

.2 Phase 2b REZOLVE-AA Topline Results from 36-Week Induction Treatment Period Rezpegaldesleukin in Patients with Severe-to-Very-Severe Alopecia Areata December 16, 2025

Forward-Looking Statements Safe Harbor Statement This presentation and any accompanying oral discussion contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding Nektar Therapeutics (the “Company” or “Nektar”)’s plans, progress, and timing relating to the Company’s rezpegaldesleukin program in alopecia areata, timing for the 52-week extension data from the Phase 2b REZOLVE-AA (alopecia areata) trial, timing for the 52-week maintenance data and 52-week off drug durability data from the Phase 2b REZOLVE-AD (atopic dermatitis) trial, and the presentation of data, rezpegaldesleukin’s potential to be a first-in-class T regulatory cell therapy, the potential market opportunity in alopecia areata and high unmet need for a new mechanism of action, the Company’s current and future research and development plans or expectations, the structure, timing and success of the Company’s planned clinical trials, the potential benefits of any of the Company’s current or future product candidates in treating patients, and the Company’s goals and strategy. Nektar intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan,” “on track,” or similar expressions or the negative of those terms. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. The express or implied forward- looking statements included in this presentation are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation: risks related to the success, cost, and timing of the Company’s development activities and clinical trials, risks related to the Company’s dependence on the success of rezpegaldesleukin, the outcomes of competitive immunotherapy clinical trials, significant competition for the Company’s product candidates, the risk that preliminary and interim data from the Company’s clinical studies are subject to audit and verification procedures that could result in material changes in the final data and may change as more patient data become available, risks related to delays in clinical trials, risks related to dependence on third parties to conduct clinical trials, risks regarding future capital requirements, risks related to dependence on the Company’s collaboration agreements, risks related to the Company’s reliance on contract manufacturers and suppliers, risks related to obtaining regulatory approval for the Company’s drug candidates, risks related to the Company’s ability to protect and maintain its intellectual property position, risks related to legal proceedings and related litigation costs and liabilities and other risk factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Nektar’s most recent Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q and any other filings that Nektar has made or may make with the U.S. Securities and Exchange Commission in the future. Any forward-looking statements contained in this presentation represent Nektar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, Nektar explicitly disclaims any obligation to update any forward-looking statements. Certain information contained in this presentation may be derived from information provided by industry sources. The Company believes such information is accurate and that the sources from which it has been obtained are reliable. However, the Company cannot guarantee the accuracy of, and has not independently verified, such information. 2
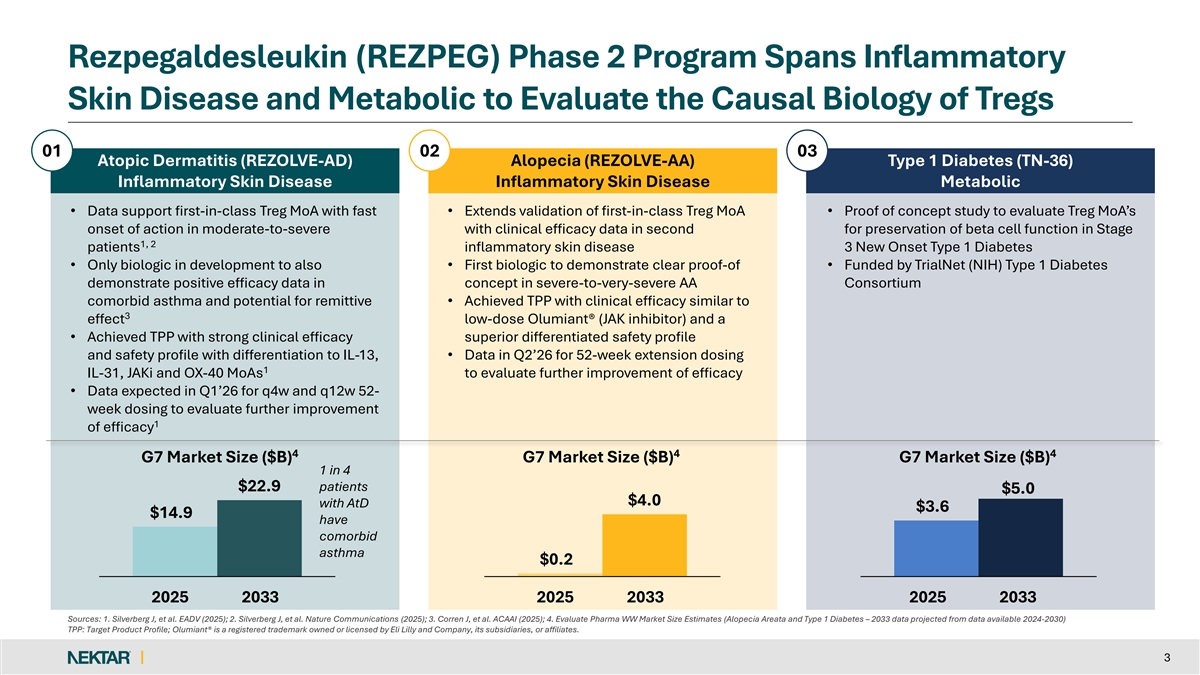
Rezpegaldesleukin (REZPEG) Phase 2 Program Spans Inflammatory Skin Disease and Metabolic to Evaluate the Causal Biology of Tregs 01 02 03 Atopic Dermatitis (REZOLVE-AD) Alopecia (REZOLVE-AA) Type 1 Diabetes (TN-36) Inflammatory Skin Disease Inflammatory Skin Disease Metabolic • Data support first-in-class Treg MoA with fast • Extends validation of first-in-class Treg MoA • Proof of concept study to evaluate Treg MoA’s onset of action in moderate-to-severe with clinical efficacy data in second for preservation of beta cell function in Stage 1, 2 patients inflammatory skin disease 3 New Onset Type 1 Diabetes • Only biologic in development to also • First biologic to demonstrate clear proof-of • Funded by TrialNet (NIH) Type 1 Diabetes demonstrate positive efficacy data in concept in severe-to-very-severe AA Consortium comorbid asthma and potential for remittive • Achieved TPP with clinical efficacy similar to 3 effect low-dose Olumiant® (JAK inhibitor) and a • Achieved TPP with strong clinical efficacy superior differentiated safety profile and safety profile with differentiation to IL-13, • Data in Q2’26 for 52-week extension dosing 1 IL-31, JAKi and OX-40 MoAs to evaluate further improvement of efficacy • Data expected in Q1’26 for q4w and q12w 52- week dosing to evaluate further improvement 1 of efficacy 4 4 4 G7 Market Size ($B) G7 Market Size ($B) G7 Market Size ($B) 1 in 4 $22.9 patients $5.0 $4.0 with AtD $3.6 $14.9 have comorbid asthma $0.2 2025 2033 2025 2033 2025 2033 Sources: 1. Silverberg J, et al. EADV (2025); 2. Silverberg J, et al. Nature Communications (2025); 3. Corren J, et al. ACAAI (2025); 4. Evaluate Pharma WW Market Size Estimates (Alopecia Areata and Type 1 Diabetes – 2033 data projected from data available 2024-2030) TPP: Target Product Profile; Olumiant® is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates. 3
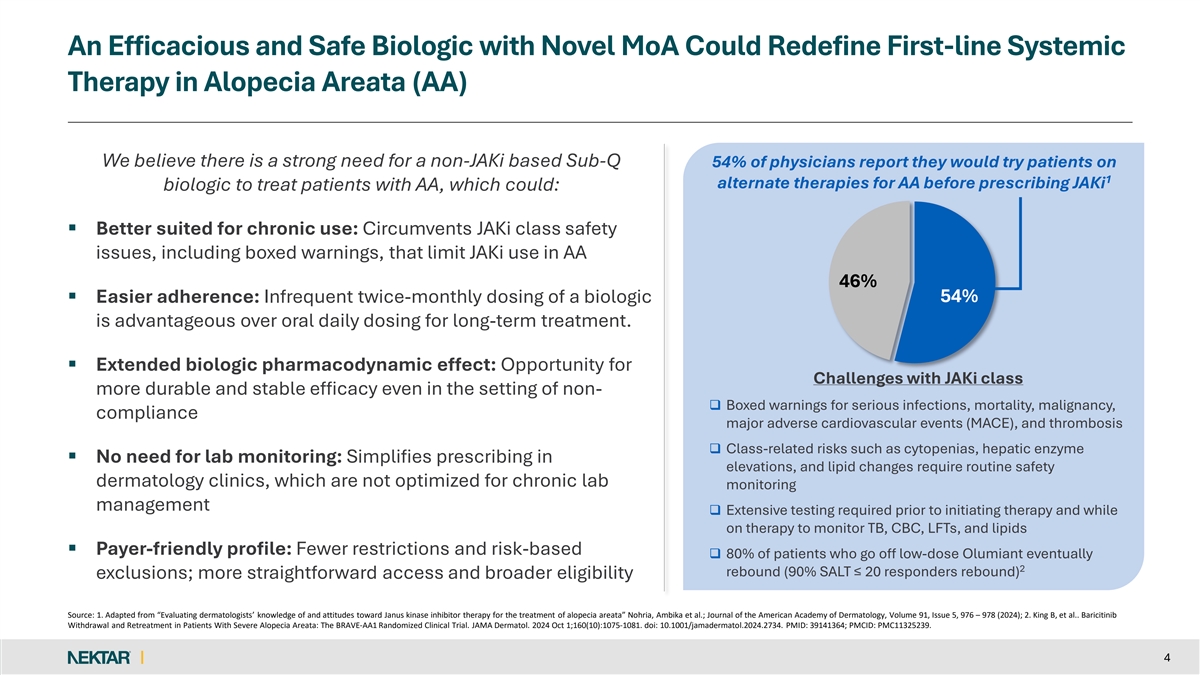
An Efficacious and Safe Biologic with Novel MoA Could Redefine First-line Systemic Therapy in Alopecia Areata (AA) We believe there is a strong need for a non-JAKi based Sub-Q 54% of physicians report they would try patients on 1 alternate therapies for AA before prescribing JAKi biologic to treat patients with AA, which could: § Better suited for chronic use: Circumvents JAKi class safety issues, including boxed warnings, that limit JAKi use in AA 46% § Easier adherence: Infrequent twice-monthly dosing of a biologic 54% is advantageous over oral daily dosing for long-term treatment. § Extended biologic pharmacodynamic effect: Opportunity for Challenges with JAKi class more durable and stable efficacy even in the setting of non- q Boxed warnings for serious infections, mortality, malignancy, compliance major adverse cardiovascular events (MACE), and thrombosis q Class-related risks such as cytopenias, hepatic enzyme § No need for lab monitoring: Simplifies prescribing in elevations, and lipid changes require routine safety dermatology clinics, which are not optimized for chronic lab monitoring management q Extensive testing required prior to initiating therapy and while on therapy to monitor TB, CBC, LFTs, and lipids § Payer-friendly profile: Fewer restrictions and risk-based q 80% of patients who go off low-dose Olumiant eventually 2 rebound (90% SALT ≤ 20 responders rebound) exclusions; more straightforward access and broader eligibility Source: 1. Adapted from “Evaluating dermatologists’ knowledge of and attitudes toward Janus kinase inhibitor therapy for the treatment of alopecia areata” Nohria, Ambika et al.; Journal of the American Academy of Dermatology, Volume 91, Issue 5, 976 – 978 (2024); 2. King B, et al.. Baricitinib Withdrawal and Retreatment in Patients With Severe Alopecia Areata: The BRAVE-AA1 Randomized Clinical Trial. JAMA Dermatol. 2024 Oct 1;160(10):1075-1081. doi: 10.1001/jamadermatol.2024.2734. PMID: 39141364; PMCID: PMC11325239. 4
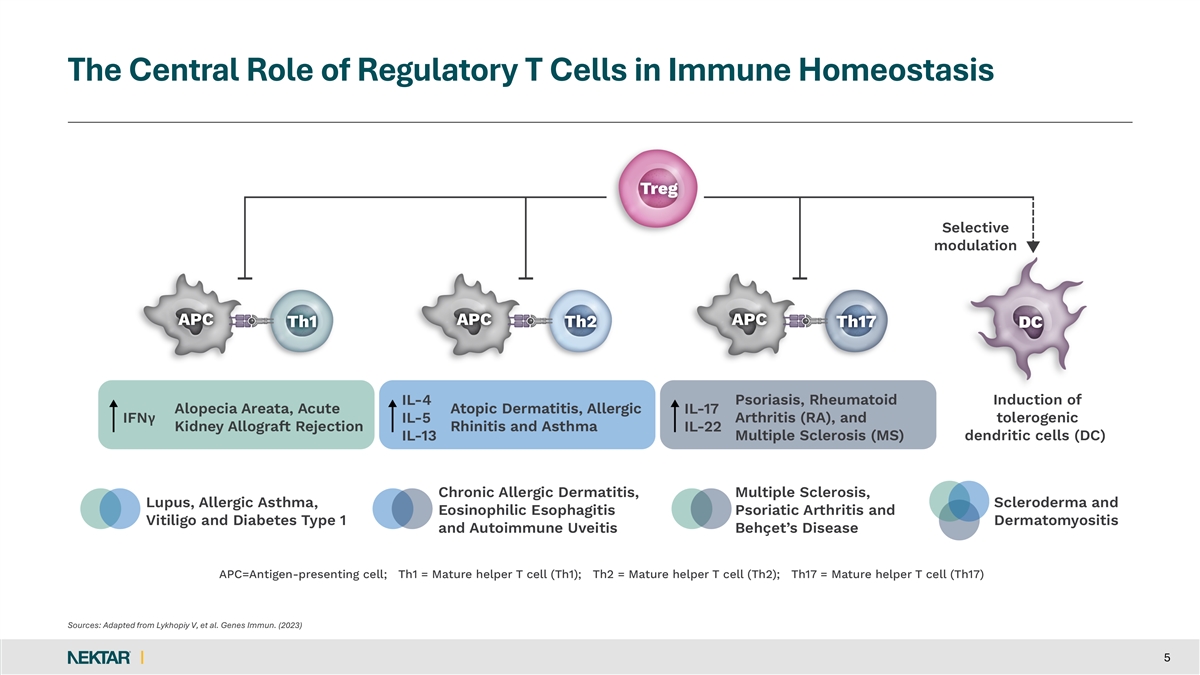
The Central Role of Regulatory T Cells in Immune Homeostasis Sources: Adapted from Lykhopiy V, et al. Genes Immun. (2023) 5
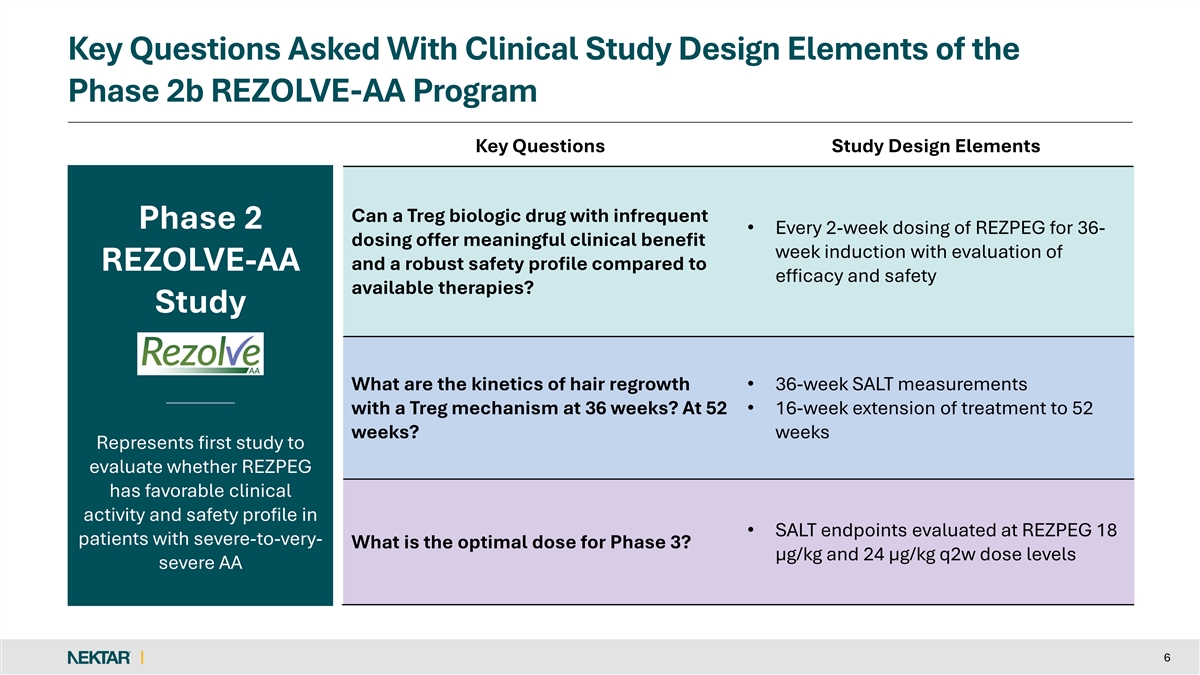
Key Questions Asked With Clinical Study Design Elements of the Phase 2b REZOLVE-AA Program Key Questions Study Design Elements Can a Treg biologic drug with infrequent Phase 2 • Every 2-week dosing of REZPEG for 36- dosing offer meaningful clinical benefit week induction with evaluation of and a robust safety profile compared to REZOLVE-AA efficacy and safety available therapies? Study What are the kinetics of hair regrowth • 36-week SALT measurements with a Treg mechanism at 36 weeks? At 52 • 16-week extension of treatment to 52 weeks? weeks Represents first study to evaluate whether REZPEG has favorable clinical activity and safety profile in • SALT endpoints evaluated at REZPEG 18 patients with severe-to-very- What is the optimal dose for Phase 3? µg/kg and 24 µg/kg q2w dose levels severe AA 6
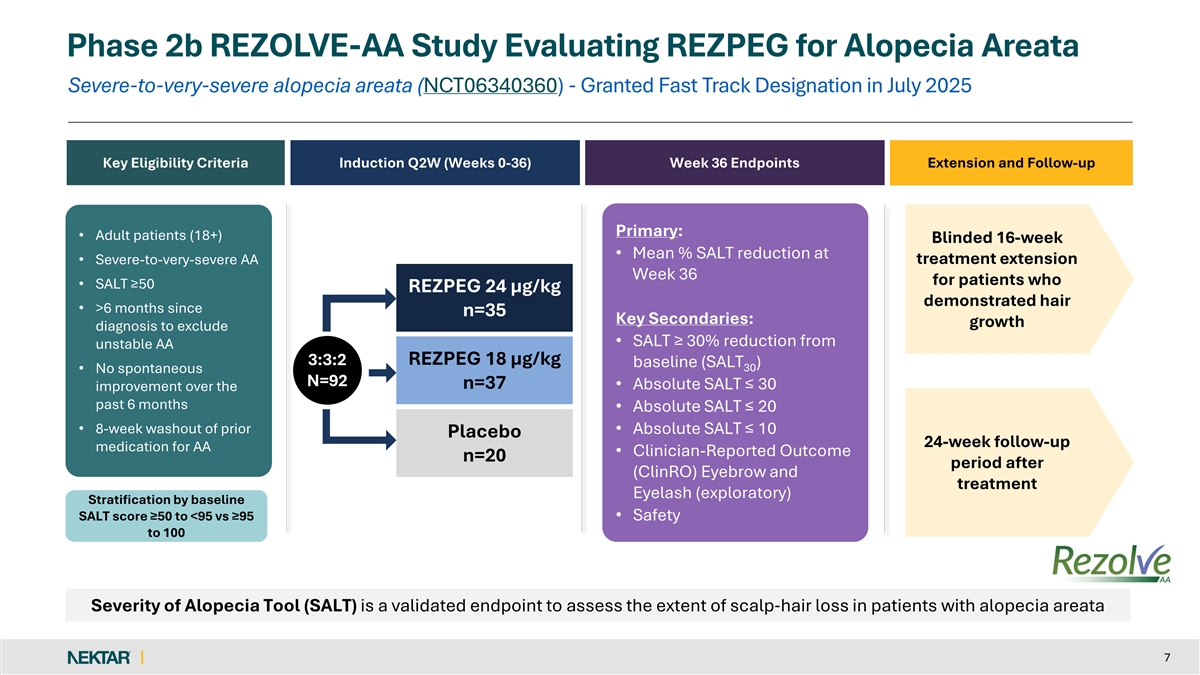
Phase 2b REZOLVE-AA Study Evaluating REZPEG for Alopecia Areata Severe-to-very-severe alopecia areata (NCT06340360) - Granted Fast Track Designation in July 2025 Key Eligibility Criteria Induction Q2W (Weeks 0-36) Week 36 Endpoints Extension and Follow-up Primary: • Adult patients (18+) Blinded 16-week • Mean % SALT reduction at treatment extension • Severe-to-very-severe AA Week 36 for patients who • SALT ≥50 REZPEG 24 µg/kg demonstrated hair • >6 months since n=35 Key Secondaries: growth diagnosis to exclude • SALT ≥ 30% reduction from unstable AA 3:3:2 REZPEG 18 µg/kg baseline (SALT ) 30 • No spontaneous N=92 n=37 • Absolute SALT ≤ 30 improvement over the past 6 months • Absolute SALT ≤ 20 • 8-week washout of prior • Absolute SALT ≤ 10 Placebo 24-week follow-up medication for AA • Clinician-Reported Outcome n=20 period after (ClinRO) Eyebrow and treatment Eyelash (exploratory) Stratification by baseline SALT score ≥50 to <95 vs ≥95 • Safety to 100 Severity of Alopecia Tool (SALT) is a validated endpoint to assess the extent of scalp‐ hair loss in patients with alopecia areata 7
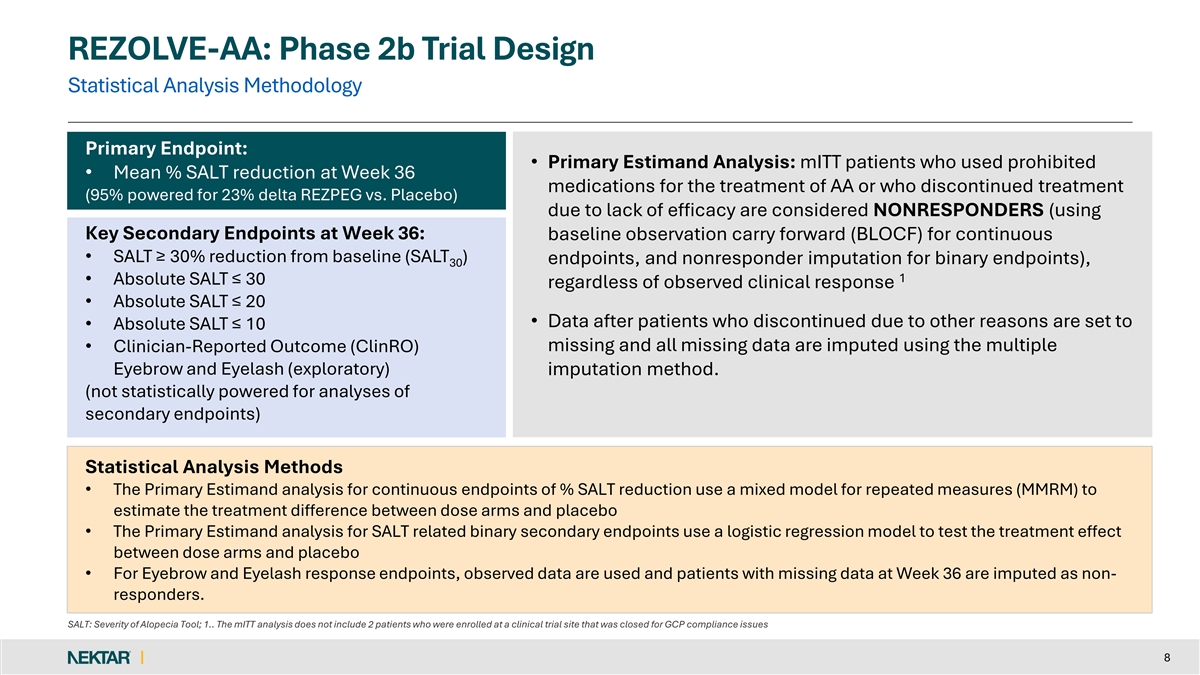
REZOLVE-AA: Phase 2b Trial Design Statistical Analysis Methodology Primary Endpoint: • Primary Estimand Analysis: mITT patients who used prohibited • Mean % SALT reduction at Week 36 medications for the treatment of AA or who discontinued treatment (95% powered for 23% delta REZPEG vs. Placebo) due to lack of efficacy are considered NONRESPONDERS (using Key Secondary Endpoints at Week 36: baseline observation carry forward (BLOCF) for continuous • SALT ≥ 30% reduction from baseline (SALT ) endpoints, and nonresponder imputation for binary endpoints), 30 1 • Absolute SALT ≤ 30 regardless of observed clinical response • Absolute SALT ≤ 20 • Data after patients who discontinued due to other reasons are set to • Absolute SALT ≤ 10 missing and all missing data are imputed using the multiple • Clinician-Reported Outcome (ClinRO) Eyebrow and Eyelash (exploratory) imputation method. (not statistically powered for analyses of secondary endpoints) Statistical Analysis Methods • The Primary Estimand analysis for continuous endpoints of % SALT reduction use a mixed model for repeated measures (MMRM) to estimate the treatment difference between dose arms and placebo • The Primary Estimand analysis for SALT related binary secondary endpoints use a logistic regression model to test the treatment effect between dose arms and placebo • For Eyebrow and Eyelash response endpoints, observed data are used and patients with missing data at Week 36 are imputed as non- responders. SALT: Severity of Alopecia Tool; 1.. The mITT analysis does not include 2 patients who were enrolled at a clinical trial site that was closed for GCP compliance issues 8
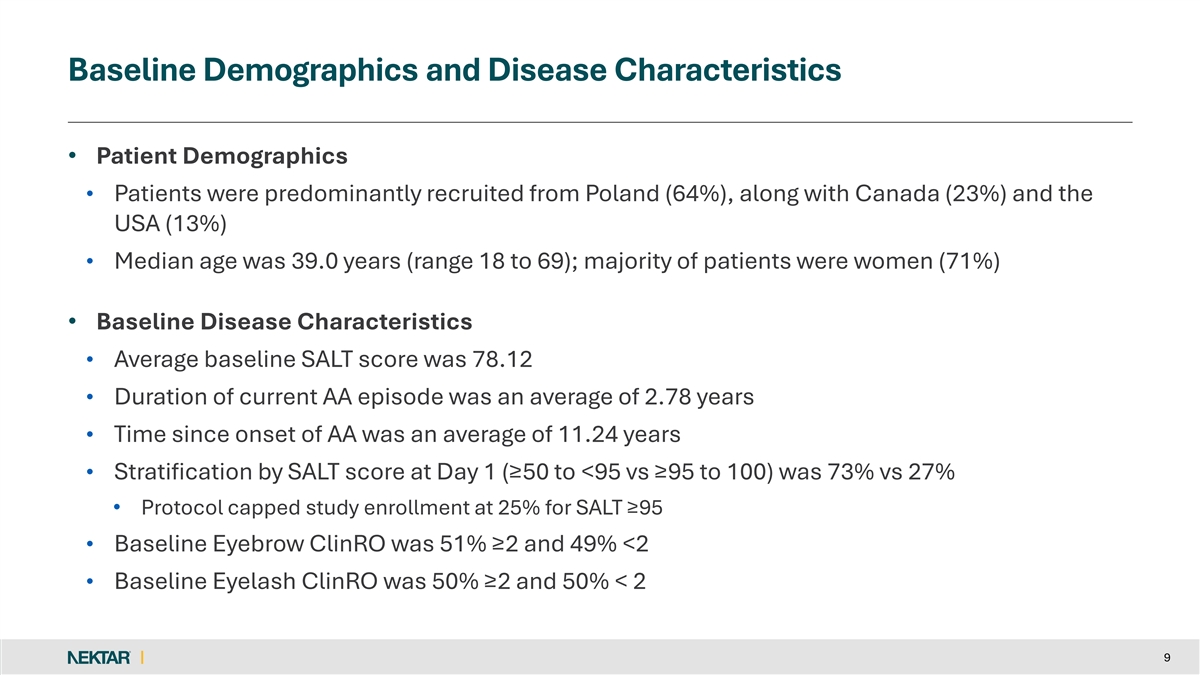
Baseline Demographics and Disease Characteristics • Patient Demographics • Patients were predominantly recruited from Poland (64%), along with Canada (23%) and the USA (13%) • Median age was 39.0 years (range 18 to 69); majority of patients were women (71%) • Baseline Disease Characteristics • Average baseline SALT score was 78.12 • Duration of current AA episode was an average of 2.78 years • Time since onset of AA was an average of 11.24 years • Stratification by SALT score at Day 1 (≥50 to <95 vs ≥95 to 100) was 73% vs 27% • Protocol capped study enrollment at 25% for SALT ≥95 • Baseline Eyebrow ClinRO was 51% ≥2 and 49% <2 • Baseline Eyelash ClinRO was 50% ≥2 and 50% < 2 9
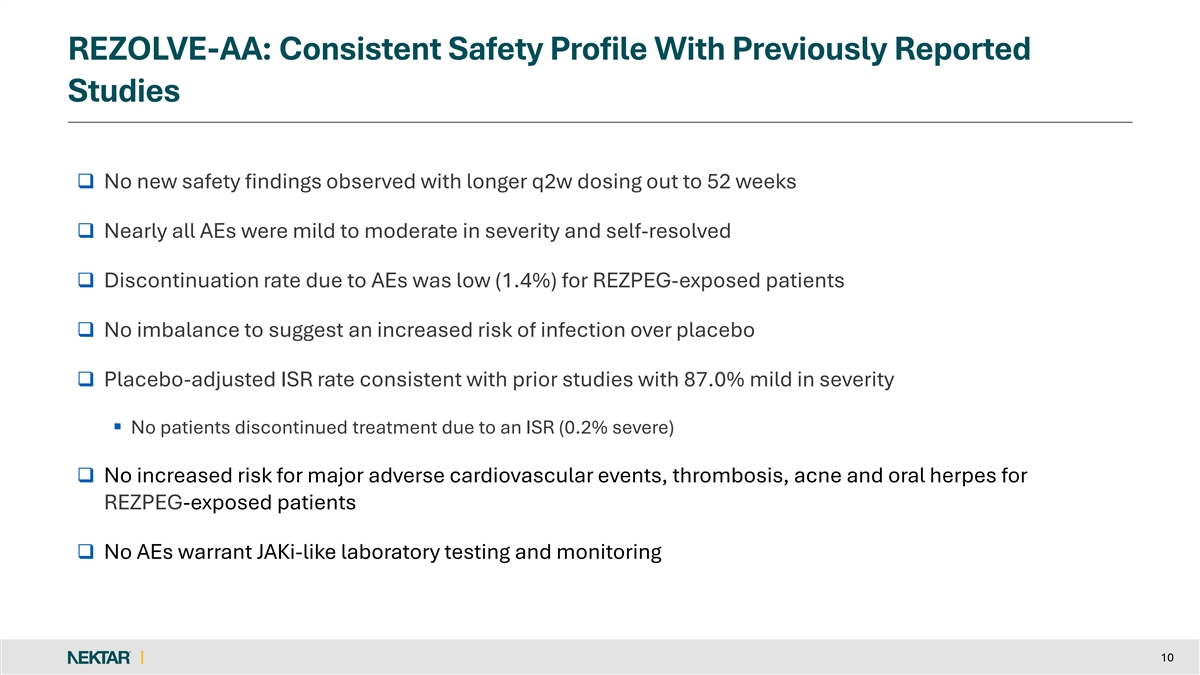
REZOLVE-AA: Consistent Safety Profile With Previously Reported Studies q No new safety findings observed with longer q2w dosing out to 52 weeks q Nearly all AEs were mild to moderate in severity and self-resolved q Discontinuation rate due to AEs was low (1.4%) for REZPEG-exposed patients q No imbalance to suggest an increased risk of infection over placebo q Placebo-adjusted ISR rate consistent with prior studies with 87.0% mild in severity § No patients discontinued treatment due to an ISR (0.2% severe) q No increased risk for major adverse cardiovascular events, thrombosis, acne and oral herpes for REZPEG-exposed patients q No AEs warrant JAKi-like laboratory testing and monitoring 10
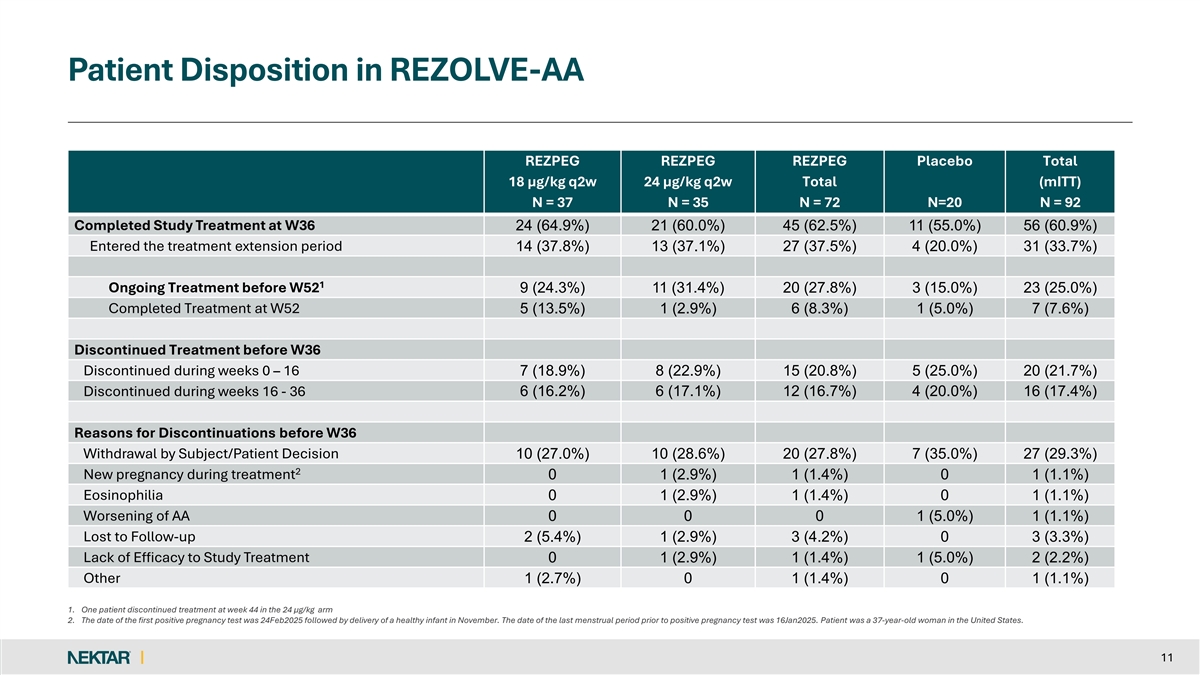
Patient Disposition in REZOLVE-AA REZPEG REZPEG REZPEG Placebo Total 18 µg/kg q2w 24 µg/kg q2w Total (mITT) N = 37 N = 35 N = 72 N=20 N = 92 Completed Study Treatment at W36 24 (64.9%) 21 (60.0%) 45 (62.5%) 11 (55.0%) 56 (60.9%) Entered the treatment extension period 14 (37.8%) 13 (37.1%) 27 (37.5%) 4 (20.0%) 31 (33.7%) 1 Ongoing Treatment before W52 9 (24.3%) 11 (31.4%) 20 (27.8%) 3 (15.0%) 23 (25.0%) Completed Treatment at W52 5 (13.5%) 1 (2.9%) 6 (8.3%) 1 (5.0%) 7 (7.6%) Discontinued Treatment before W36 Discontinued during weeks 0 – 16 7 (18.9%) 8 (22.9%) 15 (20.8%) 5 (25.0%) 20 (21.7%) Discontinued during weeks 16 - 36 6 (16.2%) 6 (17.1%) 12 (16.7%) 4 (20.0%) 16 (17.4%) Reasons for Discontinuations before W36 Withdrawal by Subject/Patient Decision 10 (27.0%) 10 (28.6%) 20 (27.8%) 7 (35.0%) 27 (29.3%) 2 New pregnancy during treatment 0 1 (2.9%) 1 (1.4%) 0 1 (1.1%) Eosinophilia 0 1 (2.9%) 1 (1.4%) 0 1 (1.1%) Worsening of AA 0 0 0 1 (5.0%) 1 (1.1%) Lost to Follow-up 2 (5.4%) 1 (2.9%) 3 (4.2%) 0 3 (3.3%) Lack of Efficacy to Study Treatment 0 1 (2.9%) 1 (1.4%) 1 (5.0%) 2 (2.2%) Other 1 (2.7%) 0 1 (1.4%) 0 1 (1.1%) 1. One patient discontinued treatment at week 44 in the 24 µg/kg arm 2. The date of the first positive pregnancy test was 24Feb2025 followed by delivery of a healthy infant in November. The date of the last menstrual period prior to positive pregnancy test was 16Jan2025. Patient was a 37-year-old woman in the United States. 11
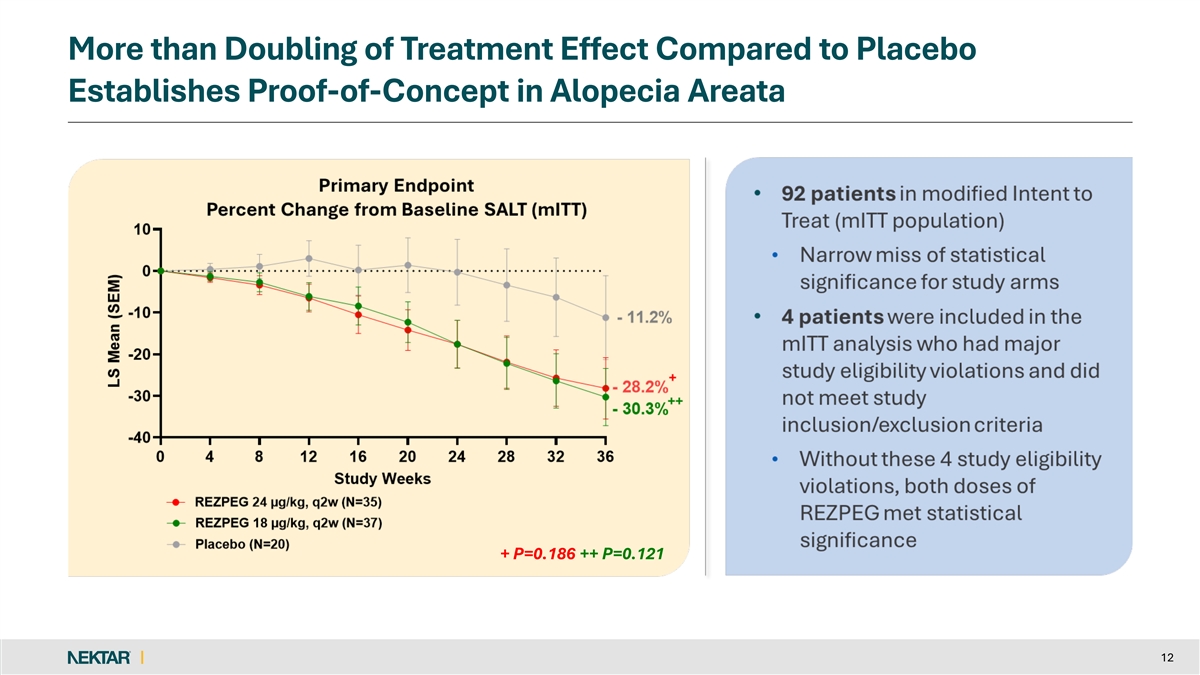
More than Doubling of Treatment Effect Compared to Placebo Establishes Proof-of-Concept in Alopecia Areata + P=0.186 ++ P=0.121 + P=0.186 ++ P=0.121 12
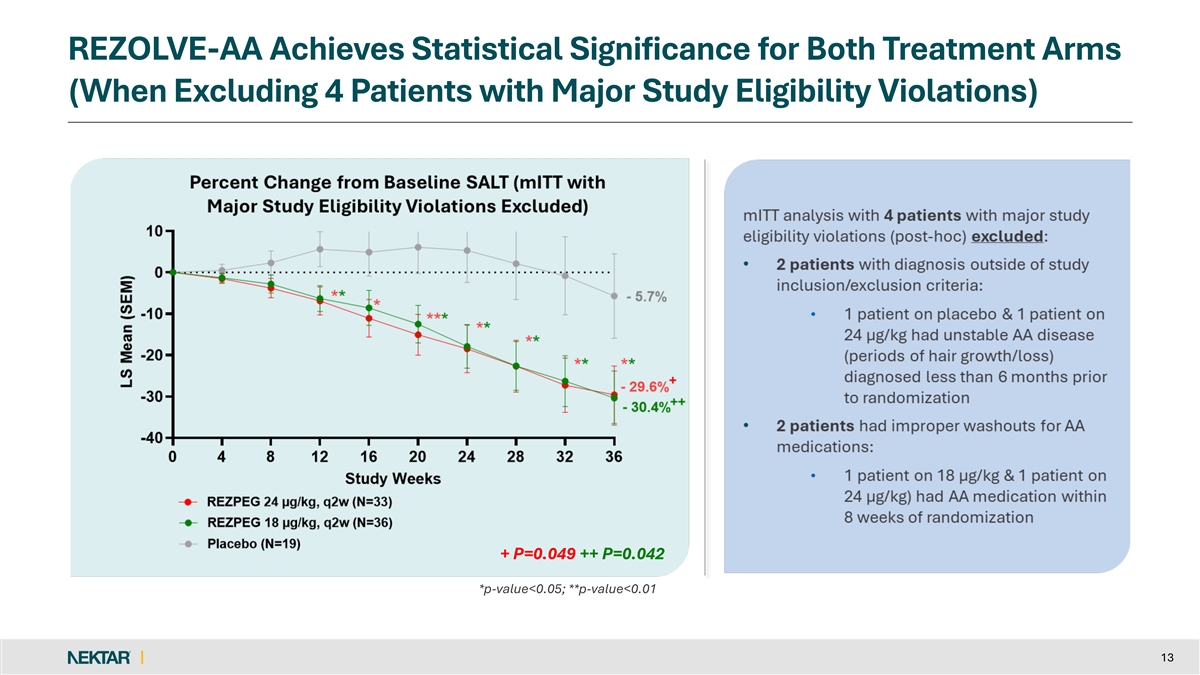
REZOLVE-AA Achieves Statistical Significance for Both Treatment Arms (When Excluding 4 Patients with Major Study Eligibility Violations) + P=0.049 ++ P=0.042 + P=0.049 ++ P=0.042 *p-value<0.05; **p-value<0.01 13
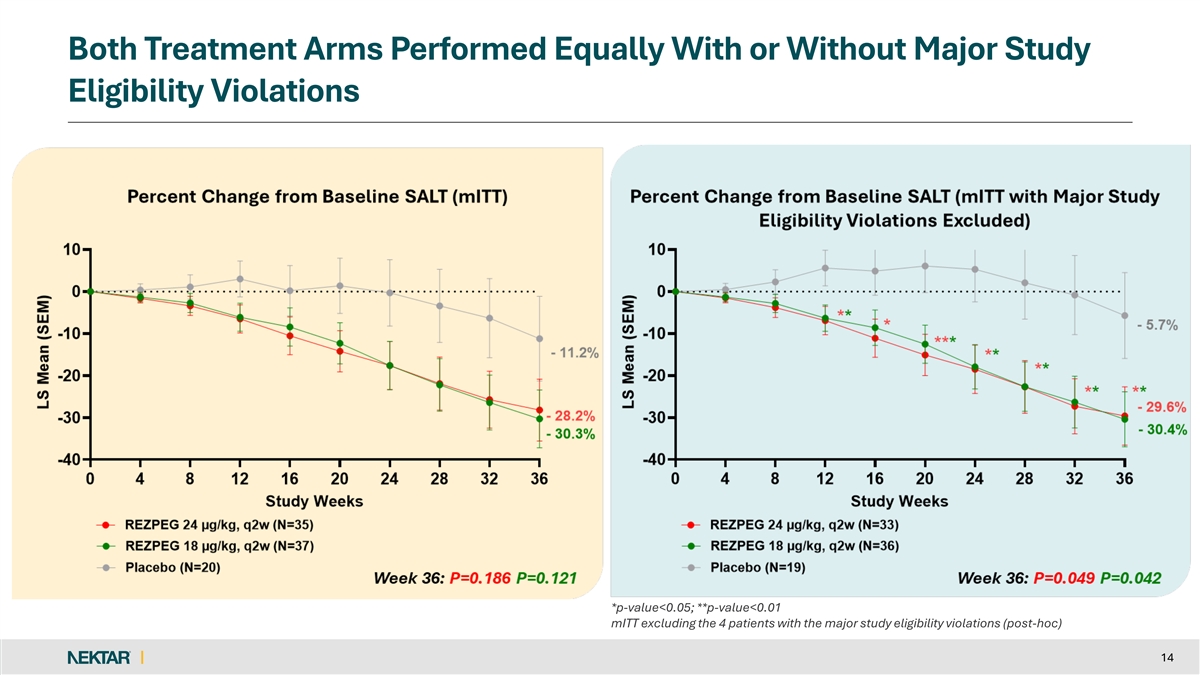
Both Treatment Arms Performed Equally With or Without Major Study Eligibility Violations *p-value<0.05; **p-value<0.01 mITT excluding the 4 patients with the major study eligibility violations (post-hoc) 14
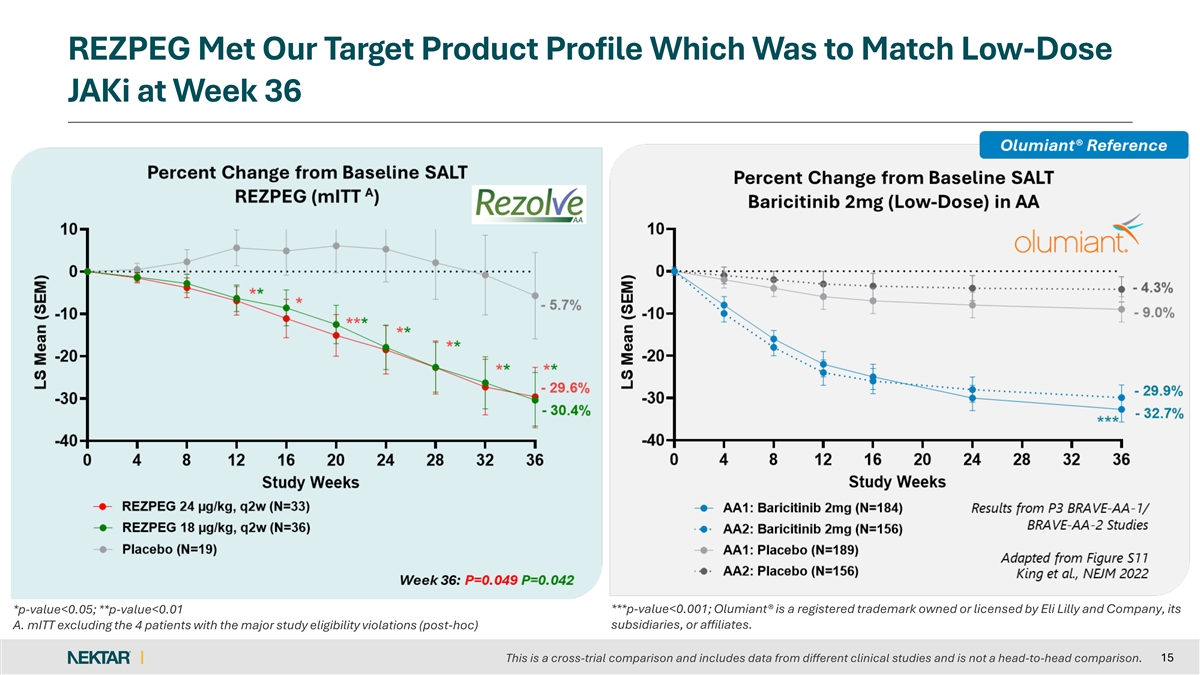
REZPEG Met Our Target Product Profile Which Was to Match Low-Dose JAKi at Week 36 *p-value<0.05; **p-value<0.01 ***p-value<0.001; Olumiant® is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates. A. mITT excluding the 4 patients with the major study eligibility violations (post-hoc) 15 This is a cross-trial comparison and includes data from different clinical studies and is not a head-to-head comparison.
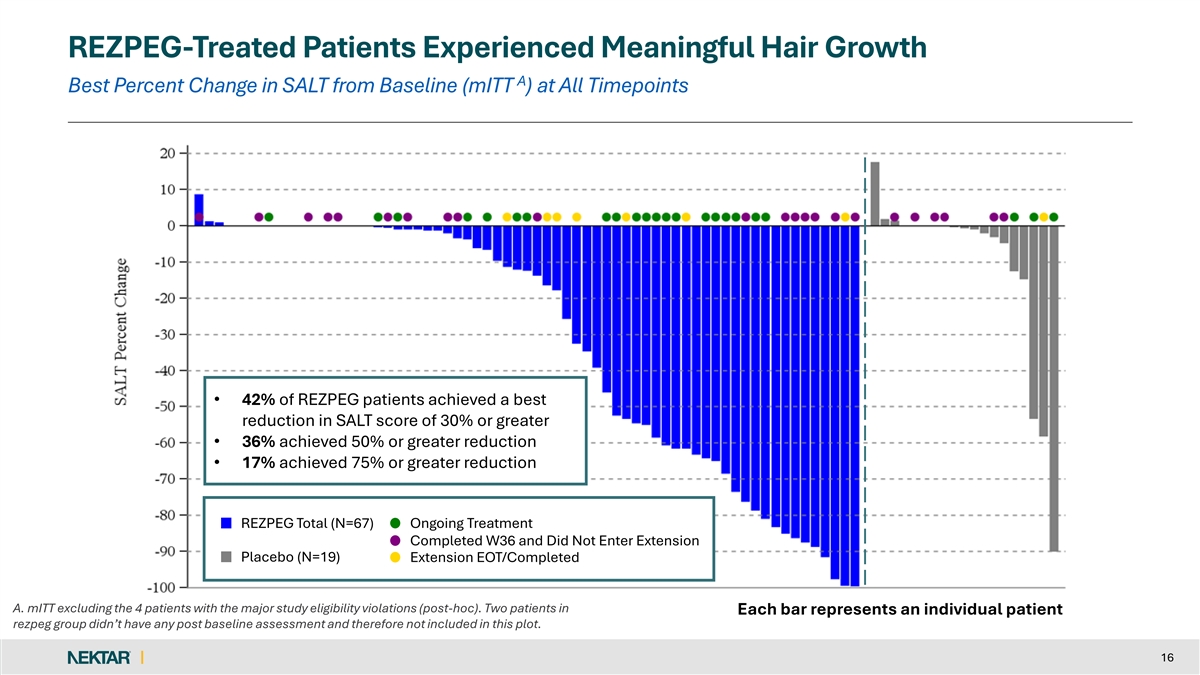
REZPEG-Treated Patients Experienced Meaningful Hair Growth A Best Percent Change in SALT from Baseline (mITT ) at All Timepoints • 42% of REZPEG patients achieved a best reduction in SALT score of 30% or greater • 36% achieved 50% or greater reduction • 17% achieved 75% or greater reduction REZPEG Total (N=67) Ongoing Treatment Completed W36 and Did Not Enter Extension Placebo (N=19) Extension EOT/Completed A. mITT excluding the 4 patients with the major study eligibility violations (post-hoc). Two patients in Each bar represents an individual patient rezpeg group didn’t have any post baseline assessment and therefore not included in this plot. 16
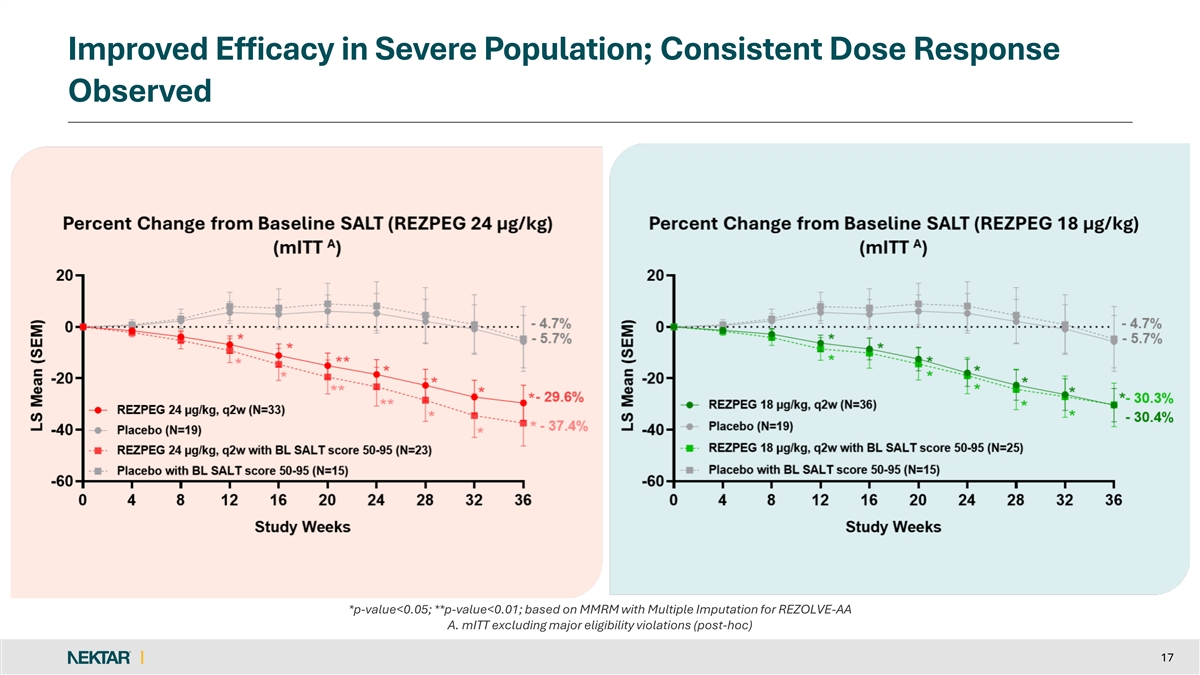
Improved Efficacy in Severe Population; Consistent Dose Response Observed *p-value<0.05; **p-value<0.01; based on MMRM with Multiple Imputation for REZOLVE-AA A. mITT excluding major eligibility violations (post-hoc) 17
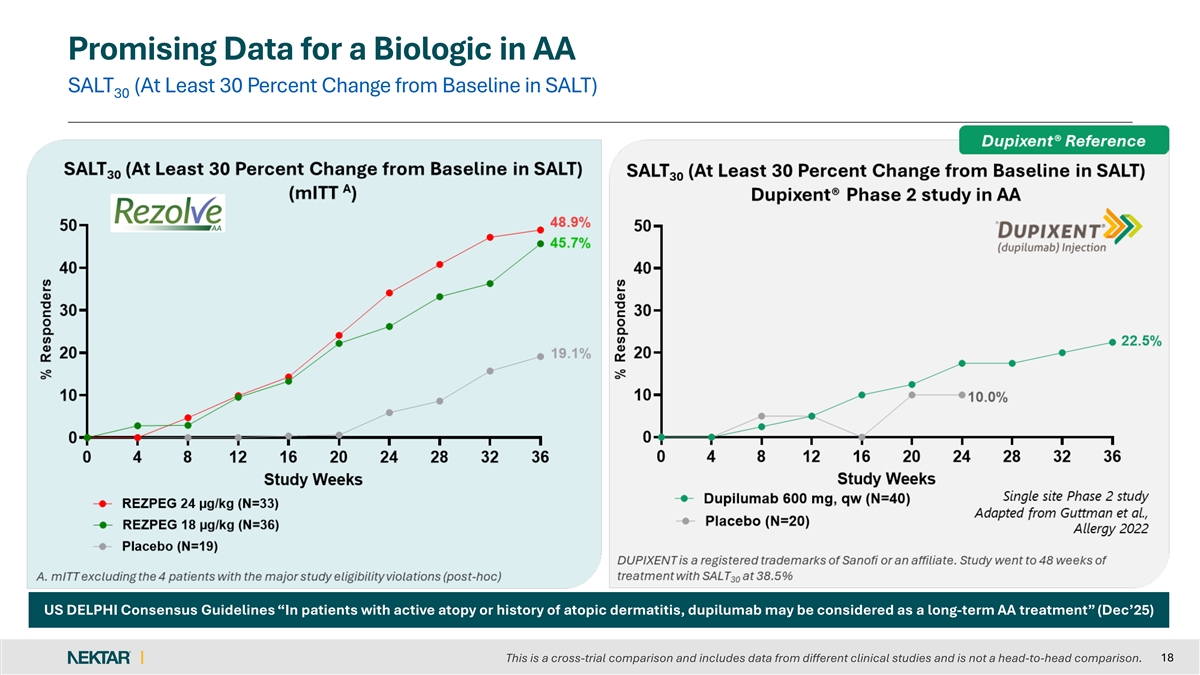
Promising Data for a Biologic in AA SALT (At Least 30 Percent Change from Baseline in SALT) 30 US DELPHI Consensus Guidelines “In patients with active atopy or history of atopic dermatitis, dupilumab may be considered as a long-term AA treatment” (Dec’25) 18 This is a cross-trial comparison and includes data from different clinical studies and is not a head-to-head comparison.
