

pro January 2026 Larimar Therapeutics Corporate Deck

Forward-Looking Statements This presentation contains forward-looking statements that are based on Larimar’s management’s beliefs and assumptions and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements, including but not limited to statements regarding Larimar’s ability to develop and commercialize nomlabofusp and any other planned product candidates, Larimar’s planned research and development efforts, including the timing of its nomlabofusp clinical trials, interactions and filings with the FDA, expectations regarding the timing of the BLA submission, the expectations of the timing of, and potential for, accelerated approval or accelerated access, time to launch and market and overall development plans and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations. In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including nomlabofusp clinical milestones and continued interactions with the FDA, and Larimar’s ability to timely implement the revised dosing regimen in its clinical program for nomlabofusp; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of nomlabofusp may not be predictive of the results or success of later non-clinical or clinical trials, and assessments; delays in patient recruitment, including as a result of changes in clinical protocols and adverse events; that the FDA may not ultimately agree with Larimar’s nomlabofusp development strategy; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale nomlabofusp’s manufacturing process; Larimar’s ability to obtain regulatory approvals for nomlabofusp and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this presentation represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law.
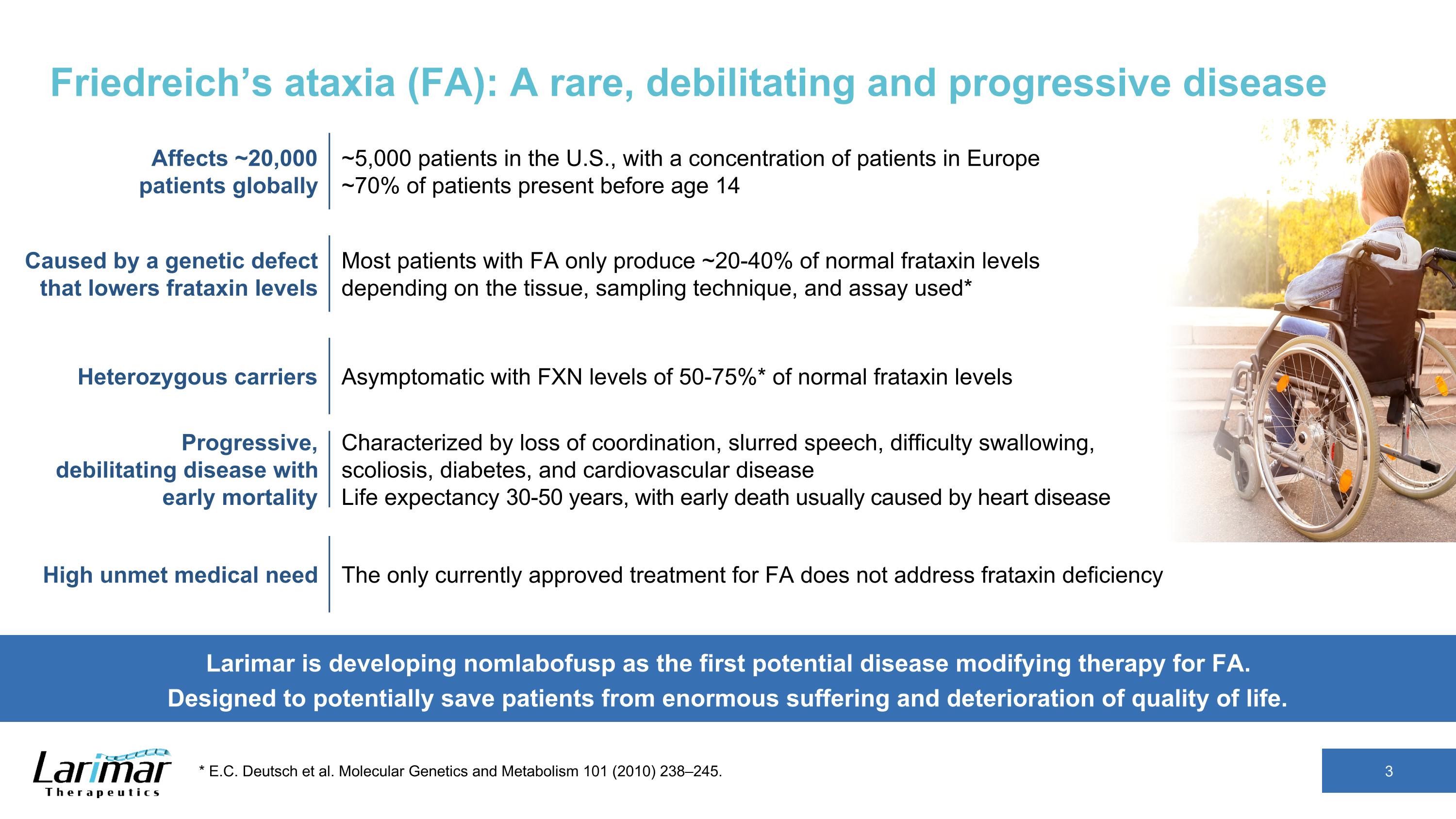
Friedreich’s ataxia (FA): A rare, debilitating and progressive disease 3 * E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245. Larimar is developing nomlabofusp as the first potential disease modifying therapy for FA. Designed to potentially save patients from enormous suffering and deterioration of quality of life. Affects ~20,000 patients globally ~5,000 patients in the U.S., with a concentration of patients in Europe ~70% of patients present before age 14 Caused by a genetic defect that lowers frataxin levels Most patients with FA only produce ~20-40% of normal frataxin levels depending on the tissue, sampling technique, and assay used* Heterozygous carriers Asymptomatic with FXN levels of 50-75%* of normal frataxin levels High unmet medical need The only currently approved treatment for FA does not address frataxin deficiency Progressive, debilitating disease with early mortality Characterized by loss of coordination, slurred speech, difficulty swallowing, scoliosis, diabetes, and cardiovascular disease Life expectancy 30-50 years, with early death usually caused by heart disease
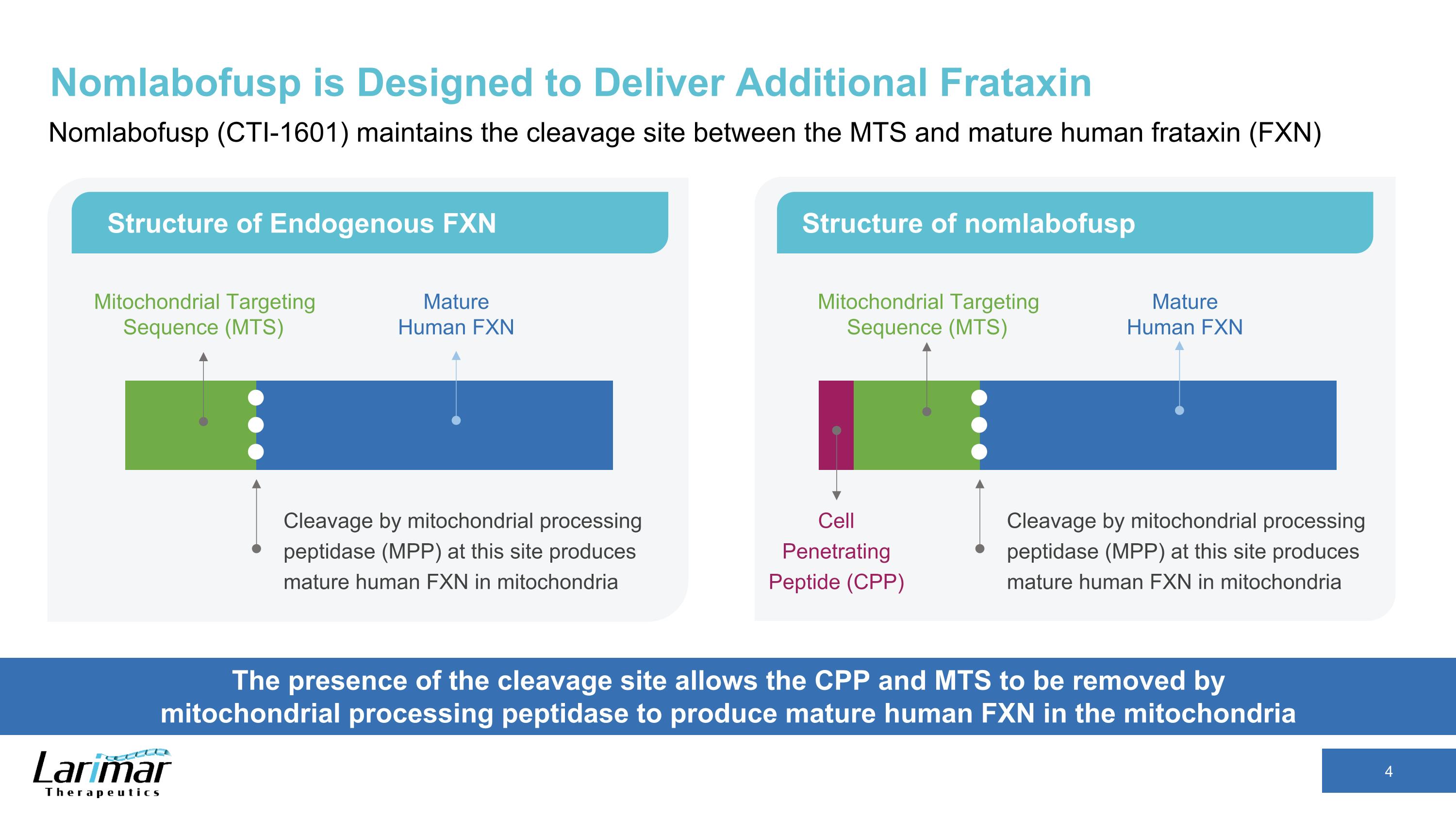
Nomlabofusp is Designed to Deliver Additional Frataxin Nomlabofusp (CTI-1601) maintains the cleavage site between the MTS and mature human frataxin (FXN) The presence of the cleavage site allows the CPP and MTS to be removed by mitochondrial processing peptidase to produce mature human FXN in the mitochondria Structure of Endogenous FXN Structure of nomlabofusp Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mitochondrial Targeting Sequence (MTS) Mature Human FXN Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mature Human FXN Cell Penetrating Peptide (CPP) Mitochondrial Targeting Sequence (MTS)
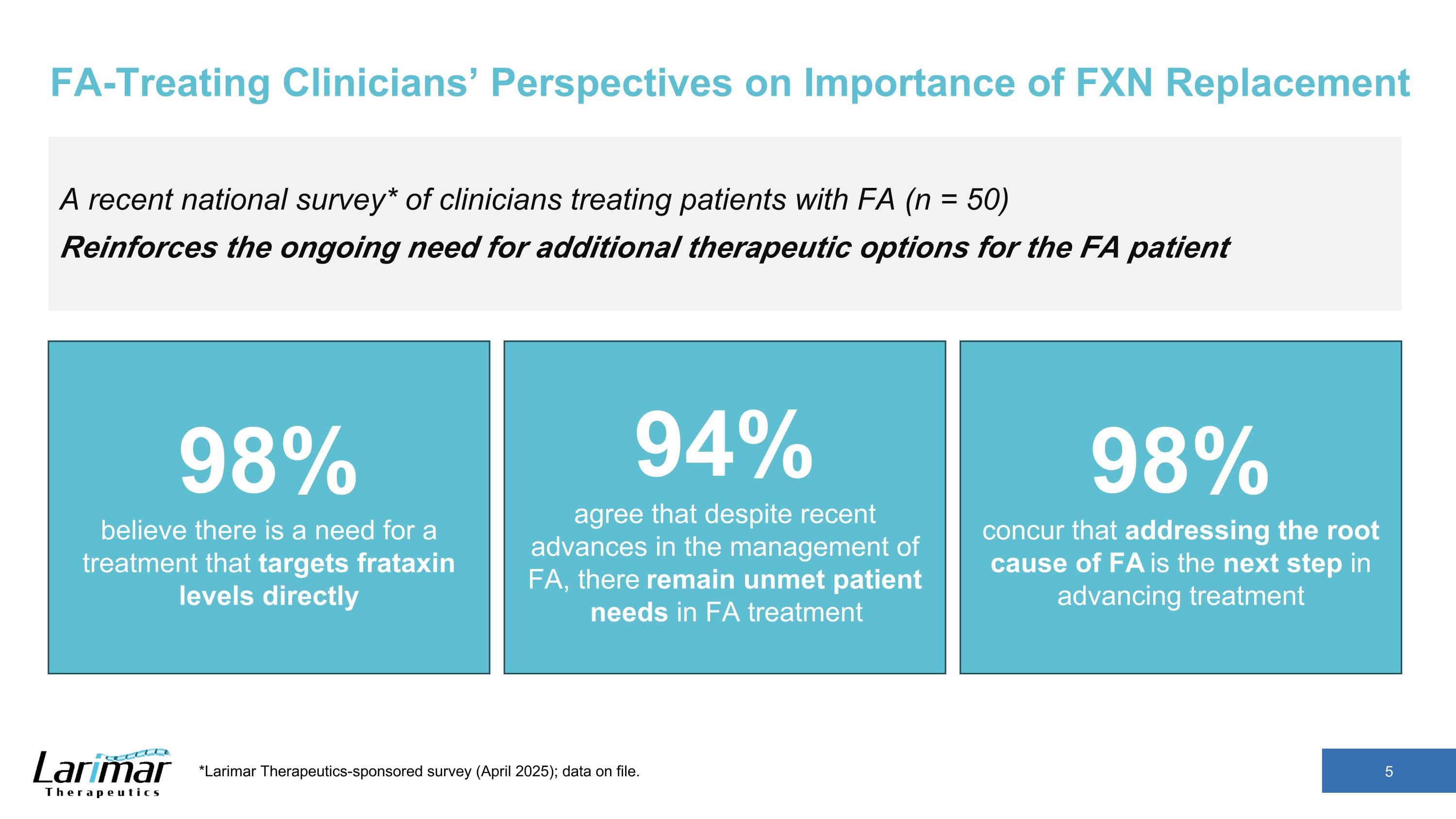
FA-Treating Clinicians’ Perspectives on Importance of FXN Replacement A recent national survey* of clinicians treating patients with FA (n = 50) Reinforces the ongoing need for additional therapeutic options for the FA patient 98% believe there is a need for a treatment that targets frataxin levels directly 94% agree that despite recent advances in the management of FA, there remain unmet patient needs in FA treatment 98% concur that addressing the root cause of FA is the next step in advancing treatment *Larimar Therapeutics-sponsored survey (April 2025); data on file.
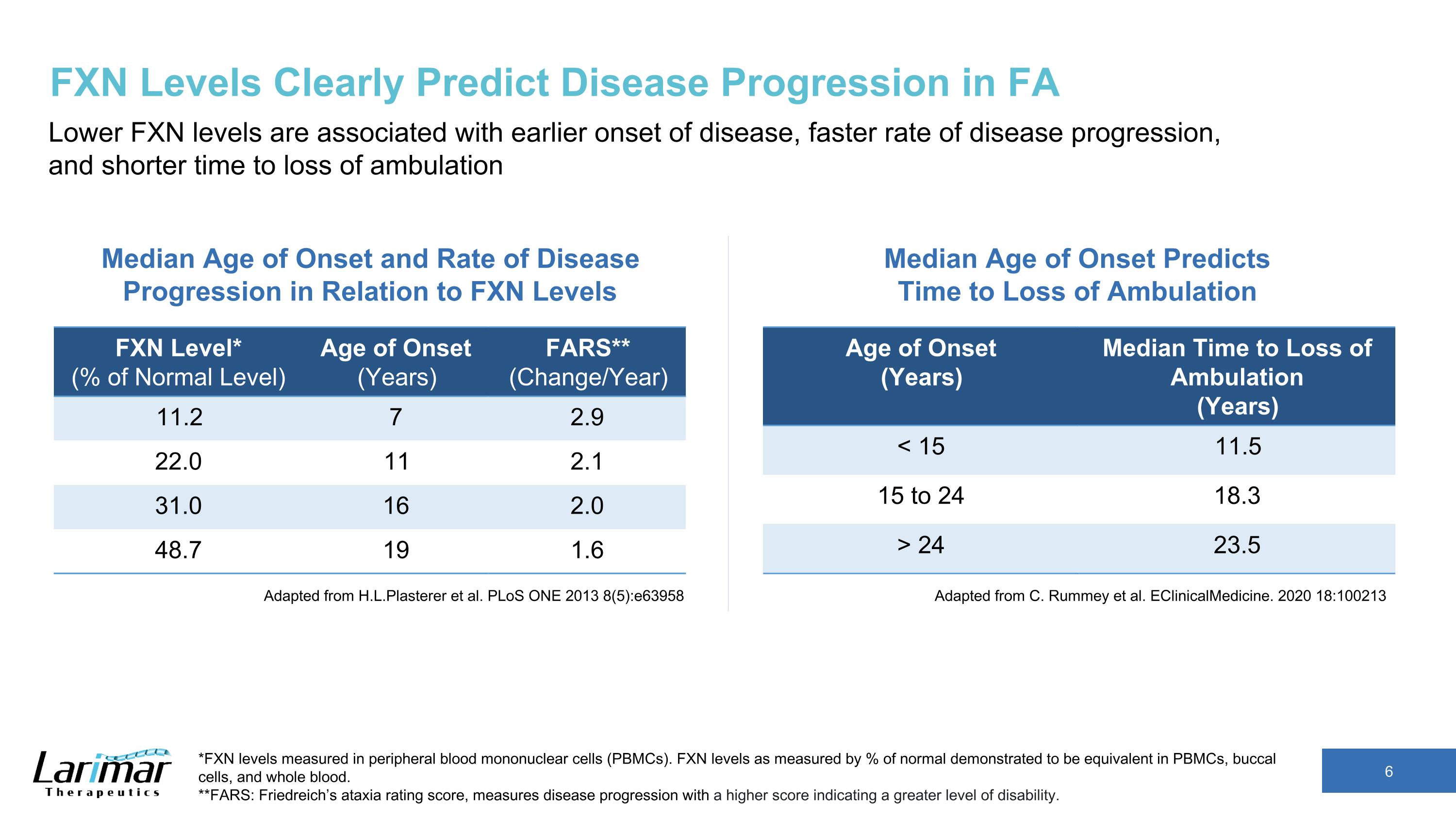
FXN Levels Clearly Predict Disease Progression in FA Lower FXN levels are associated with earlier onset of disease, faster rate of disease progression, and shorter time to loss of ambulation Adapted from H.L.Plasterer et al. PLoS ONE 2013 8(5):e63958 Age of Onset (Years) Median Time to Loss of Ambulation (Years) < 15 11.5 15 to 24 18.3 > 24 23.5 Median Age of Onset and Rate of Disease Progression in Relation to FXN Levels *FXN levels measured in peripheral blood mononuclear cells (PBMCs). FXN levels as measured by % of normal demonstrated to be equivalent in PBMCs, buccal cells, and whole blood. **FARS: Friedreich’s ataxia rating score, measures disease progression with a higher score indicating a greater level of disability. FXN Level* (% of Normal Level) Age of Onset (Years) FARS** (Change/Year) 11.2 7 2.9 22.0 11 2.1 31.0 16 2.0 48.7 19 1.6 Adapted from C. Rummey et al. EClinicalMedicine. 2020 18:100213 Median Age of Onset Predicts Time to Loss of Ambulation

