Please wait
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
Form 10-K
(Mark One)
|
| |
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2012
OR
|
| |
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 0-27188
Accelrys, Inc.
(Exact name of registrant as specified in its charter)
|
| | |
| | |
Delaware | | 33-0557266 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
10188 Telesis Court, Suite 100, San Diego, California | | 92121 |
(Address of principal executive offices) | | (Zip Code) |
(858) 799-5000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
| | |
| | |
Title of Each Class | | Name of Each Exchange on Which Registered |
Common Stock, par value $0.0001 per share | | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No ý
Note—checking the box above will not relieve any registrant required to file reports pursuant to Section 13 of 15(d) of the Exchange Act from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ¨
Indicate by checkmark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
| | | | |
Large accelerated filer | o | | Accelerated filer | x |
Non-accelerated filer | o | | Smaller reporting company | o |
(Do not check if smaller reporting company) | | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No ý
The aggregate market value of the voting stock held by non-affiliates of the registrant as of June 30, 2012 was $273.3 million (based upon the June 30, 2012 closing price for shares of the registrant’s common stock as reported by the NASDAQ Global Market). Shares of common stock held by each officer, director and holder of 5% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The number of outstanding shares of the registrant’s common stock, par value $0.0001 per share, as of March 1, 2013 was 55,612,893, net of treasury shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of Part II of this Form 10-K and Items 10, 11, 12, 13 and 14 of Part III of this Form 10-K incorporate information by reference from the registrant’s definitive proxy statement filed pursuant to Regulation 14A in connection with the registrant’s 2013 Annual Meeting of Stockholders or an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year covered by this annual report.
The Exhibit Index (Item No. 15) lists several documents incorporated by reference.
ACCELRYS, INC.
FORM 10-K—ANNUAL REPORT
For the Fiscal Year Ended December 31, 2012
Table of Contents
|
| | |
| | |
PART I | | |
Item 1 | Business | |
Item 1A | Risk Factors | |
Item 1B | Unresolved Staff Comments | |
Item 2 | Properties | |
Item 3 | Legal Proceedings | |
Item 4 | Mine Safety Disclosures | |
| | |
PART II | | |
Item 5 | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |
Item 6 | Selected Consolidated Financial Data | |
Item 7 | Management’s Discussion and Analysis of Financial Condition and Results of Operations | |
Item 7A | Quantitative and Qualitative Disclosures About Market Risk | |
Item 8 | Financial Statements and Supplementary Data | |
Item 9 | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | |
Item 9A | Controls and Procedures | |
Item 9B | Other Information | |
| | |
PART III | | |
Item 10 | Directors, Executive Officers and Corporate Governance | |
Item 11 | Executive Compensation | |
Item 12 | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |
Item 13 | Certain Relationships and Related Transactions, and Director Independence | |
Item 14 | Principal Accountant Fees and Services | |
| | |
PART IV | | |
Item 15 | Exhibits, Financial Statement Schedules | |
Signatures | |
Forward-Looking Statements
This Annual Report on Form 10-K (this “Report”) contains “forward-looking statements” that involve risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially and adversely from those expressed or implied by such forward-looking statements. These statements are often identified by the use of words such as “expect”, “believe”, “anticipate”, “estimate”, “intend”, “plan” and similar expressions and variations or negatives of these words. These forward-looking statements may include statements addressing our future financial and operating results. We have based these forward-looking statements on our current expectations about future events. Such statements are subject to certain risks and uncertainties including those related to the execution of our strategic plans, the successful release and acceptance of new products, the demand for new and existing products, additional competition, changes in economic conditions and those described in documents we have filed with the Securities and Exchange Commission (the “SEC”), including this Report in “Management's Discussion and Analysis of Financial Condition and Results of Operations” and “Risk Factors” and subsequent reports on Form 10-Q. All forward-looking statements in this document are qualified entirely by the cautionary statements included in this document and such other filings. These forward-looking statements speak only as of the date of this document. We disclaim any undertaking to publicly update or revise any forward-looking statements contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based.
Unless the context requires otherwise, in this Report the terms “we,” “us” and “our” refer to Accelrys, Inc. and its wholly owned or indirect subsidiaries, and their predecessors.
PART I
Change in Fiscal Year
On August 3, 2010, our board of directors approved a change in our fiscal year end from March 31 to December 31. The fiscal year end change was effective December 31, 2010 and resulted in a nine- month reporting period from April 1, 2010 to December 31, 2010. All references to “years”, unless otherwise noted, refer to the twelve-month fiscal year, which prior to April 1, 2010, ended on March 31, and beginning with January 1, 2011, ends on December 31, of each year.
Overview
On July 1, 2010, pursuant to the terms of the Agreement and Plan of Merger and Reorganization, dated April 5, 2010 (the “Merger Agreement”), by and among us, Alto Merger Sub, Inc., our wholly owned subsidiary (“Symyx Merger Sub”), and Symyx Technologies, Inc. (“Symyx”), Symyx Merger Sub merged with and into Symyx, with Symyx surviving as our wholly owned subsidiary (the “Symyx Merger”). Symyx's operating results are included in our consolidated financial statements and results of operations beginning July 1, 2010.
On May 19, 2011, we completed the acquisition of Contur Software AB (“Contur”), whereby Contur became our wholly owned subsidiary (the “Contur Acquisition”). Contur's operating results are included in our consolidated financial statements and results of operations beginning May 19, 2011.
On December 30, 2011, we completed the acquisition of VelQuest Corporation (“VelQuest”), whereby VelQuest became our wholly owned subsidiary (the “VelQuest Acquisition”). VelQuest's operating results are included in our consolidated financial statements and results of operations beginning December 30, 2011.
On May 17, 2012, we acquired a proprietary web-based Hit Explorer Operating System (“HEOS”) software platform from Scynexis, Inc. The operating results of the HEOS platform are included in our consolidated financial statements and results of operations beginning May 17, 2012.
On October 23, 2012, we completed the acquisition of Aegis Analytical Corporation (“Aegis”), whereby Aegis became our wholly owned subsidiary (the “Aegis Acquisition” and together with the Symyx Merger, the Contur Acquisition, the VelQuest Acquisition and the acquisition of the HEOS platform, the “Acquisitions”). Aegis's operating results are included in our consolidated financial statements and results of operations beginning October 23, 2012.
On January 11, 2013, we completed the acquisition of all of the outstanding shares of Vialis AG (“Vialis”), a joint stock company organized under the laws of Switzerland (the “Vialis Acquisition”). The information presented in this Report does not give effect to the Vialis Acquisition.
Our Business
We develop and commercialize scientific informatics software products and services for industries and organizations that rely on scientific innovation to differentiate themselves in the marketplace. Historically, our products were primarily utilized by our customers' research organizations.
As a result of the Acquisitions, we increased the breadth and depth of our scientific product portfolio by extending our offerings beyond research and into development and manufacturing. In particular, the addition of the VelQuest suite of products gives us even greater capability downstream, extending our solutions into the quality assurance and quality control areas in large pharmaceutical companies. Aegis further expands our portfolio into downstream operations with new solutions for enterprise manufacturing process intelligence. In addition, we added systems integration capabilities with the Vialis Acquisition, which strengthened our position in the laboratory informatics software market and added capabilities in the downstream analytical development, quality control, and quality assurance and manufacturing areas.
The acquisition of the HEOS platform brought a cloud-based offering that enables more efficient and streamlined drug delivery collaborations, particularly with organizations leveraging contract research organizations and other groups for externalized R&D projects.
Collectively, our products and services are intended to optimize our customers' lab-to-commercialization value chains, from early research through development into manufacturing. Our software is used by our customers' scientists, biologists, chemists, engineers and information technology professionals to design, execute and manage scientific experiments in-silico or in the lab and to aggregate, mine, manage, analyze and interactively report on the scientific data from those experiments. Our solutions also enable the development process to scale more effectively and bring increased automation to the transition from development to manufacturing, manage quality control and quality assurance operations, and monitor process and product quality, thereby reducing compliance risk. The ability to integrate and access data from diverse data sources and to make that information accessible throughout the scientific innovation value chain enables our customers to reduce costs, enhance productivity and more efficiently provide effective products to their customers that meet quality and compliance standards.
Today, we are the leading provider of scientific innovation lifecycle management software (SILM). Our customers include leaders from a variety of industries, including pharmaceutical, biotechnology, agricultural, energy, chemicals, aerospace, consumer packaged goods and industrial products, as well as various government and academic entities. We market our software products and services worldwide, principally through our direct sales force, augmented by the use of third party distributors. We are headquartered in San Diego, California and were incorporated in Delaware in 1993.
Description of Our Markets and Business
Our customers differentiate themselves through scientific innovation. As a result, innovation in the discovery, development and manufacturing of new products, compliance with applicable regulations, rapid, cost-effective commercialization of such products and the ability to protect the intellectual property therein is crucial to our customers' success. Therefore, they invest considerable resources in technologies that help identify productive new pathways for research projects, help develop new materials, increase the efficiency of discovery, development and manufacturing processes, and otherwise enable them to maximize the use of scientific data, information and knowledge. Our software solutions allow our customers to effectively design, plan and execute scientific experiments in a repeatable process and in compliance with regulations; leverage the vast amounts of information stored in both corporate databases and public data sources to optimize their processes and accelerate innovation; model, predict and analyze potential scientific outcomes; improve product and process understanding throughout development lifecycles; and access comprehensive, integrated and cross-referenced databases and reference works.
The pharmaceutical and biotechnology industries are a very important part of our business. Our products have been widely adopted within the research functions of businesses in these industries, but less widely adopted by the development, quality assurance (“QA”)/quality control (“QC”) and manufacturing functions of such businesses. In addition, these markets present challenges due to industry consolidation, the maturity of these markets, patent expirations, reduction in the level of discovery research activity, increased competition, including competition from open source software, and outsourcing of research to other entities. The other industries to which we market our products, including energy, material, chemicals, agricultural, aerospace and consumer packaged goods, are earlier in the adoption curve for such scientific software products, which we view as both a challenge and an opportunity.
Business Strategy
Scientific research and development organizations face several challenges that impact their ability to comply with applicable regulations, protect their intellectual property and rapidly and cost-effectively bring products to market. Scientific data is often found in disparate databases and research, development and manufacturing processes are disconnected, manually intensive, inefficient and repetitive. These challenges have made it incredibly difficult for organizations to manage their scientific innovation lifecycles in order to bring novel products to market faster, more efficiently and with lower compliance risk.
Our overall strategy is to deliver solutions that help organizations manage and optimize their scientific innovation lifecycles. Consequently, we are extending beyond our historically strong presence in research downstream into development and manufacturing, covering the entire lab-to-commercialization value chain; moving into new scientific domains, including biology; and expanding our presence outside of the pharmaceutical and biotech industries into other key industries such as food and beverage, fine and specialty chemicals, oil and gas and aerospace. We do this by addressing the core challenges faced by research and development and quality organizations, offering them an open enterprise-scale scientific software platform and a broad portfolio of scientific software applications leveraging our deep domain expertise in chemistry, biology and the materials sciences. We believe the combination of our enterprise platform and associated set of applications and services, such as electronic lab notebooks, the laboratory execution system (“LES”), process management informatics software, design modeling and simulation software, data management and informatics software, content and professional services, help optimize our customers' scientific innovation lifecycle.
We believe that the combination of our products with the products and domain expertise we gained as a result of the Acquisitions (most significantly the VelQuest product suite, and the Aegis process management informatics software) enables us to provide greater value to the development, QA/QC and manufacturing functions of our customers' organizations. Our plan is to continue to integrate and augment our offerings in order to further enhance the value of the products we acquired and the value of our products' collective portfolio to these organizations, thus enabling us to expand upon our presence in the research, development, quality control and quality assurance organizations of our existing customers. The VelQuest Acquisition extended our software portfolio into pharmaceutical development, QA/QC, and manufacturing, offering significant productivity improvements, faster cycle times, lower operational costs and reduced compliance risks for regulated life sciences organizations. The Aegis Acquisition brought products that complement the Velquest product line by expanding our footprint in downstream operations with solutions for process intelligence. We also intend to continue to develop advanced analysis, scientific and reporting component collections in order to extend our platform's value to and use by our customers.
Our strategy also includes offering professional services to further tailor our enterprise platform to our customers' individual business needs, thereby increasing its utility and value. Because our enterprise platform is the underlying operating platform for many products in our broad portfolio, and integration with the applications obtained as part of the Acquisitions continues to be a development priority, we expect the use of these products to expand as the use of our platform grows, thus further increasing our sales and value to our customers. Our acquisition of Vialis, a leading systems integrator based in Liestal, Switzerland, added new services capabilities to our portfolio and further strengthened our position in the laboratory informatics software market
Our enterprise platform is an open, scientifically aware platform. We partner with third party organizations and academic institutions which develop scientific software and services, and we enable and encourage these companies to develop applications that operate on our platform, further proliferating its utility and value to our customers.
We also focus on industries in markets where scientific innovation is a key differentiator, but the use of scientific software solutions has not been widely adopted. As we develop a greater presence in these markets, we believe our ability to attract additional customers will increase.
Products and Services
The following is a brief description of each of our product lines:
Accelrys Enterprise Platform and Pipeline Pilot. Our enterprise platform forms the basis of our software and services offerings and is central to our strategy to develop and deliver scientific innovation lifecycle management software. Its open, scientifically aware service-oriented architecture allows users to integrate and deploy broad scientific solutions spanning data management and informatics, enterprise lab management, modeling and simulation, and workflow automation. The Accelrys Enterprise Platform leverages our deep domain expertise in chemistry, biology and materials science, informatics, predictive science and electronic laboratory management, which includes late-stage laboratory execution for regulated industries. The enterprise
platform can manage structured and unstructured information encountered early in the innovation cycle, as well as highly structured, automated batch records required by lock-down production.
Additionally, the enterprise platform filters, normalizes and performs statistical analysis on scientific data and provides interactive visual reports to both scientists and scientific managers. We continue to develop component collections that operate with our enterprise platform to offer our customers advanced scientific functions such as image, text and chemical analysis. Our enterprise platform provides the underlying platform on which we have built many of our modeling and simulation and informatics solutions. We are integrating many of the products we have acquired in order to enhance the value of both these products and our platform.
The openness of the enterprise platform allows our users to integrate not only Accelrys solutions, but also best of breed scientific applications they have built themselves, or solutions they have acquired from academic collaborators or other commercial independent software vendors (“ISVs”). We have built an ecosystem of scientific innovation by partnering with many ISVs who build scientific and technology solutions based on our enterprise platform, including downstream enterprise resource planning and product lifecycle management systems. The enterprise platform's ability to integrate with existing infrastructure, as well as handle both structured and unstructured scientific data, enables organizations to gain efficiencies from their legacy systems, reduce complexity and improve productivity from innovation through commercialization.
Modeling and Simulation Software. We have a broad array of software products based on proprietary and licensed technologies that employ fundamental scientific principles, advanced computer visualization, molecular modeling techniques and computational chemistry. These products allow scientists to perform computations of chemical, biological and materials properties, to simulate, visualize and analyze chemical and biological systems, and to communicate the results to other scientists. Our modeling and simulation products are used by scientists in the life science industry, primarily by pharmaceutical and biotechnology companies, and scientists in materials sciences industries, such as energy, chemicals, aerospace and consumer packaged goods companies.
Discovery Studio. Our Discovery Studio life science modeling and simulation application features an open architecture allowing users to “plug and play” best of breed computational solutions from Accelrys, academia, other ISVs or solutions the customers have developed themselves. This open architecture also helps expert modelers to connect and communicate more effectively to the wider research organization. Our modeling and simulation offerings are intended to assist our customers in the discovery of new materials or the enhancement of existing materials.
Materials Studio. Our Materials Studio modeling and simulation application enables our customers to introduce innovation in their products by engineering new, high-performance materials. Materials Studio is now integrated with our enterprise platform to enable customers to extend scientific capabilities and deploy modeling and simulation to the enterprise.
Process Management and Compliance Software. We have software solutions that support rapid process development, optimization, validation and scale-up all the way to highly efficient process execution for manufacturing and product commercialization. These solutions connect the lab to plant workflows and information across the innovation and commercialization life cycle. Scientists can rapidly explore and document early investigations while developing new methods, recipes, and processes with domain-specific templates and capabilities for process, formulation, and analytical scientists. Once new operating procedures have been developed, these can be passed into a lock-down automated environment for right-first-time compliant execution by analysts, operators and engineers in QA/QC and manufacturing operations.
Enterprise Lab Notebooks. Electronic lab notebooks (“ELN”) replace paper notebooks traditionally used by scientists, providing a digital environment in which scientists can plan, execute, record, store, back-up and share their daily research activities. Our ELNs in combination with the Accelrys Enterprise Platform offer scientists the opportunity to streamline the processes associated with experiment planning, design, execution and analysis by standardizing on a single notebook that handles multiple scientific disciplines and bringing the scientific analysis and reporting capabilities from the Pipeline Pilot platform into the notebook. We currently have two ELNs, as described below, and we continue to develop domain-specific capabilities for scientists engaged in discovery, process, analytical, formulations and biology functions that are designed with their specific functions in mind.
*The Accelrys ELN is designed to be used across multiple scientific disciplines- from discovery through manufacturing - and is particularly suited for regulated environments.
*The Contur ELN is an easy-to-deploy, easy-to- use ELN that is for organizations focused on intellectual property capture and information sharing, process documentation and overall lower total cost of ownership. It is offered as both an enterprise level on-premise server-based installation (Accelrys iLabber Server) and via a SaaS model (Accelrys iLabber Cloud), which is targeted for the small- and medium-sized business and academic markets.
Whether customers deploy the Accelrys ELN or the Contur ELN, they see significant productivity gains resulting from the development of domain-specific capabilities that optimize the use of research data across the scientific enterprise, improve data and documentation quality and ensure compliance with standard operating procedures.
Accelrys Lab Execution System (“Accelrys LES”). The VelQuest Acquisition allowed us to broaden our solutions in the development and manufacturing segments. The Accelrys LES (formerly VelQuest SmartLab) provides an electronic environment removing paper process and automating the interaction with methods, instruments, and supplies that scientists and analysts use every day in regulated QA and QC environments. By automating the execution of procedures, the Accelrys LES removes paper-based inefficiencies, increases resources to improve capacity, eliminates transcription and calculation errors that are common in paper notebooks and logbooks, and accelerates reporting and review cycles. The Accelrys LES is included in the Accelrys Process Management and Compliance Suite, a comprehensive informatics platform for capturing, managing, and analyzing development and process data for operational excellence in new product development and commercial quality operations.
Accelrys Electronic Batch Records (“Accelrys EBR”). The Acclerys EBR (formerly VelQuest SmartBatch EBR) is an out-of-the-box procedure execution and electronic batch record software system designed to provide a low cost of ownership electronic environment to efficiently and compliantly document the execution of any procedure or recipe. Accelrys EBR is designed for organizations that want improved batch record keeping and compliance without the large overheads of a complex manufacturing execution system.
Accelrys Discoverant. The Aegis Acquisition added the Discoverant solution, a process management informatics software that aggregates, contextualizes and analyzes manufacturing, quality and product development data, enabling quality and manufacturing organizations to gain visibility to their process data to improve overall process understanding.
Content Databases. To support their research activities, scientists can access our comprehensive, integrated and cross-referenced collection of factual databases and reference works covering bioactivity, chemical sourcing, molecular properties, synthetic methodology, metabolism and toxicology information. These resources can be accessed easily through the ELN, data management and informatics applications or via the Internet through the Discovery Gate portal and chemicals marketplace.
Data Management & Informatics Software. We are a leading provider of software-based solutions designed to capture, store, manage and mine scientific data information. Our data management and informatics software is based on standard database architectures such as Oracle ®. As leaders in the space, we have developed a go-forward roadmap that leverages the best technology from each of the product portfolios gained through the acquisitions over the past few years. This roadmap preserves our customers' investments, while offering a solution that addresses the needs of today's scientific organizations. Through this combined portfolio, we provide comprehensive data visualization and analysis software components based on a services oriented architecture enabling users to search, retrieve and use chemical structures, biological and chemical data, experimental data and registration information. Many of these tools use industry standard software components and are compatible with applications such as Microsoft Excel ® and Microsoft SharePoint ® , allowing chemists to interact with chemical data within familiar productivity tools. We also use this component technology, combined with our enterprise platform and our database architectures, to build enterprise-wide systems for our clients.
Services. The value of our software is enhanced by robust service offerings that provide a variety of options to our clients and create greater returns on their investments.
Our professional services team delivers integrated scientific solutions and enhances the value of our software products by creating extensions to functionality to address a client's specific business needs. Our scientific informatics platform is highlighted as one of the primary technologies used in these solutions. The group delivers a range of solutions, from software wrappers that allow customers to run their own algorithms on our enterprise platform to enterprise-wide informatics systems that integrate customers' internal systems with software from many vendors, including Accelrys. The group specializes in deploying systems for use by customers in all scientific domains, from early research to early product manufacturing.
Our contract research services team provides solutions to clients' proprietary business-critical scientific problems. The world-wide client base for this service ranges from those who do not have expert modeling resources on staff to those who have expert modelers, but need to accommodate peaks in resource requirements or to fill a special skill set. We work on a project-by-project basis, or provide longer term resources that are located at client sites for a year or more. This scientific collaboration enhances our partnerships with our clients and helps them achieve their research goals. Our value is enhanced by the broad set of scientific domain expertise among our staff, our years of experience solving industrial “real-world” application problems, our commitment to maintaining confidentiality and to protecting our clients' intellectual property, the accessibility we offer to the extensive Accelrys ecosystem and all of our modeling tools.
Both internally and through our distribution channels, we offer training conducted by staff knowledgeable in both the theory and application of our products. Our implementation and training services ensure that our clients are able to quickly install our products and become expert users of our solutions. We offer onsite training and implementation services, web-based training and data migration services.
Our support services give customers access to new releases, technical notes, documentation addenda and other support which collectively enable customers to utilize our products more effectively, including access to our technical and scientific support personnel during extended business hours. Technical newsletters and bulletins are sent to customers to keep them informed and to help them with resource allocation and scheduling. To maintain an ongoing understanding of customer requirements, we hold user group meetings throughout the year.
We are committed to providing customers with superior support, including: telephone, e-mail, fax and Internet-based technical support services; training; user group conferences; and targeted contract services involving application of our technology and scientific expertise to particular research needs of customers. We believe that customer service, support and training are part of the successful adoption and utilization of our products.
Customers
Our customer base consists of companies that differentiate themselves from the competition on the basis of scientific innovation. These customers include commercial, governmental and academic organizations, including many of the world's largest pharmaceutical, biotechnology, energy, chemicals, aerospace, consumer packaged goods and industrial products companies. No single customer accounted for more than 10% of our revenue during any of the three most recently completed fiscal years. Our three largest customers accounted for approximately 11% of total revenue during the year ended December 31, 2012.
Strategic and Academic Alliances
We have entered into a number of strategic alliances relating to product development, product distribution and joint marketing. We plan to continue to cultivate relationships with academic, governmental and commercial research organizations for purposes of identifying and licensing new technology to use in product development. In addition, we plan to maintain and expand our alliances focusing on the compatibility of our products with databases and database management systems, other computational chemistry and molecular simulation products, and products in related markets such as imaging and clinical informatics. We also intend to continue to enter into integration and joint marketing arrangements with systems integrators and original equipment manufacturer partners.
Sales and Marketing
We market our products and services worldwide. During the year ended December 31, 2012, we generated 49%, 28% and 23% of our revenues from the U.S., Europe and the Asia/Pacific region, respectively. Please refer to Note 1 to our consolidated financial statements included elsewhere in this Report for a breakdown of revenues and long-lived assets by geographic region. Our continuing reliance on sales in international markets exposes us to risks attendant to foreign sales, as described more fully in Item 1A of this Report.
We sell our products and services through our direct sales force, telesales and, in some markets, arrangements with distributors. Our direct sales representatives focus on a defined list of customers or cover an assigned geographic territory. These direct sales representatives typically work closely with our pre-sales scientists in order to demonstrate our products and their applicability to various research and development efforts. Our telesales effort is directed at smaller sized commercial accounts and academic institutions. Our distributor relationships primarily exist in the Asia/Pacific region, specifically in Japan, China and Korea, and complement our direct sales force in those markets.
In support of our sales activities, we participate in industry trade shows, publish our own newsletter, advertise in industry publications and via Internet search engines, publish articles in industrial and scientific publications, conduct direct e-mail campaigns, sponsor industry conferences and seminars, and maintain a website that contains information about us and our product and service offerings.
Our products, together with the associated product literature, are generally delivered to our customers at the time of placing and processing their order. Our electronic software distribution program allows our customers to download our products over the Internet.
Our customers' buying habits have historically resulted in a higher concentration of sales in the fourth quarter of the calendar year, due to traditionally higher purchasing activity in the month of December in many commercial organizations.
Product Development
Development of our software is focused on expanding product lines, designing enhancements to our core technology and integrating existing and new products into our principal software architecture and platform technology. We intend to offer regular updates to our products and to continue to look for opportunities to expand our existing product suite.
We develop most of our products internally and, during the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010, we incurred product development expenses of $38.8 million, $34.0 and $22.1 million, respectively. We have also licensed products or have otherwise acquired products, or portions of our products, from the open source community, as well as corporate, governmental and academic institutions. These arrangements sometimes involve joint development efforts and frequently require the payment of royalties by us. The development and royalty obligations, scope of distribution rights, duration and other terms of these arrangements vary depending on the product, the market, resource requirements, the other parties with which we contract and other factors. We intend to continue to license or otherwise acquire technology or products from third parties.
Competitors
We believe our scientific informatics platform and our approach to managing the scientific innovation lifecycle is unique, and that the market for the platform is at an early stage, with limited established competition from commercial software vendors. We also believe that industry trends such as externalization, and increased cost and regulatory pressures will continue to drive organizations to optimize their lab-to-commercialization value chains and to seek ways to better innovate. The competition for our enterprise platform includes our customers' own proprietary software, open source software such as Knime, and workflow and data-pipeline applications of smaller commercial software vendors such as IDBS.
The markets for our informatics software products and our modeling and simulation products for the pharmaceutical and biotechnology industries are mature and intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. Our competitors offer a variety of products and services to address this market. We believe that the principal competitive factors in this market are product quality, flexibility, ease-of-use, scientific validation and performance, functionality and features, open architecture, quality of support and service, reputation and price. Competition currently comes from open source software, our customers' internal tools, and the following commercial software vendors (listed by product type and competitors that supply such products): other software packages for analysis of chemical and biological data (IDBS); desktop and web-based software applications (Perkin Elmer/CambridgeSoft and Tripos), including chemical drawing applications (Perkin Elmer/CambridgeSoft); molecular modeling and analytical data simulation applications (Chemical Computing Group, OpenEye, Schrodinger and Tripos); and database management systems and information technology (IDBS).
Our ELN and LES solutions face competition from commercial software vendors in two primary areas: enterprise-wide deployments of ELN or laboratory information management systems (Perkin Elmer/CambridgeSoft, IDBS, LabWare, LabVantage, Waters); and smaller, department-focused initiatives (Perkin Elmer/CambridgeSoft, IDBS and Recentris).
The market for our modeling and simulation offerings aimed at the energy, chemicals, aerospace and consumer packaged goods industries is in its early stages with limited and fragmented competition from established software vendors. The industries that these offerings target generally rely on data from experiments, rather than utilizing computer aided design modeling and simulation software to obtain such data. Our limited competition from commercial software vendors in these industries currently comes from smaller competitors such as Gaussian Inc., Materials Design Inc., Schrödinger Atomistix and Scienomics.
Backlog of Committed Revenue
As of December 31, 2012, our backlog was approximately $153.7 million, compared with $145.1 million as of December 31, 2011. We expect to recognize approximately $124.2 million of our December 31, 2012 backlog during fiscal year 2013. Our backlog consists of contractual commitments from our customers for products to be shipped, research, consulting and maintenance services to be provided, and license and content revenue to be recognized. Because of the occasional customer-driven changes in delivery schedules or cancellation of orders without significant penalty, we do not believe our backlog, as of any particular date, is necessarily indicative of actual revenue for any future period.
Intellectual Property and Other Proprietary Rights
We rely primarily on a combination of copyright, trademark and trade secret laws, confidentiality procedures and contractual provisions to protect our proprietary rights. We also have 127 U.S. and foreign patents and several pending patent applications. We believe that factors such as the technological and creative skills of our personnel, new product development, frequent product enhancements, name recognition and reliable product maintenance are essential to establishing and maintaining a technological leadership position. There can be no assurance that our means of protecting our proprietary rights in the U.S. or abroad will be adequate. We seek to protect our software, documentation and other written materials under trade secret and copyright laws, which afford only limited protection. Further, there can be no assurance that our patents will offer any protection or that they will not be challenged, invalidated or circumvented.
Employees
As of December 31, 2012, we had 647 full-time employees. None of our employees are covered by collective bargaining agreements. Substantially all of our employees, other than certain of our executive officers and employees with customary employment arrangements within Europe and Asia, are at will employees, which means that each employee can terminate his or her relationship with us and we can terminate our relationship with him or her at any time. We offer industry competitive wages and benefits and are committed to maintaining a workplace environment that promotes employee productivity and satisfaction. We believe our relations with our employees are good.
Website Access to SEC Filings
We are subject to the reporting requirements under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Consequently, we are required to file reports and information with the SEC, including reports on the following forms: annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. These reports and other information concerning us may be accessed through the SEC’s website at http://www.sec.gov and on our website at www.accelrys.com. Such filings are placed on our website as soon as reasonably practical after they are filed with the SEC. Any information contained in, or that can be accessed through our website, is not incorporated by reference into, nor is it in any way part of this Report.
You should carefully consider the risks described below before investing in our publicly traded securities. The risks described below are not the only ones facing us. Our business is also subject to the risks that affect many other companies, such as competition, technological obsolescence, labor relations, general economic conditions, geopolitical changes and international operations. Additional risks not currently known to us or that we currently believe are immaterial also may impair our business operations and our liquidity. The risks described below could cause our actual results to differ materially from those contained in the forward-looking statements we have made in this Report, the information incorporated herein by reference and those forward-looking statements we may make from time to time.
Certain Risks Related to Our Marketplace and Environment
Our revenue from the sale of computer aided design modeling and simulation software to the life science discovery research marketplace, which has historically constituted a significant portion of our overall revenue, has been declining over the past several years and may continue to decline in future years. Historically we have derived a significant portion of our revenue from the sale of computer aided design modeling and simulation software to the discovery research departments in pharmaceutical and biotechnology companies. Based on recurring analyses of incoming orders typically conducted by our sales and marketing departments, we estimate that orders for our computer aided design modeling and simulation software for the life science industry declined approximately 18% between fiscal year 2008 and 2009, approximately 16% between fiscal year 2009 and 2010, approximately 21% during the nine months ended December 31, 2010. Although revenues remained relatively constant for the years ended December 31, 2012 and December 31, 2011, we may experience a decline in revenues in the future. While there is not a linear correlation between product orders and resulting revenue, we believe that our estimates of decreased product orders ultimately reflect a decline in revenue attributable to sales of our computer aided design modeling and simulation software products. We believe this decline is due to several factors, including industry consolidation, a general reduction in the level of discovery research activity by our customers, increased competition, including competition from open source software, and reductions in profit and related information technology spending by our customers. If such declines continue and we do not increase the revenue we derive from our other product and service offerings, our business could be adversely impacted.
Our ability to sustain or increase revenues will depend upon our success in entering new markets and in deriving additional revenues from our existing customers. Our products are currently used primarily by molecular modeling and simulation specialists in discovery research organizations. One component of our overall business strategy is to derive more revenues from our existing customers by expanding their usage of our products and services. Such strategy would have our customers utilize our scientific informatics platform and our tools and components to leverage vast amounts of information stored in both corporate databases and public data sources in order to make informed scientific and business decisions during the research and development process. In addition, we seek to expand into new markets and new areas within our existing markets by acquiring businesses in these markets, attracting and retaining personnel knowledgeable in these markets, identifying the needs of these markets and developing marketing programs to address these needs. If successfully implemented, these strategies would increase the usage of our software and services by biologists, chemists, engineers and informaticians operating within our existing pharmaceutical and biotechnology customers, as well as by new customers in other industries. However, if our strategies are not successfully implemented, our products and services may not achieve market acceptance or penetration in targeted new departments within our existing customers or in new industries. As a result, we may incur additional costs and expend additional resources without being able to sustain or increase revenue.
Our focus on offering scientific business intelligence solutions to both existing and new customers and markets may make it more difficult for us to sustain our revenue from the sale of computer aided design modeling and simulation products to the life science discovery research marketplace. Our strategy involves transforming our product and service offerings by utilizing our scientific informatics platform and our tools and components in order to enable our customers to more effectively utilize the vast amounts of information stored in both their databases and public data sources in order to make informed scientific and business decisions during the research and development process. This strategy is intended to lead us to new and different markets and customers, as well as increase the usage of our offerings by existing customers. Executing this strategy will require significant management focus and utilization of resources. Though we intend to continue to dedicate sufficient resources and management focus to our life science computer aided design modeling and simulation products, it is possible that the strategy will result in loss of management focus and resources relating to these existing products and markets, thereby resulting in decreasing revenues from these markets. If these revenues are not offset by increasing revenues from new markets and/or customers, our overall revenues will suffer.
We may be unable to develop strategic relationships with our customers. Our overall business strategy is to expand usage of our products and services by expanding our current customers' usage of our products and by marketing and distributing our solutions to a broader, more diversified group of biologists, chemists, engineers and informaticians operating throughout our customers' research and development organizations. A key component of this strategy is to become a preferred provider of scientific software and solutions. Becoming a preferred provider will require substantial re-training and new skills development within our sales and service personnel and deployment of a successful account-management sales model. We believe that developing strategic relationships with our customers may lead to additional revenue opportunities. However, executing this strategy may require significant expense, and there can be no assurance that any such relationships will develop or that such relationships will produce additional revenue or profit opportunities.
Consolidation within the pharmaceutical and biotechnology industries may continue to lead to fewer potential customers for our products and services. A significant portion of our customer base consists of pharmaceutical and biotechnology companies. Continued consolidation within the pharmaceutical and biotechnology industries may result in fewer customers for our products and services. If one of the parties to a consolidation uses the products or services of our competitors, we may lose existing customers as a result of such consolidation.
Increasing competition and increasing costs within the pharmaceutical and biotechnology industries may affect the demand for our products and services, which may affect our results of operations and financial condition. Our pharmaceutical and biotechnology customers' demand for our products is impacted by continued demand for their products and by our customers' research and development costs. Demand for our customers' products could decline, and prices charged by our customers for their products may decline, as a result of increasing competition, including competition from companies manufacturing generic drugs. In addition, our customers' expenses could continue to increase as a result of increasing costs of complying with government regulations and other factors. A decrease in demand for our customers' products, pricing pressures associated with the sales of these products and additional costs associated with product development could cause our customers to reduce research and development expenditures. Because our products and services depend on such research and development expenditures, our revenues may be significantly reduced.
Health care reform and restrictions on reimbursement may affect the pharmaceutical, biotechnology and industrial chemical companies that purchase or license our products or services, which may affect our results of operations and financial condition. The continuing efforts of government and third party payers in the markets we serve to contain or reduce the cost of health care may reduce the profitability of pharmaceutical, biotechnology and industrial chemical companies causing them to reduce research and development expenditures. Because some of our products and services depend on such
research and development expenditures, our revenues may be significantly reduced. We cannot predict what actions federal, state or private payers for health care goods and services may take in response to any health care reform proposals or legislation.
We face strong competition in the life science market for computer aided design modeling and simulation software and for cheminformatics products. The market for our computer aided design modeling and simulation software products for the life science market is intensely competitive. We currently face competition from other scientific software providers, larger technology and solutions companies, in-house development by our customers and academic and government institutions and the open source community.
Some of our competitors and potential competitors in this sector have longer operating histories than we do and could have greater financial, technical, marketing, research and development and other resources. Many of our competitors offer products and services directed at more specific markets than those we target, enabling these competitors to focus a greater proportion of their efforts and resources on these markets. Some offerings that compete with our products are developed and made available at lower cost by government organizations and academic institutions, and these entities may be able to devote substantial resources to product development and also offer their products to users for little or no charge. We also face competition from open source software initiatives, in which developers provide software and intellectual property free over the Internet. In addition, many of our customers spend significant internal resources in order to develop their own software. Moreover, we intend to leverage our scientific informatics platform in order to enable our customers to more effectively utilize the vast amounts of information stored in both their databases and public data sources in order to make informed scientific and business decisions during the research and development process. This strategy could lead to competition from much larger companies which provide general data storage and management software.
There can be no assurance that our current or potential competitors will not develop products, services or technologies that are comparable to, superior to or render obsolete, the products, services and technologies we offer. There can be no assurance that our competitors will not adapt more quickly than us to technological advances and customer demands, thereby increasing such competitors' market share relative to ours. Any material decrease in demand for our technologies or services may have a material adverse effect on our business, financial condition and results of operations.
