

Corporate Overview January 2026 Developing new therapies for rare respiratory diseases

Safe Harbor Statement © Savara Inc. All Rights Reserved. Savara Inc. (“Savara” or the “Company”) cautions you that statements in this presentation that are not a description of historical fact are forward-looking statements which may be identified by the use of words such as “expect,” “intend,” “plan,” “anticipate,” “believe,” and “will,” among others. Such statements include, but are not limited to, statements regarding the potential health benefits and risks and projected development timeline of MOLBREEVI; the timing of regulatory submissions; the potential for and impact of regulatory approval; the potential addressable patient population, market size, commercial opportunity, and competitive landscape for MOLBREEVI; Savara’s commercial launch planning activities, including disease awareness campaign, planned infrastructure, and anticipated hiring and the potential impact of those activities; GM-CSF autoantibody testing and its potential impact; and the sufficiency of our resources to fund the advancement of our development program and potential sources of additional capital. Savara may not actually achieve any of its plans or product development goals in a timely manner, if at all, or otherwise carry out its current intentions or meet the expectations or projections disclosed in its forward-looking statements, and you should not place undue reliance on these forward-looking statements. These forward-looking statements are based upon Savara's current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risks associated with our ability to successfully develop, obtain regulatory approval for and commercialize MOLBREEVI for autoimmune PAP; the risks and uncertainties related to the impact of widespread health concerns and geopolitical conditions on our business and operations; risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations; the ability to successfully conduct clinical trials for our product candidate; the availability of sufficient resources and the timing and ability of Savara to raise additional capital as needed to fund continued operations. The risks and uncertainties facing Savara are described more fully in Savara's filings with the Securities and Exchange Commission, including our filings on Form 8-K, our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and our Quarterly Report on Form 10-Q for the quarter ended September 30, 2025. You are cautioned not to place undue reliance on our forward-looking statements, which speak only as of the date on which they were made. Savara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as may be required by law. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company. Additionally, this presentation includes internal research and estimates performed by the Company, which have not been independently verified. MOLBREEVI (molgramostim inhalation solution) is an investigational product that has not been approved for sale or determined to be safe or effective by the U.S. Food & Drug Administration or any regulatory authority. MOLBREEVI, MY MOLBREEVI and aPAP ClearPath are trademarks of Savara. All other trademarks included herein are the property of the owners thereof and are used for reference purposes only.
Executive Leadership Team © Savara Inc. All Rights Reserved. 1zxza Matthew Pauls, J.D., M.B.A. Chair & Chief Executive Officer Anne Erickson Chief Business Officer Dave Lowrance Chief Financial & Administrative Officer Rob Lutz, M.B.A. Chief Operating Officer Braden Parker, M.B.A. Chief Commercial Officer Yasmine Wasfi, M.D., Ph.D., FCCP Chief Medical Officer Sid Advant, Ph.D. EVP, Global Technical Operations Brian Robinson, M.D. EVP, Global Medical Affairs Kate McCabe, J.D. Chief Legal Officer Charles LaPree EVP, Global Regulatory Affairs

Autoimmune Pulmonary Alveolar Proteinosis (autoimmune PAP) Overview and Burden of Disease
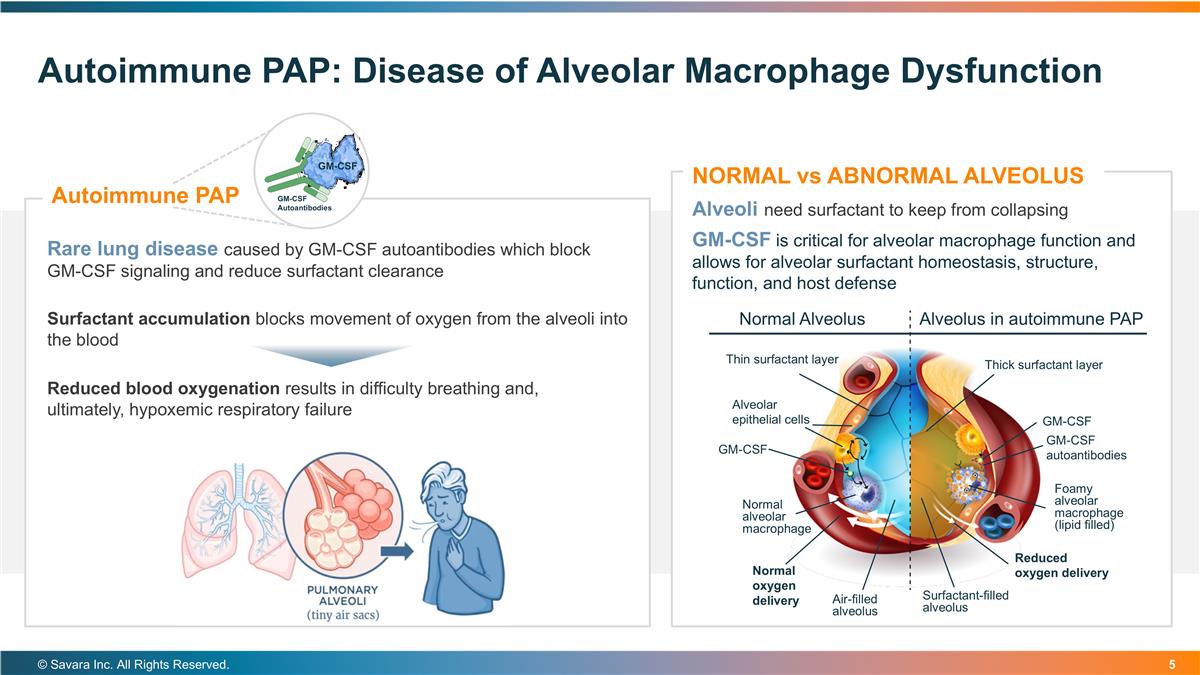
Autoimmune PAP: Disease of Alveolar Macrophage Dysfunction © Savara Inc. All Rights Reserved. Alveoli need surfactant to keep from collapsing GM-CSF is critical for alveolar macrophage function and allows for alveolar surfactant homeostasis, structure, function, and host defense NORMAL vs ABNORMAL ALVEOLUS Surfactant accumulation blocks movement of oxygen from the alveoli into the blood Reduced blood oxygenation results in difficulty breathing and, ultimately, hypoxemic respiratory failure Rare lung disease caused by GM-CSF autoantibodies which block GM-CSF signaling and reduce surfactant clearance Autoimmune PAP GM-CSF Autoantibodies GM-CSF GM-CSF autoantibodies Alveolus in autoimmune PAP Normal Alveolus Thin surfactant layer Thick surfactant layer Alveolar epithelial cells GM-CSF GM-CSF Normal oxygen delivery Reduced oxygen delivery Foamy alveolar macrophage (lipid filled) Normal alveolar macrophage Air-filled alveolus Surfactant-filled alveolus
Autoimmune PAP is a Rare, Long-Term, Chronic Lung Disease © Savara Inc. All Rights Reserved. Progressive Shortness of Breath With impaired gas exchange, patients may experience shortness of breath At first it occurs upon exertion, but as disease progresses, it can occur even when a person is at rest Cough and Episodes of Fever Cough, sputum production, and episodes of fever, especially if secondary lung infection develops Serious infections, the most common and threatening complications of autoimmune PAP, occur in 5–13% of patients and account for 18–20% of deaths1-4 Increased Risk of Infection Over time, autoimmune PAP can lead to pulmonary fibrosis and respiratory failure which can be fatal and may require lung transplantation 1. Trapnell Nat Rev Dis Primers 2019; 2. Seymour AJRCCM 2002; 3. Inoue AJRCCM 2008; 4. Jouneau Respirology 2020 Fibrosis and Lung Transplant Fatigue, Decreased Exercise Tolerance Fatigue and significantly reduced exercise capacity can dramatically impact the simplest of daily activities, e.g., getting winded walking up a flight of stairs No approved drugs in the U.S. or Europe for autoimmune PAP, only treatment option is an invasive procedure
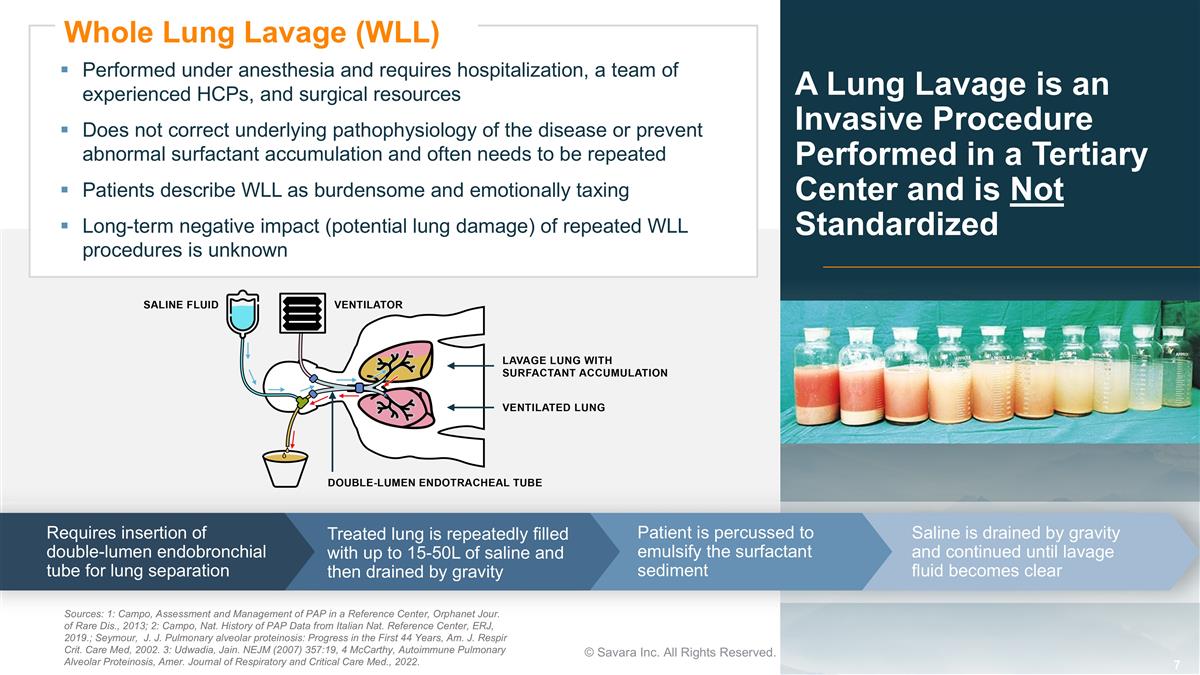
A Lung Lavage is an Invasive Procedure Performed in a Tertiary Center and is Not Standardized © Savara Inc. All Rights Reserved. Sources: 1: Campo, Assessment and Management of PAP in a Reference Center, Orphanet Jour. of Rare Dis., 2013; 2: Campo, Nat. History of PAP Data from Italian Nat. Reference Center, ERJ, 2019.; Seymour, J. J. Pulmonary alveolar proteinosis: Progress in the First 44 Years, Am. J. Respir Crit. Care Med, 2002. 3: Udwadia, Jain. NEJM (2007) 357:19, 4 McCarthy, Autoimmune Pulmonary Alveolar Proteinosis, Amer. Journal of Respiratory and Critical Care Med., 2022. Performed under anesthesia and requires hospitalization, a team of experienced HCPs, and surgical resources Does not correct underlying pathophysiology of the disease or prevent abnormal surfactant accumulation and often needs to be repeated Patients describe WLL as burdensome and emotionally taxing Long-term negative impact (potential lung damage) of repeated WLL procedures is unknown Whole Lung Lavage (WLL) Requires insertion of double-lumen endobronchial tube for lung separation Treated lung is repeatedly filled with up to 15-50L of saline and then drained by gravity Patient is percussed to emulsify the surfactant sediment Saline is drained by gravity and continued until lavage fluid becomes clear
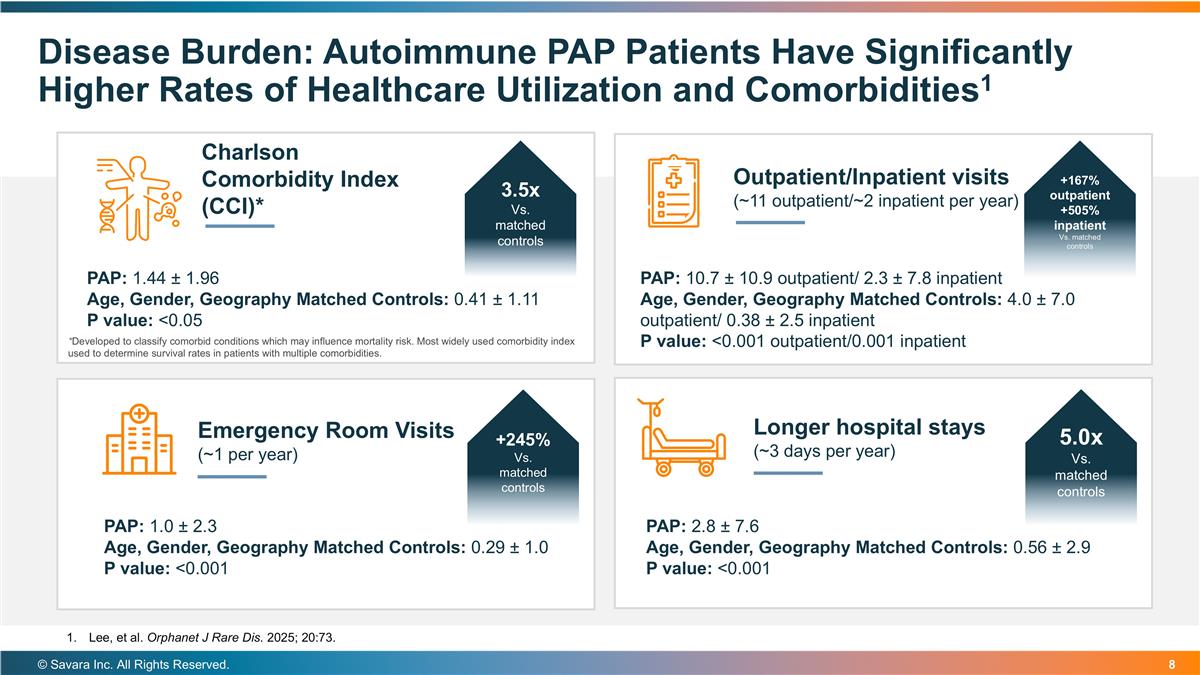
© Savara Inc. All Rights Reserved. Disease Burden: Autoimmune PAP Patients Have Significantly Higher Rates of Healthcare Utilization and Comorbidities1 Charlson Comorbidity Index (CCI)* Outpatient/Inpatient visits (~11 outpatient/~2 inpatient per year) Emergency Room Visits (~1 per year) Longer hospital stays (~3 days per year) PAP: 1.44 ± 1.96 Age, Gender, Geography Matched Controls: 0.41 ± 1.11 P value: <0.05 PAP: 10.7 ± 10.9 outpatient/ 2.3 ± 7.8 inpatient Age, Gender, Geography Matched Controls: 4.0 ± 7.0 outpatient/ 0.38 ± 2.5 inpatient P value: <0.001 outpatient/0.001 inpatient PAP: 1.0 ± 2.3 Age, Gender, Geography Matched Controls: 0.29 ± 1.0 P value: <0.001 PAP: 2.8 ± 7.6 Age, Gender, Geography Matched Controls: 0.56 ± 2.9 P value: <0.001 *Developed to classify comorbid conditions which may influence mortality risk. Most widely used comorbidity index used to determine survival rates in patients with multiple comorbidities. 3.5x Vs. matched controls +245% Vs. matched controls +167% outpatient +505% inpatient Vs. matched controls 5.0x Vs. matched controls Lee, et al. Orphanet J Rare Dis. 2025; 20:73.

