

1 © 2026, Iovance Biotherapeutics, Inc. © 2026, Iovance Biotherapeutics, Inc. Corporate Overview January 2026 1

2 © 2026, Iovance Biotherapeutics, Inc. Forward - Looking Statements Certain matters discussed in this presentation are “forward - looking statements” of Iovance Biotherapeutics, Inc. (hereinafter referred to as the “Company,” “we,” “us,” or “our”) within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLRA”). Without limiting the foregoi ng, we may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “forecast,” “guid anc e,” “outlook,” “may,” “can,” “could,” “might,” “will,” “should,” or other words that convey uncertainty of future events or outcomes and are intended to identify forward - looki ng statements. Forward - looking statements are based on assumptions and assessments made in light of management’s experience and perception of historical trends, current conditions, expected future developments, and other factors believed to be appropriate. Forward - looking statements in this presentation are made as of the d ate of this presentation, and we undertake no duty to update or revise any such statements, whether as a result of new information, future events or otherwise. Forward - looking statements are not guarantees of future performance and are subject to risks, uncertainties, and other factors, many of which are outside of our co ntrol, that may cause actual results, levels of activity, performance, achievements, and developments to be materially different from those expressed in or implied by the se forward - looking statements. Important factors that could cause actual results, developments, and business decisions to differ materially from forward - looking statemen ts are described in the sections titled "Risk Factors" in our filings with the U.S. Securities and Exchange Commission, including our most recent Annual Report on Fo rm 10 - K and Quarterly Reports on Form 10 - Q, and include, but are not limited to, the following substantial known and unknown risks and uncertainties inherent in our busi nes s: the risks related to our ability to successfully commercialize our products; the acceptance by the market of our products and product candidates, if approved, and their potential pricing and/or reimbursement by payors and whether such acceptance is sufficient to support continued commercialization or development of ou r p roducts or product candidates; the risk regarding our ability to manufacture our therapies using third party manufacturers or at our own facility, including our ab ility to increase manufacturing capacity at such third party manufacturers and our own facility, may adversely affect our commercial launch; the risk that the successful deve lop ment or commercialization of our products may not generate sufficient revenue from product sales, and we may not become profitable in the near term, or at all; the risks r el ated to the timing of and our ability to successfully develop, submit, obtain, or maintain regulatory authority approval of our product candidates; whether clinical t ria l results from our pivotal studies and cohorts, and meetings with regulatory authorities may support registrational studies and subsequent approvals by regulatory a uth orities ; preliminary and interim clinical results, including the interim results for the IOV - LUN - 202 trial, which may include efficacy and safety results, from ongoing clinical trials or cohorts may not be reflected in the final analyses of our ongoing clinical trials or subgroups within these trials or in other prior trials or cohorts; the r isk that we may be required to conduct additional clinical trials or modify ongoing or future clinical trials based on feedback from regulatory authorities; the risk that our int erpretation of the results of our clinical trials or communications with regulatory authorities may differ from the interpretation