June 2025 Corporate Presentation

2 Disclaimer Forward Looking Statements This communication contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current expectations and beliefs of the management of Neurogene, as well as assumptions made by, and information currently available to, management of Neurogene, including, but not limited to, statements regarding: the therapeutic potential and utility, efficacy and clinical benefits of its programs, including its EXACTTM technology and NGN-401; market opportunities for Neurogene's product candidates; the safety and tolerability profile of NGN-401; trial designs, clinical development plans and timing for NGN-401, including enrollment and dosing in both the pediatric and adolescent/adult cohorts of the NGN-401 Phase 1/2 clinical trial for Rett syndrome, the expected durability and deepening of clinical data results from that trial, and potential impacts of adding an adolescent/adult cohort to the Phase 1/2 trial for NGN-401; the benefits of Neurogene's in-house manufacturing capabilities; the ability of Neurogene to identify future development plans for NGN-101; future interactions with U.S. or foreign regulatory authorities, including the timing and outcome of any such interaction and anticipated benefits of the FDA's RMAT designation as well as participation in the FDA's START program with respect to NGN-401; anticipated early-stage discovery and expectations regarding the initiation of future clinical trials for programs in development; and Neurogene's cash runway. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions or the negative or plural of these words, or other similar expressions that are predictions or indicate future events or prospects, although not all forward-looking statements contain these words. Statements that are not historical facts are forward-looking statements. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: Neurogene’s limited operating history; the significant net losses incurred since inception of Neurogene; the ability to raise additional capital to finance operations; the ability of Neurogene to report its data on the predicted timeline; the ability of Neurogene to effectively use the RMAT designation or START program to accelerate development of NGN-401; the potential for negative impacts to patients dosed in the ongoing clinical trials for NGN-401; the ability to advance product candidates through non-clinical and clinical development; the ability to obtain regulatory approval for, and ultimately commercialize, Neurogene’s product candidates; Neurogene’s limited experience in designing and conducting clinical trials; the ability to identify and pivot to other programs, product candidates, or indications that may be more profitable or successful than Neurogene’s current product candidates; expectations regarding the market and potential for Neurogene’s current product candidates; expectations regarding the potential tolerability, safety or efficacy for Neurogene’s current product candidates; the ability to attract, hire, and retain skilled executive officers and employees; reliance on third parties, contract manufacturers, and contract research organizations; the ability of Neurogene to protect its intellectual property and proprietary technologies; risks related to Neurogene’s ability to correctly estimate its operating expenses, including its projected cash runway; and legislative, regulatory, political and economic developments and general market conditions. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in the Company’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission, as well as risk factors associated with companies, such as Neurogene, that operate in the biopharma industry. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond Neurogene’s control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. Nothing in this communication should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that the contemplated results of any such forward-looking statements will be achieved. Forward-looking statements in this communication speak only as of the day they are made and are qualified in their entirety by reference to the cautionary statements herein. Except as required by applicable law, Neurogene undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise. Industry and Market Data Certain information contained in this Presentation relates to or is based on studies, publications, surveys and Neurogene’s own internal estimates and research. In this Presentation, Neurogene relies on, and refers to, publicly available information and statistics regarding market participants in the sector in which Neurogene competes and other industry data. Any comparison of Neurogene to any other entity assumes the reliability of the information available to Neurogene. Neurogene obtained this information and statistics from third-party sources, including reports by market research firms and company filings. In addition, all of the market data included in this Presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while Neurogene believes its internal research is reliable, such research has not been verified by any independent source and Neurogene has not independently verified the information. Trademarks This Presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the TM, SM © or ® symbols, but Neurogene will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade names and copyrights.
