
Corporate Presentation January 2026

2 Forward-Looking Statements Except for the historical information contained herein, this presentation contains forward-looking statements made pursuant to the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that such statements, include, without limitation, those regarding: (i) Geron’s beliefs, plans and expectations regarding the growth prospects for RYTELO and future financial and operating results, including expected 2026 net revenue and forecasted 2026 operating expenses; (ii) Geron’s beliefs, plans, assumptions and expectations regarding its goal of building Geron into a sustainable hematology powerhouse and the path that may achieve such goal; (iii) Geron’s beliefs regarding its ability to drive strong commercial execution and target appropriate second-line U.S. patients; (iv) Geron’s beliefs regarding the potential to bring RYTELO ex-US to LR-MDS patients; (v) Geron’s beliefs, assumptions and expectations regarding its ability to expand into relapsed/refractory myelofibrosis (R/R MF) based on the results of the Phase 3 IMpactMF trial and that such expansion will double RYTELO’s market opportunity; (vi) Geron’s expectations regarding its ability to manage its operating expenses within the forecasted range in 2026; (vii) Geron’s beliefs regarding the potential to leverage its balance sheet to pursue innovation; (viii) GeronGeron’s belief that RYTELO is a highly differentiated treatment for eligible lower-risk MDS patients; (ix) Geron’s plans, views and expectation regarding RYTELO’s ability to meet core unmet needs in LR-MDS based on Geron’s beliefs regarding RYTELO’s efficacy, safety and the potential benefit of the inclusion of RYTELO in the NCCN Guidelines as a Category 1 and 2A treatment of symptomatic anemia in certain patients with lower-risk MDS; (x) the suggestion that treatment-emergent cytopenias reflect on-target activity and are potentially associated with transfusion independence and hemoglobin improvement; (xi) Geron’s beliefs, plans and expectations regarding specific opportunities to expand awareness, confidence and appropriate use of RYTELO through its commercial, medical affairs and patient advocacy strategies, and the anticipated success of those efforts; (xii) Geron’s beliefs regarding RYTELO’s unique mechanism of action and the suggestion that it induces apoptosis in malignant, disease-causing cells; (xiii) the status, plans and expected timing of results from Geron’s clinical programs, including as set forth on its pipeline chart; (xiv) Geron’s beliefs regarding the progress and status of the Phase 3 IMpactMF trial and expectations regarding the interim analysis occurring in the second half of 2026 and the final analysis occurring in the second half of 2028, together with the assumptions used in making these estimates; (xv) Geron’s beliefs, assumptions, and expectations regarding the potential addressable market opportunity for RYTELO in R/R MF, including estimates regarding the potential addressable patient population; (xvi) Geron’s prior guidance for total operating expenses in fiscal year 2025; (xvii) Geron’s beliefs and expectations regarding the strength of Geron’s intellectual property position and the extent to which is supports RYTELO’s commercial opportunity, including the expected length of regulatory, market and patent exclusivity for RYTELO; (xviii) any projections of revenue, patient populations, commercial opportunity and similar forecasts, along with the underlying assumptions; and (xix) other statements that are not historical facts, including statements of past performance, efforts, trends, or results of Geron’s clinical trials, commercialization efforts or performance indicators, about which inferences or assumptions may be made, also constitute forward-looking statements and are not indicative of future performance or results. These forward-looking statements involve risks and uncertainties that can cause actual results to differ materially from those in such forward-looking statements. These risks and uncertainties, include, without limitation, risks and uncertainties related to: (a) whether Geron is successful in commercializing RYTELO for the treatment of certain patients with lower-risk MDS with transfusion dependent anemia and achieves increased market acceptance across the breadth of the eligible patient segments in RYTELO’s approved indication, including in appropriate second-line U.S. patients; (b) whether the FDA and European Commission will approve imetelstat for other indications on the timelines expected, or at all; (c) Geron’s plans to pursue paths for RYTELO outside the U.S. for LR-MDS patients and risks related to operating outside of the U.S.; (d) Geron’s future opportunities and plans, including the uncertainty of future revenues, expenses and other financial performance and results, and the related risk Geron may be unable to meet its 2026 financial guidance; (e) whether regulatory authorities permit the further development of imetelstat on a timely basis, or at all, without any clinical holds; (f) whether RYTELO (imetelstat) may cause, or have attributed to it, adverse events that could delay or prevent the commencement and/or completion of clinical trials, impact its regulatory approval, or limit its commercial potential; (g) whether the Phase 3 IMpactMF trial in R/R MF has a positive outcome and demonstrates safety and effectiveness to the satisfaction of the FDA and international regulatory authorities, and whether Geron’s projected rates for death events differ from actual rates, which may cause the interim and final analyses to occur later than anticipated; (h) if the Phase 3 IMpactMF trial is positive, whether the FDA and international regulatory authorities approve RYTELO in the R/R MF indication with the labeling claims necessary or desirable for the successful commercialization of RYTELO in that indication; (i) whether any future safety or efficacy results of RYTELO treatment cause its benefit-risk profile to become unacceptable; (j) whether imetelstat actually demonstrates disease-modifying activity in patients and the ability to target the malignant stem and progenitor cells of the underlying disease; (k) whether Geron meets its post-marketing requirements and commitments for RYTELO; (l) whether there are failures or delays in manufacturing or supplying sufficient quantities of RYTELO (imetelstat) or other clinical trial materials that impact commercialization of RYTELO or the continuation of the IMpactMF trial and other trials; (m) whether Geron is able to establish and maintain effective sales, marketing and distribution capabilities, obtain adequate coverage and third-party payor reimbursement, and achieve adequate acceptance in the marketplace; (n) whether Geron is able to obtain and maintain the exclusivity terms and scopes provided by patent and patent term extensions, regulatory exclusivity, and have freedom to operate; (o) that Geron may be unable to successfully commercialize RYTELO due to competitive products, or otherwise; (p) that Geron’s past performance, clinical data and results, or commercial performance indicators and demand trends, may not be replicated in or predictive of future results, performance or trends; (q) whether Geron stays in compliance with and satisfies its obligations under its debt and synthetic royalty financing agreements; (r) whether Geron successfully completes its restructuring plan, manages the changes in its workforce, and realizes expected operating expense saving; and (s) the impact of general economic, industry or political climate in the U.S. or internationally and the effects of macroeconomic conditions on Geron’s business and business prospects, financial condition and results of operations. Additional information on the above risks and uncertainties and additional risks, uncertainties and factors that could cause actual results to differ materially from those in the forward-looking statements are contained in Geron’s filings and periodic reports filed with the Securities and Exchange Commission under the heading “Risk Factors” and elsewhere in such filings and reports, including Geron’s quarterly report on Form 10-Q for the period ended September 30, 2025, and subsequent filings and reports by Geron. Undue reliance should not be placed on forward-looking statements, which speak only as of the date they are made, and the facts and assumptions underlying the forward-looking statements may change. Except as required by law, Geron disclaims any obligation to update these forward-looking statements to reflect future information, events, or circumstances.
