
© 2026 ANI Pharmaceuticals, Inc. 1 Corporate Presentation January 2026

© 2026 ANI Pharmaceuticals, Inc. 2 Disclaimers Forward-Looking Statements This presentation contains forward-looking statements within the meaning of Section 27A of the Act, and Section 21E of the Securities Exchange Act of 1934, as amended. The guidance included herein is from or supplemental to the Company’s press release on January 12, 2026. The Company is neither reconfirming this guidance as of the date of this investor presentation nor assuming any obligation to update or revise such guidance. Any statements about our expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and may be forward-looking. These statements are often, but are not always, made through the use of words or phrases such as “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “target,” “will,” “would,” or the negative of these words or other comparable terminology. These statements may include, but are not limited to, those relating to the commercialization and potential sales of the product and any additional product launches from the Company’s generic pipeline, 2026 financial guidance, expansion plans for the Rare Disease business and transformation of ANI into a leading rare disease company, growth opportunities for Cortrophin Gel and ILUVIEN and anticipated R&D developments and clinical trial advances, and other statements that are not historical in nature. These statements involve estimates, assumptions and uncertainties which could cause actual results to differ materially from those expressed in them Uncertainties and risks include, but are not limited to: the ability of our approved products, including Cortrophin Gel and ILUVIEN, to achieve commercialization at levels of market acceptance that will continue to allow us to achieve continued profitability; our ability to complete or achieve any, or all of the intended benefits of acquisitions and investments, in a timely manner or at all; the limitation of our cash flow as a result of the indebtedness and liabilities incurred from the acquisition of Alimera; the risks that our acquisitions and investments, could disrupt our business and harm our financial position and operating results; delays and disruptions in production of our approved products, increased costs and potential loss of revenues if we need to change suppliers due to the limited number of suppliers for our raw materials, active pharmaceutical ingredients, expedients, and other materials; delays and disruptions in production of our approved products as a result of our reliance on single source third party contract manufacturing supply for certain of our key products, including Cortrophin Gel and ILUVIEN; delays or failure in obtaining and maintaining approvals by the FDA of the products we sell; changes in policy or actions that may be taken by the FDA, United States Drug Enforcement Administration and other regulatory agencies, and the focus of the current U.S. presidential administration, including among other things, drug recalls, regulatory approvals, facility inspections and potential enforcement actions; risks that we may face with respect to importing raw materials and delays in delivery of raw materials and other ingredients and supplies necessary for the manufacture of our products from both domestic and overseas sources due to supply chain disruptions or for any other reason, including increased costs due to tariffs; the ability of our manufacturing partners to meet our product demands and timelines; the impact of changes or fluctuations in exchange rates; our ability to develop, license or acquire, and commercialize new products; our obligations in agreements under which we license, develop or commercialize rights to products or technology from third parties and our ability to maintain such licenses; the level of competition we face and the legal, regulatory and/or legislative strategies employed by our competitors to prevent or delay competition from generic alternatives to branded products; our ability to protect our intellectual property rights; the impact of legislative or regulatory reform on the pricing for pharmaceutical products; the impact of any litigation to which we are, or may become, a party; our ability, and that of our suppliers, development partners, and manufacturing partners, to comply with laws, regulations and standards that govern or affect the pharmaceutical and biotechnology industries; our ability to maintain the services of our key executives and other personnel; the potential impact of new U.S. tax legislation on our business; and general business and economic conditions, such as inflationary pressures, geopolitical conflicts and conditions, and other risks and uncertainties that are described in the Company’s most recent Annual Report on Form 10-K, any subsequent quarterly reports filed by the Company on Form 10-Q, and other periodic reports filed with the Securities and Exchange Commission. You should not rely upon forward-looking statements as predictions of future events. Such statements are based on management’s expectations as of the date of this presentation and involve many risks and uncertainties that could cause our actual results, events or circumstances to differ materially from those expressed or implied in our forward-looking statements. We undertake no obligation to update any forward-looking statements made in this presentation to reflect events or circumstances after the date of this presentation or to reflect new information or the occurrence of unanticipated events, except as required by law. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements. Our forward- looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.

© 2026 ANI Pharmaceuticals, Inc. 3 Presentation of financial information Non-GAAP Financial Measures Adjusted non-GAAP EBITDA ANI’s management considers adjusted non-GAAP EBITDA to be an important financial indicator of ANI’s operating performance, providing investors and analysts with a useful measure of operating results unaffected by non- cash stock-based compensation and differences in capital structures, tax structures, capital investment cycles, ages of related assets, and compensation structures among otherwise comparable companies. Management uses adjusted non-GAAP EBITDA when analyzing Company performance. Adjusted non-GAAP EBITDA is defined as net income (loss), excluding tax expense (benefit), interest expense, net, other (income) expense, net, depreciation and amortization expense, non-cash stock-based compensation expense, M&A transaction and integration expenses, contingent consideration fair value adjustments, unrealized (gain) loss on our investment in equity securities, loss (gain) on disposal of assets, intangible asset impairment charges, litigation expenses related to certain matters, severance expense, and certain other items that vary in frequency and impact on ANI’s results of operations. Adjusted non-GAAP EBITDA should be considered in addition to, but not in lieu of, net income or loss reported under GAAP. ANI is not providing a reconciliation for the forward-looking full year 2026 adjusted EBITDA guidance because it does not currently have sufficient information to accurately estimate all of the variables and individual adjustments for such reconciliation, including “with” and “without” tax provision information. As such, ANI’s management cannot estimate on a forward-looking basis without unreasonable effort the impact these variables and individual adjustments will have on its reported results. Adjusted non-GAAP Diluted Earnings per Share ANI’s management considers adjusted non-GAAP diluted earnings per share to be an important financial indicator of ANI’s operating performance, providing investors and analysts with a useful measure of operating results unaffected by the non-cash stock-based compensation, non-cash interest expense, depreciation and amortization, M&A transaction and integration expenses, contingent consideration fair value adjustment, unrealized (gain) loss on our investment in equity securities, loss (gain) on disposal of assets, intangible asset impairment charges, litigation expenses related to certain matters, severance expense, and certain other items that vary in frequency and impact on ANI’s results of operations. Management uses adjusted non-GAAP diluted earnings per share when analyzing Company performance. Non-GAAP Adjusted Diluted Weighted-Average Shares Outstanding excludes certain dilutive shares related to the 2029 senior convertible notes as they are intended to be covered by our capped call transactions. Our outstanding capped call transactions are intended to offset the dilutive effect of the 2029 convertible senior notes recognized in the calculation of GAAP diluted EPS and have been excluded from the calculation of the Non- GAAP Adjusted Diluted Weighted-Average Shares outstanding. Adjusted non-GAAP diluted earnings per share is defined as adjusted non-GAAP net income, as defined above, divided by the diluted weighted average shares outstanding during the period. Management will continually analyze this metric and may include additional adjustments in the calculation in order to provide further understanding of ANI’s results. Adjusted non-GAAP diluted earnings per share should be considered in addition to, but not in lieu of, diluted earnings (loss) per share reported under GAAP. ANI is not providing a reconciliation for the forward-looking full year 2026 adjusted diluted earnings per share guidance because it does not currently have sufficient information to accurately estimate all of the variables and individual adjustments for such reconciliation, including “with” and “without” tax provision information. As such, ANI’s management cannot estimate on a forward-looking basis without unreasonable effort the impact these variables and individual adjustments will have on its reported results. Please refer to the Company's quarterly earnings releases filed with the SEC and that are linked on our website at https://investor.anipharmaceuticals.com/ for the reconciliations of Adjusted Non-GAAP EBITDA and Adjusted Non-GAAP Diluted Earnings per Share.
