UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
(Amendment No. )
Filed by the Registrant x Filed by a Party other than the Registrant ¨
Check the appropriate box:
| ¨ | Preliminary Proxy Statement |
| ¨ | CONFIDENTIAL, FOR USE OF THE COMMISSION ONLY (AS PERMITTED BY RULE 14a-6(e)(2)) |
| x | Definitive Proxy Statement |
| ¨ | Definitive Additional Materials |
| ¨ | Soliciting Material Pursuant to Rule 14a-12 |
Myrexis, Inc.
(Name of Registrant as Specified in its Charter)
(Name of Person(s) Filing Proxy Statement if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ¨ | No fee required. |
| ¨ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1) | Title of each class of securities to which transaction applies: |
| (2) | Aggregate number of securities to which transaction applies: |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| (4) | Proposed maximum aggregate value of transaction: |
| (5) | Total fee paid: |
| x | Fee paid previously with preliminary materials. |
| ¨ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number or the Form or Schedule and the date of its filing. |
| (1) | Amount Previously Paid: |
| (2) | Form, Schedule or Registration Statement No.: |
| (3) | Filing Party: |
| (4) | Date Filed: |
MYREXIS, INC.
305 CHIPETA WAY
SALT LAKE CITY, UTAH 84108
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS
| TIME: | 9:00 a.m., local time | |
| DATE: | January 23, 2013 | |
| PLACE: | The offices of Myrexis, Inc. located at 305 Chipeta Way, Salt Lake City, Utah 84108 | |
| PURPOSES: | ||
| 1. | To approve the dissolution and liquidation of the Company pursuant to the Plan of Complete Liquidation and Dissolution in the form attached to the accompanying proxy statement as Appendix A. |
| 2. | To grant discretionary authority to the Board of Directors to adjourn the Special Meeting, even if a quorum is present, to solicit additional proxies in the event that there are insufficient shares present in person or by proxy voting in favor of the dissolution and liquidation of the Company pursuant to the Plan of Complete Liquidation and Dissolution. |
| 3. | To approve, by non-binding, advisory vote, certain compensation arrangements for certain executive officers in connection with the dissolution and liquidation of the Company. |
| 4. | To transact such other business as may properly come before the Special Meeting or any postponements or adjournments thereof. |
WHO MAY VOTE:
You may vote if you were the record owner of Myrexis, Inc. common stock at the close of business on November 28, 2012. On or about December 26, 2012, we will begin sending this notice, the attached proxy statement and the enclosed proxy card to all stockholders entitled to vote at the Special Meeting. A list of stockholders of record will be available at the Special Meeting and, during the 10 days prior to the Special Meeting, at our principal executive offices located at 305 Chipeta Way, Salt Lake City, Utah 84108.
A copy of Myrexis’ annual report on Form 10-K for the year ended June 30, 2012, as amended by the Form 10-K/A filed on October 29, 2012, and a copy of our quarterly report on Form 10-Q for the quarter ended September 30, 2012, which contain important business and financial information about Myrexis and should be read carefully, are attached to this notice and proxy statement as Appendices B and C, respectively.
All stockholders are cordially invited to attend the Special Meeting. Whether you plan to attend the Special Meeting or not, please vote by completing, dating, signing and returning the enclosed proxy card, or by voting over the telephone or the Internet as instructed in these materials. You may change or revoke your proxy at any time before it is voted.
BY ORDER OF THE BOARD OF DIRECTORS
Andrea Kendell
Secretary
December 14, 2012
IMPORTANT NOTICE REGARDING THE AVAILABILITY OF PROXY MATERIALS FOR THE
SPECIAL STOCKHOLDER MEETING TO BE HELD ON JANUARY 23, 2013
This proxy statement is available for viewing, printing and downloading at
http://www.amstock.com/ProxyServices/ViewMaterial.asp?CoNumber=16182.
YOUR VOTE IS IMPORTANT. WHETHER OR NOT YOU EXPECT TO ATTEND THE SPECIAL MEETING, PLEASE COMPLETE, DATE AND SIGN THE ENCLOSED PROXY CARD AND MAIL IT PROMPTLY IN THE ENCLOSED POSTAGE-PREPAID ENVELOPE OR FOLLOW THE INSTRUCTIONS SET FORTH ON THE PROXY CARD TO SUBMIT YOUR VOTE BY PROXY OVER THE INTERNET OR BY TELEPHONE IN ORDER TO ASSURE REPRESENTATION OF YOUR SHARES. ADDITIONAL POSTAGE MAY BE REQUIRED IF THE PROXY CARD IS MAILED OUTSIDE OF THE UNITED STATES.
305 CHIPETA WAY
SALT LAKE CITY, UTAH 84108
801-214-7800
PROXY STATEMENT FOR THE MYREXIS, INC.
SPECIAL MEETING OF STOCKHOLDERS TO BE HELD ON JANUARY 23, 2013
Summary Term Sheet
The following is a summary of the most material terms of the Plan of Complete Liquidation and Dissolution of Myrexis, Inc. (the “Plan of Dissolution”) detailed in this proxy statement and attached as Appendix A. This summary does not contain all of the information that is important to you. We urge you to read this entire document (including the appendices) before you decide whether to vote to approve the Plan of Dissolution. As used in this proxy statement, unless the context otherwise requires, the terms “we,” “us,” “our,” “the Company,” and “Myrexis” refer to Myrexis, Inc., a Delaware corporation.
We are a biopharmaceutical company that has generated a pipeline of differentiated drug candidates in oncology and autoimmune diseases. We have no products approved for commercial sale and have incurred significant losses since our inception.
We were incorporated as Myriad Pharmaceuticals, Inc. in Delaware in January 2009, as a new, wholly owned subsidiary of Myriad Genetics, Inc. in order to effect the separation and spin-off of Myriad Genetics’ research and drug development businesses as a stand-alone, independent, publicly traded company. In connection with the formation of this new subsidiary, Myriad Genetics’ existing subsidiary, Myriad Pharmaceuticals, Inc., changed its corporate name to Myriad Therapeutics, Inc. On June 30, 2009, Myriad Genetics contributed substantially all of the assets and certain liabilities of its research and drug development businesses as well as $188 million in cash and marketable securities to us and effected the spin-off of our company through the pro rata dividend distribution to its stockholders of all outstanding shares of our common stock. Effective July 1, 2010, we changed our name from Myriad Pharmaceuticals, Inc. to Myrexis, Inc. Our principal executive offices are located at 305 Chipeta Way, Salt Lake City, Utah 84108, and our telephone number is 801-214-7800. All of our public filings with the Securities and Exchange Commission (the “SEC”), are accessible from our corporate website at www.myrexis.com. Information contained on the website is not a part of this proxy statement.
General (see page 17)
On November 9, 2012, our Board of Directors unanimously approved, subject to stockholder approval, the dissolution and liquidation of the Company pursuant to the Plan of Dissolution. A Special Meeting of our stockholders will be held at our principal executive offices located at 305 Chipeta Way, Salt Lake City, Utah, on Wednesday, January 23, 2013, at 9:00 a.m., local time. At the Special Meeting, you will be asked to consider and vote upon proposals to (1) approve the dissolution and liquidation of the Company pursuant to the Plan of Dissolution; (2) adjourn the Special Meeting, if necessary or appropriate, to permit further solicitation of proxies if there are not sufficient votes at the time of the Special Meeting to approve the dissolution and liquidation of the Company pursuant to the Plan of Dissolution (the “Meeting Adjournment Proposal”); (3) approve, by non-binding, advisory vote, certain compensation arrangements for certain executive officers in connection with the dissolution and liquidation of the Company; and (4) transact such other business as may properly come before the Special Meeting or any adjournments or postponements of the Special Meeting.
1
If the Plan of Dissolution is approved by our stockholders, we will file a Certificate of Dissolution with the Delaware Secretary of State dissolving Myrexis, Inc. Pursuant to the Delaware General Corporation Law (the “DGCL”), we will continue to exist for three years after our dissolution or for such longer period as the Delaware Court of Chancery shall direct, for the purpose of prosecuting and defending suits against us and enabling us gradually to close our business, to dispose of our property, to discharge our liabilities and to distribute to our stockholders any remaining assets. The proportionate interests of all of our stockholders will be fixed on the basis of their respective stock holdings at the close of business on the date we file the Certificate of Dissolution with the Delaware Secretary of State, which is referred to herein as the “Final Record Date.” We intend to delist our common stock from the NASDAQ Global Market and close our stock transfer books and discontinue recording transfers of shares of our common stock on the Final Record Date, and thereafter certificates representing shares of our common stock will not be assignable or transferable on our books except by will, intestate succession or operation of law. After the Final Record Date, any distributions made by us will be made solely to the stockholders of record as of the close of business on the Final Record Date, except as may be necessary to reflect subsequent transfers recorded on our books as a result of any assignments by will, intestate succession or operation of law.
Reasons for the Plan of Dissolution (see page 21)
Our Board of Directors believes that the dissolution and liquidation of the Company is advisable and in the best interests of the Company and our stockholders. Our Board of Directors considered at length, with the assistance of legal and financial advisors, potential strategic alternatives available to us, including the acquisition of one or more commercial-stage biopharmaceutical assets, a possible business combination with another company, and business development opportunities for each of our programs. In making its determination, our Board of Directors considered, in addition to other pertinent factors:
| • | the potential enhanced stockholder value that might be derived if we were to continue to pursue strategic alternatives to the development of our product candidates; |
| • | the risks, time and expenses associated with our ongoing business operations, including those associated with continuing to pursue strategic alternatives; |
| • | the fact that we have been actively exploring and evaluating strategic alternatives for some time and engaged Stifel Nicolaus Weisel as our financial advisor in February 2012 to assist in the evaluation of strategic options; and |
| • | the fact that our extensive efforts to identify and evaluate one or more strategic transactions that would have a reasonable likelihood of providing value to our stockholders in excess of the amount the stockholders would receive in a liquidation, or that would mitigate the risks of our ongoing operations, did not result in the identification of any likely transactions. |
Our Board of Directors concluded that dissolution and liquidation under Delaware law is the preferred strategy among the alternatives now available to us and is in the best interests of our stockholders. Accordingly, our Board of Directors unanimously approved the dissolution and liquidation of the Company pursuant to the Plan of Dissolution and recommends that our stockholders approve the dissolution and liquidation of the Company pursuant to the Plan of Dissolution.
Our Activities Following the Approval of the Plan of Dissolution (see page 24)
If the Plan of Dissolution is approved by the requisite vote of our stockholders, the steps set forth below will be completed at such times as our Board of Directors, in its discretion and in accordance with the DGCL, deems necessary, appropriate or advisable in our best interests and the best interests of our stockholders:
| • | the filing of a Certificate of Dissolution with the Delaware Secretary of State after obtaining a revenue clearance certificate from the Department of Finance; |
2
| • | the cessation of all of Myrexis’ business activities except those relating to winding up and liquidating Myrexis’ business and affairs, including, but not limited to, prosecuting and defending suits by or against us; |
| • | the collection, sale, exchange or other disposition of all or substantially all of Myrexis’ non-cash property and assets, in one transaction or in several transactions; |
| • | the payment of or the making of reasonable provision to pay all claims and obligations, including all contingent, conditional or un-matured contractual claims known to us; |
| • | the making of such provision as will be reasonably likely to be sufficient to provide compensation for any claim against us which is the subject of a pending action, suit or proceeding to which we are a party; |
| • | the making of such provision as will be reasonably likely to be sufficient to provide compensation for claims that have not been made known to us or that have not arisen but that, based on facts known to us, are likely to arise or become known to us within ten years after the date of dissolution; |
| • | the setting aside of a contingency reserve consisting of cash and/or property to satisfy such claims and contingent obligations of Myrexis; |
| • | the making of an initial liquidating distribution to our stockholders of record determined as of the Final Record Date; |
| • | the pro rata distribution to our stockholders, or the transfer to one or more liquidating trustees for the benefit of our stockholders under a liquidating trust, of the remaining assets of Myrexis after payment or provision for payment of claims against and obligations of Myrexis; and |
| • | the taking of any and all other actions permitted or required by the DGCL and any other applicable laws and regulations. |
Our Board of Directors may, to the full extent permitted by law, amend the Plan of Dissolution without any further stockholder approval if it determines that such amendment is in the best interest of our stockholders. In addition, if the Board of Directors determines that dissolution and liquidation are not in the best interests of our stockholders, the Board of Directors may direct that the Plan of Dissolution be abandoned, either before or after stockholder approval.
Sale of Our Remaining Assets (see page 27)
The Plan of Dissolution contemplates the sale of all of our remaining non-cash assets without further stockholder approval. Stockholder approval of the Plan of Dissolution will constitute approval of any and all such future asset dispositions on such terms as are approved by our Board of Directors in its sole discretion. The prices at which we will be able to sell our various assets will depend largely on factors beyond our control, including, without limitation, the condition of financial markets, the availability of financing to prospective purchasers of our assets, any required United States and foreign regulatory approvals, public market perceptions and limitations on transferability of certain assets.
Expected Distributions to Stockholders; Timing (see page 24)
As of October 31, 2012, we had approximately $84.9 million in cash, cash equivalents and marketable securities. We currently estimate that we will reserve between $7.0 million and $12.0 million, which will be used to pay all expenses (including operating expenses up until the filing of the Certificate of Dissolution) and other known, non-contingent liabilities, and which also includes reasonable provision for expenses of liquidation and contingent and unknown liabilities as required by Delaware law. Based on this estimated reserve, we currently estimate that the aggregate amount of an initial liquidating distribution to stockholders will be between $72.9 million and $77.9 million, or between $2.67 and $2.83 per share (based on 27,026,549 shares outstanding as of
3
December 6, 2012, plus an estimate of the number of options that will be exercised and the number of shares issuable upon the vesting of restricted stock units prior to the dissolution). We intend to make this initial distribution as soon as practicable following the filing of the Certificate of Dissolution, however, we are unable to predict the precise amount or timing of the initial distribution or of any additional liquidating distributions following the initial liquidating distribution. The timing and amount of the initial distribution and any such additional liquidating distributions will depend upon the actual expenses incurred, the timing of the resolution of matters for which we have established the contingency reserve, the amount to be paid in satisfaction of such contingencies, as well as our ability to convert our remaining assets to cash. Although our Board of Directors has not established a firm timetable for the liquidating distributions, subject to contingencies inherent in winding up our business, the Board of Directors intends to make such distributions as promptly as practicable. Subject to the requirements of Delaware law, we expect to make a final distribution prior to the third anniversary of the filing of the Certificate of Dissolution.
Due to the uncertainty of the value of our non-cash assets, we have not provided any estimate of the proceeds of a sale of such assets in the amount of liquidating distributions. Furthermore, we can provide no assurance that we will be able to sell any of our non-cash assets and receive additional proceeds for distribution. Many of the factors influencing the amount of cash distributed to our stockholders as a liquidating distribution cannot be currently quantified with certainty and are subject to change. The actual amounts of liquidating distributions may vary substantially, depending on whether we discover additional liabilities or claims, or if we incur unexpected or greater than expected losses with respect to contingent liabilities. Accordingly, you will not know the exact amount of any liquidating distributions you may receive as a result of the Plan of Dissolution when you vote on the proposal to approve the Plan of Dissolution. You may receive substantially less than the amount we currently estimate or that you otherwise expect to receive. See “Risk Factors to be Considered by Stockholders in Deciding Whether to Approve the Plan of Dissolution” below.
Contingent Liabilities; Contingency Reserve (see page 27)
In connection with our dissolution, we are required by Delaware law to pay or provide for payment of all of our liabilities and obligations, including making reasonable provision for the payment of contingent obligations. Following the date we file our Certificate of Dissolution with the Delaware Secretary of State, we will pay all expenses and other known liabilities and establish a reserve, consisting of cash or other assets, that we believe will be adequate for the satisfaction of all current contingent or conditional claims and liabilities. We also may take other steps we determine are reasonably calculated to provide for the satisfaction of the reasonably estimated amount of such liabilities, including possibly seeking to acquire insurance coverage with respect to certain contingent liabilities. We currently estimate that we will establish a contingency reserve of between $2.8 million and $6.5 million to satisfy contingent liabilities, including our obligation to indemnify Myriad Genetics with respect to the AIA Litigation described below. From time to time, we may distribute to our stockholders on a pro rata basis any portions of the contingency reserve that we deem no longer to be required. In the event we fail to create an adequate contingency reserve for the payment of our expenses and liabilities, and amounts have been distributed to the stockholders under the Plan of Dissolution, each stockholder could be held liable for the repayment to creditors of such stockholder’s pro rata portion of the shortfall out of the liquidating distributions received by such stockholder from us under the Plan of Dissolution.
Pursuant to our Separation and Distribution Agreement with Myriad Genetics, at the time of our separation from Myriad Genetics, we assumed liability for certain pending or threatened legal matters related to our business, and we are obligated to indemnify Myriad Genetics for any liability arising out of such matters, including any costs and expenses of litigating such matters, including payment of attorneys’ fees incurred to defend against claims. One such matter, a lawsuit brought by the Alzheimer’s Institute of America, Inc. (“AIA”) against Myriad Genetics and the Mayo Clinic Jacksonville and Mayo Foundation for Medical Education and Research (collectively, “Mayo”), asserts that Myriad Genetics and Mayo infringed certain patents allegedly owned by AIA in connection with Myriad Genetics’ research and development of its failed Alzheimer’s drug candidate Flurizan (hereinafter referred to as the “AIA Litigation”). Based on discussions with outside legal
4
counsel, including an independent law firm hired by the Board of Directors to review the case, we do not believe that the AIA Litigation will result in material liability for Myrexis. However, the costs of defending intellectual property litigation are substantial, and our Board recently authorized management to pursue a settlement of the AIA Litigation in order to reduce the portion of our otherwise distributable cash to our stockholders which would need to be set aside, if a dissolution and liquidation were pursued, in order to comply with the Company’s obligation to make reasonable provision for contingent obligations. Such settlement efforts have been unsuccessful, and accordingly, we have determined to reserve between $2.5 million and $5.0 million in connection with this ongoing legal proceeding, which the Board believes is a reasonable provision for this contingency. Based on a review and analysis of this matter by litigation counsel and independent counsel, we believe that, absent further developments, the current most likely outcome of this litigation will be a dismissal of the case and an unsuccessful appeal by AIA of that dismissal. Such an outcome would result in our ability to distribute a substantial portion of this reserve to our stockholders upon the final resolution of this case, which could occur within 18-24 months. However, there can be no assurance of the timing of such resolution or the ultimate outcome of the litigation. It is possible, therefore, that no portion of this reserve will ultimately be available for distribution, and it is even possible that a portion of the initial and any subsequent distributions to the stockholders in liquidation could be subject to repayment by the stockholders to satisfy a shortfall in the amount set aside for this contingency.
See “Risk Factors to be Considered by Stockholders in Deciding Whether to Approve the Plan of Dissolution.”
Treatment of Equity Awards and Employee Stock Purchase Plan (see page 30)
All outstanding restricted stock units and outstanding options, whether currently vested or unvested, will terminate immediately prior to our dissolution in accordance with the terms of our 2009 Employee, Director and Consultant Equity Incentive Plan, as amended (the “Equity Incentive Plan”), and we intend to terminate the Equity Incentive Plan effective upon our dissolution.
We have suspended the commencement of any future offering periods under our 2009 Employee Stock Purchase Plan (the “ESPP”) following completion of the offering period currently in effect, which ends on November 30, 2012, and intend to terminate the ESPP effective upon our dissolution.
Reporting Requirements (see page 30)
Whether or not the Plan of Dissolution is approved, we have an obligation to continue to comply with the applicable reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), even though compliance with such reporting requirements may be economically burdensome and of minimal value to our stockholders. If the Plan of Dissolution is approved by our stockholders, in order to curtail expenses, we intend to seek relief from the SEC to suspend our reporting obligations under the Exchange Act, and ultimately to terminate the registration of our common stock. However, the SEC may not grant us the requested relief.
Cessation of Trading of Common Stock; Closing of Stock Transfer Books (see page 29)
We anticipate that we will request that our common stock be delisted from the NASDAQ Global Market at the close of business on the date that we file the Certificate of Dissolution with the Delaware Secretary of State and that trading will be suspended on that date or as soon thereafter as is reasonably practicable. We also currently expect to close our stock transfer books at the close of business on the date that we file the Certificate of Dissolution with the Delaware Secretary of State and to discontinue recording transfers and issuing stock certificates (other than replacement certificates) at that time. Accordingly, it is expected that trading in our shares of common stock will cease after the date that we file the Certificate of Dissolution with the Delaware Secretary of State.
5
Certain Material U.S. Federal Income Tax Considerations (see page 37)
As described in “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Material United States Federal Income Tax Consequences of Our Dissolution and Liquidation” and subject to the limitations, assumptions and qualifications therein, amounts distributed to holders of our common stock pursuant to the Plan of Dissolution will be taxable to U.S. holders of our common stock for U.S. federal income tax purposes. We expect that such U.S. stockholders will realize taxable gain or loss on any liquidating distributions. Stockholders are urged to carefully review the discussion of tax matters within this proxy statement and to consult their own tax advisors as to the specific tax consequences to them of our dissolution and liquidation pursuant to the Plan of Dissolution.
Interests of Directors and Executive Officers in Approval of the Plan of Dissolution (see page 32)
In considering the recommendation of our Board of Directors, you should be aware that our directors and executive officers may have interests in the Plan of Dissolution that are different from or in addition to your interests as a stockholder and that may present actual or potential conflicts of interest. Our Board of Directors was aware of these interests and considered them, among other matters, in approving the Plan of Dissolution and the transactions contemplated thereby and in determining to recommend that Myrexis stockholders vote FOR the approval of the dissolution and liquidation of the Company pursuant to the Plan of Dissolution. You should consider these and other interests of our directors and executive officers that are described in this proxy statement.
Required Stockholder Vote (see page 38)
The approval of the dissolution and liquidation of the Company pursuant to the Plan of Dissolution requires the affirmative vote of a majority of the outstanding shares of our common stock. Abstentions and broker non-votes will have the same effect as votes against the proposal to approve the dissolution and liquidation of the Company pursuant to the Plan of Dissolution.
Recommendation of Our Board of Directors (see page 38)
On November 9, 2012, our Board of Directors unanimously: (1) determined that the dissolution and liquidation of the Company, and the other transactions contemplated thereby, are advisable and in the best interests of us and our stockholders, (2) approved in all respects the Plan of Dissolution and the other transactions contemplated thereby, and (3) recommended that our stockholders vote FOR the approval of the dissolution and liquidation of the Company pursuant to the Plan of Dissolution.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This proxy statement contains certain forward-looking statements. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995 and are including this statement for purposes of invoking these safe harbor provisions. Such forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause our actual results, performance or achievements, or industry results, to differ materially from our expectations of future results, performance or achievements expressed or implied by such forward-looking statements. These risks include the risk that we may incur additional liabilities, that we may have liabilities about which we are not currently aware, and that the cost of settlement of our liabilities and contingent obligations could be higher than expected, all of which would impact our ability to make any liquidating distributions to our stockholders. Although we believe that the expectations reflected in any forward-looking statements are reasonable, we cannot guarantee future events or results. Except as may be required under federal law, we undertake no obligation to update publicly any forward-looking statements for any reason, even if new information becomes available or other events occur.
6
QUESTIONS AND ANSWERS ABOUT THIS PROXY MATERIAL AND VOTING
Why am I receiving these materials?
We have sent you this proxy statement and the enclosed proxy card because the Board of Directors of Myrexis, Inc. is soliciting your proxy to vote at the Special Meeting. You are invited to attend the Special Meeting to vote on the proposals described in this proxy statement. However, you do not need to attend the meeting to vote your shares. Instead, you may simply complete, sign and return the enclosed proxy card, or follow the instructions below to submit your proxy over the telephone or on the Internet.
Who can vote at the Special Meeting?
Only stockholders of record at the close of business on November 28, 2012, the record date of the Special Meeting, will be entitled to vote at the Special Meeting. At the close of business on this record date, there were 27,011,631 shares of common stock outstanding and entitled to vote. If, at the close of business on November 28, 2012, your shares were registered directly in your name with our transfer agent, American Stock Transfer and Trust Company, then you are a stockholder of record. As a stockholder of record, you may vote in person at the meeting or vote by proxy. Whether or not you plan to attend the meeting, we urge you to fill out and return the enclosed proxy card or vote by proxy over the telephone or on the Internet as instructed below to ensure your vote is counted. If, at the close of business on November 28, 2012, your shares were held, not in your name, but rather in an account at a brokerage firm, bank, dealer, or other similar organization, then you are the beneficial owner of shares held in “street name” and these proxy materials are being forwarded to you by that organization. The organization holding your account is considered to be the stockholder of record for purposes of voting at the Special Meeting. As a beneficial owner, you have the right to direct your broker or other agent regarding how to vote the shares in your account. You are also invited to attend the Special Meeting. However, because you are not the stockholder of record, you may not vote your shares in person at the meeting unless you request and obtain a valid proxy from your broker or other agent.
What am I voting on?
You are voting on whether to approve the dissolution and liquidation of Myrexis pursuant to the Plan of Dissolution attached as Appendix A to this proxy statement. Additionally, stockholders will consider and vote on proposals to adjourn the Special Meeting to another date, time or place, if necessary in the judgment of the proxy holders, for the purpose of soliciting additional proxies to vote in favor of the proposed Plan of Dissolution and to approve, by non-binding, advisory vote, certain compensation arrangements for certain executive officers in connection with the dissolution and liquidation. Any other matter that is properly presented at the meeting will also be voted upon at that time.
How do I vote?
Whether you plan to attend the Special Meeting or not, we urge you to vote by proxy. All shares represented by valid proxies that we receive through this solicitation, and that are not revoked, will be voted in accordance with your instructions on the proxy card or as instructed via Internet or telephone. You may specify whether your shares should be voted for, against or abstain with respect to each of the proposals. Voting by proxy will not affect your right to attend the Special Meeting. If your shares are registered directly in your name through our stock transfer agent, American Stock Transfer and Trust Company, or you have stock certificates registered in your name, you may vote:
| • | By Internet or by telephone. Follow the instructions included in the proxy card, to vote by Internet or telephone. |
| • | By mail. You can vote by mail by completing, signing, dating and returning the proxy card as instructed on the card. If you sign the proxy card but do not specify how you want your shares voted, they will be voted in accordance with the recommendations of the Board of Directors as noted below. |
7
| • | In person at the meeting. If you attend the Special Meeting, you may deliver a completed proxy card in person or you may vote by completing a ballot, which will be available at the Special Meeting. |
Telephone and Internet voting facilities for stockholders of record will be available 24-hours a day and will close at 11:59 p.m. Eastern Time on January 22, 2013.
If your shares are held in “street name,” you will receive instructions from the holder of record. You must follow the instructions of the holder of record in order for your shares to be voted. Telephone and Internet voting also will be offered to stockholders owning shares through certain banks and brokers. If your shares are not registered in your own name and you plan to vote your shares in person at the meeting, you must contact your broker or agent to obtain a legal proxy or broker’s proxy card and bring it to the meeting in order to vote.
How many votes do I have?
On each matter to be voted upon, you have one vote for each share of our common stock you own as of November 28, 2012.
What if I return a proxy card but do not make specific choices?
If you return a signed and dated proxy card without marking any voting selections, your shares will be voted “FOR” the dissolution and liquidation of the Company pursuant to the Plan of Dissolution, “FOR” the Meeting Adjournment Proposal, and “FOR” the compensation arrangements of certain executive officers in connection with the dissolution and liquidation. If any other matter is properly presented at the meeting, your proxy holder (one of the individuals named on your proxy card) will vote your shares using his or her best judgment.
Who is paying for this proxy solicitation?
We will pay for the entire cost of soliciting proxies. In addition to these mailed proxy materials, our directors and employees may also solicit proxies in person, by telephone, or by other means of communication. Directors and employees will not be paid any additional compensation for soliciting proxies. We may also reimburse brokerage firms, banks and other agents for the cost of forwarding proxy materials to beneficial owners. In addition, we have engaged The Proxy Advisory Group, LLC, to assist in the solicitation of proxies and provide related advice and informational support, for a services fee and the reimbursement of customary disbursements, which in total is not expected to exceed $17,000.
What does it mean if I receive more than one proxy card?
If you receive more than one proxy card, your shares are registered in more than one name or are registered in different accounts. Please complete, sign and return each proxy card to ensure that all of your shares are voted.
Can I change my vote after submitting my proxy?
If you give us your proxy, you may change or revoke it at any time before the Special Meeting. You may change or revoke your proxy in any one of the following ways:
| • | by re-voting by Internet or by telephone as instructed above; |
| • | if you received printed proxy materials, by signing a new proxy card with a date later than your previously delivered proxy card and submitting it as instructed above; |
| • | by notifying our Secretary in writing before the Special Meeting that you have revoked your proxy; or |
| • | by attending the meeting in person and voting in person. Attending the meeting in person will not in and of itself revoke a previously submitted proxy unless you specifically request it. |
Your most current vote, whether by telephone, Internet or proxy card, is the one that will be counted.
8
What vote is required to approve each proposal and how are votes counted?
| Proposal 1: | Dissolution and Liquidation of the Company | The affirmative vote of a majority of our outstanding shares of common stock is required to approve the dissolution and liquidation of Myrexis. Banks and brokerage firms do not have authority to vote customers’ unvoted shares held by the firms in street name on this proposal. Broker non-votes and abstentions will have the same effect as a vote against this proposal. |
| Proposal 2: | Meeting Adjournment Proposal | The affirmative vote of a majority of the votes cast affirmatively or negatively for this proposal is required to approve the Meeting Adjournment Proposal. Abstentions will have no effect on the results of this vote. Banks and brokerage firms have authority to vote customers’ unvoted shares held by the firms in street name on this proposal. If a broker does not exercise this authority, such broker non-votes will have no effect on the results of this vote. |
| Proposal 3: | Non-binding, Advisory Vote on Certain Compensation Arrangements | The affirmative vote of a majority of the votes cast affirmatively or negatively for this proposal is required to approve the proposal relating to the non-binding, advisory vote on certain compensation arrangements for certain executive officers in connection with the dissolution and liquidation. Abstentions will have no effect on the results of this vote. Banks and brokerage firms do not have authority to vote customers’ unvoted shares held by the firms in street name on this proposal. Broker non-votes will have no effect on the results of this vote. |
Can I still sell my shares of Myrexis, Inc. common stock?
Yes, but only until the filing of our Certificate of Dissolution with the Delaware Secretary of State. Our common stock is listed on the NASDAQ Global Market. We anticipate that we will request that our common stock be delisted at the close of business on the date we file the Certificate of Dissolution and that trading will be suspended on that date or as soon thereafter as is reasonably practicable. In addition, we intend to close our stock transfer books and discontinue recording transfers of shares of our common stock at the close of business on the date we file the Certificate of Dissolution. Thereafter, certificates representing shares of our common stock will not be assignable or transferable on our books except by will, intestate succession or operation of law. See “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Description of the Plan of Dissolution and Dissolution Process—Listing and Trading of the Common Stock and Interests in the Liquidating Trust or Trusts.”
Questions and Answers Concerning the Plan of Dissolution
How does the Board of Directors recommend I vote on the proposal to approve the dissolution and liquidation of the Company pursuant to the Plan of Dissolution and the Meeting Adjournment Proposal?
The Board of Directors recommends that you vote FOR the authorization and approval of the dissolution and liquidation of the Company pursuant to the Plan of Dissolution and FOR the Meeting Adjournment Proposal.
9
Why is the Board of Directors recommending approval of the Plan of Dissolution?
In September 2011, we announced that we had completed an in-depth review of our drug development pipeline, incorporating extensive inputs from both internal and independent external analyses. As a result, we made a strategic business decision to suspend any further development of our lead drug candidate Azixa, which was in Phase 2 development for the treatment of advanced primary and metastatic tumors with brain involvement, as we concluded that completing the Phase 2b clinical trial we had underway would require a disproportionate investment of time and resources relative to its likelihood of technical and regulatory success. Following this decision, in November 2011, we announced a corporate reorganization to realign our resources with our development strategy and clinical initiatives following the suspension of further development of Azixa. The reorganization included an immediate reduction in our workforce by 15 employees or approximately 20%.
In February 2012, we announced that we had suspended development activity on all of our preclinical and clinical programs and retained Stifel Nicolaus Weisel, an investment banking firm, to assist in reviewing and evaluating a full range of strategic alternatives to enhance stockholder value. Despite these efforts, we have been unsuccessful in identifying and completing a strategic transaction that would have a reasonable likelihood of providing value to our stockholders in excess of the amount the stockholders would receive in a liquidation. Accordingly, our Board of Directors believes that the dissolution and liquidation of the Company is advisable and in the best interests of the Company and our stockholders. See “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Background of the Plan of Dissolution” and “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Reasons for the Plan of Dissolution.”
What will happen if the Plan of Dissolution is approved?
If the Plan of Dissolution is approved, we plan to file a Certificate of Dissolution with the Delaware Secretary of State. As of October 31, 2012, we had approximately $84.9 million in cash, cash equivalents and marketable securities. We currently estimate that we will reserve between $7.0 million and $12.0 million, which will be used to pay all expenses (including operating expenses up until the filing of the Certificate of Dissolution) and other known, non-contingent liabilities, and which also includes reasonable provision for expenses of liquidation and contingent and unknown liabilities as required by Delaware law. Based on this estimated reserve, we currently estimate that the aggregate amount of an initial liquidating distribution to stockholders will be between $72.9 million and $77.9 million, or between $2.67 and $2.83 per share (based on 27,026,549 shares outstanding as of December 6, 2012, plus an estimate of the number of options that will be exercised and the number of shares issuable upon the vesting of restricted stock units prior to the dissolution). We intend to make this initial distribution as soon as practicable following the filing of the Certificate of Dissolution, however, we are unable to predict the precise amount or timing of the initial distribution or of any additional liquidating distributions following the initial liquidating distribution. The timing and amount of the initial distribution and any such additional liquidating distributions will depend upon the actual expenses incurred, the timing of the resolution of matters for which we have established the contingency reserve, the amount to be paid in satisfaction of such contingencies, as well as our ability to convert our remaining assets to cash.
In addition to the satisfaction of our liabilities, we have used and anticipate continuing to use cash in the next several months for a number of items, including, but not limited to, the following:
| • | ongoing operating expenses; |
| • | expenses incurred in connection with extending our directors’ and officers’ insurance coverage; |
| • | expenses incurred in connection with the dissolution and liquidation process; |
| • | severance and related costs; and |
| • | professional, legal, consulting and accounting fees. |
We may, at any time, turn our management over to a third party to complete the liquidation of our remaining assets and distribute the available liquidation proceeds, if any, to our stockholders, pursuant to the
10
Plan of Dissolution, including an assignment for the benefit of creditors. This third-party management may be in the form of a liquidating trust, which, if adopted, would succeed to all of our assets, liabilities and obligations. Our Board of Directors may appoint one or more of its members, one or more of our officers or a third party to act as trustee or trustees of such liquidating trust. See “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Description of the Plan of Dissolution and Dissolution Process—Liquidating Trust.”
What is the total amount of the payments, if any, that stockholders will receive?
We currently estimate that the aggregate amount of an initial liquidating distribution to stockholders will be between $72.9 million and $77.9 million, or between $2.67 and $2.83 per share (based on 27,026,549 shares outstanding as of December 6, 2012 plus an estimate of the number of options that will be exercised and the number of shares issuable upon the vesting of restricted stock units prior to the dissolution). We intend to make this initial distribution as soon as practicable following the filing of the Certificate of Dissolution, however, we are unable to predict the precise amount or timing of the initial distribution or of any additional liquidating distributions following the initial liquidating distribution. The timing and amount of the initial distribution and any such additional liquidating distributions will depend upon the actual expenses incurred, the timing of the resolution of matters for which we have established the contingency reserve, the amount to be paid in satisfaction of such contingencies, as well as our ability to convert our remaining assets to cash.
Due to the uncertainty of the value of our non-cash assets, we have not provided any estimate of the proceeds of a sale of such assets in the amount of liquidating distributions. Furthermore, we can provide no assurance that we will be able to sell any of our non-cash assets and receive additional proceeds for distribution. Many of the factors influencing the amount of cash distributed to our stockholders as a liquidating distribution cannot be currently quantified with certainty and are subject to change. The actual amounts of liquidating distributions may vary substantially, depending on whether we discover additional liabilities or claims, or if we incur unexpected or greater than expected losses with respect to contingent liabilities. Accordingly, you will not know the exact amount of any liquidating distributions you may receive as a result of the Plan of Dissolution when you vote on the proposal to approve the Plan of Dissolution. You may receive substantially less than the amount we currently estimate or that you otherwise expect to receive. See “Risk Factors to be Considered by Stockholders in Deciding Whether to Approve the Plan of Dissolution” below.
When do you expect the dissolution process to be completed?
Upon approval of the Plan of Dissolution by our stockholders, we will work toward an orderly wind down of our business and operations. Subject to stockholder approval of the Plan of Dissolution, we currently expect to file a Certificate of Dissolution as soon as reasonably practicable following stockholder approval of the Plan of Dissolution and expect to have the majority of our business operations completed by that time. Additionally, pursuant to the Delaware law, our corporate existence will continue for a period of at least three years, but we would not be permitted to carry on any business except that appropriate to wind up and liquidate our business and affairs.
Should I send in my stock certificates now?
No. You should not forward your stock certificates before receiving instructions to do so. As a condition to the receipt of any distribution to the stockholders, we may, in our discretion, require stockholders to (i) surrender their certificates evidencing their shares of our common stock to us or (ii) furnish us with evidence satisfactory to us of the loss, theft or destruction of such certificates, together with such surety bond or other security or indemnity as may be required by and satisfactory to us. If surrender of stock certificates will be required following the dissolution, we will send you written instructions regarding such surrender. Any distributions otherwise payable by us to stockholders who have not surrendered their stock certificates, if requested to do so, may be held in trust for such stockholders, without interest, pending the surrender of such certificates (subject to escheat pursuant to the law relating to unclaimed property).
11
What are the material United States federal income tax consequences of the dissolution and liquidation?
Liquidating distributions received by our stockholders, if any, will be applied against and reduce each stockholder’s tax basis in such stockholder’s shares of stock. Gain will be recognized as a result of a liquidating distribution to the extent that the aggregate value of the distribution and any prior liquidating distributions received by a stockholder with respect to a share exceeds such stockholder’s basis for that share. A loss will generally be recognized if the aggregate value of all liquidating distributions with respect to a share is less than the stockholder’s tax basis for that share. Gain or loss recognized by a stockholder will be capital gain or loss provided the shares are held as capital assets, and will generally be long-term capital gain or loss if the stock has been held for more than one year. See “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Material United States Federal Income Tax Consequences of Our Dissolution and Liquidation.”
The tax consequences of the Plan of Dissolution may vary depending upon the particular circumstances of each stockholder. We recommend that each stockholder consult its own tax advisor regarding the federal income tax consequences of the Plan of Dissolution as well as the state, local and foreign tax consequences.
What will happen if the Plan of Dissolution is not approved?
If the Plan of Dissolution is not approved by our stockholders, the dissolution and liquidation of the Company will not occur and our Board of Directors and management will continue to explore other strategic alternatives. However, our management and Board of Directors have considered at length, with the assistance of legal and financial advisors, potential strategic alternatives available to us and have been unable to identify any alternative or transaction that they believe would have a reasonable likelihood of providing greater value to our stockholders than our stockholders would receive in a liquidation. It is possible that the Company would seek voluntary dissolution at a later time and potentially with diminished assets. In addition, we could cease operations, make an assignment for the benefit of creditors, turn the Company over to a third-party management company or liquidator or file for bankruptcy protection.
Do I have appraisal rights?
Under Delaware law, you do not have appraisal rights in connection with any of the proposals.
Who can help answer my questions?
If you would like additional copies, without charge, of this proxy statement or if you have questions about the Plan of Dissolution or the transactions contemplated thereby, including the procedures for voting your shares, you should contact The Proxy Advisory Group, who is assisting us in this matter, at 888-557-7699 (888-55PROXY).
12
Risk Factors to be Considered by Stockholders in Deciding Whether to Approve the Plan of Dissolution
There are many factors that our stockholders should consider when deciding whether to vote to approve the Plan of Dissolution. Such factors include those risk factors set forth below. You should carefully consider the following risk factors, together with all of the other information included in this proxy statement, before you decide whether to vote or instruct your vote to be cast to approve the proposals described in this proxy statement.
The amount we distribute to our stockholders in the initial liquidating distribution may be substantially less than the amount we currently estimate if the amounts of our liabilities, other obligations and expenses or claims against us are higher than we currently anticipate.
As of October 31, 2012, we had approximately $84.9 million in cash, cash equivalents and marketable securities. We currently estimate that we will reserve between $7.0 million and $12.0 million, which will be used to pay all expenses (including operating expenses up until the filing of the Certificate of Dissolution) and other known, non-contingent liabilities, and which also includes reasonable provision for expenses of liquidation and contingent and unknown liabilities as required by Delaware law. Based on this estimated reserve, we currently estimate that the aggregate amount of an initial liquidating distribution to stockholders will be between $72.9 million and $77.9 million, or between $2.67 and $2.83 per share (based on 27,026,549 shares outstanding as of December 6, 2012, plus an estimate of the number of options that will be exercised and the number of shares issuable upon the vesting of restricted stock units prior to the dissolution). The amount of cash ultimately distributed to our stockholders in the initial liquidating distribution depends on the amount of our liabilities, obligations and expenses and claims against us, and contingency reserves that we establish during the liquidation process. We have attempted to estimate reasonable reserves for such liabilities, obligations, expenses and claims against us. However, those estimates may be inaccurate. Factors that could impact our estimates include the following:
| • | if any of the estimates regarding the Plan of Dissolution, including the expenses to satisfy outstanding obligations, liabilities and claims during the liquidation process, are inaccurate; |
| • | if unforeseen claims are asserted against us, we will have to defend or resolve such claims or establish a reasonable reserve before making distributions to our stockholders; and |
| • | if the estimates regarding the expenses to be incurred in the liquidation process, including expenses of personnel required and other operating expenses (including legal, accounting and other professional fees) necessary to dissolve and liquidate the Company, are inaccurate. |
If any of the foregoing occurs, the amount we initially distribute to our stockholders may be substantially less than the amount we currently estimate.
We cannot assure you of the exact amount or timing of any additional liquidating distributions to our stockholders under the Plan of Dissolution.
The dissolution and liquidation process is subject to numerous uncertainties, and may not result in any remaining capital for additional liquidating distributions to our stockholders following the initial liquidating distribution. The precise amount and timing of any additional liquidating distribution to our stockholders will depend on and could be delayed by, among other things, sales of our non-cash assets, claim settlements with creditors, resolution of the AIA Litigation with respect to which we have an obligation to indemnify our former parent, Myriad Genetics, and unexpected or greater than expected expenses.
If our stockholders vote against the Plan of Dissolution, it would be very difficult for us to continue our business operations.
If our stockholders do not approve the Plan of Dissolution, we would have to continue our business operations from a difficult position, in light of our announced intent to dissolve and liquidate. We are not actively conducting any clinical development programs and have generally ceased normal business operations and
13
terminated all but ten of our employees. Prospective employees, vendors and other third parties may refuse to form relationships or conduct business with us if they do not believe we will continue to operate as a going concern.
Our Board of Directors may abandon or delay implementation of the Plan of Dissolution even if approved by our stockholders.
Even if our stockholders approve the Plan of Dissolution, our Board of Directors has reserved the right, in its discretion, to the extent permitted by Delaware law, to abandon or delay implementation of the Plan of Dissolution if such action is determined to be in the best interests of our stockholders, in order, for example, to permit us to pursue new business opportunities or strategic transactions.
The payment of liquidating distributions, if any, to our stockholders could be delayed.
Although our Board of Directors has not established a firm timetable for liquidating distributions to our stockholders, the Board of Directors intends, subject to contingencies inherent in winding down our business, to make such liquidating distributions, if any, as promptly as practicable as creditor claims and contingent liabilities are paid or settled. However, we are currently unable to predict the precise timing of any such liquidating distributions or whether any liquidating distributions will occur at all. The timing of any such liquidating distributions will depend on and could be delayed by, among other things, the timing of sales of our non-cash assets and claim settlements with creditors. Additionally, a creditor could seek an injunction against the making of such distributions to our stockholders on the ground that the amounts to be distributed were needed to provide for the payment of our liabilities and expenses. Any action of this type could delay or substantially diminish the amount available for such distribution to our stockholders.
We will continue to incur claims, liabilities and expenses that will reduce the amount available for distribution out of the liquidation to stockholders.
Claims, liabilities and expenses from operations, such as operating costs, salaries, directors’ and officers’ insurance, payroll and local taxes, legal, accounting and consulting fees and miscellaneous office expenses, will continue to be incurred as we wind down. These expenses will reduce the amount of assets available for ultimate distribution to stockholders.
If we fail to create an adequate contingency reserve for payment of our expenses and liabilities, each stockholder receiving liquidating distributions could be held liable for payment to our creditors of his, her or its pro rata share of amounts owed to creditors in excess of the contingency reserve, up to the amount actually distributed to such stockholder in dissolution.
If the Plan of Dissolution is approved by our stockholders, we will file a Certificate of Dissolution with the Delaware Secretary of State dissolving Myrexis, Inc. Pursuant to the DGCL, we will continue to exist for three years after our dissolution or for such longer period as the Delaware Court of Chancery shall direct, for the purpose of prosecuting and defending suits against us and enabling us gradually to close our business, to dispose of our property, to discharge our liabilities and to distribute to our stockholders any remaining assets. Under the DGCL, in the event we fail to create during this three-year period an adequate contingency reserve for payment of our expenses and liabilities, each stockholder of record as of the Final Record Date could be held liable for payment to our creditors of such stockholder’s pro rata share of amounts owed to creditors in excess of the contingency reserve, up to the amount actually distributed to such stockholder in dissolution.
Although the liability of any stockholder is limited to the amounts previously received by such stockholder from us (and from any liquidating trust or trusts) pursuant to the Plan of Dissolution, this means that a stockholder could be required to return all liquidating distributions previously made to such stockholder and receive nothing from us under the Plan of Dissolution. Moreover, in the event a stockholder has paid taxes on
14
amounts previously received, a repayment of all or a portion of such amount could result in a stockholder incurring a net tax cost if the stockholder’s repayment of an amount previously distributed does not cause a commensurate reduction in taxes payable. While we will endeavor to make adequate reserves for all known, contingent, and unknown liabilities, there is no guarantee that the reserves established by us will be adequate to cover all such expenses and liabilities.
No further stockholder approval will be required.
Approval of the Plan of Dissolution and the actions contemplated thereby requires the affirmative vote of a majority of our outstanding shares of common stock. If our stockholders approve the Plan of Dissolution, we will be authorized to cease operations, sell, license or otherwise dispose of our non-cash assets and dissolve the Company without further approval of our stockholders, unless required to do so by Delaware law.
We intend to have our common stock delisted from the NASDAQ Global Market and our stock transfer books closed at the close of business on the date we file the Certificate of Dissolution with the Delaware Secretary of State, after which it will not be possible for stockholders to publicly trade our stock.
We will request that our common stock be delisted from the NASDAQ Global Market at the close of business on the date we file the Certificate of Dissolution with the Delaware Secretary of State and intend to close our stock transfer books and discontinue recording transfers of our common stock at that time. Thereafter, certificates representing our common stock will not be assignable or transferable on our books except by will, intestate succession or operation of law. The proportionate interests of all of our stockholders will be fixed on the basis of their respective stock holdings at the close of business on the Final Record Date, and, after the Final Record Date, any distributions made by us will be made solely to the stockholders of record at the close of business on the Final Record Date, except as may be necessary to reflect subsequent transfers recorded on our books as a result of any assignments by will, intestate succession or operation of law. It is possible that the trading of our common stock on the NASDAQ Global Market will effectively terminate before the Final Record Date if we are unable to meet NASDAQ’s requirements for continued listing.
We will continue to incur the expenses of complying with public company reporting requirements.
We have an obligation to continue to comply with the applicable reporting requirements of the Exchange Act even though compliance with such reporting requirements is economically burdensome. In order to curtail expenses, we intend, after filing our Certificate of Dissolution, to seek relief from the SEC from the reporting requirements under the Exchange Act. However, the SEC may not grant any such relief, in which case we would be required to continue to bear the expense of being a public reporting company until at least the filing of our Annual Report on Form 10-K for the fiscal year ending June 30, 2013.
Our Board of Directors may at any time turn management of the liquidation over to a third party, and some or all of our directors may resign from our Board of Directors at any time.
Our Board of Directors may at any time turn our management over to a third party to complete the liquidation of our remaining assets and distribute the available proceeds to our stockholders, and a number of our directors have indicated that they expect to resign from our Board of Directors prior to or in connection with the filing of the Certificate of Dissolution, while others may resign at any time. If management is turned over to a third party and all of our directors resign from our Board of Directors, the third party would have sole control over the liquidation process, including the sale or distribution of any remaining assets.
If we decide to use a liquidating trust, interests of our stockholders in such a trust may not be transferable.
The interests of our stockholders in a liquidating trust set up by us may not be transferable, which could adversely affect your ability to realize the value of such interests. Even if transferable, the interests are not expected to be listed on a national securities exchange, and the extent of any trading market therein cannot be
15
predicted. Moreover, the interests may not be accepted by commercial lenders as security for loans as readily as more conventional securities with established trading markets. In addition, as stockholders will be deemed to have received a liquidating distribution equal to their pro rata share of the value of the net assets distributed to an entity which is treated as a liquidating trust for tax purposes, the distribution of non-transferable interests would result in tax liability to the interest holders without their being readily able to realize the value of such interest to pay such taxes or otherwise.
Stockholders may not be able to recognize a loss for U.S. federal income tax purposes until they receive a final distribution from us.
As a result of our dissolution and liquidation, for U.S. federal income tax purposes, our stockholders generally will recognize gain or loss equal to the difference between (1) the sum of the amount of cash and the fair market value (at the time of distribution) of property, if any, distributed to them, and (2) their tax basis for their shares of our common stock. Liquidating distributions pursuant to the Plan of Dissolution may occur at various times and in more than one tax year. Any loss generally will be recognized by a stockholder only when the stockholder receives our final liquidating distribution to stockholders, and then only if the aggregate value of all liquidating distributions with respect to a share is less than the stockholder’s tax basis for that share. Stockholders are urged to consult their own tax advisors as to the specific tax consequences to them of our dissolution and liquidation pursuant to the Plan of Dissolution. See “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Material United States Federal Income Tax Consequences of Our Dissolution and Liquidation.”
16
APPROVAL OF THE PLAN OF COMPLETE LIQUIDATION AND DISSOLUTION
Our Board of Directors is presenting the Plan of Dissolution for approval by our stockholders at the Special Meeting. The Plan of Dissolution was unanimously approved by the Board of Directors, subject to stockholder approval, on November 9, 2012. A copy of the Plan of Dissolution is attached as Appendix A to this proxy statement. All material features of the Plan of Dissolution are summarized below. We encourage you to read the Plan of Dissolution in its entirety.
Summary of the Plan of Dissolution and Dissolution Process
After stockholder approval of the Plan of Dissolution, our activities will be limited to:
| • | filing a Certificate of Dissolution with the Delaware Secretary of State and thereafter remaining in existence as a non-operating entity; |
| • | paying all of our known obligations and liabilities; |
| • | establishing a contingency reserve, consisting of cash or other assets, that we believe will be adequate for the satisfaction of all contingent or conditional claims and liabilities, including the AIA Litigation, for which we have an obligation to indemnify our former parent, Myriad Genetics; |
| • | making an initial liquidating distribution to our stockholders of record determined as of the Final Record Date; |
| • | attempting to convert, sell or otherwise dispose of all of our remaining non-cash assets for cash or cash equivalents in an orderly fashion; |
| • | terminating any of our remaining commercial agreements, relationships or outstanding obligations; |
| • | paying operating and liquidation expenses and satisfying any contingent liabilities as they become due out of funds available in the contingency reserve; |
| • | distributing pro rata in one or more additional liquidating distributions all of our remaining assets, if any, to our stockholders of record as of the Final Record Date; |
| • | complying with SEC reporting requirements, as necessary; and |
| • | completing tax filings. |
Delaware law provides that, following the approval of the Plan of Dissolution by our stockholders, the Board of Directors may take such actions as it deems necessary in furtherance of the dissolution of the Company and the winding up of its operations and affairs.
As of October 31, 2012, we had approximately $84.9 million in cash, cash equivalents and marketable securities. We currently estimate that we will reserve between $7.0 million and $12.0 million, which will be used to pay all expenses (including operating expenses up until the filing of the Certificate of Dissolution) and other known, non-contingent liabilities, and which also includes reasonable provision for expenses of liquidation and contingent and unknown liabilities as required by Delaware law. Based on this estimated reserve, we currently estimate that the aggregate amount of an initial liquidating distribution to stockholders will be between $72.9 million and $77.9 million, or between $2.67 and $2.83 per share (based on 27,026,549 shares outstanding as of December 6, 2012, plus an estimate of the number of options that will be exercised and the number of shares issuable upon the vesting of restricted stock units prior to the dissolution). We intend to make this initial distribution as soon as practicable following the filing of the Certificate of Dissolution, however, we are unable
17
to predict the precise amount or timing of the initial distribution or of any additional liquidating distributions following the initial liquidating distribution. The timing and amount of the initial distribution and any such additional liquidating distributions will depend upon the actual expenses incurred, the timing of the resolution of matters for which we have established the contingency reserve, the amount to be paid in satisfaction of such contingencies, as well as our ability to convert our remaining assets to cash. Any liquidating distributions from us will be made to stockholders according to their holdings of common stock as of the Final Record Date, which shall be the date on which we close our stock transfer books and discontinue recording transfers of our common stock except for transfers by will, intestate succession or operation of law.
In addition to the satisfaction of liabilities, we have used and anticipate continuing to use cash in the next several months for a number of items, including, but not limited to, the following:
| • | ongoing operating expenses up until the filing of the Certificate of Dissolution; |
| • | expenses incurred in connection with extending our directors’ and officers’ insurance coverage; |
| • | expenses incurred in connection with the dissolution and liquidation; |
| • | severance and related costs; and |
| • | professional, legal, consulting and accounting fees. |
We may, at any time, turn our management over to a third party to complete the liquidation of our remaining assets and distribute the proceeds from the sale of assets to our stockholders pursuant to the Plan of Dissolution, including an assignment for the benefit of creditors. This third-party management may also be in the form of a liquidating trust, which, if adopted, would succeed to all of our assets, liabilities and obligations. Our Board of Directors may appoint one or more of its members, one or more of our officers, or a third party to act as trustee or trustees of such liquidating trust. If all of our assets are not distributed within three years after the date our dissolution, we expect to transfer our remaining assets to a liquidating trust at such time.
During the liquidation of our assets, we may pay our officers, directors, employees and agents, or any of them, compensation for services rendered in connection with the implementation of the Plan of Dissolution. See “Interests of Directors and Executive Officers in Approval of the Plan of Dissolution.” Such compensation is not expected to be materially different from the compensation that would be paid to an outside party for similar services.
Background of the Plan of Dissolution
On June 30, 2009, in order to effect the separation and spin-off of the research and drug development businesses of Myriad Genetics as a stand-alone, independent, publicly traded company, Myriad Genetics contributed to us substantially all of the assets and certain liabilities of its research and drug development businesses as well as approximately $188 million in cash and marketable securities, subject to an allocation to us of certain liabilities and obligations, and effected the spin-off of our company through the pro rata dividend distribution to its stockholders of shares of our common stock. At the time, Myriad Genetics stated that its Board of Directors believed that the separation of its research and drug development businesses from its molecular diagnostic business was the best way to unlock the full value of Myriad Genetics’ businesses.
Following the spin-off, as a biopharmaceutical company we continued to carry out research and development of our pipeline of differentiated drug candidates in oncology and autoimmune diseases, including Azixa, our lead drug candidate, for the treatment of advanced primary and metastatic tumors. In 2009, we initiated a 56-patient, open-label Phase 2 clinical trial to evaluate Azixa as monotherapy in patients with recurrence of Glioblastoma multiforme, or GBM, an aggressive type of brain cancer, and in December of 2010, we initiated a two-arm Phase 2b trial of Azixa in patients newly diagnosed with GBM. Following the spin-off, we also continued to carry out research and development activities with respect to our HIV maturation inhibitor programs and three other clinical-stage and preclinical drug candidates. We suspended our HIV maturation inhibitor programs in June 2010 for strategic and business reasons.
18
In the latter half of 2009, while we were continuing our research and drug development activities, after extensive due diligence and analysis, we pursued an opportunity to acquire another company, Javelin Pharmaceuticals, Inc. (“Javelin”). Javelin had recently submitted a New Drug Application to the U.S. Food and Drug Administration, for its drug candidate Dyloject for the management of acute, moderate to severe pain in adults. Our objectives in seeking to acquire Javelin were to provide an accelerated path to commercialization; enhance our development pipeline to include early, mid- and late-stage product candidates; use a portion of our cash resources to build a focused commercial infrastructure to support and accelerate commercial opportunities; and acquire an anticipated source of relatively near-term product revenues that could generate ongoing funding for our research and development activities. The opportunity to acquire Javelin had arisen earlier as the result of Javelin’s own attempts to market itself and its product portfolio, among other possible strategic courses of action. After negotiation, on December 18, 2009, we entered into a merger agreement to acquire Javelin. However, effective April 16, 2010, Javelin terminated the merger agreement based on its Board of Directors’ exercise of a fiduciary right of termination to accept a superior proposal from another party. We received the termination fee and a stipulated expense reimbursement which were provided for under the merger agreement in such a circumstance, as well as repayment of amounts which we had loaned to Javelin and which Javelin owed to us under a loan and security agreement.
In August 2011, we began an in-depth review of our drug development pipeline in order to objectively assess the technical, regulatory and economic potential of each of our drug development programs and to facilitate an effective allocation of the Company’s resources. This review incorporated both internal and independent external analyses of preclinical and clinical data, and clinical trial designs, including the trial size and access to patients, required trial endpoints and estimated costs. In addition, we reviewed regulatory requirements, evolving competitive environments and potential market sizes for each of our development programs, and held discussions with key opinion leaders. The review was completed in September 2011, and on the basis of the inputs from the review, our Board of Directors made a strategic business decision to suspend any further development of Azixa in order to reallocate resources towards advancing lead candidates from earlier stage programs through preclinical and clinical development. We concluded that completing the Phase 2b clinical trial for Azixa would require a disproportionate investment of time and resources relative to the likelihood of technical and regulatory success.
Following this decision, in November 2011, we announced a corporate reorganization to realign our resources with our development strategy and clinical initiatives following the suspension of further development of Azixa. The reorganization included an immediate reduction in our workforce by 15 employees, or approximately 20%.
In February 2012, following further consideration by our management and Board of Directors of our remaining preclinical and clinical programs, including review of in-depth analyses by external consultants, we announced that we had suspended development activities on all of our clinical and preclinical programs and that we had retained Stifel Nicolaus Weisel (“Stifel”), an investment banking firm, to assist in reviewing and evaluating a full range of strategic alternatives to enhance stockholder value. In March 2012, we initiated a further alignment of our resources, involving a phased reduction in our workforce from approximately 59 employees to our ten current employees.
On March 29, 2012, our Board of Directors adopted a Tax Benefits Preservation Rights Plan (the “Rights Agreement”) designed to help protect and preserve the Company’s substantial tax attributes primarily associated with net operating loss carryforwards (“NOLs”) and research tax credits, under Section 382 and 383 of the Internal Revenue Code (the “Code”). The Rights Agreement replaced the Shareholder Rights Plan that the Company adopted in 2009, which the Board of Directors terminated immediately prior to the adoption of the Rights Agreement. In order to maintain our ability to realize value from, and otherwise preserve and utilize, our NOLs and research tax credits, which the Rights Agreement was designed to protect, we have continued to monitor the likelihood of an “ownership change” of the Company under Sections 382 and 383 of the Code, which would substantially limit our ability to generate a tax benefit through the use of these tax attributes.
19
Based on an evaluation in March and April 2012 of our strategic alternatives, our Board of Directors determined to pursue the acquisition of one or more commercial-stage biopharmaceutical assets, with the goal of building a commercial-stage biopharmaceutical company by optimizing their performance and profitability. Integral to these efforts, on May 11, 2012, we announced a change in management, including the hiring of Richard B. Brewer as our President and Chief Executive Officer and David W. Gryska as our Chief Operating Officer, who brought an extensive record of acquiring, commercializing and marketing pharmaceutical products. In addition, they were appointed as members of our Board of Directors. Messrs. Brewer and Gryska, with the assistance of Stifel, proceeded to canvas the market for available commercial-stage biopharmaceutical products, eventually identifying a limited number of acquisition candidates to analyze in greater depth and to discuss with their respective owners. This process continued throughout the spring and summer of 2012, including explorations, evaluations and discussions regarding several potential candidates. Our Board of Directors received progress reports during this period, including at its regularly scheduled meeting in June 2012.
On August 15, 2012, we announced the death of Mr. Brewer and that our Board of Directors had appointed Mr. Gryska as acting President and Chief Executive Officer. Mr. Gryska thereafter, with the assistance of Stifel and outside consultants, continued to seek to identify and evaluate acquisition candidates and to discuss internally and with them possible acquisition transactions.
On September 21, 2012, our Board of Directors met and received reports from representatives of Stifel and from Mr. Gryska regarding the efforts to identify and pursue commercial-stage biopharmaceutical asset acquisition candidates, as well as other business combination transactional possibilities of which they had become aware in the course of carrying out their efforts to pursue our strategy. Having received such reports and advice from our financial and legal advisors, our Board of Directors determined that the current strategic course had not yet yielded a transaction that the Board believed would be in the best interests of the Company and its stockholders to pursue, and therefore, the full range of strategic alternatives should be reviewed again, including but not limited to continued pursuit of an acquisition transaction, a voluntary dissolution and liquidation of the Company, the possible sale of the Company, and any other transaction, whether initiated by us or by another party, which would maximize value for our stockholders.
Thereafter, with the assistance of Stifel and through management’s continuing efforts, we continued to analyze our possible strategic courses, including voluntary dissolution and liquidation but without prejudice to any other alternative which would confer greater value on our stockholders. Our Board held several telephonic meetings during this period in which it received updates from management and advice from its legal counsel on the process of dissolution and liquidation of the Company if the Board were to determine such course to be in the best interests of the Company and its stockholders. Included in our consideration of the alternative of voluntary dissolution and liquidation has been a careful analysis of the Company’s obligation pursuant to the Delaware General Corporation Law to make reasonable provision, if a dissolution and liquidation were pursued, for outstanding, contingent and unknown obligations. In particular, as previously disclosed, pursuant to our Separation and Distribution Agreement with Myriad Genetics, at the time of our separation from Myriad Genetics, we assumed liability for certain pending or threatened legal matters related to our business, including the AIA Litigation. Although we do not believe that any obligation we assumed under the Separation and Distribution Agreement, including the AIA Litigation, will ultimately result in a material liability, the costs of defending intellectual property litigation are substantial. Accordingly, our Board recently authorized management to pursue a settlement of the AIA Litigation in order to reduce the portion of our otherwise distributable cash to our stockholders which would need to be set aside, if a dissolution and liquidation were pursued, in order to comply with the Company’s obligation to make reasonable provision for contingent obligations. Such settlement efforts have been unsuccessful, and accordingly, we have included a reserve for the AIA Litigation that we believe makes reasonable provision for this contingency. Based on a review and analysis of this matter by litigation counsel and independent counsel, we believe that, absent further developments, the current most likely outcome of this litigation will be a dismissal of the case and an unsuccessful appeal by AIA
20
of that dismissal. Such an outcome would result in our ability to distribute a substantial portion of this reserve to our stockholders upon the final resolution of this case, which could occur within 18-24 months. However, there can be no assurance of the timing of such resolution or the ultimate outcome of the litigation. It is possible, therefore, that no portion of this reserve will ultimately be available for distribution, and it is even possible that a portion of the initial and any subsequent distributions to the stockholders in liquidation could be subject to repayment by the stockholders to satisfy a shortfall in the amount set aside for this contingency.
On November 8, 2012, our Board of Directors held a meeting for the purpose of considering the dissolution and liquidation of the Company pursuant to the Plan of Dissolution and other alternatives available to us. Also present at the meeting were representatives of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., our outside legal counsel (“Mintz Levin”). Mintz Levin reviewed with the Board of Directors the fiduciary duties of the Board of Directors, the extensive process Myrexis had undertaken in connection with its review of strategic alternatives and the terms of the proposed Plan of Dissolution. Management presented its analysis of the alternatives available to dissolution and liquidation, noting that the Company had been unsuccessful in identifying and completing a strategic transaction that would have a reasonable likelihood of providing value to stockholders in excess of the amount the stockholders would receive in a liquidation. Management also presented its analysis of the net assets it believed would be available for distribution to stockholders pursuant to the Plan of Dissolution.
After discussion, the Board of Directors determined that the dissolution and liquidation of the Company were advisable and in the best interests of Myrexis and its stockholders. Because one of the directors was unable to attend the meeting, the dissolution and liquidation of the Company pursuant to the Plan of Dissolution was unanimously approved by the Board of Directors, subject to stockholder approval, via written consent on November 9, 2012.
If, prior to the dissolution, the Company receives an offer for a transaction that will, in the view of the Board, provide superior value to stockholders than the value of the estimated distributions under the Plan of Dissolution, taking into account all factors that could affect valuation, including timing and certainty of payment or closing, credit market risks, proposed terms and other factors, the dissolution and liquidation pursuant to the Plan of Dissolution could be abandoned in favor of such a transaction.
Reasons for the Plan of Dissolution
In arriving at its determination that the Plan of Dissolution is advisable and in the best interests of the Company and its stockholders and is the preferred strategic option for Myrexis, our Board of Directors carefully considered the terms of the Plan of Dissolution and the dissolution and liquidation process under Delaware law, as well as other available strategic alternatives. As part of the evaluation process, the Board of Directors considered the risks and timing of each alternative available to Myrexis and consulted with management and our legal and financial advisors. In approving the Plan of Dissolution, our Board of Directors considered several of the factors set out above as well as the following factors:
| • | the fact that we have engaged Stifel Nicolaus Weisel as our financial advisor since February 2012 to assist in reviewing and evaluating a full range of strategic alternatives to enhance stockholder value, and despite these efforts, we have been unsuccessful in identifying and completing a strategic transaction that would have a reasonable likelihood of providing value to our stockholders in excess of the amount the stockholders would receive in a liquidation; |
| • | the fact that, in addition to the efforts made by Stifel Nicolaus Weisel, our management actively canvassed the market for possible transactions and evaluated numerous potential transactions with the help of our financial advisors and other consultants, however, these efforts also failed to identify a potential strategic transaction that would have a reasonable likelihood of providing value to our stockholders in excess of the amount the stockholders would receive in a liquidation; |
21
| • | the death of Richard B. Brewer, our President and Chief Executive Officer, in August 2012, who would have had an instrumental role in the execution of any strategic acquisition; |
| • | the low probability that we would be presented with, or otherwise identify, within a reasonable period of time under current circumstances, any viable opportunities to engage in an attractive alternative strategic transaction that would provide value to our stockholders; |
| • | the substantial accounting, legal and other expenses associated with being a publicly traded company; |
| • | the terms and conditions of the Plan of Dissolution, including the provisions that permit our Board of Directors to revoke the plan if our Board of Directors determines that, in light of any new proposals presented or changes in circumstances, dissolution and liquidation are no longer advisable and in our best interests and the best interests of our stockholders; |
| • | the fact that Delaware law requires that the Plan of Dissolution be approved by the affirmative vote of holders of a majority of the outstanding shares of our common stock, which ensures that our Board of Directors will not be taking actions of which a significant portion of our stockholders disapprove; and |
| • | the fact that approval of the Plan of Dissolution by the requisite vote of our stockholders authorizes our Board of Directors and officers to implement the Plan of Dissolution without further stockholder approval. |
Our Board of Directors also considered the following negative factors in arriving at its conclusion that dissolving and liquidating Myrexis is in the best interests of the Company and its stockholders:
| • | the uncertainty of the timing and amount of any liquidating distributions to stockholders; |
| • | the risks associated with the sale of our remaining non-cash assets as part of the Plan of Dissolution; |
| • | that a strategic transaction could possibly provide greater value to our stockholders than a liquidation; |
| • | the risk that, under Delaware law, our stockholders may be required to return to creditors some or all of the liquidating distributions; and |
| • | the fact that, if the Plan of Dissolution is approved by our stockholders, stockholders would generally not be permitted to transfer shares of our common stock after the Final Record Date. |
Our Board of Directors also considered the other factors described in the section entitled “Risk Factors to be Considered by Stockholders in Deciding Whether to Approve the Plan of Dissolution” in this proxy statement in deciding to approve, and recommend that our stockholders approve, the dissolution and liquidation of the Company pursuant to the Plan of Dissolution.
In view of the variety of factors considered in connection with its evaluation of the Plan of Dissolution, our Board of Directors did not find it practical, and did not quantify or otherwise attempt, to assign relative weight to the specific factors considered in reaching its conclusions. In addition, our Board of Directors did not undertake to make any specific determination as to whether any particular factor, or any aspect of any particular factor, was favorable or unfavorable to its ultimate determination, but rather conducted an overall analysis of the factors described above. In considering the factors described above, individual directors may have given different weight to different factors.
We cannot offer any assurance that the liquidation value per share of our common stock will equal or exceed the price or prices at which such shares recently have traded or could trade in the future. However, our Board of Directors believes that it is in the best interests of the Company and its stockholders to distribute to the stockholders our net assets pursuant to the Plan of Dissolution.
If the dissolution and liquidation of the Company pursuant to the Plan of Dissolution is not approved by our stockholders, the dissolution and liquidation of the Company will not occur and our Board of Directors and
22
management will continue to explore other strategic alternatives. However, our management and Board of Directors have considered at length, with the assistance of legal and financial advisors, potential strategic alternatives available to us and have been unable to identify any alternative or transaction that they believe would have a reasonable likelihood of providing greater value to our stockholders than our stockholders would receive in a liquidation. It is possible that the Company would seek voluntary dissolution at a later time and potentially with diminished assets. In addition, we could cease operations, make an assignment for the benefit of creditors, turn the Company over to a third-party management company or liquidator or file for bankruptcy protection. At this time, our Board of Directors has considered these and other options and has determined that it is in the best interests of the Company and of our stockholders to dissolve Myrexis and return the cash to our stockholders. The Board of Directors, however, retains the right to consider other alternatives should a more attractive offer arise before the filing of the Certificate of Dissolution.
Dissolution Under Delaware Law
Delaware law provides that a corporation may dissolve upon the recommendation of the board of directors of the corporation, followed by the approval of its stockholders. Following such approval, the dissolution is effected by filing a Certificate of Dissolution with the Delaware Secretary of State. The corporation is dissolved upon the effective date of its Certificate of Dissolution.
Section 278 of the DGCL provides that once a corporation is dissolved, it continues its corporate existence but may not carry on any business except that appropriate to wind up and liquidate its business and affairs. The process of winding up includes:
| • | the collection of assets and the disposal of properties that will be applied toward the satisfaction, or making reasonable provision for the satisfaction, of liabilities and claims or will not otherwise be distributed in kind to the corporation’s stockholders; |
| • | the satisfaction, or making reasonable provision for the satisfaction, of liabilities and claims; |
| • | subject to statutory limitations, the distribution of any remaining assets to the stockholders of the corporation; and |
| • | the taking of all other actions necessary to wind up and liquidate the corporation’s business and affairs. |
Description of the Plan of Dissolution and Dissolution Process
This section of the proxy statement describes material aspects of the proposed Plan of Dissolution. While we believe that the description covers the material terms of the Plan of Dissolution, this summary may not contain all of the information that is important to you. You should carefully read this entire proxy statement, including the Plan of Dissolution attached as Appendix A to this proxy statement, and the other documents delivered with this proxy statement for a more complete understanding of the Plan of Dissolution.
Approval of Plan of Dissolution
The Plan of Dissolution must be approved by the affirmative vote of a majority of the outstanding shares of our common stock. The approval of the Plan of Dissolution by the requisite vote of the holders of our common stock will constitute adoption of the Plan of Dissolution and a grant of full and complete authority for our Board of Directors and officers, without further stockholder action, to proceed with the dissolution and liquidation of the Company in accordance with any applicable provision of the Delaware law, including the authority to dispose of all of our remaining non-cash assets.
23
Dissolution and Liquidation
If the Plan of Dissolution is approved by the requisite vote of our stockholders, the steps set forth below will be completed at such times as our Board of Directors, in its discretion and in accordance with the DGCL, deems necessary, appropriate or advisable in our best interests and the best interests of our stockholders:
| • | the filing of a Certificate of Dissolution with the Delaware Secretary of State after obtaining a revenue clearance certificate from the Department of Finance; |
| • | the cessation of all of Myrexis’ business activities except those relating to winding up and liquidating Myrexis’ business and affairs, including, but not limited to, prosecuting and defending suits by or against us; |
| • | the collection, sale, exchange or other disposition of all or substantially all of Myrexis’ non-cash property and assets, in one transaction or in several transactions; |
| • | the payment of or the making of reasonable provision to pay all claims and obligations, including all contingent, conditional or un-matured contractual claims known to us; |
| • | the making of such provision as will be reasonably likely to be sufficient to provide compensation for any claim against us which is the subject of a pending action, suit or proceeding to which we are a party; |
| • | the making of such provision as will be reasonably likely to be sufficient to provide compensation for claims that have not been made known to us or that have not arisen but that, based on facts known to us, are likely to arise or become known to us within ten years after the date of dissolution; |
| • | the setting aside of a contingency reserve consisting of cash and/or property to satisfy such claims and contingent obligations of Myrexis; |
| • | the making of an initial liquidating distribution to our stockholders of record determined as of the Final Record Date; |
| • | the pro rata distribution to our stockholders, or the transfer to one or more liquidating trustees for the benefit of our stockholders under a liquidating trust, of the remaining assets of Myrexis after payment or provision for payment of claims against and obligations of Myrexis; and |
| • | the taking of any and all other actions permitted or required by the DGCL and any other applicable laws and regulations. |
Liquidating Distributions; Amount; Timing
It is our current intention to make an initial liquidating distribution to our stockholders of record as of the Final Record Date as soon as practicable following the filing of the Certificate of Dissolution with the Delaware Secretary of State. Prior to the initial liquidating distribution, under the DGCL, we are required to pay or provide for payment of all of our liabilities and obligations, including contingent liabilities. In determining whether adequate provision is being made for any outstanding liabilities or wind up costs, the Board of Directors may consider a variety of factors. For example, in the case of outstanding disputed or contingent liabilities, considerations may include the estimated maximum amount of the claim and the likelihood that the claim will be resolved in the claimant’s favor or that the contingency will occur. Further, our ability to make a liquidating distribution could be adversely affected if any unanticipated liabilities or claims arise prior to the anticipated distribution.
Uncertainties as to the amount of liabilities make it impossible to predict precisely the aggregate amount that will ultimately be available for distribution. We will continue to incur claims, liabilities and expenses (including operating costs, salaries, income taxes, payroll and local taxes, legal and accounting fees and miscellaneous office expenses) following the approval of the dissolution and liquidation of the Company
pursuant to the Plan of Dissolution. These claims, liabilities and expenses will reduce the amount of cash and assets available for ultimate distribution to our stockholders.
24
Based on the assumptions set forth below, among others, we estimate that the amount available for the initial liquidating distribution to our stockholders will be approximately between $72.9 million and $77.9 million in the aggregate, or approximately between $2.67 and $2.83 per share of common stock (based on 27,026,549 shares outstanding as of December 6, 2012, plus an estimate of the number of options that will be exercised and the number of shares issuable upon the vesting of restricted stock units prior to the dissolution). This estimate of the amount that may be available for the initial liquidating distribution assumes, among other things:
| • | that there will be no lawsuits filed against us or our officers or directors prior to or following the approval of the dissolution and liquidation pursuant to the Plan of Dissolution; |
| • | that the dissolution and wind up of Myrexis will be completed within three years; |
| • | a reserve of $2.5 million to $5.0 million for the AIA Litigation with respect to which we have an obligation to indemnify our former parent, Myriad Genetics; |
| • | a reserve of $300,000 to $1.5 million for all unknown claims and contingencies that could arise after the filing of the Certificate of Dissolution; and |
| • | that the amount of our anticipated liabilities as of the approval of the Plan of Dissolution will not exceed the estimates contained in the table below. |
Any one or more of these assumptions may prove to be wrong, which could reduce the amount available to distribute to our stockholders.
The following table sets forth our basis for calculating our estimate of the initial liquidating distribution. The following table is based upon the assumptions set forth above and estimates of certain liabilities. If our assumptions or estimates contained therein prove to be incorrect, our stockholders may ultimately receive substantially more or less. We do not plan to resolicit stockholder approval for the dissolution and liquidation of the Company pursuant to the Plan of Dissolution even if the amount ultimately distributed to our stockholders changes significantly from the estimates set forth in this proxy statement.
Estimated Initial Liquidating Distribution to Stockholders
(in millions, except for share and per share amounts)
| Low | High | |||||||
| Cash, cash equivalents and marketable securities as of October 31, 2012 (1) |
$ | 84.9 | $ | 84.9 | ||||
| Estimated Proceeds, Expenses and Cash Reserves |
||||||||
| Proceeds from assumed exercises of in-the-money options (2) |
$ | 0.6 | $ | 0.9 | ||||
| Proceeds from assumed sale of equipment (3) |
$ | 0.4 | $ | 0.6 | ||||
| Proceeds from sale of non-cash assets (4) |
$ | — | $ | — | ||||
| Operating expenses after October 31, 2012 (5) |
$ | (0.7 | ) | $ | (0.6 | ) | ||
| Severance (6) |
$ | (1.0 | ) | $ | (0.9 | ) | ||
| Accounts payable and accrued liabilities (7) |
$ | (0.7 | ) | $ | (0.6 | ) | ||
| Real estate and equipment lease termination costs (8) |
$ | (0.8 | ) | $ | (0.7 | ) | ||
| Insurance (9) |
$ | (0.6 | ) | $ | (0.5 | ) | ||
| Professional fees (attorneys, bankers, accountants, consultants) (10) |
$ | (2.7 | ) | $ | (2.4 | ) | ||
| Reserve for AIA Litigation |
$ | (5.0 | ) | $ | (2.5 | ) | ||
| Reserve for unanticipated claims and contingencies |
$ | (1.5 | ) | $ | (0.3 | ) | ||
|
|
|
|
|
|||||
| Total |
$ | (12.0 | ) | $ | (7.0 | ) | ||
| Estimated cash to distribute to stockholders |
$ | 72.9 | $ | 77.9 | ||||
|
|
|
|
|
|||||
| Assumed shares outstanding (11) |
27,289,087 | 27,552,707 | ||||||
| Estimated initial liquidating distribution per share |
$ | 2.67 | $ | 2.83 | ||||
25
| (1) | Cash, cash equivalents and marketable securities as of October 31, 2012, prior to payment of known, unknown and contingent liabilities and wind up expenses and receipt of estimated future proceeds from option exercises and sale of equipment. |
| (2) | Estimated proceeds from the exercise of options that are in-the-money, which options are assumed to be exercised prior to the Final Record Date. |
| (3) | Estimated proceeds from the sale of equipment that was not sold on or before October 31, 2012. |
| (4) | Due to the uncertainty of the value of our non-cash assets, we have not provided any estimate of the proceeds of a sale of such assets in the amount of liquidating distributions. Furthermore, we can provide no assurance that we will be able to sell any of our non-cash assets and receive additional proceeds for distribution. |
| (5) | Estimated operating expenses following October 31, 2012, for personnel, facilities and other expenses to conduct our wind up operations but exclusive of all other line items specifically allocated in the table above. |
| (6) | Estimated severance costs for remaining employees involved in the wind up operations. |
| (7) | Estimated accounts payable and accrued liabilities as of October 31, 2012. |
| (8) | Estimated range of cash payments associated primarily with lease obligations that end January 4, 2013, and the termination of ongoing leases. |
| (9) | Estimated range of cash use for the purchase of insurance, including directors’ and officers’ liability insurance covering a six-year extended reported period. |
| (10) | Estimated use of cash for professional fees related to our dissolution and liquidation, as well as ongoing SEC reporting requirements. |
| (11) | Based on (a) 27,026,549 shares of common stock outstanding as of December 6, 2012, (b) with respect to the lower end of the estimated distribution, 218,228 shares of common stock issuable upon the assumed exercise of options that are in-the-money, and with respect to the higher end of the estimated distribution, 481,848 shares of common stock issuable upon the assumed exercise of options that are in-the-money, which options are assumed to be exercised prior to the Final Record Date, and (c) an estimated 44,310 shares of common stock issuable upon the vesting of restricted stock units prior to the Final Record Date. |
The amount of cash ultimately distributed to our stockholders in the initial liquidating distribution depends on the accuracy of the assumptions and estimates set forth above. We have attempted to make reasonable estimates and assumptions, however, if any of such estimates or assumptions are inaccurate, the amount we initially distribute to our stockholders may be substantially less than the amount we currently estimate. See “Risk Factors to be Considered by Stockholders in Deciding Whether to Approve the Plan of Dissolution—The amount we distribute to our stockholders in the initial liquidating distribution may be substantially less than the amount we currently estimate if the amounts of our liabilities, other obligations and expenses or claims against us are higher than we currently anticipate.”
We are unable to predict the precise amount or timing of any additional liquidating distributions following the initial liquidating distribution. The timing and amount of any such additional liquidating distributions will depend upon the actual expenses incurred, the timing of the resolution of matters for which we have established the contingency reserve, the amount to be paid in satisfaction of such contingencies as well as our ability to convert our remaining assets to cash. Although our Board of Directors has not established a firm timetable for the liquidating distributions, subject to contingencies inherent in winding up our business, the Board of Directors intends to make such distributions as promptly as practicable. Subject to the requirements of Delaware law, we expect to make a final distribution prior to the third anniversary of our dissolution. See “Risk Factors to be Considered by Stockholders in Deciding Whether to Approve the Plan of Dissolution—We cannot assure you of the exact amount or timing of any additional liquidating distributions to our stockholders under the Plan of Dissolution.”
Final Record Date
The Final Record Date will be the date upon which we file the Certificate of Dissolution with the Delaware Secretary of State. We intend to close our stock transfer books and discontinue recording transfers of shares of
26
our common stock on the Final Record Date, and thereafter certificates representing shares of our common stock will not be assignable or transferable on our books except by will, intestate succession or operation of law. After the Final Record Date, we will not issue any new stock certificates, other than replacement certificates. It is anticipated that no further trading of our shares will occur after the Final Record Date. See “—Description of the Plan of Dissolution and Dissolution Process—Listing and Trading of the Common Stock and Interests in the Liquidating Trust or Trusts.”
All liquidating distributions from us or a liquidating trust on or after the Final Record Date, if any, will be made to stockholders of record as of the Final Record Date according to their holdings of common stock as of the Final Record Date. Subsequent to the Final Record Date, we may at our election require stockholders to surrender certificates representing their shares of common stock in order to receive subsequent distributions. Stockholders should not forward their stock certificates before receiving instructions to do so. If surrender of stock certificates should be required, all distributions otherwise payable by us or the liquidating trust, if any, to stockholders who have not surrendered their stock certificates may be held in trust for such stockholders, without interest, until the surrender of their certificates (subject to escheat pursuant to the laws relating to unclaimed property). If a stockholder’s certificate evidencing the common stock has been lost, stolen or destroyed, the stockholder may be required to furnish us with satisfactory evidence of the loss, theft or destruction thereof, together with a surety bond or other indemnity, as a condition to the receipt of any distribution.
Sale of Our Remaining Assets
The Plan of Dissolution contemplates the sale of all of our remaining non-cash assets. The Plan of Dissolution does not specify the manner in which we may sell our assets. Such sales could take the form of individual sales of assets, sales of groups of assets organized by type of asset or otherwise, a single sale of all or substantially all of our assets, or some other form of sale. Sales of our assets will be made on such terms as are approved by the Board of Directors in its sole discretion. The assets may be sold to one or more purchasers in one or more transactions over a period of time.
It is not anticipated that any further stockholder votes will be solicited with respect to the approval of the specific terms of any particular sales of assets approved by the Board of Directors. We do not anticipate amending or supplementing this proxy statement to reflect any such agreement or sale, unless required by applicable law. The prices at which we will be able to sell our various assets will depend largely on factors beyond our control, including, without limitation, the condition of financial markets, the availability of financing to prospective purchasers of the assets, United States and foreign regulatory approvals, public market perceptions, and limitations on transferability of certain assets. In addition, we may not obtain as high a price for a particular asset as we might secure if we were not in liquidation.
Contingent Liabilities; Contingency Reserve
Under the DGCL, we are required, in connection with our dissolution and liquidation, to pay or make reasonable provision for payment of all of our liabilities and obligations. Following the approval of the Plan of Dissolution by our stockholders, we will pay all known liabilities. In addition, we currently estimate that we will establish a contingency reserve of between $2.8 million and $6.5 million, which will be used to satisfy contingent liabilities as they become due.
Pursuant to our Separation and Distribution Agreement with Myriad Genetics, at the time of our separation from Myriad Genetics, we assumed liability for certain pending or threatened legal matters related to our business, including the AIA Litigation, and we are obligated to indemnify Myriad Genetics for any liability arising out of such matters, including any costs and expenses of litigating such matters, including payment of attorneys’ fees incurred to defend against claims. Based on discussions with outside legal counsel, including an independent law firm hired by the Board of Directors to review the case, we do not believe that the AIA Litigation will result in material liability for Myrexis. However, the costs of defending intellectual property litigation are substantial, and our Board recently authorized
27
management to pursue a settlement of the AIA Litigation in order to reduce the portion of our otherwise distributable cash to our stockholders which would need to be set aside, if a dissolution and liquidation were pursued, in order to comply with the Company’s obligation to make reasonable provision for contingent obligations. Such settlement efforts have been unsuccessful, and accordingly, we have determined to reserve between $2.5 million and $5.0 million in connection with this ongoing legal proceeding, which the Board believes is a reasonable provision for this contingency. Based on a review and analysis of this matter by litigation counsel and independent counsel, we believe that, absent further developments, the current most likely outcome of this litigation will be a dismissal of the case and an unsuccessful appeal by AIA of that dismissal. Such an outcome would result in our ability to distribute a substantial portion of this reserve to our stockholders upon the final resolution of this case, which could occur within 18-24 months. However, there can be no assurance of the timing of such resolution or the ultimate outcome of the litigation. It is possible, therefore, that no portion of this reserve will ultimately be available for distribution, and it is even possible that a portion of the initial and any subsequent distributions to the stockholders in liquidation could be subject to repayment by the stockholders to satisfy a shortfall in the amount set aside for this contingency.
The estimated amount of the contingency reserve is based upon estimates and opinions of management and the Board of Directors and derived from consultations with outside experts and a review of our estimated operating expenses and future estimated liabilities, including, without limitation, any payments related to resolution of the outstanding litigation (including the AIA Litigation), estimated legal, accounting and consulting fees, operating lease expenses, payroll and other taxes payable, miscellaneous office expenses, and expenses accrued in our financial statements. There can be no assurance that the contingency reserve will be sufficient. If any of our estimates, including estimates relating to the costs of the liquidation process and of satisfying outstanding obligations, liabilities and claims during the liquidation process, are inaccurate, we may be required to increase the amount of the contingency reserve. After the liabilities, expenses and obligations for which the contingency reserve is established have been satisfied in full, we will distribute to our stockholders any remaining portion of the contingency reserve.
Under the DGCL, in the event we fail to create an adequate contingency reserve for payment of our expenses and liabilities, or should such contingency reserve and the assets held by any liquidating trust or trusts be exceeded by the amount ultimately found payable in respect of expenses and liabilities, each stockholder could be held liable for the repayment to creditors, out of the amounts theretofore received by such stockholder from us or from any liquidating trust or trusts, of such stockholder’s pro rata share of such excess.
If we were held by a court to have failed to make adequate provision for our expenses and liabilities or if the amount required to be paid in respect of such liabilities exceeded the amount available from the contingency reserve and the assets of the liquidating trust or trusts, a creditor of ours could seek an injunction against the making of liquidating distributions under the Plan of Dissolution on the grounds that the amounts to be distributed were needed to provide for the payment of our expenses and liabilities. Any such action could delay or substantially diminish the cash distributions to be made to stockholders under the Plan of Dissolution.
Liquidating Trust
If deemed necessary, appropriate or desirable by the Board of Directors for any reason, we may, from time to time, transfer any of our unsold assets and any portion of our contingency reserve to one or more liquidating trusts, or other structure we deem appropriate, established for the benefit of our stockholders, which property would thereafter be sold or distributed on terms approved by its trustee(s). Our Board of Directors may determine to transfer assets to a liquidating trust in circumstances where the nature of an asset is not susceptible to distribution (for example, interests in intangibles) or where our Board of Directors determines that it would not be in the best interests of us and our stockholders for such assets to be distributed directly to the stockholders at such time. If all of our assets (other than the contingency reserve) are not sold or distributed prior to the third anniversary of the effectiveness of the dissolution, we must transfer in final distribution such remaining assets to a liquidating trust. Our Board of Directors may also elect in its discretion, as applicable, to transfer the contingency reserve, if any, or any portion thereof, to such a liquidating trust.
28
The purpose of a liquidating trust would be to distribute such property, or to sell such property on terms satisfactory to the liquidating trustee(s) and distribute the proceeds of such sale, after paying our liabilities, if any, assumed by the trust, to our stockholders, based on their proportionate ownership interest in the trust. Any liquidating trust acquiring all of our unsold assets will assume all of our liabilities and obligations and will be obligated to pay any of our expenses and liabilities that remain unsatisfied. If the contingency reserve transferred to the liquidating trust is exhausted, such expenses and liabilities will be satisfied out of the liquidating trust’s other unsold assets.
The Plan of Dissolution authorizes our Board of Directors to appoint one or more individuals, who may include persons who are also officers or directors of the Company, or entities to act as trustee or trustees of the liquidating trust or trusts and to cause us to enter into a liquidating trust agreement or agreements with such trustee or trustees on such terms and conditions as may be approved by our Board of Directors. It is anticipated that our Board of Directors will select such trustee or trustees on the basis of the experience of such individual or entity in administering and disposing of assets and discharging liabilities of the kind to be held by the liquidating trust or trusts and the ability of such individual or entity to serve the best interests of our stockholders.
The trust would be evidenced by a trust agreement between us and the trustees. Pursuant to the trust agreement, the trust property would be transferred to the trustees immediately prior to the distribution of interests in the trust to our stockholders, to be held in trust for the benefit of the stockholder beneficiaries subject to the terms of the trust agreement. It is anticipated that the interests would be evidenced only by the records of the trust, there would be no certificates or other tangible evidence of such interests and no holder of our common stock would be required to pay any cash or other consideration for the interests to be received in the distribution or to surrender or exchange shares of our common stock in order to receive the interests.
It is further anticipated that, pursuant to the trust agreements:
| • | a majority of the trustees would be required to be independent of our management; |
| • | approval of a majority of the trustees would be required to take any action; and |
| • | the trust would be irrevocable and would terminate after, the earliest of (a) the trust property having been fully distributed, or (b) a majority in interest of the beneficiaries of the trust, or a majority of the trustees, having approved of such termination, or (c) a specified number of years having elapsed after the creation of the trust. |
Listing and Trading of the Common Stock and Interests in the Liquidating Trust or Trusts
If our stockholders approve the Plan of Dissolution, we intend to close our stock transfer books and delist our stock from the NASDAQ Global Market on the Final Record Date. We will cease recording stock transfers and issuing stock certificates (other than replacement certificates) at such time. Accordingly, it is expected that trading in the shares will cease after the Final Record Date. It is possible that the trading of our common stock on the NASDAQ Global Market will effectively terminate before then if we are unable to meet NASDAQ’s requirements for continued listing. See “Risk Factors to Be Considered by Stockholders in Deciding Whether to Approve the Plan of Dissolution.”
After delisting and closing of our transfer books, our stockholders will not be able to transfer their shares. It is anticipated that the interests in a liquidating trust or trusts will not be transferable, although no determination has yet been made. Such determination will be made by our Board of Directors and management prior to the transfer of unsold assets to the liquidating trust and will be based on, among other things, our Board of Directors’ and management’s estimate of the value of the assets being transferred to the liquidating trust or trusts, tax matters and the impact of compliance with applicable securities laws.
The costs of compliance with such requirements would reduce the amount which otherwise could be distributed to interest holders. Even if transferable, the interests are not expected to be listed on a national securities exchange or quoted through NASDAQ, and the extent of any trading market therein cannot be
29
predicted. Moreover, the interests may not be accepted by commercial lenders as security for loans as readily as more conventional securities with established trading markets. As stockholders will be deemed to have received a liquidating distribution equal to their pro rata share of the value of the net assets distributed to an entity which is treated as a liquidating trust for tax purposes (see “Material United States Federal Income Tax Consequences of Our Dissolution and Liquidation”), the distribution of non-transferable interests could result in tax liability to the interest holders without their being readily able to realize the value of such interests to pay such taxes or otherwise.
Absence of Appraisal Rights
Under the DGCL, our stockholders are not entitled to appraisal rights for their shares of common stock in connection with the transactions contemplated by the Plan of Dissolution.
Reporting Requirements
Whether or not the Plan of Dissolution is approved, we have an obligation to continue to comply with the applicable reporting requirements of the Exchange Act, even though compliance with such reporting requirements is economically burdensome. If the Plan of Dissolution is approved, in order to curtail expenses, we will, after filing our Certificate of Dissolution, seek relief from the SEC from the reporting requirements under the Exchange Act. However, the SEC may not grant any such relief, in which case we would be required to continue to bear the expense of being a public reporting company until at least the filing of our Annual Report on Form 10-K for the fiscal year ending June 30, 2013.
Treatment of Equity Awards and Employee Stock Purchase Plan
Pursuant to the terms of our 2009 Employee, Director and Consultant Equity Incentive Plan, as amended (the “Equity Incentive Plan”), under which all restricted stock units and options to acquire shares of our common stock have been granted, all outstanding restricted stock units and outstanding options, whether currently vested or unvested, will terminate immediately prior to our dissolution. On November 9, 2012, our Board of Directors, based on the recommendation of our Compensation Committee, accelerated the vesting of an aggregate of 45,124 restricted stock units issued to our employees, other than our executive officers, effective on the second business day following the public announcement of the Board’s decision to approve the dissolution of the Company, and 64,250 stock options held by our employees, other than our executive officers, effective on the opening of business on the date of the filing of the Certificate of Dissolution. Information regarding the treatment of the equity awards held by our executive officers is set forth under the heading “Interests of Directors and Executive Officers in Approval of the Plan of Dissolution.” In accordance with the Equity Incentive Plan, we will give notice to holders of outstanding stock options of our plans to dissolve and liquidate, and allow these holders to exercise their vested stock options prior to our dissolution. Unless and until an option is exercised and payment of the applicable exercise price is made, option holders are not entitled to any cash distributions with respect to their options payable under the Plan of Dissolution.
We intend to terminate the Equity Incentive Plan effective upon our dissolution.
In connection with our dissolution, we have suspended the commencement of any future offering periods under our 2009 Employee Stock Purchase Plan (the “ESPP”) following completion of the offering period currently in effect, which ends on November 30, 2012, and intend to terminate the ESPP effective upon our dissolution.
Authority of Officers and Directors
After the Final Record Date, we expect that our Board of Directors (or some subset thereof) and our officers will continue in their positions for the purpose of winding up the business and affairs of Myrexis. Our Board of Directors may appoint officers, hire employees and retain independent contractors and agents in connection with
30
the winding up process, and is authorized to pay compensation to or otherwise compensate our directors, officers, employees, independent contractors and agents above their regular compensation in recognition of the extraordinary efforts they may be required to undertake in connection with the successful implementation of the Plan of Dissolution. Adoption of the Plan of Dissolution by the requisite vote of our stockholders will constitute approval by our stockholders of any such compensation.
The approval of the Plan of Dissolution by our stockholders also will authorize, without further stockholder action, our Board of Directors to do and perform, or to cause our officers to do and perform, any and all acts and to make, execute, deliver or adopt any and all agreements, resolutions, conveyances, certificates and other documents of every kind that our Board of Directors deems necessary, appropriate or desirable, in the absolute discretion of the Board of Directors, to implement the Plan of Dissolution and the transactions contemplated thereby, including, without limitation, all filings or acts required by any state or federal law or regulation to wind up its affairs.
Professional Fees and Expenses
It is specifically contemplated that we will obtain legal and accounting advice and guidance from one or more law and accounting firms in implementing the Plan of Dissolution, and we will pay all fees and expenses reasonably incurred by us in connection with or arising out of the implementation of the Plan of Dissolution, including the prosecution, defense, settlement or other resolution of any claims or suits by or against us, the discharge, filing and disclosure of outstanding obligations, liabilities and claims, filing and resolution of claims with local, county, state and federal tax authorities, and the advancement and reimbursement of any fees and expenses payable by us pursuant to the indemnification we provide in our Certificate of Incorporation and Bylaws, the DGCL or otherwise. In addition, in connection with and for the purpose of implementing and assuring completion of the Plan of Dissolution, we may, in the absolute discretion of the Board of Directors, pay any brokerage, agency, professional and other fees and expenses of persons rendering services to us in connection with the collection, sale, exchange or other disposition of our property and assets and the implementation of the Plan of Dissolution.
Indemnification and Insurance
We will continue to indemnify our officers, directors, employees, agents and the liquidating trustee(s), if any, to the maximum extent permitted in accordance with applicable law, our Certificate of Incorporation and Bylaws, and any contractual arrangements, for actions taken in connection with the Plan of Dissolution and the winding up of our business and affairs. Our Board of Directors is authorized to obtain and maintain insurance as may be necessary, appropriate or advisable to cover such indemnification obligations, including seeking an extension in time and coverage of our insurance policies currently in effect.
Amendment, Modification or Revocation of Plan of Dissolution
If for any reason our Board of Directors determines that such action would be in the best interest of Myrexis and its stockholders, our Board may, in its sole discretion and without requiring further stockholder approval, revoke the Plan of Dissolution and all action contemplated thereunder, to the extent permitted by the DGCL. Our Board of Directors may not amend or modify the Plan of Dissolution under circumstances that would require additional stockholder approval under the DGCL and federal securities laws without complying with such requirements. The Plan of Dissolution would be void upon the effective date of any such revocation.
Regulatory Approvals
No United States federal or state regulatory requirements must be complied with or approvals obtained in connection with the liquidation.
31
Interests of Directors and Executive Officers in Approval of the Plan of Dissolution
Members of our Board of Directors and our executive officers may have interests in the approval of the Plan of Dissolution that are different from, or are in addition to, the interests of our stockholders generally. Our Board of Directors was aware of these interests and considered them, among other matters, in approving the Plan of Dissolution.
In connection with our liquidating distributions the members of our Board of Directors and our executive officers will be entitled to the same cash distributions as our stockholders based on their ownership of shares of our common stock, which is detailed below.
Equity Ownership
Members of our Board of Directors and our executive officers own, as of the date of this filing, an aggregate of 644,259 shares of our outstanding common stock as follows:
| Name |
No. of Shares of Outstanding Common Stock Owned |
|||
| Gerald P. Belle Director |
77,230 | |||
| Jason M. Aryeh Director |
555,145 | |||
| Robert Forrester, LL.B. Director |
0 | |||
| Timothy R. Franson, M.D. Director |
0 | |||
| John T. Henderson, M.D. Director |
1,075 | |||
| Dennis H. Langer, M.D., J.D. Director |
0 | |||
| David W. Gryska Acting President and Chief Executive Officer, Chief Operating Officer and Director |
0 | |||
| Andrea Kendell Chief Financial Officer |
10,809 | |||
32
The following table identifies, for each of our directors and our executive officers, the number of shares of our common stock subject to outstanding vested and exercisable options as of the anticipated date of the dissolution and the exercise price of such options, and restricted stock units outstanding and vested immediately prior to the dissolution.
| Name |
No. of Shares of Common Stock Underlying Vested and Exercisable Options as of the Anticipated Dissolution Date |
Option Exercise Price |
Restricted Stock Units Outstanding and Vested Immediately Prior to the Dissolution |
|||||||||
| Gerald P. Belle |
25,000 | $ | 4.03 | — | ||||||||
| Director |
7,500 | $ | 3.78 | — | ||||||||
| 16,250 | $ | 3.71 | — | |||||||||
| 16,250 | $ | 2.82 | — | |||||||||
| 7,500 | $ | 2.55 | — | |||||||||
| — | — | 0 | ||||||||||
| Jason M. Aryeh |
25,000 | $ | 2.74 | — | ||||||||
| Director |
— | — | 0 | |||||||||
| Robert Forrester, LL.B. |
25,000 | $ | 4.03 | — | ||||||||
| Director |
16,250 | $ | 3.71 | — | ||||||||
| 16,250 | $ | 2.82 | — | |||||||||
| — | — | 0 | ||||||||||
| Timothy R. Franson, M.D. |
25,000 | $ | 4.63 | — | ||||||||
| Director |
16,250 | $ | 3.71 | — | ||||||||
| 16,250 | $ | 2.82 | — | |||||||||
| — | — | 0 | ||||||||||
| John T. Henderson, M.D. |
25,000 | $ | 4.03 | — | ||||||||
| Director |
7,500 | $ | 3.78 | — | ||||||||
| 16,250 | $ | 3.71 | — | |||||||||
| 16,250 | $ | 2.82 | — | |||||||||
| 7,500 | $ | 2.55 | — | |||||||||
| 7,500 | $ | 1.65 | — | |||||||||
| 7,500 | $ | 1.18 | — | |||||||||
| 7,500 | $ | 1.07 | — | |||||||||
| 2,500 | $ | 0.95 | — | |||||||||
| — | — | 0 | ||||||||||
| Dennis H. Langer, M.D., J.D. |
25,000 | $ | 4.03 | — | ||||||||
| Director |
7,500 | $ | 3.78 | — | ||||||||
| 16,250 | $ | 3.71 | — | |||||||||
| 16,250 | $ | 2.82 | — | |||||||||
| 7,500 | $ | 2.55 | — | |||||||||
| 2,500 | $ | 1.65 | — | |||||||||
| — | — | 0 | ||||||||||
| David W. Gryska |
0 | 0 | — | |||||||||
| Acting President and Chief Executive Officer, Chief Operating Officer and Director | — | — | 0 | (1) | ||||||||
| Andrea Kendell |
3,750 | $ | 4.03 | — | ||||||||
| Chief Financial Officer |
7,965 | $ | 4.63 | — | ||||||||
| 5,538 | $ | 4.83 | — | |||||||||
| 8,630 | $ | 3.86 | — | |||||||||
| 80,000 | (2) | $ | 2.75 | (2) | — | |||||||
| 60,000 | (2) | $ | 2.65 | (2) | — | |||||||
| — | — | 24,310 | (3) | |||||||||
33
| (1) | Mr. Gryska’s restricted stock units will expire unvested by their terms upon our dissolution. |
| (2) | See the disclosure below under the heading “Compensation Arrangements with Andrea Kendell, Chief Financial Officer” for further information with respect to the treatment of these stock options in connection with our dissolution. |
| (3) | Ms. Kendell’s restricted stock units will vest and convert into shares of common stock on the date of our dissolution. |
Unless and until any such options are exercised and payment of the applicable exercise price is made, our directors and our Chief Financial Officer are not entitled to any cash distributions with respect to their options under the Plan of Dissolution. In accordance with our Equity Incentive Plan, all outstanding restricted stock units and outstanding options, whether currently vested or unvested, will terminate immediately prior to the effectiveness of the dissolution.
Compensation Arrangements with David W. Gryska, Acting President and Chief Executive Officer, and Chief Operating Officer
We do not have any severance or change in control arrangements in place with David W. Gryska, our Acting President and Chief Executive Officer and Chief Operating Officer. Mr. Gryska does not own any shares of our common stock or options to purchase shares of our common stock, and, as none of the milestones that would have triggered the vesting of his restricted stock units has occurred, his restricted stock units will expire unvested upon our dissolution.
Compensation Arrangements with Andrea Kendell, Chief Financial Officer
In connection with the proposed dissolution, our Board of Directors, based on the recommendation of the Compensation Committee and subject to stockholder approval of the dissolution, approved modifications of certain equity awards held by Andrea Kendell, our Chief Financial Officer, as well as compensation arrangements with Ms. Kendell following the dissolution in order to incentivize her to continue her services with us during the dissolution process, the terms of which are summarized below.
Treatment of Equity Awards
Restricted Stock Units
The following restricted stock units (“RSUs”) will vest immediately prior to the effectiveness of the dissolution:
| • | 20,000 restricted stock units granted on July 2, 2012, scheduled to vest on November 1, 2013; and |
| • | 4,310 restricted stock units granted on September 24, 2010, scheduled to vest as to 2,155 shares on each of September 25, 2013 and September 25, 2014. |
Stock Options
The stock options set forth below will convert on the day prior to the dissolution into a number of RSUs that equals the quotient of (A) the aggregate intrinsic value (as hereinafter defined) of such options divided by (B) the Fair Market Value (as defined in our Equity Incentive Plan) of our common stock on the date of issuance of the RSUs, which RSUs will vest immediately prior to the effectiveness of the dissolution. For each option the intrinsic value per share will be calculated by subtracting the exercise price of such option from the sum of the estimated per share initial liquidating distribution amount to be paid to stockholders plus the estimated per share additional liquidating distribution amounts, if any, anticipated to be paid to stockholders, each as estimated by the Company on the day prior to the dissolution (provided that if the exercise price of such option is greater than the sum of these amounts, the intrinsic value of such option shall be $0):
| • | 60,000 options with an exercise price of $2.65 per share granted on July 2, 2012, which vest quarterly as to 10,000 shares through November 1, 2013; and |
34
| • | 80,000 of the 160,000 options granted on September 22, 2011, with an exercise price of $2.75 per share, 40,000 of which are vested and 40,000 of which vest on September 22, 2013. |
Severance and Post-Dissolution Compensation
In consideration of Ms. Kendell’s continued service to us following the dissolution, the following compensation arrangements with Ms. Kendell were approved which will be set forth in an agreement between the Company and Ms. Kendell to be effective as of the filing of the Certificate of Dissolution and which agreement will replace Ms. Kendell’s Executive Severance and Change in Control Agreement, dated September 22, 2011 (the “Severance Agreement”), and Retention Bonus Agreement, entered into effective July 2, 2012 (the “Retention Bonus Agreement”):
| • | Ms. Kendell will continue as the Company’s Chief Financial Officer on a full time basis compensated at her current salary through February 28, 2013 (the “Employment Term”). |
| • | If Ms. Kendell’s employment is terminated by the Company without cause (as defined in the Severance Agreement) prior to the expiration of the Employment Term or if Ms. Kendell is then still employed, her employment will be terminated as of the end of the Employment Term, and, in either case, she will receive, conditioned upon her execution of a release of claims: |
| • | The payments and benefits currently due to her under her Severance Agreement in connection with a termination without “Cause” (as defined therein) comprised of: |
| • | payment in a lump sum amount equal to one times her then current annual base salary; |
| • | payment in a lump sum amount equal to one times her then current fiscal year target bonus amount; and |
| • | continuation of health benefits for up to 12 months. |
| • | The $100,000 retention bonus payable to her under her Retention Bonus Agreement. |
| • | From March 1, 2013 through June 30, 2013, Ms. Kendell will be engaged as a consultant to the Company on a regular basis providing such services as the Company may request from time to time at a rate of $14,000 per month. |
| • | Beginning July 1, 2013, Ms. Kendell may be engaged as a consultant to the Company on an as-needed hourly basis at a rate of $175 per hour. |
The compensation arrangements described above are quantified below under the heading “Golden Parachute Compensation.”
As defined in the Severance Agreement, “Cause” means the executive’s willful and continued failure to substantially perform her reasonable assigned duties (other than any such failure resulting from incapacity due to physical or mental illness), which failure is not cured within 30 days after a written demand for substantial performance is received by the executive from the Board of Directors of the Company which specifically identifies the manner in which the Board of Directors believes the executive has not substantially performed the executive’s duties; or the executive’s willful engagement in illegal conduct or gross misconduct which is materially and demonstrably injurious to the Company.
Golden Parachute Compensation
SEC rules require us to disclose and conduct an advisory vote on the compensation that would be payable to our named executive officers based on or that otherwise relates to the dissolution and liquidation of the Company. The following table sets forth the amounts Andrea Kendell, our Chief Financial Officer, is entitled to under her arrangements with the Company described above. This compensation is referred to as “golden parachute” compensation and is subject to a non-binding advisory vote of the stockholders described under
35
“Proposal 3—Advisory Vote on Compensation of Certain Executive Officers in Connection with the Dissolution and Liquidation of the Company” on page 40 of this proxy statement. Ms. Kendell is our only named executive officer entitled to “golden parachute” compensation. David W. Gryska, our Acting President and Chief Executive Officer and Chief Operating Officer and our only other executive officer, will receive no compensation based on or that otherwise relates to the dissolution and liquidation of the Company.
| Name |
Cash ($)(1) | Equity ($)(2) |
Pension/ NQDC ($) |
Perquisites/ Benefits ($)(3) |
Tax Reimburse- ment ($) |
Other ($)(4) |
Total ($) |
|||||||||||||||||||||
| Andrea Kendell |
$ | 544,417 | $ | 73,181 | $ | 0 | $ | 16,435 | $ | 0 | $ | 56,000 | $ | 690,033 | ||||||||||||||
| Chief Financial Officer |
||||||||||||||||||||||||||||
| (1) | Consists of (a) one times Ms. Kendell’s annual base salary ($280,000), (b) one times Ms. Kendell’s target bonus amount for the 2013 fiscal year ($112,000), (c) a retention payment ($100,000), and (d) estimated accrued vacation pay assuming a termination date of February 28, 2013 ($52,417). |
| (2) | Represents an estimate of the total aggregate value of 24,310 accelerated restricted stock units (“RSUs”) ($66,901) and 60,000 stock options with an exercise price of $2.65 per share and 80,000 stock options with an exercise price of $2.75 per share to be converted on the day prior to the effective date of the dissolution into RSUs that will vest immediately prior to the effectiveness of dissolution, as described above ($6,280). For purposes of this disclosure, the value of the RSUs and stock options to be converted into RSUs has been calculated based on the average closing price of the common stock on the five trading days subsequent to November 9, 2012 ($2.752), which was the date of the first public announcement of the Company’s plans to dissolve. The stock options described herein will convert on the day prior to the dissolution into RSUs calculated in the manner described above under the heading “—Treatment of Equity Awards—Stock Options.” |
| (3) | Represents the estimated cost of the continuation of health benefits for 12 months. |
| (4) | Represents retainer payments of $14,000 per month from March 2013 through June 2013 for Ms. Kendell’s service as a consultant. Beginning on July 1, 2013, Ms. Kendell may be engaged as a consultant on an as-needed hourly basis at a rate of $175 per hour. |
Board Compensation
Pursuant to our Director Compensation Policy, our non-employee directors currently receive annual cash retainers for their service on the Board of Directors and committee service, 25% of which are paid following each quarter of service, and meeting attendance fees following a certain number of meetings being held. The Board of Directors has authorized that the cash retainer otherwise due and payable on December 31, 2012, for Board and committee service during the Company’s second fiscal quarter will be paid in full as of the dissolution and that the Director Compensation Policy shall cease and be terminated as of the dissolution, provided that the Company may adopt a new cash-based Director Compensation Policy if necessary to take effect for non-employee directors performing services in 2013 and beyond.
Indemnification and Insurance
In connection with the dissolution and liquidation of the Company pursuant to the Plan of Dissolution, we will continue to indemnify our directors and officers to the maximum extent permitted in accordance with applicable law, our Certificate of Incorporation and Bylaws, and any contractual arrangements, for actions taken in connection with the Plan of Dissolution and the winding up of our business and affairs. Our Board of Directors is authorized to obtain and maintain insurance as may be necessary, appropriate or advisable to cover such indemnification obligations, including seeking an extension in time and coverage of our insurance policies currently in effect.
As a result of these benefits, our directors and executive officers generally could be more likely to vote to approve the Plan of Dissolution, including the dissolution and liquidation of the Company contemplated thereby, than our other stockholders.
36
Other than as set forth above, it is not currently anticipated that our dissolution and liquidation will result in any material benefit to any of our executive officers or to directors who participated in the vote to adopt the Plan of Dissolution.
Material United States Federal Income Tax Consequences of Our Dissolution and Liquidation
Federal Income Taxation of our Stockholders
Amounts received by stockholders pursuant to the Plan of Dissolution will be applied against and reduce a stockholder’s tax basis in his, her or its shares of stock. Gain will be recognized as a result of a liquidating distribution to the extent that the aggregate value of the distribution and any prior liquidating distributions received by a stockholder with respect to a share exceeds his or her tax basis for that share. If we make more than one liquidating distribution, each liquidating distribution will be allocated proportionately to each share of stock owned by a stockholder. Any loss will generally be recognized only when the final distribution from us has been received and then only if the aggregate value of all liquidating distributions with respect to a share is less than the stockholder’s tax basis for that share. Gain or loss recognized by a stockholder will be capital gain or loss provided the shares are held as capital assets, and will be long-term capital gain or loss if the stock has been held for more than one year.
Although we currently do not intend to make distributions of property other than cash, in the event of a distribution of property, the stockholder’s tax basis in such property immediately after the distribution will be the fair market value of such property at the time of distribution.
After the close of our taxable year, we will provide stockholders and the Internal Revenue Service with a statement of the amount of cash distributed to our stockholders and our best estimate as to the value of any property distributed to them during that year. There is no assurance that the Internal Revenue Service will not challenge our valuation of any property. As a result of such a challenge, the amount of gain or loss recognized by stockholders might be changed. Distributions of property other than cash to stockholders could result in tax liability to any given stockholder exceeding the amount of cash received, requiring the stockholder to meet the tax obligations from other sources or by selling all or a portion of the assets received.
If a stockholder is required to satisfy any liability of ours not fully covered by our contingency reserve (see “Description of the Plan of Dissolution and Dissolution Process—Contingent Liabilities; Contingency Reserve”), payments by stockholders in satisfaction of such liabilities would generally produce a capital loss, which, in the hands of individual stockholders, could not be carried back to prior years to offset capital gains realized from liquidating distributions in those years.
Liquidating Trusts
If we transfer assets to a liquidating trust or trusts, we intend to structure such trust or trusts so that stockholders will be treated for tax purposes as having received their pro rata share of the property transferred to the liquidating trust or trusts, reduced by the amount of known liabilities assumed by the liquidating trust or trusts or to which the property transferred is subject. Assets transferred to a liquidating trust will cause the stockholder to be treated in the same manner for federal income tax purposes as if the stockholder had received a distribution directly from us. The liquidating trust or trusts themselves will not be subject to federal income tax. After formation of the liquidating trust or trusts, the stockholders must take into account for federal income tax purposes their allocable portion of any income, gain or loss recognized by the liquidating trust or trusts. As a result of the transfer of property to the liquidating trust or trusts and the ongoing operations of the liquidating trust or trusts, stockholders should be aware that they may be subject to tax, whether or not they have received any actual distributions from the liquidating trust or trusts with which to pay such tax.
The tax consequences of the Plan of Dissolution may vary depending upon the particular circumstances of the stockholder. We recommend that each stockholder consult his, her or its own tax advisor regarding the federal income tax consequences of the Plan of Dissolution as well as the state, local and foreign tax consequences.
37
If our stockholders approve the dissolution and liquidation of the Company pursuant to the Plan of Dissolution, we will change our basis of accounting to the liquidation basis of accounting. Under the liquidation basis of accounting, assets are stated at their estimated net realizable values, and liabilities are stated at their estimated settlement amounts. Recorded liabilities will include the estimated expenses associated with carrying out the Plan of Dissolution. For periodic reporting, a statement of net assets in liquidation will summarize the liquidation value per outstanding share of common stock. Valuations presented in the statement will represent management’s estimates, based on present facts and circumstances, of the net realizable values of assets, satisfaction amounts of liabilities, and expenses associated with carrying out the Plan of Dissolution based upon management assumptions.
The valuation of assets and liabilities will necessarily require many estimates and assumptions, and there will be substantial uncertainties in carrying out the provisions of the Plan of Dissolution. Ultimate values realized for our assets and ultimate amounts paid to satisfy our liabilities are expected to differ from estimates recorded in annual or interim financial statements.
The affirmative vote of the holders of a majority of our outstanding common stock entitled to vote is required for approval of the dissolution and liquidation of the Company pursuant to the Plan of Dissolution.
Recommendation of our Board of Directors
On November 9, 2012, our Board of Directors unanimously: (1) determined that the dissolution and liquidation of the Company, and the other transactions contemplated thereby, are advisable and in the best interests of us and our stockholders, (2) approved in all respects the Plan of Dissolution and the other transactions contemplated thereby, and (3) recommended that our stockholders vote FOR the approval of the dissolution and liquidation of the Company pursuant to the Plan of Dissolution.
38
APPROVAL OF ADJOURNMENT OF SPECIAL MEETING TO SOLICIT ADDITIONAL PROXIES
At the Special Meeting, we may ask our stockholders to consider and vote on a proposal to adjourn the Special Meeting to another date, time or place, if necessary, for the purpose of soliciting additional proxies to vote in favor of Proposal 1 if there are not sufficient votes at the Special Meeting to approve Proposal 1. Any adjournment of the Special Meeting may be made without notice, other than by the announcement made at the Special Meeting, if a majority of the votes cast affirmatively or negatively for this proposal vote in favor of the Meeting Adjournment Proposal at the Special Meeting. However, if the adjournment is for more than 30 days from the date set for the original meeting, a new notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the adjourned meeting. If we adjourn the Special Meeting to a later date, we will transact the same business and, unless we must fix a new record date, only the stockholders who were eligible to vote at the original meeting will be permitted to vote at the adjourned meeting.
The approval of any adjournment of the Special Meeting requires the affirmative vote of a majority of the votes cast affirmatively or negatively for this proposal at the Special Meeting.
Recommendation of our Board of Directors
Our Board of Directors recommends that our stockholders vote FOR the approval of Proposal 2.
39
ADVISORY VOTE ON COMPENSATION OF CERTAIN EXECUTIVE OFFICERS IN CONNECTION WITH THE DISSOLUTION AND LIQUIDATION OF THE COMPANY
Section 14A of the Exchange Act, which was enacted as part of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, requires that we provide our stockholders with the opportunity to vote to approve, on an advisory, non-binding basis, the “golden parachute” compensation arrangements for certain executive officers. We are asking our stockholders to indicate their approval of the various payments which Andrea Kendell, our Chief Financial Officer, will or may be eligible to receive in connection with the dissolution and liquidation of the Company. These payments are set forth above under “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Interests of Directors and Executive Officers in Approval of the Plan of Dissolution—Golden Parachute Compensation.” These arrangements were adopted and approved by our Board of Directors, based on the recommendation of the Compensation Committee of our Board, which is composed solely of independent directors, and are believed to be reasonable and in line with marketplace norms.
Accordingly we are seeking approval of the following resolution at the Special Meeting:
“RESOLVED, that the stockholders of Myrexis approve, solely on an advisory basis, the golden parachute compensation which may be paid to Andrea Kendell, Chief Financial Officer of Myrexis, in connection with the dissolution and liquidation of Myrexis, as disclosed pursuant to Item 402(t) of Regulation S-K in the section entitled “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Interests of Directors and Executive Officers in Approval of the Plan of Dissolution—Golden Parachute Compensation,” in the proxy statement for the Special Meeting.”
Stockholders should note that this non-binding proposal regarding certain compensation arrangements is merely an advisory vote which will not be binding on Myrexis or our Board of Directors. Further, the underlying plans and arrangements are contractual in nature and not, by their terms, subject to stockholder approval. Accordingly, regardless of the outcome of the advisory vote, if the Plan of Dissolution is approved by the stockholders and we go forward with the filing of the Certificate of Dissolution with the Delaware Secretary of State, Andrea Kendell will be eligible to receive the various payments in accordance with the terms and conditions applicable to those payments.
The approval of this proposal requires the affirmative vote of a majority of the votes cast affirmatively or negatively for this proposal at the Special Meeting.
Recommendation of our Board of Directors
Our Board of Directors recommends that our stockholders vote FOR the approval of Proposal 3.
40
IMPORTANT INFORMATION CONCERNING MYREXIS, INC.
For a description of our business, see our Annual Report on Form 10-K for the fiscal year ended June 30, 2012, as amended by the Form 10-K/A filed on October 29, 2012 (the “Form 10-K”), which is attached as Appendix B to this proxy statement and the Quarterly Report on Form 10-Q for the quarter ended September 30, 2012 (the “Form 10-Q”), which is attached as Appendix C to this proxy statement. The Form 10-K and Form 10-Q, which are attached to this proxy statement as appendices, do not include the exhibits originally filed with such reports.
For a description of our properties, see the Form 10-K and Form 10-Q, which are attached as Appendix B and Appendix C to this proxy statement, respectively.
For a description of our legal proceedings, see the Form 10-K and Form 10-Q, which are attached as Appendix B and Appendix C to this proxy statement, respectively.
Our financial statements as of and for the years ended June 30, 2012, 2011 and 2010 and the notes thereto, are included in the Form 10-K, which is attached as Appendix B to this proxy statement. Our financial statements as of and for the three months ended September 30, 2012 and 2011, and the notes thereto, are included in the Form 10-Q, which is attached as Appendix B to this proxy statement.
The following table sets forth selected financial information as of and for each of the periods presented. The statement of operations data for the fiscal years ended June 30, 2012, 2011 and 2010, and the balance sheet data as of June 30, 2012 and 2011 have been derived from our audited financial statements set forth in the Form 10-K, which is attached as Appendix B to this proxy statement. The statement of operations data for the fiscal years ended June 30, 2009 and 2008 have been derived from our audited financial statements, which are not included elsewhere in this proxy statement. The statement of operations and comprehensive loss data for the three months ended September 30, 2012 and 2011 and the balance sheet data as of September 30, 2012, have been derived from our unaudited financial statements set forth in the Form 10-Q, which is attached as Appendix C to this proxy statement. Because our historical financial information for periods ending on or prior to June 30, 2009 reflects allocations for services historically provided to us by Myriad Genetics, Inc., the selected financial information
41
presented below for such periods may not be indicative of our results of operations and financial position as an independent company. The selected financial information presented for the years ended June 30, 2012, 2011 and 2010, reflects our performance as an independent company.
| Three months ended September 30, |
Years ended June 30, | |||||||||||||||||||||||||||
| In thousands (except per share data)
Statement of Operations Data: |
2012 | 2011 | 2012 | 2011 | 2010 | 2009 | 2008 | |||||||||||||||||||||
| Research revenue |
$ | — | $ | — | $ | — | $ | 185 | $ | 90 | $ | 5,456 | $ | 6,774 | ||||||||||||||
| Pharmaceutical revenue |
— | — | — | — | — | — | 100,000 | (1) | ||||||||||||||||||||
| Other revenues |
— | — | — | — | — | — | 4,000 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total revenues |
— | — | — | 185 | 90 | 5,456 | 110,774 | |||||||||||||||||||||
| Costs and expenses: |
||||||||||||||||||||||||||||
| Research and development expense |
291 | 4,300 | 14,230 | 22,296 | 28,222 | 54,611 | (7) | 121,526 | (2) | |||||||||||||||||||
| General and administrative expense |
3,485 | 4,385 | 17,571 | 18,339 | (8) | 19,984 | 8,981 | 20,600 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total costs and expenses |
3,776 | 8,685 | 31,801 | 40,635 | 48,206 | 63,592 | 142,126 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Operating loss |
(3,776 | ) | (8,685 | ) | (31,801 | ) | (40,450 | ) | (48,116 | ) | (58,136 | ) | (31,352 | ) | ||||||||||||||
| Other income (expense), net |
355 | 99 | 592 | 1,742 | 1,165 | — | (3,017 | )(3) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss |
$ | (3,421 | ) | $ | (8,586 | ) | $ | (31,209 | ) | $ | (38,708 | ) | $ | (46,951 | ) | $ | (58,136 | ) | $ | (34,369 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss per basic and diluted share (4) |
$ | (0.13 | ) | $ | (0.33 | ) | $ | (1.18 | ) | $ | (1.52 | ) | $ | (1.91 | ) | $ | (2.43 | ) | $ | (1.43 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| In thousands | As of September 30, | As of June 30, | ||||||||||||||||||||||||||
| Balance Sheet Data: | 2012 | 2012 | 2011 | 2010 | 2009 | 2008 | ||||||||||||||||||||||
| Cash, cash equivalents and marketable securities (5) |
$85,459 | $ | 89,626 | $ | 115,878 | $ | 147,453 | $ | 188,005 | $ | — | |||||||||||||||||
| Current liabilities |
1,521 | 2,279 | 3,310 | 4,250 | 4,576 | 46,568 | ||||||||||||||||||||||
| Total assets |
87,670 | 91,651 | 121,260 | 154,108 | 193,677 | 15,746 | ||||||||||||||||||||||
| Total stockholders’/parent equity (6) |
86,149 | 89,372 | 117,950 | 149,858 | 189,101 | (30,822 | ) | |||||||||||||||||||||
| (1) | Represents a nonrefundable upfront payment from A/S Lundbeck for the former drug candidate Flurizan. |
| (2) | Amount includes an accrued $20 million sublicense fee payable related to Flurizan. |
| (3) | Amount includes the write-off of the cost basis investment in Encore Pharmaceuticals. |
| (4) | For years ended June 30, 2009 and 2008, pro forma net loss per share calculated based on the 23,974,211 shares issued in connection with the spin-off. |
| (5) | Prior to June 30, 2009, all cash and investments were held and managed by Myriad Genetics. |
| (6) | Balances prior to June 30, 2009 represent Myriad Genetics’ net investment (or capital deficiency) in Myrexis. |
| (7) | Amount includes a $9.0 million credit recorded in fiscal 2009, resulting from the difference in an estimated sub-license fee accrual recorded in fiscal 2008 and amounts actually paid in 2009. |
| (8) | Includes a $1.1 million impairment loss on certain fixed assets. For the year ended June 30, 2011, the Company reclassified the $1.1 million in impairment charges from other income (expense) to general and administrative expense. |
42
Supplementary Financial Information
The following tables summarize our quarterly results of operations for each quarter in the fiscal years ended June 30, 2011 and 2012 and for the quarter ended September 30, 2012. These quarterly results are unaudited, but in the opinion of management have been prepared on the same basis as our audited financial information and include all adjustments (consisting only of normal recurring adjustments) necessary for a fair presentation of our results of operations.
Quarterly Financial Data (Unaudited)
|
|
Quarter Ended | |||||||||||||||||||
| In thousands | September 30, 2012 |
June 30, 2012 |
March 31, 2012(3) |
December 31, 2011 |
September 30, 2011 |
|||||||||||||||
| Research revenue |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Costs and expenses: |
||||||||||||||||||||
| Research and development expense |
291 | 558 | 5,603 | 3,769 | 4,300 | |||||||||||||||
| General and administrative expense |
3,485 | 4,129 | 5,216 | 3,841 | 4,385 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
3,776 | 4,687 | 10,819 | 7,610 | 8,685 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(3,776 | ) | (4,687 | ) | (10,819 | ) | (7,610 | ) | (8,685 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other income, net |
355 | 266 | 127 | 100 | 99 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (3,421 | ) | $ | (4,421 | ) | $ | (10,692 | ) | $ | (7,510 | ) | $ | (8,586 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Quarter Ended | ||||||||||||||||
| June 30, 2011 |
March 31, 2011(3) |
December 31, 2010 |
September 30, 2010 |
|||||||||||||
| Research revenue |
$ | — | $ | 55 | $ | 23 | $ | 107 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
— | 55 | 23 | 107 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and expenses: |
||||||||||||||||
| Research and development expense |
3,651 | 7,935 | 4,995 | 5,715 | ||||||||||||
| General and administrative expense |
4,449 | (1) | 5,088 | 4,240 | 4,562 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
8,100 | 13,023 | 9,235 | 10,277 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(8,100 | ) | (12,968 | ) | (9,212 | ) | (10,170 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income, net |
108 | 125 | 1,349 | (2) | 160 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (7,992 | ) | $ | (12,843 | ) | $ | (7,863 | ) | $ | (10,010 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Includes a $1.1 million impairment loss on certain fixed assets. For the year ended June 30, 2011, the Company reclassified the $1.1 million in impairment charges from other income (expense) to general and administrative expense. |
| (2) | Includes a one-time $1.2 million grant received in November 2010 as a part of the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code. |
| (3) | Includes one-time severance costs related to corporate reorganizations of $3.6 million for the period ending March 31, 2012 and $3.0 million for the period ended March 31, 2011. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations
Management’s Discussion and Analysis of Financial Condition and Results of Operations is included in the Form 10-K, which is attached as Appendix B to this proxy statement, and the Form 10-Q, which is attached as Appendix C to this proxy statement.
43
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
There were no changes in or disagreements with accountants on matters of accounting principles or practices or financial disclosures for the periods covered by the Form 10-K, which is attached as Appendix B to this proxy statement, and the Form 10-Q, which is attached as Appendix C to this proxy statement.
Market Price of our Common Stock
Our common stock currently trades on the NASDAQ Global Market under the symbol “MYRX.” The following table sets forth the range of high and low sales prices of our common stock for the quarterly periods indicated, as reported by NASDAQ:
| High | Low | |||||||
| Fiscal Year Ending June 30, 2013: |
||||||||
| First Quarter |
$ | 2.74 | $ | 2.34 | ||||
| Second Quarter (through December 13, 2012) |
$ | 2.84 | 2.30 | |||||
| Fiscal Year Ended June 30, 2012: |
||||||||
| First Quarter |
$ | 3.70 | $ | 2.64 | ||||
| Second Quarter |
2.88 | 2.41 | ||||||
| Third Quarter |
3.32 | 2.58 | ||||||
| Fourth Quarter |
3.20 | 2.26 | ||||||
| Fiscal Year Ended June 30, 2011: |
||||||||
| First Quarter |
$ | 3.98 | $ | 3.56 | ||||
| Second Quarter |
4.50 | 3.57 | ||||||
| Third Quarter |
4.26 | 3.72 | ||||||
| Fourth Quarter |
4.52 | 3.29 | ||||||
On December 13, 2012, the closing price of a share of our common stock on the NASDAQ Global Market was $2.78. You are encouraged to obtain current market quotations for our common stock in connection with voting your shares.
As of December 6, 2012, there were 100 registered holders of our common stock.
We have not paid any cash dividends on our common stock since our inception. In accordance with the Plan of Dissolution, it is anticipated that, if the Plan of Dissolution is approved by our stockholders, we will, to the extent permitted by law, make one or more liquidating distributions to our stockholders.
Security Ownership of Certain Beneficial Owners, Directors and Management
The following table sets forth information regarding beneficial ownership of our common stock as of December 6, 2012 (except as otherwise indicated), based on information available to us and filings with the SEC, by:
| • | each person or group of affiliated persons known by us to be the beneficial owner of more than 5% of our common stock; |
| • | each of our “named executive officers” as defined under SEC rules; |
| • | each of our directors; and |
| • | all of our current directors and executive officers as a group. |
Beneficial ownership and percentage ownership are determined in accordance with the rules of the SEC and include voting or investment power with respect to shares of stock. This information does not necessarily indicate beneficial ownership for any other purpose. Under these rules, shares of common stock issuable under stock options that are exercisable or restricted stock units that vest within 60 days of December 6, 2012 are deemed outstanding for the purpose of computing the percentage ownership of such individual or group, but are
44
not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Certain of the shares listed as beneficially owned are pursuant to stock options which were “out-of-the-money” as of such date. Percentage of ownership is based on 27,026,549 shares of common stock outstanding on December 6, 2012. Attached to each share of common stock is a Preferred Share Purchase Right to acquire one one-thousandth of a share of our Series A Junior Participating Preferred Stock, par value $.01 per share, which Preferred Share Purchase Rights are not presently exercisable. Unless otherwise noted below, the address of each person listed on the table is c/o Myrexis, Inc., 305 Chipeta Way, Salt Lake City, Utah 84108.
| Shares of Common Stock Beneficially Owned |
||||||||
| Beneficial Owner |
Number | Percentage | ||||||
| Principal Stockholders: |
||||||||
| Biotechnology Value Fund, L.P. (1) 900 North Michigan Avenue, Suite 1100 Chicago, IL 60611 |
1,344,900 | 5.02 | % | |||||
| Bulldog Investors (2) Park 80 West, 250 Pehle Avenue, Suite 708 Saddle Brook, NJ 07663 |
1,936,564 | 7.22 | % | |||||
| ICS Opportunities, Ltd. (3) c/o Millennium International Management LP 666 Fifth Avenue New York, New York 10103 |
1,413,300 | 5.27 | % | |||||
| Executive Officers and Directors: |
||||||||
| Andrea Kendell (4) |
96,692 | * | ||||||
| Robert J. Lollini (5) |
343,683 | 1.27 | % | |||||
| Adrian N. Hobden, Ph.D. (6) |
131,140 | * | ||||||
| Wayne Laslie (7) |
70,036 | * | ||||||
| Gerald P. Belle (8) |
149,730 | * | ||||||
| Jason M. Aryeh (9) |
580,145 | 2.16 | % | |||||
| Robert Forrester (10) |
57,500 | * | ||||||
| Timothy R. Franson, M.D. (10) |
57,500 | * | ||||||
| David W. Gryska |
0 | 0 | ||||||
| John T. Henderson, M.D. (11) |
98,575 | * | ||||||
| Dennis H. Langer, M.D., J.D. (10) |
75,000 | * | ||||||
| All current executive officers and directors as a group (8 persons) (12) |
1,115,142 | 4.09 | % | |||||
| * | Represents beneficial ownership of less than 1% of the shares of common stock. |
| (1) | This information is based on a Schedule 13G filed with the SEC on October 28, 2011. As of the close of business on October 27, 2011, (i) Biotechnology Value Fund, L.P. (“BVF”) beneficially owned 280,500 shares of common stock, (ii) Biotechnology Value Fund II, L.P. (“BVF2”) beneficially owned 171,900 shares of common stock, (iii) BVF Investments, L.L.C. (“BVLLC”) beneficially owned 800,200 shares of common stock, and (iv) Investment 10, L.L.C. (“ILL10”) beneficially owned 92,300 shares of common stock. BVF Partners L.P. (“Partners”), as the general partner of BVF and BVF2, the manager of BVLLC and the investment adviser of ILL10, may be deemed to beneficially own the 1,344,900 shares of common stock beneficially owned in the aggregate by BVF, BVF2, BVLLC and ILL10. BVF Inc., as the general partner of Partners, may be deemed to beneficially own the 1,344,900 shares of common stock beneficially owned by Partners. Mark N. Lampert (“Mr. Lampert”), as a director and officer of BVF Inc., may be deemed to beneficially own the 1,344,900 shares of common stock beneficially owned by BVF Inc. Partners, BVF Inc. and Mr. Lampert share voting and dispositive power over the shares of common stock beneficially owned by BVF, BVF2, BVLLC and ILL10. |
45
| (2) | This information is based on a Schedule 13G filed with the SEC on February 27, 2012 by Bulldog Investors, Brooklyn Capital Management, Phillip Goldstein and Andrew Dakos, reporting sole power to dispose of all 1,936,564 shares of common stock beneficially owned and shared power to vote certain of the shares of common stock beneficially owned. Phillip Goldstein and Andrew Dakos are principals of Bulldog Investors. On August 7, 2012, we entered into a letter agreement dated August 6, 2012 (the “Bulldog Shareholders Letter Agreement”) with Bulldog Investors and Brooklyn Capital Management LLC (collectively, the “Bulldog Shareholders”). The Bulldog Shareholders Letter Agreement granted to the Bulldog Shareholders an exemption under Section 29 of our Tax Benefits Shareholder Rights Agreement, embodying a shareholder rights plan adopted on March 29, 2012 to protect the use of our net operating losses and certain other tax attributes (the “Plan”). Under the exemption, through the completion of our 2013 annual meeting of shareholders (the “2013 Annual Meeting”), the Bulldog Shareholders’ shareholdings must not at any time represent more than 9.9% ownership in Myrexis. Also through the completion of the 2013 annual meeting, the Bulldog Shareholders must cause the shares of common stock they own to be voted on any matter presented to our stockholders for their vote as our Board of Directors recommends. The voting requirement is subject to the condition that Jason Aryeh is included in the Board of Directors majority approving the recommendation or, if he abstains or otherwise does not vote as a member of the Board of Directors on the matter, that he otherwise concurs with the Board’s recommendation. |
| (3) | This information is based on a Schedule 13D/A filed with the SEC on February 1, 2012 filed by ICS Opportunities, Ltd. (“ICS Opportunities”). ICS Opportunities is the beneficial owner of 1,413,300 shares of our common stock. Millennium International Management LP, a Delaware limited partnership (“Millennium International Management”), is the investment manager to ICS Opportunities and may be deemed to have shared voting control and investment discretion over securities owned by ICS Opportunities. Millennium International Management GP LLC, a Delaware limited liability company (“Millennium International Management GP”), is the general partner of Millennium International Management and may also be deemed to have shared voting control and investment discretion over securities owned by ICS Opportunities. Millennium Management LLC, a Delaware limited liability company (“Millennium Management”), is the general partner of the 100% shareholder of ICS Opportunities and may be deemed to have shared voting control and investment discretion over securities owned by ICS Opportunities. Israel A. Englander, a United States citizen (“Mr. Englander”), is the managing member of Millennium International Management GP and Millennium Management and consequently may also be deemed to have shared voting control and investment discretion over securities owned by ICS Opportunities. |
| (4) | Includes 85,883 shares of common stock issuable upon the exercise of options currently exercisable or exercisable within 60 days of December 6, 2012. For information regarding the treatment of the equity awards held by Ms. Kendell in connection with the dissolution, please see the disclosure under the heading “Proposal 1—Approval of the Plan of Complete Liquidation and Dissolution—Interests of Directors and Executive Officers in Approval of the Plan of Dissolution—Compensation Arrangements with Andrea Kendell, Chief Financial Officer.” |
| (5) | Includes 299,500 shares of common stock issuable upon the exercise of options currently exercisable or exercisable within 60 days of December 6, 2012. |
| (6) | Includes 39,375 shares of common stock issuable upon the exercise of currently exercisable options. |
| (7) | Includes 20,500 shares of common stock issuable upon the exercise of currently exercisable options. |
| (8) | Includes 72,500 shares of common stock issuable upon the exercise of currently exercisable options. |
| (9) | Represents 545,245 shares of common stock held by JALAA Equities, LP, of which Mr. Aryeh is the founder and general partner, 9,900 shares of common stock held in trust, and 25,000 shares of common stock issuable upon the exercise of currently exercisable options. |
| (10) | Represents shares of common stock issuable upon the exercise of currently exercisable options. |
| (11) | Represents 1,000 shares of common stock beneficially owned directly by Dr. Henderson, 75 shares of common stock owned by Dr. Henderson’s spouse, and 97,500 shares of common stock issuable upon the exercise of currently exercisable options. |
| (12) | Consists of the shares of common stock and shares of common stock issuable upon the exercise options held by Ms. Kendell and Messrs. Belle, Aryeh, Forrester, Franson, Henderson, and Langer. |
46
| resigned as a member of the Board of Directors effective November 15, 2012, his ownership has not been included in this calculation. |
Based on the decision to proceed with the Plan of Dissolution, we do not intend to hold a 2012 annual meeting of stockholders. If we do, however, decide to hold an annual meeting and if the date of the 2012 annual meeting is changed by more than 30 days from the date of the 2011 annual meeting (December 8, 2011), such stockholder proposals must be received a reasonable time before we begin to print and send proxy materials. If we do decide to hold an annual meeting and if the date of the 2012 annual meeting is changed by more than 30 days from the date of the 2011 annual meeting (December 8, 2011), to be considered for presentation at the annual meeting, although not included in the proxy statement, stockholder proposals must be received no earlier than the 90th day prior to the annual meeting and no later than the later of the 60th day prior to the annual meeting or the 10th day following the day on which public announcement of the date of the annual meeting is first made by us. Proposals that are not received in a timely manner will not be voted on at the annual meeting. If a proposal is received on time, the proxies that management solicits for the meeting may still exercise discretionary voting authority on the proposal under circumstances consistent with the proxy rules of the SEC. All stockholder proposals should be marked for the attention of: Secretary, Myrexis, Inc., 305 Chipeta Way, Salt Lake City, Utah 84108.
Where You Can Find More Information
We are subject to the reporting requirements of the Exchange Act and we file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy the reports, proxy statements and other information that we file at the SEC’s Public Reference Room at 100 F Street NE, Washington, D.C. 20549 at prescribed rates. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. Our filings are also available free of charge at the SEC’s website at http://www.sec.gov.
You should rely only on the information contained in this proxy statement. No one has been authorized to provide you with information that is different from what is contained in this proxy statement. The date of this proxy statement is December 14, 2012. You should not assume that the information contained in this proxy statement is accurate as of any date other than that date. The mailing of this proxy statement will not create any implication to the contrary.
HOUSEHOLDING OF PROXY MATERIALS
SEC rules concerning the delivery of disclosure documents allow us or your broker to send a single set of our proxy materials to any household at which two or more of our stockholders reside, if we or your broker believe that the stockholders are members of the same family. This practice, referred to as “householding,” benefits both you and us. It reduces the volume of duplicate information received at your household and helps to reduce our expenses. The rule applies to our notices, annual reports, proxy statements and information statements. Once you receive notice from your broker or from us that communications to your address will be “householded,” the practice will continue until you are otherwise notified or until you revoke your consent to the practice. Stockholders who participate in householding will continue to have access to and utilize separate proxy voting instructions.
If your household received a single set of proxy materials for the Special Meeting, but you would prefer to receive your own copy, please contact our transfer agent, American Stock Transfer and Trust Company, by calling their toll free number, 1-800-937-5449.
47
If you do not wish to participate in “householding” and would like to receive your own set of proxy materials in future years, follow the instructions described below. Conversely, if you share an address with another Myrexis stockholder and together both of you would like to receive only a single set of proxy materials, follow these instructions:
| • | If your Myrexis shares are registered in your own name, please contact our transfer agent, American Stock Transfer and Trust Company, and inform them of your request by (i) calling them at 1-800-937-5449; (ii) visiting their website at www.amstock.com; or (iii) writing them at American Stock Transfer and Trust Company, 6201 15th Avenue, 2nd Floor, Brooklyn, New York 11219. |
| • | If a broker or other holder of record holds your Myrexis shares, please contact the broker or other nominee directly and inform them of your request. Be sure to include your name, the name of your brokerage firm and your account number. |
The Board of Directors knows of no other matters that will be presented for consideration at the Special Meeting. If any other matters are properly brought before the meeting, it is the intention of the persons named in the accompanying proxy to vote on such matters in accordance with their best judgment.
BY ORDER OF THE BOARD OF DIRECTORS
Andrea Kendell
Secretary
December 14, 2012
Our Annual Report on Form 10-K, as amended, which includes our financial statements for the fiscal year ended June 30, 2012, filed with the SEC, is available on the website of the SEC at www.sec.gov, or in the “SEC Filings” section of the “Investors—Financial Information” section of our website at www.myrexis.com. You may also obtain a printed copy of our Annual Report on Form 10-K, as amended, free of charge from us by sending a written request to: Myrexis, Inc., 305 Chipeta Way, Salt Lake City, Utah 84108, Attn: Secretary. Exhibits will be provided upon written request and payment of an appropriate processing fee.
48
PLAN OF COMPLETE LIQUIDATION AND DISSOLUTION
OF
MYREXIS, INC.
The following Plan of Complete Liquidation and Dissolution (the “Plan of Dissolution”), and the actions described in this Plan of Dissolution are intended to effect the dissolution and complete liquidation of Myrexis, Inc., a Delaware corporation (the “Company”), in accordance with Section 275 and other applicable provisions of the Delaware General Corporation Law (the “DGCL”) and Sections 331 and 336 of the Internal Revenue Code of 1986, as amended (the “Code”).
1. Adoption of Plan. The board of directors of the Company (the “Board of Directors”) has adopted resolutions deeming it advisable and in the best interest of the stockholders of the Company to dissolve and liquidate the Company, adopt the Plan of Dissolution, and call a special meeting (the “Meeting”) of the holders of the Company’s common stock, $0.01 par value per share (the “Common Stock”), to approve the dissolution and liquidation of the Company (including the sale of all or substantially all of the Company’s assets) and adopt the Plan of Dissolution. If stockholders holding a majority of the outstanding shares of Common Stock on the record date fixed by the Board of Directors vote in favor of the proposed dissolution and liquidation of the Company (including the sale of all or substantially all of the Company’s assets) and the adoption of the Plan of Dissolution at the Meeting, the Plan of Dissolution shall constitute the adopted Plan of Dissolution of the Company as of the date of the Meeting, or such later date on which the stockholders may approve the Plan of Dissolution if the Meeting is adjourned to a later date.
2. Cessation of Business Activities. After the Effective Date (as defined below) and in accordance with Section 278 of the DGCL, the Company shall not engage in any business activities except for the purpose of preserving the value of its assets, winding up and liquidating its business and affairs, including, but not limited to, prosecuting and defending suits, whether civil, criminal or administrative, by or against the Company, collecting its assets, converting its assets into cash or cash equivalents, discharging or making provision for discharging its liabilities, withdrawing from all jurisdictions in which it is qualified to do business, distributing its remaining property among its stockholders according to their interests, and doing every other act necessary to wind up and liquidate its business and affairs, but not for the purpose of continuing the business for which the Company was organized.
3. Certificate of Dissolution. Following the receipt of stockholder approval of the dissolution of the Company and subject to Section 13 hereof, the officers of the Company shall, at such time as the Board of Directors, in its absolute discretion, deems necessary, appropriate or desirable, obtain any certificates required from the Delaware tax authorities or any other governmental authority and, upon obtaining such certificates and paying such taxes as may be owing, the Company shall file with the Secretary of State of the State of Delaware a certificate of dissolution (the “Certificate of Dissolution”) in accordance with the DGCL (the effective time of such filing, or such later time as stated therein, the “Effective Date”).
4. Liquidation Process. From and after the Effective Date and subject to the provisions hereof, the Company shall complete the following corporate actions:
a. Sale of All or Substantially All of the Non-Cash Assets. The Company shall determine whether and when to collect, sell, exchange or otherwise dispose of all or substantially all of its non-cash property and assets, including but not limited to all tangible assets, intellectual property and other intangible assets, in one or more transactions upon such terms and conditions as the Board of Directors, in its absolute discretion, deems expedient and in the best interests of the Company and its stockholders, without any further vote or
A-1
action by the Company’s stockholders. It is understood that, to the extent that the Company has already commenced the sale and disposition of its assets, such sales and dispositions are hereby ratified and approved. The Company’s non-cash assets and properties may be sold in one transaction or in several transactions to one or more buyers. The Company shall not be required to obtain appraisals, fairness opinions or other third-party opinions as to the value of its properties and assets in connection with the liquidation. In connection with such collection, sale, exchange and other disposition, the Company shall collect or make provision for the collection of all accounts receivable, debts and claims owing to the Company.
b. Liquidation of Assets. The Company shall determine whether and when to transfer the Company’s property and assets to a liquidating trust (established pursuant to Section 6 hereof).
c. Payment Obligations. The Company shall, as determined by the Board of Directors, (i) pay or make reasonable provision to pay all claims and obligations, including all contingent, conditional or unmatured contractual claims known to the Company, (ii) make such provisions as will be reasonably likely to be sufficient to provide compensation for any claim against the Company which is the subject of a pending action, suit or proceeding to which the Company is a party and (iii) make such provision as will be reasonably likely to be sufficient to provide compensation for claims that have not been made known to the Company or that have not arisen but that, based on facts known to the Company or successor entity, are likely to arise or to become known to the Company or successor entity within ten (10) years after the Effective Date, if any. All such claims shall be paid in full and any such provision for payment made shall be made in full if there are sufficient assets. If there are insufficient assets of the Company, such claims and obligations of the Company shall be paid or provided for in accordance with their priority and, among claims of equal priority, ratably to the extent of assets of the Company legally available therefor. If and to the extent deemed necessary, appropriate or desirable by the Board of Directors or the Trustees (as defined in Section 6 below), in their absolute discretion, the Company may establish and set aside a reasonable amount of cash and/or property (the “Contingency Reserve”) to satisfy such claims and obligations against the Company, including, without limitation, tax obligations, and all expenses related to the sale of the Company’s property and assets, all expenses related to the collection and defense of the Company’s property and assets, and the liquidation and dissolution provided for in this Plan of Dissolution.
d. Distributions to Stockholders. Any assets of the Company remaining after the payment of claims or the provision for payment of claims and obligations of the Company as provided in subsection (c) above shall be distributed by the Company pro rata to its stockholders. Such distribution may occur all at once or in a series of distributions and shall be in cash or assets, in such amounts, and at such time or times, as the Board of Directors or the Trustees, in their absolute discretion, may determine.
e. Notwithstanding the foregoing provisions of this Section 4 and without limiting the flexibility of the Board of Directors, the Board of Directors may, at its option, elect, but shall not be required, to follow the procedures for liquidating the Company set forth in Sections 280 and 281(a) of the DGCL.
5. Cancellation of Common Stock. The distributions to stockholders pursuant to Sections 4, 6 and 8 hereof (the “Liquidating Distribution”) shall be in complete cancellation of all of the outstanding shares of Common Stock. From and after the Effective Date, and subject to applicable law, each holder of shares of Common Stock shall cease to have any rights in respect thereof, except the right to receive Liquidating Distributions pursuant to the terms hereof. As a condition to receipt of the Liquidating Distribution, the Board of Directors or the Trustees, in their absolute discretion, may require the stockholders to (i) surrender their certificates evidencing the Common Stock to the Company or its agents for recording of such distributions thereon, or (ii) furnish the Company with evidence satisfactory to the Board of Directors or the Trustees of the loss, theft or destruction of their certificates evidencing the Common Stock, together with such surety bond or other security or indemnity as may be required by and satisfactory to the Board of Directors or the Trustees. The Company will close its stock transfer books and discontinue recording transfers of shares of Common Stock on the Effective Date, and thereafter certificates representing shares of Common Stock of the Company will not be assignable or transferable on the books of the Company except by will, intestate succession, or operation of law.
A-2
6. Liquidating Trust. If deemed necessary, appropriate or desirable by the Board of Directors, in its absolute discretion, in furtherance of the liquidation and distribution of the Company’s assets to the stockholders in accordance with the provisions hereof, as a final Liquidating Distribution or from time to time, the Company may transfer to one or more liquidating trustees, for the benefit of its stockholders and/or creditors (the “Trustees”) under a liquidating trust (the “Trust”), any assets of the Company, including cash, intended for distribution to creditors and stockholders not disposed of at the time of dissolution of the Company, including the Contingency Reserve. The Board of Directors is hereby authorized to appoint one or more individuals, corporations, partnerships or other persons or entities, or any combination thereof, including, without limitation, any one or more officers, directors, employees, agents or representatives of the Company, to act as the initial Trustee or Trustees for the benefit of the stockholders and to receive any assets of the Company. Any Trustees appointed as provided in the preceding sentence shall succeed to all right, title and interest of the Company of any kind and character with respect to such transferred assets and, to the extent of the assets so transferred and solely in their capacity as Trustees, shall assume all of the claims and obligations of the Company as provided in Section 4(b) hereof, including, without limitation, any unsatisfied claims and unknown or contingent liabilities. Further, any conveyance of assets to the Trustees shall be deemed to be a distribution of property and assets by the Company to the stockholders for the purposes of Section 4(d) of this Plan of Dissolution. Any such conveyance to the Trustees shall be treated for U.S federal and state income tax purposes as if the Company made such distribution to the stockholders and the assets conveyed shall be held in trust for the stockholders and creditors of the Company. The Company, subject to this Section 6 and as authorized by the Board of Directors, in its absolute discretion, may enter into a liquidating trust agreement with the Trustees, on such terms and conditions as the Board of Directors, in its absolute discretion, may deem necessary, appropriate or desirable. Adoption of the Plan of Dissolution by holders of a majority of the outstanding shares of Common Stock shall constitute the approval of the stockholders of any such appointment, any such liquidating trust agreement and any transfer of assets by the Company to the Trust as their act and as a part hereof as if herein written.
7. Abandoned Property. If any Liquidating Distribution to a stockholder cannot be made, whether because the stockholder cannot be located, has not surrendered its certificates evidencing the Common Stock as required hereunder or for any other reason, then the distribution to which such stockholder is entitled (unless transferred to the Trust established pursuant to Section 6) shall be transferred, at such time as the final Liquidating Distribution is made by the Company, to the extent permitted by law, to the official of such state or other jurisdiction authorized by applicable law to receive the proceeds of such distribution. The proceeds of such distribution shall thereafter be held solely for the benefit of and for ultimate distribution to such stockholder as the sole equitable owner thereof and shall be treated as abandoned property and escheat to the applicable state or other jurisdiction in accordance with applicable law. In no event shall the proceeds of any such distribution revert to or become the property of the Company.
8. Final Liquidating Distribution. Whether or not a Trust shall have been previously established pursuant to Section 6 hereof, if it should not be feasible for the Company to make the final Liquidating Distribution to its stockholders of all assets and all properties of the Company (excluding amounts set aside for unknown claims) prior to the third anniversary of the Effective Date, then, on or before such date, the Company shall be required to establish a Trust and transfer any remaining assets and properties (including, without limitation, any uncollected claims, contingent assets and the Contingency Reserve) to the Trustees as set forth in Section 6.
9. Stockholder Approval of Sale of Assets. Approval of the proposed dissolution and adoption of the Plan of Dissolution by holders of a majority of the outstanding shares of Common Stock shall constitute the approval of the stockholders of the Company of the dissolution of the Company and the sale, exchange or other disposition in liquidation of all or substantially all of the property and assets of the Company pursuant to the terms hereof, whether such sale, exchange or other disposition occurs in one transaction or a series of transactions, and shall constitute ratification of all contracts for sale, exchange or other disposition which are conditioned on adoption of the Plan of Dissolution.
10. Expenses of Dissolution. In connection with and for the purposes of implementing and assuring completion of the Plan of Dissolution, the Company may, in the absolute discretion of the Board of Directors,
A-3
pay any brokerage, agency, professional, legal and other fees and expenses of persons rendering services to the Company in connection with the collection, sale, exchange or other disposition of the Company’s property and assets and the implementation of the Plan of Dissolution. Adoption of the Plan of Dissolution shall constitute approval of such payments by the stockholders of the Company.
11. Employees and Independent Contractors. In connection with effecting the dissolution of the Company and for the purpose of implementing and assuring completion of the Plan of Dissolution, the Company may, in the absolute discretion of the Board of Directors, hire or retain such employees, consultants, independent contractors, agents and advisors as the Board of Directors deems necessary or desirable to supervise or facilitate the dissolution and liquidation. The Company may, in the absolute discretion of the Board of Directors, but subject to applicable legal and regulatory requirements, pay the Company’s officers, directors, employees, consultants, independent contractors, agents, advisors and representatives, or any of them, compensation or additional compensation above their regular compensation, in money or other property, as severance, bonus, or in any other form, in recognition of the extraordinary efforts they, or any of them, will be required to undertake, or actually undertake, in connection with the implementation of the Plan of Dissolution. Adoption of the Plan of Dissolution shall constitute approval of any such compensation by the stockholders of the Company.
12. Indemnification. The Company shall continue to indemnify its officers, directors, employees, agents and Trustee in accordance with its certificate of incorporation, bylaws, and contractual arrangements as therein or elsewhere provided, the Company’s existing directors’ and officers’ liability insurance policy and applicable law, and such indemnification shall apply to acts or omissions of such persons in connection with the implementation of this Plan of Dissolution and the winding up of the affairs of the Company. The Board or the Trustee is authorized to obtain and maintain insurance as may be necessary to cover the Company’s indemnification obligations.
13. Amendment, Modification or Abandonment of Plan. Notwithstanding stockholder approval of the Plan of Dissolution and the transactions contemplated hereby, if for any reason the Board of Directors determines that such action would be in the best interest of the Company, the Board of Directors may, in its sole discretion and without requiring further stockholder approval, revoke the Plan of Dissolution and all action contemplated thereunder, to the extent permitted by the DGCL.
14. Tax Matters. It is intended that this Plan of Dissolution shall be a plan of complete liquidation of the Company in accordance with the terms of Sections 331 and 336 of the Code. The Plan of Dissolution shall be deemed to authorize the taking of such action as, in the opinion of counsel for the Company, may be necessary to conform with the provisions of said Sections 331 and 336 and the regulations promulgated thereunder. The Company’s officers shall be authorized to cause the Company to make such elections for tax purposes as are deemed appropriate and in the best interest of the Company. Within thirty (30) days after the Effective Date, the Company shall file with the Internal Revenue Service an appropriate statement of corporate dissolution on IRS Form 966, as required by Section 6043 of the Code, and such additional forms and reports with the Internal Revenue Service as may be necessary or appropriate in connection with the Plan of Dissolution and the carrying out thereof. The Company shall notify all jurisdictions of any withdrawals related to qualification to do business. The Company shall make arrangements authorizing one or more representatives or agents to maintain such Company records as may be appropriate for purposes of any tax audit of the Company occurring during the process of dissolution or after liquidation.
15. Power of Board of Directors and Officers. The Board of Directors is hereby authorized, without further action by the Company’s stockholders, to do and perform, or cause the officers of the Company, subject to approval of the Board of Directors, to do and perform, any and all acts, and to make, execute, deliver or adopt any and all agreements, resolutions, conveyances, certificates and other documents of every kind that are deemed necessary, appropriate or desirable, in the absolute discretion of the Board of Directors, to implement the Plan of Dissolution and the transactions contemplated hereby, including, without limitation, all filings or acts required by any state or federal law or regulation to wind up its affairs.
A-4
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2012
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-34275
MYREXIS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 26-3996918 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 305 Chipeta Way Salt Lake City, Utah |
84108 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code (801) 214-7800
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of each class |
Name of each exchange on which registered | |
| Common Stock, $0.01 Par Value Per Share Preferred Share Purchase Rights |
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | x | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common stock was last sold on December 31, 2011, the last business day of the registrant’s most recently completed second fiscal quarter, was $69,028,808. As of September 6, 2012 the registrant had 26,798,833 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the registrant’s Proxy Statement for the 2012 Annual Meeting of Stockholders.
B-2
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934, as amended. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including any statements relating to our plans and objectives of management regarding strategic alternatives, our intention to acquire one or more commercial-stage biopharmaceutical assets, the pursuit of business development activities for our programs, future economic conditions or performance, and any statement of assumptions underlying any of the foregoing. In some cases, forward-looking statements can be identified by the use of terminology such as “may,” “will,” “expects,” “plans,” “anticipates,” “estimates,” “potential,” or “continue” or the negative thereof or other comparable terminology. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, there can be no assurance that such expectations or any of the forward-looking statements will prove to be correct, and actual results could differ materially from those projected or assumed in the forward-looking statements. Our future financial condition and results of operations, as well as any forward-looking statements, are subject to inherent risks and uncertainties, including but not limited to the risk factors set forth in Item 1A “Risk Factors” below, and for the reasons described elsewhere in this Annual Report. All forward-looking statements and reasons why results may differ included in this Annual Report are made as of the date hereof, and we assume no obligation to update these forward-looking statements or reasons why actual results might differ.
As used in this Annual Report on Form 10-K, the terms “we,” “us,” “our,” the “Company,” and “Myrexis” mean Myrexis, Inc. (unless the context indicates a different meaning). In addition, these terms refer to the former research and drug development businesses that were integrated with and operated by Myriad Genetics, Inc. prior to June 30, 2009, which are now operated by Myrexis, Inc.
B-3
| Item 1. | BUSINESS |
Overview
We are a biopharmaceutical company that has generated a pipeline of differentiated drug candidates in oncology and autoimmune diseases. We currently retain all rights to all of our drug candidates and programs across all geographic markets and therapeutic indications.
In September 2011, we announced that we had completed an in-depth review of our drug development pipeline, incorporating extensive inputs from both internal and independent external analyses. As a result, we made a strategic business decision to suspend any further development of our lead drug candidate Azixa, which was in Phase 2 development for the treatment of advanced primary and metastatic tumors with brain involvement. This decision was not based on any single factor. Our review took into consideration the accumulated data from our clinical trials, the evolving competitive environment in Glioblastoma multiforme, or GBM, including ongoing studies of competitive drug candidates that are in more advanced stages of development, inputs from key opinion leaders, updated cost and timing estimates, and other factors affecting the risks and opportunities relating to the development of Azixa. On the basis of these inputs, we concluded that completing the Phase 2b clinical trial we had underway would require a disproportionate investment of time and resources relative to its likelihood of technical and regulatory success, when compared to our other programs. Following this decision, in November 2011, we announced a corporate reorganization to realign our resources with our development strategy and clinical initiatives following the suspension of further development of Azixa. The reorganization included an immediate reduction in our workforce by 15 employees or approximately 20%.
In February 2012, we announced that we had suspended development activity on all of our preclinical and clinical programs and retained Stifel Nicolaus Weisel, an investment banking firm, to assist in reviewing and evaluating a full range of strategic alternatives to enhance shareholder value. Thereafter, in March 2012, we initiated an alignment of our resources involving a phased reduction in our workforce from approximately 59 employees to 10 current employees.
Based on our evaluation of strategic alternatives, we determined to pursue the acquisition of one or more commercial-stage biopharmaceutical assets, with the goal of building a commercial-stage biopharmaceutical company by optimizing their performance and profitability. Integral to these efforts, on May 11, 2012, we announced a change in management, including the appointment of Richard B. Brewer as President and Chief Executive Officer and David W. Gryska as Chief Operating Officer, collectively bringing an extensive track record of commercializing, acquiring and marketing pharmaceutical products throughout their careers. In addition, both Mr. Brewer and Mr. Gryska were appointed as members of the Board of Directors.
On August 15, 2012 we announced the death of Richard B. Brewer, our President and Chief Executive Officer. The Board of Directors appointed David W. Gryska as the acting President and Chief Executive Officer while considering succession plans. In addition, the Board of Directors is further evaluating our strategic direction in light of this development and our progress to date in identifying attractive biopharmaceutical assets.
We do not know if we will be successful in pursuing any strategic alternative or that any transaction will occur; however, we are committed to pursuing a strategic direction that our Board of Directors believes is in the best interests of our shareholders. During this period, we continue to actively pursue business development opportunities for each of our programs. However, despite our significant efforts to identify and attract third parties to whom we could out-license or sell these assets for further development, we have been unsuccessful to date.
We currently do not have any drugs that are commercially available and none of our drug candidates have obtained approval of the U.S. Food and Drug Administration, or FDA, or any similar foreign regulatory authority.
B-4
Our Oncology Programs
We have two programs in oncology. As indicated in our February 2012 announcement, we have suspended development activities in these programs and are actively pursuing business development opportunities with respect to them. Despite significant efforts, however, we have been unsuccessful to date in identifying and attracting third parties to whom we could out-license or sell these assets for further development.
| • | Hsp90 Inhibitor Program. Our compound MPC-3100 is an Hsp90 inhibitor for the treatment of cancer. In November 2011, we presented the results of an open-label, dose-escalating, multiple-dose, Phase 1 study of MPC-3100 in 26 patients with recurrent cancer or cancer refractory to available systemic therapy. Our compound MPC-0767 is a novel L-alanine ester pro-drug of MPC-3100 that was designed to have improved aqueous solubility compared to MPC-3100. We have completed all preclinical activities required for submission of an investigational new drug application, or IND, for MPC-0767. |
| • | Cancer Metabolism Inhibitor Program. MPC-8640 is our lead preclinical compound for the Cancer Metabolism Inhibitor, or CMI, program. As of June 2012, we have completed certain IND enabling studies. |
Oncology Background
Cancers are diseases characterized by abnormal and uncontrolled cell growth and division, typically leading to tumor formation. As a tumor grows, it can directly disrupt organ function at its site of origin. In addition, cancer cells can also spread to other organs, such as the brain, bones and liver, by a process called metastasis. The growth of metastatic tumors at these new sites can disrupt the function of these other organs. There are many kinds of cancer, but all are characterized by uncontrolled growth of abnormal cells.
Our Hsp90 Inhibitor Program for the Treatment of Cancer
Background
Heat shock protein 90, or Hsp90, is involved in the folding and stabilization of many proteins, including mutant oncogenes that become reliant on Hsp90 to maintain their activity, making them particularly sensitive to Hsp90 inhibition. Targeted therapies against such mutant oncogenes, such as ALK, HER2, FLT3 and B-RAF, have proven to be efficacious in the clinic and we believe that by inhibiting these targets through the different mechanism of Hsp90 inhibition, either as monotherapy or in combination with these targeted therapies, clinical efficacy and the duration of response can be improved.
We believe that the potential for Hsp90 inhibitors to improve therapeutic outcomes across a number of oncogene “addicted” cancers, coupled with the oral bioavailability, long half-life and the relative safety profile of our compounds makes our Hsp90 inhibitor program a potentially attractive program.
MPC-3100 and MPC-0767: Preclinical Development
MPC-3100 and MPC-0767, a pro-drug of MPC-3100, are fully synthetic, orally bio-available, non-geldanamycin Hsp90 inhibitors that have shown significant and broad preclinical anti-tumor activity in mouse models of human cancers. These unique molecules are structurally distinct from the geldanamycin family of early Hsp90 inhibitors, which are associated with certain toxicities. MPC-3100 inhibits Hsp90 by binding to the same site as geldanamycin and has displayed potent anti-cancer activity in multiple in vitro and in vivo models. MPC-3100 significantly and dose-dependently reduced tumor growth in studies conducted in mice implanted with a variety of human cancer cell lines, including colon, prostate, myeloid leukemia, small-cell lung, gastric, breast, and ovarian cancers. In April 2011, we reported additional preclinical data on our Hsp90 inhibitor program at the annual meeting of the American Association for Cancer Research in Orlando, Florida. The data presented included a demonstration that the combination of MPC-3100 with other targeted therapies, including erlotinib and sorafenib, showed greater in vivo anti-tumor activity compared to either agent alone, suggesting the
B-5
potential of combining MPC-3100 with these targeted cancer therapies in the clinic. We also presented a preliminary assessment of MPC-0767, a novel L-alanine ester pro-drug of MPC-3100, which is designed to have improved aqueous solubility. Animal studies showed that MPC-0767 displayed pharmacokinetics comparable to MPC-3100 and equivalent efficacy, inducing significant tumor regressions.
MPC-3100: Clinical Development
We submitted an IND application for MPC-3100 in the first quarter of 2009 and initiated patient enrollment of a Phase 1 clinical trial in the second quarter of 2009 to investigate the safety and tolerability of MPC-3100, pharmacokinetics, and the potential for anti-tumor activity. The Phase 1 study was an open-label, dose-escalating, multiple-dose study in which 26 patients aged 45-85 years with recurrent cancer or cancer refractory to available systemic therapy were treated with MPC-3100. Patients received oral MPC-3100 either once daily for 21 days followed by seven days off (cohorts 1-5, at doses of 50, 100, 165, 245, and 340mg/m2, respectively) or continuously for a 28-day cycle at doses spaced 12 hours apart (cohorts 6-7, at total daily doses of 480mg/kg and 640mg/kg, respectively). The primary objective of the Phase 1 study was to determine the safety and tolerability of MPC-3100 in cancer patients. The study also included secondary objectives such as characterization of the pharmacokinetic parameters, determining anti-tumor activity of MPC-3100, and evaluating certain pharmacodynamic biomarkers. In November 2011, we presented the results of this study at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics meeting in San Francisco. The study demonstrated that MPC-3100 was generally safe and well tolerated at doses below 600mg/kg per day. The most common adverse events were gastrointestinal, including diarrhea, nausea, and vomiting. Pharmacokinetic analysis indicated that the maximum plasma concentration, or Cmax, and the area under the curve, or AUC (0-12h), increased proportional to the dose of MPC-3100. The terminal plasma half-life of MPC-3100 ranged from 4.8 to 21.4 hours with a mean half-life of 11.2 hours. The best clinical response was stable disease (12/26; 46%), with a median duration of 11.1 weeks (range 3.0-52.3 weeks). On target activity of MPC-3100 was confirmed by biomarker analysis, which suggested effective and persistent in vivo inhibition of Hsp90.
MPC-3100 and MPC-0767: Future Clinical Development
We have conducted non-clinical studies as well as other technical, regulatory and market assessments with the objective of identifying optimal cancer indications and drug combination regimens to potentially advance one or both of our Hsp90 inhibitor compounds into Phase 2 clinical development. We believe we have completed all preclinical activities required for submission of an IND for MPC-0767.
Our Cancer Metabolism Inhibitor Program
Our CMI program is focused on the inhibition of Nicotinamide phosphoribosyltransferase, or Nampt, an enzyme involved in the production of Nicotinamide Adenine Dinucleotide, or NAD, which is an essential cofactor for the production of cellular energy that is critical for cell survival, growth, and DNA repair.
Cancer cells, in addition to spending energy on rapid, unregulated growth, must also invest significant energy on DNA synthesis and repair mechanisms to cope with the DNA damage. As a result, cancer cells are more susceptible to metabolic downshifts than healthy cells, and the NAD depletion caused by Nampt inhibitors has a greater effect on tumors versus normal tissue.
MPC-8640 is our lead preclinical compound for our CMI program. MPC-8640 is an orally bio-available pro-drug of a follow-on molecule to our prior CMI drug candidate, MPC-9528, that has enhanced solubility and distinct pharmacokinetic advantages and is being developed for the treatment of cancer. Both the active moiety of MPC-8640 (MPI-0487316) as well as MPC-9528 inhibit Nampt in vitro and in cells at picomolar drug concentrations and are tumoricidal in every cancer line tested to date representing 17 different tumor tissue types. Both compounds display on-target activity by potently reducing NAD levels, which leads to inhibition of glycolysis, energy deprivation and cell death in tumor cells, while NAD levels in normal tissues are less affected.
B-6
In preclinical efficacy studies, MPC-8640 and MPC-9528 causes dramatic tumor regressions in multiple tumor types when administered orally with either a low daily dosing or a higher intermittent dose regimen and are well tolerated. This anti-tumor activity is dose-dependent and tightly correlated to the level of NAD depletion, confirming the on-target mechanism of action. The sensitivity of tumor cells to our Nampt inhibitors in vitro appears to parallel their anti-tumor potency in xenograft models and is linked to basal Nampt expression levels. Nampt expression levels may therefore have utility for predicting tumor response to Nampt inhibitors. Nicotinic acid is converted to NAD through an alternative pathway that is dependent upon the enzyme Nicotinic acid phosphoribosyltransferase (Naprt1) which does not involve Nampt. In tumor cell types with sufficient Naprt expression to support this NAD biosynthetic pathway, nicotinic acid (niacin, Vitamin B3) can completely block the NAD-reducing and tumoricidal activity of MPC-9528. Our studies have found that approximately 40% of tumor cell lines are deficient in Naprt1 and in these cells, nicotinic acid has little to no effect on MPC-9528 tumoricidal activity. Furthermore, in animal model studies, a combination of nicotinic acid with MPC-9528 increases the tolerated dose of MPC-9528, while still causing growth inhibition of tumors deficient in Naprt1. This demonstrates the potential to increase the therapeutic index and efficacy of a Nampt inhibitor by combining it with nicotinic acid to treat patients with tumors that are deficient in Naprt1. A diagnostic method designed to measure Naprt1 expression could be used to identify those patients with Naprt1 deficient tumors that are most likely to benefit from this combination therapy.
Additional preclinical studies of MPC-9528 support the potential of Nampt inhibitors for broad spectrum tumoricidal activity as monotherapy and in a variety of combinations with other agents. Inhibition of Nampt by MPC-9528 was shown to exhibit synergistic anti-tumor activity when coupled with DNA damaging agents, such as alkylating agents and thymidylate synthase inhibitors. These common classes of chemotherapy drugs also reduce NAD cellular levels as a result of their mechanism of action, specifically by activating the NAD-consuming enzyme poly (ADP-ribose) polymerase (PARP). The mechanism of action of our Nampt inhibitors is distinct from these other agents, leading to a combined effect on cellular NAD levels and synergistic anti-tumor activity.
In June 2011, preclinical studies on MPC-9528 and MPI-0487316, a structurally distinct Nampt inhibitor and the active moiety of MPC-8640, were presented at the annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. Oral administration of either compound resulted in tumor regressions in animal model studies across multiple dosing schedules. MPC-8640 is a pro-drug of MPI-0487316 with enhanced solubility and distinct pharmacokinetic advantages. In November 2011, we presented data from preclinical studies of MPC-8640 at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics meeting in San Francisco. In these studies, mice with HT1080 human fibrosarcoma xenograft tumors were treated orally with MPC-8640 on either a once-daily or twice-daily dosing schedule. After one week of treatment, the mice demonstrated complete tumor growth inhibition at lower doses and substantial tumor regression at higher doses. Significantly, tumor regression could be achieved well below the maximum tolerated dose of MPC-8640, and the anti-tumor response observed after one week of dosing was maintained for at least one week without further treatment. The results also demonstrated that MPC-8640 is effectively converted into active Nampt inhibitor, either in the gut or immediately upon absorption, as evidenced by the lack of significant plasma concentrations of intact MPC-8640. Taken together, these results demonstrate that oral treatment with MPC-8640 is an effective mode of delivery of active Nampt inhibitor and that administration of this drug results in significant anti-tumor activity in animal models of cancer. We have completed certain IND enabling studies on MPC-8640 and have suspended any new development activities on this program.
Our Small-Molecule Autoimmune Disease Program
Oral Anti-interferon Program for the Treatment of Autoimmune Diseases
MPI-0485520 is our lead preclinical compound in our small-molecule anti-interferon program for autoimmune diseases. It has demonstrated proof of concept activity in an animal model of the autoimmune disease rheumatoid arthritis, or RA. As of June 2012, we have concluded all lead optimization activities and have
B-7
suspended any new development activities on this program. We continue to actively pursue business development opportunities for this program. Despite significant efforts, however, we have been unsuccessful to date in identifying and attracting third parties to whom we could out-license or sell these assets for further development.
IKKe and TBK1 are kinases that serve as key regulators of the pathway that activates alpha and beta interferon expression. Inhibition of these kinases thereby inhibits a major pro-inflammatory pathway involved in a number of autoimmune diseases, including RA, Lupus and psoriasis. We have demonstrated in preclinical studies that treatment with our oral anti-interferons, or OAI’s, inhibits the interferon response in several animal models, including significant inhibition of this response and reduction in the severity of clinical symptoms in a mouse model of RA.
MPI-0485520 is an orally-available small molecule that potently and selectively inhibits IKKe and TBK1 and is our lead preclinical compound in our small molecule anti-interferon program for autoimmune diseases. MPI-0485520 exhibits high oral bio-availability, favorable absorption, distribution, metabolism, and excretion pharmacokinetic properties and efficacy in an in vivo mouse model of RA. In cellular models of type I interferon production, MPI-0485520 potently inhibits induction of type I interferons (IFNa/b) following stimulation of a variety of receptors that mediate the type-I interferon to pathogens, such as TLR3, TLR4, RIG-I, and MDA-5. Inhibition of type I interferon production by IKKe/TBK1 inhibitors may benefit patients with autoimmune disorders such as RA, systemic lupus erythematosus (SLE), scleroderma, Sjögren’s syndrome, and polymyositis. In April 2011, results from preclinical studies of MPI-0485520 were presented at the European League Against Rheumatism (EULAR) Annual European Congress of Rheumatology in London. In a proof of concept study, in the well characterized collagen-induced mouse model of arthritis, mice treated with MPI-0485520 show a dose-dependent and statistically significant reduction in the severity of clinical symptoms and paw and joint histopathology, as well as lower weight loss compared to control mice. MPI-0485520 is one compound out of an extensive portfolio of potent and selective IKK epsilon/TBK1 inhibitors identified by our oral anti-interferon program.
Intellectual Property
As a biopharmaceutical company, our success will depend in part on our ability to obtain and maintain proprietary protection for our intellectual property, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. Our policy has been to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions, and improvements that have been important to the development of our business. We have also relied on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
We own or have licensed rights to over 33 issued patents and over 148 patent applications in the United States and foreign countries. Issued patents expire between 2015 and 2029. Any patent applications which we have filed or will file or to which we have licensed or will license rights may not issue, and patents that do issue may not contain commercially valuable claims. In addition, any patents issued to us or our licensors may not afford meaningful protection for our products or technology, or may be subsequently circumvented, invalidated or narrowed, or found unenforceable. Our processes and potential products may also conflict with patents which have been or may be granted to competitors, academic institutions or others. As the pharmaceutical industry expands and more patents are issued, the risk increases that our processes and potential products may give rise to claims of patent infringement by other companies, institutions or individuals. These entities or persons could bring legal actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the related product or process. If any of these actions are successful, in addition to any potential liability for damages, we could be required to cease the infringing activity or obtain a license in order to continue to manufacture or market the relevant product or process. We may not prevail in any such action and any license required under any such patent may not be made available on acceptable terms, if at all. Our failure to obtain a license to any technology that we may require to commercialize our technologies or potential products could have a materially adverse effect on our business.
B-8
Licenses
Our rights to certain patents and technologies have been acquired through license agreements with other corporations or academic institutions.
License and Collaboration Agreement with EpiCept Corporation
In November 2003, Myriad Genetics, Inc., our former parent company, entered into a license and collaboration agreement with Maxim Pharmaceuticals, Inc. and Cytovia, Inc. All licensed rights of Maxim and Cytovia were subsequently acquired by EpiCept Corporation, and we refer to Maxim, Cytovia and EpiCept collectively herein as EpiCept. In connection with our separation from Myriad Genetics on June 30, 2009, Myriad Genetics assigned this agreement to us. Pursuant to this agreement, we were granted an exclusive, worldwide right to utilize certain intellectual property rights of EpiCept, including patents, patent applications and know-how that relate to Azixa, in the development and commercialization of products for the treatment or prevention of any disease or disorder in exchange for, among other things, certain royalty and milestone payment obligations.
In September 2011, we announced that we had suspended any further development of Azixa. On August 28, 2012, we provided EpiCept notice of termination of the license and collaboration agreement following our election to terminate all of our efforts to develop and commercialize Azixa in any major market as such products and markets are defined in the agreement. As a result of the termination of the agreement, all rights and licenses granted under the agreement by EpiCept have terminated and reverted to EpiCept. We have no further obligation for royalty or milestone payments to EpiCept as a result of this notice to terminate. As of June 30, 2009, Myriad Genetics had made payments totaling $4 million under the EpiCept license and collaboration agreement. As of June 30, 2012, we have made no payments under the EpiCept license and collaboration agreement.
Manufacturing and Supply
Prior to the suspension of our development activities, we relied on contract manufacturers to produce drug substances and drug products required for our clinical trials under current good manufacturing practices, or cGMP, with oversight by internal managers. As a result of the decision to suspend all further development activity on all of our preclinical and clinical programs, we have ceased production of additional drug substance for clinical trials.
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, packaging, promotion, storage, advertising, distribution, marketing and export and import of drugs. Drugs must be approved by the FDA through the new drug application, or NDA, process before they may be legally marketed in the United States.
United States Government Regulation
NDA Approval Processes
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, or the FDCA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statues and regulation require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| • | completion of preclinical laboratory tests, animal studies and formulation studies conducted according to Good Laboratory Practices, or GLPs, or other applicable regulations; |
B-9
| • | submission to the FDA of an IND, which must become effective before human clinical trials may begin; |
| • | performance of adequate and well-controlled human clinical trials according to Good Clinical Practices, or GCPs, to establish the safety and efficacy of the proposed drug for its intended use; |
| • | submission to the FDA of an NDA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current Good Manufacturing Practices, or cGMPs, to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| • | FDA review and approval of the NDA. |
Once a pharmaceutical candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| • | Phase 1. The drug is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and elimination. In the case of some products for severe or life-threatening diseases, such as cancer, especially when the product may be inherently too toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| • | Phase 2. Clinical trials in a limited patient population intended to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| • | Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide, an adequate basis for product labeling. |
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. It may request additional information rather than accept a NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity.
Satisfaction of FDA requirements or similar requirements of foreign regulatory authorities typically takes at least several years and the actual time required may vary substantially, based upon, among other things, the indication and the type, complexity and novelty of the product. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly requirements. Success in early stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval.
B-10
Reimbursement
Sales of pharmaceutical products will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly challenging the prices charged for medical products and services. Additionally, the containment of healthcare costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, imposed new requirements for the distribution and pricing of prescription drugs for Medicare beneficiaries and included a major expansion of the prescription drug benefit under Medicare Part D. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities which will provide coverage of outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Part A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee.
The American Recovery and Reinvestment Act of 2009 provides funding for the federal government to compare the effectiveness of different treatments for the same illness. A plan for the research will be developed by the Department of Health and Human Services, the Agency for Healthcare Research and Quality and the National Institutes for Health, and periodic reports on the status of the research and related expenditures will be made to Congress. Although the results of the comparative effectiveness studies are not intended to mandate coverage policies for public or private payors, it is not clear how such a result could be avoided and what if any effect the research will have on the sales of our product candidates, if any such product or the condition that it is intended to treat is the subject of a study. It is also possible that comparative effectiveness research demonstrating benefits in a competitor's product could adversely affect the sales of our product candidates. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payor to not cover our product candidates could reduce physician usage of the product candidate and have a material adverse effect on our sales, results of operations and financial condition.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the ACA) enacted in March 2010, is expected to have a significant impact on the health care industry. The ACA is expected to expand coverage for the uninsured while at the same time contain overall healthcare costs. With regard to pharmaceutical products, among other things, the ACA is expected to expand and increase industry rebates for drugs covered under Medicaid programs and make changes to the coverage requirements under the Medicare D program. We cannot predict the impact of the ACA on pharmaceutical companies as many of the ACA reforms require the promulgation of detailed regulations implementing the statutory provisions which has not yet occurred. In addition, although the United States Supreme Court recently upheld the constitutionality of most of the ACA, some states have stated their intentions to not implement certain sections of the ACA and some members of Congress are still working to repeal the ACA. These challenges add to the uncertainty of the changes enacted as part of the ACA.
B-11
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
Employees
As of the date of this filing, we had 10 full-time employees, 3 of whom hold an M.D., Ph.D, or combined M.D./Ph.D. All 10 employees are in general and administrative functions. Our workforce is non-unionized, and we believe our employee relations are good.
Corporate History and Available Information
We were incorporated as Myriad Pharmaceuticals, Inc. in Delaware in January 2009 as a new, wholly owned subsidiary of Myriad Genetics, Inc. in order to effect the separation and spin-off of Myriad Genetics’ research and drug development businesses as a stand-alone, independent, publicly traded company. In connection with the formation of this new subsidiary, Myriad Genetics’ existing subsidiary, Myriad Pharmaceuticals, Inc., changed its corporate name to Myriad Therapeutics, Inc. On June 30, 2009, Myriad Genetics contributed substantially all of the assets and certain liabilities of its research and drug development businesses as well as $188 million in cash and marketable securities to us and effected the spin-off of our company through the pro rata dividend distribution to its stockholders of all outstanding shares of our common stock. Effective July 1, 2010, we changed our name from Myriad Pharmaceuticals, Inc. to Myrexis, Inc. Our principal executive offices are located at 305 Chipeta Way, Salt Lake City, Utah 84108. Our telephone number is 801-214-7800 and our web site address is www.myrexis.com. We include our web site address in this Annual Report on Form 10-K only as an inactive textual reference and do not intend it to be an active link to our web site. We make available free of charge through the “Investors” section of our web site our Corporate Code of Conduct and Ethics, our Audit Committee and other committee charters and our other corporate governance policies, as well as our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission.
| Item 1A. | RISK FACTORS |
The risks and uncertainties described below are those that we currently believe may materially affect our company. If any of the following risks actually occur, they may materially harm our business, our financial condition and our results of operations.
Risks Relating to Our Evaluation of Strategic Alternatives and Our Business
The impact and results of our previously announced strategic direction are uncertain and may not be successful.
As announced earlier in 2012, we have suspended development activities with respect to all of our preclinical and clinical programs, and after an evaluation of various strategic alternatives, we have focused our efforts on licensing or acquiring one or more commercial-stage biopharmaceutical assets that are under-performing, with the goal of enhancing their performance and profitability. In May 2012, we announced a change
B-12
in management to facilitate the pursuit of our new strategic direction, including the appointment of Richard B. Brewer as President and Chief Executive Officer and David W. Gryska as Chief Operating Officer. Messrs. Brewer and Gryska were recruited because of their extensive track record in acquiring and commercializing biopharmaceutical products throughout their careers. During the subsequent months, Messrs. Brewer and Gryska have been actively engaged in identifying and evaluating numerous biopharmaceutical assets for their potential fit with our corporate objectives.
On August 15, 2012, we announced the death of Mr. Brewer and the appointment of Mr. Gryska as acting President and Chief Executive Officer while our Board of Directors considers succession plans. In addition, the Board has begun and is continuing a further evaluation of our strategic alternatives in light of Mr. Brewer’s death, progress to date in identifying attractive biopharmaceutical assets, and other factors. The outcome of that further evaluation will be announced upon its completion.
Our Board remains dedicated to diligently deliberating upon and making informed decisions that the directors believe are in the best interests of the company and its shareholders. There can be no assurance, however, that the company’s current strategic direction, or the Board’s evaluation of strategic alternatives, will result in any initiatives, agreements, transactions or plans that will enhance shareholder value.
One of the strategic alternatives that our Board of Directors could pursue is the dissolution and liquidation of the Company. If our Board of Directors and shareholders were to approve a Plan of Liquidation and Dissolution, we would be required, as part of the liquidation process under Delaware law, to pay our outstanding obligations and to make reasonable provision for contingent obligations, as well as unknown obligations that could arise during the post-dissolution period. While we do not believe that any of our contingent obligations is material, the requirement under Delaware law to make reasonable provision for contingent and unknown obligations would impact the amount and timing of a portion of the distributions in liquidation to our shareholders.
Following the death of our President and Chief Executive Officer on August 15, 2012, our Board of Directors has undertaken a review of the range of possible strategic alternatives available to us, including, but not limited to, the dissolution and liquidation of the Company. If our Board of Directors were to approve and recommend, and the shareholders approved, a dissolution and liquidation, we would be required under Delaware law to pay our outstanding obligations, as well as to make reasonable provision for contingent and unknown obligations, prior to making any distributions in liquidation to our shareholders. In particular, as previously disclosed, pursuant to our Separation and Distribution Agreement with our former parent, Myriad Genetics, Inc., at the time of our separation from Myriad Genetics in 2009, we assumed liability for certain pending or threatened legal matters related to our business, and we are obligated to indemnify Myriad Genetics for any liability arising out of such matters, including any costs of litigating such matters. Although we do not believe that any obligation we assumed under the Separation and Distribution Agreement will result in a material liability, we cannot predict with certainty the amount or timing of such liability, if any. However, as a result of the requirement under Delaware law that our Board of Directors make reasonable provision for contingent and unknown obligations in connection with a dissolution and liquidation of the Company, a portion of our assets would need to be reserved until the resolution of such matters. This would impact the amount and timing of a portion of the distributions in liquidation to our shareholders. If a dissolution and liquidation were pursued, our Board of Directors, in consultation with its advisors, would need to evaluate these matters and make a determination about a reasonable amount to reserve.
We anticipate that we will incur losses for the foreseeable future and we may never achieve or sustain profitability.
We incurred losses of $31.2 million, $38.7 million and $46.9 million for the years ended June 30, 2012, 2011 and 2010, respectively. We expect to continue to incur operating expenses and anticipate that we will continue to have losses in the foreseeable future as we pursue our current strategic direction. We expect to
B-13
continue to incur operating losses and anticipate that we will have net losses for the foreseeable future as we pursue our current strategic direction. Moreover, even if our Board determines to pursue a different strategic alternative, we expect that significant expenses will be involved in implementing any such strategic path, which will further reduce our existing capital. We may never achieve or sustain profitability as a business.
We will require additional capital to fund our pursuit and consummation of the acquisition of one or more biopharmaceutical assets.
The pursuit of our strategy to acquire one or more under-performing biopharmaceutical assets involves significant management time, effort and associated expense, and if such assets are identified, will require us to incur significant additional expenses to consummate. Moreover, we expect to require substantial additional funding to finance such acquisitions and to optimize the commercialization of such assets, and there can be no assurance that such additional funding will be available on terms that are acceptable to us, or at all. If adequate funds are not available on a timely basis, we may not be able to effectively implement our strategic plan.
We may seek to raise any necessary funds through public or private equity offerings, debt financings or strategic alliances and licensing arrangements. We currently have an effective universal shelf registration statement pursuant to which we have $80 million in securities available for sale. We may not be able to obtain additional financing on terms favorable to us, if at all. General market conditions may make it very difficult for us to seek financing from the capital markets. We may be required to relinquish rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us.
There can be no assurance that we will be successful in our pursuit of business development opportunities for our suspended clinical and preclinical programs.
We are actively pursuing business development opportunities, including out-licenses, for each of our now suspended clinical and preclinical programs. We face significant competition in our pursuit of these opportunities, and any arrangements will likely be complex and time-consuming to negotiate and document. We may not be able to negotiate any such arrangements on acceptable terms, or at all.
The absence of a permanent Chief Executive Officer may disrupt our operations and our future success depends on our ability to attract a highly qualified permanent Chief Executive Officer.
Richard B. Brewer, our President and Chief Executive Officer, died on August 15, 2012. David W. Gryska, our Chief Operating Officer, is currently serving as acting President and Chief Executive Officer while the Board of Directors considers succession plans. The appointment of Mr. Brewer as our President and Chief Executive Officer in May 2012 was an integral part of our change in strategic direction, and, unless a suitable replacement can promptly be identified and recruited, his loss will delay and impede our efforts. A search for a permanent Chief Executive Officer may take longer than we expect, and there can be no assurance that we will be able to attract a permanent Chief Executive Officer on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for such leadership. During this leadership transition, Mr. Gryska will bear substantial additional leadership responsibilities, which may present challenges as we seek to respond to business opportunities and make significant business decisions with a smaller executive team. Any failure to manage this leadership transition successfully could have a material adverse effect on our business.
Our future success depends on our ability to retain our key executives.
The competition for qualified personnel in the biopharmaceutical field is intense and we must retain and motivate our key executives. In the aftermath of Richard B. Brewer’s death, we remain even more dependent on David W. Gryska, our Chief Operating Officer and acting President and Chief Executive Officer, and Andrea Kendell, our Chief Financial Officer. There can be no assurance that we will be able to retain either Mr. Gryska or Ms. Kendell due in part to the fact that the agreements we have entered into with each of them provide for
B-14
employment that can be terminated by either party without cause at any time. Although we do not have any reason to believe that we may lose the services of either Mr. Gryska or Ms. Kendell in the foreseeable future, the loss of the services of either of them may impede our efforts to pursue a new strategic direction.
Changes in healthcare policy could adversely affect our business.
U.S. and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. For example, the Medicare Prescription Drug Improvement and Modernization Act of 2003, or MMA, expanded Medicare coverage for drugs purchased by Medicare beneficiaries and introduced new reimbursement methodologies. In addition, this law provided authority for limiting the number of drugs that will be covered in any therapeutic class. We do not know what impact the MMA and similar laws will have on the availability of coverage for and the price that we receive for any approved products. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare policies in setting their own reimbursement policies, and any reduction in reimbursement that results from the MMA may result in similar reductions by private payors.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or the ACA, enacted in March 2010, is expected to result in an increase in the number of people who are covered by both public and private insurance and is also expected to substantially change the way health care is financed by both government health program and private insurers, and significantly impact the pharmaceutical industry. The ACA contains a number of provisions that are expected to impact our business and operations in ways that may negatively affect our potential revenues in the future. While it is too early to predict all the specific effects the ACA or any future healthcare reform legislation will have on our business, they could have a material adverse effect on our business and financial condition. In addition, although the United States Supreme Court recently upheld the constitutionality of most of the ACA, some states have stated their intentions not to implement certain sections of the ACA, and some members of Congress are still working to repeal the ACA. These challenges add to the uncertainty of the changes enacted as part of the ACA.
The availability of government reimbursement for prescription drugs is also likely to be impacted by the Budget Control Act of 2011, which was signed into law on August 2, 2011. This law is expected to result in federal spending cuts totaling between $1.2 trillion and $1.5 trillion over the next decade over half of which will include cuts in Medicare and other health related spending.
If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, we could incur substantial liability.
The use of our drug candidates in clinical trials and the sale of any products for which marketing approval has been obtained expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, health care providers or others selling or otherwise coming into contact with our products. If we cannot successfully defend ourselves against product liability claims, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| • | decreased demand for any approved drug candidates; |
| • | impairment of our business reputation; |
| • | costs of related litigation; |
| • | distraction of management’s attention from our primary business; |
| • | substantial monetary awards to patients or other claimants; |
| • | loss of revenues; and |
| • | limitations on the commercialization of any approved drug candidates. |
B-15
We have obtained product liability insurance coverage for our previously conducted clinical trials with a $5.0 million annual aggregate coverage limit. However, our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we acquire any approved drug candidates, we intend to expand our insurance coverage to include the sale of commercial products; however, we may be unable to obtain this product liability insurance on commercially reasonable terms. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
Risks Related to Our Intellectual Property
If we are unable to adequately protect the intellectual property relating to our drug candidates, or if we infringe the rights of others, our ability to successfully commercialize our drug candidates will be harmed.
We own or hold licenses to a number of issued patents and U.S. pending patent applications, as well as foreign patents and pending PCT applications and foreign counterparts. As a biopharmaceutical company, our success will depend in part on our ability to obtain and maintain proprietary protection for our intellectual property, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, our ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions. Accordingly, rights under any issued patents may not provide us with sufficient protection for our drug candidates or provide sufficient protection to afford us a commercial advantage against competitive products or processes.
In addition, we cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to us. Even if patents have issued or will issue, we cannot guarantee that the claims of these patents are or will be valid or enforceable or will provide us with any significant protection against competitive products or otherwise be commercially valuable to us. Patent applications in the United States are maintained in confidence for up to 18 months after their filing. In some cases, however, patent applications remain confidential in the U.S. Patent and Trademark Office, or the U.S. Patent Office, for the entire time prior to issuance as a U.S. patent. Similarly, publication of discoveries in the scientific or patent literature often lag behind actual discoveries. Consequently, we cannot be certain that we or our licensors or co-owners were the first to invent, or the first to file patent applications on, our drug candidates or their use as drugs. In the event that a third party has also filed a U.S. patent application relating to our drug candidates or a similar invention, we may have to participate in interference proceedings declared by the U.S. Patent Office to determine priority of invention in the United States. The costs of these proceedings could be substantial and it is possible that our efforts would be unsuccessful, resulting in a loss of our U.S. patent position. Furthermore, we may not have identified all U.S. and foreign patents or published applications that affect our business either by blocking our ability to commercialize our drugs or by covering similar technologies.
The laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as in the United States and many companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. If we encounter such difficulties in protecting or are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business prospects could be substantially harmed.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated, or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary
B-16
technologies, drug candidates, and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
We license patent rights from third-party owners. Our licenses may be subject to early termination if we fail to comply with our obligations in our licenses with third parties.
We are party to a number of licenses that give us rights to third-party intellectual property. We may also enter into additional licenses to third-party intellectual property in the future. Our licensors may terminate their agreements with us in the event we breach the applicable license agreement and fail to cure the breach within a specified period of time. Under our existing license agreements we are obligated to pay the licensor fees, which may include annual license fees, royalties, a percentage of revenues associated with the licensed technology and a percentage of sublicensing revenue. In addition, under our existing license agreements, we are required to diligently pursue the development of products using the licensed technology. If we breach any of the terms of our licenses, the licensors may terminate the agreements.
Litigation regarding patents, patent applications and other proprietary rights may be expensive and time consuming. If we are involved in such litigation, it could cause delays in bringing drug candidates to market and harm our ability to operate.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may obtain patents in the future and allege that the use of our technologies infringes these patent claims or that we are employing their proprietary technology without authorization.
In addition, third parties may challenge or infringe upon our existing or future patents. Proceedings involving our patents or patent applications or those of others could result in adverse decisions regarding:
| • | the patentability of our inventions relating to our drug candidates; and/or |
| • | the enforceability, validity or scope of protection offered by our patents relating to our drug candidates. |
Even if we are successful in these proceedings, we may incur substantial costs and divert management time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may:
| • | incur substantial monetary damages; and/or |
| • | be precluded from participating in the manufacture, use or sale of our drug candidates or methods of treatment requiring licenses. |
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce
B-17
and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Risks Related to Our Common Stock
Our stock price has been and is likely to continue to be volatile and the market price of our common stock may drop.
On June 12, 2009, trading of shares of our common stock began on The NASDAQ Global Market on a “when-issued” basis and has continued on a “regular” basis since July 1, 2009. However, there can be no assurance that an active trading market for our common stock will continue or be sustained in the future. There is a limited history on which to gauge the volatility of our stock price; however, since our common stock began “regular” trading on The NASDAQ Global Market on July 1, 2009 through June 30, 2012, our stock price has fluctuated from a low of $2.26 to a high of $6.81. Furthermore, the stock market has recently experienced significant volatility, particularly with respect to pharmaceutical, biotechnology, and other life sciences company stocks. The volatility of pharmaceutical, biotechnology, and other life sciences company stocks often does not relate to the operating performance of the companies represented by the stock. We cannot predict the prices at which our common stock may trade in the future. The market price of our common stock may continue to fluctuate widely, depending upon many factors, some of which may be beyond our control, including:
| • | the outcome of our review and evaluation of strategic alternatives; |
| • | changes in our business strategy; |
| • | regulatory developments or enforcement in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | changes in estimates or recommendations by securities analysts, if any cover our common stock; |
| • | failure to secure adequate capital to fund our operations if and when needed, or the issuance of equity securities at prices below the current market price; |
| • | the ability to partner, sell or out-license rights to our programs on favorable terms; |
| • | litigation; |
| • | future sales of our common stock; |
| • | general market conditions; |
| • | economic and other external factors or other disasters or crises; |
| • | period-to-period fluctuations in our financial results; and |
| • | overall fluctuations in U.S. equity markets. |
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
Provisions of our charter and bylaws and Delaware law and our tax benefits preservation rights plan may make an acquisition of us or a change in our management more difficult.
Certain provisions of our restated certificate of incorporation and restated bylaws could discourage, delay, or prevent a merger, acquisition, or other change in control that stockholders may consider favorable, including
B-18
transactions in which you might otherwise receive a premium for your shares. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions also could limit the price that investors might be willing to pay for shares of our common stock, thereby depressing the market price of our common stock. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions:
| • | allow the authorized number of directors to be changed only by resolution of our board of directors; |
| • | establish a classified board of directors, providing that not all members of our board be elected at one time; |
| • | authorize our board of directors to issue without stockholder approval blank check preferred stock; |
| • | require that stockholder actions must be effected at a duly called stockholder meeting and prohibit stockholder action by written consent; |
| • | establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings; |
| • | limit who may call stockholder meetings; and |
| • | require the approval of the holders of 80% of the outstanding shares of our capital stock entitled to vote in order to amend certain provisions of our restated certificate of incorporation and restated bylaws. |
We have also adopted a Tax Benefits Preservation Rights Plan in the form of a rights agreement designed to help protect and preserve our substantial tax attributes primarily associated with net operating loss carryforwards (NOLs) and research tax credits, under Sections 382 and 383 of the Internal Revenue Code (the NOL Plan). Although this is not the purpose of the NOL Plan, it could have the effect of making it uneconomical for a third party to acquire us on a hostile basis. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or combining with us for a prescribed period of time.
We do not anticipate paying cash dividends, and accordingly, stockholders must rely on stock appreciation for any return on their investment.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
A failure to maintain adequate internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 or prevent or detect material misstatements in our annual or interim consolidated financial statements in the future could materially harm our business and cause our stock price to decline.
As a public company, our internal control over financial reporting is required to comply with the standards adopted by the Public Company Accounting Oversight Board in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002. Accordingly, we are currently required to document and test our internal controls and procedures to assess the effectiveness of our internal control over financial reporting. In addition, our independent registered public accounting firm is currently required to report on management’s assessment of the effectiveness of our internal control over financial reporting and the effectiveness of our internal control over financial reporting. If we are unable to maintain effective control over financial reporting, such conclusion would be disclosed in this and/or subsequent Annual Reports on Form 10-K. In the future, we may identify material weaknesses and deficiencies which we may not be able to remediate in a timely manner. If
B-19
we fail to maintain effective internal control over financial reporting in accordance with Section 404, we will not be able to conclude that we have and maintain effective internal control over financial reporting or our independent registered accounting firm may not be able to issue an unqualified report on the effectiveness of our internal control over financial reporting. As a result, our ability to report our financial results on a timely and accurate basis may be adversely affected, we may be subject to sanctions or investigation by regulatory authorities, including the SEC or The NASDAQ Global Market and investors may lose confidence in our financial information, which in turn could cause the market price of our common stock to decrease. We may also be required to restate our financial statements from prior periods. In addition, testing and maintaining internal control in accordance with Section 404 requires increased management time and resources. Any failure to maintain effective internal control over financial reporting could impair the success of our business and harm our financial results.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| Item 2. | PROPERTIES |
Our headquarters and facilities are located in Salt Lake City, Utah. We currently lease 87,000 square feet of office and laboratory space from Myriad Genetics, Inc., our former parent company, under a sublease with an initial term expiring January 2013 renewable at our election for a total of an additional 12 years in three-year increments.
We believe our existing facilities and equipment are well maintained and in good working condition and that our current facilities will provide adequate capacity and that additional space, if needed, will be available in the future on commercially reasonable terms.
| Item 3. | LEGAL PROCEEDINGS |
In the ordinary course of business, various legal claims have been asserted, and in the future may be asserted, against Myrexis. In addition, as previously disclosed, under the terms of our Separation and Distribution Agreement with our former parent Myriad Genetics, Inc. we have the obligation to indemnify Myriad Genetics with respect to certain legal claims against Myriad Genetics which we assumed in connection with our spin-out from Myriad Genetics.
| Item 4. | MINE SAFETY DISCLOSURES |
None.
B-20
| Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on The NASDAQ Global Market under the symbol “MYRX.” The following table sets forth the high and low sales prices for our common stock as reported by The NASDAQ Global Market for the periods indicated.
| High | Low | |||||||
| Fiscal Year Ended June 30, 2012: |
||||||||
| Fourth Quarter |
$ | 3.20 | $ | 2.26 | ||||
| Third Quarter |
$ | 3.32 | $ | 2.58 | ||||
| Second Quarter |
$ | 2.88 | $ | 2.41 | ||||
| First Quarter |
$ | 3.70 | $ | 2.64 | ||||
| High | Low | |||||||
| Fiscal Year Ended June 30, 2011: |
||||||||
| Fourth Quarter |
$ | 4.52 | $ | 3.29 | ||||
| Third Quarter |
$ | 4.26 | $ | 3.72 | ||||
| Second Quarter |
$ | 4.50 | $ | 3.57 | ||||
| First Quarter |
$ | 3.98 | $ | 3.56 | ||||
Stockholders
As of September 6, 2012, there were approximately 101 stockholders of record of our common stock and, according to our estimates, approximately 8,435 beneficial owners of our common stock.
Dividends
We have not paid cash dividends to our stockholders since our inception and we do not currently plan to pay cash dividends in the foreseeable future.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
None.
Stock Performance Graph
The graph set forth below compares the annual percentage change in our cumulative total stockholder return on our common stock, during a period commencing on June 12, 2009 (the first day of trading of our common stock on The NASDAQ Global Market) and ending on June 29, 2012 (as measured by dividing (A) the difference between our share price at the end and the beginning of the measurement period; by (B) our share price at the beginning of the measurement period) with the cumulative total return of The NASDAQ Stock Market, Inc. and the NASDAQ Biotech Index during such period. We have not paid any cash dividends on our common stock, and we do not include cash dividends in the representation of our performance. The price of a share of common stock is based upon the closing price per share as quoted on The NASDAQ Global Market on the last trading day of the
B-21
year shown. The graph lines merely connect quarter-end values and do not reflect fluctuations between those dates. The comparison assumes $100 was invested on June 12, 2009 in our common stock and in each of the foregoing indices. The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock.
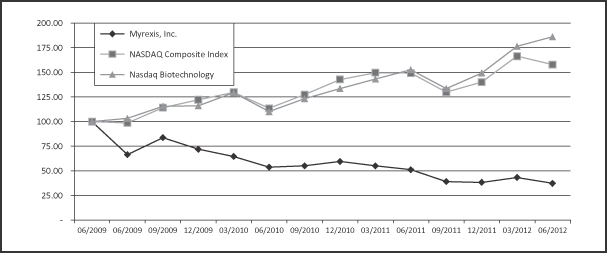
| June 12, 2009 |
June 30, 2009 |
Sept 30, 2009 |
Dec 31, 2009 |
Mar 31, 2010 |
June 30, 2010 |
Sept 30, 2010 |
Dec 31, 2010 |
Mar 31, 2011 |
June 30, 2011 |
Sept 30, 2011 |
Dec 30, 2011 |
Mar 30, 2012 |
June 29, 2012 |
|||||||||||||||||||||||||||||||||||||||||||
| Myrexis, Inc. |
100.00 | 66.43 | 83.71 | 71.86 | 64.57 | 53.71 | 55.14 | 59.43 | 55.00 | 51.14 | 39.14 | 38.29 | 43.29 | 37.29 | ||||||||||||||||||||||||||||||||||||||||||
| NASDAQ Biotechnology Index |
100.00 | 103.19 | 115.59 | 116.13 | 129.27 | 110.13 | 123.26 | 133.56 | 143.29 | 152.59 | 133.49 | 149.33 | 176.37 | 186.08 | ||||||||||||||||||||||||||||||||||||||||||
| NASDAQ Composite Index |
100.00 | 98.72 | 114.18 | 122.08 | 129.69 | 113.47 | 127.43 | 142.72 | 149.62 | 149.21 | 129.94 | 140.15 | 166.32 | 157.90 | ||||||||||||||||||||||||||||||||||||||||||
The performance graph shall not be deemed to be incorporated by reference by means of any general statement incorporating by reference this Form 10-K into any filing under the Securities Act of 1933, as amended or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate such information by reference, and shall not otherwise be deemed filed or soliciting material under such acts.
| Item 6. | SELECTED FINANCIAL DATA |
The following table sets forth selected financial information as of and for each of the years in the five-year period ended June 30, 2012, which has been derived from our (1) audited financial statements as of June 30, 2012 and 2011 and for the years ended June 30, 2012, 2011 and 2010, which are included elsewhere in this Form 10-K; and (2) audited financial statements as of June 30, 2010, 2009 and 2008 and for the years ended June 30, 2009 and 2008, which are not included elsewhere in this Form 10-K. Because our historical financial information for periods ending on or prior to June 30, 2009 reflects allocations for services historically provided to us by Myriad Genetics, the selected financial information presented below for such periods may not be indicative of our results of operations and financial position as an independent company. The selected financial information presented for the years ended June 30, 2012, 2011 and 2010, reflects our performance as an independent company.
B-22
The selected financial data below should be read in conjunction with our audited financial statements (and notes thereon) and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” included elsewhere in this Form 10-K.
| Years ended June 30, | ||||||||||||||||||||
| In thousands (except per share data) | 2012 | 2011 | 2010 | 2009 | 2008 | |||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||
| Research revenue |
$ | — | $ | 185 | $ | 90 | $ | 5,456 | $ | 6,774 | ||||||||||
| Pharmaceutical revenue |
— | — | — | — | 100,000 | (1) | ||||||||||||||
| Other revenue |
— | — | — | — | 4,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenues |
— | 185 | 90 | 5,456 | 110,774 | |||||||||||||||
| Costs and expenses: |
||||||||||||||||||||
| Research and development expense |
14,230 | 22,296 | 28,222 | 54,611 | (7) | 121,526 | (2) | |||||||||||||
| General and administrative expense |
17,571 | 18,339 | (8) | 19,984 | 8,981 | 20,600 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
31,801 | 40,635 | 48,206 | 63,592 | 142,126 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(31,801 | ) | (40,450 | ) | (48,116 | ) | (58,136 | ) | (31,352 | ) | ||||||||||
| Other income (expense), net |
592 | 1,742 | 1,165 | — | (3,017 | )(3) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (31,209 | ) | $ | (38,708 | ) | $ | (46,951 | ) | $ | (58,136 | ) | $ | (34,369 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per basic and diluted share (4) |
$ | (1.18 | ) | $ | (1.52 | ) | $ | (1.91 | ) | $ | (2.43 | ) | $ | (1.43 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| As of June 30, | ||||||||||||||||||||
| In thousands | 2012 | 2011 | 2010 | 2009 | 2008 | |||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents and marketable securities (5) |
$ | 89,626 | $ | 115,878 | $ | 147,453 | $ | 188,005 | $ | — | ||||||||||
| Current liabilities |
2,279 | 3,310 | 4,250 | 4,576 | 46,568 | |||||||||||||||
| Total assets |
91,651 | 121,260 | 154,108 | 193,677 | 15,746 | |||||||||||||||
| Total stockholders’ / parent equity (6) |
89,372 | 117,950 | 149,858 | 189,101 | (30,822 | ) | ||||||||||||||
| (1) | Represents a nonrefundable upfront payment from A/S Lundbeck for the former drug candidate Flurizan. |
| (2) | Amount includes an accrued $20 million sublicense fee payable related to Flurizan. |
| (3) | Amount includes the write-off of the cost basis investment in Encore Pharmaceuticals. |
| (4) | For years ended June 30, 2009 and 2008, pro forma net loss per share calculated based on the 23,974,211 shares issued in connection with the spin-off. |
| (5) | Prior to June 30, 2009, all cash and investments were held and managed by Myriad Genetics. |
| (6) | Balances prior to June 30, 2009 represent Myriad Genetics’ net investment (or capital deficiency) in Myrexis. |
| (7) | Amount includes a $9.0 million credit recorded in fiscal 2009, resulting from the difference in an estimated sub-license fee accrual recorded in fiscal 2008 and amounts actually paid in 2009. |
| (8) | Includes a $1.1 million impairment loss on certain fixed assets. For the year ended June 30, 2011, the Company reclassified the $1.1 million in impairment charges from other income (expense) to general and administrative expense. |
B-23
Quarterly Financial Data (Unaudited)
| Quarter Ended | ||||||||||||||||
| In thousands | June 30, 2012 |
March 31, 2012(3) |
December 31, 2011 |
September 30, 2011 |
||||||||||||
| Research revenue |
$ | — | $ | — | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and expenses: |
||||||||||||||||
| Research and development expense |
558 | 5,603 | 3,769 | 4,300 | ||||||||||||
| General and administrative expense |
4,129 | 5,216 | 3,841 | 4,385 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
4,687 | 10,819 | 7,610 | 8,685 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(4,687 | ) | (10,819 | ) | (7,610 | ) | (8,685 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income, net |
266 | 127 | 100 | 99 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (4,421 | ) | $ | (10,692 | ) | $ | (7,510 | ) | $ | (8,586 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Quarter Ended | ||||||||||||||||
| June 30, 2011 |
March 31, 2011(3) |
December 31, 2010 |
September 30, 2010 |
|||||||||||||
| Research revenue |
$ | — | $ | 55 | $ | 23 | $ | 107 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
— | 55 | 23 | 107 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and expenses: |
||||||||||||||||
| Research and development expense |
3,651 | 7,935 | 4,995 | 5,715 | ||||||||||||
| General and administrative expense |
4,449 | (1) | 5,088 | 4,240 | 4,562 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
8,100 | 13,023 | 9,235 | 10,277 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(8,100 | ) | (12,968 | ) | (9,212 | ) | (10,170 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income, net |
108 | 125 | 1,349 | (2) | 160 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (7,992 | ) | $ | (12,843 | ) | $ | (7,863 | ) | $ | (10,010 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Includes a $1.1 million impairment loss on certain fixed assets. For the year ended June 30, 2011, the Company reclassified the $1.1 million in impairment charges from other income (expense) to general and administrative expense. |
| (2) | Includes a one-time $1.2 million grant received in November 2010 as a part of the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code. |
| (3) | Includes one-time severance costs related to corporate reorganizations of $3.6 million for the period ending March 31, 2012 and $3.0 million for the period ended March 31, 2011. |
| Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis should be read together with “Selected Financial Data,” and the financial statements and the related notes appearing elsewhere in this Form 10-K. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Information regarding these forward-looking statements can be found in the preface to Part I, Item 1 “Business” of this Form 10-K. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including, but not limited to, those set forth under “Risk Factors” and elsewhere in this Form 10-K.
B-24
Overview
We are a biopharmaceutical company that has generated a pipeline of differentiated drug candidates in oncology and autoimmune diseases. We currently retain all rights to all of our drug candidates and programs across all geographic markets and therapeutic indications.
In September 2011, we announced that we had completed an in-depth review of our drug development pipeline, incorporating extensive inputs from both internal and independent external analyses. As a result, we made a strategic business decision to suspend any further development of our lead drug candidate Azixa, which was in Phase 2 development for the treatment of advanced primary and metastatic tumors with brain involvement. This decision was not based on any single factor. Our review took into consideration the accumulated data from our clinical trials, the evolving competitive environment in Glioblastoma multiforme, or GBM, including ongoing studies of competitive drug candidates that are in more advanced stages of development, inputs from key opinion leaders, updated cost and timing estimates, and other factors affecting the risks and opportunities relating to the development of Azixa. On the basis of these inputs, we concluded that completing the Phase 2b clinical trial we had underway would require a disproportionate investment of time and resources relative to its likelihood of technical and regulatory success, when compared to our other programs. Following this decision, in November 2011, we announced a corporate reorganization to realign our resources with our development strategy and clinical initiatives following the suspension of further development of Azixa. The reorganization included an immediate reduction in our workforce by 15 employees or approximately 20%.
In February 2012, we announced that we had suspended development activity on all of our preclinical and clinical programs and retained Stifel Nicolaus Weisel, an investment banking firm, to assist in reviewing and evaluating a full range of strategic alternatives to enhance shareholder value. Thereafter, in March 2012, we initiated an alignment of our resources involving a phased reduction in our workforce from approximately 59 employees to 10 current employees.
Based on our evaluation of strategic alternatives, we determined to pursue the acquisition of one or more commercial-stage biopharmaceutical assets, with the goal of building a commercial-stage biopharmaceutical company by optimizing their performance and profitability. Integral to these efforts, on May 11, 2012, we announced a change in management, including the appointment of Richard B. Brewer as President and Chief Executive Officer and David W. Gryska as Chief Operating Officer, collectively bringing an extensive track record of commercializing, acquiring and marketing pharmaceutical products throughout their careers. In addition, both Mr. Brewer and Mr. Gryska were appointed as members of the Board of Directors.
On August 15, 2012, we announced the death of Richard B. Brewer, our President and Chief Executive Officer. The Board of Directors appointed David W. Gryska as the acting President and Chief Executive Officer while considering succession plans. In addition, the Board of Directors is further evaluating our strategic direction in light of this development and our progress to date in identifying attractive biopharmaceutical assets.
We do not know if we will be successful in pursuing any strategic alternative or that any transaction will occur; however, we are committed to pursuing a strategic direction that our Board of Directors believes is in the best interests of our shareholders. During this period, we continue to actively pursue business development opportunities for each of our programs. However, despite our significant efforts to identify and attract third parties to whom we could out-license or sell these assets for further development, we have been unsuccessful to date.
We were incorporated as Myriad Pharmaceuticals, Inc. in Delaware in January 2009 as a new, wholly owned subsidiary of Myriad Genetics, Inc. in order to effect the separation and spin-off of Myriad Genetics’ research and drug development businesses as a stand-alone, independent, publicly traded company. In connection with the formation of this new subsidiary, Myriad Genetics’ existing subsidiary, Myriad Pharmaceuticals, Inc., changed its corporate name to Myriad Therapeutics, Inc., and we adopted the name of Myriad Pharmaceuticals, Inc. which was subsequently changed to Myrexis, Inc. effective July 1, 2010. On June 30, 2009, Myriad Genetics
B-25
contributed substantially all of the assets and certain liabilities of its research and drug development businesses as well as $188 million in cash and marketable securities to us and effected the spin-off of our company through a pro rata dividend distribution to its stockholders of all outstanding shares of our common stock.
We operate in one reportable business segment, pharmaceutical development and related research activities.
During the years ended June 30, 2012, 2011 and 2010, we reported $0, $185,000 and $90,000, respectively in revenues associated with research services related to short-term research agreements and a net loss of $31.2 million, $38.7 million and $46.9 million, respectively.
We expect to incur net losses for the foreseeable future and that such losses will fluctuate from quarter to quarter.
Our drug research and development expenses include costs incurred for our drug candidates. The only costs we track by each drug candidate are external costs such as services provided to us by clinical research organizations, manufacturing of drug supply, and other outsourced research. We do not assign or allocate internal costs such as salaries and benefits, facilities costs, lab supplies and the costs of preclinical research and studies to individual development programs. We also incurred costs related to external research collaborations from our research services business. We track all underlying principal costs associated with our research collaborations. All development costs for our drug candidates and external research collaborations are expensed as incurred. Our research and development expense for Azixa (for which development was suspended in September 2011), our clinical-stage drug candidate, MPC-3100, our preclinical-stage drug candidates, MPC-9528, MPC-8640, IKKe and MPC-0767 (for which development was suspended in February 2012), and our discontinued drug candidate MPC-4326 during the fiscal years ended June 30, 2012, 2011 and 2010 are as follows:
| Years Ended June 30, | ||||||||||||
| (In thousands) | 2012 | 2011 | 2010 | |||||||||
| External costs, drug candidates: |
||||||||||||
| Azixa |
$ | 1,367 | $ | 1,388 | $ | 2,998 | ||||||
| MPC-4326 |
40 | (144 | )(1) | 1,720 | ||||||||
| MPC-3100 |
214 | 1,202 | 2,568 | |||||||||
| MPC-0767 |
980 | 278 | — | |||||||||
| MPC-9528 |
— | 264 | 14 | |||||||||
| MPC-8640 |
1,124 | 121 | — | |||||||||
| IKKe |
269 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Sub-total direct costs |
3,994 | 3,109 | 7,300 | |||||||||
| Internal costs, drug candidates |
4,645 | 5,318 | 5,965 | |||||||||
| Preclinical development costs |
5,591 | 13,157 | 13,812 | |||||||||
| External research collaborations |
— | 712 | 1,145 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total research and development |
$ | 14,230 | $ | 22,296 | $ | 28,222 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Amount includes a $0.2 million credit recorded in fiscal 2011 resulting from a favorable change in estimate for outside clinical services which were later terminated due to the discontinuation of the program. |
We expect to see reduced research and development costs as a result of the decision to suspend further development activities for all preclinical and clinical programs.
B-26
Critical Accounting Policies and Use of Estimates
Critical accounting policies are those policies which are both important to the portrayal of a company’s financial condition and results and require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies are as follows:
| • | income taxes; |
| • | clinical trial expenses; |
| • | share-based payment expense; and |
| • | impairment of long-lived assets. |
Income Taxes
Our income tax provision is based on income before taxes and is computed using the liability method in accordance with Accounting Standards Codification, or ASC, 740—Income Taxes. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using tax rates projected to be in effect for the year in which the differences are expected to reverse. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations, or the expected results from any future tax examinations. Various internal and external factors may have favorable or unfavorable effects on our future provision for income taxes. Those factors include, but are not limited to, changes in tax laws, regulations and/or rates, the results of any future tax examinations, changing interpretations of existing tax laws or regulations, changes in estimates of prior years’ items, past levels of R&D spending, acquisitions, changes in our corporate structure, and changes in overall levels of income before taxes all of which may result in periodic revisions to our provision for income taxes.
Developing our provision for income taxes, including our effective tax rate and analysis of potential uncertain tax positions, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowance we deem necessary to offset deferred tax assets. We have established a valuation allowance to fully offset our deferred tax assets. Our judgment and tax strategies are subject to audit by various taxing authorities. While we believe we have provided adequately for our uncertain income tax positions in our consolidated financial statements, an adverse determination by these taxing authorities could have a material adverse effect on our consolidated financial condition, results of operations or cash flows. Interest and penalties on income tax items are included as a component of overall income tax expense.
Clinical Trial Expenses
Prior to our suspension of drug development activities, the cost of our clinical trials was based, in part, on estimates of the services received and efforts expended pursuant to contracts with numerous clinical trial centers and clinical research organizations, or the CROs. We contracted with third parties to perform various clinical trial activities in the development of our drug candidates. The financial terms of the agreements varied from contract to contract, were subject to negotiation and resulted in uneven payment flows. Payments under the contracts depended on factors such as the achievement of certain events, the successful enrollment of patients or the completion of portions of the clinical trial or similar conditions. The objective of our accrual policy was to match the recording of expenses in our financial statements to the actual services received and efforts expended. As such, we recognized direct expenses related to each patient enrolled in a clinical trial on an estimated cost-per-patient basis as services were performed. In addition, we considered information from our clinical operations group regarding the status of our clinical trials, we relied on information from CROs, such as estimated costs per patient, to calculate our accrual for direct clinical expenses at the end of each reporting
B-27
period. For indirect expenses, which related to site and other administrative costs to manage our clinical trials, we relied on information provided by the CRO, including costs incurred by the CRO as of a particular reporting date, to calculate our indirect clinical expenses. In connection with the early termination of a clinical trial, we recognized expenses in an amount based on our estimate of the remaining non-cancelable obligations associated with the winding down of the clinical trial, which we confirmed directly with the CRO.
If our CROs were to have either under or over reported the costs that they had incurred or if there was a change in the estimated per patient costs, it could have had an impact on our clinical trial expenses during the period in which they reported a change in estimated costs to us. Adjustments to our clinical trial accruals primarily relate to indirect costs, for which we placed significant reliance on our CROs for accurate information at the end of each reporting period.
Share-Based Payment Expense
Share-based compensation expense standards set accounting requirements for “share-based” compensation to employees, including employee stock purchase plans, and require us to recognize, as expense, in our statements of operations, the grant date fair value of our stock options and other equity-based compensation. The determination of grant date fair value is estimated using an option-pricing model, which includes variables such as the terms of each grant, the expected volatility of our share price, the exercise behavior of our employees, interest rates, and dividend yields. These variables are projected based on our historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based payments.
In connection with the separation and spin-off from Myriad Genetics and related transactions, each outstanding Myriad Genetics stock option was converted into an adjusted Myriad Genetics common stock option, exercisable for the same number of shares of common stock as the original Myriad Genetics option, and a new Myrexis common stock option, exercisable for one-fourth of the number of shares of common stock as the original Myriad Genetics option. An adjusted exercise price of each converted option was determined in accordance with Section 409A and Section 422 of the Internal Revenue Code of 1986, as amended. All other terms of the converted options remain the same however; the vesting and expiration of the converted options will be based on the optionholder’s continuing employment with Myriad Genetics or Myrexis, as applicable, following the separation.
As a result of the option modifications that occurred in connection with the separation from Myriad Genetics, Myriad Genetics measured the potential accounting impact of these option modifications. Based upon the analysis, which included a comparison of the fair value of the modified options granted to our employees and directors immediately after the modification with the fair value of the original option immediately prior to the modification, it was determined that there was no incremental compensation expense. All unrecognized compensation expense at June 30, 2009 that is related to Myriad Genetics options and Myrexis options that are held by current Myrexis employees and directors will be recognized by us over the remaining vesting term of the option. All such expense relating to Myrexis options held by current and former Myriad Genetics employees, directors or consultants will not be recognized by us.
Impairment of Long-Lived Assets
We assess the impairment of long-lived assets when events or changes in circumstances indicate that the carrying value of the assets or the asset grouping may not be recoverable. Factors that we consider in deciding when to perform an impairment review include significant negative industry or economic trends, and significant changes or planned changes in our use of the assets. We measure the recoverability of assets that will continue to be used in our operations by comparing the carrying value of the asset grouping to our estimate of the related total future undiscounted net cash flows. If an asset grouping’s carrying value is not recoverable through the related undiscounted cash flows, the asset grouping is considered to be impaired. The impairment is measured by comparing the difference between the asset grouping’s carrying value and its fair value. Fair value is the price
B-28
that would be received from selling an asset in an orderly transaction between market participants at the measurement date. Long-lived assets such as intangible assets and property, plant and equipment are considered non-financial assets, and are recorded at fair value only when an impairment charge is recognized. We recorded impairment charges for the years ended June 30, 2012 and 2011 of $0.3 million and $1.1 million, respectively. These charges are reflected in the statement of operations in general and administrative expenses.
We have evaluated our equipment and management has committed to a plan to sell our laboratory equipment. Equipment categorized as equipment held for sale on the balance sheet at June 30, 2012 totaled $1.0 million. Equipment held for sale is no longer subject to depreciation, and is recorded at the lower of depreciated carrying value or fair market value less costs to sell. We expect to sell these assets by the end of calendar year 2012.
Results of Operations
Years ended June 30, 2012 and 2011
Research revenue is comprised of research services related to short-term research agreements. Research revenue for the fiscal year ended June 30, 2012 was $0 compared to $185,000 in the prior year. The decrease in research revenue was primarily attributable to stopping all contract research services activity in March 2011 as a result of a corporate reorganization.
Research and development expenses are comprised primarily of salaries and related personnel costs, laboratory supplies, equipments costs, and costs associated with our clinical trials. Research and development expenses for the fiscal year ended June 30, 2012 were $14.2 million compared to $22.3 million in 2011. This 36% decrease was primarily due to:
| • | decreased internal preclinical developments costs of approximately $7.6 million resulting from a reduction in headcount; |
| • | increased external drug candidate costs associated with our Nampt and Hsp90 drug candidates of $1.9 million, partially offset by decreased costs of $1.1 million associated with the development of other drug candidates and the timing of the trial initiations and completions; and |
| • | decreased external research collaboration costs of $0.7 million associated with a reduction in headcount. |
We expect to see reduced research and development costs as a result of the decision to suspend further activities for all preclinical and clinical programs.
General and administrative expenses consist primarily of salaries and related personnel costs for business development, executive, legal, finance and accounting, information technology, human resources, and allocated facilities expenses. General and administrative expenses for the fiscal year ended June 30, 2012 were $17.6 million, compared to $18.3 million in 2011. The decrease in general and administrative expenses of 4% was due primarily to a decrease in the loss on impairment of assets from $1.1 million to $0.3 million, share-based compensation and depreciation expense, partially offset by increased severance and professional fees during the year ended June 30, 2012. We recognized $2.5 million in severance expenses recorded in general and administrative for the year ended June 30, 2012 in connection with executive management changes, the November 2011 corporate reorganization and the March 2012 resource alignment. We recognized $0.5 million in severance expenses recorded in general and administrative for the year ended June 30, 2011. We expect our general and administrative expenses to decrease as a result of these changes.
Other income (expense) for the fiscal year ended June 30, 2012 was $0.6 million compared to $1.7 million for the fiscal year ended June 30, 2011. Other income for the year ended June 30, 2012, reflects interest income and realized gains on our marketable securities and a gain on sale of assets of $0.3 million. Other income for the
B-29
year ended June 30, 2011 reflects interest income and realized gains on our marketable securities. Other income for the year ended June 30, 2011, includes a $1.2 million one-time grant received in November 2010 as a part of the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code.
Years ended June 30, 2011 and 2010
Research revenue for the fiscal year ended June 30, 2011 was $185,000 compared to $90,000 in 2010. The 105% increase in research revenue was primarily attributable to increased research services related to short-term research agreements that were completed during the 2011 fiscal year. As a result of the March 2011 corporate reorganization, we have stopped all contract research services activity going forward.
Research and development expenses for the fiscal year ended June 30, 2011 were $22.3 million compared to $28.2 million in 2010. This 21% decrease was primarily due to:
| • | decreased internal preclinical developments costs of approximately $0.7 million resulting from a reduction in headcount; |
| • | decreased external drug candidate costs associated with our HIV drug candidate of $1.9 million, decreased costs of $1.6 million associated with the development of Azixa and the timing of the Phase 2b trial initiation, and decreased costs of $1.4 million associated with MPC-3100 due to the completion of current studies; and |
| • | decreased external research collaboration costs of $0.4 million associated with a reduction in headcount. |
General and administrative expenses for the fiscal year ended June 30, 2011 were $17.2 million, compared to $20.0 million in 2010. The decrease in general and administrative expenses of 14% was due primarily to a decrease in expenses as a result of a reduction in headcount in June 2010. We incurred $3.1 million in external acquisition expenses in connection with the proposed merger with Javelin Pharmaceuticals, Inc. that was terminated in April 2010. These expenses were offset by $1.5 million in stipulated expenses reimbursed by Javelin plus a termination fee of $2.9 million. These reimbursed expenses are presented in the financials for the year ended June 30, 2010, as an offset to total general and administrative costs.
Other income (expense) for the fiscal year ended June 30, 2011 was $1.7 million compared to $1.2 million for the fiscal year ended June 30, 2010. Other income in the year ended June 30, 2011 includes a one-time $1.2 million grant received in November 2010 as a part of the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code and interest income and realized gains on our marketable securities. Other income for the same period in 2010 reflects interest income and realized gains on our marketable securities, offset by a loss on disposal of assets of $0.2 million.
Liquidity and Capital Resources
Net cash used in operating activities was $27.8 million during the fiscal year ended June 30, 2012 compared to $33.5 million used by operating activities during the prior fiscal year. The change in cash flow from operating activity can be attributed primarily to the higher net loss in fiscal 2011 offset, in part, by higher non-cash charges associated with share-based compensation recorded in fiscal 2011.
Our investing activities provided $27.1 million in cash during the fiscal year ended June 30, 2012 compared to $14.8 million during the prior fiscal year. The change is primarily due to the maturities and selling of our marketable investment securities. In addition, we received $0.5 million in proceeds from the sale of assets during the year ended June 30, 2012. We anticipate our investment in additional equipment and leasehold improvements will be minimal in the future.
Approximately $1.2 million in cash was provided by financing activities during fiscal 2012, compared to $1.9 million during the prior fiscal year. Financing activities in fiscal 2012 and 2011 were comprised primarily of cash proceeds from the exercise of stock awards.
B-30
As of June 30, 2012, we had $89.6 million in cash, cash equivalents and marketable securities, a decrease of $26.3 million from $115.9 million as of June 30, 2011. Notwithstanding the factors listed below, we believe our cash, cash equivalents and marketable securities are sufficient for at least the next 12 months. Our future capital requirements, cash flows, and results of operations could be affected by and will depend on many factors that are currently unknown to us, including:
| • | the outcome of our review and evaluation of strategic alternatives; |
| • | changes in our business strategy; |
| • | regulatory developments or enforcement in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | changes in estimates or recommendations by securities analysts, if any cover our common stock; |
| • | the ability to partner, sell or out-license rights to our programs on favorable terms; |
| • | failure to secure adequate capital to fund our operations if and when needed, or the issuance of equity securities at prices below the current market price; |
| • | litigation; |
| • | future sales of our common stock; |
| • | general market conditions; |
| • | economic and other external factors or other disasters or crises; |
| • | period-to-period fluctuations in our financial results; and |
| • | overall fluctuations in U.S. equity markets. |
To the extent our capital resources are insufficient to meet our future capital requirements, we will need to finance our future cash needs through public or private equity offerings, collaboration agreements, debt financings or licensing arrangements. However, the credit markets and the financial services industry have recently been experiencing a period of unprecedented turmoil and upheaval that have made equity and debt financing more difficult to obtain. Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. For example, if we raise additional funds by issuing equity securities or by selling convertible debt securities, further dilution to our existing stockholders may result. If we raise funds through licensing arrangements, we may be required to relinquish rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us.
We may elect to raise additional funds even before we need them if the conditions for raising capital are favorable. We have an effective universal shelf registration statement on Form S-3 pursuant to which we have up to $80 million of securities available for issuance.
Off-Balance Sheet Arrangements
None.
Contractual Obligations
The following table represents our contractual obligations as of June 30, 2012 (in thousands):
| Total | Less than one year |
1-3 Years | 4-5 Years | More than 5 years |
||||||||||||||||
| Lease Obligations |
$ | 2,104 | $ | 2,104 | $ | — | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 2,104 | $ | 2,104 | $ | — | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
B-31
The table above only includes payment obligations that are fixed or determinable. The table excludes potential milestone payments we may have been required to pay under the now terminated EpiCept license agreement in the aggregate of up to $23 million.
Effects of Inflation
We do not believe that inflation has had a material impact on our business, revenues, or operating results during the periods presented.
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We maintain a portfolio of cash, cash equivalents and short term and long term marketable securities which are subject to interest rate risk. Our investments consist primarily of highly liquid securities of various types and maturities of two years or less, with a maximum average maturity of 12 months. Changes in interest rates affect the fair market value of these marketable investment securities. After a review of our marketable securities as of June 30, 2012 and 2011, we have determined, hypothetically, that in the event of a change of 100 basis points in a key market interest rate, the resulting change in fair market value of our marketable investment securities would not have a material effect on our financial condition or on our financial statements as a whole.
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The information required by this Item 8 is included at the end of this Annual Report on Form 10-K beginning on page F-1.
MYREXIS, INC.
| Index to Financial Statements |
Number | |||
| B-40 | ||||
| B-41 | ||||
| Statements of Operations for the Years Ended June 30, 2012, 2011 and 2010 |
B-42 | |||
| B-43 | ||||
| Statements of Cash Flows for the Years Ended June 30, 2012, 2011 and 2010 |
B-44 | |||
| B-45 | ||||
B-32
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| Item 9A. | CONTROLS AND PROCEDURES |
1. Disclosure Controls and Procedures
We maintain disclosure controls and procedures (Disclosure Controls) within the meaning of Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Our Disclosure Controls are designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act, such as this Annual Report on Form 10-K, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Our Disclosure Controls are also designed to ensure that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Our acting Chief Executive Officer and Chief Financial Officer, after evaluating the effectiveness of our Disclosure Controls as of the end of the period covered by this Annual Report on Form 10-K, have concluded that, based on such evaluation, our Disclosure Controls were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
2. Internal Control Over Financial Reporting
(a) Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, a company’s principal executive and principal financial officers and effected by the company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that:
| • | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| • | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| • | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of those inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Our management assessed the effectiveness of our internal control over financial reporting as of June 30, 2012. In making their assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework. Based on our assessment, management believes that, as of June 30, 2012, our internal control over financial reporting is effective based on those criteria.
Our independent registered public accounting firm has issued its report on the effectiveness of our internal control over financial reporting. This report appears below.
B-33
(b) Attestation Report of the Registered Public Accounting Firm
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Myrexis, Inc.
We have audited Myrexis, Inc.’s internal control over financial reporting as of June 30, 2012, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Myrexis, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Myrexis, Inc. maintained, in all material respects, effective internal control over financial reporting as of June 30, 2012, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Myrexis, Inc. as of June 30, 2012 and 2011, and the related statements of operations, stockholders’ equity and comprehensive loss, and cash flows for each of the three years in the period ended June 30, 2012, and our report dated September 13, 2012 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Salt Lake City, Utah
September 13, 2012
B-34
(c) Change in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during the fourth quarter of our last fiscal year that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | OTHER INFORMATION |
Not applicable.
B-35
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Management and Corporate Governance,” “Section 16(a) Beneficial Ownership Reporting Compliance” and “Code of Conduct and Ethics” in our Proxy Statement for the 2012 Annual Meeting of Stockholders.
| Item 11. | EXECUTIVE COMPENSATION |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Compensation Discussion and Analysis,” “Executive Officer and Director Compensation,” “Management and Corporate Governance-Committees of the Board of Directors and Meetings,” “Management and Corporate Governance-Compensation Committee Interlocks and Insider Participation,” “Compensation Committee Report” and “Risks Related to Compensation Practices and Policies” in our Proxy Statement for the 2012 Annual Meeting of Stockholders.
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in our Proxy Statement for the 2012 Annual Meeting of Stockholders.
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Certain Relationships and Related Person Transactions” and “Management and Corporate Governance-Director Independence” in our Proxy Statement for the 2012 Annual Meeting of Stockholders.
| Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The response to this item is incorporated by reference to the discussion responsive thereto in the proposal entitled “Independent Registered Public Accounting Firm” in our Proxy Statement for the 2012 Annual Meeting of Stockholders.
B-36
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are filed as part of this Annual Report on Form 10-K:
1. Financial Statements
See “Index to Financial Statements” at Item 8 of this Annual Report on Form 10-K.
2. Financial Statement Schedules
The financial statements beginning on page F-1 are filed as part of this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
3. Exhibits
The exhibits which are filed with or incorporated by reference into this Annual Report on Form 10-K are set forth in the Exhibit Index to this Annual Report on Form 10-K, which is incorporated herein by reference.
B-37
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on September 13, 2012.
| MYREXIS, INC. | ||
| By: |
/S/ DAVID W. GRYSKA | |
| David W. Gryska | ||
| Acting President and Chief Executive Officer and Chief Operating Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated below and on the dates indicated.
| Signatures |
Title |
Date | ||||
| By: |
/s/ DAVID W. GRYSKA |
Acting President and Chief Executive Officer, Chief Operating Officer and Director (principal executive officer) |
September 13, 2012 | |||
| David W. Gryska | ||||||
| By: |
/s/ ANDREA KENDELL |
Chief Financial Officer (principal financial and accounting officer) |
September 13, 2012 | |||
| Andrea Kendell | ||||||
| By: |
/s/ GERALD P. BELLE |
Chairman of the Board | September 13, 2012 | |||
| Gerald P. Belle | ||||||
| By: |
/s/ ROBERT FORRESTER |
Director | September 13, 2012 | |||
| Robert Forrester | ||||||
| By: |
/s/ ROBERT J. LOLLINI |
Director | September 13, 2012 | |||
| Robert J. Lollini | ||||||
| By: |
/s/ JOHN T. HENDERSON |
Director | September 13, 2012 | |||
| John T. Henderson, M.D. | ||||||
| By: |
/s/ DENNIS H. LANGER |
Director | September 13, 2012 | |||
| Dennis H. Langer, M.D., J.D. | ||||||
| By: |
/s/ JASON M. ARYEH |
Director | September 13, 2012 | |||
| Jason M. Aryeh | ||||||
| By: |
/s/ TIMOTHY R. FRANSON |
Director | September 13, 2012 | |||
| Timothy R. Franson, M.D. | ||||||
B-38
Myrexis, Inc.
Years Ended June 30, 2012, 2011 and 2010
| Page | ||||
| B-40 | ||||
| Financial Statements: |
||||
| B-41 | ||||
| Statements of Operations for the Years Ended June 30, 2012, 2011 and 2010 |
B-42 | |||
| B-43 | ||||
| Statements of Cash Flows for the Years Ended June 30, 2012, 2011 and 2010 |
B-44 | |||
| B-45 | ||||
B-39
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Myrexis, Inc.
We have audited the accompanying balance sheets of Myrexis, Inc. as of June 30, 2012 and 2011, and the related statements of operations, stockholders’ equity and comprehensive loss, and cash flows for each of the three years in the period ended June 30, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Myrexis, Inc. at June 30, 2012 and 2011, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2012, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Myrexis, Inc.’s internal control over financial reporting as of June 30, 2012, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated September 13, 2012 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Salt Lake City, Utah
September 13, 2012
B-40
MYREXIS, INC.
June 30, 2012 and 2011
(In thousands, except per share amounts)
| 2012 | 2011 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 19,707 | $ | 19,189 | ||||
| Marketable investment securities |
68,671 | 86,446 | ||||||
| Equipment held for sale |
974 | — | ||||||
| Prepaid expenses and other assets |
192 | 1,861 | ||||||
|
|
|
|
|
|||||
| Total current assets |
89,544 | 107,496 | ||||||
|
|
|
|
|
|||||
| Equipment and leasehold improvements: |
||||||||
| Equipment |
1,298 | 4,320 | ||||||
| Leasehold improvements |
1,197 | 1,192 | ||||||
|
|
|
|
|
|||||
| 2,495 | 5,512 | |||||||
| Less accumulated depreciation |
1,846 | 2,197 | ||||||
|
|
|
|
|
|||||
| Net equipment and leasehold improvements |
649 | 3,315 | ||||||
|
|
|
|
|
|||||
| Long-term marketable investment securities |
1,248 | 10,243 | ||||||
| Other assets |
210 | 206 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 91,651 | $ | 121,260 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 197 | $ | 1,210 | ||||
| Accrued liabilities |
2,082 | 2,100 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
2,279 | 3,310 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.01 par value, authorized 5,000 shares; no shares issued and outstanding |
— | — | ||||||
| Common stock, $0.01 par value, authorized 60,000 shares; issued and outstanding 26,794 shares at June 30, 2012; issued and outstanding 26,053 shares at June 30, 2011 |
268 | 261 | ||||||
| Additional paid-in capital |
205,968 | 203,301 | ||||||
| Accumulated other comprehensive income |
4 | 47 | ||||||
| Accumulated deficit |
(116,868 | ) | (85,659 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
89,372 | 117,950 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 91,651 | $ | 121,260 | ||||
|
|
|
|
|
|||||
See accompanying notes to financial statements.
B-41
MYREXIS, INC.
Years ended June 30, 2012, 2011 and 2010
(In thousands, except per share amounts)
| 2012 | 2011 | 2010 | ||||||||||
| Research revenue |
$ | — | $ | 185 | $ | 90 | ||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
— | 185 | 90 | |||||||||
|
|
|
|
|
|
|
|||||||
| Costs and expenses: |
||||||||||||
| Research and development expense |
14,230 | 22,296 | 28,222 | |||||||||
| General and administrative expense |
17,571 | 18,339 | 19,984 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total costs and expenses |
31,801 | 40,635 | 48,206 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(31,801 | ) | (40,450 | ) | (48,116 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income, net |
592 | 1,742 | 1,165 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (31,209 | ) | $ | (38,708 | ) | $ | (46,951 | ) | |||
|
|
|
|
|
|
|
|||||||
| Loss per basic and diluted share |
$ | (1.18 | ) | $ | (1.52 | ) | $ | (1.91 | ) | |||
| Weighted-average shares used to compute net loss per basic and diluted share |
26,387 | 25,513 | 24,545 | |||||||||
See accompanying notes to financial statements.
B-42
MYREXIS, INC.
Statements of Stockholders’ Equity and Comprehensive Loss
Years ended June 30, 2012, 2011 and 2010
(In thousands)
| Common Stock | Additional Paid-In Capital |
Accumulated Deficit |
Unrealized Gain on Available-for- sale securities |
Total Stockholders’ Equity |
||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Balance at June 30, 2009 |
23,974 | $ | 240 | $ | 188,400 | $ | — | $ | 461 | $ | 189,101 | |||||||||||||
| Comprehensive Income: |
||||||||||||||||||||||||
| Net loss |
— | — | — | (46,951 | ) | — | (46,951 | ) | ||||||||||||||||
| Change in unrealized gains on marketable investment securities |
— | — | — | — | (436 | ) | (436 | ) | ||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive loss |
(47,387 | ) | ||||||||||||||||||||||
| Issuance of common stock for cash upon exercise of options and employee stock purchase plan |
1,240 | 12 | 2,332 | — | — | 2,344 | ||||||||||||||||||
| Share-based payment expense |
— | — | 5,800 | — | — | 5,800 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2010 |
25,214 | 252 | 196,532 | (46,951 | ) | 25 | 149,858 | |||||||||||||||||
| Comprehensive Income: |
||||||||||||||||||||||||
| Net loss |
— | — | — | (38,708 | ) | — | (38,708 | ) | ||||||||||||||||
| Change in unrealized gains on marketable investment securities |
— | — | — | — | 22 | 22 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive loss |
(38,686 | ) | ||||||||||||||||||||||
| Issuance of common stock for cash upon exercise of options and employee stock purchase plan |
839 | 9 | 1,937 | — | — | 1,946 | ||||||||||||||||||
| Share-based payment expense |
— | — | 5,800 | — | — | 5,800 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2011 |
26,053 | 261 | 203,301 | (85,659 | ) | 47 | 117,950 | |||||||||||||||||
| Comprehensive Income: |
||||||||||||||||||||||||
| Net loss |
— | — | — | (31,209 | ) | — | (31,209 | ) | ||||||||||||||||
| Change in unrealized gains on marketable investment securities |
— | — | — | — | (43 | ) | (43 | ) | ||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive loss |
(31,252 | ) | ||||||||||||||||||||||
| Issuance of common stock for cash upon exercise of options and employee stock purchase plan |
741 | 7 | 1,160 | — | — | 1,167 | ||||||||||||||||||
| Share-based payment expense |
— | — | 1,507 | — | — | 1,507 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2012 |
26,794 | $ | 268 | $ | 205,968 | $ | (116,868 | ) | $ | 4 | $ | 89,372 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
See accompanying notes to financial statements.
B-43
MYREXIS, INC.
Years ended June 30, 2012, 2011 and 2010
(In thousands)
| 2012 | 2011 | 2010 | ||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (31,209 | ) | $ | (38,708 | ) | $ | (46,951 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
1,282 | 1,661 | 1,347 | |||||||||
| Loss on impairment of assets |
281 | 1,112 | 224 | |||||||||
| Share-based compensation expense |
1,507 | 4,832 | 5,800 | |||||||||
| Gain on sale of marketable investment securities |
(3 | ) | (5 | ) | (43 | ) | ||||||
| Gain on sale of assets |
(266 | ) | — | — | ||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Prepaid expenses |
1,669 | (1,414 | ) | (213 | ) | |||||||
| Other assets |
(4 | ) | — | (206 | ) | |||||||
| Accounts payable |
(1,013 | ) | (711 | ) | 1,927 | |||||||
| Accrued liabilities |
(18 | ) | (222 | ) | (2,253 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(27,774 | ) | (33,455 | ) | (40,368 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Capital expenditures for equipment and leasehold improvements |
(55 | ) | (93 | ) | (2,135 | ) | ||||||
| Proceeds from sale of assets |
450 | — | — | |||||||||
| Purchase of marketable investment securities |
(232,439 | ) | (142,428 | ) | (183,875 | ) | ||||||
| Proceeds from sale of marketable investment securities |
82,500 | 29,099 | 32,794 | |||||||||
| Proceeds from maturity of marketable investment securities |
176,669 | 128,209 | 98,779 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
27,125 | 14,787 | (54,437 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Net proceeds from common stock issued under share-based compensation plans |
1,167 | 1,946 | 2,344 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
1,167 | 1,946 | 2,344 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
518 | (16,722 | ) | (92,461 | ) | |||||||
| Cash and cash equivalents at beginning of year |
19,189 | 35,911 | 128,372 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of year |
$ | 19,707 | $ | 19,189 | $ | 35,911 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental cash flow information: |
||||||||||||
| Fair value adjustment on marketable investment securities recorded to stockholders’ equity |
43 | 22 | (436 | ) | ||||||||
See accompanying notes to financial statements.
B-44
MYREXIS, INC.
June 30, 2012, 2011, and 2010
| (1) | Organization and Summary of Significant Accounting Policies |
| (a) | Organization and Business Description |
Myrexis, Inc. (“Myrexis” or the “Company”) is a biopharmaceutical company that has generated a pipeline of differentiated drug candidates in oncology and autoimmune diseases. The Company currently retains all rights to all of its drug candidates and programs across all geographic markets and therapeutic indications.
In September 2011, the Company announced that it had completed an in-depth review of our drug development pipeline, incorporating extensive inputs from both internal and independent external analyses. As a result, the Company made a strategic business decision to suspend any further development of its lead drug candidate Azixa, which was in Phase 2 development for the treatment of advanced primary and metastatic tumors with brain involvement. This decision was not based on any single factor. The review took into consideration the accumulated data from its clinical trials, the evolving competitive environment in Glioblastoma multiforme, or GBM, including ongoing studies of competitive drug candidates that are in more advanced stages of development, inputs from key opinion leaders, updated cost and timing estimates, and other factors affecting the risks and opportunities relating to the development of Azixa. On the basis of these inputs, the Company concluded that completing the Phase 2b clinical trial it had underway would require a disproportionate investment of time and resources relative to its likelihood of technical and regulatory success, when compared to the Company’s other programs. Following this decision, in November 2011, Myrexis announced a corporate reorganization to realign its resources with its development strategy and clinical initiatives following the suspension of further development of Azixa. The reorganization included an immediate reduction in its workforce by 15 employees or approximately 20%.
In February 2012, the Company announced that it had suspended development activity on all of its preclinical and clinical programs and retained Stifel Nicolaus Weisel, an investment banking firm, to assist in reviewing and evaluating a full range of strategic alternatives to enhance shareholder value. Thereafter, in March 2012, the Company initiated an alignment of its resources involving a phased reduction in its workforce from approximately 59 employees to 10 current employees.
Based on the Company’s evaluation of strategic alternatives, it determined to pursue the acquisition of one or more commercial-stage biopharmaceutical assets, with the goal of building a commercial-stage biopharmaceutical company by optimizing their performance and profitability. Integral to these efforts, on May 11, 2012, the Company announced a change in management, including the appointment of Richard B. Brewer as President and Chief Executive Officer and David W. Gryska as Chief Operating Officer, collectively bringing an extensive track record of commercializing, acquiring and marketing pharmaceutical products throughout their careers. In addition, both Mr. Brewer and Mr. Gryska were appointed as members of the Board of Directors.
On August 15, 2012, the Company announced the death of Richard B. Brewer, its President and Chief Executive Officer. The Board of Directors appointed David W. Gryska as the acting President and Chief Executive Officer while considering succession plans. In addition, the Board of Directors is further evaluating our strategic direction in light of this development and the Company’s progress to date in identifying attractive biopharmaceutical assets.
The Company does not know if it will be successful in pursuing any strategic alternative or that any transaction will occur; however, the Company is committed to pursuing a strategic direction that its Board of Directors believes is in the best interests of its shareholders. During this period, the Company
B-45
continues to actively pursue business development opportunities for each of its programs. However, despite its significant efforts to identify and attract third parties to whom it could out-license or sell these assets for further development, the Company has been unsuccessful to date.
| (b) | Use of Estimates |
The preparation of the financial statements in accordance with U.S. generally accepted accounting principles requires Myrexis management to make estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the period. Significant items subject to such estimates and assumptions include assessment of impairment of long-lived assets, the carrying amount of certain accrued liabilities and share-based compensation. Actual results could differ from those estimates presented herein.
| (c) | Cash and Cash Equivalents |
The Company considers all cash on deposit, money market accounts, and highly liquid debt instruments purchased with original maturities of three months or less to be cash and cash equivalents. The Company maintains cash and cash equivalents in bank deposit and other investment accounts which, at times, may exceed federally insured limits.
| (d) | Loss Per Share |
The loss per basic and diluted share is calculated by dividing net loss by the weighted-average number of shares outstanding during the reported period.
For the year ended June 30, 2012, there were outstanding potential common equivalent shares of 2,648,774 compared to 2,613,945 and 2,004,904, in the same periods in 2011 and 2010 which were excluded from the computation of diluted earnings per share because the effect would have been anti-dilutive. These potential dilutive common equivalent shares may be dilutive to basic earnings per share in future periods.
The calculation of diluted loss per share is the same as the basic loss per share since the inclusion of any potentially dilutive securities would be anti-dilutive.
| (e) | Fair Value Disclosure |
At June 30, 2012 and 2011, the carrying value of the Company’s other receivables, accounts payable and accrued expenses approximates fair value, principally because of the short term nature of the assets and liabilities.
| (f) | Revenue Recognition |
Research revenue is comprised of research services related to short-term research agreements. Research revenue reflects revenues earned utilizing the Company’s prior expertise to identify and characterize protein-protein interactions. In connection with the Company’s March 2011 corporate reorganization, it stopped all contract research services activity.
| (g) | Research and Development Expenses |
Research and development expenses consist primarily of costs associated with the clinical trials of the Company’s product candidates, development materials, compensation and related benefits for research and development personnel, costs for consultants, and various overhead costs. Research and development costs are expensed as incurred. In February 2012, the Company suspended activity on all of its preclinical and clinical programs.
B-46
| (h) | General and Administrative Expenses |
General and administrative expenses for the year ended June 30, 2010, include $1.5 million in reimbursed stipulated expenses and a $2.9 million termination fee in connection with the proposed merger with Javelin Pharmaceuticals, Inc. that was terminated in April 2010. For the year ended June 30, 2010, the Company incurred expenses of $3.1 million in external acquisition expenses which are offset by the fees described above.
| (i) | Equipment Held for Sale |
In conjunction with the suspension of all development activities, the Company has evaluated its equipment and management has committed to a plan to sell the Company’s laboratory equipment. Equipment categorized as equipment held for sale on the balance sheet at June 30, 2012 totaled $1.0 million. Equipment held for sale is no longer subject to depreciation, and is recorded at the lower of depreciated carrying value or fair market value less costs to sell. The Company expects to sell these assets by the end of calendar year 2012.
| (j) | Equipment and Leasehold Improvements |
Equipment and leasehold improvements are stated at cost. Depreciation and amortization are computed using the straight-line method based on the lesser of estimated useful lives of the related assets or lease terms. Equipment items have depreciable lives of five years. Leasehold improvements are depreciated over the shorter of the estimated useful lives or the associated lease terms, which range from three to fifteen years. For the years ended June 30, 2012, 2011, and 2010, the Company recorded depreciation expense of $1.3 million, $1.7 million, and $1.3 million, respectively.
| (k) | Impairment of Long-Lived Assets |
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. For the years ended June 30, 2012, 2011 and 2010, $0.3 million, $1.1 million and $0.2 million, respectively, was recorded for impairment of assets, and is included in general and administrative expenses in the statement of operations.
| (l) | Other Assets |
Other assets are comprised of purchased intellectual property, a purchased library of chemical compounds and a security deposit for the sublease agreement entered into with Myriad Genetics, Inc. (“MGI”), the Company’s former parent, to provide for the lease of office and laboratory space and a sublease for office space in Monterey, CA. Management reviews the valuation of these investments for possible impairment as changes in facts and circumstances indicate that potential impairment should be assessed.
The library of chemical compounds and related purchased intellectual property were fully amortized during the year ended June 30, 2010. Myrexis has also reassessed the useful lives of its other assets and has determined that the estimated useful lives are appropriate.
For the years ended June 30, 2012, 2011, and 2010, the Company recorded amortization expense of $0, $0, and $95,000, respectively, related to these assets.
As of June 30, 2012, other assets is comprised of only security deposits for the sublease agreement entered into with MGI and a sublease for office space in Monterey, CA.
B-47
| (m) | Income Taxes |
The Company recognizes income taxes under the liability method. This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities.
The provision for income taxes, including the effective tax rate and analysis of potential tax exposure items, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowances deemed necessary to recognize deferred tax assets at an amount that is more likely than not to be realized. The Company’s filings, including the positions taken therein, are subject to audit by various taxing authorities. While the Company believes it has provided adequately for its income tax liabilities in the consolidated financial statements, adverse determinations by these taxing authorities could have a material adverse effect on the consolidated financial condition, results of operations or cash flows.
| (n) | Share-based Compensation |
The Company recognizes compensation expense using a fair-value based method for costs related to stock options and other equity-based compensation. The expense is measured based on the grant date fair value of the awards that are expected to vest, and the expense is recorded over the applicable requisite service period. For time-based stock options and restricted stock, compensation expense is recognized over the vesting period from the vesting commencement date using the straight-line method. In the absence of an observable market price for a share-based award, the fair value is based upon a valuation methodology that takes into consideration various factors, including the exercise price of the award, the expected term of the award, the current price of the underlying shares, the expected volatility of the underlying share price based on peer companies, the expected dividends on the underlying shares and the risk-free interest rate.
| (o) | Marketable Investment Securities |
The Company has classified its marketable investment securities as available-for-sale. These securities are carried at estimated fair value with unrealized holding gains and losses, net of the related tax effect, included in accumulated other comprehensive loss in stockholders’ equity until realized. Gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned.
A decline in the market value of any available-for-sale security below cost that is deemed other than temporary results in a charge to earnings and establishes a new cost basis for the security. Losses are charged against “Other income (expense)” when a decline in fair value is determined to be other than temporary. The Company reviews several factors to determine whether a loss is other than temporary. These factors include but are not limited to: (i) the extent to which the fair value is less than cost and the cause for the fair value decline, (ii) the financial condition and near term prospects of the issuer or declines in credit risk, (iii) the length of time a security is in an unrealized loss position and (iv) the Company more likely than not, holding securities for a period of time sufficient to allow for any anticipated recovery in fair value. The Company recognized no impairments on available-for-sale securities for the years ended June 30, 2012, 2011 and 2010.
| (p) | Segment and Related Information |
The Company operates in one reportable business segment, pharmaceutical development and related research activities.
The Company’s revenues were derived from research performed in the United States. Additionally, all of the Company’s long-lived assets are located in the United States.
B-48
| (q) | Reclassifications |
Certain amounts for prior periods have been reclassified to conform to the current year presentation. For the year ended June 30, 2011, the Company reclassified $1.1 million in impairment charges from other income (expense) to general and administrative in the statement of operations.
| (2) | Marketable Investment Securities |
The amortized cost, gross unrealized holding gains, gross unrealized holding losses, and fair value of available-for-sale securities by major security type and class of security at June 30, 2012 and 2011 were as follows (in thousands):
| Amortized cost |
Gross unrealized holding gains |
Gross unrealized holding losses |
Estimated fair value |
|||||||||||||
| June 30, 2012: |
||||||||||||||||
| Available-for-sale: |
||||||||||||||||
| Money market funds |
$ | 19,707 | $ | — | $ | — | $ | 19,707 | ||||||||
| Corporate bonds and notes |
53,989 | 2 | — | 53,991 | ||||||||||||
| Federal agency issues |
15,679 | 2 | — | 15,681 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 89,375 | $ | 4 | $ | — | $ | 89,379 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Amortized cost |
Gross unrealized holding gains |
Gross unrealized holding losses |
Estimated fair value |
|||||||||||||
| June 30, 2011: |
||||||||||||||||
| Available-for-sale: |
||||||||||||||||
| Money market funds |
$ | 18,071 | $ | — | $ | — | $ | 18,071 | ||||||||
| Corporate bonds and notes |
13,963 | 12 | — | 13,975 | ||||||||||||
| Federal agency issues |
82,431 | 40 | (6 | ) | 82,465 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 114,465 | $ | 52 | $ | (6 | ) | $ | 114,511 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
Cash and cash equivalents of $19.7 million at June 30, 2012 consist of cash and money market funds. In addition, the Company holds $200,000 restricted cash in an 18-month certificate of deposit as collateral for a corporate purchasing card program and $48,000 in a restricted cash account as collateral for office equipment. These amounts are included in long-term marketable securities on the balance sheet as of June 30, 2012 and 2011. Maturities of debt securities classified as available-for-sale are as follows at June 30, 2012 (in thousands):
| Amortized cost |
Estimated fair value |
|||||||
| Available-for-sale: |
||||||||
| Due within one year |
$ | 68,668 | $ | 68,670 | ||||
| Due after one year through three years |
1,000 | 1,002 | ||||||
|
|
|
|
|
|||||
| $ | 69,668 | $ | 69,672 | |||||
|
|
|
|
|
|||||
B-49
| (3) | Fair Value Measurements |
The fair value of the Company’s financial instruments reflects the amounts that the Company estimates to receive in connection with the sale of an asset or paid in connection with the transfer of a liability in an orderly transaction between market participants at the measurement date (exit price). The fair value hierarchy prioritizes the use of inputs used in valuation techniques into the following three levels:
Level 1—quoted prices in active markets for identical assets and liabilities.
Level 2—observable inputs other than quoted prices in active markets for identical assets and liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Some of the Company’s marketable securities primarily utilize broker quotes in a non-active market for valuation of these securities.
Level 3—unobservable inputs.
The majority of the Company’s financial instruments are valued using quoted prices in active markets or based on other observable inputs. The following table sets forth the fair value of the Company’s financial assets that the Company re-measured:
| (In thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| June 30, 2012 |
||||||||||||||||
| Money market funds |
$ | 19,707 | $ | — | $ | — | $ | 19,707 | ||||||||
| Corporate bonds and notes |
— | 53,991 | — | 53,991 | ||||||||||||
| Federal agency issues |
— | 15,681 | — | 15,681 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 19,707 | $ | 69,671 | $ | — | $ | 89,379 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| June 30, 2011 |
||||||||||||||||
| Money market funds |
$ | 18,071 | $ | — | $ | — | $ | 18,071 | ||||||||
| Corporate bonds and notes |
— | 13,975 | — | 13,975 | ||||||||||||
| Federal agency issues |
— | 82,465 | — | 82,465 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 18,071 | $ | 96,440 | $ | — | $ | 114,511 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
As of June 30, 2012 and 2011, the Company has no investments which were measured using unobservable (Level 3) inputs.
In conjunction with the suspension of all development activities, the Company has evaluated its equipment and management has committed to a plan to sell the Company’s laboratory equipment. Equipment categorized as equipment held for sale on the balance sheet at June 30, 2012 totaled $1.0 million. Equipment held for sale is no longer subject to depreciation, and is recorded at the lower of depreciated carrying value or fair market value less costs to sell. The fair value of the equipment was determined by using broker quotes for similar assets. The Company has classified the inputs used for determining the fair value of these assets as Level II in the fair value hierarchy.
| (4) | Leases |
The Company entered into a sublease agreement with MGI effective July 1, 2009, as amended on November 11, 2009, and February 19, 2010, that provides for the sublease of certain office and laboratory space. The sublease for the Company’s facility took effect January 4, 2010 for a period of three years from the commencement date with the option to extend for an additional four three-year periods. In addition, the Company entered into a sublease agreement on June 1, 2012 that provides for the sublease of certain office space in Monterey, CA. The sublease for this office space took effect July 1, 2012 for a period of one year
B-50
from the commencement date with the option to extend for an additional two one year periods. Rental expense for the years ended June 30, 2012, 2011 and 2010 was $3.8 million, $3.8 million and $3.6 million, respectively. The table below is reflective of the facility subleases. As of June 30, 2012 the future minimum lease payments under the sublease agreements are as follows (in thousands):
| Fiscal year ending: |
||||
| 2013 |
$ | 2,104 | ||
|
|
|
|||
| $ | 2,104 | |||
|
|
|
| (5) | Share-Based Compensation |
Myrexis Share-Based Compensation Plans
The Company adopted two equity incentive plans, the Myrexis, Inc. 2009 Employee, Director and Consultant Equity Incentive Plan (the “Equity Incentive Plan”) and the Myrexis, Inc. 2009 Employee Stock Purchase Plan (the “ESPP”). At June 30, 2012, the Company was authorized to issue a total of 8.6 million shares under the plans. The number of shares of common stock reserved for issuance under the Equity Incentive Plan is subject to increase pursuant to an “evergreen” provision, which provides for an annual increase equal to the lesser of 2,400,000 shares, 5% of the Company’s then outstanding shares of common stock, or such other amount as the Board of Directors may determine. The Board of Directors determined not to increase the number of shares reserved under the Equity Incentive Plan as of July 1, 2012. The number of shares of common stock reserved for issuance under the ESPP is subject to increase pursuant to an “evergreen” provision, which provides for an annual increase equal to the lesser of 500,000 shares, 2% of the Company’s then outstanding shares of common stock, or such other amount as the Board of Directors may determine. The Board of Directors determined not to increase the number of shares reserved under the ESPP as of July 1, 2012.
The Equity Incentive Plan provides for the issuance of common stock based awards, including restricted stock, restricted stock units, stock options, stock appreciation rights and other equity based awards to the Company’s directors, officers, employees and consultants.
The ESPP is intended to qualify as an “employee stock purchase plan” under Section 423 of the Internal Revenue Code of 1986, as amended. Full-time employees of Myrexis who will own less than five percent of Myrexis, Inc’s outstanding shares of common stock will be eligible to contribute a percentage of their base salary, subject to certain limitations, over the course of six-month offering periods for the purchase of shares of common stock. The purchase price for shares of common stock purchased under the ESPP will equal 85 percent of the fair market value of a share of common stock at the beginning or end of the relevant six-month offering period, whichever is lower.
In connection with the separation from MGI and related transactions, each outstanding MGI stock option was converted into an adjusted MGI common stock option, exercisable for the same number of shares of common stock as the original MGI option, and a new Myrexis common stock option, exercisable for one-fourth of the number of shares of common stock as the original MGI option. All other terms of the converted options remained the same. However, the vesting and expiration of the converted options is based on the optionholder’s continuing employment with either MGI or Myrexis, as applicable, following the separation. The adjusted exercise price of each converted option was determined in accordance with Section 409A and Section 422 of the Code, as follows:
| • | The per share exercise price of each such MGI converted option is equal to the product of (i) the per share exercise price of the original MGI option multiplied by (ii) a fraction, the numerator of which is the closing MGI’s stock price on the day after the distribution, and the denominator of which is the ex-dividend closing stock price of MGI on the day of the distribution plus one-quarter of the “when-issued” Myrexis stock price on the day of the distribution. |
B-51
| • | The per share exercise price of each such Myrexis converted option is equal to the product of (i) the per share exercise price of the original MGI option multiplied by (ii) a fraction, the numerator of which is the closing Myrexis stock price on the day after the distribution, and the denominator of which is the ex-dividend closing stock price of MGI on the day of the distribution plus one-quarter of the “when-issued” Myrexis stock price on the day of the distribution. |
Accordingly, in connection with the separation and related transactions, the Company issued stock options to current and former directors, officers, employees and consultants of MGI and Myrexis.
The exercise price of options granted during the period ended June 30, 2012, 2011 and 2010 was equivalent to the fair value of the stock on the date of grant. The number of shares, terms, and vesting periods are determined by the Company’s Board of Directors or a committee thereof on an option-by-option basis. Options generally vest ratably over service periods of four years and expire ten years from the date of grant. As of June 30, 2012, 1,487,299 shares were available for future grant under the Equity Incentive Plan and 949,850 shares were available for purchase under the Myrexis ESPP.
The fair value of each option grant is estimated on the date of the grant using the Black-Scholes option-pricing model with the following weighted-average assumptions used for grants for the fiscal years ended June 30:
| 2012 | 2011 | 2010 | ||||||||||
| Risk-free interest rate |
0.9 | % | 1.4 | % | 2.1 | % | ||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Expected lives (in years) |
6.0 -7.0 | 6.0 -7.0 | 3.6 -4.0 | |||||||||
| Expected volatility |
77.5 | % | 75.4 | % | 65.7 | % | ||||||
Expected option lives and volatilities are based on historical data and other factors.
A summary of option activity is as follows:
| 2012 | 2011 | 2010 | ||||||||||||||||||||||
| Number of shares |
Weighted average exercise price |
Number of shares |
Weighted average exercise price |
Number of shares |
Weighted average exercise price |
|||||||||||||||||||
| Options outstanding at beginning of year |
3,248,984 | $ | 3.42 | 3,592,227 | $ | 3.28 | 3,592,372 | $ | 2.42 | |||||||||||||||
| Options granted |
1,097,400 | 2.79 | 844,060 | 3.84 | 1,326,064 | 4.61 | ||||||||||||||||||
| Less: |
||||||||||||||||||||||||
| Options exercised |
(536,985 | ) | 1.63 | (562,562 | ) | 2.22 | (954,522 | ) | 1.73 | |||||||||||||||
| Options canceled or expired |
(1,181,390 | ) | 3.92 | (624,741 | ) | 4.26 | (371,687 | ) | 3.80 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Options outstanding at end of year |
2,628,009 | 3.30 | 3,248,984 | 3.42 | 3,592,227 | 3.28 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Options exercisable at end of year |
1,409,760 | 3.38 | 1,542,307 | 2.76 | 1,525,053 | 2.36 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Options vested and expected to vest |
2,450,823 | 3.33 | 3,086,509 | 3.38 | 3,316,003 | 3.19 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Weighted average fair value of options granted during the year |
1.84 | 2.53 | 2.33 | |||||||||||||||||||||
B-52
The following table summarizes information about the stock options outstanding under the Equity Incentive Plan for both Myrexis and MGI employees at June 30, 2012:
| Range
of |
Options outstanding |
Options exercisable | ||||||||
| Number |
Weighted |
Weighted |
Number |
Weighted | ||||||
| $ 0.59 - 2.75 |
884,287 | 7.00 | $2.29 | 399,287 | $1.74 | |||||
| 2.78 - 3.56 |
702,224 | 4.86 | 3.08 | 279,532 | 3.30 | |||||
| 3.65 - 4.67 |
931,740 | 6.36 | 4.24 | 661,645 | 4.25 | |||||
| 4.73 - 4.83 |
109,758 | 4.57 | 4.83 | 69,296 | 4.83 | |||||
|
|
|
|||||||||
| 2,628,009 | 6.10 | 3.30 | 1,409,760 | 3.37 | ||||||
|
|
|
|||||||||
The fair-value of each Myrexis stock option issued pursuant to the separation was based on an allocation of the unamortized fair-value of the original MGI stock option from which it was derived. Myrexis recognizes share-based compensation expense relating to both Myrexis and MGI options held by current directors, officers, employees and consultants of Myrexis. Share-based compensation expense relating to Myrexis options held by current and former directors, officers, employees and consultants of MGI will be recognized by MGI.
As of June 30, 2012, unrecognized compensation expense related to the unvested portion of MGI’s stock options granted to Myrexis employees and the unvested portion of Myrexis stock options granted was approximately $1.3 million and will be recognized over a weighted-average period of 2.41 years.
The total intrinsic value of options exercised during the fiscal year ended June 30, 2012, 2011 and 2010 was $0.7 million, $1.0 million and $3.4 million, respectively. The aggregate intrinsic value of options outstanding was approximately $0.3 million and the aggregate intrinsic value for options fully vested was approximately $0.3 million as of June 30, 2012.
On December 8, 2011, the Company issued 53,400 restricted stock units under the Equity Incentive Plan at a fair value of $2.82. On May 11, 2012, the Company issued 2,139,230 restricted stock units (1,069,615 units to each of the Company’s then serving President and Chief Executive Officer and its Chief Operating Officer) under the Equity Incentive Plan at a fair value of $2.75. The restricted stock units issued on May 11, 2012 include certain performance conditions as well as market conditions. As of June 30, 2012, the performance criteria were not probable of being achieved; therefore, no stock-based compensation expense recorded during the year ended June 30, 2012. The units that were issued to the Company’s then serving President and Chief Executive Officer, Richard B. Brewer, expired by their terms upon his death on August 15, 2012. If specific performance conditions are met with respect to the restricted stock units issued to the Company’s Chief Operating Officer, a substantial expense could be incurred. As of June 30, 2012, the unrecognized compensation expense related to unvested restricted stock units was approximately $0.1 million and will be recognized over a weighted-average period of 1.0 year.
Activity with respect to outstanding restricted stock units for the fiscal years ended June 30, 2012, 2011 and 2010 is as follows:
| Number of shares |
Weighted average grant date fair value |
|||||||
| Balance at June 30, 2009 |
— | $ | — | |||||
| Granted |
284,740 | 4.03 | ||||||
| Cancelled |
(52,550 | ) | 4.03 | |||||
| Vested |
(87,724 | ) | 4.03 | |||||
|
|
|
|||||||
| Balance at June 30, 2010 |
144,466 | 4.03 | ||||||
|
|
|
|||||||
B-53
| Number of shares |
Weighted average grant date fair value |
|||||||
| Balance at June 30, 2010 |
144,466 | $ | 4.03 | |||||
| Granted |
141,094 | 3.86 | ||||||
| Cancelled |
(54,657 | ) | 3.97 | |||||
| Vested |
(55,302 | ) | 4.03 | |||||
|
|
|
|||||||
| Balance at June 30, 2011 |
175,601 | 3.91 | ||||||
|
|
|
|||||||
| Number of shares |
Weighted average grant date fair value |
|||||||
| Balance at June 30, 2011 |
175,601 | $ | 3.91 | |||||
| Granted |
2,192,630 | 2.75 | ||||||
| Cancelled |
(114,277 | ) | 3.47 | |||||
| Vested |
(72,302 | ) | 3.94 | |||||
|
|
|
|||||||
| Balance at June 30, 2012 |
2,181,652 | 2.77 | ||||||
|
|
|
|||||||
For the years ended June 30, 2012, 2011 and 2010, Myrexis employees purchased 131,617, 221,191 and 197,342 shares, respectively, under the Myrexis ESPP. Compensation expenses associated with Myrexis employees participating in the Myrexis ESPP for the years ended June 30, 2012, 2011 and 2010 were approximately $206,000, $360,000, and $318,000, respectively. The fair value of shares issued under the Myrexis ESPP was calculated using the Black-Scholes option-pricing model with the following weighted-average assumptions for the fiscal years ended June 30:
| 2012 | 2011 | 2010 | ||||||||||
| Risk-free interest rate |
0.05 | % | 0.2 | % | 0.2 | % | ||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Expected lives (in years) |
0.5 | 0.5 | 0.5 | |||||||||
| Expected volatility |
77 | % | 75 | % | 75 | % | ||||||
Share-based compensation expense recognized for Myrexis employees included in the statement of operations for the fiscal years ended June 30, 2012, 2011 and 2010 is as follows (in thousands):
| 2012 | 2011 | 2010 | ||||||||||
| Research and development |
$ | 595 | $ | 2,086 | $ | 2,455 | ||||||
| General and administrative |
912 | 2,746 | 3,345 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total employee stock-based compensation expense |
$ | 1,507 | $ | 4,832 | $ | 5,800 | ||||||
|
|
|
|
|
|
|
|||||||
B-54
| (6) | Income Taxes |
Income tax expense (benefit) consists of the following:
| (In thousands) | Year ended June 30, | |||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| Current: |
||||||||||||
| Federal |
$ | — | $ | — | $ | — | ||||||
| State |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Current |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred: |
||||||||||||
| Federal |
(9,991 | ) | (13,032 | ) | (18,007 | ) | ||||||
| State |
(1,606 | ) | (2,380 | ) | (2,900 | ) | ||||||
| Change in valuation allowance |
11,597 | 15,412 | 20,907 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Deferred |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total income tax expense (benefit) |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
The differences between income taxes at the statutory federal income tax rate and income taxes reported in the consolidated statements of operations were as follows:
| Year ended June 30, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| Federal income tax expense at the statutory rate |
(34.0 | )% | (34.0 | )% | (34.0 | )% | ||||||
| State income taxes, net of federal benefit |
(3.3 | ) | (3.3 | ) | (3.3 | ) | ||||||
| Research and development credits, net of the federal tax on state credits |
(0.9 | ) | (5.9 | ) | (1.7 | ) | ||||||
| Tax basis differences from spin off transaction |
— | — | (5.7 | ) | ||||||||
| Incentive stock option and employee stock purchase plan expense |
0.8 | 2.3 | — | |||||||||
| Uncertain tax positions, net of federal benefit on state positions |
0.3 | 1.1 | 0.2 | |||||||||
| Change in valuation allowance |
37.1 | 39.8 | 44.5 | |||||||||
|
|
|
|
|
|
|
|||||||
| — | % | — | % | — | % | |||||||
|
|
|
|
|
|
|
|||||||
The significant components of the Company’s deferred tax assets and liabilities were comprised of the following at June 30,:
| (In thousands) | Year ended June 30, | |||||||
| 2012 | 2011 | |||||||
| Net operating loss carry-forwards |
$ | 40,026 | $ | 28,699 | ||||
| Intangible asset basis difference |
1,216 | 1,319 | ||||||
| Accrued vacation |
44 | 236 | ||||||
| Stock compensation expense |
3,057 | 2,914 | ||||||
| Research and development credits |
3,364 | 3,069 | ||||||
| Property, plant and equipment |
794 | 589 | ||||||
| Other, net |
82 | 35 | ||||||
| Liability for unrecognized tax benefits |
(666 | ) | (541 | ) | ||||
|
|
|
|
|
|||||
| Total net deferred tax assets before valuation allowance |
47,917 | 36,320 | ||||||
| Less valuation allowance |
(47,917 | ) | (36,320 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
Due to losses incurred, the Company has determined that it is not more likely than not that the Company’s deferred tax assets will be realized. Accordingly, a valuation allowance has been established for the full amount of the Company’s deferred tax assets. The valuation allowance increased $11.6 million, $15.4 million and $20.9 million for the years ended June 30, 2012, 2011 and 2010, respectively.
B-55
At June 30, 2012, the Company had total federal and state tax net operating loss carry-forwards of approximately $107.3 million. If not utilized, the federal operating loss carry-forwards will expire beginning in 2030 through 2032, and the state net operating loss carry-forwards will expire beginning in 2025 through 2027. The Company had approximately $2.7 million of federal research tax credits, which can be carried forward to reduce federal income taxes. If not utilized, the federal research credits will expire beginning in 2030 through 2032. Additionally, the Company had approximately $1.1 million of Utah research tax credits, which can be carried forward to reduce Utah income taxes. If not utilized, the Utah research tax credit carry-forwards will expire beginning in 2024 through 2026. The net operating loss and research credit carry-forwards are not subject to the limitations imposed by Section 382 of the Internal Revenue Code.
Approximately $15.8 million of net operating losses result from ‘excess tax benefits’ as defined by ASC guidance. As such, they are not included in deferred tax assets. They will be recognized as additional paid-in-capital only upon realization of the tax benefit.
On March 29, 2012, in an effort to protect the use of its carry-forward tax benefits, the Company adopted a Tax Benefits Preservation Rights Plan that discourages significant changes in ownership of the Company’s stock that might limit the use of its carry-forward tax benefits.
The Company has adopted the provisions of ASC Topic 740 Subtopic 10 Section 05, which addresses the accounting for uncertainty in tax positions. The guidance requires that the impact of a tax position be recognized in the financial statements if that position is more likely than not of being sustained on audit, based on the technical merits of the position.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Year ended June 30, | ||||||||||||
| (In thousands) | 2012 | 2011 | 2010 | |||||||||
| Unrecognized tax benefits at beginning of year |
$ | 541 | $ | 90 | $ | — | ||||||
| Gross increases—current year tax positions |
125 | 451 | 90 | |||||||||
|
|
|
|
|
|
|
|||||||
| Unrecognized tax benefits at end of year |
$ | 666 | $ | 541 | $ | 90 | ||||||
|
|
|
|
|
|
|
|||||||
Approximately $666,000 of the total unrecognized tax benefits as of June 30, 2012, if recognized, would affect the effective tax rate. The Company does not anticipate that unrecognized tax benefits will significantly increase or decrease within 12 months of the reporting date. Interest and penalties related to uncertain tax positions are included as a component of income tax expense.
The Company files U.S. and various state income tax returns. The 2009, 2010 and 2011 tax years remain subject to examination by the respective tax authorities. The Company’s federal tax return and state tax returns are not currently under examination. Annual tax provisions include amounts considered necessary to pay assessments that may result from examination of the Company’s tax returns. However, the amount ultimately paid upon resolution of issues may differ materially from the amount accrued.
| (7) | Stockholders’ Equity |
Comprehensive loss
The components of the Company’s comprehensive loss are as follows:
| June 30, | ||||||||
| (In thousands) | 2012 | 2011 | ||||||
| Net loss |
$ | (31,209 | ) | $ | (38,708 | ) | ||
| Other comprehensive loss: |
||||||||
| Change in unrealized gain on marketable securities |
(43 | ) | 22 | |||||
|
|
|
|
|
|||||
| Total comprehensive loss |
$ | (31,252 | ) | $ | (38,686 | ) | ||
|
|
|
|
|
|||||
B-56
| (8) | Employee Deferred Savings Plan |
During fiscal years 2012, 2011 and 2010, Myrexis employees participated in a deferred savings plan which qualifies under Section 401(k) of the Internal Revenue Code. Substantially all of the Myrexis employees were covered by the plan. Myrexis made matching contributions of 50% of each employee’s contribution with the employer’s contribution not to exceed 4% of the employee’s compensation. Myrexis contributions to the plan were $240,000, $470,000, and $552,000, for the years ended June 30, 2012, 2011, and 2010, respectively.
| (9) | NOL Rights Agreement |
In March 2012, the Company adopted a Tax Benefits Preservation Rights Plan in the form of a Rights Agreement designed to help protect and preserve its substantial tax attributes primarily associated with net operating loss carryforwards (NOLs) and research tax credits, under Sections 382 and 383 of the Internal Revenue Code. The Tax Benefits Preservation Rights Plan is similar to plans adopted by numerous other public companies with significant NOLs. The Tax Benefits Preservation Rights Plan replaces the Shareholder Rights Plan that Myrexis adopted in 2009, which the Myrexis Board of Directors terminated immediately prior to the adoption of the Rights Agreement.
Myrexis’ ability to generate a tax benefit through the use of its tax attributes would be substantially limited in the event of an “ownership change” under Sections 382 and 383 of the Internal Revenue Code, including if shareholders who own (or are deemed to own) 5% or more of Myrexis’ stock increase their collective ownership in Myrexis by more than 50 percentage points over a rolling three-year period. The Tax Benefits Preservation Rights Plan is intended to reduce the likelihood of an unintended 50% “ownership change” occurring through the buying and selling of Myrexis common stock. The Board of Directors believes that the plan serves the interests of all shareholders as it is designed to protect the use of its tax attributes.
As part of the plan, on March 29, 2012, Myrexis’ Board of Directors declared a dividend of one preferred share purchase right for each share of Myrexis common stock outstanding as of April 9, 2012. