

Unique Oncolytic Virus Therapies for Multiple Solid Tumors January 2026

2 FORWARD LOOKING STATEMENTS This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . In some cases forward - looking statements can be identified by terminology such as “may,” “should,” “potential,” “continue,” “expects,” “anticipates ,” “intends,” “plans,” “believes,” “estimates,” and similar expressions, and include statements regarding oncolytic viruses (OVs) being promising cancer therapeutics; the multiple potential value opportunities for VCN - 01; the regulatory status expected to facilitate VCN - 01 development; potential access to a priority review voucher; the therapeutic potential of VCN - 01 and other Theriva OVs; the ability of VCN - 01 and other Theriva OVs to overcome key OV challenges ; the potential of VCN - 01 to enable immuno - oncology therapies in refractory tumors; the clinical advancement of VCN - 01 and other Theriva OVs in div erse cancer indications (including pancreatic ductal adenocarcinoma, head and neck cancer, ovarian cancer, colorectal cancer, and retinob las toma) and the projected milestones . Important factors that could cause actual results to differ materially from current expectations include, among others, the Co mpany’s ability to enroll patients as planned and reach clinical trial milestones when anticipated ; the Company’s ability to complete clinical trials on time and achieve the desired results and benefits ; the Company’s product candidates demonstrating safety and effectiveness, including positive clinical data that demonstrates VCN - 01 may lead to improved clinical outcomes for patients; the Company’s ability to obtain regulatory approval for commercializ ation of product candidates or to comply with ongoing regulatory requirements; regulatory limitations relating to the Company’s ability to pro mot e or commercialize their product candidates for the specific indications; acceptance of product candidates in the marketplace and the successful devel opm ent, marketing or sale of the Company’s products; developments by competitors that render such products obsolete or non - competitive; the Company’s ability to maintain license agreements; the continued maintenance and growth of the Company’s patent estate; the ability to continue to remain well finan ced ; and other factors described in the Company’s Annual Report on Form 10 - K for the year ended December 31, 2024 and its other filings with the SEC, i ncluding subsequent periodic reports on Forms 10 - Q and current reports on Form 8 - K. The information in this release is provided only as of the date of this release, and Theriva Biologics undertakes no obligation to update any forward - looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
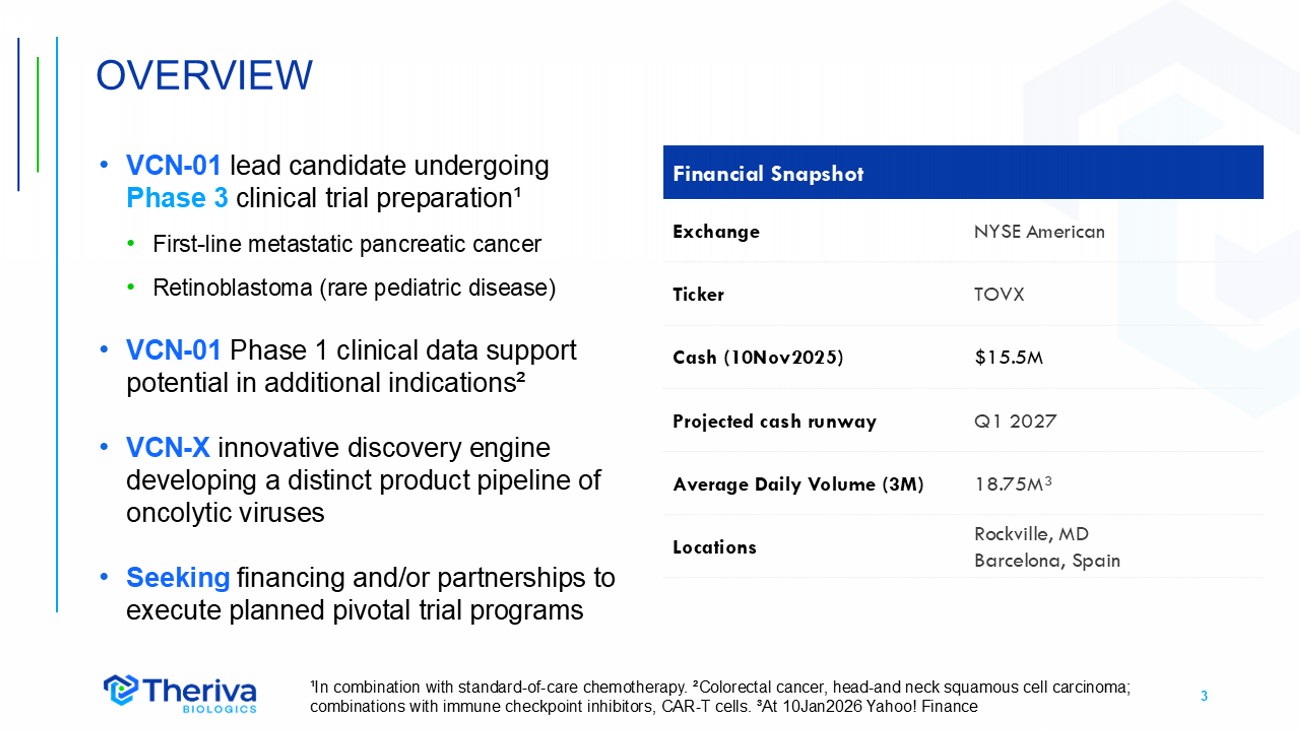
3 • VCN - 01 lead candidate undergoing Phase 3 clinical trial preparation¹ • First - line metastatic pancreatic cancer • Retinoblastoma (rare pediatric disease) • VCN - 01 Phase 1 clinical data support potential in additional indications² • VCN - X innovative discovery engine developing a distinct product pipeline of oncolytic viruses • Seeking financing and/or partnerships to execute planned pivotal trial programs OVERVIEW Financial Snapshot NYSE American Exchange TOVX Ticker $15.5M Cash (10Nov2025) Q1 2027 Projected cash runway 18.75M³ Average Daily Volume (3M) Rockville, MD Barcelona, Spain Locations ¹In combination with standard - of - care chemotherapy. ²Colorectal cancer, head - and neck squamous cell carcinoma; combinations with immune checkpoint inhibitors, CAR - T cells. ³At 10Jan2026 Yahoo! Finance
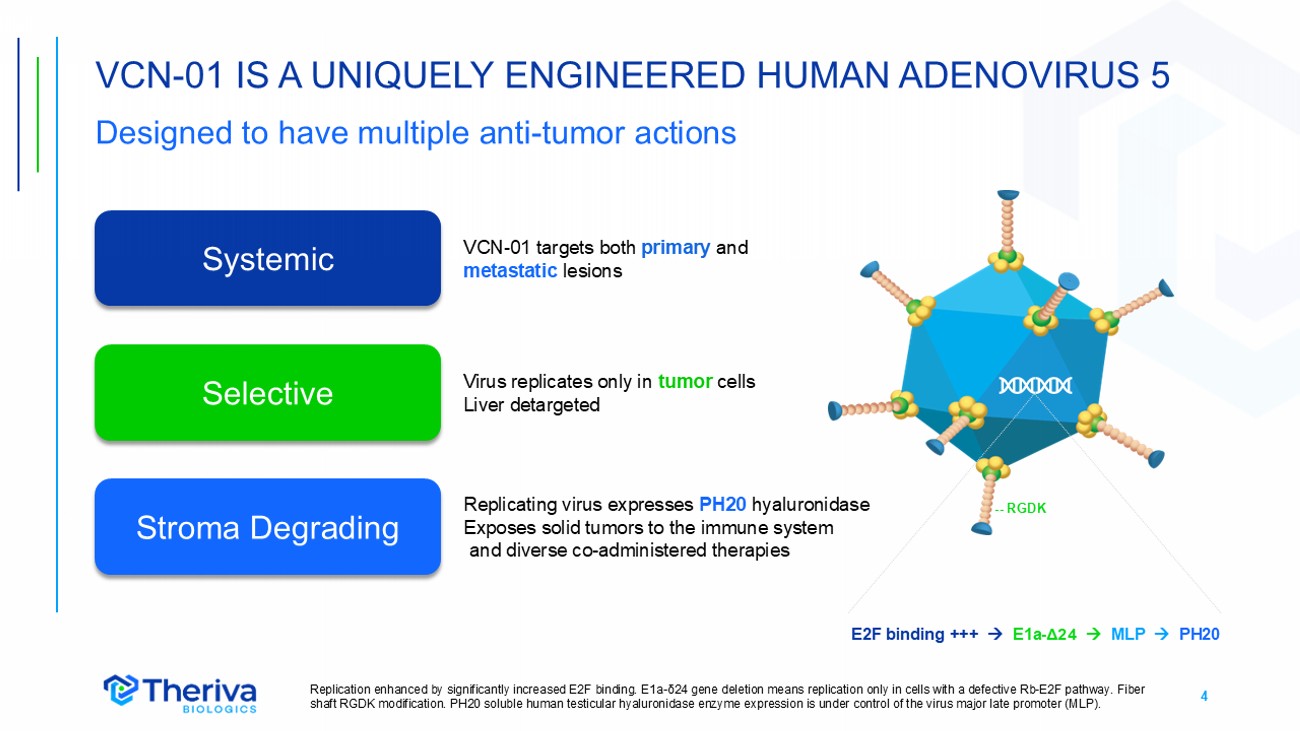
4 VCN - 01 IS A UNIQUELY ENGINEERED HUMAN ADENOVIRUS 5 Designed to have multiple anti - tumor actions Replication enhanced by significantly increased E2F binding. E1a - δ24 gene deletion means replication only in cells with a defective Rb - E2F pathway. Fiber shaft RGDK modification. PH20 soluble human testicular hyaluronidase enzyme expression is under control of the virus major la te promoter (MLP). Systemic Selective Stroma Degrading E2F binding +++ E1a - Δ24 MLP PH20 -- RGDK VCN - 01 targets both primary and metastatic lesions Replicating virus expresses PH20 hyaluronidase Exposes solid tumors to the immune system and diverse co - administered therapies Virus replicates only in tumor cells Liver detargeted
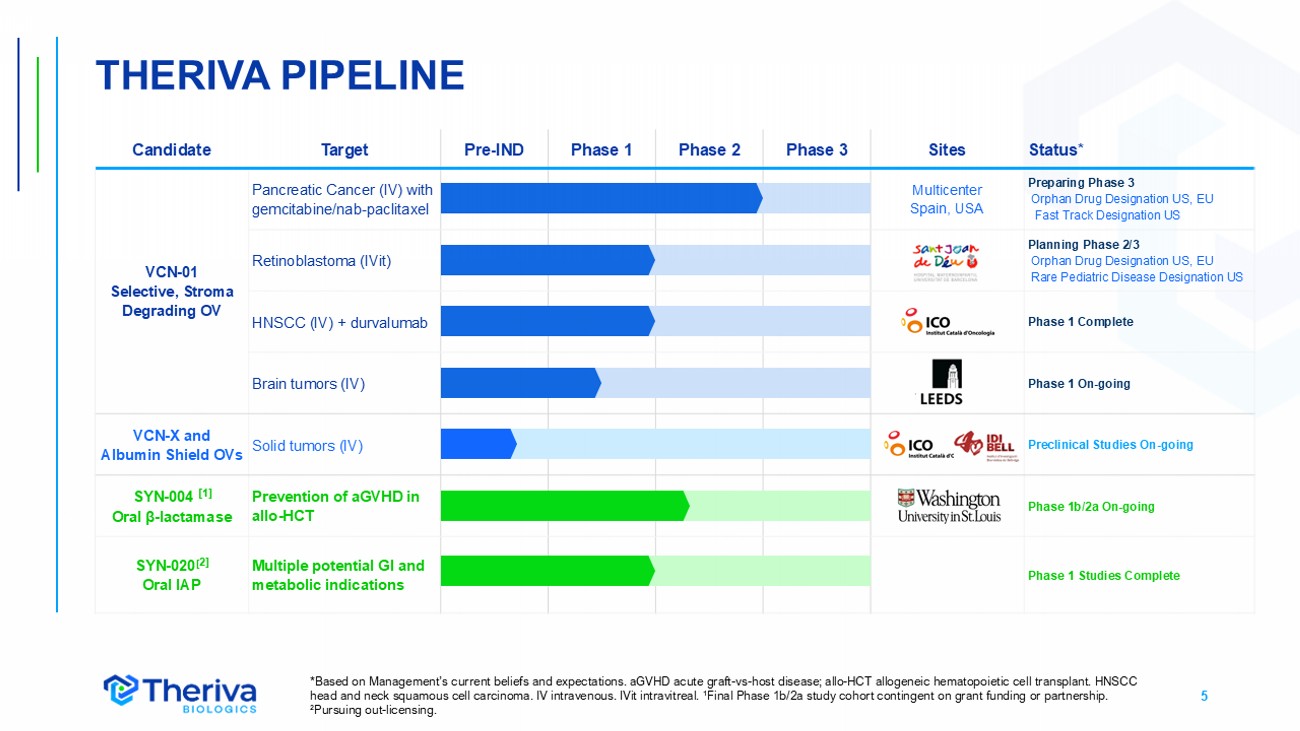
5 THERIVA PIPELINE Status* Sites Phase 3 Phase 2 Phase 1 Pre - IND Target Candidate Preparing Phase 3 Orphan Drug Designation US, EU Fast Track Designation US Multicenter Spain, USA Pancreatic Cancer (IV) with gemcitabine/nab - paclitaxel VCN - 01 Selective, Stroma Degrading OV Planning Phase 2/3 Orphan Drug Designation US, EU Rare Pediatric Disease Designation US Retinoblastoma (IVit) Phase 1 Complete HNSCC (IV) + durvalumab Phase 1 On - going Brain tumors (IV) Preclinical Studies On - going Solid tumors (IV) VCN - X and Albumin Shield OVs Phase 1b/2a On - going Prevention of aGVHD in allo - HCT SYN - 004 [1] Oral β - lactamase Phase 1 Studies Complete Multiple potential GI and metabolic indications SYN - 020 [ 2] Oral IAP *Based on Management’s current beliefs and expectations. aGVHD acute graft - vs - host disease; allo - HCT allogeneic hematopoietic ce ll transplant. HNSCC head and neck squamous cell carcinoma. IV intravenous. IVit intravitreal. ¹Final Phase 1b/2a study cohort contingen t on grant funding or partnership. ² Pursuing out - licensing.
6 VCN - 01 LEAD INDICATION PANCREATIC CANCER Highly fatal cancer protected by dense tumor stroma • Orphan disease, highest mortality of all solid tum ors • Median survival 8 - 11 months for metastatic disease 1,2 • USA est. 6 7,440 new cases and 51,980 deaths in 2025 3 • Hyaluronic acid in stroma is associated with reduced treatment efficacy and poor prognosis 4 • VCN - 01 designed to degrade hyaluronic acid • Incidence is growing worldwide • Est. treatment market ~$2.9B (2024) ~$6.0B (2030) 5 ¹ Michael (2019) BMC Palliat Care 18:13, Bengtsson (2020) Sci Rep 10:16425, Carioli (2021) Ann Oncol 32:478, ASCO Pancreatic Cancer Statistics . ² SEER Cancer Stat Facts: Pancreatic Cancer website . ³ American Cancer Society. Cancer Facts & Figures 2025. Atlanta: American Cancer Society; 2025. ⁴ Tahkola (2021) Sci Rep 11:12216, Placencio - Hickok (2022) Pancreatology 22:92. ⁵Grand View Research website . Pancreatic adenocarcinoma resected from the pancreas body and tail
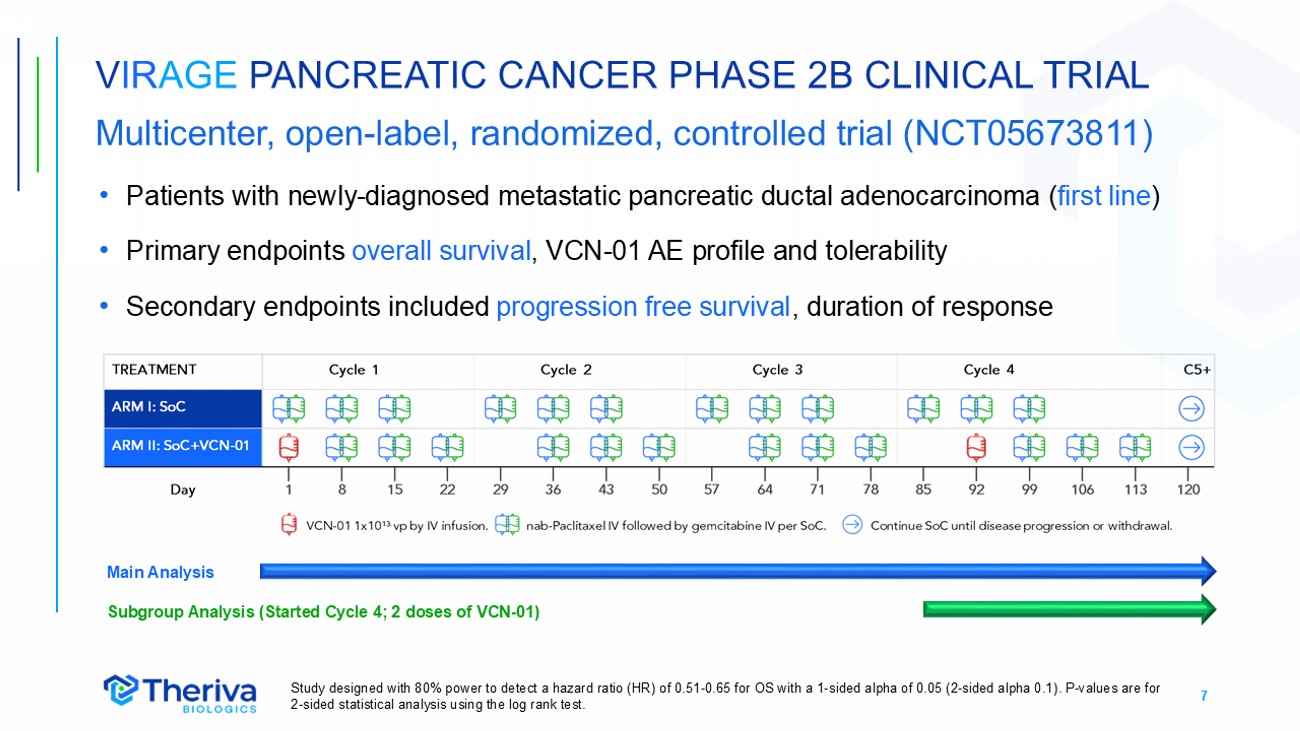
7 V IR AGE PANCREATIC CANCER PHASE 2B CLINICAL TRIAL Multicenter, open - label, randomized, controlled trial (NCT05673811) • Patients with newly - diagnosed metastatic pancreatic ductal adenocarcinoma ( first line ) • Primary endpoints overall survival , VCN - 01 AE profile and tolerability • Secondary endpoints included progression free survival , duration of response Study designed with 80% power to detect a hazard ratio (HR) of 0.51 - 0.65 for OS with a 1 - sided alpha of 0.05 (2 - sided alpha 0.1) . P - values are for 2 - sided statistical analysis using the log rank test . Main Analysis Subgroup Analysis (Started Cycle 4; 2 doses of VCN - 01)
8 V IR AGE PHASE 2B TRIAL KEY FINDINGS Data provide strong support for Phase 3 trial • Enrolled a “real world” population of older and more fragile patients • Increased overall and progression free survival (OS, PFS) and duration of response (DoR) observed in VCN - 01 plus gemcitabine/nab - paclitaxel SoC treatment group compared to SoC alone • Additional survival benefit observed in patients receiving two doses VCN - 01 • Greater improvements at later timepoints consistent with immune MOA • Acceptable AE profile consistent with prior VCN - 01 clinical trials • Better hazard ratios for OS, PFS, DoR vs gemcitabine/nab - paclitaxel than reported in NALIRIFOX Phase 3 trial ¹ ¹Wainberg (2023) Lancet 402:1272. NALIRIFOX leucovorin+ 5 - FU+liposomal irinotecan+oxaliplatin .
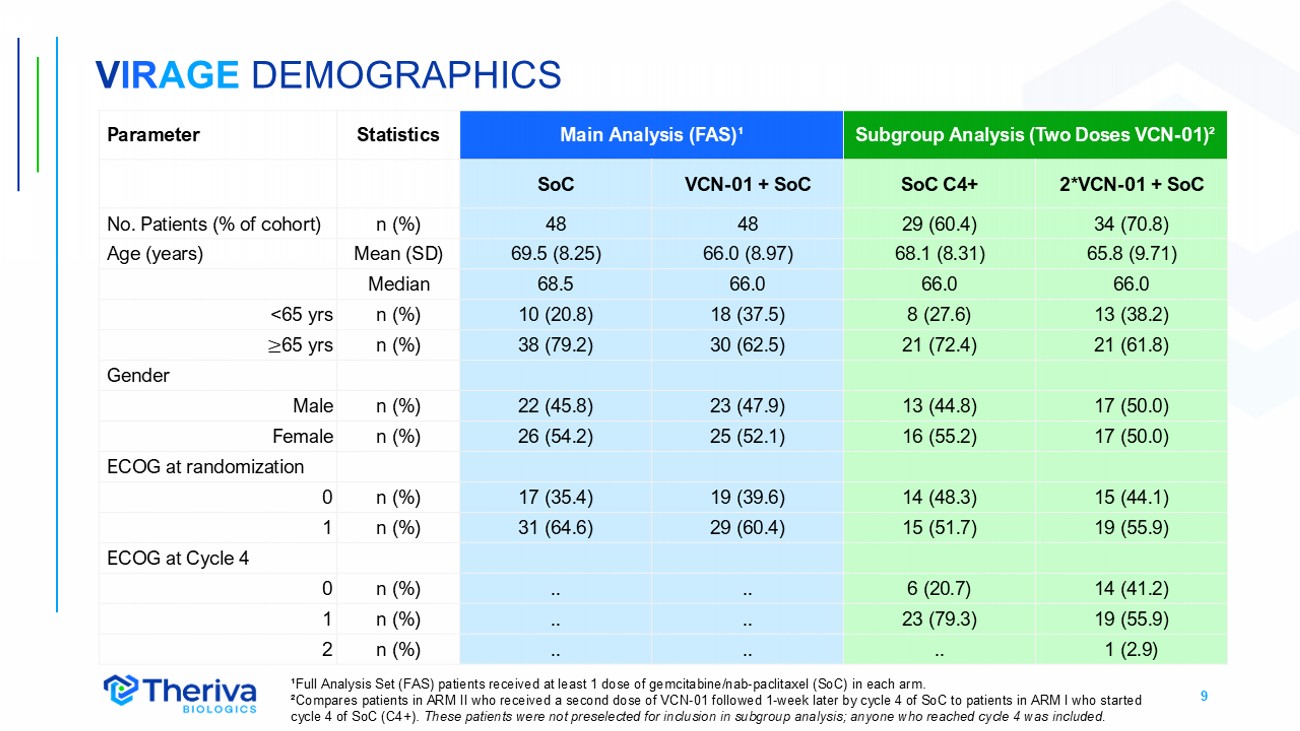
9 V IR AGE DEMOGRAPHICS ¹Full Analysis Set (FAS) patients received at least 1 dose of gemcitabine/nab - paclitaxel (SoC) in each arm. ²Compares patients in ARM II who received a second dose of VCN - 01 followed 1 - week later by cycle 4 of SoC to patients in ARM I w ho started cycle 4 of SoC (C4+). These patients were not preselected for inclusion in subgroup analysis; anyone who reached cycle 4 was included. Subgroup Analysis (Two Doses VCN - 01) ² Main Analysis (FAS) ¹ Statistics Parameter 2*VCN - 01 + SoC SoC C4+ VCN - 01 + SoC SoC 34 (70.8) 29 (60.4) 48 48 n (%) No. Patients (% of cohort) 65.8 (9.71) 68.1 (8.31) 66.0 (8.97) 69.5 (8.25) Mean (SD) Age (years) 66.0 66.0 66.0 68.5 Median 13 (38.2) 8 (27.6) 18 (37.5) 10 (20.8) n (%) <65 yrs 21 (61.8) 21 (72.4) 30 (62.5) 38 (79.2) n (%) ≥ 65 yrs Gender 17 (50.0) 13 (44.8) 23 (47.9) 22 (45.8) n (%) Male 17 (50.0) 16 (55.2) 25 (52.1) 26 (54.2) n (%) Female ECOG at randomization 15 (44.1) 14 (48.3) 19 (39.6) 17 (35.4) n (%) 0 19 (55.9) 15 (51.7) 29 (60.4) 31 (64.6) n (%) 1 ECOG at Cycle 4 14 (41.2) 6 (20.7) .. .. n (%) 0 19 (55.9) 23 (79.3) .. .. n (%) 1 1 (2.9) .. .. .. n (%) 2
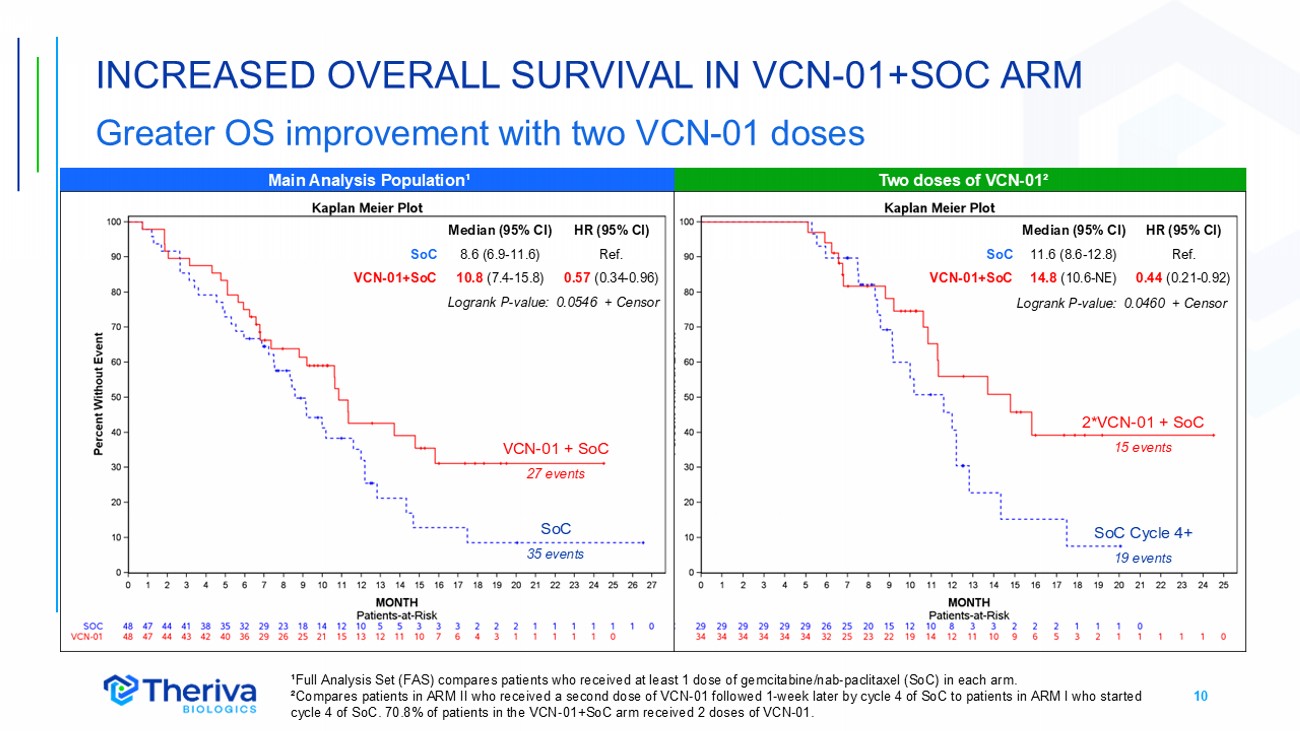
10 INCREASED OVERALL SURVIVAL IN VCN - 01+SOC ARM Greater OS improvement with two VCN - 01 doses ¹Full Analysis Set (FAS) compares patients who received at least 1 dose of gemcitabine/nab - paclitaxel (SoC) in each arm. ²Compares patients in ARM II who received a second dose of VCN - 01 followed 1 - week later by cycle 4 of SoC to patients in ARM I w ho started cycle 4 of SoC. 70.8% of patients in the VCN - 01+SoC arm received 2 doses of VCN - 01. SoC 3 5 events VCN - 01 + SoC 27 events 2*VCN - 01 + SoC 15 events SoC Cycle 4+ 19 events Two doses of VCN - 01² Main Analysis Population¹ HR (95% CI) Median (95% CI) Ref. 8.6 (6.9 - 11.6) SoC 0.57 (0.34 - 0.96) 10.8 (7.4 - 15.8) VCN - 01+SoC Logrank P - value: 0.0546 + Censor HR (95% CI) Median (95% CI) Ref. 11.6 (8.6 - 12.8) SoC 0.44 (0.21 - 0.92) 14.8 (10.6 - NE) VCN - 01+SoC Logrank P - value: 0.0460 + Censor
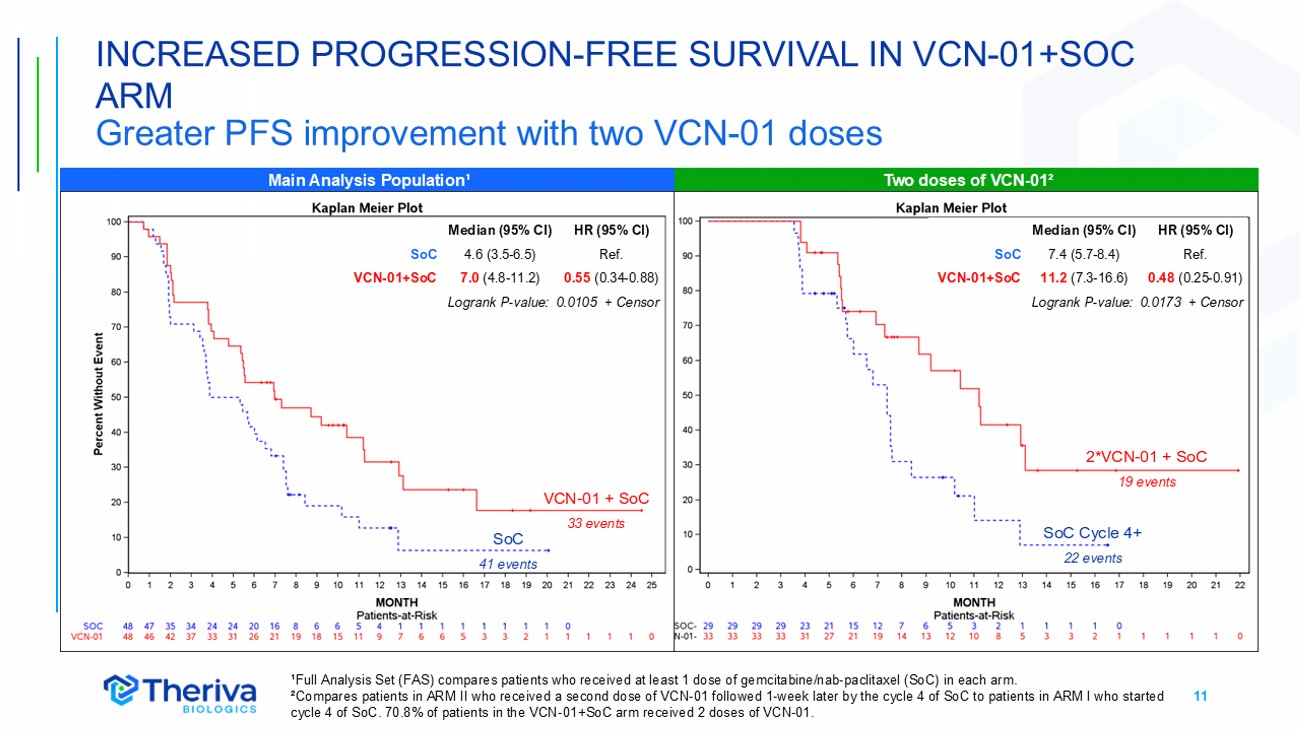
11 INCREASED PROGRESSION - FREE SURVIVAL IN VCN - 01+SOC ARM Greater PFS improvement with two VCN - 01 doses SoC VCN - 01 + SoC ¹Full Analysis Set (FAS) compares patients who received at least 1 dose of gemcitabine/nab - paclitaxel (SoC) in each arm. ²Compares patients in ARM II who received a second dose of VCN - 01 followed 1 - week later by the cycle 4 of SoC to patients in ARM I who started cycle 4 of SoC. 70.8% of patients in the VCN - 01+SoC arm received 2 doses of VCN - 01. Two doses of VCN - 01² Main Analysis Population¹ HR (95% CI) Median (95% CI) Ref. 4.6 (3.5 - 6.5) SoC 0.55 (0.34 - 0.88) 7.0 (4.8 - 11.2) VCN - 01+SoC Logrank P - value: 0.0105 + Censor SoC 41 events VCN - 01 + SoC 33 events 2*VCN - 01 + SoC 19 events SoC Cycle 4+ 22 events HR (95% CI) Median (95% CI) Ref. 7.4 (5.7 - 8.4) SoC 0.48 (0.25 - 0.91) 11.2 (7.3 - 16.6) VCN - 01+SoC Logrank P - value: 0.0173 + Censor
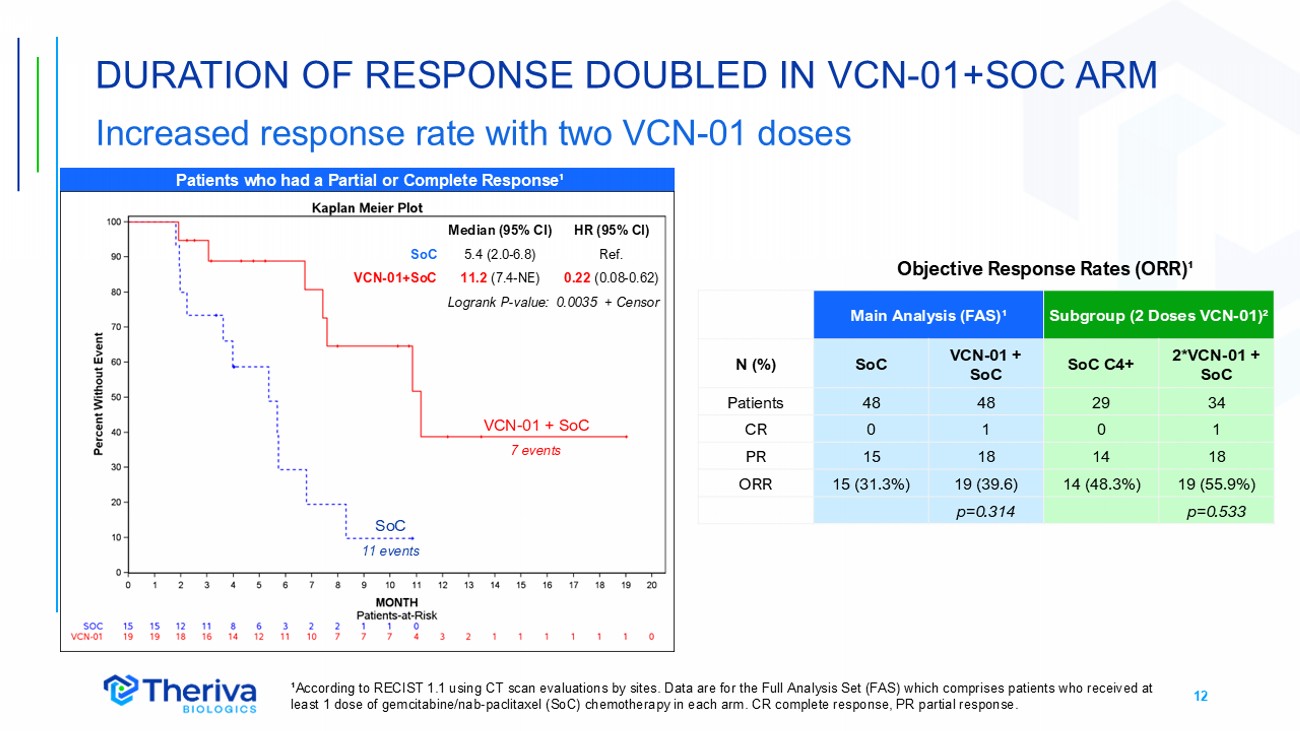
12 DURATION OF RESPONSE DOUBLED IN VCN - 01+SOC ARM Increased response rate with two VCN - 01 doses ¹According to RECIST 1.1 using CT scan evaluations by sites. Data are for the Full Analysis Set (FAS) which comprises patients who received at least 1 dose of gemcitabine/nab - paclitaxel (SoC) chemotherapy in each arm. CR complete response, PR partial response. Patients who had a Partial or Complete Response¹ HR (95% CI) Median (95% CI) Ref. 5.4 (2.0 - 6.8) SoC 0.22 (0.08 - 0.62) 11.2 (7.4 - NE) VCN - 01+SoC Logrank P - value: 0.0035 + Censor SoC 11 events VCN - 01 + SoC 7 events Subgroup (2 Doses VCN - 01) ² Main Analysis (FAS) ¹ 2*VCN - 01 + SoC SoC C4+ VCN - 01 + SoC SoC N (%) 34 29 48 48 Patients 1 0 1 0 CR 18 14 18 15 PR 19 (55.9%) 14 (48.3%) 19 (39.6) 15 (31.3%) ORR p=0.533 p=0.314 Objective Response Rates (ORR) ¹
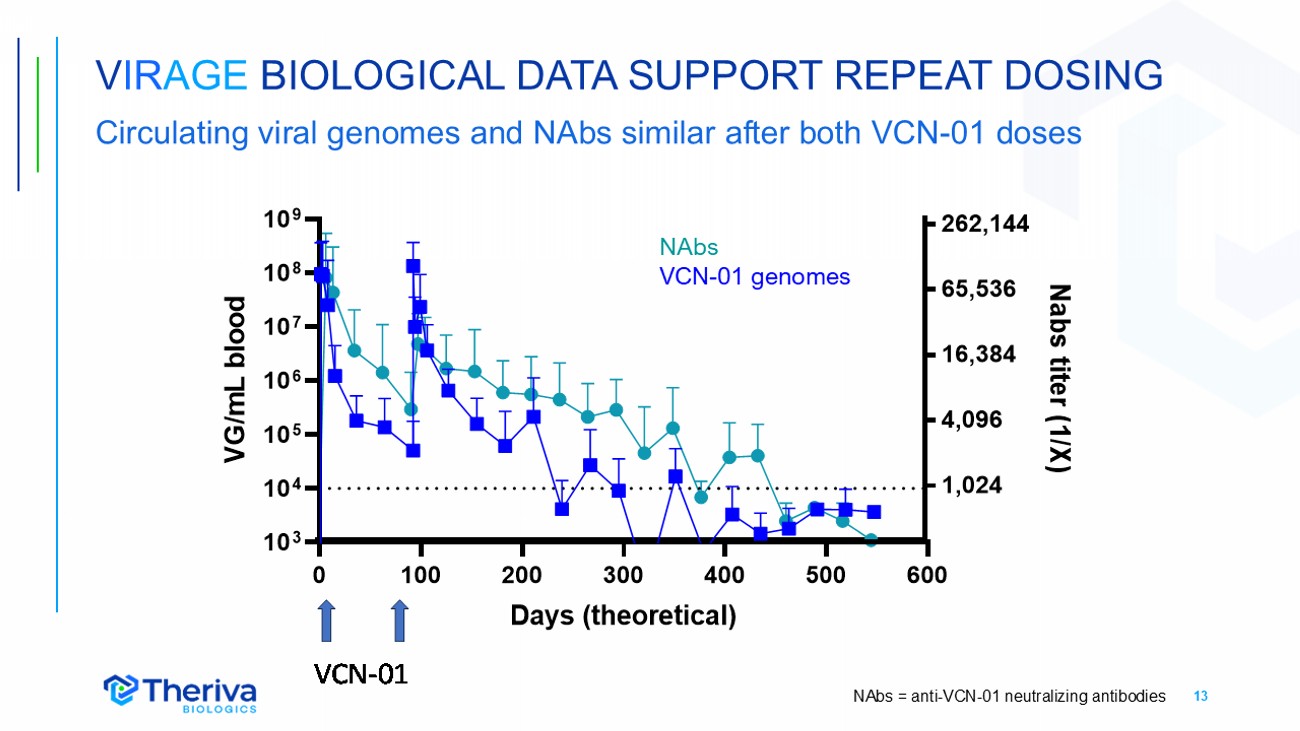
13 V IR AGE BIOLOGICAL DATA SUPPORT REPEAT DOSING Circulating viral genomes and NAbs similar after both VCN - 01 doses NAbs VCN - 01 genomes NAbs = anti - VCN - 01 neutralizing antibodies
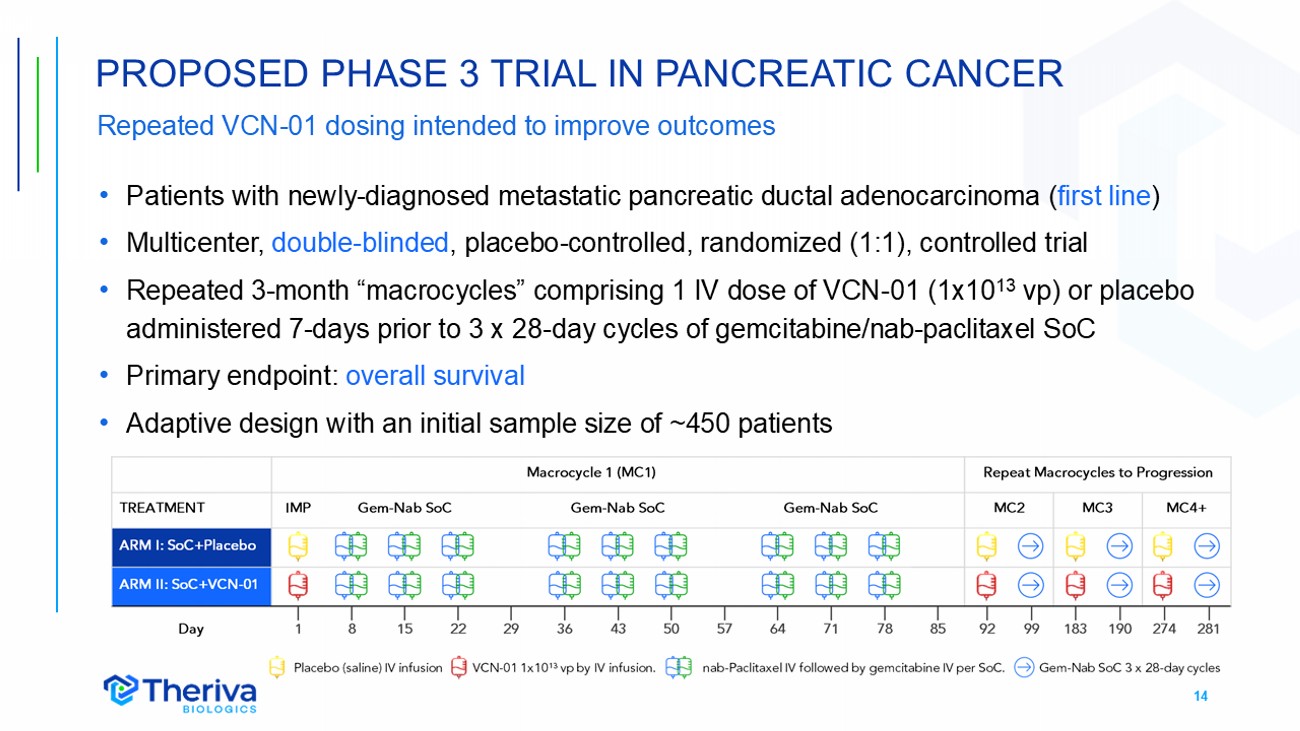
14 Repeated VCN - 01 dosing intended to improve outcomes • Patients with newly - diagnosed metastatic pancreatic ductal adenocarcinoma ( first line ) • Multicenter, double - blinded , placebo - controlled, randomized (1:1), controlled trial • Repeated 3 - month “macrocycles” comprising 1 IV dose of VCN - 01 (1x10 13 vp) or placebo administered 7 - days prior to 3 x 28 - day cycles of gemcitabine/nab - paclitaxel SoC • Primary endpoint: overall survival • Adaptive design with an initial sample size of ~450 patients PROPOSED PHASE 3 TRIAL IN PANCREATIC CANCER

