

Initial Public Offering – June 2014
Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration No. 333-194606 June 30, 2014

Forward Looking Statements
Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s Tarmogen technology, for the timing and outcome of the Company’s clinical trials, the potential approval to market any Tarmogens, and the Company’s capital needs. Actual events could differ materially from those projected and the Company cautions investors not to rely on the forward-looking statements contained in, or made in connection with, the presentation.
Among other things, the Company’s, or it’s collaborators’ clinical trials may be delayed or may eventually be unsuccessful. The Company may consume more cash than it currently anticipates and faster than projected or the Company’s collaborators may terminate or amend their agreements with the Company with terms which are adverse to the Company’s prospects. Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates. If the FDA or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. The Company may not enter into any additional strategic collaboration agreements. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and competitive developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations.
Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Registration Statement on Form S-1, as amended and the Company’s other Periodic and Current Reports filed with the SEC, if any. GlobeImmune undertakes no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations.
| 2 |
|

Free Writing Prospectus Statement
• This presentation highlights basic information about us and the offering.
Because it is a summary, it does not contain all of the information that you
should consider before investing in our common stock.
• We have filed a registration statement (including prospectus) with the United
States Securities and Exchange Commission (SEC) for the offering to which this
presentation relates. The registration statement has not yet become effective.
Before you invest, you should read the prospectus in the registration statement
(including the risk factors described therein) and other documents we have filed
with the SEC for more complete information about us and the offering. You
may get these documents, including the preliminary prospectus dated June 27,
2014 for free by visiting EDGAR on the SEC website at http://www.sec.gov.
| • |
|
Alternatively, we or any underwriter participating in the offering will arrange to |
send you the prospectus if you contact Aegis Capital Corp., Prospectus
Department, 810 Seventh Avenue, 18th Floor, New York, NY 10019, telephone
212-813-1010, email: prospectus@aegiscap.com
| 3 |
|
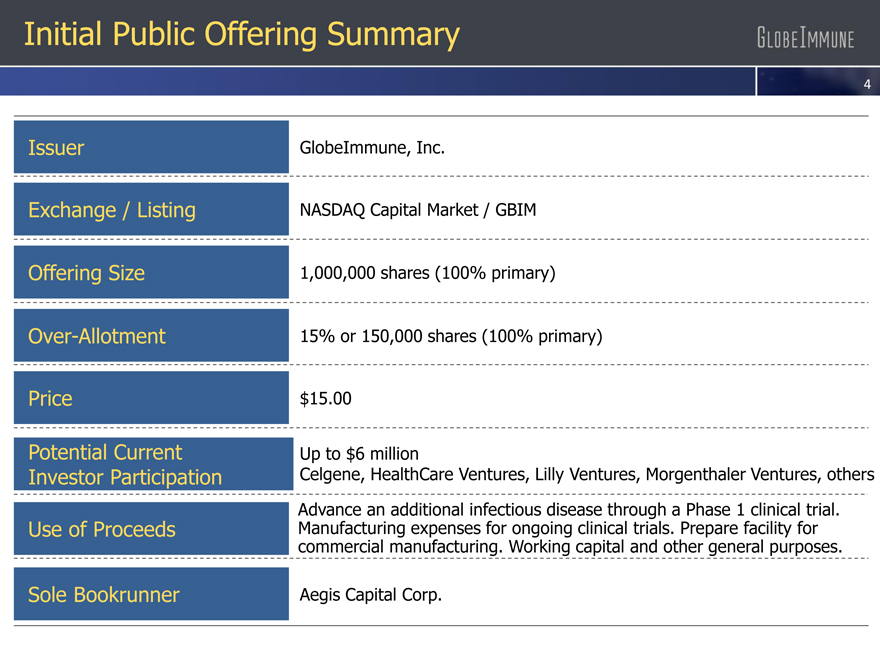
Initial Public Offering Summary
Issuer GlobeImmune, Inc.
Exchange / Listing NASDAQ Capital Market / GBIM
Offering Size 1,000,000 shares (100% primary)
Over-Allotment 15% or 150,000 shares (100% primary)
Price $15.00
Potential Current Up to $6 million
Investor Participation Celgene, HealthCare Ventures, Lilly Ventures, Morgenthaler Ventures, others
Advance an additional infectious disease through a Phase 1 clinical trial.
Use of Proceeds Manufacturing expenses for ongoing clinical trials. Prepare facility for
commercial manufacturing. Working capital and other general purposes.
Sole Bookrunner Aegis Capital Corp.
| 4 |
|

GlobeImmune
| • |
|
Differentiated immunotherapeutic platform |
| • |
|
Proof of concept in infectious disease & oncology |
– 4 ongoing phase 2 studies with multiple upcoming readouts
| • |
|
Major alliances with Gilead and Celgene |
– Over $60 million in partnership revenue to date
– Upcoming data from randomized phase 2 HBV trial
– Upcoming complete data from chordoma phase 1 patients
| • |
|
Multiple additional proprietary programs |
| 5 |
|
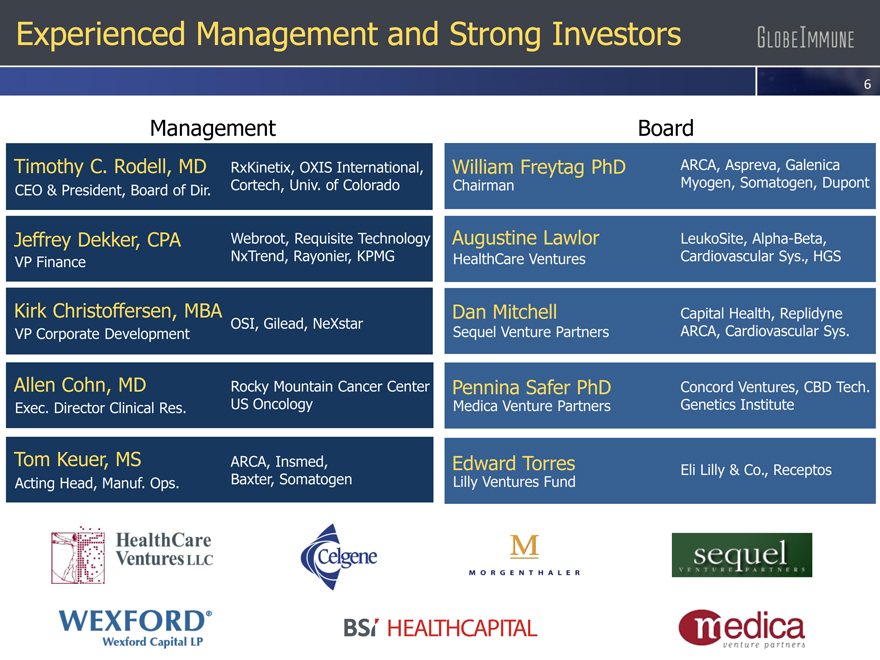
Experienced Management and Strong Investors
Management
Timothy C. Rodell, MD
RxKinetix, OXIS International,
CEO & President, Board of Dir.
