Ascending Dose; SAD = Single Ascending Dose; T2DM = Type 2 Diabetes Mellitus; TZP = Tirzepatide Cohort 3b (100 mg ARO-ALK7) (n=12) Cohort 3c (100 mg ARO-ALK7 + TZP 5 mg vs. TZP 5 mg alone) (n=12) Cohort 4b (200 mg ARO-ALK7) (n=12) Cohort 4c (200 mg ARO-ALK7 + TZP 5 mg vs. TZP 5 mg alone) (n=12) Cohort 4d (200 mg ARO-ALK7 + TZP 5 mg vs. TZP 5mg alone) [T2DM] (n=12) Cohort 5b (400 mg ARO-ALK7) (n=12) Cohort 5c (400 mg ARO-ALK7 + TZP 5 mg vs. TZP 5 mg alone) (n=12) Cohort 5d (400 mg ARO-ALK7 + TZP 5 mg vs. TZP 5mg alone) [T2DM] (n=12) Part 1 Cohorts Fully Enrolled Cohort 2a (30 mg ARO-ALK7) (n=6) Cohort 3a (100 mg ARO-ALK7) (n=6) Cohort 4a (200 mg ARO-ALK7) (n=6) Cohort 5a (400 mg ARO-ALK7) (n=6) Cohort 1a (10 mg ARO-ALK7) (n=6) Part 1A SAD (4 Active : 2 Placebo) Part 1B MAD (8 Active : 4 Placebo) Part 2 (8 Active : 4 Placebo)
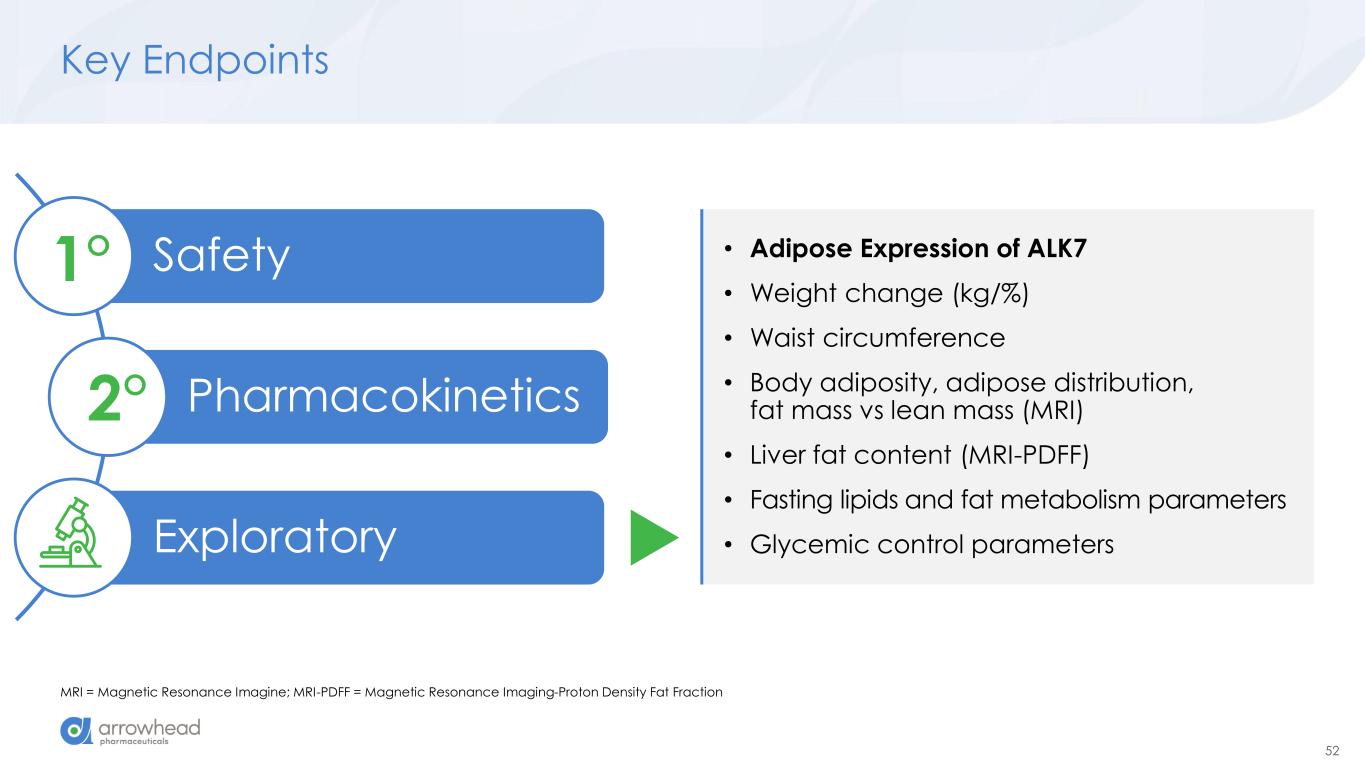