Our Path to Building a Sustainable Hematology Powerhouse o Plan to pursue ex-U.S. RYTELO paths for LR-MDS patients o Expand into relapsed/refractory myelofibrosis if Phase 3 IMpactMF trial is successful and RYTELO is approved in this indication o Potential to leverage balance sheet to pursue innovation Maximize the Value of RYTELO® (imetelstat) - Wholly-Owned, First-in-Class Telomerase Inhibitor Financial Discipline and Opportunistic Innovation o $220M-$240M in expected 2026 RYTELO net product revenue, expected to be driven by strong commercial execution and targeting of appropriate second-line U.S. patients* o Guiding to total operating expenses of $230M-$240M in 2026 * RYTELO® (imetelstat) is approved in the U.S. and the EU for the treatment of certain adult patients with lower-risk myelodysplastic syndromes (LR-MDS) with transfusion-dependent anemia. See U.S. Prescribing Information and Medication Guide: https://pi.geron.com/products/US/pi/rytelo_pi.pdf; see Summary of Product Characteristics for RYTELO in the EU: https://pi.geron.com/products/rytelo/eu/rytelo_smpc_eu.pdf
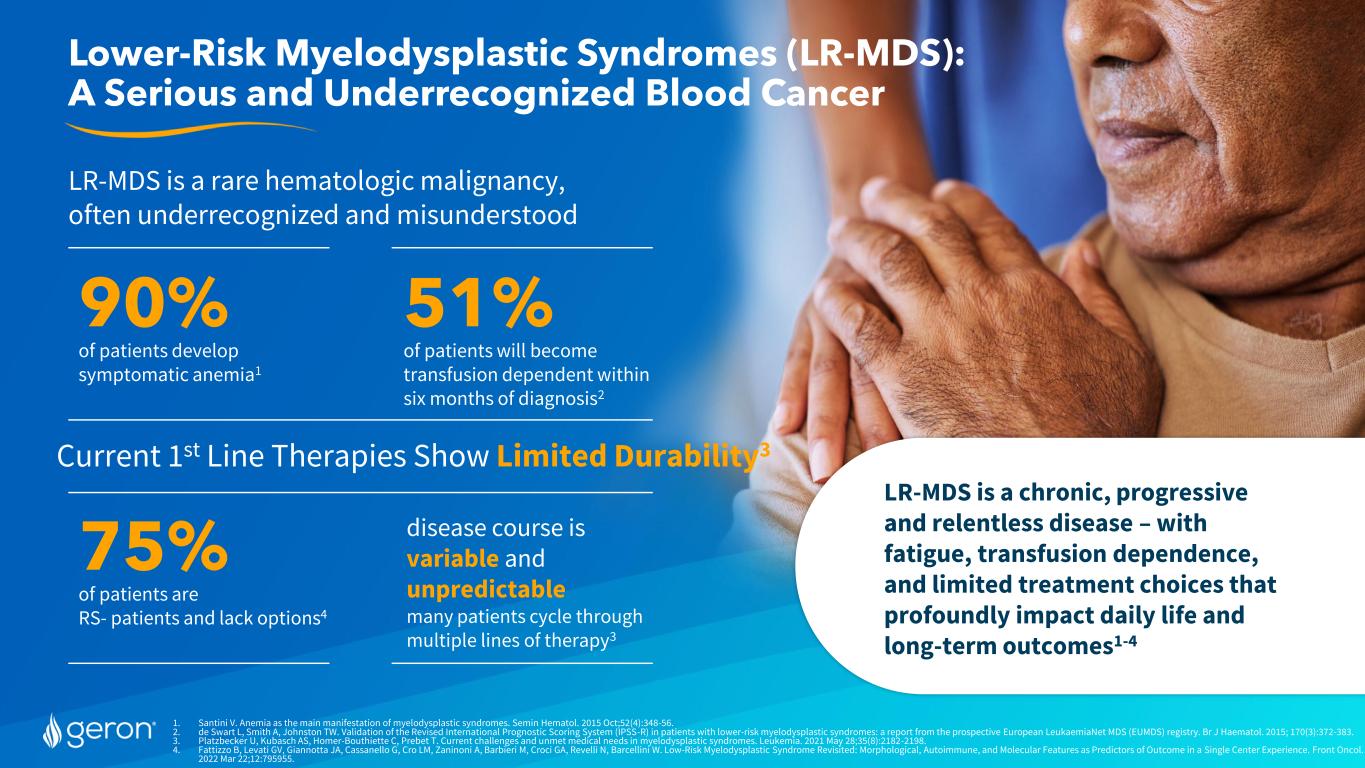
1. Santini V. Anemia as the main manifestation of myelodysplastic syndromes. Semin Hematol. 2015 Oct;52(4):348-56. 2. de Swart L, Smith A, Johnston TW. Validation of the Revised International Prognostic Scoring System (IPSS-R) in patients with lower-risk myelodysplastic syndromes: a report from the prospective European LeukaemiaNet MDS (EUMDS) registry. Br J Haematol. 2015; 170(3):372-383. 3. Platzbecker U, Kubasch AS, Homer-Bouthiette C, Prebet T. Current challenges and unmet medical needs in myelodysplastic syndromes. Leukemia. 2021 May 28;35(8):2182-2198. 4. Fattizzo B, Levati GV, Giannotta JA, Cassanello G, Cro LM, Zaninoni A, Barbieri M, Croci GA, Revelli N, Barcellini W. Low-Risk Myelodysplastic Syndrome Revisited: Morphological, Autoimmune, and Molecular Features as Predictors of Outcome in a Single Center Experience. Front Oncol. 2022 Mar 22;12:795955. Lower-Risk Myelodysplastic Syndromes (LR-MDS): A Serious and Underrecognized Blood Cancer LR-MDS is a chronic, progressive and relentless disease – with fatigue, transfusion dependence, and limited treatment choices that profoundly impact daily life and long-term outcomes1-4 LR-MDS is a rare hematologic malignancy, often underrecognized and misunderstood 90% of patients develop symptomatic anemia1 51% of patients will become transfusion dependent within six months of diagnosis2 Current 1st Line Therapies Show Limited Durability3 75% of patients are RS- patients and lack options4 disease course is variable and unpredictable many patients cycle through multiple lines of therapy3

Inspired by the real experiences of people living with blood cancer, Geron is advancing science that aims to meaningfully improve patients’ lives. Living with lower-risk myelodysplastic syndrome is a trip you wish you didn't have to take. Some days I can’t even do normal things –grocery shopping, tidying up, even walking to the door to hang a holiday wreath. I’m just so persistently and relentlessly tired. It feels like trying to walk through cement." – LINDA, LIVING WITH LR-MDS