We are subject to pricing pressures in some of the markets we serve. The market for computer aided design modeling and simulation products for the life science industry is intensely competitive, which has led to significant pricing pressure and declines in average selling price over the past several years. Based on recurring analyses of incoming orders typically conducted by our sales and marketing departments, we estimate that the average sales price of our computer aided design modeling and simulation products for the life science industry declined approximately 10% between fiscal year 2008 and 2009 and approximately 14% between fiscal year 2009 and 2010. Although the average price remained relatively constant for the years ended December 31, 2012 and December 31, 2011, we may experience a decline in the future. While there is not a linear correlation between our product pricing and competition in the marketplace, we believe that our estimates of decreased prices ultimately reflect increased competition that has led and may continue to lead to pricing pressure with respect to sales of these products. In response to such increased competition and general adverse economic conditions in this market, we may be required to further modify our pricing practices. Changes in our pricing model could adversely affect our revenue and earnings.
Revenue from our database content business has been declining and may continue to decline. As a result of the Symyx Merger, we have acquired a database content business. This business faces intense competition from both third parties and the increasing availability of freely-available content databases. As a result, revenue from this database content business has been declining. If we are unable to reverse the decline in this business or to increase revenues from our other businesses in order to offset the decline, our revenues and results of operations may suffer.
Our operations may be interrupted by the occurrence of a natural disaster or other catastrophic event at our primary facilities. Our research and development operations and administrative functions are primarily conducted at our facilities in San Diego, California, San Ramon, California, Bend, Oregon, Milford, Massachusetts, Lafayette, Colorado, Durham, North Carolina and Cambridge, United Kingdom. We also conduct sales and customer support activities at our facilities in Santa Clara, California, Bedminster, New Jersey, Burlington, Massachusetts, Paris, France, Basel, Switzerland, Cologne, Germany, Stockholm, Sweden Bangalore, India and Tokyo, Japan. Although we have contingency plans in effect for natural disasters or other catastrophic events, the occurrence of such events could still disrupt our operations. For example, our San Diego, San Ramon, and Tokyo facilities are located in areas that are particularly susceptible to earthquakes. Any natural disaster or catastrophic event in our facilities or the areas in which they are located could have a significant negative impact on our operations.
Our insurance coverage may not be sufficient to avoid material impact on our financial position or results of operations resulting from claims or liabilities against us, and we may not be able to obtain insurance coverage in the future.
We maintain insurance coverage for protection against many risks of liability. The extent of our insurance coverage is under continuous review and is modified as we deem it necessary. Despite this insurance, it is possible that claims or liabilities against us may have a material adverse impact on our financial position or results of operations. In addition, we may not be able to obtain any insurance coverage, or adequate insurance coverage, when our existing insurance coverage expires. For example, we do not carry earthquake insurance for our facilities in Tokyo, Japan, San Ramon, California, or San Diego, California, because we do not believe the costs of such insurance are reasonable in relation to the potential risk.
Certain Risks Related to Our Operations
Defects or malfunctions in our products could hurt our reputation among our customers, result in delayed or lost revenue and expose us to liability. Our business and the level of customer acceptance of our products depend upon the continuous, effective and reliable operation of our software and related tools and functions. To the extent that defects cause our software to malfunction and our customers' use of our products is interrupted, our reputation could suffer and our revenue could decline or be delayed while such defects are remedied. We may also be subject to liability for the defects and malfunctions of third party technology partners and others with whom our products and services are integrated.
Delays in the release of new or enhanced products or services or undetected errors in our products or services may result in increased cost to us, delayed market acceptance of our products and delayed or lost revenue. To achieve market acceptance, new or enhanced products or services can require long development and testing periods, which may result in delays in scheduled introduction. Any delays in the release schedule for new or enhanced products or services may delay market acceptance of these products or services and may result in delays in new customer orders for these new or enhanced products or services or the loss of customer orders. In addition, new or enhanced products or services may contain a number of undetected errors or “bugs” when they are first released. Although we test each new or enhanced software product or service before it is released to the market, there can be no assurance that significant errors will not be found in existing or future releases. As a result, in the months following the introduction of certain releases, we may need to devote significant resources to correct these errors. There can be no assurance, however, that all of these errors can be corrected.
We are subject to risks associated with the operation of a global business. We derive a significant portion of our total revenue from our operations in international markets. During the years ended December 31, 2012 and 2011, and the nine months ended December 31, 2010, 51%, 53% and 51% respectively, of our total revenue was derived from our international operations. Our global business may be affected by local economic conditions, including inflation, recession and currency exchange rate fluctuations. In addition, political and economic changes throughout the world may interfere with our or our customers' activities in particular locations and result in a material adverse effect on our business, financial condition and operating results. We anticipate that revenue from operations in international markets will continue to account for a significant percentage of future revenue. The following table depicts our region-specific revenue as a percentage of total revenue for the periods referenced:
|
| | | | | | | | | |
| Year Ended | | Year Ended | | Nine Months Ended | |
Region | December 31,
2012 | | December 31,
2011 | | December 31,
2010 | |
U.S. | 49 | % | | 47 | % | | 49 | % | |
Europe | 28 | % | | 29 | % | | 28 | % | |
Asia-Pacific | 23 | % | | 24 | % | | 23 | % | |
Other potential risks inherent in our international business include:
| |
• | unexpected changes in regulatory requirements; |
| |
• | currency exchange rate fluctuations; |
| |
• | import and export license requirements; |
| |
• | tariffs and other barriers; |
| |
• | political unrest, terrorism and economic instability; |
| |
• | disruption of our operations due to local labor conditions; |
| |
• | limited intellectual property protection; |
| |
• | difficulties in collecting trade receivables; |
| |
• | difficulties in managing distributors or representatives; |
| |
• | difficulties in managing an organization spread over various countries; |
| |
• | difficulties in staffing foreign subsidiary or joint venture operations; and |
| |
• | potentially adverse tax consequences. |
Our success depends, in part, on our ability to anticipate and address these risks. There can be no assurance that we will do so effectively, or that these or other factors relating to our international operations will not adversely affect our business or operating results.
In order to improve our financial position, we have reduced our headcount, both prior to and as a result of the Symyx Merger, which could negatively impact our business. We have undergone several reductions-in-force over the past several years, and our workforce has declined approximately 25% from March 31, 2006 to July 1, 2010 when we completed the Symyx Merger. In addition, as a result of the Symyx Merger, we implemented a reduction in force of approximately 15% of the combined employee workforce after the Symyx Merger. We do not believe that these reductions have had a material adverse effect on our operations or our ability to generate revenue to date. However, while we believe we have sufficient staff to operate our business, we do not have duplicative or redundant resources in many of our functions or operations. As a result, there can be no assurance that further attrition will not impact our ability to operate our business, or adversely impact our revenues.
Failure to attract and retain skilled personnel could have a material adverse effect on us. Our success depends in part on the continued service of key scientific, sales, business development, marketing, engineering, management and accounting personnel and our ability to identify, hire and retain additional personnel. There is intense competition for qualified personnel. Immigration laws may further restrict our ability to attract or hire qualified personnel. We may not be able to continue to attract and retain the personnel necessary for the development of our business. Failure to attract and retain key personnel could have a material adverse effect on our business, financial condition and results of operations. Further, we are highly dependent on the principal members of our technical, scientific and management staff and certain groups of consultants. One or more of these key employees or consultants could retire or otherwise leave our employ within the foreseeable future, and the loss of any of these people could have a material adverse effect on our business, financial condition or results of operations. We do not intend to maintain key person life insurance on the life of any employee.
Continued turmoil in the worldwide financial markets may negatively impact our business, results of operations, and financial condition. As widely reported, financial markets in the U.S., Europe and Asia have been experiencing disruption in recent years, including, among other things, volatility in security prices, declining valuations of certain investments, severely diminished liquidity and credit availability, the failure or sale of various financial institutions and an unprecedented level of government intervention. Many economists believe that the U.S. economy, and possibly the global economy, is in the midst of a prolonged recession. This protracted downturn may hurt our business in a number of ways, including through general decreases in spending and the adverse impact on our customers' ability to obtain financing, which may lead to delays or failures in our signing customer agreements or signing customer agreements at reduced purchase levels. While we are unable to predict the likely duration and severity of the current disruption in the financial markets and the adverse economic conditions in the U.S. and other countries, any of the circumstances mentioned above could have a material adverse effect on our revenues, financial condition and results of operations.
Recent acquisitions and potential future acquisitions could prove difficult to integrate, disrupt our business, dilute stockholder value and strain our resources. Since July 2010, we have acquired five companies: Symyx, Contur , VelQuest, Aegis and Vialis. We continually evaluate opportunities to acquire new businesses as part of our ongoing strategy and we may in the future acquire additional businesses that we believe could complement or expand our business or increase our customer base. We do not know if we will be able to complete any future acquisitions, or whether we will be able to successfully integrate any acquired businesses, operate them profitably or retain their key employees. Integrating the operations of acquired businesses successfully or otherwise realizing any of the anticipated benefits of any acquisitions, including anticipated cost savings and additional revenue opportunities, involves a number of potential challenges. The failure to meet these integration challenges could seriously harm our financial condition and results of operations. Realizing the benefits of our acquisitions or any future acquisitions depends in part on the integration of operations and personnel. These integration activities are complex, expensive and time-consuming and we may encounter unexpected difficulties or incur unexpected costs, including:
| |
• | our inability to achieve the operating synergies anticipated in the acquisitions; |
| |
• | lost sales and customers as a result of our customers or customers of any acquired company deciding not to do business us; |
| |
• | complexities associated with managing the larger, more complex, combined business; |
| |
• | integrating personnel from acquired companies while maintaining focus on providing consistent, high quality products; |
| |
• | potential unknown liabilities and unforeseen expenses, delays or regulatory conditions associated with acquisitions; and |
| |
• | diversion of management attention from ongoing business concerns to integration matters. |
Acquired businesses may have liabilities or adverse operating issues that we fail to discover through due diligence prior to the acquisition. In particular, to the extent that prior owners of any acquired businesses or properties failed to comply with or otherwise violated applicable laws or regulations, or failed to fulfill their contractual obligations, we, as the successor owner, may be financially responsible for these violations and failures and may suffer reputational harm or otherwise be adversely affected. Acquisitions also frequently result in the recording of goodwill and other intangible assets which are subject to potential impairment in the future that could harm our financial results. As a result, if we fail to properly evaluate acquisitions or investments, we may not achieve the anticipated benefits of any such acquisitions, and we may incur costs in excess of what we anticipate.
In addition, in order to finance any acquisition, we may utilize our existing funds, thereby lowering the amount of funds we currently have, or might need to raise additional funds through public or private equity or debt financings. Prolonged tightening of the financial markets may impact our ability to obtain financing to fund future acquisitions and we could be forced to obtain financing on less than favorable terms. Additionally, equity financings may result in dilution to our stockholders, which could affect the market price of our stock.
Our ability to utilize our net operating loss (“NOL”) and tax credit carryforwards in the future is limited by Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (the “Code”). In general, under Sections 382 and 383 of the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change NOL and tax credit carryforwards to offset future taxable income. In general, an ownership change occurs if the aggregate stock ownership of certain stockholders increases by more than 50 percentage points over such stockholders' lowest percentage ownership during the testing period (which is generally three years). The amount of the annual limitation is generally equal to the value of the stock of the corporation immediately prior to the ownership change, multiplied by the adjusted federal tax-exempt rate set by the Internal Revenue Service.
As a result of the Symyx Merger, Accelrys and Symyx have undergone an “ownership change” for purposes of Section 382 of the Code. Accordingly, the combined company's ability to utilize Accelrys' and Symyx's NOLs and tax credit carryforwards will be limited as described in the preceding paragraph. These limitations could in turn result in increased future tax payments for the combined company, which could have a material adverse effect on the business, financial condition or results of operations of the combined company.
Certain Risks Related To Our Financial Performance
We have a history of losses and our future profitability is uncertain. We generated net losses for the year ended December 31, 2012, the nine months ended December 31, 2010, and the years ended March 31, 2007 and 2006. Although we generated net income for the years ended December 31, 2011, March 31, 2010, 2009 and 2008, we may experience future net losses which may limit our ability to fund our operations and we may not generate income from operations in the future. Our future profitability depends upon many factors, including several that are beyond our control. These factors include without limitation:
| |
• | changes in the demand for our products and services; |
| |
• | the introduction of competitive software; |
| |
• | our ability to license desirable technologies; |
| |
• | changes in the research and development budgets of our customers and potential customers; |
| |
• | our ability to successfully, cost effectively and timely develop, introduce and market new products, services and product enhancements; and |
| |
• | the acquisition of any new entities or businesses which may have a dilutive effect upon our earnings. |
Our sales forecast and/or revenue projections may not be accurate. We use a “pipeline” system, a common industry practice, to forecast sales and trends in our business. Our sales personnel monitor the status of proposals, including the date when they estimate a customer will make a purchase decision and the potential size of the order. We aggregate these estimates on a quarterly basis in order to generate a sales pipeline. While the pipeline process provides us with some guidance in business planning and forecasting, it is based on estimates only and is therefore subject to risks and uncertainties. Any variation in the conversion of the pipeline into revenue or the pipeline itself could cause us to improperly plan or budget and thereby adversely affect our business, results of operations and financial condition.
If we are unable to license software to, or collect receivables from, our customers, our operating results may be adversely affected. While the majority of our current customers are well-established, large companies and universities, we also provide products and services to smaller companies. Our financial success depends upon the creditworthiness and ultimate collection of amounts due from our customers, including our smaller customers with fewer financial resources. While we have not experienced significant customer defaults during the 2009, 2010, 2011 and the 2012 fiscal years and the nine months ended December 31, 2010, if we are not able to collect from our customers, we may be required to write-off significant accounts receivable and recognize bad debt expenses which could materially and adversely affect our operating results.
Our quarterly operating results, particularly our quarterly cash flows, may fluctuate. Quarterly operating results may fluctuate as a result of a number of factors, including lengthy sales cycles, market acceptance of new products and upgrades, timing of new product introductions, changes in pricing policies, changes in general economic and competitive conditions, and the timing and integration of acquisitions. We may also experience fluctuations in quarterly operating results due to general and industry specific economic conditions that may affect the research and development expenditures of pharmaceutical and biotechnology companies. In particular, historically we have received approximately two-thirds of our annual customer orders in the quarters ended December 31 and March 31. In accordance with our revenue recognition policies, the revenue associated with these orders is generally recognized over the contractual license term. Therefore, because we accrue sales commissions and royalties upon the receipt of customer orders, we have generally experienced an increase in operating costs and expenses during the quarters ended December 31 and March 31 with only a minimal corresponding incremental increase in revenue. Additionally, our cash flows from operations have historically been positive in the fiscal quarter ended March 31 as we collect the accounts receivable generated from these customer orders, while we have historically experienced negative cash flows from operations in the other three fiscal quarters. As a result of these seasonal variations, we believe that quarter-to-quarter comparisons of our operating results are not a good indication of our future performance and that our interim financial results are not necessarily indicative of results for a full year or for any subsequent interim period.
We may be required to indemnify Pharmacopeia Drug Discovery, Inc. (“PDD”), or may not be able to collect on indemnification rights from PDD. On April 30, 2004, we spun-off our drug discovery subsidiary, PDD, into an independent,
separately traded, publicly held company through the distribution to our stockholders of a dividend of one share of PDD common stock for every two shares of our common stock. As part of the spin-off, we agreed to indemnify the indebtedness, liabilities and obligations of PDD. One such obligation includes a guarantee by us to the landlord of PDD’s obligations under certain facility leases, with respect to which PDD will indemnify us should we be required to make any payment under the guarantee. These indemnification obligations could be as significant as the remaining future minimum lease payments, which totaled approximately $8.5 million as of December 31, 2012. PDD’s ability to satisfy any such indemnification obligations (including, without limitation, PDD’s commitment to indemnify us in the event of our payment under our guarantee of its leases) will depend upon PDD’s future financial strength. We cannot assure you that, if PDD becomes obligated to indemnify us for any substantial obligations, PDD will have the ability to do so. There also can be no assurance that we will be able to satisfy any indemnification obligations to PDD. Any failure by PDD to satisfy its obligations and any required payment by us could have a material adverse effect on our business.
Enacted and proposed changes in securities laws and regulations have increased our costs and may continue to increase our costs in the future. In recent years, there have been several changes in laws, rules, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”), the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley”) and various other new regulations promulgated by the SEC and rules promulgated by the national securities exchanges.
The Dodd-Frank Act, enacted in July 2010, expands federal regulation of corporate governance matters and imposes requirements on publicly-held companies, including us, to, among other things, provide stockholders with a periodic advisory vote on executive compensation and also adds compensation committee reforms and enhanced pay-for-performance disclosures. While some provisions of the Dodd-Frank Act are effective upon enactment, others will be implemented upon the SEC's adoption of related rules and regulations. The scope and timing of the adoption of such rules and regulations is uncertain and accordingly, the cost of compliance with the Dodd-Frank Act is also uncertain.
Sarbanes-Oxley required changes in some of our corporate governance and securities disclosure and compliance practices. Under Sarbanes-Oxley, publicly-held companies, including us, are required to, among other things, furnish independent annual audit reports regarding the existence and reliability of their internal control over financial reporting and have their chief executive officer and chief financial officer certify as to the accuracy and completeness of their financial reports.
These and other new or changed laws, rules, regulations and standards are, or will be, subject to varying interpretations in many cases due to their lack of specificity. As a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Our efforts to comply with evolving laws, regulations and standards are likely to continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. Further, compliance with new and existing laws, rules, regulations and standards may make it more difficult and expensive for us to maintain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. Members of our board of directors and our principal executive officer and principal financial officer could face an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty attracting and retaining qualified directors and executive officers, which could harm our business. We continually evaluate and monitor regulatory developments and cannot estimate the timing or magnitude of additional costs we may incur as a result.
Our business, financial condition and results of operations may be adversely impacted by fluctuations in foreign currency exchange rates. Our international sales generally are denominated in local currencies. Fluctuations in the value of currencies in which we conduct business relative to the U.S. dollar result in currency transaction gains and losses, and the impact of future exchange rate fluctuations cannot be accurately predicted. Future fluctuations in currency exchange rates may have a material adverse impact on revenue from international sales, and thus on our business, financial condition and results of operations. When deemed appropriate, we may engage in currency exchange rate hedging transactions in an attempt to mitigate the impact of adverse exchange rate fluctuations. However, currency hedging policies may not be successful, and they may increase the negative impact of exchange rate fluctuations.
If we consume cash more quickly than expected, we may be unable to raise additional capital and we may be forced to curtail operations. We anticipate that our existing capital resources will be adequate to fund our operations for at least the next twelve months. However, our capital requirements will depend on many factors, including potential acquisitions of other businesses or technologies. If we determine that we must raise additional capital, we may attempt to do so through public or private financings involving debt or equity. However, additional capital may not be available on favorable terms, or at all. If adequate funds are not available, we may be required to curtail operations significantly or to obtain funds by entering into arrangements with collaborative partners or others that may require us to relinquish rights to certain of our technologies, products or potential markets that we would not otherwise relinquish.
Certain Risks Related to Owning Our Stock
We expect that our stock price will fluctuate significantly, and as a result, our stockholders may not be able to resell their shares at or above their original investment price. The stock market, historically and in recent years, has experienced significant volatility particularly with technology company stocks. The volatility of technology company stock prices often does not relate to the operating performance of the companies represented by the stock. Factors that could cause volatility in the price of our common stock include without limitation:
| |
• | actual and anticipated fluctuations in our quarterly financial and operating results; |
| |
• | market conditions in the technology and software sectors; |
| |
• | issuance of new or changed securities analysts’ reports or recommendations; |
| |
• | developments or disputes concerning our intellectual property or other proprietary rights or other legal claims; |
| |
• | introduction of technological innovations or new commercial products by us or our competitors; |
| |
• | market acceptance of our products and services; |
| |
• | additions or departures of key personnel; and |
| |
• | the acquisition of new businesses or technologies |
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock.
As institutions hold the majority of our common stock in large blocks, substantial sales by these stockholders could depress the market price for our shares. As of March 1, 2013, the top ten institutional holders of our common stock held approximately 54.1% of our outstanding common stock. As a result, if one or more of these major stockholders were to sell all or a portion of their holdings, or if the market were to perceive that such sale or sales may occur, the market price of our common stock may fall significantly.
Because we do not intend to pay dividends, our stockholders will benefit from an investment in our common stock only if our stock price appreciates in value. We have never declared or paid any cash dividends on our common stock. We currently intend to retain our future earnings, if any, to finance the expansion of our business and do not expect to pay any cash dividends in the foreseeable future. As a result, the success of an investment in our common stock will depend entirely upon any future appreciation in its value. There is no guarantee that our common stock will appreciate in value or even maintain the price at which it was purchased.
Anti-takeover provisions under the Delaware General Corporation Law and provisions in our certificate of incorporation and bylaws may make the accomplishment of mergers or the assumption of control by a principal stockholder more difficult, thereby making the removal of management more difficult. Certain provisions of the Delaware General Corporation Law may delay or deter attempts to secure control of our company without the consent of our management. Also, our governing documents provide for a staggered board of directors, which will make it more difficult for a potential acquirer to gain control of our board of directors. The provisions of our governing documents and of Delaware law could have the effect of delaying, deferring or preventing a change of control, including without limitation a proxy contest, making the acquisition of a substantial block of our common stock more difficult. The provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock. Further, the existence of these anti-takeover measures may cause potential bidders to look elsewhere, rather than initiating acquisition discussions with us.
Certain Risks Related to Intellectual Property
We may not be able to protect adequately the trade secrets and confidential information that we disclose to our employees. We rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our competitive position. Competitors through their independent discovery (or improper means, such as unauthorized disclosure or industrial espionage) may come to know our proprietary information. We generally require employees and consultants to execute confidentiality and assignment-of-inventions agreements. These agreements typically provide that all materials and confidential information developed by or made known to the employee or consultant during his, her or its relationship with us are to be kept confidential, and that all inventions arising out of the employee's or consultant's relationship with us are our exclusive property. Our employees and consultants may breach these agreements, and in some instances we may not have an adequate remedy. Additionally, in some instances, we may have failed to require that employees and consultants execute confidentiality and assignment-of-inventions agreements.
Foreign laws may not afford us sufficient protections for our intellectual property, and we may not seek patent protection outside the U.S. We believe that our success depends, in part, upon our ability to protect our intellectual property throughout the world. However, the laws of some foreign countries may not be as comprehensive as those of the U.S. and may not be sufficient to protect our proprietary rights abroad. In addition, we generally do not pursue patent protection outside the U.S. because of cost and confidentiality concerns. Accordingly, our international competitors could obtain foreign patent protection for, and market overseas, products and technologies for which we are seeking patent protection in the U.S.
A patent issued to us may not be sufficiently broad to protect adequately our rights in intellectual property to which the patent relates. Due to cost and other considerations, we generally do not rely on patent protection to enforce our intellectual property rights, and have filed only a limited number of patent applications. Even if patents are issued to us, these patents may not sufficiently protect our interest in our software or other technologies because the scope of protection provided by any patents issued to or licensed by us are subject to the uncertainty inherent in patent law. Third parties may be able to design
around these patents or develop unique products providing effects similar to our products. In addition, others may discover uses for our software or technologies other than those uses covered in our patents, and these other uses may be separately patentable. A number of pharmaceutical and biotechnology companies, software organizations and research and academic institutions, have developed technologies, filed patent applications or received patents on various technologies that may be related to our business. Some of these technologies, patent applications or patents may conflict with our technologies, patent applications or patents. These conflicts could also limit the scope of patents, if any, that we may be able to obtain, or result in the denial of our patent applications.
We may be subject to claims of infringement by third parties. A number of patents may have been issued or may be issued in the future that could cover certain aspects of our technology or functionality of our products and could prevent us from using technology that we use or expect to use, or making or selling certain of our products. In addition, to the extent our employees are involved in research areas similar to those areas in which they were involved at their former employers, we may inadvertently use or disclose alleged trade secrets or other proprietary information of their former employer. Thus, our products may infringe patent or other intellectual property rights of third parties, and we may be subject to infringement claims by third parties. Any such claims, with or without merit, could be time consuming to defend, result in costly litigation, divert management's attention and resources, cause product shipment delays or require us to enter into royalty or licensing agreements. Such licenses may not be available on terms acceptable to us, if at all. In the event of a successful claim of product infringement against us, our failure or inability to license or design around the infringed technology could have a material adverse effect on our business, financial condition and results of operations.
Third party software codes incorporated into our products could subject us to liability or limit our ability to sell such products. Some of our products include software codes licensed from third parties, including the open source community. Some of these licenses impose certain obligations upon us, including royalty and indemnification obligations. In the case of codes licensed from the open source community, the licenses may also limit our ability to sell products containing such code. Though we generally review the applicable licenses prior to incorporating third party code into our software products, there can be no assurance that such third party codes incorporated into our products would not subject us to liability or limit our ability to sell the products containing such code, thereby having a material adverse effect upon our business.
|
| |
Item 1B. | Unresolved Staff Comments |
None
The following is a summary of our principal business locations:
|
| | | | | | | |
Location | | Principal activities | | Lease Ends | | Square Feet |
U.S | | | | | | |
San Diego, California, | | Corporate headquarters, sales, customer support, marketing, product development and administration | | 2013 | | 68,436 |
|
San Ramon, California | | Product development, customer support, sales, marketing and administration | | 2016 | | 64,409 |
|
Santa Clara, California | | Customer support | | 2013 | | 3,046 |
|
Bend, Oregon | | Customer support, product development and marketing | | 2014 | | 7,292 |
|
Bedminster, New Jersey | | Customer support | | 2015 | | 7,855 |
|
Burlington, Massachusetts | | Sales | | 2014 | | 4,830 |
|
Milford, Massachusetts | | Customer support, product development, sales and marketing | | 2019 | | 19,556 |
|
Lafayette, Colorado | | Customer support, product development and sales | | 2013 | | 8,309 |
|
Durham, North Carolina | | Product development, sales and marketing | | 2017 | | 4,015 |
|
| | | | | | |
Europe | | | | | | |
Cambridge, U.K. | | Sales, customer support, marketing, product development and administration | | 2022 | | 24,451 |
|
Paris, France | | Sales | | 2015 | | 2,486 |
|
Basel, Switzerland | | Sales, customer support | | 2013 | | 8,148 |
|
Cologne, Germany | | Sales, customer support | | 2013 | | 2,325 |
|
Stockholm, Sweden | | Customer support | | 2014 | | 3,014 |
|
Asia | | | | | | |
Tokyo, Japan | | Sales, customer support and administration | | 2014 | | 9,001 |
|
Bangalore, India | | Sales | | 2013 | | 2,500 |
|
In December 2012, we entered into a lease agreement for a facility with 61,460 square feet of office space which will serve as our new corporate headquarters in San Diego, California. The lease has a 10-year term with an option to terminate after five years. We excluded this lease from the table above as the lease will not commence until the second half of 2013.
We believe our existing properties, including both owned and leased sites, are in good condition and are adequate to meet our current and reasonably foreseeable future requirements.
In July 2012, a customer of one of our subsidiaries provided us with a request for arbitration, and on October 5, 2012, served us with its Statement of Case in connection with the arbitration, which is pending before the London Court of International Arbitration. The dispute relates to certain software and professional services arrangements that the customer claims were not completed pursuant to the terms of the underlying agreements. We intend to vigorously defend against such claims and to assert counterclaims for payments owed to us. Because of the uncertainties related to this arbitration, we are currently unable to predict the ultimate outcome. However, we believe that it is possible that an unfavorable outcome could result in a material adverse effect on our results of operations, liquidity or financial position.
We are also subject to various other claims and legal proceedings arising in the ordinary course of our business. In connection with our regular assessments of such other claims and legal proceedings, we accrue an estimated loss contingency in our financial statements if it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. However, a determination as to the amount of the accrual required for such contingencies is highly subjective and requires judgments about future events. As a result, the amount of ultimate loss, if any, may differ from our estimates. Nevertheless, management believes that the disposition of such matters, in the aggregate, will not have a material adverse effect on our results of operations, liquidity or financial position.
|
| |
Item 4. | Mine Safety Disclosures
|
Not applicable.
PART II
|
| |
Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market for Common Stock
Our common stock trades on the NASDAQ Global Market under the symbol “ACCL”. The following table sets forth, for the periods indicated, the range of high and low sale prices per share of our common stock:
|
| | | | | | | | |
| | High | | Low |
Year Ended December 31, 2012 | | | | |
First Quarter | | $ | 8.45 |
| | $ | 6.43 |
|
Second Quarter | | $ | 8.31 |
| | $ | 7.44 |
|
Third Quarter | | $ | 8.91 |
| | $ | 7.59 |
|
Fourth Quarter | | $ | 9.59 |
| | $ | 8.45 |
|
Year Ended December 31, 2011 | | | | |
First Quarter | | $ | 8.90 |
| | $ | 7.00 |
|
Second Quarter | | $ | 8.16 |
| | $ | 6.65 |
|
Third Quarter | | $ | 7.57 |
| | $ | 5.80 |
|
Fourth Quarter | | $ | 7.17 |
| | $ | 5.68 |
|
From the date of our initial public offering on December 5, 1995 until February 26, 2004, our stock traded under the symbol “PCOP”. Since February 27, 2004, our common stock has traded under the symbol “ACCL”.
Holders of Record
As of March 1, 2013 there were 337 holders of record of our common stock.
Dividends
No cash dividends have been paid on the common stock to date, nor do we anticipate paying dividends in the foreseeable future.
Equity Compensation Plan Information
The information required by Item 201(d) of Regulation S-K will be included in the definitive proxy statement for our 2012 annual meeting of stockholders or an amendment to this Report (either of the foregoing, a “Subsequent Filing”) to be filed with the SEC within 120 days after our fiscal period ended December 31, 2012 and is incorporated into this Report by reference.
Issuer Purchases of Equity Securities
The following table sets forth information concerning purchases of our common stock, in each fiscal month within the fourth quarter of the fiscal year ended December 31, 2012:
|
| | | | | | | | | | | | |
| | Total number of shares purchased (1) | | Average price paid per share | | Total number of shares purchased as part of publicly announced plans or programs | | Approximate dollar value of shares that may yet be purchased under the plan or programs |
| | | | | | | | (in thousands) |
10/1/2012 – 10/31/2012 | | 77,600 |
| | 8.71 |
| | 77,600 |
| | 17,540 |
|
11/1/2012 – 11/30/2012 | | 118,098 |
| | 8.72 |
| | 118,098 |
| | 16,510 |
|
12/1/2012 – 12/31/2012 | | 34,345 |
| | 8.77 |
| | 34,345 |
| | 15,000 |
|
(1) On August 3, 2011 and February 29, 2012, our board of directors authorized the Company to commit up to $8.0 million and $2.0 million, respectively, for an aggregate of $10.0 million, for the repurchase of the Company's common stock during fiscal 2012 pursuant to a program to be executed in accordance with a Rule 10b5-1 trading plan. On March 14, 2012, we entered into a stock repurchase plan (the “2012 Repurchase Plan”) in accordance with Rule 10b5-1 of Exchange Act. Pursuant to the 2012 Repurchase Plan, and subject to the conditions set forth therein and the provisions of Rule 10b-18 of the Exchange Act, we instructed the broker to purchase for our account up to $10.0 million worth of our common stock by December 31, 2012. During the quarter ended December 31, 2012, we repurchased 230,043 shares of our common stock for approximately $2.0 million at an average cost of $8.72 per share.
Comparison of Stockholder Return
The following information shall not be deemed to be “filed” with the SEC nor shall this information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent that we specifically incorporate it by reference into a filing.
The following graph compares the five-year cumulative total return through December 31, 2012 for our common stock, which is traded under the symbol “ACCL” on the NASDAQ Global Market, to a broad market index, namely the NASDAQ U.S. Composite Index (the “NASDAQ Index”) and an industry index, namely the NASDAQ Computer and Data Processing Services Index (the “Industry Index”). This graph assumes an investment of $100 was made in both our common stock and in each index on March 31, 2008 and further assumes that all dividends were reinvested. The stock price performance on the
graph is not necessarily indicative of future stock price performance.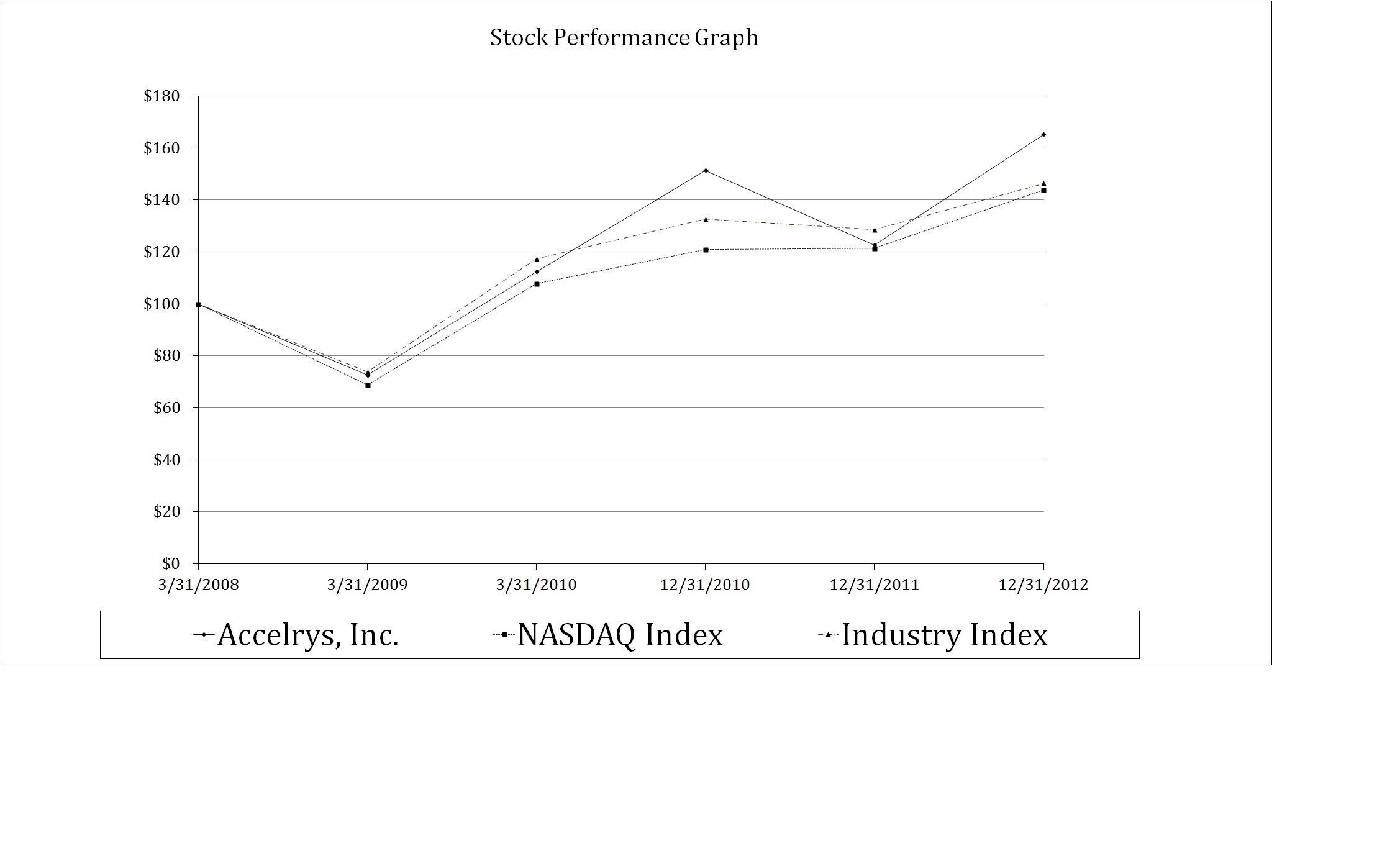
|
| | | | | | | | | |
| | Accelrys, Inc. | | NASDAQ Index | | Industry Index |
March 31, 2008 | | 100.00 |
| | 100.00 |
| | 100.00 |
|
March 31, 2009 | | 62.38 |
| | 64.18 |
| | 72.78 |
|
March 31, 2010 | | 96.55 |
| | 100.68 |
| | 115.61 |
|
December 31, 2010 | | 130.09 |
| | 112.78 |
| | 130.65 |
|
December 31, 2011 | | 105.33 |
| | 113.39 |
| | 126.62 |
|
December 31, 2012 | | 141.85 |
| | 134.14 |
| | 144.26 |
|
|
| |
Item 6. | Selected Consolidated Financial Data |
Change in Fiscal Year End
On August 3, 2010, our board of directors approved a change in our fiscal year end from March 31 to December 31. The fiscal year end change was effective December 31, 2010 and resulted in a nine-month reporting period from April 1, 2010 to December 31, 2010, which is included in the accompanying consolidated financial statements. All references to “years,” unless otherwise noted, refer to the twelve-month fiscal year, which prior to April 1, 2010, ended on March 31, and beginning with January 1, 2011, ends on December 31, of each year.
