
Creating a World Class Platform January 2025 © 2025 AtriCure, Inc. All rights reserved. J.P. MORGAN HEALTHCARE CONFERENCE .2

Forward Looking Statements and Non-GAAP Financial Measures This presentation and oral statements made in connection with this presentation contain “forward-looking statements,” which are statements related to future events that by their nature address matters that are uncertain. Forward-looking statements address, among other things, AtriCure’s expected market opportunity, future business, financial performance, financial condition, and results of operations, and often contain words such as “intends,” “estimates,” “anticipates,” “hopes,” “projects,” “plans,” “expects,” “drives,” “seek,” “believes,” "see," “focus,” “should,” “will,” “would,” “can,” “opportunity,” “target,” “outlook,” and similar expressions and the negative versions thereof. Such statements are based only upon current expectations of AtriCure. All forward-looking information is inherently uncertain and actual results may differ materially from assumptions, estimates, projections or expectations reflected or contained in the forward-looking statements as a result of various risk factors. Reliance should not be placed on forward-looking statements because they involve known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to differ materially from those expressed or implied. These risks, uncertainties and other factors include, but are not limited to, those identified at http://www.atricure.com/forward-looking- statements and/or described in AtriCure’s Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q, particularly the “Risk Factors” sections thereof, as filed with the U.S. Securities and Exchange Commission and available at http://www.sec.gov. With respect to all forward-looking statements, AtriCure claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. Forward- looking statements speak only as of the date they are made. AtriCure undertakes no obligation, and does not expect, to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise unless required by law. To supplement AtriCure’s consolidated financial statements prepared in accordance with accounting principles generally accepted in the United States of America, or GAAP, AtriCure provides certain non- GAAP financial measures as supplemental financial metrics in this presentation. Adjusted EBITDA is calculated as net income (loss) before other income/expense (including interest), income tax expense, depreciation and amortization expense, share-based compensation expense, acquisition costs, acquired in-process research and development, legal settlements, impairment of intangible assets and change in fair value of contingent consideration liabilities. Management believes in order to properly understand short-term and long-term financial trends, investors may wish to consider the impact of these excluded items in addition to GAAP measures. The excluded items vary in frequency and/or impact on our continuing results of operations and management believes that the excluded items are typically not reflective of our ongoing core business operations and financial condition. Further, management uses adjusted EBITDA for both strategic and annual operating planning. Adjusted loss per share is a non-GAAP measure which calculates the net loss per share before non-cash adjustments in fair value of contingent consideration liabilities, impairment of intangible assets, acquired in-process research and development, debt extinguishment and legal settlements. The non-GAAP financial measures used by AtriCure may not be the same or calculated in the same manner as those used and calculated by other companies. Non-GAAP financial measures have limitations as analytical tools and should not be considered in isolation or as a substitute for AtriCure’s financial results prepared and reported in accordance with GAAP. We urge investors to review the reconciliation of these non-GAAP financial measures to the comparable GAAP financials measures, and not to rely on any single financial measure to evaluate our business. © 2025 AtriCure, Inc. All rights reserved. 2