Cortech, Univ. of Colorado
Jeffrey Dekker, CPA Webroot, Requisite Technology
VP Finance NxTrend, Rayonier, KPMG
Kirk Christoffersen, MBA
VP Corporate Development OSI, Gilead, NeXstar
Allen Cohn, MD Rocky Mountain Cancer Center
Exec. Director Clinical Res. US Oncology
Tom Keuer, MS ARCA, Insmed,
Acting Head, Manuf. Ops. Baxter, Somatogen
William Freytag PhD
Chairman
Augustine Lawlor
HealthCare Ventures
Dan Mitchell
Sequel Venture Partners
Pennina Safer PhD
Medica Venture Partners
Edward Torres
Lilly Ventures Fund
Board
ARCA, Aspreva, Galenica Myogen, Somatogen, Dupont
LeukoSite, Alpha-Beta, Cardiovascular Sys., HGS
Capital Health, Replidyne ARCA, Cardiovascular Sys.
Concord Ventures, CBD Tech. Genetics Institute
Eli Lilly & Co., Receptos
| 6 |
|
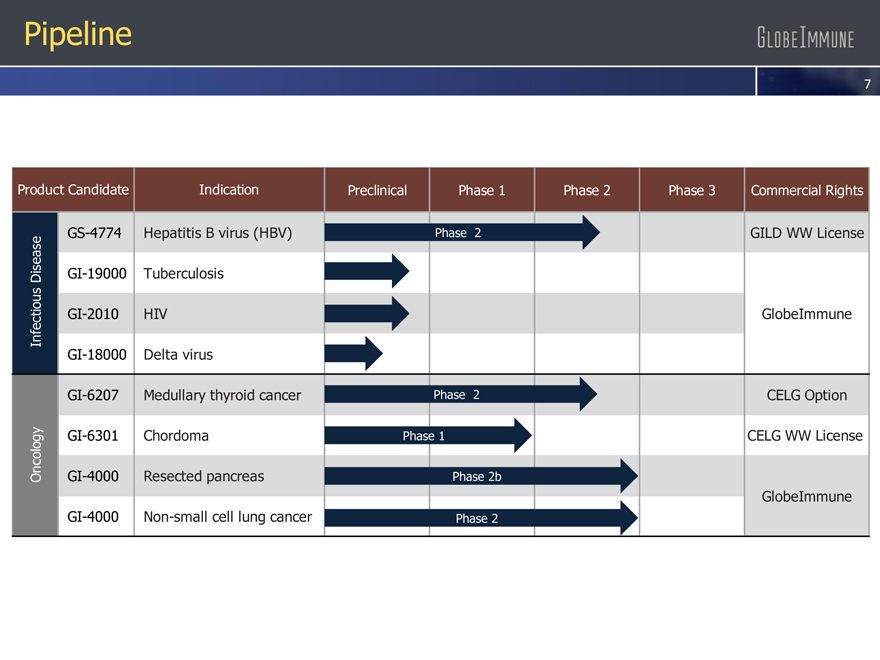
Pipeline
Product Candidate Indication Preclinical Phase 1 Phase 2 Phase 3 Commercial Rights
GS-4774 Hepatitis B virus (HBV) Phase 2 GILD WW License
ease
s
i GI-19000 Tuberculosis
D
s
iou
nfect GI-2010 HIV GlobeImmune
I
GI-18000 Delta virus
GI-6207 Medullary thyroid cancer Phase 2 CELG Option
y GI-6301 Chordoma Phase 1 CELG WW License
og
ol
c
On GI-4000 Resected pancreas Phase 2b
GlobeImmune
GI-4000 Non-small cell lung cancer Phase 2
| 7 |
|
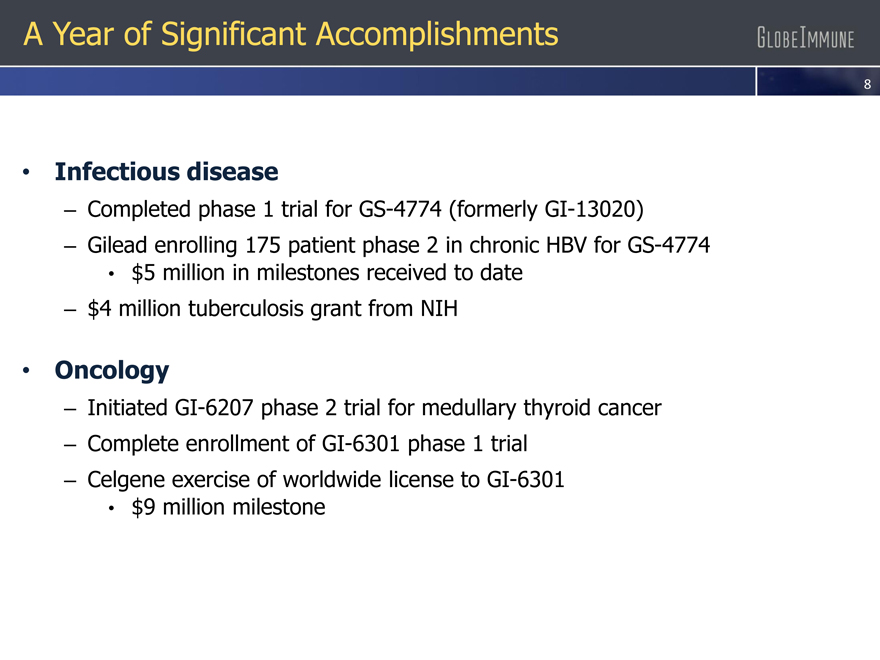
A Year of Significant Accomplishments
| • |
|
Infectious disease |