Advancing Nomlabofusp Towards Registration as the First Potential Disease Modifying Therapy for Friedreich’s Ataxia Nomlabofusp is a first-in-class mitochondrial protein replacement therapy designed to directly address systemic frataxin deficiency in patients with FA, a rare neurodegenerative disease Targeting the Root Cause of Disease Strong and Consistent Data Package Data from 4 successfully completed studies (Phase 1 SAD and MAD, Phase 2 dose-exploration, and adolescent PK); ongoing long-term open label study supports dose-dependent increases in tissue FXN Clear FDA Expectations Pursuing an accelerated approval using FXN levels as a novel surrogate endpoint with written FDA recommendations on key elements for BLA submission Registrational Near-Term Catalysts Regulatory update and timeline confirmation expected in Q1 2026; BLA submission seeking potential accelerated approval targeted in Q2 2026 with a U.S. launch targeted for early 2027 Regulatory Designations Orphan Drug (US & EU), Rare Pediatric Disease (US), Fast Track (US), PRIME (EU) and ILAP (UK) designations. Selected by FDA to participate in its START pilot program
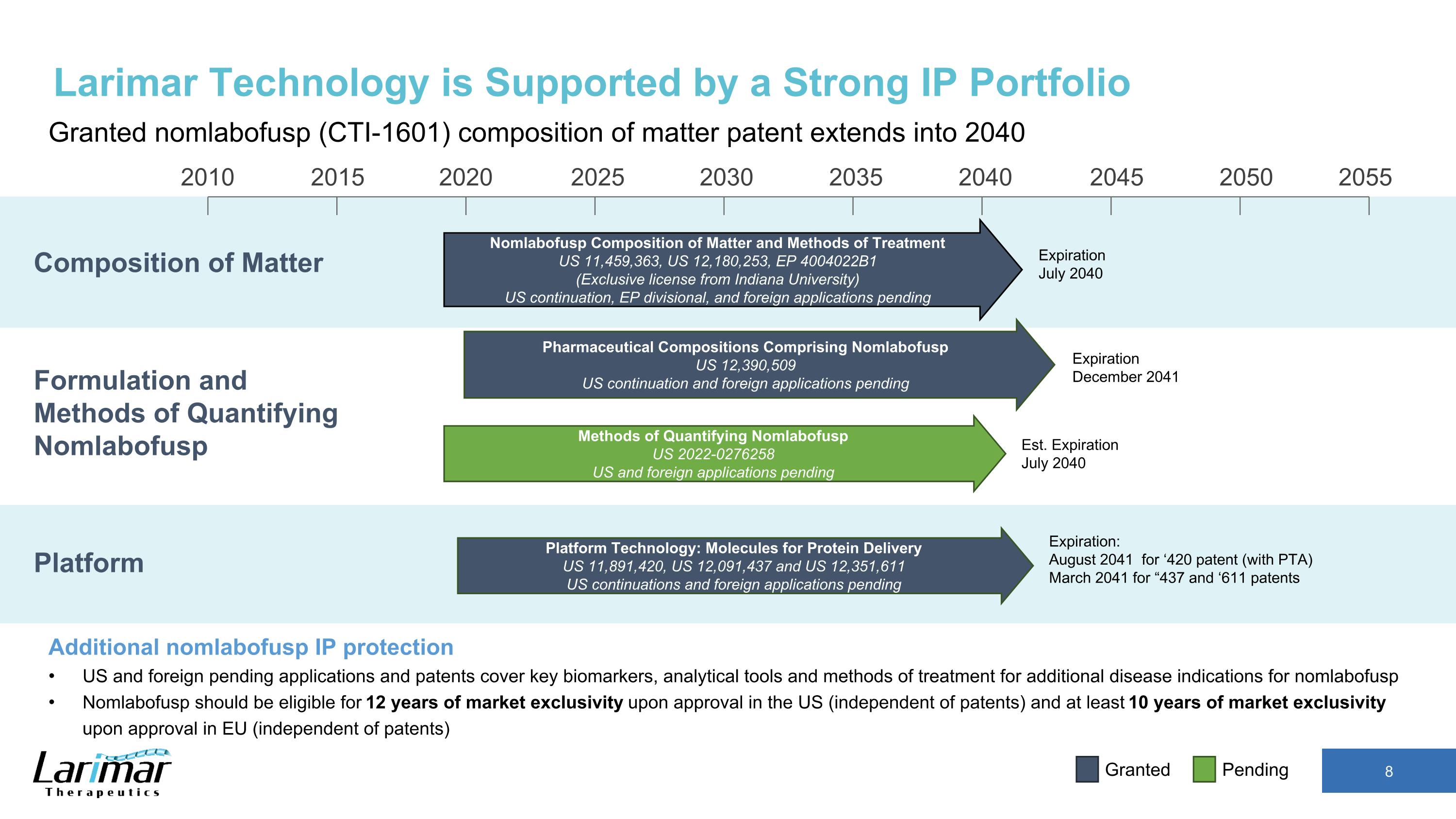
2015 2020 2030 2035 2040 2045 2050 2055 2010 2025 Nomlabofusp Composition of Matter and Methods of Treatment US 11,459,363, US 12,180,253, EP 4004022B1 (Exclusive license from Indiana University) US continuation, EP divisional, and foreign applications pending Expiration July 2040 Composition of Matter Larimar Technology is Supported by a Strong IP Portfolio Granted nomlabofusp (CTI-1601) composition of matter patent extends into 2040 Additional nomlabofusp IP protection US and foreign pending applications and patents cover key biomarkers, analytical tools and methods of treatment for additional disease indications for nomlabofusp Nomlabofusp should be eligible for 12 years of market exclusivity upon approval in the US (independent of patents) and at least 10 years of market exclusivity upon approval in EU (independent of patents) Pending Granted Platform Formulation and Methods of Quantifying Nomlabofusp Platform Technology: Molecules for Protein Delivery US 11,891,420, US 12,091,437 and US 12,351,611 US continuations and foreign applications pending Pharmaceutical Compositions Comprising Nomlabofusp US 12,390,509 US continuation and foreign applications pending Methods of Quantifying Nomlabofusp US 2022-0276258 US and foreign applications pending Expiration December 2041 Est. Expiration July 2040 Expiration: August 2041 for ‘420 patent (with PTA) March 2041 for “437 and ‘611 patents

Nomlabofusp Long-term Open Label Study
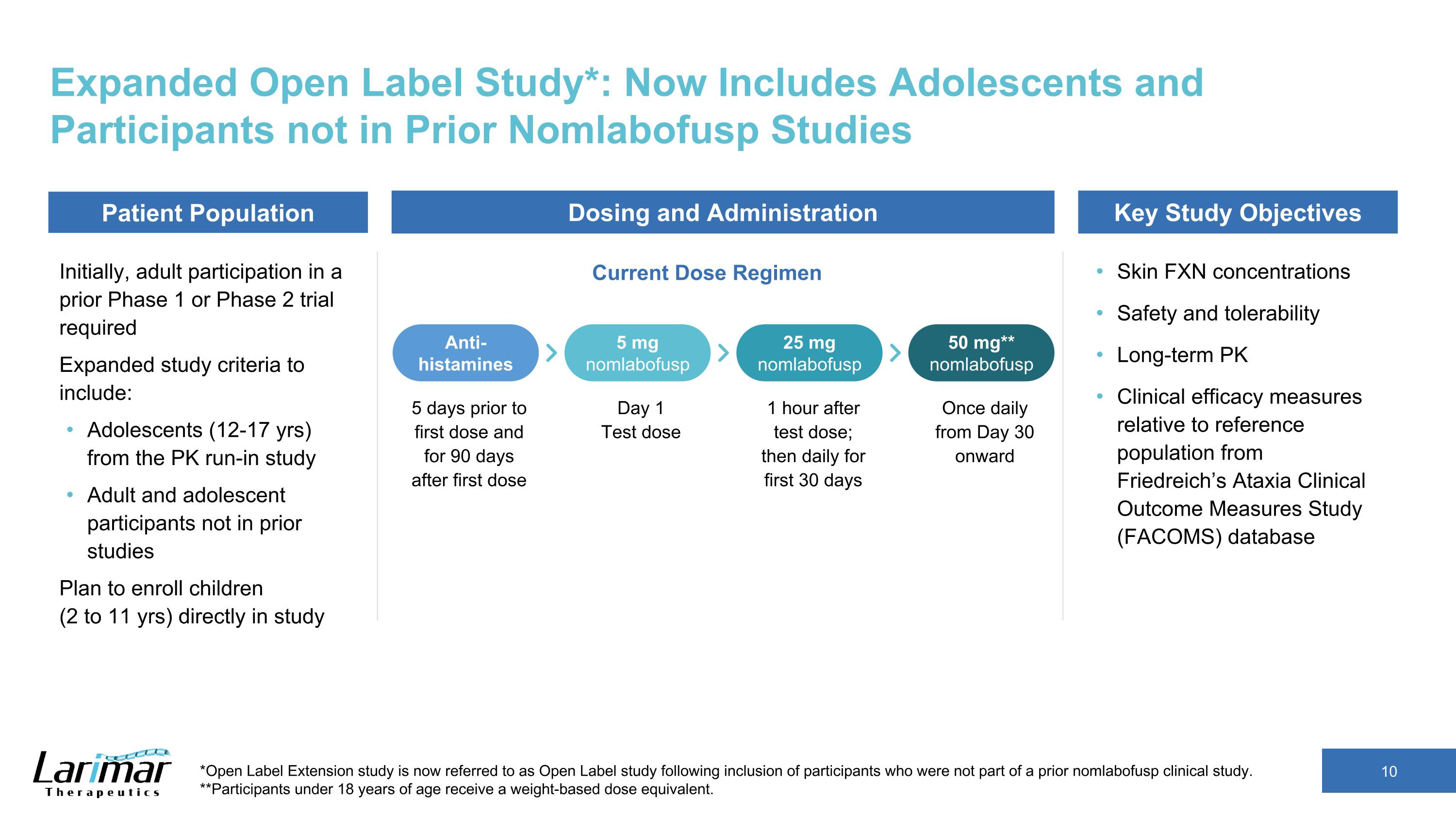
Expanded Open Label Study*: Now Includes Adolescents and Participants not in Prior Nomlabofusp Studies Initially, adult participation in a prior Phase 1 or Phase 2 trial required Expanded study criteria to include: Adolescents (12-17 yrs) from the PK run-in study Adult and adolescent participants not in prior studies Plan to enroll children (2 to 11 yrs) directly in study Skin FXN concentrations Safety and tolerability Long-term PK Clinical efficacy measures relative to reference population from Friedreich’s Ataxia Clinical Outcome Measures Study (FACOMS) database *Open Label Extension study is now referred to as Open Label study following inclusion of participants who were not part of a prior nomlabofusp clinical study. **Participants under 18 years of age receive a weight-based dose equivalent. Patient Population Current Dose Regimen Dosing and Administration Key Study Objectives 5 days prior to first dose and for 90 days after first dose Anti-histamines 5 mg nomlabofusp 25 mg nomlabofusp 50 mg** nomlabofusp Day 1 Test dose 1 hour after test dose; then daily for first 30 days Once daily from Day 30 onward
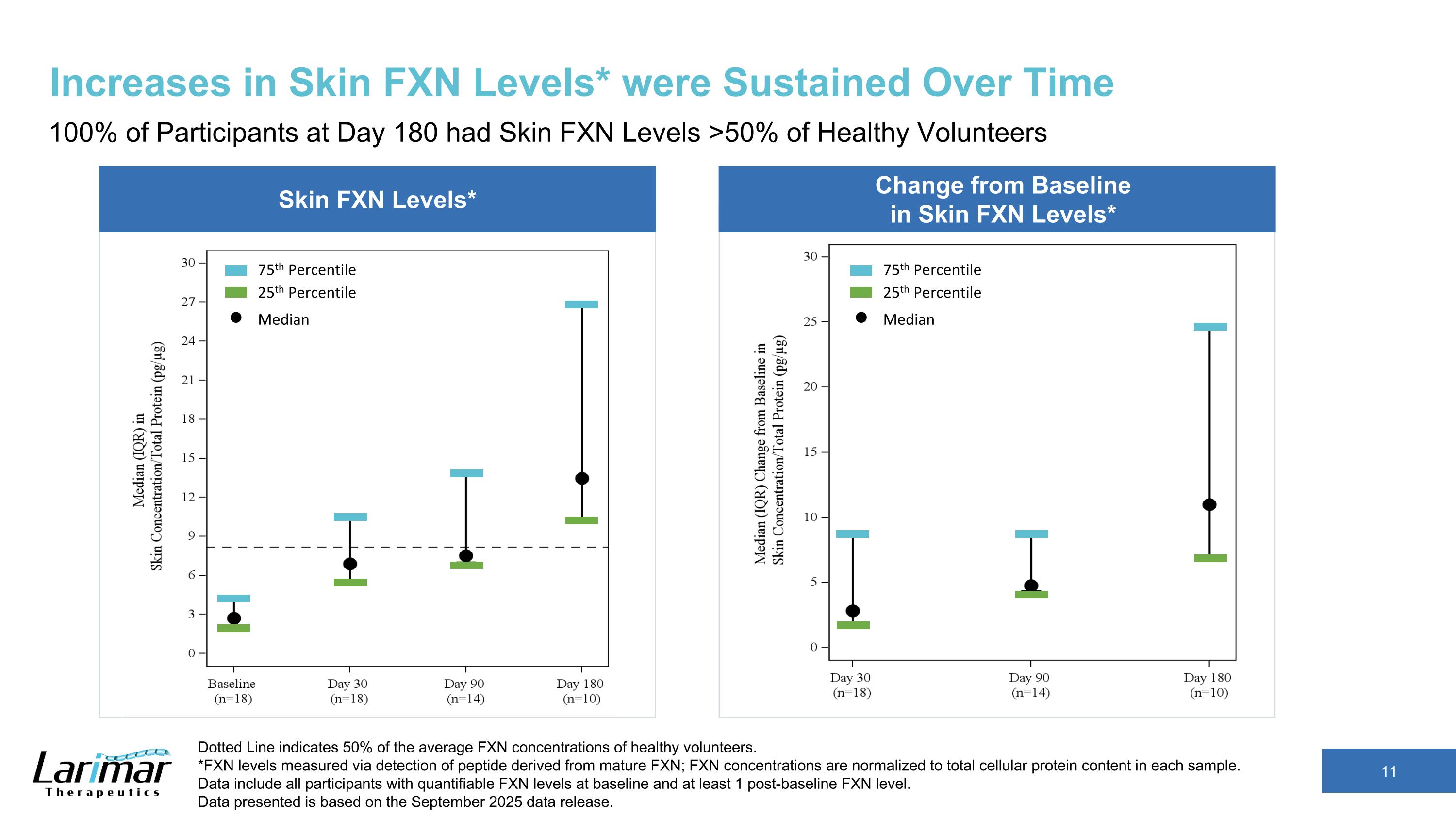
Change from Baseline in Skin FXN Levels* \ Skin FXN Levels* Increases in Skin FXN Levels* were Sustained Over Time 100% of Participants at Day 180 had Skin FXN Levels >50% of Healthy Volunteers Dotted Line indicates 50% of the average FXN concentrations of healthy volunteers. *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample. Data include all participants with quantifiable FXN levels at baseline and at least 1 post-baseline FXN level. Data presented is based on the September 2025 data release. 25th Percentile 75th Percentile Median 25th Percentile 75th Percentile Median
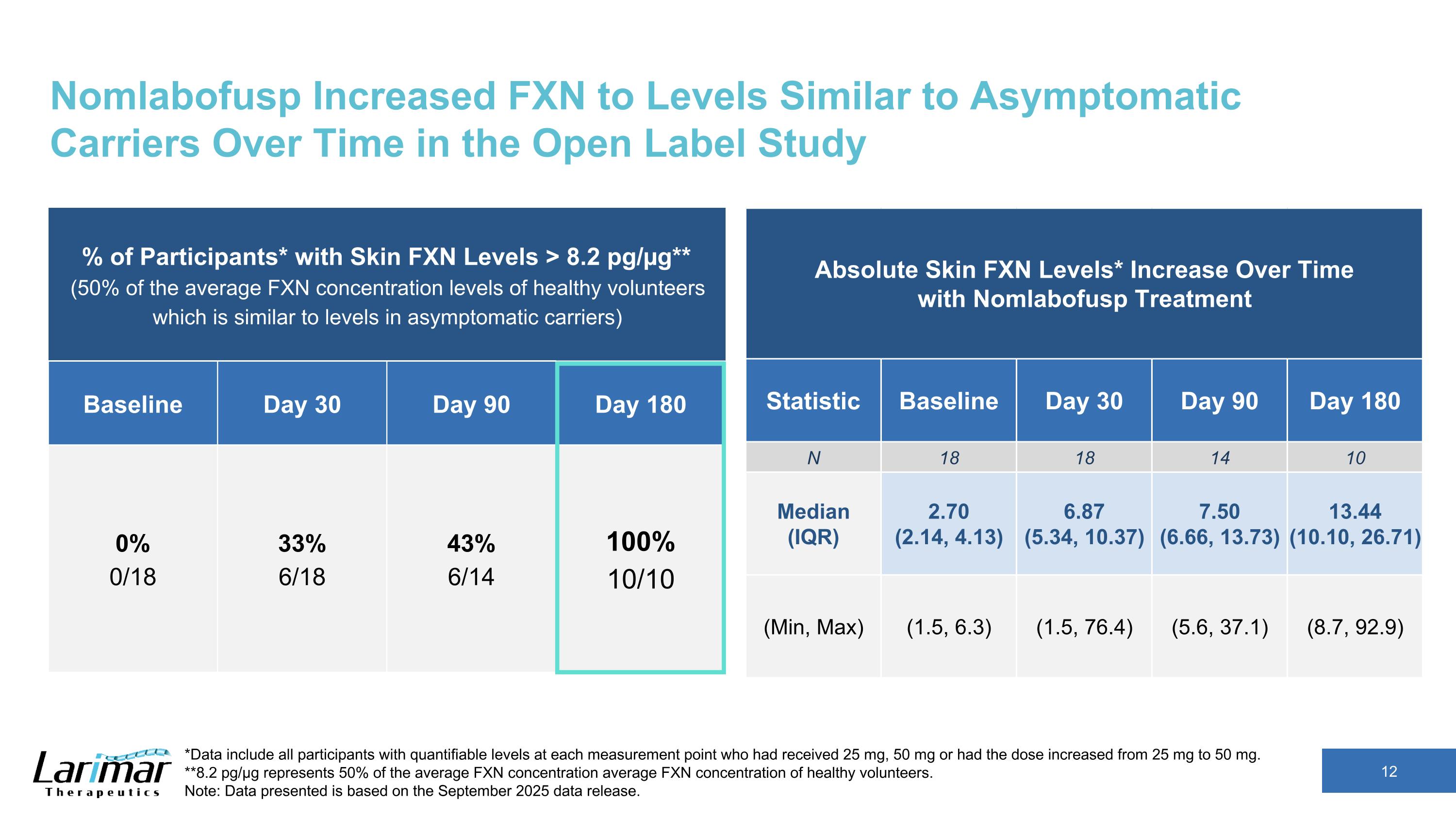
Nomlabofusp Increased FXN to Levels Similar to Asymptomatic Carriers Over Time in the Open Label Study % of Participants* with Skin FXN Levels > 8.2 pg/µg** (50% of the average FXN concentration levels of healthy volunteers which is similar to levels in asymptomatic carriers) Baseline Day 30 Day 90 Day 180 0% 0/18 33% 6/18 43% 6/14 100% 10/10 *Data include all participants with quantifiable levels at each measurement point who had received 25 mg, 50 mg or had the dose increased from 25 mg to 50 mg. **8.2 pg/µg represents 50% of the average FXN concentration average FXN concentration of healthy volunteers. Note: Data presented is based on the September 2025 data release. Absolute Skin FXN Levels* Increase Over Time with Nomlabofusp Treatment Statistic Baseline Day 30 Day 90 Day 180 N 18 18 14 10 Median (IQR) 2.70 (2.14, 4.13) 6.87 (5.34, 10.37) 7.50 (6.66, 13.73) 13.44 (10.10, 26.71) (Min, Max) (1.5, 6.3) (1.5, 76.4) (5.6, 37.1) (8.7, 92.9)
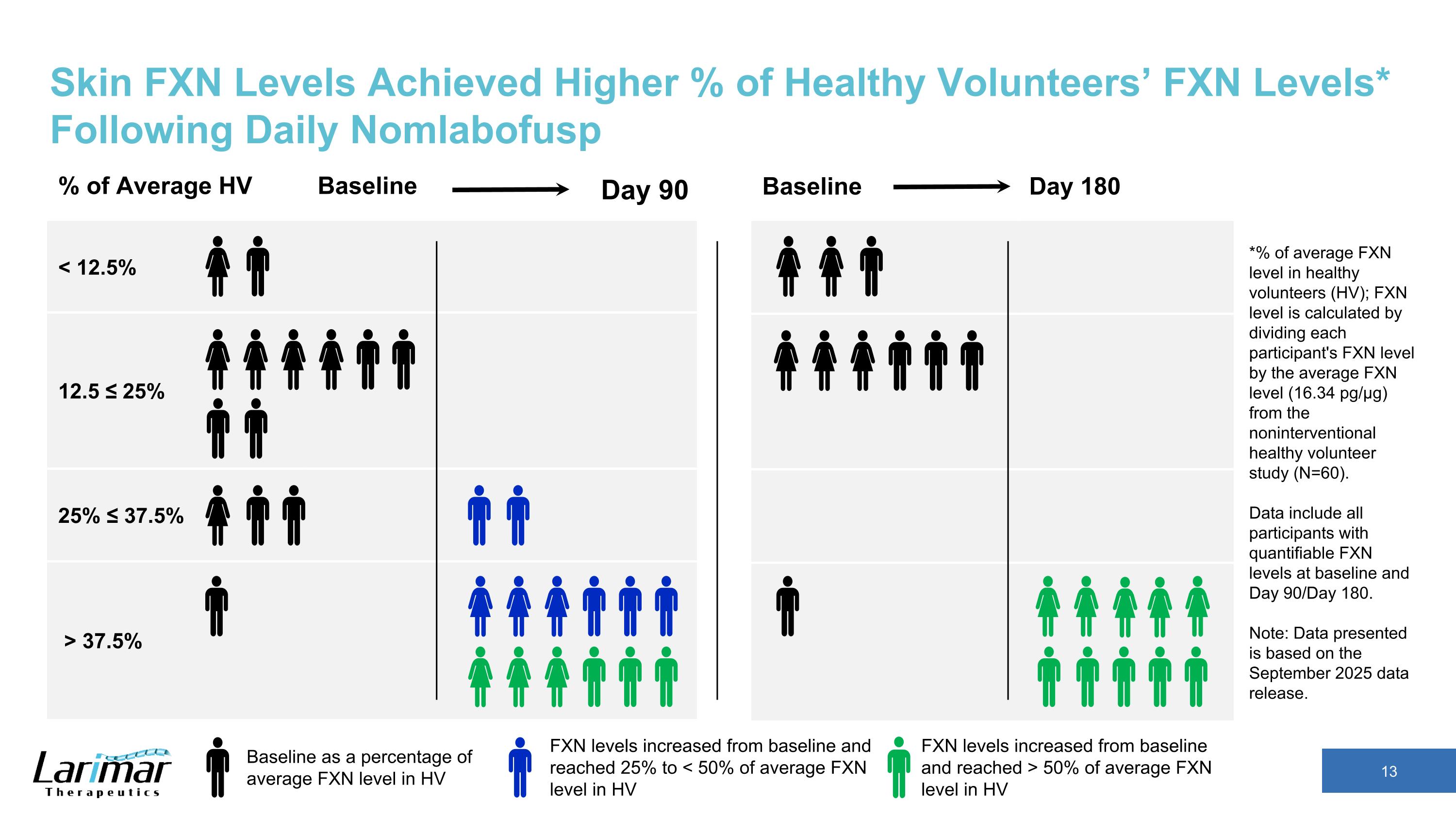
Skin FXN Levels Achieved Higher % of Healthy Volunteers’ FXN Levels* Following Daily Nomlabofusp % of Average HV Baseline < 12.5% 12.5 ≤ 25% 25% ≤ 37.5% > 37.5% *% of average FXN level in healthy volunteers (HV); FXN level is calculated by dividing each participant's FXN level by the average FXN level (16.34 pg/µg) from the noninterventional healthy volunteer study (N=60). Data include all participants with quantifiable FXN levels at baseline and Day 90/Day 180. Note: Data presented is based on the September 2025 data release. Baseline as a percentage of average FXN level in HV FXN levels increased from baseline and reached > 50% of average FXN level in HV FXN levels increased from baseline and reached 25% to < 50% of average FXN level in HV Day 90 Baseline Day 180

