
Pioneering mRNA Cell Therapy for Autoimmunity January 2026

Disclosures For the purposes of this notice, the “presentation” that follows shall mean and include the slides that follow, the oral presentation of the slides by members of management of Cartesian Therapeutics, Inc. (the “Company”) or any person on their behalf, any question-and-answer session that follows such oral presentation, hard copies of this document and any materials distributed at, or in connection with, such oral presentation. Information in this presentation (including market data and statistical information) has been obtained from various sources (including third-party sources) and the Company does not guarantee the accuracy or completeness of such information. All projections, valuations and statistical analyses are provided for informational purposes only. They may be based on subjective assessments and assumptions and may use one among many alternative methodologies that produce different results and, to the extent they are based on historical information, they should not be relied upon as an accurate prediction of future performance, and you are cautioned not to give undue weight to them. The Company’s product candidates are investigational clinical product candidates currently under clinical evaluation and study. The Company’s product candidates have not been approved for use by the U.S. Food and Drug Administration ("FDA"). Any reference to the Company’s product candidates’ potential benefits, safety, or efficacy is based on observations from ongoing clinical research and should not be interpreted as definitive clinical evidence. Use or discussion of the Company’s product candidates is limited to the context of clinical research and free scientific exchange of information and is not intended for the general public, as medical advice, nor as any suggestion or indication that the Company’s product candidates have been found by the FDA to be safe or effective or approved for use outside of clinical trials. Forward-looking Statements Any statements in this presentation about the future expectations, plans and prospects of the Company, including without limitation, statements about the Company’s expected cash resources and cash runway, statements regarding the ability of the Company’s product candidates to be administered in an outpatient setting or without the need for preconditioning lymphodepleting chemotherapy, the potential of the Company’s product candidates to treat myasthenia gravis, juvenile myasthenia gravis, systemic lupus erythematosus, myositis, juvenile systemic lupus erythematosus, juvenile dermatomyositis, or any other disease, the anticipated timing or the outcome of ongoing and planned clinical trials, studies and data readouts, the anticipated timing or the outcome of the FDA’s review of the Company’s regulatory filings, including the number of trials that may be necessary in order to obtain marketing approval, the Company’s ability to conduct its clinical trials and preclinical studies, the timing or making of any regulatory filings, the anticipated timing or outcome of selection of developmental product candidates, the novelty of treatment paradigms that the Company is able to develop, the potential of any therapies developed by the Company to fulfill unmet medical needs, enrollment in the Company’s clinical trials, expectations regarding manufacturing and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical trials including proof of concept trials, including uncertain outcomes, the availability and timing of data from ongoing and future clinical trials and the results of such trials, whether preliminary results from a particular clinical trial will be predictive of the final results of that trial, whether results of early clinical trials will be indicative of the results of later clinical trials and whether results observed in certain patient subgroups will be indicative of the results in such subgroups in later clinical trials or are reflective of a product candidate's overall characteristics, the ability to predict results of studies performed on human beings based on results of studies performed on non-human subjects, the unproven approach of the Company’s technology, potential delays in enrollment of patients, undesirable side effects of the Company’s product candidates, political uncertainty, the Company’s reliance on third parties to conduct its clinical trials, the Company’s inability to maintain its existing or future collaborations, licenses or contractual relationships, its inability to protect its proprietary technology and intellectual property, potential delays in regulatory approvals, the availability of funding sufficient for its foreseeable and unforeseeable operating expenses and capital expenditure requirements, the Company’s recurring losses from operations and negative cash flows, substantial fluctuation in the price of the Company’s common stock, risks related to geopolitical conflicts and pandemics and other important factors discussed in the “Risk Factors” section of the Company’s most recent Annual Report on Form 10-K and subsequently filed Quarterly Reports on Form 10-Q, and in other filings that the Company makes with the Securities and Exchange Commission. In addition, any forward-looking statements included in this presentation represent the Company’s views only as of the date of its publication and should not be relied upon as representing its views as of any subsequent date. The Company specifically disclaims any intention to update any forward- looking statements included in this presentation, except as required by law. Forward-looking statements 2 PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
Executive summary 3 Cartesian’s lead asset, Descartes-08, delivers deep and durable responses in MG through 12 months following a single course of therapy—administered outpatient without lymphodepletion—positioning it to transform the current treatment landscape. The AURORA Phase 3 trial positions Descartes-08 to capture a $1B+ market opportunity in MG*, differentiated from chronic biologic therapies—backed by leadership with a strong commercial track record. US-based in-house manufacturing supports commercial readiness for MG launch with potential biologic-like margins and full supply chain control – ongoing process optimization creates opportunity for further margin expansion. Currently planned expansion into myositis positions Descartes-08 to address significant unmet medical need in a sizeable patient population. Strong efficacy signal observed in patients treated with Descartes-08 in Phase 2 SLE trial, supporting broad applicability across autoimmune diseases and enabling expansion into multiple indications beyond MG. *Internal company projectionsPIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY MG, myasthenia gravis

4 Late-stage clinical company pioneering mRNA cell therapy specifically designed to expand the reach of cell therapy to autoimmunity • mRNA cell therapy designed to be dosed reliably and safely in an outpatient setting without lymphodepletion • Descartes-08: Investigational mRNA CAR T-cell (CAR-T) with deep and durable responses through 12 months observed in randomized, double-blind, placebo-controlled Phase 2b trial in patients with myasthenia gravis (MG) • US-based in-house manufacturing supports commercial readiness with potential for biologic- like margins * As of September 30, 2025; includes cash, cash equivalents and restricted cash (unaudited) SLE, Systemic Lupus Erythematosus CAR, Chimeric antigen receptor JDM, juvenile dermatomyositis • Phase 3 AURORA trial initiated in May 2025 positions Descartes-08 to potentially access $1B+1 market opportunity in MG • Strong efficacy signal observed in patients treated with Descartes- 08 in Phase 2 SLE trial supports potential applicability across autoimmune diseases • Currently planned expansion into myositis positions Descartes-08 to address significant unmet medical need2 • Initiated Phase 1/2 pediatric trial in children and young adults with autoimmune diseases (HELIOS), including JDM DESCARTES-08 RECENT AND PLANNED ACTIVITY • Strong balance sheet with approximately $145 million* • Expected to support planned operations, including completion of ongoing Phase 3 trial of Descartes-08 for MG, into mid-2027 CASH RESOURCES 1. Internal company projections 2. Kronzer et al., 2021; Coffey et al., 2021; Marotta et al., 2020; OCTAGAM efficacy data PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY5 Cartesian’s mRNA approach is designed to expand the reach of potent cell therapy products to address autoimmunity No Lymphodepletion No associated cytopenia, secondary malignancies, or other chemotherapy toxicities Administered Outpatient Convenient dosing schedule Delivered at Therapeutic Levels Administered at therapeutic doses without uncontrollable proliferation Transient Cell Modification Does not carry risk of genomic integration
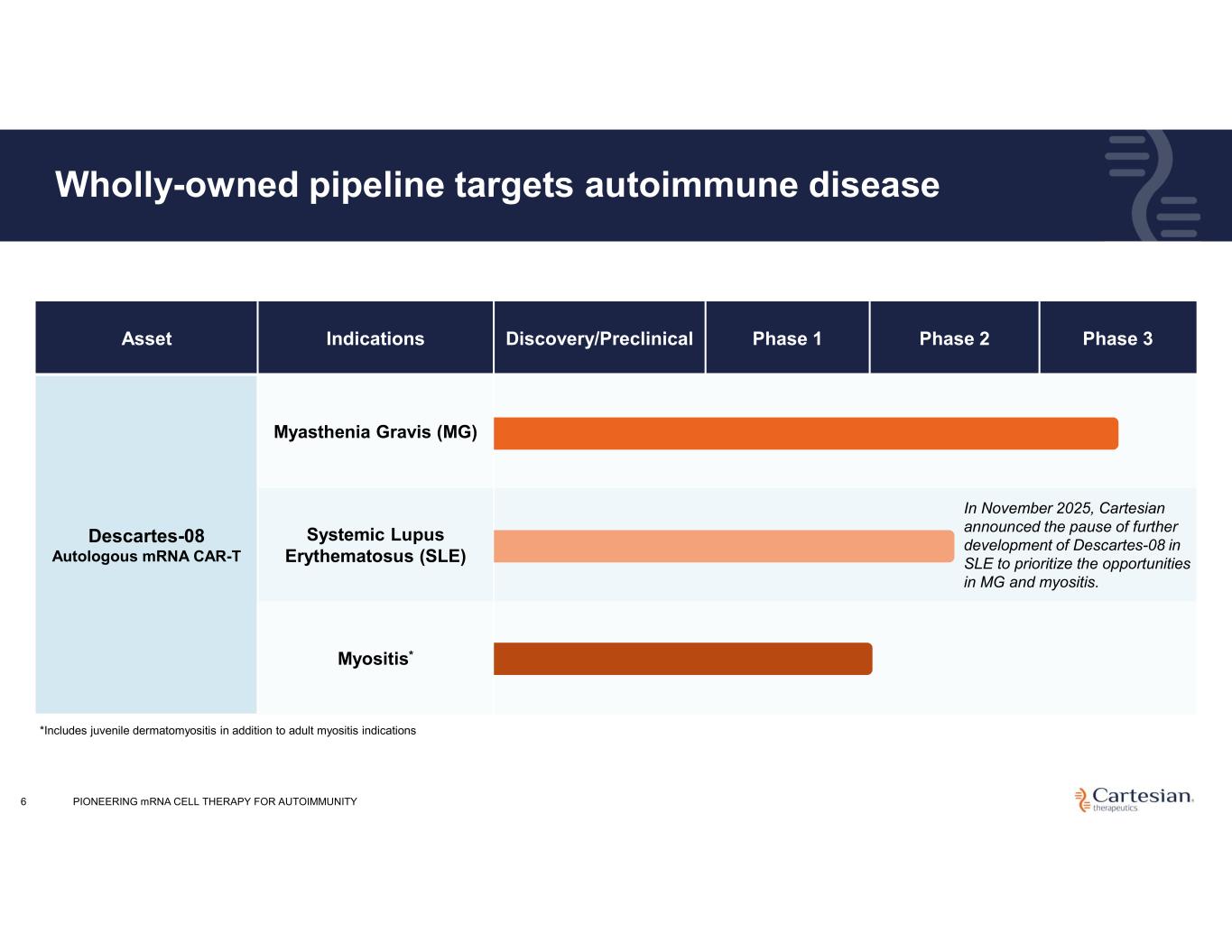
Phase 3Phase 2Phase 1Discovery/PreclinicalIndicationsAsset Myasthenia Gravis (MG) Descartes-08 Autologous mRNA CAR-T Systemic Lupus Erythematosus (SLE) Myositis* Wholly-owned pipeline targets autoimmune disease 6 *Includes juvenile dermatomyositis in addition to adult myositis indications PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY In November 2025, Cartesian announced the pause of further development of Descartes-08 in SLE to prioritize the opportunities in MG and myositis.