15 • Positive Scientific Advice from European Medicines Agency (EMA) • Agreed on proposed inclusion/exclusion criteria, primary endpoint (OS), and secondary endpoints (including PFS, DoR, and patient reported outcomes) • Agreed on proposed sample size and adaptive trial design with two interim analyses • Agreed that a single study, if successful, could support a marketing authorization • Recognized the survival benefit of the second VCN - 01 dose in the VIRAGE trial; suggested potentially more frequent dosing • Requested additional VCN - 01 genome and anti - VCN - 01 Ab measurements for the additional doses • FDA End - of - Phase 2 Meeting Requested • Meeting to review proposed Phase 3 trial design anticipated Q1 2026 PURSUING REGULATORY AGREEMENT ON PHASE 3 DESIGN
16 • Potential Phase 1b study in PDAC to explore more frequent VCN - 01 dosing (q2 months) to potentially improve outcomes • Builds on EMA suggestion and recognition of the benefit of multiple VCN - 01 doses • VCN - 01 dosing q2 months means at least two doses could be administered to most patients (PFS 3.5 - 6.5 months in VIRAGE study) • Small study (n=6 - 10) could be conducted with existing cash and available clinical drug product • Planning a potential pivotal trial in retinoblastoma (Rb) • Intravitreal VCN - 01 plus topotecan in children with refractory vitreous seeds • Extremely rare pediatric disease may permit a relatively small, single - arm study • Rare Pediatric Disease designation may enable access to monetizable Priority Review Voucher • No prior approvals in Rb, pursuing close collaboration with regulators VCN - 01 ADDITIONAL CLINICAL ACTIVITIES
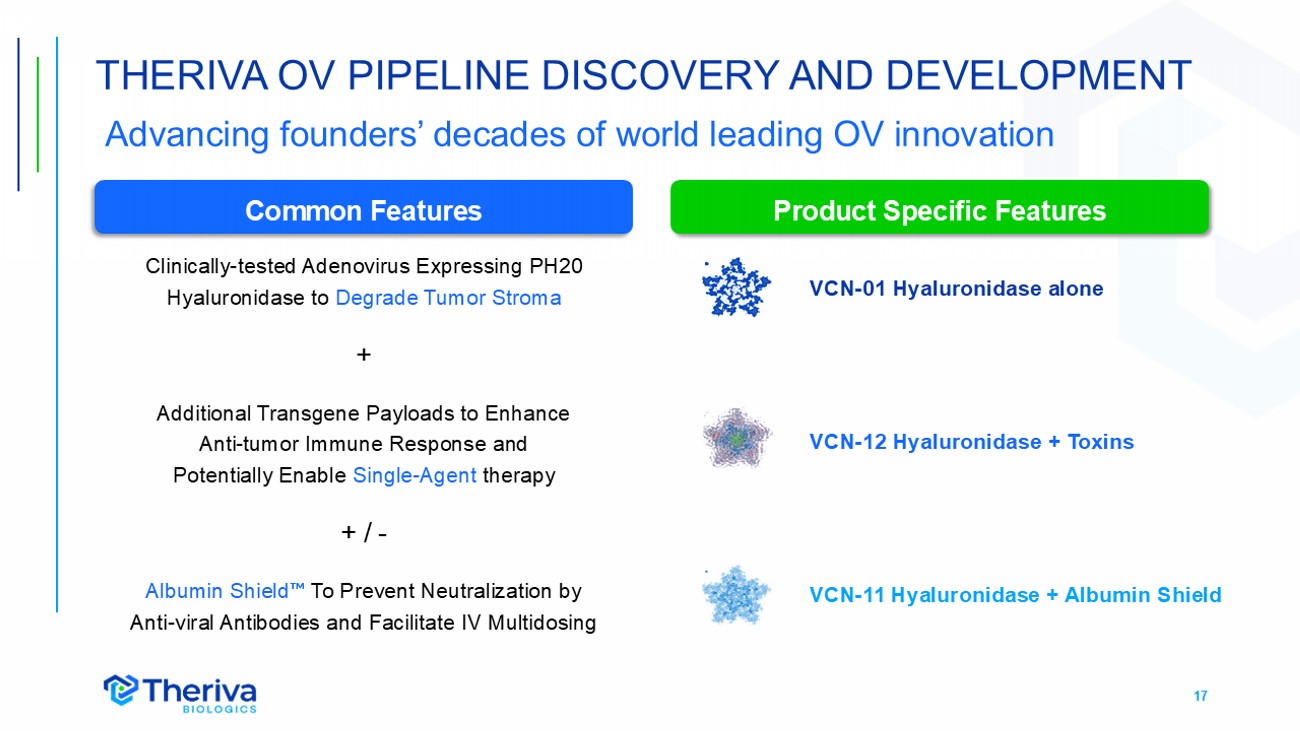
17 Clinically - tested Adenovirus Expressing PH20 Hyaluronidase to Degrade Tumor Stroma + Additional Transgene Payloads to Enhance Anti - tumor Immune Response and Potentially Enable Single - Agent therapy + / - Albumin Shield To Prevent Neutralization by Anti - viral Antibodies and Facilitate IV Multidosing THERIVA OV PIPELINE DISCOVERY AND DEVELOPMENT Common Features Product Specific Features VCN - 01 Hyaluronidase alone VCN - 12 Hyaluronidase + Toxins VCN - 11 Hyaluronidase + Albumin Shield Advancing founders’ decades of world leading OV innovation
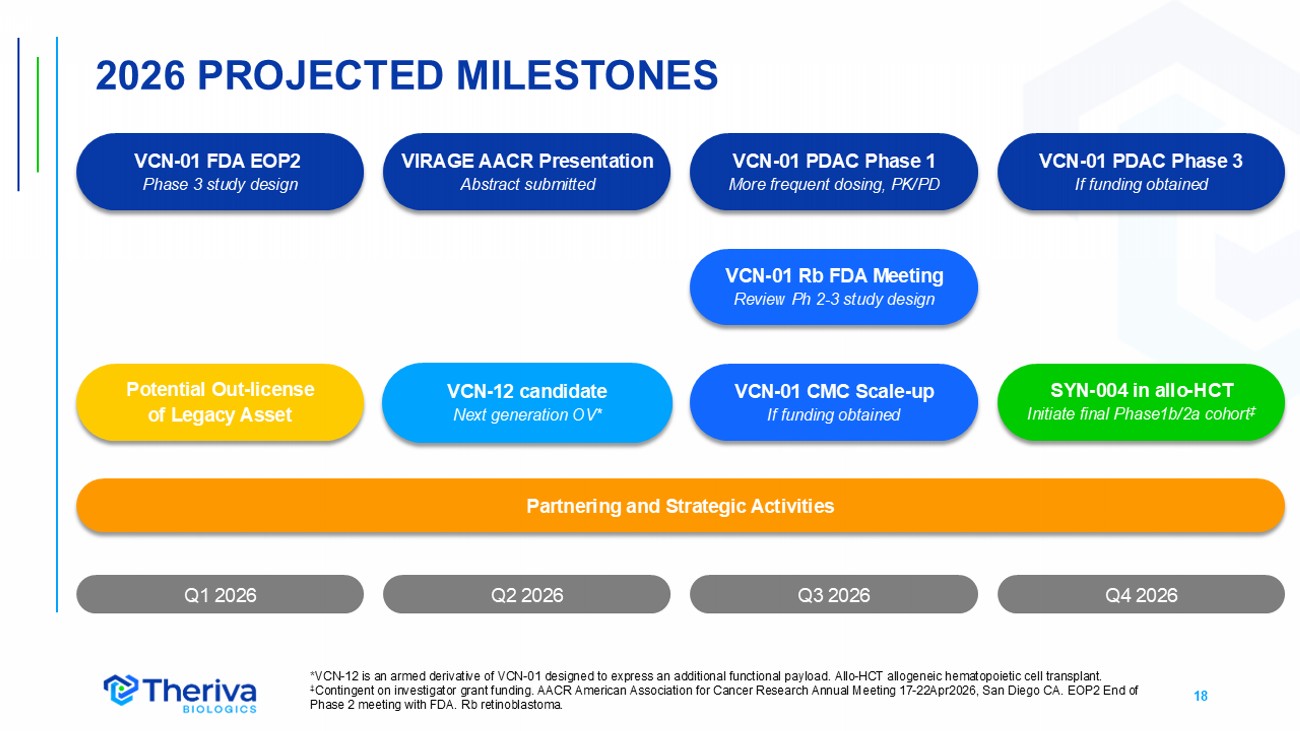
18 Q4 2026 2026 PROJECTED MILESTONES Q1 2026 Q2 2026 * VCN - 12 is an armed derivative of VCN - 01 designed to express an additional functional payload . Allo - HCT allogeneic hematopoietic cell transplant. ‡ Contingent on investigator grant funding. AACR American Association for Cancer Research Annual Meeting 17 - 22Apr2026, San Diego C A. EOP2 End of Phase 2 meeting with FDA. Rb retinoblastoma. Q3 2026 VCN - 12 candidate Next generation OV* VCN - 01 Rb FDA Meeting Review Ph 2 - 3 study design VCN - 01 PDAC Phase 1 More frequent dosing, PK/PD SYN - 004 in allo - HCT Initiate final Phase1b/2a cohort ‡ VCN - 01 FDA EOP2 Phase 3 study design Partnering and Strategic Activities Potential Out - license of Legacy Asset VCN - 01 CMC Scale - up If funding obtained VIRAGE AACR Presentation Abstract submitted VCN - 01 PDAC Phase 3 If funding obtained

