| Investor Presentation OTCQX: AWHL |
| Forward -looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts contained in this presentation, including statements regarding Aspira Women’s Health, Inc.’s (the “Company’s” or Aspira”) strategy, future, operations, future financial position, projected costs, prospects, plans and objectives of management are, forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “depends,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “will,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of forward-looking statements include but are not limited to our projections or expectations regarding our future test volumes, revenue, average unit price, cost of revenue, operating expenses, research and development expenses, gross profit margin, cash flow, results of operations and financial condition ; our plan to broaden our commercial focus from ovarian cancer to differential diagnosis of women with a range of gynecological diseases, including additional pelvic disease conditions such as endometriosis and benign pelvic mass monitoring ; our plan to address our liquidity needs and capital requirements; our anticipated future losses and our ability to continue as a going concern; and our expectations regarding raising capital and the amount of financing anticipated to be required to fund our planned operations. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Such differences may result from a variety of factors, including, but not limited to, those described under the heading “Risk Factors” in the Company’s most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and other filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent the Company’s views. Subsequent events and developments may cause the Company’s views to change. The Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representations of the Company’s views as of any date subsequent to the date of this presentation. |
| Experienced Women’s Health Executive Team Mike Buhle Chief Executive Officer Brian Hungerford Chief Financial Officer Todd Pappas, PhD Chief Scientific Officer, VP of R&D and Lab Operations Michelle Snider SVP, Commercialization and Integration Matt Ramey SVP, Commercial & Corporate Accounts |
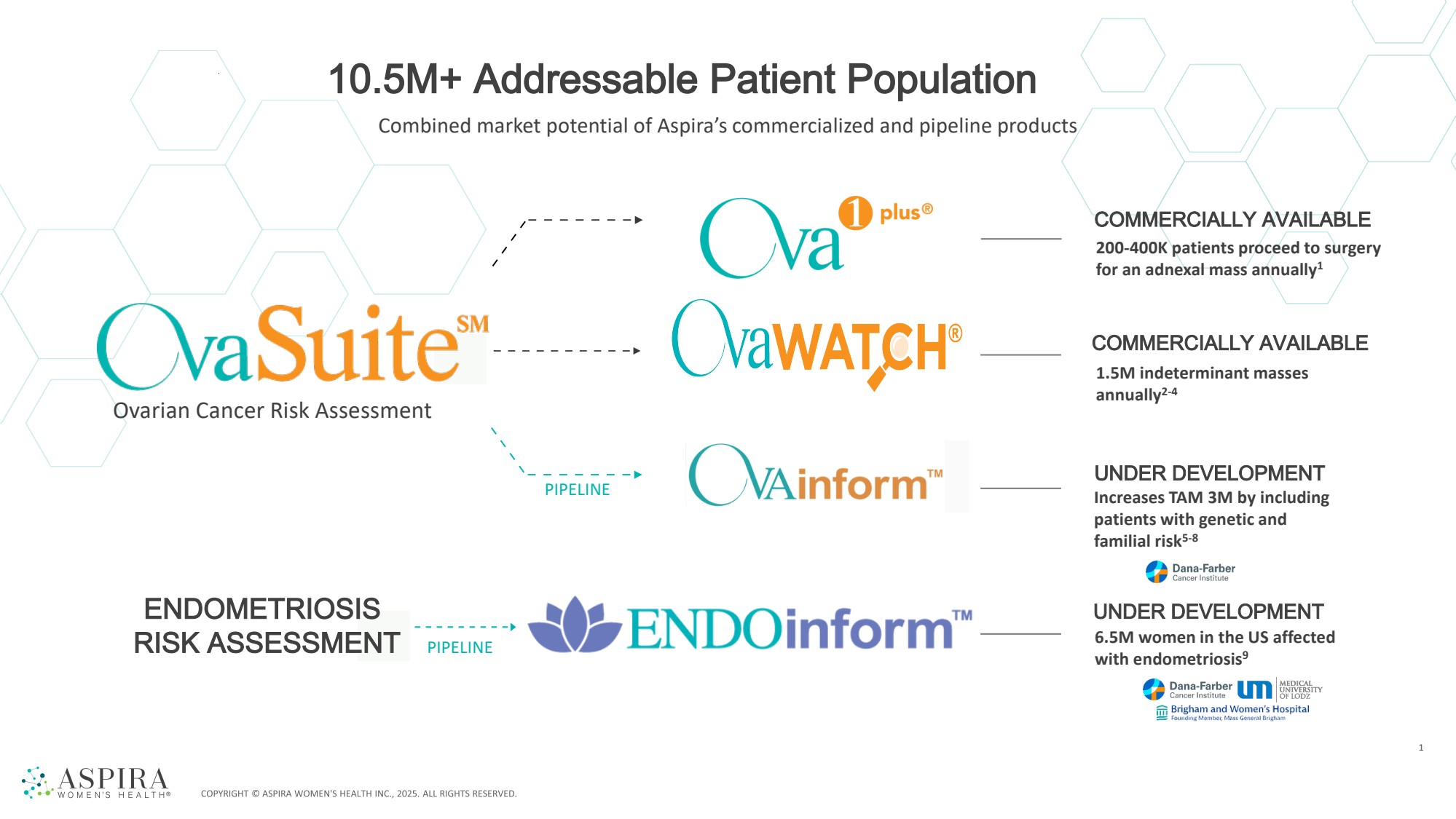
| UNDER DEVELOPMENT PIPELINE PIPELINE COMMERCIALLY AVAILABLE COMMERCIALLY AVAILABLE UNDER DEVELOPMENT 1 200-400K patients proceed to surgery for an adnexal mass annually1 1.5M indeterminant masses annually2-4 Increases TAM 3M by including patients with genetic and familial risk5-8 6.5M women in the US affected with endometriosis9 ENDOMETRIOSIS RISK ASSESSMENT Combined market potential of Aspira’s commercialized and pipeline products 10.5M+ Addressable Patient Population Ovarian Cancer Risk Assessment |
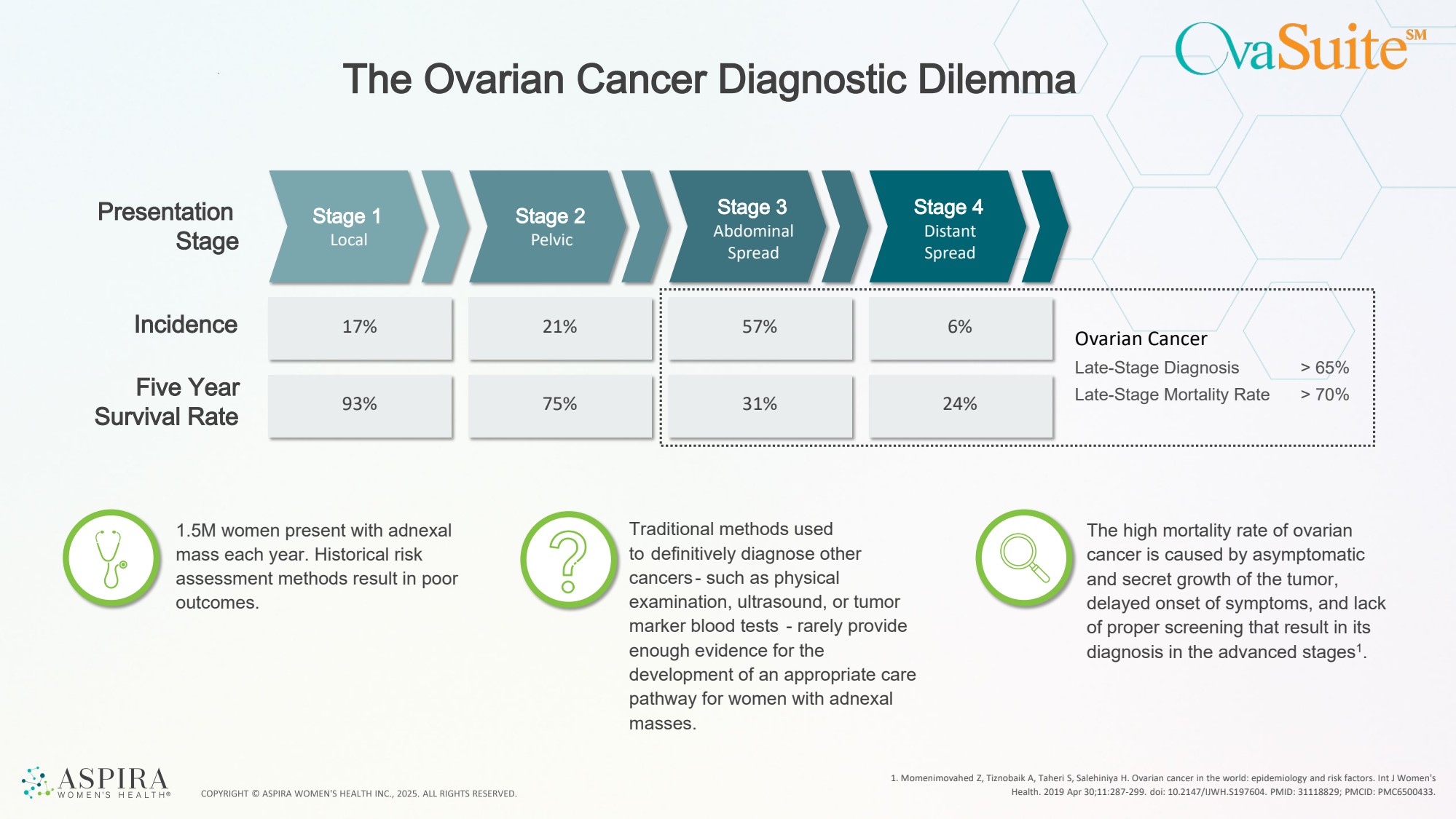
