

PLUS THERAPEUTICS C Corporate orporate Up Update date Ja January nuary 2 2026 026 P Power ower a and nd p precision recision iin n c cancer ancer rradiotherapeutics adiotherapeutics N NASDAQ: ASDAQ: P PSTV STV

Cautionary Note Regarding Forward Looking Statements This presentation contains statements that may be deemed “forward-looking statements” within the meaning of U.S. securities laws, including statements regarding clinical trials, expected operations and upcoming developments. All statements in this presentation other than statements of historical fact are forward-looking statements. These forward-looking statements may be identified by future verbs, as well as terms such as “potential,” “anticipating,” “planning”, “projecting”, “expecting” and similar expressions or the negatives thereof. Such statements are based upon certain assumptions and assessments made by management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate. These statements include, without limitation, statements regarding the following: the potential promise of REYOBIQ including the ability of REYOBIQ to safely and effectively deliver radiation directly to the tumor at high doses; expectations as to the Company’s future performance including the next steps in developing the Company’s current assets, which include the Company’s nanomedicine platform and commercializing CNSide, REYOBIQ and 188RNL-BAM; the Company’s manufacturing capabilities and commercial scalability of the Company’s product candidates; the Company’s clinical trials including statements regarding the timing and characteristics of the ReSPECT-GBM, ReSPECT-LM and ReSPECT-PBC clinical trials; possible negative effects of REYOBIQ ; the continued evaluation of REYOBIQ , including through evaluations in additional patient cohorts; the intended functions of the Company’s platform and expected benefits from such functions; development and utility of the CNSide leptomeningeal metastases diagnostic test; and upcoming catalysts and cash runway. The forward-looking statements included in this presentation could differ materially from those expressed or implied by these forward-looking statements because of risks, uncertainties, and other factors that include, but are not limited to, the following: the early stage of the Company’s product candidates and therapies; the results of the Company’s research and development activities, including uncertainties relating to the clinical trials of its product candidates and therapies; the Company’s liquidity and capital resources and its ability to raise additional cash; to fund its operations in the near-term and long-term, on terms acceptable to us or at all; the outcome of the Company’s partnering/licensing efforts; risks associated with laws or regulatory requirements applicable to the Company, including the ability to come into compliance with The Nasdaq Capital Market listing requirements; market conditions; product performance; litigation or potential litigation; and competition within the cancer diagnostics and therapeutics field; ability to develop and protect proprietary intellectual property or obtain licenses to intellectual property developed by others on commercially reasonable and competitive terms; manufacturing supply chain risks; and material security breach or cybersecurity attack affecting the Company’s operations or property. This list of risks, uncertainties, and other factors is not complete. Plus Therapeutics discusses some of these