1.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
AMENDED AND RESTATED COLLABORATION AND LICENSE AGREEMENT
This Amended and Restated Collaboration and License Agreement (the
“Agreement”) is entered into as of December 17, 2025 (the “Restatement Date”), by and
between Exelixis, Inc., a Delaware company having an address at 1851 Harbor Bay Parkway,
Alameda, CA 94502, USA (“Exelixis”) and Ipsen Pharma SAS, a French corporation having an
address at 70 rue Balard, 75015 Paris, France (“Licensee”). Exelixis and Licensee may be
referred to herein individually as a “Party” or collectively as the “Parties”.
Recitals
Whereas, Exelixis, a biopharmaceutical company, is developing its proprietary
compound known as cabozantinib for the treatment of cancer, and owns or controls certain
patents, know-how and other intellectual property relating to such compound;
Whereas, Licensee, a fully-integrated pharmaceutical company, possesses substantial
resources and expertise in the development and commercialization of pharmaceutical products;
and
Whereas, Exelixis and Licensee are parties to that certain Collaboration and License
Agreement dated February 29, 2016 (the “Effective Date”), as subsequently amended by the
First Amendment dated effective December 20, 2016, Second Amendment dated effective
September 14, 2017, Third Amendment dated effective October 26, 2017, Fourth Amendment
dated effective October 11, 2022 (the “Fourth Amendment Effective Date”), and Fifth
Amendment dated effective August 24, 2023 (the “Fifth Amendment Effective Date”)
(collectively, the “Original Agreement”) under which the Parties have been collaborating on the
development and commercialization of cabozantinib;
Whereas, the Parties wish to amend and restate the Original Agreement in accordance
with Section 17.2 thereof to incorporate the amendments, all under the terms and conditions
hereof.
Agreement
Now, Therefore, in consideration of the foregoing premises and the mutual covenants
contained herein, and for other good and valuable consideration, the receipt and sufficiency of
which are hereby acknowledged, Exelixis and Licensee hereby agree to amend and restate the
Original Agreement as of the Restatement Date, so that it reads in its entirety as follows:
1.Definitions
1.1“Additional Markets” means [ * ].
1.2“Affiliate” means, with respect to any party, any entity that, directly or indirectly
through one or more intermediaries, controls, is controlled by or is under common control with
such party, but for only so long as such control exists. As used in this Section 1.2, “control”
2.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
means (a) to possess, directly or indirectly, the power to direct the management or policies of an
entity, whether through ownership of voting securities, by contract relating to voting rights or
corporate governance; or (b) direct or indirect beneficial ownership of more than fifty percent
(50%) (or such lesser percentage which is the maximum allowed to be owned by a foreign
corporation in a particular jurisdiction) of the voting share capital or other equity interest in such
entity.
1.3“Applicable Laws” means the applicable provisions of any and all national,
supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative
codes, guidance, ordinances, judgments, decrees, directives, injunctions, orders, permits
(including MAAs) of or from any court, Regulatory Authority or governmental agency or
authority having jurisdiction over or related to the subject item.
1.4“Calendar Quarter” means each respective period of three (3) consecutive
months ending on March 31, June 30, September 30, and December 31.
1.5“Calendar Year” means each respective period of twelve (12) consecutive
months ending on December 31.
1.6 “Clinical Trial” or “Clinical Trials” means Phase 1 Clinical Trial, Phase 2
Clinical Trial, Phase 3 Clinical Trial or Phase 4 Clinical Trial as the context dictates.
1.7“cGCP” shall mean the current clinical practice as set out in (i) ICH Harmonized
Guidance on current Good Clinical Practice (CPMP/ICH/135/95), (ii) US Code of Federal
Regulations, Title 21, Chapters 50, 54, 56, 58, 210, 211 and 312, as may be amended from time
to time, (iii) EU Directive 2001/20/EC and related guidelines, and (iv) the equivalent law or
regulation in any other applicable jurisdiction in the Territory.
1.8“cGLP” shall mean current good laboratory practice standards promulgated or
endorsed by the FDA, as defined in U.S. 21 C.F.R. Part 58 (or such other comparable regulatory
standards in jurisdictions outside the U.S.), as they may be updated from time to time.
1.9“cGMP” shall mean the current minimum standards for methods to be used in,
and the facilities or controls to be used for, the manufacture, processing, packing, or holding of a
drug as specified by applicable laws of the relevant countries at the time of manufacturing
conducted in accordance with this Agreement, defined under (i) 21 C.F.R. Part 210 and 211, (ii)
Directive 2003/94/EC, (iii) Volume 4, Rules Governing Medicinal Products in the EU, Part I and
II, in each case, as amended from time to time, and (iv) equivalent law or regulations in any other
applicable jurisdiction in the Territory.
1.10“Cometriq” means that certain pharmaceutical product containing the Compound
in capsule formulation and known as Cometriq®, which has been developed and commercialized
by Exelixis as of the Effective Date for the treatment of progressive, metastatic medullary
thyroid cancer (MTC).
1.11“Commercialization” means the conduct of all activities undertaken before and
after Regulatory Approval relating to the promotion, sales, marketing, medical support, and
distribution (including importing, exporting, transporting, customs clearance, warehousing,
invoicing, handling and delivering Products to customers) of Products in the Field in or outside
of the Licensee Territory, including sales force efforts, detailing, advertising, market research,
market access (including price and reimbursement activities), medical education and information
3.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
services, publication, scientific and medical affairs; advisory and collaborative activities with
opinion leaders and professional societies including symposia, marketing, sales force training,
and sales (including receiving, accepting and filling Product orders) and distribution.
“Commercialize” and “Commercializing” have correlative meanings.
1.12“Commercially Reasonable Efforts” means, with respect to a Party and its
obligations under this Agreement, those commercially reasonable efforts and resources
consistent with the usual practices of a similarly situated company for the development and
commercialization of a pharmaceutical product originating from its own research and
development department without a royalty obligation to others, which is at a similar stage of
research, development or commercialization, taking into account that product’s profile of
efficacy and safety; proprietary position, including patent and regulatory exclusivity; regulatory
status, including anticipated or approved labeling and anticipated or approved post-approval
requirements; present and future market and commercial potential, including competitive market
conditions (but not taking into account any payment owed to the other Party under this
Agreement), and all other relevant factors, including technical, legal, scientific and/or medical
factors. Commercially Reasonable Efforts requires that a Party: (i) at a minimum establish a
plan to achieve objectives and assign specific responsibilities for the achievement of that plan
and (ii) make and implement decisions and allocate resources designed to advance progress with
respect to such objectives.
1.13“Committee” means the JSC, JDC, JCC or any subcommittee established by the
JSC, as applicable.
1.14“Competing Product” means any product or compound, other than the
Compound and Products: (a) for which the mechanism of action includes modulation of the
kinase activities of cMET, VEGFR2, Ret or any combination of these targets; and (b) which
directly binds and modulates the activity of: (i) VEGFR2; (ii) cMET; and/or (iii) Ret, [ * ].
1.15“Compound” means cabozantinib, having the chemical structure set forth in
Exhibit A, including [ * ].
1.16“Confidentiality Agreement” means that certain Confidential Disclosure
Agreement between Exelixis and Licensee dated as of February 10, 2015.
1.17“Confidential Information” means all Know-How and other proprietary
scientific, marketing, financial or commercial information or data that is generated by or on
behalf of a Party or its Affiliates or which one Party or any of its Affiliates has supplied or
otherwise made available to the other Party or its Affiliates, whether made available orally, in
writing, or in electronic form, including information comprising or relating to concepts,
discoveries, inventions, data, designs or formulae in relation to this Agreement; provided that all
Exelixis Technology will be deemed Exelixis’ Confidential Information, all Licensee
Technology will be deemed Licensee’s Confidential Information, and all Joint Inventions and
Joint Patents will be deemed both Parties’ Confidential Information.
1.18“Control” or “Controlled” means, with respect to any Know-How, Patents or
other intellectual property rights, the legal authority or right (whether by ownership, license or
otherwise but without taking into account any rights granted by one Party to the other Party
pursuant to this Agreement) of a Party to grant access, a license or a sublicense of or under such
Know-How, Patents or other intellectual property rights to another Party, or to otherwise disclose
proprietary or trade secret information to such other Party, without breaching the terms of any
4.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
agreement with a Third Party, or misappropriating the proprietary or trade secret information of a
Third Party.
1.19“Cost of Goods” means, with respect to any Compound or Product, the fully
burdened cost to manufacture such Compound or Product, which means: (a) in the case of [ * ];
and (b) in the case of [ * ]. Actual unit costs shall consist of [ * ]. Direct material costs shall
include the [ * ]. Direct labor costs shall include the cost of: [ * ]. [ * ].
1.20“Data” means any and all scientific, technical, test, marketing or sales data
pertaining to any Product that is generated by or on behalf of Exelixis, Licensee, their respective
Affiliates and Sublicensees, including research data, clinical pharmacology data, pre-clinical
data, clinical data, clinical study reports or submissions made in association with an IND or
MAA with respect to any Product.
1.21“Development” means all development activities for the Compound and Product
(whether alone or for use together, or in combination, with another active agent or
pharmaceutical product as a combination product or combination therapy) that are directed to
obtaining Regulatory Approval(s) of the Product and lifecycle management of the Product in any
country in the world, including all non-clinical, preclinical and clinical testing and studies of the
Product; toxicology, pharmacokinetic and pharmacological studies; statistical analyses; assay
development; protocol design and development; the preparation, filing and prosecution of any
MAA for the Product; development activities directed to label expansion and/or obtaining
Regulatory Approval for one or more additional indications following initial Regulatory
Approval; development activities conducted after receipt of Regulatory Approval, including
Phase 4 Clinical Trials; and all regulatory affairs related to any of the foregoing. “Develop” and
“Developing” have correlative meanings.
1.22“Development Costs” means the costs incurred by a Party or for its account,
during the Term and pursuant to this Agreement, that are specifically directed (or reasonably
allocable) to the Development of a Product. The Development Costs shall include amounts that
a Party pays to Third Parties involved in the Development of a Product (at cost, and excluding
any Third Party Royalties), and all internal costs (calculated on an FTE basis at the then-current
FTE Rate) and out-of-pocket costs incurred by or on account of a Party in performing
Development in accordance with the GDP.
1.23“Drug Master File” means any (a) drug master files filed with the FDA with
respect to the Product, (b) active substance master file (ASMF) filed with the EMA, and (c)
equivalent filing in other countries in the Licensee Territory.
1.24“EMA” means the European Medicines Agency or its successor.
1.25“EU” means the European Economic Area and Switzerland.
1.26“Executive Officers” the Chief Executive Officer of Exelixis and the Chief
Executive Officer of Licensee.
1.27“Exelixis Know-How” means all Know-How that Exelixis Controls as of the
Effective Date or during the Term, including any Joint Inventions, that is necessary or reasonably
useful for the Development, use, importation, offer for sale or sale of any Compound or Product
in the Field in the Licensee Territory. The Exelixis Know-How includes the Exelixis Data.
5.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
1.28“Exelixis Patents” means all Patents in the Licensee Territory that Exelixis
Controls as of the Effective Date or during the Term (including any Joint Patents) that would be
infringed, absent a license or other right to practice granted under such Patents, by the
Development, use, importation, offer for sale or sale of any Compound or Product in the Field in
the Licensee Territory (considering patent applications to be issued with the then-pending claims
and considering Joint Patents as if owned solely by Exelixis), but excluding those Patents set
forth in Exhibit B-2. The Exelixis Patents existing as of the Restatement Date are set forth in
Exhibit B-1.
1.29“Exelixis [ * ]” means the [ * ] in Exhibit F.
1.30“Exelixis Technology” means the Exelixis Know-How and the Exelixis Patents,
including Exelixis’ interest in the Joint Inventions and Joint Patents.
1.31“Exelixis Territory” means the U.S. and Japan.
1.32“Expanded Access Program” means the administration of the Product to named
individuals who do not meet the clinical trial enrollment criteria either outside of a clinical trial
or after the completion of a clinical trial. Expanded Access Programs are also known as named
patient programs, named patient supply, and temporary authorization for use.
1.33“Export Control Laws” means all applicable U.S. laws and regulations relating
to (a) sanctions and embargoes imposed by the Office of Foreign Assets Control of the U.S.
Department of Treasury or (b) the export or re-export of commodities, technologies, or services,
including the Export Administration Act of 1979, 24 U.S.C. §§ 2401-2420, the International
Emergency Economic Powers Act, 50 U.S.C. §§ 1701-1706, the Trading with the Enemy Act, 50
U.S.C. §§ 1 et. seq., the Arms Export Control Act, 22 U.S.C. §§ 2778 and 2779, and the
International Boycott Provisions of Section 999 of the U.S. Internal Revenue Code of 1986 (as
amended).
1.34“FCPA” means the U.S. Foreign Corrupt Practices Act (15 U.S.C. Section
78dd-1, et. seq.), as amended.
1.35“FDA” means the U.S. Food and Drug Administration or its successor.
1.36“Field” means all indications and uses in humans and animals.
1.37“First Commercial Sale” means, on a Product-by-Product and country-by-
country basis, the earlier of (i) the First Commercial RCC Sale or (ii) first sale by Licensee or
any of its Affiliates or Sublicensees to a Third Party for end use of Cometriq for the MTC
indication in a given country in the Licensee Territory after Regulatory Approval has been
granted with respect to such Product in such country.
1.38“First Commercial RCC Sale” means, on a Product-by-Product and country-by-
country basis, the first sale by Licensee or any of its Affiliates or Sublicensees to a Third Party
for end use of a Product in a given country in the Licensee Territory after Regulatory Approval
has been granted with respect to such Product in such country for the first indication approved by
the relevant Regulatory Authority in the treatment of RCC (e.g., 2nd line therapy for RCC).
6.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
1.39“FTE” means the equivalent of full-time individual’s work, performed by one or
more individuals, in an Exelixis fiscal year (which fiscal year is, in most years, 52 weeks exactly
consisting of [ * ] working hours [ * ].
1.40“FTE Rate” means an initial rate of (a) with respect to Exelixis’ personnel, [ * ]
per FTE per 52-week Exelixis Fiscal Year and (b) with respect to Licensee’s personnel, [ * ],
which rate shall apply through December 31, 2016. Thereafter, the FTE Rate shall be changed
annually on a Calendar Year basis to reflect any year-to-year percentage increase or decrease (as
the case may be) (i) with respect to Exelixis, in the Consumer Price Index for All Urban
Consumers for the U.S., as published by the U.S. Department of Labor, Bureau of Labor
Statistics (“CPI”), and (ii) with respect to Licensee, in the French consumer price index as
published by the French National Institute of Statistics and Economic Studies (“INSEE”)
available at insee.fr (both changes based on the change in the CPI from the most recent
applicable index available as of the Effective Date to the most recent applicable index available
as of the date of the calculation of such revised FTE Rate). [ * ] The Parties acknowledge that
the FTE Rate calculated in accordance with Section 1.39 with respect to all Development Costs
shall be effective as from [ * ].
1.41“Future Exelixis Licensee” means any licensee or Sublicensee of Exelixis (other
than Licensee) to which a license or a sublicense with respect to Products is granted by Exelixis
for all or any portion of the Exelixis Territory (e.g., the U.S., Canada and/or Japan) or will be
granted after the Effective Date.
1.42“Generic Product” means, with respect to a Product in a particular regulatory
jurisdiction, any pharmaceutical product that (a) contains the same active pharmaceutical
ingredient(s) as such Product; (b) is approved by the Regulatory Authority in such country as a
substitutable generic for such Product (for an indication for which such Product obtained
Regulatory Approval from the applicable Regulatory Authority in such jurisdiction) on an
expedited or abbreviated basis based on bioequivalence or interchangeability with the Product;
and (c) is sold in such jurisdiction by a Third Party that is not a Sublicensee and did not purchase
such product in a chain of distribution that included any of Exelixis, Licensee, or their respective
Affiliates, licensees, or sublicensees.
1.43“Governmental Authority” means any national, international, federal, state,
provincial or local government, or political subdivision thereof, or any multinational organization
or any authority, agency or commission entitled to exercise any administrative, executive,
judicial, legislative, police, regulatory or taxing authority or power, any court or tribunal (or any
department, bureau or division thereof, or any governmental arbitrator or arbitral body).
1.44“HCC” means hepatocellular carcinoma.
1.45“Health Canada” means the federal department of the government of Canada
having the authority to regulate the sale of medicinal or pharmaceutical products, or any
successor agency thereof.
1.46“ICH” means the International Conference on Harmonization (of Technical
Requirements for Registration of Pharmaceuticals for Human Use).
1.47“IND” means an investigational new drug application or equivalent application
filed with the applicable Regulatory Authority, which application is required to commence
human clinical trials in the applicable country.
7.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
1.48“Initiation” means, with respect to a Clinical Trial, the first dosing of the first
human subject in such Clinical Trial.
1.49“Inventions” means all inventions, whether or not patentable, discovered, made,
conceived, or reduced to practice, in the course of activities contemplated by this Agreement.
1.50“Know-How” means all technical information, know-how and data, including
inventions, discoveries, trade secrets, specifications, instructions, processes, formulae,
compositions of matter, cells, cell lines, assays, animal models and other physical, biological, or
chemical materials, expertise and other technology applicable to, development, registration, use
or marketing or to methods of assaying or testing them, and including all biological, chemical,
pharmacological, biochemical, toxicological, pharmaceutical, physical and analytical, safety,
nonclinical and clinical data, regulatory documents, data and filings, instructions, processes,
formulae, expertise and information, relevant to the research, development, use, importation,
offering for sale or sale of, or which may be useful in studying, testing, developing, Products.
Know-How excludes Patents and manufacturing know-how of Compound or Product.
1.51“Licensee Know-How” means all Know-How that Licensee or its Affiliate
Controls as of the Effective Date or during the Term, including any Joint Inventions, that is [ * ]
for the research, Development, manufacture, use, importation, offer for sale or sale of any
Compound or Product in the Field. The Licensee Know-How includes the Licensee Data.
1.52 “Licensee Patents” means all Patents that Licensee or its Affiliate Controls as of
the Effective Date or during the Term (including any Joint Patents) that would be infringed,
absent a license or other right to practice granted under such Patents, by the research,
Development, manufacture, use, importation, offer for sale or sale of any Compound or Product
(considering patent applications to be issued with the then-pending claims and considering Joint
Patents as if owned solely by Licensee or its Affiliate).
1.53“Licensee Technology” means the Licensee Know-How and the Licensee
Patents, including Licensee’s interest in the Joint Inventions and Joint Patents.
1.54“Licensee Territory” means the world outside the Exelixis Territory.
1.55“MAA” means a marketing authorization application or equivalent application,
and all amendments and supplements thereto, filed with the applicable Regulatory Authority in
any country or jurisdiction. For clarity, MAA does not include any application for Pricing and
Reimbursement Approval.
1.56“MAA Approval” means approval of an MAA by the applicable Regulatory
Authority for marketing and sale of a Product in the applicable country or jurisdiction, but
excluding any pricing and/or reimbursement approval.
1.57“Major Market Countries” means [ * ].
1.58“Medical Affairs” or “Medical Affairs Activities” means activities designed to
ensure or improve appropriate medical use of, conduct medical education of, or further research
regarding, the Product, including by way of example: (a) activities of medical scientific liaisons
who, among their other functions, may: (i) conduct service based medical activities including
providing input and assistance with consultancy meetings, proposing investigators for clinical
trials sponsored or co-sponsored by a Party or Affiliate, and providing input in the design of such
8.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
trials and other research related activities; and/or (ii) deliver non-promotional communications
and conduct non-promotional activities; (b) grants to support continuing medical education,
symposia, or Third Party research related to the Product; (c) development, publication and
dissemination of publications relating to the Products; (d) medical information services provided
in response to inquiries communicated via sales representatives or received by letter, phone call
or email; (e) conducting advisory board meetings, international advisory board activities or other
consultant programs, including the engagement of key opinion leaders and health care
professional in individual or group advisory and consulting arrangements; and (f) the evaluation
of applications submitted to Licensee for support of investigator-initiated trials.
1.59“MTC” means medullary thyroid cancer.
1.60“Net Sales” means, with respect to any Product, the gross amounts invoiced for
sales or other dispositions of such Product by or on behalf of Licensee and its Affiliates and
Sublicensees to Third Parties, less the following deductions to the extent included in the gross
invoiced sales price for such Product or otherwise directly paid or incurred by Licensee or its
Affiliates or Sublicensees, as applicable, with respect to the sale or other disposition of such
Product:
(a)normal and customary trade and quantity discounts actually allowed and
properly taken directly with respect to sales of such Product (provided that such discounts are not
applied disproportionately to such Product when compared to the other products of Licensee or
its Affiliate or Sublicensee, as applicable);
(b)credits or allowances given or made for rejection or return of previously
sold Products or for retroactive price reductions and billing errors;
(c)rebates and chargeback payments granted to managed health care
organizations, pharmacy benefit managers (or equivalents thereof), national, state/provincial,
local, and other governments, their agencies and purchasers and reimbursors, or to trade
customers;
(d)[ * ] costs of freight, carrier insurance, and other transportation charges
directly related to the distribution of such Product. [ * ]; and
(e)taxes, duties or other governmental charges (including any tax such as a
value added or similar tax, other than any taxes based on income) directly levied on or measured
by the billing amount for such Product, as adjusted for rebates and refunds.
Upon any sale or other disposition of any Product that should be included within Net
Sales for any consideration other than exclusively monetary consideration on bona fide arms’-
length terms, then for purposes of calculating Net Sales under this Agreement, such Product shall
be deemed to be sold exclusively for money at the average sales price of the relevant Product in
arm’s length transactions during the applicable reporting period generally achieved for such
Product in the country in which such sale or other disposition occurred when such Product is sold
alone and not with other products (average sales price to be measured as the aggregate Product
Net Sales divided by the aggregate number of units sold in such country).
In no event will any particular amount identified above be deducted more than once in
calculating Net Sales. Sales of a Product between Licensee and its Affiliates or Sublicensees for
9.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
resale shall be excluded from the computation of Net Sales, but the subsequent resale of such
Product to a Third Party shall be included within the computation of Net Sales.
The supply of Product as samples, for use in non-clinical or clinical trials, or for use in
any test or studies reasonably necessary to comply with any applicable laws, rules, or regulations
or as is otherwise normal and customary in the industry shall not be included in the computation
of Net Sales, so long as Licensee, its Affiliates, and Sublicensees do not receive payment for
such Product in excess of the Cost of Goods of such Product.
1.61 “NSCLC” means non-small cell lung cancer.
1.62“Patents” means (a) all patents, certificates of invention, applications for
certificates of invention, priority patent filings and patent applications, and (b) any renewals,
divisions, continuations (in whole or in part), or requests for continued examination of any of
such patents, certificates of invention and patent applications, and any all patents or certificates
of invention issuing thereon, and any and all reissues, reexaminations, extensions, supplementary
protection certificates, divisions, renewals, substitutions, confirmations, registrations,
revalidations, revisions, and additions of or to any of the foregoing.
1.63“Phase 1 Clinical Trial” means a clinical trial in any country conducted in a
small number of human volunteers designed or intended to establish an initial safety profile,
pharmacodynamics, or pharmacokinetics of a Product. For clarity, a Phase 1 Clinical Trial may
include studies conducted in oncology patients.
1.64“Phase 2 Clinical Trial” means a clinical trial of a Product in human patients in
any country to determine initial efficacy and safety and dose range finding. A Phase 2 Clinical
Trial is typically conducted before embarking on a Phase 3 Clinical Trial, but may be
registrational.
1.65“Phase 3 Clinical Trial” means a pivotal clinical trial of a Product in human
patients in any country with a defined dose or a set of defined doses of a Product designed to
ascertain efficacy and safety of such Product for the purpose of submitting applications for
Regulatory Approval to the competent Regulatory Authorities.
1.66“Phase 4 Clinical Trial” means a product support clinical trial of a Product that
is commenced after receipt of MAA Approval in the country where such trial is conducted.
Phase 4 Clinical Trial may include epidemiological studies, modeling and pharmacoeconomic
studies, post-marketing surveillance trials, and any such trials conducted as part of an Expanded
Access Program.
1.67 “Pricing and Reimbursement Approval” means, with respect to a Product, the
approval, agreement, determination or decision of any Governmental Authority establishing the
price or level of reimbursement for such Product, as required in a given country or jurisdiction
prior to sale of such Product in such jurisdiction.
1.68“Product” means any pharmaceutical product containing the Compound as an
active ingredient, in any form, presentations, dosage or formulation, including but not limited to
Cometriq. For purposes of this Agreement, all formulations of single-agent Product containing
the Compound shall be considered the same Product, and all formulations of combination
product, if any, containing the same set of active agents shall be considered the same Product.
10.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
1.69“Public Official or Entity” means (a) any officer, employee (including
physicians, hospital administrators, or other healthcare professionals), agent, representative,
department, agency, de facto official, representative, corporate entity, instrumentality or
subdivision of any government, military or international organization, including any ministry or
department of health or any state-owned or affiliated company or hospital, or (b) any candidate
for political office, any political party or any official of a political party.
1.70“RCC” means renal cell carcinoma.
1.71“Region” means, individually and collectively, the following regions: [ * ].
1.72“Regulatory Approval” means any and all approvals (including MAA Approval,
and Pricing and Reimbursement Approval, if applicable), licenses, registrations, permits,
notifications and authorizations (or waivers) of any Regulatory Authority that are necessary for
the manufacture, use, storage, import, transport, promotion, marketing, distribution, offer for
sale, sale or other commercialization of a Product in any country or jurisdiction.
1.73“Regulatory Authority” means any Governmental Authority that has
responsibility in its applicable jurisdiction over the testing, development, manufacture, use,
storage, import, transport, promotion, marketing, distribution, offer for sale, sale or other
commercialization of pharmaceutical products in a given jurisdiction, including the FDA, the
EMA and Health Canada or other foreign equivalent. For countries where governmental
approval is required for pricing or reimbursement for a pharmaceutical product to be reimbursed
by national health insurance (or its local equivalent), Regulatory Authority shall also include any
Governmental Authority whose review or approval of pricing or reimbursement of such product
is required.
1.74“Regulatory Exclusivity” means any exclusive marketing rights or data
exclusivity rights conferred by any Regulatory Authority with respect to a Product other than
patents, including, without limitation, rights conferred in the U.S. under the Hatch-Waxman Act
or the FDA Modernization Act of 1997 (including pediatric exclusivity), or rights similar thereto
outside the U.S., such as Directive 2001/83/EC (as amended) in the EU.
1.75“Regulatory Filing” means all applications, filings, submissions, approvals,
licenses, registrations, permits, notifications and authorizations (or waivers) with respect to the
testing, Development, manufacture or Commercialization of any Product made to or received
from any Regulatory Authority in a given country, including any INDs and MAAs.
1.76“Safety Data” means Data related solely to any adverse drug experiences and
serious adverse drug experience as such information is reportable to Regulatory Authorities.
Safety Data also includes “adverse events”, “adverse drug reactions” and “unexpected adverse
drug reactions” as defined in the ICH Harmonised Tripartite Guideline for Clinical Safety Data
Management: Definitions and Standards for Expedited Reporting.
1.77“SEC” means the U.S. Securities and Exchange Commission, or any successor
entity or its foreign equivalent such as the French Autorités des Marchés Financiers or
otherwise, as applicable.
1.78“Sponsor” means the Party that takes the ultimate responsibility for the initiation,
performance and management of, including financing or arranging the financing for, the
appropriate Clinical Trial.
11.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
1.79“Stockout Period” means a period during which Licensee, as a result of failure of
Exelixis to supply Product, has no commercial inventory available to supply the market in the
Licensee Territory. Inventory stockouts arising from Licensee’s failure to maintain the [ * ]
safety stock in accordance with the Supply Agreement shall not give rise to a Stockout Period.
1.80“Sublicensee” means a Third Party to whom Licensee grants a sublicense to
Develop, use, import, promote, offer for sale or sell any Product in the Field in the Licensee
Territory, beyond the mere right to purchase Products from Licensee and its Affiliates, and
excluding wholesalers, full-service distributors that do not promote the sale of the Product, and
other similar physical distributors. In no event shall Exelixis or any of its Affiliates be deemed a
Sublicensee.
1.81“Third Party” means any entity other than Exelixis or Licensee or an Affiliate of
Exelixis or Licensee.
1.82“Tier 1 Additional Indication” means RCC (1st line), HCC (1st line), [ * ].
1.83“Tier 2 Additional Indication” means any line of therapy for [ * ].
1.84“Top 5 EU” means the United Kingdom, Germany, France, Spain, and Italy.
1.85“U.S.” means the United States of America, including its territories and
possessions (including Puerto Rico).
1.86“Valid Claim” means (a) a claim of an issued and unexpired patent that has not
been revoked or held unenforceable, unpatentable or invalid by a decision of a court or other
governmental agency of competent jurisdiction that is not appealable or has not been appealed
within the time allowed for appeal, and that has not been abandoned, disclaimed, denied or
admitted to be invalid or unenforceable through reissue, re-examination or disclaimer or
otherwise, or (b) a claim of a pending patent application that has not been cancelled, withdrawn
or abandoned or finally rejected by an administrative agency action from which no appeal can be
taken and that has not been pending for more than [ * ].