– Completed phase 1 trial for GS-4774 (formerly GI-13020)
– Gilead enrolling 175 patient phase 2 in chronic HBV for GS-4774
| • |
|
$5 million in milestones received to date |
– $4 million tuberculosis grant from NIH
| • |
|
Oncology |
– Initiated GI-6207 phase 2 trial for medullary thyroid cancer
– Complete enrollment of GI-6301 phase 1 trial
– Celgene exercise of worldwide license to GI-6301
| • |
|
$9 million milestone |
| 8 |
|
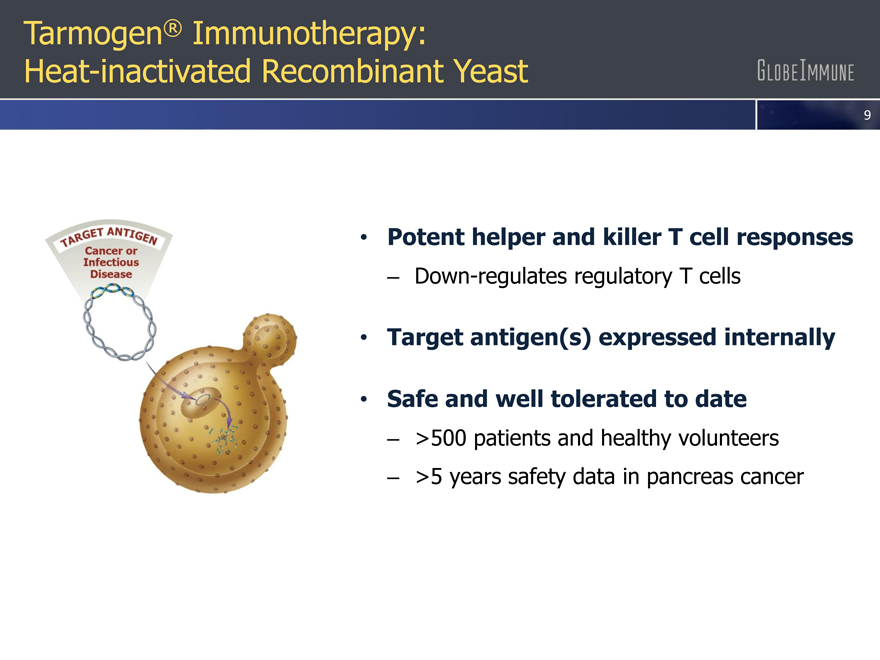
Tarmogen® Immunotherapy: Heat-inactivated Recombinant Yeast
| • |
|
Potent helper and killer T cell responses |
– Down-regulates regulatory T cells
| • |
|
Target antigen(s) expressed internally |
| • |
|
Safe and well tolerated to date |
– >500 patients and healthy volunteers
– >5 years safety data in pancreas cancer
9
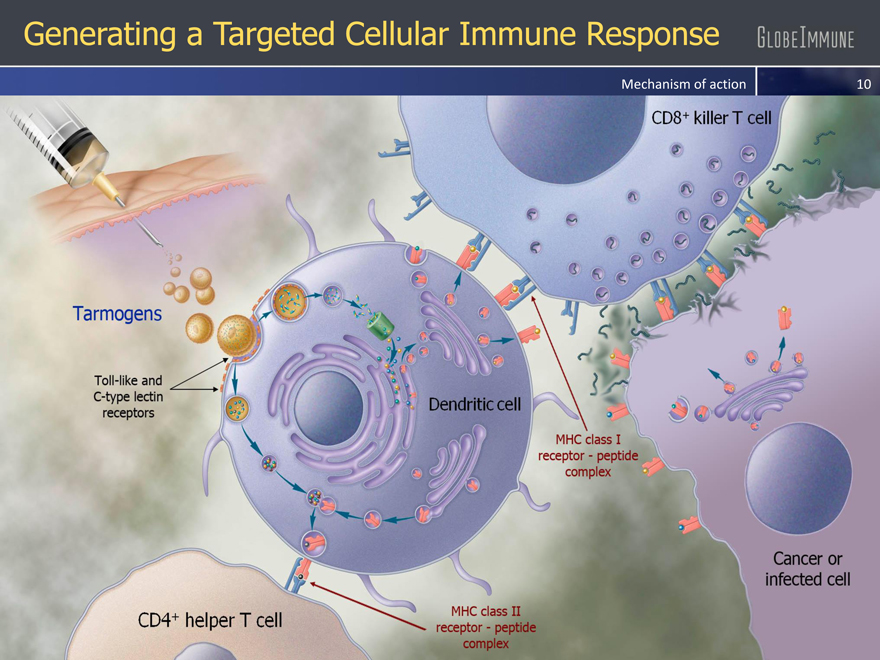
Generating a Targeted Cellular Immune Response
Mechanism of action
10
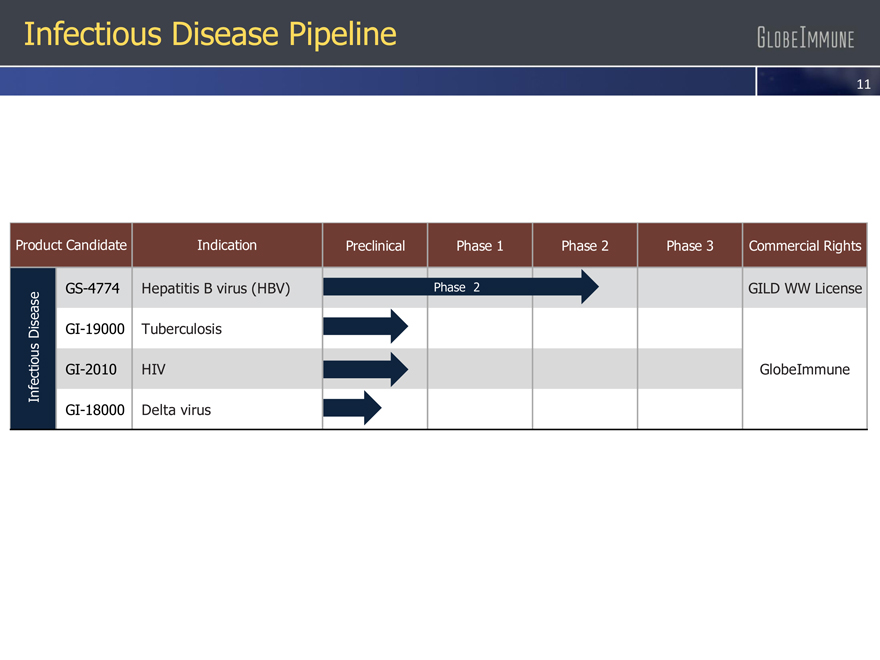
Infectious Disease Pipeline
Product Candidate Indication Preclinical Phase 1 Phase 2 Phase 3 Commercial Rights
GS-4774 Hepatitis B virus (HBV) Phase 2 GILD WW License
GI-19000 Tuberculosis
Infectious Disease GI-2010 HIV GlobeImmune
GI-18000 Delta virus
11
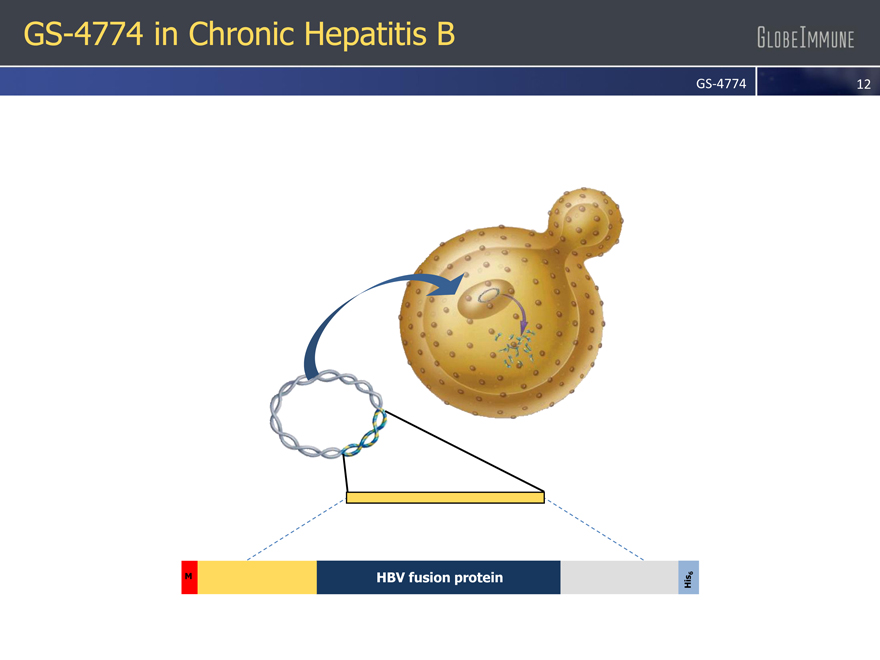
GS-4774 in Chronic Hepatitis B
GS-4774
HBV fusion protein
M
His 6
12
Hepatitis B Market
GS-4774