52 Key Endpoints MRI = Magnetic Resonance Imagine; MRI-PDFF = Magnetic Resonance Imaging-Proton Density Fat Fraction Safety Pharmacokinetics Exploratory 1° 2° • Adipose Expression of ALK7 • Weight change (kg/%) • Waist circumference • Body adiposity, adipose distribution, fat mass vs lean mass (MRI) • Liver fat content (MRI-PDFF) • Fasting lipids and fat metabolism parameters • Glycemic control parameters
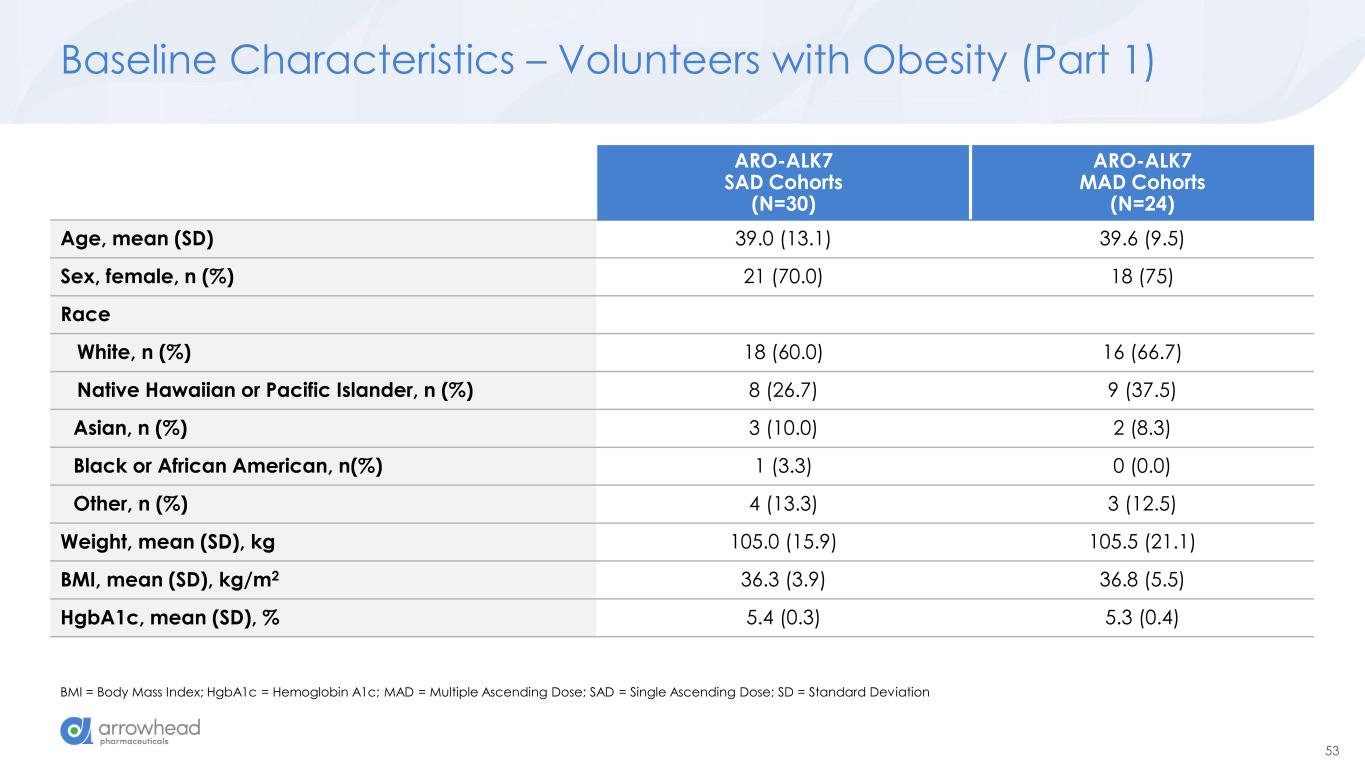
53 Baseline Characteristics – Volunteers with Obesity (Part 1) BMI = Body Mass Index; HgbA1c = Hemoglobin A1c; MAD = Multiple Ascending Dose; SAD = Single Ascending Dose; SD = Standard Deviation ARO-ALK7 SAD Cohorts (N=30) ARO-ALK7 MAD Cohorts (N=24) Age, mean (SD) 39.0 (13.1) 39.6 (9.5) Sex, female, n (%) 21 (70.0) 18 (75) Race White, n (%) 18 (60.0) 16 (66.7) Native Hawaiian or Pacific Islander, n (%) 8 (26.7) 9 (37.5) Asian, n (%) 3 (10.0) 2 (8.3) Black or African American, n(%) 1 (3.3) 0 (0.0) Other, n (%) 4 (13.3) 3 (12.5) Weight, mean (SD), kg 105.0 (15.9) 105.5 (21.1) BMI, mean (SD), kg/m2 36.3 (3.9) 36.8 (5.5) HgbA1c, mean (SD), % 5.4 (0.3) 5.3 (0.4)