The following selected consolidated financial data have been derived from our audited consolidated financial statements. This data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes thereto included elsewhere in this Report. The following table sets forth our selected consolidated financial data as of and for the years ended December 31, 2012 and 2011, the nine months ended December 31, 2010 and the years ended March 31, 2010 and 2009.
|
| | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended | | For the Nine Months Ended | | For the Years Ended |
| | December 31,
2012 | | December 31, 2011 | | December 31, 2010 | | March 31, 2010 | | March 31, 2009 |
| | | | | | | | | | |
Consolidated Statements of Operations: | | | | | | | | | | |
Revenue: | | | | | | | | | | |
License and subscription revenue | | $ | 89,440 |
| | $ | 79,425 |
| | $ | 51,003 |
| | $ | 64,854 |
| | $ | 62,426 |
|
Maintenance on perpetual licenses | | 38,254 |
| | 34,862 |
| | 12,512 |
| | 10,499 |
| | 11,093 |
|
Content | | 12,485 |
| | 16,838 |
| | 6,592 |
| | — |
| | — |
|
Professional services and other | | 22,347 |
| | 13,214 |
| | 10,098 |
| | 7,606 |
| | 7,462 |
|
Total revenue | | 162,526 |
| | 144,339 |
| | 80,205 |
| | 82,959 |
| | 80,981 |
|
Cost of revenue: | | | | | | | | | | |
Cost of revenue | | 41,695 |
| | 36,065 |
| | 23,528 |
| | 14,136 |
| | 14,749 |
|
Amortization of completed technology | | 8,843 |
| | 8,393 |
| | 4,887 |
| | 844 |
| | 1,524 |
|
Total cost of revenue | | 50,538 |
| | 44,458 |
| | 28,415 |
| | 14,980 |
| | 16,273 |
|
Gross profit | | 111,988 |
| | 99,881 |
| | 51,790 |
| | 67,979 |
| | 64,708 |
|
Operating expenses: | | | | | | | | | | |
Product development | | 38,849 |
| | 33,977 |
| | 22,080 |
| | 16,045 |
| | 15,978 |
|
Sales and marketing | | 57,971 |
| | 51,517 |
| | 34,848 |
| | 38,042 |
| | 36,083 |
|
General and administrative | | 17,480 |
| | 16,467 |
| | 11,697 |
| | 12,479 |
| | 12,070 |
|
Business consolidation, transaction and restructuring costs (recoveries) | | 7,803 |
| | 7,775 |
| | 16,493 |
| | (86 | ) | | 896 |
|
Purchased intangible asset amortization | | 8,939 |
| | 9,846 |
| | 2,712 |
| | — |
| | — |
|
Total operating expenses | | 131,042 |
| | 119,582 |
| | 87,830 |
| | 66,480 |
| | 65,027 |
|
Operating income (loss) | | (19,054 | ) | | (19,701 | ) | | (36,040 | ) | | 1,499 |
| | (319 | ) |
Net gain on sale of cost method investment | | — |
| | 18,970 |
| | — |
| | — |
| | — |
|
Royalty and other income, net | | 8,870 |
| | 1,740 |
| | 1,377 |
| | 914 |
| | 1,608 |
|
Income (loss) before income taxes | | (10,184 | ) | | 1,009 |
| | (34,663 | ) | | 2,413 |
| | 1,289 |
|
Income tax expense (benefit) | | 218 |
| | (756 | ) | | (14,055 | ) | | 1,218 |
| | 1,195 |
|
Net income (loss) | | $ | (10,402 | ) | | $ | 1,765 |
| | $ | (20,608 | ) | | $ | 1,195 |
| | $ | 94 |
|
Net income (loss) per share amounts: | | | | | | | | | | |
Basic | | $ | (0.19 | ) | | $ | 0.03 |
| | $ | (0.44 | ) | | $ | 0.04 |
| | $0.00 |
Diluted | | $ | (0.19 | ) | | $ | 0.03 |
| | $ | (0.44 | ) | | $ | 0.04 |
| | $0.00 |
| | | | | | | | | | |
Weighted average shares used to compute net income (loss) per share: | | | | | | | | | | |
Basic | | 55,696 |
| | 55,489 |
| | 46,446 |
| | 27,504 |
| | 27,093 |
|
Diluted | | 55,696 |
| | 56,037 |
| | 46,446 |
| | 27,760 |
| | 27,203 |
|
| | | | | | | | | | |
| | December 31,
2012 | | December 31, 2011 | | December 31, 2010 | | March 31, 2010 | | March 31, 2009 |
Consolidated Balance Sheet Data: | | | | | | | | | | |
Cash, cash equivalents, marketable securities and restricted cash | | 115,646 |
| | $ | 143,624 |
| | $ | 141,052 |
| | $ | 93,082 |
| | $ | 81,769 |
|
Promissory note receivable | | 34,796 |
| | 34,720 |
| | — |
| | — |
| | — |
|
Total assets | | 405,842 |
| | 407,886 |
| | 363,282 |
| | 171,205 |
| | 160,614 |
|
Total deferred revenue | | 89,151 |
| | 86,012 |
| | 67,459 |
| | 61,325 |
| | 57,224 |
|
Deferred gain on sale of intellectual property | | 25,895 |
| | 25,974 |
| | — |
| | — |
| | — |
|
Noncurrent liabilities(1) | | 10,098 |
| | 10,634 |
| | 11,331 |
| | 6,953 |
| | 7,204 |
|
Accumulated deficit | | (238,240 | ) | | (198,208 | ) | | (199,973 | ) | | (179,365 | ) | | (180,560 | ) |
Total stockholders’ equity | | 242,821 |
| | 248,684 |
| | 247,646 |
| | 86,104 |
| | 80,759 |
|
| |
(1) | Noncurrent liabilities primarily consist of long-term, lease-related liabilities and accrued income tax. |
|
| |
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with “Selected Consolidated Financial Data” and our consolidated financial statements and related notes thereto included elsewhere in this Report.
In addition to historical information, the following discussion contains forward-looking statements that are subject to certain risks and uncertainties, including those risks and uncertainties described in Item 1A of this Report. Actual results may differ substantially from those referred to herein due to a number of factors, including but not limited to those risks and uncertainties.
Overview
On July 1, 2010, pursuant to the terms of the Merger Agreement, by and among us, Symyx Merger Sub and Symyx , Symyx Merger Sub merged with and into Symyx, with Symyx surviving as our wholly owned subsidiary. Symyx's operating results are included in our consolidated financial statements and results of operations beginning July 1, 2010.
On May 19, 2011, we completed the Contur Acquisition, whereby Contur became our wholly owned subsidiary. Contur's operating results are included in our consolidated financial statements and results of operations beginning May 19, 2011.
On December 30, 2011, we completed the Velquest Acquisition, whereby VelQuest became our wholly owned subsidiary. VelQuest's operating results are included in our consolidated financial statements and results of operations beginning December 30, 2011.
On May 17, 2012, we acquired the HEOS software platform from Scynexis, Inc. The operating results of the HEOS platform are included in our consolidated financial statements and results of operations beginning May 17, 2012.
On October 23, 2012, we completed the Aegis Acquisition, whereby Aegis became our wholly owned subsidiary. Aegis's operating results are included in our consolidated financial statements and results of operations beginning October 23, 2012.
On January 11, 2013, we completed the Vialis Acquisition. The information presented in this Report does not give effect to the Vialis Acquisition.
Our Business
We develop and commercialize scientific informatics software products and services for industries and organizations that rely on scientific innovation to differentiate themselves in the marketplace. Historically, our products were primarily utilized by our customers' research organizations.
As a result of the Acquisitions, we increased the breadth and depth of our scientific product portfolio by extending our offerings beyond research and into development and manufacturing. In particular, the addition of the VelQuest suite of products gives us even greater capability downstream, extending our solutions into the quality assurance and quality control areas in large pharmaceutical companies. Aegis further expands our portfolio into downstream operations with new solutions for enterprise manufacturing process intelligence. In addition, we added systems integration capabilities with the Vialis Acquisition, which strengthened our position in the laboratory informatics software market and added capabilities in the downstream analytical development, quality control, and quality assurance and manufacturing areas.
The acquisition of the HEOS platform brought a cloud-based offering that enables more efficient and streamlined drug delivery collaborations, particularly with organizations leveraging contract research organizations and other groups for externalized R&D projects.
Collectively, our products and services are intended to optimize our customers' lab-to-commercialization value chains, from early research through development into manufacturing. Our software is used by our customers' scientists, biologists, chemists, engineers and information technology professionals to design, execute and manage scientific experiments in-silico or in the lab and to aggregate, mine, manage, analyze and interactively report on the scientific data from those experiments. Our solutions also enable the development process to scale more effectively and bring increased automation to the transition from development to manufacturing, manage quality control and quality assurance operations, and monitor process and product quality, thereby reducing compliance risk. The ability to integrate and access data from diverse data sources and to make that
information accessible throughout the scientific innovation value chain enables our customers to reduce costs, enhance productivity and more efficiently provide effective products to their customers that meet quality and compliance standards.
Today, we are the leading provider of scientific innovation lifecycle management software (SILM). Our customers include leaders from a variety of industries, including pharmaceutical, biotechnology, agricultural, energy, chemicals, aerospace, consumer packaged goods and industrial products, as well as various government and academic entities. We market our software products and services worldwide, principally through our direct sales force, augmented by the use of third party distributors. We are headquartered in San Diego, California and were incorporated in Delaware in 1993.
Description of Our Markets and Business
Our customers differentiate themselves through scientific innovation. As a result, innovation in the discovery, development and manufacturing of new products, compliance with applicable regulations, rapid, cost-effective commercialization of such products and the ability to protect the intellectual property therein is crucial to our customers' success. Therefore, they invest considerable resources in technologies that help identify productive new pathways for research projects, help develop new materials, increase the efficiency of discovery, development and manufacturing processes, and otherwise enable them to maximize the use of scientific data, information and knowledge. Our software solutions allow our customers to effectively design, plan and execute scientific experiments in a repeatable process and in compliance with regulations; leverage the vast amounts of information stored in both corporate databases and public data sources to optimize their processes and accelerate innovation; model, predict and analyze potential scientific outcomes; improve product and process understanding throughout development lifecycles; and access comprehensive, integrated and cross-referenced databases and reference works.
The pharmaceutical and biotechnology industries are a very important part of our business. Our products have been widely adopted within the research functions of businesses in these industries, but less widely adopted by the development, quality assurance (“QA”)/quality control (“QC”) and manufacturing functions of such businesses. In addition, these markets present challenges due to industry consolidation, the maturity of these markets, patent expirations, reduction in the level of discovery research activity, increased competition, including competition from open source software, and outsourcing of research to other entities. The other industries to which we market our products, including energy, material, chemicals, agricultural, aerospace and consumer packaged goods, are earlier in the adoption curve for such scientific software products, which we view as both a challenge and an opportunity.
Business Strategy
Scientific research and development organizations face several challenges that impact their ability to comply with applicable regulations, protect their intellectual property and rapidly and cost-effectively bring products to market. Scientific data is often found in disparate databases and research, development and manufacturing processes are disconnected, manually intensive, inefficient and repetitive. These challenges have made it incredibly difficult for organizations to manage their scientific innovation lifecycles in order to bring novel products to market faster, more efficiently and with lower compliance risk.
Our overall strategy is to deliver solutions that help organizations manage and optimize their scientific innovation lifecycles. Consequently, we are extending beyond our historically strong presence in research downstream into development and manufacturing, covering the entire lab-to-commercialization value chain; moving into new scientific domains, including biology; and expanding our presence outside of the pharmaceutical and biotech industries into other key industries such as food and beverage, fine and specialty chemicals, oil and gas and aerospace. We do this by addressing the core challenges faced by research and development and quality organizations, offering them an open enterprise-scale scientific software platform and a broad portfolio of scientific software applications leveraging our deep domain expertise in chemistry, biology and the materials sciences. We believe the combination of our enterprise platform and associated set of applications and services, such as electronic lab notebooks, the laboratory execution system (“LES”), process management informatics software, design modeling and simulation software, data management and informatics software, content and professional services, help optimize our customers' scientific innovation lifecycle.
We believe that the combination of our products with the products and domain expertise we gained as a result of the Acquisitions (most significantly the VelQuest product suite, and the Aegis process management informatics software) enables us to provide greater value to the development, QA/QC and manufacturing functions of our customers' organizations. Our plan is to continue to integrate and augment our offerings in order to further enhance the value of the products we acquired and the value of our products' collective portfolio to these organizations, thus enabling us to expand upon our presence in the research, development, quality control and quality assurance organizations of our existing customers. The VelQuest Acquisition extended
our software portfolio into pharmaceutical development, QA/QC, and manufacturing, offering significant productivity improvements, faster cycle times, lower operational costs and reduced compliance risks for regulated life sciences organizations. The Aegis Acquisition brought products that complement the Velquest product line by expanding our footprint in downstream operations with solutions for process intelligence. We also intend to continue to develop advanced analysis, scientific and reporting component collections in order to extend our platform's value to and use by our customers.
Our strategy also includes offering professional services to further tailor our enterprise platform to our customers' individual business needs, thereby increasing its utility and value. Because our enterprise platform is the underlying operating platform for many products in our broad portfolio, and integration with the applications obtained as part of the Acquisitions continues to be a development priority, we expect the use of these products to expand as the use of our platform grows, thus further increasing our sales and value to our customers. Our acquisition of Vialis, a leading systems integrator based in Liestal, Switzerland, added new services capabilities to our portfolio and further strengthened our position in the laboratory informatics software market
Our enterprise platform is an open, scientifically aware platform. We partner with third party organizations and academic institutions which develop scientific software and services, and we enable and encourage these companies to develop applications that operate on our platform, further proliferating its utility and value to our customers.
We also focus on industries in markets where scientific innovation is a key differentiator, but the use of scientific software solutions has not been widely adopted. As we develop a greater presence in these markets, we believe our ability to attract additional customers will increase.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. (“GAAP”). The preparation of the consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the related disclosure of contingent assets and liabilities. We review our estimates on an on-going basis, including those related to income taxes and the valuation of goodwill, intangibles and other long-lived assets. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. Our accounting policies are described in more detail in Note 1 to our consolidated financial statements included elsewhere in this Report. We have identified the following as the most critical accounting policies and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We generate revenue from the following primary sources:
| |
• | post-contract customer support and maintenance services on licensed software (collectively referred to as “PCS”), |
Customer billings issued in connection with our revenue-generating activities are initially recorded as deferred revenue. We then recognize the revenue from these customer billings as set forth below and when all of the following criteria are met:
| |
• | a fully executed written contract or purchase order has been obtained from the customer (i.e., persuasive evidence of an arrangement exists), |
| |
• | the contractual price of the product or services has been defined and agreed to in the contract or purchase order (i.e., price is fixed or determinable), |
| |
• | delivery of the product or service has occurred and no material uncertainties regarding customer acceptance of the delivered product or service exist, and |
| |
• | collection of the purchase price from the customer is considered probable. |
Software Licenses. We license software on a term and perpetual basis. When sold perpetually, our standard perpetual software licensing arrangements generally include twelve months of bundled PCS, while our standard term-based software licensing arrangements typically include PCS for the full duration of the term license. Because we do not have vendor-specific objective evidence (“VSOE”) of the selling price of these undelivered elements, we recognize as revenue the entire fee for such perpetual and term-based licenses ratably over the term of the bundled PCS.
Renewal of PCS Under Perpetual Software Licenses. Our PCS includes the right to receive unspecified upgrades or enhancements and technical support. Fees from customer renewals of PCS related to previously purchased perpetual licenses are recognized ratably over the term of the PCS.
Content. Content is licensed on a term basis and provides customers with access to the licensed content over the term of the agreement. Revenue from these licensing arrangements is recognized ratably over the term of the agreement, which is typically twelve months and coincides with the term of the PCS and/or licensed software.
Professional Services. We provide certain services to our customers, including non-complex product training, installation, implementation and other professional services which are non-essential to the operation of the software. We also perform professional services for our customers designed to enhance the value of our software products by creating extensions to functionality to address a client's specific business needs. When sold separately, revenue from time and materials service engagements are generally recognized as the services are performed. Revenue from fixed fee service engagements are typically recognized as the services are performed using the percentage-of-completion method when we can reasonably estimate the level of effort required to complete our performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. For service arrangements that include provisions that create significant uncertainties (e.g. customer acceptance) or service deliverables wherein the level of effort to complete the services cannot be reasonably estimated, the associated revenue is recognized using the completed performance method.
Hosting Services. We are an application service provider (“ASP”), where we provide hosting services that allow customers access to software that resides on our servers. The ASP model typically includes an up-front fee and a monthly commitment from the customer that commences upon completion of the implementation through the remainder of the customer life. The up-front fee is the initial setup fee, or the implementation fee. The monthly commitment includes, but is not limited to, a fixed monthly fee or a transactional fee based on system usage that exceeds monthly minimums. We do not view the activities of signing the contract or providing initial setup services as discrete earnings events. Revenue is typically deferred until the date the customer commences use of our services, at which point the up-front fees are recognized ratably over the customer life of the customer arrangement.
Multi-Element Arrangements. For multi-element arrangements that include software licenses, PCS, and non-essential installation and implementation services, the entire fee for such arrangements is recognized as revenue ratably over the term of the PCS or delivery of the services, whichever is longer. For multi-element arrangements that also include services that are essential to the operation of the software, the fee for such arrangements is generally deferred until the services essential to the operation of the software have been performed, at which point the entire fee for such arrangements is recognized as revenue ratably over the remaining term of the PCS or the delivery of the non-essential services, whichever is longer. We allocate the arrangement fee to the software-related elements and the non-software-related elements based upon the relative standard list price of the products and/or services that comprise each element, which is our best estimate of selling price.
Royalty Income
We recognize royalty income based on reported sales by third party licensees of products containing the applicable licensed materials and intellectual property. Royalty revenue is recognized as these payments become due. Non-refundable royalties, for which there are no further performance obligations, are recognized when due under the terms of the applicable agreements. Royalty income is included in the royalty and other income, net line on the consolidated statements of operations.
Goodwill and Purchased Intangible Assets
Our goodwill resulted from acquisitions in fiscal years 1999 through 2012. Goodwill and intangible assets with indefinite useful lives are not amortized, but are tested for impairment at least annually or as circumstances indicate that their value may no longer be recoverable. In accordance with ASC Topic 350, Intangibles—Goodwill and Other (“ASC Topic 350”), we review our goodwill and indefinite-lived intangible asset for impairment at least annually in our fiscal fourth quarter and more frequently if events or changes in circumstances occur that indicate a potential reduction in the fair value of our reporting unit and/or our indefinite-lived intangible asset below their respective carrying values. Examples of such events or circumstances include: a significant adverse change in legal factors or in the business climate, a significant decline in our stock price, a significant decline in our projected revenue or cash flows, an adverse action or assessment by a regulator, unanticipated
competition, a loss of key personnel, or the presence of other indicators that would indicate a reduction in the fair value of a reporting unit.
Our goodwill is considered to be impaired if we determine that the carrying value of the reporting unit to which the goodwill has been assigned exceeds management’s estimate of its fair value. Based on the guidance provided by ASC Topic 350 and ASC Topic 280, Segment Reporting, (“ASC Topic 280”) management has determined that our company consists of one reporting unit given the similarities in economic characteristics between our operations and the common nature of our products, services and customers. Because we have only one reporting unit, and because we are publicly traded, we determine the fair value of the reporting unit based on our market capitalization as we believe this represents the best evidence of fair value. In the fourth quarter of fiscal year 2012, we completed our annual goodwill impairment test for the fiscal year ended December 31, 2012 and concluded that our goodwill was not impaired. Our conclusion that goodwill was not impaired was based on a comparison of our net assets as of December 31, 2012 to our market capitalization.
Because we determine the fair value of our reporting unit based on our market capitalization, our future reviews of goodwill for impairment may be impacted by changes in the price of our common stock. For example, a significant decline in the price of our common stock may cause the fair value of our goodwill to fall below its carrying value. Therefore, we cannot assure you that when we complete our future reviews of goodwill for impairment a material impairment charge will not be recorded.
Valuation of Indefinite-Lived Intangible Asset
In connection with our acquisition of SciTegic in September 2004, we acquired the SciTegic trade name which was determined to be indefinitely lived. In accordance with ASC Topic 350, we review our indefinite-lived intangible asset for impairment at least annually in our fiscal fourth quarter and more frequently if events or changes in circumstances occur that indicate a potential reduction in the fair value of the asset below its carrying value. Examples of such events or circumstances include: a significant adverse change in legal factors or in the business climate, a significant decline in our stock price, a significant decline in our projected revenue or cash flows, an adverse action or assessment by a regulator, unanticipated competition, a loss of key personnel, or the presence of other indicators that would indicate a reduction in the fair value of an asset.
Our indefinite-lived intangible asset is considered to be impaired if we determine that the carrying value of the asset exceeds its estimated fair value. In the quarter ended December 31, 2012 we completed our annual indefinite-lived intangible asset impairment test and concluded that our indefinite-lived intangible asset was not impaired. We estimated the fair value of our indefinite-lived intangible asset utilizing a discounted cash flow analysis which considers the estimated future customer orders for our scientific informatics platform product line and the associated direct and incremental selling, marketing and development costs. Key assumptions included in the discounted cash flow analysis include projections of future customer order growth for our scientific informatics platform product line and developing an appropriate discount rate.
We cannot assure you that the underlying assumptions used to forecast the cash flows will materialize as estimated. For example, if our projections of future customer order growth do not materialize, the fair value of our indefinite-lived intangible asset may fall below its carrying value. Therefore, we cannot assure you that when we complete our future reviews of our indefinite-lived intangible asset for impairment that a material impairment charge will not be recorded.
Impairment of Long-Lived Assets
We review long-lived assets to be held and used, including acquired intangible assets subject to amortization and property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. Conditions that would necessitate an impairment assessment include a significant decline in the market price of an asset or asset group, a significant adverse change in the extent or manner in which an asset or asset group is being used, the loss of legal ownership or title to the asset, significant negative industry or economic trends or the presence of other indicators that would indicate that the carrying amount of an asset or asset group is not recoverable.
A long-lived asset is considered to be impaired if the estimated undiscounted future cash flows resulting from the use of the asset and its eventual disposition are not sufficient to recover the carrying value of the asset. In order to estimate an asset’s undiscounted future cash flows, we utilize our internal forecast of our future operating results and cash flows, our strategic business plans and anticipated future economic and market conditions. There are inherent estimates and assumptions underlying this information and management’s judgment is required in the application of this information to the determination of an asset’s undiscounted future cash flows. No assurance can be given that the underlying estimates and assumptions utilized in our determination of an asset’s undiscounted future cash flows will materialize as anticipated.
Valuation of Marketable Securities
We invest in various types of securities, including obligations of U.S. government sponsored enterprises, commercial paper, certificates of deposits, corporate and municipal debt. In accordance with the accounting standard for fair value measurements, we classify our investments as Level 1, 2 or 3 within the fair value hierarchy. Fair values determined by Level 1 inputs utilize quoted prices that are observable for the asset or liability, either directly or indirectly. Fair values determined by Level 2 inputs utilize data points that are observable, and have active markets, for the asset or liability, either directly or indirectly. These might include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (such as interest rates, volatilities, prepayment speeds, credit risks, etc.) or inputs that are derived principally from or corroborated by market data by correlation or other means. Fair values determined by Level 3 inputs utilize model-derived valuations in which one or more significant inputs or significant value-drivers are unobservable. As noted in note “6. Fair Value Measurements” in Part II, Item 8 of this Form 10-K, a majority of our security holdings have been classified as Level 2.
We assess our marketable securities for impairment under the guidance provided by ASC Topic 320, Investments—Debt and Equity Securities. Accordingly, we review the fair value of our marketable securities at least quarterly to determine if declines in the fair value of individual securities are other-than-temporary in nature. If we believe the decline in the fair value of an individual security is other-than-temporary, we write-down the carrying value of the security to its estimated fair value, with a corresponding charge against income. To determine if a decline in the fair value of an investment is other-than-temporary, we consider several factors including, among others, the period of time and extent to which the estimated fair value has been less than cost, overall market conditions, the historical and projected future financial condition of the issuer of the security and our ability and intent to hold the security for a period of time sufficient to allow for a recovery of the market value.
Valuation of Share-Based Awards
We account for our share-based awards in accordance with ASC Topic 718, Compensation—Stock Compensation (“ASC Topic 718”). We estimate the fair value of our share-based stock awards on the date of grant using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the use of certain input variables, as follows:
Expected Volatility. Volatility is a measure of the amount the stock price will fluctuate during the expected life of an award. We determine volatility based on our historical stock price volatility over the most recent period equivalent to the expected life of the award, giving consideration to company-specific events impacting our historical volatility that are unlikely to occur in the future, as well as anticipated future events that may impact volatility. We also consider the historical stock price volatilities of comparable publicly traded companies.
Risk-Free Interest Rate. Our assumption of the risk-free interest rate is based on the interest rates on U.S. constant rate treasury securities with contractual terms approximately equal to the expected life of the award.
Expected Dividend Yield. Because we have not paid any cash dividends since our inception and do not anticipate paying dividends in the foreseeable future, we assume a dividend yield of zero.
Expected Award Life. We determine the expected life of an award by considering various relevant factors, including the vesting period and contractual term of the award, our employees’ historical exercise patterns and length of service and employee characteristics. We also consider the expected award lives of comparable publicly traded companies. For stock purchase plan purchase rights, the expected life is equal to the current offering period under the stock purchase plan.
Under ASC Topic 718, we are also required to estimate at grant the likelihood that the award will ultimately vest (the “pre-vesting forfeiture rate”), and to revise the estimate, if necessary, in future periods if the actual forfeiture rate differs. We determine the pre-vesting forfeiture rate of an award based on our historical pre-vesting award forfeiture experience, giving consideration to company-specific events impacting historical pre-vesting award forfeiture experience that are unlikely to occur in the future as well as anticipated future events that may impact forfeiture rates.
Our determination of the input variables used in the Black-Scholes option pricing model as well as the pre-vesting forfeiture rate is based on various underlying estimates and assumptions that are highly subjective and are affected by our stock price, among other factors. Changes in these underlying estimates and assumptions could materially affect the fair value of our share-based awards and, therefore, the amount of share-based compensation expense to be recognized in our results of operations.
Long Term Investments
We determine the accounting method used to account for investments in equity securities of other business entities in which we do not have a controlling interest primarily based on our ownership of each such business entity and whether we have the ability to exercise significant influence over the strategic, operating, investing and financing activities of each such business entity. We monitor these investments for impairment by considering current factors including economic environment, market conditions and operational performance and other specific factors relating to the business underlying the investment.
Income Taxes
We account for income taxes in accordance with ASC Topic 740, Income Taxes. Deferred tax assets and liabilities are recognized for the tax consequences of temporary differences between the financial carrying amounts and the tax basis of existing assets and liabilities by applying enacted statutory tax rates applicable to future years. The provision for income taxes is based on our estimates for taxable income of the various legal entities we operate and the various jurisdictions in which we operate. As such, income tax expense may vary from the customary relationship between income tax expense and pre-tax income. We establish a valuation allowance against our net deferred tax assets to reduce them to the amount expected to be realized.
We assess the recoverability of our deferred tax assets on an ongoing basis. In making this assessment we are required to consider all available positive and negative evidence to determine whether, based on such evidence, it is more likely than not that some portion or all of our net deferred assets will be realized in future periods. This assessment requires significant judgment. We do not recognize current and future tax benefits until it is more likely than not that our tax positions will be sustained. In general, any realization of our net deferred tax assets will reduce our effective rate in future periods. However, the realization of deferred tax assets that are related to net NOLs that were generated by tax deductions resulting from the exercise of non-qualified stock options will be a direct increase to stockholders’ equity.
We determine whether the benefits of our tax positions are more-likely-than-not to be sustained upon audit based on the technical merits of the tax position. We recognize the impact of an uncertain income tax position taken on our income tax return at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position is not recognized if it has less than a 50% likelihood of being sustained.
Change in Fiscal Year and Comparative Financial Information
On August 3, 2010, our board of directors approved a change in our fiscal year end from March 31 to December 31. The fiscal year end change was effective December 31, 2010 and resulted in a nine-month reporting period from April 1, 2010 to December 31, 2010, which is included in the accompanying consolidated financial statements. All references to “years”, unless otherwise noted, refer to the twelve-month fiscal year, which prior to April 1, 2010, ended on March 31, and beginning with January 1, 2011, ends on December 31, of each year. As a result of the fiscal year change, the unaudited comparative information for the nine months ended December 31, 2009 is included in the consolidated statement of operations and consolidated statement of cash flows.
Results of Operations
The following table sets forth the consolidated statements of operations data for the periods presented (amounts in thousands, except per share data). Financial information for the years ended December 31, 2012 and 2011, the nine month transition period ended December 31, 2010 and the years ended March 31, 2010 and 2009, respectively, were derived from audited financial statements. Financial information for the year ended December 31, 2010 and the nine months ended December 31, 2009 were derived from unaudited financial statements.
|
| | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended | | For the Year Ended | | For the Nine Months Ended |
| | December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2010 | | December 31, 2009 |
| | | | | | (unaudited) | | | | (unaudited) |
Consolidated Statements of Operations: | | | | | | | | | | |
Revenue: | | | | | | | | | | |
License and subscription revenue | | $ | 89,440 |
| | $ | 79,425 |
| | $ | 66,363 |
| | $ | 51,003 |
| | $ | 48,949 |
|
Maintenance on perpetual licenses | | 38,254 |
| | 34,862 |
| | 15,717 |
| | 12,512 |
| | 7,839 |
|
Content | | 12,485 |
| | 16,838 |
| | 6,592 |
| | 6,592 |
| | — |
|
Professional services and other | | 22,347 |
| | 13,214 |
| | 12,292 |
| | 10,098 |
| | 5,412 |
|
Total revenue | | 162,526 |
| | 144,339 |
| | 100,964 |
| | 80,205 |
| | 62,200 |
|
Cost of revenue: | | | | | | | | | | |
Cost of revenue | | 41,695 |
| | 36,065 |
| | 27,140 |
| | 23,528 |
| | 10,524 |
|
Amortization of completed technology | | 8,843 |
| | 8,393 |
| | 4,928 |
| | 4,887 |
| | 803 |
|
Total cost of revenue | | 50,538 |
| | 44,458 |
| | 32,068 |
| | 28,415 |
| | 11,327 |
|
Gross profit | | 111,988 |
| | 99,881 |
| | 68,896 |
| | 51,790 |
| | 50,873 |
|
Operating expenses: | | | | | | | | | | |
Product development | | 38,849 |
| | 33,977 |
| | 26,492 |
| | 22,080 |
| | 11,633 |
|
Sales and marketing | | 57,971 |
| | 51,517 |
| | 46,522 |
| | 34,848 |
| | 26,338 |
|
General and administrative | | 17,480 |
| | 16,467 |
| | 14,528 |
| | 11,697 |
| | 8,946 |
|
Business consolidation, transaction and restructuring costs (recoveries) | | 7,803 |
| | 7,775 |
| | 17,199 |
| | 16,493 |
| | (90 | ) |
Purchased intangible asset amortization | | 8,939 |
| | 9,846 |
| | 2,712 |
| | 2,712 |
| | — |
|
Total operating expenses | | 131,042 |
| | 119,582 |
| | 107,483 |
| | 87,830 |
| | 46,827 |
|
Operating income (loss) | | (19,054 | ) | | (19,701 | ) | | (38,587 | ) | | (36,040 | ) | | 4,046 |
|
Net gain on sale of cost method investment | | — |
| | 18,970 |
| | — |
| | — |
| | — |
|
Royalty and other income, net | | 8,870 |
| | 1,740 |
| | 1,671 |
| | 1,377 |
| | 620 |
|
Income (loss) before income taxes | | (10,184 | ) | | 1,009 |
| | (39,916 | ) | | (34,663 | ) | | 4,666 |
|
Income tax expense (benefit) | | 218 |
| | (756 | ) | | (13,934 | ) | | (14,055 | ) | | 1,097 |
|
Net income (loss) | | $ | (10,402 | ) | | $ | 1,765 |
| | $ | (22,982 | ) | | $ | (20,608 | ) | | $ | 3,569 |
|
Net income (loss) per share amounts: | | | | | | | | | | |
Basic | | $ | (0.19 | ) | | $ | 0.03 |
| | $ | (0.55 | ) | | $ | (0.44 | ) | | $ | 0.13 |
|
Diluted | | $ | (0.19 | ) | | $ | 0.03 |
| | $ | (0.55 | ) | | $ | (0.44 | ) | | $ | 0.13 |
|
Weighted average shares used to compute net income (loss) per share: | | | | | | | | | | |
Basic | | 55,696 |
| | 55,489 |
| | 41,823 |
| | 46,446 |
| | 27,470 |
|
Diluted | | 55,696 |
| | 56,037 |
| | 41,823 |
| | 46,446 |
| | 27,704 |
|
Comparison of the Year Ended December 31, 2012 and 2011
The following table summarizes our results of operations as a percentage of revenue for the respective periods:
|
| | | | | |
| Year Ended December 31, |
| 2012 | | 2011 |
Revenue: | | | |
License and subscription revenue | 55 | % | | 55 | % |
Maintenance on perpetual licenses | 24 | % | | 24 | % |
Content | 8 | % | | 12 | % |
Professional services and other | 14 | % | | 9 | % |
Total revenue | 100 | % | | 100 | % |
Cost of revenue: | | | |
Cost of revenue | 26 | % | | 25 | % |
Amortization of completed technology | 5 | % | | 6 | % |
Total cost of revenue | 31 | % | | 31 | % |
Gross profit | 69 | % | | 69 | % |
Operating expenses: | | | |
Product development | 24 | % | | 24 | % |
Sales and marketing | 36 | % | | 36 | % |
General and administrative | 11 | % | | 11 | % |
Business consolidation, transaction and restructuring costs (recoveries) | 5 | % | | 5 | % |
Purchased intangible asset amortization | 6 | % | | 7 | % |
Total operating expenses | 81 | % | | 83 | % |
Operating loss | (12 | )% | | (14 | )% |
Net gain on sale of cost method investment | — | % | | 13 | % |
Royalty and other income, net | 5 | % | | 1 | % |
Income (loss) before income taxes | (6 | )% | | 1 | % |
Income tax expense (benefit) | — | % | | (1 | )% |
Net income (loss) | (6 | )% | | 1 | % |
Due to rounding to the nearest percent, totals may not equal the sum of the line items in the table above.
Revenue
Total revenue increased to $162.5 million for the year ended December 31, 2012, as compared to $144.3 million for the year ended December 31, 2011. The increase in total revenue during the year ended December 31, 2012 was attributable to a decrease in the impact of acquisition-related valuation adjustments on revenue recognition related to the Symyx Merger, combined with incremental revenue from the VelQuest Acquisition and the Contur Acquisition. Total revenue generated in the U.S. accounted for $79.6 million, or 49% of total revenue for the year ended December 31, 2012, compared with $67.8 million, or 47% of total revenue for the year ended December 31, 2011. International revenue accounted for $82.9 million, or 51% of total revenue for the year ended December 31, 2012, compared with $76.6 million, or 53% of total revenue for the year ended December 31, 2011.
License and subscription revenue. License and subscription revenue increased to $89.4 million for the year ended December 31, 2012, as compared to $79.4 million for the year ended December 31, 2011. As a percentage of total revenue, license and subscription revenue remained constant at 55% for both the year ended December 31, 2012 and 2011. The increase in license and subscription revenue during the year ended December 31, 2012 was primarily attributable to additional revenue resulting from the Contur Acquisition and the VelQuest Acquisition, combined with an incremental increase as a result of a decrease in revenue impacted by acquisition-related valuation adjustments related to the Symyx Merger.
Maintenance on perpetual licenses. Maintenance on perpetual licenses revenue increased to $38.3 million for the year ended December 31, 2012, as compared to $34.9 million for the year ended December 31, 2011. As a percentage of total revenue, revenue from maintenance on perpetual licenses remained constant at 24% for both the years ended December 31, 2012 and 2011. The increase in revenue from maintenance on perpetual licenses during the year ended December 31, 2012 was
primarily attributable to incremental revenue from the VelQuest Acquisition, combined with an incremental increase as a result of a decrease in revenue impacted by acquisition-related valuation adjustments related to the Symyx Merger.
Content. Content revenue decreased to $12.5 million for the year ended December 31, 2012, as compared to $16.8 million for the year ended December 31, 2011. As a percentage of total revenue, content revenue decreased to 8% for the year ended December 31, 2012, as compared to 12% for the year ended December 31, 2011. The decrease in content revenue during the year ended December 31, 2012 is attributable to the previously announced phase-out of certain content product lines.
Professional services and other. Professional services and other revenue increased to $22.3 million for the year ended December 31, 2012, as compared to $13.2 million for the year ended December 31, 2011. As a percentage of total revenue, professional services and other revenue increased to 14% for the year ended December 31, 2012, as compared to 9% for the year ended December 31, 2011. The increase in revenue from professional services and other revenue during the year ended December 31, 2012 was primarily attributable to an increase in revenue from solution consulting and contract research engagements.
Total Cost of Revenue
Cost of Revenue. Cost of revenue increased to $41.7 million for the year ended December 31, 2012, as compared to $36.1 million for the year ended December 31, 2011. As a percentage of revenue, cost of revenue increased to 26% for the year ended December 31, 2012, as compared to 25% for the year ended December 31, 2011. The increase in cost of revenue during the year ended December 31, 2012 was primarily attributable to increases in personnel and related expenses in our services department of approximately $4.2 million from higher headcount, consulting and professional fees of approximately $0.9 million, overhead expense of approximately $0.4 and capitalized labor costs of $0.6 million, partially offset by a decrease in software and content royalties of approximately $0.4 million and a decrease in distributor commissions of approximately $0.1 million.
Amortization of Completed Technology. Amortization of completed technology was $8.8 million for the year ended December 31, 2012, as compared to $8.4 million for the year ended December 31, 2011. As a percentage of revenue, amortization of completed technology decreased slightly to 5% for the year ended December 31, 2012, as compared to 6% for the year ended December 31, 2011. The increase in amortization of completed technology during the year ended December 31, 2012 was attributable primarily to $1.8 million increase in amortization related to the VelQuest, the Aegis and the Contur Acquisitions, offset by a decrease in amortization of approximately $1.4 million related to the intangibles acquired in the Symyx Merger.
Operating Expenses
Product Development Expenses. Product development expenses increased to $38.8 million for the year ended December 31, 2012, as compared to $34.0 million for the year ended December 31, 2011. As a percentage of revenue, product development expenses remained consistent at 24% for each of the years ended December 31, 2012 and 2011. The increase in product development expenses during the year ended December 31, 2012 was primarily attributable to an increase in personnel and related expenses of approximately $4.6 million as a result of higher headcount and an increase in professional fees of approximately $0.4 million, partially offset by a decrease in overhead expense of approximately $0.1 million.
Sales and Marketing Expenses. Sales and marketing expenses increased to $58.0 million for the year ended December 31, 2012, as compared to $51.5 million for the year ended December 31, 2011. As a percentage of revenue, sales and marketing expenses remained consistent at 36% for each of the years ended December 31, 2012 and 2011. The increase in sales and marketing expenses for the year ended December 31, 2012 was primarily attributable to an increase in personnel-related expenses of approximately $6.0 million and an increase in professional fees of approximately $0.8 million, partially offset by a decrease in overhead expense of approximately $0.4 million.
General and Administrative Expenses. General and administrative expenses increased to $17.5 million for the year ended December 31, 2012, as compared to $16.5 million for the year ended December 31, 2011. As a percentage of revenue, general and administrative expenses remained consistent at 11% for each of the years ended December 31, 2012 and 2011. The increase in general and administrative expenses during the year ended December 31, 2012 was primarily attributable to an increase in personnel-related expenses of $1.3 million, partially offset by a decrease in overhead expense of approximately $0.3 million.
Business Consolidation, Transaction and Restructuring Costs. Business consolidation, transaction and restructuring costs of $7.8 million for the year ended December 31, 2012 consisted primarily of business consolidation costs related to recent acquisitions including accounting, legal, litigation and costs incurred to integrate acquired companies, including consultant and employee related costs incurred during integration and transition periods.
As a percentage of revenue, business consolidation, transaction and restructuring costs were at 5% for both the year ended December 31, 2012 and 2011. We have classified all transaction and integration-related costs to business consolidation costs, transaction and restructuring costs, which for the year ended December 31, 2012 includes $1.0 million of acquisition-related contingent compensation and professional services, legal, litigation and other costs of $7.1 million directly related to our acquisitions and integrating them into our company, partially offset by a write off of lease-related obligations of $0.4 million.
Business consolidation, transaction and restructuring costs of $7.8 million for the year ended December 31, 2011 consisted of $5.9 million in business consolidation and transaction costs and $1.9 million in restructuring costs. We have classified all transaction and integration related costs to business consolidation costs, which include personnel costs of $1.9 million related to employees who were notified that their employment with us was being terminated, $0.9 million of acquisition related contingent compensation related to the Contur Acquisition and professional service and other costs of $3.1 million directly related to our acquisition activities, including the Symyx Merger and Contur Acquisition, related integrations of Symyx’s and Contur’s businesses into ours and other non-operating investing activities.
Purchased Intangible Asset Amortization. Purchased intangible asset amortization decreased to $8.9 million for the year ended December 31, 2012, as compared to $9.8 million for the year ended December 31, 2011. As a percentage of revenue, amortization of purchased intangible assets decreased to 6% for the year ended December 31, 2012, as compared to 7% for the year ended December 31, 2011 and is related solely to the amortization of backlog, customer relationship and trademark intangible assets acquired in the Acquisitions. The decrease in purchased intangible asset amortization during the year ended December 31, 2012 was attributable primarily to $1.2 million increase in amortization related to the VelQuest, the Aegis and the Contur Acquisitions, offset by a decrease in amortization of approximately $2.1 million related to the intangibles acquired in the Symyx Merger.
Net Gain on Sale of Cost Method Investment
Pursuant to the terms of our July 28, 2011 agreement (the “IM Agreement”) between Symyx and Intermolecular, Inc. (“IM”), we sold our equity interest in IM in their November 18, 2011 initial public offering (the “IPO”). Upon consummation of the IPO, we received proceeds of approximately $37.3 million, net of transaction and consulting costs and commissions of $2.4 million. The carrying value of our investment in IM prior to the IPO was $18.3 million. As a result, during the fourth quarter of fiscal year 2011, we recognized a net gain of approximately $19.0 million on the sale of our equity investment in IM.
Royalty and Other Income, net
Royalty and other income, net increased to $8.9 million for the year ended December 31, 2012, as compared to $1.7 million for the year ended December 31, 2011. Significant components of royalty and other income, net for the year ended December 31, 2012 included net royalty revenue of $5.4 million, interest income of $2.3 million, amortization of the discount on promissory notes receivable of $0.9 million and the net gain on the sale of real estate of $2.7 million, partially offset by a foreign currency exchange loss of $0.6 million, amortization of purchased intangible assets of $1.7 million and $0.3 million net loss from rental activities primarily related to write off of certain lease-related assets.
Significant components of royalty and other income, net for the year ended December 31, 2011 included royalty revenue of $6.8 million, interest income of $1.5 million, amortization of the discount on promissory notes receivable of $0.2 million, and a foreign currency exchange gain of $0.5 million, partially offset by a $4.3 million write-off of intangible assets in connection with the sale of intellectual property and related deferred gain on sale as described in detail in Note 5 to the consolidated financial statements included elsewhere in this Report, royalty expense of $0.6 million and amortization of purchased intangible assets of $2.4 million
Income Tax Expense
Income tax expense increased to $0.2 million for the year ended December 31, 2012, as compared to income tax benefit of $0.8 million for the year ended December 31, 2011. Income tax expense consists of provision for income taxes for federal and state income taxes in the U.S. and income taxes in certain foreign tax jurisdictions. Income tax benefit related to U.S. jurisdictions was $0.7 million for the year ended December 31, 2012 compared to income tax expense of $0.4 million for the year ended December 31, 2011. The income tax benefit for the year ended December 31, 2012 resulted from a release in the fourth quarter of 2012 of $2.2 million of liability for unrecognized tax benefits due to the expiration of the statute of limitations applicable to the 2007 tax year, offset by $1.5 million of income tax expense.
Income tax expense related to foreign jurisdictions was $0.9 million for the year ended December 31, 2012 compared to income tax benefit of $1.2 million for the year ended December 31, 2011, which resulted primarily from a valuation allowance release for one of our United Kingdom subsidiaries.
The American Taxpayer Relief Act of 2012, which reinstated the U.S. federal research and development tax credit retroactively from January 1, 2012 through December 31, 2013, was not enacted into law until the first quarter of 2013. Therefore, the expected tax benefit resulting from such reinstatement for 2012 will not be reflected in the Company's estimated annual effective tax rate until 2013.
Comparison of the Year Ended December 31, 2011 and 2010
The following table summarizes our results of operations as a percentage of revenue for the respective periods:
|
| | | | | | |
| | Year Ended December 31, |
| | 2011 | | 2010 |
| | | | (unaudited) |
Revenue: | | | | |
License and subscription revenue | | 55 | % | | 66 | % |
Maintenance on perpetual licenses | | 24 | % | | 16 | % |
Content | | 12 | % | | 7 | % |
Professional services and other | | 9 | % | | 12 | % |
Total revenue | | 100 | % | | 100 | % |
Cost of revenue: | | | | |
Cost of revenue | | 25 | % | | 27 | % |
Amortization of completed technology | | 6 | % | | 5 | % |
Total cost of revenue | | 31 | % | | 32 | % |
Gross profit | | 69 | % | | 68 | % |
Operating expenses: | | | | |
Product development | | 24 | % | | 26 | % |
Sales and marketing | | 36 | % | | 46 | % |
General and administrative | | 11 | % | | 14 | % |
Business consolidation, transaction and restructuring costs | | 5 | % | | 17 | % |
Purchased intangible asset amortization | | 7 | % | | 3 | % |
Total operating expenses | | 83 | % | | 106 | % |
Operating loss | | (14 | )% | | (38 | )% |
Net gain on sale of cost method investment | | 13 | % | | — |
|
Royalty and other income, net | | 1 | % | | 2 | % |
Loss before income taxes | | 1 | % | | (37 | )% |
Income tax benefit | | (1 | )% | | (14 | )% |
Net income (loss) | | 1 | % | | (23 | )% |
Due to rounding to the nearest percent, totals may not equal the sum of the line items in the table above.
Revenue
Total revenue increased 43% to $144.3 million for the year ended December 31, 2011, as compared to approximately $101.0 million for the year ended December 31, 2010. The increase in total revenue was attributable to the Symyx Merger.
License and Subscription Revenue. License and subscription revenue increased to $79.4 million for the year ended December 31, 2011, as compared to $66.4 million for the year ended December 31, 2010. As a percentage of revenue, license and subscription revenue decreased to 55% for the year ended December 31, 2011, as compared to 66% for the year ended December 31, 2010. The increase in license and subscription revenue was attributable to the Symyx Merger.
Maintenance on Perpetual Licenses. Maintenance on perpetual licenses revenue increased to $34.9 million for the year ended December 31, 2011, as compared to $15.7 million for the year ended December 31, 2010. As a
percentage of revenue, revenue from maintenance on perpetual licenses increased to 24% for the year ended December 31, 2011, as compared to 16% for the year ended December 31, 2010. The increase in revenue from maintenance on perpetual licenses was attributable to the Symyx Merger.
Content. Content revenue increased to $16.8 million for the year ended December 31, 2011, as compared to $6.6 million for the year ended December 31, 2010. As a percentage of revenue, content revenue increased to 12% for the year ended December 31, 2011, as compared to 7% for the year ended December 31, 2010. The increase in content revenue was attributable to the Symyx Merger.
Professional Services and Other. Professional services and other revenue increased to $13.2 million for the year ended December 31, 2011, as compared to $12.3 million for the year ended December 31, 2010. As a percentage of revenue, professional services and other revenue decreased to 9% for the year ended December 31, 2011, as compared to 12% for the year ended December 31, 2010. The increase in professional services and other revenue was attributable to the Symyx Merger.
Total Cost of Revenue
Cost of Revenue. Cost of revenue increased to $36.1 million for the year ended December 31, 2011, as compared to $27.1 million for the year ended December 31, 2010. As a percentage of revenue, cost of revenue decreased to 25% for the year ended December 31, 2011, as compared to 27% for the year ended December 31, 2010. The increase in cost of revenue was primarily attributable to an increase in software and content royalties of approximately $1.4 million, an increase in personnel and related expenses in our services department of approximately $5.3 million, an increase in professional fees of approximately $0.7 million and an increase in overhead expense of approximately $1.6 million, each as a result of the Symyx Merger.
Amortization of Completed Technology. Amortization of completed technology increased to $8.4 million for the year ended December 31, 2011, as compared to $4.9 million for the year ended December 31, 2010. As a percentage of revenue, amortization of completed technology increased to 6% for the year ended December 31, 2011, as compared to 5% for the year ended December 31, 2010. The increase in amortization of completed technology was due to amortization of completed technology intangible assets acquired in the Symyx Merger and the Contur Acquisition.
Operating Expenses
Product Development Expenses. Product development expenses increased to approximately $34.0 million for the year ended December 31, 2011, as compared to $26.5 million for the year ended December 31, 2010. As a percentage of revenue, product development expenses decreased to 24% for the year ended December 31, 2011, as compared to 26% for the year ended December 31, 2010. The increase in product development expenses was primarily attributable to an increase in personnel and related expenses of approximately $4.8 million, an increase in professional fees of approximately $0.4 million and an increase in overhead expense of approximately $2.3 million, each as a result of the Symyx Merger.
Sales and Marketing Expenses. Sales and marketing expenses increased to $51.5 million for the year ended December 31, 2011, as compared to $46.6 million for the year ended December 31, 2010. As a percentage of revenue, sales and marketing expenses decreased to 36% for the year ended December 31, 2011, as compared to 46% for the year ended December 31, 2010. The increase in sales and marketing expenses was primarily attributable to an increase in personnel and related expenses of approximately $3.4 million and an increase in overhead expense of approximately $1.5 million, partially offset by a decrease in professional fees of approximately $0.1 million, each as a result of the Symyx Merger.
General and Administrative Expenses. General and administrative expenses increased to $16.5 million for the year ended December 31, 2011, as compared to $14.5 million for the year ended December 31, 2010. As a percentage of revenue, general and administrative expenses decreased to 11% for the year ended December 31, 2011, as compared to 14% for the year ended December 31, 2010. The increase in general and administrative expenses was primarily attributable to an increase in personnel and related expenses of approximately $1.2 million, an increase in professional fees of approximately $0.2 million and an increase in overhead expense of approximately $0.6 million, each as a result of the Symyx Merger.
Business Consolidation, Transaction and Restructuring Costs. Business consolidation, transaction and restructuring costs of $7.8 million for the year ended December 31, 2011 consisted of $5.9 million in business consolidation and transaction costs and $1.9 million in restructuring costs. As a percentage of revenue, business consolidation, transaction and restructuring costs decreased to 5% for the year ended December 31, 2011, as compared to 17% for the year ended December 31, 2010. We have classified all transaction and integration related costs to business consolidation costs, which include personnel costs of $1.9 million related to employees who were notified that their employment with us was being terminated, $0.9 million of acquisition related contingent compensation related to the Contur Acquisition and professional service and other costs of $3.1 million directly related to our acquisition activities, including the Symyx Merger and Contur Acquisition, related integrations of
Symyx’s and Contur’s businesses into ours and other non-operating investing activities. Business consolidation, transaction and restructuring costs of $17.2 million for the year ended December 31, 2010 consisted of $4.6 million in restructuring costs and $12.6 million in business consolidation and transaction costs, including $5.4 million related to employees who were notified that their employment with us was being terminated and professional service and other costs of $7.2 million directly related to the Symyx Merger and related integration of Symyx’s business into ours.
Purchased Intangible Asset Amortization. Purchased intangible assets amortization increased to $9.8 million for the year ended December 31, 2011, as compared to $2.7 million for the year ended December 31, 2010. As a percentage of revenue, purchased intangible asset amortization increased to 7% for the year ended December 31, 2011, as compared to 3% for the year ended December 31, 2010 and was related solely to the amortization of backlog and customer relationship and trademark intangible assets acquired in the Symyx Merger.
Net Gain on Sale of Cost Method Investment. Pursuant to the terms of the IM Agreement, we sold our equity interest in IM in the IPO. Upon consummation of the IPO, we received proceeds of approximately $37.3 million, net of transaction and consulting costs and commissions of $2.4 million. The carrying value of our investment in IM prior to the IPO was $18.3 million. As a result, during the fourth quarter of fiscal year 2011, we recognized a net gain of approximately $19.0 million on the sale of our equity investment in IM.
Royalty and Other Income, net. Royalty and other income, net was approximately $1.7 million for both the year ended December 31, 2011 and 2010. Significant components of royalty and other income, net for the year ended December 31, 2011 included interest income of $1.5 million, royalty revenue of $6.8 million and a foreign currency exchange gain of $0.5 million, partially offset by a $4.3 million write-off of intangible assets in connection with the sale of intellectual property and related deferred gain on sale as described in detail in Note 5 to the consolidated financial statements included elsewhere in this Report, royalty expense of $0.6 million and amortization of purchased intangible assets of $2.4 million. Significant components of royalty and other income, net for the year ended December 31, 2010 included net interest income of $0.8 million, royalty revenue of $2.0 million and foreign currency exchange gain of $0.4 million, partially offset by royalty expense of $0.3 million and amortization of purchased intangible assets of $1.2 million.
Income Tax Benefit
Income tax benefit was $0.8 million for the year ended December 31, 2011, as compared to income tax benefit of $13.9 million for the year ended December 31, 2010. In connection with the Symyx Merger, we released $14.9 million of our valuation allowance against our deferred tax assets, as we have concluded that a portion of our NOLs can be utilized against the deferred tax liabilities assumed in connection with the Symyx Merger.
Comparison of the Nine Months Ended December 31, 2010 and 2009
The following table summarizes our results of operations as a percentage of revenue for the respective periods:
|
| | | | | | |
| | Nine Months Ended December 31, |
| | 2010 | | 2009 |
| | | | (unaudited) |
Revenue | | 100 | % | | 100 | % |
Cost of revenue: | | | | |
Cost of revenue | | 29 | % | | 17 | % |
Amortization of completed technology | | 6 | % | | 1 | % |
Total cost of revenue | | 35 | % | | 18 | % |
Gross profit | | 65 | % | | 82 | % |
Operating expenses: | | | | |
Product development | | 28 | % | | 19 | % |
Sales and marketing | | 43 | % | | 42 | % |
General and administrative | | 15 | % | | 14 | % |
Business consolidation, transaction and restructuring costs (recoveries) | | 21 | % | | *% |
|
Purchased intangible asset amortization | | 3 | % | | — |
|
Total operating expenses | | 110 | % | | 75 | % |
Operating income (loss) | | (45 | )% | | 7 | % |
Royalty and other income, net | | 2 | % | | 1 | % |
Income (loss) before income taxes | | (43 | )% | | 8 | % |
Income tax expense (benefit) | | (18 | )% | | 2 | % |
Net income (loss) | | (25 | )% | | 6 | % |
Due to rounding to the nearest percent, totals may not equal the sum of the line items in the table above.
Revenue
Revenue increased 29% to $80.2 million for the nine months ended December 31, 2010 compared to $62.2 million for the nine months ended December 31, 2009. The increase in total revenue was primarily attributable to the Symyx Merger, and includes Symyx revenue of approximately $17.9 million.
Total Cost of Revenue
Cost of Revenue. Cost of revenue increased to $23.5 million for the nine months ended December 31, 2010, as compared to $10.5 million for the nine months ended December 31, 2009. As a percentage of revenue, cost of revenue increased to 29% for the nine months ended December 31, 2010, as compared to 17% for the nine months ended December 31, 2009. The increase in cost of revenue was primarily attributable to an increase in software and content royalties of approximately $3.9 million, an increase in personnel and related expenses in our services department of approximately $4.8 million, an increase in professional fees of approximately $0.9 million and an increase in overhead allocated expense of approximately $3.4 million, each as a result of the Merger.
Amortization of Completed Technology. Amortization of completed technology increased to $4.9 million for the nine months ended December 31, 2010, as compared to $0.8 million for the nine months ended December 31, 2009. As a percentage of revenue, amortization of completed technology increased to 6% for the nine months ended December 31, 2010, as compared to 1% for the nine months ended December 31, 2009. The increase in amortization of completed technology was due to amortization of completed technology intangible assets acquired in the Symyx Merger.
Operating Expenses
Product Development Expenses. Product development expenses increased to $22.1 million for the nine months ended December 31, 2010, as compared to $11.6 million for the nine months ended December 31, 2009. As a percentage of revenue,
product development expenses increased to 28% for the nine months ended December 31, 2010, as compared to 19% for the nine months ended December 31, 2009. The increase in product development expenses was primarily attributable to an increase in personnel and related expenses of approximately $5.3 million, an increase in professional fees of approximately $2.3 million and an increase in overhead expense of approximately $2.9 million, each as a result of the Symyx Merger.
Sales and Marketing Expenses. Sales and marketing expenses increased to $34.8 million for the nine months ended December 31, 2010, as compared to $26.3 million for the nine months ended December 31, 2009. As a percentage of revenue, sales and marketing expenses increased to 43% for the nine months ended December 31, 2010, as compared to 42% for the nine months ended December 31, 2009. The increase in sales and marketing expenses was primarily attributable to an increase in personnel and related expenses of approximately $5.7 million, an increase in professional fees of approximately $0.9 million and an increase in overhead expense of approximately $2.0 million, each as a result of the Symyx Merger.
General and Administrative Expenses. General and administrative expenses increased to $11.7 million for the nine months ended December 31, 2010, as compared to $8.9 million for the nine months ended December 31, 2009. As a percentage of revenue, general and administrative expenses increased to 15% for the nine months ended December 31, 2010, as compared to 14% for the nine months ended December 31, 2009. The increase in general and administrative expenses was primarily attributable to an increase in personnel and related expenses of approximately $3.0 million, an increase in professional fees of approximately $1.5 million and an increase in overhead expenses of approximately $1.8 million, each as a result of the Symyx Merger.
Business Consolidation, Transaction and Restructuring Costs. Business consolidation, transaction and restructuring costs of $16.5 million for the nine months ended December 31, 2010, were comprised of $11.8 million in business consolidation costs and $4.7 million in restructuring costs. As a percentage of revenue, business consolidation, transaction and restructuring costs were 21% for the nine months ended December 31, 2010. We have classified all transaction and integration related costs to business consolidation costs, which includes personnel costs of $5.4 million related to current employees who have been terminated or notified that their employment with us was being terminated and professional service and other costs of $6.4 million directly related to the Symyx Merger and integration of Symyx’s business into ours.
Purchased Intangible Asset Amortization. Purchased intangible asset amortization was $2.7 million for the nine months ended December 31, 2010. As a percentage of revenue, purchased intangible asset amortization was 3% for the nine months ended December 31, 2010, and was related solely to the amortization of backlog and customer relationship and trademark intangible assets acquired in the Symyx Merger.
Royalty and Other Income, net
Royalty and other income, net was $1.4 million for the nine months ended December 31, 2010, as compared to $0.6 million for the nine months ended December 31, 2009. Significant components of interest and other income, net for the nine months ended December 31, 2010 included interest income of $0.7 million, royalty income of $2.0 million and a foreign currency exchange gain of $0.2 million, partially offset by royalty expense of $0.3 million and amortization of purchased intangible assets of $1.2 million. Significant components of interest and other income, net for the nine months ended December 31, 2009 included interest income of $0.2 million, foreign currency exchange gain of $0.2 million and a gain on our auction rate securities of $0.2 million.
Income Tax Expense (Benefit)
Income tax benefit was $14.1 million for the nine months ended December 31, 2010, as compared to income tax expense of $1.1 million for the nine months ended December 31, 2009. In connection with the Symyx Merger, we released $14.9 million of our valuation allowance against our deferred tax assets, as we have concluded that our NOLs can be utilized against the deferred tax liabilities assumed in connection with the Symyx Merger.
Liquidity and Capital Resources
We had cash, cash equivalents, marketable securities, and restricted cash of $115.6 million as of December 31, 2012, as compared to $143.6 million as of December 31, 2011, a decrease of $28.0 million. Of these amounts, $16.1 million and $21.8 million as of December 31, 2012 and December 31, 2011, respectively, were held by our foreign subsidiaries. If such funds are needed for our U.S. operations, the repatriation of cash balances from our foreign subsidiaries would result in the recognition and payment of U.S. income taxes. We generally invest our cash and cash equivalents in investment grade, highly liquid fixed income securities. Our marketable securities portfolio is denominated in U.S. Dollars and consists of investment grade, highly liquid securities of various holdings including obligations of U.S. government sponsored enterprises, commercial paper, certificates of deposit, corporate and municipal debt. Our primary objective for holding fixed income securities is to achieve an appropriate investment return consistent with preserving principal and managing risk.
The decrease in cash, cash equivalents, marketable securities and restricted cash during the year ended December 31, 2012 was primarily attributable to cash provided by operations of $17.0 million, net proceeds from the sale of real estate of $6.8 million and proceeds from the sale of common stock of $6.2 million, net of shares tendered for payment of withholding tax, partially offset by $40.7 million in cash paid for acquisitions, purchases of property and equipment of $6.3 million, payment of contingent consideration liabilities related to the Contur Acquisition of $0.3 million and repurchases of our common stock of $11.3 million.
The following table sets forth a summary of our cash flows for the periods indicated (in thousands):
|
| | | | | | | |
| Year Ended December 31, |
| 2012 | | 2011 |
| |
Net cash provided by operating activities | $ | 18,247 |
| | $ | 23,735 |
|
Net cash used in investing activities | (9,510 | ) | | (35,509 | ) |
Net cash used in financing activities | (5,355 | ) | | (4,754 | ) |
Net cash provided by operating activities was $18.2 million for the year ended December 31, 2012, as compared to $23.7 million for the year ended December 31, 2011. The decrease in cash provided by operating activities during the year ended December 31, 2012 was primarily attributable to changes in working capital, partially offset by an increase in our net loss for the year ended December 31, 2012.
Net cash used in investing activities was $9.5 million for the year ended December 31, 2012, as compared to net cash used in investing activities of $35.5 million for the year ended December 31, 2011. Significant components of cash flows from investing activities for the year ended December 31, 2012 included $30.7 million in cash paid for the Aegis Acquisition, $4.5 million in cash paid for the acquisition of the HEOS platform, $5.4 million of acquisition related deposit paid for the acquisition of Vialis, net purchases of property and equipment of $6.3 million and purchases of marketable securities of approximately $51.0 million, partially offset by proceeds from maturities of marketable securities of approximately $80.6 million, net proceeds from the sale of real estate of $6.8 million and payments on promissory notes receivable of $0.8 million. Significant components of cash flows from investing activities for the year ended December 31, 2011 included cash paid for the VelQuest Acquisition of $35.0 million, cash paid for the Contur Acquisition of $9.9 million, funding of acquisition related restricted cash of $0.5 million and an acquisition related prepaid contingent compensation of $2.0 million, net of purchases of property and equipment of $3.9 million and purchases of marketable securities of approximately $104.1 million, partially offset by proceeds from the sale of our equity interest in IM in their IPO of $37.3 million, net of transaction and consulting costs and commissions of $2.4 million, proceeds from maturities of marketable securities of approximately $81.5 million and net decrease of restricted cash of $ 0.3 million.
Net cash used in financing activities was $5.4 million for the year ended December 31, 2012, as compared to net cash used in financing activities of $4.8 million for the year ended December 31, 2011. Significant components of cash used in financing activities for the year ended December 31, 2012 included repurchases of our common stock of $11.3 million, payment of contingent consideration liabilities related to the Contur Acquisition of $0.3 million, partially offset by $6.2 million of proceeds from the issuance of common stock under our equity incentive plans, reduced by payments made to taxing authorities on behalf of our employees when tendering stock to us for settlement of minimum income tax liability upon vesting of restricted stock units (“RSUs”). Significant components of cash flows from financing activities for the year ended December 31, 2011 included repurchases of our common stock of $7.0 million partially offset by $0.2 million excess tax benefit from exercise of stock options and $2.1 million in proceeds from the issuance of our common stock under employee stock plans, reduced by payments made to taxing authorities on behalf of our employees when tendering stock to us for settlement of minimum income tax liability upon vesting RSUs.
We have funded our activities to date primarily through the sales of software licenses and related services and the issuance of equity securities.
We anticipate that our capital requirements may increase in future periods as a result of seasonal sales trends, additional product development activities and infrastructure investments. Our capital requirements may also increase in future periods as we seek to expand our technology platform through investments, licensing arrangements, technology alliances or acquisitions.
On October 26, 2012, our board of directors authorized us to commit up to $15.0 million for the repurchase of our common stock pursuant to a program to be executed in accordance with a Rule 10b5-1 trading plan during fiscal year 2013.
On January 11, 2013, we acquired Vialis for aggregate consideration consisting of: (i) an upfront payment in an amount equal to five million Swiss francs (or approximately $5.4 million), subject to certain adjustments and escrow holdbacks set forth in the purchase agreement; and (ii) certain contingent earn-out consideration payable during the next three fiscal years in an aggregate amount of up to five million Swiss francs (or approximately $5.4 million), subject to the satisfaction of certain milestones set forth in the purchase agreement. Vialis is a leading systems integrator based in Liestal, Switzerland that serves the pharmaceutical, biotechnology, chemicals and agro-science industries. We acquired Vialis as part of our strategy to expand our professional services offerings internationally. We funded the purchase price with cash on hand.
We anticipate that our existing capital resources will be adequate to fund our operations for at least the next twelve months. However, there can be no assurance that changes will not occur that would consume our available capital resources before that time. Our capital requirements depend on numerous factors, including our ability to continue to generate software sales, the purchase of additional capital equipment and acquisitions of other businesses or technologies. There can be no assurance that additional funding, if necessary, will be available to us on favorable terms, if at all. Our forecast for the period of time through which our financial resources will be adequate to support our operations is forward-looking information, and actual results could vary.
Contractual Obligations
Our long-term contractual obligations as of December 31, 2012 are as follows (in thousands):
|
| | | | | | | | | | | | | | | | | | | | |
| | | | Payment Due by Period |
Contractual Obligations | | Total | | Less than 1 Year | | 1 to 3 Years | | 3 to 5 Years | | After 5 Years |
Operating leases, net of subleases | | $ | 40,745 |
| | $ | 6,234 |
| | $ | 10,963 |
| | $ | 6,920 |
| | $ | 16,628 |
|
Minimum royalty payments(1) | | 4,422 |
| | 1,008 |
| | 872 |
| | 872 |
| | 1,670 |
|
Total | | $ | 45,167 |
| | $ | 7,242 |
| | $ | 11,835 |
| | $ | 7,792 |
| | $ | 18,298 |
|
| |
(1) | Includes indefinite-lived contractual minimum royalty payments due in the ten fiscal years following December 31, 2012. |
We are obligated to pay royalties for licenses to enhance and market certain software used in connection with our product offerings. In addition to the contractual minimum royalties included in the table above, we have several royalty agreements that are long term or perpetual in nature with royalty obligations that are generally based on a percentage of revenues derived from certain software license sales and associated sales of PCS. The royalty agreements require quarterly payments and generally do not limit the maximum royalties owed. We incurred royalty related expenses of $5.5 million, $5.8 million, $4.6 million and $1.8 million for the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010 and December 31, 2009, respectively. Our future annual royalty obligations will vary based on the volume and mix of our product sales and, as a result, may increase in the future.
On April 30, 2004, in connection with the spin-off of PDD, the landlords of our New Jersey facilities, which were used by our PDD operations, consented to the assignment of the leases to PDD. Despite the assignment, the landlords required us to guarantee the remaining lease obligations, which totaled approximately $8.5 million as of December 31, 2012. In accordance with ASC Topic 460, Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others, we recognized a liability and corresponding charge to stockholders’ equity for the probability-weighted fair market value of the guarantee. Changes to the fair market value of the liability are recognized in stockholders’ equity. The liability for our guarantee of the lease obligation was $0.2 million and $0.3 million at December 31, 2012 and December 31, 2011, respectively.
Off-Balance Sheet Arrangements and Related Party Transactions
As of December 31, 2012 we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As such, we are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in such relationships.
We do not have relationships or transactions with persons or entities that derive benefits from their non-independent relationship with us or our related parties.
Recently Issued Accounting Standards
See Note 1 to our audited consolidated financial statements included elsewhere in this Report for recently issued accounting standards, including the expected dates of adoption and expected impact on our consolidated financial statements upon adoption.
Common Stock Repurchases
On November 10, 2010, we entered into a stock repurchase plan (the “2010 Repurchase Plan”) in accordance with Rule 10b5-1 of the Exchange Act. Pursuant to the 2010 Repurchase Plan, and subject to the conditions set forth therein and the provisions of Rule 10b-18 of the Exchange Act, we instructed the broker to purchase for our account up to $6.0 million worth of our common stock by March 31, 2011. We purchased 353,955 shares of our common stock for approximately $3.0 million at an average cost of $8.40 per share during the quarter ended December 31, 2010, and we completed the repurchase of the remaining $3.0 million of our common stock by purchasing 350,553 shares at an average cost of $8.50 per share during the first quarter ended March 31, 2011.
On March 11, 2011, we entered into a second repurchase plan (the “2011 Repurchase Plan”) in accordance with Rule 10b5-1 of Exchange Act. Pursuant to the 2011 Repurchase Plan, and subject to the conditions set forth therein and the provisions of Rule 10b-18 of the Exchange Act, we instructed the broker to purchase for our account up to an additional $4.0 million worth of our common stock by September 30, 2011. We purchased 253,624 shares of our common stock for $2.0 million during the quarter ended June 30, 2011, and we completed the repurchase of the remaining $2.0 million of our common stock by purchasing 273,141 shares at an average cost of $7.29 per share during the quarter ended September 30, 2011.
On August 3, 2011 and February 29, 2012, our board of directors authorized the Company to commit up to $8.0 million and $2.0 million, respectively, for an aggregate of $10.0 million, for the repurchase of the Company's common stock during fiscal 2012 pursuant to a program to be executed in accordance with a Rule 10b5-1 trading plan. On March 14, 2012, we entered into the 2012 Repurchase Plan in accordance with Rule 10b5-1 of Exchange Act. Pursuant to the 2012 Repurchase Plan, and subject to the conditions set forth therein and the provisions of Rule 10b-18 of the Exchange Act, we instructed the broker to purchase for our account up to $10.0 million worth of our common stock by December 31, 2012.
As of December 31, 2012, we have repurchased 1,373,355 shares of our common stock under the 2012 Repurchase Plan for approximately $11.3 million at an average cost of $8.22 per share. In addition, as of such date, we have made cumulative repurchases of 2,604,628 shares, at a cost of approximately $21.3 million (including commissions), since we began our repurchase program in November 2010. Stock repurchases were funded principally from operating cash flows. All repurchased shares of common stock have been retired.
On October 26, 2012, our board of directors authorized us to commit up to $15.0 million for the repurchase of our common stock pursuant to a program to be executed in accordance with a Rule 10b5-1 trading plan during fiscal year 2013. On December 13, 2012, we entered into a stock repurchase plan (the “2013 Repurchase Plan”) in accordance with Rule 10b5-1 of Exchange Act. Pursuant to the 2013 Repurchase Plan, and subject to the conditions set forth therein and the provisions of Rule 10b-18 of the Exchange Act, we instructed the broker to purchase for our account up to $15.0 million worth of our common stock by December 31, 2013.
As of March 1, 2013, we have repurchased 353,949 shares of our common stock for approximately $3.3 million at a weighted-average cost of $9.39 per share, pursuant to the 2013 Repurchase Plan.
|
| |
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
In addition to our U.S. operations, we have operations in Europe and the Asia/Pacific region. As a result, our financial position, results of operations and cash flows can be affected by fluctuations in foreign currency exchange rates, particularly fluctuations in the pound sterling, euro and yen exchange rates. Many of our reporting entities conduct a portion of their business in currencies other than the entity’s functional currency. These transactions give rise to receivables and payables that are denominated in currencies other than the entity’s functional currency. The value of these receivables and payables is subject to changes in exchange rates because the receivables and payables may become worth more or less than they were worth at the time we entered into the transaction due to changes in currency exchange rates. The majority of our software license agreements are denominated in U.S. dollars. Both realized and unrealized gains or losses on the value of these receivables and payables, along with the settlement of certain intercompany transactions, are included in the determination of net income.
To mitigate our risk from foreign currency fluctuations, we launched a foreign currency hedging program in December 2011 whereby we will periodically enter into currency derivative contracts, principally forward contracts with short term
maturities. Our intent is to offset gains and losses that occur from the underlying exposure with gains and losses on the derivative contracts used to offset them. We do not hold or purchase any foreign currency contracts for trading or speculative purposes and we do not designate these forward contracts or swaps as hedging instruments. Accordingly, we report the fair value of these contracts in the consolidated balance sheet with changes in fair value recorded in the consolidated statements of operations. The fair value of foreign currency contracts is estimated based on the prevailing exchange rates of the various hedged currencies as of the end of the period.
Our investment portfolio is maintained in accordance with our investment policy that defines allowable investments, specifies credit quality standards and limits the credit exposure of any single issuer. None of our investments are held for trading or speculative purposes. The fair value of our cash equivalents and marketable securities is subject to change as a result of changes in market interest rates and investment risk related to the issuers’ credit worthiness. We do not utilize financial contracts to manage our exposure in our investment portfolio to changes in interest rates. A hypothetical 100 basis point increase or decrease in market interest rates would not have a material impact on the fair value of our cash equivalents and marketable securities due to the relatively short maturities of these investments. While changes in interest rates may affect the fair value of our investment portfolio, any gains or losses will not be recognized in our results of operations until the investment is sold or if the reduction in fair value was determined to be an other-than-temporary impairment.
|
| |
Item 8. | Financial Statements and Supplementary Data |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Accelrys, Inc.
We have audited the accompanying consolidated balance sheets of Accelrys, Inc. as of December 31, 2012 and 2011, and the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2012 and the nine month period ended December 31, 2010. Our audits also included the financial statement schedule listed in the index at Item 15(a)(2). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Accelrys, Inc. at December 31, 2012 and 2011, and the consolidated results of its operations and its cash flows for each of the two years in the period ended December 31, 2012 and the nine month period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Accelrys, Inc.’s internal control over financial reporting as of December 31, 2012, based on the criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 6, 2013 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
San Diego, California
March 6, 2013
Accelrys, Inc.