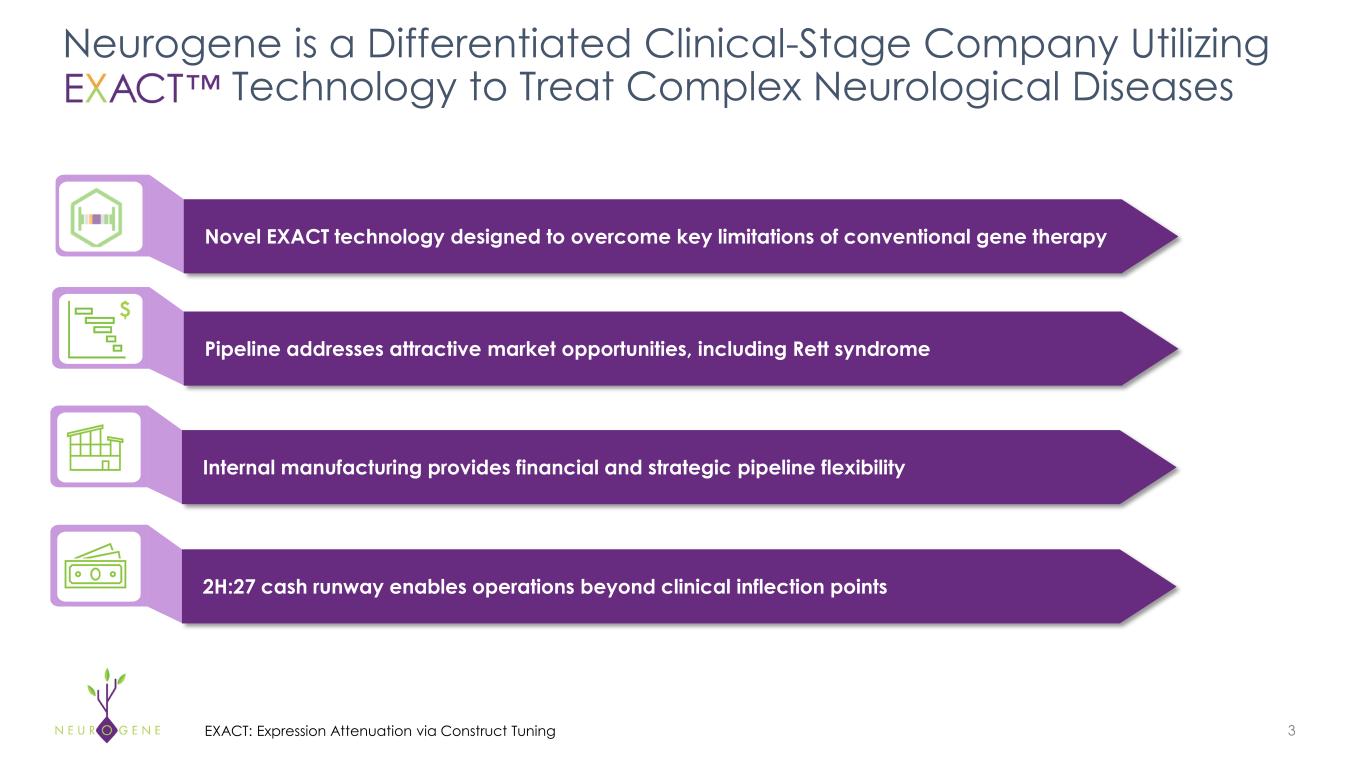
Neurogene is a Differentiated Clinical-Stage Company Utilizing EXACT Technology to Treat Complex Neurological Diseases 3 Novel EXACT technology designed to overcome key limitations of conventional gene therapy Internal manufacturing provides financial and strategic pipeline flexibility Pipeline addresses attractive market opportunities, including Rett syndrome Internal manufacturing provides financial and strategic pipeline flexibility 2H:27 cash runway enables operations beyond clinical inflection points $ EXACT: Expression Attenuation via Construct Tuning
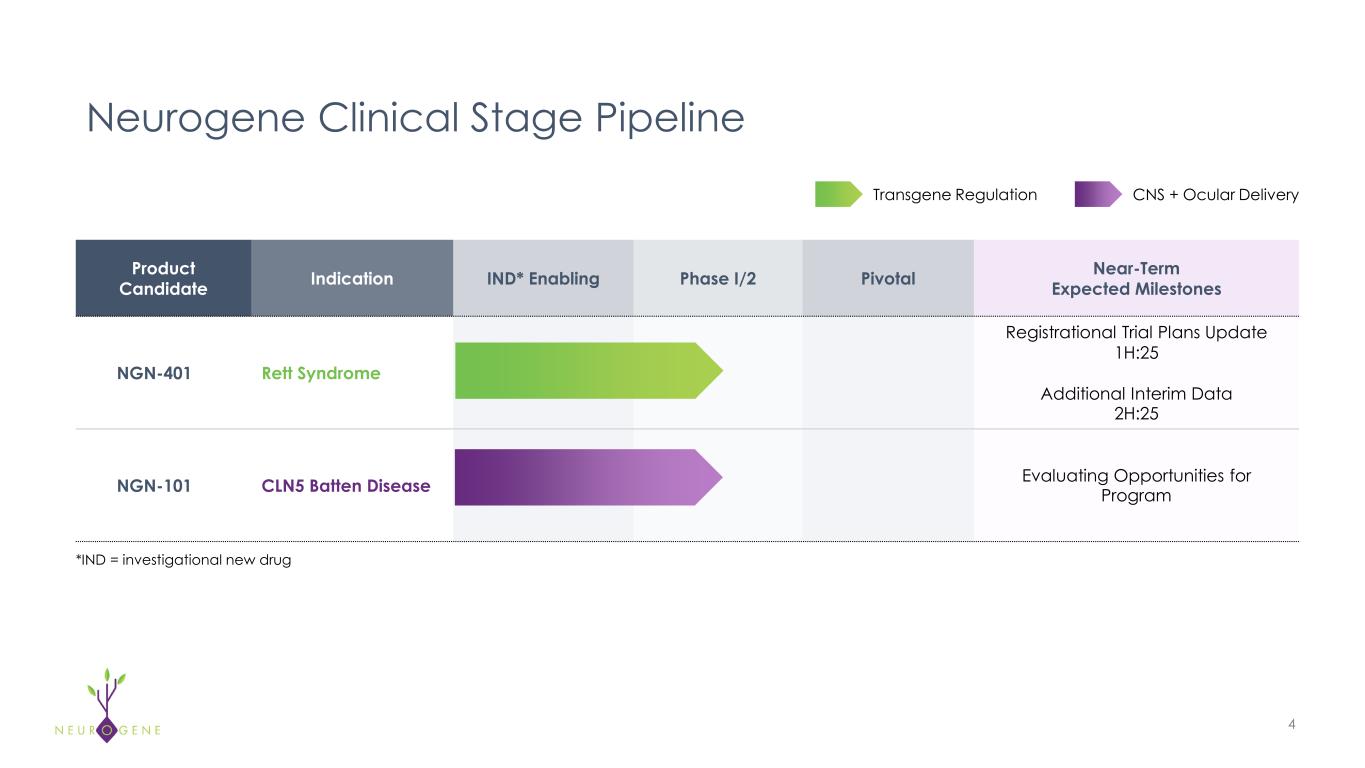
Product Candidate Indication IND* Enabling Phase I/2 Pivotal Near-Term Expected Milestones NGN-401 Rett Syndrome Registrational Trial Plans Update 1H:25 Additional Interim Data 2H:25 NGN-101 CLN5 Batten Disease Evaluating Opportunities for Program Neurogene Clinical Stage Pipeline 4 *IND = investigational new drug Transgene Regulation CNS + Ocular Delivery
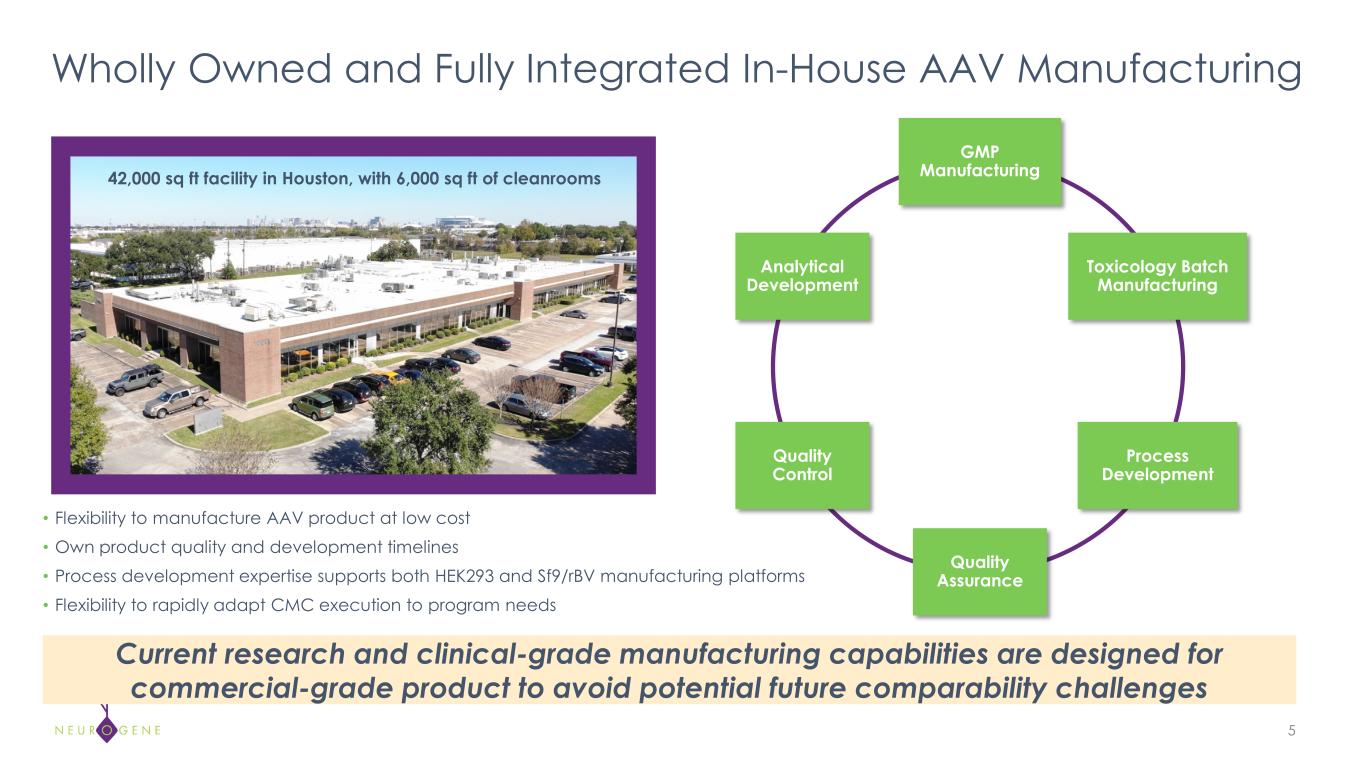
Wholly Owned and Fully Integrated In-House AAV Manufacturing 5 GMP Manufacturing Toxicology Batch Manufacturing Process Development Quality Assurance Quality Control Analytical Development • Flexibility to manufacture AAV product at low cost • Own product quality and development timelines • Process development expertise supports both HEK293 and Sf9/rBV manufacturing platforms • Flexibility to rapidly adapt CMC execution to program needs 42,000 sq ft facility in Houston, with 6,000 sq ft of cleanrooms Current research and clinical-grade manufacturing capabilities are designed for commercial-grade product to avoid potential future comparability challenges

NGN-401 for Rett Syndrome Leveraging EXACT transgene regulation technology
Rett Syndrome – Devastating Disorder with High Unmet Need 7 Genetics • X-Linked disorder causing mutations in the gene encoding for methyl-CpG binding protein 2 (MeCP2) • Unknown incidence in boys, but typically lethal by ~3 years of age due to no healthy copy of MeCP2 U.S. prevalence estimate based on published incidence rates; Laurvick CL, et al. J Pediatr 2006;148(3):347–35. WW incidence estimate based on published incidence rates; Pini G, et al. Orphanet Journal of Rare Diseases (2016) 11:132. High Unmet Need • There are no approved treatments that address root cause of disease • Significant unmet need remains for new treatment options Compelling Market Opportunity • U.S. prevalence - ~6,000-9,000 patients • WW incidence - 1:10,000 females
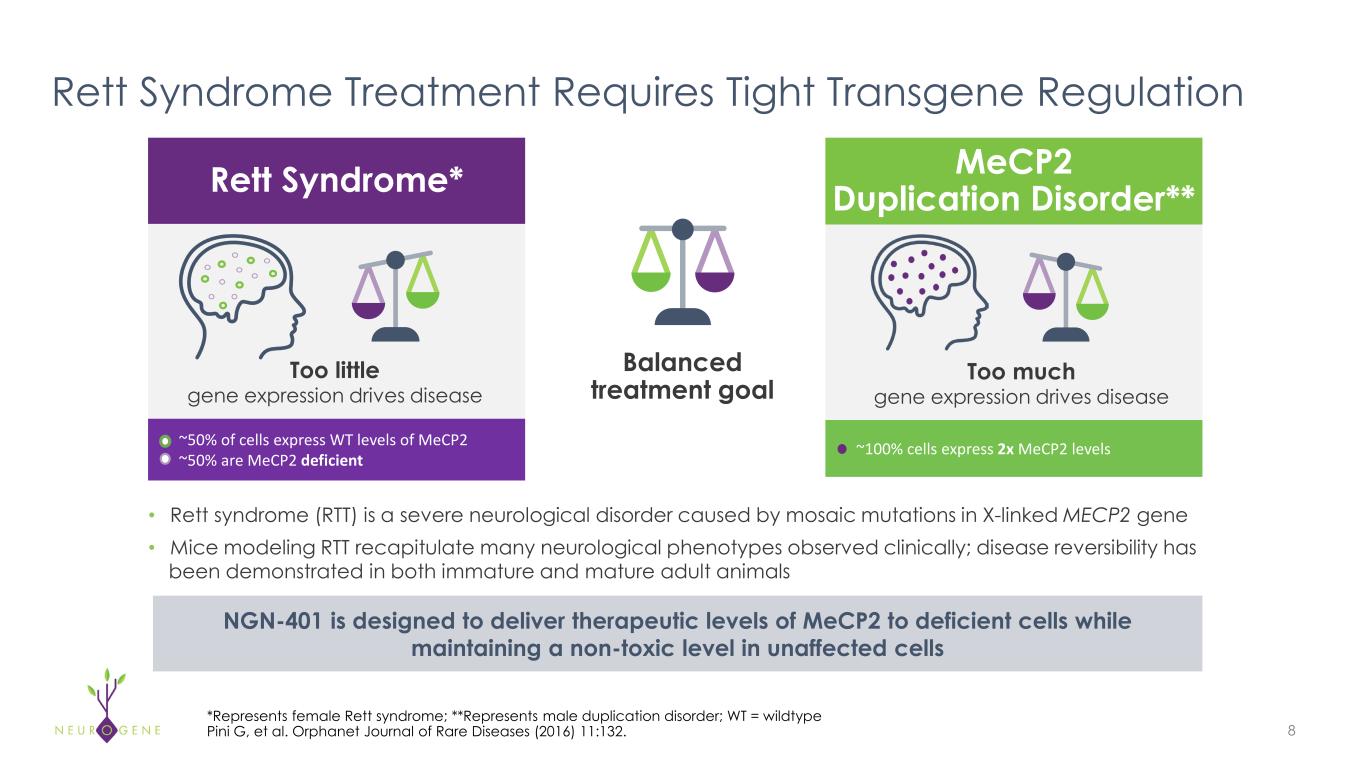
• Rett syndrome (RTT) is a severe neurological disorder caused by mosaic mutations in X-linked MECP2 gene • Mice modeling RTT recapitulate many neurological phenotypes observed clinically; disease reversibility has been demonstrated in both immature and mature adult animals 8 ~100% cells express 2x MeCP2 levels~50% of cells express WT levels of MeCP2 ~50% are MeCP2 deficient Too little gene expression drives disease Too much gene expression drives disease Balanced treatment goal Rett Syndrome Treatment Requires Tight Transgene Regulation *Represents female Rett syndrome; **Represents male duplication disorder; WT = wildtype Pini G, et al. Orphanet Journal of Rare Diseases (2016) 11:132. Rett Syndrome* MeCP2 Duplication Disorder** NGN-401 is designed to deliver therapeutic levels of MeCP2 to deficient cells while maintaining a non-toxic level in unaffected cells
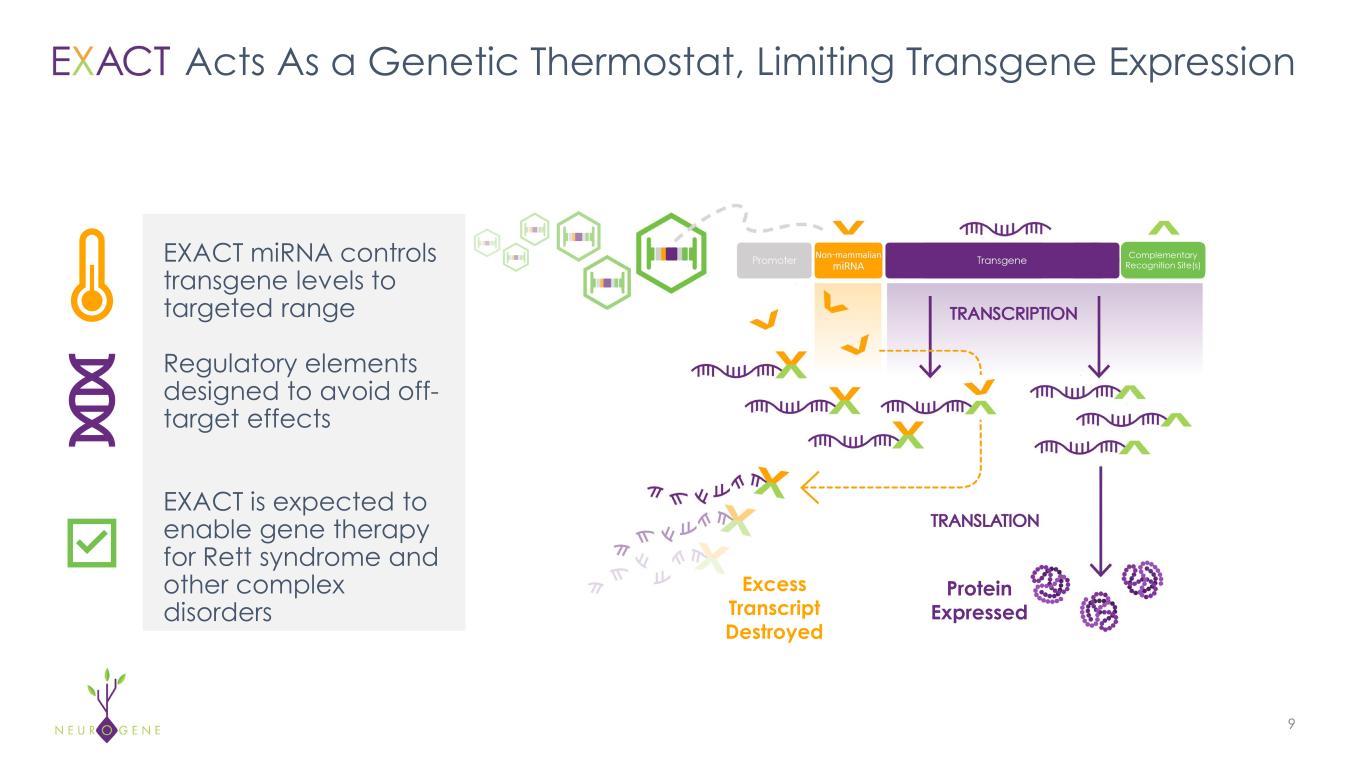
Acts As a Genetic Thermostat, Limiting Transgene Expression 9 Protein Expressed EXACT miRNA controls transgene levels to targeted range Regulatory elements designed to avoid off- target effects EXACT is expected to enable gene therapy for Rett syndrome and other complex disorders Excess Transcript Destroyed Complementary Recognition Site(s)
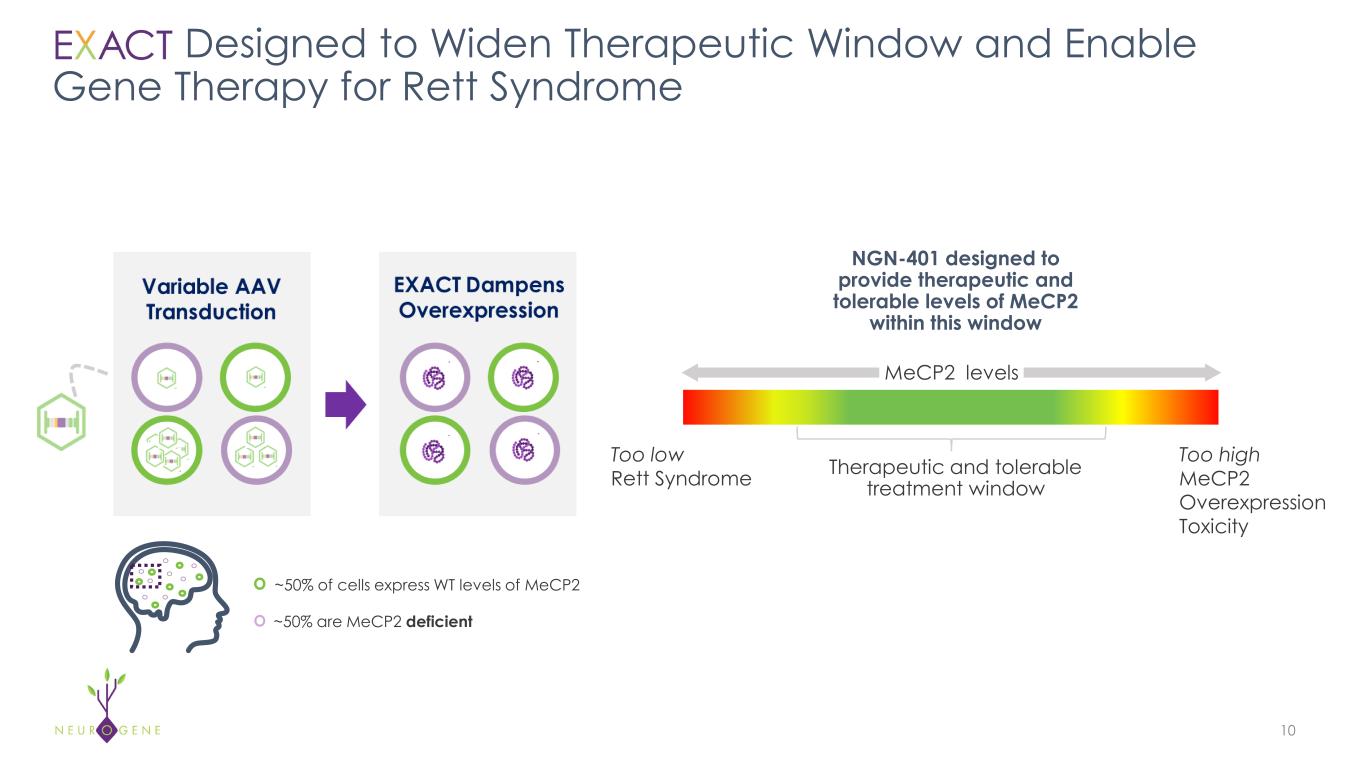
10 Designed to Widen Therapeutic Window and Enable Gene Therapy for Rett Syndrome ~50% of cells express WT levels of MeCP2 ~50% are MeCP2 deficient NGN-401 designed to provide therapeutic and tolerable levels of MeCP2 within this window Too low Rett Syndrome Too high MeCP2 Overexpression Toxicity Therapeutic and tolerable treatment window MeCP2 levels
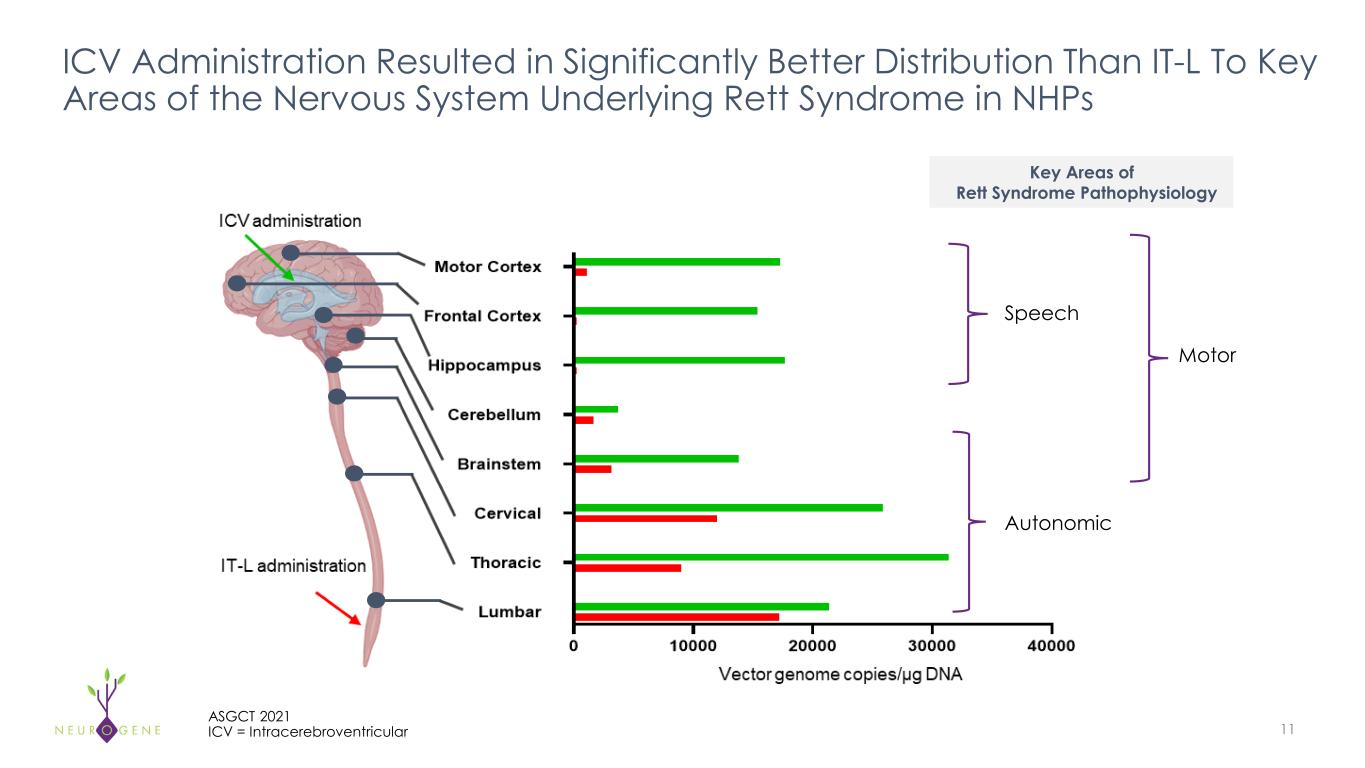
11 ASGCT 2021 ICV = Intracerebroventricular ICV Administration Resulted in Significantly Better Distribution Than IT-L To Key Areas of the Nervous System Underlying Rett Syndrome in NHPs Key Areas of Rett Syndrome Pathophysiology Autonomic Motor Speech Speech Motor utonomic
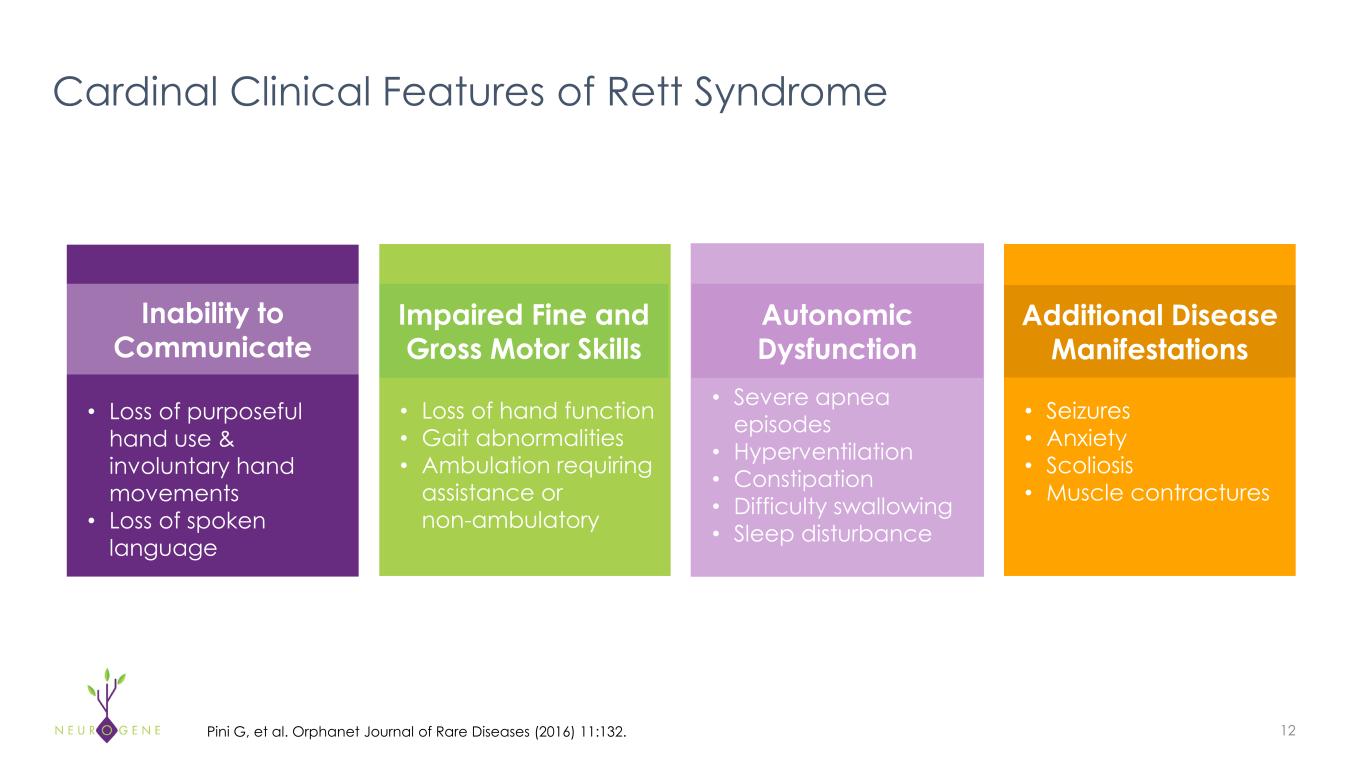
Cardinal Clinical Features of Rett Syndrome • Loss of purposeful hand use & involuntary hand movements • Loss of spoken language • Loss of hand function • Gait abnormalities • Ambulation requiring assistance or non-ambulatory • Severe apnea episodes • Hyperventilation • Constipation • Difficulty swallowing • Sleep disturbance • Seizures • Anxiety • Scoliosis • Muscle contractures Inability to Communicate Impaired Fine and Gross Motor Skills Autonomic Dysfunction Additional Disease Manifestations 12Pini G, et al. Orphanet Journal of Rare Diseases (2016) 11:132.