Large Markets Addressing an underserved and growing patient population Strong Portfolio Existing products and solutions and continuous innovation driving consistent growth Bright Future Novel therapies supported by growing body of clinical evidence We are passionately focused on healing the lives of those affected by Afib and pain after surgery © 2025 AtriCure, Inc. All rights reserved. 3
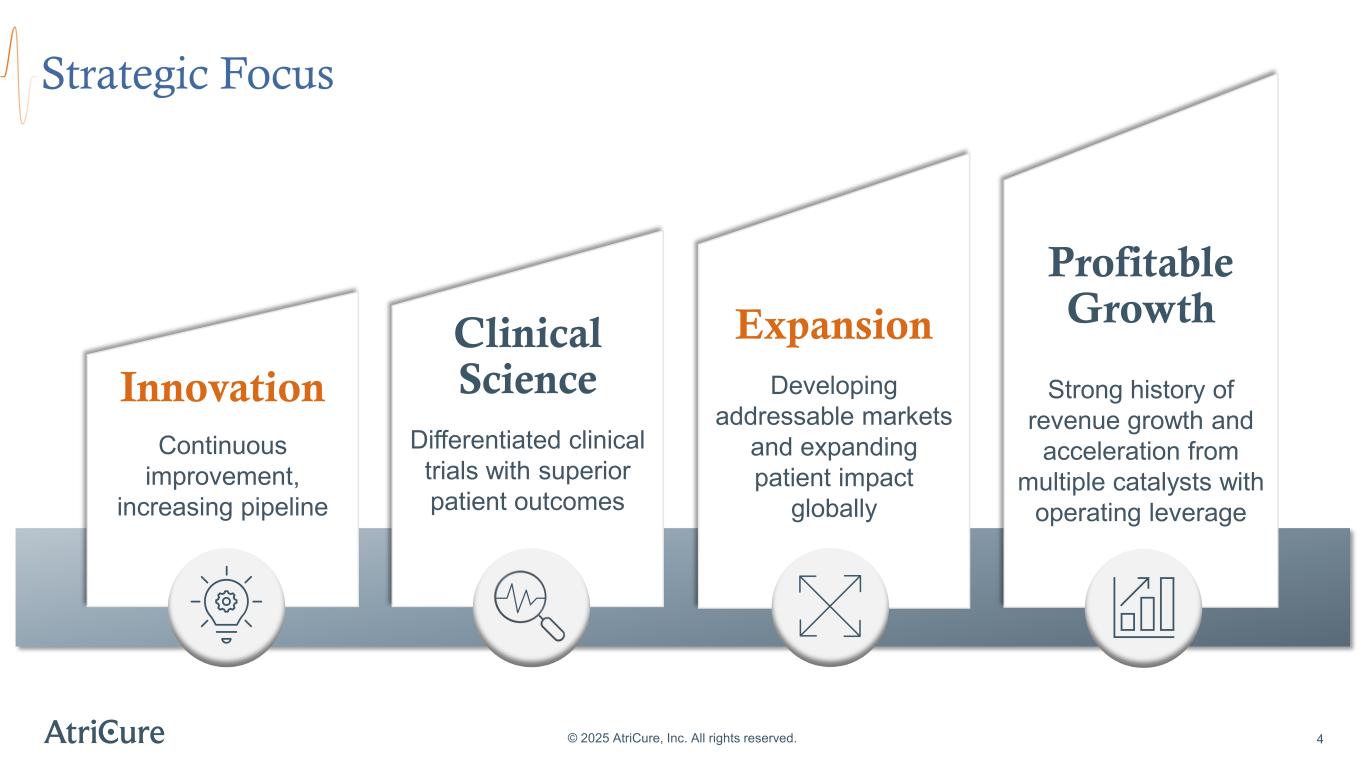
Profitable Growth Strong history of revenue growth and acceleration from multiple catalysts with operating leverage Expansion Developing addressable markets and expanding patient impact globally Innovation Continuous improvement, increasing pipeline Strategic Focus © 2025 AtriCure, Inc. All rights reserved. 4 Clinical Science Differentiated clinical trials with superior patient outcomes
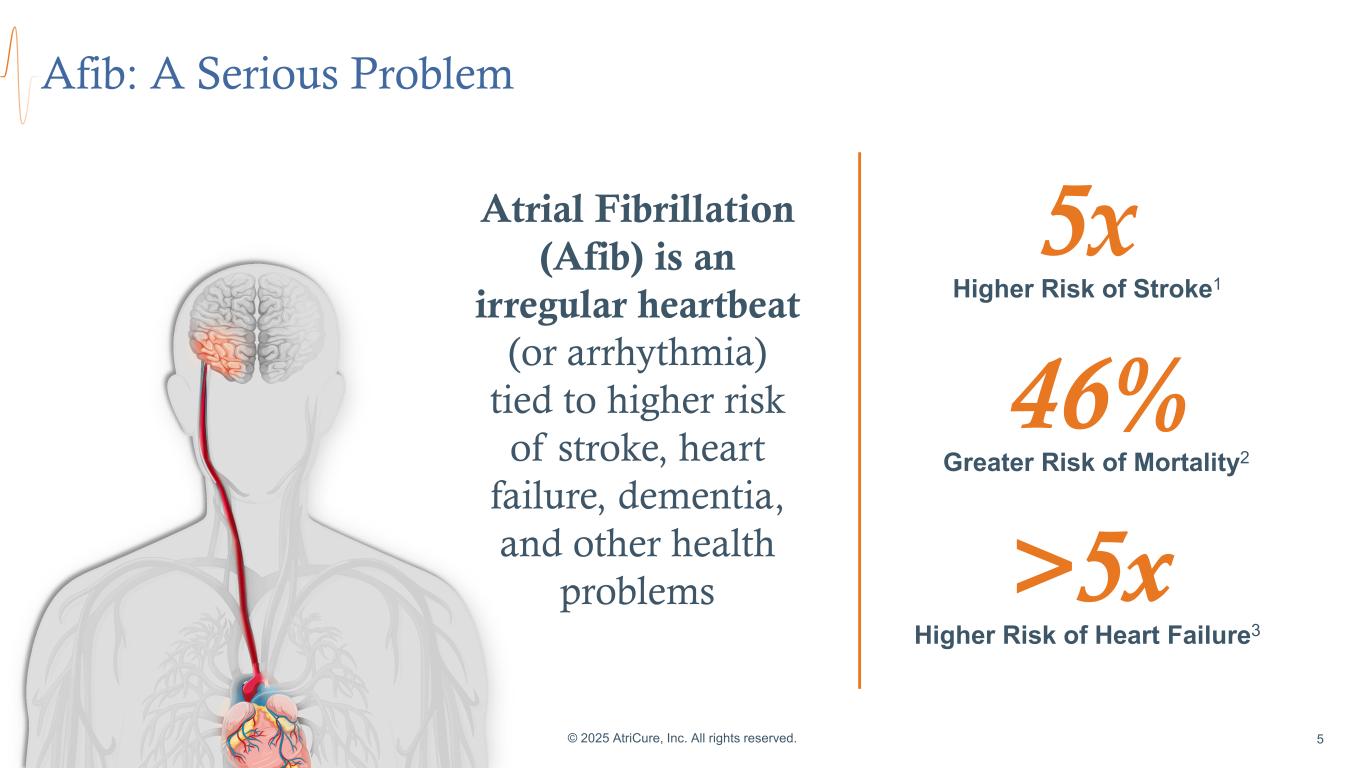
Afib: A Serious Problem © 2025 AtriCure, Inc. All rights reserved. 5 5x Higher Risk of Stroke1 46% Greater Risk of Mortality2 >5x Higher Risk of Heart Failure3 Atrial Fibrillation (Afib) is an irregular heartbeat (or arrhythmia) tied to higher risk of stroke, heart failure, dementia, and other health problems
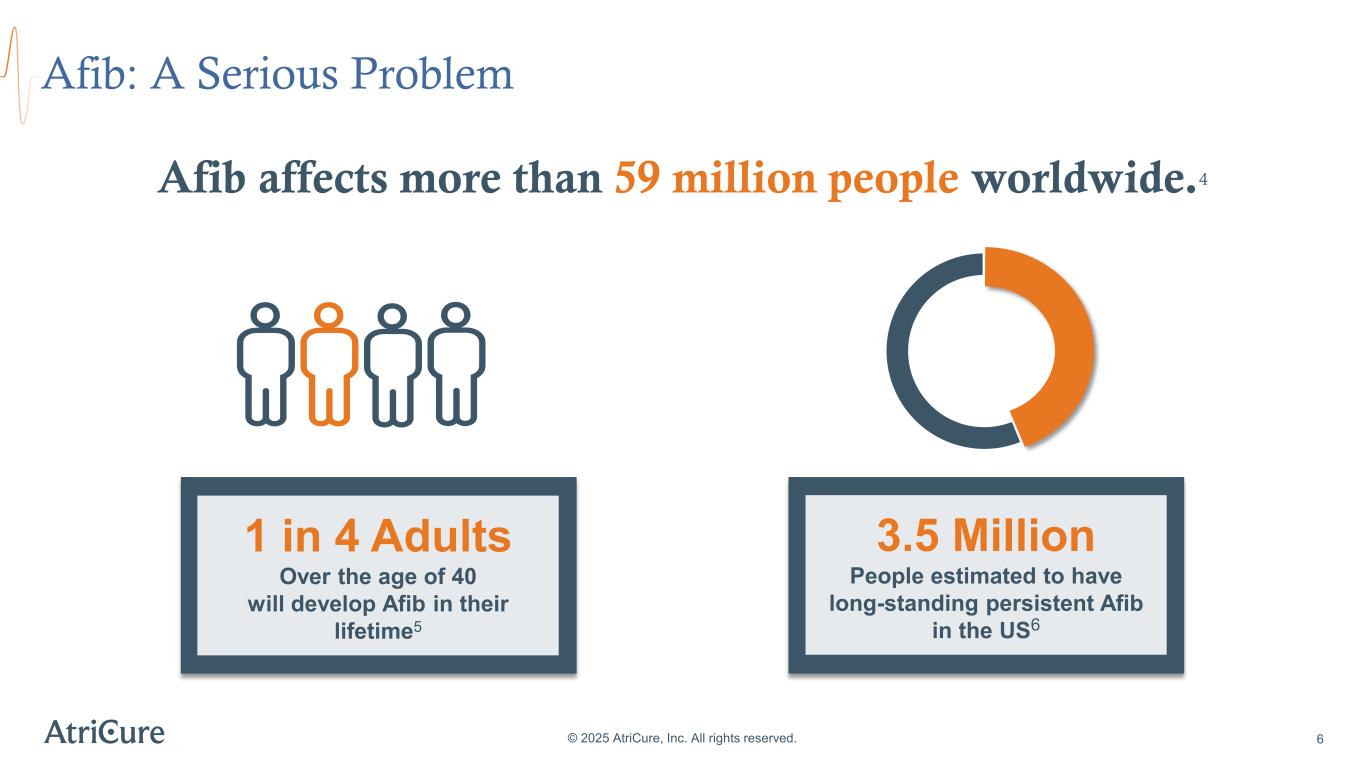
Afib: A Serious Problem © 2025 AtriCure, Inc. All rights reserved. 6 Afib affects more than 59 million people worldwide.4 3.5 Million People estimated to have long-standing persistent Afib in the US6 1 in 4 Adults Over the age of 40 will develop Afib in their lifetime5
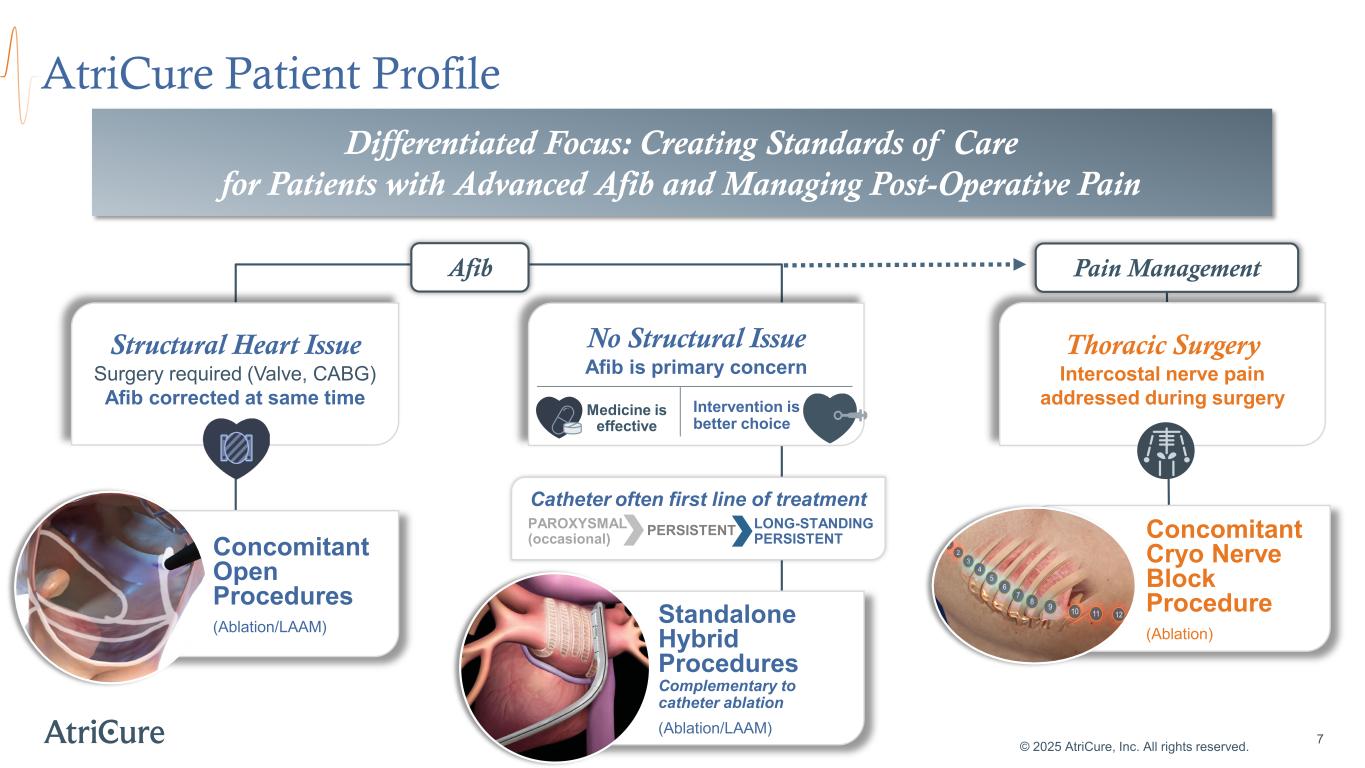
Standalone Hybrid Procedures Complementary to catheter ablation (Ablation/LAAM) No Structural Issue Afib is primary concern AtriCure Patient Profile © 2025 AtriCure, Inc. All rights reserved. Intervention is better choice Medicine is effective PAROXYSMAL (occasional) LONG-STANDING PERSISTENTPERSISTENT Afib 7 Catheter often first line of treatment Concomitant Open Procedures (Ablation/LAAM) Structural Heart Issue Surgery required (Valve, CABG) Afib corrected at same time Differentiated Focus: Creating Standards of Care for Patients with Advanced Afib and Managing Post-Operative Pain Thoracic Surgery Intercostal nerve pain addressed during surgery Pain Management Concomitant Cryo Nerve Block Procedure (Ablation)