* RYTELO® (imetelstat) is approved in the U.S. and the EU for the treatment of certain adult patients with lower-risk myelodysplastic syndromes (LR-MDS) with transfusion-dependent anemia. See U.S. Prescribing Information and Medication Guide: https://pi.geron.com/products/US/pi/rytelo_pi.pdf; see Summary of Product Characteristics for RYTELO in the EU: https://pi.geron.com/products/rytelo/eu/rytelo_smpc_eu.pdf RYTELO® (imetelstat) Approved in the U.S. and EU for Eligible Lower Risk Myelodysplastic Syndrome* A first-in-class telomerase inhibitor with a unique mechanism of action representing a highly differentiated treatment approved for eligible patients with lower-risk myelodysplastic syndromes (LR-MDS)*
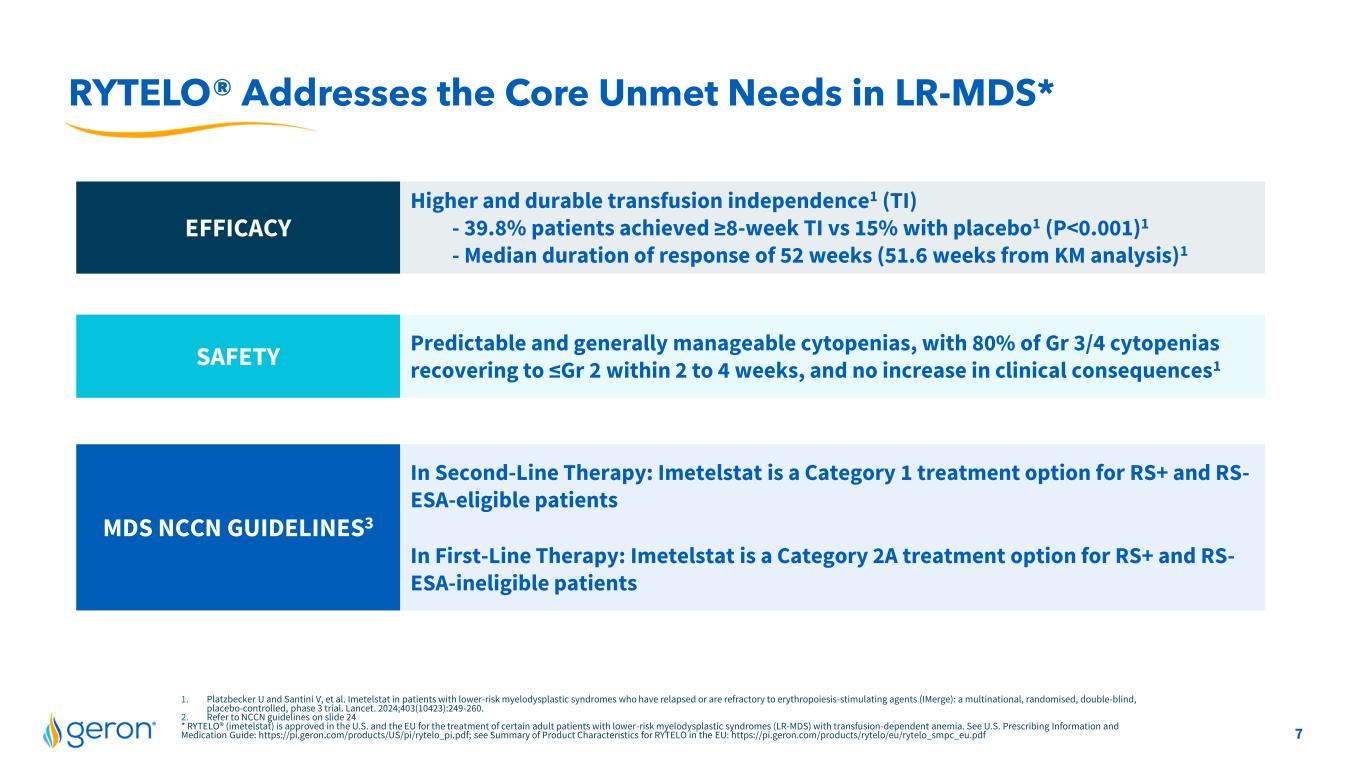
7 1. Platzbecker U and Santini V, et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): a multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024;403(10423):249-260. 2. Refer to NCCN guidelines on slide 24 * RYTELO® (imetelstat) is approved in the U.S. and the EU for the treatment of certain adult patients with lower-risk myelodysplastic syndromes (LR-MDS) with transfusion-dependent anemia. See U.S. Prescribing Information and Medication Guide: https://pi.geron.com/products/US/pi/rytelo_pi.pdf; see Summary of Product Characteristics for RYTELO in the EU: https://pi.geron.com/products/rytelo/eu/rytelo_smpc_eu.pdf RYTELO® Addresses the Core Unmet Needs in LR-MDS* EFFICACY Higher and durable transfusion independence1 (TI) - 39.8% patients achieved ≥8-week TI vs 15% with placebo1 (P<0.001)1 - Median duration of response of 52 weeks (51.6 weeks from KM analysis)1 SAFETY Predictable and generally manageable cytopenias, with 80% of Gr 3/4 cytopenias recovering to ≤Gr 2 within 2 to 4 weeks, and no increase in clinical consequences1 MDS NCCN GUIDELINES3 In Second-Line Therapy: Imetelstat is a Category 1 treatment option for RS+ and RS- ESA-eligible patients In First-Line Therapy: Imetelstat is a Category 2A treatment option for RS+ and RS- ESA-ineligible patients
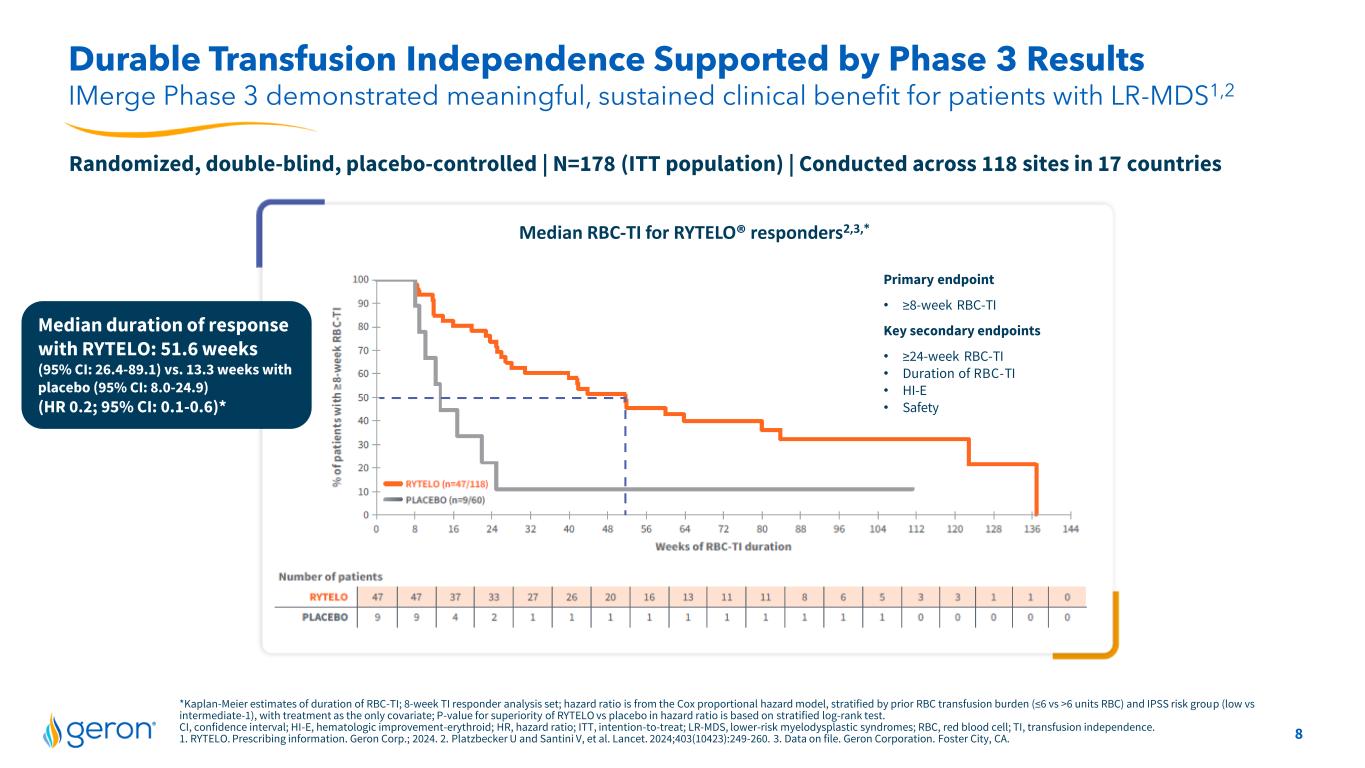
8 Durable Transfusion Independence Supported by Phase 3 Results IMerge Phase 3 demonstrated meaningful, sustained clinical benefit for patients with LR-MDS1,2 Median duration of response with RYTELO: 51.6 weeks (95% CI: 26.4-89.1) vs. 13.3 weeks with placebo (95% CI: 8.0-24.9) (HR 0.2; 95% CI: 0.1-0.6)* Primary endpoint • ≥8-week RBC-TI Key secondary endpoints • ≥24-week RBC-TI • Duration of RBC-TI • HI-E • Safety Randomized, double-blind, placebo-controlled | N=178 (ITT population) | Conducted across 118 sites in 17 countries *Kaplan-Meier estimates of duration of RBC-TI; 8-week TI responder analysis set; hazard ratio is from the Cox proportional hazard