Consolidated Balance Sheets
(In thousands)
|
| | | | | | | |
| December 31,
2012 | | December 31, 2011 |
| | | |
Assets | | | |
Current assets: | | | |
Cash and cash equivalents | $ | 72,747 |
| | $ | 69,610 |
|
Restricted cash | 3,227 |
| | — |
|
Marketable securities | 26,851 |
| | 52,976 |
|
Trade receivables, net of allowance for doubtful accounts of $143 as of December 31, 2012 and December 31, 2011 | 47,196 |
| | 40,706 |
|
Promissory note receivable | 25,895 |
| | 921 |
|
Prepaid expenses, deferred tax assets and other current assets | 10,492 |
| | 11,090 |
|
Total current assets | 186,408 |
| | 175,303 |
|
Marketable securities, net of current portion | 12,548 |
| | 17,224 |
|
Restricted cash, net of current portion | 273 |
| | 3,814 |
|
Property and equipment, net | 10,888 |
| | 12,108 |
|
Goodwill | 123,670 |
| | 100,429 |
|
Purchased intangible assets, net | 53,223 |
| | 60,361 |
|
Promissory notes receivable, net of current portion | 8,901 |
| | 33,799 |
|
Other assets | 9,931 |
| | 4,848 |
|
Total assets | $ | 405,842 |
| | $ | 407,886 |
|
Liabilities and stockholders’ equity | | | |
Current liabilities: | | | |
Accounts payable | $ | 3,279 |
| | $ | 3,527 |
|
Accrued liabilities | 19,760 |
| | 19,361 |
|
Accrued compensation and benefits | 14,514 |
| | 12,372 |
|
Current portion of accrued restructuring charges | 324 |
| | 1,322 |
|
Current portion of deferred gain on sale of intellectual property | 25,895 |
| | 921 |
|
Current portion of deferred revenue | 86,340 |
| | 81,151 |
|
Total current liabilities | 150,112 |
| | 118,654 |
|
Deferred revenue, net of current portion | 2,811 |
| | 4,861 |
|
Accrued income tax | 8,710 |
| | 8,095 |
|
Deferred gain on sale of intellectual property, net of current portion | — |
| | 25,053 |
|
Accrued restructuring charges, net of current portion | 367 |
| | 388 |
|
Lease-related liabilities, net of current portion | 1,021 |
| | 2,151 |
|
Stockholders’ equity: | | | |
Preferred stock, $.0001 par value; 2,000 shares authorized; no shares issued and outstanding | — |
| | — |
|
Common stock, $.0001 par value; 100,000 shares authorized; 55,879 and 57,588 shares issued and outstanding at December 31, 2012 and December 31, 2011, respectively | 6 |
| | 6 |
|
Additional paid-in capital | 480,001 |
| | 465,701 |
|
Lease guarantee | (210 | ) | | (273 | ) |
Treasury stock; zero and 1,875 shares at December 31, 2012 and December 31, 2011, respectively | — |
| | (18,340 | ) |
Accumulated deficit | (238,240 | ) | | (198,208 | ) |
Accumulated other comprehensive income (loss) | 1,264 |
| | (202 | ) |
Total stockholders’ equity | 242,821 |
| | 248,684 |
|
Total liabilities and stockholders’ equity | $ | 405,842 |
| | $ | 407,886 |
|
See accompanying notes to these consolidated financial statements.
Accelrys, Inc.
Consolidated Statements of Operations
(In thousands, except per share amounts)
|
| | | | | | | | | | | | | | | |
| For the Years Ended | | For the Nine Months Ended |
| December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2009 |
| | | | | | | (unaudited) |
Revenue: | | | | | | | |
License and subscription revenue | $ | 89,440 |
| | $ | 79,425 |
| | $ | 51,003 |
| | $ | 48,949 |
|
Maintenance on perpetual licenses | 38,254 |
| | 34,862 |
| | 12,512 |
| | 7,839 |
|
Content | 12,485 |
| | 16,838 |
| | 6,592 |
| | — |
|
Professional services and other | 22,347 |
| | 13,214 |
| | 10,098 |
| | 5,412 |
|
Total revenue | 162,526 |
| | 144,339 |
| | 80,205 |
| | 62,200 |
|
Cost of revenue: | | | | | | | |
Cost of revenue | 41,695 |
| | 36,065 |
| | 23,528 |
| | 10,524 |
|
Amortization of completed technology | 8,843 |
| | 8,393 |
| | 4,887 |
| | 803 |
|
Total cost of revenue | 50,538 |
| | 44,458 |
| | 28,415 |
| | 11,327 |
|
Gross profit | 111,988 |
| | 99,881 |
| | 51,790 |
| | 50,873 |
|
Operating expenses: | | | | | | | |
Product development | 38,849 |
| | 33,977 |
| | 22,080 |
| | 11,633 |
|
Sales and marketing | 57,971 |
| | 51,517 |
| | 34,848 |
| | 26,338 |
|
General and administrative | 17,480 |
| | 16,467 |
| | 11,697 |
| | 8,946 |
|
Business consolidation, transaction and restructuring costs (recoveries) | 7,803 |
| | 7,775 |
| | 16,493 |
| | (90 | ) |
Purchased intangible asset amortization | 8,939 |
| | 9,846 |
| | 2,712 |
| | — |
|
Total operating expenses | 131,042 |
| | 119,582 |
| | 87,830 |
| | 46,827 |
|
Operating income (loss) | (19,054 | ) | | (19,701 | ) | | (36,040 | ) | | 4,046 |
|
Net gain on sale of cost method investment | — |
| | 18,970 |
| | — |
| | — |
|
Royalty and other income, net | 8,870 |
| | 1,740 |
| | 1,377 |
| | 620 |
|
Income (loss) before income taxes | (10,184 | ) | | 1,009 |
| | (34,663 | ) | | 4,666 |
|
Income tax expense (benefit) | 218 |
| | (756 | ) | | (14,055 | ) | | 1,097 |
|
Net income (loss) | $ | (10,402 | ) | | $ | 1,765 |
| | $ | (20,608 | ) | | $ | 3,569 |
|
| | | | | | | |
Net income (loss) per share amounts: | | | | | | | |
Basic | $ | (0.19 | ) | | $ | 0.03 |
| | $ | (0.44 | ) | | $ | 0.13 |
|
Diluted | $ | (0.19 | ) | | $ | 0.03 |
| | $ | (0.44 | ) | | $ | 0.13 |
|
Weighted average shares used to compute net income (loss) per share: | | | | | | | |
Basic | 55,696 |
| | 55,489 |
| | 46,446 |
| | 27,470 |
|
Diluted | 55,696 |
| | 56,037 |
| | 46,446 |
| | 27,704 |
|
See accompanying notes to these consolidated financial statements.
Accelrys, Inc.
Consolidated Statements of Comprehensive Income
(In thousands)
|
| | | | | | | | | | | | | | | |
| For the Years Ended | | For the Nine Months Ended |
| December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2009 |
| | | | | | | (unaudited) |
Net income (loss) | $ | (10,402 | ) | | $ | 1,765 |
| | $ | (20,608 | ) | | $ | 3,569 |
|
Other comprehensive income (loss): | | | | | | | |
Foreign currency translation adjustment | 1,479 |
| | (1,726 | ) | | (28 | ) | | (748 | ) |
Unrealized gain (loss) on marketable securities, net of tax | (13 | ) | | 118 |
| | (29 | ) | | 25 |
|
Total other comprehensive income (loss) | 1,466 |
| | (1,608 | ) | | (57 | ) | | (723 | ) |
Comprehensive income (loss) | $ | (8,936 | ) | | $ | 157 |
| | $ | (20,665 | ) | | $ | 2,846 |
|
See accompanying notes to these consolidated financial statements.
Accelrys, Inc.
Consolidated Statements of Stockholders’ Equity
(In thousands)
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional Paid-In Capital | | Lease Guarantee | | Treasury Stock | | Accumulated Deficit | | Accumulated Other Comprehensive Income (Loss) | | Total Stockholders’ Equity |
| | Shares | | Amount | | Shares | | Amount | |
Balance at March 31, 2010 | | 28,409 |
| | $3 | | $272,726 | | $ | (383 | ) | | 644 |
| | (8,340 | ) | | (179,365 | ) | | $1,463 | | $86,104 |
Net loss | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (20,608 | ) | | — |
| | (20,608 | ) |
Other comprehensive loss | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (57 | ) | | (57 | ) |
Change in value of lease guarantee | | — |
| | — |
| | — |
| | 47 |
| | — |
| | — |
| | — |
| | — |
| | 47 |
|
Stock-based compensation expense | | — |
| | — |
| | 4,298 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 4,298 |
|
Equity instruments issued in connection with the Symyx Merger | | 27,502 |
| | 3 |
| | 179,294 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 179,297 |
|
Equity issuance costs | | — |
| | — |
| | (783 | ) | | — |
| | — |
| | — |
| | — |
| | — |
| | (783 | ) |
Repurchase of common stock | | — |
| | — |
| | — |
| | — |
| | 354 |
| | (3,000 | ) | | — |
| | — |
| | (3,000 | ) |
Issuance of common stock upon exercise of stock options and under employee stock purchase plan, net of shares tendered | | 822 |
| | — |
| | 2,348 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 2,348 |
|
Balance at December 31, 2010 | | 56,733 |
| | $ | 6 |
| | $ | 457,883 |
| | $ | (336 | ) | | 998 |
| | $ | (11,340 | ) | | $ | (199,973 | ) | | $ | 1,406 |
| | $ | 247,646 |
|
Net income | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 1,765 |
| | — |
| | 1,765 |
|
Other comprehensive loss | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (1,608 | ) | | (1,608 | ) |
Change in value of lease guarantee | | — |
| | — |
| | — |
| | 63 |
| | — |
| | — |
| | — |
| | — |
| | 63 |
|
Stock-based compensation expense | | — |
| | — |
| | 5,572 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 5,572 |
|
Excess tax benefit from exercise of stock options | | — |
| | — |
| | 169 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 169 |
|
Repurchase of common stock | | — |
| | — |
| | — |
| | — |
| | 877 |
| | (7,000 | ) | | — |
| | — |
| | (7,000 | ) |
Issuance of common stock upon exercise of stock options and under employee stock purchase plan, net of shares tendered | | 855 |
| | — |
| | 2,077 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 2,077 |
|
Balance at December 31, 2011 | | 57,588 |
| | $ | 6 |
| | $ | 465,701 |
| | $ | (273 | ) | | 1,875 |
| | $ | (18,340 | ) | | $ | (198,208 | ) | | $ | (202 | ) | | $ | 248,684 |
|
Net loss | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (10,402 | ) | | — |
| | (10,402 | ) |
Other comprehensive income | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 1,466 |
| | 1,466 |
|
Change in value of lease guarantee | | — |
| | — |
| | — |
| | 63 |
| | — |
| | — |
| | — |
| | — |
| | 63 |
|
Stock-based compensation expense | | — |
| | — |
| | 8,115 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 8,115 |
|
Excess tax benefit from exercise of stock options | | — |
| | — |
| | 19 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 19 |
|
Repurchase of common stock | | — |
| | — |
| | — |
| | — |
| | 1,374 |
| | (11,290 | ) | | — |
| | — |
| | (11,290 | ) |
Retirement of treasury stock | | (3,249 | ) | | — |
| | — |
| | — |
| | (3,249 | ) | | 29,630 |
| | (29,630 | ) | | — |
| | — |
|
Issuance of common stock upon exercise of stock options and under employee stock purchase plan, net of shares tendered | | 1,540 |
| | — |
| | 6,166 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 6,166 |
|
Balance at December 31, 2012 | | 55,879 |
| | $ | 6 |
| | $ | 480,001 |
| | $ | (210 | ) | | — |
| | $ | — |
| | $ | (238,240 | ) | | $ | 1,264 |
| | $ | 242,821 |
|
Accelrys, Inc.
Consolidated Statements of Cash Flows
(In thousands)
|
| | | | | | | | | | | | | | | |
| For the Years Ended | | For the Nine Months Ended |
| December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2009 |
Cash flows from operating activities: | | | | | | | (unaudited) |
Net income (loss) | $ | (10,402 | ) | | $ | 1,765 |
| | $ | (20,608 | ) | | $ | 3,569 |
|
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | |
Depreciation | 3,325 |
| | 3,800 |
| | 2,220 |
| | 1,182 |
|
Amortization | 19,889 |
| | 21,016 |
| | 9,156 |
| | 1,087 |
|
Gain on sale of real estate | (2,744 | ) | | — |
| | — |
| | — |
|
Share-based compensation | 8,115 |
| | 5,572 |
| | 4,298 |
| | 2,916 |
|
Change in fair value of contingent consideration liabilities | 78 |
| | 78 |
| | — |
| | — |
|
Prepaid contingent compensation amortization | 897 |
| | 847 |
| | — |
| | — |
|
Amortization of premium on marketable debt securities | 1,244 |
| | 1,739 |
| | 72 |
| | — |
|
Amortization of discount on promissory notes receivable | (892 | ) | | (155 | ) | | (78 | ) | | — |
|
Release of valuation allowance against deferred tax assets | (334 | ) | | (1,120 | ) | | (14,921 | ) | | — |
|
Deferred income taxes | (1,100 | ) | | (2,105 | ) | | 213 |
| | — |
|
Excess tax benefit from exercise of stock options | (19 | ) | | (169 | ) | | — |
| | — |
|
Net gain on sale of cost method investment | — |
| | (18,970 | ) | | — |
| | — |
|
Sale of intellectual property | — |
| | 4,303 |
| | — |
| | — |
|
Unrealized losses on foreign currency forward contracts | (18 | ) | | 117 |
| | — |
| | — |
|
Other | (685 | ) | | (390 | ) | | (521 | ) | | (408 | ) |
Changes in operating assets and liabilities: | | | | | | | |
Trade receivables | (5,781 | ) | | (6,304 | ) | | 8,022 |
| | (8,646 | ) |
Prepaid expense and other current assets | 1,769 |
| | 1,578 |
| | 1,619 |
| | (309 | ) |
Other assets | 82 |
| | 1,444 |
| | (453 | ) | | (77 | ) |
Accounts payable | (437 | ) | | (802 | ) | | 1,187 |
| | (1,020 | ) |
Accrued liabilities, compensation and benefits | 1,469 |
| | (2,579 | ) | | (4,371 | ) | | (895 | ) |
Deferred revenue | 3,791 |
| | 14,070 |
| | (9,968 | ) | | (2,889 | ) |
Net cash provided by (used in) operating activities | 18,247 |
| | 23,735 |
| | (24,133 | ) | | (5,490 | ) |
Cash flows from investing activities: | | | | | | | |
Cash paid for acquisitions, net of cash acquired | (35,229 | ) | | (44,564 | ) | | 74,497 |
| | — |
|
Deposit for business acquisitions
| (5,476 | ) | | — |
| | — |
| | — |
|
Equity issuance costs in connection with the Symyx Merger | — |
| | — |
| | (783 | ) | | — |
|
Acquisition-related prepaid contingent compensation | — |
| | (2,000 | ) | | — |
| | — |
|
Purchases of property and equipment, net | (6,332 | ) | | (3,908 | ) | | (3,061 | ) | | (472 | ) |
Net proceeds from sale of real estate | 6,800 |
| | — |
| | — |
| | — |
|
|
| | | | | | | | | | | | | | | |
Purchases of marketable securities | (51,031 | ) | | (104,110 | ) | | (49,312 | ) | | (19,192 | ) |
Proceeds from maturities and sales of marketable securities | 80,613 |
| | 81,505 |
| | 11,750 |
| | 43,966 |
|
Net proceeds from sale of cost method investment | — |
| | 37,293 |
| | — |
| | — |
|
Proceeds from promissory notes receivable | 816 |
| | — |
| | — |
| | — |
|
Net decrease in restricted cash | 329 |
| | 275 |
| | — |
| | — |
|
Net cash provided by (used in) investing activities | (9,510 | ) | | (35,509 | ) | | 33,091 |
| | 24,302 |
|
Cash flows from financing activities: | | | | | | | |
Payment of contingent consideration liabilities | (250 | ) | | — |
| | — |
| | — |
|
Proceeds from issuance of common stock | 7,356 |
| | 2,991 |
| | 3,027 |
| | 615 |
|
Common stock tendered for payment of withholding taxes | (1,190 | ) | | (914 | ) | | (679 | ) | | (541 | ) |
Excess tax benefit from exercise of stock options | 19 |
| | 169 |
| | — |
| | — |
|
Repurchases of common stock | (11,290 | ) | | (7,000 | ) | | (3,000 | ) | | — |
|
Net cash (used in) provided by financing activities | (5,355 | ) | | (4,754 | ) | | (652 | ) | | 74 |
|
Effect of changes in exchange rates on cash and cash equivalents | (245 | ) | | (178 | ) | | 889 |
| | 948 |
|
Increase (decrease) in cash and cash equivalents | 3,137 |
| | (16,706 | ) | | 9,195 |
| | 19,834 |
|
Cash and cash equivalents at beginning of period | 69,610 |
| | 86,316 |
| | 77,121 |
| | 40,595 |
|
Cash and cash equivalents at end of period | $ | 72,747 |
| | $ | 69,610 |
| | $ | 86,316 |
| | $ | 60,429 |
|
Supplemental cash flow information: | | | | | | | |
Cash (received) paid during the year for income taxes, net of refunds | $ | 2,690 |
| | $ | (1,181 | ) | | $ | 280 |
| | $ | 770 |
|
Non-cash investing and financing activities: | | | | | | | |
Equity instruments issued in connection with Merger | $ | — |
| | $ | — |
| | $ | 179,297 |
| | $ | — |
|
Note receivable received in sale of intellectual property | $ | — |
| | $ | 25,974 |
| | $ | — |
| | $ | — |
|
Deferred gain on sale of intellectual property | $ | — |
| | $ | (25,974 | ) | | $ | — |
| | $ | — |
|
| | | | | | | |
See accompanying notes to these consolidated financial statements.
Accelrys, Inc.
Notes to Consolidated Financial Statements
|
| |
1. | Organization and Summary of Significant Accounting Policies
|
Organization and Business
Accelrys, Inc. (“we”, “our”, or “us”) develops and commercializes scientific business intelligence software and solutions that enable our customers to accelerate the discovery, development and manufacturing of new products and materials. Our customers include pharmaceutical, biotechnology and other life science companies, as well as companies that are in the energy, chemicals, aerospace and consumer packaged goods markets. Our software and service solutions are used by our customers’ scientists, biologists, chemists and information technology professionals in order to aggregate, mine, integrate, analyze, simulate, manage and interactively report scientific data.
On July 1, 2010, Alto Merger Sub, Inc., our wholly owned subsidiary (“Symyx Merger Sub”), merged with and into Symyx Technologies, Inc. (“Symyx”), with Symyx surviving as our wholly owned subsidiary (the “Symyx Merger”). Symyx's results of operations are included in our consolidated financial statements beginning July 1, 2010. On May 19, 2011, we completed the acquisition of Contur Software AB (“Contur”), whereby Contur became our wholly owned subsidiary (the “Contur Acquisition”). Contur's operating results are included in our consolidated financial statements and results of operations beginning May 19, 2011. On December 30, 2011 we completed the acquisition of VelQuest Corporation (“VelQuest”), whereby VelQuest became our wholly owned subsidiary (the “VelQuest Acquisition”). VelQuest's operating results are included in our consolidated financial statements and results of operations beginning December 30, 2011. On May 17, 2012, we acquired a proprietary web-based Hit Explorer Operating System (“HEOS”) software platform from Scynexis, Inc. The operating results of the HEOS platform are included in our consolidated financial statements and results of operations beginning May 17, 2012. On October 23, 2012, we completed the acquisition of Aegis Analytical Corporation (“Aegis”), whereby Aegis became our wholly owned subsidiary (the “Aegis Acquisition” and together with the Symyx Merger, the Contur Acquisition, the VelQuest Acquisition and the acquisition of the HEOS platform, the “Acquisitions”). Aegis's operating results are included in our consolidated financial statements and results of operations beginning October 23, 2012.
Principles of Consolidation
These consolidated financial statements include our accounts and those of our wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
Fiscal Year
On August 3, 2010, our board of directors approved a change in our fiscal year end from March 31 to December 31. The fiscal year end change was effective December 31, 2010 and resulted in a nine month reporting period from April 1, 2010 to December 31, 2010, which is included in the accompanying consolidated financial statements. All references to “years”, unless otherwise noted, refer to the twelve-month fiscal year, which prior to April 1, 2010, ended on March 31, and beginning with January 1, 2011, ends on December 31, of each year.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and the disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to share-based compensation, income taxes, contingent consideration, loss contingencies related to legal proceedings and the valuation of goodwill, intangibles and other long-lived assets. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual future results could differ from those estimates.
Cash, Cash Equivalents and Marketable Securities
We consider all highly liquid investments with an original maturity of three months or less to be cash equivalents. We invest our cash with financial institutions in money market funds and other investment grade securities such as certificates of deposits, commercial paper, corporate and municipal bonds and obligations of U.S. government sponsored enterprises.
Our marketable securities consist of fixed income securities with original maturities of greater than three months and include obligations of U.S. government sponsored enterprises, commercial paper, certificates of deposit, corporate and municipal debt. We account for our investments in marketable securities in accordance with Financial Accounting Standards
Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 320, Investments-Debt and Equity Securities (“ASC Topic 320”). Accordingly, our marketable securities have been classified as available-for-sale and are recorded at their estimated fair value, with unrealized gains and losses recorded in stockholders equity and included in accumulated other comprehensive income. Realized gains and losses on the sale of available-for-sale marketable securities are recorded in royalty and other income, net. Available-for-sale marketable securities with original maturities greater than three months and remaining maturities of one year or less are classified as short-term available-for-sale marketable securities. Available-for-sale marketable securities with remaining maturities of greater than one year are classified as long-term available-for-sale marketable securities. Unrealized losses that are not considered other than temporary and unrealized gains are included in accumulated other comprehensive income in stockholders’ equity. Unrealized losses that are determined to be other-than-temporary are recorded as a charge against income. The cost of marketable securities sold is determined based on the specific identification method.
Fair Value of Financial Instruments
We carry our cash equivalents, marketable securities and foreign currency forward contracts at market value. The carrying amount of accounts receivable, accounts payable and accrued liabilities are considered to be representative of their respective fair values due to their short-term nature. The fair value of our foreign currency contracts is estimated based on the prevailing exchange rates of the various hedged currencies as of the end of the period. Our investments in privately-held companies are carried at cost. Our notes receivable are carried at net realizable value. It is impracticable for us to estimate the fair value of these instruments on a recurring basis if there are no identified events or changes in circumstances that may have a significant adverse effect on their fair value.
Concentrations of Risk
We maintain deposits in federally insured financial institutions in excess of federally insured limits. We do not believe we are exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. Additionally, we have established guidelines regarding diversification of our investment portfolio, credit quality of issuers and maturities of investments, which are designed to maintain the safety and liquidity of our deposits and investments.
In addition to our U.S. operations, we conduct business in Europe and in the Asia/Pacific region, primarily in Japan. Any significant decline in the economies or the value of the currencies in these regions could have a material adverse impact on us.
Allowance for Doubtful Accounts
We maintain an allowance for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments on accounts receivable. This estimate is based on a specific customer analysis and an analysis of our historical write-offs as a percent of outstanding accounts receivable balances. If the financial condition of a customer were to deteriorate, resulting in an impairment of its ability to make payments against outstanding balances owed to us, an additional allowance may be required.
Property and Equipment
Property and equipment consists of the following:
|
| | | | | | | | | | |
| | Useful Life (Yrs) | | December 31, 2012 | | December 31, 2011 |
| | | | (In thousands) |
Computers and software | | 1-3 | | 18,244 |
| | 14,984 |
|
Furniture and fixtures | | 1-3 | | 1,637 |
| | 1,228 |
|
Leasehold improvements | | 1-5 | | 6,533 |
| | 5,758 |
|
Total property and equipment, gross | | | | 26,414 |
| | 21,970 |
|
Less accumulated depreciation and amortization | | | | (15,526 | ) | | (14,155 | ) |
Land and Building held for sale | | | | — |
| | 4,293 |
|
Total property and equipment, net | | | | $ | 10,888 |
| | $ | 12,108 |
|
In June 2012, we sold real property comprised of land and an office building located in Santa Clara, California, which we reclassified as “held for sale” during the year ended December 31, 2011, for a net sales price of $6.8 million. We recorded a pre-tax gain of approximately $2.7 million on the sale, which is included in royalty and other income, net, as this property was not utilized in our ongoing operations since its acquisition in the Symyx Merger.
During the year ended December 31, 2012, we wrote off fixed assets which were no longer in use with a total cost of $1.8 million and associated accumulated depreciation of $1.7 million, resulting in a loss of $0.1 million. During the year ended December 31, 2011, we wrote off fixed assets which were no longer in use with a total cost of $1.2 million and associated accumulated depreciation of $1.1 million, resulting in a loss of $0.1 million. During the nine months ended December 31, 2010 and 2009, we wrote off the cost and associated accumulated depreciation of approximately $3.6 million and $0.1 million (unaudited), respectively, of fully depreciated assets which were no longer in use.
Depreciation is recorded on the straight-line method over the estimated useful lives of the related assets. Leasehold improvements are amortized over the shorter of their estimated useful lives or the remaining lease term. Repair and maintenance costs are charged to expense as incurred. Depreciation expense was $3.3 million and $3.8 million for the years ended December 31, 2012 and December 31, 2011, respectively. Depreciation expense was $2.2 million and $1.2 million (unaudited) for the nine months ended December 31, 2010 and December 31, 2009, respectively.
Software Development Costs
We account for costs incurred for computer software to be sold in accordance with ASC Topic 985, Software. Accordingly, costs incurred internally in the research and development of new software products and significant enhancements to existing software products are expensed as incurred until the technological feasibility of the product has been established. Technological feasibility occurs shortly before our internally developed software products are available for general release. We have determined that the internal costs eligible for capitalization are not material. Costs paid to third parties for products in which technological feasibility has been established are capitalized upon purchase of the software.
As of December 31, 2012 and December 31, 2011, we had $0.1 million and $0.6 million, respectively, of capitalized software purchased from third parties. This amount is included in purchased intangible assets, net, in the accompanying consolidated balance sheet. These costs are being amortized to cost of revenue on a straight-line basis over their estimated useful life of five years. Amortization expense was $0.4 million for each of the years ended December 31, 2012 and December 31, 2011. Amortization expense was $0.3 million for each of the nine months ended December 31, 2010 and 2009 (unaudited).
Goodwill and Purchased Intangible Assets
Our goodwill resulted from acquisitions in fiscal years 1999 through 2012. Goodwill and intangible assets with indefinite useful lives are not amortized, but are tested for impairment at least annually or as circumstances indicate that their value may no longer be recoverable. In accordance with ASC Topic 350, Intangibles—Goodwill and Other (“ASC Topic 350”), we review our goodwill and indefinite-lived intangible asset for impairment at least annually in our fiscal fourth quarter and more frequently if events or changes in circumstances occur that indicate a potential reduction in the fair value of our reporting unit and/or our indefinite-lived intangible asset below their respective carrying values. Examples of such events or circumstances include: a significant adverse change in legal factors or in the business climate, a significant decline in our stock price, a significant decline in our projected revenue or cash flows, an adverse action or assessment by a regulator, unanticipated competition, a loss of key personnel, or the presence of other indicators that would indicate a reduction in the fair value of a reporting unit.
Our goodwill is considered to be impaired if we determine that the carrying value of the reporting unit to which the goodwill has been assigned exceeds management’s estimate of its fair value. Based on the guidance provided by ASC Topic 350 and ASC Topic 280, Segment Reporting, (“ASC Topic 280”) management has determined that our company consists of one reporting unit given the similarities in economic characteristics between our operations and the common nature of our products, services and customers. Because we have only one reporting unit, and because we are publicly traded, we determine the fair value of the reporting unit based on our market capitalization as we believe this represents the best evidence of fair value. In the fourth quarter of fiscal year 2012, we completed our annual goodwill impairment test for the fiscal year ended December 31, 2012 and concluded that our goodwill was not impaired. Our conclusion that goodwill was not impaired was based on a comparison of our net assets as of December 31, 2012 to our market capitalization.
Valuation of Indefinite-Lived Intangible Asset
In connection with our acquisition of SciTegic in September 2004, we acquired the SciTegic trade name which was determined to be indefinitely lived. In accordance with ASC Topic 350, we review our indefinite-lived intangible asset for impairment at least annually in our fiscal fourth quarter and more frequently if events or changes in circumstances occur that indicate a potential reduction in the fair value of the asset below its carrying value. Examples of such events or circumstances include: a significant adverse change in legal factors or in the business climate, a significant decline in our stock price, a significant decline in our projected revenue or cash flows, an adverse action or assessment by a regulator, unanticipated competition, a loss of key personnel, or the presence of other indicators that would indicate a reduction in the fair value of an asset.
Our indefinite-lived intangible asset is considered to be impaired if we determine that the carrying value of the asset exceeds its estimated fair value. In the quarter ended December 31, 2012 we completed our annual indefinite-lived intangible asset impairment test and concluded that our indefinite-lived intangible asset was not impaired. We estimated the fair value of our indefinite-lived intangible asset utilizing a discounted cash flow analysis which considers the estimated future customer orders for our scientific informatics platform product line and the associated direct and incremental selling, marketing and development costs. Key assumptions included in the discounted cash flow analysis include projections of future customer order growth for our scientific informatics platform product line and developing an appropriate discount rate.
Impairment of Long-Lived Assets
We review long-lived assets to be held and used, including acquired intangible assets subject to amortization and property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. Conditions that would necessitate an impairment assessment include a significant decline in the market price of an asset or asset group, a significant adverse change in the extent or manner in which an asset or asset group is being used, the loss of legal ownership or title to the asset, significant negative industry or economic trends or the presence of other indicators that would indicate that the carrying amount of an asset or asset group is not recoverable. A long-lived asset is considered to be impaired if management’s estimate of the undiscounted future cash flows anticipated to result from the use of the asset and its eventual disposition are not sufficient to recover the carrying value of the asset.
Acquisitions
Under the accounting guidance for business combinations we recognize separately from goodwill the assets acquired and the liabilities assumed, at their fair values as of the date of the acquisition. Goodwill as of the acquisition date is measured as the excess of consideration transferred and the net of the acquisition date fair values of the assets acquired and the liabilities assumed.
While we use our best estimates and assumptions as a part of the purchase price allocation process to accurately value assets acquired and liabilities assumed at the business combination date, our estimates and assumptions are inherently uncertain and subject to refinement. As a result, during the preliminary purchase price allocation period, which may be up to one year from the business combination date, we record adjustments to the assets acquired and liabilities assumed, with the corresponding offset to goodwill. After the preliminary purchase price allocation period, we record adjustments to assets acquired or liabilities assumed subsequent to the purchase price allocation period in our operating results in the period in which the adjustments were determined.
Revenue Recognition
We generate revenue from the following primary sources:
| |
• | post-contract customer support and maintenance services on licensed software (collectively referred to as “PCS”), |
Customer billings issued in connection with our revenue-generating activities are initially recorded as deferred revenue. We then recognize the revenue from these customer billings as set forth below and when all of the following criteria are met:
| |
• | a fully executed written contract or purchase order has been obtained from the customer (i.e., persuasive evidence of an arrangement exists), |
| |
• | the contractual price of the product or services has been defined and agreed to in the contract or purchase order (i.e., price is fixed or determinable), |
| |
• | delivery of the product or service has occurred and no material uncertainties regarding customer acceptance of the delivered product or service exist, and |
| |
• | collection of the purchase price from the customer is considered probable. |
Software Licenses. We license software on a term and perpetual basis. When sold perpetually, our standard perpetual software licensing arrangements generally include twelve months of bundled PCS, while our standard term-based software licensing arrangements typically include PCS for the full duration of the term license. Because we do not have vendor-specific objective evidence (“VSOE”) of the selling price of these undelivered elements, we recognize as revenue the entire fee for such perpetual and term-based licenses ratably over the term of the bundled PCS.
Renewal of PCS Under Perpetual Software Licenses. Our PCS includes the right to receive unspecified upgrades or enhancements and technical support. Fees from customer renewals of PCS related to previously purchased perpetual licenses are recognized ratably over the term of the PCS.
Content. Content is licensed on a term basis and provides customers with access to the licensed content over the term of the agreement. Revenue from these licensing arrangements is recognized ratably over the term of the agreement, which is typically twelve months and coincides with the term of the PCS and/or licensed software.
Professional Services. We provide certain services to our customers, including non-complex product training, installation, implementation and other professional services which are non-essential to the operation of the software. We also perform professional services for our customers designed to enhance the value of our software products by creating extensions to functionality to address a client's specific business needs. When sold separately, revenue from time and materials service engagements are generally recognized as the services are performed. Revenue from fixed fee service engagements are typically recognized as the services are performed using the percentage-of-completion method when we can reasonably estimate the level of effort required to complete our performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. For service arrangements that include provisions that create significant uncertainties (e.g. customer acceptance) or service deliverables wherein the level of effort to complete the services cannot be reasonably estimated, the associated revenue is recognized using the completed performance method.
Hosting Services. We are an application service provider (“ASP”), where we provide hosting services that allow customers access to software that resides on our servers. The ASP model typically includes an up-front fee and a monthly commitment from the customer that commences upon completion of the implementation through the remainder of the customer life. The up-front fee is the initial setup fee, or the implementation fee. The monthly commitment includes, but is not limited to, a fixed monthly fee or a transactional fee based on system usage that exceeds monthly minimums. We do not view the activities of signing the contract or providing initial setup services as discrete earnings events. Revenue is typically deferred until the date the customer commences use of our services, at which point the up-front fees are recognized ratably over the customer life of the customer arrangement.
Multi-Element Arrangements. For multi-element arrangements that include software licenses, PCS, and non-essential installation and implementation services, the entire fee for such arrangements is recognized as revenue ratably over the term of the PCS or delivery of the services, whichever is longer. For multi-element arrangements that also include services that are essential to the operation of the software, the fee for such arrangements is generally deferred until the services essential to the operation of the software have been performed, at which point the entire fee for such arrangements is recognized as revenue ratably over the remaining term of the PCS or the delivery of the non-essential services, whichever is longer. We allocate the arrangement fee to the software-related elements and the non-software-related elements based upon the relative standard list price of the products and/or services that comprise each element, which is our best estimate of selling price.
Sales Taxes Collected
Sales taxes collected from customers and remitted to various governmental agencies are excluded from revenues in our consolidated statements of operations.
Shipping Costs
The costs of shipping products to our customers are expensed as incurred and are included in cost of revenue in our consolidated statements of operations. We incurred shipping costs of $0.4 million during both the years ended December 31,
2012 and December 31, 2011. We incurred shipping costs of $0.6 million and $0.3 million(unaudited), during the nine months ended December 31, 2010 and 2009, respectively.
Royalty Income
We recognize royalty income based on reported sales by third party licensees of products containing the applicable licensed materials and intellectual property. Royalty revenue is recognized as these payments become due. Non-refundable royalties, for which there are no further performance obligations, are recognized when due under the terms of the applicable agreements. Royalty income is included in the royalty and other income, net line on the consolidated statements of operations.
Advertising Costs
The costs of advertising are expensed as incurred and are included in sales and marketing expenses in our consolidated statements of operations. We incurred advertising costs of $0.1 million and $0.2 million during the years ended December 31, 2012 and December 31, 2011, respectively. We incurred advertising costs of $0.1 million during each of the nine months ended December 31, 2010 and 2009 (unaudited).
Deferred Costs
Occasionally, we enter into professional service arrangements under which resulting revenue is deferred until certain elements of the arrangements are delivered. As a result, if deemed material, we defer the direct variable expense, not exceeding the revenue deferred, in other current assets on the balance sheet until the period when revenue is recognized. Direct and incremental variable expenses include direct labor costs and direct services contracts with third parties working on the software service arrangements. As of December 31, 2012 and 2011, we deferred approximately $0.8 million and $0.9 million, respectively, of direct and incremental variable costs related to software service arrangements where the revenue is deferred until future periods.
Income Taxes
We account for income taxes in accordance with ASC Topic 740, Income Taxes. Deferred tax assets and liabilities are recognized for the tax consequences of temporary differences between the financial carrying amounts and the tax basis of existing assets and liabilities by applying enacted statutory tax rates applicable to future years. The provision for income taxes is based on our estimates for taxable income of the various legal entities we operate and the various jurisdictions in which we operate. As such, income tax expense may vary from the customary relationship between income tax expense and pre-tax income. We establish a valuation allowance against our net deferred tax assets to reduce them to the amount expected to be realized.
We assess the recoverability of our deferred tax assets on an on-going basis. In making this assessment we are required to consider all available positive and negative evidence to determine whether, based on such evidence, it is more-likely-than-not that some portion or all of our net deferred assets will be realized in future periods. This assessment requires significant judgment. We do not recognize current and future tax benefits until it is more-likely-than-not that our tax positions will be sustained. In general, any realization of our net deferred tax assets will reduce our effective rate in future periods. However, the realization of deferred tax assets that are related to net operating losses (“NOLs”) that were generated by tax deductions resulting from the exercise of non-qualified stock options will be a direct increase to stockholders’ equity.