matters more fully, as well as certain risk factors that could affect Plus Therapeutics’ business, financial condition, results of operations, and prospects, in its reports filed with the SEC, including Plus Therapeutics’ annual report on Form 10-K for the fiscal year ended December 31, 2024, quarterly reports on Form 10-Q, and current reports on Form 8-K. These filings are available for review through the SEC’s website at www.sec.gov. Any or all forward-looking statements Plus Therapeutics makes may turn out to be wrong and can be affected by inaccurate assumptions Plus Therapeutics might make or by known or unknown risks, uncertainties, and other factors, including those identified in this presentation. Accordingly, you should not place undue reliance on the forward-looking statements made in this presentation, which speak only as of its date. The Company assumes no responsibility to update or revise any forward-looking statements to reflect events, trends or circumstances after the date they are made unless the Company has an obligation under U.S. federal securities laws to do so. 2 Plus Therapeutics, Inc. NASDAQ: PSTV

Execut > Clava ive Summary Summary ve THERAPEUTICS obisbemeda injection Contact us at investor@plustherapeutics.com Contact us at investor@plustherapeutics.com Basrect evn CNSIde
Plus Therapeutics (PSTV) Overview Best-In-Class Diagnostic for CNS Mets - CNSide®: >$6B TAM + U.S. commercial relaunch Q3 2025 – Projected 500K patients / year + Announced first of planned national coverage agreements, with UnitedHealthcare and Humana + Previously commercialized, ordered by 120 U.S. institutions + Data and analytics for personalized therapeutic approach and predictive models First-In-Class Radiotherapeutic for CNS Cancers: >$16B TAM + Safe, precision therapy with up to 20x radiation delivery vs. standard of care + FDA Fast Track & Orphan Designations facilitate expeditious approval process Multiple Mid-Stage ReSPECT Clinical Trials of REYOBIQ + First-in-class drug treating leptomeningeal mets (LM), Dosing/Phase 2 trial enrolling + Completing recurrent glioblastoma (rGBM) Phase 2 trial in 2025 Glossary: rGBM – recurrent glioblastoma Dx – diagnostic TAM – total addressable market CTC – circulating tumor cells LM – leptomeningeal metastases mets – metastases BBB – blood brain barrier CSF – cerebral spinal fluid PBC – pediatric brain cancer mOS – median overall survival MTD – maximum tolerated dose 4 Plus Therapeutics, Inc. NASDAQ: PSTV Tx – radiotherapeutics CNS – central nervous system SOC – standard of care

CLINICAL TRIALS obisbemeda injection Company Company Overview Overview ve PLUS THERAPEUTICS Contact us at investor@plustherapeutics.com Contact us at investor@plustherapeutics.com Basrect evn CNSIde
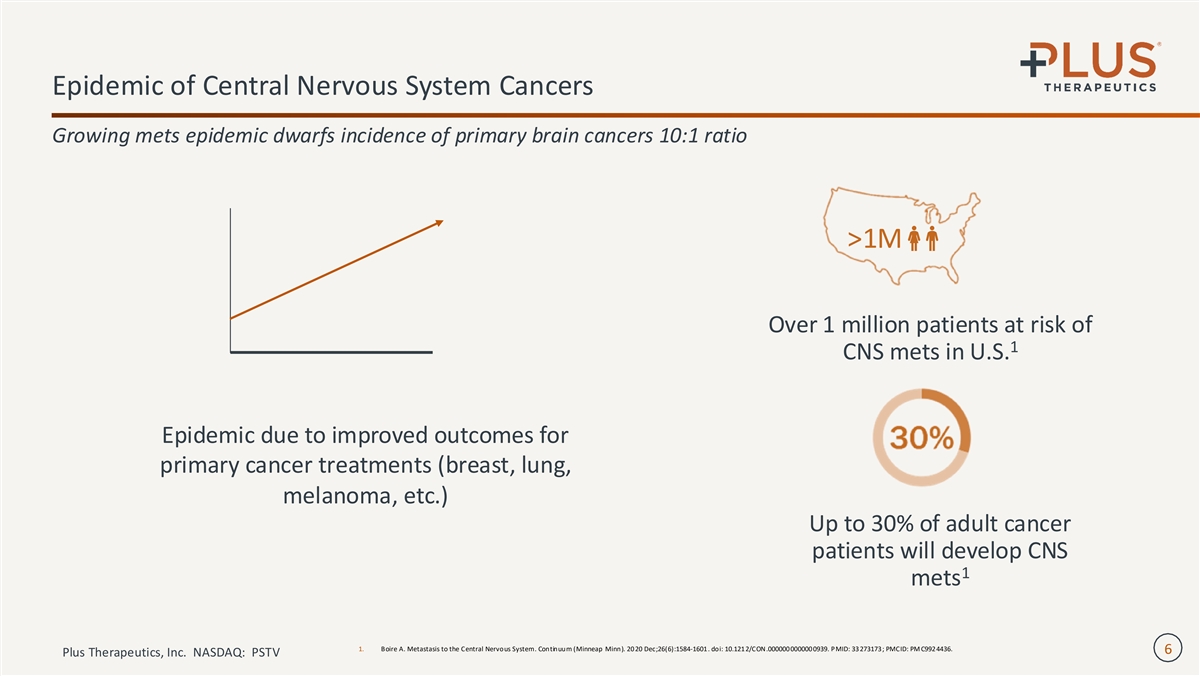
Epidemic of Central Nervous System Cancers Growing mets epidemic dwarfs incidence of primary brain cancers 10:1 ratio >1M Over 1 million patients at risk of 1 CNS mets in U.S. Epidemic due to improved outcomes for primary cancer treatments (breast, lung, melanoma, etc.) Up to 30% of adult cancer patients will develop CNS 1 mets 1. Boire A. Metastasis to the Central Nervous System. Continuum (Minneap Minn). 20 20 Dec;26(6):1584-1601 . doi: 10.121 2/CON.000000 000000 0939. PMID: 33 273173 ; PMCID: PM C992 4436. 6 Plus Therapeutics, Inc. NASDAQ: PSTV
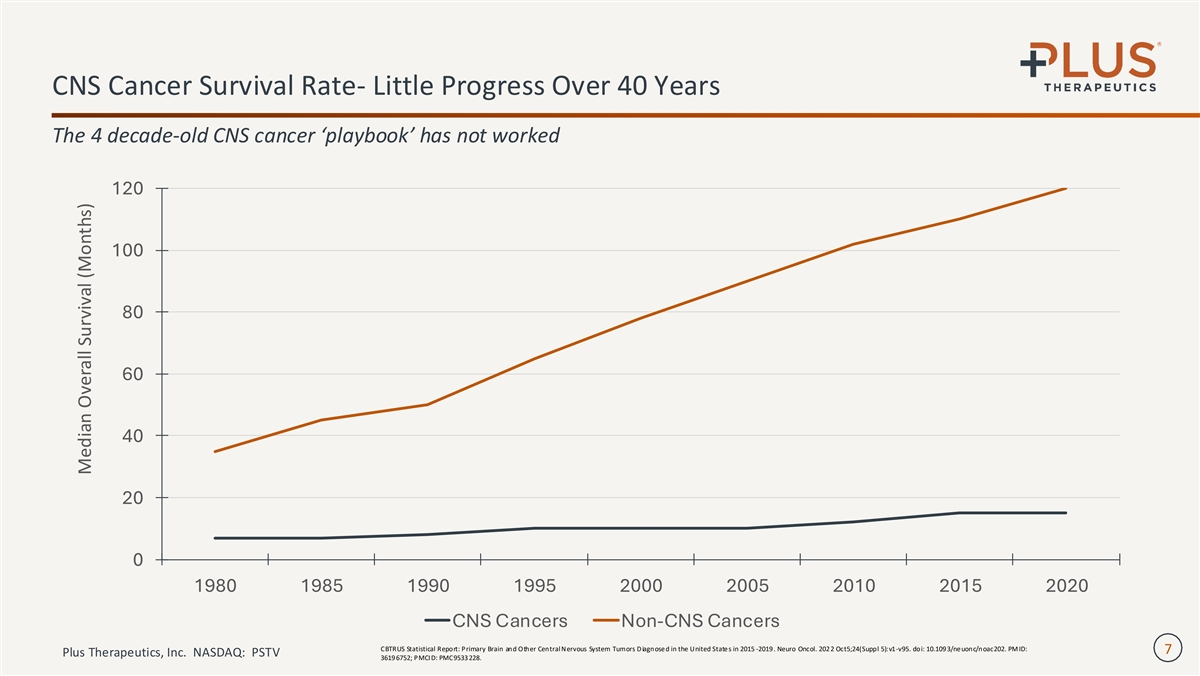
