| J.P. Morgan 2026 Healthcare Conference PTC Therapeutics 2026 Matthew B. Klein, MD CEO |
| J.P. Morgan 2026 Healthcare Conference FDA and EMA approvals of Sephience Advancement of early-stage R&D programs Strong start to Sephience global launch Drive revenue and effectively manage OpEx 2025: A Year of Focus and Execution 2 |
| J.P. Morgan 2026 Healthcare Conference This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. All statements contained in this presentation, other than statements of historic fact, are forward-looking statements, including statements with respect to 2026 total product revenue guidance and 2026 operating expense guidance, and statements regarding: the future expectations, plans and prospects for PTC, including with respect to the expected timing of clinical trials and studies, availability of data, regulatory submissions and responses, meetings with regulatory agencies, commercialization and other matters with respect to its products and product candidates; PTC's strategy, future operations, future financial position, future revenues, projected costs; and the objectives of management. Other forward-looking statements may be identified by the words, "guidance," "plan," "anticipate," "believe," "estimate," "expect," "intend," "may," "target," "potential," "will," "would," "could," "should," "continue," "aim," and similar expressions. PTC's actual results, performance or achievements could differ materially from those expressed or implied by forward-looking statements it makes as a result of a variety of risks and uncertainties, including those related to: the outcome of pricing, coverage