Engineered by transfection of autologous CD8+ T cells with mRNA encoding anti-BCMA CAR Granted U.S. FDA orphan and RMAT designations for generalized myasthenia gravis, and RPDD for juvenile dermatomyositis Typical lot processed for infusion within as little as ~3 weeks RMAT, Regenerative Medicine Advanced Therapy RPDD, Rare Pediatric Disease Designation PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY7 Descartes-08 is an mRNA CAR T-cell therapy in clinical development for autoimmune disease FDA, U.S. Food and Drug Administration BCMA, B-cell maturation antigen

8 Descartes-08 in Myasthenia Gravis PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
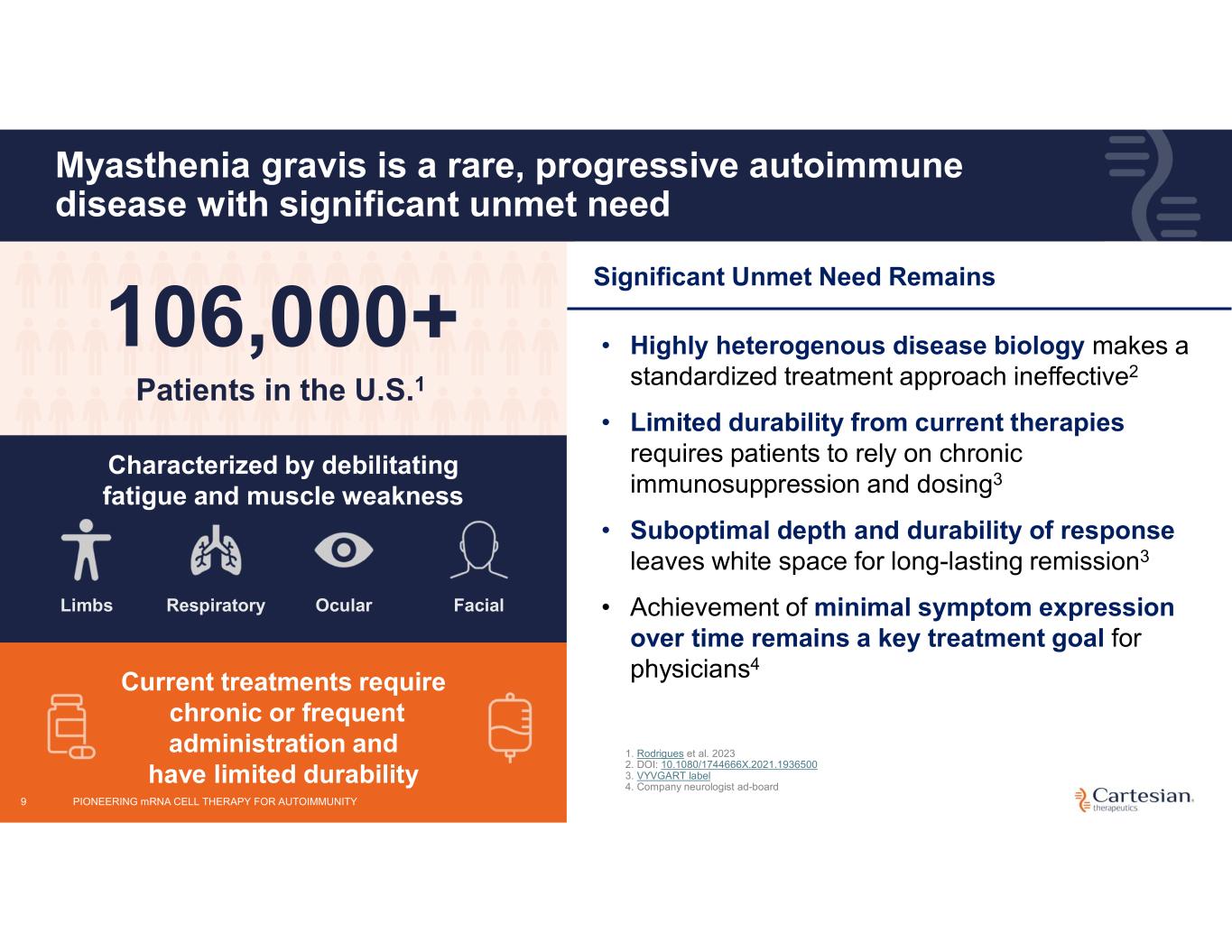
9 Myasthenia gravis is a rare, progressive autoimmune disease with significant unmet need Characterized by debilitating fatigue and muscle weakness Limbs Respiratory Ocular Facial 106,000+ Patients in the U.S.1 Current treatments require chronic or frequent administration and have limited durability Significant Unmet Need Remains • Highly heterogenous disease biology makes a standardized treatment approach ineffective2 • Limited durability from current therapies requires patients to rely on chronic immunosuppression and dosing3 • Suboptimal depth and durability of response leaves white space for long-lasting remission3 • Achievement of minimal symptom expression over time remains a key treatment goal for physicians4 1. Rodrigues et al. 2023 2. DOI: 10.1080/1744666X.2021.1936500 3. VYVGART label 4. Company neurologist ad-board PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
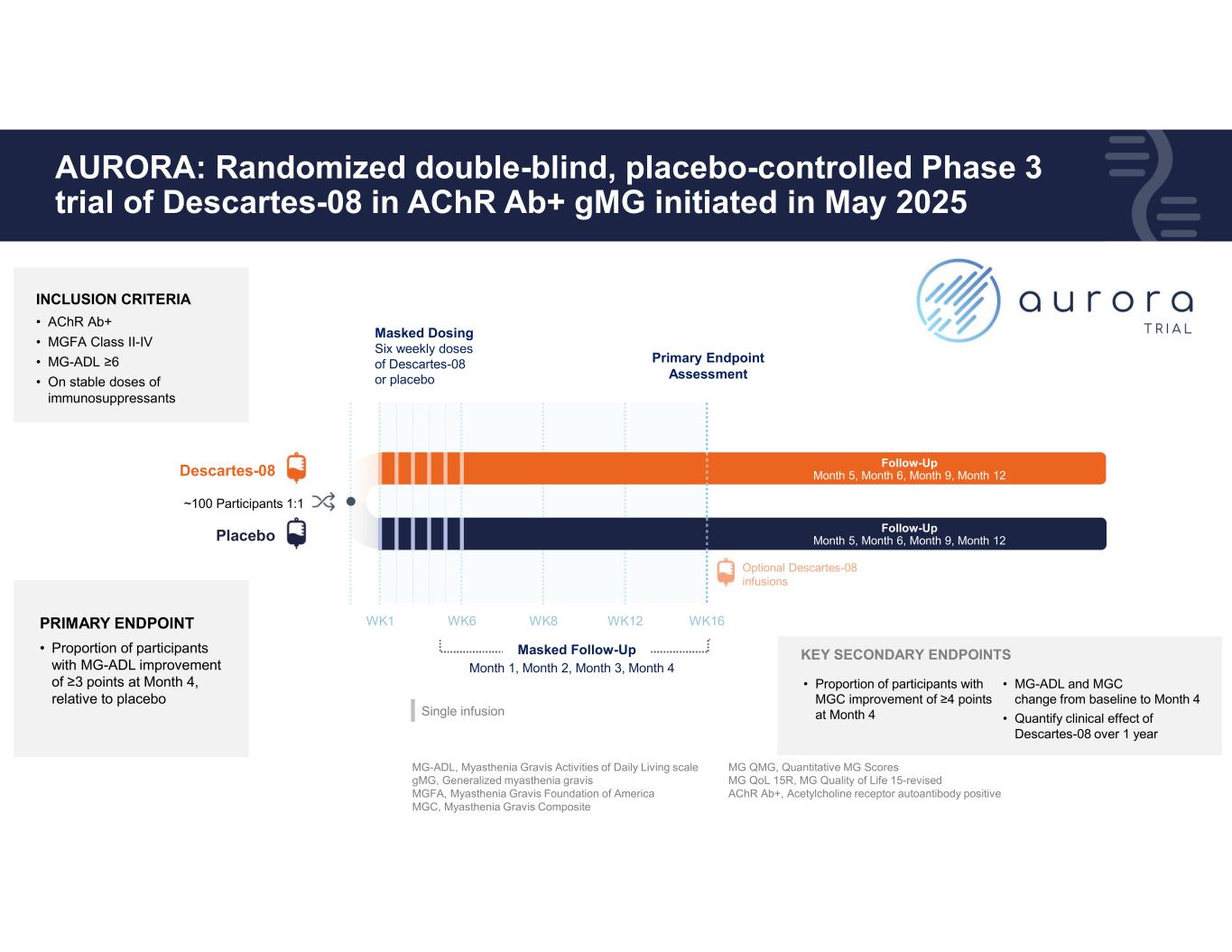
10 AURORA: Randomized double-blind, placebo-controlled Phase 3 trial of Descartes-08 in AChR Ab+ gMG initiated in May 2025 WK1 Masked Dosing Six weekly doses of Descartes-08 or placebo PRIMARY ENDPOINT • Proportion of participants with MG-ADL improvement of ≥3 points at Month 4, relative to placebo • Proportion of participants with MGC improvement of ≥4 points at Month 4 WK6 WK8 WK12 WK16 Masked Follow-Up Month 1, Month 2, Month 3, Month 4 Primary Endpoint Assessment Optional Descartes-08 infusions Follow-Up Month 5, Month 6, Month 9, Month 12 Follow-Up Month 5, Month 6, Month 9, Month 12Descartes-08 Placebo ~100 Participants 1:1 INCLUSION CRITERIA • AChR Ab+ • MGFA Class II-IV • MG-ADL ≥6 • On stable doses of immunosuppressants Single infusion MG-ADL, Myasthenia Gravis Activities of Daily Living scale gMG, Generalized myasthenia gravis MGFA, Myasthenia Gravis Foundation of America MGC, Myasthenia Gravis Composite MG QMG, Quantitative MG Scores MG QoL 15R, MG Quality of Life 15-revised AChR Ab+, Acetylcholine receptor autoantibody positive KEY SECONDARY ENDPOINTS • MG-ADL and MGC change from baseline to Month 4 • Quantify clinical effect of Descartes-08 over 1 year PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
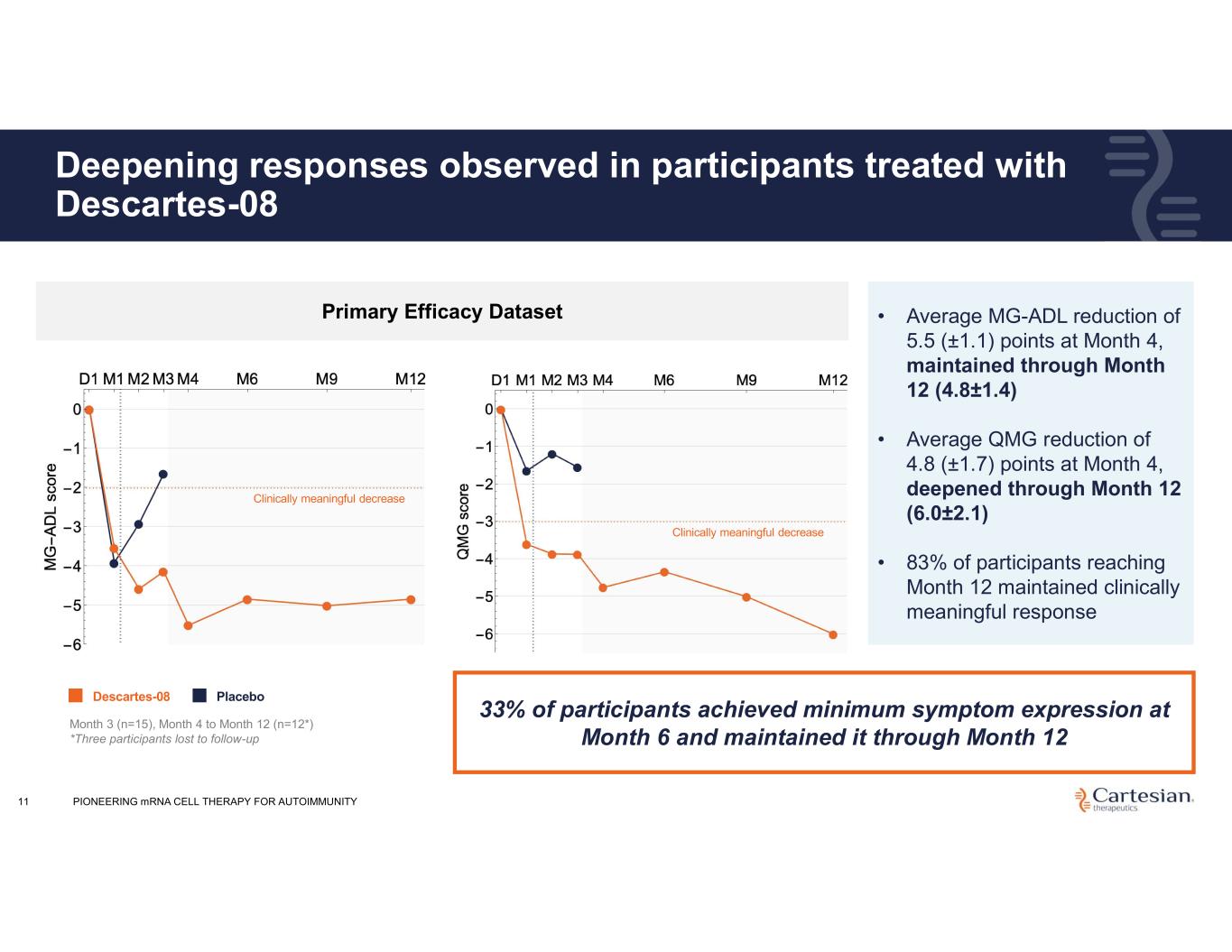
Deepening responses observed in participants treated with Descartes-08 11 • Average MG-ADL reduction of 5.5 (±1.1) points at Month 4, maintained through Month 12 (4.8±1.4) • Average QMG reduction of 4.8 (±1.7) points at Month 4, deepened through Month 12 (6.0±2.1) • 83% of participants reaching Month 12 maintained clinically meaningful response Primary Efficacy Dataset Month 3 (n=15), Month 4 to Month 12 (n=12*) *Three participants lost to follow-up Descartes-08 Placebo Clinically meaningful decrease Clinically meaningful decrease PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY 33% of participants achieved minimum symptom expression at Month 6 and maintained it through Month 12