“When You can’t Breathe, Nothing Else Matters:” Karli’s Story © Savara Inc. All Rights Reserved. Karli saw 9 specialists before a pulmonologist diagnosed her with autoimmune PAP She wishes her doctors had used the simple blood test to confirm or rule out a diagnosis earlier I would cough until I couldn’t see anything. I would cough until I couldn’t swallow the spit in my mouth. Trying to move fast, lift something, do household tasks, and even showering would trigger my symptoms. Doing the simplest things became a minefield. I was convinced that I was going to die before anyone figured out what was wrong with me at that point. The day I was diagnosed was one of the best days that I've had in my entire life, which sounds weird from the outside, but when you're looking for so long for an answer it was just the most relief I've ever felt. “ ” “ ” “ ” “ ” Karli “missed out on 3 years of her life” searching for a diagnosis
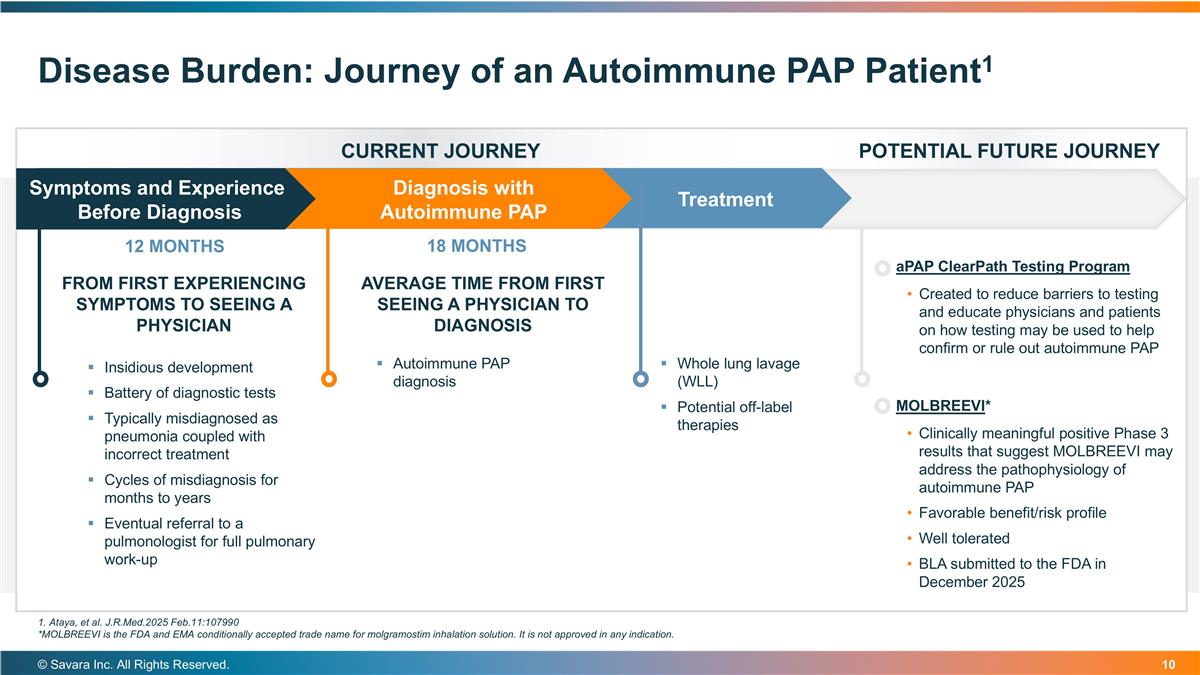
Disease Burden: Journey of an Autoimmune PAP Patient1 © Savara Inc. All Rights Reserved. MOLBREEVI* Clinically meaningful positive Phase 3 results that suggest MOLBREEVI may address the pathophysiology of autoimmune PAP Favorable benefit/risk profile Well tolerated BLA submitted to the FDA in December 2025 Symptoms and Experience Before Diagnosis Diagnosis with Autoimmune PAP Treatment CURRENT JOURNEY POTENTIAL FUTURE JOURNEY FROM FIRST EXPERIENCING SYMPTOMS TO SEEING A PHYSICIAN AVERAGE TIME FROM FIRST SEEING A PHYSICIAN TO DIAGNOSIS 12 MONTHS 18 MONTHS Autoimmune PAP diagnosis Insidious development Battery of diagnostic tests Typically misdiagnosed as pneumonia coupled with incorrect treatment Cycles of misdiagnosis for months to years Eventual referral to a pulmonologist for full pulmonary work-up Whole lung lavage (WLL) Potential off-label therapies aPAP ClearPath Testing Program Created to reduce barriers to testing and educate physicians and patients on how testing may be used to help confirm or rule out autoimmune PAP 1. Ataya, et al. J.R.Med.2025 Feb.11:107990 *MOLBREEVI is the FDA and EMA conditionally accepted trade name for molgramostim inhalation solution. It is not approved in any indication.

MOLBREEVI* *MOLBREEVI is the FDA and EMA conditionally accepted trade name for molgramostim inhalation solution. It is not approved in any indication. (molgramostim inhalation solution)
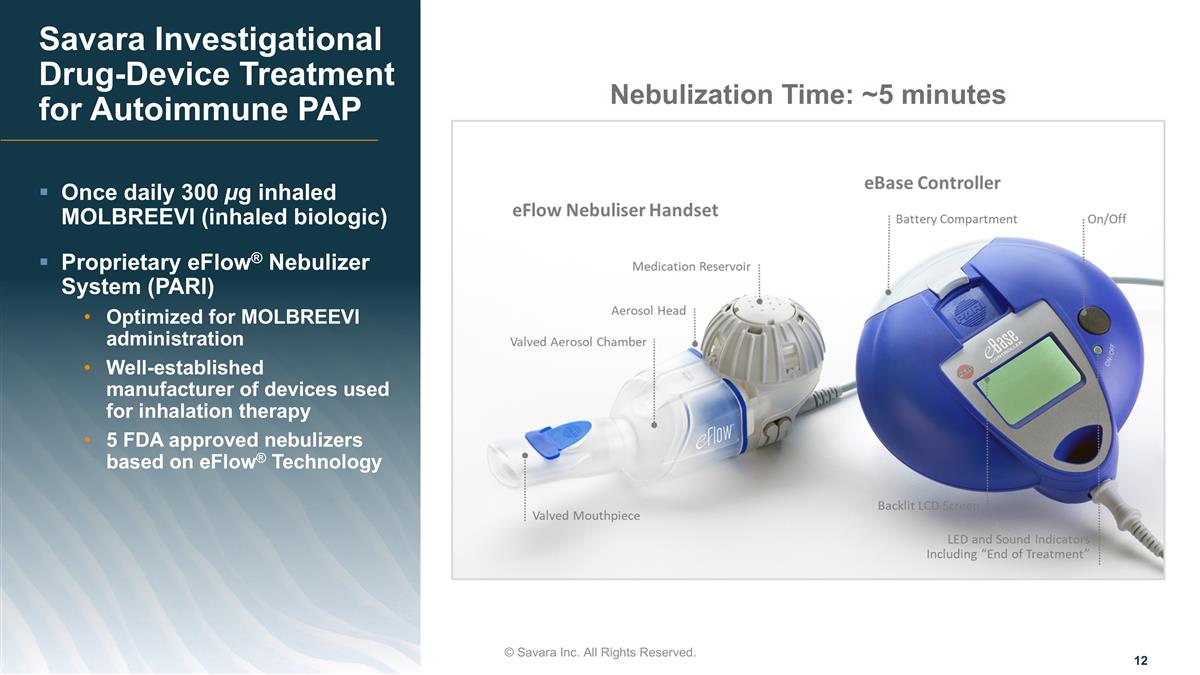
© Savara Inc. All Rights Reserved. Once daily 300 µg inhaled MOLBREEVI (inhaled biologic) Proprietary eFlow® Nebulizer System (PARI) Optimized for MOLBREEVI administration Well-established manufacturer of devices used for inhalation therapy 5 FDA approved nebulizers based on eFlow® Technology Savara Investigational Drug-Device Treatment for Autoimmune PAP Nebulization Time: ~5 minutes