| 57% 31% 6% The Ovarian Cancer Diagnostic Dilemma 1. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Women's Health. 2019 Apr 30;11:287-299. doi: 10.2147/IJWH.S197604. PMID: 31118829; PMCID: PMC6500433. Presentation Stage 1 Stage 2 Stage Stage 3 Stage 4 Local Pelvic Abdominal Spread Distant Spread Incidence Five Year Survival Rate 17% 93% 21% 75% 1.5M women present with adnexal mass each year. Historical risk assessment methods result in poor outcomes. Traditional methods used to definitively diagnose other cancers - such as physical examination, ultrasound, or tumor marker blood tests - rarely provide enough evidence for the development of an appropriate care pathway for women with adnexal masses. The high mortality rate of ovarian cancer is caused by asymptomatic and secret growth of the tumor, delayed onset of symptoms, and lack of proper screening that result in its diagnosis in the advanced stages1. 24% Ovarian Cancer Late-Stage Diagnosis Late-Stage Mortality Rate > 65% > 70% |
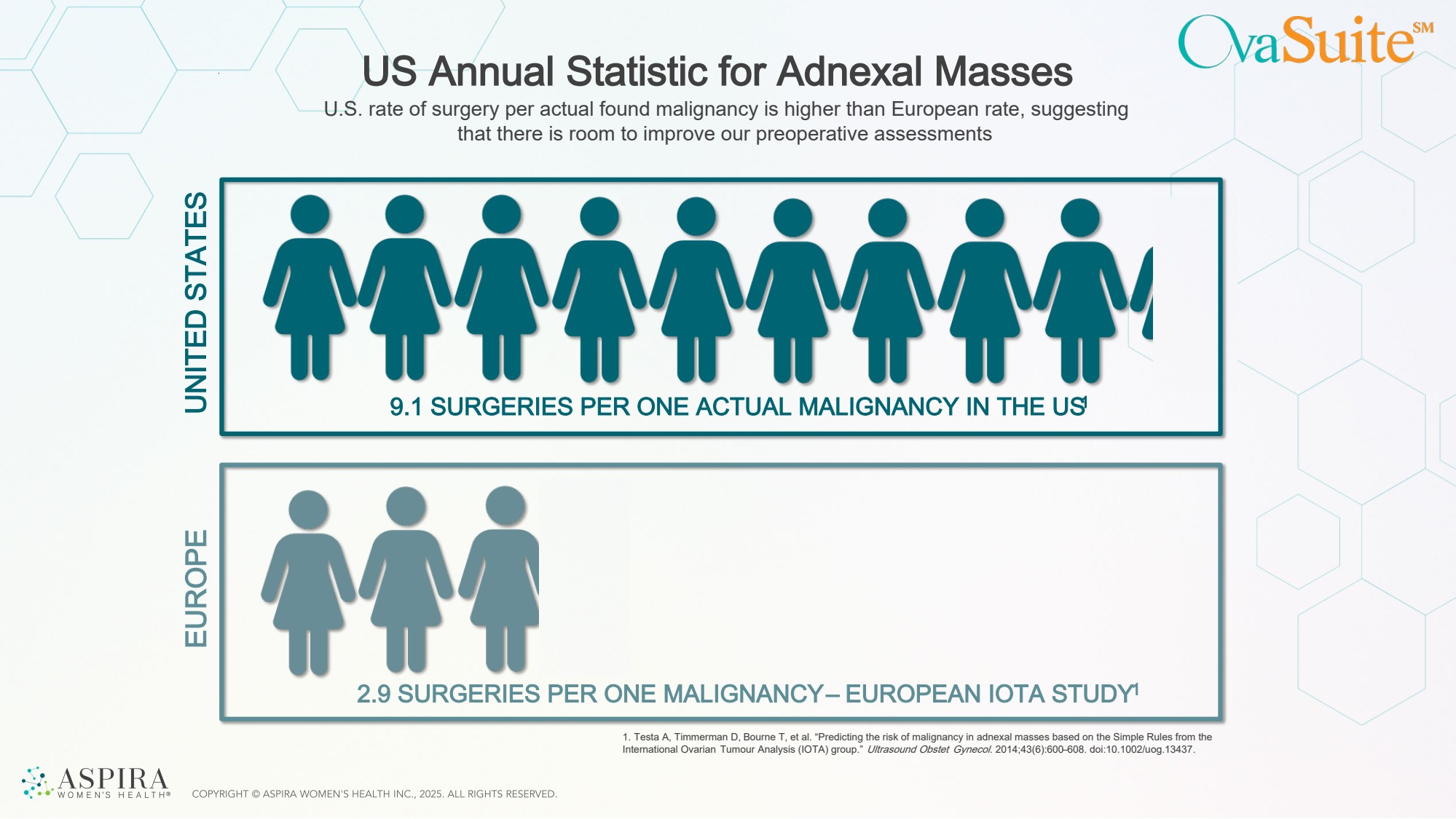
| US Annual Statistic for Adnexal Masses 9.1 SURGERIES PER ONE ACTUAL MALIGNANCY IN THE US1 2.9 SURGERIES PER ONE MALIGNANCY – EUROPEAN IOTA STUDY1 U.S. rate of surgery per actual found malignancy is higher than European rate, suggesting that there is room to improve our preoperative assessments UNITED STATES EUROPE 1. Testa A, Timmerman D, Bourne T, et al. “Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumour Analysis (IOTA) group.” Ultrasound Obstet Gynecol. 2014;43(6):600–608. doi:10.1002/uog.13437. |
| Biomarkers (including CA-125), menopausal status, and ultrasound for a personalized risk score A Test for Women for Every Adnexal Mass 1. Through September 2025 2. Bristow, R. E., Smith, A., Zhang, Z., Chan, D. W., Crutcher, G., Fung, E. T., & Munroe, D. G. (2013). Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecologic oncology, 128(2), 252-259 3.Dunton C, Bullock RG, Twiggs L et al. Improvement in MIA testing for detection of ovarian malignancy. Poster presentation at the SGO 2020 meeting. 4. Reilly, G., Bullock, R. G., Greenwood, J., Ure, D. R., Stewart, E., Davidoff, P., ... & Northrop, L. E. (2022). Analytical Validation of a Deep Neural Network Algorithm for the Detection of Ovarian Cancer. JCO Clinical Cancer Informatics, 6, e2100192. Ova1® has a greater than 98% sensitivity with clinical assessment2 Negative Predictive Value of over 99%4 For adnexal masses initially evaluated as indeterminate or benign Longitudinal monitoring feature to help providers track risk over time MULTIVARIATE INDEX ASSAY OPTIMIZED FOR SURGICAL TRIAGING MACHINE LEARNING ALGORITHM OPTIMIZED TOWARDS NEGATIVE PREDICTIVE VALUE (NPV) TO ASSESS OVARIAN CANCER RISK PLANNED FOR SURGERY PLANNED FOR CLINICAL MANAGEMENT Ova1Plus is a reflex process: Ova1® is performed3 first. If an intermediate risk is detected, Overa ® is automatically performed. Physicians have ordered ~240,000 OvaSuite tests since launch1 |
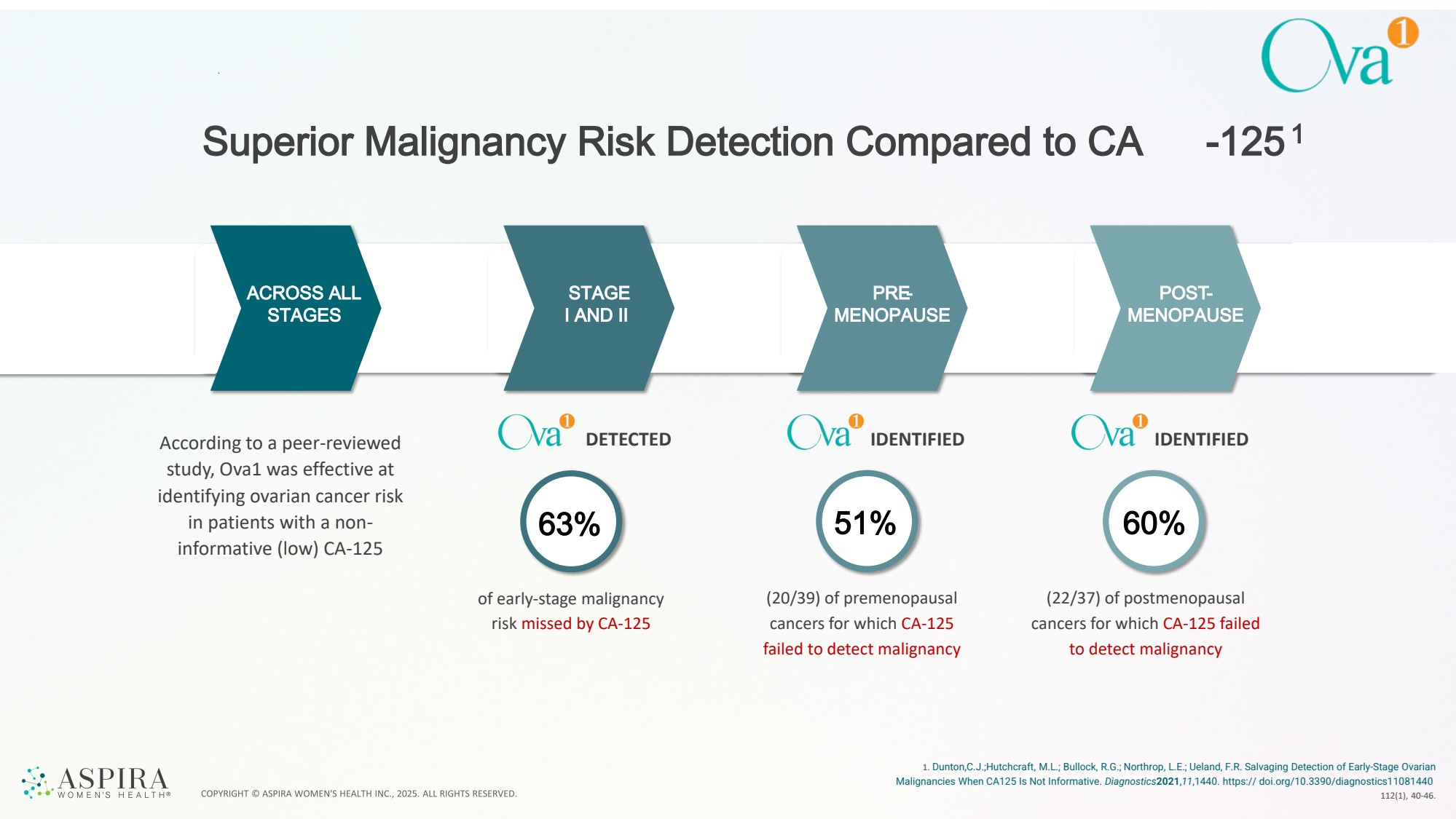
