
SEIZE THE MOMENT for a brighter future CRESCENT BIOPHARMA STRATEGIC PARTNERSHIP & PIPELINE UPDATE DECEMBER 4, 2025 NASDAQ: CBIO

Disclaimers 2 Forward-Looking Statements Certain statements in this presentation, other than purely historical information, may constitute "forward-looking statements" within the meaning of the federal securities laws, including for purposes of the "safe harbor" provisions under the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, express or implied statements relating to the expectations, hopes, beliefs, intentions or strategies of Crescent Biopharma, Inc. ("Crescent") regarding the future of its pipeline and business including, without limitation: statements regarding the strategic partnership with Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech"), including the potential synergies and benefits of the partnership; the expected benefits or opportunities with respect to CR-001, CR-002 and CR-003, including the expected timelines of regulatory filings, including the acceptance thereof, and initial clinical data; the potential for CR-001 to replicate the cooperative pharmacology of ivonescimab in clinical trials; the potential for CR-001 to replicate preclinical demonstration of cooperative pharmacology and in vivo anti-tumor activity in clinical trials; the proposed Phase 1/2 trial design and indication selection for CR-001; the potential for CR-002 and CR-003 to act as single agents and in combination with CR-001; and Crescent’s anticipated cash runway. The words "opportunity," "potential," "milestones," "pipeline," "can," "goal," "strategy," "target," "anticipate," "achieve," "believe," "contemplate," "continue," "could," "estimate," "expect," "intends," "may," "plan," "possible," "project," "should," "will," "would" and similar expressions (including the negatives of these terms or variations of them) may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements are based on current expectations and beliefs concerning future developments and their potential effects. There can be no assurance that future developments affecting Crescent will be those that have been anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond Crescent’s control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: the expected benefits of, and opportunities related to, the strategic partnership between Crescent and Kelun-Biotech may not be realized by either party or may take longer to realize than anticipated; that the potential of CR-001 and/or CR-003 may change; that either party may fail to discover and develop any commercially successful product candidates through the strategic partnership; that such product candidates may not receive regulatory approval for the indications contemplated in this presentation and, if approved, such product candidates may not be commercially successful; Crescent’s limited operating history, including with respect to clinical trials; Crescent’s historical losses and any future ability to generate revenue; Crescent’s ability to raise capital to support its business plans; risks associated with clinical development and regulatory approval; risks related to Crescent’s intellectual property; Crescent’s reliance on third parties, including to help develop its product candidates and run its clinical trials, as well as to manufacture its product candidates; Crescent’s dependence on key personnel; Crescent’s estimates of market opportunity may prove to be inaccurate; significant disruptions of information technology systems or breaches of data security, litigation and regulatory risks; as well as those factors more fully described in Crescent’s most recent filings with the Securities and Exchange Commission (including its Quarterly Report on Form 10-Q), and Crescent’s other filings with the Securities and Exchange Commission. Should one or more of these risks or uncertainties materialize, or should any of Crescent’s assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements in this press release, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Crescent does not undertake or accept any duty to release publicly any updates or revisions to any forward-looking statements. This press release does not purport to summarize all of the conditions, risks and other attributes of an investment in Crescent. Industry and Market Data Market and industry data and forecasts used in and made orally during this presentation have been obtained from independent industry sources and from research reports prepared for other purposes as well as our own internal estimates and research. Although we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified the data obtained from these sources and we cannot assure you of the accuracy, adequacy, fairness or completeness of the data. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and uncertainties as the other forward-looking statements in this presentation. Statements as to our market and competitive position data are based on market data currently available to us, as well as management’s internal analyses and assumptions regarding the Company, which involve certain assumptions and estimates. These internal analyses have not been verified by any independent sources and there can be no assurance that the assumptions or estimates are accurate. While we are not aware of any misstatements regarding our industry data presented herein, our estimates involve risks and uncertainties and are subject to change based on various factors. As a result, we cannot guarantee the accuracy or completeness of such information contained in this presentation.

Opening Remarks Joshua Brumm CEO Overview of Kelun-Biotech Partnership Jonathan McNeill, M.D. President & COO Clinical Development Plan for CR-001, PD-1 x VEGF Bispecific Antibody Ellie Im, M.D. Chief Medical Officer ADC Programs: CR-002 and CR-003 (SKB105) Jan Pinkas, Ph.D. Chief Scientific Officer Q&A 3 Program

Opening Remarks Joshua Brumm CEO Overview of Kelun-Biotech Partnership Jonathan McNeill, M.D. President & COO Clinical Development Plan for CR-001, PD-1 x VEGF Bispecific Antibody Ellie Im, M.D. Chief Medical Officer ADC Programs: CR-002 and CR-003 (SKB105) Jan Pinkas, Ph.D. Chief Scientific Officer Q&A 4 Program

5 DELIVERING THE NEXT WAVE OF TRANSFORMATIVE THERAPIES to bring a brighter future for people living with cancer MISSION
Our bold vision is to build a world-leading oncology company 6 PD-1 x VEGF cooperativity Novel ADCs Synergistic combinations Crescent is advancing the next wave of innovative I/O and ADC therapies to transform cancer care
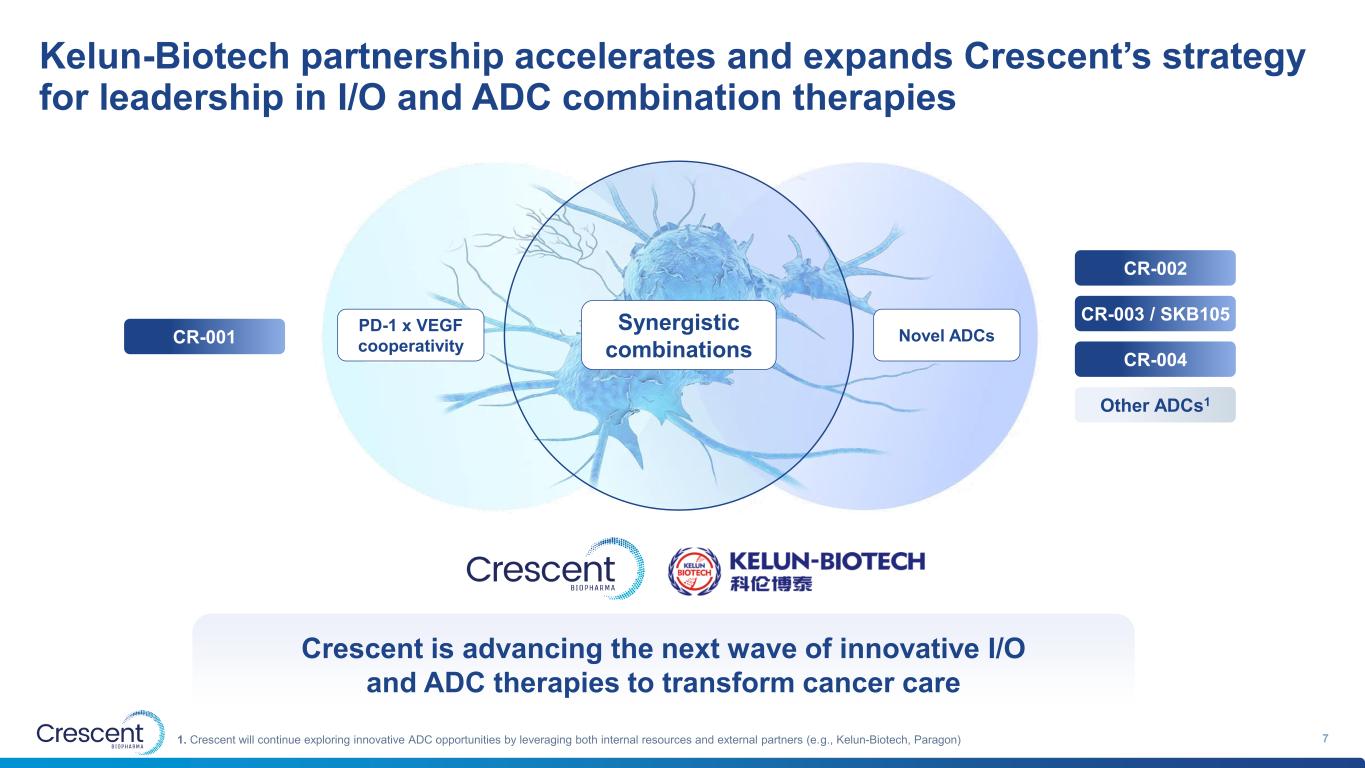
Kelun-Biotech partnership accelerates and expands Crescent’s strategy for leadership in I/O and ADC combination therapies 71. Crescent will continue exploring innovative ADC opportunities by leveraging both internal resources and external partners (e.g., Kelun-Biotech, Paragon) PD-1 x VEGF cooperativity Novel ADCs Synergistic combinations CR-001 CR-002 CR-003 / SKB105 CR-004 Other ADCs1 Crescent is advancing the next wave of innovative I/O and ADC therapies to transform cancer care
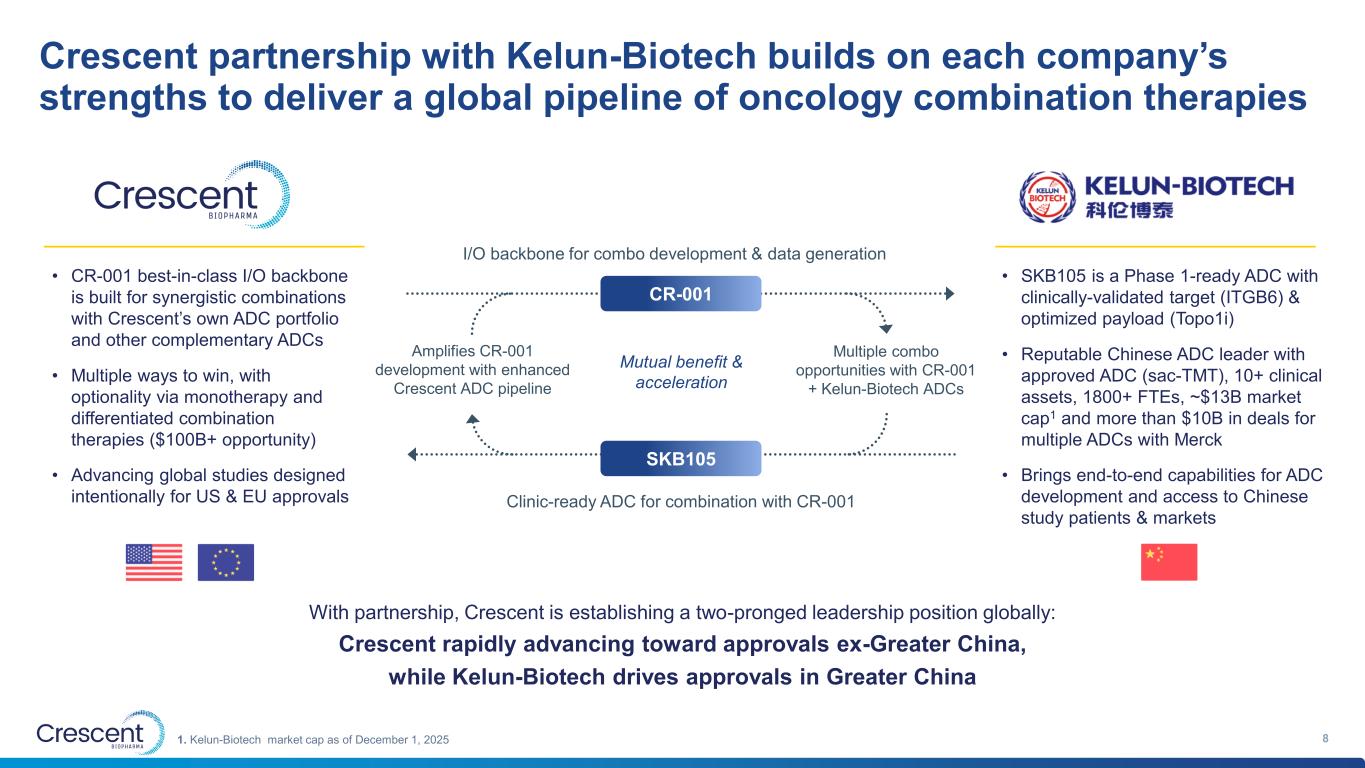
Crescent partnership with Kelun-Biotech builds on each company’s strengths to deliver a global pipeline of oncology combination therapies 8 • CR-001 best-in-class I/O backbone is built for synergistic combinations with Crescent’s own ADC portfolio and other complementary ADCs • Multiple ways to win, with optionality via monotherapy and differentiated combination therapies ($100B+ opportunity) • Advancing global studies designed intentionally for US & EU approvals • SKB105 is a Phase 1-ready ADC with clinically-validated target (ITGB6) & optimized payload (Topo1i) • Reputable Chinese ADC leader with approved ADC (sac-TMT), 10+ clinical assets, 1800+ FTEs, ~$13B market cap1 and more than $10B in deals for multiple ADCs with Merck • Brings end-to-end capabilities for ADC development and access to Chinese study patients & markets With partnership, Crescent is establishing a two-pronged leadership position globally: Crescent rapidly advancing toward approvals ex-Greater China, while Kelun-Biotech drives approvals in Greater China CR-001 I/O backbone for combo development & data generation SKB105 Clinic-ready ADC for combination with CR-001 Multiple combo opportunities with CR-001 + Kelun-Biotech ADCs Amplifies CR-001 development with enhanced Crescent ADC pipeline Mutual benefit & acceleration 1. Kelun-Biotech market cap as of December 1, 2025

Transformative Kelun-Biotech partnership accelerates & expands Crescent’s strategy to deliver next-generation therapies for solid tumors 9 Accelerates & expands CR-001 development Partnership enables parallel generation of clinical data in US/EU (Crescent) and Greater China (Kelun-Biotech) Enhances clinical-stage pipeline Expands Crescent's 2026 clinical stage pipeline to three programs with addition of CR-003 (SKB105), further broadening pipeline of ADCs directed against validated targets (PD-L1, ITGB6) Speeds time to CR-001 + ADC combo data Rapidly generates combination data for CR-001 + ADCs Expands scope of CR-001 + ADC combo studies Sets the stage for future ADC combinations involving CR-001 Enables generation of multiple clinical data readouts by end of 2027 across I/O, ADCs and combination therapies
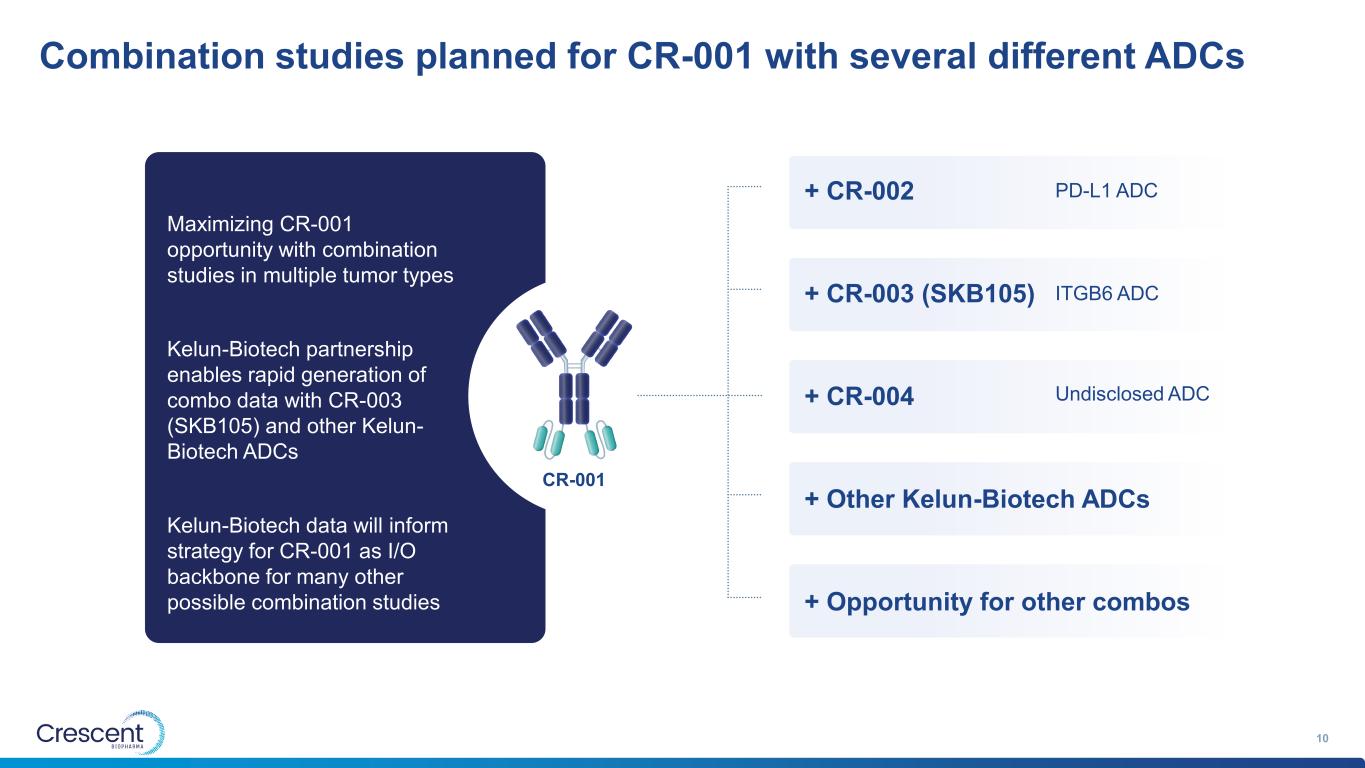
Combination studies planned for CR-001 with several different ADCs 10 Maximizing CR-001 opportunity with combination studies in multiple tumor types Kelun-Biotech partnership enables rapid generation of combo data with CR-003 (SKB105) and other Kelun- Biotech ADCs Kelun-Biotech data will inform strategy for CR-001 as I/O backbone for many other possible combination studies + CR-002 + CR-003 (SKB105) + CR-004 + Other Kelun-Biotech ADCs + Opportunity for other combos PD-L1 ADC ITGB6 ADC CR-001 Undisclosed ADC
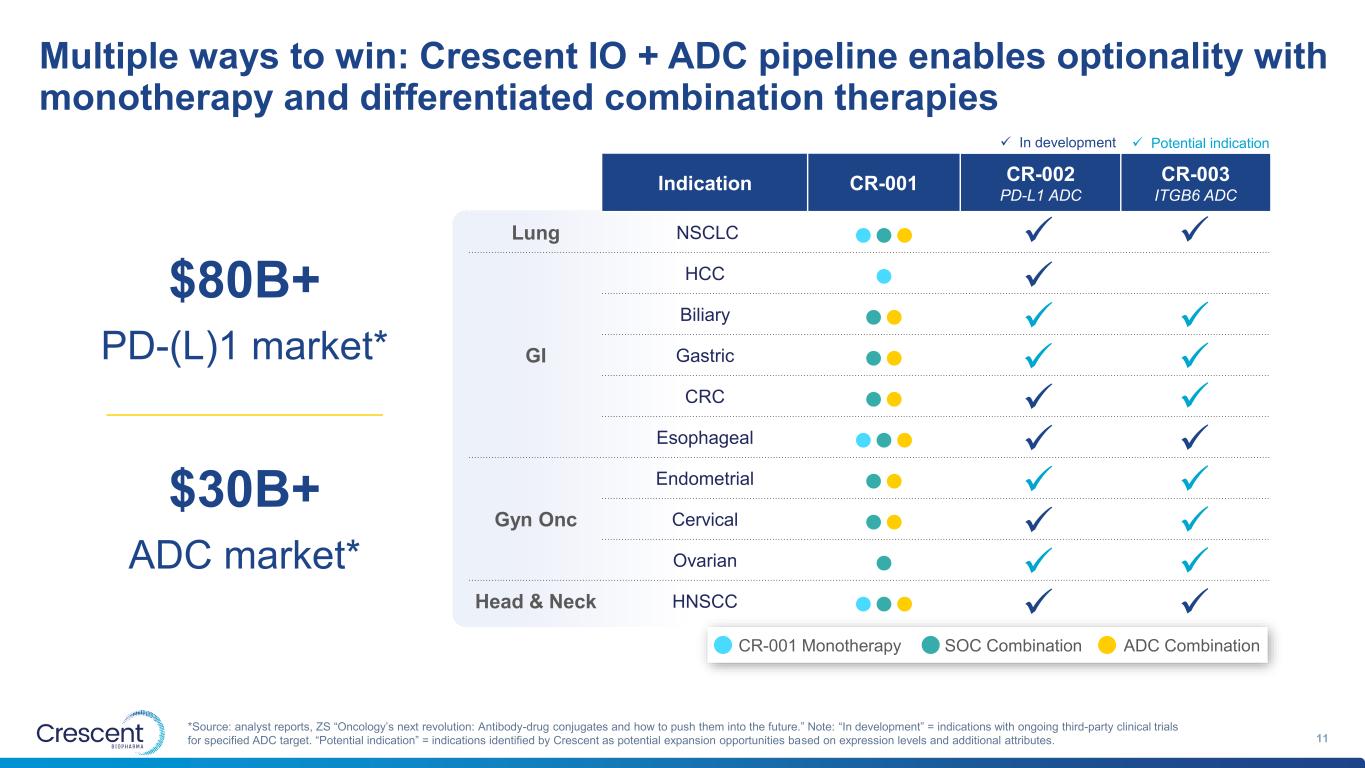
Multiple ways to win: Crescent IO + ADC pipeline enables optionality with monotherapy and differentiated combination therapies 11 *Source: analyst reports, ZS “Oncology’s next revolution: Antibody-drug conjugates and how to push them into the future.” Note: “In development” = indications with ongoing third-party clinical trials for specified ADC target. “Potential indication” = indications identified by Crescent as potential expansion opportunities based on expression levels and additional attributes. Indication CR-001 CR-002 CR-003 PD-L1 ADC ITGB6 ADC Lung NSCLC ●●● GI HCC ● Biliary ●● Gastric ●● CRC ●● Esophageal ●●● Gyn Onc Endometrial ●● Cervical ●● Ovarian ● Head & Neck HNSCC ●●● ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ $30B+ ADC market* $80B+ PD-(L)1 market* ✓ ✓ In development ✓ Potential indication CR-001 Monotherapy SOC Combination ADC Combination ✓
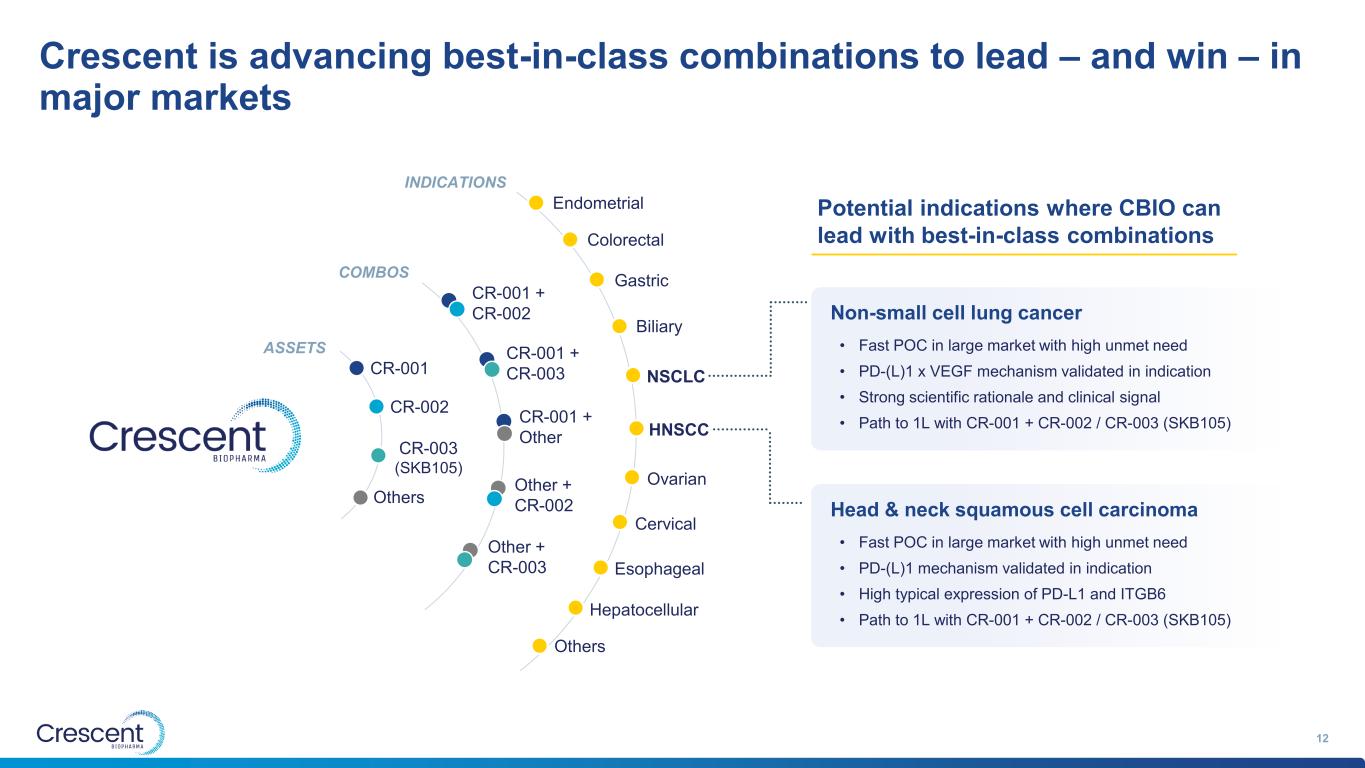
Crescent is advancing best-in-class combinations to lead – and win – in major markets 12 CR-001 CR-002 CR-003 (SKB105) Others ASSETS COMBOS INDICATIONS CR-001 + CR-002 CR-001 + CR-003 CR-001 + Other Other + CR-002 Other + CR-003 NSCLC HNSCC Biliary Gastric Ovarian Cervical Esophageal Colorectal Endometrial Hepatocellular Others • Fast POC in large market with high unmet need • PD-(L)1 x VEGF mechanism validated in indication • Strong scientific rationale and clinical signal • Path to 1L with CR-001 + CR-002 / CR-003 (SKB105) Non-small cell lung cancer • Fast POC in large market with high unmet need • PD-(L)1 mechanism validated in indication • High typical expression of PD-L1 and ITGB6 • Path to 1L with CR-001 + CR-002 / CR-003 (SKB105) Head & neck squamous cell carcinoma Potential indications where CBIO can lead with best-in-class combinations
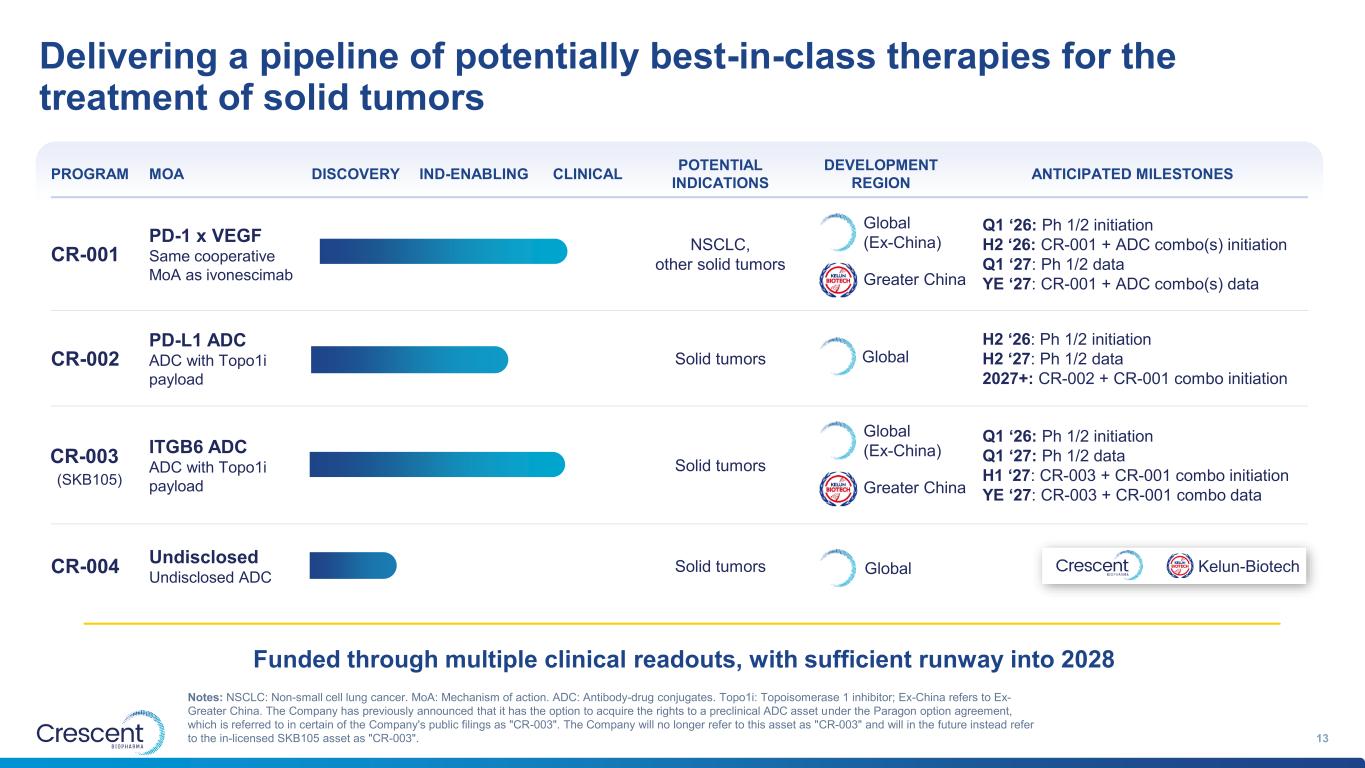
Delivering a pipeline of potentially best-in-class therapies for the treatment of solid tumors 13 PROGRAM MOA DISCOVERY IND-ENABLING CLINICAL POTENTIAL INDICATIONS DEVELOPMENT REGION ANTICIPATED MILESTONES CR-001 PD-1 x VEGF Same cooperative MoA as ivonescimab NSCLC, other solid tumors Q1 ‘26: Ph 1/2 initiation H2 ‘26: CR-001 + ADC combo(s) initiation Q1 ‘27: Ph 1/2 data YE ‘27: CR-001 + ADC combo(s) data CR-002 PD-L1 ADC ADC with Topo1i payload Solid tumors H2 ‘26: Ph 1/2 initiation H2 ‘27: Ph 1/2 data 2027+: CR-002 + CR-001 combo initiation ITGB6 ADC ADC with Topo1i payload Solid tumors Q1 ‘26: Ph 1/2 initiation Q1 ‘27: Ph 1/2 data H1 ‘27: CR-003 + CR-001 combo initiation YE ‘27: CR-003 + CR-001 combo data CR-004 Undisclosed Undisclosed ADC Solid tumors Global (Ex-China) Greater China Global Notes: NSCLC: Non-small cell lung cancer. MoA: Mechanism of action. ADC: Antibody-drug conjugates. Topo1i: Topoisomerase 1 inhibitor; Ex-China refers to Ex- Greater China. The Company has previously announced that it has the option to acquire the rights to a preclinical ADC asset under the Paragon option agreement, which is referred to in certain of the Company's public filings as "CR-003". The Company will no longer refer to this asset as "CR-003" and will in the future instead refer to the in-licensed SKB105 asset as "CR-003". Funded through multiple clinical readouts, with sufficient runway into 2028 Kelun-Biotech (SKB105) CR-003 Global (Ex-China) Greater China Global
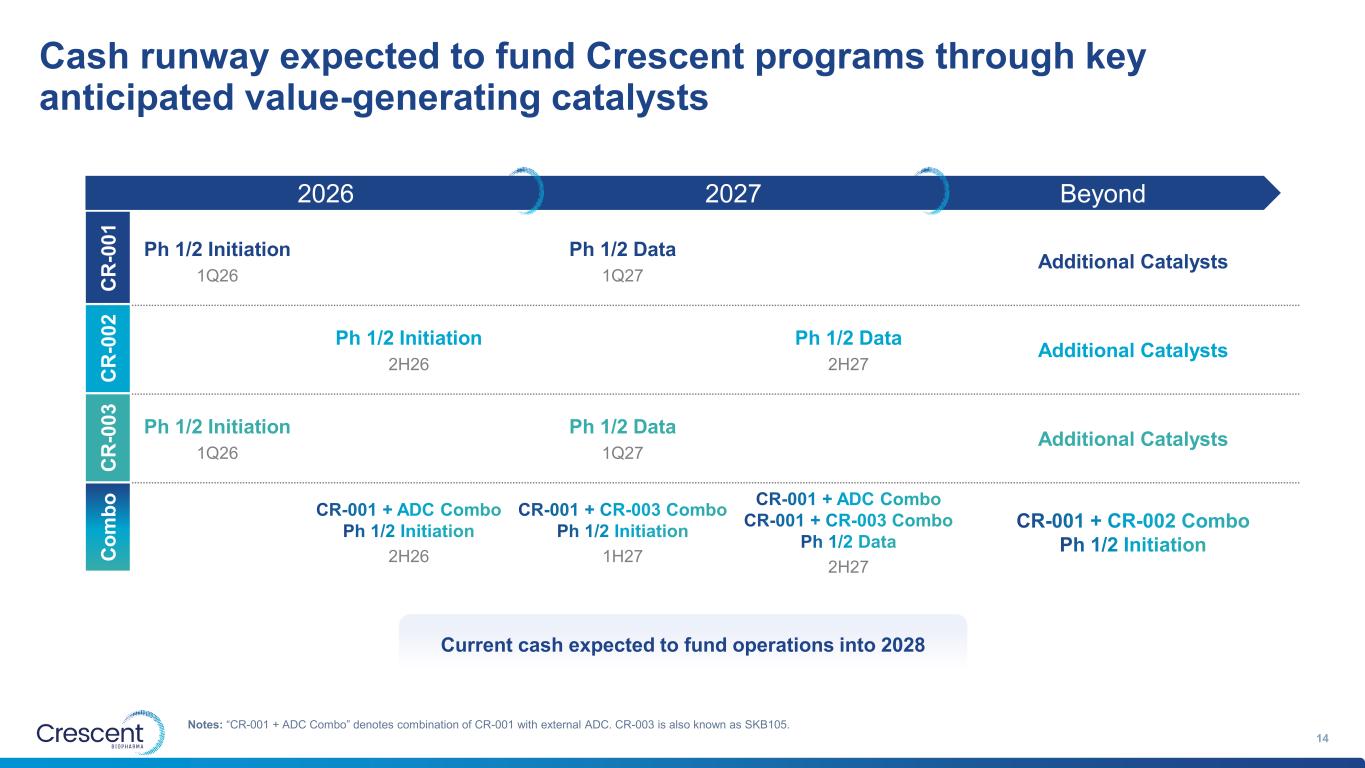
Current cash expected to fund operations into 2028 2027 Cash runway expected to fund Crescent programs through key anticipated value-generating catalysts 14 Notes: “CR-001 + ADC Combo” denotes combination of CR-001 with external ADC. CR-003 is also known as SKB105. 2026 Beyond Ph 1/2 Initiation 1Q26 Ph 1/2 Data 1Q27 Additional Catalysts Ph 1/2 Initiation 2H26 Ph 1/2 Data 2H27 Additional Catalysts Ph 1/2 Initiation 1Q26 Ph 1/2 Data 1Q27 Additional Catalysts CR-001 + ADC Combo Ph 1/2 Initiation 2H26 CR-001 + CR-003 Combo Ph 1/2 Initiation 1H27 CR-001 + ADC Combo CR-001 + CR-003 Combo Ph 1/2 Data 2H27 CR-001 + CR-002 Combo Ph 1/2 Initiation C R -0 0 1 C R -0 0 2 C R -0 0 3 C o m b o

Opening Remarks Joshua Brumm CEO Overview of Kelun-Biotech Partnership Jonathan McNeill, M.D. President & COO Clinical Development Plan for CR-001, PD-1 x VEGF Bispecific Antibody Ellie Im, M.D. Chief Medical Officer ADC Programs: CR-002 and CR-003 (SKB105) Jan Pinkas, Ph.D. Chief Scientific Officer Q&A 15 Program
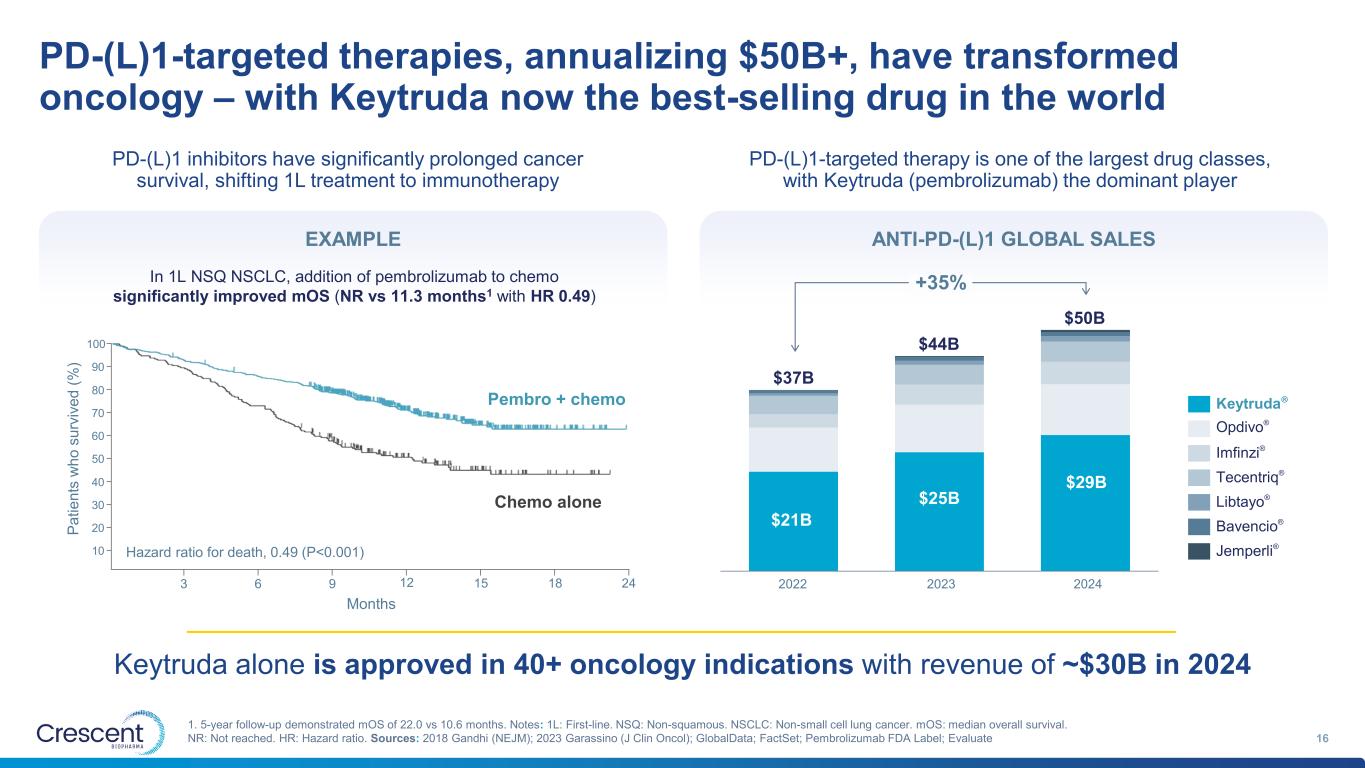
PD-(L)1-targeted therapies, annualizing $50B+, have transformed oncology – with Keytruda now the best-selling drug in the world 16 1. 5-year follow-up demonstrated mOS of 22.0 vs 10.6 months. Notes: 1L: First-line. NSQ: Non-squamous. NSCLC: Non-small cell lung cancer. mOS: median overall survival. NR: Not reached. HR: Hazard ratio. Sources: 2018 Gandhi (NEJM); 2023 Garassino (J Clin Oncol); GlobalData; FactSet; Pembrolizumab FDA Label; Evaluate 2022 2023 2024 $37B $44B $50B Keytruda® Opdivo® Imfinzi® Tecentriq® Libtayo® Bavencio® Jemperli® $21B $25B $29B PD-(L)1 inhibitors have significantly prolonged cancer survival, shifting 1L treatment to immunotherapy Pembro + chemo Chemo alone 3 6 9 12 15 18 24 100 90 80 70 60 50 40 30 20 10 Hazard ratio for death, 0.49 (P<0.001) Months P a ti e n ts w h o s u rv iv e d ( % ) PD-(L)1-targeted therapy is one of the largest drug classes, with Keytruda (pembrolizumab) the dominant player Keytruda alone is approved in 40+ oncology indications with revenue of ~$30B in 2024 ANTI-PD-(L)1 GLOBAL SALES In 1L NSQ NSCLC, addition of pembrolizumab to chemo significantly improved mOS (NR vs 11.3 months1 with HR 0.49) EXAMPLE +35%
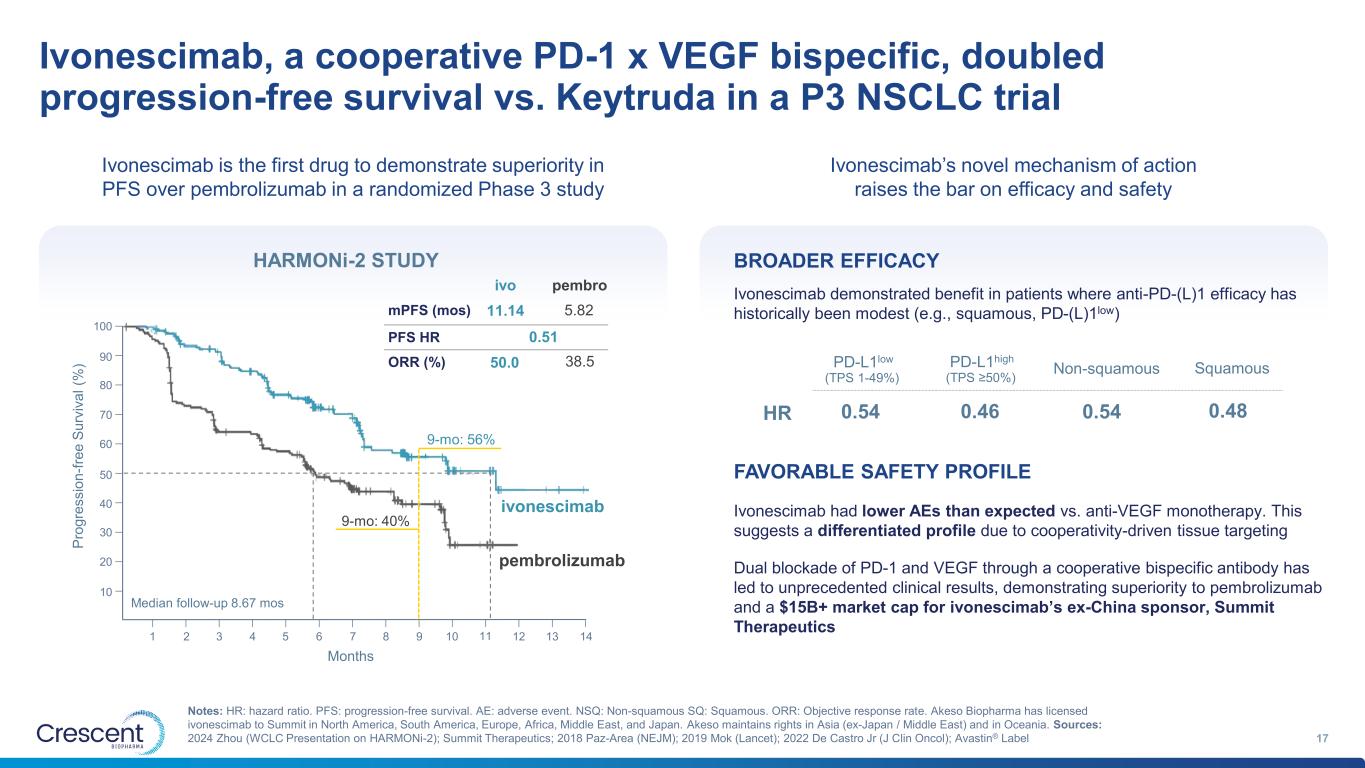
Ivonescimab, a cooperative PD-1 x VEGF bispecific, doubled progression-free survival vs. Keytruda in a P3 NSCLC trial 17 Notes: HR: hazard ratio. PFS: progression-free survival. AE: adverse event. NSQ: Non-squamous SQ: Squamous. ORR: Objective response rate. Akeso Biopharma has licensed ivonescimab to Summit in North America, South America, Europe, Africa, Middle East, and Japan. Akeso maintains rights in Asia (ex-Japan / Middle East) and in Oceania. Sources: 2024 Zhou (WCLC Presentation on HARMONi-2); Summit Therapeutics; 2018 Paz-Area (NEJM); 2019 Mok (Lancet); 2022 De Castro Jr (J Clin Oncol); Avastin® Label 2 4 7 9 12 14 100 90 80 70 60 50 40 30 20 10 Months P ro g re s s io n -f re e S u rv iv a l (% ) 1 3 5 6 8 10 1311 ivonescimab pembrolizumab 9-mo: 56% 9-mo: 40% Median follow-up 8.67 mos mPFS (mos) PFS HR ORR (%) ivo pembro 11.14 0.51 50.0 38.5 5.82 HARMONi-2 STUDY Dual blockade of PD-1 and VEGF through a cooperative bispecific antibody has led to unprecedented clinical results, demonstrating superiority to pembrolizumab and a $15B+ market cap for ivonescimab’s ex-China sponsor, Summit Therapeutics BROADER EFFICACY FAVORABLE SAFETY PROFILE Ivonescimab demonstrated benefit in patients where anti-PD-(L)1 efficacy has historically been modest (e.g., squamous, PD-(L)1low) Ivonescimab had lower AEs than expected vs. anti-VEGF monotherapy. This suggests a differentiated profile due to cooperativity-driven tissue targeting HR PD-L1low 0.54 (TPS 1-49%) PD-L1high (TPS ≥50%) Non-squamous Squamous 0.46 0.54 0.48 Ivonescimab is the first drug to demonstrate superiority in PFS over pembrolizumab in a randomized Phase 3 study Ivonescimab’s novel mechanism of action raises the bar on efficacy and safety
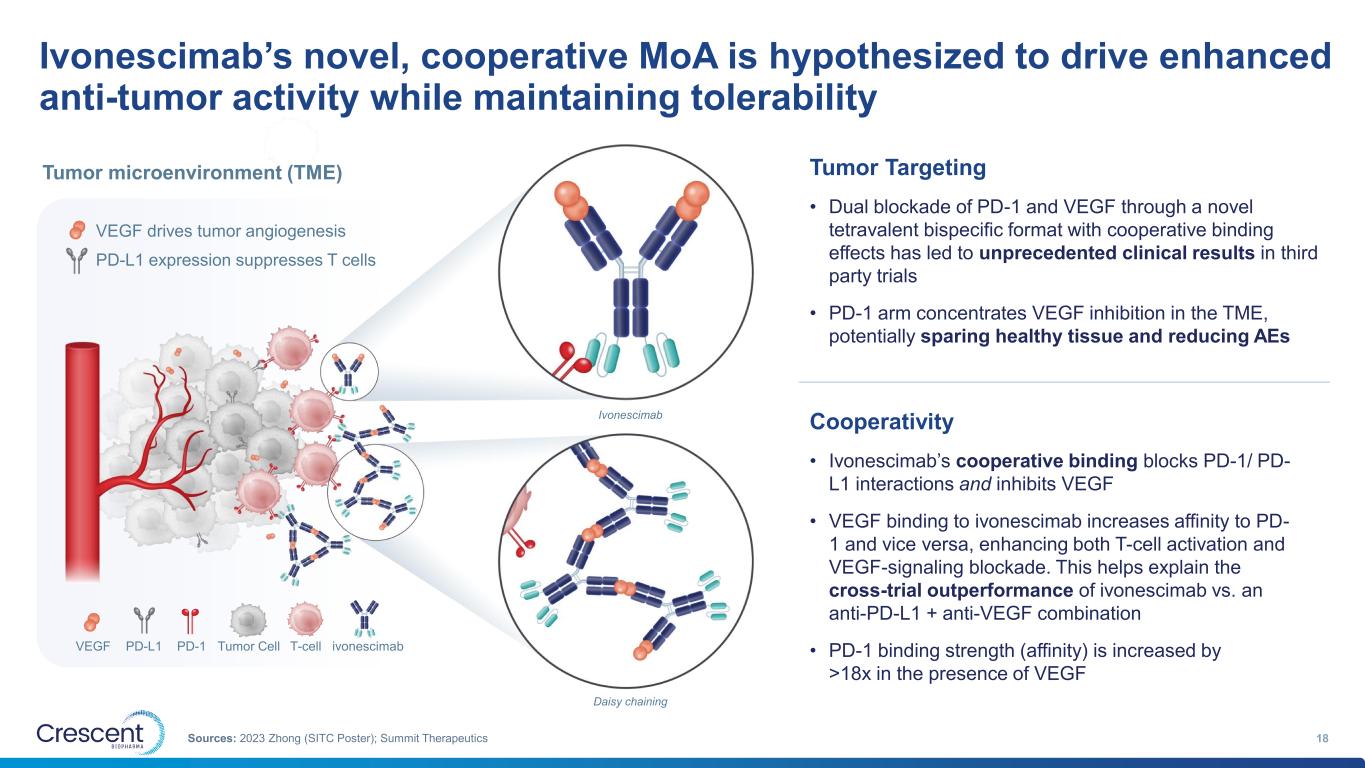
Ivonescimab’s novel, cooperative MoA is hypothesized to drive enhanced anti-tumor activity while maintaining tolerability 18Sources: 2023 Zhong (SITC Poster); Summit Therapeutics Cooperativity • Ivonescimab’s cooperative binding blocks PD-1/ PD- L1 interactions and inhibits VEGF • VEGF binding to ivonescimab increases affinity to PD- 1 and vice versa, enhancing both T-cell activation and VEGF-signaling blockade. This helps explain the cross-trial outperformance of ivonescimab vs. an anti-PD-L1 + anti-VEGF combination • PD-1 binding strength (affinity) is increased by >18x in the presence of VEGF Tumor Targeting • Dual blockade of PD-1 and VEGF through a novel tetravalent bispecific format with cooperative binding effects has led to unprecedented clinical results in third party trials • PD-1 arm concentrates VEGF inhibition in the TME, potentially sparing healthy tissue and reducing AEs VEGF drives tumor angiogenesis PD-L1 expression suppresses T cells Tumor microenvironment (TME) VEGF PD-L1 PD-1 Tumor Cell T-cell ivonescimab Ivonescimab Daisy chaining
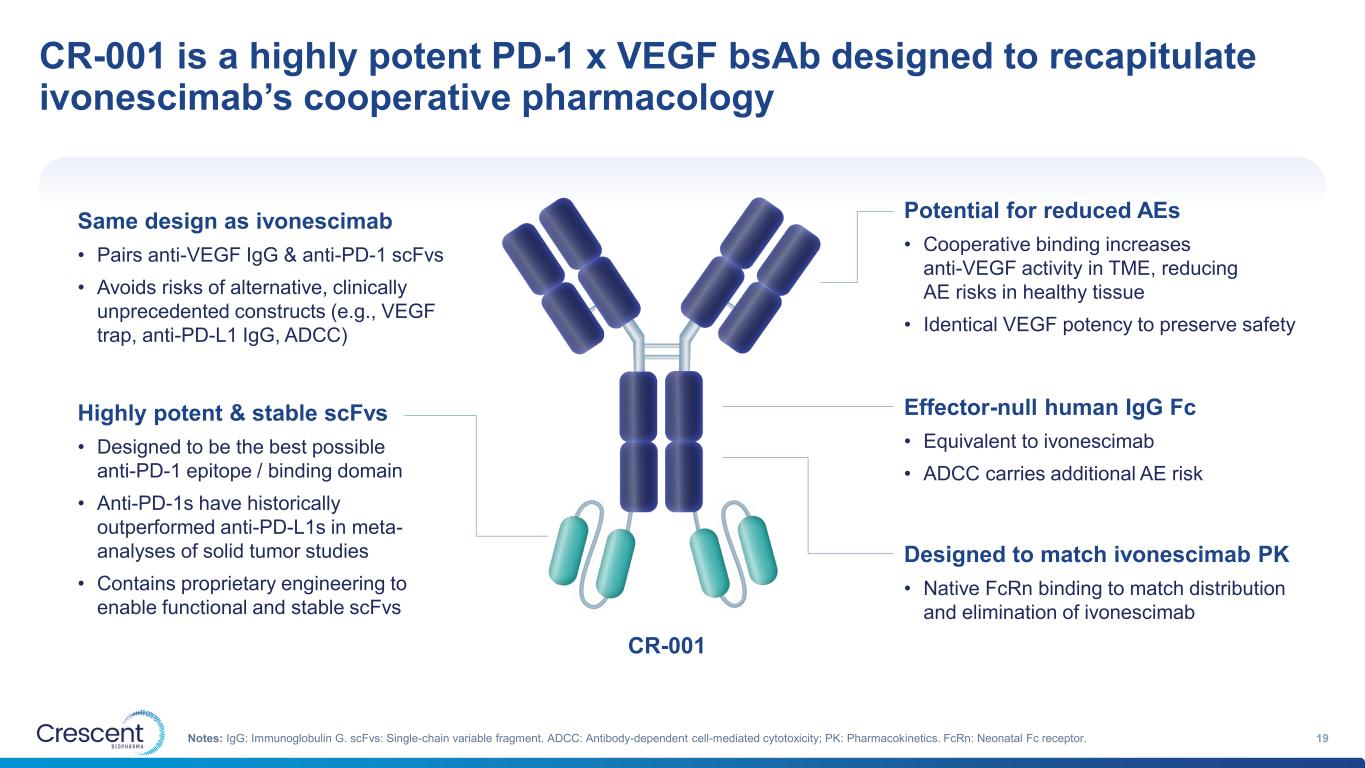
CR-001 is a highly potent PD-1 x VEGF bsAb designed to recapitulate ivonescimab’s cooperative pharmacology 19Notes: IgG: Immunoglobulin G. scFvs: Single-chain variable fragment. ADCC: Antibody-dependent cell-mediated cytotoxicity; PK: Pharmacokinetics. FcRn: Neonatal Fc receptor. Same design as ivonescimab • Pairs anti-VEGF IgG & anti-PD-1 scFvs • Avoids risks of alternative, clinically unprecedented constructs (e.g., VEGF trap, anti-PD-L1 IgG, ADCC) Highly potent & stable scFvs • Designed to be the best possible anti-PD-1 epitope / binding domain • Anti-PD-1s have historically outperformed anti-PD-L1s in meta- analyses of solid tumor studies • Contains proprietary engineering to enable functional and stable scFvs Potential for reduced AEs • Cooperative binding increases anti-VEGF activity in TME, reducing AE risks in healthy tissue • Identical VEGF potency to preserve safety Effector-null human IgG Fc • Equivalent to ivonescimab • ADCC carries additional AE risk Designed to match ivonescimab PK • Native FcRn binding to match distribution and elimination of ivonescimab CR-001
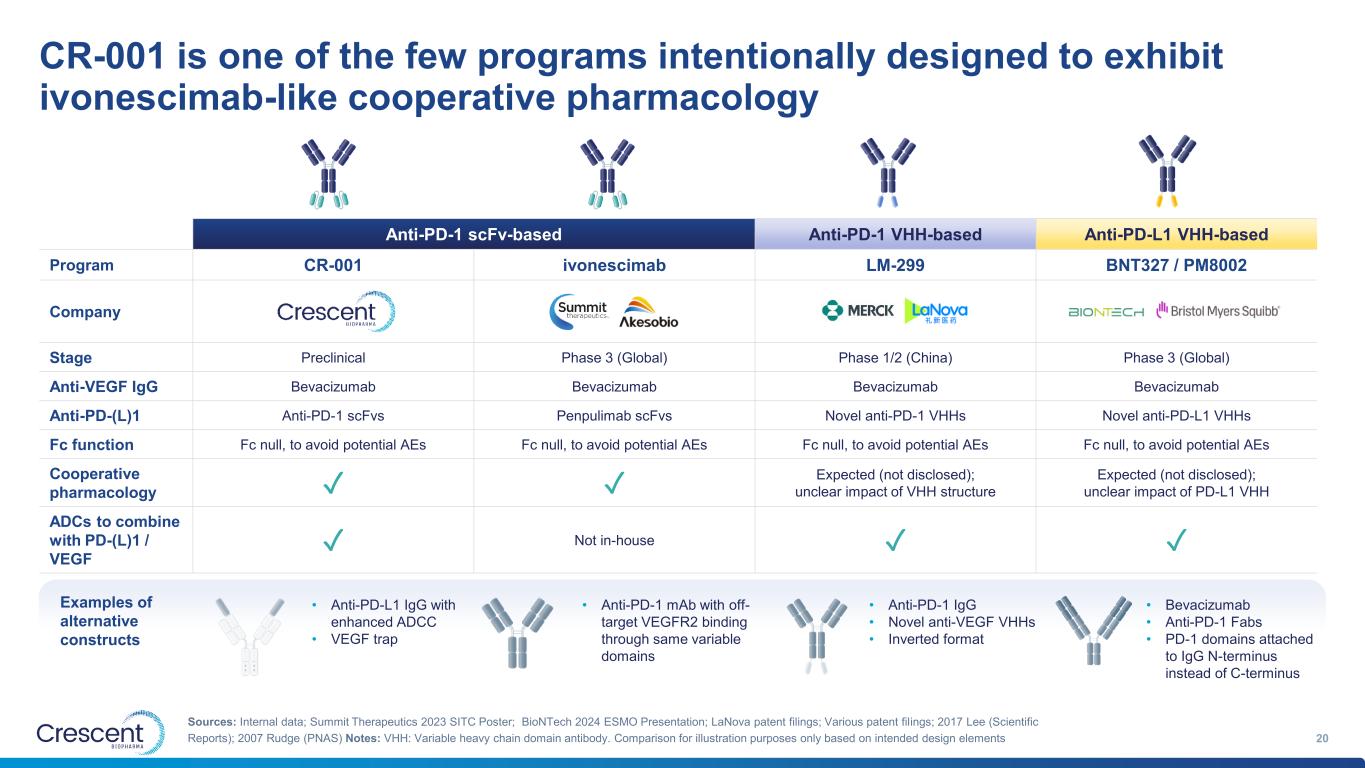
CR-001 is one of the few programs intentionally designed to exhibit ivonescimab-like cooperative pharmacology 20 Sources: Internal data; Summit Therapeutics 2023 SITC Poster; BioNTech 2024 ESMO Presentation; LaNova patent filings; Various patent filings; 2017 Lee (Scientific Reports); 2007 Rudge (PNAS) Notes: VHH: Variable heavy chain domain antibody. Comparison for illustration purposes only based on intended design elements • Anti-PD-L1 IgG with enhanced ADCC • VEGF trap Anti-PD-1 scFv-based Anti-PD-1 VHH-based Anti-PD-L1 VHH-based Program CR-001 ivonescimab LM-299 BNT327 / PM8002 Company Stage Preclinical Phase 3 (Global) Phase 1/2 (China) Phase 3 (Global) Anti-VEGF IgG Bevacizumab Bevacizumab Bevacizumab Bevacizumab Anti-PD-(L)1 Anti-PD-1 scFvs Penpulimab scFvs Novel anti-PD-1 VHHs Novel anti-PD-L1 VHHs Fc function Fc null, to avoid potential AEs Fc null, to avoid potential AEs Fc null, to avoid potential AEs Fc null, to avoid potential AEs Cooperative pharmacology ✓ ✓ Expected (not disclosed); unclear impact of VHH structure Expected (not disclosed); unclear impact of PD-L1 VHH ADCs to combine with PD-(L)1 / VEGF ✓ Not in-house ✓ ✓ • Anti-PD-1 mAb with off- target VEGFR2 binding through same variable domains • Anti-PD-1 IgG • Novel anti-VEGF VHHs • Inverted format • Bevacizumab • Anti-PD-1 Fabs • PD-1 domains attached to IgG N-terminus instead of C-terminus Examples of alternative constructs
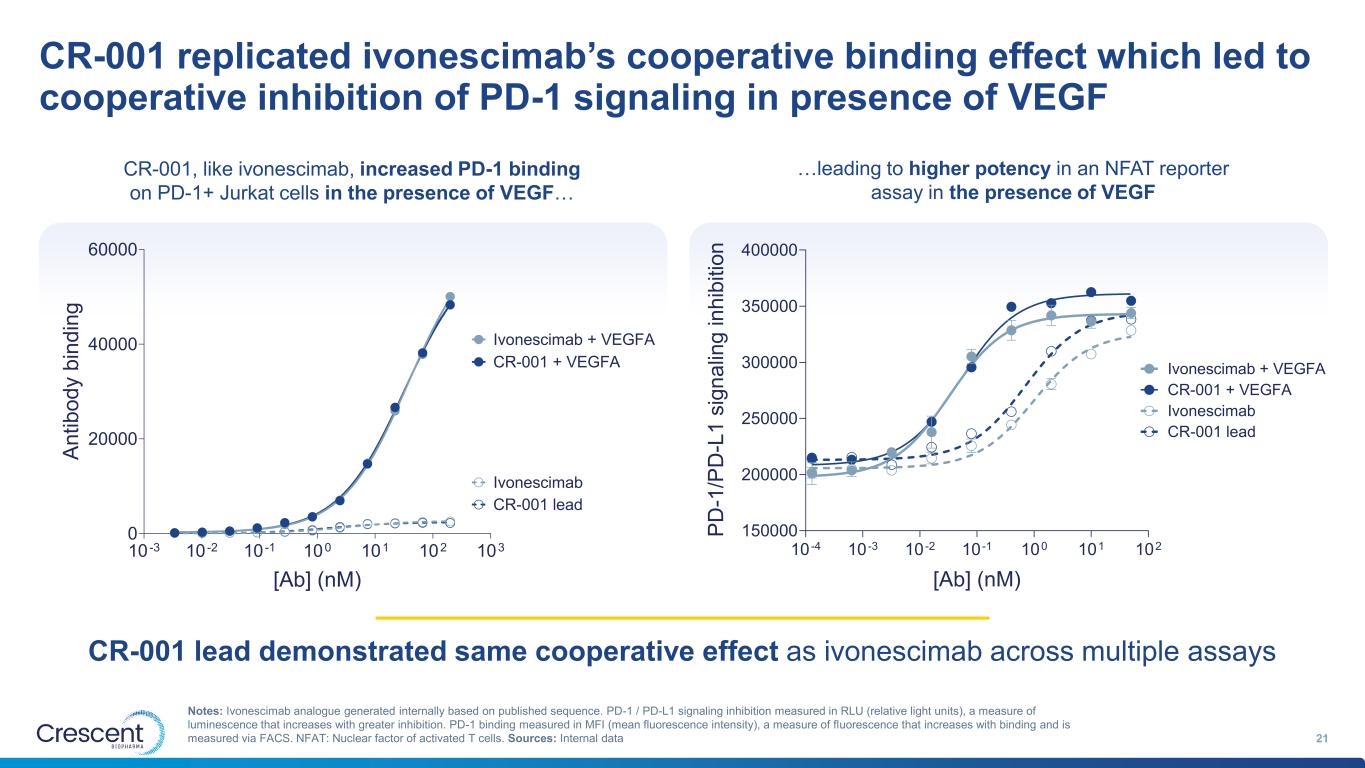
CR-001 replicated ivonescimab’s cooperative binding effect which led to cooperative inhibition of PD-1 signaling in presence of VEGF 21 Notes: Ivonescimab analogue generated internally based on published sequence. PD-1 / PD-L1 signaling inhibition measured in RLU (relative light units), a measure of luminescence that increases with greater inhibition. PD-1 binding measured in MFI (mean fluorescence intensity), a measure of fluorescence that increases with binding and is measured via FACS. NFAT: Nuclear factor of activated T cells. Sources: Internal data CR-001 lead demonstrated same cooperative effect as ivonescimab across multiple assays 10 -3 10 -2 10 -1 100 101 102 103 0 20000 40000 60000 [Ab] (nM) A n ti b o d y b in d in g Ivonescimab + VEGFA Ivonescimab CR-001 + VEGFA CR-001 lead 10 -4 10 -3 10 -2 10 -1 100 101 102 150000 200000 250000 300000 350000 400000 [Ab] (nM) P D -1 /P D -L 1 s ig n a lin g i n h ib it io n Ivonescimab Ivonescimab + VEGFA CR-001 lead CR-001 + VEGFA …leading to higher potency in an NFAT reporter assay in the presence of VEGF CR-001, like ivonescimab, increased PD-1 binding on PD-1+ Jurkat cells in the presence of VEGF…
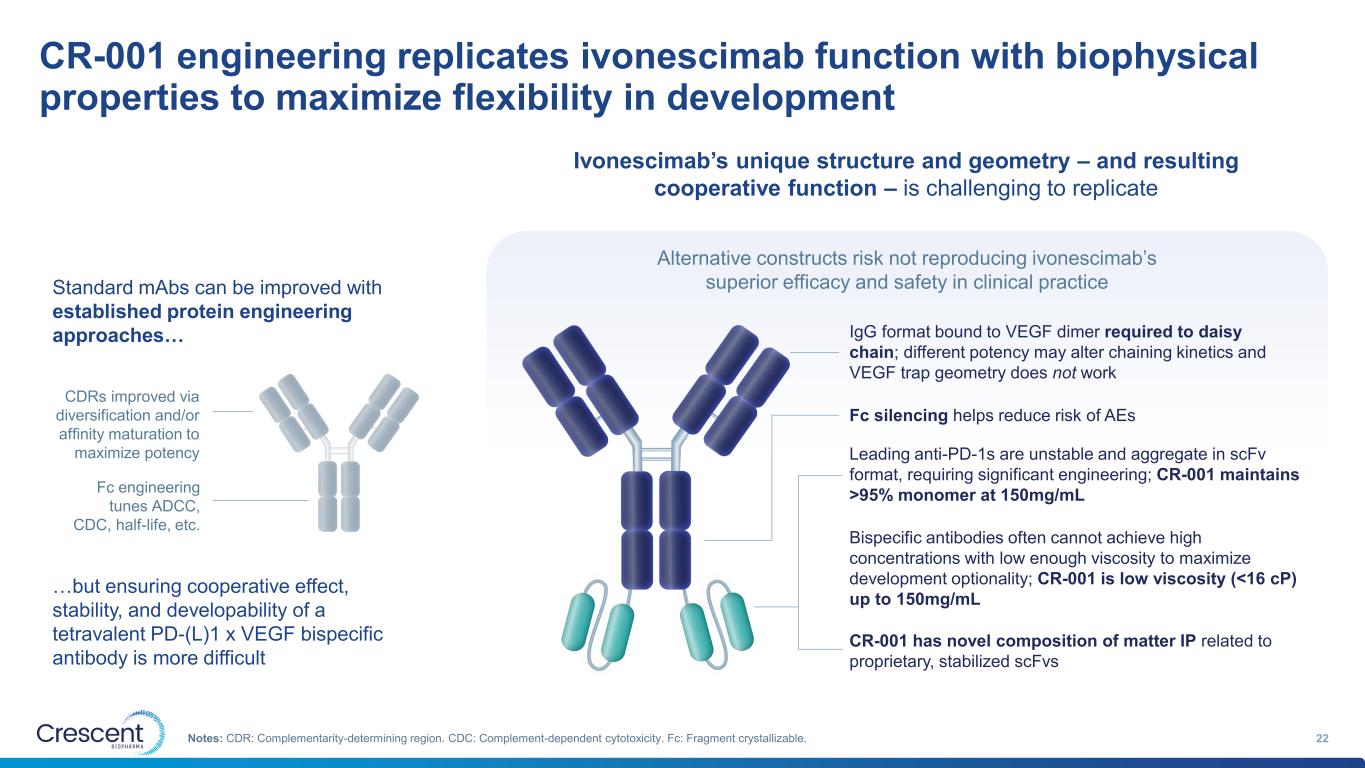
CR-001 engineering replicates ivonescimab function with biophysical properties to maximize flexibility in development 22Notes: CDR: Complementarity-determining region. CDC: Complement-dependent cytotoxicity. Fc: Fragment crystallizable. Ivonescimab’s unique structure and geometry – and resulting cooperative function – is challenging to replicate Standard mAbs can be improved with established protein engineering approaches… CDRs improved via diversification and/or affinity maturation to maximize potency Fc engineering tunes ADCC, CDC, half-life, etc. …but ensuring cooperative effect, stability, and developability of a tetravalent PD-(L)1 x VEGF bispecific antibody is more difficult IgG format bound to VEGF dimer required to daisy chain; different potency may alter chaining kinetics and VEGF trap geometry does not work Fc silencing helps reduce risk of AEs Leading anti-PD-1s are unstable and aggregate in scFv format, requiring significant engineering; CR-001 maintains >95% monomer at 150mg/mL Bispecific antibodies often cannot achieve high concentrations with low enough viscosity to maximize development optionality; CR-001 is low viscosity (<16 cP) up to 150mg/mL CR-001 has novel composition of matter IP related to proprietary, stabilized scFvs Alternative constructs risk not reproducing ivonescimab’s superior efficacy and safety in clinical practice
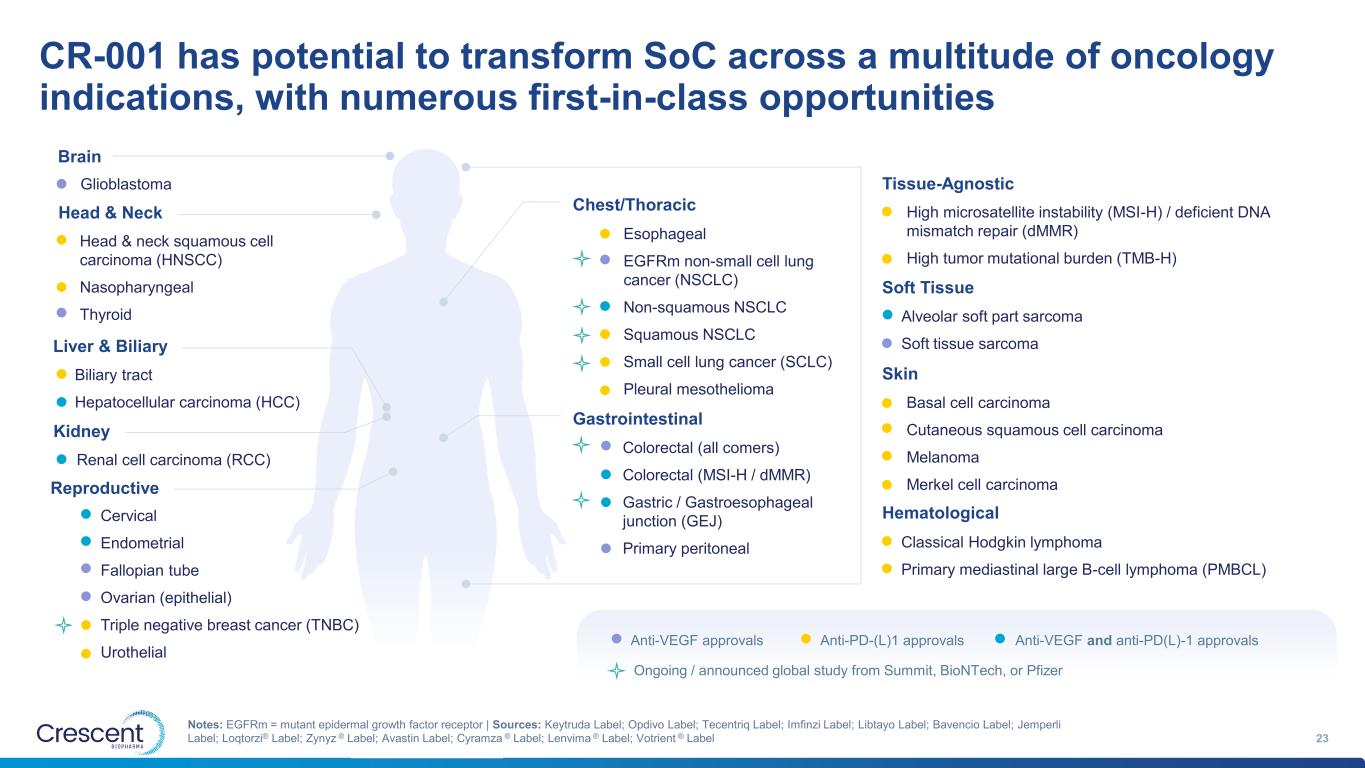
CR-001 has potential to transform SoC across a multitude of oncology indications, with numerous first-in-class opportunities 23 Notes: EGFRm = mutant epidermal growth factor receptor | Sources: Keytruda Label; Opdivo Label; Tecentriq Label; Imfinzi Label; Libtayo Label; Bavencio Label; Jemperli Label; Loqtorzi® Label; Zynyz ® Label; Avastin Label; Cyramza ® Label; Lenvima ® Label; Votrient ® Label Cervical Endometrial Fallopian tube Ovarian (epithelial) Triple negative breast cancer (TNBC) Urothelial Colorectal (all comers) Colorectal (MSI-H / dMMR) Gastric / Gastroesophageal junction (GEJ) Primary peritoneal Glioblastoma Classical Hodgkin lymphoma Primary mediastinal large B-cell lymphoma (PMBCL) Biliary tract Hepatocellular carcinoma (HCC) Alveolar soft part sarcoma Soft tissue sarcoma High microsatellite instability (MSI-H) / deficient DNA mismatch repair (dMMR) High tumor mutational burden (TMB-H) Basal cell carcinoma Cutaneous squamous cell carcinoma Melanoma Merkel cell carcinoma Renal cell carcinoma (RCC) Head & neck squamous cell carcinoma (HNSCC) Nasopharyngeal Thyroid Brain Gastrointestinal Head & Neck Chest/Thoracic Liver & Biliary Kidney Hematological Reproductive Soft Tissue Skin Tissue-Agnostic Anti-VEGF approvals Anti-PD-(L)1 approvals Anti-VEGF and anti-PD(L)-1 approvals Ongoing / announced global study from Summit, BioNTech, or Pfizer Esophageal EGFRm non-small cell lung cancer (NSCLC) Non-squamous NSCLC Squamous NSCLC Small cell lung cancer (SCLC) Pleural mesothelioma
Parallel clinical development paths offer potential for both first-in-class and lower risk opportunities for CR-001 24 Two parallel development plans for CR-001 First-in-class opportunities • Numerous indications with clinically meaningful anti-PD-(L)1 +/- VEGF efficacy and potential to combine with chemo / orthogonal MoAs • Focus on potential first-in-class opportunities with rapid path to market (i.e., efficient development strategy, anticipated high likelihood of PFS and OS success) Fast-follower in clinically validated indications TNBCNSCLC OTHERS • Plan to rapidly follow ivonescimab in indications where clinical validation vs. anti-PD-(L)1 is highly differentiating • High conviction CR-001 can replicate ivonescimab’s efficacy given similar construct and equivalent MoA Potential indications based on ongoing Phase 3 trials Illustrative
Tumor types selected for CR-001 Phase 1/2 study 25 Three therapeutic areas with high POS and multiple opportunities for first-in-class and fast-follower approach Maintaining optionality to allow data driven indication selection for registrational studies Thoracic GI Gyn Onc NSCLC Selected tumor types have clinical validation of PD-(L)1 and/or VEGF inhibitors with opportunity to improve on standard of care with dual targeting Hepatocellular (HCC) Colorectal (CRC) Gastric Biliary Endometrial Cervical Ovarian

CR-001 Phase 1/2 data offer potential for rapid clinical development – a rarity for a solid tumor oncology program 26 Phase 1/2 proof-of-concept readout is a potentially significant value-generating event for CR-001 IND PoC PHASE 1/2 MULTIPLE SOLID TUMOR TYPES PHASE 3 STUDIES (validated indications) PHASE 2/3 STUDIES (first-in-class opportunities) 4Q25 1Q27 Key accelerating preliminary data: PK, safety, efficacy (e.g., ORR) Higher confidence to fund and accelerate CR-001 into P3s after P1 data, given replication of ivonescimab’s cooperative pharmacology ILLUSTRATIVE Preliminary data from Phase 1/2 cohorts provides substantial validation of program because CR-001’s structural design and preclinical data are similar to those of ivonescimab Early Phase 1/2 data, as single agent and in combination with SoC, enables rapid late-stage development in multiple solid tumor types, unlocking broad first-in-class and fast-follower opportunities and supporting combinations studies CR-001 is markedly differentiated from novel constructs disconnected from ivonescimab’s MoA; alternative formats may require significantly more patients worth of safety and efficacy data in tumor-specific expansion cohorts and/or Phase 2s to establish conviction before initiating Phase 3s High conviction in CR-001’s clinical profile can be reached in ~12 months from Phase 1/2 initiation, offering potential for significant early value inflection
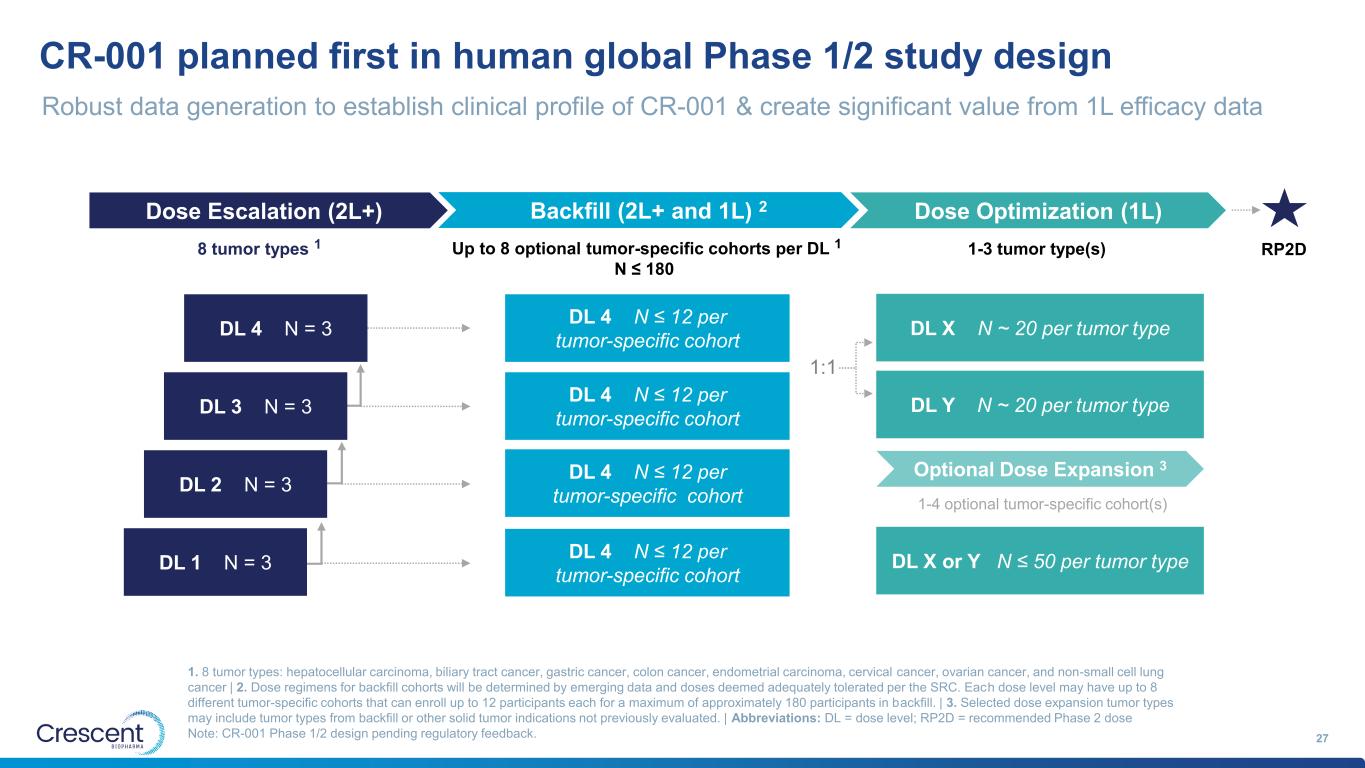
CR-001 planned first in human global Phase 1/2 study design 27 1. 8 tumor types: hepatocellular carcinoma, biliary tract cancer, gastric cancer, colon cancer, endometrial carcinoma, cervical cancer, ovarian cancer, and non-small cell lung cancer | 2. Dose regimens for backfill cohorts will be determined by emerging data and doses deemed adequately tolerated per the SRC. Each dose level may have up to 8 different tumor-specific cohorts that can enroll up to 12 participants each for a maximum of approximately 180 participants in backfill. | 3. Selected dose expansion tumor types may include tumor types from backfill or other solid tumor indications not previously evaluated. | Abbreviations: DL = dose level; RP2D = recommended Phase 2 dose Note: CR-001 Phase 1/2 design pending regulatory feedback. Robust data generation to establish clinical profile of CR-001 & create significant value from 1L efficacy data DL 1 N = 3 DL 2 N = 3 DL 3 N = 3 DL 4 N = 3 RP2D Dose Escalation (2L+) Backfill (2L+ and 1L) 2 Dose Optimization (1L) 1:1 Optional Dose Expansion 3 8 tumor types 1 1-3 tumor type(s)Up to 8 optional tumor-specific cohorts per DL 1 N ≤ 180 1-4 optional tumor-specific cohort(s) DL 4 N ≤ 12 per tumor-specific cohort DL 4 N ≤ 12 per tumor-specific cohort DL 4 N ≤ 12 per tumor-specific cohort DL 4 N ≤ 12 per tumor-specific cohort DL X or Y N ≤ 50 per tumor type DL X N ~ 20 per tumor type DL Y N ~ 20 per tumor type
Combination studies planned for CR-001 with several different ADCs 28 Maximizing CR-001 opportunity with combination studies in multiple tumor types Kelun-Biotech partnership enables rapid generation of combo data with CR-003 (SKB105) and other Kelun- Biotech ADCs Kelun-Biotech data will inform strategy for CR-001 as I/O backbone for many other possible combination studies + CR-002 + CR-003 (SKB105) + CR-004 + Other Kelun-Biotech ADCs + Opportunity for other combos PD-L1 ADC ITGB6 ADC CR-001 Undisclosed ADC
CR-001: opportunity to rapidly generate significant value 29 CR-001 is a highly potent PD-1 x VEGF bsAb reproducing cooperative binding qualities critical to ivonescimab CR-002 (PD-L1), CR-003 (ITGB6) and additional ADCs offer complementary development opportunities for CR-001 Ivonescimab significantly improved PFS versus pembrolizumab in Phase 3 in 1L NSCLC – the first therapy to do so head-to-head Poised to transform NSCLC standard of care, with broad application across $50B+ anti-PD-(L)1 market validate PD-1 x VEGF cooperativity for $50B+ market is designed to replicate ivonescimab with compelling pipeline of ADCs Unprecedented third-party data Transformative MoA CR-001’s proprietary engineering Synergistic combinations

Opening Remarks Joshua Brumm CEO Overview of Kelun-Biotech Partnership Jonathan McNeill, M.D. President & COO Clinical Development Plan for CR-001, PD-1 x VEGF Bispecific Antibody Ellie Im, M.D. Chief Medical Officer ADC Programs: CR-002 and CR-003 (SKB105) Jan Pinkas, Ph.D. Chief Scientific Officer Q&A 30 Program
Crescent is executing a comprehensive strategy to develop best-in- class ADCs from both internal and external sources 31 Targeting Payloads Sourcing Mono-specific Bi-specific Multi-specific Single payload Dual payload New payloads Evaluated for: performance, differentiation, portfolio fit, combo synergies, developability, market opportunity Internal development Paragon relationship External (the right partners) Multiple opportunities for market leadership based on differentiated ADC in-house development plus sourcing from innovative external partners, combined with best-in-class immuno-oncology backbone
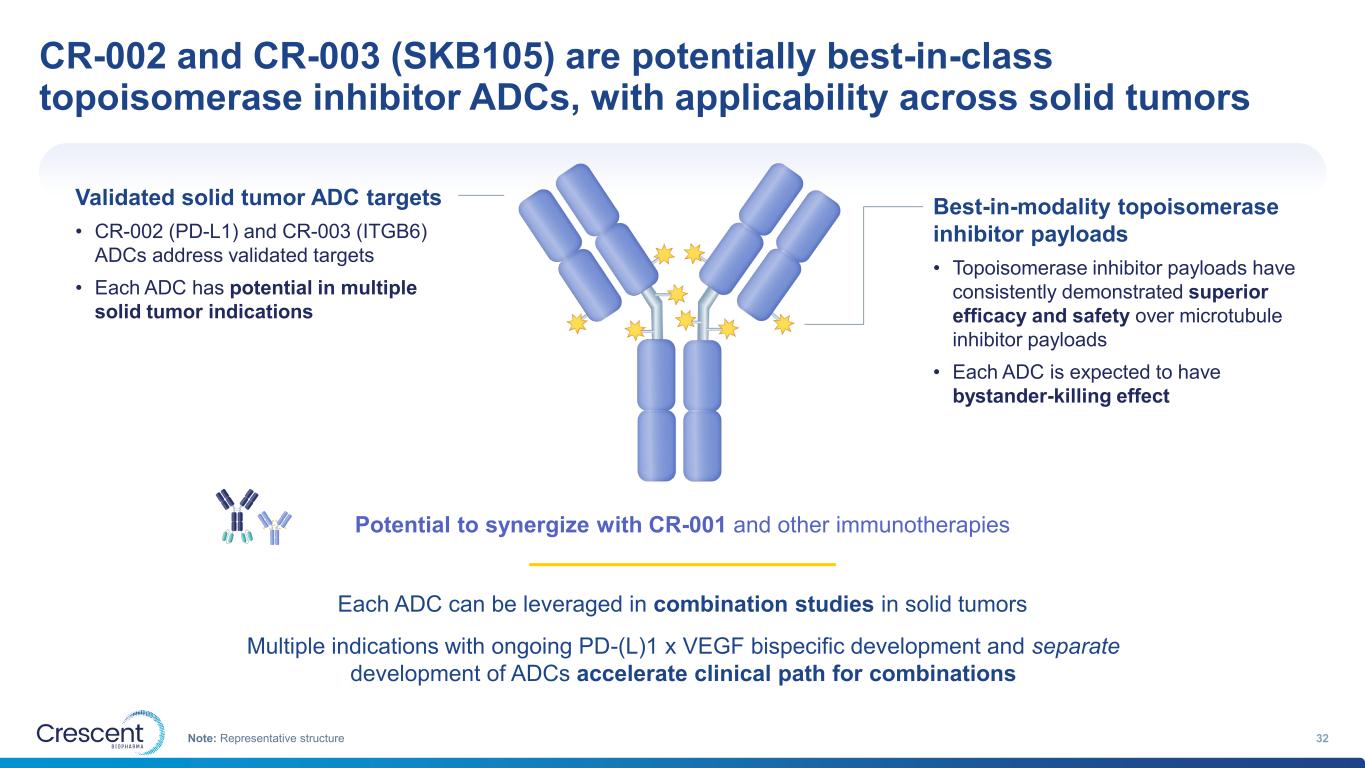
CR-002 and CR-003 (SKB105) are potentially best-in-class topoisomerase inhibitor ADCs, with applicability across solid tumors 32Note: Representative structure Each ADC can be leveraged in combination studies in solid tumors Multiple indications with ongoing PD-(L)1 x VEGF bispecific development and separate development of ADCs accelerate clinical path for combinations Validated solid tumor ADC targets • CR-002 (PD-L1) and CR-003 (ITGB6) ADCs address validated targets • Each ADC has potential in multiple solid tumor indications Best-in-modality topoisomerase inhibitor payloads • Topoisomerase inhibitor payloads have consistently demonstrated superior efficacy and safety over microtubule inhibitor payloads • Each ADC is expected to have bystander-killing effect Potential to synergize with CR-001 and other immunotherapies
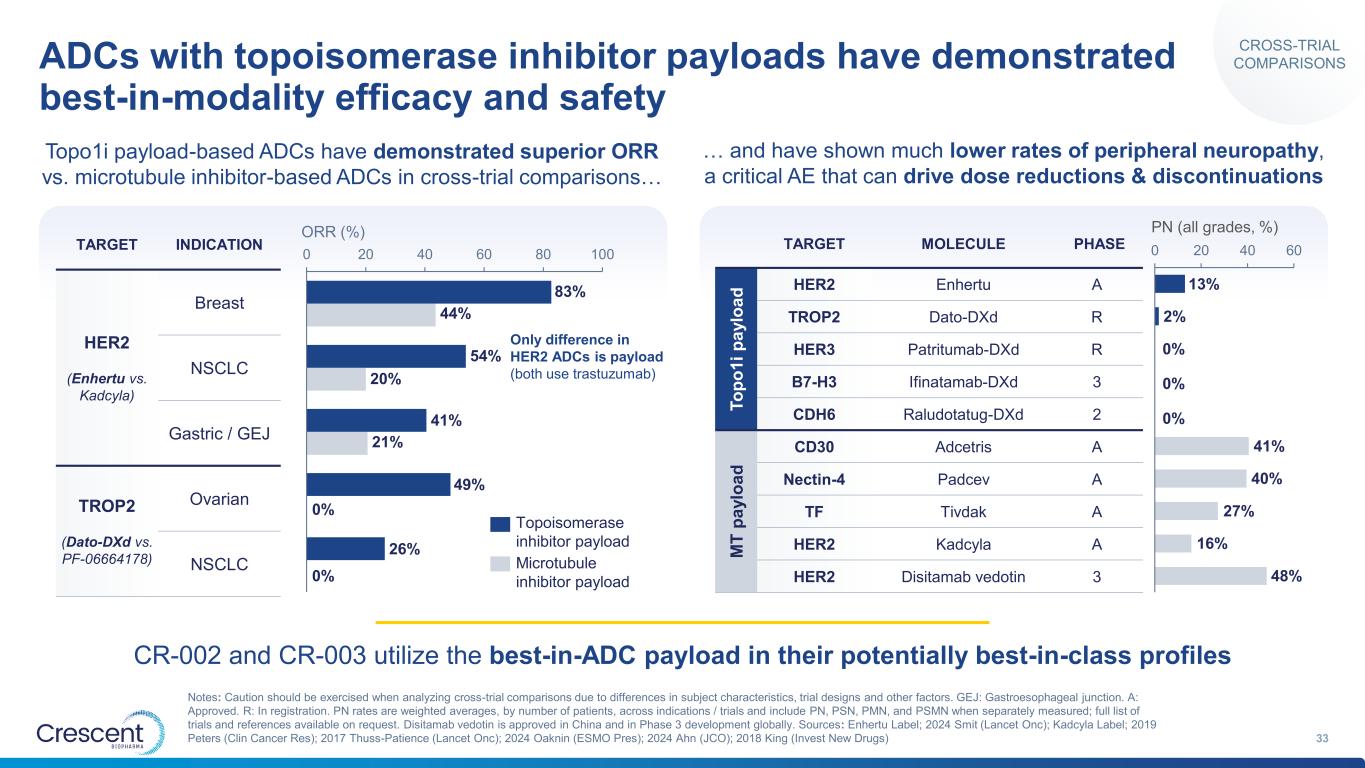
ADCs with topoisomerase inhibitor payloads have demonstrated best-in-modality efficacy and safety 33 Notes: Caution should be exercised when analyzing cross-trial comparisons due to differences in subject characteristics, trial designs and other factors. GEJ: Gastroesophageal junction. A: Approved. R: In registration. PN rates are weighted averages, by number of patients, across indications / trials and include PN, PSN, PMN, and PSMN when separately measured; full list of trials and references available on request. Disitamab vedotin is approved in China and in Phase 3 development globally. Sources: Enhertu Label; 2024 Smit (Lancet Onc); Kadcyla Label; 2019 Peters (Clin Cancer Res); 2017 Thuss-Patience (Lancet Onc); 2024 Oaknin (ESMO Pres); 2024 Ahn (JCO); 2018 King (Invest New Drugs) Topo1i payload-based ADCs have demonstrated superior ORR vs. microtubule inhibitor-based ADCs in cross-trial comparisons… … and have shown much lower rates of peripheral neuropathy, a critical AE that can drive dose reductions & discontinuations 0 20 40 60 80 100 ORR (%) 83% 44% 54% 20% 41% 21% 49% 0% 26% 0% Topoisomerase inhibitor payload Microtubule inhibitor payload 0 20 40 60 PN (all grades, %) 13% 2% 0% 0% 0% 41% 40% 27% 16% 48% TARGET INDICATION HER2 (Enhertu vs. Kadcyla) Breast NSCLC Gastric / GEJ TROP2 (Dato-DXd vs. PF-06664178) Ovarian NSCLC TARGET MOLECULE PHASE T o p o 1 i p a y lo a d HER2 Enhertu A TROP2 Dato-DXd R HER3 Patritumab-DXd R B7-H3 Ifinatamab-DXd 3 CDH6 Raludotatug-DXd 2 M T p a y lo a d CD30 Adcetris A Nectin-4 Padcev A TF Tivdak A HER2 Kadcyla A HER2 Disitamab vedotin 3 CR-002 and CR-003 utilize the best-in-ADC payload in their potentially best-in-class profiles Only difference in HER2 ADCs is payload (both use trastuzumab) CROSS-TRIAL COMPARISONS
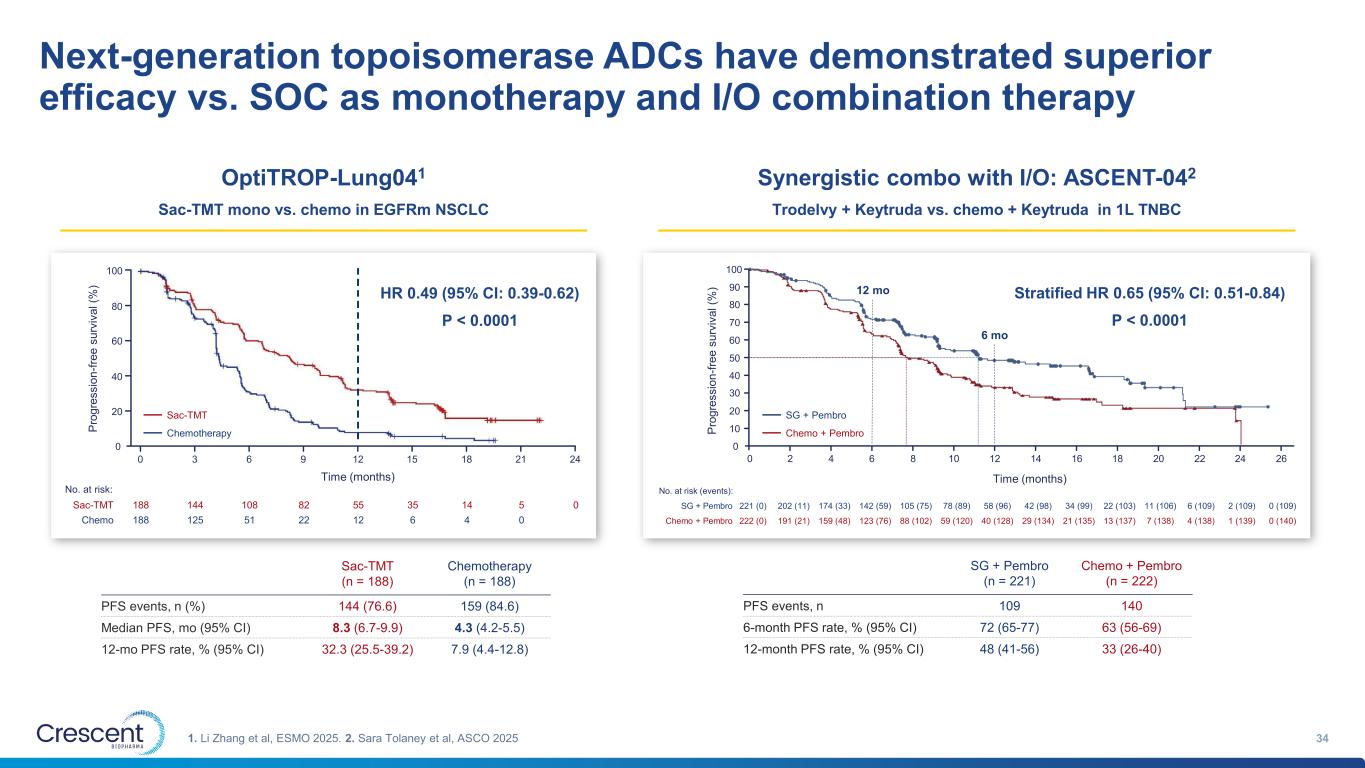
Next-generation topoisomerase ADCs have demonstrated superior efficacy vs. SOC as monotherapy and I/O combination therapy 341. Li Zhang et al, ESMO 2025. 2. Sara Tolaney et al, ASCO 2025 32.3% 7.9% 0 2 4 6 8 10 12 14 16 18 20 22 24 26 0 10 20 30 40 50 60 70 80 90 100 P ro g re s s io n -f re e s u rv iv a l (% ) Time (months) 12 mo 6 mo Sac-TMT 188 144 108 82 55 35 14 5 0 Chemo 188 125 51 22 12 6 4 0 0 3 6 9 12 15 18 21 0 20 40 60 80 100 P ro g re s s io n -f re e s u rv iv a l (% ) Time (months) 24 No. at risk: Sac-TMT Chemotherapy Sac-TMT (n = 188) Chemotherapy (n = 188) PFS events, n (%) 144 (76.6) 159 (84.6) Median PFS, mo (95% CI) 8.3 (6.7-9.9) 4.3 (4.2-5.5) 12-mo PFS rate, % (95% CI) 32.3 (25.5-39.2) 7.9 (4.4-12.8) HR 0.49 (95% CI: 0.39-0.62) P < 0.0001 OptiTROP-Lung041 Sac-TMT mono vs. chemo in EGFRm NSCLC SG + Pembro 221 (0) 202 (11) 174 (33) 142 (59) 105 (75) 78 (89) 58 (96) 42 (98) 34 (99) 22 (103) 11 (106) 6 (109) 2 (109) 0 (109) Chemo + Pembro 222 (0) 191 (21) 159 (48) 123 (76) 88 (102) 59 (120) 40 (128) 29 (134) 21 (135) 13 (137) 7 (138) 4 (138) 1 (139) 0 (140) No. at risk (events): SG + Pembro Chemo + Pembro Stratified HR 0.65 (95% CI: 0.51-0.84) P < 0.0001 SG + Pembro (n = 221) Chemo + Pembro (n = 222) PFS events, n 109 140 6-month PFS rate, % (95% CI) 72 (65-77) 63 (56-69) 12-month PFS rate, % (95% CI) 48 (41-56) 33 (26-40) Synergistic combo with I/O: ASCENT-042 Trodelvy + Keytruda vs. chemo + Keytruda in 1L TNBC
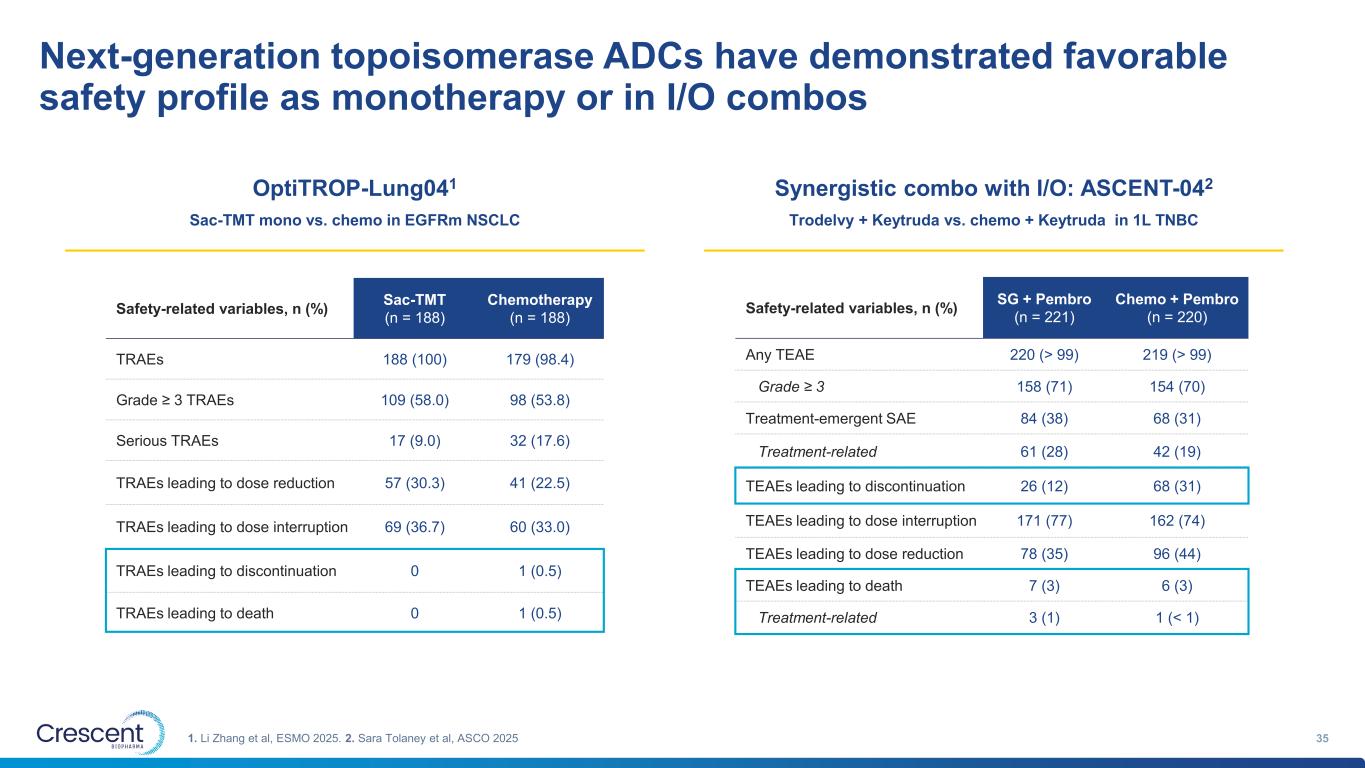
Next-generation topoisomerase ADCs have demonstrated favorable safety profile as monotherapy or in I/O combos 351. Li Zhang et al, ESMO 2025. 2. Sara Tolaney et al, ASCO 2025 OptiTROP-Lung041 Sac-TMT mono vs. chemo in EGFRm NSCLC Synergistic combo with I/O: ASCENT-042 Trodelvy + Keytruda vs. chemo + Keytruda in 1L TNBC Safety-related variables, n (%) Sac-TMT (n = 188) Chemotherapy (n = 188) TRAEs 188 (100) 179 (98.4) Grade ≥ 3 TRAEs 109 (58.0) 98 (53.8) Serious TRAEs 17 (9.0) 32 (17.6) TRAEs leading to dose reduction 57 (30.3) 41 (22.5) TRAEs leading to dose interruption 69 (36.7) 60 (33.0) TRAEs leading to discontinuation 0 1 (0.5) TRAEs leading to death 0 1 (0.5) Safety-related variables, n (%) SG + Pembro (n = 221) Chemo + Pembro (n = 220) Any TEAE 220 (> 99) 219 (> 99) Grade ≥ 3 158 (71) 154 (70) Treatment-emergent SAE 84 (38) 68 (31) Treatment-related 61 (28) 42 (19) TEAEs leading to discontinuation 26 (12) 68 (31) TEAEs leading to dose interruption 171 (77) 162 (74) TEAEs leading to dose reduction 78 (35) 96 (44) TEAEs leading to death 7 (3) 6 (3) Treatment-related 3 (1) 1 (< 1)
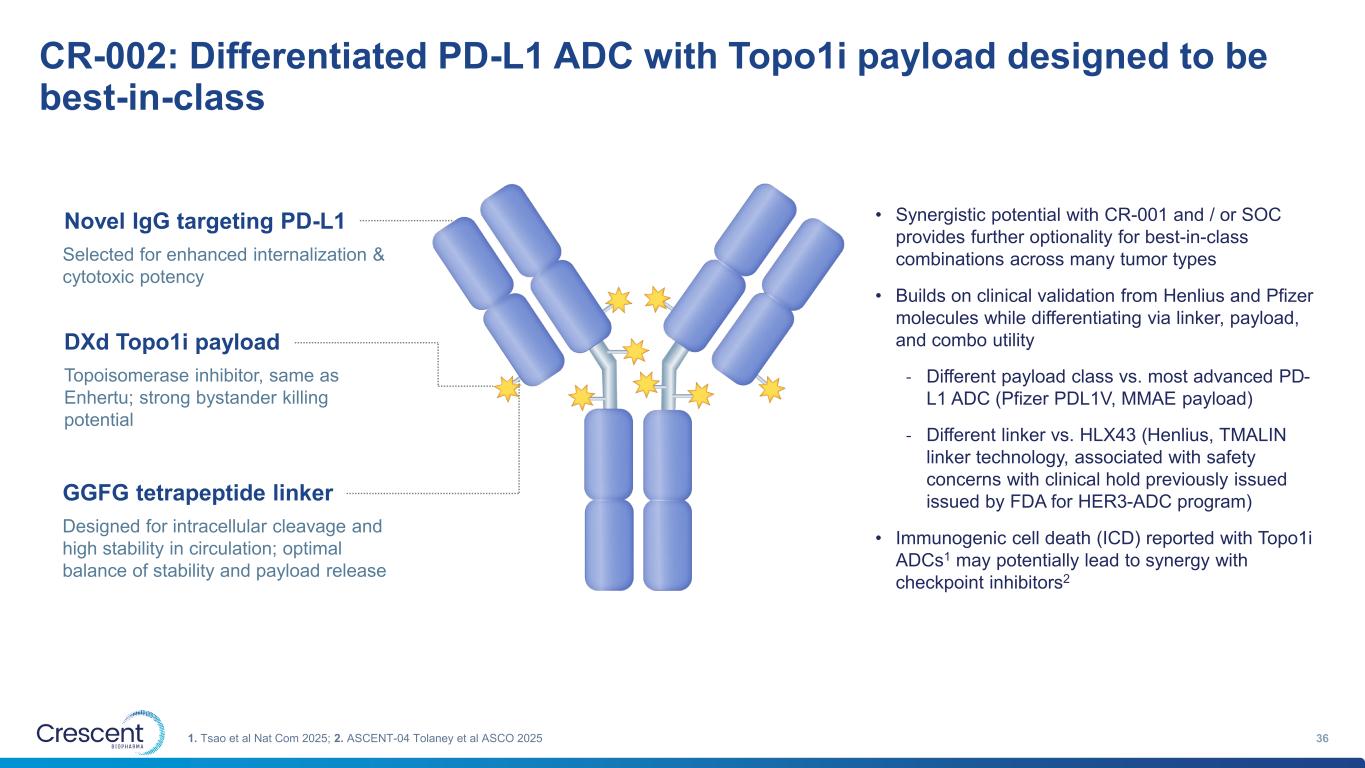
CR-002: Differentiated PD-L1 ADC with Topo1i payload designed to be best-in-class 361. Tsao et al Nat Com 2025; 2. ASCENT-04 Tolaney et al ASCO 2025 Novel IgG targeting PD-L1 Selected for enhanced internalization & cytotoxic potency DXd Topo1i payload Topoisomerase inhibitor, same as Enhertu; strong bystander killing potential GGFG tetrapeptide linker Designed for intracellular cleavage and high stability in circulation; optimal balance of stability and payload release • Synergistic potential with CR-001 and / or SOC provides further optionality for best-in-class combinations across many tumor types • Builds on clinical validation from Henlius and Pfizer molecules while differentiating via linker, payload, and combo utility - Different payload class vs. most advanced PD- L1 ADC (Pfizer PDL1V, MMAE payload) - Different linker vs. HLX43 (Henlius, TMALIN linker technology, associated with safety concerns with clinical hold previously issued issued by FDA for HER3-ADC program) • Immunogenic cell death (ICD) reported with Topo1i ADCs1 may potentially lead to synergy with checkpoint inhibitors2
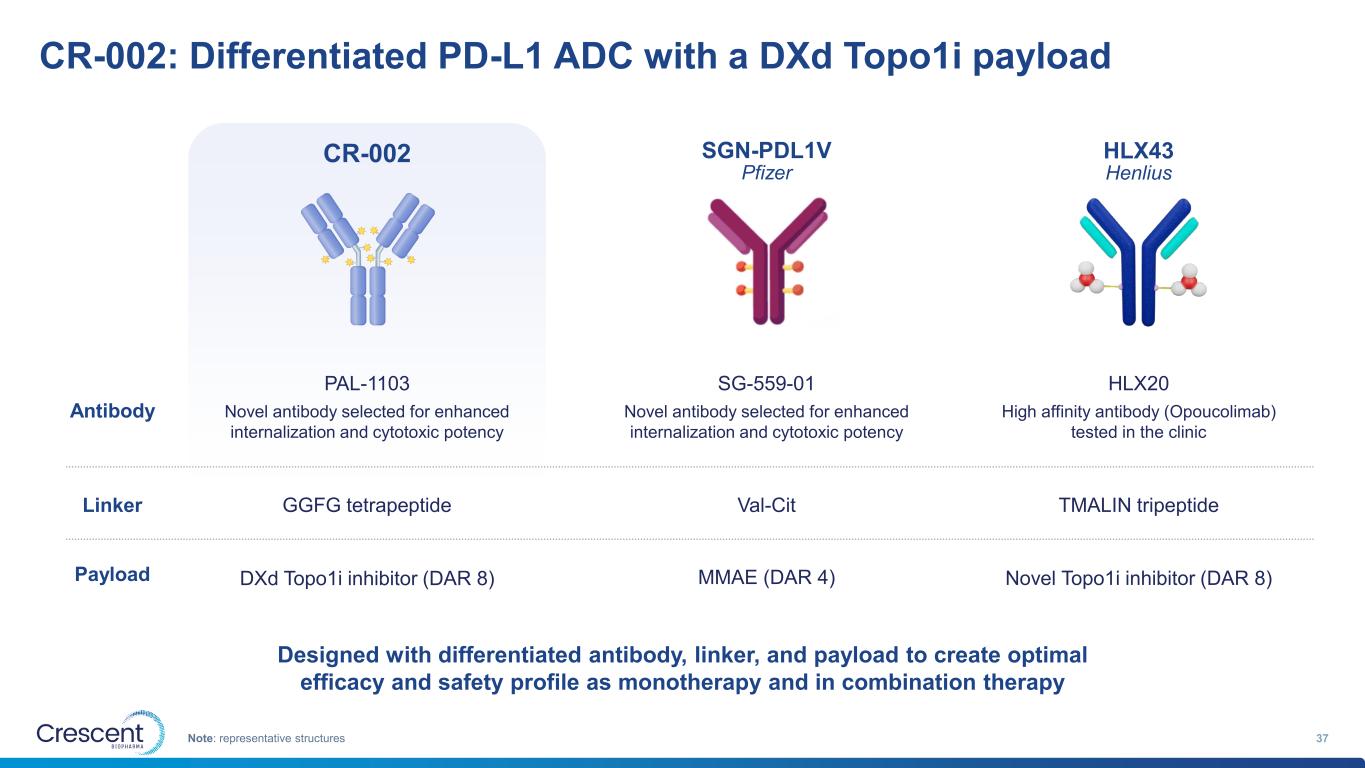
CR-002: Differentiated PD-L1 ADC with a DXd Topo1i payload 37Note: representative structures CR-002 SGN-PDL1V Pfizer HLX43 Henlius GGFG tetrapeptide DXd Topo1i inhibitor (DAR 8) Val-Cit MMAE (DAR 4) TMALIN tripeptide Novel Topo1i inhibitor (DAR 8) PAL-1103 SG-559-01 HLX20 Antibody Linker Payload Novel antibody selected for enhanced internalization and cytotoxic potency Novel antibody selected for enhanced internalization and cytotoxic potency High affinity antibody (Opoucolimab) tested in the clinic Designed with differentiated antibody, linker, and payload to create optimal efficacy and safety profile as monotherapy and in combination therapy
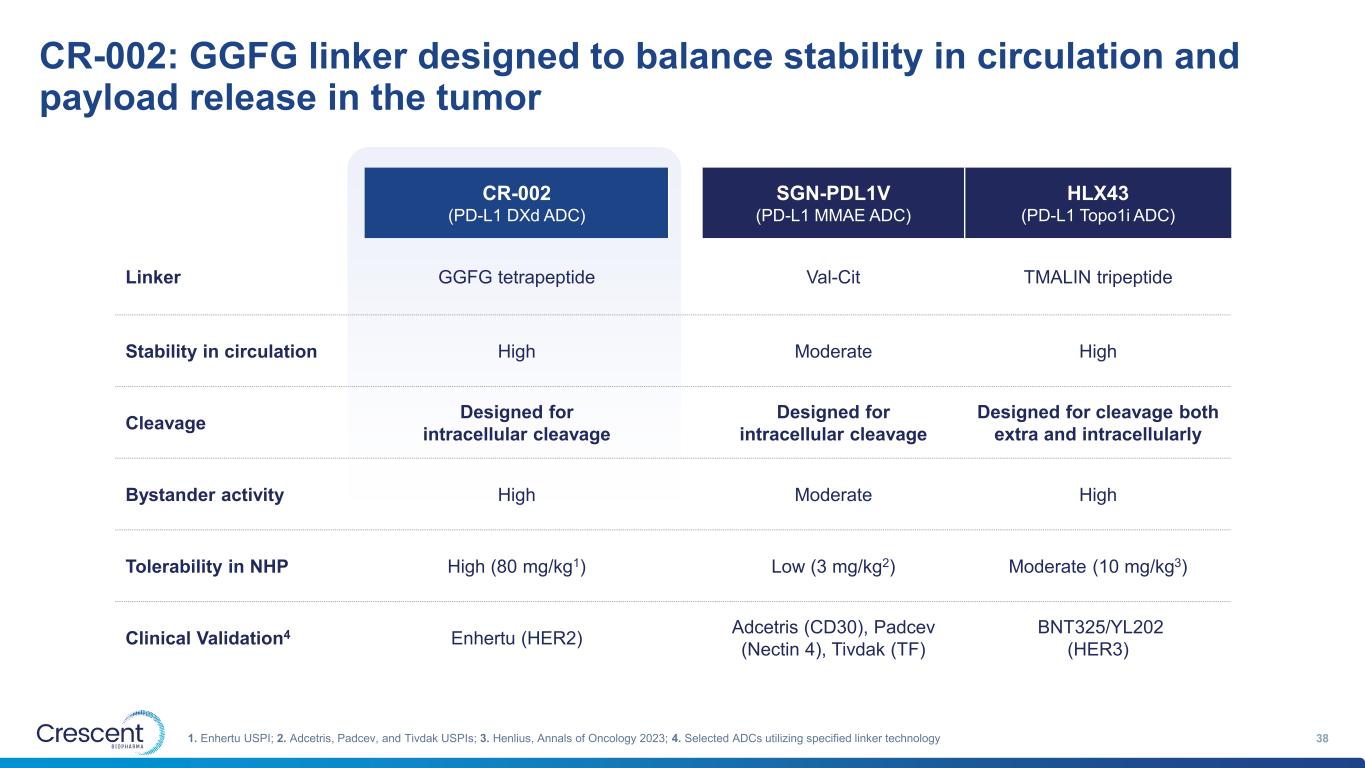
CR-002: GGFG linker designed to balance stability in circulation and payload release in the tumor 381. Enhertu USPI; 2. Adcetris, Padcev, and Tivdak USPIs; 3. Henlius, Annals of Oncology 2023; 4. Selected ADCs utilizing specified linker technology CR-002 (PD-L1 DXd ADC) SGN-PDL1V (PD-L1 MMAE ADC) HLX43 (PD-L1 Topo1i ADC) Linker GGFG tetrapeptide Val-Cit TMALIN tripeptide Stability in circulation High Moderate High Cleavage Designed for intracellular cleavage Designed for intracellular cleavage Designed for cleavage both extra and intracellularly Bystander activity High Moderate High Tolerability in NHP High (80 mg/kg1) Low (3 mg/kg2) Moderate (10 mg/kg3) Clinical Validation4 Enhertu (HER2) Adcetris (CD30), Padcev (Nectin 4), Tivdak (TF) BNT325/YL202 (HER3)
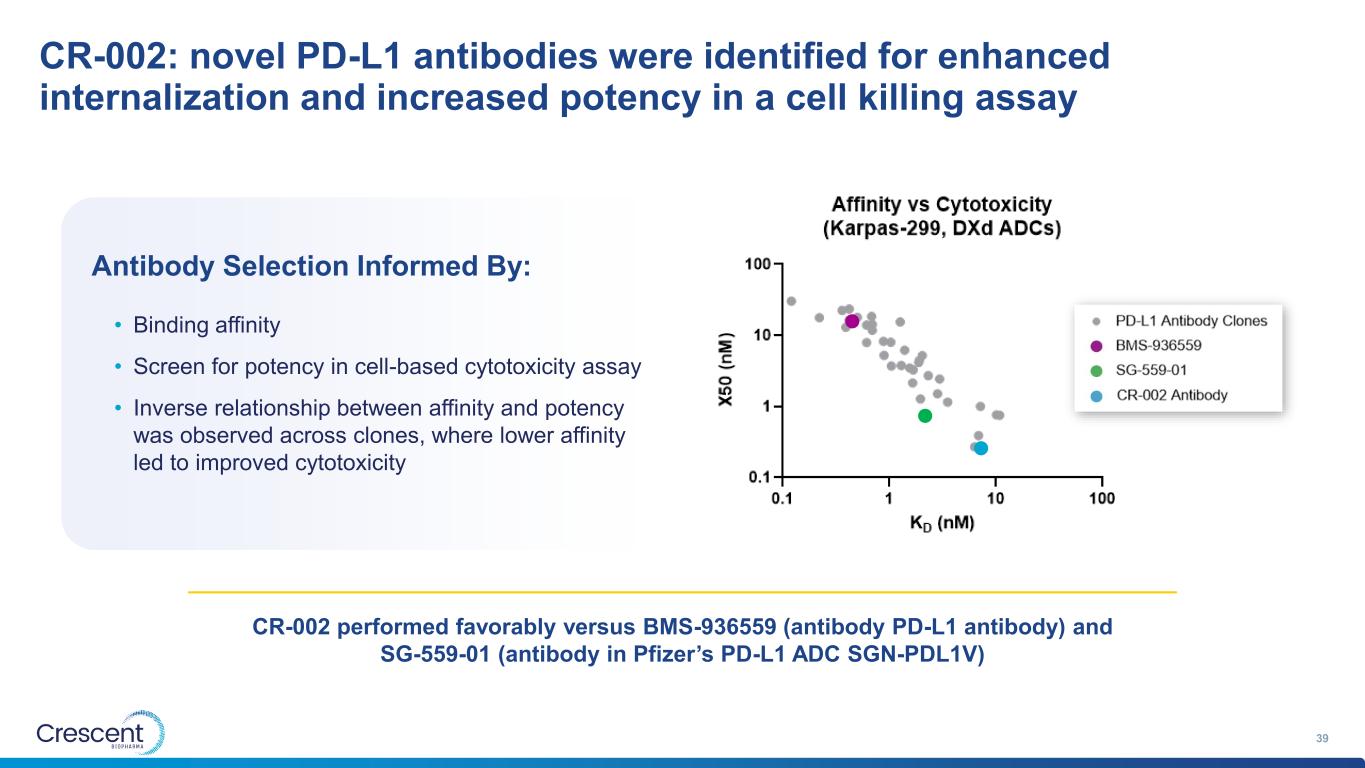
CR-002: novel PD-L1 antibodies were identified for enhanced internalization and increased potency in a cell killing assay 39 Antibody Selection Informed By: • Binding affinity • Screen for potency in cell-based cytotoxicity assay • Inverse relationship between affinity and potency was observed across clones, where lower affinity led to improved cytotoxicity CR-002 performed favorably versus BMS-936559 (antibody PD-L1 antibody) and SG-559-01 (antibody in Pfizer’s PD-L1 ADC SGN-PDL1V)
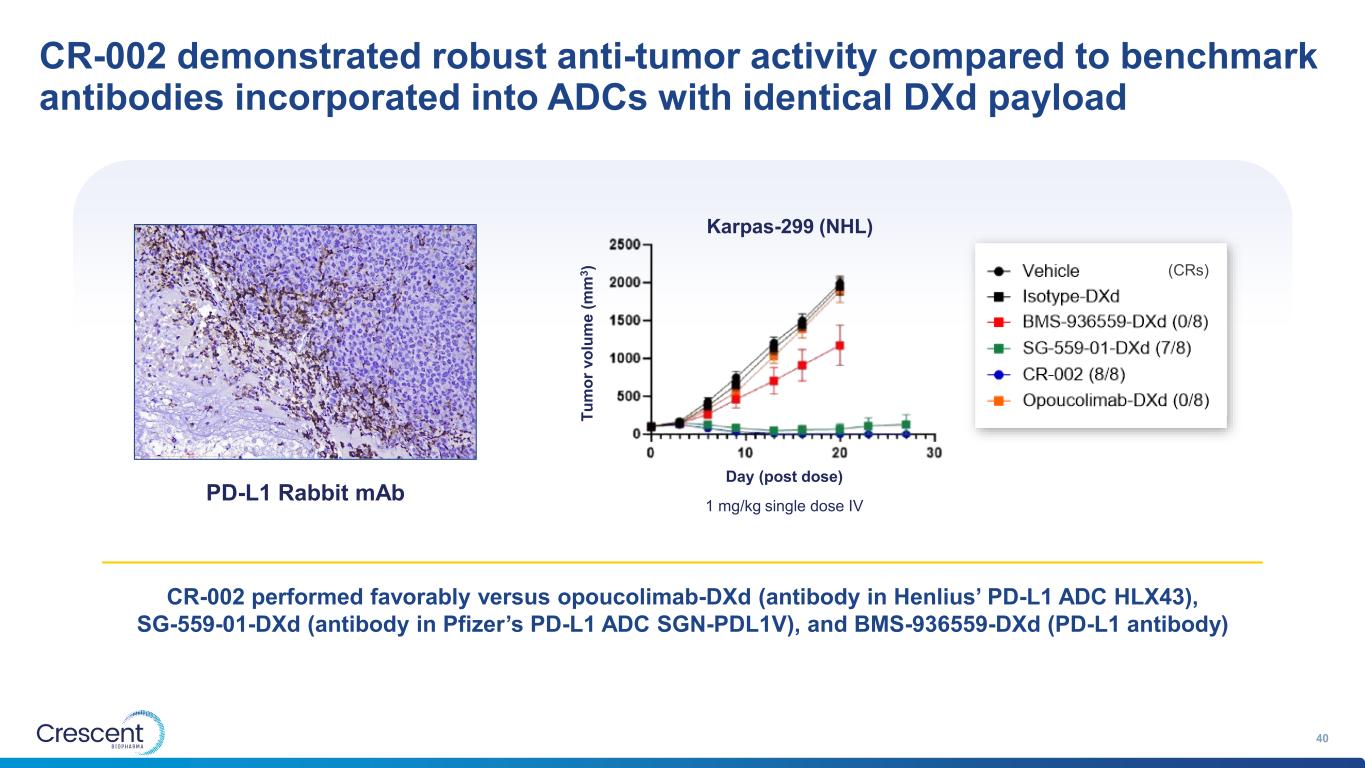
CR-002 demonstrated robust anti-tumor activity compared to benchmark antibodies incorporated into ADCs with identical DXd payload 40 PD-L1 Rabbit mAb (CRs) 1 mg/kg single dose IV Karpas-299 (NHL) Day (post dose) T u m o r v o lu m e ( m m 3 ) CR-002 performed favorably versus opoucolimab-DXd (antibody in Henlius’ PD-L1 ADC HLX43), SG-559-01-DXd (antibody in Pfizer’s PD-L1 ADC SGN-PDL1V), and BMS-936559-DXd (PD-L1 antibody)
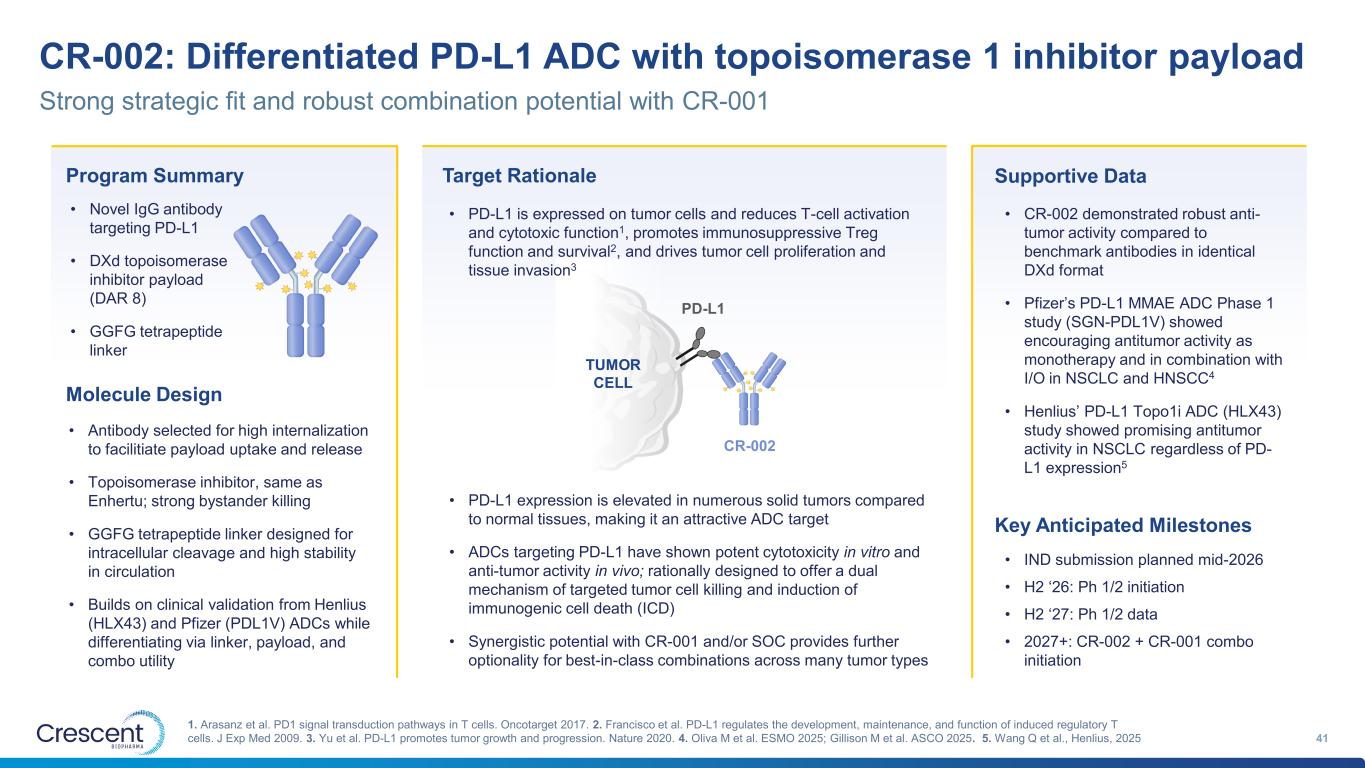
CR-002: Differentiated PD-L1 ADC with topoisomerase 1 inhibitor payload 41 1. Arasanz et al. PD1 signal transduction pathways in T cells. Oncotarget 2017. 2. Francisco et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009. 3. Yu et al. PD-L1 promotes tumor growth and progression. Nature 2020. 4. Oliva M et al. ESMO 2025; Gillison M et al. ASCO 2025. 5. Wang Q et al., Henlius, 2025 Strong strategic fit and robust combination potential with CR-001 Program Summary • Novel IgG antibody targeting PD-L1 • DXd topoisomerase inhibitor payload (DAR 8) • GGFG tetrapeptide linker Molecule Design • Antibody selected for high internalization to facilitiate payload uptake and release • Topoisomerase inhibitor, same as Enhertu; strong bystander killing • GGFG tetrapeptide linker designed for intracellular cleavage and high stability in circulation • Builds on clinical validation from Henlius (HLX43) and Pfizer (PDL1V) ADCs while differentiating via linker, payload, and combo utility Target Rationale • PD-L1 expression is elevated in numerous solid tumors compared to normal tissues, making it an attractive ADC target • ADCs targeting PD-L1 have shown potent cytotoxicity in vitro and anti-tumor activity in vivo; rationally designed to offer a dual mechanism of targeted tumor cell killing and induction of immunogenic cell death (ICD) • Synergistic potential with CR-001 and/or SOC provides further optionality for best-in-class combinations across many tumor types PD-L1 TUMOR CELL CR-002 • PD-L1 is expressed on tumor cells and reduces T-cell activation and cytotoxic function1, promotes immunosuppressive Treg function and survival2, and drives tumor cell proliferation and tissue invasion3 Supportive Data • CR-002 demonstrated robust anti- tumor activity compared to benchmark antibodies in identical DXd format • Pfizer’s PD-L1 MMAE ADC Phase 1 study (SGN-PDL1V) showed encouraging antitumor activity as monotherapy and in combination with I/O in NSCLC and HNSCC4 • Henlius’ PD-L1 Topo1i ADC (HLX43) study showed promising antitumor activity in NSCLC regardless of PD- L1 expression5 Key Anticipated Milestones • IND submission planned mid-2026 • H2 ‘26: Ph 1/2 initiation • H2 ‘27: Ph 1/2 data • 2027+: CR-002 + CR-001 combo initiation
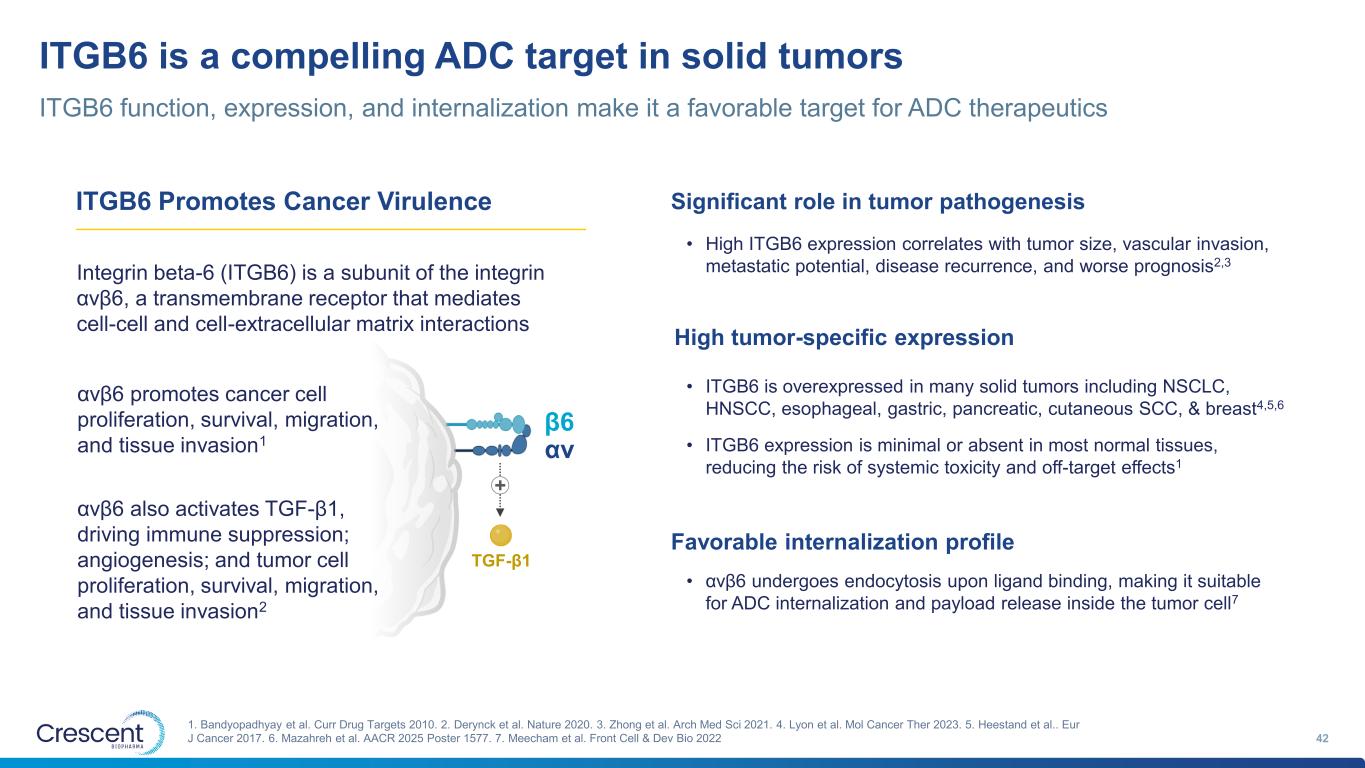
ITGB6 is a compelling ADC target in solid tumors ITGB6 function, expression, and internalization make it a favorable target for ADC therapeutics 42 1. Bandyopadhyay et al. Curr Drug Targets 2010. 2. Derynck et al. Nature 2020. 3. Zhong et al. Arch Med Sci 2021. 4. Lyon et al. Mol Cancer Ther 2023. 5. Heestand et al.. Eur J Cancer 2017. 6. Mazahreh et al. AACR 2025 Poster 1577. 7. Meecham et al. Front Cell & Dev Bio 2022 β6 αv TGF-β1 + αvβ6 promotes cancer cell proliferation, survival, migration, and tissue invasion1 Integrin beta-6 (ITGB6) is a subunit of the integrin αvβ6, a transmembrane receptor that mediates cell-cell and cell-extracellular matrix interactions αvβ6 also activates TGF-β1, driving immune suppression; angiogenesis; and tumor cell proliferation, survival, migration, and tissue invasion2 ITGB6 Promotes Cancer Virulence Significant role in tumor pathogenesis High tumor-specific expression Favorable internalization profile • ITGB6 is overexpressed in many solid tumors including NSCLC, HNSCC, esophageal, gastric, pancreatic, cutaneous SCC, & breast4,5,6 • ITGB6 expression is minimal or absent in most normal tissues, reducing the risk of systemic toxicity and off-target effects1 • High ITGB6 expression correlates with tumor size, vascular invasion, metastatic potential, disease recurrence, and worse prognosis2,3 • αvβ6 undergoes endocytosis upon ligand binding, making it suitable for ADC internalization and payload release inside the tumor cell7
CR-003 (SKB105): Differentiated ITGB6 ADC with Topo1i payload designed to be best-in-class 43Source: Kelun-Biotech Internal Data; DAR = drug-antibody ratio SKB105 from Kelun-Biotech is a Phase 1-ready ADC with strong preclinical data anti-β6 Fc silenced IgG1 mAb Antibody binding epitope distinct from SGN-B6A Topo1i payload Strong bystander killing effects Optimized linker-payload Differentiated version from other Kelun-Biotech ADCs, optimized for target Favorable safety in NHP toxicology Well-tolerated in cyno to 80 mpk/dose Q2W Higher internalization vs. SGN-B6A Superior payload delivery vs SGN-B6A, an ITGB6-directed ADC with MMAE payload Superior anti-tumor activity Superior anti-tumor response vs. SGN-B6A in CDX & PDX models of NSCLC & other cancers Superior PK profile vs. SGN-B6A Longer half life with minimal payload falloff; higher ADC AUC exposure improves efficacy Kthiol Oligopeptide linker Stable, cleavable, clinically-validated linker
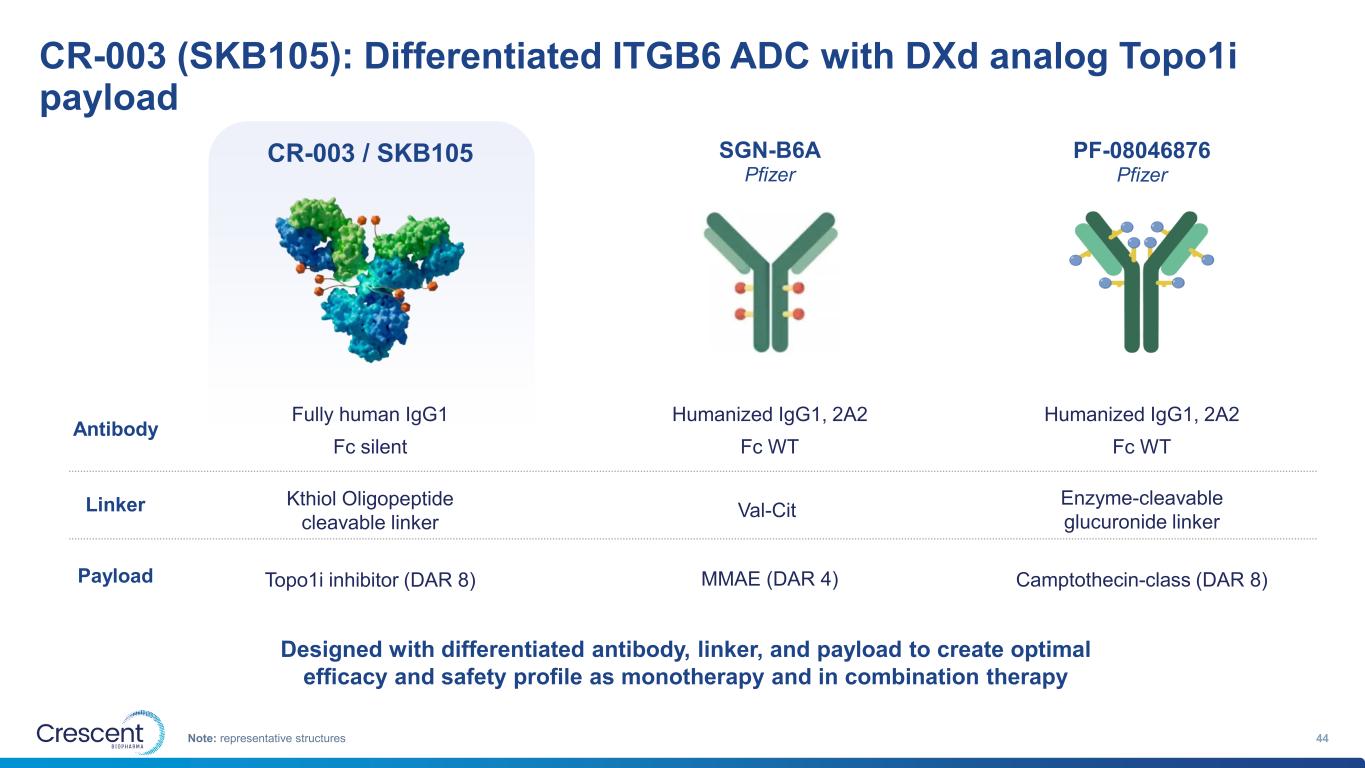
CR-003 (SKB105): Differentiated ITGB6 ADC with DXd analog Topo1i payload 44Note: representative structures CR-003 / SKB105 SGN-B6A Pfizer PF-08046876 Pfizer Kthiol Oligopeptide cleavable linker Topo1i inhibitor (DAR 8) Val-Cit MMAE (DAR 4) Enzyme-cleavable glucuronide linker Camptothecin-class (DAR 8) Fully human IgG1 Fc silent Humanized IgG1, 2A2 Fc WT Humanized IgG1, 2A2 Fc WT Antibody Linker Payload Designed with differentiated antibody, linker, and payload to create optimal efficacy and safety profile as monotherapy and in combination therapy
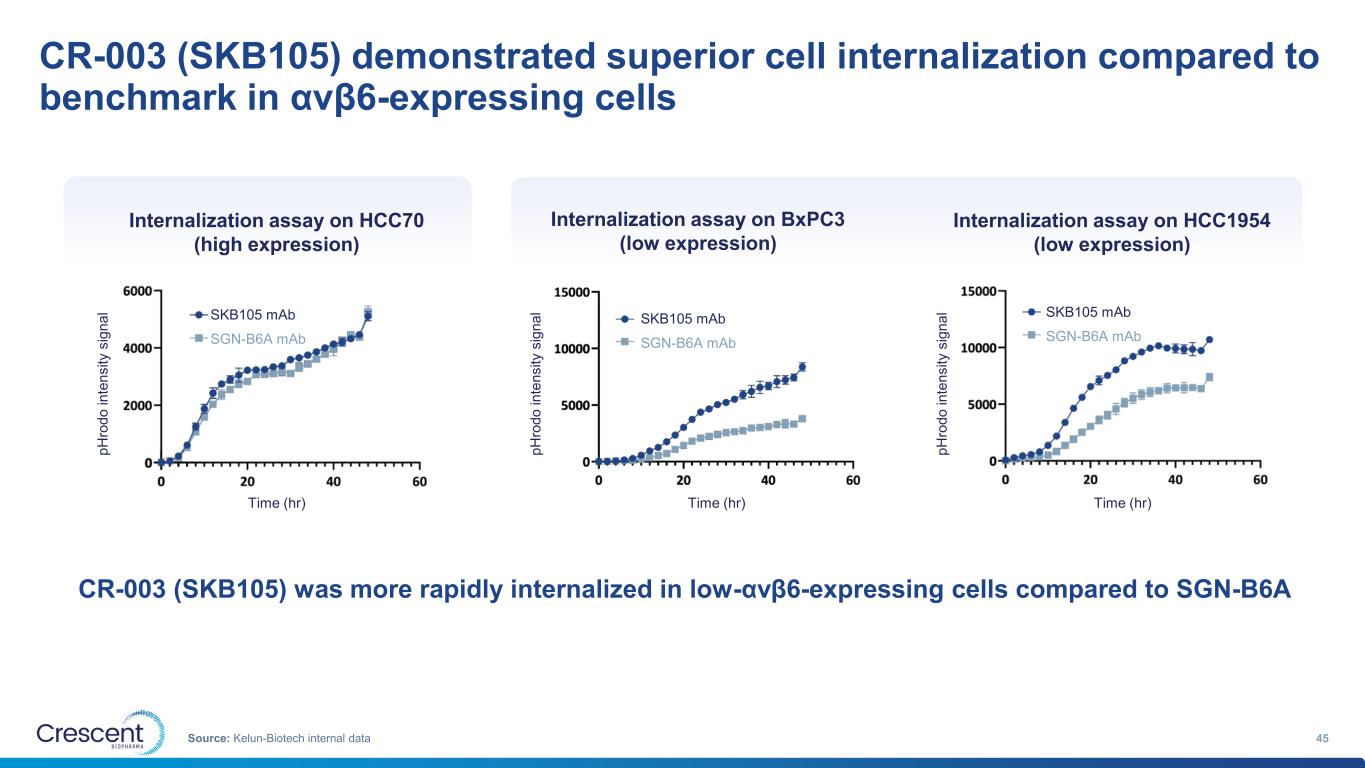
CR-003 (SKB105) demonstrated superior cell internalization compared to benchmark in αvβ6-expressing cells 45Source: Kelun-Biotech internal data CR-003 (SKB105) was more rapidly internalized in low-αvβ6-expressing cells compared to SGN-B6A Internalization assay on HCC70 (high expression) Internalization assay on BxPC3 (low expression) Internalization assay on HCC1954 (low expression) p H ro d o i n te n s it y s ig n a l p H ro d o i n te n s it y s ig n a l p H ro d o i n te n s it y s ig n a l Time (hr) Time (hr) Time (hr) SKB105 mAb SGN-B6A mAb SKB105 mAb SGN-B6A mAb SKB105 mAb SGN-B6A mAb
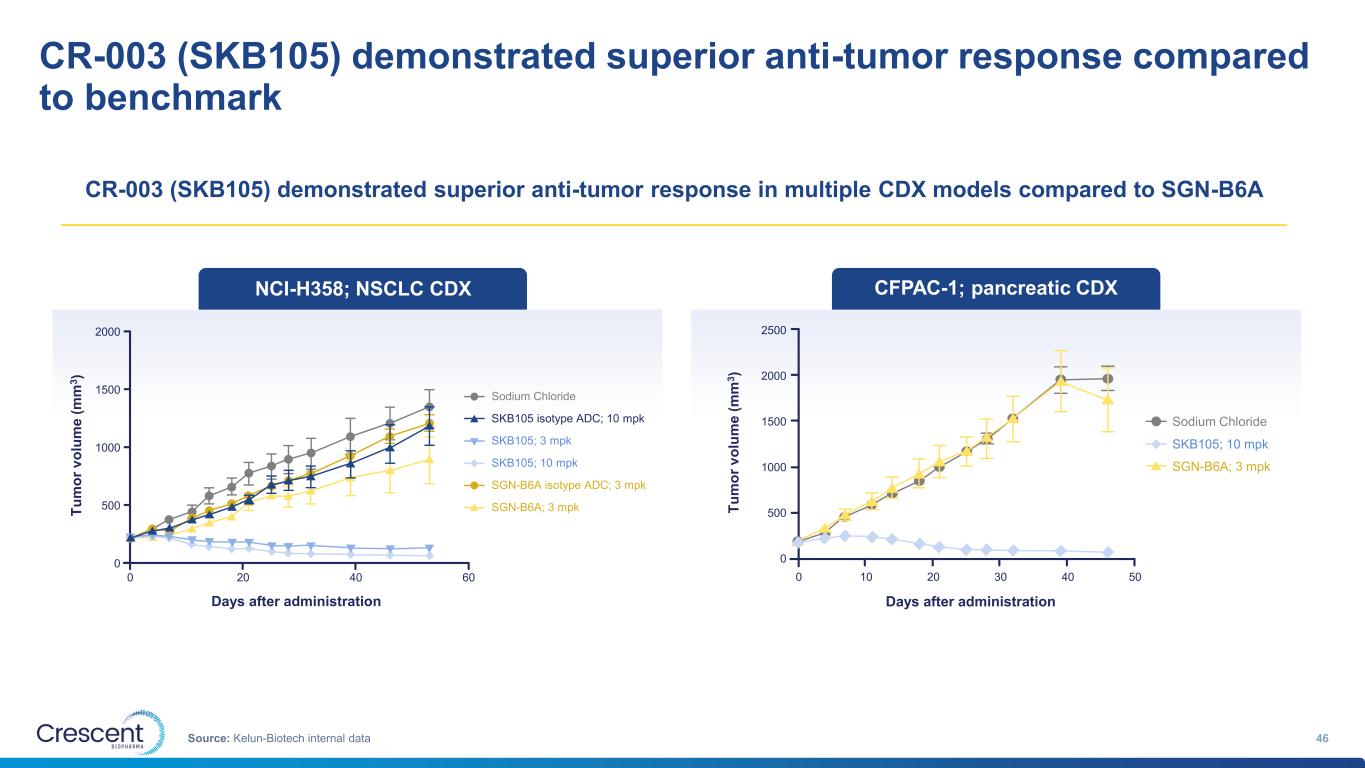
CR-003 (SKB105) demonstrated superior anti-tumor response compared to benchmark 46Source: Kelun-Biotech internal data NCI-H358; NSCLC CDX Sodium Chloride SKB105 isotype ADC; 10 mpk SKB105; 3 mpk SKB105; 10 mpk SGN-B6A isotype ADC; 3 mpk SGN-B6A; 3 mpk 0 20 40 60 0 500 1000 1500 2000 T u m o r v o lu m e ( m m 3 ) Days after administration CFPAC-1; pancreatic CDX T u m o r v o lu m e ( m m 3 ) Days after administration 0 10 20 30 40 50 500 1000 1500 2000 2500 0 CR-003 (SKB105) demonstrated superior anti-tumor response in multiple CDX models compared to SGN-B6A Sodium Chloride SKB105; 10 mpk SGN-B6A; 3 mpk
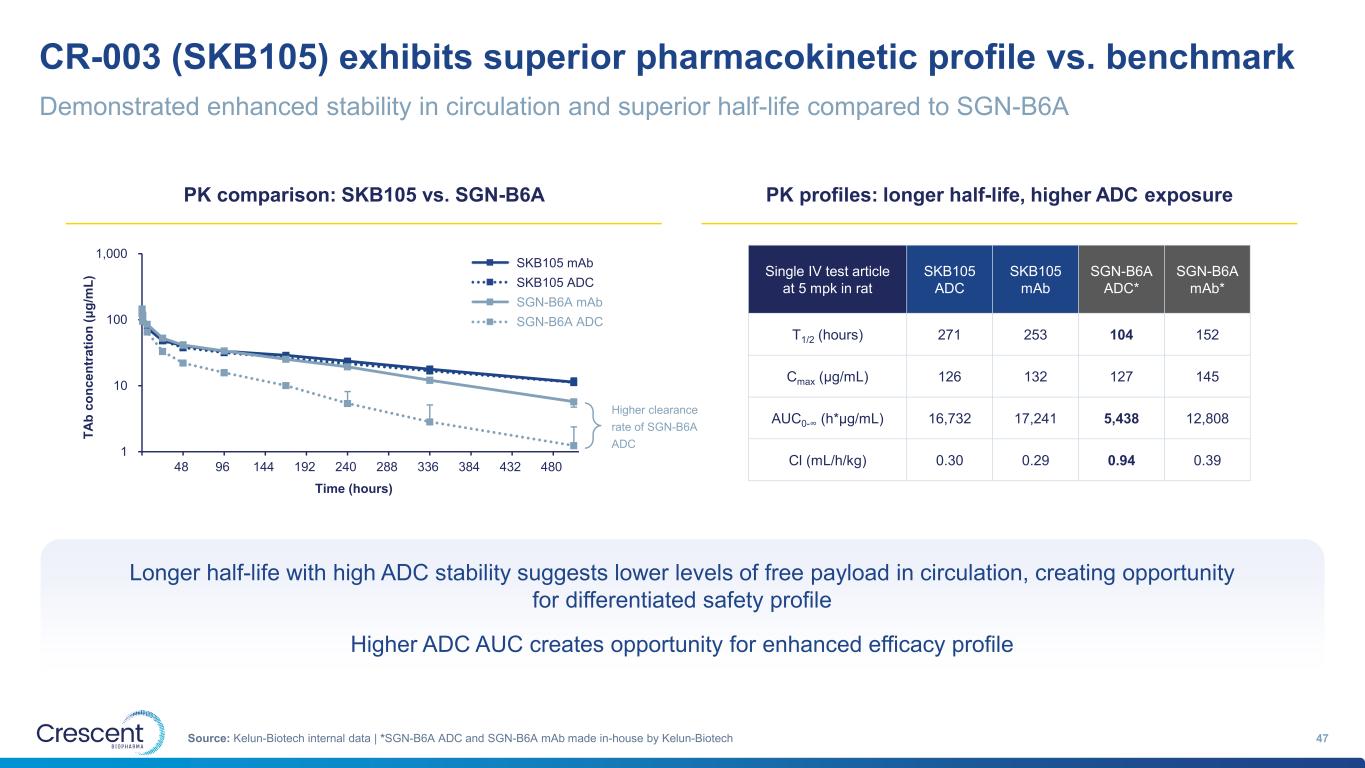