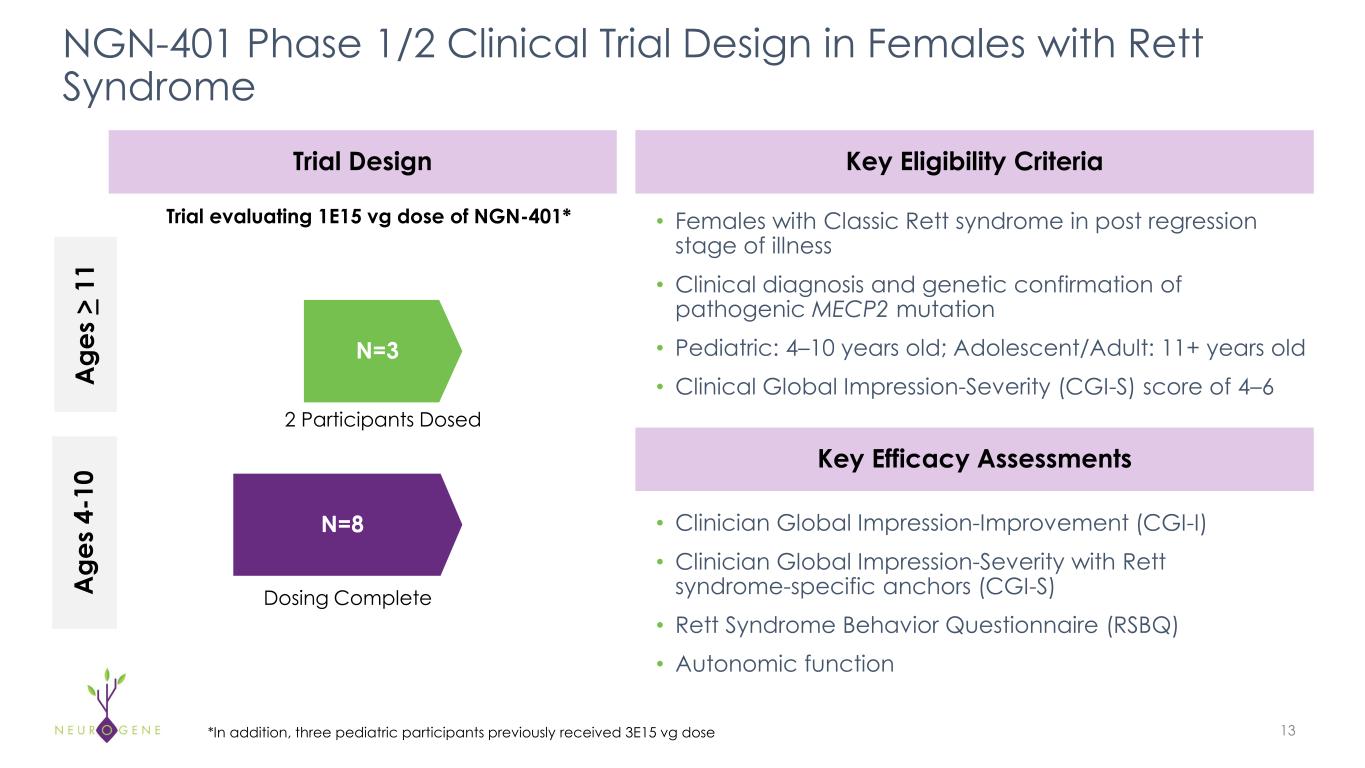
NGN-401 Phase 1/2 Clinical Trial Design in Females with Rett Syndrome 13*In addition, three pediatric participants previously received 3E15 vg dose Trial Design • Females with Classic Rett syndrome in post regression stage of illness • Clinical diagnosis and genetic confirmation of pathogenic MECP2 mutation • Pediatric: 4–10 years old; Adolescent/Adult: 11+ years old • Clinical Global Impression-Severity (CGI-S) score of 4–6 N=8 N=3 Key Eligibility Criteria • Clinician Global Impression-Improvement (CGI-I) • Clinician Global Impression-Severity with Rett syndrome-specific anchors (CGI-S) • Rett Syndrome Behavior Questionnaire (RSBQ) • Autonomic function Key Efficacy Assessments Trial evaluating 1E15 vg dose of NGN-401* A ge s 4- 10 A ge s > 11 Dosing Complete 2 Participants Dosed
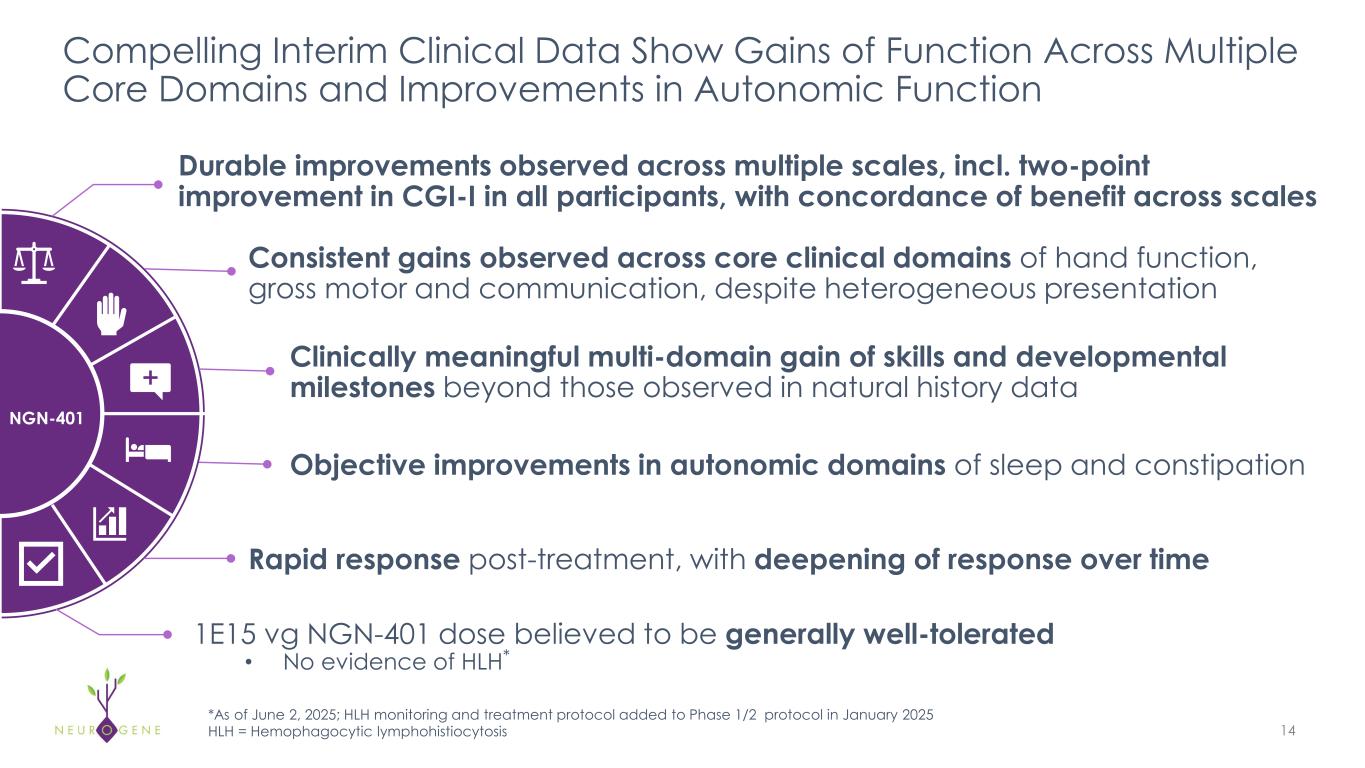
Compelling Interim Clinical Data Show Gains of Function Across Multiple Core Domains and Improvements in Autonomic Function 14 NGN-401 Durable improvements observed across multiple scales, incl. two-point improvement in CGI-I in all participants, with concordance of benefit across scales Consistent gains observed across core clinical domains of hand function, gross motor and communication, despite heterogeneous presentation Objective improvements in autonomic domains of sleep and constipation Clinically meaningful multi-domain gain of skills and developmental milestones beyond those observed in natural history data Rapid response post-treatment, with deepening of response over time 1E15 vg NGN-401 dose believed to be generally well-tolerated • No evidence of HLH* *As of June 2, 2025; HLH monitoring and treatment protocol added to Phase 1/2 protocol in January 2025 HLH = Hemophagocytic lymphohistiocytosis
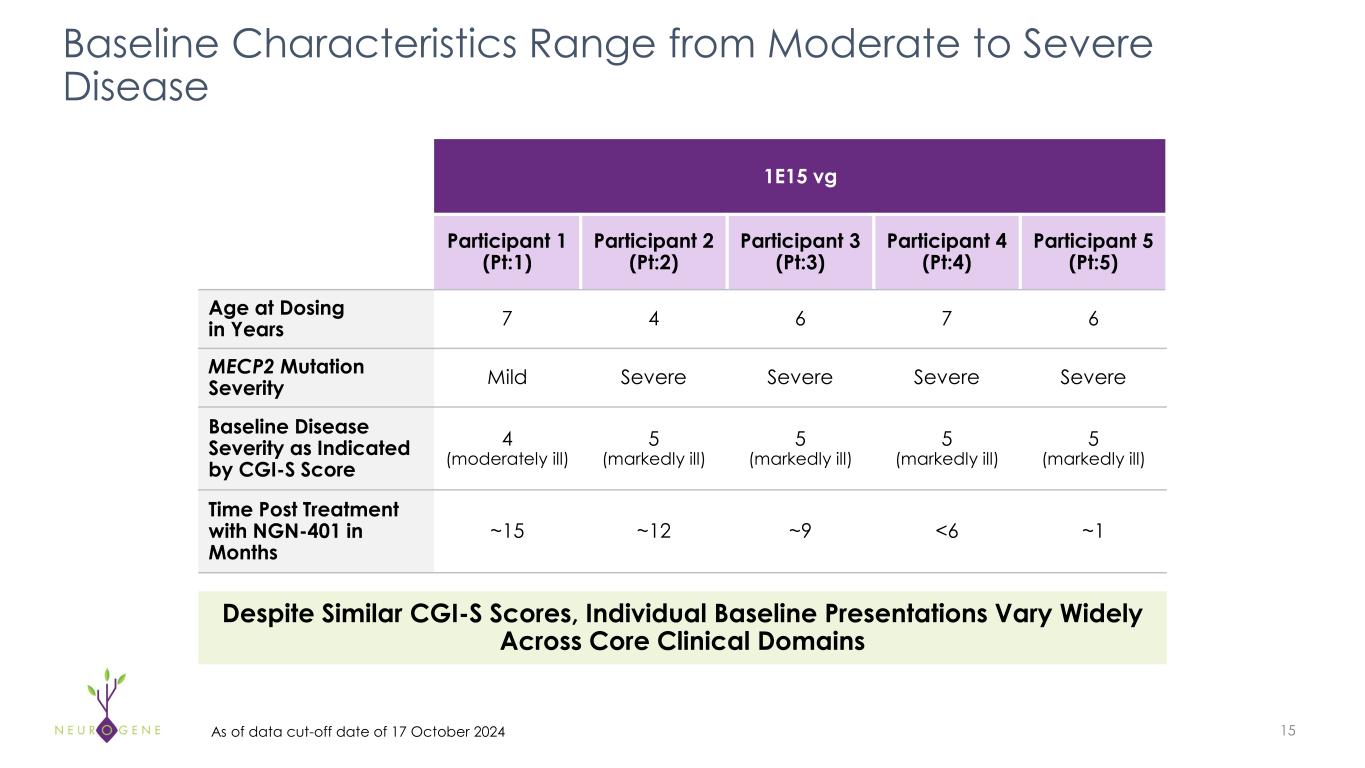
15 Baseline Characteristics Range from Moderate to Severe Disease 1E15 vg Participant 1 (Pt:1) Participant 2 (Pt:2) Participant 3 (Pt:3) Participant 4 (Pt:4) Participant 5 (Pt:5) Age at Dosing in Years 7 4 6 7 6 MECP2 Mutation Severity Mild Severe Severe Severe Severe Baseline Disease Severity as Indicated by CGI-S Score 4 (moderately ill) 5 (markedly ill) 5 (markedly ill) 5 (markedly ill) 5 (markedly ill) Time Post Treatment with NGN-401 in Months ~15 ~12 ~9 <6 ~1 Despite Similar CGI-S Scores, Individual Baseline Presentations Vary Widely Across Core Clinical Domains As of data cut-off date of 17 October 2024
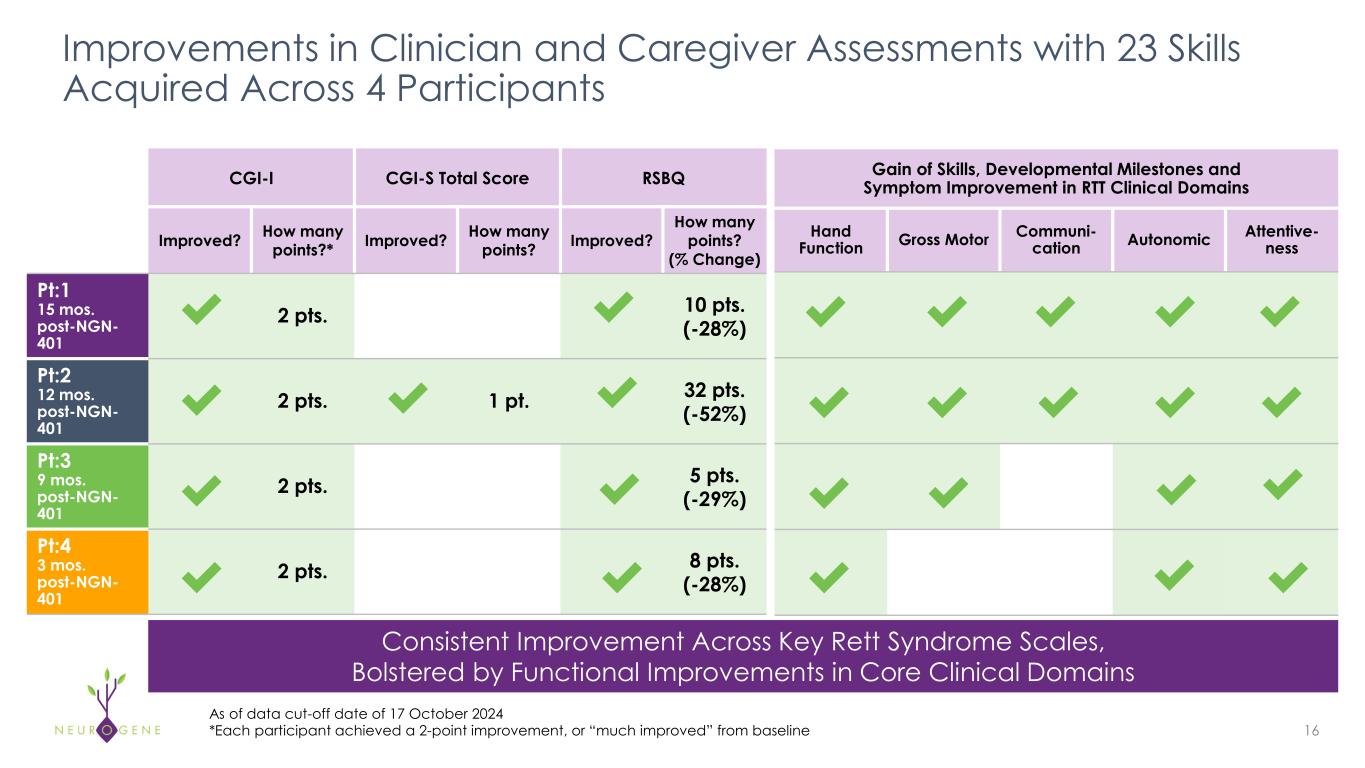
Improvements in Clinician and Caregiver Assessments with 23 Skills Acquired Across 4 Participants 16 As of data cut-off date of 17 October 2024 *Each participant achieved a 2-point improvement, or “much improved” from baseline CGI-I CGI-S Total Score RSBQ Improved? How many points?* Improved? How many points? Improved? How many points? (% Change) Pt:1 15 mos. post-NGN- 401 2 pts. 10 pts. (-28%) Pt:2 12 mos. post-NGN- 401 2 pts. 1 pt. 32 pts. (-52%) Pt:3 9 mos. post-NGN- 401 2 pts. 5 pts. (-29%) Pt:4 3 mos. post-NGN- 401 2 pts. 8 pts. (-28%) Gain of Skills, Developmental Milestones and Symptom Improvement in RTT Clinical Domains Hand Function Gross Motor Communi- cation Autonomic Attentive- ness Consistent Improvement Across Key Rett Syndrome Scales, Bolstered by Functional Improvements in Core Clinical Domains
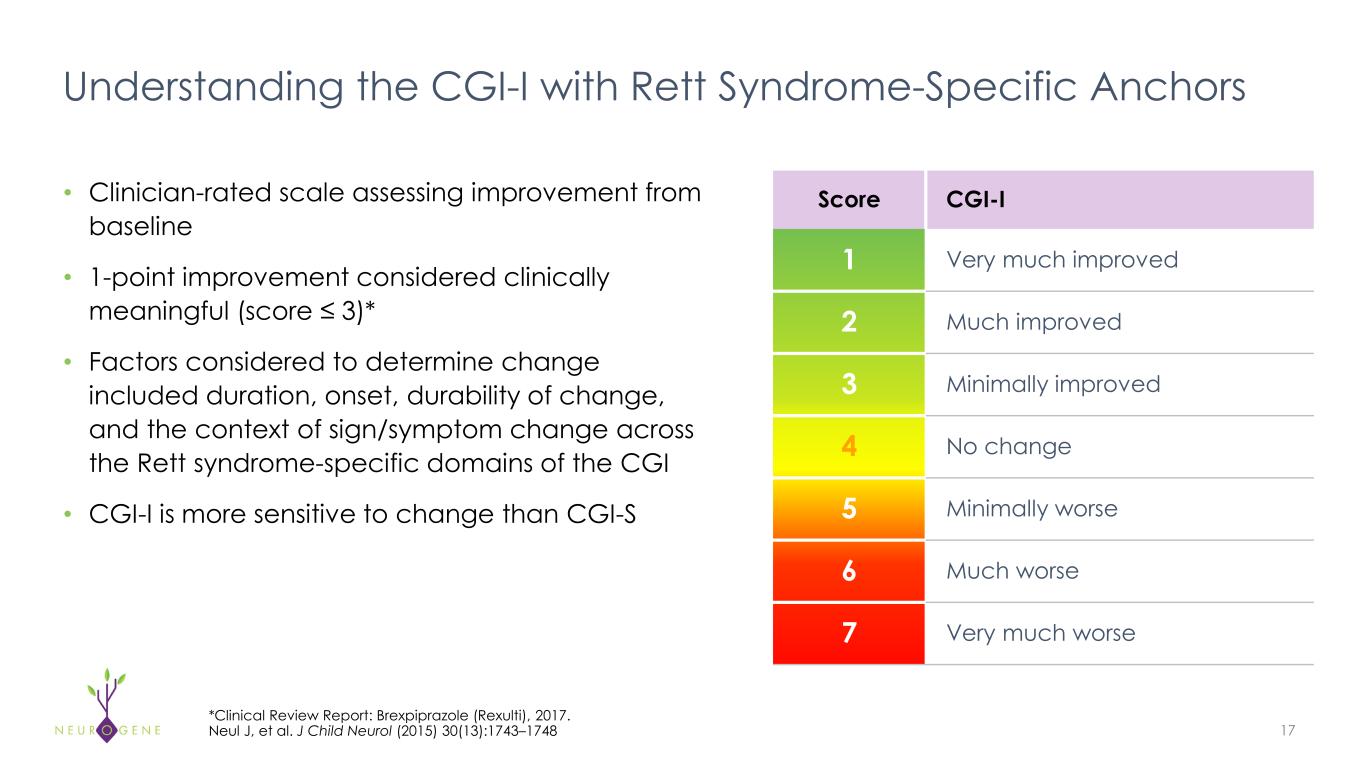
Understanding the CGI-I with Rett Syndrome-Specific Anchors 17 *Clinical Review Report: Brexpiprazole (Rexulti), 2017. Neul J, et al. J Child Neurol (2015) 30(13):1743–1748 • Clinician-rated scale assessing improvement from baseline • 1-point improvement considered clinically meaningful (score ≤ 3)* • Factors considered to determine change included duration, onset, durability of change, and the context of sign/symptom change across the Rett syndrome-specific domains of the CGI • CGI-I is more sensitive to change than CGI-S Score CGI-I 1 Very much improved 2 Much improved 3 Minimally improved 4 No change 5 Minimally worse 6 Much worse 7 Very much worse
