| Targeting MetabolicDysfunction with Novel Therapies to Treat MASH, Acne&Cancer October 2025 |
| October 2025 2 Forward-Looking Statements and Disclaimer This presentation contains forward-looking statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act of 1995. All statements contained in this document, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding possible or assumed future results of operations, business strategies, research and development plans, regulatory activities, the presentation of data from clinical trials, Sagimet’s clinical development plans and related anticipated clinical development milestones, market opportunity, competitive position and potential growth opportunities are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “would,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “believe,” “estimate,” “predict,” “potential,” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this presentation are only predictions. These forward-looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control, including, among others: the clinical development and therapeutic potential of denifanstat, TVB-3567 or any other drug candidates or combination therapies we may develop; our ability to advance drug candidates into and successfully complete clinical trials, the risk the topline clinical trials may not be predictive of, and may differ from final clinical data and later-stage clinical trials; our ability to advance drug candidates into and successfully complete clinical trials within anticipated timelines; that unfavorable new clinical trial data may emerge in other clinical trials of our product candidates; that clinical trial data are subject to differing interpretations and assessments, including by regulatory authorities; our relationship with Ascletis, and the success of its development efforts for denifanstat; the accuracy of our estimates regarding our capital requirements; and our ability to maintain and successfully enforce adequate intellectual property protection. These and otherrisks and uncertainties are described more fully in the “Risk Factors”section of our most recent filings with the Securities and Exchange Commission (SEC) and available at www.sec.gov. You should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-lookingstatements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update or revise any forward-lookingstatements contained herein,whether as a result of any new information,future events,changedcircumstances or otherwise. |
| October 2025 3 Leadership Team with Proven Development and Commercialization Experience DaveHappel President&CEO >20 years of experience in executive leadership in biotech and pharma Brought multiple innovative healthcare products to the market Eduardo Martins CMO >20 years of leadership of large-scale multinational clinical trials & global teams in pharma and biotech Led clinical development team of cenicriviroc for MASH Thierry ChaucheCFO >20 years of financial and operational leadership experience in finance and healthcare companies Elizabeth Rozek General Counsel >20 years of legal experience including executive leadership of legal, IP and compliance functions in biopharma and biotech Rob D’Urso Senior Vice President of New Products >20 years of US and global leadership experience in dermatology Marie O'Farrell Senior Vice President of R&D >20 years of experience in R&D and translational medicine in biopharma and biotech Successfully guided development for multiple clinical programs |
| October 2025 4 Sagimet at a Glance • Our lead molecule, denifanstat, is a novel fatty acid synthase (FASN) inhibitor with a differentiated MOA with the potential to target multiple underserved diseases • Strong clinical data demonstrates denifanstat’s proof of concept across multiple disease states Unique MOA: FASN Inhibition • Denifanstat directly targets the 3 key drivers of MASH (metabolic dysfunction-associated steatohepatitis) – liver fat, inflammation, and fibrosis • Successful outcome of Phase 2b trial; met both primary endpoints with significant reduction in fibrosis • Pre-clinical data demonstrated synergistic effect of combination of FASN inhibitor and resmetirom • Phase 1 clinical trial to evaluate the pharmacokinetics (PK) and tolerability of a combination of denifanstat and resmetirom initiated in September 2025; data readout expected 1H 2026 Denifanstat in MASH TVB-3567 in Acne • Our follow-on FASN inhibitor, TVB 3567, received Investigational New Drug (IND) clearance in March 2025 • First-in-human Phase 1 clinical trial initiated in June 2025 for development of an acne indication |
| October 2025 5 Strong IP, Cash Position, and Collaboration Potential • Denifanstat met all primary and secondary endpoints in Phase 3 clinical trial in patients with moderate to severe acne vulgaris in China conducted by license partner for China, Ascletis • Ascletis announced that it completed its pre-New Drug Application (NDA) consultation with China National Medical Products Administration (NMPA) and plans to submit an NDA soon • Denifanstat: • Method of use patent—2036; potential PTE to 2041 • Composition of matter patent—2032 • Combination of denifanstat and resmetirom: • Application filed 2024; if granted—2044; potential PTE to 2048 • TVB-3567: • Method of use application for TVB-3567 for acne filed 2025; if granted—2046 • Composition of matter patent—2035; potential PTE to 2038 Cash Position • Nasdaq: SGMT; $135.5M cash on hand*, expected to fund current operations through 2027 Strategic Collaboration with Ascletis in Acne IP Portfolio *Cash, cash equivalents and marketable securities as of 06/30/2025 |