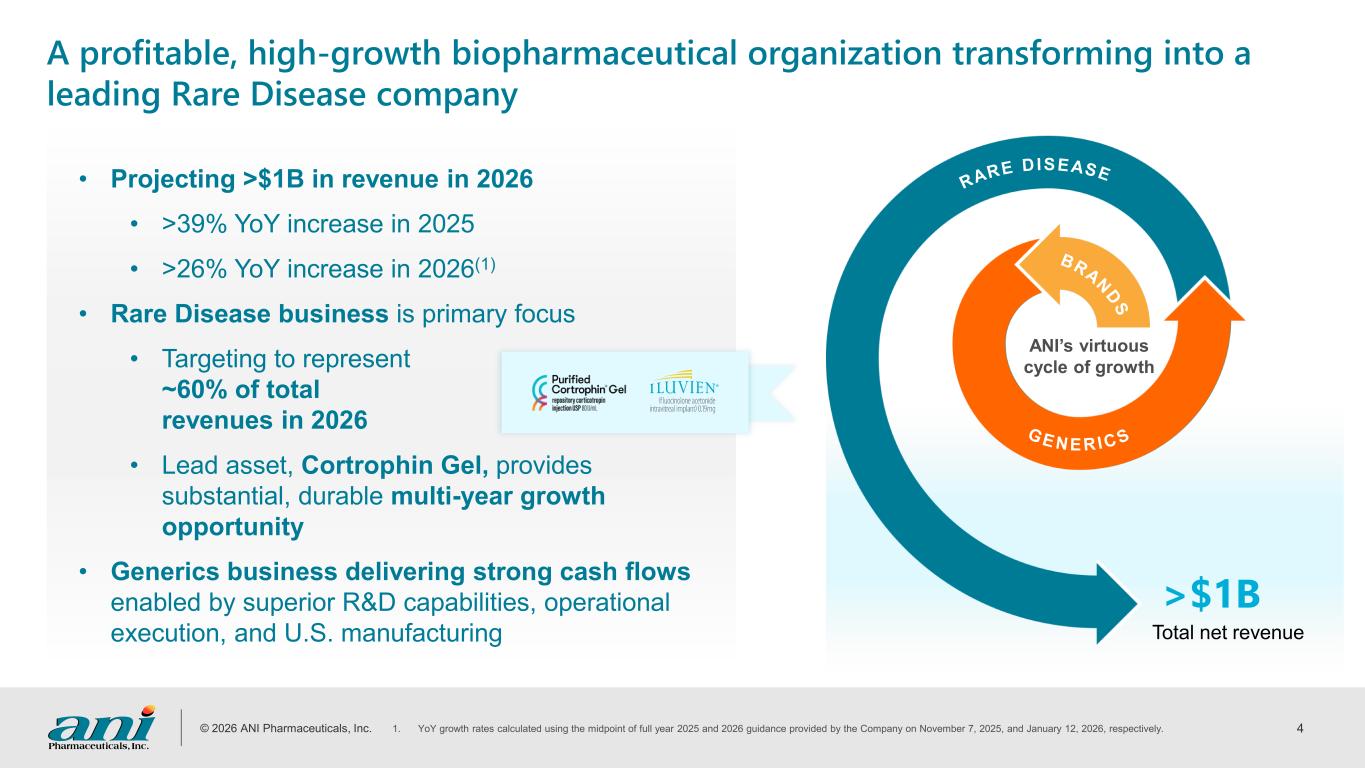
© 2026 ANI Pharmaceuticals, Inc. 4 >$1B Total net revenue A profitable, high-growth biopharmaceutical organization transforming into a leading Rare Disease company • Projecting >$1B in revenue in 2026 • >39% YoY increase in 2025 • >26% YoY increase in 2026(1) • Rare Disease business is primary focus • Targeting to represent ~60% of total revenues in 2026 • Lead asset, Cortrophin Gel, provides substantial, durable multi-year growth opportunity • Generics business delivering strong cash flows enabled by superior R&D capabilities, operational execution, and U.S. manufacturing 1. YoY growth rates calculated using the midpoint of full year 2025 and 2026 guidance provided by the Company on November 7, 2025, and January 12, 2026, respectively. RARE DISEASE BRA N D S G E N E R I C S ANI’s virtuous cycle of growth
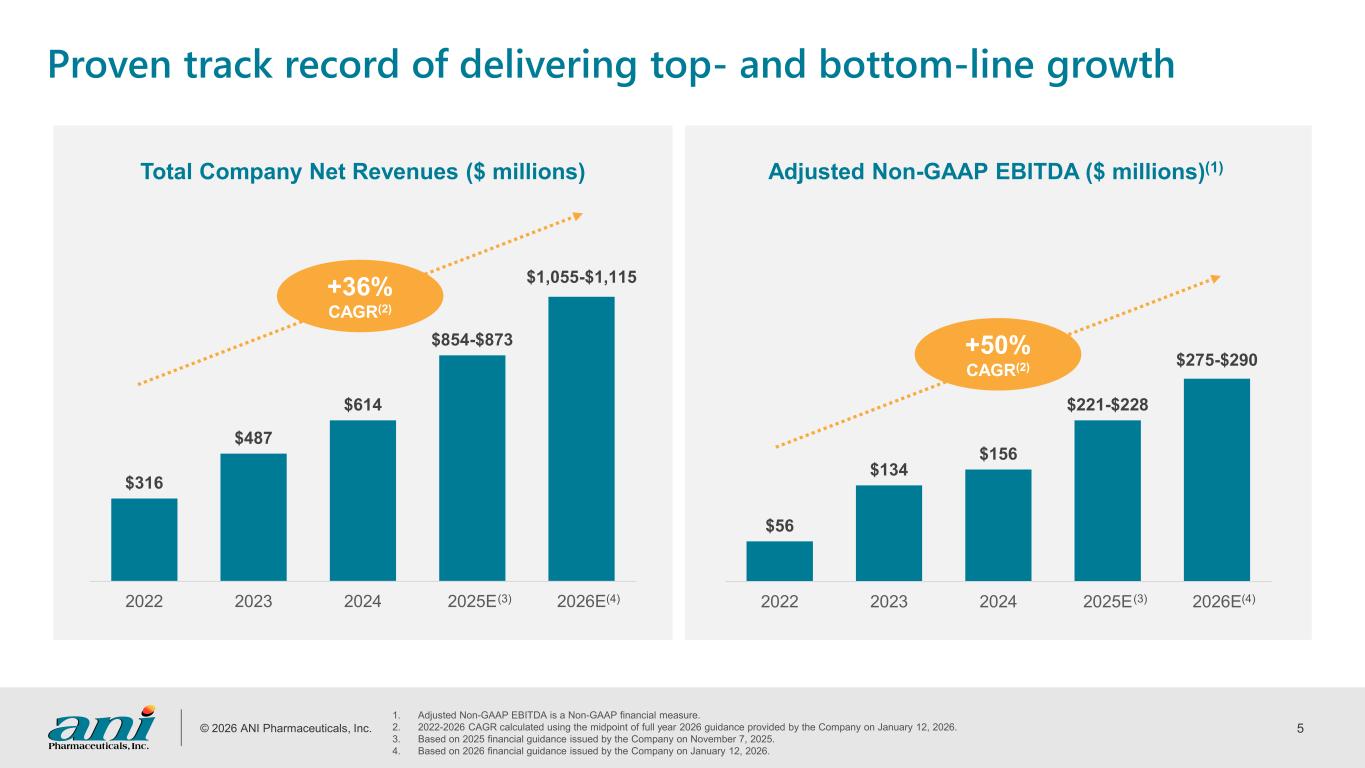
© 2026 ANI Pharmaceuticals, Inc. 5 Proven track record of delivering top- and bottom-line growth Adjusted Non-GAAP EBITDA ($ millions)(1)Total Company Net Revenues ($ millions) 1. Adjusted Non-GAAP EBITDA is a Non-GAAP financial measure. 2. 2022-2026 CAGR calculated using the midpoint of full year 2026 guidance provided by the Company on January 12, 2026. 3. Based on 2025 financial guidance issued by the Company on November 7, 2025. 4. Based on 2026 financial guidance issued by the Company on January 12, 2026. $316 $487 $614 $854-$873 $1,055-$1,115 2022 2023 2024 2025E 2026E +36% CAGR(2) $56 $134 $156 $221-$228 $275-$290 2022 2023 2024 2025E 2026E +50% CAGR(2) (3)(3) (4) (4)
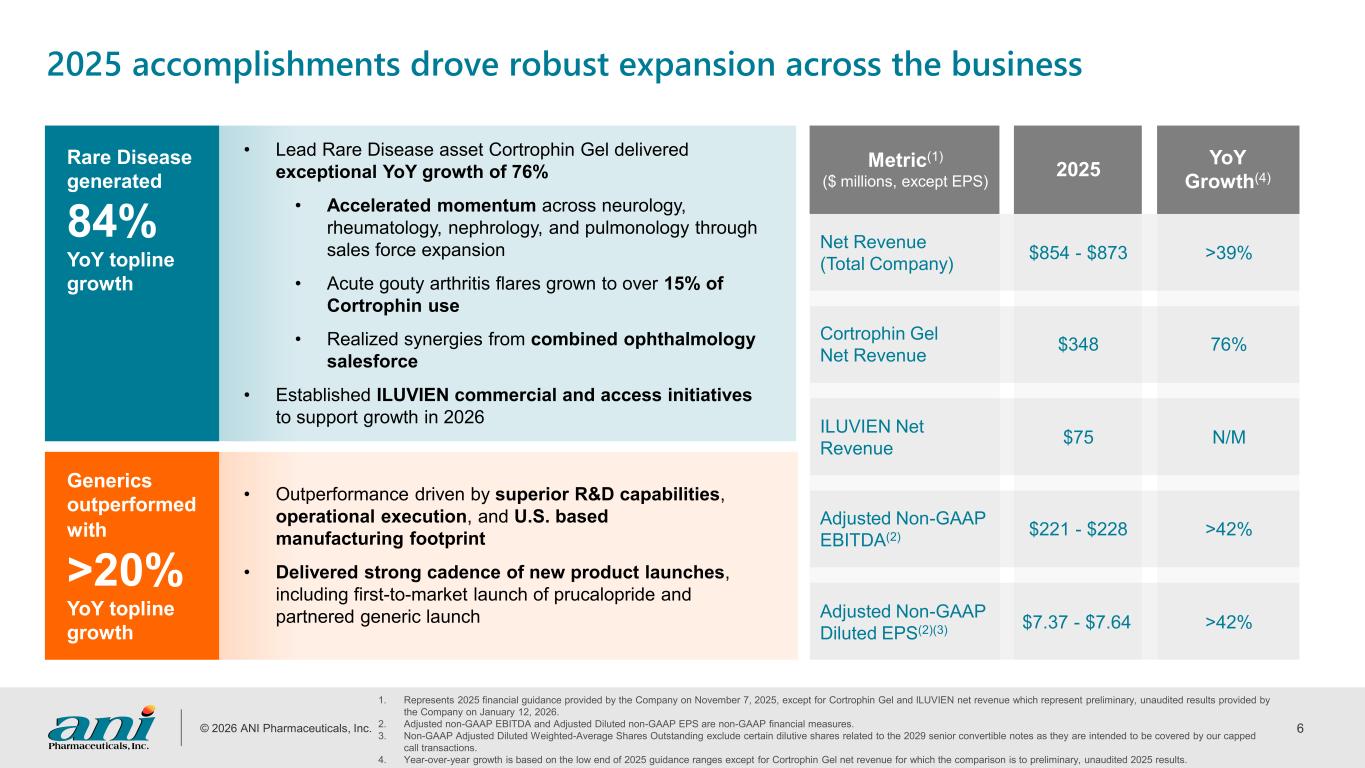
© 2026 ANI Pharmaceuticals, Inc. 6 2025 accomplishments drove robust expansion across the business Metric(1) ($ millions, except EPS) 2025 YoY Growth(4) Net Revenue (Total Company) $854 - $873 >39% Cortrophin Gel Net Revenue $348 76% ILUVIEN Net Revenue $75 N/M Adjusted Non-GAAP EBITDA(2) $221 - $228 >42% Adjusted Non-GAAP Diluted EPS(2)(3) $7.37 - $7.64 >42% Rare Disease generated 84% YoY topline growth Generics outperformed with >20% YoY topline growth • Outperformance driven by superior R&D capabilities, operational execution, and U.S. based manufacturing footprint • Delivered strong cadence of new product launches, including first-to-market launch of prucalopride and partnered generic launch 1. Represents 2025 financial guidance provided by the Company on November 7, 2025, except for Cortrophin Gel and ILUVIEN net revenue which represent preliminary, unaudited results provided by the Company on January 12, 2026. 