Please wait
UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Form 10-K
| |
|
|
|
þ
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the fiscal year ended
December 31, 2006
|
|
or
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the transition period
from to
|
Commission File Number:
000-51709
Iomai Corporation
(exact name of registrant as
specified in its charter)
| |
|
|
|
Delaware
|
|
52-2049149
|
(State or other jurisdiction
of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
20 Firstfield Road, Suite 250, Gaithersburg, Maryland
20878
(Address of principal executive
offices including zip code)
Registrant’s telephone number, including area code:
(301) 556-4500
Securities registered pursuant to Section 12(b) of the
Act:
| |
|
|
|
None
|
|
None
|
|
(Title of each Class)
|
|
(Name of each exchange on which
registered)
|
Securities registered pursuant to Section 12(g) of the
Act:
Common Stock, $.01 Par Value Per Share
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities
Act. Yes o No þ
Indicate by check mark if the registrant is not required to file
reports pursuant to Section 13 or Section 15(d) of the
Act. Yes þ No
o
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant
was required to file such reports), and (2) has been
subject to such filing requirements for the past
90 days. Yes þ No
o
Indicate by check mark if disclosure of delinquent filers
pursuant to Item 405 of
Regulation S-K
is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in
Part III of this
Form 10-K
or any amendment to this
Form 10-K.
o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, or a non-accelerated
filer. See definition of “accelerated filer and large
accelerated filer” in
Rule 12b-2
of the Exchange Act.
|
|
|
| Large
accelerated filer o
|
Accelerated
Filer o
|
Non-accelerated
filer þ
|
Indicate by check mark whether the registrant is a shell company
(as defined in
Rule 12b-2
of the Exchange
Act). Yes o No þ
The aggregate market value of voting stock held by
non-affiliates of the registrant as of March 14, 2007 was
$79,518,299. There were 25,509,045 shares of the
registrant’s Common Stock outstanding as of March 14,
2007.
DOCUMENTS
INCORPORATED BY REFERENCE
Portions of the definitive proxy statement for the
registrant’s 2007 Annual Meeting of Stockholders to be held
on June 5 2007, which definitive proxy statement will be filed
with the Securities and Exchange Commission not later than
120 days after the registrant’s fiscal year of
December 31, 2006, are incorporated by reference into
Part III of this Annual Report on
Form 10-K.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This annual report on
Form 10-K
contains forward-looking statements. All statements contained in
this annual report other than statements of historical fact are
forward-looking statements. Forward-looking statements include
statements regarding our future financial position, business
strategy, budgets, projected costs, plans and objectives of
management for future operations. The words “may,”
“continue,” “estimate,” “intend,”
“plan,” “will,” “believe,”
“project,” “expect,” “seek,”
“anticipate” and similar expressions may identify
forward-looking statements, but the absence of these words does
not necessarily mean that a statement is not forward-looking.
These forward-looking statements include, among other things,
statements about:
|
|
|
| |
•
|
the implementation of our corporate strategy;
|
| |
| |
•
|
our future financial performance and projected expenditures;
|
| |
| |
•
|
our ability to enter into future collaborations with
pharmaceutical, biopharmaceutical and biotechnology companies
and academic institutions or to obtain funding from government
agencies;
|
| |
| |
•
|
our product research and development activities, including the
timing and progress of our clinical trials, and projected
expenditures;
|
| |
| |
•
|
our technology’s potential efficacy, advantages over
current approaches to vaccination and broad applicability to
infectious diseases;
|
| |
| |
•
|
our ability to receive regulatory approvals to develop and
commercialize our product candidates;
|
| |
| |
•
|
our ability to increase our manufacturing capabilities for our
product candidates;
|
| |
| |
•
|
our ability to develop or obtain funding for our immunostimulant
patch for pandemic flu applications;
|
| |
| |
•
|
our projected markets and growth in markets;
|
| |
| |
•
|
our product formulations and patient needs and potential funding
sources;
|
| |
| |
•
|
our staffing needs; and
|
| |
| |
•
|
our plans for sales and marketing.
|
The forward-looking statements in this annual report are subject
to risks and uncertainties that may cause actual results to
differ materially. These risks and uncertainties include those
described in Item 1A. “Risk Factors.” In light of
these risks and uncertainties, the forward-looking events and
circumstances discussed in this annual report may not occur as
contemplated, and actual results could differ materially from
those anticipated or implied by the forward-looking statements.
You should not unduly rely on these forward-looking statements,
which speak only as of the date of this annual report. Unless
required by law, we undertake no obligation to publicly update
or revise any forward-looking statements to reflect new
information or future events or otherwise. You should, however,
review the factors and risks we describe in the reports we file
from time to time with the U.S. Securities and Exchange
Commission (SEC) after the date of this annual report. See
“Availability of Periodic SEC Reports” in Item 1.
“Business.”
Iomai®,
Transcutaneous
Immunizationtm,
TCItm,
Immunity That’s More Than Skin
Deeptm
and the Iomai logo are trademarks or service marks of Iomai
Corporation. All other trademarks and service marks appearing in
this report are the property of their respective holders.
ii
PART I
OVERVIEW
We are a biopharmaceutical company focused on the discovery,
development and commercialization of vaccines and immune system
stimulants delivered to the skin via a novel, needle-free
technology called transcutaneous immunization, or TCI. TCI taps
into the unique benefits of a major group of antigen-presenting
cells found in the outer layers of the skin (Langerhans cells)
to generate an enhanced immune response. Our plans are to
leverage TCI to enhance the efficacy of existing vaccines,
develop new vaccines that are viable only through transcutaneous
administration and expand the global vaccine market.
We are developing two distinct product applications for our TCI
technology. The first application is an immunostimulant, or IS,
patch that is applied to the skin and contains only an adjuvant,
which is a compound used to enhance a vaccine’s ability to
elicit a stronger immune response, thereby making the vaccine
more effective. In this application, our IS patch is placed over
the vaccine injection site in order to stimulate a stronger
immune response to the injectable vaccine. Initially we expect
to target our IS patch to extend vaccine supply in the event of
an influenza pandemic and for use in patient populations with
weakened or impaired immune systems, such as the elderly, for
whom existing vaccines often do not elicit an adequate immune
response. The second application is a needle-free vaccine patch
that is applied to the skin and delivers both pathogen-specific
antigens and an adjuvant. We are using this application to
develop needle-free vaccines to replace currently injectable
vaccines as well as novel vaccines that cannot be delivered by
injection.
We currently have five product candidates in development: four
targeting influenza and pandemic flu and one to prevent E.
coli-related travelers’ diarrhea:
|
|
|
| |
•
|
IS patch for pandemic flu program. We are
developing an IS patch that, when used in conjunction with an
injectable flu vaccine, is designed to stimulate an immune
response to even small doses of vaccine, thereby allowing public
health officials to extend vaccine supply in the event of an
influenza pandemic. In January 2007, we were awarded a
five-year, cost-plus reimbursement contract by the Department of
Health and Human Services, or DHHS, to fund our development of a
dose-sparing patch for use with a pandemic flu vaccine. If the
product is developed through licensure, the total cost
reimbursed by DHHS, plus a fixed fee, is estimated to be
$128 million. During the first 15 months of the
contract, DHHS has allotted approximately $14.5 million for
us to assess the safety and immunogenicity of the patch in two
clinical trials and to develop plans on how we would produce
150 million IS patches in a six-month period, as required
under the contract. Once we demonstrate the safety and
dose-sparing capability of our IS patch, we hope to sell up to
150 million IS patches to the United States government for
its stockpile of pandemic flu products.
|
| |
| |
•
|
IS patch for elderly receiving flu
vaccines. We are developing our IS patch to
improve the immune response of the elderly to existing
injectable influenza vaccines. We have completed a number of
studies in the United States and Europe, including a European
Phase 2 clinical trial that demonstrated the
proof-of-concept
with a previous patch formulation. In February 2007, we
commenced a Phase 1 safety study in Australia with our
current dry patch formulation, and we plan to commence a
Phase 2 study in the United States in the second half of
2007 to confirm the results observed in our prior European
Phase 2 study. Approximately 36,000 individuals aged 65 and
over die from influenza, influenza-related pneumonia and related
respiratory complications in the United States each year, making
influenza one of the leading causes of death among the elderly.
According to the United States Centers for Disease Control and
Prevention, or CDC, over 65% of the elderly population in the
United States receive influenza vaccination, and public health
officials have stated the goal of achieving 90% vaccination
rates by 2010.
|
| |
| |
•
|
Needle-free flu vaccine patch. We are
developing a needle-free flu vaccine patch that combines flu
antigens with an adjuvant in a single patch. During the fall of
2006, we initiated a large, multi-center Phase 1 trial for
our needle-free flu vaccine patch. This study is designed to
test the safety and immunogenicity of our needle-free flu
vaccine in comparison with the traditional injected vaccine.
Vaccinations were completed during the first quarter of 2007,
and we expect interim data from this trial to be available by
the middle of
|
1
|
|
|
| |
|
2007. We expect this data to guide us in our design for a
Phase 2 program that could potentially support licensure of
a self-administered, needle-free flu vaccine patch. As we do not
manufacture commercial flu antigens, we are also actively
seeking to collaborate with suppliers of commercial flu antigens
in order to advance further development of this program. Each
flu season, approximately 15 million to 60 million
people in the United States become ill with influenza. This
infection rate results in approximately 36,000 deaths and
approximately 200,000 hospitalizations in the United States
annually. Approximately 80 million people in the United
States are vaccinated against the flu annually.
|
|
|
|
| |
•
|
Needle-free pandemic flu vaccine patch. We are
in preclinical development of a needle-free pandemic flu vaccine
patch that combines pandemic flu antigen with an adjuvant in a
single patch. This vaccine patch could solve a great many issues
regarding mass vaccination in the event of an outbreak of
pandemic flu. Specifically, we believe that based on data from
our other programs, it might be possible to develop a
needle-free pandemic flu vaccine that (1) is stable at room
temperature, which could eliminate the need for refrigeration
and allow for distribution through the mail, and (2) can be
self-applied without the assistance of a healthcare worker.
Researchers at the CDC have estimated that the possible effects
of the next influenza pandemic in the United States would result
in 89,000 to 207,000 deaths; 314,000 to 734,000
hospitalizations; 18 to 42 million outpatient visits; and
20 to 47 million additional illnesses, and the estimated
economic impact would be US$71.3 to $166.5 billion,
excluding disruptions to commerce and society. Leveraging our
expertise from the needle-free seasonal flu vaccine, we are
currently conducting preclinical testing of various needle-free
pandemic flu formulations.
|
| |
| |
•
|
Needle-free travelers’ diarrhea vaccine
patch. We are also developing a needle-free
travelers’ diarrhea vaccine patch. We have completed a
double-blind, placebo-controlled challenge trial in the United
States, the results of which demonstrated the product
candidate’s ability to induce a strong immune response that
diminished the severity and delayed the onset of the most common
form of travelers’ diarrhea. During 2006, we completed a
number of Phase 1 and 2 studies to further advance the
program. These trials demonstrated, among other things, that our
current dry patch formulation achieved high immune responses in
those vaccinated and outperformed our earlier liquid-based patch
and that our travelers’ diarrhea vaccine patch can be
self-applied by individuals, not just by healthcare providers.
We also have gathered data that shows that research-grade dry
patches are stable for at least 12 months at room
temperature. All this data could have important implications for
our other programs and could form the basis for our development
of vaccine patches that could be stockpiled, mailed and
self-applied. We are currently completing a Phase 2
dose-ranging study, as well as a Phase 2 field study
designed to evaluate the logistics around how to best conduct a
larger pivotal field study. We expect data from these two trials
to be available during the second and third quarters of 2007.
Based on these data, we will select a dose and study the final
formulation in a Phase 2 trial designed to confirm safety
and immunogenicity of the final patch configuration. That trial
is expected to commence in the fourth quarter of 2007. If we
successfully complete these Phase 2 trials in a timely
fashion, we expect to begin our Phase 3 clinical trials in
late 2008. Travelers’ diarrhea usually is contracted by the
ingestion of food or water contaminated with Enterotoxigenic
E. coli bacteria (ETEC). The CDC estimates that 20% to
50% of international travelers contract travelers’
diarrhea. Currently, in the United States there are no approved
vaccines against travelers’ diarrhea caused by ETEC.
|
We expect that each of our product candidates, consisting of a
patch and one or more active ingredients, will be treated as a
separate investigational drug by the FDA, even if any active
ingredient is part of an existing approved product. None of the
active ingredients in our product candidates have been approved
by the FDA for commercial sale in any product.
We believe that TCI technology is broadly applicable and may
provide the means to prevent or mitigate many other diseases.
For example, we have compiled Phase 1 data supporting the
ability of TCI-based vaccines to stimulate an immune response
against anthrax exposure. On the basis of our preclinical and
clinical findings to date, we believe products based on our TCI
technology have the potential to address major unmet needs in
preventing infectious diseases, and would represent a
fundamental change in the way vaccines are administered.
2
OUR
STRATEGY
Our goal is to become a leader in the discovery, development and
commercialization of vaccines and immunostimulants delivered to
the skin. The key elements of our strategy are as follows:
|
|
|
| |
•
|
Develop our IS patch for pandemic flu
applications. Under the DHHS contract, we plan to
perform preclinical and clinical testing of the patch and to
develop a plan to bring the IS patch to licensure for
dose-sparing of pandemic flu vaccines. Once we demonstrate the
safety and dose-sparing capability of our IS patch, we hope to
sell up to 150 million IS patches to the United States
government for its stockpile of pandemic flu products.
|
| |
| |
•
|
Commercialize our IS patch for elderly receiving flu
vaccines. We intend to advance our IS patch for
elderly receiving flu vaccines into and through Phase 3
clinical trials ourselves, and then into commercialization,
possibly with a partner. To date, we have retained all of our
rights to this product candidate, and we believe this will
enable us to obtain advantageous terms in any potential
collaboration.
|
| |
| |
•
|
Seek a strategic collaboration for the development, marketing
and commercialization of our needle-free flu vaccine
patch. As we do not intend to manufacture the flu
antigens for our needle-free flu vaccine patch, we are actively
exploring collaborations with large pharmaceutical companies in
the United States and Europe that would provide the flu antigens
(including pandemic flu antigens) and financial resources to
develop and, if approved, commercialize our needle-free flu
vaccine patch. To date, we have retained all of our rights to
this product, and we believe this will enable us to obtain
advantageous terms in any potential collaboration.
|
| |
| |
•
|
Commercialize our needle-free travelers’ diarrhea
vaccine patch. We intend to advance our
needle-free travelers’ diarrhea vaccine patch into and
through Phase 3 clinical trials ourselves, and then into
commercialization, possibly with a partner. To date, we have
retained all of our rights to this product candidate, and we
believe this will enable us to obtain advantageous terms in any
potential collaboration.
|
| |
| |
•
|
Continue to develop and expand our manufacturing capabilities
to retain manufacturing rights. We intend to
retain manufacturing rights for our adjuvant and patch
technologies. To the extent practicable, we intend to continue
to develop and expand our manufacturing capabilities to meet our
expected demands. We are manufacturing all of our patches for
our clinical trials in our pilot manufacturing facility, and we
believe we currently have the capacity to produce anticipated
needs through initial commercialization of our first product.
|
| |
| |
•
|
Leverage our broad technology platform to develop additional
product candidates. As our TCI technology has the
potential to be useful with many different types of vaccines,
other companies developing vaccines represent possible partners
with which to develop new products. We intend to continue to
evaluate and develop additional product candidates to expand our
pipeline where we perceive a significant unmet medical need and
commercial potential.
|
MARKET
OVERVIEW
The
immune system and vaccines
The immune system is the body’s natural defense mechanism
for fighting disease caused by infectious pathogens, which are
organisms such as bacteria, viruses and other microbes. The skin
is one of the body’s first lines of defense against
pathogens. In addition to presenting a barrier to entry, the
skin contains specialized immune surveillance cells called
Langerhans cells, a subset of the body’s main class of
antigen-presenting cells, that serve an important early-warning
function. The role of Langerhans cells is to detect antigens
embodied in pathogens and present them to other components of
the immune system, which in turn produce antibodies that bind to
and destroy the invading pathogen or render it harmless. Once
produced, these antibodies remain in the body to ward off future
infections by the same pathogen, causing the individual to
become immune for some period of time after initial exposure.
An immune response may be induced by natural exposure to a
pathogen or by a controlled exposure to the antigens embodied in
a pathogen by using a vaccine. A vaccine contains a dead or
weakened form, or a derivative, of
3
an infectious pathogen, including the antigens that allow the
body to recognize the pathogen. Exposure to a vaccine induces
the immune system to destroy the pathogen and prevent it from
subsequently infecting the body. Several doses of a vaccine may
be needed for a full immune response. In addition, the immunity
provided by some vaccines is not lifelong. In such cases, the
immune response may decrease over time, requiring subsequent
doses of a vaccine, or boosters, to restore or increase immunity.
In order to increase the effectiveness of the body’s immune
response to a vaccine, vaccines are often administered in
combination with an adjuvant. Adjuvants are substances that act
as danger signals to the immune system and increase the ability
of vaccines to stimulate the immune system. The use of adjuvants
in combination with a vaccine has a variety of advantages over
the use of a vaccine by itself. For instance, adjuvants may be
used to help generate a meaningful immune response in groups of
individuals whose immune systems may not react effectively to a
vaccine alone, such as the elderly and individuals whose immune
systems have been weakened by disease. Adjuvants may also be
used to lower the required dose of a vaccine necessary to
stimulate an effective immune response in healthy individuals.
Our
initial target markets
Influenza
Influenza, commonly called the flu, is an infection of the
respiratory tract caused by an influenza virus. Influenza is
highly contagious and occurs mainly in the late fall, winter and
early spring. Influenza affects all age groups and commonly
causes moderate to severe illness that results in absences from
school and work and lost productivity. Some individuals,
however, develop serious complications, such as pneumonia, that
require hospitalization and may even result in death.
Flu-related complications can occur at any age; however, the
elderly and people with weakened or impaired immune systems are
much more likely to develop serious complications than are
younger, healthier people.
Pandemic influenza
When a new, highly infectious and dangerous strain of the
influenza virus appears, the general lack of immunity to this
strain in the general population can lead to a pandemic outbreak
that quickly spreads. Three influenza pandemics have occurred in
the 20th century, the most recent of which occurred in
1968. By United States government estimates, pandemic flu has a
greater potential to cause more deaths and illnesses than
virtually any other natural health threat. Signs of a possible
pandemic flu have emerged in Southeast Asia, as lethal
infections of poultry and humans with avian influenza virus
continue. The current virus is now endemic in bird populations,
having spread to more than 40 countries and causing the deaths
of hundreds of millions of birds. Furthermore, the World Health
Organization, or WHO, reports that the number of avian flu cases
in humans has reached more than 275 cases in 12 countries. The
spread of the virus amongst birds increases the likelihood of
continued human exposure and the risk of a pandemic flu outbreak.
In response, the United States government has been actively
trying to address this risk through the development of
pre-pandemic vaccines based on current lethal strains of
pandemic flu, collaboration with industry to increase the
nation’s vaccine production capacity and to expand or
extend the existing supply. As part of this, DHHS announced in
May 2006 the award of $1 billion in contracts to encourage
companies to support the advanced development of cell-based
production technologies for influenza vaccines and will help to
modernize and strengthen the nation’s influenza vaccine
production by creating an alternative to producing influenza
vaccines in eggs. The U.S. Government’s stated goal
for all of its pandemic flu programs is to expand domestic
influenza vaccine surge capacity for the production of pandemic
influenza vaccines for the entire U.S. population within
six months of a pandemic declaration, but this expansion in
domestic manufacturing capacity may take many years to
accomplish.
Potentially compounding the strain on manufacturing capacity,
initial clinical studies of a pandemic flu vaccine candidate
have shown that two 90-microgram doses of the vaccine, which is
twelve times the single 15-microgram dose of a seasonal flu
vaccine, are required to stimulate a level of immune response
that researchers anticipate would provide protection for an
individual against the pandemic flu strains that have been
spreading among birds in Asia. Because of public safety
concerns, and given that there are no pandemic flu vaccines
currently
4
approved for use in the United States, an advisory committee of
vaccine experts recommended in February 2007 that the FDA
approve this candidate pandemic flu vaccine, even though it is
expected to protect less then half of the individuals who
receive the large two-dose regimen. The FDA usually follows the
recommendations of its advisory committees, although it is not
bound to do so.
If there is an outbreak of pandemic flu, vaccine manufacturers
using current techniques will likely not be able to rapidly
scale up production to meet increased demand, as the manufacture
of flu vaccine is a complex and time-consuming process and the
vaccine is generally manufactured well before the relevant flu
season. Because of these manufacturing limitations, governments
and vaccine manufacturers are exploring dose-sparing strategies
by which a smaller dose of vaccine could produce an effective
immune response. With pressure on governments to develop
dose-sparing strategies, we believe that there may be an
important role for an IS patch, when administered in combination
with injected pandemic flu vaccines being developed by different
manufacturers, either to reduce the required dose of, or improve
the timing or magnitude of the immune response to, a pandemic
flu vaccine.
In January 2007, the DHHS awarded us a five-year,
$128 million contract to develop a “dose-sparing”
patch for use in the event of an influenza pandemic. Our key
requirements under the contract are to assess the safety and
immunogenicity of the patch, to perform preclinical and clinical
testing of the patch and to develop a plan to bring the product
to licensure.
Seasonal influenza
Each flu season approximately 15 million to 60 million
people in the United States become ill with influenza. This
infection rate results in approximately 36,000 deaths and
approximately 200,000 hospitalizations in the United States
annually. Approximately 80 million people in the United
States are vaccinated against the flu annually. The CDC has
indicated that it wants to increase this figure to
185 million annually by 2010. According to a study
conducted by Kalorama Information, the worldwide market for
influenza vaccines was estimated to be in excess of
$1.4 billion in 2004 and is expected to grow to
$1.8 billion in 2006. In addition, Kalorama estimates that
the worldwide market for influenza vaccines has the potential to
reach approximately $3 billion by 2010. This market growth
is expected to be driven principally by expanded supply,
heightened public awareness and increased advocacy for
vaccination by public health organizations, particularly for the
elderly and young children, as well as higher prices. We believe
that this market can be further expanded by providing an
alternate vaccine delivery method, such as a needle-free flu
vaccine patch, that is more convenient and less painful than a
hypodermic needle, and has the potential to be mailed and
self-applied.
Influenza in the elderly
The CDC has highlighted the relatively low effectiveness of
existing flu vaccines for persons aged 65 and over. Studies show
that the efficacy of flu vaccines decreases from the 70% to 90%
range observed in healthy individuals who are less than
65 years of age, down to the 30% to 40% range for
individuals 65 years of age and over. Most of the elderly
population receives a flu shot annually, yet in the United
States influenza is one of the leading causes of death in
persons 65 years of age and older, and approximately
40,000 people in this age group die from influenza,
influenza-related pneumonia and related respiratory
complications each year. This significant number of deaths
reaffirms the need for a more efficacious vaccine. As a result,
we believe there is an unmet need for immunostimulants that can
be delivered in conjunction with a flu vaccine to help persons
with weakened or impaired immune systems, such as the elderly,
develop adequate immune responses.
Travelers’
diarrhea
Travelers’ diarrhea is a disease caused by a bacterial,
viral or other microbial infection contracted by the ingestion
of contaminated food or water while abroad. According to the
United States Centers for Disease Control and Prevention, or
CDC, travelers’ diarrhea is the most common illness
affecting travelers, with an estimated 20% to 50% of
international travelers contracting travelers’ diarrhea.
Enterotoxigenic E coli bacteria (ETEC) is believed to be
the most common cause of travelers’ diarrhea. Ingested ETEC
bacteria move to the small intestine where they grow and secrete
toxins which induce massive fluid secretion from the lining of
the small intestine. Common symptoms associated with
ETEC-induced diarrhea include
5
frequent loose or watery bowel movements, nausea, vomiting,
abdominal cramping, bloating and fever. A majority of affected
individuals tend to have six or more loose stools per day.
Severe infections that cause large fluid loss may cause
dehydration and require hospitalization in order to administer
intravenous fluids. ETEC secretes one or both of two different
toxins: heat labile toxin (LT) and heat stabile toxin (ST). LT,
either acting alone or in combination with ST, is responsible
for approximately two-thirds of all cases of ETEC disease. Each
year more than 50 million individuals from the United
States, Western Europe and Japan/Australia visit regions where
infection by ETEC is common, resulting in an estimated
10 million cases of travelers’ diarrhea caused by
LT-secreting ETEC strains. Our travelers’ diarrhea program
focuses on ETEC disease caused by LT-secreting strains.
Currently, in the United States there are no approved vaccines
against travelers’ diarrhea caused by ETEC infection.
Travelers’ diarrhea, consequently, is generally treated
with antibiotics, either prophylactically or following onset, or
with
over-the-counter
products that alleviate symptoms but do not treat the underlying
infection. Use of antibiotics either as a treatment or as a
prophylaxis can be effective but has many drawbacks. Multi-drug
resistance in ETEC infection is common, especially in the
developing world, and prophylactic antibiotic use is implicated
in causing patterns of widespread resistance. In addition,
patients must take many doses over several days or weeks for
antibiotics to be effective and this poses potential compliance
problems. Antibiotics also destroy beneficial resident
intestinal bacteria that normally keep in check any ingested
disease-causing bacteria. Following antibiotic treatment, these
toxic bacteria may grow rapidly leading to severe intestinal
inflammation and additional episodes of diarrhea. For these
reasons, health authorities generally prefer vaccination to
treatment with antibiotics for diseases preventable by vaccine.
We believe a vaccine that reduces the risk of contracting
travelers’ diarrhea caused by ETEC, or that reduces the
severity of the symptoms associated with such an infection,
would be appealing to travelers. According to a market study,
approximately 25% of United States travelers already seek
medications and vaccinations for other diseases, such as
hepatitis A and B, prior to traveling internationally.
Additional studies estimate that the worldwide market for a
vaccine to combat travelers’ diarrhea caused by ETEC
infection is approximately $450 million.
THE TCI
SOLUTION
TCI is a proprietary technology designed to trigger an immune
response by targeting Langerhans cells. After contact with an
infectious pathogen and its associated antigens, Langerhans
cells leave the skin and migrate to the nearest lymph node via
the lymphatic system. Upon arrival, the Langerhans cells present
the antigens in the lymph node, which triggers a potent and
highly specific immune response that persists to combat future
attacks by the invading pathogen. Because Langerhans cells are
evenly distributed throughout the human skin surface, we believe
that TCI-based immunizations may be delivered through a patch to
a variety of locations on the body.
Our research to date suggests that the immune response to
TCI-delivered vaccines may be improved by applying adjuvants
along with antigens to the skin. Specifically, we are exploring
the use of the LT toxin from ETEC as an adjuvant. While LT is
not currently approved by the FDA for any indication nor as a
component of any product approved by the FDA, LT has a long
history of experimental use as an adjuvant because of its
potency as a general immune system stimulant and has been used
preclinically with a wide variety of vaccine formulations,
including those utilizing peptides, proteins, conjugates, whole
killed cells and live vectors. Other means of delivery such as
injection, intranasal and oral delivery methods act
systemically, preventing the use of LT as an adjuvant without
the occurrence of significant side effects. TCI avoids these
side effects by delivering LT to the skin, where the LT is
sequestered, rather than delivered systemically.
6
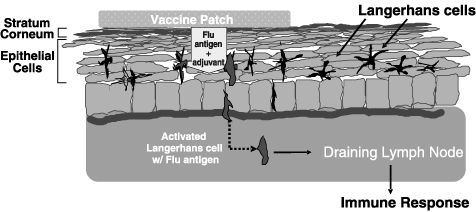
The diagram below highlights the use of TCI in a needle-free
vaccine patch.
Our needle-free vaccine patch is designed to work as follows:
|
|
|
| |
•
|
Following pretreatment that involves a mild abrasion of the skin
at the application point to disrupt the outer dead layer of
skin, known as the stratum corneum, a patch containing an
adjuvant and one or more antigens from the target pathogen (the
three antigens associated with the flu vaccine each year, for
example) is applied.
|
| |
| |
•
|
After permeating the disrupted stratum corneum, the adjuvant and
antigens reach and activate the underlying Langerhans cells.
|
| |
| |
•
|
The Langerhans cells take up the adjuvant and antigens and
migrate through the skin and the lymphatic system to the nearest
lymph node, where they stimulate a systemic immune response.
|
We believe that our needle-free vaccine patch offers the
following potential advantages over traditional, injection-based
approaches to vaccination:
|
|
|
| |
•
|
Increased efficacy with reduced risk of side
effects. Because the adjuvant and antigens
delivered by our needle-free vaccine patch cannot penetrate
further than the outer layers of the skin, neither the adjuvant
nor the antigens circulate systemically as they would if
delivered through injection. Preventing a systemic distribution
of adjuvants and antigens reduces the risk of side effects. As a
result, our needle-free vaccine patch permits the use of more
potent adjuvants relative to other systemic means of delivery
(such as injection, intranasal and oral delivery), which
increases the overall level of immune response. We and our
collaborators have conducted over 30 trials, including three
double-blind, placebo-controlled trials, with over 3,500
volunteers receiving the LT, and no vaccine-related serious
adverse events from applying this adjuvant to the skin were
observed.
|
| |
| |
•
|
Stable at ambient temperatures. Most vaccines
must be stored in refrigerated environments. We believe our
vaccines will be stable at room temperature, making both
transport and storage without refrigeration simple and
inexpensive. We now have data that demonstrates research-grade
patches for our needle-free travelers’ diarrhea and flu
vaccine programs are stable at room temperature for at least
12 months.
|
| |
| |
•
|
Self-application: All but a handful of
vaccines must be administered by a healthcare worker. We believe
that our patch system has the potential to be self-administered,
thereby expanding the market opportunities for existing vaccines
and solving many of the logistical issues associated with
governmental mass vaccination programs as part of biodefense and
pandemic flu preparedness. In 2006, we demonstrated in a
Phase 1 needle-free travelers’ diarrhea trial that
there was no difference in immune response between
self-application and a patch administered by a healthcare worker.
|
| |
| |
•
|
Increased compliance. We believe needle-free
administration has the potential to increase acceptance by
patients who dislike the use of needles and desire a more
convenient and less painful means of vaccination.
|
7
|
|
|
| |
•
|
Safer administration and disposal. Needle-free
administration eliminates the risk to the health care provider
of inadvertent needle sticks and avoids the need to dispose of
contaminated needles.
|
| |
| |
•
|
Reduced dosages. By targeting the skin and
eliciting a stronger immune response, we believe the amount of
antigen required for immunization may be reduced.
|
Our IS patch uses TCI-administered adjuvants to boost the immune
response to traditional, injectable vaccines in specific patient
populations. Our IS patch would work similarly to our
needle-free vaccine patch, except that a needle would be used to
inject the pathogenic antigens and a patch containing only an
adjuvant would be applied to the skin over the injection site.
We believe our IS patch could be applied to boost the immune
response in individuals who might not otherwise achieve immunity
through the injected vaccine alone. In a European Phase 2
trial, elderly subjects who received an IS patch in combination
with an injected flu vaccination generated antibody levels that
were significantly higher than the antibody level generated with
an injected flu vaccine alone. We also believe our IS patch
could be used to lower the injected vaccine dosage necessary to
immunize individuals with healthy immune responses.
PRODUCTS
IN DEVELOPMENT
We are developing two distinct product applications for our TCI
technology. The first application is a needle-free vaccine patch
applied to the skin that contains both pathogen-specific
antigens and an adjuvant. We are using this application to
develop needle-free vaccines to replace currently injectable
vaccines as well as novel vaccines that cannot be delivered by
injection. The second application is an immunostimulant, or IS,
patch for use in combination with an injected vaccine and
through which only an adjuvant is delivered by the patch. In
this second application, we expect that our IS patch would be
placed over the injection site of an existing injectable vaccine
in order to stimulate a stronger immune response to that
vaccine. Initially we expect to target our IS patch for use in
patient populations with weakened or impaired immune systems,
such as the elderly, for whom existing vaccines often do not
elicit an adequate immune response or in pandemic flu
applications to expand the supply of vaccine. For the years
ended December 31, 2006, 2005 and 2004, our research and
development costs were approximately $28.0 million,
$16.5 million and $14.3 million, respectively.
