| Overview January 2026 |
| This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements about our expectations regarding the potential benefits, activity, effectiveness, and safety of our product candidates, our expectations with regard to the design and results of our research and development programs, preclinical studies, and clinical trials, including the timing and availability of data from such studies and trials, our preclinical, clinical, and regulatory development plans for our product candidates, including the timing or likelihood of regulatory filings and approvals for our product candidates, our expectations with regard to our ability to license, acquire, discover, and develop additional products candidates and advance such product candidates into, and successfully complete, preclinical studies and clinical trials, the potential market size and size of the potential patient populations for our product candidates and any future product candidates, our ability to maintain existing, and establish new, strategic collaborations, licensing, or other arrangements, the scope of protection we are able to establish and maintain for intellectual property rights covering our initial product candidates and any future product candidates, our business strategy, and our future results of operations and financial position, and our anticipated cash runway are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, and our other filings with the SEC, which are available on the SEC’s website at www.sec.gov. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. This presentation contains trademarks, service marks, trade names and copyrights of Tvardi and other companies which are the property of their respective owners. 2 Disclaimer and Forward-Looking Statements |
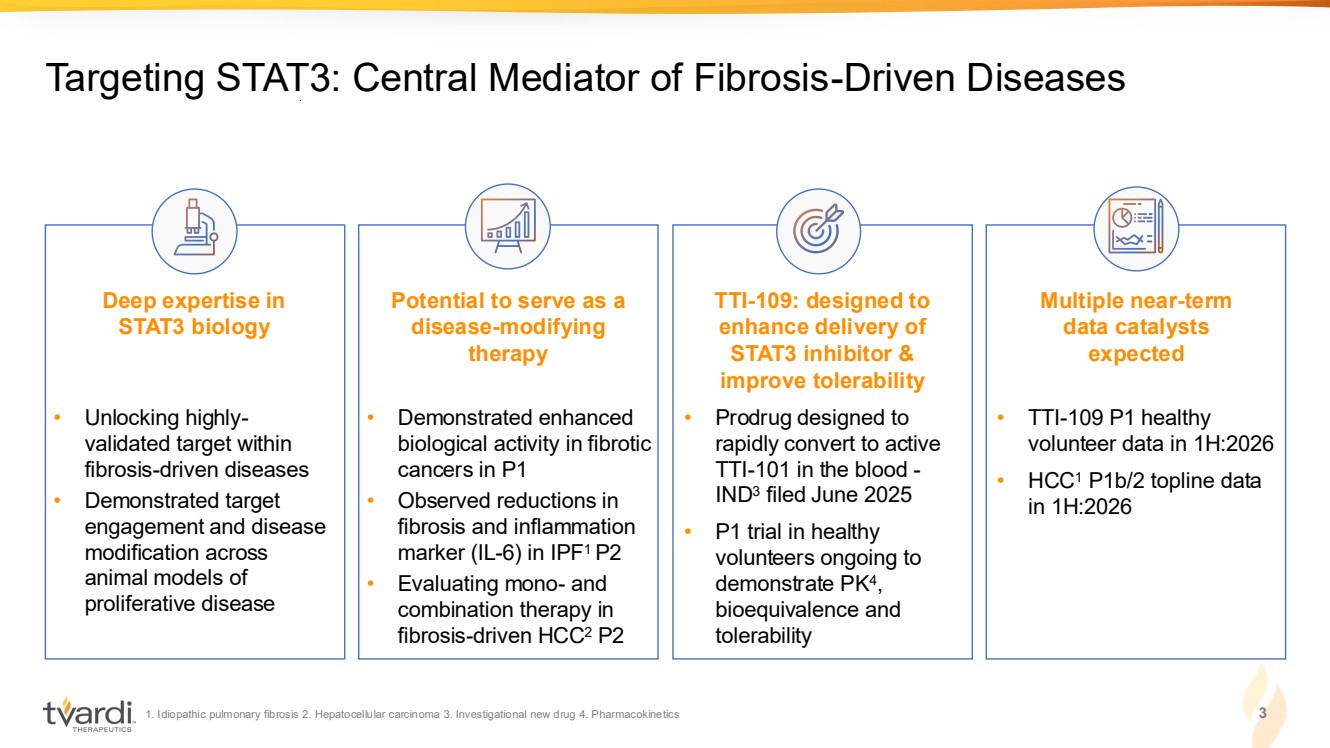
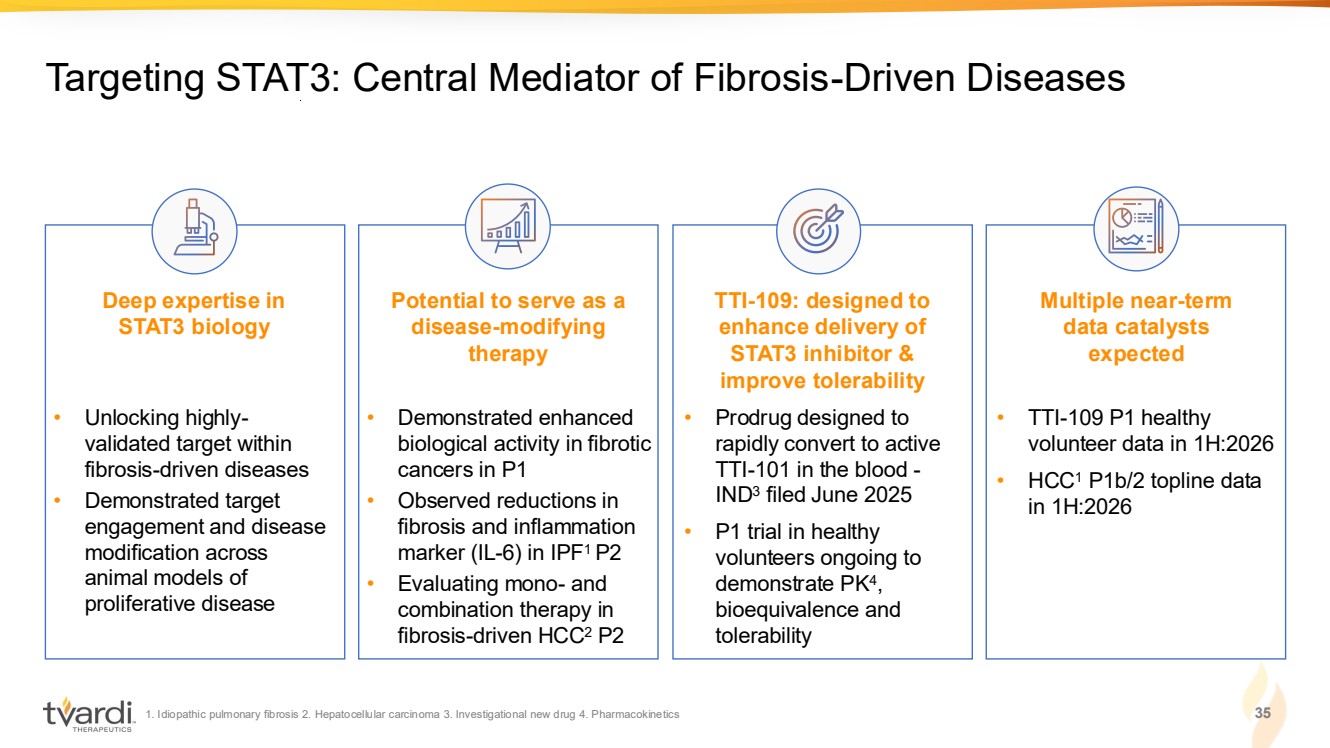
| 1. Idiopathic pulmonary fibrosis 2. Hepatocellular carcinoma 3. Investigational new drug 4. Pharmacokinetics 3 Targeting STAT3: Central Mediator of Fibrosis-Driven Diseases Deep expertise in STAT3 biology Potential to serve as a disease-modifying therapy TTI-109: designed to enhance delivery of STAT3 inhibitor & improve tolerability Multiple near-term data catalysts expected • Unlocking highly-validated target within fibrosis-driven diseases • Demonstrated target engagement and disease modification across animal models of proliferative disease • Demonstrated enhanced biological activity in fibrotic cancers in P1 • Observed reductions in fibrosis and inflammation marker (IL-6) in IPF1 P2 • Evaluating mono- and combination therapy in fibrosis-driven HCC2 P2 • TTI-109 P1 healthy volunteer data in 1H:2026 • HCC1 P1b/2 topline data in 1H:2026 • Prodrug designed to rapidly convert to active TTI-101 in the blood - IND3 filed June 2025 • P1 trial in healthy volunteers ongoing to demonstrate PK4 , bioequivalence and tolerability |
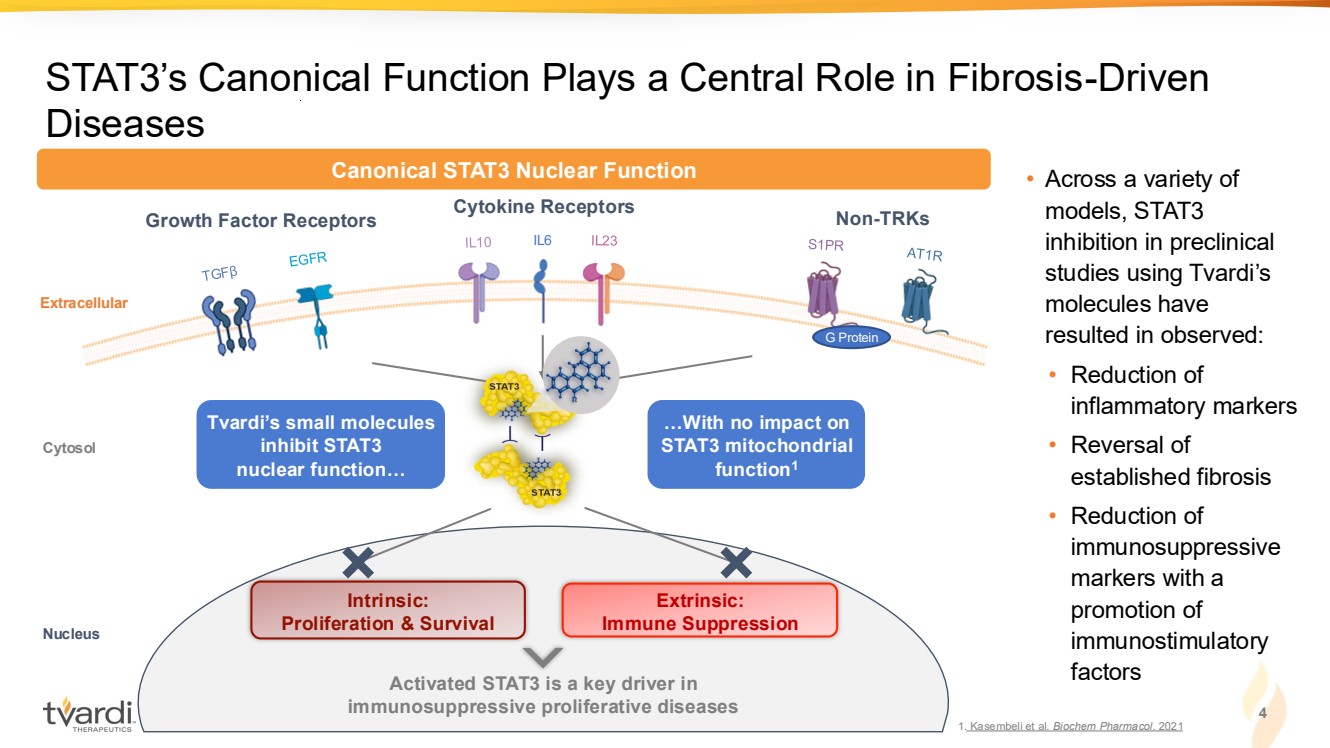
| STAT3’s Canonical Function Plays a Central Role in Fibrosis-Driven Diseases IL6 Growth Factor Receptors Cytokine Receptors Non-TRKs Extracellular Intrinsic: Proliferation & Survival Extrinsic: Immune Suppression Activated STAT3 is a key driver in immunosuppressive proliferative diseases Cytosol Nucleus G Protein Canonical STAT3 Nuclear Function Tvardi’s small molecules inhibit STAT3 nuclear function… …With no impact on STAT3 mitochondrial function1 • Across a variety of models, STAT3 inhibition in preclinical studies using Tvardi’s molecules have resulted in observed: • Reduction of inflammatory markers • Reversal of established fibrosis • Reduction of immunosuppressive markers with a promotion of immunostimulatory factors 4 1. Kasembeli et al. Biochem Pharmacol. 2021 |