and reimbursement negotiations with third party payors for PTC's products or product candidates that PTC commercializes or may commercialize in the future; expectations with respect to Sephience, including any regulatory submissions and potential approvals, commercialization, and the potential achievement of sales milestones and contingent payments that PTC may be obligated to make; PTC's ability to maintain its marketing authorization of Translarna for the treatment of nmDMD in Brazil, Russia and other regions; the effect of the European Commission's adoption of the negative opinion from the Committee for Medicinal Products for Human Use (CHMP) on Translarna on other regulatory bodies; PTC's ability to use the results of Study 041, a randomized, 18-month, placebo-controlled clinical trial of Translarna for the treatment of nmDMD followed by an 18-month open-label extension, and from its international drug registry study to support a marketing approval for Translarna for the treatment of nmDMD in the United States; whether investigators agree with PTC's interpretation of the results of clinical trials and the totality of clinical data from its trials in Translarna; expectations with respect to PTC's license and collaboration agreement with Novartis Pharmaceuticals Corporation for votoplam for the treatment of Huntington's disease including its right to receive development, regulatory and sales milestones, profit sharing and royalty payments from Novartis the design and expected timing of clinical trials and studies, the availability of data, and regulatory submissions and responses, including potential accelerated approval; expectations with respect to Upstaza/Kebilidi, including commercialization, manufacturing capabilities, and the potential achievement of sales milestones and contingent payments that PTC may be obligated to make; expectations with respect to vatiquinone, including with respect to the design and expected timing of clinical trials and studies, the availability of data, and regulatory submissions and responses and potential approvals and other matters; expectations with respect to the commercialization of Evrysdi under PTC's SMA collaboration; expectations with respect to the commercialization of Tegsedi and Waylivra; significant business effects, including the effects of industry, market, economic, political or regulatory conditions; changes in tax and other laws, regulations, rates and policies; the eligible patient base and commercial potential of PTC's products and product candidates; PTC's scientific approach and general development progress; PTC's ability to satisfy its obligations under the terms of its lease agreements; the sufficiency of PTC's cash resources and its ability to obtain adequate financing in the future for its foreseeable and unforeseeable operating expenses and capital expenditures; and the factors discussed in the "Risk Factors" section of PTC's Annual Report on Form 10-K, as well as any updates to these risk factors filed from time to time in PTC's other filings with the SEC. You are urged to carefully consider all such factors. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. There are no guarantees that any product will receive or maintain regulatory approval in any territory, or prove to be commercially successful, including Sephience, Translarna, Emflaza, Upstaza, Kebilidi, Evrysdi, Tegsedi or Waylivra. The forward-looking statements contained herein represent PTC's views only as of the date of this presentation and PTC does not undertake or plan to update or revise any such forward-looking statements to reflect actual results or changes in plans, prospects, assumptions, estimates or projections, or other circumstances occurring after the date of this presentation except as required by law. Forward Looking Statements 3 |
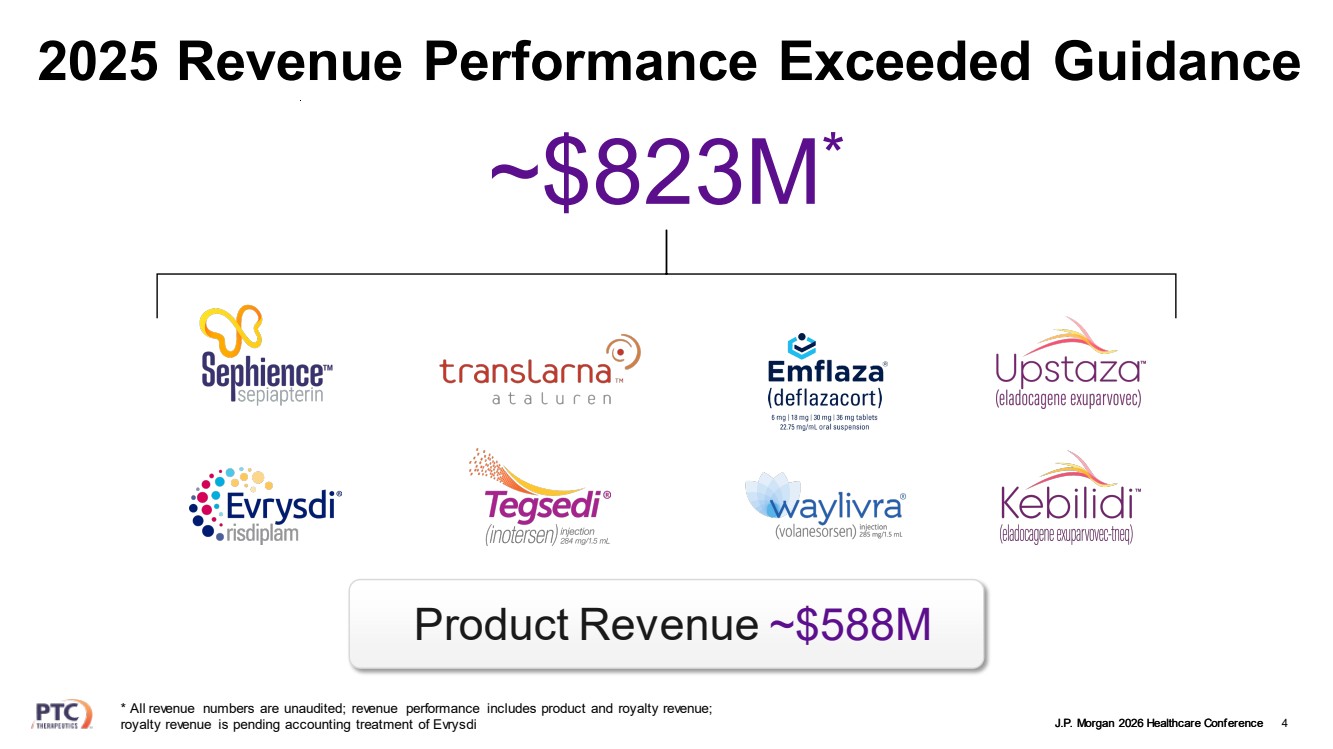
| J.P. Morgan 2026 Healthcare Conference 2025 Revenue Performance Exceeded Guidance 4 ~$823M* Product Revenue ~$588M * All revenue numbers are unaudited; revenue performance includes product and royalty revenue; royalty revenue is pending accounting treatment of Evrysdi |
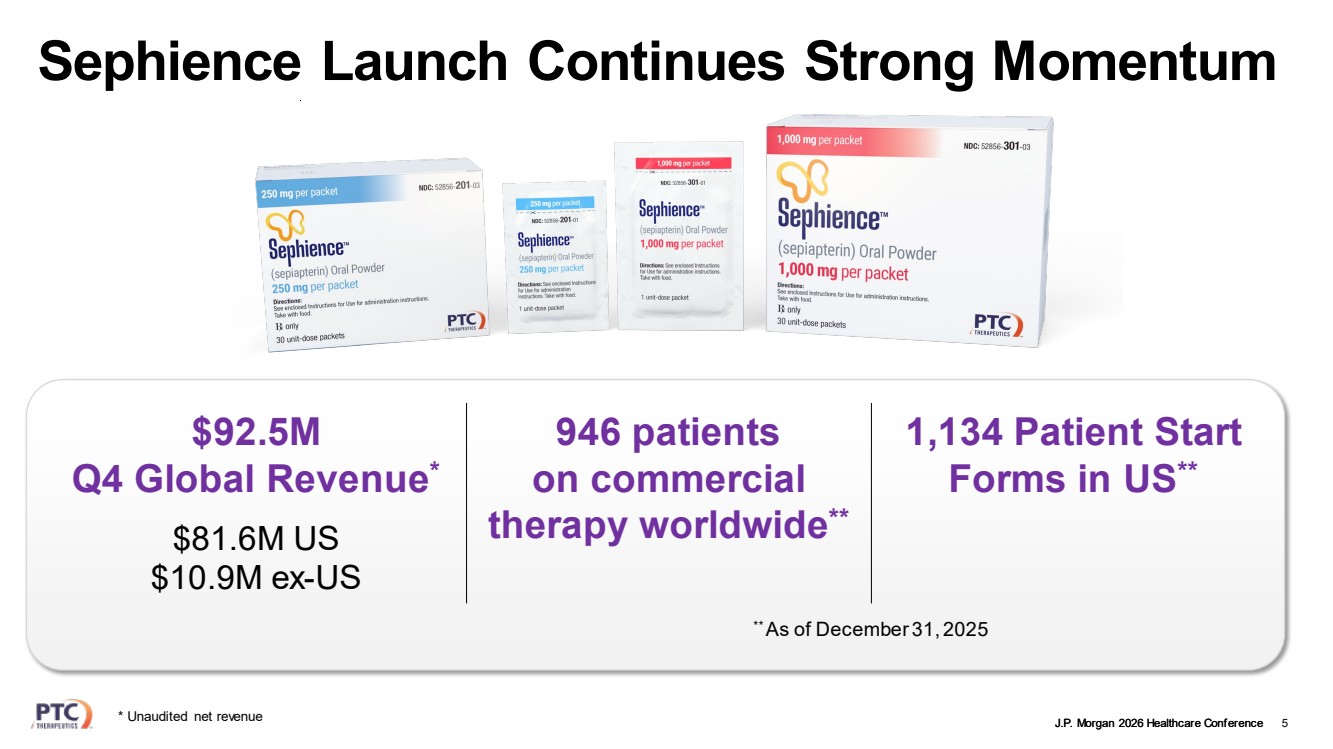
| J.P. Morgan 2026 Healthcare Conference Sephience Launch Continues Strong Momentum 5 $92.5M Q4 Global Revenue* $81.6M US $10.9M ex-US 946 patients on commercial therapy worldwide** 1,134 Patient Start Forms in US** ** As of December 31, 2025 * Unaudited net revenue |
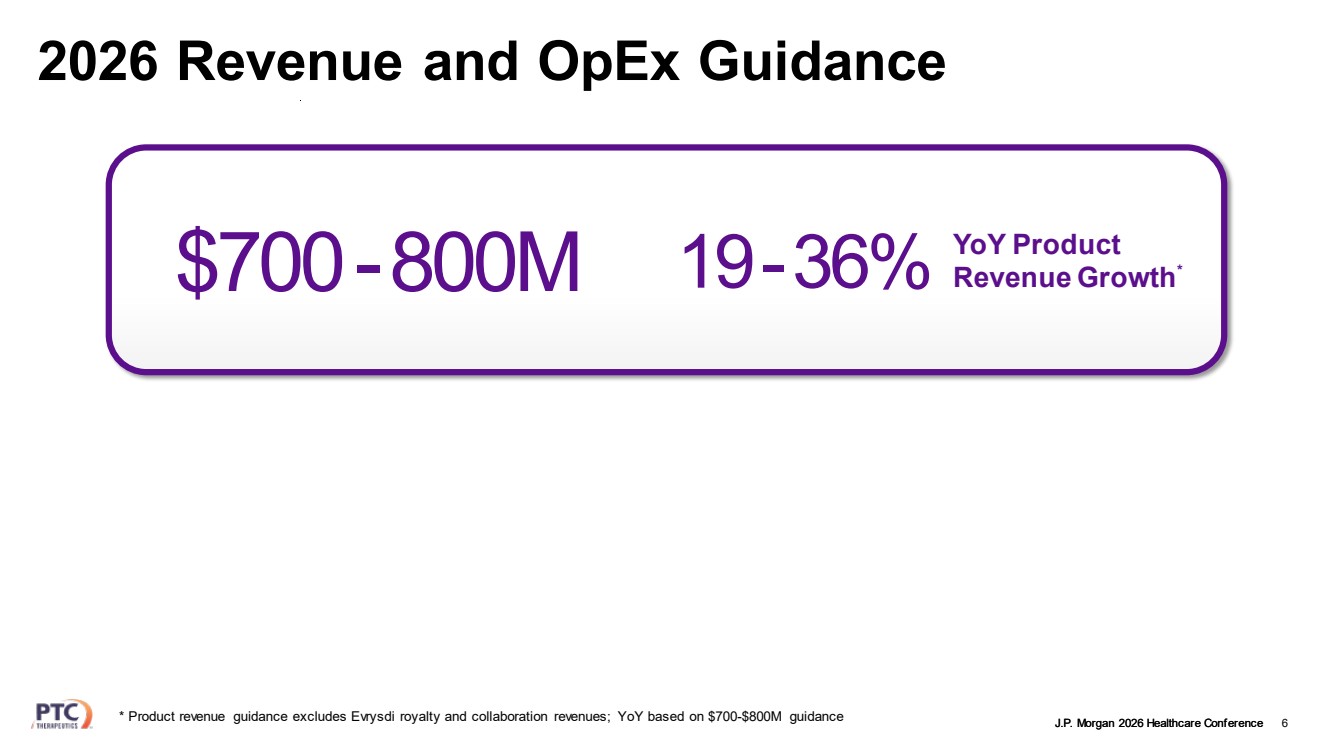
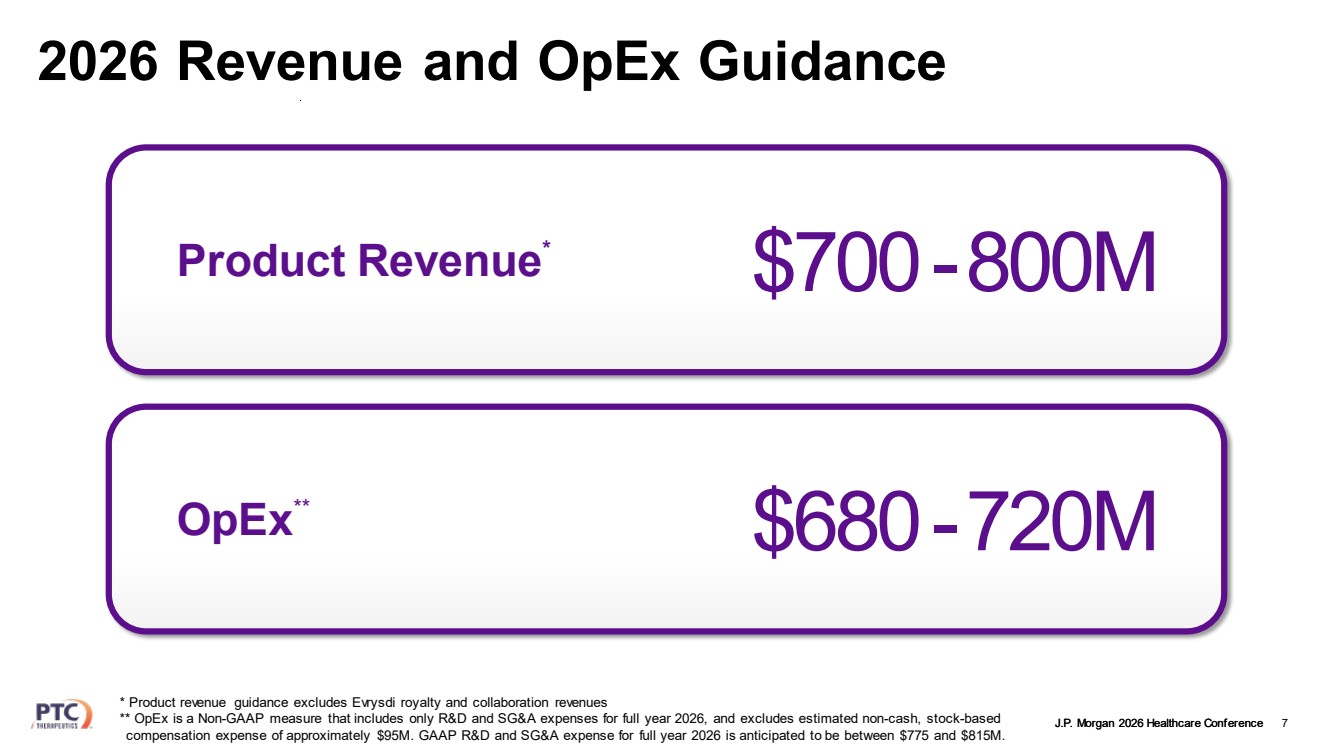
| J.P. Morgan 2026 Healthcare Conference 2026 Revenue and OpEx Guidance 6 YoY Product Revenue Growth $700 19-36% * -800M * Product revenue guidance excludes Evrysdi royalty and collaboration revenues; YoY based on $700-$800M guidance |