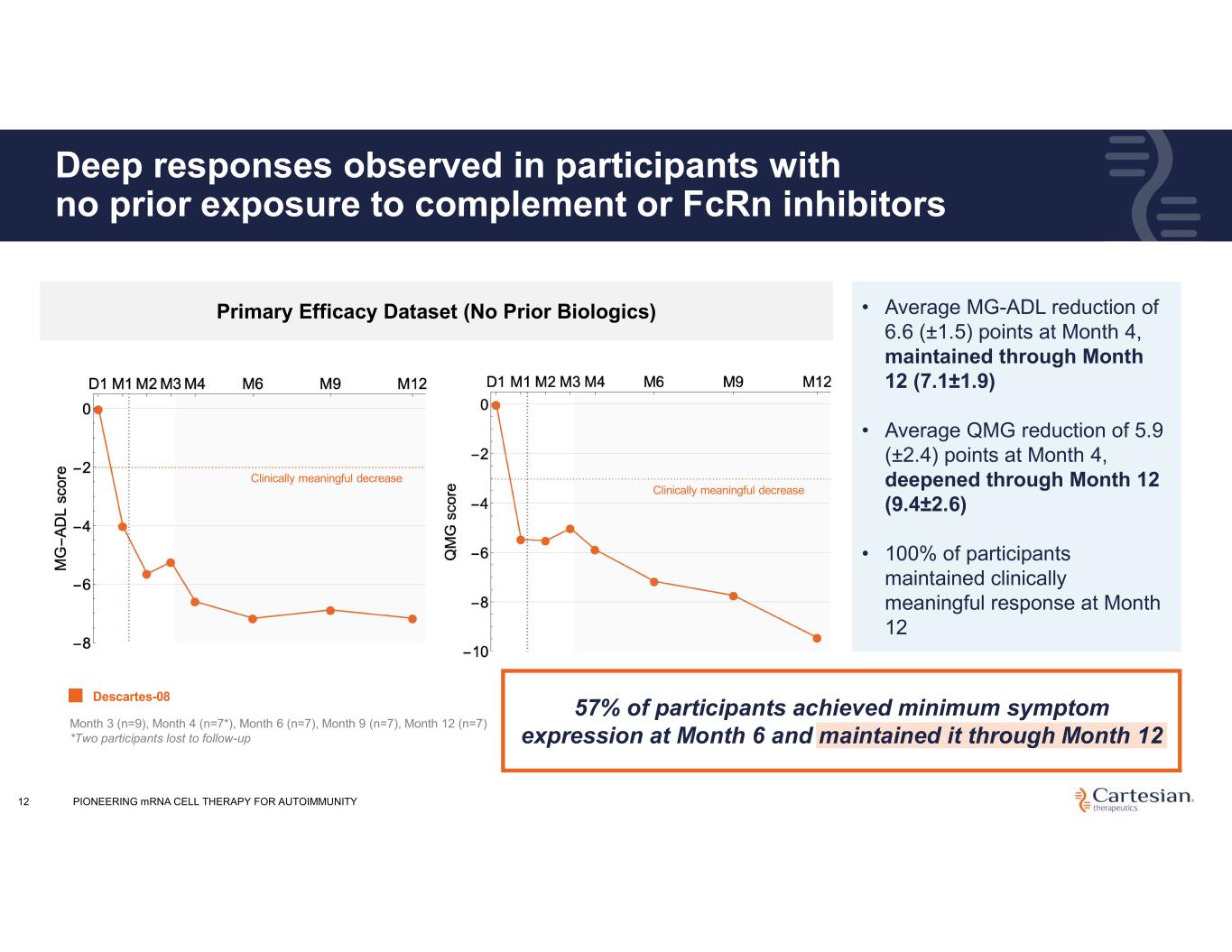
Deep responses observed in participants with no prior exposure to complement or FcRn inhibitors 12 • Average MG-ADL reduction of 6.6 (±1.5) points at Month 4, maintained through Month 12 (7.1±1.9) • Average QMG reduction of 5.9 (±2.4) points at Month 4, deepened through Month 12 (9.4±2.6) • 100% of participants maintained clinically meaningful response at Month 12 Primary Efficacy Dataset (No Prior Biologics) Month 3 (n=9), Month 4 (n=7*), Month 6 (n=7), Month 9 (n=7), Month 12 (n=7) *Two participants lost to follow-up Descartes-08 Clinically meaningful decrease Clinically meaningful decrease 57% of participants achieved minimum symptom expression at Month 6 and maintained it through Month 12 PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
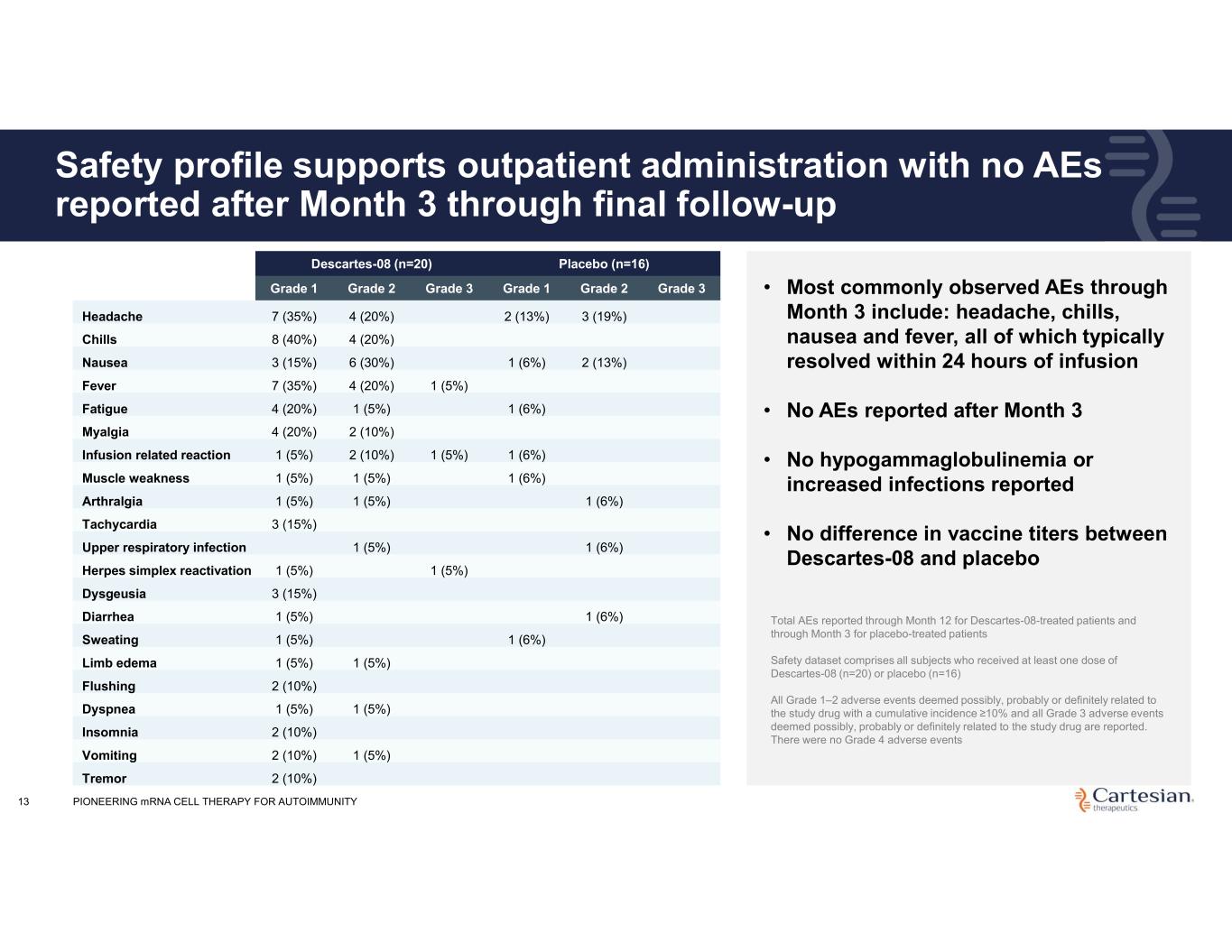
13 Safety profile supports outpatient administration with no AEs reported after Month 3 through final follow-up Placebo (n=16)Descartes-08 (n=20) Grade 3Grade 2Grade 1Grade 3Grade 2Grade 1 3 (19%)2 (13%)4 (20%)7 (35%)Headache 4 (20%)8 (40%)Chills 2 (13%)1 (6%)6 (30%)3 (15%)Nausea 1 (5%)4 (20%)7 (35%)Fever 1 (6%)1 (5%)4 (20%)Fatigue 2 (10%)4 (20%)Myalgia 1 (6%)1 (5%)2 (10%)1 (5%)Infusion related reaction 1 (6%)1 (5%)1 (5%)Muscle weakness 1 (6%)1 (5%)1 (5%)Arthralgia 3 (15%)Tachycardia 1 (6%)1 (5%)Upper respiratory infection 1 (5%)1 (5%)Herpes simplex reactivation 3 (15%)Dysgeusia 1 (6%)1 (5%)Diarrhea 1 (6%)1 (5%)Sweating 1 (5%)1 (5%)Limb edema 2 (10%)Flushing 1 (5%)1 (5%)Dyspnea 2 (10%)Insomnia 1 (5%)2 (10%)Vomiting 2 (10%)Tremor • Most commonly observed AEs through Month 3 include: headache, chills, nausea and fever, all of which typically resolved within 24 hours of infusion • No AEs reported after Month 3 • No hypogammaglobulinemia or increased infections reported • No difference in vaccine titers between Descartes-08 and placebo Total AEs reported through Month 12 for Descartes-08-treated patients and through Month 3 for placebo-treated patients Safety dataset comprises all subjects who received at least one dose of Descartes-08 (n=20) or placebo (n=16) All Grade 1–2 adverse events deemed possibly, probably or definitely related to the study drug with a cumulative incidence ≥10% and all Grade 3 adverse events deemed possibly, probably or definitely related to the study drug are reported. There were no Grade 4 adverse events PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
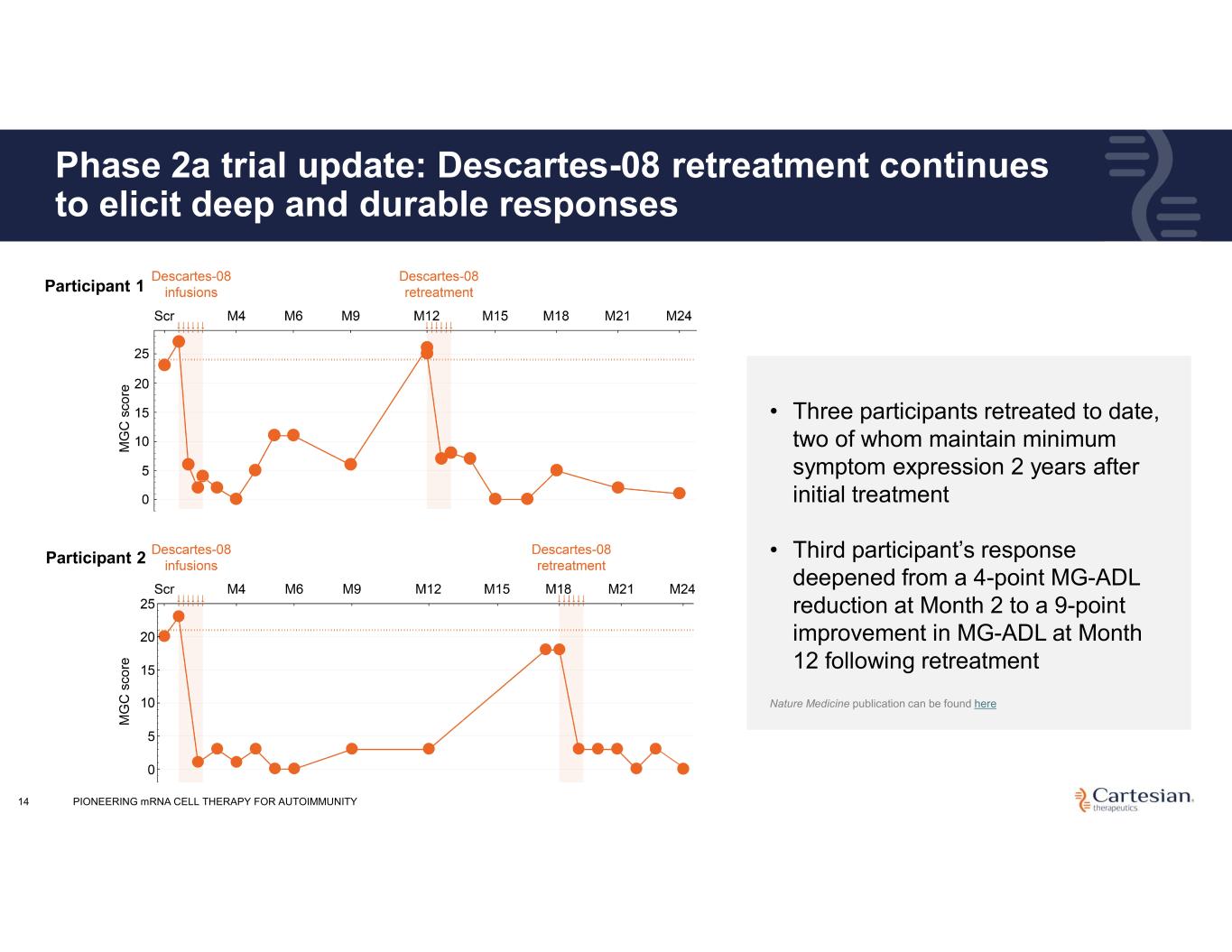
Phase 2a trial update: Descartes-08 retreatment continues to elicit deep and durable responses Nature Medicine publication can be found here • Three participants retreated to date, two of whom maintain minimum symptom expression 2 years after initial treatment • Third participant’s response deepened from a 4-point MG-ADL reduction at Month 2 to a 9-point improvement in MG-ADL at Month 12 following retreatment 14 Participant 1 Participant 2 PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