| Superior Malignancy Risk Detection Compared to CA -1251 1. Dunton,C.J.;Hutchcraft, M.L.; Bullock, R.G.; Northrop, L.E.; Ueland, F.R. Salvaging Detection of Early-Stage Ovarian Malignancies When CA125 Is Not Informative. Diagnostics2021,11,1440. https:// doi.org/10.3390/diagnostics11081440 112(1), 40-46. 63% 51% 60% of early-stage malignancy risk missed by CA-125 (20/39) of premenopausal cancers for which CA-125 failed to detect malignancy (22/37) of postmenopausal cancers for which CA-125 failed to detect malignancy ACROSS ALL STAGES STAGE I AND II PRE-MENOPAUSE POST-MENOPAUSE According to a peer-reviewed DETECTED study, Ova1 was effective at identifying ovarian cancer risk in patients with a non-informative (low) CA-125 IDENTIFIED IDENTIFIED |
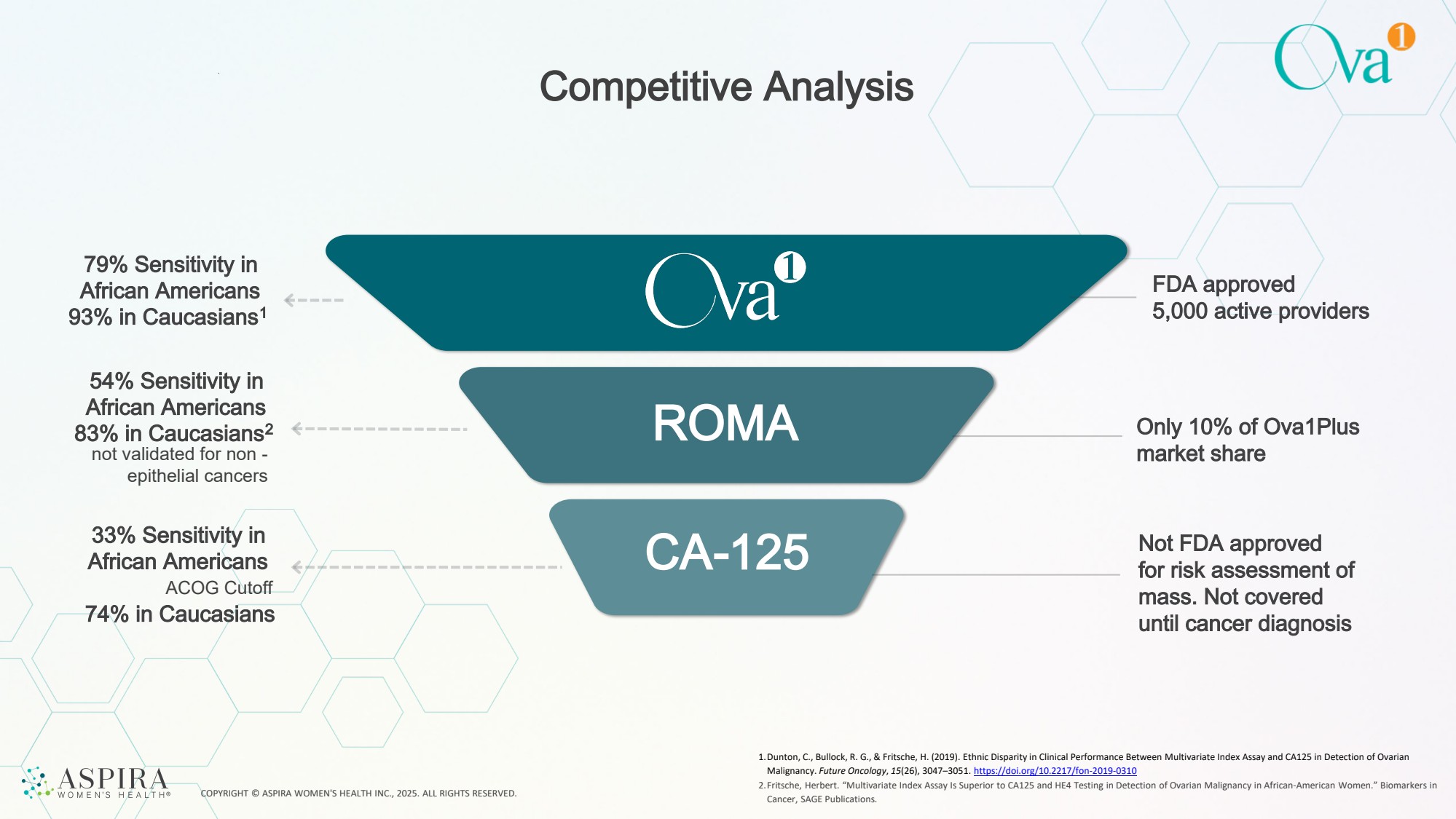
| COPYRIGHT © ASPIRA WOMEN'S HEALTH INC., 2025. ALL RIGHTS RESERVED. Competitive Analysis 54% Sensitivity in African Americans 83% in Caucasians2 ROMA 79% Sensitivity in African Americans 93% in Caucasians1 33% Sensitivity in African Americans ACOG Cutoff 74% in Caucasians CA-125 not validated for non - epithelial cancers FDA approved 5,000 active providers Only 10% of Ova1Plus market share Not FDA approved for risk assessment of mass. Not covered until cancer diagnosis 1.Dunton, C., Bullock, R. G., & Fritsche, H. (2019). Ethnic Disparity in Clinical Performance Between Multivariate Index Assay and CA125 in Detection of Ovarian Malignancy. Future Oncology, 15(26), 3047–3051. https://doi.org/10.2217/fon-2019-0310 2.Fritsche, Herbert. “Multivariate Index Assay Is Superior to CA125 and HE4 Testing in Detection of Ovarian Malignancy in African-American Women.” Biomarkers in Cancer, SAGE Publications. |
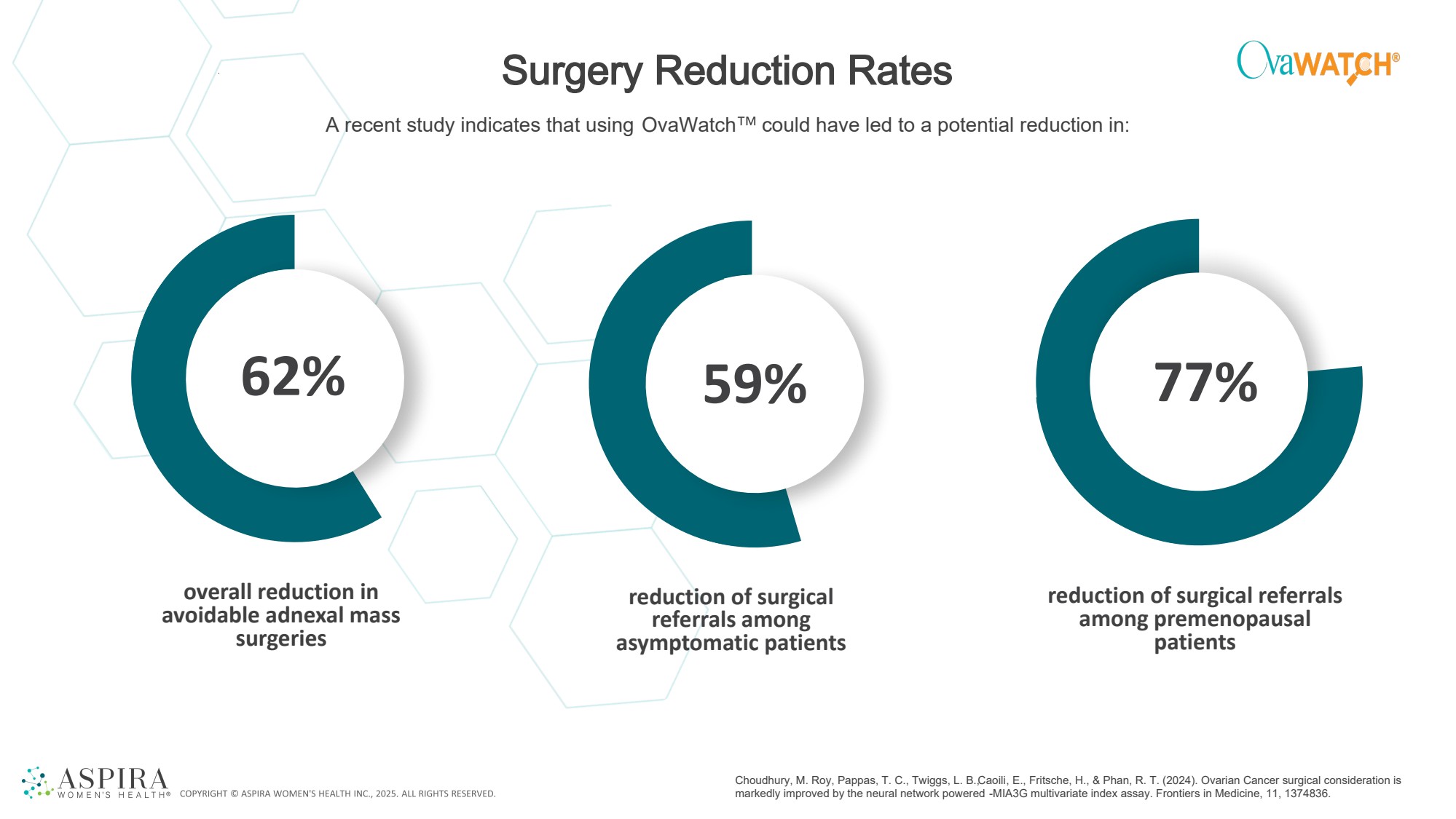
| Surgery Reduction Rates overall reduction in avoidable adnexal mass surgeries reduction of surgical referrals among asymptomatic patients reduction of surgical referrals among premenopausal patients 62% 59% 77% A recent study indicates that using OvaWatch could have led to a potential reduction in: Choudhury, M. Roy, Pappas, T. C., Twiggs, L. B., Caoili, E., Fritsche, H., & Phan, R. T. (2024). Ovarian Cancer surgical consideration is markedly improved by the neural network powered -MIA3G multivariate index assay. Frontiers in Medicine, 11, 1374836. |
| Product Pipeline Ad vancing Wome n’s He alth d iag nostics b y inte g rating AI-p owe re d , multi-omic te chnolog y to e nhance risk asse ssme nt p e rformance and clinical utility |
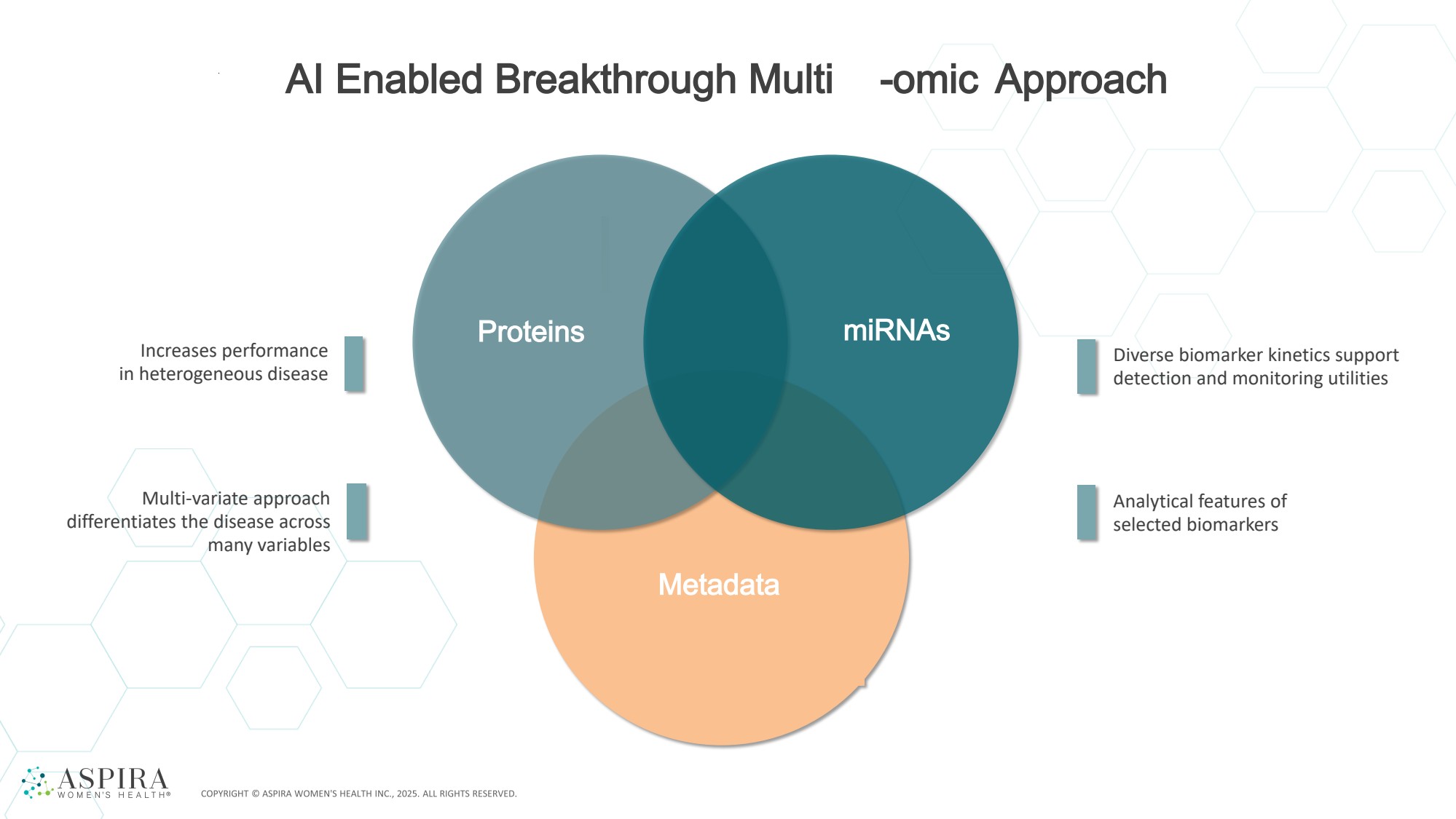
| AI Enabled Breakthrough Multi -omic Approach Proteins miRNAs Metadata Increases performance in heterogeneous disease Multi-variate approach differentiates the disease across many variables Diverse biomarker kinetics support detection and monitoring utilities Analytical features of selected biomarkers |
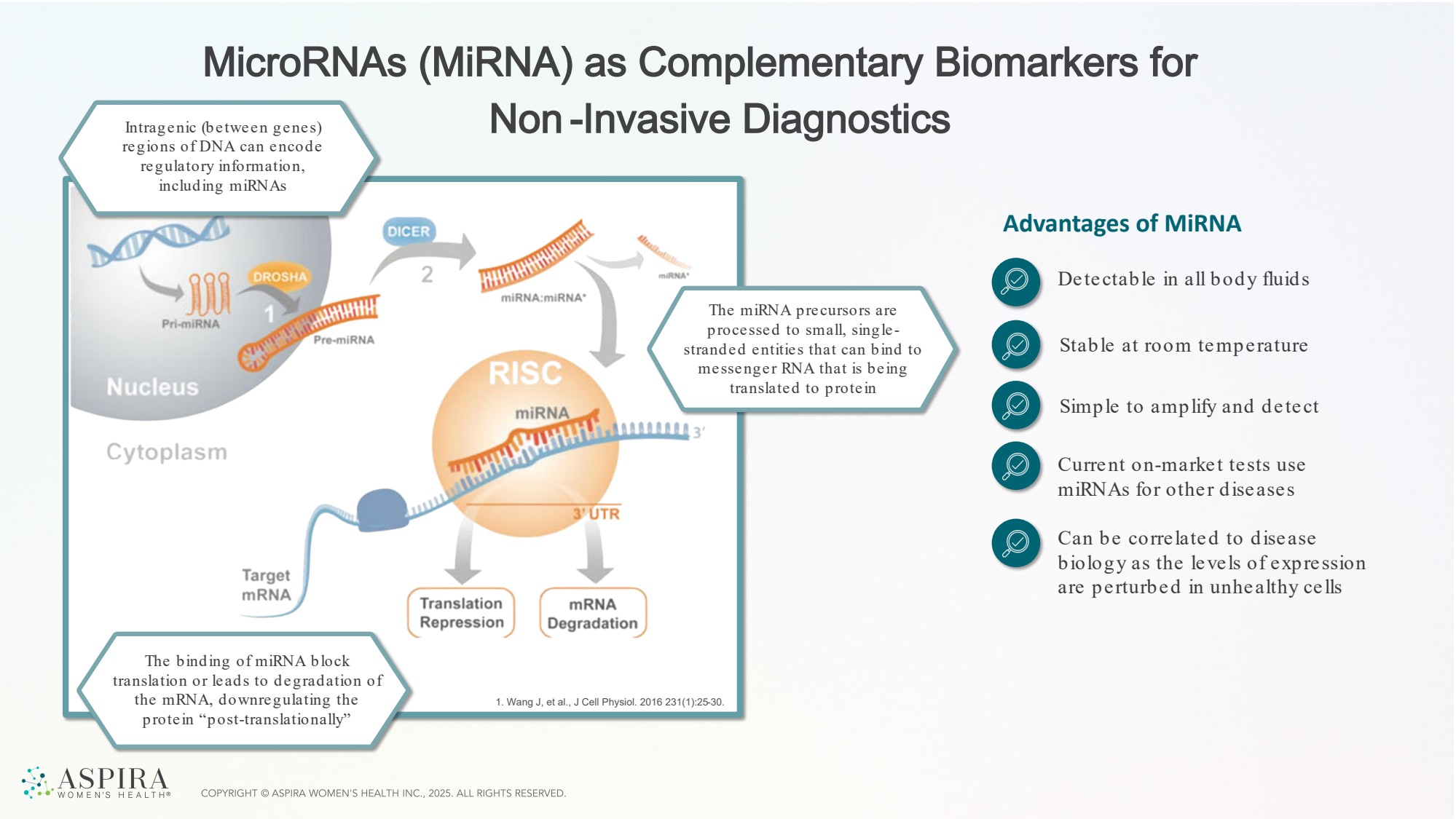
| MicroRNAs (MiRNA) as Complementary Biomarkers for Non -Invasive Diagnostics 1. Wang J, et al., J Cell Physiol. 2016 231(1):25-30. Advantages of MiRNA Detectable in all body fluids Stable at room temperature Simple to amplify and detect Current on-market tests use miRNAs for other diseases Can be correlated to disease biology as the levels of expression are perturbed in unhealthy cells Intragenic (between genes) regions of DNA can encode regulatory information, including miRNAs The binding of miRNA block translation or leads to degradation of the mRNA, downregulating the protein “post-translationally” The miRNA precursors are processed to small, single - stranded entities that can bind to messenger RNA that is being translated to protein |