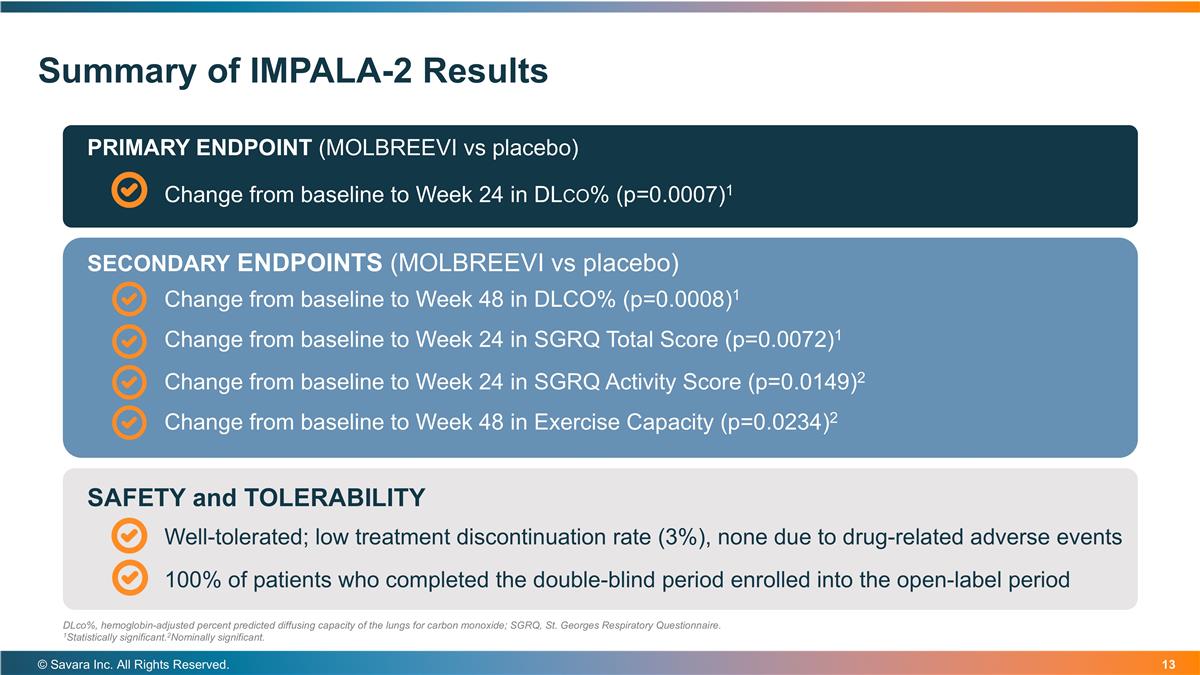
Summary of IMPALA-2 Results © Savara Inc. All Rights Reserved. DLco%, hemoglobin-adjusted percent predicted diffusing capacity of the lungs for carbon monoxide; SGRQ, St. Georges Respiratory Questionnaire. 1Statistically significant.2Nominally significant. Change from baseline to Week 24 in DLco% (p=0.0007)1 PRIMARY ENDPOINT (MOLBREEVI vs placebo) Change from baseline to Week 48 in DLCO% (p=0.0008)1 SECONDARY ENDPOINTS (MOLBREEVI vs placebo) Change from baseline to Week 24 in SGRQ Total Score (p=0.0072)1 Change from baseline to Week 24 in SGRQ Activity Score (p=0.0149)2 Change from baseline to Week 48 in Exercise Capacity (p=0.0234)2 Well-tolerated; low treatment discontinuation rate (3%), none due to drug-related adverse events SAFETY and TOLERABILITY 100% of patients who completed the double-blind period enrolled into the open-label period
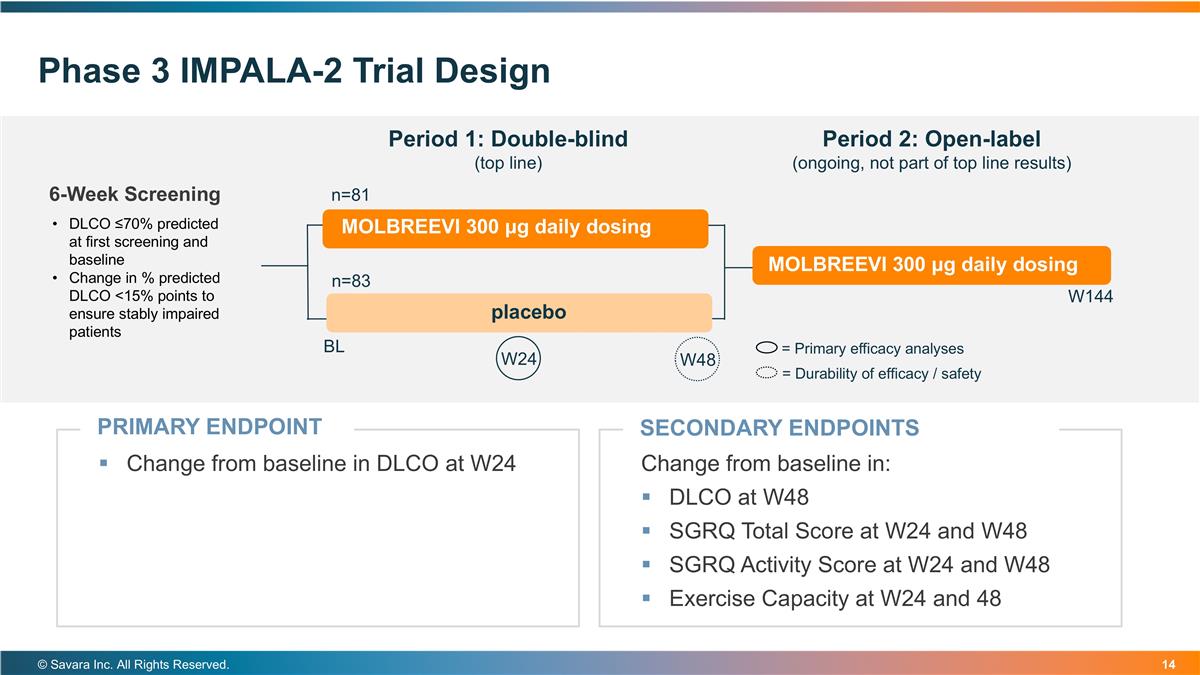
Phase 3 IMPALA-2 Trial Design © Savara Inc. All Rights Reserved. PRIMARY ENDPOINT Change from baseline in DLCO at W24 6-Week Screening Period 1: Double-blind (top line) Period 2: Open-label (ongoing, not part of top line results) SECONDARY ENDPOINTS Change from baseline in: DLCO at W48 SGRQ Total Score at W24 and W48 SGRQ Activity Score at W24 and W48 Exercise Capacity at W24 and 48 DLCO ≤70% predicted at first screening and baseline Change in % predicted DLCO <15% points to ensure stably impaired patients n=81 n=83 BL W48 W24 = Primary efficacy analyses MOLBREEVI 300 μg daily dosing placebo MOLBREEVI 300 μg daily dosing = Durability of efficacy / safety W144
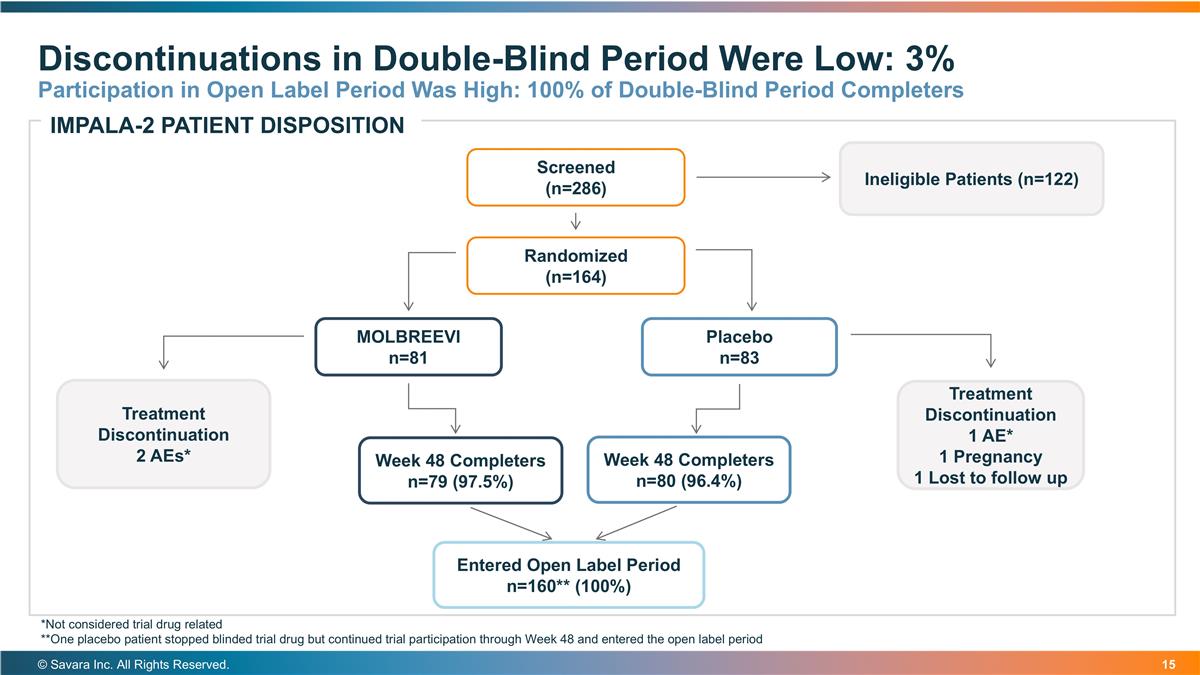
Discontinuations in Double-Blind Period Were Low: 3% Participation in Open Label Period Was High: 100% of Double-Blind Period Completers © Savara Inc. All Rights Reserved. Screened (n=286) Randomized (n=164) MOLBREEVI n=81 Placebo n=83 Ineligible Patients (n=122) Week 48 Completers n=79 (97.5%) Week 48 Completers n=80 (96.4%) Treatment Discontinuation 2 AEs* Treatment Discontinuation 1 AE* 1 Pregnancy 1 Lost to follow up Entered Open Label Period n=160** (100%) IMPALA-2 PATIENT DISPOSITION *Not considered trial drug related **One placebo patient stopped blinded trial drug but continued trial participation through Week 48 and entered the open label period
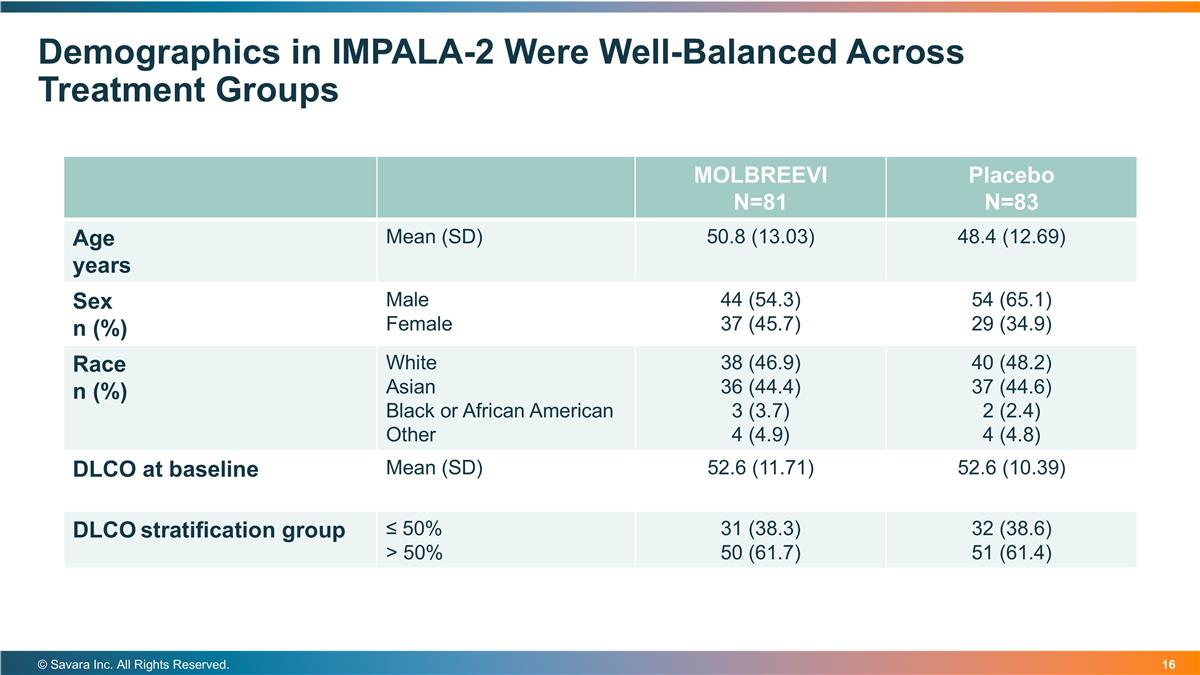