of such results or communications by such regul ato ry authorities ; the risk that clinical data from ongoing clinical trials of Amtagvi will not continue or be repeated in ongoing or planned clinical trials or may not support regulatory approval or renewal of authorization; the risk that unanticipated expenses may decrease our estimated cash balances and forecasts and increase our e sti mated capital requirements; the risk that we may not be able to recognize revenue for our products; the risk that Proleukin revenues may not continue to serve as a leading indicator for Amtagvi revenues; the risks regarding our anticipated operating and financial performance, including our financial guidance and projections; the effects of global and domestic geopolitical factors or public health events ; and other factors, including general economic conditions and regulatory developments, not within our control.
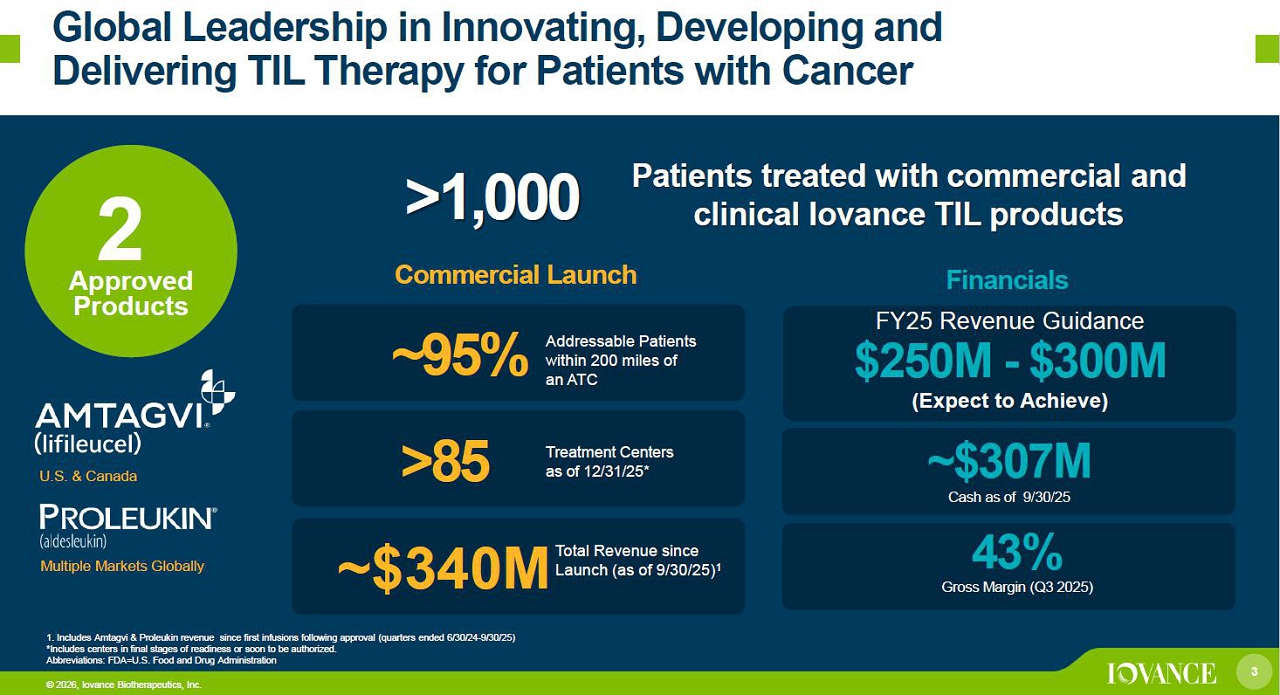
3 © 2026, Iovance Biotherapeutics, Inc. Global Leadership in Innovating, Developing and Delivering TIL Therapy for Patients with Cancer 3 Approved Products Commercial Launch Financials >85 ~$307M Cash as of 9/30/25 Treatment Centers as of 12/31/25* 2 ~95% 43% Gross Margin (Q3 2025) © 2026, Iovance Biotherapeutics, Inc. 1. Includes Amtagvi & Proleukin revenue for quarters ended 3/31/24 - 9/30/25 *Includes center s in final stages of readiness or soon to be authorized. Abbreviations: BTD=Breakthrough Therapy Designation; FDA=U.S. Food and Drug Administration; RMAT=Regenerative Medicine Advanc ed Therapy Designation >1,000 Patients treated with commercial and clinical Iovance TIL products U.S. & Canada Multiple Markets Globally $340M FY25 Revenue Guidance $250M - $300M (Expect to Achieve) Addressable Patients within 200 miles of an ATC Total Revenue as of 9/30/2025 since Q1 2024 Amtagvi FDA Approval 1
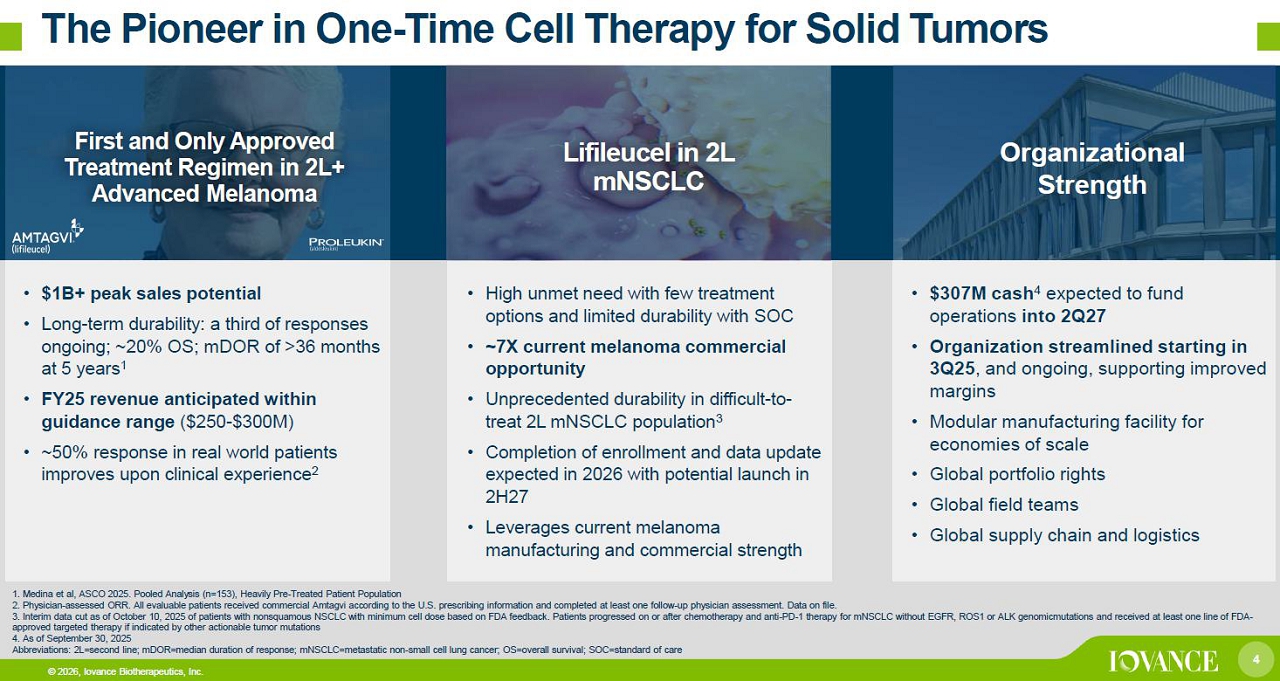
4 © 2026, Iovance Biotherapeutics, Inc. The Pioneer in One - Time Cell Therapy for Solid Tumors 4 Abbreviations: FTD, fast track designation; ICI, immune checkpoint inhibitor; NSCLC, non - small cell lung cancer; PD - 1, programme d cell death protein - 1 1. Medina et al, ASCO 2025. Pooled Analysis (n=153), Heavily Pre - Treated Patient Population 2. Physician - assessed ORR. All evaluable patients received commercial Amtagvi according to the U.S. prescribing information and completed at least one follow - up physician assessment. Data on file. 3. Interim data cut as of October 10, 2025 of patients with nonsquamous NSCLC with minimum cell dose based on FDA feedback. Patients progressed on or after chemotherapy and anti - PD - 1 therapy for mNSCLC without EGFR, ROS1 or ALK genomicmutations and received at least one line of FDA - approved targeted therapy if indicated by other actionable tumor mutations 4. As of September 30, 2025 Abbreviations: 2L=second line; mDOR =median duration of response; mNSCLC =metastatic non - small cell lung cancer; OS=overall survival; NR=not reached; ORR=objective response rate; SOC=standard of care Organizational Strength Lifileucel in 2L mNSCLC First and Only Approved Treatment Regimen in 2L+ Advanced Melanoma • $307M cash 4 expected to fund operations into 2Q27 • Organization streamlined in 3Q25 supporting improved margins • Modular manufacturing facility for economies of scale • Global portfolio rights • Global field teams • Global supply chain and logistics • High unmet need with few treatment options and limited durability with SOC • ~7X current melanoma opportunity • Unprecedented durability in difficult - to - treat 2L mNSCLC population 3 • Completion of enrollment and data update expected in 2026 with potential launch in 2H27 • Leverages current melanoma manufacturing and commercial strength • $1B+ peak sales potential • Long - term durability: a third of responses ongoing; ~20% OS; mDOR of >36 months at 5 years 1 • FY25 revenue anticipated within guidance range ($250 - $300M) • ~50% response in real world patients improves upon clinical experience 2
