
Creating New Possibilities for Patients to Live Their Lives August 2022 © 2022 Concert Pharmaceuticals, Inc. All Rights Reserved. .2

Forward-Looking Statements Any statements in this presentation about our future expectations, plans and prospects, including, among others, statements about our expectations regarding the development of CTP-543, the potential for CTP-543 to be a best-in-class treatment for the treatment of alopecia areata, the planned timing for filing an NDA for CTP-543 and the sufficiency of our cash, cash equivalents and investments to fund our operations, and any other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties inherent in the initiation, timing and design of future clinical trials, the availability and timing of data from ongoing and future clinical trials and the results of such trials, whether preliminary results, including safety profiles, from a clinical trial will be predictive of the final results of that trial or whether results of early clinical trials will be indicative of the results of later clinical trials, expectations for the timing of the submission of an NDA, the availability of regulatory approvals, the availability of funding sufficient for our foreseeable and unforeseeable operating expenses and capital expenditure requirements, expectations with respect to the protection of our intellectual property afforded by our patents and other factors discussed in the “Risk Factors” section of our most recent Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission and in other filings that we make with the Securities and Exchange Commission. In addition, any forward-looking statements included in this presentation represent our views only as of the date of this presentation and should not be relied upon as representing our views as of any subsequent date. We specifically disclaim any obligation to update any forward-looking statements included in this presentation. 2