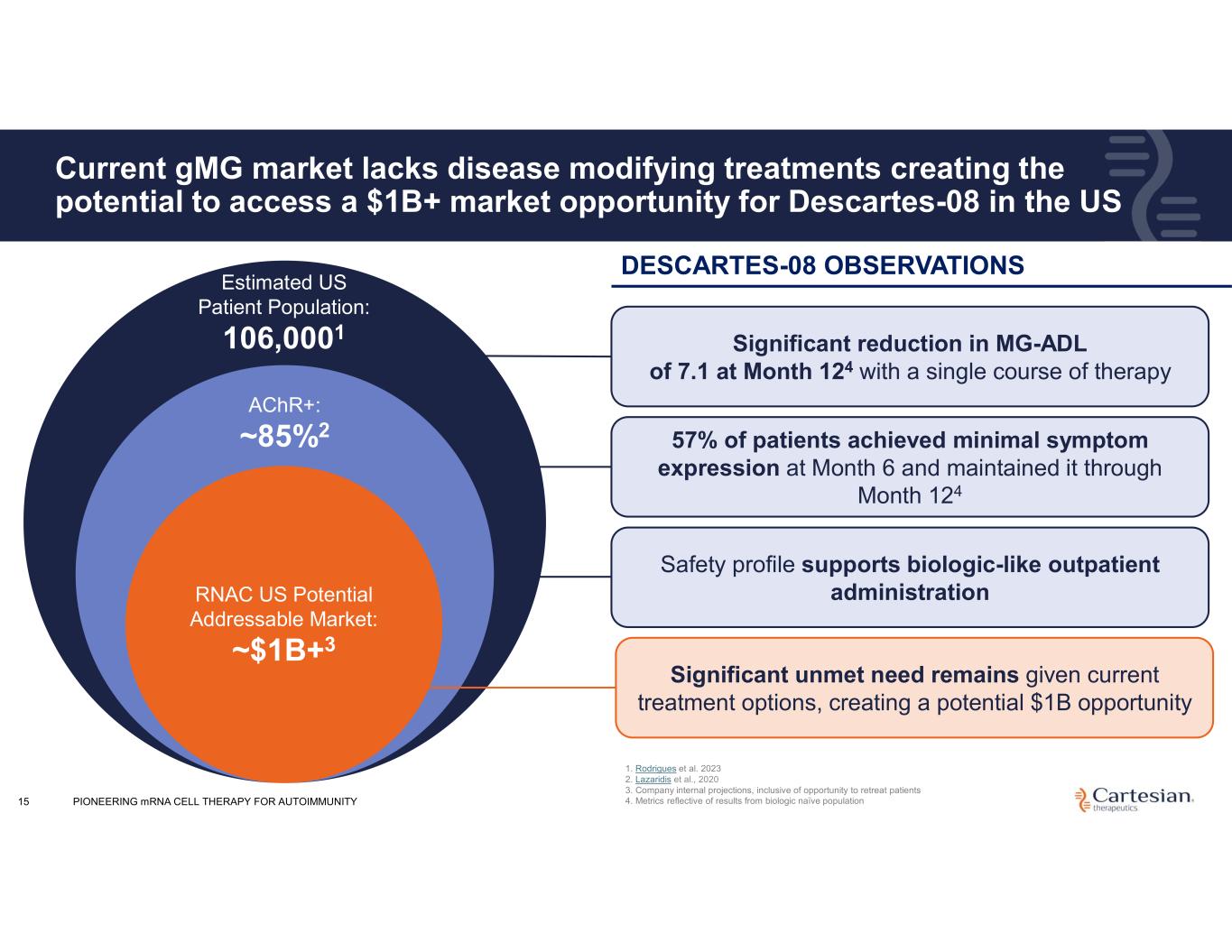
15 Current gMG market lacks disease modifying treatments creating the potential to access a $1B+ market opportunity for Descartes-08 in the US Estimated US Patient Population: 106,0001 AChR+: ~85%2 RNAC US Potential Addressable Market: ~$1B+3 Significant reduction in MG-ADL of 7.1 at Month 124 with a single course of therapy 57% of patients achieved minimal symptom expression at Month 6 and maintained it through Month 124 Safety profile supports biologic-like outpatient administration Significant unmet need remains given current treatment options, creating a potential $1B opportunity 1. Rodrigues et al. 2023 2. Lazaridis et al., 2020 3. Company internal projections, inclusive of opportunity to retreat patients 4. Metrics reflective of results from biologic naïve population DESCARTES-08 OBSERVATIONS PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
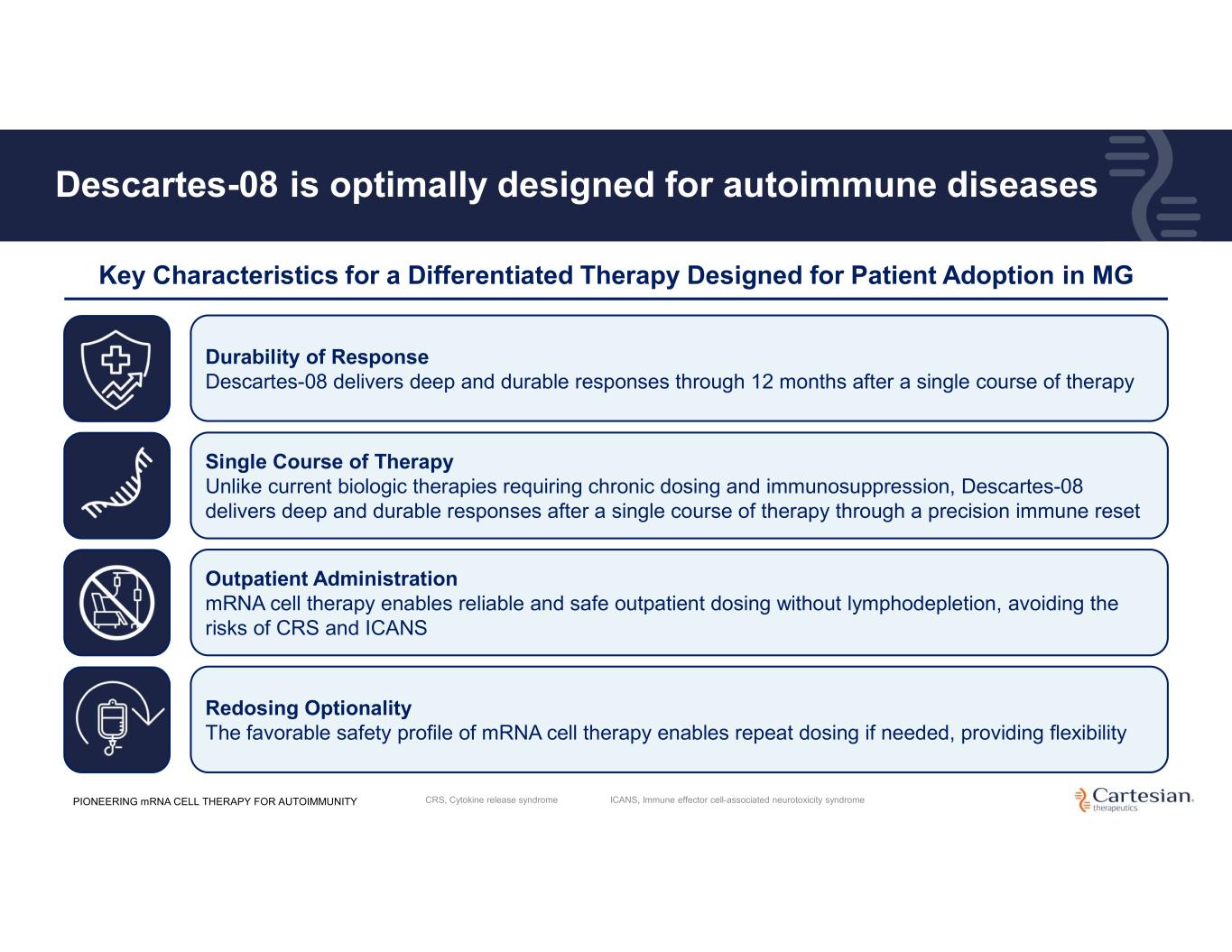
Descartes-08 is optimally designed for autoimmune diseases Key Characteristics for a Differentiated Therapy Designed for Patient Adoption in MG Durability of Response Descartes-08 delivers deep and durable responses through 12 months after a single course of therapy Single Course of Therapy Unlike current biologic therapies requiring chronic dosing and immunosuppression, Descartes-08 delivers deep and durable responses after a single course of therapy through a precision immune reset Outpatient Administration mRNA cell therapy enables reliable and safe outpatient dosing without lymphodepletion, avoiding the risks of CRS and ICANS Redosing Optionality The favorable safety profile of mRNA cell therapy enables repeat dosing if needed, providing flexibility PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY CRS, Cytokine release syndrome ICANS, Immune effector cell-associated neurotoxicity syndrome
17 Descartes-08 in SLE PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
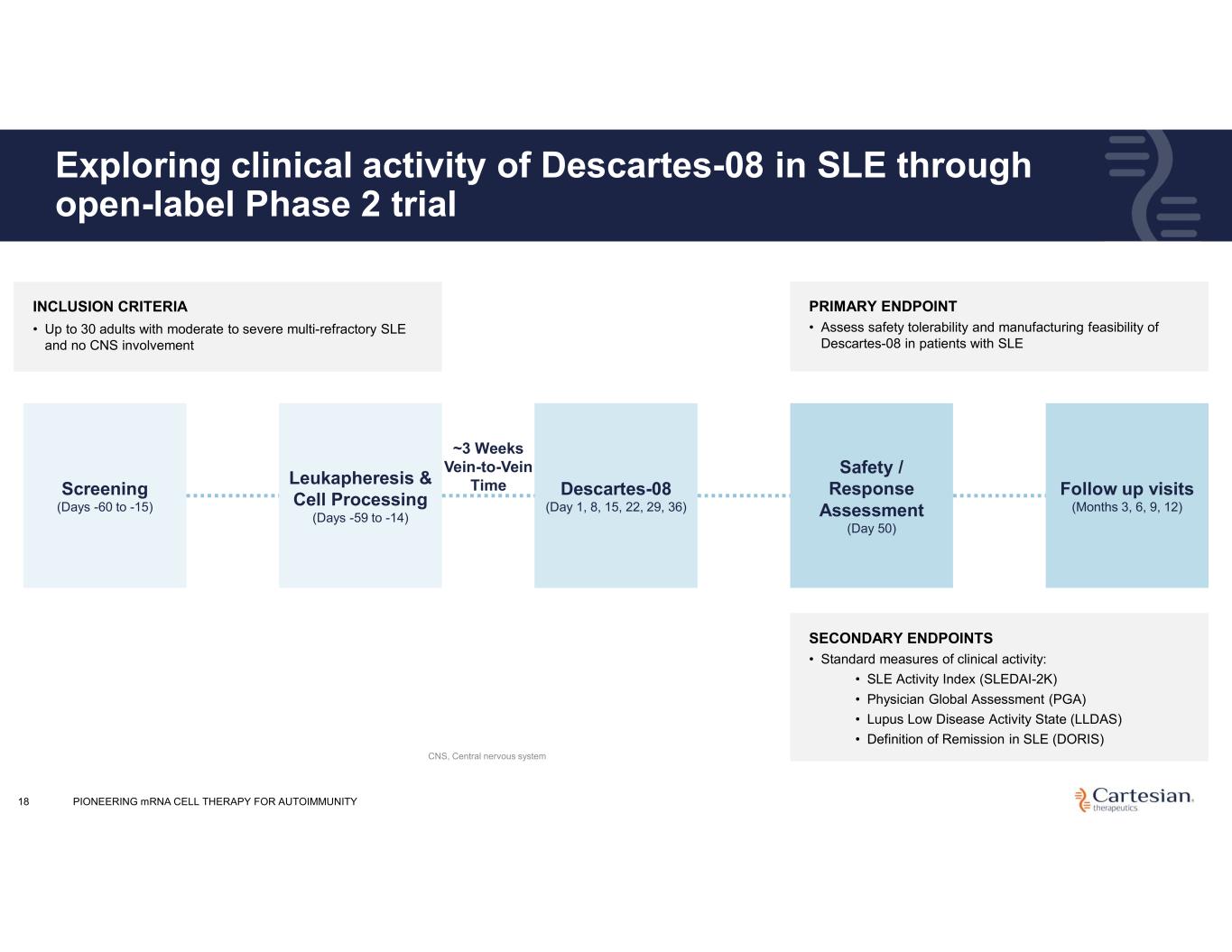
18 Exploring clinical activity of Descartes-08 in SLE through open-label Phase 2 trial Screening (Days -60 to -15) Leukapheresis & Cell Processing (Days -59 to -14) ~3 Weeks Vein-to-Vein Time Descartes-08 (Day 1, 8, 15, 22, 29, 36) Safety / Response Assessment (Day 50) Follow up visits (Months 3, 6, 9, 12) INCLUSION CRITERIA • Up to 30 adults with moderate to severe multi-refractory SLE and no CNS involvement PRIMARY ENDPOINT • Assess safety tolerability and manufacturing feasibility of Descartes-08 in patients with SLE SECONDARY ENDPOINTS • Standard measures of clinical activity: • SLE Activity Index (SLEDAI-2K) • Physician Global Assessment (PGA) • Lupus Low Disease Activity State (LLDAS) • Definition of Remission in SLE (DORIS) PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY CNS, Central nervous system
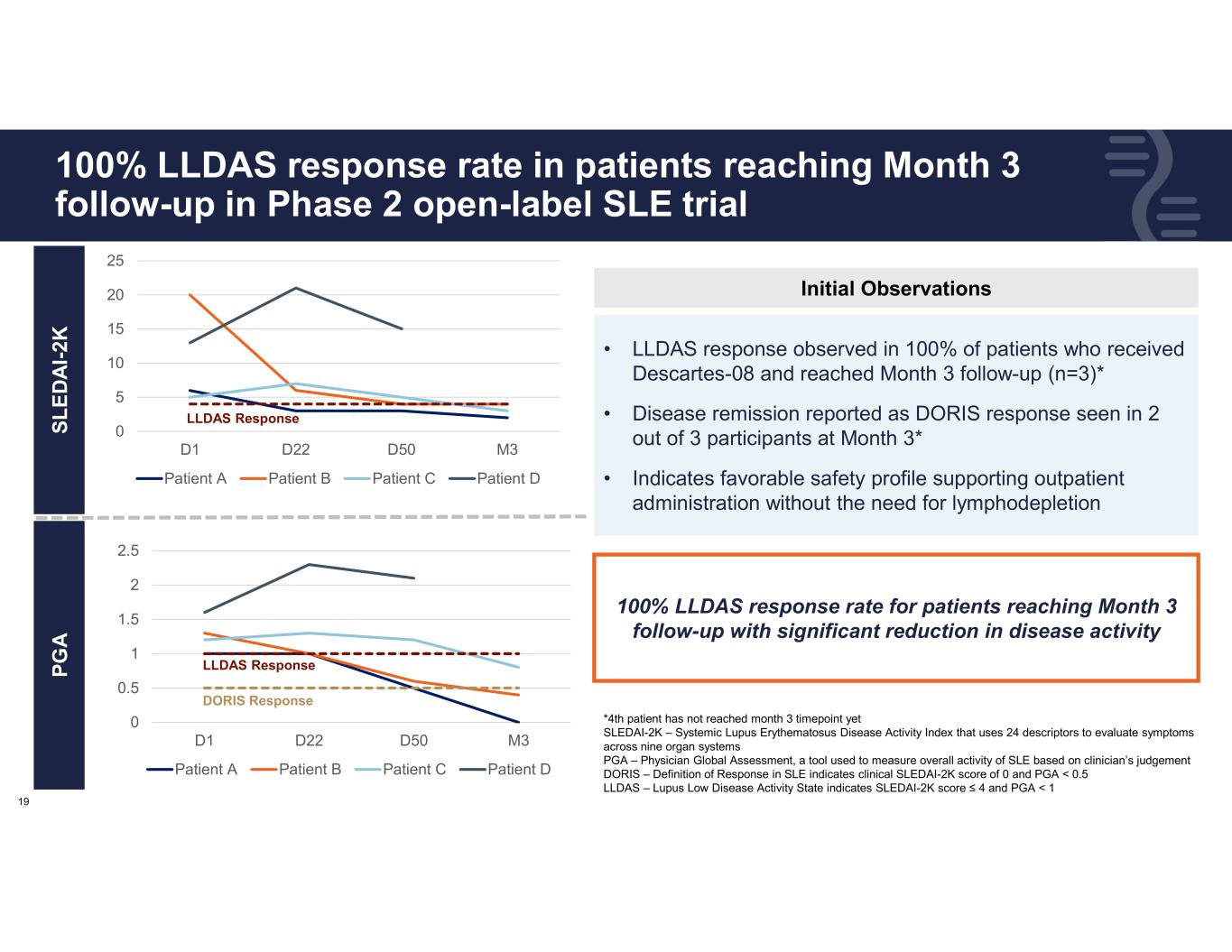