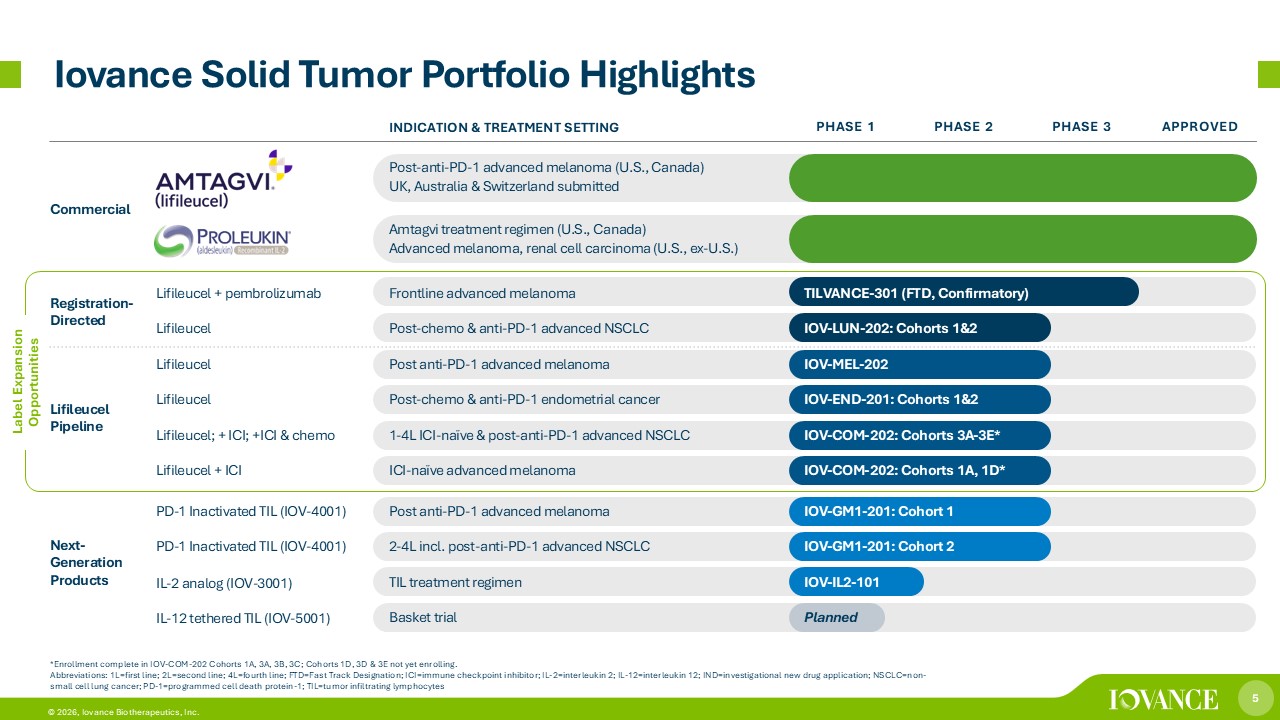
5 © 2026, Iovance Biotherapeutics, Inc. Iovance Solid Tumor Portfolio Highlights *Enrollment complete in IOV - COM - 202 Cohorts 1A, 3A, 3B, 3C ; Cohorts 1D, 3D & 3E not yet enrolling. Abbreviations: 1L=first line; 2L=second line; 4L=fourth line; FTD=Fast Track Designation; ICI=immune checkpoint inhibitor; IL - 2= interleukin 2; IL - 12=interleukin 12; IND=investigational new drug application; NSCLC=non - small cell lung cancer; PD - 1=programmed cell death protein - 1; TIL=tumor infiltrating lymphocytes Label Expansion Opportunities APPROVED PHASE 3 PHASE 2 PHASE 1 INDICATION & TREATMENT SETTING Post - anti - PD - 1 advanced melanoma (U.S., Canada) UK, Australia & Switzerland submitted Commercial Amtagvi treatment regimen (U.S., Canada) Advanced melanoma, renal cell carcinoma (U.S., ex - U.S.) TILVANCE - 301 (FTD, Confirmatory) Frontline advanced melanoma Lifileucel + pembrolizumab Registration - Directed IOV - LUN - 202: Cohorts 1&2 Post - chemo & anti - PD - 1 advanced NSCLC Lifileucel IOV - MEL - 202 Post anti - PD - 1 advanced melanoma Lifileucel Lifileucel Pipeline IOV - END - 201: Cohorts 1&2 Post - chemo & anti - PD - 1 endometrial cancer Lifileucel IOV - COM - 202: Cohorts 3A - 3E* 1 - 4L ICI - naïve & post - anti - PD - 1 advanced NSCLC Lifileucel; + ICI; +ICI & chemo IOV - COM - 202: Cohorts 1A, 1D* ICI - naïve advanced melanoma Lifileucel + ICI IOV - GM1 - 201: Cohort 1 Post anti - PD - 1 advanced melanoma PD - 1 Inactivated TIL (IOV - 4001) Next - Generation Products IOV - GM1 - 201: Cohort 2 2 - 4L incl. post - anti - PD - 1 advanced NSCLC PD - 1 Inactivated TIL (IOV - 4001) IOV - IL2 - 101 TIL treatment regimen IL - 2 analog (IOV - 3001) Planned Basket trial IL - 12 tethered TIL (IOV - 5001)
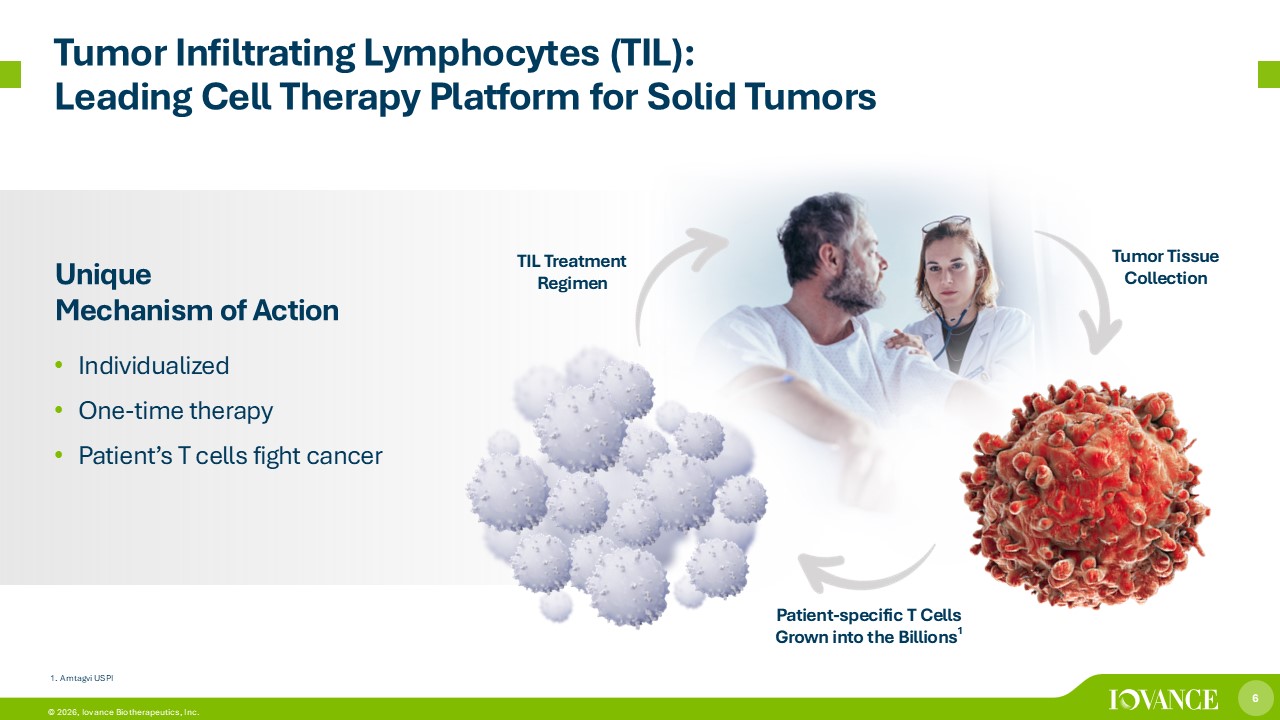
6 © 2026, Iovance Biotherapeutics, Inc. Tumor Infiltrating Lymphocytes (TIL): Leading Cell Therapy Platform for Solid Tumors Unique Mechanism of Action • Individualized • One - time therapy • Patient’s T cells fight cancer Tumor Tissue Collection Patient - specific T Cells Grown into the Billions 1 TIL Treatment Regimen 1. Amtagvi USPI
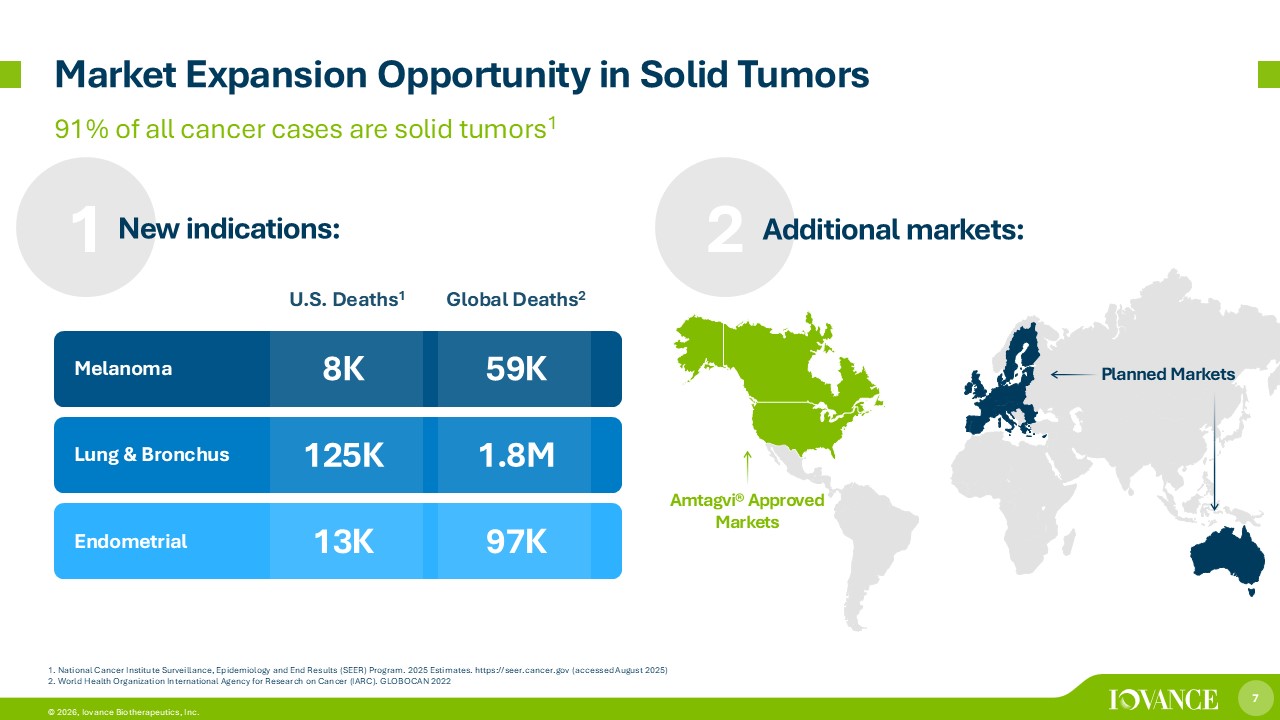
7 © 2026, Iovance Biotherapeutics, Inc. Global Deaths 2 U.S. Deaths 1 59K 8K Melanoma 1.8M 125K Lung & Bronchus 97K 13K Endometrial 1. National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program. 2025 Estimates. https://seer.cancer.g ov (accessed August 2025) 2. World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2022 91% of all cancer cases are solid tumors 1 Market Expansion Opportunity in Solid Tumors 1 New indications: 2 Additional markets: Amtagvi® Approved Markets Planned Markets

8 © 2026, Iovance Biotherapeutics, Inc. 8 First FDA - approved One - time, Individualized T cell Therapy for a Solid Tumor Cancer
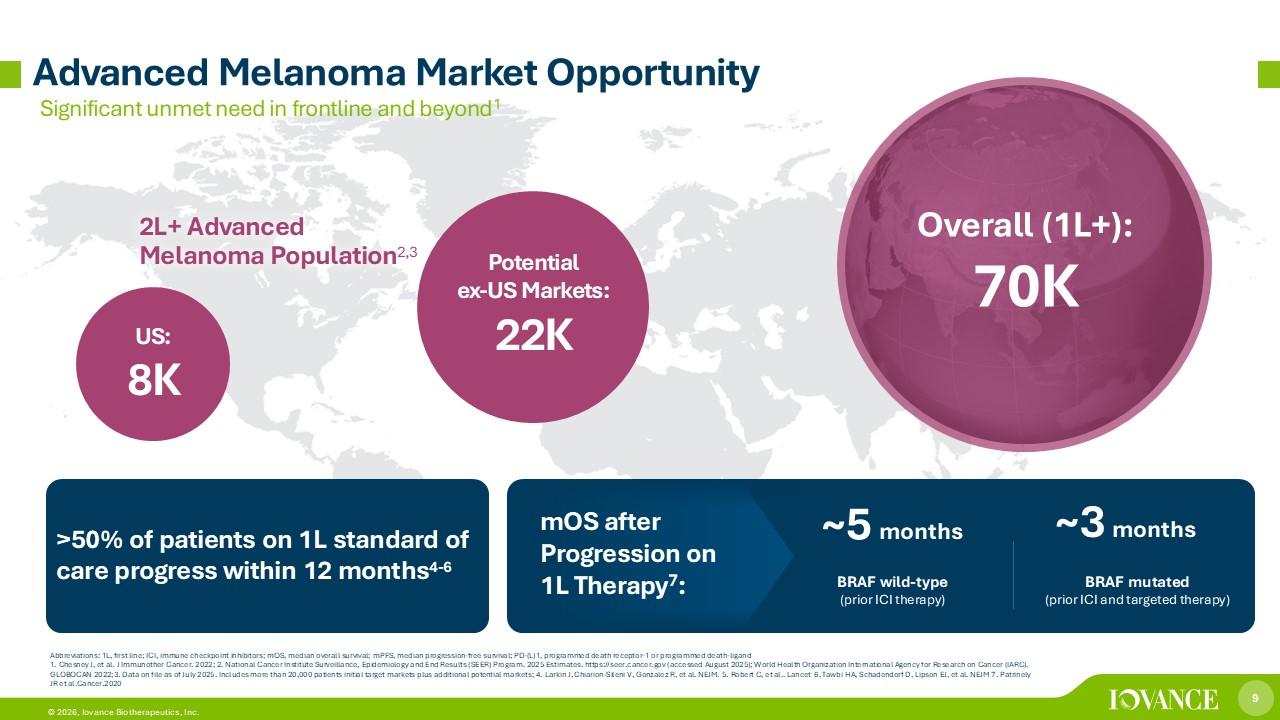
9 © 2026, Iovance Biotherapeutics, Inc. Significant unmet need in frontline and beyond 1 Advanced Melanoma Market Opportunity 2L+ Advanced Melanoma Population 2,3 US: 8K Potential ex - US Markets: 22 K Overall (1L+): 7 0K BRAF wild - type (prior ICI therapy ) ~ 5 months BRAF mutated (prior ICI and targeted therapy) ~ 3 months Abbreviations: 1L , first line; ICI, immune checkpoint inhibitors; mOS , median overall survival; mPFS , median progression - free survival; PD - (L)1, programmed death receptor - 1 or programmed death - ligand 1. Chesney J, et al. J Immunother Cancer. 2022; 2. National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program. 2025 Estimates. https:/ /s eer.cancer.gov (accessed August 2025); World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2022;3. Data on file as of July 2025. Includes more than 20,000 patients initial target markets plus additional pote nti al markets; 4. Larkin J, Chiarion - Sileni V, Gonzalez R, et al. NEJM. 5. Robert C, et al.. Lancet 6. Tawbi HA, Schadendorf D, Lipson EJ, et al. NEJM 7. Patrinely JR et al.Cancer.2020 >50% of patients on 1L standard of care progress within 12 months 4 - 6 mOS after Progression on 1L Therapy 7 :