| First-of-its-kind, AI -powered, non -invasive blood test for endometriosis |
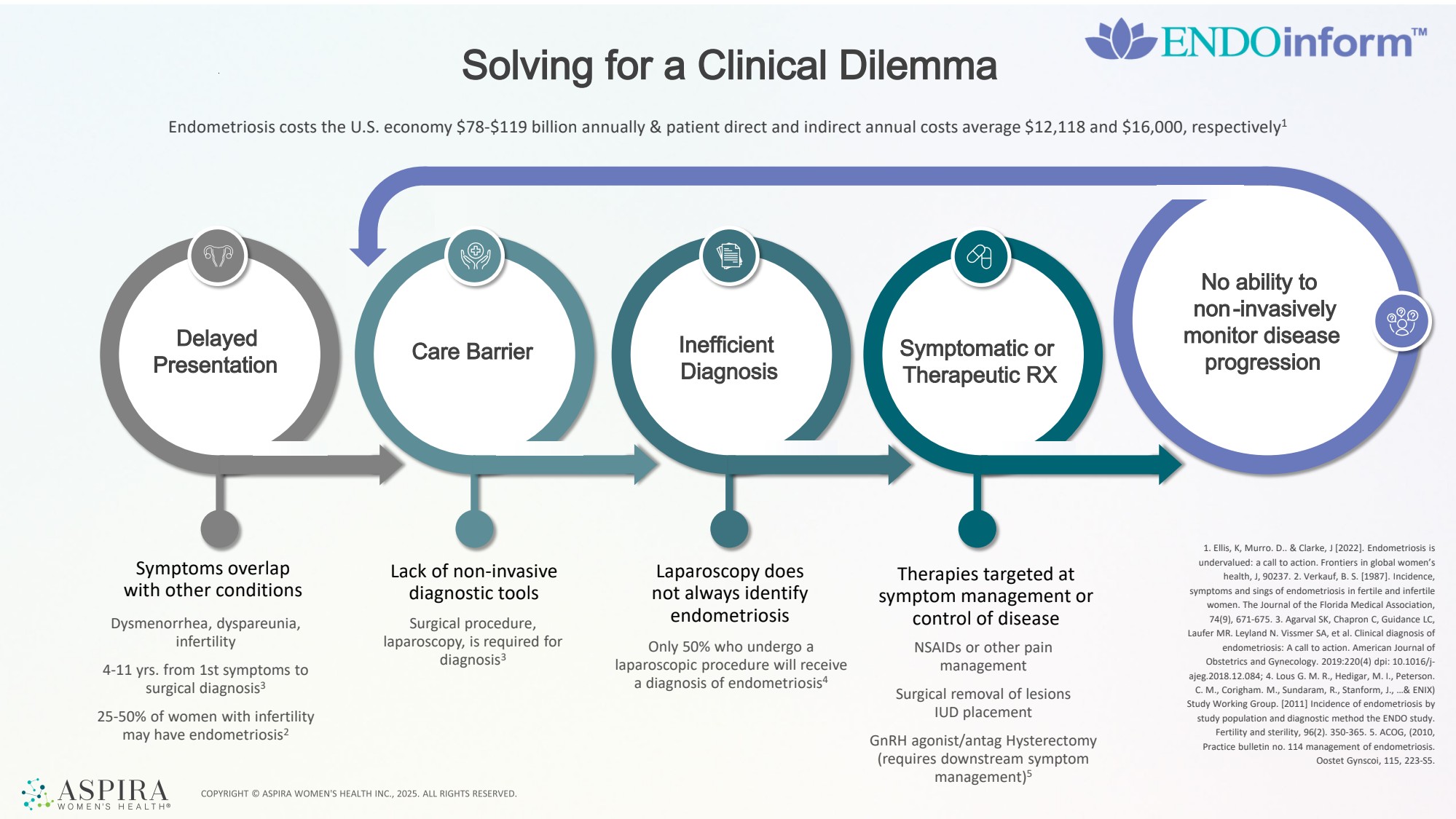
| Symptoms overlap with other conditions Lack of non-invasive diagnostic tools Dysmenorrhea, dyspareunia, infertility 4-11 yrs. from 1st symptoms to surgical diagnosis3 25-50% of women with infertility may have endometriosis2 Surgical procedure, laparoscopy, is required for diagnosis3 Laparoscopy does not always identify endometriosis Only 50% who undergo a laparoscopic procedure will receive a diagnosis of endometriosis4 Therapies targeted at symptom management or control of disease NSAIDs or other pain management Surgical removal of lesions IUD placement GnRH agonist/antag Hysterectomy (requires downstream symptom management)5 Solving for a Clinical Dilemma Endometriosis costs the U.S. economy $78-$119 billion annually & patient direct and indirect annual costs average $12,118 and $16,000, respectively1 Delayed Presentation Care Barrier Inefficient Diagnosis Symptomatic or Therapeutic RX No ability to non-invasively monitor disease progression 1. Ellis, K, Murro. D.. & Clarke, J [2022]. Endometriosis is undervalued: a call to action. Frontiers in global women’s health, J, 90237. 2. Verkauf, B. S. [1987]. Incidence, symptoms and sings of endometriosis in fertile and infertile women. The Journal of the Florida Medical Association, 74(9), 671-675. 3. Agarval SK, Chapron C, Guidance LC, Laufer MR. Leyland N. Vissmer SA, et al. Clinical diagnosis of endometriosis: A call to action. American Journal of Obstetrics and Gynecology. 2019:220(4) dpi: 10.1016/j-ajeg.2018.12.084; 4. Lous G. M. R., Hedigar, M. I., Peterson. C. M., Corigham. M., Sundaram, R., Stanform, J., …& ENIX) Study Working Group. [2011] Incidence of endometriosis by study population and diagnostic method the ENDO study. Fertility and sterility, 96(2). 350-365. 5. ACOG, (2010, Practice bulletin no. 114 management of endometriosis. Oostet Gynscoi, 115, 223-S5. |
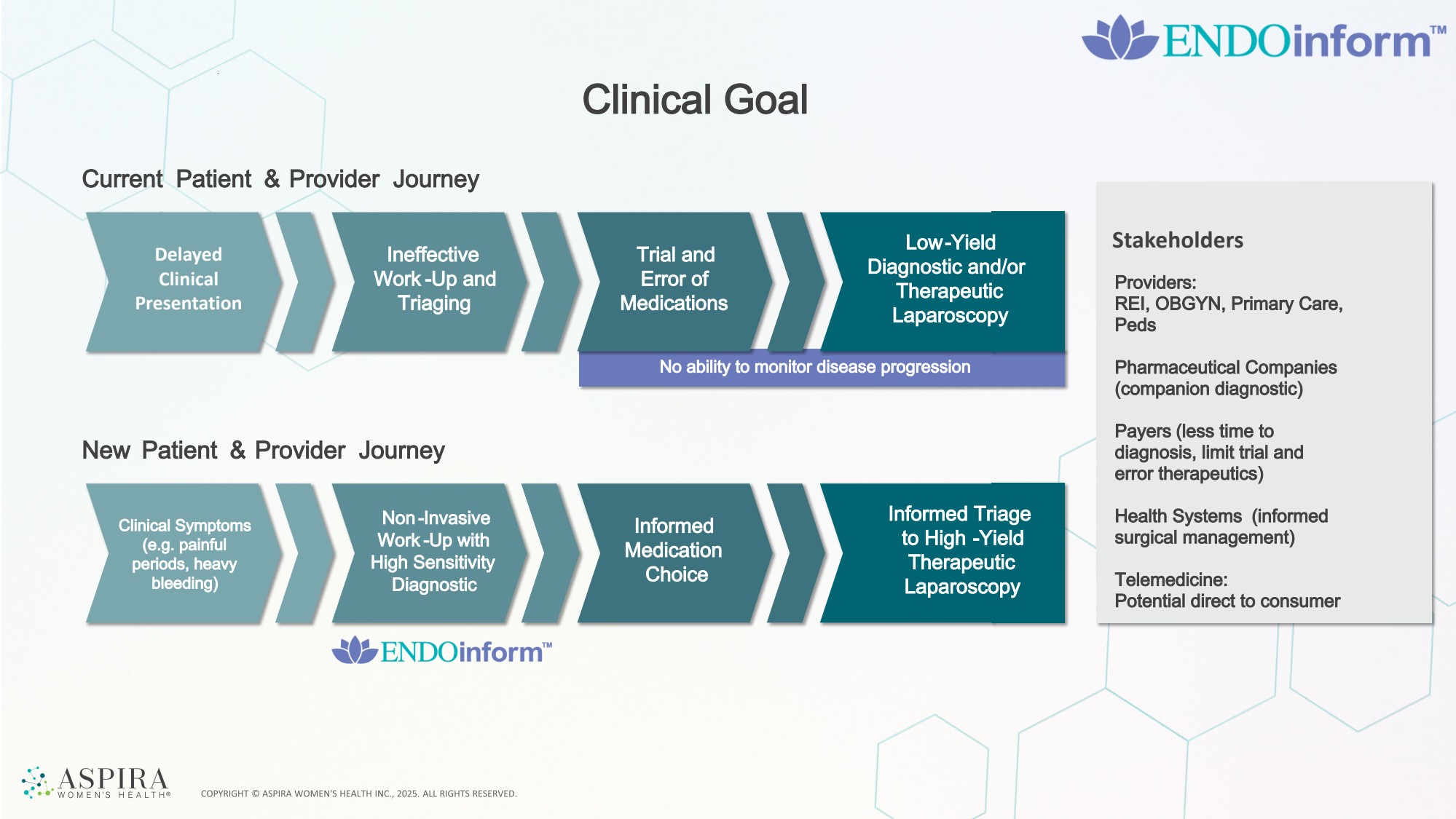
| Clinical Goal Delayed Clinical Presentation Ineffective Work -Up and Triaging Current Patient & Provider Journey Trial and Error of Medications Low-Yield Diagnostic and/or Therapeutic Laparoscopy New Patient & Provider Journey Clinical Symptoms (e.g. painful periods, heavy bleeding) Non -Invasive Work -Up with High Sensitivity Diagnostic Informed Medication Choice Informed Triage to High -Yield Therapeutic Laparoscopy Stakeholders No ability to monitor disease progression Providers: REI, OBGYN, Primary Care, Peds Pharmaceutical Companies (companion diagnostic) Payers (less time to diagnosis, limit trial and error therapeutics) Health Systems (informed surgical management) Telemedicine: Potential direct to consumer Informed Triage to High -Yield Therapeutic Laparoscopy |
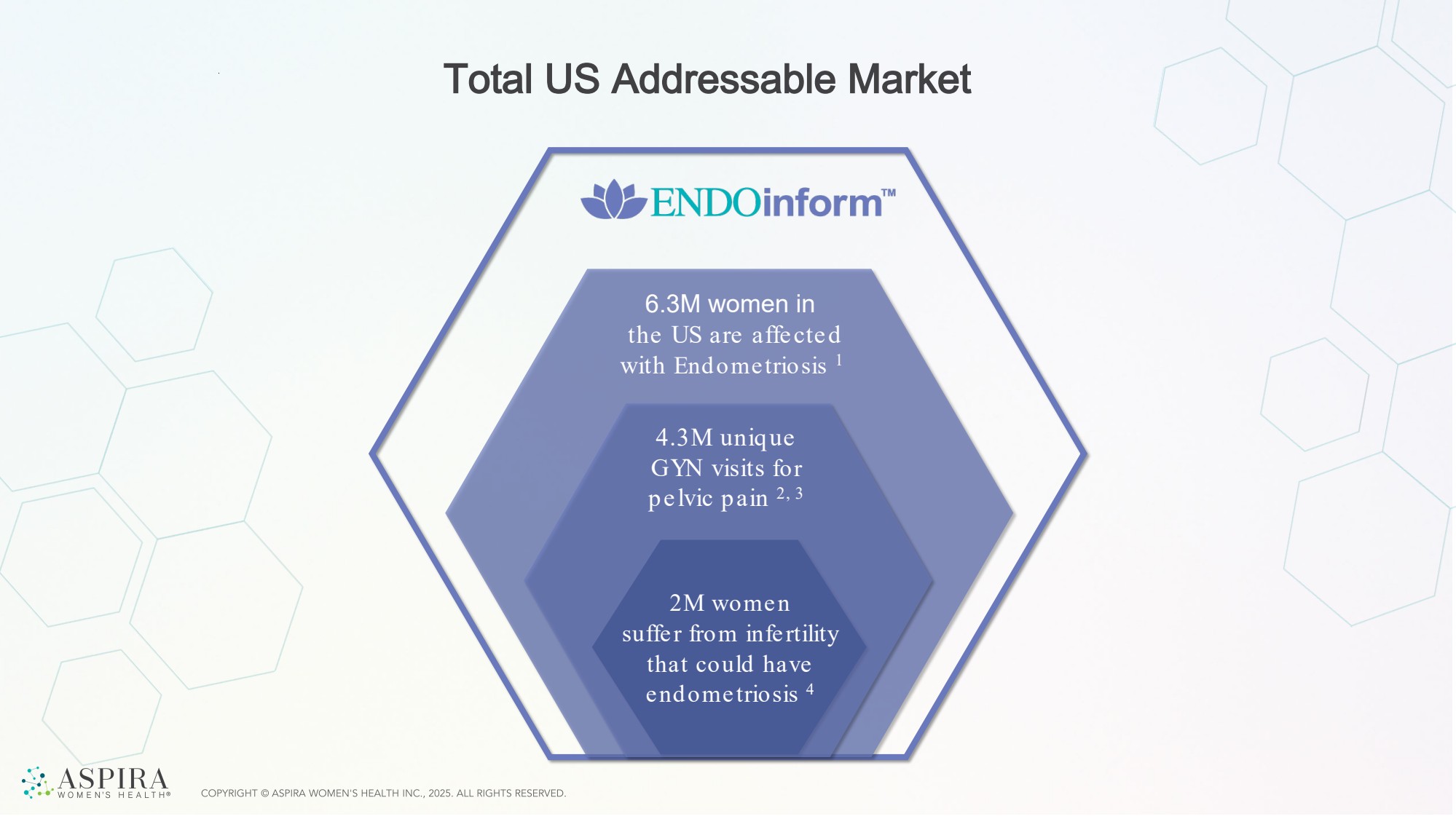
