

Investor Presentation October 2025 .2

Legal Disclaimers Various statements made in this presentation are forward-looking, within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, are forward-looking statements, including but not limited to statements regarding: our expectations regarding our clinical development and regulatory plans; our belief that DURAVYU™ is on track to be the first-to-market of the current investigational sustained release treatments for wet AMD; our belief that DURAVYU has two potential blockbuster indications; our belief that DURAVYU’s potential real-world application in multiple retinal disease indications and de-risked trial designs position DURAVYU for clinical and commercial success; our expected timing for the availability and release of clinical data; our expected cash runway; our belief that DURAVYU has the potential to maintain a majority of patients with active disease with no supplemental anti-VEGF therapy for six months or longer; our expectations regarding our manufacturing capabilities; and our expectations regarding the timing and clinical development of our other product candidates, including EYP-2301. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause EyePoint’s actual results to be materially different than those expressed in or implied by EyePoint’s forward-looking statements. For EyePoint, these risks and uncertainties include the timing, progress and results of the company’s clinical development activities, including DURAVYU; uncertainties and delays relating to communications with the U.S. Food and Drug Administration and the ability to obtain regulatory approval from FDA for the commercialization of DURAVYU; unanticipated costs and expenses; the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the risk that results of clinical trials may not be predictive of future results, and interim and preliminary data are subject to further analysis and may change as more data becomes available; unexpected safety or efficacy data observed during clinical trials; uncertainties related to the regulatory authorization or approval process, and available development and regulatory pathways for approval of the Company’s product candidates; changes in the regulatory environment; disruptions at the FDA, including due to a reduction in the FDA’s workforce and/or inadequate funding for the FDA; the impact of the government shutdown on our business operations; changes in U.S. and international trade policies; changes in expected or existing competition; the success of current and future license agreements; our dependence on contract research organizations, and other outside vendors and service providers; product liability; the impact of general business and economic conditions; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; delays, interruptions or failures in the manufacture and supply of our product candidates; the availability of and the need for additional financing; our ability to obtain additional funding to support our clinical development programs; uncertainties regarding the timing and results of the August 2022 subpoena from the U.S. Attorney’s Office for the District of Massachusetts; uncertainties regarding the FDA warning letter pertaining to our Watertown, MA manufacturing facility; and other factors described in our filings with the Securities and Exchange Commission (SEC). More detailed information on these and additional factors that could affect our actual results are described in our filings with the SEC, including our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, as revised or supplemented by our Quarterly Reports on Form 10-Q and other documents filed with the SEC. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. EyePoint undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise.
A Leader in Sustained Release Drug Delivery for Retinal Disease 1. Unaudited estimate for September 30, 2025. Our actual results as of September 30, 2025, may differ from the preliminary financial data due to the completion of our closing procedures with respect to the fiscal quarter ended September 30, 2025, final adjustments and other developments that may arise between now and the time the financial results for the fiscal quarter are finalized. wet AMD, wet age-related macular degeneration; DME, diabetic macular edema Veteran leadership team with 3+ decades of clinical drug development, commercial, manufacturing and ophthalmology experience Durasert® delivery technology with strong safety profile across multiple FDA approved products and indications Commercial scale-up underway in state-of-art integrated US manufacturing facility $200M+1 at 9/30/25 - runway into 2027 beyond Phase 3 wet AMD data DURAVYU™ Phase 3 DME program underway supported by new data demonstrating dual inhibition of both VEGF and IL-6 Two Phase 3 trials fully enrolled for DURAVYU™ in wet AMD with data anticipated in mid-2026 DURAVYUTM has been conditionally accepted by the FDA as the proprietary name for DURAVYU. DURAVYU is an investigational product; it has not been approved by the FDA. FDA approval and the timeline for potential approval is uncertain. These data are preliminary. Conclusive evidence of efficacy and safety of DURAVYU will require further investigation in well-controlled Phase 3 clinical trials.
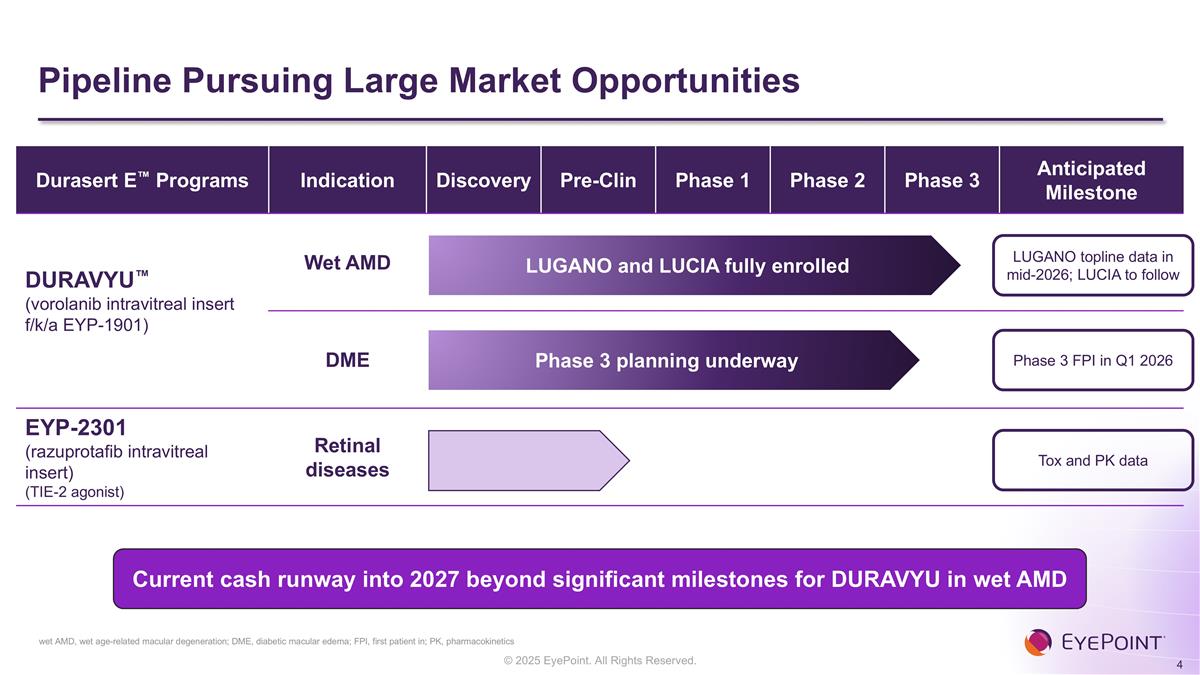
Pipeline Pursuing Large Market Opportunities wet AMD, wet age-related macular degeneration; DME, diabetic macular edema; FPI, first patient in; PK, pharmacokinetics Durasert E™ Programs Indication Discovery Pre-Clin Phase 1 Phase 2 Phase 3 Anticipated Milestone DURAVYU™ (vorolanib intravitreal insert f/k/a EYP-1901) Wet AMD DME EYP-2301 (razuprotafib intravitreal insert) (TIE-2 agonist) Retinal diseases LUGANO topline data in mid-2026; LUCIA to follow Phase 3 FPI in Q1 2026 Tox and PK data LUGANO and LUCIA fully enrolled Phase 3 planning underway Current cash runway into 2027 beyond significant milestones for DURAVYU in wet AMD
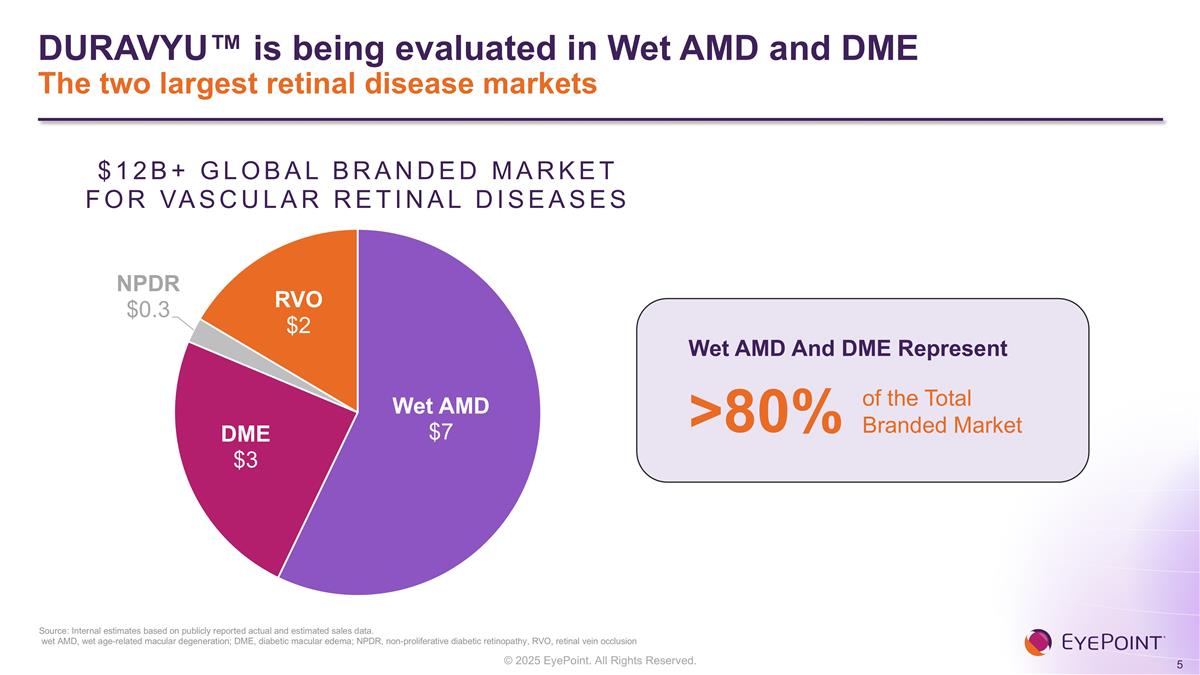
Source: Internal estimates based on publicly reported actual and estimated sales data. wet AMD, wet age-related macular degeneration; DME, diabetic macular edema; NPDR, non-proliferative diabetic retinopathy, RVO, retinal vein occlusion Wet AMD And DME Represent >80% of the Total Branded Market DURAVYU™ is being evaluated in Wet AMD and DME The two largest retinal disease markets
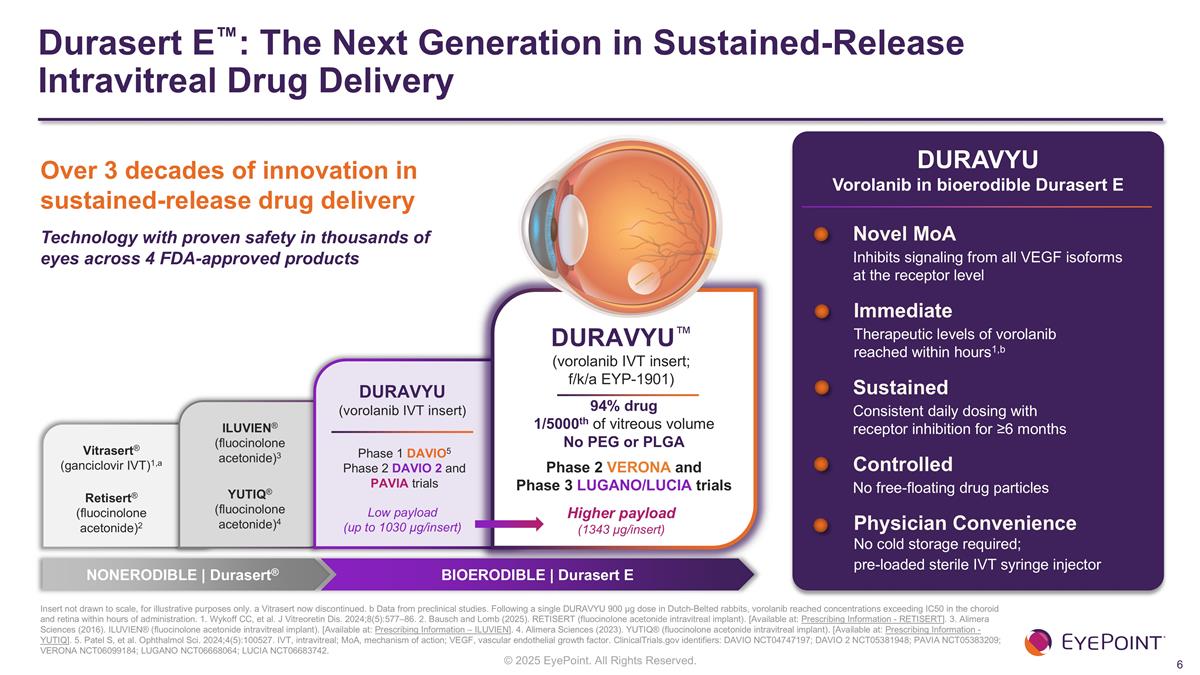
Durasert E™: The Next Generation in Sustained-Release Intravitreal Drug Delivery Insert not drawn to scale, for illustrative purposes only. a Vitrasert now discontinued. b Data from preclinical studies. Following a single DURAVYU 900 µg dose in Dutch-Belted rabbits, vorolanib reached concentrations exceeding IC50 in the choroid and retina within hours of administration. 1. Wykoff CC, et al. J Vitreoretin Dis. 2024;8(5):577–86. 2. Bausch and Lomb (2025). RETISERT (fluocinolone acetonide intravitreal implant). [Available at: Prescribing Information - RETISERT]. 3. Alimera Sciences (2016). ILUVIEN® (fluocinolone acetonide intravitreal implant). [Available at: Prescribing Information – ILUVIEN]. 4. Alimera Sciences (2023). YUTIQ® (fluocinolone acetonide intravitreal implant). [Available at: Prescribing Information - YUTIQ]. 5. Patel S, et al. Ophthalmol Sci. 2024;4(5):100527. IVT, intravitreal; MoA, mechanism of action; VEGF, vascular endothelial growth factor. ClinicalTrials.gov identifiers: DAVIO NCT04747197; DAVIO 2 NCT05381948; PAVIA NCT05383209; VERONA NCT06099184; LUGANO NCT06668064; LUCIA NCT06683742. 94% drug 1/5000th of vitreous volume No PEG or PLGA Phase 2 VERONA and Phase 3 LUGANO/LUCIA trials Vitrasert® (ganciclovir IVT)1,a Retisert® (fluocinolone acetonide)2 ILUVIEN® (fluocinolone acetonide)3 YUTIQ® (fluocinolone acetonide)4 DURAVYU™ (vorolanib IVT insert; f/k/a EYP-1901) NONERODIBLE | Durasert® BIOERODIBLE | Durasert E Over 3 decades of innovation in sustained-release drug delivery Technology with proven safety in thousands of eyes across 4 FDA-approved products Immediate Therapeutic levels of vorolanib reached within hours1,b Sustained Consistent daily dosing with receptor inhibition for ≥6 months Controlled No free-floating drug particles Physician Convenience No cold storage required; pre-loaded sterile IVT syringe injector DURAVYU Vorolanib in bioerodible Durasert E DURAVYU (vorolanib IVT insert) Low payload (up to 1030 µg/insert) Higher payload (1343 µg/insert) Phase 1 DAVIO5 Phase 2 DAVIO 2 and PAVIA trials Novel MoA Inhibits signaling from all VEGF isoforms at the receptor level
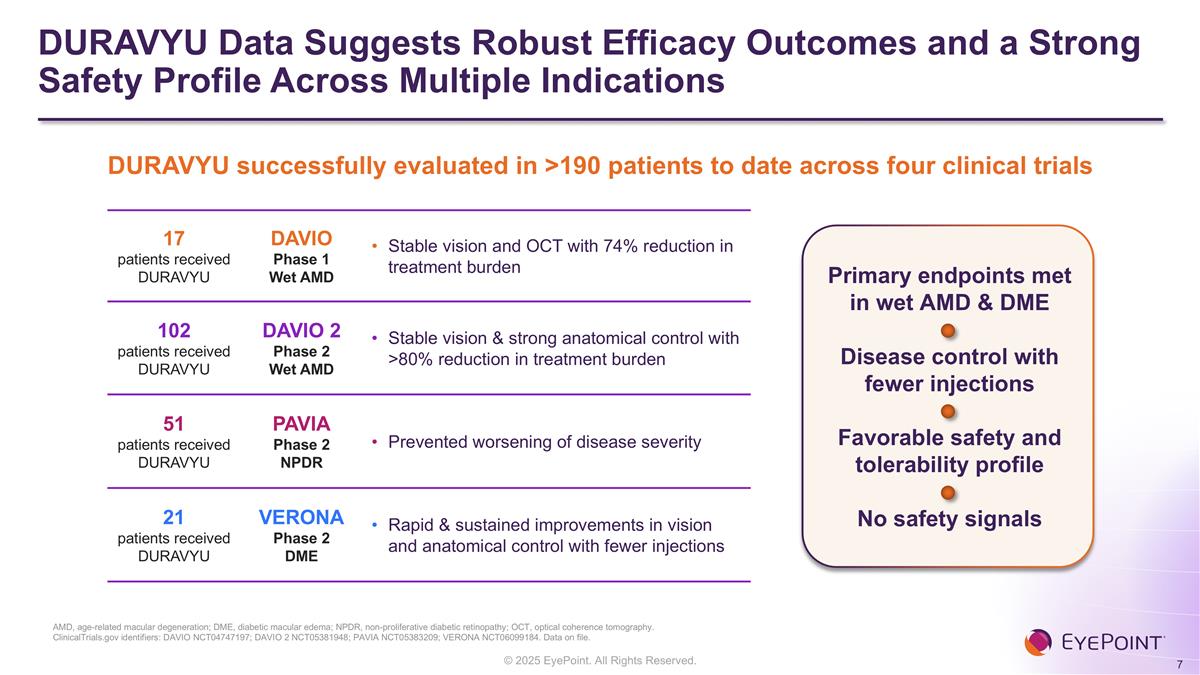
DURAVYU Data Suggests Robust Efficacy Outcomes and a Strong Safety Profile Across Multiple Indications AMD, age-related macular degeneration; DME, diabetic macular edema; NPDR, non-proliferative diabetic retinopathy; OCT, optical coherence tomography. ClinicalTrials.gov identifiers: DAVIO NCT04747197; DAVIO 2 NCT05381948; PAVIA NCT05383209; VERONA NCT06099184. Data on file. DURAVYU successfully evaluated in >190 patients to date across four clinical trials 17 patients received DURAVYU DAVIO Phase 1 Wet AMD Stable vision and OCT with 74% reduction in treatment burden 102 patients received DURAVYU DAVIO 2 Phase 2 Wet AMD Stable vision & strong anatomical control with >80% reduction in treatment burden 51 patients received DURAVYU PAVIA Phase 2 NPDR Prevented worsening of disease severity 21 patients received DURAVYU VERONA Phase 2 DME Rapid & sustained improvements in vision and anatomical control with fewer injections Primary endpoints met in wet AMD & DME Disease control with fewer injections Favorable safety and tolerability profile No safety signals

DURAVYU for Wet AMD Fully enrolled Phase 3 pivotal program on track to be first sustained-release TKI to market
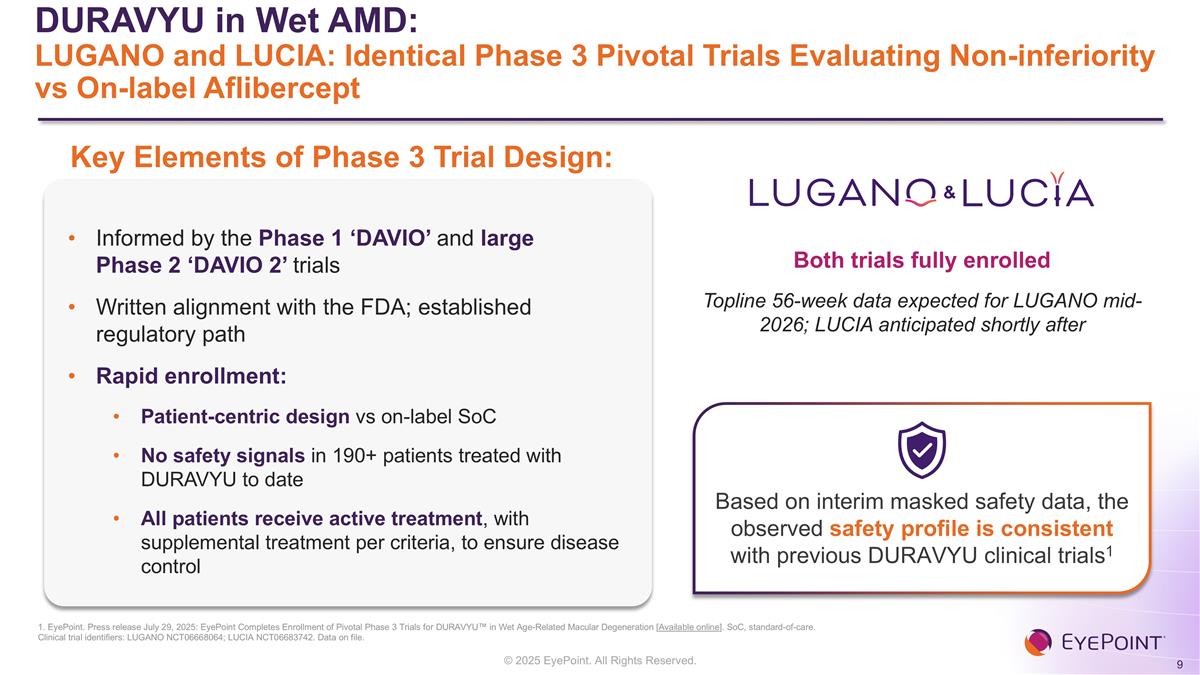
DURAVYU in Wet AMD: LUGANO and LUCIA: Identical Phase 3 Pivotal Trials Evaluating Non-inferiority vs On-label Aflibercept & Based on interim masked safety data, the observed safety profile is consistent with previous DURAVYU clinical trials1 Both trials fully enrolled Topline 56-week data expected for LUGANO mid-2026; LUCIA anticipated shortly after Informed by the Phase 1 ‘DAVIO’ and large Phase 2 ‘DAVIO 2’ trials Written alignment with the FDA; established regulatory path Rapid enrollment: Patient-centric design vs on-label SoC No safety signals in 190+ patients treated with DURAVYU to date All patients receive active treatment, with supplemental treatment per criteria, to ensure disease control Key Elements of Phase 3 Trial Design: 1. EyePoint. Press release July 29, 2025: EyePoint Completes Enrollment of Pivotal Phase 3 Trials for DURAVYU™ in Wet Age-Related Macular Degeneration [Available online]. SoC, standard-of-care. Clinical trial identifiers: LUGANO NCT06668064; LUCIA NCT06683742. Data on file.
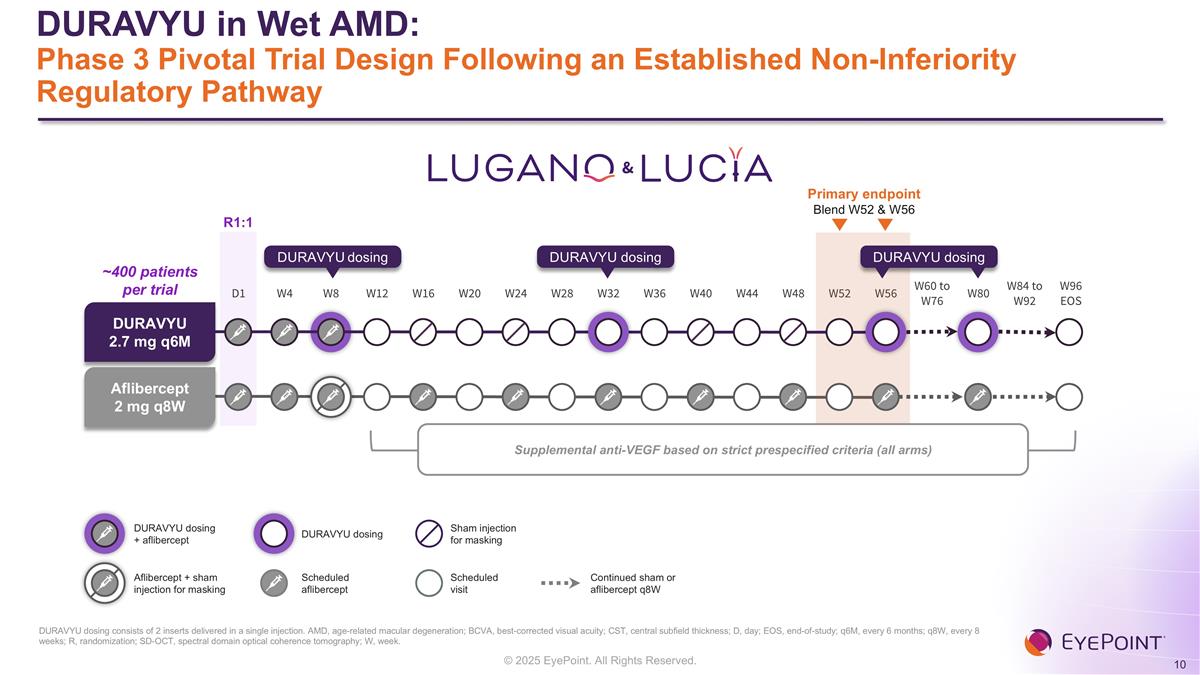
DURAVYU in Wet AMD: Phase 3 Pivotal Trial Design Following an Established Non-Inferiority Regulatory Pathway DURAVYU dosing consists of 2 inserts delivered in a single injection. AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; CST, central subfield thickness; D, day; EOS, end-of-study; q6M, every 6 months; q8W, every 8 weeks; R, randomization; SD-OCT, spectral domain optical coherence tomography; W, week. & Scheduled aflibercept Scheduled visit Sham injection for masking Continued sham or aflibercept q8W D1 W4 W8 W12 W16 W20 W24 W28 W32 W36 W40 W44 W48 W52 W56 W60 to W76 W80 W84 to W92 W96 EOS Primary endpoint Blend W52 & W56 DURAVYU 2.7 mg q6M Aflibercept 2 mg q8W DURAVYU dosing DURAVYU dosing DURAVYU dosing ~400 patients per trial Supplemental anti-VEGF based on strict prespecified criteria (all arms) R1:1 DURAVYU dosing + aflibercept Aflibercept + sham injection for masking DURAVYU dosing

Commercial Manufacturing Facility to Support DURAVYU through Potential NDA Approval and Commercial Launch cGMP, current good manufacturing practices; FDA, Federal Drug Administration; EMA, European Medicines Agency; cGMP, current good manufacturing practice USA based in Northbridge, MA Built to US FDA and EU EMA standards DURAVYU registration batches underway to support future NDA filing Built to EYPT specifications by landlord preserving upfront cash investment 41,000sf commercial facility

DURAVYU for DME Phase 3 DME program plan underway Supported by Multi-MOA inhibition of both VEGF and IL-6
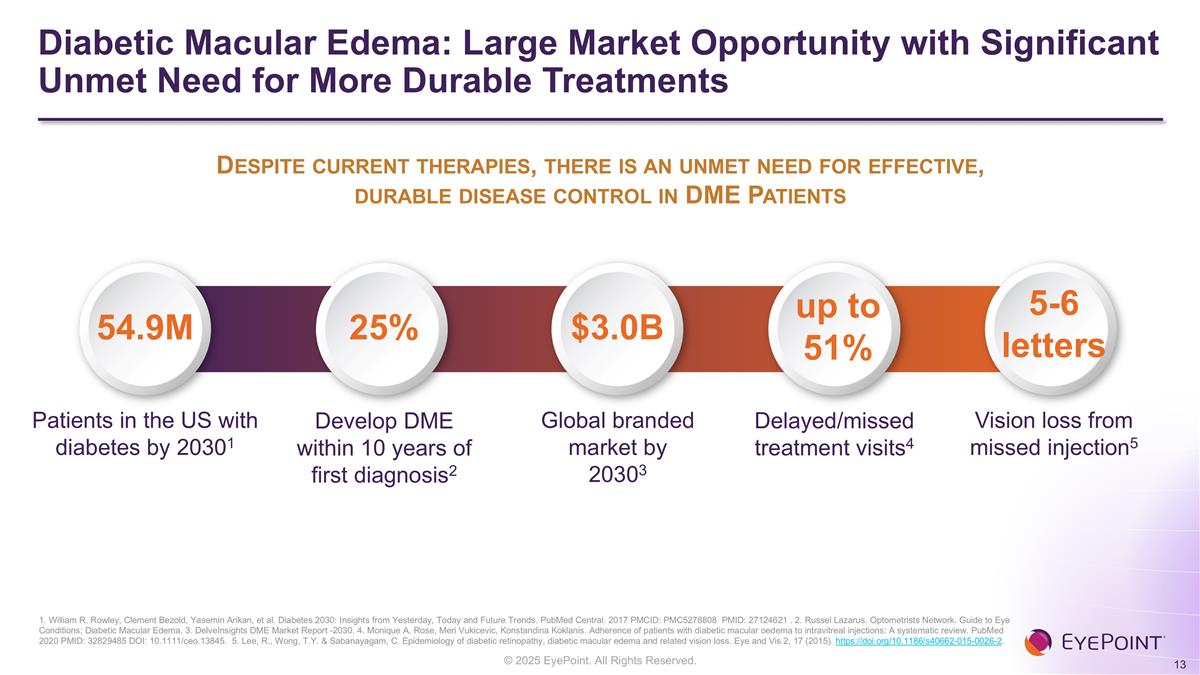
Diabetic Macular Edema: Large Market Opportunity with Significant Unmet Need for More Durable Treatments 1. William R. Rowley, Clement Bezold, Yasemin Arikan, et al. Diabetes 2030: Insights from Yesterday, Today and Future Trends. PubMed Central. 2017 PMCID: PMC5278808 PMID: 27124621 . 2. Russel Lazarus. Optometrists Network. Guide to Eye Conditions; Diabetic Macular Edema. 3. DelveInsights DME Market Report -2030. 4. Monique A. Rose, Meri Vukicevic, Konstandina Koklanis. Adherence of patients with diabetic macular oedema to intravitreal injections: A systematic review. PubMed 2020 PMID: 32829485 DOI: 10.1111/ceo.13845. 5. Lee, R., Wong, T.Y. & Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and Vis 2, 17 (2015). https://doi.org/10.1186/s40662-015-0026-2. Patients in the US with diabetes by 20301 Global branded market by 20303 Delayed/missed treatment visits4 54.9M 25% up to 51% $3.0B Develop DME within 10 years of first diagnosis2 Vision loss from missed injection5 5-6 letters Despite current therapies, there is an unmet need for effective, durable disease control in DME Patients
DME is the Second-Largest Retinal Disease Market Unmet Needs: Longer-Duration Therapies AND Inflammation Control Insert not drawn to scale, for illustrative purposes only. 1. Kuo BL et al. Ophthalmol Retina. 2024;8:1074–1082. 2. Rose MA et al. Clin Exp Ophthalmol. 2020;48(9):1286–1298. 3. Bressler NM, et al. JAMA Ophthalmol. 2018;136(3):257–69. 4. Maturi RK, et al. JAMA Ophthalmol. 2017;136(1):29–38. DME, diabetic macular edema; VEGF, vascular endothelial growth factor. Despite current therapies, there are two unmet needs in DME therapy: DURAVYU provides sustained drug release for ≥6 months with Vorolanib, a multi-target TKI Effective, durable disease control via sustained release Many patients still lose vision despite available therapies1,2 AND The need to treat inflammation VEGF suppression is not the entire story3,4 The Solution
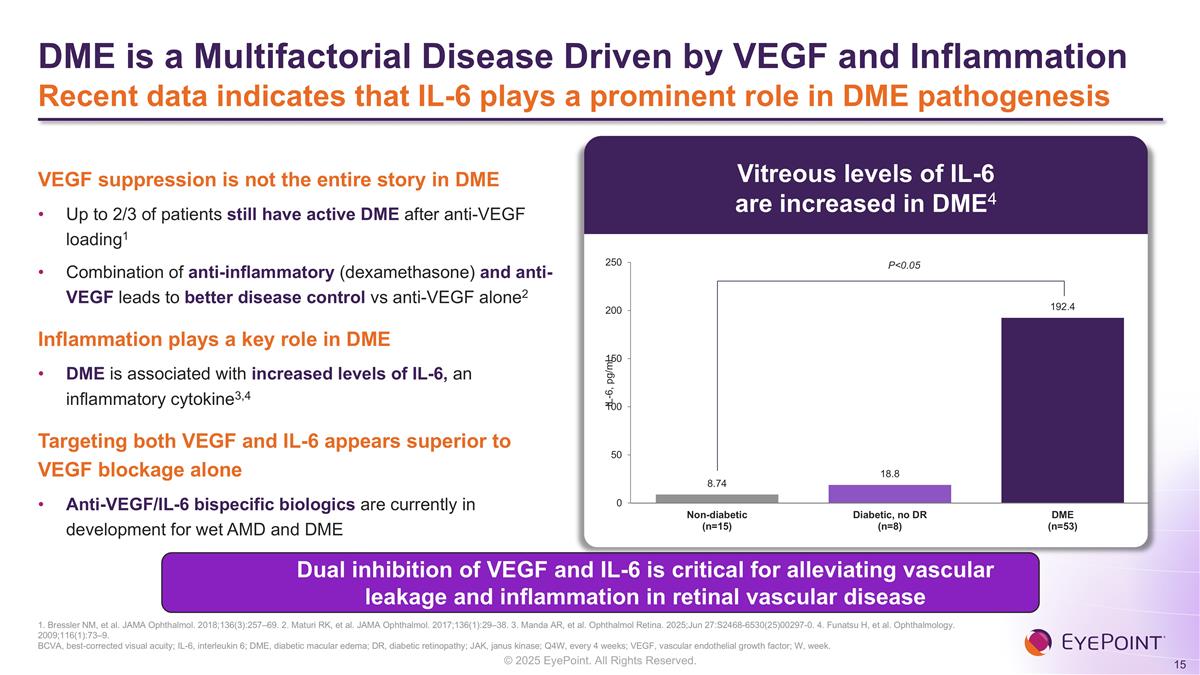
DME is a Multifactorial Disease Driven by VEGF and Inflammation Recent data indicates that IL-6 plays a prominent role in DME pathogenesis Dual inhibition of VEGF and IL-6 is critical for alleviating vascular leakage and inflammation in retinal vascular disease VEGF suppression is not the entire story in DME Up to 2/3 of patients still have active DME after anti-VEGF loading1 Combination of anti-inflammatory (dexamethasone) and anti-VEGF leads to better disease control vs anti-VEGF alone2 Inflammation plays a key role in DME DME is associated with increased levels of IL-6, an inflammatory cytokine3,4 Targeting both VEGF and IL-6 appears superior to VEGF blockage alone Anti-VEGF/IL-6 bispecific biologics are currently in development for wet AMD and DME Vitreous levels of IL-6 are increased in DME4 P<0.05 1. Bressler NM, et al. JAMA Ophthalmol. 2018;136(3):257–69. 2. Maturi RK, et al. JAMA Ophthalmol. 2017;136(1):29–38. 3. Manda AR, et al. Ophthalmol Retina. 2025;Jun 27:S2468-6530(25)00297-0. 4. Funatsu H, et al. Ophthalmology. 2009;116(1):73–9. BCVA, best-corrected visual acuity; IL-6, interleukin 6; DME, diabetic macular edema; DR, diabetic retinopathy; JAK, janus kinase; Q4W, every 4 weeks; VEGF, vascular endothelial growth factor; W, week.
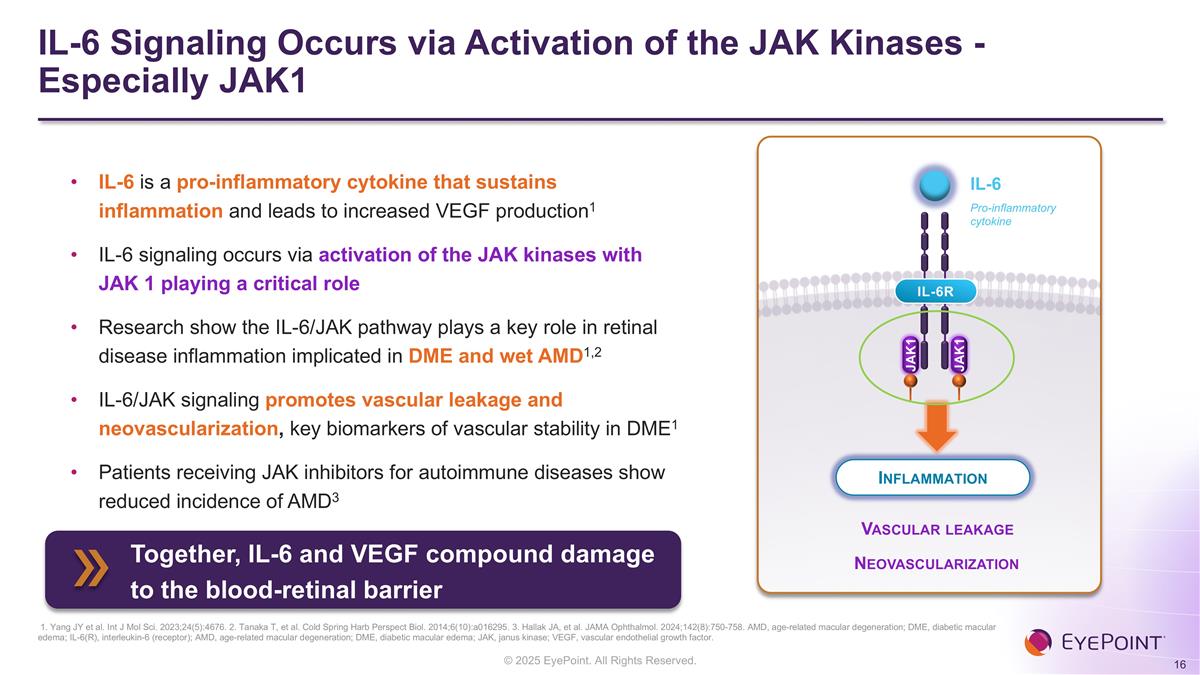
IL-6 Signaling Occurs via Activation of the JAK Kinases - Especially JAK1 1. Yang JY et al. Int J Mol Sci. 2023;24(5):4676. 2. Tanaka T, et al. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. 3. Hallak JA, et al. JAMA Ophthalmol. 2024;142(8):750-758. AMD, age-related macular degeneration; DME, diabetic macular edema; IL-6(R), interleukin-6 (receptor); AMD, age-related macular degeneration; DME, diabetic macular edema; JAK, janus kinase; VEGF, vascular endothelial growth factor. IL-6 is a pro-inflammatory cytokine that sustains inflammation and leads to increased VEGF production1 IL-6 signaling occurs via activation of the JAK kinases with JAK 1 playing a critical role Research show the IL-6/JAK pathway plays a key role in retinal disease inflammation implicated in DME and wet AMD1,2 IL-6/JAK signaling promotes vascular leakage and neovascularization, key biomarkers of vascular stability in DME1 Patients receiving JAK inhibitors for autoimmune diseases show reduced incidence of AMD3 Inflammation Vascular leakage Neovascularization JAK1 IL-6R JAK1 IL-6 Pro-inflammatory cytokine Together, IL-6 and VEGF compound damage to the blood-retinal barrier
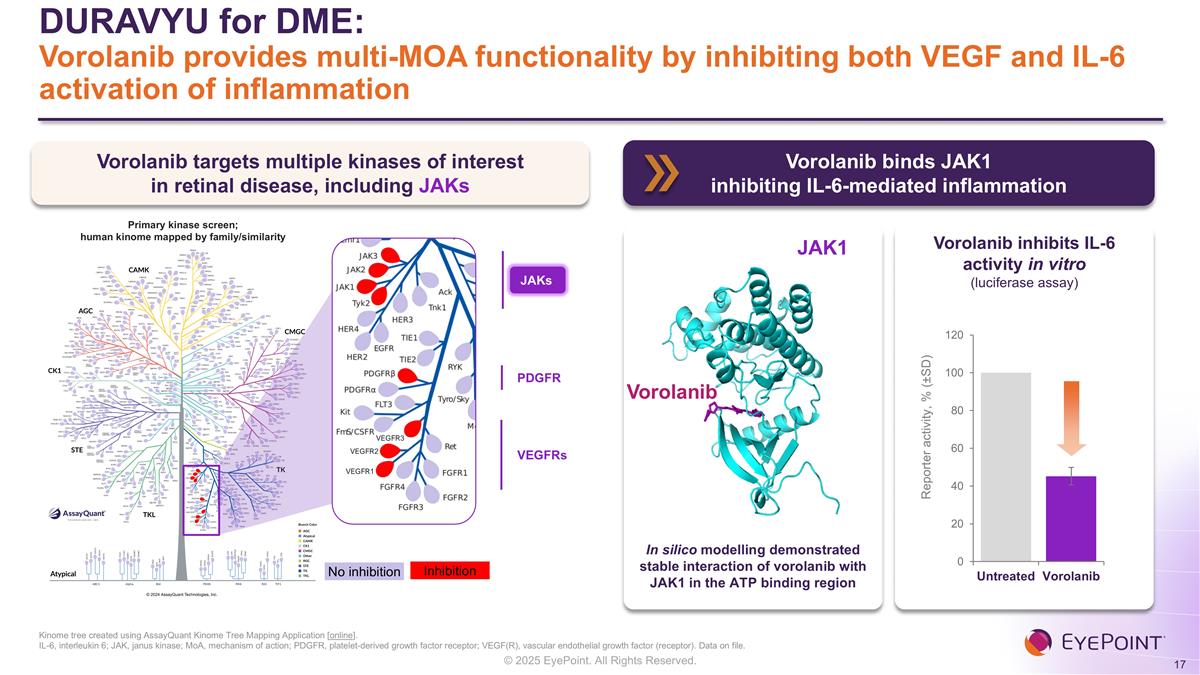
DURAVYU for DME: Vorolanib provides multi-MOA functionality by inhibiting both VEGF and IL-6 activation of inflammation Kinome tree created using AssayQuant Kinome Tree Mapping Application [online]. IL-6, interleukin 6; JAK, janus kinase; MoA, mechanism of action; PDGFR, platelet-derived growth factor receptor; VEGF(R), vascular endothelial growth factor (receptor). Data on file. Vorolanib targets multiple kinases of interest in retinal disease, including JAKs JAK1 Vorolanib In silico modelling demonstrated stable interaction of vorolanib with JAK1 in the ATP binding region Vorolanib inhibits IL-6 activity in vitro (luciferase assay) Vorolanib binds JAK1 inhibiting IL-6-mediated inflammation JAKs VEGFRs PDGFR Inhibition No inhibition Primary kinase screen; human kinome mapped by family/similarity VEGFR1 VEGFR2 VEGFR3
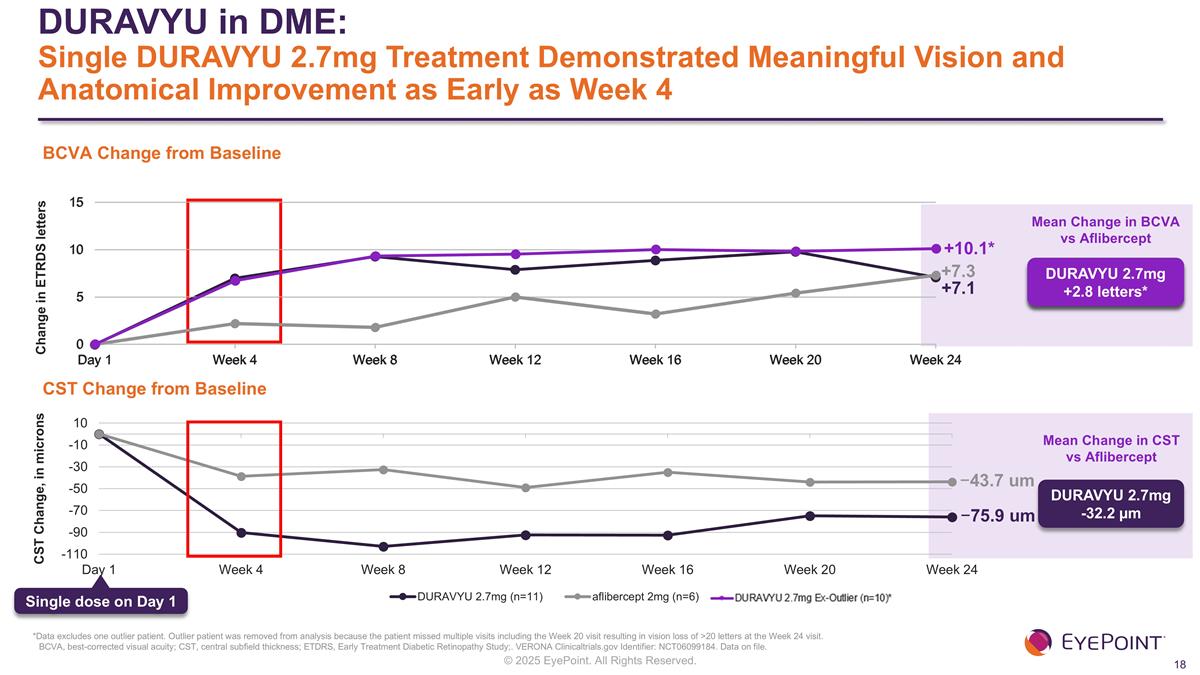
DURAVYU in DME: Single DURAVYU 2.7mg Treatment Demonstrated Meaningful Vision and Anatomical Improvement as Early as Week 4 BCVA, best-corrected visual acuity; CST, central subfield thickness; ETDRS, Early Treatment Diabetic Retinopathy Study;. VERONA Clinicaltrials.gov Identifier: NCT06099184. Data on file. Mean Change in BCVA vs Aflibercept +7.1 +7.3 −75.9 um −43.7 um Mean Change in CST vs Aflibercept DURAVYU 2.7mg -32.2 µm Single dose on Day 1 BCVA Change from Baseline CST Change from Baseline DURAVYU 2.7mg -0.2 letters +10.1* DURAVYU 2.7mg +2.8 letters* *Data excludes one outlier patient. Outlier patient was removed from analysis because the patient missed multiple visits including the Week 20 visit resulting in vision loss of >20 letters at the Week 24 visit.
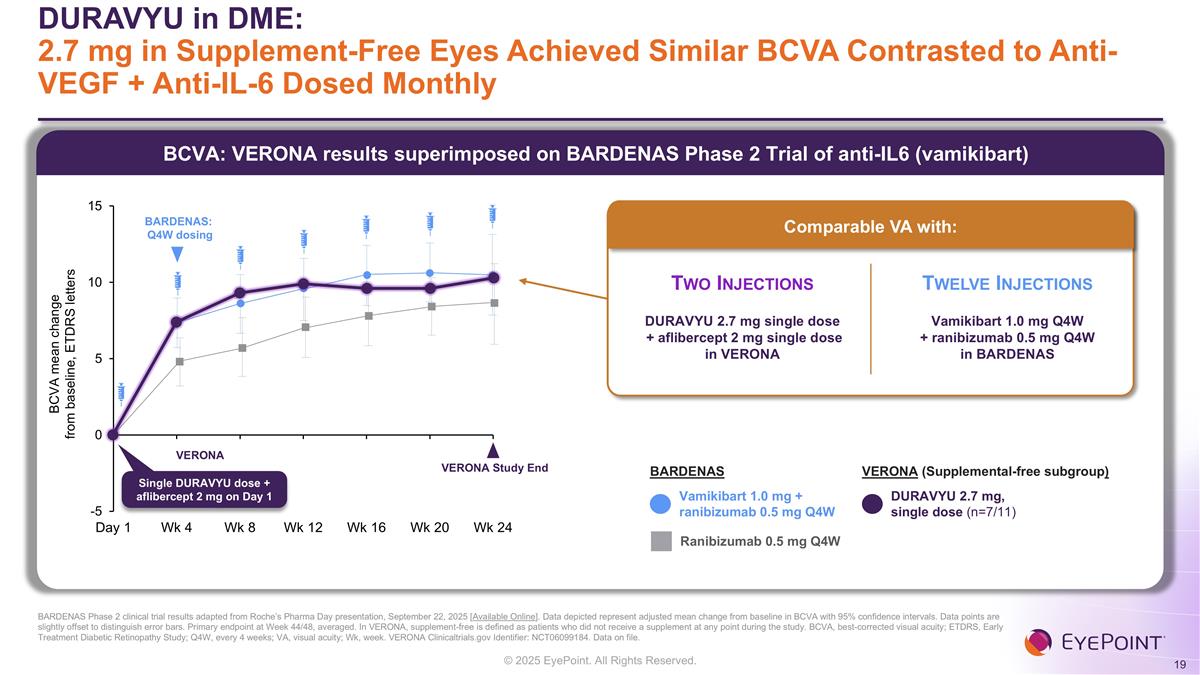
BCVA: VERONA results superimposed on BARDENAS Phase 2 Trial of anti-IL6 (vamikibart) DURAVYU in DME: 2.7 mg in Supplement-Free Eyes Achieved Similar BCVA Contrasted to Anti-VEGF + Anti-IL-6 Dosed Monthly BARDENAS Phase 2 clinical trial results adapted from Roche’s Pharma Day presentation, September 22, 2025 [Available Online]. Data depicted represent adjusted mean change from baseline in BCVA with 95% confidence intervals. Data points are slightly offset to distinguish error bars. Primary endpoint at Week 44/48, averaged. In VERONA, supplement-free is defined as patients who did not receive a supplement at any point during the study. BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; Q4W, every 4 weeks; VA, visual acuity; Wk, week. VERONA Clinicaltrials.gov Identifier: NCT06099184. Data on file. Vamikibart 1.0 mg + ranibizumab 0.5 mg Q4W DURAVYU 2.7 mg, single dose (n=7/11) Ranibizumab 0.5 mg Q4W BARDENAS VERONA (Supplemental-free subgroup) VERONA Study End Comparable VA with: Two Injections DURAVYU 2.7 mg single dose + aflibercept 2 mg single dose in VERONA Twelve Injections Vamikibart 1.0 mg Q4W + ranibizumab 0.5 mg Q4W in BARDENAS BARDENAS: Q4W dosing VERONA Single DURAVYU dose + aflibercept 2 mg on Day 1 BCVA mean change from baseline, ETDRS letters
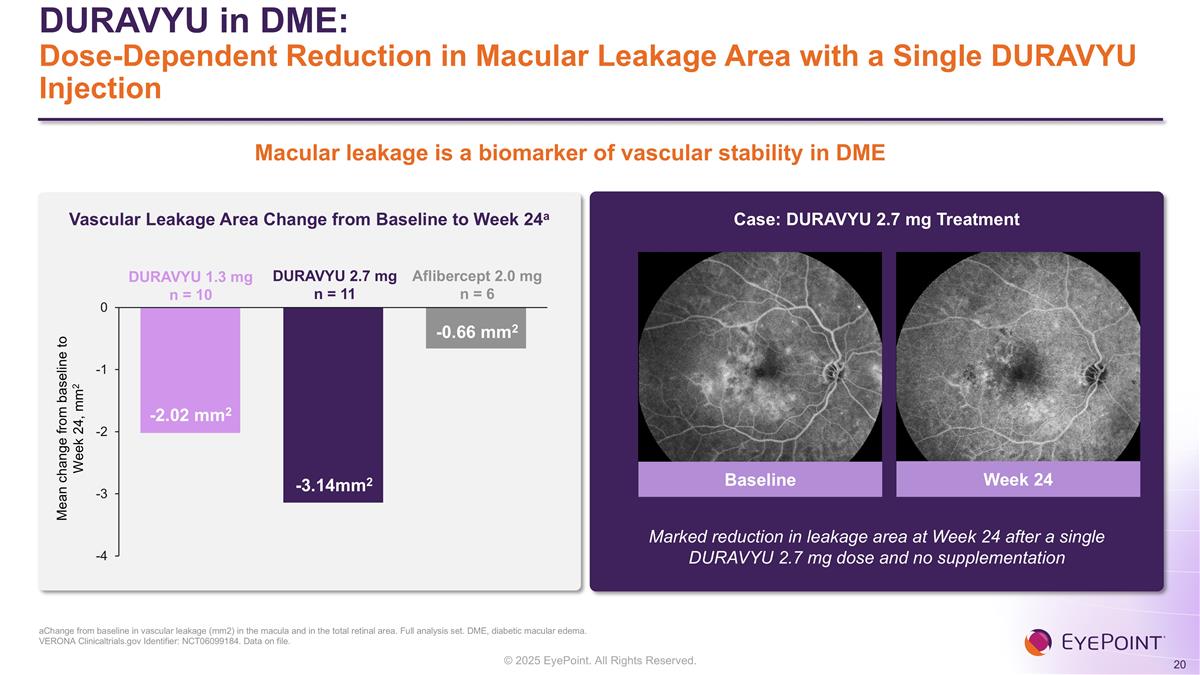
DURAVYU in DME: Dose-Dependent Reduction in Macular Leakage Area with a Single DURAVYU Injection aChange from baseline in vascular leakage (mm2) in the macula and in the total retinal area. Full analysis set. DME, diabetic macular edema. VERONA Clinicaltrials.gov Identifier: NCT06099184. Data on file. Vascular Leakage Area Change from Baseline to Week 24a Case: DURAVYU 2.7 mg Treatment Mean change from baseline to Week 24, mm2 DURAVYU 1.3 mg n = 10 Baseline Week 24 Marked reduction in leakage area at Week 24 after a single DURAVYU 2.7 mg dose and no supplementation DURAVYU 2.7 mg n = 11 Aflibercept 2.0 mg n = 6 Macular leakage is a biomarker of vascular stability in DME -2.02 mm2 -3.14mm2 -0.66 mm2
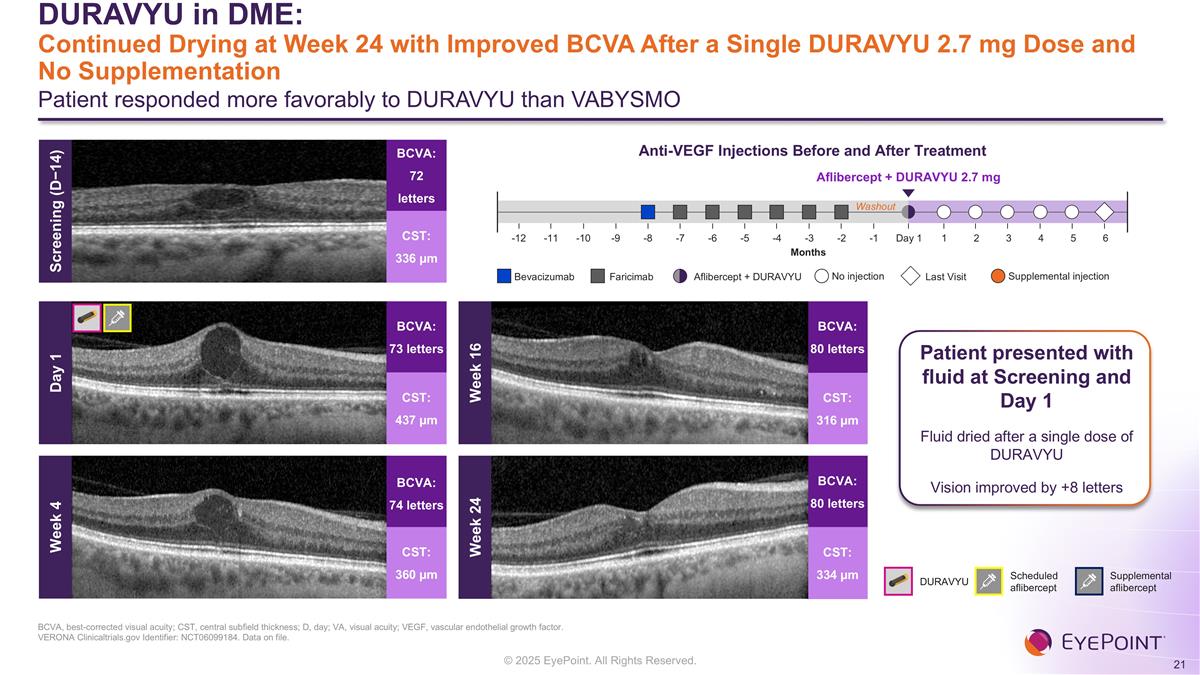
DURAVYU in DME: Continued Drying at Week 24 with Improved BCVA After a Single DURAVYU 2.7 mg Dose and No Supplementation Patient responded more favorably to DURAVYU than VABYSMO BCVA, best-corrected visual acuity; CST, central subfield thickness; D, day; VA, visual acuity; VEGF, vascular endothelial growth factor. VERONA Clinicaltrials.gov Identifier: NCT06099184. Data on file. Week 4 BCVA: 74 letters CST: 360 µm Week 16 BCVA: 80 letters CST: 316 µm Week 24 BCVA: 80 letters CST: 334 µm Anti-VEGF Injections Before and After Treatment Aflibercept + DURAVYU 2.7 mg Months Washout Day 1 2 1 4 5 6 3 -1 -3 -2 -5 -4 -7 -6 -8 -10 -9 -12 -11 Bevacizumab No injection Supplemental injection Faricimab Last Visit Aflibercept + DURAVYU Patient presented with fluid at Screening and Day 1 Fluid dried after a single dose of DURAVYU Vision improved by +8 letters Screening (D−14) BCVA: 72 letters CST: 336 µm DURAVYU Scheduled aflibercept Supplemental aflibercept BCVA: 73 letters CST: 437 µm Day 1

DURAVYU for DME Phase 3 PIVOTAL Plans
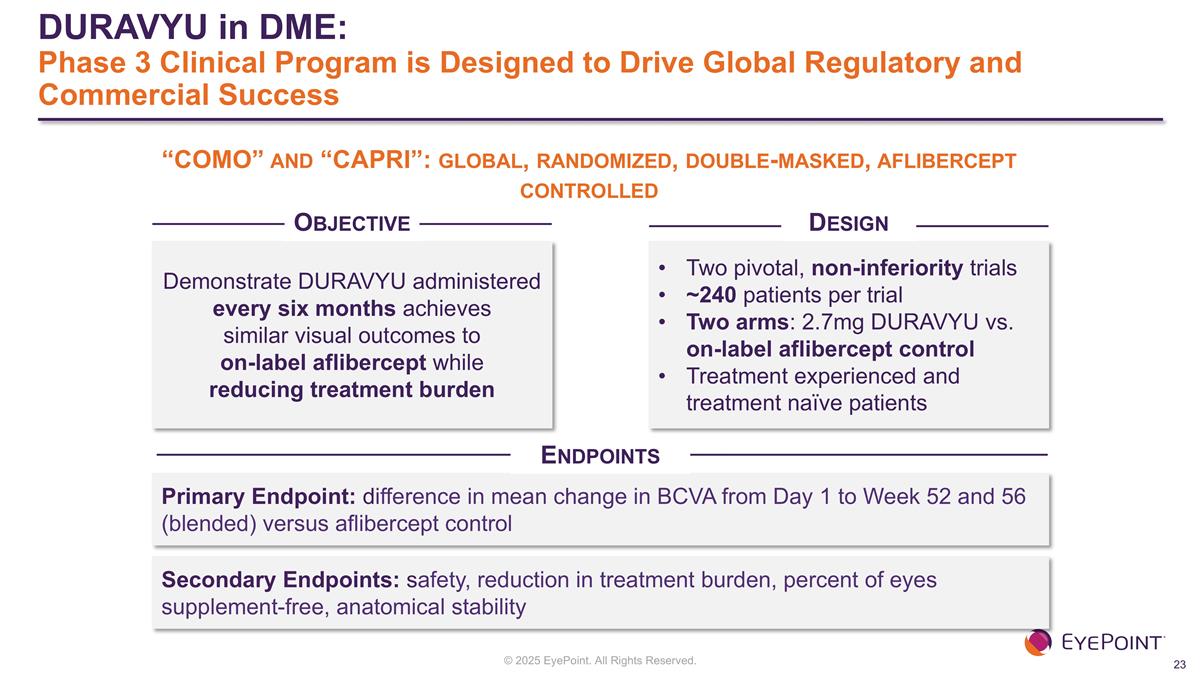
DURAVYU in DME: Phase 3 Clinical Program is Designed to Drive Global Regulatory and Commercial Success Demonstrate DURAVYU administered every six months achieves similar visual outcomes to on-label aflibercept while reducing treatment burden Two pivotal, non-inferiority trials ~240 patients per trial Two arms: 2.7mg DURAVYU vs. on-label aflibercept control Treatment experienced and treatment naïve patients Primary Endpoint: difference in mean change in BCVA from Day 1 to Week 52 and 56 (blended) versus aflibercept control Secondary Endpoints: safety, reduction in treatment burden, percent of eyes supplement-free, anatomical stability “COMO” and “CAPRI”: global, randomized, double-masked, aflibercept controlled Objective Design Endpoints
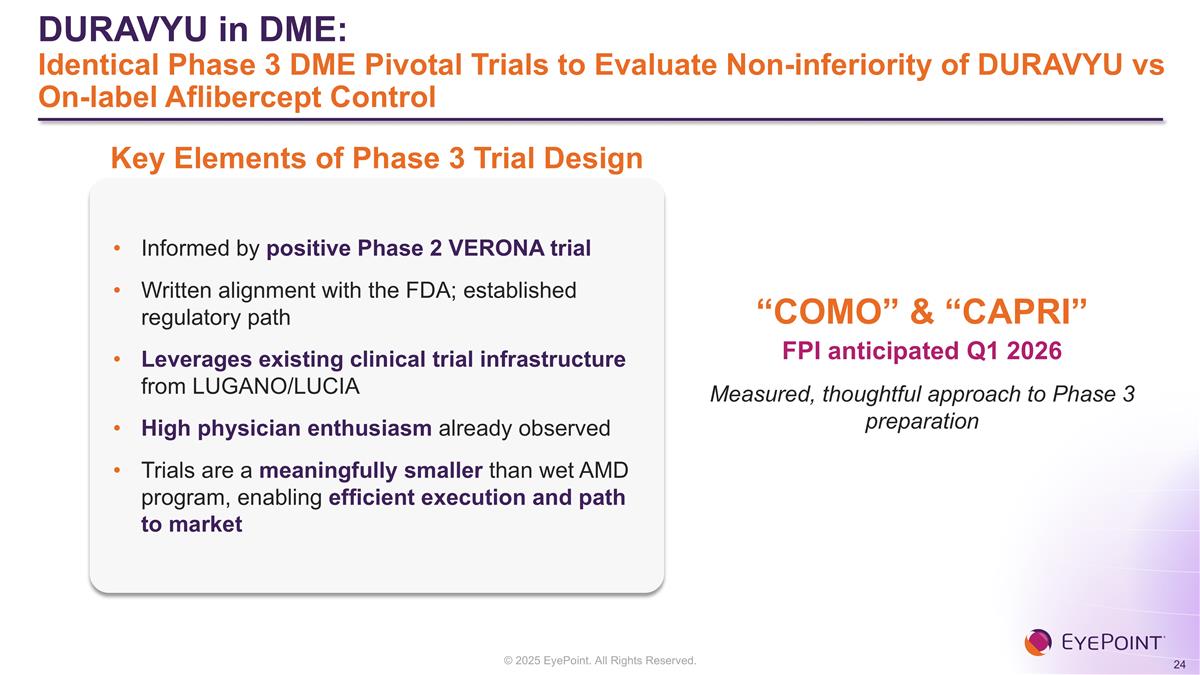
DURAVYU in DME: Identical Phase 3 DME Pivotal Trials to Evaluate Non-inferiority of DURAVYU vs On-label Aflibercept Control Informed by positive Phase 2 VERONA trial Written alignment with the FDA; established regulatory path Leverages existing clinical trial infrastructure from LUGANO/LUCIA High physician enthusiasm already observed Trials are a meaningfully smaller than wet AMD program, enabling efficient execution and path to market Key Elements of Phase 3 Trial Design FPI anticipated Q1 2026 Measured, thoughtful approach to Phase 3 preparation “COMO” & “CAPRI”
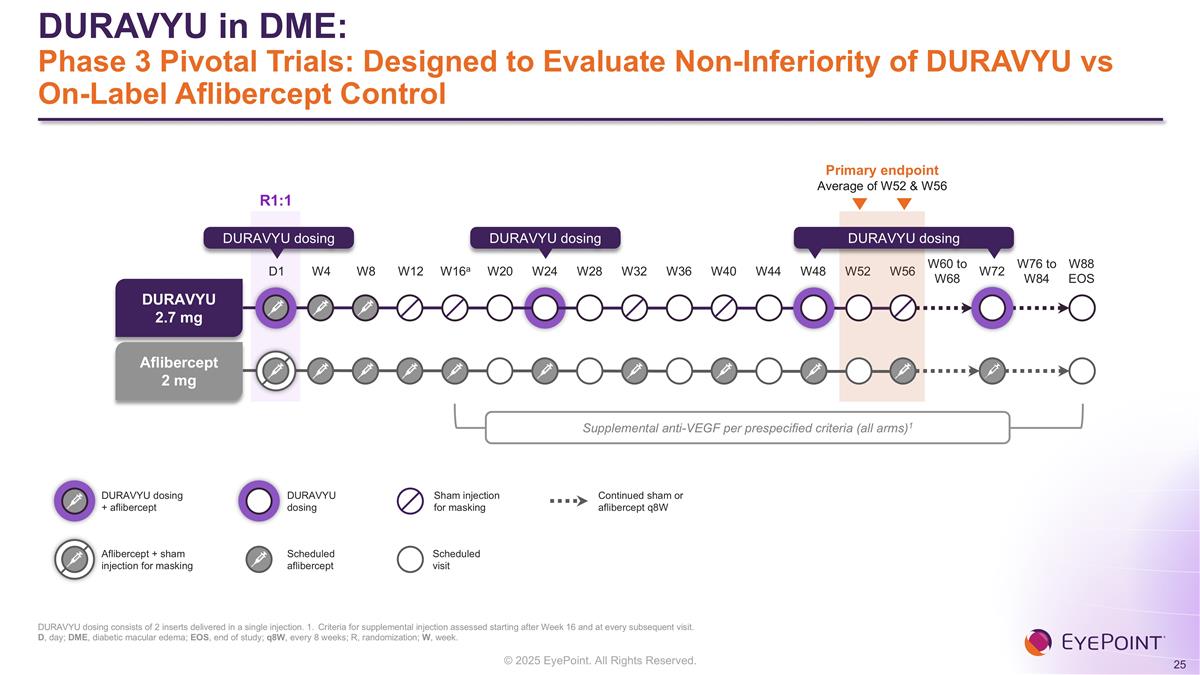
DURAVYU in DME: Phase 3 Pivotal Trials: Designed to Evaluate Non-Inferiority of DURAVYU vs On-Label Aflibercept Control D1 W4 W8 W12 W16a W20 W24 W28 W32 W36 W40 W44 W48 W52 W56 W60 to W68 W72 W76 to W84 W88 EOS Primary endpoint Average of W52 & W56 DURAVYU 2.7 mg Aflibercept 2 mg DURAVYU dosing DURAVYU dosing DURAVYU dosing Scheduled aflibercept Scheduled visit Sham injection for masking Continued sham or aflibercept q8W Supplemental anti-VEGF per prespecified criteria (all arms)1 Aflibercept + sham injection for masking DURAVYU dosing + aflibercept DURAVYU dosing R1:1 DURAVYU dosing consists of 2 inserts delivered in a single injection. 1. Criteria for supplemental injection assessed starting after Week 16 and at every subsequent visit. D, day; DME, diabetic macular edema; EOS, end of study; q8W, every 8 weeks; R, randomization; W, week.

DURAVYU™: The Only TKI in Development for DME Dual anti-VEGF and anti-IL-6 inhibition alleviates both vascular leakage and inflammation in retinal vascular disease Vorolanib features a novel, multi-MOA inhibition of both VEGF and IL-6, enabling a potential synergistic approach to treating DME Positive results from the Phase 2 VERONA trial showed early and sustained improvements in vision and anatomy underscoring the potential clinical utility of vorolanib’s multi-MOA Following a positive End-of-Phase 2 meeting with the FDA, initiation of Phase 3 pivotal trials in DME expected in Q1 2026 Efficient Phase 3 program consists of two pivotal non-inferiority trials (n=~240 patients per trial) evaluating 2.7mg DURAVYU vs. on label aflibercept

Investor Presentation October 2025