Exhibit (a)(5)(a)
Agreement to acquire RAPT Therapeutics
Lead asset: ozureprubart, a
potential best-in-class, long-acting anti-IgE monoclonal antibody for food allergy
Exhibit (a)(5)(a)

Agreement to acquire RAPT Therapeutics
Lead asset: ozureprubart, a
potential best-in-class, long-acting anti-IgE monoclonal antibody for food allergy

Speakers
Luke Miels
Chief Executive Officer
Nina Mojas
President, Global Product
Strategy
Tony Wood
Chief Scientific Officer

Disclosure statement
This communication is for informational purposes
only and is neither an offer to purchase nor a solicitation of an offer or a recommendation to sell securities, nor is it a substitute for the tender offer materials that Redrose Acquisition Co., GlaxoSmithKline LLC and GSK plc will file with the
Securities and Exchange Commission (the SEC). The tender offer for the outstanding shares of RAPT Therapeutics, Inc.’s (the Company) common stock described in this communication has not commenced. At the time the tender offer is commenced,
Redrose Acquisition Co., GlaxoSmithKline LLC and GSK plc will file, or will cause to be filed, a Schedule TO Tender Offer Statement with the SEC and the Company will file a Schedule 14D-9
Solicitation/Recommendation Statement with the SEC, in each case with respect to the tender offer. The Schedule TO Tender Offer Statement (including an offer to purchase, a related letter of transmittal and other offer documents) and the Schedule 14D-9 Solicitation/Recommendation Statement will contain important information that should be read carefully before any decision is made with respect to the tender offer. Those materials (once they become available)
will be made available to the Company’s stockholders at no expense to them by the information agent for the tender offer, which will be announced. In addition, those materials and all other documents filed, or caused to be filed, by Redrose
Acquisition Co., GlaxoSmithKline LLC and GSK plc with the SEC will be available at no charge on the SEC’s website at www.sec.gov

4 Cautionary statement regarding forward-looking statements GSK plc cautions investors that any forward-looking statements or projections made by GSK plc, including those made in this communication, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described in the “Risk Factors” section in GSK plc’s Annual Report on Form 20-F for the year ended December 31, 2024. This communication includes forward-looking statements related to the Company, ozureprubart and the acquisition of the Company by GSK plc, Redrose Acquisition Co. and GlaxoSmithKline LLC that are subject to risks, uncertainties and other factors. All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including all statements regarding the intent, belief or current expectation of the Company and members of its senior management team and can typically be identified by words such as “believe,” “expect,” “estimate,” “predict,” “target,” “potential,” “likely,” “continue,” “ongoing,” “could,” “should,” “intend,” “may,” “might,” “plan,” “seek,” “anticipate,” “project” and similar expressions, as well as variations or negatives of these words. Forward-looking statements include, without limitation, statements regarding the business combination, similar transactions, prospective performance, future plans, events, expectations, performance, objectives and opportunities and the outlook for the Company’s business; the commercial success of the Company’s products; the anticipated timing of clinical data and regulatory filings or approvals relating to products; the possibility of favourable or unfavourable results from clinical trials; the anticipated benefits of the acquisition; filings and approvals relating to the transaction; the expected timing of the completion of the transaction; the parties’ ability to complete the transaction; and the accuracy of any assumptions underlying any of the foregoing. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties and are cautioned not to place undue reliance on these forward-looking statements. Actual results may differ materially from those currently anticipated due to a number of risks and uncertainties. Risks and uncertainties that could cause the actual results to differ from expectations contemplated by forward-looking statements include: uncertainties as to the timing of the tender offer and completion of the merger; the possibility that various closing conditions for the transaction may not be satisfied or waived, including that the Company stockholders may not tender into the offer a majority of the shares outstanding at the time of the expiration of the offer or that required regulatory approvals may not be obtained or are obtained subject to conditions that are not anticipated; the occurrence of any event, change or other circumstance that could give rise to the termination of the agreement and plan of merger; the failure to realize anticipated benefits of the proposed acquisition when expected or at all; potential adverse reactions or changes to business relationships resulting from the proposed acquisition, including the effect of the announcement, pendency or consummation of the acquisition on the ability of the Company to retain and hire key personnel or maintain key vendor, supplier or partner relationships; risks that the proposed acquisition disrupts the current plans and operations of the Company; transaction costs; risks associated with potential litigation or regulatory actions related to the transaction; and other risks and uncertainties described from time to time in documents filed with the SEC by the Company, including current reports on Form 8-K, quarterly reports on Form 10-Q and annual reports on Form 10-K, as well as the Solicitation/Recommendation Statement to be filed by the Company, or in GSK plc’s Annual Report on Form 20-F for the year ended December 31, 2024 filed with the SEC by GSK plc, as well as the Tender Offer Statement to be filed by GSK plc, Redrose Acquisition Co. and GlaxoSmithKline LLC. All forward-looking statements are based on information currently available to GSK plc and the Company neither GSK plc nor the Company assumes any obligation to update any forward-looking statements.