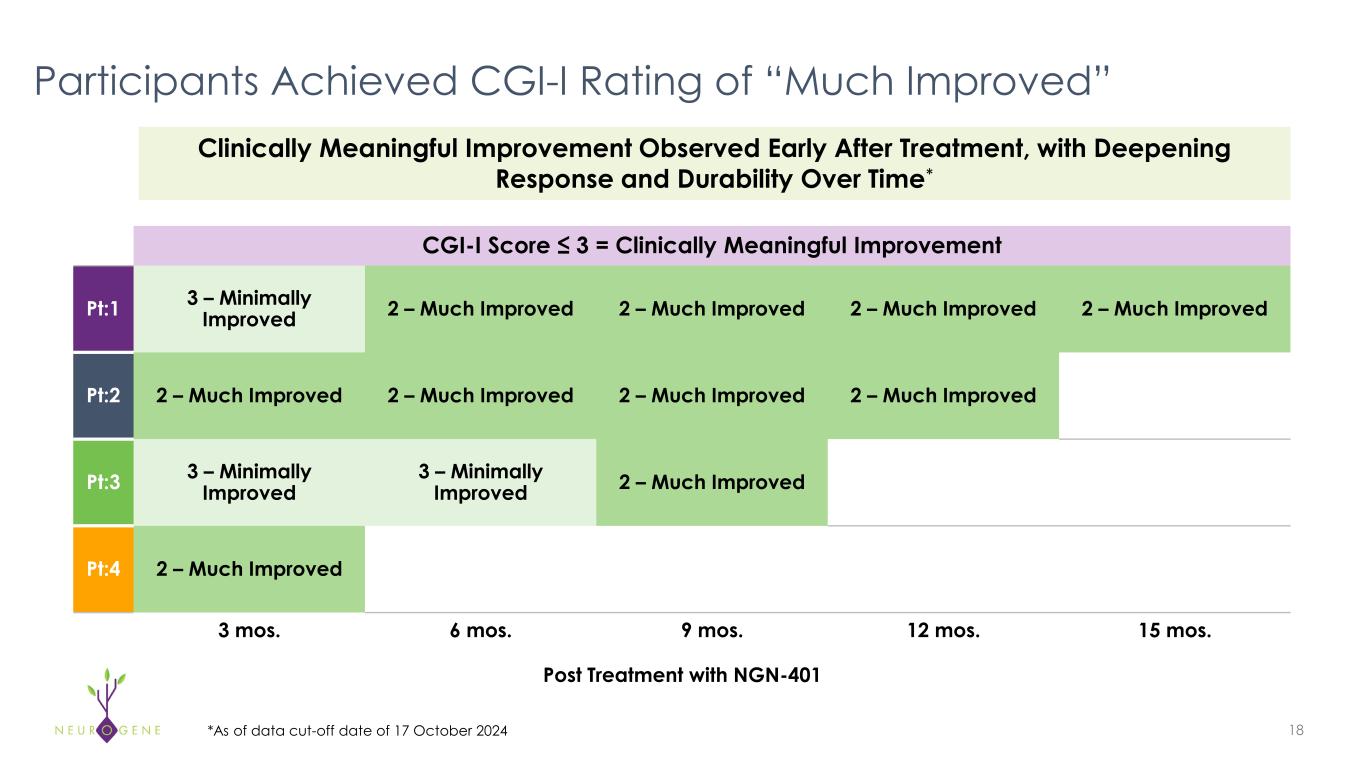
Participants Achieved CGI-I Rating of “Much Improved” CGI-I Score ≤ 3 = Clinically Meaningful Improvement Pt:1 3 – Minimally Improved 2 – Much Improved 2 – Much Improved 2 – Much Improved 2 – Much Improved Pt:2 2 – Much Improved 2 – Much Improved 2 – Much Improved 2 – Much Improved Pt:3 3 – Minimally Improved 3 – Minimally Improved 2 – Much Improved Pt:4 2 – Much Improved 3 mos. 6 mos. 9 mos. 12 mos. 15 mos. 18 Post Treatment with NGN-401 *As of data cut-off date of 17 October 2024 Clinically Meaningful Improvement Observed Early After Treatment, with Deepening Response and Durability Over Time*
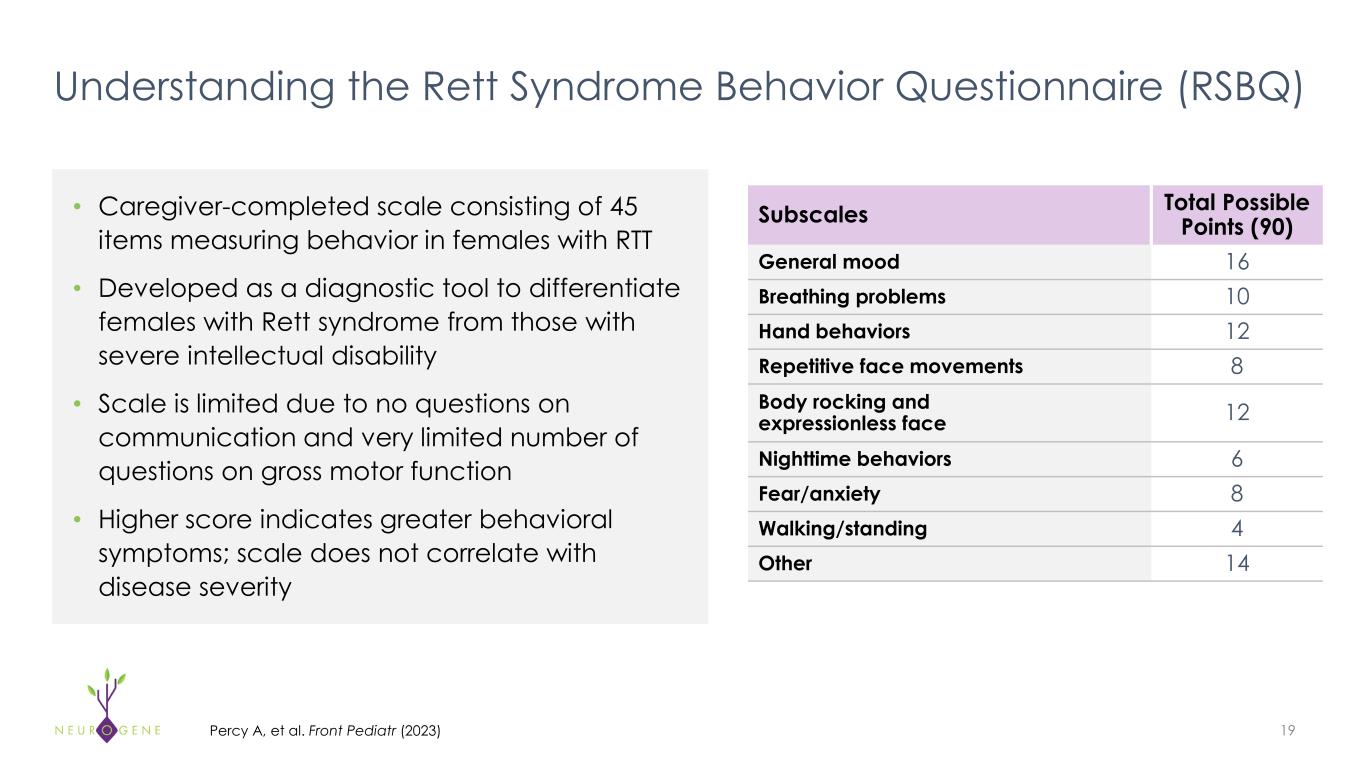
Understanding the Rett Syndrome Behavior Questionnaire (RSBQ) • Caregiver-completed scale consisting of 45 items measuring behavior in females with RTT • Developed as a diagnostic tool to differentiate females with Rett syndrome from those with severe intellectual disability • Scale is limited due to no questions on communication and very limited number of questions on gross motor function • Higher score indicates greater behavioral symptoms; scale does not correlate with disease severity 19Percy A, et al. Front Pediatr (2023) Subscales Total Possible Points (90) General mood 16 Breathing problems 10 Hand behaviors 12 Repetitive face movements 8 Body rocking and expressionless face 12 Nighttime behaviors 6 Fear/anxiety 8 Walking/standing 4 Other 14
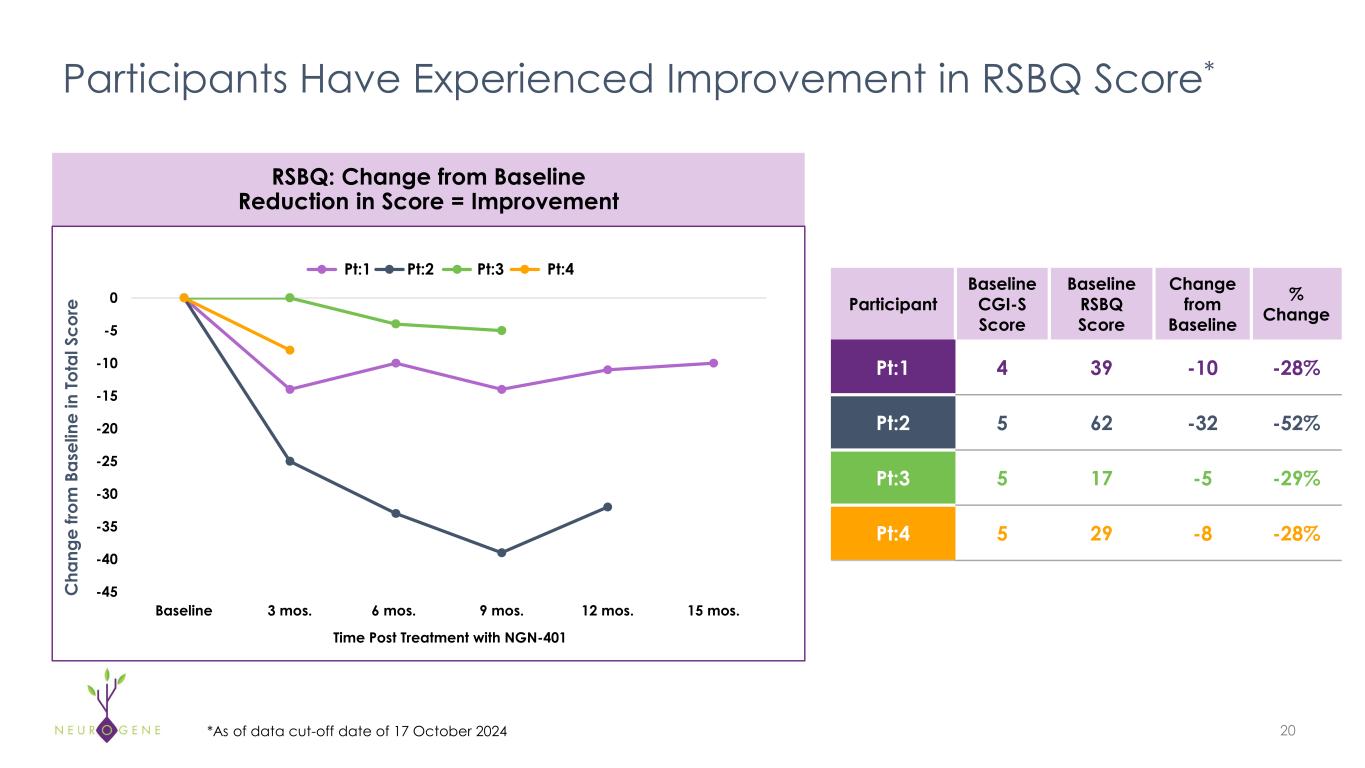
Participants Have Experienced Improvement in RSBQ Score* 20 Participant Baseline CGI-S Score Baseline RSBQ Score Change from Baseline % Change Pt:1 4 39 -10 -28% Pt:2 5 62 -32 -52% Pt:3 5 17 -5 -29% Pt:4 5 29 -8 -28% *As of data cut-off date of 17 October 2024 C ha ng e fro m B as el in e in To ta l S co re RSBQ: Change from Baseline Reduction in Score = Improvement -45 -40 -35 -30 -25 -20 -15 -10 -5 0 Baseline 3 mos. 6 mos. 9 mos. 12 mos. 15 mos. LD1 LD2 LD3 LD4 Time Post Treatment with NGN-401 Pt:1 Pt: Pt:3 Pt:4

Participants Experienced Improvements in Autonomic Function, as Measured by Objective Assessments* 21Detailed data provided in Appendix • Pt:1 and Pt:2, who had sleep deficits at Baseline, experienced improvements in sleep parameters, as measured by a wearable device • Pt:1 sleep efficiency increased from 83% to 90% at 6 months • Pt:2 sleep efficiency increased from 90% to >95% at 6 months, considered ideal • Pt:1, Pt:2 and Pt:4 had constipation at Baseline, and experienced improvements over time as measured by the caregiver-reported modified Bristol Stool Form Scale • Pt:3 had dysphagia, or difficulty swallowing, at Baseline, requiring a pureed diet and had to be spoon-fed by caregiver due to aspiration; at 9 months after dosing, she gained the ability to swallow liquids from a cup and chew and swallow food items *As of data cut-off date of 17 October 2024

Participant Vignettes
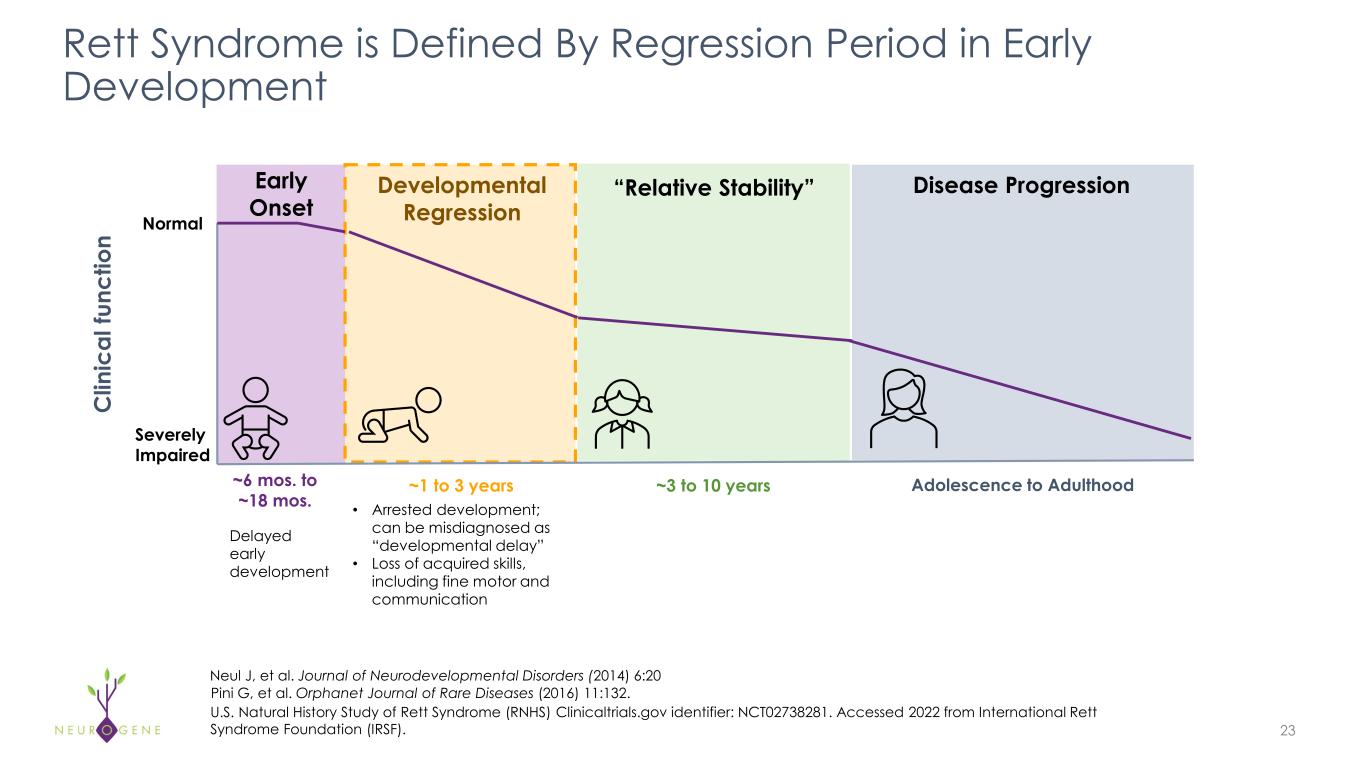
Rett Syndrome is Defined By Regression Period in Early Development 23 Pini G, et al. Orphanet Journal of Rare Diseases (2016) 11:132. C lin ic al fu nc tio n ~6 mos. to ~18 mos. ~1 to 3 years ~3 to 10 years Normal Adolescence to Adulthood Developmental Regression Early Onset Disease Progression“Relative Stability” Severely Impaired Delayed early development • Arrested development; can be misdiagnosed as “developmental delay” • Loss of acquired skills, including fine motor and communication U.S. Natural History Study of Rett Syndrome (RNHS) Clinicaltrials.gov identifier: NCT02738281. Accessed 2022 from International Rett Syndrome Foundation (IRSF). Neul J, et al. Journal of Neurodevelopmental Disorders (2014) 6:20
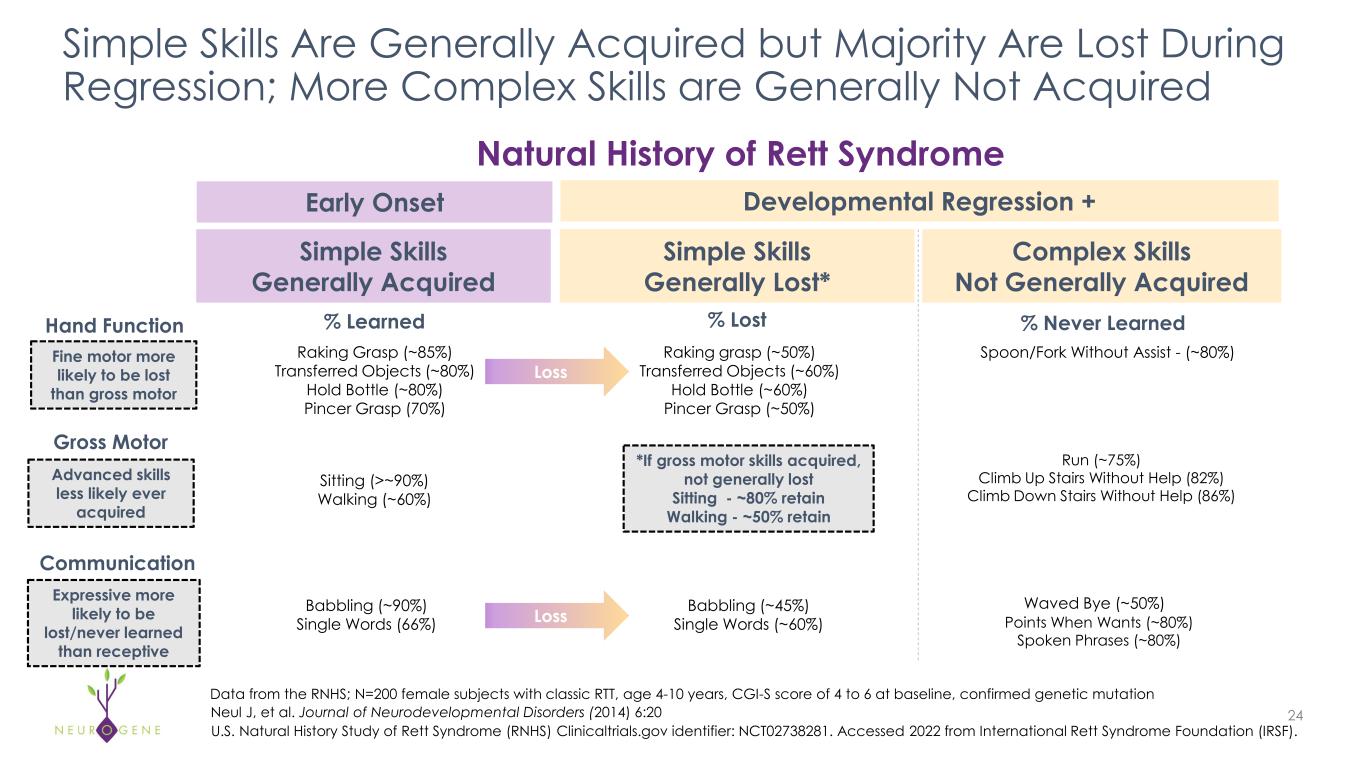