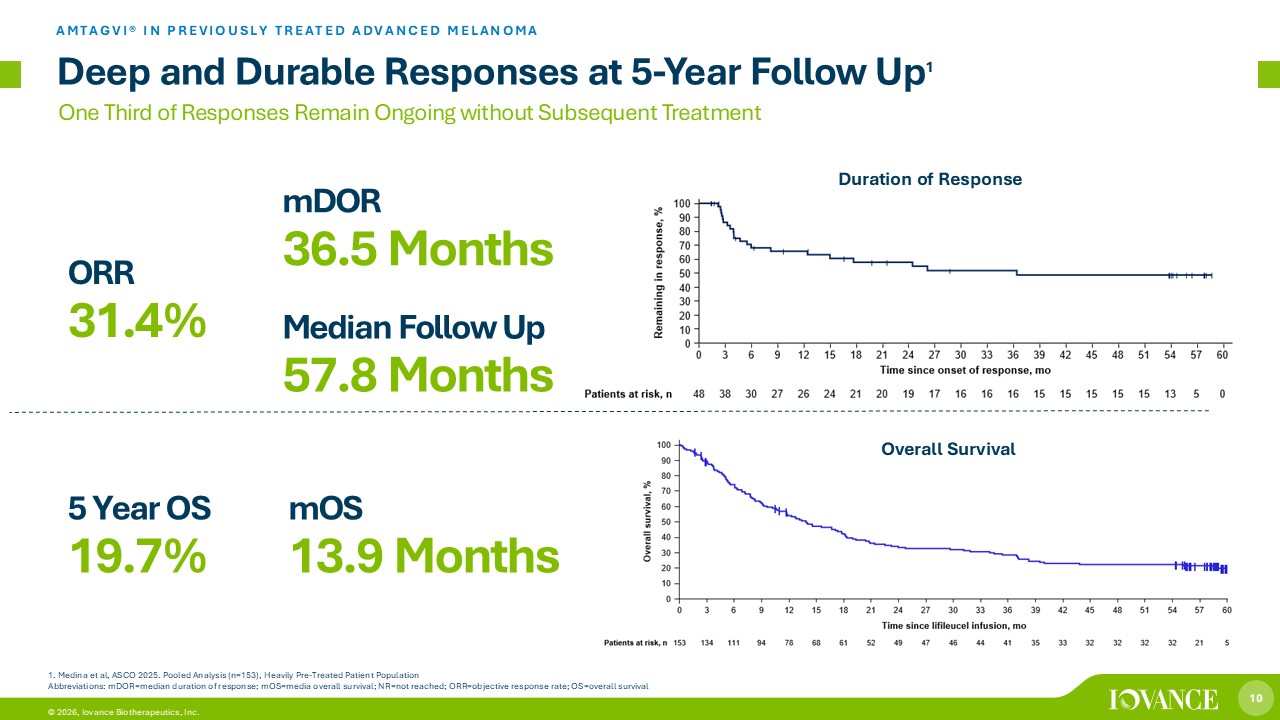
10 © 2026, Iovance Biotherapeutics, Inc. One Third of Responses Remain Ongoing without Subsequent Treatment 5 Year OS 19.7% mDOR 36.5 Months ORR 31.4% mOS 13.9 Months 1. Medina et al, ASCO 2025. Pooled Analysis (n=153), Heavily Pre - Treated Patient Population Abbreviations: mDOR=median duration of response; mOS =media overall survival; NR=not reached; ORR=objective response rate; OS=overall survival Duration of Response Overall Survival Deep and Durable Responses at 5 - Year Follow Up 1 AMTAGVI® IN PREVIOUSLY TREATED ADVANCED MELANOMA Median Follow Up 57.8 Months
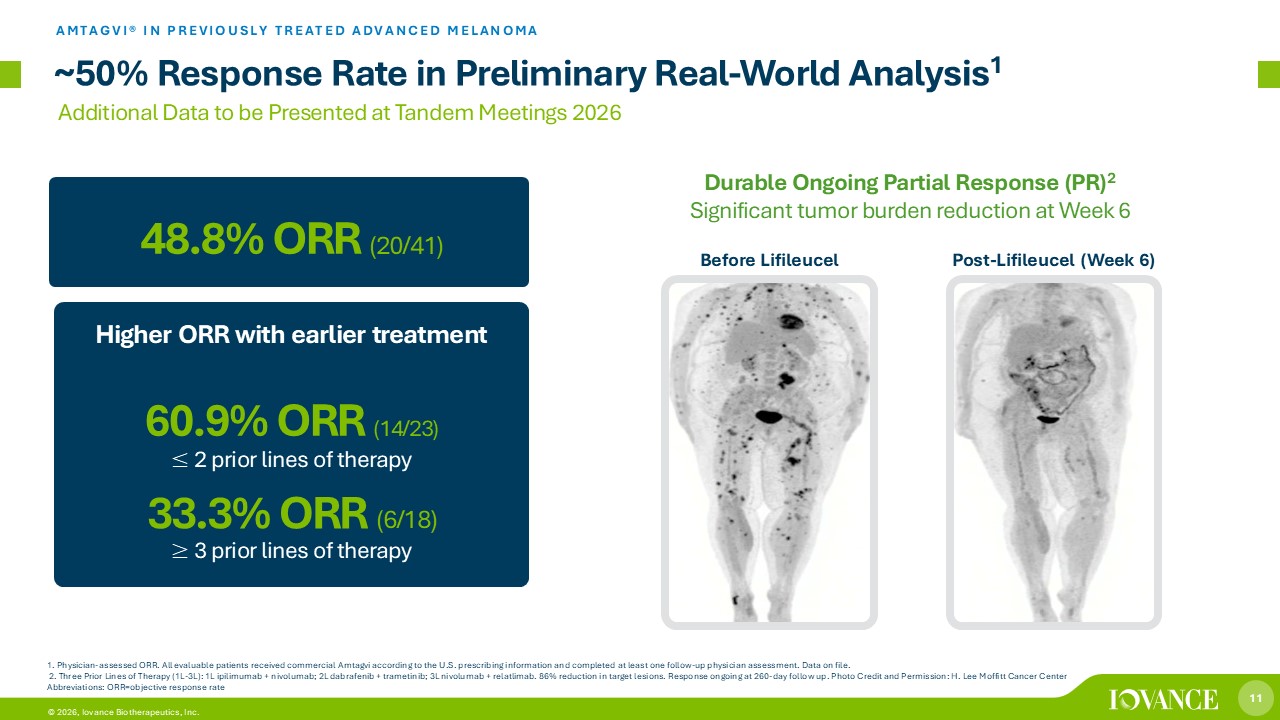
11 © 2026, Iovance Biotherapeutics, Inc. Higher ORR with earlier treatment 60.9% ORR (14/23) ~50% Response Rate in Preliminary Real - World Analysis 1 AMTAGVI® IN PREVIOUSLY TREATED ADVANCED MELANOMA 1. Physician - assessed ORR. All evaluable patients received commercial Amtagvi according to the U.S. prescribing information and completed at least one follow - up physician assessment. Data on file. 2. Three Prior Lines of Therapy (1L - 3L): 1L ipilimumab + nivolumab; 2L dabrafenib + trametinib; 3L nivolumab + relatlimab . 86% reduction in target lesions. Response ongoing at 260 - day follow up. Photo Credit and Permission: H. Lee Moffitt Cancer Cen ter Abbreviations: ORR=objective response rate 48.8 % ORR (20/41) 33.3% ORR (6/18) Before Lifileucel Post - Lifileucel (Week 6) Durable Ongoing Partial Response (PR) 2 Significant tumor burden reduction at Week 6 ≤ 2 prior lines of therapy ≥ 3 prior lines of therapy Additional Data to be Presented at Tandem Meetings 2026
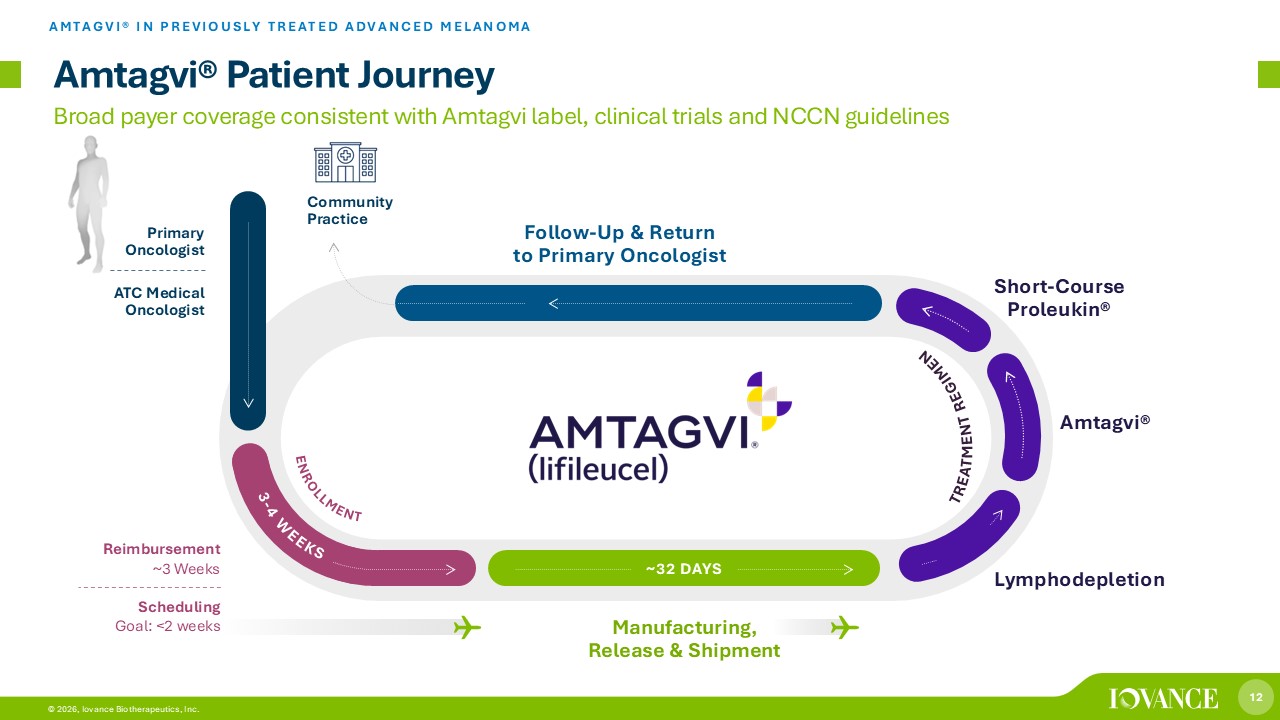
12 © 2026, Iovance Biotherapeutics, Inc. 12 © 2026, Iovance Biotherapeutics, Inc. Lymphodepletion Reimbursement ~3 Weeks Manufacturing, Release & Shipment Short - Course Proleukin® Scheduling Goal: <2 weeks Primary Oncologist ATC Medical Oncologist Follow - Up & Return to Primary Oncologist ~ 32 DAYS Community Practice Amtagvi® Patient Journey AMTAGVI® IN PREVIOUSLY TREATED ADVANCED MELANOMA Amtagvi® Broad payer coverage consistent with Amtagvi label, clinical trials and NCCN guidelines

13 © 2026, Iovance Biotherapeutics, Inc. • Modular design • Global supply and logistics • C apacity for up to 5K patients/year • Optimal utilization , quality & COS Manufacturing Facility Dedicated to Commercial and Clinical TIL Cell Therapies COS = cost of sales Philadelphia, PA
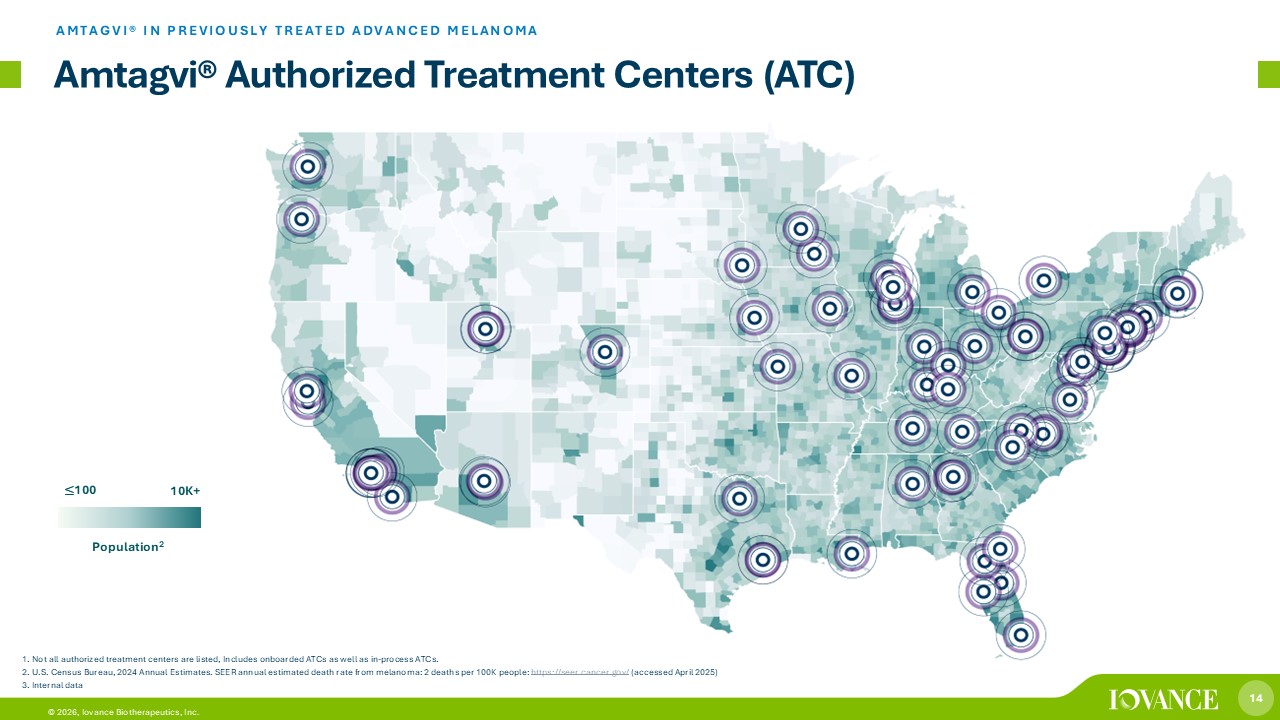
14 © 2026, Iovance Biotherapeutics, Inc. Amtagvi ® Authorized Treatment Centers (ATC) AMTAGVI® IN PREVIOUSLY TREATED ADVANCED MELANOMA 10K+ ≤ 100 Population 2 1. Not all authorized treatment centers are listed, Includes onboarded ATCs as well as in - process ATCs. 2. U.S. Census Bureau, 2024 Annual Estimates. SEER annual estimated death rate from melanoma: 2 deaths per 100K people: https://seer.cancer.gov/ (accessed April 2025) 3. Internal data
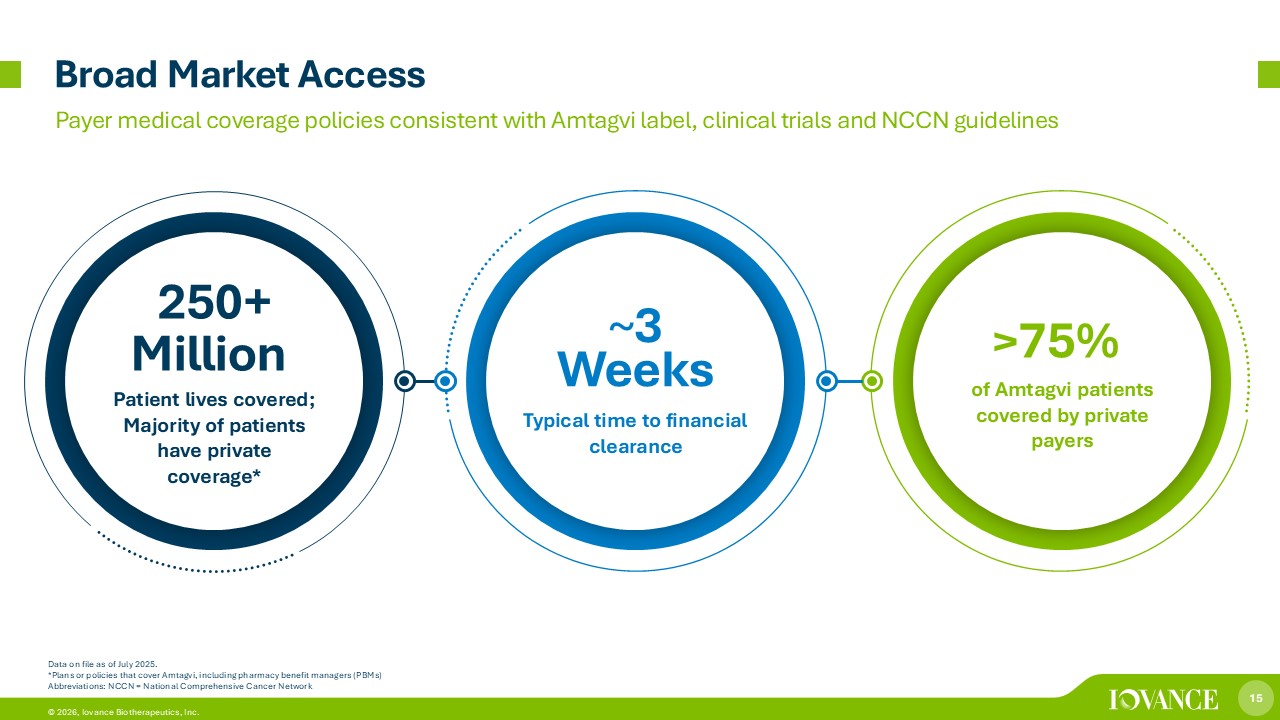
15 © 2026, Iovance Biotherapeutics, Inc. Broad Market Access Data on file as of July 2025. *Plans or policies that cover Amtagvi, including pharmacy benefit managers (PBMs) Abbreviations: NCCN = National Comprehensive Cancer Network Payer medical coverage policies consistent with Amtagvi label, clinical trials and NCCN guidelines Patient lives covered; Majority of patients have private coverage* Typical time to financial clearance 250+ Million ~ 3 Weeks > 75% of Amtagvi patients covered by private payers

16 © 2026, Iovance Biotherapeutics, Inc. 16 Amtagvi® Expansion Plans in Advanced Melanoma
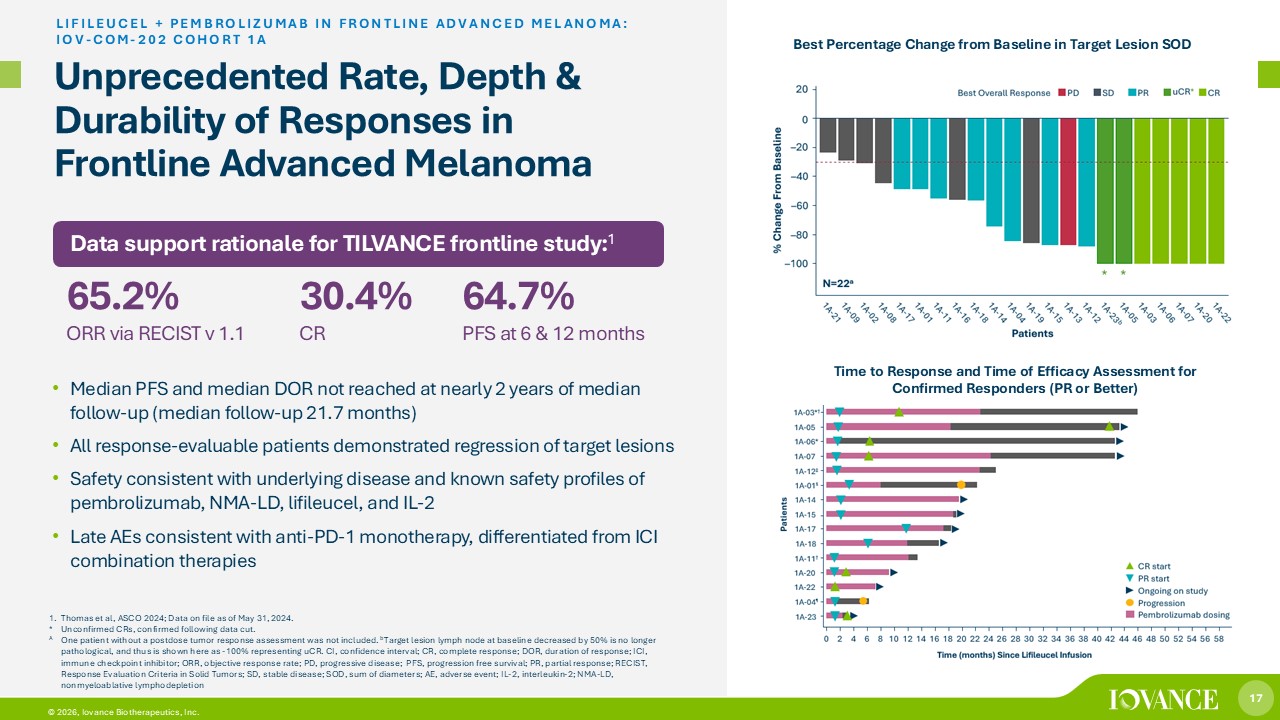