Nomlabofusp Safety Observations* with Long-term Treatment 65 total participants received at least 1 dose of nomlabofusp across all studies with 39 participants in OL study Most common AEs were mild/moderate local ISRs and did not lead to any withdrawals 7 of 39 participants in OL study experienced anaphylaxis Standard treatment with epinephrine resulted in reversal of symptoms and no late phase response or complications were observed All affected participants returned to usual state of health with no further sequelae 10 participants in OL Study did not have prior exposure to nomlabofusp; 1 of these 10 experienced anaphylaxis Long-term daily dosing was generally well tolerated, including 14 on treatment for at least 6 months and 8 for over 1 year * Data presented is based on the September 2025 data release. AE: Adverse events; ISR: Injection site reaction
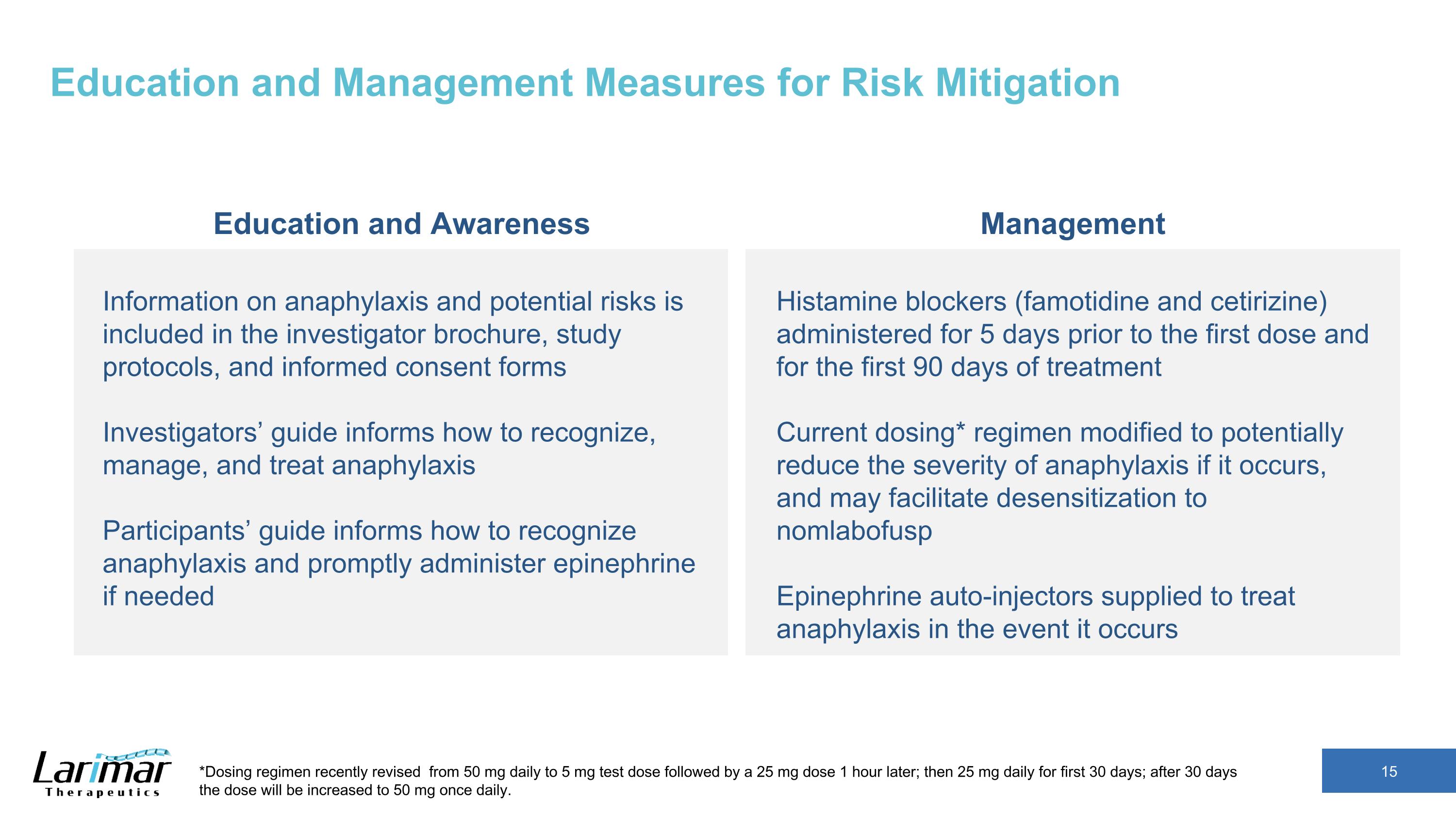
Education and Management Measures for Risk Mitigation Education and Awareness Management Information on anaphylaxis and potential risks is included in the investigator brochure, study protocols, and informed consent forms Investigators’ guide informs how to recognize, manage, and treat anaphylaxis Participants’ guide informs how to recognize anaphylaxis and promptly administer epinephrine if needed Histamine blockers (famotidine and cetirizine) administered for 5 days prior to the first dose and for the first 90 days of treatment Current dosing* regimen modified to potentially reduce the severity of anaphylaxis if it occurs, and may facilitate desensitization to nomlabofusp Epinephrine auto-injectors supplied to treat anaphylaxis in the event it occurs *Dosing regimen recently revised from 50 mg daily to 5 mg test dose followed by a 25 mg dose 1 hour later; then 25 mg daily for first 30 days; after 30 days the dose will be increased to 50 mg once daily.
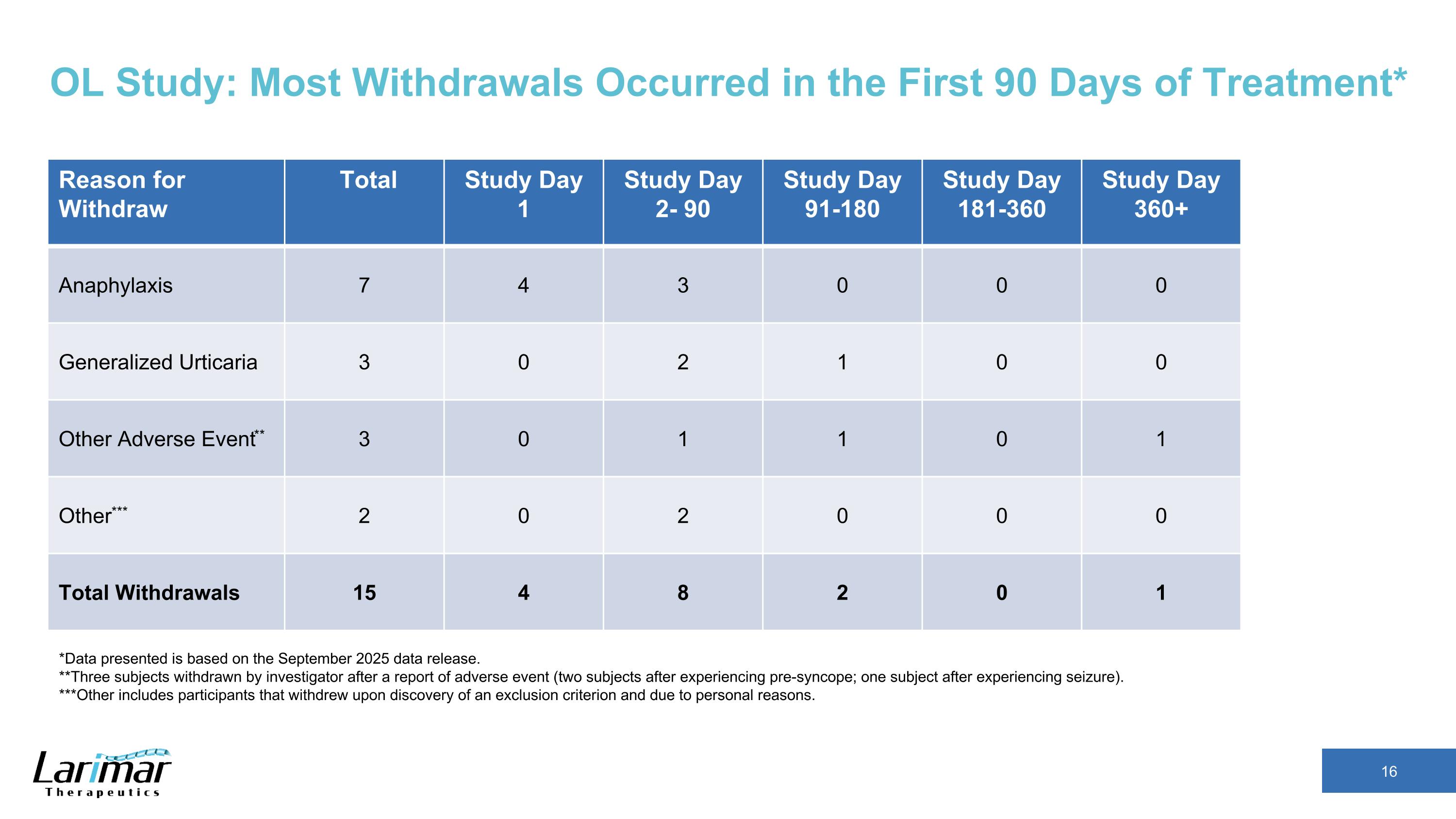
OL Study: Most Withdrawals Occurred in the First 90 Days of Treatment* Reason for Withdraw Total Study Day 1 Study Day 2- 90 Study Day 91-180 Study Day 181-360 Study Day 360+ Anaphylaxis 7 4 3 0 0 0 Generalized Urticaria 3 0 2 1 0 0 Other Adverse Event** 3 0 1 1 0 1 Other*** 2 0 2 0 0 0 Total Withdrawals 15 4 8 2 0 1 *Data presented is based on the September 2025 data release. **Three subjects withdrawn by investigator after a report of adverse event (two subjects after experiencing pre-syncope; one subject after experiencing seizure). ***Other includes participants that withdrew upon discovery of an exclusion criterion and due to personal reasons.
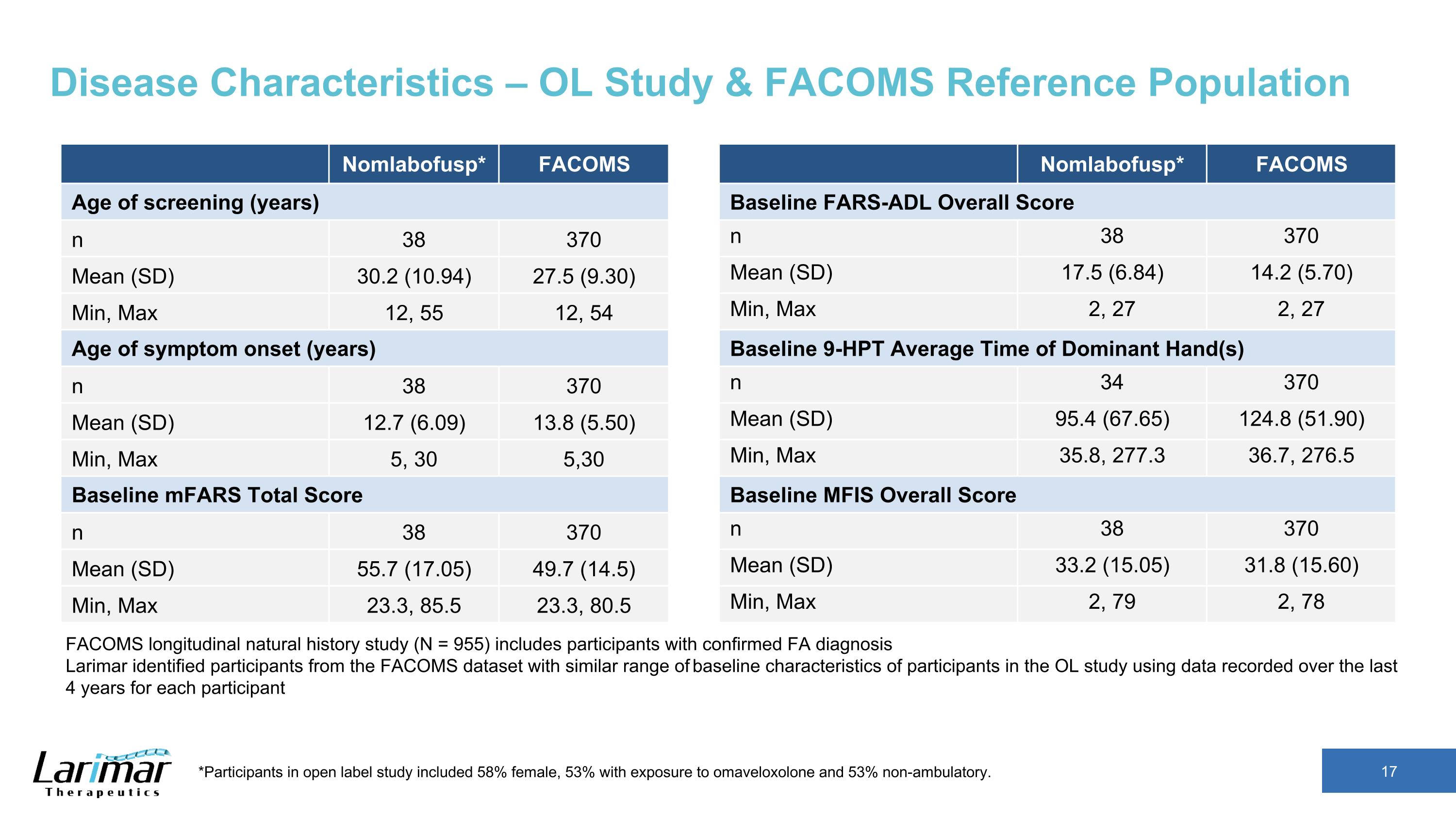
Disease Characteristics – OL Study & FACOMS Reference Population Nomlabofusp* FACOMS Age of screening (years) n 38 370 Mean (SD) 30.2 (10.94) 27.5 (9.30) Min, Max 12, 55 12, 54 Age of symptom onset (years) n 38 370 Mean (SD) 12.7 (6.09) 13.8 (5.50) Min, Max 5, 30 5,30 Baseline mFARS Total Score n 38 370 Mean (SD) 55.7 (17.05) 49.7 (14.5) Min, Max 23.3, 85.5 23.3, 80.5 Nomlabofusp* FACOMS Baseline FARS-ADL Overall Score n 38 370 Mean (SD) 17.5 (6.84) 14.2 (5.70) Min, Max 2, 27 2, 27 Baseline 9-HPT Average Time of Dominant Hand(s) n 34 370 Mean (SD) 95.4 (67.65) 124.8 (51.90) Min, Max 35.8, 277.3 36.7, 276.5 Baseline MFIS Overall Score n 38 370 Mean (SD) 33.2 (15.05) 31.8 (15.60) Min, Max 2, 79 2, 78 FACOMS longitudinal natural history study (N = 955) includes participants with confirmed FA diagnosis Larimar identified participants from the FACOMS dataset with similar range of baseline characteristics of participants in the OL study using data recorded over the last 4 years for each participant *Participants in open label study included 58% female, 53% with exposure to omaveloxolone and 53% non-ambulatory.
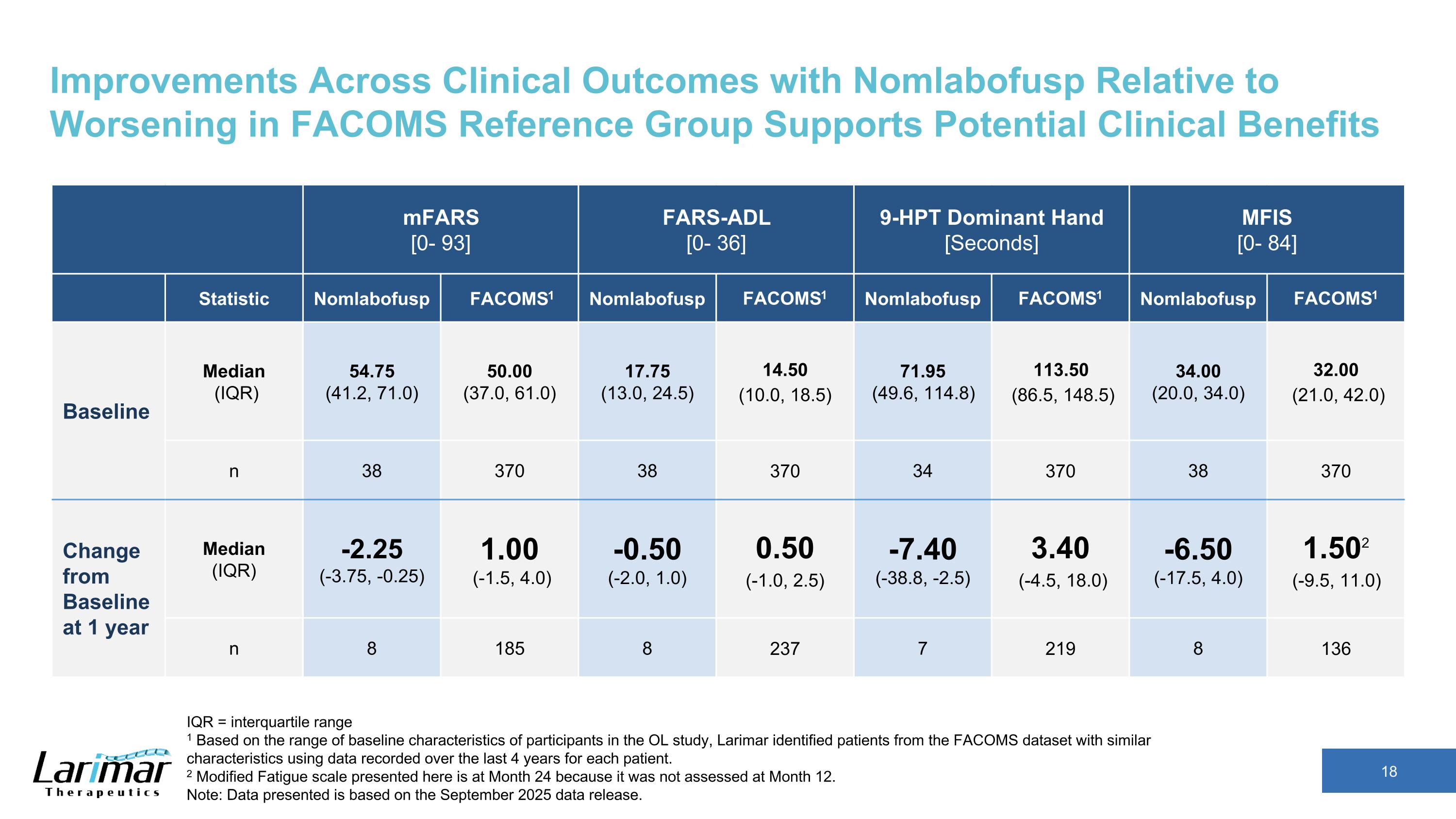
Improvements Across Clinical Outcomes with Nomlabofusp Relative to Worsening in FACOMS Reference Group Supports Potential Clinical Benefits mFARS [0- 93] FARS-ADL [0- 36] 9-HPT Dominant Hand [Seconds] MFIS [0- 84] Statistic Nomlabofusp FACOMS1 Nomlabofusp FACOMS1 Nomlabofusp FACOMS1 Nomlabofusp FACOMS1 Baseline Median (IQR) 54.75 (41.2, 71.0) 50.00 (37.0, 61.0) 17.75 (13.0, 24.5) 14.50 (10.0, 18.5) 71.95 (49.6, 114.8) 113.50 (86.5, 148.5) 34.00 (20.0, 34.0) 32.00 (21.0, 42.0) n 38 370 38 370 34 370 38 370 Change from Baseline at 1 year Median (IQR) -2.25 (-3.75, -0.25) 1.00 (-1.5, 4.0) -0.50 (-2.0, 1.0) 0.50 (-1.0, 2.5) -7.40 (-38.8, -2.5) 3.40 (-4.5, 18.0) -6.50 (-17.5, 4.0) 1.502 (-9.5, 11.0) n 8 185 8 237 7 219 8 136 IQR = interquartile range 1 Based on the range of baseline characteristics of participants in the OL study, Larimar identified patients from the FACOMS dataset with similar characteristics using data recorded over the last 4 years for each patient. 2 Modified Fatigue scale presented here is at Month 24 because it was not assessed at Month 12. Note: Data presented is based on the September 2025 data release.
Benefit-Risk Profile Underpins the Potential of Nomlabofusp Based on FDA October 2023 Guidance on Benefit-Risk Assessment Framework for Biological Products Clinical data suggests increases in frataxin levels may result in slowing or halting disease progression 2 Anaphylaxis occurred in some patients and was treated with epinephrine, resulting in reversal of symptoms and return to usual state of health with no further sequelae 3 Generally well-tolerated long-term safety profile in complex patients; most common AEs were mild/moderate local ISRs and did not lead to any withdrawals 4 1 Potentially addresses unmet need by increasing tissue frataxin levels to treat the underlying cause of FA in both adults and children with this progressive and debilitating disease