CNS Cancer Survival Rate- Little Progress Over 40 Years The 4 decade-old CNS cancer ‘playbook’ has not worked 120 100 80 60 40 20 0 1980 1985 1990 1995 2000 2005 2010 2015 2020 CNS Cancers Non-CNS Cancers CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors D iagnose d in the United State s in 2015 -2019 . Neuro Oncol. 202 2 Oct5;24(Suppl 5):v1-v95. doi: 10.109 3/ne uonc/noac202. PM ID: 7 Plus Therapeutics, Inc. NASDAQ: PSTV 3619 6752; PMCI D: PMC9533 228. Median Overall Survival (Months)
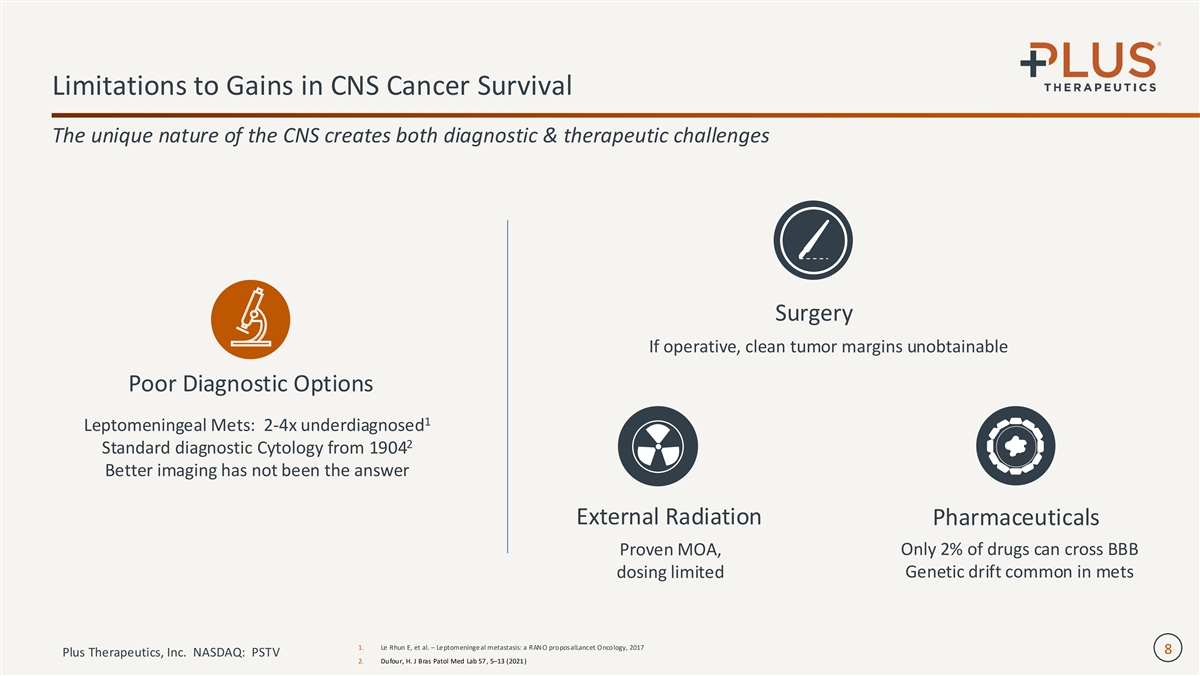
Limitations to Gains in CNS Cancer Survival The unique nature of the CNS creates both diagnostic & therapeutic challenges Surgery If operative, clean tumor margins unobtainable Poor Diagnostic Options 1 Leptomeningeal Mets: 2-4x underdiagnosed 2 Standard diagnostic Cytology from 1904 Better imaging has not been the answer External Radiation Pharmaceuticals Proven MOA, Only 2% of drugs can cross BBB dosing limited Genetic drift common in mets 1. Le Rhun E, et al. – Le ptomeninge al metastasis: a RANO proposalLancet Oncology, 2017 8 Plus Therapeutics, Inc. NASDAQ: PSTV 2. Dufour, H. J Bras Patol Med Lab 57 , 5–13 (2021 )
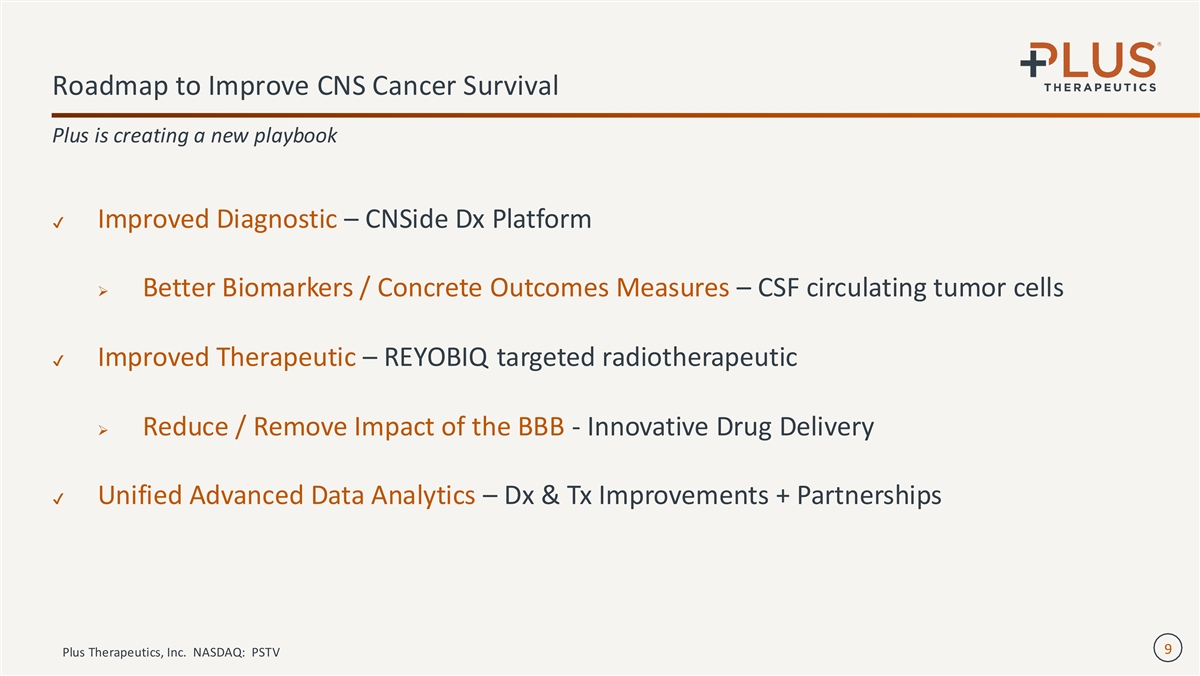
Roadmap to Improve CNS Cancer Survival Plus is creating a new playbook ✔ Improved Diagnostic – CNSide Dx Platform ➢ Better Biomarkers / Concrete Outcomes Measures – CSF circulating tumor cells ✔ Improved Therapeutic – REYOBIQ targeted radiotherapeutic ➢ Reduce / Remove Impact of the BBB - Innovative Drug Delivery ✔ Unified Advanced Data Analytics – Dx & Tx Improvements + Partnerships 9 Plus Therapeutics, Inc. NASDAQ: PSTV
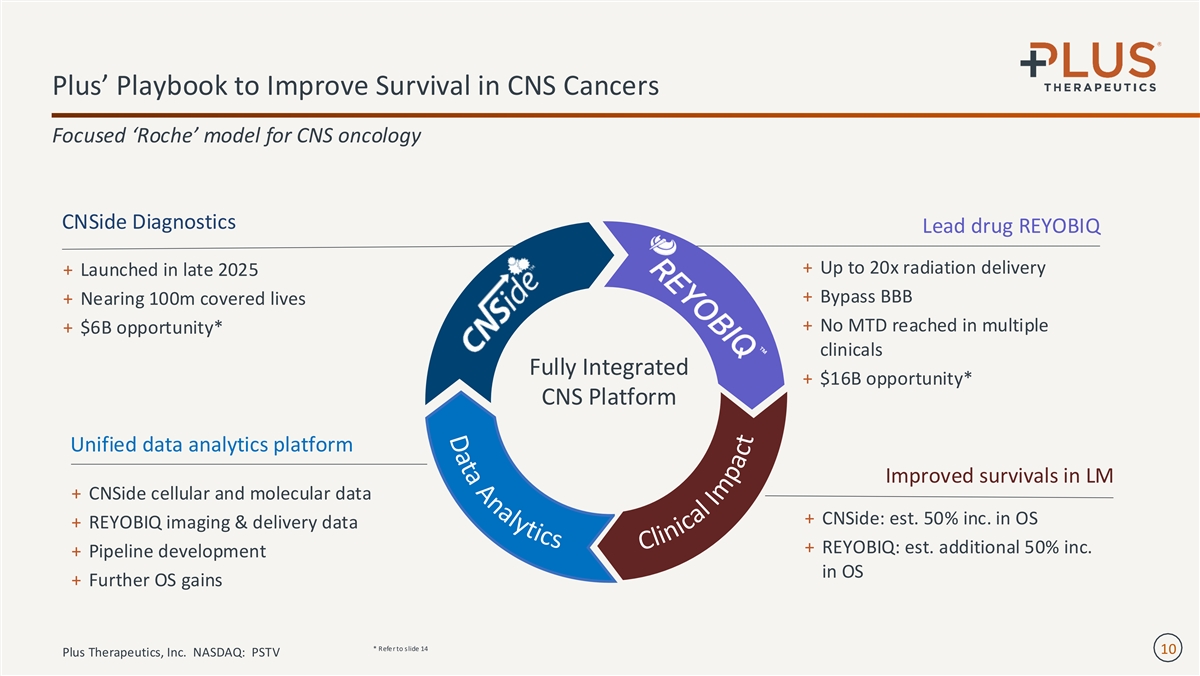
D a t a A n a l y t i c s Plus’ Playbook to Improve Survival in CNS Cancers Focused ‘Roche’ model for CNS oncology CNSide Diagnostics Lead drug REYOBIQ + Up to 20x radiation delivery + Launched in late 2025 + Bypass BBB + Nearing 100m covered lives + No MTD reached in multiple + $6B opportunity* clinicals Fully Integrated + $16B opportunity* CNS Platform Unified data analytics platform Improved survivals in LM + CNSide cellular and molecular data + CNSide: est. 50% inc. in OS + REYOBIQ imaging & delivery data + REYOBIQ: est. additional 50% inc. + Pipeline development in OS + Further OS gains * Refe r to slide 14 10 Plus Therapeutics, Inc. NASDAQ: PSTV C l i n i c a l I m p a c t
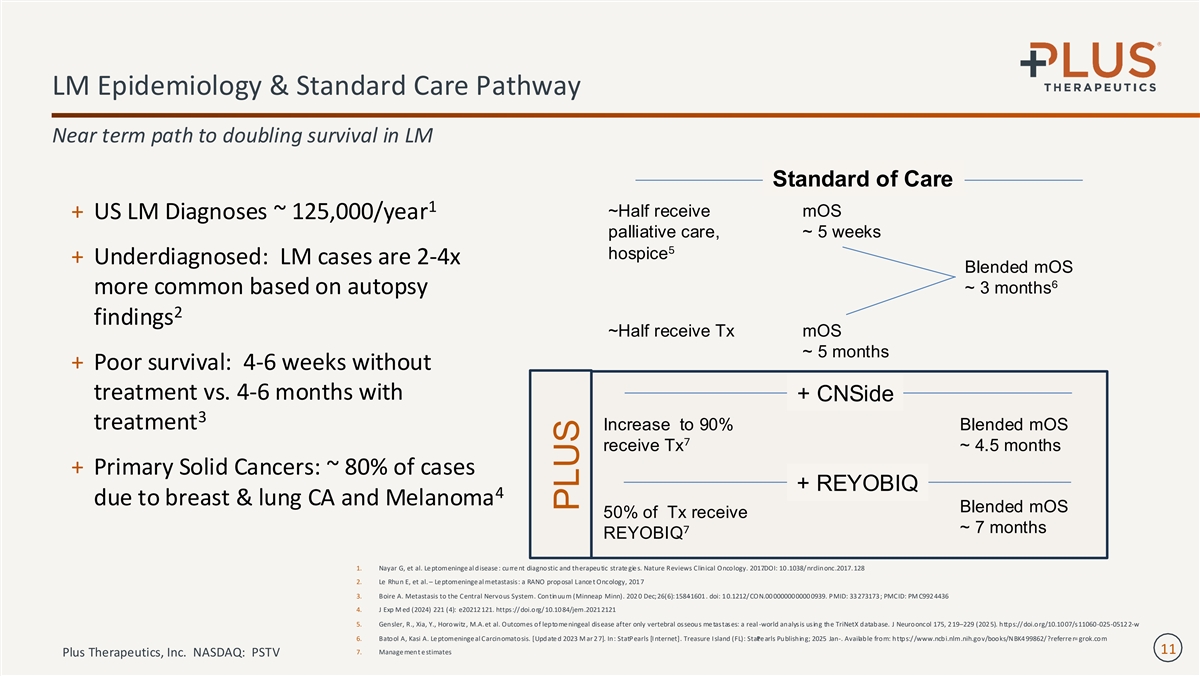
LM Epidemiology & Standard Care Pathway Near term path to doubling survival in LM Standard of Care 1 ~Half receive mOS + US LM Diagnoses ~ 125,000/year palliative care, ~ 5 weeks 5 hospice + Underdiagnosed: LM cases are 2-4x Blended mOS 6 more common based on autopsy ~ 3 months 2 findings ~Half receive Tx mOS ~ 5 months + Poor survival: 4-6 weeks without treatment vs. 4-6 months with + CNSide 3 treatment Increase to 90% Blended mOS 7 receive Tx ~ 4.5 months + Primary Solid Cancers: ~ 80% of cases + REYOBIQ 4 due to breast & lung CA and Melanoma Blended mOS 50% of Tx receive 7 ~ 7 months REYOBIQ 1. Nayar G, et al. Le ptomeninge al disease : curre nt diagnostic and therapeutic strate gie s. Nature Reviews Clinical Oncology. 2017 .DOI: 10 .1038/nrclinonc.2017. 128 2. Le Rhun E, et al. – Le ptomeninge al metastasis: a RANO proposal Lance t Oncology, 201 7 3. Boire A. Metastasis to the Central Nervous System. Continuum (Minneap Minn). 202 0 Dec;26(6):1584 -1601 . doi: 1 0.1212/CON.00 0000000000 0939. PMID: 33 273173 ; PMCID: PM C992 4436 4. J Exp M ed (2024) 221 (4): e20212 121. https:// doi.org/ 10.10 84/jem.2021 2121 5. Gensler, R., Xia, Y., Horowitz, M.A.et al. Outcomes of leptome ningeal disease after only vertebral osseous me tastases: a real -world analysis using the TriNetX database. J Neurooncol 175, 2 19–229 (202 5). https://doi.org/10.10 07/s11060-025-0512 2-w 6. Batool A, Kasi A. Le ptomeninge al Carcinomatosis. [Update d 2023 M ar 2 7]. In: StatPearls [I nternet]. Treasure I sland (FL): StaP te arls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK4 99862/?referre r= grok.com 11 7. Manage ment e stimates Plus Therapeutics, Inc. NASDAQ: PSTV PLUS