1.87Additional Definitions. The following table identifies the location of definitions set
forth in various Sections of the Agreement:
Defined Terms | Section |
Acquisition Transaction | 17.8(b) |
Alliance Manager | 3.9 |
Allowable Increases | 4.5(b) |
Auditor | 10.4 |
Beneficial Party | 9.2(d) |
Canada Studies | 4.15 |
Change of Control | 2.8(c) |
Claim | 13.3 |
Commercialization Plan | 6.2 |
Competing Program | 2.8(a) |
12.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Defined Terms | Section |
Compound Invention | 11.1(b)(i) |
Development Budget | 4.2 |
Disputed Matter | 16.2 |
Divest | 2.8(c) |
EU LOE Event | 5.5(a)(iii)(B) |
Excess Funds | 4.5(a) |
Exelixis Fiscal Year | 1.38 |
Exelixis Data | 11.1(a) |
Exelixis Entity | 17.8(a)(i)(1) |
Exelixis Indemnitee | 13.2 |
Exelixis Only Development Work | 4.5(e) |
First LOE Event | 5.5(a)(iii)(C) |
Generic Entry | 5.5(a)(iii)(A) |
Global Development Plan or GDP | 4.2 |
Indemnitee | 13.3 |
Indemnitor | 13.3 |
Independent Work | 4.3 |
Independent Work Cost | 9.2(b) |
Initial Committed Studies | 4.5(a) |
Injunctive Relief | 16.3(b) |
Licensee Data | 11.1(a) |
Licensee Indemnitee | 13.1 |
Licensee Only Development Work | 4.5(e) |
Joint Commercialization Committee or JDC | 3.3 |
Joint Development Committee or JDC | 3.2 |
Joint Steering Committee or JSC | 3.1 |
Joint Inventions | 11.1(b)(ii) |
Joint Patents | 11.1(b)(ii) |
Losses | 13.1 |
Loss of Exclusivity | 5.5(a)(iii)(A) |
Materials | 4.14 |
PV Contribution Fee | 5.5(a)(ii) |
PV Costs | 5.5(a)(i) |
Pharmacovigilance Agreement | 5.5 |
Previously Achieved Commercial Milestone | 9.4(b)(ii) |
Previously Achieved Commercial Milestone for Canada | 9.4(b)(iii) |
13.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Defined Terms | Section |
Product Infringement | 11.3(a) |
Product Marks | 11.6(a) |
Promotional Materials | 6.4(c) |
Recall | 5.9 |
Regulatory Meeting | 5.4 |
Royalty Term | 9.5(b) |
Sales Forecast | 6.3(c) |
Second LOE Event | 5.5(a)(iii)(C) |
Sobi | 5.2 |
Sobi Agreement | 8.1 |
Sole Inventions | 11.1(b)(ii) |
Standstill Period | 17.8(a) |
Sunshine Reporting Laws | 5.10 |
Supply Agreement | 7.1 |
Supply Contacts | 3.10 |
Term | 15.1(a) |
TMC | 5.2 |
U.S. LOE Event | 5.5(a)(iii)(B) |
Withholding Tax Action | 10.3(c) |
2.Grant of Licenses
2.1Licenses Granted to Licensee. Subject to the terms and conditions of this
Agreement (including Section 8.1), Exelixis hereby grants to Licensee, during the Term:
(a)an exclusive (even as to Exelixis, except as expressly set forth herein),
royalty-bearing license, with the right to grant sublicenses solely as provided in Section 2.2,
under the Exelixis Technology to use, sell, offer for sale, import and otherwise Commercialize
(but not to make or have made) the Products in the Field and in the Licensee Territory; and
(b)a non-exclusive license, with the right to grant sublicenses solely as
provided in Section 2.2, under the Exelixis Technology to Develop (but not to make or have
made) the Products on a worldwide basis under the GDP, and to use the Products for that
purpose. Exelixis agrees not to grant any further license to Develop the Products except to
Future Exelixis Licensees.
2.2Sublicenses. Licensee shall have the right to grant sublicenses under the licenses
granted in Section 2.1:
(a)to an Affiliate of Licensee without Exelixis’ express prior written consent
and without providing any written notice to Exelixis, provided that such sublicense will
terminate if such sublicensee no longer qualifies as an Affiliate of Licensee.
14.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(b)to any Third Party distributor identified on Exhibit C attached hereto
(which list of approved distributors shall be agreed upon by the Parties within thirty (30) days
following the Effective Date) without Exelixis’ express prior written consent, provided that
Licensee does not have an Affiliate that is then engaged in selling pharmaceutical products in
such sublicensed territory.
(c)to any Third Party distributor not listed in Exhibit C without Exelixis’
express prior written consent, provided that (i) Licensee does not have an Affiliate that is then
engaged in selling pharmaceutical products in such sublicensed territory; (ii) Licensee has
conducted a reasonable investigation of such Third Party and believes that such Third Party is
qualified and competent, and such Third Party annually certifies its compliance with, and
actually complies with, Applicable Laws and other applicable requirements, (iii) such Third
Party is then engaged in the promotion and commercialization of oncology products, and (iv)
Licensee is then using such Third Party for distribution of pharmaceutical products other than
Products; and provided further that Licensee notifies Exelixis in writing [ * ] days’ in advance of
granting such sublicense specifying (x) the name of such Third Party and the country(ies) such
sublicense will cover, and (y) that Licensee has met the conditions set forth in (ii) – (iv). If
Exelixis believes Licensee should not grant such sublicense to such Third Party, it may direct
such concern and any documentation supporting such concern to the JSC for discussion.
(d)to a Third Party other than as set forth in (b) and (c) with Exelixis’ express
prior written consent.
(e)All sublicenses granted under the licenses granted in Section 2.1 shall be in writing and
shall be subject to, and consistent with, the terms and conditions of this Agreement and shall
provide that any such Sublicensee (for clarity, including any distributor) shall not further
sublicense except with the consent of Licensee and Exelixis. Licensee shall ensure that each
agreement with a Sublicensee grants Exelixis all rights with respect to Data, Inventions and
Regulatory Filings made or generated by such Sublicensee as if such Data, Inventions and
Regulatory Filings were made or generated by Licensee. Licensee shall be responsible for the
compliance of its Affiliates, Sublicensees (for clarity, including any distributors), and
subcontractors with the terms and conditions of this Agreement. Licensee shall provide written
notice to Exelixis of each sublicense granted to a Third Party hereunder, specifying the name of
the Sublicensee, the territory, and the duration of the sublicense.
Licensee agrees that in countries where it is not Commercializing Products through its Affiliates,
it will only contract with Third Party distributors who satisfy the conditions of paragraphs (b),
(c), or (d) above, whether or not a sublicense of rights hereunder is actually required.
2.3Reserved Rights. Exelixis hereby expressly reserves:
(a)the right under Exelixis Technology to exercise its rights and perform its
obligations under this Agreement, whether directly or through one or more licensees or
subcontractors, including the right to Develop the Compound and Products in the Licensee
Territory under the GDP; and
(b)subject to Section 2.8, all rights to practice, and to grant licenses under,
the Exelixis Technology outside of the scope of the licenses granted in Section 2.1, including the
exclusive right to make and have made the Compound and Products anywhere in the world, and
15.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
the exclusive rights to practice the Exelixis Patents and Exelixis Know-How with respect to
compounds and products other than Compound and Products.
2.4Licenses Granted to Exelixis. Subject to the terms and conditions of this
Agreement, Licensee hereby grants to Exelixis:
(a)an exclusive (even as to Licensee, except as expressly set forth herein),
royalty-free, fully paid-up license, with the right to sublicense (provided that any such
sublicensee may only grant a further sublicense at two tiers), under the Licensee Technology to
use, sell, offer for sale, import and otherwise Commercialize the Products in the Field in the
Exelixis Territory;
(b)a co-exclusive, royalty-free, fully paid-up license, with the right to
sublicense (provided that any such sublicensee may only grant a further sublicense at two tiers),
under the Licensee Technology to Develop the Compound and Products on a worldwide basis
under the GDP; and
(c)an exclusive (even as to Licensee), royalty-free, fully paid-up license, with
the right to sublicense (provided that any such sublicensee may only grant a further sublicense at
two tiers), under the Licensee Technology to make and have made the Compound and Products
anywhere in the world.
(d)Sublicenses: Exelixis shall have the right to grant sublicenses under the
licenses granted in Section 2.4
A.without Licensee’s consent and without providing any
written notice to Licensee if such sublicense is granted to an Affiliate; and
B.without Licensee’s prior written consent, provided however
that a written notice is sent to Licensee for Licensee’s information if such sublicense is granted
to Third Parties to manufacture the Product and provided further that such Third Party is
qualified and certified to manufacture the Product in such country in accordance with Applicable
Laws and other applicable requirements.
2.5No Implied Licenses; Negative Covenant. Except as set forth in this
Agreement, neither Party shall acquire any license or other intellectual property interest, by
implication or otherwise, under or to any Patents, Know-How or other intellectual property
owned or controlled by the other Party. Neither Party shall, nor shall it permit any of its
Affiliates or sublicensees to, practice any Patents or Know-How licensed to it by the other Party
outside the scope of the licenses granted to it under this Agreement.
2.6Disclosure of Know-How. For as long as the Parties are conducting
Development activities under the GDP, Exelixis shall, without additional compensation, disclose
and make available to Licensee, in electronic form where, all Exelixis Know-How that comes
into existence after the Effective Date and that was not previously provided to Licensee,
promptly after the development, making, conception or reduction to practice of such Exelixis
Know-How. For as long as the Parties are conducting Development activities under the GDP,
Licensee shall and shall cause its Affiliates to, without additional compensation, disclose and
make available to Exelixis, in electronic form where possible, any Licensee Know-How not
previously provided to Exelixis, and promptly after the earlier of the development, making,
conception or reduction to practice of such Licensee Know-How. The JDC and JCC shall each
16.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
establish a mechanism for the reciprocal disclosure of Know-How within its respective area of
responsibility.
2.7Third Party Licenses.
(a)If Exelixis enters into any agreement with a Third Party after the Effective
Date that includes a license from such Third Party to Exelixis under any Know-How or Patents
that are necessary or reasonably useful to Develop, use, sell, offer for sale or import the Products
in the Field and in the Licensee Territory, then Exelixis shall notify Licensee, identifying the
relevant Know-How or Patents, by providing Licensee with the substantive terms of the
applicable Third Party license agreement to Licensee, to the extent applicable to the rights that
would be sublicensed to Licensee, which Exelixis hereby agrees to do. Such Know-How and
Patents, to the extent falling within the definition of Exelixis Technology, will be sublicensed to
Licensee if Licensee provides Exelixis with written notice in which (i) Licensee consents to
adding such Patents and Know-How to the definition of Exelixis Technology, (ii) Exelixis and
Licensee, acting reasonably in good faith, agree on the terms and conditions of the payments that
would be owed under such license agreement as a result of Exelixis’ granting a sublicense to
Licensee or Licensee’s practice thereunder, including Licensee’s and its Affiliates’ and
Sublicensees’ Development, use, sale, offer for sale and importation of the Compound and
Products in the Field and in the Licensee Territory, and a reasonable allocation of all other
payments under such license agreement, and to make all payments when due and provide all
reports required under such license agreement; and (iii) Licensee acknowledges in writing that its
sublicense under such license agreement is subject to the terms and conditions of such license
agreement.
(b)Licensee shall promptly notify Exelixis if it becomes aware of any Third
Party Know-How or Patents that are necessary or reasonably useful to Develop, make, have
made, use, sell, offer for sale or import the Compound and Products in the Field, and shall give
Exelixis the first right to negotiate and obtain a license from such Third Party under such Know-
How or Patents. Except with the prior written consent of the other Party, neither Party shall
obtain a license to Third Party Patents or Know-How that is necessary or reasonably useful to
Develop, make, have made, use, sell, offer for sale or import the Products, for use with the
Products in the other Party’s territory, unless it obtains the right to sublicense such rights to the
other Party.
2.8Exclusivity.
(a)Subject to Section 2.8(c) below, for the period starting from the Effective
Date and for ten (10) years following the first Regulatory Approval of the Product in the first
indication other than MTC, neither Party (nor any of its Affiliates) shall, directly or indirectly
(including through a Third Party), commercialize any Competing Product for therapeutic or
prophylactic use (a “Competing Program”).
(b)Subject to Section 2.8(c) below, for the period starting from the Effective
Date and for five (5) years following the first Regulatory Approval of the Product in the first
indication other than MTC, neither Party (nor any of its Affiliates) shall, directly or indirectly
(including through a Third Party) develop any Competing Program [ * ].
(c)In the event that a Third Party becomes an assignee of this Agreement, or
an Affiliate of a Party after the Effective Date through merger, acquisition, consolidation or other
17.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
similar transaction, and such Third Party, as of the closing date of such transaction, is engaged in
the conduct of a Competing Program:
(i)if such transaction constitutes a Change of Control of [ * ], [ * ]
shall have the right to terminate the Agreement as provided herein. [ * ] shall have [ * ]
following the announcement of such transaction to give written notice to [ * ] of its intent to
terminate the Agreement, such termination to be effective [ * ] after receipt of notice of
termination (but only after completion of the transaction with such entity having a Competing
Product) unless [ * ] notifies [ * ] within [ * ] of receipt of the notice of termination of its
decision to (a) Divest any such Competing Product to a Third Party, (b) discontinue the
Competing Program, or (c) acting reasonably and in good faith agree with [ * ] and such assignee
or new Affiliate to find a mutually acceptable agreement whereby they can, on compliance with
Applicable Laws, jointly exploit such Competing Product together with the Product. Such
disposition shall be completed within [ * ] of completion of any such sale or Change of Control
transaction. During the [ * ], such assignee or new Affiliate (as the case may be) shall have the
right to continue the Competing Program and such continuation shall not constitute a breach of
such Party’s exclusivity obligations set forth above; provided that such assignee or new Affiliate
(as the case may be) conducts the Competing Program independently of the activities of this
Agreement and does not use any [ * ] in the conduct of the Competing Program. In the event this
Agreement is terminated in accordance with the foregoing, neither Party shall [ * ];
(ii)if such transaction constitutes a Change of Control of [ * ], then
such assignee or new Affiliate shall continue to Develop and Commercialize the Product using a
level of Commercially Reasonable Efforts that assumes the Competing Program was not
acquired and shall, within [ * ] after the closing of such Change of Control transaction: (a) Divest
the Competing Program to a Third Party, or (b) discontinue the Competing Program. During the
[ * ] period, such assignee or new Affiliate (as the case may be) shall continue to fulfill its
obligations under this Agreement in all respects, shall conduct Competing Program activities
independently of the activities pursuant to this Agreement and shall not use any [ * ] in the
conduct of the Competing Program;
(iii)if such transaction does not constitute a Change of Control of such
Party, then such Party and its new Affiliate shall have [ * ] from the closing date of such
transaction to wind down or complete the Divesture of the Competing Program; during this
period, the Party’s conduct of the Competing Program shall not deemed a breach of the
exclusivity obligations set forth above, provided that the Party continues to fulfill its obligations
under this Agreement in all respects, conducts its Competing Program activities independently of
the activities pursuant to this Agreement and does not use: (A) any [ * ] or (B) [ * ], in each case
in the conduct of such Competing Program. For clarity, if such Party completely winds down the
Competing Program within the [ * ] time period, it shall be allowed to divest the Competing
Program later, provided that it does not restart the Competing Program.
As used in this Section 2.8(c), “Change of Control” means, with respect to a Party: (1) a
merger, reorganization or consolidation involving such Party in which the voting securities of
such Party outstanding immediately prior thereto cease to represent at least fifty percent (50%) of
the combined voting power of the surviving entity immediately after such merger, reorganization
or consolidation; or (2) a person or entity, or group of persons or entities acting in concert,
acquire more than fifty percent (50%) of the voting equity securities or management control of
such Party; and “Divest” means the sale or transfer of rights to the Competing Program to a
18.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Third Party without receiving a continuing share of profit, royalty payment or other economic
interest in the success of such Competing Program.
(d)During the Term of this Agreement, neither Party (nor any of its
Affiliates) shall, directly or indirectly (including through a Third Party), commercialize the
Product or any Generic Product of any Product in the other party’s territory.
(e)To enforce the Parties’ respective obligations set forth in this Section
2.8(e), to the extent permitted by Applicable Law, neither Party shall, and shall ensure that its
respective Affiliates, permitted Sublicensees, and Third Party distributors will not, either directly
or indirectly, advertise, promote, or market Products, including via the Internet, to any Third
Party or place of business, residence, or shipping address in the other Party’s territory for the
duration of the Royalty Term. The foregoing shall restrict either Party, to the extent permitted by
Applicable Law, from engaging in any form of direct or indirect solicitation, advertisement, or
promotion in the other Party’s territory. Each Party shall promptly, without any right to
remuneration or compensation, forward to the other Party all inquiries regarding the Product by
persons or entities whose place of business, residence, or shipping address is in the other Party’s
territory.
(f)Licensee will [ * ]. In the event that Exelixis or Licensee [ * ], Licensee
shall [ * ].
3.Governance
3.1Joint Steering Committee. As of the Effective Date, the Parties have established
a joint steering committee (the “Joint Steering Committee” or the “JSC”), composed of an
equal number of up to [ * ] senior officers of each Party, to oversee and guide the strategic
direction of the collaboration of the Parties under this Agreement. The JSC shall act as a joint
consultative body and to the extent expressly provided herein, a joint decision-making body.
The JSC shall in particular:
(a)provide a forum for discussion of the Development and
Commercialization of the Compound and Products in the Licensee Territory and the Exelixis
Territory;
(b)review and approve the global strategy for the Development of the Product
worldwide and review and approve any proposed amendments to the GDP, including
corresponding budgets, following recommendation by the JDC;
(c)review and approve the Commercialization Plans for the Licensee
Territory, including proposed amendments, following recommendation by the JCC;
(d)review and approve Sales Forecasts (and corrective plans, if any)
submitted by Licensee pursuant to Section 6.3(c), following recommendation by the JCC;
(e)review the manufacturing and supply strategy, supply performance and
Cost of Goods, including periodic review of worldwide order forecasts for the Product to avoid
supply shortage and unfavorable treatment of Licensee’s supply requirements disproportionate to
those of Exelixis and Future Exelixis Licensees on the basis of their respective volumes;
19.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(f)review and approve any recommendations of the JCC not to launch (or to
significantly delay the launch of) a Product in a particular country of the Licensee Territory;
(g)review and approve coordinated activities under global brand strategies for
the Products in each of the Parties’ territories, following recommendation by the JCC;
(h)approve decisions of the JDC, JCC and any other joint subcommittee
established by JSC, including appointment of memberships, membership changes, and resolving
any disputed matter submitted to it by such Committees;
(i)establish additional joint subcommittees as it deems necessary or advisable
to further the purpose of this Agreement, including approving establishment and membership of
subcommittees if proposed by the JDC or JCC; and
(j)perform such other functions as appropriate to further the purposes of this
Agreement, as expressly set forth in this Agreement or allocated to it by the Parties’ written
agreement, including providing financial oversight of the activities conducted pursuant to this
Agreement.
For clarity, any information sharing of Commercialization matters regarding the Exelixis
Territory shall be for solely for purposes of the coordination of the Parties’ activities, and
Exelixis shall retain all decision making authority with respect to such matters without requiring
any approvals except as expressly provided in Sections 14.4 and 14.5.
3.2Joint Development Committee. As of the Effective Date, the Parties have
established a joint Development, Medical Affairs, and regulatory committee (the “Joint
Development Committee” or the “JDC”), composed of up to [ * ] representatives of each Party,
to monitor and coordinate the Development of, and Medical Affairs Activities connected with,
the Compound and Products at the operational level. Each JDC representative shall have
knowledge and expertise in the clinical development of products similar to the Products. The
JDC shall in particular:
(a)report to the JSC on all significant Development activities, including
implementation of the GDP, and on the activities of the JDC;
(b)coordinate and monitor the Development activities of the Parties under the
GDP and oversee implementation of the GDP;
(c)provide a forum for and facilitate communications between the Parties
with respect to the Development of Products in the Licensee Territory and the Exelixis Territory,
including sharing of Development information and Data in accordance with Section 4.7(a);
(d)elaborate, review and approve clinical trial protocols, including
investigator-initiated and cooperative group clinical trial plans and protocols, and statistical
analysis plans for Clinical Trials (and any amendments thereto) in the Exelixis and Licensee
Territories and monitor the progress of the clinical studies;
(e)define areas of permissible scientific and medical inquiry and parameters
for Phase 4 Clinical Trials in the Exelixis and Licensee Territories;
20.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(f)review Data resulting from Phase 1/1b/2 Clinical Trials against go/no-go
criteria in the GDP to determine progression to a Phase 3 Clinical Trial;
(g)review Data resulting from Phase 3 Clinical Trials against go/no-go
criteria in the GDP to determine progression to submission of Regulatory Filing;
(h)prepare amendments to the GDP (including the Development Budget) and
submit such amendments to the JSC for approval;
(i)monitor and coordinate all regulatory actions worldwide, communications
and submissions for the Compound and Products under the GDP and pharmacovigilance and
safety matters worldwide;
(j)establish joint working groups (such clinical, regulatory and safety) as it
deems necessary or appropriate to oversee the day-to-day management of different aspects of the
Development work under the GDP;
(k)oversee and coordinate the Medical Affairs Activities for the Product in all
indications, which shall be subject to a Medical Affairs portion of the GDP and may be
coordinated through a Medical Affairs working group established and overseen by the JDC;
(l)oversee and coordinate decisions related to research or Development of
new indications, characterization and Development of bio-markers (if any), which may be
coordinated through a Medical Affairs working group established and overseen by the JDC;
(m)review activities related to pharmaceutical development, Phase 3 Clinical
Trial active ingredient and drug product new campaigns (i.e., chemical process scale-up/
optimization (if needed) and micronization process study, manufacturing, QC testing and release
of GMP batches of active ingredient and drug product as needed for Phase 3 Clinical Trial, in
particular, review and approval of the protocols on manufacturing, micronization, scale-up plan
and process optimization);
(n)maintain and review the “Company Core Data Sheet”, which shall cover
material relating to safety, indications, dosing, pharmacology and other information concerning
the Product including Company Core Safety Information;
(o)coordinate the supply of the Compound and Products to Licensee for
Development use;
(p)oversee and facilitate the Parties’ communications and activities with
respect to publications under Section 14.4;
(q)establish and supervise the global publication strategy with respect to the
Compound and Products;
(r)perform such other functions as may be appropriate to further the purposes
of this Agreement with respect to the Development of Products, including endeavoring to resolve
any disputes between the Parties arising from the deliberations of the JDC, or as otherwise
directed by the JSC.
21.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
3.3Joint Commercialization Committee. As of the Effective Date, the Parties have
established a joint commercialization committee (the “Joint Commercialization Committee” or
the “JCC”), composed of up to [ * ] representatives of each Party, to monitor and discuss the
Commercialization of Products at the operational level. Each JCC representative shall have
knowledge and expertise in the commercialization of products similar to Products. The JCC
shall in particular:
(a)report to the JSC on all significant Commercialization activities in the
Licensee Territory, including implementation of the Commercialization Plan, and on the
activities of the JCC;
(b)review, discuss and approve the Commercialization Plans and related
activities with respect to the Commercialization of Products in the Licensee Territory;
(c)provide a forum for and facilitate communications and coordination
between the Parties with respect to the Commercialization of Products in the Licensee Territory
and the Exelixis Territory;
(d)on an annual basis, review and approve Licensee’s Sales Forecast prepared
pursuant to Section 6.3(c) as well as any corrective plans submitted thereunder;
(e)review and approve any recommendation by Licensee not to launch (or to
significantly delay the launch of) any Product in any country of the Licensee Territory;
(f)review and discuss the major findings of Licensee’s market research with
respect to any Product in the Licensee Territory;
(g)provide input to the JDC on the global publication strategy with respect to
the Products and implement such strategy under supervision of the JDC once it has been
established;
(h)review and oversee the branding and product positioning strategy for
Products in the Licensee Territory;
(i)establish pricing corridors for Products in the Licensee Territory for the
purpose of reimbursement and potential international pricing reference by relevant Regulatory
Authorities;
(j)define and coordinate medical messaging worldwide with respect to the
Products;
(k)oversee and facilitate the Parties’ communications and activities with
respect to publications under Section 14.4;
(l)design a global brand strategy for the Licensee Territory (e.g., a four-year
brand plan, resource plan, etc.) and submit such strategy to the JSC for review and approval;
(m)discuss and coordinate the manufacture and supply of the Products to
Licensee for Commercial use; and
22.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(n)perform such other functions as may be appropriate to further the purposes
of this Agreement with respect to the Commercialization of Products, including endeavoring to
resolve any disputes between the Parties arising from the deliberations of the JCC, or as
otherwise directed by the JSC.
3.4Executive Committee. Each Party shall designate an appropriate senior
executive officer of Exelixis and/or Licensee (e.g., CEO or members of each Party’s executive
committee) to meet once a year to discuss strategic issues and other issues that either Party
deems important to maintain a successful partnership and collaboration.
3.5Committee Membership and Meetings.
(a)Committee Members. Each Committee representative shall have
appropriate knowledge and expertise and sufficient seniority within the applicable Party to make
decisions arising within the scope of the applicable Committee’s responsibilities. Each Party
may replace its representatives on any Committee on written notice to the other Party, but each
Party shall strive to maintain continuity in the representation of its Committee members. The
[ * ]. [ * ]. The chairperson shall prepare and circulate agendas to Committee members at least
seven (7) days before each Committee meeting and shall direct the preparation of reasonably
detailed minutes for each Committee meeting, which shall be approved by the chairperson and
circulated to Committee members within thirty (30) days of such meeting. The initial members
of each of the JSC, JCC and JDC shall be determined by the Parties promptly following the
Effective Date.
(b)Meetings. Each Committee shall hold meetings at such times as it elects
to do so, but in no event shall meetings of the JDC and JCC be held less frequently than once
every [ * ], and meetings of the JSC once every [ * ], during the [ * ] following the Effective
Date and then the Parties may decide to reduce the frequency of the Committee meetings. The
first JSC meeting, first JDC meeting, and first JCC meeting shall be held within [ * ] after the
Effective Date, at which meetings the dates for the first calendar year shall be set. Meetings of
any Committee may be held in person, or by audio or video teleconference; provided that unless
otherwise agreed by both Parties at least [ * ] meetings per year shall be held in person during the
first [ * ] following the Effective Date, and, for the subsequent years of the Term, at least one (1)
meeting per year of each Committee shall be held in person. In-person Committees shall be held
at locations alternately selected by the Parties. Each Party shall be responsible for all of its own
expenses of participating in any Committee meetings. No action taken at any meeting of a
Committee shall be effective unless at least one (1) representative of each Party is participating.
In addition, upon written notice to the other Party, either Party may request that a special ad hoc
meeting of the JSC be convened for the purpose of resolving any disputes in connection with, or
for the purpose of reviewing or making a decision pertaining to any material subject-matter
within the scope of the JSC, the review or resolution of which cannot be reasonably postponed
until the following scheduled JSC meeting. Such ad hoc meeting shall be convened at such time
as may be mutually agreed by the Parties, but no later than [ * ] following the notification date of
request that such meeting be held.
(c)Non-Member Attendance. Each Party may from time to time invite a
reasonable number of participants, in addition to its representatives, to attend the Committee
meetings in a non-voting capacity; provided that if either Party intends to have any Third Party
(including any consultant) attend such a meeting, such Party shall provide reasonable prior
written notice to the other Party and obtain the other Party’s approval for such Third Party to
attend such meeting, which approval shall not be unreasonably withheld or delayed. Such Party
23.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
shall ensure that such Third Party is bound by written confidentiality and non-use obligations
consistent with the terms of this Agreement.
3.6Decision-Making.
(a)All decisions of each Committee shall be made by unanimous vote, with
each Party’s representatives collectively having one (1) vote. If after reasonable discussion and
good faith consideration of each Party’s view on a particular matter before a Committee, the
representatives of the Parties cannot reach an agreement as to such matter within [ * ] after such
matter was brought to such Committee for resolution, then, except as provided in Section 3.6(c),
if such disagreement arose within the JDC or JCC, it shall be referred to the JSC for resolution.
If the JSC cannot resolve such matter within [ * ], or if the disagreement first arose within the
JSC, then either Party at any time may refer such issue to the Executive Officers for resolution.
(b)If the Executive Officers cannot resolve such matter within [ * ] after such
matter has been referred to them, then:
(i)Exelixis shall have the final decision making authority, which shall
be exercised in its reasonable discretion, with respect to Development matters, except for:
A.the addition of [ * ], the cost of which would be [ * ]; and,
B.any material modification to a [ * ]; for the purpose of this
clause, “material modification” means any material changes to the agreed upon [ * ].