Simple Skills Are Generally Acquired but Majority Are Lost During Regression; More Complex Skills are Generally Not Acquired 24 Data from the RNHS; N=200 female subjects with classic RTT, age 4-10 years, CGI-S score of 4 to 6 at baseline, confirmed genetic mutation Raking Grasp (~85%) Transferred Objects (~80%) Hold Bottle (~80%) Pincer Grasp (70%) Points When Wants (~80%) Spoken Phrases (~80%) Hand Function Gross Motor Communication Simple Skills Generally Acquired Simple Skills Generally Lost* Complex Skills Not Generally Acquired Natural History of Rett Syndrome Early Onset Developmental Regression + % Never Learned Spoon/Fork Without Assist - (~80%) % Learned U.S. Natural History Study of Rett Syndrome (RNHS) Clinicaltrials.gov identifier: NCT02738281. Accessed 2022 from International Rett Syndrome Foundation (IRSF). % Lost Sitting (>~90%) Walking (~60%) Run (~75%) Climb Up Stairs Without Help (82%) Climb Down Stairs Without Help (86%) Babbling (~90%) Single Words (66%) Raking grasp (~50%) Transferred Objects (~60%) Hold Bottle (~60%) Pincer Grasp (~50%) Babbling (~45%) Single Words (~60%) *If gross motor skills acquired, not generally lost Sitting - ~80% retain Walking - ~50% retain Fine motor more likely to be lost than gross motor Advanced skills less likely ever acquired Expressive more likely to be lost/never learned than receptive Loss Waved Bye (~50%) Loss Neul J, et al. Journal of Neurodevelopmental Disorders (2014) 6:20
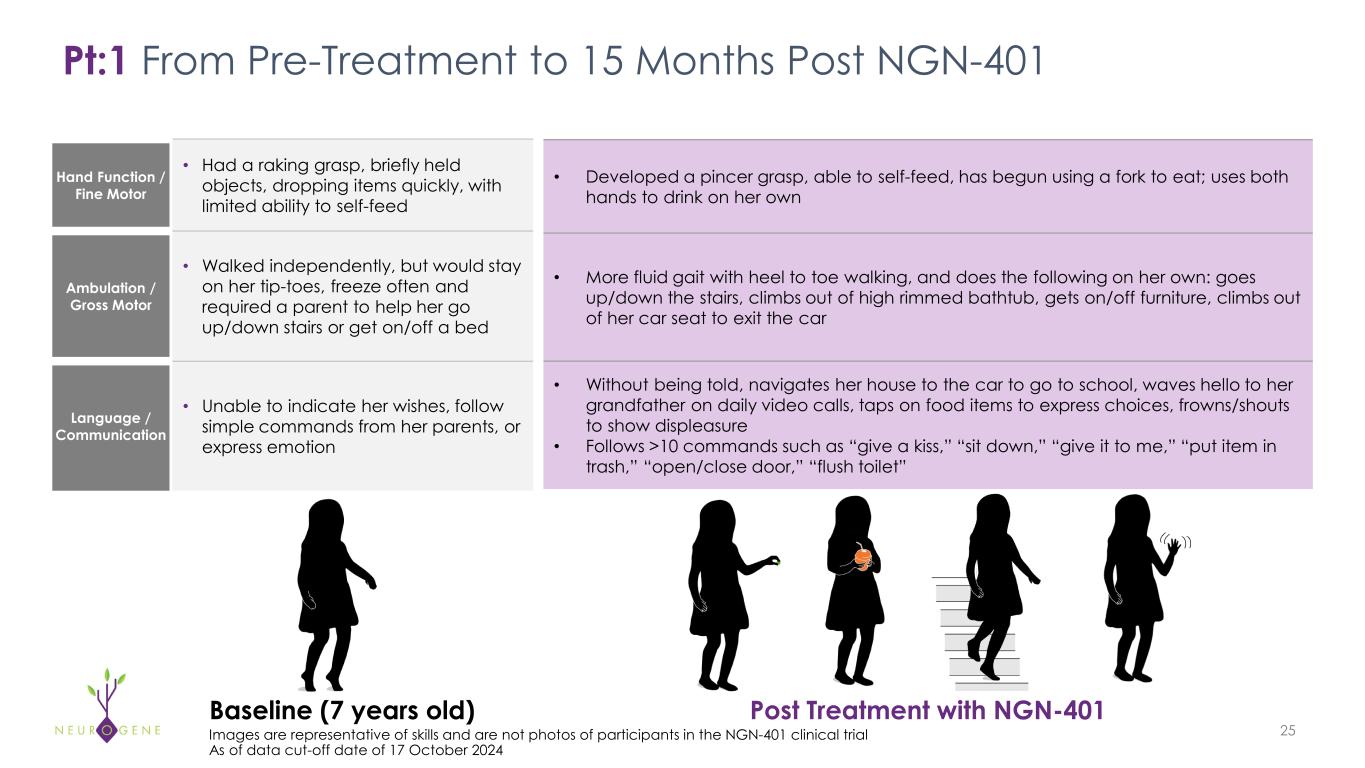
Hand Function / Fine Motor • Had a raking grasp, briefly held objects, dropping items quickly, with limited ability to self-feed Ambulation / Gross Motor • Walked independently, but would stay on her tip-toes, freeze often and required a parent to help her go up/down stairs or get on/off a bed Language / Communication • Unable to indicate her wishes, follow simple commands from her parents, or express emotion Pt:1 From Pre-Treatment to 15 Months Post NGN-401 25 Baseline (7 years old) Post Treatment with NGN-401 Images are representative of skills and are not photos of participants in the NGN-401 clinical trial As of data cut-off date of 17 October 2024 • Developed a pincer grasp, able to self-feed, has begun using a fork to eat; uses both hands to drink on her own • More fluid gait with heel to toe walking, and does the following on her own: goes up/down the stairs, climbs out of high rimmed bathtub, gets on/off furniture, climbs out of her car seat to exit the car • Without being told, navigates her house to the car to go to school, waves hello to her grandfather on daily video calls, taps on food items to express choices, frowns/shouts to show displeasure • Follows >10 commands such as “give a kiss,” “sit down,” “give it to me,” “put item in trash,” “open/close door,” “flush toilet”
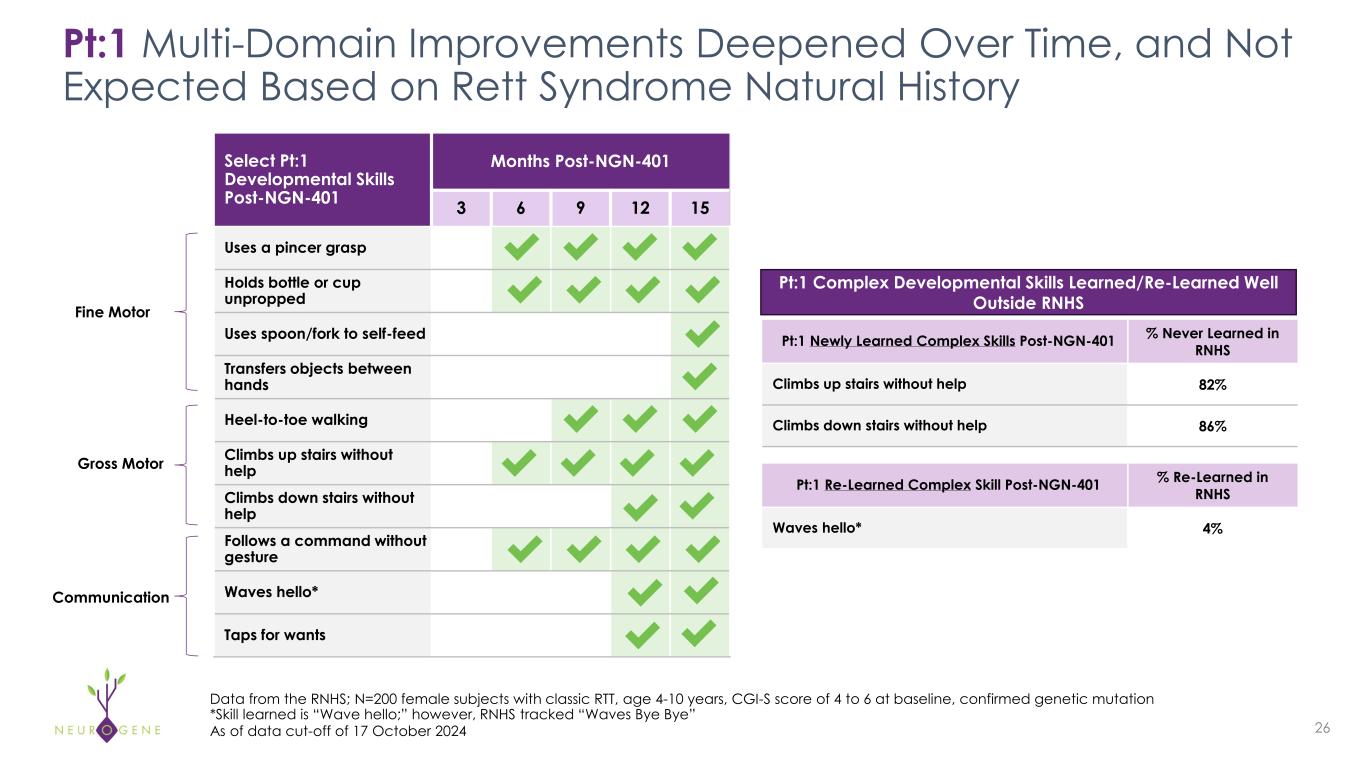
Pt:1 Multi-Domain Improvements Deepened Over Time, and Not Expected Based on Rett Syndrome Natural History 26 Select Pt:1 Developmental Skills Post-NGN-401 Months Post-NGN-401 3 6 9 12 15 Uses a pincer grasp Holds bottle or cup unpropped Uses spoon/fork to self-feed Transfers objects between hands Heel-to-toe walking Climbs up stairs without help Climbs down stairs without help Follows a command without gesture Waves hello* Taps for wants Data from the RNHS; N=200 female subjects with classic RTT, age 4-10 years, CGI-S score of 4 to 6 at baseline, confirmed genetic mutation *Skill learned is “Wave hello;” however, RNHS tracked “Waves Bye Bye” As of data cut-off of 17 October 2024 Pt:1 Newly Learned Complex Skills Post-NGN-401 % Never Learned in RNHS Climbs up stairs without help 82% Climbs down stairs without help 86% Pt:1 Re-Learned Complex Skill Post-NGN-401 % Re-Learned in RNHS Waves hello* 4% Pt:1 Complex Developmental Skills Learned/Re-Learned Well Outside RNHSFine Motor Gross Motor Communication
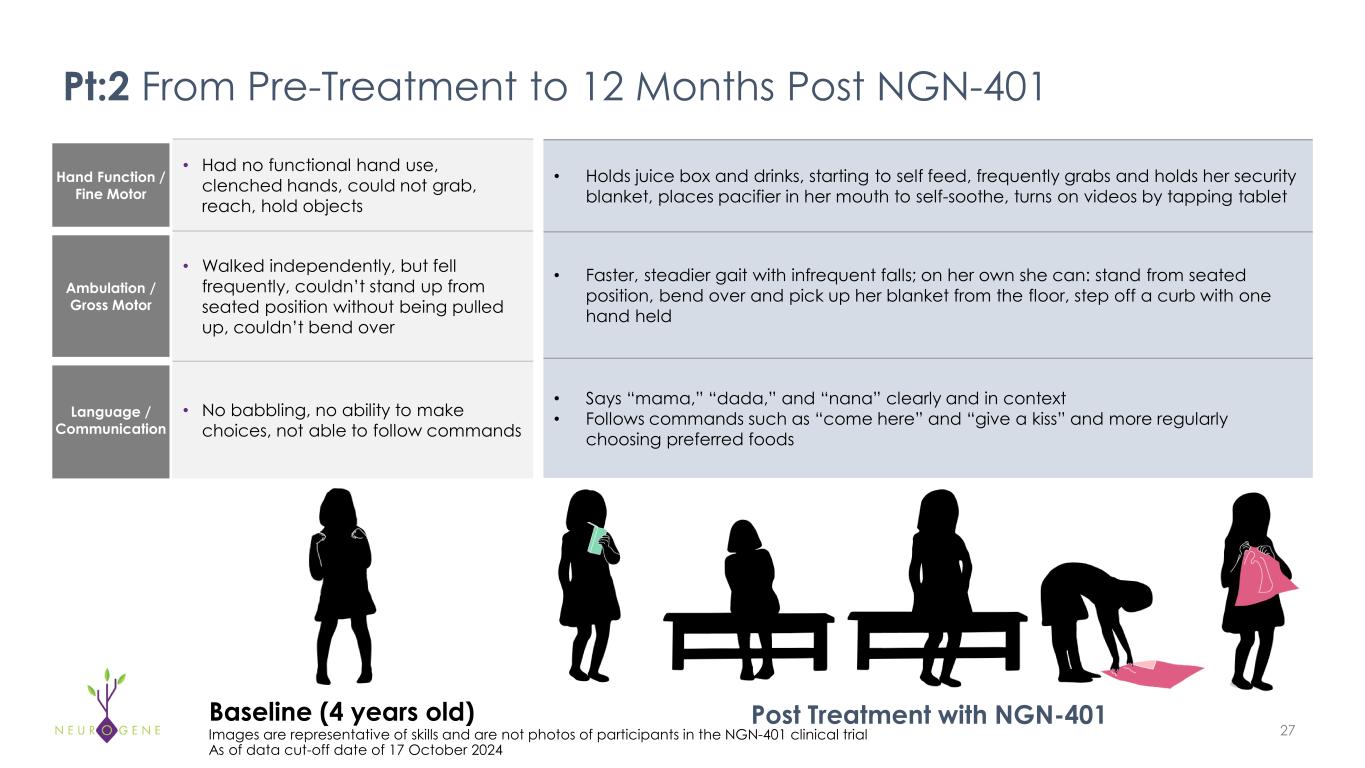