19 100% LLDAS response rate in patients reaching Month 3 follow-up in Phase 2 open-label SLE trial • LLDAS response observed in 100% of patients who received Descartes-08 and reached Month 3 follow-up (n=3)* • Disease remission reported as DORIS response seen in 2 out of 3 participants at Month 3* • Indicates favorable safety profile supporting outpatient administration without the need for lymphodepletion *4th patient has not reached month 3 timepoint yet SLEDAI-2K – Systemic Lupus Erythematosus Disease Activity Index that uses 24 descriptors to evaluate symptoms across nine organ systems PGA – Physician Global Assessment, a tool used to measure overall activity of SLE based on clinician’s judgement DORIS – Definition of Response in SLE indicates clinical SLEDAI-2K score of 0 and PGA < 0.5 LLDAS – Lupus Low Disease Activity State indicates SLEDAI-2K score ≤ 4 and PGA < 1 100% LLDAS response rate for patients reaching Month 3 follow-up with significant reduction in disease activity S L E D A I- 2 K P G A Initial Observations 0 5 10 15 20 25 D1 D22 D50 M3 Patient A Patient B Patient C Patient D 0 0.5 1 1.5 2 2.5 D1 D22 D50 M3 Patient A Patient B Patient C Patient D LLDAS Response LLDAS Response DORIS Response
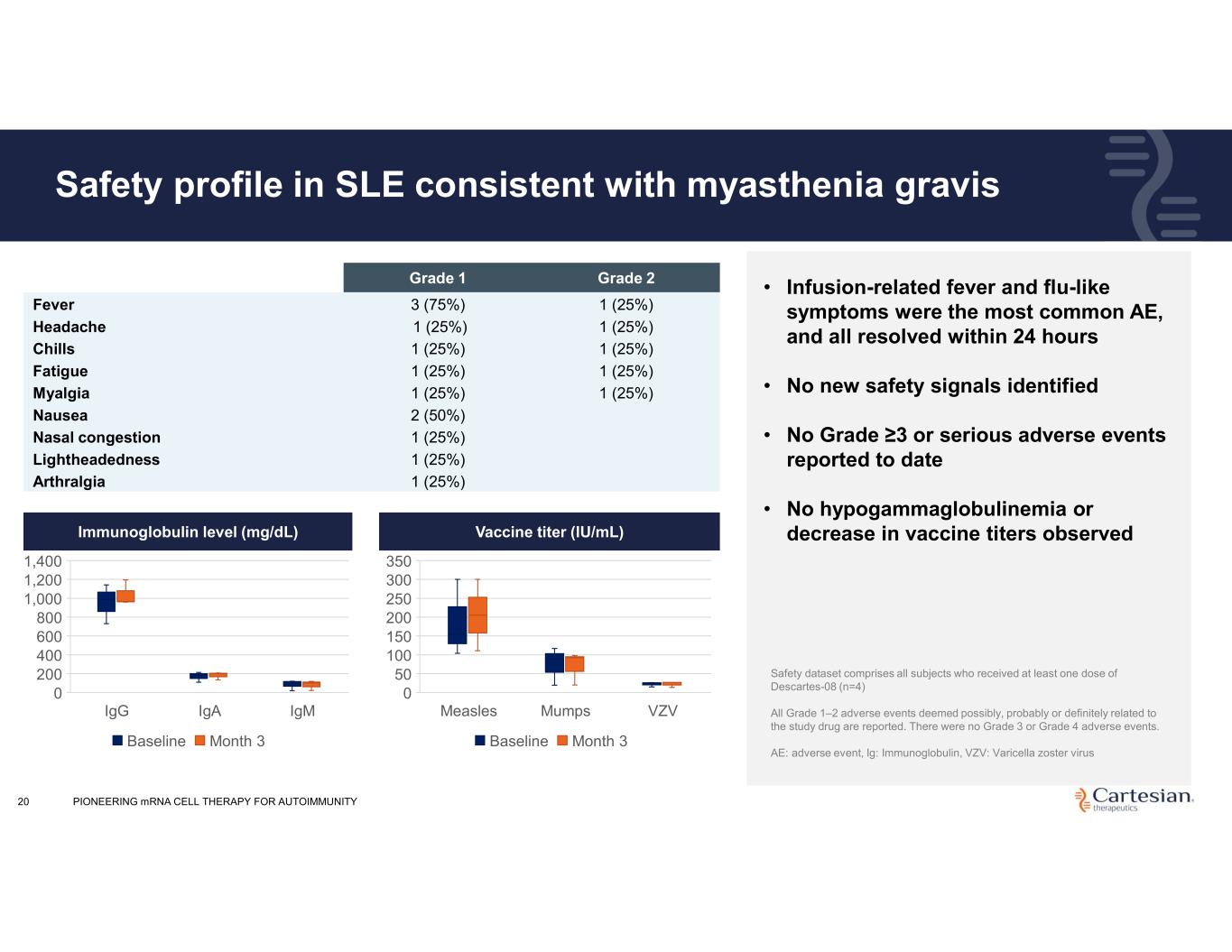
20 Safety profile in SLE consistent with myasthenia gravis Grade 2Grade 1 1 (25%)3 (75%)Fever 1 (25%)1 (25%)Headache 1 (25%)1 (25%)Chills 1 (25%)1 (25%)Fatigue 1 (25%)1 (25%)Myalgia 2 (50%)Nausea 1 (25%)Nasal congestion 1 (25%)Lightheadedness 1 (25%)Arthralgia • Infusion-related fever and flu-like symptoms were the most common AE, and all resolved within 24 hours • No new safety signals identified • No Grade ≥3 or serious adverse events reported to date • No hypogammaglobulinemia or decrease in vaccine titers observed Safety dataset comprises all subjects who received at least one dose of Descartes-08 (n=4) All Grade 1–2 adverse events deemed possibly, probably or definitely related to the study drug are reported. There were no Grade 3 or Grade 4 adverse events. AE: adverse event, Ig: Immunoglobulin, VZV: Varicella zoster virus IgG IgA IgM 0 200 400 600 800 1,000 1,200 1,400 Immunoglobulin level (mg/dL) Baseline Month 3 Measles Mumps VZV 0 50 100 150 200 250 300 350 Vaccine titer (IU/mL) Baseline Month 3 Immunoglobulin level (mg/dL) Vaccine titer (IU/mL) PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
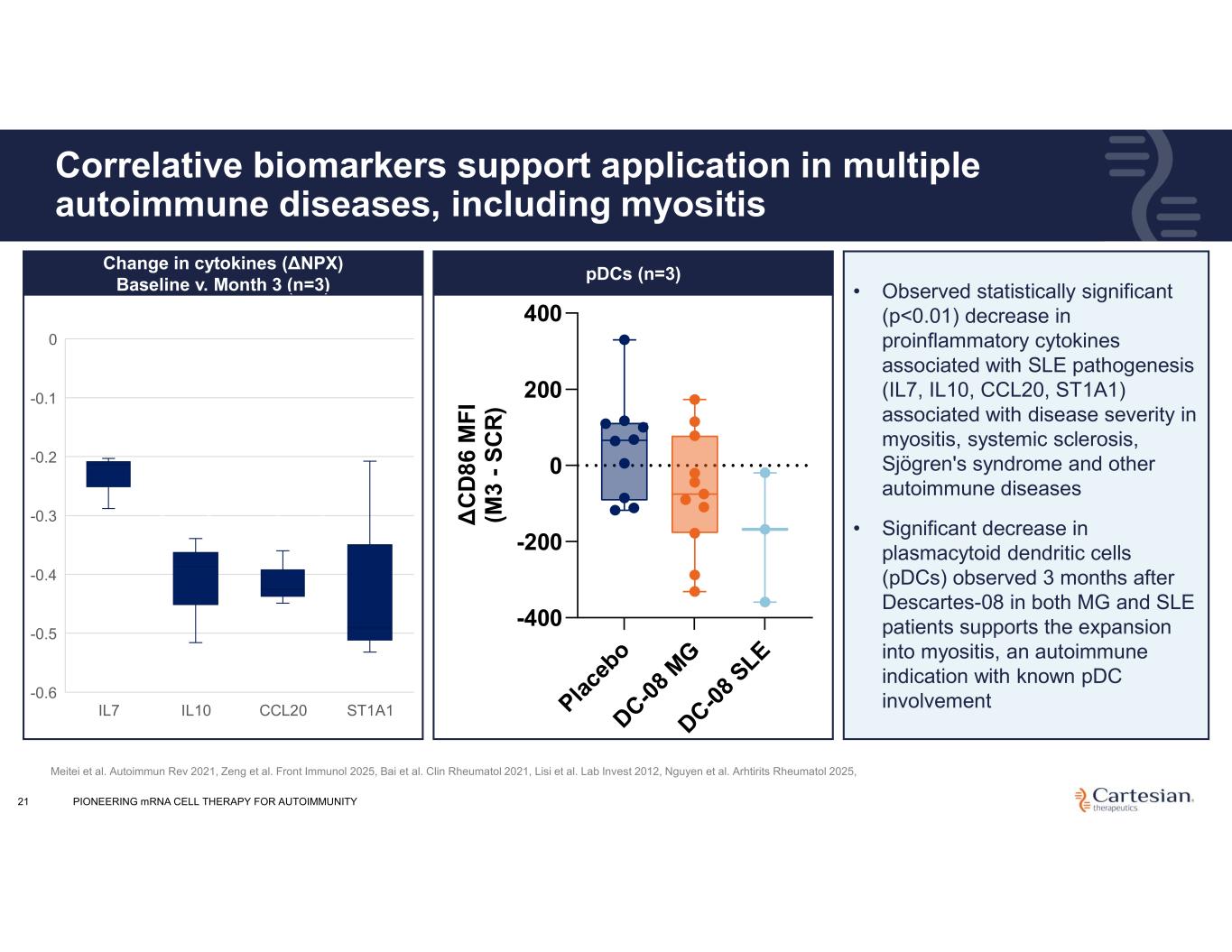
Pla ce bo DC-0 8 M G DC-0 8 SLE -400 -200 0 200 400 pDCs Δ C D 8 6 M F I (M 3 - S C R ) 21 Correlative biomarkers support application in multiple autoimmune diseases, including myositis • Observed statistically significant (p<0.01) decrease in proinflammatory cytokines associated with SLE pathogenesis (IL7, IL10, CCL20, ST1A1) associated with disease severity in myositis, systemic sclerosis, Sjögren's syndrome and other autoimmune diseases • Significant decrease in plasmacytoid dendritic cells (pDCs) observed 3 months after Descartes-08 in both MG and SLE patients supports the expansion into myositis, an autoimmune indication with known pDC involvement IL7 IL10 CCL20 ST1A1 0 -0.1 -0.2 -0.3 -0.4 -0.5 -0.6 Change in cytokines (ΔNPX) Baseline v. Month 3 (n=3) Meitei et al. Autoimmun Rev 2021, Zeng et al. Front Immunol 2025, Bai et al. Clin Rheumatol 2021, Lisi et al. Lab Invest 2012, Nguyen et al. Arhtirits Rheumatol 2025, pDCs (n=3) Change in cytokines (ΔNPX) Baseline v. Month 3 PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY

22 Descartes-08 Expansion into Myositis PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
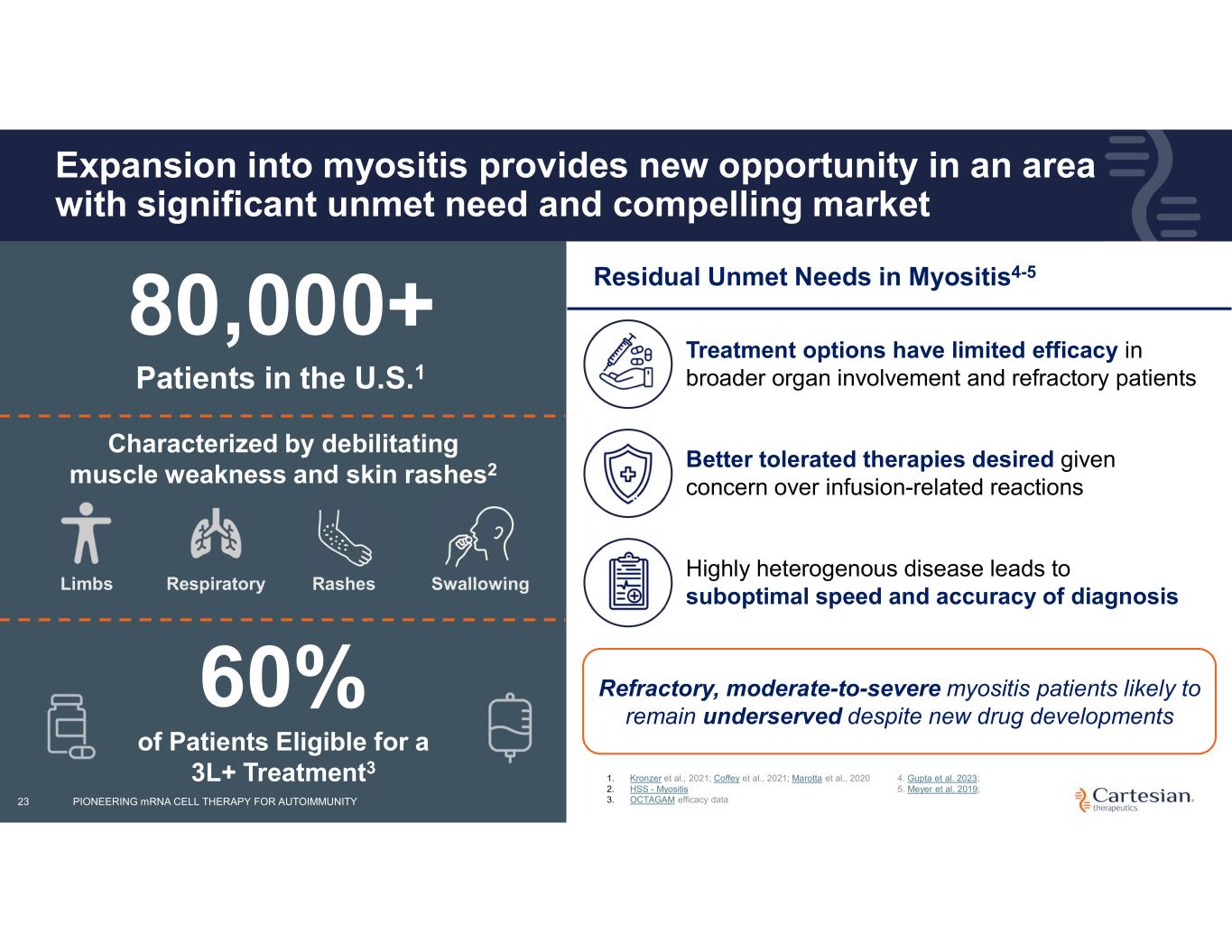