2. Adjusted non-GAAP EBITDA and Adjusted Diluted non-GAAP EPS are non-GAAP financial measures. 3. Non-GAAP Adjusted Diluted Weighted-Average Shares Outstanding exclude certain dilutive shares related to the 2029 senior convertible notes as they are intended to be covered by our capped call transactions. 4. Year-over-year growth is based on the low end of 2025 guidance ranges except for Cortrophin Gel net revenue for which the comparison is to preliminary, unaudited 2025 results. • Lead Rare Disease asset Cortrophin Gel delivered exceptional YoY growth of 76% • Accelerated momentum across neurology, rheumatology, nephrology, and pulmonology through sales force expansion • Acute gouty arthritis flares grown to over 15% of Cortrophin use • Realized synergies from combined ophthalmology salesforce • Established ILUVIEN commercial and access initiatives to support growth in 2026
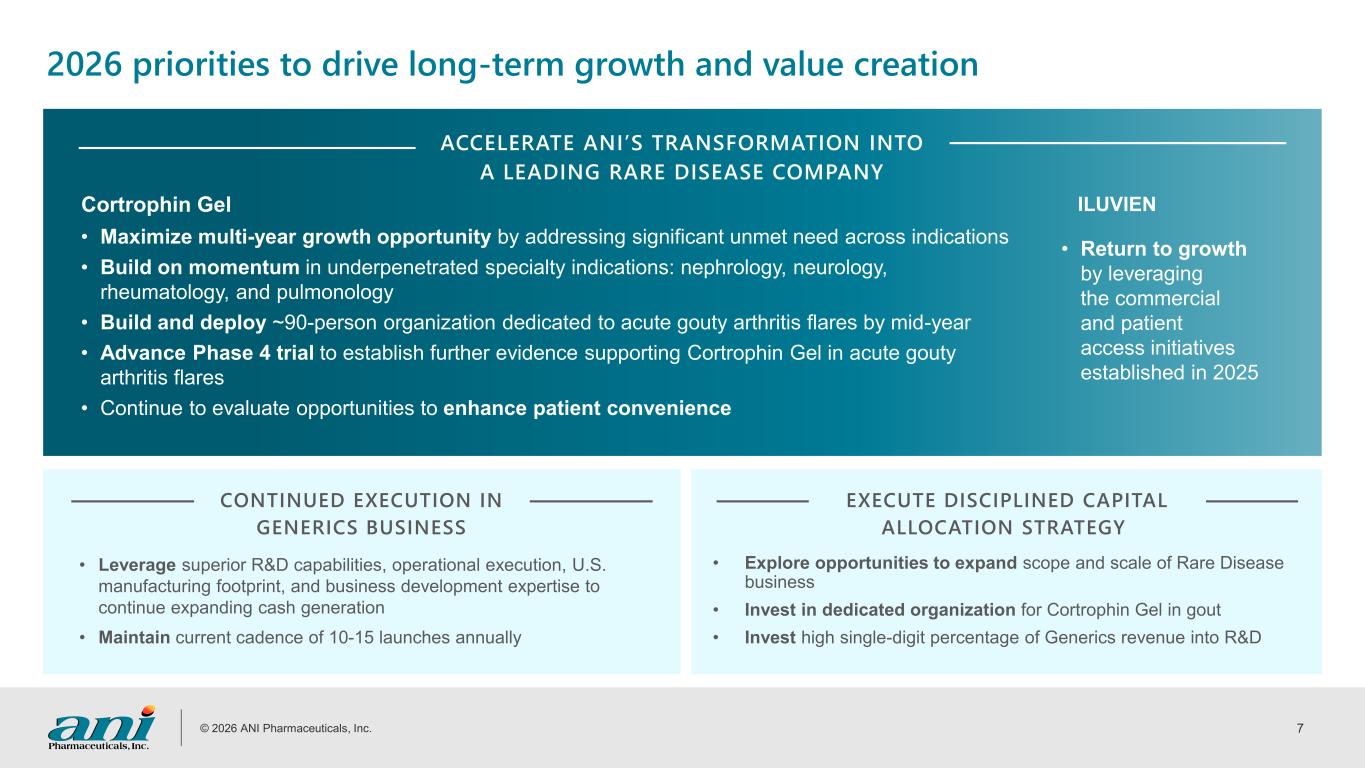
© 2026 ANI Pharmaceuticals, Inc. 7 ACCELERATE ANI’S TRANSFORMATION INTO A LEADING RARE DISEASE COMPANY Cortrophin Gel 2026 priorities to drive long-term growth and value creation • Maximize multi-year growth opportunity by addressing significant unmet need across indications • Build on momentum in underpenetrated specialty indications: nephrology, neurology, rheumatology, and pulmonology • Build and deploy ~90-person organization dedicated to acute gouty arthritis flares by mid-year • Advance Phase 4 trial to establish further evidence supporting Cortrophin Gel in acute gouty arthritis flares • Continue to evaluate opportunities to enhance patient convenience ILUVIEN • Return to growth by leveraging the commercial and patient access initiatives established in 2025 CONTINUED EXECUTION IN GENERICS BUSINESS EXECUTE DISCIPLINED CAPITAL ALLOCATION STRATEGY • Leverage superior R&D capabilities, operational execution, U.S. manufacturing footprint, and business development expertise to continue expanding cash generation • Maintain current cadence of 10-15 launches annually • Explore opportunities to expand scope and scale of Rare Disease business • Invest in dedicated organization for Cortrophin Gel in gout • Invest high single-digit percentage of Generics revenue into R&D
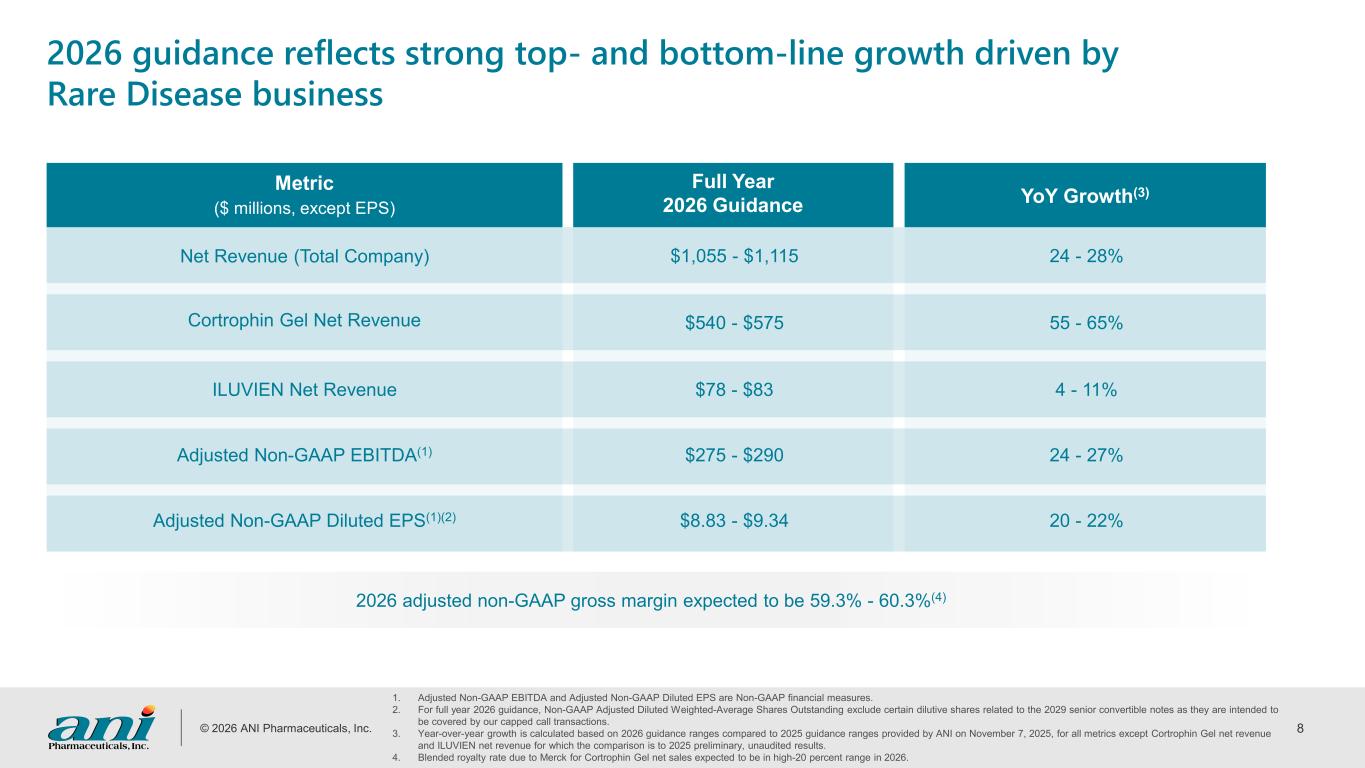