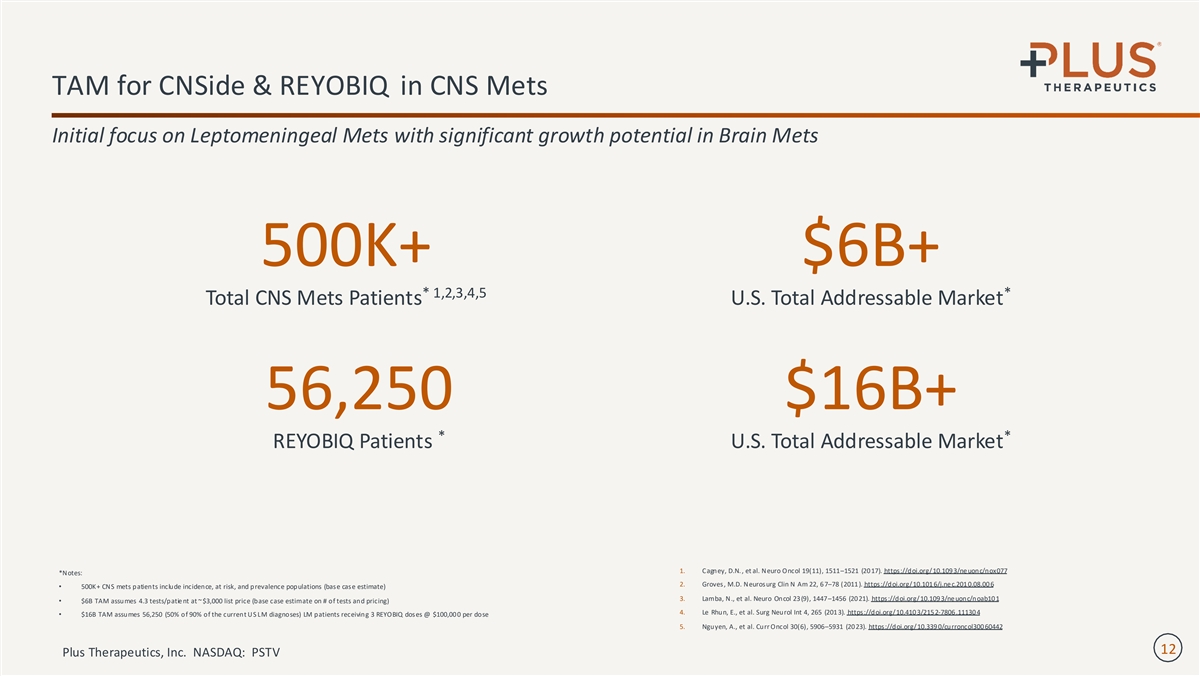
TAM for CNSide & REYOBIQ in CNS Mets Initial focus on Leptomeningeal Mets with significant growth potential in Brain Mets 500K+ $6B+ * 1,2,3,4,5 * Total CNS Mets Patients U.S. Total Addressable Market 56,250 $16B+ * * REYOBIQ Patients U.S. Total Addressable Market 1. Cagney, D.N. , et al. Neuro Oncol 19(11), 1511–1521 (20 17). https://doi.org/10.109 3/ne uonc/nox077 *Notes: 2. Groves, M.D. Neurosurg Clin N Am 22, 6 7–78 (2011 ). https://doi.org/10.101 6/j.ne c.201 0.08.00 6 • 500K+ CNS mets patients include incidence, at risk, and prevalence populations (base case estimate) 3. Lamba, N., et al. Neuro Oncol 23 (9), 1447–1456 (20 21). https://doi.org/10.109 3/ne uonc/noab10 1 • $6B TAM assumes 4.3 tests/patie nt at ~ $3,000 list price (base case estimate on # of tests and pricing) 4. Le Rhun, E., et al. Surg Neurol Int 4, 265 (201 3). https://doi.org/10.410 3/215 2-7806 .11130 4 • $16B TAM assumes 56,250 (50% of 90% of the current US LM diagnoses) LM patients receiving 3 REYOBIQ doses @ $100,00 0 per dose 5. Nguyen, A., et al. Curr Oncol 30(6), 5906–5931 (20 23). https://doi.org/10.339 0/curroncol300 60442 12 Plus Therapeutics, Inc. NASDAQ: PSTV
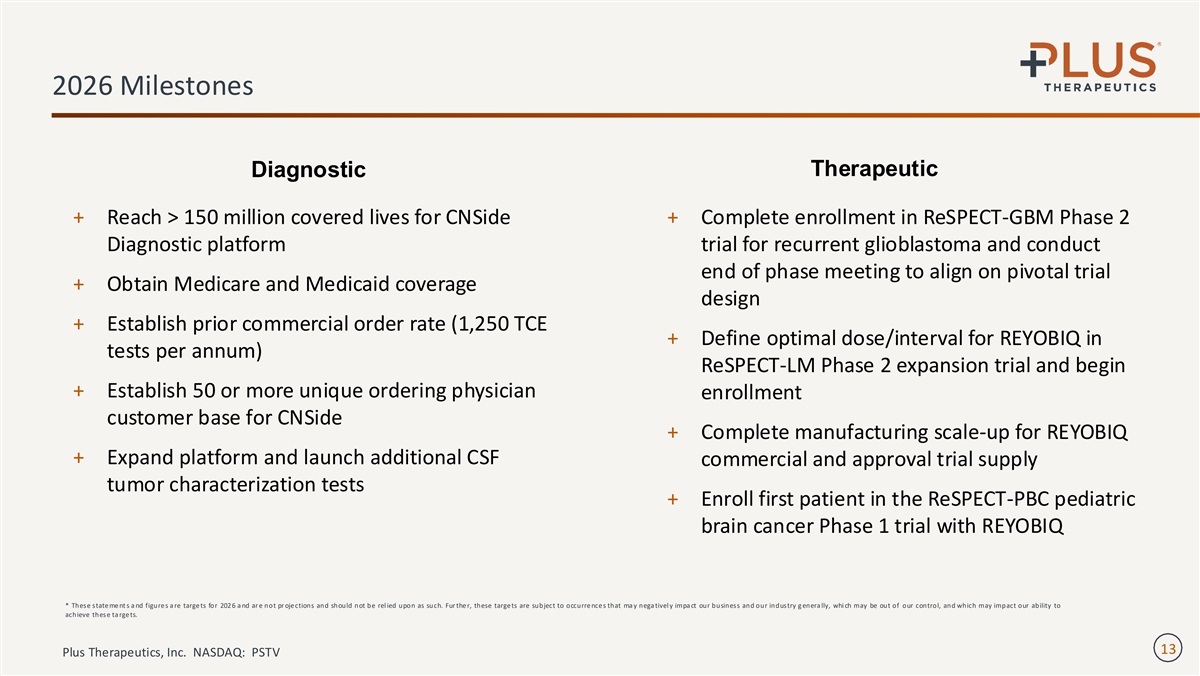
2026 Milestones Therapeutic Diagnostic + Reach > 150 million covered lives for CNSide + Complete enrollment in ReSPECT-GBM Phase 2 Diagnostic platform trial for recurrent glioblastoma and conduct end of phase meeting to align on pivotal trial + Obtain Medicare and Medicaid coverage design + Establish prior commercial order rate (1,250 TCE + Define optimal dose/interval for REYOBIQ in tests per annum) ReSPECT-LM Phase 2 expansion trial and begin + Establish 50 or more unique ordering physician enrollment customer base for CNSide + Complete manufacturing scale-up for REYOBIQ + Expand platform and launch additional CSF commercial and approval trial supply tumor characterization tests + Enroll first patient in the ReSPECT-PBC pediatric brain cancer Phase 1 trial with REYOBIQ * These state me nt s a nd figure s a re targe ts for 202 6 a nd ar e not pr oje ctions and should not be rel ied upon as such. Fur the r, these targe ts are subject to occurre nce s that ma y nega tivel y impa ct our business and our industry g enera lly, whi ch may be out of our control, and which may impact our ability to achieve these ta rge ts. 13 Plus Therapeutics, Inc. NASDAQ: PSTV

Executive Management Team Marc H. Hedrick, MD MBA President & CEO, Plus Therapeutics 30+ years of clinical development and business leadership Russell Bradley Russ Havranek, MS MBA Andrew Brenner, MD PhD Andrew Sims, CPA President & General Manager Executive VP Corporate Strategy & Interim CMO CFO & VP of Finance (CNSide) Business Development Professor of Medicine and Neuro-Oncology 25+ years of Financial 30+ years of diagnostic & life 25+ years of development & Leadership Clinical Investigator sciences leadership commercialization experience Endowed Chair, Mays Cancer Center, UT Health San Antonio 14 Plus Therapeutics, Inc. NASDAQ: PSTV

