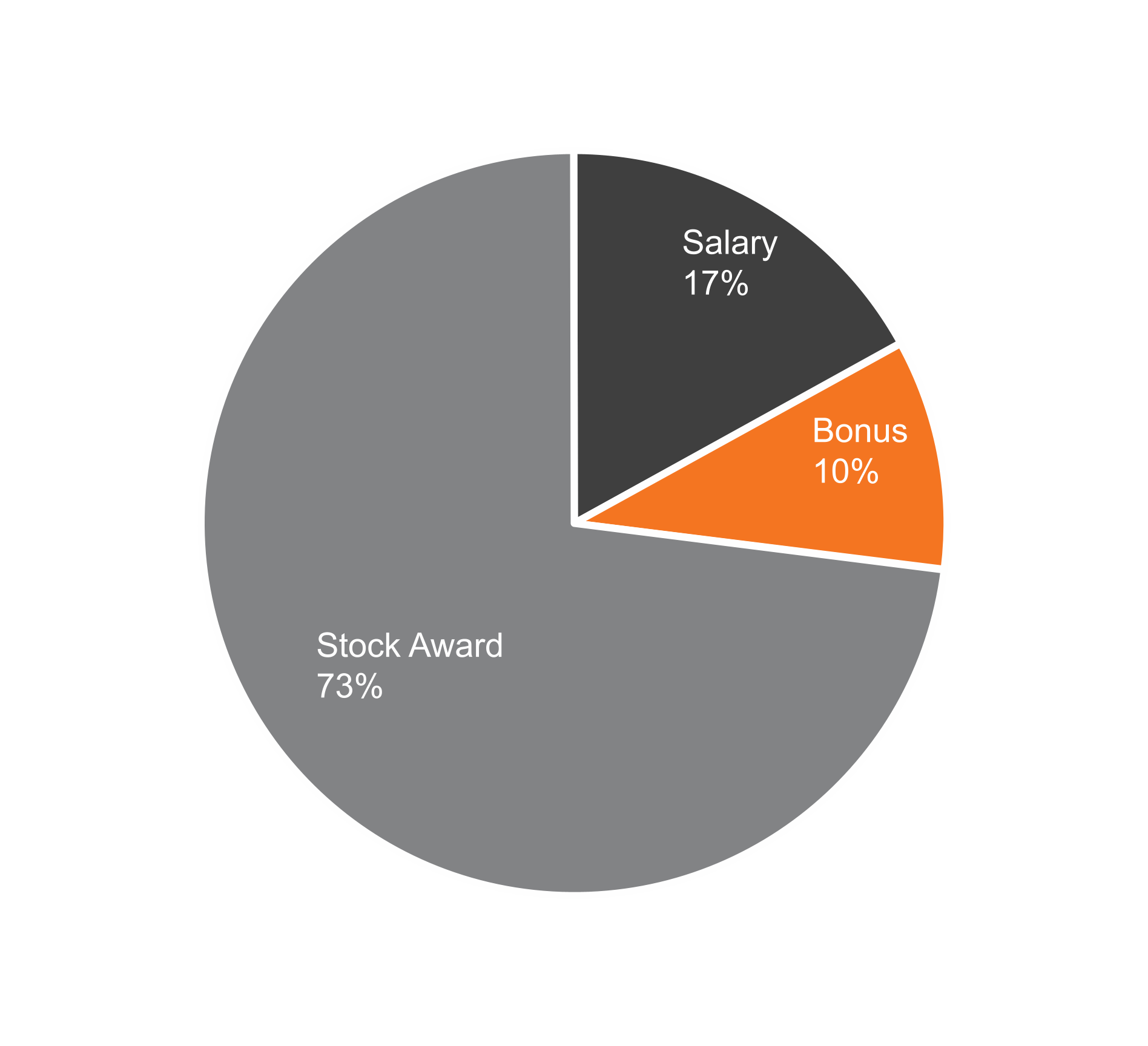
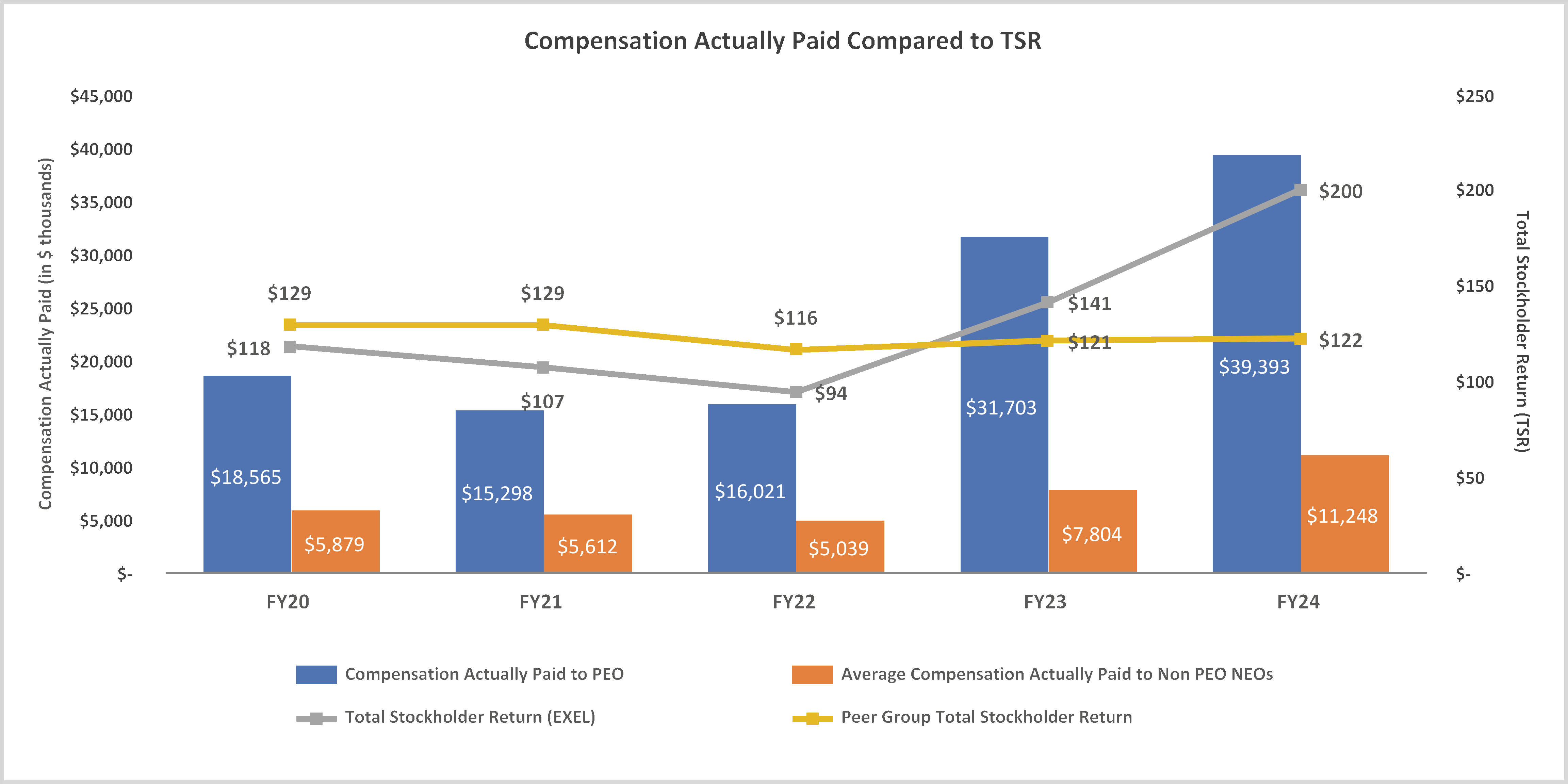
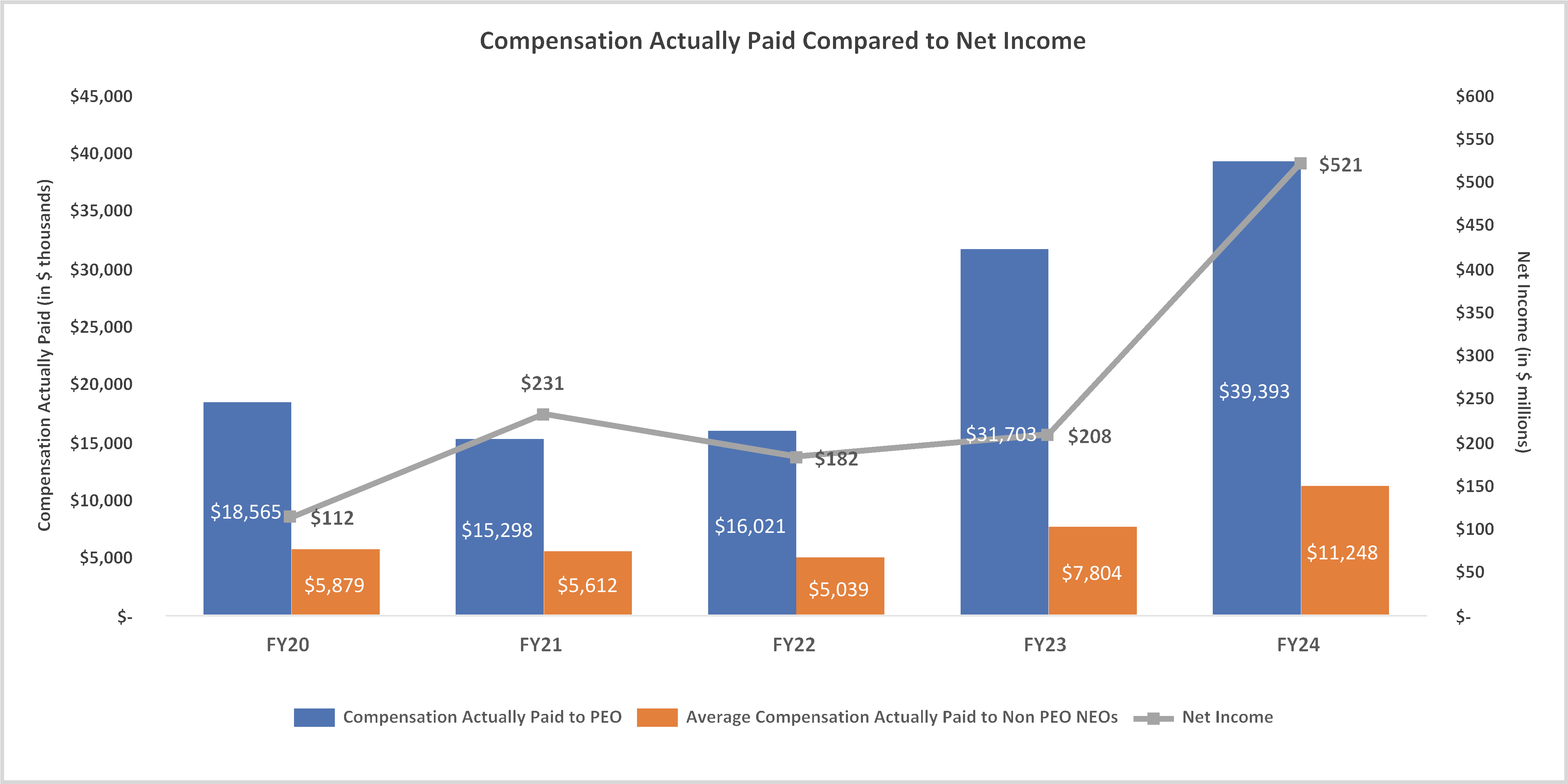
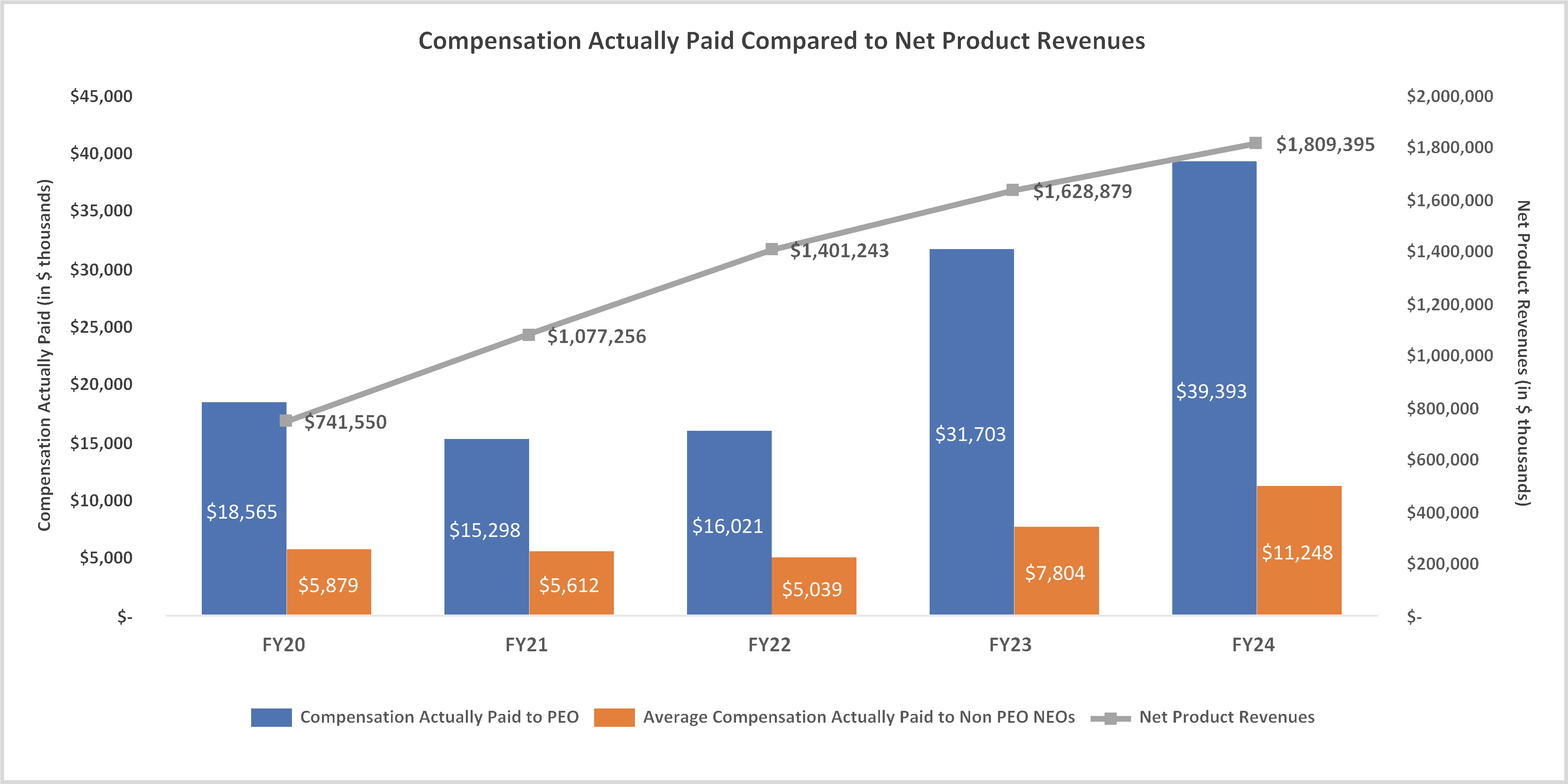
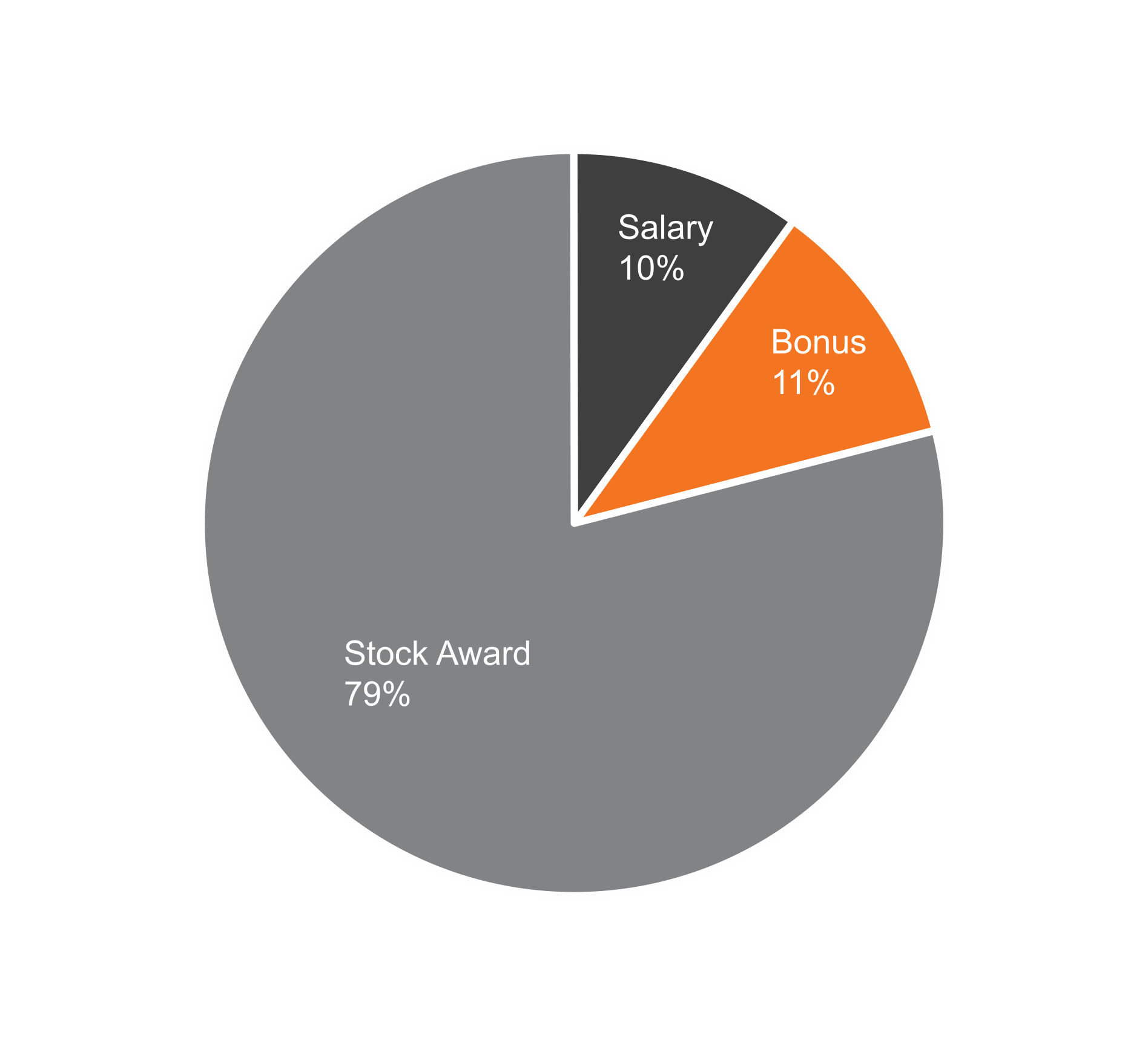February 28, 2025, by the sum of the number of shares outstanding as of such date plus the number of shares as to which such