Path to Planned BLA Submission in Q2 2026 Seeking Accelerated Approval Expected Elements of BLA Submission Use of Skin FXN Levels as a Surrogate Endpoint Justification for using increases in skin FXN concentrations from participants in the OL study as a reasonably likely surrogate endpoint (RLSE) FDA has stated that acceptability of increases in skin FXN for accelerated approval will be decided during future BLA review Safety Database At least 30 participants with continuous exposure for 6-months A subset of at least 10 participants with continuous exposure for 1-year A large majority of the exposure should be on the 50 mg dose Clinical Data Package & Global Phase 3 Study Data from 4 successfully completed studies (Phase 1 SAD and MAD, Phase 2 dose-exploration, and adolescent PK) and an ongoing long-term OL study OL study data Results to date: 10/10 participants in OL study with data at 6 months achieved skin FXN levels over 50% of median levels in healthy volunteers (which is similar to levels in asymptomatic carriers) Consistent directional improvement in OL study across 4 key clinical outcomes (mFARS, FARS-ADL, 9-HPT, MFIS) observed after 1 year of nomlabofusp treatment relative to a worsening in a FACOMS natural history study reference population could suggest potential for clinical benefit Global Phase 3 study, intended as the confirmatory study, to evaluate clinical outcomes including upright stability and mFARS expected to be underway at the time of BLA submission Pharmacology & Toxicology Nonclinical data supporting a relationship between increased skin FXN and relevant tissues such as the heart, dorsal root ganglion, and skeletal muscle Complete toxicology package including juvenile toxicology study Chemistry Manufacturing & Controls Data supporting the lyophilized drug product with stability at room temperature Data on batches manufactured at a commercial scale Analytical methods and proposed specifications FXN: Frataxin; OL: Open label study; BLA: Biologics License Application; FDA: Food and Drug Administration; RLSE: reasonably likely surrogate endpoint; SAD: Single ascending-dose ; MAD: Multiple ascending dose
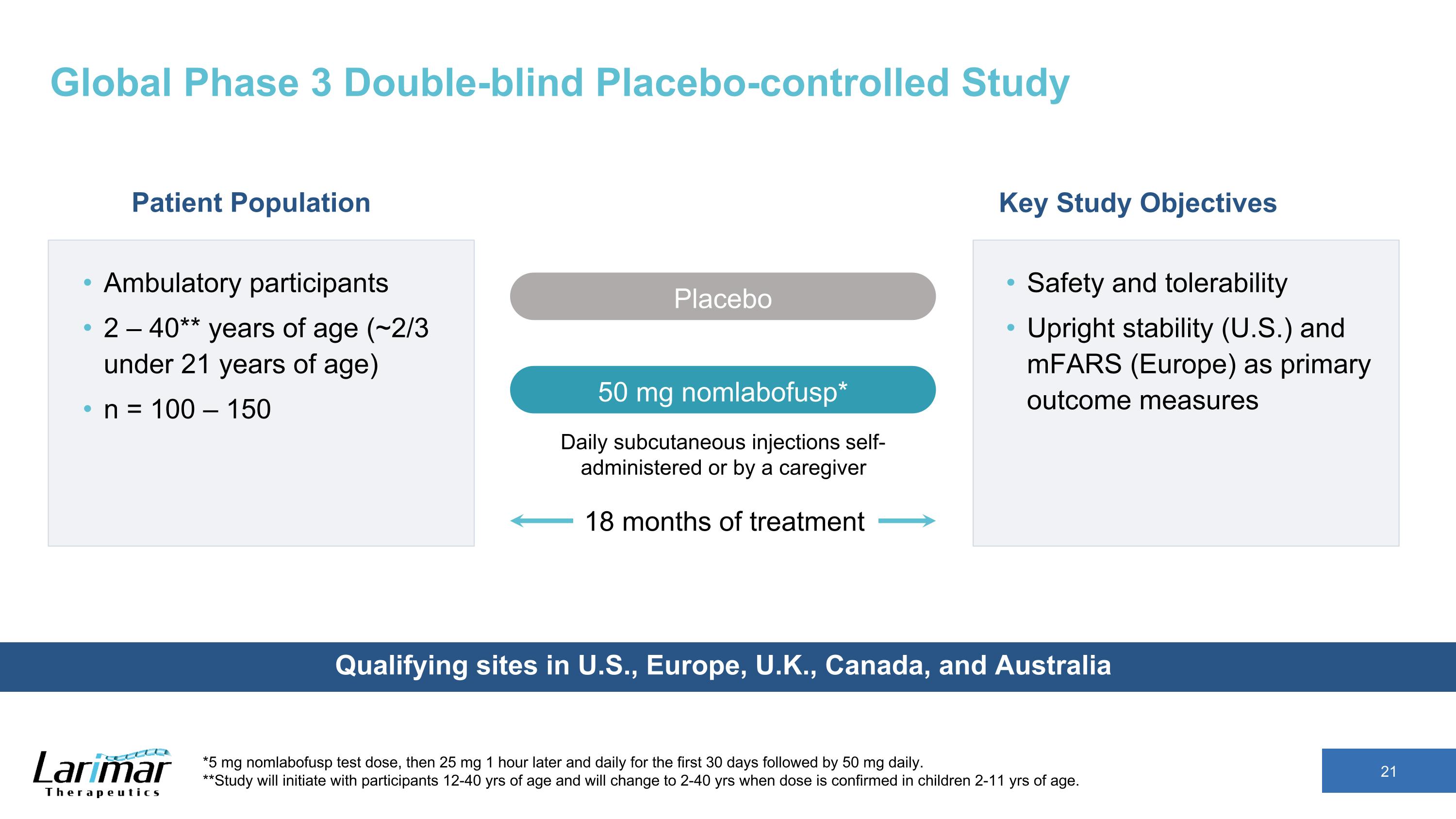
Global Phase 3 Double-blind Placebo-controlled Study Qualifying sites in U.S., Europe, U.K., Canada, and Australia 18 months of treatment Ambulatory participants 2 – 40** years of age (~2/3 under 21 years of age) n = 100 – 150 Key Study Objectives Safety and tolerability Upright stability (U.S.) and mFARS (Europe) as primary outcome measures Daily subcutaneous injections self-administered or by a caregiver Placebo 50 mg nomlabofusp* Patient Population *5 mg nomlabofusp test dose, then 25 mg 1 hour later and daily for the first 30 days followed by 50 mg daily. **Study will initiate with participants 12-40 yrs of age and will change to 2-40 yrs when dose is confirmed in children 2-11 yrs of age.
Nomlabofusp Advancing Towards BLA Submission for FA First potential disease modifying therapy Designed to systemically address FXN deficiency in FA FDA clarity on key BLA elements BLA based on skin FXN levels as potential novel surrogate endpoint Positive long-term data Increased skin FXN levels similar to levels expected in asymptomatic carriers and consistent directional improvement across 4 key clinical outcome measures BLA submission seeking accelerated approval targeted Q2 2026 U.S. launch targeted for early 2027 $136.9 million* in estimated cash and investments at 12/31/25 with projected runway into Q4 2026 . *Estimate is unaudited and preliminary and actual results may differ due to the completion of our December 31, 2025 closing procedures and issuance of our December 31, 2025 financial statements prepared in accordance with U.S. generally accepted accounting principles.

Appendix Larimar Therapeutics

Mitochondrial Localization and Preclinical Data
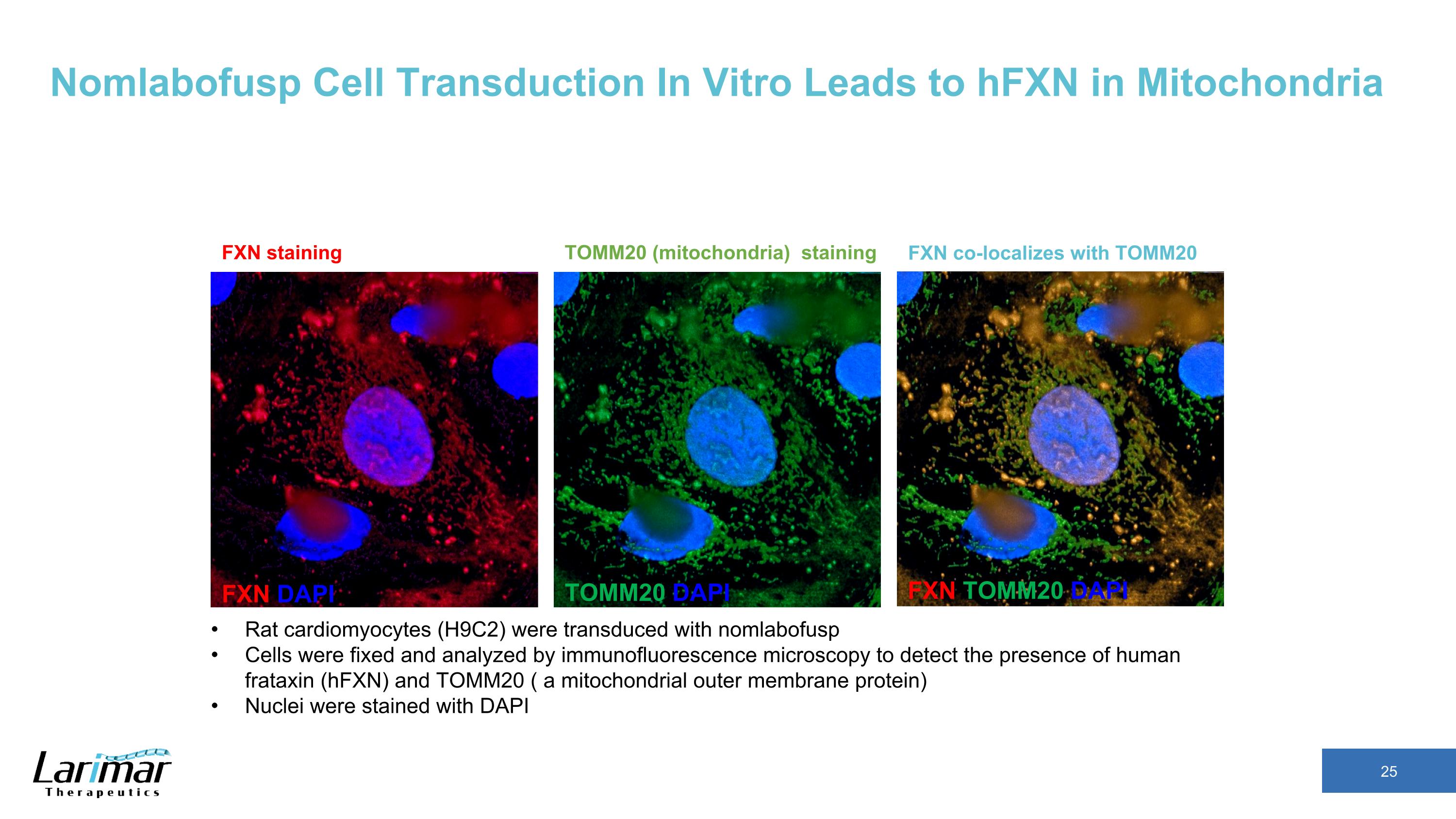
Nomlabofusp Cell Transduction In Vitro Leads to hFXN in Mitochondria FXN DAPI TOMM20 DAPI FXN TOMM20 DAPI FXN co-localizes with TOMM20 FXN staining TOMM20 (mitochondria) staining Rat cardiomyocytes (H9C2) were transduced with nomlabofusp Cells were fixed and analyzed by immunofluorescence microscopy to detect the presence of human frataxin (hFXN) and TOMM20 ( a mitochondrial outer membrane protein) Nuclei were stained with DAPI
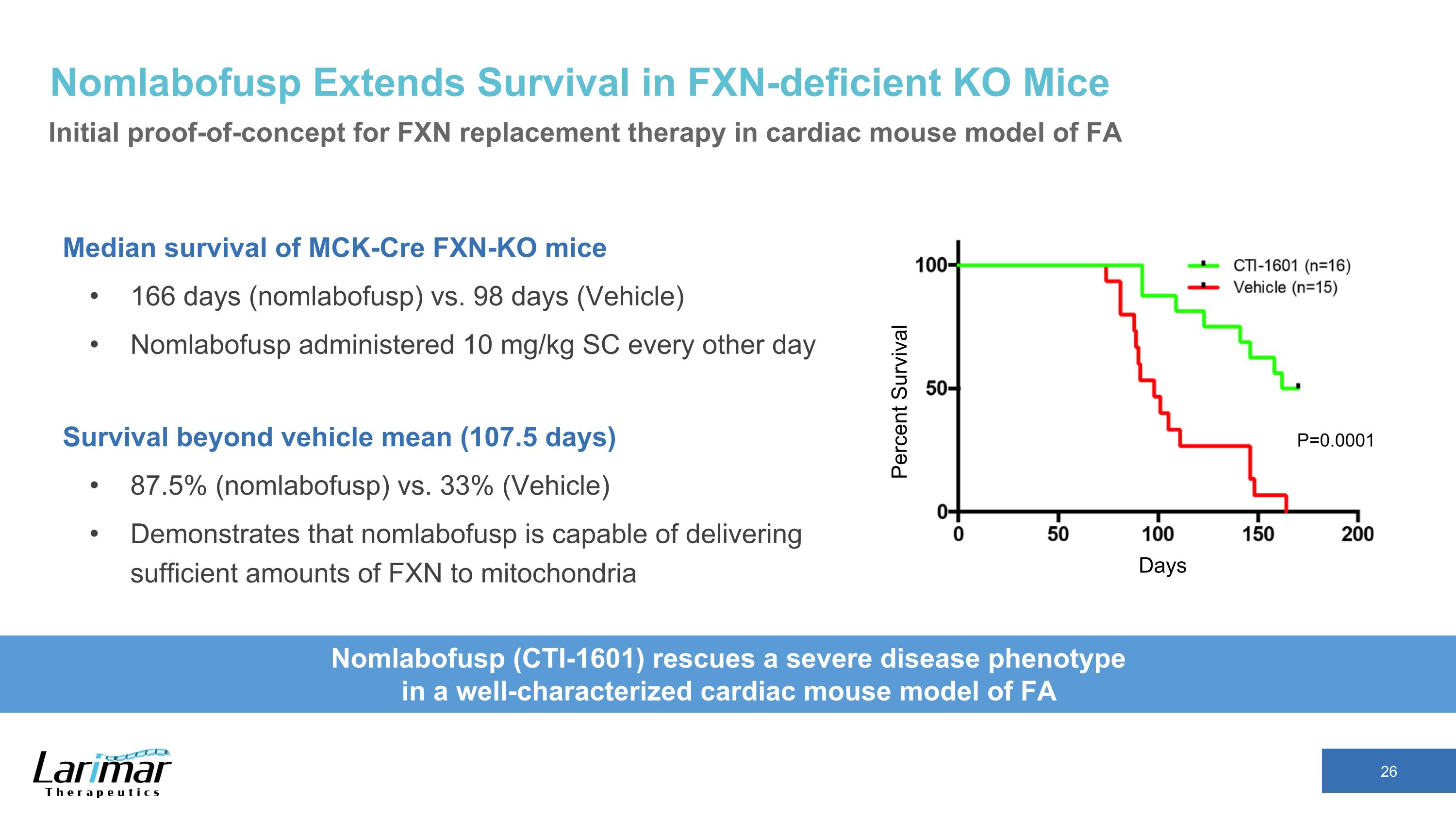
Nomlabofusp Extends Survival in FXN-deficient KO Mice Initial proof-of-concept for FXN replacement therapy in cardiac mouse model of FA Median survival of MCK-Cre FXN-KO mice 166 days (nomlabofusp) vs. 98 days (Vehicle) Nomlabofusp administered 10 mg/kg SC every other day Survival beyond vehicle mean (107.5 days) 87.5% (nomlabofusp) vs. 33% (Vehicle) Demonstrates that nomlabofusp is capable of delivering sufficient amounts of FXN to mitochondria Days Percent Survival Nomlabofusp (CTI-1601) rescues a severe disease phenotype in a well-characterized cardiac mouse model of FA P=0.0001
Nomlabofusp Prevents Development of Ataxic Gait in Neurologic KO Mouse Model hFXN replacement with nomlabofusp prevents development of ataxic gait Nomlabofusp-treated mice survive longer than untreated mice Human frataxin present in brain, dorsal root ganglia and spinal cord demonstrating central nervous system penetration In-Vivo Efficacy Data in Pvalb-Cre FXN-KO Mouse Model Single dose level: 10 mg/kg nomlabofusp or vehicle given intraperitoneally three times per week
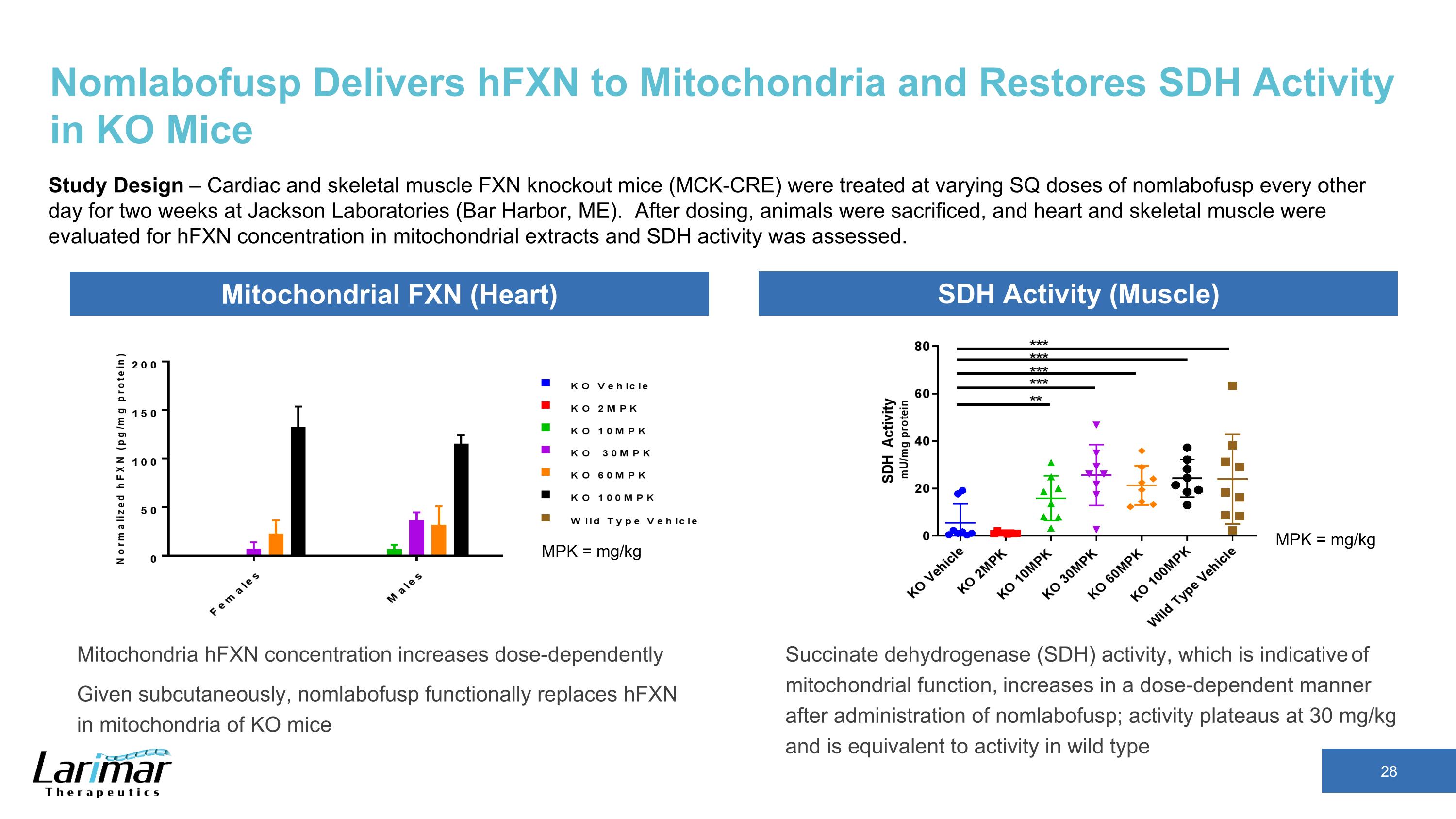
Nomlabofusp Delivers hFXN to Mitochondria and Restores SDH Activity in KO Mice Study Design – Cardiac and skeletal muscle FXN knockout mice (MCK-CRE) were treated at varying SQ doses of nomlabofusp every other day for two weeks at Jackson Laboratories (Bar Harbor, ME). After dosing, animals were sacrificed, and heart and skeletal muscle were evaluated for hFXN concentration in mitochondrial extracts and SDH activity was assessed. Mitochondria hFXN concentration increases dose-dependently Given subcutaneously, nomlabofusp functionally replaces hFXN in mitochondria of KO mice MPK = mg/kg MPK = mg/kg Mitochondrial FXN (Heart) SDH Activity (Muscle) Succinate dehydrogenase (SDH) activity, which is indicative of mitochondrial function, increases in a dose-dependent manner after administration of nomlabofusp; activity plateaus at 30 mg/kg and is equivalent to activity in wild type
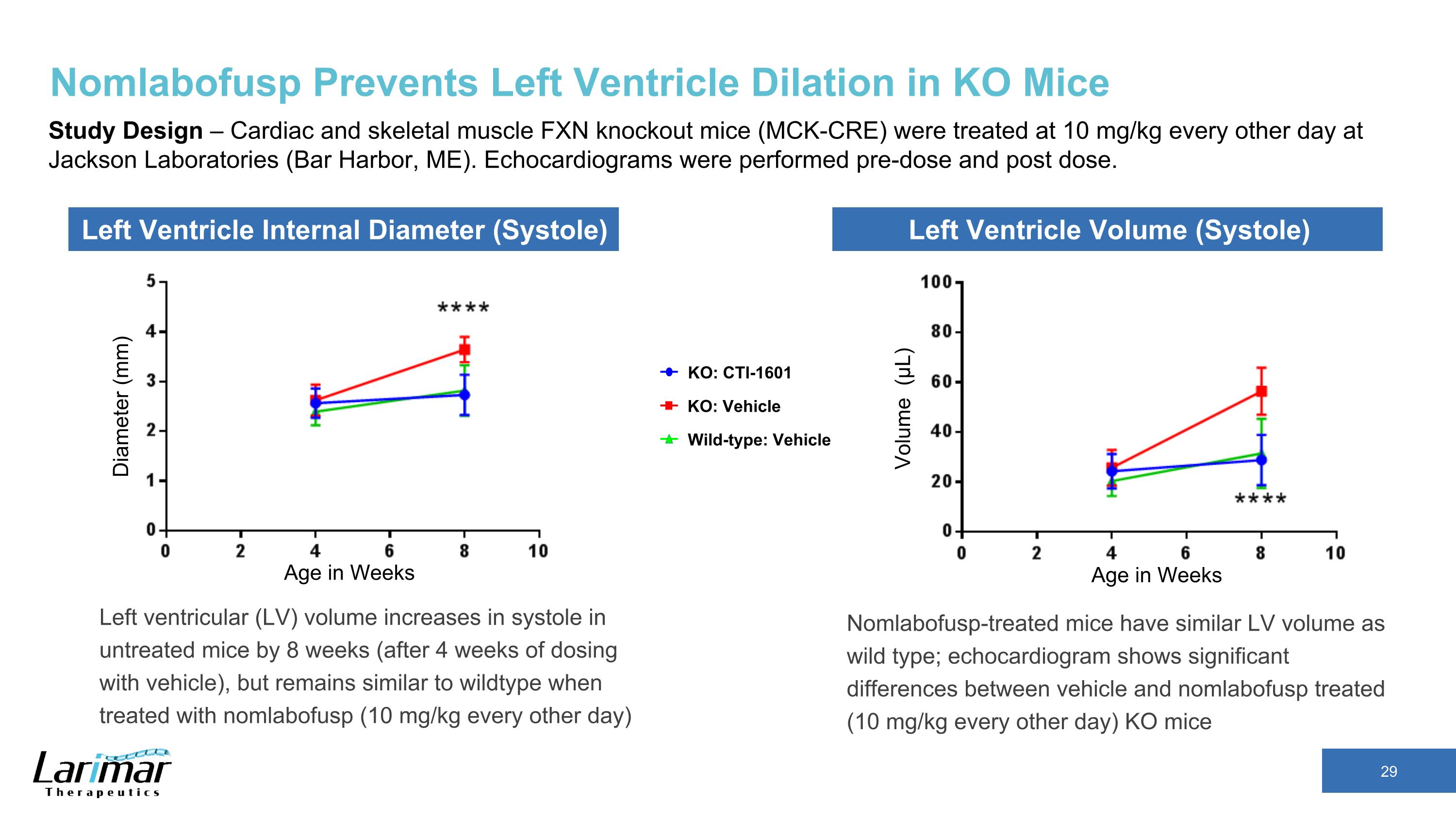
Nomlabofusp Prevents Left Ventricle Dilation in KO Mice Study Design – Cardiac and skeletal muscle FXN knockout mice (MCK-CRE) were treated at 10 mg/kg every other day at Jackson Laboratories (Bar Harbor, ME). Echocardiograms were performed pre-dose and post dose. Left ventricular (LV) volume increases in systole in untreated mice by 8 weeks (after 4 weeks of dosing with vehicle), but remains similar to wildtype when treated with nomlabofusp (10 mg/kg every other day) Diameter (mm) Age in Weeks Age in Weeks Volume (μL) KO: CTI-1601 Wild-type: Vehicle KO: Vehicle Left Ventricle Internal Diameter (Systole) Left Ventricle Volume (Systole) Nomlabofusp-treated mice have similar LV volume as wild type; echocardiogram shows significant differences between vehicle and nomlabofusp treated (10 mg/kg every other day) KO mice
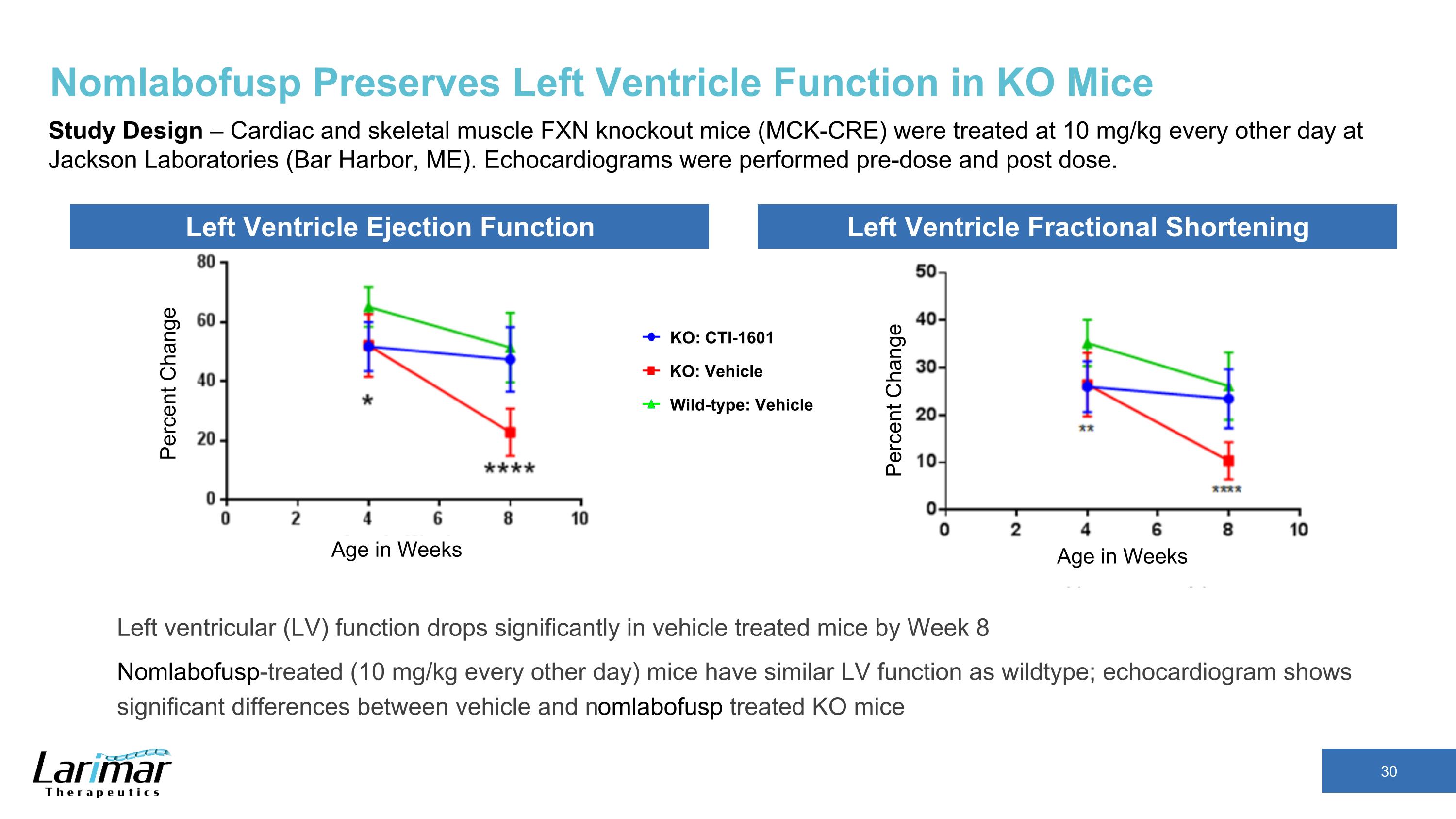
Nomlabofusp Preserves Left Ventricle Function in KO Mice Study Design – Cardiac and skeletal muscle FXN knockout mice (MCK-CRE) were treated at 10 mg/kg every other day at Jackson Laboratories (Bar Harbor, ME). Echocardiograms were performed pre-dose and post dose. Percent Change Age in Weeks Left Ventricle Ejection Function Left Ventricle Fractional Shortening Percent Change Age in Weeks KO: CTI-1601 Wild-type: Vehicle KO: Vehicle Left ventricular (LV) function drops significantly in vehicle treated mice by Week 8 Nomlabofusp-treated (10 mg/kg every other day) mice have similar LV function as wildtype; echocardiogram shows significant differences between vehicle and nomlabofusp treated KO mice