D DIET iag =dalexya nostic es s ra Ges Contact us at investor@plustherapeutics.com Contact us at investor@plustherapeutics.com
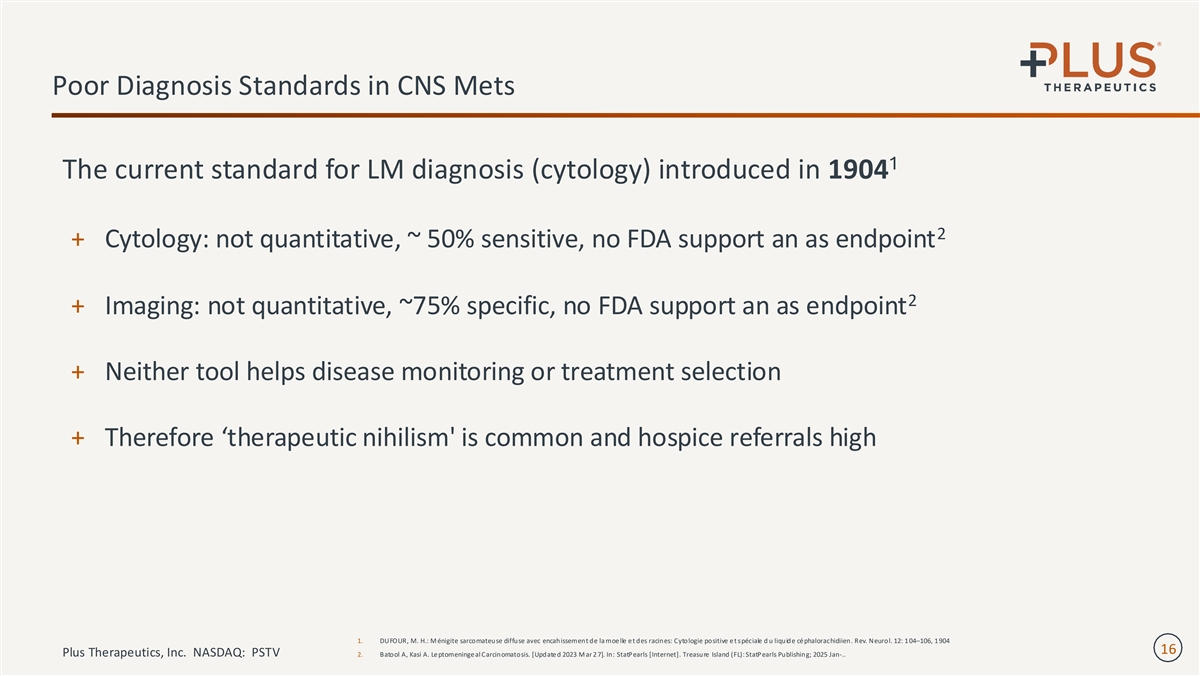
Poor Diagnosis Standards in CNS Mets 1 The current standard for LM diagnosis (cytology) introduced in 1904 2 + Cytology: not quantitative, ~ 50% sensitive, no FDA support an as endpoint 2 + Imaging: not quantitative, ~75% specific, no FDA support an as endpoint + Neither tool helps disease monitoring or treatment selection + Therefore ‘therapeutic nihilism' is common and hospice referrals high 1. DUFOUR, M. H.: M énigite sarcomateuse diffuse avec encahissement de la moe lle e t des racines: Cytologie positive e t spéciale d u liquide cé phalorachidiien. Rev. Neurol. 12: 1 04–106, 1 904 16 Plus Therapeutics, Inc. NASDAQ: PSTV 2. Batool A, Kasi A. Le ptomeninge al Carcinomatosis. [Update d 2023 M ar 2 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-..
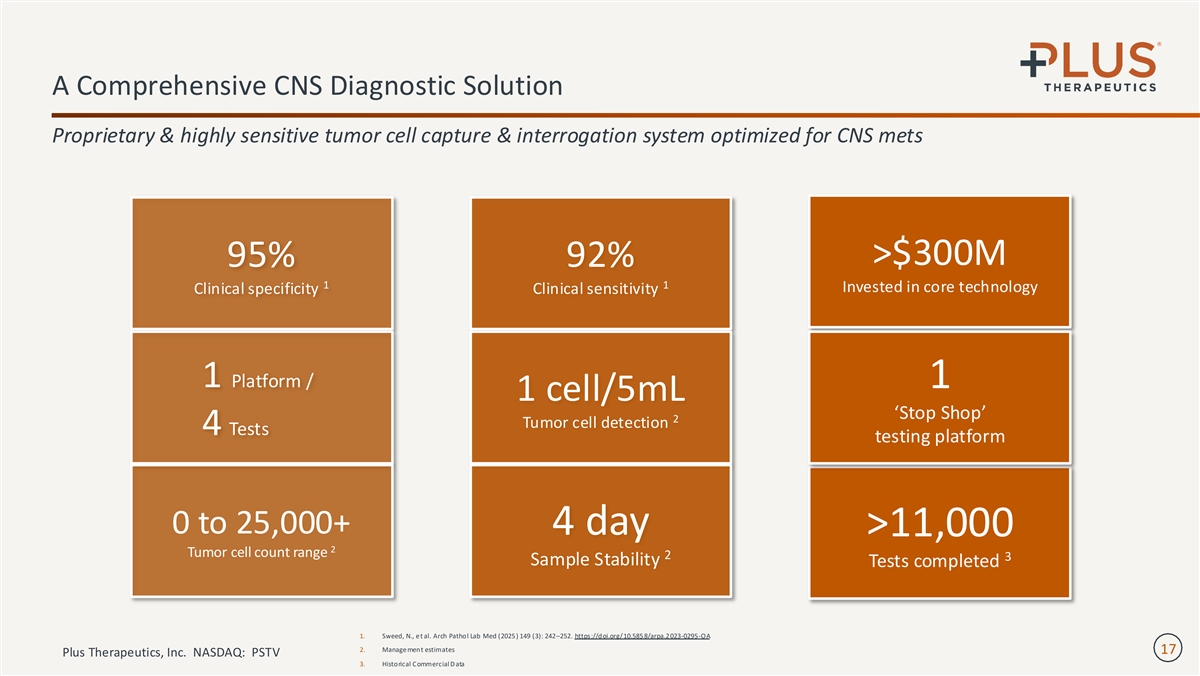
A Comprehensive CNS Diagnostic Solution Proprietary & highly sensitive tumor cell capture & interrogation system optimized for CNS mets >$300M 95% 92% 1 1 Invested in core technology Clinical specificity Clinical sensitivity 1 Platform / 1 1 cell/5mL ‘Stop Shop’ 2 Tumor cell detection 4 Tests testing platform 0 to 25,000+ 4 day >11,000 2 Tumor cell count range 2 3 Sample Stability Tests completed 1. Sweed, N., e t al. Arch Pathol Lab Med (2025 ) 149 (3): 242–252. https://doi.org/10.585 8/arpa.2 023-0295-OA 2. Manage ment estimates 17 Plus Therapeutics, Inc. NASDAQ: PSTV 3. Historical Commercial D ata
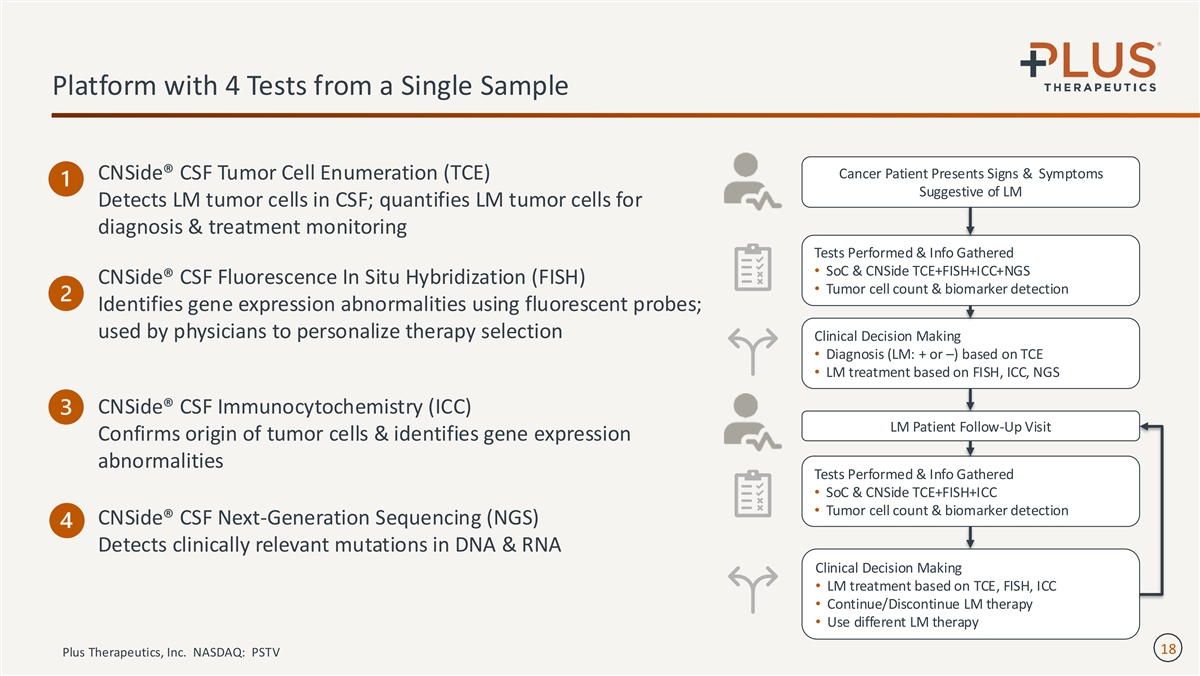
Platform with 4 Tests from a Single Sample Cancer Patient Presents Signs & Symptoms CNSide® CSF Tumor Cell Enumeration (TCE) Suggestive of LM Detects LM tumor cells in CSF; quantifies LM tumor cells for diagnosis & treatment monitoring Tests Performed & Info Gathered • SoC & CNSide TCE+FISH+ICC+NGS CNSide® CSF Fluorescence In Situ Hybridization (FISH) • Tumor cell count & biomarker detection Identifies gene expression abnormalities using fluorescent probes; used by physicians to personalize therapy selection Clinical Decision Making • Diagnosis (LM: + or –) based on TCE • LM treatment based on FISH, ICC, NGS CNSide® CSF Immunocytochemistry (ICC) LM Patient Follow-Up Visit Confirms origin of tumor cells & identifies gene expression abnormalities Tests Performed & Info Gathered • SoC & CNSide TCE+FISH+ICC • Tumor cell count & biomarker detection CNSide® CSF Next-Generation Sequencing (NGS) Detects clinically relevant mutations in DNA & RNA Clinical Decision Making • LM treatment based on TCE, FISH, ICC • Continue/Discontinue LM therapy • Use different LM therapy 18 Plus Therapeutics, Inc. NASDAQ: PSTV
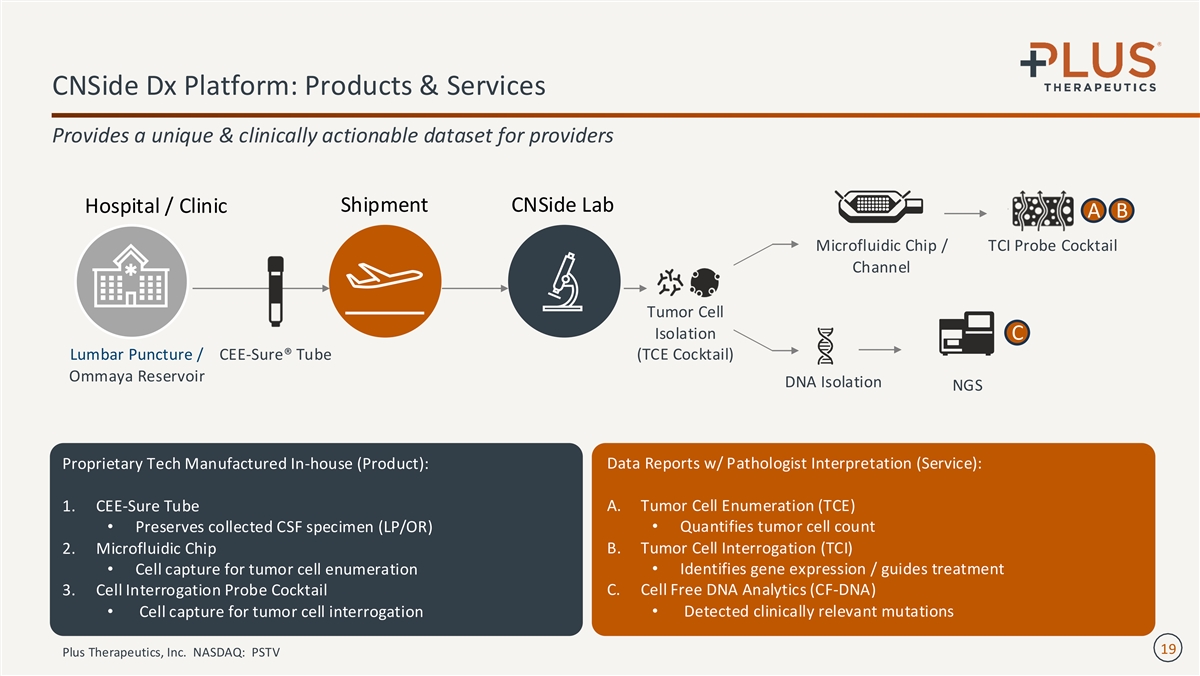
CNSide Dx Platform: Products & Services Provides a unique & clinically actionable dataset for providers Shipment CNSide Lab Hospital / Clinic A B Microfluidic Chip / TCI Probe Cocktail Channel Tumor Cell Isolation C (TCE Cocktail) Lumbar Puncture / CEE-Sure® Tube Ommaya Reservoir DNA Isolation NGS Data Reports w/ Pathologist Interpretation (Service): Proprietary Tech Manufactured In-house (Product): A. Tumor Cell Enumeration (TCE) 1. CEE-Sure Tube • Preserves collected CSF specimen (LP/OR)• Quantifies tumor cell count B. Tumor Cell Interrogation (TCI) 2. Microfluidic Chip • Cell capture for tumor cell enumeration• Identifies gene expression / guides treatment C. Cell Free DNA Analytics (CF-DNA) 3. Cell Interrogation Probe Cocktail • Cell capture for tumor cell interrogation• Detected clinically relevant mutations 19 Plus Therapeutics, Inc. NASDAQ: PSTV