person has the right to acquire voting or investment power within 60 days of February 28, 2025. Consequently, the
denominator for calculating beneficial ownership percentages may be different for each beneficial owner.
(2) Includes 1,764,985 shares held by Michael M. Morrissey and Meghan D. Morrissey, Trustees of the Morrissey Family Living Trust
dated July 21, 1994, as amended. Also includes 803,065 shares Dr. Morrissey has the right to acquire pursuant to options
exercisable within 60 days of February 28, 2025. Also includes 17,728 shares held by Dr. Morrissey under our 401(k) Plan,
determined based upon information provided in plan statements.
(3) Includes 100,000 shares Mr. Senner has the right to acquire pursuant to options exercisable within 60 days of February 28, 2025.
Also includes 2,723 shares held by Mr. Senner under our 401(k) Plan, determined based upon information provided in plan
statements.
(4) Includes 5,835 shares held by Dr. Aftab under our 401(k) Plan, determined based upon information provided in plan statements.
(5) Includes 999 shares held by Mr. Hessekiel under our 401(k) Plan, determined based upon information provided in plan
statements.
(6) Includes 66,666 shares Dr. Peterson has the right to acquire pursuant to options exercisable within 60 days of February 28,
2025.
(7) Includes 22,494 shares Dr. Beckerle has the right to acquire pursuant to options exercisable within 60 days of February 28,
2025, all of which would be subject to repurchase by us, if so exercised.
