
Advancing medicines. Solving problems. Improving lives. September 2024 Advancing medicines. Solving problems. Improving lives. Advancing medicines. Solving problems. Improving lives. 1 Anaphylm (dibutepinephrine) Sublingual Film Complete Response Letter Supplemental Material February 2, 2026

© 2026 Aquestive Therapeutics, Inc. 2 ® This presentation has been prepared by Aquestive Therapeutics, Inc . (“Aquestive”, the “Company”, “our” or “us”) and contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “believe,” “anticipate,” “plan,” “expect,” “estimate,” “intend,” “may,” “will,” or the negative of those terms, and similar expressions, are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the advancement and related timing of our product candidate Anaphylm (dibutepinephrine) Sublingual Film through clinical development and approval by the FDA, including: whether our clinical and other data will be adequate enough to address the concerns raised by the FDA in the Complete Response Letter dated January 30, 2026 (CRL) provided to the Company and for the FDA to finally approve Anaphylm or whether the FDA may request further information from us, disagree with our findings or otherwise undertake a lengthy review of our resubmission, and challenges regarding the following commercial launch of Anaphylm, if approved by the FDA; the advancement and related timing of potential international regulatory filings and marketing authorization of Anaphylm outside of the U.S.; that Anaphylm will be the first and only oral administration of epinephrine and accepted as an alternative to existing standards of care, if Anaphylm is approved by the FDA; the potential benefits Anaphylm could bring to patients, if approved by the FDA; the advancement and related timing of our product candidate AQST-108 (epinephrine) Topical Gel through clinical development and FDA regulatory approval process, including design and timing of clinical studies including those necessary to support