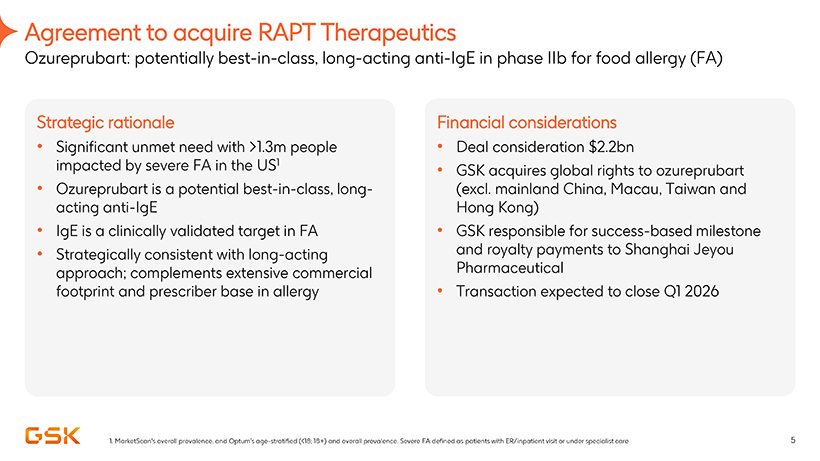
Agreement to acquire RAPT Therapeutics
Ozureprubart: potentially best-in-class, long-acting anti-IgE in phase IIb for food allergy (FA)
Strategic rationale
• Significant unmet need with >1.3m people impacted by severe FA in the US1
• Ozureprubart is a potential best-in-class, longacting
anti-IgE
• IgE is a clinically validated target in FA
• Strategically consistent with long-acting approach; complements extensive commercial footprint and prescriber base in allergy
Financial considerations
Deal consideration $2.2bn
GSK acquires global rights to ozureprubart (excl. mainland China, Macau, Taiwan and Hong Kong)
GSK responsible for
success-based milestone and royalty payments to Shanghai Jeyou Pharmaceutical
Transaction expected to close Q1 2026
1. MarketScan’s overall prevalence, and Optum’s age-stratified (<18; 18+) and overall prevalence. Severe FA defined as patients with
ER/inpatient visit or under specialist care
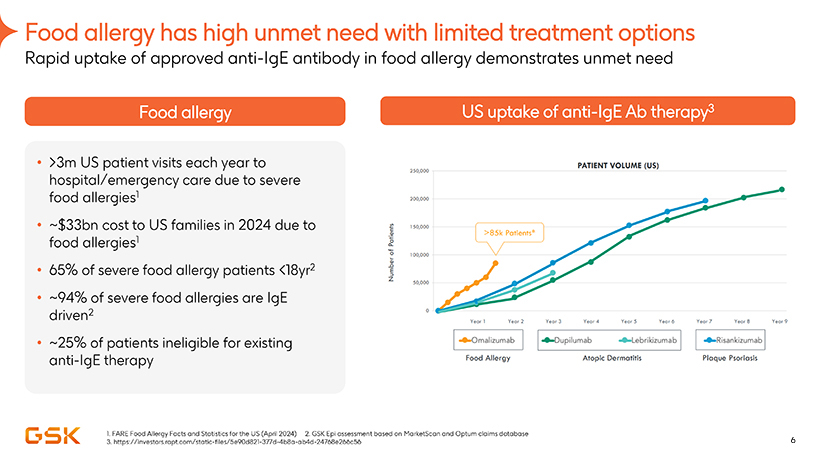
Food allergy has high unmet need with limited treatment options
Food
allergy
>3m US patient visits each year to hospital/emergency care due to severe food allergies1
~$33bn cost to US families in 2024 due to food allergies1
65% of severe food allergy patients
<18yr2
~94% of severe food allergies are IgE driven2
~25% of patients ineligible for existing anti-IgE therapy
US uptake of anti-IgE Ab therapy3
1. FARE Food Allergy Facts and Statistics for the US (April 2024) 2. GSK Epi assessment based on MarketScan and Optum claims database