| • |
|
400 million patients chronically infected with HBV worldwide |
– Current treatment is lifelong treatment with antivirals
– Antivirals control viremia but long term cure rates are <8%
| • |
|
80% of acutely infected patients clear virus spontaneously |
– Clearance is mediated by cellular immunity
– Proof of concept in GlobeImmune hepatitis C program
| • |
|
Combine GS-4774 with oral antivirals |
– Improved cellular immune responses to increase cure rates
| • |
|
Phase 1 trial conducted in 60 healthy volunteers |
– Completed 1H2013
– Safe and well tolerated
– Cellular immune responses in 88% of subjects
13
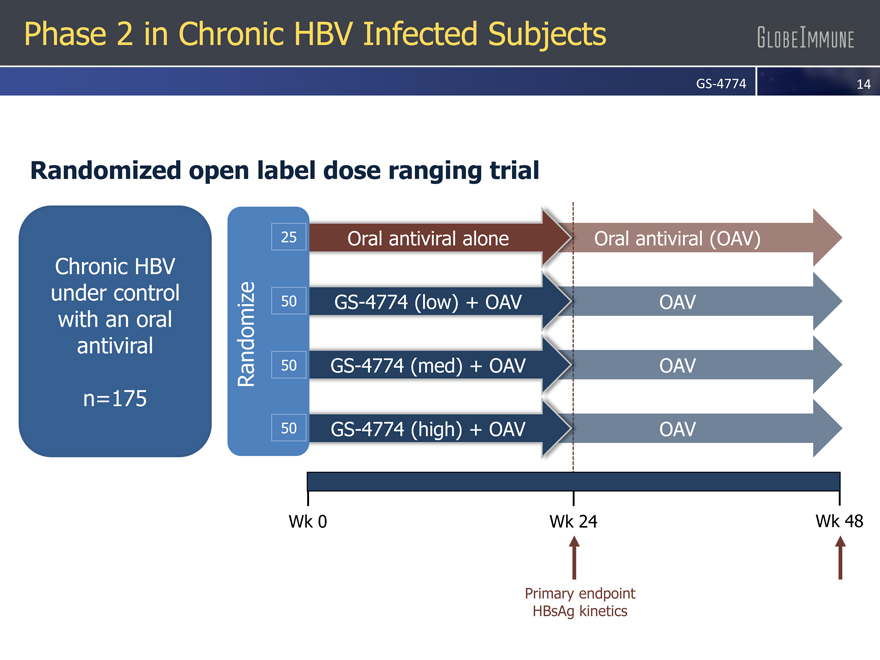
Phase 2 in Chronic HBV Infected Subjects
GS-4774
Randomized open label dose ranging trial
Chronic HBV under control with an oral antiviral
n=175
25 Oral antiviral alone Oral antiviral (OAV)
50 GS-4774 (low) + OAV OAV
50 GS-4774 (med) + OAV OAV
50 GS-4774 (high) + OAV OAV
Wk 0 Wk 24 Wk 48
Primary endpoint
HBsAg kinetics Randomize
14
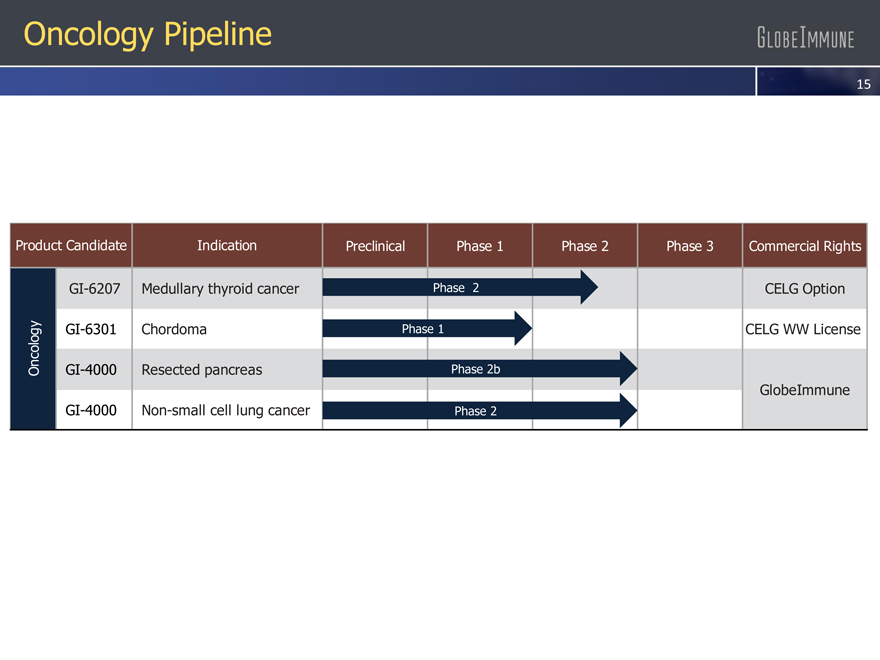
Oncology Pipeline
Medullary thyroid cancer Chordoma Resected pancreas Non-small cell lung cancer
Product Candidate
GI-6207
GI-6301
Indication
Oncology GI-4000
GI-4000
Preclinical Phase 1 Phase 2 Phase 3
Phase 2 Phase 1 Phase 2b Phase 2
Commercial Rights
CELG Option
CELG WW License
GlobeImmune
15