the targeted indication of alopecia areata for AQST-108; our future financial and operating results and financial position, including with respect to our 2025 financial outlook, estimated cash runway and sufficiency to support the Company’s long-term growth strategy for the potential regulatory approval and subsequent launch of Anaphylm in the U.S. and around the world, if approved by the respective regulatory authorities in such jurisdictions; and business strategies, market opportunities, and other statements that are not historical facts. These forward-looking statements are based on our current expectations and beliefs and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Such risks and uncertainties include, but are not limited to, risks associated with our development work, including any delays or changes to the timing, cost and success of our product development activities and clinical trials and plans for Anaphylm; risk of delays in advancement of the regulatory approval process through the FDA of our product candidate Anaphylm, or failure to receive FDA approval at all; risk of FDA inspections of manufacturing and clinical study sites for any of our product candidates, including Anaphylm; risk of government shutdowns or actions to reduce government workforces on the ability of the FDA to act on the approval of our product candidates, including Anaphylm; risk of the Company’s ability to generate sufficient clinical and other human factor data, including with respect to our submission of pharmacokinetic and pharmacodynamic (PK/PD) comparability data for FDA approval of Anaphylm; risks associated with our ability to address the FDA’s comments on and identified deficiencies in our NDA, including the concerns raised by the FDA in the Complete Response Letter dated January 30 2026 issued to the Company for approval of Anaphylm; risk that the FDA may consider issues raised in the citizen petition submitted to the FDA regarding Anaphylm on October 1, 