We are focusing our current product development efforts on the
product candidates set forth in the chart below.
| |
|
|
|
Product Candidates
|
|
Clinical Status in 2007
|
|
|
|
Influenza
|
|
|
|
IS patch for pandemic flu
(under DHHS contract)
|
|
Phase 1
|
|
IS patch for elderly receiving flu
vaccines
|
|
Phase 2
|
|
Needle-free flu vaccine patch
|
|
Phase 1
|
|
Needle-free pandemic flu vaccine
patch
|
|
Preclinical
|
|
Travelers’
Diarrhea
|
|
|
|
Needle-free travelers’
diarrhea vaccine patch
|
|
Phase 2
|
We view our IS patches and our needle-free travelers’
diarrhea vaccine patch as distinct products. Although both
patches contain the same active ingredient, they will have
distinct formulations and require separate regulatory approvals.
IS
patch for pandemic flu
We anticipate that the IS patch could play a role in containing
pandemic flu by helping governments execute dose-sparing
strategies by which a smaller dose of injected vaccine could
produce an effective immune response. We believe our IS patch,
when administered in combination with an injected vaccine, may
be able to achieve this either by reducing the required dose of
injected vaccine, or improving the timing
and/or
magnitude of the immune response to an injected pandemic flu
vaccine. We believe that our IS patch for pandemic flu presents
a particularly attractive dose-sparing alternative because it
has the potential to be used with pandemic flu vaccines being
developed by different manufacturers without requiring different
formulations or packaging.
8
In January 2007, the DHHS awarded us a five-year, cost-plus
reimbursement contract to fund our development of a dose-sparing
patch for use with a pandemic flu vaccine. If the product is
developed through licensure, the total cost reimbursed by DHHS,
plus a fixed fee, is estimated to be $128 million. During
the first 15 months of the contract, DHHS has allotted
approximately $14.5 million for us to assess the safety and
immunogenicity of the IS patch in clinical trials. To do this,
we expect to initiate a Phase 1 safety trial during the
third quarter of 2007 and then follow up that trial with a
larger Phase 1/2 trial, which is expected to start in the
fourth quarter of 2007, to test the safety and immunogenicity of
the IS patch with an injectable pandemic flu vaccine. We will be
testing our IS patch with an injectable pandemic flu vaccine
provided by our subcontractor, Solvay Pharmaceuticals. Also,
during the initial fifteen-month period, we are expected to
provide DHHS with a plan on how we would build up our capacity
to produce, within six months after the onset of an influenza
pandemic, 150 million doses of our IS patches.
In support of our application for the DHHS contract, we
conducted preclinical studies in animals that highlight the
dose-sparing potential of our IS patch. The goal of these
preclinical studies was to demonstrate that the use of our IS
patch, if confirmed in larger scale human studies, could
effectively expand the nation’s vaccine supply quickly. In
one study, we examined the effects of our IS patch when used in
combination with an avian flu antigen delivered either
intramuscularly, into the muscle tissue, or intradermally, just
below the skin surface. In this study, we immunized mice
intradermally with injections of an avian flu strain isolated
from Vietnam (H5N1) to look at the effect of immunizing with
declining flu doses with and without our IS patch. Accordingly,
the first group received 1.5 ug of vaccine and the other two
groups received doses ten times lower (0.15 ug) and 100 times
lower (0.015 ug), and half of each of these three groups had an
IS patch placed over the injection site. After two weeks, we
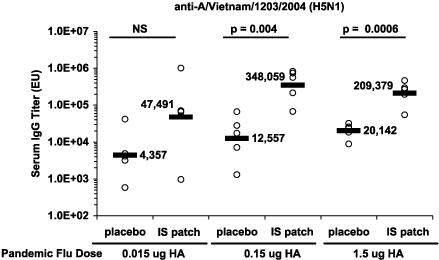
then measured antibody titers to the avian flu strain. As
indicated in the following chart, the group receiving the 0.015
ug dose with our IS patch had an antibody response (47,491)
comparable to the group receiving the 1.5 ug dose intradermally
alone (20,142). This data suggests that a 100-fold reduction in
vaccine may be achievable when the vaccine is delivered
intradermally in conjunction with our IS patch. Data that we
collected in the same study suggests that a ten-fold reduction
in vaccine may be achievable when the vaccine is delivered
intramuscularly, which is the current standard of care, in
conjunction with our IS patch. While intramuscular
administration of flu vaccines is the current standard of care,
recent studies have shown that flu vaccines administered
intradermally can achieve comparable immune response by using
less vaccine.
This work was preceded by a two-year, $2.9 million grant in
January 2005 from the National Institutes of Health, or NIH, for
further development of our IS patch technology for pandemic flu
applications.
Because pandemic flu vaccines and alternative strategies for
“dose-sparing” are still being developed by third
parties and no pandemic flu vaccines have been approved for use
by the FDA, and because our IS patch for pandemic flu is in the
preclinical development stage and extensive additional
preclinical and clinical testing would be required before
approval, this product candidate may not be available for the
potential treatment of pandemic flu in the next few years, if
ever.
9
IS
patch for elderly receiving flu vaccines
We are currently investigating ways to improve the immune
response in the elderly to standard influenza vaccination
through the use of adjuvants. We believe that the placement of
an IS patch over the injection site of an injected flu vaccine
will provide a stronger immune response in elderly recipients of
the flu vaccine.
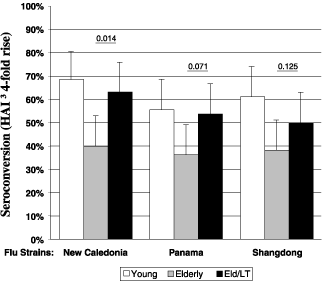
In 2002, we completed a European Phase 2 clinical study in
which elderly individuals who were vaccinated with an injected
flu vaccine in combination with our IS patch had a higher
antibody response, on average, than those receiving the standard
flu vaccine without an IS patch. The following chart describes
the immune response achieved in that clinical study, which had
three groups of approximately 55 volunteers each vaccinated with
a standard injected flu vaccine consisting of three strains of
flu antigen. The first group of volunteers consisted of healthy
young adults. The second and third groups consisted of elderly
(over 60 years old) volunteers. In the third group, the
volunteers were given both the injected vaccine and an IS patch.
In this trial, elderly individuals who were vaccinated with a
standard flu vaccine in combination with our IS patch had a
higher antibody response, on average, than those receiving the
flu vaccine without a patch. In addition, we did not observe any
systemic vaccine-related side effects in this trial.
Enhanced
Immune Responses In Elderly After IS Patch Application
We conducted a subsequent study in which we utilized a prior dry
patch formulation and were unable to demonstrate this same
effect. After analysis, we concluded that we were not able to
replicate the previous results due to a change in formulation,
which we believe caused the active agent, our LT adjuvant, to
remain in the patch and, therefore, not to be delivered to the
skin. Based on data from animal studies and data from a
travelers’ diarrhea clinical trials that have indicated
that our current dry patch formulation achieves high immune
responses in those vaccinated and outperforms our earlier
liquid-based patch, we expect our new dry patch formulations
will be at least equivalent to the wet patches used in the study
above; however, we will not know whether these dry patch
formulations will perform adequately to boost immune response in
the elderly to standard influenza vaccination until we obtain
clinical data in humans.
In February 2007, we commenced a Phase 1 safety study in
Australia with our current dry patch formulation, and we plan to
commence a Phase 2 study in the United States in the second
half of 2007 to confirm the results observed in our prior
European study. Following this trial, we expect to conduct
additional Phase 2 trials to evaluate dose ranging and
time-of-patch-wear.
We intend to request discussions with regulatory agencies in the
United States prior to initiating any Phase 3 studies in
order to finalize endpoints and design for our Phase 3
studies, which we expect to commence in 2008. Our IS patch, when
administered in combination with existing commercially available
injectable flu vaccines distributed by different flu vaccine
manufacturers, will require individual testing and FDA approval
with our IS patch, and is designed to improve the immune
response of the elderly to these flu vaccines.
10
Needle-free
flu vaccine patch
Our needle-free flu vaccine patch combines seasonal flu antigens
and an adjuvant in a single patch. We believe this approach
offers the potential for effective flu vaccination without the
use of needles.
We completed a Phase 1 study of our needle-free flu vaccine
patch in Europe during the fourth quarter of 2004 using our
earlier liquid-based patch. The data from our Phase 1 trial
demonstrated that the needle-free flu vaccine patch stimulated
an immune response to antigens from all three flu strains
recommended by the World Health Organization for the 2004 flu
season and demonstrated a positive effect from using our
adjuvant, although the observed immune response was not as great
as that of existing injectable flu vaccines. During 2005 and
2006, we worked to optimize our formulation and product design
in an effort to achieve an immune response equal to or greater
than that of existing injectable vaccines, which is one of the
requirements necessary in order to achieve regulatory approval
for this product candidate. Based on preclinical studies, we
believe we have reformulated this product candidate in a dry
patch formulation that will result in a greater immune response
than that observed in the 2004 Phase 1 study.
In the fall of 2006, we initiated a 300-person, multi-center
Phase 1 trial for our needle-free flu vaccine patch with
the new dry patch formulation. This study is designed to test
the safety and immunogenicity of our needle-free flu vaccine in
comparison with the traditional injected vaccine. Vaccinations
were completed during the first quarter of 2007, and we expect
interim data from this trial to be available by the middle of
2007. We expect this data to guide us in our design for a
Phase 2 program that could potentially support licensure of
a self-administered, needle-free flu vaccine patch.
Because we do not manufacture our own flu antigen, we conducted
our clinical trials for this using flu antigen supplied to us by
a flu vaccine manufacturer under a material transfer agreement.
We plan to work with companies who can provide a source of
vaccine antigen and complementary commercial capabilities, such
as sales and marketing expertise. We expect that we will not
advance our needle-free flu vaccine product candidate into
additional clinical trials after the current Phase 1 trial
until we enter into a long-term supply arrangement for flu
antigen.
Needle-free
pandemic flu vaccine patch
Our needle-free pandemic flu vaccine patch also combines
pandemic flu antigens and an adjuvant in a single patch. We are
in preclinical development of a needle-free pandemic flu vaccine
patch that combines pandemic flu antigen with an adjuvant in a
single patch. Leveraging our expertise from the needle-free
seasonal flu vaccine, we are currently conducting preclinical
testing of various needle-free pandemic flu formulations. We
believe that this vaccine patch could solve a great many issues
regarding mass vaccination in the event of an outbreak of
pandemic flu. Specifically, we believe that based on data from
our other programs, it might be possible to develop a
needle-free pandemic flu vaccine that (1) is stable at room
temperature, which could eliminate the need for refrigeration
and allow for distribution through the mail, and (2) can be
self-applied without the assistance of a healthcare worker.
Needle-free
travelers’ diarrhea vaccine patch
Our travelers’ diarrhea program is designed to reduce the
risk of contracting travelers’ diarrhea or reduce the
severity of symptoms in the event of infection. Our vaccine
candidate targets disease caused by LT-secreting ETEC. The LT
toxin alone or in conjunction with the ST toxin is the
pathogenic agent in approximately two-thirds of the cases of
ETEC infection. Immunity against LT has been shown to mitigate
travelers’ diarrhea caused by ETEC infection, which we
believe makes LT an excellent vaccine candidate. For our
needle-free travelers’ diarrhea vaccine patch, LT acts both
as the antigen and the adjuvant. Because the LT toxin is
sequestered in the skin, it is not delivered systemically in a
way that might cause diarrhea.
We have conducted several clinical studies of our
travelers’ diarrhea vaccine patch. In our initial
proof-of-principle
trial, a simple gauze patch containing a 500ug LT dose was
applied to the skin for six hours with no pretreatment. Three
such doses resulted in robust immune responses with no systemic
side effects. This initial finding, robust immunity without
systemic effects, for an LT-containing patch has been replicated
in all of our
11
subsequent trials in over 3,500 healthy volunteers, including
our recent trials using our current dry patch formulation, which
is about the size of a U.S. quarter and contains much
smaller doses of LT.
In other trials we have studied the effects of multiple doses
and the durability of response. For example, in a study
involving 60 volunteers where a needle-free travelers’
diarrhea vaccine patch was given on the first testing day and
again 21 days later, test subjects experienced a 12-fold
rise in antibody levels on the day the second patch was
administered and a 23-fold rise in antibody
levels 21 days after the second patch was administered
(see figure, below). The subjects did not experience any
vaccine-related systemic adverse events. Ninety-three percent of
subjects experienced a mild rash which was limited to the skin
that came in direct contact with the patch. The incidence of
this mild rash decreased during the second application. Three
hundred sixty days after receiving the second patch, 52 of the
53 returning test subjects retained LT antibodies in their
systems, suggesting that the vaccine effects will last for at
least one year.
Antibody
Response to Toxin After Vaccination
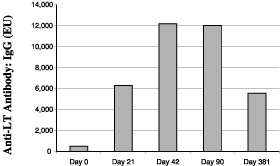
During the first quarter of 2005, we completed a clinical study
designed to test whether high antibody responses to LT delivered
in a patch could prevent or reduce the severity of
travelers’ diarrhea caused by oral ingestion of ETEC
bacteria. The clinical study found that volunteers who received
the vaccine before being exposed to high levels of ETEC bacteria
had less severe diarrhea and were significantly less likely to
require intravenous fluids than patients who were not
vaccinated. We conducted the trial with the assistance of
investigators at the Johns Hopkins School of Public Health.
Forty-seven volunteers participated in this double-blind,
placebo controlled clinical trial. Twenty-seven of the
volunteers were vaccinated with a liquid-based, or
“wet,” patch formulation of our needle-free
travelers’ diarrhea vaccine patch and 20 were given
placebos. At the end of the vaccination period, the volunteers
from both vaccinated and unvaccinated groups ingested
approximately one-half billion ETEC organisms. Although experts
estimate that between 1,000 and 100,000 bacteria can cause an
ETEC infection, we chose a considerably larger amount of ETEC
organisms to ensure exposure in substantially all of our
volunteers to an infectious dose. In this trial we also gave our
volunteers live bacteria that secreted both the LT and ST
toxins. This combination of high doses of ETEC bacteria and the
introduction of two toxins resulted in a severe test of our
vaccine candidate. After ingestion of the ETEC organisms, the
volunteers were carefully monitored for diarrhea. Similar
numbers in both vaccine and control groups met the definition of
moderate to severe illness, but subjects who received the
vaccine had significantly fewer loose stools (p=0.04) and lower
mean weights of the loose stools (p<0.05). In addition,
volunteers who did not receive the vaccine became ill more
quickly and were more likely to require intravenous fluids, with
40 percent of the control group receiving fluids compared
with only 14 percent of the vaccine group (p=0.03). Members
of the vaccine group also saw increases in two antibodies, IgA
and IgG, associated with protection against ETEC. After three
doses, all patients had a four-fold increase in serum IgG
antibodies and 97 percent had a four-fold increase in serum
IgA antibodies.
During 2006, we completed a Phase 1/2 travelers’
diarrhea human clinical trial involving 160 subjects in the
United States. In this study, we compared our new dry patch
formulation with our prior wet patch. One hundred subjects were
dosed using a dry formulation and 60 subjects have been dosed
using the prior wet formulation. The study was designed to
determine whether immune responses after wearing the dry patch
formulation were equal to those obtained with wet patches.
Subjects were tested weekly for immune responses to ETEC. By the
second week,
12
the fold rise in antibodies to the ETEC antigen in the dry patch
groups were significantly greater than those in the wet patch
group. In addition, dry patch recipients had at least
90 percent seroconversion rates with one dose. In another
Phase 1/2 trial, we demonstrated that in two groups of 20
subjects each, antibody responses to a dry LT patch self-applied
were not statistically different from a dry LT patch applied by
a healthcare worker. We also have gathered data that shows that
research-grade dry patches are stable for at least
12 months at room temperature. All this data could also
have important implications not only for our travelers’
diarrhea program, but for our other programs, particularly our
influenza programs, and could form the basis for our development
of vaccine patches that could be stockpiled, mailed and
self-applied — all important considerations
particularly in the event of a pandemic influenza outbreak.
In the second half of 2006, we also commenced two additional
Phase 2 studies for our travelers’ diarrhea vaccine
patch: a logistics study and a dose-ranging study. We designed
the Phase 2 logistics trial in approximately 200 subjects
to test our vaccine patch in volunteers traveling to sites in
Mexico and Guatemala. The placebo-controlled study is designed
to assess the safety of the vaccine and the frequency of ETEC
infection in volunteers traveling to sites where the disease is
endemic and to provide other operational information that will
serve as the groundwork for designing and executing our pivotal
field trial for this vaccine. The Phase 2 dose-ranging
study is examining four different LT doses in approximately 400
subjects and is expected to provide us with data to determine
our final dose formulation for the Phase 3 studies. We
expect interim data from both of these trials to be available
during the second and third quarters of 2007. Based on these
data, we will select a dose and study the final formulation in a
Phase 2 trial designed to confirm safety and immunogenicity
of the final patch configuration. That trial is expected to
commence in the fourth quarter of 2007. If we successfully
complete these Phase 2 trials in a timely fashion, we
expect to begin our Phase 3 clinical trials in late 2008.
Additional
indications
While we are currently focusing our resources on the five
programs described above, we believe that our needle-free
vaccine patch and IS patch technologies may be broadly
applicable. If we have adequate resources, we plan to explore
application of our technology to anthrax and other infectious
diseases, as well as to cancer, allergic diseases and other
indications. We have compiled Phase 1 data supporting the
ability of TCI-based vaccines to stimulate an immune response
against anthrax exposure. Anthrax is a rare, acute infectious
disease caused by bacteria that is contracted through the skin,
lungs or gastrointestinal system. The anthrax bioterrorism
attacks of 2001 heightened the urgency to develop a safe and
effective anthrax vaccine for use in the general population. The
only anthrax vaccine currently approved by the FDA requires six
injections over 18 months, has limited availability and
distribution, and is only indicated for at-risk individuals,
such as members of the military. We intend to seek a government
grant to fund the development of a vaccine patch candidate. At
this time, the United States government is not offering such a
grant. If we are successful, we would hope to have the vaccine
developed to the stage where it would be eligible for purchase
under the biodefense stockpile program.
INTELLECTUAL
PROPERTY AND PROPRIETARY TECHNOLOGY
Protection of our intellectual property and proprietary
technology is a strategic priority for our business. We rely on
a combination of patent, trademark, copyright and trade secret
laws along with institutional know-how and continuing
technological advancement to develop and maintain our
competitive position. Our ability to protect and use our
intellectual property rights in the continued development and
commercialization of our technologies and products, operate
without infringing the proprietary rights of others, and prevent
others from infringing our proprietary rights, is crucial to our
potential success. We will be able to protect our products and
technologies from unauthorized use by third parties only to the
extent that they are covered by valid and enforceable patents,
trademarks or copyrights, or are effectively maintained as trade
secrets, know-how or other proprietary information.
Our policy is to seek US and international patent protection for
technological developments that we believe will enhance the
market position of our product candidates and methods of using
our product candidates. As of March 8, 2007, we held
exclusive rights, either through our license from the Walter
Reed Army Institute of
13
Research (WRAIR) or on our own, to a patent portfolio consisting
of 68 issued patents or patent applications as follows:
|
|
|
| |
•
|
4 issued US patents;
|
| |
| |
•
|
13 US non-provisional patent applications;
|
| |
| |
•
|
23 issued foreign patents;
|
| |
| |
•
|
27 foreign patent applications that are in various national
stages of prosecution; and
|
| |
| |
•
|
1 foreign patent applications not at the national stage.
|
The issued United States patents will expire between November
2016 and February 2019. The issued foreign patents will expire
between November 2016 and February 2019. Our core patents claim
the composition of matter of the skin patch system as well as
methods for inducing an antigen specific immune response from
applying antigen and adjuvant to the skin. The first of these
patents expires in November 2016, but subsequent patents provide
additional coverage until February 2019. In addition, we are
prosecuting patent applications relating to the use of different
antigen and adjuvant combinations on the skin and other
improvements to our TCI technology, such as methods for inducing
an immune response by applying antigens and adjuvants to the
skin in dry form.
We license substantially all of our TCI technology from WRAIR.
Pursuant to a license agreement dated April 6, 2001, WRAIR
has granted us an exclusive, worldwide license to use the TCI
technology in all fields of use, including the right to grant
sublicenses subject to WRAIR’s approval. Each of our
current product candidates is dependent on TCI technology
subject to this license.
The terms of the WRAIR license require us to pay WRAIR royalties
equal to a percentage of:
|
|
|
| |
•
|
net sales of licensed products by us and by our
affiliates; and
|
| |
| |
•
|
revenues received by us from the licensed TCI technology and by
our affiliates under any sublicenses of the licensed TCI
technology.
|
In addition, we are required to pay to WRAIR:
|
|
|
| |
•
|
a minimum annual payment of $15,000 as a non-recoverable advance
against royalty and milestone payments;
|
| |
| |
•
|
milestone payments upon the achievement of specified regulatory
approval based milestones, which may total up to $1,560,000 in
the aggregate if we secure regulatory approval for six or more
products; and
|
| |
| |
•
|
all costs and expenses relating to prosecution of patent
protection for the licensed TCI technology.
|
We paid WRAIR a license issue fee of $150,000, and continue to
pay WRAIR minimum annual fees and milestone payments. We also
issued to WRAIR 57,500 shares of our common stock valued at
$34.125 per share on the date these shares were issued. The
common stock issued to WRAIR was subject to a put option, which
expired by its terms on February 1, 2006, the date of our
initial public offering. The common shares issued to WRAIR are
held in trust for WRAIR by MdBio, Inc. pursuant to a voting
trust and escrow agreement dated April 6, 2001. Under the
terms of this agreement, we are required to pay MdBio an annual
service fee of $5,000.
Our license is subject to a non-exclusive, non-transferable,
royalty-free right of the United States government to practice
the TCI technology for research and other governmental purposes
on behalf of the United States and on behalf of any foreign
government or international organization pursuant to an existing
or future treaty or agreement with the United States.
Additionally, WRAIR reserves the right to require us to grant
sublicenses to third parties if WRAIR determines that:
|
|
|
| |
•
|
such sublicenses are necessary to fulfill public health and
safety needs that we are not reasonably addressing;
|
| |
| |
•
|
such sublicenses are necessary to meet requirements for public
use specified by applicable United States government regulations
with which we are not reasonably in compliance; or
|
| |
| |
•
|
we are not manufacturing our products substantially in the
United States.
|
14
The license agreement with WRAIR will automatically terminate
upon the expiration of the last licensed patent which, based on
our current portfolio of issued patents and our assumption that
such patents will not be invalidated, will occur no earlier than
February 2019. WRAIR may unilaterally terminate or modify the
license if we:
|
|
|
| |
•
|
do not expend reasonable efforts and resources to carry out the
development and marketing of the licensed TCI technology and do
not manufacture, use or operate products that use the TCI
technology by April 2008 (subject to extension based upon a
showing of reasonable diligence in developing the technology);
|
| |
| |
•
|
do not continue to make our TCI-based products available to the
public on commercially reasonable terms after we have developed
such products;
|
| |
| |
•
|
misuse the licensed TCI technology or permit any of our
affiliates or
sub-licensees
to do so;
|
| |
| |
•
|
fail to pay royalties or meet our other payment or reporting
obligations under the license;
|
| |
| |
•
|
become bankrupt; or
|
| |
| |
•
|
otherwise materially breach our obligations under the license.
|
As we do not expect to have regulatory approval for any of our
TCI-based product candidates by April 2008, we may need to seek
an extension of the April 2008 clinical milestone.
We retain the right to terminate the WRAIR license with respect
to any or all countries covered by the license at any time, and
for any reason upon 60 days prior written notice. Upon
termination of the WRAIR license, other than by expiration, we
will be permitted to sell our existing inventory of licensed
products for a period of 180 days, after which time we will
no longer retain the right to make, use or sell products
licensed under the WRAIR license. Our right to make, use or sell
products subject to the WRAIR license will end immediately upon
expiration of the WRAIR license.
In June 2005, we licensed from DowPharma, on a non-exclusive,
world-wide basis, rights to the proprietary expression system
that we plan to use to manufacture the LT to be used in each of
our current product candidates. Pursuant to an existing letter
agreement for services with DowPharma, DowPharma has provided us
with technological assistance, as well as the master and working
cell banks of the expression system that we plan to use to
produce our LT.
In exchange for these licensed rights and such technical
assistance as DowPharma has and is expected to continue to
provide, the terms of the DowPharma license require us to pay
DowPharma:
|
|
|
| |
•
|
royalties equal to a percentage of net sales of products
incorporating LT produced under the license;
|
| |
| |
•
|
milestone payments based upon regulatory approval of products
incorporating LT produced under the license, which may total up
to $500,000 for each approved product; and
|
| |
| |
•
|
time-based fees for any continuing technical assistance we may
request at DowPharma’s prevailing hourly rate, with fees
for the first 500 hours of technical assistance due only
upon the date the first milestone payment, if any, is due.
|
Our payment obligations under the license do not go into effect
until our first product receives regulatory approval.
The DowPharma license also requires us to grant DowPharma and
its affiliates an irrevocable, world-wide, non-exclusive,
royalty-free license to any improvements to the expression
technology that we or our permitted sublicensees may create
during the term of the license.
The DowPharma license will automatically terminate upon the
earlier of (i) ten years from the date we first sell a
product incorporating LT produced under the license and
(ii) the end of the term of the last to expire patent
subject to the license which, assuming the relevant DowPharma
patent applications are issued and the resulting patents are not
invalidated, is expected to occur no earlier than
November 19, 2024. After expiration of the DowPharma
license,
15
we will retain an irrevocable and fully
paid-up
license to produce LT using DowPharma’s proprietary
expression system. DowPharma may unilaterally terminate the
license agreement prior to expiration if we:
|
|
|
| |
•
|
at any time during the term of the license agreement, reasonably
agree with Dow that there are material health and safety risks
associated with products produced under the license which create
a material risk of liability to DowPharma;
|
| |
| |
•
|
fail to meet our payment obligations under the license;
|
| |
| |
•
|
become bankrupt; or
|
| |
| |
•
|
otherwise materially breach our obligations under the license.
|
In the event DowPharma unilaterally terminates the license as
described above, we will not have any continuing right to make,
use or sell LT produced using DowPharma’s proprietary
expression technology.
Our contract with DHHS includes certain patent and data rights
provisions governing our rights and those of the
U.S. government in respect of patentable processes and
inventions and works subject to copyright, including software,
that we may develop under the contract and that are paid for by
DHHS. While we do not believe that our performance under the
DHHS contract will result in patentable processes or inventions,
or works subject to copyright, including software, that we would
need to market our IS patch technology in the future, our
performance under the DHHS contract subjects us to the risk that
the U.S. government may claim rights or interests in such
patentable processes or inventions or works subject to copyright.
We also rely on trade secrets and know-how, which are not
protected by patents, to maintain our competitive position. We
have implemented trade secret protection policies to actively
protect and secure the technology and proprietary information
that we develop. We require our officers, employees and
consultants to execute confidentiality agreements upon
commencement of their relationships with us. These agreements
also provide that all inventions developed by the individual on
behalf of us must be assigned to us and that the individual will
cooperate with us to secure patent protection for these
inventions if we wish to pursue such protection. There can be no
assurance, however, that these agreements will provide
meaningful protection for our inventions, trade secrets or other
proprietary information in the event of unauthorized use or
disclosure of such information.
We also execute confidentiality agreements with outside
collaborators. However, disputes may arise as to the ownership
of proprietary rights to the extent that outside collaborators
apply technological information developed independently by them
or others to projects involving our technology and there can be
no assurance that any such disputes would be resolved in our
favor. In addition, any of these parties may breach the
agreements and disclose our confidential information or our
competitors might learn of the information in some other way. If
any of our trade secrets, know-how or other proprietary
information that is not protected by a patent were to be
disclosed to or independently developed by a competitor, our
business, results of operations and financial condition could be
adversely affected.
MANUFACTURING
AND COMMERCIAL SUPPLY
To maintain control over the manufacture of our product
candidates during development and possibly into
commercialization, we constructed a pilot production plant in
which we currently manufacture the LT and the finished patch
formulations for our clinical products. In connection with the
construction of our pilot plant, we engaged DowPharma, a
business unit within The Dow Chemical Company, to assist us with
the development of our own LT strain and associated
manufacturing process. In June 2005, we licensed from DowPharma
a proprietary expression system for our LT strain. As part of
the technology transfer under the license, DowPharma provided us
with technological assistance, as well as the master and working
cell banks of the expression system that we currently use to
produce our LT in our pilot plant.
Our LT requires precise, high quality manufacturing that is
subject to current Good Manufacturing Practices, or cGMP. Since
the third quarter of 2005, we have been actively producing LT
and investigational patch formulations for use in clinical
trials in accordance with cGMP standards. We believe that our
pilot plant has adequate capacity to meet our currently
anticipated clinical trial needs, and we currently intend to use
this facility for all future clinical production of LT adjuvant
and formulated patches. To the extent practicable, we also
intend to
16
use this facility for initial commercial launch of our
needle-free travelers’ diarrhea vaccine patch, if we obtain
marketing approval.
The only product components that we currently intend to
manufacture at our pilot plant are our LT and formulated
patches. We currently intend to rely on either a collaborator or
third-party supplier to produce other product components, such
as the flu antigens contained in our needle-free flu vaccine
patch. A flu vaccine manufacturer is currently supplying flu
antigens for our Phase 1 clinical trial for our needle-free
flu vaccine patch under a material transfer agreement. As
variability in product specifications and characteristics
generally is not acceptable to regulatory authorities, our
primary sourcing strategy will be to establish long-term,
single-source relationships with suppliers of these other
components. For this reason, we expect that we will need to
enter into a long-term supply arrangement for flu antigen with a
flu vaccine manufacturer before advancing our needle-free flu
vaccine patch product candidate into additional clinical trials.
If there are no existing suppliers or collaborators able or
willing to meet our selection criteria, then we will consider
contracting the manufacture of key components that we do not
manufacture ourselves to a contract manufacturer. At this time,
we have not entered into any agreements that provide us
assurance of continued supply of any of these other product
components.
COMPETITION
The pharmaceutical, biopharmaceutical and biotechnology
industries are intensely competitive and are characterized by
rapid and significant technological progress. Our competitors
include large integrated pharmaceutical and biotechnology
companies, universities, and public and private research
institutions which currently engage in, have engaged in or may
engage in efforts related to the discovery and development of
new biopharmaceuticals and vaccines, some of which may be
competitive. Almost all of these entities have substantially
greater research and development capabilities and financial,
scientific, manufacturing, marketing and sales resources than we
do, as well as more experience in research and development,
clinical trials, regulatory matters, manufacturing, marketing
and sales.
If approved for marketing, our product candidates may compete
with existing drugs and vaccines. For example, there are
multiple influenza vaccines approved for sale in both the United
States and Europe. These vaccines are marketed by companies that
include Novartis AG, GlaxoSmithKline plc, MedImmune, Inc.,
sanofi-aventis S.A. and Solvay S.A. In addition, we know of
multiple flu vaccine candidates that incorporate adjuvants to
enhance immune responses, particularly in the elderly and to
extend the supply of pandemic flu vaccines. Some of these flu
vaccines with adjuvants are being developed by pharmaceutical
companies that have substantial resources and well-established
reputations among physicians and patients, such as
sanofi-aventis, GlaxoSmithKline and Novartis. These adjuvanted
flu vaccines would compete against our IS patch for dose-sparing
of pandemic flu vaccines and improved flu vaccination in the
elderly. For example, both GlaxoSmithKline and Novartis were
awarded “dose-sparing” contracts by DHHS totaling
$63.3 million and $54.8 million, respectively, in
January 2007 to develop pandemic flu vaccines with their
proprietary adjuvant systems. Both GlaxoSmithKline and Novartis
have separately reported initial data indicating that low doses
(3.8 ug and 7.5 ug, respectively) of their adjuvanted pandemic
flu vaccines have elicited immune responses at levels considered
protective by health authorities, as well as strong immune
responses against “drifted” strains of pandemic flu
that have changed over time.