Demographics in IMPALA-2 Were Well-Balanced Across Treatment Groups © Savara Inc. All Rights Reserved. MOLBREEVI N=81 Placebo N=83 Age years Mean (SD) 50.8 (13.03) 48.4 (12.69) Sex n (%) Male Female 44 (54.3) 37 (45.7) 54 (65.1) 29 (34.9) Race n (%) White Asian Black or African American Other 38 (46.9) 36 (44.4) 3 (3.7) 4 (4.9) 40 (48.2) 37 (44.6) 2 (2.4) 4 (4.8) DLCO at baseline Mean (SD) 52.6 (11.71) 52.6 (10.39) DLCO stratification group ≤ 50% > 50% 31 (38.3) 50 (61.7) 32 (38.6) 51 (61.4)
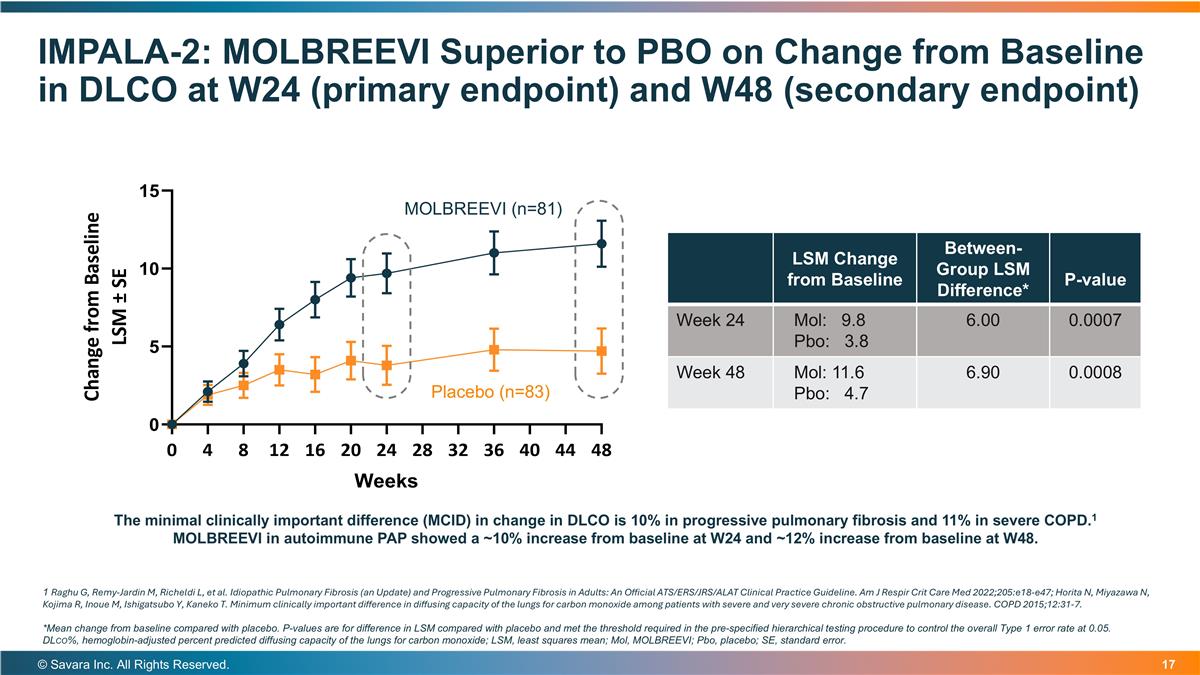
IMPALA-2: MOLBREEVI Superior to PBO on Change from Baseline in DLCO at W24 (primary endpoint) and W48 (secondary endpoint) © Savara Inc. All Rights Reserved. The minimal clinically important difference (MCID) in change in DLCO is 10% in progressive pulmonary fibrosis and 11% in severe COPD.1 MOLBREEVI in autoimmune PAP showed a ~10% increase from baseline at W24 and ~12% increase from baseline at W48. LSM Change from Baseline Between-Group LSM Difference* P-value Week 24 Mol: 9.8 Pbo: 3.8 6.00 0.0007 Week 48 Mol: 11.6 Pbo: 4.7 6.90 0.0008 1 Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-e47; Horita N, Miyazawa N, Kojima R, Inoue M, Ishigatsubo Y, Kaneko T. Minimum clinically important difference in diffusing capacity of the lungs for carbon monoxide among patients with severe and very severe chronic obstructive pulmonary disease. COPD 2015;12:31-7. *Mean change from baseline compared with placebo. P-values are for difference in LSM compared with placebo and met the threshold required in the pre-specified hierarchical testing procedure to control the overall Type 1 error rate at 0.05. DLco%, hemoglobin-adjusted percent predicted diffusing capacity of the lungs for carbon monoxide; LSM, least squares mean; Mol, MOLBREEVI; Pbo, placebo; SE, standard error. Placebo (n=83) MOLBREEVI (n=81)
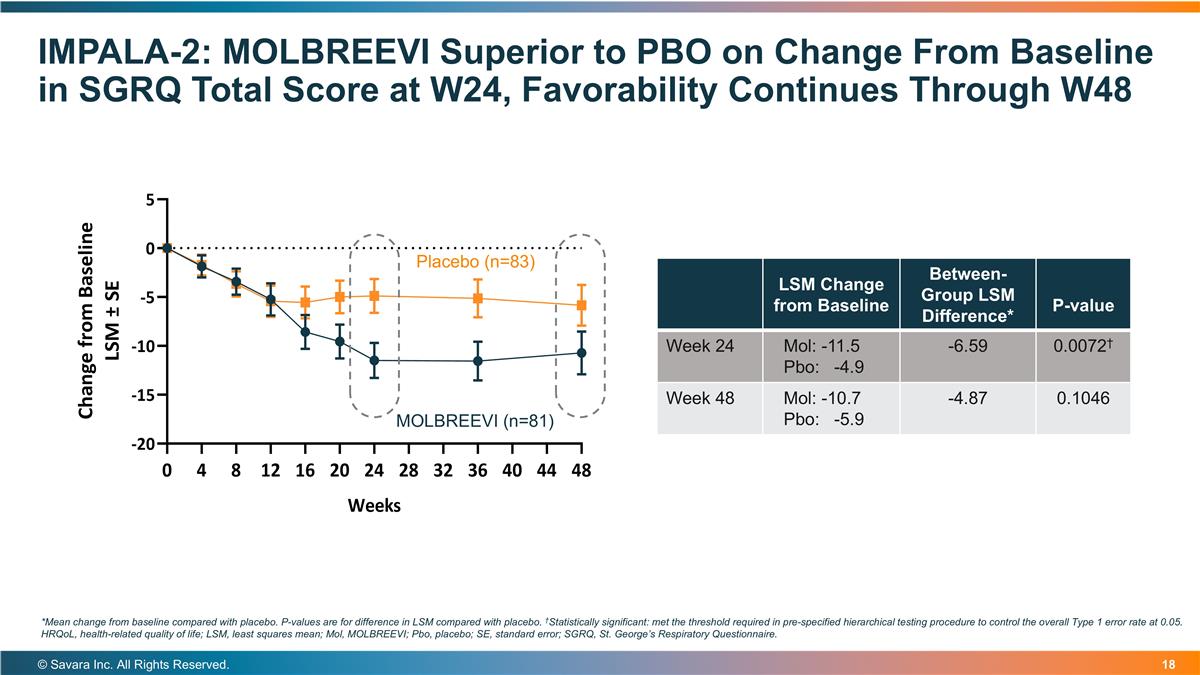
IMPALA-2: MOLBREEVI Superior to PBO on Change From Baseline in SGRQ Total Score at W24, Favorability Continues Through W48 © Savara Inc. All Rights Reserved. LSM Change from Baseline Between-Group LSM Difference* P-value Week 24 Mol: -11.5 Pbo: -4.9 -6.59 0.0072† Week 48 Mol: -10.7 Pbo: -5.9 -4.87 0.1046 *Mean change from baseline compared with placebo. P-values are for difference in LSM compared with placebo. †Statistically significant: met the threshold required in pre-specified hierarchical testing procedure to control the overall Type 1 error rate at 0.05. HRQoL, health-related quality of life; LSM, least squares mean; Mol, MOLBREEVI; Pbo, placebo; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire. Placebo (n=83) MOLBREEVI (n=81)
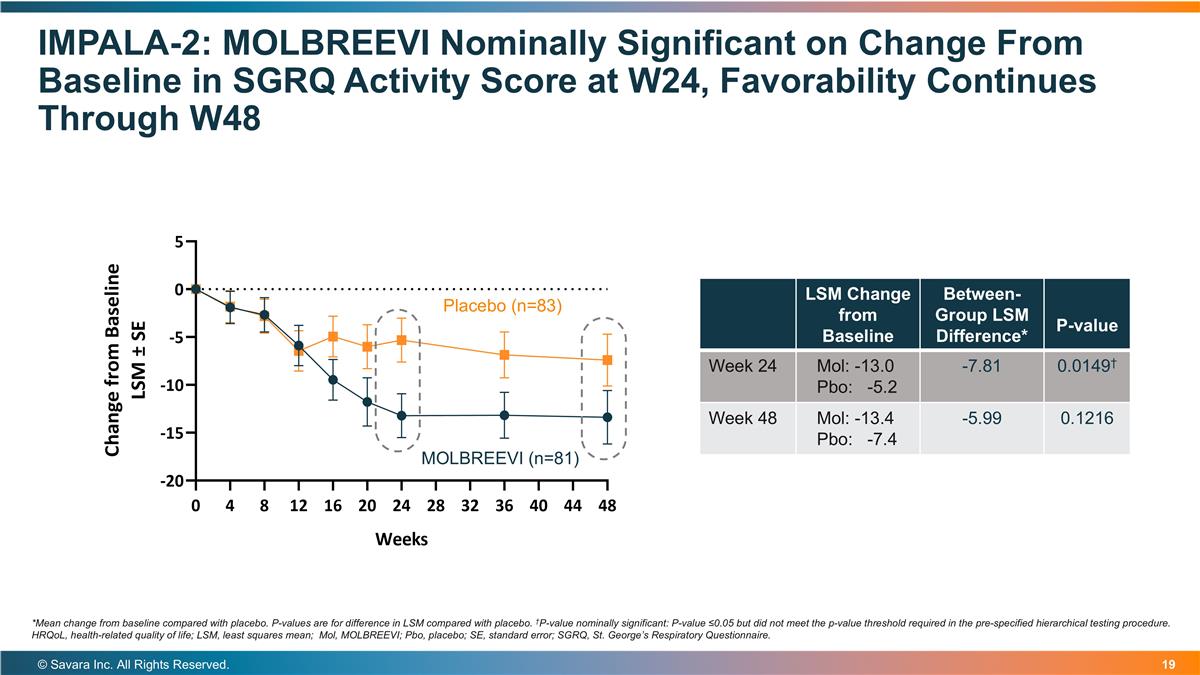