CR-003 (SKB105) exhibits superior pharmacokinetic profile vs. benchmark 47Source: Kelun-Biotech internal data | *SGN-B6A ADC and SGN-B6A mAb made in-house by Kelun-Biotech PK comparison: SKB105 vs. SGN-B6A PK profiles: longer half-life, higher ADC exposure Single IV test article at 5 mpk in rat SKB105 ADC SKB105 mAb SGN-B6A ADC* SGN-B6A mAb* T1/2 (hours) 271 253 104 152 Cmax (μg/mL) 126 132 127 145 AUC0-∞ (h*μg/mL) 16,732 17,241 5,438 12,808 Cl (mL/h/kg) 0.30 0.29 0.94 0.39 Longer half-life with high ADC stability suggests lower levels of free payload in circulation, creating opportunity for differentiated safety profile Higher ADC AUC creates opportunity for enhanced efficacy profile Demonstrated enhanced stability in circulation and superior half-life compared to SGN-B6A 1 10 100 1,000 48 96 144 192 240 288 336 384 432 480 G1: RL12 (5 mg/kg) G2: RL12-566 (5 mg/kg) G5: 2A2 (5 mg/kg) G6: 2A2-220 (5 mg/kg) Higher clearance rate of SGN-B6A ADC T A b c o n c e n tr a ti o n ( μ g /m L ) Time (hours) SKB105 mAb SKB105 ADC SGN-B6A mAb SGN-B6A ADC
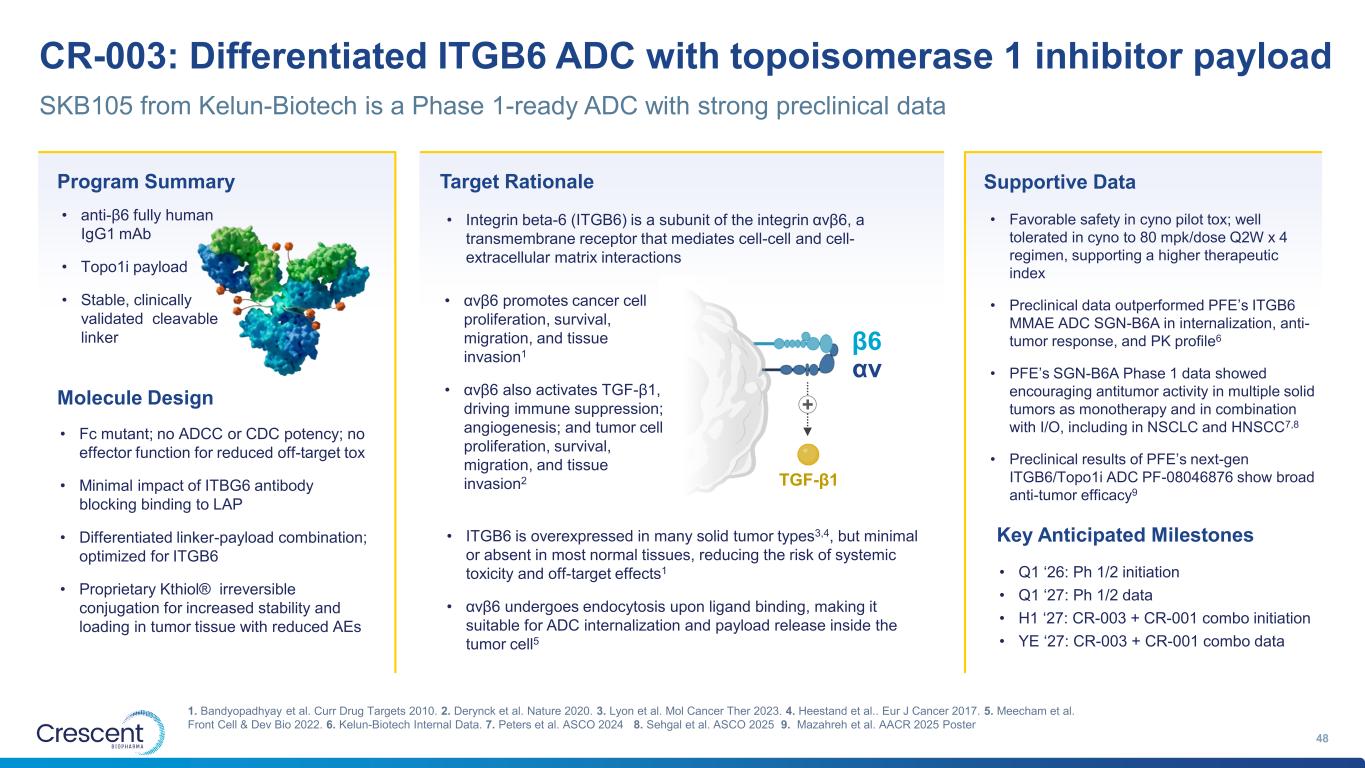
CR-003: Differentiated ITGB6 ADC with topoisomerase 1 inhibitor payload 48 1. Bandyopadhyay et al. Curr Drug Targets 2010. 2. Derynck et al. Nature 2020. 3. Lyon et al. Mol Cancer Ther 2023. 4. Heestand et al.. Eur J Cancer 2017. 5. Meecham et al. Front Cell & Dev Bio 2022. 6. Kelun-Biotech Internal Data. 7. Peters et al. ASCO 2024 8. Sehgal et al. ASCO 2025 9. Mazahreh et al. AACR 2025 Poster SKB105 from Kelun-Biotech is a Phase 1-ready ADC with strong preclinical data Program Summary • anti-β6 fully human IgG1 mAb • Topo1i payload • Stable, clinically validated cleavable linker Molecule Design • Fc mutant; no ADCC or CDC potency; no effector function for reduced off-target tox • Minimal impact of ITBG6 antibody blocking binding to LAP • Differentiated linker-payload combination; optimized for ITGB6 • Proprietary Kthiol® irreversible conjugation for increased stability and loading in tumor tissue with reduced AEs Target Rationale • ITGB6 is overexpressed in many solid tumor types3,4, but minimal or absent in most normal tissues, reducing the risk of systemic toxicity and off-target effects1 • αvβ6 undergoes endocytosis upon ligand binding, making it suitable for ADC internalization and payload release inside the tumor cell5 • Integrin beta-6 (ITGB6) is a subunit of the integrin αvβ6, a transmembrane receptor that mediates cell-cell and cell- extracellular matrix interactions Supportive Data • Favorable safety in cyno pilot tox; well tolerated in cyno to 80 mpk/dose Q2W x 4 regimen, supporting a higher therapeutic index • Preclinical data outperformed PFE’s ITGB6 MMAE ADC SGN-B6A in internalization, anti- tumor response, and PK profile6 • PFE’s SGN-B6A Phase 1 data showed encouraging antitumor activity in multiple solid tumors as monotherapy and in combination with I/O, including in NSCLC and HNSCC7,8 • Preclinical results of PFE’s next-gen ITGB6/Topo1i ADC PF-08046876 show broad anti-tumor efficacy9 Key Anticipated Milestones • Q1 ‘26: Ph 1/2 initiation • Q1 ‘27: Ph 1/2 data • H1 ‘27: CR-003 + CR-001 combo initiation • YE ‘27: CR-003 + CR-001 combo data β6 αv TGF-β1 + • αvβ6 promotes cancer cell proliferation, survival, migration, and tissue invasion1 • αvβ6 also activates TGF-β1, driving immune suppression; angiogenesis; and tumor cell proliferation, survival, migration, and tissue invasion2
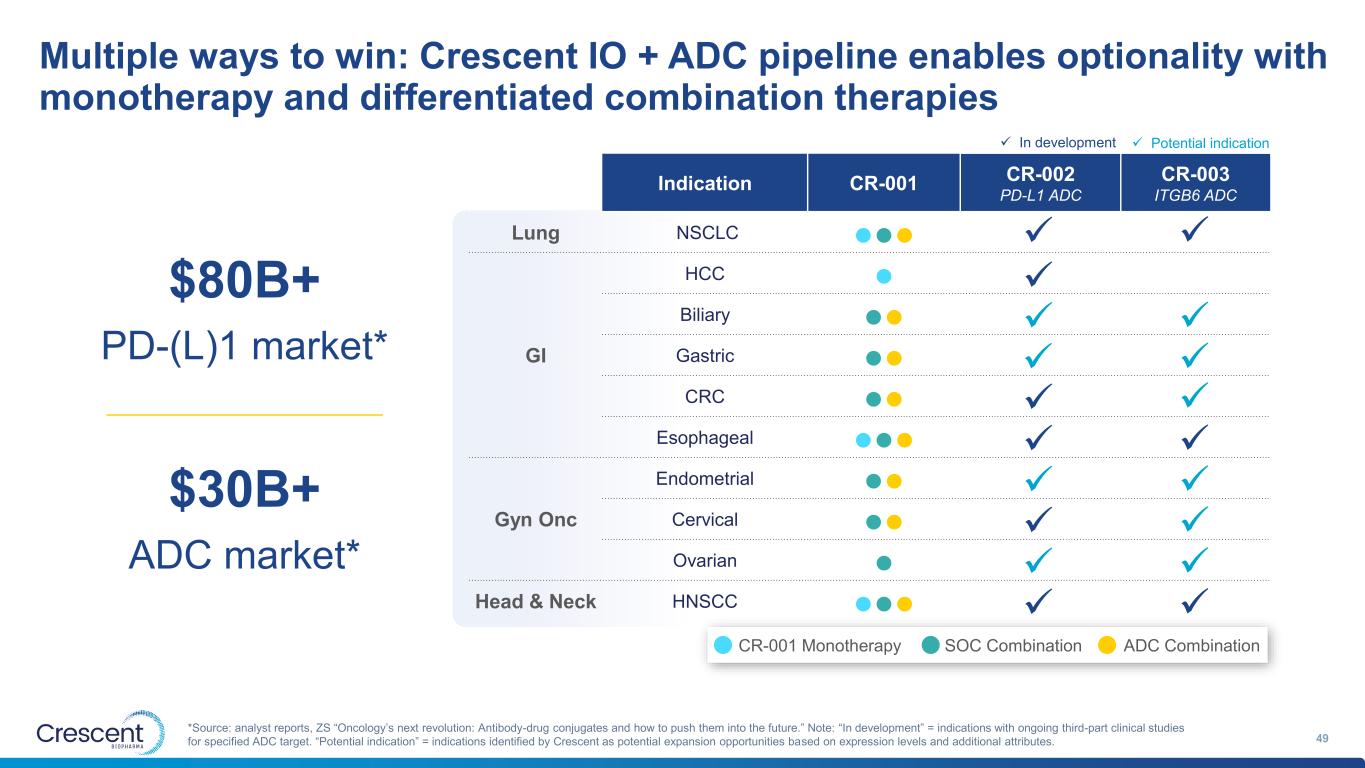
Multiple ways to win: Crescent IO + ADC pipeline enables optionality with monotherapy and differentiated combination therapies 49 *Source: analyst reports, ZS “Oncology’s next revolution: Antibody-drug conjugates and how to push them into the future.” Note: “In development” = indications with ongoing third-part clinical studies for specified ADC target. “Potential indication” = indications identified by Crescent as potential expansion opportunities based on expression levels and additional attributes. Indication CR-001 CR-002 CR-003 PD-L1 ADC ITGB6 ADC Lung NSCLC ●●● GI HCC ● Biliary ●● Gastric ●● CRC ●● Esophageal ●●● Gyn Onc Endometrial ●● Cervical ●● Ovarian ● Head & Neck HNSCC ●●● ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ $30B+ ADC market* $80B+ PD-(L)1 market* ✓ ✓ In development ✓ Potential indication CR-001 Monotherapy SOC Combination ADC Combination ✓
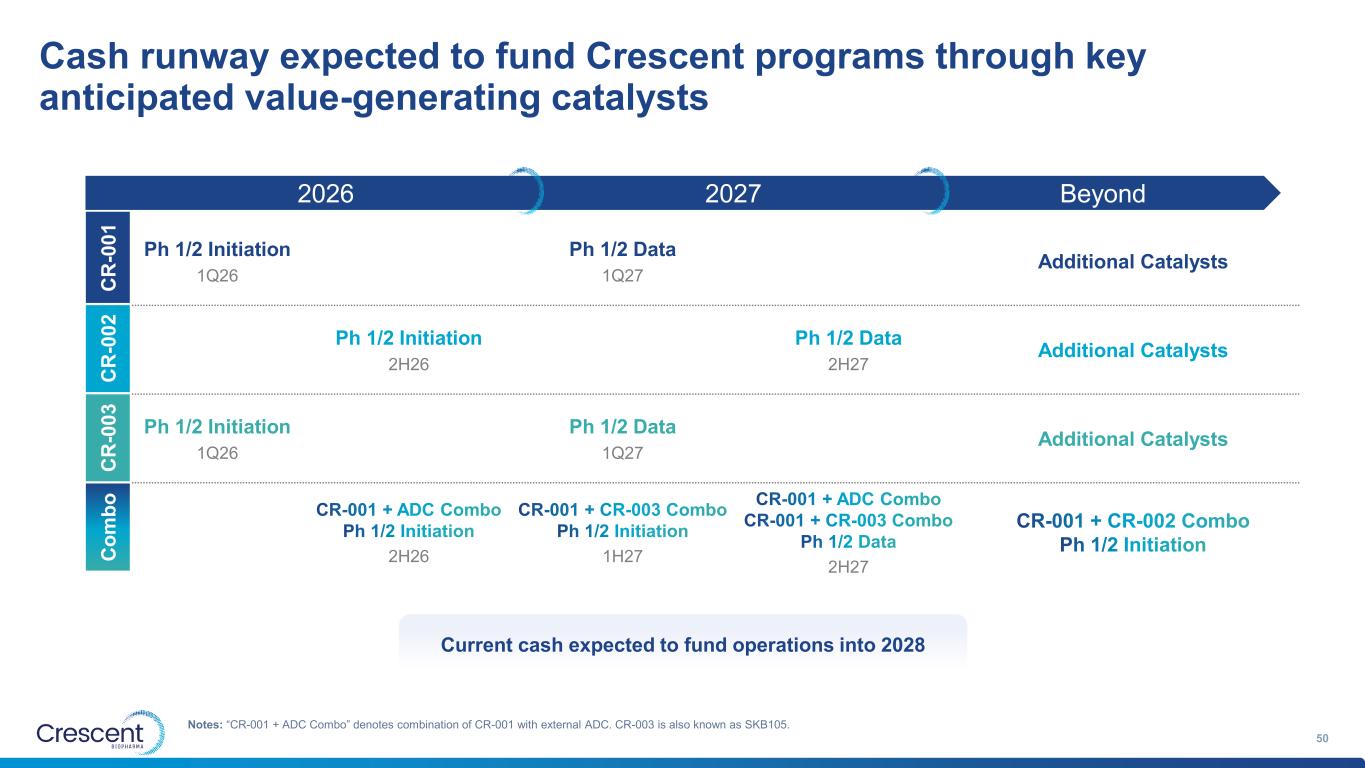
Current cash expected to fund operations into 2028 2027 Cash runway expected to fund Crescent programs through key anticipated value-generating catalysts 50 Notes: “CR-001 + ADC Combo” denotes combination of CR-001 with external ADC. CR-003 is also known as SKB105. 2026 Beyond Ph 1/2 Initiation 1Q26 Ph 1/2 Data 1Q27 Additional Catalysts Ph 1/2 Initiation 2H26 Ph 1/2 Data 2H27 Additional Catalysts Ph 1/2 Initiation 1Q26 Ph 1/2 Data 1Q27 Additional Catalysts CR-001 + ADC Combo Ph 1/2 Initiation 2H26 CR-001 + CR-003 Combo Ph 1/2 Initiation 1H27 CR-001 + ADC Combo CR-001 + CR-003 Combo Ph 1/2 Data 2H27 CR-001 + CR-002 Combo Ph 1/2 Initiation C R -0 0 1 C R -0 0 2 C R -0 0 3 C o m b o

Opening Remarks Joshua Brumm CEO Overview of Kelun-Biotech Partnership Jonathan McNeill, M.D. President & COO Clinical Development Plan for CR-001, PD-1 x VEGF Bispecific Antibody Ellie Im, M.D. Chief Medical Officer ADC Programs: CR-002 and CR-003 (SKB105) Jan Pinkas, Ph.D. Chief Scientific Officer Q&A 51 Program

SEIZE THE MOMENT for a brighter future Delivering the next wave of cancer therapies
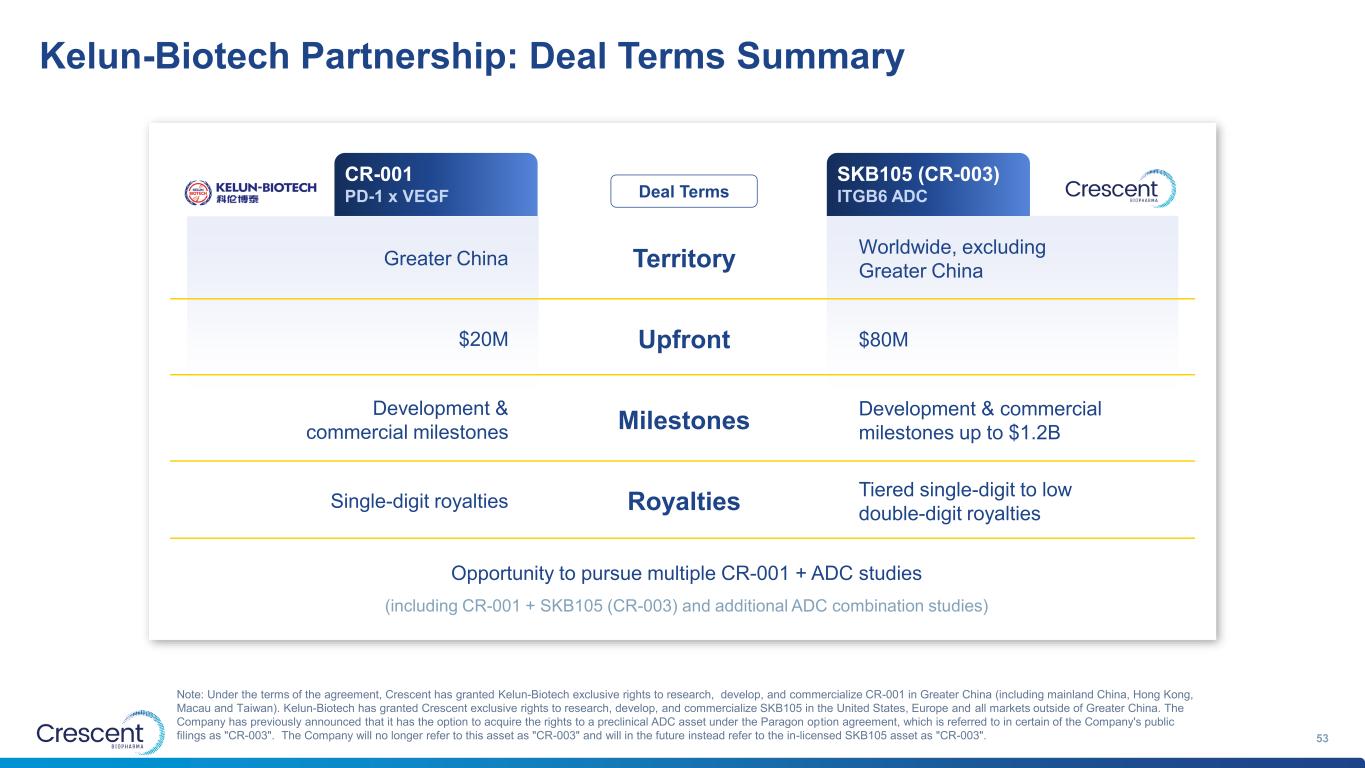
Kelun-Biotech Partnership: Deal Terms Summary 53 Note: Under the terms of the agreement, Crescent has granted Kelun-Biotech exclusive rights to research, develop, and commercialize CR-001 in Greater China (including mainland China, Hong Kong, Macau and Taiwan). Kelun-Biotech has granted Crescent exclusive rights to research, develop, and commercialize SKB105 in the United States, Europe and all markets outside of Greater China. The Company has previously announced that it has the option to acquire the rights to a preclinical ADC asset under the Paragon option agreement, which is referred to in certain of the Company's public filings as "CR-003". The Company will no longer refer to this asset as "CR-003" and will in the future instead refer to the in-licensed SKB105 asset as "CR-003". Territory Upfront Milestones Royalties Greater China $20M Single-digit royalties Development & commercial milestones Opportunity to pursue multiple CR-001 + ADC studies Worldwide, excluding Greater China $80M Tiered single-digit to low double-digit royalties Development & commercial milestones up to $1.2B SKB105 (CR-003) ITGB6 ADCDeal Terms CR-001 PD-1 x VEGF (including CR-001 + SKB105 (CR-003) and additional ADC combination studies)