| J.P. Morgan 2026 Healthcare Conference 2026 Revenue and OpEx Guidance 7 * Product revenue guidance excludes Evrysdi royalty and collaboration revenues ** OpEx is a Non-GAAP measure that includes only R&D and SG&A expenses for full year 2026, and excludes estimated non-cash, stock-based compensation expense of approximately $95M. GAAP R&D and SG&A expense for full year 2026 is anticipated to be between $775 and $815M. Product Revenue* $700-800M OpEx $680-720M ** |
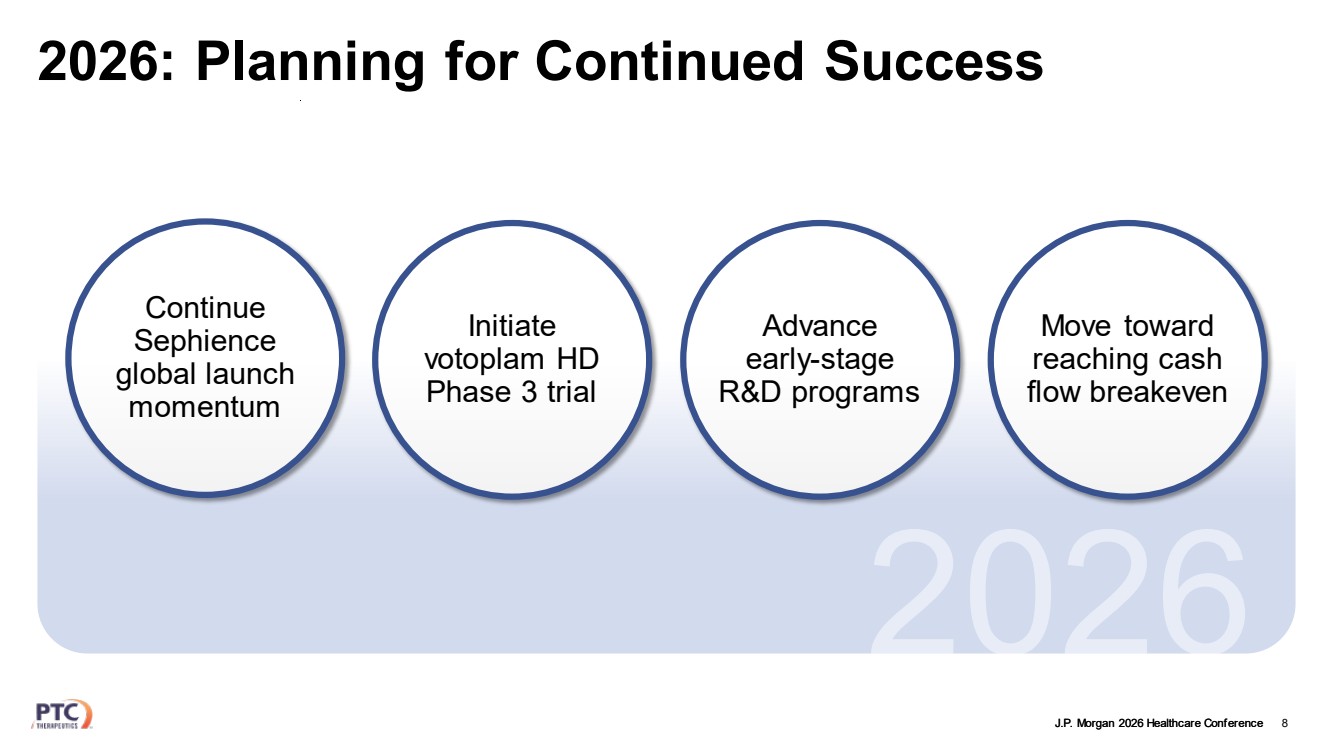
| J.P. Morgan 2026 Healthcare Conference 2026: Planning for Continued Success 8 Continue Sephience global launch momentum Move toward reaching cash flow breakeven Initiate votoplam HD Phase 3 trial Advance early-stage R&D programs |
| J.P. Morgan 2026 Healthcare Conference 9 Patient living w ith PKU Sephience PKU Program |
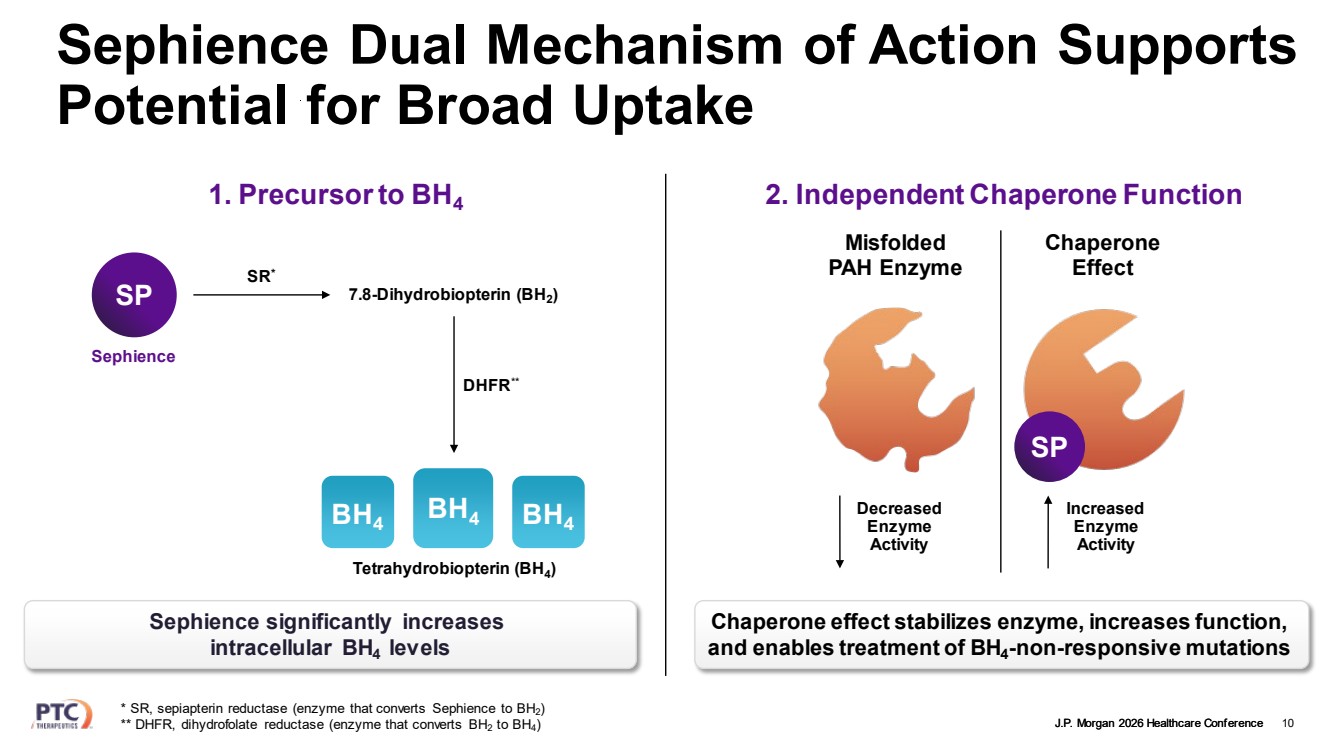
| J.P. Morgan 2026 Healthcare Conference Sephience Dual Mechanism of Action Supports Potential for Broad Uptake 10 * SR, sepiapterin reductase (enzyme that converts Sephience to BH2) ** DHFR, dihydrofolate reductase (enzyme that converts BH2 to BH4) 1. Precursor to BH4 SP Sephience 7.8-Dihydrobiopterin (BH2) Tetrahydrobiopterin (BH4) SR* DHFR** BH BH4 4 BH4 Sephience significantly increases intracellular BH4 levels 2. Independent Chaperone Function Misfolded PAH Enzyme Chaperone Effect Decreased Enzyme Activity Increased Enzyme Activity SP Chaperone effect stabilizes enzyme, increases function, and enables treatment of BH4-non-responsive mutations |
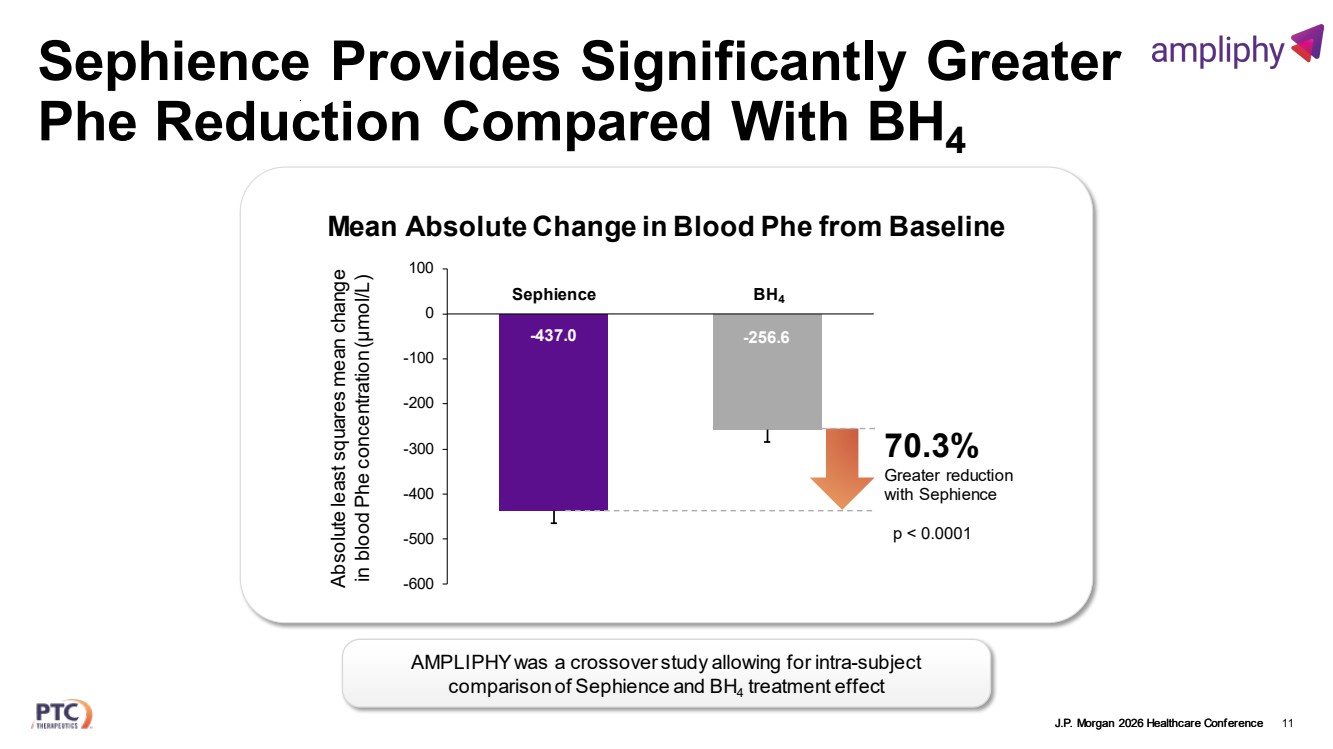