APPENDIX

20 SEASONED LEADERSHIP TEAM Manel Cascall ó PhD General Director, EU Subsidiary Expertise in oncolytic adenovirus clinical development, received several patents for the use of adenovirus as antitumoral agents and authored many peer - reviewed scientific publications Deep regulatory experience and serves as an independent expert for the European Medicines Agencies (EMA) Steven Shallcross Chief Executive Officer, Chief Financial Officer Served as the Company’s CEO since 2018 and CFO since joining the Company in 2015 Deep operational, financial and international biotech industry experience and proven track record of leading the financial development and strategy in the public sector Vince Wacher PhD Head Corporate Development Nearly 30 years leading corporate strategy, partnering, research, clinical development, and intellectual property programs for start - ups, small companies, and new business units within large companies Development experience across oncology, infection, GI, metabolic diseases, transplantation, and drug delivery
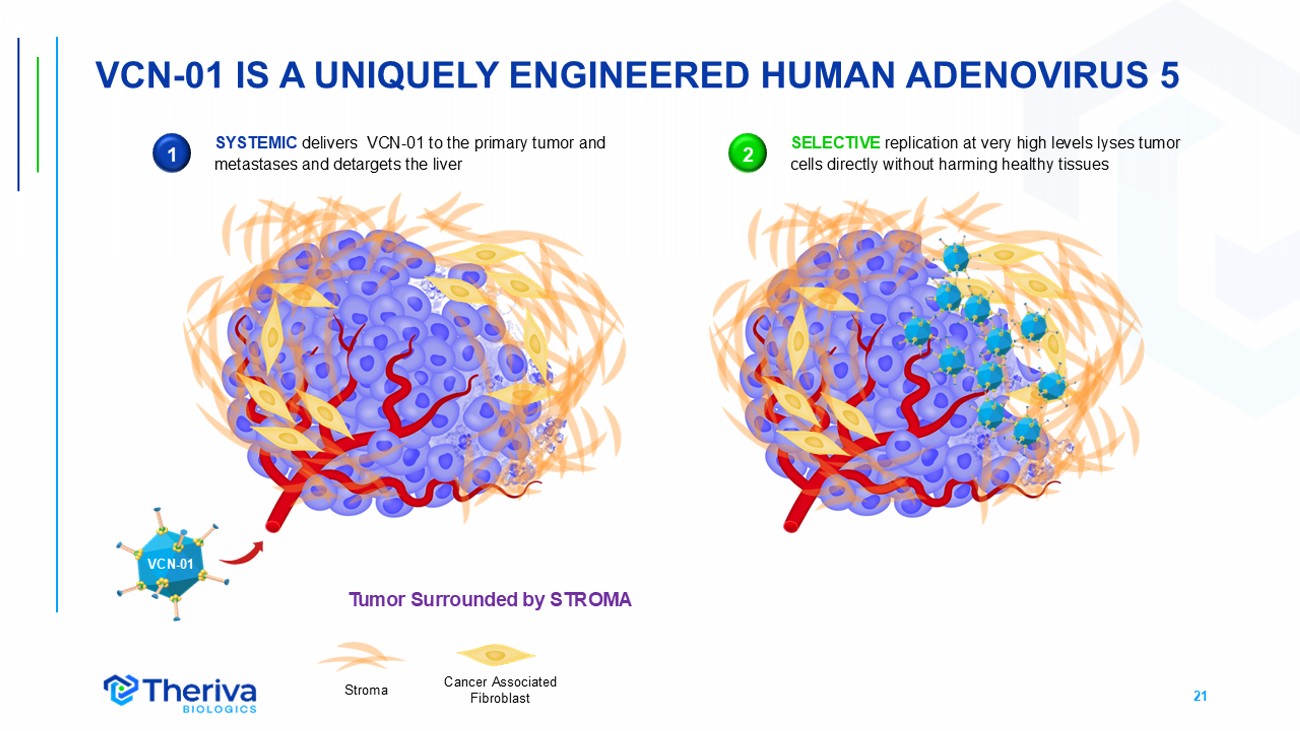
21 VCN - 01 IS A UNIQUELY ENGINEERED HUMAN ADENOVIRUS 5 Cancer Associated Fibroblast Stroma Tumor Surrounded by STROMA VCN - 01 1 SYSTEMIC delivers VCN - 01 to the primary tumor and metastases and detargets the liver SELECTIVE replication at very high levels lyses tumor cells directly without harming healthy tissues 2
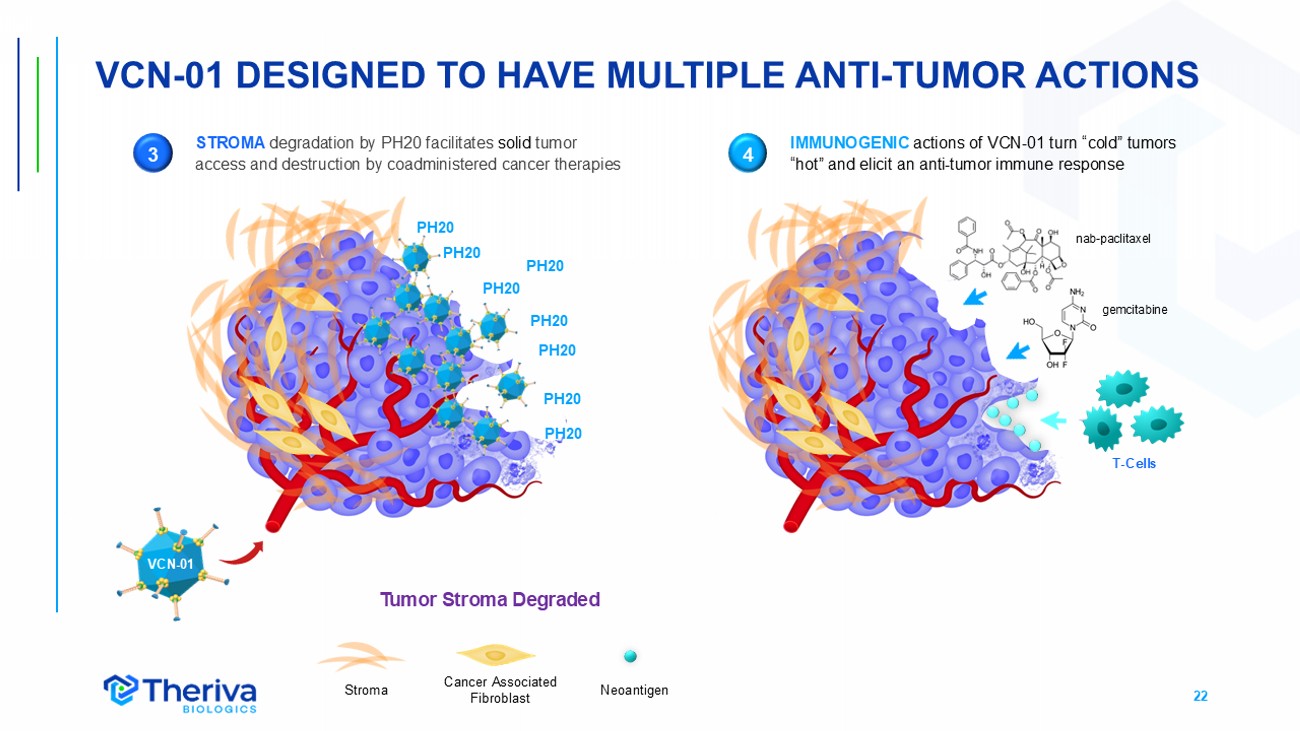
22 VCN - 01 DESIGNED TO HAVE MULTIPLE ANTI - TUMOR ACTIONS PH20 PH20 PH20 PH20 PH20 PH20 PH20 PH20 Neoantigen Cancer Associated Fibroblast Stroma STROMA degradation by PH20 facilitates solid tumor access and destruction by coadministered cancer therapies 3 4 IMMUNOGENIC actions of VCN - 01 turn “cold” tumors “hot” and elicit an anti - tumor immune response VCN - 01 nab - paclitaxel gemcitabine T - Cells Tumor Stroma Degraded
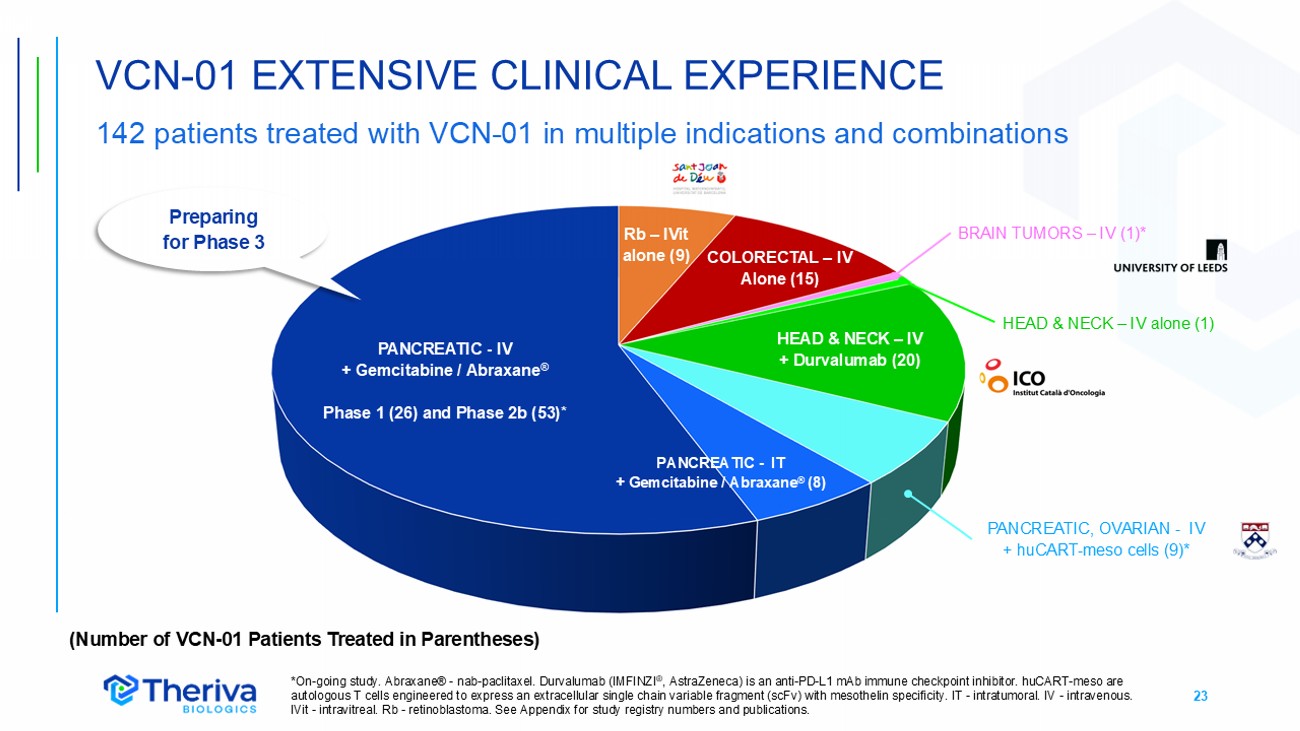
23 VCN - 01 EXTENSIVE CLINICAL EXPERIENCE 142 patients treated with VCN - 01 in multiple indications and combinations HEAD & NECK – IV + Durvalumab (20) PANCREATIC - IV + Gemcitabine / Abraxane ® Phase 1 (26) and Phase 2b (53)* COLORECTAL – IV Alone (15) PANCREATIC - IT + Gemcitabine / Abraxane ® (8) PANCREATIC, OVARIAN - IV + huCART - meso cells (9)* BRAIN TUMORS – IV (1)* HEAD & NECK – IV alone (1) *On - going study. Abraxane® - nab - paclitaxel. Durvalumab (IMFINZI ® , AstraZeneca) is an anti - PD - L1 mAb immune checkpoint inhibitor. huCART - meso are autologous T cells engineered to express an extracellular single chain variable fragment (scFv) with mesothelin specificity. IT - intratumoral. IV - intravenous. IVit - intravitreal. Rb - retinoblastoma. See Appendix for study registry numbers and publications. Rb – IVit alone (9) (Number of VCN - 01 Patients Treated in Parentheses) Preparing for Phase 3
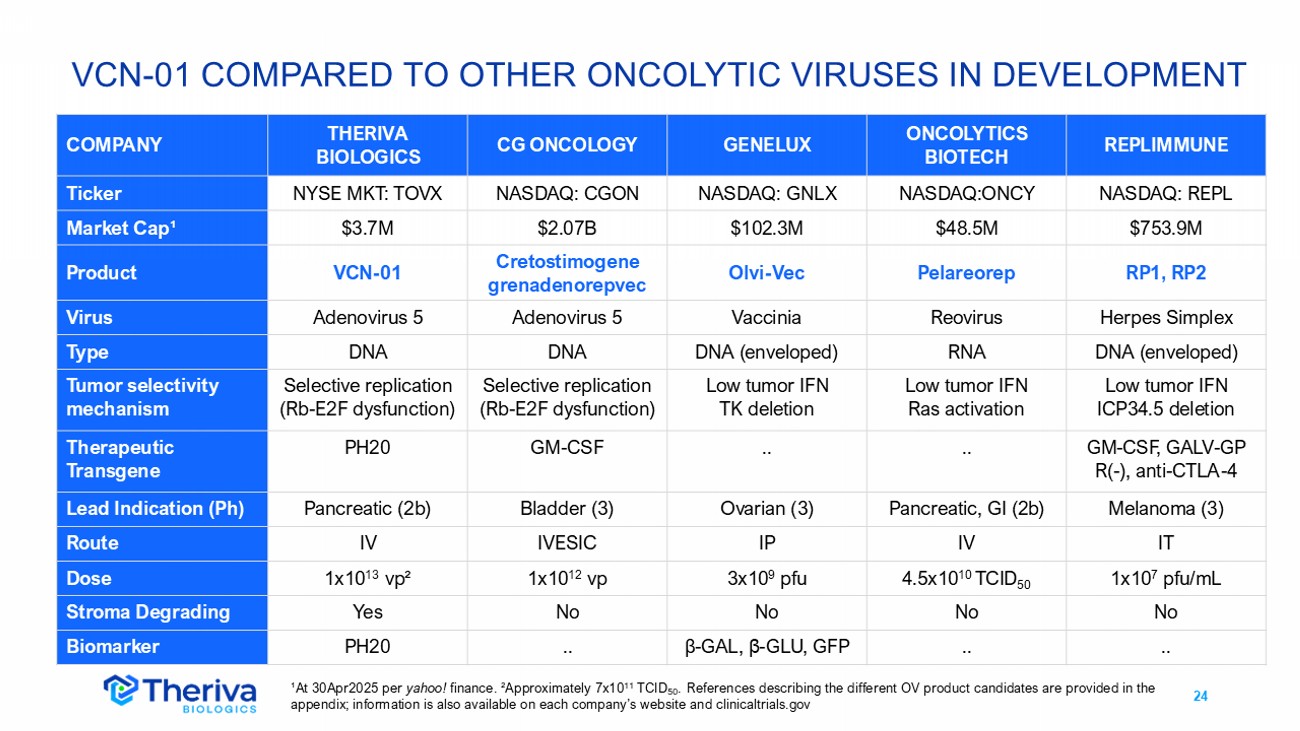
24 VCN - 01 COMPARED TO OTHER ONCOLYTIC VIRUSES IN DEVELOPMENT REPLIMMUNE ONCOLYTICS BIOTECH GENELUX CG ONCOLOGY THERIVA BIOLOGICS COMPANY NASDAQ: REPL NASDAQ:ONCY NASDAQ: GNLX NASDAQ: CGON NYSE MKT: TOVX Ticker $753.9M $48.5M $102.3M $2.07B $3.7M Market Cap ¹ RP1, RP2 Pelareorep Olvi - Vec Cretostimogene grenadenorepvec VCN - 01 Product Herpes Simplex Reovirus Vaccinia Adenovirus 5 Adenovirus 5 Virus DNA (enveloped) RNA DNA (enveloped) DNA DNA Type Low tumor IFN ICP34.5 deletion Low tumor IFN Ras activation Low tumor IFN TK deletion Selective replication (Rb - E2F dysfunction) Selective replication (Rb - E2F dysfunction) Tumor selectivity mechanism GM - CSF, GALV - GP R( - ), anti - CTLA - 4 .. .. GM - CSF PH20 Therapeutic Transgene Melanoma (3) Pancreatic, GI (2b) Ovarian (3) Bladder (3) Pancreatic (2b) Lead Indication (Ph) IT IV IP IVESIC IV Route 1x10 7 pfu/mL 4.5x10 10 TCID 50 3x10 9 pfu 1x10 12 vp 1x10 13 vp ² Dose No No No No Yes Stroma Degrading .. .. β - GAL, β - GLU, GFP .. PH20 Biomarker ¹At 30Apr2025 per yahoo! finance. ² Approximately 7x10 11 TCID 50 . References describing the different OV product candidates are provided in the appendix; information is also available on each company’s website and clinicaltrials.gov

25 THERIVA OV PORTFOLIO HIGHLIGHTS Multiple modes of action, indications and combinations • Highly differentiated OVs designed to have multiple antitumor effects • Systemic administration, selective tumor replication, stroma degradation • Multiple potential value opportunities for lead asset VCN - 01 • Preparing Phase 3 trial with SoC in first - line metastatic PDAC; planning Phase 2/3 trial in retinoblastoma • Phase 1 data support additional indications (HNSCC, CRC) and diverse combinations (chemotherapy, CPI, CAR - T) • Regulatory status expected to facilitate VCN - 01 development • PDAC: Orphan Drug Designation (FDA, EMA), Fast Track designation (FDA) • Retinoblastoma: Orphan Drug Designation (EMA; FDA); Rare Pediatric Disease Designation (FDA: potential access to priority review voucher) • Leading OV discovery engine advancing diverse new product candidates • Potent tumor killing with potential single agent efficacy CPI immune checkpoint inhibitor. CRC colorectal cancer. HNSCC head and neck squamous cell carcinoma. PDAC pancreatic ductal a den ocarcinoma. SoC standard of care (gemcitabine + nab - paclitaxel in PDAC).