While there are no vaccines against ETEC infection that have
been approved for sale in the United States, we are aware of
several companies with ETEC vaccine product candidates that are
under development, which, if approved, would compete against our
needle-free travelers’ diarrhea vaccine patch. Those
companies with potential ETEC vaccine candidates include Avant
Immunotherapeutics, Inc., Cambridge Biostability Ltd., Crucell,
N.V., SBL Vaccin AB (acquired by Crucell) and Emergent
BioSolutions, Inc. In the absence of vaccines, travelers’
diarrhea is generally treated, either prophylactically or
following onset, with antibiotics or
over-the-counter
products that alleviate symptoms. Some of these antibiotics and
OTC products are marketed by pharmaceutical companies, such as
Bayer. In addition, Salix Pharmaceuticals, Inc. has announced
that it has completed a Phase 3 study and initiated another
to evaluate the efficacy and safety of an antibiotic
specifically designed to be taken prophylactically for
travelers’ diarrhea.
We are aware of companies that are developing alternative
methods to the syringe for delivering vaccines and
biopharmaceutical products. These alternative methods include
microneedles, electroporation, microporation, jet injectors,
nasal sprays and oral delivery. For example, 3M Drug Delivery
Systems, MacroFlux Corporation and
17
Becton, Dickinson and Company are developing microneedles and
intradermal devices to deliver drugs to the skin. PowderMed
Limited, which was recently acquired by Pfizer, Inc., has
developed a hand-held jet injector unit, MedImmune, Inc. has a
nasal flu spray that has been approved for sale in the United
States, ID Biomedical Corporation, which was acquired by
GlaxoSmithKline, has a nasal flu spray under development, and
sanofi-aventis has a micro-injection flu vaccine in Phase 3
clinical trials aimed at providing a superior immune response in
the elderly.. There are also a number of companies developing
drug delivery technologies that make microchannels in the skin,
including Altea Corporation, TransPharma Medical Ltd. and Sontra
Medical Corporation.
We believe that the principal competitive factors in the markets
for our product candidates will include:
|
|
|
| |
•
|
safety and efficacy profile;
|
| |
| |
•
|
product price;
|
| |
| |
•
|
ease of application;
|
| |
| |
•
|
length of time to receive regulatory approval;
|
| |
| |
•
|
product supply;
|
| |
| |
•
|
enforceability of patent and other proprietary rights; and
|
| |
| |
•
|
marketing and sales capability.
|
Because our product candidates are in early stages of
development, our relative competitive position in the future is
difficult to predict precisely.
GOVERNMENT
REGULATION
The testing, approval, manufacturing, labeling, advertising and
marketing, post-approval safety reporting, and export, among
other things, of our product candidates are extensively
regulated by governmental authorities in the United States and
other countries. In the United States, the FDA regulates
biopharmaceutical products under the Federal Food, Drug, and
Cosmetic Act, or FDCA, and other laws, including, in the case of
biologics, the Public Health Service Act. Each of our product
candidates consists of a patch and one or more active
ingredients, such as vaccines or adjuvants, and we expect that
each product candidate will be considered a separate
investigational vaccine by the FDA, even if any of its active
ingredients is part of an existing approved product. None of the
active ingredients in our product candidates have been approved
by the FDA for commercial sale in any product. We believe that
most of our product candidates will be regulated by the FDA as
biologics. We cannot market a biologic until we have submitted a
Biologics License Application, or BLA, to the FDA, and the FDA
has approved it. Both before and after approval is obtained,
violations of regulatory requirements may result in various
adverse consequences, including the FDA’s suspension of
clinical studies, delay in approving or refusal to approve a
product, suspension or withdrawal of an approved product from
the market, operating restrictions, and the imposition of civil
or criminal penalties.
The steps required before the FDA may approve a product for
marketing in the United States generally include:
|
|
|
| |
•
|
preclinical laboratory tests and animal tests;
|
| |
| |
•
|
the submission to the FDA of an investigational new drug
application, or IND, for human clinical testing, which must
become effective before human clinical trials may begin;
|
| |
| |
•
|
adequate and well-controlled human clinical trials to establish
the safety and efficacy of the product for its specific intended
use(s);
|
| |
| |
•
|
the submission to the FDA of a BLA;
|
| |
| |
•
|
satisfactory completion of an FDA inspection of the
manufacturing facility or facilities at which the product is
made to assess compliance with current good manufacturing
practices, or cGMP; and
|
| |
| |
•
|
FDA review and approval of the BLA.
|
18
Preclinical tests include laboratory evaluation of the product
candidate, as well as animal studies to assess the potential
safety and efficacy of the product candidate. We must submit the
results of the preclinical tests, together with manufacturing
information and analytical data, to the FDA as part of an IND,
which must become effective before we may commence human
clinical trials. The IND will automatically become effective
30 days after its receipt by the FDA, unless the FDA before
that time raises concerns or questions about the conduct of the
trials as outlined in the IND. In such a case, the IND sponsor
and the FDA must resolve any outstanding concerns before
clinical trials can proceed. We cannot be sure that submission
of an IND will result in the FDA allowing clinical trials to
begin, or that, once begun, issues will not arise that suspend
such trials.
Clinical trials involve the administration of the product
candidate to healthy volunteers or patients under the
supervision of principal investigators, generally physicians who
are not employed by or under the trial sponsor’s control.
An institutional review board must review and approve each
clinical study. Clinical trials typically are conducted in three
sequential phases, but the phases may overlap or be combined. In
Phase 1, the initial introduction of the drug into human
subjects, the drug is usually tested for safety (adverse
effects), dosage tolerance, and pharmacologic action.
Phase 2 usually involves studies in a limited patient
population to:
|
|
|
| |
•
|
evaluate preliminarily the efficacy of the drug for specific,
targeted conditions;
|
| |
| |
•
|
determine dosage tolerance and appropriate dosage; and
|
| |
| |
•
|
identify possible adverse effects and safety risks.
|
Phase 3 trials generally further evaluate clinical efficacy
and test further for safety within an expanded patient
population. We or the FDA may suspend clinical trials at any
time on various grounds, including a finding that subjects are
being exposed to an unacceptable health risk.
The results of the preclinical and clinical studies, together
with other detailed information, including information on the
manufacture and composition of the product candidate, are
submitted to the FDA in the form of a BLA requesting approval to
market the product. If the BLA contains all pertinent
information and data, the FDA will “file” the
application and begin review. The FDA may “refuse to
file” the BLA if it does not contain all pertinent
information and data. In that case, the applicant may resubmit
the BLA when it contains the missing information and data.
Before approving a BLA, the FDA will inspect the facilities at
which the product candidate is manufactured, and will not
approve the product candidate unless cGMP compliance is
satisfactory. The FDA may refuse to approve a BLA if applicable
regulatory criteria are not satisfied, require additional
testing or information, limit the indications for use
and/or
require postmarketing testing and surveillance to monitor the
safety or efficacy of a product.
The testing and approval process requires substantial time,
effort and financial resources, and we cannot be sure that any
of our product candidates will be approved on a timely basis, if
at all. The results of preclinical studies and initial clinical
trials are not necessarily predictive of the results from
large-scale clinical trials, and clinical trials may be subject
to additional costs, delays or modifications due to a number of
factors, including difficulty in obtaining enough patients,
investigators or product candidate supply. Failure by us or our
collaborators, licensors or licensees to obtain, or any delay in
obtaining, regulatory approvals or in complying with
requirements could adversely affect the commercialization of
product candidates and our ability to receive product or royalty
revenues. Also, if regulatory approval is granted, it is granted
for use of the product in a specific dosage form for a specific
indication. If a BLA holder wishes to market a product for a
different dosage form or indication, it is generally required to
submit and have approved a supplementary application.
Once the FDA approves a product, the BLA holder and
product’s manufacturers are required to comply with a
number of post-approval requirements. For example, we may be
required to conduct postmarketing testing to monitor the safety
and the efficacy of the marketed product, and we will be
required to report certain adverse reactions to the FDA and to
comply with certain requirements concerning advertising and
promotional labeling for our products. Also, quality control and
manufacturing procedures must continue to conform to cGMP
regulations after approval, and the FDA periodically inspects
manufacturing facilities to assess compliance with cGMP.
Accordingly, the manufacturer must continue to expend time,
monies and effort in the area of production and quality control
to maintain cGMP compliance. We expect to rely on third parties
to manufacture some of our product candidates after FDA
approval, which will also be required to comply with cGMP. In
addition, discovery of
19
problems may result in marketing restrictions on a product.
Also, new federal, state or local government requirements may be
established that could delay or prevent regulatory approval of
our product candidates or impose other additional restrictions
on our business.
Whether or not FDA approval has been obtained, approval of a
product by the comparable regulatory authorities of foreign
countries must be obtained prior to commencement of marketing of
the product in those countries. The approval process varies from
country to country and the time may be longer or shorter than
that required for FDA approval. In those countries, we also are
subject to foreign regulatory requirements governing clinical
trials, labeling, promotion and marketing, post-approval safety
reporting, export, pricing and reimbursement, which also vary
greatly from country to country.
We are also subject to various federal, state and local laws,
rules, regulations and policies relating to safe working
conditions, laboratory and manufacturing practices, the
experimental use of animals and the use and disposal of
hazardous or potentially hazardous substances used in connection
with our research work. Although we have developed safety
procedures for handling and disposing of such materials, the
risk of accidental injury or contamination from these materials
cannot be entirely eliminated.
SALES AND
MARKETING
We currently have no sales, marketing or distribution
capability. In order to commercialize any of our product
candidates, we must either internally develop sales, marketing
and distribution capabilities or make arrangements with a third
party to perform these services. We believe that by presenting a
distinctive route of administration, TCI technology offers
marketing and branding opportunities for us and our potential
strategic partners. We intend to sell, market and distribute
some products directly and rely on relationships with third
parties to sell, market and distribute other products. To market
any of our products directly, we must develop a marketing and
sales force with technical and regulatory expertise and with
supporting distribution capabilities.
EMPLOYEES
As of December 31, 2006, we directly employed
101 people full-time, of whom one has an M.D. degree, 19
have Ph.D. degrees, and two have DVM degrees. Of our workforce,
86 employees are engaged in research and development and 15 are
engaged in business development, finance and administration.
None of our employees is represented by a labor union or covered
by a collective bargaining agreement. We consider our
relationship with our employees to be good.
CORPORATE
INFORMATION
We were incorporated in Delaware on May 14, 1997. Our
address is 20 Firstfield Road, Suite 250, Gaithersburg,
Maryland 20878, U.S.A. Our telephone number is
(301) 556-4500.
AVAILABILITY
OF PERIODIC SEC REPORTS
Our Internet website address is www.iomai.com. We make
available free of charge through our website our annual reports
on
Form 10-K,
quarterly reports on
Form 10-Q,
current reports on
Form 8-K,
and amendments to those reports filed or furnished pursuant to
Section 13(a) or 15(d) of the Exchange Act as soon as
reasonably practicable after we electronically file such
material with, or furnish such material to, the SEC. The
contents of our website are not part of, or incorporated into,
this document. The reports and other information we file with
the SEC can be read and copied at the SEC’s Public
Reference Room at 100 F Street, N.E., Washington D.C. 20549.
Copies of these materials can be obtained at prescribed rates
from the SEC’s Public Reference Room at such address. You
may obtain information regarding the operation of the public
reference room by calling
1-800-SEC-0330.
The SEC also maintains a web site (http://www.sec.gov) that
contains reports, proxy and information statements and other
information regarding issuers that file electronically with the
SEC.
20
Our future operating results may differ materially from the
results described in this annual report due to the risks and
uncertainties related to our business and our industry,
including those discussed below. In addition, these factors
represent risks and uncertainties that could cause actual
results to differ materially from those implied by
forward-looking statements in this report. We refer you to our
“Cautionary Statement Regarding Forward-Looking
Statements,” at the beginning of this report, which
identifies forward-looking statements in this report. The risks
described below are not the only risks we face. Additional risk
and uncertainties not currently known to us or that we currently
deem to be immaterial may also materially and adversely affect
our business, financial condition or results of operations.
Risks
Relating to Our Business
We are
a biopharmaceutical company with a limited operating history and
have generated no revenue from product sales. As a development
stage company, we face many risks inherent in our business. If
we do not overcome these risks, our business will not
succeed.
Biopharmaceutical product development is a highly speculative
undertaking and involves a substantial degree of risk. We
commenced operations in May 1997, and since that time we have
been engaged in research and development activities in
connection with our product candidates. We have never generated
any revenue from product sales. All of our current product
candidates are at an early stage of development, and we do not
expect to realize revenue from product sales for several more
years, if at all. In addition, we are not currently receiving
any significant funding for our research and development
programs from third parties through collaborations or grants,
except for our DHHS contract to develop a
“dose-sparing” IS patch in the event of a pandemic flu
outbreak. We are seeking to create a business based upon new
technology that is intended to change existing practices of
vaccine delivery. As such, we are subject to all the risks
incident to the creation of new products and may encounter
unforeseen expenses, difficulties, complications and delays and
other unknown factors. You also should consider that we will
need to:
|
|
|
| |
•
|
obtain sufficient capital to support our efforts to develop our
technology and commercialize our product candidates;
|
| |
| |
•
|
complete and continue to enhance the product characteristics and
development of our product candidates; and
|
| |
| |
•
|
attempt to transition from a development stage company to a
company capable of supporting commercial activities.
|
We
have a history of operating losses and may never be
profitable.
We have incurred substantial losses since our inception, and we
expect to continue to incur substantial losses for the
foreseeable future. Our net loss for the twelve months ended
December 31, 2006 was $31.8 million. As of
December 31, 2006, we had an accumulated deficit of
approximately $100.8 million. These losses have resulted
principally from costs incurred in our research and development
programs and from our general and administrative costs. We
expect to incur additional operating losses in the future, and
we expect these losses to increase significantly, whether or not
we generate revenue, as we expand our product development and
clinical trial efforts. These losses have had, and are expected
to continue to have, an adverse impact on our working capital,
total assets and stockholders’ equity.
To date, we have generated no revenue from product sales or
royalties. We do not expect to achieve any revenue from product
sales or royalties unless and until we receive regulatory
approval and begin commercialization of our product candidates.
We are not certain of when, if ever, that will occur.
Even if the regulatory authorities approve any of our product
candidates and we commercialize these candidates, we may never
be profitable. Even if we achieve profitability for a particular
period, we may not be able to sustain or increase profitability.
21
We
will need additional funding, and we cannot guarantee that we
will find adequate sources of capital in the
future.
As of December 31, 2006, we had approximately
$15.3 million in unrestricted cash, cash equivalents and
marketable securities. We have incurred negative cash flows from
operations since inception and have expended, and expect to
continue to expend, substantial funds to conduct our research
and development programs. On January 17, 2007, we were
awarded a five-year, $128 million contract by DHHS to fund
our development of a dose-sparing patch for use with a pandemic
flu vaccine. During the first 15 months of the contract,
DHHS has allotted approximately $14.5 million to reimburse
us for our activities under that contract. Also, on
March 2, 2007, we closed a private placement in which we
raised approximately $30.3 million in net proceeds after
expenses. We believe that our current working capital and
reimbursement of expenses under our existing government grants
and contracts will be sufficient to fund our operating expenses
and capital requirements through the second quarter of 2008,
although we cannot assure you that we will not require
additional funds before then. We have based this estimate on
assumptions that may prove to be wrong. Our future capital
requirements will depend on many factors, including:
|
|
|
| |
•
|
the number, size and complexity of our clinical trials;
|
| |
| |
•
|
our progress in developing our product candidates;
|
| |
| |
•
|
the timing of and costs involved in obtaining regulatory
approvals;
|
| |
| |
•
|
costs of manufacturing our product candidates;
|
| |
| |
•
|
costs to maintain, expand and protect our intellectual property
portfolio; and
|
| |
| |
•
|
costs to develop our sales and marketing capability.
|
We expect to seek to raise additional funds in advance of when
we exhaust our cash resources, which, if we raise additional
funds by issuing equity securities, will result in further
dilution to our stockholders. Any equity securities issued also
may provide for rights, preferences or privileges senior to
those of holders of our common stock. If we raise additional
funds by issuing debt securities, these debt securities would
have rights, preferences and privileges senior to those of
holders of our common stock, and the terms of the debt
securities issued could impose significant restrictions on our
operations. If we raise additional funds through collaborations
and licensing arrangements, we might be required to relinquish
significant rights to our technology, product candidates or
products that we would otherwise seek to develop or
commercialize ourselves, or to grant licenses on terms that are
not favorable to us.
We do not know whether additional financing will be available on
commercially acceptable terms when needed. If adequate funds are
not available or are not available on commercially acceptable
terms, we may need to downsize or halt our operations and may be
unable to continue developing our products.
The
success of some programs, such as our needle-free flu vaccine
patch, may depend on licensing biologics from, and entering into
collaboration arrangements with, third parties. We cannot be
certain that our licensing or collaboration efforts will succeed
or that we will realize any revenue from them.
The success of our business strategy is, in part, dependent on
our ability to enter into multiple licensing and collaboration
arrangements and to manage effectively the numerous
relationships that will result. For example, we currently do not
intend to manufacture any product components other than heat
labile toxin (LT) adjuvant and formulated patches. Therefore, we
will need to negotiate agreements to acquire biologics, such as
the flu antigens contained in our needle-free flu vaccine patch,
and other product components from third parties in order to
develop some of our vaccine candidates (other than our
needle-free travelers’ diarrhea vaccine patch and IS
patch). We are seeking a collaboration with respect to our
needle-free flu patch. A failure to enter into a collaboration
could delay development of this product candidate. Also, we may
not establish a direct sales force for our products and,
therefore, may need to establish marketing arrangements with
third parties or major pharmaceutical companies.
Our ability to enter into agreements with commercial partners
depends in part on convincing them that our TCI technology can
help achieve and accelerate their goals or efforts. This may
require substantial time and effort on our
22
part. While we anticipate expending substantial funds and
management effort, we cannot assure you that a strategic
relationship will result or that we will be able to negotiate
additional strategic agreements in the future on acceptable
terms, if at all. Furthermore, we may incur significant
financial commitments to collaborators in connection with
potential licenses and sponsored research agreements. Generally,
we will not be able to directly control the areas of
responsibility undertaken by our strategic partners and will be
adversely affected should these partners prove unable to carry
the product candidates forward to full commercialization or
should they lose interest in dedicating the necessary resources
toward developing such products quickly.
Third parties may terminate our licensing and other strategic
arrangements if we do not perform as required under these
arrangements. Generally, we expect that agreements for rights to
develop technologies will require us to exercise diligence in
bringing product candidates to market and will require us to
make milestone and royalty payments that, in some instances, are
substantial. Our failure to exercise the required diligence or
make any required milestone or royalty payment could result in
the termination of the relevant license agreement, which could
have a material adverse effect on us and our operations. In
addition, these third parties may also breach or terminate their
agreements with us or otherwise fail to conduct their activities
in connection with our relationships in a timely manner. If we
or our partners terminate or breach any of our licenses or
relationships, we:
|
|
|
| |
•
|
may lose our rights to develop and market our product candidates;
|
| |
| |
•
|
may lose patent
and/or trade
secret protection for our product candidates;
|
| |
| |
•
|
may experience significant delays in the development or
commercialization of our product candidates;
|
| |
| |
•
|
may not be able to obtain any other licenses on acceptable
terms, if at all; and
|
| |
| |
•
|
may incur liability for damages.
|
Licensing arrangements and strategic relationships in our
industry can be very complex, particularly with respect to
intellectual property rights. Disputes may arise in the future
regarding ownership rights to technology developed by or with
other parties. These and other possible disagreements between us
and third parties with respect to our licenses or our strategic
relationships could lead to delays in the research, development,
manufacture and commercialization of our product candidates.
These disputes could also result in litigation or arbitration,
both of which are time-consuming and expensive. These third
parties also may pursue alternative technologies or product
candidates either on their own or in strategic relationships
with others in direct competition with us.
Our IS
patch for pandemic flu program relies on government health
organizations for funding clinical development and for
ultimately procuring the product, if approved.
We are currently relying upon the United States government to
fund our IS patch for pandemic flu. In January 2007, we entered
into a five-year, $128 million cost-reimbursement contract
with the DHHS to develop a “dose-sparing” patch for
use in the event of an influenza pandemic where the DHHS will
fund our costs for research, development and capital and will
pay a fixed fee. Currently, DHHS has allocated
$14.5 million through the first 15 months of the
contract for us to assess the safety and immunogenicity of the
patch in two clinical trials and to develop plans on how we
would produce 150 million IS patches in a six-month period,
as required under the contract. If the results for either of
these clinical trials are not deemed adequate by DHHS or we fail
to satisfy, from DHHS’s perspective, the requirements
regarding our product development and manufacturing plans, DHHS
may not extend the contract through its full term, in which
event we may not be able to proceed with the balance of the
contract.
In the performance under the DHHS contract, we are highly
dependent on timely and adequate performance of our
subcontractors, including Solvay Pharmaceuticals, which is
supplying us with injectable pandemic flu vaccine for our
clinical trials, and our architectural design and engineering
firm. If the performance of our subcontractors is not adequate
or timely, our performance under our DHHS contract may be
delayed, and we therefore may not be able to satisfy the
contract’s requirements, which could cause us to be in
breach under those contracts and cause those contracts to be
terminated. We cannot assure you that one or more of these
subcontractors will not be delayed in performing, or fail to
perform their obligations under these contracts in compliance
with applicable legal requirements.
23
Although we have been awarded the DHHS contract, government
funding is subject to annual appropriations and other political
risks. As a result, the receipt by us of funds from the United
States government to fund our research and development of our IS
patch for pandemic flu beyond the $14.5 million already
allotted cannot be assured. In addition, we expect that United
States and foreign governments would be the entities procuring
any pandemic flu products, if approved, and not individual
consumers. While there has recently been increased public
awareness of the risks of pandemic flu, government health
organizations may not devote significant resources to the
prevention of an outbreak and may not seek to procure pandemic
flu products from us. The acceptance of our product candidate
for pandemic flu may depend on whether government health
organizations adopt a dose-sparing strategy to prevent pandemic
flu and whether a competing technology for dose sparing is
adopted. If a dose-sparing strategy is not endorsed or a
competing technology for dose sparing is adopted, our product
candidate will not yield any revenues.
The development and clinical testing of our IS patch for
pandemic flu product candidate will likely take several years.
Even if our IS patch for pandemic flu obtains regulatory
approval, by that time the threat of a pandemic flu outbreak may
be reduced or government health organizations may have adequate
stockpiles of flu vaccine or have adopted other technologies or
strategies to prevent or limit outbreaks.
Even if our IS patch for pandemic flu gains regulatory approval
and governmental health organizations choose to stockpile the
product, we may be not be able to produce enough of the product
to fulfill public health and safety needs.
U.S. government
agencies have special contracting requirements, which create
additional risks.
We have entered into a contract with DHHS, which is a
U.S. government agency. Currently, DHHS has allocated
$14.5 million through the first 15 months of the
contract for us to assess the safety and immunogenicity of the
patch in two clinical trials and to develop plans on how we
would produce 150 million IS patches in a six-month period,
as required under the contract. In contracting with government
agencies, we are subject to various U.S. government agency
contract requirements. Future revenues from DHHS and other
U.S. government agencies will depend, in part, on our
ability to meet U.S. government agency contract
requirements, certain of which we may not be able to satisfy.
U.S. government contracts typically contain unfavorable
termination provisions allowing the U.S. government to
terminate the contract at any time and are subject to audit and
modification by the government at its sole discretion, which
subjects us to additional risks. These risks include the ability
of the U.S. government to unilaterally:
|
|
|
| |
•
|
suspend or prevent us for a set period of time from receiving
new contracts or extending existing contracts based on
violations or suspected violations of laws or regulations;
|
| |
| |
•
|
terminate our existing contracts;
|
| |
| |
•
|
reduce the scope and value of our existing contracts;
|
| |
| |
•
|
audit and object to our contract-related costs and fees,
including allocated indirect costs;
|
| |
| |
•
|
control and potentially prohibit the export of our products; and
|
| |
| |
•
|
change certain terms and conditions in our contracts.
|
The U.S. government may terminate any of its contracts with
us either for its convenience or if we default by failing to
perform in accordance with the contract schedule and terms.
Termination for convenience provisions generally enable us to
recover only our costs incurred or committed and profit on the
work completed prior to termination, and settlement expenses
incurred in the termination for convenience process. Termination
for default provisions do not permit these recoveries and may
make us liable for excess costs incurred by the
U.S. government in procuring undelivered items from another
source.
As a U.S. government contractor, we are required to comply
with applicable laws, regulations and standards relating to our
accounting practices and are subject to periodic audits and
reviews. As part of any such audit or review, the
U.S. government may review the adequacy of, and our
compliance with, our internal control systems and
24
policies, including those relating to our purchasing, property,
estimating, compensation and management information systems.
Based on the results of its audits, the U.S. government may
adjust our contract-related costs and fees, including allocated
indirect costs. In addition, if an audit or review uncovers any
improper or illegal activity, we may be subject to civil and
criminal penalties and administrative sanctions, including
termination of our contracts, forfeiture of profits, suspension
of payments, fines and suspension or prohibition from doing
business with the U.S. government. We could also suffer
serious harm to our reputation if allegations of impropriety
were made against us. Although adjustments arising from
government audits and reviews have not seriously harmed our
business in the past, future audits and reviews could cause
adverse effects. In addition, under U.S. government
purchasing regulations, some of our costs, including most
financing costs, amortization of intangible assets, portions of
our research and development costs, and some marketing expenses,
may not be reimbursable or allowed under our contracts. Further,
as a U.S. government contractor, we are subject to an
increased risk of investigations, criminal prosecution, civil
fraud, whistleblower lawsuits and other legal actions and
liabilities to which purely private sector companies are not.
Our
technology is unproven and presents challenges for our product
development efforts.
Our TCI technology represents a novel approach to stimulating
the body’s immune system. There is no precedent for the
successful commercialization of product candidates based on our
technology. Developing biopharmaceuticals based on new
technologies presents inherent risks of failure, including:
|
|
|
| |
•
|
research could demonstrate that the scientific basis of our
technology is incorrect or less sound than we had believed;
|
| |
| |
•
|
unexpected research results could reveal side effects or other
unsatisfactory conditions that either will add cost to
development of our product candidates or jeopardize our ability
to complete the necessary clinical trials; and
|
| |
| |
•
|
time and effort required to solve technical problems could
sufficiently delay the development of product candidates such
that any competitive advantage that we may enjoy is lost.
|
All of our product candidates currently in development rely on
LT, a naturally occurring bacterial toxin, as an adjuvant. LT
has been shown to cause undesirable side effects when delivered
through conventional mechanisms such as injection, intranasal
and oral delivery methods. LT cannot be administered orally as
an adjuvant and was linked to an increase in the risk of
Bell’s palsy, a temporary paralysis of the facial muscles,
when used in a nasal flu vaccine that was on the market in
Europe during the 2000/2001 flu season. If we find that LT is
not safe when administered to patients through the skin using
our TCI technology, we will not be able to use LT as an adjuvant
in our products. This would have a material adverse effect on
our product development efforts.
Successful commercialization of our TCI technology will require
integration of multiple dynamic and evolving components, such as
antigens, adjuvants and a delivery mechanism, into finished
product candidates. This complexity will likely increase the
number of technical problems that we can expect to confront in
the clinical and product development processes and, therefore,
add to the cost and time required to commercialize each product
candidate.
As
part of our product development efforts, we may make significant
changes to our product candidates. These changes may not yield
the benefits that we expect.
We have made changes in the design or application of our product
candidates in response to preclinical and clinical trial results
and technological changes, and we may make additional changes in
the future before we are able to commercialize our products.
These and other changes to our product candidates may not yield
the benefits that we expect and may result in complications and
additional expenses that delay the development of our product
candidates. In addition, changes to our product candidates may
not be covered by our existing patents and patent applications,
and may not qualify for patent protection, which could have a
material adverse effect on our ability to commercialize our
product candidates. See the risk factor entitled “If we are
unable to protect our intellectual property, we may not be able
to operate our business profitably.”
25
We may
not be able to manufacture our product candidates in commercial
quantities, which would prevent us from initiating Phase 3
trials and commercializing our product candidates.
To date, our product candidates have been manufactured in small
quantities by us and third party manufacturers for preclinical
and clinical trials. As we prepare our facility for Phase 3
and commercial manufacturing, this will require scale up of our
fermentation, downstream processing and patch manufacturing as
compared to our current levels. For example, we have recently
installed manufacturing equipment capable of producing initial
commercial quantities of our vaccine patches. These projects may
result in unanticipated delays and cost more than expected due
to a number of factors, including complying with cGMP
regulations, such as qualification and validation of these
equipment, and this could result in the delay of initiation of
our planned Phase 3 trials and commercial launch of
products.
If any of our product candidates is approved by the FDA or other
regulatory agencies for commercial sale, we will need to
manufacture it in larger quantities. We or our third party
manufacturers may not be able to successfully increase the
manufacturing capacity for any of our product candidates in a
timely or economic manner, or at all. If we are unable to
successfully increase the manufacturing capacity for a product
candidate, the regulatory approval or commercial launch of that
product candidate may be delayed or there may be a shortage in
the supply of the product candidate. Our product candidates
require precise, high quality manufacturing that is subject to
cGMP. Our failure or the failure of our third party
manufacturers to achieve and maintain these high manufacturing
standards and comply with the cGMP, including the incidence of
manufacturing errors, could result in patient injury or death,
product recalls or withdrawals, delays or failures in product
testing or delivery, cost overruns or other problems that could
seriously harm our business. In addition, our manufacturing
facilities will be subject to unannounced inspections by the
FDA, and significant
scale-up of
manufacturing may require additional validation studies, which
the FDA must review and approve.
We
rely on third parties to conduct our clinical trials, and those
third-parties may not perform satisfactorily, including failing
to meet established deadlines for the completion of such
trials.
We do not have the ability to independently conduct clinical
trials for our vaccine candidates, and we rely on third parties
such as contract research organizations, medical institutions
and clinical investigators to enroll qualified patients and
conduct our clinical trials. Our reliance on these third parties
for clinical development activities reduces our control over
these activities. Furthermore, these third parties may also have
relationships with other entities, some of which may be our
competitors. If these third parties do not successfully carry
out their contractual duties or meet expected deadlines, our
efforts to obtain regulatory approvals for and commercialize our
vaccine candidates may be delayed or prevented.
We may
be required to conduct clinical trials of our IS patch with flu
vaccines developed by different manufacturers, which may lead to
added cost and delay in approval and commercialization of our IS
patch.
In order for our IS patch to gain approval from the FDA and to
be used with commercially available injectable flu vaccines, we
may need to conduct multiple clinical trials of our IS patch
with multiple flu vaccines developed by different manufacturers.
This may substantially expand the cost and time required for the
clinical trials of our IS patch, which could delay its potential
approval by the FDA and its commercialization.
The
skin preparation systems used in connection with our product
candidates may make our products less attractive to
consumers.
We have observed in our product development efforts that the
delivery of antigens and adjuvants to Langerhans cells through
the skin is significantly improved by prepping the skin to
disrupt the outer dead layer of skin, known as the stratum
corneum. Based on animal studies, we believe that the method and
level of skin preparation may differ from one product candidate
to another because of the different physical properties of the
active ingredients. Therefore, more than one skin preparation
system (SPS) may be required for our different products to be
effective. In recent clinical studies for our needle-free
travelers’ diarrhea and flu vaccine patches, we have tested
simple abrasive materials to disrupt the stratum corneum and are
now working to design and test improved embodiments of
26
these materials for use in our upcoming clinical trials for our
product candidates. We believe our SPS will be simple to perform
and will not involve discomfort to the patient. However, to the
degree that our SPS is not perceived to be simple to perform or
involve patient discomfort, it could reduce the attractiveness
of our products since alternative vaccines or treatments are
available for many of the indications that our product
candidates under development seek to address.
None
of our product candidates has been approved for commercial sale,
and we may never receive such approval.