17 © 2026, Iovance Biotherapeutics, Inc. Unprecedented Rate, Depth & Durability of Responses in Frontline Advanced Melanoma • Median PFS and median DOR not reached at nearly 2 years of median follow - up (median follow - up 21.7 months) • All response - evaluable patients demonstrated regression of target lesions • Safety consistent with underlying disease and known safety profiles of pembrolizumab, NMA - LD, lifileucel, and IL - 2 • Late AEs consistent with anti - PD - 1 monotherapy, differentiated from ICI combination therapies LIFILEUCEL + PEMBROLIZUMAB IN FRONTLINE ADVANCED MELANOMA: IOV - COM - 202 COHORT 1A 1. Thomas et al, ASCO 2024; Data on file as of May 31, 2024. * Unconfirmed CRs, confirmed following data cut. A One patient without a postdose tumor response assessment was not included. b Target lesion lymph node at baseline decreased by 50% is no longer pathological, and thus is shown here as - 100% representing uCR. CI, confidence interval; CR, complete response; DOR, duration of response; ICI, immune checkpoint inhibitor; ORR, objective response rate; PD, progressive disease; PFS, progression free survival; PR, part ial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SOD, sum of diameters; AE, adverse event; IL - 2, interleukin - 2; NMA - LD, nonmyeloablative lymphodepletion Data support rationale for TILVANCE frontline study: 1 65.2% ORR via RECIST v 1.1 30.4% CR 64.7% PFS at 6 & 12 months Best Percentage Change from Baseline in Target Lesion SOD Time to Response and Time of Efficacy Assessment for Confirmed Responders (PR or Better)
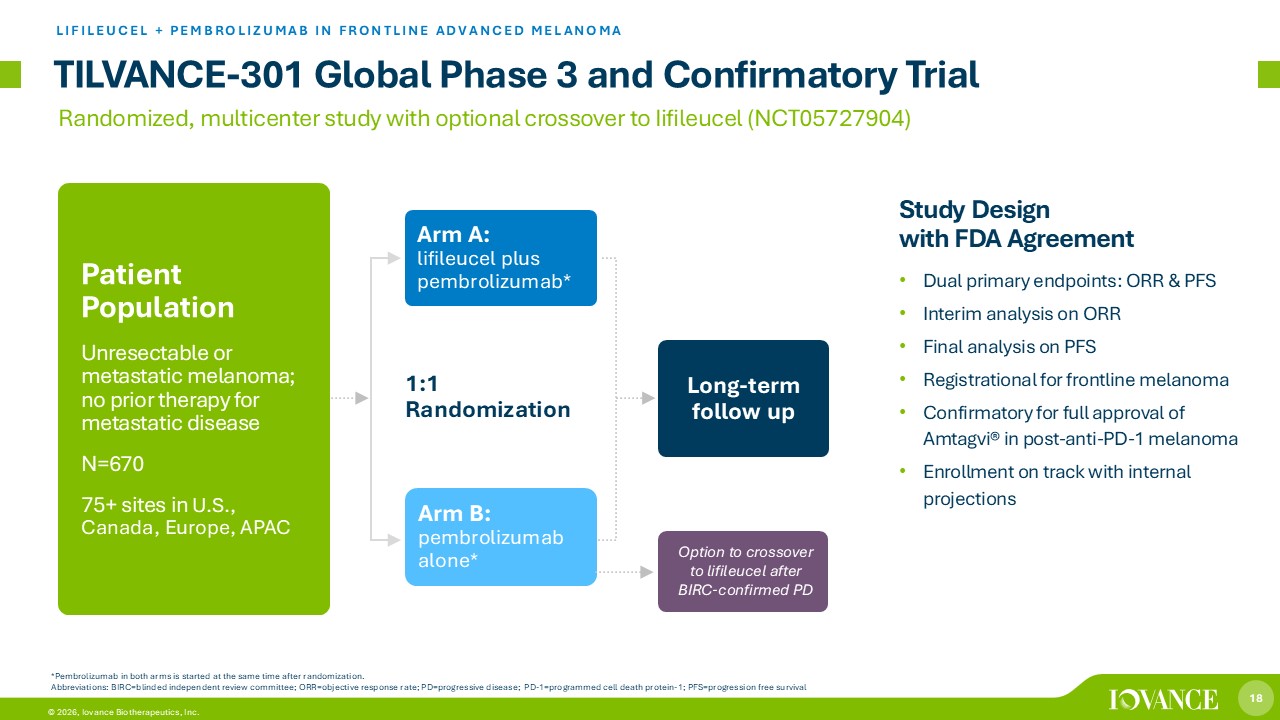
18 © 2026, Iovance Biotherapeutics, Inc. Option to crossover to lifileucel after BIRC - confirmed PD 1:1 Randomization TILVANCE - 301 Global Phase 3 and Confirmatory Trial LIFILEUCEL + PEMBROLIZUMAB IN FRONTLINE ADVANCED MELANOMA *Pembrolizumab in both arms is started at the same time after randomization. Abbreviations: BIRC=blinded independent review committee; ORR=objective response rate; PD=progressive disease; PD - 1=programmed cell death protein - 1; PFS=progression free survival Arm A: lifileucel plus pembrolizumab* Long - term follow up Patient Population Unresectable or metastatic melanoma; no prior therapy for metastatic disease N=670 75+ sites in U.S., Canada, Europe, APAC Arm B: pembrolizumab alone* Study Design with FDA Agreement • Dual primary endpoints: ORR & PFS • Interim analysis on ORR • Final analysis on PFS • Registrational for frontline melanoma • Confirmatory for full approval of Amtagvi ® in post - anti - PD - 1 melanoma • Enrollment on track with internal projections Randomized, multicenter study with optional crossover to Iifileucel (NCT05727904)

19 © 2026, Iovance Biotherapeutics, Inc. 19 TIL Therapy Pipeline
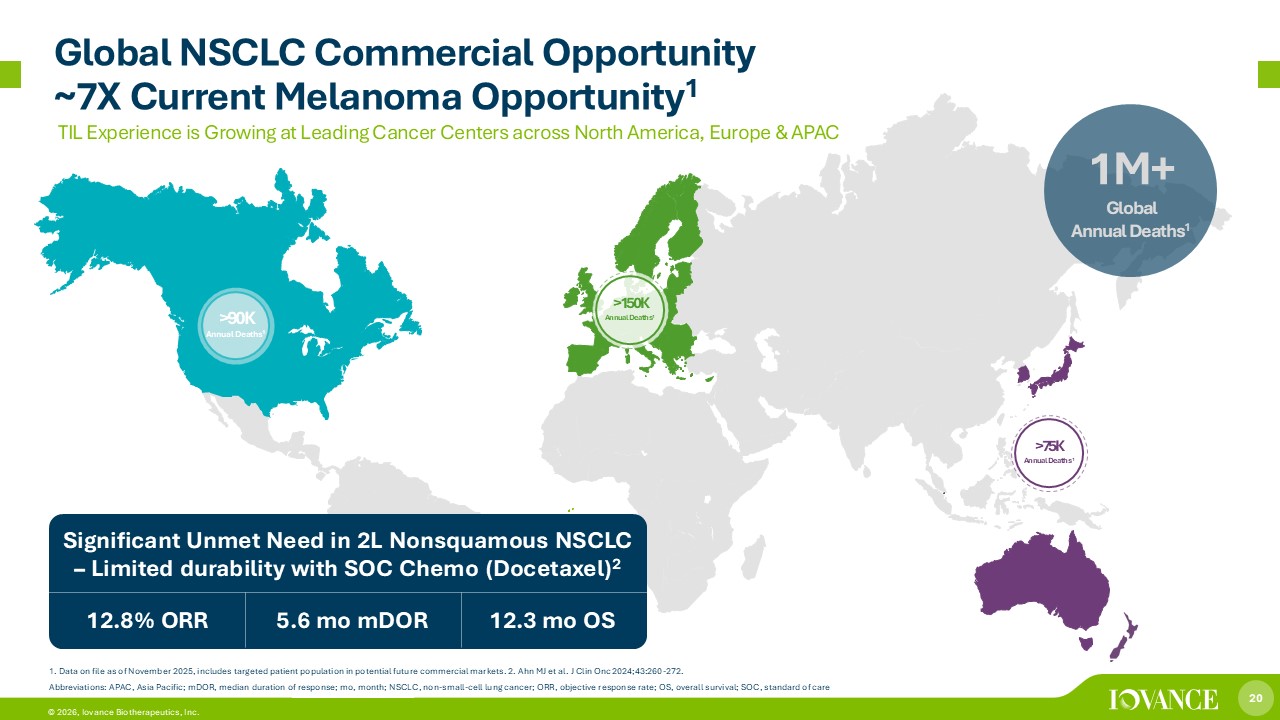
20 © 2026, Iovance Biotherapeutics, Inc. 1M+ Global Annual Deaths 1 Significant Unmet Need in 2L Nonsquamous NSCLC – Limited durability with SOC Chemo (Docetaxel) 2 5.6 mo mDOR 12.3 mo OS 12.8% ORR >90K Annual Deaths 1 1. Data on file as of November 2025, includes targeted patient population in potential future commercial markets. 2. Ahn MJ et al. J Clin Onc 2024;43:260 - 272. Abbreviations: APAC, Asia Pacific; mDOR, median duration of response; mo , month; NSCLC, non - small - cell lung cancer; ORR, objective response rate; OS, overall survival; SOC, standard of care >150K Annual Deaths 1 >75K Annual Deaths 1 TIL Experience is Growing at Leading Cancer Centers across North America, Europe & APAC Global NSCLC Commercial Opportunity ~7X Current Melanoma Opportunity 1
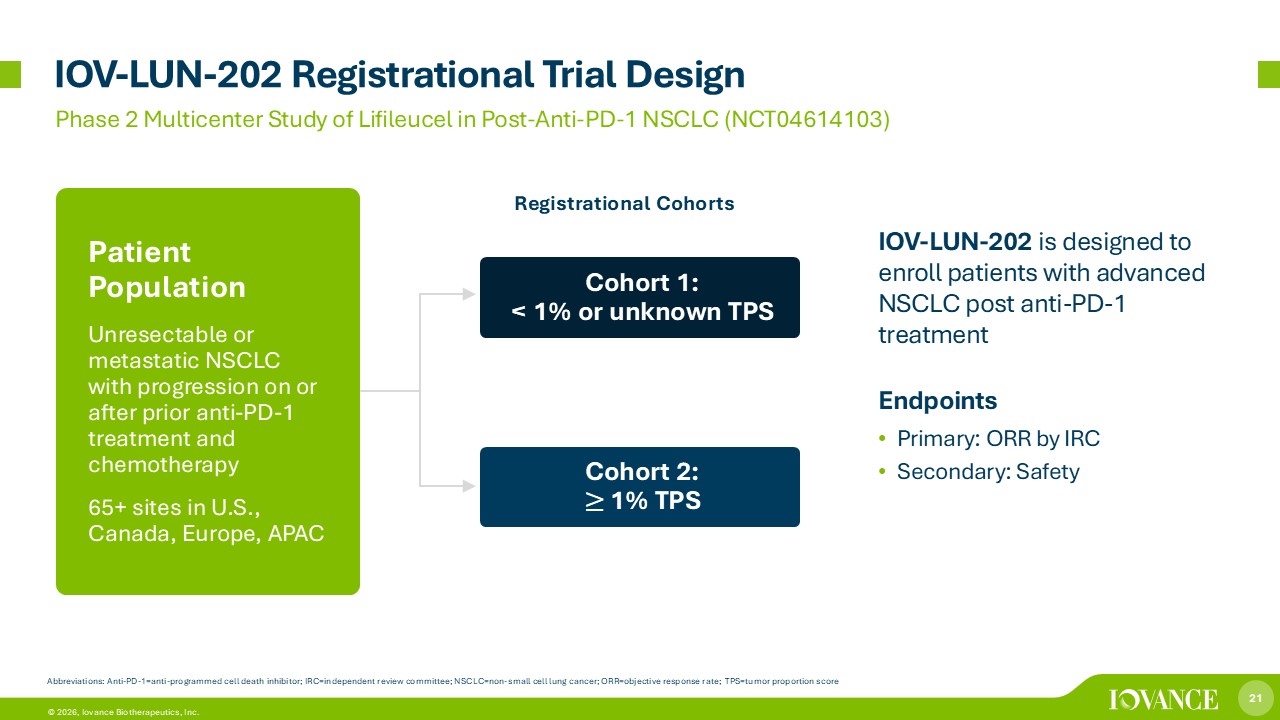