2025; risks associated with the success of any competing products, including generics; risks and uncertainties inherent in commercializing a new product (including technology risks, financial risks, market risks and implementation risks and regulatory limitations); risk of development of a sales and marketing capability for commercialization of our product candidates, including Anaphylm, if approved by the FDA; risks associated with the potential impact on the value of the Company of the sale or outlicensing of our product and product candidates, including Anaphylm; risk of sufficient capital and cash resources, including sufficient access to available debt and equity financing, including under our ATM facility, and revenues from operations, to satisfy all of our short-term and longer-term liquidity and cash requirements to support our growth strategy, and other cash needs, at the times and in the amounts needed, including to commence principal payments on our 13.5% Senior Secured Notes in 2026, and to fund future clinical development and commercial activities for our product candidates, including Anaphylm, should these product candidates be approved by the FDA; risk of the impact of our obligations under the Company's Purchase Agreement and the Royalty Rights Agreement with third parties, each of which agreements requires the Company to make payments to each counterparty thereof, respectively, of a portion of our revenues, on our ability to contribute to the funding of our operations and the payment of principal and interest on our debt; the risk of our obligations under such Purchase Agreement and Royalty Rights Agreement impacting our ability to refinance our 13.5% Senior Secured Notes; risk that our manufacturing capabilities will be sufficient to support demand of our product candidates in the U.S. and abroad, including Anaphylm, if such product candidates should be approved by the FDA and other regulatory authorities, and our licensed products in the