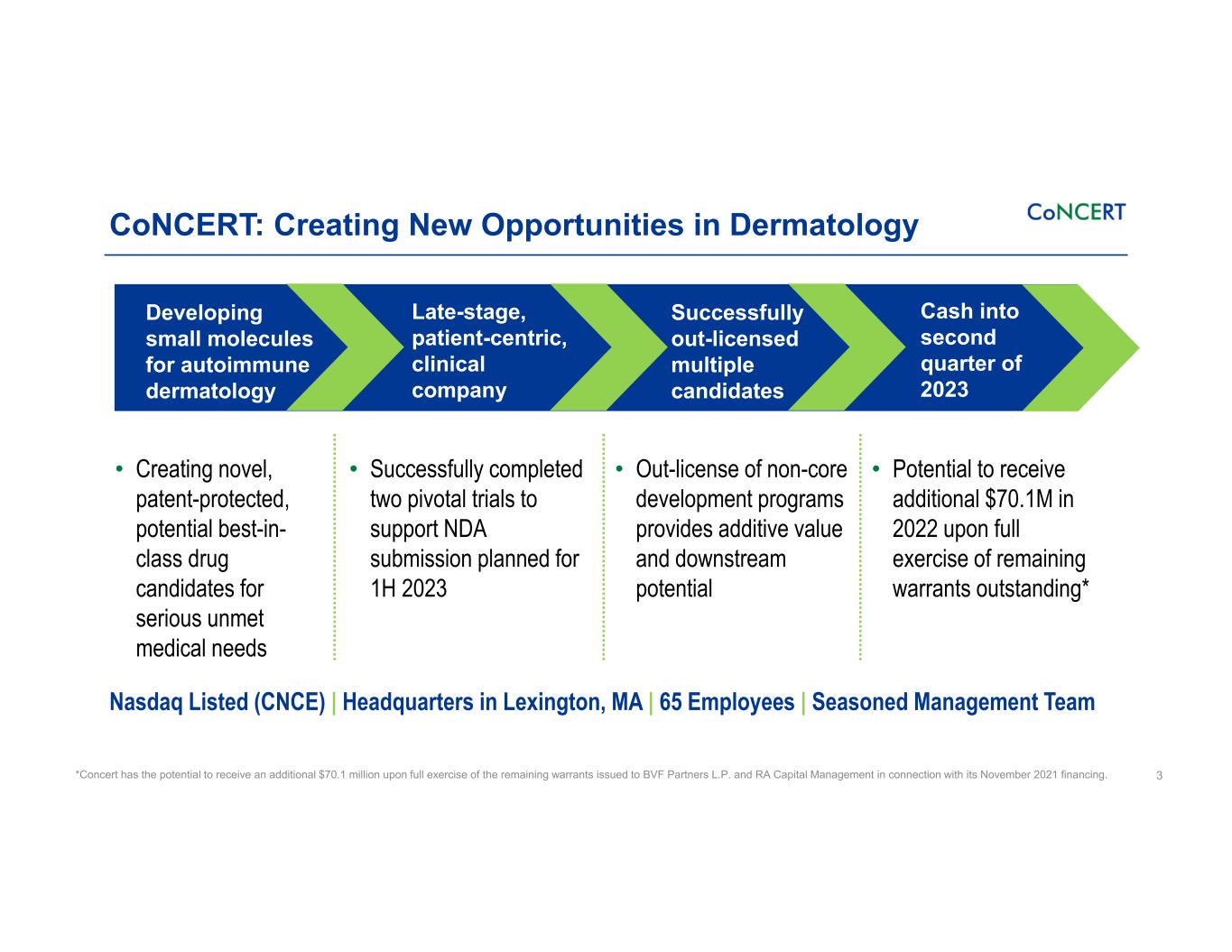
CoNCERT: Creating New Opportunities in Dermatology 3 Developing small molecules for autoimmune dermatology Late-stage, patient-centric, clinical company Successfully out-licensed multiple candidates Cash into second quarter of 2023 • Successfully completed two pivotal trials to support NDA submission planned for 1H 2023 • Creating novel, patent-protected, potential best-in- class drug candidates for serious unmet medical needs • Potential to receive additional $70.1M in 2022 upon full exercise of remaining warrants outstanding* *Concert has the potential to receive an additional $70.1 million upon full exercise of the remaining warrants issued to BVF Partners L.P. and RA Capital Management in connection with its November 2021 financing. • Out-license of non-core development programs provides additive value and downstream potential Nasdaq Listed (CNCE) | Headquarters in Lexington, MA | 65 Employees | Seasoned Management Team
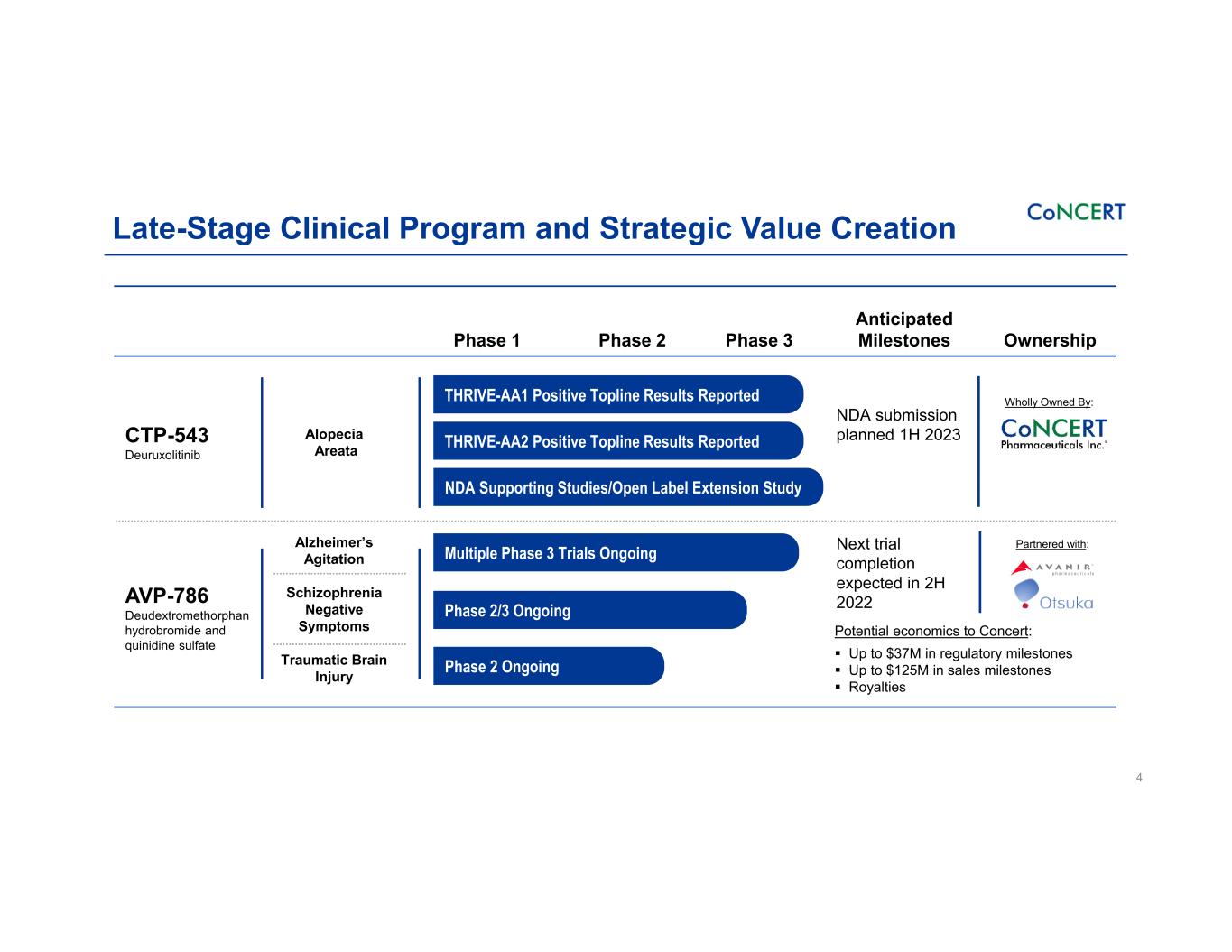
Late-Stage Clinical Program and Strategic Value Creation 4 Phase 1 Phase 2 Phase 3 Anticipated Milestones Ownership CTP-543 Deuruxolitinib Alopecia Areata NDA submission planned 1H 2023 AVP-786 Deudextromethorphan hydrobromide and quinidine sulfate Alzheimer’s Agitation Schizophrenia Negative Symptoms Traumatic Brain Injury Next trial completion expected in 2H 2022 THRIVE-AA1 Positive Topline Results Reported THRIVE-AA2 Positive Topline Results Reported NDA Supporting Studies/Open Label Extension Study Multiple Phase 3 Trials Ongoing Phase 2/3 Ongoing Phase 2 Ongoing Potential economics to Concert: Up to $37M in regulatory milestones Up to $125M in sales milestones Royalties Wholly Owned By: Partnered with:

CTP-543: Compelling Opportunity for Alopecia Areata 5 • Serious autoimmune disorder with significant unmet patient need • CTP-543 has Breakthrough Therapy designation Large Market Opportunity • Plan to file NDA in 1H 2023 • Pre-commercial initiatives underway Preparing for Commercialization • Orange Book-eligible patent protection into 2037 • Patent portfolio expansion ongoing Strong Patent Protection • Phase 3 program: robust hair regrowth, met primary and key secondary endpoints • 3+ year extension study supports favorable safety/tolerability profile Potential Best-in-Class

Alopecia Areata: A Devasting Autoimmune Disease 6 Limited Treatment Options Available • Strong patient advocacy • FDA PFDDI meeting held September 2017 • CTP-543 potentially best- in-class ‒ Breakthrough Therapy designation granted by FDA Common Disorder 1 Est. 700,000-1,600,000 patients: Benigno M. Clinical, Cosmetic and Investigational Dermatology 2020 2 Mesinkovska N. Journal of Investigative Dermatology Symposium Proceedings 2020 Significant Burden of Disease • Disease profoundly impacts patients • Patients suffer increased burdens, including significant psychosocial impact2 ‒ Major impact on self esteem and self confidence • Associated with anxiety, depression and other autoimmune conditions • Up to approximately 1.5 million patients affected with alopecia areata in the U.S. at any given time1 • Estimated 40+% of patients reported to have ≥ 50% loss of scalp hair1 • Chronic condition affecting women, men and children of all ages