21 © 2026, Iovance Biotherapeutics, Inc. IOV - LUN - 202 Registrational Trial Design Phase 2 Multicenter Study of Lifileucel in Post - Anti - PD - 1 NSCLC (NCT04614103) Abbreviations: Anti - PD - 1 = anti - programmed cell death inhibitor; IRC=independent review committee; NSCLC=non - small cell lung cancer; ORR = objective response rate; TPS=tumor proportion score Iovance TIL Therapy Lifileucel in NSCLC IOV - LUN - 202 is designed to enroll patients with advanced NSCLC post anti - PD - 1 treatment Endpoints • Primary: ORR by IRC • Secondary: Safety Patient Population Unresectable or metastatic NSCLC with progression on or after prior anti - PD - 1 treatment and chemotherapy 65+ sites in U.S., Canada, Europe, APAC Cohort 1: < 1% or unknown TPS Cohort 2: ≥ 1% TPS Registrational Cohorts
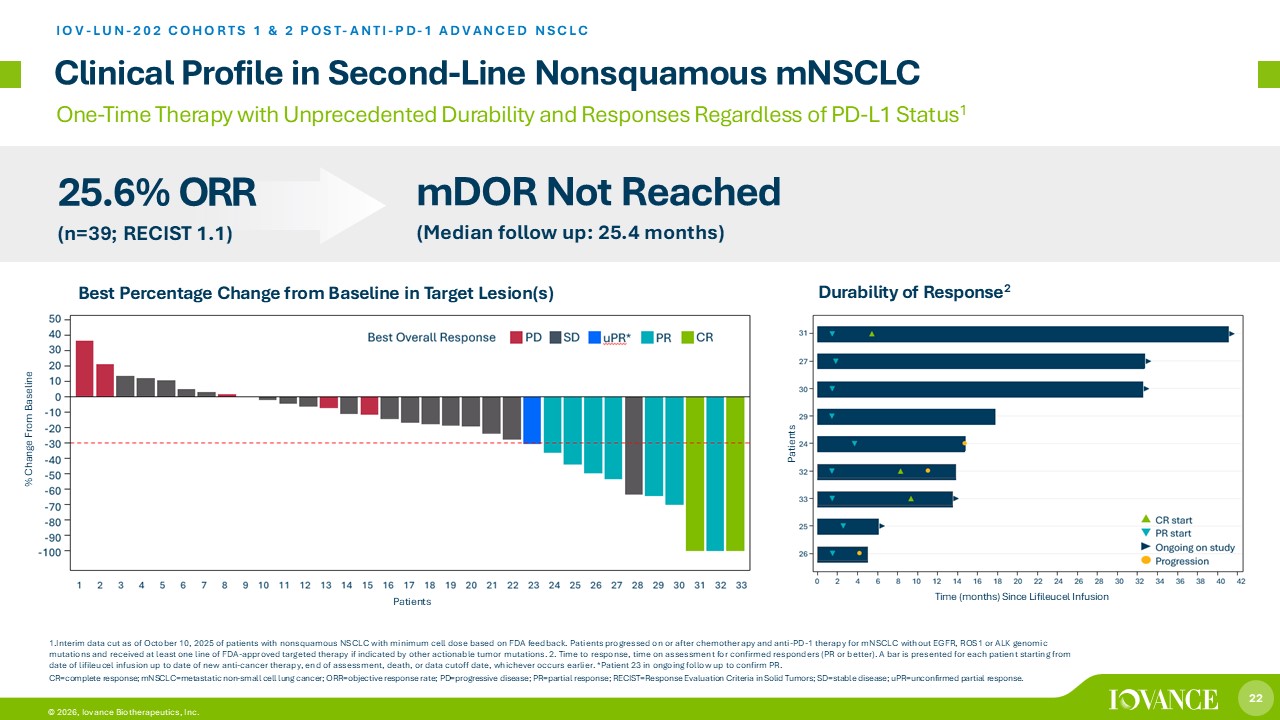
22 © 2026, Iovance Biotherapeutics, Inc. Clinical Profile in Second - Line Nonsquamous mNSCLC IOV - LUN - 202 COHORTS 1 & 2 POST - ANTI - PD - 1 ADVANCED NSCLC 1. Interim data cut as of October 10, 2025 of patients with nonsquamous NSCLC with minimum cell dose based on FDA feedback. Pati ent s progressed on or after chemotherapy and anti - PD - 1 therapy for mNSCLC without EGFR, ROS1 or ALK genomic mutations and received at least one line of FDA - approved targeted therapy if indicated by other actionable tumor mutations. 2. Time to response, time on assessment for confirmed responders (PR or better). A bar is presented for each patient starting f rom date of lifileucel infusion up to date of new anti - cancer therapy, end of assessment, death, or data cutoff date, whichever occu rs earlier. *Patient 23 in ongoing follow up to confirm PR. CR=complete response; mNSCLC =metastatic non - small cell lung cancer; ORR=objective response rate; PD=progressive disease; PR=partial response; RECIST=Respons e Evaluation Criteria in Solid Tumors; SD=stable disease; uPR =unconfirmed partial response. One - Time Therapy with Unprecedented Durability and Responses Regardless of PD - L1 Status 1 25.6% ORR (n=39; RECIST 1.1) mDOR Not Reached (Median follow up: 25.4 months) Patients Time (months) Since Lifileucel Infusion % Change From Baseline Durability of Response 2 Patients Best Percentage Change from Baseline in Target Lesion(s)
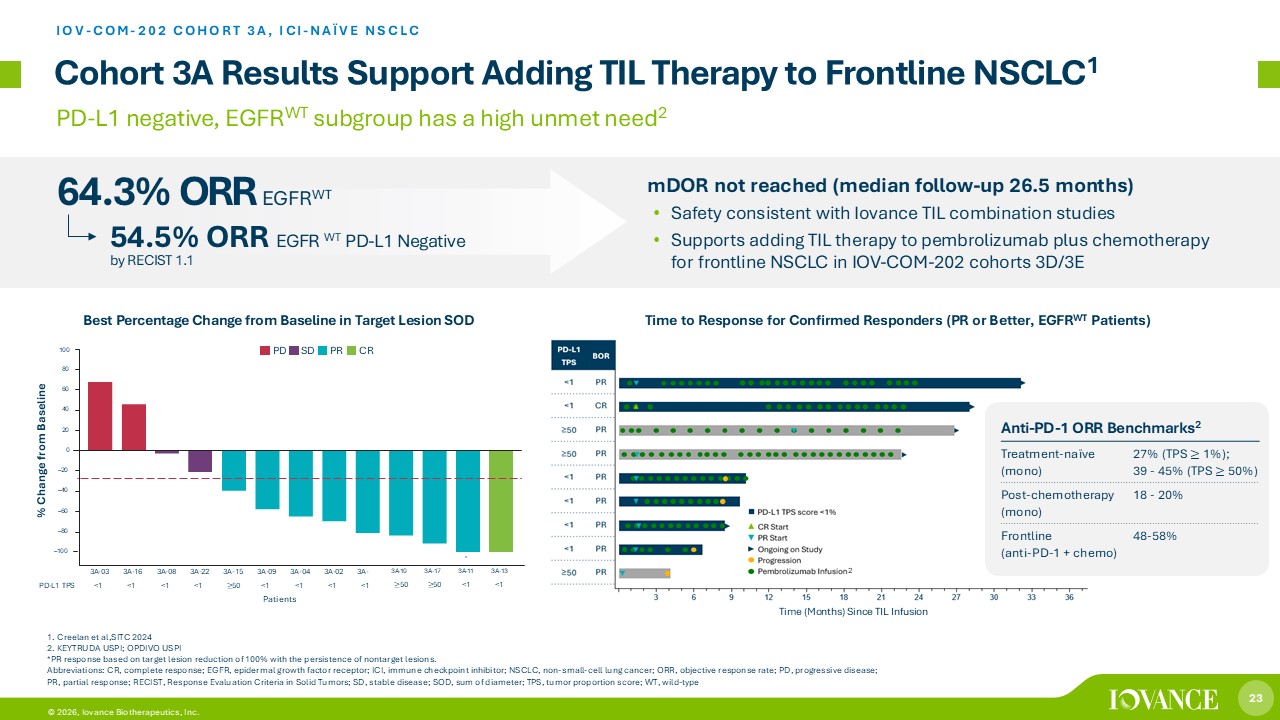
23 © 2026, Iovance Biotherapeutics, Inc. Cohort 3A Results Support Adding TIL Therapy to Frontline NSCLC 1 IOV - COM - 202 COHORT 3A, ICI - NAÏVE NSCLC % Change from Baseline Time (Months) Since TIL Infusion PD - L1 negative, EGFR WT subgroup has a high unmet need 2 Best Percentage Change from Baseline in Target Lesion SOD 64.3% ORR EGFR WT Time to Response for Confirmed Responders (PR or Better, EGFR WT Patients) mDOR not reached (median follow - up 26.5 months) • Safety consistent with Iovance TIL combination studies • Supports adding TIL therapy to pembrolizumab plus chemotherapy for frontline NSCLC in IOV - COM - 202 cohorts 3D/3E 1. Creelan et al,SITC 2024 2 . KEYTRUDA USPI; OPDIVO USPI *PR response based on target lesion reduction of 100% with the persistence of nontarget lesions. Abbreviations: CR, complete response; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; NSCLC, non - small - cell lung cancer; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SOD, sum of diameter; TPS, tumor proportion score; WT, wild - type 54.5% ORR EGFR WT PD - L1 Negative by RECIST 1.1 Anti - PD - 1 ORR Benchmarks 2 27% (TPS ≥ 1%); 39 - 45% (TPS ≥ 50%) Treatment - naïve (mono) 18 - 20% Post - chemotherapy (mono) 48 - 58% Frontline (anti - PD - 1 + chemo) 2 Patients 60 40 20 0 – 20 – 40 – 60 – 80 – 100 a 3A - 03 3A - 16 3A - 08 3A - 22 3A - 15 3A - 09 3A - 04 3A - 02 3A - PD - L1 TPS <1 <1 <1 <1 ≥ 50 <1 <1 <1 <1 3A - 10 ≥ 50 3A - 17 ≥ 50 3A - 11 <1 3A - 13 <1 8 0 10 0 PD SD PR CR
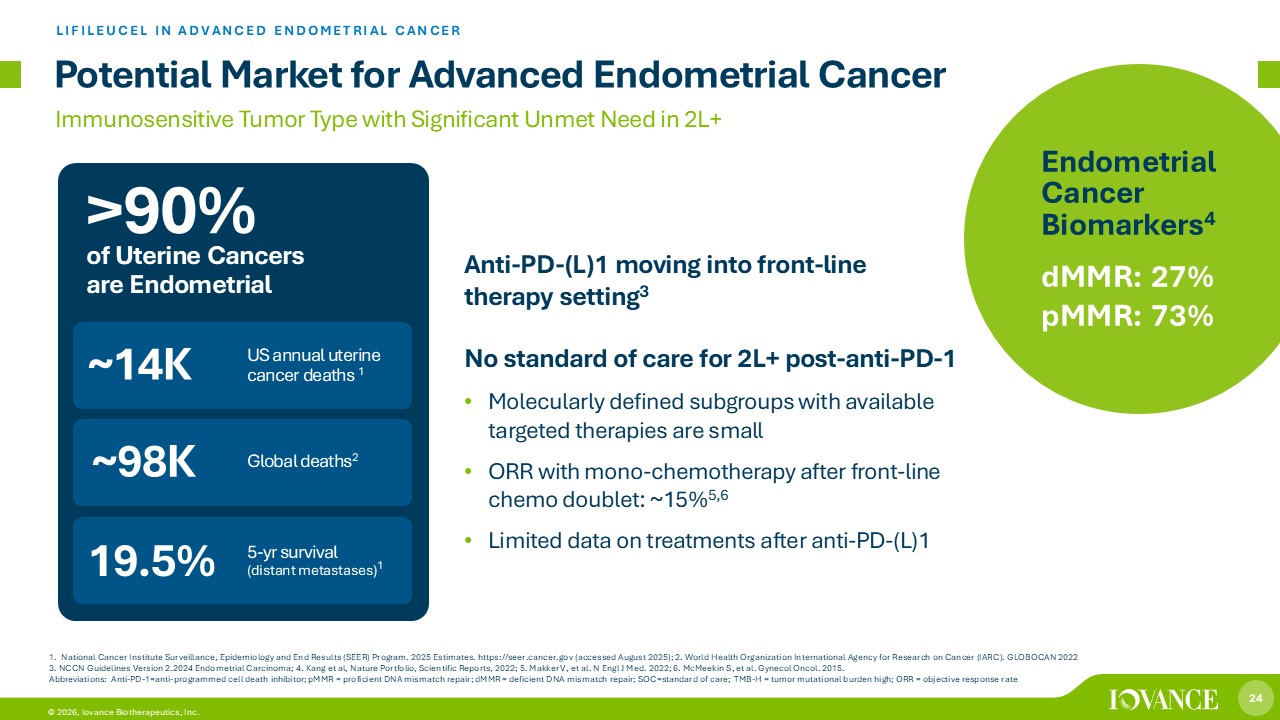