We determine whether the benefits of our tax positions are more-likely-than-not to be sustained upon audit based on the technical merits of the tax position. We recognize the impact of an uncertain income tax position taken on our income tax return at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position is not recognized if it has less than a 50% likelihood of being sustained.
Interest and penalties related to income tax matters are recognized in income tax expense. During the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010 we incurred interest and penalties of approximately $0.1 million, $0.1 million and $0.5 million, respectively. During the nine months ended December 31, 2009 (unaudited) we did not incur any expense related to interest or penalties for income tax matters. Interest and penalties of $0.1 million remained accrued as of December 31, 2012.
Foreign Currency Translation
In accordance with ASC Topic 830, Foreign Currency Matters, we translate the financial statements of our foreign subsidiaries into U.S. dollars using period-end exchange rates for assets and liabilities and average exchange rates during each
reporting period for results of operations. Gains and losses resulting from foreign currency translation are excluded from results of operations and recorded as accumulated other comprehensive income in stockholders’ equity.
Gains and losses resulting from the cash settlement of certain intercompany transactions as well as transactions with customers and vendors that are denominated in currencies other than the functional currency of each entity give rise to foreign exchange gains or losses. We realized foreign exchange loss of $0.6 million during the year ended December 31, 2012 and foreign exchange gains of $0.5 million during the year ended December 31, 2011. We realized foreign exchange gains of $0.2 million and $0.1 million (unaudited) during the nine months ended December 31, 2010 and 2009, respectively. These amounts are included in royalty and other income, net in our consolidated statements of operations.
Foreign Currency Forward Contracts Not Designated as Hedges
We transact business in various currencies other than the U.S. dollar and have significant international sales, expenses and intercompany transactions denominated in currencies other than the U.S. dollar, subjecting us to currency exchange rate risks. To mitigate our risk from foreign currency fluctuations, we launched a foreign currency hedging program in November 2011, whereby we will periodically enter into currency derivative contracts, principally forward contracts with-short term maturities. Our intent is to offset gains and losses that occur from the underlying exposure with gains and losses on the derivative contracts used to offset them. We do not hold or purchase any foreign currency contracts for trading or speculative purposes and we do not designate these forward contracts or swaps as hedging instruments. Accordingly, we report the fair value of these contracts in the consolidated balance sheet with changes in fair value recorded in royalty and other income, net in the consolidated statement of operations. The fair value of foreign currency contracts is estimated based on the prevailing exchange rates of the various hedged currencies as of the end of the period.
Share Based Compensation
We use the Black-Scholes-Merton option-pricing model to estimate the fair value of stock options granted and stock purchases under the Employee Stock Purchase Plan (“ESPP”). This model incorporates various assumptions including expected volatility, expected term of an award, expected dividends, and the risk-free interest rates discussed below. The fair value of restricted stock units granted is based on the market price of our common stock on the date of grant. We recognize the fair value of share-based compensation on a straight-line basis over the requisite service periods of the awards.
Expected Volatility. Volatility is a measure of the amount the stock price will fluctuate during the expected life of an award. We determine volatility based on our historical stock price volatility over the most recent period equivalent to the expected life of the award, giving consideration to company-specific events impacting our historical volatility that are unlikely to occur in the future, as well as anticipated future events that may impact volatility. We also consider the historical stock price volatilities of comparable publicly traded companies.
Risk-Free Interest Rate. Our assumption of the risk-free interest rate is based on the interest rates on U.S. constant rate treasury securities with contractual terms approximately equal to the expected life of the award.
Expected Dividend Yield. Because we have not paid any cash dividends since our inception and do not anticipate paying dividends in the foreseeable future, we assume a dividend yield of zero.
Expected Award Life. We determine the expected life of an award by considering various relevant factors, including the vesting period and contractual term of the award, our employees’ historical exercise patterns and length of service and employee characteristics. We also consider the expected award lives of comparable publicly traded companies. For stock purchase plan purchase rights, the expected life is equal to the current offering period under the stock purchase plan.
Under ASC Topic 718, we are also required to estimate at grant the likelihood that the award will ultimately vest (the “pre-vesting forfeiture rate”), and to revise the estimate, if necessary, in future periods if the actual forfeiture rate differs. We determine the pre-vesting forfeiture rate of an award based on our historical pre-vesting award forfeiture experience, giving consideration to company-specific events impacting historical pre-vesting award forfeiture experience that are unlikely to occur in the future as well as anticipated future events that may impact forfeiture rates.
Our determination of the input variables used in the Black-Scholes option pricing model as well as the pre-vesting forfeiture rate is based on various underlying estimates and assumptions that are highly subjective and are affected by our stock price, among other factors. Changes in these underlying estimates and assumptions could materially affect the fair value of our share-based awards and, therefore, the amount of share-based compensation expense to be recognized in our results of operations.
Segment and Geographic Information
In accordance with ASC Topic 280, our operations have been aggregated into one reportable segment given the similarities in economic characteristics between our operations and the common nature of our products, services and customers.
Financial information by geographic region is as follows:
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended | | For the Nine Months Ended |
| | December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2009 |
| | Amount | | % of Total | | Amount | | % of Total | | Amount | | % of Total | | Amount | | % of Total |
| | (In thousands, except percentages) |
| | | | | | | | | | | | (unaudited) |
Revenues: | | | | | | | | | | | | | | | | |
U.S. | | $ | 79,635 |
| | 49 | % | | $ | 67,756 |
| | 47 | % | | $ | 39,271 |
| | 49 | % | | $ | 28,480 |
| | 46 | % |
Europe | | 46,005 |
| | 28 | % | | 42,339 |
| | 29 | % | | 22,679 |
| | 28 | % | | 19,276 |
| | 31 | % |
Asia-Pacific | | 36,886 |
| | 23 | % | | 34,244 |
| | 24 | % | | 18,255 |
| | 23 | % | | 14,444 |
| | 23 | % |
Total | | $ | 162,526 |
| | 100 | % | | $ | 144,339 |
| | 100 | % | | $ | 80,205 |
| | 100 | % | | $ | 62,200 |
| | 100 | % |
Revenues are attributable to geographic areas based on the region of destination.
|
| | | | | | | | | | | | | | | |
| | December 31, 2012 | | December 31, 2011 | |
| | Amount | | Percentage of Total | | Amount | | Percentage of Total | |
| | (In thousands, except percentages) |
Long-lived assets: | | | | | | | | | |
U.S. | | $ | 176,611 |
| | 93 | % | | $ | 160,884 |
| | 92 | % | |
Europe | | 11,234 |
| | 6 | % | | 12,204 |
| | 7 | % | |
Asia-Pacific | | 1,361 |
| | 1 | % | | 1,802 |
| | 1 | % | |
Total | | $ | 189,206 |
| | 100 | % | | $ | 174,890 |
| | 100 | % | |
Guarantees
We account for guarantees in accordance with ASC Topic 460, Guarantees (“ASC Topic 460”). ASC Topic 460 requires that a guarantor recognize, at the inception of a guarantee, a liability for the fair value of certain guarantees and requires certain disclosures to be made by a guarantor about its obligations under certain guarantees that it has issued.
Effect of New Accounting Standards
In September 2011, the FASB issued ASU No. 2011-08, Testing Goodwill for Impairment (“ASU 2011-08”), which gives an entity the option of performing a qualitative assessment to determine whether it is necessary to perform step 1 of the annual goodwill impairment test. An entity is required to perform step 1 only if it concludes that it is more likely than not that a reporting unit’s fair value is less than its carrying amount. An entity may choose to perform the qualitative assessment on none, some or all of its reporting units or an entity may bypass the qualitative assessment for any reporting unit in any period and proceed directly to step 1 of the impairment test. This new guidance is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. Early adoption is permitted. The adoption of this accounting pronouncement did not have a material impact on our consolidated financial statements.
2. Business Combinations
Aegis Acquisition
On October 23, 2012, we acquired Aegis as part of our strategy to expand our product portfolio to complement our current software offerings, expand our footprint in downstream operations and reinforce our position as a leader in scientific innovation lifecycle management software.
The total consideration for the net assets acquired was $30.7 million, consisting of an initial cash payment of $26.1 million, which was paid in connection with the signing and simultaneous closing of the acquisition, and additional consideration of $4.6 million which was deposited into an escrow account in the name of the selling shareholders on the acquisition date. The escrowed funds will be maintained until December 27, 2013 if, and to the extent that, certain breaches of the representations and warranties have not occurred.
We funded the purchase price with cash on hand. All acquisition costs related to the transaction were expensed as incurred. The total acquisition date fair value of the consideration was $30.7 million.
The preliminary determination of fair value is as follows:
|
| | | |
| (in thousands) |
Fair Value of Consideration Transferred | $ | 30,729 |
|
Less: Recognized amounts of identifiable assets acquired and liabilities assumed: | |
Accounts receivable | 661 |
|
Other assets | 311 |
|
Property, plant and equipment | 79 |
|
Intangible assets | 11,830 |
|
Other current liabilities | (602 | ) |
Deferred revenue | (510 | ) |
Goodwill | $ | 18,960 |
|
The above purchase price determination is considered preliminary and is subject to revision during the measurement period. We are awaiting the third party analysis of the valuation of acquired intangible assets and deferred revenue. Additionally, we are in the process of obtaining support for the net present value of other assets and obligations related to income tax and other liabilities. There is no tax deductible goodwill as a result of the Aegis Acquisition.
We have determined that the acquisition of Aegis was a non-material business combination. As such, pro forma disclosures are not required and are not presented in this Annual Report on Form 10-K. The consolidated financial statements include the operating results of Aegis from the date of acquisition.
Acquisition of the HEOS Platform
On May 17, 2012, we acquired the HEOS software platform from Scynexis, Inc. as part of our strategy to expand our product portfolio to complement our current software offerings. Enabled by our applications, HEOS is a proven Software-as-a-Service ("SaaS") workspace that accelerates and streamlines collaborative drug discovery by providing secure, real-time access to chemical registration, biological assay results, computational and visual analytics, safety assessment and pharmacokinetics data, and other project information in the Cloud with minimal IT overhead and effort.
The total consideration for the net assets acquired was $4.5 million, of which, based on our determination of fair value completed during the third quarter of fiscal year 2012, $3.8 million was allocated to goodwill and $0.7 million was allocated to purchased technology intangible assets. We funded the purchase price with cash on hand. All acquisition costs related to the transaction were expensed as incurred. The goodwill related to the acquisition of the HEOS platform is deductible over a 15-year period for U.S. income tax purposes.
We have determined that the acquisition of the HEOS platform was a non-material business combination. As such, pro forma disclosures are not required and are not presented in this Annual Report on Form 10-K. The consolidated financial statements include the operating results of the HEOS platform from the date of acquisition.
VelQuest Acquisition
On December 30, 2011, we entered into an Agreement and Plan of Merger pursuant to which VelQuest became our wholly owned subsidiary. We acquired VelQuest as part of our strategy to expand our product portfolio to complement our current software offerings. VelQuest extends our software portfolio into pharmaceutical development quality assurance and quality control, offering significant productivity improvements, faster cycle times, lower operational costs and reduced compliance risks for regulated life sciences organizations.
The total consideration for the net assets acquired was $35.0 million, consisting of an initial cash payment of $29.8 million, which was paid in connection with the signing and simultaneous closing of the acquisition, and an additional consideration of $5.3 million which was deposited into an escrow account in the name of the selling shareholders on the acquisition date. The escrowed funds will be released 15 months following the acquisition date if, and to the extent that, certain breaches of the representations and warranties have not occurred.
We funded the purchase price with cash on hand. All acquisition costs related to the transaction were expensed as incurred. The total acquisition date fair value of the consideration was $35.0 million.
The determination of fair value is as follows:
|
| | | |
| (in thousands) |
Fair Value of Consideration Transferred | $ | 35,000 |
|
Less: Recognized amounts of identifiable assets acquired and liabilities assumed: | |
Cash and cash equivalents | 299 |
|
Accounts receivable | 4,336 |
|
Other assets | 23 |
|
Property, plant and equipment | 14 |
|
Intangible assets | 10,880 |
|
Other current liabilities | (1,086 | ) |
Deferred revenue | (3,446 | ) |
Goodwill | $ | 23,980 |
|
During the year ended December 31, 2012, we made measurement period adjustments totaling $0.2 million related primarily to accrued liabilities. We did not recast the December 31, 2011 balance sheet as a result of these measurement period adjustments as we did not consider them to be material. There is no tax deductible goodwill as a result of the VelQuest Acquisition.
We have determined that the acquisition of VelQuest was a non-material business combination. As such, pro forma disclosures are not required and are not presented in this Annual Report on Form 10-K. The consolidated financial statements include the operating results of VelQuest from the date of acquisition.
Contur Acquisition
On May 19, 2011, by and through our subsidiary, Accelrys Software Inc., we entered into a Sale and Purchase Agreement (the “Contur Purchase Agreement”) pursuant to which we acquired all outstanding equity interests in Contur. We acquired Contur as part of our strategy to expand our product portfolio to complement our current software offerings. Based in Stockholm, Sweden, Contur licenses electronic laboratory notebook software solutions which enable scientific organizations to document their research and development processes and to capture their intellectual property. Contur is a provider of electronic lab notebook capabilities via a SaaS model.
The total consideration for the net assets acquired is up to $11.1 million, consisting of an initial cash payment of $10.6 million, which was paid in connection with the signing and simultaneous closing of the acquisition, and additional consideration of up to $0.5 million upon the achievement of certain agreed-upon performance milestones, payable in equal increments upon each of the first and second anniversaries of the date of the acquisition. In accordance with the terms of the Contur Purchase Agreement, we deposited $0.5 million in an escrow account in our name on the acquisition date for the potential payments of the contingent consideration obligation. During the quarter ended June 30, 2012 the first year performance milestone was met. Accordingly, $0.3 million was released in accordance with the escrow agreement during the
quarter ended June 30, 2012. As of December 31, 2012 and December 31, 2011, we had cash balances of $0.3 million and $0.5 million, respectively, related to the escrow account, which are included in restricted cash in the consolidated balance sheets.
We funded the purchase price with cash on hand. All acquisition costs related to the transaction were expensed as incurred. The total acquisition date fair value of the consideration was estimated at approximately $11.0 million as follows (in thousands):
|
| | | |
| (in thousands) |
Initial cash payment to Contur | $ | 10,624 |
|
Estimated fair value of contingent milestone consideration | 335 |
|
Total consideration | $ | 10,959 |
|
As of the acquisition date, a liability of $0.3 million was recognized for the estimated fair value of the contingent milestone consideration. Changes in the fair value of the liability subsequent to the acquisition date are recognized in earnings. We have determined the fair value of the contingent consideration obligation based on a discounted probability-weighted income approach derived from revenue estimates and a probability assessment with respect to the likelihood of achieving the milestone criteria.
The determination of fair value is as follows:
|
| | | |
| (in thousands) |
Fair Value of Consideration Transferred | $ | 10,959 |
|
Less: Recognized amounts of identifiable assets acquired and liabilities assumed: | |
Cash and cash equivalents | 761 |
|
Accounts receivable | 641 |
|
Other current assets | 240 |
|
Property, plant and equipment | 51 |
|
Intangible assets | 4,410 |
|
Other current liabilities | (698 | ) |
Deferred tax liabilities | (1,582 | ) |
Deferred revenue | (353 | ) |
Goodwill | $ | 7,489 |
|
We made measurement period adjustments totaling $29,000 to accrued income tax balances in the first quarter of fiscal year 2012. We did not recast the December 31, 2011 balance sheet as a result of these measurement period adjustments as we did not consider them to be material. There is no tax deductible goodwill as a result of the Contur Acquisition.
In addition to the initial purchase price, we have deposited $2.0 million in an escrow account in the name of the six former equity holders of Contur. The escrowed funds were scheduled to be released two years following the acquisition date, in $1.0 million increments upon each of the first and second anniversaries of the date of the acquisition if, and to the extent that, no breaches of the representations and warranties have occurred. The $2.0 million is also subject to forfeiture in equal amounts by each of the former equity holders if employment is terminated prior to a two-year retention period. Accordingly, the payment was determined to be compensation for post-acquisition service, the cost of which will be recognized as compensation expense over the two-year retention period. During the quarter ended June 30, 2012, $1.0 million of the escrowed funds were released in accordance with the escrow agreement. The unrecognized contingent compensation expense was $0.3 million as of December 31, 2012, which is expected to be recognized in the first half of fiscal year 2013.
We have determined that the acquisition of Contur was a non-material business combination. As such, pro forma disclosures are not required and are not presented in this Annual Report on Form 10-K. The consolidated financial statements include the operating results of Contur from the date of acquisition.
Symyx Merger
On July 1, 2010, we completed the Symyx Merger, whereby Symyx became our wholly owned subsidiary. Pursuant to the terms of the Agreement and Plan of Merger and Reorganization, dated April 5, 2010, by and among Accelrys, Symyx Merger Sub and Symyx, Symyx stockholders received 0.7802 shares of our common stock (the “Exchange Ratio”) for each share of Symyx common stock held on July 1, 2010. As a result, we issued an aggregate of approximately 27.5 million shares
of our common stock to Symyx stockholders on July 1, 2010. In addition, outstanding options to purchase Symyx common stock were assumed by us and converted into options to purchase 2,441,274 shares of our common stock based on the Exchange Ratio. Based on criteria under business combination accounting guidance, Accelrys was deemed to be the accounting acquirer.
These transactions resulted in a total consideration transferred of $179.3 million. In allocating the purchase price based on estimated fair values, we recorded approximately $27.1 million of goodwill and $75.4 million of identifiable intangible assets.
3. Share-Based Payments
2011 Stock Incentive Plan
On August 3, 2011, our stockholders approved the adoption of our 2011 Stock Incentive Plan (the “2011 Plan”). The total number of shares of our common stock that are reserved for issuance under the 2011 Plan is 17.8 million shares. The 2011 Plan also provides that the following shares of our common stock will not be returned to the 2011 Plan or otherwise become available for issuance under the 2011 Plan: (i) shares of common stock tendered by participants as full or partial payment to the Company upon exercise of options granted under the 2011 Plan; (ii) shares of common stock withheld by, or otherwise remitted to, the Company to satisfy a participant’s tax withholding obligations; and (iii) shares of common stock covered by the portion of any stock appreciation right (“SAR”) that is exercised (whether or not such shares of common stock are actually issued to a participant upon exercise of the SAR).
Notwithstanding the foregoing, any shares of common stock issued in connection with awards other than stock options and SARs will be counted against the total share limit reserved under the 2011 Plan by applying the Multiplier (as defined below) to the shares of common stock covered by each such award; conversely, shares of common stock returned to or deemed not to have been issued from the 2011 Plan under awards other than stock options and SARs will return to the share reserve at a rate determined by multiplying such number of shares by the Multiplier. The “Multiplier” for awards other than stock options and SARs will be 2.22 shares of common stock for every 1.0 share of common stock issued in connection with such award. For example, a grant of a restricted stock award for 1,000 shares would count as 2,220 shares against the reserve, and if returned to the 2011 Plan, would count as 2,220 shares returned to the 2011 Plan. As of December 31, 2012, there are approximately 12,597,585 shares of our common stock available for issuance under the 2011 Plan.
On August 3, 2011, our stockholders also approved an amendment to our 2005 Employee Stock Purchase Plan (the “ESPP”), to increase the number of shares available for future grant under the 2005 ESPP to 2,500,000. Under the ESPP, employees may defer up to 10% of their base salary to purchase shares of our common stock, up to a maximum of 1,000 shares per offering period. The purchase price of the common stock is equal to 85% of the lower of the fair market value per share of our common stock on the commencement date of the applicable offering period or the applicable purchase date. To date, we have issued 1,073,134 shares under the ESPP and1,426,866 shares remain available for future issuance under the ESPP as of December 31, 2012.
Share-Based Award Activity
Our stock options generally vest over four years and have a contractual term of seven to ten years. A summary of stock option activity under our share-based compensation plans is as follows:
|
| | | | | | | | | | | | | |
| Number of Shares | | Weighted Average Exercise Price | | Aggregate Intrinsic Value | | Weighted Average Remaining Contractual Life |
| (In thousands, except per share amounts) |
Outstanding at December 31, 2011 | 6,021 |
| | $ | 7.64 |
| |
| |
|
Granted | 1,840 |
| | 8.20 |
| |
| |
|
Exercised | (1,003 | ) | | 6.07 |
| |
| |
|
Expired/Forfeited | (950 | ) | | 12.98 |
| |
| |
|
Outstanding at December 31, 2012 | 5,908 |
| | 7.23 |
| | 12,014 |
| | 7.08 |
Vested and expected to vest at December 31, 2012 | 5,412 |
| | 7.17 |
| | 11,385 |
| | 6.92 |
Exercisable at December 31, 2012 | 2,661 |
| | $ | 6.84 |
| | $ | 7,109 |
| | 5.38 |
|
The aggregate intrinsic value of stock options outstanding and exercisable at December 31, 2012 was based on the closing price of our common stock on December 31, 2012 of $9.05 per share.
The total intrinsic value of stock options exercised during the years ended December 31, 2012 and December 31, 2011 was $2.0 million and $1.0 million, respectively. The total intrinsic value of stock options exercised during the nine months ended December 31, 2010 and December 31, 2009 was $1.0 million and $26,000 (unaudited), respectively. The intrinsic value of exercised stock options is calculated based on the difference between the exercise price and the quoted market price of our common stock as of the close of the exercise date.
Restricted stock units (“RSUs”) granted under our Amended and Restated 2004 Stock Incentive Plan, as amended (the “2004 Plan”), the Symyx 2007 Stock Incentive Plan (the “2007 Plan”), as amended, and the 2011 Plan generally vest annually over three years and, once vested, do not expire. A limited number of RSUs granted under the 2011 Plan vest over one year and, once vested, do not expire.
Upon vesting of an RSU, employees are generally issued an equivalent number of shares of our common stock. A summary of RSU activity under our share-based compensation plans is as follows:
|
| | | | | | |
| Number of Shares | | Weighted Average Grant Date Fair Value Per Share |
| (In thousands, except per share amounts) |
Unvested at December 31, 2011 | 1,432 |
| | $ | 6.82 |
|
Granted | 866 |
| | 8.20 |
|
Vested | (480 | ) | | 6.58 |
|
Forfeited | (165 | ) | | 7.03 |
|
Unvested at December 31, 2012 | 1,653 |
| | $ | 7.53 |
|
Included in the vested shares for the period are approximately148,643shares tendered to us by employees for payment of minimum income tax obligations upon vesting of RSUs.
The total fair value of RSUs vested was $3.8 million and $3.0 million for the years ended December 31, 2012 and December 31, 2011, respectively. The total fair value of RSUs vested during the nine months ended December 31, 2010 and December 31, 2009 was $2.3 million and $1.8 million (unaudited), respectively.
Valuation Assumptions of Share-Based Awards
The fair value of stock options and ESPP purchase rights granted during the years ended December 31, 2012 and 2011, and the nine months ended December 31, 2010 and 2009 was estimated at the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions:
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended | | Nine Months Ended | |
| December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2009 | |
| | | | | | | | | | | | | (unaudited) | |
| Stock
Options | | ESPP
Purchase
Rights | | Stock
Options | | ESPP
Purchase
Rights | | Stock
Options | | ESPP
Purchase
Rights | | Stock
Options | | ESPP
Purchase
Rights | |
Expected volatility | 50 | % | | 34 | % | | 50 | % | | 35 | % | | 51 | % | | 28 | % | | 58 | % | | 64 | % | |
Risk-free interest rate | 0.7 | % | | 0.1 | % | | 0.8 | % | | 0.1 | % | | 1.5 | % | | 0.2 | % | | 2.5 | % | | 0.3 | % | |
Expected option life in years | 5 |
| | 0.5 |
| | 5 |
| | 0.5 |
| | 5 |
| | 0.5 |
| | 5 |
| | 0.5 |
| |
Expected dividend yield | — | % | | — | % | | — | % | | — | % | | — | % | | — | % | | — | % | | — | % | |
Weighted average per share grant date fair value | $ | 3.57 |
| | $ | 1.77 |
| | $ | 3.04 |
| | $ | 1.72 |
| | $ | 3.06 |
| | $ | 1.64 |
| | $ | 2.92 |
| | $ | 1.22 |
| |
The fair value of RSUs granted during the years ended December 31, 2012 and December 31, 2011, and the nine months ended December 31, 2010 and 2009 was based on the market price of our common stock on the date of grant.
Share-Based Compensation Expense
The estimated fair value of our share-based awards is recognized as a charge against income on a straight-line basis over the requisite service period, which is typically the vesting period of the award. Total share-based compensation expense recognized in our consolidated statements of operations for the years ended December 31, 2012 and 2011, and the nine months ended December 31, 2010 and 2009 was as follows:
|
| | | | | | | | | | | | | | | |
| Year Ended December 31, | | Nine Months Ended December 31, |
| 2012 | | 2011 | | 2010 | | 2009 |
| | | | | | | (unaudited) |
| (in thousands) |
Cost of revenue | $ | 755 |
| | $ | 333 |
| | $ | 179 |
| | $ | 198 |
|
Product development | 1,763 |
| | 1,136 |
| | 794 |
| | 704 |
|
Sales and marketing | 2,545 |
| | 1,672 |
| | 940 |
| | 763 |
|
General and administrative | 3,093 |
| | 2,428 |
| | 1,506 |
| | 1,251 |
|
Business consolidation, transaction and restructuring costs | (41 | ) | | 3 |
| | 879 |
| | — |
|
Total stock-based compensation expense | $ | 8,115 |
| | $ | 5,572 |
| | $ | 4,298 |
| | $ | 2,916 |
|
No share-based compensation expense was capitalized in the periods presented. At December 31, 2012, the gross amount of unrecognized share-based compensation expense relating to unvested share-based stock options, restricted stock units and ESPP shares was approximately $19.6 million, which we anticipate recognizing as a charge against operations over a weighted average period of 2.5 years.
Stock Repurchases
Our Board of Directors approves common stock repurchase programs from time to time authorizing management to repurchase shares of our common stock in open market transactions in accordance with Rule 10b-18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). We repurchase shares primarily to offset dilution caused by ongoing stock issuances from existing equity compensation plans and to improve stockholders’ returns. Repurchased shares are recorded as treasury stock upon repurchase and are subsequently retired.
As of December 31, 2012, we have an outstanding authorization, approved by our board of directors on October 26, 2012, of up to $15.0 million for the repurchase of our common during fiscal year 2013.
On December 13, 2012, we entered into a stock repurchase plan in accordance with Rule 10b5-1 of the Exchange Act, instructing our broker, subject to the conditions set forth therein and the provisions of Rule 10b-18 of the Exchange Act, to purchase for our account up to $15.0 million worth of our common stock by December 31, 2013.
As of December 31, 2012, we have made cumulative repurchases of 2,605,273 shares, at a cost of approximately $21.3 million (including commissions) since we began our repurchase program in November 2010. Stock repurchases were funded principally from operating cash flows. All repurchased shares of common stock have been retired.
The following table provides a summary of the repurchase activity for the periods indicated under our stock repurchase program which started in November 2010 (in thousands, except per share data):
|
| | | | | | | | | | | |
| Year Ended December 31, | | Nine Months Ended December 31, |
| 2012 | | 2011 | | 2010 |
| (in thousands, except per share price) |
Shares repurchased | 1,374 |
| | 877 |
| | 354 |
|
Average purchase price | $ | 8.22 |
| | $ | 7.98 |
| | $ | 8.48 |
|
Aggregate purchase price | $ | 11,290 |
| | $ | 7,000 |
| | $ | 3,000 |
|
Retirement of Treasury Stock
During the year ended December 31, 2012, we retired 3.2 million shares of our treasury stock. These shares remain as authorized shares; however they are now considered unissued. In accordance with ASC Topic 505, Equity, the retirement of our treasury stock resulted in a reduction in our treasury stock and a corresponding increase in our accumulated deficit of $29.6 million. There was no effect on the total stockholders' equity position as a result of the retirement.
4. Marketable Securities
Our marketable securities consist of fixed income securities with original maturities of greater than three months and include obligations of U.S. government sponsored enterprises, commercial paper, certificates of deposit, corporate and municipal debt. We account for our investments in marketable securities in accordance with ASC Topic 320. Accordingly, our marketable securities have been classified as available-for-sale and are recorded at their estimated fair value, with unrealized gains and losses recorded in stockholders equity and included in accumulated other comprehensive income. Realized gains and losses on the sale of available-for-sale marketable securities are recorded in royalty and other income, net. Available-for-sale marketable securities with original maturities greater than 3 months and remaining maturities of one year or less are classified as short-term available-for-sale marketable securities. Available-for-sale marketable securities with remaining maturities of greater than one year are classified as long-term available-for-sale marketable securities, as we intend to hold these securities until maturity. Unrealized losses that are not considered other than temporary and unrealized gains are included in accumulated other comprehensive income in stockholders’ equity. Unrealized losses that are determined to be other-than-temporary are recorded as a charge against income. The cost of marketable securities sold is determined based on the specific identification method.
Marketable securities as of December 31, 2012 and December 31, 2011 consisted of the following:
|
| | | | | | | | | | | | | | | |
| Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| (In thousands) |
December 31, 2012: | | | | | | | |
Short-Term Marketable Securities: | | | | | | | |
U.S. Treasury securities | $ | 1,800 |
| | $ | 5 |
| | $ | — |
| | $ | 1,805 |
|
U.S. government and agency securities | 3,018 |
| | 5 |
| | — |
| | 3,023 |
|
Commercial paper and certificates of deposit | 6,001 |
| | 2 |
| | — |
| | 6,003 |
|
Corporate debt securities | 16,010 |
| | 11 |
| | (1 | ) | | 16,020 |
|
Total Short-Term Marketable Securities | $ | 26,829 |
| | $ | 23 |
| | $ | (1 | ) | | $ | 26,851 |
|
Long-Term Marketable Securities: | | | | | | | |
U.S. Treasury securities | $ | 1,502 |
| | $ | 19 |
| | $ | — |
| | $ | 1,521 |
|
U.S. government and agency securities | 156 |
| | 2 |
| | — |
| | 158 |
|
Corporate debt securities | 10,835 |
| | 34 |
| | — |
| | 10,869 |
|
Total Long-Term Marketable Securities | $ | 12,493 |
| | $ | 55 |
| | $ | — |
| | $ | 12,548 |
|
|
| | | | | | | | | | | | | | | |
| Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| (In thousands) |
December 31, 2011: | | | | | | | |
Short-Term Marketable Securities: | | | | | | | |
U.S. Treasury securities | $ | 2,505 |
| | $ | 8 |
| | $ | — |
| | $ | 2,513 |
|
U.S. government and agency securities | 9,516 |
| | 10 |
| | — |
| | 9,526 |
|
Commercial paper and certificates of deposit | 3,449 |
| | — |
| | — |
| | 3,449 |
|
Corporate debt securities | 33,780 |
| | 35 |
| | (22 | ) | | 33,793 |
|
Municipal debt securities | 3,695 |
| | — |
| | — |
| | 3,695 |
|
Total Short-Term Marketable Securities | $ | 52,945 |
| | $ | 53 |
| | $ | (22 | ) | | $ | 52,976 |
|
Long-Term Marketable Securities: | | | | | | | |
U.S. Treasury securities | $ | 3,307 |
| | $ | 49 |
| | $ | — |
| | $ | 3,356 |
|
U.S. government and agency securities | 1,759 |
| | 25 |
| | — |
| | 1,784 |
|
Corporate debt securities | 12,100 |
| | 13 |
| | (29 | ) | | 12,084 |
|
Total Long-Term Marketable Securities | $ | 17,166 |
| | $ | 87 |
| | $ | (29 | ) | | $ | 17,224 |
|
As of December 31, 2012 and December 31, 2011, we did not have any investments in marketable securities with material unrealized loss position for twelve months or greater.
We assess our marketable securities for impairment under the guidance provided by ASC Topic 320. Accordingly, we review the fair value of our marketable securities at least quarterly to determine if declines in the fair value of individual securities are other-than-temporary in nature. If we believe the decline in the fair value of an individual security is other-than-temporary, we write-down the carrying value of the security to its estimated fair value, with a corresponding charge against income. To determine if a decline in the fair value of an investment is other-than-temporary, we consider several factors, including, among others, the period of time and extent to which the estimated fair value has been less than cost, overall market conditions, the historical and projected future financial condition of the issuer of the security and our ability and intent to hold the security for a period of time sufficient to allow for a recovery of the market value. The unrealized losses related to our marketable securities held as of December 31, 2012 and December 31, 2011 were primarily caused by recent fluctuations in market interest rates, and not the credit quality of the issuer, and we have the ability and intent to hold these securities until a recovery of fair value, which may be at maturity. As a result, we do not believe these securities to be other-than-temporarily impaired as of December 31, 2012.
The contractual maturities of our marketable securities at December 31, 2012 are as follows (in thousands):
|
| | | |
| |
Due within one year | $ | 26,851 |
|
Due in one to five years | 12,548 |
|
Total | $ | 39,399 |
|
| |
5. Long -Term Investments and Promissory Notes Receivable
Our long-term investments consist of equity investments in privately held companies. We determine the accounting method used to account for investments in equity securities of other business entities in which we do not have a controlling interest primarily based on our ownership of each such business entity and whether we have the ability to exercise significant influence over the strategic, operating, investing, and financing activities of each such business entity. As we do not have a controlling interest or have the ability to exercise significant influence over the companies, we account for our investments using the cost method of accounting. We monitor these investments for impairment by considering current factors including economic environment, market conditions and operational performance and other specific factors relating to the business underlying the investment.
Freeslate
We have determined that one of the entities, Freeslate Inc. (“Freeslate”), in which we carry an approximately 19.5% equity interest, is a variable interest entity in which we are not the primary beneficiary. The key factors in our assessment were that Freeslate’s management team and board of directors were solely responsible for all economic aspects of the entity, including key business decisions that impact Freeslate’s economic performance, and we do not have power, through our variable interest, to direct the activities that most significantly impact the economic performance of Freeslate. We also do not hold any seats on Freeslate’s board of directors. Accordingly, we account for our investment in Freeslate as a cost method investment. The carrying value of our investment in Freeslate was $1.0 million as of both December 31, 2012 and December 31, 2011 and is included in the other assets line on our consolidated balance sheets. In addition to our equity interest, we have a warrant allowing us to retain our 19.5% interest in the event of dilution through future stock issuances under Freeslate’s existing stock plans. We have determined that as of December 31, 2012, our investment in Freeslate is not impaired.
We also hold an unsecured promissory note receivable with a face value of $10.0 million from Freeslate, which bears interest at 8% per annum payable annually in arrears. The note requires principal payments in the amount of $1.0 million starting on March 1, 2014 and continuing annually until March 1, 2019, with the final principal payment of $4.0 million due on March 1, 2020. As the note receivable was acquired in the Symyx Merger, it was revalued in purchase accounting to its then fair value of $8.8 million. The note receivable is not measured at fair value on a recurring basis, but is periodically reviewed for possible impairment. The note receivable had a carrying amount of $8.9 million and $8.7 million as of December 31, 2012 and December 31, 2011, respectively.
Our maximum exposure to loss as a result of our interests in Freeslate is limited to the aggregate of the carrying value of our equity investment and promissory note receivable. There were no future funding commitments as of December 31, 2012 related to Freeslate.
Intermolecular
On July 28, 2011, Symyx entered into an agreement (the “IM Agreement”) with Intermolecular, Inc. (“IM”). Symyx was a stockholder of IM, holding 3,968,204 shares of IM common stock (on an as-converted to common stock basis), after giving effect to a 1-for-2 reverse stock split on November 15, 2011. By virtue of its stock ownership, pursuant to a voting agreement, Symyx was also entitled to appoint one member of the IM board of directors.
Pursuant to the terms of the IM Agreement, subject to the transactions contemplated thereby, Symyx agreed to: (i) terminate all royalty obligations of IM accruing after December 31, 2011 pursuant to an existing Alliance Agreement and an existing Collaborative Development and License Agreement between Symyx and IM; and (ii) transfer to IM, subject to a license grant back to Symyx, certain patents relating to Symyx’s legacy high-throughput research business.
As consideration for the foregoing, IM agreed to: (i) use its commercially reasonable efforts to allow Symyx to be included as a selling stockholder in IM’s initial public offering (the “IPO”) with respect to not less than all of the IM shares held by Symyx (with Symyx being obligated to sell such shares); (ii) to the extent the gross proceeds from Symyx’s sale of all
of its IM shares in the IPO were less than $67.0 million, issue a secured promissory note to Symyx upon the consummation of the IPO in an amount equal to such shortfall; and (iii) reimburse Symyx for 50% of the underwriting discounts and commission payable by Symyx with respect to the sale of such shares in the IPO. In addition, pursuant to the IM Agreement, IM agreed to continue to sublicense from Symyx its rights to certain patents and to assume certain royalty and license fee obligations relating thereto.
On November 18, 2011, IM filed its final prospectus relating to the IPO and began trading on the NASDAQ Global Select Market. As disclosed in the final prospectus, Symyx participated as a selling stockholder in the IPO by selling 3,968,204 shares of IM common stock at price per share equal to $10.00, resulting in aggregate proceeds of $39.7 million. In addition, IM reimbursed Symyx $1.4 million of the underwriting discounts and commissions payable by Symyx in connection with the IPO, representing 50% of such fees.