U.S. and abroad; risk of eroding market share for Suboxone® as a sunsetting product, which accounts for a substantial part of our current operating revenue; risk of default of our debt instruments; risks related to the outsourcing of certain sales, marketing and other operational and staff functions to third parties; risk of the rate and degree of market acceptance in the U.S. and abroad of Anaphylm and our other product candidates, should these product candidates be approved by the FDA and other regulatory authorities, and for our licensed products in the U.S. and abroad; risk associated with the size and growth of our product markets; risk associated with our compliance with all FDA and other governmental and customer requirements for our manufacturing facilities; risks associated with intellectual property rights and infringement claims relating to our products; risk that our patent applications for our product candidates, including for Anaphylm, will not be timely issued, or issued at all, by the U.S. Patent and Trademark Office or, if issued, will be sufficient to provide long-term commercial success of these product candidates; risk of unexpected patent developments; risk of legislation and regulatory actions and changes in laws or regulations affecting our business, including relating to our products and product candidates and product pricing, reimbursement or access therefor; risk of loss of significant customers; risks related to claims and legal proceedings against us including patent infringement, securities, business torts, investigative, product safety or efficacy and antitrust litigation matters; risk of product recalls and withdrawals; risks related to any disruptions in our information technology networks and systems, including the impact of cybersecurity attacks; risk of increased cybersecurity attacks and data accessibility disruptions due to remote working arrangements; risk of adverse developments affecting the financial services