(ii)Licensee shall have the final decision making authority, which
shall be exercised in its reasonable discretion, with respect to (1) Commercialization in the
Licensee Territory, except with respect to the decision [ * ] a particular Product in a country, (2)
Medical Affairs in the Licensee Territory, and (3) regulatory matters in the Licensee Territory
that do not affect the Exelixis Territory; provided that Licensee’s decision shall be consistent
with the terms and conditions of this Agreement, including without limitation Section 6.4(b)
regarding pricing, and Section 6.3(c) regarding sales forecasts.
(iii)Neither Party shall have the final decision making authority with
respect to the matters in Sections 3.6(b)(i)(1) and 3.6(b)(i)(2) or with respect to the decision not
to [ * ] a particular Product in a particular country [ * ], and the status quo shall persist with
respect to such matter if the Parties are unable to agree.
(c)Notwithstanding Section 3.6(a), [ * ] representative shall have the deciding
vote on all tactical [ * ] matters for the Products [ * ], and such matter shall not be subject to
escalation to [ * ]; provided that such decision does not directly affect [ * ] and such decision
shall be consistent with the terms and conditions of this Agreement.
3.7Limitations on Authority. Each Committee shall have only such powers as are
expressly assigned to it in this Agreement, and such powers shall be subject to the terms and
conditions of this Agreement. Without limiting the generality of the foregoing, no Committee
will have the power to amend this Agreement, and no decision of a Committee may be in
contravention of any terms and conditions of this Agreement.
3.8Discontinuation of Committees. The activities to be performed by each
Committee shall solely relate to governance under this Agreement, and are not intended to be or
24.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
involve the delivery of services. Each Committee shall continue to exist until the first to occur
of: (a) the Parties mutually agree to disband such Committee; or (b) Exelixis provides written
notice to Licensee of its intention to disband and no longer participate in such Committee. Once
the Parties mutually agree or Exelixis has provided written notice to disband such Committee,
such Committee shall have no further obligations under this Agreement and, thereafter, each
Party shall designate a contact person for the exchange of information under this Agreement or
such exchange of information shall be made through Alliance Managers, and decisions of such
Committee shall be decisions as between the Parties, subject to the other terms and conditions of
this Agreement.
3.9Alliance Managers. Promptly after the Effective Date, each Party shall appoint
an individual who shall be an employee of such Party having appropriate qualification and
experience to act as the alliance manager for such Party (the “Alliance Manager”). Each
Alliance Manager shall be responsible for coordinating and managing processes and interfacing
between the Parties on a day-to-day basis throughout the Term. The Alliance Manager will
ensure communication to the JSC of all relevant matters raised at the JDC, the JCC and at any
joint subcommittees and project teams. Each Alliance Manager shall be permitted to attend
meetings of the JSC and other Committees as appropriate as non-voting participants. The
Alliance Managers shall be the primary contact for the Parties regarding the activities
contemplated by this Agreement and shall facilitate all such activities hereunder. Each Party
may replace its Alliance Manager with an alternative representative at any time with prior
written notice to the other Party. Any Alliance Manager may designate a substitute to
temporarily perform the functions of that Alliance Manager. Each Alliance Manager shall be
charged with creating and maintaining a collaborative work environment within the JSC and its
subcommittees. Each Party shall bear its own costs of its Alliance Manager, which costs shall be
excluded from the Parties’ respective Development and manufacturing costs.
3.10Supply Contacts. Each Party shall designate one (1) qualified and experienced
supply chain professional to serve as that Party's primary supply contact regarding the supply of
Compound and Products within this Agreement (“Supply Contacts”) and under the direction of
the JCC. Each Party may replace its Supply Contact with an alternative representative at any
time with prior written notice to the other Party. Supply Contacts shall be responsible for
facilitating information exchange and discussion between the Parties regarding the supply of
Compound and Products under this Agreement. [ * ]. Each Party shall bear its own costs of its
Supply Contact, which costs shall be excluded from the Parties’ respective Development and
Cost of Goods.
4.Development
4.1Overview. Subject to the terms and conditions of this Agreement, the Parties will
collaborate with respect to the Development of the Compound and Products and share the Data
resulting from such collaboration to facilitate the Development of the Compound and Products
throughout the Licensee Territory and the Exelixis Territory.
4.2Development Plan. The Development of the Compound and Products under this
Agreement (including the development of the Compound and any Product as a combination
product or combination therapy with another product and/or therapy), including Independent
Work and Licensee Only Development Work, shall be conducted only pursuant to a
comprehensive written global Development plan (the “Global Development Plan” or “GDP”),
which shall be incorporated by reference as part of this Agreement. The GDP shall set forth the
timeline and details (including line of therapy, tumor type, primary endpoints, approximate
25.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
patient size, combination agents and comparator agents) of all preclinical and clinical
Development activities to be conducted by the Parties as necessary to generate Data sufficient to
meet the common requirements of both the EMA and FDA for MAA Approval of the Compound
and Products for RCC, HCC, and other indications agreed upon by the Parties. The GDP may
also include any other Development activities approved by the JSC, including parameters for
permissible scientific inquiry in Phase 4 Clinical Trials. The GDP will include Clinical Trials
that the Parties are committed to conducting (unless modification is required by a Regulatory
Authority or any local or regional IRB/ethics committee, or is reasonably necessary to protect
patient safety) as well as Clinical Trials that will be decided by the JDC and JSC based on Data
and results obtained after the Effective Date and the Parties’ review of the future competitive
landscape. The GDP shall include a coordinated Development and regulatory strategy, including
the Parties’ respective roles in the Development of the registration dossier and Regulatory Filings
for the Products and the countries in which Development of the Products will occur. The GDP
shall also set forth the detailed budget of the anticipated costs for such Development activities
(the “Development Budget”) on a study-by-study or Clinical Trial-by-Clinical Trial basis. As
of the Effective Date, the Parties have agreed upon an initial GDP and Development Budget,
attached to this Agreement as Exhibit D. If the terms of the GDP contradict, or create
inconsistencies or ambiguities with, the terms of this Agreement, then the terms of this
Agreement shall govern. From time to time during the Term (at least on [ * ] basis), the JDC
shall prepare updates and amendments, as appropriate, to the then-current GDP, including
budgets, and shall submit such updates and amendments to the JSC for review and approval
before such updates and amendments are adopted. If upon the determination by the JDC as
reviewed and approved by the JSC, any pre-clinical, or Clinical Trials not included in the GDP
(i) are required in order to obtain and/or maintain MAA Approval for a Product in the EU and in
one or all the countries of the Exelixis Territory, or (ii) are otherwise recommended by the EMA
or the FDA in the EU and in one or all of the countries of the Exelixis Territory, then the JDC
shall review and recommend and the JSC shall review and approve an amendment to the GDP
reflecting such additional studies, including associated budget. The costs of such additional
studies shall be borne by the Parties as provided in Section 4.5(a).
4.3Independent Work. If either Party is interested in pursuing additional
Development work on a Product (the “Developing Party”) for the benefit of the Exelixis
Territory (in the case of Exelixis) or the Licensee Territory (in the case of Licensee) beyond what
is set forth in the then current GDP, then such Party shall provide the other Party with a written
detailed plan and budget for such additional work (the “Proposal”). Within [ * ] of receipt of the
Proposal, the JDC or delegated team shall meet to review the Proposal and to permit the other
Party (“Non-Developing Party”) an opportunity to ask questions and request additional
information from the Developing Party related to the Proposal, including whether such Proposal
is reasonably likely to have a material and adverse effect on the Product in the Non-Developing
Party’s territory. The Parties acknowledge that it is their intent to collaborate in good faith to
establish a similar review and approval process with any Future Exelixis Licensee. No additional
Development work shall proceed without the approval of the JSC, and following each such
approval such additional Development work and corresponding budget shall be incorporated into
the GDP by the JDC (the “Newly-Proposed Development”). For any Newly-Proposed
Development work, the Non-Developing Party that did not propose such work originally may
elect, at its discretion, to share the Development Costs with respect to such Development work
under Section 9.2(b). If the Non-Developing Party does not decide to pursue the Newly-
Proposed Development work jointly with the Developing Party or does not share the
Development Costs with respect to such Development work, in which event such Development
work shall be deemed “Independent Work” and the Developing Party may pursue such work in
the Field in its respective territory and the Development Costs with respect thereto shall be
26.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
deemed Independent Work Costs and subject to Sections 4.5(d) and 9.2(b). Notwithstanding the
foregoing, following the approval of the Independent Work by the JSC, the Party proposing the
Independent Work may conduct such Independent Work, provided that: (A) it shall do so in
accordance with the amended GDP; (B) such Independent Work shall be conducted under the
oversight of the JDC and the JSC; and (C) neither Party shall conduct Independent Work in a
manner that would have a material adverse effect on the Products in either Party’s territory.
4.4Annual Update to Development Budget. The JDC shall discuss and agree upon
the subsequent year’s Development Budget on an annual basis no later than November 1 of each
year. The JDC shall report any significant changes in the annual budgets to the JSC for approval
at the next scheduled JSC meeting.
4.5Development Cost.
(a)Committed Studies As Of The Effective Date (Current Budget).
Except as set forth in Section 4.5(b) below, Exelixis shall bear one hundred percent (100%) of all
Development Costs for the first [ * ] dollars ($[ * ]) of Development Costs for all Clinical Trials
that are committed studies in the GDP [ * ] as of the Effective Date (“Initial Committed
Studies”). Thereafter, except as set forth in Section 4.5(b) below, (i) Exelixis shall bear sixty-
five percent (65%) and Licensee shall bear thirty-five percent (35%) of all Development Costs
for such Clinical Trials [ * ] until the aggregate Development Costs of such Clinical Trials equals
[ * ] dollars ($[ * ]), and (ii) if aggregate Development Costs for such Clinical Trials [ * ] exceed
[ * ] dollars ($[ * ]), Exelixis shall bear [ * ] percent ([ * ]%) and Licensee shall bear [ * ] percent
([ * ]%) of all remaining Development Costs for such Clinical Trials. For Clinical Trials that
become committed studies in the GDP after the Effective Date, Exelixis shall bear sixty-five
percent (65%) and Licensee shall bear thirty-five percent (35%) of all Development Costs of
such Clinical Trials. If Exelixis completes the Initial Committed Studies for an amount less than
[ * ] dollars ($[ * ]), any amount not spent (“Excess Funds”) shall be credited against the Parties’
respective share of Clinical Trials that become committed studies in the GDP after the Effective
Date. Without limiting the foregoing, if any [ * ].
(b)Allowable Increases. Separate from the cost allocation provided for in
Section 4.5(a), Exelixis shall bear sixty-five percent (65%) and Licensee shall bear thirty-five
percent (35%) of all Allowable Increases in Development Costs for all Clinical Trials that are
committed studies in the GDP as of the Effective Date. “Allowable Increases” are defined as
increased Development Costs resulting from (i) changes in study design after the Effective Date
that are approved by the JDC and JSC [ * ] (up to the amount of a mutually-agreed budget
increase), (ii) changes in regulatory requirements arising after the Effective Date (including
changes required or recommended by Regulatory Authorities, but excluding changes required or
recommended specifically by a Regulatory Authority of the Exelixis Territory solely for the
benefit of the Exelixis Territory), and (iii) extensions in the duration of Clinical Trials resulting
from a lower than anticipated rate of clinical events or higher rates of survival.
(c)Expanded Access Program 214. Exelixis shall bear the first [ * ] dollars
($[ * ]) of Development Costs (excluding the costs of Licensee FTEs and other internal costs of
Licensee) associated with Expanded Access Program 214. Licensee shall bear one hundred
percent (100%) of its internal costs of such program, inclusive of its FTEs, as well as one
hundred percent (100%) of all Development Costs of such program in excess of the [ * ] dollars
($[ * ]) borne by Exelixis. If such program is completed for an amount of Development Costs
less than [ * ] dollars ($[ * ]), no financial adjustment shall be made.
27.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(d)Independent Work Cost. Notwithstanding Section 4.5(a), the Party
conducting the Independent Work approved by the JSC under Section 4.3 shall be solely
responsible for the Development Costs with respect to such Independent Work, subject to
Section 9.2(c).
(e)Country-Specific Development Work. Notwithstanding Section 4.5(a),
each Party shall be solely responsible for all Development Costs with respect to Development
activities that are exclusively for the benefit of the countries within such Party’s Territory,
including: (i) any and all country-specific activities (e.g., a Japan only trial for Exelixis or China
only trial and Canada Studies for Licensee, Expanded Access Programs); (ii) all Phase 4 Clinical
Trials solely benefiting such Party’s territory; (iii) any and all Development activities required
for any pricing and/or reimbursement approvals in such Party’s territory (but are not required for
the MAA Approval in such territory). The Development work set forth in this Section 4.5(e)
pertaining to Licensee shall be deemed the “Licensee Only Development Work” and the
Development work set forth in this Section 4.5(e) pertaining to Exelixis shall be deemed the
“Exelixis Only Development Work.” All planned and in-process Licensee Only Development
Work and Exelixis Only Development Work shall be included in and conducted in accordance
with the GDP, to be performed reasonably and subject to the oversight of the JDC and the JSC.
4.6Development Responsibilities. The JDC shall reasonably allocate Development
responsibilities of the Compound and Products under the GDP between the Parties and such
allocation shall be set forth in the GDP, provided that: (a) Exelixis shall be the Sponsor and have
the operational responsibility for all Development work under the GDP that is ongoing as of the
Effective Date; (b) each Party shall have the operational responsibility for its own Independent
Work; and (c) Licensee shall be the Sponsor and have the operational responsibility for the
Licensee Only Development Work and Exelixis shall be the Sponsor and have the operational
responsibility for the Exelixis Only Development Work.
4.7Data Exchange and Use.
(a)General. In addition to its adverse event and Safety Data reporting
obligations pursuant to Section 5.5, each Party shall promptly provide the other Party with (i)
[ * ] status reports on trial recruitment and other metrics consistent with the performing Party’s
internal reporting for clinical studies and Development activities, provided however that in case
of unexpected events that may have any impact on safety and recruitment, each Party shall
inform the other Party within forty-eight (48) hours from knowledge of the occurrence of such
event; (ii) supporting documentation (e.g. protocols, CRFs, analysis plans, etc.); (iii) preliminary
and final Data, and interim, preliminary and final results and reports; and (iv) output from
advisory committees and investigator meetings, any and all such documentation generated by
each Party (including by any Sublicensee or any Future Exelixis Licensee) from its Development
activities under this Agreement as such documentation could reasonably be deemed to affect the
Development or Commercialization activities of the Product in each Party’s territory. As time
may be of the essence, each Party shall collaborate in good faith in the exchange of any such
Data set forth in this Section within [ * ] of receipt. The Parties shall cooperate on a secure
website to facilitate the sharing of reports, Data and other information on a routine basis. Except
as set forth in Section 4.7(b) below, each Party shall have the right to use and reference, without
additional consideration, any and all Data generated by or on behalf of the other Party (including
by any Sublicensee or any Future Exelixis Licensee) under this Agreement for obtaining and
maintaining Regulatory Approval for the Products and otherwise Commercializing the Products
in its territory in accordance with the terms of this Agreement. For clarity, this Section 4.7(a)
28.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
shall apply to all Development under the GDP, including Independent Work (but subject to
Section 4.7(b) below), Exelixis Only Development Work and Licensee Only Development
Work. Notwithstanding the foregoing, should either Party fail to obtain such use and reference
rights from any Sublicensee or Future Exelixis Licensee, such Party shall not have the right to
grant use and access or rights to such Sublicensee or Future Exelixis Licensee to any
documentation listed in this Section 4.7(a) generated by or on behalf of the other Party.
(b)Independent Work. Notwithstanding the foregoing, the Party receiving
Data resulting from the other Party’s Independent Work shall have the right to use such Data
only to the extent reasonably necessary for the receiving Party to comply with its regulatory
reporting and compliance obligations, including safety reporting obligations, but shall not have
the right to use such Data to support its own Development, Regulatory Approval or
Commercialization except pursuant to Section 9.2(c).
4.8Diligence. Each Party shall use Commercially Reasonable Efforts to perform the
Development activities assigned to such Party under and in accordance with the GDP. Unless
otherwise agreed by the Parties, Exelixis shall be the Sponsor and be responsible for conducting
all Clinical Trials that are required to obtain MAA Approvals by both the EMA and FDA for
RCC, HCC, NSCLC, and other indications in the GDP. In addition, Licensee shall also use
Commercially Reasonable Efforts to Develop Licensee Only Development Work and any
Licensee Independent Work, file MAAs and seek and maintain Regulatory Approval (including
Pricing and Reimbursement Approval, as applicable) for the Products throughout the Licensee
Territory.
4.9Compliance. Each Party shall Develop the Compound and Products in
compliance with all Applicable Laws, including good scientific and clinical practices under the
Applicable Laws of the country in which such activities are conducted.
4.10Development Records. Each Party shall maintain complete, current and accurate
records of all Development activities conducted by it hereunder, and all data and other
information resulting from such activities. Such records shall fully and properly reflect all work
done and results achieved in the performance of the Development activities in good scientific
manner appropriate for regulatory and patent purposes. Each Party shall document all non-
clinical studies and Clinical Trials in formal written study reports according to Applicable Laws
and national and international guidelines (e.g., ICH, cGCP, cGLP, and cGMP).
4.11Development Reports. At [ * ] JDC meeting, each Party shall provide the JDC
with regular reports detailing its Development activities for the Products under this Agreement,
and the results of such activities. In addition, after the completion of any Clinical Trial or other
study of the Products, the Party responsible for the conduct of such Clinical Trial or study shall
promptly provide the other Party (but in no event more than [ * ] following receipt) with a data
package consisting of, at a minimum, tables, lists and figures, as well as any other Data specified
in the GDP or otherwise agreed by the Parties. The Parties shall discuss the status, progress and
results of each Party’s Development activities under this Agreement at such JDC meetings.
4.12Use of Subcontractors. Each Party may perform its Development activities
under this Agreement through one or more subcontractors, provided that (a) such Party will
remain responsible for the work allocated to, and payment to, such subcontractors to the same
extent it would if it had done such work itself; (b) each subcontractor undertakes in writing
obligations of confidentiality and non-use regarding Confidential Information that are
substantially the same as those undertaken by the Parties pursuant to Article 14, and (c) each
29.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
subcontractor agrees in writing to assign all intellectual property developed in the course of
performing any such work to such Party (or, in the event such assignment is not feasible, a
license to such intellectual property with the right to sublicense to such other Party). The Parties
may also subcontract work on terms other than those set forth in this Section 4.12 with the prior
approval of the JDC.
4.13[This Section has been intentionally omitted.]
4.14Materials Transfer. In order to facilitate the Development activities
contemplated by this Agreement, either Party may provide to the other Party certain biological
materials or chemical compounds Controlled by the supplying Party (collectively, “Materials”)
for use by the other Party in furtherance of such Development activities. Except as otherwise
provided for under this Agreement, all such Materials delivered to the other Party will remain the
sole property of the supplying Party, will be used only in furtherance of the Development
activities conducted in accordance with this Agreement, will not be used or delivered to or for
the benefit of any Third Party, except to subcontractors, without the prior written consent of the
supplying Party, and will be used in compliance with all Applicable Laws. The Materials
supplied under this Agreement must be used with prudence and appropriate caution in any
experimental work because not all of their characteristics may be known. Except as expressly set
forth in this Agreement, THE MATERIALS ARE PROVIDED “AS IS” AND WITHOUT ANY
REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, INCLUDING WITHOUT
LIMITATION ANY IMPLIED WARRANTY OF MERCHANTABILITY OR OF FITNESS
FOR ANY PARTICULAR PURPOSE OR ANY WARRANTY THAT THE USE OF THE
MATERIALS WILL NOT INFRINGE OR VIOLATE ANY PATENT OR OTHER
PROPRIETARY RIGHTS OF ANY THIRD PARTY.
4.15Canada Studies. If any Regulatory Authority in Canada requires one or more
additional studies to support any MAA submitted by Licensee for the Product in Canada that are
exclusively for the benefit of Canada (the “Canada Studies”), such studies shall be deemed
Licensee Only Development Work and subject to Licensee’s applicable obligations set forth
herein including, without limitation, those obligations set forth in Sections 4.2, 4.5(e), 4.6, 4.7(a),
and 4.8. In accordance with Section 3.2(h), the JDC shall prepare an amendment(s) to the GDP
with respect to any Canada Studies and submit such amendment(s) to the JSC for approval.
Exelixis shall, as may be required to enable Licensee to be the Sponsor of the Canada Studies, be
subject to Exelixis’ applicable obligations set forth in Section 5.1(b). Exelixis shall have the
right to use the Data of the Canada Studies generated by Licensee to support its own
Development, Regulatory Approval or Commercialization in the Exelixis Territory subject to
Section 9.2(b).
5.Regulatory Activities
5.1Regulatory Responsibilities.
(a)General.
(i)The GDP shall set forth the regulatory strategy for seeking
Regulatory Approval for the Compound and Products by the appropriate Regulatory Authorities
in the Licensee Territory and Exelixis Territory. The GDP shall also specify which Party shall
apply for and hold Regulatory Filings in each country with respect to the conduct of
Development activities; provided that if any Canada Studies are included in the GDP, the GDP
shall specify that Licensee shall apply for and hold Regulatory Filings in Canada. Subject to the
30.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
direction and oversight of the JDC, each Party shall be responsible for implementing such
regulatory strategy in its territory. Except as otherwise provided herein or required by
Applicable Law, each Party shall be responsible for the preparation and submission of any and
all Product registrations and marketing approvals in its territory and shall own and hold all such
Regulatory Filings (including Regulatory Approvals), and neither Party shall submit any
application for Product registration or marketing approval in the other Party’s territory.
(ii)Each Party shall be responsible for the cost and expense of all
regulatory activities in its territory.
(iii)Licensee acknowledges that Exelixis may be required to
communicate with Regulatory Authorities in the Licensee Territory as a result of Development
and manufacturing activities in such territory. Exelixis shall notify Licensee as soon as
reasonably possible of such communication with Regulatory Authorities and seek to incorporate
input from Licensee in preparation for such communication. Exelixis shall then keep Licensee
informed of any such communications.
(b)Transfer of Regulatory Filings. Except as set forth in Section 5.2,
Exelixis shall, in each case as may be required to enable Licensee to submit and file Regulatory
Filings and obtain MAA Approvals for Products in the Licensee Territory:
(i)transfer to Licensee all Regulatory Approvals and Regulatory
Filings submitted to any Regulatory Authority in the Licensee Territory for the Compound and
Products that are in Exelixis’ name and Controlled by Exelixis, other than INDs relating to
Clinical Trials conducted and sponsored by Exelixis pursuant to the GDP;
(ii)to the extent that such transfer is not permitted under Applicable
Laws, Exelixis shall provide to Licensee a right of reference or use to such Regulatory Approvals
and Regulatory Filings. Exelixis shall provide appropriate notification of Licensee’s access and
reference rights to the applicable Regulatory Authorities (including, to the extent applicable, an
informed consent letter under Article 10c of Directive 2001/83/EC as amended), at the expense
of Licensee seeking such right of reference. For the purposes of this Agreement, “right of
reference” shall mean the “right of reference or use” as defined in 21 C.F.R. §314.3(b) and any
equivalent regulation outside the US, including Article 10c of Directive 2001/83/EC, as each
may be amended from time to time;
(iii)provide to Licensee copies in electronic form of all Regulatory
Approvals and Regulatory Filings submitted to any Regulatory Authority in the Licensee
Territory including those related to CMC, manufacturing and product development, validation
and manufacturing for the Compound and Products that are in Exelixis’ name and Controlled by
Exelixis, regulatory dossiers in Exelixis’ possession or Control, and the Drug Master File; and
(iv)to the extent any variations to the chemistry, manufacturing, and
controls (“CMC”) section of the Regulatory Filing are required to conform with a variation that
is initiated by Exelixis at its sole discretion, Exelixis shall reimburse Licensee for all associated
fees that are paid by Licensee in filing such variations; provided that, for variations required to
comply with Applicable Laws or any requirement of a Regulatory Authority, (a) Exelixis shall
remain responsible for submissions and associated fees for all CMC variations originally
attributable to a Regulatory Authority in the Exelixis Territory, and (b) Licensee shall be
responsible for submissions and associated fees for all CMC variations originally attributable to a
Regulatory Authority in the Licensee Territory.
31.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
5.2Existing Arrangements. The Parties acknowledge that as of the Effective Date,
Exelixis; its regulatory agent, TMC Pharma Services (“TMC”); and its authorized distributor,
Swedish Orphan Biovitrum AB (“Sobi”), hold certain Regulatory Filings, licenses, and MAA
Approvals related to Cometriq for MTC in the EU. Exelixis, and Exelixis on behalf of TMC and
Sobi, will ensure that Exelixis, TMC and Sobi will transfer Regulatory Filings, licenses, and
MAA Approvals for Cometriq for MTC to Licensee in accordance with Article 8. In addition,
Exelixis holds certain EMA Regulatory Filings, including the EMA MAA filing, for the Product
in RCC. As set forth in Section 5.1(b), the Parties shall cooperate to be ready to transfer and
assign these EMA Regulatory Filings to Licensee and Exelixis shall notify the EMA promptly
after the Effective Date that Licensee shall be the Marketing Authorization Holder as from the
date of the transfer of the MAA. The Parties agree to work toward the transfer of MAA holder
status to Licensee by [ * ]. Until the MAA transfer is accepted by the EMA, Exelixis shall be
responsible for preparing and filing the MAA for the Product in RCC.
5.3Regulatory Information Sharing. Each Party shall, upon the other Party’s
reasonable request, promptly provide the other Party (but in no event more than [ * ]) with copies
of any Regulatory Filings prepared (including any drafts), submitted or received by such Party in
the U.S. and the Licensee Territory pertaining to the Compound and Products, and such other
Party shall have the right to review and comment on drafts of such Regulatory Filings, provided
that such review and comment shall not delay the submission of any Regulatory Filings. The
sharing of Regulatory Filings shall, as applicable, be the following communications/
correspondence with the Regulatory Authority: (i) summary of contact reports either Party
receives concerning substantive conversations or substantive meetings in its respective territory
with the FDA, EMA, CFDA and PMDA with respect to the Product or if contacts with those
Regulatory Authorities are made orally, to be reduced in writing, (ii) documents related to
regulatory milestones and dates (e.g., submission, validations, agency review questions, CHMP
opinion and FDA complete response letter and their equivalent), (iii) IND annual reports and
cover letters of all agency submissions relating to the Compound or any Product. If any
Regulatory Filing to be provided under this Section 5.3 was originally created in a language
other than the English language, then at the receiving Party’s request and to the extent already
existing and readily available, the providing Party shall provide an English translation along with
the original document to the receiving Party. The Parties acknowledge that it is their intent to
collaborate in good faith in the exchange of such Regulatory communications including with any
Sublicensee or Future Exelixis Licensee. Each of Licensee and Exelixis shall use Commercially
Reasonable Efforts to grant the other Party access and rights to use any such communications
with any Regulatory Authority generated by or on behalf of any Sublicensee or Future Exelixis
Licensee, respectively. Should either Party fail to obtain such access and rights from any
Sublicensee or Future Exelixis Licensee, such Party shall not have the right to grant access or
rights to such Sublicensee or Future Exelixis Licensee to any such communications with any
Regulatory Authority generated by or on behalf of the other Party.
5.4Meetings with Regulatory Authorities. On a current and ongoing basis, each
Party shall provide the other Party with a list and schedule of any in-person meeting or material
teleconference with the Regulatory Authorities (or related advisory committees) in the Licensee
Territory planned for the next Calendar Quarter that relates to the Development of the
Compound and Products under the GDP in the Licensee Territory (each, a “Regulatory
Meeting”). In addition, each Party shall notify the other Party as soon as reasonably possible if
such Party becomes aware of any additional Regulatory Meetings that become scheduled for
such Calendar Quarter and will keep the other Party informed of any significant interface or
communication with any Regulatory Authority which might affect efforts to obtain Regulatory
Approval for the Product. Licensee shall be solely responsible for any communications with the
32.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Regulatory Authorities occurring or required in connection with performing its regulatory
responsibilities set forth in this Article 5 with respect to the Product in the Licensee Territory,
and Exelixis shall have the right to provide input in preparation for all Regulatory Meetings and,
with the consent of Licensee, not to be unreasonably withheld, the right, but not the obligation, to
have its representatives attend (but, unless otherwise requested by the other Party, not participate
in) the Regulatory Meetings. Licensee shall have these same rights with respect to any such
Regulatory Meetings before such Regulatory Filings are transferred to Licensee under Sections
5.1(b) and 5.2.