IMPALA-2: MOLBREEVI Nominally Significant on Change From Baseline in SGRQ Activity Score at W24, Favorability Continues Through W48 © Savara Inc. All Rights Reserved. *Mean change from baseline compared with placebo. P-values are for difference in LSM compared with placebo. †P-value nominally significant: P-value ≤0.05 but did not meet the p-value threshold required in the pre-specified hierarchical testing procedure. HRQoL, health-related quality of life; LSM, least squares mean; Mol, MOLBREEVI; Pbo, placebo; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire. LSM Change from Baseline Between-Group LSM Difference* P-value Week 24 Mol: -13.0 Pbo: -5.2 -7.81 0.0149† Week 48 Mol: -13.4 Pbo: -7.4 -5.99 0.1216 Placebo (n=83) MOLBREEVI (n=81)
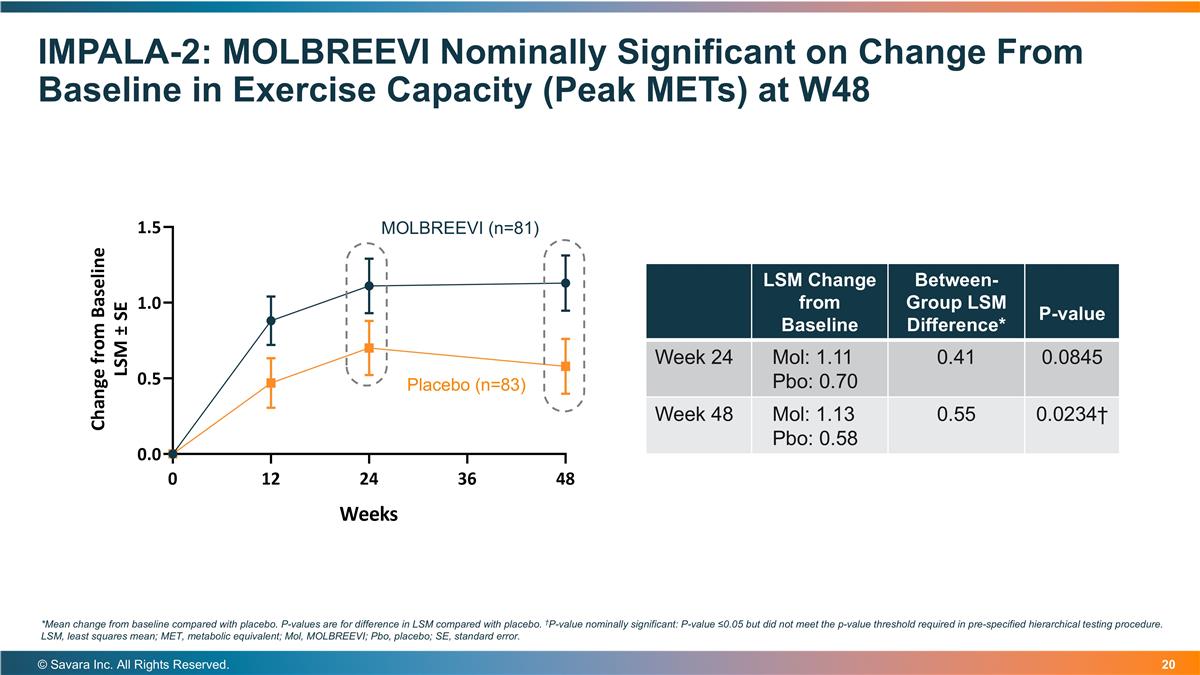
IMPALA-2: MOLBREEVI Nominally Significant on Change From Baseline in Exercise Capacity (Peak METs) at W48 © Savara Inc. All Rights Reserved. *Mean change from baseline compared with placebo. P-values are for difference in LSM compared with placebo. †P-value nominally significant: P-value ≤0.05 but did not meet the p-value threshold required in pre-specified hierarchical testing procedure. LSM, least squares mean; MET, metabolic equivalent; Mol, MOLBREEVI; Pbo, placebo; SE, standard error. Placebo (n=83) MOLBREEVI (n=81) LSM Change from Baseline Between-Group LSM Difference* P-value Week 24 Mol: 1.11 Pbo: 0.70 0.41 0.0845 Week 48 Mol: 1.13 Pbo: 0.58 0.55 0.0234†
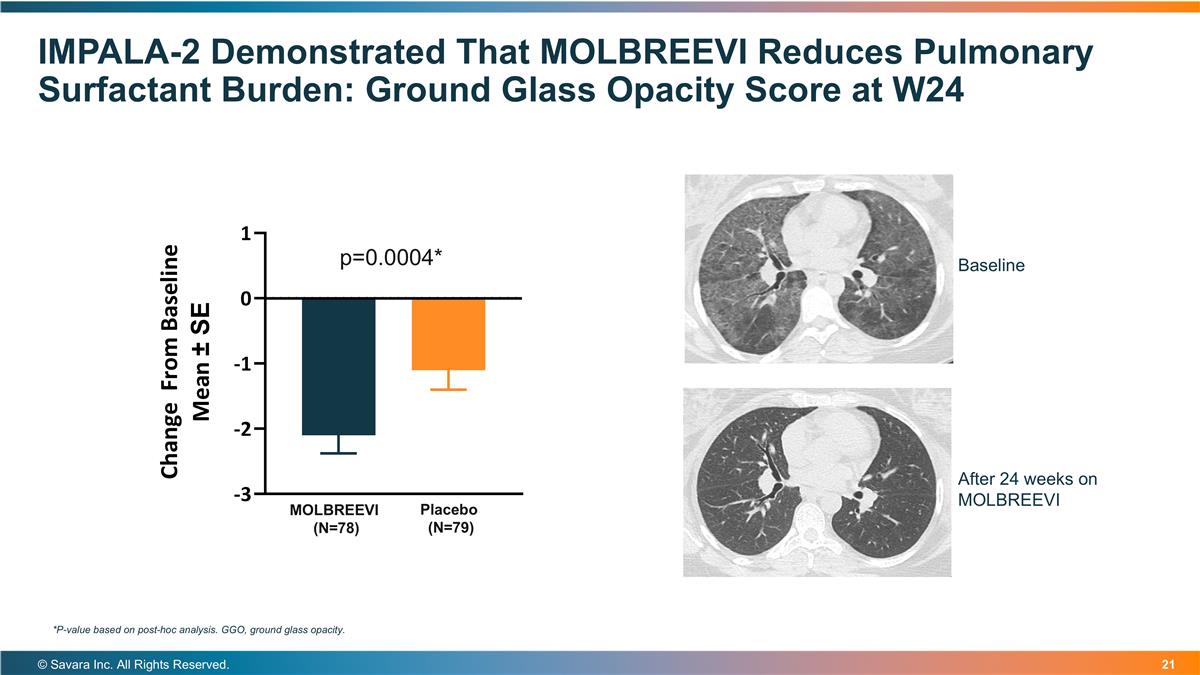
IMPALA-2 Demonstrated That MOLBREEVI Reduces Pulmonary Surfactant Burden: Ground Glass Opacity Score at W24 © Savara Inc. All Rights Reserved. *P-value based on post-hoc analysis. GGO, ground glass opacity. MOLBREEVI (N=78) Placebo (N=79) p=0.0004* Baseline After 24 weeks on MOLBREEVI
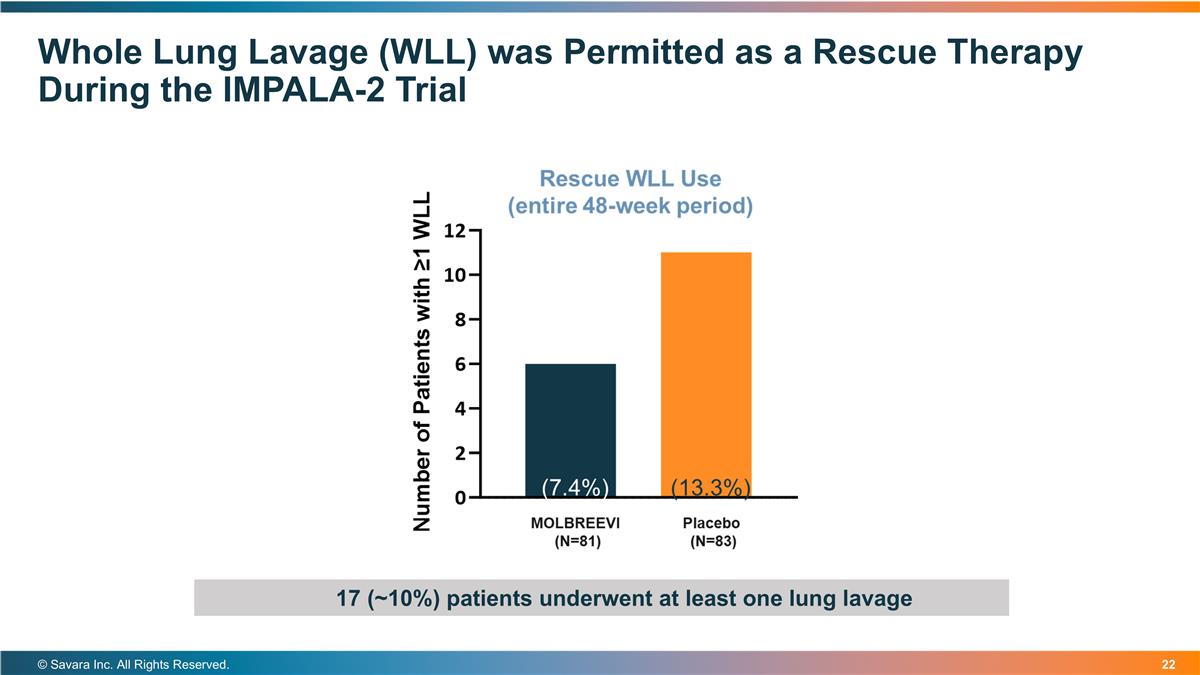
Whole Lung Lavage (WLL) was Permitted as a Rescue Therapy During the IMPALA-2 Trial © Savara Inc. All Rights Reserved. 17 (~10%) patients underwent at least one lung lavage
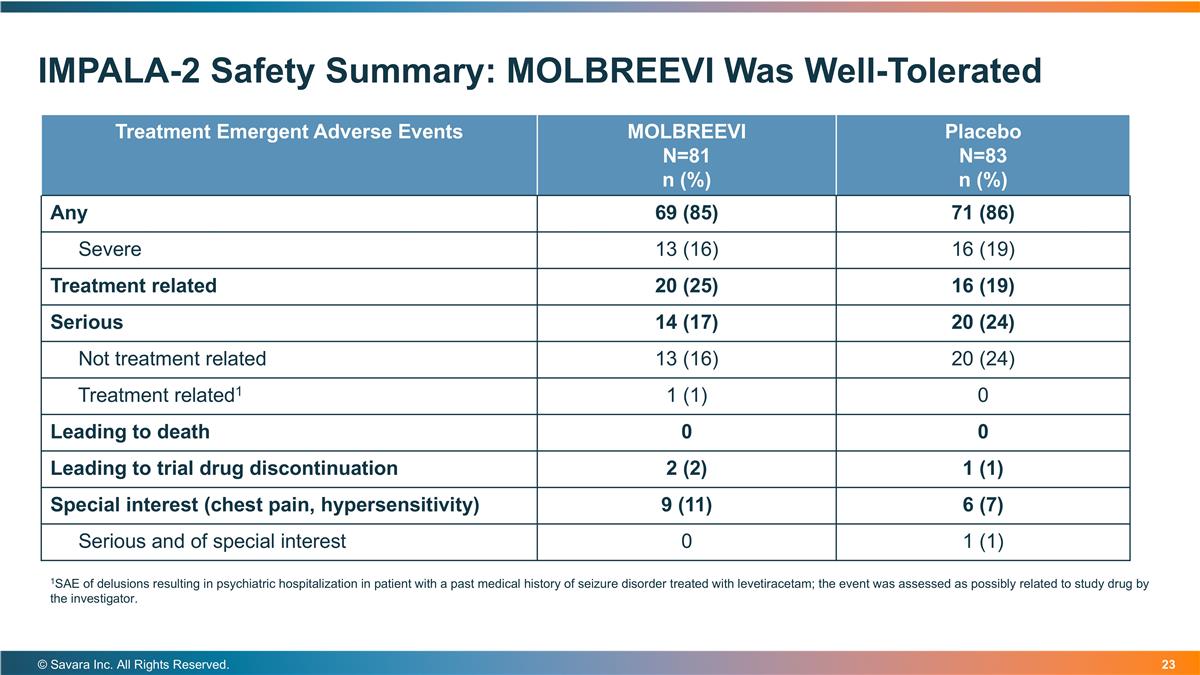
IMPALA-2 Safety Summary: MOLBREEVI Was Well-Tolerated © Savara Inc. All Rights Reserved. Treatment Emergent Adverse Events MOLBREEVI N=81 n (%) Placebo N=83 n (%) Any 69 (85) 71 (86) Severe 13 (16) 16 (19) Treatment related 20 (25) 16 (19) Serious 14 (17) 20 (24) Not treatment related 13 (16) 20 (24) Treatment related1 1 (1) 0 Leading to death 0 0 Leading to trial drug discontinuation 2 (2) 1 (1) Special interest (chest pain, hypersensitivity) 9 (11) 6 (7) Serious and of special interest 0 1 (1) 1SAE of delusions resulting in psychiatric hospitalization in patient with a past medical history of seizure disorder treated with levetiracetam; the event was assessed as possibly related to study drug by the investigator.