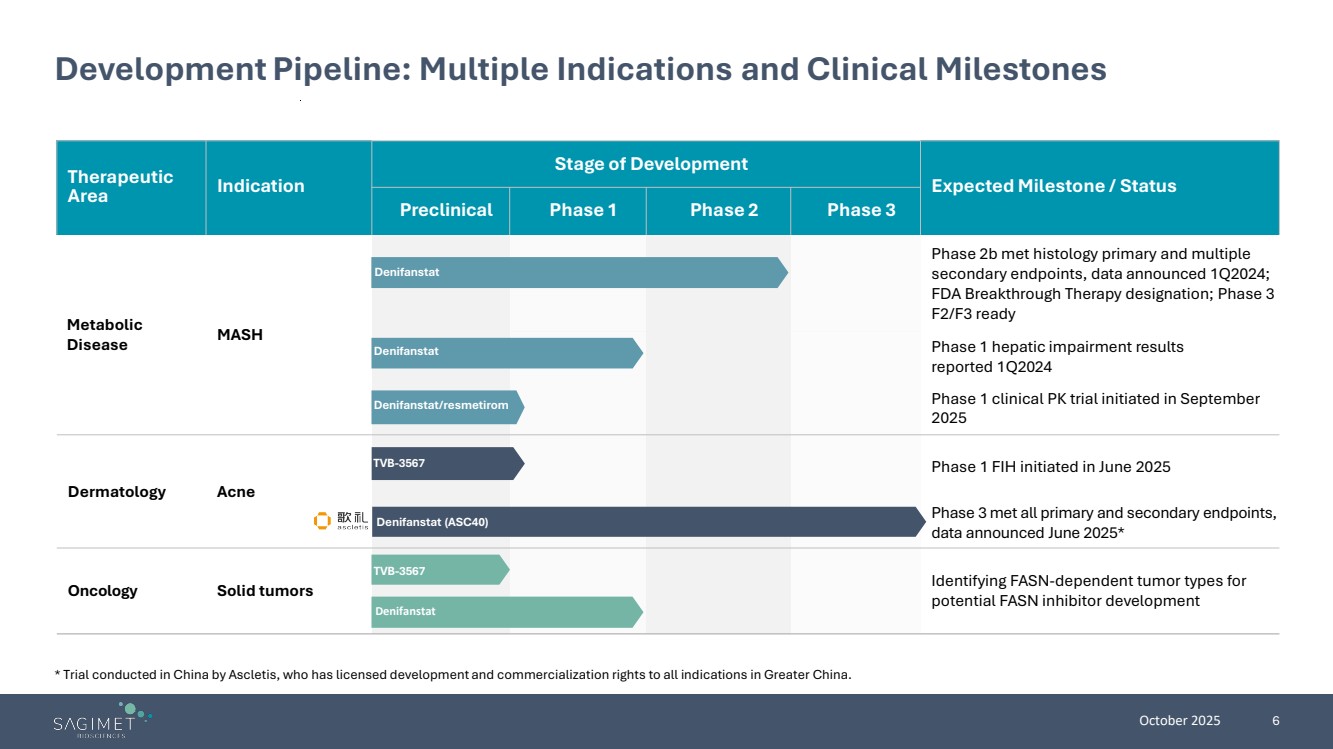
| October 2025 6 Therapeutic Area Indication Stage of Development Expected Milestone / Status Preclinical Phase 1 Phase 2 Phase 3 Metabolic Disease MASH Phase 2b met histology primary and multiple secondary endpoints, data announced 1Q2024; FDA Breakthrough Therapy designation; Phase 3 F2/F3 ready Phase 1 hepatic impairment results reported 1Q2024 Phase 1 clinical PK trial initiated in September 2025 Dermatology Acne Phase 1 FIH initiated in June 2025 Phase 3 met all primary and secondary endpoints, data announced June 2025* Oncology Solid tumors Identifying FASN-dependent tumor types for potential FASN inhibitor development Development Pipeline: Multiple Indications and Clinical Milestones * Trial conducted in China by Ascletis, who has licensed development and commercialization rights to all indications in Greater China. Denifanstat Denifanstat TVB-3567 Denifanstat (ASC40) TVB-3567 Denifanstat Denifanstat/resmetirom |
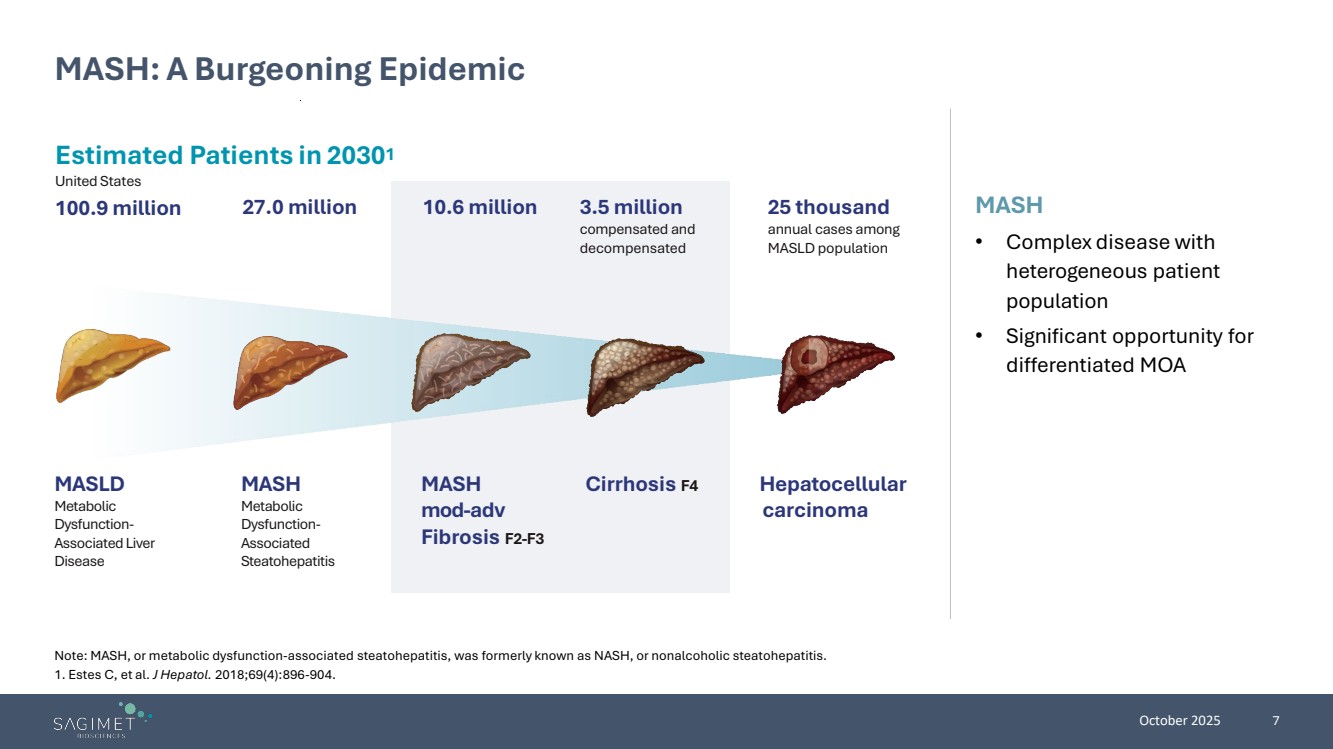
| October 2025 7 MASH • Complex disease with heterogeneous patient population • Significant opportunity for differentiated MOA MASH: A Burgeoning Epidemic Note: MASH, or metabolic dysfunction-associated steatohepatitis, was formerly known as NASH, or nonalcoholic steatohepatitis. 1. Estes C, et al. J Hepatol. 2018;69(4):896-904. Estimated Patients in 20301 United States 100.9 million Hepatocellular carcinoma Cirrhosis F4 25 thousand annual cases among MASLDpopulation 3.5 million compensated and decompensated 27.0 million 10.6 million MASLD Metabolic Dysfunction-Associated Liver Disease MASH Metabolic Dysfunction-Associated Steatohepatitis MASH mod-adv Fibrosis F2-F3 |
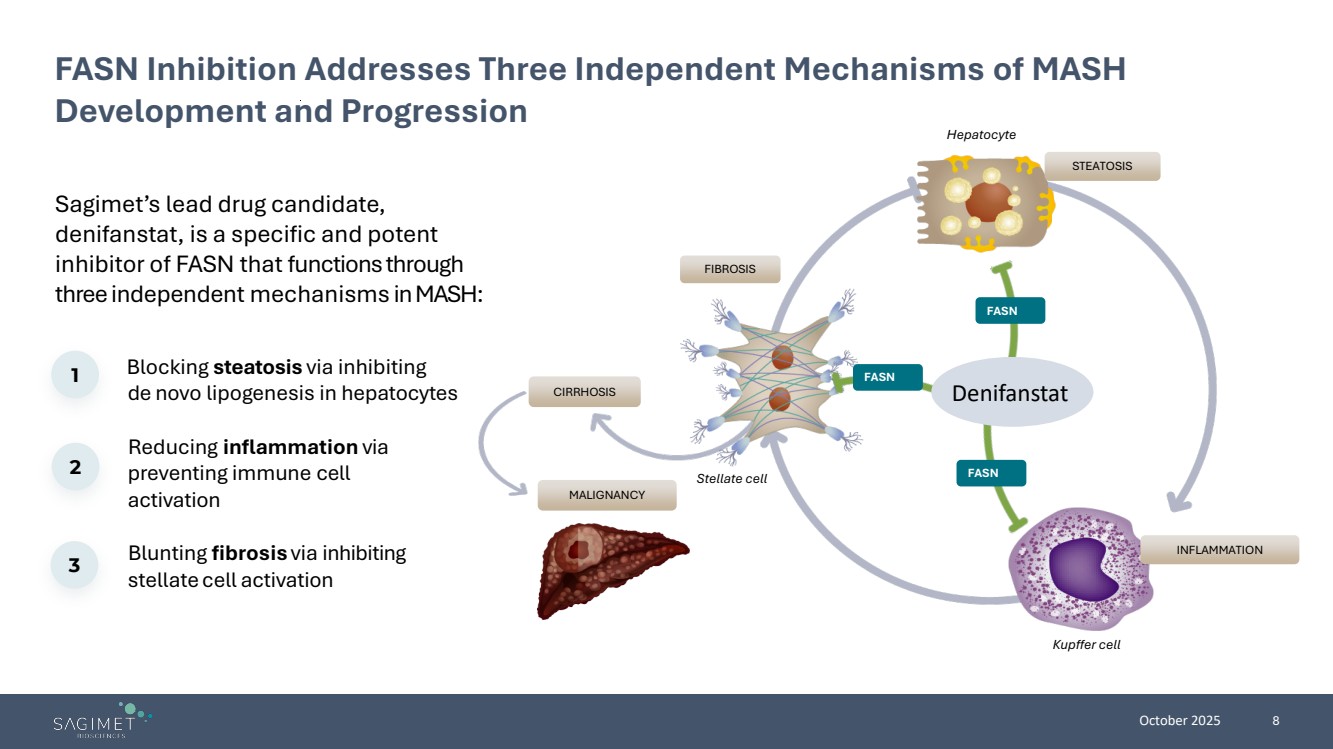
| October 2025 8 Sagimet’s lead drug candidate, denifanstat, is a specific and potent inhibitor of FASN that functions through three independent mechanisms in MASH: FASN Inhibition Addresses Three Independent Mechanisms of MASH Development and Progression Blocking steatosis via inhibiting de novo lipogenesis in hepatocytes Reducing inflammation via preventing immune cell activation Blunting fibrosis via inhibiting stellate cell activation 1 2 3 INFLAMMATION FIBROSIS STEATOSIS Hepatocyte Kupffer cell Stellate cell CIRRHOSIS MALIGNANCY FASN FASN FASN Denifanstat |
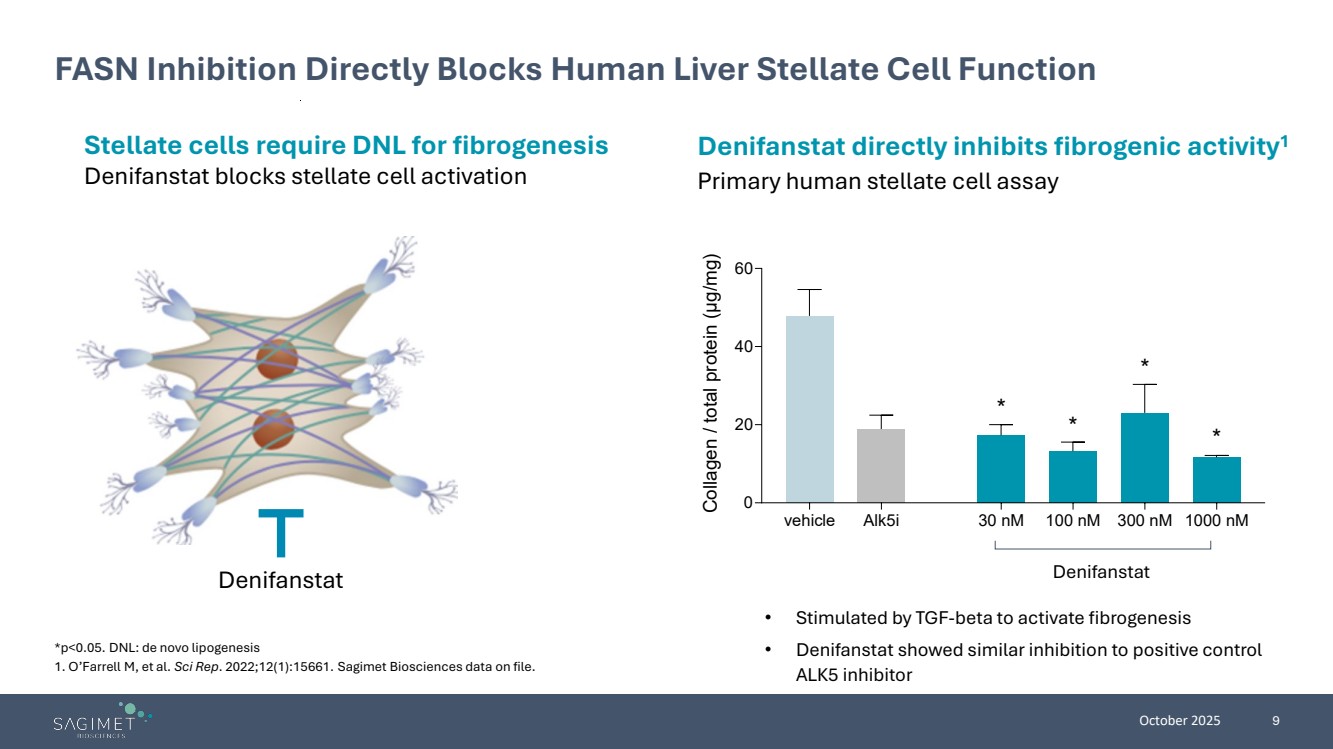
| October 2025 9 FASN Inhibition Directly Blocks Human Liver Stellate Cell Function Stellate cells require DNL for fibrogenesis Denifanstat blocks stellate cell activation Denifanstat directly inhibits fibrogenic activity1 Primary human stellate cell assay Denifanstat • Stimulated by TGF-beta to activate fibrogenesis • Denifanstat showed similar inhibition to positive control ALK5 inhibitor *p<0.05. DNL: de novo lipogenesis 1. O’Farrell M, et al. Sci Rep. 2022;12(1):15661. Sagimet Biosciences data on file. vehicle Alk5i 30 nM 100 nM 300 nM 1000 nM 0 20 40 60 Collagen / total protein (μg/mg) * * * * Denifanstat |
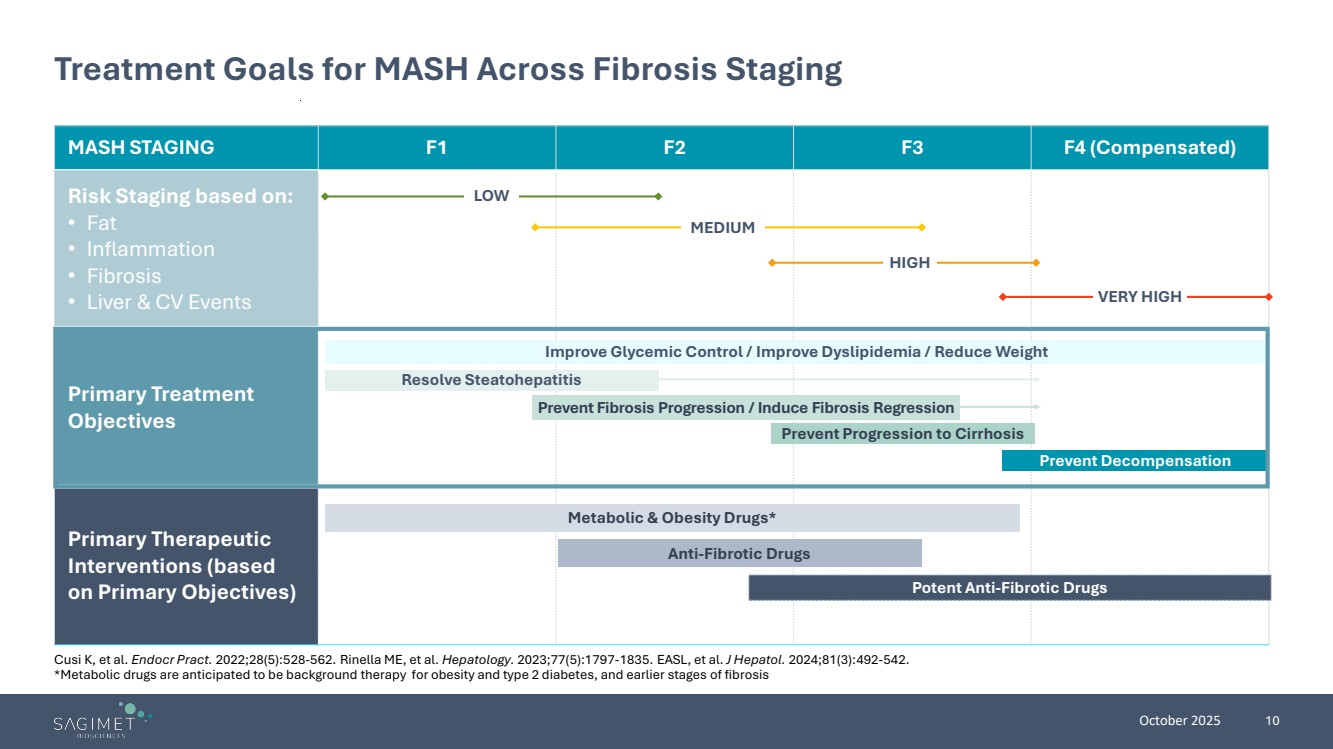
| October 2025 10 Treatment Goals for MASH Across Fibrosis Staging MASH STAGING F1 F2 F3 F4 (Compensated) Risk Staging based on: • Fat • Inflammation • Fibrosis • Liver & CV Events Primary Treatment Objectives Primary Therapeutic Interventions (based on Primary Objectives) Improve Glycemic Control / Improve Dyslipidemia / Reduce Weight Resolve Steatohepatitis Prevent Progression to Cirrhosis Prevent Decompensation Metabolic & Obesity Drugs* Anti-Fibrotic Drugs Potent Anti-Fibrotic Drugs Prevent Fibrosis Progression / Induce Fibrosis Regression LOW MEDIUM HIGH VERY HIGH Cusi K, et al. Endocr Pract. 2022;28(5):528-562. Rinella ME, et al. Hepatology. 2023;77(5):1797-1835. EASL, et al. J Hepatol. 2024;81(3):492-542. *Metabolic drugs are anticipated to be background therapy for obesity and type 2 diabetes, and earlier stages of fibrosis |
| Strong MASH Data Creates Opportunities to Reach Advanced Patient Populations |
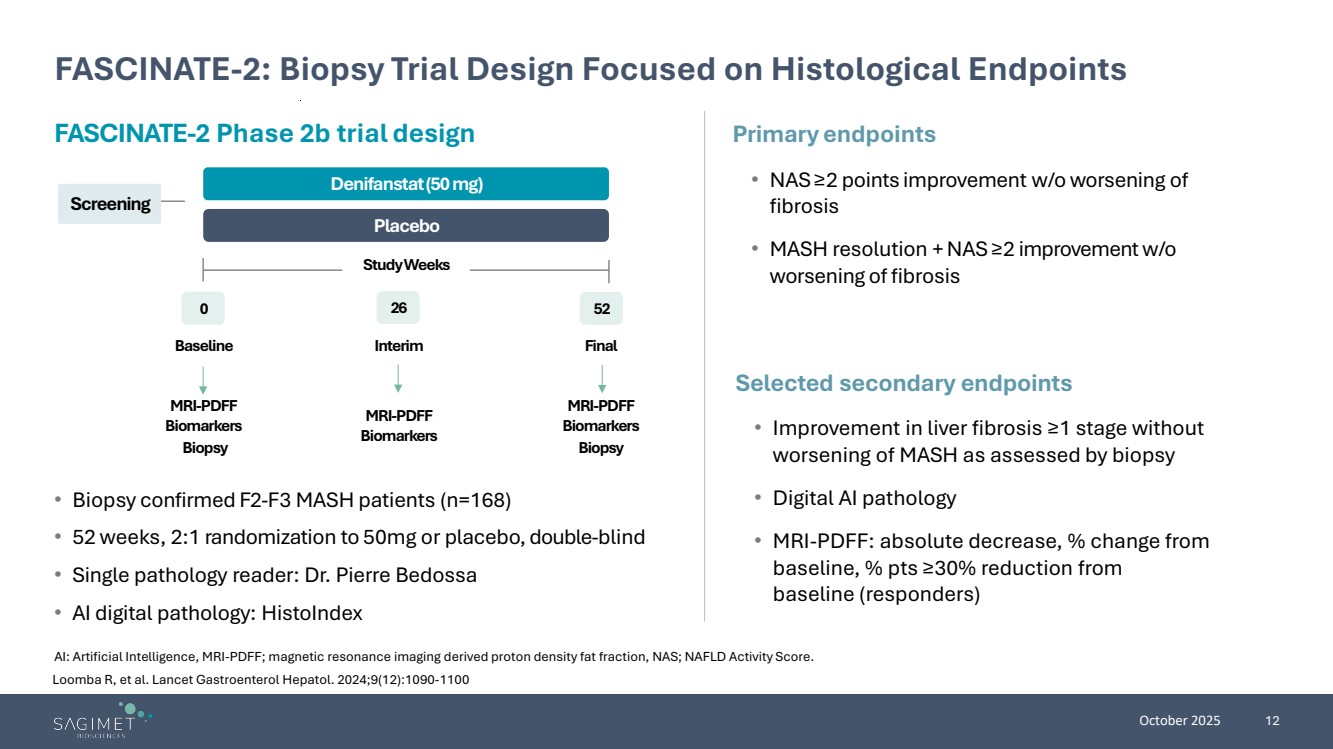
| October 2025 12 FASCINATE-2: Biopsy Trial Design Focused on Histological Endpoints AI: Artificial Intelligence, MRI-PDFF; magnetic resonance imaging derived proton density fat fraction, NAS; NAFLD Activity Score. • Biopsy confirmed F2-F3 MASH patients (n=168) • 52 weeks, 2:1 randomization to 50mg or placebo, double-blind • Single pathology reader: Dr. Pierre Bedossa • AI digital pathology: HistoIndex Primary endpoints • NAS≥2 points improvement w/o worsening of fibrosis • MASH resolution + NAS ≥2 improvement w/o worsening of fibrosis Selected secondary endpoints • Improvement in liver fibrosis ≥1 stage without worsening of MASH as assessed by biopsy • Digital AI pathology • MRI-PDFF: absolute decrease, % change from baseline, % pts ≥30% reduction from baseline (responders) FASCINATE-2 Phase 2b trial design Screening Placebo Denifanstat(50 mg) 0 26 52 Study Weeks Baseline Interim Final MRI-PDFF Biomarkers Biopsy MRI-PDFF Biomarkers MRI-PDFF Biomarkers Biopsy Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100 |
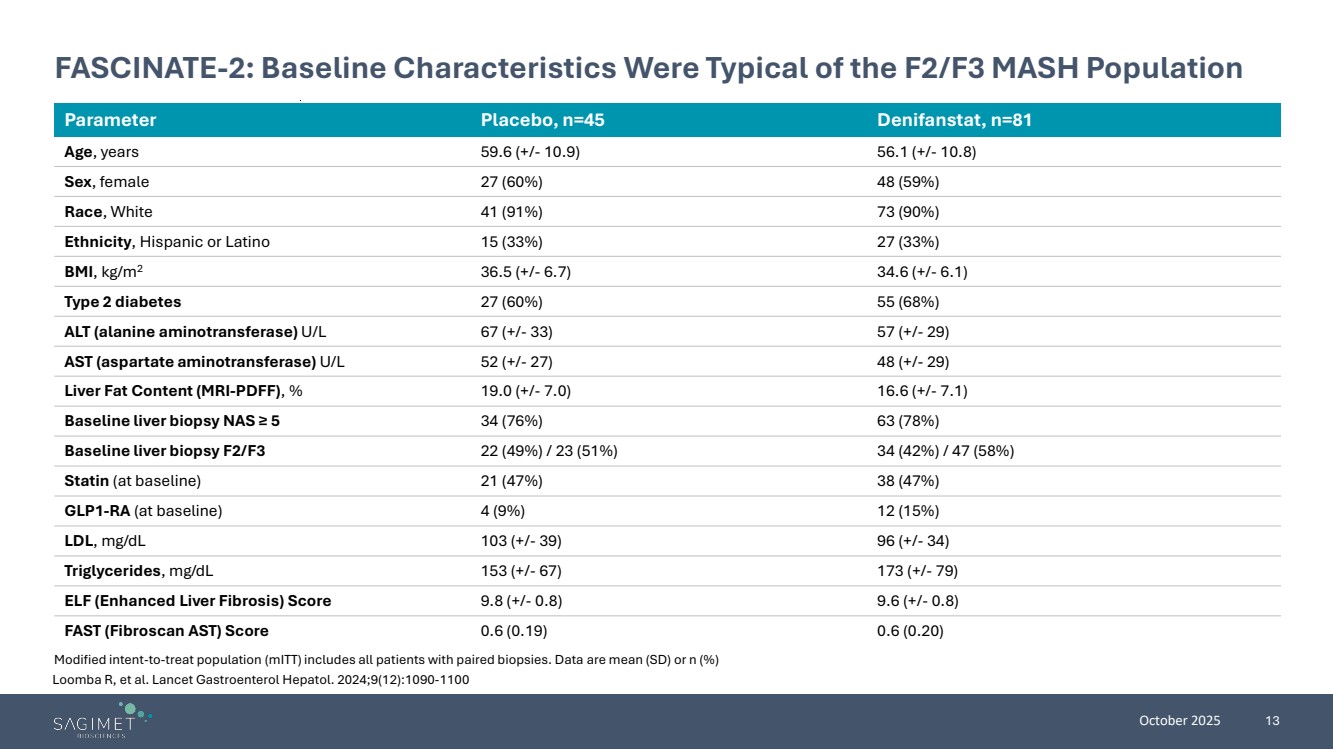