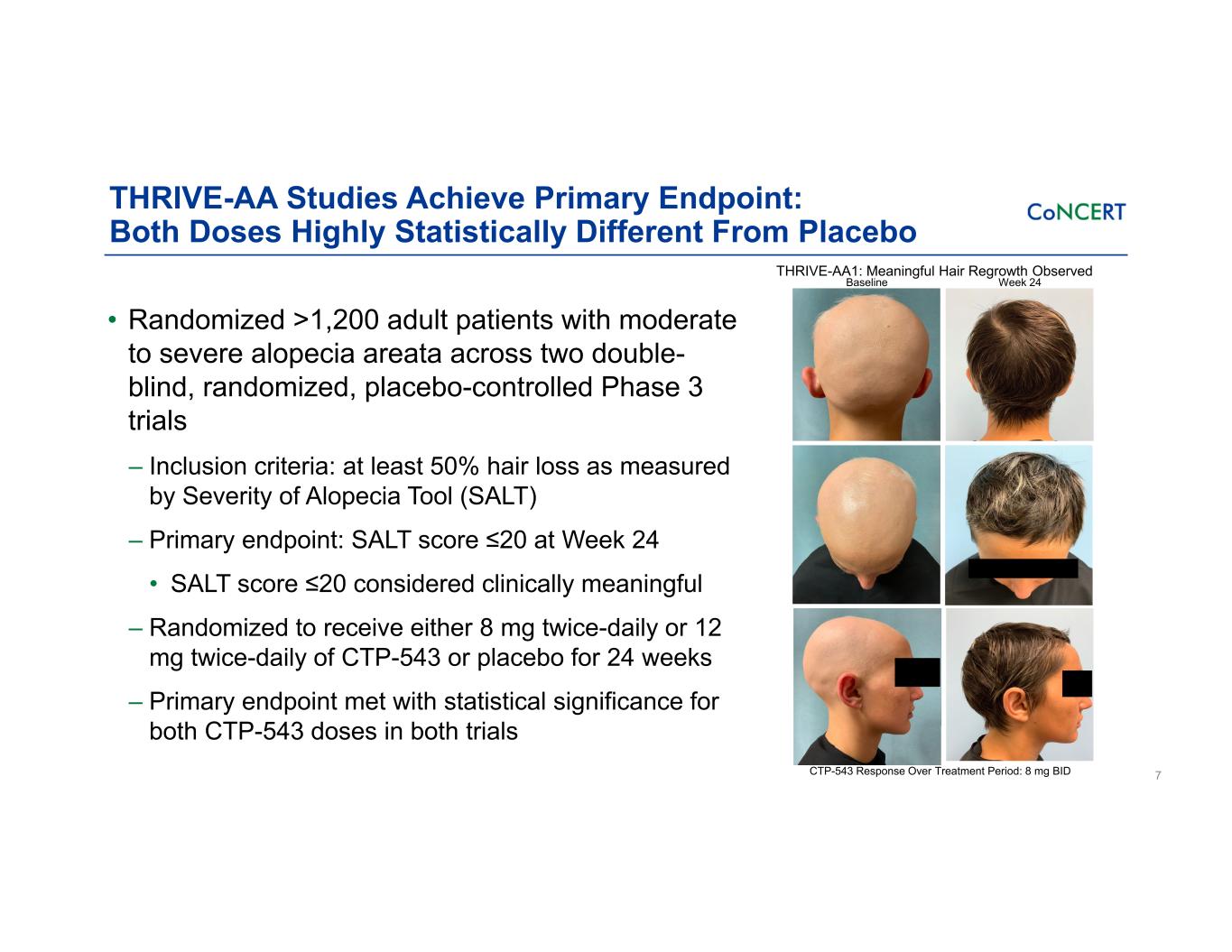
THRIVE-AA Studies Achieve Primary Endpoint: Both Doses Highly Statistically Different From Placebo • Randomized >1,200 adult patients with moderate to severe alopecia areata across two double- blind, randomized, placebo-controlled Phase 3 trials ‒ Inclusion criteria: at least 50% hair loss as measured by Severity of Alopecia Tool (SALT) ‒ Primary endpoint: SALT score ≤20 at Week 24 • SALT score ≤20 considered clinically meaningful ‒ Randomized to receive either 8 mg twice-daily or 12 mg twice-daily of CTP-543 or placebo for 24 weeks ‒ Primary endpoint met with statistical significance for both CTP-543 doses in both trials 7CTP-543 Response Over Treatment Period: 8 mg BID THRIVE-AA1: Meaningful Hair Regrowth Observed Baseline Week 24
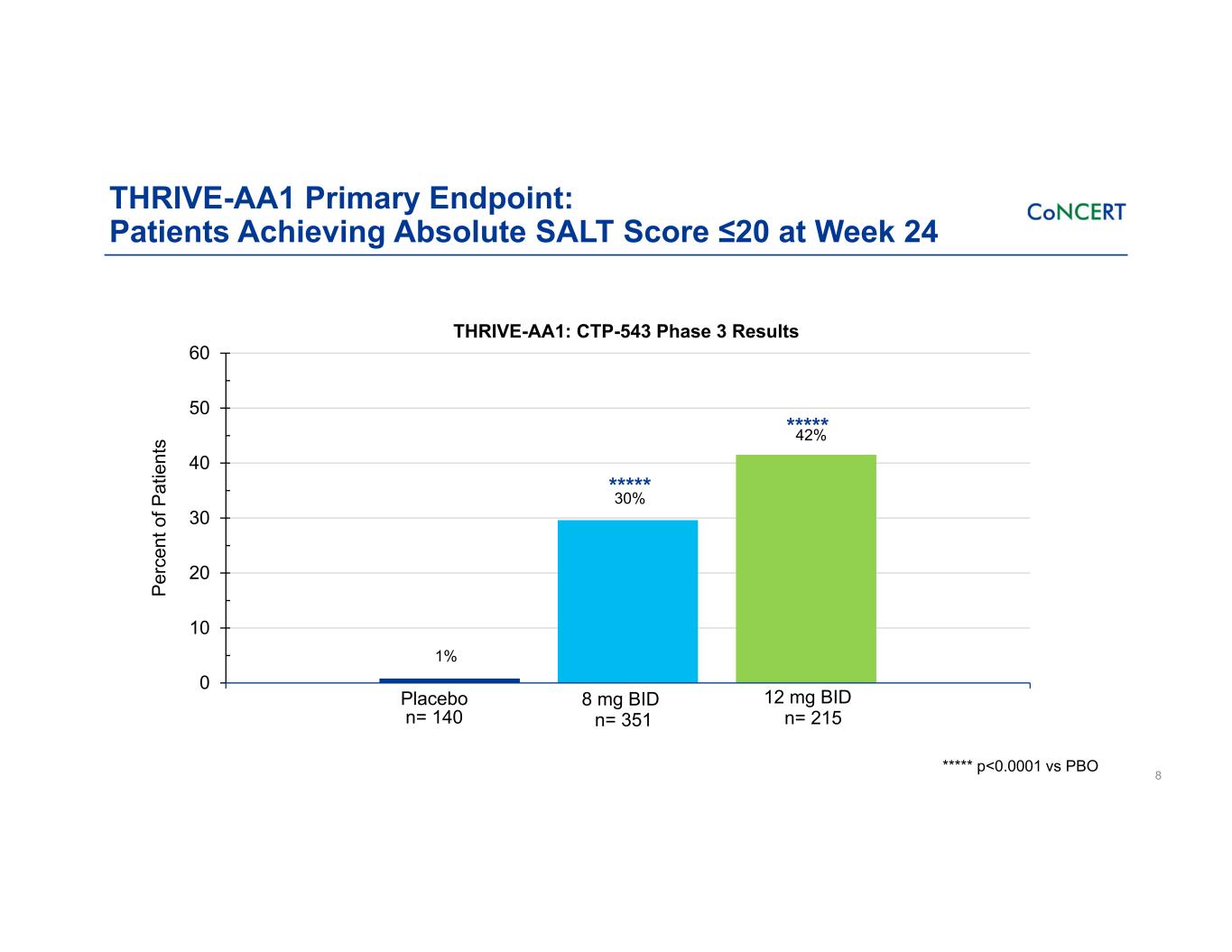
THRIVE-AA1 Primary Endpoint: Patients Achieving Absolute SALT Score ≤20 at Week 24 8 0 10 20 30 40 50 60 8 mg BID 12 mg BID 42% 1% Placebo ***** 30%***** ***** p<0.0001 vs PBO THRIVE-AA1: CTP-543 Phase 3 Results n= 140 n= 351 n= 215 Pe rc en t o f P at ie nt s
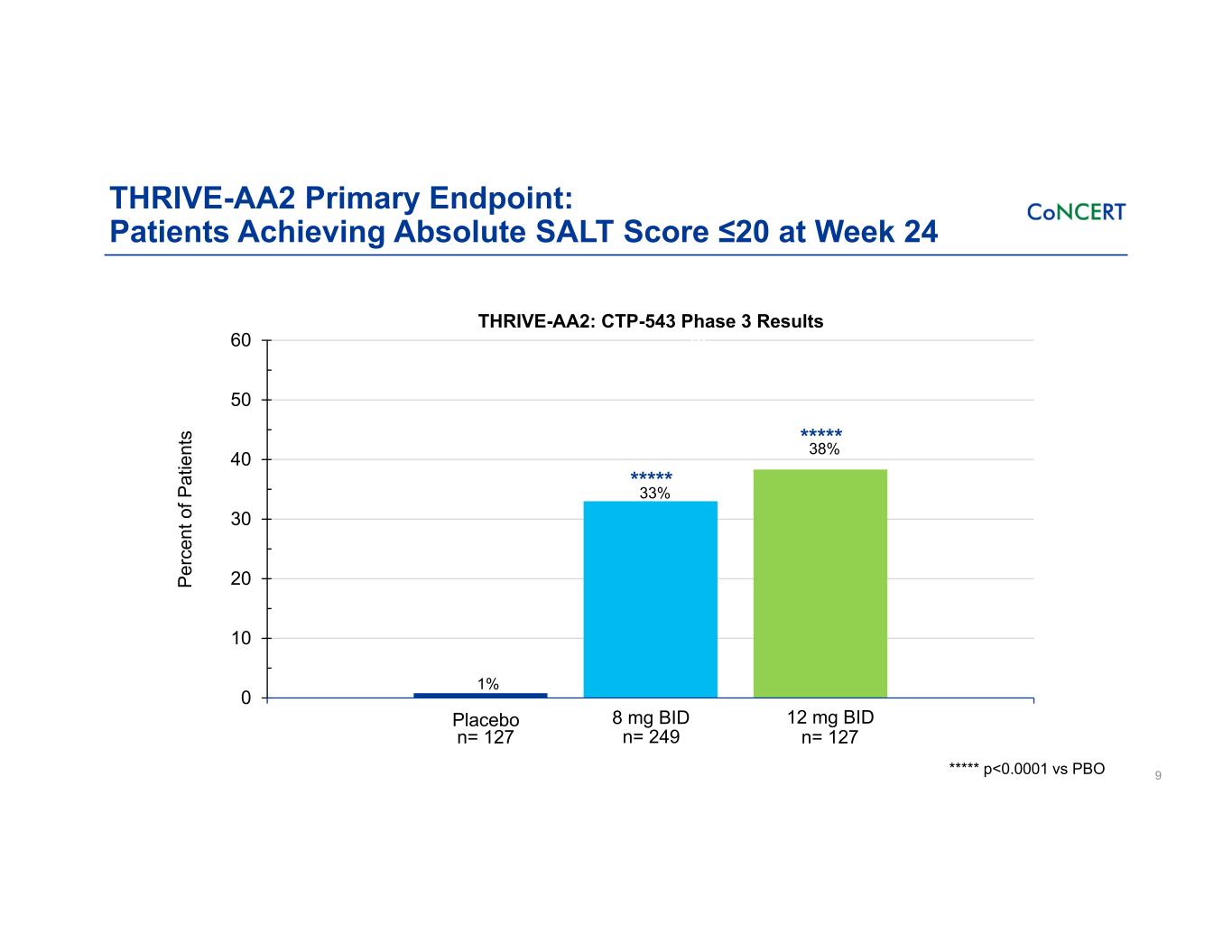
0 10 20 30 40 50 60 8 mg BID 12 mg BID 38% 1% Placebo 33% 60 % THRIVE-AA2 Primary Endpoint: Patients Achieving Absolute SALT Score ≤20 at Week 24 9***** p<0.0001 vs PBO THRIVE-AA2: CTP-543 Phase 3 Results ***** ***** n= 127 n= 249 n= 127 Pe rc en t o f P at ie nt s
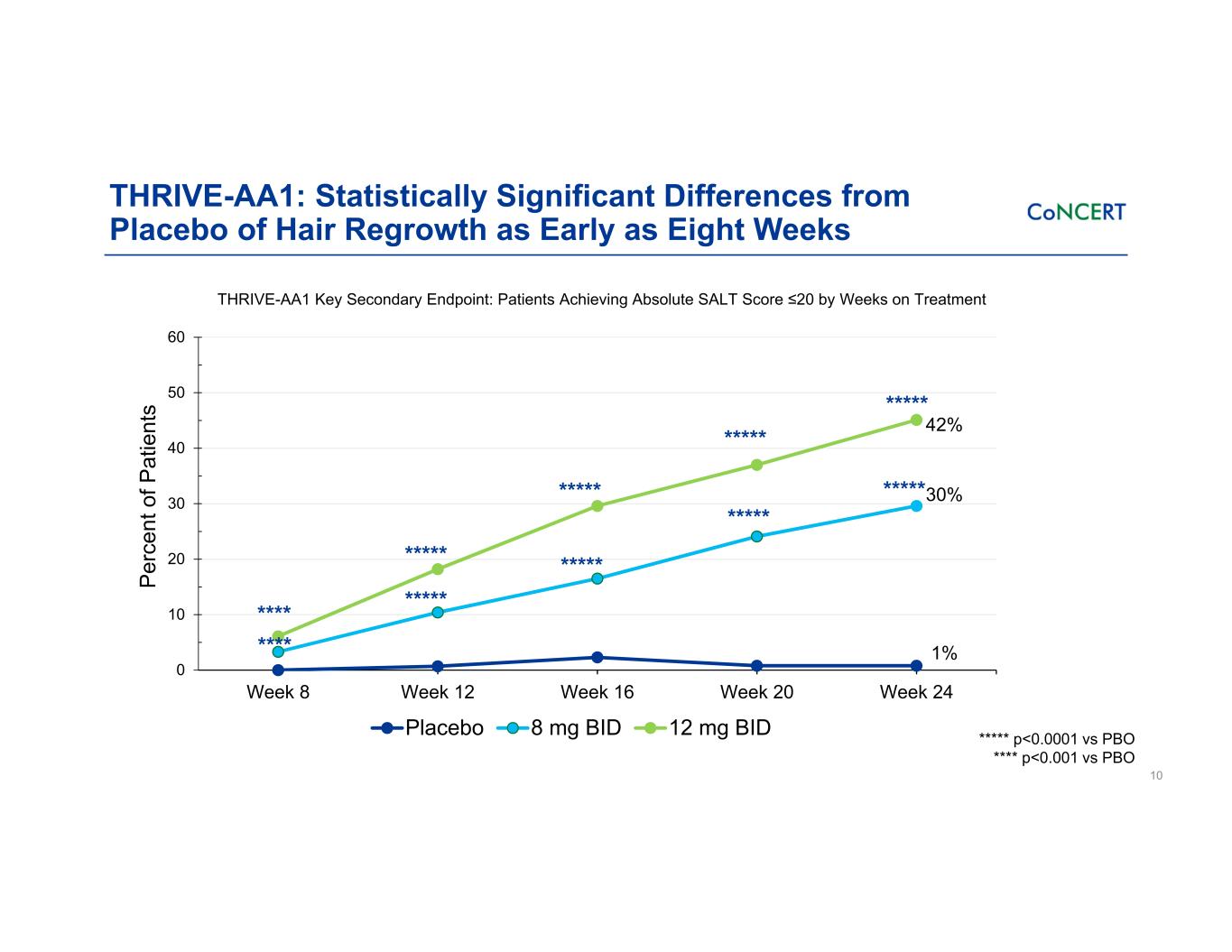
THRIVE-AA1: Statistically Significant Differences from Placebo of Hair Regrowth as Early as Eight Weeks 10 THRIVE-AA1 Key Secondary Endpoint: Patients Achieving Absolute SALT Score ≤20 by Weeks on Treatment 0 10 20 30 40 50 60 Week 8 Week 12 Week 16 Week 20 Week 24 Pe rc en t o f P at ie nt s Placebo 8 mg BID 12 mg BID ***** **** ***** ***** **** ***** ***** ***** 42% 30% 1% ********** ***** p<0.0001 vs PBO **** p<0.001 vs PBO
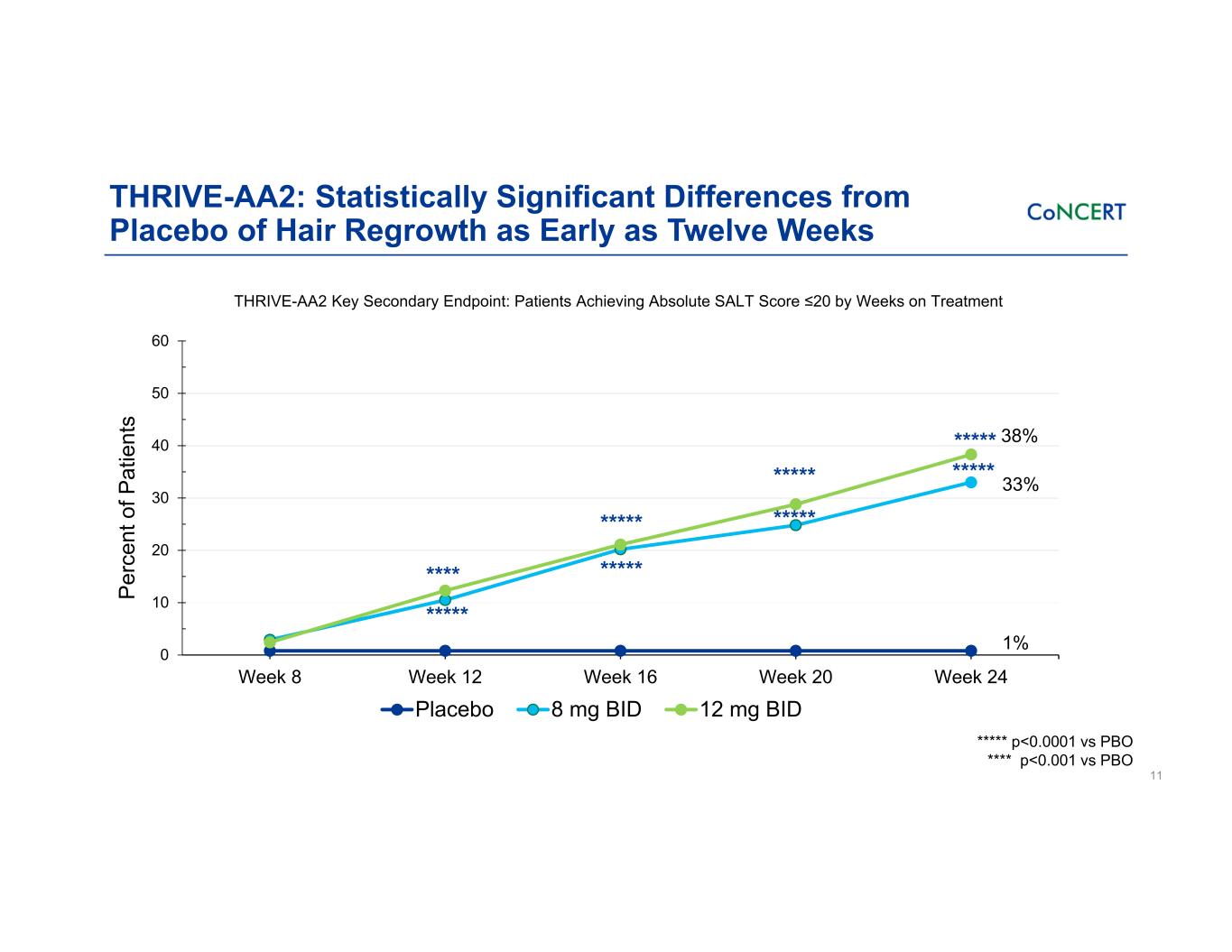
0 10 20 30 40 50 60 Week 8 Week 12 Week 16 Week 20 Week 24 Pe rc en t o f P at ie nt s Placebo 8 mg BID 12 mg BID ***** THRIVE-AA2: Statistically Significant Differences from Placebo of Hair Regrowth as Early as Twelve Weeks 11 THRIVE-AA2 Key Secondary Endpoint: Patients Achieving Absolute SALT Score ≤20 by Weeks on Treatment ***** ***** ***** ***** ***** 38% 33% 1% ***** **** ***** p<0.0001 vs PBO **** p<0.001 vs PBO

THRIVE-AA1: Statistically Significant Differences in Relative Change in SALT from Baseline as Early as 4 Weeks 12 0 10 20 30 40 50 60 Week 4 Week 8 Week 12 Week 16 Week 20 Week 24 M ea n % R el at iv e C ha ng e in S AL T Sc or e Placebo 8 mg BID 12 mg BID ***** ***** 2% 41% 50% ***** p<0.0001 vs PBO *** p<0.01 vs PBO *** ***** ***** ***** ********** ***** ***** ***** THRIVE-AA1 Secondary Endpoint: Relative Change in SALT Scores from Baseline by Weeks on Treatment This graph represents a decrease (improvement) in SALT score relative to baseline.
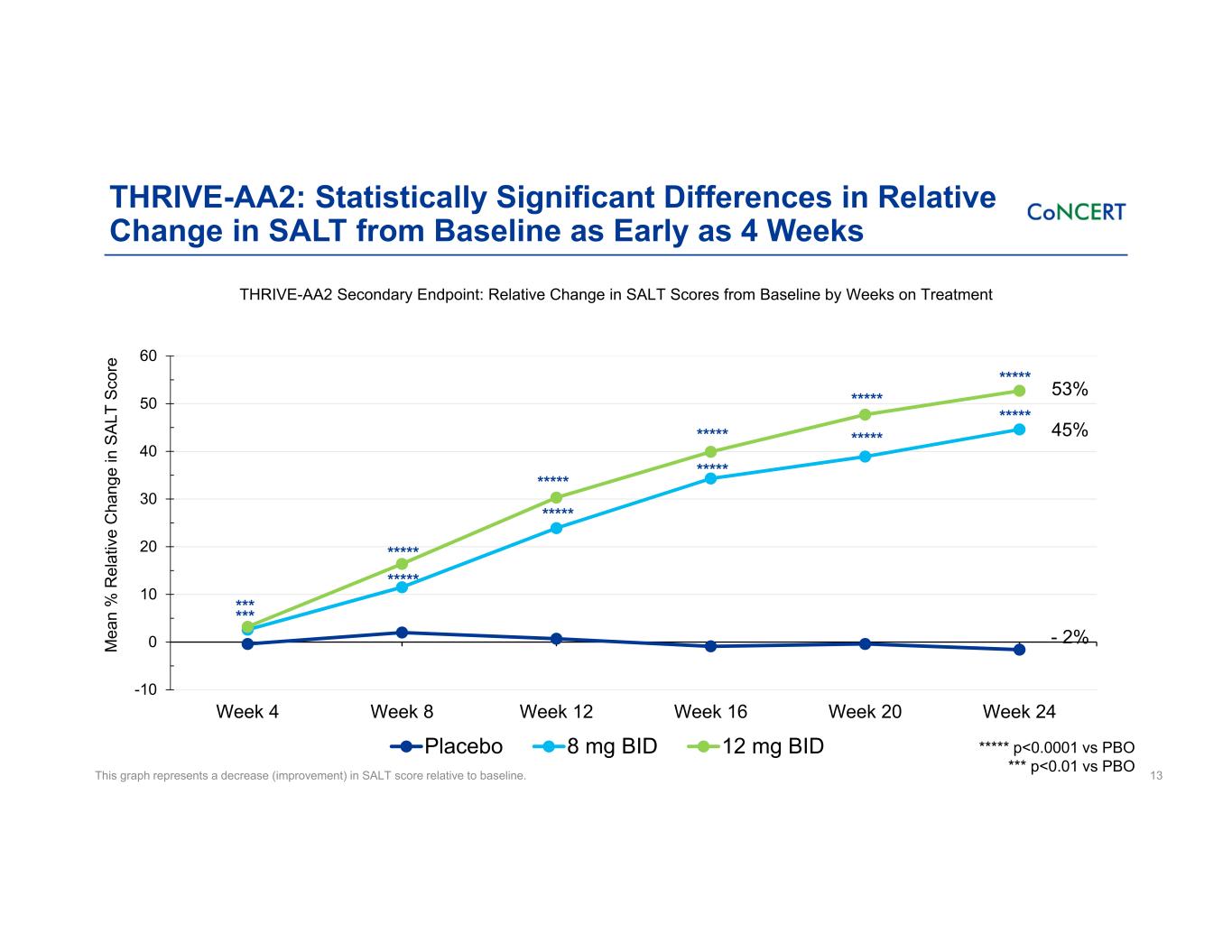
THRIVE-AA2: Statistically Significant Differences in Relative Change in SALT from Baseline as Early as 4 Weeks 13 -10 0 10 20 30 40 50 60 Week 4 Week 8 Week 12 Week 16 Week 20 Week 24 M ea n % R el at iv e C ha ng e in S AL T Sc or e Placebo 8 mg BID 12 mg BID ***** ***** - 2% ***** p<0.0001 vs PBO *** p<0.01 vs PBO 45% 53% *** ***** ********** ***** ***** ***** ***** ***** *** THRIVE-AA2 Secondary Endpoint: Relative Change in SALT Scores from Baseline by Weeks on Treatment This graph represents a decrease (improvement) in SALT score relative to baseline.