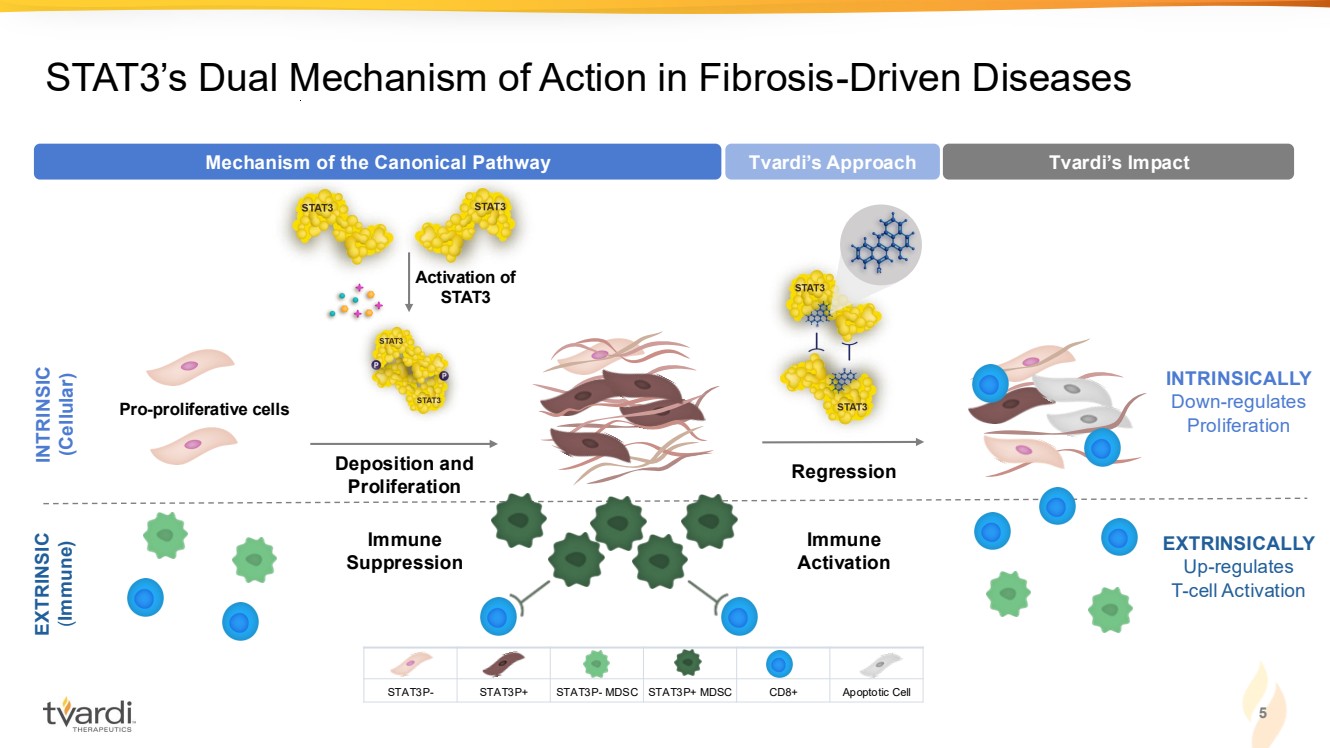
| 5 STAT3’s Dual Mechanism of Action in Fibrosis-Driven Diseases EXTRINSICALLY Up-regulates T-cell Activation Immune Activation Regression Tvardi’s Approach Tvardi’s Impact INTRINSICALLY Down-regulates Proliferation INTRINSIC (Cellular) EXTRINSIC (Immune) Immune Suppression Pro-proliferative cells Activation of STAT3 Deposition and Proliferation Mechanism of the Canonical Pathway STAT3P- STAT3P+ STAT3P- MDSC STAT3P+ MDSC CD8+ Apoptotic Cell |
| 6 Seasoned Leadership: Deep R&D and Operational Expertise Sujal Shah Chairman Michael Wyzga Director Cynthia Smith Director Susan Shiff, PhD Director Wallace Hall Director Imran Alibhai, PhD CEO & Director Dan Conn, JD, MBA CFO John Kauh, MD CMO David Tweardy, MD Founder & Advisor Ron DePinho, MD Founder & Advisor Keith Flaherty, MD Advisor (Oncology) Jeff Swigris, DO Advisor (Fibrosis) Management Team Scientific Advisory Board Board of Directors BioMatrix Partners |
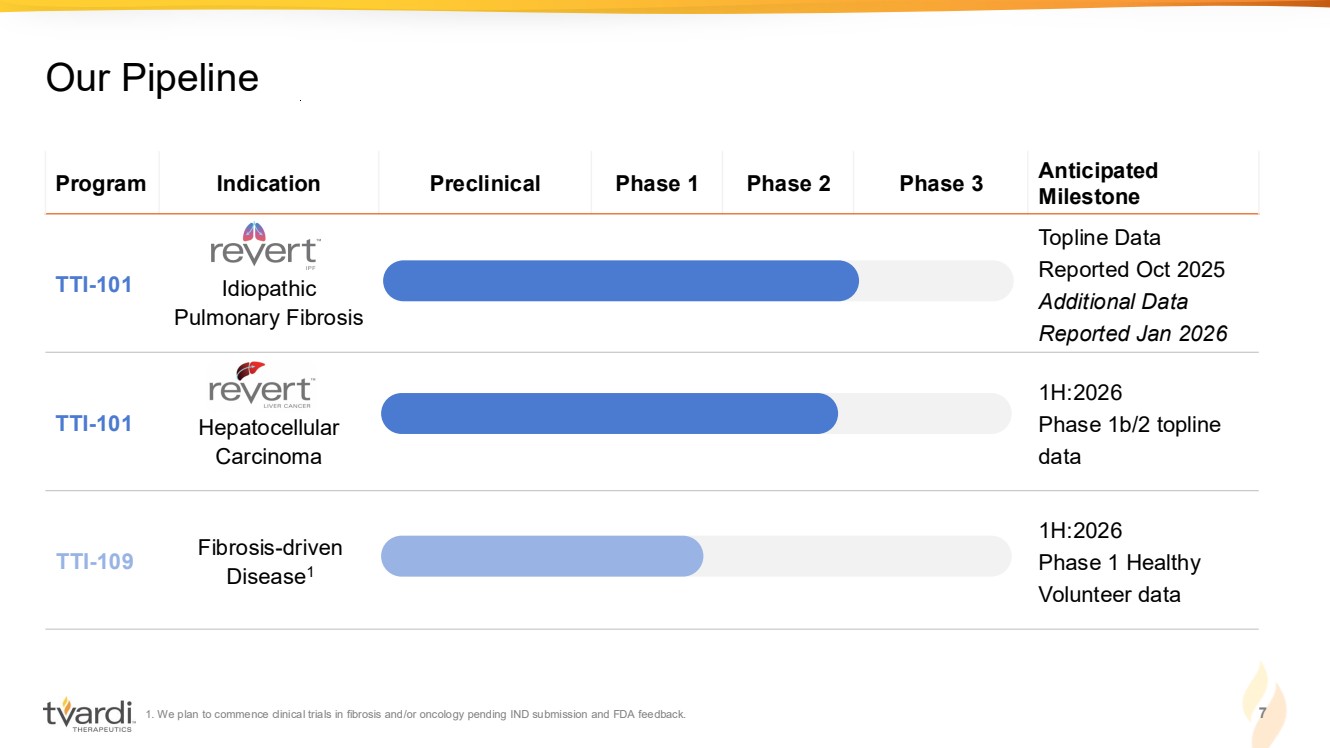
| 1. We plan to commence clinical trials in fibrosis and/or oncology pending IND submission and FDA feedback. 7 Our Pipeline Program Indication Preclinical Phase 1 Phase 2 Phase 3 Anticipated Milestone TTI-101 Idiopathic Pulmonary Fibrosis Topline Data Reported Oct 2025 Additional Data Reported Jan 2026 TTI-101 Hepatocellular Carcinoma 1H:2026 Phase 1b/2 topline data TTI-109 Fibrosis-driven Disease1 1H:2026 Phase 1 Healthy Volunteer data |
| TTI-101 in IPF |
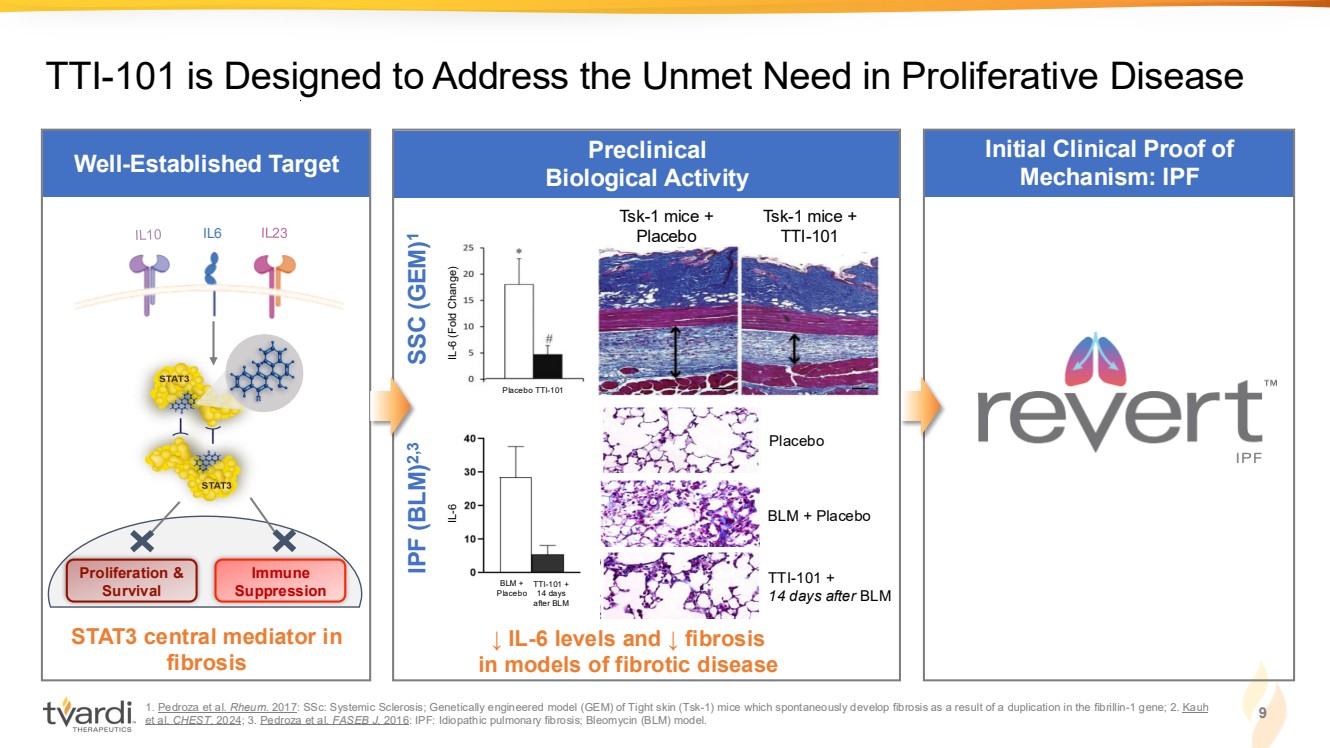
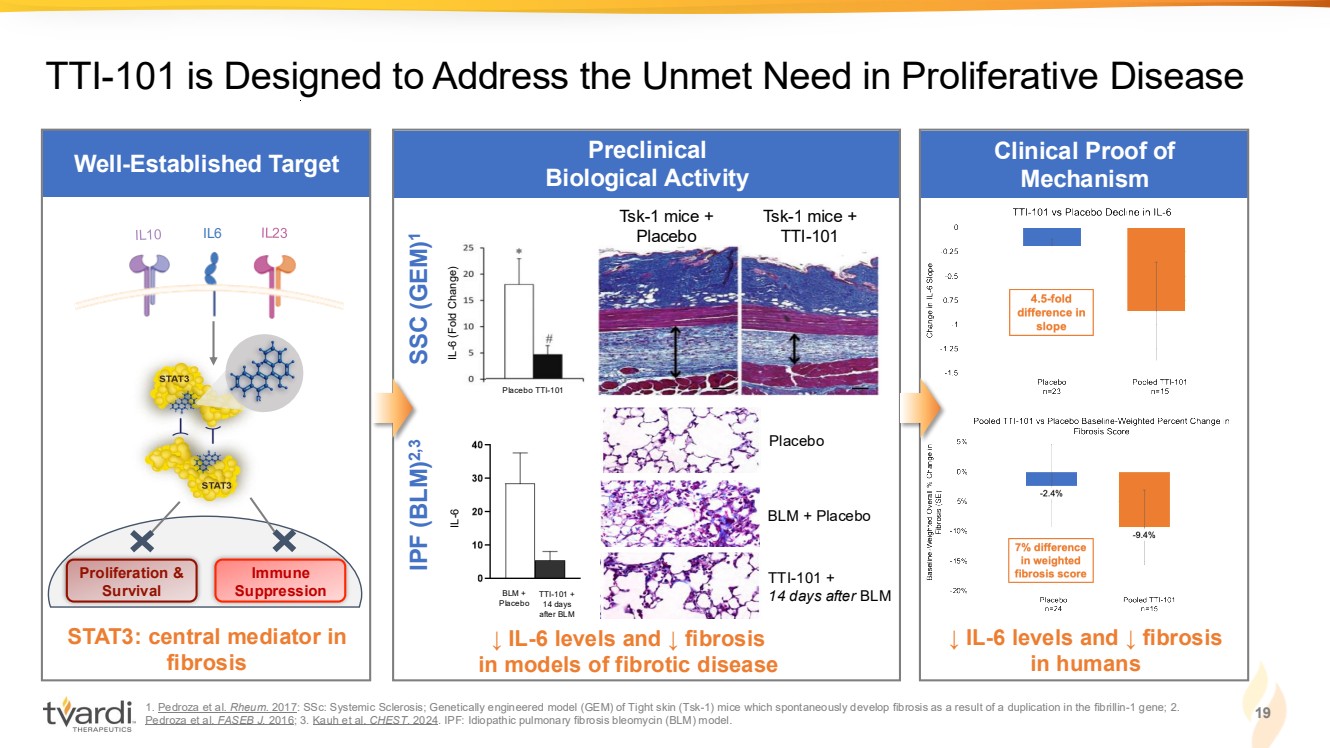
| Well-Established Target 1. Pedroza et al. Rheum. 2017: SSc: Systemic Sclerosis; Genetically engineered model (GEM) of Tight skin (Tsk-1) mice which spontaneously develop fibrosis as a result of a duplication in the fibrillin-1 gene; 2. Kauh et al. CHEST. 2024; 3. Pedroza et al. FASEB J. 2016: IPF: Idiopathic pulmonary fibrosis; Bleomycin (BLM) model. Preclinical Biological Activity STAT3 central mediator in fibrosis TTI-101 is Designed to Address the Unmet Need in Proliferative Disease Tsk-1 mice + Placebo Tsk-1 mice + IL6 TTI-101 Proliferation & Survival Immune Suppression ↓ IL-6 levels and ↓ fibrosis in models of fibrotic disease IL-6 (Fold Change) Placebo TTI-101 BLM + Placebo Placebo BLM + Placebo TTI-101 + 14 days after BLM IPF (BLM)2,3 SSC (GEM) 1 IL-6 TTI-101 + 14 days after BLM 9 Initial Clinical Proof of Mechanism: IPF |
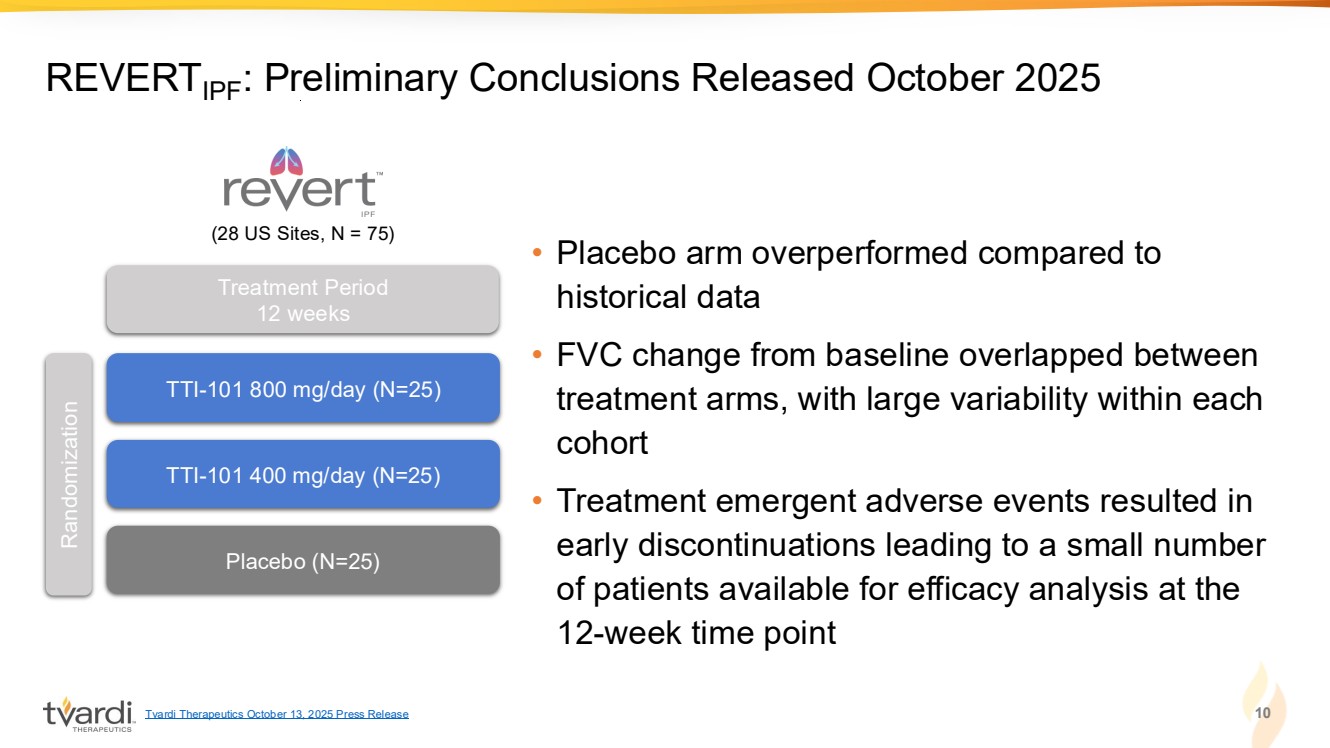
| • Placebo arm overperformed compared to historical data • FVC change from baseline overlapped between treatment arms, with large variability within each cohort • Treatment emergent adverse events resulted in early discontinuations leading to a small number of patients available for efficacy analysis at the 12-week time point 10 REVERTIPF: Preliminary Conclusions Released October 2025 TTI-101 800 mg/day (N=25) TTI-101 400 mg/day (N=25) Placebo (N=25) Randomization Treatment Period 12 weeks (28 US Sites, N = 75) Tvardi Therapeutics October 13, 2025 Press Release |
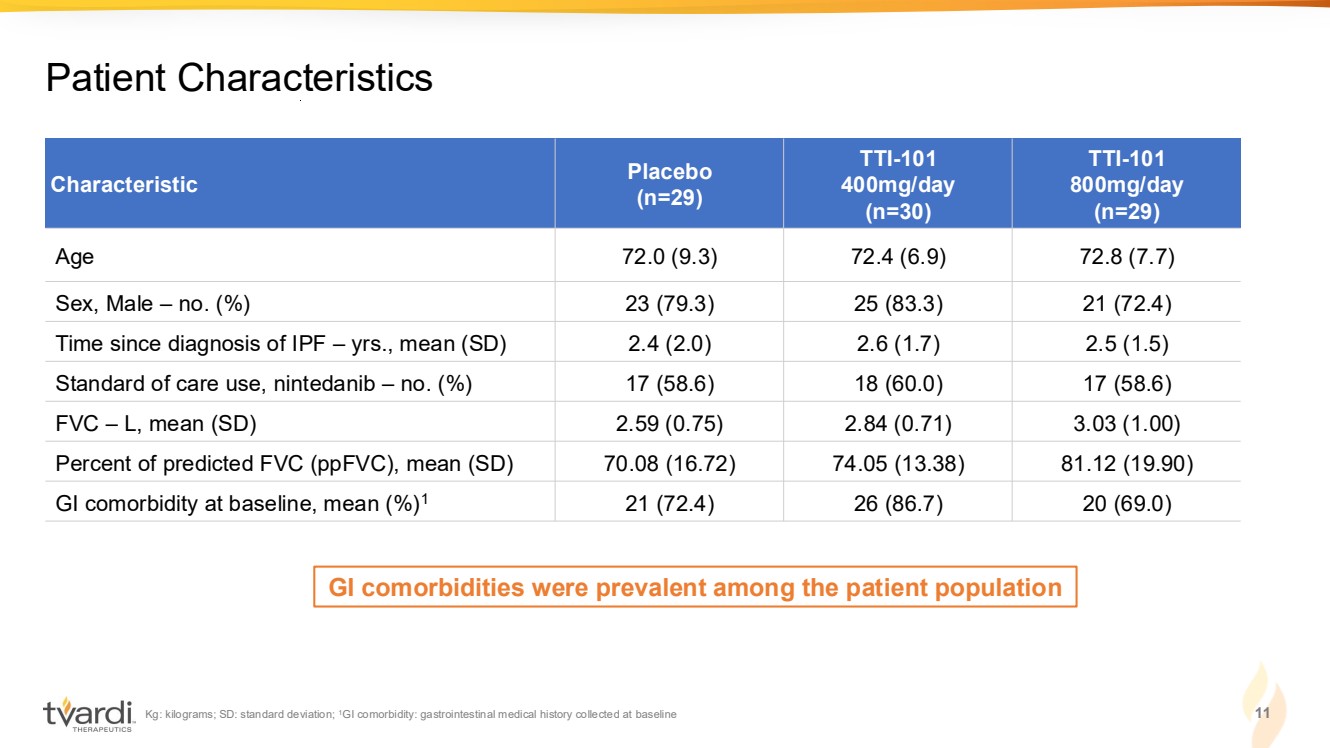
| Kg: kilograms; SD: standard deviation; 1GI comorbidity: gastrointestinal medical history collected at baseline 11 Patient Characteristics Characteristic Placebo (n=29) TTI-101 400mg/day (n=30) TTI-101 800mg/day (n=29) Age 72.0 (9.3) 72.4 (6.9) 72.8 (7.7) Sex, Male – no. (%) 23 (79.3) 25 (83.3) 21 (72.4) Time since diagnosis of IPF – yrs., mean (SD) 2.4 (2.0) 2.6 (1.7) 2.5 (1.5) Standard of care use, nintedanib – no. (%) 17 (58.6) 18 (60.0) 17 (58.6) FVC – L, mean (SD) 2.59 (0.75) 2.84 (0.71) 3.03 (1.00) Percent of predicted FVC (ppFVC), mean (SD) 70.08 (16.72) 74.05 (13.38) 81.12 (19.90) GI comorbidity at baseline, mean (%)1 21 (72.4) 26 (86.7) 20 (69.0) GI comorbidities were prevalent among the patient population |
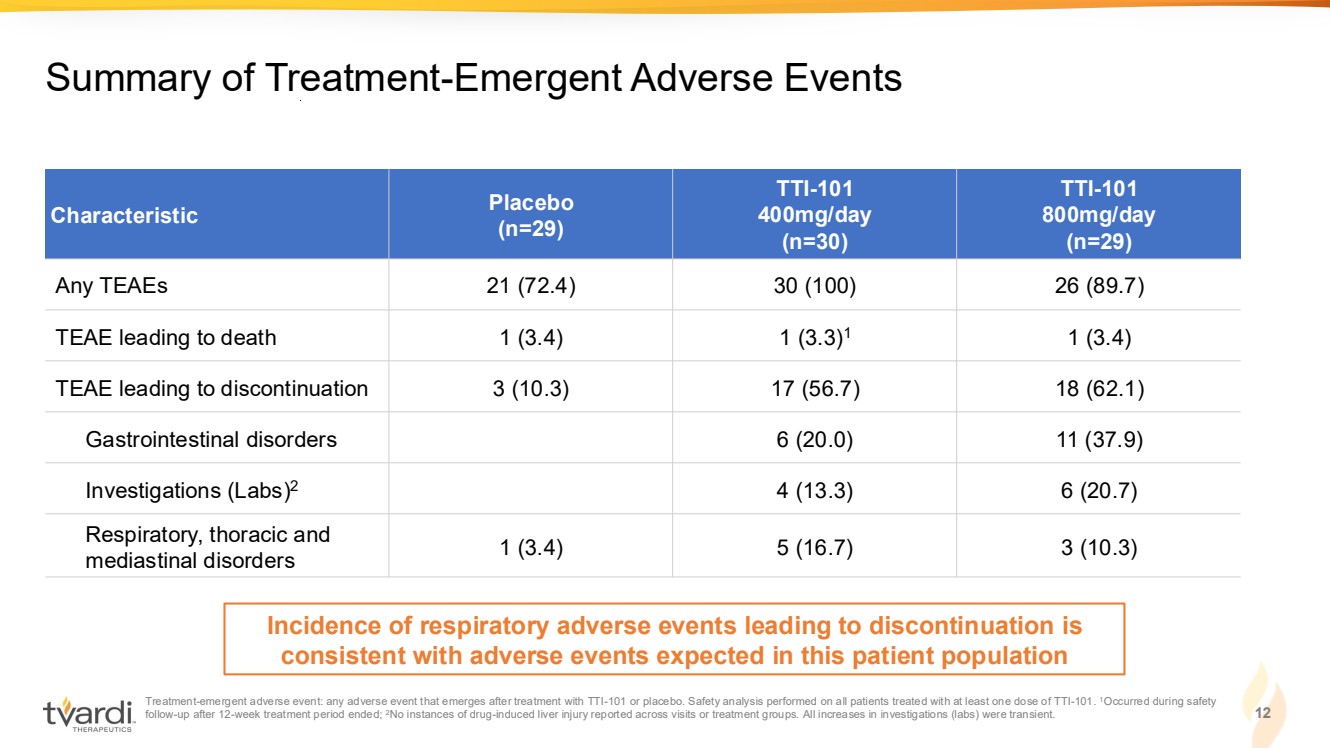
| Treatment-emergent adverse event: any adverse event that emerges after treatment with TTI-101 or placebo. Safety analysis performed on all patients treated with at least one dose of TTI-101. 1Occurred during safety follow-up after 12-week treatment period ended; 2No instances of drug-induced liver injury reported across visits or treatment groups. All increases in investigations (labs) were transient. 12 Summary of Treatment-Emergent Adverse Events Characteristic Placebo (n=29) TTI-101 400mg/day (n=30) TTI-101 800mg/day (n=29) Any TEAEs 21 (72.4) 30 (100) 26 (89.7) TEAE leading to death 1 (3.4) 1 (3.3)1 1 (3.4) TEAE leading to discontinuation 3 (10.3) 17 (56.7) 18 (62.1) Gastrointestinal disorders 6 (20.0) 11 (37.9) Investigations (Labs)2 4 (13.3) 6 (20.7) Respiratory, thoracic and mediastinal disorders 1 (3.4) 5 (16.7) 3 (10.3) Incidence of respiratory adverse events leading to discontinuation is consistent with adverse events expected in this patient population |
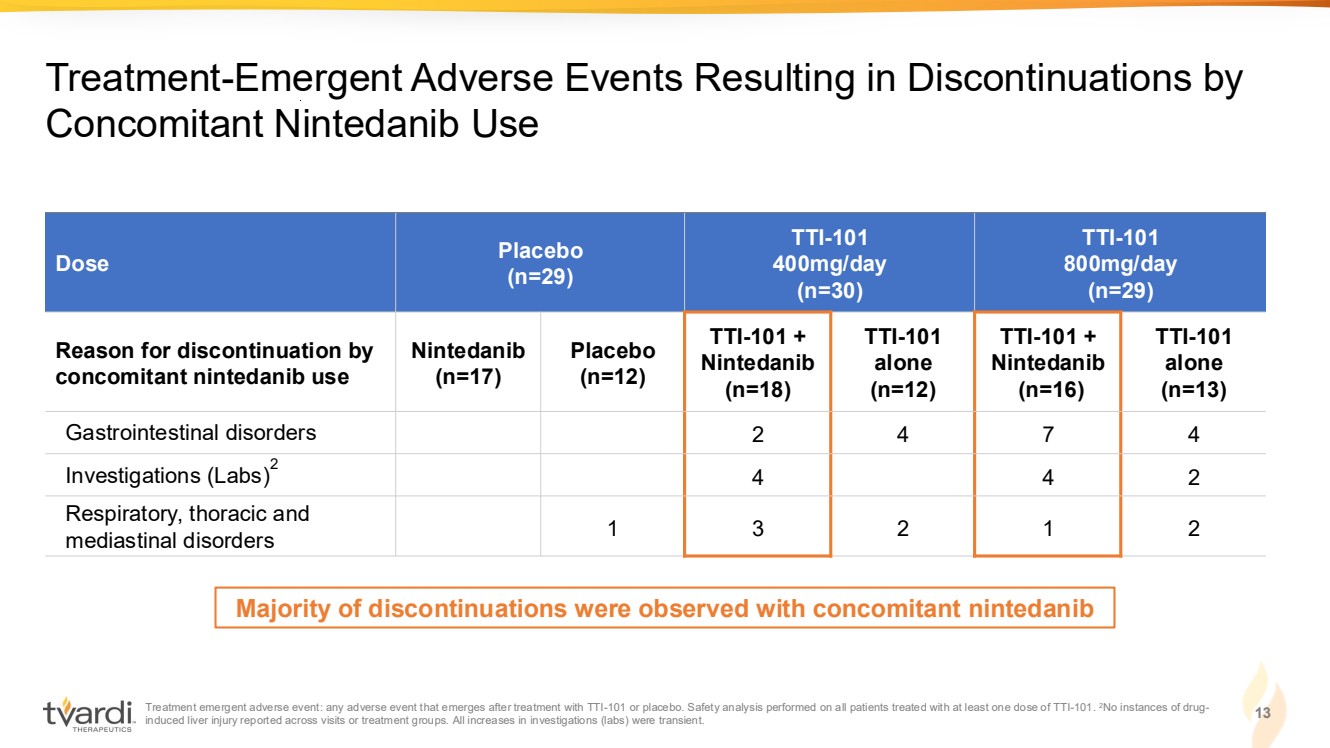
| Treatment emergent adverse event: any adverse event that emerges after treatment with TTI-101 or placebo. Safety analysis performed on all patients treated with at least one dose of TTI-101. 2No instances of drug-induced liver injury reported across visits or treatment groups. All increases in investigations (labs) were transient. 13 Treatment-Emergent Adverse Events Resulting in Discontinuations by Concomitant Nintedanib Use Dose Placebo (n=29) TTI-101 400mg/day (n=30) TTI-101 800mg/day (n=29) Reason for discontinuation by concomitant nintedanib use Nintedanib (n=17) Placebo (n=12) TTI-101 + Nintedanib (n=18) TTI-101 alone (n=12) TTI-101 + Nintedanib (n=16) TTI-101 alone (n=13) Gastrointestinal disorders 2 4 7 4 Investigations (Labs)2 4 4 2 Respiratory, thoracic and mediastinal disorders 1 3 2 1 2 Majority of discontinuations were observed with concomitant nintedanib |
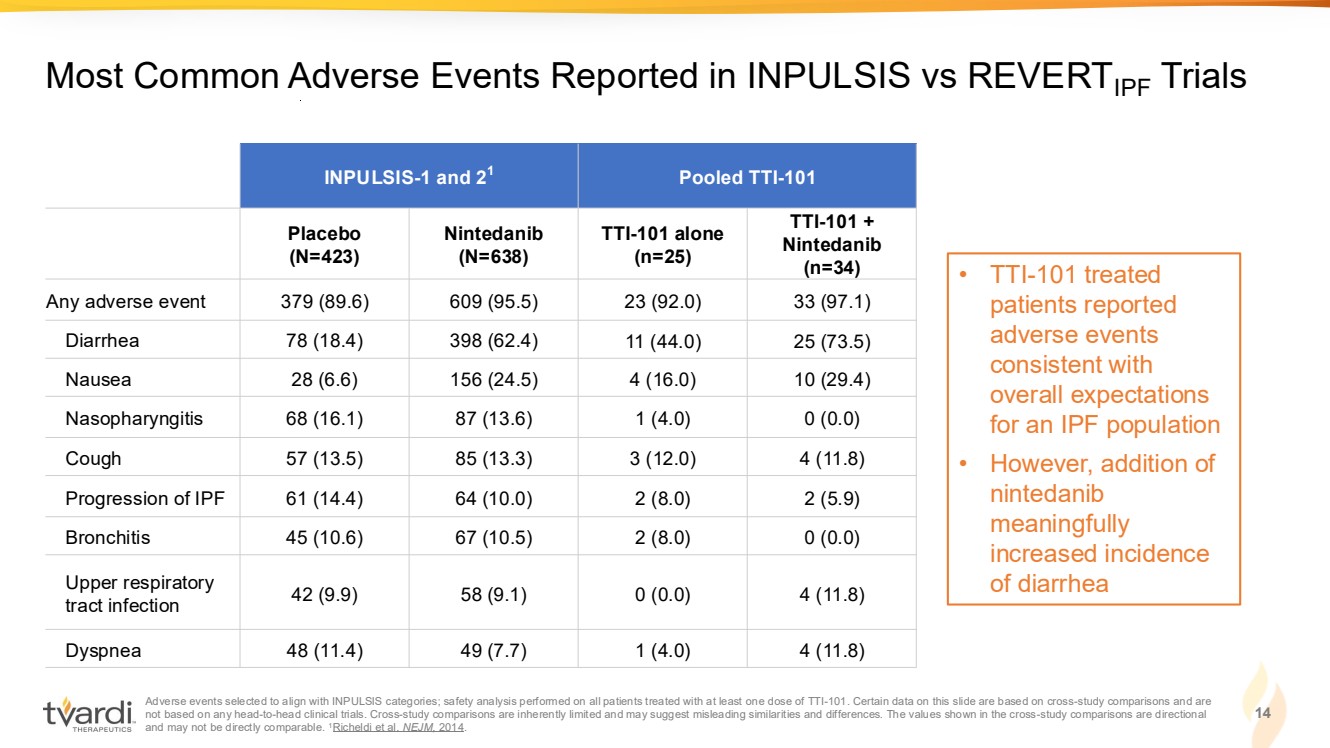
| Adverse events selected to align with INPULSIS categories; safety analysis performed on all patients treated with at least one dose of TTI-101. Certain data on this slide are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly comparable. 1Richeldi et al. NEJM, 2014. 14 Most Common Adverse Events Reported in INPULSIS vs REVERTIPF Trials INPULSIS-1 and 21 Pooled TTI-101 Placebo (N=423) Nintedanib (N=638) TTI-101 alone (n=25) TTI-101 + Nintedanib (n=34) Any adverse event 379 (89.6) 609 (95.5) 23 (92.0) 33 (97.1) Diarrhea 78 (18.4) 398 (62.4) 11 (44.0) 25 (73.5) Nausea 28 (6.6) 156 (24.5) 4 (16.0) 10 (29.4) Nasopharyngitis 68 (16.1) 87 (13.6) 1 (4.0) 0 (0.0) Cough 57 (13.5) 85 (13.3) 3 (12.0) 4 (11.8) Progression of IPF 61 (14.4) 64 (10.0) 2 (8.0) 2 (5.9) Bronchitis 45 (10.6) 67 (10.5) 2 (8.0) 0 (0.0) Upper respiratory tract infection 42 (9.9) 58 (9.1) 0 (0.0) 4 (11.8) Dyspnea 48 (11.4) 49 (7.7) 1 (4.0) 4 (11.8) • TTI-101 treated patients reported adverse events consistent with overall expectations for an IPF population • However, addition of nintedanib meaningfully increased incidence of diarrhea |
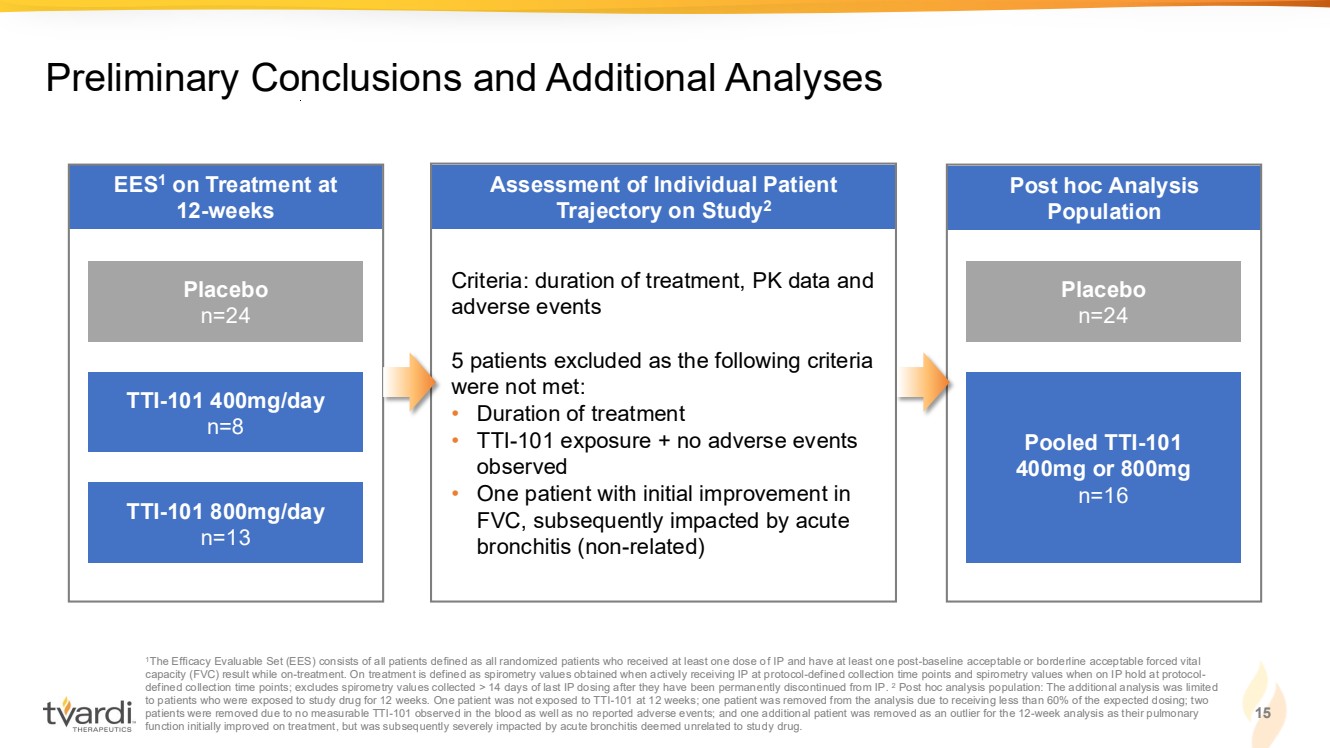
| Post hoc Analysis Population Assessment of Individual Patient Trajectory on Study2 EES1 on Treatment at 12-weeks 15 Preliminary Conclusions and Additional Analyses 1The Efficacy Evaluable Set (EES) consists of all patients defined as all randomized patients who received at least one dose of IP and have at least one post-baseline acceptable or borderline acceptable forced vital capacity (FVC) result while on-treatment. On treatment is defined as spirometry values obtained when actively receiving IP at protocol-defined collection time points and spirometry values when on IP hold at protocol-defined collection time points; excludes spirometry values collected > 14 days of last IP dosing after they have been permanently discontinued from IP. 2 Post hoc analysis population: The additional analysis was limited to patients who were exposed to study drug for 12 weeks. One patient was not exposed to TTI-101 at 12 weeks; one patient was removed from the analysis due to receiving less than 60% of the expected dosing; two patients were removed due to no measurable TTI-101 observed in the blood as well as no reported adverse events; and one additional patient was removed as an outlier for the 12-week analysis as their pulmonary function initially improved on treatment, but was subsequently severely impacted by acute bronchitis deemed unrelated to study drug. Placebo n=24 TTI-101 400mg/day n=8 TTI-101 800mg/day n=13 Placebo n=24 Pooled TTI-101 400mg or 800mg n=16 Criteria: duration of treatment, PK data and adverse events 5 patients excluded as the following criteria were not met: • Duration of treatment • TTI-101 exposure + no adverse events observed • One patient with initial improvement in FVC, subsequently impacted by acute bronchitis (non-related) |
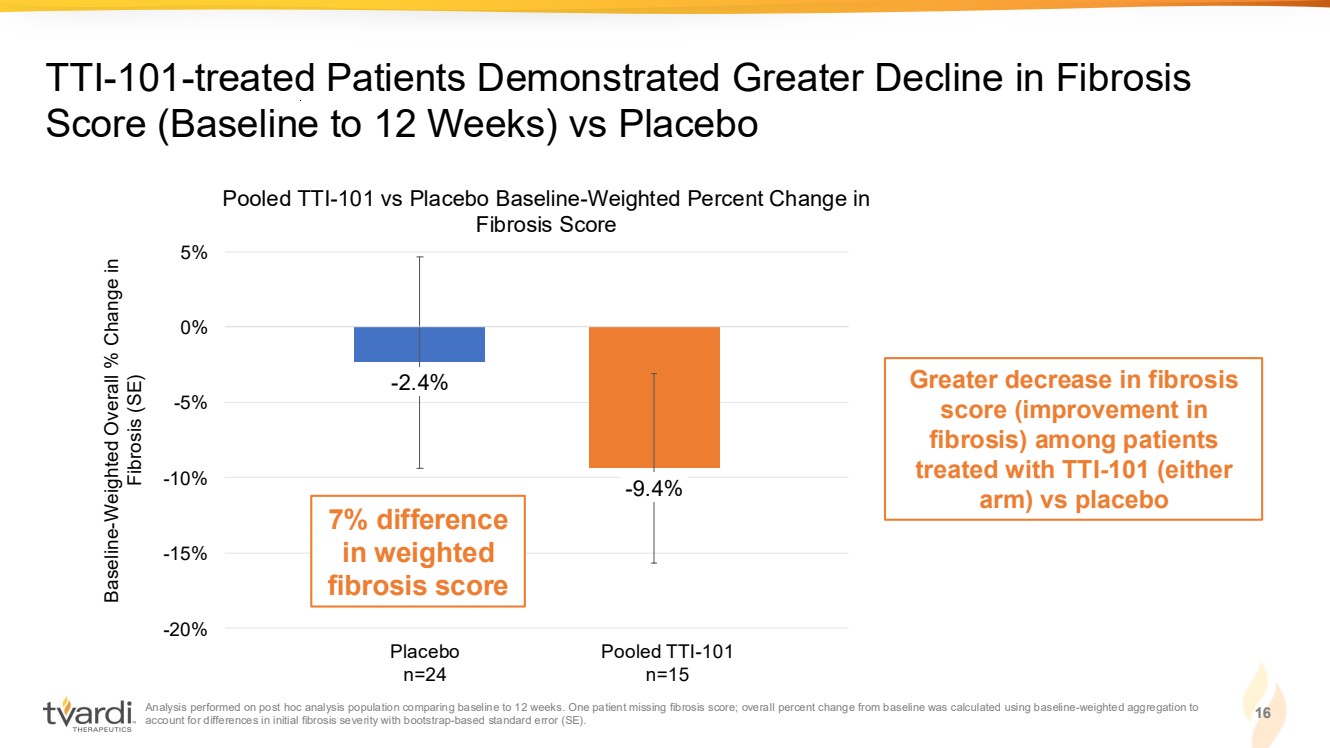
| Analysis performed on post hoc analysis population comparing baseline to 12 weeks. One patient missing fibrosis score; overall percent change from baseline was calculated using baseline-weighted aggregation to account for differences in initial fibrosis severity with bootstrap-based standard error (SE). 16 TTI-101-treated Patients Demonstrated Greater Decline in Fibrosis Score (Baseline to 12 Weeks) vs Placebo 24 24 20* 15* Greater decrease in fibrosis score (improvement in fibrosis) among patients treated with TTI-101 (either arm) vs placebo -2.4% -9.4% -20% -15% -10% -5% 0% 5% Pooled TTI-101 vs Placebo Baseline-Weighted Percent Change in Fibrosis Score Baseline-Weighted Overall % Change in Fibrosis (SE) Placebo n=24 Pooled TTI-101 n=15 7% difference in weighted fibrosis score |
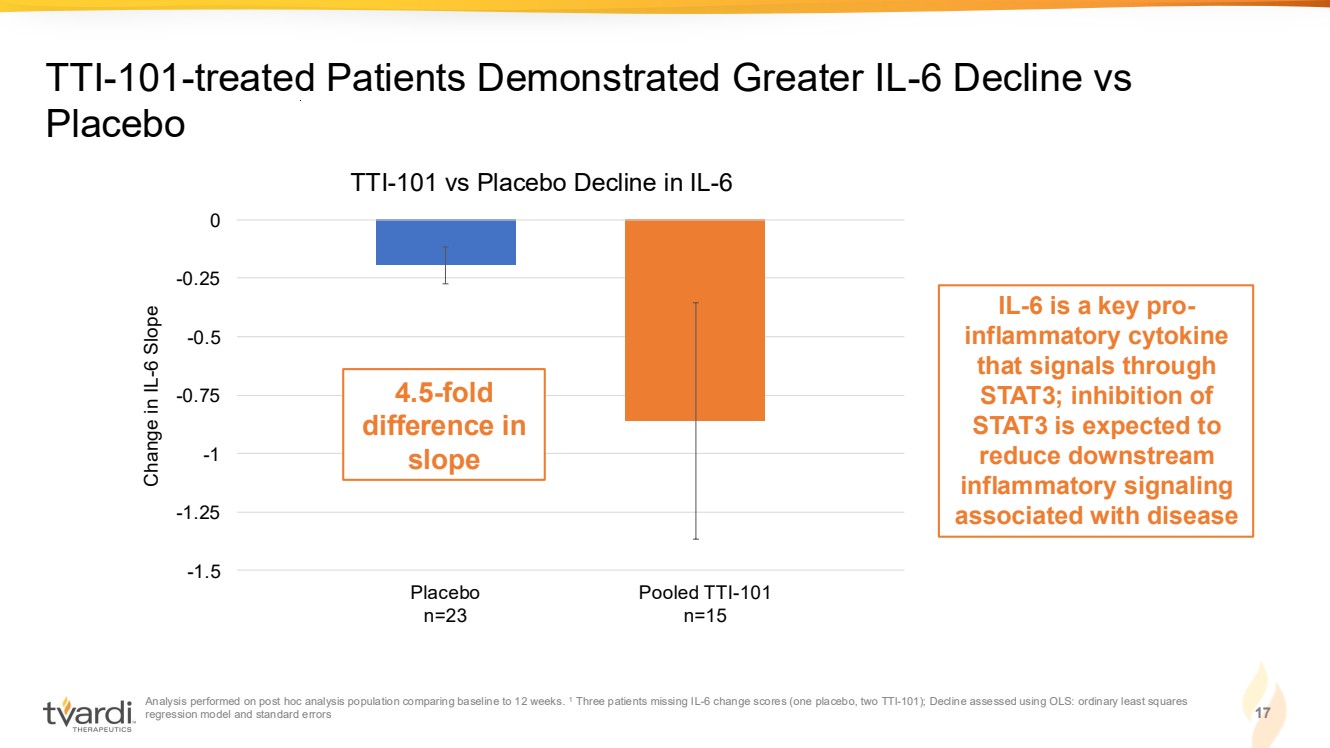
| Analysis performed on post hoc analysis population comparing baseline to 12 weeks. 1 Three patients missing IL-6 change scores (one placebo, two TTI-101); Decline assessed using OLS: ordinary least squares regression model and standard errors 17 TTI-101-treated Patients Demonstrated Greater IL-6 Decline vs Placebo -1.5 -1.25 -1 -0.75 -0.5 -0.25 0 TTI-101 vs Placebo Decline in IL-6 Change in IL-6 Slope Placebo n=23 Pooled TTI-101 n=15 4.5-fold difference in slope IL-6 is a key pro-inflammatory cytokine that signals through STAT3; inhibition of STAT3 is expected to reduce downstream inflammatory signaling associated with disease |
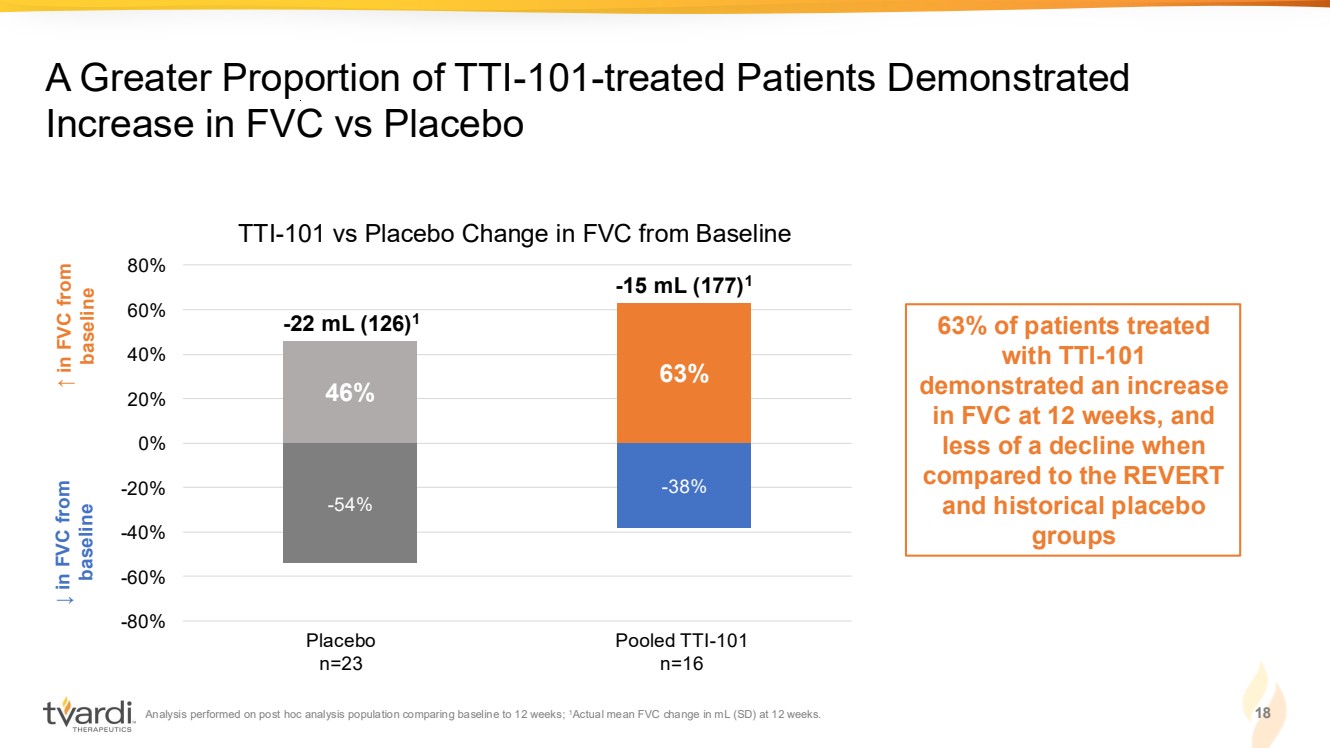
| Analysis performed on post hoc analysis population comparing baseline to 12 weeks; 1Actual mean FVC change in mL (SD) at 12 weeks. 18 A Greater Proportion of TTI-101-treated Patients Demonstrated Increase in FVC vs Placebo ↓ in FVC from baseline ↑ in FVC from baseline 63% of patients treated with TTI-101 demonstrated an increase in FVC at 12 weeks, and less of a decline when compared to the REVERT and historical placebo groups -54% -38% 46% 63% -80% -60% -40% -20% 0% 20% 40% 60% 80% Placebo n=23 Pooled TTI-101 n=16 TTI-101 vs Placebo Change in FVC from Baseline -22 mL (126)1 -15 mL (177)1 |
| IPF (BLM)2,3 Clinical Proof of Mechanism Well-Established Target 1. Pedroza et al. Rheum. 2017: SSc: Systemic Sclerosis; Genetically engineered model (GEM) of Tight skin (Tsk-1) mice which spontaneously develop fibrosis as a result of a duplication in the fibrillin-1 gene; 2. Pedroza et al. FASEB J. 2016; 3. Kauh et al. CHEST. 2024. IPF: Idiopathic pulmonary fibrosis bleomycin (BLM) model. Preclinical Biological Activity STAT3: central mediator in fibrosis ↓ IL-6 levels and ↓ fibrosis in humans TTI-101 is Designed to Address the Unmet Need in Proliferative Disease Tsk-1 mice + Placebo Tsk-1 mice + IL6 TTI-101 Proliferation & Survival Immune Suppression ↓ IL-6 levels and ↓ fibrosis in models of fibrotic disease IL-6 (Fold Change) Placebo TTI-101 BLM + Placebo Placebo BLM + Placebo TTI-101 + 14 days after BLM SSC (GEM) 1 IL-6 TTI-101 + 14 days after BLM 19 |
| Certain information on this slide are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. 20 REVERTIPF Provided Additional Proof of Mechanism for STAT3 Inhibition in Fibrotic-driven Diseases IL-6, a key pro-inflammatory cytokine that signals through STAT3, was observed to have a greater decline among TTI-101- treated patients vs placebo Placebo overperformed vs historical controls; TTI-101-treated patients demonstrated less of an FVC decline vs REVERT and historical trial placebo groups Adverse events in TTI-101-treated patients were consistent with IPF population; however, addition of nintedanib resulted in increased diarrhea incidence Patients treated with TTI-101 demonstrated a ~9% vs ~2% placebo decrease from baseline fibrosis score Fibrosis IL-6 Biomarker Pulmonary Function Safety TTI-109 is designed to enhance delivery of STAT3 inhibitor and improve tolerability for future studies |
| TTI-109 |
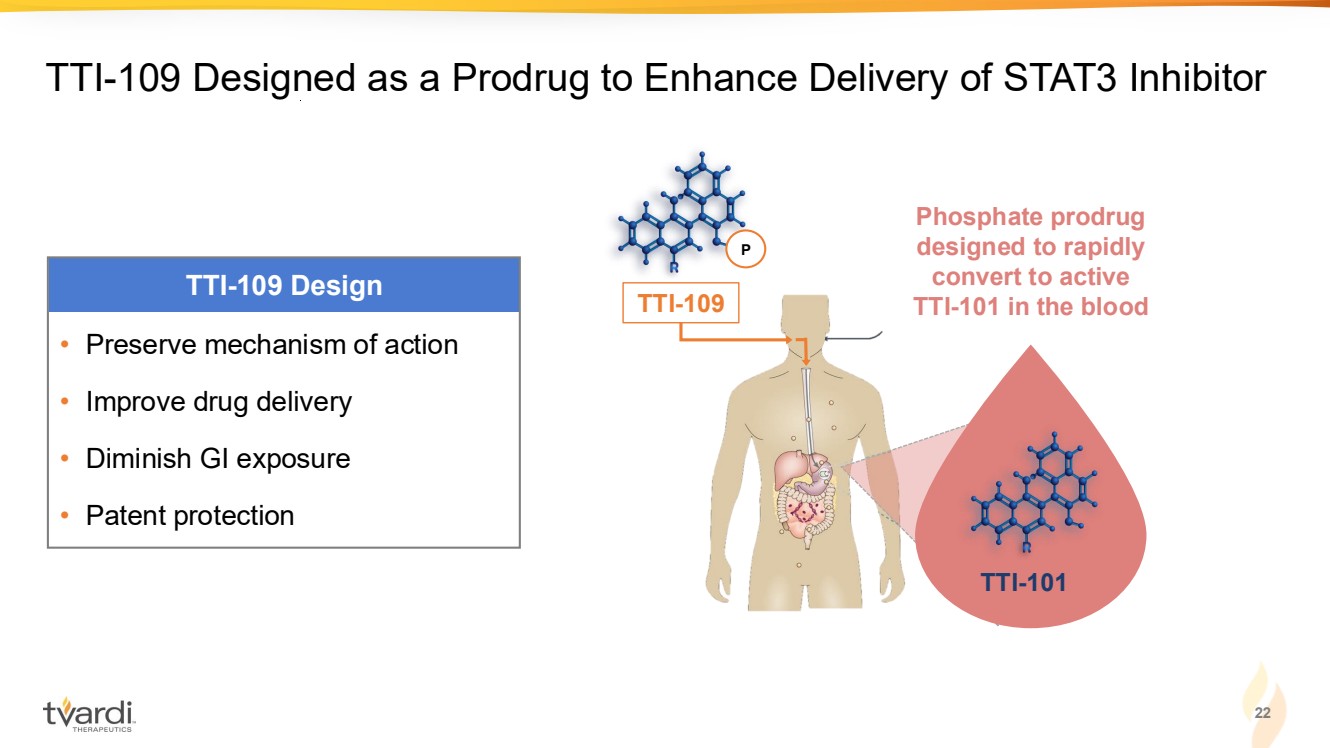
| TTI-101 TTI-109 Designed as a Prodrug to Enhance Delivery of STAT3 Inhibitor TTI-101 Phosphate prodrug designed to rapidly convert to active TTI-101 in the blood P TTI-109 • Preserve mechanism of action • Improve drug delivery • Diminish GI exposure • Patent protection TTI-109 Design 22 |
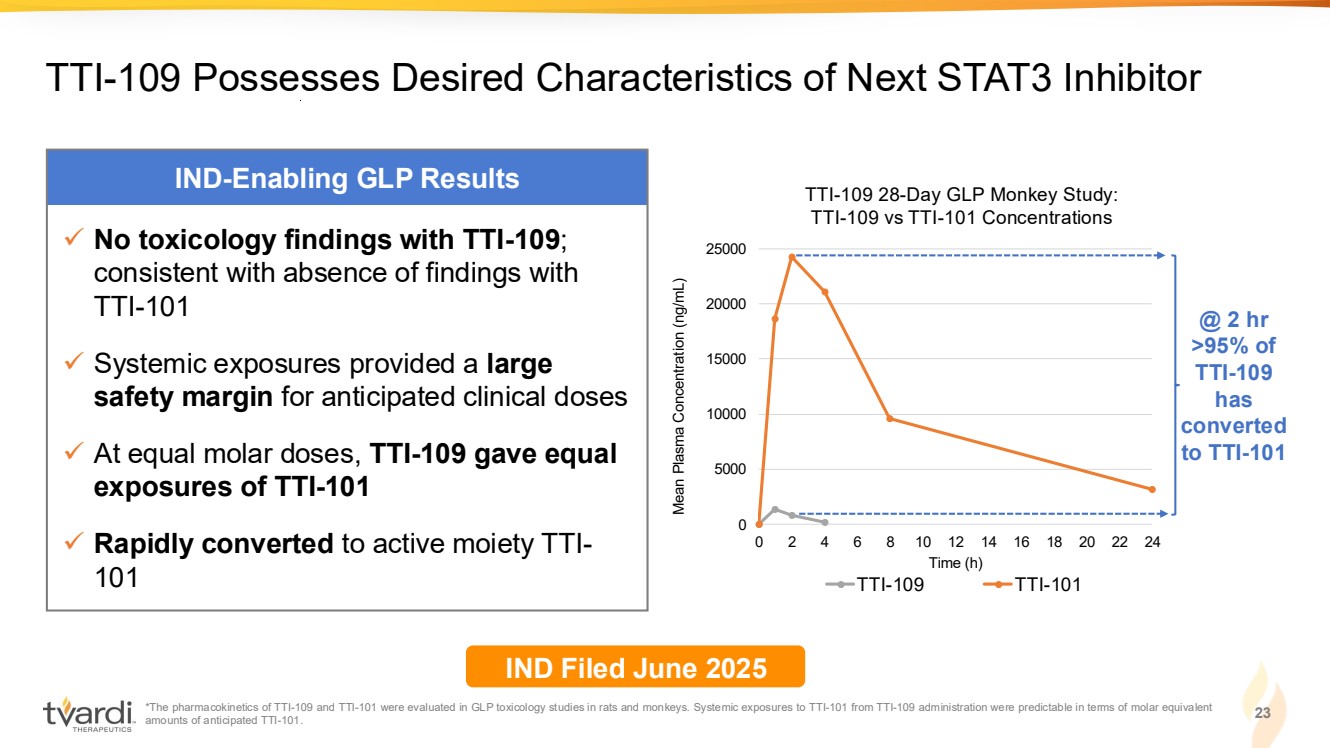
| 23 TTI-109 Possesses Desired Characteristics of Next STAT3 Inhibitor IND-Enabling GLP Results ✓ No toxicology findings with TTI-109; consistent with absence of findings with TTI-101 ✓ Systemic exposures provided a large safety margin for anticipated clinical doses ✓ At equal molar doses, TTI-109 gave equal exposures of TTI-101 ✓ Rapidly converted to active moiety TTI-101 IND Filed June 2025 *The pharmacokinetics of TTI-109 and TTI-101 were evaluated in GLP toxicology studies in rats and monkeys. Systemic exposures to TTI-101 from TTI-109 administration were predictable in terms of molar equivalent amounts of anticipated TTI-101. 0 5000 10000 15000 20000 25000 0 2 4 6 8 10 12 14 16 18 20 22 24 Mean Plasma Concentration (ng/mL) Time (h) TTI-109 28-Day GLP Monkey Study: TTI-109 vs TTI-101 Concentrations TTI-109 TTI-101 @ 2 hr >95% of TTI-109 has converted to TTI-101 |
| • Confirm key findings from IND-enabling studies in a 3-part Phase 1 healthy volunteer study: Single Ascending Dose, Bio-equivalence, Multiple Ascending Dose • Pharmacokinetics • Rapid conversion of TTI-109 to TTI-101 • Dose-dependent increase in systemic exposures • TTI-109 vs TTI-101 cross-over to demonstrate similar exposures of active moiety • Safety and tolerability of TTI-109 vs TTI-101 24 Overall Goals for Ongoing Phase 1 Healthy Volunteer Study with TTI-109 Phase 1 Data Expected 1H 2026 |
| TTI-101 in HCC |
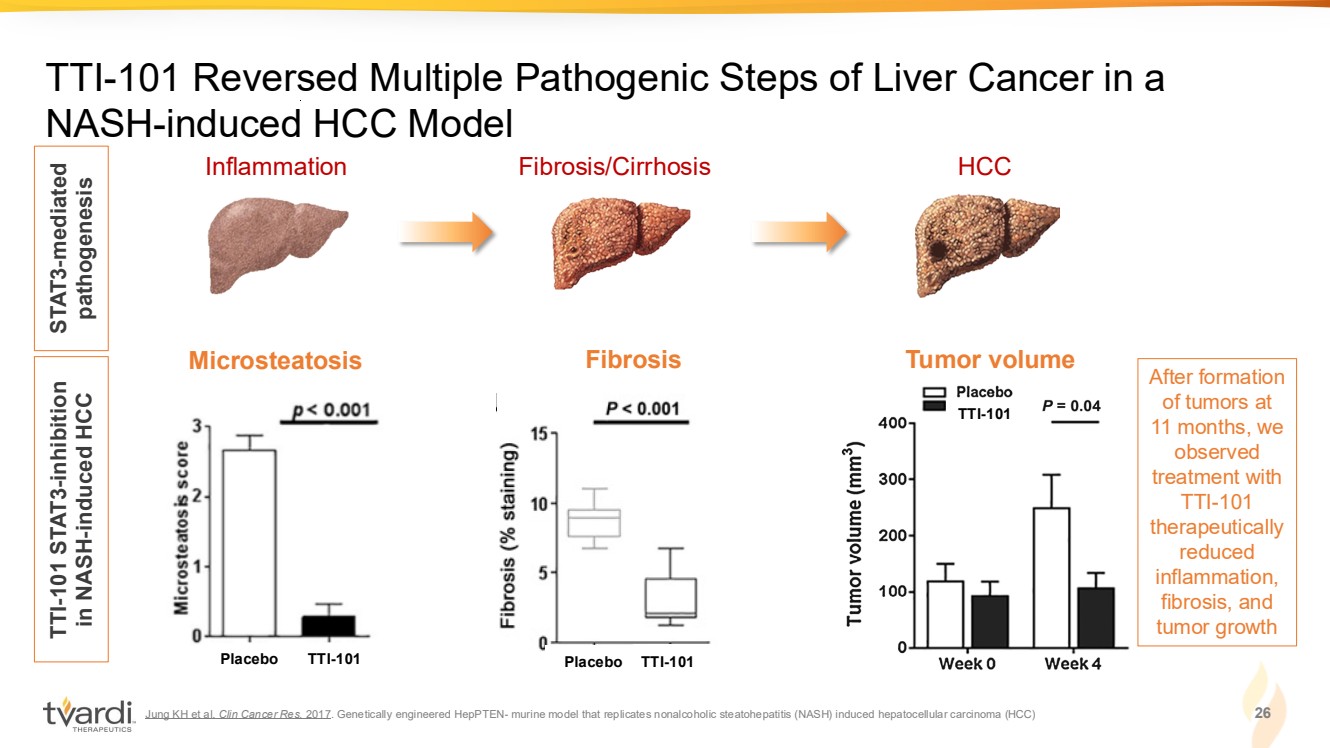
| Jung KH et al. Clin Cancer Res. 2017. Genetically engineered HepPTEN- murine model that replicates nonalcoholic steatohepatitis (NASH) induced hepatocellular carcinoma (HCC) 26 TTI-101 Reversed Multiple Pathogenic Steps of Liver Cancer in a NASH-induced HCC Model Inflammation Fibrosis/Cirrhosis HCC STAT3-mediated pathogenesis TTI-101 STAT3-inhibition in NASH-induced HCC TTI-101 Microsteatosis Tumor volume Placebo TTI-101 TTI-101 Fibrosis Placebo TTI-101 After formation of tumors at 11 months, we observed treatment with TTI-101 therapeutically reduced inflammation, fibrosis, and tumor growth |
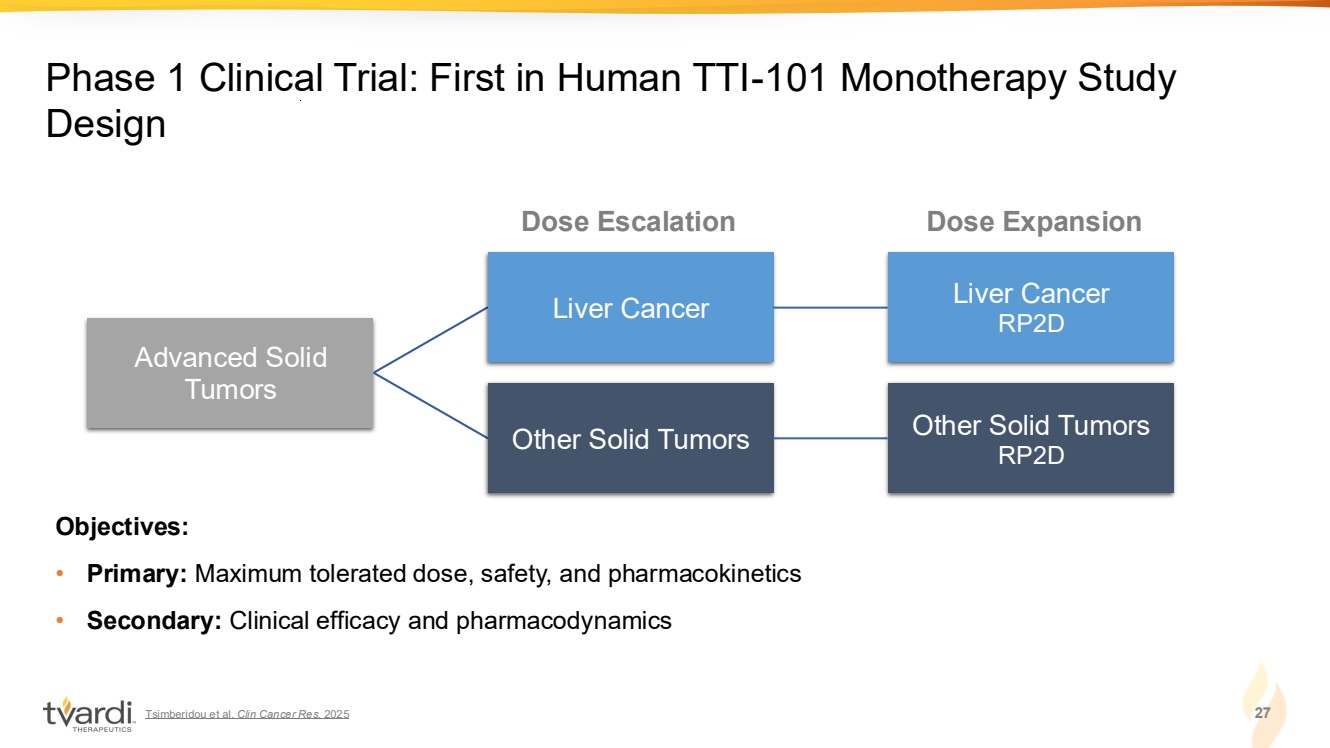
| Tsimberidou et al. Clin Cancer Res. 2025 27 Phase 1 Clinical Trial: First in Human TTI-101 Monotherapy Study Design Advanced Solid Tumors Liver Cancer Liver Cancer RP2D Other Solid Tumors Other Solid Tumors RP2D Objectives: • Primary: Maximum tolerated dose, safety, and pharmacokinetics • Secondary: Clinical efficacy and pharmacodynamics Dose Escalation Dose Expansion |
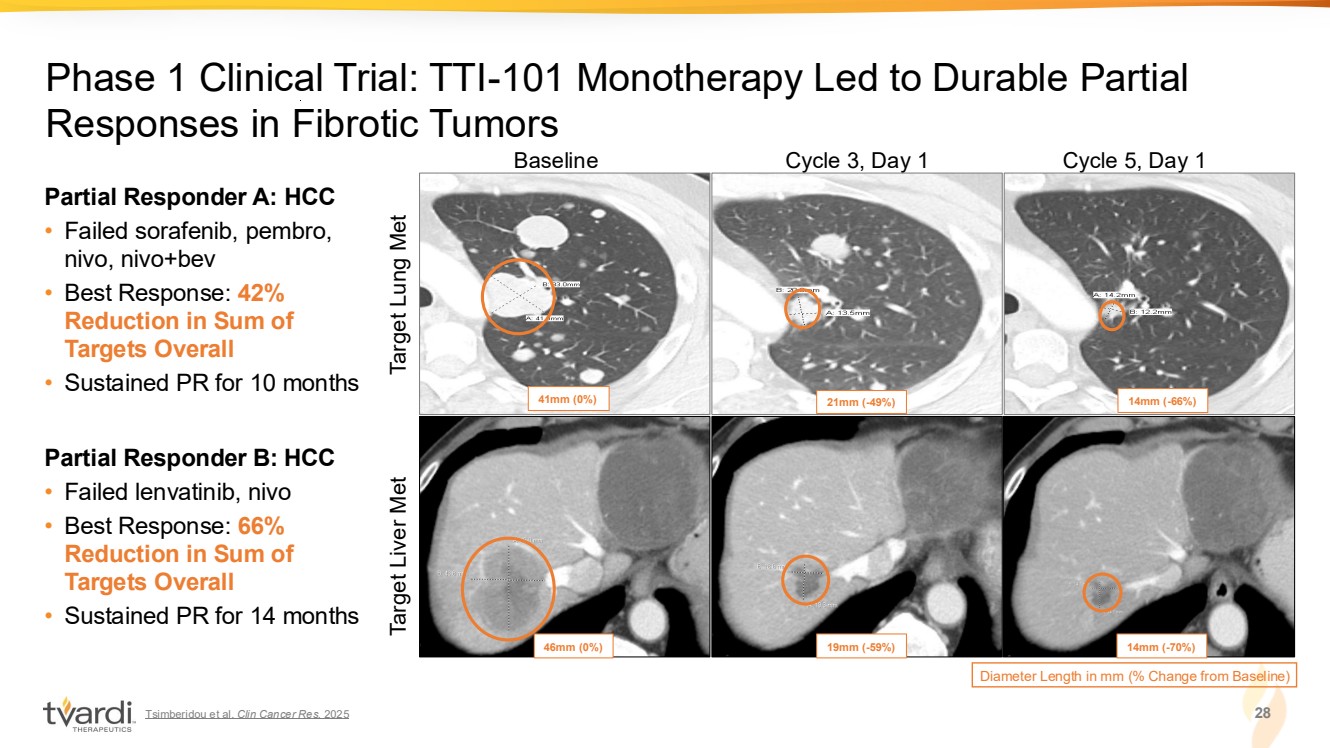
| Tsimberidou et al. Clin Cancer Res. 2025 28 Phase 1 Clinical Trial: TTI-101 Monotherapy Led to Durable Partial Responses in Fibrotic Tumors Baseline Cycle 3, Day 1 Cycle 5, Day 1 Diameter Length in mm (% Change from Baseline) 41mm (0%) 21mm (-49%) 14mm (-66%) 46mm (0%) 19mm (-59%) 14mm (-70%) Target Lung Met Target Liver Met Partial Responder A: HCC • Failed sorafenib, pembro, nivo, nivo+bev • Best Response: 42% Reduction in Sum of Targets Overall • Sustained PR for 10 months Partial Responder B: HCC • Failed lenvatinib, nivo • Best Response: 66% Reduction in Sum of Targets Overall • Sustained PR for 14 months |
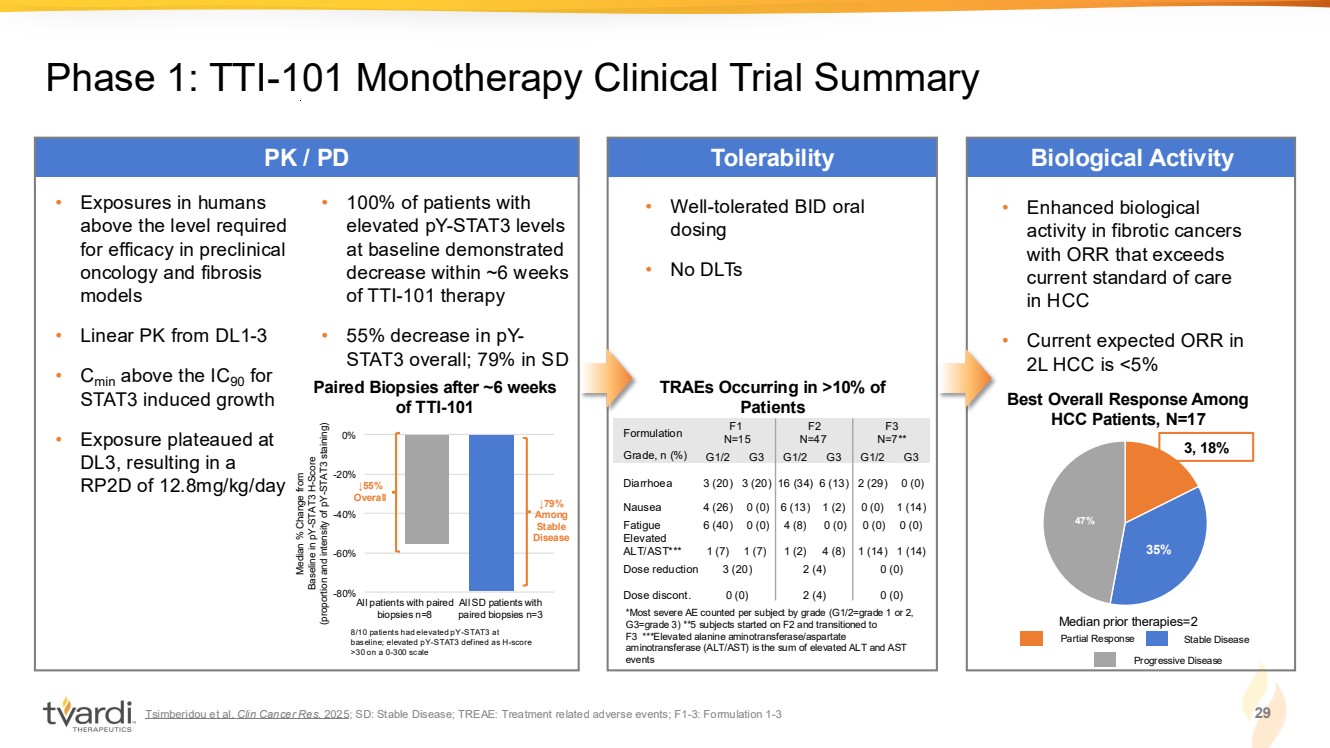
| Tsimberidou et al. Clin Cancer Res. 2025; SD: Stable Disease; TREAE: Treatment related adverse events; F1-3: Formulation 1-3 29 Phase 1: TTI-101 Monotherapy Clinical Trial Summary PK / PD Tolerability Biological Activity • Well-tolerated BID oral dosing • No DLTs *Most severe AE counted per subject by grade (G1/2=grade 1 or 2, G3=grade 3) **5 subjects started on F2 and transitioned to F3 ***Elevated alanine aminotransferase/aspartate aminotransferase (ALT/AST) is the sum of elevated ALT and AST events TRAEs Occurring in >10% of Patients • Exposures in humans above the level required for efficacy in preclinical oncology and fibrosis models • Linear PK from DL1-3 • Cmin above the IC90 for STAT3 induced growth • Exposure plateaued at DL3, resulting in a RP2D of 12.8mg/kg/day Median % Change from Baseline in pY-STAT3 H-Score (proportion and intensity of pY-STAT3 staining) All patients with paired biopsies n=8 All SD patients with paired biopsies n=3 -80% -60% -40% -20% 0% ↓79% Among Stable Disease 8/10 patients had elevated pY-STAT3 at baseline; elevated pY-STAT3 defined as H-score >30 on a 0-300 scale ↓55% Overall • 100% of patients with elevated pY-STAT3 levels at baseline demonstrated decrease within ~6 weeks of TTI-101 therapy • 55% decrease in pY-STAT3 overall; 79% in SD • Enhanced biological activity in fibrotic cancers with ORR that exceeds current standard of care in HCC • Current expected ORR in 2L HCC is <5% 35% 47% 3, 18% Best Overall Response Among HCC Patients, N=17 Median prior therapies=2 Paired Biopsies after ~6 weeks of TTI-101 Partial Response Stable Disease Progressive Disease Formulation F1 N=15 F2 N=47 F3 N=7** Grade, n (%) G1/2 G3 G1/2 G3 G1/2 G3 Diarrhoea 3 (20) 3 (20) 16 (34) 6 (13) 2 (29) 0 (0) Nausea 4 (26) 0 (0) 6 (13) 1 (2) 0 (0) 1 (14) Fatigue 6 (40) 0 (0) 4 (8) 0 (0) 0 (0) 0 (0) Elevated ALT/AST*** 1 (7) 1 (7) 1 (2) 4 (8) 1 (14) 1 (14) Dose reduction 3 (20) 2 (4) 0 (0) Dose discont. 0 (0) 2 (4) 0 (0) |
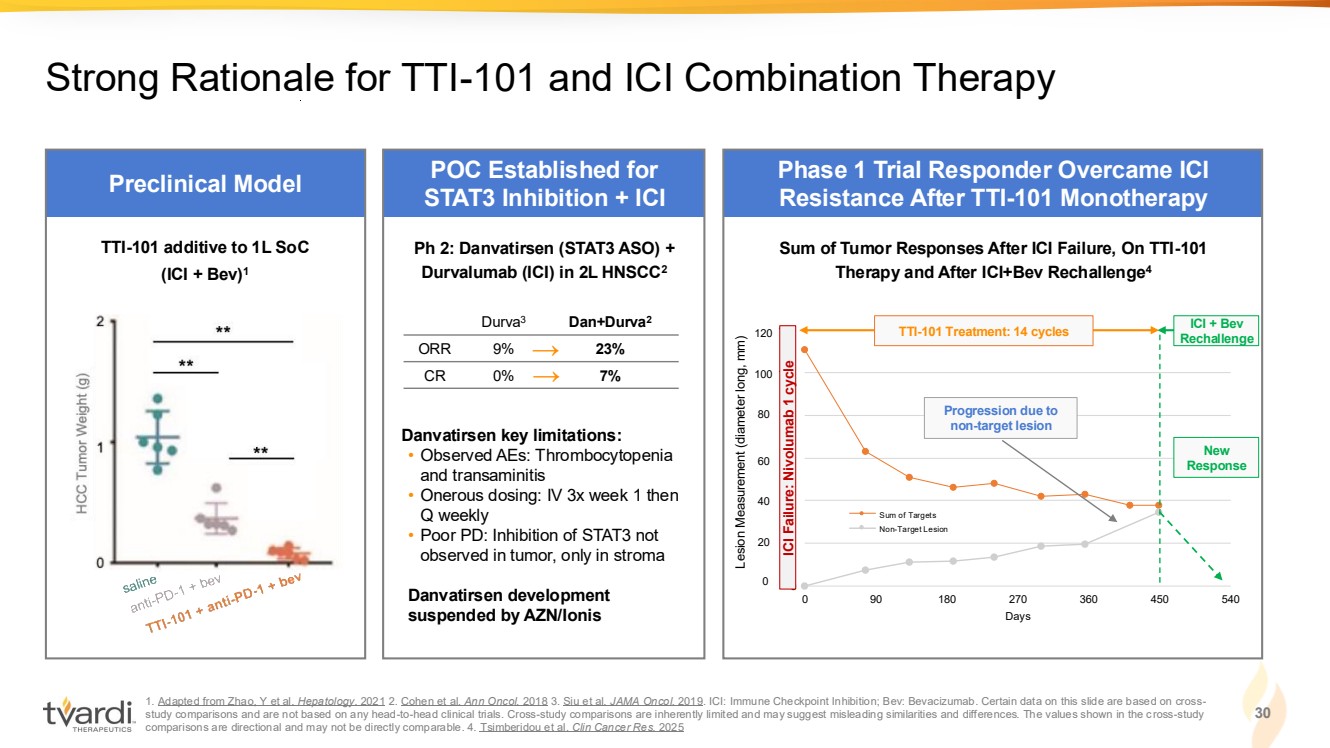
| 1. Adapted from Zhao, Y et al. Hepatology. 2021 2. Cohen et al. Ann Oncol. 2018 3. Siu et al. JAMA Oncol. 2019. ICI: Immune Checkpoint Inhibition; Bev: Bevacizumab. Certain data on this slide are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly comparable. 4. Tsimberidou et al. Clin Cancer Res. 2025 30 Strong Rationale for TTI-101 and ICI Combination Therapy 0 20 40 60 80 100 120 0 90 180 270 360 450 540 Days Sum of Targets Non-Target Lesion Preclinical Model POC Established for STAT3 Inhibition + ICI Phase 1 Trial Responder Overcame ICI Resistance After TTI-101 Monotherapy → → Ph 2: Danvatirsen (STAT3 ASO) + Durvalumab (ICI) in 2L HNSCC2 Danvatirsen key limitations: • Observed AEs: Thrombocytopenia and transaminitis • Onerous dosing: IV 3x week 1 then Q weekly • Poor PD: Inhibition of STAT3 not observed in tumor, only in stroma Danvatirsen development suspended by AZN/Ionis Durva3 Dan+Durva2 ORR 9% 23% CR 0% 7% TTI-101 additive to 1L SoC (ICI + Bev)1 Progression due to non-target lesion New Response ICI + Bev Rechallenge Sum of Tumor Responses After ICI Failure, On TTI-101 Therapy and After ICI+Bev Rechallenge4 0 20 40 60 80 100 120 ICI Failure: Nivolumab 1 cycle Lesion Measurement (diameter long, mm) TTI-101 Treatment: 14 cycles |
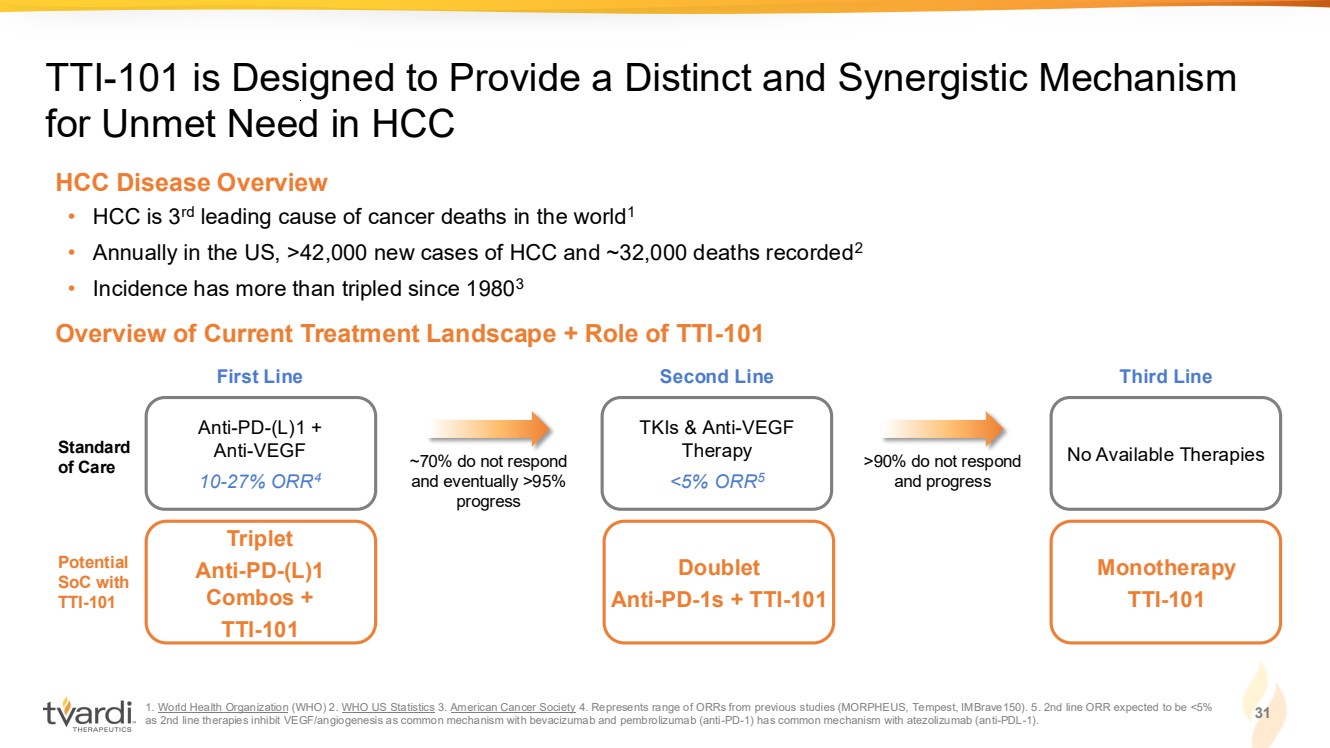
| 1. World Health Organization (WHO) 2. WHO US Statistics 3. American Cancer Society 4. Represents range of ORRs from previous studies (MORPHEUS, Tempest, IMBrave150). 5. 2nd line ORR expected to be <5% as 2nd line therapies inhibit VEGF/angiogenesis as common mechanism with bevacizumab and pembrolizumab (anti-PD-1) has common mechanism with atezolizumab (anti-PDL-1). 31 TTI-101 is Designed to Provide a Distinct and Synergistic Mechanism for Unmet Need in HCC Overview of Current Treatment Landscape + Role of TTI-101 • HCC is 3rd leading cause of cancer deaths in the world1 • Annually in the US, >42,000 new cases of HCC and ~32,000 deaths recorded2 • Incidence has more than tripled since 19803 HCC Disease Overview Triplet Anti-PD-(L)1 Combos + TTI-101 Potential SoC with TTI-101 Doublet Anti-PD-1s + TTI-101 Monotherapy TTI-101 Anti-PD-(L)1 + Anti-VEGF 10-27% ORR4 Standard of Care First Line TKIs & Anti-VEGF Therapy <5% ORR5 Second Line No Available Therapies Third Line ~70% do not respond and eventually >95% progress >90% do not respond and progress |
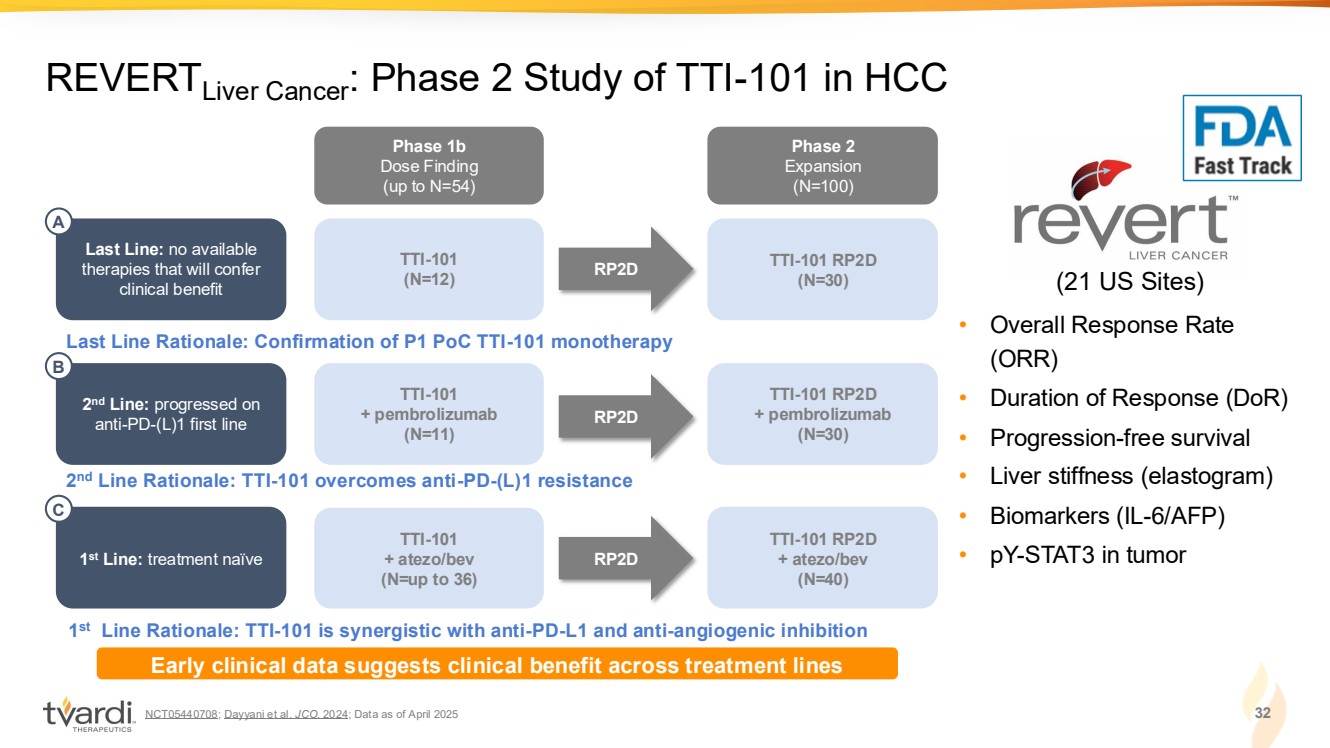
| NCT05440708; Dayyani et al. JCO. 2024; Data as of April 2025 32 REVERTLiver Cancer: Phase 2 Study of TTI-101 in HCC • Overall Response Rate (ORR) • Duration of Response (DoR) • Progression-free survival • Liver stiffness (elastogram) • Biomarkers (IL-6/AFP) • pY-STAT3 in tumor (21 US Sites) Phase 1b Dose Finding (up to N=54) Phase 2 Expansion (N=100) TTI-101 RP2D (N=30) TTI-101 (N=12) Last Line: no available therapies that will confer clinical benefit RP2D Last Line Rationale: Confirmation of P1 PoC TTI-101 monotherapy A TTI-101 RP2D + pembrolizumab (N=30) TTI-101 + pembrolizumab (N=11) RP2D 2 nd Line Rationale: TTI-101 overcomes anti-PD-(L)1 resistance 2 nd Line: progressed on anti-PD-(L)1 first line B TTI-101 RP2D + atezo/bev (N=40) TTI-101 + atezo/bev (N=up to 36) RP2D 1 st Line Rationale: TTI-101 is synergistic with anti-PD-L1 and anti-angiogenic inhibition 1 st Line: treatment naïve C Early clinical data suggests clinical benefit across treatment lines |
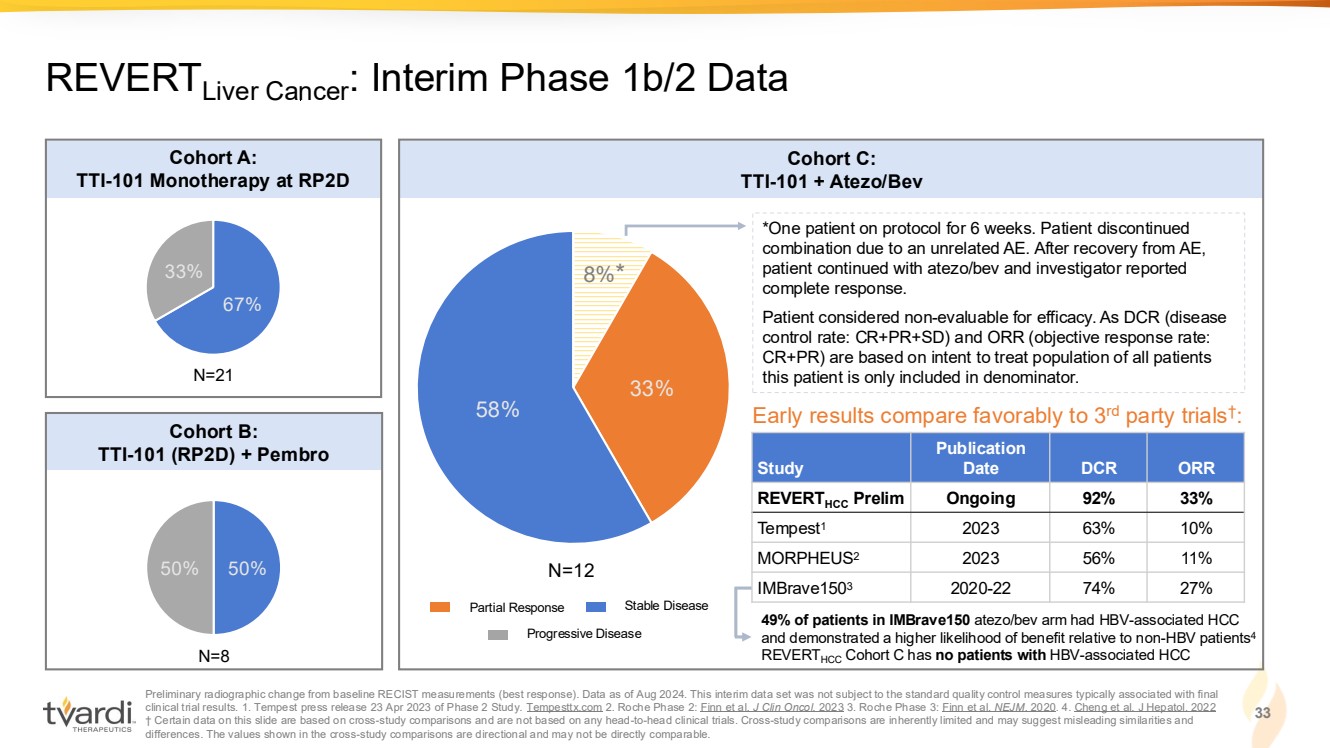