23 Expansion into myositis provides new opportunity in an area with significant unmet need and compelling market Characterized by debilitating muscle weakness and skin rashes2 Limbs Respiratory Rashes Swallowing 80,000+ Patients in the U.S.1 60% of Patients Eligible for a 3L+ Treatment3 Residual Unmet Needs in Myositis4-5 Refractory, moderate-to-severe myositis patients likely to remain underserved despite new drug developments Treatment options have limited efficacy in broader organ involvement and refractory patients Better tolerated therapies desired given concern over infusion-related reactions Highly heterogenous disease leads to suboptimal speed and accuracy of diagnosis 1. Kronzer et al., 2021; Coffey et al., 2021; Marotta et al., 2020 2. HSS - Myositis 3. OCTAGAM efficacy data 4. Gupta et al. 2023; 5. Meyer et al. 2019; PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
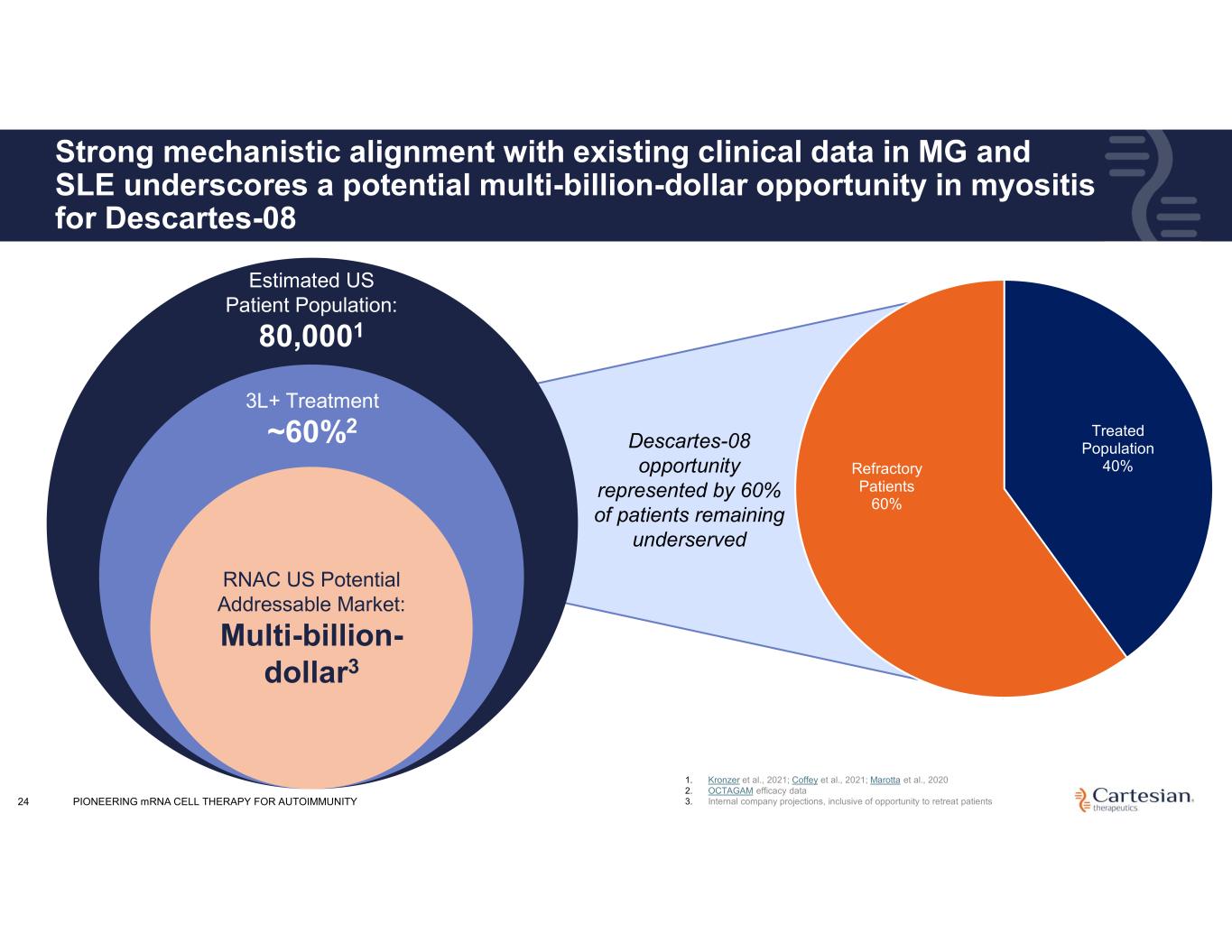
24 Strong mechanistic alignment with existing clinical data in MG and SLE underscores a potential multi-billion-dollar opportunity in myositis for Descartes-08 Estimated US Patient Population: 80,0001 3L+ Treatment ~60%2 RNAC US Potential Addressable Market: Multi-billion- dollar3 Descartes-08 opportunity represented by 60% of patients remaining underserved Treated Population 40%Refractory Patients 60% 1. Kronzer et al., 2021; Coffey et al., 2021; Marotta et al., 2020 2. OCTAGAM efficacy data 3. Internal company projections, inclusive of opportunity to retreat patientsPIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
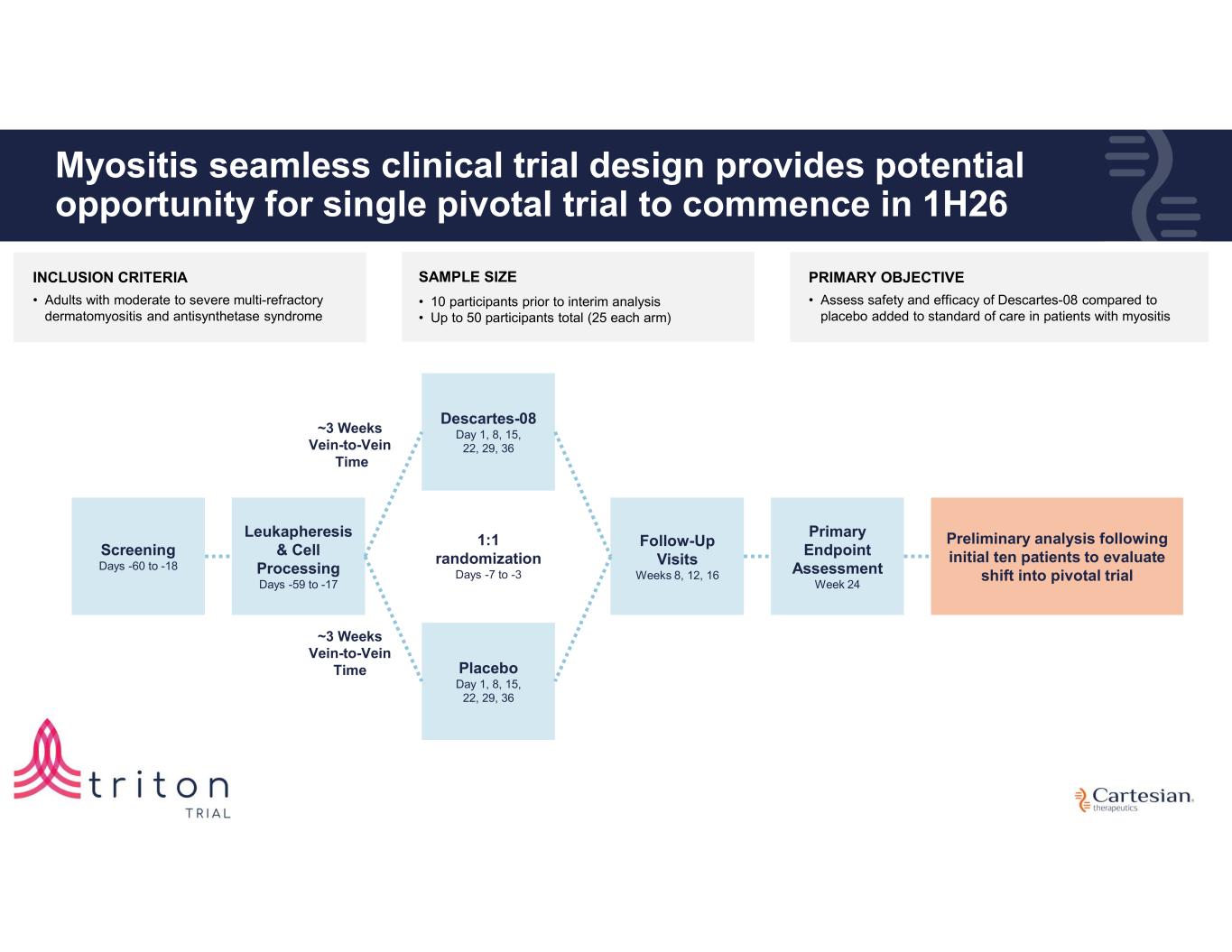
25 Myositis seamless clinical trial design provides potential opportunity for single pivotal trial to commence in 1H26 Screening Days -60 to -18 Leukapheresis & Cell Processing Days -59 to -17 ~3 Weeks Vein-to-Vein Time Descartes-08 Day 1, 8, 15, 22, 29, 36 Follow-Up Visits Weeks 8, 12, 16 Primary Endpoint Assessment Week 24 Placebo Day 1, 8, 15, 22, 29, 36 ~3 Weeks Vein-to-Vein Time 1:1 randomization Days -7 to -3 Preliminary analysis following initial ten patients to evaluate shift into pivotal trial INCLUSION CRITERIA • Adults with moderate to severe multi-refractory dermatomyositis and antisynthetase syndrome PRIMARY OBJECTIVE • Assess safety and efficacy of Descartes-08 compared to placebo added to standard of care in patients with myositis SAMPLE SIZE • 10 participants prior to interim analysis • Up to 50 participants total (25 each arm)
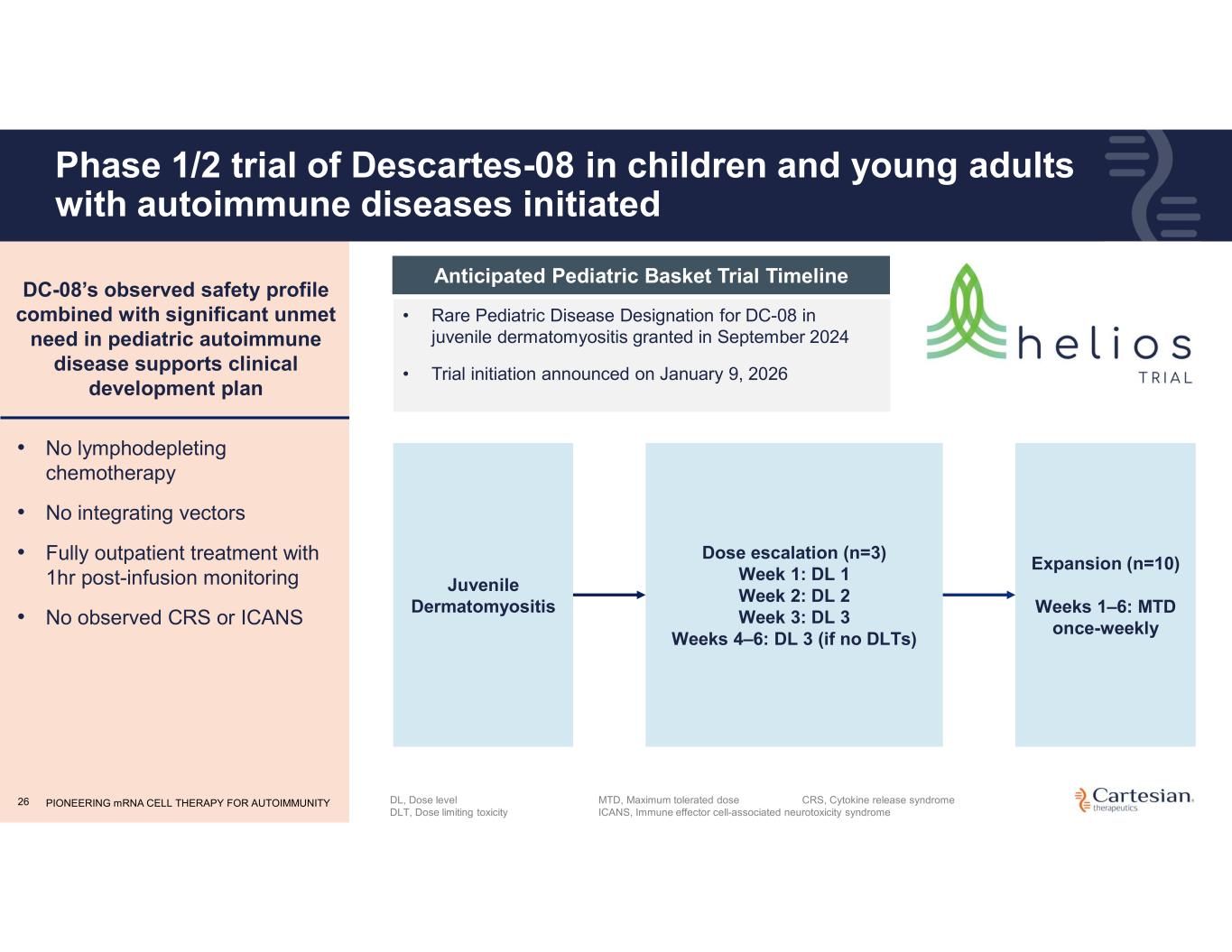
PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY26 Phase 1/2 trial of Descartes-08 in children and young adults with autoimmune diseases initiated • Rare Pediatric Disease Designation for DC-08 in juvenile dermatomyositis granted in September 2024 • Trial initiation announced on January 9, 2026 DL, Dose level DLT, Dose limiting toxicity Dose escalation (n=3) Week 1: DL 1 Week 2: DL 2 Week 3: DL 3 Weeks 4–6: DL 3 (if no DLTs) Juvenile Dermatomyositis Expansion (n=10) Weeks 1–6: MTD once-weekly • No lymphodepleting chemotherapy • No integrating vectors • Fully outpatient treatment with 1hr post-infusion monitoring • No observed CRS or ICANS DC-08’s observed safety profile combined with significant unmet need in pediatric autoimmune disease supports clinical development plan Anticipated Pediatric Basket Trial Timeline MTD, Maximum tolerated dose ICANS, Immune effector cell-associated neurotoxicity syndrome CRS, Cytokine release syndrome