(a)Regulatory Inspections. Licensee shall permit the Regulatory
Authority(ies) in the Exelixis Territory to conduct inspections of Licensee, its Affiliates, and
acting reasonably and in good faith of Sublicensees or subcontractors (including Clinical Trial
sites) relating to the Development of the Product under the GDP, and shall ensure that such
Affiliates, and acting reasonably and on good faith, such Sublicensees and subcontractors permit
such inspections. In addition, Licensee shall promptly notify Exelixis of any such inspection and
shall supply Exelixis with all information pertinent thereto. Licensee shall use Commercially
Reasonable Efforts to allow an Exelixis representative to attend any such inspection with the
presence of Licensee. Exelixis shall permit the Regulatory Authority(ies) in the Licensee
Territory to conduct inspections of Exelixis, its Affiliates, and acting reasonably and in good
faith of Sublicensees or subcontractors (including Clinical Trial sites) relating to the
Development of the Product under the GDP for the Licensee Territory, and shall ensure that such
Affiliates, and acting reasonably and on good faith, such Sublicensees and subcontractors permit
such inspections. In addition, Exelixis shall promptly notify Licensee of any such inspection and
shall supply Licensee with all information pertinent thereto. Exelixis shall use Commercially
Reasonable Efforts to allow a Licensee representative to attend any such inspection with the
presence of Exelixis.
5.5Adverse Event Reporting; Pharmacovigilance Agreement. Within sixty (60)
days after the Effective Date, but in any case prior to transfer of the marketing authorization, the
Parties shall enter into a pharmacovigilance agreement setting forth the worldwide
pharmacovigilance procedures for the Parties with respect to the Products, such as Safety Data
sharing, adverse events reporting and safety signal and risk management (the
“Pharmacovigilance Agreement”), which agreement shall be amended by the Parties [ * ] to
comply with any changes in Applicable Laws or any guidance received from Regulatory
Authorities. Such procedures shall be in accordance with, and enable the Parties to fulfill, local
and national regulatory reporting obligations under Applicable Laws (including to the extent
applicable, those obligations contained in ICH guidelines, E2A, E2B, E2C, E2D and E2F) to
monitor the patients’ safety. Exelixis has established and shall continue to hold at its costs and
expenses the global safety database for the Products, and shall maintain such global safety
database for so long as such Product is under Development and/or Commercialization by the
Parties. The Parties will collaboratively agree on data cut points for periodic aggregate safety
reports and Exelixis will author such reports; the Parties will jointly review and approve such
reports before submission to worldwide Regulatory Authorities as required. The Parties
acknowledge that the Parties amended the Pharmacovigilance Agreement to address the
modification to the Parties’ respective territories as outlined in the First Amendment.
(a)Parties’ Respective Contributions to PV Costs.
33.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(i)From the Effective Date through [ * ], Exelixis shall bear one
hundred percent (100%) of the cost and expense for establishing and maintaining such global
database and the preparation of periodic aggregate safety reports (“PV Costs”).
(ii)For the [ * ] commencing on [ * ], Licensee shall pay Exelixis, as a
contribution towards the PV Costs, an amount equal to [ * ] (the “PV Contribution Fee”), in the
manner set forth in clauses (A) and (B) immediately below, which amount shall not be subject in
any respect to audit or renegotiation.
A.Within [ * ] of the Fourth Amendment Effective Date, Licensee
shall pay Exelixis the previously invoiced and outstanding PV
Costs for the Calendar Quarters ending [ * ], which aggregate
amount shall equal [ * ].
B.Licensee shall pay Exelixis an amount equal to [ * ] within [ * ]
of receipt of the invoice for such amount.
(iii)For each Calendar Quarter commencing on [ * ] through the end of
the Calendar Quarter in which the First LOE Event (as defined below) occurs, Licensee shall pay
Exelixis an amount equal to [ * ] within [ * ] of the end of such quarter, which amount shall be
invoiced and shall not be subject in any respect to audit or renegotiation; provided, however, that
in consideration of the entry into this Agreement as of the Restatement Date, for each Calendar
Year commencing on [ * ], the PV Contribution Fee shall be reduced to [ * ]. LOE Event, U.S.
LOE Event, EU LOE Event, First LOE Event and Second LOE Event shall be defined as
follows:
A.Loss of exclusivity (“LOE”) occurs at the earliest of (i) the
latest of expiration of the last-to-expire Valid Claim of the
Exelixis Patents or Licensee Patents covering the Product in
either the US or the EU, as applicable, [ * ] and (ii) the first
time there is a Generic Entry with respect to such Product [ * ].
For purposes of this definition, “Generic Entry” means, with
respect to a particular Product, that one or more Generic
Products to such Product [ * ].
B.There are two separate LOE Events based on the following
Territories: the U.S. (“U.S. LOE Event”), and the EU (“EU
LOE Event”).
C.The earliest to occur of either the U.S. LOE Event or the EU
LOE Event is the “First LOE Event.” The latest to occur of
either the U.S. LOE Event or the EU LOE Event is the
“Second LOE Event.”
(iv)For each Calendar Quarter following the First LOE Event through
the end of the Calendar Quarter in which the Second LOE Event occurs, Licensee shall pay
Exelixis an amount equal to [ * ] within [ * ] of the end of such quarter, which amount shall be
[ * ] at the end of [ * ] Calendar Quarters, which shall be invoiced and shall not be subject in any
respect to audit or renegotiation. For the sake of clarity, by way of example, after the occurrence
of the First LOE Event, Licensee pays Exelixis [ * ] for [ * ] Calendar Quarters, then [ * ] for
34.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
[ * ] Calendar Quarters, and so on until the end of the Calendar Quarter in which the Second
LOE Event occurs.
(v)Further, for the sake of clarity, Licensee shall have no obligation to
pay Exelixis [ * ] under this Section 5.5(a) for any Calendar Quarter from, and after, the end of
the Calendar Quarter in which the Second LOE Event occurs.
(b)Exelixis will ensure that each Party and any Future Exelixis Licensee are
able to access the data, if necessary indirectly, from the global safety database in order to meet
legal and regulatory obligations. The Parties agree that Exelixis shall not transfer the
responsibility or holding of the global safety database to any CRO, sublicensee, Future Exelixis
Licensee or any Third Party without Licensee’s prior written consent and approval, which shall
not be unreasonably withheld, conditioned or delayed if such transferee (and its Affiliates) is a
pharmaceutical company of comparable size as Licensee and agrees to grant Licensee access and
other rights to the global safety database substantially equivalent to those granted by Exelixis
under the Pharmacovigilance Agreement. The use by Exelixis of a CRO, sublicensee, Future
Exelixis Licensee shall be at Exelixis’ sole cost and expenses.
(c)The JDC shall establish a joint safety subcommittee which shall have the
role and responsibility of reviewing and maintaining up to date the Pharmacovigilance
Agreement. As per the applicable Pharmacovigilance Agreement each Party shall be primarily
responsible for reporting quality complaints, adverse events and Safety Data related to the
Products to any Regulatory Authorities and responding to safety issues and to all requests of
Regulatory Authorities related to the Products under any MAA or Regulatory Approval for the
Product held by such Party and filed with such Regulatory Authorities, in each case at its own
cost. Each Party agrees to comply with its respective obligations under the Pharmacovigilance
Agreement and to cause its Affiliates, licensees and sublicensees to comply with such
obligations.
5.6No Harmful Actions. If a Party believes that the other Party is taking or intends
to take any action with respect to a Product that could reasonably be expected to have a material
adverse impact upon the regulatory status of such Product in the first Party’s territory, then such
Party may bring the matter to the attention of the JDC and the Parties shall discuss in good faith
to resolve such concern.
5.7Notification of Threatened Action. Each Party shall notify the other Party
within [ * ] of any information it receives regarding any threatened or pending action, inspection
or communication by any Regulatory Authority, which may affect the safety or efficacy claims
of any Product or the continued Development or Commercialization of any Product. Upon
receipt of such information, the Parties shall promptly consult with each other in an effort to
arrive at a mutually acceptable procedure for taking appropriate action.
5.8Right of Reference to Regulatory Materials. Each Party hereby grants to the
other Party the right of reference to all Regulatory Filings pertaining to the Compound and
Products submitted by or on behalf of such Party. The receiving Party may use such right of
reference solely for the purpose of seeking, obtaining and maintaining Regulatory Approval of
the Products for use in its territory in accordance with this Agreement. Notwithstanding the
foregoing, the receiving Party may use such right of reference to any Regulatory Filings based on
Data resulting from the other Party’s Independent Work only to comply with its safety reporting
obligations, unless the receiving Party pays the other Party for such work pursuant to Section
9.2(c).
35.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
5.9Recalls. In the event that a recall, withdrawal or correction (including the
dissemination of relevant information) of any Product in a Party’s territory is required by a
Regulatory Authority of competent jurisdiction, or if any Regulatory Authority requires or
advises either Party or such Party’s Affiliates or Sublicensees to distribute a “Dear Doctor” letter
or its equivalent regarding use of such Product in a Party’s territory or if a recall, withdraw or
correction of a Product in its territory is deemed advisable by such Party in its sole discretion,
such Party shall so notify the other Party no later than [ * ] in advance of the earlier of (i)
initiation of a recall, withdrawal or correction; or (ii) the submission of plans for such an action
to a Regulatory Authority. Any such recall, withdrawal, correction, or dissemination of
information (e.g., “Dear Doctor” letter) shall be referred to herein as a “Recall”. Promptly after
being notified of a Recall, each Party shall provide the other Party with such assistance in
connection with such Recall as may be reasonably requested by such other Party. All costs and
expenses in connection with a Recall in a Party’s territory shall be paid by such Party, including
without limitation the costs and expenses related to the dissemination of relevant information.
Each Party shall handle exclusively the organization and implementation of all Recalls of
Products in its territory. Notwithstanding the foregoing, any Recall related to the manufacture
and supply of the Product by Exelixis to Licensee shall be governed by the terms and conditions
of the Supply Agreement.
5.10Sunshine Reporting Laws. Each Party acknowledges that the other Party may
be subject to federal, state, local and international laws, regulations and rules related to the
tracking and reporting of payments and transfers of value provided to health care professionals,
health care organizations, and other relevant individuals and entities (collectively, “Sunshine
Reporting Laws”), and agrees to provide the other Party with all information regarding such
payments or transfers of value by such Party as necessary for such other Party to comply in a
timely manner with its reporting obligations under the Sunshine Reporting Law.
6.Commercialization
6.1General. Subject to the terms and conditions of this Article 6 (including Section
6.7), Licensee shall have the sole and exclusive responsibility, at its own expense, for all aspects
of the Commercialization of the Products in the Licensee Territory, including: (a) developing and
executing a commercial launch and pre-launch plan, (b) negotiating with applicable
Governmental Authorities and other payors regarding the price and reimbursement status of the
Products; (c) marketing and promotion; (d) booking sales and distribution and performance of
related services; (e) handling all aspects of order processing, invoicing and collection, inventory
and receivables; (f) providing customer support, including handling medical queries, and
performing other related functions; and (g) conforming its practices and procedures to
Applicable Laws relating to the promotion, sales and marketing, access, and distribution of the
Products.
6.2Commercialization Plan. As soon as practical after the Effective Date, Licensee
shall prepare and present to the JCC a Commercialization plan for Products in the Licensee
Territory, including a reasonably detailed description and an anticipated timeline for Licensee’s
significant Commercialization activities for the Products for the next [ * ] commencing with the
[ * ] (the “Commercialization Plan”). Taking into consideration the requirements of Section
6.3(c), the Commercialization Plan shall include such information on a country-by-country basis
for each of the Major Market Countries. Licensee shall update and amend the
Commercialization Plan annually starting in November 2017 and each subsequent November,
shall present such updates and amendments to the JCC for review and discussion. Without
limiting the provisions of this Section 6.2, through the JCC, Licensee shall consult with and
36.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
provide updates to Exelixis ([ * ]) regarding the commercial strategy and Commercialization of
Products in the Licensee Territory. Subject to the provisions of this Agreement and compliance
with the Commercialization Plan, Licensee shall have full Control and authority with respect to
the day-to-day Commercialization of the Products and implementation of the Commercialization
Plan.
6.3 Diligence.
(a)General. During the Term, Licensee shall use Commercially Reasonable
Efforts to Commercialize the Products for all indications that have received or will receive
Regulatory Approval throughout the Licensee Territory. In addition, and without limitation of
the foregoing, Licensee shall, as soon as possible following each MAA Approval(s), subject to
Section 6.4(b), launch the Product for such indication and obtain all necessary Price and
Reimbursement Approvals at least in [ * ] (subject to the business judgment to delay or not to
launch a particular Product in a particular country of the EU because of adverse pricing or other
business considerations). In the event that Licensee recommends not to launch a particular
Product in a particular country of the Licensee Territory, or to deliberately defer such launch, it
shall advise the JCC at the next meeting of such Committee and provide a reasonably detailed
rationale for such determination. Thereafter, Licensee shall utilize Commercially Reasonable
Efforts in the ongoing support for the Product in each such country. Licensee shall report to the
JCC its efforts in each of these countries at least [ * ] at meetings of the JCC [ * ].
(b)Additional Markets. Promptly after the Effective Date, Licensee shall
commence preparation of a reasonably detailed Commercialization plan, sales forecast, and
launch timing for Commercialization of the Product, using Commercially Reasonable Efforts, in
the Additional Markets. On or before December 31, 2016, Licensee shall present to the JCC
such reasonably detailed Commercialization plan, sales forecast, and launch timing for
Commercialization of the Product, using Commercially Reasonable Efforts, in the Additional
Markets. Such report shall specifically assess the opportunity and plans for [ * ].
(c)Minimum Commercial Performance. In addition to the foregoing
general commitments, and subject to Section 6.3(e), for each Calendar Year for [ * ] full
Calendar Years commencing [ * ], Licensee shall prepare a commercially reasonable forecast of
commercial sales of Product in the Licensee Territory (“Sales Forecast”) and submit the Sales
Forecast to the JCC with sufficient time for the JCC to review and finalize such Sales Forecast
by [ * ] of the year immediately preceding the year covered in such Sales Forecast. The Sales
Forecast shall be based upon the same market share trajectory as the Product achieved in the U.S.
for the same time period following Regulatory Approval (including Pricing and Reimbursement
Approval, if required) for each indication, as may be modified on the basis of other relevant
commercial considerations, including other comparable product experience in Europe compared
to the U.S. For the first [ * ] Calendar Years following [ * ], Sales Forecasts will be used solely
for management purposes and have no effect under this Agreement. If in any Calendar Year
during the remaining [ * ] Calendar Years (the “Minimum Commercial Performance Period”)
Net Sales realized in the Licensee Territory are less than [ * ] percent ([ * ]%) of forecasted sales
for such year, then Licensee shall submit a corrective plan to the JCC for review and approval for
the next Calendar Year in order to achieve forecasted sales and such corrective plan shall be
incorporated into the Commercialization Plan. If, for a second Calendar Year during the
Minimum Commercial Performance Period, Net Sales realized are again less than [ * ] percent
([ * ]%) of forecasted sales then:
37.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(i) if Licensee failed to execute the corrective plan submitted to the
JCC, it shall be considered a material breach giving rise to Exelixis’ right to terminate this
Agreement pursuant to Section 15.2;
(ii)if Licensee did execute the corrective plan submitted to the JCC,
but still failed to achieve at least [ * ] percent ([ * ]%) of forecasted sales, then Licensee must
submit a new corrective plan to the JCC; and
(iii)if during the Minimum Commercial Performance Period, Licensee
fails to achieve at least [ * ] percent ([ * ]%) of forecasted sales in [ * ] of the [ * ] Calendar
Years during the Minimum Commercial Performance Period, it shall be considered a material
breach after the [ * ] of such Calendar Years giving rise to Exelixis’ right to terminate this
Agreement pursuant to Section 15.2.
(d)Minimum Commercial Performance Compensation. If in any
Calendar Year during the Minimum Commercial Performance Period Net Sales realized in the
Licensee Territory are less than [ * ] percent ([ * ]%) of forecasted sales for such year, then
Licensee shall owe to Exelixis the Minimum Commercial Performance Compensation in respect
of such year, to be paid within [ * ] of the end of the relevant Calendar Year. For the purposes of
this Agreement, the “Minimum Commercial Performance Compensation” shall be equal to
the royalty payments due on the difference between [ * ] percent ([ * ]%) of the forecasted sales
for the applicable Calendar Year and the Net Sales realized during the applicable Calendar Year.
(e)Minimum Commercial Performance Relief. In the event of conditions
that give rise to a Stockout Period, Licensee shall be relieved of the obligation to meet minimum
commercial performance obligations pursuant to Section 6.3(c) for the Calendar Year in which
such Stockout Period occurs.
(f)Commercial Updates. Licensee shall update the JCC on a [ * ] basis
regarding its Commercialization activities with respect to the Products in the Licensee Territory.
Each such update shall be in a form to be agreed by the JCC and shall summarize Licensee’s, its
Affiliates’ and Sublicensees’ significant Commercialization activities with respect to the
Products in the Licensee Territory, and shall contain at least such information at such level of
detail reasonably required by Exelixis to determine Licensee’s compliance with its diligence
obligations set forth herein. Such updates shall include, on a [ * ] basis, Licensee’s sales
activities, marketing activities and Medical Affairs Activities. In addition, if Licensee is then
working under a corrective plan under Section 6.3(c), such updates shall also include the budget
and actual cost and expense (including FTE levels) for such activities in [ * ] for the current year
and previous year.
6.4Coordination of Commercialization Activities.
(a)Generally. The Parties recognize that their collaboration may benefit
from the coordination of certain activities in support of the Commercialization of the Products in
both the Licensee Territory and the Exelixis Territory. As such, the Parties, through the JCC,
shall develop and coordinate Commercialization strategies for the Product (e.g., for branding and
messaging, international congresses, advisory boards), and the Parties shall conduct
Commercialization activities for the Product in their respective territories consistent with such
global strategy. The foregoing shall not be construed as requiring Exelixis to seek Licensee’s
consent in connection with the establishment and/or implementation of any sales, marketing, or
medical affairs practices in the Exelixis Territory.
38.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(b)Pricing. Licensee shall keep Exelixis timely informed on the status of any
application for Pricing and Reimbursement Approval or material updates to an existing Pricing
and Reimbursement Approval in the Licensee Territory, including any discussion with a
Regulatory Authority with respect thereto. Licensee shall have the right to determine the price of
the Product sold in the Licensee Territory [ * ]. [ * ]. In the event the Pricing and
Reimbursement Approvals in a given country of the Licensee Territory [ * ], Licensee shall have
no obligation to launch the Product in such country. Licensee and its Affiliates and Sublicensees
shall not sell any Product in combination with, as part of a bundle with, or as a combination
therapy with other products, or offer packaged arrangements to customers that include a Product,
in such a manner as to disproportionately discount the selling price of the Product [ * ]. For
clarification, should Licensee derive direct economic benefit from the sale of another
pharmaceutical product that is approved to be used in combination with Product, [ * ].
(c)Sharing of Promotional Materials. Licensee shall, at its own expense,
prepare, develop, produce or otherwise obtain, and utilize sales, promotional, advertising,
marketing, website, educational and training materials (the “Promotional Materials”) to support
its Commercialization activities in the Licensee Territory. The Parties shall share samples of
Promotional Materials (including English translation, if available) with respect to the
Commercialization of the Products with one another. Additional materials, including medical
education and medical information, sales force and sales force training materials, will be made
available to the other Party upon request.
(d)Commercialization in Exelixis Territory. Subject to the terms and
conditions of this Agreement (including Section 6.7), Exelixis shall have the exclusive right to
Commercialize the Product in the Exelixis Territory at its own cost and expense, with or without
Third Party(ies).
6.5Detailing and Promotion. Licensee shall not engage any contract sales
organization to conduct sales activities for the Product in the Licensee Territory without written
JCC approval, nor shall Licensee use the same sales force to promote the Product and a separate
product that is indicated for the same indication without written JCC approval.
6.6Medical Affairs Activities.
(a)Coordination of Global Medical Affairs Activities. Commencing with
transfer of the RCC MAA to Licensee, but subject to the final sentence of this Section 6.6(a),
Licensee shall lead and conduct all Medical Affairs Activities for the Product in the Licensee
Territory in accordance with the medical affairs portion of the GDP. From such date, Licensee
shall be responsible for Medical Affairs Activities in the Licensee Territory, provided however,
that Exelixis shall have the right, but not the obligation, to also conduct Medical Affairs
Activities in the Licensee Territory in global support of the Product consistent with the medical
affairs portion of the GDP and in coordination with Licensee. Exelixis will not undertake
Medical Affairs Activities in the Licensee Territory without prior coordination with Licensee.
(b)Advisory Panels. To the extent practicable, each Party shall give the
other Party written notice at least [ * ] in advance of any major market or international level
advisory panel meetings with key opinion leaders with respect to the Commercialization of the
Products in the Licensee Territory and the Exelixis Territory that are held, sponsored or attended
by either Party or its Affiliate or sublicensee, and each Party shall have the right to attend and
participate in such meetings.
39.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
6.7Diversion. Each Party hereby covenants and agrees that it and its Affiliates shall
not, and it shall contractually obligate (and use Commercially Reasonable Efforts to enforce such
contractual obligation) its sublicensees not to, directly or indirectly, promote, market, distribute,
import, sell or have sold any Product, including via the Internet or mail order, to any Third Party
or to any address or Internet Protocol address or the like in the other Party’s territory. Neither
Party shall engage, nor permit its Affiliates and sublicensees to engage, in any advertising or
promotional activities relating to any Product for use directed primarily to customers or other
buyers or users of such Product located in any country or jurisdiction in the other Party’s
territory, or solicit orders from any prospective purchaser located in any country or jurisdiction in
the other Party’s territory. If a Party or its Affiliates or sublicensees receives any order for a
Product for use from a prospective purchaser located in a country or jurisdiction in the other
Party’s territory, such Party shall immediately refer that order to such other Party and shall not
accept any such orders. Neither Party shall, nor permit its Affiliates and sublicensees to, deliver
or tender (or cause to be delivered or tendered) any Product for use in the other Party’s territory.
7.Manufacture and Supply.
7.1Manufacture and Supply. Exelixis will manufacture and supply, itself and/or
through a Third Party contract manufacturer, all Compound and Products for use in the
Development and Commercialization of the Products under this Agreement. All Products
supplied by Exelixis to Licensee shall be at a price equal to [ * ]. It is anticipated that Exelixis
will supply commercial Product to Licensee in final, labeled packaged form. Exelixis shall be
responsible for packaging and labeling for all countries in the Licensee Territory. The Cost of
Goods of the Compound and Products used in the Development work under the GDP shall be
included in the Development Cost and shared by the Parties in accordance with Sections 4.5 and
9.2. Exelixis shall source such Product supply for both Parties either from a facility owned by
Exelixis or from a reputable, qualified and certified Third Party and, in the event Licensee is
responsible for conducting any Clinical Studies pursuant to Section 4.3, 4.5(d) or 4.5(e), Exelixis
shall provide such supply to Licensee for such Clinical Studies in accordance with the GDP.
Within two (2) months of the Effective Date, the Parties shall enter into a Supply Agreement for
the manufacture and supply of the Compound and Products to Licensee (the “Supply
Agreement”). The Parties acknowledge that the Parties amended the Supply Agreement to
address the modification to the Parties’ respective territories as outlined in the First Amendment
and added [ * ] reports from the tracking system as set forth in Section 2.8(f) detailing the
distribution and sale of product supplied for Canada.
8.Transition of EU Regulatory and Commercialization Operation.
8.1Termination of Sobi Agreement. Licensee acknowledges that as of the
Effective Date, Exelixis has entered into an Amended and Restated Commercialization
Agreement with Swedish Orphan Biovitrum AB (“Sobi”) for the distribution of the Product in
the EU in MTC, effective January 1, 2015 (the “Sobi Agreement”). No later than March 4,
2016, Exelixis shall exercise its right to terminate the Sobi Agreement and Exelixis shall bear the
cost of any resulting termination payment to Sobi under Section 8.3(g) of the Sobi Agreement.
Prior to the effective date of the termination of the Sobi Agreement, Licensee acknowledges and
agrees that the licenses granted by Exelixis to Licensee hereunder are subject to the rights
granted by Exelixis to Sobi under the Sobi agreement. Exelixis shall ensure that a meeting be
held with Sobi and Licensee within sixty (60) days of the Effective Date to achieve a smooth
transition from Sobi to Licensee for the distribution of the Product in MTC in the EU. Exelixis
agrees, if necessary, to enforce the obligations of the Sobi Agreement as against Sobi to provide
40.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
for a smooth transition of commercial responsibility for the distribution of the Product in the EU
in MTC as contemplated by the Sobi Agreement.
8.2Transfer of Regulatory Filings. As soon as practicable, but no later than [ * ],
the Parties shall cooperate to transfer the EMA MAA filing from TMC for Cometriq in MTC to
Licensee, including Marketing Authorization Holder status (including commitments and
obligations listed in the MAA, and Exelixis shall ensure with TMC that such transfer shall occur,
except that Exelixis shall complete the EMA post-marketing commitment of Study XL184-401,
with the costs of such study to be shared in accordance with Section 4.5), and the Pediatric
Investigation Plan, provided that Licensee shall be responsible for all Regulatory Filings and
interactions with the EMA with respect to such studies and Regulatory Filings, maintaining
Orphan Drug Status, and all further EMA requirements with respect to such studies and
Regulatory Filings. Without limiting the foregoing, such transfer efforts shall include (a)
providing supporting documentation, responding to requests by applicable Regulatory
Authorities and other reasonable efforts in connection with the MAA Approvals, (b) preparing
and filing Regulatory Filings in countries of the Licensee Territory where Sobi and/or TMC has
not as of the Effective Date filed Regulatory Filings and it is or it becomes commercially
reasonable to do so. Licensee’s rights and obligations as a regulatory sponsor with respect to
each particular Regulatory Filing under Article 5 shall commence upon the completion of such
transfer.
8.3Transition of Commercial Responsibilities for Cometriq. Licensee and
Exelixis acknowledge and agree that Licensee shall assume the rights and responsibilities for the
Commercialization of Cometriq in the EU concurrent with the effective date of the termination
of the Sobi Agreement. Consistent with Section 8.2, the Parties shall cooperate to effectuate the
transfer of such rights and responsibilities to Licensee in a manner that minimizes any delay or
interruption of the Commercialization of Cometriq in the EU.
9.Financial Provisions
9.1Upfront Payment. Licensee shall make a one-time, non-refundable, non-
creditable upfront payment to Exelixis of two hundred million dollars ($200,000,000) within five
(5) business days after the Effective Date. In consideration of the expanded license rights
granted by Exelixis to Licensee to include Canada as part of the Licensee Territory, by virtue of
the First Amendment, Licensee shall make a one-time, non-refundable, non-creditable payment
to Exelixis of ten million dollars ($10,000,000) within five (5) days after execution of First
Amendment.
9.2Sharing/Reimbursements of Development Costs and PV Costs.
(a)Future Development Costs. No later than [ * ] after the beginning of
each Calendar Quarter during which a Party will perform any Development activity (other than
the Independent Work and Licensee Only Development Work) in such Calendar Quarter
pursuant to the GDP, such Party shall submit to the other Party a statement setting forth the
Development Costs incurred, including the other Party’s share (calculated in accordance with
Section 4.5) of (i) estimated Development Costs for the then current quarter; (ii) variances from
prior invoiced estimates and actual Development Costs; and (iii) Development Costs incurred by
or on account of such Party in the past quarter not previously invoiced. Such invoice shall
include a reasonably detailed report for such Development Costs, including supporting
documents. To the extent provided in Section 4.5, the other Party shall pay the amount invoiced
within [ * ] after the receipt of the invoice, subject to the other Party’s right to audit the invoicing
41.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Party’s records and books related to such costs as provided in Section 10.4. For clarity, making
such a payment does not preempt the paying Party’s audit rights under Section 10.4, which
remain in full force and effect. If both Parties will perform Development activities under the
GDP in such Calendar Quarter, the Parties shall consolidate the payments for such Calendar
Quarter into a single payment from one Party to the other Party.
(b)Independent Work. Except as set forth below in this Section 9.2(b), each
Party shall bear all the internal (calculated on an FTE basis using the then current FTE Rate) and
out-of-pocket costs and expenses incurred by or on account of such Party in performing its own
Independent Work (the “Independent Work Costs”). After the completion of such Independent
Work, such Party shall provide the other Party with a report of such Independent Work Costs. If
a Party desires to submit any portion of the Data resulting from any Independent Work
conducted by the other Party and related Regulatory Filings generated by the other Party to
support Regulatory Approval in its territory, then such Party shall notify the other Party in
writing at any time upon the completion of such Independent Work. Within [ * ] after its receipt
of such notice, the Party conducting or having conducted such Independent Work shall submit to
the other Party a reasonably detailed invoice setting forth [ * ] ([ * ]%) of the Independent Work
Costs that would have been incurred by or on account of such other Party in connection with the
generation of such Data under Section 9.2(b) as if such Independent Work Costs were
Development Costs. If the Party seeking to use such Data decides to use such Data to support
Regulatory Approval in its territory, then such Party shall notify the other Party in writing and
pay the amount invoiced within [ * ] after the receipt of such invoice, subject to such Party’s
right to audit the invoicing Party’s records and books related to such costs as provided in Section
10.4. For clarity, making such a payment does not preempt the paying Party’s audit rights under
Section 10.4, which remain in full force and effect.