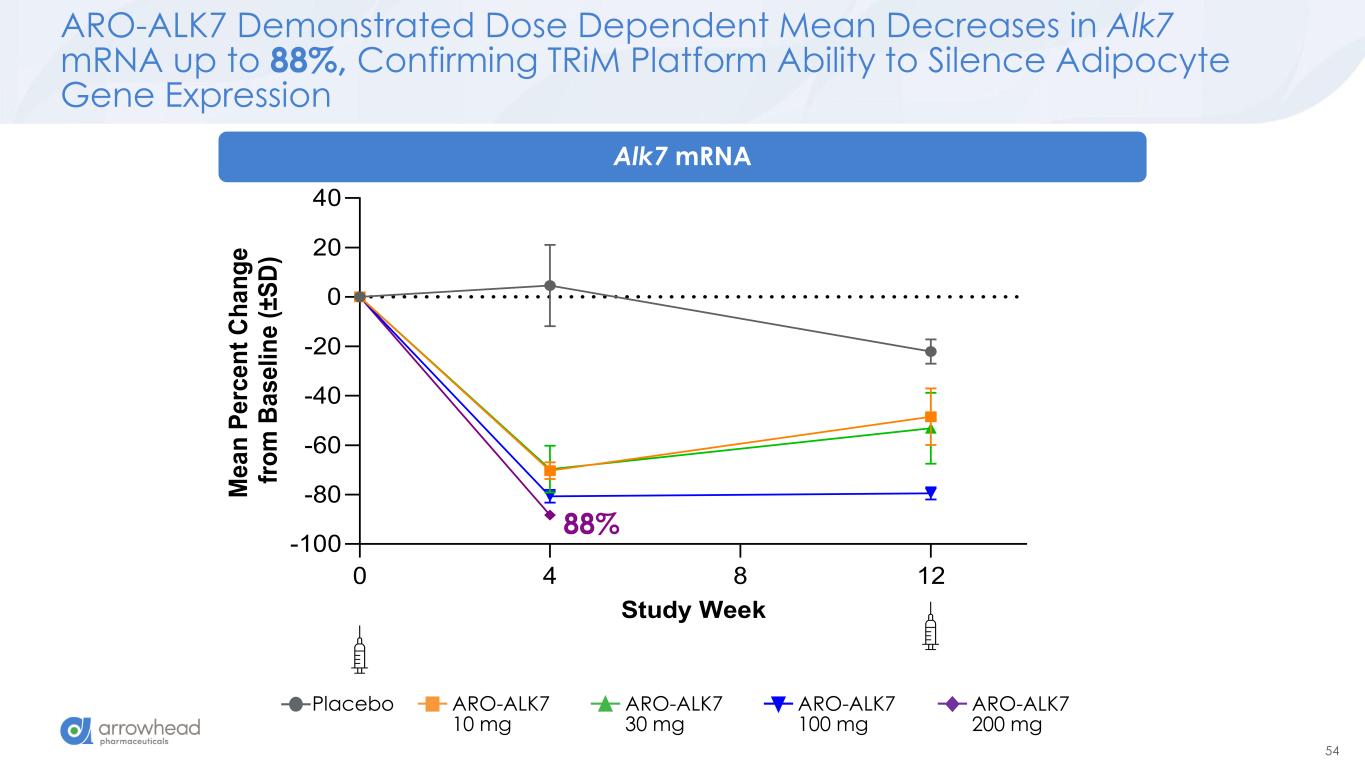
54 0 4 8 12 -100 -80 -60 -40 -20 0 20 40 Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e ( ± S D ) ARO-ALK7 Demonstrated Dose Dependent Mean Decreases in Alk7 mRNA up to 88%, Confirming TRiM Platform Ability to Silence Adipocyte Gene Expression 88% Placebo ARO-ALK7 10 mg ARO-ALK7 30 mg ARO-ALK7 100 mg ARO-ALK7 200 mg Alk7 mRNA
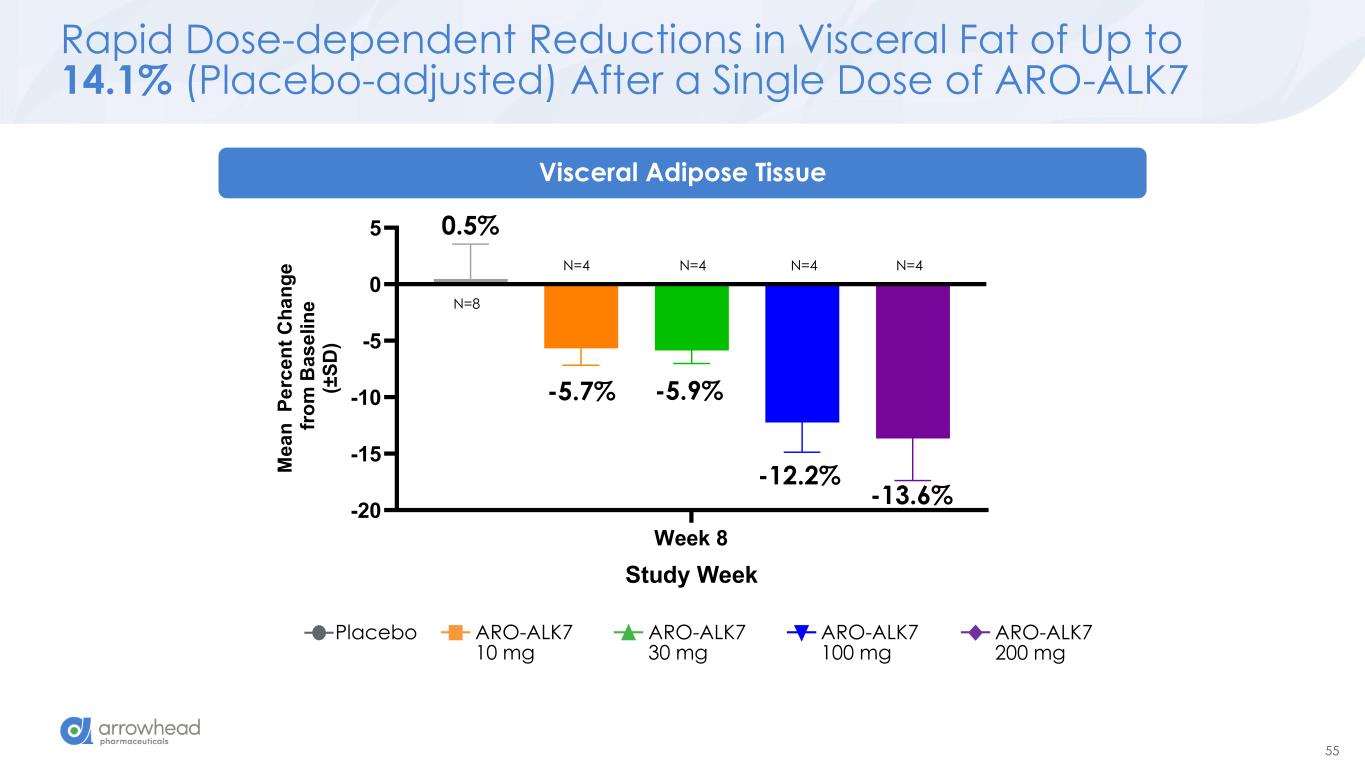
55 Rapid Dose-dependent Reductions in Visceral Fat of Up to 14.1% (Placebo-adjusted) After a Single Dose of ARO-ALK7 Placebo ARO-ALK7 10 mg ARO-ALK7 30 mg ARO-ALK7 100 mg ARO-ALK7 200 mg Week 8 -20 -15 -10 -5 0 5 Visceral Adipose Tissue Study Week M e a n P e rc e n t C h a n g e f ro m B a s e li n e (± S D ) -5.7% -5.9% -12.2% -13.6% 0.5% N=4 N=4 N=4 N=4 N=8 Visceral Adipose Tissue
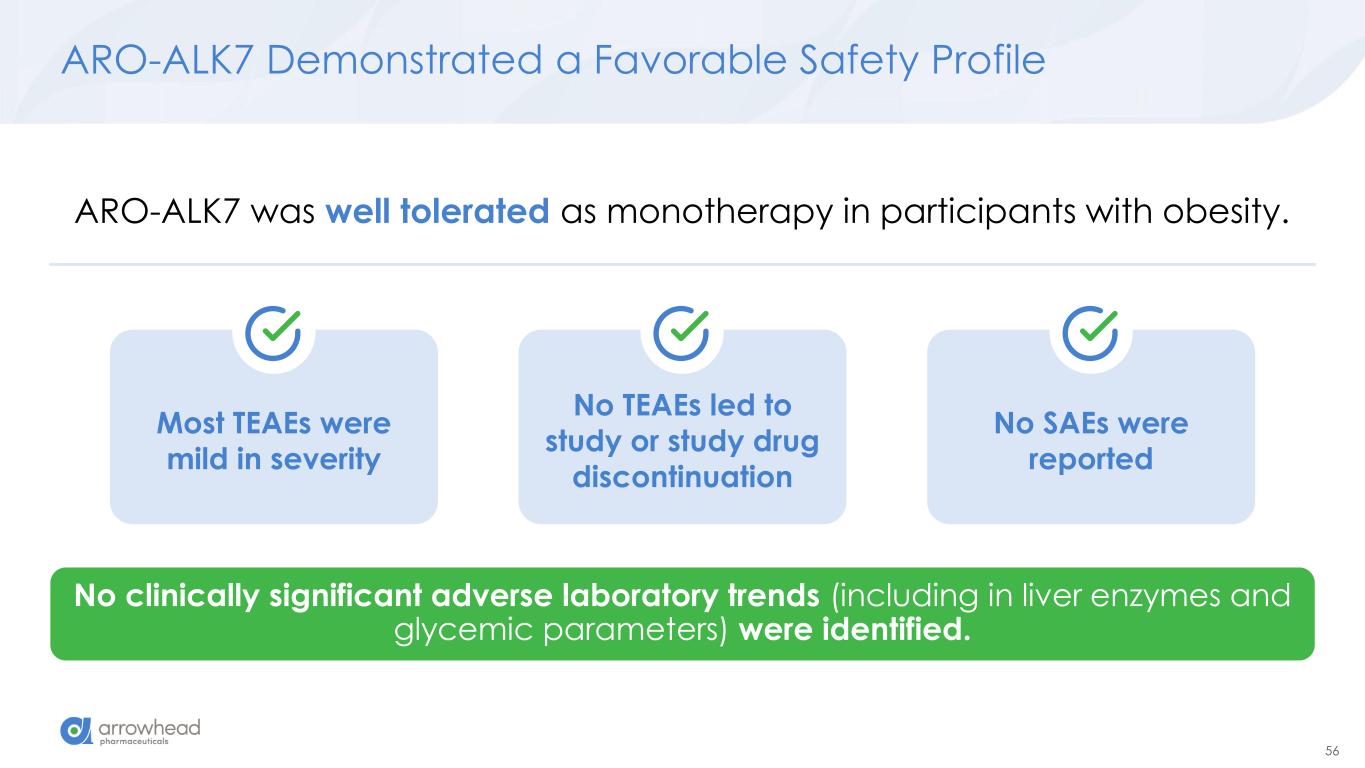