| October 2025 13 FASCINATE-2: Baseline Characteristics Were Typical of the F2/F3 MASH Population Parameter Placebo, n=45 Denifanstat, n=81 Age, years 59.6 (+/- 10.9) 56.1 (+/- 10.8) Sex, female 27 (60%) 48 (59%) Race, White 41 (91%) 73 (90%) Ethnicity, Hispanic or Latino 15 (33%) 27 (33%) BMI, kg/m2 36.5 (+/- 6.7) 34.6 (+/- 6.1) Type 2 diabetes 27 (60%) 55 (68%) ALT (alanine aminotransferase) U/L 67 (+/- 33) 57 (+/- 29) AST (aspartate aminotransferase) U/L 52 (+/- 27) 48 (+/- 29) Liver Fat Content (MRI-PDFF), % 19.0 (+/- 7.0) 16.6 (+/- 7.1) Baseline liver biopsy NAS ≥ 5 34 (76%) 63 (78%) Baseline liver biopsy F2/F3 22 (49%) / 23 (51%) 34 (42%) / 47 (58%) Statin (at baseline) 21 (47%) 38 (47%) GLP1-RA (at baseline) 4 (9%) 12 (15%) LDL, mg/dL 103 (+/- 39) 96 (+/- 34) Triglycerides, mg/dL 153 (+/- 67) 173 (+/- 79) ELF (Enhanced Liver Fibrosis) Score 9.8 (+/- 0.8) 9.6 (+/- 0.8) FAST (Fibroscan AST) Score 0.6 (0.19) 0.6 (0.20) Modified intent-to-treat population (mITT) includes all patients with paired biopsies. Data are mean (SD) or n (%) Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100 |
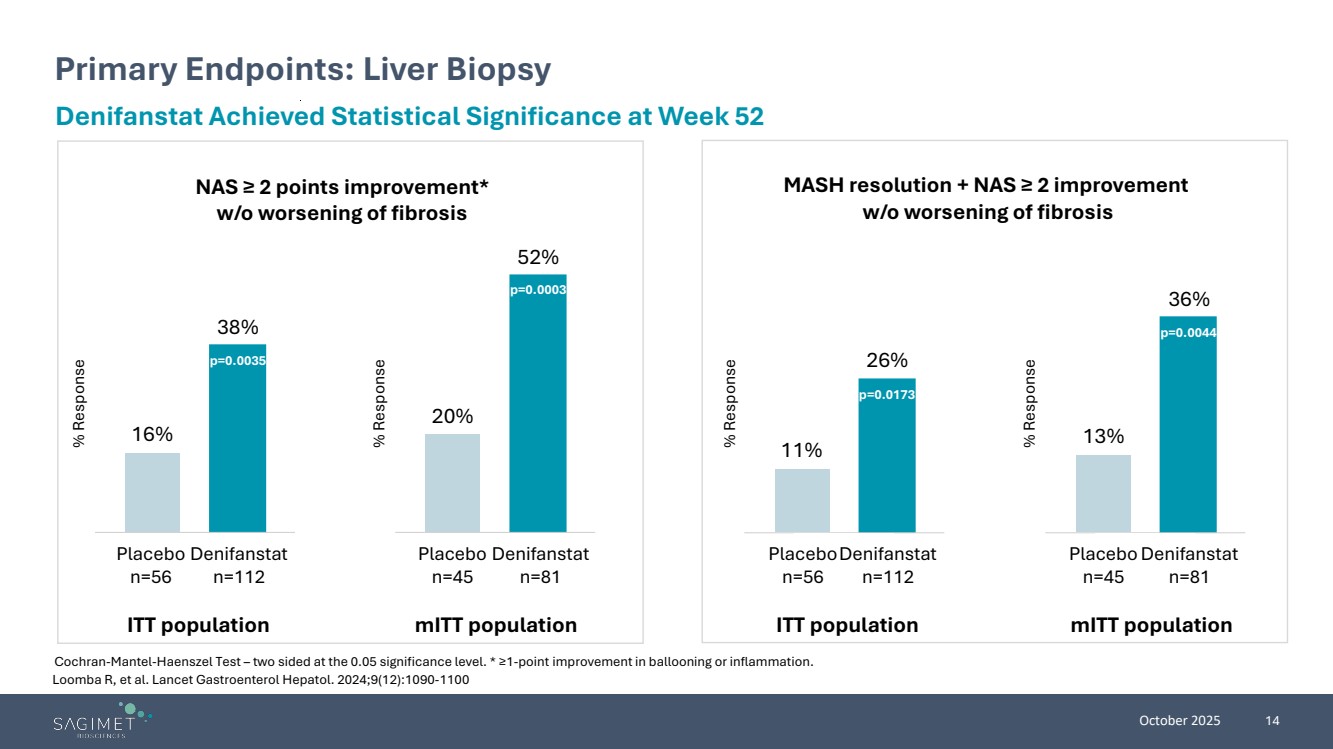
| October 2025 14 Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100 Primary Endpoints: Liver Biopsy Cochran-Mantel-Haenszel Test – two sided at the 0.05 significance level. * ≥1-point improvement in ballooning or inflammation. Denifanstat Achieved Statistical Significance at Week 52 NAS ≥ 2 points improvement* w/o worsening of fibrosis MASH resolution + NAS ≥ 2 improvement w/o worsening of fibrosis ITT population mITT population Placebo n=56 Denifanstat n=112 16% 38% Placebo n=56 Denifanstat n=112 11% 26% Placebo n=45 Denifanstat n=81 20% 52% Placebo n=45 Denifanstat n=81 13% 36% ITT population mITT population % Response % Response % Response % Response p=0.0173 p=0.0044 p=0.0003 p=0.0035 |
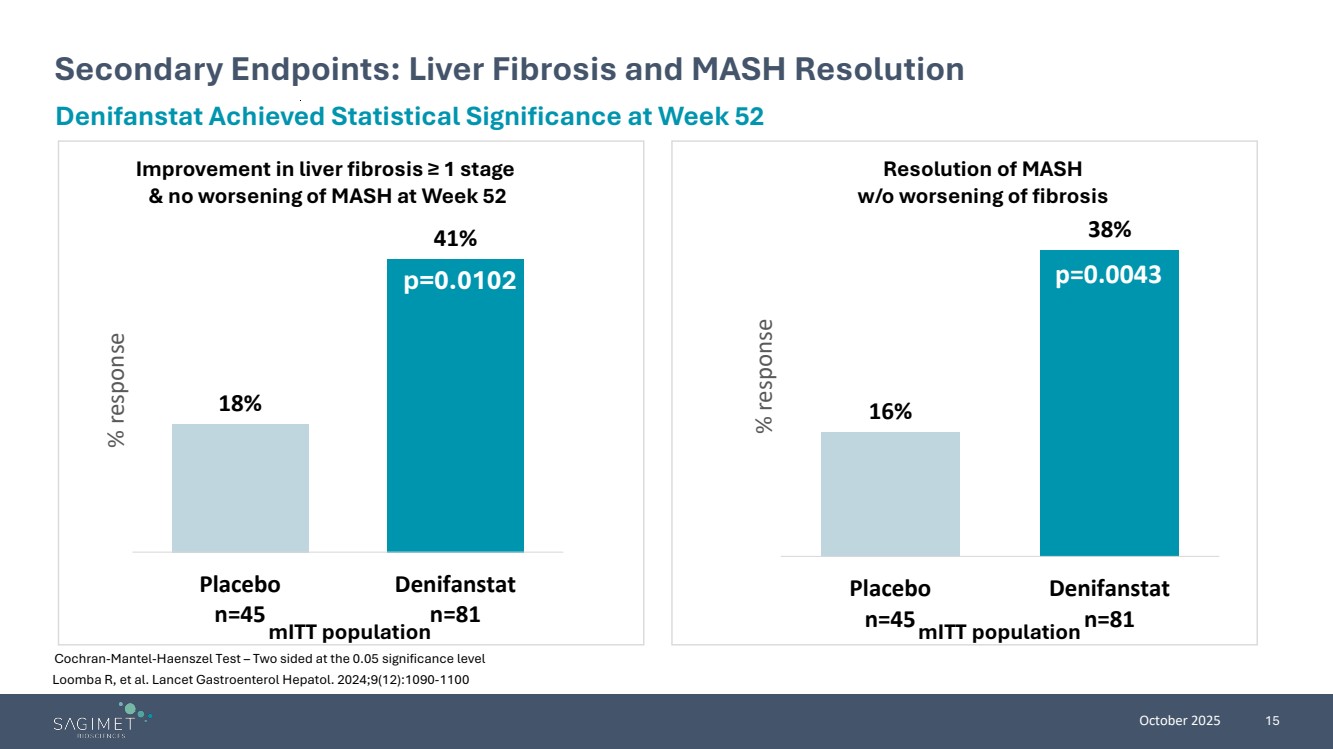
| October 2025 15 Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100 Secondary Endpoints: Liver Fibrosis and MASH Resolution Cochran-Mantel-Haenszel Test – Two sided at the 0.05 significance level Denifanstat Achieved Statistical Significance at Week 52 18% 41% Placebo n=45 Denifanstat n=81 % response Improvement in liver fibrosis ≥ 1 stage & no worsening of MASH at Week 52 p=0.0102 mITT population 16% 38% Placebo n=45 Denifanstat n=81 % response Resolution of MASH w/o worsening of fibrosis p=0.0043 mITT population |
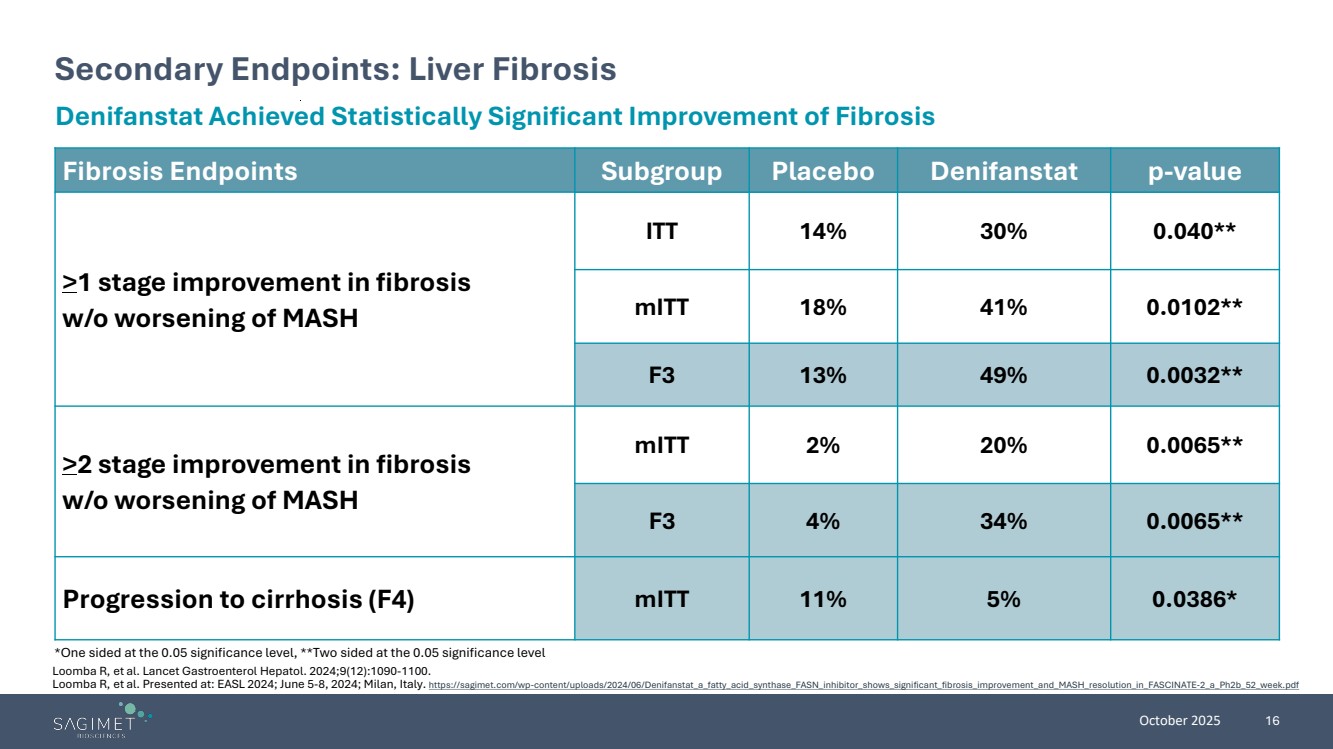
| October 2025 16 Secondary Endpoints: Liver Fibrosis *One sided at the 0.05 significance level, **Two sided at the 0.05 significance level Denifanstat Achieved Statistically Significant Improvement of Fibrosis Fibrosis Endpoints Subgroup Placebo Denifanstat p-value >1 stage improvement in fibrosis w/o worsening of MASH ITT 14% 30% 0.040** mITT 18% 41% 0.0102** F3 13% 49% 0.0032** >2 stage improvement in fibrosis w/o worsening of MASH mITT 2% 20% 0.0065** F3 4% 34% 0.0065** Progression to cirrhosis (F4) mITT 11% 5% 0.0386* Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100. Loomba R, et al. Presented at: EASL 2024; June 5-8, 2024; Milan, Italy. https://sagimet.com/wp-content/uploads/2024/06/Denifanstat_a_fatty_acid_synthase_FASN_inhibitor_shows_significant_fibrosis_improvement_and_MASH_resolution_in_FASCINATE-2_a_Ph2b_52_week.pdf |
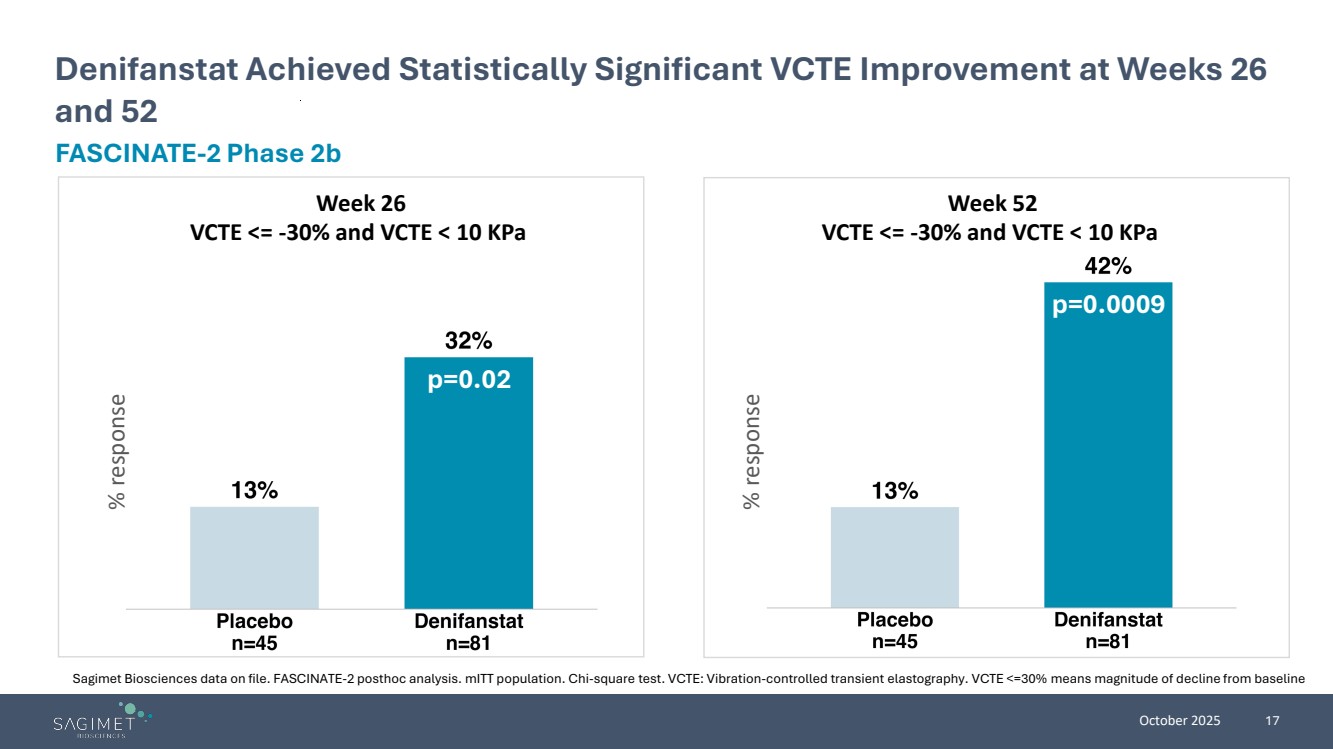
| October 2025 17 Denifanstat Achieved Statistically Significant VCTE Improvement at Weeks 26 and 52 FASCINATE-2 Phase 2b Sagimet Biosciences data on file. FASCINATE-2 posthoc analysis. mITT population. Chi-square test. VCTE: Vibration-controlled transient elastography. VCTE <=30% means magnitude of decline from baseline p=0.0009 % response % response p=0.02 Week 26 VCTE <= -30% and VCTE < 10 KPa Week 52 VCTE <= -30% and VCTE < 10 KPa |
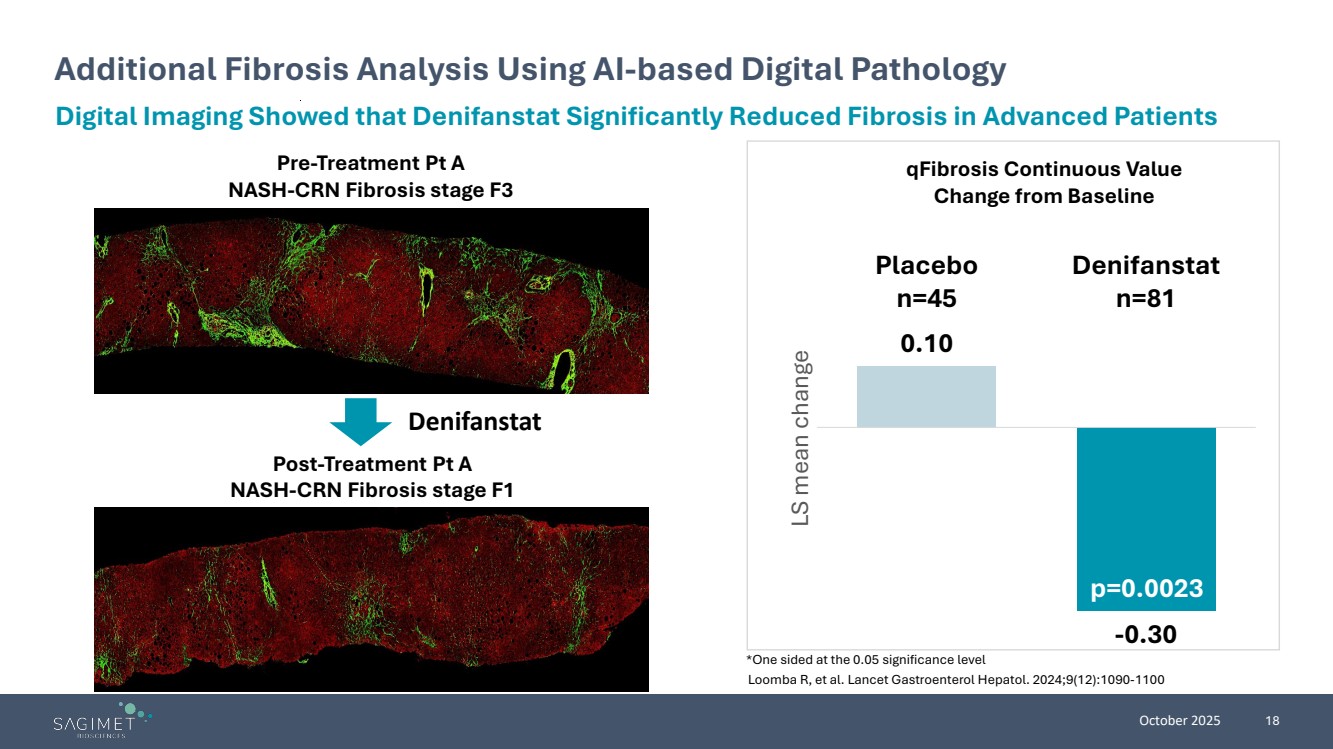
| October 2025 18 Additional Fibrosis Analysis Using AI-based Digital Pathology Digital Imaging Showed that Denifanstat Significantly Reduced Fibrosis in Advanced Patients Pre-Treatment Pt A NASH-CRN Fibrosis stage F3 Post-Treatment Pt A NASH-CRN Fibrosis stage F1 Denifanstat 0.10 -0.30 Placebo n=45 Denifanstat n=81 LS mean change qFibrosis Continuous Value Change from Baseline p=0.0023 *One sided at the 0.05 significance level Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100 |
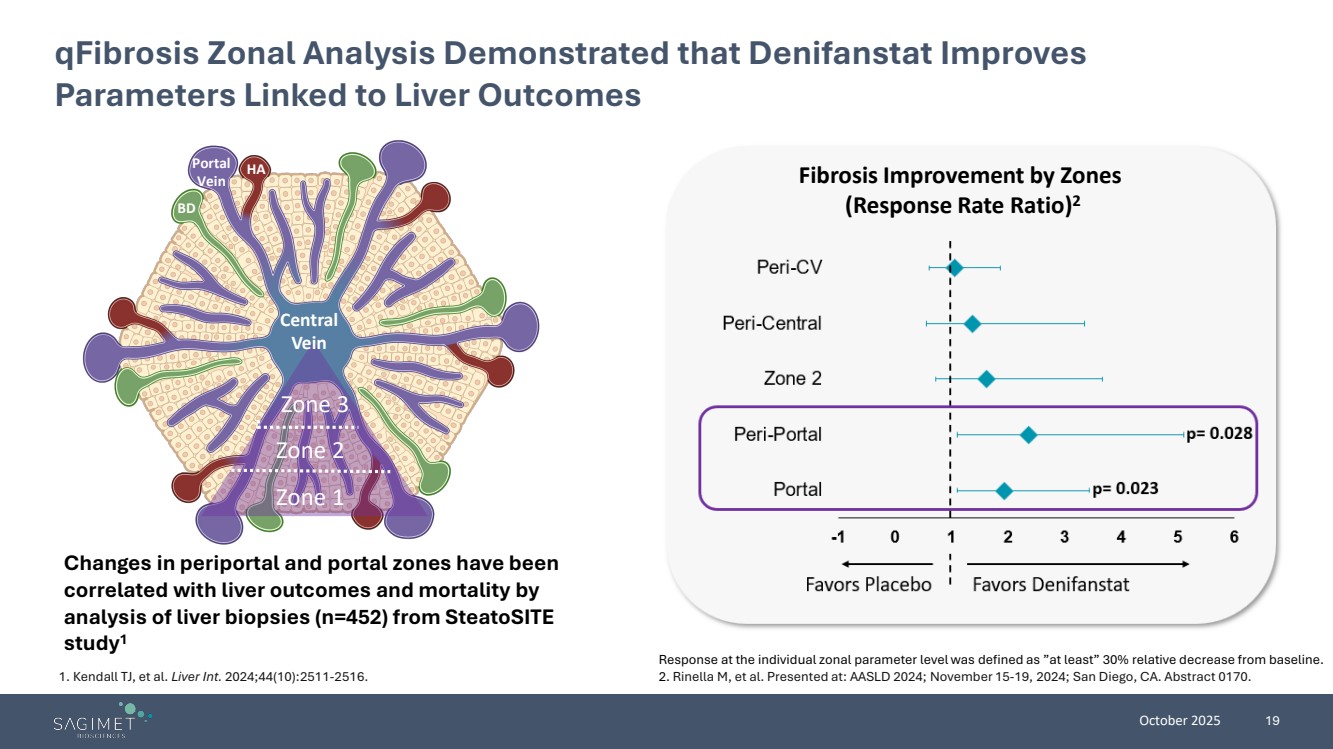
| October 2025 19 qFibrosis Zonal Analysis Demonstrated that Denifanstat Improves Parameters Linked to Liver Outcomes Zone 1 Zone 2 Zone 3 Central Vein Portal Vein HA BD Response at the individual zonal parameter level was defined as ”at least” 30% relative decrease from baseline. 2. Rinella M, et al. Presented at: AASLD 2024; November 15-19, 2024; San Diego, CA. Abstract 0170. Fibrosis Improvement by Zones (Response Rate Ratio)2 Changes in periportal and portal zones have been correlated with liver outcomes and mortality by analysis of liver biopsies (n=452) from SteatoSITE study1 1. Kendall TJ, et al. Liver Int. 2024;44(10):2511-2516. |
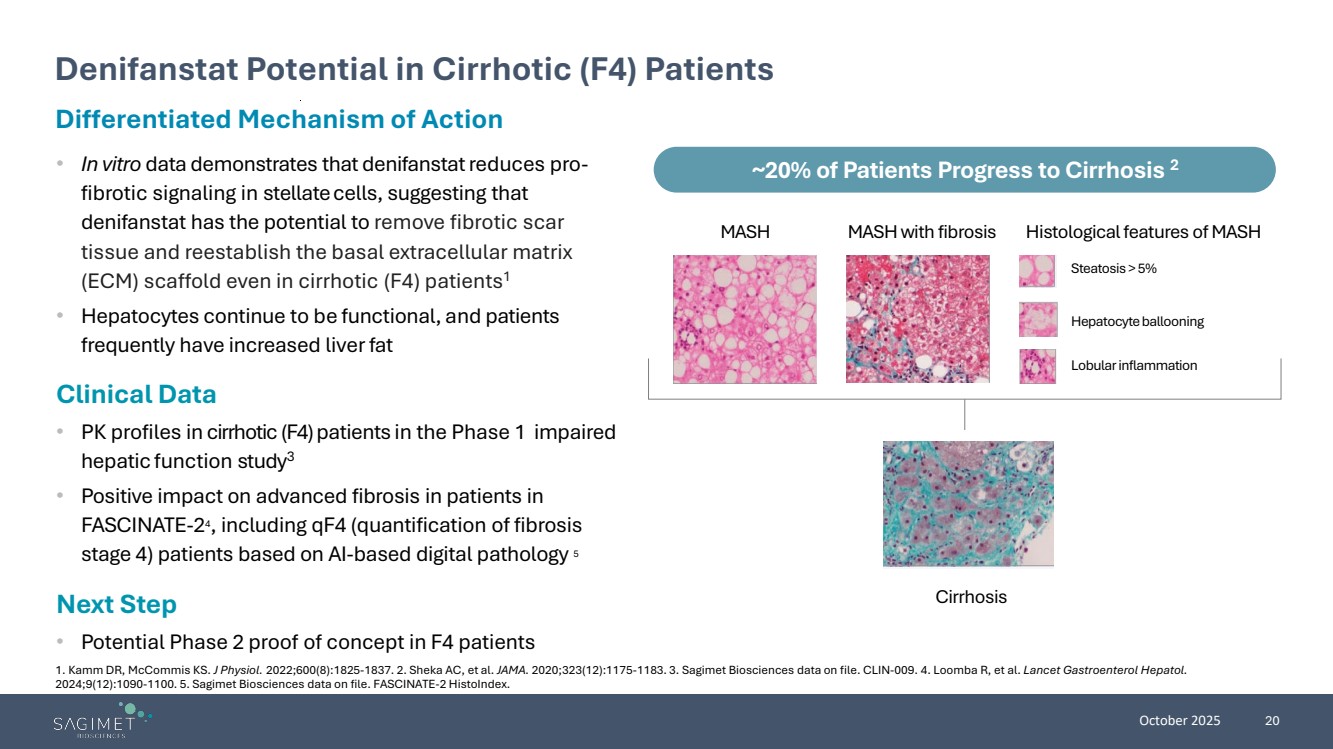
| October 2025 20 Differentiated Mechanism of Action • In vitro data demonstrates that denifanstatreduces pro-fibrotic signaling in stellatecells, suggesting that denifanstat has the potential to remove fibrotic scar tissue and reestablish the basal extracellular matrix (ECM) scaffold even in cirrhotic (F4) patients1 • Hepatocytes continue to be functional, and patients frequently have increased liverfat Clinical Data • PK profiles in cirrhotic (F4) patients in the Phase 1 impaired hepatic function study3 • Positive impact on advanced fibrosis in patients in FASCINATE-24, including qF4 (quantification of fibrosis stage 4) patients based on AI-based digital pathology 5 Next Step • Potential Phase 2 proof of concept in F4 patients Denifanstat Potential in Cirrhotic (F4) Patients ~20% of Patients Progress to Cirrhosis 2 MASH MASH with fibrosis Histological features of MASH Steatosis > 5% Hepatocyte ballooning Lobular inflammation Cirrhosis 1. Kamm DR, McCommis KS. J Physiol. 2022;600(8):1825-1837. 2. Sheka AC, et al. JAMA. 2020;323(12):1175-1183. 3. Sagimet Biosciences data on file. CLIN-009. 4. Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100. 5. Sagimet Biosciences data on file. FASCINATE-2 HistoIndex. |