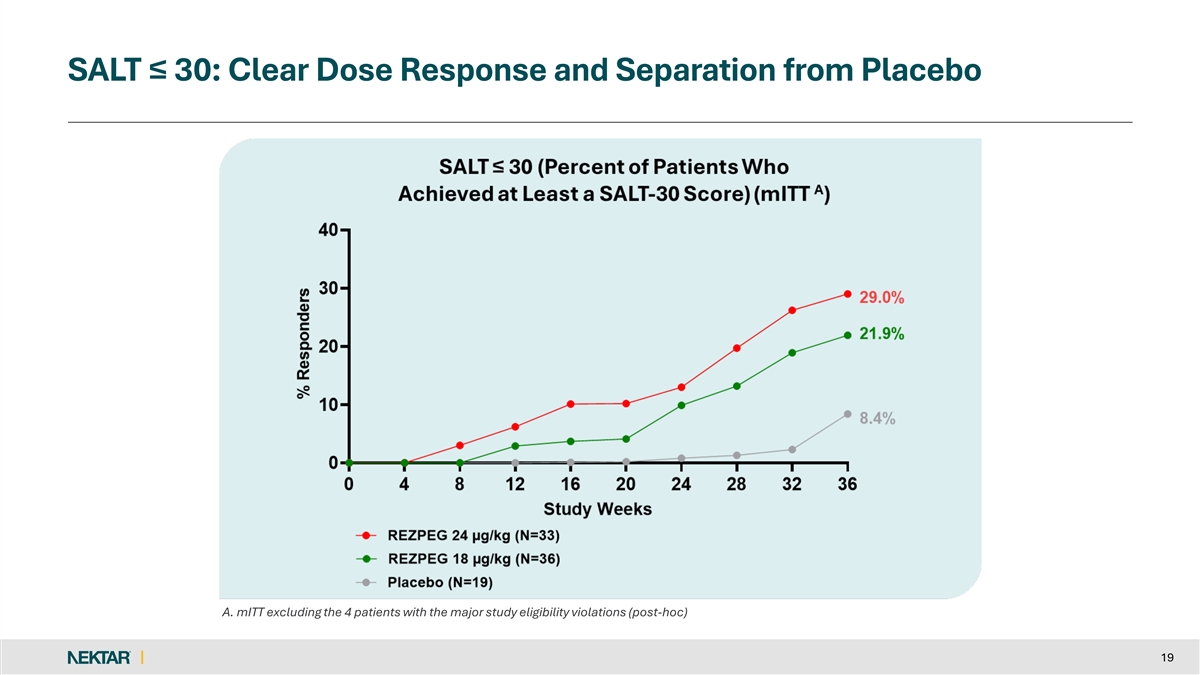
SALT ≤ 30: Clear Dose Response and Separation from Placebo A. mITT excluding the 4 patients with the major study eligibility violations (post-hoc) 19
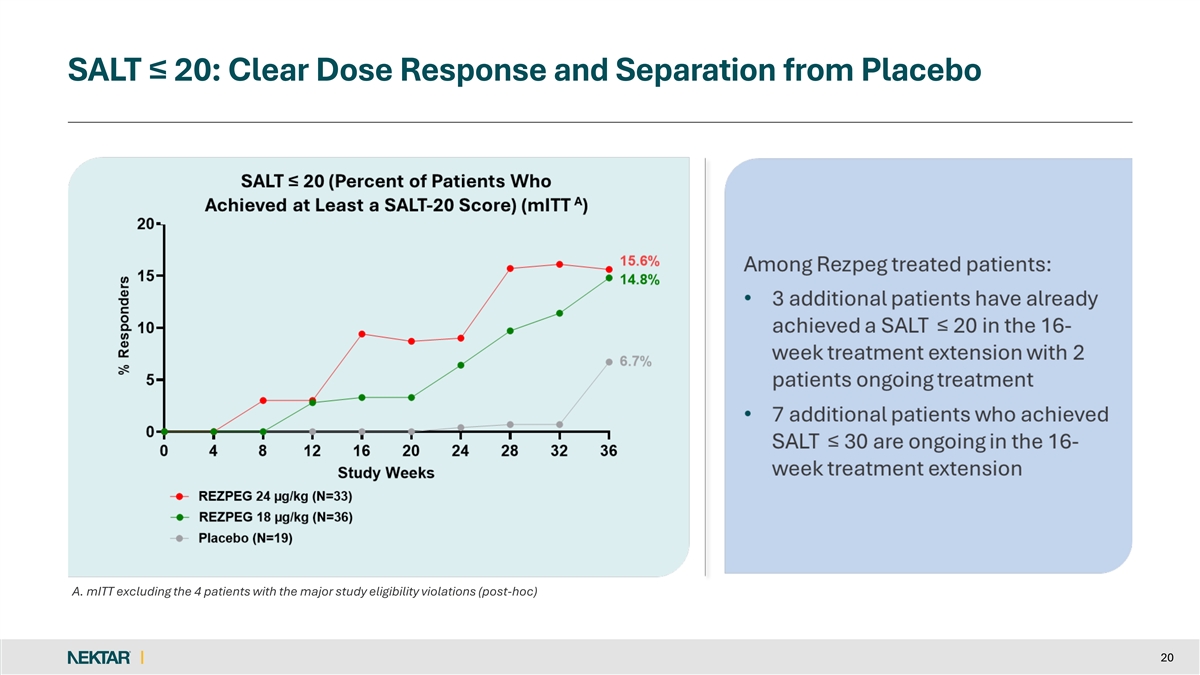
SALT ≤ 20: Clear Dose Response and Separation from Placebo A. mITT excluding the 4 patients with the major study eligibility violations (post-hoc) 20
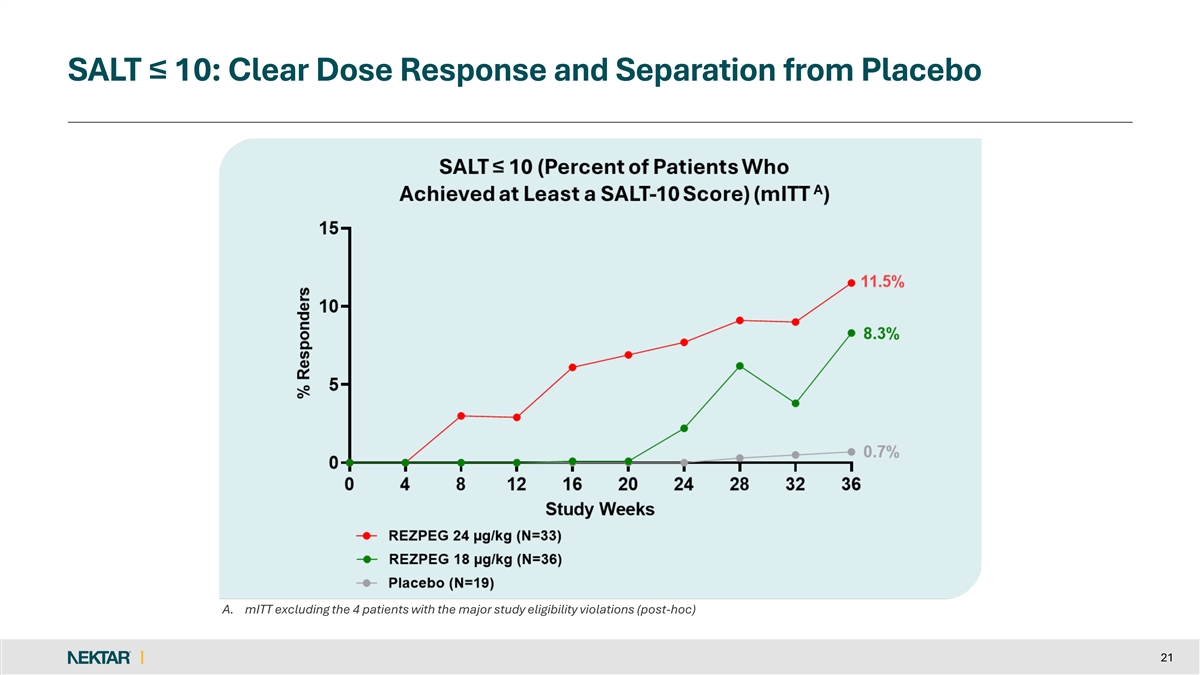
SALT ≤ 10: Clear Dose Response and Separation from Placebo A. mITT excluding the 4 patients with the major study eligibility violations (post-hoc) 21
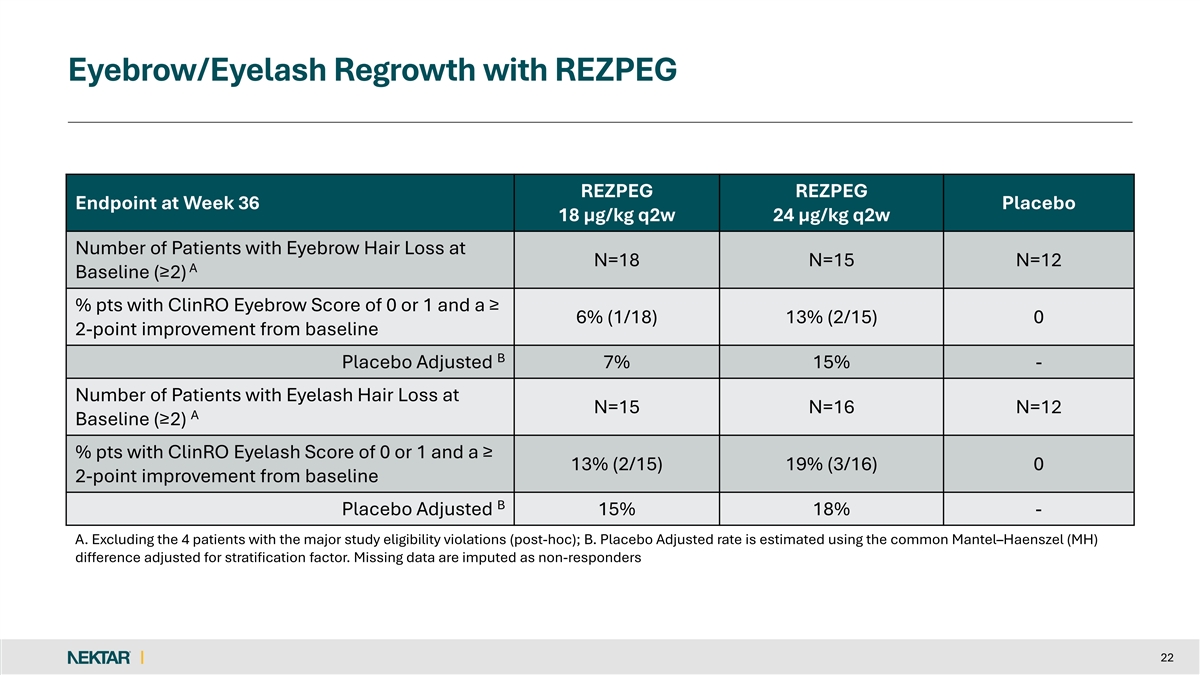
Eyebrow/Eyelash Regrowth with REZPEG REZPEG REZPEG Endpoint at Week 36 Placebo 18 µg/kg q2w 24 µg/kg q2w Number of Patients with Eyebrow Hair Loss at N=18 N=15 N=12 A Baseline (≥2) % pts with ClinRO Eyebrow Score of 0 or 1 and a ≥ 6% (1/18) 13% (2/15) 0 2-point improvement from baseline B Placebo Adjusted 7% 15% - Number of Patients with Eyelash Hair Loss at N=15 N=16 N=12 A Baseline (≥2) % pts with ClinRO Eyelash Score of 0 or 1 and a ≥ 13% (2/15) 19% (3/16) 0 2-point improvement from baseline B Placebo Adjusted 15% 18% - A. Excluding the 4 patients with the major study eligibility violations (post-hoc); B. Placebo Adjusted rate is estimated using the common Mantel–Haenszel (MH) difference adjusted for stratification factor. Missing data are imputed as non-responders 22
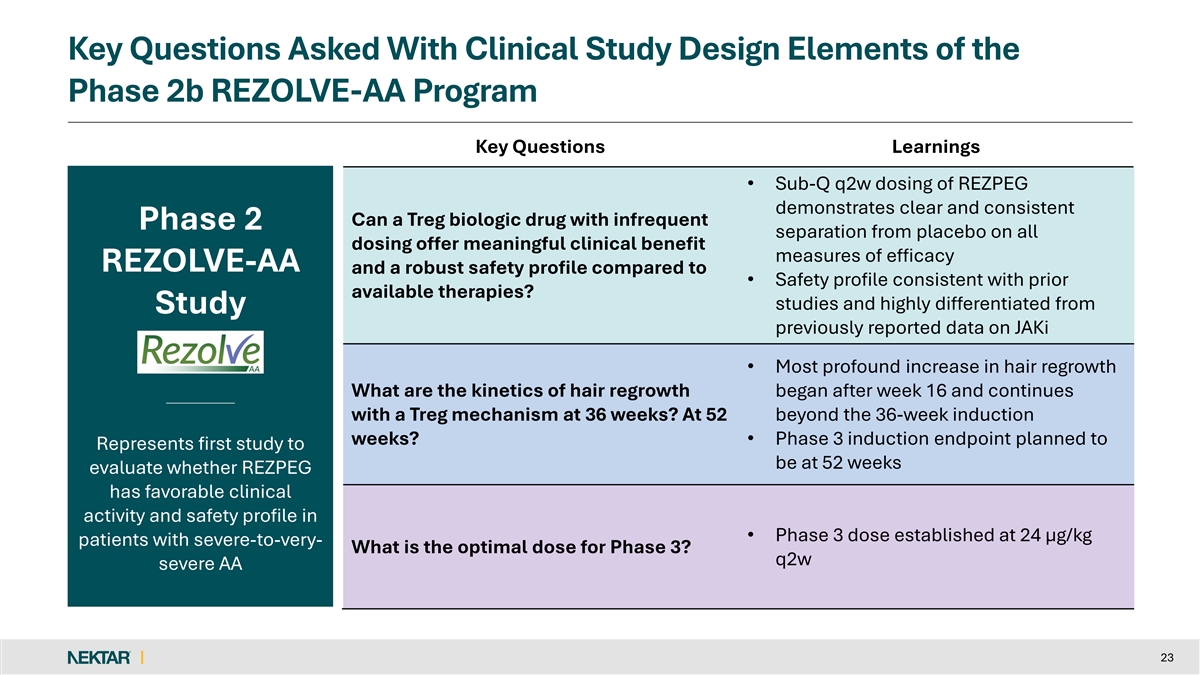
Key Questions Asked With Clinical Study Design Elements of the Phase 2b REZOLVE-AA Program Key Questions Learnings • Sub-Q q2w dosing of REZPEG demonstrates clear and consistent Can a Treg biologic drug with infrequent Phase 2 separation from placebo on all dosing offer meaningful clinical benefit measures of efficacy REZOLVE-AA and a robust safety profile compared to • Safety profile consistent with prior available therapies? studies and highly differentiated from Study previously reported data on JAKi • Most profound increase in hair regrowth What are the kinetics of hair regrowth began after week 16 and continues with a Treg mechanism at 36 weeks? At 52 beyond the 36-week induction weeks? • Phase 3 induction endpoint planned to Represents first study to be at 52 weeks evaluate whether REZPEG has favorable clinical activity and safety profile in • Phase 3 dose established at 24 µg/kg patients with severe-to-very- What is the optimal dose for Phase 3? q2w severe AA 23
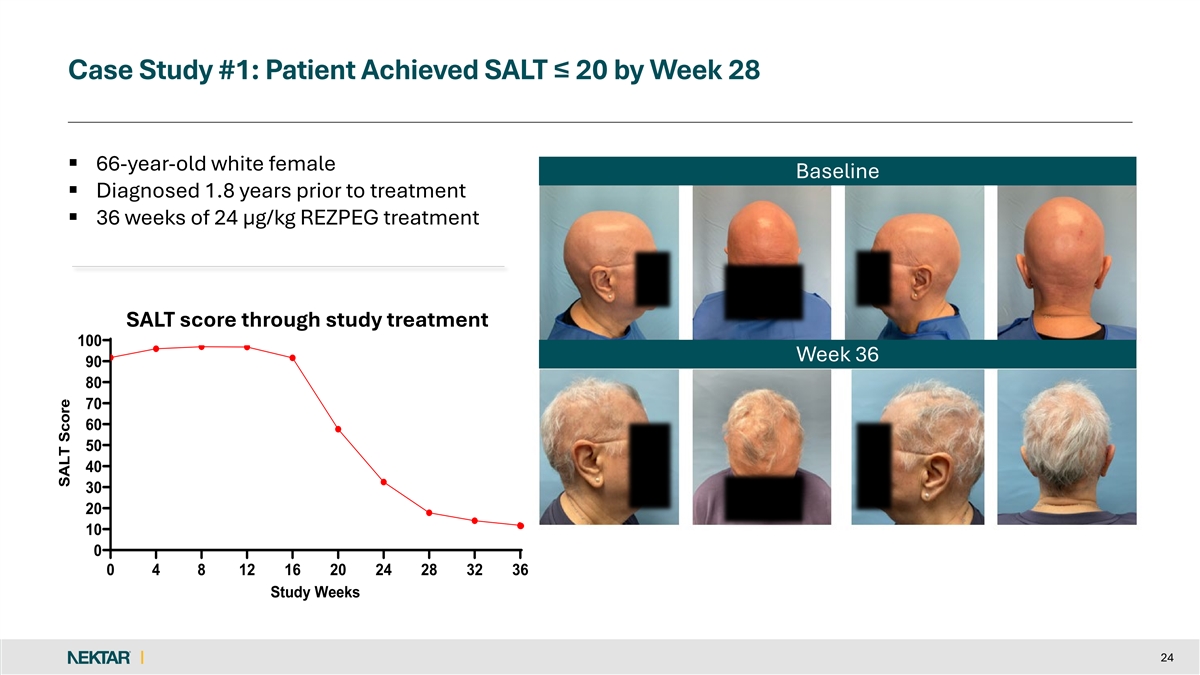
Case Study #1: Patient Achieved SALT ≤ 20 by Week 28 § 66-year-old white female Baseline § Diagnosed 1.8 years prior to treatment § 36 weeks of 24 µg/kg REZPEG treatment SALT score through study treatment 100 Week 36 90 80 70 60 50 40 30 20 10 0 0 4 8 12 16 20 24 28 32 36 Study Weeks 24 SALT Score
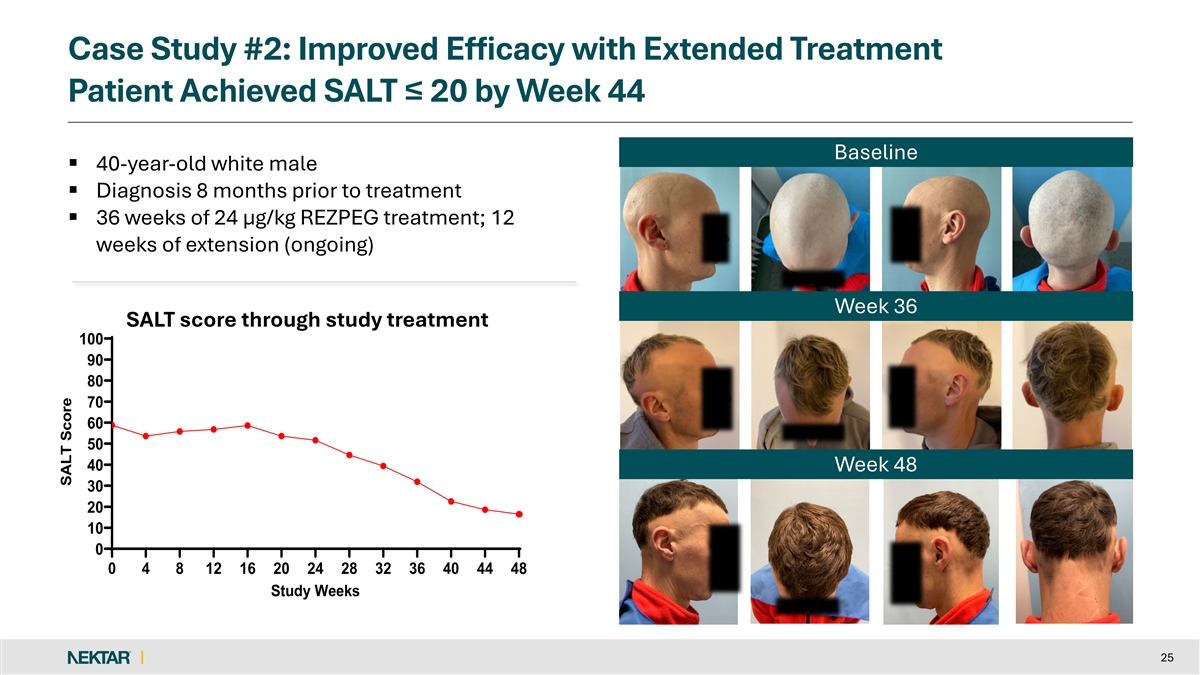
Case Study #2: Improved Efficacy with Extended Treatment Patient Achieved SALT ≤ 20 by Week 44 Baseline § 40-year-old white male § Diagnosis 8 months prior to treatment § 36 weeks of 24 µg/kg REZPEG treatment; 12 weeks of extension (ongoing) Week 36 SALT score through study treatment 100 90 80 70 60 50 40 Week 48 30 20 10 0 0 4 8 12 16 20 24 28 32 36 40 44 48 Study Weeks 25 SALT Score
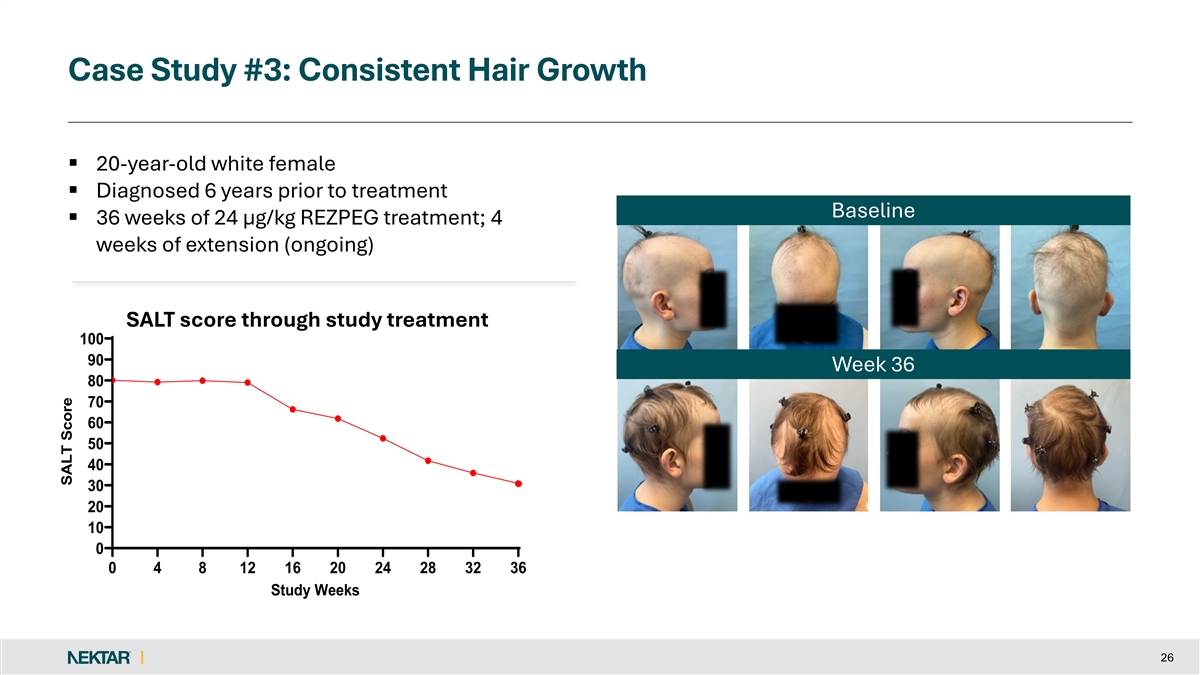
Case Study #3: Consistent Hair Growth § 20-year-old white female § Diagnosed 6 years prior to treatment Baseline § 36 weeks of 24 µg/kg REZPEG treatment; 4 weeks of extension (ongoing) SALT score through study treatment 100 90 Week 36 80 70 60 50 40 30 20 10 0 0 4 8 12 16 20 24 28 32 36 Study Weeks 26 SALT Score
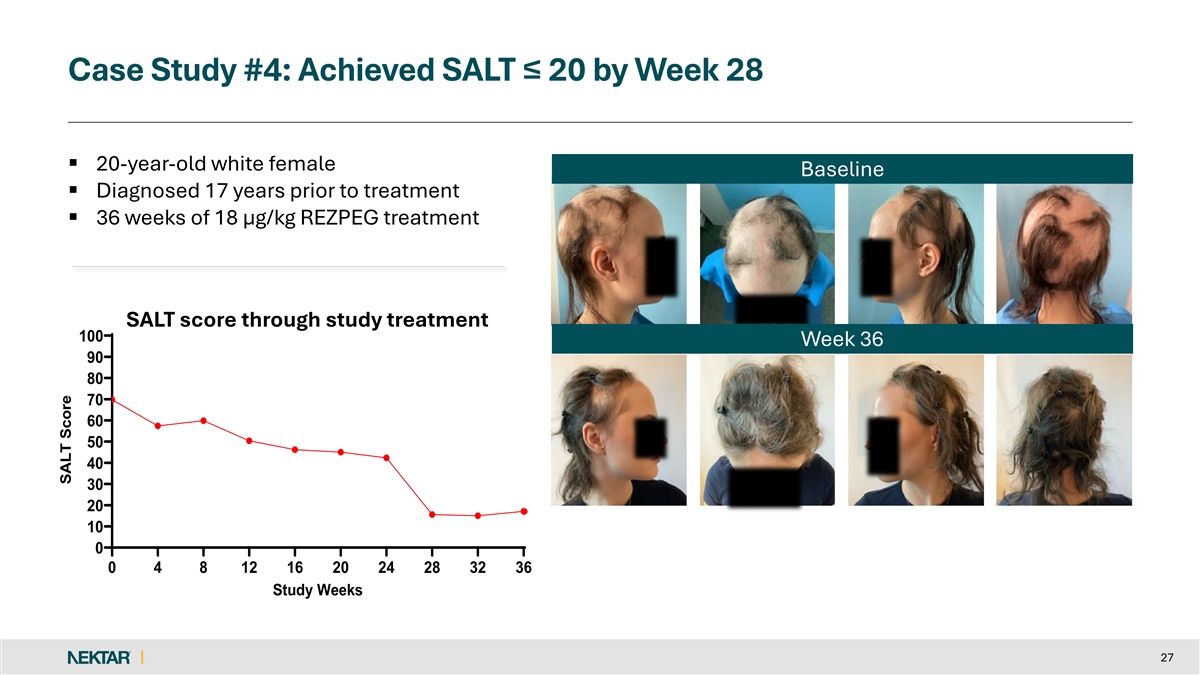
Case Study #4: Achieved SALT ≤ 20 by Week 28 § 20-year-old white female Baseline § Diagnosed 17 years prior to treatment § 36 weeks of 18 µg/kg REZPEG treatment SALT score through study treatment 100 Week 36 90 80 70 60 50 40 30 20 10 0 0 4 8 12 16 20 24 28 32 36 Study Weeks 27 SALT Score
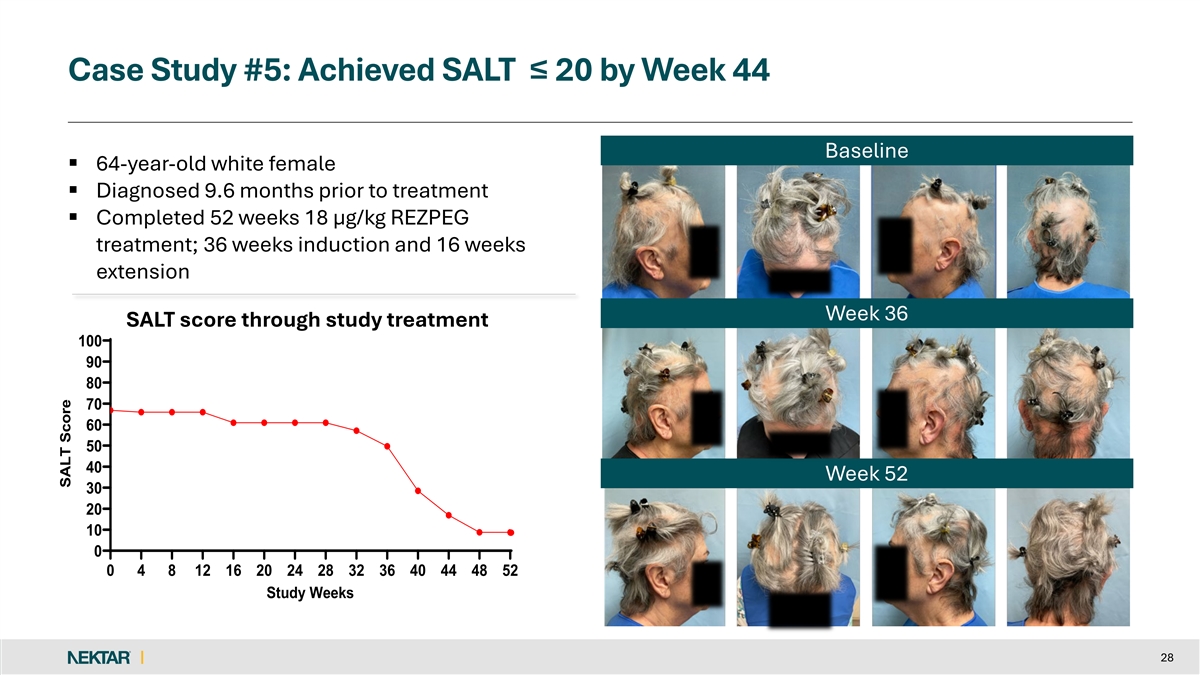
Case Study #5: Achieved SALT ≤ 20 by Week 44 Baseline § 64-year-old white female § Diagnosed 9.6 months prior to treatment § Completed 52 weeks 18 µg/kg REZPEG treatment; 36 weeks induction and 16 weeks extension Week 36 SALT score through study treatment 100 90 80 70 60 50 40 Week 52 30 20 10 0 0 4 8 12 16 20 24 28 32 36 40 44 48 52 Study Weeks 28 SALT Score
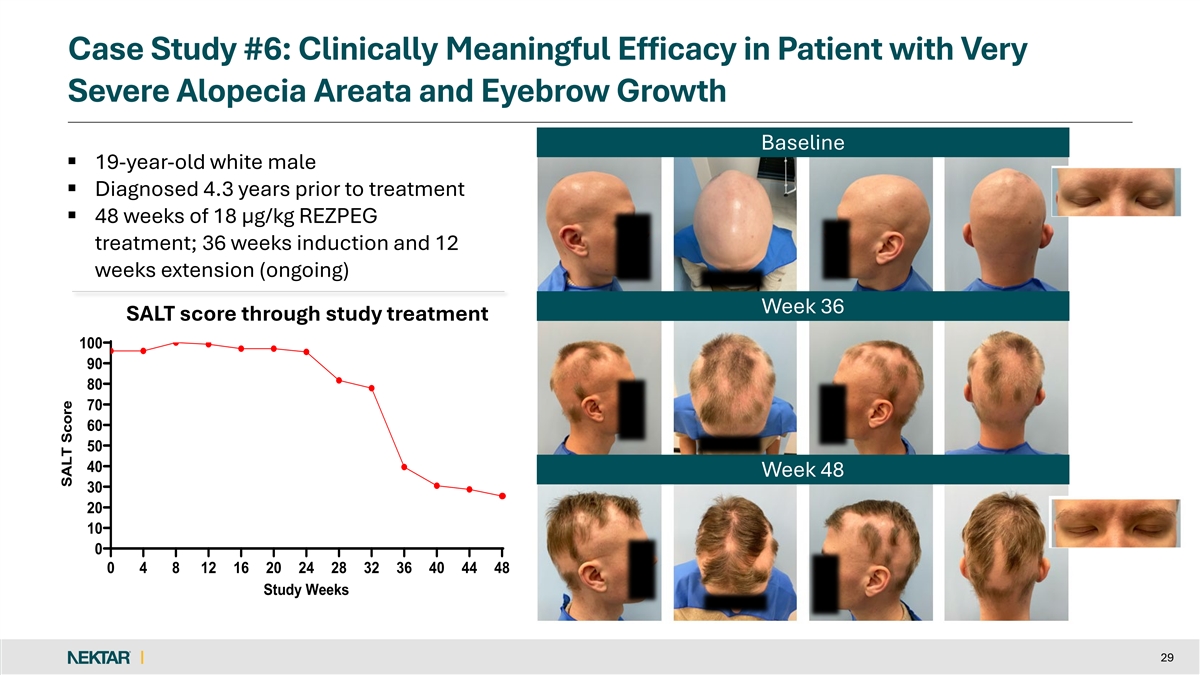
Case Study #6: Clinically Meaningful Efficacy in Patient with Very Severe Alopecia Areata and Eyebrow Growth Baseline § 19-year-old white male § Diagnosed 4.3 years prior to treatment § 48 weeks of 18 µg/kg REZPEG treatment; 36 weeks induction and 12 weeks extension (ongoing) Week 36 SALT score through study SALT score through study treatment 100 90 80 70 60 50 40 Week 48 30 20 10 0 0 4 8 12 16 20 24 28 32 36 40 44 48 Study Weeks 29 SALT Score
Study Results Demonstrate Proof of Concept for REZPEG as a Potential First-in-Class Biologic in Alopecia Areata Achieved our target product profile for favorable clinical efficacy data and tolerability with differentiated safety Profile meets urgent unmet medical need for a safe alternative to JAKi class in AA Represents a second Phase 2b study identifying 24 µg/kg as optimal dose for inflammatory skin diseases Recommended dose established for Phase 3 Mean % change SALT from baseline to week 36 was -30% in REZPEG arms vs placebo -6% (p<0.05) with consistent A separation at each time point from placebo B Majority of REZPEG-treated patients regrew hair No plateau of SALT reduction by week 36 SALT≤30, SALT≤20 and SALT≤10 treatment effects showed consistent dose response and separation from placebo Improvements observed in regrowth of eyebrows and eyelashes Safety profile highly differentiated from JAKi Class • No increased risk of major adverse cardiovascular events (MACE) events, thrombosis, acne and infections including oral herpes observed • No JAK-inhibitor AEs requiring laboratory testing and monitoring, no malignancies • No drop-outs for ISRs, only one patient discontinued due to a TEAE A. mITT excluding the 4 patients with the major study eligibility violation; B. 54% of REZPEG-treated patients achieved a best reduction in SALT score of 10% or greater vs. 26% for placebo 30

Jonathan Silverberg, MD, PhD, MPH Professor of Dermatology at The George Washington University School of Medicine and Health Sciences Director of Clinical Research and Contact Dermatitis Dr. Silverberg is the Director of Clinical Research and Contact Dermatitis. Dr. Silverberg's area of clinical subspecialty is inflammatory skin disease. Dr. Silverberg has also been a local, national and/or international principal investigator for numerous clinical trials for novel treatments in inflammatory skin disorders. Dr. Silverberg's research interests include drug development, clinical trial design, biomarkers, dermato-epidemiology, health services research, patient-reported outcomes, comorbidities and burden of inflammatory skin disease and evidence-based dermatology. His publications include more than 1,000 peer-reviewed articles, abstracts and book chapters. He is an associate editor for the Journal of the American Academy of Dermatology, British Journal of Dermatology and Current Dermatology Reports. David Rosmarin, MD Chair of the Department of Dermatology at Indiana University School of Medicine Kampen-Norins Scholar in Dermatology Dr. Rosmarin is nationally recognized and serves as a