| 6.3M women in the US are affected with Endometriosis 1 Total US Addressable Market 4.3M unique GYN visits for pelvic pain 2, 3 2M women suffer from infertility that could have endometriosis 4 |
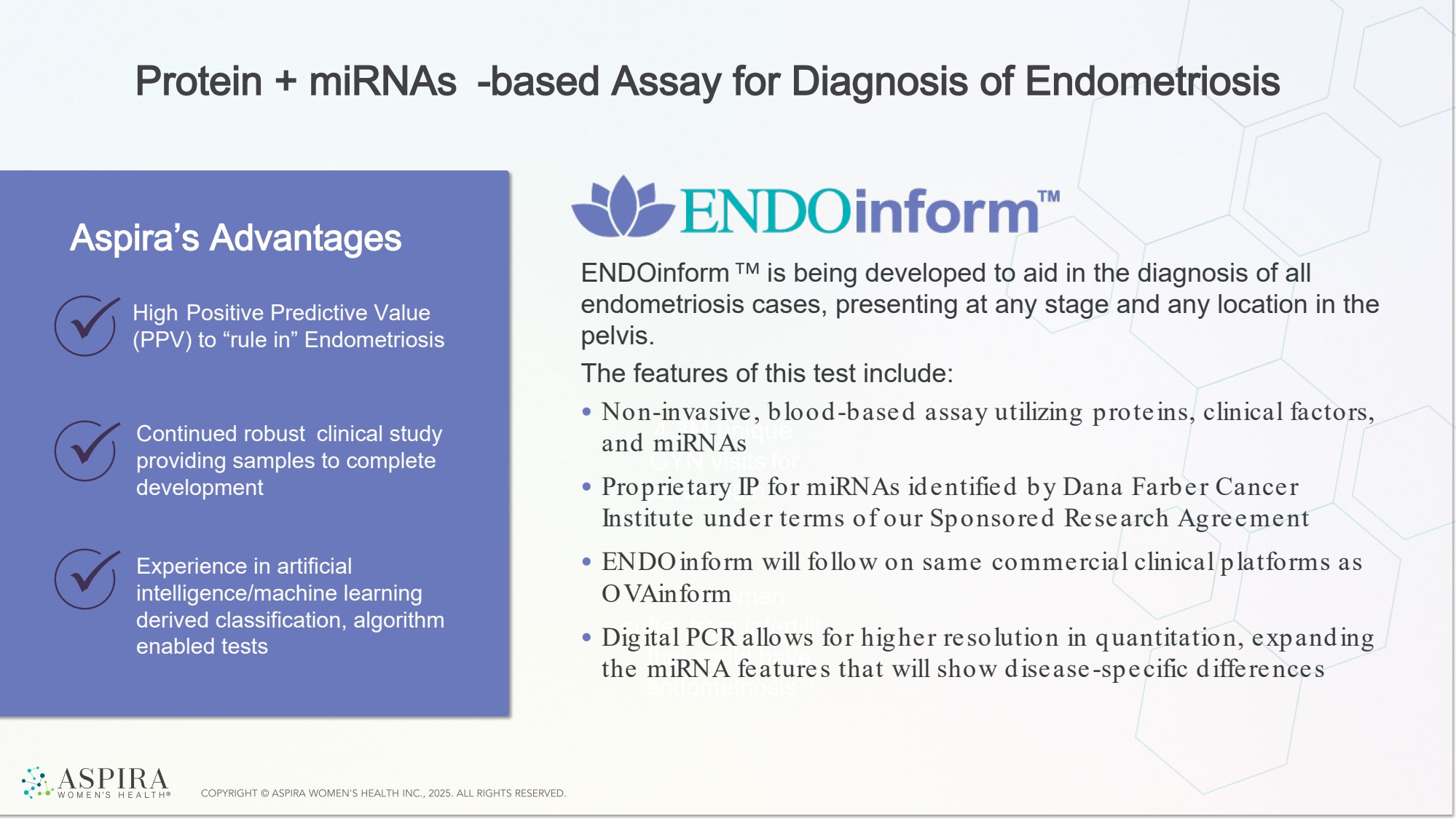
| Protein + miRNAs -based Assay for Diagnosis of Endometriosis 4.3M unique GYN visits for pelvic pain 2, 3 2Mwomen suffer from infertility that could have endometriosis 4 High Positive Predictive Value (PPV) to “rule in” Endometriosis Continued robust clinical study providing samples to complete development Experience in artificial intelligence/machine learning derived classification, algorithm enabled tests Aspira’s Advantages ENDOinform is being developed to aid in the diagnosis of all endometriosis cases, presenting at any stage and any location in the pelvis. The features of this test include: • Non-invasive, blood-based assay utilizing proteins, clinical factors, and miRNAs • Proprietary IP for miRNAs identified by Dana Farber Cancer Institute under terms of our Sponsored Research Agreement • ENDOinform will follow on same commercial clinical platforms as OVAinform • Digital PCR allows for higher resolution in quantitation, expanding the miRNA features that will show disease -specific differences |
| First-of-its-kind , AI-powered next-g e ne ration ovarian cance r asse ssme nt for ad ne xal mass, g e rmline , and familial risk. |
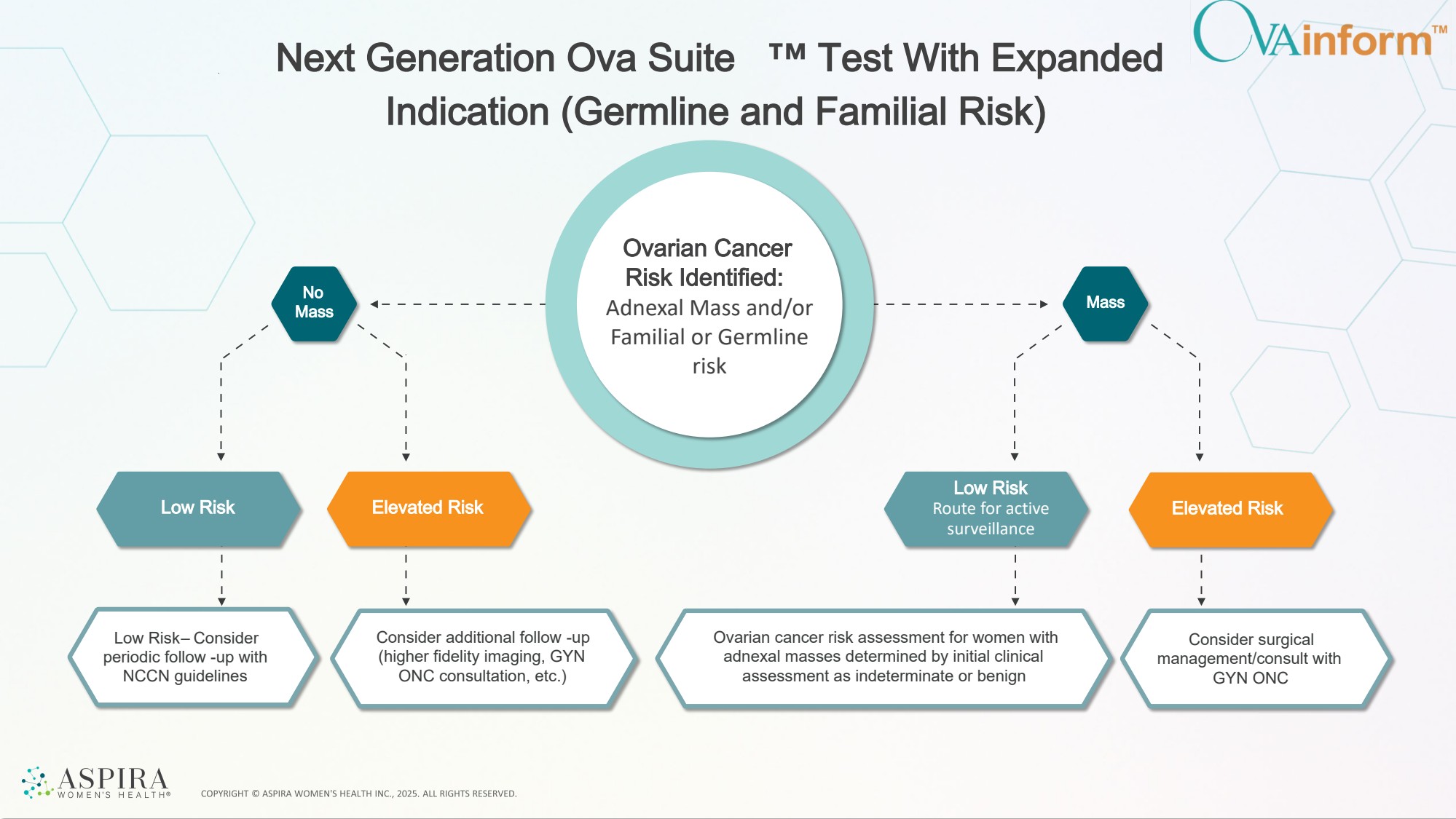
| No Mass Mass Next Generation Ova Suite Test With Expanded Indication (Germline and Familial Risk) Low Risk Low Risk Route for active surveillance Elevated Risk Elevated Risk Ovarian cancer risk assessment for women with adnexal masses determined by initial clinical assessment as indeterminate or benign Consider surgical management/consult with GYN ONC Consider additional follow -up (higher fidelity imaging, GYN ONC consultation, etc.) Low Risk – Consider periodic follow -up with NCCN guidelines Ovarian Cancer Risk Identified: Adnexal Mass and/or Familial or Germline risk |
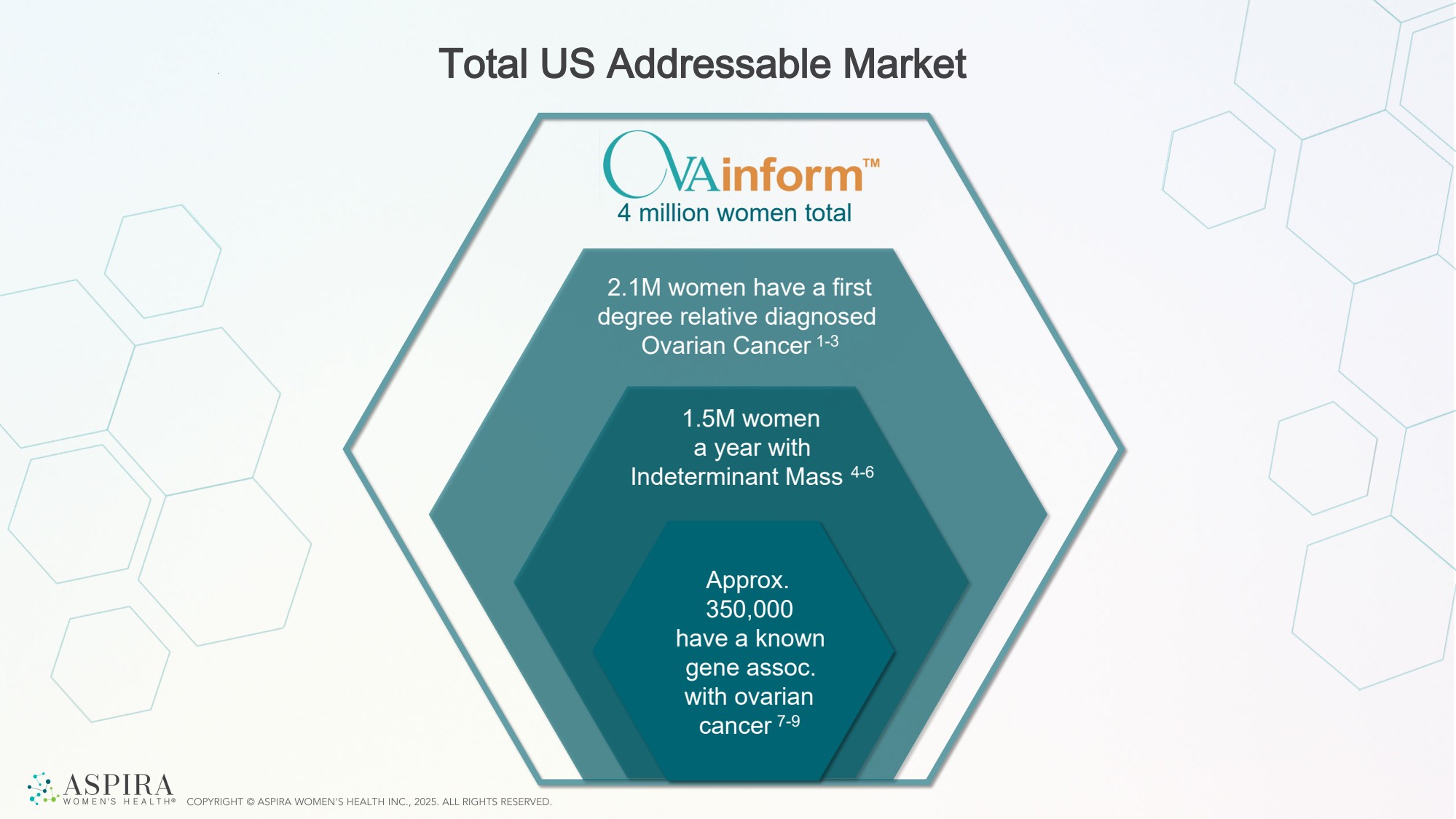
| Total US Addressable Market Approx. 350,000 have a known gene assoc. with ovarian cancer 7-9 4 million women total 1.5M women a year with Indeterminant Mass 4-6 2.1M women have a first degree relative diagnosed Ovarian Cancer 1-3 |
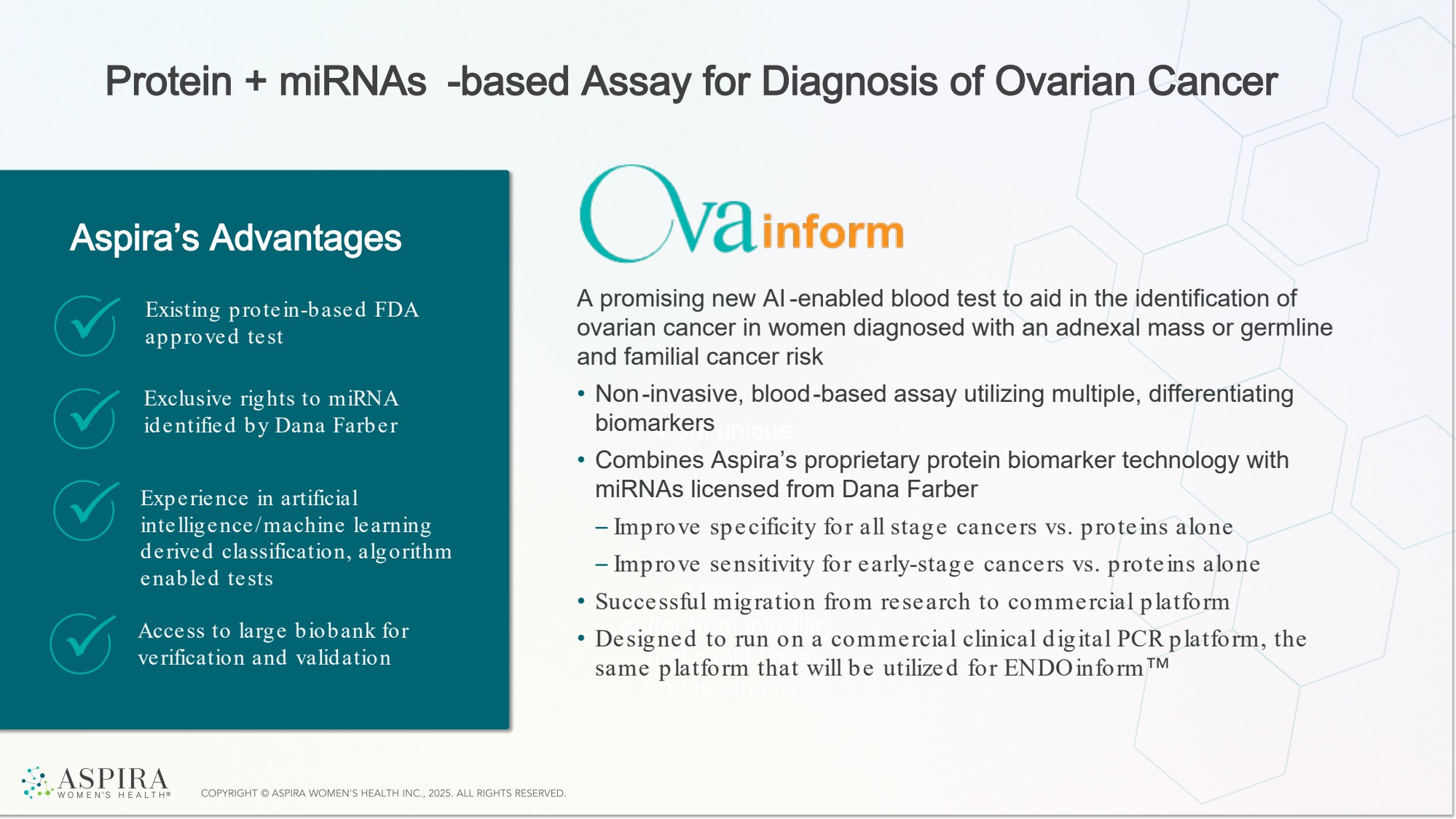
| Protein + miRNAs -based Assay for Diagnosis of Ovarian Cancer 4.3M unique GYN visits for pelvic pain 2, 3 2Mwomen suffer from infertility that could have endometriosis 4 Aspira’s Advantages A promising new AI-enabled blood test to aid in the identification of ovarian cancer in women diagnosed with an adnexal mass or germline and familial cancer risk • Non-invasive, blood-based assay utilizing multiple, differentiating biomarkers • Combines Aspira’s proprietary protein biomarker technology with miRNAs licensed from Dana Farber – Improve specificity for all stage cancers vs. proteins alone – Improve sensitivity for early-stage cancers vs. proteins alone • Successful migration from research to commercial platform • Designed to run on a commercial clinical digital PCR platform, the same platform that will be utilized for ENDOinform Existing protein-based FDA approved test Exclusive rights to miRNA identified by Dana Farber Experience in artificial intelligence/machine learning derived classification, algorithm enabled tests Access to large biobank for verification and validation |