GI-6207 Targets Carcinoembryonic Antigen
GI-6207
| • |
|
CEA over-expressed in > 500,000 cases/year in US |
NSCLC, colorectal, breast, gastric, pancreas, medullary thyroid cancers
| • |
|
Phase 1 complete at NCI |
n=25
Monotherapy dose escalation trial
Advanced metastatic cancers
Stable disease in 20% of subjects
CEA
16

Medullary Thyroid Cancer
GI-6207
GI-6207
| • |
|
MTC represents 8% of all thyroid cancer cases annually in US |
| • |
|
Resection is the only opportunity for cure |
– In less symptomatic metastatic disease SoC is observation
| • |
|
25% and 10% survival at 5 and 10 years with metastases |
| • |
|
Vandetanib and cabozantinib approved for metastatic disease |
– Due to toxicity used only in very symptomatic patients
Vandetanib Cabozantinib
WARNING:
QT PROLONGATION, TORSADES DE POINTES, AND SUDDEN DEATH
See full prescribing information for full boxed warning.
WARNING:
PERFORATIONS AND FISTULAS, and HEMORRHAGE
See full prescribing information for full boxed warning.
17
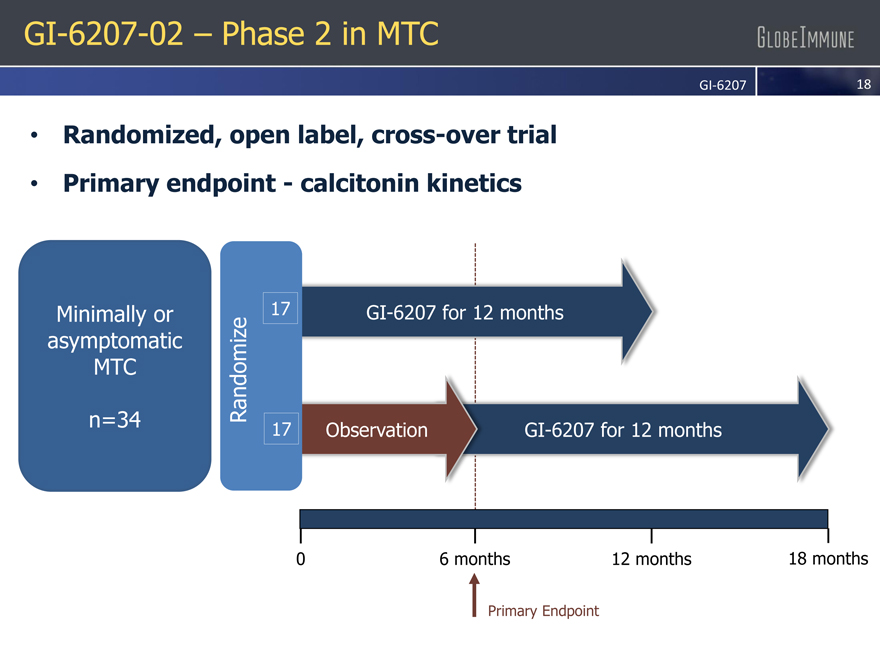
GI-6207-02 – Phase 2 in MTC
GI-6207
| • |
|
Randomized, open label, cross-over trial |
GI-6207 for 12 months
Observation GI-6207 for 12 months
| 6 |
|
months 12 months 18 months |
Primary Endpoint
| • |
|
Primary endpoint—calcitonin kinetics |
Minimally or 17 asymptomatic
MTC Randomize n=34 17
0
18