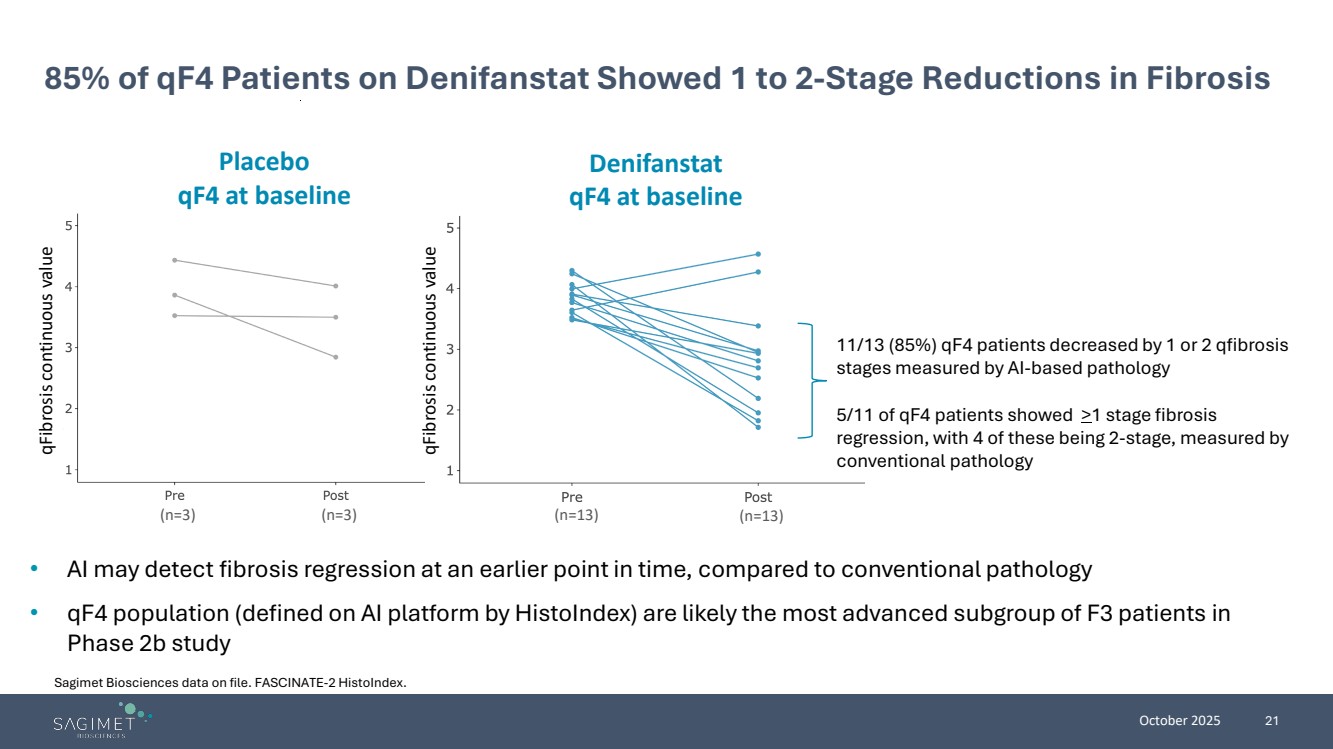
| October 2025 21 85% of qF4 Patients on Denifanstat Showed 1 to 2-Stage Reductions in Fibrosis • AI may detect fibrosis regression at an earlier point in time, compared to conventional pathology • qF4 population (defined on AI platform by HistoIndex) are likely the most advanced subgroup of F3 patients in Phase 2b study (n=3) (n=3) (n=13) (n=13) Placebo qF4 at baseline Denifanstat qF4 at baseline qFibrosis continuous value qFibrosis continuous value 11/13 (85%) qF4 patients decreased by 1 or 2 qfibrosis stages measured by AI-based pathology 5/11 of qF4 patients showed >1 stage fibrosis regression, with 4 of these being 2-stage, measured by conventional pathology Sagimet Biosciences data on file. FASCINATE-2 HistoIndex. |
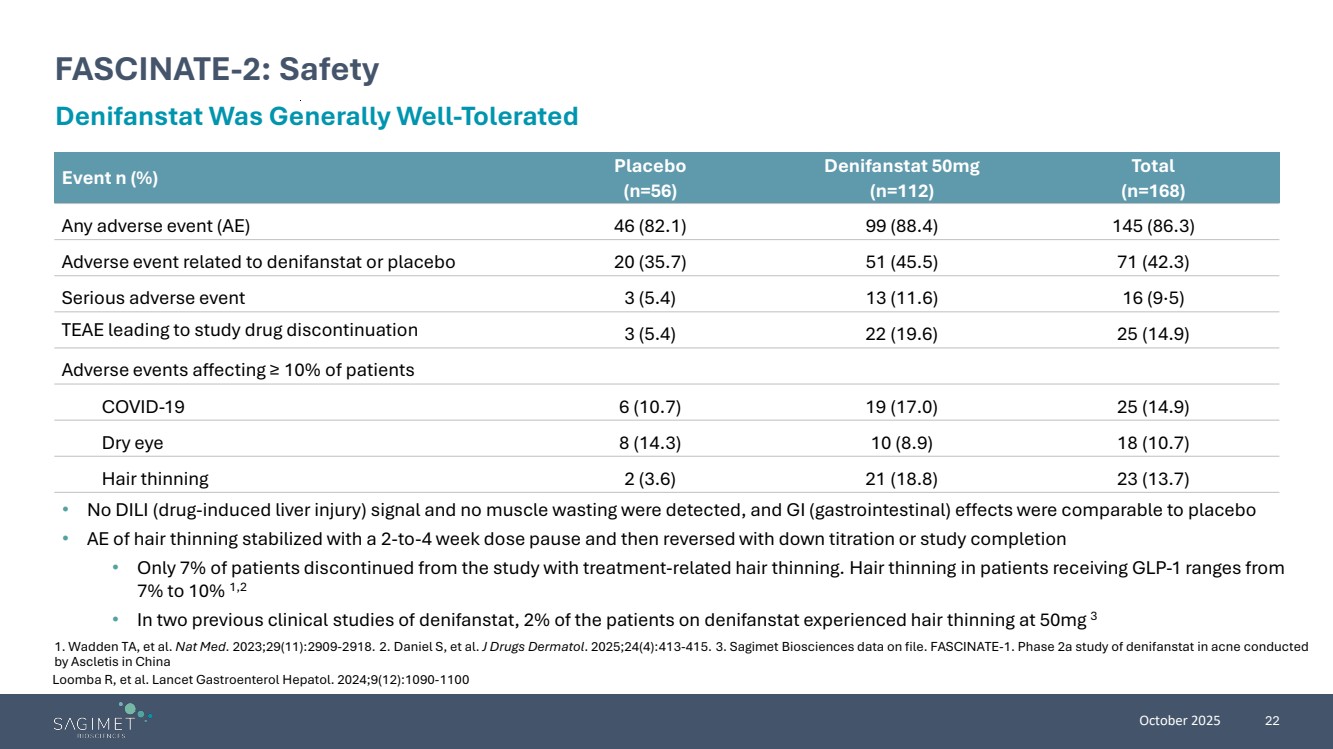
| October 2025 22 FASCINATE-2: Safety Denifanstat Was Generally Well-Tolerated • No DILI (drug-induced liver injury) signal and no muscle wasting were detected, and GI (gastrointestinal) effects were comparable to placebo • AE of hair thinning stabilized with a 2-to-4 week dose pause and then reversed with down titration or study completion • Only 7% of patients discontinued from the study with treatment-related hair thinning. Hair thinning in patients receiving GLP-1 ranges from 7% to 10% 1,2 • In two previous clinical studies of denifanstat, 2% of the patients on denifanstat experienced hair thinning at 50mg 3 Event n (%) Placebo (n=56) Denifanstat 50mg (n=112) Total (n=168) Any adverse event (AE) 46 (82.1) 99 (88.4) 145 (86.3) Adverse event related to denifanstat or placebo 20 (35.7) 51 (45.5) 71 (42.3) Serious adverse event 3 (5.4) 13 (11.6) 16 (9·5) TEAE leading to study drug discontinuation 3 (5.4) 22 (19.6) 25 (14.9) Adverse events affecting ≥ 10% of patients COVID-19 6 (10.7) 19 (17.0) 25 (14.9) Dry eye 8 (14.3) 10 (8.9) 18 (10.7) Hair thinning 2 (3.6) 21 (18.8) 23 (13.7) 1. Wadden TA, et al. Nat Med. 2023;29(11):2909-2918. 2. Daniel S, et al. J Drugs Dermatol. 2025;24(4):413-415. 3. Sagimet Biosciences data on file. FASCINATE-1. Phase 2a study of denifanstat in acne conducted by Ascletis in China Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100 |
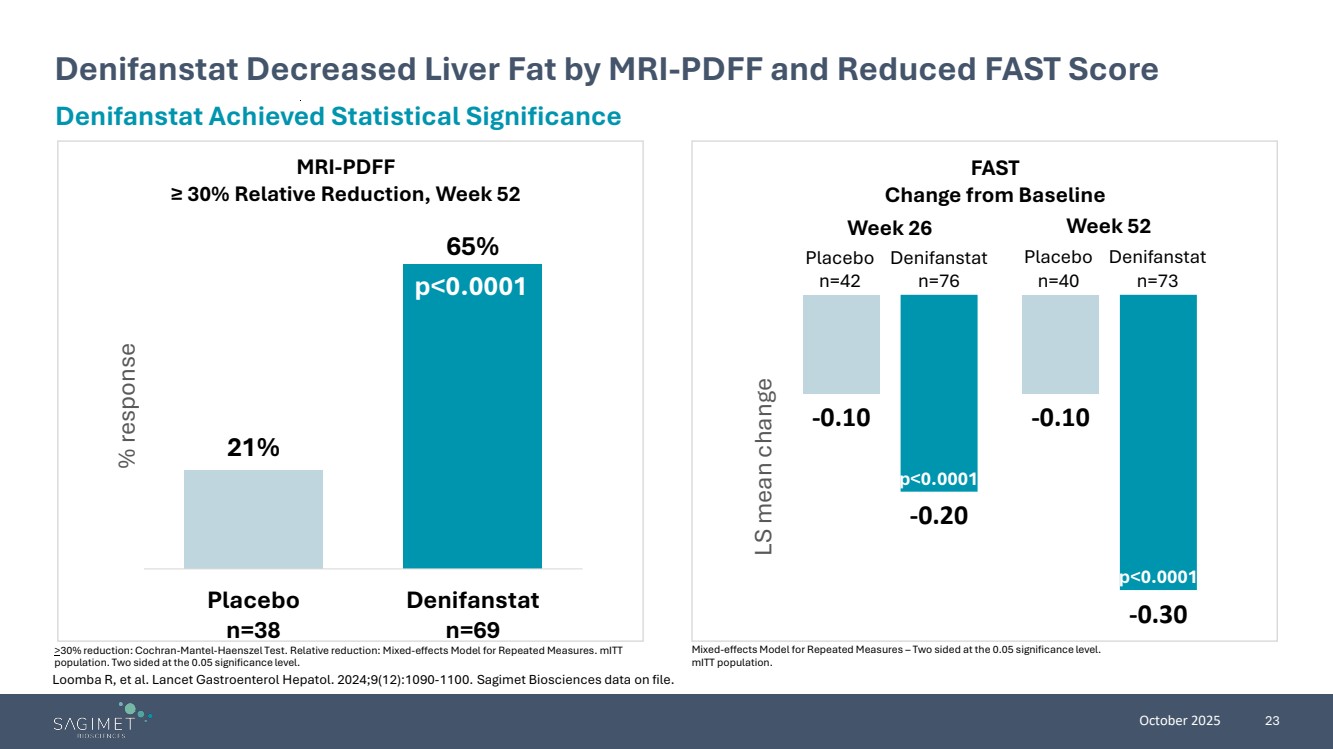
| October 2025 23 Denifanstat Decreased Liver Fat by MRI-PDFF and Reduced FAST Score >30% reduction: Cochran-Mantel-Haenszel Test. Relative reduction: Mixed-effects Model for Repeated Measures. mITT population. Two sided at the 0.05 significance level. Denifanstat Achieved Statistical Significance 21% 65% Placebo n=38 Denifanstat n=69 % response p<0.0001 MRI-PDFF ≥ 30% Relative Reduction, Week 52 FAST Change from Baseline -0.10 -0.10 -0.20 -0.30 LS mean change p<0.0001 p<0.0001 Week 26 Week 52 Placebo n=42 Denifanstat n=76 Placebo n=40 Denifanstat n=73 Mixed-effects Model for Repeated Measures – Two sided at the 0.05 significance level. mITT population. Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100. Sagimet Biosciences data on file. |
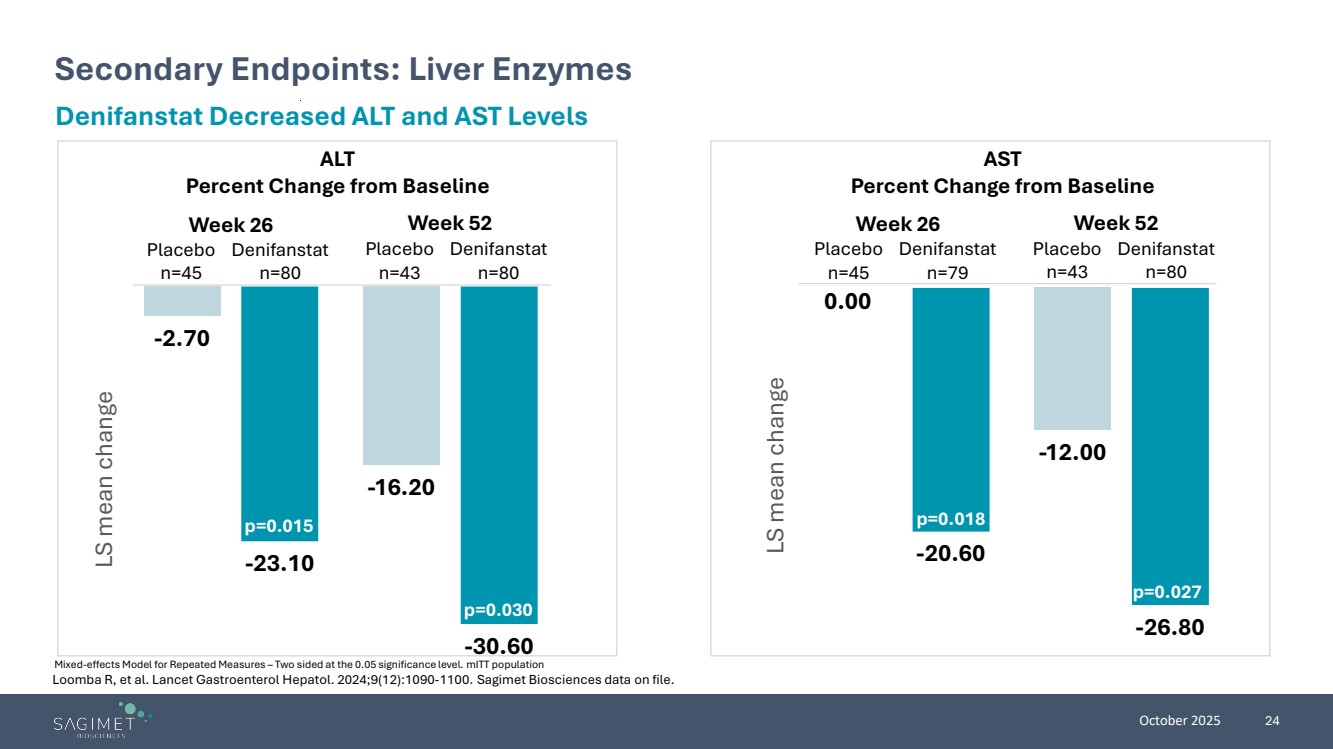
| October 2025 24 Secondary Endpoints: Liver Enzymes Mixed-effects Model for Repeated Measures – Two sided at the 0.05 significance level. mITT population Denifanstat Decreased ALT and AST Levels ALT Percent Change from Baseline AST Percent Change from Baseline -2.70 -16.20 -23.10 -30.60 LS mean change p=0.015 p=0.030 0.00 -12.00 -20.60 -26.80 LS mean change p=0.018 p=0.027 Week 26 Week 52 Week 26 Week 52 Placebo n=45 Denifanstat n=80 Placebo n=43 Denifanstat n=80 Placebo n=45 Denifanstat n=79 Placebo n=43 Denifanstat n=80 Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100. Sagimet Biosciences data on file. |
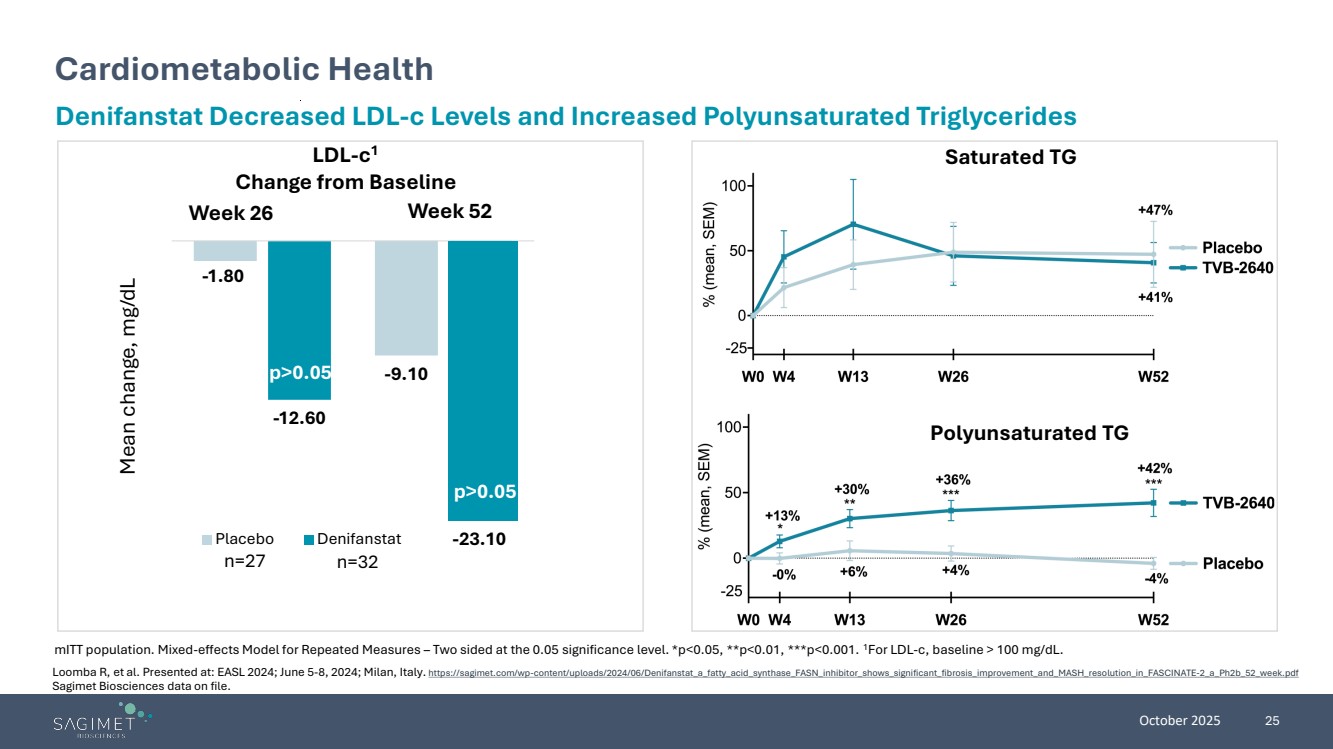
| October 2025 25 Cardiometabolic Health mITT population. Mixed-effects Model for Repeated Measures – Two sided at the 0.05 significance level. *p<0.05, **p<0.01, ***p<0.001. 1For LDL-c, baseline > 100 mg/dL. Denifanstat Decreased LDL-c Levels and Increased Polyunsaturated Triglycerides LDL-c1 Change from Baseline Saturated TG Polyunsaturated TG Week 26 Week 52 p>0.05 Mean change, mg/dL n=27 n=32 -1.80 -9.10 -12.60 Placebo Denifanstat -23.10 p>0.05 -1.80 n=27 n=32 p>0.05 Loomba R, et al. Presented at: EASL 2024; June 5-8, 2024; Milan, Italy. https://sagimet.com/wp-content/uploads/2024/06/Denifanstat_a_fatty_acid_synthase_FASN_inhibitor_shows_significant_fibrosis_improvement_and_MASH_resolution_in_FASCINATE-2_a_Ph2b_52_week.pdf Sagimet Biosciences data on file. |
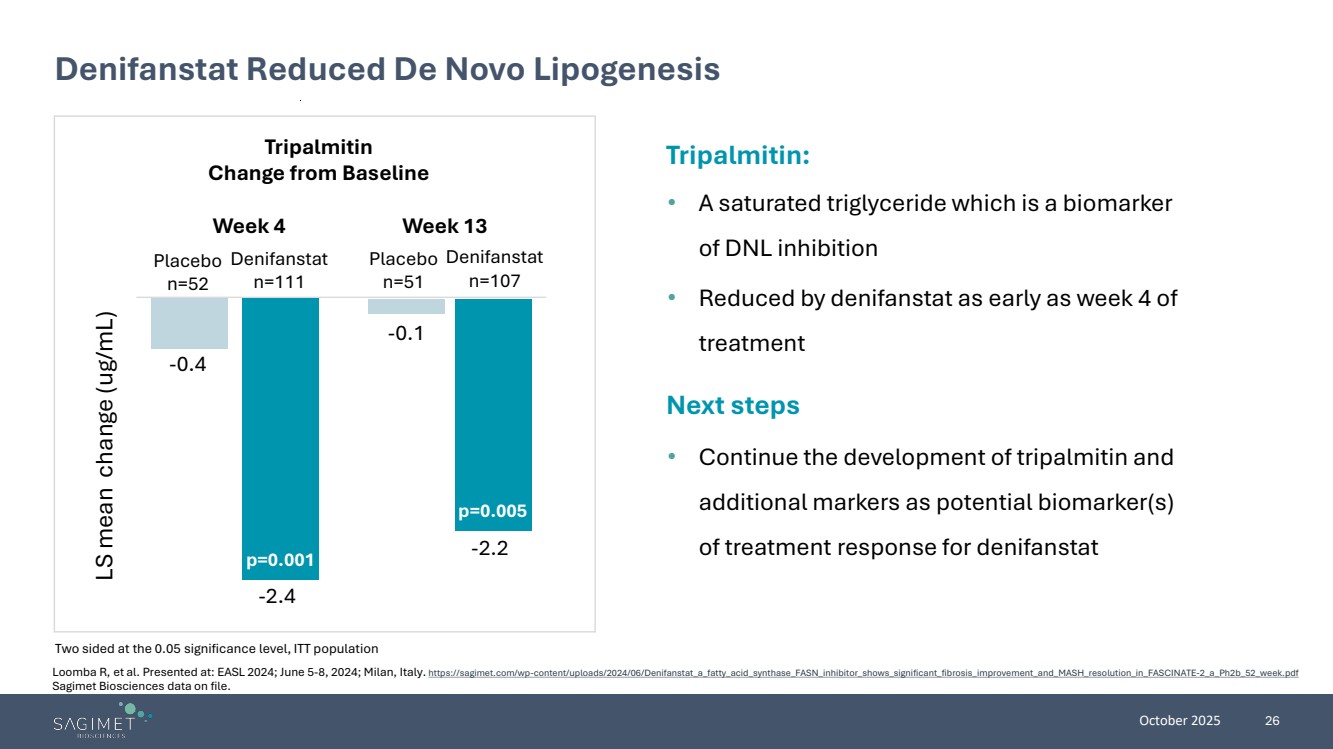
| October 2025 26 Denifanstat Reduced De Novo Lipogenesis Two sided at the 0.05 significance level, ITT population Tripalmitin: • A saturated triglyceride which is a biomarker of DNL inhibition • Reduced by denifanstat as early as week 4 of treatment Next steps • Continue the development of tripalmitin and additional markers as potential biomarker(s) of treatment response for denifanstat Tripalmitin Change from Baseline Placebo n=52 Denifanstat n=111 Placebo n=51 Denifanstat n=107 -2.2 LS mean change (ug/mL) -2.4 -0.4 -0.1 Week 4 Week 13 p=0.001 p=0.005 Loomba R, et al. Presented at: EASL 2024; June 5-8, 2024; Milan, Italy. https://sagimet.com/wp-content/uploads/2024/06/Denifanstat_a_fatty_acid_synthase_FASN_inhibitor_shows_significant_fibrosis_improvement_and_MASH_resolution_in_FASCINATE-2_a_Ph2b_52_week.pdf Sagimet Biosciences data on file. |
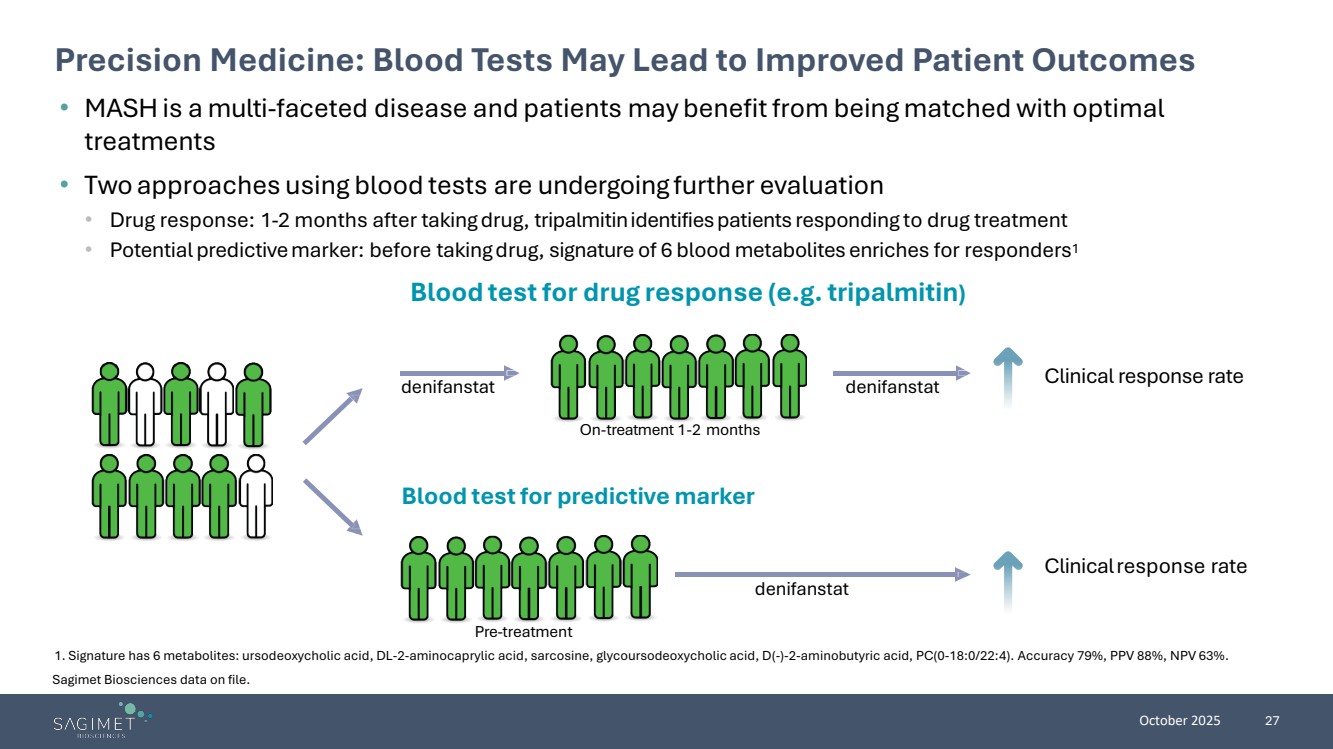