3. https://investors.rapt.com/static-files/5e90d821-377d-4b8a-ab4d-24768e266c56
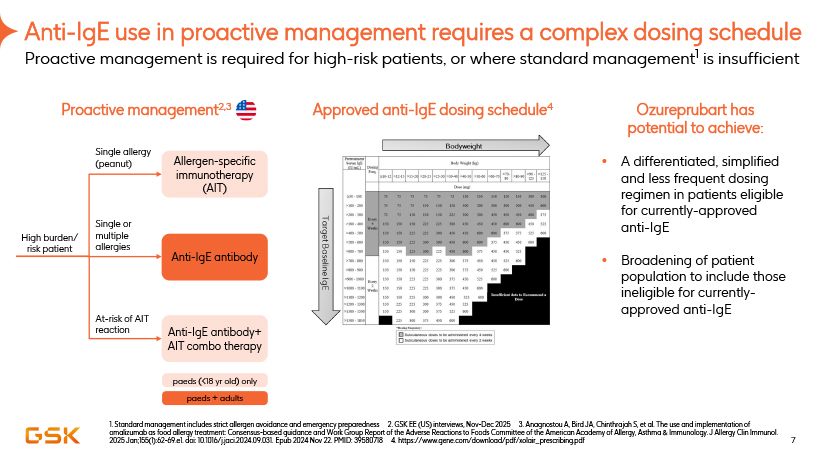
Anti-IgE use in proactive management requires a complex dosing schedule Proactive management is required for high-risk patients, or where standard management1 is insufficient Proactive management2,3 Approved anti-IgE dosing schedule4 Ozureprubart has potential to achieve: A differentiated, simplified and less frequent dosing regimen in patients eligible for currently-approved anti-IgE Broadening of patient population to include those ineligible for currently-approved anti-IgE 1. Standard management includes strict allergen avoidance and emergency preparedness 2. GSK EE (US) interviews, Nov-Dec 2025 3. Anagnostou A, Bird JA, Chinthrajah S, et al. The use and implementation of omalizumab as food allergy treatment: Consensus-based guidance and Work Group Report of the Adverse Reactions to Foods Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2025 Jan;155(1):62-69.e1. doi: 10.1016/j.jaci.2024.09.031. Epub 2024 Nov 22. PMID: 39580718 4. https://www.gene.com/download/pdf/xolair_prescribing.pdf Single allergy (peanut) Allergen-specific Immunotherapy (AIT) High burden/ risk patient Single or multiple allergies Anti-IgE antibody At-risk of AIT reaction Anti-IgE antibody+ AIT combo therapy paeds (<18 yr old) only paeds + adults 7
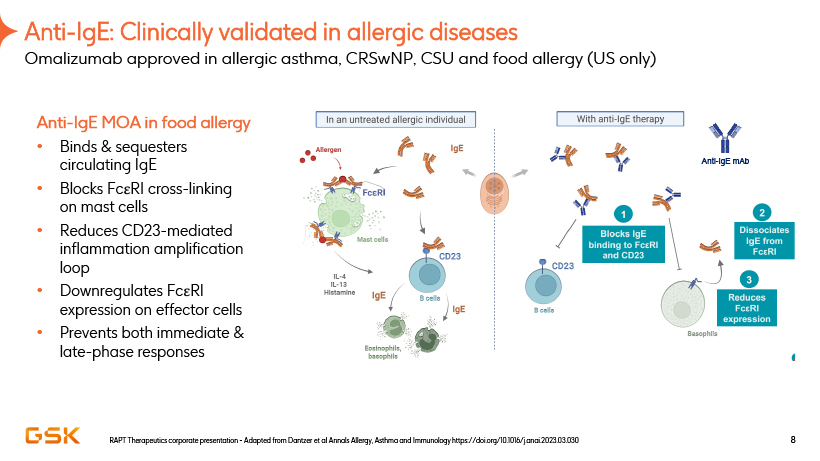