GI-6301 Targets Brachyury Expressing Cancers
GI-6301
Brachyury
Lung, breast, colon, bladder, kidney, ovary, pancreas, chordoma
Transcription factor involved in normal embryogenesis
Important driver of metastasis
Brachyury
19
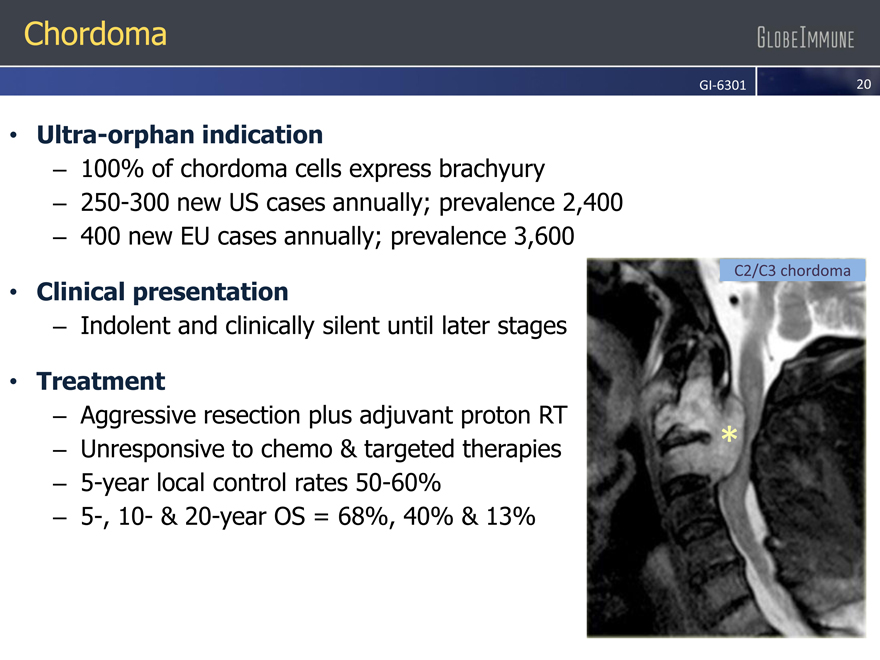
Chordoma
Ultra-orphan indication
– 100% of chordoma cells express brachyury
– 250-300 new US cases annually; prevalence 2,400
– 400 new EU cases annually; prevalence 3,600
Clinical presentation
– Indolent and clinically silent until later stages
Treatment
– Aggressive resection plus adjuvant proton RT
– Unresponsive to chemo & targeted therapies
– 5-year local control rates 50-60%
– 5-, 10- & 20-year OS = 68%, 40% & 13%
GI-6301 20
C2/C3 chordoma
20
GI-6301 Clinical Summary
GI-6301
NCI sponsored phase 1
34 subject, open label dose escalation trial
4 dose groups
Fully enrolled
Ten chordoma patients enrolled in Phase 1
Initial chordoma results presented at ASCO 2014
Phase 2 trial being designed
21
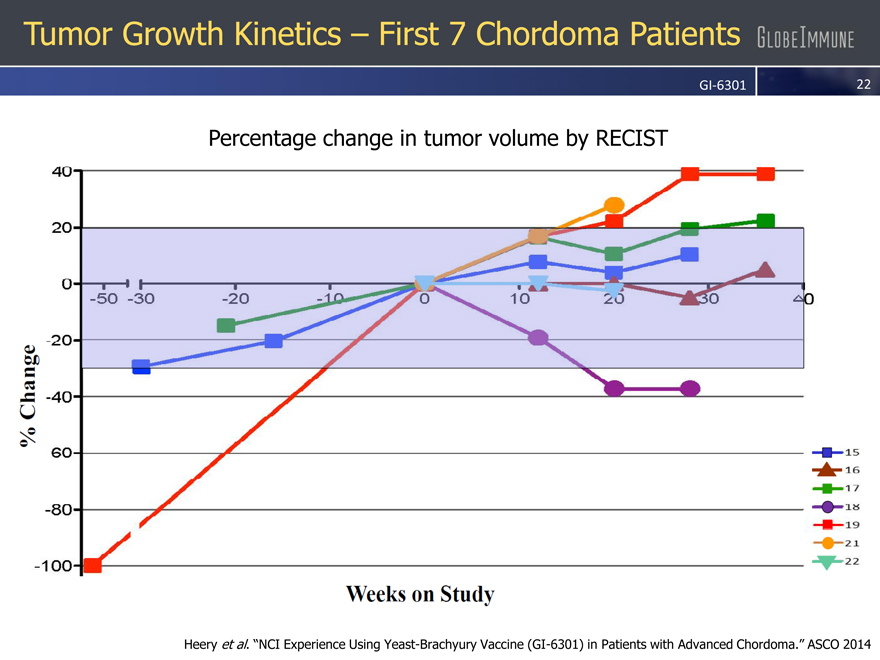
Tumor Growth Kinetics – First 7 Chordoma Patients
GI-6301
Percentage change in tumor volume by RECIST
Heery et al. “NCI Experience Using Yeast-Brachyury Vaccine (GI-6301) in Patients with Advanced Chordoma.” ASCO 2014
22
GI-4000 Targets Mutated Ras
GI-4000
Critical molecular target in multiple cancers
~200,000 Ras mutation+ cancer cases/year in U.S.