© 2026 ANI Pharmaceuticals, Inc. 8 2026 adjusted non-GAAP gross margin expected to be 59.3% - 60.3%(4) 1. Adjusted Non-GAAP EBITDA and Adjusted Non-GAAP Diluted EPS are Non-GAAP financial measures. 2. For full year 2026 guidance, Non-GAAP Adjusted Diluted Weighted-Average Shares Outstanding exclude certain dilutive shares related to the 2029 senior convertible notes as they are intended to be covered by our capped call transactions. 3. Year-over-year growth is calculated based on 2026 guidance ranges compared to 2025 guidance ranges provided by ANI on November 7, 2025, for all metrics except Cortrophin Gel net revenue and ILUVIEN net revenue for which the comparison is to 2025 preliminary, unaudited results. 4. Blended royalty rate due to Merck for Cortrophin Gel net sales expected to be in high-20 percent range in 2026. YoY Growth(3) $1,055 - $1,115 24 - 28% $540 - $575 55 - 65% $78 - $83 4 - 11% $275 - $290 24 - 27% $8.83 - $9.34 20 - 22% Metric ($ millions, except EPS) Net Revenue (Total Company) Cortrophin Gel Net Revenue ILUVIEN Net Revenue Adjusted Non-GAAP EBITDA(1) Adjusted Non-GAAP Diluted EPS(1)(2) Full Year 2026 Guidance 2026 guidance reflects strong top- and bottom-line growth driven by Rare Disease business

© 2026 ANI Pharmaceuticals, Inc. 95 Rare Disease Business
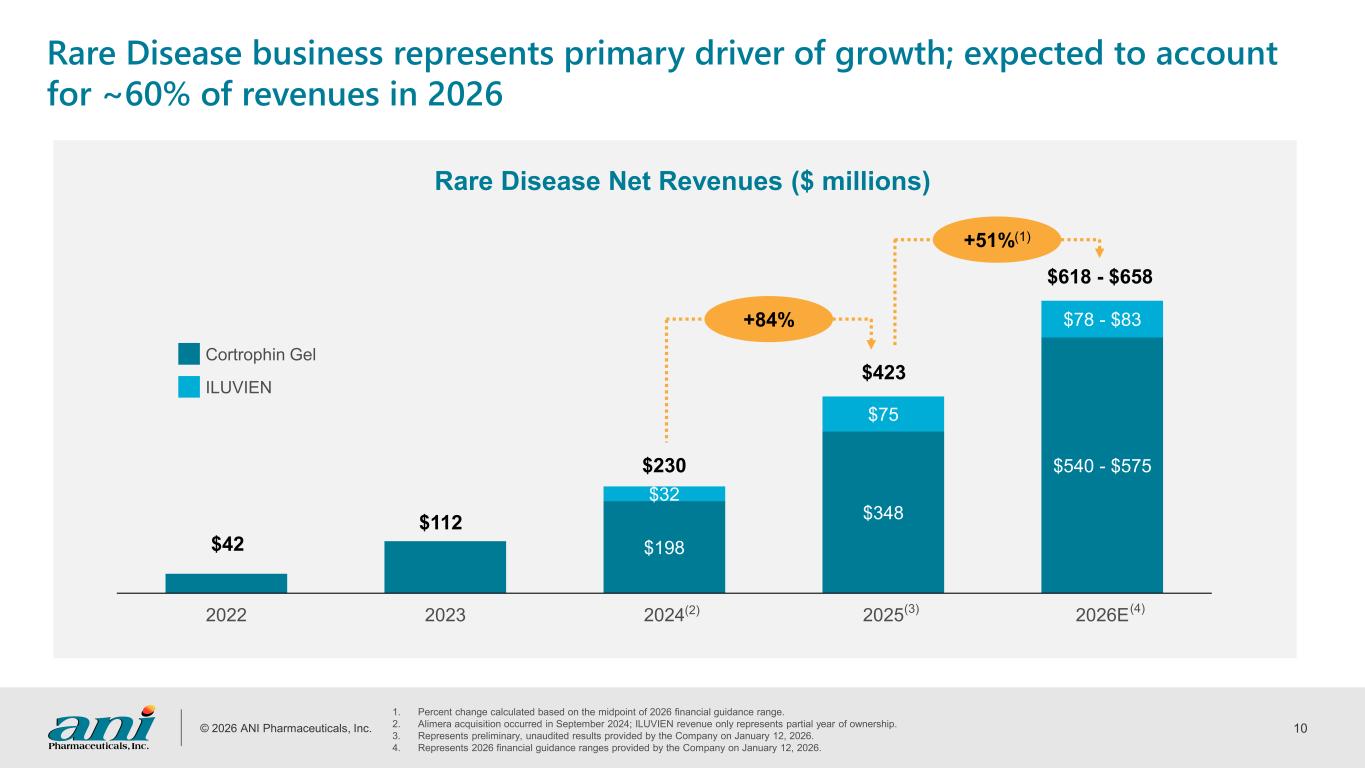
© 2026 ANI Pharmaceuticals, Inc. 10 Rare Disease business represents primary driver of growth; expected to account for ~60% of revenues in 2026 1. Percent change calculated based on the midpoint of 2026 financial guidance range. 2. Alimera acquisition occurred in September 2024; ILUVIEN revenue only represents partial year of ownership. 3. Represents preliminary, unaudited results provided by the Company on January 12, 2026. 4. Represents 2026 financial guidance ranges provided by the Company on January 12, 2026. Rare Disease Net Revenues ($ millions) $198 $348 $540 - $575 $32 $75 $78 - $83 2022 2023 2024 2025 2026E $230 $423 $42 $112 $618 - $658 (3) (4) Cortrophin Gel ILUVIEN +84% +51%(1) (2)
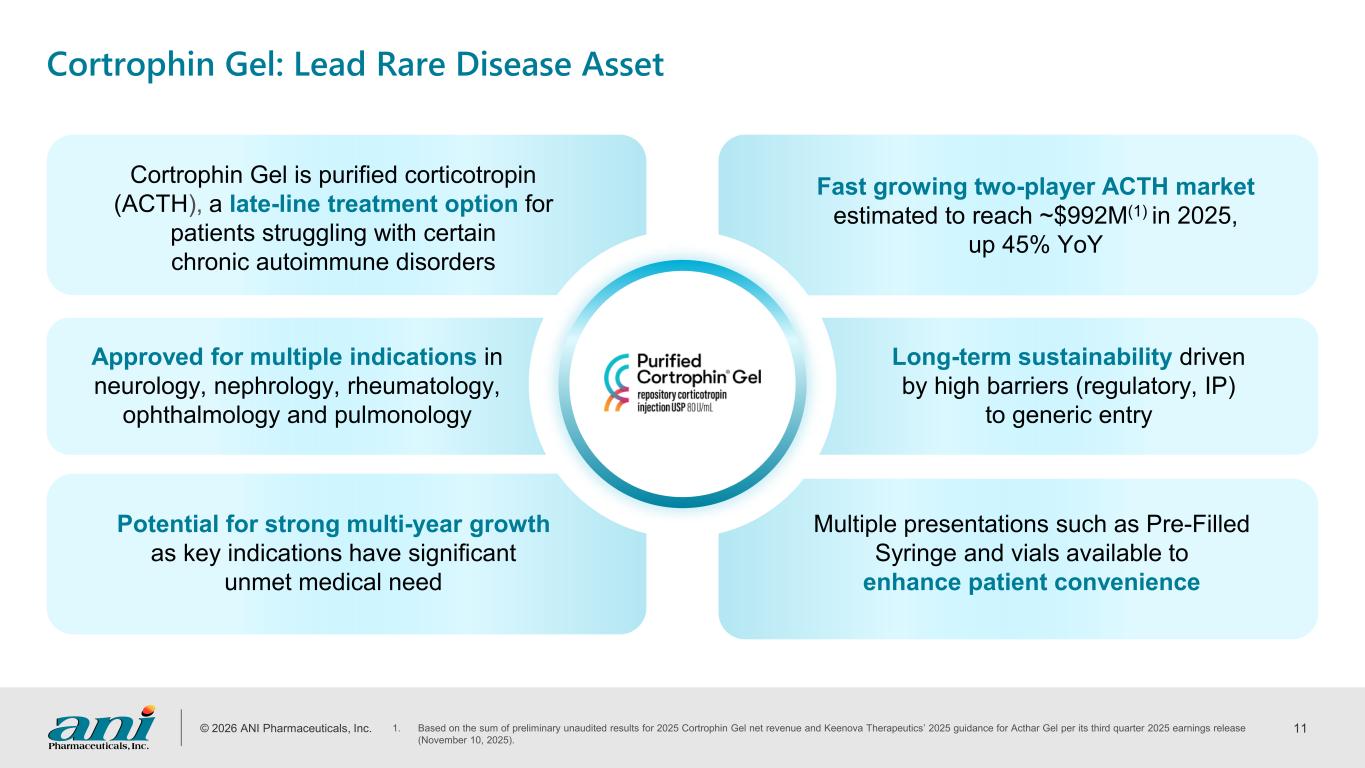
© 2026 ANI Pharmaceuticals, Inc. 11 Cortrophin Gel: Lead Rare Disease Asset Cortrophin Gel is purified corticotropin (ACTH), a late-line treatment option for patients struggling with certain chronic autoimmune disorders Approved for multiple indications in neurology, nephrology, rheumatology, ophthalmology and pulmonology Potential for strong multi-year growth as key indications have significant unmet medical need Multiple presentations such as Pre-Filled Syringe and vials available to enhance patient convenience Long-term sustainability driven by high barriers (regulatory, IP) to generic entry Fast growing two-player ACTH market estimated to reach ~$992M(1) in 2025, up 45% YoY 1. Based on the sum of preliminary unaudited results for 2025 Cortrophin Gel net revenue and Keenova Therapeutics’ 2025 guidance for Acthar Gel per its third quarter 2025 earnings release (November 10, 2025).