(c)Internal Development Cost. Each Party shall record and calculate its
internal Development Costs on an FTE basis at the FTE Rate.
(d)Development Cost for Products in Combination. If any Product is
Developed under this Agreement in combination with a Party’s proprietary product (the
“Beneficial Party”), either as a combination product or combination therapy, then such
Development work shall be conducted in accordance with the GDP and the Development Costs
with respect to such Development shall be included in the Development Budget, provided that
only [ * ] percent ([ * ]%) of the Development Cost with respect to such Development shall be
subject to the Parties’ cost sharing under Section 9.2(b) and the Beneficial Party shall be solely
responsible for the other [ * ] percent ([ * ]%) of the Development Costs.
9.3Development Milestone Payments.
(a)Development Milestones. Subject to the remainder of this Section 9.3,
Licensee shall pay to Exelixis the non-refundable, non-creditable payment set forth in the table
below upon the achievement of the applicable milestone event (whether by or on behalf of
Licensee, Exelixis, or their Affiliates, licensee(s) of Exelixis or Sublicensees):
42.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Milestone Event | Milestone Payments | |||
For RCC (2nd line) | For HCC (2nd line) | Tier 1 Additional Indication | Tier 2 Additional Indication | |
Milestone #1: Initiation of first Phase 3 Clinical Trial | n.a. | n.a. | $20 million | $[ * ] |
Milestone #2: First MAA filing with the EMA | n.a | $ 10 million | $25 million | $[ * ] |
Milestone #3: First MAA Approval by EMA | $60 million | $40 million | $50 million | $[ * ] |
TOTAL | $60 million | $50 million | $95 million | $[ * ] |
(i)For RCC (2nd line) and for HCC (2nd line), each milestone payment
shall be paid once for the applicable events described above for each different applicable
Product.
(ii)For Tier 1 Additional Indications and Tier 2 Additional
Indications, each milestone payment shall be paid once for the applicable milestone events
described above for a total payment of [ * ] for up to [ * ]. For clarity, such milestone payments
may be earned if [ * ]. In the event that any indication [ * ].
(iii) Milestone #1 shall be deemed achieved and payable, if not already
achieved, upon achievement of any of Milestone #2 and/or Milestone #3 for the same indication.
(iv)Milestone #2 shall be deemed achieved and payable, if not already
achieved, upon achievement of Milestone #3 for the same indication.
(b)Notice and Payment. Each Party shall notify the other Party in writing
within [ * ] after the achievement of any milestone set forth in this Section 9.3 by such Party, its
Affiliates, or its Sublicensees. Licensee shall pay to Exelixis the applicable development
milestone payments within [ * ] after the delivery or receipt of such notice. Notwithstanding the
foregoing sentence, Licensee shall pay to Exelixis the Milestone #2 payment (First MAA filing
with the EMA) for the Tier 1 Additional Indication ($25,000,000) either within [ * ] after the
delivery or receipt of notice, or on [ * ], whichever is later.
43.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(c)Development Milestones Specific to Canada. Subject to the remainder
of this Section 9.3(c), Licensee shall pay to Exelixis the non-refundable, non-creditable payment
set forth in the table below upon the achievement of the applicable milestone event (whether by
or on behalf of Licensee, Exelixis, or their Affiliates, licensee(s) of Exelixis or Sublicensees):
Milestone Event | Milestone Payment |
Milestone A: MAA Approval by Health Canada (i.e., receipt of a “Notice of Compliance”) for a Product for RCC (2nd line) | $5,000,000 |
Milestone B: MAA Approval by Health Canada for a Product for RCC (1st line) | $3,000,000* |
Milestone C: MAA Approval by Health Canada (i.e., receipt of a “Notice of Compliance”) for a Product for HCC (2nd line) | $2,000,000 |
Milestone D: MAA Approval by Health Canada (i.e., receipt of a “Notice of Compliance”) for a Product for the first indication other than RCC or HCC | $[ * ] |
Milestone E: MAA Approval by Health Canada (i.e., receipt of a “Notice of Compliance”) for a Product for the second indication other than RCC or HCC | $[ * ] |
(i)*With respect to a Product, if Licensee achieves Milestone A, and
as part of such Milestone A, RCC (1st line) is also included in the claims section of the approved
label of Milestone A and allows Licensee to promote the Product for use in RCC (1st line), then
Licensee shall pay Exelixis the milestone payment corresponding to Milestone B in addition to
the milestone amount owed for achievement of Milestone A. For clarity, in no event shall
Licensee be obligated to pay to Exelixis more than a total of $8,000,000 for the achievement of
Milestones A and B with respect to any one Product.
(ii)Subject to Section 9.3(c)(i), each milestone payment shall be paid
once for the applicable events described above for each different applicable Product.
9.4Commercial Milestones Payments.
(a)EU Launch Milestones. Licensee shall pay to Exelixis the non-
refundable, non-creditable payment set forth in the table below upon the achievement of the
applicable milestone event (whether by or on behalf of Licensee, its Affiliates, or Sublicensees):
44.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
EU Launch Milestones | Milestone Payments |
First commercial sale of a Product in any country in the Top 5 EU | $10 million |
First commercial sale of a Product in any second country in the Top 5 EU | $10 million |
(b)Net Sales Milestones.
(i)The Parties acknowledge that the Net Sales shall be calculated in
accordance with Section 1.60 with respect to all sales for the Products in the Licensee Territory
made on or after [ * ]. In the event of overpayment or underpayment by Licensee of royalties to
Exelixis on the Net Sales of the Products sold in the Licensee Territory for the Calendar Years
[ * ] due to change in subparagraph (d) of Section 1.60, the Parties hereby agree that any
remaining balance due by or owed to Licensee shall be debited or credited to Licensee, as
applicable pursuant to payment terms to be mutually agreed by the Parties in writing.
(ii)Net Sales Milestones for Licensee Territory Excluding Canada.
Licensee shall pay to Exelixis the one-time, non-refundable, non-creditable payments set forth in
the table below when the aggregated Net Sales of all Products in the Licensee Territory, but
excluding the Net Sales of all Products in Canada, in any period of four (4) consecutive Calendar
Quarters first reach the values indicated in the table below. Once one of the values indicated in
the table below is first reached and the corresponding milestone payment is paid by Licensee
under this Section 9.4(b)(ii) (the “Previously Achieved Commercial Milestone”), the period of
four (4) consecutive Calendar Quarters to be applied to determine the reaching of a subsequent
Net Sales amount in the table below shall only start at the Calendar Quarter immediately
following the fourth (4th) Calendar Quarter which served as the period to determine the reaching
of the Net Sales amount triggering the Previously Achieved Commercial Milestone. For the
avoidance of doubt, each payment in this Section 9.4(b)(ii) shall be payable once only, regardless
of the number of times such milestone is subsequently achieved.
Aggregate Net Sales of all Products in the Licensee Territory Excluding Canada in Any 4 Consecutive Calendar Quarters | Milestone Payments |
Equal or exceed$100 million | $25 million |
Equal or exceed$250 million | $50 million |
Equal or exceed $400 million | $100 million |
Equal or exceed $600 million | $150 million |
Equal or exceed $[ * ] | $[ * ] |
(iii)Net Sales Milestones for Canada. Licensee shall pay to Exelixis
the one-time, non-refundable, non-creditable payments set forth in the table below when the
45.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
aggregated Net Sales of all Products in Canada in any period of four (4) consecutive Calendar
Quarters first reach the values indicated in the table below. Once one of the values indicated in
the table below is first reached and the corresponding milestone payment is paid by Licensee
under this Section 9.4(b)(iii) (the “Previously Achieved Commercial Milestone for Canada”),
the period of four (4) consecutive Calendar Quarters to be applied to determine the reaching of a
subsequent Net Sales amount in the table below shall only start at the Calendar Quarter
immediately following the fourth (4th) Calendar Quarter which served as the period to determine
the reaching of the Net Sales amount triggering the Previously Achieved Commercial Milestone
for Canada. For the avoidance of doubt, each payment in this Section 9.4(b)(iii) shall be payable
once only, regardless of the number of times such milestone is subsequently achieved. For
clarity, the amounts set forth in this Section 9.4(b)(iii) refer to Canadian dollars.
Aggregate Net Sales of all Products in Canada in Any 4 Consecutive Calendar Quarters | Milestone Payments |
Equal or exceedCAD$30 million | CAD$3 million |
Equal or exceedCAD$[ * ] | CAD$[ * ] |
Equal or exceed CAD$[ * ] | CAD$[ * ] |
(c)Notice and Payment.
(i)Licensee shall notify Exelixis in writing within [ * ] after the
achievement of any EU launch milestone set forth in Section 9.4(a) above by Licensee, its
Affiliates or its Sublicensees. Licensee shall pay to Exelixis the applicable EU launch milestone
payments within [ * ] after the delivery or receipt of such notice.
(ii)As part of the report in Section 10.1, Licensee shall provide written
notice to Exelixis if (1) the aggregated Net Sales of all Products in the Licensee Territory, but
excluding the Net Sales of all Products in Canada, in any four (4) consecutive Calendar Quarters
first reach the values set forth in Section 9.4(b)(ii), or (2) the aggregated Net Sales of all Products
in Canada in any four (4) consecutive Calendar Quarters first reach the values set forth in Section
9.4(b)(iii), and Licensee shall pay to Exelixis the corresponding Net Sales milestone payment
within [ * ] after the end of the Calendar Quarter.
9.5Royalty Payments.
(a)Royalty Rate.
(i)Royalty Rate for Licensee Territory Excluding Canada.
Subject to the other terms of this Section 9.5, during the Royalty Term, Licensee shall make
quarterly non-refundable, non-creditable royalty payments to Exelixis on the annual Net Sales of
all Products sold in the Licensee Territory, but excluding the annual Net Sales of all Products
sold in Canada, at the applicable rate set forth below:
46.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Annual Net Sales of all Products in the Licensee Territory Excluding Canada | Royalty Rate |
Portion less than or equal to $[ * ] | 22% |
Portion greater than $[ * ] and less than or equal to $[ * ] | [ * ]% |
Portion greater than $[ * ] | 26% |
(ii)Royalty Rate for Canada. Subject to the other terms of this
Section 9.5, during the Royalty Term, Licensee shall make quarterly non-refundable, non-
creditable royalty payments to Exelixis on the annual Net Sales of all Products sold in Canada at
the applicable rate set forth below:
Annual Net Sales of all Products in Canada | Royalty Rate |
Portion less than or equal to CAD$30 million | 22% |
Portion greater than CAD$30 million and less than or equal to CAD$[ * ] | [ * ]% |
Portion greater than CAD$[ * ] | 26% |
For clarity, the annual Net Sales amounts set forth in this Section 9.5(a)(ii) refer to
Canadian dollars.
(b)Royalty Term. Royalties shall be paid on a Product-by-Product and
country-by-country basis in the Licensee Territory from the First Commercial Sale of such
Product in such country by or on behalf of Licensee, its Affiliates or Sublicensees, until the latest
of (i) expiration of the last-to-expire Valid Claim of the Exelixis Patents and Licensee Patents
covering such Product in such country, including its composition, method of manufacture or
method of use, each covering the Product as Commercialized; (ii) the expiration of any
Regulatory Exclusivity covering such Product in such country; or (iii) ten (10) years after the
First Commercial Sale of such Product in such country for the first indication to obtain
Regulatory Approval in the Licensee Territory other than MTC (the “Royalty Term”).
(c)Royalty Reductions
(i)If one or more Generic Products to a Product is sold in any country
in the Licensee Territory during the Royalty Term for such Product in such country, and [ * ], the
royalty rates provided in Section 9.5(a) for such Product shall be reduced in such country by [ * ]
percent ([ * ]%) [ * ].
47.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(ii)If it is [ * ] for Licensee to obtain a license from a Third Party
under any Patent in a particular country in the Licensee Territory in order to sell a Product in
such country and Licensee obtains such a license, Licensee may deduct, from the royalty
payment that would otherwise have been due pursuant to Section 9.5(a) with respect to Net Sales
of such Product in such country in a particular Calendar Quarter, an amount equal to [ * ] percent
([ * ]%) of the royalties paid by Licensee to such Third Party pursuant to such license on account
of the sale of such Product in such country during such Calendar Quarter.
(iii)If the Applicable Laws (including legal doctrine) in a particular
country or jurisdiction requires a royalty reduction after the expiration of the relevant patents,
and the Royalty Term for a particular Product in such country or jurisdiction extends beyond the
time period set forth in Section 9.5(b)(i), then the royalty rates provided in Section 9.5(a) shall be
reduced by [ * ] percent ([ * ]%) for such Product in such country (e.g., a reduction from [ * ]%
to [ * ]%) during the remainder of the Royalty Term that extends beyond the time period set forth
in Section 9.5(b)(i) unless and until the royalty reduction set forth in Section 9.5(c)(i) becomes
applicable. For the same period of time, if neither Exelixis nor Licensee has [ * ] in such
country, such royalty reduction shall be [ * ] percent ([ * ]%) instead of [ * ] percent ([ * ]%).
(iv)If [ * ] any and all approvals (including pricing and reimbursement
approval, if applicable), licenses, registrations, permits, notifications, and authorizations (or
waivers) of any Regulatory Authority that are necessary for the promotion, marketing,
distribution, offer for sale, sale, or other commercialization [ * ] (“Approval”) in any country in
the Licensee Territory [ * ], and such Approval [ * ], and [ * ], then, [ * ] the royalty rates
provided in Section 9.5(a) for such Product shall be reduced in such country by [ * ] percent
([ * ]%) for the remainder of the Royalty Term for such Product in such country. For clarity,
[ * ]for the purposes of this Section 9.5(c)(iv). Further, if the Approval is [ * ], then the royalty
reduction will only apply [ * ]; and, where [ * ], the Parties will discuss in good faith to promptly
agree upon a reasonable method for [ * ] for the purpose of calculating the royalty reduction
pursuant to this Section 9.5(c)(iv).
(v)Notwithstanding the foregoing, during any Calendar Quarter in the
Royalty Term for a Product in a country, the operation of clause 9.5(c)(i), 9.5(c)(ii), 9.5(c)(iii),
and 9.5(c)(iv) above, individually or in combination, shall not reduce by more than [ * ] percent
([ * ]%) the royalties that would otherwise have been due under Section 9.5(a) with respect to
Net Sales of such Product in such country during such Calendar Quarter.
(d)Basis of Payment. This Section 9.5 is intended to provide for royalty
payments to Exelixis equal to the percentages of Net Sales set forth in this Section 9.5 for the
entire duration of the Royalty Term. In establishing this payment structure, Licensee recognizes
and acknowledges the substantial value of the various actions and investments that Exelixis has
taken and will undertake under this Agreement, as well as the fact that the value of the license
granted hereunder resides substantially in the Know-How. Therefore, Licensee agrees that the
royalty payments set forth above are appropriate for the entire duration of such payment
obligation. The Parties have agreed to the payment structure set forth herein as a convenient and
fair mechanism for both Parties to be compensated for the value of their actions and investments
under this Agreement.
(e)Launch Period Adjustment. For the first fifty million dollars
($50,000,000) of cumulative Net Sales, Licensee shall make quarterly non-refundable, non-
creditable royalty payments to Exelixis on the Net Sales of all Products sold in the Licensee
Territory at the rate of two percent (2%) rather than at the rate set forth in Section 9.5(a). For the
48.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
first one hundred million dollars ($100,000,000) of cumulative Net Sales immediately following
the initial fifty million dollars ($50,000,000) of cumulative Net Sales, Licensee shall make
quarterly non-refundable, non-creditable royalty payments to Exelixis on the Net Sales of all
Products sold in the Licensee Territory at the rate of twelve percent (12%) rather than at the rate
set forth in Section 9.5(a). Thereafter, the royalty rate for all Net Sales shall be at the applicable
rate set forth in Section 9.5(a).
(f)Stockout Holiday. In the event of conditions that give rise to a Stockout
Period, Licensee shall be relieved of the obligation to pay royalties pursuant to Section 9.5(a) on
Net Sales occurring for a period of time, commencing with the first commercial sale following
the end of the Stockout Period, equal in duration to the Stockout Period.
9.6Exelixis Payments to Third Party. Exelixis shall be solely responsible for all
payments, including royalties and milestone payments, due with respect to Compound and
Products pursuant to any Third Party agreement that Exelixis entered into prior to or as of the
Effective Date, including any obligations surviving the termination of the Product Development
and Commercialization Agreement between [ * ], as set forth in such Collaboration Agreement.
9.7Supply Payments. Licensee shall pay Exelixis for Compound and Product
Exelixis supplies to Licensee an amount equal to [ * ], all as provided in the Supply Agreement.
10.Payment; Records; Audits
10.1Payment; Reports. Royalty payments due by Licensee to Exelixis under Section
9.5 shall be calculated and reported for each Calendar Quarter. All royalty payments due under
Section 9.5 shall be paid within [ * ] after the end of each Calendar Quarter and shall be
accompanied by a report setting forth, on a country-by-country basis, Net Sales of the Products
by Licensee and its Affiliates and Sublicensees in the Licensee Territory in sufficient detail to
permit confirmation of the accuracy of the royalty payment made, including, for each country,
the number of Products sold, the gross sales and Net Sales of Products, including the deductions
from gross sales to arrive at Net Sales, the royalties payable, the method used to calculate the
royalties, the exchange rates used, any adjustments to royalties in accordance with Section 9.5,
and whether any commercial milestone under Section 9.4 has been achieved. Promptly after the
Effective Date, the Parties will agree on the form of royalty report. Licensee shall submit a
single report for all Net Sales during the Calendar Quarter, including all Licensee’s, Affiliates’
and Sublicensees’ Net Sales but shall separately identify the Net Sales and other information
applicable to each entity.
10.2Exchange Rate; Manner and Place of Payment. Except as provided in
Sections 9.4(b)(iii) and 9.5(a)(ii), all references to dollars and “$” herein shall refer to U.S.
dollars. All payments hereunder shall be payable in U.S. dollars. When conversion of Net Sales
from any currency other than U.S. dollars is required, such conversion shall be at the exchange
rate [ * ]. All payments owed under this Agreement shall be made by wire transfer in
immediately available funds to a bank and account designated in writing by Exelixis, unless
otherwise specified in writing by Exelixis.
10.3Taxes.
(a)Taxes on Income. Each Party shall be solely responsible for the payment
of all taxes imposed on its share of income arising directly or indirectly from the activities of the
Parties under this Agreement.
49.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(b)Tax Cooperation. The Parties agree to cooperate with one another and
use reasonable efforts to avoid or reduce tax withholding or similar obligations in respect of the
milestone payments, milestone payments and other payments made by Licensee to Exelixis
under this Agreement. To the extent Licensee is required by Applicable Laws to deduct and
withhold taxes on any payment to Exelixis, Licensee shall pay the amounts of such taxes to the
proper Governmental Authority in a timely manner and promptly transmit to Exelixis an official
tax certificate or other evidence of such payment sufficient to enable Exelixis to claim such
payment of taxes. Exelixis shall provide Licensee any tax forms that may be reasonably
necessary in order for Licensee to not withhold tax or to withhold tax at a reduced rate under an
applicable bilateral income tax treaty, to the extent legally able to do so. Exelixis shall use
reasonable efforts to provide any such tax forms to Licensee in advance of the due date.
Licensee shall provide Exelixis with reasonable assistance to enable the recovery, as permitted
by Applicable Laws, of withholding taxes or similar obligations resulting from payments made
under this Agreement, such recovery to be for the benefit of Exelixis. Licensee shall have the
right to deduct any such tax, levy or charge actually paid from payment due to Exelixis. Each
Party agrees to assist the other Party in claiming exemption from such deductions or
withholdings under double taxation or similar agreement or treaty from time to time in force and
in minimizing the amount required to be so withheld or deducted.
(c)Taxes Resulting From Licensee’s Action. Licensee represents and
warrants that, as of the Effective Date, Licensee is not required by Applicable Law to deduct or
withhold taxes on the upfront payment, milestone payments, royalty payments, and other
payments payable to Exelixis under this Agreement. If a Party takes any action of its own
discretion (not required by a Regulatory Authority), including any assignment, sublicense,
change of place of incorporation, or failure to comply with Applicable Laws or filing or record
retention requirements, which results in a withholding or deduction obligation (“Withholding
Tax Action”), then such Party shall pay the sum associated with such Withholding Tax Action.
For clarity, if Licensee undertakes a Withholding Tax Action, then the sum payable by Licensee
(in respect of which such deduction or withholding is required to be made) shall be increased to
the extent necessary to ensure that Exelixis receives a sum equal to the sum which it would have
received had no such Withholding Tax Action occurred. Otherwise, the sum payable by
Licensee (in respect of which such deduction or withholding is required to be made) shall be
made to Exelixis after deduction of the amount required to be so withheld or deducted. If a
change in Applicable Laws results in a withholding or deduction obligation absent either Party
taking a Withholding Tax Action, then the amount of such withholding or deduction obligation
shall be paid by Licensee to the applicable Governmental Authority on behalf of Exelixis,
provided that Licensee shall assist Exelixis in minimizing or recovering such withholding or
deduction obligation. The Parties shall use commercially reasonable efforts to invoke the
application of any applicable bilateral income tax treaty that would reduce or eliminate otherwise
applicable taxes with respect to payments payable pursuant to this Agreement.
10.4Records; Audit. Each Party shall maintain complete and accurate records in
sufficient detail in relation to this Agreement to permit the other Party to confirm the accuracy of
the amount of Development Costs and the Cost of Goods to be reimbursed or shared,
achievement of commercial milestones, the amount of royalty and other payments under this
Agreement. Each Party will keep such books and records for at least [ * ] following the Calendar
Year to which they pertain. Upon reasonable prior notice, such records shall be inspected during
regular business hours at such place or places where such records are customarily kept by an
independent certified public accountant (the “Auditor”) selected by the auditing Party and
reasonably acceptable to the audited Party for the sole purpose of verifying for the auditing Party
the accuracy of the financial reports furnished by the audited Party pursuant to this Agreement or
50.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
of any payments made, or required to be made, by or to the audited Party pursuant to this
Agreement. Before beginning its audit, the Auditor shall execute an undertaking acceptable to
each Party by which the Auditor agrees to keep confidential all information reviewed during the
audit. Such audits may occur no more often than once each Calendar Year and not more
frequently than once with respect to records covering any specific period of time. Each Party
shall only be entitled to audit the books and records from the [ * ] Calendar Years prior to the
Calendar Year in which the audit request is made. Such auditor shall not disclose the audited
Party’s Confidential Information to the auditing Party, except to the extent such disclosure is
necessary to verify the accuracy of the financial reports furnished by the audited Party or the
amount of payments to or by the audited Party under this Agreement. In the event that the final
result of the inspection reveals an undisputed underpayment or overpayment, the underpaid or
overpaid amount shall be settled within [ * ] after the Auditor’s report. The auditing Party shall
bear the full cost of such audit unless such audit reveals an overpayment to, or an underpayment
by, the audited Party that resulted from a discrepancy in the financial report provided by the
audited Party for the audited period, which underpayment or overpayment was more than [ * ]
percent ([ * ]%) of the amount set forth in such report, in which case the audited Party shall
reimburse the auditing Party for the costs for such audit. With respect more specifically to the
Development Costs to be paid or shared pursuant to Section 9.2, in addition to the right of
inspection and audit by an Auditor, the Party making the payment (the “Payor”) shall have the
right at its expense to review any records of out-of-pocket costs and expenses incurred by the
Party requesting the payment (the “Payee”) and time-keeping logs of Payee sufficient to justify
the work-time spent by each FTE of the Payee as well as the books of the Payee upon reasonable
notice sent by Payor to Payee and during regular business hours. For clarity, making such a
payment does not preempt the paying Party’s audit rights under this Section 10.4, which remain
in full force and effect. Payee’s FTE’s work-time shall be appropriately allocated between the
other product and the Product for purpose of calculating the internal costs specifically dedicated
to the Product.
10.5Late Payments. In the event that any payment due under this Agreement is not
paid when due in accordance with the applicable provisions of this Agreement, the payment shall
accrue interest from the date due at the [ * ] interest rate of [ * ] percent ([ * ]%) [ * ]; provided,
however, that in no event shall such rate exceed the maximum legal annual interest rate. The
payment of such interest shall not limit the Party entitled to receive payment from exercising any
other rights it may have as a consequence of the lateness of any payment.
11.Intellectual Property
11.1Ownership.
(a)Data. All Data generated in connection with any Development or
Commercial activities with respect to any Product conducted by or on behalf of Exelixis and its
Affiliates and licensees (other than Licensee) (the “Exelixis Data”) shall be the sole and
exclusive property of Exelixis or its Affiliates or licensees, as applicable. All Data generated in
connection with any Development or Commercial activities with respect to any Product
conducted by or on behalf of Licensee or its Affiliates or Sublicensees (the “Licensee Data”)
shall be the sole and exclusive property of Licensee or of its Affiliates or Sublicensees, as
applicable. For clarity, each Party shall have access and right to use and reference the other
Party’s Data as and to the extent set forth in this Agreement.
(b)Inventions. Inventorship of any Inventions will be determined in
accordance with the standards of inventorship and conception under U.S. patent laws. The
51.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Parties will work together to resolve any issues regarding inventorship or ownership of
Inventions. Ownership of Inventions will be allocated as follows:
(i)Exelixis will solely own all data, Inventions, and Patents claiming
such Inventions that relate to the composition, manufacture or use of any Compound, or any
improvement of any such composition, manufacture or use (each, a “Compound Invention”).
All Compound Inventions will be included in the Exelixis Know-How, and Patents in the
Licensee Territory claiming such Inventions will be included in the Exelixis Patents. To the
extent any Compound Invention is made by Licensee, whether solely or jointly with Exelixis,
Licensee shall, and hereby does, transfer and assign to Exelixis, without additional consideration,
all of its interest in such Compound Invention.
(ii)Except for Compound Inventions, each Party shall solely own any
Inventions made solely by its and its Affiliates’ employees, agents, or independent contractors
(“Sole Inventions”), and the Parties shall jointly own any Inventions that are made jointly by
employees, agents, or independent contractors of one Party and its Affiliates together with
employees, agents, or independent contractors of the other Party and its Affiliates (“Joint
Inventions”). All Patents claiming patentable Joint Inventions shall be referred to herein as
“Joint Patents.” Except to the extent either Party is restricted by the licenses granted to the
other Party under this Agreement, each Party shall be entitled to practice, license, assign and
otherwise exploit its interest under the Joint Inventions and Joint Patents without the duty of
accounting or seeking consent from the other Party.
11.2Patent Prosecution and Maintenance.
(a)Exelixis Patents.
(i)Subject to this Section 11.2(a), Exelixis shall have the sole right,
but not the obligation, to control the preparation, filing, prosecution and maintenance (including
any interferences, reissue proceedings, reexaminations, inter partes review, patent term
extensions, applications for supplementary protection certificates, oppositions, invalidation
proceedings and defense of validity or enforceability challenges) of the Exelixis Patents (other
than Joint Patents) worldwide, using counsel of its own choice in the Exelixis Territory and
counsel mutually agreed to by the Parties in the Licensee Territory. Licensee shall reimburse
Exelixis for all costs and expenses incurred with respect to the preparation, filing, prosecution
and maintenance of Exelixis Patents in the Licensee Territory after the Effective Date, within
[ * ] from the date of invoice for such costs and expenses provided by Exelixis. In the event that
Licensee does not reimburse Exelixis for such costs and expenses for any Exelixis Patent or
notifies Exelixis in writing that it elects to cease reimbursing Exelixis for such costs and
expenses for any Exelixis Patent, such Patent shall cease to be an Exelixis Patent and shall no
longer be subject to the licenses and other rights granted by Exelixis to Licensee under this
Agreement. Exelixis shall keep Licensee informed of material progress with regard to the
preparation, filing, prosecution and maintenance of Exelixis Patents in the Licensee Territory,
sufficiently in advance for Licensee to be able to review any material documents, including
content, timing and jurisdiction of the filing of such Exelixis Patents in the Licensee Territory,
and Exelixis shall consult with, and consider in good faith the requests and suggestions of,
Licensee with respect to strategies for filing, prosecuting and defending, if any, Exelixis Patents
in the Licensee Territory.
(ii)In the event that Exelixis desires to abandon or cease prosecution
or maintenance of any Exelixis Patent in any country in the Licensee Territory, Exelixis shall
52.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
provide reasonable prior written notice to Licensee of such intention to abandon (which notice
shall, to the extent possible, be given no later than [ * ] prior to the next deadline for any action
that must be taken with respect to any such Exelixis Patent in the relevant patent office). In such
case, upon Licensee’s written election provided no later than [ * ] after such notice from
Exelixis, Exelixis shall continue prosecution and maintenance of such Exelixis Patent at
Licensee’s direction and expense. If Licensee does not provide such election within [ * ] after
such notice from Exelixis, Exelixis may, in its sole discretion, continue prosecution and
maintenance of such Exelixis Patent or discontinue prosecution and maintenance of such
Exelixis Patent.