A Derisked Commercial Opportunity 2025 commercial relaunch: clinically proven, market traction, & positioned for scale + Proven high throughput & scalable technology • ~$300M investment in core tech made by original developer • 9 peer-reviewed studies and publications • Clinically validated (FORESEE trial completed) • CSF CTCs in NCCN guidelines* + CNSide commercially available since 2020 by original developer (paused 2023) • Previously launched & accepted by CMS in 2020 • 11,000+ tests ordered • 200+ unique physician users across 120+ institutions • Avg. 4.3 tests/patient st + 1 national policy coverages: United Healthcare and Humana • 51 million covered lives with United Healthcare • 16 million covered lives with Humana *Jl Clin Oncology, vol 42,No. 16 suppl, Jume 2024 20 Plus Therapeutics, Inc. NASDAQ: PSTV
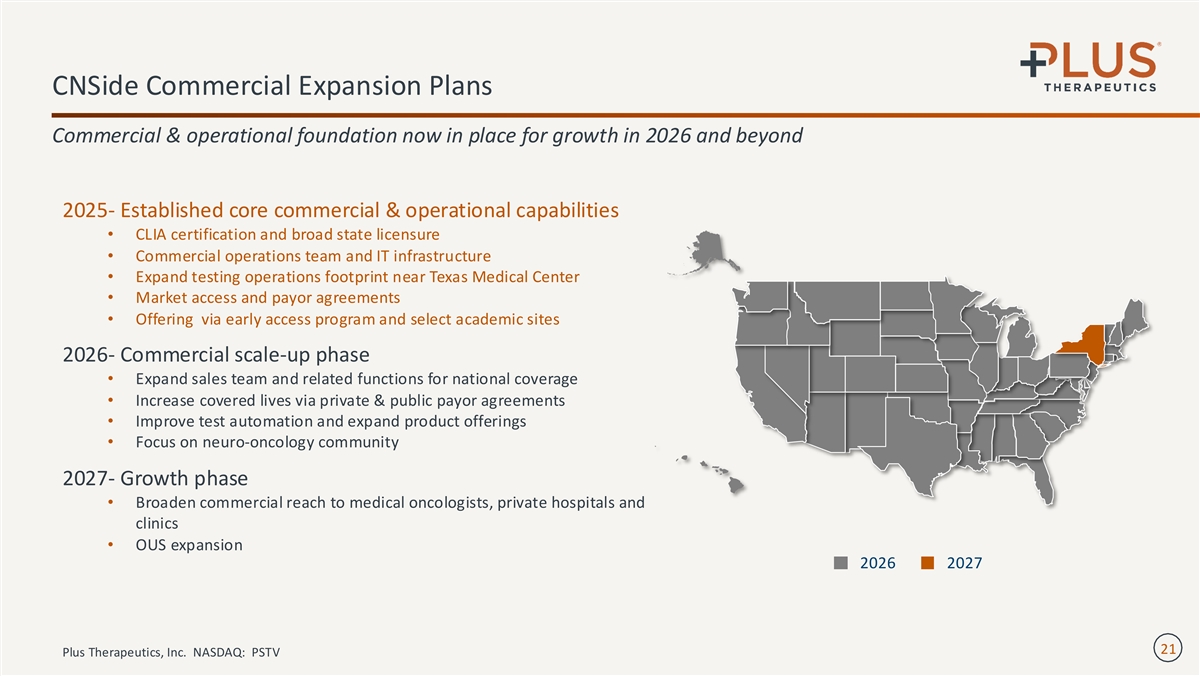
CNSide Commercial Expansion Plans Commercial & operational foundation now in place for growth in 2026 and beyond 2025- Established core commercial & operational capabilities • CLIA certification and broad state licensure • Commercial operations team and IT infrastructure • Expand testing operations footprint near Texas Medical Center • Market access and payor agreements • Offering via early access program and select academic sites 2026- Commercial scale-up phase • Expand sales team and related functions for national coverage • Increase covered lives via private & public payor agreements • Improve test automation and expand product offerings • Focus on neuro-oncology community 2027- Growth phase • Broaden commercial reach to medical oncologists, private hospitals and clinics • OUS expansion 2026 2027 21 Plus Therapeutics, Inc. NASDAQ: PSTV

CLINICAL TRIALS obisbemeda injection Tar Targeted geted R Radiotherapeutics adiotherapeutics vy . Ret xeay REYOBIQ Contact us at investor@plustherapeutics.com Contact us at investor@plustherapeutics.com
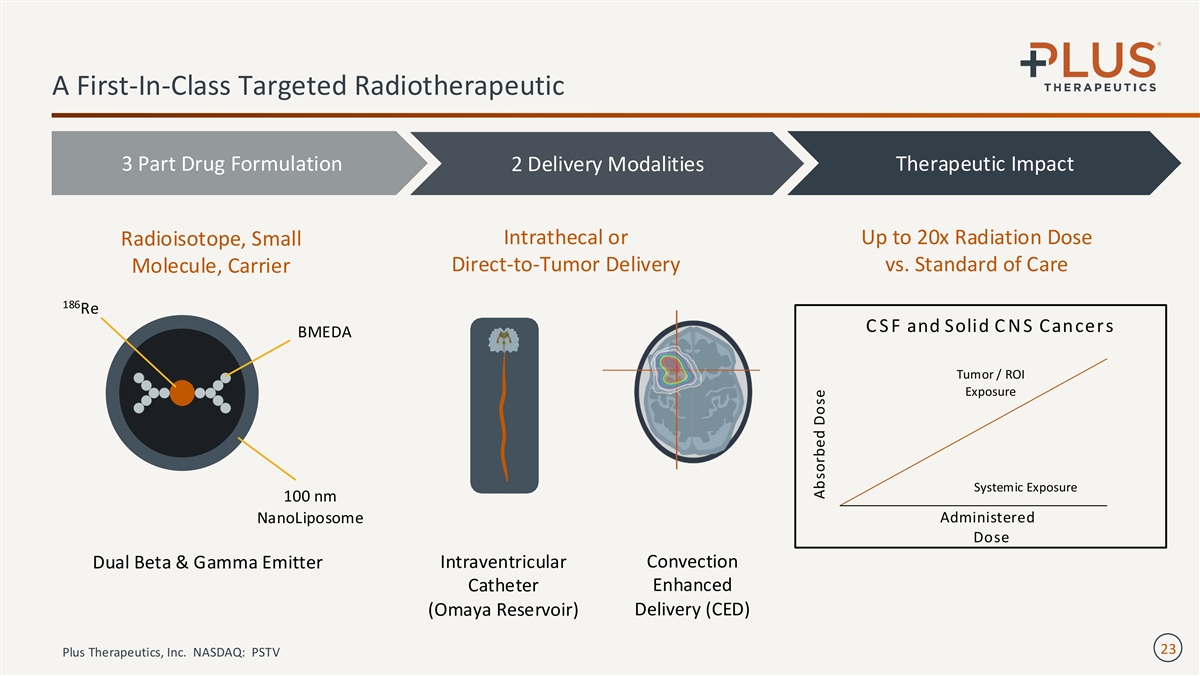
A First-In-Class Targeted Radiotherapeutic 3 Part Drug Formulation 2 Delivery Modalities Therapeutic Impact Intrathecal or Up to 20x Radiation Dose Radioisotope, Small Direct-to-Tumor Delivery vs. Standard of Care Molecule, Carrier 186 Re C S F and Solid C N S Cancers BMEDA Tumor / ROI Exposure Systemic Exposure 100 nm Administered NanoLiposome Dose Dual Beta & Gamma Emitter Intraventricular Convection Enhanced Catheter (Omaya Reservoir) Delivery (CED) 23 Plus Therapeutics, Inc. NASDAQ: PSTV Absorbed Dose
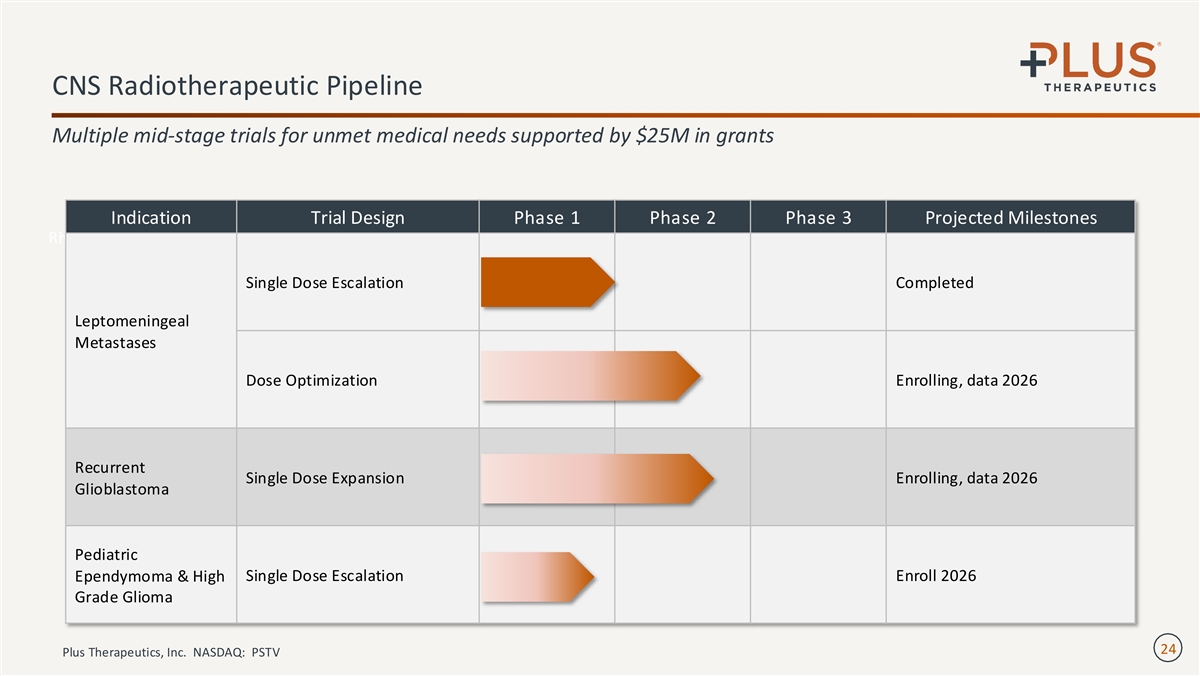
CNS Radiotherapeutic Pipeline Multiple mid-stage trials for unmet medical needs supported by $25M in grants Indication Trial Design Phase 1 Phase 2 Phase 3 Projected Milestones 186 Rhenium ( Re) Obisbemeda Single Dose Escalation Completed Leptomeningeal Metastases Dose Optimization Enrolling, data 2026 Recurrent Single Dose Expansion Enrolling, data 2026 Glioblastoma Pediatric Single Dose Escalation Enroll 2026 Ependymoma & High Grade Glioma 24 Plus Therapeutics, Inc. NASDAQ: PSTV
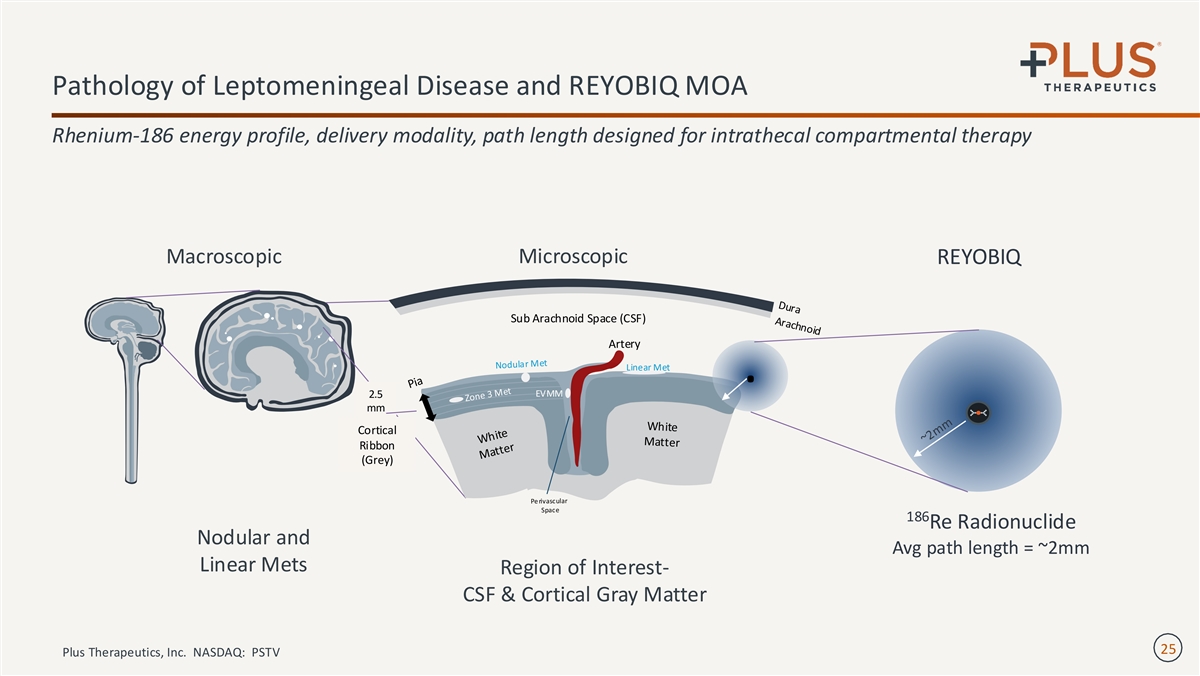
Pathology of Leptomeningeal Disease and REYOBIQ MOA Rhenium-186 energy profile, delivery modality, path length designed for intrathecal compartmental therapy Microscopic Macroscopic REYOBIQ Sub Arachnoid Space (CSF) Linear Met . 2.5 mm Cortical Ribbon (Grey) 186 Re Radionuclide Nodular and Avg path length = ~2mm Linear Mets Region of Interest- CSF & Cortical Gray Matter 25 Plus Therapeutics, Inc. NASDAQ: PSTV