27 Manufacturing PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
28 Wholly-owned, in-house, US-based manufacturing FUTURE GROWTH Clinical and commercial manufacturing scale capabilities support maturing pipeline and future growth QUICK TO ADAPT Continuous process optimization creates opportunity for progressive margin expansion WHOLLY-OWNED Ownership of quality control and production timelines COST EFFICIENT Supports commercial readiness for MG launch with potential for biologic-like margins Facility located in Frederick, MD Over 35,000 sq. ft. state- of-the-art cGMP facility PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY cGMP, current good manufacturing practice
29 FINANCIAL POSITION: Current Cash and Cash Equivalents Expected to Support Pipeline Through Key Milestones All metrics as of 9/30/25 *Includes Series A Non-Voting Convertible Preferred Stock and Series B Non-Voting Convertible Preferred Stock that remain subject to beneficial ownership limitations that are convertible into shares of common stock and includes outstanding options, RSUs and warrants. $145.1M <75 FULL TIME EMPLOYEES Based in Gaithersburg, MD and Frederick, MD 26.0M Basic shares outstanding 34.1M Fully diluted shares outstanding* In cash, cash equivalents and restricted cash (unaudited) PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY

PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY30 Our team | Management Carsten Brunn, PhD PRESIDENT AND CEO Blaine Davis CHIEF FINANCIAL OFFICER Miloš Miljković, MD CHIEF MEDICAL OFFICER Emily English, PhD CHIEF OPERATIONS OFFICER Matthew Bartholomae GENERAL COUNSEL, SECRETARY
Key takeaways 31 Cartesian’s lead asset, Descartes-08, delivers deep and durable responses in MG through 12 months following a single course of therapy—administered outpatient without lymphodepletion—positioning it to transform the current treatment landscape. The AURORA Phase 3 trial positions Descartes-08 to capture a $1B+ market opportunity in MG*, differentiated from chronic biologic therapies—backed by leadership with a strong commercial track record. US-based in-house manufacturing supports commercial readiness for MG launch with potential biologic-like margins and full supply chain control – ongoing process optimization creates opportunity for further margin expansion. Currently planned expansion into myositis positions Descartes-08 to address significant unmet medical need in a sizeable patient population. Strong efficacy signal observed in patients treated with Descartes-08 in Phase 2 SLE trial, supporting broad applicability across autoimmune diseases and enabling expansion into multiple indications beyond MG. *Internal company projectionsPIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY MG, myasthenia gravis

32 Appendix PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
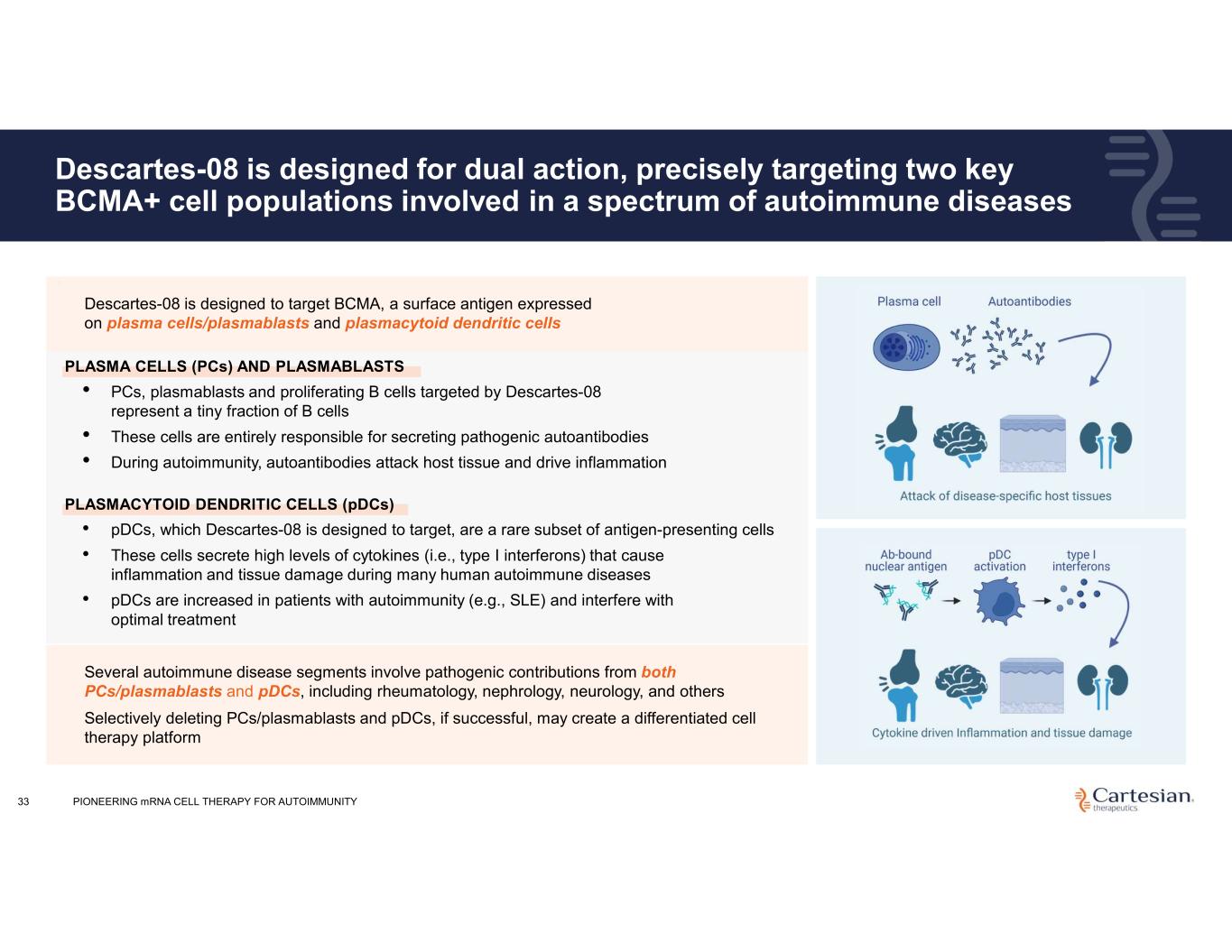