(b)Licensee Patents.
(i)Subject to this Section 11.2(b), Licensee shall have the first right,
but not the obligation, to control the preparation, filing, prosecution and maintenance (including
any interferences, reissue proceedings, reexaminations, patent term extensions, applications for
supplementary protection certificates, oppositions, invalidation proceedings and defense of
validity or enforceability challenges) of all Licensee Patents (other than Joint Patents)
worldwide, at its sole cost and expense and by counsel of its own choice in the Licensee
Territory and by counsel mutually agreed to by the Parties in the Exelixis Territory. Licensee
shall keep Exelixis informed of the status of filing, prosecution, maintenance and defense, if any,
of the Licensee Patents, and Licensee shall consult with, and consider in good faith the requests
and suggestions of, Exelixis with respect to strategies for filing, prosecuting and defending, if
any, Licensee Patents.
(ii)In the event that Licensee desires to abandon or cease prosecution
or maintenance of any Licensee Patent, Licensee shall provide reasonable prior written notice to
Exelixis of such intention to abandon (which notice shall, to the extent possible, be given no later
than [ * ] prior to the next deadline for any action that must be taken with respect to any such
Licensee Patent in the relevant patent office). In such case, upon Exelixis’ written election
provided no later than [ * ] after such notice from Licensee, Exelixis shall have the right to
assume prosecution and maintenance of such Licensee Patent at Exelixis’ expense and Licensee
shall assign to Exelixis all of its rights, title and interest in and to such Licensee Patent. If
Exelixis does not provide such election within [ * ] after such notice from Licensee, Licensee
may, in its sole discretion, continue prosecution and maintenance of such Licensee Patent or
discontinue prosecution and maintenance of such Licensee Patent.
(c)Joint Patents.
(i)Subject to this Section 11.2(c), Exelixis shall have the first right,
but not the obligation, to prepare, file, prosecute and maintain (including any interferences,
reissue proceedings, reexaminations, patent term extensions, applications for supplementary
protection certificates, oppositions, invalidation proceedings and defense of validity or
enforceability challenges) Joint Patents using a patent counsel selected by Exelixis in the
Exelixis Territory and counsel mutually agreed to by the Parties in the Licensee Territory.
Licensee shall reimburse Exelixis for all costs and expenses incurred with respect to the
preparation, filing, prosecution and maintenance of Joint Patents in the Licensee Territory, within
[ * ] from the date of invoice for such costs and expenses provided by Exelixis. In the event that
Licensee does not reimburse Exelixis for such costs and expense for any Joint Patent or notifies
Exelixis in writing that it elects to cease reimbursing Exelixis for such costs and expense for any
Joint Patent, Licensee shall execute such documents and perform such acts, at Licensee’s
expense, as may be reasonably necessary to effect an assignment of Licensee’s entire right, title,
53.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
and interest in and to such Joint Patent to Exelixis, and such Patent shall cease to be either a Joint
Patent or a Exelixis Patent and shall no longer be subject to the licenses and other rights granted
by Exelixis to Licensee under this Agreement. Exelixis shall keep Licensee informed of material
progress with regard to the preparation, filing, prosecution, maintenance and defense, if any of
Joint Patents, including content, timing and jurisdiction of the filing of such Joint Patents, and
Exelixis shall consult with, and consider in good faith the requests and suggestions of, Licensee
with respect to filing, prosecuting and defending, if any, Joint Patents in the Licensee Territory.
(ii)In the event that Exelixis desires to abandon or cease prosecution
or maintenance of any Joint Patent in any country in the Licensee Territory, Exelixis shall
provide reasonable prior written notice to Licensee of such intention to abandon (which notice
shall, to the extent possible, be given no later than [ * ] prior to the next deadline for any action
that must be taken with respect to any such Joint Patent in the relevant patent office). In such
case, at Licensee’s sole discretion, upon written notice from Licensee to Exelixis, Licensee may
elect to continue prosecution or maintenance of any such Joint Patent at its own expense, and
Exelixis shall execute such documents and perform such acts, at Licensee’s expense, as may be
reasonably necessary to allow Licensee to continue the prosecution and maintenance of such
Joint Patent in such country in the Licensee Territory. Any such assignment shall be completed
in a timely manner to allow Licensee to continue prosecution and maintenance of any such Joint
Patent and any such Patent so assigned shall cease to be either a Joint Patent or a Licensee Patent
and shall no longer be subject to the licenses and other rights granted by Licensee to Exelixis
under this Agreement.
(d)Cooperation. Each Party agrees to cooperate fully in the preparation,
filing, prosecution, maintenance and defense, if any, of Patents under Section 11.2 and in the
obtaining and maintenance of any patent term extensions, supplementary protection certificates
and their equivalent with respect thereto respectively, at its own cost (except as expressly set
forth otherwise in this Article 11). Such cooperation includes: (i) executing all papers and
instruments, or requiring its employees or contractors, to execute such papers and instruments, so
as enable the other Party to apply for and to prosecute patent applications in any country as
permitted by Section 11.2; and (ii) promptly informing the other Party of any matters coming to
such Party’s attention that may affect the preparation, filing, prosecution or maintenance of any
such patent application and the obtaining of any patent term extensions, supplementary
protection certificates and their equivalent.
11.3Patent Enforcement.
(a)Notice. Each Party shall notify the other within [ * ] of becoming aware
of any alleged or threatened infringement by a Third Party of any of the Exelixis Patents
(including Joint Patents) in the Licensee Territory, which infringement adversely affects or is
expected to adversely affect any Product, including any declaratory judgment, opposition, or
similar action alleging the invalidity, unenforceability or non-infringement of any of the Exelixis
Patents (collectively “Product Infringement”).
(b)Enforcement Right. Exelixis shall have the first right to bring and
control any legal action in connection with such Product Infringement at its own expense as it
reasonably determines appropriate. If Exelixis (i) decides not to bring such legal action against a
Product Infringement (the decision of which Exelixis shall inform Licensee promptly) or (ii)
Exelixis otherwise fails to bring such legal action against a Product Infringement within [ * ] of
first becoming aware of such Product Infringement, Licensee shall have the right to bring and
54.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
control any legal action in connection with such Product Infringement at its own expense as it
reasonably determines appropriate after consultation with Exelixis.
(c)Collaboration. Each Party shall provide to the enforcing Party reasonable
assistance in such enforcement, at such enforcing Party’s request and expense, including to be
named in such action if required by Applicable Laws to pursue such action. The enforcing Party
shall keep the other Party regularly informed of the status and progress of such enforcement
efforts, shall reasonably consider the other Party’s comments on any such efforts, including,
without limitation, determination of litigation strategy, filing of material papers to the competent
court. The non-enforcing Party shall be entitled to separate representation in such matter by
counsel of its own choice and at its own expense, but such Party shall at all times cooperate fully
with the enforcing Party.
(d)Expense and Recovery.
(i)Except as set forth in clause (ii) below, the enforcing Party shall be
solely responsible for any cost and expenses incurred by such Party as a result of such
enforcement action. If such Party recovers monetary damages in such enforcement action, such
recovery shall be allocated first to the reimbursement of any expenses incurred by the enforcing
Party in such enforcement action, second to the reimbursement of any expenses incurred by the
other Party in such enforcement action, and any remaining amounts shall be retained by the
enforcing Party.
(ii)Notwithstanding the foregoing, if Exelixis is the enforcing Party
against a Product Infringement in the Licensee Territory, Licensee shall have the option to share
[ * ] percent ([ * ]%) of the cost and expense incurred by Exelixis in such enforcement action,
which option may be exercised by Licensee by providing written notice to Exelixis within [ * ]
after receiving a notice from Exelixis that Exelixis decides to bring such action. If Licensee
exercises such option, then (1) Licensee shall reimburse Exelixis for [ * ] percent ([ * ]%) of all
costs and expenses incurred by Exelixis in such enforcement action, within [ * ] from the date of
invoice for such costs and expenses provided by Exelixis; (2) If Exelixis recovers any monetary
damages in such enforcement action, such recovery shall be allocated [ * ] percent ([ * ]%) to
Exelixis and [ * ] percent ([ * ]%) to Licensee.
(e)Other Infringement. Except for Product Infringement as set forth above,
each Party shall have the exclusive right to enforce its own Patent against any infringement
anywhere in the world. For clarity, Exelixis shall have the exclusive right to enforce (i) the
Exelixis Patents against any infringement in the Licensee Territory that is not a Product
Infringement, and (ii) the Exelixis Patents and Joint Patents against any infringement in the
Exelixis Territory, in each case at its own expense as it reasonably determines appropriate. The
Parties shall discuss global enforcement strategy for the Exelixis Patents and Licensee Patents,
including the defense of validity and enforceability challenges arising from any enforcement
action.
11.4Infringement of Third Party Rights. If any Product used or sold by Licensee,
its Affiliates or Sublicensees becomes the subject of a Third Party’s claim or assertion of
infringement of any intellectual property rights in a jurisdiction within the Licensee Territory,
Licensee shall promptly notify Exelixis and the Parties shall promptly meet to consider the claim
or assertion and the appropriate course of action and may, if appropriate, agree on and enter into
a “common interest agreement” wherein the Parties agree to their shared, mutual interest in the
outcome of such potential dispute. Absent any agreement to the contrary, and subject to claims
55.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
for indemnification under Article 13, each Party defend itself from any such Third Party claim at
its own cost and expense, provided, however, that the provisions of Section 11.3 shall govern the
right of Licensee to assert a counterclaim of infringement of any Exelixis Patents.
11.5Patents Licensed From Third Parties. Each Party’s rights under this Article 11
with respect to the prosecution and enforcement of any Exelixis Patent and Licensee Patent shall
be subject to the rights: (a) retained by any upstream licensor to prosecute and enforce such
Patent Right, if such Patent Right is subject to an upstream license agreement; and (b) granted to
any Third Party prior to such Patent Right becoming subject to the license grant under this
Agreement.
11.6Trademarks.
(a)Product Trademarks. Exelixis shall develop and adopt trademarks,
including trade names, trade dresses, branding, and logos, to be used for the Products (the
“Product Marks”). Exelixis shall own the Product Marks throughout the world and all goodwill
in the Product Marks shall accrue to Exelixis. The Parties (including any Future Exelixis
Licensee to the extent feasible) shall collaborate to have a global, worldwide trademark to be
used on the Product. The Parties acknowledge that Exelixis has been using the trademark
Cometriq® for the Product in MTC, and unless otherwise mutually agreed, the Parties shall
continue to use Cometriq® in MTC. Exelixis shall select another Product Mark for the Product
to be used for all other indications. In the event Exelixis is unable to obtain or maintain the
Product Marks for the Product in the Licensee Territory or in some countries in the Licensee
Territory, the Parties shall collaborate to select such other Product Marks (i.e., back-up names) as
may be available for registration and marketing of the Product in those countries. Exelixis shall
be responsible for the registration, maintenance, defense and enforcement of the Product Marks
using counsel of its own choice in the Exelixis Territory and counsel mutually agreed to by the
Parties in the Licensee Territory. Licensee shall reimburse Exelixis for all costs and expenses
incurred with respect to the registration and maintenance of the Product Marks in the Licensee
Territory, within [ * ] from the date of invoice for such costs and expenses provided by Exelixis.
Exelixis shall keep Licensee informed of material progress with regard to the registration,
prosecution, maintenance and defense, if any, of Exelixis Trademarks in the Licensee Territory,
including content, timing and jurisdiction of the filing of such Exelixis Trademarks in the
Licensee Territory, sufficiently in advance for Licensee to be able to review any material
documents, and Exelixis shall consult with, and consider in good faith the requests and
suggestions of, Licensee with respect to strategies for filing, prosecuting and defending, if any,
Exelixis Trademarks in the Licensee Territory.
(i)Without limiting the generality of the foregoing Section 11.6(a),
the Parties shall use the trademark Cabometyx® for the Product in Canada to the extent that such
trademark is approved for use with the Product by Health Canada or other applicable Regulatory
Authority. If Exelixis is unable to obtain or register Cabometyx® for use with the Product in
Canada, the Parties shall collaborate to select another Product Mark to be used for the Product in
Canada. In accordance with Section 11.6(a), Exelixis shall own the Product Marks used for the
Product in Canada and all goodwill in such Products Marks shall accrue to Exelixis.
(b)Trademark License. Licensee shall use the Product Marks selected by
Exelixis to Commercialize the Product in the Licensee Territory. Where Licensee reasonably
believes the Product Mark is not appropriate for commercial use in a specific country, the Parties
shall agree on an alternative product trademark for such country and such alternative product
trademark shall be included in Product Mark. In addition, unless prohibited by Applicable Laws,
56.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Licensee shall use Commercially Reasonable Effort to include Exelixis’ corporate trademark on
the packaging and product information (i.e. SmPC) of the Products sold in the Licensee Territory
to indicate that the Product is licensed from Exelixis. Exelixis hereby grants to Licensee a
limited royalty-free license to use such Product Marks and Exelixis’ corporate trademark solely
in connection with the Commercialization of the Product in the Licensee Territory under this
Agreement. All use of the Product Marks and Exelixis’ corporate trademark shall comply with
Applicable Laws and regulations and shall be subject to Exelixis’ review and approval. For
clarity, Licensee shall also include its (or its Affiliate’s or Sublicensee’s) corporate logo in the
Product sold in the Licensee Territory.
12.Representations and Warranties
12.1Mutual Representations and Warranties. Each Party represents and warrants
to the other that, as of the Effective Date: (a) it is duly organized and validly existing under the
laws of its jurisdiction of incorporation or formation, and has full corporate or other power and
authority to enter into this Agreement and to carry out the provisions hereof, (b) it is duly
authorized to execute and deliver this Agreement and to perform its obligations hereunder, and
the person or persons executing this Agreement on its behalf has been duly authorized to do so
by all requisite corporate or partnership action, (c) this Agreement is legally binding upon it,
enforceable in accordance with its terms, and does not conflict with any agreement, instrument or
understanding, oral or written, to which it is a Party or by which it may be bound, nor violate any
material law or regulation of any court, governmental body or administrative or other agency
having jurisdiction over it, and (d) it has the right to grant the licenses granted by it under this
Agreement.
12.2Covenants.
(a)Employees, Consultants and Contractors. Each Party covenants that it
has obtained or will obtain written agreements from each of its employees, consultants and
contractors who perform Development activities pursuant to this Agreement, which agreements
will obligate such persons to obligations of confidentiality and non-use and to assign (or, in the
case of contractor, grant a license under) Inventions in a manner consistent with the provisions of
this Agreement.
(b)Debarment. Each Party represents, warrants and covenants to the other
Party that it is not debarred or disqualified under the U.S. Federal Food, Drug and Cosmetic Act,
as may be amended, or comparable laws in any country or jurisdiction other than the U.S., and it
does not, and will not during the Term, employ or use the services of any person who is debarred
or disqualified, in connection with activities relating to any Product. In the event that either
Party becomes aware of the debarment or disqualification or threatened debarment or
disqualification of any person providing services to such Party, including the Party itself or its
Affiliates or Sublicensees, that directly or indirectly relate to activities contemplated by this
Agreement, such Party shall immediately notify the other Party in writing and such Party shall
cease employing, contracting with, or retaining any such person to perform any such services.
(c)Compliance. Licensee covenants as follows:
(i)In the performance of its obligations under this Agreement,
Licensee shall comply and shall cause its and its Affiliates’ employees and contractors to comply
with all Applicable Laws.
57.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(ii)Licensee and its Affiliates’ employees and contractors shall not, in
connection with the performance of their respective obligations under this Agreement, directly or
indirectly through Third Parties, pay, promise or offer to pay, or authorize the payment of, any
money or give any promise or offer to give, or authorize the giving of anything of value to a
Public Official or Entity or other person for purpose of obtaining or retaining business for or
with, or directing business to, any person, including, Licensee (and Licensee represents and
warrants that as of the Effective Date, Licensee, and to its knowledge, its and its Affiliates’
employees and contractors, have not directly or indirectly promised, offered or provided any
corrupt payment, gratuity, emolument, bribe, kickback, illicit gift or hospitality or other illegal or
unethical benefit to a Public Official or Entity or any other person in connection with the
performance of Licensee’s obligations under this Agreement, and Licensee covenants that it and
its Affiliates’ employees and contractors shall not, directly or indirectly, engage in any of the
foregoing).
(iii)Licensee and its Affiliates, and their respective employees and
contractors, in connection with the performance of their respective obligations under this
Agreement, shall not cause its Indemnitees to be in violation of the FCPA, Export Control Laws,
or any other Applicable Laws, rules or regulations or otherwise cause any reputational harm to
Exelixis.
(iv)Licensee shall immediately notify Exelixis if Licensee has any
information or suspicion that there may be a violation of the FCPA, Export Control Laws, or any
other Applicable Laws, rules or regulations in connection with the performance of this
Agreement or the Development, manufacture or Commercialization of any Product.
(v)In connection with the performance of its obligations under this
Agreement, Licensee shall comply and shall cause its and its Affiliates’ employees and
contractors to comply with Licensee’s own anti-corruption and anti-bribery policy, a copy of
which has been provided to Exelixis prior to the Effective Date.
(vi)Exelixis will have the right, upon reasonable prior written notice
and during Licensee’s regular business hours, to conduct at its own cost and expenses inspections
of and to audit Licensee’s books and records in the event of a suspected violation or to ensure
compliance with the representations, warranties or covenants of this Section 12.2(c); provided,
however, that in the absence of good cause for such inspections and audits, Exelixis exercise this
right no more than annually.
(vii)In the event that Licensee has violated or been suspected of
violating any of the representations, warranties, or covenants in this Section 12.2(c), Licensee
will cause its or its Affiliates’ personnel or others working under its direction or control to
submit to periodic training that Licensee will provide on anti-corruption law compliance.
(viii)Licensee will, at Exelixis’ request, annually certify to Exelixis in
writing Licensee’s compliance, in connection with the performance of Licensee’s obligations
under this Agreement, with the representations, warranties, or covenants in Section 12.2(c),
which certification shall be issued by Licensee’s global commercial head for the Product.
(ix)Exelixis shall have the right to suspend or terminate this
Agreement in its entirety where there is a credible finding, after a reasonable investigation, that
Licensee, its Affiliates, or its Sublicensees, in connection with performance of Licensee’s
obligations under this Agreement, has engaged in chronic or material violations of the FCPA.
58.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
12.3Additional Exelixis Representations, Warranties and Covenants. Exelixis
represents, warrants and covenants, as applicable, to Licensee that, as of the Effective Date:
(a)Exhibit B-1 lists all Patents Controlled by Exelixis in the Licensee
Territory as of the Effective Date that claim the composition of matter or use of the Compound
and have been filed, prosecuted and maintained in a manner consistent with Exelixis’ standard
practice, in each applicable jurisdiction in which such Patent have been filed, that no official
final deadlines with respect to prosecution thereof have been missed and all applicable fees have
been paid on or before the due date for payment;
(b)All inventors of Inventions claimed in the Patent listed on Exhibit B-1
have assigned their entire right, title and interest in and to such inventions to Exelixis and the
inventors listed are correct and there are no claims or assertions in writing received by Exelixis
regarding the inventorship of such Patent alleging that additional or alternative Inventors ought
to be listed;
(c)Exelixis has the right to grant all rights and licenses it purports to grant to
Licensee with respect to the Exelixis Technology under this Agreement;
(d)Exelixis has not granted any liens or security interests on the Exelixis
Technology;
(e)Exelixis has not received any written notice from a Third Party that the
Development of any Product conducted by Exelixis prior to the Effective Date has infringed any
Patents of any Third Party;
(f)Exelixis has not as of the Effective Date, and will not during the Term,
grant any right to any Third Party under the Exelixis Technology that would conflict with the
rights granted to Licensee hereunder;
(g)no claim or action has been brought or, to Exelixis’ knowledge, threatened
in writing, by any Third Party alleging that the Exelixis Patents are invalid or unenforceable, and
no Exelixis Patent is the subject of any interference, opposition, cancellation or other protest
proceeding [ * ];
(h)to Exelixis’ knowledge, no Third Party is infringing or misappropriating
or has infringed or misappropriated the Exelixis Technology in the Licensee Territory;
(i)Exelixis has disclosed to Licensee all clinical and non-clinical data in the
Control of Exelixis that is material to the evaluation of the safety, efficacy and manufacturing
process of the Product; and
(j)to Exelixis’ knowledge, there are no issues or information, which to
Exelixis’ knowledge and reasonable opinion, are reasonably likely to have a material impact on
the Development of the Product that have not been fully disclosed to Licensee in the course of
Licensee’s due diligence.
12.4Additional Licensee Representations, Warranties and Covenants. Licensee
represents, warrants and covenants to Exelixis that, as of the Effective Date, Licensee has not
granted, and will not grant during the Term, any right to any Third Party under the Licensee
Technology that would conflict with the rights granted to Exelixis hereunder. Licensee further
59.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
represents, warrants and covenants to Exelixis that, as of the Effective Date, Licensee does not
own or control any Licensee Patents.
12.5Disclaimer. Except as expressly set forth in this Agreement, THE
TECHNOLOGY AND INTELLECTUAL PROPERTY RIGHTS PROVIDED BY EACH
PARTY HEREUNDER ARE PROVIDED “AS IS” AND EACH PARTY EXPRESSLY
DISCLAIMS ANY AND ALL WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED,
INCLUDING THE WARRANTIES OF DESIGN, MERCHANTABILITY, FITNESS FOR A
PARTICULAR PURPOSE, NONINFRINGEMENT OF THE INTELLECTUAL PROPERTY
RIGHTS OF THIRD PARTIES, OR ARISING FROM A COURSE OF DEALING, USAGE OR
TRADE PRACTICES, IN ALL CASES WITH RESPECT THERETO. Without limiting the
foregoing, (a) neither Party represents or warrants that any data obtained from conducting
Clinical Trials in one country or jurisdiction will comply with the laws and regulations of any
other country or jurisdiction, and (b) neither Party represents or warrants the success of any study
or test conducted by pursuant to this Agreement or the safety or usefulness for any purpose of the
technology it provides hereunder.
13.Indemnification
13.1Indemnification by Exelixis. Exelixis hereby agrees to defend, indemnify and
hold harmless Licensee and its Affiliates and their respective directors, officers, employees and
agents (each, an “Licensee Indemnitee”) from and against any and all liabilities, expenses and
losses including any product liability, personal injury, property damage, including reasonable
legal expenses and attorneys’ fees (collectively, “Losses”), to which any Licensee Indemnitee
may become subject as a result of any claim, demand, action or other proceeding by any Third
Party to the extent such Losses arise out of: (a) the Development, use, handling, storage,
Commercialization or other disposition of any Compound or Product by Exelixis or its Affiliates
or licensees or the contractors of any of them (excluding any activities by or on behalf of
Licensee or its Affiliates or Sublicensees), (b) the gross negligence or willful misconduct of any
Exelixis Indemnitee, or (c) the breach by Exelixis of any warranty, representation, covenant or
agreement made by Exelixis in this Agreement; except, in each case (a)-(c), to the extent such
Losses arise out of any activities set forth in Section 13.2(a), (b) or (c) for which Licensee is
obligated to indemnify the Exelixis Indemnitee under Section 13.2.
13.2Indemnification by Licensee. Licensee hereby agrees to defend, indemnify and
hold harmless Exelixis, its Affiliates and licensees and their respective directors, officers,
employees and agents (each, a “Exelixis Indemnitee”) from and against any and all Losses to
which any Exelixis Indemnitee may become subject as a result of any claim, demand, action or
other proceeding by any Third Party to the extent such Losses arise out of: (a) the Development,
use, handling, storage, Commercialization or other disposition of any Compound or Product by
Licensee or its Affiliates or Sublicensees or the contractor of any of them, (b) the gross
negligence or willful misconduct of any Licensee Indemnitee, or (c) the breach by Licensee of
any warranty, representation, covenant or agreement made by Licensee in this Agreement;
except, in each case (a)-(c), to the extent such Losses arise out of any activities set forth in
Section 13.1(a), (b) or (c) for which Exelixis is obligated to indemnify the Licensee Indemnitee
under Section 13.1.
13.3Procedure. A party that intends to claim indemnification under this Article 13
(the “Indemnitee”) shall promptly notify the indemnifying Party (the “Indemnitor”) in writing
of any Third Party claim, demand, action or other proceeding (each, a “Claim”) in respect of
which the Indemnitee intends to claim such indemnification, and the Indemnitor shall have sole
60.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
control of the defense or settlement thereof. The Indemnitee may participate at its expense in the
Indemnitor’s defense of and settlement negotiations for any Claim with counsel of the
Indemnitee’s own selection. The indemnity arrangement in this Article 13 shall not apply to
amounts paid in settlement of any action with respect to a Claim, if such settlement is effected
without the consent of the Indemnitor, which consent shall not be withheld or delayed
unreasonably. The failure to deliver written notice to the Indemnitor within a reasonable time
after the commencement of any action with respect to a Third Party Claim shall only relieve the
Indemnitor of its indemnification obligations under this Article 13 if and to the extent the
Indemnitor is actually prejudiced thereby. The Indemnitee shall cooperate fully with the
Indemnitor and its legal representatives in the investigation of any action with respect to a Claim
covered by this indemnification.
13.4Insurance. Each Party, at its own expense, for a period until [ * ] after expiration
or termination of this Agreement, shall maintain commercial general liability insurance,
including public and product liability and other appropriate insurance (e.g., contractual liability,
bodily injury, property damage and personal injury coverage) (or self-insure) in an amount
consistent with sound business practice and reasonable in light of its obligations under this
Agreement during the Term, at a minimum equivalent to [ * ] dollars ($[ * ]) for any one claim or
in the aggregate. Each Party shall provide a certificate of insurance (or evidence of self-
insurance) evidencing such coverage to the other Party upon request. It is understood that such
insurance shall not be construed to create any limit of either Party’s obligations or liabilities with
respect to its indemnification obligations hereunder. In the event of use by either Party of
subcontractors, Sublicensees or any Third Party in the performance of such Party’s obligations
under the Agreement, such Party shall ensure that its subcontractor, Sublicensee or Third Party
shall have a proper and adequate general liability insurance to cover its risks with respect to the
other Party for damages mentioned above.
13.5Limitation of Liability. EXCEPT FOR LIABILITY FOR BREACH OF
ARTICLE 14, NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE
OTHER PARTY ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE
DAMAGES, INCLUDING LOST PROFITS IN CONNECTION WITH THIS AGREEMENT
OR ANY LICENSE GRANTED HEREUNDER; provided, however, that this Section 13.5 shall
not be construed to limit either Party’s indemnification obligations under this Article 13.
14.Confidentiality
14.1Confidential Information. Except to the extent expressly authorized by this
Agreement or otherwise agreed in writing by the Parties, the Parties agree that, during the Term
and for [ * ] thereafter, the receiving Party shall keep confidential and shall not publish or
otherwise disclose and shall not use for any purpose other than as expressly provided for in this
Agreement any Confidential Information of the other Party, and both Parties shall keep
confidential and, subject to Sections 14.2, 14.3 and 14.5, shall not publish or otherwise disclose
the terms of this Agreement. Each Party may use the other Party’s Confidential Information only
to the extent required to accomplish the purposes of this Agreement, including exercising its
rights or performing its obligations under this Agreement. Each Party will use at least the same
standard of care as it uses to protect proprietary or confidential information of its own (but no
less than reasonable care) to ensure that its employees, agents, consultants, contractors and other
representatives do not disclose or make any unauthorized use of the Confidential Information of
the other Party. Each Party will promptly notify the other upon discovery of any unauthorized
use or disclosure of the Confidential Information of the other Party.
61.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
14.2Exceptions. The obligations of confidentiality and restriction on use under
Section 14.1 will not apply to any information that the receiving Party can prove by competent
written evidence: (a) is now, or hereafter becomes, through no act or failure to act on the part of
the receiving Party, generally known or available to the public; (b) is known by the receiving
Party at the time of receiving such information, other than by previous disclosure of the
disclosing Party, or its Affiliates, employees, agents, consultants, or contractors; (c) is hereafter
furnished to the receiving Party without restriction by a Third Party who has no obligation of
confidentiality or limitations on use with respect thereto, as a matter of right; or (d) is
independently discovered or developed by the receiving Party without the use of Confidential
Information belonging to the disclosing Party.
14.3Authorized Disclosure. Each Party may disclose Confidential Information
belonging to the other Party as expressly permitted by this Agreement or if and to the extent such
disclosure is reasonably necessary in the following instances:
(a)filing, prosecuting, or maintaining Patents as permitted by this Agreement;
(b)regulatory filings for Products that such Party has a license or right to
Develop and Commercialize hereunder in a given country or jurisdiction;
(c)prosecuting or defending litigation as permitted by this Agreement;
(d)complying with applicable court orders or governmental regulations; and
(e)disclosure to its and its Affiliates’ employees, consultants, contractors and
agents, to its licensees and sublicensees, in each case on a need-to-know basis in connection with
the Development, manufacture and Commercialization of the Compound and Products in
accordance with the terms of this Agreement, in each case under written obligations of
confidentiality and non-use at least as stringent as those herein; and
(f)disclosure to potential and actual investors, acquirors, licensees and other
financial or commercial partners solely for the purpose of evaluating or carrying out an actual or
potential investment, acquisition or collaboration, in each case under written obligations of
confidentiality and non-use at least as stringent as those herein, provided that the disclosing Party
redacts the financial terms and other provisions of this Agreement that are not reasonably
required to be disclosed in connection with such potential investment, acquisition or
collaboration, which redaction shall be prepared in consultation with the other Party.