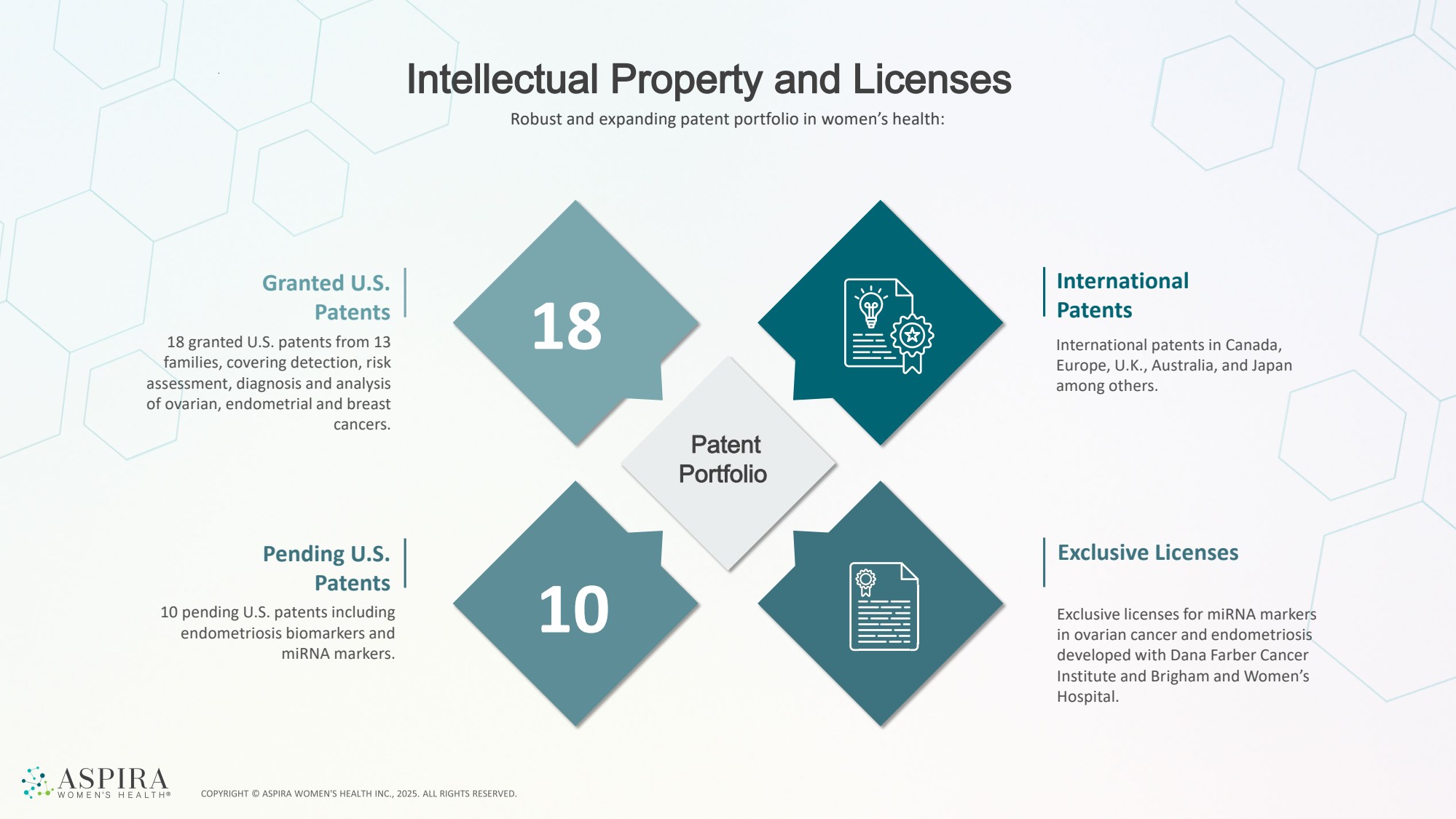
| Intellectual Property and Licenses Patent Portfolio Granted U.S. Patents 18 granted U.S. patents from 13 families, covering detection, risk assessment, diagnosis and analysis of ovarian, endometrial and breast cancers. Pending U.S. Patents 10 pending U.S. patents including endometriosis biomarkers and miRNA markers. Exclusive licenses for miRNA markers in ovarian cancer and endometriosis developed with Dana Farber Cancer Institute and Brigham and Women’s Hospital. Exclusive Licenses International patents in Canada, Europe, U.K., Australia, and Japan among others. International Patents 18 10 Robust and expanding patent portfolio in women’s health: |
| Commercial & Financials |
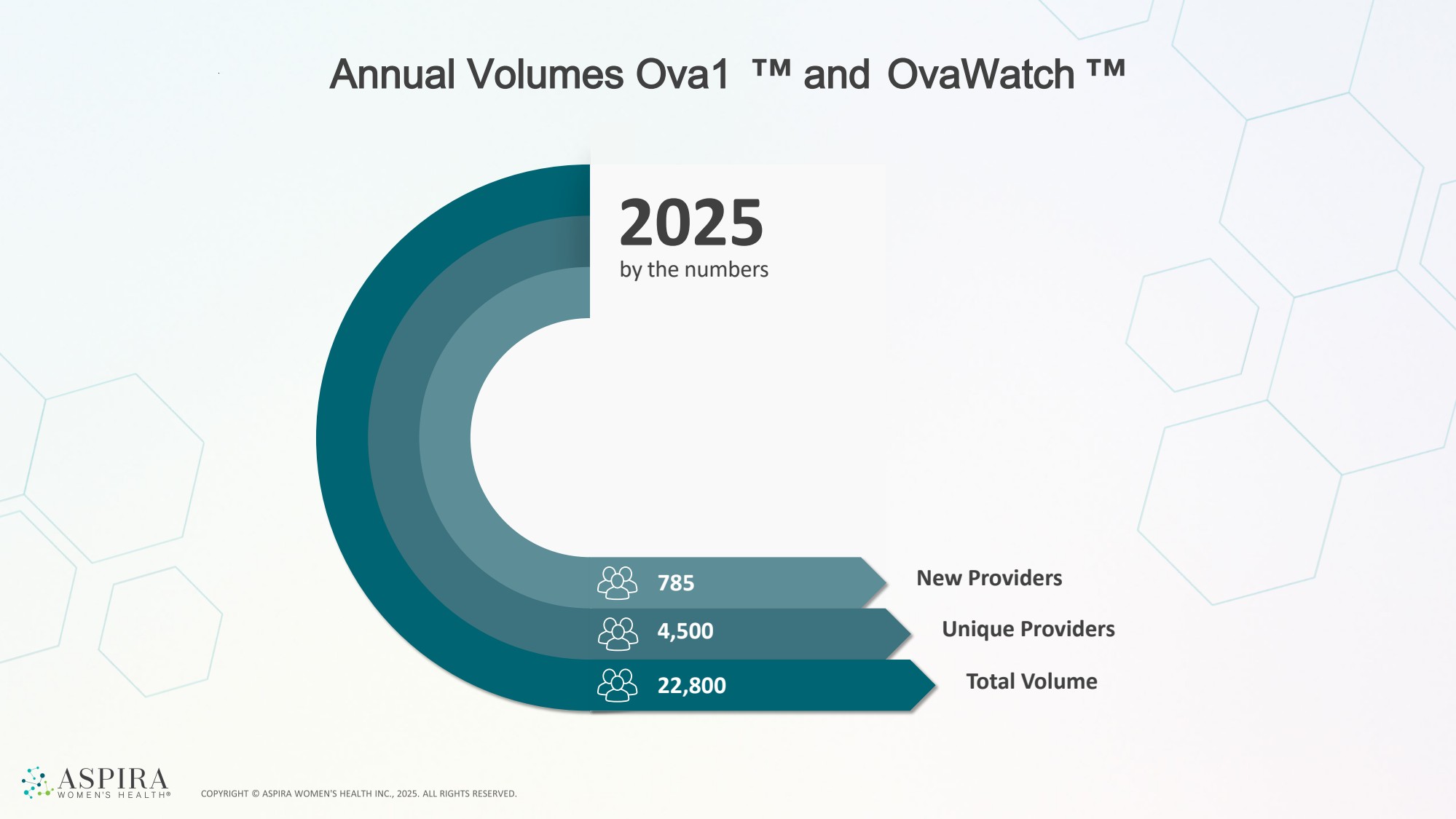
| Annual Volumes Ova1 and OvaWatch 785 New Providers 4,500 22,800 Unique Providers Total Volume by the numbers 2025 |
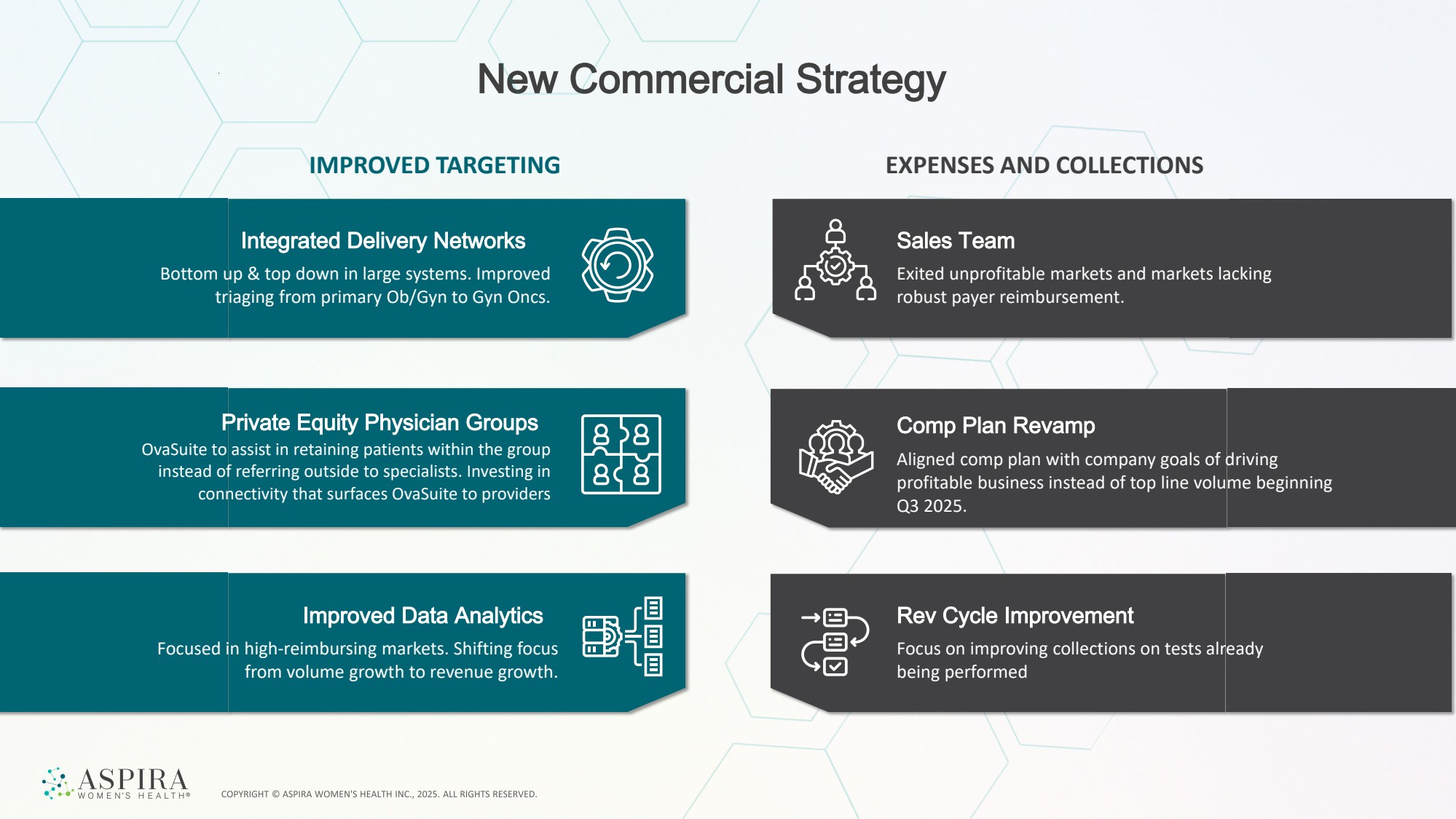
| New Commercial Strategy IMPROVED TARGETING EXPENSES AND COLLECTIONS Integrated Delivery Networks Bottom up & top down in large systems. Improved triaging from primary Ob/Gyn to Gyn Oncs. Improved Data Analytics Focused in high-reimbursing markets. Shifting focus from volume growth to revenue growth. Sales Team Exited unprofitable markets and markets lacking robust payer reimbursement. Comp Plan Revamp Aligned comp plan with company goals of driving profitable business instead of top line volume beginning Q3 2025. Rev Cycle Improvement Focus on improving collections on tests already being performed Private Equity Physician Groups OvaSuite to assist in retaining patients within the group instead of referring outside to specialists. Investing in connectivity that surfaces OvaSuite to providers |
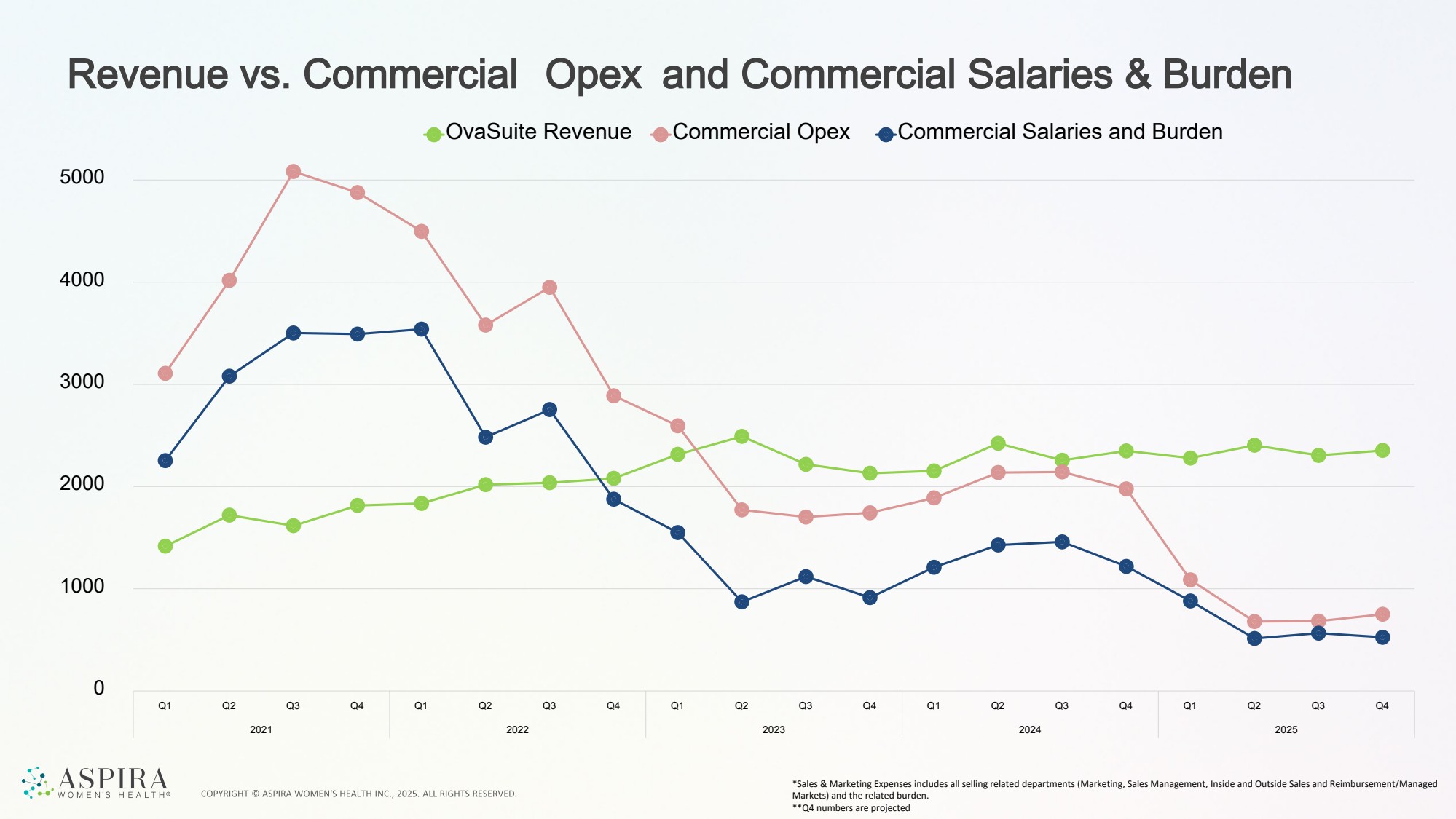
| Revenue vs. Commercial Opex and Commercial Salaries & Burden *Sales & Marketing Expenses includes all selling related departments (Marketing, Sales Management, Inside and Outside Sales and Reimbursement/Managed Markets) and the related burden. **Q4 numbers are projected 0 1000 2000 3000 4000 5000 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 2021 2022 2023 2024 2025 OvaSuite Revenue Commercial Opex Commercial Salaries and Burden |
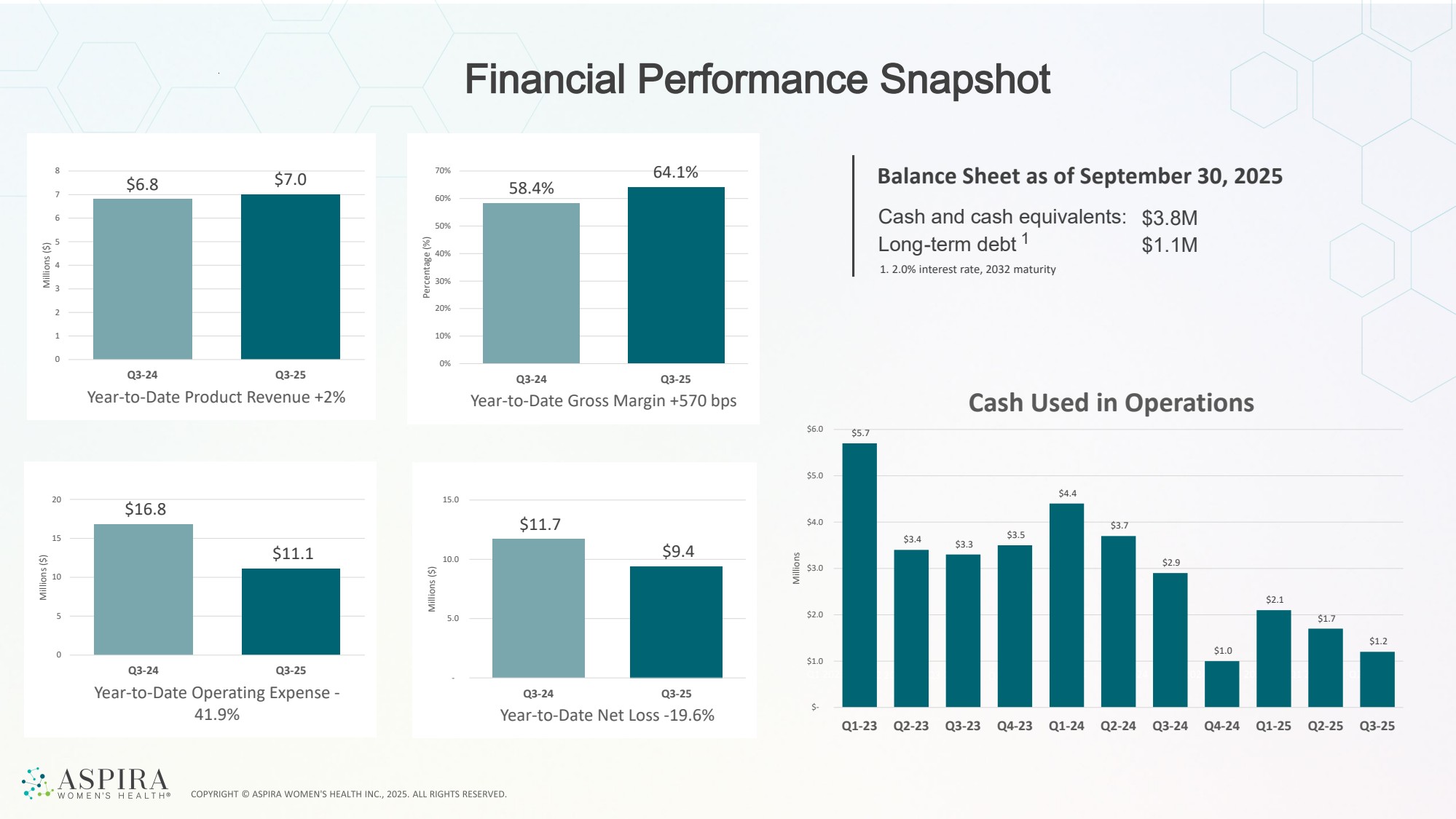
| Financial Performance Snapshot Balance Sheet as of September 30, 2025 Cash and cash equivalents: Long-term debt 1 $3.8M $1.1M 1. 2.0% interest rate, 2032 maturity Q2 2024 Q2 2025 Q2 2024 Q2 2025 Q2 2024 Q2 2025 Q2 2024 Q2 2025 Q1 2023 Q2 2023 Q3 2023 Q4 2023 Q1 2024 Q2 2024 Q3 2024 Q4 2024 Q1 2025 Q2 2025 $5.7 $3.4 $3.3 $3.5 $4.4 $3.7 $2.9 $1.0 $2.1 $1.7 $1.2 $- $1.0 $2.0 $3.0 $4.0 $5.0 $6.0 Q1-23 Q2-23 Q3-23 Q4-23 Q1-24 Q2-24 Q3-24 Q4-24 Q1-25 Q2-25 Q3-25 Millions Cash Used in Operations $6.8 $7.0 0 1 2 3 4 5 6 7 8 Q3-24 Q3-25 Millions ($) Year-to-Date Product Revenue +2% 58.4% 64.1% 0% 10% 20% 30% 40% 50% 60% 70% Q3-24 Q3-25 Percentage (%) Year-to-Date Gross Margin +570 bps $16.8 $11.1 0 5 10 15 20 Q3-24 Q3-25 Millions ($) Year-to-Date Operating Expense - 41.9% $11.7 $9.4 - 5.0 10.0 15.0 Q3-24 Q3-25 Millions ($) Year-to-Date Net Loss -19.6% |