– Poor prognosis
– Less responsive to chemotherapy and targeted agents
176 patient Phase 2 in resected pancreas cancer
– 2.6 month improvement in OS in subjects with residual disease
– Companion diagnostic BDX-001 appears to predict response to GI-4000
BDX-001 signature seen in ~50% of tested samples
Predicts 16.6 month improvement in overall survival with GI-4000
Phase 2 in non-small cell lung cancer
– 24 patient case-controlled study at Memorial Sloan Kettering
– 43% reduction in the risk of mortality compared with case-matched controls
23
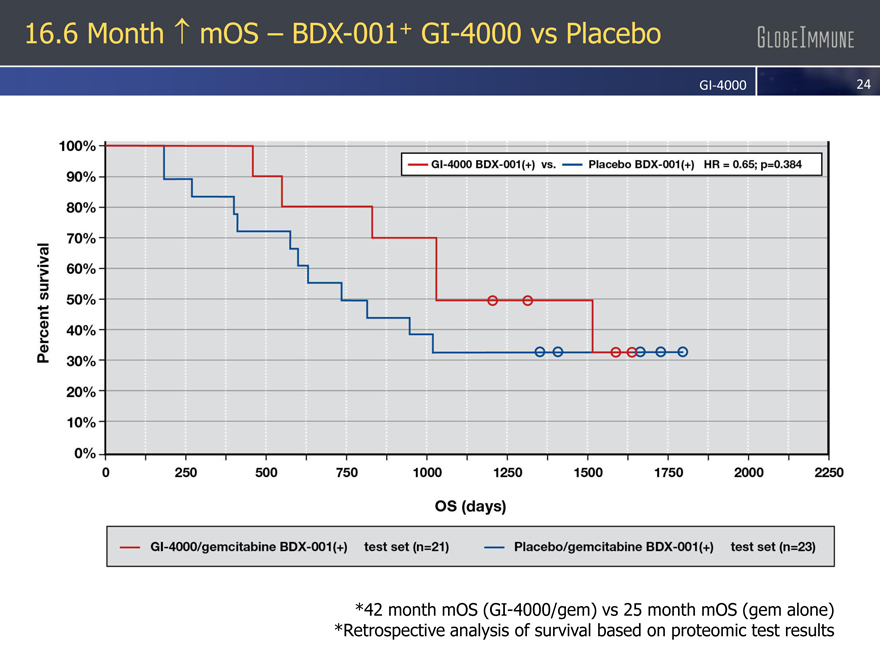
16.6 Month mOS – BDX-001+ GI-4000 vs Placebo
GI-4000
*42 month mOS (GI-4000/gem) vs 25 month mOS (gem alone)
*Retrospective analysis of survival based on proteomic test results
24
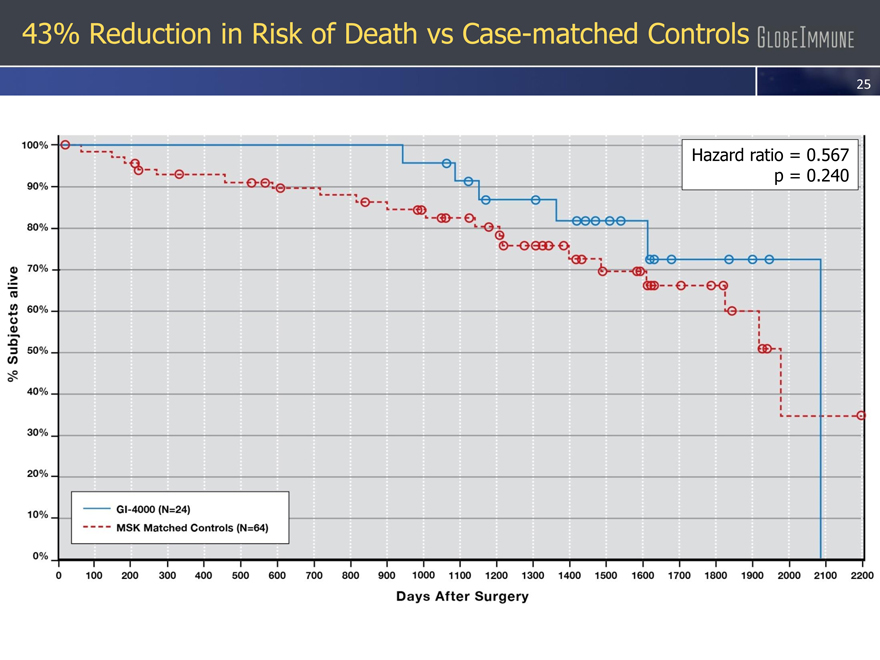
43% Reduction in Risk of Death vs Case-matched Controls
25

Key strategic collaborations
GlobeImmune

Gilead Alliance
GlobeImmune
GI-13020
27
Exclusive license to all HBV Tarmogens
– Signed in October 2011
GILEAD
Economics
– $10 million upfront
– Up to $135 million in potential clinical and regulatory milestones
– Up to $40 million in potential sales milestones
– Tiered royalties from high single digits to mid teens
Gilead funds clinical development and manufacturing
– Randomized phase 2 ongoing

Celgene Collaboration & Option Agreement
GlobeImmune
28
Exclusive option to oncology products
– Program-by-program basis
– GI-6301 licensed by Celgene in 2013
Celgene
Option agreement economics
– $40 million upfront
– Clinical, regulatory and commercial milestones for each product
– Celgene pays all future expenses for licensed products
– Royalties in the teens for products subject to option
Option structure
– GlobeImmune conducts early development through predefined endpoints
– Celgene has option to obtain an exclusive WW license
GI-6301 license
– $9M upfront; $145M in future milestones
– Tiered royalties high single to low double digits

Operations and financial
GlobeImmune

Financial Highlights
GlobeImmune
30
>$60 million partnership revenue to date from Gilead & Celgene
$119 million in 5
private rounds from premier VCs
$7.5 million convertible note, January 2014
– Converts to common stock at IPO
~$10 million in grants
$9.2 million in cash (3/31/14)
$15 million IPO provides funding through 2015
Summary Financials
GlobeImmune
31
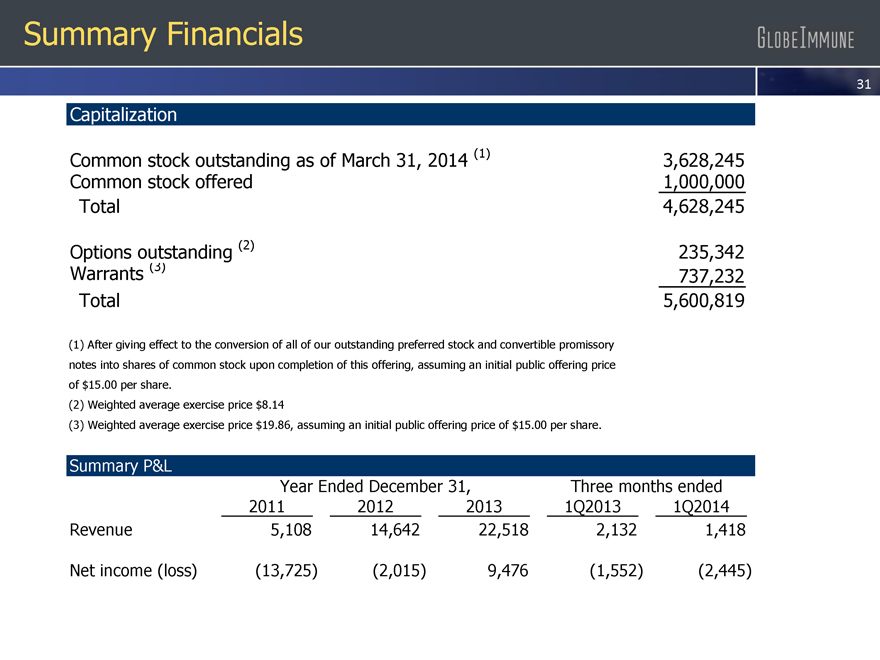
Capitalization
Common stock outstanding as of March 31,
2014 (1)
3,628,245
Common stock offered
1,000,000
Total
4,628,245
Options outstanding (2)
235,342
Warrants (3)
737,232
Total
5,600,819
(1) After giving effect to the conversion of all of our outstanding preferred stock and convertible promissory notes into shares of common stock upon completion of this offering,
assuming an initial public offering price of $15.00 per share.