Notwithstanding the foregoing, in the event a Party is required to make a disclosure of the other
Party’s Confidential Information pursuant to Section 14.3(c) or 14.3(d), it will, except where
impracticable, give reasonable advance notice to the other Party of such disclosure and use
efforts to secure confidential treatment of such Confidential Information at least as diligent as
such Party would use to protect its own confidential information, but in no event less than
reasonable efforts. In any event, the Parties agree to take all reasonable action to avoid
disclosure of Confidential Information hereunder. Any information disclosed pursuant to
Section 14.3(c) or 14.3(d) shall remain Confidential Information and subject to the restrictions
set forth in this Agreement, including the foregoing provisions of this Article 14.
14.4Publications.
62.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(a)Each Party shall have the right to review and comment on any material
proposed for disclosure or publication by the other Party regarding results of and other
information regarding the other Party’s Development activities with respect to [ * ], whether by
oral presentation, manuscript or abstract. Before any such material is submitted for publication,
or presentation of any such material is made, each Party shall deliver a complete copy of the
material proposed for disclosure to the other Party at least three (3) weeks (for oral presentations
or abstracts) or five (5) weeks (for manuscripts) prior to submitting the material to a publisher or
initiating any other disclosure. Each Party shall review any such material and give its comments
to the other Party within two (2) weeks (for oral presentations or abstracts) or twenty (20) days
(for manuscripts) of the receipt of such material. With respect to oral presentation materials and
abstracts, each Party shall make reasonable efforts to expedite review of such materials and
abstracts, and shall return such items as soon as practicable to the other Party with appropriate
comments, if any. Each Party shall comply with the other Party’s request to delete references to
its Confidential Information in any such material and agrees to not make any submission for
publication or other public disclosure in order not to jeopardize the patentability of any results or
data for the purpose of preparing and filing appropriate patent applications as provided in Section
14.4(b).
(b)If the non-Publishing Party notifies the Publishing Party that such
publication or presentation, in the non-Publishing Party’s reasonable judgment, (i) contains an
invention for which such Party desires to obtain patent protection, (ii) contains any Confidential
Information of such Party, or (iii) could be expected to have an adverse effect on the commercial
value of any Confidential Information disclosed by such Party to the Publishing Party, the
Publishing Party shall delete such Confidential Information from the proposed publication or
presentation.
(c)For as long as the JDC or JCC remains in place, the JDC or JCC shall be
responsible for overseeing and facilitating the Parties’ communications and activities with
respect to publications and presentations under this Section, and for serving as the initial forum
for resolving any disputes between the Parties arising under this Section.
14.5Publicity; Public Disclosures. The Parties agree to issue a joint press release
substantially in a form agreed by the Parties and attached to this Agreement as Exhibit E
announcing the signature of this Agreement at or shortly after the Effective Date within the time-
period as required by relevant securities laws. It is understood that each Party may desire or be
required to issue subsequent press releases relating to this Agreement or activities hereunder.
The Parties agree to consult with each other reasonably and in good faith with respect to the text
and timing of such press releases prior to the issuance thereof, to the extent practicable, provided
that a Party may not unreasonably withhold, condition or delay consent to such releases by more
than [ * ], and that either Party may issue such press releases or make such disclosures to the
SEC or other applicable agency as it determines, based on advice of counsel, as reasonably
necessary to comply with laws or regulations or for appropriate market disclosure. Each Party
shall provide the other Party with advance notice of legally required disclosures to the extent
practicable. The Parties will consult with each other on the provisions of this Agreement to be
redacted in any filings made by a Party with the SEC or as otherwise required by Applicable
Laws; provided that each Party shall have the right to make any such filing as it reasonably
determines necessary under Applicable Laws. In addition, following the initial joint press
release announcing this Agreement, either Party shall be free to disclose, without the other
Party’s prior written consent, the existence of this Agreement, the identity of the other Party and
those terms of the Agreement which have already been publicly disclosed in accordance
herewith.
63.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
14.6Prior Confidentiality Agreement. As of the Effective Date, the terms of this
Article 14 shall supersede any prior non-disclosure, secrecy or confidentiality agreement
between the Parties (or their Affiliates) relating to the subject of this Agreement, including the
Confidentiality Agreement. Any information disclosed pursuant to any such prior agreement
shall be deemed Confidential Information for purposes of this Agreement.
14.7Equitable Relief. Given the nature of the Confidential Information and the
competitive damage that a Party would suffer upon unauthorized disclosure, use or transfer of its
Confidential Information to any Third Party, the Parties agree that monetary damages may not be
a sufficient remedy for any breach of this Article 14. In addition to all other remedies, a Party
shall be entitled to seek specific performance and injunctive and other equitable relief as a
remedy for any breach or threatened breach of this Article 14.
15.Term and Termination
15.1Term.
(a)This Agreement shall commence on the Restatement Date and, unless
terminated earlier as provided in this Article 15 or by mutual written agreement of the Parties,
shall continue until the expiration of the last Royalty Term in the Licensee Territory (the
“Term”).
(b)Notwithstanding anything herein, on a Product-by-Product and country-
by-country basis, upon the expiration of the Royalty Term (i.e., all royalty payment obligations
for a Product in a country), the licenses granted to Licensee in Section 2.1 shall be deemed to be
perpetual and fully paid-up with respect to such Product in such country, but thereafter shall be
on a non-exclusive basis.
15.2Termination for Cause.
(a)Material Breach. Each Party shall have the right to terminate this
Agreement immediately in its entirety upon written notice to the other Party if such other Party
materially breaches this Agreement and has not cured such breach to the reasonable satisfaction
of the other Party within [ * ] ([ * ] with respect to any payment breach) after notice of such
breach from the non-breaching Party. If the alleged breaching Party disputes in good faith the
existence or materiality of a breach specified in a notice provided by the other Party, and such
alleged breaching Party provides the other Party notice of such dispute within [ * ], then the other
Party shall not have the right to terminate this Agreement under this Section 15.2 unless and until
an arbitral panel, in accordance with Article 16, has determined that the alleged breaching Party
has materially breached the Agreement and that such Party fails to cure such breach within the
applicable cure period set forth above following such decision. In the event Exelixis commences
an arbitration alleging material breach by Licensee and Licensee later delivers notice of
voluntary termination under Section 15.3(b), then, at the election of Exelixis, the period of time
set forth in Section 15.3(b) shall be reduced by an amount of time equal to the duration of time
from the commencement of the arbitration to the delivery of such notice, [ * ].
(b)Bankruptcy. Each Party shall have the right to terminate this Agreement
immediately in its entirety upon written notice to the other Party if such other Party makes a
general assignment for the benefit of creditors, files an insolvency petition in bankruptcy,
petitions for or acquiesces in the appointment of any receiver, trustee or similar officer to
liquidate or conserve its business or any substantial part of its assets, commences under the laws
64.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
of any jurisdiction any proceeding involving its insolvency, bankruptcy, reorganization,
adjustment of debt, dissolution, liquidation or any other similar proceeding for the release of
financially distressed debtors or becomes a party to any proceeding or action of the type
described above and such proceeding is not dismissed within [ * ] after the commencement
thereof.
(c)Patent Challenge. Exelixis shall have the right to terminate this
Agreement immediately in its entirety upon written notice to Licensee if Licensee or any of its
Affiliates or Sublicensees directly, or indirectly through any Third Party, commences any
interference or opposition proceeding with respect to, challenges the validity or enforceability of,
or opposes any extension of or the grant of a supplementary protection certificate with respect to,
any Exelixis Patent.
(d)Safety Reasons. Either Party shall have the right to terminate this
Agreement upon written notice to the other Party if the terminating Party reasonably determines,
based upon additional information that becomes available or an analysis of the existing
information at any time, that the medical risk/benefit of such Product is so unfavorable that it
would be incompatible with the welfare of patients to Develop or Commercialize or to continue
to Develop or Commercialize such Product. Prior to any such termination, the terminating Party
shall comply with such internal review and management approval processes as it would normally
follow in connection with the termination of the development and commercialization of its own
products for safety reasons. The terminating Party shall document the decisions of such
committees or members of management and the basis therefor and shall make such minutes and
documentation available to the other Party promptly upon written request.
(e)Discontinuation of Clinical Trials. Licensee may terminate this
Agreement upon [ * ] advance written notice to Exelixis, if substantially all ongoing Clinical
Trials of the Product are ordered or required to be terminated by the FDA or the EMA.
15.3Termination without Cause.
(a)Termination in Its Entirety by Licensee. Licensee shall have the right
to terminate this Agreement in its entirety, or for only the countries that are under the EMA
jurisdiction, without cause upon [ * ] prior written notice to Exelixis if the EMA refuses to
approve the MAA for the Product in Renal Cell Carcinoma (2nd line therapy). For the purpose
of this Section 15.3(a), if EMA conditions such MAA Approval on the performance of additional
Phase 3b or other studies, then EMA shall not be deemed to have refused the approval of such
MAA.
(b)Termination by Region by Licensee. Licensee shall have the right to
terminate this Agreement on a Region-by-Region basis without cause upon [ * ] prior written
notice to Exelixis following the First Commercial RCC Sale of any Product in a given Region;
provided however that Licensee may not provide such notice of termination of this Agreement in
a Region prior to the [ * ] anniversary of the First Commercial RCC Sale of any Product (other
than Cometriq) in such Region. In the event that termination occurs for the EU, then termination
shall automatically be considered to have occurred for the entire Licensee Territory.
15.4Effects of Termination. Upon any termination of this Agreement by either
Party, the following will apply: If this Agreement is terminated only with respect to a particular
Region, then the following shall apply to the terminated Region and the terminated Region shall
be included in Exelixis Territory. For clarity, during the pendency of any dispute regarding
65.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
material breach and/or any termination notice period, all of the terms and conditions of this
Agreement shall remain in effect and the Parties shall continue to perform all of their respective
obligations hereunder.
(a)Licenses. All licenses granted by Exelixis to Licensee will automatically
terminate, including all sublicenses granted by Licensee to any Sublicensee. Except in the event
of termination by Licensee under Section 15.2(a) for material breach by Exelixis, the licenses
granted by Licensee to Exelixis shall survive such termination and shall automatically become
worldwide or for the terminated Region if the Agreement is terminated only for a particular
Region.
(b)Regulatory Materials; Data. Except in the event of termination by
Licensee under Section 15.2(a) for material breach by Exelixis, within [ * ] of the effective date
of such termination, Licensee shall transfer and assign to Exelixis, at no cost to Exelixis, all
Regulatory Filings and Regulatory Approvals for the Products, Data from all preclinical, non-
clinical and clinical studies conducted by or on behalf of Licensee, its Affiliates or Sublicensees
on the Product and all pharmacovigilance data (including all adverse event database) on the
Products. In addition, at Exelixis’ request, Licensee shall provide Exelixis with reasonable
assistance with any inquiries and correspondence with Regulatory Authorities regarding the
Product in the Licensee Territory, such assistance shall be limited to a period of [ * ] after such
termination and not to exceed a total of [ * ] of working time without charge (with any additional
time to be charged at the FTE Rate). The transfer and assignment under this Section 15.4(b)
shall apply with respect to the terminated Region if the Agreement is terminated only for a
particular Region.
(c)Development Wind-Down. Licensee shall either, as directed by Exelixis,
(i) wind-down any ongoing Development activities (including any Clinical Trials) of Licensee
and its Affiliates and Sublicensees with respect to any Product in the Licensee Territory in an
orderly fashion or (ii) promptly transfer such Development activities to Exelixis or its designee,
in compliance with all Applicable Laws.
(d)Cost of Ongoing Trials. If there is any ongoing Clinical Trial of the
Product under the GDP for which the Parties are sharing cost, then Licensee shall continue to
share the cost of such Clinical Trial until the effective date of termination. The remaining costs
from the effective date of termination until completion of such Clinical Trial (or early
termination of such Clinical Trial by Exelixis) shall be either (i) borne entirely by Exelixis
following the effective date of termination if termination occurs as a result of Exelixis’ breach, or
(ii) shared by Licensee for the duration of such Clinical Trial if termination occurs as a result of
Licensee’s breach, or pursuant to Section 15.3 or Section 2.8(c)(i).
(e)Commercial Wind-Down. Licensee shall, as directed by Exelixis, (i)
continue certain ongoing Commercial activities of Licensee and its Affiliates and Sublicensees
with respect to any Product in the Licensee Territory for a period of up to [ * ] as determined by
Exelixis, and (ii) handoff such Commercial activities to Exelixis or its designee, on a timetable to
be set by Exelixis, not to exceed [ * ], and in compliance with all Applicable Laws. During such
commercial wind-down period, the Licensee shall continue to book sales and pay royalties to
Exelixis. Except as necessary to conduct the foregoing activities as directed by Exelixis,
Licensee shall immediately discontinue its (and shall ensure that its Affiliates and Sublicensees
immediately discontinue their) promotion, marketing, offering for sale, and servicing of the
Product and its use of all Product Marks. In addition, Licensee shall immediately deliver to
66.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Exelixis (at Licensee’s expense) all samples, demonstration equipment, sales materials, catalogs,
and literature of Exelixis in Licensee’s possession or control.
(f)Transition Assistance. Licensee shall use Commercially Reasonable
Efforts to seek an orderly transition of the Development and Commercialization of the
Compound and Products to Exelixis or its designee. Except for termination by Licensee under
Section 15.2, Exelixis may, in its sole discretion, postpone the effective date of any termination
for a period of up to [ * ]. Except in the event of termination by Licensee under Section 15.2(a)
for material breach by Exelixis, Licensee shall, at no cost to Exelixis, provide reasonable
consultation and assistance for a period of no more than [ * ] after termination (and in any case
not to exceed a total of [ * ] of working time including the assistance provided under Section
15.4(b)) for the purpose of transferring or transitioning to Exelixis all Licensee Know-How not
already in Exelixis’ possession and, at Exelixis’ request, all then-existing commercial
arrangements relating to the Products that Licensee is able, using Commercially Reasonable
Efforts, to transfer or transition to Exelixis or its designee, in each case, to the extent reasonably
necessary or for Exelixis to continue the Development and/or Commercialization of the
Compound and Products in the Licensee Territory. If any such contract between Licensee and a
Third Party is not assignable to Exelixis or its designee (whether by such contract’s terms or
because such contract does not relate specifically to the Products) but is otherwise reasonably
necessary for Exelixis to continue the Development and/or Commercialization of the Compound
and Products in the Licensee Territory, or if Licensee is performing such work for the Compound
and Product itself (and thus there is no contract to assign), then Licensee shall reasonably
cooperate with Exelixis to negotiate for the continuation of such services for Exelixis from such
entity, or Licensee shall continue to perform such work for Exelixis, as applicable, for a
reasonable period (not to exceed [ * ]) after termination at Exelixis’ cost until Exelixis
establishes an alternate, validated source of such services.
(g)Remaining Inventories. Exelixis shall have the right, at its discretion, to
purchase from Licensee any or all of the inventory of the Products held by Licensee as of the
date of termination at a price equal to the transfer price paid by Licensee to acquire such
inventory from Exelixis. Exelixis shall notify Licensee within [ * ] after the date of termination
whether Exelixis elects to exercise such right.
(h)Non-Compete. Following any termination of this Agreement by Licensee
pursuant to Section 2.8(c)(i) or Section 15.3, or by Exelixis pursuant to Section 15.2, neither
Licensee nor any of its Affiliates shall (directly or indirectly, either with or without a bona fide
collaborator or any other Third Party) commercialize any Competing Product for either (i) a
period of [ * ] (in case of termination pursuant to Section 2.8(c)(i)) or [ * ] (in case of termination
by Licensee pursuant to Section 15.3 or Exelixis pursuant to Section 15.2) following the
effective date of such termination, or (ii) [ * ], whichever is shorter.
15.5Confidential Information. Upon expiration or termination of this Agreement in
its entirety, except to the extent that a Party obtains or retains the right to use the other Party’s
Confidential Information, each Party shall promptly return to the other Party, or delete or
destroy, all relevant records and materials in such Party’s possession or control containing
Confidential Information of the other Party; provided that such Party may keep one copy of such
materials for archival purposes only subject to continuing confidentiality obligations. All
Licensee Data and Regulatory Filings assigned to Exelixis upon termination of this Agreement
will be deemed Exelixis’ Confidential Information and no longer Licensee’s Confidential
Information.
67.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
15.6Survival. Expiration or termination of this Agreement shall not relieve the
Parties of any obligation or right accruing prior to such expiration or termination. Except as set
forth below or elsewhere in this Agreement, the obligations and rights of the Parties under the
following provisions of this Agreement shall survive expiration or termination of this
Agreement: Article 1 (Definitions); Article 10 (Payments, Records, Audits); Article 13
(Indemnification); Article 15 (Dispute Resolution); Article 17 (General Provisions); Section 5.10
(Sunshine Reporting Laws); Section 11.1 (IP Ownership); Sections 14.1, 14.2, 14.3, 14.6, 14.7
(Confidentiality); and Section 15.4 (Effects of Termination).
15.7Exercise of Right to Terminate. All rights and obligations of a Party accrued
prior to the effective date of a termination (including the rights to receive reimbursement for
costs incurred prior to the effective date of such termination and payments accrued or due prior
to the effective date of such termination) shall survive such termination.
15.8Rights in Bankruptcy. All rights and licenses granted under or pursuant to this
Agreement by one Party to the other Party are, and will otherwise be deemed to be, for purposes
of Section 365(n) of the U.S. Bankruptcy Code or comparable provision of applicable
bankruptcy or insolvency laws, licenses of right to “intellectual property” as defined under
Section 101 of the U.S. Bankruptcy Code or comparable provision of applicable bankruptcy or
insolvency laws. The Parties agree that a Party that is a licensee of such rights under this
Agreement will retain and may fully exercise all of its rights and elections under the U.S.
Bankruptcy Code or comparable provision of applicable bankruptcy or insolvency laws. The
Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or
against a Party to this Agreement under the U.S. Bankruptcy Code or comparable provision of
applicable bankruptcy or insolvency laws, the other Party will be entitled to a complete duplicate
of (or complete access to, as appropriate) any such intellectual property and all embodiments of
such intellectual property, and same, if not already in its possession, will be promptly delivered
to it (a) upon any such commencement of a bankruptcy or insolvency proceeding upon its written
request therefor, unless the bankrupt Party elects to continue to perform all of its obligations
under this Agreement, or (b) if not delivered under (a) above, following the rejection of this
Agreement by or on behalf of the bankrupt Party upon written request therefor by the other Party.
16.Dispute Resolution
16.1Objective. The Parties recognize that disputes as to matters arising under or
relating to this Agreement or either Party’s rights and obligations hereunder may arise from time
to time. It is the objective of the Parties to establish procedures to facilitate the resolution of
such disputes in an expedient manner by mutual cooperation and without resort to litigation. To
accomplish this objective, the Parties agree to follow the procedures set forth in this Article 16 to
resolve any such dispute if and when it arises.
16.2Executive Mediation. The Parties will try to settle any dispute, controversy or
claim that arises out of, or relates to, any provision of the Agreement (“Disputed Matter”) by
first referring the Disputed Matter to the CEO of Exelixis (or his designee) and the CEO of
Licensee (or his designee). Either Party may initiate such informal dispute resolution by sending
written notice of the Disputed Matter to the other Party, and, within [ * ] after such notice, such
CEOs (or their respective designees having the authority to settle such Disputed Matter) of the
Parties will meet for attempted resolution by good faith negotiations. If such CEOs (or their
respective designees) are unable to resolve such dispute within [ * ] of their first meeting for such
negotiations, either Party may seek to have such dispute resolved in accordance with Section
16.3 below.
68.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
16.3Dispute Resolution.
(a)If the Parties are unable to resolve a Disputed Matter using the process
described in Section 16.2, then a Party seeking further resolution of the Disputed Matter will
submit the Disputed Matter to resolution by final and binding arbitration. Whenever a Party will
decide to institute arbitration proceedings, it will give written notice to that effect to the other
Party. Arbitration will be held in London, the United Kingdom, and administered by the
International Chamber of Commerce pursuant to its ICC International Arbitration Rules then in
effect (the “Rules”), except as otherwise provided herein and applying the substantive law
specified in Section 16.1. The arbitration will be conducted by a panel of three (3) arbitrators
appointed in accordance with the Rules; provided that each Party will, within [ * ] after the
institution of the arbitration proceedings, appoint an arbitrator, and such arbitrators will together,
within [ * ], select a third (3rd) arbitrator as the chairman of the arbitration panel. Each arbitrator
must have significant business or legal experience in the pharmaceutical business. If the two (2)
initial arbitrators are unable to select a third (3rd) arbitrator within such [ * ] period, the third
(3rd) arbitrator will be appointed in accordance with Rules. The Parties hereby agree to engage
in discovery of information and evidence that is or might be relevant to the claims, defenses, and
issues in the dispute, including by means of [ * ]. The Parties further agree to the ability, right,
and power to subpoena Third Party witnesses for both discovery and hearing purposes. The
discovery provided for herein may commence once the Terms of Reference have been signed by
the Parties and the panel of arbitrators. The panel of arbitrators shall address the time required
for the completion of discovery at the initial case management conference and shall address any
discovery issues if any arise based on motion and arbitral order. After conducting any hearing
and taking any evidence deemed appropriate for consideration, the arbitrators will be requested
to render their opinion within [ * ] of the final arbitration hearing. No panel of arbitrators will
have the power to award damages excluded pursuant to Section 13.5 under this Agreement and
any arbitral award that purports to award such damages is expressly prohibited and void ab
initio. Decisions of the panel of arbitrators that conform to the terms of this Section 16.3 will be
final and binding on the Parties and judgment on the award so rendered may be entered in any
court of competent jurisdiction. The losing Party, as determined by the panel of arbitrators, will
pay all of the ICC administrative costs and fees of the arbitration and the fees and costs of the
arbitrators, and the arbitrators will be directed to provide for payment or reimbursement of such
fees and costs by the losing Party. If the panel of arbitrators determines that there is no losing
Party, the Parties will each bear or pay one-half of those costs and fees and the arbitrators’ award
will so provide. Notwithstanding the foregoing, each Party is to bear or pay its own attorneys’
fees, expert or witness fees, and any other fees and costs, and no such fees or costs will be shifted
to the other Party.
(b)Notwithstanding the terms of and procedures set forth in Section 16.2 or
16.3(a), any applications, motions or orders to show cause seeking temporary restraining orders,
preliminary injunctions or other similar preliminary or temporary legal or equitable relief
(“Injunctive Relief”) concerning a Disputed Matter (including, but not limited to, Disputed
Matters arising out of a potential or actual breach of the confidentiality and non-use provisions in
Article 14) may immediately be brought in the first instance and without invocation or
exhaustion of the procedures set forth in subsections 16.3(a) and 16.3(b) for hearing and
resolution in and by a court of competent jurisdiction. Alternatively, a party seeking Injunctive
Relief may immediately institute arbitral proceedings without invocation or exhaustion of the
procedures set forth in subsections 16.3(a) and 16.3(b), and any such Injunctive Relief
proceedings will be administered in accordance with by the ICC pursuant to its ICC emergency
arbitration procedures then in effect and applying the substantive law specified in Section 16.2.
In either event, once the Injunctive Relief proceedings have been conducted and a decision
69.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
rendered thereon by the court or arbitral forum, the Parties will, if the Disputed Matter is not
finally resolved by the Injunctive Relief, proceed to resolve the Disputed Matter in accordance
with the terms of Section 16.2 and 16.3(a).
(c)Notwithstanding the foregoing, this Section 16.3 shall not apply to any
dispute, controversy or claim that concerns (i) the validity, enforceability or infringement of a
patent, trademark or copyright; or (ii) any antitrust, anti-monopoly or competition law or
regulation, whether or not statutory.
17.General Provisions
17.1Governing Law. This Agreement, and all questions regarding the existence,
validity, interpretation, breach or performance of this Agreement, shall be governed by, and
construed and enforced in accordance with, the laws of the State of New York, United States,
without reference to its conflicts of law principles.
17.2Entire Agreement; Modification. This Agreement, including the exhibits, is
both a final expression of the Parties’ agreement and a complete and exclusive statement with
respect to all of its terms. This Agreement supersedes all prior and contemporaneous agreements
and communications, whether oral, written or otherwise, concerning any and all matters
contained herein, other than the Original Agreement, which governs the Parties’ rights and
obligations thereunder during the period from the Effective Date until the Restatement Date.
This Agreement may only be modified or supplemented in a writing expressly stated for such
purpose and signed by the Parties to this Agreement.
17.3Relationship Between the Parties. The Parties’ relationship, as established by
this Agreement, is solely that of independent contractors. This Agreement does not create any
partnership, joint venture or similar business relationship between the Parties. Neither Party is a
legal representative of the other Party, and neither Party can assume or create any obligation,
representation, warranty or guarantee, express or implied, on behalf of the other Party for any
purpose whatsoever.
17.4Non-Waiver. The failure of a Party to insist upon strict performance of any
provision of this Agreement or to exercise any right arising out of this Agreement shall neither
impair that provision or right nor constitute a waiver of that provision or right, in whole or in
part, in that instance or in any other instance. Any waiver by a Party of a particular provision or
right shall be in writing, shall be as to a particular matter and, if applicable, for a particular
period of time and shall be signed by such Party.
17.5Assignment. Except as expressly provided hereunder, neither this Agreement nor
any rights or obligations hereunder may be assigned or otherwise transferred by either Party
without the prior written consent of the other Party (which consent shall not be unreasonably
withheld); provided, however, that either Party may assign or otherwise transfer this Agreement
and its rights and obligations hereunder without the other Party’s consent:
(a)in connection with the transfer or sale of all or substantially all of the
business or assets of such Party relating to the Compound and Products to a Third Party, whether
by merger, consolidation, divesture, restructure, sale of stock, sale of assets or otherwise,
provided that in the event of any such transaction (whether this Agreement is actually assigned or
is assumed by the acquiring Party by operation of law (e.g., in the context of a reverse triangular
70.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
merger)), intellectual property rights of the acquiring Party to such transaction (if other than one
of the Parties to this Agreement) shall not be included in the technology licensed hereunder; or
(b)to an Affiliate, provided that the assigning Party shall remain liable and
responsible to the non-assigning Party hereto for the performance and observance of all such
duties and obligations by such Affiliate.
The rights and obligations of the Parties under this Agreement shall be binding upon and inure to
the benefit of the successors and permitted assigns of the Parties specified above, and the name
of a Party appearing herein will be deemed to include the name of such Party’s successors and
permitted assigns to the extent necessary to carry out the intent of this Section 17.5. Any
assignment not in accordance with this Section 17.5 shall be null and void.
17.6Severability. If, for any reason, any part of this Agreement is adjudicated invalid,
unenforceable or illegal by a court of competent jurisdiction, such adjudication shall not, to the
extent feasible, affect or impair, in whole or in part, the validity, enforceability, or legality of any
remaining portions of this Agreement. All remaining portions shall remain in full force and
effect as if the original Agreement had been executed without the invalidated, unenforceable or
illegal part.
17.7Notices. Any notice to be given under this Agreement must be in writing and
delivered either in person, by (a) air mail (postage prepaid) requiring return receipt, (b) overnight
courier, or (c) facsimile confirmed thereafter by any of the foregoing, to the Party to be notified
at its address(es) given below, or at any address such Party may designate by prior written notice
to the other in accordance with this Section 17.7. Notice shall be deemed sufficiently given for
all purposes upon the earliest of: (i) the date of actual receipt; (ii) if air mailed, five (5) days
after the date of postmark; (iii) if delivered by overnight courier, the next day the overnight
courier regularly makes deliveries or (iv) if sent by facsimile, the date of confirmation of receipt
if during the recipient’s normal business hours, otherwise the next business day.
If to Licensee, notices must be addressed to:
Ipsen Pharma SAS
70 rue Balard
75015 Paris
France
Attention: Executive VP, General Counsel
Facsimile: [ * ]
If to Exelixis, notices must be addressed to:
Exelixis, Inc.
1851 Harbor Bay
Parkway, Alameda, CA 94502
USA
Attention: General Counsel
Facsimile: [ * ]
71.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
17.8Standstill.