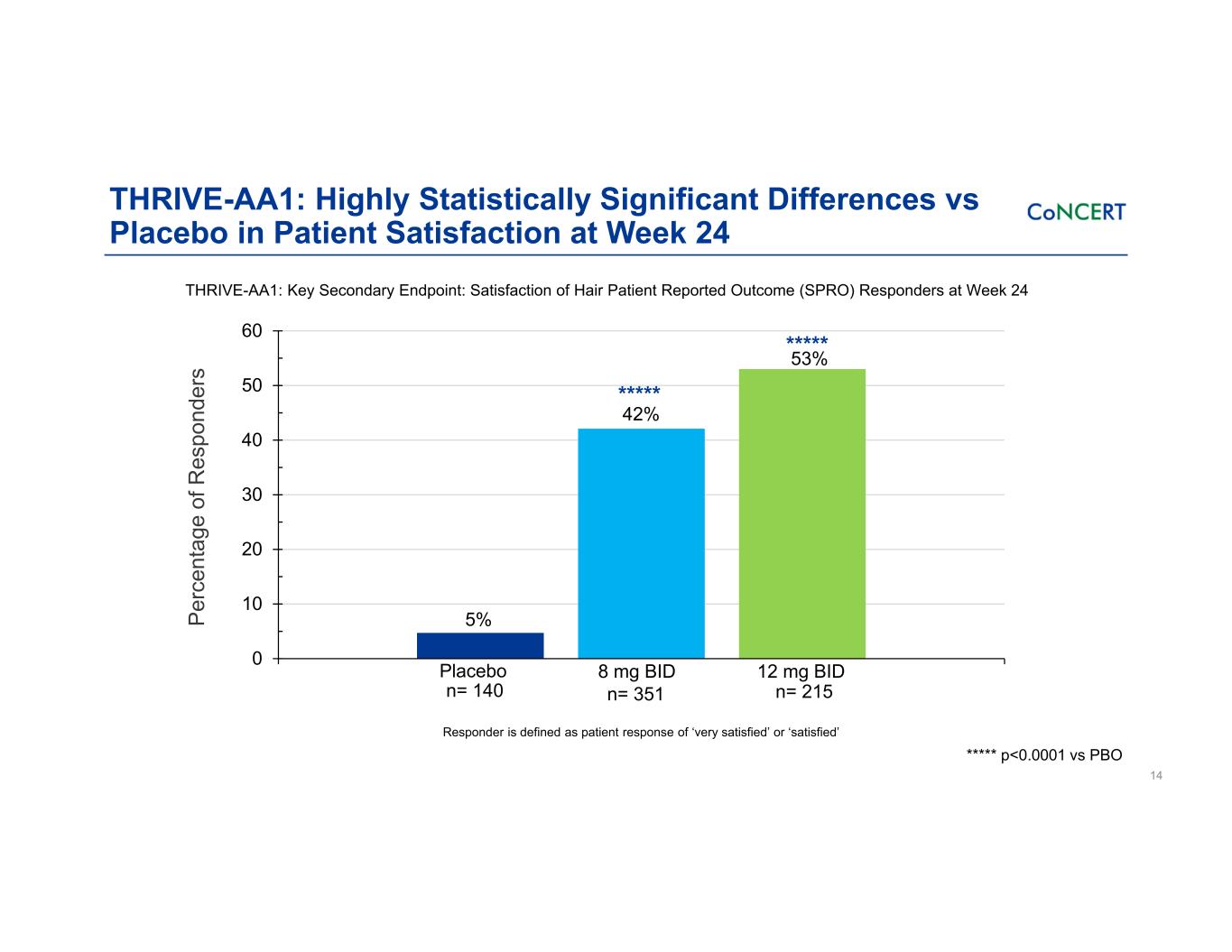
THRIVE-AA1: Highly Statistically Significant Differences vs Placebo in Patient Satisfaction at Week 24 14 THRIVE-AA1: Key Secondary Endpoint: Satisfaction of Hair Patient Reported Outcome (SPRO) Responders at Week 24 ***** p<0.0001 vs PBO 0 10 20 30 40 50 60 8 mg BID 12 mg BIDPlacebo Pe rc en ta ge o f R es po nd er s 53% 42% 5% ***** ***** Responder is defined as patient response of ‘very satisfied’ or ‘satisfied’ n= 140 n= 351 n= 215
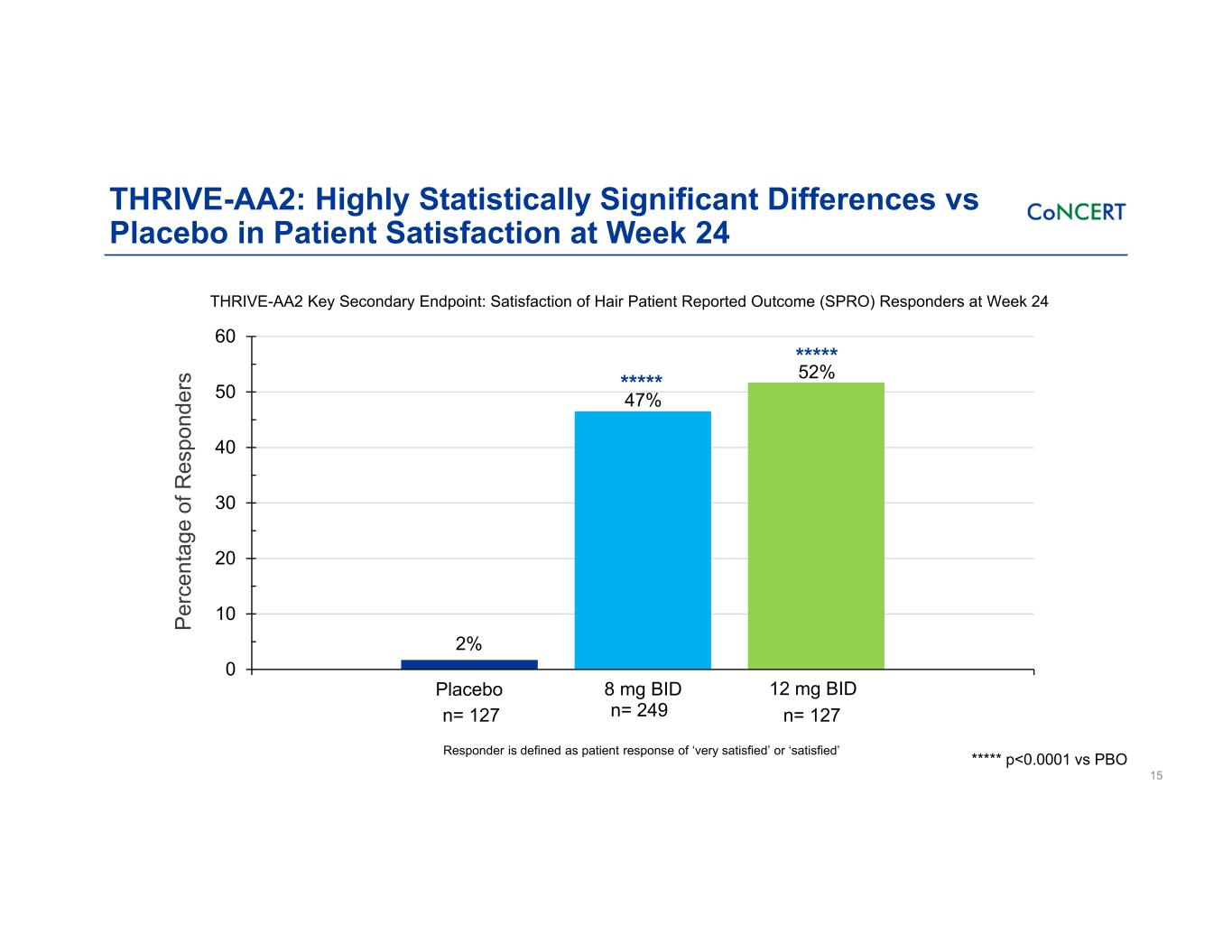
THRIVE-AA2: Highly Statistically Significant Differences vs Placebo in Patient Satisfaction at Week 24 15 THRIVE-AA2 Key Secondary Endpoint: Satisfaction of Hair Patient Reported Outcome (SPRO) Responders at Week 24 Responder is defined as patient response of ‘very satisfied’ or ‘satisfied’ 0 10 20 30 40 50 60 8 mg BID 12 mg BIDPlacebo 60% 2% 47% 52% n= 127 n= 249 n= 127 ***** ***** ***** p<0.0001 vs PBO Pe rc en ta ge o f R es po nd er s
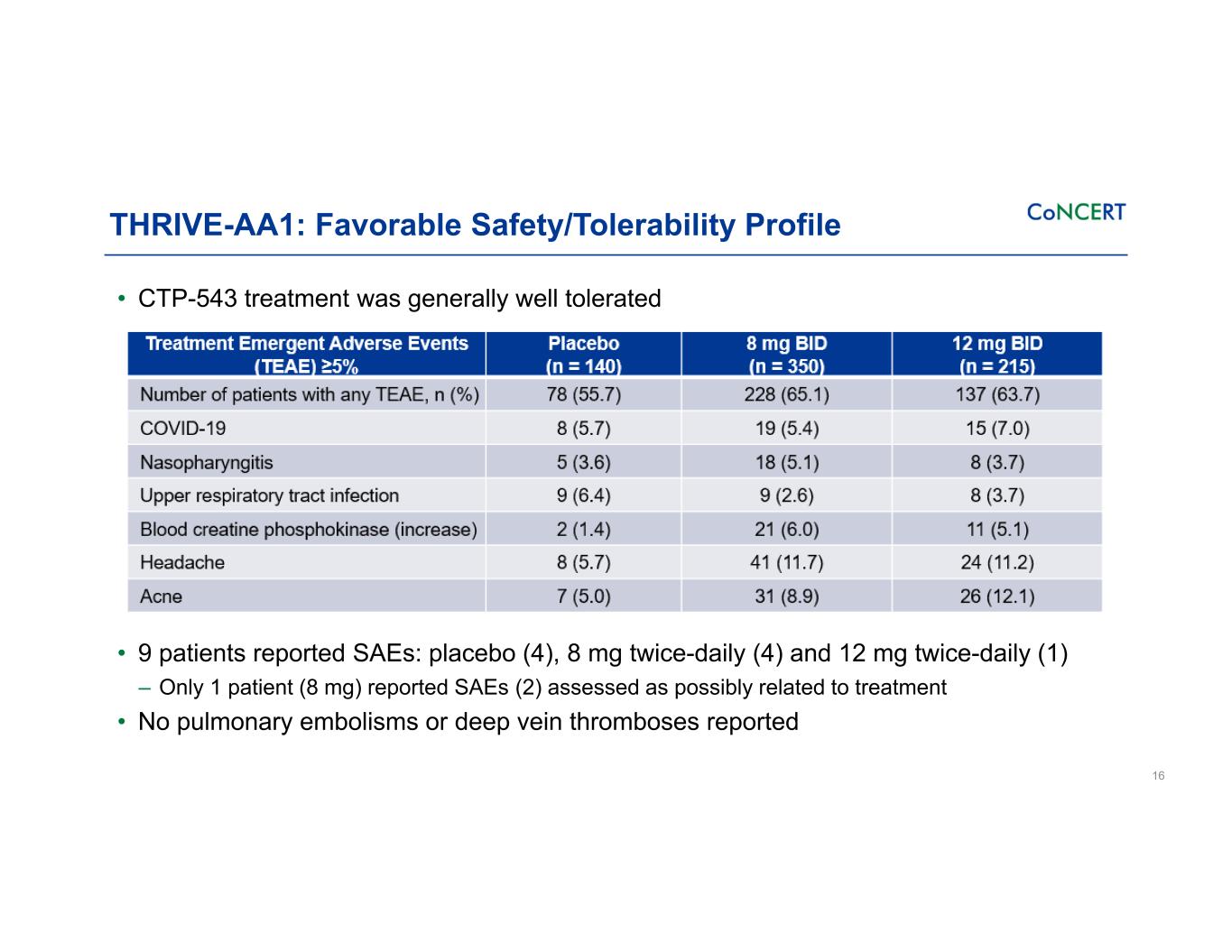
THRIVE-AA1: Favorable Safety/Tolerability Profile • CTP-543 treatment was generally well tolerated • 9 patients reported SAEs: placebo (4), 8 mg twice-daily (4) and 12 mg twice-daily (1) ‒ Only 1 patient (8 mg) reported SAEs (2) assessed as possibly related to treatment • No pulmonary embolisms or deep vein thromboses reported 16
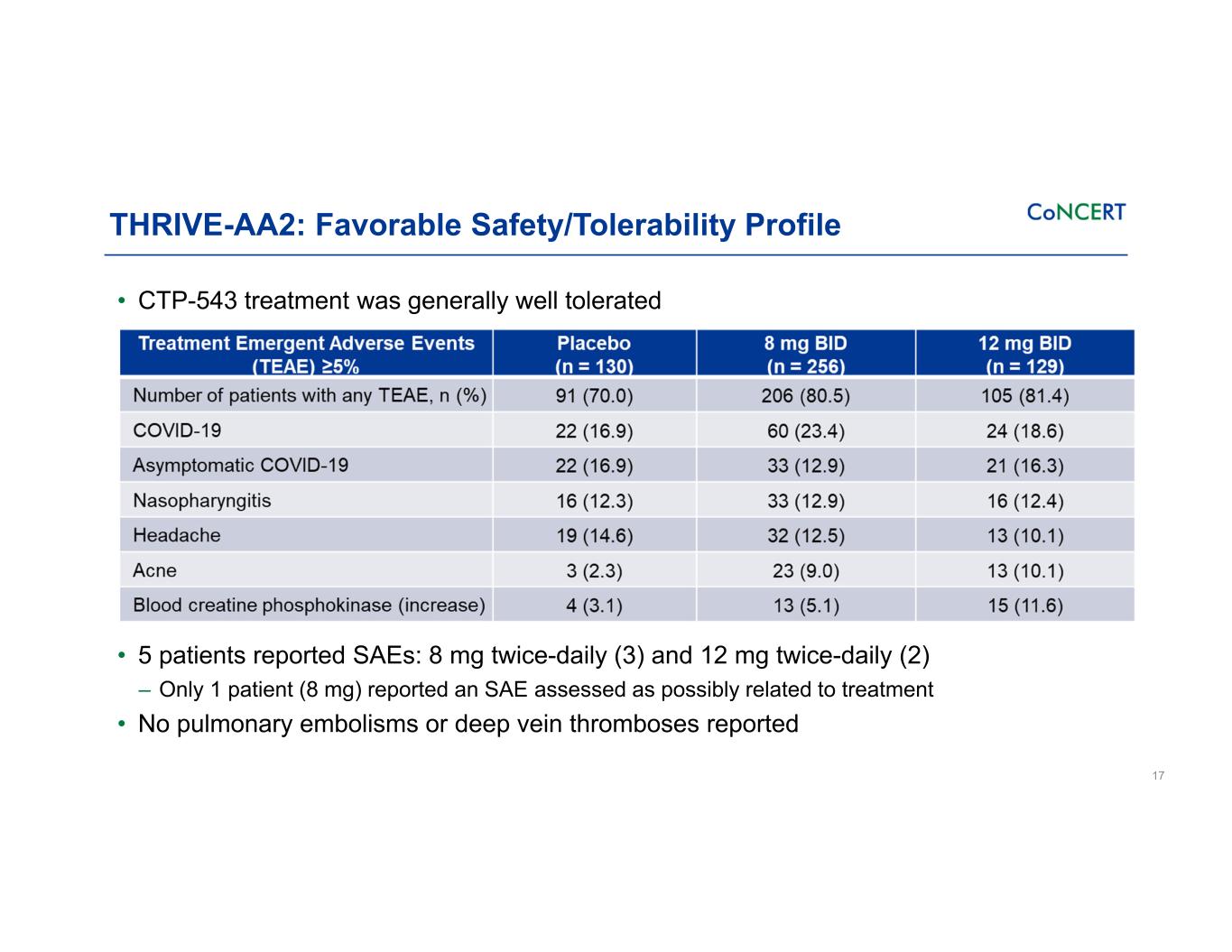
THRIVE-AA2: Favorable Safety/Tolerability Profile • CTP-543 treatment was generally well tolerated • 5 patients reported SAEs: 8 mg twice-daily (3) and 12 mg twice-daily (2) ‒ Only 1 patient (8 mg) reported an SAE assessed as possibly related to treatment • No pulmonary embolisms or deep vein thromboses reported 17