VCN - 01 IN PANCREATIC CANCER
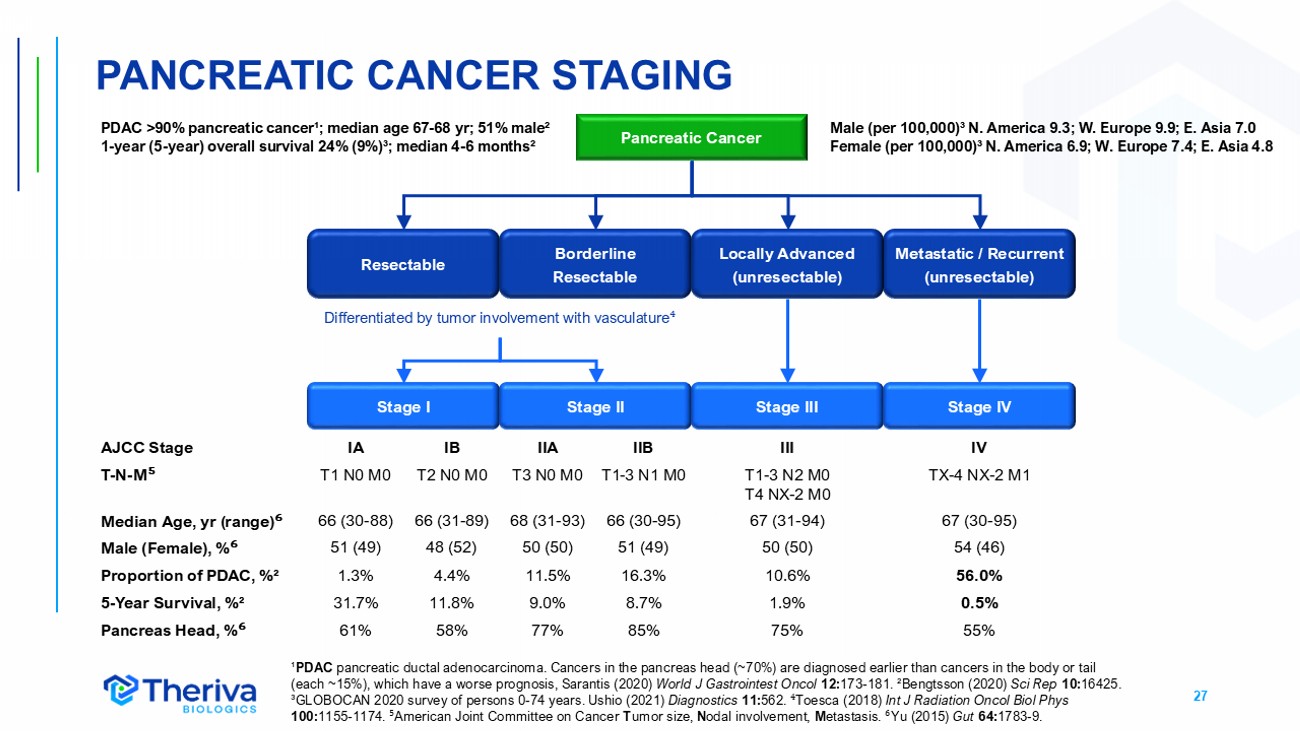
27 ¹ PDAC pancreatic ductal adenocarcinoma. Cancers in the pancreas head (~70%) are diagnosed earlier than cancers in the body or tail (each ~15%), which have a worse prognosis, Sarantis (2020) World J Gastrointest Oncol 12: 173 - 181. ²Bengtsson (2020) Sci Rep 10: 16425. ³GLOBOCAN 2020 survey of persons 0 - 74 years. Ushio (2021) Diagnostics 11: 562. ⁴ Toesca (2018) Int J Radiation Oncol Biol Phys 100: 1155 - 1174. ⁵ American Joint Committee on Cancer T umor size, N odal involvement, M etastasis. ⁶ Yu (2015) Gut 64: 1783 - 9. Pancreatic Cancer Resectable Borderline Resectable Metastatic / Recurrent (unresectable) Stage I Stage II Stage III Stage IV PDAC >90% pancreatic cancer¹; median age 67 - 68 yr ; 51% male² 1 - year (5 - year) overall survival 24% (9%)³; median 4 - 6 months² Locally Advanced (unresectable) Differentiated by tumor involvement with vasculature ⁴ IV III IIB IIA IB IA AJCC Stage TX - 4 NX - 2 M1 T1 - 3 N2 M0 T4 NX - 2 M0 T1 - 3 N1 M0 T3 N0 M0 T2 N0 M0 T1 N0 M0 T - N - M ⁵ 67 (30 - 95) 67 (31 - 94) 66 (30 - 95) 68 (31 - 93) 66 (31 - 89) 66 (30 - 88) Median Age, yr (range) ⁶ 54 (46) 50 (50) 51 (49) 50 (50) 48 (52) 51 (49) Male (Female), % ⁶ 56.0% 10.6% 16.3% 11.5% 4.4% 1.3% Proportion of PDAC, %² 0.5% 1.9% 8.7% 9.0% 11.8% 31.7% 5 - Year Survival, %² 55% 75% 85% 77% 58% 61% Pancreas Head, % ⁶ Male (per 100,000)³ N. America 9.3; W. Europe 9.9; E. Asia 7.0 Female (per 100,000)³ N. America 6.9; W. Europe 7.4; E. Asia 4.8 PANCREATIC CANCER STAGING
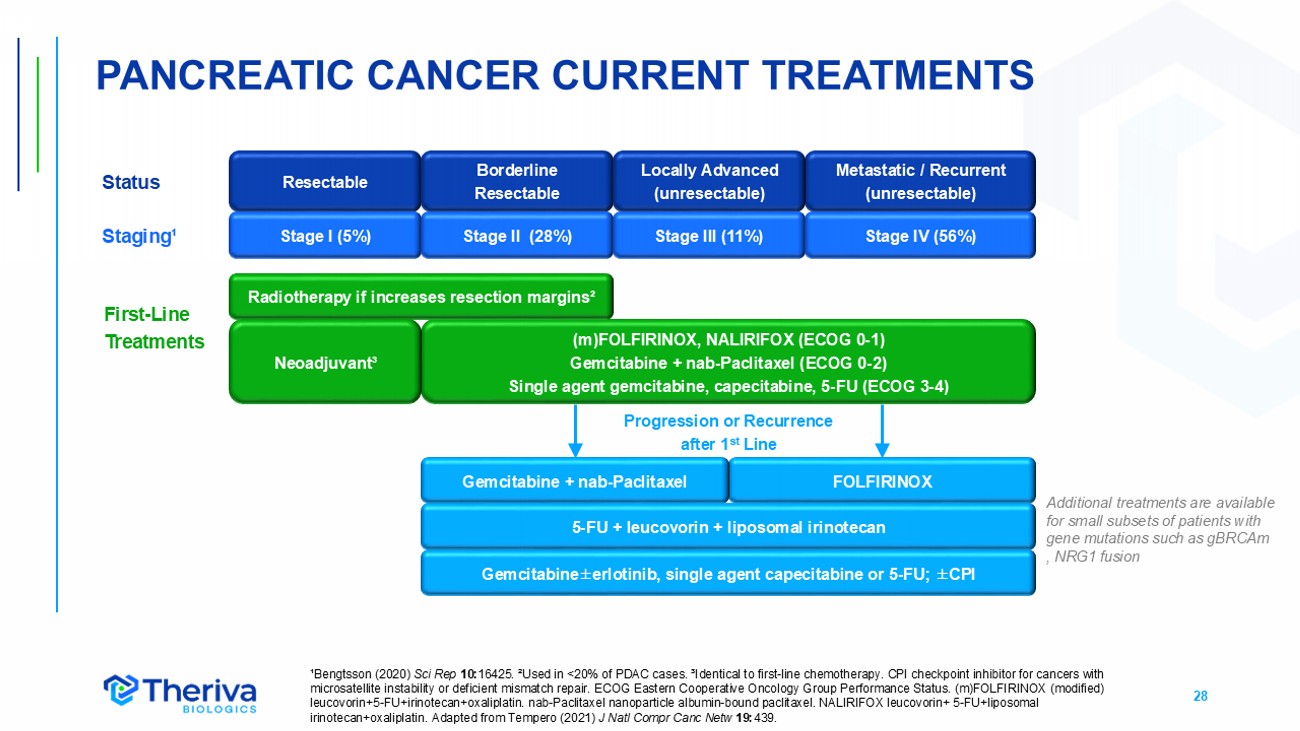
28 (m)FOLFIRINOX, NALIRIFOX (ECOG 0 - 1) Gemcitabine + nab - Paclitaxel (ECOG 0 - 2) S ingle agent gemcitabine, capecitabine, 5 - FU (ECOG 3 - 4) PANCREATIC CANCER CURRENT TREATMENTS ¹Bengtsson (2020) Sci Rep 10: 16425. ²Used in <20% of PDAC cases. ³Identical to first - line chemotherapy. CPI checkpoint inhibitor for cancers with microsatellite instability or deficient mismatch repair. ECOG Eastern Cooperative Oncology Group Performance Status. (m)FOLFI RIN OX (modified) leucovorin+5 - FU+irinotecan+oxaliplatin. nab - Paclitaxel nanoparticle albumin - bound paclitaxel. NALIRIFOX leucovorin+ 5 - FU+liposom al irinotecan+oxaliplatin . Adapted from Tempero (2021) J Natl Compr Canc Netw 19: 439. Resectable Borderline Resectable Metastatic / Recurrent (unresectable) Locally Advanced (unresectable) FOLFIRINOX Gemcitabine + nab - Paclitaxel 5 - FU + leucovorin + liposomal i rinotecan Radiotherapy if increases resection margins² Neoadjuvant³ Gemcitabine ± erlotinib , single agent capecitabine or 5 - FU; ± CPI Stage I (5%) Stage II (28%) Stage III (11%) Stage IV (56%) Progression or Recurrence after 1 st Line First - Line Treatments Additional treatment s are available for small subsets of patients with gene mutations such as gBRCAm , NRG1 fusion Status Staging¹
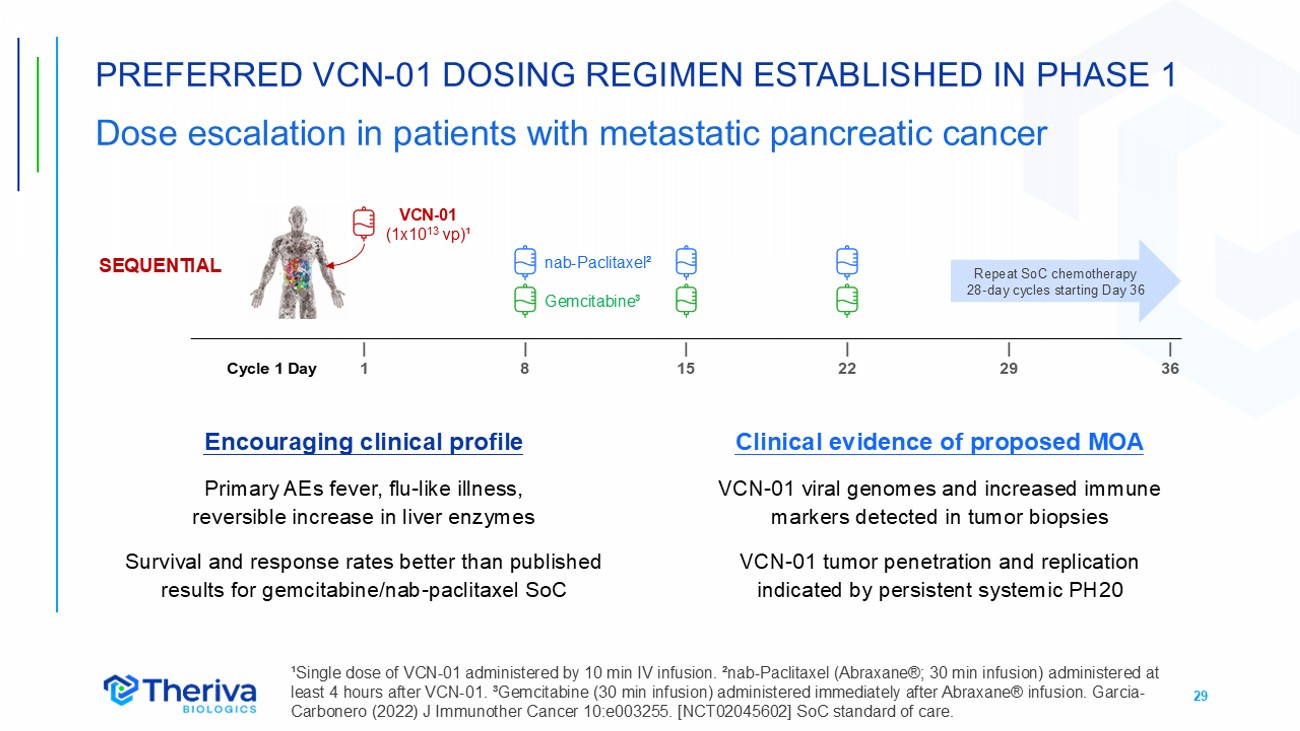
29 PREFERRED VCN - 01 DOSING REGIMEN ESTABLISHED IN PHASE 1 Dose escalation in patients with metastatic pancreatic cancer ¹Single dose of VCN - 01 administered by 10 min IV infusion. ²nab - Paclitaxel (Abraxane®; 30 min infusion) administered at least 4 hours after VCN - 01. ³Gemcitabine (30 min infusion) administered immediately after Abraxane® infusion. Garcia - Carbonero (2022) J Immunother Cancer 10:e003255. [NCT02045602] SoC standard of care. SEQUENTIAL | 36 | 29 | 22 | 15 | 8 | 1 Cycle 1 Day VCN - 01 (1x10 13 vp) ¹ nab - Paclitaxel ² Gemcitabine ³ Repeat SoC chemotherapy 28 - day cycles starting Day 36 Encouraging clinical profile Primary AEs fever, flu - like illness, reversible increase in liver enzymes Survival and response rates better than published results for gemcitabine/nab - paclitaxel SoC Clinical evidence of proposed MOA VCN - 01 viral genomes and increased immune markers detected in tumor biopsies VCN - 01 tumor penetration and replication indicated by persistent systemic PH20
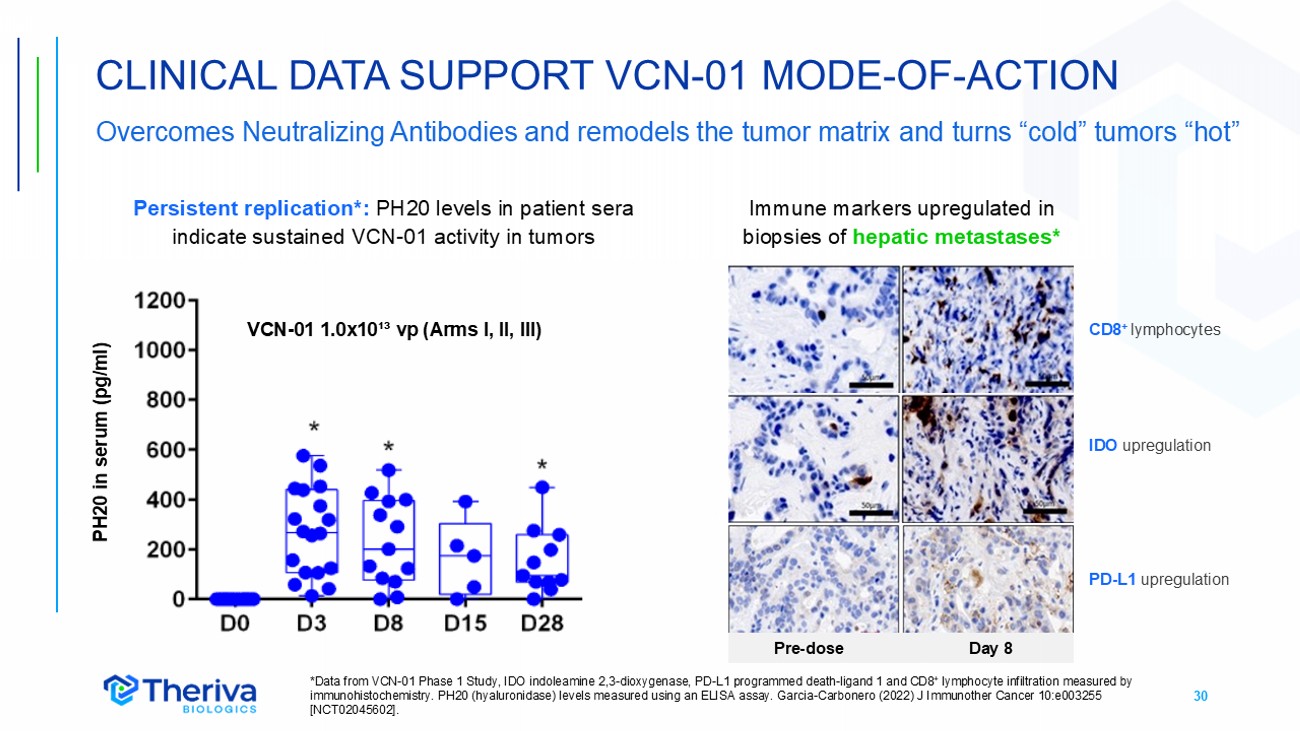
30 CLINICAL DATA SUPPORT VCN - 01 MODE - OF - ACTION Overcomes Neutralizing Antibodies and remodels the tumor matrix and turns “cold” tumors “hot” *Data from VCN - 01 Phase 1 Study, IDO indoleamine 2,3 - dioxygenase, PD - L1 programmed death - ligand 1 and CD8 + lymphocyte infiltration measured by immunohistochemistry. PH20 (hyaluronidase) levels measured using an ELISA assay. Garcia - Carbonero (2022) J Immunother Cancer 10: e003255 [NCT02045602]. PH20 in serum (pg/ml) CD8 + lymphocytes IDO upregulation PD - L1 upregulation VCN - 01 1.0x10 ¹³ vp (Arms I, II, III) Pre - dose Day 8 Persistent replication*: PH20 levels in patient sera indicate sustained VCN - 01 activity in tumors Immune markers upregulated in biopsies of hepatic metastases*
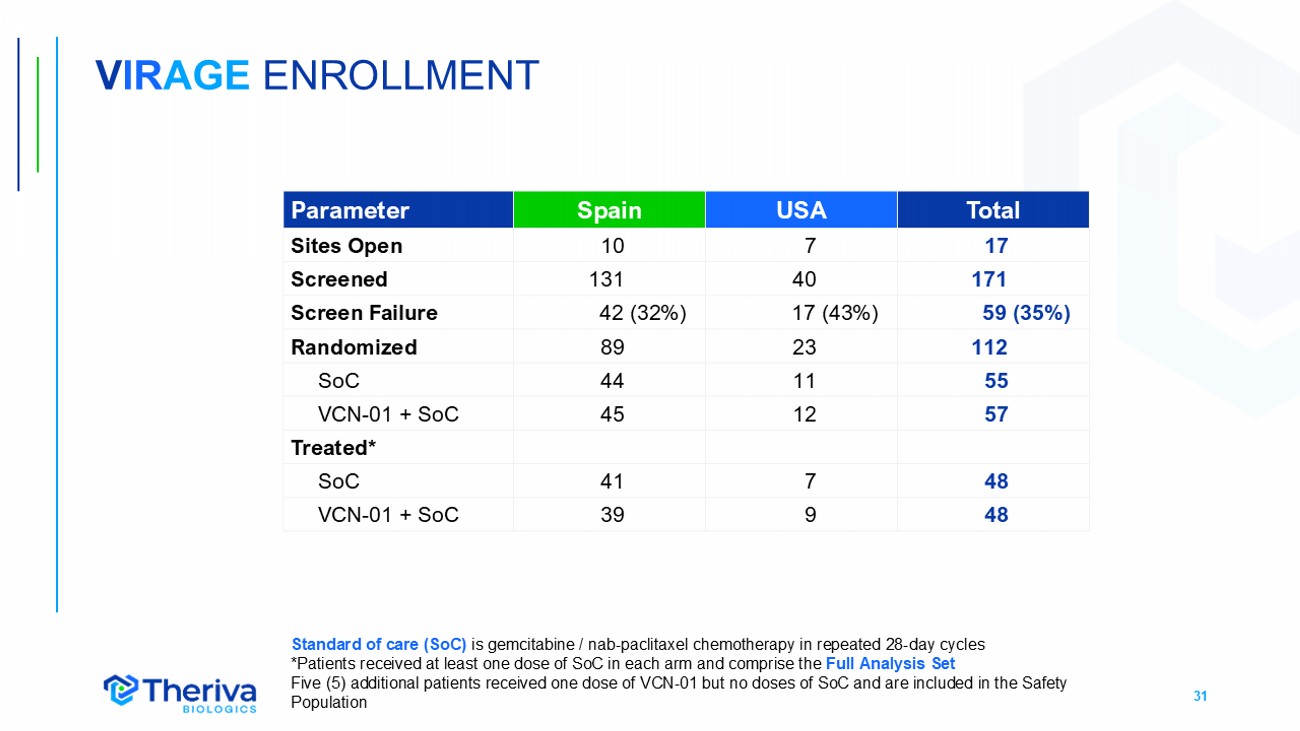
31 V IR AGE ENROLLMENT Total USA Spain Parameter 17 7 10 Sites Open 171 40 131 Screened 59 (35%) 17 (43%) 42 (32%) Screen Failure 112 23 89 Randomized 55 11 44 SoC 57 12 45 VCN - 01 + SoC Treated* 48 7 41 SoC 48 9 39 VCN - 01 + SoC Standard of care (SoC) is gemcitabine / nab - paclitaxel chemotherapy in repeated 28 - day cycles *Patients received at least one dose of SoC in each arm and comprise the Full Analysis Set Five (5) additional patients received one dose of VCN - 01 but no doses of SoC and are included in the Safety P opulation
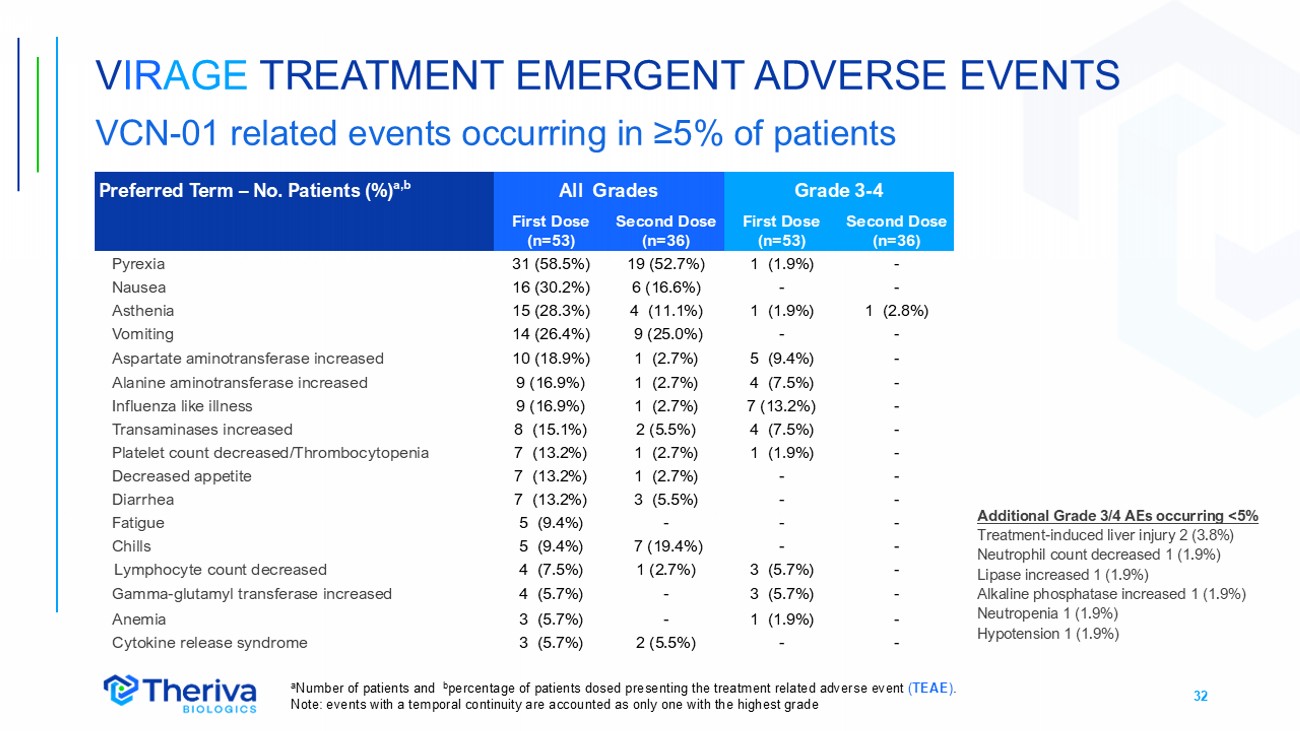
32 Grade 3 - 4 All Grades Preferred Term – No. Patients (%) a,b Second Dose (n=36) First Dose (n=53) Second Dose (n=36) First Dose (n=53) - 1 (1.9%) 19 (52.7%) 31 (58.5%) Pyrexia - - 6 (16.6%) 16 (30.2%) Nausea 1 (2.8%) 1 (1.9%) 4 (11.1%) 15 (28.3%) Asthenia - - 9 (25.0%) 14 (26.4%) Vomiting - 5 (9.4%) 1 (2.7%) 10 (18.9%) Aspartate aminotransferase increased - 4 (7.5%) 1 (2.7%) 9 (16.9%) Alanine aminotransferase increased - 7 (13.2%) 1 (2.7%) 9 (16.9%) Influenza like illness - 4 (7.5%) 2 (5.5%) 8 (15.1%) Transaminases increased - 1 (1.9%) 1 (2.7%) 7 (13.2%) Platelet count decreased/Thrombocytopenia - - 1 (2.7%) 7 (13.2%) Decreased appetite - - 3 (5.5%) 7 (13.2%) Diarrhea - - - 5 (9.4%) Fatigue - - 7 (19.4%) 5 (9.4%) Chills - 3 (5.7%) 1 (2.7%) 4 (7.5%) Lymphocyte count decreased - 3 (5.7%) - 4 (5.7%) Gamma - glutamyl transferase increased - 1 (1.9%) - 3 (5.7%) Anemia - - 2 (5.5%) 3 (5.7%) Cytokine release syndrome a Number of patients and b percentage of patients dosed presenting the treatment related adverse event ( TEAE ) . Note: events with a temporal continuity are accounte d as only one with the highest grade V IR AGE TREATMENT EMERGENT ADVERSE EVENTS VCN - 01 related events occurring in ≥5% of patients Additional Grade 3/4 AEs occurring <5% Treatment - induced liver injury 2 (3.8%) Neutrophil count decreased 1 (1.9%) Lipase increased 1 (1.9%) Alkaline phosphatase increased 1 (1.9%) Neutropenia 1 (1.9%) Hypotension 1 (1.9%)