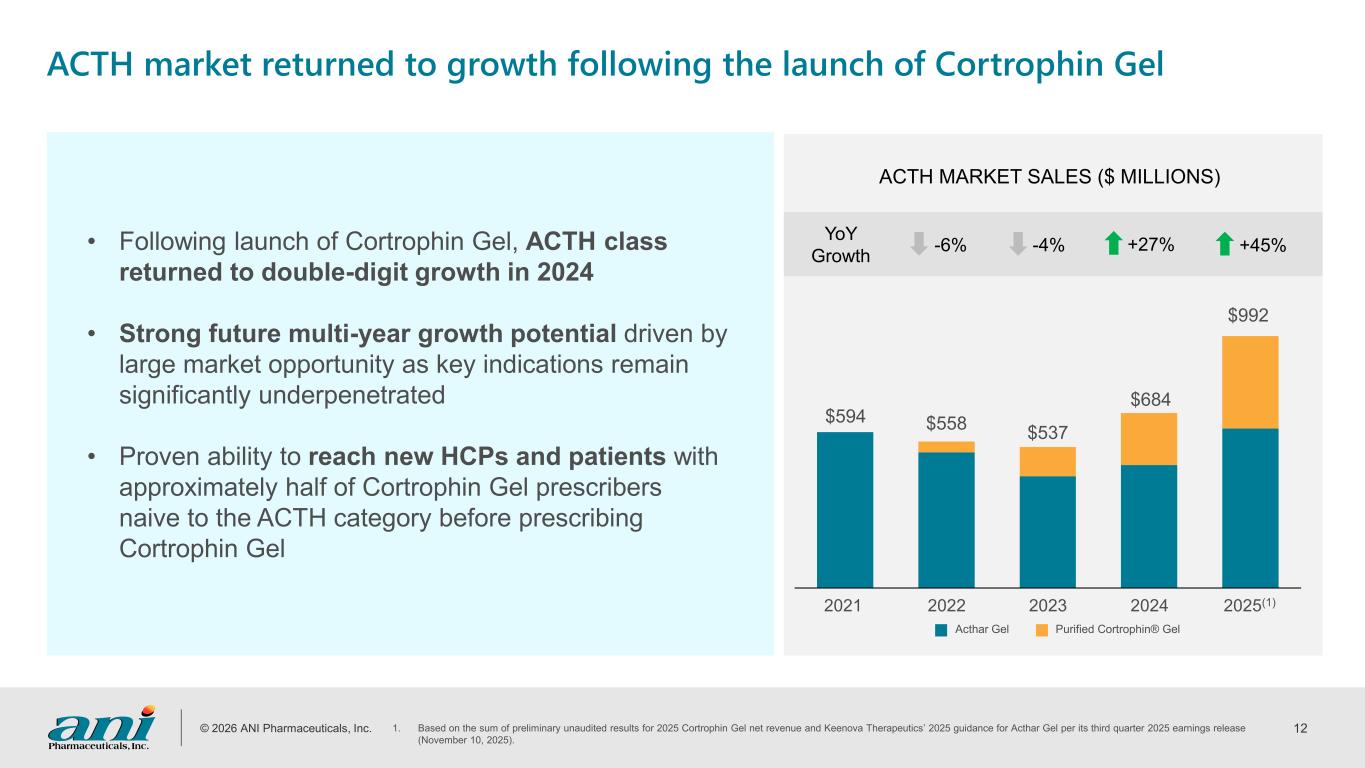
© 2026 ANI Pharmaceuticals, Inc. 12 2021 2022 2023 2024 2025(1) $594 $558 $537 $684 $992 ACTH MARKET SALES ($ MILLIONS) -6% -4% +27% YoY Growth +45% Acthar Gel Purified Cortrophin® Gel • Following launch of Cortrophin Gel, ACTH class returned to double-digit growth in 2024 • Strong future multi-year growth potential driven by large market opportunity as key indications remain significantly underpenetrated • Proven ability to reach new HCPs and patients with approximately half of Cortrophin Gel prescribers naive to the ACTH category before prescribing Cortrophin Gel ACTH market returned to growth following the launch of Cortrophin Gel 1. Based on the sum of preliminary unaudited results for 2025 Cortrophin Gel net revenue and Keenova Therapeutics’ 2025 guidance for Acthar Gel per its third quarter 2025 earnings release (November 10, 2025).
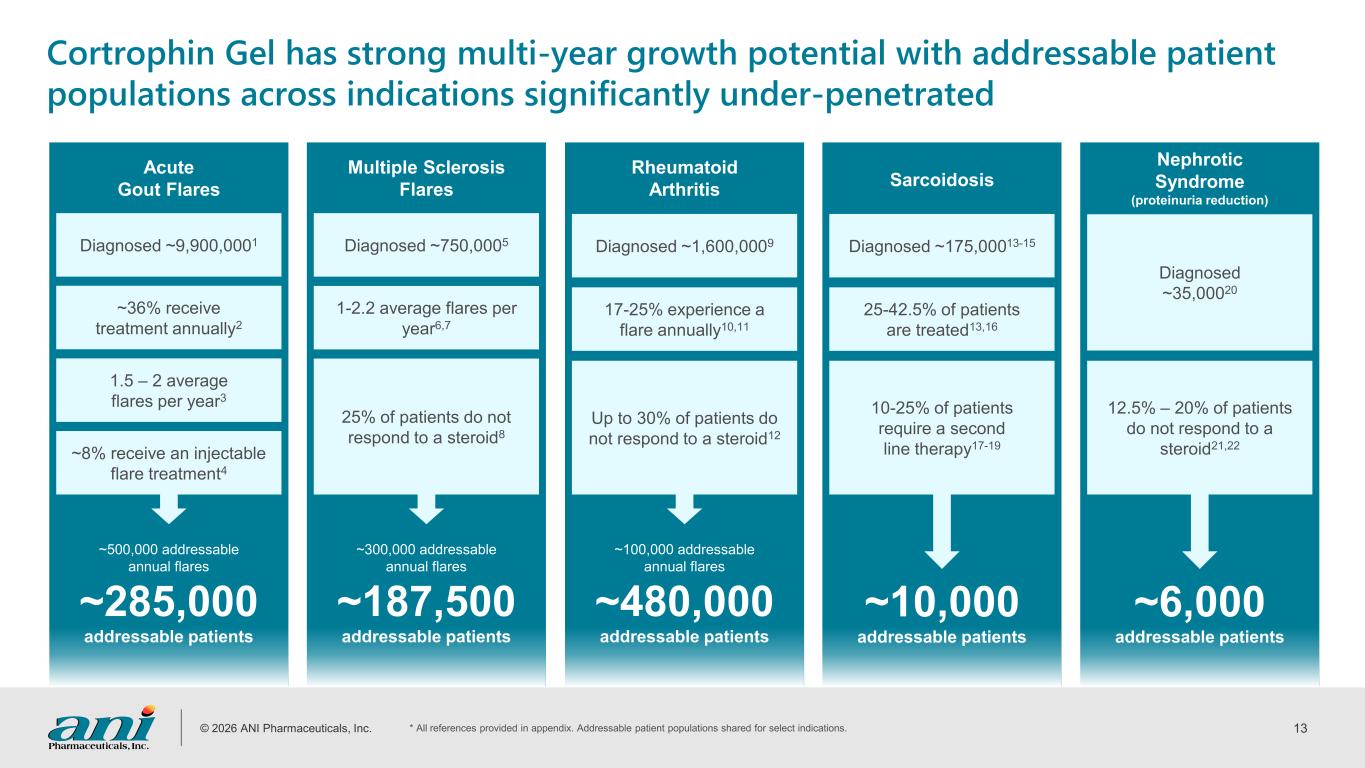
© 2026 ANI Pharmaceuticals, Inc. 13 Diagnosed ~9,900,0001 ~500,000 addressable annual flares ~285,000 addressable patients Acute Gout Flares Cortrophin Gel has strong multi-year growth potential with addressable patient populations across indications significantly under-penetrated ~36% receive treatment annually2 1.5 – 2 average flares per year3 ~8% receive an injectable flare treatment4 Diagnosed ~750,0005 Multiple Sclerosis Flares 1-2.2 average flares per year6,7 25% of patients do not respond to a steroid8 Diagnosed ~1,600,0009 Rheumatoid Arthritis 17-25% experience a flare annually10,11 Up to 30% of patients do not respond to a steroid12 Diagnosed ~35,00020 Nephrotic Syndrome (proteinuria reduction) 12.5% – 20% of patients do not respond to a steroid21,22 Diagnosed ~175,00013-15 Sarcoidosis 25-42.5% of patients are treated13,16 10-25% of patients require a second line therapy17-19 ~300,000 addressable annual flares ~187,500 addressable patients ~100,000 addressable annual flares ~480,000 addressable patients ~10,000 addressable patients ~6,000 addressable patients * All references provided in appendix. Addressable patient populations shared for select indications.
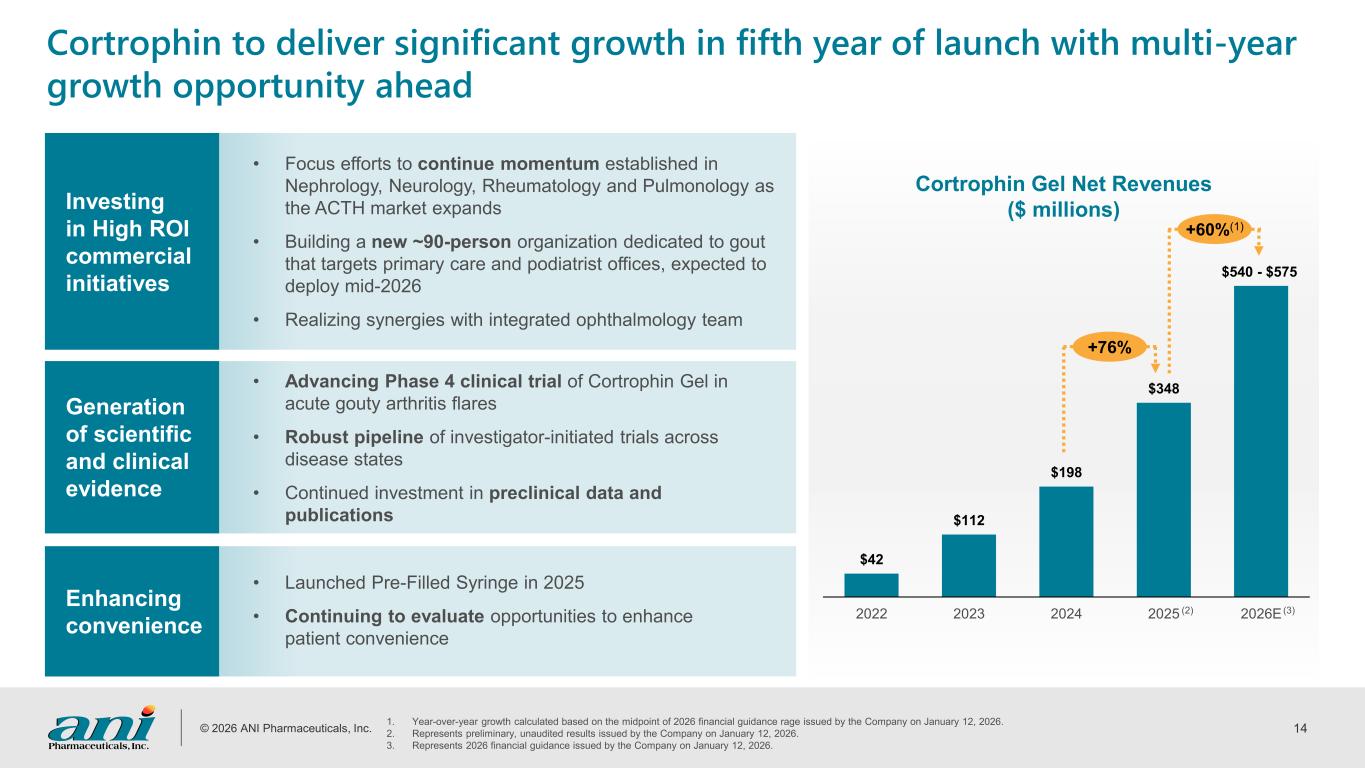
© 2026 ANI Pharmaceuticals, Inc. 14 $42 $112 $198 $348 $540 - $575 2022 2023 2024 2025 2026E Cortrophin Gel Net Revenues ($ millions) Cortrophin to deliver significant growth in fifth year of launch with multi-year growth opportunity ahead 1. Year-over-year growth calculated based on the midpoint of 2026 financial guidance rage issued by the Company on January 12, 2026. 2. Represents preliminary, unaudited results issued by the Company on January 12, 2026. 3. Represents 2026 financial guidance issued by the Company on January 12, 2026. (2) (3) Investing in High ROI commercial initiatives • Focus efforts to continue momentum established in Nephrology, Neurology, Rheumatology and Pulmonology as the ACTH market expands • Building a new ~90-person organization dedicated to gout that targets primary care and podiatrist offices, expected to deploy mid-2026 • Realizing synergies with integrated ophthalmology team Generation of scientific and clinical evidence • Advancing Phase 4 clinical trial of Cortrophin Gel in acute gouty arthritis flares • Robust pipeline of investigator-initiated trials across disease states • Continued investment in preclinical data and publications Enhancing convenience • Launched Pre-Filled Syringe in 2025 • Continuing to evaluate opportunities to enhance patient convenience +76% +60%(1)

© 2026 ANI Pharmaceuticals, Inc. 15 Capturing sizable additional opportunity in gout through commercial organization expansion * All references provided in appendix. Addressable patient populations shared for select indications. • Focus on most severe patients: ~285,000 who are currently treated with injectables for their flares • Need for new treatments for those patients not well served by first line options • Co-morbidities may limit certain treatment options • Only approved ACTH therapy to treat acute gouty arthritis flares • Gout indication generated ~15% of Cortrophin Gel volume in 2025, largely from nephrologists and rheumatologists • Prefilled syringe and subcutaneous option for Cortrophin self- administration enables flare readiness, unlike most injectable options • In 2025, ran successful pilots across 10 territories in primary care and podiatry • By mid-2026, deploying new ~90- person commercial organization to target high priority PCPs and podiatrists who see most severe acute gouty arthritis patients • Investing in evidence generation through Phase 4 trial to expand use over time Diagnosed ~9,900,0001 Acute Gout Flares ~36% receive treatment annually2 1.5 – 2 average flares per year3 Large underpenetrated market opportunity Successful track record in Gout Our approach to serving more patients ~8% receive an injectable flare treatment4 ~285,000 patients ~500,000 addressable annual flares
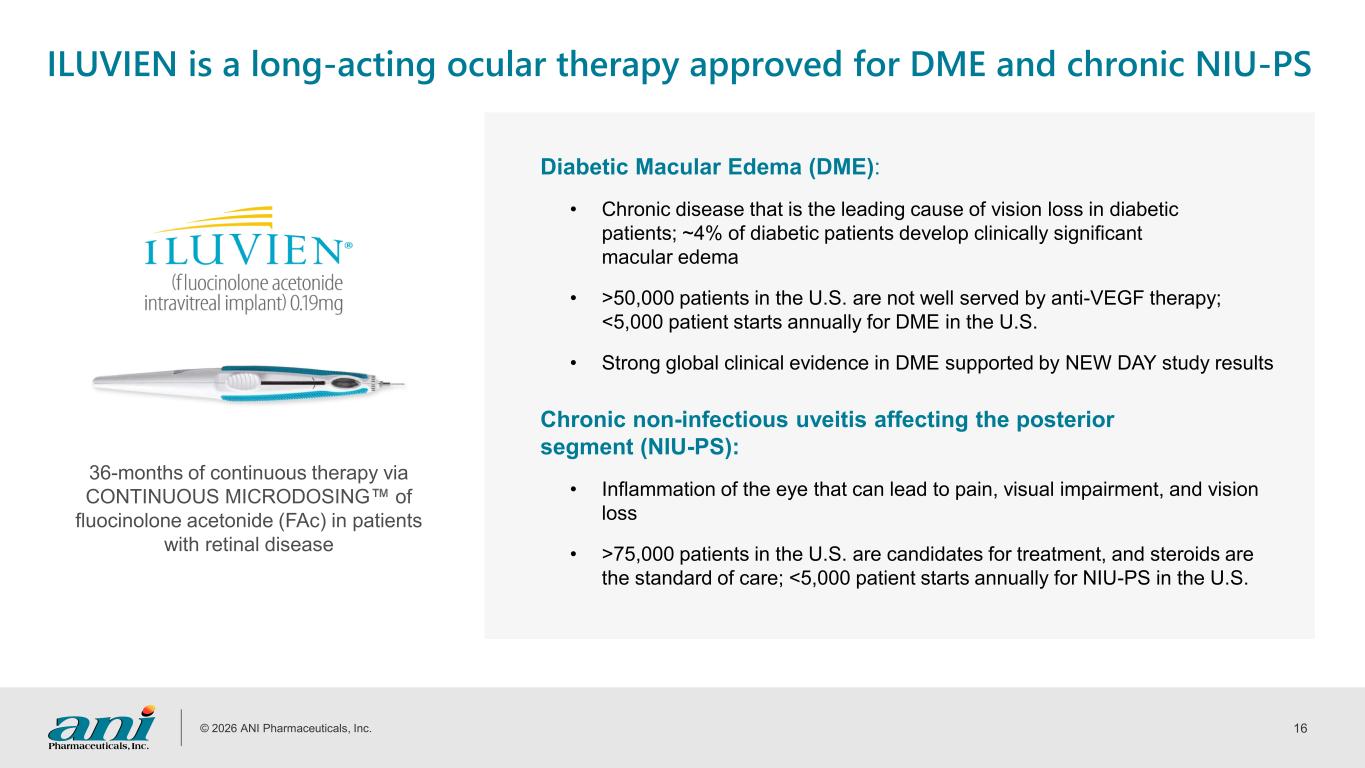
© 2026 ANI Pharmaceuticals, Inc. 16 ILUVIEN is a long-acting ocular therapy approved for DME and chronic NIU-PS 36-months of continuous therapy via CONTINUOUS MICRODOSING of fluocinolone acetonide (FAc) in patients with retinal disease Diabetic Macular Edema (DME): • Chronic disease that is the leading cause of vision loss in diabetic patients; ~4% of diabetic patients develop clinically significant macular edema • >50,000 patients in the U.S. are not well served by anti-VEGF therapy; <5,000 patient starts annually for DME in the U.S. • Strong global clinical evidence in DME supported by NEW DAY study results Chronic non-infectious uveitis affecting the posterior segment (NIU-PS): • Inflammation of the eye that can lead to pain, visual impairment, and vision loss • >75,000 patients in the U.S. are candidates for treatment, and steroids are the standard of care; <5,000 patient starts annually for NIU-PS in the U.S.