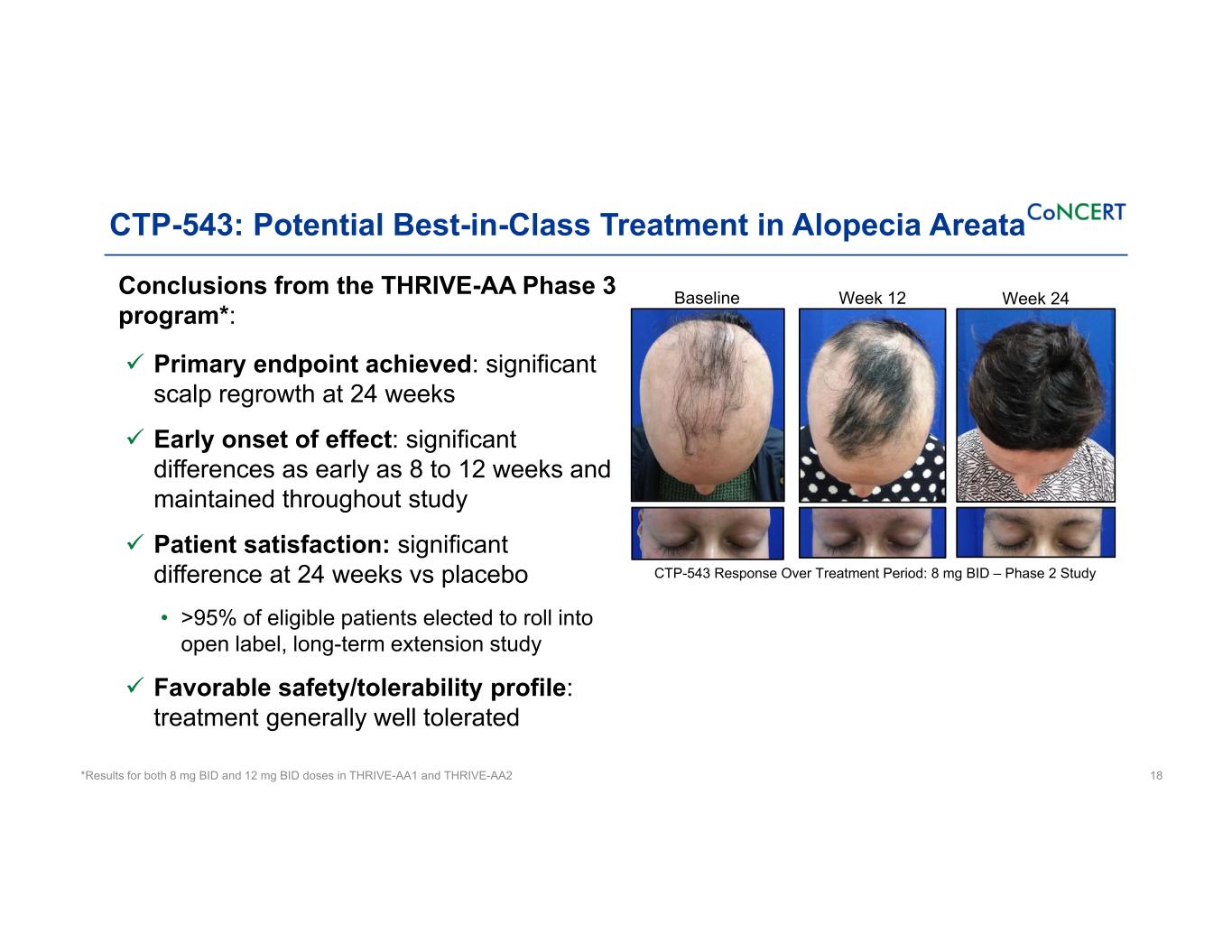
CTP-543: Potential Best-in-Class Treatment in Alopecia Areata Conclusions from the THRIVE-AA Phase 3 program*: Primary endpoint achieved: significant scalp regrowth at 24 weeks Early onset of effect: significant differences as early as 8 to 12 weeks and maintained throughout study Patient satisfaction: significant difference at 24 weeks vs placebo • >95% of eligible patients elected to roll into open label, long-term extension study Favorable safety/tolerability profile: treatment generally well tolerated 18*Results for both 8 mg BID and 12 mg BID doses in THRIVE-AA1 and THRIVE-AA2 Baseline Week 12 Week 24 CTP-543 Response Over Treatment Period: 8 mg BID – Phase 2 Study
CTP-543 for Alopecia Areata: Pre-Commercial Planning • CTP-543 has highly competitive profile based on Phase 3 results • Alopecia areata represents potential blockbuster opportunity • High unmet need with strong patient advocacy and demand • Multiple pre-commercial initiatives underway ‒ Payor evidence and reimbursement strategy ‒ KOL segmentation and targeting ‒ Medical communication planning ‒ Patient journey 19 Large, chronic disease now recognized as autoimmune disorder A ready segment of motivated patients with high burden of disease Clear market opportunity with significant growth potential