Phase 1 Clinical Data
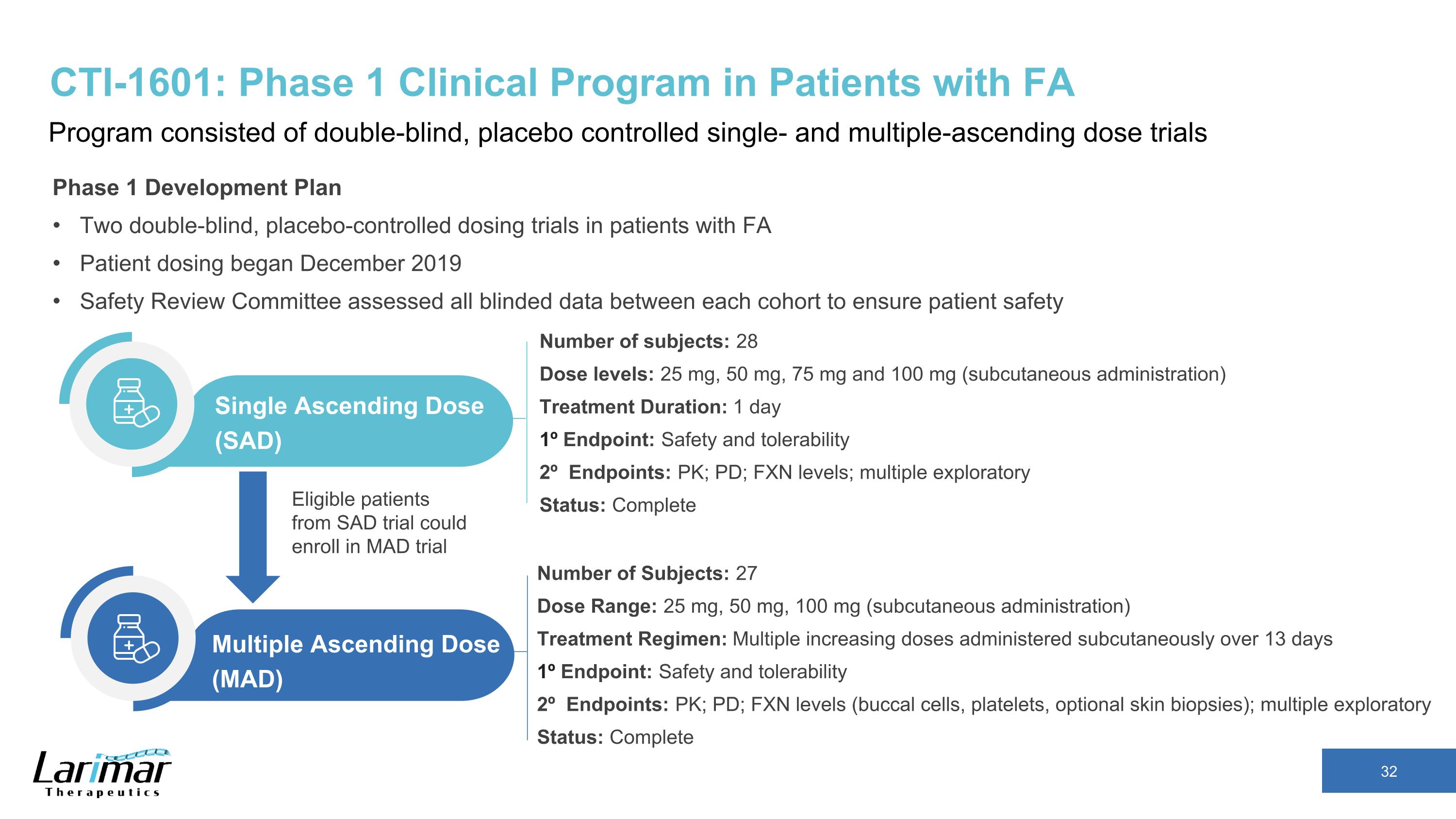
CTI-1601: Phase 1 Clinical Program in Patients with FA Program consisted of double-blind, placebo controlled single- and multiple-ascending dose trials Phase 1 Development Plan Two double-blind, placebo-controlled dosing trials in patients with FA Patient dosing began December 2019 Safety Review Committee assessed all blinded data between each cohort to ensure patient safety Number of subjects: 28 Dose levels: 25 mg, 50 mg, 75 mg and 100 mg (subcutaneous administration) Treatment Duration: 1 day 1º Endpoint: Safety and tolerability 2º Endpoints: PK; PD; FXN levels; multiple exploratory Status: Complete Single Ascending Dose (SAD) Number of Subjects: 27 Dose Range: 25 mg, 50 mg, 100 mg (subcutaneous administration) Treatment Regimen: Multiple increasing doses administered subcutaneously over 13 days 1º Endpoint: Safety and tolerability 2º Endpoints: PK; PD; FXN levels (buccal cells, platelets, optional skin biopsies); multiple exploratory Status: Complete Multiple Ascending Dose (MAD) Eligible patients from SAD trial could enroll in MAD trial
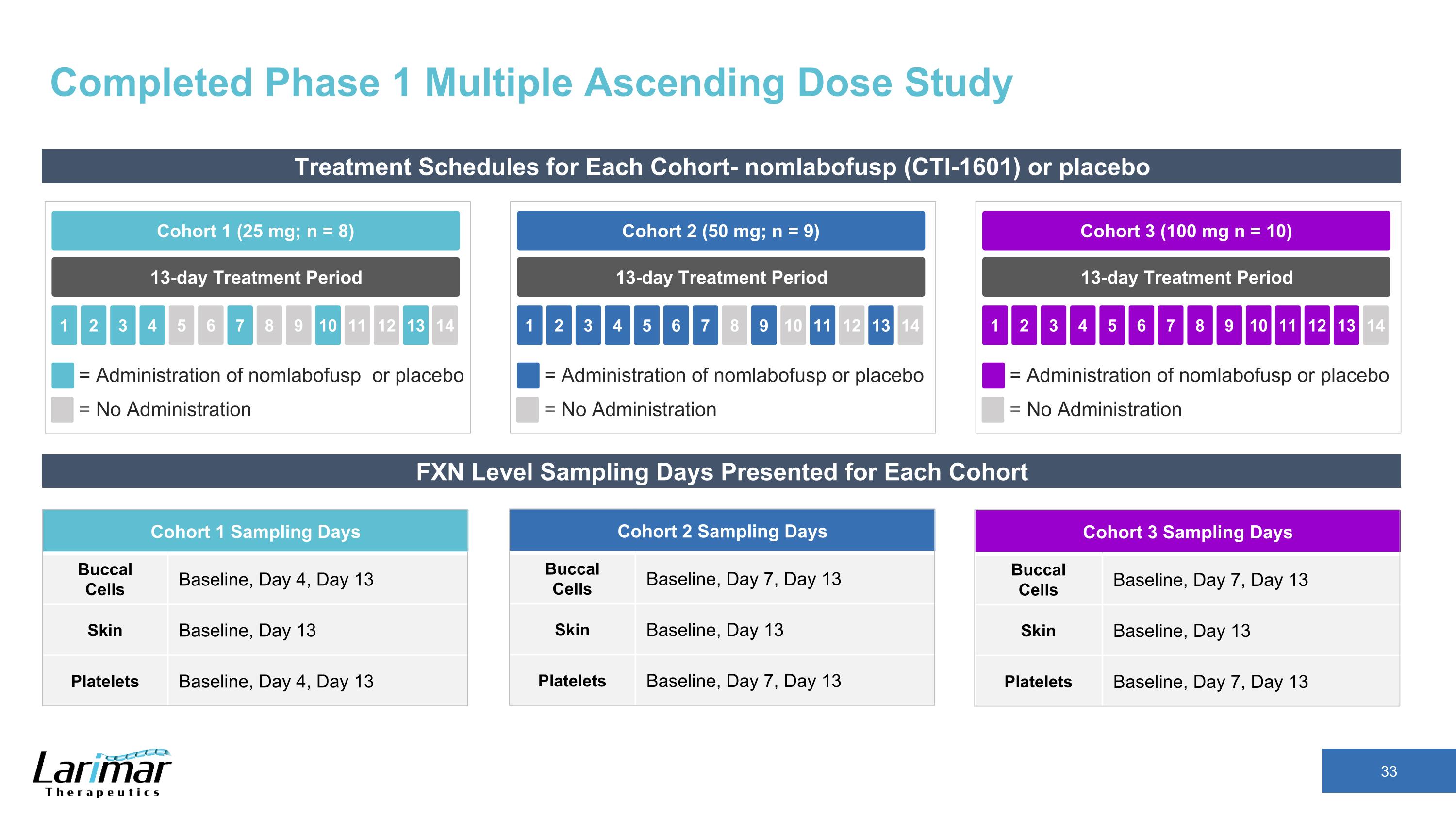
Completed Phase 1 Multiple Ascending Dose Study Treatment Schedules for Each Cohort- nomlabofusp (CTI-1601) or placebo 13-day Treatment Period Cohort 2 (50 mg; n = 9) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of nomlabofusp or placebo = No Administration 13-day Treatment Period Cohort 1 (25 mg; n = 8) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of nomlabofusp or placebo = No Administration 13-day Treatment Period Cohort 3 (100 mg n = 10) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of nomlabofusp or placebo = No Administration FXN Level Sampling Days Presented for Each Cohort Cohort 1 Sampling Days Buccal Cells Baseline, Day 4, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 4, Day 13 Cohort 2 Sampling Days Buccal Cells Baseline, Day 7, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 7, Day 13 Cohort 3 Sampling Days Buccal Cells Baseline, Day 7, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 7, Day 13
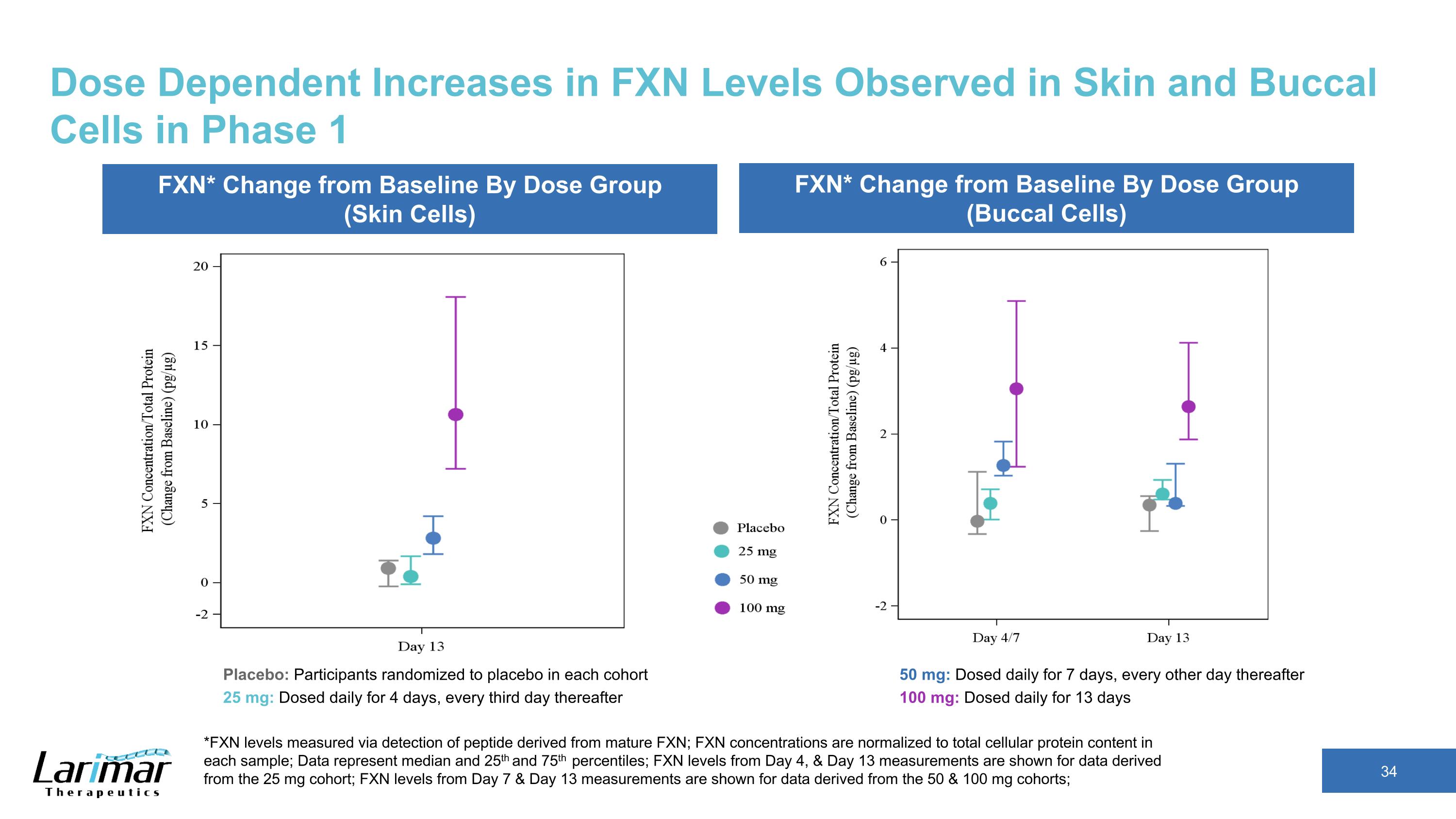
Dose Dependent Increases in FXN Levels Observed in Skin and Buccal Cells in Phase 1 *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; Data represent median and 25th and 75th percentiles; FXN levels from Day 4, & Day 13 measurements are shown for data derived from the 25 mg cohort; FXN levels from Day 7 & Day 13 measurements are shown for data derived from the 50 & 100 mg cohorts; FXN* Change from Baseline By Dose Group (Skin Cells) FXN* Change from Baseline By Dose Group (Buccal Cells) Placebo: Participants randomized to placebo in each cohort 25 mg: Dosed daily for 4 days, every third day thereafter 50 mg: Dosed daily for 7 days, every other day thereafter 100 mg: Dosed daily for 13 days
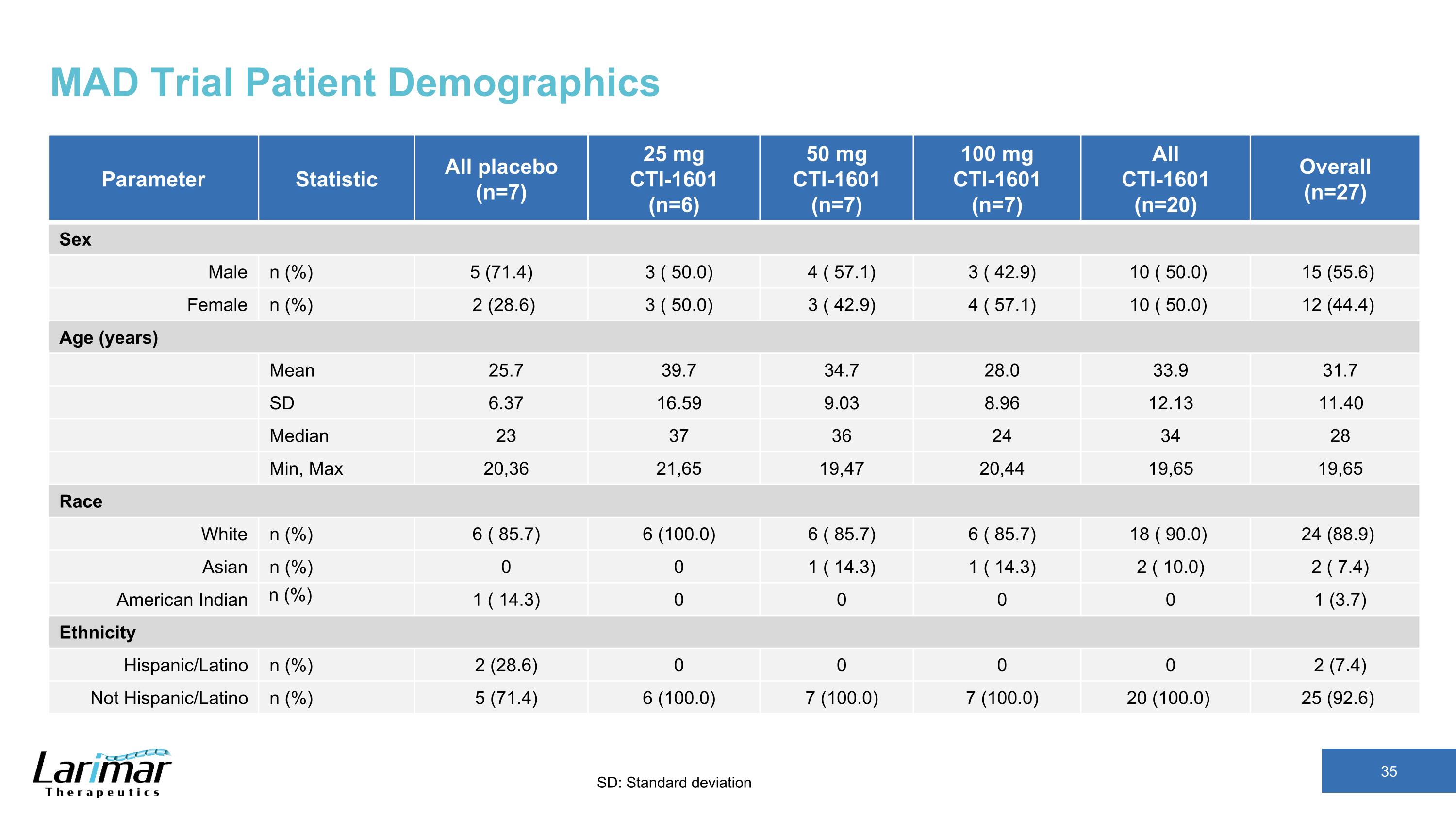
MAD Trial Patient Demographics Parameter Statistic All placebo (n=7) 25 mg CTI-1601 (n=6) 50 mg CTI-1601 (n=7) 100 mg CTI-1601 (n=7) All CTI-1601 (n=20) Overall (n=27) Sex Male n (%) 5 (71.4) 3 ( 50.0) 4 ( 57.1) 3 ( 42.9) 10 ( 50.0) 15 (55.6) Female n (%) 2 (28.6) 3 ( 50.0) 3 ( 42.9) 4 ( 57.1) 10 ( 50.0) 12 (44.4) Age (years) Mean 25.7 39.7 34.7 28.0 33.9 31.7 SD 6.37 16.59 9.03 8.96 12.13 11.40 Median 23 37 36 24 34 28 Min, Max 20,36 21,65 19,47 20,44 19,65 19,65 Race White n (%) 6 ( 85.7) 6 (100.0) 6 ( 85.7) 6 ( 85.7) 18 ( 90.0) 24 (88.9) Asian n (%) 0 0 1 ( 14.3) 1 ( 14.3) 2 ( 10.0) 2 ( 7.4) American Indian n (%) 1 ( 14.3) 0 0 0 0 1 (3.7) Ethnicity Hispanic/Latino n (%) 2 (28.6) 0 0 0 0 2 (7.4) Not Hispanic/Latino n (%) 5 (71.4) 6 (100.0) 7 (100.0) 7 (100.0) 20 (100.0) 25 (92.6) SD: Standard deviation
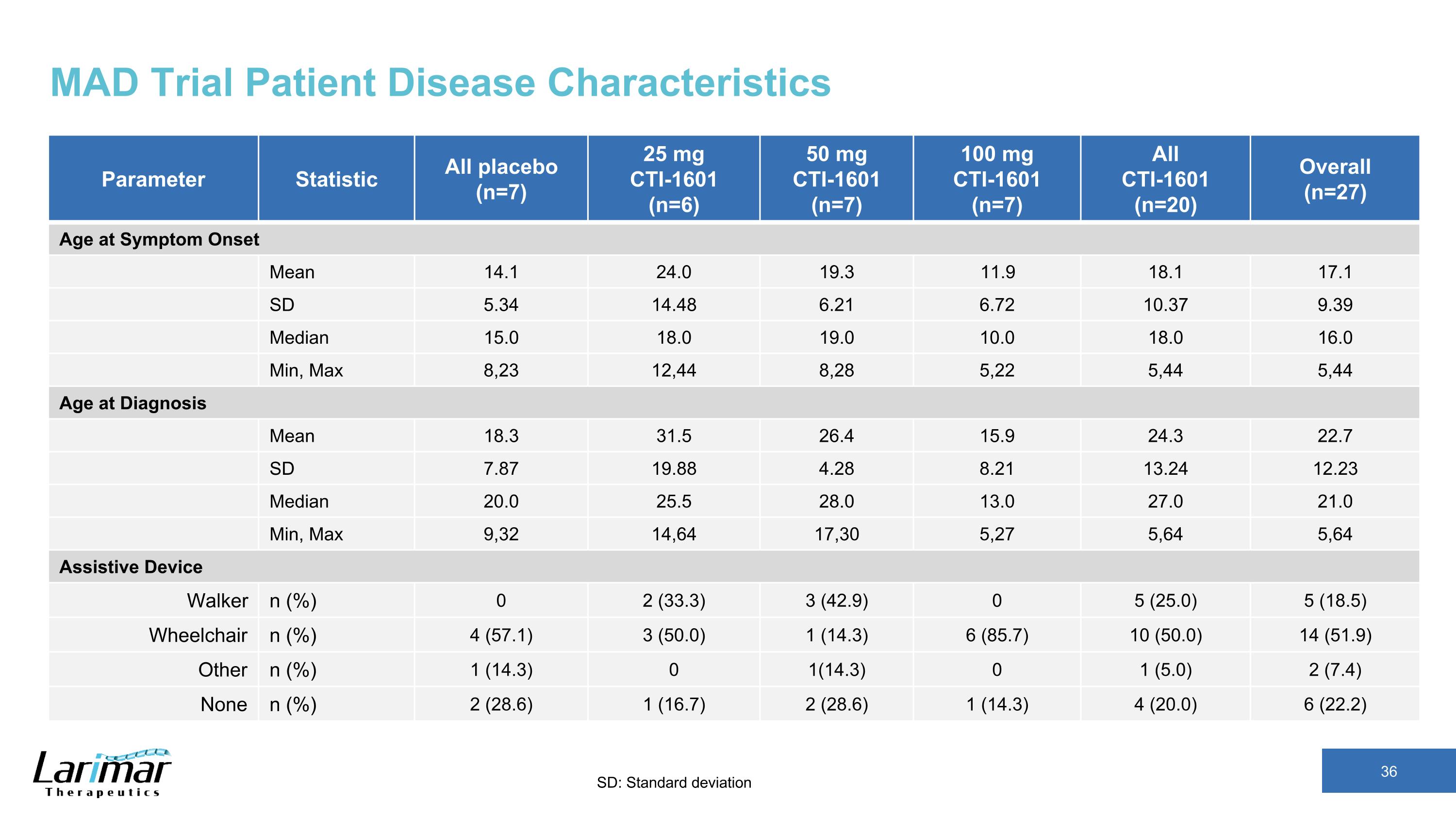
MAD Trial Patient Disease Characteristics Parameter Statistic All placebo (n=7) 25 mg CTI-1601 (n=6) 50 mg CTI-1601 (n=7) 100 mg CTI-1601 (n=7) All CTI-1601 (n=20) Overall (n=27) Age at Symptom Onset Mean 14.1 24.0 19.3 11.9 18.1 17.1 SD 5.34 14.48 6.21 6.72 10.37 9.39 Median 15.0 18.0 19.0 10.0 18.0 16.0 Min, Max 8,23 12,44 8,28 5,22 5,44 5,44 Age at Diagnosis Mean 18.3 31.5 26.4 15.9 24.3 22.7 SD 7.87 19.88 4.28 8.21 13.24 12.23 Median 20.0 25.5 28.0 13.0 27.0 21.0 Min, Max 9,32 14,64 17,30 5,27 5,64 5,64 Assistive Device Walker n (%) 0 2 (33.3) 3 (42.9) 0 5 (25.0) 5 (18.5) Wheelchair n (%) 4 (57.1) 3 (50.0) 1 (14.3) 6 (85.7) 10 (50.0) 14 (51.9) Other n (%) 1 (14.3) 0 1(14.3) 0 1 (5.0) 2 (7.4) None n (%) 2 (28.6) 1 (16.7) 2 (28.6) 1 (14.3) 4 (20.0) 6 (22.2) SD: Standard deviation
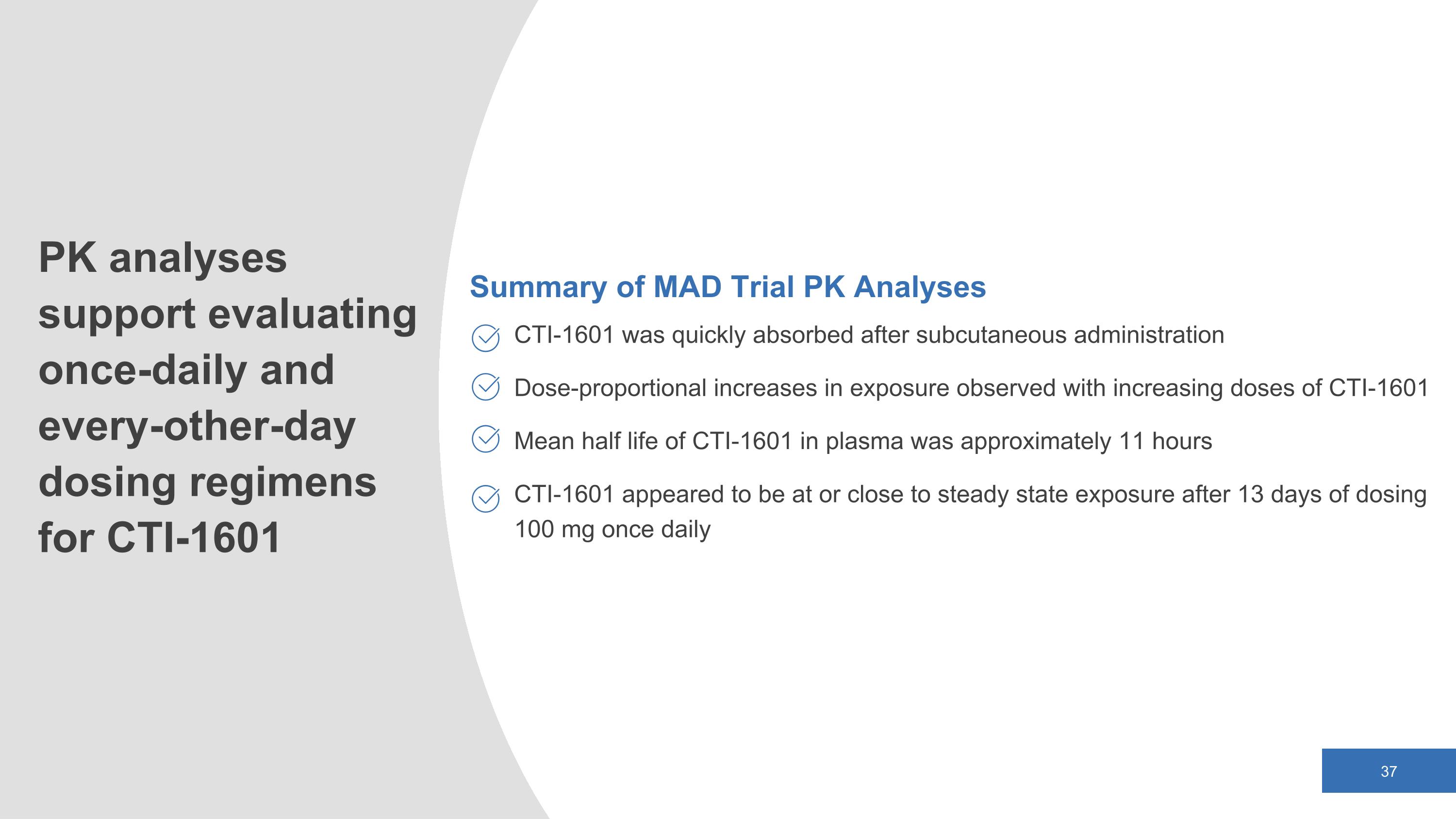
Summary of MAD Trial PK Analyses CTI-1601 was quickly absorbed after subcutaneous administration Dose-proportional increases in exposure observed with increasing doses of CTI-1601 Mean half life of CTI-1601 in plasma was approximately 11 hours CTI-1601 appeared to be at or close to steady state exposure after 13 days of dosing 100 mg once daily PK analyses support evaluating once-daily and every-other-day dosing regimens for CTI-1601