All of our product candidates are in early pre-commercial
stages, and we do not expect our product candidates to be
commercially available for several years, if at all. We expect
that each of our product candidates, consisting of a patch and
one or more active ingredients, will be treated together as a
separate investigational product by the FDA, even if any active
ingredient is part of an existing approved product. None of the
active ingredients in our product candidates have been approved
by the FDA for commercial sale in any product. Our product
candidates are subject to stringent regulation by regulatory
authorities in the United States and in other countries. We
cannot market any product candidate until we have completed our
clinical trials and have obtained the necessary regulatory
approvals for that product candidate. We do not know whether
regulatory authorities will grant approval for any of our
product candidates.
Conducting clinical trials and obtaining regulatory approvals
are uncertain, time consuming and expensive processes. Our
product candidates must complete rigorous preclinical testing
and clinical trials. It will take us many years to complete our
testing, and failure could occur at any stage of testing.
Results of early trials frequently do not predict results of
later trials, and acceptable results in early trials may not be
repeated.
Even if we complete preclinical and clinical trials
successfully, we may not be able to obtain regulatory approvals.
Data obtained from preclinical and clinical studies are subject
to varying interpretations that could delay, limit or prevent
regulatory approval, and failure to observe regulatory
requirements or inadequate manufacturing processes are examples
of other problems that could prevent approval. In addition, we
may encounter delays or rejections due to additional government
regulation from future legislation, administrative action or
changes in FDA policy. Even if the FDA approves a product, the
approval will be limited to those indications encompassed in the
approval; marketing the product for a different indication would
require a supplementary application.
Outside the United States, our ability to market any of our
potential products is contingent upon receiving marketing
authorizations from the appropriate regulatory authorities.
These foreign regulatory approval processes include all of the
risks associated with the FDA approval process described above.
If our research and testing is not successful, or if we cannot
show that our product candidates are safe and effective, we will
be unable to commercialize our product candidates, and our
business may fail.
If
physicians and patients do not accept our products, we may be
unable to generate significant revenue.
Even if any of our vaccine candidates obtain regulatory
approval, they still may not gain market acceptance among
physicians, patients and the medical community, which would
limit our ability to generate revenue and would adversely affect
our results of operations. Physicians will not recommend
products developed by us or our collaborators until clinical
data or other factors demonstrate the safety and efficacy of our
products as compared to other available treatments. Even if the
clinical safety and efficacy of our products is established,
physicians may elect not to recommend these products for a
variety of factors, including the reimbursement policies of
government and third-party payors. There are other established
treatment options for the diseases that many of our product
candidates target, such as travelers’ diarrhea and the flu.
In order to successfully launch a product based on our TCI
technology, we must educate physicians and patients about the
relative benefits of our products. If our products are not
perceived as easy and convenient to use, for example as compared
to an injectable vaccine or antibiotics, or are perceived to
present a greater risk of side effects or are not perceived to
be as effective as other available treatments, physicians and
patients might not adopt our products. A failure of our
technology to gain commercial acceptance would have a material
adverse effect on our business. We expect that, if approved for
commercialization, our needle-free travelers’ diarrhea
vaccine patch will be paid for by patients out of pocket. Our
needle-free travelers’ diarrhea vaccine patch is not
designed to protect against all forms of travelers’
diarrhea, rather it is designed to
27
protect against only those cases of travelers’ diarrhea
caused by enterotoxigenic E. coli bacteria, or ETEC, and
in which the LT toxin is present. We estimate this to be
approximately one-third of all cases of travelers’
diarrhea. This could limit commercial acceptance of this
product. Because our needle-free flu vaccine patch and IS patch
candidates are targeted at preventing or ameliorating the
effects of flu infection, if the launch of these products for a
particular flu season fails, we may not receive significant
revenues from that product until the next season, if at all. See
the risk factors set forth below entitled “Our competitors
in the biopharmaceutical industry may have superior products,
manufacturing capability or marketing expertise,” “Our
ability to generate revenues will be diminished if we fail to
obtain acceptable prices or an adequate level of reimbursement
for our products,” and “We have no experience in
sales, marketing and distribution and will depend on the sales
and marketing efforts of third parties.”
Our
license to use TCI technology from The Walter Reed Army
Institute of Research, or WRAIR, is critical to our success.
Under some circumstances, WRAIR may modify or terminate our
license or sublicense the TCI technology to third parties which
could adversely affect our business.
We license substantially all of our patented and patentable TCI
technology through an exclusive license from WRAIR, a federal
government entity. This license will terminate automatically on
the expiration date of the last to expire patent subject to the
license, which, based on currently issued patents and our
assumption that such patents will not be invalidated, would be
February 2019. WRAIR may unilaterally modify or terminate the
license if we, among other things:
|
|
|
| |
•
|
do not expend reasonable efforts and resources to carry out the
development and marketing of the licensed TCI technology and do
not manufacture, use, or operate products that use the TCI
technology by April 2008 (subject to extension based upon a
showing of reasonable diligence in developing the technology);
|
| |
| |
•
|
do not continue to make our TCI-based products available to the
public on commercially reasonable terms after we have developed
such products;
|
| |
| |
•
|
misuse the licensed TCI technology or permit any of our
affiliates or
sub-licensees
to do so;
|
| |
| |
•
|
fail to pay royalties or meet our other payment or reporting
obligations under the license;
|
| |
| |
•
|
become bankrupt; or
|
| |
| |
•
|
otherwise materially breach our obligations under the license.
|
As we do not expect to have regulatory approval for any of our
TCI-based product candidates by April 2008, we may need to seek
an extension of the April 2008 milestone. If we violate the
terms of the WRAIR license, or otherwise lose our licensed
rights to the TCI technology, we would likely be unable to
continue to develop our products. WRAIR and third parties may
dispute the scope of our rights to the TCI technology under the
license. Additionally, WRAIR may breach the terms of its
obligations under the license or fail to prevent infringement or
fail to assist us to prevent infringement by third parties of
the patents underlying the licensed TCI technology. Loss or
impairment of the WRAIR license for any reason could materially
harm our financial condition and operating results.
In addition to WRAIR’s termination and modification rights
described above, our license is subject to a non-exclusive,
non-transferable, royalty-free right of the United States
government to practice the licensed TCI technology for research
and other governmental purposes on behalf of the United States
and on behalf of any foreign government or international
organization pursuant to any existing or future treaty or
agreement with the United States. WRAIR also reserves the right
to require us to grant sublicenses to third parties if WRAIR
determines that:
|
|
|
| |
•
|
such sublicenses are necessary to fulfill public health and
safety needs that we are not reasonably addressing;
|
| |
| |
•
|
such sublicenses are necessary to meet requirements for public
use specified by applicable United States government regulations
with which we are not reasonably in compliance; or
|
| |
| |
•
|
we are not manufacturing our products substantially in the
United States.
|
Although we are currently the only party licensed to actively
develop the TCI technology, we cannot assure you that WRAIR will
not in the future require us to sublicense the TCI technology.
Any action by WRAIR to force
28
us to issue such sublicenses or development activities
instituted by the United States government pursuant to its
reserved rights in the TCI technology would erode our ability to
exclusively develop products based on the TCI technology and
could materially harm our financial condition and operating
results.
Licenses of technology owned by agencies of the United States
government, including the WRAIR license, require that
licensees — in this case, us — and our
affiliates and
sub-licensees
agree that products covered by the license will be manufactured
substantially in the United States. This may restrict our
ability to contract for manufacturing facilities, if we attempt
to do so, outside the United States and we may risk losing our
rights under the WRAIR license, which could materially harm our
financial condition and operating results.
If we
are unable to protect our intellectual property, we may not be
able to operate our business profitably.
We base our TCI technology in large part on innovations for
which WRAIR has sought protection under the United States and
certain foreign patent laws. We consider patent protection of
our TCI technology to be critical to our business prospects. As
of March 8, 2007, there are four issued United States
patents, 23 issued foreign patents and 41 United States and
foreign patent applications relating to TCI and improvements on
the technology. The four issued United States patents will
expire between November 2016 and February 2019. The issued
foreign patents will expire between November 2016 and February
2019. Under our license agreement with WRAIR, we bear financial
responsibility for the preparation, filing, prosecution and
maintenance of any and all patents and patent applications
licensed. With respect to enforcement, we have the right to
bring actions to enforce patents licensed under the WRAIR
license agreement, subject to WRAIR’s continuing right to
intervene, and WRAIR maintains the right to bring enforcement
actions if we fail to do so.
Our contract with DHHS includes certain patent and data rights
provisions governing our rights and those of the
U.S. government in respect of patentable processes and
inventions and works subject to copyright, including software,
that we may develop under the contract and that are paid for by
DHHS. While we do not believe that our performance under the
DHHS contract will result in patentable processes or inventions,
or works subject to copyright, including software, that we would
need to market our IS patch technology in the future, our
performance under the DHHS contract subjects us to the risk that
the U.S. government may claim rights or interests in such
patentable processes or inventions or works subject to copyright.
Patent protection in the field of biopharmaceuticals is highly
uncertain and involves complex legal and scientific questions
and has recently been the subject of much litigation. We cannot
control when or if any patent applications will result in issued
patents. Even if issued, our patents may not afford us
protection against competitors marketing similar products.
Neither the US Patent and Trademark Office nor the courts have a
consistent policy regarding the breadth of claims allowed or the
degree of protection afforded under many biopharmaceutical
patents. The claims of our existing US patents and those that
may issue in the future, or those licensed to us, may not offer
significant protection of our TCI technology and other
technologies. Our patents on transcutaneous immunization, in
particular, are broad in that they cover the delivery of
antigens and adjuvants to the skin to induce an immune response.
While our TCI technology is covered by issued patents and we are
not aware of any challenges, patents with broad claims tend to
be more vulnerable to challenge by other parties than patents
with more narrowly written claims. Patent applications in the
United States and many foreign jurisdictions are typically not
published until 18 months following their priority filing
date, and in some cases not at all. In addition, publication of
discoveries in scientific literature often lags significantly
behind actual discoveries. Therefore, neither we nor our
licensors can be certain that we or they were the first to make
the inventions claimed in our issued patents or pending patent
applications, or that we or they were the first to file for
protection of the inventions set forth in these patent
applications. In addition, changes in either patent laws or in
interpretations of patent laws in the United States and other
countries may diminish the value of our intellectual property or
narrow the scope of our patent protection. Furthermore, our
competitors may independently develop similar technologies or
duplicate technology developed by us in a manner that does not
infringe our patents or other intellectual property.
29
If we
are unable to protect the confidentiality of our proprietary
information and know-how, the value of our technology and
products could be adversely affected.
In addition to patented technology, we rely upon unpatented
proprietary technology, processes and know-how. We and our
licensors seek to protect this information in part by
confidentiality agreements with employees, consultants and third
parties. These agreements may be breached, and there may not be
adequate remedies for any such breach. In addition, our trade
secrets may otherwise become known or be independently developed
by competitors. If we are unable to protect the confidentiality
of our proprietary information and know-how, competitors may be
able to use this information to develop products that compete
with our products, which could adversely impact our business.
If the
use of our technology conflicts with the intellectual property
rights of third parties, we may incur substantial liabilities,
and we may be unable to commercialize products based on this
technology in a profitable manner, if at all.
Our competitors or others may have or acquire patent rights that
they could enforce against us. In addition, we may be subject to
claims from others that we are misappropriating their trade
secrets or confidential proprietary information. If our
technology conflicts with the intellectual property rights of
others, they could bring legal action against us or our
licensors, licensees, suppliers, customers or collaborators. If
a third party claims that we infringe upon its proprietary
rights, any of the following may occur:
|
|
|
| |
•
|
we may become involved in time-consuming and expensive
litigation, even if the claim is without merit;
|
| |
| |
•
|
we may become liable for substantial damages for past
infringement if a court decides that our technology infringes
upon a competitor’s patent;
|
| |
| |
•
|
a court may prohibit us from selling or licensing our product
without a license from the patent holder, which may not be
available on commercially acceptable terms, if at all, or which
may require us to pay substantial royalties or grant cross
licenses to our patents; and
|
| |
| |
•
|
we may have to redesign our product so that it does not infringe
upon others’ patent rights, which may not be possible or
could be very expensive and time-consuming.
|
If any of these events occurs, our business will suffer and the
market price of our common stock will likely decline.
We may
be involved in lawsuits to protect or enforce our patents or the
patents of our collaborators or licensors, which could be
expensive and time consuming.
Competitors may infringe our patents or the patents of our
collaborators or licensors. To counter infringement or
unauthorized use, we may be required to file infringement
claims, which can be expensive and time-consuming. In addition,
in an infringement proceeding, a court may decide that a patent
of ours is not valid or is unenforceable, or may refuse to stop
the other party from using the technology at issue on the
grounds that our patents do not cover the technology in
question. An adverse result in any litigation or defense
proceedings could put one or more of our patents at risk of
being invalidated or interpreted narrowly and could put our
patent applications at risk of not issuing.
Interference proceedings brought by the US Patent and Trademark
Office may be necessary to determine the priority of inventions
with respect to our patent applications or those of our
collaborators or licensors. Litigation or interference
proceedings may fail and, even if successful, may result in
substantial costs and be a distraction to our management. We may
not be able, alone or with our collaborators and licensors, to
prevent misappropriation of our proprietary rights, particularly
in countries where the laws may not protect such rights as fully
as in the United States.
Furthermore, because of the substantial amount of discovery
required in connection with intellectual property litigation,
there is a risk that some of our confidential information could
be compromised by disclosure during this type of litigation. In
addition, there could be public announcements of the results of
hearings, motions or other
30
interim proceedings or developments. If securities analysts or
investors perceive these results to be negative, it could have a
substantial adverse effect on the price of our common stock.
We
depend on our key personnel, the loss of whom would adversely
affect our operations. If we fail to attract and retain the
talent required for our business, our business will be
materially harmed.
We are a small company with 101 employees as of
December 31, 2006, and we depend to a great extent on
principal members of our management and scientific staff. If we
lose the services of any key personnel, in particular, Stanley
Erck, our President and Chief Executive Officer, or Gregory
Glenn, our Chief Scientific Officer, it could significantly
impede the achievement of our research and development
objectives and could delay our product development programs and
approval and commercialization of our product candidates. We do
not currently have any key man life insurance policies. While we
have entered into agreements with certain of our executive
officers relating to severance after a change of control and
these officers have agreed to restrictive covenants relating to
non-competition and non solicitation, we have not entered into
employment agreements with members of our senior management team
other than Stanley Erck. Our agreement with Stanley Erck does
not ensure that we will retain his services for any period of
time in the future. Our success depends on our ability to
attract and retain highly qualified scientific, technical and
managerial personnel and research partners. Competition among
biopharmaceutical and biotechnology companies for qualified
employees is intense, and we may not be able to retain existing
personnel or attract and retain qualified staff in the future.
If we fail to hire and retain personnel in key positions, we
will be unable to develop or commercialize our product
candidates in a timely manner.
Our
competitors may have superior products, manufacturing capability
or marketing expertise.
Our business may fail because it faces intense competition from
major pharmaceutical companies, specialized biopharmaceutical
and biotechnology companies and drug development companies
engaged in the development and production of vaccines, vaccine
delivery technologies and other biopharmaceutical products.
Several companies are pursuing programs that target the same
markets we are targeting. In addition to pharmaceutical,
biopharmaceutical and biotechnology companies, our competitors
include academic and scientific institutions, government
agencies and other public and private research organizations.
Many of our competitors have greater financial and human
resources and more experience. Our competitors may:
|
|
|
| |
•
|
develop products or product candidates earlier than we do;
|
| |
| |
•
|
form collaborations before we do, or preclude us from forming
collaborations with others;
|
| |
| |
•
|
obtain approvals from the FDA or other regulatory agencies for
such products more rapidly than we do;
|
| |
| |
•
|
develop and validate manufacturing processes more rapidly than
we do;
|
| |
| |
•
|
obtain patent protection or other intellectual property rights
that would limit our ability to use our technologies or develop
our product candidates;
|
| |
| |
•
|
develop products that are safer or more effective than those we
develop or propose to develop; or
|
| |
| |
•
|
implement more effective approaches to sales and marketing.
|
Alternative competitive technologies and products could render
our TCI technology and our product candidates based on this
technology obsolete and non-competitive. Presently, there are a
number of companies developing alternative methods to the
syringe for delivering vaccines. These alternative methods
include microneedles, electroporations, microporations, jet
injectors, nasal sprays and oral delivery, and several of these
delivery mechanisms are in clinical trials.
While there are no vaccines against infection by ETEC that have
been approved for sale in the United States, we are aware of
several companies with ETEC vaccine product candidates that are
in development, which, if approved, would compete against our
needle-free travelers’ diarrhea vaccine patch. Those
companies with potential ETEC vaccine candidates include Avant
Immunotherapeutics, Inc., Crucell, N.V., Cambridge Biostability
Ltd., SBL Vaccin AB (acquired by Crucell) and Emergent
BioSolutions, Inc. One of our competitors, SBL Vaccin AB, has
announced the results from a study of an ETEC vaccine indicating
the vaccine may be effective in preventing
31
diarrhea caused by ETEC. In the absence of vaccines,
travelers’ diarrhea is generally treated, either
prophylactically or following onset, with antibiotics or
over-the-counter,
or OTC, products that alleviate symptoms. Some of these OTC
products and antibiotics, such as Cipro, are marketed by
pharmaceutical companies with substantial resources and enjoy
widespread acceptance among physicians and patients. In
addition, Salix Pharmaceuticals, Inc. has announced that it has
completed a Phase 3 study and initiated another to evaluate
the efficacy and safety of an antibiotic specifically designed
to be taken prophylactically for the prevention of
travelers’ diarrhea, and is targeting filing a license
application in the first half of 2008.
There are multiple influenza vaccines approved for sale in both
the United States and Europe. In many cases, these products are
manufactured and distributed by pharmaceutical companies with
substantial resources, such as Novartis AG, GlaxoSmithKline plc,
sanofi-aventis SA, Solvay SA and MedImmune, Inc. FluMist, a
nasal flu vaccine, has received marketing approval from the FDA
and would compete against our needle-free flu vaccine. We are
also aware of other flu vaccine candidates to be delivered by
alternative methods, such as nasal spray and skin delivery,
which, if approved, would compete against our needle-free flu
vaccine. In addition, we know of multiple flu vaccine candidates
that incorporate adjuvants to enhance immune responses,
particularly in the elderly. Some of these adjuvanted flu
vaccines are being developed by pharmaceutical companies with
substantial resources, such as sanofi-aventis, GlaxoSmithKline
and Novartis. These adjuvanted vaccines would compete against
our IS patch for the elderly and for pandemic flu applications.
For example, both GlaxoSmithKline and Novartis were awarded
“dose-sparing” contracts by DHHS totaling
$63.3 million and $54.8 million, respectively, in
January 2007 to develop pandemic flu vaccines with their
proprietary adjuvant systems. Both GlaxoSmithKline and Novartis
have reported separately initial data indicating that low doses
(3.8 ug and 7.5 ug, respectively) of their respective adjuvanted
pandemic flu vaccines have elicited immune responses at levels
considered protective by health authorities, as well as strong
immune responses against “drifted” strains of pandemic
flu that have changed over time. In addition, sanofi-aventis has
a micro-injection flu vaccine in Phase 3 clinical trials
aimed at providing a superior immune response in the elderly.
Our
ability to generate revenues will be diminished if we fail to
obtain acceptable prices or an adequate level of reimbursement
for our products.
We expect that most patients will rely on private health
insurers, Medicare and Medicaid and other third party payors to
pay for any products that we or our collaborators may market,
other than our needle-free travelers’ diarrhea vaccine
patch for which we expect patients will pay
out-of-pocket.
The continuing efforts of government and third party payors to
contain or reduce the costs of health care through various means
may limit our commercial opportunity. For example, in some
foreign markets, pricing and profitability of prescription
biopharmaceuticals are subject to government control. In the
United States, we expect that there will continue to be a number
of federal and state proposals to implement similar government
controls. In addition, increasing emphasis on managed care in
the United States will continue to put pressure on the pricing
of biopharmaceutical products. Cost control initiatives could
decrease the price that we would receive for any products in the
future.
Our ability to commercialize biopharmaceutical product
candidates, alone or with third parties, could be adversely
affected by cost control initiatives and also may depend in part
on the extent to which reimbursement for the product candidates
will be available from:
|
|
|
| |
•
|
government and health administration authorities;
|
| |
| |
•
|
private health insurers; and
|
| |
| |
•
|
other third party payors.
|
Significant uncertainty exists as to the reimbursement status of
newly approved health care products. Third party payors,
including Medicare, are challenging the prices charged for
medical products and services. Government and other third party
payors increasingly attempt to contain health care costs by
limiting both coverage and the level of reimbursement for new
biopharmaceutical products and by refusing, in some cases, to
provide coverage for uses of approved products for disease
indications for which the FDA has not granted labeling approval.
In the United States, there have been a number of legislative
and regulatory proposals to change the healthcare system in ways
that could affect our ability to market and sell our product
candidates profitably. In particular, in December
32
2003, President Bush signed into law new Medicare prescription
drug coverage legislation which went into effect on
January 1, 2006. Under this legislation, the Centers for
Medicare and Medicaid Services, or CMS, the agency within the
Department of Health and Human Services that administers
Medicare and is responsible for reimbursement of the cost of
drugs, has asserted the authority of Medicare to elect not to
cover particular drugs if CMS determines that the drugs are not
“reasonable and necessary” for Medicare beneficiaries
or to elect to cover a drug at a lower rate similar to that of
drugs that CMS considers to be “therapeutically
comparable.” Changes in reimbursement policies or health
care cost containment initiatives that limit or restrict
reimbursement for our products may cause our potential revenues
to decline. Third party insurance coverage may not be available
to patients for any product candidates we discover and develop,
alone or through our strategic relationships. If government and
other third party payors do not provide adequate coverage and
reimbursement levels for our product candidates, the market
acceptance of these product candidates may be reduced.
We
have no experience in sales, marketing and distribution and will
depend on the sales and marketing efforts of third
parties.
We plan to establish marketing arrangements with third parties
or major pharmaceutical companies and do not expect to establish
direct sales capability for several years. However, these types
of marketing arrangements might not be available on acceptable
terms, or at all. In the future, to market any of our product
candidates directly, we will need to develop a marketing and
sales force with technical expertise and distribution
capability. To the extent that we enter into marketing or
distribution arrangements, any revenues we receive will depend
upon the efforts of third parties. We cannot assure you that we
will be successful in gaining market acceptance for any products
we may develop.
Our
business exposes us to potential product liability
claims.
Our proposed products could be the subject of product liability
claims. A failure of our product candidates to function as
anticipated, whether as a result of the design of these
products, unanticipated health consequences or side effects, or
misuse or mishandling by third parties of such products, could
result in injury. Claims also could be based on failure to
immunize as anticipated. Tort claims could be substantial in
size and could include punitive damages. We cannot assure you
that any warranty disclaimers provided with our proposed
products would be successful in protecting us from product
liability exposure. Damages from any such claims could be
substantial and could affect our financial condition.
Our business exposes us to potential product liability risks
that are inherent in the testing, manufacturing and marketing of
biopharmaceutical products. We have obtained clinical trial
liability insurance for our clinical trials in the aggregate
amount of $10 million. We cannot be certain that we will be
able to maintain adequate insurance for our clinical trials. We
also intend to seek product liability insurance in the future
for products approved for marketing, if any. However, we may not
be able to acquire or maintain adequate insurance at a
reasonable cost. Any insurance coverage may not be sufficient to
satisfy any liability resulting from product liability claims. A
successful product liability claim or series of claims could
have a material adverse impact on our operations.
We
deal with hazardous materials that may cause injury to others
and are regulated by environmental laws that may impose
significant costs and restrictions on our
business.
Our research and development programs and manufacturing
operations involve the controlled use of potentially harmful
biological materials such as toxins from E. coli and
other hazardous materials. We cannot completely eliminate the
risk of accidental contamination or injury to others from the
use, manufacture, storage, handling or disposal of these
materials. In the event of contamination or injury, we could be
held liable for any resulting damages, and any liability could
exceed our resources or any applicable insurance coverage we may
have. We are also subject to federal, state and local laws and
regulations governing the use, manufacture, storage, handling or
disposal of hazardous materials and waste products. If we fail
to comply with these laws and regulations or with the conditions
attached to our operating licenses, then our operating licenses
could be revoked, we could be subjected to criminal sanctions
and substantial liability and we could be required to suspend,
modify or terminate our operations. We may also have to incur
significant costs to comply with future environmental laws and
regulations. We do not currently have a pollution and
remediation insurance policy.
33
Until
recently, we have operated as a private company and as a result,
we have limited experience attempting to comply with public
company obligations. Attempting to comply with these
requirements will increase our costs and require additional
management resources, and we still may fail to
comply.
In February 2006, we closed our initial public offering.
Previously, as a private company, we maintained a small finance
and accounting staff. While we expect to continue to expand our
staff, we may encounter substantial difficulty attracting
qualified staff with requisite experience due to the high level
of competition for experienced financial professionals.
We face increased legal, accounting, administrative and other
costs and expenses as a public company that we did not incur as
a private company. Compliance with the Sarbanes Oxley Act of
2002, as well as other rules of the SEC, the Public Company
Accounting Oversight Board and The Nasdaq Stock Market will
result in a significant initial cost to us as well as an ongoing
increase in our legal, audit and financial compliance costs. As
a public company, we expect to become subject to
Section 404 of the Sarbanes Oxley Act relating to internal
control over financial reporting for the year ended
December 31, 2007. We have only recently begun a formal
process to evaluate our internal controls for purposes of
Section 404, and we cannot assure that our internal control
over financial reporting will prove to be effective.
If we
fail to maintain an effective system of internal control over
financial reporting, we may not be able to accurately report our
financial results or prevent fraud. As a result, stockholders
could lose confidence in our financial and other public
reporting, which would harm our business and the trading price
of our common stock.
Effective internal controls over financial reporting are
necessary for us to provide reliable financial reports and,
together with adequate disclosure controls and procedures, are
designed to prevent fraud. If we cannot provide reliable
financial reports or prevent fraud, our operating results could
be harmed. Also, to account for our continued growth, as well
the additional reporting obligations under the DHHS contract, we
have installed a new accounting software system, which went live
on January 1, 2007. Our experience with this new system is
limited and could impact our ability to provide timely financial
reports. We have only recently begun a formal process to
evaluate our internal control over financial reporting. Given
the status of our efforts, coupled with the fact that guidance
from regulatory authorities in the area of internal controls
continues to evolve, substantial uncertainty exists regarding
our ability to comply by applicable deadlines. Any failure to
implement required new or improved controls, or difficulties
encountered in their implementation, could harm our operating
results or cause us to fail to meet our reporting obligations.
Inferior internal controls could also cause investors to lose
confidence in our reported financial information, which could
have a negative effect on the trading price of our common stock.
Investment
Risks
We
expect that our stock price will fluctuate significantly, which
may adversely affect holders of our stock and our ability to
raise capital.
The stock market, particularly in recent years, has experienced
significant volatility particularly with respect to
pharmaceutical, biopharmaceutical and biotechnology stocks. The
volatility of pharmaceutical, biopharmaceutical and
biotechnology stocks often does not relate to the operating
performance of the companies represented by the shares. Factors
that could cause volatility in the market price of our common
stock include:
|
|
|
| |
•
|
the timing and the results from our clinical trial programs;
|
| |
| |
•
|
FDA or international regulatory actions;
|
| |
| |
•
|
failure of any of our product candidates, if approved, to
achieve commercial success;
|
| |
| |
•
|
announcements of clinical trial results or new product
introductions by our competitors;
|
| |
| |
•
|
market conditions in the pharmaceutical, biopharmaceutical and
biotechnology sectors;
|
| |
| |
•
|
developments concerning intellectual property rights;
|
| |
| |
•
|
litigation or public concern about the safety of our potential
products;
|
34
|
|
|
| |
•
|
actual and anticipated fluctuations in our quarterly operating
results;
|
| |
| |
•
|
deviations in our operating results from the estimates of
securities analysts;
|
| |
| |
•
|
additions or departures of key personnel;
|
| |
| |
•
|
third party reimbursement policies; and
|
| |
| |
•
|
developments concerning current or future strategic alliances.
|
These and other factors may cause the market price and demand
for our common stock to fluctuate substantially, which may limit
or prevent investors from readily selling their shares of common
stock and may otherwise negatively affect the liquidity of our
common stock and our ability to raise capital.
If the
price and volume of our common stock experience extreme
fluctuations, then we could face costly
litigation.
In the past, companies that experience volatility in the market
price of their securities have often faced securities class
action litigation. Whether or not meritorious, if any of our
stockholders brought a lawsuit against us, we could incur
substantial costs defending the lawsuit. Such a lawsuit could
also divert the time and attention of our management and harm
our ability to grow our business.
Our
directors and management exercise significant control over our
company.
Our directors and executive officers and their affiliates
collectively control approximately 43.5% of our outstanding
common stock. These stockholders, if they act together, may be
able to influence our management and affairs and all matters
requiring stockholder approval, including the election of
directors and approval of significant corporate transactions.
The concentration of ownership may have the effect of delaying
or preventing a change in control of our company and might
affect the market price of our common stock.
We may
not achieve our projected development goals in the time frames
we announce and expect.
We set goals for and make public statements regarding timing of
the accomplishment of objectives material to our success, such
as the commencement and completion of clinical trials. The
actual timing of these events can vary dramatically due to
factors such as delays or failures in our clinical trials, the
uncertainties inherent in the regulatory approval process and
delays in achieving manufacturing or marketing arrangements
sufficient to commercialize our products. There can be no
assurance that our clinical trials will be completed, that we
will make regulatory submissions or receive regulatory approvals
as planned or that we will be able to adhere to our current
schedule for the launch of any of our products. If we fail to
achieve one or more of these milestones as planned, the market
price of our shares could decline.
Provisions
of Delaware law or our charter documents could delay or prevent
an acquisition of our company, even if the acquisition would be
beneficial to our stockholders, and could make it more difficult
for you to change management.
Provisions of our certificate of incorporation and bylaws may
discourage, delay or prevent a merger, acquisition or other
change in control that stockholders may consider favorable,
including transactions in which stockholders might otherwise
receive a premium for their shares. This is because these
provisions may prevent or frustrate attempts by stockholders to
replace or remove our current management or members of our board
of directors.
These provisions include:
|
|
|
| |
•
|
a staggered board of directors;
|
| |
| |
•
|
a prohibition on stockholder action through written consent;
|
35
|
|
|
| |
•
|
a requirement that special meetings of stockholders be called
only by the chairman of the board of directors, the chief
executive officer, or the board of directors pursuant to a
resolution adopted by a majority of the total number of
authorized directors;
|
| |
| |
•
|
advance notice requirements for stockholder proposals and
nominations; and
|
| |
| |
•
|
the authority of the board of directors to issue preferred stock
with such terms as it may determine.
|
As a result, these provisions and others available under
Delaware law could limit the price that investors are willing to
pay in the future for shares of our common stock.
Because
we do not expect to pay dividends in the foreseeable future, you
must rely on stock appreciation for any return on your
investment.
We have paid no cash dividends on any of our capital stock to
date, and we currently intend to retain our future earnings, if
any, to fund the development and growth of our business. As a
result, we do not expect to pay any cash dividends in the
foreseeable future, and payment of cash dividends, if any, will
also depend on our financial condition, results of operations,
capital requirements and other factors and will be at the
discretion of our board of directors. Furthermore, we may in the
future become subject to contractual restrictions on, or
prohibitions against, the payment of dividends. Accordingly, the
success of your investment in our common stock will likely
depend entirely upon any future appreciation. There is no
guarantee that our common stock will appreciate in value after
you purchase them or even maintain the price at which you
purchased your shares, and you may not realize a return on your
investment in our common stock.
|
|
|
Item 1B.
|
Unresolved
Staff Comments
|
Not applicable.
We currently lease approximately 46,449 square feet of
research, manufacturing and administrative space in
Gaithersburg, Maryland for our principal laboratories, pilot
manufacturing facility and corporate offices. In January 2007,
we signed a lease amendment under which we will lease an
additional 1,365 square feet in our current facility
beginning on or about July 1, 2007 and an additional
5,650 square feet in our current facility beginning on our
about September 1, 2007. At that time, we will occupy the
entire facility. The lease expires in May 2013 and has a
five-year renewal option.
|
|
|
Item 3.
|
Legal
Proceedings
|
We are not party to any material legal proceedings.
|
|
|
Item 4.
|
Submission
of Matters to a Vote of Security Holders
|
No matters were submitted to stockholders for a vote during the
fourth quarter of 2006.