model, stratified by prior RBC transfusion burden (≤6 vs >6 units RBC) and IPSS risk group (low vs intermediate-1), with treatment as the only covariate; P-value for superiority of RYTELO vs placebo in hazard ratio is based on stratified log-rank test. CI, confidence interval; HI-E, hematologic improvement-erythroid; HR, hazard ratio; ITT, intention-to-treat; LR-MDS, lower-risk myelodysplastic syndromes; RBC, red blood cell; TI, transfusion independence. 1. RYTELO. Prescribing information. Geron Corp.; 2024. 2. Platzbecker U and Santini V, et al. Lancet. 2024;403(10423):249-260. 3. Data on file. Geron Corporation. Foster City, CA. Median RBC-TI for RYTELO® responders2,3,*
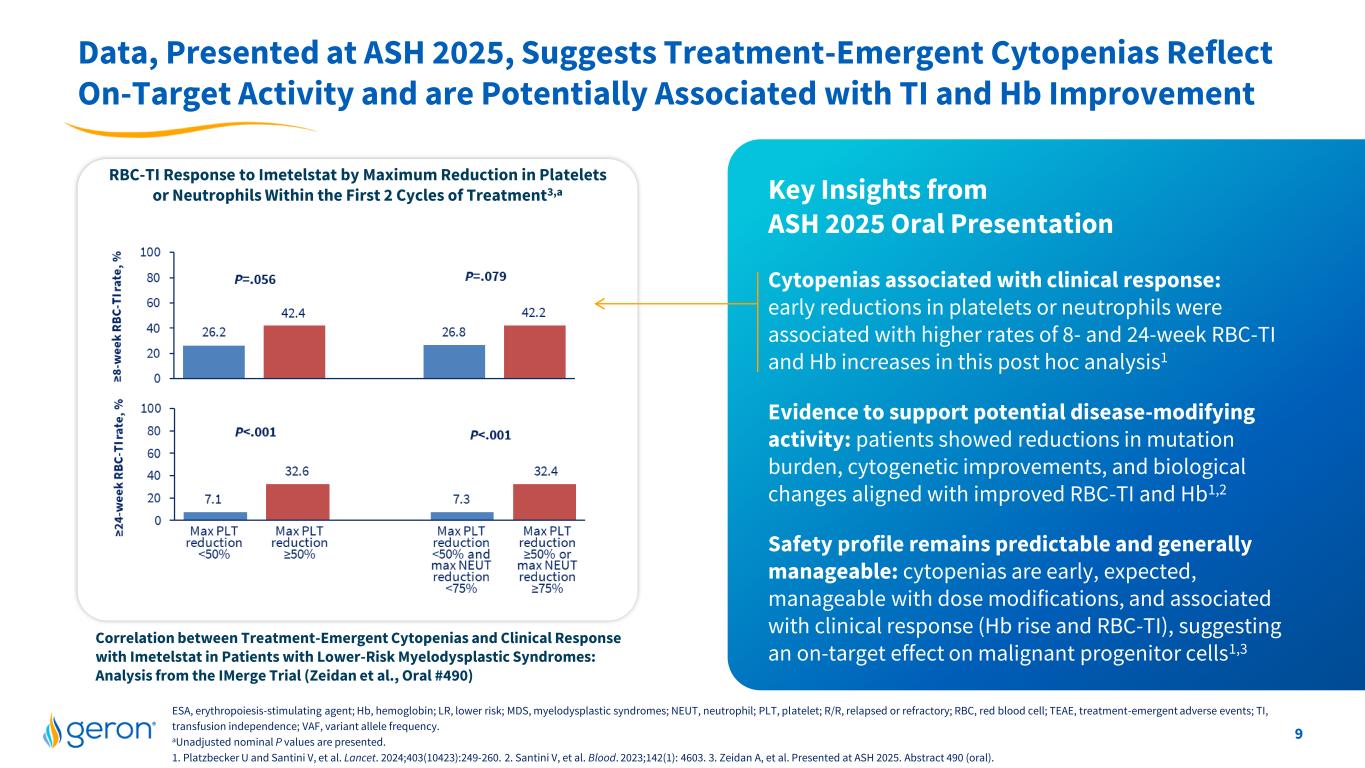
9 Data, Presented at ASH 2025, Suggests Treatment-Emergent Cytopenias Reflect On-Target Activity and are Potentially Associated with TI and Hb Improvement RBC-TI Response to Imetelstat by Maximum Reduction in Platelets or Neutrophils Within the First 2 Cycles of Treatment3,a Cytopenias associated with clinical response: early reductions in platelets or neutrophils were associated with higher rates of 8- and 24-week RBC-TI and Hb increases in this post hoc analysis1 Evidence to support potential disease-modifying activity: patients showed reductions in mutation burden, cytogenetic improvements, and biological changes aligned with improved RBC-TI and Hb1,2 Safety profile remains predictable and generally manageable: cytopenias are early, expected, manageable with dose modifications, and associated with clinical response (Hb rise and RBC-TI), suggesting an on-target effect on malignant progenitor cells1,3 ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; LR, lower risk; MDS, myelodysplastic syndromes; NEUT, neutrophil; PLT, platelet; R/R, relapsed or refractory; RBC, red blood cell; TEAE, treatment-emergent adverse events; TI, transfusion independence; VAF, variant allele frequency. aUnadjusted nominal P values are presented. 1. Platzbecker U and Santini V, et al. Lancet. 2024;403(10423):249-260. 2. Santini V, et al. Blood. 2023;142(1): 4603. 3. Zeidan A, et al. Presented at ASH 2025. Abstract 490 (oral). Key Insights from ASH 2025 Oral Presentation Correlation between Treatment-Emergent Cytopenias and Clinical Response with Imetelstat in Patients with Lower-Risk Myelodysplastic Syndromes: Analysis from the IMerge Trial (Zeidan et al., Oral #490)

Digital and Non- Personal Campaigns HCP Education Patient Community Engagement Disease Awareness Presence at Hematology Forums Third- Party Education Engaging the LR-MDS Community in Surround Sound Commercial Medical Affairs Patient Advocacy
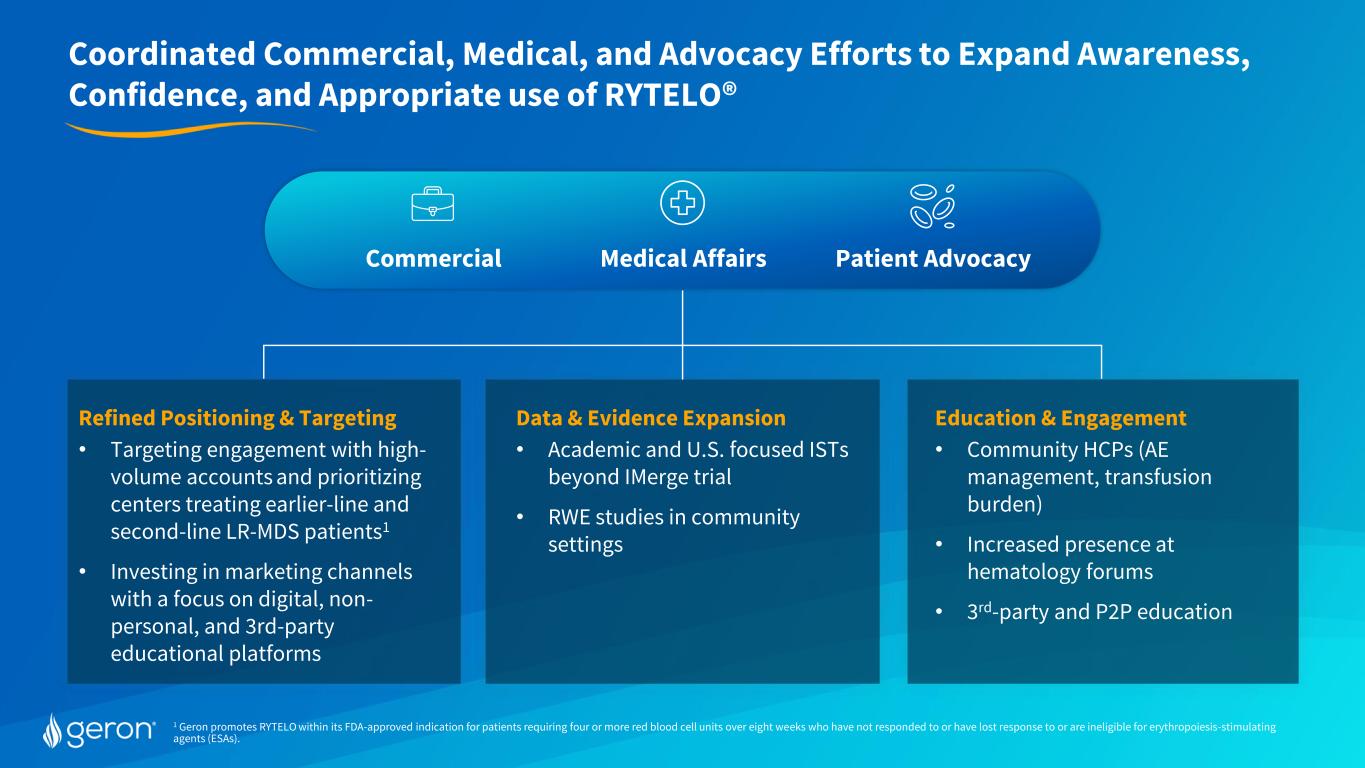