industry; risks related to inflation and changing interest rates; risks related to the impact of pandemic diseases on our business; risks and uncertainties related to general economic, political (including the Ukraine and Israel wars and other acts of war and terrorism), business, industry, regulatory, financial and market conditions and other unusual items; risks related to uncertainty about presidential administration initiatives and their impact on our business, including imposition of government tariffs and other trade restrictions; and other uncertainties affecting the Company including those described in the "Risk Factors" section and in other sections included in the Company’s Annual Report on 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the U.S. Securities and Exchange Commission. Given those uncertainties, you should not place undue reliance on these forward-looking statements, which speak only as of the date made. All subsequent forward-looking statements attributable to the Company or any person acting on its behalf are expressly qualified in their entirety by this cautionary statement. The Company assumes no obligation to update forward-looking statements or outlook or guidance after the date of this presentation whether as a result of new information, future events or otherwise, except as may be required by applicable law. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any of the Company’s securities, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction. The Aquestive logo is a registered trademark of Aquestive Therapeutics, Inc. and has been conditionally approved by the FDA. Final approval of the Anaphylm proprietary name is conditioned on FDA approval of the product candidate, AQST-109. All other registered trademarks referenced herein are the property of their respective owners. Disclaimer

3 In the following slides, we provide exact language in the comments contained in the CRL and Aquestive’s viewpoint. Of note: • Deficiencies limited to packaging and administration • Company believes it can rapidly resolve deficiencies and expects to resubmit as early as Q3 2026, assuming completion of planned responsive Human Factor (HF) study and pharmacokinetic (PK) study and typical response times from the FDA • Remains well-capitalized and anticipates ending 2026 with significant cash • Reiterates plans to submit for regulatory approval in Canada and EU in the second half of 2026 Key takeaways from the FDA’s Complete Response Letter (CRL)
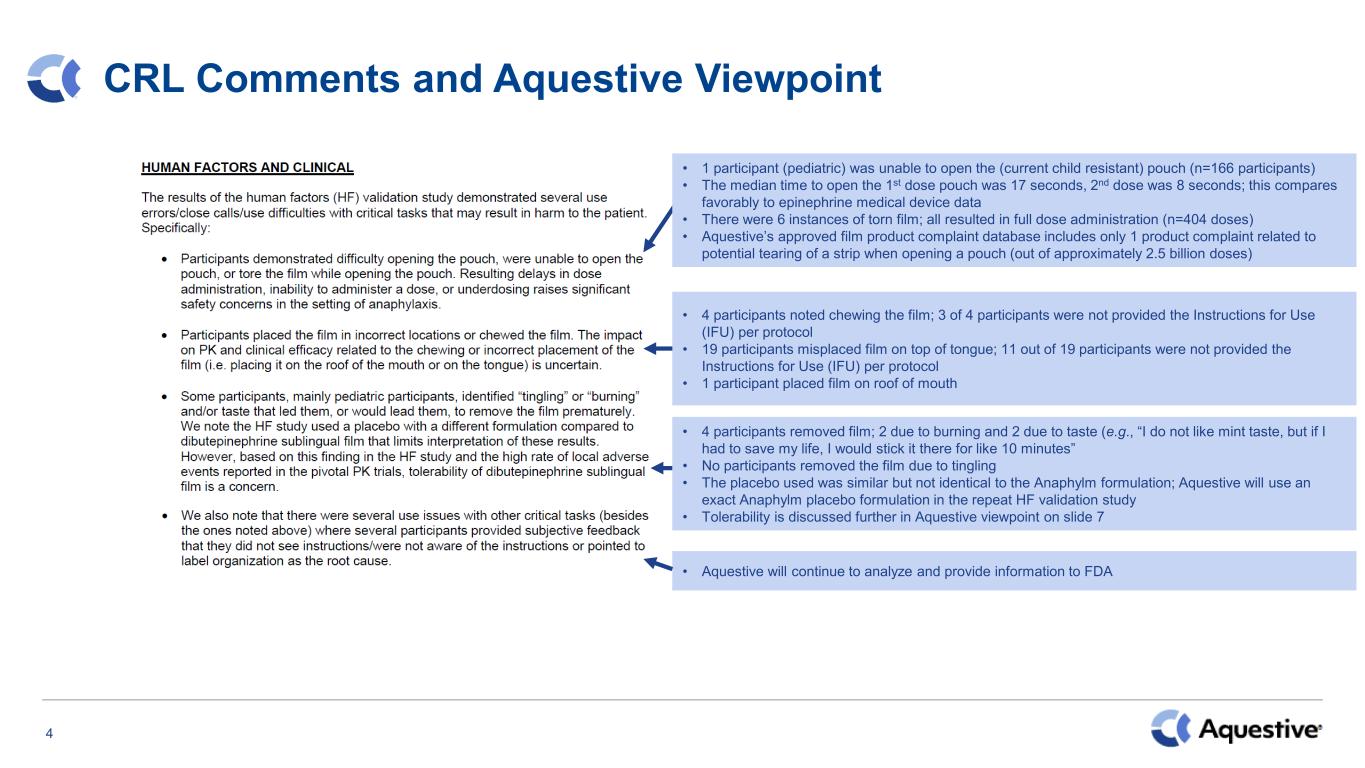
4 CRL Comments and Aquestive Viewpoint • 1 participant (pediatric) was unable to open the (current child resistant) pouch (n=166 participants) • The median time to open the 1st dose pouch was 17 seconds, 2nd dose was 8 seconds; this compares favorably to epinephrine medical device data • There were 6 instances of torn film; all resulted in full dose administration (n=404 doses) • Aquestive’s approved film product complaint database includes only 1 product complaint related to potential tearing of a strip when opening a pouch (out of approximately 2.5 billion doses) • 4 participants noted chewing the film; 3 of 4 participants were not provided the Instructions for Use (IFU) per protocol • 19 participants misplaced film on top of tongue; 11 out of 19 participants were not provided the Instructions for Use (IFU) per protocol • 1 participant placed film on roof of mouth • 4 participants removed film; 2 due to burning and 2 due to taste (e.g., “I do not like mint taste, but if I had to save my life, I would stick it there for like 10 minutes” • No participants removed the film due to tingling • The placebo used was similar but not identical to the Anaphylm formulation; Aquestive will use an exact Anaphylm placebo formulation in the repeat HF validation study • Tolerability is discussed further in Aquestive viewpoint on slide 7 • Aquestive will continue to analyze and provide information to FDA