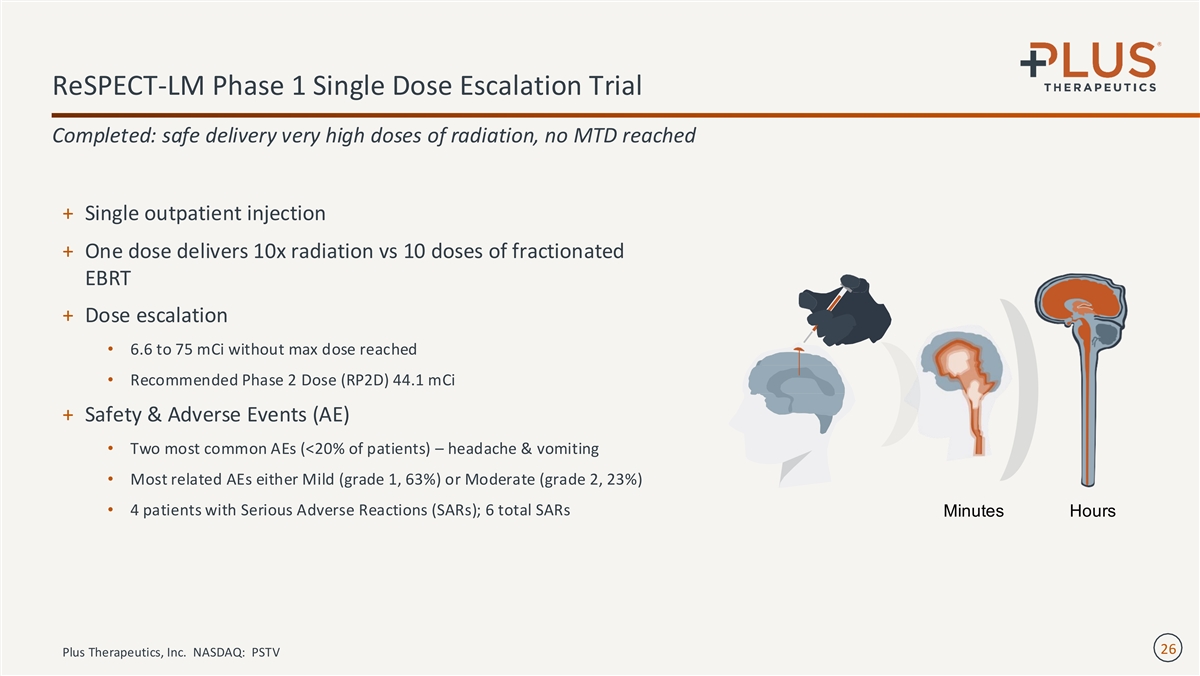
ReSPECT-LM Phase 1 Single Dose Escalation Trial Completed: safe delivery very high doses of radiation, no MTD reached + Single outpatient injection + One dose delivers 10x radiation vs 10 doses of fractionated EBRT + Dose escalation • 6.6 to 75 mCi without max dose reached • Recommended Phase 2 Dose (RP2D) 44.1 mCi + Safety & Adverse Events (AE) • Two most common AEs (<20% of patients) – headache & vomiting • Most related AEs either Mild (grade 1, 63%) or Moderate (grade 2, 23%) • 4 patients with Serious Adverse Reactions (SARs); 6 total SARs Minutes Hours 26 Plus Therapeutics, Inc. NASDAQ: PSTV
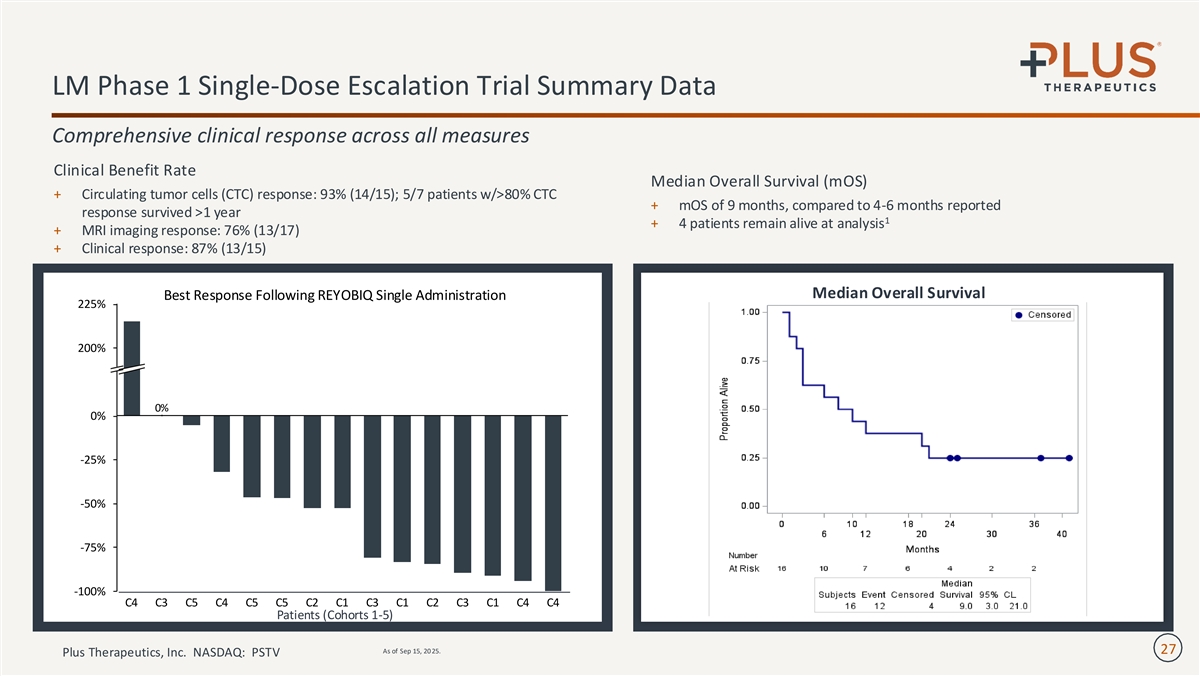
LM Phase 1 Single-Dose Escalation Trial Summary Data Comprehensive clinical response across all measures Clinical Benefit Rate Median Overall Survival (mOS) + Circulating tumor cells (CTC) response: 93% (14/15); 5/7 patients w/>80% CTC + mOS of 9 months, compared to 4-6 months reported response survived >1 year 1 + 4 patients remain alive at analysis + MRI imaging response: 76% (13/17) + Clinical response: 87% (13/15) Median Overall Survival Best Response Following REYOBIQ Single Administration 225% 200% 0% 0% -25% -50% -75% -100% C4 C3 C5 C4 C5 C5 C2 C1 C3 C1 C2 C3 C1 C4 C4 Patients (Cohorts 1-5) As of Sep 15, 20 25. 27 Plus Therapeutics, Inc. NASDAQ: PSTV

LM Clinical Development Plan Establishing RP2D and planning for pivotal trial Completed- Phase 1 Single Dose Safety Trial + No MTD, MFD of 66 mCi + MFD 44.1 mCi + Safety & evidence of clinical benefit at 13.2, 26.4 & 44.1 mCi Completed FDA End of Phase Meeting NOV 2025 Enrolling- Multiple Dose Phase 1/2 Trial + 3 intervals: every 56, 28 & 15 days (13.2 & 26.4 mCi doses only) + 3 doses: 13.2, 26.4 & 44.1 mCi + Phase 2 safety/efficacy expansion at 1 or more doses to plan/power pivotal trial + Assess improvement in overall survival, patient reported outcomes and neurologic improvement over standard of care 28 Plus Therapeutics, Inc. NASDAQ: PSTV

FDA Meeting and Feedback FDA ReSPECT-LM end of phase meeting November 2025 Responses to key sponsor enquiries • Accelerated approval- May be appropriate for this indication. • Primary & key endpoints- FDA stated that there are insufficient data to use CTCs as a surrogate endpoint but provided feedback on additional steps to support validation of CTCs to support future applications. Agency encouraged study of patient reported outcomes, neurologic function as well as overall survival as potential basis of marketing approval. Agency & Sponsor aligned that CTCs could be considered for use as a key secondary endpoint. • Trial design & comparator group- Agency & Sponsor aligned on a randomized controlled design approach. Agency & sponsor further discussed that the study may include an intrathecal chemotherapeutic as a comparator and approaches to standardize the available comparator chemotherapy regimens as well as any background and rescue interventions under the trial protocol in both active and control arms. • Treated populations- Agency conveyed that it may be reasonable to incorporate multiple histologies in a single trial, i.e. multiple underlying etiologies in LM. 29 Plus Therapeutics, Inc. NASDAQ: PSTV
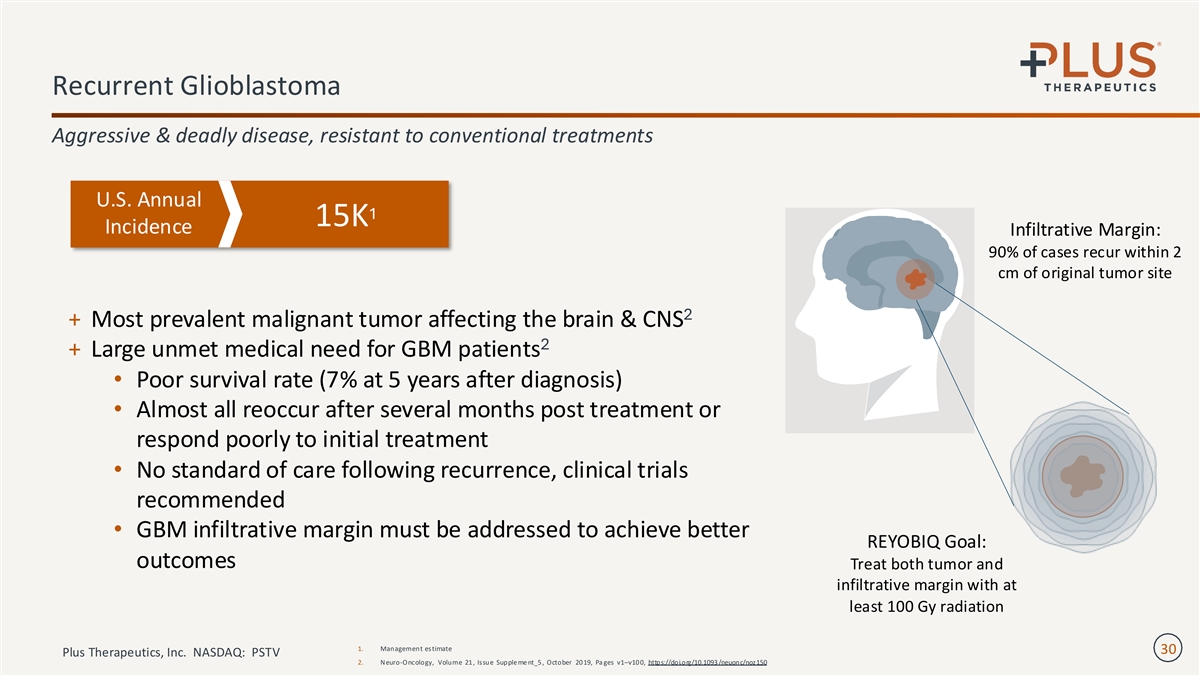