| October 2025 27 Precision Medicine: Blood Tests May Lead to Improved Patient Outcomes 1. Signature has 6 metabolites: ursodeoxycholic acid, DL-2-aminocaprylic acid, sarcosine, glycoursodeoxycholic acid, D(-)-2-aminobutyric acid, PC(0-18:0/22:4). Accuracy 79%, PPV 88%, NPV 63%. • MASH is a multi-faceted disease and patients may benefit from being matched with optimal treatments • Two approaches using blood tests are undergoing further evaluation • Drug response: 1-2 months after takingdrug, tripalmitinidentifiespatients responding to drug treatment • Potential predictivemarker: before takingdrug, signature of 6 blood metabolites enriches for responders1 Blood testfor predictive marker denifanstat denifanstat denifanstat Clinicalresponse rate On-treatment 1-2 months Pre-treatment Clinical response rate Blood testfor drug response (e.g.tripalmitin) Sagimet Biosciences data on file. |
| Combination Therapy Development Program for MASH |
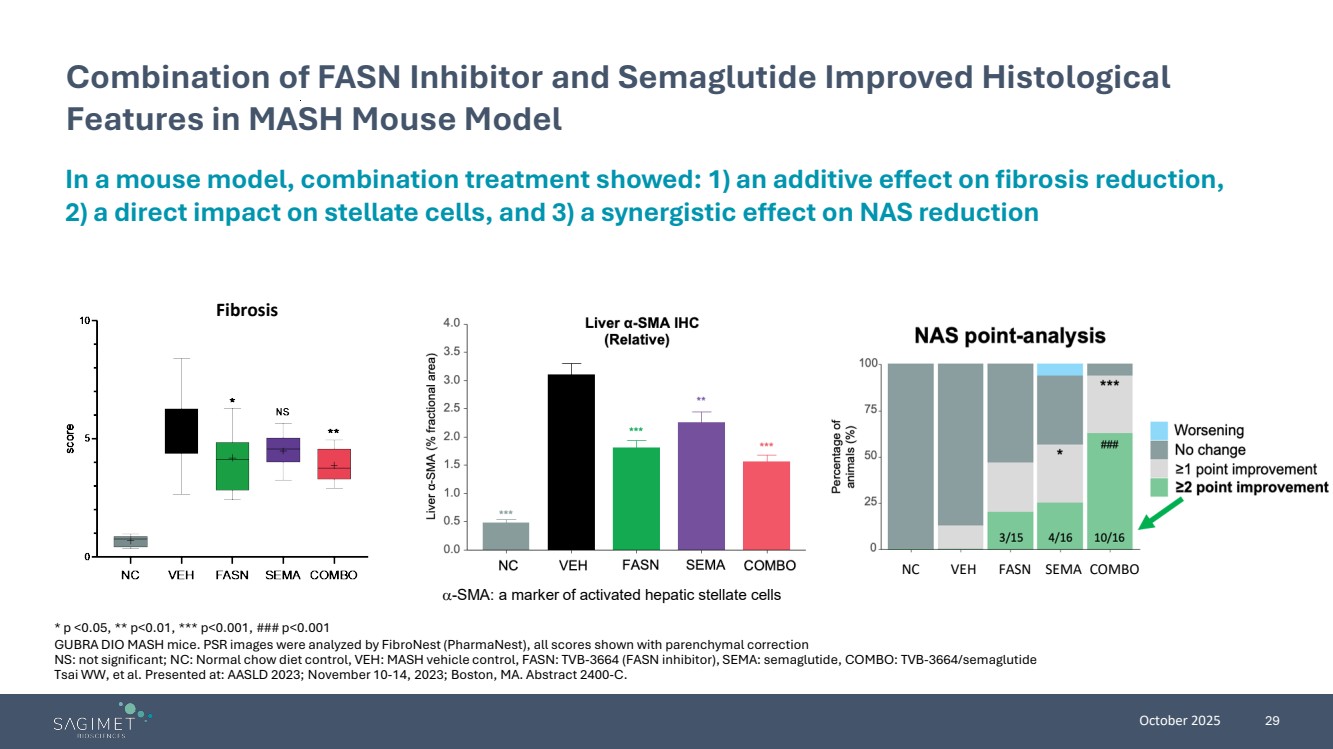
| October 2025 29 Combination of FASN Inhibitor and Semaglutide Improved Histological Features in MASH Mouse Model α-SMA: a marker of activated hepatic stellate cells Fibrosis NC VEH FASN SEMA COMBO In a mouse model, combination treatment showed: 1) an additive effect on fibrosis reduction, 2) a direct impact on stellate cells, and 3) a synergistic effect on NAS reduction * p <0.05, ** p<0.01, *** p<0.001, ### p<0.001 GUBRA DIO MASH mice. PSR images were analyzed by FibroNest (PharmaNest), all scores shown with parenchymal correction NS: not significant; NC: Normal chow diet control, VEH: MASH vehicle control, FASN: TVB-3664 (FASN inhibitor), SEMA: semaglutide, COMBO: TVB-3664/semaglutide Tsai WW, et al. Presented at: AASLD 2023; November 10-14, 2023; Boston, MA. Abstract 2400-C. |
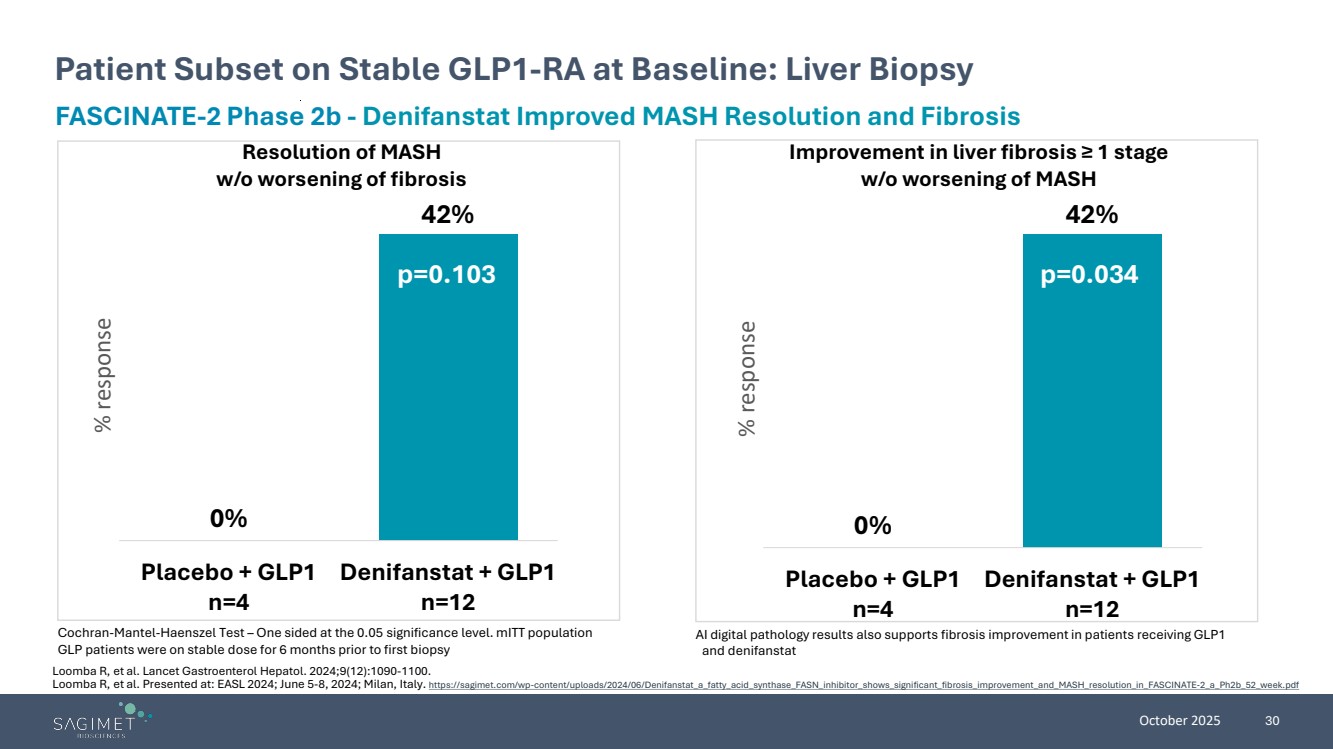
| October 2025 30 Patient Subset on Stable GLP1-RA at Baseline: Liver Biopsy Cochran-Mantel-Haenszel Test – One sided at the 0.05 significance level. mITT population GLP patients were on stable dose for 6 months prior to first biopsy FASCINATE-2 Phase 2b - Denifanstat Improved MASH Resolution and Fibrosis Resolution of MASH w/o worsening of fibrosis Improvement in liver fibrosis ≥ 1 stage w/o worsening of MASH 0% 42% Placebo + GLP1 n=4 Denifanstat + GLP1 n=12 % response 0% 42% Placebo + GLP1 n=4 Denifanstat + GLP1 n=12 % response p=0.103 p=0.034 AI digital pathology results also supports fibrosis improvement in patients receiving GLP1 and denifanstat Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100. Loomba R, et al. Presented at: EASL 2024; June 5-8, 2024; Milan, Italy. https://sagimet.com/wp-content/uploads/2024/06/Denifanstat_a_fatty_acid_synthase_FASN_inhibitor_shows_significant_fibrosis_improvement_and_MASH_resolution_in_FASCINATE-2_a_Ph2b_52_week.pdf |
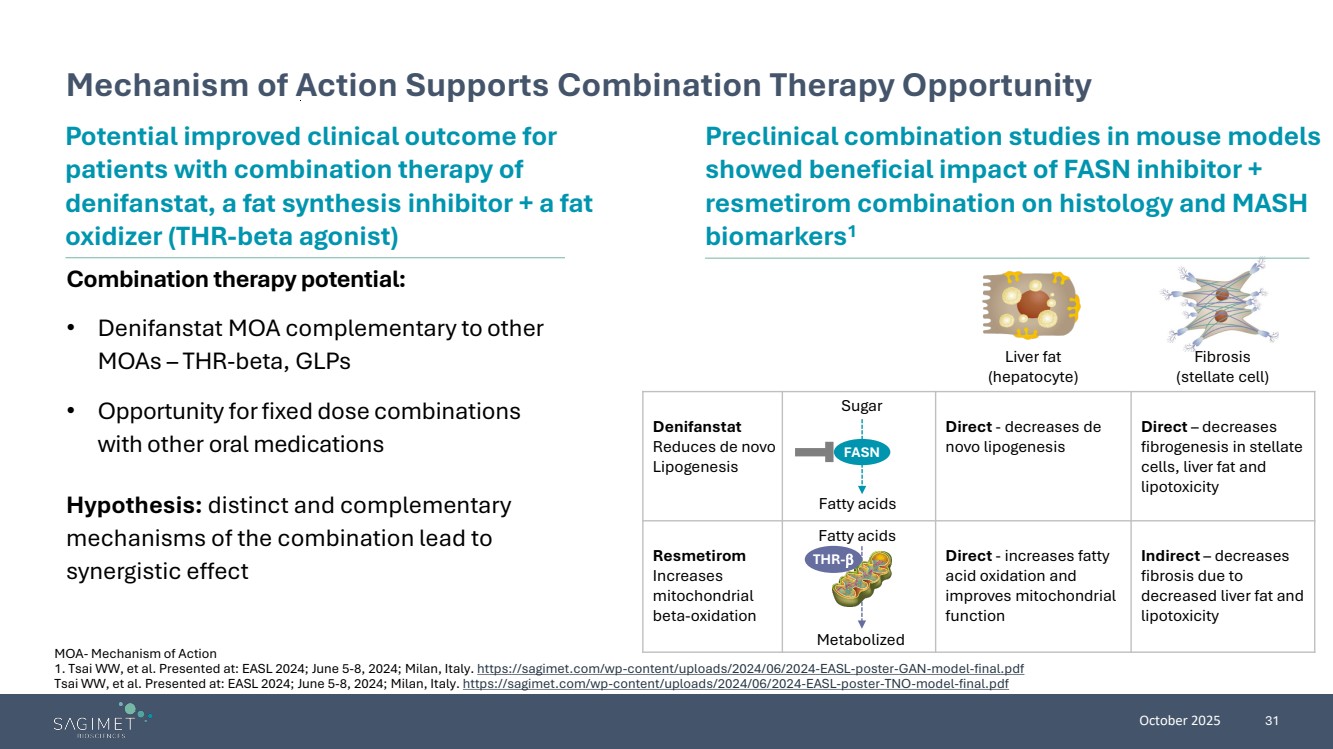
| October 2025 31 Mechanism of Action Supports Combination Therapy Opportunity MOA- Mechanism of Action 1. Tsai WW, et al. Presented at: EASL 2024; June 5-8, 2024; Milan, Italy. https://sagimet.com/wp-content/uploads/2024/06/2024-EASL-poster-GAN-model-final.pdf Tsai WW, et al. Presented at: EASL 2024; June 5-8, 2024; Milan, Italy. https://sagimet.com/wp-content/uploads/2024/06/2024-EASL-poster-TNO-model-final.pdf Combination therapy potential: • Denifanstat MOA complementary to other MOAs – THR-beta, GLPs • Opportunity forfixed dose combinations with other oral medications Potential improved clinical outcome for patients with combination therapy of denifanstat, a fat synthesis inhibitor + a fat oxidizer (THR-beta agonist) Hypothesis: distinct and complementary mechanisms of the combination lead to synergistic effect Preclinical combination studies in mouse models showed beneficial impact of FASN inhibitor + resmetirom combination on histology and MASH biomarkers1 Liver fat (hepatocyte) Fibrosis (stellate cell) Denifanstat Reduces de novo Lipogenesis Direct - decreases de novo lipogenesis Direct – decreases fibrogenesis in stellate cells, liver fat and lipotoxicity Resmetirom Increases mitochondrial beta-oxidation Direct - increases fatty acid oxidation and improves mitochondrial function Indirect – decreases fibrosis due to decreased liver fat and lipotoxicity FASN THR-β Fatty acids Metabolized Sugar Fatty acids |
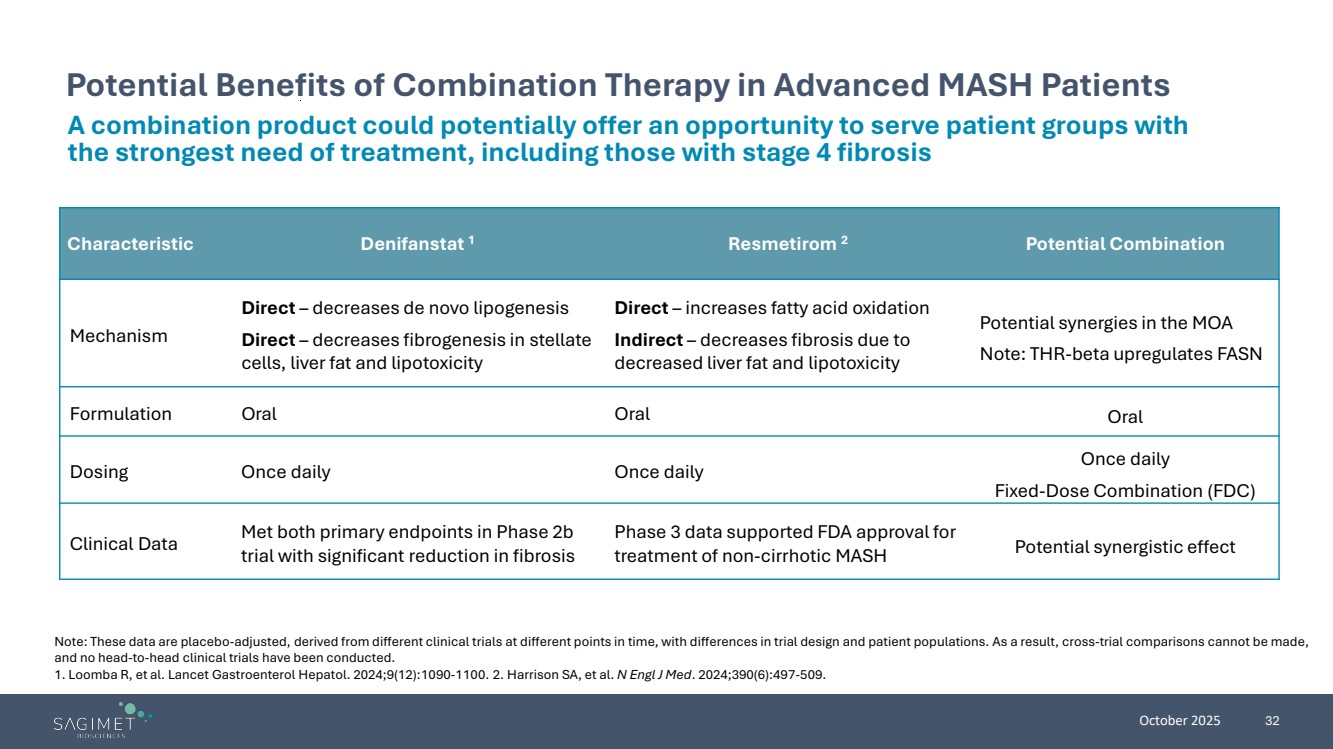
| October 2025 32 Potential Benefits of Combination Therapy in Advanced MASH Patients Note: These data are placebo-adjusted, derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. 1. Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100. 2. Harrison SA, et al. N Engl J Med. 2024;390(6):497-509. Characteristic Denifanstat 1 Resmetirom 2 Potential Combination Mechanism Direct – decreases de novo lipogenesis Direct – decreases fibrogenesis in stellate cells, liver fat and lipotoxicity Direct – increases fatty acid oxidation Indirect – decreases fibrosis due to decreased liver fat and lipotoxicity Potential synergies in the MOA Note: THR-beta upregulates FASN Formulation Oral Oral Oral Dosing Once daily Once daily Once daily Fixed-Dose Combination (FDC) Clinical Data Met both primary endpoints in Phase 2b trial with significant reduction in fibrosis Phase 3 data supported FDA approval for treatment of non-cirrhotic MASH Potential synergistic effect A combination product could potentially offer an opportunity to serve patient groups with the strongest need of treatment, including those with stage 4 fibrosis |