As a result of the sale of Symyx’s equity interest in IM in the IPO and the cash proceeds of $39.7 million, during the quarter ended December 31, 2011 we recognized a gain on sale of approximately $19.0 million, net of transaction costs and commissions of $1.4 million related to the IPO and consulting costs of $1.0 million we incurred in connection with the IM Agreement.
Pursuant to the terms of the IM Agreement, IM issued to Symyx a secured promissory note receivable in a principal amount equal to $27.3 million, representing the difference between $67.0 million and the aggregate gross proceeds from Symyx’s sale of its IM common stock in the IPO. The note receivable has a term of 24 months and an interest rate equal to 4% and is payable in quarterly installments such that an amount equal to the greater of $500,000 per quarter or the amount of accrued interest for such quarter would be payable at the end of each such quarter, with a balloon payment due at maturity. The note is pre-payable by IM at any time without penalty or premium and is secured by a proportionate amount of IM’s tangible fixed assets, excluding intellectual property.
The note receivable was discounted to a fair value of $26.0 million, representing the consideration for the patents transferred to IM, which we carried as purchased technology intangible assets acquired in the Symyx Merger, and the termination of IM’s royalty obligations to Symyx. In connection with the sale of the intellectual property we wrote off intangible assets sold to IM with a net book value of $4.3 million in the fourth quarter of fiscal 2011 and, based on our assessment of the relevant gain recognition guidance and other factors, we deferred the recognition of the portion of the net gain equal to the fair value of the note receivable of $26.0 million. We will recognize this gain when payments become due on the note receivable to the extent they exceed interest due and the amortization of the discount on the note receivable. During the year ended December 31, 2012, we received four quarterly installments due on the note of $500,000 each, which were applied to interest and discount and as such, no gain on sale was recognized. In addition, based on our evaluation of the contractual terms of the promissory note receivable and its collectability as of December 31, 2012, we believe the promissory note receivable meets the definition of a financial asset, therefore we have recorded the note receivable at expected net realizable value on our consolidated balance sheet. The fair value of the note was based on a market interest rate of 6.89% for comparable instruments.
6. Fair Value
Financial Assets and Liabilities
We carry our cash equivalents, marketable securities and foreign currency forward contracts at market value. Cash equivalents are comprised of short-term, highly liquid investments including money market funds and other investment grade securities such as certificates of deposit, commercial paper, corporate and municipal bonds and obligations of U.S. government sponsored enterprises. Our marketable securities consist of debt securities with original maturities of greater than three months and include obligations of U.S. government sponsored enterprises, commercial paper, certificates of deposit and corporate and municipal debt. The fair value of our foreign currency contracts is estimated based on the prevailing exchange rates of the various hedged currencies as of the end of the period.
Fair value accounting provides a definition of fair value, establishes acceptable methods of measuring fair value and expands disclosures for fair value measurements. The principles apply under accounting pronouncements that require measurement of fair value and do not require any new fair value measurements in accounting pronouncements where fair value is the relevant measurement attribute. Fair value accounting also specifies a fair value hierarchy based upon the observability of inputs used in valuation techniques. Observable inputs (highest level) reflect market data obtained from independent sources, while unobservable inputs (lowest level) reflect internally developed market assumptions.
In accordance with fair value accounting, fair value measurements are classified under the following hierarchy:
Level 1—Quoted prices for identical instruments in active markets.
Level 2—Inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly. These might include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (such as interest rates, volatilities, prepayment speeds, credit risks, etc.) or inputs that are derived principally from or corroborated by market data by correlation or other means.
Level 3—Model-derived valuations in which one or more significant inputs or significant value-drivers are unobservable.
Fair value measurements are classified according to the lowest level input or value-driver that is significant to the valuation. A measurement may therefore be classified within Level 3 even though there may be significant inputs that are readily observable.
Where applicable, the determination of fair values is based on unadjusted quoted prices that are available in active markets for the identical assets or liabilities at the measurement date. This pricing methodology applies to our Level 1 investments. To the extent quoted prices in active markets are not available at the measurement date, fair values can be based on usage of observable market prices in less active markets, use of broker/dealer quotes and alternative pricing sources with reasonable levels of price transparency.
Because many fixed income securities do not trade on a daily basis or have market prices from multiple sources, the pricing applications may apply available information as applicable to determine the fair value as of the measurement date. This methodology applies to our Level 2 investments. Currently we do not hold any Level 3 investments.
We recorded an acquisition-related liability for contingent consideration representing the amounts payable to former Contur equity holders, as outlined under the terms of the Contur Purchase Agreement, upon the achievement of certain agreed-upon performance milestones. The fair value of this Level 3 liability is estimated based on a discounted probability-weighted income approach derived from revenue estimates and a probability assessment with respect to the likelihood of achieving the milestone criteria. Subsequent changes in the fair value of the contingent consideration liability are recorded in the statement of operations and result from updates to assumed probability of achievement of milestones and adjustments to the discount periods and rates. Since the May 19, 2011 acquisition date, we have recognized a $0.2 million increase to the $0.3 million acquisition date liability for contingent consideration, to a balance of $0.2 million as of December 31, 2012, after the payment of the first year milestone as discussed in Note 2.
The following table summarizes our assets and liabilities that require fair value measurements on a recurring basis and their respective input levels based on the fair value hierarchy:
|
| | | | | | | | | | | | | | | |
| Carrying
Value at December 31,
2012 | | Quoted Market Prices for Identical Assets (Level 1) | | Significant Other Observable Inputs (Level 2) | | Significant Unobservable Inputs (Level 3) |
| (In thousands) |
December 31, 2012: | | | | | | | |
Cash equivalents (1) | $ | 46,043 |
| | $ | 46,043 |
| | $ | — |
| | $ | — |
|
Short-Term Marketable Securities: | | | | | | | |
U.S. Treasury securities | 1,805 |
| | 1,805 |
| | — |
| | — |
|
U.S. government and agency securities | 3,023 |
| | — |
| | 3,023 |
| | — |
|
Commercial paper and certificates of deposit | 6,003 |
| | — |
| | 6,003 |
| | — |
|
Corporate debt securities | 16,020 |
| | — |
| | 16,020 |
| | — |
|
Municipal debt securities | — |
| | — |
| | — |
| | — |
|
Long-Term Marketable Securities: | | | | | | | |
U.S. Treasury securities | 1,521 |
| | 1,521 |
| | — |
| | — |
|
U.S. government and agency securities | 158 |
| | — |
| | 158 |
| | — |
|
Corporate debt securities | 10,869 |
| | — |
| | 10,869 |
| | — |
|
Total assets at fair value | $ | 85,442 |
| | $ | 49,369 |
| | $ | 36,073 |
| | $ | — |
|
Liabilities: | | | | | | | |
Acquisition-related contingent consideration | $ | 241 |
| | $ | — |
| | $ | — |
| | $ | 241 |
|
Foreign currency forward contracts not designated as hedges | $ | 99 |
| | $ | — |
| | $ | 99 |
| | $ | — |
|
Total liabilities at fair value | $ | 340 |
| | $ | — |
| | $ | 99 |
| | $ | 241 |
|
| |
(1) | Cash equivalents are included in the Cash and cash equivalents line in the consolidated balance sheet |
There were no transfers between Level 1 and Level 2 securities during the year ended December 31, 2012.
|
| | | | | | | | | | | | | | | |
| Carrying
Value at
December 31,
2011 | | Quoted Market Prices for Identical Assets (Level 1) | | Significant Other Observable Inputs (Level 2) | | Significant Unobservable Inputs (Level 3) |
| (In thousands) |
December 31, 2011: | | | | | | | |
Cash equivalents (1) | $ | 30,541 |
| | $ | 27,440 |
| | $ | 3,101 |
| | $ | — |
|
Short-Term Marketable Securities: | | | | | | | |
U.S. Treasury securities | 2,513 |
| | 2,513 |
| | — |
| | — |
|
U.S. government and agency securities | 9,526 |
| | — |
| | 9,526 |
| | — |
|
Commercial paper and certificates of deposit | 3,449 |
| | — |
| | 3,449 |
| | — |
|
Corporate debt securities | 33,793 |
| | — |
| | 33,793 |
| | — |
|
Municipal debt securities | 3,695 |
| | — |
| | 3,695 |
| | — |
|
Long-Term Marketable Securities: | | | | | | | |
U.S. Treasury securities | 3,356 |
| | 3,356 |
| | — |
| | — |
|
U.S. government and agency securities | 1,784 |
| | — |
| | 1,784 |
| | — |
|
Corporate debt securities | 12,084 |
| | — |
| | 12,084 |
| | — |
|
Total assets at fair value | $ | 100,741 |
| | $ | 33,309 |
| | $ | 67,432 |
| | — |
|
Liabilities: | | | | | | | |
Acquisition-related contingent consideration | $ | 413 |
| | $ | — |
| | $ | — |
| | $ | 413 |
|
Foreign currency forward contracts not designated as hedges | 117 |
| | — |
| | 117 |
| | — |
|
Total liabilities at fair value | $ | 530 |
| | $ | — |
| | $ | 117 |
| | $ | 413 |
|
| |
(1) | Cash equivalents are included in the Cash and cash equivalents line in the consolidated balance sheet |
The following table includes a summary of the contingent consideration measured at fair value using significant unobservable inputs (Level 3) during the year ended December 31, 2012 (in thousands):
|
| | | |
Balance at December 31, 2011 | $ | 413 |
|
Expenses recorded due to changes in fair value | 78 |
|
Payments | (250 | ) |
Balance at December 31, 2012 | $ | 241 |
|
The carrying amount of accounts receivable, accounts payable and accrued liabilities are considered to be representative of their respective fair values due to their short-term nature. Our investments in privately-held companies are carried at cost. Our notes receivable are carried at the expected net realizable value. It is impracticable for us to estimate the fair value of these instruments on a recurring basis if there are no identified events or changes in circumstances that may have a significant adverse effect on their fair value.
Non-Financial Assets and Liabilities Measured at Fair Value on a Nonrecurring Basis
The non-financial assets and liabilities are recognized at fair value subsequent to initial recognition when they are deemed to be other-than-temporarily impaired. There were no non-financial assets and liabilities deemed to be other-than-temporarily impaired and measured at fair value on a nonrecurring basis for the periods presented.
7. Goodwill and Purchased Intangible Assets
Intangible assets consist of the following as of December 31, 2012 and December 31, 2011
|
| | | | | | | | | | | | | | | | | | | |
| December 31, 2012 | | December 31, 2011 |
| Weighted Average Life (yrs) | | Gross Carrying Amount | | Accumulated Amortization | | Weighted Average Life (yrs) | | Gross Carrying Amount | | Accumulated Amortization |
| | | (In thousands) | | | | (In thousands) |
Intangible assets subject to amortization: | | | | | | | | | | | |
Purchased technology | 8 | | $ | 61,309 |
| | $ | 32,569 |
| | 8 | | $ | 53,509 |
| | $ | 22,160 |
|
Purchased customer relationships | 7 | | 30,729 |
| | 13,991 |
| | 7 | | 25,906 |
| | 8,158 |
|
Purchased backlog | 3 | | 7,800 |
| | 6,370 |
| | 3 | | 7,740 |
| | 4,390 |
|
Purchased contract base | 5 | | 50 |
| | 50 |
| | 5 | | 50 |
| | 50 |
|
Purchased intellectual property | 5 | | 1,998 |
| | 1,860 |
| | 5 | | 1,998 |
| | 1,448 |
|
Purchased trademark/tradename | 5 | | 6,850 |
| | 3,173 |
| | 5 | | 6,712 |
| | 1,848 |
|
| | | $ | 108,736 |
| | $ | 58,013 |
| | | | $ | 95,915 |
| | $ | 38,054 |
|
Intangible assets not subject to amortization: | | | | | | | | | | | |
Purchased trademark/tradename | | | $ | 2,500 |
| | | | | | $ | 2,500 |
| | |
As discussed in Note 3, intangible assets related to the Aegis Acquisition are included in our preliminary purchase price allocation and are subject to change during the measurement period. Intangible assets are amortized over their estimated useful lives either on a straight-line or accelerated basis that reflects the pattern in which the economic benefits of the intangible assets are expected to be realized, which range from two to fifteen years, with no residual value.
Intangible asset amortization expense was $19.9 million and $21.0 million for the years ended December 31, 2012 and December 31, 2011, respectively. Intangible asset amortization expense was $9.2 million and $1.1 million (unaudited) for the nine months ended December 31, 2010 and December 31, 2009, respectively.
Future estimated amortization expense for intangible assets as of December 31, 2012 is as follows and is subject to change as a result of measurement period adjustments (in thousands):
|
| | | |
2013 | $ | 19,079 |
|
2014 | 13,421 |
|
2015 | 8,058 |
|
2016 | 4,593 |
|
2017 | 2,939 |
|
Thereafter | 2,633 |
|
Total | $ | 50,723 |
|
The changes to the carrying amount of goodwill for the year ended December 31, 2012, are as follows (in thousands):
|
| | | | |
Balance at December 31, 2011 | $ | 100,429 |
| |
Goodwill acquired in the Aegis Acquisition | 18,960 |
| |
Goodwill acquired in the acquisition of the HEOS platform
| 3,750 |
| |
Goodwill measurement period adjustments | 118 |
| (1) |
Effect of foreign exchange | 413 |
| |
Balance at December 31, 2012 | $ | 123,670 |
| |
(1) As discussed in Note 2, we made total measurement period adjustments related to the VelQuest Acquisition of $0.2 million related primarily to accrued liabilities, offset by a measurement period adjustment of $29,000 to accrued income tax balances related to the Contur Acquisition.
8. Restructuring Activities
The following summarizes the changes in our accrued restructuring charges liability:
|
| | | | | | | | | | | |
| Severance and Related Costs | | Lease Obligation Exit and Facility Closure Costs | | Total |
| (In thousands) |
Balance at December 31, 2010 | $ | 2,866 |
| | $ | 1,241 |
| | $ | 4,107 |
|
Additional severance and lease abandonment charges | 1,765 |
| | 187 |
| | 1,952 |
|
Adjustments to liability | (36 | ) | | (155 | ) | | (191 | ) |
Cash payments | (3,563 | ) | | (600 | ) | | (4,163 | ) |
Effect of foreign exchange | 5 |
| | — |
| | 5 |
|
Balance at December 31, 2011 | $ | 1,037 |
| | $ | 673 |
| | $ | 1,710 |
|
Additional severance and lease abandonment charges | 22 |
| | — |
| | 22 |
|
Adjustments to liability | — |
| | 156 |
| | 156 |
|
Cash payments | (978 | ) | | (230 | ) | | (1,208 | ) |
Effect of foreign exchange | (16 | ) | | 27 |
| | 11 |
|
Balance at December 31, 2012 | $ | 65 |
| | $ | 626 |
| | $ | 691 |
|
On April 28, 2011, we renegotiated a sublease for a facility in the UK that was abandoned in 2006 and for which the tenant had exercised a break clause, whereby the tenant reduced the amount of floor space sublet. Additionally, we negotiated the sublease of the remaining floor space with another party. As a result of the change in estimate of sublease income for this facility, we recorded a net additional $0.2 million in restructuring expense during the year ended December 31, 2011.
On April 28, 2011, we committed to the implementation of a reduction in force of approximately ten to fifteen employees, in connection with the streamlining of our operations and the integration of our Content business. As a result, we incurred total severance charges of approximately $1.0 million during the year ended December 31, 2011, of which $0.6 million was paid during fiscal year 2011 and $0.4 million was paid during the year ended December 31, 2012.
In July 2010, as a result of the Symyx Merger, we implemented a plan to terminate the employment of approximately 80 employees in the U.S., Europe and the Asia-Pacific region. As a result of such terminations, we have incurred total severance charges of approximately $5.3 million, with $0.8 million incurred during the year ended December 31, 2011 and $4.5 million incurred during the year ended December 31, 2010. As of December 31, 2012, approximately $5.2 million in cash payments had been made with the remaining balance of $0.1 million expected to be paid during fiscal year 2013.
Prior to the Symyx Merger, Symyx had implemented various restructuring plans under which lease obligations of approximately $0.1 million remain as of December 31, 2012 and terminate in 2016. Additionally, prior to 2007, we had implemented a restructuring plan under which we have remaining lease obligations of approximately $0.5 million as of December 31, 2012. These lease obligations terminate in 2022.
All amounts incurred in connection with the above activities are recorded in the “Business consolidation, transaction and restructuring costs” line in the accompanying consolidated statements of operations.
9. Royalty and Other Income, Net
Royalty and other income, net includes non-operating items such as interest income, amortization of discount on promissory notes receivable, realized investment gains and losses, foreign currency exchange gains and losses, non-operating gain on real estate and other non-operating gains and losses. It also includes royalty income (net of royalty expense) and amortization of purchased intangible assets related to products that are not part of current operations. As part of the Symyx Merger, we have retained the rights to receive royalty income related to the divestiture of the High Productivity Research business unit previously divested by Symyx.
Royalty and other income, net consisted of the following:
|
| | | | | | | | | | | | | | | |
| Years Ended | | Nine Months Ended |
| December 31
2012 | | December 31,
2011 | | December 31
2010 | | December 31,
2009 |
| | | | | | | (unaudited) |
| (In thousands) |
Royalty income, net | $ | 5,428 |
| | $ | 6,167 |
| | $ | 1,722 |
| | $ | — |
|
Net interest income | 2,296 |
| | 1,486 |
| | 739 |
| | 237 |
|
Realized gain on investments | — |
| | — |
| | — |
| | 248 |
|
Amortization of discount on promissory notes receivable | 892 |
| | 155 |
| | 78 |
| | — |
|
Foreign currency transaction gain (loss) | (558 | ) | | 530 |
| | 171 |
| | 145 |
|
Purchased intangible assets amortization | (1,696 | ) | | (2,365 | ) | | (1,247 | ) | | — |
|
Net gain on sale of non-operating real estate | 2,744 |
| | — |
| | — |
| | — |
|
Net income (loss) from rental activities | (338 | ) | | 311 |
| | — |
| | — |
|
Write-off of intangible asset in connection with the sale of intellectual property | — |
| | (4,303 | ) | | — |
| | — |
|
Other | 102 |
| | (241 | ) | | (86 | ) | | (10 | ) |
Total royalty and other income, net | $ | 8,870 |
| | $ | 1,740 |
| | $ | 1,377 |
| | $ | 620 |
|
In June 2012, we sold real property comprised of land and an office building located in Santa Clara, California, for a net sales price of $6.8 million. We recorded a pre-tax gain of approximately $2.7 million on the sale in royalty and other income, net, as this property was not utilized in our ongoing operations since its acquisition in the Symyx Merger.
Under our royalty income agreements, we have contractual minimum royalty income payments as follows (in thousands):
|
| | | |
| |
Fiscal year 2013 | $ | 6,000 |
|
Fiscal year 2014 | 5,000 |
|
Fiscal year 2015 | 5,000 |
|
Total | $ | 16,000 |
|
| |
As these amounts are related to intellectual property acquired in the Symyx Merger as described above, they will be partially offset by amortization expense related to such intellectual property. The income related to these agreements for the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010 is included in the net royalty income presented above of $5.4 million , $6.2 million $1.7 million, respectively.
10. Income Taxes
Income (loss) from continuing operations before taxes was as follows:
|
| | | | | | | | | | | | | | | | |
| | Years Ended | | Nine Months Ended |
| | December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2009 |
| | (In thousands) |
| | | | | | | | (unaudited) |
U.S. | | $ | (12,827 | ) | | $ | 3,372 |
| | $ | (32,841 | ) | | $ | 3,668 |
|
Foreign | | 2,643 |
| | (2,363 | ) | | (1,822 | ) | | 998 |
|
Total | | $ | (10,184 | ) | | $ | 1,009 |
| | $ | (34,663 | ) | | $ | 4,666 |
|
Income tax expense consists of the following:
|
| | | | | | | | | | | | | | | | |
| | Years Ended | | Nine Months Ended |
| | December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2009 |
| | | | (In thousands) | | |
| | | | | | | | (unaudited) |
Current: | | | | | | | | |
Federal | | $ | 72 |
| | $ | 1,358 |
| | $ | — |
| | $ | (15 | ) |
State | | (1,518 | ) | | (152 | ) | | (336 | ) | | 129 |
|
Foreign | | 763 |
| | 950 |
| | 454 |
| | 419 |
|
Total current income tax expense (benefit) | | $ | (683 | ) | | $ | 2,156 |
| | $ | 118 |
| | $ | 533 |
|
Deferred: | | | | | | |
Federal | | $ | 781 |
| | $ | (652 | ) | | $ | (11,366 | ) | | $ | 530 |
|
State | | 10 |
| | (116 | ) | | (2,669 | ) | | — |
|
Foreign | | 110 |
| | (2,144 | ) | | (138 | ) | | 34 |
|
Total deferred income tax expense (benefit) | | $ | 901 |
| | $ | (2,912 | ) | | $ | (14,173 | ) | | $ | 564 |
|
Total income tax expense (benefit) | | $ | 218 |
| | $ | (756 | ) | | $ | (14,055 | ) | | $ | 1,097 |
|
The following reconciles income taxes computed at the U.S. statutory federal tax rate to income tax expense (benefit):
|
| | | | | | | | | | | | | | | | |
| | Years Ended | | Nine Months Ended |
| | December 31, 2012 | | December 31, 2011 | | December 31, 2010 | | December 31, 2009 |
| | |
| | | | | | | | (unaudited) |
Income tax at U.S. statutory rate | | $ | (3,463 | ) | | $ | 343 |
| | $ | (11,786 | ) | | $ | 1,586 |
|
State income taxes, net of federal benefit | | (52 | ) | | 193 |
| | (1,692 | ) | | 187 |
|
Foreign taxes, rate differential | | 163 |
| | (26 | ) | | (49 | ) | | 178 |
|
Transaction Costs | | 3 |
| | 15 |
| | 894 |
| | — |
|
Indefinite life goodwill | | 732 |
| | 540 |
| | — |
| | 439 |
|
Research and Developmental Credits | | (1,763 | ) | | (1,047 | ) | | (1,072 | ) | | (674 | ) |
Equity compensation | | 413 |
| | 145 |
| | (83 | ) | | 35 |
|
Change in state rate and expired state NOL’s | | (48 | ) | | (386 | ) | | (300 | ) | | 2,125 |
|
Expired/Section382 limited net operating losses & credits | | (330 | ) | | (185 | ) | | 9,383 |
| | — |
|
Other | | 1,663 |
| | 141 |
| | 747 |
| | 2 |
|
Change in UK NOL | | (2,252 | ) | | 650 |
| | — |
| | — |
|
Reversal of uncertain tax positions | | (2,168 | ) | | (433 | ) | | — |
| | — |
|
Interested related to uncertain tax positions | | 177 |
| | 117 |
| | — |
| | — |
|
Installment sale interest | | 66 |
| | 96 |
| | — |
| | — |
|
Contingent consideration | | 366 |
| | 340 |
| | — |
| | — |
|
Change in UK branch loss benefit | | 1,964 |
| | 1,719 |
| | — |
| | — |
|
Reduction in valuation allowance – expired net operating losses & credits | | — |
| | — |
| | (9,383 | ) | | — |
|
Tax benefit from business combination – reduction in valuation allowance | | — |
| | — |
| | (14,921 | ) | | — |
|
Change in valuation allowance – current year operating loss | | 4,747 |
| | (2,978 | ) | | 14,207 |
| | (2,781 | ) |
Income tax expense (benefit) | | $ | 218 |
| | $ | (756 | ) | | $ | (14,055 | ) | | $ | 1,097 |
|
Significant components of our deferred tax assets and liabilities are as follows:
|
| | | | | | | | | |
| | December 31, 2012 | | December 31, 2011 | |
| | (In thousands) |
Deferred tax assets: | | | | | |
Net operating loss carryforwards | | $ | 34,700 |
| | $ | 32,909 |
| |
Goodwill and intangibles | | 2,505 |
| | 3,277 |
| |
Property and equipment | | 3,512 |
| | 3,727 |
| |
Unused tax credits | | 10,249 |
| | 8,254 |
| |
Accruals and reserves | | 23,102 |
| | 20,664 |
| |
Gross deferred tax assets | | 74,068 |
| | 68,831 |
| |
Deferred tax liabilities: | | | | | |
Intangible assets | | 14,836 |
| | 16,357 |
| |
Other deferred tax liabilities | | 4,044 |
| | 1,719 |
| |
Indefinite lived intangible assets | | 4,187 |
| | 3,375 |
| |
Land and Other Long Term Investments | | 346 |
| | 712 |
| |
Gross deferred tax liabilities | | 23,413 |
| | 22,163 |
| |
Total net deferred tax assets | | 50,655 |
| | 46,668 |
| |
Valuation allowance for deferred tax assets | | (52,670 | ) | | (48,253 | ) | |
Net deferred taxes | | $ | (2,015 | ) | | $ | (1,585 | ) | |
At December 31, 2012 and December 31, 2011, we had $4.2 million and $3.4 million, respectively, of net deferred tax liabilities in the U.S. related to indefinite-lived goodwill and intangible assets that are amortized for income tax purposes. For book purposes, the indefinite-lived goodwill and intangible assets will be charged to results of operations when they are determined to be impaired in accordance with ASC Topic 350. Because we cannot predict when these assets will be impaired and when the associated deferred tax liabilities will reverse, the deferred tax liabilities related to these assets are not considered a source of income to support the realization of our deferred tax assets. Additionally, we have deferred tax liabilities of $0.3 million related to the Symyx purchase price adjustments for acquired investments. Upon disposition, we will have a lower tax basis in these assets. As we cannot predict when these assets will be disposed of, the deferred tax liabilities associated with these assets are also not considered a source of income to support the realization of our deferred tax assets. We have provided a full valuation allowance for our remaining net U.S. deferred tax assets as we do not believe that the ultimate realization of such deferred tax assets is more-likely-than-not.
At December 31, 2012 and December 31, 2011, we had $1.2 million and $1.3 million, respectively, of net deferred tax assets related to Accelrys, K.K. As of December 31, 2012 we also have $0.1 million and $0.4 million of deferred tax assets for Symyx KK and Symyx Switzerland, respectively. As of December 31, 2012 , we have a $1.5 million deferred tax asset for Accelrys UK related to net operating loss carryforwards and other deferred tax assets. We believe that the ultimate realization of these deferred tax assets is more-likely-than-not since our intercompany transfer pricing arrangements provide for Accelrys, K.K., Symyx KK , Symyx Switzerland, and Accelrys UK to achieve an annual operating profit margin.
Due to uncertainties surrounding our ability to generate future taxable income, a valuation allowance has been established against our domestic and certain foreign deferred tax assets. As of December 31, 2012 the valuation allowance was $52.7 million. For the year ended December 31, 2012, the valuation allowance increased by $4.4 million primarily as a result of the Velquest Acquisition. For the year ended December 31, 2011, the valuation allowance decreased by $3.0 million primarily as a result of the recognition of a deferred tax asset for net operating losses in the UK and the netting of deferred tax liabilities for land and other investments.
For the nine months ended December 31, 2010, the valuation allowance decreased by $14.9 million as a result of net deferred tax liabilities recorded for the Symyx acquisition, which were considered a source of income to reduce the valuation allowance on our existing deferred tax assets. In addition, during the nine months ended December 31, 2010, the valuation allowance increased by $14.2 million as a result of operating losses for which no tax benefit was recorded and decreased by $9.4 million related to NOLs and tax credits which either expired or were limited under Internal Revenue Code section 382.
We do not record U.S. income taxes on the undistributed earnings of our foreign subsidiaries based upon the Company's intention to permanently reinvest undistributed earnings to ensure sufficient working capital and further expansion of existing operations outside the U.S. The undistributed earnings of the foreign subsidiaries as of December 31, 2012 are approximately $5.5 million. In the event we are required to repatriate funds from outside of the U. S., such repatriation would be subject to local laws, customs, and tax consequences. We cannot practicably estimate the amount of additional taxes that might be payable on the unremitted earnings.
As of December 31, 2012, we had U.S. federal NOLs of approximately $84.4 million and tax credit carryforwards of approximately $3.2 million. We also had California NOLs of approximately $67.7 million and tax credit carryforwards of approximately $13.5 million. We also had approximately $4.3 million of UK NOLs. Approximately $46.7 million of the federal NOLs relate to stock option deductions which, upon realization, will result in an increase to paid-in capital and a decrease in income taxes payable. If not fully utilized, portions of our U.S. federal NOLs will expire in varying amounts from 2019 through 2030. Federal tax credit carryforwards will expire in varying amounts from 2020 through 2031. Portions of our California NOLs will expire in varying amounts from 2014 through 2030. The California tax credit carryforwards do not expire. Realization of our NOLs and credits is dependent upon our generating sufficient taxable income prior to expiration of those attributes.
In addition to the NOLs reflected above, as of December 31, 2012, we had pre-acquisition NOLs of $41.5 million from acquisitions outside the U.S. which have been reserved as unrecognized tax benefits. The pre- acquisition NOLs, if ultimately realized, will result in an increase to income tax benefit. No pre-acquisition NOLs were utilized during the years ended December 31, 2012 and December 31, 2011 or the nine months ended December 31, 2010.
We recognize windfall tax benefits associated with the exercise of stock options directly to stockholders’ equity only when realized. Accordingly, deferred tax assets are not recognized for NOLs resulting from windfall tax benefits. At December 31, 2012, deferred tax assets do not include $0.4 million of excess tax benefits from share-based compensation.
The future utilization of our NOLs to offset future taxable income may be subject to a substantial annual limitation as a result of changes in ownership by our stockholders that hold 5% or more of our common stock. An assessment of such ownership changes under Sections 382 and 383 of the Internal Revenue Code (the “Code”) was completed through December 31, 2010. As a result of this assessment, we determined that we experienced an ownership change during 2010 related to the acquisition of Symyx which will limit the future utilization of our NOLs and credit carryforwards. U.S. federal NOLs of approximately $17.1 million will expire due to limitations under Sections 382 and U.S. federal R&D credits of approximately $2.2 million will expire due to limitations under Section 383. Future ownership changes may further impact the utilization of existing NOLs and tax credit carryforwards. We acquired Velquest on December 30, 2011. The acquisition generated an ownership change under Sections 382 and 383 of the Code. A Section 382 and Section 382 analysis was completed for such acquisition and we recorded a deferred tax asset of federal NOLs of $11.8 million and state NOLs of $2.6 million.
Upon the adoption of new accounting principles relating to uncertain tax positions as of April 1, 2007, Accelrys did not record any reserves for uncertain tax positions. In the third quarter for the year ended March 31, 2010 Accelrys identified an uncertain tax position of $10.8 million relating to the NOL carryovers obtained in connection with the acquisition of one of our foreign subsidiaries. Additionally, during the nine months ended December 31, 2010 we identified an additional $7.3 million in uncertain tax positions.
Following is a tabular reconciliation of the Unrecognized Tax Benefits (“UTB”) activity during the year ended December 31, 2012 (excluding interest and penalties):
|
| | | |
| |
| (In thousands) |
Beginning balance, April 1, 2009 | $ | — |
|
Additions based on tax positions related to prior years | 10,800 |
|
Balance at March 31, 2010 | $ | 10,800 |
|
Additions based on tax positions related to the current year | 7,259 |
|
Reductions for tax positions of prior year | — |
|
Settlements | — |
|
Reductions due to lapse of applicable statute of limitations | (584 | ) |
Impact of foreign currency fluctuations | 286 |
|
| |
Balance at December 31, 2010 | $ | 17,761 |
|
Additions based on tax positions related to the current year | 189 |
|
Reductions for tax positions of prior year | — |
|
Settlements | — |
|
Reductions due to lapse of applicable statute of limitations | (747 | ) |
Impact of foreign currency fluctuations | (1,559 | ) |
| |
Balance at December 31, 2011 | $ | 15,644 |
|
Additions based on tax positions related to the current year | 909 |
|
Additions for tax positions of prior year
| 611 |
|
Settlements | — |
|
Reductions due to lapse of applicable statute of limitations | (2,393 | ) |
Impact of foreign currency fluctuations | 95 |
|
| |
Balance at December 31, 2012 | $ | 14,866 |
|
Included in the balance of unrecognized tax benefits as of December 31, 2012, are $10.4 million of tax benefits that, if recognized, would affect the effective tax rate. Also included in the balance of unrecognized tax benefits at December 31, 2012, are $4.4 million of tax benefits that, if recognized, would result in adjustments to other tax accounts, primarily deferred taxes.
We have elected to include interest expense and penalties accrued on unrecognized tax benefits as a component of our provision for income taxes. The Company has approximately $0.1 million of accrued interest and penalties related to unrecognized tax benefits in the provision for income taxes at December 31, 2012.
We are subject to taxation in the U.S. and various state and foreign jurisdictions. Our tax years from 1996 and forward are subject to examination by the U.S. and various other state jurisdictions due to the carryforward of unutilized NOLs and research and development credits. With respect to significant foreign jurisdictions, tax years from 2006 and forward are subject to examinations by various foreign tax authorities in Sweden, and Japan. Tax years from 2007 and forward are subject to examination by foreign tax authorities in Germany. Tax years from 2008 and forward are subject to examination in France and Switzerland and tax years from 2009 and forward are subject to examination by tax authorities in United Kingdom. In the fourth quarter of fiscal 2013, we may release $0.5 million of our liability for unrecognized tax benefits related to positions taken applicable to the prior taxable years which would have an impact to our current taxes.
11. Retirement Savings Plans
U.S. Based Employees
We have an employee savings and retirement plan (the “401(K) Plan”). The 401(K) Plan is intended to be a tax-qualified plan covering substantially all U.S.-based employees. Under the terms of the 401(K) Plan, employees may elect to contribute up to 20% of their compensation, or the statutory prescribed limit, if less. We may, at our discretion, match employee contributions up to a maximum of 4% of the employee’s compensation. Employer matching contributions totaled $1.7 million and $1.3 million for the years ended December 31, 2012 and December 31, 2011, respectively. Employer matching contributions totaled $0.8 million and $0.5 million for the nine months ended December 31, 2010 and December 31, 2009(unaudited), respectively.
Non-U.S. Based Employees
We provide retirement benefits for employees located outside the U.S. commensurate with other similar companies in those respective locations. Employer contributions, in the form of cash, totaled $1.2 million and $1.1 million for the years ended December 31, 2012 and December 31, 2011, respectively. Employer contributions, in the form of cash, totaled $0.6 million and $0.5 million for the nine months ended December 31, 2010 and December 31, 2009 (unaudited), respectively.
12. Net Income (Loss) Per Share
We compute net income (loss) per share pursuant to FASB ASC Topic 260, Earnings Per Share. Accordingly, basic net income (loss) per share and diluted net income (loss) per share are computed by dividing the net income (loss) for the period by the weighted average number of common shares outstanding during the period, without consideration for common stock equivalents. Diluted net income per share is computed by dividing the net income for the period by the weighted average number of common stock and common stock equivalents outstanding during the period. Potentially dilutive common stock equivalents consist of common stock options and unvested RSUs.
|
| | | | | | | | | | | | | | | |
| Year Ended December 31, | | Nine Months Ended December 31, |
| 2012 | | 2011 | | 2010 | | 2009 |
| | | | | | | (unaudited) |
Numerator: | | | | | | | |
Net income (loss) | (10,402 | ) | | 1,765 |
| | (20,608 | ) | | 3,569 |
|
Denominator for basic and diluted net income (loss) per share: | | | | | | | |
Weighted average common shares outstanding for basic | 55,696 |
| | 55,489 |
| | 46,446 |
| | 27,470 |
|
Dilutive potential common stock equivalents | — |
| | 548 |
| | — |
| | 234 |
|
Weighted average common shares outstanding for diluted | 55,696 |
| | 56,037 |
| | 46,446 |
| | 27,704 |
|
Basic net income (loss) per share | $ | (0.19 | ) | | $ | 0.03 |
| | $ | (0.44 | ) | | $ | 0.13 |
|
Diluted net income (loss) per share | $ | (0.19 | ) | | $ | 0.03 |
| | $ | (0.44 | ) | | $ | 0.13 |
|
As we reported a net loss for the year ended December 31, 2012 and the nine months ended December 31, 2010, basic and diluted net loss per share were the same. Potentially dilutive securities not included in the computation of diluted net income (loss) per share totaled approximately 7.6 million, 3.8 million and 6.9 million shares for the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010 and, respectively. Potentially dilutive common stock equivalents that were excluded from the diluted net income per share calculations since their effect would be anti-dilutive totaled approximately 3.1 million shares for the nine months ended December 31, 2009 (unaudited).
13. Guarantees
Guarantee of Lease Obligation of Others
On April 30, 2004, we spun-off our drug discovery subsidiary, Pharmacopeia Drug Discovery, Inc. (“PDD”), into an independent, separately traded, publicly held company through the distribution to our stockholders of a dividend of one share of PDD common stock for every two shares of our common stock. The landlords of our New Jersey facilities, which were used by our PDD operations, consented to the assignment of the leases to PDD. Despite the assignment, the landlords required us to guarantee the remaining lease obligations, which totaled approximately $8.5 million as of December 31, 2012. In the event that PDD defaults on its lease commitment, we are permitted under the guarantee to sublease the facility in order to mitigate our lease obligation. In fiscal year 2005, we recognized a liability and corresponding charge to stockholders’ equity for the probability-weighted fair value of the guarantee. Changes to the fair value of the liability are recognized in stockholders’ equity. The liability for our guarantee of the lease obligation was $0.2 million and $0.3 million at December 31, 2012 and December 31, 2011, respectively.