(2) Weighted average exercise price $8.14
(3) Weighted average exercise price $19.86, assuming an initial public offering price of $15.00 per share.
Summary P&L
Year Ended December 31,
Three months ended
2011
2012
2013
1Q2013
1Q2014
Revenue
5,108
14,642
22,518
2,132
1,418
Net income (loss)
(13,725)
(2,015)
9,476(1,552)
(2,445)

Efficient and Scalable Manufacturing
GlobeImmune
32
Established manufacturing
– Scaleable efficient process
250L commercial scale projected
Low capital cost
Portable equipment
– Facilitates technology transfer
Intellectual Property
GlobeImmune
33
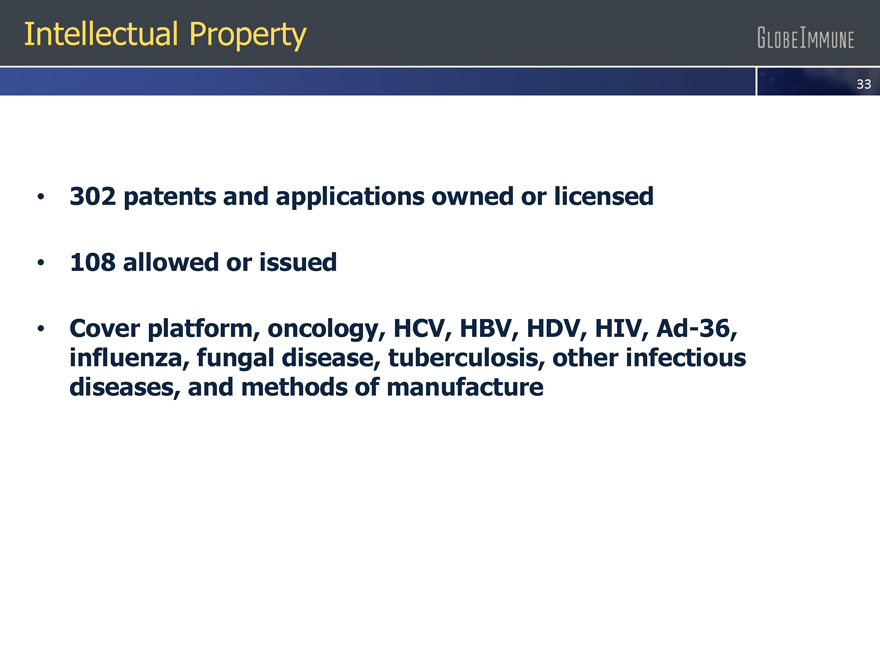
302 patents and applications owned or licensed
108 allowed or issued
Cover platform, oncology, HCV, HBV, HDV, HIV, Ad-36,
influenza, fungal disease, tuberculosis,
other infectious
diseases, and methods of manufacture
GBIM Value Drivers
GlobeImmune
34
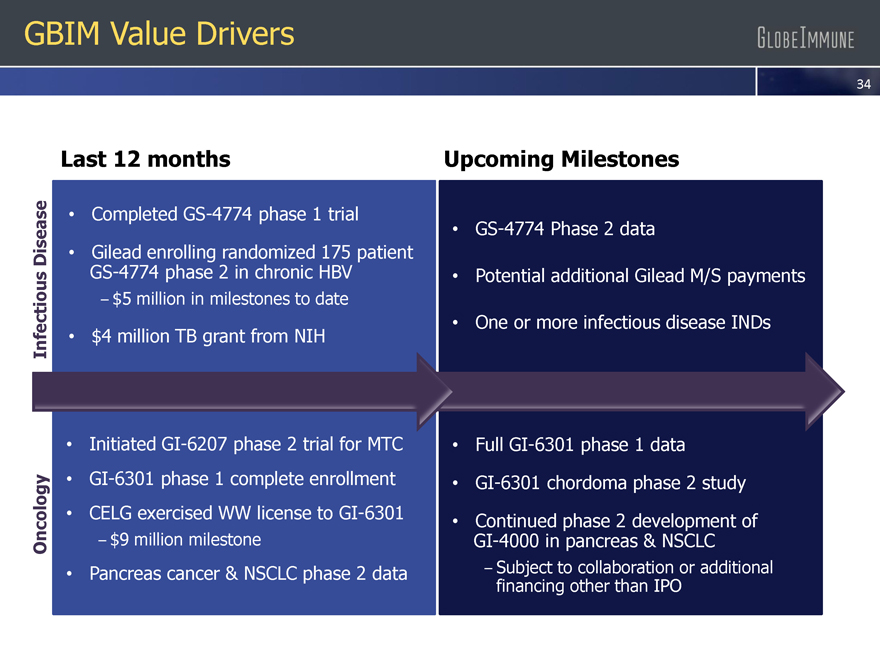
Last 12 months Upcoming Milestones
Infectious Disease
Completed GS-4774 phase 1 trial
Gilead enrolling randomized 175 patient GS-4774 phase 2 in chronic HBV
– $5 million in milestones to date
$4 million TB grant from NIH
GS-4774 Phase 2 data
Potential additional Gilead M/S payments
One or more infectious disease INDs
Oncology
Initiated GI-6207 phase 2 trial for MTC
GI-6301 phase 1 complete enrollment
CELG exercised WW license to GI-6301
– $9 million milestone
Pancreas cancer & NSCLC phase 2 data
Full GI-6301 phase 1 data
GI-6301 chordoma phase 2 study
Continued phase 2 development of
GI-4000 in pancreas & NSCLC
– Subject to collaboration or additional financing other than IPO

Investment Overview
GlobeImmune
35
Targeting major diseases including HBV and multiple cancers
Alliances with Gilead and Celgene
– >$60 million in partnership revenue to date
Proof of concept in multiple diseases
Experienced management team
Multiple potential near term value drivers