(8) Includes 23,136 shares Dr. Eckhardt has the right to acquire pursuant to options exercisable within 60 days of February 28,
2025, all of which would be subject to repurchase by us, if so exercised.
(9) Includes 52,688 shares Dr. Freire has the right to acquire pursuant to options exercisable within 60 days of February 28, 2025.
(10) Includes 36,353 shares Mr. Heyman has the right to acquire pursuant to options exercisable within 60 days of February 28,
2025, all of which would be subject to repurchase by us, if so exercised.
(11) Includes 79,120 shares Mr. Johnson has the right to acquire pursuant to options exercisable within 60 days of February 28,
2025, all of which would be subject to repurchase by us, if so exercised, and Mr. Johnson is deemed to hold such options for the
benefit of the Caligan Fund and Accounts, and may, after the exercise of such options, if applicable, transfer the underlying
shares directly to the Caligan Fund and Accounts. Also includes 1,525,730 shares held by Caligan Partners Master Fund LP
(Caligan Master Fund), a Cayman Islands limited partnership, and managed accounts (Caligan Accounts, together with the
Caligan Master Fund, the Caligan Fund and Accounts) to which Caligan Partners LP (Caligan) serves as investment manager. Mr.
Johnson is the Managing Partner of Caligan and a Managing Member of Caligan Partners GP LLC, the general partner of Caligan.
The above information is based solely on a Form 4, filed jointly by Mr. Johnson and Caligan with the SEC on May 30, 2024.
(12) Includes 21,206 shares Mr. Oliver has the right to acquire pursuant to options exercisable within 60 days of February 28, 2025,
all of which would be subject to repurchase by us, if so exercised.
(13) Includes 36,508 shares Dr. Papadopoulos has the right to acquire pursuant to options exercisable within 60 days of February 28,
2025.
(14) Includes 20,634 shares Dr. Poste has the right to acquire pursuant to options exercisable within 60 days of February 28, 2025.
(15) Includes 106,539 shares Ms. Smith has the right to acquire pursuant to options exercisable within 60 days of February 28, 2025,
all of which would be subject to repurchase by us, if so exercised.
(16) Includes 52,688 shares Mr. Wyszomierski has the right to acquire pursuant to options exercisable within 60 days of February 28,
2025.
(17) Total number of shares includes 7,902,512 shares of common stock held by our current directors and executive officers as of
February 28, 2025, and entities affiliated with such directors and executive officers. Also includes 1,537,480 shares our current
directors and executive officers have the right to acquire pursuant to options exercisable within 60 days of February 28, 2025,
164,286 of which would be subject to repurchase by us if so exercised. Also includes 37,933 shares held by our current directors
and executive officers under our 401(k) Plan, determined based upon information provided in plan statements.
(18) BlackRock, Inc. (BlackRock) reported that it has sole voting power over 34,488,790 of such shares and sole dispositive power
over 36,013,885 of such shares. BlackRock also reported that BlackRock Fund Advisors beneficially owns 5% or more of our
outstanding common stock. The information is based solely on a Schedule 13G/A, filed with the SEC on January 24, 2024, which
provides information only as of December 31, 2023, and, consequently, the beneficial ownership of BlackRock may have
changed between December 31, 2023 and February 28, 2025.
(19) The Vanguard Group (Vanguard) reported that it has shared voting power over 126,364 of such shares, sole dispositive power
over 31,219,906 of such shares, and shared dispositive power over 451,184 of such shares. The information is based solely on a
Schedule 13G/A, filed with the SEC on February 13, 2024, which provides information only as of December 29, 2023, and,
consequently, the beneficial ownership of Vanguard may have changed between December 29, 2023 and February 28, 2025.
(20) Includes shares held by Farallon Capital Partners, L.P. (FCP), Farallon Capital Institutional Partners, L.P. (FCIP), Farallon Capital
Institutional Partners II, L.P. (FCIP II), Farallon Capital Institutional Partners III, L.P. (FCIP III), Four Crossings Institutional Partners
V, L.P. (FCIP V), Farallon Capital Offshore Investors II, L.P. (FCOI II), Farallon Capital (AM) Investors, L.P. (FCAMI), Farallon Capital