24 © 2026, Iovance Biotherapeutics, Inc. 1. National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program. 2025 Estimates. https://seer.cancer.gov (accessed August 2025); 2. World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2022 3. NCCN Guidelines Version 2.2024 Endometrial Carcinoma; 4. Kang et al, Nature Portfolio, Scientific Reports, 2022; 5. Makker V, et al. N Engl J Med. 2022; 6. McMeekin S, et al. Gynecol Oncol. 2015. Abbreviations: Anti - PD - 1 = anti - programmed cell death inhibitor; pMMR = proficient DNA mismatch repair; dMMR = deficient DNA mismatch repair; SOC=standard of care; TMB - H = tumor mutational burden high; ORR = objective response rate Potential Market for Advanced Endometrial Cancer LIFILEUCEL IN ADVANCED ENDOMETRIAL CANCER Immunosensitive Tumor Type with Significant Unmet Need in 2L+ Anti - PD - (L)1 moving into front - line therapy setting 3 No standard of care for 2L+ post - anti - PD - 1 • Molecularly defined subgroups with available targeted therapies are small • ORR with mono - chemotherapy after front - line chemo doublet: ~15% 5,6 • Limited data on treatments after anti - PD - (L)1 ~14K ~98K US annual uterine cancer deaths 1 5 - yr survival ( distant metastases ) 1 Global deaths 2 19.5% Endometrial Cancer Biomarkers 4 dMMR : 27% pMMR : 73% >90% of Uterine Cancers are Endometrial
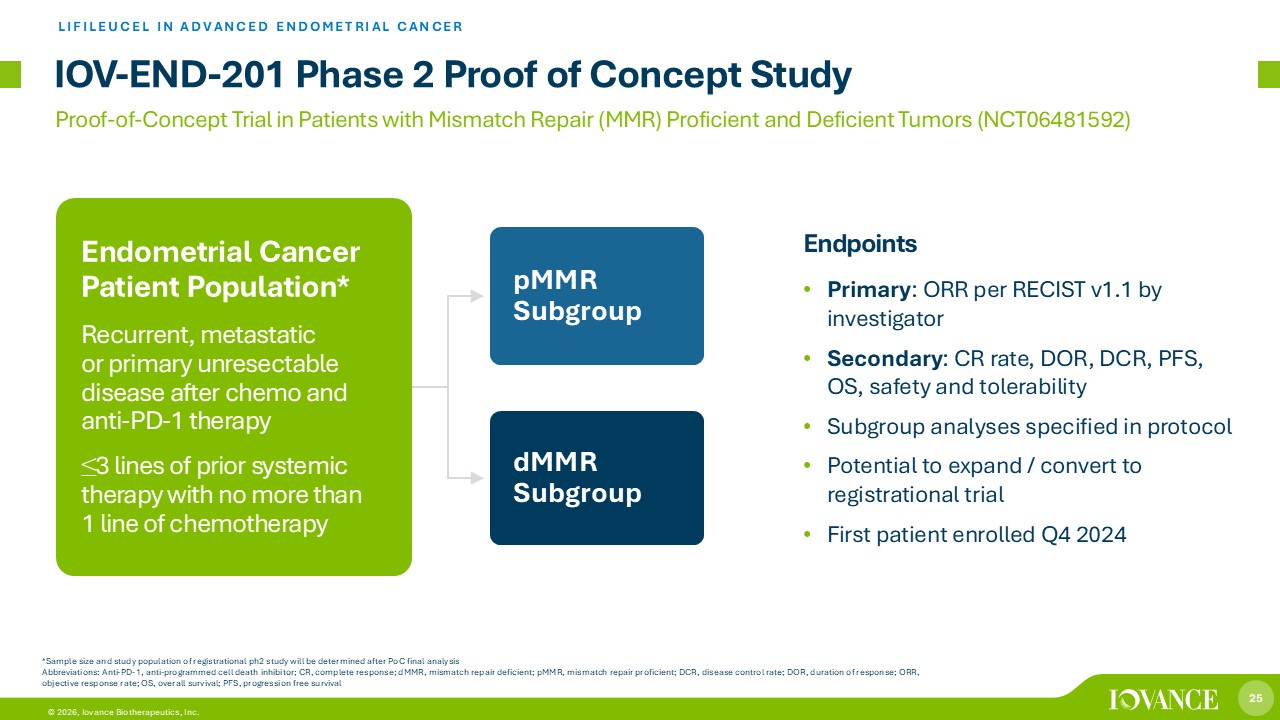
25 © 2026, Iovance Biotherapeutics, Inc. pMMR Subgroup dMMR Subgroup Endpoints • Primary : ORR per RECIST v1.1 by investigator • Secondary : CR rate , DOR, DCR, PFS, OS, safety and tolerability • Subgroup analyses specified in protocol • Potential to expand / convert to registrational trial • First patient enrolled Q4 2024 IOV - END - 201 Phase 2 Proof of Concept Study Endometrial Cancer Patient Population * Recurrent, metastatic or primary unresectable disease after chemo and anti - PD - 1 therapy ≤ 3 lines of prior systemic therapy with no more than 1 line of chemotherapy *Sample size and study population of registrational ph2 study will be determined after PoC final analysis Abbreviations: Anti - PD - 1, anti - programmed cell death inhibitor; CR, complete response; dMMR, mismatch repair deficient; pMMR, mi smatch repair proficient; DCR, disease control rate; DOR, duration of response; ORR, objective response rate; OS, overall survival; PFS, progression free survival LIFILEUCEL IN ADVANCED ENDOMETRIAL CANCER Proof - of - Concept Trial in Patients with Mismatch Repair (MMR) Proficient and Deficient Tumors (NCT06481592)
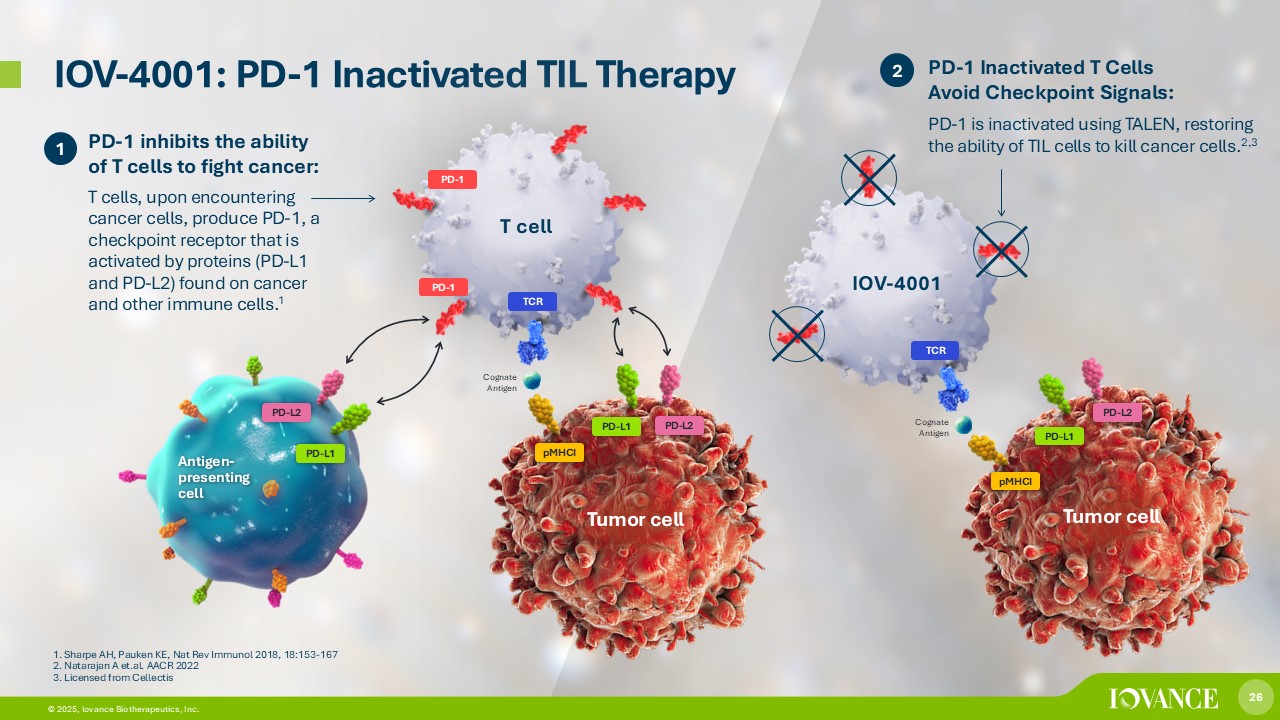
26 © 2026, Iovance Biotherapeutics, Inc. 26 © 2025, Iovance Biotherapeutics, Inc. IOV - 4001: PD - 1 Inactivated TIL Therapy 2 T cell PD - 1 PD - 1 inhibits the ability of T cells to fight cancer: T cells, upon encountering cancer cells, produce PD - 1, a checkpoint receptor that is activated by proteins (PD - L1 and PD - L2) found on cancer and other immune cells. 1 PD - L2 PD - L1 PD - 1 TCR pMHCI PD - L2 PD - L1 Tumor cell Antigen - presenting cell 1. Sharpe AH, Pauken KE, Nat Rev Immunol 2018, 18:153 - 167 2. Natarajan A et.al. AACR 2022 3. Licensed from Cellectis 1 IOV - 4001 TCR pMHCI PD - L2 PD - L1 Tumor cell PD - 1 Inactivated T Cells Avoid Checkpoint Signals: PD - 1 is inactivated using TALEN, restoring the ability of TIL cells to kill cancer cells. 2,3 Cognate Antigen Cognate Antigen
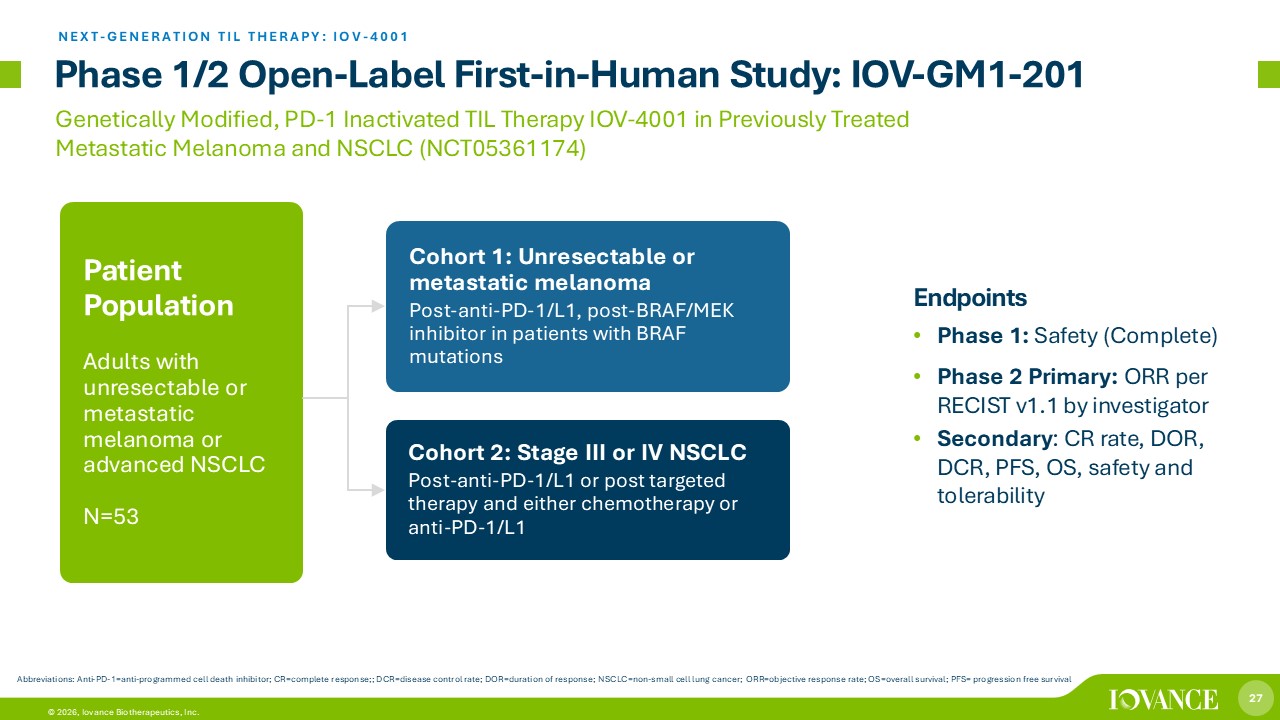
27 © 2026, Iovance Biotherapeutics, Inc. Phase 1/2 Open - Label First - in - Human Study: IOV - GM1 - 201 Endpoints • Phase 1: Safety (Complete) • Phase 2 Primary: ORR per RECIST v1.1 by investigator • Secondary : CR rate , DOR, DCR, PFS, OS, safety and tolerability Genetically Modified, PD - 1 Inactivated TIL Therapy IOV - 4001 in Previously Treated Metastatic Melanoma and NSCLC (NCT05361174) Cohort 1: Unresectable or metastatic melanoma Post - anti - PD - 1/L1, post - BRAF/MEK inhibitor in patients with BRAF mutations Cohort 2: Stage III or IV NSCLC Post - anti - PD - 1/L1 or post targeted therapy and either chemotherapy or anti - PD - 1/L1 Patient Population Adults with unresectable or metastatic melanoma or advanced NSCLC N=53 NEXT - GENERATION TIL THERAPY: IOV - 4001 Abbreviations: Anti - PD - 1 = anti - programmed cell death inhibitor; CR=complete response;; DCR=disease control rate; DOR=duration of response; NSCLC=non - small cell lung cancer; ORR=objective response rate; OS=overall survival; PFS= progression free survival