Other Guarantees and Indemnifications
We provide indemnifications of varying scope and size to certain customers against claims of intellectual property infringement made by third parties arising from the use of our products. We have also entered into indemnification agreements with our officers and directors. Although the maximum potential amount of future payments we could be required to make under these indemnifications is unlimited, to date we have not incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. Additionally, we have insurance policies that, in most cases, would limit our exposure and enable us to recover a portion of any amounts paid. Therefore, we believe the estimated fair value of these agreements is minimal and the likelihood of incurring an obligation is remote. Accordingly, we have not accrued any liabilities in connection with these indemnification obligations as of December 31, 2012.
14. Commitments and Contingencies
Operating Leases
We lease office space in several facilities under operating leases that expire through 2023. Certain of our lease agreements contain renewal options, rent holidays or rent escalation clauses. In accordance with ASC Topic 840, Leases, we recognize rent expense on a straight-line basis over the term of the related operating leases, accounting for scheduled rent escalation clauses during the lease terms or for rental payments commencing at a date other than the date of initial occupancy and including any cancelable option periods where failure to exercise such options would result in economic penalty. Rent expense recognized in excess of rent paid is reflected as a deferred rent liability, which is included in lease-related liabilities in the accompanying consolidated balance sheets. Rent expense under our operating leases was $5.7 million, $6.2 million, $3.9 million and $2.5 million for the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010 and December 31, 2009 (unaudited), respectively.
In December 2012, we entered into a lease agreement for a facility with 61,460 square feet of office space, which will serve as our new corporate headquarters in San Diego, California. The lease commences on July 1, 2013 and has a 10-year term (with an option to terminate after five years as discussed below). The annual base rent for the first year of the lease is $1.6 million and is subject to annual increases of approximately three to four percent per year, with the rent in the first and sixth years subject to partial abatement. Pursuant to the terms of the lease, we will have: (i) two five-year options to renew the lease (with the base rent subject to adjustment based on certain comparable metrics set forth in the lease); and (ii) a one-time right to terminate the lease effective as of June 30, 2018 upon written notice to the landlord on or before January 1, 2018. In the event that we exercise our rights to terminate the lease in accordance with the foregoing, we will be obligated to pay to the landlord a termination fee of $2.3 million.
In addition, the lease provides that if our cash on hand, earnings before interest, taxes, depreciation and amortization or market capitalization fall below certain thresholds, we will be required to deposit with the landlord a letter of credit equal to 12 times the then-current monthly installment of base rent, subject to certain adjustments and exceptions. The landlord will have the right to draw down on the letter of credit upon the occurrence of certain events, including, among other things, in connection with (i) the bankruptcy of Accelrys (whether voluntary or involuntary); (ii) landlord's receipt of notice that the bank extending the letter of credit will not renew or extend such letter of credit as provided for under the lease; or (iii) a material adverse change in the financial condition of the bank extending the letter of credit.
Additionally, under the terms of the lease for our current San Diego, California corporate office, we were required to obtain an irrevocable letter of credit for $6.6 million to ensure our performance under the lease agreement. The letter of credit requirement decreased to $3.5 million on September 1, 2011 and will expire upon termination of the lease on September 1, 2013. The letter of credit is fully secured by cash and cash equivalents, which are included in restricted cash in the accompanying consolidated balance sheets.
We sublease certain facilities abandoned in connection with previous restructuring activities, and sublease income is recorded as a reduction of the restructuring liability and related restructuring expense upon abandonment of the facility.
Future minimum lease commitments and future committed sublease income as of December 31, 2012 are as follows:
|
| | | | | | | | | | | | |
| | Operating Lease Commitments | | Sublease Income | | Net |
| | (In thousands) |
Fiscal Year 2013 | | $ | 6,902 |
| | $ | 668 |
| | $ | 6,234 |
|
Fiscal Year 2014 | | 6,536 |
| | 498 |
| | 6,038 |
|
Fiscal Year 2015 | | 5,423 |
| | 498 |
| | 4,925 |
|
Fiscal Year 2016 | | 3,726 |
| | 341 |
| | 3,385 |
|
Fiscal Year 2017 | | 3,535 |
| | — |
| | 3,535 |
|
Thereafter | | 16,628 |
| | — |
| | 16,628 |
|
Total | | $ | 42,750 |
| | $ | 2,005 |
| | $ | 40,745 |
|
Royalties
We are obligated to pay royalties for licenses to enhance and market certain software used in connection with our product offerings. Annual contractual minimum royalty payments as of December 31, 2012 are as follows (in thousands):
|
| | | | |
| | |
Fiscal Year 2013 | | $ | 1,008 |
|
Fiscal Year 2014 | | 436 |
|
Fiscal Year 2015 | | 436 |
|
Fiscal Year 2016 | | 436 |
|
Fiscal Year 2017 | | 436 |
|
Thereafter(1) | | 1,670 |
|
Total minimum royalty commitments | | $ | 4,422 |
|
|
(1) For purposes of the contractual minimum royalty payments table, we have included indefinite-lived contractual minimum royalty payments due in the ten fiscal years following December 31, 2012.
In addition to the contractual minimum royalties included in the table above, we have several royalty agreements that are long term or perpetual in nature and the royalty obligations are generally based on a percentage of revenues derived from certain software license sales and associated sales of PCS. The royalty agreements require quarterly payments and generally do not limit the maximum royalties owed. We incurred royalty related expenses of $5.5 million, $5.8 million, $4.6 million and $1.8 million (unaudited) for the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010 and December 31, 2009, respectively.
15. Legal Proceedings
In July 2012, a customer of one of our subsidiaries provided us with a request for arbitration, and on October 5, 2012, served us with its Statement of Case in connection with the arbitration, which is pending before the London Court of International Arbitration. The dispute relates to certain software and professional services arrangements that the customer claims were not completed pursuant to the terms of the underlying agreements. We intend to vigorously defend against such claims and to assert counterclaims for payments owed to us. Because of the uncertainties related to this arbitration, we are currently unable to predict the ultimate outcome. However, we believe that it is possible that an unfavorable outcome could result in a material adverse effect on our results of operations, liquidity or financial position.
We are also subject to various other claims and legal proceedings arising in the ordinary course of our business. In connection with our regular assessments of such other claims and legal proceedings, we accrue an estimated loss contingency in our financial statements if it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. However, a determination as to the amount of the accrual required for such contingencies is highly subjective and requires judgments about future events. As a result, the amount of ultimate loss, if any, may differ from our estimates. Nevertheless, management believes that the disposition of such matters, in the aggregate, will not have a material adverse effect on our results of operations, liquidity or financial position.
16. Subsequent events
On January 11, 2013, we acquired Vialis AG, a joint stock company organized under the laws of Switzerland (“Vialis”). We acquired Vialis for aggregate consideration consisting of: (i) an upfront payment in an amount equal to five million Swiss francs (or approximately $5.4 million), subject to certain adjustments and escrow holdbacks set forth in the purchase agreement; and (ii) certain contingent earn-out consideration payable during the next three fiscal years in an aggregate amount of up to an five million Swiss francs, subject to the satisfaction of certain milestones set forth in the purchase agreement. Vialis is a systems integrator based in Liestal, Switzerland that serves the pharmaceutical, biotechnology, chemicals and agro-science industries. We acquired Vialis as part of our strategy to expand our professional services offerings internationally. We funded the purchase price with cash on hand.
On December 31, 2012, prior to the close of the Vialis Acquisition we advanced toVialis purchase consideration in the amount of $5.4 million as a deposit, with such funds to be returned to us if the acquisition was not completed. This deposit is included in other assets in our consolidated balance sheet.
As of March 1, 2013, we have repurchased 353,949 shares of our common stock for approximately $3.3 million at a weighted-average cost of $9.39 per share, pursuant to our $15.0 million repurchase authorization for fiscal 2013.
17. Selected Quarterly Financial Information (unaudited)
The following quarterly financial data, in the opinion of management, reflects all adjustments, consisting of normal recurring adjustments necessary for a fair presentation of results for the periods presented (in thousands, except per share amounts).
|
| | | | | | | | | | | | | | | | |
| | Year Ended December 31, 2012 |
| | First Quarter | | Second Quarter | | Third Quarter | | Fourth Quarter |
Revenue: | | | | | | | | |
License and subscription revenue | | $ | 21,697 |
| | $ | 21,399 |
| | $ | 23,195 |
| | $ | 23,149 |
|
Maintenance on perpetual licenses | | 9,491 |
| | 9,128 |
| | 9,600 |
| | 10,035 |
|
Content | | 3,543 |
| | 3,032 |
| | 2,919 |
| | 2,991 |
|
Professional services and other | | 4,708 |
| | 4,835 |
| | 4,785 |
| | 8,019 |
|
Total revenue | | 39,439 |
| | 38,394 |
| | 40,499 |
| | 44,194 |
|
Cost of revenue: | | | | | | | | |
Cost of revenue | | 9,878 |
| | 10,017 |
| | 9,839 |
| | 11,961 |
|
Amortization of completed technology | | 2,081 |
| | 2,074 |
| | 2,108 |
| | 2,580 |
|
Total cost of revenue | | 11,959 |
| | 12,091 |
| | 11,947 |
| | 14,541 |
|
Gross profit | | 27,480 |
| | 26,303 |
| | 28,552 |
| | 29,653 |
|
Operating expenses: | | | | | | |
Product development | | 9,552 |
| | 9,747 |
| | 9,658 |
| | 9,892 |
|
Sales and marketing | | 13,865 |
| | 13,813 |
| | 12,765 |
| | 17,528 |
|
General and administrative | | 4,526 |
| | 4,367 |
| | 4,358 |
| | 4,229 |
|
Business consolidation, transaction and restructuring costs | | 630 |
| | (21 | ) | | 606 |
| | 6,588 |
|
Purchased intangible asset amortization | | 2,095 |
| | 2,118 |
| | 2,107 |
| | 2,619 |
|
Total operating expenses | | 30,668 |
| | 30,024 |
| | 29,494 |
| | 40,856 |
|
Operating loss | | (3,188 | ) | | (3,721 | ) | | (942 | ) | | (11,203 | ) |
Royalty and other income, net | | 1,631 |
| | 3,613 |
| | 1,863 |
| | 1,763 |
|
Income (loss) before taxes | | (1,557 | ) | | (108 | ) | | 921 |
| | (9,440 | ) |
Income tax expense (benefit) | | 697 |
| | 412 |
| | 320 |
| | (1,211 | ) |
Net income (loss) | | $ | (2,254 | ) | | $ | (520 | ) | | $ | 601 |
| | $ | (8,229 | ) |
Basic net income (loss) per share | | $ | (0.04 | ) | | $ | (0.01 | ) | | $ | 0.01 |
| | $ | (0.15 | ) |
Diluted net income (loss) per share | | $ | (0.04 | ) | | $ | (0.01 | ) | | $ | 0.01 |
| | $ | (0.15 | ) |
|
| | | | | | | | | | | | | | | | |
| | Year Ended December 31, 2011 |
| | First Quarter | | Second Quarter | | Third Quarter | | Fourth Quarter |
Revenue: | | | | | | | | |
License and subscription revenue | | $ | 19,480 |
| | $ | 18,587 |
| | $ | 20,127 |
| | $ | 21,231 |
|
Maintenance on perpetual licenses | | 7,814 |
| | 8,581 |
| | 9,166 |
| | 9,301 |
|
Content | | 4,048 |
| | 4,272 |
| | 4,248 |
| | 4,270 |
|
Professional services and other | | 3,258 |
| | 2,286 |
| | 2,710 |
| | 4,960 |
|
Total revenue | | 34,600 |
| | 33,726 |
| | 36,251 |
| | 39,762 |
|
Cost of revenue: | | | | | | | | |
Cost of revenue | | 9,380 |
| | 8,835 |
| | 8,349 |
| | 9,501 |
|
Amortization of completed technology | | 2,037 |
| | 2,037 |
| | 2,184 |
| | 2,135 |
|
Total cost of revenue | | 11,417 |
| | 10,872 |
| | 10,533 |
| | 11,636 |
|
Gross profit | | 23,183 |
| | 22,854 |
| | 25,718 |
| | 28,126 |
|
Operating expenses: | | | | | | |
Product development | | 8,535 |
| | 8,402 |
| | 8,261 |
| | 8,779 |
|
Sales and marketing | | 13,489 |
| | 12,339 |
| | 11,516 |
| | 14,173 |
|
General and administrative | | 4,381 |
| | 3,960 |
| | 4,079 |
| | 4,047 |
|
Business consolidation, transaction and restructuring costs | | 1,602 |
| | 2,418 |
| | 2,205 |
| | 1,550 |
|
Purchased intangible asset amortization | | 2,394 |
| | 2,394 |
| | 2,555 |
| | 2,503 |
|
Total operating expenses | | 30,401 |
| | 29,513 |
| | 28,616 |
| | 31,052 |
|
Operating loss | | (7,218 | ) | | (6,659 | ) | | (2,898 | ) | | (2,926 | ) |
Net gain on sale of cost method investment | | — |
| | — |
| | — |
| | 18,970 |
|
Royalty and other (expense) income, net | | 2,280 |
| | 1,837 |
| | 882 |
| | (3,259 | ) |
Income (loss) before taxes | | (4,938 | ) | | (4,822 | ) | | (2,016 | ) | | 12,785 |
|
Income tax expense (benefit) | | 507 |
| | (33 | ) | | 190 |
| | (1,420 | ) |
Net income (loss) | | $ | (5,445 | ) | | $ | (4,789 | ) | | $ | (2,206 | ) | | $ | 14,205 |
|
Basic net income (loss) per share | | $ | (0.10 | ) | | $ | (0.09 | ) | | $ | (0.04 | ) | | $ | 0.26 |
|
Diluted net income (loss) per share | | $ | (0.10 | ) | | $ | (0.09 | ) | | $ | (0.04 | ) | | $ | 0.25 |
|
|
| | | | | | | | | | | | |
| | Nine Months Ended December 31, 2010 |
| | Quarter ended | | Quarter ended | | Quarter ended |
Revenue | | $ | 19,761 |
| | $ | 29,114 |
| | $ | 31,330 |
|
Cost of revenue: | | | | | | |
Cost of revenue | | 3,955 |
| | 9,117 |
| | 10,456 |
|
Amortization of completed technology | | 41 |
| | 2,373 |
| | 2,473 |
|
Total cost of revenue | | 3,996 |
| | 11,490 |
| | 12,929 |
|
Gross profit | | 15,765 |
| | 17,624 |
| | 18,401 |
|
Operating expenses: | | | | | | |
Product development | | 3,982 |
| | 9,208 |
| | 8,890 |
|
Sales and marketing | | 8,740 |
| | 12,171 |
| | 13,937 |
|
General and administrative | | 2,580 |
| | 4,635 |
| | 4,482 |
|
Business consolidation, transaction and restructuring costs | | 1,686 |
| | 10,683 |
| | 4,124 |
|
Purchased intangible asset amortization | | — |
| | 1,356 |
| | 1,356 |
|
Total operating expenses | | 16,988 |
| | 38,053 |
| | 32,789 |
|
Operating loss | | (1,223 | ) | | (20,429 | ) | | (14,388 | ) |
Royalty and other (expense) income, net | | (134 | ) | | 934 |
| | 577 |
|
Loss before taxes | | (1,357 | ) | | (19,495 | ) | | (13,811 | ) |
Income tax expense (benefit) | | 225 |
| | (16,116 | ) | | 1,836 |
|
Net loss | | (1,582 | ) | | (3,379 | ) | | (15,647 | ) |
Basic and diluted net loss per share | | (0.06 | ) | | (0.06 | ) | | (0.28 | ) |
|
| |
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None
|
| |
Item 9A. | Controls and Procedures |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
We maintain disclosure controls and procedures, as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, that are designed to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required financial disclosures.
As of the end of the period covered by this Report, we conducted an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Exchange Act Rules 13a-15(b) and 15d-15(b). Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of December 31, 2012.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that internal controls may become inadequate because of changes in conditions, or that the degree of compliance with policies and procedures may deteriorate.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework set forth in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations
of the Treadway Commission. Based on our evaluation under such framework , our management concluded that our internal control over financial reporting was effective as of December 31, 2012.
Management’s assessment of and conclusion on the effectiveness of internal controls over financial reporting did not include the internal controls of Aegis which we acquired on October 23, 2012 and which was included in our 2012 consolidated financial statements, and constituted $2.5 million and $2.9 million of total assets and total liabilities, respectively, as of December 31, 2012 and $0.7 million and $1.9 million of revenues and net loss before income taxes, respectively, for the year ended December 31, 2012.
Ernst & Young LLP, the independent registered public accounting firm that audited the consolidated financial statements included in this Report, has also audited our internal control over financial reporting as of December 31, 2012, as stated in their report which is included herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2012 that materially affected, or are reasonably likely to materially affect, those controls.
|
| |
Item 9B. | Other Information |
On March 1, 2013, we entered into a separate but identical Indemnification Agreement (each, an “Indemnification Agreement”) with each of Max Carnecchia, Michael A. Piraino, Dr. Matt Hahn, Judith Ohrn Hicks, Mollie Hunter, Leif Pedersen, Anil Thakur, Chris van Ingen, Kenneth L. Coleman, Larry Ferguson, Tim Harkness, Dr. Ricardo Levy and Jeffrey Rodek (each, an “Indemnitee”).
Under each Indemnification Agreement, each Indemnitee is entitled to be indemnified against all expenses, judgments, penalties, fines, and amounts paid in settlement actually and reasonably incurred by or on behalf of such Indemnitee in connection with any claims, proceedings or other actions brought against Indemnitee as a result of such Indemnitee's service to the Company, provided that such Indemnitee (i) acted in good faith; (ii) reasonably believed the action was in the best interest of the Company; and (iii) in criminal proceedings, reasonably believed his or her conduct was not unlawful. Additionally, the Indemnification Agreement entitles the Indemnitee to a contribution of expenses from the Company in any proceeding in which the Company is jointly liable with Indemnitee, but for which indemnification is not otherwise available. Finally, the Indemnification Agreement also entitles each Indemnitee to advancement of expenses incurred by an Indemnitee in connection with any claim, proceeding or other action in advance of the final adjudication of any such claim, proceeding or other action, provided Indemnitee agrees to reimburse the Company for all such advances if it shall ultimately be determined that Indemnitee is not entitled to indemnification.
The foregoing summary of the Indemnification Agreements qualified in its entirety by reference to the form of Indemnification Agreement that is attached as Exhibit 10.41 of this Annual Report on Form 10-K and is incorporated herein by reference.
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Accelrys, Inc.
We have audited Accelrys Inc.’s internal control over financial reporting as of December 31, 2012, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Accelrys, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As indicated in the accompanying Management's Report on Internal Control over Financial Reporting, management's assessment of and conclusion on the effectiveness of internal control over financial reporting did not include the internal controls of Aegis Analytical Corporation, which is included in the 2012 consolidated financial statements of Accelrys, Inc. and constituted $2.5 million and $2.9 million of total assets and total liabilities, respectively, as of December 31, 2012 and $0.7 million and $1.9 million of revenues and net loss before income taxes , respectively, for the year then ended. Our audit of internal control over financial reporting of Accelrys, Inc. also did not include an evaluation of the internal control over financial reporting of Aegis Analytical Corporation.
In our opinion, Accelrys, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2012, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Accelrys, Inc. as of December 31, 2012 and 2011, and the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2012, and for the nine month period ended December 31, 2010 of Accelrys, Inc. and our report dated March 6, 2013 expressed an unqualified opinion thereon.
/s/Ernst & Young LLP
San Diego, California
March 6, 2013
PART III
|
| |
Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this item will be included in the applicable Subsequent Filing to be filed within 120 days of our fiscal year ended December 31, 2012.
|
| |
Item 11. | Executive Compensation |
The information required by this item will be included in the applicable Subsequent Filing to be filed within 120 days of our fiscal year ended December 31, 2012.
|
| |
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item will be included in the applicable Subsequent Filing to be filed within 120 days of our fiscal year ended December 31, 2012.
|
| |
Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item will be included in the applicable Subsequent Filing to be filed within 120 days of our fiscal year ended December 31, 2012.
|
| |
Item 14. | Principal Accountant Fees and Services |
The information required by this item will be included in the applicable Subsequent Filing to be filed within 120 days of our fiscal year ended December 31, 2012.
PART IV
|
| |
Item 15. | Exhibits, Financial Statement Schedules |
(a)(1) Financial Statements
The following Consolidated Financial Statements are included:
|
| |
| |
Report of Independent Registered Public Accounting Firm | |
Consolidated Balance Sheets as of December 31, 2012 and 2011 | |
Consolidated Statements of Operations for the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010 and 2009 (unaudited) | |
Consolidated Statements of Comprehensive Income for the years ended December 31, 2012 and December 31, 2011 and the nine months ended December 31, 2010 and 2009 (unaudited) | |
Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2012, December 31, 2011, March 31, 2010 and 2009 (unaudited) | |
Consolidated Statements of Cash Flows for the years ended December 31, 2012, December 31, 2011 and the nine months ended December 31, 2010 and 2009 (unaudited) | |
Notes to Consolidated Financial Statements | |
(a)(2) Financial Statement Schedules
Schedule II—Valuation and Qualifying Accounts
|
| | | | | | | | | | | | | | | | |
Description | | Balance at Beginning of Year | | Additions Charged to Costs and Expenses | | Deductions(1) | | Balance at End of Year |
| | (In thousands) |
Allowance for doubtful accounts: | | | | | | | | |
Year ended December 31, 2012 | | $ | 143 |
| | $ | — |
| | $ | — |
| | $ | 143 |
|
Year ended December 31, 2011 | | $ | 163 |
| | $ | — |
| | $ | (20 | ) | | $ | 143 |
|
Nine months ended December 31, 2010 | | $ | 163 |
| | $ | — |
| | $ | — |
| | $ | 163 |
|
| |
(1) | Represents write-offs, net of recoveries. |
All other schedules are omitted because they are not applicable, or not required, or because the required information is included in the financial statements or notes thereto.
(a)(3) Exhibits:
|
| | |
Exhibit Number | | Description |
|
| | |
2.1 | | Agreement and Plan of Merger and Reorganization, dated April 5, 2010, by and among Accelrys, Inc., Alto Merger Sub, Inc. and Symyx Technologies, Inc. (incorporated by reference to Exhibit 2.1 of Accelrys, Inc.'s Current Report on Form 8-K filed on April 6, 2010). |
| | |
2.2 | | Sale and Purchase Agreement Regarding All Shares in Contur Industry Holding AB and Warrants in Contur Software AB, dated 19 May 2011 (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.’s Current Report on Form 8-K filed on May 24, 2011). |
| | |
2.3 | | Asset Purchase Agreement, dated as of July 28, 2011, by and between Intermolecular, Inc. and Symyx Technologies, Inc. (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on July 29, 2011). |
| | |
2.4 | | Agreement and Plan of Merger, dated as of December 30, 2011, by and among Accelrys, Inc., Velocity Acquisition Corp., VelQuest Corporation and Laurel Services, LLC (incorporated by reference to Exhibit 2.1 to Accelrys, Inc.’s Current Report on Form 8-K filed on January 3, 2012). |
| | |
2.5 | | Agreement and Plan of Merger, dated as of October 23, 2012, by and among Accelrys, Inc., Aardvark Acquisition Corp., Aegis Analytical Corporation and Shareholder Representative Services LLC (incorporated by reference to Exhibit 2.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on October 29, 2012. |
| | |
3.1 | | Restated Certificate of Incorporation of Pharmacopeia, Inc., as amended (including a Certificate of Designation, Preferences and Rights of Series A Junior Participating Preferred Stock) (incorporated by reference to Exhibit 3.2 to Accelrys, Inc.’s Annual Report on Form 10-K for the year ended March 31, 2005). |
| | |
3.2 | | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Accelrys, Inc. (incorporated by reference to Exhibit 3.4 to Accelrys, Inc.’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2007). |
| | |
3.3 | | Certificate of Amendment of the Restated Certificate of Incorporation of Accelrys, Inc. (incorporated by reference to Exhibit 3.1 to Accelrys, Inc.’s Current Report on Form 8-K filed on July 2, 2010). |
| | |
3.4 | | Amended and Restated Bylaws of Accelrys, Inc. (incorporated by reference to Exhibit 3.3 to Accelrys, Inc.’s Annual Report on Form 10-K for the year ended March 31, 2005). |
| | |
10.1# | | Amended 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.5 to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended December 31, 1995). |
| | |
10.1(a)# | | Amendment No. 1 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.1(a) to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2000). |
| | |
10.1(b)# | | Amendment No. 2 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.1(b) to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2000). |
| | |
10.1(c)# | | Amendment No. 3 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.5(a) to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended June 30, 1997). |
| | |
10.1(d)# | | Amendment No. 4 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.1(d) to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2000). |
| | |
10.1(e)# | | Amendment No. 5 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.1(e) to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2000). |
| | |
10.1(f)# | | Amendment No. 6 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.1(f) to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2000). |
| | |
10.1(g)# | | Amendment No. 7 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.1(g) to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2000). |
| | |
10.1(h)# | | Amendment No. 8 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.54 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended June 30, 2000). |
| | |
10.1(i)# | | Amendment No. 9 to the 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.1(i) to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended March 31, 2002). |
| | |
10.2# | | 1995 Director Option Plan (incorporated by reference to Exhibit 10.7 to Accelrys, Inc.'s Registration Statement on Form S-1 (Reg. No. 33-98246)). |
| | |
|
| | |
10.2(a)# | | Amendment No. 1 to 1995 Director Option Plan (incorporated by reference to Exhibit 10.3(a) to Accelrys, Inc.'s Annual Report on form 10-K for the year ended December 31, 2000). |
| | |
10.2(b)# | | Amendment No. 2 to the 1995 Director Option Plan (incorporated by reference to Exhibit 10.3(b) to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended March 31, 2001). |
| |
10.3# | | Accelrys, Inc. 2000 Stock Option Plan (incorporated by reference to Exhibit 10.23 to Accelrys, Inc.'s Annual Report on form 10-K for the year ended December 31, 2000). |
| | |
10.4# | | Accelrys, Inc. 2004 New Hire Equity Incentive Plan (incorporated by reference to Exhibit 10.3(c) to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended March 31, 2005). |
| |
10.5# | | Accelrys, Inc. Amended and Restated 2004 Stock Incentive Plan (incorporated by reference to Exhibit 4.2 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 26, 2005). |
| |
10.5(a)# | | Accelrys, Inc. Amendment to Amended and Restated 2004 Stock Incentive Plan, dated August 28, 2008 (incorporated by reference to Exhibit 10.2 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended September 30, 2008). |
| |
10.6# | | Form of Stock Option Award Agreement pursuant to Amended and Restated 2004 Stock Incentive Plan (incorporated by reference to Exhibit 4.3 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 26, 2005). |
| |
10.7# | | Form of SAR Award Agreement pursuant to Amended and Restated 2004 Stock Incentive Plan (incorporated by reference to Exhibit 4.4 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 26, 2005). |
| |
10.8# | | Form of Restricted Stock Award Agreement pursuant to Amended and Restated 2004 Stock Incentive Plan (incorporated by reference to Exhibit 4.5 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 26, 2005). |
| | |
10.9# | | Form of Restricted Stock Unit Award Agreement pursuant to Amended and Restated 2004 Stock Incentive Plan (incorporated by reference to Exhibit 4.6 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 26, 2005). |
| |
10.10# | | Form of Director Restricted Stock Unit Award Agreement pursuant to Amended and Restated 2004 Stock Incentive Plan (incorporated by reference to Exhibit 99.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on August 21, 2007). |
| |
10.11# | | Form of Performance Based Restricted Stock Unit Award Agreement pursuant to Amended and Restated 2004 Stock Incentive Plan (incorporated by reference to Exhibit 10.3 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008). |
| |
10.12# | | Stock Option Agreement, dated June 15, 2009, between Max Carnecchia and Accelrys, Inc. (incorporated by reference to Exhibit 10.4 to Accelrys, Inc.'s Current Report on Form 8-K filed on June 16, 2009). |
| |
10.13# | | Symyx Technologies, Inc. 2007 Stock Incentive Plan, as amended, and the forms of agreement related thereto (incorporated by reference to Exhibit 4.3 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 9, 2010). |
| | |
10.14# | | Intellichem, Inc. 2003 Stock Option Plan, as amended (incorporated by reference to Exhibit 4.4 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 9, 2010). |
| |
10.15# | | Symyx Technologies, Inc. 2001 Nonstatutory Stock Option Plan, as amended (incorporated by reference to Exhibit 4.5 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 9, 2010). |
| |
10.16# | | Synthematix, Inc. Amended and Restated 2000 Equity Compensation Plan (incorporated by reference to Exhibit 4.6 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 9, 2010). |
| |
10.17# | | Symyx Technologies, Inc. 1997 Stock Plan, as amended (incorporated by reference to Exhibit 4.7 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 9, 2010). |
| | |
10.18 | | 2011 Management Incentive Plan for the Calendar Year Ending December 31, 2011 (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on March 7, 2011). |
| | |
10.19 | | Accelrys, Inc. 2011 Stock Incentive Plan (incorporated by reference to Exhibit 4.3 to Accelrys, Inc.'s Registration Statement on Form S-8 filed on August 10, 2011). |
| | |
10.20 | | Lease, dated August 20, 2003, between Accelrys, Inc. and Eastpark at 8A (Building 1000) (incorporated by reference to Exhibit 10.35 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended September 30, 2003). |
| | |
|
| | |
10.21 | | Lease, dated August 20, 2003, between Accelrys, Inc. and Eastpark at 8A (Building 3000) (incorporated by reference to Exhibit 10.36 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended September 30, 2003). |
| | |
10.22 | | Sublease, dated May 18, 2004, between Accelrys, Inc. and Pfizer Inc. (incorporated by reference to Exhibit 10.18 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended December 31, 2004). |
| | |
10.23 | | Lease, dated May 18, 2004, between Accelrys, Inc. and AGRRI Seaview, L.LC. (incorporated by reference to Exhibit 10.19 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended December 31, 2004). |
| | |
10.24 | | Sublease, dated May 10, 2006, between Accelrys, Inc., Accelrys, Ltd., QUALCOMM Inc., and QUALCOMM UK Ltd. (incorporated by reference to Exhibit 10.33 to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended March 31, 2006). |
| | |
10.25 | | Agreement for Purchase and Sale of Real Property, dated as of March 23, 2012, by and between Symyx Solutions, Inc., Olga Issakova and Nikolai Sepetov, as amended. (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.'s Quarterly Report on Form 10-Q filed on August 3, 2012). |
| | |
10.26 | | Office Lease, dated as of December 27, 2012, by and between Kilroy Realty, L.P. and Accelrys, Inc. (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on December 27, 2012). |
| | |
10.27# | | Form of Indemnity Agreement for Directors and Executive Officers (incorporated by reference to Exhibit 10.48 to Accelrys, Inc.'s Annual Report on Form 10-K for the year ended December 31, 1998). |
| | |
10.28# | | Terms of continuing employment of Matthew Hahn, dated as of August 12, 2005 (incorporated by reference to Exhibit 10.3 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended September 30, 2005). |
| | |
10.29# | | Second Amendment to Employment Agreement, dated September 26, 2006, between Accelrys, Inc. and Mathew Hahn, Ph.D. (incorporated by reference to Exhibit 99.2 to Accelrys, Inc.'s Current Report on Form 8-K filed on September 28, 2006). |
| | |
10.30# | | Form of Employment Agreement between Accelrys, Inc. and certain of its executives (incorporated by reference to Exhibit 10.5 to Accelrys, Inc.'s Quarterly Report on Form 10-Q for the quarter ended September 30, 2006) . |
| | |
10.31# | | Letter of Employment, dated January 5, 2009, between Accelrys, Inc. and Todd Johnson (incorporated by reference to Exhibit 10.2 to Accelrys, Inc.'s Current Report on Form 8-K filed on January 5, 2009). |
| | |
10.32 | | Agreement between Accelrys and UBS dated November 11, 2008 (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on November 11, 2008). |
| | |
10.33# | | Employment Agreement, dated June 15, 2009, between Max Carnecchia and Accelrys, Inc. (incorporated by reference to Exhibit 10.2 to Accelrys, Inc.'s Current Report on Form 8-K filed on June 16, 2009). |
| | |
10.34# | | Letter of Employment, dated June 15, 2009, between Max Carnecchia and Accelrys, Inc. (incorporated by reference to Exhibit 10.3 to Accelrys, Inc.'s Current Report on Form 8-K filed on June 16, 2009). |
| | |
10.35# | | Letter Agreement, dated July 15, 2009, between Todd Johnson and Accelrys, Inc. (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on July 20, 2009). |
| | |
10.36# | | Letter of Employment, dated December 23, 2009, between Accelrys, Inc. and Michael Piraino (incorporated by reference to Exhibit 10.2 to Accelrys, Inc.'s Current Report on Form 8-K filed on January 5, 2010). |
| | |
10.37# | | Employment Agreement, dated January 5, 2010, between Michael Piraino and Accelrys, Inc. (incorporated by reference to Exhibit 10.3 to Accelrys, Inc.'s Current Report on Form 8-K filed on January 5, 2010). |
| | |
10.38# | | Offer Letter from Accelrys, Inc. to Todd Johnson (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on April 22, 2010). |
| | |
10.39# | | Employment Agreement, dated as of April 21, 2010, by and between the Accerlys, Inc. and Todd Johnson (incorporated by reference to Exhibit 10.2 to Accelrys, Inc.'s Current Report on Form 8-K filed on April 22, 2010). |
| | |
10.40# | | Separation Agreement and Release, dated as of December 27, 2011, by and between the Accelrys, Inc. and Trevor Heritage (incorporated by reference to Exhibit 10.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on December 27, 2011). Note: This exhibit is being retained because Trevor is still bound by the 2 year non-solicitation provision of this agreement. |
|
| | |
| | |
10.41* | | Form of Indemnification Agreement between Accelrys Inc. and its Directors and Executive officers. |
| | |
14.1 | | Code of Ethics (incorporated by reference to Exhibit 14.1 to Accelrys, Inc.'s Current Report on Form 8-K filed on February 5, 2005). |
| | |
21.1* | | Subsidiaries of Accelrys, Inc. |
| |
23.1* | | Consent of Independent Registered Public Accounting Firm |
| |
31.1* | | Section 302 Certification of the Principal Executive Officer |
| | |
31.2* | | Section 302 Certification of the Principal Financial Officer |
| | |
32.1* | | Section 906 Certification of the Chief Executive Officer and Chief Financial Officer |
| | |
101.INS** | | XBRL Instance Document. |
| | |
101.SCH** | | XBRL Taxonomy Extension Schema Document. |
| | |
101.DEF** | | XBRL Taxonomy Extension Definition Linkbase Document. |
| | |
101.CAL** | | XBRL Taxonomy Extension Calculation Linkbase Document |
| | |
101.LAB** | | XBRL Taxonomy Extension Label Linkbase Document. |
| | |
101.PRE** | | XBRL Taxonomy Extension Presentation Linkbase Database. |
| | |
|
| |
# | Represents a management contract or compensatory plan or arrangement. |
See Item 15(a)(3) above.
| |
(c) | Financial Statement Schedules |
See Item 15(a)(2) above.
SIGNATURE
Pursuant to the requirements of Section 13 or 15(d) the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | | |
ACCELRYS, INC. |
| | | |
By: | | /s/ MICHAEL A. PIRAINO |
| | Michael A. Piraino |
| | Executive Vice President and Chief Financial |
| | Officer (Duly Authorized Officer, Principal |
| | Financial Officer and Principal Accounting |
| | Officer) |
| | Date: | March 6, 2013 |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Max Carnecchia and Michael A. Piraino, jointly and severally, as his or her attorney-in-fact, each with full power of substitution, for him or her, in any and all capacities, to sign each amendment to this report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact or his or her substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
|
| | | | |
| | | | |
Signatures | | Title | | Date |
| | |
/s/ MAX CARNECCHIA Max Carnecchia | | President and Chief Executive Officer (Principal Executive Officer) | | February 20, 2013 |
| | |
/s/ MICHAEL A. PIRAINO Michael A. Piraino | | Executive Vice President and Chief Financial Officer (Principal Accounting and Financial Officer) | | February 20, 2013 |
| | |
/s/ CHRIS VAN INGEN Chris van Ingen | | Chairman of the Board of Directors | | February 20, 2013 |
| | |
/s/ JEFFREY RODEK Jeffrey Rodek | | Director | | February 20, 2013 |
| | |
/s/ RICARDO B. LEVY, Ph.D. Ricardo B. Levy, Ph.D. | | Director | | February 20, 2013 |
| | |
/s/ TIMOTHY HARKNESS Timothy Harkness | | Director | | February 20, 2013 |
| | |
/s/ KENNETH L. COLEMAN Kenneth L. Coleman | | Director | | February 20, 2013 |
| | |
/s/ LARRY FERGUSON Larry Ferguson | | Director | | February 20, 2013 |