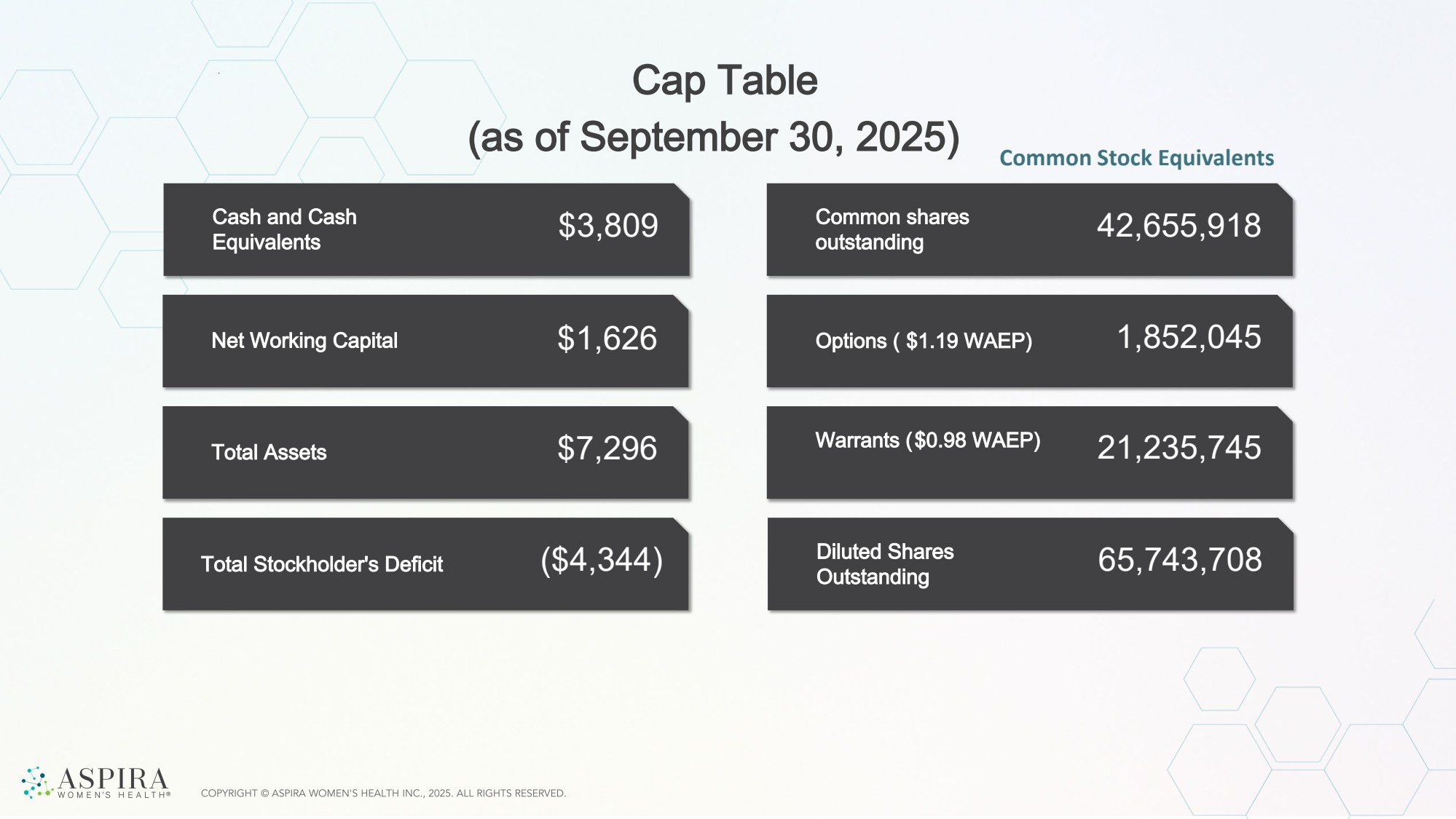
| Net Working Capital Total Assets Cash and Cash Equivalents Total Stockholder's Deficit $3,809 $1,626 $7,296 ($4,344) Options ( $1.19 WAEP) Common shares outstanding Warrants ($0.98 WAEP) 42,655,918 1,852,045 21,235,745 Diluted Shares Outstanding 65,743,708 Cap Table (as of September 30, 2025) Common Stock Equivalents |
| Contact Us investors@aspirawh.com |
| Appendix |
| OvaSuite Market Access 2024 CMS Clinical Lab Fee Schedule for OvaWatch and Ova1Plus Advantage $897 On Fee Schedule in 10 States |
| 1. Moore, R. G., McMeekin, D. S., Brown, A. K., DiSilvestro, P., Miller, M. C., Allard, W. J., ... & Skates, S. J. (2009). A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecologic oncology, 112(1), 40-46 2. Ueland, F. R., & Fredericks, T. I. (2018). Ovarian masses: Surgery or surveillance. OBG Management, 30(6), 17–26. 3. Pavlik, E. J., Ueland, F. R., Miller, R. W., Ubellacker, J. M., DeSimone, C. P., Elder, J., … van Nagell, J. R. (2013). Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstetrics & Gynecology, 122(2 Pt 1), 210–217. 4. U.S. Census Bureau. (2019). Annual estimates of the resident population: April 1, 2010 to July 1, 2018 (PEPANNRES). Retrieved from https:// www2.census.gov/programs-surveys/popest/tables/2010-2018/state/totals/ PEPANNRES.pdf 5. About 10–15% of ovarian cancers are hereditary. BRCA1/2 carriers face up to 45% lifetime ovarian cancer risk, Lynch syndrome confers a 6–20% lifetime risk, depending on which MMR gene is affected. BRIP1/RAD51C/D carriers have intermediate risk (5–10%) https://www.cancer.gov/publications/pdq/ information-summaries/genetics/brca-genes-hp-pdq? 6. Nearly 90% of carriers of CDC Tier 1 variants were previously undiagnosed. (MyCode Community Health Initiative — genomic screening at one health system: ~87.6% unaware) 7. 1st degree relative with OC quadruples your risk: https://www.cancer.org/ research/acs-research-highlights/ovarian-cancer-research-highlights/ ovarian-cancer-special-section-of-cancer-facts-and-figures-helps-policy-makers-and-others.html 8. About 1.8% of U.S. women report a first-degree relative https:// seer.cancer.gov/statfacts/html/ovary.html?utm_source=chatgpt.com 9. Ellis, K., Munro, D., & Clarke, J. (2022). Endometriosis is undervalued: a call to action. Frontiers in global women's health, 3, 902371 Notes: Slide 4 |