1 Geron promotes RYTELO within its FDA-approved indication for patients requiring four or more red blood cell units over eight weeks who have not responded to or have lost response to or are ineligible for erythropoiesis-stimulating agents (ESAs). Refined Positioning & Targeting • Targeting engagement with high- volume accounts and prioritizing centers treating earlier-line and second-line LR-MDS patients1 • Investing in marketing channels with a focus on digital, non- personal, and 3rd-party educational platforms Education & Engagement • Community HCPs (AE management, transfusion burden) • Increased presence at hematology forums • 3rd-party and P2P education Data & Evidence Expansion • Academic and U.S. focused ISTs beyond IMerge trial • RWE studies in community settings Commercial Medical Affairs Patient Advocacy Coordinated Commercial, Medical, and Advocacy Efforts to Expand Awareness, Confidence, and Appropriate use of RYTELO®

12 1. Platzbecker U and Santini V, et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): a multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024;403(10423):249-260. 2. In the primary analysis, mOS was not reached at a median study follow-up of 18 months. The study was not statistically powered to detect significant OS. 3. Zeidan A, et al. Correlation between treatment-emergent cytopenias and clinical response with imetelstat (IME) in patients (Pts) with lower-risk myelodysplastic syndromes (LR-MDS): Analysis from the IMerge Trial. Blood 2025; 146 (Supplement 1): 490. A Field Ready Scientific Story RYTELO® Key Messages Unique MOA Efficacy Durability Safety Long-Term Follow-Up Cytopenia as a Predictor of Response Targets telomerase and is thought to induce apoptosis in malignant, disease-causing cells, unlike ESAs, EMAs and HMAs 39.8% patients achieved ≥8-week TI vs 15% with placebo (P<0.001)1 Median duration of response of 52 weeks (51.6 weeks from KM analysis)1 Almost 1 year of ZERO transfusions observed for nearly half of RYTELO responders, with a median rise in HG of +3.6 g/dL1 In a post hoc analysis, 45 months IMerge follow up suggests no increase in risk of death with HR of 0.82, and progression to AML similar to placebo arm2 Proactive education and simplified management algorithm provided to HCPS for management of expected and generally short duration cytopenias potentially associated with efficacy3 Predictable and generally manageable cytopenias, with 80% of cytopenias recovering within 2 to 4 weeks with no increase in clinical consequences1
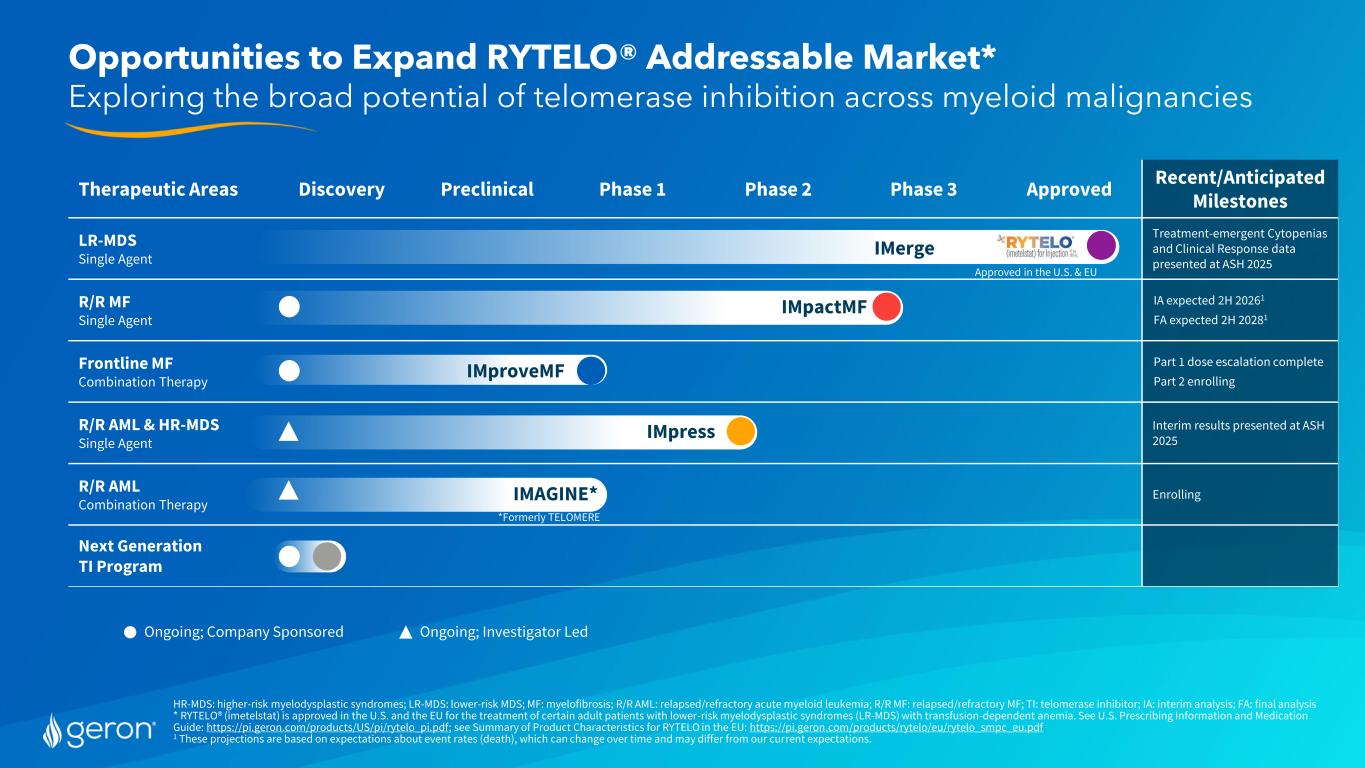
HR-MDS: higher-risk myelodysplastic syndromes; LR-MDS: lower-risk MDS; MF: myelofibrosis; R/R AML: relapsed/refractory acute myeloid leukemia; R/R MF: relapsed/refractory MF; TI: telomerase inhibitor; IA: interim analysis; FA: final analysis * RYTELO® (imetelstat) is approved in the U.S. and the EU for the treatment of certain adult patients with lower-risk myelodysplastic syndromes (LR-MDS) with transfusion-dependent anemia. See U.S. Prescribing Information and Medication Guide: https://pi.geron.com/products/US/pi/rytelo_pi.pdf; see Summary of Product Characteristics for RYTELO in the EU: https://pi.geron.com/products/rytelo/eu/rytelo_smpc_eu.pdf 1 These