Phase 2 Dose Exploration Data
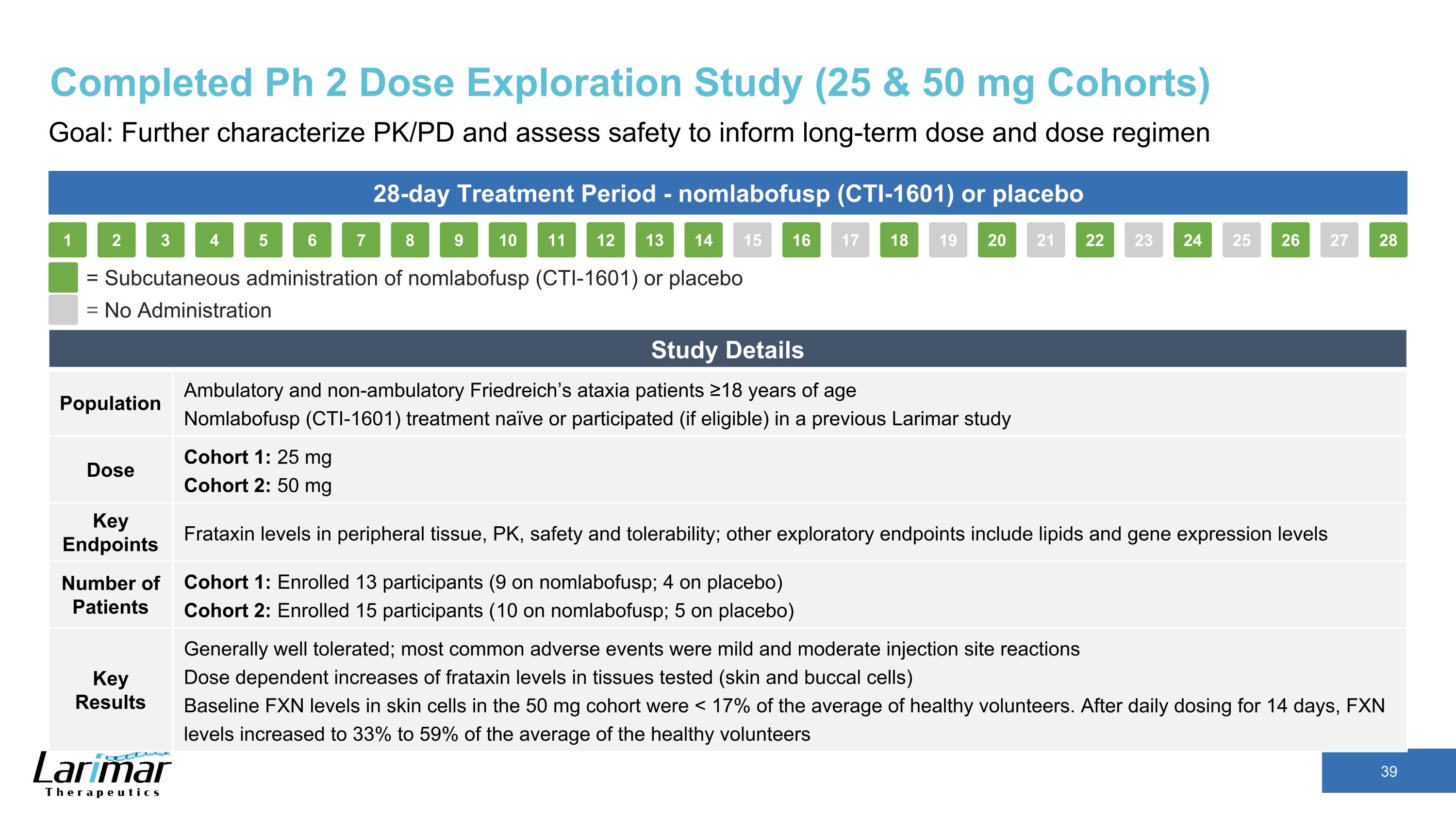
Completed Ph 2 Dose Exploration Study (25 & 50 mg Cohorts) Goal: Further characterize PK/PD and assess safety to inform long-term dose and dose regimen 28-day Treatment Period - nomlabofusp (CTI-1601) or placebo 16 17 18 19 15 20 21 22 23 24 25 26 27 28 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Subcutaneous administration of nomlabofusp (CTI-1601) or placebo = No Administration Study Details Population Ambulatory and non-ambulatory Friedreich’s ataxia patients ≥18 years of age Nomlabofusp (CTI-1601) treatment naïve or participated (if eligible) in a previous Larimar study Dose Cohort 1: 25 mg Cohort 2: 50 mg Key Endpoints Frataxin levels in peripheral tissue, PK, safety and tolerability; other exploratory endpoints include lipids and gene expression levels Number of Patients Cohort 1: Enrolled 13 participants (9 on nomlabofusp; 4 on placebo) Cohort 2: Enrolled 15 participants (10 on nomlabofusp; 5 on placebo) Key Results Generally well tolerated; most common adverse events were mild and moderate injection site reactions Dose dependent increases of frataxin levels in tissues tested (skin and buccal cells) Baseline FXN levels in skin cells in the 50 mg cohort were < 17% of the average of healthy volunteers. After daily dosing for 14 days, FXN levels increased to 33% to 59% of the average of the healthy volunteers
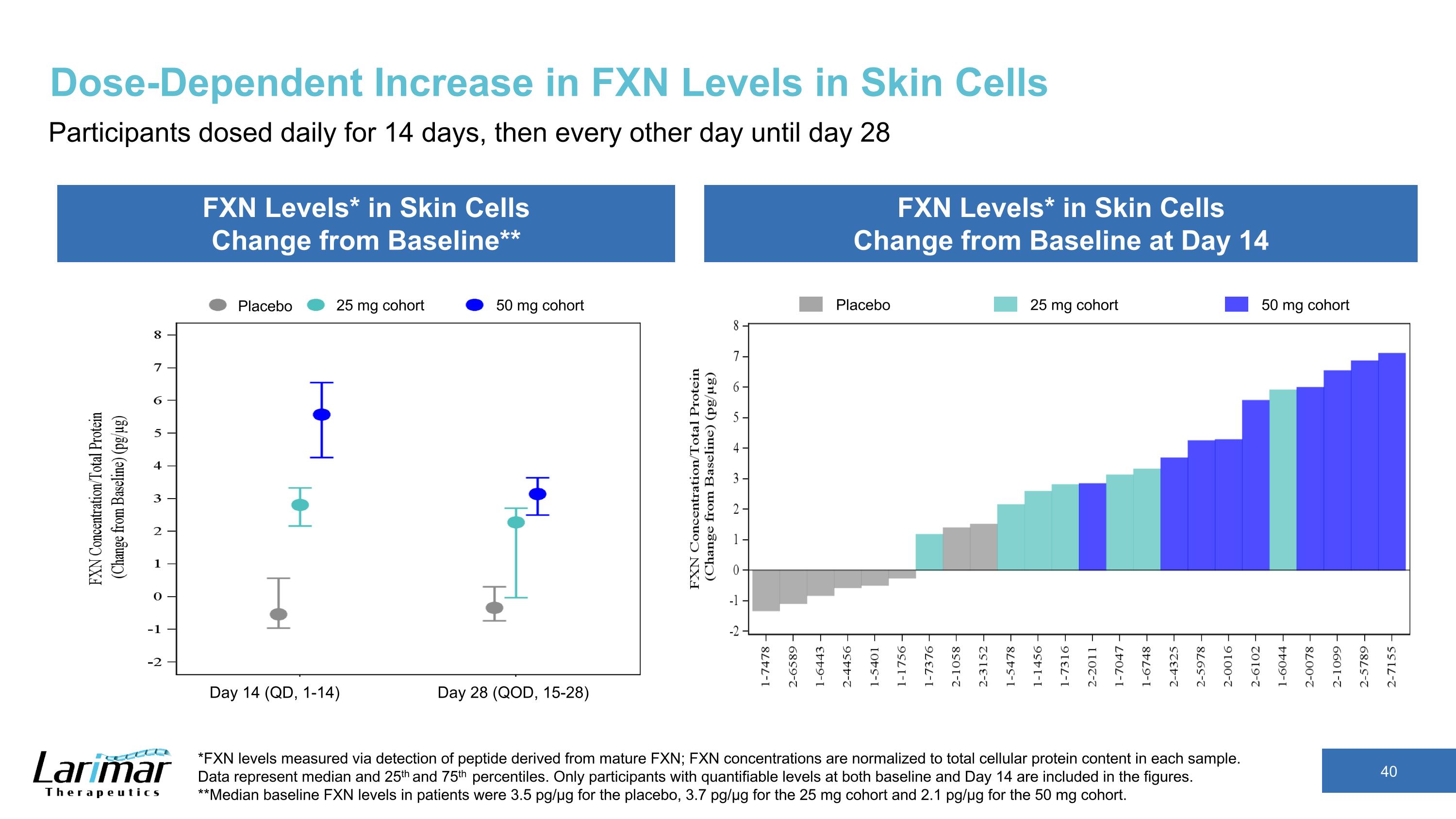
Dose-Dependent Increase in FXN Levels in Skin Cells Participants dosed daily for 14 days, then every other day until day 28 FXN Levels* in Skin Cells Change from Baseline** FXN Levels* in Skin Cells Change from Baseline at Day 14 *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample. Data represent median and 25th and 75th percentiles. Only participants with quantifiable levels at both baseline and Day 14 are included in the figures. **Median baseline FXN levels in patients were 3.5 pg/µg for the placebo, 3.7 pg/µg for the 25 mg cohort and 2.1 pg/µg for the 50 mg cohort. Placebo 25 mg cohort 50 mg cohort Placebo 25 mg cohort 50 mg cohort Day 14 (QD, 1-14) Day 28 (QOD, 15-28)
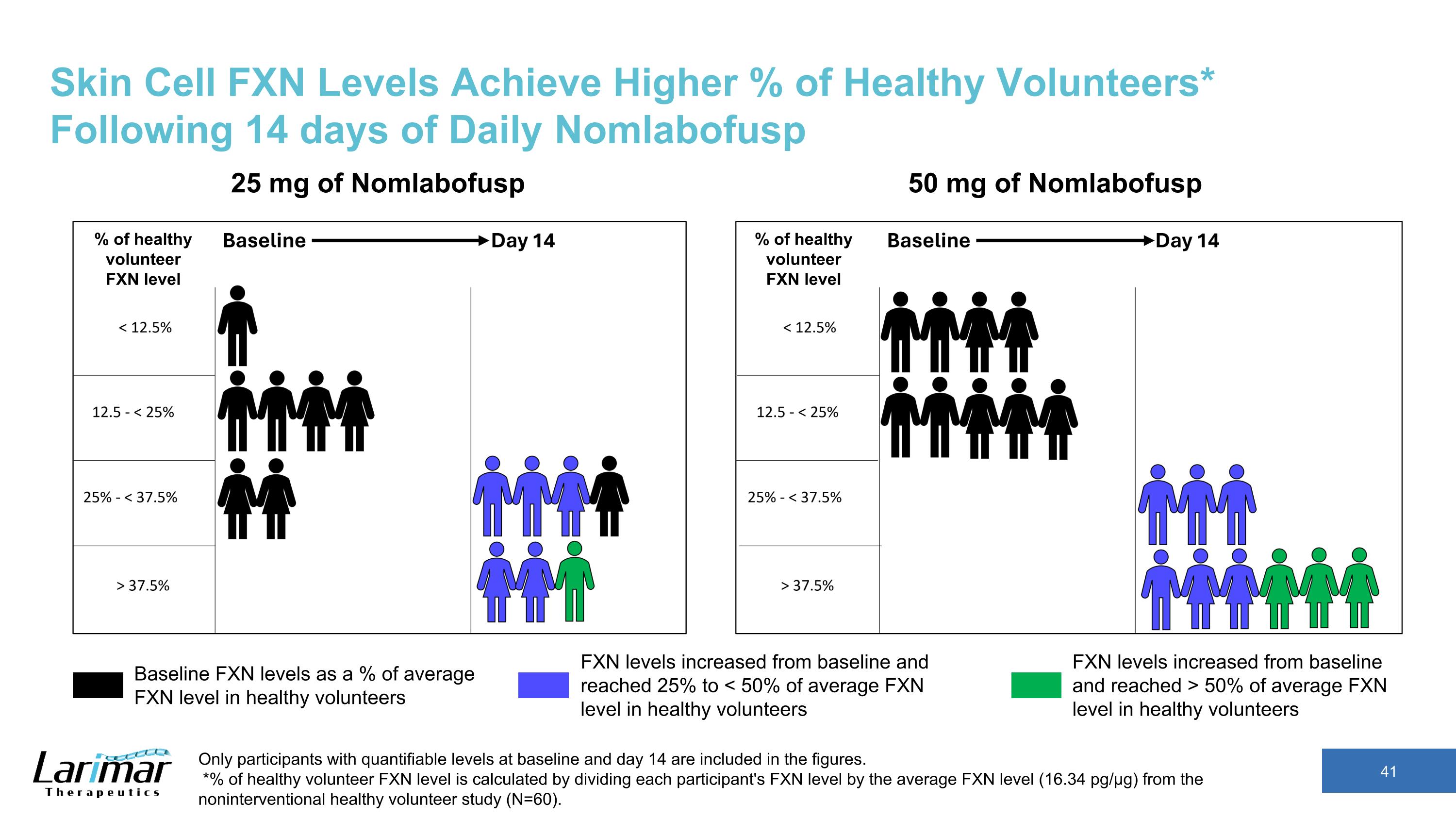
Skin Cell FXN Levels Achieve Higher % of Healthy Volunteers* Following 14 days of Daily Nomlabofusp Only participants with quantifiable levels at baseline and day 14 are included in the figures. *% of healthy volunteer FXN level is calculated by dividing each participant's FXN level by the average FXN level (16.34 pg/µg) from the noninterventional healthy volunteer study (N=60). 25 mg of Nomlabofusp 50 mg of Nomlabofusp Baseline FXN levels as a % of average FXN level in healthy volunteers FXN levels increased from baseline and reached > 50% of average FXN level in healthy volunteers FXN levels increased from baseline and reached 25% to < 50% of average FXN level in healthy volunteers % of healthy volunteer FXN level % of healthy volunteer FXN level
Nomlabofusp PK Profile Consistent Across Studies Rapid absorption after subcutaneous administration Steady state reached by Day 30 at both the 25 mg and 50 mg doses with no further accumulation Pharmacokinetic profile consistent with Phase 1 and Phase 2 studies Long-term PK Profile Consistent with Phase 1 and Phase 2 Studies Adolescents 12 to 17 years of age received a weight-based equivalent of 50 mg for 7 days Exposure and PK in 9 adolescents 12 to 17 years of age on nomlabofusp was similar to adults on 50 mg of nomlabofusp Adolescent PK Profile Consistent with Adult
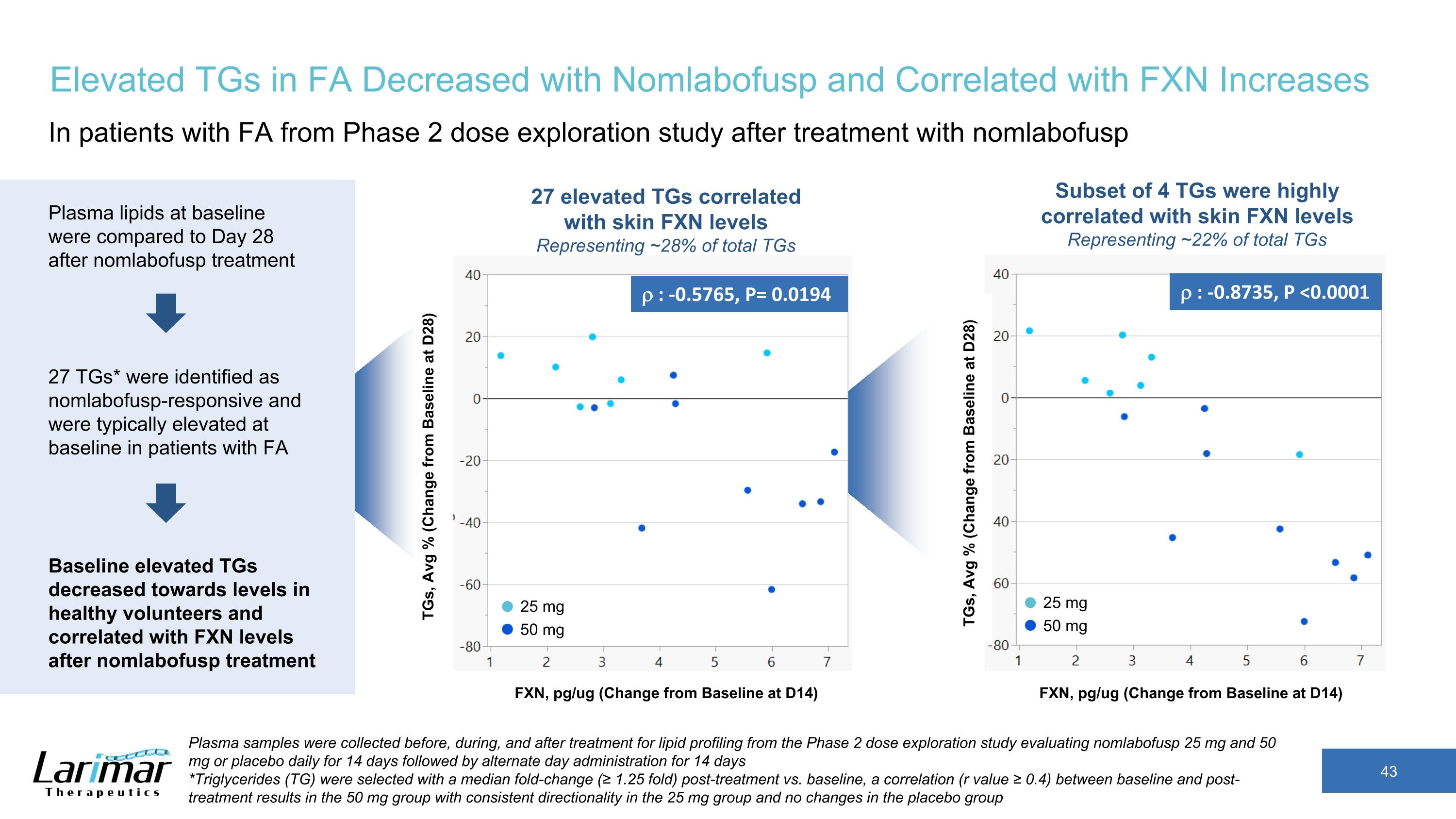
Elevated TGs in FA Decreased with Nomlabofusp and Correlated with FXN Increases In patients with FA from Phase 2 dose exploration study after treatment with nomlabofusp r : -0.8735, P <0.0001 r : -0.5765, P= 0.0194 TGs, Avg % (Change from Baseline at D28) FXN, pg/ug (Change from Baseline at D14) 25 mg 50 mg 25 mg 50 mg 27 elevated TGs correlated with skin FXN levels Representing ~28% of total TGs Subset of 4 TGs were highly correlated with skin FXN levels Representing ~22% of total TGs FXN, pg/ug (Change from Baseline at D14) Plasma lipids at baseline were compared to Day 28 after nomlabofusp treatment Baseline elevated TGs decreased towards levels in healthy volunteers and correlated with FXN levels after nomlabofusp treatment 27 TGs* were identified as nomlabofusp-responsive and were typically elevated at baseline in patients with FA TGs, Avg % (Change from Baseline at D28) Plasma samples were collected before, during, and after treatment for lipid profiling from the Phase 2 dose exploration study evaluating nomlabofusp 25 mg and 50 mg or placebo daily for 14 days followed by alternate day administration for 14 days *Triglycerides (TG) were selected with a median fold-change (≥ 1.25 fold) post-treatment vs. baseline, a correlation (r value ≥ 0.4) between baseline and post-treatment results in the 50 mg group with consistent directionality in the 25 mg group and no changes in the placebo group
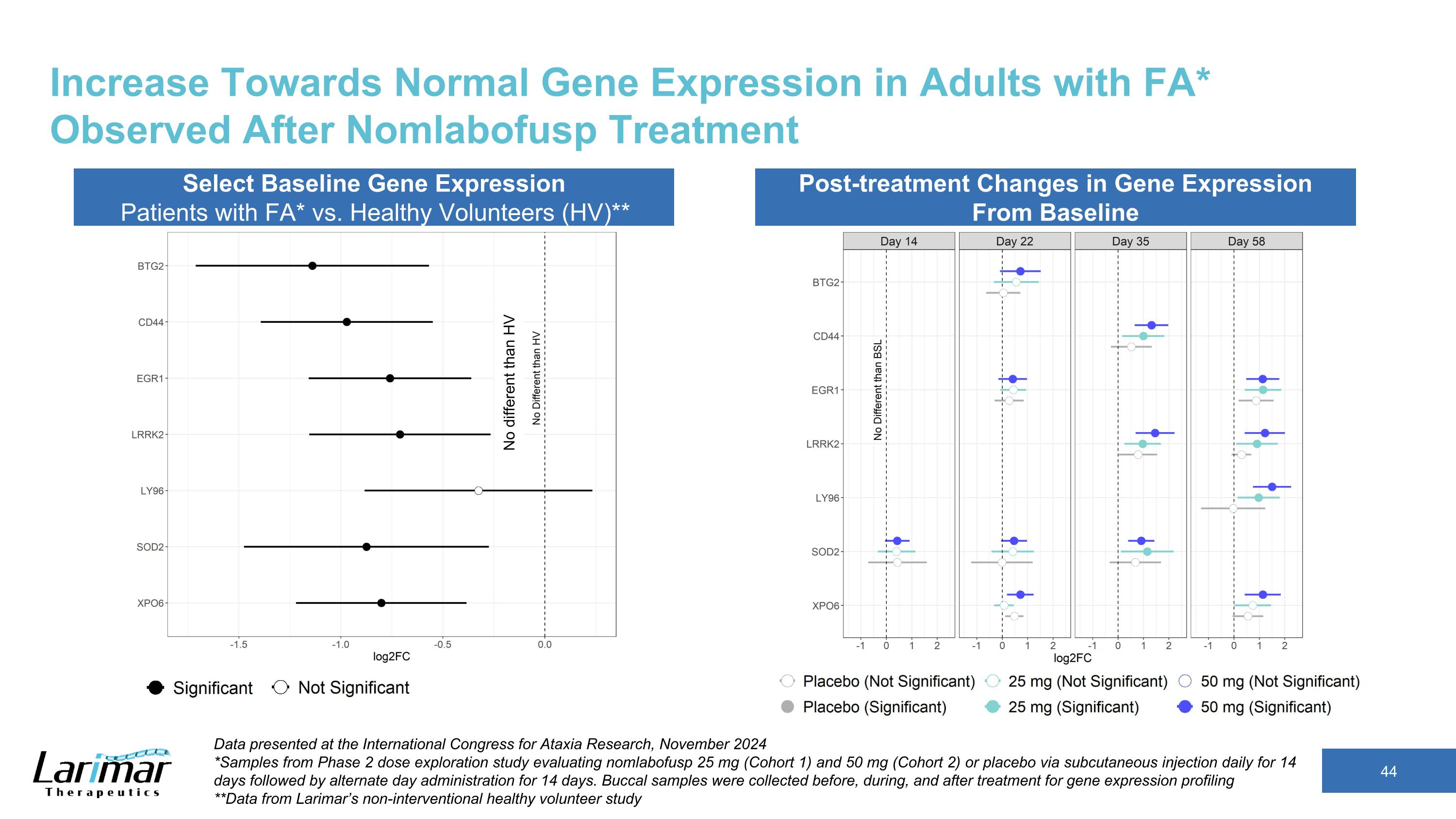
Increase Towards Normal Gene Expression in Adults with FA* Observed After Nomlabofusp Treatment Select Baseline Gene Expression Patients with FA* vs. Healthy Volunteers (HV)** Post-treatment Changes in Gene Expression From Baseline Data presented at the International Congress for Ataxia Research, November 2024 *Samples from Phase 2 dose exploration study evaluating nomlabofusp 25 mg (Cohort 1) and 50 mg (Cohort 2) or placebo via subcutaneous injection daily for 14 days followed by alternate day administration for 14 days. Buccal samples were collected before, during, and after treatment for gene expression profiling **Data from Larimar’s non-interventional healthy volunteer study No different than HV