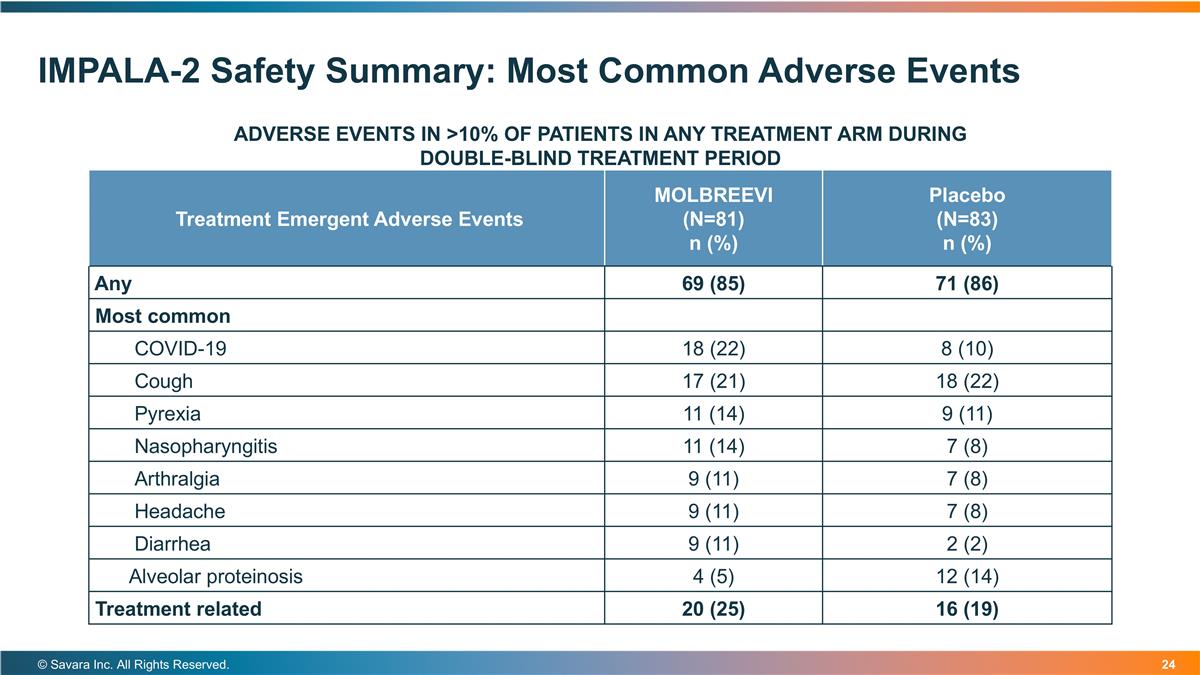
IMPALA-2 Safety Summary: Most Common Adverse Events © Savara Inc. All Rights Reserved. ADVERSE EVENTS IN >10% OF PATIENTS IN ANY TREATMENT ARM DURING DOUBLE-BLIND TREATMENT PERIOD Treatment Emergent Adverse Events MOLBREEVI (N=81) n (%) Placebo (N=83) n (%) Any 69 (85) 71 (86) Most common COVID-19 18 (22) 8 (10) Cough 17 (21) 18 (22) Pyrexia 11 (14) 9 (11) Nasopharyngitis 11 (14) 7 (8) Arthralgia 9 (11) 7 (8) Headache 9 (11) 7 (8) Diarrhea 9 (11) 2 (2) Alveolar proteinosis 4 (5) 12 (14) Treatment related 20 (25) 16 (19)
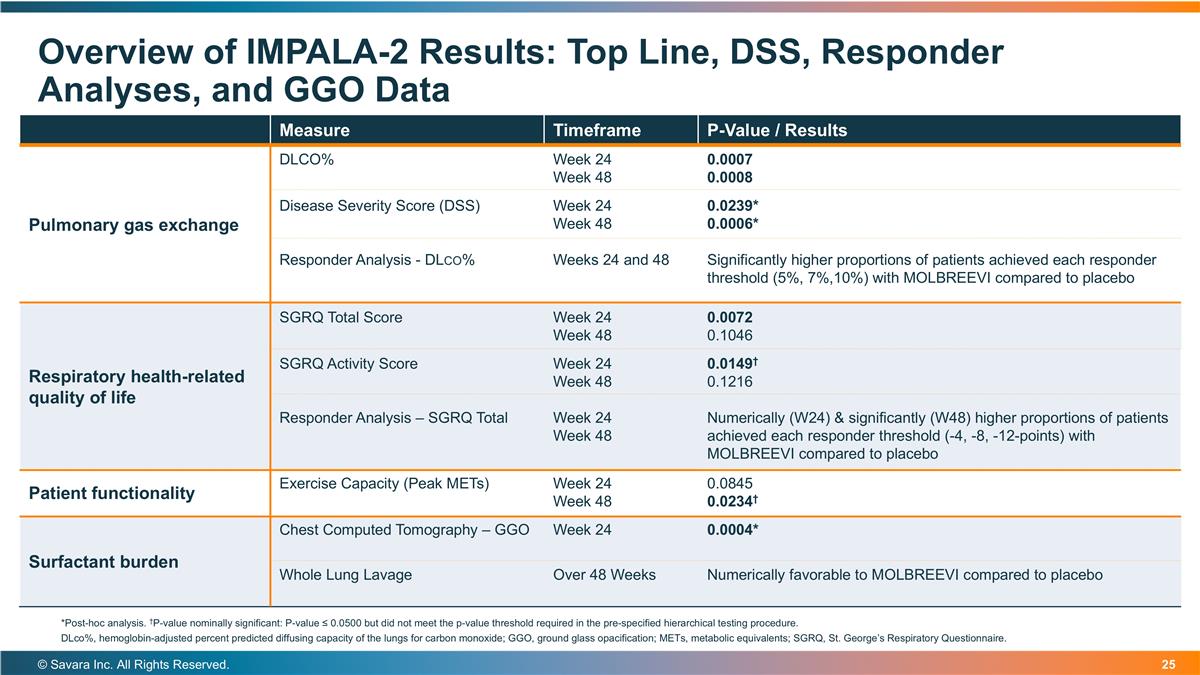
Overview of IMPALA-2 Results: Top Line, DSS, Responder Analyses, and GGO Data © Savara Inc. All Rights Reserved. Measure Timeframe P-Value / Results Pulmonary gas exchange DLCO% Week 24 Week 48 0.0007 0.0008 Disease Severity Score (DSS) Responder Analysis - DLco% Week 24 Week 48 Weeks 24 and 48 0.0239* 0.0006* Significantly higher proportions of patients achieved each responder threshold (5%, 7%,10%) with MOLBREEVI compared to placebo Respiratory health-related quality of life SGRQ Total Score Week 24 Week 48 0.0072 0.1046 SGRQ Activity Score Responder Analysis – SGRQ Total Week 24 Week 48 Week 24 Week 48 0.0149† 0.1216 Numerically (W24) & significantly (W48) higher proportions of patients achieved each responder threshold (-4, -8, -12-points) with MOLBREEVI compared to placebo Patient functionality Exercise Capacity (Peak METs) Week 24 Week 48 0.0845 0.0234† Surfactant burden Chest Computed Tomography – GGO Week 24 0.0004* Whole Lung Lavage Over 48 Weeks Numerically favorable to MOLBREEVI compared to placebo *Post-hoc analysis. †P-value nominally significant: P-value ≤ 0.0500 but did not meet the p-value threshold required in the pre-specified hierarchical testing procedure. DLco%, hemoglobin-adjusted percent predicted diffusing capacity of the lungs for carbon monoxide; GGO, ground glass opacification; METs, metabolic equivalents; SGRQ, St. George’s Respiratory Questionnaire.
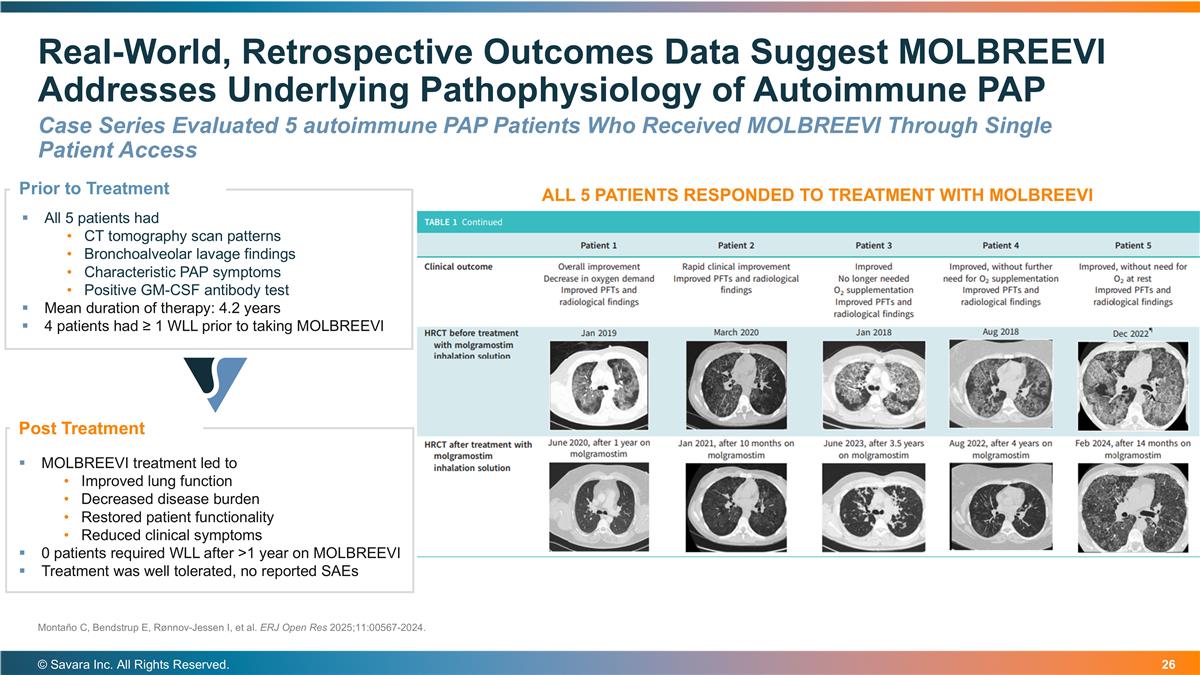
Real-World, Retrospective Outcomes Data Suggest MOLBREEVI Addresses Underlying Pathophysiology of Autoimmune PAP © Savara Inc. All Rights Reserved. Montaño C, Bendstrup E, Rønnov-Jessen I, et al. ERJ Open Res 2025;11:00567-2024. MOLBREEVI treatment led to Improved lung function Decreased disease burden Restored patient functionality Reduced clinical symptoms 0 patients required WLL after >1 year on MOLBREEVI Treatment was well tolerated, no reported SAEs Prior to Treatment Post Treatment All 5 patients had CT tomography scan patterns Bronchoalveolar lavage findings Characteristic PAP symptoms Positive GM-CSF antibody test Mean duration of therapy: 4.2 years 4 patients had ≥ 1 WLL prior to taking MOLBREEVI ALL 5 PATIENTS RESPONDED TO TREATMENT WITH MOLBREEVI Case Series Evaluated 5 autoimmune PAP Patients Who Received MOLBREEVI Through Single Patient Access

Results from IMPALA and IMPALA-2 Clinical Trials Were Published in the New England Journal of Medicine © Savara Inc. All Rights Reserved. Published online on 9/2/2020. Published online on 8/21/2025. IMPALA RESULTS IMPALA-2 RESULTS

Regulatory and Intellectual Property © Savara Inc. All Rights Reserved.
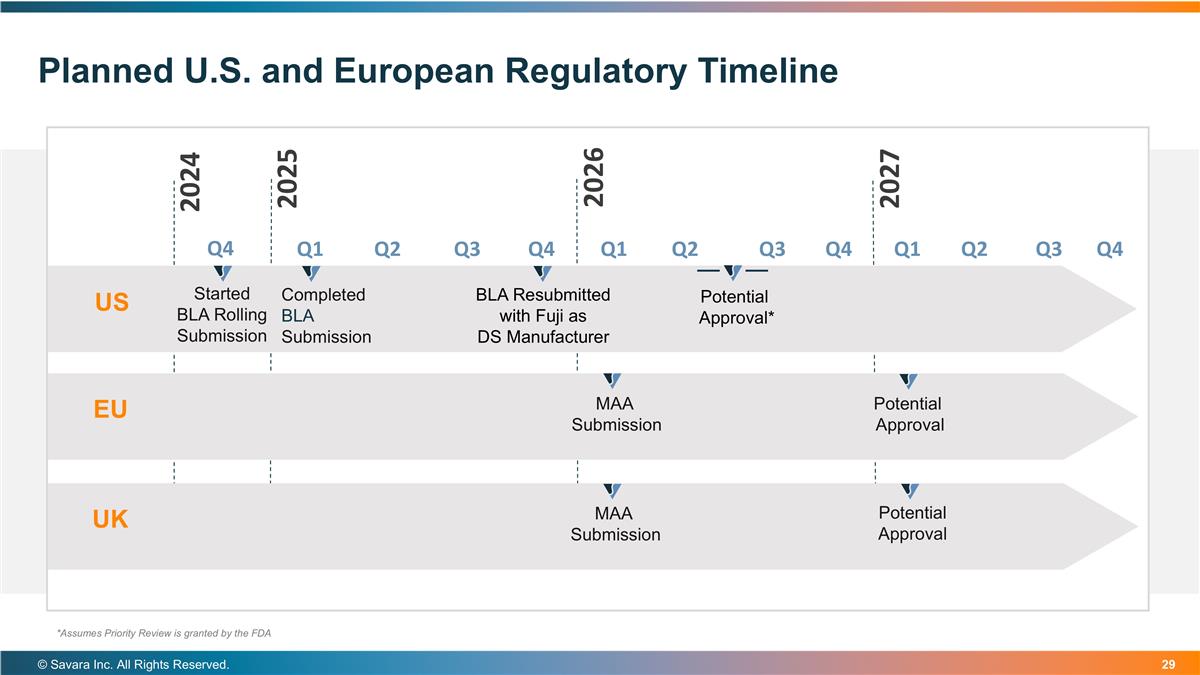
Planned U.S. and European Regulatory Timeline © Savara Inc. All Rights Reserved. US Started BLA Rolling Submission EU Completed BLA Submission BLA Resubmitted with Fuji as DS Manufacturer Potential Approval* MAA Submission Potential Approval *Assumes Priority Review is granted by the FDA UK MAA Submission Potential Approval

MOLBREEVI in Autoimmune PAP Regulatory and IP Summary © Savara Inc. All Rights Reserved. Upon Biologics License Application (BLA) approval FDA would grant 12 years marketing exclusivity BIOLOGIC EXCLUSIVITY US Orphan Drug Designation (eligible for 7 years exclusivity) Fast Track Designation Breakthrough Therapy Designation EUROPE Orphan Drug Designation (eligible for 10 years exclusivity) UK Innovation Passport Designation Promising Innovative Medicine Designation REGULATORY DESIGNATIONS INTELLECTUAL PROPERTY Pending patent applications for drug formulation of MOLBREEVI Notified that European Patent Office intends to grant patent Worldwide exclusive license to proprietary eFlow® Nebulizer System (PARI) for MOLBREEVI in autoimmune PAP and pending joint patent application with PARI for the drug/device combination European patent granted US patent pending Proprietary cell bank

Commercial Update © Savara Inc. All Rights Reserved.