| October 2025 33 Step 1 - Phase 1 PK trial for combination of denifanstat and resmetirom • PK to evaluate any drug/drug interaction • Assess safety/tolerability • Confirm optimal combination dose levels for later clinical efficacy study in MASH Step 2, subject to consultation with regulatory authorities - Phase 2 clinical combination study with denifanstat and resmetirom in F4 MASH patients • At least 52 weeks combination treatment • Non-invasive biomarkers for early readout to show potential beneficial impact of the combination • Primary endpoint: liver biopsies Potential Clinical Development Program for Denifanstat and Resmetirom Combination Phase 1 started in September 2025 Phase 2 trial design will be informed by the results of the Phase 1 trial |
| October 2025 34 Attractiveness of a Potential Denifanstat/Resmetirom Combination • Phase 1 clinical trial to evaluate the pharmacokinetics (PK) and tolerability of a combination of denifanstat and resmetirom initiated in September 2025; data readout expected 1H 2026 • If the outcome of this Phase 1 trial is positive, will explore moving into the development of a combination product for patients living with MASH • Prudent deployment of resources with quick path to go/no go decision Denifanstat in MASH Potential of a Fixed Dose Combination Next Steps • Denifanstat directly targets the 3 key drivers of MASH (metabolic dysfunction-associated steatohepatitis) – liver fat, inflammation, and fibrosis • Successful outcome of Phase 2b trial; met both primary endpoints with significant reduction in fibrosis 1 • Pre-clinical data demonstrated synergistic effect of combination of FASN inhibitor and resmetirom 2 • Combination of a Phase 3-ready drug candidate, with the first drug approved for MASH • IP for the combination of denifanstat and resmetirom: • Application filed 2024; if granted—2044; potential PTE to 2048 • Potential oral, once-daily product • Potential to address an unmet need in MASH advanced patients (F4) 1. Loomba R, et al. Lancet Gastroenterol Hepatol. 2024;9(12):1090-1100. 2. Tsai WW, et al. Presented at: EASL 2024; June 5-8, 2024; Milan, Italy. https://sagimet.com/wp-content/uploads/2024/06/2024-EASL-poster-GAN-model-final.pdf Tsai WW, et al. Presented at: EASL 2024; June 5-8, 2024; Milan, Italy. https://sagimet.com/wp-content/uploads/2024/06/2024-EASL-poster-TNO-model-final.pdf |
| FASN Inhibition Offers Potential Benefit in Multiple Indications: Acne |
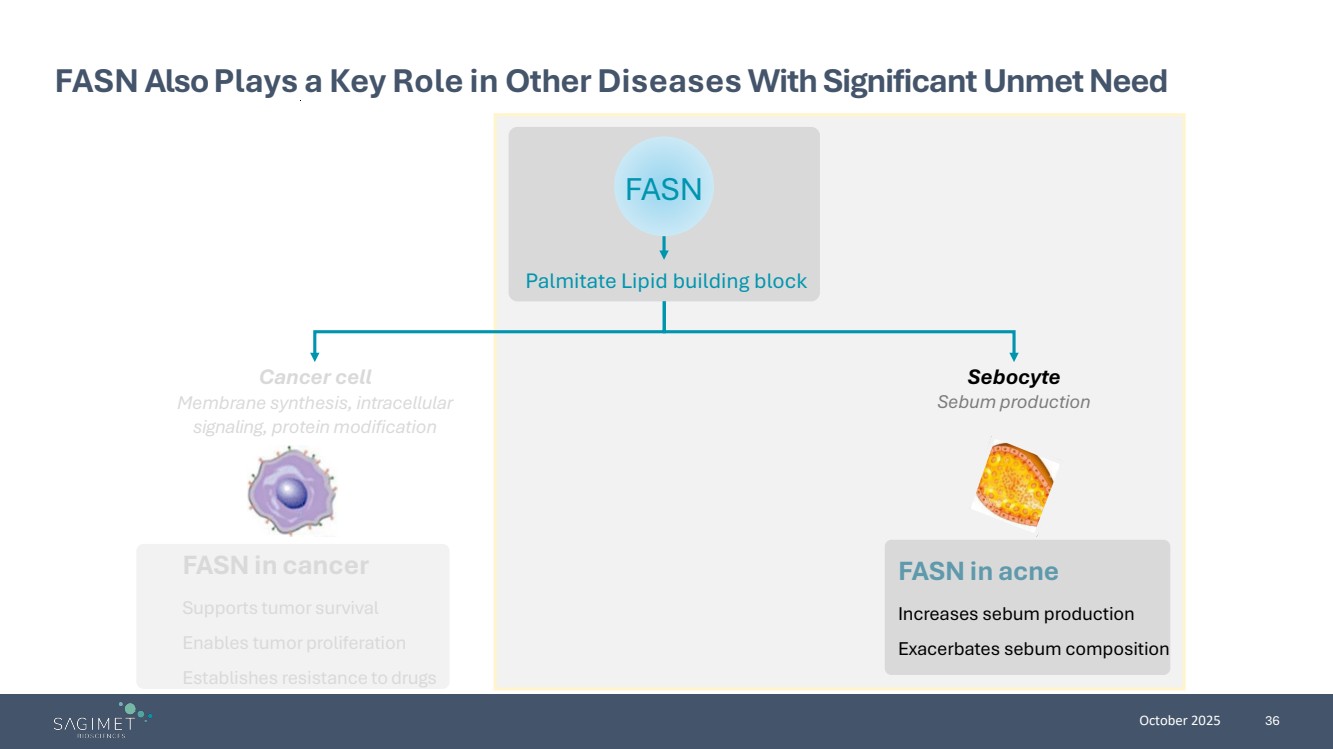
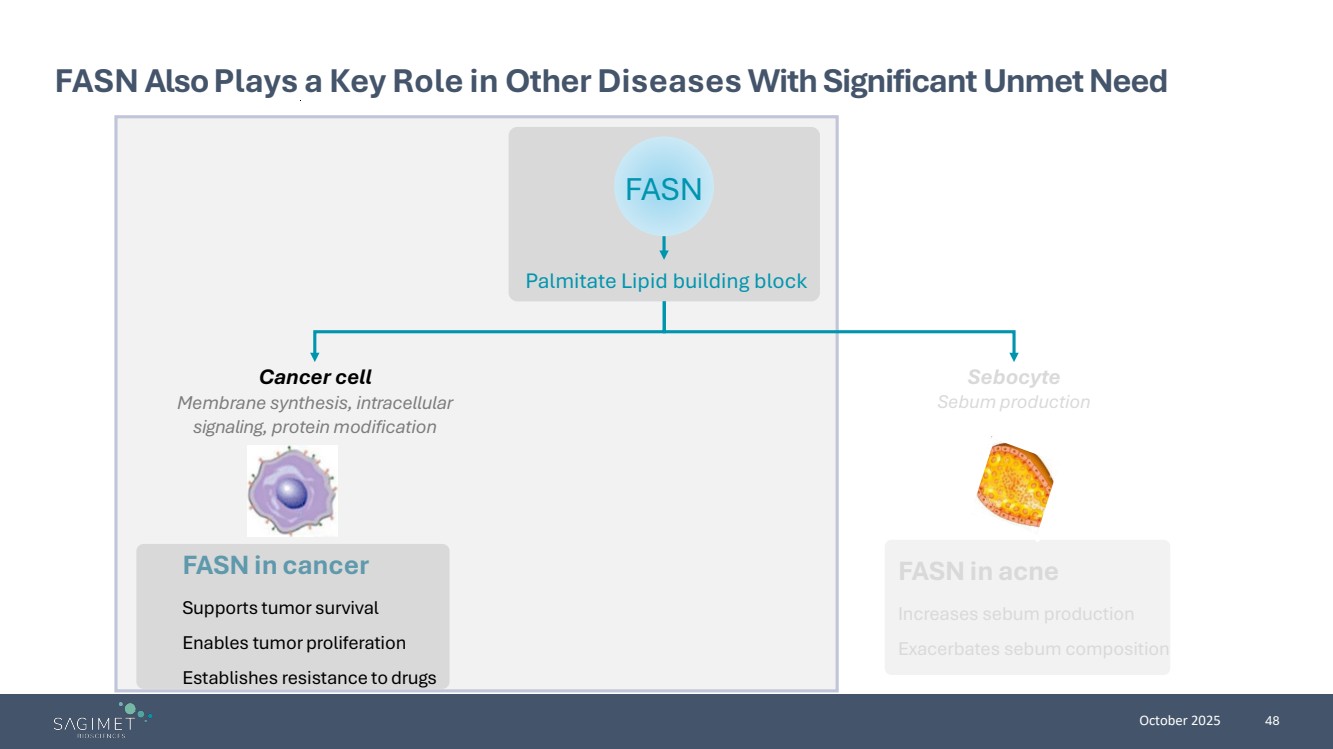
| October 2025 36 FASN Also Plays a Key Role in Other Diseases With Significant Unmet Need FASN in cancer Supports tumor survival Enables tumor proliferation Establishes resistance to drugs FASN in acne Increases sebum production Exacerbates sebum composition Cancer cell Membrane synthesis, intracellular signaling, protein modification Sebocyte Sebum production Palmitate Lipid building block FASN |
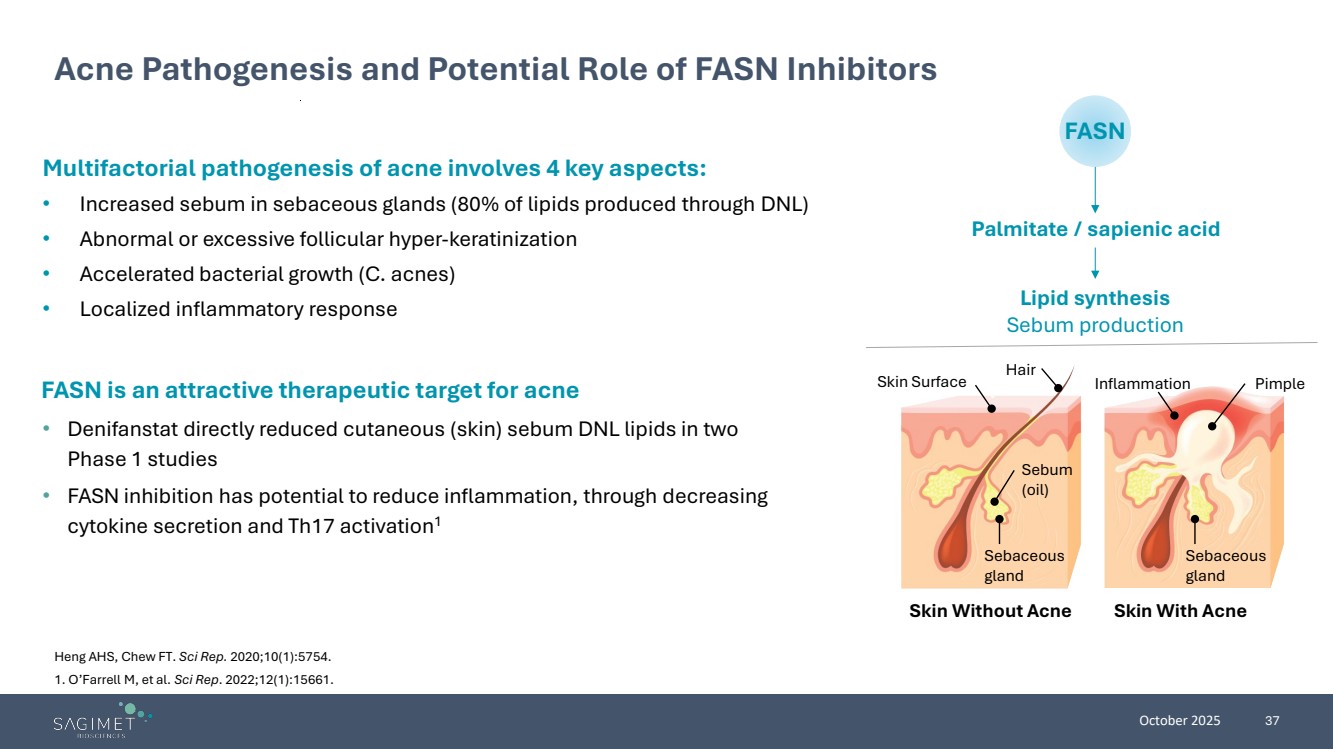
| October 2025 37 Multifactorial pathogenesis of acne involves 4 key aspects: • Increased sebum in sebaceous glands (80% of lipids produced through DNL) • Abnormal or excessive follicular hyper-keratinization • Accelerated bacterial growth (C. acnes) • Localized inflammatory response Acne Pathogenesis and Potential Role of FASN Inhibitors Heng AHS, Chew FT. Sci Rep. 2020;10(1):5754. 1. O’Farrell M, et al. Sci Rep. 2022;12(1):15661. FASN Palmitate / sapienic acid Lipid synthesis Sebum production Hair Skin Surface Sebum (oil) Inflammation Sebaceous gland Skin Without Acne Skin With Acne Pimple Sebaceous gland FASN is an attractive therapeutic target for acne • Denifanstat directly reduced cutaneous (skin) sebum DNL lipids in two Phase 1 studies • FASN inhibition has potential to reduce inflammation, through decreasing cytokine secretion and Th17 activation1 |
| October 2025 38 Blackheads Whiteheads Papules & Pustules Cysts & Nodules Acne Market Overview Global acne market is expected to reach $17B in next decade1 5.1 million US acne patients are treated by dermatologists annually (total US acne market is 50 million people) 2,3 • Acne is the #1 or #2 patient concern in dermatology offices and 65%+ of patients in dermatology offices have private insurance4 • Although acne treatments are currently available, dermatologists are open to new therapies (Seysara® Tablets & Winlevi® Cream) • There is no cure for acne; due to its pathology, most patients require chronic management and multiple courses for flare control annually Acne patients visiting a dermatologist are aligned to potential positioning of FASN inhibitor4 • 70% of patients presenting to dermatologists have moderate to severe disease4 • Approximately 70% of patients have inflammatory lesions, and 16% of patients are post-menopausal women3 1. www.expertmarketresearch.com/reports/acne-treatment-market 2. Bickers DR, et al. J Am Acad Dermatol. 2006;55(3):490-500. 3. American Academy of Dermatology. Burden of skin disease. 2017. www.aad.org/BSD. 4. Sagimet Biosciences data on file. Market research conducted in July 2024 among 50 dermatologists. |
| October 2025 39 Mild Disease Moderate to Severe Disease Acne Treatment Algorithm • Most acne patients receive skin care routines that include OTC cleansers and moisturizers to address AEs associated with their treatment Disease management involves flare and prevention intervention Treatment includes topical agents used as mono-therapy, combination therapy, or with fixed dosed combination products Main topical therapy categories • Retinoids • Benzoyl Peroxide • Antibiotics • Clascoterone • Salicylic Acid • Azelaic Acid Treatment approach adds oral products on top of the topical agents Main oral therapy categories: • Antibiotics (tetracyclines, sarecycline) • Hormonal contraceptives • Spironolactone (off-label) • Intralesional corticosteroids Severe (cystic) patients are generally managed with isotretinoin (Accutane®) Main therapy categories: • Isotretinoin Severe (Cystic) Disease |