projections are based on expectations about event rates (death), which can change over time and may differ from our current expectations. Opportunities to Expand RYTELO® Addressable Market* Exploring the broad potential of telomerase inhibition across myeloid malignancies Therapeutic Areas Discovery Preclinical Phase 1 Phase 2 Phase 3 Approved Recent/Anticipated Milestones LR-MDS Single Agent Treatment-emergent Cytopenias and Clinical Response data presented at ASH 2025 R/R MF Single Agent IA expected 2H 20261 FA expected 2H 20281 Frontline MF Combination Therapy Part 1 dose escalation complete Part 2 enrolling R/R AML & HR-MDS Single Agent Interim results presented at ASH 2025 R/R AML Combination Therapy Enrolling Next Generation TI Program IMpactMF IMproveMF IMpress IMAGINE* Ongoing; Investigator LedOngoing; Company Sponsored Approved in the U.S. & EU *Formerly TELOMERE IMerge

JAKi: Janus kinase inhibitor; MF: myelofibrosis; R/R MF: relapsed/refractory. Source: US IQVIA Claims through 2023 (MF Market Sizing Report delivered March 2024); DRG Epi (2022 Report) Growth Rates applied through 2040 *Patient estimates as of 2028. 1 Subject to positive results in the Phase 3 IMpactMF trial and FDA approval of RYTELO label expansion to treat addressable U.S. JAKi R/R MF patients. Potential to Double RYTELO Addressable Market with JAK Inhibitor Relapsed Refractory Myelofibrosis Opportunity1 Potential to treat U.S. JAKi R/R MF patients*~10,000 ~12,000 U.S. JAKi naïve / well-controlled MF patients (currently only 3 approved treatments – all JAK inhibitors) 75% of those JAKi treated patients fail or discontinue treatment
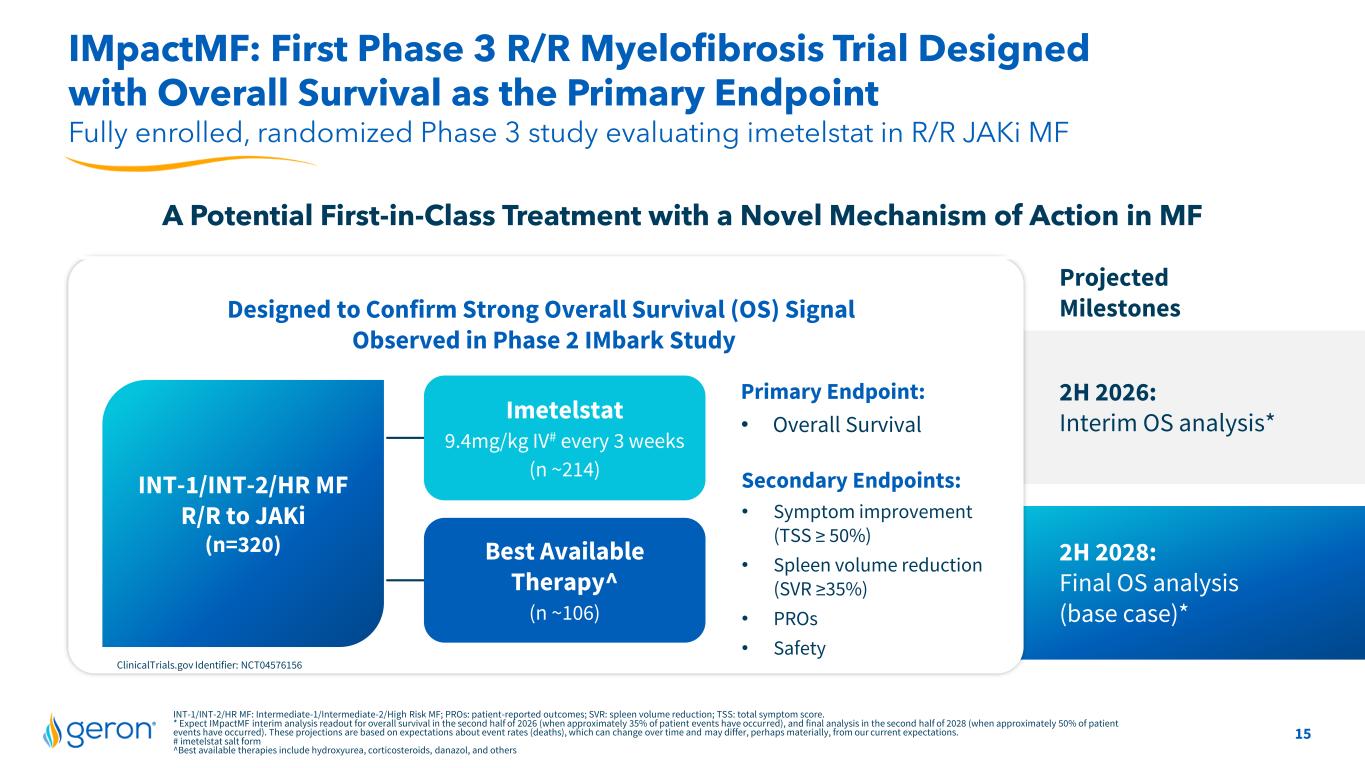
15 INT-1/INT-2/HR MF: Intermediate-1/Intermediate-2/High Risk MF; PROs: patient-reported outcomes; SVR: spleen volume reduction; TSS: total symptom score. * Expect IMpactMF interim analysis readout for overall survival in the second half of 2026 (when approximately 35% of patient events have occurred), and final analysis in the second half of 2028 (when approximately 50% of patient events have occurred). These projections are based on expectations about event rates (deaths), which can change over time and may differ, perhaps materially, from our current expectations. # imetelstat salt form ^Best available therapies include hydroxyurea, corticosteroids, danazol, and others Fully enrolled, randomized Phase 3 study evaluating imetelstat in R/R JAKi MF IMpactMF: First Phase 3 R/R Myelofibrosis Trial Designed with Overall Survival as the Primary Endpoint Primary Endpoint: • Overall Survival Imetelstat 9.4mg/kg IV# every 3 weeks (n ~214) Best Available Therapy^ (n ~106) INT-1/INT-2/HR MF R/R to JAKi (n=320) Designed to Confirm Strong Overall Survival (OS) Signal Observed in Phase 2 IMbark Study ClinicalTrials.gov Identifier: NCT04576156 Projected Milestones 2H 2026: Interim OS analysis* A Potential First-in-Class Treatment with a Novel Mechanism of Action in MF Secondary Endpoints: • Symptom improvement (TSS ≥ 50%) • Spleen volume reduction (SVR ≥35%) • PROs • Safety 2H 2028: Final OS analysis (base case)*