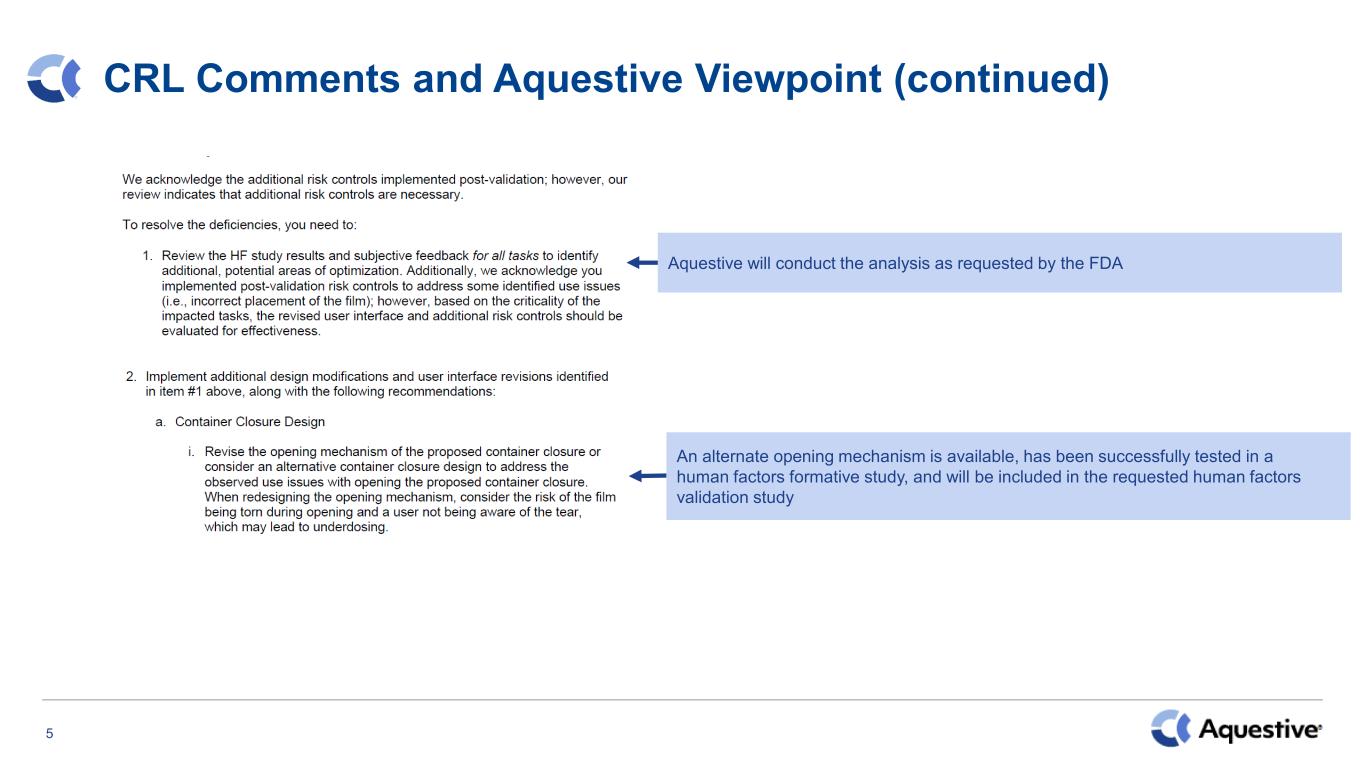
5 CRL Comments and Aquestive Viewpoint (continued) Aquestive will conduct the analysis as requested by the FDA An alternate opening mechanism is available, has been successfully tested in a human factors formative study, and will be included in the requested human factors validation study
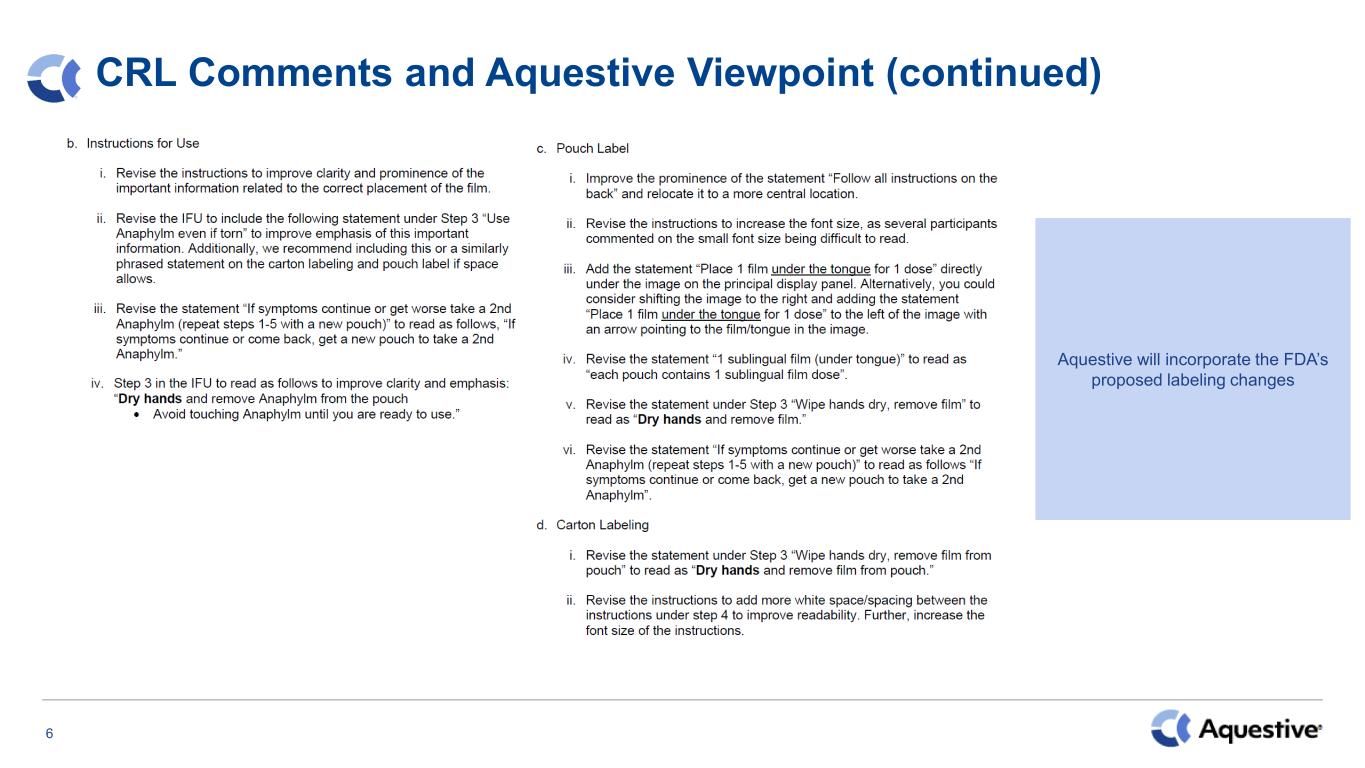
6 CRL Comments and Aquestive Viewpoint (continued) Aquestive will incorporate the FDA’s proposed labeling changes
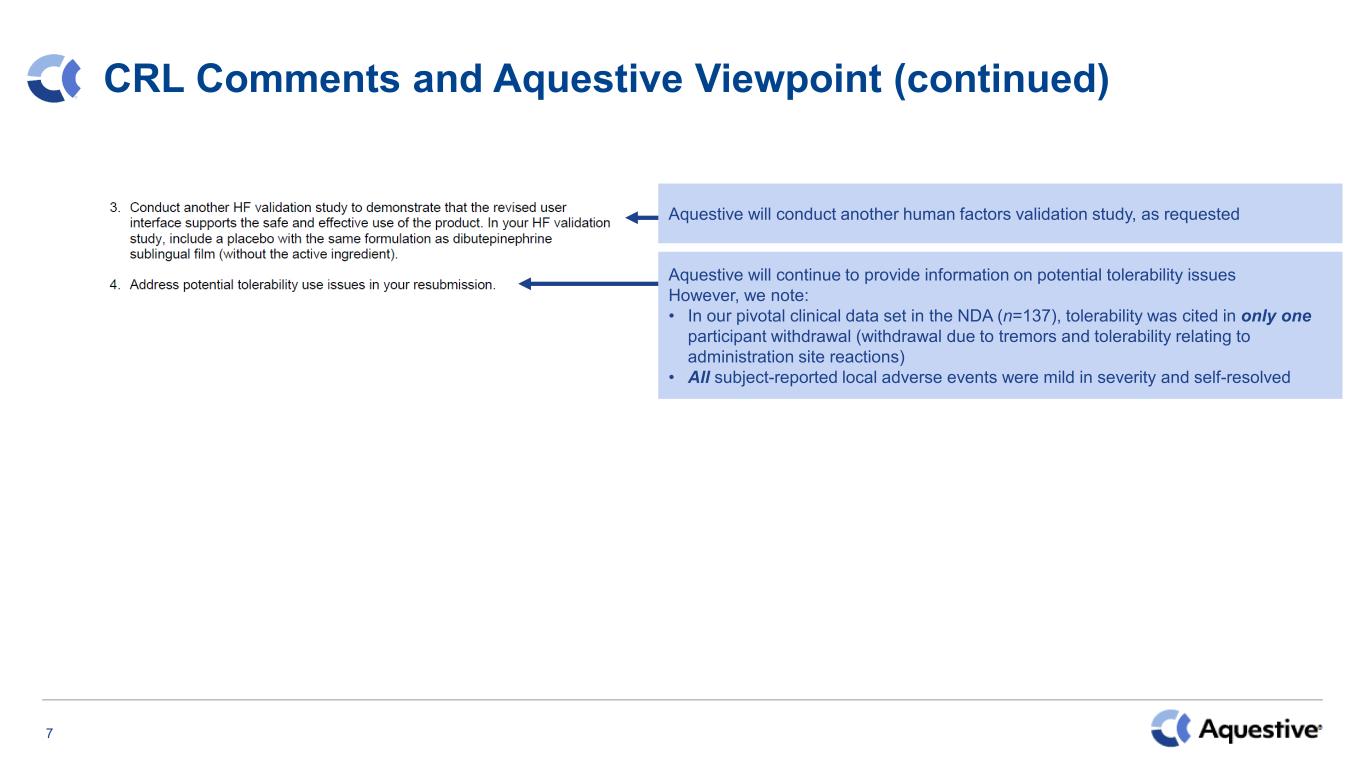
7 CRL Comments and Aquestive Viewpoint (continued) Aquestive will conduct another human factors validation study, as requested Aquestive will continue to provide information on potential tolerability issues However, we note: • In our pivotal clinical data set in the NDA (n=137), tolerability was cited in only one participant withdrawal (withdrawal due to tremors and tolerability relating to administration site reactions) • All subject-reported local adverse events were mild in severity and self-resolved

8 CRL Comments and Aquestive Viewpoint (continued) Aquestive will discuss with the clinical pharmacology team at the Type A meeting and will conduct the study as requested

9 CRL Comments and Aquestive Viewpoint (continued) Aquestive is assessing the potential label impact and if additional clinical data generation is warranted Language matches boilerplate language provided to nasal spray manufacturer in response to auto-injector manufacturer submitted citizen petition (CP), and other FDA issued CRLs which referenced unresolved pre-submitted CPs
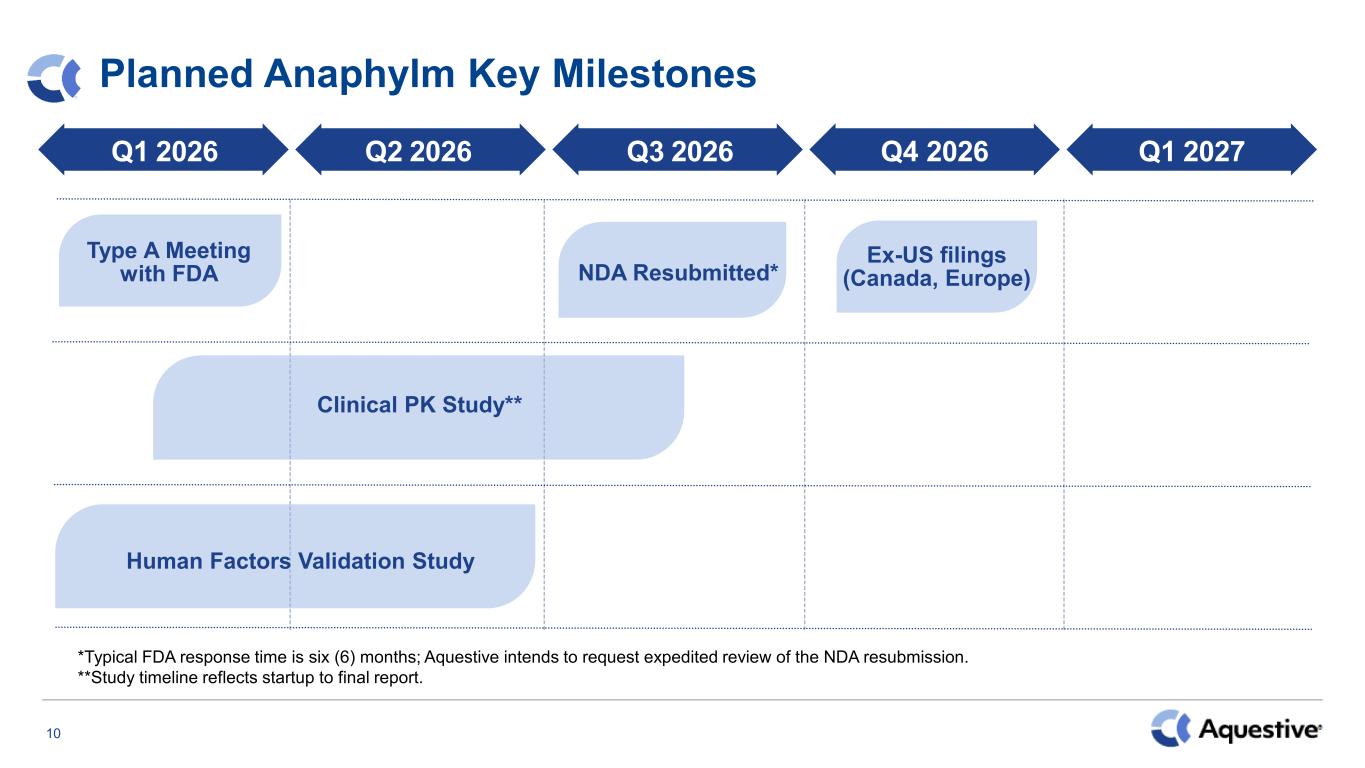
10 Planned Anaphylm Key Milestones Q1 2026 Type A Meeting with FDA Q2 2026 Q3 2026 Q4 2026 Q1 2027 Human Factors Validation Study NDA Resubmitted* Ex-US filings (Canada, Europe) Clinical PK Study** *Typical FDA response time is six (6) months; Aquestive intends to request expedited review of the NDA resubmission. **Study timeline reflects startup to final report.

Thank You 11