| Slide 6 1. In the US, there are 9.1 surgeries per malignancy compared to the European International Ovarian Tumor Analysis center trials, where only 2.3 (oncology centers) and 5.9 (other centers) reported surgeries per malignancy. Slide 13 Victor Ambros and Gary Ruvkun were awarded the 2024 Nobel Prize in Physiology or Medicine for their discovery of microRNAs in the 1990’s. Since then, numerous studies have demonstrated that they are involved in development and disease progression and can function as biomarkers for disease. Slide 17 1. Ellis, K., Munro, D., & Clarke, J. (2022). Endometriosis is undervalued: a call to action. Frontiers in global women's health, 3, 902371 2. https://www.contemporaryobgyn.net/view/acog-releases-new-study-obgyn-workforce 3. Mathias, S. D., Kuppermann, M., Liberman, R. F., Lipschutz, R. C., & Steege, J. F. (1996). Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstetrics & Gynecology, 87(3), 321-327 4. Eisenberg VH, Weil C, Chodick G, Shalev V. Epidemiology of endometriosis: a large population-based database study from a healthcare provider with 2 million members. BJOG 2018; 125: 55–62. Slide 21 1. 2.1M women in the US have a FDR with OC: Kumerow MT, Rodriguez JL, Dai S, Kolor K, Rotunno M, Peipins LA. Prevalence of Americans reporting a family history of cancer indicative of increased cancer risk: Estimates from the 2015 National Health Interview Survey. Prev Med. 2022 Jun;159:107062. doi:10.1016/j.ypmed.2022.107062. PMCID: PMC9162122. 2. 1.3M women in US over 18 y.o.: U.S. Census Bureau. (2024, June 1). Population estimates — total resident population and resident population age 18 years and older: United States (Table SCPRC-EST2024-18+POP). Retrieved from https://www.census.gov/ data/tables/time-series/demo/popest/2020s-national-detail.html 3. About 1.8% of U.S. women report a first-degree relative (mother, sister, or daughter) with ovarian cancer. That estimate comes straight from an NHIS-based, population study (Genetics in Medicine), which tabulated first-degree family history by site; ovarian cancer was 1.79% among respondents. Nature Headcount ~169M U.S. women today, ~3.0 million women (≈1.79% × 169M) https:// www.nature.com/articles/gim200695.pdf 4. Ueland, F. R., & Fredericks, T. I. (2018). Ovarian masses: Surgery or surveillance. OBG Management, 30(6), 17–26. 5. Pavlik, E. J., Ueland, F. R., Miller, R. W., Ubellacker, J. M., DeSimone, C. P., Elder, J., … van Nagell, J. R. (2013). Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstetrics & Gynecology, 122(2 Pt 1), 210–217. |
| 6. U.S. Census Bureau. (2019). Annual estimates of the resident population: April 1, 2010 to July 1, 2018 (PEPANNRES). Retrieved from https:// www2.census.gov/programs-surveys/popest/tables/2010-2018/state/totals/ PEPANNRES.pdf 7. About 10–15% of ovarian cancers are hereditary. BRCA1/2 carriers face up to 45% lifetime ovarian cancer risk, Lynch syndrome confers a 6–20% lifetime risk, depending on which MMR gene is affected. 8. BRIP1/RAD51C/D carriers have intermediate risk (5–10%) https:// www.cancer.gov/publications/pdq/information-summaries/genetics/brca-genes-hp-pdq? 9. Diagnosed HCRA: ~165,000–350,000 women, Undiagnosed: ~850,000– 1,000,000 women, Nearly 90% of carriers of CDC Tier 1 variants were previously undiagnosed. (MyCode Community Health Initiative — genomic screening at one health system: ~87.6% unaware) |