(a)Commencing the Effective Date and expiring on the fifth (5th) anniversary
date of the Effective Date, unless such provision is terminated earlier (the “Standstill Period”),
neither Licensee nor any of its Affiliates, without the prior consent of Exelixis or except as
provided for in this Agreement or in any agreement referred to herein, or in any agreement
executed after the Effective Date by Exelixis with Licensee or any of its Affiliates, will:
(i)make, effect, initiate, cause or participate in:
A.any acquisition of beneficial ownership of any securities of
Exelixis or any securities of any subsidiary or other Affiliate of Exelixis (each, a “Exelixis
Entity”) such that following any such acquisition, Licensee and its Affiliates then own more than
five percent (5%) of the securities of such Exelixis Entity;
B.any acquisition of any assets of any Exelixis Entity;
C.any tender offer, exchange offer, merger, business
combination, recapitalization, restructuring, liquidation, dissolution or extraordinary transaction
involving a Exelixis Entity, or involving any securities or assets of a Exelixis Entity; or
D.any “solicitation” of “proxies” (as those terms are used in
the proxy rules of the Securities and Exchange Commission) or consents with respect to any
securities of a Exelixis Entity;
(ii)form, join or participate in a “group” (as defined in the Securities
Exchange Act of 1934 and the rules promulgated thereunder) with respect to the beneficial
ownership of any securities of a Exelixis Entity;
(iii)act, alone or in concert with others, to seek to control or influence
the management, board of directors or policies of a Exelixis Entity;
(iv)take any action that might require a Exelixis Entity to make a
public announcement regarding any of the types of matters set forth in clause “(i)” of this Section
17.8(a);
(v)agree or offer to take, or encourage or propose (publicly or
otherwise) the taking of, any action referred to in clause “(i)”, “(ii)”, “(iii)” or “(iv)” of this
Section 17.8(a);
(vi)assist, induce or encourage any other person or entity to take any
action of the type referred to in clause “(i)”, “(ii)”, “(iii)”, “(iv)” or “(v)” of this Section 17.8(a);
or
(vii)enter into any discussions, negotiations, arrangement or agreement
with any other person or entity relating to any of the foregoing.
(viii)For clarity, the expiration of the Standstill Period will not terminate or otherwise affect
any of the other provisions of this Agreement.
72.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
(b)Notwithstanding the foregoing provisions, Licensee or its Affiliates will
not be subject to any of the restrictions set forth in this Section 17.8 with respect to a Exelixis
Entity if either:
(i)such Exelixis Entity publicly announces its intention to pursue a
proposed Acquisition Transaction (as defined below);
(ii)such Exelixis Entity shall have entered into an agreement in
principle or definitive agreement providing for an Acquisition Transaction;
(iii)the board of directors of such Exelixis Entity shall have adopted a
formal plan of liquidation or dissolution;
(iv)if a Third Party commences a tender or exchange offer or bid
which, if successful, would result in such Third Party beneficially owning not less than thirty five
percent (35%) of the voting securities or equity interest in such Exelixis Entity; or
(v)if a Third Party makes a public announcement of a bone fide
takeover bid to acquire the outstanding voting securities or equity interest in such Exelixis Entity.
(vi)“Acquisition Transaction” means (A) any direct or indirect acquisition or purchase of
assets of the applicable Exelixis Entity at a purchase price representing [ * ] ([ * ]%) of the
voting securities of or equity interest in such Exelixis Entity by any person or “group”; (B) any
tender offer or exchange offer that if consummated would result in any person or “group”
beneficially owning [ * ] ([ * ]%) or more of any class of equity securities of such Exelixis
Entity; or (C) any merger, consolidation, business combination, sale of assets, recapitalization or
similar transaction involving such Exelixis Entity representing more than [ * ] ([ * ]%) of the
market capitalization of such Exelixis Entity.
(c)Notwithstanding the foregoing, the Parties agree that Licensee or its
Affiliates shall not be prohibited from (i) initiating private discussions with, and submitting
confidential private proposals to, the management or Chief Executive Officer of any acquisition
of beneficial ownership of any securities or any assets of any Exelixis Entity, including
discussing a right of first refusal before a Exelixis Entity intends to pursue any Acquisition
Transaction; or (ii) proposing other collaborative research agreements or other commercial
license agreements to Exelixis.
(d)the Parties agree to discuss whether to terminate the Standstill Period on a
biennial basis at the anniversary date of the Effective Date.
17.9Force Majeure. Each Party shall be excused from liability for the failure or
delay in performance of any obligation under this Agreement (other than failure to make
payment when due) by reason of any event beyond such Party’s reasonable control including
Acts of God, fire, flood, explosion, earthquake, pandemic flu, or other natural forces, war, civil
unrest, acts of terrorism, accident, destruction or other casualty, any lack or failure of
transportation facilities, any lack or failure of supply of raw materials, or any other event similar
to those enumerated above. Such excuse from liability shall be effective only to the extent and
duration of the event(s) causing the failure or delay in performance and provided that the Party
has not caused such event(s) to occur. Notice of a Party’s failure or delay in performance due to
force majeure must be given to the other Party within [ * ] after its occurrence. All delivery
73.
Exhibit 10.42
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
dates under this Agreement that have been affected by force majeure shall be tolled for the
duration of such force majeure. In no event shall any Party be required to prevent or settle any
labor disturbance or dispute.
17.10Interpretation. The headings of clauses contained in this Agreement preceding
the text of the sections, subsections and paragraphs hereof are inserted solely for convenience
and ease of reference only and shall not constitute any part of this Agreement, or have any effect
on its interpretation or construction. All references in this Agreement to the singular shall
include the plural where applicable. Unless otherwise specified, references in this Agreement to
any Article shall include all Sections, subsections and paragraphs in such Article, references to
any Section shall include all subsections and paragraphs in such Section, and references in this
Agreement to any subsection shall include all paragraphs in such subsection. The word
“including” and similar words means including without limitation. The word “or” means “and/
or” unless the context dictates otherwise because the subject of the conjunction are mutually
exclusive. The words “herein,” “hereof” and “hereunder” and other words of similar import
refer to this Agreement as a whole and not to any particular Section or other subdivision. All
references to days in this Agreement mean calendar days, unless otherwise specified.
Ambiguities and uncertainties in this Agreement, if any, shall not be interpreted against either
Party, irrespective of which Party may be deemed to have caused the ambiguity or uncertainty to
exist. This Agreement has been prepared in the English language and the English language shall
control its interpretation. In addition, all notices required or permitted to be given hereunder, and
all written, electronic, oral or other communications between the Parties regarding this
Agreement shall be in the English language.
17.11Counterparts; Electronic or Facsimile Signatures. This Agreement may be
executed in any number of counterparts, each of which shall be an original, but all of which
together shall constitute one instrument. This Agreement may be executed and delivered
electronically or by facsimile and upon such delivery such electronic or facsimile signature will
be deemed to have the same effect as if the original signature had been delivered to the other
Party.
{Signature Page Follows}
[Signature Page to A&R Collabaoration and License Agreement]
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
In Witness Whereof, the Parties hereto have caused this Amended and Restated
Collaboration and License Agreement to be executed and entered into by their duly authorized
representatives as of the Restatement Date.
Exelixis, Inc. By: /s/ Christopher Senner Name: Christopher Senner Title: EVP and CFO | Ipsen Pharma S.A.S By: /s/ Aymeric Le Chatelier Name: Aymeric Le Chatelier Title: EVP CFO |
A-1
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
List of Exhibits:
Exhibit A:Chemical Structure of cabozantinib
Exhibit B-1:List of Exelixis Patents as of the Restatement Date
Exhibit B-2:List of Excluded Patents
Exhibit C:Approved Distributors
Exhibit D:Initial Global Development Plan and Budget
Exhibit E:Press Release
Exhibit F: Exelixis [ * ]
A-1A-1
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Exhibit A
CHEMICAL STRUCTURE OF CABOZANTINIB
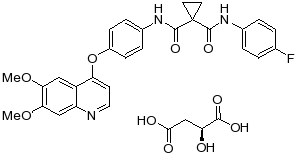
Cabozantinib (S)-malate salt
A-1A-1
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
B1-1
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I)
NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Exhibit B-1
LIST OF EXELIXIS PATENTS AS OF THE RESTATEMENT DATE
{redacted Exhibit B-1 content comprises approximately 33 pages}
[ * ]
B2-1
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I)
NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Exhibit B-2
LIST OF EXCLUDED PATENTS
{redacted Exhibit B-2 content comprises approximately 15 pages}
[ * ]
C-1
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Exhibit C
APPROVED DISTRIBUTORS
[ * ]
2
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Exhibit D
GLOBAL DEVELOPMENT PLAN
{redacted Exhibit D content comprises 2 pages}
[ * ]
E-1
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Exhibit E
PRESS RELEASE
 |  |
Exelixis Contacts Financial Community: Susan Hubbard Investor Relations and Corporate Communications (650) 837-8194 shubbard@exelixis.com Media: Hal Mackins For Exelixis, Inc. (415) 994-0040 hal@torchcomllc.com | Ipsen Contacts Media: Didier Véron Senior Vice-Président, Public Affairs and Communication Tel.: +33 (0)1 58 33 51 16 Fax: +33 (0)1 58 33 50 58 E-mail: didier.veron@ipsen.com Financial Community: Stéphane Durant des Aulnois Vice President, Investor Relations Tel.: +33 (0)1 58 33 60 09 Fax: +33 (0)1 58 33 50 63 E-mail: stephane.durant.des.aulnois@ipsen.com |
EXELIXIS AND IPSEN ENTER INTO EXCLUSIVE LICENSING
AGREEMENT TO COMMERCIALIZE AND DEVELOP NOVEL CANCER THERAPY
CABOZANTINIB IN REGIONS OUTSIDE THE UNITED STATES, CANADA AND JAPAN
- Cabozantinib commercialized for medullary thyroid cancer (MTC)
and filed for advanced renal cell carcinoma (RCC) -
- $200 million upfront payment and subsequent regulatory and commercial milestones -
South San Francisco, Calif. and Paris, France – February 29, 2016 – Exelixis, Inc. (NASDAQ:EXEL)
and Ipsen (Euronext: IPN; ADR: IPSEY) today jointly announced an exclusive licensing agreement for the
commercialization and further development of cabozantinib, Exelixis’ lead oncology drug. Under the
agreement, Ipsen will have exclusive commercialization rights for current and potential future cabozantinib
indications outside of the United States, Canada and Japan. This agreement includes rights to
COMETRIQ®, which is currently approved in the European Union (EU) for the treatment of adult patients
with progressive, unresectable, locally advanced or metastatic medullary thyroid cancer (MTC). The
companies have agreed to collaborate on the development of cabozantinib for current and potential future
indications. Exelixis will maintain exclusive commercial rights for cabozantinib in the United States and
Canada, and continue its discussions to partner commercial rights in Japan.
Under the agreement, Exelixis will receive a $200 million upfront payment. Exelixis is eligible to receive
regulatory milestones, including $60 million upon the approval of cabozantinib in Europe for advanced
renal cell carcinoma (RCC) and $50 million upon the filing and approval of cabozantinib in Europe for
advanced hepatocellular carcinoma (HCC), as well as additional regulatory milestones for potential further
E-2
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
indications. The agreement also includes up to $545 million of potential commercial milestones and
provides for Exelixis to receive tiered royalties up to 26% on Ipsen’s net sales of cabozantinib in its
territories.
Marc de Garidel, Chairman and Chief Executive Officer of Ipsen said: “The robust results from the
METEOR study in advanced renal cell carcinoma demonstrate that cabozantinib has the potential to become
a key oncology product in Europe. This transaction will help Ipsen accelerate the growth of the company
and strengthen its oncology footprint in Europe. We are excited to bring cabozantinib to patients and
clinicians around the world.”
Future commercial indications for cabozantinib could include advanced HCC, the subject of CELESTIAL,
an Exelixis-sponsored phase 3 pivotal trial for which top-line results are anticipated in 2017. Additional
earlier-stage studies are under way through Exelixis’ collaboration with the National Cancer Institute’s
Cancer Therapy Evaluation Program (NCI-CTEP), and its ongoing Investigator-Sponsored Trial (IST)
program. Through these two programs, there are more than 45 ongoing or planned studies including trials
in advanced RCC, bladder cancer, colorectal cancer, non-small cell lung cancer, and endometrial cancer.
“In Ipsen, Exelixis has an ideal partner to maximize the potential for cabozantinib to have a positive impact
on the treatment of cancer on a global basis,” said Michael M. Morrissey, Ph.D., President and Chief
Executive Officer of Exelixis. “Ipsen’s established international oncology marketing presence, late-stage
clinical development expertise and shared vision with Exelixis for the franchise potential of cabozantinib
will accelerate cabozantinib’s commercialization in its territories, while Exelixis remains focused on our
launch in the United States. While our immediate priority will be on advanced renal cell carcinoma,
Exelixis and Ipsen are committed to exploring and potentially developing cabozantinib in a variety of
cancer settings.”
Cabozantinib is a small molecule therapy that inhibits the activity of tyrosine kinases including VEGF
receptors, MET, AXL, and RET. Following positive results from the METEOR global phase 3 pivotal trial,
the tablet form of cabozantinib is the subject of pending U.S. and EU regulatory applications for use as a
treatment for advanced RCC in patients who have received one prior therapy. In the EU, the Marketing
Authorization Application (MAA) for cabozantinib in advanced RCC has been accepted and granted
accelerated assessment. With this designation, the MAA is eligible for a 150-day review, versus the
standard 210 days (excluding clock stops when information is requested by the EMA). Exelixis plans to
transfer sponsorship of this MAA to Ipsen. Exelixis also anticipates transitioning the commercialization
rights to COMETRIQ® outside the U.S. from Exelixis’ current international partner for COMETRIQ®,
Swedish Orphan Biovitrum AB (Sobi), to Ipsen, in accordance with the terms of its agreement with Sobi. In
March 2014, the capsule form of cabozantinib was approved by the European Commission under the trade
name COMETRIQ for the treatment of patients with progressive, unresectable, locally advanced or
metastatic MTC.
About the METEOR Phase 3 Clinical Trial
METEOR is a global, randomized open-label trial that compares cabozantinib to everolimus, a standard of
care therapy, in 658 patients with advanced RCC whose disease progressed following treatment with a
VEGF receptor (VEGFR) tyrosine kinase inhibitor (TKI). The trial’s primary endpoint is progression-free
survival (PFS), and secondary endpoints include overall survival (OS) and objective response rate (ORR).
Patients were randomized 1:1 to receive 60 mg of cabozantinib or 10 mg of everolimus daily, and were
1 Choueiri T.K. et al. N Engl J Med 2015;373:1814-23.
2 Cancer Facts & Figures 2015. American Cancer Society. Available at
http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
3 Jonasch et al., BMJ (2014) vol. 349, g4797.
4 http://www.cancer.org/cancer/kidneycancer/detailedguide/kidney-cancer-adult-survival-rates
5 ACS Cancer Facts and Figures 2015; Heng et al., Ann Oncol (2012) vol. 23 no. 6; internal data on file; Motzer et al., N Engl
J Med (2007) vol. 356 no. 2; NCIN (UK) report, April 2014, Available at http://www.ncin.org.uk/view?rid=2676.
E-3
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
stratified based on number of prior VEGFR TKI therapies and on commonly applied RCC risk criteria. No
crossover was allowed.
As published in the New England Journal of Medicine, the trial met its primary PFS and secondary ORR
endpoints.1 Cabozantinib demonstrated a 42% reduction in the rate of disease progression or death as
compared with everolimus, with median PFS of 7.4 months versus 3.8 months for everolimus (Hazard Ratio
[HR]=0.58, 95% Confidence Interval [CI] 0.45-0.75, p<0.001).
Following a pre-planned interim analysis that showed a strong trend in OS favoring cabozantinib (HR=0.67,
95% CI 0.51-0.89, p=0.005) but did not reach statistical significance, Exelixis undertook a second interim
analysis after consulting with regulatory authorities. The results of this second interim analysis
demonstrated a highly statistically significant and clinically meaningful increase in OS for cabozantinib.
Exelixis has shared these data with regulators and intends to present them at a medical conference later this
year.
Cabozantinib’s safety profile was similar to that of other VEGFR TKIs in this patient population. The
incidence of adverse events (any grade), regardless of causality, was 100% with cabozantinib and more than
99% with everolimus. Serious adverse events occurred in 40% of cabozantinib patients and 43% of
everolimus patients. The rate of treatment discontinuation due to adverse events was low (~10%) in both
treatment arms.
About Advanced Renal Cell Carcinoma
The American Cancer Society’s 2015 statistics cite kidney cancer as among the top ten most commonly
diagnosed forms of cancer among both men and women in the U.S.2 Clear cell RCC is the most common
type of kidney cancer in adults.3 If detected in its early stages, the five-year survival rate for RCC is high;
however, the five-year survival rate for patients with advanced or late-stage metastatic RCC is under 10
percent, with no identified cure for the disease.4
Until the introduction of targeted therapies into the RCC setting a decade ago, treatments for metastatic
RCC had historically been limited to cytokine therapy (e.g., interleukin-2 and interferon). In the second-
and later-line settings, which encompass approximately 17,000 drug-eligible patients in the U.S. and 37,000
globally,5 two small-molecule therapies and an immune checkpoint inhibitor have been approved. The
currently approved small-molecule agents have shown little differentiation in terms of efficacy,
demonstrating only modest PFS benefit in patients refractory to sunitinib, a commonly-used first-line
therapy.
About Cabozantinib
Cabozantinib is currently marketed in capsule form under the brand name COMETRIQ® in the United
States for the treatment of progressive, metastatic MTC, and in the European Union for the treatment of
adult patients with progressive, unresectable locally advanced or metastatic MTC. COMETRIQ is not
E-4
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
indicated for patients with RCC. In the METEOR trial, and all other cancer trials currently underway,
Exelixis is investigating a tablet formulation of cabozantinib distinct from the COMETRIQ capsule form.
The tablet formulation of cabozantinib is the subject of the NDA and MAA for advanced RCC.
Cabozantinib inhibits the activity of tyrosine kinases including VEGF receptors, MET, AXL and RET.
These receptor tyrosine kinases are involved in both normal cellular function and in pathologic processes
such as oncogenesis, metastasis, tumor angiogenesis and maintenance of the tumor microenvironment.
The European Commission granted COMETRIQ conditional approval for the treatment of adult patients
with progressive, unresectable locally advanced or metastatic MTC. Similar to another drug approved in
this setting, the approved indication states that for patients in whom Rearranged during Transfection (RET)
mutation status is not known or is negative, a possible lower benefit should be taken into account before
individual treatment decisions.
Important Safety Information, including Boxed WARNINGS
WARNING: PERFORATIONS AND FISTULAS, and HEMORRHAGE
•Serious and sometimes fatal gastrointestinal perforations and fistulas occur in COMETRIQ-treated
patients.
•Severe and sometimes fatal hemorrhage occurs in COMETRIQ-treated patients.
•COMETRIQ treatment results in an increase in thrombotic events, such as heart attacks.
•Wound complications have been reported with COMETRIQ.
•COMETRIQ treatment results in an increase in hypertension.
•Osteonecrosis of the jaw has been observed in COMETRIQ-treated patients.
•Palmar-Plantar Erythrodysesthesia Syndrome (PPES) occurs in patients treated with COMETRIQ.
•The kidneys can be adversely affected by COMETRIQ. Proteinuria and nephrotic syndrome have
been reported in patients receiving COMETRIQ.
•Reversible Posterior Leukoencephalopathy Syndrome has been observed with COMETRIQ.
•Avoid administration of COMETRIQ with agents that are strong CYP3A4 inducers or inhibitors.
•COMETRIQ is not recommended for use in patients with moderate or severe hepatic impairment.
•COMETRIQ can cause fetal harm when administered to a pregnant woman.
Adverse Reactions – The most commonly reported adverse drug reactions (≥25%) are diarrhea, stomatitis,
palmar-plantar erythrodysesthesia syndrome (PPES), decreased weight, decreased appetite, nausea, fatigue,
oral pain, hair color changes, dysgeusia, hypertension, abdominal pain, and constipation. The most
common laboratory abnormalities (≥25%) are increased AST, increased ALT, lymphopenia, increased
alkaline phosphatase, hypocalcemia, neutropenia, thrombocytopenia, hypophosphatemia, and
hyperbilirubinemia.
Please see full U.S. prescribing information, including Boxed WARNINGS, at www.COMETRIQ.com/
downloads/Cometriq_Full_Prescribing_Information.pdf
Please refer to the full European Summary of Product Characteristics for full European Union prescribing
information, including contraindication, special warnings and precautions for use at www.sobi.com once
posted.
About Ipsen
E-5
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Ipsen is a global specialty-driven biotechnological group with total sales exceeding €1.4 billion in 2015.
Ipsen sells more than 20 drugs in more than 115 countries, with a direct commercial presence in more than
30 countries. Ipsen’s ambition is to become a leader in specialty healthcare solutions for targeted
debilitating diseases. Its fields of expertise cover oncology, neurosciences and endocrinology (adult &
pediatric). Ipsen’s commitment to oncology is exemplified through its growing portfolio of key therapies
improving the care of patients suffering from prostate cancer, bladder cancer and neuro-endocrine tumors.
Ipsen also has a significant presence in primary care. Moreover, the Group has an active policy of
partnerships. Ipsen's R&D is focused on its innovative and differentiated technological platforms, peptides
and toxins, located in the heart of the leading biotechnological and life sciences hubs (Les Ulis/Paris-
Saclay, France; Slough/Oxford, UK; Cambridge, US). In 2015, R&D expenditure totaled close to €193
million, representing about 13% of Group sales. The Group has more than 4,600 employees worldwide.
Ipsen’s shares are traded on segment A of Euronext Paris (stock code: IPN, ISIN code: FR0010259150) and
eligible to the “Service de Règlement Différé” (“SRD”). The Group is part of the SBF 120 index. Ipsen
has implemented a Sponsored Level I American Depositary Receipt (ADR) program, which trade on the
over-the-counter market in the United States under the symbol IPSEY. For more information on Ipsen, visit
www.ipsen.com.
About Exelixis
Exelixis, Inc. is a biopharmaceutical company committed to developing small molecule therapies for the
treatment of cancer. Exelixis is focusing its development and commercialization efforts primarily on
cabozantinib, an internally discovered inhibitor of multiple receptor tyrosine kinases. Another Exelixis-
discovered compound, COTELLIC™ (cobimetinib), a selective inhibitor of MEK, has been approved in
Switzerland, the United States, the European Union, and Canada, and is being evaluated by Roche and
Genentech (a member of the Roche Group) in a broad global development program under a collaboration
with Exelixis. For more information, please visit the company’s website at www.exelixis.com.
Exelixis Forward-Looking Statement Disclaimer
This press release contains forward-looking statements, including, without limitation, statements related to:
the business and financial terms of the collaboration agreement for cabozantinib with Ipsen, including, the
division of commercialization rights, development plans and Exelixis’ eligibility to receive regulatory and
commercial milestones and royalties; Exelixis’ plan to continue its discussions to partner commercial rights
for cabozantinib in Japan; the potential for cabozantinib to become a key oncology product in Europe and
the impact of the transaction on the growth of Ipsen; advanced HCC as a future potential commercial
indication for cabozantinib and the timing for anticipated top-line results from CELESTIAL; the impact of
the collaboration with Ipsen on Exelixis’ plan to maximize the potential for cabozantinib on a global basis;
Exelixis’ plan to stay focused on the potential launch of cabozantinib in advanced RCC in the United States;
advanced RCC as Exelixis’ immediate priority; Exelixis’ and Ipsen’s commitment to exploring and
potentially developing cabozantinib in a variety of cancers; the eligibility for an expedited review of
Exelixis’ MAA for cabozantinib in advanced RCC by the EMA and Exelixis’ plans to transfer sponsorship
of the MAA to Ipsen; Exelixis’ plans to transition the commercialization rights to COMETRIQ outside of
the U.S. from Sobi to Ipsen; and Exelixis’ intent to present data from the second interim analysis of OS for
METEOR at a medical conference later this year. Words such as “will,” “potential,” “future,” “continue,”
“eligible,” “priority,” “committed,” “plans,” “anticipates,” “intends,” or other similar expressions identify
forward-looking statements, but the absence of these words does not necessarily mean that a statement is
not forward-looking. In addition, any statements that refer to expectations, projections or other
characterizations of future events or circumstances are forward-looking statements. These forward-looking
statements are based upon Exelixis’ current plans, assumptions, beliefs, expectations, estimates and
E-6
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
projections. Forward-looking statements involve risks and uncertainties. Actual results and the timing of
events could differ materially from those anticipated in the forward-looking statements as a result of these
risks and uncertainties, which include, without limitation: the clinical, therapeutic and commercial potential
of cabozantinib; Exelixis’ dependence on its relationship with Ipsen, including, the level of Ipsen’s
investment in the resources necessary to successfully commercialize cabozantinib in the territories where it
is approved; Exelixis’ ability to maintain its rights under the Ipsen collaboration; risks and uncertainties
related to regulatory review and approval processes and Exelixis' compliance with applicable legal and
regulatory requirements; the ability to conduct clinical trials of cabozantinib sufficient to achieve a positive
completion; Exelixis’ ability to judge the proper size and level of experience of the commercialization
teams required to support the launch of cabozantinib for advanced RCC; unanticipated complications
associated with the transition of the COMETRIQ commercialization rights from Sobi to Ipsen; the
availability of data at the referenced times; Exelixis’ ability to protect the company’s intellectual property
rights; market competition; changes in economic and business conditions, and other factors discussed under
the caption “Risk Factors” in Exelixis’ quarterly report on Form 10-Q filed with the Securities and
Exchange Commission (SEC) on November 10, 2015, and in Exelixis’ future filings with the SEC,
including, without limitation, Exelixis’ annual report on Form 10-K expected to be filed with the SEC on
February 29, 2016. The forward-looking statements made in this press release speak only as of the date of
this press release. Exelixis expressly disclaims any duty, obligation or undertaking to release publicly any
updates or revisions to any forward-looking statements contained herein to reflect any change in Exelixis’
expectations with regard thereto or any change in events, conditions or circumstances on which any such
statements are based.
Ipsen Forward-Looking Statement Disclaimer
The forward-looking statements, objectives and targets contained herein are based on the Group’s
management strategy, current views and assumptions. Such statements involve known and unknown risks
and uncertainties that may cause actual results, performance or events to differ materially from those
anticipated herein. All of the above risks could affect the Group’s future ability to achieve its financial
targets, which were set assuming reasonable macroeconomic conditions based on the information available
today. Use of the words "believes," "anticipates" and "expects" and similar expressions are intended to
identify forward-looking statements, including the Group’s expectations regarding future events, including
regulatory filings and determinations. Moreover, the targets described in this document were prepared
without taking into account external growth assumptions and potential future acquisitions, which may alter
these parameters. These objectives are based on data and assumptions regarded as reasonable by the
Group. These targets depend on conditions or facts likely to happen in the future, and not exclusively on
historical data. Actual results may depart significantly from these targets given the occurrence of certain
risks and uncertainties, notably the fact that a promising product in early development phase or clinical trial
may end up never being launched on the market or reaching its commercial targets, notably for regulatory
or competition reasons. The Group must face or might face competition from generic products that might
translate into a loss of market share. Furthermore, the Research and Development process involves several
stages each of which involves the substantial risk that the Group may fail to achieve its objectives and be
forced to abandon its efforts with regards to a product in which it has invested significant sums. Therefore,
the Group cannot be certain that favorable results obtained during pre-clinical trials will be confirmed
subsequently during clinical trials, or that the results of clinical trials will be sufficient to demonstrate the
safe and effective nature of the product concerned. There can be no guarantees a product will receive the
necessary regulatory approvals or that the product will prove to be commercially successful. If underlying
assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from
those set forth in the forward-looking statements. Other risks and uncertainties include but are not limited
E-7
Ipsen Confidential |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
to, general industry conditions and competition; general economic factors, including interest rate and
currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care
legislation; global trends toward health care cost containment; technological advances, new products and
patents attained by competitors; challenges inherent in new product development, including obtaining
regulatory approval; the Group's ability to accurately predict future market conditions; manufacturing
difficulties or delays; financial instability of international economies and sovereign risk; dependence on the
effectiveness of the Group’s patents and other protections for innovative products; and the exposure to
litigation, including patent litigation, and/or regulatory actions. The Group also depends on third parties to
develop and market some of its products which could potentially generate substantial royalties; these
partners could behave in such ways which could cause damage to the Group’s activities and financial
results. The Group cannot be certain that its partners will fulfill their obligations. It might be unable to
obtain any benefit from those agreements. A default by any of the Group’s partners could generate lower
revenues than expected. Such situations could have a negative impact on the Group’s business, financial
position or performance. The Group expressly disclaims any obligation or undertaking to update or revise
any forward looking statements, targets or estimates contained in this press release to reflect any change in
events, conditions, assumptions or circumstances on which any such statements are based, unless so
required by applicable law. The Group’s business is subject to the risk factors outlined in its registration
documents filed with the French Autorité des Marchés Financiers.
Exelixis, the Exelixis logo, and COMETRIQ are registered U.S. trademarks,
and COTELLIC is a U.S. trademark.
# # #
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY
BRACKETS, HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE
COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
Exhibit F
Exelixis [ * ]
[ * ]