Additional Phase 1 and 2 Data Presented at the International Congress for Ataxia Research, November 2024
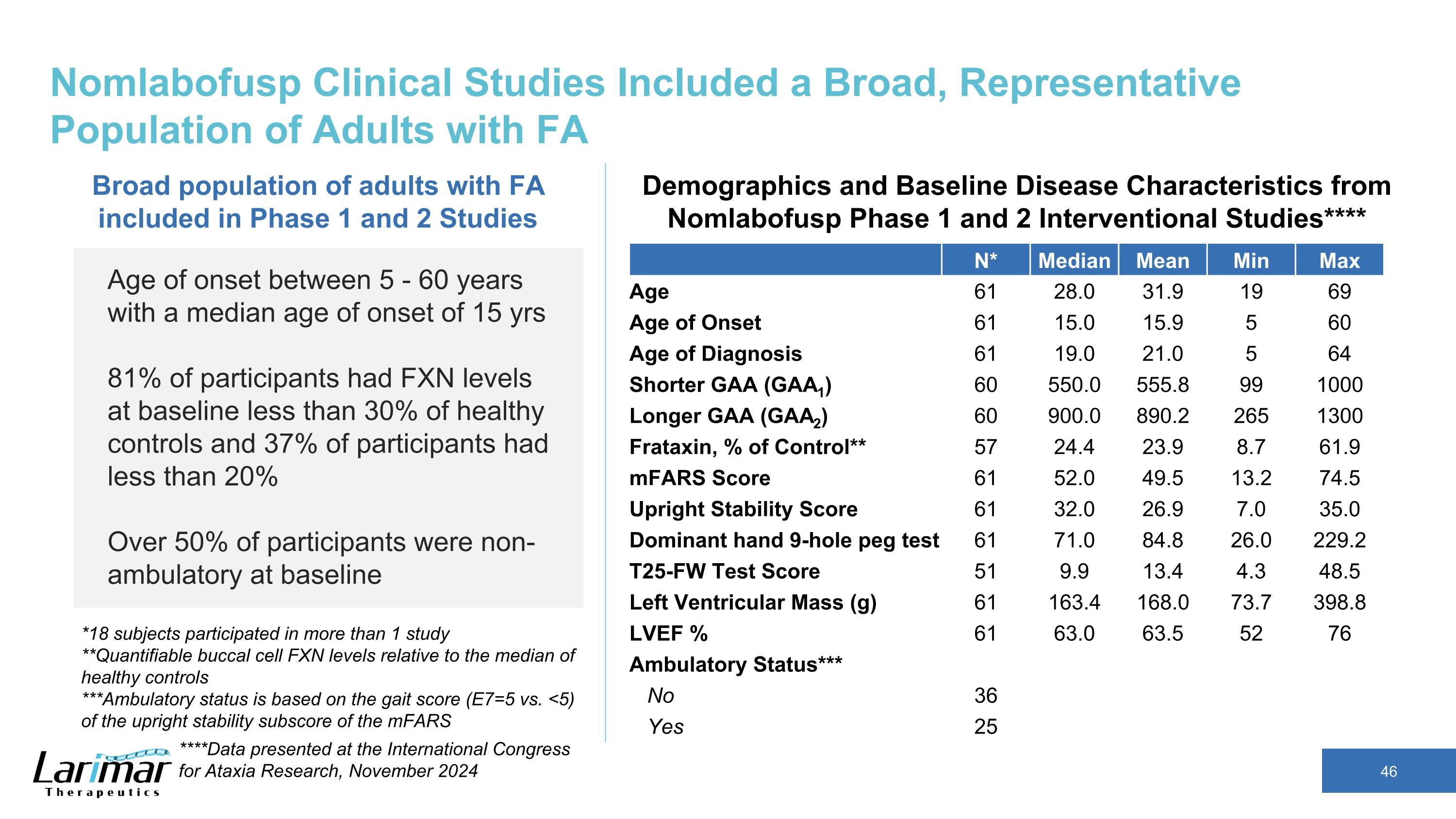
Nomlabofusp Clinical Studies Included a Broad, Representative Population of Adults with FA N* Median Mean Min Max Age 61 28.0 31.9 19 69 Age of Onset 61 15.0 15.9 5 60 Age of Diagnosis 61 19.0 21.0 5 64 Shorter GAA (GAA1) 60 550.0 555.8 99 1000 Longer GAA (GAA2) 60 900.0 890.2 265 1300 Frataxin, % of Control** 57 24.4 23.9 8.7 61.9 mFARS Score 61 52.0 49.5 13.2 74.5 Upright Stability Score 61 32.0 26.9 7.0 35.0 Dominant hand 9-hole peg test 61 71.0 84.8 26.0 229.2 T25-FW Test Score 51 9.9 13.4 4.3 48.5 Left Ventricular Mass (g) 61 163.4 168.0 73.7 398.8 LVEF % 61 63.0 63.5 52 76 Ambulatory Status*** No 36 Yes 25 Age of onset between 5 - 60 years with a median age of onset of 15 yrs 81% of participants had FXN levels at baseline less than 30% of healthy controls and 37% of participants had less than 20% Over 50% of participants were non-ambulatory at baseline Broad population of adults with FA included in Phase 1 and 2 Studies Demographics and Baseline Disease Characteristics from Nomlabofusp Phase 1 and 2 Interventional Studies**** *18 subjects participated in more than 1 study **Quantifiable buccal cell FXN levels relative to the median of healthy controls ***Ambulatory status is based on the gait score (E7=5 vs. <5) of the upright stability subscore of the mFARS ****Data presented at the International Congress for Ataxia Research, November 2024
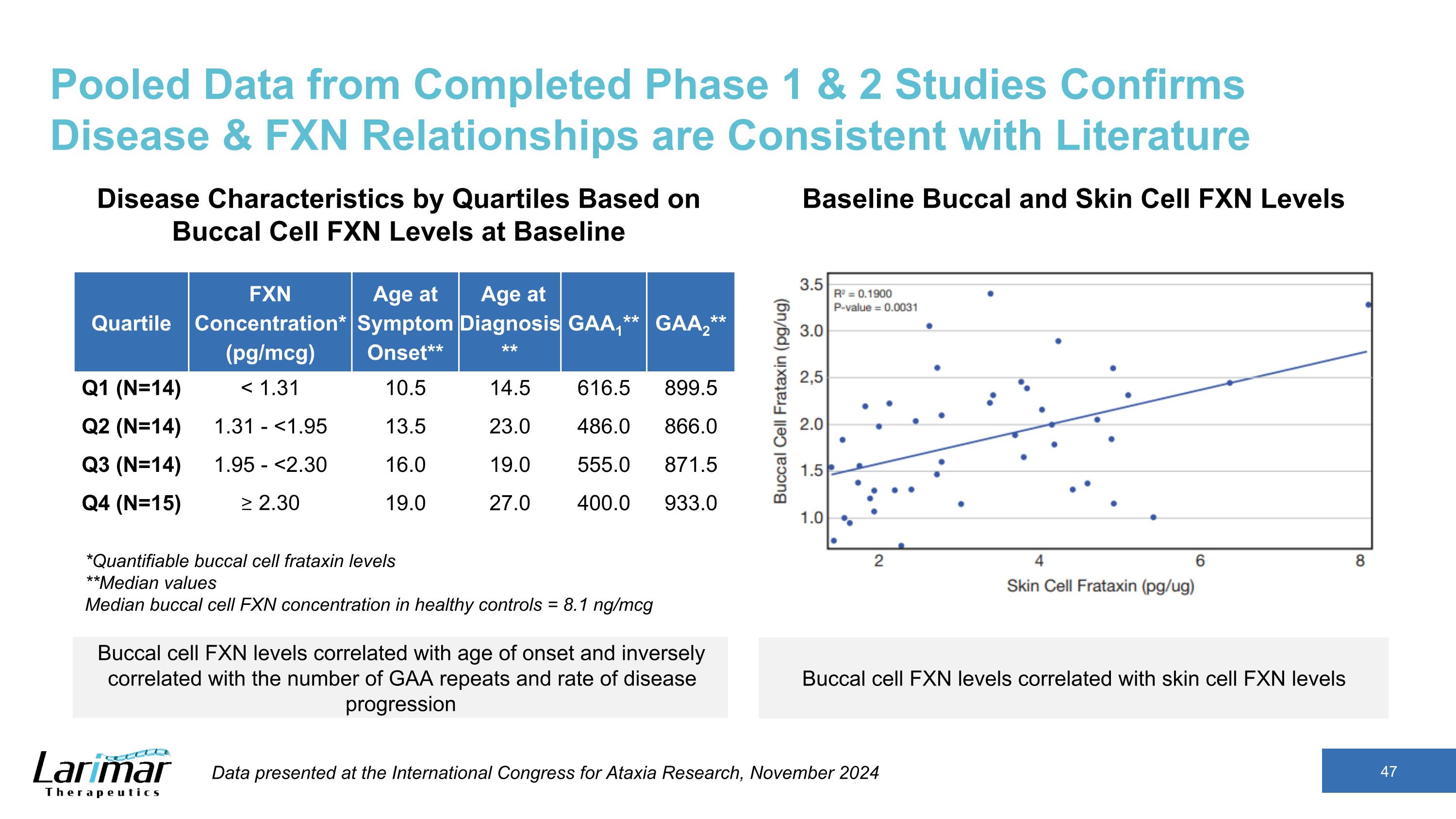
Pooled Data from Completed Phase 1 & 2 Studies Confirms Disease & FXN Relationships are Consistent with Literature Quartile FXN Concentration* (pg/mcg) Age at Symptom Onset** Age at Diagnosis** GAA1** GAA2** Q1 (N=14) < 1.31 10.5 14.5 616.5 899.5 Q2 (N=14) 1.31 - <1.95 13.5 23.0 486.0 866.0 Q3 (N=14) 1.95 - <2.30 16.0 19.0 555.0 871.5 Q4 (N=15) ≥ 2.30 19.0 27.0 400.0 933.0 *Quantifiable buccal cell frataxin levels **Median values Median buccal cell FXN concentration in healthy controls = 8.1 ng/mcg Disease Characteristics by Quartiles Based on Buccal Cell FXN Levels at Baseline Baseline Buccal and Skin Cell FXN Levels Buccal cell FXN levels correlated with age of onset and inversely correlated with the number of GAA repeats and rate of disease progression Buccal cell FXN levels correlated with skin cell FXN levels Data presented at the International Congress for Ataxia Research, November 2024
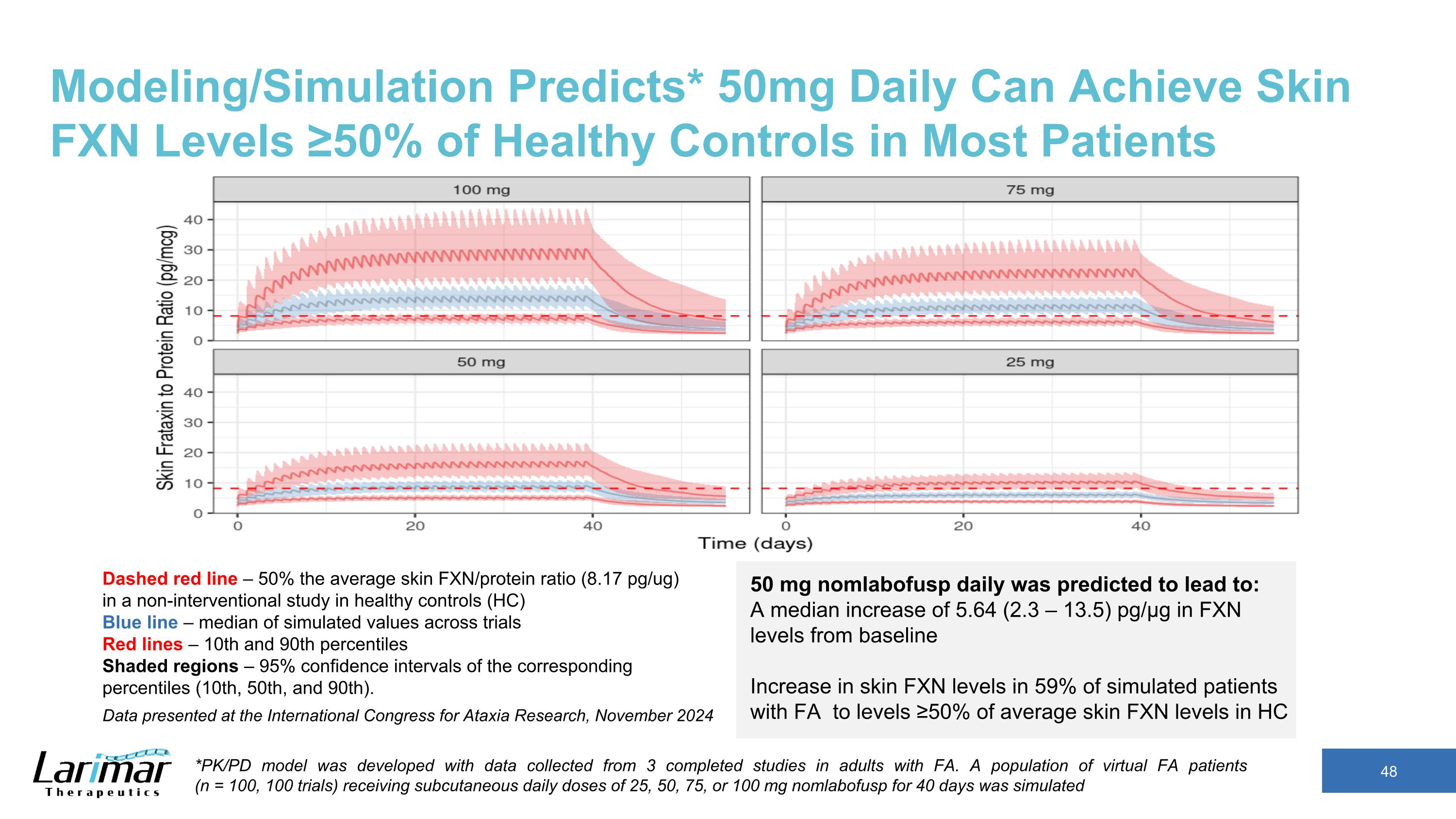
Modeling/Simulation Predicts* 50mg Daily Can Achieve Skin FXN Levels ≥50% of Healthy Controls in Most Patients Dashed red line – 50% the average skin FXN/protein ratio (8.17 pg/ug) in a non-interventional study in healthy controls (HC) Blue line – median of simulated values across trials Red lines – 10th and 90th percentiles Shaded regions – 95% confidence intervals of the corresponding percentiles (10th, 50th, and 90th). 50 mg nomlabofusp daily was predicted to lead to: A median increase of 5.64 (2.3 – 13.5) pg/µg in FXN levels from baseline Increase in skin FXN levels in 59% of simulated patients with FA to levels ≥50% of average skin FXN levels in HC *PK/PD model was developed with data collected from 3 completed studies in adults with FA. A population of virtual FA patients (n = 100, 100 trials) receiving subcutaneous daily doses of 25, 50, 75, or 100 mg nomlabofusp for 40 days was simulated Data presented at the International Congress for Ataxia Research, November 2024