Hand Function / Fine Motor • Had no functional hand use, clenched hands, could not grab, reach, hold objects Ambulation / Gross Motor • Walked independently, but fell frequently, couldn’t stand up from seated position without being pulled up, couldn’t bend over Language / Communication • No babbling, no ability to make choices, not able to follow commands 27Images are representative of skills and are not photos of participants in the NGN-401 clinical trial As of data cut-off date of 17 October 2024 • Holds juice box and drinks, starting to self feed, frequently grabs and holds her security blanket, places pacifier in her mouth to self-soothe, turns on videos by tapping tablet • Faster, steadier gait with infrequent falls; on her own she can: stand from seated position, bend over and pick up her blanket from the floor, step off a curb with one hand held • Says “mama,” “dada,” and “nana” clearly and in context • Follows commands such as “come here” and “give a kiss” and more regularly choosing preferred foods Pt:2 From Pre-Treatment to 12 Months Post NGN-401 Post Treatment with NGN-401Baseline (4 years old)
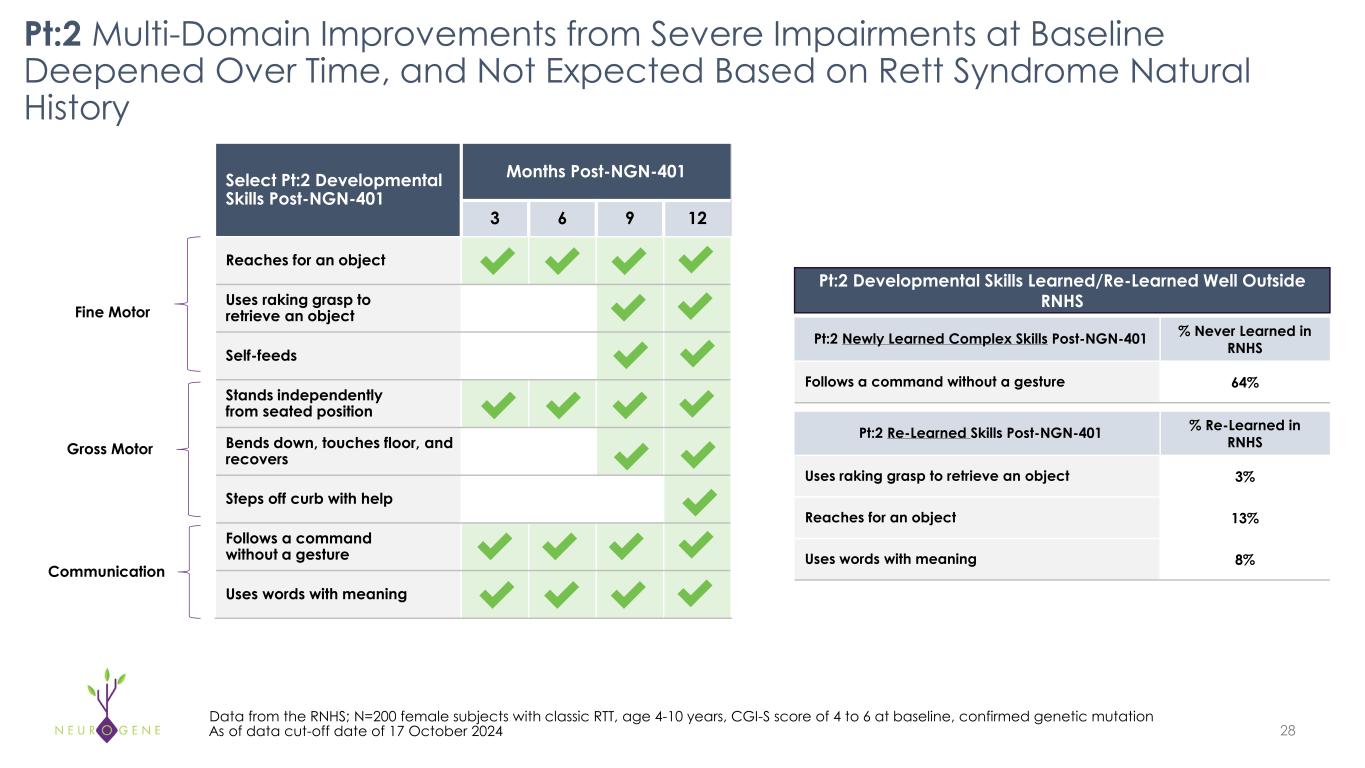
Pt:2 Multi-Domain Improvements from Severe Impairments at Baseline Deepened Over Time, and Not Expected Based on Rett Syndrome Natural History 28 Data from the RNHS; N=200 female subjects with classic RTT, age 4-10 years, CGI-S score of 4 to 6 at baseline, confirmed genetic mutation As of data cut-off date of 17 October 2024 Select Pt:2 Developmental Skills Post-NGN-401 Months Post-NGN-401 3 6 9 12 Reaches for an object Uses raking grasp to retrieve an object Self-feeds Stands independently from seated position Bends down, touches floor, and recovers Steps off curb with help Follows a command without a gesture Uses words with meaning Pt:2 Newly Learned Complex Skills Post-NGN-401 % Never Learned in RNHS Follows a command without a gesture 64% Pt:2 Re-Learned Skills Post-NGN-401 % Re-Learned in RNHS Uses raking grasp to retrieve an object 3% Reaches for an object 13% Uses words with meaning 8% Pt:2 Developmental Skills Learned/Re-Learned Well Outside RNHSFine Motor Gross Motor Communication
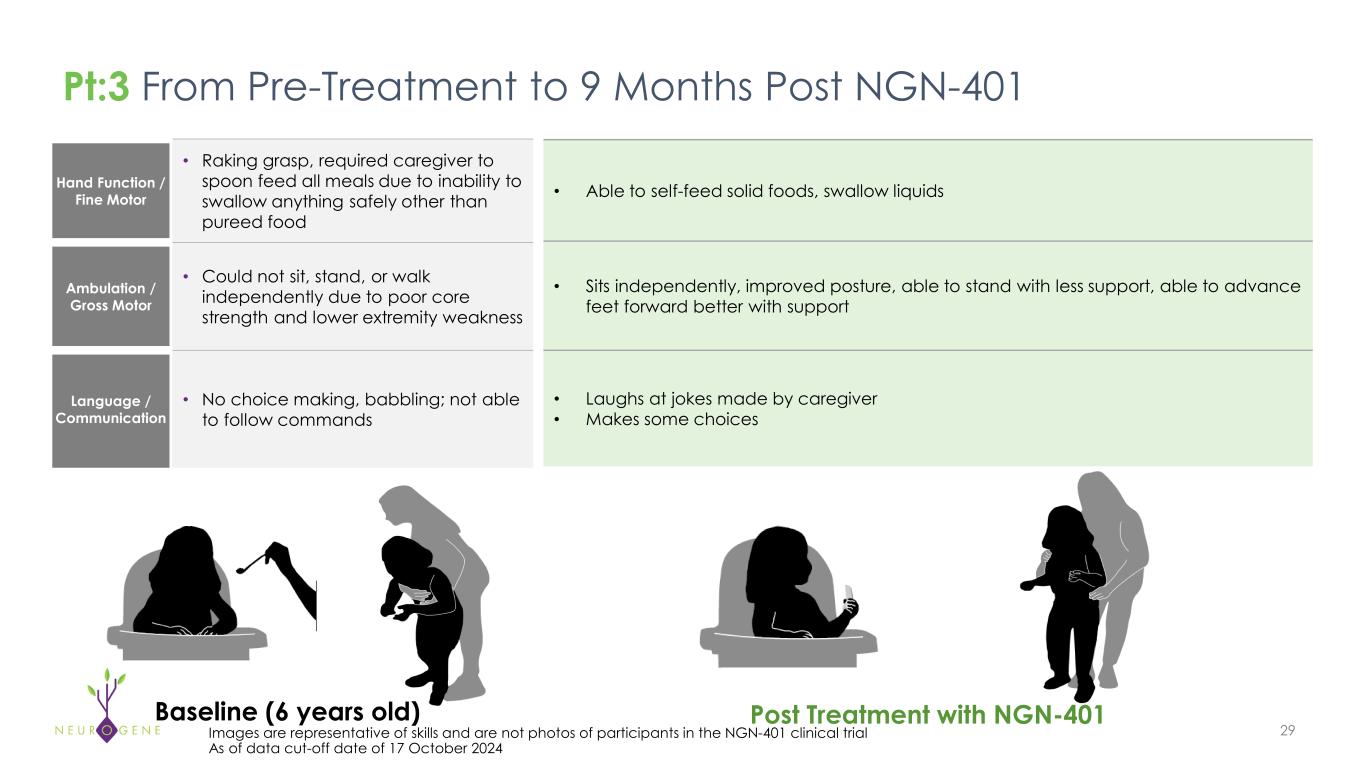
Hand Function / Fine Motor • Raking grasp, required caregiver to spoon feed all meals due to inability to swallow anything safely other than pureed food Ambulation / Gross Motor • Could not sit, stand, or walk independently due to poor core strength and lower extremity weakness Language / Communication • No choice making, babbling; not able to follow commands 29Images are representative of skills and are not photos of participants in the NGN-401 clinical trial As of data cut-off date of 17 October 2024 • Able to self-feed solid foods, swallow liquids • Sits independently, improved posture, able to stand with less support, able to advance feet forward better with support • Laughs at jokes made by caregiver • Makes some choices Pt:3 From Pre-Treatment to 9 Months Post NGN-401 Baseline (6 years old) Post Treatment with NGN-401
Pt:3 Multi-Domain Improvements Not Expected Based on Rett Syndrome Natural History 30 Select Pt:3 Developmental Skills Months Post-NGN-401 3 6 9 Uses a pincer grasp Able to self-feed Sits independently Data from the RNHS; N=200 female subjects with classic RTT, age 4-10 years, CGI-S score of 4 to 6 at baseline, confirmed genetic mutation As of data cut-off date of 17 October 2024 Pt:3 Re-Learned Skills Post-NGN-401 % Re-Learned in RNHS Uses a pincer grasp 6% Able to self-feed 8% Sits independently 7% Pt:3 Developmental Re-Learned Well Outside RNHS Fine Motor Gross Motor
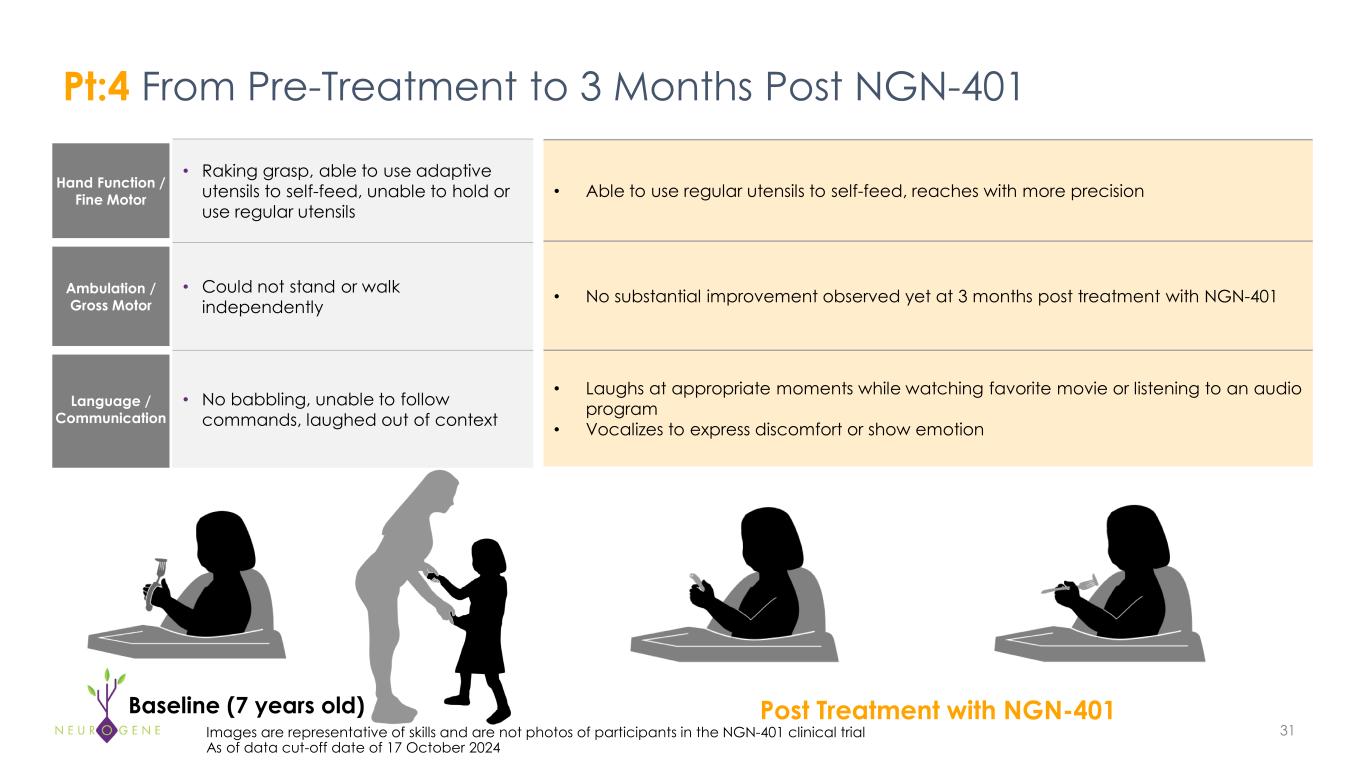