| J.P. Morgan 2026 Healthcare Conference Sephience Provides Significantly Greater Phe Reduction Compared With BH4 11 -437.0 -256.6 -600 -500 -400 -300 -200 -100 0 100 Absolute least squares mean change in blood Phe concentration (µmol/L) Sephience BH4 70.3% Greater reduction with Sephience p < 0.0001 Mean Absolute Change in Blood Phe from Baseline AMPLIPHY was a crossover study allowing for intra-subject comparison of Sephience and BH4 treatment effect |
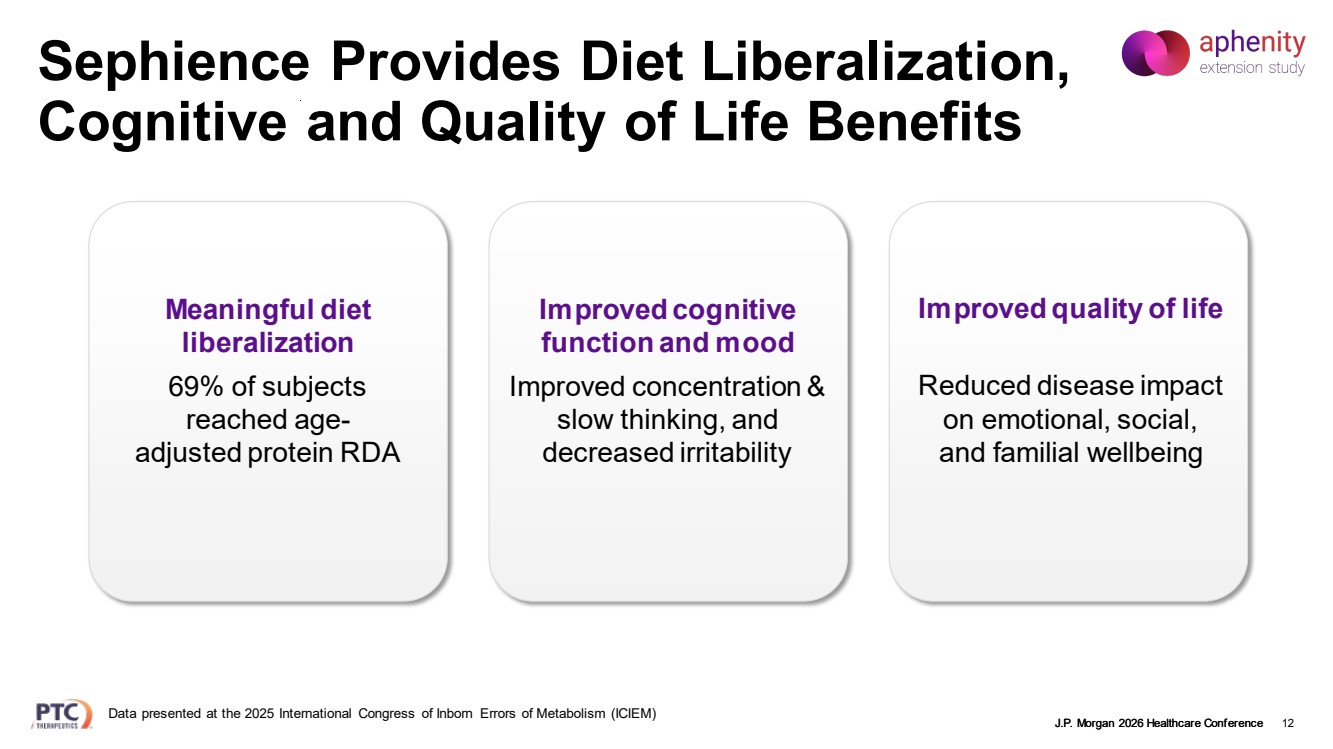
| J.P. Morgan 2026 Healthcare Conference Sephience Provides Diet Liberalization, Cognitive and Quality of Life Benefits 12 Improved cognitive function and mood Improved concentration & slow thinking, and decreased irritability Meaningful diet liberalization 69% of subjects reached age-adjusted protein RDA Improved quality of life Reduced disease impact on emotional, social, and familial wellbeing Data presented at the 2025 International Congress of Inborn Errors of Metabolism (ICIEM) |
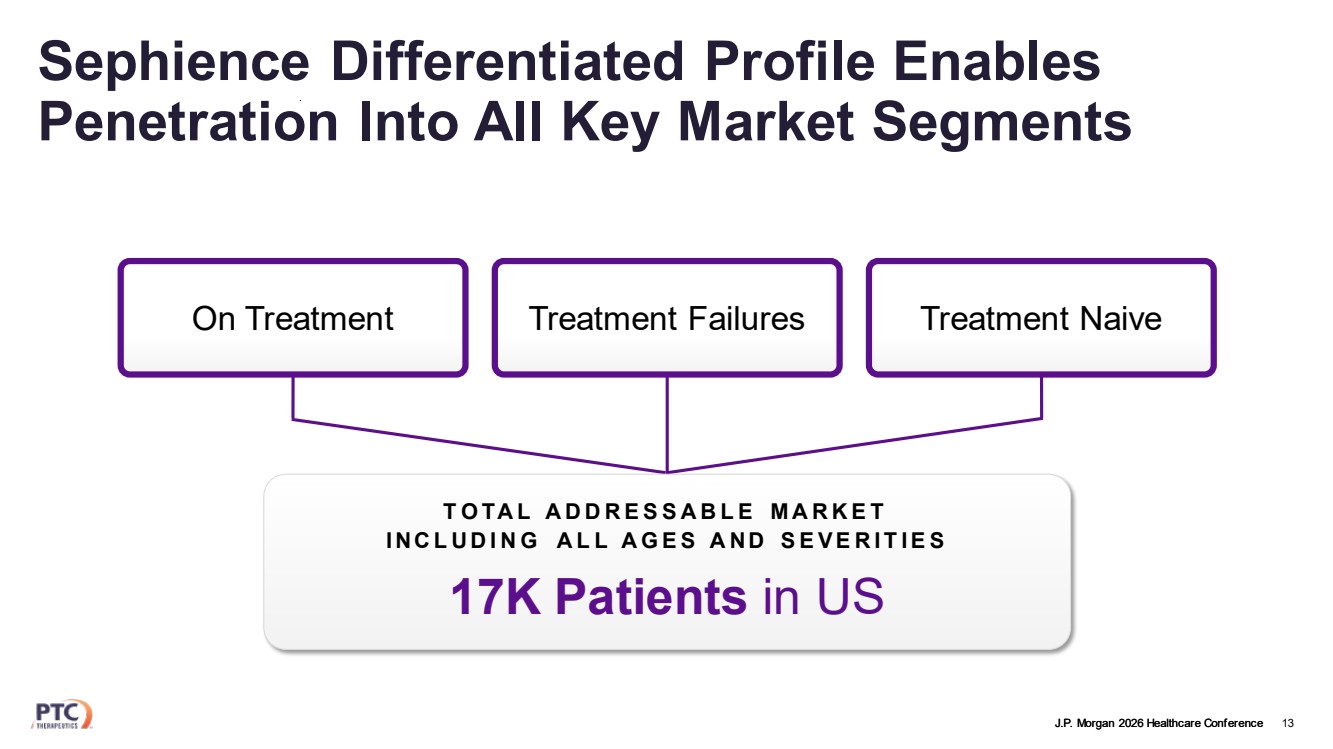
| J.P. Morgan 2026 Healthcare Conference Sephience Differentiated Profile Enables Penetration Into All Key Market Segments 13 On Treatment Treatment Failures Treatment Naive 17K Patients in US TOTAL ADDRESSABLE MARKET INCLUDING ALL AGES AND SEVERITIES |
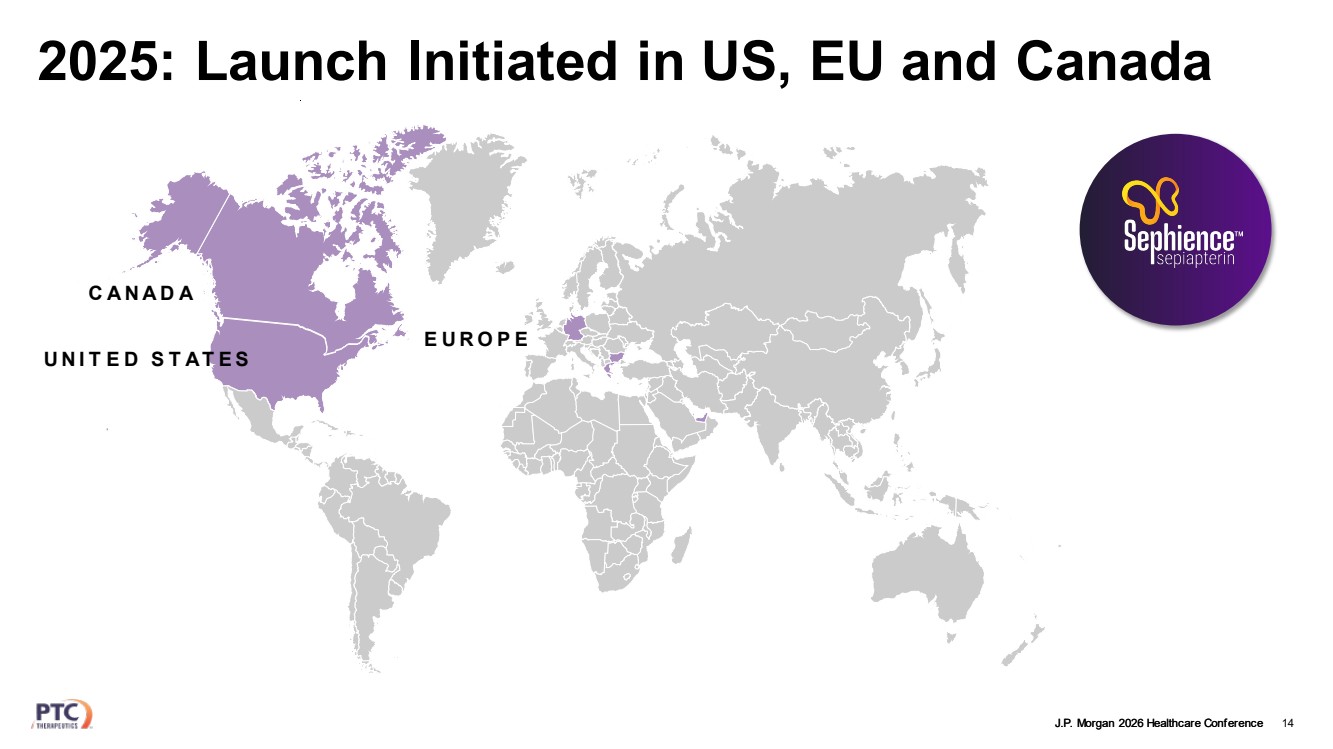
| J.P. Morgan 2026 Healthcare Conference 2025: Launch Initiated in US, EU and Canada 14 UNIT ED STATES CANADA EUROPE |
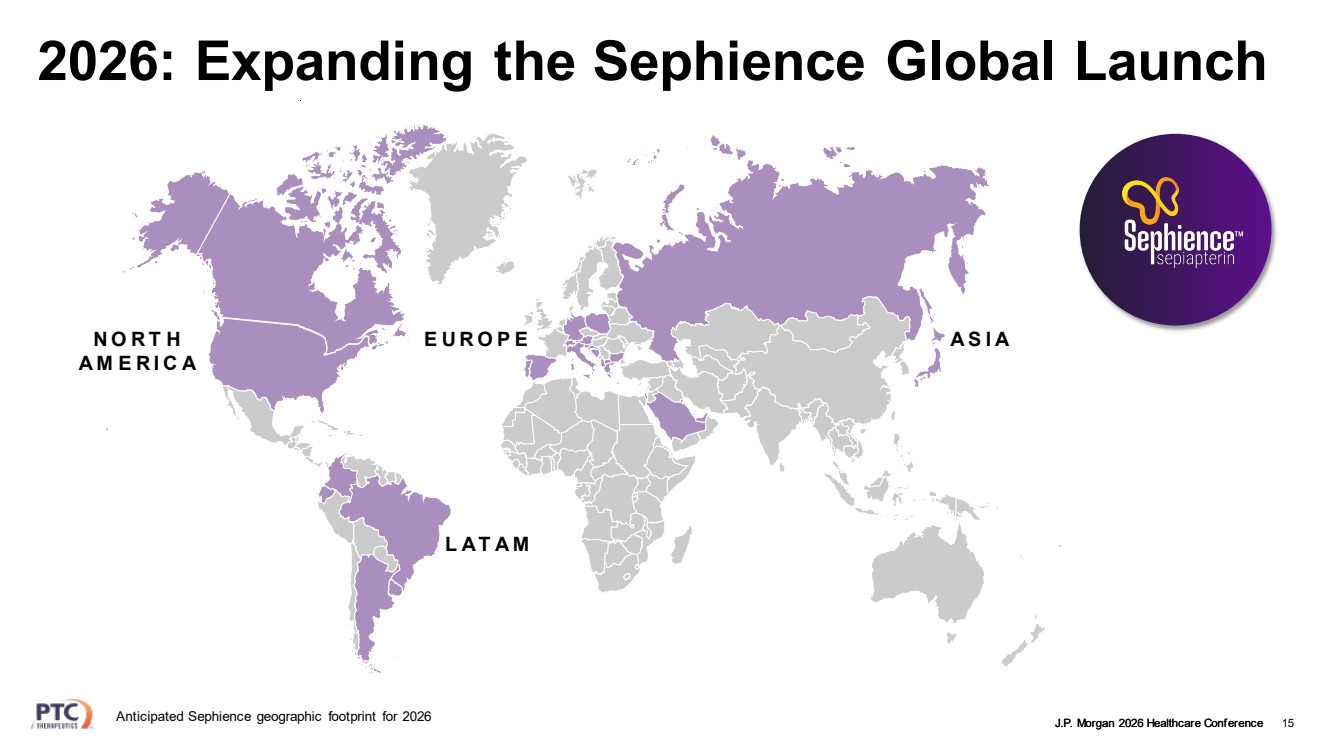
| J.P. Morgan 2026 Healthcare Conference 15 2026: Expanding the Sephience Global Launch NORTH AMERICA EUROPE ASIA LATAM Anticipated Sephience geographic footprint for 2026 |
| J.P. Morgan 2026 Healthcare Conference 16 Votoplam Huntington’s Disease Program Patient living w ith HD |
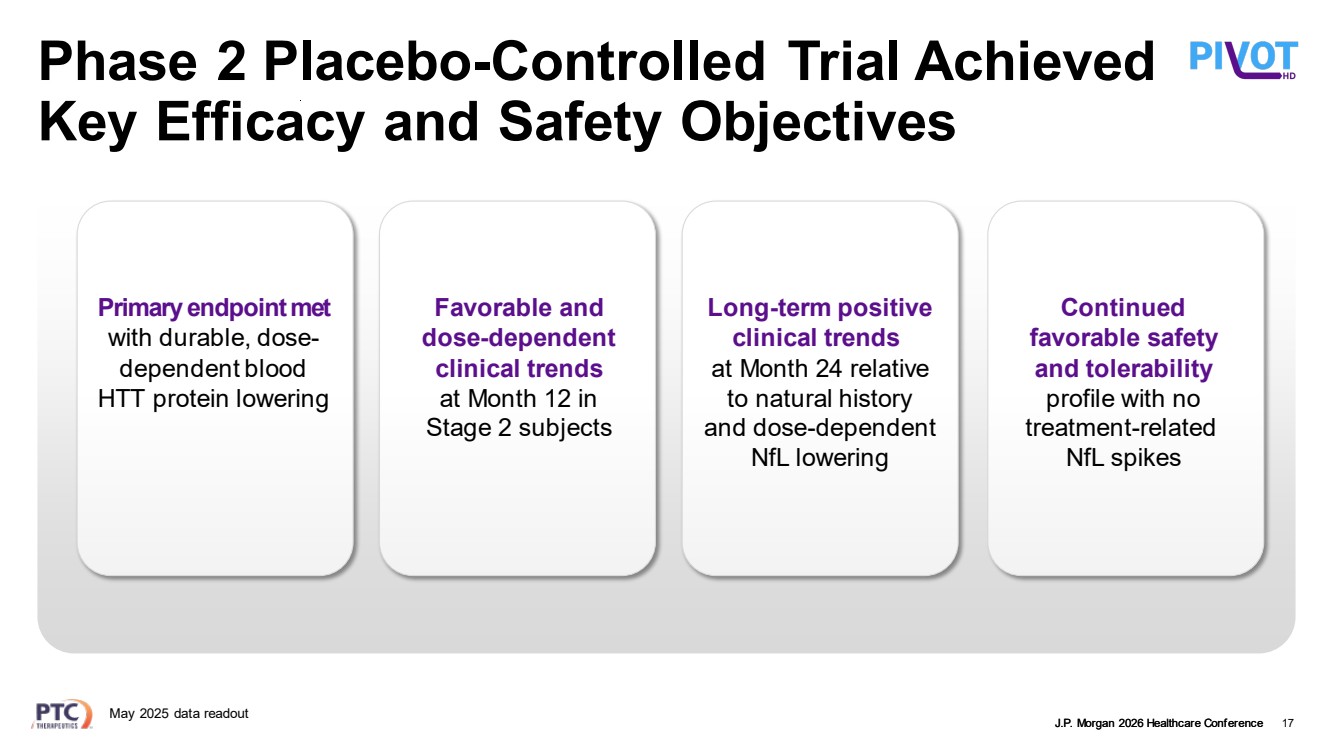