33 • VIRAGE clinical data was reviewed on two occasions by an independent Data Monitoring Committee (DMC) who noted the following: • Intravenous VCN - 01 was well tolerated in patients treated in this study • The most common VCN - 01 related AEs (pyrexia, flu - like illness, vomiting, nausea, and elevated transaminases) were transient and reversible. • AEs were observed to be less frequent and of reduced CTCAE grade after the second VCN - 01 dose compared to the first VCN - 01 dose • The overall type and number of AEs in the VCN - 01+SoC treatment group was as expected for the pancreatic cancer population, the duration of treatment, and the administration of an oncolytic virus V IR AGE SAFETY REVIEW BY INDEPENDENT DMC
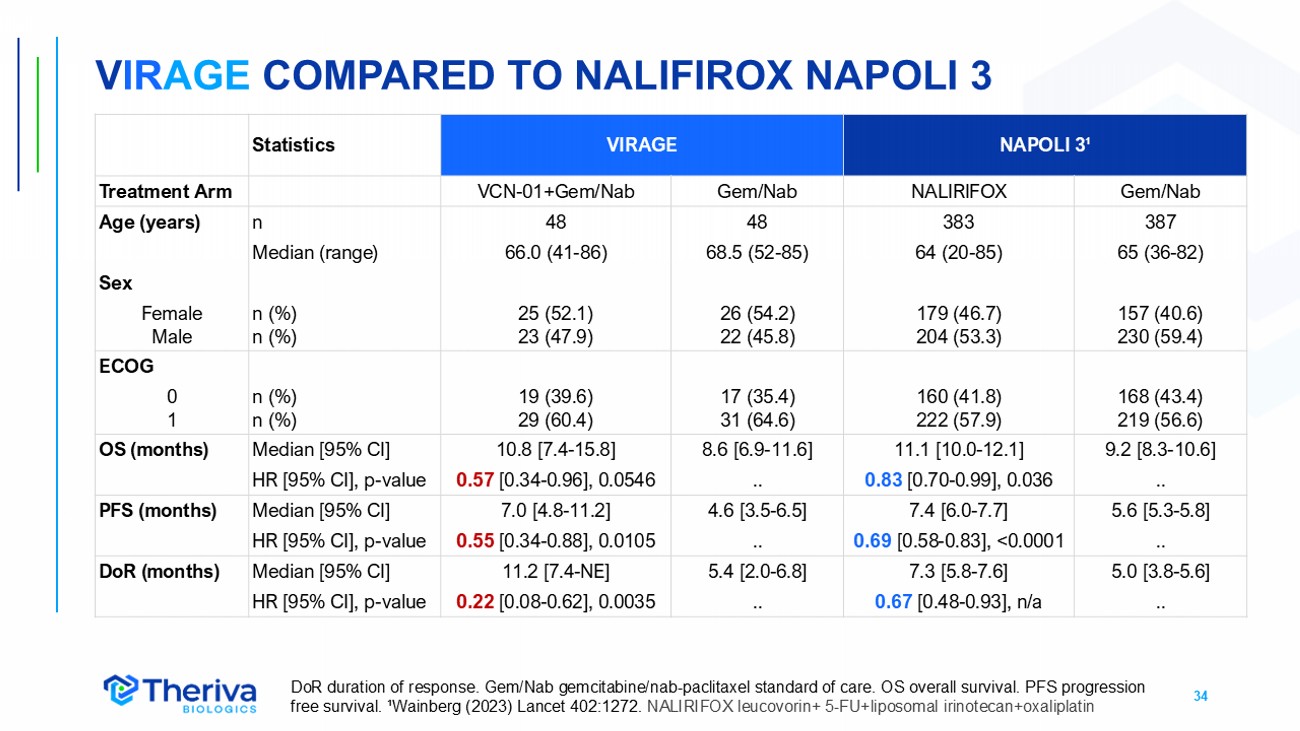
34 V IR AGE COMPARED TO NALIFIROX NAPOLI 3 NAPOLI 3 ¹ VIRAGE Statistics Gem/Nab NALIRIFOX Gem/Nab VCN - 01+Gem/Nab Treatment Arm 387 383 48 48 n Age (years) 65 (36 - 82) 64 (20 - 85) 68.5 (52 - 85) 66.0 (41 - 86) Median (range) Sex 157 (40.6) 230 (59.4) 179 (46.7) 204 (53.3) 26 (54.2) 22 (45.8) 25 (52.1) 23 (47.9) n (%) n (%) Female Male ECOG 168 (43.4) 219 (56.6) 160 (41.8) 222 (57.9) 17 (35.4) 31 (64.6) 19 (39.6) 29 (60.4) n (%) n (%) 0 1 9.2 [8.3 - 10.6] 11.1 [10.0 - 12.1] 8.6 [6.9 - 11.6] 10.8 [7.4 - 15.8] Median [95% CI] OS (months) .. 0.83 [0.70 - 0.99], 0.036 .. 0.57 [0.34 - 0.96], 0.0546 HR [95% CI], p - value 5.6 [5.3 - 5.8] 7.4 [6.0 - 7.7] 4.6 [3.5 - 6.5] 7.0 [4.8 - 11.2] Median [95% CI] PFS (months) .. 0.69 [0.58 - 0.83], <0.0001 .. 0.55 [0.34 - 0.88], 0.0105 HR [95% CI], p - value 5.0 [3.8 - 5.6] 7.3 [5.8 - 7.6] 5.4 [2.0 - 6.8] 11.2 [7.4 - NE] Median [95% CI] DoR (months) .. 0.67 [0.48 - 0.93], n/a .. 0.22 [0.08 - 0.62], 0.0035 HR [95% CI], p - value DoR duration of response. Gem/Nab gemcitabine/nab - paclitaxel standard of care. OS overall survival. PFS progression free survival. ¹Wainberg (2023) Lancet 402:1272. NALIRIFOX leucovorin+ 5 - FU+liposomal irinotecan+oxaliplatin

VCN - 01 IN HEAD & NECK CANCER
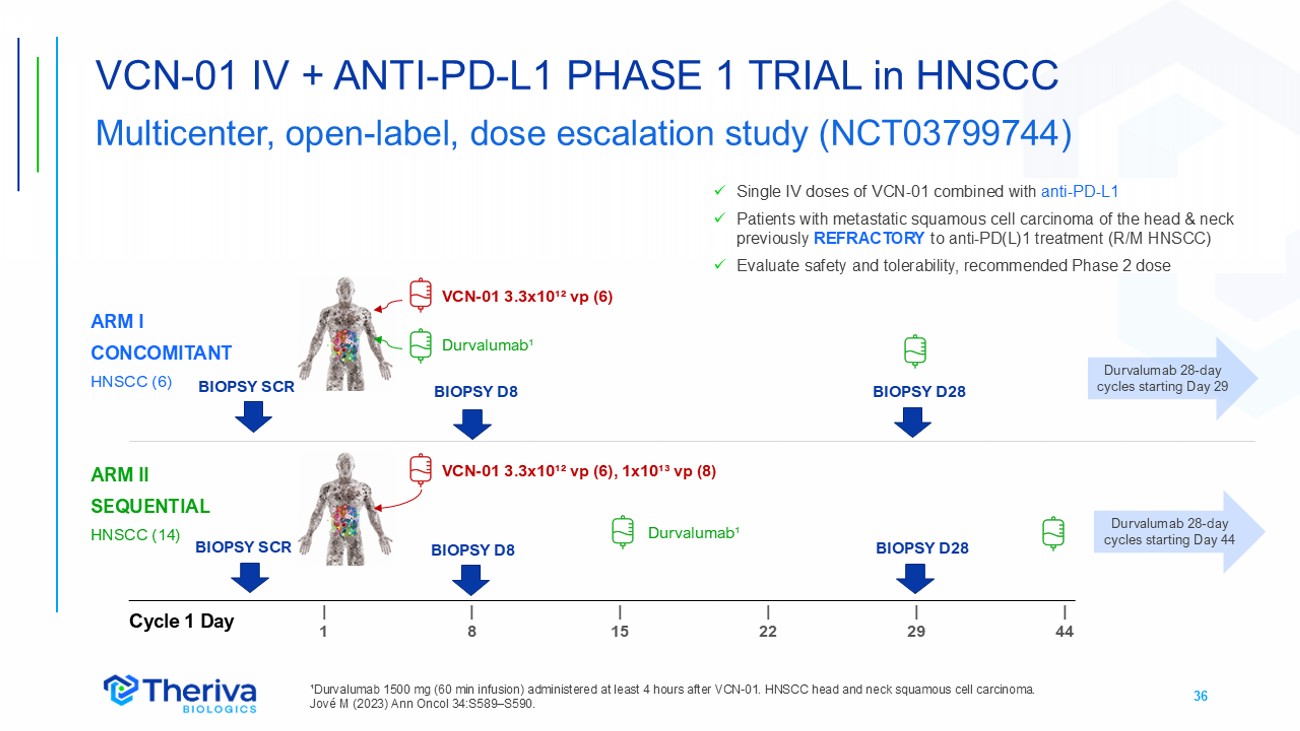
36 VCN - 01 IV + ANTI - PD - L1 PHASE 1 TRIAL in HNSCC Multicenter, open - label, dose escalation study (NCT03799744) ¹Durvalumab 1500 mg (60 min infusion) administered at least 4 hours after VCN - 01. HNSCC head and neck squamous cell carcinoma. Jové M (2023) Ann Oncol 34:S589 – S590. x Single IV doses of VCN - 01 combined with anti - PD - L1 x Patients with metastatic squamous cell carcinoma of the head & neck previously REFRACTORY to anti - PD(L)1 treatment (R/M HNSCC) x Evaluate safety and tolerability, recommended Phase 2 dose | 44 | 29 | 22 | 15 | 8 | 1 Cycle 1 Day ARM I CONCOMITANT HNSCC (6) ARM II SEQUENTIAL HNSCC (14) VCN - 01 3.3x10¹² vp (6) VCN - 01 3.3x10¹² vp (6), 1x10¹³ vp (8) Durvalumab¹ Durvalumab¹ Durvalumab 28 - day cycles starting Day 29 Durvalumab 28 - day cycles starting Day 44 BIOPSY D8 BIOPSY D8 BIOPSY SCR BIOPSY SCR BIOPSY D28 BIOPSY D28
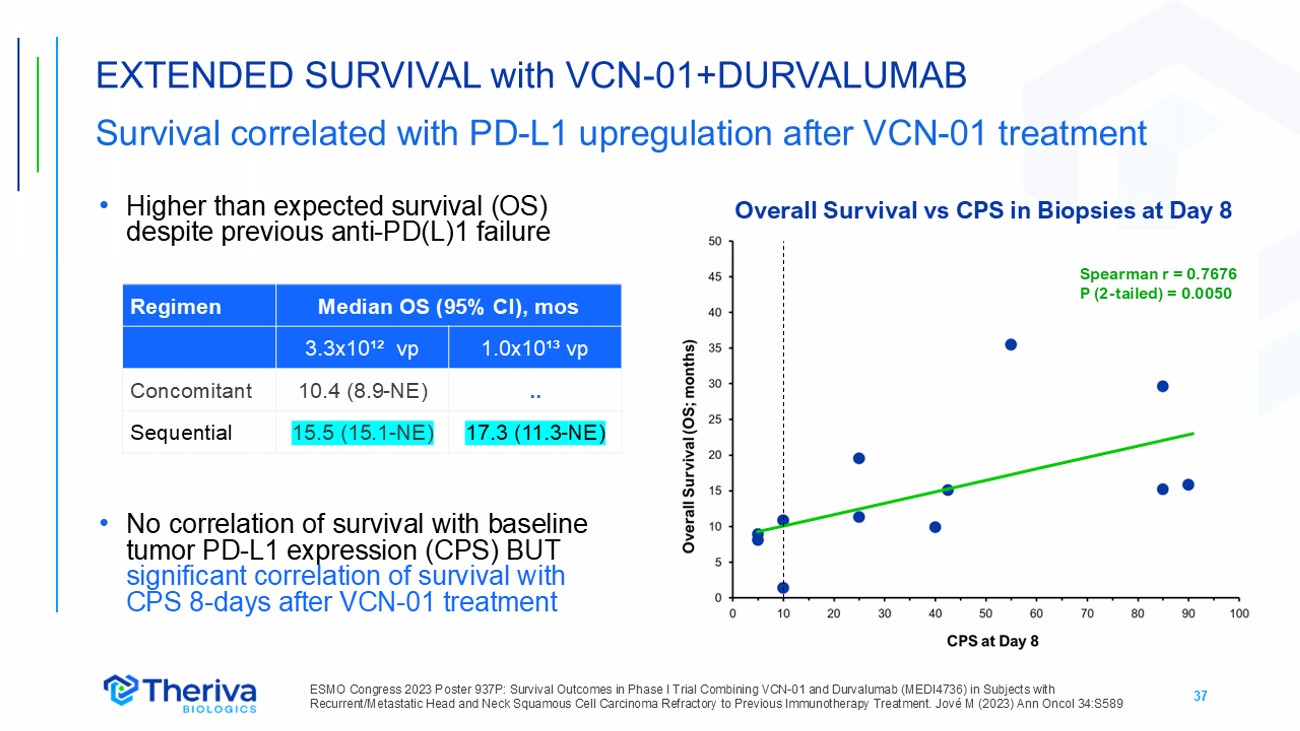
37 • Higher than expected survival (OS) despite previous anti - PD(L)1 failure • No correlation of survival with baseline tumor PD - L1 expression (CPS) BUT significant correlation of survival with CPS 8 - days after VCN - 01 treatment EXTENDED SURVIVAL with VCN - 01+DURVALUMAB Survival correlated with PD - L1 upregulation after VCN - 01 treatment ESMO Congress 2023 P oster 937 P: Survival Outcomes in Phase I Trial Combining VCN - 01 and Durvalumab (MEDI4736) in Subjects with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma Refractory to Previous Immunotherapy Treatment. Jové M (2023) Ann Oncol 34:S589 Median OS (95% CI), mos Regimen 1.0x10¹³ vp 3.3x10¹² vp .. 10.4 (8.9 - NE) Concomitant 17.3 (11.3 - NE) 15.5 (15.1 - NE) Sequential Overall Survival vs CPS in Biopsies at Day 8 Spearman r = 0.7676 P (2 - tailed) = 0.0050
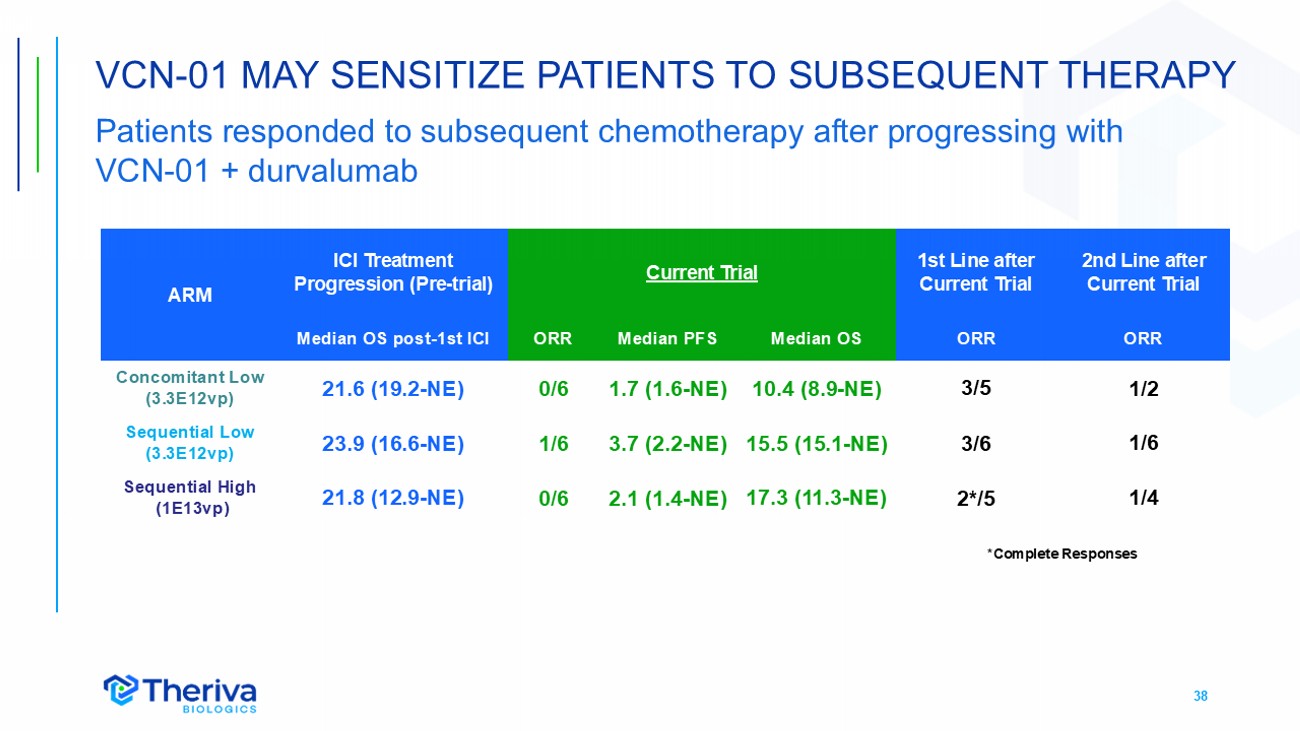
38 VCN - 01 MAY SENSITIZE PATIENTS TO SUBSEQUENT THERAPY Patients responded to subsequent chemotherapy after progressing with VCN - 01 + durvalumab 2nd Line after Current Trial 1st Line after Current Trial Current Trial ICI Treatment Progression ( Pre - trial ) ARM ORR ORR Median OS Median PFS ORR Median OS post - 1st ICI 1/2 3/5 10.4 (8.9 - NE) 1.7 (1.6 - NE) 0/6 21.6 (19.2 - NE) Concomitant Low (3.3E12vp) 1/6 3/6 15.5 (15.1 - NE) 3.7 (2.2 - NE) 1/6 23.9 (16.6 - NE) Sequential Low (3.3E12vp) 1/4 2*/5 17.3 (11.3 - NE) 2.1 (1.4 - NE) 0 /6 21.8 (12.9 - NE) Sequential High (1E13vp) *Complete Responses
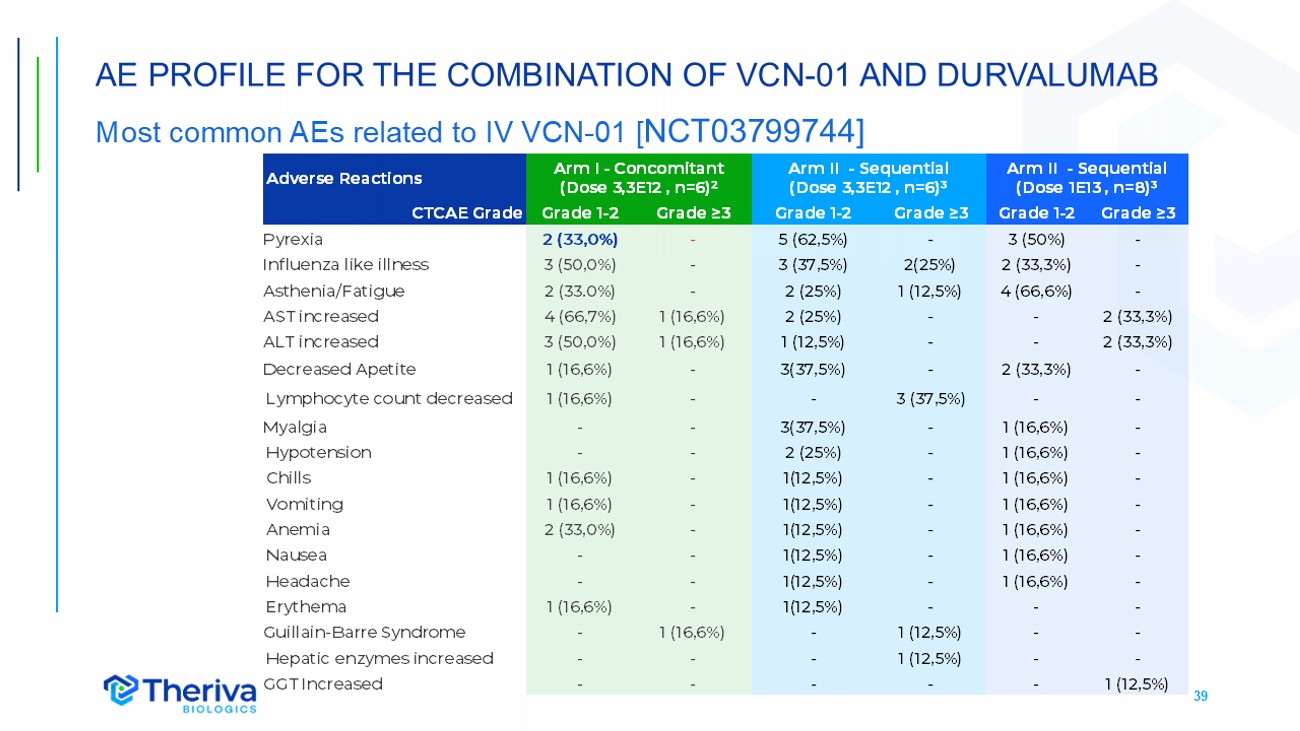
39 AE PROFILE FOR THE COMBINATION OF VCN - 01 AND DURVALUMAB Most common AEs related to IV VCN - 01 [ NCT03799744] Arm II - Sequential (Dose 1E13 , n=8)³ Arm II - Sequential (Dose 3,3E12 , n=6)³ Arm I - Concomitant (Dose 3,3E12 , n=6)² Adverse Reactions Grade ≥3 Grade 1 - 2 Grade ≥3 Grade 1 - 2 Grade ≥3 Grade 1 - 2 CTCAE Grade - 3 (50%) - 5 (62,5%) - 2 (33,0%) Pyrexia - 2 (33,3%) 2(25%) 3 (37,5%) - 3 (50,0%) Influenza like illness - 4 (66,6%) 1 (12,5%) 2 (25%) - 2 (33.0%) Asthenia /Fatigue 2 (33,3%) - - 2 (25%) 1 (16,6%) 4 (66,7%) AST increased 2 (33,3%) - - 1 (12,5%) 1 (16,6%) 3 (50,0%) ALT increased - 2 (33,3%) - 3(37,5%) - 1 (16,6%) Decreased Apetite - - 3 (37,5%) - - 1 (16,6%) Lymphocyte count decreased - 1 (16,6%) - 3(37,5%) - - Myalgia - 1 (16,6%) - 2 (25%) - - Hypotension - 1 (16,6%) - 1(12,5%) - 1 (16,6%) Chills - 1 (16,6%) - 1(12,5%) - 1 (16,6%) Vomiting - 1 (16,6%) - 1(12,5%) - 2 (33,0%) Anemia - 1 (16,6%) - 1(12,5%) - - Nausea - 1 (16,6%) - 1(12,5%) - - Headache - - - 1(12,5%) - 1 (16,6%) Erythema - - 1 (12,5%) - 1 (16,6%) - Guillain - Barre Syndrome - - 1 (12,5%) - - - Hepatic enzymes increased 1 (12,5%) - - - - - GGT Increased
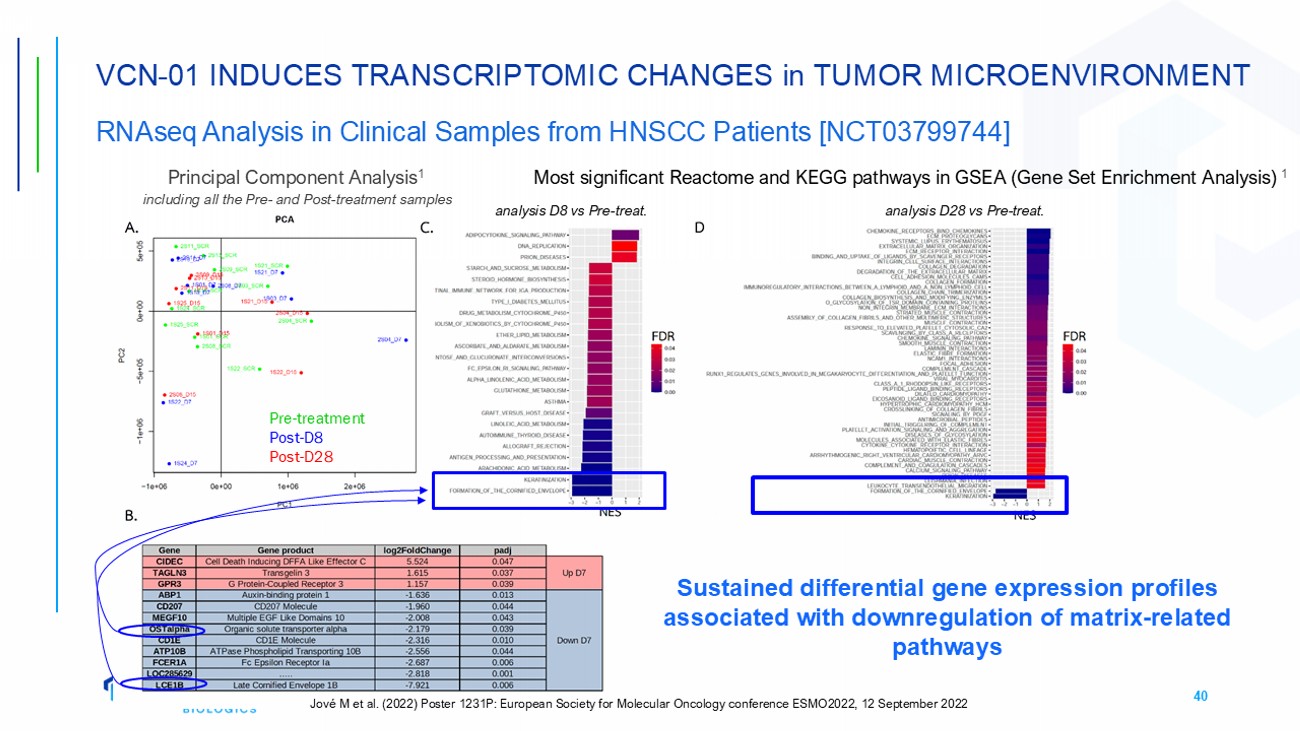
40 VCN - 01 INDUCES TRANSCRIPTOMIC CHANGES in TUMOR MICROENVIRONMENT RNAseq Analysis in Clinical Samples from HNSCC Patients [NCT03799744] Principal Component Analysis 1 including all the Pre - and Post - treatment samples Most significant Reactome and KEGG pathways in GSEA (Gene Set Enrichment Analysis) 1 analysis D8 vs Pre - treat. analysis D28 vs Pre - treat. Pre - treatment Post - D8 Post - D28 Sustained differential gene expression profiles associated with downregulation of matrix - related pathways Jové M et al. (2022) Poster 1231P: European Society for Molecular Oncology conference ESMO2022, 12 September 2022
41 VCN - 01 FINDINGS in R/M HNSCC Data support VCN - 01 MOA and immune enhancing effects • VCN - 01 has an acceptable adverse event profile when administered prior to durvalumab (Imfinzi ® ) • VCN - 01 reaches tumors, has sustained replication and PH20 expression • VCN - 01 treatment led to downregulation of tumor matrix genes and increased levels of immune markers in tumor biopsies (CD8, PD - L1 , IDO) • VCN - 01 - treated patients showed increased response to subsequent chemotherapy treatment lines after progressing on this trial ESMO Congress 2022 P oster 1231P: Phase I Study to Evaluate the Safety, Tolerability, and Efficacy of VCN - 01 in Combination With Durvalumab (MEDI4736) in Subjects With Recurrent/ Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M HNSCC). Jove M (2022) Ann On col 33:S1112. IDO Indoleamine - 2,3 - dioxygenase.