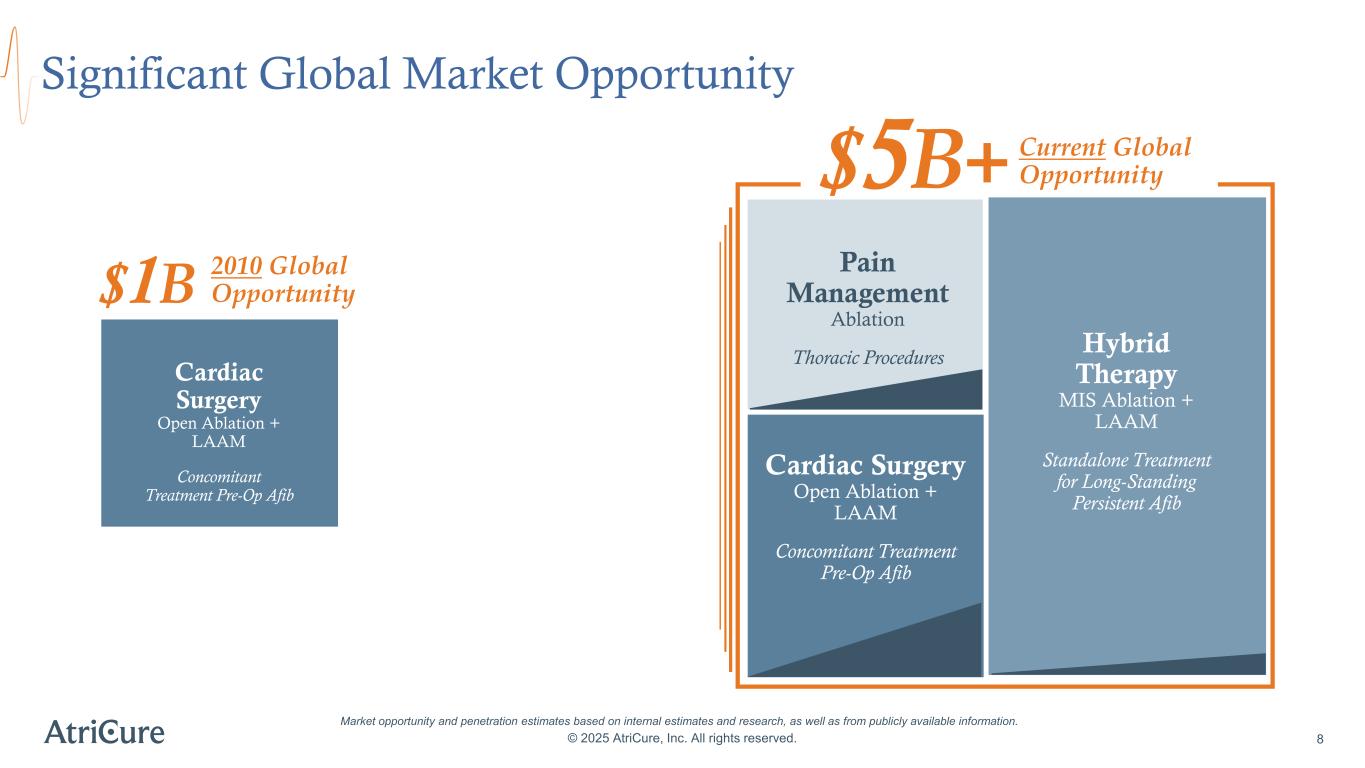
Significant Global Market Opportunity © 2025 AtriCure, Inc. All rights reserved. 8 Market opportunity and penetration estimates based on internal estimates and research, as well as from publicly available information. Pain Management Ablation Thoracic Procedures Hybrid Therapy MIS Ablation + LAAM Standalone Treatment for Long-Standing Persistent Afib Cardiac Surgery Open Ablation + LAAM Concomitant Treatment Pre-Op Afib $1B Cardiac Surgery Open Ablation + LAAM Concomitant Treatment Pre-Op Afib Current Global Opportunity$5B+ 2010 Global Opportunity
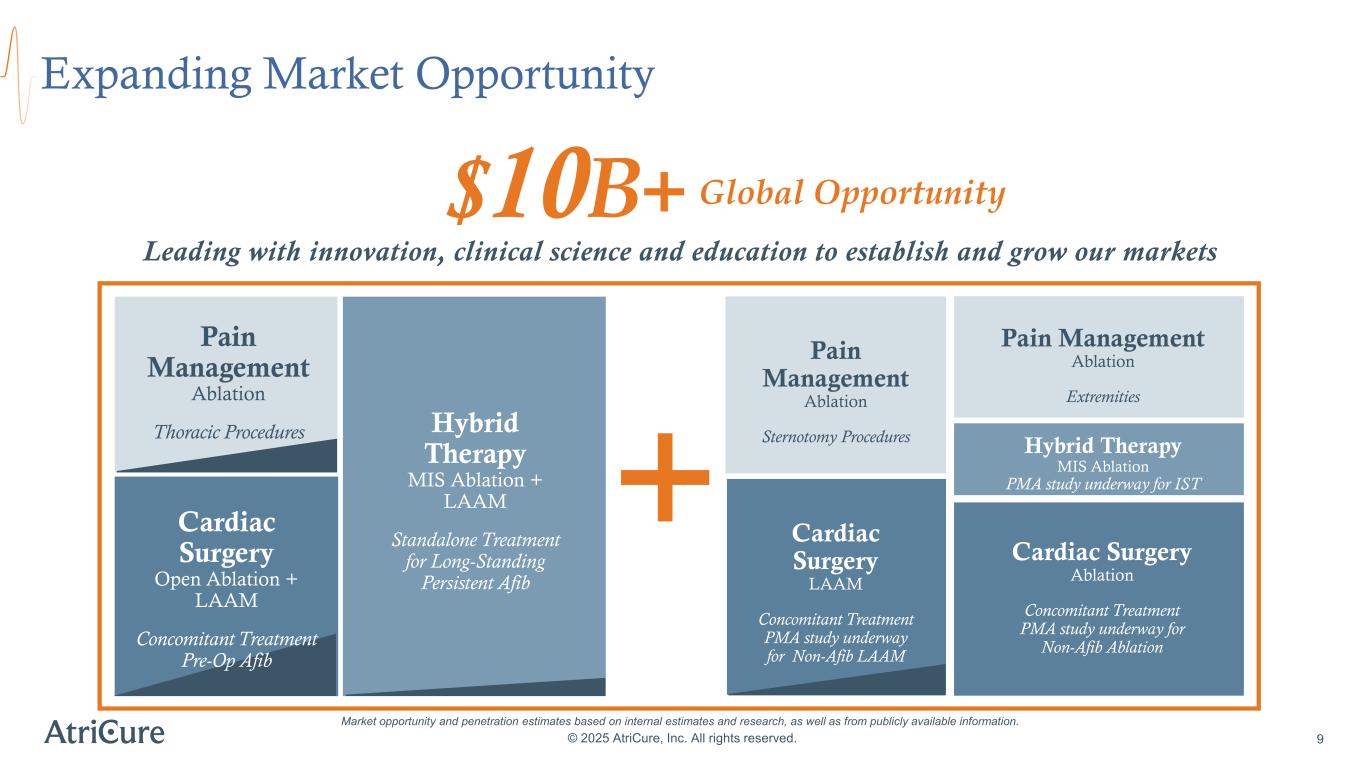
Expanding Market Opportunity © 2025 AtriCure, Inc. All rights reserved. 9 Market opportunity and penetration estimates based on internal estimates and research, as well as from publicly available information. Leading with innovation, clinical science and education to establish and grow our markets $10B+ Pain Management Ablation Thoracic Procedures Cardiac Surgery Open Ablation + LAAM Concomitant Treatment Pre-Op Afib Hybrid Therapy MIS Ablation + LAAM Standalone Treatment for Long-Standing Persistent Afib Cardiac Surgery LAAM Concomitant Treatment PMA study underway for Non-Afib LAAM Pain Management Ablation Sternotomy Procedures Hybrid Therapy MIS Ablation PMA study underway for IST Pain Management Ablation Extremities Cardiac Surgery Ablation Concomitant Treatment PMA study underway for Non-Afib Ablation Global Opportunity +
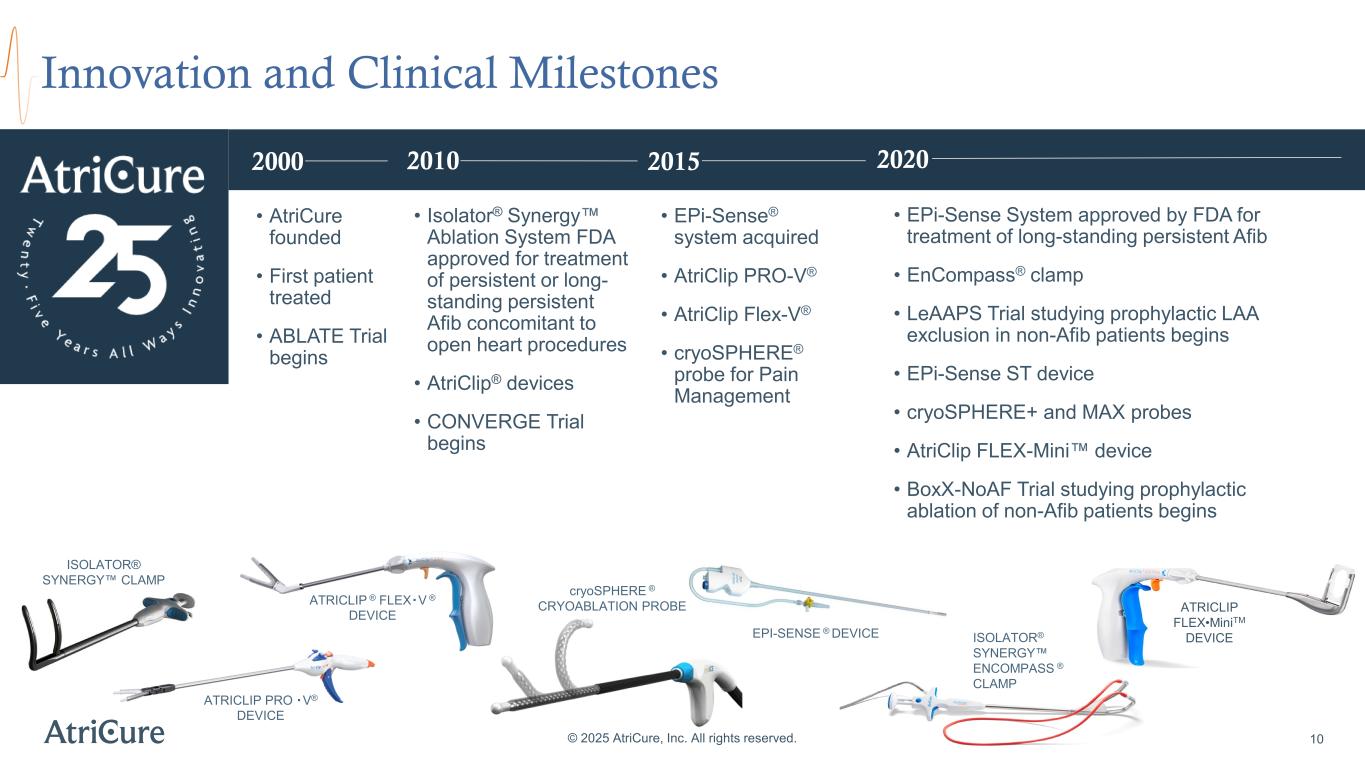
Innovation and Clinical Milestones © 2025 AtriCure, Inc. All rights reserved. 10 EPI-SENSE ® DEVICE ATRICLIP PRO・V® DEVICE ATRICLIP ® FLEX・V ® DEVICE ISOLATOR® SYNERGY CLAMP cryoSPHERE ® CRYOABLATION PROBE ISOLATOR® SYNERGY ENCOMPASS ® CLAMP ATRICLIP FLEX•MiniTM DEVICE • Isolator® Synergy Ablation System FDA approved for treatment of persistent or long- standing persistent Afib concomitant to open heart procedures • AtriClip® devices • CONVERGE Trial begins • AtriCure founded • First patient treated • ABLATE Trial begins • EPi-Sense® system acquired • AtriClip PRO-V® • AtriClip Flex-V® • cryoSPHERE® probe for Pain Management • EPi-Sense System approved by FDA for treatment of long-standing persistent Afib • EnCompass® clamp • LeAAPS Trial studying prophylactic LAA exclusion in non-Afib patients begins • EPi-Sense ST device • cryoSPHERE+ and MAX probes • AtriClip FLEX-Mini device • BoxX-NoAF Trial studying prophylactic ablation of non-Afib patients begins 2000 2010 2015 2020
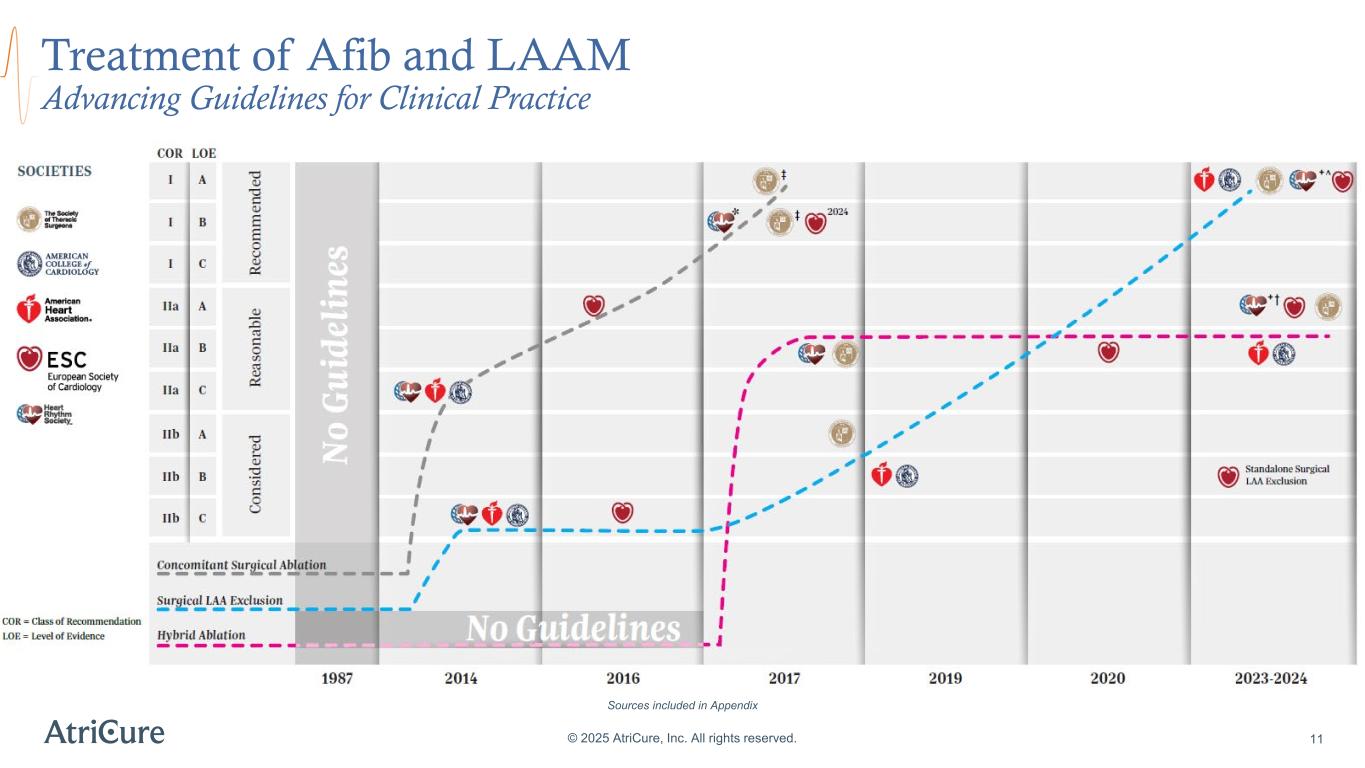
Treatment of Afib and LAAM Advancing Guidelines for Clinical Practice © 2025 AtriCure, Inc. All rights reserved. 11 Lasts beyond 7 days and as long as 1 year Lasts longer than 1 year without stopping Sources included in Appendix
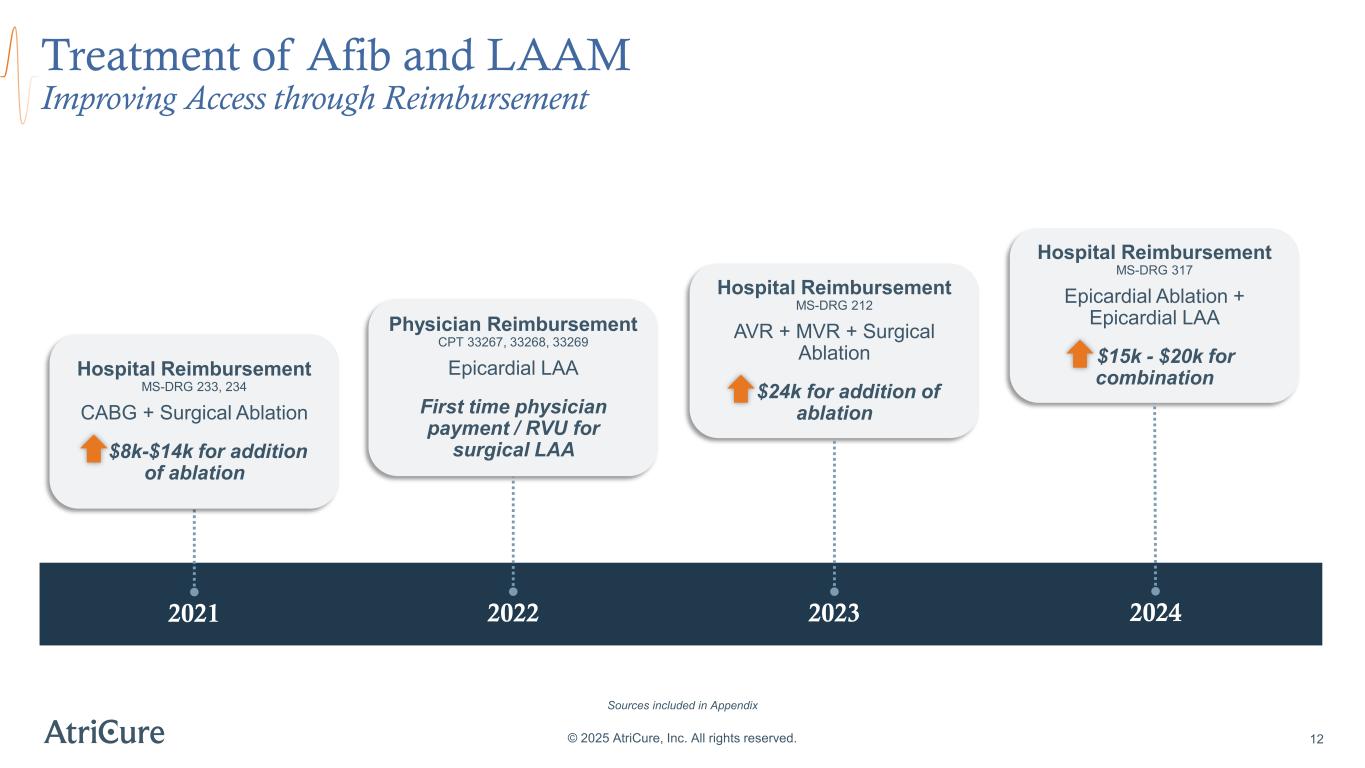
© 2025 AtriCure, Inc. All rights reserved. 12 Lasts longer than 1 year without stopping Treatment of Afib and LAAM Improving Access through Reimbursement 2021 2022 2023 2024 Hospital Reimbursement MS-DRG 233, 234 CABG + Surgical Ablation $8k-$14k for addition of ablation Physician Reimbursement CPT 33267, 33268, 33269 Epicardial LAA First time physician payment / RVU for surgical LAA Hospital Reimbursement MS-DRG 212 AVR + MVR + Surgical Ablation $24k for addition of ablation Hospital Reimbursement MS-DRG 317 Epicardial Ablation + Epicardial LAA $15k - $20k for combination Sources included in Appendix
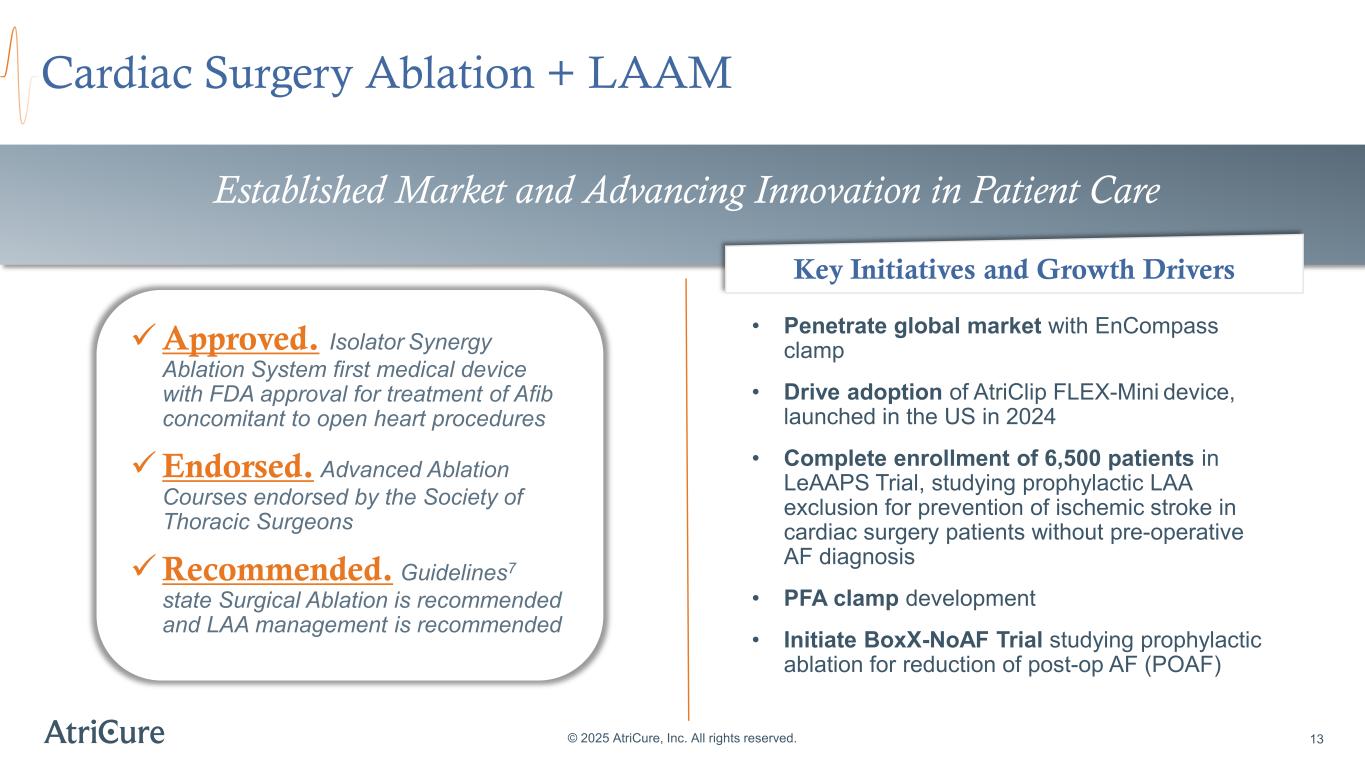
Cardiac Surgery Ablation + LAAM © 2025 AtriCure, Inc. All rights reserved. 13 • Penetrate global market with EnCompass clamp • Drive adoption of AtriClip FLEX-Mini device, launched in the US in 2024 • Complete enrollment of 6,500 patients in LeAAPS Trial, studying prophylactic LAA exclusion for prevention of ischemic stroke in cardiac surgery patients without pre-operative AF diagnosis • PFA clamp development • Initiate BoxX-NoAF Trial studying prophylactic ablation for reduction of post-op AF (POAF) Established Market and Advancing Innovation in Patient Care Approved. Isolator Synergy Ablation System first medical device with FDA approval for treatment of Afib concomitant to open heart procedures Endorsed. Advanced Ablation Courses endorsed by the Society of Thoracic Surgeons Recommended. Guidelines7 state Surgical Ablation is recommended and LAA management is recommended Key Initiatives and Growth Drivers
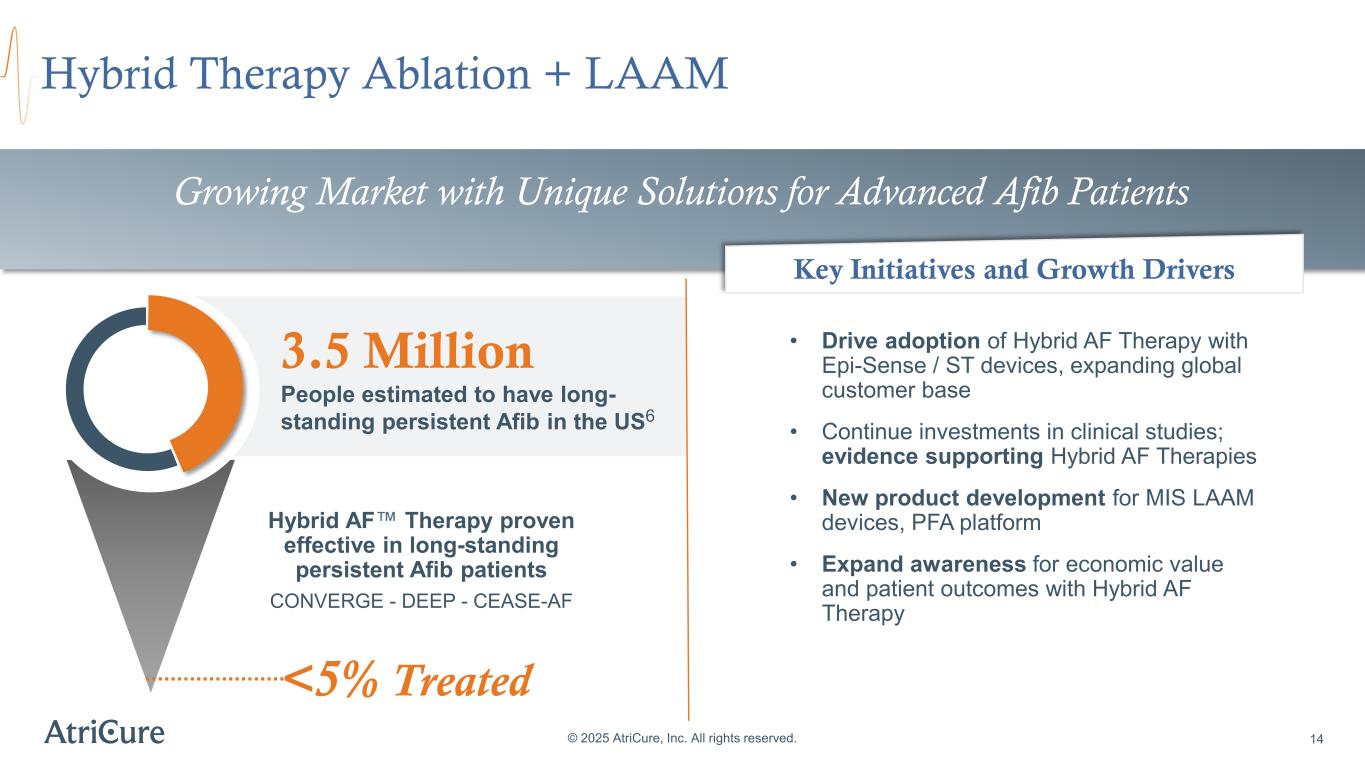
3.5 Million People estimated to have long- standing persistent Afib in the US6 Hybrid Therapy Ablation + LAAM © 2025 AtriCure, Inc. All rights reserved. 14 Hybrid AF Therapy proven effective in long-standing persistent Afib patients CONVERGE - DEEP - CEASE-AF Growing Market with Unique Solutions for Advanced Afib Patients • Drive adoption of Hybrid AF Therapy with Epi-Sense / ST devices, expanding global customer base • Continue investments in clinical studies; evidence supporting Hybrid AF Therapies • New product development for MIS LAAM devices, PFA platform • Expand awareness for economic value and patient outcomes with Hybrid AF Therapy Key Initiatives and Growth Drivers <5% Treated

Cryo Nerve Block Therapy can be an important tool in combatting the opioid epidemic – 1 in 7 thoracic surgery patients become reliant upon opioids after their procedure8 Pain Management © 2025 AtriCure, Inc. All rights reserved. 15 • Expand adoption of 2024 new product launches: cryoSPHERE+ probe and cryoSPHERE MAX probe • Reduced freeze times by 25% (cryoSPHERE+) and 50% (cryoSPHERE MAX) compared to first generation technology • Continue investments in registries and studies to support economic benefit of Cryo Nerve Block therapy • Product development in new therapy areas Key Initiatives and Growth Drivers Leading Market Development through Ablation Expertise

Key New Product Launches 2024 Highlights and Accomplishments 17% 4,200 Patients Enrolled To Date>190,000 Patients Treated Improving guidelines in cardiac surgery globally Annual Revenue Growth* Top Workplace Honors Cincinnati, Minneapolis, Netherlands, Australia, United Kingdom +42% Increase in Positive Adjusted EBITDA** Improving profitability while driving growth *2024 Revenue is preliminary and unaudited. ** 2024 Positive Adjusted EBITDA based on mid-point of 2024 guidance range of $26 million to $29 million. © 2025 AtriCure, Inc. All rights reserved. Fast Company’s 100 Best Workplaces for Innovators ATRICLIP FLEX•Mini cryoS+ cryoS MAX Pain Management LAAM International Products Expanding • EnCompass clamp approved in E.U. • Expanded labeling for AtriClip devices in E.U. • AtriClip devices approved in China Grants to support facility expansion, jobs growth BoxX-NoAF Trial Protocol Approved 26% International Revenue Growth* State of Ohio + City of Mason 16
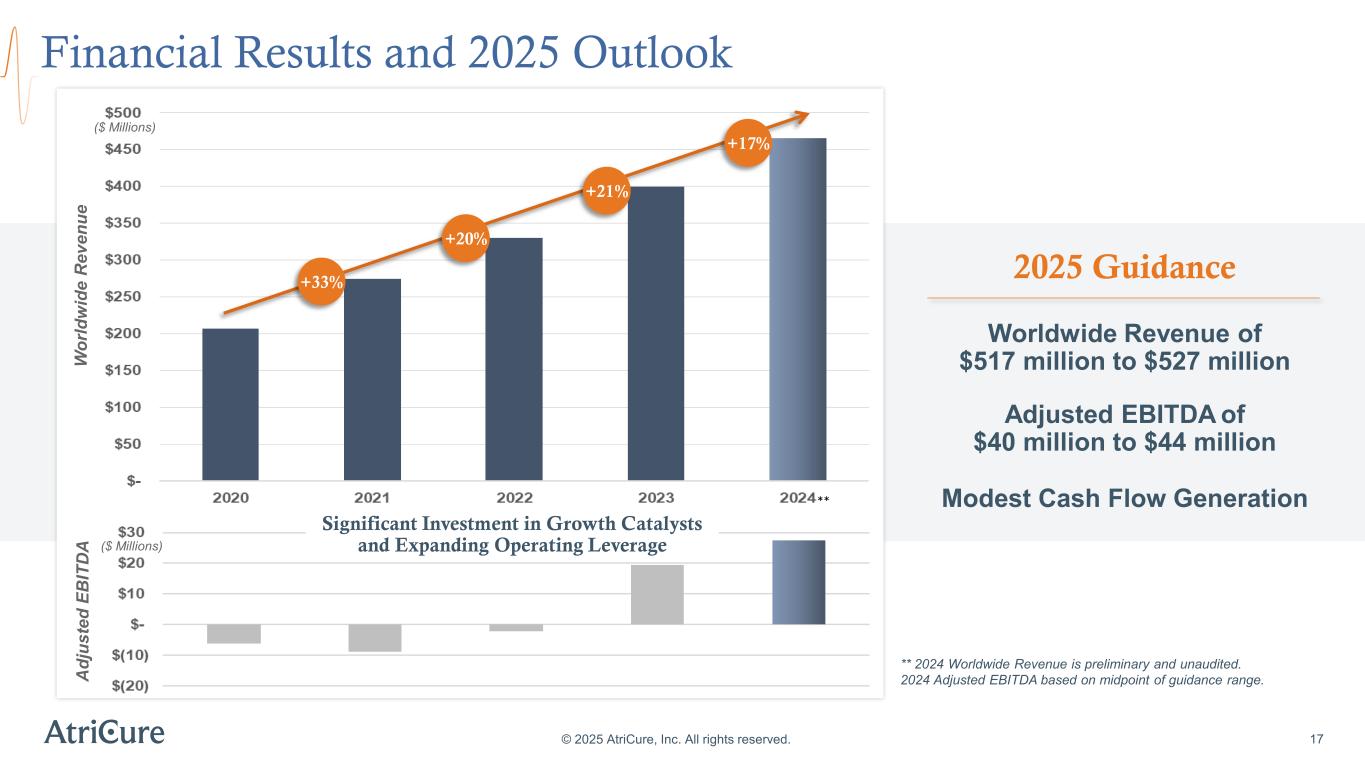
Financial Results and 2025 Outlook © 2025 AtriCure, Inc. All rights reserved. ** 2024 Worldwide Revenue is preliminary and unaudited. 2024 Adjusted EBITDA based on midpoint of guidance range. 17 W or ld w id e R ev en ue ($ Millions) 2025 Guidance Worldwide Revenue of $517 million to $527 million Adjusted EBITDA of $40 million to $44 million Modest Cash Flow Generation +33% +20% +21% A dj us te d EB IT D A ($ Millions) Significant Investment in Growth Catalysts and Expanding Operating Leverage ** +17%

[ 18 ] Key Takeaways Strong Q4 2024 and FY 2024 Q4 Worldwide Revenue $124.3M (~17% Growth) 2024 Worldwide Revenue $465.3M (~17% Growth) 2025 Guidance Worldwide Revenue $517M to $527M Positive Adjusted EBITDA $40M to $44M Positive Cash Flow Focused on Market Penetration + Expansion cryoSPHERE probes EnCompass clamp AtriClip devices HybridTherapies PFA platform development LeAAPS Clinical Trial BoxX-NoAF Clinical Trial Analyst & Investor Day March 26, 2025 Headquarters (Mason, Ohio) Our Vision Portfolio and Pipeline Financial Goals KOL perspectives 18 © 2025 AtriCure, Inc. All rights reserved.

Thank You! © 2025 AtriCure, Inc. All rights reserved.