Executive
Officers of the Registrant
The following table sets forth the names and ages of our
executive officers as of December 31, 2006.
| |
|
|
|
|
|
|
|
Name
|
|
Age
|
|
Position(s)
|
|
|
|
Stanley C. Erck
|
|
|
58
|
|
|
President, Chief Executive
Officer, Treasurer and Director
|
|
Gregory M. Glenn, M.D.
|
|
|
52
|
|
|
Senior Vice President and Chief
Scientific Officer
|
|
Russell P. Wilson
|
|
|
47
|
|
|
Senior Vice President, Chief
Financial Officer, General Counsel and Secretary
|
Stanley C. Erck. Mr. Erck has served as
President, Chief Executive Officer, Treasurer and Director since
May 2000. Mr. Erck has 30 years of management
experience in healthcare and biotechnology. Mr. Erck has
worked at
36
Baxter International, Procept, and Integrated Genetics.
Mr. Erck has a B.S. from the University of Illinois and an
M.B.A. from the University of Chicago.
Gregory M. Glenn, M.D. Dr. Glenn has
served as Senior Vice President and Chief Scientific Officer
since September 1997 and was a Director from February 1998
through May 2000. Dr. Glenn is the co-discoverer of the TCI
technology and a co-founder of Iomai. He has been responsible
for the conception, implementation and development of the basic
science and early clinical trials relating to TCI, and has
multiple patents, publications and book chapters describing TCI.
Dr. Glenn is a pediatrician who completed the Medical
Research Fellowship at the Walter Reed Army Institute of
Research, or WRAIR, where he continued his research in vaccine
delivery while on active duty. He received a B.A. from Whitman
College and his M.D. from Oral Roberts University School of
Medicine, where he received the Pediatrics Award and Dean’s
Award for Academic Excellence.
Russell P. Wilson. Mr. Wilson has served
as Senior Vice President since May 2005, Chief Financial Officer
since June 2002, General Counsel since March 2000 and Secretary
since May 2000, and served as Vice President, Business
Development from March 2000 to June 2002. Mr. Wilson
received a B.A. from Princeton University and holds a joint
M.B.A./J.D. degree from the University of Virginia.
PART II
|
|
|
Item 5.
|
Market
for the Registrant’s Common Equity, Related Stockholder
Matters and Issuer Purchases of Equity Securities
|
Our common stock has been traded on The NASDAQ Global Market
under the symbol “IOMI” since February 1, 2006.
The following table sets forth, for the periods indicated, the
high and low sale prices per share of our common stock as
reported on the NASDAQ Global Market.
| |
|
|
|
|
|
|
|
|
|
|
|
High
|
|
|
Low
|
|
|
|
|
2006
|
|
|
|
|
|
|
|
|
|
First Quarter (beginning
February 1, 2006)
|
|
$
|
7.34
|
|
|
$
|
5.22
|
|
|
Second Quarter
|
|
|
6.00
|
|
|
|
3.91
|
|
|
Third Quarter
|
|
|
4.81
|
|
|
|
2.61
|
|
|
Fourth Quarter
|
|
|
6.40
|
|
|
|
4.60
|
|
As of March 14, 2007, there were approximately 166
registered holders and approximately 1,290 beneficial owners of
our common stock.
We have never declared or paid any cash dividends on our capital
stock and we do not currently anticipate declaring or paying
cash dividends on our capital stock in the foreseeable future.
We currently intend to retain all of our future earnings, if
any, to finance operations. Any future determination relating to
our dividend policy will be made at the discretion of our board
of directors and will depend on a number of factors, including
future earnings, capital requirements, financial conditions,
future prospects, contractual restrictions and covenants and
other factors that our board of directors may deem relevant.
37
Securities
Authorized For Issuance Under Equity Compensation
Plans
The following table summarizes the number of securities issuable
under our incentive compensation plans as of December 31,
2006.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Securities
|
|
|
|
|
Number of Securities
|
|
|
|
|
|
Remaining Available for
|
|
|
|
|
to Be Issued
|
|
|
Weighted-average
|
|
|
Future Issuance Under
|
|
|
|
|
Upon Exercise of
|
|
|
Exercise Price of
|
|
|
Equity Compensation Plan
|
|
|
|
|
Outstanding Options,
|
|
|
Outstanding Options,
|
|
|
(Excluding Securities
|
|
|
|
|
Warrants and Rights(1)
|
|
|
Warrants and Rights
|
|
|
Reflected in Column(a))(2)
|
|
|
Plan Category
|
|
(a)
|
|
|
(b)
|
|
|
(c)
|
|
|
|
|
Equity compensation plans approved
by security holders
|
|
|
2,918,805
|
|
|
$
|
2.93
|
|
|
|
182,648
|
|
|
Equity compensation plans not
approved by security holders
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
2,918,805
|
|
|
|
|
|
|
|
182,648
|
|
|
|
|
|
(1) |
|
Includes (i) outstanding options to purchase
50,380 shares of common stock under the 1998 Plan, of which
50,380 have been vested; and (ii) outstanding awards to
purchase 1,808,664 shares of common stock under the 1999
Plan, of which 1,137,396 have been vested; and
(iii) outstanding awards to purchase 1,059,761 shares
of common stock under the 2005 Plan, of which 7,775 have been
vested. Options granted under the 1999 Plan to purchase
52,199 shares of common stock were exercised prior to
December 31, 2006. |
| |
|
(2) |
|
Includes (i) up to 26,543 shares of common stock that
may be issued under the 1998 Plan; (ii) up to
156,105 shares of common stock that may be issued under the
1999 Plan; and (iii) no shares of common stock that may be
issued under the 2005 Plan. Does not include up to
80,000 shares that may be issued under the 2006 Employee
Stock Purchase Plan which became effective upon our initial
public offering. |
38
Stock
Performance Graph
The following graph shows the cumulative total stockholder
return on our common stock over the period from February 1,
2006 to December 29, 2006, as compared with that of the
NASDAQ Stock Market (U.S. Companies) Index and the NASDAQ
Pharmaceuticals Index, based on an initial investment of $100 in
each on February 1, 2006. Total stockholder return is
measured by dividing share price change plus dividends, if any,
for each period by the share price at the beginning of the
respective period, and assumes reinvestment of dividends.
COMPARISON
OF CUMULATIVE TOTAL RETURN OF IOMAI CORPORATION
NASDAQ STOCK MARKET (U.S. COMPANIES) INDEX AND
AND NASDAQ PHARMACEUTICAL STOCKS INDEX
39
|
|
|
Item 6.
|
Selected
Financial Data
|
The following table sets forth selected financial data that is
qualified in its entirety by and should be read in conjunction
with Item 7. “Management’s Discussion and
Analysis of Financial Condition and Results of Operations”
and our financial statements and notes thereto appearing
elsewhere in this annual report on
Form 10-K.
The financial data for the fiscal years ended December 31,
2006, 2005 and 2004 are derived from our audited financial
statements appearing elsewhere in this document. The financial
data for the fiscal years ended December 31, 2003 and 2002
are derived from our audited financial statements not included
in this document.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
Statement of Operations
Data:
|
|
2002
|
|
|
2003
|
|
|
2004
|
|
|
2005
|
|
|
2006
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License, option agreements and
grants
|
|
$
|
995
|
|
|
$
|
1,601
|
|
|
$
|
2,345
|
|
|
$
|
2,371
|
|
|
$
|
1,475
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
995
|
|
|
|
1,601
|
|
|
|
2,345
|
|
|
|
2,371
|
|
|
|
1,475
|
|
|
Cost and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
7,354
|
|
|
|
13,332
|
|
|
|
14,349
|
|
|
|
16,529
|
|
|
|
28,041
|
|
|
General and administrative
|
|
|
2,411
|
|
|
|
3,348
|
|
|
|
3,206
|
|
|
|
3,780
|
|
|
|
5,857
|
|
|
Purchased in-process research and
development
|
|
|
2,517
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reimbursed by related party
|
|
|
(426
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total costs and expenses
|
|
|
11,856
|
|
|
|
16,680
|
|
|
|
17,555
|
|
|
|
20,309
|
|
|
|
33,898
|
|
|
Loss from operations
|
|
|
(10,861
|
)
|
|
|
(15,079
|
)
|
|
|
(15,210
|
)
|
|
|
(17,938
|
)
|
|
|
(32,423
|
)
|
|
Other (expense)
income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on discharge of convertible
notes payable
|
|
|
11,219
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
57
|
|
|
|
953
|
|
|
|
620
|
|
|
|
392
|
|
|
|
1,006
|
|
|
Interest expense
|
|
|
(2,120
|
)
|
|
|
(128
|
)
|
|
|
(265
|
)
|
|
|
(467
|
)
|
|
|
(377
|
)
|
|
Other expense, net
|
|
|
(75
|
)
|
|
|
(448
|
)
|
|
|
(225
|
)
|
|
|
(17
|
)
|
|
|
(8
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other (expense) income, net
|
|
|
9,081
|
|
|
|
377
|
|
|
|
130
|
|
|
|
(92
|
)
|
|
|
621
|
|
|
Cumulative effect of change in
accounting principle
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17
|
|
|
Net loss
|
|
|
(1,780
|
)
|
|
|
(14,702
|
)
|
|
|
(15,080
|
)
|
|
|
(18,030
|
)
|
|
|
(31,785
|
)
|
|
Dividends on and accretion of
convertible preferred stock
|
|
|
(380
|
)
|
|
|
(5,466
|
)
|
|
|
(5,525
|
)
|
|
|
(5,562
|
)
|
|
|
(471
|
)
|
|
Carrying value of preferred stock
in excess of fair value transferred
|
|
|
11,092
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) available to
common stockholders
|
|
$
|
8,932
|
|
|
$
|
(20,168
|
)
|
|
$
|
(20,605
|
)
|
|
$
|
(23,592
|
)
|
|
$
|
(32,256
|
)
|
|
Net income (loss) per share of
common stock — basic and diluted
|
|
$
|
11.79
|
|
|
$
|
(26.43
|
)
|
|
$
|
(26.90
|
)
|
|
$
|
(30.14
|
)
|
|
$
|
(2.03
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares of common
stock outstanding — basic and diluted
|
|
|
757,402
|
|
|
|
763,075
|
|
|
|
765,945
|
|
|
|
782,715
|
|
|
|
15,915,797
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
40
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
Balance Sheet Data:
|
|
2002
|
|
|
2003
|
|
|
2004
|
|
|
2005
|
|
|
2006
|
|
|
|
|
(In thousands)
|
|
|
|
|
Cash, cash equivalents and
marketable securities
|
|
$
|
49,493
|
|
|
$
|
38,213
|
|
|
$
|
22,397
|
|
|
$
|
5,190
|
|
|
$
|
15,336
|
|
|
Working capital
|
|
|
47,263
|
|
|
|
34,945
|
|
|
|
18,878
|
|
|
|
270
|
|
|
|
10,401
|
|
|
Total assets(1)
|
|
|
51,398
|
|
|
|
41,511
|
|
|
|
28,942
|
|
|
|
11,861
|
|
|
|
23,085
|
|
|
Debt and capital lease obligations
|
|
|
706
|
|
|
|
2,966
|
|
|
|
5,273
|
|
|
|
4,239
|
|
|
|
4,759
|
|
|
Redeemable convertible preferred
stock and Series C warrant
|
|
|
52,435
|
|
|
|
59,555
|
|
|
|
65,357
|
|
|
|
70,386
|
|
|
|
—
|
|
|
Common stock subject to put right
|
|
|
1,958
|
|
|
|
1,958
|
|
|
|
1,958
|
|
|
|
1,959
|
|
|
|
—
|
|
|
Total stockholders’ equity
(deficit)
|
|
|
(6,533
|
)
|
|
|
(26,038
|
)
|
|
|
(45,993
|
)
|
|
|
(68,458
|
)
|
|
|
14,035
|
|
|
|
|
|
(1) |
|
As of December 31, 2006, we had net operating loss (NOL)
and research and development credit carryforwards of
approximately $83.7 million. Tax benefits may arise from
these carryforwards in the future in the event that we realize
U.S. taxable income. Potential tax benefits arising from
these carryforwards are not reflected in our total assets.
Despite the NOL carryforward, we may have an income tax
liability in future years due to the application of the
alternative minimum tax rules. The NOL may also be limited in
its ability to offset future losses in the event that there is a
change in the stock ownership as defined by federal tax
regulations. |
|
|
|
Item 7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
The following discussion of our financial condition and
results of operations should be read in conjunction with our
financial statements and the notes to those financial statements
appearing elsewhere in this annual report on
Form 10-K.
This discussion contains forward-looking statements, which are
identified under our “Cautionary Statement Regarding
Forward-Looking Statements” at the beginning of this
report, that involve significant risks and uncertainties. As a
result of many factors, such as those set forth under “Risk
Factors” in Item 1A and elsewhere in this annual
report on
Form 10-K,
our actual results may differ materially from those anticipated
in these forward-looking statements.
OVERVIEW
We are a biopharmaceutical company focused on the discovery,
development and commercialization of vaccines and immune system
stimulants delivered to the skin via a novel, needle-free
technology called transcutaneous immunization (TCI). TCI
exploits the unique benefits of a major group of
antigen-presenting cells found in the outer layers of the skin
(Langerhans cells) to generate an enhanced immune response. TCI
has the potential to enhance the efficacy of existing vaccines,
enable new vaccines that are viable only through transcutaneous
administration and expand the global vaccine market. We are
developing two distinct product applications: (1) an
immunostimulant, or IS, patch and (2) a needle-free vaccine
patch. We currently have five product candidates in development:
four targeting influenza and pandemic flu and one to prevent
E. coli-related travelers’ diarrhea.
Our five product candidates are (1) an IS patch intended to
stimulate an immune response to even small doses of a pandemic
flu vaccine, thereby extending vaccine supply in the event of an
influenza pandemic, (2) an IS patch intended to boost the
immune response of the elderly to the standard flu vaccine, (3)
a needle-free vaccine patch for seasonal flu, (4) a
needle-free pandemic flu vaccine patch, and (5) a
needle-free travelers’ diarrhea vaccine patch. None of our
product candidates has been approved for commercial sale by the
FDA or any comparable foreign agencies.
As of December 31, 2006, we had an accumulated deficit of
$100.8 million. We expect to incur substantial expenses
over the next several years as we continue the clinical
development of our IS patch to extend the supply of pandemic flu
vaccines, our needle-free flu vaccine patch, IS patch for
elderly receiving flu vaccines; expand the clinical trial
program for our needle-free travelers’ diarrhea vaccine
patch; continue our existing preclinical development programs,
including our needle-free pandemic flu vaccine program, and
commence new programs; increase our manufacturing capabilities
for product candidates; and expand our research and development
activities.
41
We anticipate that a substantial portion of our efforts
throughout 2007 will be focused on conducting additional
development for our four product candidates in clinical
development, as well as continue to build up our infrastructure
to support our operations as a public company.
Management
Review of 2006
The following is a summary of key events that occurred during
2006:
|
|
|
| |
•
|
On February 6, 2006, we closed our initial public offering,
in which we sold 5,000,000 shares of common stock at a
public offering price of $7.00 per share. Net proceeds,
before expenses, were $32,853,574. Our total expenses for the
initial public offering were $1.9 million, so our total net
proceeds, after expenses, were $30.9 million.
|
| |
| |
•
|
In February 2006, the National Institutes of Health, or NIH,
awarded us the remaining $1.4 million available under the
last year of a two-year grant to fund up to $2.9 million
for further development of our IS technology for pandemic flu
applications. We completed work under this grant in the second
quarter of 2006.
|
| |
| |
•
|
In May 2006, we submitted to the Department of Health and Human
Services, or DHHS, a response to the government’s request
for proposal (RFP) for further development of our IS patch for
pandemic influenza vaccines. This patch is designed to enhance
the immune response to any manufactured pandemic influenza
vaccine, an advantage that may allow the public health service
to employ a universal dose-sparing strategy to extend the supply
of vaccine. In January 2007, DHHS awarded us a five-year,
cost-plus reimbursement contract to fund our development of a
dose-sparing patch for use with a pandemic flu vaccine. If the
product is developed through licensure, the total cost
reimbursed by DHHS, plus a fixed fee, is estimated to be
$128 million. During the first 15 months of the
contract, DHHS has allotted approximately $14.5 million for
us to assess the safety and immunogenicity of the patch in two
clinical trials and to develop plans on how we would produce
150 million IS patches in a six-month period, as required
under the contract. Once we demonstrate the safety and
dose-sparing capability of our IS patch, we hope to sell up to
150 million IS patches to the United States government for
its stockpile of pandemic flu products.
|
| |
| |
•
|
During 2006, we completed a number of Phase 1 and 2 studies
to further advance our dry vaccine patch for travelers’
diarrhea. These trials demonstrated, among other things, that
our current dry patch formulation achieved high immune responses
in those vaccinated and outperformed our earlier liquid-based
patch and that our travelers’ diarrhea vaccine patch can be
self-applied by individuals, not just by healthcare providers.
We also have gathered data that shows that the dry patch is
stable for more than 12 months at room temperature. All of
this data could also have important implications not only for
our travelers’ diarrhea program, but for our other
programs, particularly our influenza programs, and could form
the basis for our development of vaccine patches that could be
stockpiled, mailed and self-applied — all important
considerations in the event of a pandemic influenza outbreak. We
also initiated during 2006 a Phase 2 dose-ranging study for
our travelers’ diarrhea vaccine patch, as well as a
Phase 2 field study designed to evaluate the logistics
around how to best conduct a larger pivotal field study for our
travelers’ diarrhea program. We expect data from these two
trials to be available during the second quarter of 2007. Based
on these data, we will design an additional Phase 2 trial
to determine the final dry patch formulation, and that trial is
expected to commence in the fourth quarter of 2007.
|
| |
| |
•
|
During the fall of 2006, we initiated a large, multi-center
Phase 1 trial for our needle-free flu vaccine patch. This
study is designed to test the safety and immunogenicity of our
needle-free flu vaccine in comparison with the traditional
injected vaccine. Vaccinations were completed during the first
quarter of 2007, and we expect interim data from this trial to
be available by the middle of 2007. We expect this data to guide
us in our design for a Phase 2 program that could
potentially support licensure of a self-administered,
needle-free flu vaccine patch. As we do not manufacture
commercial flu antigens, we are also actively seeking to
collaborate with suppliers of commercial flu antigens in order
to advance further development of this program.
|
42
|
|
|
| |
•
|
In May 2006, we negotiated an extension of our credit facility
to finance up to $2 million of additional capital
expenditures for our pilot manufacturing plant, as well as for
our research and development laboratories. We drew down the
entire $2 million during 2006.
|
| |
| |
•
|
By the end of third quarter of 2007, we will occupy the entire
facility which houses our principal laboratories, pilot
manufacturing facility and corporate offices in approximately
53,500 square feet. In July 2006, we amended our lease for
the facility to add approximately 12,000 square feet, and
in January 2007, we signed another amendment to lease the
remaining approximately 7,000 square feet in the building
over the course of first nine months of 2007.
|
| |
| |
•
|
Over the past six months, we have raised approximately
$40.2 million in net proceeds through two separate private
placements. In October 2006, we raised approximately
$9.9 million in net proceeds when we sold
2,283,106 shares of our common stock at a price of
$4.38 per share in a private placement to existing
stockholders, Essex Woodlands Health Ventures and New Enterprise
Associates (NEA). Then in early March 2007, we raised
$30.3 million in net proceeds when we sold
6,291,828 units, each unit consisting of one share of our
common stock and two warrants to purchase, in total, 0.7
additional shares of Common Stock, at a purchase price of
$5.0675 per unit. The purchase price for the share
component of each unit is $4.98 per share. Each warrant
provides the right to acquire 0.35 shares of Common Stock
at an exercise price of $5.25 per full share. One warrant
is exercisable at any time until March 2, 2012, and the
other warrant is exercisable at any time until the date four
months after a registration statement for the resale of the
shares and the common stock underlying the warrants is declared
effective by the SEC.
|
HISTORICAL
RESULTS OF OPERATIONS / LIQUIDITY & CAPITAL
RESOURCES
Revenues
Our revenues to date have principally been limited to amounts we
have received under U.S. federal grant programs. During
2006, we had one principal active grant, which the NIH
originally awarded us in January 2005 to fund over two years up
to $2.9 million for further development of our IS
technology for pandemic influenza applications. In the first
quarter of 2006, the NIH awarded us the $1.4 million
remaining to be received under this grant for 2006. Through
December 31, 2006, we have been reimbursed for the full
$1.4 million of expenses under this grant. These amounts
include reimbursement for our employees’ time and benefits
and other expenses related to performance under the relevant
grants.
Research
and development expenses
Our research and development expenses consist primarily of:
|
|
|
| |
•
|
salaries and related expenses for personnel;
|
| |
| |
•
|
fees paid to consultants and clinical research organizations in
conjunction with their monitoring our clinical trials and
acquiring and evaluating data in conjunction with our clinical
trials;
|
| |
| |
•
|
consulting fees paid to third parties in connection with other
aspects of our product development efforts;
|
| |
| |
•
|
fees paid to research organizations in conjunction with
preclinical animal studies;
|
| |
| |
•
|
costs of materials used in research and development;
|
| |
| |
•
|
depreciation of facilities and equipment used to develop our
product candidates; and
|
| |
| |
•
|
milestone payments, license fees, and royalty payments for
technology licenses.
|
We expense both internal and external research and development
costs as incurred, other than those capital expenditures that
have alternative future uses, such as the build-out of our pilot
plant. Due to the risks inherent in the clinical trial process
and the early stage of development of our product candidates, we
do not currently track our internal research and development
costs by program and cannot state precisely the costs incurred
for each of our
43
research and development programs. However, the following table
shows, for the periods presented, our estimate of the total
costs that have been incurred for our lead product candidates:
from January 1, 2003 to December 31, 2006:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
Cumulative Since
|
|
|
Product Candidate
|
|
2006
|
|
|
2005
|
|
|
January 1, 2003
|
|
|
|
|
(In thousands)
|
|
|
|
|
|
|
|
IS patch for pandemic flu
|
|
$
|
2,593
|
|
|
$
|
2,409
|
|
|
$
|
5,006
|
|
|
IS patch for elderly receiving flu
vaccines
|
|
|
1,262
|
|
|
|
1,545
|
|
|
|
15,816
|
|
|
Needle-free flu vaccine patch
|
|
|
5,889
|
|
|
|
4,895
|
|
|
|
13,093
|
|
|
Needle-free travelers’
diarrhea vaccine patch
|
|
|
16,726
|
|
|
|
5,641
|
|
|
|
26,927
|
|
|
Other programs
|
|
|
1,571
|
|
|
|
2,039
|
|
|
|
11,407
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL
|
|
|
28,041
|
|
|
|
16,529
|
|
|
|
72,249
|
|
We expect our research and development costs will continue to be
substantial and that they will increase as we advance our
current portfolio of product candidates through clinical trials
and move other product candidates into preclinical and clinical
trials.
General
and administrative expense
General and administrative expense consists primarily of
compensation for employees in executive and operational
functions, including finance and accounting, business
development, and corporate development. Other significant costs
include facilities costs and professional fees for accounting
and legal services. With the completion of our initial public
offering, our general and administrative expenses have increased
due to increased costs for insurance, professional fees, public
company reporting requirements, and investor relations costs
associated with operating as a publicly-traded company. In
addition, there will likely be further increases going forward
related to the hiring of additional personnel.
RESULTS
OF OPERATIONS
Comparison
of the years ended December 31, 2006 and 2005
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
2006
|
|
|
2005
|
|
|
$ Change
|
|
|
% Change
|
|
|
|
|
(In thousands, except percentages)
|
|
|
|
|
Revenues
|
|
$
|
1,475
|
|
|
$
|
2,371
|
|
|
$
|
(896
|
)
|
|
|
(37.8
|
)%
|
|
Costs & Expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research & development
|
|
|
28,041
|
|
|
|
16,529
|
|
|
|
11,512
|
|
|
|
69.6
|
%
|
|
General & administrative
|
|
|
5,857
|
|
|
|
3,780
|
|
|
|
2,077
|
|
|
|
54.9
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total costs & expenses
|
|
|
33,898
|
|
|
|
20,309
|
|
|
|
13,589
|
|
|
|
66.9
|
%
|
|
Other income (expense)
|
|
|
621
|
|
|
|
(92
|
)
|
|
|
713
|
|
|
|
775.0
|
%
|
|
Cumulative effect of a change in
accounting principle
|
|
|
17
|
|
|
|
—
|
|
|
|
17
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(31,785
|
)
|
|
$
|
(18,030
|
)
|
|
$
|
(13,755
|
)
|
|
|
76.3
|
%
|
Revenues. The $0.9 million decrease in
grant revenues was principally the result of fewer active grants
in 2006. The principal grant revenue in 2006 was from the
second-year award under our NIH grant to further development of
our IS patch technology for pandemic flu applications, which
ended in the second quarter of 2006. There was only one other
small active grant in the second half of 2006. Grant revenues in
2005 were principally from reimbursable expenses incurred under
the first year of our NIH grant to further development of our IS
patch technology for pandemic flu applications, as well as
revenues from a cancer grant that ended in the fourth quarter of
2005.
Research and Development Expenses. The
$11.5 million increase in research and development
expenditures was driven by three major factors associated with
supporting our clinical and product development programs:
44
(1) increased clinical trial activity, (2) increased
development costs for our skin preparation system; and
(3) higher payroll costs associated with a 41% increase in
headcount and the expensing of stock options. These increased
costs were partially offset by lower depreciation and
amortization costs associated with our pilot manufacturing plant
in 2006. Prior to signing the lease extension for our facility
in October 2005, we amortized all leasehold improvements for the
facility over the lesser of the useful life or the expiration of
the lease term, which was May 2006. Once we entered into the
lease extension, the amortization period for the remaining
unamortized leasehold improvements was extended to May 2013.
General and Administrative Expenses. The
$2.1 million increase in general and administrative
expenses was principally due to (1) higher payroll costs
associated with expensing stock options, (2) higher
insurance, legal, and investor relation costs associated with
being a public company, and (3) higher facilities cost
associated with leasing additional space in our facility.
Interest Income (Expense) and Other —
Net. The net interest and other income reflects
the interest received on our cash and marketable securities,
offset by interest expense on financing to purchase equipment
and leasehold improvements, and the amortization income /
expense associated with the purchase of marketable securities at
a discount or premium. The increase in other income was
primarily the result of amortization income associated with
securities purchased at a discount after our initial public
offering in February 2006.
Net Loss. The $13.8 million increase in
our net loss was principally a result of increased research and
development expenses in the year ended December 31, 2006.
Comparison
of the years ended December 31, 2005 and 2004
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
2005
|
|
|
2004
|
|
|
$ Change
|
|
|
% Change
|
|
|
|
|
(In thousands, except percentages)
|
|
|
|
|
Revenues
|
|
$
|
2,371
|
|
|
$
|
2,345
|
|
|
$
|
26
|
|
|
|
1.1
|
%
|
|
Costs & Expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research & development
|
|
|
16,529
|
|
|
|
14,349
|
|
|
|
2,180
|
|
|
|
15.2
|
%
|
|
General & administrative
|
|
|
3,780
|
|
|
|
3,206
|
|
|
|
574
|
|
|
|
17.9
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total costs & expenses
|
|
|
20,309
|
|
|
|
17,555
|
|
|
|
2,754
|
|
|
|
15.7
|
%
|
|
Other (expense) income
|
|
|
(92
|
)
|
|
|
130
|
|
|
|
(222
|
)
|
|
|
(170.8
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(18,030
|
)
|
|
$
|
(15,080
|
)
|
|
$
|
(2,950
|
)
|
|
|
19.6
|
%
|
Revenues. The slight increase in grant
revenues is principally the result of revenues from the NIH
grant awarded in January 2005 for further development of our IS
patch technology for pandemic flu applications, which was offset
by a decline in reimbursable activities in 2005 under other
grants for our needle-free patch programs.
Research and Development Expenses. The
$2.2 million increase in research expenditures was driven
by three major factors associated with supporting our clinical
and product development programs: (1) increased preclinical
study costs as a result of higher charges for services and
continuing development of preclinical models; (2) increased
depreciation and amortization costs associated with our pilot
manufacturing facility, which was put into service in July 2004;
and (3) higher payroll costs associated with a 25% increase
in headcount and expensing of stock options. These increased
costs were partially offset by lower clinical trial costs and
contract manufacturing expenses, both of which were higher
during 2004 as we initiated five clinical trials during 2004.
General and Administrative Expenses. The
$0.6 million increase in general and administrative
expenses was principally due to consulting expenses associated
with evaluating potential corporate collaborations, higher legal
costs associated with prosecuting our patent portfolio, and
higher payroll costs associated with expensing stock options.
Interest Income (Expense) and Other —
Net. Our net interest and other expense was
approximately $92,000 for 2005, as compared to net interest and
other income of approximately $130,000 for 2004. The net
interest and other income reflects the interest received on our
cash and marketable securities, offset by interest expense on
financing to purchase equipment and leasehold improvements. The
decrease in net interest and other income was a
45
result of lower interest income because of declining cash
balances and higher interest expense associated funds borrowed
to finance the construction of our pilot manufacturing facility.
Net Loss. The $3.0 million increase in
our net loss was principally a result of increased research and
development expenses in 2005.
Liquidity
and capital resources
We have incurred annual operating losses since inception, and,
as of December 31, 2006, we had an accumulated deficit of
$100.8 million. We expect to incur increasing and
significant losses over the next several years as we continue
our clinical trials, apply for regulatory approvals, continue
development of our technologies, and expand our operations.
Since our inception, we have financed our operations primarily
through the sale of equity securities, interest income earned on
cash, cash equivalents, and short-term investment balances, and
debt. We have also generated funds from collaborative partners
and research grants.
As of December 31, 2006 we had approximately
$15.3 million in unrestricted cash, cash equivalents, and
marketable securities. We invest in cash equivalents and US
government and agency obligations. Our investment objectives
are, primarily, to assure liquidity and preservation of capital
and, secondarily, to obtain investment income. All of our
marketable securities are classified as
available-for-sale.
These securities are carried at fair value, plus any accrued
interest. We have used cash primarily to finance our research
operations, including clinical trials. These costs will be
offset by reimbursements from DHHS under our pandemic flu
contract, which was awarded in January 2007. DHHS has allotted
approximately $14.5 million in the first 15 months of
that contract and we expect to be reimbursed for research,
development and capital costs associated with the preclinical
and clinical testing of the IS patch under the contract on a
monthly basis. Also, in March 2007, we closed a private
placement in our common stock in which we raised approximately
$30.3 million in net proceeds.
We expect that we will be able to fund our capital expenditures
and growing operations with our current working capital through
the second quarter of 2008. In order to fund our needs
subsequently, we will need to raise additional money and may
seek to do so by: (1) out-licensing technologies or product
candidates to one or more corporate partners,
(2) completing an outright sale of assets,
(3) securing debt financing,
and/or
(4) selling additional equity securities. Our ability to
successfully enter into any such arrangements is uncertain, and,
if funds are not available, or not available on terms acceptable
to us, we may be required to revise our planned clinical trials,
other development activities, capital expenditure requirements,
and the scale of our operations. We expect to attempt to raise
additional funds in advance of depleting our existing cash
balances; however, we may not be able to raise funds or raise
amounts sufficient to meet the long-term needs of the business.
Satisfying long-term needs will require the successful
commercialization of our product candidates and, at this time,
we cannot reliably estimate if or when that will occur.
Our future cash requirements include, but are not limited to,
supporting our clinical trial efforts and continuing our other
research and development programs. We have entered into various
agreements with institutions and clinical research organizations
to conduct and monitor our current clinical studies. Under these
agreements, subject to the enrollment of patients and
performance by the applicable institution of certain services,
we have estimated our potential payments to be
$14.3 million over the term of currently ongoing studies.