VCN - 01 IN RETINOBLASTOMA
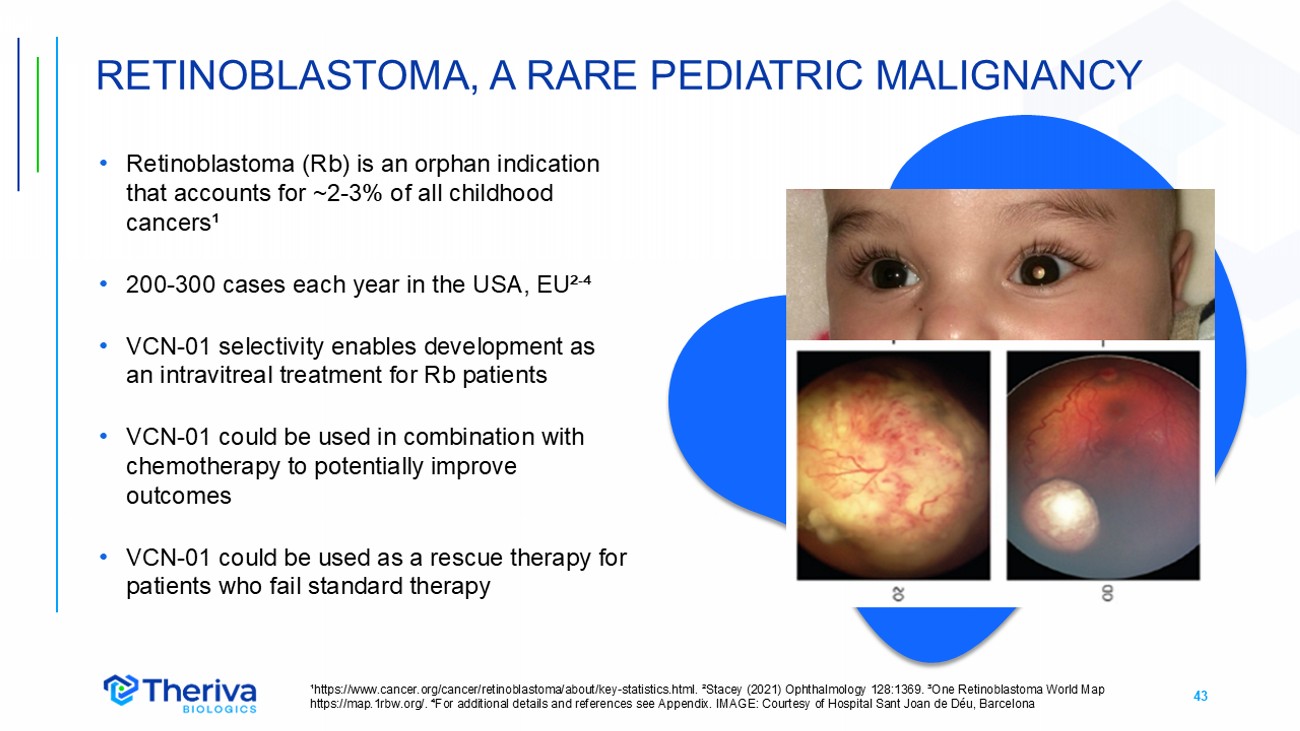
43 • Retinoblastoma (Rb) is an orphan indication that accounts for ~2 - 3% of all childhood cancers¹ • 200 - 300 cases each year in the USA, EU² - ⁴ • VCN - 01 selectivity enables development as an intravitreal treatment for Rb patients • VCN - 01 could be used in combination with chemotherapy to potentially improve outcomes • VCN - 01 could be used as a rescue therapy for patients who fail standard therapy RETINOBLASTOMA, A RARE PEDIATRIC MALIGNANCY ¹https://www.cancer.org/cancer/retinoblastoma/about/key - statistics.html. ²Stacey (2021) Ophthalmology 128:1369. ³One Retinoblast oma World Map https://map.1rbw.org/. ⁴For additional details and references see Appendix. IMAGE: Courtesy of Hospital Sant Joan de Déu, Bar cel ona
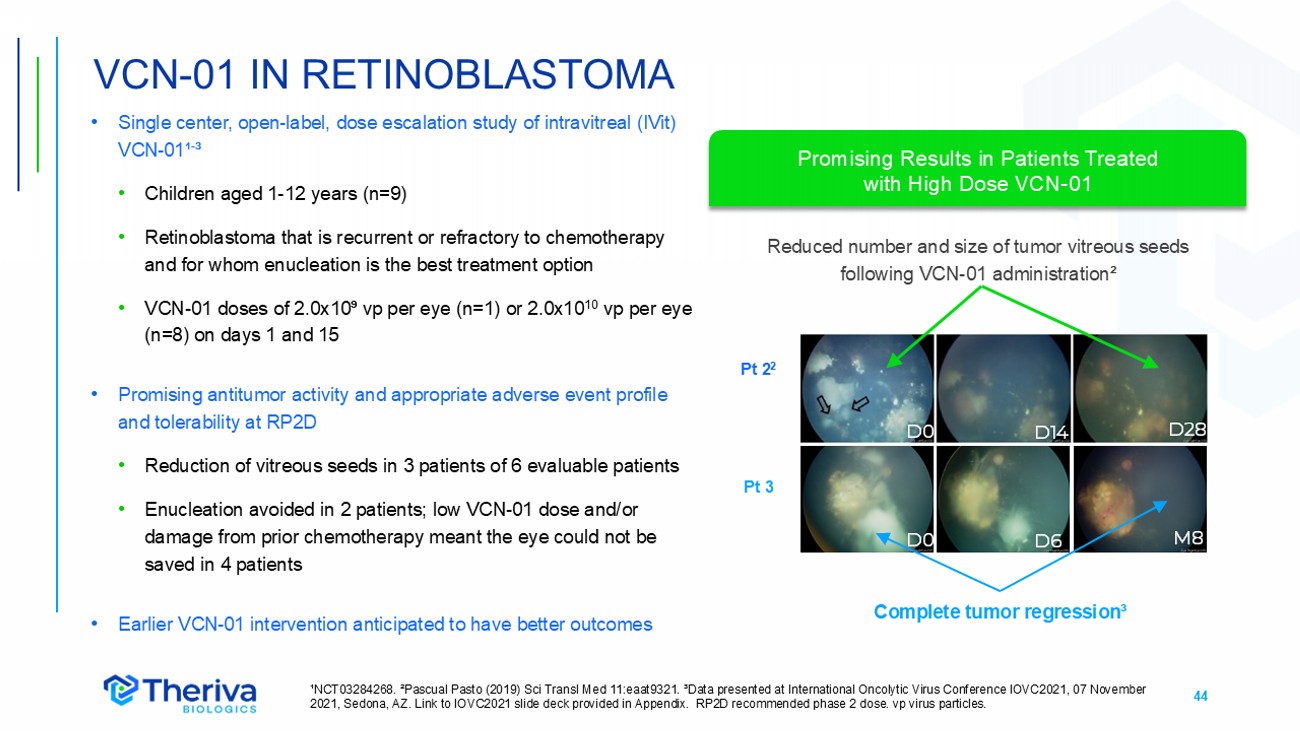
44 • Single center, open - label, dose escalation study of intravitreal (IVit) VCN - 01¹ - ³ • Children aged 1 - 12 years (n=9) • Retinoblastoma that is recurrent or refractory to chemotherapy and for whom enucleation is the best treatment option • VCN - 01 doses of 2.0x10⁹ vp per eye (n=1) or 2.0x10 10 vp per eye (n=8) on days 1 and 15 • Promising antitumor activity and appropriate adverse event profile and tolerability at RP2D • Reduction of vitreous seeds in 3 patients of 6 evaluable patients • Enucleation avoided in 2 patients; low VCN - 01 dose and/or damage from prior chemotherapy meant the eye could not be saved in 4 patients • Earlier VCN - 01 intervention anticipated to have better outcomes VCN - 01 IN RETINOBLASTOMA ¹NCT03284268. ²Pascual Pasto (2019) Sci Transl Med 11:eaat9321. ³Data presented at International Oncolytic Virus Conference IOVC2021, 07 November 2021, Sedona, AZ. Link to IOVC2021 slide deck provided in Appendix. RP2D recommended phase 2 dose. vp virus particles. Pt 2 2 Pt 3 Reduced number and size of tumor vitreous seeds following VCN - 01 administration² Complete tumor regression³ D14 D28 D0 D6 4 M8 D0 Promising Results in Patients Treated with High Dose VCN - 01
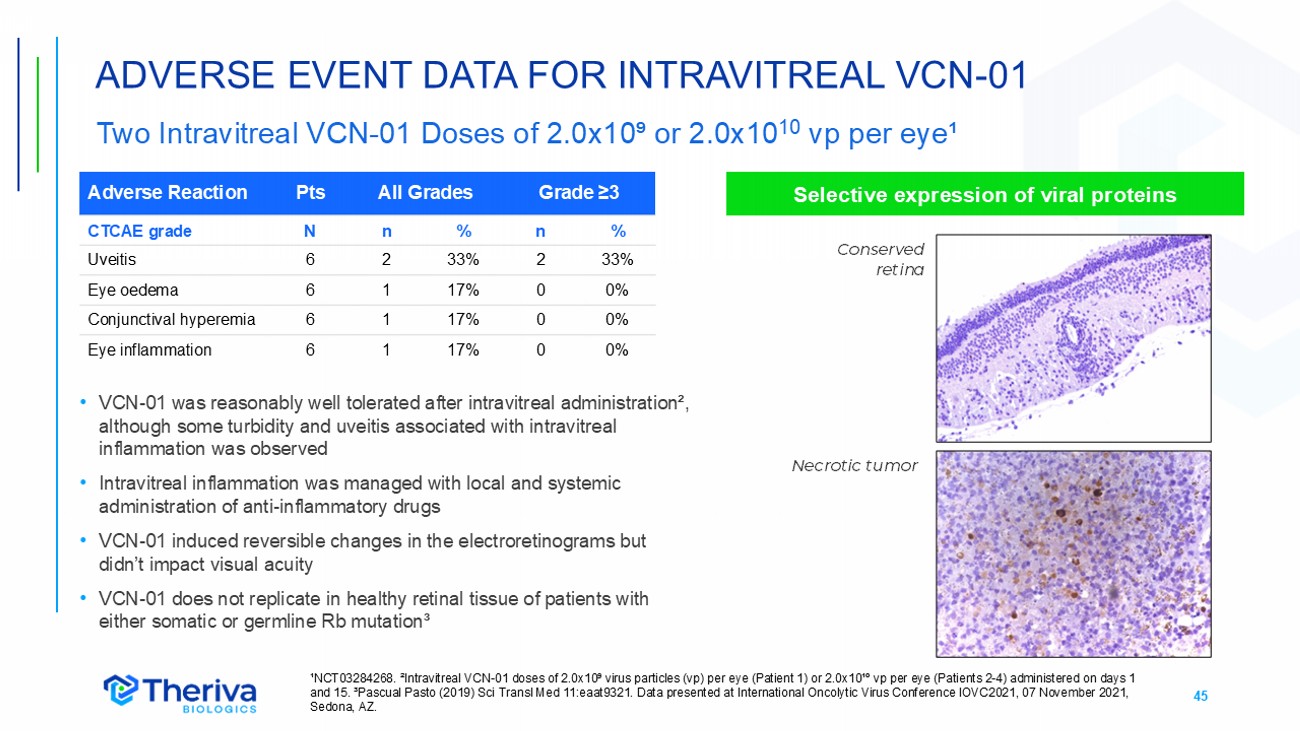
45 ADVERSE EVENT DATA FOR INTRAVITREAL VCN - 01 Two Intravitreal VCN - 01 Doses of 2.0x10⁹ or 2.0x10 10 vp per eye¹ ¹NCT03284268. ²Intravitreal VCN - 01 doses of 2.0x10⁹ virus particles (vp) per eye (Patient 1) or 2.0x10¹º vp per eye (Patients 2 - 4) administered on days 1 and 15. ³Pascual Pasto (2019) Sci Transl Med 11:eaat9321. Data presented at International Oncolytic Virus Conference IOVC2021, 07 November 2021, Sedona, AZ. Grade ≥3 All Grades Pts Adverse Reaction % n % n N CTCAE grade 33% 2 33% 2 6 Uveitis 0% 0 17% 1 6 Eye oedema 0% 0 17% 1 6 Conjunctival hyperemia 0% 0 17% 1 6 Eye inflammation • VCN - 01 was reasonably well tolerated after intravitreal administration², although some turbidity and uveitis associated with intravitreal inflammation was observed • Intravitreal inflammation was managed with local and systemic administration of anti - inflammatory drugs • VCN - 01 induced reversible changes in the electroretinograms but didn’t impact visual acuity • VCN - 01 does not replicate in healthy retinal tissue of patients with either somatic or germline Rb mutation³ Selective expression of viral proteins Necrotic tumor Conserved retina