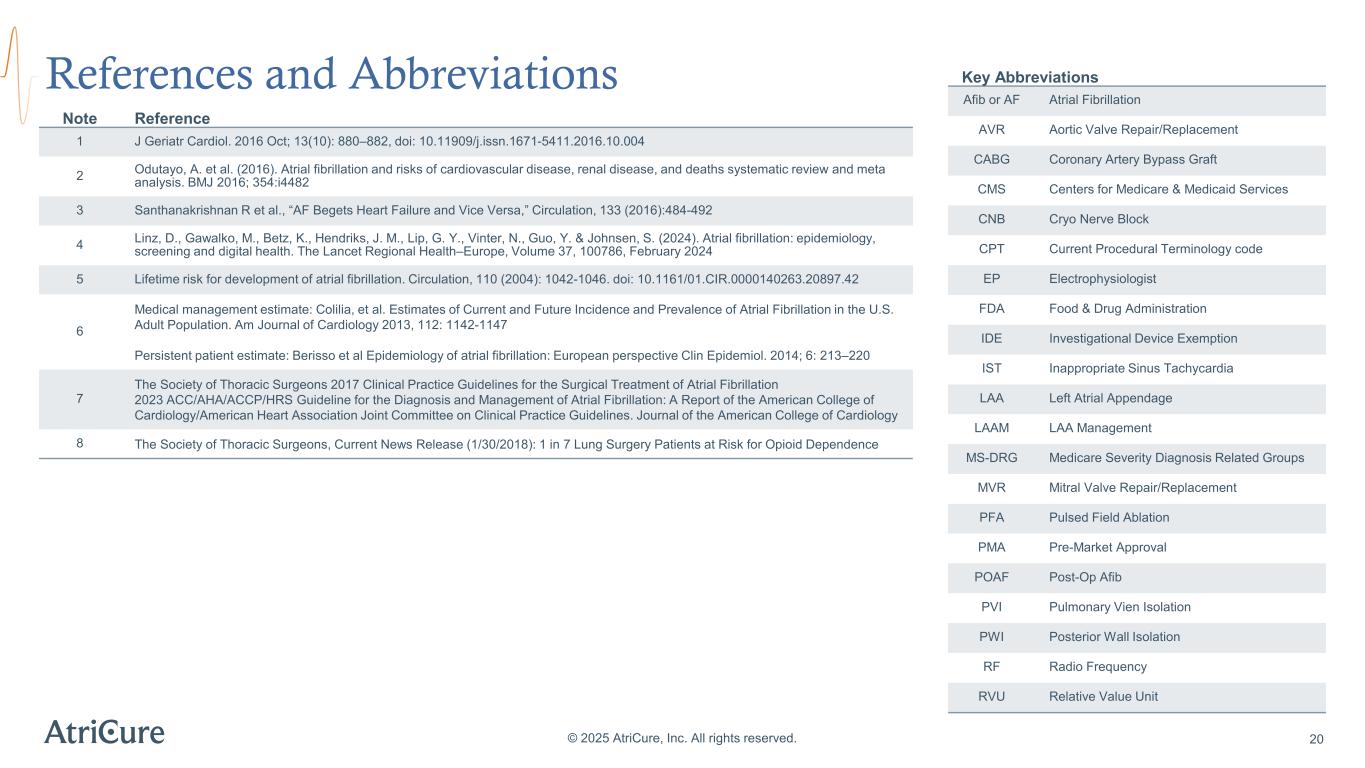
References and Abbreviations © 2025 AtriCure, Inc. All rights reserved. Note Reference 1 J Geriatr Cardiol. 2016 Oct; 13(10): 880–882, doi: 10.11909/j.issn.1671-5411.2016.10.004 2 Odutayo, A. et al. (2016). Atrial fibrillation and risks of cardiovascular disease, renal disease, and deaths systematic review and meta analysis. BMJ 2016; 354:i4482 3 Santhanakrishnan R et al., “AF Begets Heart Failure and Vice Versa,” Circulation, 133 (2016):484-492 4 Linz, D., Gawalko, M., Betz, K., Hendriks, J. M., Lip, G. Y., Vinter, N., Guo, Y. & Johnsen, S. (2024). Atrial fibrillation: epidemiology, screening and digital health. The Lancet Regional Health–Europe, Volume 37, 100786, February 2024 5 Lifetime risk for development of atrial fibrillation. Circulation, 110 (2004): 1042-1046. doi: 10.1161/01.CIR.0000140263.20897.42 6 Medical management estimate: Colilia, et al. Estimates of Current and Future Incidence and Prevalence of Atrial Fibrillation in the U.S. Adult Population. Am Journal of Cardiology 2013, 112: 1142-1147 Persistent patient estimate: Berisso et al Epidemiology of atrial fibrillation: European perspective Clin Epidemiol. 2014; 6: 213–220 7 The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology 8 The Society of Thoracic Surgeons, Current News Release (1/30/2018): 1 in 7 Lung Surgery Patients at Risk for Opioid Dependence Key Abbreviations Afib or AF Atrial Fibrillation AVR Aortic Valve Repair/Replacement CABG Coronary Artery Bypass Graft CMS Centers for Medicare & Medicaid Services CNB Cryo Nerve Block CPT Current Procedural Terminology code EP Electrophysiologist FDA Food & Drug Administration IDE Investigational Device Exemption IST Inappropriate Sinus Tachycardia LAA Left Atrial Appendage LAAM LAA Management MS-DRG Medicare Severity Diagnosis Related Groups MVR Mitral Valve Repair/Replacement PFA Pulsed Field Ablation PMA Pre-Market Approval POAF Post-Op Afib PVI Pulmonary Vien Isolation PWI Posterior Wall Isolation RF Radio Frequency RVU Relative Value Unit 20

© 2025 AtriCure, Inc. All rights reserved. 21 Sources Tables +Article in Press. https://www.heartrhythmjournal.com/article/S1547-5271(24)00261-3/fulltext (accessed 4/10/2024). Heart Rhythm Society, the European Society of Cardiology, the Asia Pacific Heart Rhythm Society, and the Latin American Heart Rhythm Society 2024. +Hybrid ablation type of evidence META (meta-analysis); LAAE type of evidence RAND (randomized controlled); nomenclature did not use LOE classification. ^Advice TO DO/RAND. †Advice TO DO/META. Wyler von Ballmoos, M.C. et al. (2024). The Society of Thoracic Surgeons 2023 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Members, W. C., et al. (2023). 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology. January, C. T., et al. (2019). 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation, CIR-0000000000000665. Badhwar, et al. (2017). The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg, 103(1):329-41. ‡MVR LOE A; AVR,CABG LOE B. January, C.T., et al. (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol, 64(21):e1-76. *Calkins, H., et al. (2017). 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm, 14(10):e275-444. AVR/CABG concomitant ablation Class I LOR for symptomatic persistent and long-standing persistent “refractory or intolerant to at least one Class 1 or 3 antiarrhythmic medication.” Meier, B., et al. (2014). EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace, 16(10):1397-416. Cox, J.L., et al. (1991). Dr. Cox performed first surgical ablation using maze I; Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA, 266 (14):1976-80. Isabelle C Van Gelder, Michiel Rienstra, Karina V Bunting, Ruben Casado-Arroyo, Valeria Caso, Harry J G M Crijns, Tom J R De Potter, Jeremy Dwight, Luigina Guasti, Thorsten Hanke, et al. (2024). 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO), European Heart Journal, ehae176. Sources: Treatment of Afib and LAAM Advancing Guidelines for Clinical Practice Sources: Treatment of Afib and LAAM Improving Access through Reimbursement In 2021, CMS moved CABG plus ablation cases to MS-DRGs 223/234 from MS-DRGs 235/236. In 2022, CMS physician payment rates included new surgical LAA codes (CPT 33267, 33268, 33269). In 2023, CMS created MS-DRG 212 which moves cases with an AVR plus and MVR plus an ablation from MS-DRGs 216-221 to MS-DRG 212. In 2024, CMS created MS-DRG 317 which moves cases with ablation plus LAAM from MS-DRG 228/229 to MS-DRG 317. Healthcare providers are solely responsible for the accuracy of codes selected for the services rendered and reported. AtriCure does not assume responsibility for coding decisions, nor recommend codes for specific cases. AtriCure also does not promote off-label use of its devices.