Descartes-08 is designed for dual action, precisely targeting two key BCMA+ cell populations involved in a spectrum of autoimmune diseases 33 Several autoimmune disease segments involve pathogenic contributions from both PCs/plasmablasts and pDCs, including rheumatology, nephrology, neurology, and others Selectively deleting PCs/plasmablasts and pDCs, if successful, may create a differentiated cell therapy platform • Descartes-08 is designed to target BCMA, a surface antigen expressed on plasma cells/plasmablasts and plasmacytoid dendritic cells PLASMA CELLS (PCs) AND PLASMABLASTS • PCs, plasmablasts and proliferating B cells targeted by Descartes-08 represent a tiny fraction of B cells • These cells are entirely responsible for secreting pathogenic autoantibodies • During autoimmunity, autoantibodies attack host tissue and drive inflammation PLASMACYTOID DENDRITIC CELLS (pDCs) • pDCs, which Descartes-08 is designed to target, are a rare subset of antigen-presenting cells • These cells secrete high levels of cytokines (i.e., type I interferons) that cause inflammation and tissue damage during many human autoimmune diseases • pDCs are increased in patients with autoimmunity (e.g., SLE) and interfere with optimal treatment PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY
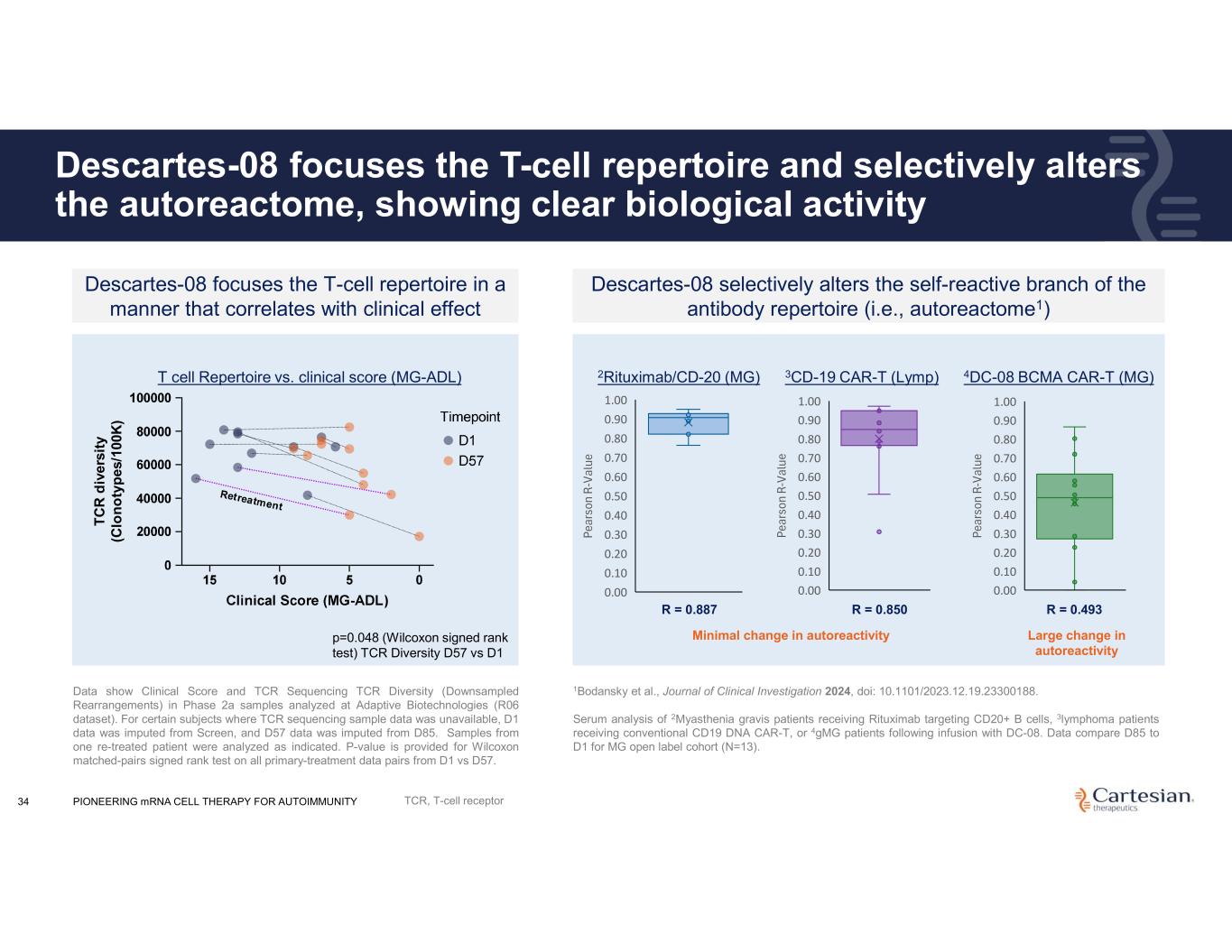
PIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY34 Descartes-08 focuses the T-cell repertoire and selectively alters the autoreactome, showing clear biological activity Descartes-08 focuses the T-cell repertoire in a manner that correlates with clinical effect p=0.048 (Wilcoxon signed rank test) TCR Diversity D57 vs D1 Data show Clinical Score and TCR Sequencing TCR Diversity (Downsampled Rearrangements) in Phase 2a samples analyzed at Adaptive Biotechnologies (R06 dataset). For certain subjects where TCR sequencing sample data was unavailable, D1 data was imputed from Screen, and D57 data was imputed from D85. Samples from one re-treated patient were analyzed as indicated. P-value is provided for Wilcoxon matched-pairs signed rank test on all primary-treatment data pairs from D1 vs D57. Descartes-08 selectively alters the self-reactive branch of the antibody repertoire (i.e., autoreactome1) 1Bodansky et al., Journal of Clinical Investigation 2024, doi: 10.1101/2023.12.19.23300188. Serum analysis of 2Myasthenia gravis patients receiving Rituximab targeting CD20+ B cells, 3lymphoma patients receiving conventional CD19 DNA CAR-T, or 4gMG patients following infusion with DC-08. Data compare D85 to D1 for MG open label cohort (N=13). Pe ar so n R- Va lu e 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 T C R d iv er si ty (C lo n o ty p es /1 00 K ) Minimal change in autoreactivity Pe ar so n R- Va lu e 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 R = 0.887 3CD-19 CAR-T (Lymp) Pe ar so n R- Va lu e 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 R = 0.850 4DC-08 BCMA CAR-T (MG) R = 0.493 2Rituximab/CD-20 (MG)T cell Repertoire vs. clinical score (MG-ADL) Large change in autoreactivity TCR, T-cell receptor
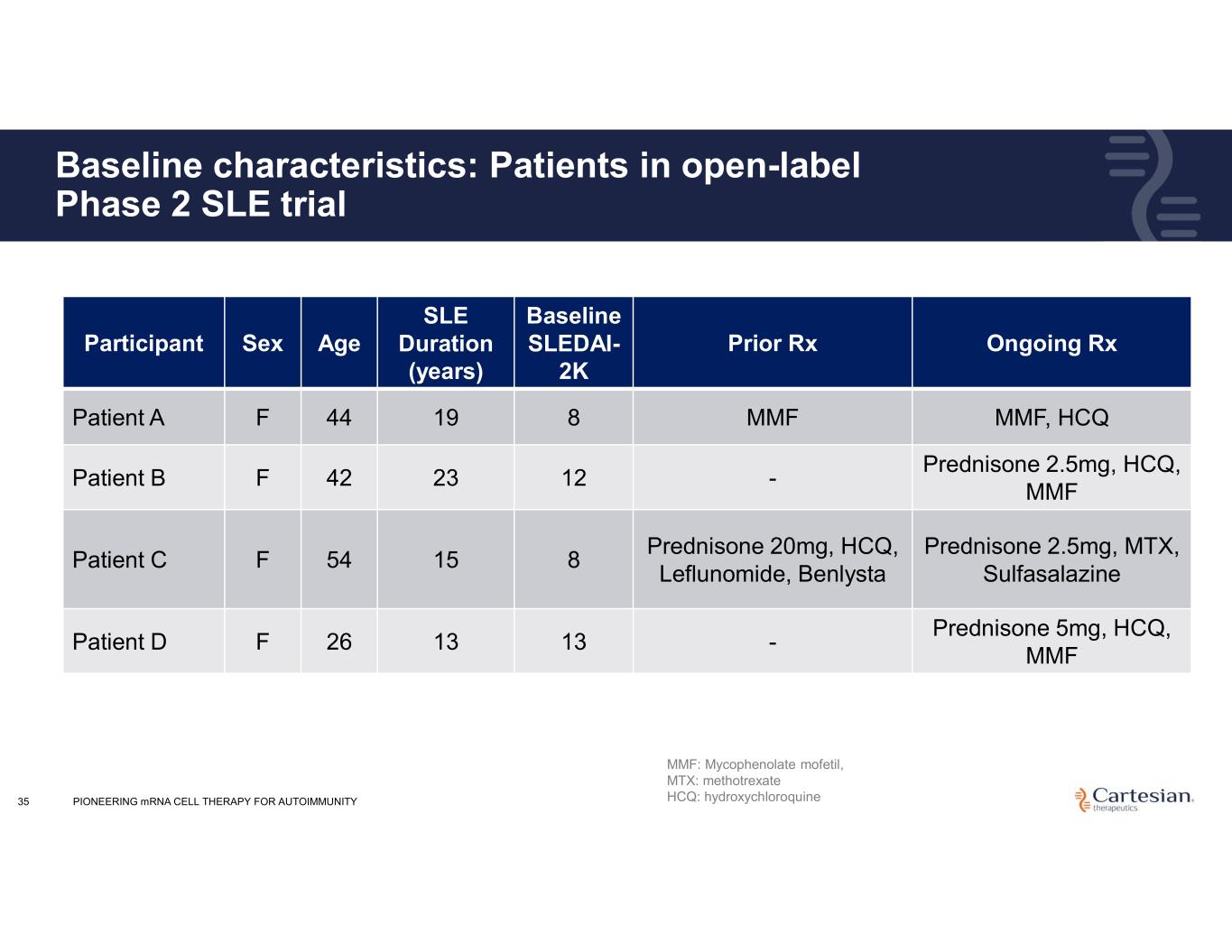
35 Baseline characteristics: Patients in open-label Phase 2 SLE trial Ongoing RxPrior Rx Baseline SLEDAI- 2K SLE Duration (years) AgeSexParticipant MMF, HCQMMF81944FPatient A Prednisone 2.5mg, HCQ, MMF -122342FPatient B Prednisone 2.5mg, MTX, Sulfasalazine Prednisone 20mg, HCQ, Leflunomide, Benlysta 81554FPatient C Prednisone 5mg, HCQ, MMF -131326FPatient D MMF: Mycophenolate mofetil, MTX: methotrexate HCQ: hydroxychloroquinePIONEERING mRNA CELL THERAPY FOR AUTOIMMUNITY