| J.P. Morgan 2026 Healthcare Conference Phase 2 Placebo-Controlled Trial Achieved Key Efficacy and Safety Objectives 17 Long-term positive clinical trends at Month 24 relative to natural history and dose-dependent NfL lowering Continued favorable safety and tolerability profile with no treatment-related NfL spikes Primary endpoint met with durable, dose-dependent blood HTT protein lowering Favorable and dose-dependent clinical trends at Month 12 in Stage 2 subjects May 2025 data readout |
| J.P. Morgan 2026 Healthcare Conference Votoplam Phase 3 Trial Expected to be Initiated by Novartis in H1 2026 18 INVEST-HD Global Phase 3 Study • 3:2 randomization of votoplam: placebo • Target enrollment: ~770 participants in >30 countries • Primary endpoint: Change in cUHDRS from Baseline up to 36 months • Interim analysis planned for efficacy and futility |
| J.P. Morgan 2026 Healthcare Conference 19 Vatiquinone Friedreich’s Ataxia Program Patient living w ith FA |
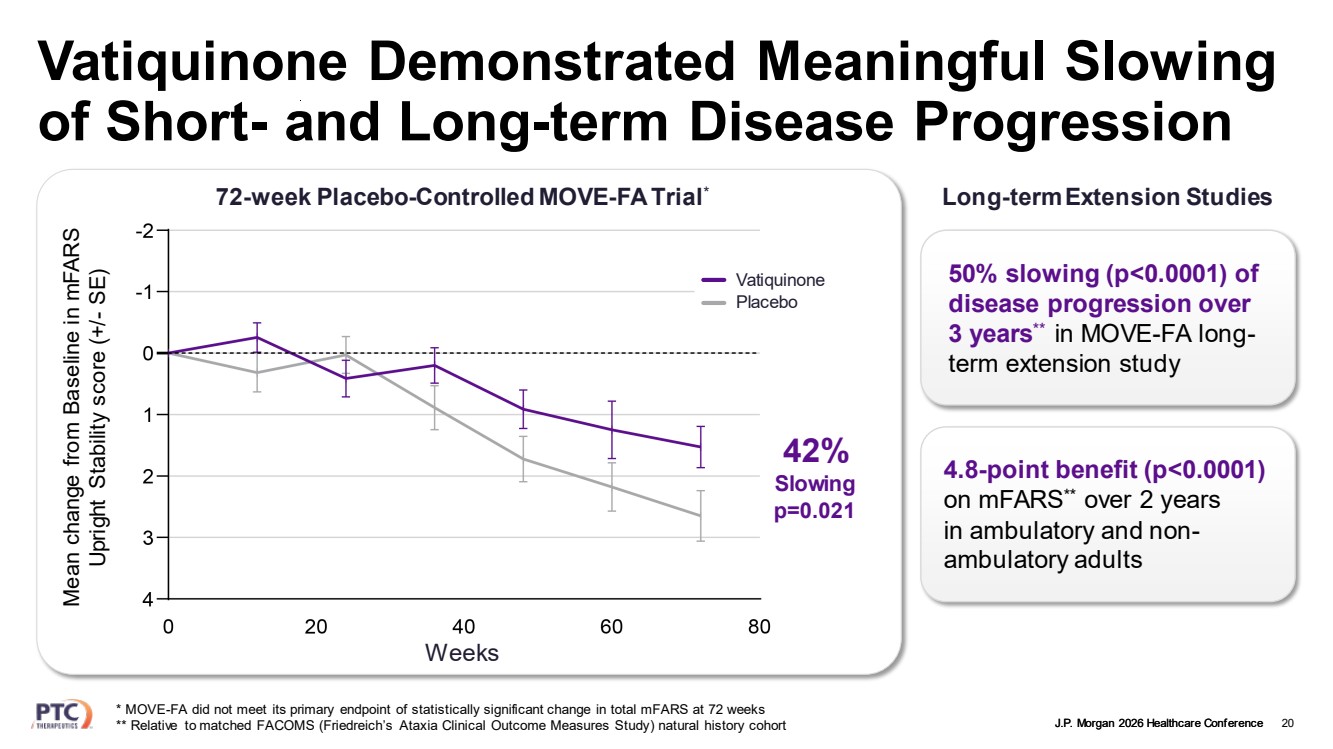
| J.P. Morgan 2026 Healthcare Conference Vatiquinone Demonstrated Meaningful Slowing of Short- and Long-term Disease Progression 20 Vatiquinone Placebo Long-term Extension Studies 4.8-point benefit (p<0.0001) on mFARS** over 2 years in ambulatory and non-ambulatory adults 50% slowing (p<0.0001) of disease progression over 3 years** in MOVE-FA long-term extension study * MOVE-FA did not meet its primary endpoint of statistically significant change in total mFARS at 72 weeks ** Relative to matched FACOMS (Friedreich’s Ataxia Clinical Outcome Measures Study) natural history cohort 72-week Placebo-Controlled MOVE-FA Trial* Weeks Mean change from Baseline in mFARS Upright Stability score (+/- SE) 42% Slowing p=0.021 |
| J.P. Morgan 2026 Healthcare Conference 21 Innovative Research Platforms |
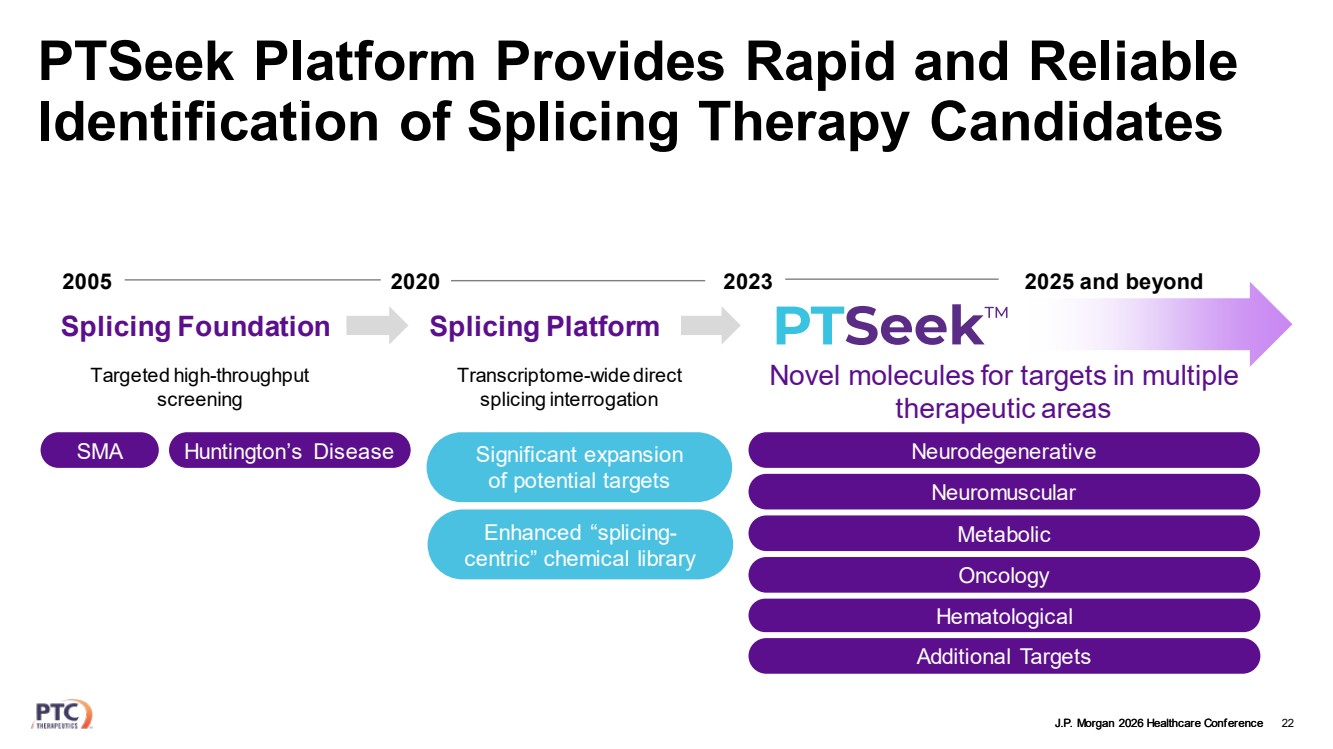
| J.P. Morgan 2026 Healthcare Conference PTSeek Platform Provides Rapid and Reliable Identification of Splicing Therapy Candidates 22 2005 2020 2023 Splicing Foundation Splicing Platform 2025 and beyond Targeted high-throughput screening Transcriptome-wide direct splicing interrogation Novel molecules for targets in multiple therapeutic areas SMA Huntington’s Disease Enhanced “splicing-centric” chemical library Significant expansion of potential targets Neurodegenerative Hematological Additional Targets Metabolic Oncology Neuromuscular |
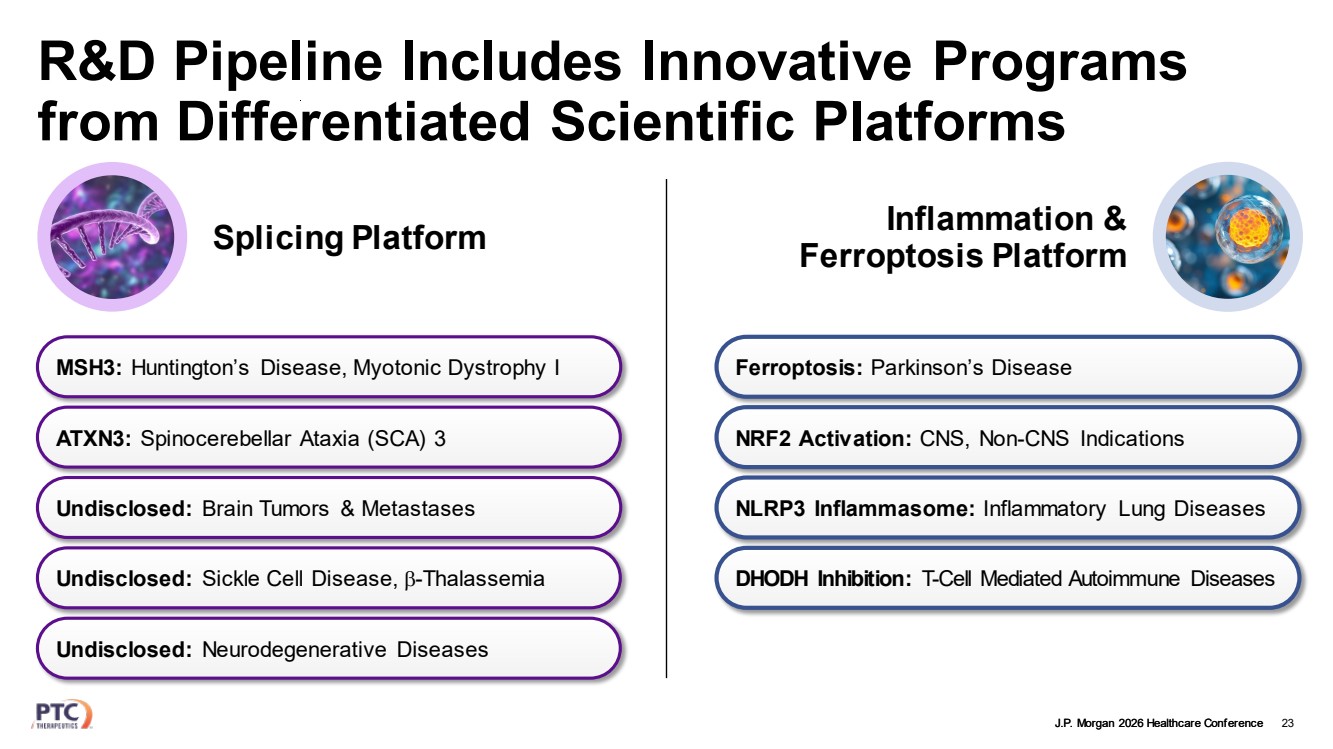
| J.P. Morgan 2026 Healthcare Conference R&D Pipeline Includes Innovative Programs from Differentiated Scientific Platforms 23 MSH3: Huntington’s Disease, Myotonic Dystrophy I Undisclosed: Neurodegenerative Diseases Undisclosed: Brain Tumors & Metastases Undisclosed: Sickle Cell Disease, β-Thalassemia ATXN3: Spinocerebellar Ataxia (SCA) 3 Splicing Platform Inflammation & Ferroptosis Platform Ferroptosis: Parkinson’s Disease NLRP3 Inflammasome: Inflammatory Lung Diseases DHODH Inhibition: T-Cell Mediated Autoimmune Diseases NRF2 Activation: CNS, Non-CNS Indications |
| J.P. Morgan 2026 Healthcare Conference Well Positioned for Success in 2026 & Beyond 24 Strong Financial Position Robust Global Commercial Engine Innovative R&D Platforms |
| J.P. Morgan 2026 Healthcare Conference PTC Therapeutics 2026 Matthew B. Klein, MD CEO |