| Preliminary radiographic change from baseline RECIST measurements (best response). Data as of Aug 2024. This interim data set was not subject to the standard quality control measures typically associated with final clinical trial results. 1. Tempest press release 23 Apr 2023 of Phase 2 Study. Tempesttx.com 2. Roche Phase 2: Finn et al. J Clin Oncol. 2023 3. Roche Phase 3: Finn et al. NEJM. 2020. 4. Cheng et al. J Hepatol. 2022 † Certain data on this slide are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly comparable. 33 REVERTLiver Cancer: Interim Phase 1b/2 Data 67% 33% 50% 50% 8% 33% 58% Cohort A: TTI-101 Monotherapy at RP2D Cohort B: TTI-101 (RP2D) + Pembro Cohort C: TTI-101 + Atezo/Bev *One patient on protocol for 6 weeks. Patient discontinued combination due to an unrelated AE. After recovery from AE, patient continued with atezo/bev and investigator reported complete response. Patient considered non-evaluable for efficacy. As DCR (disease control rate: CR+PR+SD) and ORR (objective response rate: CR+PR) are based on intent to treat population of all patients N=21 this patient is only included in denominator. N=8 N=12 Study Publication Date DCR ORR REVERTHCC Prelim Ongoing 92% 33% Tempest1 2023 63% 10% MORPHEUS2 2023 56% 11% IMBrave1503 2020-22 74% 27% Early results compare favorably to 3rd party trials† : Partial Response Stable Disease Progressive Disease * 49% of patients in IMBrave150 atezo/bev arm had HBV-associated HCC and demonstrated a higher likelihood of benefit relative to non-HBV patients4 REVERTHCC Cohort C has no patients with HBV-associated HCC |
| 34 Key Takeaways: TTI-101 in HCC Inhibition of STAT3 activation to have dual therapeutic effect on cancer cells – overcoming tumorigenesis and immune suppression Clinically meaningful activity in both monotherapy and combination therapy in areas of unmet need Topline results from ongoing Phase 2 REVERTLIVER CANCER trial expected in 1H:2026 STAT3 long recognized as prime target in oncology; >95% of patients with HCC have activated STAT3 in their tumors STAT3: Well-Established Biology Differentiated Approach Encouraging Clinical Activity Near-Term Clinical Milestone |
| 1. Idiopathic pulmonary fibrosis 2. Hepatocellular carcinoma 3. Investigational new drug 4. Pharmacokinetics 35 Targeting STAT3: Central Mediator of Fibrosis-Driven Diseases Deep expertise in STAT3 biology Potential to serve as a disease-modifying therapy TTI-109: designed to enhance delivery of STAT3 inhibitor & improve tolerability Multiple near-term data catalysts expected • Unlocking highly-validated target within fibrosis-driven diseases • Demonstrated target engagement and disease modification across animal models of proliferative disease • Demonstrated enhanced biological activity in fibrotic cancers in P1 • Observed reductions in fibrosis and inflammation marker (IL-6) in IPF1 P2 • Evaluating mono- and combination therapy in fibrosis-driven HCC2 P2 • TTI-109 P1 healthy volunteer data in 1H:2026 • HCC1 P1b/2 topline data in 1H:2026 • Prodrug designed to rapidly convert to active TTI-101 in the blood - IND3 filed June 2025 • P1 trial in healthy volunteers ongoing to demonstrate PK4 , bioequivalence and tolerability |
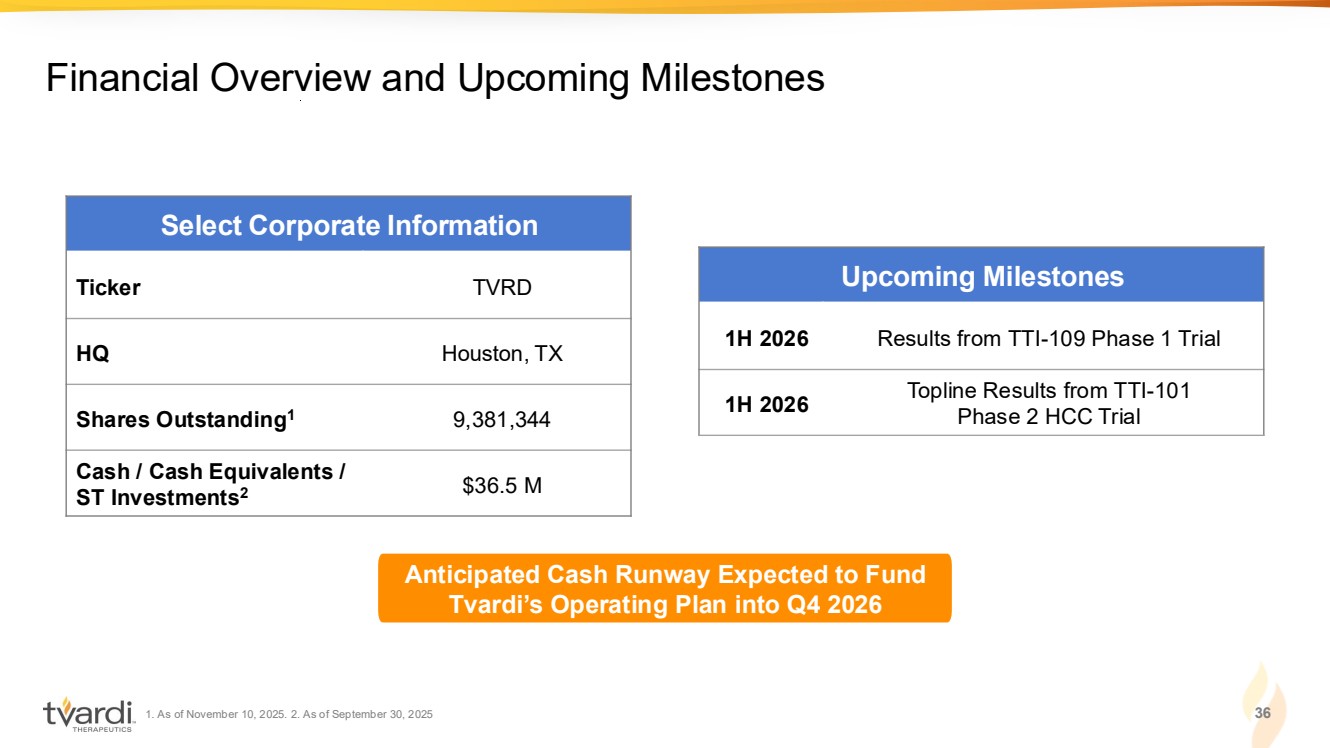
| 1. As of November 10, 2025. 2. As of September 30, 2025 36 Financial Overview and Upcoming Milestones Select Corporate Information Ticker TVRD HQ Houston, TX Shares Outstanding1 9,381,344 Cash / Cash Equivalents / ST Investments2 $36.5 M Upcoming Milestones 1H 2026 Results from TTI-109 Phase 1 Trial 1H 2026 Topline Results from TTI-101 Phase 2 HCC Trial Anticipated Cash Runway Expected to Fund Tvardi’s Operating Plan into Q4 2026 |