56 Most TEAEs were mild in severity ARO-ALK7 Demonstrated a Favorable Safety Profile No clinically significant adverse laboratory trends (including in liver enzymes and glycemic parameters) were identified. ARO-ALK7 was well tolerated as monotherapy in participants with obesity. No TEAEs led to study or study drug discontinuation No SAEs were reported

57 Summary and Next Steps MAD = Multiple Ascending Dose; SAD = Single Ascending Dose ARO-ALK7 was safe, well-tolerated, and led to deep reductions in adipose ALK7 expression at all clinical doses SAD and MAD Cohorts fully enrolled and Part 2 Cohorts, recruiting participants with obesity with and without type 2 diabetes, are actively enrolling Additional AROALK7-1001 data release throughout 2026

We would like to thank the patients, investigators, and site personnel who participated in the studies

Key Takeaways Chris Anzalone, Ph.D. President and CEO ARO-INHBE and ARO-ALK7 Interim Clinical Data Update January 2026
60 Key Early Findings from Ongoing Phase 1/2a Studies ➢ ARO-INHBE: dose-dependent reductions in serum Activin E o mean max reduction of -85% after a single 400 mg dose o max observed reduction of -94% ➢ ARO-INHBE + TZP drove high quality weight loss in obese diabetic patients o -23.2% visceral fat reduction o -15.4% total fat reduction o -76.7% liver fat reduction o Approximately 3-fold improvement versus TZP alone across these measures ➢ ARO-INHBE monotherapy reduced visceral fat o -9.9% after a single dose at week 16 o -15.6% after 2 doses at week 24 ➢ ARO-INHBE + tirzepatide doubled weight loss in obese diabetic patients versus TZP alone o -9.4% weight loss at week at week 16 with ARO-INHBE + TZP o -4.8% weight loss at week 16 with TZP alone ➢ ARO-ALK7 is the first RNAi therapeutic to show knockdown of an adipocyte-expressed target in humans o mean reduction of -88% ALK7 mRNA o max reduction of -94% ➢ Early data, but ARO-ALK7 could be more active than ARO-INHBE o -14% placebo-adjusted reduction in visceral fat after single dose monotherapy at week 8