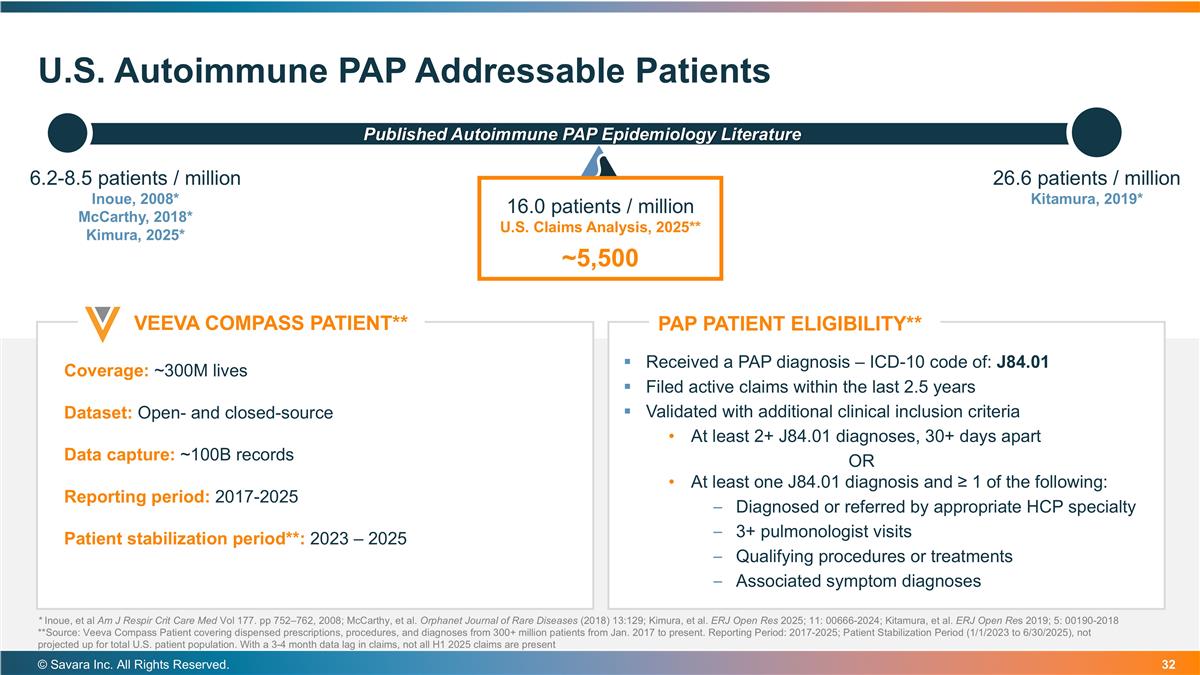
U.S. Autoimmune PAP Addressable Patients © Savara Inc. All Rights Reserved. 26.6 patients / million Kitamura, 2019* Published Autoimmune PAP Epidemiology Literature Coverage: ~300M lives Dataset: Open- and closed-source Data capture: ~100B records Reporting period: 2017-2025 Patient stabilization period**: 2023 – 2025 PAP PATIENT ELIGIBILITY** VEEVA COMPASS PATIENT** Received a PAP diagnosis – ICD-10 code of: J84.01 Filed active claims within the last 2.5 years Validated with additional clinical inclusion criteria At least 2+ J84.01 diagnoses, 30+ days apart OR At least one J84.01 diagnosis and ≥ 1 of the following: Diagnosed or referred by appropriate HCP specialty 3+ pulmonologist visits Qualifying procedures or treatments Associated symptom diagnoses 16.0 patients / million U.S. Claims Analysis, 2025** ~5,500 6.2-8.5 patients / million Inoue, 2008* McCarthy, 2018* Kimura, 2025* * Inoue, et al Am J Respir Crit Care Med Vol 177. pp 752–762, 2008; McCarthy, et al. Orphanet Journal of Rare Diseases (2018) 13:129; Kimura, et al. ERJ Open Res 2025; 11: 00666-2024; Kitamura, et al. ERJ Open Res 2019; 5: 00190-2018 **Source: Veeva Compass Patient covering dispensed prescriptions, procedures, and diagnoses from 300+ million patients from Jan. 2017 to present. Reporting Period: 2017-2025; Patient Stabilization Period (1/1/2023 to 6/30/2025), not projected up for total U.S. patient population. With a 3-4 month data lag in claims, not all H1 2025 claims are present
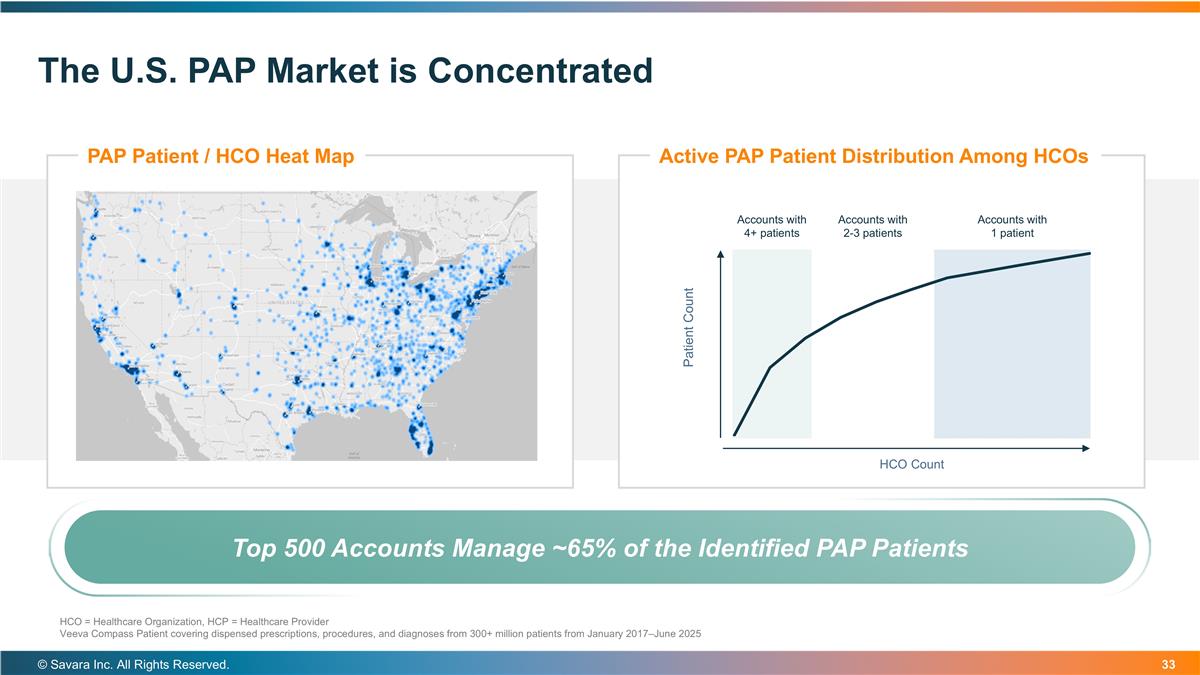
The U.S. PAP Market is Concentrated © Savara Inc. All Rights Reserved. PAP Patient / HCO Heat Map Active PAP Patient Distribution Among HCOs Accounts with 1 patient Accounts with 2-3 patients Accounts with 4+ patients HCO = Healthcare Organization, HCP = Healthcare Provider Veeva Compass Patient covering dispensed prescriptions, procedures, and diagnoses from 300+ million patients from January 2017–June 2025 Top 500 Accounts Manage ~65% of the Identified PAP Patients
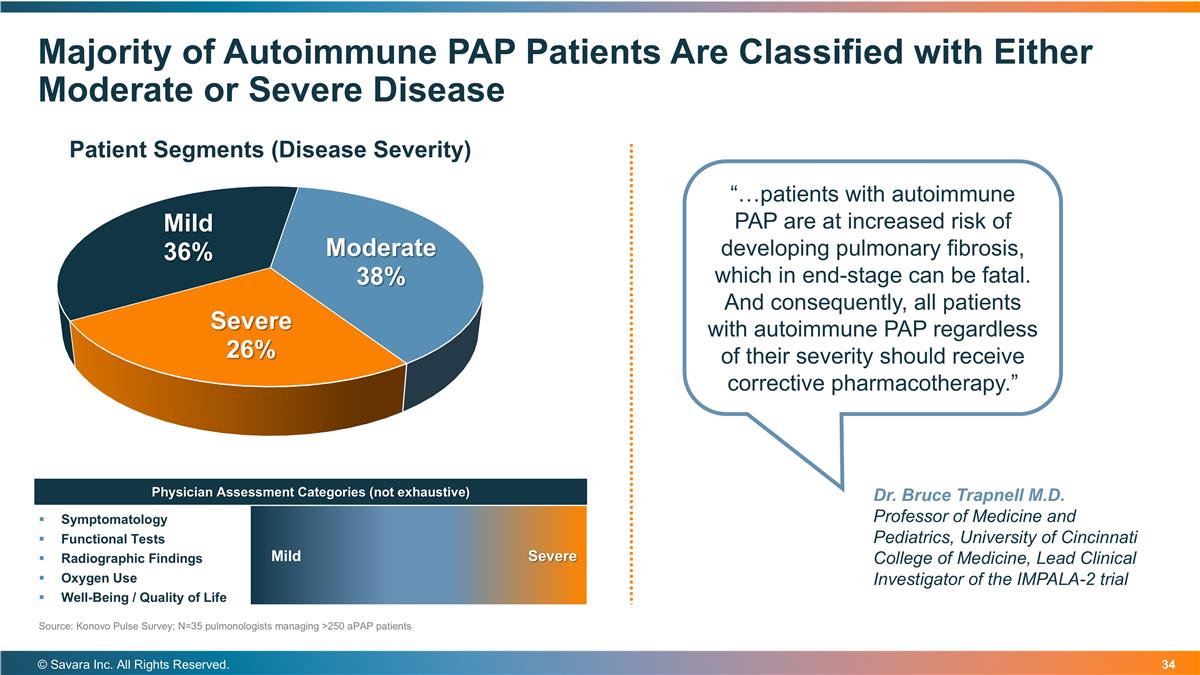
Majority of Autoimmune PAP Patients Are Classified with Either Moderate or Severe Disease © Savara Inc. All Rights Reserved. Dr. Bruce Trapnell M.D. Professor of Medicine and Pediatrics, University of Cincinnati College of Medicine, Lead Clinical Investigator of the IMPALA-2 trial Symptomatology Functional Tests Radiographic Findings Oxygen Use Well-Being / Quality of Life Mild Severe Physician Assessment Categories (not exhaustive) “…patients with autoimmune PAP are at increased risk of developing pulmonary fibrosis, which in end-stage can be fatal. And consequently, all patients with autoimmune PAP regardless of their severity should receive corrective pharmacotherapy.” Source: Konovo Pulse Survey; N=35 pulmonologists managing >250 aPAP patients
Commercial Planning Advancing Against U.S. Launch Objectives © Savara Inc. All Rights Reserved. Expand awareness of autoimmune PAP and educate on the importance of early testing Hire and onboard key commercial roles to expand core activities AWARENESS PERSONNEL Build critical capabilities to facilitate access to MOLBREEVI post approval INFRASTRUCTURE

Autoimmune PAP Disease State Awareness Campaign Multi-channel effort across healthcare professionals (HCP) and patients © Savara Inc. All Rights Reserved. HCP DSA Campaign Patient DSA Campaign
aPAP ClearPath Testing Program: No Charge Third-Party Testing Program in the U.S.* © Savara Inc. All Rights Reserved. Created to reduce barriers to GM-CSF autoantibody testing and educate physicians/patients on how testing may be used to help confirm or rule out PAP** * Patients, healthcare professionals, or payers are not charged for this program. The aPAP ClearPath Testing Program is not yet offered in NY State. The test kit can only be ordered by a healthcare professional for eligible patients. ** Physicians and patients who use this program have no obligation to recommend, purchase, order, prescribe, promote, administer, use, or support any Savara product or product candidate. GM-CSF autoantibody blood test available in two forms: Dried blood spot Serum-based Same test used in Interstitial Lung Disease (ILD) Clinic Program**