Through December 31, 2006, approximately $7.7 million
of this amount has been expensed as research and development
expenses and $6.3 million has been paid related to these
clinical studies. The timing of our expense recognition and
future payments related to these agreements are subject to the
enrollment of patients and performance by the applicable
institutions of certain services. The actual amounts we pay out,
if any, will depend on a range of factors outside of our
control, including the success of our pre-clinical and clinical
development efforts with respect to any products being
developed, the content and timing of decisions made by the FDA
and other regulatory authorities, and other factors affecting
future operating results. As we expand our clinical studies, we
plan to enter into additional agreements.
46
The following table summarizes sources and uses of cash and cash
equivalents for the years ended December 31, 2006 and 2005.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
|
|
|
2006
|
|
|
2005
|
|
|
$ Change
|
|
|
|
|
(In thousands)
|
|
|
|
|
Net cash used in operating
activities
|
|
$
|
(28,866
|
)
|
|
$
|
(14,304
|
)
|
|
$
|
(14,562
|
)
|
|
Net cash (used in) provided by
investing activities
|
|
|
(4,357
|
)
|
|
|
14,056
|
|
|
|
(18,413
|
)
|
|
Net cash provided by (used in)
financing activities
|
|
|
41,880
|
|
|
|
(1,504
|
)
|
|
|
43,384
|
|
|
Net increase (decrease) in cash
and cash equivalents
|
|
|
8,657
|
|
|
|
(1,752
|
)
|
|
|
10,409
|
|
|
Cash and cash equivalents at end
of period
|
|
$
|
13,847
|
|
|
$
|
5,190
|
|
|
$
|
8,657
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The $14.6 million increase in net cash used in operations
was primarily attributable to the increase in net loss from the
prior period. As we develop our technologies and further our
clinical trial programs, we expect to increase our spending. Our
future ability to generate cash from operations will depend on
achieving regulatory approval of our products, market acceptance
of such products, and our ability to enter into collaborations.
The $18.4 million increase in net cash used in investing
activities was principally the result of investing the proceeds
from our initial public offering in February 2006. For the year
ended December 31, 2006, we invested $17.7 million of
our available cash in marketable securities and received
proceeds of $16.5 million from the maturity of such
investments, as compared to $6.8 million of our available
cash invested in marketable securities and $22.1 million
received in proceeds from the maturity of such investments for
the year ended December 31, 2005. Additionally, for the
year ended December 31, 2006, we invested approximately
$3.5 million in the purchase of equipment, furniture, and
fixtures, for our pilot manufacturing facility and our build-out
of additional office and laboratory space, as compared to
approximately $1.3 million for such purchases in 2005.
The $43.3 million increase in net cash provided by
financing activities was principally the result of the
$30.9 million in net proceeds from the company’s
initial public offering in February 2006, and from
$9.9 million in net proceeds from a private placement to
existing stockholders in October 2006. During 2006 and 2005, net
proceeds from debt borrowings, including landlord leasehold
financing, totaled $2.3 million and $0.5 million,
respectively, as we financed the build-out of additional office
space and the purchase of additional equipment. During 2006, we
repaid $1.8 million of our debt, as compared to
$1.5 million during the comparable period in 2005.
The following summarizes our long-term contractual obligations
as of December 31, 2006:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due by Period
|
|
|
|
|
|
|
|
Less Than
|
|
|
|
|
|
|
|
|
More Than
|
|
|
Contractual Obligations
|
|
Total
|
|
|
1 Year
|
|
|
1-3 Years
|
|
|
3-5 Years
|
|
|
5 Years
|
|
|
|
|
(In thousands)
|
|
|
|
|
Long-Term Debt(1)
|
|
$
|
5,772
|
|
|
$
|
1,964
|
|
|
$
|
2,410
|
|
|
$
|
935
|
|
|
$
|
463
|
|
|
Capital Lease Obligations
|
|
|
1
|
|
|
|
1
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Operating Lease Obligations(2)
|
|
|
7,921
|
|
|
|
1,074
|
|
|
|
2,433
|
|
|
|
2,532
|
|
|
|
1,882
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
13,694
|
|
|
$
|
3,039
|
|
|
$
|
4,843
|
|
|
$
|
3,467
|
|
|
$
|
2,345
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Includes interest payable in the period. |
| |
|
(2) |
|
These amounts include payments for additional facility space
leased under a lease amendment signed in January 2007. See
discussion of amended lease terms below. |
In January 2007, we amended our lease to acquire access to
approximately 7,000 square feet in the building over the
course of first nine months of 2007. Upon leasing the additional
space, we will lease the entire building.
Under our existing license agreements, we could be required to
pay up to a total of $800,000 for each product candidate in
milestone payments through product approval, in addition to
sales milestones, and royalties on commercial sales, if any
occur.
47
Our future capital uses and requirements depend on numerous
forward-looking factors. These factors include but are not
limited to the following:
|
|
|
| |
•
|
the progress and costs of preclinical development and laboratory
testing and clinical trials;
|
| |
| |
•
|
the time and costs involved in obtaining regulatory approvals;
|
| |
| |
•
|
delays that may be caused by evolving requirements of regulatory
agencies;
|
| |
| |
•
|
our ability to establish, enforce, and maintain collaborations
required for product commercialization;
|
| |
| |
•
|
the number of product candidates we pursue;
|
| |
| |
•
|
the costs involved in filing and prosecuting patent applications
and enforcing or defending patent claims;
|
| |
| |
•
|
our plans to establish sales, marketing,
and/or
manufacturing capabilities;
|
| |
| |
•
|
the acquisition of technologies, products, and other business
opportunities that require financial commitments; and
|
| |
| |
•
|
our revenues, if any, from successful development and
commercialization of our products.
|
As of December 31, 2006, we did not have any relationships
with unconsolidated entities or financial partnerships, such as
entities often referred to as structured finance or special
purpose entities, which would have been established for the
purpose of facilitating off-balance sheet arrangements or other
contractually narrow or limited purposes. In addition, we do not
engage in trading activities involving non-exchange traded
contracts. Therefore, we are not materially exposed to any
financing, liquidity, market, or credit risk that could arise if
we had engaged in these relationships.
CRITICAL
ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and
results of operations are based on our financial statements,
which have been prepared in accordance with accounting
principles generally accepted in the United States. The
preparation of these financial statements requires us to make
estimates and judgments that affect the reported amounts of
assets, liabilities, and expenses and related disclosure of
contingent assets and liabilities. We review our estimates on an
ongoing basis. We base our estimates on historical experience
and on various other assumptions that we believe to be
reasonable under the circumstances. Actual results may differ
from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more
detail in the notes to our financial statements included in this
report, we believe the following accounting policies to be
critical to the judgments and estimates used in the preparation
of our financial statements.
Revenue Recognition. We recognize revenue when
all terms and conditions of the agreements have been met,
including persuasive evidence of an arrangement, services have
been rendered, price is fixed or determinable, and
collectability is reasonably assured. For reimbursable cost
research grants, we recognize revenue as costs are incurred once
the grant funding is authorized. Funding of government grants
beyond the U.S. government’s current fiscal year is
subject to annual congressional appropriations, and the Company
cannot recognize revenue for subsequent years until the period
in which such funding is duly authorized. Provisions for
estimated losses on research grant projects and any other
contracts are made in the period such losses are determined.
Research and Development Costs. We expense our
research and development costs as incurred.
Stock-Based Compensation. We have four
stock-based employee compensation plans, described more fully in
Note 5 to the Financial Statements, and we record
compensation expense based upon the fair value of stock-based
awards. Effective January 1, 2003, we adopted the fair
value recognition provisions of SFAS No. 123,
Accounting for Stock-Based Compensation (SFAS 123).
The recognition provisions have been applied to all employee
awards granted, modified, or settled after January 1, 2003.
Effective January 1, 2006, the Company adopted the fair
value recognition provisions of FASB Statement No. 123(R),
“Share-Based Payment” (Statement 123(R)), using
the modified-prospective-transition method. As we have expensed
options since 2003, the adoption of Statement 123(R) did
not have a material impact on our stock-
48
based compensation expenses for the first quarter of 2006, and
management believes that the adoption of Statement 123(R)
will not have a material impact on its financial statements
going forward. We have continued to use the Black-Scholes
formula to estimate the value of stock-based awards with the
adoption of Statement 123(R).
With the adoption of Statement 123(R), we recognized in our
statement of operations for the year ended December 31,
2006 a one-time charge recorded as the cumulative effect of a
change in accounting principle, which reflects an estimate of
forfeitures for unvested awards outstanding as of the adoption
of Statement 123(R). This charge represents a reduction in
the compensation cost for prior periods for any unvested options
remaining that would not have been recognized in those prior
periods had forfeitures for such unvested options been estimated
during those prior periods. The cumulative effect for this
change in accounting principle totaled $16,726 and is recorded
on the statement of operations for the year ended
December 31, 2006.
As of December 31, 2006, we anticipate recognizing
approximately $3.5 million of total unrecognized
compensation expense, less estimated forfeitures, related to
nonvested options under the stock compensation plans in future
periods. These expenses are expected to be recognized over a
weighted-average period of 2.1 years.
Based on the closing price of our stock on December 29,
2006, the intrinsic value of options outstanding as of that date
was $6.0 million, of which $3.6 million related to
vested options and $2.4 million related to unvested options.
We account for equity instruments issued to nonemployees under
the provisions of SFAS 123 and Emerging Issues Task Force
Issue
No. 96-18,
Accounting for Equity Instruments That are Issued to Other
Than Employees for Acquiring, or in conjunction with, Selling,
Goods and Services. Accordingly, the estimated fair value of
the equity instrument is recorded on the earlier of the
performance commitment date or the date the services required
are completed.
|
|
|
Item 7A.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
Our primary exposure to market risk is interest income
sensitivity, which is affected by changes in the general level
of U.S. interest rates, particularly because the majority
of our investments are in short-term US government and agency
debt securities. The market value of these investments
fluctuates with changes in current market interest rates. In
general, as rates increase, the market value of a debt
investment would be expected to decrease. Likewise, as rates
decrease, the market value of a debt investment would be
expected to increase. To minimize such market risk, we generally
hold these instruments to maturity at which time they are
redeemed at their stated or face value. Due to the nature of our
short-term investments, we believe that we are not subject to
any material market risk exposure.
The interest rates on our debt obligations are fixed so
repayment of these obligations is not subject to any material
market risk exposure.
We do not have any foreign currency or other derivative
financial instruments.
|
|
|
Item 8.
|
Financial
Statements and Supplementary Data
|
Financial Statements and Supplementary Data are submitted as a
separate section of this report commencing on
Page F-1.
|
|
|
Item 9.
|
Changes
in and Disagreements With Accountants on Accounting and
Financial Disclosure
|
Not Applicable.
|
|
|
Item 9A.
|
Controls
and Procedures
|
Disclosure
Controls and Procedures
We currently have in place systems relating to disclosure
controls and procedures (as defined in
Rules 13a-15(e)
and
15d-15(e) of
the Securities Exchange Act of 1934). Our principal executive
officer and our principal financial officer evaluated the
effectiveness of these disclosure controls and procedures as of
the end of
49
our 2006 fiscal year in connection with the preparation of this
annual report. They concluded that the controls and procedures
are effective and adequate at that time.
Changes
in Internal Controls Over Financial Reporting
There have been no significant changes in our internal control
over financial reporting during the quarter ended
December 31, 2006, that have materially affected, or are
reasonably likely to materially affect, our internal control
over financial reporting.
|
|
|
Item 9B.
|
Other
Information
|
None.
PART III
The information required by Item 10 — Directors,
Executive Officers and Corporate Governance;
Item 11 — Executive Compensation;
Item 12 — Security Ownership of Certain
Beneficial Owners and Management and Related Stockholder
Matters; Item 13 — Certain Relationships and
Related Transactions and Director Independence; and
Item 14 — Principal Accounting Fees and Services
is incorporated into Part III of this Annual Report on
Form 10-K
by reference to our Proxy Statement for the Annual Meeting of
Stockholders scheduled to be held on June 5, 2007, except
that the information required by Item 10 pertaining to our
executive officers is contained in Part I of this report.
PART IV
|
|
|
Item 15.
|
Exhibits,
Financial Statement Schedules
|
(a) 1. Financial Statements
The financial statements are listed under Item 8 of this
report.
2. Financial Statement Schedules
The financial statement schedules required under this Item and
Item 8 are omitted because they are not applicable or the
required information is shown in the financial statements or the
footnotes thereto.
3. Exhibits
The exhibits are listed below under Part IV Item 15(b).
(b) Exhibits
The exhibits are set forth in the Exhibit Index.
EXHIBIT INDEX
| |
|
|
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
Location
|
|
|
|
|
3
|
.1.3
|
|
Third Amended and Restated
Certificate of Incorporation
|
|
|
(1)
|
|
|
|
3
|
.2.3
|
|
Third Amended and Restated By-laws
|
|
|
(2)
|
|
|
|
4
|
.2
|
|
Grant of Put Option dated
April 6, 2001 by Iomai Corporation to the Walter Reed Army
Institute of Research as representative of the United States of
America.
|
|
|
(1)
|
|
|
|
4
|
.3
|
|
Option Agreement dated
December 4, 2002 by and between Iomai Corporation and Elan
Corporation, plc.
|
|
|
(1)
|
|
|
|
4
|
.4
|
|
Stock Purchase Warrant dated
December 4, 2002 issued by Iomai Corporation to Friedman
Billings Ramsey & Co., Inc.
|
|
|
(1)
|
|
50
| |
|
|
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
Location
|
|
|
|
|
4
|
.5.1
|
|
Investor Rights Agreement dated
December 4, 2002 between Iomai Corporation and the
individuals specified in Exhibit A thereto.
|
|
|
(1)
|
|
|
|
4
|
.5.2
|
|
Amendment to the Investor Rights
Agreement dated March 27, 2003 among Iomai Corporation and
the Purchasers listed on the signature pages thereto.
|
|
|
(1)
|
|
|
|
4
|
.5.3
|
|
Amendment to Investor Rights
Agreement and Consent dated May 30, 2003 among Iomai
Corporation and the Purchasers listed on the signature pages
thereto.
|
|
|
(1)
|
|
|
|
4
|
.5.4
|
|
Amendment to Investor Rights
Agreement and Consent dated December 1, 2005 among Iomai
Corporation and the purchasers listed on the signature pages
thereto.
|
|
|
(1)
|
|
|
|
4
|
.6
|
|
Registration Rights Agreement
dated April 6, 2001 by and between Iomai Corporation and
MdBio, Inc. as trustee for and on behalf of the Walter Reed Army
Institute of Research, a representative of the United States of
America.
|
|
|
(1)
|
|
|
|
4
|
.7
|
|
Registration Rights Agreement
dated March 30, 1999 by and between Iomai Corporation and
Maryland Health Care Product Development Corporation.
|
|
|
(1)
|
|
|
|
4
|
.8
|
|
Registration Rights Agreement
dated July 18, 2000 by and between Iomai Corporation and
Eiicha Ida.
|
|
|
(1)
|
|
|
|
4
|
.9
|
|
Registration Rights Agreement
dated July 18, 2000 by and between Iomai Corporation and
Yuichi Suzuki.
|
|
|
(1)
|
|
|
|
4
|
.10
|
|
Registration Rights Agreement
dated July 18, 2000 by and between Iomai Corporation and
Toshiro Osoegawa.
|
|
|
(1)
|
|
|
|
4
|
.11
|
|
Registration Rights Agreement
dated August 15, 2000 by and between Iomai Corporation and
CZ Venture Operations, Inc.
|
|
|
(1)
|
|
|
|
4
|
.12
|
|
Registration Rights Agreement
dated January 4, 2001 by and between Iomai Corporation and
Alexandria Real Estate Equities, L.P.
|
|
|
(1)
|
|
|
|
4
|
.13
|
|
Form of common stock warrant dated
March 2, 2007
|
|
|
(7)
|
|
|
|
4
|
.14
|
|
Form of common stock warrant dated
March 2, 2007
|
|
|
(7)
|
|
|
|
9
|
.1
|
|
Voting Trust and Escrow Agreement
dated April 6, 2001 between Iomai Corporation and MdBio,
Inc., as trustee for and on behalf of Walter Reed Army Institute
of Research, a representative of the United States of America.
|
|
|
(1)
|
|
|
|
10
|
.1
|
|
Employment Agreement dated
May 18, 2002 between Iomai Corporation and Stanley Erck, as
amended on October 25, 2002.**
|
|
|
(1)
|
|
|
|
10
|
.1.1
|
|
Amendment No. 2, dated
December 1, 2005, to the Employment Agreement between Iomai
Corporation and Stanley Erck.**
|
|
|
(1)
|
|
|
|
10
|
.2
|
|
1998 Stock Option Plan, as amended
January 16, 2002.**
|
|
|
(1)
|
|
|
|
10
|
.3.1
|
|
1999 Stock Incentive Plan.
|
|
|
(1)
|
|
|
|
10
|
.3.2
|
|
Form of Incentive Stock Option
Agreement.**
|
|
|
(1)
|
|
|
|
10
|
.3.3
|
|
Form of Nonqualified Stock Option
Agreement.**
|
|
|
(1)
|
|
|
|
10
|
.4
|
|
2005 Incentive Plan, as amended
March 6, 2007.**
|
|
|
(8)
|
|
|
|
10
|
.5
|
|
2006 Employee Stock Purchase
Plan.**
|
|
|
(1)
|
|
|
|
10
|
.6
|
|
Terms of Non-Employee Director
Compensation.**
|
|
|
(1)
|
|
|
|
10
|
.7
|
|
Amended and Restated License
Agreement dated April 6, 2001 by and between Iomai
Corporation and the Walter Reed Army Institute of Research as
representative of the United States of America.+
|
|
|
(1)
|
|
|
|
10
|
.8
|
|
Form of Subordinated Convertible
Promissory Note issued by Iomai Corporation to the Walter Reed
Army Institute of Research as representative of the United
States of America.
|
|
|
(1)
|
|
51
| |
|
|
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
Location
|
|
|
|
|
10
|
.9
|
|
Commercial License Agreement dated
June 30, 2005 by and between Iomai Corporation and Dow
Global Technologies Incorporated.+
|
|
|
(1)
|
|
|
|
10
|
.10.1
|
|
Master Security Agreement dated
September 26, 2003 by and between Iomai Corporation and
Oxford Finance Corporation.
|
|
|
(1)
|
|
|
|
10
|
.10.2
|
|
Form of Promissory Note issued by
Iomai Corporation to Oxford Finance Corporation together with
schedule identifying dates, principal amounts and interest rates
of all outstanding promissory notes.
|
|
|
(1)
|
|
|
|
10
|
.11.1
|
|
Lease Agreement dated
December 18, 2000 by and between Iomai Corporation and
ARE-20/22/1300
Firstfield Quince Orchard, LLC.
|
|
|
(1)
|
|
|
|
10
|
.11.2
|
|
First Amendment to Lease dated
November 29, 2001 by and between Iomai Corporation and
ARE-20/22/1300
Firstfield Quince Orchard, LLC.
|
|
|
(1)
|
|
|
|
10
|
.11.3
|
|
Second Amendment to Lease dated
April 14, 2003 by and between Iomai Corporation and
ARE-20/22/1300
Firstfield Quince Orchard, LLC.
|
|
|
(1)
|
|
|
|
10
|
.11.4
|
|
Third Amendment to Lease dated
August 28, 2003 by and between Iomai Corporation and
ARE-20/22/1300
Firstfield Quince Orchard, LLC.
|
|
|
(1)
|
|
|
|
10
|
.11.5
|
|
Fourth Amendment to Lease dated
October 26, 2005 by and between Iomai Corporation and ARE
20/22/1300
Firstfield Quince Orchard, LLC.
|
|
|
(1)
|
|
|
|
10
|
.11.6
|
|
Letter Agreement amending Fourth
Amendment to Lease dated January 3, 2006 by and between
Iomai Corporation and ARE
20/22/1300
Firstfield Quince Orchard, LLC.
|
|
|
(3)
|
|
|
|
10
|
.11.7
|
|
Sublease Agreement dated
February 28, 2006 by and between Geomet Technologies, LLC
and Iomai Corporation.
|
|
|
(3)
|
|
|
|
10
|
.11.8
|
|
Fifth Amendment to Lease dated
July 25, 2006 by and between Iomai Corporation and ARE
20/22/1300
Firstfield Quince Orchard, LLC.
|
|
|
(4)
|
|
|
|
10
|
.11.9
|
|
Sixth Amendment to Lease dated
January 26, 2007 by and between Iomai Corporation and ARE
20/22/1300
Firstfield Quince Orchard, LLC.
|
|
|
(6)
|
|
|
|
10
|
.12
|
|
Form of Change in Control
Agreement, between Iomai Corporation and certain officers.**
|
|
|
(1)
|
|
|
|
10
|
.13
|
|
Securities Purchase Agreement
dated October 23, 2006 by and among Iomai Corporation and
the Purchasers thereto.
|
|
|
(5)
|
|
|
|
10
|
.14
|
|
Securities Purchase Agreement
dated March 2, 2007 by and among Iomai Corporation and
Investors thereto.
|
|
|
(7)
|
|
|
|
23
|
.1
|
|
Consent of Independent Registered
Public Accounting Firm.
|
|
|
|
|
|
|
31
|
.1
|
|
Certification of Chief Executive
Officer pursuant to
Rules 13a-14(a)
and
15d-14(a)
under the Securities Exchange Act of 1934, as amended.
|
|
|
|
|
|
|
31
|
.2
|
|
Certification of Chief Financial
Officer pursuant to
Rules 13a-14(a)
and
15d-14(a)
under the Securities Exchange Act of 1934, as amended.
|
|
|
|
|
|
|
32
|
.1
|
|
Certification of Chief Executive
Officer and Chief Financial Officer pursuant to 18 U.S.C.
Section 1350 as adopted pursuant to Section 906 of the
Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
|
|
|
** |
|
Indicates a management contract or compensatory plan. |
| |
|
+ |
|
Confidential Treatment Requested Under 17 C.F.R.
§§ 200.80(b)(4) and 230.406. The confidential
portions of this exhibit have been omitted and are marked by an
asterisk. |
| |
|
(1) |
|
Previously filed as an exhibit to the Company’s
Form S-1/A
(File No.
333-128765)
and incorporated herein by reference thereto. |
| |
|
(2) |
|
Previously filed as an exhibit to the Company’s
Form 8-K
filed March 24, 2006 and incorporated herein by reference
thereto. |
52
|
|
|
|
(3) |
|
Previously filed as an exhibit to the Company’s Annual
Report on Form
10-K filed
March 24, 2006 and incorporated herein by reference thereto. |
| |
|
(4) |
|
Previously filed as an exhibit to the Company’s
Form 8-K
filed July 7, 2006 and incorporated herein by reference
thereto. |
| |
|
(5) |
|
Previously filed as an exhibit to the Company’s
Form 8-K
filed October 24, 2006 and incorporated herein by reference
thereto. |
| |
|
(6) |
|
Previously filed as an exhibit to the Company’s
Form 8-K
filed January 30, 2007 and incorporated herein by reference
thereto. |
| |
|
(7) |
|
Previously filed as an exhibit to the Company’s
Form 8-K
filed March 2, 2007 and incorporated herein by reference
thereto. |
| |
|
(8) |
|
Previously filed as an exhibit to the Company’s
Form 8-K
filed March 7, 2007 and incorporated herein by reference
thereto. |
53
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the
Securities Exchange Act of 1934, the registrant has duly caused
this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
IOMAI CORPORATION
Name: Stanley C. Erck
Title: President, Chief Executive Officer,
Treasurer and Director
Dated: March 22, 2007
Pursuant to the requirements of the Securities Exchange Act of
1934, this report has been signed below by the following persons
on behalf of the registrant in the capacities indicated as of
March 22, 2007.
| |
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
/s/ Stanley
C. Erck
Stanley
C. Erck
|
|
Chief Executive Officer,
President,
Treasurer and Director
(Principal Executive Officer)
|
|
|
|
|
|
/s/ Russell
P. Wilson
Russell
P. Wilson
|
|
Senior Vice President, Chief
Financial Officer,
General Counsel and Secretary
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
/s/ M.
James
Barrett
M.
James Barrett, Ph.D.
|
|
Chairman of the Board of Directors
|
|
|
|
|
|
/s/ R.
Gordon
Douglas
R.
Gordon Douglas, M.D.
|
|
Director
|
|
|
|
|
|
/s/ Richard
Douglas
Richard
Douglas, Ph.D.
|
|
Director
|
|
|
|
|
|
/s/ Jeff
Himawan
Jeff
Himawan, Ph.D.
|
|
Director
|
|
|
|
|
|
/s/ F.
Weller
Meyer
F.
Weller Meyer
|
|
Director
|
|
|
|
|
|
/s/ Thomas
Martin
Vernon
Thomas
Martin Vernon, M.D.
|
|
Director
|
54
IOMAI
CORPORATION
INDEX TO
FINANCIAL STATEMENTS
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
F-2
|
|
|
|
|
|
F-3
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
F-1
IOMAI
CORPORATION
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders of
Iomai Corporation
We have audited the accompanying balance sheets of Iomai
Corporation as of December 31, 2006 and 2005 and the
related statements of operations, changes in redeemable
preferred stock and stockholders’ equity (deficit), and
cash flows for each of the three years in the period ended
December 31, 2006. These financial statements are the
responsibility of the Company’s management. Our
responsibility is to express an opinion on these financial
statements based on our audits.
We conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. We were not engaged to perform an
audit of the Company’s internal control over financial
reporting. Our audits included consideration of internal control
over financial reporting as a basis for designing audit
procedures that are appropriate in the circumstances, but not
for the purpose of expressing an opinion on the effectiveness of
the Company’s internal control over financial reporting.
Accordingly, we express no such opinion. An audit also includes
examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements, assessing the
accounting principles used and significant estimates made by
management, and evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable
basis for our opinion.
In our opinion, the financial statements referred to above
present fairly, in all material respects, the financial position
of Iomai Corporation at December 31, 2006 and 2005, and the
results of its operations and its cash flows for each of the
three years in the period ended December 31, 2006 in
conformity with U.S. generally accepted accounting
principles.
As discussed in Note 2 to the financial statements, the
Company changed its method of accounting for stock-based
compensation in 2006 upon adoption of Statement of Financial
Accounting Standards No. 123(R), “Share-Based
Payment”.