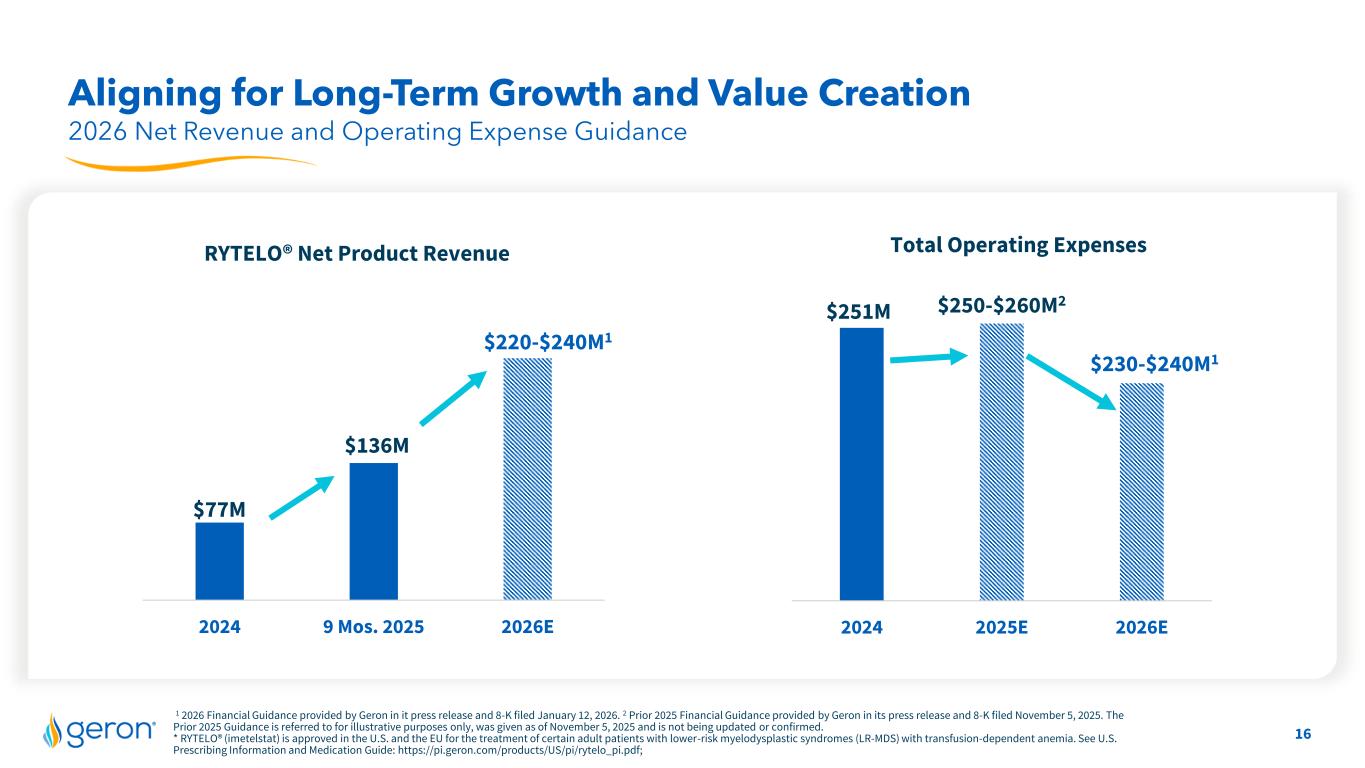
16 1 2026 Financial Guidance provided by Geron in it press release and 8-K filed January 12, 2026. 2 Prior 2025 Financial Guidance provided by Geron in its press release and 8-K filed November 5, 2025. The Prior 2025 Guidance is referred to for illustrative purposes only, was given as of November 5, 2025 and is not being updated or confirmed. * RYTELO® (imetelstat) is approved in the U.S. and the EU for the treatment of certain adult patients with lower-risk myelodysplastic syndromes (LR-MDS) with transfusion-dependent anemia. See U.S. Prescribing Information and Medication Guide: https://pi.geron.com/products/US/pi/rytelo_pi.pdf; Aligning for Long-Term Growth and Value Creation 2024 9 Mos. 2025 2026E RYTELO® Net Product Revenue $77M 2024 2025E 2026E Total Operating Expenses $251M $136M $220-$240M1 2026 Net Revenue and Operating Expense Guidance $250-$260M2 $230-$240M1
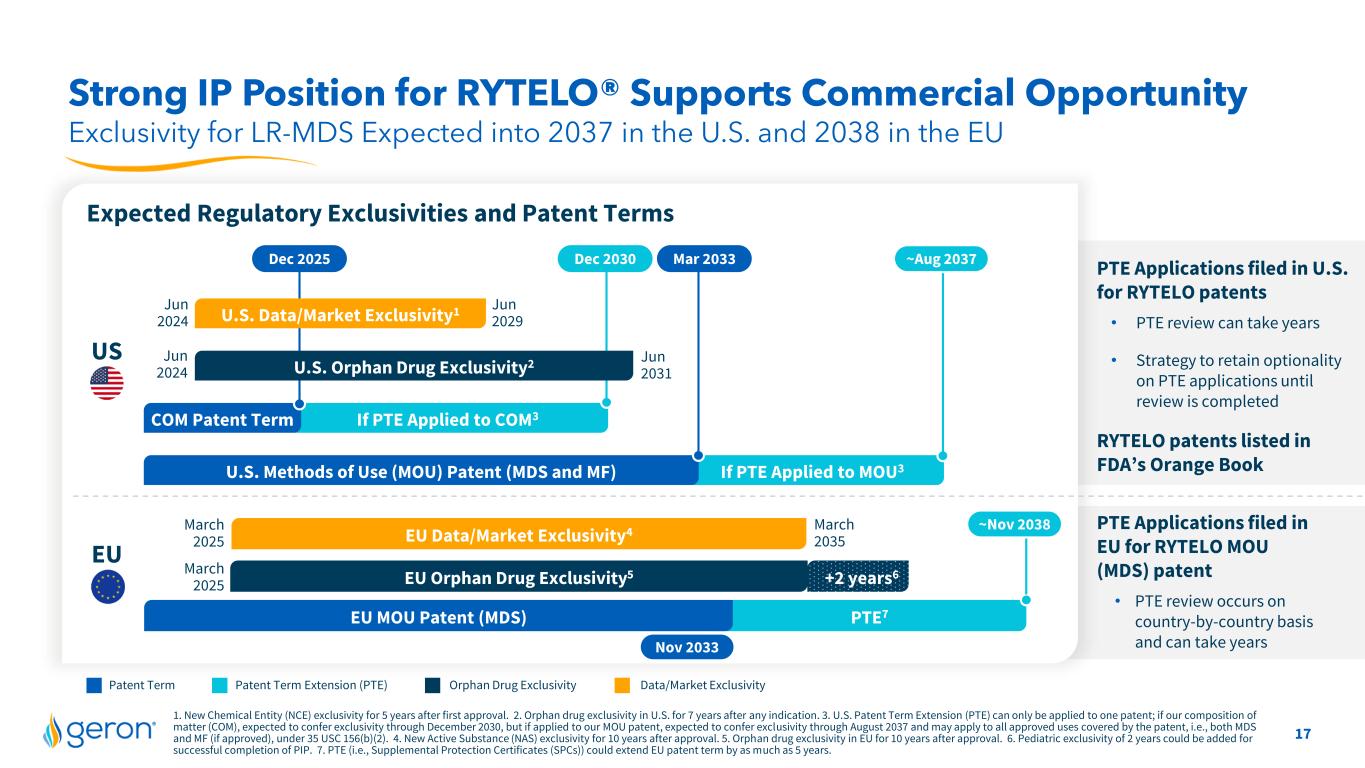
17 If PTE Applied to COM3COM Patent Term +2 years6 1. New Chemical Entity (NCE) exclusivity for 5 years after first approval. 2. Orphan drug exclusivity in U.S. for 7 years after any indication. 3. U.S. Patent Term Extension (PTE) can only be applied to one patent; if our composition of matter (COM), expected to confer exclusivity through December 2030, but if applied to our MOU patent, expected to confer exclusivity through August 2037 and may apply to all approved uses covered by the patent, i.e., both MDS and MF (if approved), under 35 USC 156(b)(2). 4. New Active Substance (NAS) exclusivity for 10 years after approval. 5. Orphan drug exclusivity in EU for 10 years after approval. 6. Pediatric exclusivity of 2 years could be added for successful completion of PIP. 7. PTE (i.e., Supplemental Protection Certificates (SPCs)) could extend EU patent term by as much as 5 years. Exclusivity for LR-MDS Expected into 2037 in the U.S. and 2038 in the EU Strong IP Position for RYTELO® Supports Commercial Opportunity Expected Regulatory Exclusivities and Patent Terms If PTE Applied to MOU3 Mar 2033Dec 2025 Dec 2030 Jun 2024 Jun 2031 Jun 2024 Jun 2029U.S. Data/Market Exclusivity1 Patent Term Patent Term Extension (PTE) Orphan Drug Exclusivity Data/Market Exclusivity EU Data/Market Exclusivity4 EU Orphan Drug Exclusivity5 March 2025 March 2025 March 2035 PTE7 US PTE Applications filed in U.S. for RYTELO patents • PTE review can take years • Strategy to retain optionality on PTE applications until review is completed RYTELO patents listed in FDA’s Orange Book PTE Applications filed in EU for RYTELO MOU (MDS) patent • PTE review occurs on country-by-country basis and can take years U.S. Methods of Use (MOU) Patent (MDS and MF) EU EU MOU Patent (MDS) ~Aug 2037 ~Nov 2038 U.S. Orphan Drug Exclusivity2 Nov 2033