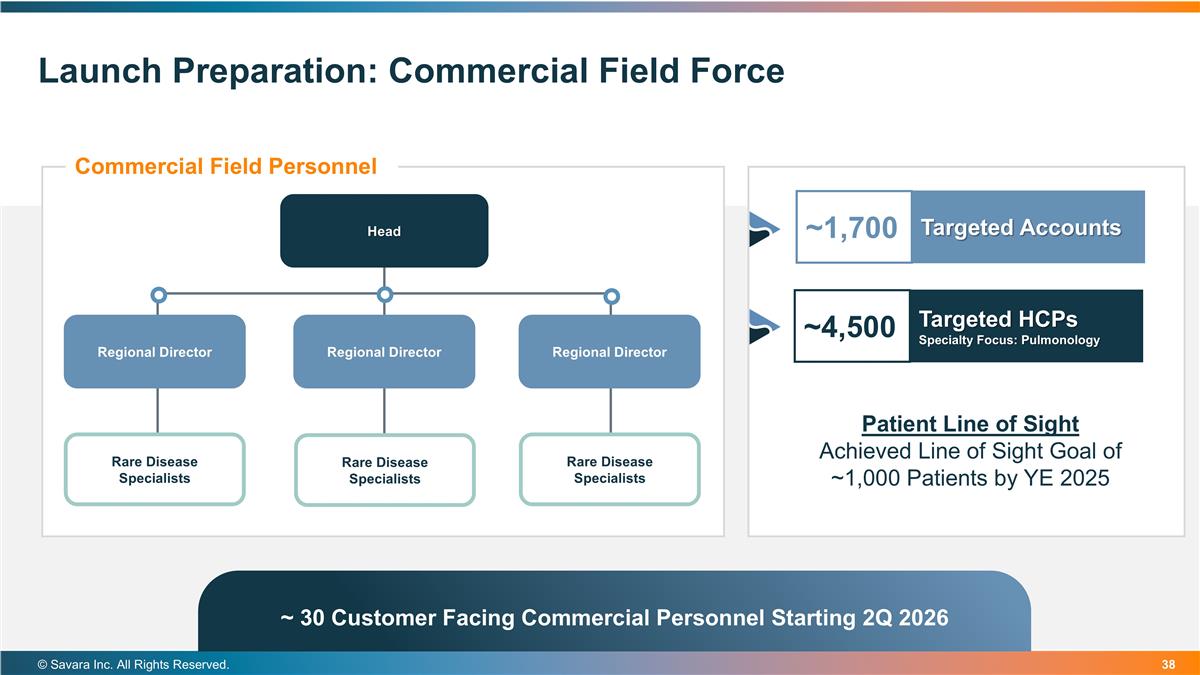
Patient Line of Sight Achieved Line of Sight Goal of ~1,000 Patients by YE 2025 Launch Preparation: Commercial Field Force © Savara Inc. All Rights Reserved. Commercial Field Personnel Head Regional Director Regional Director Regional Director Rare Disease Specialists Rare Disease Specialists Rare Disease Specialists ~1,700 Targeted Accounts ~4,500 Targeted HCPs Specialty Focus: Pulmonology ~ 30 Customer Facing Commercial Personnel Starting 2Q 2026
PANTHERx® Rare Pharmacy Selected as Savara’s U.S. Exclusive Specialty Pharmacy (SP) Partner for Distribution of MOLBREEVI © Savara Inc. All Rights Reserved. 8x Top Performing Independent SP Connectivity with Pulmonary Prescribers Relevant Device Experience Specializes in Rare/Orphan Disease Unparalleled Net Promoter Scores 8 Time Winner – Independent Specialty Pharmacy

MyMOLBREEVI: Best in Class Launch Support in Development Support programs aim to reduce access barriers for appropriate MOLBREEVI patients post approval © Savara Inc. All Rights Reserved. PATIENT RESOURCES Case management approach Financial assistance Clinical education Insurance services PRESCRIBER RESOURCES Streamlined prescribing Prior authorization checklist Sample letter of medical necessity Sample letter of appeal MyMOLBREEVI SUPPORT Specialty Pharmacy and Hub Services
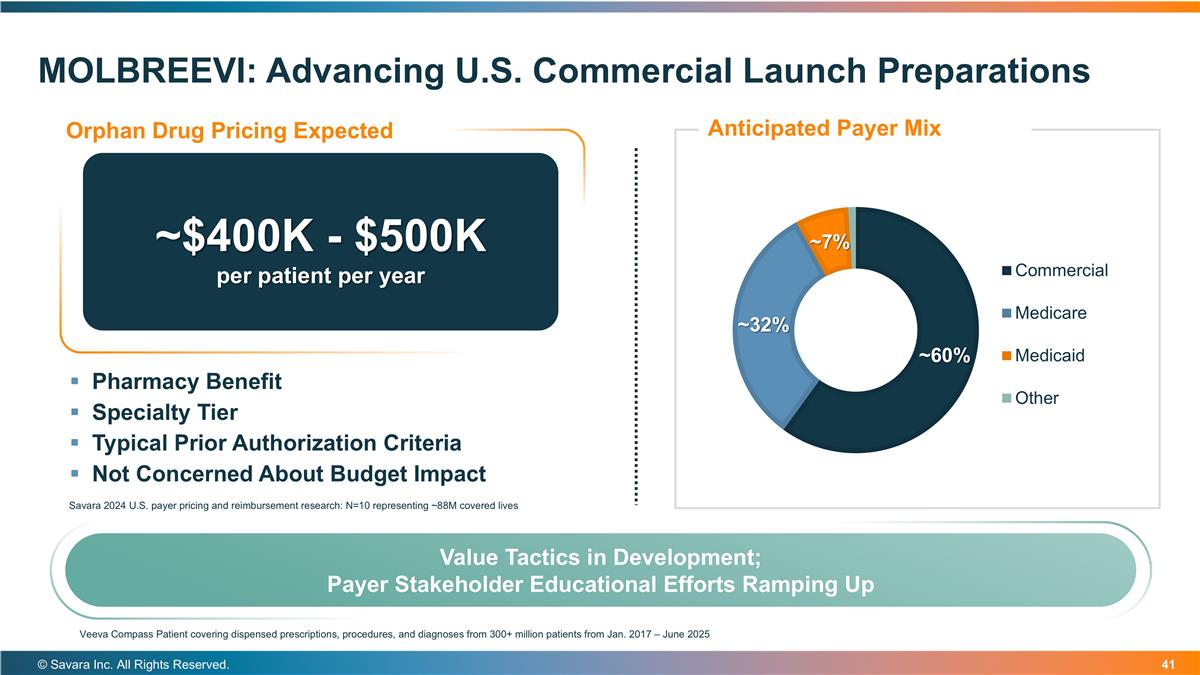
MOLBREEVI: Advancing U.S. Commercial Launch Preparations © Savara Inc. All Rights Reserved. ~$400K - $500K per patient per year Pharmacy Benefit Specialty Tier Typical Prior Authorization Criteria Not Concerned About Budget Impact Savara 2024 U.S. payer pricing and reimbursement research: N=10 representing ~88M covered lives Value Tactics in Development; Payer Stakeholder Educational Efforts Ramping Up Veeva Compass Patient covering dispensed prescriptions, procedures, and diagnoses from 300+ million patients from Jan. 2017 – June 2025 Orphan Drug Pricing Expected Anticipated Payer Mix
MOLBREEVI: U.S. Commercial Opportunity © Savara Inc. All Rights Reserved. MOLBREEVI Clinically meaningful benefit Strong stakeholder interest Orphan drug pricing potential Chronic dosing Small customer facing footprint Exclusive pharmacy network 12-year biologic exclusivity (U.S.) Biosimilar competition unlikely High disease burden No FDA approved therapies Whole lung lavage is invasive and not standardized Significant Unmet Need Significant Commercial Opportunity Efficient Rare Disease Model Long Term Exclusivity
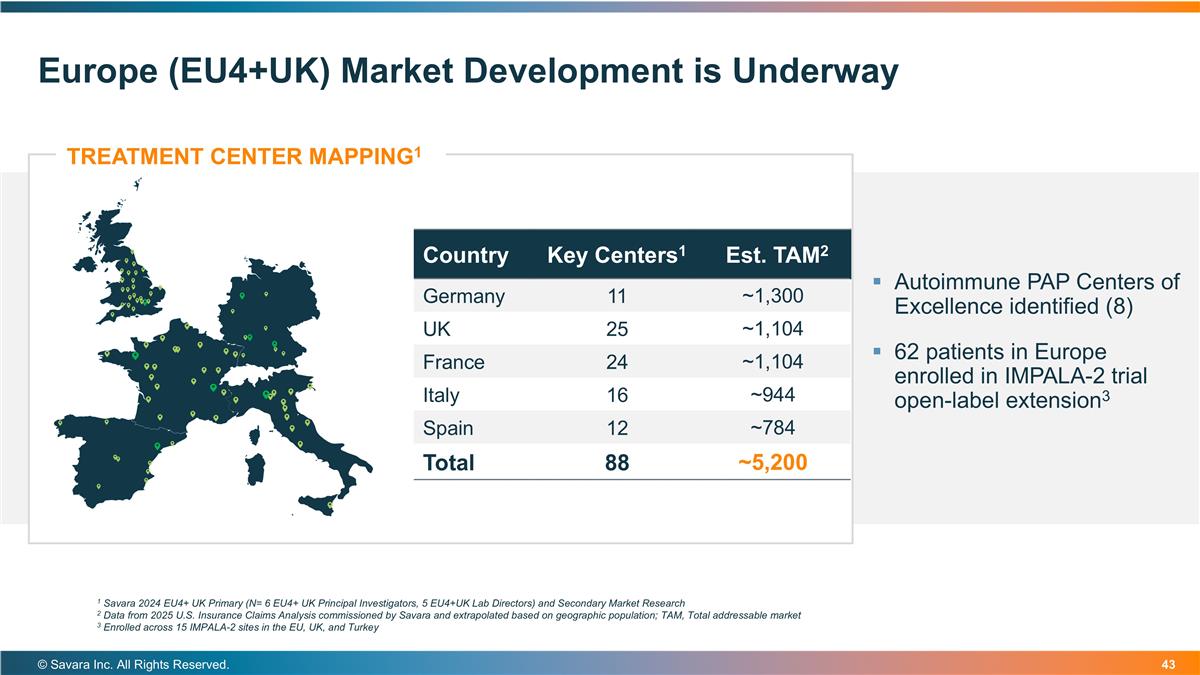
Europe (EU4+UK) Market Development is Underway © Savara Inc. All Rights Reserved. TREATMENT CENTER MAPPING1 Country Key Centers1 Est. TAM2 Germany 11 ~1,300 UK 25 ~1,104 France 24 ~1,104 Italy 16 ~944 Spain 12 ~784 Total 88 ~5,200 1 Savara 2024 EU4+ UK Primary (N= 6 EU4+ UK Principal Investigators, 5 EU4+UK Lab Directors) and Secondary Market Research 2 Data from 2025 U.S. Insurance Claims Analysis commissioned by Savara and extrapolated based on geographic population; TAM, Total addressable market 3 Enrolled across 15 IMPALA-2 sites in the EU, UK, and Turkey Autoimmune PAP Centers of Excellence identified (8) 62 patients in Europe enrolled in IMPALA-2 trial open-label extension3

Financials © Savara Inc. All Rights Reserved.
The Company is well capitalized: ~264.4M in cash* Strong investor support with coverage from 8 equity research analysts Financial Highlights © Savara Inc. All Rights Reserved. * Pro forma for cash, cash equivalents, and short-term investments as of 09/30/25, including October 2025 equity offering of $140M (net) Andrew Tsai Yasmeen Rahimi, Ph.D. Vamil Divan, M.D., M.B.A. Andreas Argyrides Jonathan Wolleben Andrew Fein Benjamin Burnett, Ph.D. Francois Brisebois
U.S. Autoimmune PAP Market Opportunity is Sizable with Blockbuster Potential © Savara Inc. All Rights Reserved. TAM = Total addressable market Current U.S. TAM of autoimmune PAP patients >$2B Potential U.S. Opportunity ~$400K-$500K ~5,500 Orphan rare disease potential pricing power Patents currently being prosecuted Multiple Biologic exclusivity in U.S. upon approval Durable revenue stream with biosimilar competition unlikely 12-years Long-term

Thank You