McLean, Virginia
March 19, 2007
F-2
IOMAI
CORPORATION
BALANCE
SHEETS
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
ASSETS
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
13,847
|
|
|
$
|
5,190
|
|
|
Marketable securities
|
|
|
1,489
|
|
|
|
—
|
|
|
Accounts receivable
|
|
|
62
|
|
|
|
12
|
|
|
Prepaid expenses and other current
assets
|
|
|
500
|
|
|
|
258
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
15,898
|
|
|
|
5,460
|
|
|
Property and equipment, net
|
|
|
6,736
|
|
|
|
4,465
|
|
|
Restricted cash
|
|
|
—
|
|
|
|
49
|
|
|
Restricted marketable securities
|
|
|
268
|
|
|
|
590
|
|
|
Deferred financing costs
|
|
|
—
|
|
|
|
1,108
|
|
|
Other noncurrent assets
|
|
|
183
|
|
|
|
189
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
23,085
|
|
|
$
|
11,861
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND
STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
|
1,598
|
|
|
|
2,012
|
|
|
Accrued expenses
|
|
|
2,353
|
|
|
|
1,611
|
|
|
Notes payable, current portion
|
|
|
1,369
|
|
|
|
1,333
|
|
|
Notes payable to related party,
current portion
|
|
|
176
|
|
|
|
229
|
|
|
Capital lease obligation, current
portion
|
|
|
1
|
|
|
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
5,497
|
|
|
|
5,190
|
|
|
Notes payable, long-term portion
|
|
|
1,866
|
|
|
|
1,397
|
|
|
Notes payable to related party,
long-term portion
|
|
|
1,347
|
|
|
|
1,264
|
|
|
Capital lease obligation,
long-term portion
|
|
|
—
|
|
|
|
1
|
|
|
Deferred rent
|
|
|
340
|
|
|
|
122
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
9,050
|
|
|
|
7,974
|
|
|
Common stock subject to put right
|
|
|
—
|
|
|
|
1,959
|
|
|
Warrant to purchase Series C
preferred stock
|
|
|
—
|
|
|
|
23
|
|
|
Series C convertible
redeemable preferred stock, $0.01 par value; 0 shares
authorized, issued and outstanding as of December 31, 2006;
150,000,000 shares authorized; 129,590,034 shares
issued and outstanding at December 31, 2005
|
|
|
—
|
|
|
|
70,363
|
|
|
Stockholders’ equity
(deficit):
|
|
|
|
|
|
|
|
|
|
Series B convertible
preferred stock; $0.01 par value; 0 shares authorized,
issued and outstanding as of December 31, 2006;
15,200,000 shares authorized; 14,734,578 shares issued
and outstanding at December 31, 2005
|
|
|
—
|
|
|
|
147
|
|
|
Common stock, $0.01 par
value; 200,000,000 shares authorized and
19,197,387 shares issued and outstanding as of
December 31, 2006; 220,000,000 shares authorized and
795,519 shares issued and outstanding at December 31,
2005
|
|
|
192
|
|
|
|
8
|
|
|
Additional paid-in capital
|
|
|
114,631
|
|
|
|
—
|
|
|
Accumulated other comprehensive
income
|
|
|
—
|
|
|
|
1
|
|
|
Accumulated deficit
|
|
|
(100,788
|
)
|
|
|
(68,614
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
(deficit)
|
|
|
14,035
|
|
|
|
(68,458
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and
stockholders’ equity (deficit)
|
|
$
|
23,085
|
|
|
$
|
11,861
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-3
IOMAI
CORPORATION
STATEMENTS
OF OPERATIONS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
2004
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Revenues
|
|
$
|
1,475
|
|
|
$
|
2,371
|
|
|
$
|
2,345
|
|
|
Cost and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
28,041
|
|
|
|
16,529
|
|
|
|
14,349
|
|
|
General and administrative
|
|
|
5,857
|
|
|
|
3,780
|
|
|
|
3,206
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total costs and expenses
|
|
|
33,898
|
|
|
|
20,309
|
|
|
|
17,555
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(32,423
|
)
|
|
|
(17,938
|
)
|
|
|
(15,210
|
)
|
|
Other income (expense)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
1,006
|
|
|
|
392
|
|
|
|
621
|
|
|
Interest expense
|
|
|
(377
|
)
|
|
|
(467
|
)
|
|
|
(266
|
)
|
|
Other expense, net
|
|
|
(8
|
)
|
|
|
(17
|
)
|
|
|
(225
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income (expense), net
|
|
|
621
|
|
|
|
(92
|
)
|
|
|
130
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss before cumulative effect
of a change in accounting principle
|
|
|
(31,802
|
)
|
|
|
(18,030
|
)
|
|
|
(15,080
|
)
|
|
Cumulative effect of change in
accounting principle
|
|
|
17
|
|
|
|
—
|
|
|
|
—
|
|
|
Net loss
|
|
|
(31,785
|
)
|
|
|
(18,030
|
)
|
|
|
(15,080
|
)
|
|
Dividends on and accretion of
convertible preferred stock
|
|
|
(471
|
)
|
|
|
(5,562
|
)
|
|
|
(5,526
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss available to common
stockholders
|
|
$
|
(32,256
|
)
|
|
$
|
(23,592
|
)
|
|
$
|
(20,606
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss available to common
stockholders per share of common stock — basic and
diluted
|
|
$
|
(2.03
|
)
|
|
$
|
(30.14
|
)
|
|
$
|
(26.90
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average number of shares
of common stock — basic and diluted
|
|
|
15,915,797
|
|
|
|
782,715
|
|
|
|
765,945
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-4
IOMAI
CORPORATION
STATEMENTS
OF CHANGES IN REDEEMABLE PREFERRED STOCK AND STOCKHOLDERS’
EQUITY (DEFICIT)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity (Deficit)
|
|
|
|
|
Series C Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
Total
|
|
|
|
|
Redeemable
|
|
|
Series B Convertible
|
|
|
|
|
|
|
|
|
Additional
|
|
|
Other
|
|
|
|
|
|
Stockholders’
|
|
|
|
|
Preferred Stock
|
|
|
Preferred Stock
|
|
|
Common Stock
|
|
|
Paid-In
|
|
|
Comprehensive
|
|
|
Accumulated
|
|
|
Equity
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Income (Loss)
|
|
|
Deficit
|
|
|
(Deficit)
|
|
|
|
|
(In thousands, except share data)
|
|
|
|
|
Balance at December 31, 2003
|
|
|
127,685,982
|
|
|
|
59,507
|
|
|
|
14,734,578
|
|
|
|
147
|
|
|
|
764,325
|
|
|
|
8
|
|
|
|
8,679
|
|
|
|
18
|
|
|
|
(34,890
|
)
|
|
|
(26,038
|
)
|
|
Issuance of Series C
convertible redeemable preferred stock, net of issuance costs of
$31,741
|
|
|
1,904,052
|
|
|
|
810
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Amortization of Series C
warrants
|
|
|
—
|
|
|
|
12
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Accretion of issuance costs
|
|
|
—
|
|
|
|
438
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(438
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(438
|
)
|
|
Accrual of dividends
|
|
|
—
|
|
|
|
4,554
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,554
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,554
|
)
|
|
Issuance of Common Stock warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1
|
|
|
Stock option compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
154
|
|
|
|
—
|
|
|
|
—
|
|
|
|
154
|
|
|
Exercise of options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,596
|
|
|
|
—
|
|
|
|
7
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7
|
|
|
Other comprehensive loss:
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(15,080
|
)
|
|
|
(15,080
|
)
|
|
Unrealized loss on investments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(44
|
)
|
|
|
—
|
|
|
|
(44
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(15,124
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2004
|
|
|
129,590,034
|
|
|
|
65,321
|
|
|
|
14,734,578
|
|
|
|
147
|
|
|
|
771,921
|
|
|
|
8
|
|
|
|
3,849
|
|
|
|
(26
|
)
|
|
|
(49,970
|
)
|
|
|
(45,992
|
)
|
|
Amortization of Series C
warrants
|
|
|
—
|
|
|
|
12
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Accretion of issuance costs
|
|
|
—
|
|
|
|
443
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(333
|
)
|
|
|
—
|
|
|
|
(110
|
)
|
|
|
(443
|
)
|
|
Accrual of dividends
|
|
|
—
|
|
|
|
4,587
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,084
|
)
|
|
|
—
|
|
|
|
(504
|
)
|
|
|
(4,588
|
)
|
|
Issuance of Common Stock warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
9
|
|
|
|
—
|
|
|
|
—
|
|
|
|
9
|
|
|
Stock option compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
538
|
|
|
|
—
|
|
|
|
—
|
|
|
|
538
|
|
|
Cash in lieu of fractional common
shares
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(60
|
)
|
|
|
—
|
|
|
|
(1
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(1
|
)
|
|
Exercise of options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
23,658
|
|
|
|
—
|
|
|
|
22
|
|
|
|
—
|
|
|
|
—
|
|
|
|
22
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(18,030
|
)
|
|
|
(18,030
|
)
|
|
Unrealized gain on investments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
27
|
|
|
|
—
|
|
|
|
27
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(18,003
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2005
|
|
|
129,590,034
|
|
|
|
70,363
|
|
|
|
14,734,578
|
|
|
|
147
|
|
|
|
795,519
|
|
|
|
8
|
|
|
|
—
|
|
|
|
1
|
|
|
|
(68,614
|
)
|
|
|
(68,458
|
)
|
|
Amortization of Series C
warrants
|
|
|
—
|
|
|
|
1
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1
|
)
|
|
|
—
|
|
|
|
(1
|
)
|
|
Accretion of issuance costs
|
|
|
—
|
|
|
|
37
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(37
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(37
|
)
|
|
Accrual of dividends
|
|
|
—
|
|
|
|
389
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(389
|
)
|
|
|
(389
|
)
|
|
Stock option compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,065
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,065
|
|
|
Exercise of options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
14,030
|
|
|
|
—
|
|
|
|
13
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13
|
|
|
Exercise of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,865
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Issuance of common stock in
connection with initial public offering, net of offering costs
of $4,012,584
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
5,000,000
|
|
|
|
50
|
|
|
|
30,892
|
|
|
|
—
|
|
|
|
—
|
|
|
|
30,942
|
|
|
Automatic conversion of common
stock subject to put right into common stock upon initial public
offering
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,958
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,958
|
|
|
Automatic conversion of preferred
stock into common stock upon initial public offering
|
|
|
(129,590,034
|
)
|
|
|
(70,790
|
)
|
|
|
(14,734,578
|
)
|
|
|
(147
|
)
|
|
|
11,101,867
|
|
|
|
111
|
|
|
|
70,849
|
|
|
|
—
|
|
|
|
—
|
|
|
|
70,813
|
|
|
Issuance of common stock, net of
offering costs of $86,593
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,283,106
|
|
|
|
23
|
|
|
|
9,891
|
|
|
|
—
|
|
|
|
—
|
|
|
|
9,914
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(31,785
|
)
|
|
|
(31,785
|
)
|
|
Unrealized gain on investments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(31,785
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2006
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
19,197,387
|
|
|
$
|
192
|
|
|
$
|
114,631
|
|
|
$
|
—
|
|
|
$
|
(100,788
|
)
|
|
$
|
14,035
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-5
IOMAI
CORPORATION
STATEMENTS
OF CASH FLOWS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
2004
|
|
|
|
|
(In thousands)
|
|
|
|
|
Cash flows from operating
activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(31,785
|
)
|
|
$
|
(18,030
|
)
|
|
$
|
(15,080
|
)
|
|
Adjustments to reconcile net loss
to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
1,261
|
|
|
|
2,670
|
|
|
|
1,627
|
|
|
Stock-based compensation expense
|
|
|
1,065
|
|
|
|
538
|
|
|
|
154
|
|
|
Non-cash interest expense and
amortization of premium/discount of marketable securities
|
|
|
(255
|
)
|
|
|
28
|
|
|
|
229
|
|
|
Deferred rent
|
|
|
194
|
|
|
|
(54
|
)
|
|
|
(36
|
)
|
|
Provision for doubtful accounts
|
|
|
—
|
|
|
|
—
|
|
|
|
(135
|
)
|
|
Loss on disposal of property and
equipment
|
|
|
10
|
|
|
|
4
|
|
|
|
—
|
|
|
Changes in operating assets and
liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(50
|
)
|
|
|
62
|
|
|
|
265
|
|
|
Prepaid expenses and other current
assets
|
|
|
(242
|
)
|
|
|
(40
|
)
|
|
|
135
|
|
|
Other noncurrent assets
|
|
|
2
|
|
|
|
(45
|
)
|
|
|
29
|
|
|
Accounts payable
|
|
|
(9
|
)
|
|
|
420
|
|
|
|
(471
|
)
|
|
Accrued expenses
|
|
|
943
|
|
|
|
143
|
|
|
|
(218
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating
activities
|
|
|
(28,866
|
)
|
|
|
(14,304
|
)
|
|
|
(13,501
|
)
|
|
Cash flows from investing
activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment
|
|
|
(3,501
|
)
|
|
|
(1,339
|
)
|
|
|
(5,075
|
)
|
|
Sale of property and equipment
|
|
|
7
|
|
|
|
—
|
|
|
|
—
|
|
|
Restricted cash and marketable
securities
|
|
|
384
|
|
|
|
35
|
|
|
|
(4
|
)
|
|
Sales/maturities of marketable
securities
|
|
|
16,500
|
|
|
|
22,150
|
|
|
|
32,450
|
|
|
Purchases of marketable securities
|
|
|
(17,747
|
)
|
|
|
(6,790
|
)
|
|
|
(18,510
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by
investing activities
|
|
|
(4,357
|
)
|
|
|
14,056
|
|
|
|
8,861
|
|
|
Cash flows from financing
activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from the exercise of
stock options
|
|
|
13
|
|
|
|
22
|
|
|
|
7
|
|
|
Cash issued in lieu of fractional
common stock
|
|
|
—
|
|
|
|
(1
|
)
|
|
|
—
|
|
|
Proceeds from issuance of
Series C convertible redeemable preferred stock, net
|
|
|
—
|
|
|
|
—
|
|
|
|
810
|
|
|
Proceeds from initial public
offering, net of underwriting commissions
|
|
|
32,854
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from private placement of
common stock
|
|
|
10,000
|
|
|
|
—
|
|
|
|
—
|
|
|
Stock issuance costs
|
|
|
(1,517
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Deferred stock issuance costs
|
|
|
—
|
|
|
|
(481
|
)
|
|
|
—
|
|
|
Proceeds from notes payable
|
|
|
2,000
|
|
|
|
468
|
|
|
|
1,902
|
|
|
Principal payments on notes payable
|
|
|
(1,495
|
)
|
|
|
(1,166
|
)
|
|
|
(820
|
)
|
|
Proceeds from notes payable to
related party
|
|
|
280
|
|
|
|
—
|
|
|
|
1,451
|
|
|
Principal payments on notes
payable to related party
|
|
|
(250
|
)
|
|
|
(333
|
)
|
|
|
(207
|
)
|
|
Payments under capital lease
obligations
|
|
|
(5
|
)
|
|
|
(13
|
)
|
|
|
(18
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in)
financing activities
|
|
|
41,880
|
|
|
|
(1,504
|
)
|
|
|
3,125
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash
and cash equivalents
|
|
|
8,657
|
|
|
|
(1,752
|
)
|
|
|
(1,515
|
)
|
|
Cash and cash equivalents at
beginning of year
|
|
|
5,190
|
|
|
|
6,942
|
|
|
|
8,457
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end
of year
|
|
$
|
13,847
|
|
|
$
|
5,190
|
|
|
$
|
6,942
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental schedule of
noncash investing and financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$
|
902
|
|
|
$
|
497
|
|
|
$
|
424
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-6
IOMAI
CORPORATION
Iomai Corporation (the Company or Iomai) was incorporated in May
1997 as a Delaware corporation to discover and develop vaccines
and immune system stimulants, delivered to the skin via a novel,
needle-free technology called transcutaneous immunization (TCI).
TCI exploits the unique benefits of a major group of
antigen-presenting cells found in the outer layers of the skin
(Langerhans cells) to generate an enhanced immune response. TCI
has the potential to enhance the efficacy of existing vaccines,
develop new vaccines that are viable only through transcutaneous
administration and expand the global vaccine market. The Company
currently has five product candidates in development: four
targeting influenza and pandemic flu and one to prevent E.
coli-related travelers’ diarrhea.
|
|
|
2.
|
SIGNIFICANT
ACCOUNTING POLICIES
|
Use of
estimates
The preparation of the financial statements in conformity with
accounting principles generally accepted in the United States
requires management to make estimates and assumptions that
affect the reported amounts of assets and liabilities, the
disclosure of contingent assets and liabilities at the date of
the financial statements, and the reported amounts of revenues
and expenses during the reporting period. Actual results could
differ from those estimates.
Cash
and cash equivalents
The Company considers demand deposits and all highly liquid
investments with original maturities of three months or less at
the date of purchase to be cash equivalents. As of
December 31, 2006 and 2005, the Company’s cash
equivalents consisted of money market accounts, an overnight
repurchase agreement and a savings account.
Marketable
securities
All marketable securities, consisting of U.S. government
obligations and U.S. agency obligations, are classified as
available-for-sale.
These securities are carried at fair value, plus accrued
interest. Any unrealized holding gains and losses are reported
as accumulated other comprehensive income (loss), which is a
separate component of stockholders’ equity (deficit).
Realized gains and losses are reported in operations, and gains
and losses on sales of securities are computed using the
specific-identification method.
The Company periodically reviews its marketable securities to
determine whether a decline in fair value below the carrying
value exists and is
other-than-temporary.
This evaluation consists of a review of several factors,
including but not limited to: the length of time and extent that
a security has been in an unrealized loss position, the
existence of an event that would impair the issuer’s future
earnings potential, the near-term prospects for recovery of the
market value of a security, and the intent and ability of the
Company to hold the security until the market value recovers.
Declines in value below cost for debt securities are not assumed
to be
other-than-temporary
where: it is considered probable that all contractual terms of
the security will be satisfied, the decline is due primarily to
changes in interest rates (and not because of increased credit
risk), or the Company intends and has the ability to hold the
investment for a period of time sufficient to allow a market
recovery. Unrealized losses related to debt securities greater
than and less than one year are not significant.
If management determines that such an impairment exists, the
carrying value of the investment will be reduced to the current
fair value of the investment and the Company will recognize a
change in the statement of operations equal to the amount of the
carrying value reduction.
F-7
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
Restricted
cash and marketable securities
Restricted cash as of December 31, 2006 and 2005 was $0 and
$49,150, respectively. Restricted cash at December 31, 2005
related to a deposit held as collateral for a letter of credit
acting as a security deposit on a facility operating lease. In
December 2006, the letter of credit was terminated.
Restricted marketable securities at December 31, 2006 and
2005 include marketable securities with a face value of $265,000
and $600,000, respectively, pledged as collateral to secure
payment of promissory notes issued to finance, in part, the
build-out of the Company’s facilities (see
Note 6 — Long-Term Debt).
Accounts
receivable
Accounts receivable that management has the intent and ability
to hold until payment are reported in the balance sheets at
outstanding amounts, less the allowance for doubtful accounts.
The Company writes off uncollectible receivables when the
likelihood of collection is remote. The Company maintains an
allowance for doubtful accounts, which is determined based on
historical experience and management’s expectations of
future losses. There was no allowance for doubtful accounts in
either 2005 or 2006.
Unbilled accounts receivable consist principally of expenses
incurred on reimbursable research grants prior to year-end that
have not yet been billed to the contracting agent.
Concentration
of credit risk and fair value of financial
instruments
Financial instruments that potentially subject the Company to
concentrations of credit risk consist primarily of cash and cash
equivalents, marketable securities, accounts receivable, and
notes payable. The Company places its cash and cash equivalents
with financial institutions, and its marketable securities
consist of U.S. government obligations and U.S. agency
obligations. Management believes that the financial risks
associated with its cash and cash equivalents and marketable
securities are minimal. Accounts receivable principally consist
of amounts due from government agencies under government grants.
The carrying amount of current assets and liabilities
approximates their fair values due to their short-term
maturities. The fair value of notes payable approximates their
carrying amount as of December 31, 2006 and 2005 based on
rates currently available to the Company for debt with similar
terms and remaining maturities.
Property
and equipment
Property and equipment are stated at cost. Depreciation is
computed using the straight-line method over the estimated
useful lives as follows:
| |
|
|
|
|
|
Asset Description
|
|
Useful Life (Years)
|
|
|
|
|
Furniture and equipment
|
|
|
3 - 7
|
|
|
Lab equipment
|
|
|
5
|
|
Leasehold improvements are amortized over the shorter of the
life of the lease or the related asset. Maintenance and repairs
are charged to expense as incurred.
Income
taxes
Income taxes are accounted for using the liability method.
Deferred tax assets and liabilities are recognized for the
future tax consequences attributable to differences between the
financial statement carrying amounts of existing assets and
liabilities, and their respective tax bases and operating loss
carryforwards. Deferred tax assets and liabilities are measured
using enacted tax rates expected to apply to taxable income in
the year in which those temporary differences are expected to be
recovered or settled. The effect on deferred tax assets and
liabilities of a change in tax rates is recognized in income in
the period that includes the enactment date.
F-8
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
Valuation allowances are established when necessary to reduce
net deferred tax assets to the amount expected to be realized.
Income tax expense is the tax payable for the period and the
change during the period in deferred tax assets and liabilities.
Revenue
recognition
The Company recognizes revenue when all terms and conditions of
the agreements have been met, including persuasive evidence of
an arrangement, services have been rendered, price is fixed or
determinable, and collectability is reasonably assured. For
reimbursable cost research grants and contracts, the Company
recognizes revenue as costs are incurred. Provisions for
estimated losses on research grant projects and any other
contracts are made in the period such losses are determined.
Research
and development costs
The Company expenses its research and development costs as
incurred; however, equipment and facilities that are acquired or
constructed for research and development activities that have
alternative future uses (in research and development projects or
otherwise) are capitalized and depreciated as tangible assets.
Comprehensive
loss
Statement of Financial Accounting Standards (SFAS) No. 130,
Reporting Comprehensive Income (SFAS No. 130),
requires the presentation of the comprehensive loss and its
components as part of the financial statements. Comprehensive
loss is comprised of net loss and other changes in equity that
are excluded from net loss. The Company includes unrealized
holding gains and losses on
available-for-sale
securities in accumulated other comprehensive loss on its
balance sheets and statements of changes in redeemable preferred
stock and stockholders’ equity (deficit).
Stock-based
compensation
The Company has four stock-based employee compensation plans,
described more fully in Note 9 —
Stockholders’ Equity (Deficit). Effective January 1,
2003, the Company adopted the fair value recognition provisions
of SFAS No. 123, Accounting for Stock-Based
Compensation (SFAS No. 123). Under the prospective
method of adoption selected by the Company under the provisions
of FASB Statement No. 148, Accounting for Stock-Based
Compensation — Transition and Disclosure, an
amendment of FASB Statement No. 123
(SFAS No. 148), the recognition provisions have been
applied to all employee awards granted, modified, or settled
after January 1, 2003. Effective January 1, 2006, the
Company adopted the fair value recognition provisions of FASB
Statement No. 123(R), “Share-Based Payment”
(Statement 123(R)), using the
modified-prospective-transition method. See
Note 9 — Stockholders’ Equity (Deficit) for
discussion of impact of adoption of Statement 123(R). The
Company uses the Black-Scholes-Merton formula to estimate the
value of stock options granted to employees.
Statement 123(R) also requires the benefits of tax
deductions in excess of recognized compensation cost to be
reported as a financing cash flow rather than as an operating
cash flow as required under current literature. This requirement
will reduce net operating cash flows and increase net financing
cash flows in periods after adoption; however, the Company
cannot estimate what those amounts will be in the future
(because they depend on, among other things, when employees
exercise stock options).
Equity instruments issued to nonemployees are accounted for
under the provisions of SFAS No. 123 and Emerging
Issues Task Force Issue
No. 96-18,
Accounting for Equity Instruments That are Issued to Other
Than Employees for Acquiring, or in conjunction with, Selling,
Goods and Services. Accordingly, the estimated fair value of
the equity instrument is recorded on the earlier of the
performance commitment date or the date the services required
are completed.
F-9
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
Prior to the Company’s initial public offering, our board
of directors determined fair value of our common stock for
options to acquire shares of our common stock. Our board of
directors made contemporaneous determinations of fair value and
did not employ a third party valuation firm to determine fair
value. In the absence of a public trading market prior to the
Company’s initial public offering, and as a clinical-stage
company with no significant revenues, the Company believes that
it was appropriate to consider a range of factors to determine
the fair value market value of the common stock at each option
grant date. These factors included: (1) the achievement of
clinical and operational milestones by the Company, (2) the
status of strategic relationships with collaborators,
(3) the significant risks associated with the
Company’s early stage of development of a new technology,
(4) capital market conditions for life science companies,
particularly similarly situated privately-held, early-stage life
science companies, (5) the Company’s available cash,
financial condition and results of operations, (6) the most
recent sales of the Company’s preferred stock and
(7) the preferential rights of the outstanding preferred
stock. In connection with the preparation of the financial
statements for the Company’s initial public offering, the
Company reassessed its estimate of the fair value for financial
reporting purposes of its common stock following its stock
option grants in March 2004. This valuation was done
retrospectively by management, a related party, and the Company
did not obtain contemporaneous valuations from an independent
valuation specialist. Based on this reassessment, the Company
determined that there were five periods between March 2004 and
October 2005 in which the Company’s reassessment of the
fair value of its common stock ranged from $2.75 to $9.00 per
share during the period options were granted.
Basic
and diluted net loss attributable to common stockholders per
share of common stock
Basic net loss attributable to common stockholders per share of
common stock excludes dilution for potential common stock
issuances and is computed by dividing net loss attributable to
common stockholders by the weighted-average number of shares
outstanding for the period. Diluted net loss attributable to
common stockholders per share reflects the potential dilution
that could occur if securities or other contracts to issue
common stock were exercised or converted into common stock.
Mandatory redeemable convertible preferred stock, stock options
and warrants were not considered in the computation of diluted
net loss attributable to common stockholders per share for the
years ended December 31, 2006, 2005 and 2004 as their
effect is antidilutive.
Reverse
stock split
All share and per share amounts have been retroactively adjusted
to give effect to a
1-for-13
reverse stock split effective on December 2, 2005.
F-10
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
Following is a summary of the
available-for-sale
marketable securities at December 31, 2006 and 2005 (in
thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2006
|
|
|
|
|
|
|
|
Gross
|
|
|
Gross
|
|
|
|
|
|
|
|
|
|
|
Unrealized
|
|
|
Unrealized
|
|
|
|
|
|
|
|
Amortized Cost
|
|
|
Gains
|
|
|
Losses
|
|
|
Fair Value
|
|
|
|
|
Available for Sale
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Treasury and agency
obligations
|
|
$
|
1,489
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
1,489
|
|
|
Accrued interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total — Marketable
securities
|
|
$
|
1,489
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
1,489
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Marketable
Securities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Treasury and agency
obligations
|
|
$
|
265
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
265
|
|
|
Accrued interest
|
|
|
3
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total — Restricted
marketable securities
|
|
$
|
268
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
268
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2005
|
|
|
|
|
|
|
|
Gross
|
|
|
Gross
|
|
|
|
|
|
|
|
|
|
|
Unrealized
|
|
|
Unrealized
|
|
|
|
|
|
|
|
Amortized Cost
|
|
|
Gains
|
|
|
Losses
|
|
|
Fair Value
|
|
|
|
|
Available for Sale
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Treasury and agency
obligations
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Accrued interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total — Marketable
securities
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Marketable
Securities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Treasury and agency
obligations
|
|
$
|
590
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
590
|
|
|
Accrued interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total — Restricted
marketable securities
|
|
$
|
590
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
590
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The contractual maturities of the Company’s marketable
securities and restricted marketable securities were less than
one year at December 31, 2006 and 2005. Actual maturities
may differ from contractual maturities because the issuers of
the securities may have the right to prepay obligations without
prepayment penalties.
The Company’s gross proceeds from maturities of its
marketable securities for the years ended December 31,
2006, 2005 and 2004 were $16,500,000 $22,150,000 and
$32,450,000, respectively. For these periods, the Company did
not realize any gains or losses related to its marketable
securities.
F-11
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
|
|
|
4.
|
PROPERTY
AND EQUIPMENT
|
Property and equipment consist of the following (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
|
|
Furniture and equipment
|
|
$
|
1,465
|
|
|
$
|
769
|
|
|
Lab equipment
|
|
|
4,225
|
|
|
|
3,327
|
|
|
Leasehold improvements
|
|
|
6,051
|
|
|
|
5,297
|
|
|
Construction-in-progress
|
|
|
1,377
|
|
|
|
208
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal
|
|
|
13,117
|
|
|
|
9,602
|
|
|
Accumulated depreciation
|
|
|
(6,381
|
)
|
|
|
(5,137
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
$
|
6,736
|
|
|
$
|
4,465
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization expense on property, plant and
equipment totaled approximately $1.3 million,
$2.7 million, and $1.6 million for the years ended
December 31, 2006, 2005 and 2004 respectively. For the
years ended December 31, 2006, 2005 and 2004, the Company
capitalized interest expense of approximately $65,000, $26,000
and $175,000, respectively.
Accrued liabilities consist of the following (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
|
|
Salary and employee benefits
|
|
$
|
1,282
|
|
|
$
|
775
|
|
|
Clinical trial obligations
|
|
|
561
|
|
|
|
395
|
|
|
Other
|
|
|
510
|
|
|
|
441
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
2,353
|
|
|
$
|
1,611
|
|
|
|
|
|
|
|
|
|
|
|
Notes
payable
In September 2003, the Company entered into a loan arrangement
with an equipment leasing company to finance up to
$5 million for the build-out of the Company’s facility
to accommodate (1) a GMP pilot plant for biological and
patch manufacturing to support clinical trials and to advance
patch formulation and development, and (2) laboratories to
support patch formulation and development, as well as
small-scale biological research. Under the terms of the loan,
the Company was permitted to draw down up to $5 million
through September 2005. In May 2006, the Company negotiated an
extension of this facility to finance up to $2 million of
additional capital expenditures. The extension is subject to a
covenant that any additional drawdowns maintain a specific
collateral mix.
In connection with the original loan agreement, the Company
entered into a master security agreement under which the Company
pledged to the equipment leasing company as collateral
(i) a security interest in existing equipment then having a
net book value of approximately $416,000, (ii) a security
interest in all laboratory/scientific and related manufacturing
equipment, together with furniture and computer hardware,
financed under the facility, and (iii) marketable
securities with a face value of $600,000, which was reduced to a
face value of $265,000 during the second quarter of 2006. As of
December 31, 2006 and 2005, the Company had aggregate
drawdowns of $6,843,672 and $4,843,672, respectively, under the
loan agreement. The lender has the right to accelerate
F-12
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
repayment of the indebtedness upon a default, including if the
lender determines in good faith that there has been a material
adverse change in the Company’s business plan which would
materially impair the ability of the Company to perform its
obligations or of the lender to enforce the indebtedness or
realize upon the collateral.
The components of notes payable are as follows (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
|
|
Promissory Note dated
September 26, 2003; 8.93%, maturing March 26, 2007
|
|
$
|
114
|
|
|
$
|
547
|
|
|
Promissory Note dated
September 26, 2003; 9.03%, maturing September 26, 2007
|
|
|
16
|
|
|
|
35
|
|
|
Promissory Note dated
December 30, 2003; 9.24%, maturing June 30, 2007
|
|
|
162
|
|
|
|
465
|
|
|
Promissory Note dated
February 26, 2004; 9.03%, maturing February 26, 2008
|
|
|
70
|
|
|
|
124
|
|
|
Promissory Note dated
April 30, 2004; 9.48%, maturing April 30, 2008
|
|
|
127
|
|
|
|
213
|
|
|
Promissory Note dated
June 30, 2004; 10.06%, maturing December 30, 2007
|
|
|
33
|
|
|
|
63
|
|
|
Promissory Note dated
June 30, 2004; 10.03%, maturing June 30, 2008
|
|
|
327
|
|
|
|
520
|
|
|
Promissory Note dated
September 30, 2004; 9.58%, maturing March 30, 2008
|
|
|
11
|
|
|
|
20
|
|
|
Promissory Note dated
September 30, 2004; 9.40%, maturing September 30, 2008
|
|
|
90
|
|
|
|
135
|
|
|
Promissory Note dated
November 30, 2004; 9.72%, maturing November 30, 2008
|
|
|
129
|
|
|
|
188
|
|
|
Promissory Note dated
March 30, 2005; 10.46%, maturing March 30, 2009
|
|
|
43
|
|
|
|
59
|
|
|
Promissory Note dated
March 30, 2005; 10.70%, maturing September 30, 2008
|
|
|
14
|
|
|
|
20
|
|
|
Promissory Note dated
July 27, 2005; 10.32%, maturing July 27, 2009
|
|
|
131
|
|
|
|
173
|
|
|
Promissory Note dated
July 27, 2005; 10.66%, maturing January 27, 2009
|
|
|
15
|
|
|
|
21
|
|
|
Promissory Note dated
September 30, 2005; 10.31%, maturing September 30, 2009
|
|
|
114
|
|
|
|
147
|
|
|
Promissory Note dated May 31,
2006; 11,38%, maturing May 31, 2010
|
|
|
381
|
|
|
|
—
|
|
|
Promissory Note dated May 31,
2006; 11.73%, maturing November 30, 2009
|
|
|
220
|
|
|
|
—
|
|
|
Promissory Note dated
August 28, 2006; 11.27%, maturing August 28, 2010
|
|
|
525
|
|
|
|
—
|
|
|
Promissory Note dated
August 28, 2006, 11.65%, maturing February 28, 2010
|
|
|
79
|
|
|
|
—
|
|
|
Promissory Note dated
December 20, 2006; 10.96%, maturing December 20, 2010
|
|
|
492
|
|
|
|
—
|
|
|
Promissory Note dated
December 20, 2006, 11.35%, maturing June 1, 2010
|
|
|
142
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Total notes payable
|
|
|
3,235
|
|
|
|
2,730
|
|
|
Less current portion of notes
payable
|
|
|
(1,369
|
)
|
|
|
(1,333
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Long-term portion of notes payable
|
|
$
|
1,866
|
|
|
$
|
1,397
|
|
|
|
|
|
|
|
|
|
|
|
F-13
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
The Company has long-term obligations to a related party (see
Note 7 — Related Party Transactions).
Related-party notes payable consist of the following at
December 31, 2006 and 2005 (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
2006
|
|
|
2005
|
|
|
|
|
Total related-party notes payable,
with interest rates ranging from 14.0% to 10.5%, maturing
May 31, 2013(1)
|
|
|
1,523
|
|
|
|
1,494
|
|
|
Less current portion of
related-party notes payable
|
|
|
(176
|
)
|
|
|
(229
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Long-term portion of related-party
notes payable
|
|
$
|
1,347
|
|
|
$
|
1,264
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
On October 26, 2005, the Company amended its lease
agreement to, among things, extend the maturity of the
related-party notes payable from May 2009 until May 2013. In
connection with this lease amendment, the then-remaining balance
owed to the landlord as of May 31, 2006 under the
related-party notes payable, which totaled $1,342,719, is being
amortized over the seven-year period until May 2013 at a revised
rate of 10.5%. See Note 7 — Related Party
Transactions. |
As of December 31, 2006, scheduled principal repayments on
notes payable are as follows (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes Payable from
|
|
|
|
|
|
|
|
Notes Payable
|
|
|
Related Party(1)
|
|
|
Total
|
|
|
|
|
Year ending:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2007
|
|
$
|
1,369
|
|
|
$
|
176
|
|
|
$
|
1,545
|
|
|
2008
|
|
|
896
|
|
|
|
195
|
|
|
|
1,091
|
|
|
2009
|
|
|
628
|
|
|
|
216
|
|
|
|
844
|
|
|
2010
|
|
|
342
|
|
|
|
240
|
|
|
|
582
|
|
|
2011 and thereafter
|
|
|
—
|
|
|
|
696
|
|
|
|
696
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
3,235
|
|
|
$
|
1,523
|
|
|
$
|
4,758
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
On October 26, 2005, the Company amended its lease
agreement to, among things, extend the maturity of the
related-party notes payable from May 2009 until May 2013. In
connection with this lease amendment, the then-remaining balance
owed to the landlord as of May 31, 2006 under the
related-party notes payable, which totaled $1,342,719, is being
amortized over the seven-year period until May 2013 at a revised
rate of 10.5%. See Note 7 — Related Party
Transactions |
|
|
|
7.
|
RELATED
PARTY TRANSACTIONS
|
The landlord for the Company’s laboratory and office
facilities participated in the Series C financing in 2002
and, therefore, is a related party. Accordingly, the
landlord’s financing of the Company’s leasehold
improvements has been recorded on the balance sheets as notes
payable to related party.
When the Company entered into its original laboratory and office
facilities lease agreement with the landlord in 2001, the
Company also entered into a participation agreement with the
landlord. The agreement provided the landlord with the right to
purchase any capital stock of Iomai or any options, warrants, or
other securities convertible into Iomai capital stock in the
Company’s next two rounds of equity financing that raise
gross proceeds of at least $1.0 million (subject to certain
limitations). The Series C Preferred Stock financing
constituted the first of these two rounds. In the Series C
financing, the landlord purchased 565,483 shares of
Series Preferred Stock for $250,000 in the aggregate (see
Note 8 — Redeemable Preferred Stock). In
connection with the Company’s initial public offering in
February 2006 (see Note 9 — Stockholders’
Equity (Deficit)), the landlord agreed to terminate this
participation agreement and its shares of Series C
Preferred Stock were automatically converted into
43,498 shares of Common Stock (see Note 8 —
Redeemable Preferred Stock).
F-14
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
Under the terms of the original lease agreement, the Company
leased its laboratory and office facilities from June 2001
through May 31, 2006, and the Company had a three-year
renewal option to extend the term through May 31, 2009. In
June 2001, the landlord financed leasehold improvements of
approximately $740,000, which amount was repayable monthly
through May 31, 2009 with imputed interest rate of 14%
(assuming exercise of the three-year renewal option). In 2003,
the Company amended its lease agreement to take over the balance
of the second floor not already leased by the Company in its
building. In conjunction with the lease amendment, the landlord
provided the Company with a tenant improvement allowance of
$29,010 and agreed to finance a portion of any additional
leasehold improvements up to approximately $1.4 million.