Hand Function / Fine Motor • Raking grasp, able to use adaptive utensils to self-feed, unable to hold or use regular utensils Ambulation / Gross Motor • Could not stand or walk independently Language / Communication • No babbling, unable to follow commands, laughed out of context 31Images are representative of skills and are not photos of participants in the NGN-401 clinical trial As of data cut-off date of 17 October 2024 • Able to use regular utensils to self-feed, reaches with more precision • No substantial improvement observed yet at 3 months post treatment with NGN-401 • Laughs at appropriate moments while watching favorite movie or listening to an audio program • Vocalizes to express discomfort or show emotion Pt:4 From Pre-Treatment to 3 Months Post NGN-401 Baseline (7 years old) Post Treatment with NGN-401
Pt:4 Early Improvements in Hand Function Not Expected Based on Rett Syndrome Natural History 32 Select Pt:4 Developmental Skills Months Post- NGN-401 3 Uses a pincer grasp Can use utensils to self-feed (without assistance) Data from the RNHS; N=200 female subjects with classic RTT, age 4-10 years, CGI-S score of 4 to 6 at baseline, confirmed genetic mutation As of data cut-off date of 17 October 2024 Pt:4 Newly Learned Complex Skill Post-NGN-401 % Never Learned in RNHS Can use utensils to self-feed (without assistance) 80% Pt:4 Developmental Skills Learned Well Outside RNHS
Leveraging START and RMAT to Accelerate Program to Registration 33 START Program participation provides clear channel of communication with FDA intended to accelerate registrational planning RMAT designation provides eligibility for an Accelerated Approval pathway and rolling BLA and potential for Priority Review FDA alignment on potency assay strategy to support future registrational trial and manufacturing scale-up plans at Neurogene Houston facility expected to support commercial launch plans Initiated ages > 11 cohort to support potential for a broad label to capture higher portion of prevalent population Multiple Touch Points with FDA Intended to Accelerate Registration .

Key Anticipated Milestone Events

Key Upcoming Anticipated Milestones and Pipeline Developments Rett syndrome (NGN-401) CLN5 Batten disease (NGN-101) Evaluate opportunities for the program 35 Cash runway expected to fund operations into 2H:27 Provide regulatory update in 1H:25 regarding pivotal trial Announce additional Phase 1/2 clinical data in 2H:25

Appendix
HLH Risk Mitigation included in NGN-401 Phase 1/2 Trial • HLH is a rare, life-threatening hyperinflammatory syndrome characterized by immune dysregulation, cytokine storm, and multi-organ damage1-3 • HLH has been rarely reported following high-dose AAV gene therapy (>1E14 vg/kg) • Despite only moving forward with the 1E15 vg dose, which translates to ~E13 vg/kg, implemented risk mitigation in ongoing Phase 1/2 trial: • Dose level above 1E14 vg/kg not allowed • In the first week post dosing: employ daily monitoring of “the three Fs” - ferritin, fever, and falling blood counts (cytopenia) • Exclude subjects with any illness within 30 days of dosing and COVID within 6 weeks of screening • Prior to dosing, require sites to have anakinra available and encourage availability of a local HLH expert • Included HLH treatment algorithm: 1st line - high-dose corticosteroids, 2nd line - anakinra 37 1Soy et al. Rheumatol Int. 2021;41(1):7-18. 2Mérad et al., 2019; 3Jordan et al. Blood. 2011;118(15):4041-52; 4Henter J et al 2007 Feb; 48(2):124-31
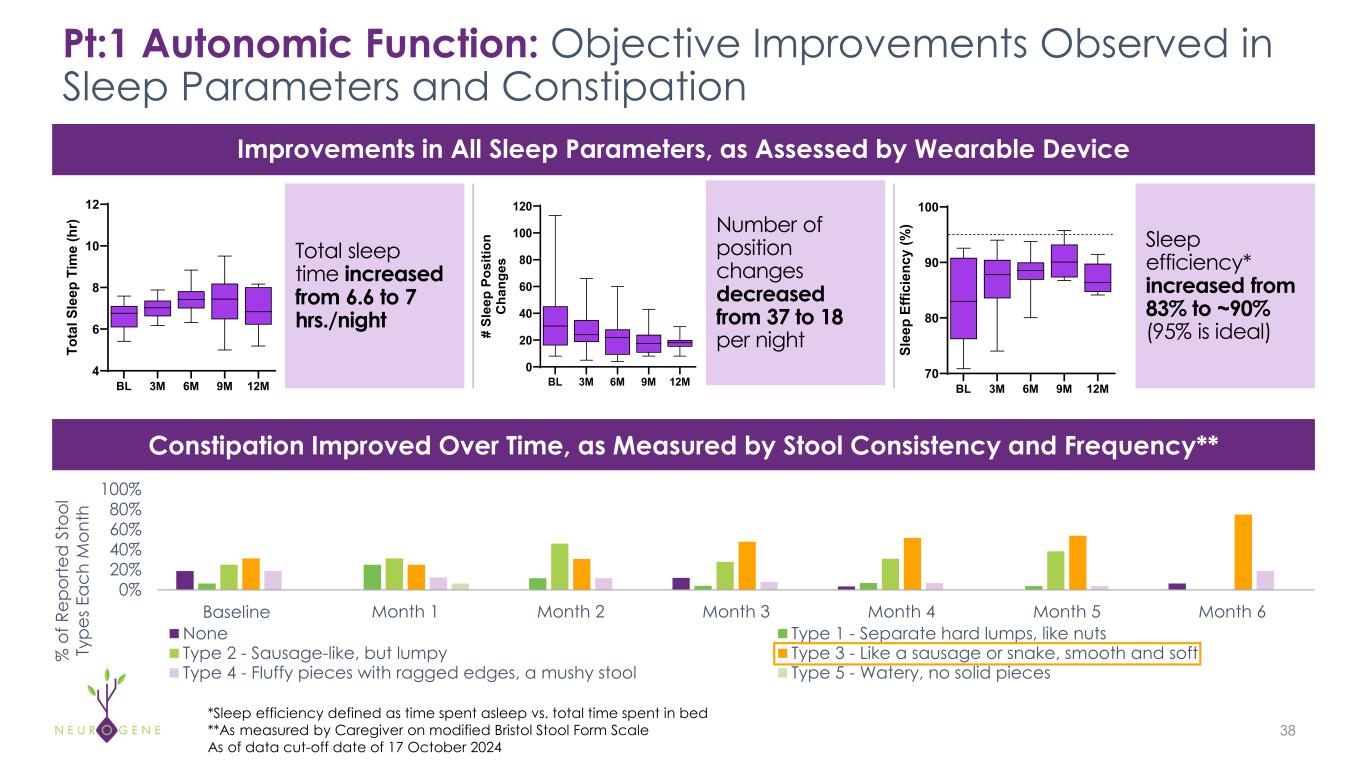
Pt:1 Autonomic Function: Objective Improvements Observed in Sleep Parameters and Constipation 38 Total sleep time increased from 6.6 to 7 hrs./night Number of position changes decreased from 37 to 18 per night Sleep efficiency* increased from 83% to ~90% (95% is ideal) Improvements in All Sleep Parameters, as Assessed by Wearable Device Constipation Improved Over Time, as Measured by Stool Consistency and Frequency** % o f R ep or te d S to ol Ty pe s E ac h M on th 0% 20% 40% 60% 80% 100% Month 0 Month 1 Month 2 Month 3 Month 4 Month 5 Month 6 None Type 1 - Separate hard lumps, like nuts Type 2 - Sausage-like, but lumpy Type 3 - Like a sausage or snake, smooth and soft Type 4 - Fluffy pieces with ragged edges, a mushy stool Type 5 - Watery, no solid pieces Baseline BL 3M 6M 9M 12M 4 6 8 10 12 To ta l S le ep T im e (h r) BL 3M 6M 9M 12M 0 20 40 60 80 100 120 # Sl ee p Po si tio n C ha ng es BL 3M 6M 9M 12M 70 80 90 100 Sl ee p Ef fic ie nc y (% ) *Sleep efficiency defined as time spent asleep vs. total time spent in bed **As measured by Caregiver on modified Bristol Stool Form Scale As of data cut-off date of 17 October 2024