Anti-IgE: Clinically validated in allergic diseases Omalizumab approved in allergic asthma, CRSwNP, CSU and food allergy (US only) Anti-IgE MOA in food allergy Binds & sequesters circulating IgE Blocks FceRI cross-linking on mast cells Reduces CD23-mediated inflammation amplification loop Downregulates FceRI expression on effector cells Prevents both immediate & late-phase responses RAPT Therapeutics corporate presentation—Adapted from Dantzer et al Annals Allergy, Asthma and Immunology https://doi.org/10.1016/j.anai.2023.03.030 8
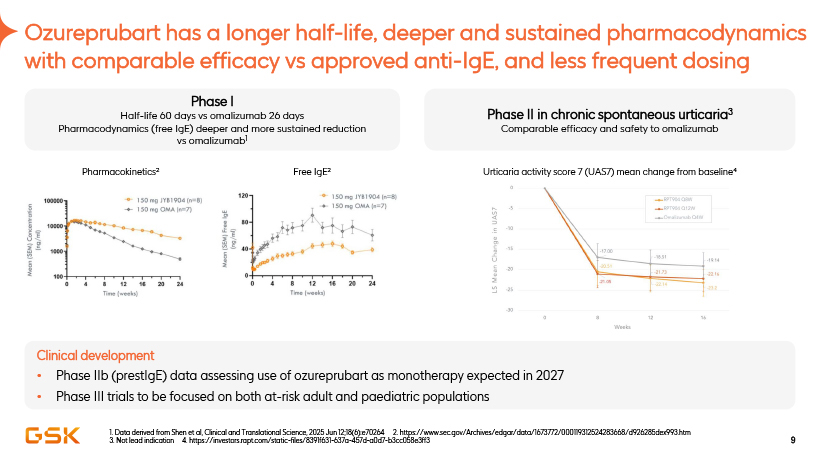
Ozureprubart has a longer half-life, deeper and sustained pharmacodynamics with comparable efficacy vs approved anti-IgE, and less frequent dosing Phase I Half-life 60 days vs omalizumab 26 days Pharmacodynamics (free IgE) deeper and more sustained reduction vs omalizumab1 Pharmacokinetics2 Phase II in chronic spontaneous urticaria3 Comparable efficacy and safety to omalizumab Urticaria activity score 7 (UAS7) mean change from baseline4 1. Data derived from Shen et al, Clinical and Translational Science, 2025 Jun 12;18(6):e70264 2. https://www.sec.gov/Archives/edgar/data/1673772/000119312524283668/d926285dex993.htm 3. Not lead indication 4. https://investors.rapt.com/static-files/8391f631-637a-457d-a0d7-b3cc058e3ff3 Clinical development • Phase IIb (prestIgE) data assessing use of ozureprubart as monotherapy expected in 2027 • Phase III trials to be focused on both at-risk adult and paediatric populations 9
Q&A GSK 10