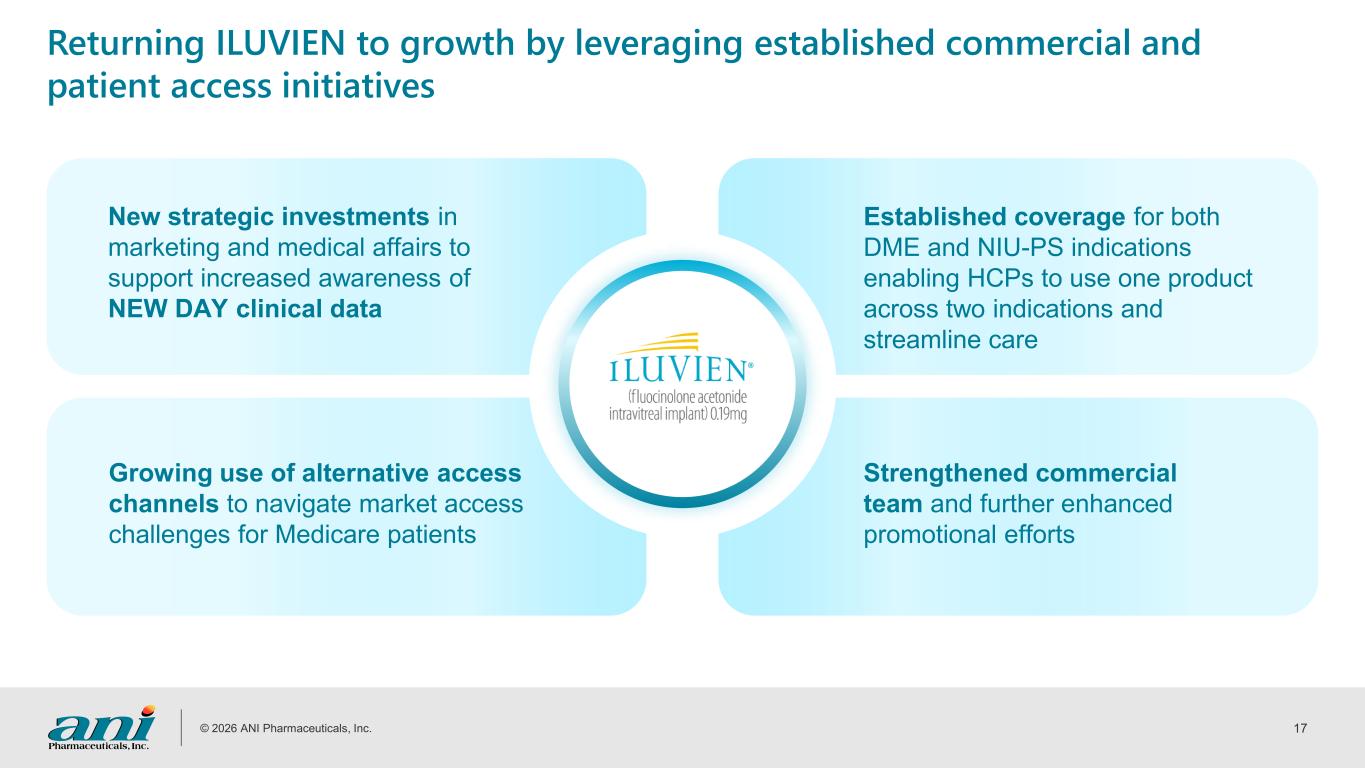
© 2026 ANI Pharmaceuticals, Inc. 17 Returning ILUVIEN to growth by leveraging established commercial and patient access initiatives New strategic investments in marketing and medical affairs to support increased awareness of NEW DAY clinical data Growing use of alternative access channels to navigate market access challenges for Medicare patients Strengthened commercial team and further enhanced promotional efforts Established coverage for both DME and NIU-PS indications enabling HCPs to use one product across two indications and streamline care

© 2026 ANI Pharmaceuticals, Inc. 185 Generics Business
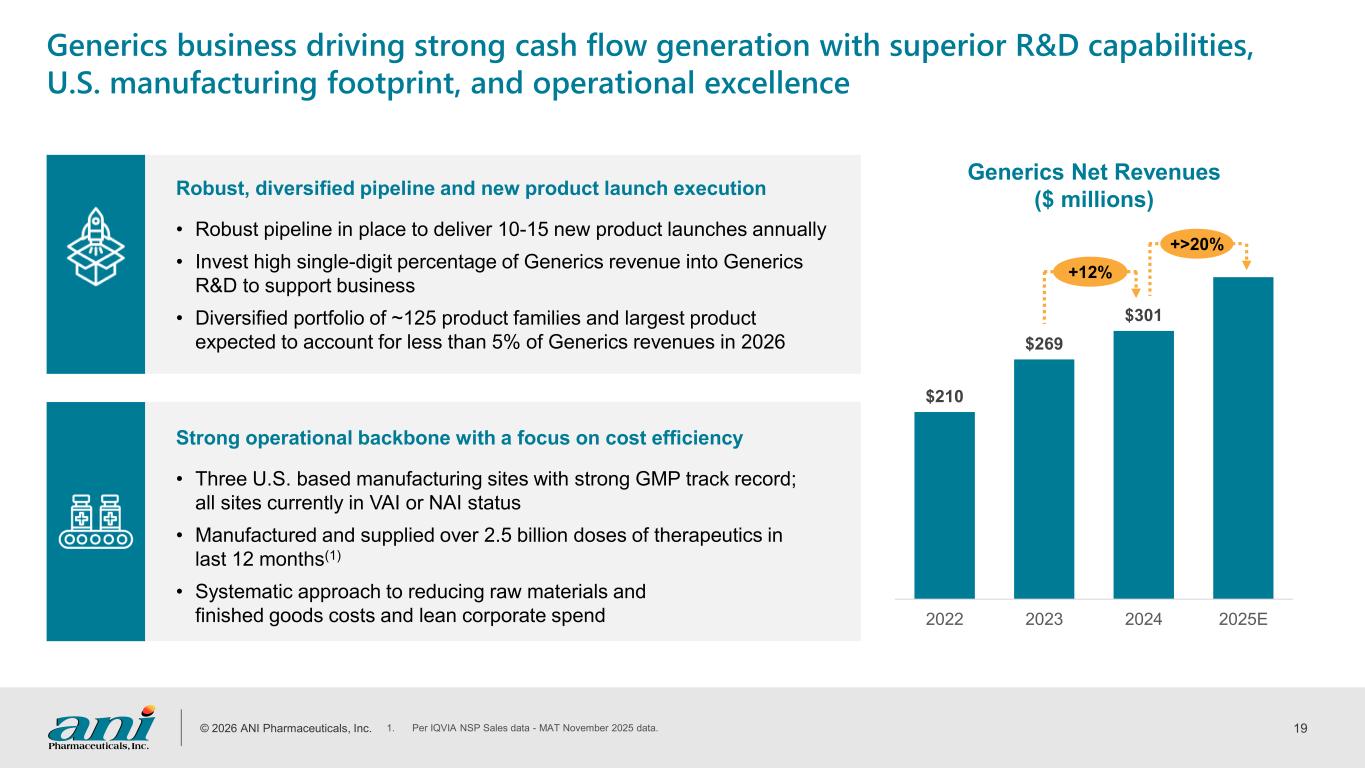
© 2026 ANI Pharmaceuticals, Inc. 19 Robust, diversified pipeline and new product launch execution • Robust pipeline in place to deliver 10-15 new product launches annually • Invest high single-digit percentage of Generics revenue into Generics R&D to support business • Diversified portfolio of ~125 product families and largest product expected to account for less than 5% of Generics revenues in 2026 Strong operational backbone with a focus on cost efficiency • Three U.S. based manufacturing sites with strong GMP track record; all sites currently in VAI or NAI status • Manufactured and supplied over 2.5 billion doses of therapeutics in last 12 months(1) • Systematic approach to reducing raw materials and finished goods costs and lean corporate spend Generics business driving strong cash flow generation with superior R&D capabilities, U.S. manufacturing footprint, and operational excellence Generics Net Revenues ($ millions) $210 $269 $301 2022 2023 2024 2025E +12% +>20% 1. Per IQVIA NSP Sales data - MAT November 2025 data.

© 2026 ANI Pharmaceuticals, Inc. 205 U.S. Manufacturing Footprint
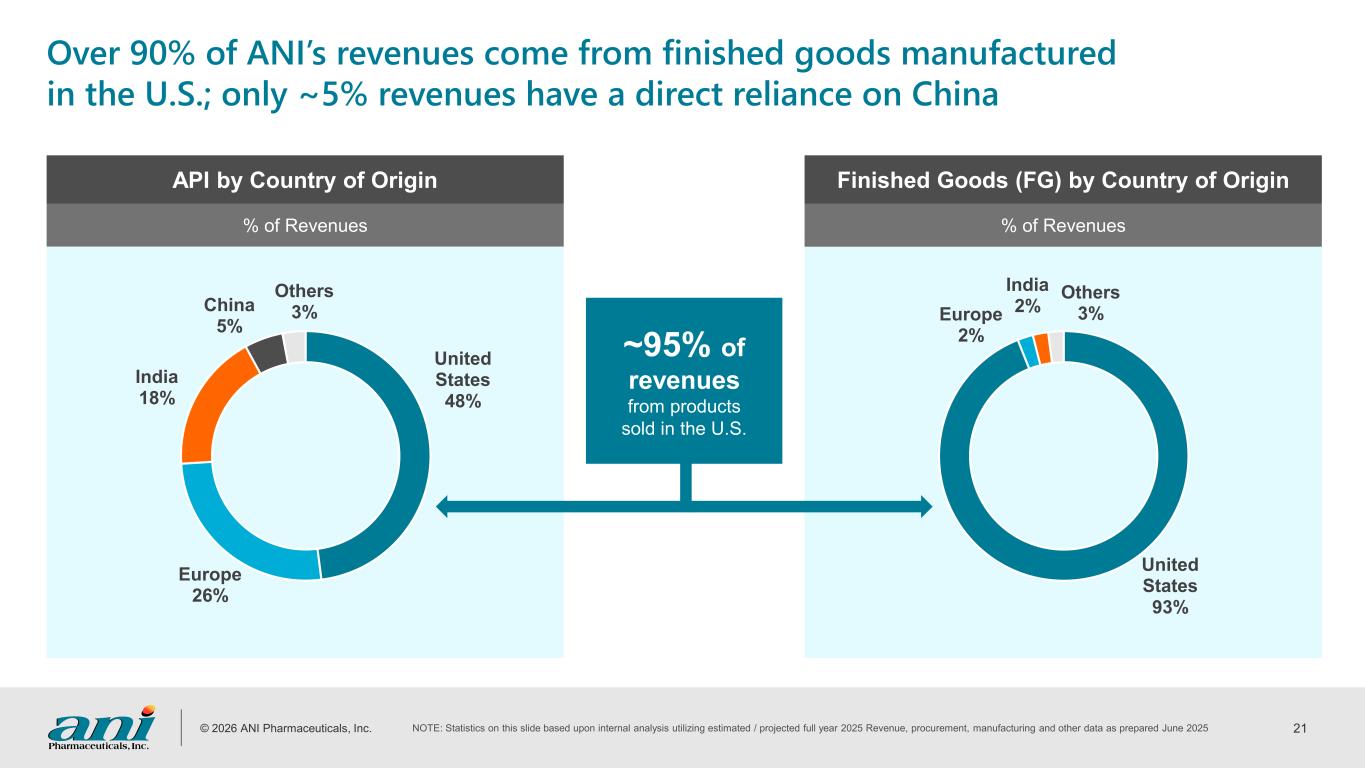
© 2026 ANI Pharmaceuticals, Inc. 21 United States 93% Europe 2% India 2% Others 3% United States 48% Europe 26% India 18% China 5% Others 3% Over 90% of ANI’s revenues come from finished goods manufactured in the U.S.; only ~5% revenues have a direct reliance on China API by Country of Origin Finished Goods (FG) by Country of Origin % of Revenues % of Revenues ~95% of revenues from products sold in the U.S. NOTE: Statistics on this slide based upon internal analysis utilizing estimated / projected full year 2025 Revenue, procurement, manufacturing and other data as prepared June 2025
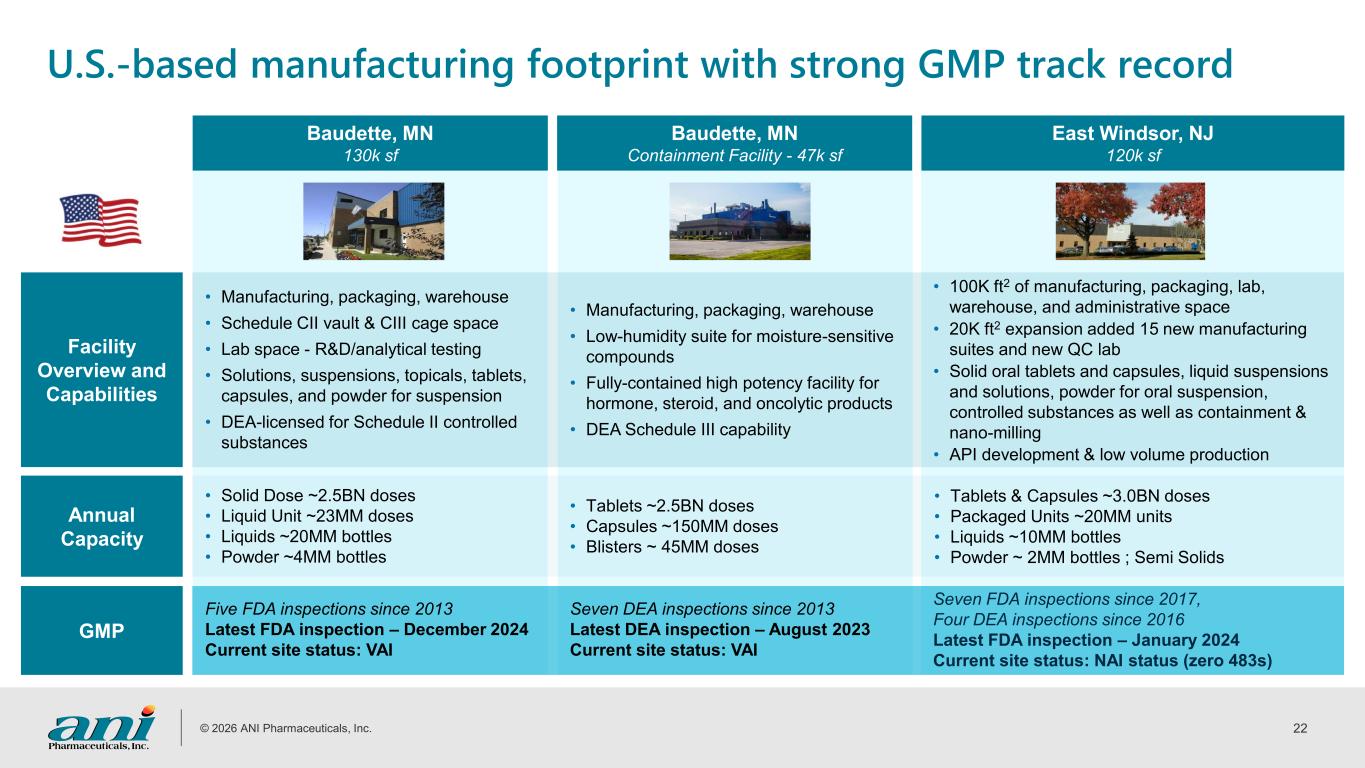
© 2026 ANI Pharmaceuticals, Inc. 22 U.S.-based manufacturing footprint with strong GMP track record Baudette, MN 130k sf Baudette, MN Containment Facility - 47k sf East Windsor, NJ 120k sf Facility Overview and Capabilities • Manufacturing, packaging, warehouse • Schedule CII vault & CIII cage space • Lab space - R&D/analytical testing • Solutions, suspensions, topicals, tablets, capsules, and powder for suspension • DEA-licensed for Schedule II controlled substances • Manufacturing, packaging, warehouse • Low-humidity suite for moisture-sensitive compounds • Fully-contained high potency facility for hormone, steroid, and oncolytic products • DEA Schedule III capability • 100K ft2 of manufacturing, packaging, lab, warehouse, and administrative space • 20K ft2 expansion added 15 new manufacturing suites and new QC lab • Solid oral tablets and capsules, liquid suspensions and solutions, powder for oral suspension, controlled substances as well as containment & nano-milling • API development & low volume production Annual Capacity • Solid Dose ~2.5BN doses • Liquid Unit ~23MM doses • Liquids ~20MM bottles • Powder ~4MM bottles • Tablets ~2.5BN doses • Capsules ~150MM doses • Blisters ~ 45MM doses • Tablets & Capsules ~3.0BN doses • Packaged Units ~20MM units • Liquids ~10MM bottles • Powder ~ 2MM bottles ; Semi Solids GMP Five FDA inspections since 2013 Latest FDA inspection – December 2024 Current site status: VAI Seven DEA inspections since 2013 Latest DEA inspection – August 2023 Current site status: VAI Seven FDA inspections since 2017, Four DEA inspections since 2016 Latest FDA inspection – January 2024 Current site status: NAI status (zero 483s)

© 2026 ANI Pharmaceuticals, Inc. 235 Summary