Our Path to Building a Sustainable Hematology Powerhouse o Plan to pursue ex-U.S. RYTELO paths for LR-MDS patients o Expand into relapsed/refractory myelofibrosis if Phase 3 IMpactMF trial is successful and RYTELO is approved in this indication o Potential to leverage balance sheet to pursue innovation Maximize the Value of RYTELO® (imetelstat) - Wholly-Owned, First-in-Class Telomerase Inhibitor Financial Discipline and Opportunistic Innovation o $220M-$240M in expected 2026 RYTELO net product revenue, expected to be driven by strong commercial execution and targeting of appropriate second-line U.S. patients* o Guiding to total operating expenses of $230M-$240M in 2026 * RYTELO® (imetelstat) is approved in the U.S. and the EU for the treatment of certain adult patients with lower-risk myelodysplastic syndromes (LR-MDS) with transfusion-dependent anemia. See U.S. Prescribing Information and Medication Guide: https://pi.geron.com/products/US/pi/rytelo_pi.pdf; see Summary of Product Characteristics for RYTELO in the EU: https://pi.geron.com/products/rytelo/eu/rytelo_smpc_eu.pdf

19 Appendix
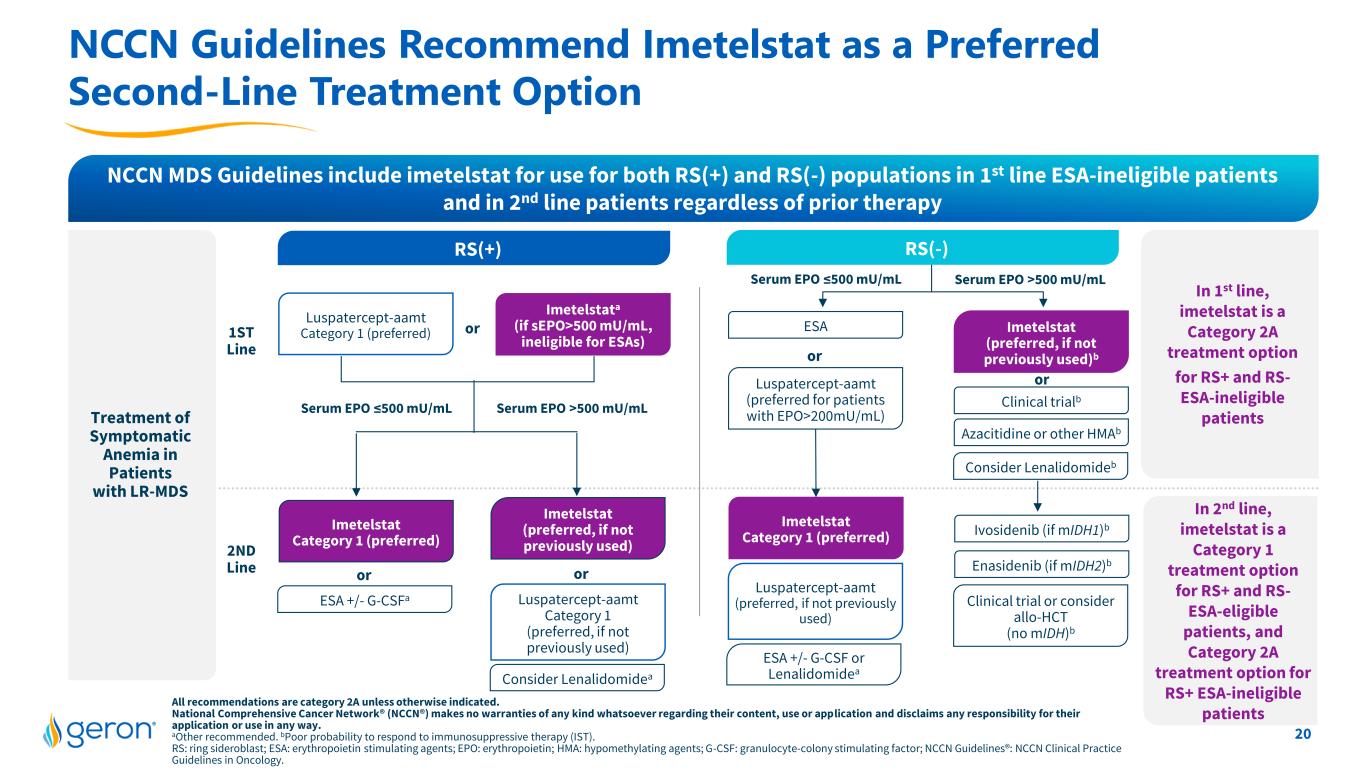
20 All recommendations are category 2A unless otherwise indicated. National Comprehensive Cancer Network® (NCCN®) makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. aOther recommended. bPoor probability to respond to immunosuppressive therapy (IST). RS: ring sideroblast; ESA: erythropoietin stimulating agents; EPO: erythropoietin; HMA: hypomethylating agents; G-CSF: granulocyte-colony stimulating factor; NCCN Guidelines®: NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines Recommend Imetelstat as a Preferred Second-Line Treatment Option NCCN MDS Guidelines include imetelstat for use for both RS(+) and RS(-) populations in 1st line ESA-ineligible patients and in 2nd line patients regardless of prior therapy Treatment of Symptomatic Anemia in Patients with LR-MDS In 1st line, imetelstat is a Category 2A treatment option for RS+ and RS- ESA-ineligible patients 1ST Line 2ND Line In 2nd line, imetelstat is a Category 1 treatment option for RS+ and RS- ESA-eligible patients, and Category 2A treatment option for RS+ ESA-ineligible patients RS(-) ESA Imetelstat Category 1 (preferred) ESA +/- G-CSF or Lenalidomidea or Luspatercept-aamt (preferred, if not previously used) Ivosidenib (if mIDH1)b Clinical trial or consider allo-HCT (no mIDH)b Enasidenib (if mIDH2)b Azacitidine or other HMAb Imetelstat (preferred, if not previously used)b Consider Lenalidomideb Clinical trialb orLuspatercept-aamt (preferred for patients with EPO>200mU/mL) Serum EPO >500 mU/mLSerum EPO ≤500 mU/mL RS(+) Imetelstata (if sEPO>500 mU/mL, ineligible for ESAs) Luspatercept-aamt Category 1 (preferred) Luspatercept-aamt Category 1 (preferred, if not previously used) Consider Lenalidomidea Imetelstat (preferred, if not previously used) or Imetelstat Category 1 (preferred) ESA +/- G-CSFa or or Serum EPO >500 mU/mLSerum EPO ≤500 mU/mL