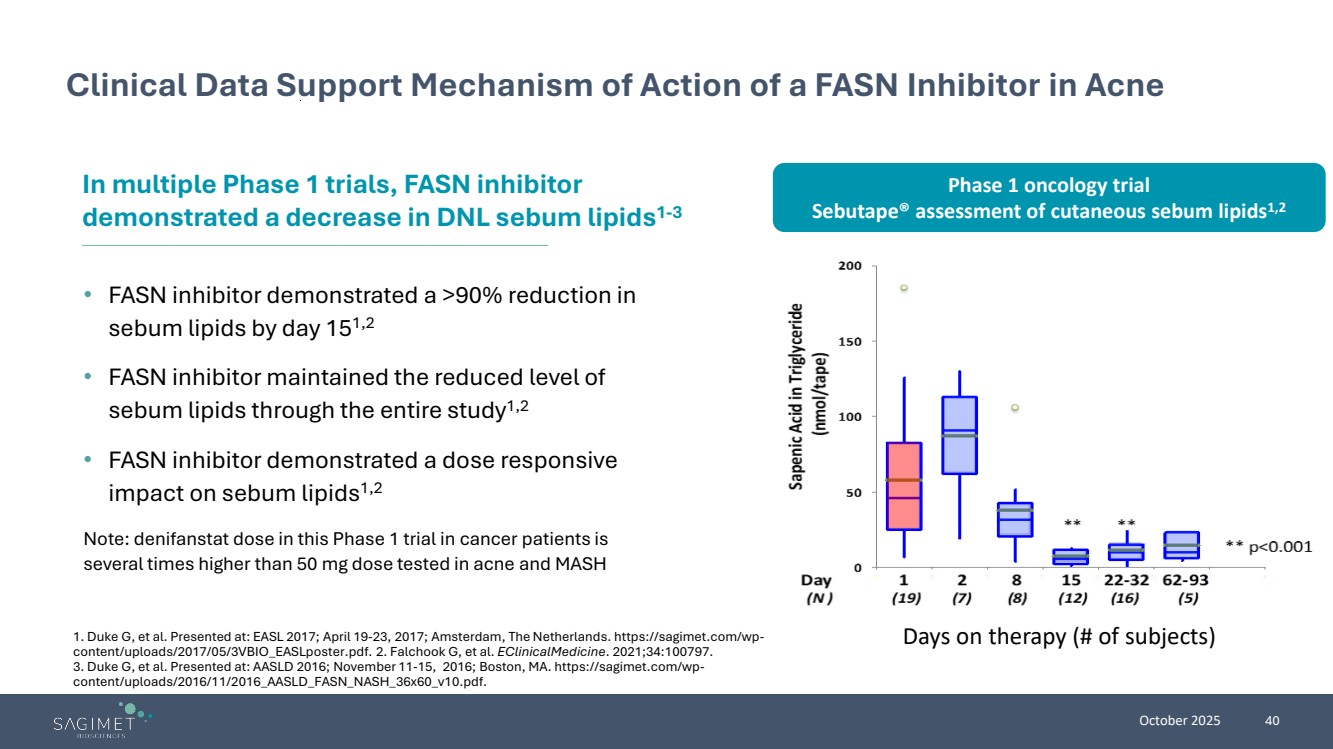
| October 2025 40 Clinical Data Support Mechanism of Action of a FASN Inhibitor in Acne • FASN inhibitor demonstrated a >90% reduction in sebum lipids by day 151,2 • FASN inhibitor maintained the reduced level of sebum lipids through the entire study1,2 • FASN inhibitor demonstrated a dose responsive impact on sebum lipids1,2 Note: denifanstat dose in this Phase 1 trial in cancer patients is several times higher than 50 mg dose tested in acne and MASH In multiple Phase 1 trials, FASN inhibitor demonstrated a decrease in DNL sebum lipids1-3 1. Duke G, et al. Presented at: EASL 2017; April 19-23, 2017; Amsterdam, The Netherlands. https://sagimet.com/wp-content/uploads/2017/05/3VBIO_EASLposter.pdf. 2. Falchook G, et al. EClinicalMedicine. 2021;34:100797. 3. Duke G, et al. Presented at: AASLD 2016; November 11-15, 2016; Boston, MA. https://sagimet.com/wp-content/uploads/2016/11/2016_AASLD_FASN_NASH_36x60_v10.pdf. Days on therapy (# of subjects) Phase 1 oncology trial Sebutape® assessment of cutaneous sebum lipids1,2 |
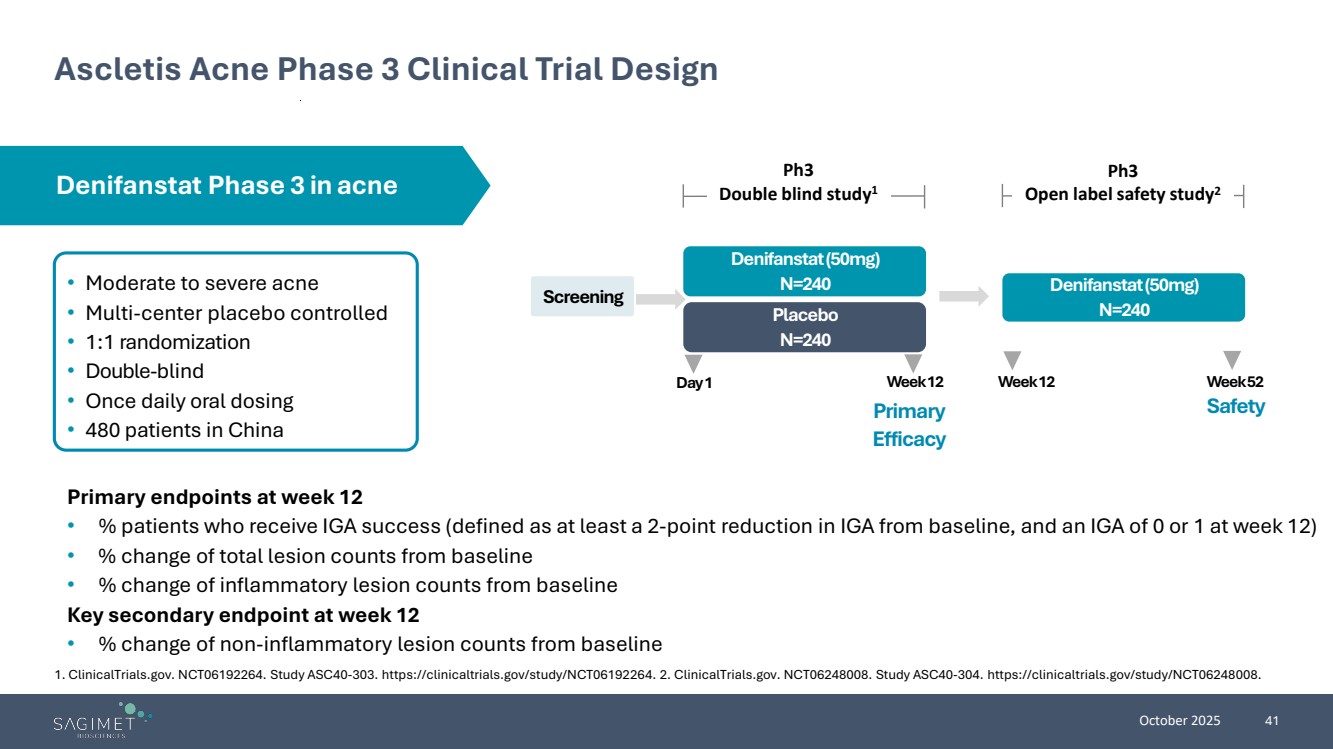
| October 2025 41 Ascletis Acne Phase 3 Clinical Trial Design • Moderate to severe acne • Multi-center placebo controlled • 1:1 randomization • Double-blind • Once daily oral dosing • 480 patients in China Primary endpoints at week 12 • % patients who receive IGA success (defined as at least a 2-point reduction in IGA from baseline, and an IGA of 0 or 1 at week 12) • % change of total lesion counts from baseline • % change of inflammatory lesion counts from baseline Key secondary endpoint at week 12 • % change of non-inflammatory lesion counts from baseline Screening Placebo N=240 Denifanstat (50mg) N=240 Day 1 Week 12 Primary Efficacy Denifanstat (50mg) N=240 Safety Ph3 Double blind study1 Ph3 Open label safety study2 Week 12 Week 52 Denifanstat Phase 3 in acne 1. ClinicalTrials.gov. NCT06192264. Study ASC40-303. https://clinicaltrials.gov/study/NCT06192264. 2. ClinicalTrials.gov. NCT06248008. Study ASC40-304. https://clinicaltrials.gov/study/NCT06248008. |
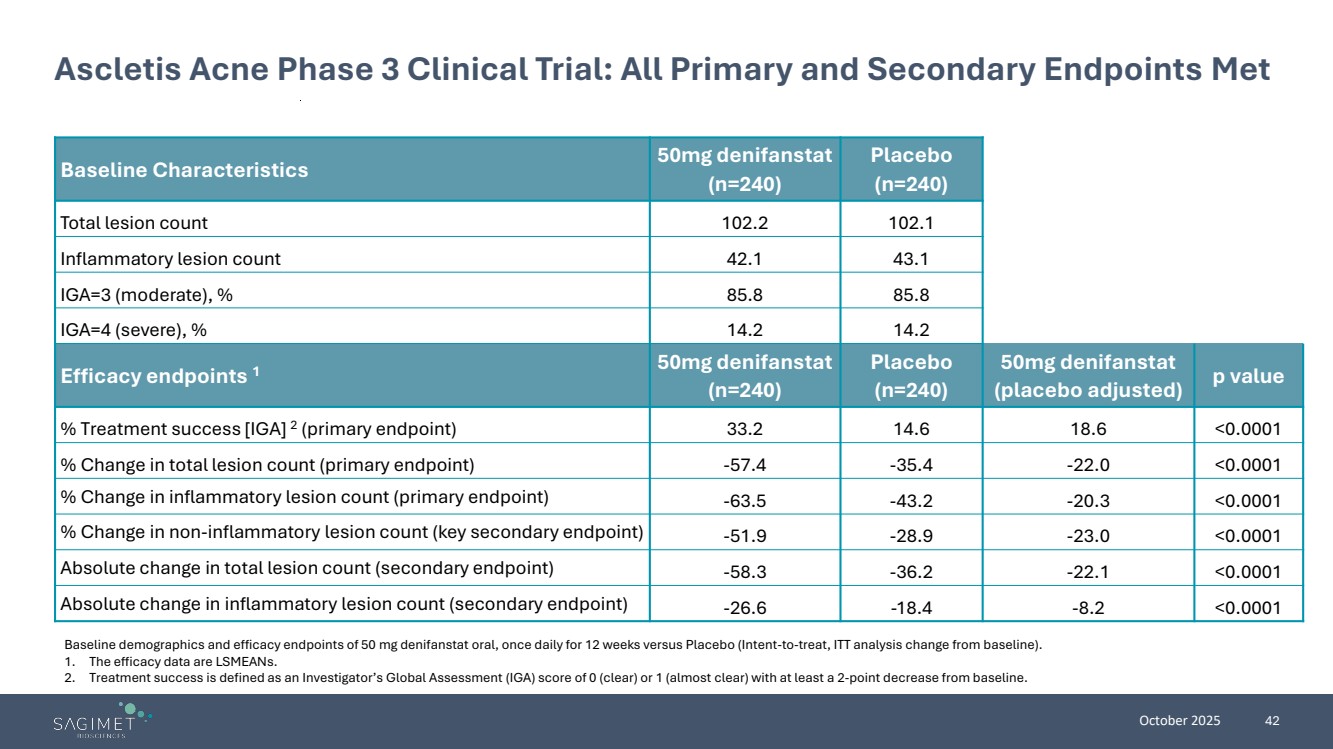
| October 2025 42 Ascletis Acne Phase 3 Clinical Trial: All Primary and Secondary Endpoints Met Baseline Characteristics 50mg denifanstat (n=240) Placebo (n=240) Total lesion count 102.2 102.1 Inflammatory lesion count 42.1 43.1 IGA=3 (moderate), % 85.8 85.8 IGA=4 (severe), % 14.2 14.2 Efficacy endpoints 1 50mg denifanstat (n=240) Placebo (n=240) 50mg denifanstat (placebo adjusted) p value % Treatment success [IGA] 2 (primary endpoint) 33.2 14.6 18.6 <0.0001 % Change in total lesion count (primary endpoint) -57.4 -35.4 -22.0 <0.0001 % Change in inflammatory lesion count (primary endpoint) -63.5 -43.2 -20.3 <0.0001 % Change in non-inflammatory lesion count (key secondary endpoint) -51.9 -28.9 -23.0 <0.0001 Absolute change in total lesion count (secondary endpoint) -58.3 -36.2 -22.1 <0.0001 Absolute change in inflammatory lesion count (secondary endpoint) -26.6 -18.4 -8.2 <0.0001 Baseline demographics and efficacy endpoints of 50 mg denifanstat oral, once daily for 12 weeks versus Placebo (Intent-to-treat, ITT analysis change from baseline). 1. The efficacy data are LSMEANs. 2. Treatment success is defined as an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2-point decrease from baseline. |
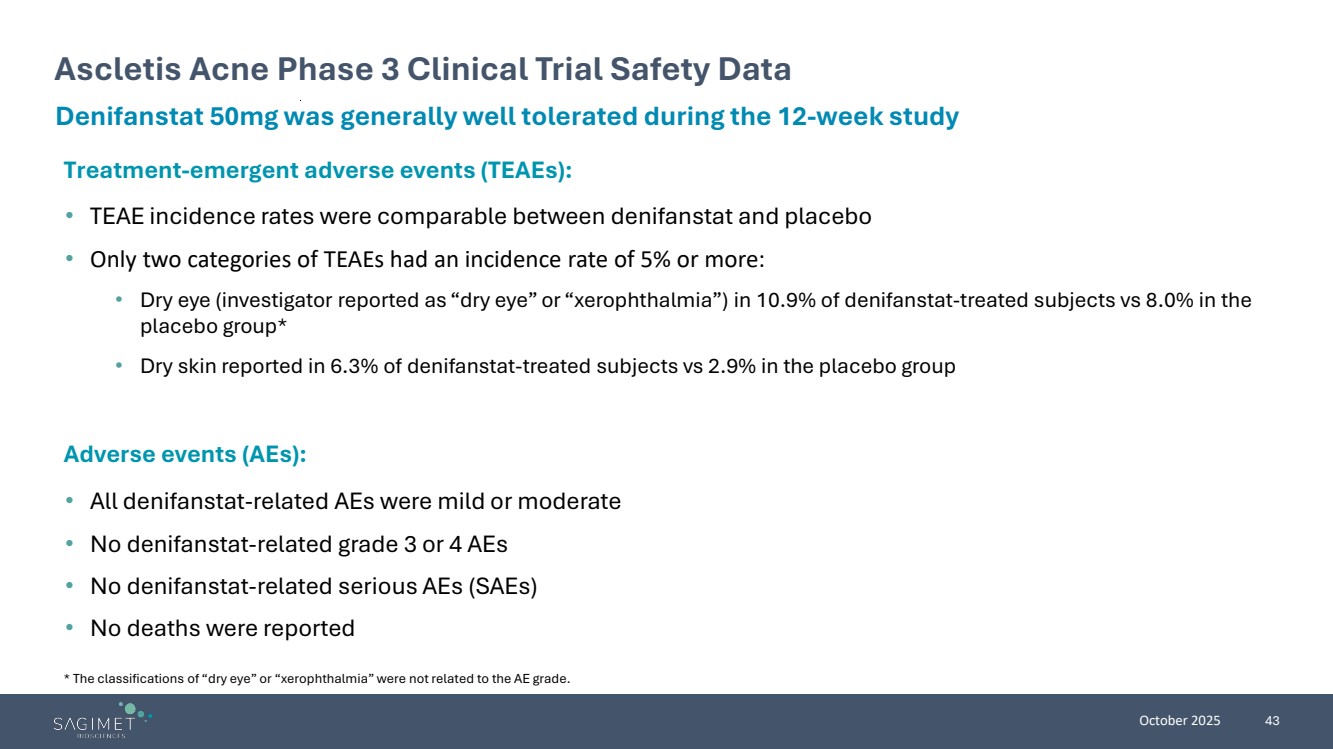
| October 2025 43 Ascletis Acne Phase 3 Clinical Trial Safety Data Denifanstat 50mg was generally well tolerated during the 12-week study Treatment-emergent adverse events (TEAEs): • TEAE incidence rates were comparable between denifanstat and placebo • Only two categories of TEAEs had an incidence rate of 5% or more: • Dry eye (investigator reported as “dry eye” or “xerophthalmia”) in 10.9% of denifanstat-treated subjects vs 8.0% in the placebo group* • Dry skin reported in 6.3% of denifanstat-treated subjects vs 2.9% in the placebo group Adverse events (AEs): • All denifanstat-related AEs were mild or moderate • No denifanstat-related grade 3 or 4 AEs • No denifanstat-related serious AEs (SAEs) • No deaths were reported * The classifications of “dry eye” or “xerophthalmia” were not related to the AE grade. |
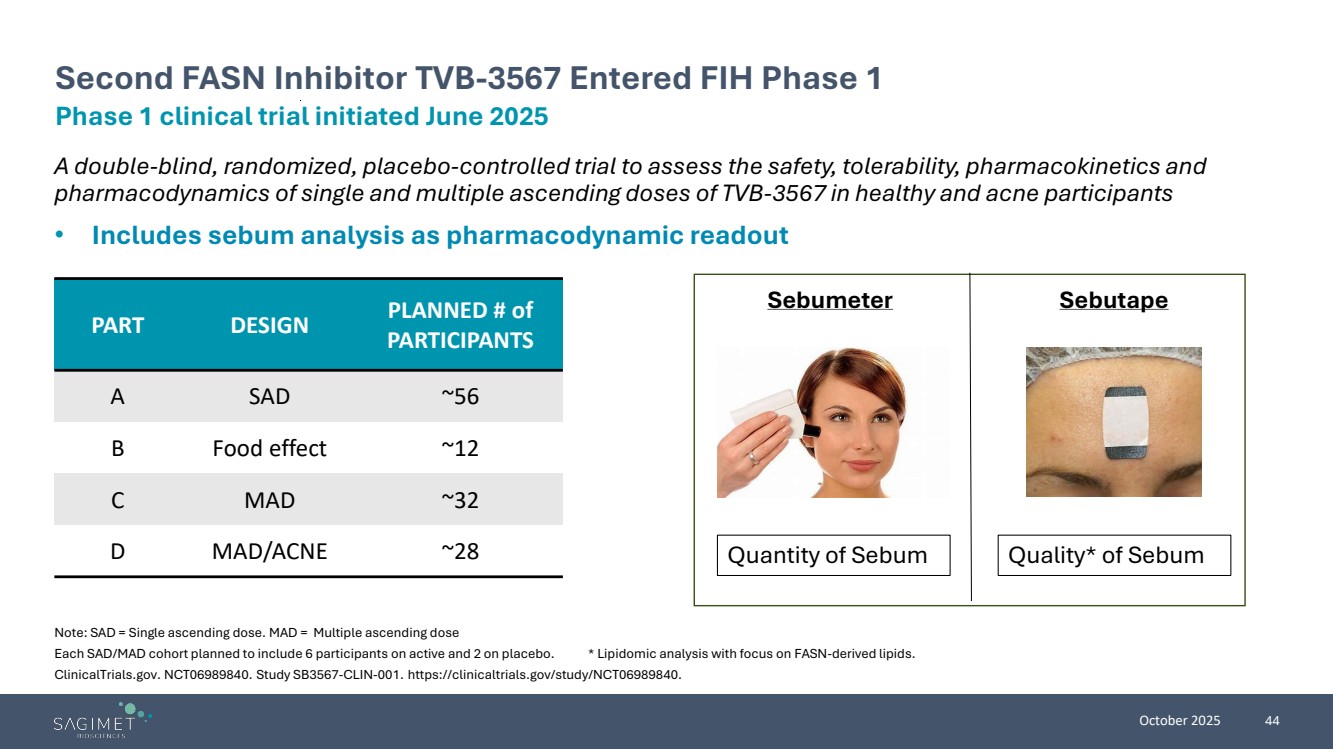
| October 2025 44 A double-blind, randomized, placebo-controlled trial to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple ascending doses of TVB-3567 in healthy and acne participants • Includes sebum analysis as pharmacodynamic readout Note: SAD = Single ascending dose. MAD = Multiple ascending dose Each SAD/MAD cohort planned to include 6 participants on active and 2 on placebo. * Lipidomic analysis with focus on FASN-derived lipids. ClinicalTrials.gov. NCT06989840. Study SB3567-CLIN-001. https://clinicaltrials.gov/study/NCT06989840. Phase 1 clinical trial initiated June 2025 Second FASN Inhibitor TVB-3567 Entered FIH Phase 1 Sebumeter Sebutape Quantity of Sebum Quality* of Sebum PART DESIGN PLANNED # of PARTICIPANTS A SAD ~56 B Food effect ~12 C MAD ~32 D MAD/ACNE ~28 |
| October 2025 45 Potential Clinical Development Program for TVB-3567 in Acne Step 1 - Phase 1 first-in-human pharmacokinetic (PK) clinical trial of TVB-3567 in healthy volunteers • PK and pharmacodynamics (PD) evaluation to confirm profile • Assess safety/tolerability • Confirm potential doses for an acne Phase 2 trial Step 2 - Phase 2 clinical trial in moderate to severe acne patients • Upon completion of Phase 1, will consult with regulatory authorities regarding Phase 2 trial design, with goal of initiating Phase 2 trial in 2026 • Phase 2 trial design will be informed by the results of the Phase 1 trial, anticipate a 12-week dose ranging study in moderate to severe acne patients with lesion reduction, treatment success (IGA) as endpoints Phase 1 trial initiated in June 2025 Goal to initiate Phase 2 trial in 2026, subject to consultation with regulatory authorities and outcome of Phase 1 trial |