CTP-543: Compelling Opportunity for Alopecia Areata 20 • Serious autoimmune disorder with significant unmet patient need • CTP-543 has Breakthrough Therapy designation Large Market Opportunity • Plan to file NDA in 1H 2023 • Pre-commercial initiatives underway Preparing for Commercialization • Orange Book-eligible patent protection into 2037 • Patent portfolio expansion ongoing Strong Patent Protection • Phase 3 program: robust hair regrowth, met primary and key secondary endpoints • 3+ year extension study supports favorable safety/tolerability profile Potential Best-in-Class
Financial Position Clinical Milestones Regulatory Milestones 21 Financial Position and Milestones Successful THRIVE-AA1 Phase 3 trial topline results reported Q2 2022 Successful THRIVE-AA2 Phase 3 trial topline results reported Q3 2022 • Cash, cash equivalents and investments: $153.7M as of June 30, 2022 • Potential to receive additional $70.1M in 2022 upon full exercise of remaining warrants outstanding* • Plan to file NDA in 1H 2023 *In June 2022, Concert closed an equity offering raising gross proceeds of $54.6 million before underwriting discounts and offering expenses. Concurrent with the initial closing of the offering, Concert received $18.9 million from the partial exercise of warrants issued to BVF Partners L.P. and RA Capital Management in connection with its November 2021 financing. Concert has the potential to receive an additional $70.1 million upon full exercise of the remaining warrants issued to BVF Partners L.P. and RA Capital Management in connection with its November 2021 financing.

© 2022 Concert Pharmaceuticals, Inc. All Rights Reserved. NASDAQ: CNCE www.concertpharma.com @ConcertPharma For additional information contact: Justine Koenigsberg ir@concertpharma.com