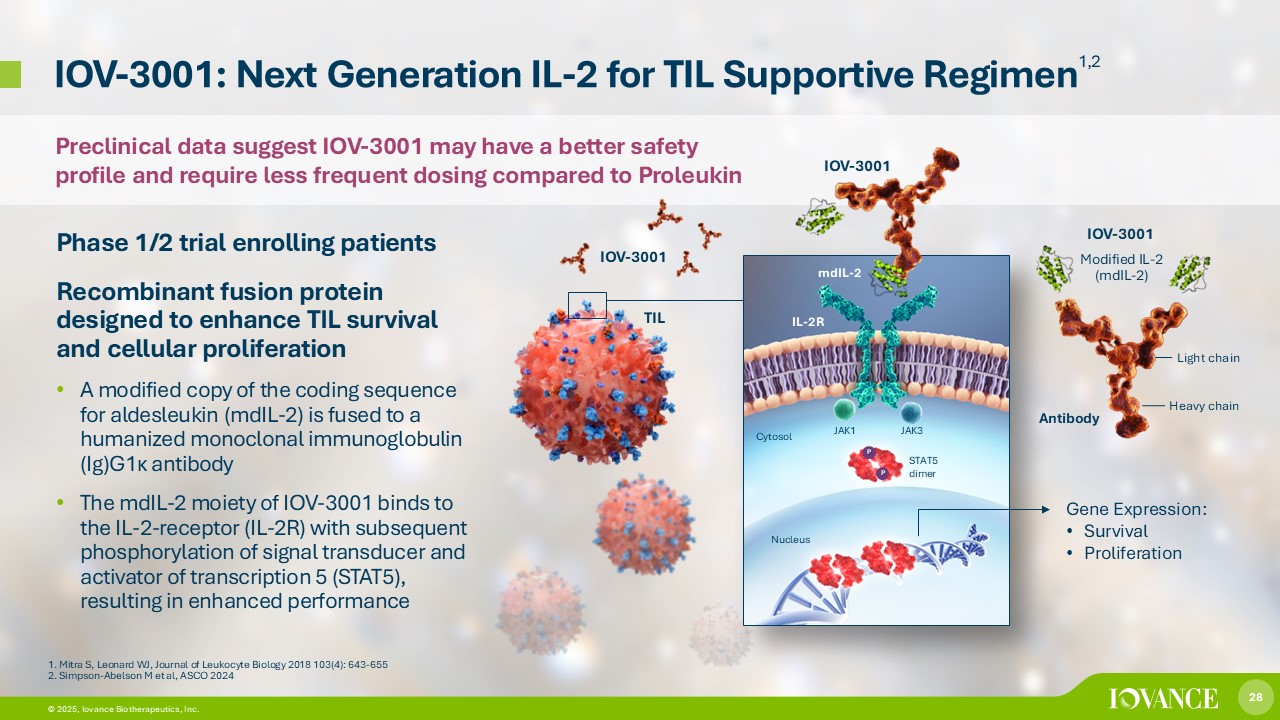
28 © 2026, Iovance Biotherapeutics, Inc. 28 © 2025, Iovance Biotherapeutics, Inc. IOV - 3001: Next Generation IL - 2 for TIL Supportive Regimen 1,2 Phase 1/2 trial enrolling patients Recombinant fusion protein designed to enhance TIL survival and cellular proliferation • A modified copy of the coding sequence for aldesleukin (mdIL - 2) is fused to a humanized monoclonal immunoglobulin (Ig)G1 κ antibody • The mdIL - 2 moiety of IOV - 3001 binds to the IL - 2 - receptor (IL - 2R) with subsequent phosphorylation of signal transducer and activator of transcription 5 (STAT5), resulting in enhanced performance 1. Mitra S, Leonard WJ, Journal of Leukocyte Biology 2018 103(4): 643 - 655 2. Simpson - Abelson M et al, ASCO 2024 Gene Expression: • Survival • Proliferation mdIL - 2 IL - 2R JAK1 P JAK3 STAT5 dimer Cytosol Nucleus P IOV - 3001 IOV - 3001 Heavy chain Light chain IOV - 3001 Modified IL - 2 (mdIL - 2) TIL Antibody Preclinical data suggest IOV - 3001 may have a better safety profile and require less frequent dosing compared to Proleukin
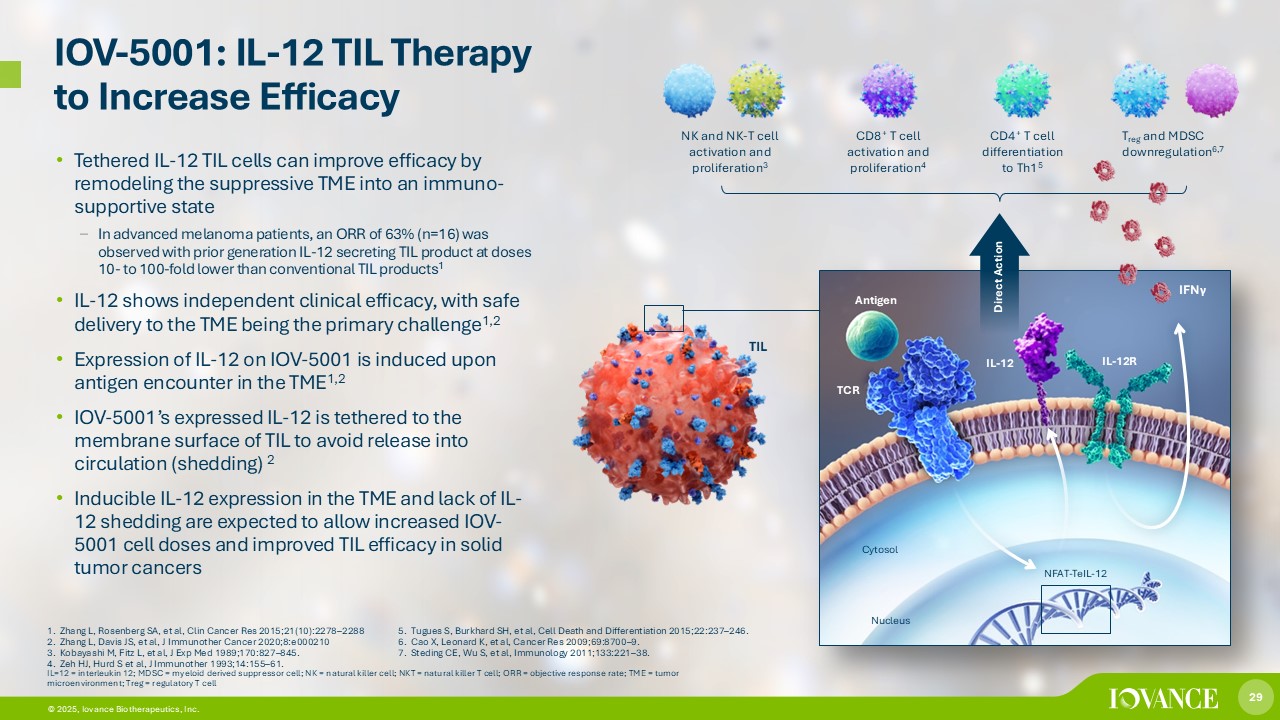
29 © 2026, Iovance Biotherapeutics, Inc. IOV - 5001: IL - 12 TIL Therapy to Increase Efficacy 1. Zhang L, Rosenberg SA, et al, Clin Cancer Res 2015;21(10):2278 – 2288 2. Zhang L, Davis JS, et al, J Immunother Cancer 2020;8:e000210 3. Kobayashi M, Fitz L, et al, J Exp Med 1989;170:827 – 845. 4. Zeh HJ, Hurd S et al, J Immunother 1993;14:155 – 61. 5. Tugues S, Burkhard SH, et al, Cell Death and Differentiation 2015;22:237 – 246. 6. Cao X, Leonard K, et al, Cancer Res 2009;69:8700 – 9. 7. Steding CE, Wu S, et al, Immunology 2011;133:221 – 38. • Tethered IL - 12 TIL cells can improve efficacy by remodeling the suppressive TME into an immuno - supportive state – In advanced melanoma patients, an ORR of 63% (n=16) was observed with prior generation IL - 12 secreting TIL product at doses 10 - to 100 - fold lower than conventional TIL products 1 • IL - 12 shows independent clinical efficacy, with safe delivery to the TME being the primary challenge 1,2 • Expression of IL - 12 on IOV - 5001 is induced upon antigen encounter in the TME 1,2 • IOV - 5001’s expressed IL - 12 is tethered to the membrane surface of TIL to avoid release into circulation (shedding) 2 • Inducible IL - 12 expression in the TME and lack of IL - 12 shedding are expected to allow increased IOV - 5001 cell doses and improved TIL efficacy in solid tumor cancers IL=12 = interleukin 12; MDSC = myeloid derived suppressor cell; NK = natural killer cell; NKT = natural killer T cell; ORR = obj ective response rate; TME = tumor microenvironment; Treg = regulatory T cell NK and NK - T cell activation and proliferation 3 CD8 + T cell activation and proliferation 4 CD4 + T cell differentiation to Th1 5 T reg and MDSC downregulation 6,7 29 Direct Action IFN γ Cytosol Nucleus TIL NFAT - TeIL - 12 IL - 12 IL - 12R TCR Antigen © 2025, Iovance Biotherapeutics, Inc.

30 © 2026, Iovance Biotherapeutics, Inc. 30 Corporate Summary

31 © 2026, Iovance Biotherapeutics, Inc. 1. Includes anticipated revenue from Amtagvi® and Proleukin® and anticipated savings from strategic restructuring announced on Aug ust 7, 2025 2. Preferred shares are shown on an as - converted basis . Financial Position & Outlook Guidance Cash runway into Q2 2027 1 FY 2025 revenue $250M - $300M (in millions) September 30, 2025 $306.8 Cash position 385.5 Common shares outstanding 2.0 2 Preferred shares outstanding 28.5 Stock options and restricted stock units outstanding Revenue Growth Margin Improvement Cost Control

32 © 2026, Iovance Biotherapeutics, Inc. © 2026, Iovance Biotherapeutics, Inc. Thank You