| October 2025 46 Attractiveness of FASN Inhibition in Acne • First-in-human Phase 1 clinical trial of TVB-3567 initiated in June 2025 for development in acne • Upon completion of TVB-3567 Phase 1, will consult with regulatory authorities regarding Phase 2 trial design, with goal of initiating TVB-3567 Phase 2 in 2026 • Plan to consult with US FDA by early 2026 on the potential use of Ascletis Phase 3 data for the development of denifanstat in acne (e.g., as one of two registrational trials) FASN Inhibition in Acne Potential of TVB-3567 in Acne Development Pathways • Oral FASN inhibitors offer a novel mechanism of action for the potential treatment of moderate to severe acne • Denifanstat met all primary and secondary endpoints in Phase 3 clinical trial in patients with moderate to severe acne vulgaris in China ; Denifanstat was generally well tolerated • Acne market in dermatology is large (>50m people in the US) and aligned to those patients most likely to be prescribed an oral FASN inhibitor • TVB-3567 IP: • Method of use application for TVB-3567 for acne filed 2025; if granted—2046 • Composition of matter patent—2035; potential PTE to 2038 |
| FASN Inhibition Offers Potential Benefit in Multiple Indications: Oncology |
| October 2025 48 FASN Also Plays a Key Role in Other Diseases With Significant Unmet Need FASN in cancer Supports tumor survival Enables tumor proliferation Establishes resistance to drugs FASN in acne Increases sebum production Exacerbates sebum composition Cancer cell Membrane synthesis, intracellular signaling, protein modification Sebocyte Sebum production Palmitate Lipid building block FASN |
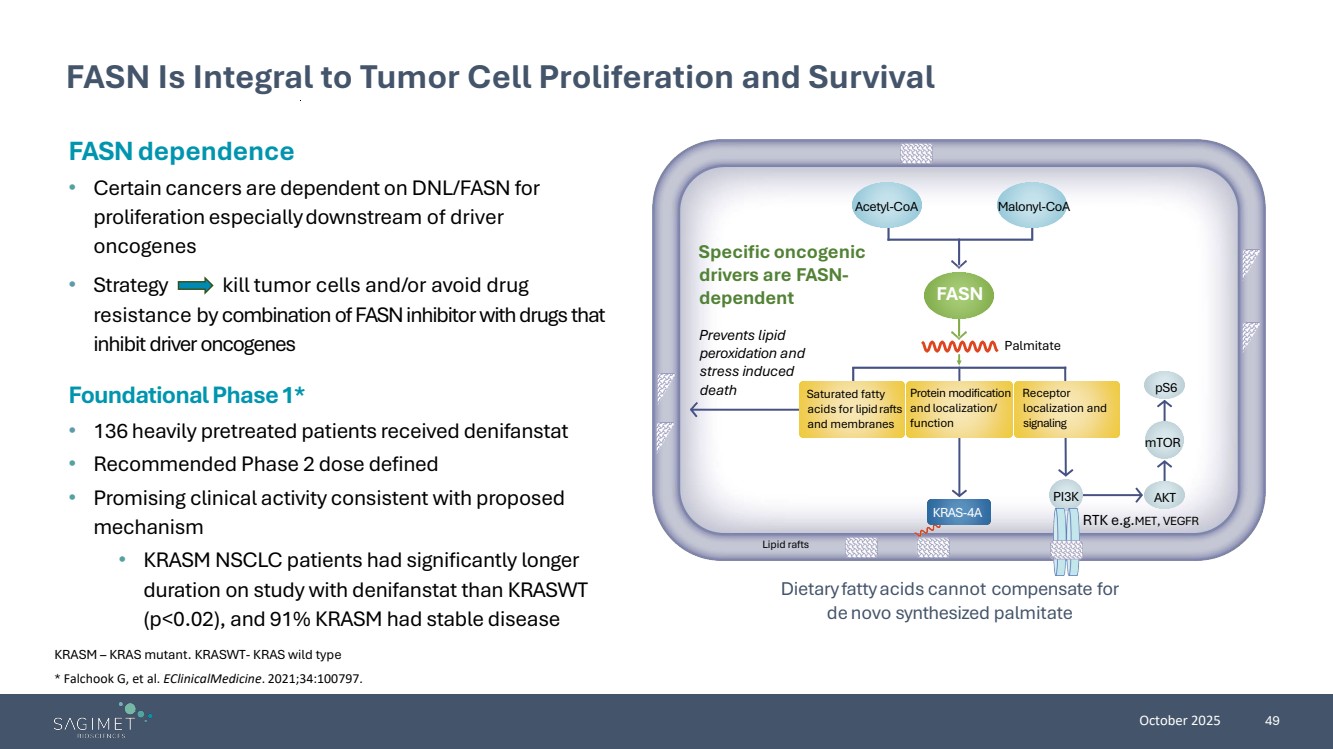
| October 2025 49 FASN Is Integral to Tumor Cell Proliferation and Survival KRASM – KRAS mutant. KRASWT- KRAS wild type FASN dependence • Certain cancers are dependent onDNL/FASN for proliferation especiallydownstream of driver oncogenes • Strategy kill tumor cells and/or avoid drug resistance by combination of FASN inhibitor with drugs that inhibit driver oncogenes Dietaryfattyacids cannot compensate for de novo synthesized palmitate Specific oncogenic drivers are FASN-dependent Prevents lipid peroxidation and stress induced death Palmitate RTK e.g.MET, VEGFR Saturated fatty acids for lipidrafts and membranes Protein modification and localization/ function Receptor localization and signaling Acetyl-CoA Malonyl-CoA pS6 mTOR PI3K AKT KRAS-4A Lipid rafts FASN Foundational Phase 1* • 136 heavily pretreated patients received denifanstat • Recommended Phase 2 dose defined • Promising clinical activity consistent with proposed mechanism • KRASM NSCLC patients had significantly longer duration on study with denifanstat than KRASWT (p<0.02), and 91% KRASM had stable disease * Falchook G, et al. EClinicalMedicine. 2021;34:100797. |
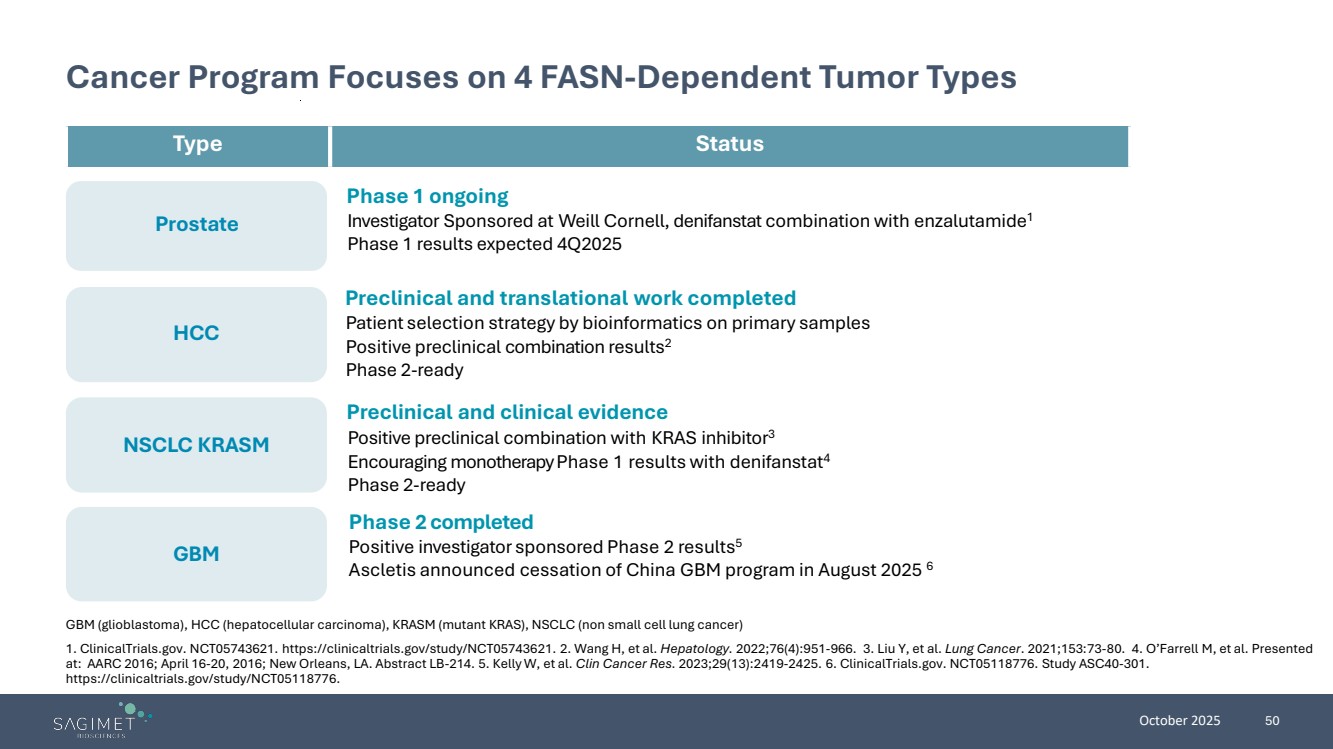
| October 2025 50 GBM Prostate HCC NSCLC KRASM Cancer Program Focuses on 4 FASN-Dependent Tumor Types GBM (glioblastoma), HCC (hepatocellular carcinoma), KRASM (mutant KRAS), NSCLC (non small cell lung cancer) 1. ClinicalTrials.gov. NCT05743621. https://clinicaltrials.gov/study/NCT05743621. 2. Wang H, et al. Hepatology. 2022;76(4):951-966. 3. Liu Y, et al. Lung Cancer. 2021;153:73-80. 4. O’Farrell M, et al. Presented at: AARC 2016; April 16-20, 2016; New Orleans, LA. Abstract LB-214. 5. Kelly W, et al. Clin Cancer Res. 2023;29(13):2419-2425. 6. ClinicalTrials.gov. NCT05118776. Study ASC40-301. https://clinicaltrials.gov/study/NCT05118776. Phase 2 completed Positive investigator sponsored Phase 2 results5 Ascletis announced cessation of China GBM program in August 2025 6 Type Status Phase 1 ongoing Investigator Sponsored at Weill Cornell, denifanstat combination with enzalutamide1 Phase 1 results expected 4Q2025 Preclinical and translational work completed Patient selection strategy by bioinformatics on primary samples Positive preclinical combination results2 Phase 2-ready Preclinical and clinical evidence Positive preclinical combination with KRAS inhibitor3 Encouraging monotherapy Phase 1 results with denifanstat4 Phase 2-ready |
| October 2025 51 Sagimet at a Glance • Our lead molecule, denifanstat, is a novel fatty acid synthase (FASN) inhibitor with a differentiated MOA with the potential to target multiple underserved diseases • Strong clinical data demonstrates denifanstat’s proof of concept across multiple disease states Unique MOA: FASN Inhibition • Denifanstat directly targets the 3 key drivers of MASH (metabolic dysfunction-associated steatohepatitis) – liver fat, inflammation, and fibrosis • Successful outcome of Phase 2b trial; met both primary endpoints with significant reduction in fibrosis • Pre-clinical data demonstrated synergistic effect of combination of FASN inhibitor and resmetirom • Phase 1 clinical trial to evaluate the pharmacokinetics (PK) and tolerability of a combination of denifanstat and resmetirom initiated in September 2025; data readout expected 1H 2026 Denifanstat in MASH TVB-3567 in Acne • Our follow-on FASN inhibitor, TVB 3567, received Investigational New Drug (IND) clearance in March 2025 • First-in-human Phase 1 clinical trial for development of an acne indication initiated in June 2025 |