© 2026 ANI Pharmaceuticals, Inc. 24 ANI well positioned to deliver long-term growth and value creation 1. Based on the midpoint of 2026 financial guidance ranges issued by the Company on January 12, 2026. 2. Adjusted Non-GAAP EBITDA is a Non-GAAP financial measure. 3. Cash is preliminary unaudited balance as of December 31, 2026. Net leverage as of September 30, 2026 was 1.7x and the Company expects or-going de-levering in the fourth quarter of 2025. 4. YoY growth rate calculated using the midpoint of 2026 financial guidance provided by the Company on January 12, 2026, compared to preliminary, unaudited results. • Rare Disease expected to represent ~60% of total revenue in 2026 • Lead asset, Cortrophin Gel, expected to deliver +60% YoY growth in 2026 with substantial, multi-year growth opportunity(4) • Strong Generics cash flows further enables investments in Rare Disease business VIRTUOUS CYCLE OF GROWTH DRIVES TRANSFORMATION INTO A LEADING RARE DISEASE COMPANY Accelerate transformation into leading Rare Disease company Continued excellence in Generics R&D and operations Execute disciplined capital allocation strategy 2026 total revenues(1) >$1B 26% YoY 2026 adjusted EBITDA(1)(2) ~$283M 26% YoY 2025 year- end cash(3) ~$285M 2025 year-end net leverage(3) <1.7x FINANCIAL STRENGTH 2026 STRATEGIC PRIORITIES

© 2026 ANI Pharmaceuticals, Inc. 255 Appendix
© 2026 ANI Pharmaceuticals, Inc. 26 References for Cortrophin Gel Addressable Patient Population Gout 1. Singh G, Lingala B, Mithal A. Gout and hyperuricaemia in the USA: prevalence and trends. Rheumatology (Oxford). 2019 Dec 1;58(12):2177-2180. doi: 10.1093/rheumatology/kez196. PMID: 31168609 2. Thorpe K. Partnership to fight chronic disease. May 21, 2018 3. Singh JA, Morlock A, Morlock R. Gout Flare Burden in the United States: A Multiyear Cross‐Sectional Survey Study. ACR Open Rheumatology. 2025 Jan;7(1):e11759, ANI claims data analysis (data on file), Proudman C, et al. Arthritis Res Ther. 2019;21:132. 4. Based on ANI claims analysis Multiple Sclerosis 5. Hittle M, Culpepper WJ, Langer-Gould A, Marrie RA, Cutter GR, Kaye WE, Wagner L, Topol B, LaRocca NG, Nelson LM, Wallin MT. Population-based estimates for the prevalence of multiple sclerosis in the United States by race, ethnicity, age, sex, and geographic region. JAMA neurology. 2023 Jul 1;80(7):693-701. 6. Nazareth TA, Rava AR, Polyakov JL, Banfe EN, Waltrip II RW, Zerkowski KB, Herbert LB. Relapse prevalence, symptoms, and health care engagement: patient insights from the Multiple Sclerosis in America 2017 survey. Multiple sclerosis and related disorders. 2018 Nov 1;26:219-34. 7. Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse: the patient’s perspective. The Patient-Patient-Centered Outcomes Research. 2012 Mar;5(1):57-69. 8. Wynn D, Goldstick L, Bauer W, Zhao E, Tarau E, Cohen JA, Robertson D, Miller A. Results from a multicenter, randomized, double‐blind, placebo‐controlled study of repository corticotropin injection for multiple sclerosis relapse that did not adequately respond to corticosteroids. CNS Neuroscience & Therapeutics. 2022 Mar;28(3):364-71. Rheumatoid Arthritis 9. Evaluate Pharma, Evaluate Epi USA Population Insight 10. Bachman K. et al. J Rheumatol. 2018;45(11):1515-1521 11. Oh YJ, Moon KW. Predictors of flares in patients with rheumatoid arthritis who exhibit low disease activity: A nationwide cohort study. Journal of Clinical Medicine. 2020 Oct 7;9(10):3219. 12. Chikanza IC, Kozaci DL. Corticosteroid resistance in rheumatoid arthritis: molecular and cellular perspectives. Rheumatology. 2004 Nov 1;43(11):1337-45. Sarcoidosis 13. Baughman RP, et al. Ann Am Thorac Soc. 2016;13(8):1244-1252 14. Gerke AK, Judson MA, Cozier YC, Culver DA, Koth LL. Disease burden and variability in sarcoidosis. Annals of the American Thoracic Society. 2017 Dec;14(Supplement 6):S421-8. 15. Nam HH, Washington A, Butt M, Maczuga S, Guck D, Yanosky JD, Helm MF. The prevalence and geographic distribution of sarcoidosis in the United States. JAAD international. 2022 Dec 1;9:30-2. 16. Sangani R, Bosch NA, Govender P, Scarpato B, Walkey AJ, Newman J, Law AC, Gillmeyer KR, Shankar DA. Sarcoidosis treatment patterns in the United States: 2016-2022. Chest. 2025 Apr 1;167(4):1099-106. 17. ANI primary market research 2023 18. El Jammal T, Jamilloux Y, Gerfaud-Valentin M, Valeyre D, Sève P. Refractory sarcoidosis: a review. Therapeutics and clinical risk management. 2020 Apr 17:323-45. 19. Mahmood K, Butt NI, Ashfaq F, Younus R. Refractory Sarcoidosis. Journal of Ayub Medical College Abbottabad. 2023 Jul 9;35(3):479-81. Nephrotic Syndrome 20. Evaluate Pharma, Evaluate Epi USA Population Insight 21. Bensimhon AR, Williams AE, Gbadegesin RA. Treatment of steroid-resistant nephrotic syndrome in the genomic era. Pediatric nephrology. 2019 Nov;34(11):2279-93. / 22. Ghedira-Besbes L, Mallek A, Guediche MN. Idiopathic nephrotic syndrome in children: report of 57 cases. La Tunisie Medicale. 2003 Sep 1;81(9):702-8.