61 What We Already Achieved in Phase 1/2a Established Safety and Tolerability of single-dose, multi-dose, and combination regimens with tirzepatide Demonstrated deep and durable knockdown of Activin E and ALK7 Identified signals of Activin E/ALK7 pathway translation in humans Measured favorable changes in body composition Showed benefit on weight loss in a specific population with unmet need

62 What We Still Hope to Achieve Next Steps • Expanding current studies, including: • Increasing numbers of patients to increase power • Extending follow-up to better understand drug durability and activity out to 1 year • Initiation of a monotherapy cohort in obese diabetic patients • Initiation of additional combination cohorts with other GLPs • Initiate Phase 2b studies ASAP (current studies and additions are not gating) • Combination studies (tirzepitide and other GLPs) in obese diabetic patients • Studies aimed at use as maintenance therapy (after GLPs are removed) • Obesity pipeline expansion • New liver and adipocyte targets • Dimers targeting 2 adipocyte targets • Dimers targeting 2 liver targets • Leveraging our sc CNS platform to address central targets
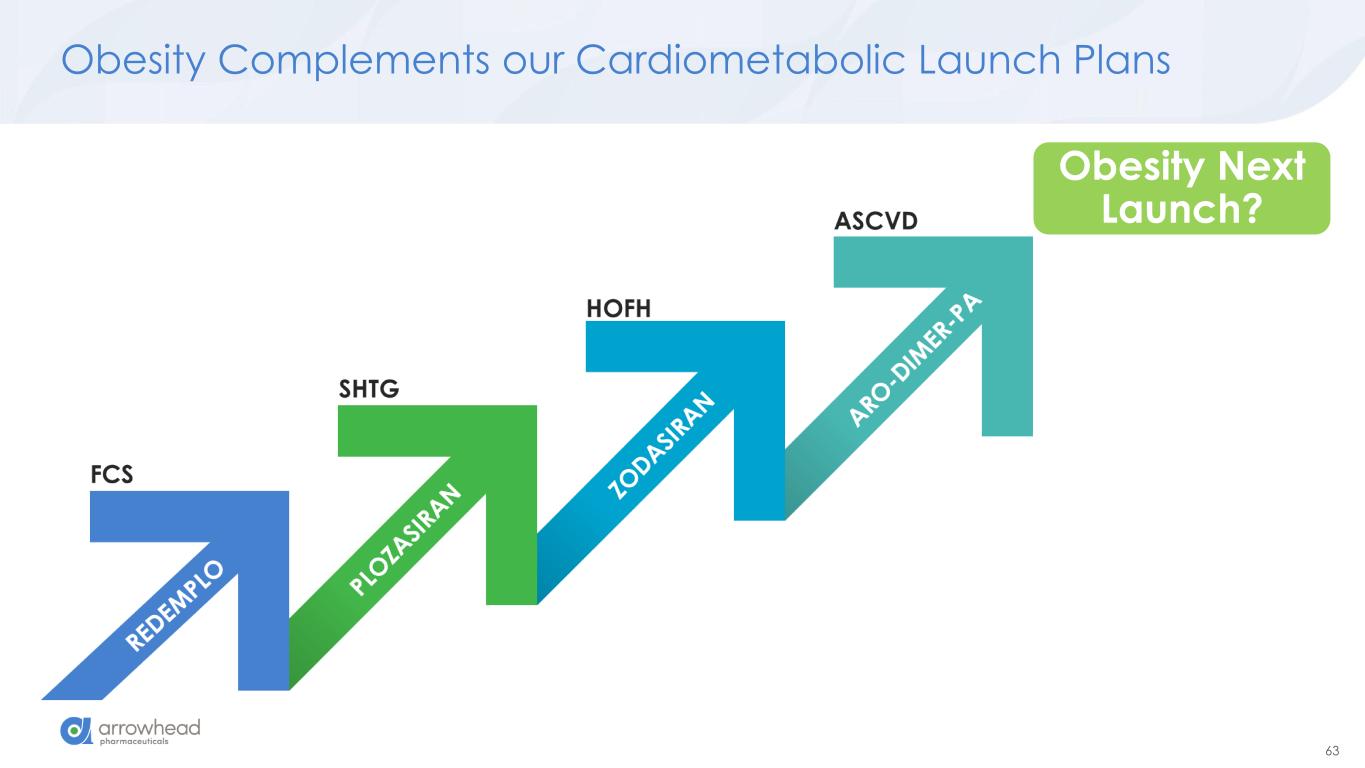
63 Obesity Complements our Cardiometabolic Launch Plans Obesity Next Launch?
64 Arrowhead’s Growth Drivers in 2026 and Beyond Arrowhead’s first commercial sales of REDEMPLO in familial chylomicronemia ARO-DIMER-PA targeting PCSK9 and APOC3 first clinical readout in 2H 2026 Phase 3 studies of plozasiran in severe hypertriglyceridemia (potential multi-billion-dollar opportunity) on pace to readout in Q3 2026 Funded into fiscal 2028, potentially through multiple independent and partner launches Emerging CNS pipeline with systemic delivery via SC administration: early ARO-MAPT data presented in 2026 Obesity franchise will grow with new targets, including dimers. Additional ARO-INHBE and ARO- ALK7 data presented in 2026
65 Questions? Answers.