Non-Interventional Study Data
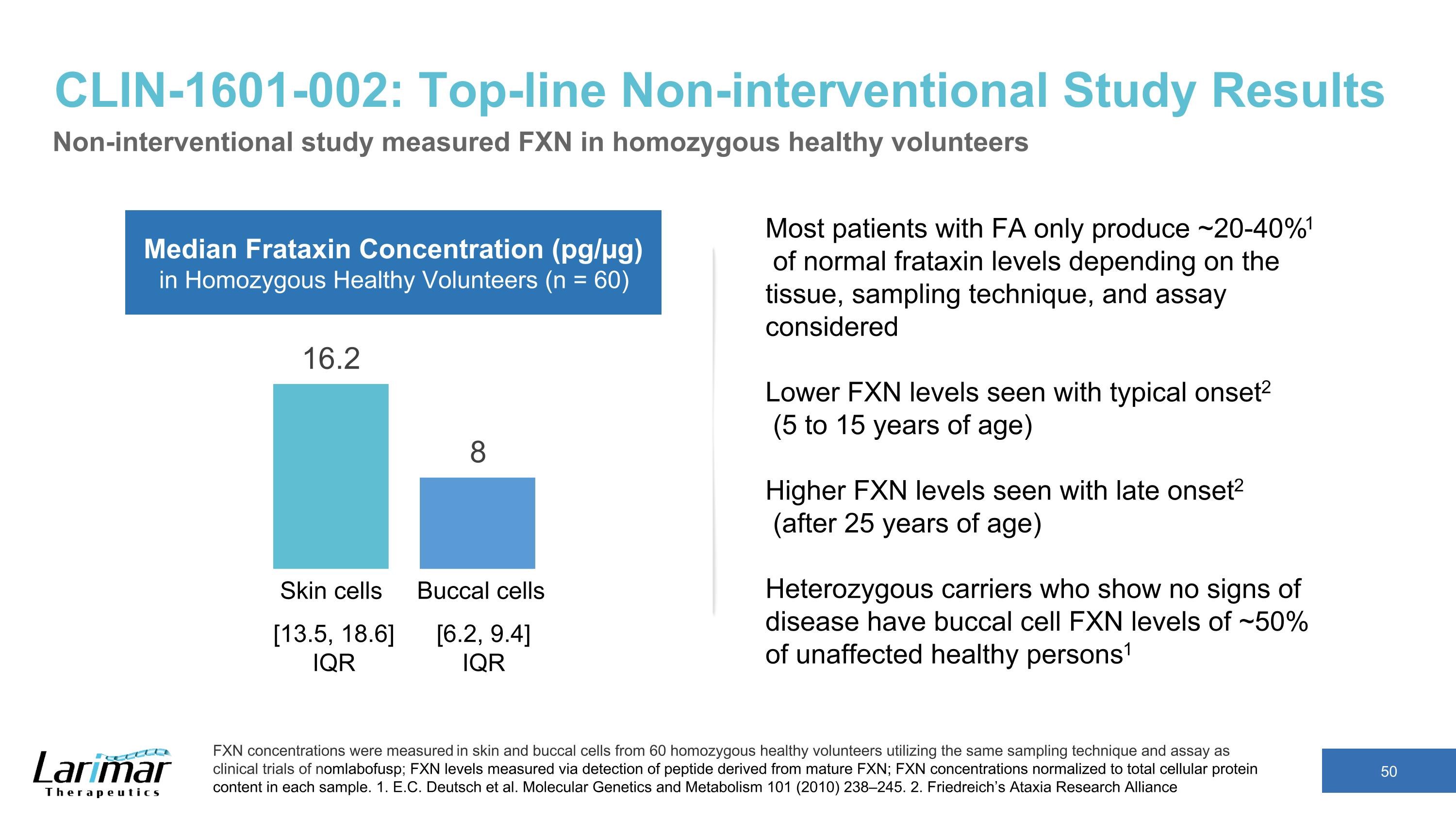
CLIN-1601-002: Top-line Non-interventional Study Results Non-interventional study measured FXN in homozygous healthy volunteers FXN concentrations were measured in skin and buccal cells from 60 homozygous healthy volunteers utilizing the same sampling technique and assay as clinical trials of nomlabofusp; FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations normalized to total cellular protein content in each sample. 1. E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245. 2. Friedreich’s Ataxia Research Alliance Skin cells Buccal cells Median Frataxin Concentration (pg/µg) in Homozygous Healthy Volunteers (n = 60) Most patients with FA only produce ~20-40%1 of normal frataxin levels depending on the tissue, sampling technique, and assay considered Lower FXN levels seen with typical onset2 (5 to 15 years of age) Higher FXN levels seen with late onset2 (after 25 years of age) Heterozygous carriers who show no signs of disease have buccal cell FXN levels of ~50% of unaffected healthy persons1 [13.5, 18.6] IQR [6.2, 9.4] IQR

FDA START Pilot Program
START Pilot Program Continues to Expedite the Clinical and Regulatory Development of Nomlabofusp START Pilot Program Support for Clinical Trials Advancing Rare Disease Therapeutics 1 of 7 novel drugs development programs selected by FDA A new milestone-driven program launched by the FDA in September 2023 Designed to accelerate the development of novel therapies for rare diseases Sponsors selected can benefit from: more frequent and rapid ad-hoc FDA interactions help facilitating the development of programs to pre-BLA meeting stage guidance on generating high-quality and reliable data intended to support a BLA CDER Selection Based On Demonstrated development program readiness Potential to address serious and unmet medical need in a rare neurodegenerative condition Alignment of CMC development timelines with clinical development plans Proposed plan where enhanced communication can improve efficiency of product development FDA: Food and Drug Administration; CDER: Center for Drug Evaluation and Research; CMC: Chemistry, Manufacturing, and Controls

FARA

Strong Relationship with FARA – Joined FARA’s TRACK-FA Neuroimaging Consortium as an Industry Partner National, non-profit organization dedicated to the pursuit of scientific research leading to treatments and a cure for FA FARA provides industry with several key items Assistance with patient recruitment and education Access to Global Patient Registry with demographic and clinical information on more than 1,000 FA patients Sponsored a Patient-Focused Drug Development Meeting in 2017 resulting in a publication titled “The Voice of the Patient” TRACK-FA collects natural history data to establish disease specific neuroimaging biomarkers for potential use in clinical trials. Larimar will have access to all study data for use in regulatory filings, as appropriate