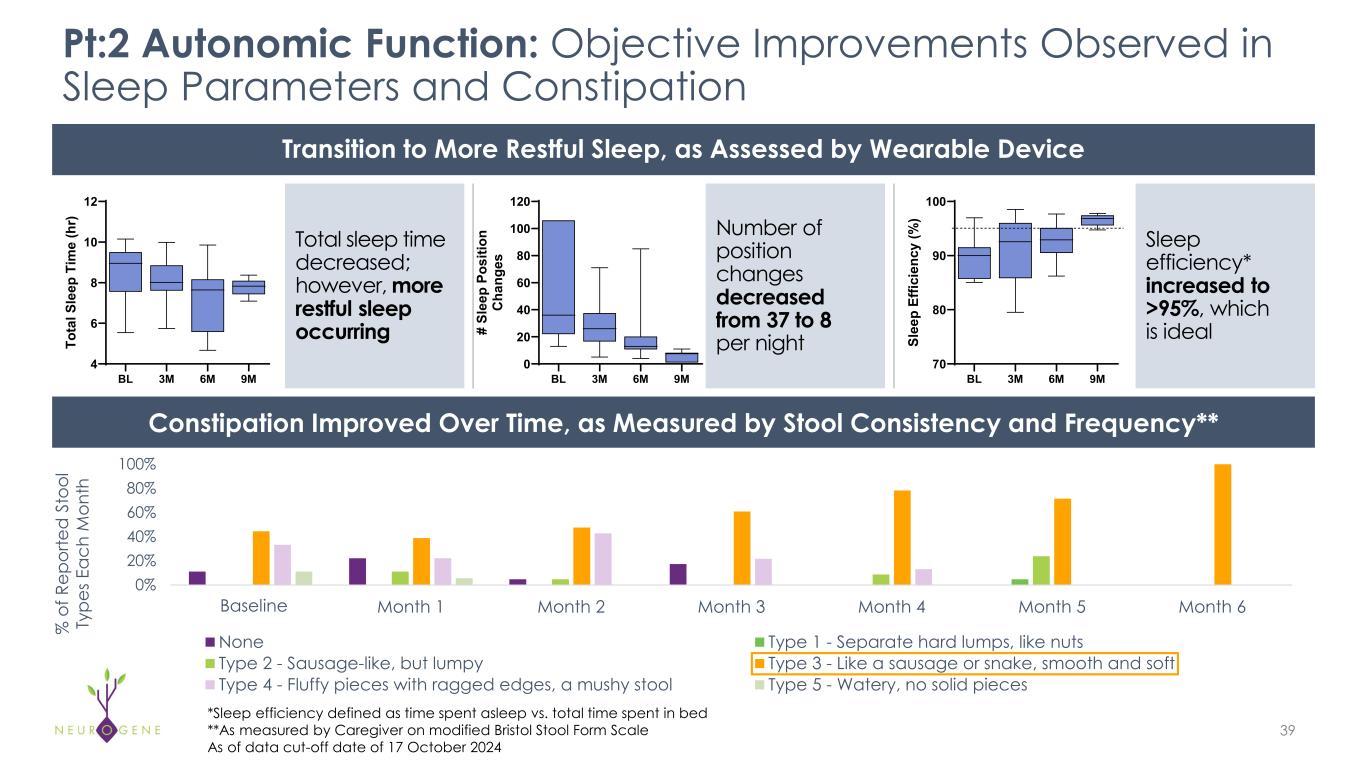
0% 20% 40% 60% 80% 100% Month 0 Month 1 Month 2 Month 3 Month 4 Month 5 Month 6 None Type 1 - Separate hard lumps, like nuts Type 2 - Sausage-like, but lumpy Type 3 - Like a sausage or snake, smooth and soft Type 4 - Fluffy pieces with ragged edges, a mushy stool Type 5 - Watery, no solid pieces Pt:2 Autonomic Function: Objective Improvements Observed in Sleep Parameters and Constipation 39 Transition to More Restful Sleep, as Assessed by Wearable Device Constipation Improved Over Time, as Measured by Stool Consistency and Frequency** Total sleep time decreased; however, more restful sleep occurring Number of position changes decreased from 37 to 8 per night Sleep efficiency* increased to >95%, which is ideal % o f R ep or te d S to ol Ty pe s E ac h M on th BL 3M 6M 9M 4 6 8 10 12 To ta l S le ep T im e (h r) BL 3M 6M 9M 0 20 40 60 80 100 120 # Sl ee p Po si tio n C ha ng es BL 3M 6M 9M 70 80 90 100 Sl ee p Ef fic ie nc y (% ) Baseline *Sleep efficiency defined as time spent asleep vs. total time spent in bed **As measured by Caregiver on modified Bristol Stool Form Scale As of data cut-off date of 17 October 2024
Beginning 3 months post-NGN-401, Pt:3 could swallow liquids, such as clear soup and water from a sippy cup, and chew and swallow soft items, such as meatballs and cooked carrots, without choking At 9 months post-NGN-401, she is now able to grasp food such as apple slices and self-feed At Baseline, Pt:3 had dysphagia requiring a pureed diet and had to be spoon- fed by caregiver due to aspiration Pt:3 Autonomic Function: Experienced Clinically Meaningful Improvement in Swallowing and Gained Ability to Self-feed 40 Pt:3 did not have Baseline deficits in autonomic categories of sleep or constipation • Sleep duration and quality maintained post-treatment • No change in Modified Bristol Stool Form Scale scores post-treatment As of data cut-off date of 17 October 2024
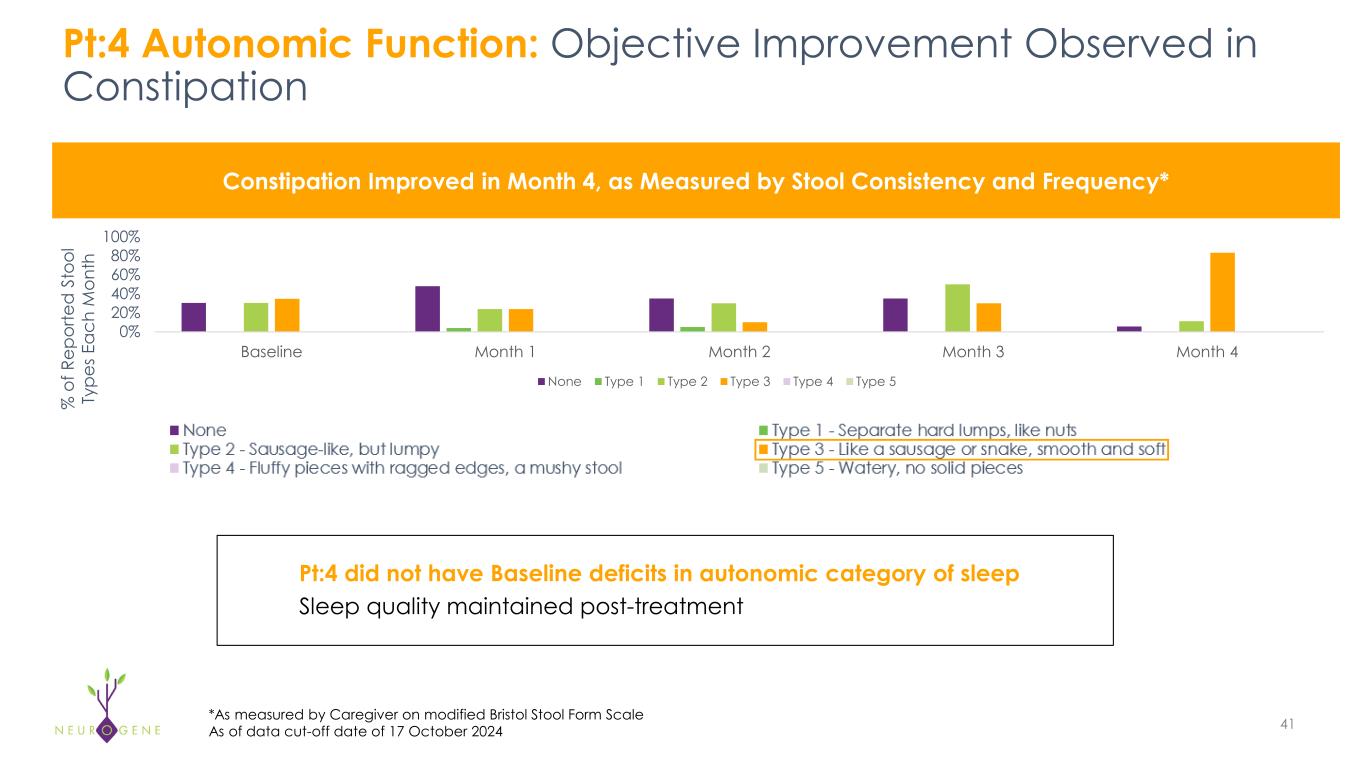
Pt:4 did not have Baseline deficits in autonomic category of sleep Sleep quality maintained post-treatment Pt:4 Autonomic Function: Objective Improvement Observed in Constipation 41 Constipation Improved in Month 4, as Measured by Stool Consistency and Frequency* *As measured by Caregiver on modified Bristol Stool Form Scale 0% 20% 40% 60% 80% 100% Baseline Month 1 Month 2 Month 3 Month 4 None Type 1 Type 2 Type 3 Type 4 Type 5 % o f R ep or te d S to ol Ty pe s E ac h M on th As of data cut-off date of 17 October 2024
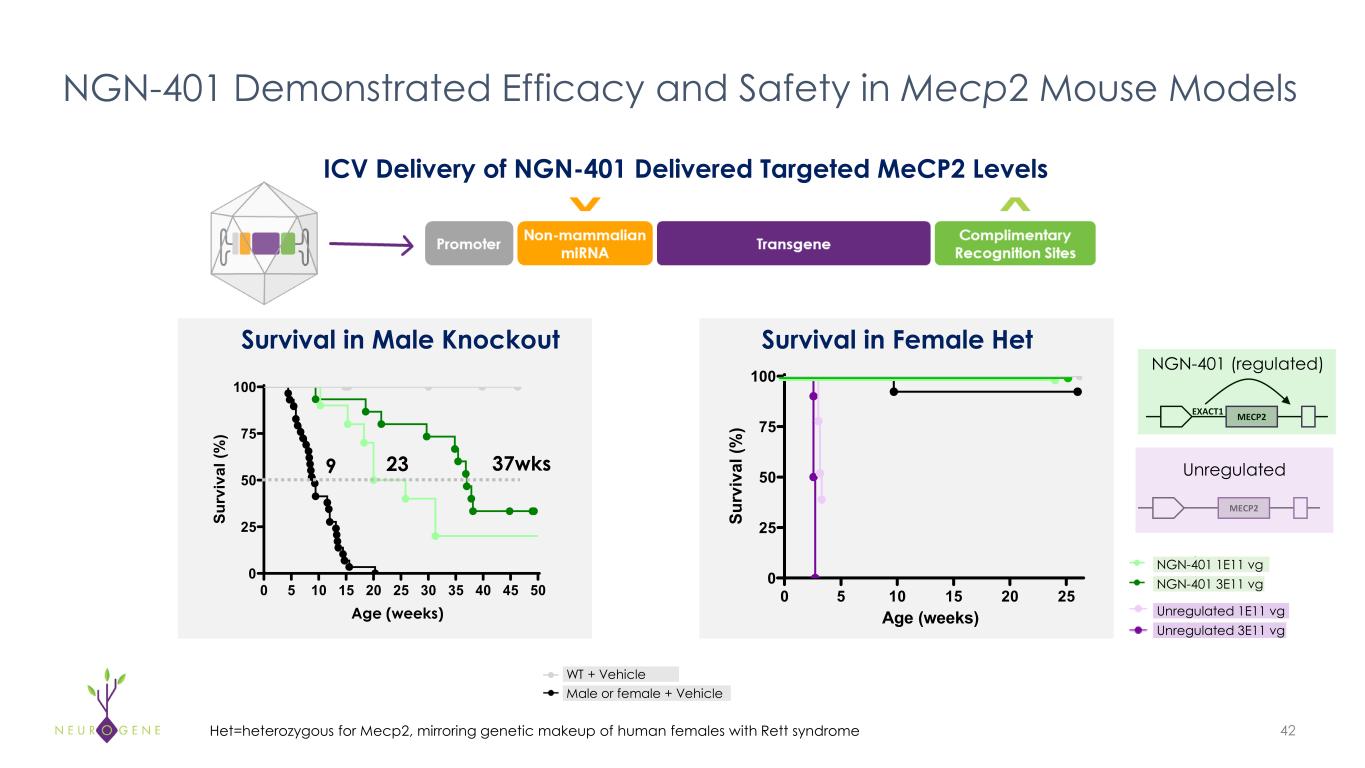
NGN-401 Demonstrated Efficacy and Safety in Mecp2 Mouse Models 42 Survival in Male Knockout 0 5 10 15 20 25 30 35 40 45 50 0 25 50 75 100 Su rv iv al (% ) 9 23 37wks Age (weeks) 0 5 10 15 20 25 0 25 50 75 100 Age (weeks) Su rv iv al (% ) Survival in Female Het NGN-401 1E11 vg NGN-401 3E11 vg MECP2EXACT1 NGN-401 (regulated) MECP2 Unregulated Unregulated 1E11 vg Unregulated 3E11 vg WT + Vehicle Male or female + Vehicle Het=heterozygous for Mecp2, mirroring genetic makeup of human females with Rett syndrome ICV Delivery of NGN-401 Delivered Targeted MeCP2 Levels
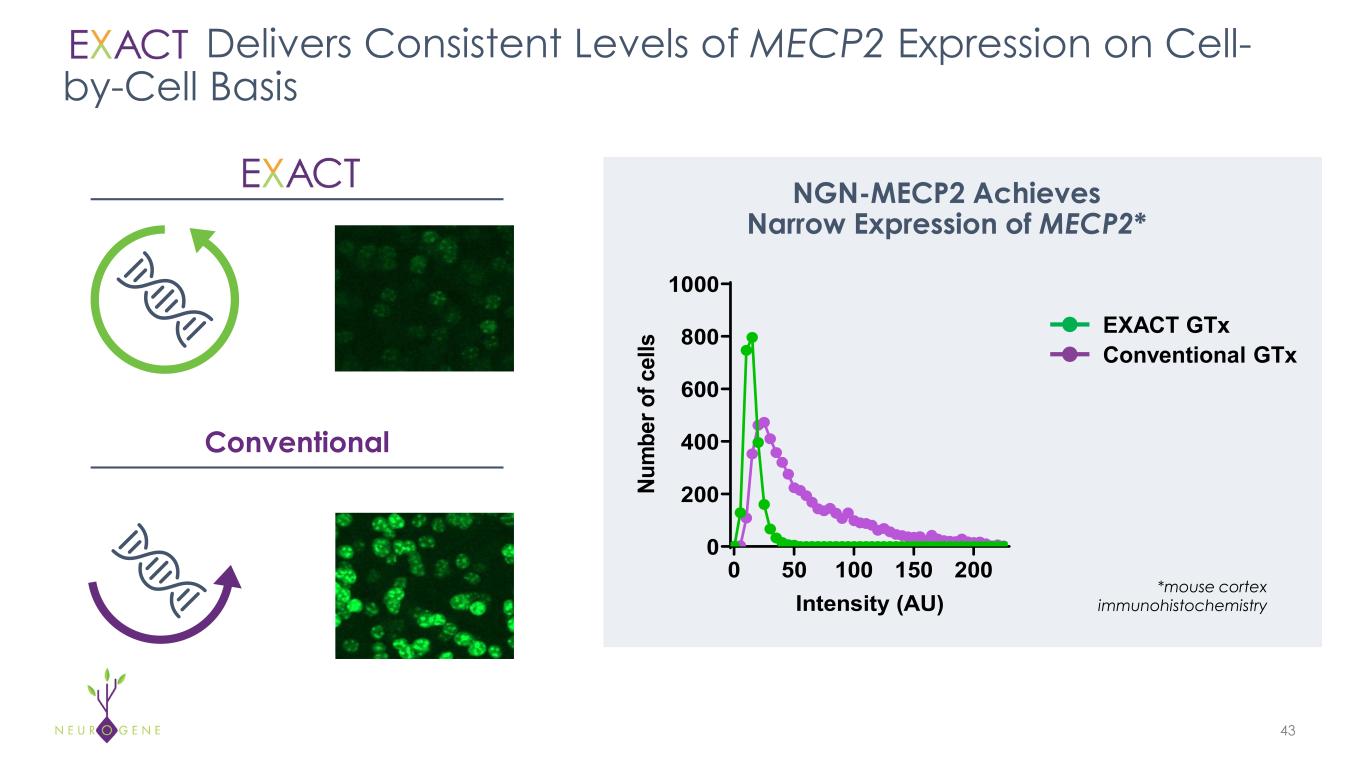
Delivers Consistent Levels of MECP2 Expression on Cell- by-Cell Basis 43 Conventional NGN-MECP2 Achieves Narrow Expression of MECP2* 0 50 100 150 200 0 200 400 600 800 1000 Intensity (AU) Nu m be r o f c el ls Unregulated Regulated EXACT GTx Conventional GTx *mouse cortex immunohistochemistry