referral for physicians with difficult to manage inflammatory diseases such as alopecia areata. Previously, Dr. Rosmarin served as the Director of the Clinical Trials Unit in the Department of Dermatology at Tufts Medical Center. His research interests focus on development of novel therapeutics and investigating novel uses of established therapies, with a particular focus on chronic skin diseases such as alopecia areata, atopic dermatitis, vitiligo, discoid lupus, and hidradenitis suppuritiva. Dr. Rosmarin went to medical school at NYU, dermatology residency at Boston University-Tufts combined training program, and fellowship at Brigham and Women’s Hospital. Benjamin N. Ungar MD Assistant Professor, Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai Director of the Alopecia Center of Excellence and as Director of the Rosacea & Seborrheic Dermatitis Clinic Dr. Ungar's clinical and research focus specialization is in inflammatory skin diseases, as well as how the immunology of the skin relates to the systemic components of the diseases he studies. His research is centered on atopic dermatitis and alopecia areata, as well as diseases such as seborrheic dermatitis. Dr. Ungar has authored or coauthored more than 75 original articles and more than 45 abstracts. He has led various talks on alopecia areata both in the United States and abroad. Dr. Ungar received his medical degree from the Icahn School of Medicine at Mount Sinai. He completed his internship at NYU Winthrop Hospital in Mineola, New York, followed by a residency in dermatology at the Icahn School of Medicine at Mount Sinai, from which he graduated as the chief resident. KOL Panel
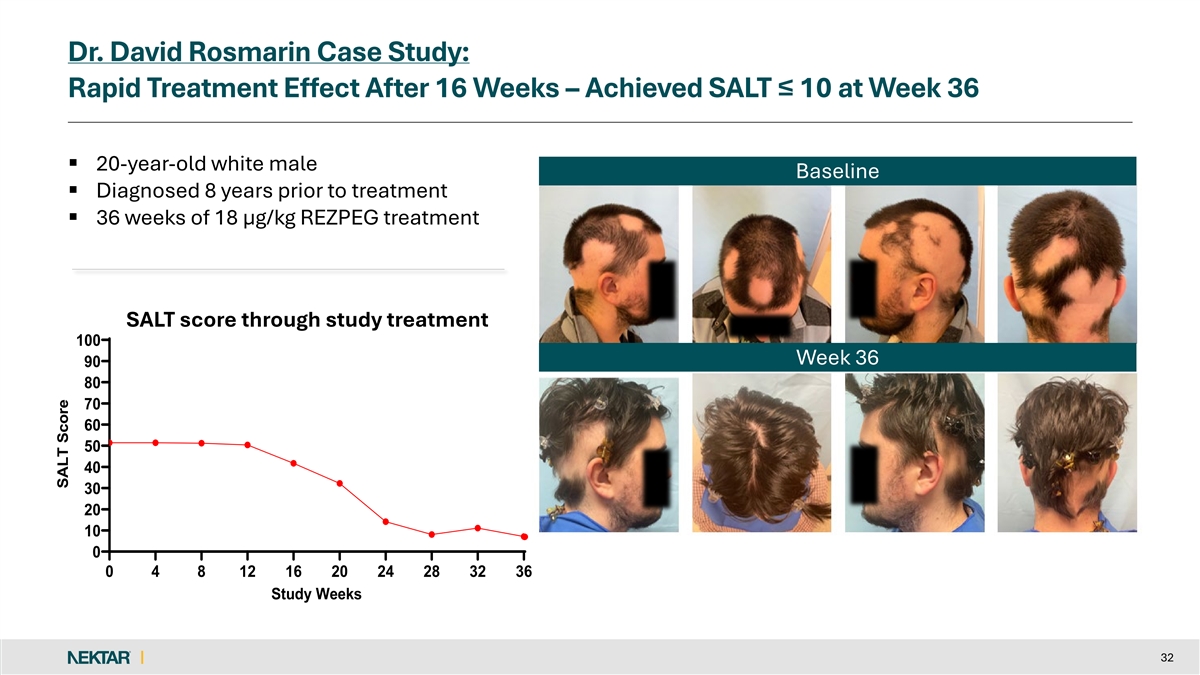
Dr. David Rosmarin Case Study: Rapid Treatment Effect After 16 Weeks – Achieved SALT ≤ 10 at Week 36 § 20-year-old white male Baseline § Diagnosed 8 years prior to treatment § 36 weeks of 18 µg/kg REZPEG treatment SALT score through study treatment 100 Week 36 90 80 70 60 50 40 30 20 10 0 0 4 8 12 16 20 24 28 32 36 Study Weeks 32 SALT Score

Appendix (Data tables, additional materials) 33
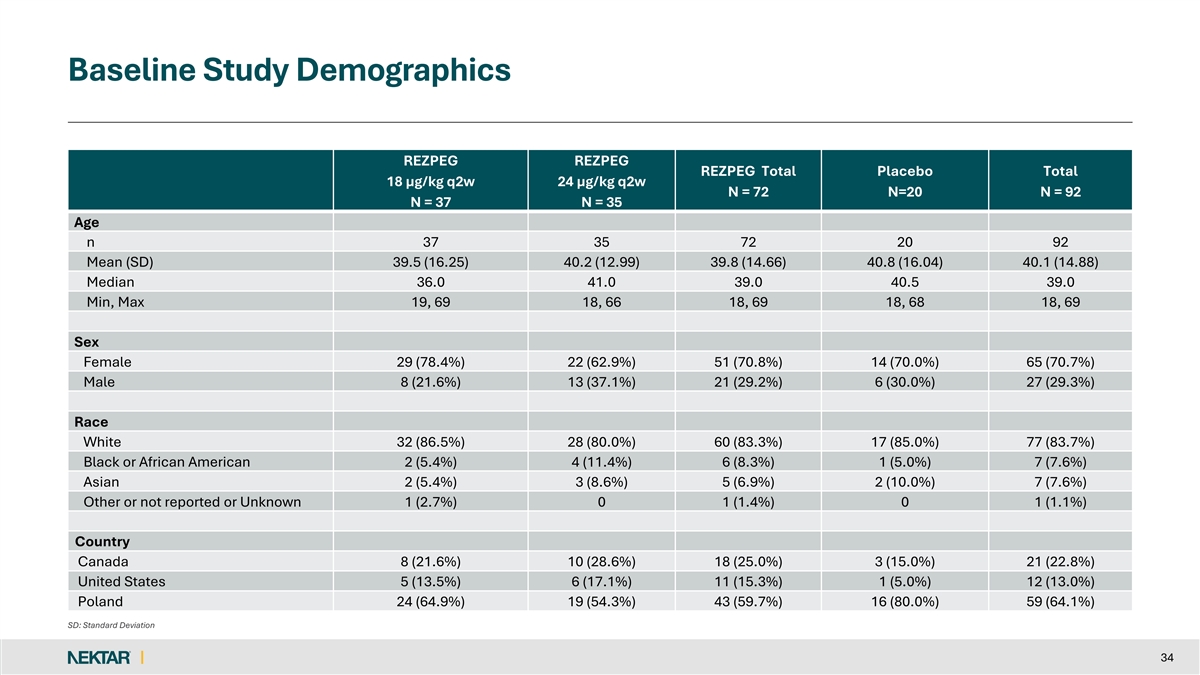
Baseline Study Demographics REZPEG REZPEG REZPEG Total Placebo Total 18 µg/kg q2w 24 µg/kg q2w N = 72 N=20 N = 92 N = 37 N = 35 Age n 37 35 72 20 92 Mean (SD) 39.5 (16.25) 40.2 (12.99) 39.8 (14.66) 40.8 (16.04) 40.1 (14.88) Median 36.0 41.0 39.0 40.5 39.0 Min, Max 19, 69 18, 66 18, 69 18, 68 18, 69 Sex Female 29 (78.4%) 22 (62.9%) 51 (70.8%) 14 (70.0%) 65 (70.7%) Male 8 (21.6%) 13 (37.1%) 21 (29.2%) 6 (30.0%) 27 (29.3%) Race White 32 (86.5%) 28 (80.0%) 60 (83.3%) 17 (85.0%) 77 (83.7%) Black or African American 2 (5.4%) 4 (11.4%) 6 (8.3%) 1 (5.0%) 7 (7.6%) Asian 2 (5.4%) 3 (8.6%) 5 (6.9%) 2 (10.0%) 7 (7.6%) Other or not reported or Unknown 1 (2.7%) 0 1 (1.4%) 0 1 (1.1%) Country Canada 8 (21.6%) 10 (28.6%) 18 (25.0%) 3 (15.0%) 21 (22.8%) United States 5 (13.5%) 6 (17.1%) 11 (15.3%) 1 (5.0%) 12 (13.0%) Poland 24 (64.9%) 19 (54.3%) 43 (59.7%) 16 (80.0%) 59 (64.1%) SD: Standard Deviation 34
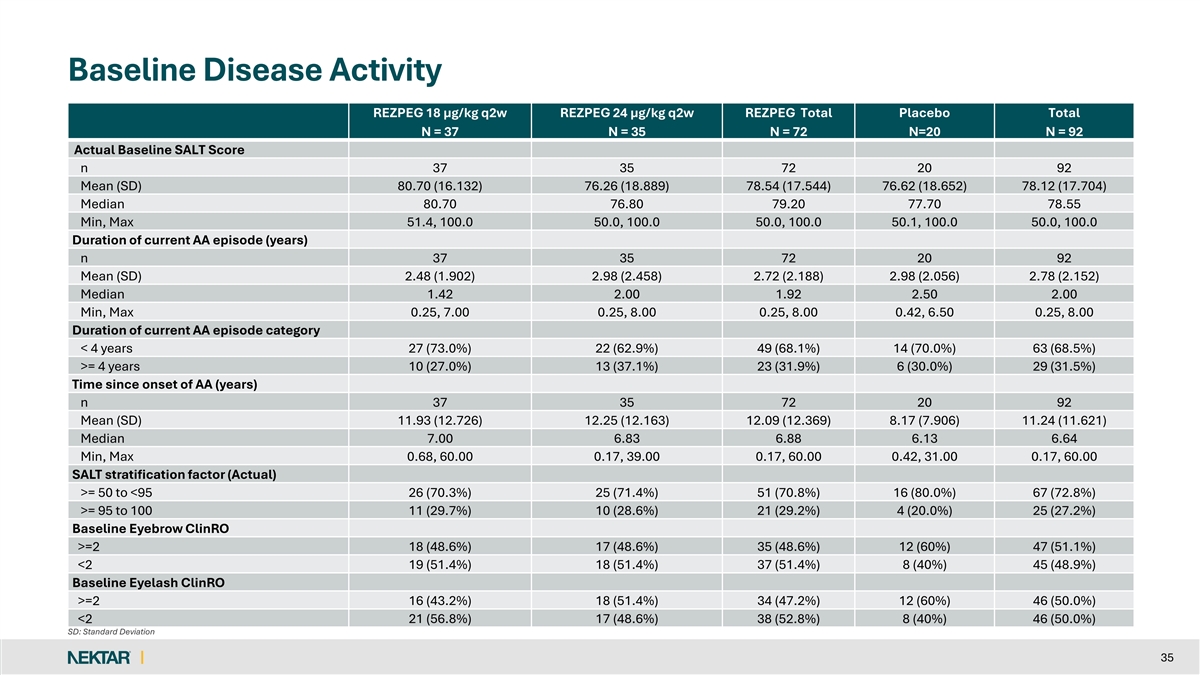
Baseline Disease Activity REZPEG 18 µg/kg q2w REZPEG 24 µg/kg q2w REZPEG Total Placebo Total N = 37 N = 35 N = 72 N=20 N = 92 Actual Baseline SALT Score n 37 35 72 20 92 Mean (SD) 80.70 (16.132) 76.26 (18.889) 78.54 (17.544) 76.62 (18.652) 78.12 (17.704) Median 80.70 76.80 79.20 77.70 78.55 Min, Max 51.4, 100.0 50.0, 100.0 50.0, 100.0 50.1, 100.0 50.0, 100.0 Duration of current AA episode (years) n 37 35 72 20 92 Mean (SD) 2.48 (1.902) 2.98 (2.458) 2.72 (2.188) 2.98 (2.056) 2.78 (2.152) Median 1.42 2.00 1.92 2.50 2.00 Min, Max 0.25, 7.00 0.25, 8.00 0.25, 8.00 0.42, 6.50 0.25, 8.00 Duration of current