46 • Phase 1 ISS Completed H1 2024 • Initial data demonstrate acceptable adverse event profile and one durable complete response • Developing a clinical protocol for an open - label, multinational study • Refractory retinoblastoma patients with vitreous seeds • IVit VCN - 01 in combination with topotecan • PI Dr. Guillermo Chantada, MD PhD ¹ • Status • US and EU Orphan Drug Designation • Pre - IND meeting with FDA completed Q4 2023 • Rare Pediatric Disease Designation (potential eligibility for Priority Review Voucher) VCN - 01 DEVELOPMENT IN RETINOBLASTOMA ¹Principal Researcher, Associate Physician, Hospital Sant Joan de Déu, Barcelona, Spain; Scientific Director, Pediatric Hemato - Oncology Service of the Hospital Universitario Austral, Argentina

VCN - X NEXT GENERATION OV DISCOVERY PLATFORM
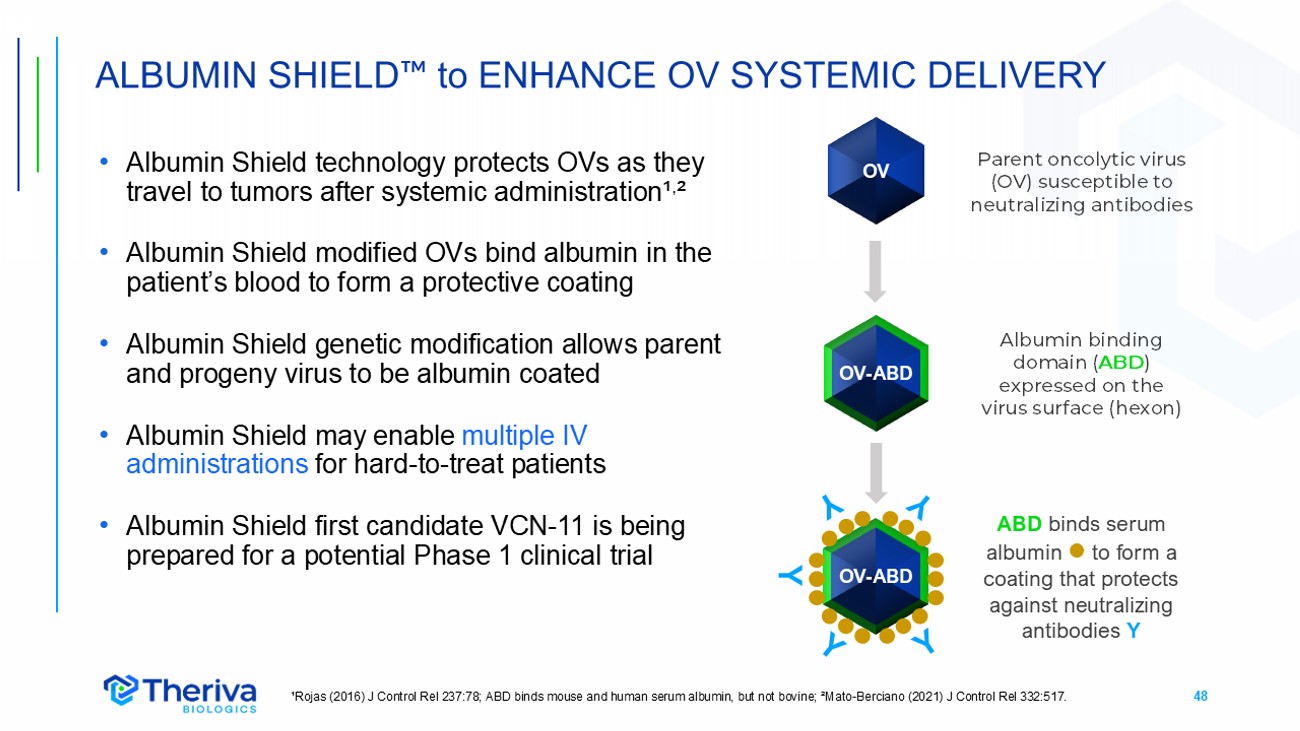
48 OV - ABD • Albumin Shield technology protects OVs as they travel to tumors after systemic administration¹ , ² • Albumin Shield modified OVs bind albumin in the patient’s blood to form a protective coating • Albumin Shield genetic modification allows parent and progeny virus to be albumin coated • Albumin Shield may enable multiple IV administrations for hard - to - treat patients • Albumin Shield first candidate VCN - 11 is being prepared for a potential Phase 1 clinical trial ALBUMIN SHIELD to ENHANCE OV SYSTEMIC DELIVERY ¹Rojas (2016) J Control Rel 237:78; ABD binds mouse and human serum albumin, but not bovine; ²Mato - Berciano (2021) J Control Rel 332:517. Albumin binding domain ( ABD ) expressed on the virus surface ( hexon ) Y ABD binds serum albumin to form a coating that protects against neutralizing antibodies Y Parent oncolytic virus (OV) susceptible to neutralizing antibodies OV - ABD OV

BIBLIOGRAPHY

50 Bayo - Puxan N et al. (2006) Role of the putative heparan sulfate glycosaminoglycan - binding site of the adenovirus type 5 fiber sh aft on liver detargeting and knob - mediated retargeting. J Gen Virol 87:2487 – 2495 Bazan - Peregrino M et al. (2021) VCN - 01 disrupts pancreatic cancer stroma and exerts antitumor effects. J ImmunoTher Cancer 9:e00 3254. Garcia - Carbonero R et al. (2019) Poster 5185: Systemic administration of the hyaluronidase - expressing oncolytic adenovirus VCN - 01 in patients with advanced or metastatic pancreatic cancer: first - in - human clinical trial. European Society for Molecular Oncology conference ESMO, 29 Septemb er 2019. Garcia - Carbonero R et al. (2022) A phase I, multicenter, open - label study of intravenous VCN - 01 oncolytic adenovirus with or without nab - paclitaxel plus gemcita bine in patients with advanced solid tumors J ImmunoTher Cancer 10:e003255 Garcia - Carbonero R et al. (2024) VIRAGE: A phase IIb, open - label, randomized study of nab - paclitaxel and gemcitabine plus/minus VCN - 01 in patients with metastatic pancreatic cancer. J Clin Oncol 42S:TPS4210. Guedan S et al. (2010) Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor gro wt h. Mol Ther 18:1275 – 1283 Hidalgo M et al. (2019) Poster 5465: Proof of concept clinical study by EUS - guided intratumor injection of VCN - 01, an oncolytic adenovirus expressing hyaluronidase i n patients with pancreatic cancer. European Society for Molecular Oncology conference ESMO, 28 September 2019. Jove M et al. (2022) Poster 1231P: Phase I study to evaluate the safety, tolerability, and efficacy of VCN - 01 in combination wit h durvalumab (MEDI4736) in subjects with recurrent/metastatic squamous cell carcinoma of the head and neck (R/M HNSCC) Ann Oncol. 33:S1112. European Society for Mol ecular Oncology conference ESMO 2022, 10 September 2022 Kiyokawa M et al. (2021) Modification of extracellular matrix enhances oncolytic adenovirus Immunotherapy in glioblastoma . Clin Cancer Res 27:889 - 902 Martínez - Vélez N et al. (2019) The oncolytic adenovirus VCN - 01 as therapeutic approach against pediatric osteosarcoma. Clin Canc er Res 22:2217 - 25 Mato - Berciano A et al. (2021) Oncolytic adenovirus with hyaluronidase activity that evades neutralizing antibodies: VCN - 11. J C ontrol Rel 332:517 - 528 Pascual Pasto G et al. (2019) Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN - 01. S ci Transl Med 11:eaat9321 Pascual - Pasto G et al. (2021) Presentation: VCN - 01 is an encouraging therapy against retinoblastoma. International Oncolytic Virus Conference IOVC2021, 07 November 2021, Sedona, AZ. Rodríguez - García A et al. (2015) Safety and efficacy of VCN - 01, an oncolytic adenovirus combining fiber HSG - binding domain repla cement with RGD and hyaluronidase expression. Clin Cancer Res 21:1406 - 18 Rojas J et al. (2012) Improved systemic antitumor therapy with oncolytic adenoviruses by replacing the fiber shaft HSG - binding d omain with RGD. Gene Ther 19:453 – 457 Rojas J et al. (2010) Minimal RB - responsive E1A promoter modification to attain potency, selectivity, and transgene - arming capac ity in oncolytic adenoviruses. 2010) Mol Ther 18:1960 – 1971 Rojas L et al. (2016) Albumin - binding adenoviruses circumvent pre - existing neutralizing antibodies upon systemic delivery. J Con trol Rel 237:78 – 88 THERIVA ONCOLYTIC VIRUSES KEY PUBLICATIONS
51 DESCRIPTION, CLASSIFICATION, STAGING, STROMA Balachandran VP et al. (2019) Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gast roe nterology 156:2056 - 72 Christenson ES et al. (2020) Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a br igh t future. Lancet Oncol 21:e135 - e145 Orth M et al. (2019) Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combi ned modality treatment approaches. Radiation Oncol 14:141 Placencio - Hickok VR et al. (2022) Hyaluronan heterogeneity in pancreatic ductal adenocarcinoma: primary tumors compared to sites of met as tasis. Pancreatology 22:92 - 97 Sarantis P et al. (2020) Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol 12:173 - 181 Tahkola K et al. (2021) Stromal hyaluronan accumulation is associated with low immune response and poor prognosis in pancreat ic cancer. Sci Rep 11:12216 Yu J et al. (2015) Time to progression of pancreatic ductal adenocarcinoma from low - to - high tumour stages. Gut 64:1783 - 9 INCIDENCE Bengtsson A et al. (2020) The actual 5 - year survivors of pancreatic ductal adenocarcinoma based on real - world data. Sci Rep 10:1 6425. Carioli G et al. (2021) European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann Oncol 32:478. da Costa WL et al. (2020) Trends in the incidence of pancreatic adenocarcinoma in all 50 United States examined through an ag e - p eriod - cohort analysis. JNCI Cancer Spectrum 4:pkaa033 GLOBOCAN International 2020 survey of persons 0 - 74 years. https://gco.iarc.fr/today/data/factsheets/cancers/13 - Pancreas - fact - sheet.pdf Michael N et al. (2019) Timing of palliative care referral and aggressive cancer care toward the end - of - life in pancreatic cance r: a retrospective, single - center observational study. BMC Palliat Care 18 :1 3. Sung H et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 18 5 Countries. CA Cancer J Clin 71:209 – 249 Ushio J et al. (2021) Pancreatic ductal adenocarcinoma: epidemiology and risk factors. Diagnostics 11:562 TREATMENT Dotan E et al. (2025) Effect of baseline geriatric and quality of life assessments on treatment outcomes in ECOG - ACRIN EA2186 (G IANT): A randomized phase II study of gemcitabine and nab - paclitaxel compared with 5 - fluorouracil, leucovorin, and liposomal irinotecan in older patients with treatment - naïve met astatic pancreatic cancer. J Clin Oncol 43S:676 Conroy T et al. (2011) FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med 364:1817 - 25. Elsayed M et al. (2021) The latest advancement in pancreatic ductal adenocarcinoma therapy: a review article for the latest guideline s and novel therapies. Biomedicines 9:389 Tempero MA et al. (2021) NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma, V2.2021. J Natl Compr Canc Netw 19:439 - 457 Toesca DAS et al. (2018) Management of borderline resectable pancreatic cancer. Int J Radiation Oncol Biol Phys 100:1155 - 74 Vogel A et al. (2016) Efficacy and safety profile of nab - paclitaxel plus gemcitabine in patients with metastatic pancreatic canc er treated to disease progression: a subanalysis from a phase 3 trial (MPACT). BMC Cancer16:817 Von Hoff DD et al. (2013) Increased survival in pancreatic cancer with nab - paclitaxel plus gemcitabine. N Engl J Med 369:1691 - 70 3 Wainberg ZA et al. (2023) NALIRIFOX versus nab - paclitaxel and gemcitabine in treatment - naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open - label, phase 3 trial. Lancet 402:1272 PANCREATIC CANCER REFERENCES

52 DESCRIPTION, CLASSIFICATION, STAGING American Academy of Ophthalmology. EyeWiki ®. Retinoblastoma. https://eyewiki.org/Retinoblastoma American Cancer Society. Key statistics for retinoblastoma. https://www.cancer.org/cancer/retinoblastoma/about/key - statistics.html Canturk S et al. (2010) Survival of retinoblastoma in less - developed countries impact of socioeconomic and health - related indicators. B r J Ophthalmol 94:1432 - 6 Fabian ID et al. (2018) Classification and staging of retinoblastoma. Community Eye Health 31:11 - 13 Fabian ID et al. (2020) Global retinoblastoma presentation and analysis by national income level. JAMA Oncol 6:685 Tomar AS et al. (2020) Multicenter, international collaborative study for American Joint Committee on Cancer Staging of Retinoblast om a/ Part I: metastasis - associated mortality. Ophthalmology 127:1719 - 32 INCIDENCE One Retinoblastoma World Map. https://map.1rbw.org/ (accessed April - November 2021) Stacey AW et al. (2021) Incidence of retinoblastoma has increased: results from 40 European countries. Ophthalmology 128:1369 - 71 TREATMENT Abramson DH et al. (2015) Advanced unilateral retinoblastoma: the impact of ophthalmic artery chemosurgery on enucleation rat e a nd patient survival at MSKCC. PLoS ONE 10:e0145436 Ancona - Lezama D et al. (2020) Modern treatment of retinoblastoma: a 2020 review. Indian J Ophthalmol 68:2356 - 65 Tomar AS et al. (2021) Global retinoblastoma treatment outcomes. Association with national income level. 128:740 - 53 RETINOBLASTOMA (Rb) REFERENCES 52

53 CG Oncology CG0070 ( cretostimogene grenadenorepvec ) https://cgoncology.com Ramesh N et al. (2006) CG0070, a conditionally r eplicating granulocyte macrophage c olony - stimulating factor - armed o ncolytic a denovirus for the treatment of bl adder c ancer. Clin Cancer Res 12:305 Svatek RS et al. (2024) PIVOT - 006: A Phase 3, Randomized Study of cretostimogene grenadenorepvec versus Observation for the Treatment of Intermediate Risk NMIBC Following TURBT. Abstract TPS715. Presentation at ASCO Genitourinary Symposium 2024. J Clin Oncol 42: TPS 715 Tyson M et al. (2023) First Results from BOND - 003: Phase 3 study of cretostimogene grenadenorepvec Monotherapy for Patients with BCG Unresponsive High - Risk NMIBC with CIS +/ - Papillary (Ta/T1) Tumors. Presentation at Society of Urologic Oncology Annual Meeting S UO 2023. Uchio EM et al. A phase 3, single - arm study of CG0070 in subjects with non - muscle invasive bladder cancer (NMIBC) unresponsive to Bac illus Calmette - Guerin (BCG). J Clin Oncol 40:TPS598 Genelux Corporation Olvi - Vec (GL - ONC1, GLV - 1h68, olvimulogene nanivacirepvec ) https://genelux.com Clinicaltrials.gov NCT05281471: Efficacy & s afety of Olvi - Vec and platinum - doublet + bevacizumab c ompared to pl atinum - doublet + bevacizumab in platinum - resistant/refractory o varian c ancer ( OnPrime , GOG - 3076) Holloway RW et al. (2023) Clinical activity of olvimulogene nanivacirepvec – primed immunochemotherapy in heavily pretreated Patients With Platinum - Resistant or Platinum - Refractory Ovarian Cancer. The Nonrandomized Phase 2 VIRO - 15 Clinical Trial. JAMA Oncol. 9:903 Lin D et al. (2023) Oncolytic virotherapy: basic principles, recent advances and future directions. Signal Transduct Target Ther. 8:156 Mell LK et al. (2017) Phase I trial of Intravenous oncolytic vaccinia virus (GL - ONC1) with cisplatin and radiotherapy in patient s with locoregionally advanced head and neck carcinoma. Clin Cancer Res 23:5696 Zhang Q et al. (2007) Eradication of solid h uman b reast t umors in nude m ice with an intravenously i njected l ight - emitting o ncolytic v accinia v irus. Cancer Res 67:10038 OV COMPANY REFERENCES 53

54 Oncolytics Biotech: Pelareorep (formerly Reolysin ®) https://oncolyticsbiotech.com Arnold D et al. Pelareorep (pela) + atezolizumab ( atezo ) and chemotherapy in first - line (1L) advanced or metastatic pancreatic ductal adenocarcinoma (PDAC) patients – Results from the GOBLET study. Poster presentation at the European Society for Molecular Oncology Annual Congr ess ESMO 2023. Clements D et al. (2014) Reovirus in cancer therapy: an evidence - based review. Oncolytic Virother 3:69 Lin D et al. (2023) Oncolytic virotherapy: basic principles, recent advances and future directions. Signal Transduct Target Ther. 8:156 Philips MB et al. (2018) Current understanding of reovirus oncolysis mechanisms Oncolytic Virother 7:53 Xie R et al. (2023) Effectiveness and safety of pelareorep plus chemotherapy versus chemotherapy alone for advanced solid tumors: a meta - analysis. Front Pharmacol 14:1228225 Replimune : RP1,RP2 ( vusolimogene oderparepvec ) https://replimune.com Chmielowski et al. (2023) Initial efficacy and safety of RP1 + nivolumab in patients with anti – PD1 – failed melanoma from the ongo ing phase 1/2 IGNYTE study. Abstract 9609. Poster presentation American Society of Clinical Oncologists Annual Meeting ASCO 2023. J Clin Oncol 41: 950 9 Sacco JJ et al. (2023) Preliminary safety and efficacy results from an open - label, multicenter, phase 1 study of RP2 as a single agent and in combination with nivolumab in a cohort of patients with uveal melanoma. Presentation at the International Congress of the Society for Mel ano ma Research SMR 2023. Thomas S et al. (2019) Development of a new fusion - enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J ImmunoTher Cancer 7:214 OV COMPANY REFERENCES 54