During 2004, the Company drew down from the landlord $1,450,500
to finance leasehold improvements for the build-out of its pilot
plant. The repayment commenced in June 2004 and was repayable
monthly through May 2009 (assuming exercise of three-year
renewal option) under the following terms: (1) $870,300 of
principal at an imputed interest rate of 12%, and
(2) $580,200 of principal at an imputed interest rate of
10.5%.
In October 2005, the Company amended its lease to extend its
term by seven years from June 2006 to May 2013. In
connection with the lease extension, the Company renegotiated
with the landlord the terms of the financing for the build-out
of its pilot plant and agreed to lease approximately
8,000 square feet of additional space in the same building.
Effective as of June 1, 2006, the then-remaining balance
owed to the landlord under all of its existing debt financing,
which totaled $1,342,719, is being amortized over the seven-year
extension period at a rate of 10.5%. This change will result in
a monthly payment of approximately $22,639 through May 2013, as
opposed to the prior monthly payment of approximately $44,689,
which was being amortized through May 2009. In addition, the
Company now has the right to prepay this debt obligation at any
time upon four months’ written notice to the landlord.
As part of the amended lease, the landlord provided the Company
with tenant improvement allowances totaling approximately
$134,000 for the existing space and approximately $120,000 for
the expansion space. As of December 31, 2006, the Company
had utilized all of these allowances. Both tenant improvement
allowances are classified as other current assets in the
accompanying balance sheets. The landlord also provided us with
up to $280,000 of additional financing to apply toward
alterations to the expansion space on the same terms and
conditions as the existing landlord financing (that is,
amortized from date of draw down through May 2013 at a rate of
10.5%). The Company drew down the full $280,000 in additional
financing during the first half of 2006.
In July 2006, the Company amended its lease again to provide
that, among other things, (a) the Company will lease an
additional 6,117 square feet in the facility and
(b) directly lease from the landlord an additional
5,572 square feet, currently subleased by the Company,
after February 28, 2007. Under the amendment, the monthly
rent for the new premises began on August 1, 2006 (although
rent was abated through December 31, 2006), while the
monthly rent for the formerly subleased space will begin upon
the expiration or earlier termination of the sublease.
The Company amended its lease in January 2007 to lease the
remaining space available in the building. See
Note 14 — Subsequent Events.
|
|
|
8.
|
REDEEMABLE
PREFERRED STOCK
|
Series C
convertible redeemable preferred stock
In December 2002, the Company issued 122,935,926 shares of
Series C Redeemable Convertible Preferred Stock
(Series C Preferred Stock) for $0.4421 per share for
net proceeds of $52,093,856, which includes $60,000 of
Series C Preferred Stock issued to an investor for
professional fees. In 2003, the Company issued an additional
4,750,056 shares of Series C Preferred Stock at
$0.4421 per share for gross proceeds of $2,100,000. In June
2004, the Company issued an additional 1,904,052 shares of
Series C Preferred Stock at $0.4421 per share for
gross proceeds of $841,781. All shares of Series C
Preferred Stock were automatically converted by their terms into
F-15
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
9,968,443 shares of Common Stock upon closing of the
Company’s initial public offering in February 2006 (see
Note 9 — Stockholders’ Equity (Deficit)).
Prior to the Company’s initial public offering, the
Series C Preferred Stock bore a cumulative annual dividend
at a rate of $0.0354 per share, and had a liquidation
preference equal to the greater of (i) $0.4421 per
share increased by an annual implicit growth factor of 20%
compounded annually, subject to certain adjustments, plus any
declared or accrued and unpaid dividends, and (ii) the
amount per share that would have been payable had each such
share of Series C Preferred Stock been converted into
common stock immediately prior to a liquidation, dissolution,
winding up, sale or merger. As of December 31, 2006 and
2005, the aggregate per share liquidation preference of the
outstanding shares of Series C Preferred Stock was $0 and
0.8783, respectively, which includes accrued and unpaid
dividends of $0 and $13,940,759, respectively.
Series C
preferred stock warrants
In connection with the Series C Preferred Stock financing,
the Company issued to its placement agent warrants to purchase
250,000 shares of Series C Preferred Stock at an
exercise price of $0.4421 per share. The fair value of the
warrants of $60,000 was recorded as a reduction to the
Series C preferred stock. The fair value was estimated
using the Black-Scholes option-pricing model assuming a
risk-free interest rate of 2.79%, dividend yield of 0%,
volatility of 60.0%, and expected term of approximately five
years. The Company accreted $1,000 and $12,000 of the
Series C warrants to the Series C Preferred Stock in
2006 and 2005, respectively. The conversion rate is
one-thirteenth
(1/13) of one share of common stock for each share of
Series C Preferred Stock, which is adjustable for certain
dilutive events, as defined. With completion of the
Company’s initial public offering in February 2006, the
warrant holder can now purchase up to 19,231 shares of
Common Stock at an exercise price of $5.7473 per share.
|
|
|
9.
|
STOCKHOLDERS’
EQUITY (DEFICIT)
|
Series B
convertible preferred stock
In connection with the closing of the Series C Preferred
Stock financing, the Company issued 14,734,578 shares of
Series B Convertible Preferred Stock (Series B
Preferred Stock) to a subsidiary of Elan Corporation plc (Elan)
as part of the unwinding of a pre-existing joint venture. On
January 5, 2006, Elan sold all of the
14,734,578 shares of our Series B Preferred Stock held
by it to certain of the holders of Series C Preferred Stock
pursuant to a contractual right of first refusal. All
Series B Preferred Stock were automatically converted into
1,133,424 shares of common stock upon closing of the
Company’s initial public offering in February 2006.
Prior to the Company’s initial public offering, the
Series B Preferred Stock bore a cumulative annual dividend
at a rate of $0.0354 per share, and a liquidation
preference equal to the greater of (i) $0.4421 per
share, subject to certain adjustments, plus any declared or
accrued and unpaid dividends, and (ii) the amount per share
that would have been payable had each such share of
Series B Preferred Stock been converted into common stock
immediately prior to a liquidation, dissolution, winding up,
sale or merger. As of December 31, 2006 and 2005, the
aggregate per share liquidation preference of the outstanding
shares of Series B Preferred Stock was $0 and $0.4421,
respectively, plus accrued and unpaid dividends of $0 and
$1,603,397, respectively.
Initial
Public Offering
On February 6, 2006, the Company closed its initial public
offering by selling 5,000,000 shares of its common stock at
a public offering price of $7.00 per share. Net proceeds,
before expenses, were $32.8 million. The Company’s
total expenses for the initial public offering, including legal,
accounting and printing fees, were $1.9 million, so total
net proceeds, after expenses, were $30.9 million.
Upon the closing of the Company’s initial public offering,
a put option on 57,500 shares of common stock terminated in
accordance with its terms without further financial obligation
to the Company. In 2001, the Company
F-16
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
granted the put option to the Walter Reed Army Institute of
Research (WRAIR) in connection with the issuance of those shares
to WRAIR as part of an amendment to the master license agreement
for the transcutaneous immunization technology. Prior to initial
public offering WRAIR had the right to put these shares to the
Company, upon certain conditions, for the greater of $1,958,450
or the then fair market value of those shares at the date of
exercise.
Also in connection with the initial public offering, all of the
Company’s preferred stock was automatically converted into
shares of Common Stock as follows:
|
|
|
| |
•
|
All shares of Series B Preferred Stock were automatically
converted into 1,133,424 shares of common stock;
|
| |
| |
•
|
All shares of Series C Preferred Stock were automatically
converted into 9,968,443 shares of common stock; and
|
| |
| |
•
|
A warrant issued to the Company’s placement agent in
connection with the Series C Stock financing in December
2002 for 250,000 shares of Series C Preferred Stock
was automatically converted into a warrant to purchase up to
19,231 shares of Common Stock at an exercise price of
$5.7473 per share.
|
In connection with the financing of the build-out of the
Company’s facility, the Company previously issued warrants
to purchase up to 16,529 shares at an exercise price of
$5.75 per share. Upon the initial public offering, the
holder exercised these warrants in accordance with their terms
in a cashless exercise for 2,865 shares of Common Stock.
Upon closing of our initial public offering, the Company amended
and restated its certificate of incorporation to reduce
(1) the number of shares of common stock authorized for
issuance from 220,000,000 shares to
200,000,000 shares, and (2) the number of shares of
preferred stock authorized for issuance from
166,700,000 shares to 25,000,000 shares.
Private
Placement in Public Equity
In October 2006, the Company closed a private placement (Private
Placement) with two existing stockholders, Essex Woodlands
Health Ventures and New Enterprise Associates (NEA), pursuant to
which the Company raised gross proceeds of approximately
$10 million through the sale of 2,283,106 shares of
the Company’s Common Stock. The price per share under the
securities purchase agreement was $4.38. The Company incurred
costs of $86,593 associated with the sale and registration of
these shares as described below.
Pursuant to the securities purchase agreement dated
October 23, 2006, the Company agreed to file a registration
statement on
Form S-1
with the SEC to register the resale of the shares. The
registration statement was declared effective on
December 8, 2006, and the Company has agreed to use
commercially reasonable efforts to keep it effective until the
fourth anniversary of such date, at the latest. If the
registration statement ceases to be effective or useable for
resales during this period, then the Company has agreed to pay
each purchaser liquidated damages at a rate equal to
2.5% per month of the total purchase price of the shares
purchased by such purchaser that are covered by the Registration
Statement immediately prior to such lapse. Notwithstanding the
foregoing provisions, in no event shall the Company be obligated
to pay such liquidated damages (a) to more than one
purchaser in respect of the same share for the same period of
time or (b) for the first two (2) years following the
closing date, in an annual aggregate amount that exceeds 18% of
the purchase price paid by the purchasers for the shares or
(c) after the first two (2) years following the
closing date, in an aggregate amount that exceeds 18% of the
purchase price paid by the purchasers for the shares. At this
time, management does not believe that the payment of liquidated
damages is probable.
F-17
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
Stock
Options
Since 2003, the Company expensed options in accordance with the
fair value recognition provisions of SFAS No. 123,
whereby the Company recognized stock-based compensation based on
the fair value of the options at the date of grant using the
Black-Scholes model.
Effective January 1, 2006, the Company adopted the fair
value recognition provisions of Statement 123(R), using the
modified-prospective-transition method. Results for prior
periods have not been restated.
Under the modified-prospective-transition method, the Company
will continue to recognize compensation as options vest. The
principal change in accounting with the adoption of
Statement 123(R) is that beginning with the first quarter
of 2006, management must estimate forfeitures of options
(resulting from the failure of optionholders to satisfy service
or performance conditions) prior to vesting, and reduce
stock-based compensation expense by the amount of these
estimated forfeitures. Previously, under Statement 123, the
Company had elected to adjust stock-based compensation expense
only for actual forfeitures. The Company will periodically
monitor the rates for actual forfeitures and, if necessary,
adjust its stock-based compensation expense for changes in its
estimated rate of forfeitures and differences between
expectations and actual experience.
Also, with the adoption of Statement 123(R), the Company
recognized in the first quarter of 2006 a one-time charge
recorded as the cumulative effect of a change in accounting
principle, which reflects an estimate of forfeitures for
unvested awards outstanding upon the adoption of
Statement 123(R). This amount represents a reduction in the
compensation cost for prior periods for any unvested options
remaining that would not have been recognized in those prior
periods had forfeitures for such unvested options been estimated
during those prior periods. The cumulative effect for this
change in accounting principle totaled $16,726 and is recorded
on the statement of operations for the year ended
December 31, 2006.
The adoption of Statement 123(R) on January 1, 2006
did not have a material impact on the Company’s net loss
and net loss per share for the year ended December 31, 2006.
Under Statement 123(R), the cumulative amount of
compensation cost recognized for instruments classified as
equity that ordinarily would result in a future tax deduction
under existing tax law shall be considered to be a deductible
temporary difference in applying FASB Statement No. 109,
Accounting for Income Taxes. The deductible temporary
difference is based on the compensation cost recognized for
financial reporting purposes; however, these provisions
currently do not impact the Company as all the deferred tax
assets have a full valuation allowance against them.
Since the Company had a net operating loss carryforward as of
December 31, 2006, no excess tax benefits for the tax
deductions related to stock-based awards were recognized in the
statement of operations. Additionally, no incremental tax
benefits were recognized from stock options exercised in the
twelve months ended December 31, 2006 which would have
resulted in a reclassification to reduce net cash used in
operating activities with an offsetting increase in net cash
provided by financing activities.
F-18
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
A summary of all stock option plan activity during the year
ended December 31, 2006 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
Weighted-
|
|
|
Remaining
|
|
|
Aggregate
|
|
|
|
|
|
|
|
Average
|
|
|
Contractual
|
|
|
Intrinsic Value(1)
|
|
|
|
|
Shares
|
|
|
Exercise Price
|
|
|
Term
|
|
|
($000’s)
|
|
|
|
|
Outstanding at December 31,
2005
|
|
|
1,893,264
|
|
|
|
1.64
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at December 31,
2005
|
|
|
784,748
|
|
|
|
2.38
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
1,075,761
|
|
|
|
5.18
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(14,030
|
)
|
|
|
0.91
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
(36,190
|
)
|
|
|
3.16
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31,
2006
|
|
|
2,918,805
|
|
|
|
2.93
|
|
|
|
7.5
|
|
|
$
|
5,991
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested or expected to vest
|
|
|
2,654,673
|
|
|
|
2.87
|
|
|
|
7.4
|
|
|
$
|
5,990
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at December 31,
2006
|
|
|
1,195,551
|
|
|
|
1.93
|
|
|
|
6.1
|
|
|
$
|
3,641
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Calculated using the per-share closing price of the
Company’s common stock on December 29, 2006, which was
$4.98. |
The following table summarizes information about stock options
outstanding under the Company’s option plans at
December 31, 2006.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Outstanding
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-Average
|
|
|
|
|
|
Options Exercisable
|
|
Range of
|
|
Number
|
|
|
Remaining Contractual
|
|
|
Weighted-Average
|
|
|
Number
|
|
|
Weighted-Average
|
|
|
Exercise Prices
|
|
Outstanding
|
|
|
Life—Years
|
|
|
Exercise Price
|
|
|
Exercisable
|
|
|
Exercise Price
|
|
|
|
|
$0.91
|
|
|
1,705,373
|
|
|
|
6.6
|
|
|
$
|
0.91
|
|
|
|
1,115,427
|
|
|
$
|
0.91
|
|
|
$2.86
|
|
|
104,258
|
|
|
|
8.7
|
|
|
|
2.86
|
|
|
|
22,936
|
|
|
|
2.86
|
|
|
$4.34-4.70
|
|
|
248,761
|
|
|
|
9.4
|
|
|
|
4.41
|
|
|
|
7,775
|
|
|
|
4.35
|
|
|
$5.04-5.85
|
|
|
811,769
|
|
|
|
9.3
|
|
|
|
5.42
|
|
|
|
769
|
|
|
|
5.85
|
|
|
$16.25-22.75
|
|
|
32,690
|
|
|
|
1.4
|
|
|
|
20.84
|
|
|
|
32,690
|
|
|
|
20.84
|
|
|
$28.99-34.19
|
|
|
15,954
|
|
|
|
2.9
|
|
|
|
32.15
|
|
|
|
15,954
|
|
|
|
32.15
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,918,805
|
|
|
|
|
|
|
|
|
|
|
|
1,195,551
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The total fair value of stock options which vested during the
year ended December 31, 2006, less estimated forfeitures,
was approximately $1,082,000. During the year ended
December 31, 2006, participants exercised stock options
totaling 14,030, and the total intrinsic value of stock options
exercised during the year ended December 31, 2006 was
approximately $74,000. Cash received from option exercise under
all stock compensation plans was $12,767 for the year ended
December 31, 2006.
As of December 31, 2006, there was approximately
$3.5 million of total unrecognized compensation expense,
less estimated forfeitures, related to unvested options under
the Company’s stock compensation plans. The expenses are
expected to be recognized over a weighted-average period of
2.1 years.
Stock options awarded to directors and employees generally are
granted with an exercise price equal to the market price of the
Company’s stock on the date of grant. Those options
generally vest in equal installments over a four-year period
based on continued service and have ten-year contractual terms.
The Company recognizes compensation costs for those options on a
straight-line basis over the requisite service period for the
entire award (that is, generally over four years).
F-19
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
During the year ended December 31, 2006, the Company issued
1,075,761 options, which vest in equal installments over a
four-year period. The weighted-average fair value of options
granted during the years ended December 31, 2006, 2005, and
2004 was $5.18, $4.62, and $0.77, respectively, applying the
Black-Scholes option-pricing model utilizing the following
weighted-average assumptions:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
2004
|
|
|
|
|
Expected dividend yield
|
|
|
0
|
%
|
|
|
0
|
%
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
77
|
%
|
|
|
60
|
%
|
|
|
56
|
%
|
|
Risk-free interest rate
|
|
|
4.66
|
%
|
|
|
3.89
|
%
|
|
|
3.03
|
%
|
|
Expected average life of options
|
|
|
5 years
|
|
|
|
5 years
|
|
|
|
5 years
|
|
Dividend Yield — The Company has never
declared or paid dividends and has no plans to do so in the
foreseeable future.
Expected Volatility — Volatility is a
measure of the amount by which a financial variable such as a
share price has fluctuated (historical volatility) or is
expected to fluctuate (expected volatility) during a period. The
Company tracks the historical volatility of its own stock, but
since the Company went public only in February 2006, the Company
also uses an estimated volatility based on the volatility of a
number of similarly situated biotechnology companies, along with
other factors deemed relevant by management. In 2006, estimated
volatility used to value options ranged between 70% and 113%.
Risk-Free Interest Rate — This is the
U.S. Treasury rate for the week of each option grant during
the quarter having a term that most closely resembles the
expected life of the option.
Expected Life of the Option Term — This
is the period of time that the options granted are expected to
remain unexercised. Options granted during the year have a
maximum term of ten years. The Company estimates the expected
life of the option term to be five years; however, now that the
Company is a public entity, management expects that over time,
management will track estimates of the expected life of the
option term so that its estimates will approximate actual past
behavior for similar options.
Expected Forfeiture Rate — The
forfeiture rate is the estimated percentage of options granted
that are expected to be forfeited or canceled on an annual basis
before becoming fully vested. The Company estimates the
forfeiture rate based on past turnover data ranging anywhere
from the past one to three years, with further consideration
given to the class of employees to whom the options were granted.
The Company recognized the following stock option compensation
expense for the years ended:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
2004
|
|
|
|
|
Employees and directors
|
|
$
|
1,077,680
|
|
|
$
|
508,575
|
|
|
$
|
152,928
|
|
|
Non-employee consultants
|
|
|
(12,664
|
)
|
|
|
29,569
|
|
|
|
777
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,065,016
|
|
|
$
|
538,144
|
|
|
$
|
153,705
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Following is a summary of the Company’s stock plans:
1998
Stock Incentive Plan
In 1998, the Company adopted a stock option plan (the 1998 Plan)
and reserved 76,923 shares of common stock pursuant to the
1998 Plan. Incentive stock awards are granted with an exercise
price equal to the fair market value of the Company’s stock
on the date of grant. Nonstatutory options are granted with an
exercise price at not less than 85% of the fair market value of
the Company’s stock on the date of grant. The options
expire 10 years from the date of grant.
F-20
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
1999
Stock Incentive Plan
In August 1999, the Company adopted the 1999 Stock Incentive
Plan (the 1999 Plan). The 1999 Plan currently provides for the
issuance of restricted common stock, stock options or the direct
award of common stock, for up to 2,001,584 shares.
Incentive stock awards are required to be granted with an
exercise price equal to the estimated fair market value of the
Company’s stock on the date of grant. Nonstatutory options
are granted with an exercise price at not less than 85% of the
fair market value of the Company’s stock on the date of
grant. The 1999 Plan options expire 10 years from the date
of grant.
In January 2003, the 1999 Plan was amended to increase the
number of shares of Common Stock available for issuance under
the 1999 Plan from 423,076 shares to 1,476,923 shares.
In September 2003, the 1999 Plan was further amended to increase
the number of shares of Common Stock available for issuance
under the 1999 Plan by an additional 524,661 shares to
2,001,584 shares, in the aggregate.
2005
Stock Incentive Plan
Under the 2005 Incentive Plan (the 2005 Plan), a total of
1,040,000 shares of common stock has been reserved for
issuance upon the exercise of awards granted under the 2005
Plan. The 2005 Plan provides for the grant of awards consisting
of any or a combination of stock options, stock appreciation
rights, restricted stock, unrestricted stock, stock unit,
performance and cash awards. The compensation committee may make
grants to key employees of Iomai and its affiliates, board
members, including outside directors, and members of an
affiliate’s board of directors, and consultants, advisors
or other independent contractors who perform services for us or
any of our affiliates. The maximum number of shares of stock for
which options and stock appreciation rights may be granted to
any particular participant in a calendar year shall be, in each
case, 780,000, and the maximum number of shares of stock subject
to other awards that may be delivered to any particular
participant in any calendar year shall be 140,000. These limits,
and the total number of shares reserved under the 2005 Plan, are
subject to adjustment in the case of stock dividends and other
transactions affecting the common stock. Options granted under
the 2005 Plan generally expire 10 years from the date of
grant. In March 2007, the 2005 Plan was amended to increase the
number of shares of Common Stock available for issuance under
the 2005 Plan from 1,040,000 shares to
1,890,000 shares (see Note 14 — Subsequent
Events).
2006
Employee Stock Purchase Plan
Under the 2006 Employee Stock Purchase Plan (the ESPP), a total
of 80,000 shares of common stock has been reserved for
issuance under the ESPP. The ESPP will permit eligible employees
to purchase shares of our common stock at a discount from fair
market value. The Company intends for the ESPP to meet the
requirements for an “employee stock purchase plan”
under Section 423 of the Internal Revenue Code.
The ESPP did not go into effect until completion of the
Company’s initial public offering in February 2006. As of
December 31, 2006, no shares of Common Stock were issued
under the ESPP. The plan is expected to become operational in
2007 and, consequently, is expected to have an impact on future
periods. The compensation committee, or a duly appointed
administrator, will determine the frequency and duration of
individual offerings under the ESPP and the dates when employees
may purchase stock.
Eligible employees participate voluntarily and may withdraw from
any offering at any time before they purchase stock.
Participation terminates automatically upon termination of
employment. The purchase price per share of common stock in an
offering will equal 85% of the fair market value of the common
stock at the first trading day of the offering period or at the
last trading day of the offering period, whichever is less.
Employees will pay through payroll deductions.
F-21
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
|
|
|
10.
|
COMMITMENTS
AND CONTINGENCIES
|
The Company leases laboratory and office facilities and
scientific equipment under operating lease agreements. For the
years ended December 31, 2006, 2005, and 2004, rent expense
was $915,719, $624,310 and $601,391, and, respectively. All base
rents will be subject to minimum rent escalation of 3.5%.
Future minimum payments under noncancelable operating lease
obligations at December 31, 2006 are as follows (in
thousands):
| |
|
|
|
|
|
|
|
Operating(1)
|
|
|
|
|
2007
|
|
$
|
1,074
|
|
|
2008
|
|
|
1,196
|
|
|
2009
|
|
|
1,236
|
|
|
2010
|
|
|
1,253
|
|
|
2011
|
|
|
1,279
|
|
|
Thereafter
|
|
|
1,883
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
$
|
7,921
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
These amounts include payments for additional facility space
leased under a lease amendment signed in January 2007 (see
Note 14 — Subsequent Events). |
At December 31, 2006, and 2005, the Company had commitments
for capital expenditures relating primarily to the expansion of
the Company’s facilities of approximately $15,767 and
$341,000, respectively.
License
agreement with the Walter Reed Army Institute of Research
(WRAIR)
On April 6, 2001, the Company entered into an Amended and
Restated License Agreement with WRAIR (the WRAIR Agreement). The
WRAIR Agreement grants an exclusive license to Iomai for its TCI
technology as of December 15, 1997. The Company paid a
license issuance fee of $150,000, annual license fees of $15,000
and additional fees upon certain milestones.
The Company may terminate the WRAIR Agreement at any time after
60 days’ notice to WRAIR without WRAIR’s consent.
The Company did not expense any milestone payments to WRAIR in
2006, 2005, or 2004. No royalty payments have been made to WRAIR
under the license.
In conjunction with the WRAIR Agreement, the Company issued
57,500 shares of common stock to WRAIR in 2001. These
shares are held in escrow by an unrelated third party under a
Voting Trust and Escrow Agreement, which provides economic
benefit to WRAIR but removes WRAIR from any voting control or
direct influence over the Company’s operations. The fair
value of the shares of common stock was charged to research and
development upon issuance.
In addition, the Company issued a put option (Put Option) to
WRAIR related to the 57,500 shares of common stock. The put
price will be the greater of $34.19 per share (or $1,958,450, in
the aggregate) or the then fair market value at the date of
exercise (Put Price). Prior to the Company’s initial public
offering, the Put Option could have been exercised immediately
had the Company fail to pay any amounts under the WRAIR
Agreement or at any time after April 6, 2005. Upon the
closing of the Company’s initial public offering in
February 2006, the Put Option terminated in accordance with its
terms without further financial obligation to the Company.
For the years ending December 31, 2006, 2005, and 2004,
there is no current provision for federal or state income taxes.
The deferred tax benefit has been entirely offset by valuation
allowances.
F-22
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
The Company’s net deferred tax asset consists of the
following (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
|
|
NOL carryforward
|
|
$
|
32,323
|
|
|
$
|
21,239
|
|
|
Research and development credit
carryforward
|
|
|
2,678
|
|
|
|
1,648
|
|
|
Deferred temporary differences
|
|
|
2,051
|
|
|
|
1,198
|
|
|
Valuation allowance
|
|
|
(37,052
|
)
|
|
|
(24,085
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax asset
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
The net operating loss carryforwards of approximately
$83.7 million will begin to expire in the year 2017 if
unused. The use of the Company’s net operating loss
carryforwards may be restricted due to changes in Company
ownership. The Company paid no income taxes in 2004, 2005, and
2006.
The provision for income taxes differs from the amount of taxes
determined by applying the U.S. federal statutory rate to
loss before provision for income taxes as a result of the
following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2006
|
|
|
2005
|
|
|
2004
|
|
|
|
|
Federal tax at statutory rates
|
|
|
34.0
|
%
|
|
|
34.0
|
%
|
|
|
34.0
|
%
|
|
State taxes, net of federal benefit
|
|
|
4.6
|
|
|
|
4.6
|
|
|
|
4.6
|
|
|
Research and development credit
|
|
|
3.2
|
|
|
|
2.8
|
|
|
|
2.5
|
|
|
Permanent difference
|
|
|
(1.0
|
)
|
|
|
(1.2
|
)
|
|
|
(0.1
|
)
|
|
Change in valuation allowance
|
|
|
(40.8
|
)
|
|
|
(40.2
|
)
|
|
|
(41.0
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes
|
|
|
0.0
|
%
|
|
|
0.0
|
%
|
|
|
0.0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12.
|
EMPLOYEE
BENEFIT PLAN
|
The Company maintains a defined contribution plan (the 401(k)
Plan) qualified under Section 401(k) of the Internal
Revenue Code. The 401(k) Plan covers substantially all
employees. Under the 401(k) Plan, employees may make elective
salary deferrals and the Company provides for a 50% matching of
qualified deferrals, up to a maximum of 6%. During the years
ended December 31, 2006, 2005, and 2004, the Company made
matching contributions of approximately $164,015, $132,999 and
$122,883, respectively.
F-23
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
|
|
|
13.
|
QUARTERLY
FINANCIAL INFORMATION (UNAUDITED)
|
Quarterly financial information for the years ended
December 31, 2005 and 2006 is presented in the following
tables (in thousands, except per share data):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1st Quarter
|
|
|
2nd Quarter
|
|
|
3rd Quarter
|
|
|
4th Quarter
|
|
|
|
|
Year ended December 31,
2005
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
971
|
|
|
$
|
856
|
|
|
$
|
504
|
|
|
$
|
41
|
|
|
Income (loss) from operations
|
|
|
(3,649
|
)
|
|
|
(3,681
|
)
|
|
|
(4,343
|
)
|
|
|
(6,266
|
)
|
|
Net income (loss)
|
|
|
(3,669
|
)
|
|
|
(3,695
|
)
|
|
|
(4,365
|
)
|
|
|
(6,301
|
)
|
|
Net income (loss) per share, basic
and diluted
|
|
$
|
(4.75
|
)
|
|
$
|
(4.78
|
)
|
|
$
|
(5.52
|
)
|
|
$
|
(7.94
|
)
|
|
Year ended December 31,
2006
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
997
|
|
|
$
|
408
|
|
|
$
|
30
|
|
|
$
|
40
|
|
|
Income (loss) from operations
|
|
|
(5,151
|
)
|
|
|
(7,763
|
)
|
|
|
(9,474
|
)
|
|
|
(10,035
|
)
|
|
Net income (loss)
|
|
|
(5,020
|
)
|
|
|
(7,544
|
)
|
|
|
(9,326
|
)
|
|
|
(9,895
|
)
|
|
Net income (loss) per share, basic
and diluted
|
|
$
|
(0.45
|
)
|
|
$
|
(0.45
|
)
|
|
$
|
(0.55
|
)
|
|
$
|
(0.53
|
)
|
Award
of Government Contract
On January 17, 2007, the Company was awarded a five-year,
cost-plus reimbursement contract by the U.S. Department of
Health and Human Services (DHHS) to fund the development of a
dose-sparing patch for use with a pandemic flu vaccine. The
immunostimulant (IS) patch is intended to stimulate an immune
response to influenza when used in conjunction with small doses
of influenza vaccine. If the product is developed through
licensure, the total cost reimbursed by DHHS, plus a fixed fee,
is estimated to be $128 million. During the first
15 months of the contract, DHHS has allotted approximately
$14.5 million for the Company to assess the safety and
immunogenicity of the patch in two clinical trials and to
develop plans on how the Company would produce 150 million
IS patches in a six-month period, as required under the contract.
Amendment
to Facility Lease
On January 26, 2007, the Company amended its lease to lease
an additional 7,015 square feet in the facility, comprised
of (a) 1,365 square feet currently under lease by a
third party until July 1, 2007 (“Expansion Space
A”), and (b) 5,650 square feet currently under
lease to another third party until September 1, 2007
(“Expansion Space B”). Upon leasing this additional
space, the Company will be leasing the entire facility.
Under the amendment, the Company is expected to begin leasing
and paying a monthly rent of $3,242 for Expansion Space A
beginning on July 1, 2007, and to begin leasing and paying
a monthly rate of $13,419 for Expansion Space B beginning on
September 1, 2007. The amendment does not change the May
2013 expiration date of the lease. The Company will continue to
pay its proportionate share of the operating expenses of the
facility.
Private
Placement
On March 2, 2007, the Company entered into a securities
purchase agreement with accredited investors pursuant to which
the Company agreed to sell, in a private placement, an aggregate
of 6,291,828 units, each unit consisting of one share of
common stock, and two warrants to purchase, in total, 0.7
additional shares of common stock, at a purchase price of
$5.0675 per unit. The Company expects to receive
approximately $30.3 million in net proceeds, after
expenses. The purchase price for the share component of each
unit is $4.98 per share, the closing bid
F-24
IOMAI
CORPORATION
NOTES TO
FINANCIAL STATEMENTS — (Continued)
price for the common stock on March 1, 2007. Each warrant
provides the right to acquire 0.35 shares of common stock
at an exercise price of $5.25 per full share. One warrant
is exercisable at any time until March 2, 2012, and the
other warrant is exercisable at any time until the date four
months after a resale registration statement is declared
effective by the SEC.
Amendment
of 2005 Stock Incentive Plan
On March 6, 2007, the Company’s stockholders approved
an amendment to the 2005 Plan to increase the number of shares
of common stock available for issuance under the plan by
850,000 shares, making a total of 1,890,000 shares of
common stock reserved and available for issuance.
F-25