AA episode category < 4 years 27 (73.0%) 22 (62.9%) 49 (68.1%) 14 (70.0%) 63 (68.5%) >= 4 years 10 (27.0%) 13 (37.1%) 23 (31.9%) 6 (30.0%) 29 (31.5%) Time since onset of AA (years) n 37 35 72 20 92 Mean (SD) 11.93 (12.726) 12.25 (12.163) 12.09 (12.369) 8.17 (7.906) 11.24 (11.621) Median 7.00 6.83 6.88 6.13 6.64 Min, Max 0.68, 60.00 0.17, 39.00 0.17, 60.00 0.42, 31.00 0.17, 60.00 SALT stratification factor (Actual) >= 50 to <95 26 (70.3%) 25 (71.4%) 51 (70.8%) 16 (80.0%) 67 (72.8%) >= 95 to 100 11 (29.7%) 10 (28.6%) 21 (29.2%) 4 (20.0%) 25 (27.2%) Baseline Eyebrow ClinRO >=2 18 (48.6%) 17 (48.6%) 35 (48.6%) 12 (60%) 47 (51.1%) <2 19 (51.4%) 18 (51.4%) 37 (51.4%) 8 (40%) 45 (48.9%) Baseline Eyelash ClinRO >=2 16 (43.2%) 18 (51.4%) 34 (47.2%) 12 (60%) 46 (50.0%) <2 21 (56.8%) 17 (48.6%) 38 (52.8%) 8 (40%) 46 (50.0%) SD: Standard Deviation 35
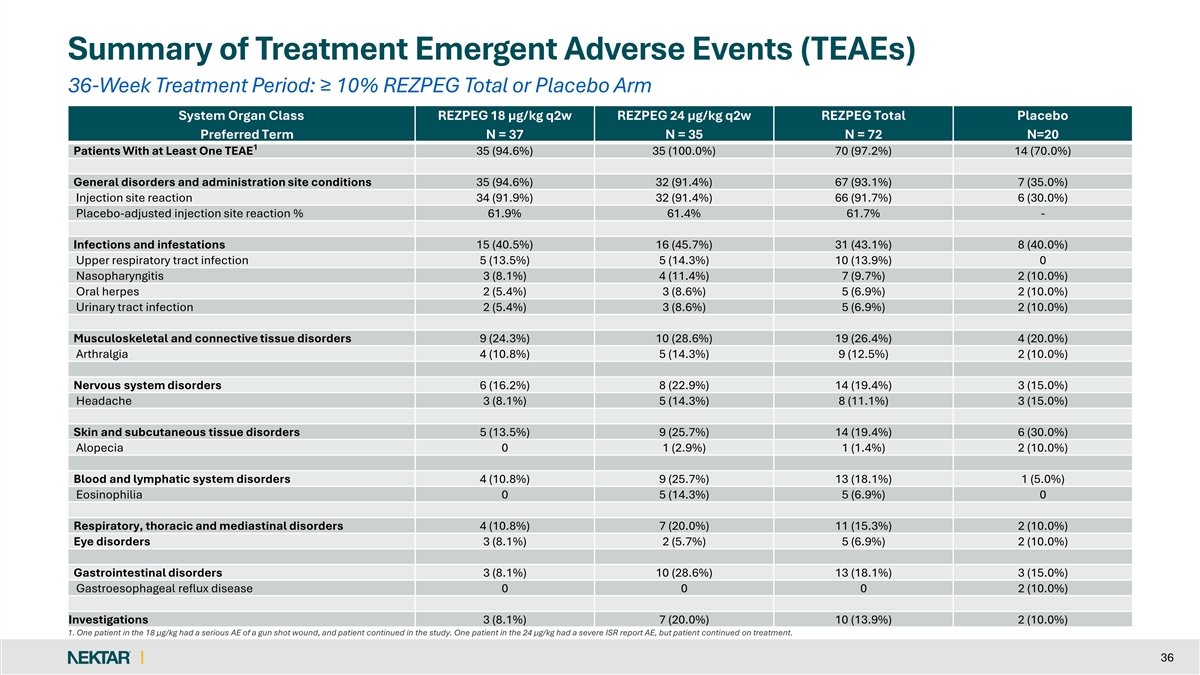
Summary of Treatment Emergent Adverse Events (TEAEs) 36-Week Treatment Period: ≥ 10% REZPEG Total or Placebo Arm System Organ Class REZPEG 18 µg/kg q2w REZPEG 24 µg/kg q2w REZPEG Total Placebo Preferred Term N = 37 N = 35 N = 72 N=20 1 Patients With at Least One TEAE 35 (94.6%) 35 (100.0%) 70 (97.2%) 14 (70.0%) General disorders and administration site conditions 35 (94.6%) 32 (91.4%) 67 (93.1%) 7 (35.0%) Injection site reaction 34 (91.9%) 32 (91.4%) 66 (91.7%) 6 (30.0%) Placebo-adjusted injection site reaction % 61.9% 61.4% 61.7% - Infections and infestations 15 (40.5%) 16 (45.7%) 31 (43.1%) 8 (40.0%) Upper respiratory tract infection 5 (13.5%) 5 (14.3%) 10 (13.9%) 0 Nasopharyngitis 3 (8.1%) 4 (11.4%) 7 (9.7%) 2 (10.0%) Oral herpes 2 (5.4%) 3 (8.6%) 5 (6.9%) 2 (10.0%) Urinary tract infection 2 (5.4%) 3 (8.6%) 5 (6.9%) 2 (10.0%) Musculoskeletal and connective tissue disorders 9 (24.3%) 10 (28.6%) 19 (26.4%) 4 (20.0%) Arthralgia 4 (10.8%) 5 (14.3%) 9 (12.5%) 2 (10.0%) Nervous system disorders 6 (16.2%) 8 (22.9%) 14 (19.4%) 3 (15.0%) Headache 3 (8.1%) 5 (14.3%) 8 (11.1%) 3 (15.0%) Skin and subcutaneous tissue disorders 5 (13.5%) 9 (25.7%) 14 (19.4%) 6 (30.0%) Alopecia 0 1 (2.9%) 1 (1.4%) 2 (10.0%) Blood and lymphatic system disorders 4 (10.8%) 9 (25.7%) 13 (18.1%) 1 (5.0%) Eosinophilia 0 5 (14.3%) 5 (6.9%) 0 Respiratory, thoracic and mediastinal disorders 4 (10.8%) 7 (20.0%) 11 (15.3%) 2 (10.0%) Eye disorders 3 (8.1%) 2 (5.7%) 5 (6.9%) 2 (10.0%) Gastrointestinal disorders 3 (8.1%) 10 (28.6%) 13 (18.1%) 3 (15.0%) Gastroesophageal reflux disease 0 0 0 2 (10.0%) Investigations 3 (8.1%) 7 (20.0%) 10 (13.9%) 2 (10.0%) 1. One patient in the 18 µg/kg had a serious AE of a gun shot wound, and patient continued in the study. One patient in the 24 µg/kg had a severe ISR report AE, but patient continued on treatment. 36
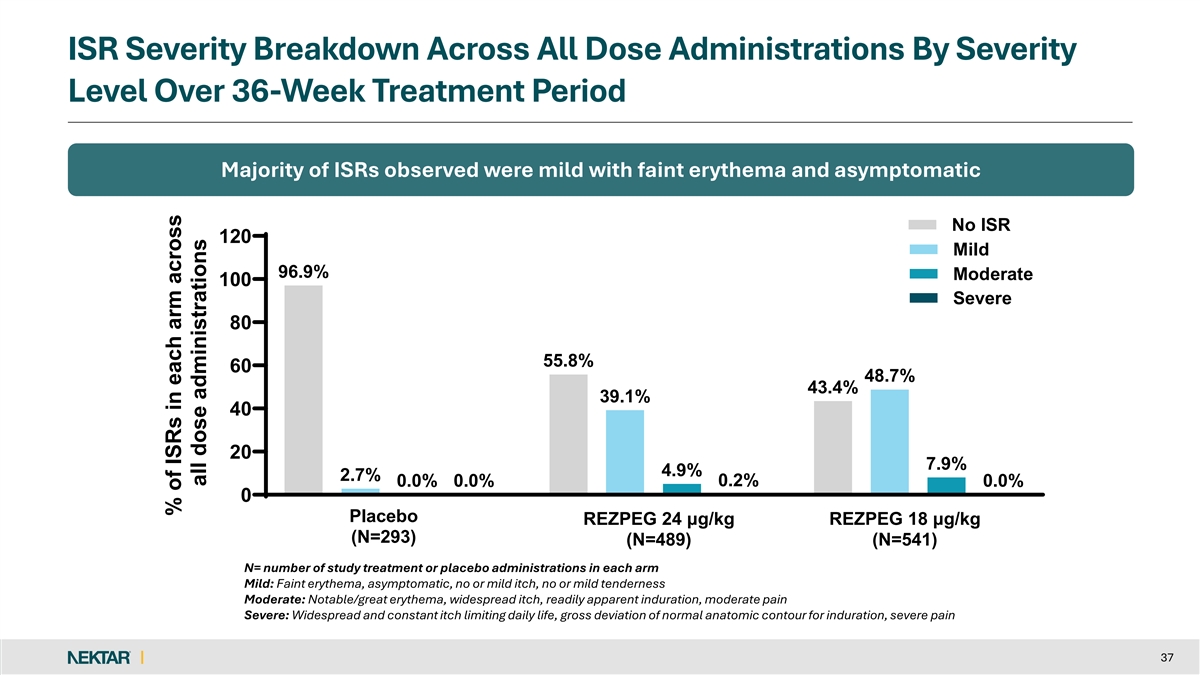
ISR Severity Breakdown Across All Dose Administrations By Severity Level Over 36-Week Treatment Period Majority of ISRs observed were mild with faint erythema and asymptomatic No ISR 120 Mild 96.9% Moderate 100 Severe 80 55.8% 60 48.7% 43.4% 39.1% 40 20 7.9% 4.9% 2.7% 0.2% 0.0% 0.0% 0.0% 0 Placebo REZPEG 24 μg/kg REZPEG 18 μg/kg (N=293) (N=489) (N=541) N= number of study treatment or placebo administrations in each arm Mild: Faint erythema, asymptomatic, no or mild itch, no or mild tenderness Moderate: Notable/great erythema, widespread itch, readily apparent induration, moderate pain Severe: Widespread and constant itch limiting daily life, gross deviation of normal anatomic contour for induration, severe pain 37 % of ISRs in each arm across all dose administrations
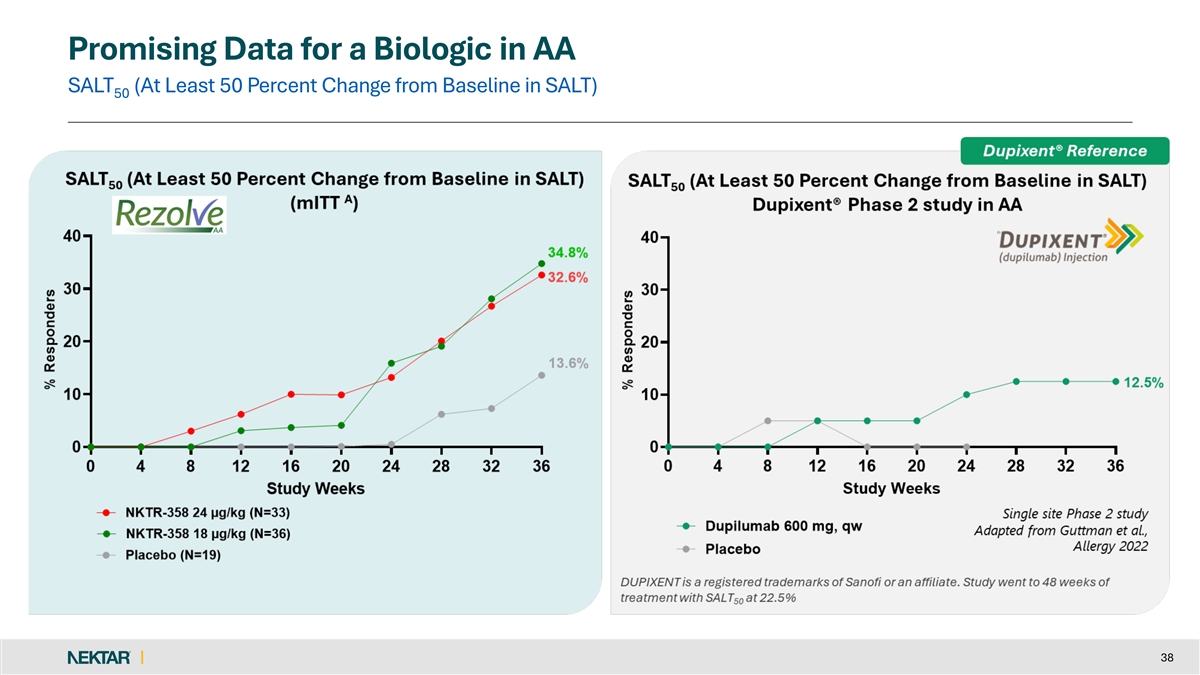
Promising Data for a Biologic in AA SALT (At Least 50 Percent Change from Baseline in SALT) 50 38