Recurrent Glioblastoma Aggressive & deadly disease, resistant to conventional treatments U.S. Annual 1 15K Incidence Infiltrative Margin: 90% of cases recur within 2 cm of original tumor site 2 + Most prevalent malignant tumor affecting the brain & CNS 2 + Large unmet medical need for GBM patients • Poor survival rate (7% at 5 years after diagnosis) • Almost all reoccur after several months post treatment or respond poorly to initial treatment • No standard of care following recurrence, clinical trials recommended • GBM infiltrative margin must be addressed to achieve better REYOBIQ Goal: outcomes Treat both tumor and infiltrative margin with at least 100 Gy radiation 1. Management estimate 30 Plus Therapeutics, Inc. NASDAQ: PSTV 2. Neuro-Oncology, Volume 21 , Issue Supple me nt_5 , October 20 19, Pa ges v1–v10 0, https://doi.org/10.1093 /neuonc/noz150
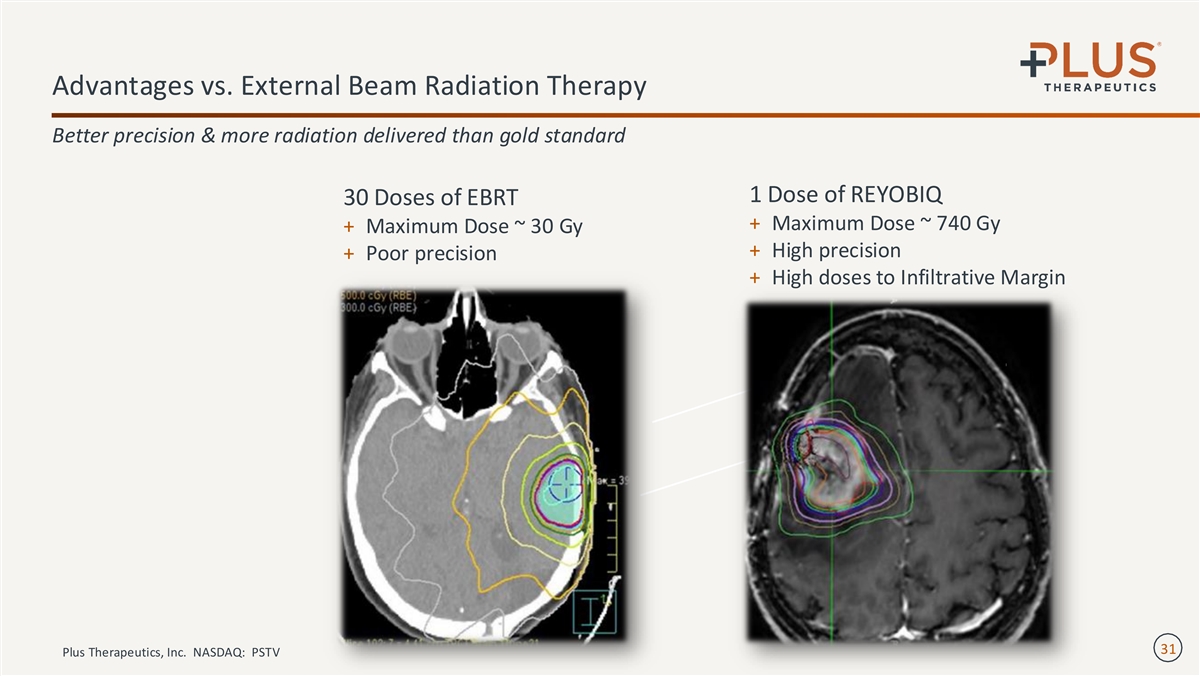
Advantages vs. External Beam Radiation Therapy Better precision & more radiation delivered than gold standard 1 Dose of REYOBIQ 30 Doses of EBRT + Maximum Dose ~ 740 Gy + Maximum Dose ~ 30 Gy + High precision + Poor precision + High doses to Infiltrative Margin 31 Plus Therapeutics, Inc. NASDAQ: PSTV
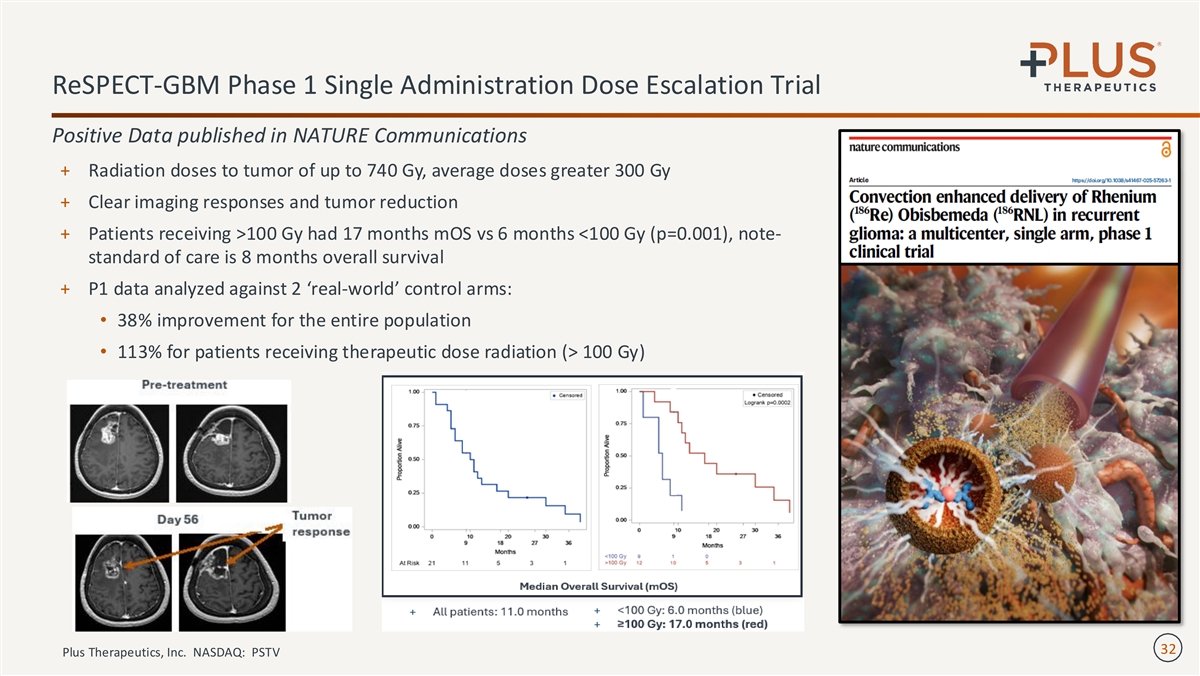
ReSPECT-GBM Phase 1 Single Administration Dose Escalation Trial Positive Data published in NATURE Communications + Radiation doses to tumor of up to 740 Gy, average doses greater 300 Gy + Clear imaging responses and tumor reduction + Patients receiving >100 Gy had 17 months mOS vs 6 months <100 Gy (p=0.001), note- standard of care is 8 months overall survival + P1 data analyzed against 2 ‘real-world’ control arms: • 38% improvement for the entire population • 113% for patients receiving therapeutic dose radiation (> 100 Gy) 32 Plus Therapeutics, Inc. NASDAQ: PSTV
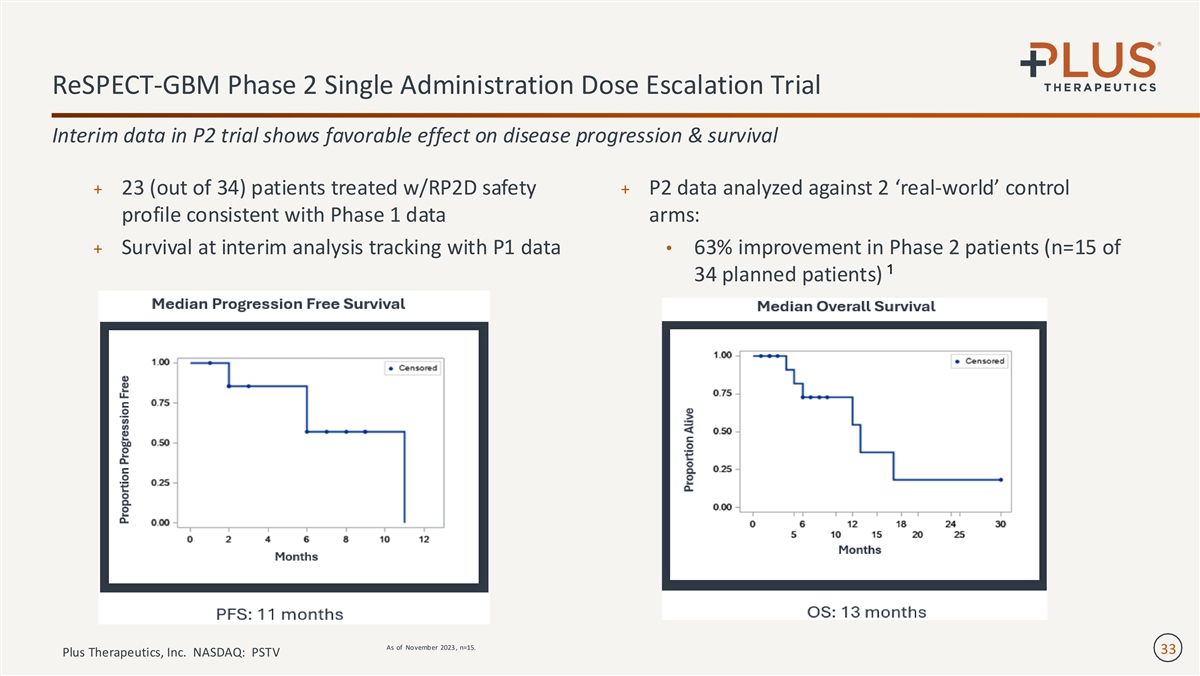
ReSPECT-GBM Phase 2 Single Administration Dose Escalation Trial Interim data in P2 trial shows favorable effect on disease progression & survival + 23 (out of 34) patients treated w/RP2D safety + P2 data analyzed against 2 ‘real-world’ control profile consistent with Phase 1 data arms: + Survival at interim analysis tracking with P1 data• 63% improvement in Phase 2 patients (n=15 of 1 34 planned patients) As of November 2023 , n=15. 33 Plus Therapeutics, Inc. NASDAQ: PSTV

ReSPECT-PBC Phase 1/2 Single Dose Escalation Trial For the treatment of rare, aggressive pediatric brain cancers + Recurrent, refractory, or progressive ependymoma and high-grade glioma + Obtained FDA IND clearance Q2 2025 + Expect to begin enrollment in 1Q 2026 + Initial site: Lurie Children’s Hospital in Chicago, IL + Funded by $3.0 million award from U.S. Department of Defense 34 Plus Therapeutics, Inc. NASDAQ: PSTV
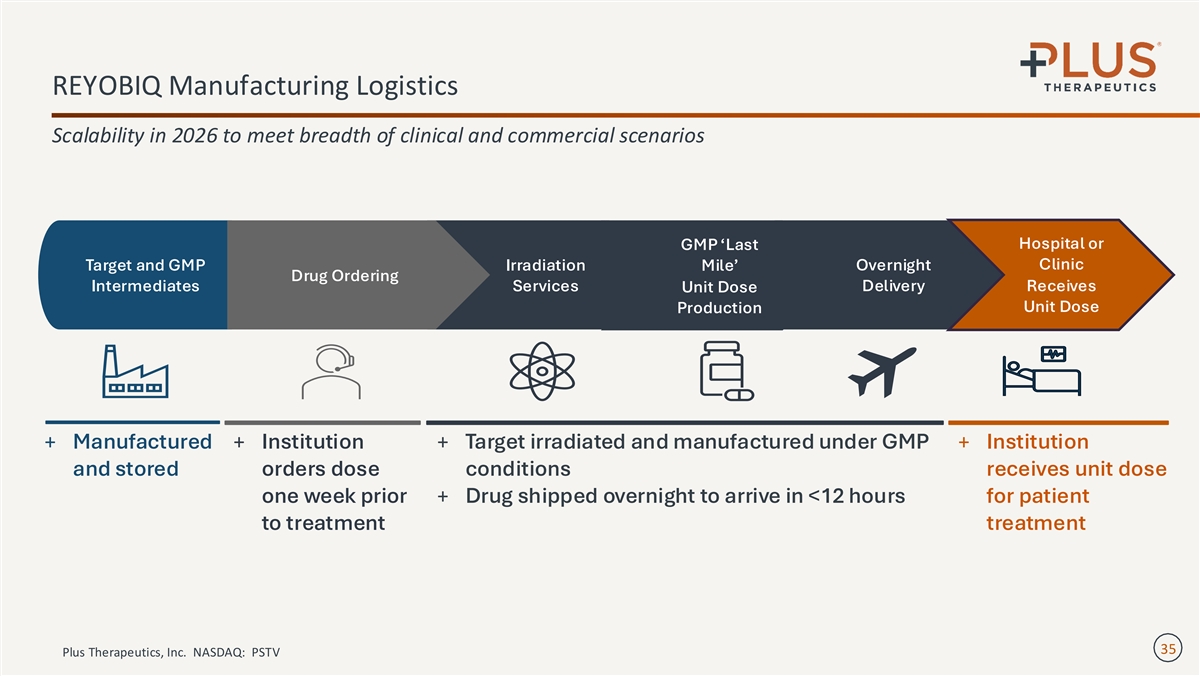
REYOBIQ Manufacturing Logistics Scalability in 2026 to meet breadth of clinical and commercial scenarios Hospital or GMP ‘Last Clinic Target and GMP Irradiation Mile’ Overnight Drug Ordering Intermediates Services Delivery Receives Unit Dose Unit Dose Production + Manufactured + Institution + Target irradiated and manufactured under GMP + Institution and stored orders dose conditions receives unit dose one week prior + Drug shipped overnight to arrive in <12 hours for patient to treatment treatment 35 Plus Therapeutics, Inc. NASDAQ: PSTV

CLINICAL TRIALS obisbemeda injection Thank You Marella dele ve PLUS THERAPEUTICS Contact us at investor@plustherapeutics.com Contact us at investor@plustherapeutics.com Basrect evn CNSIde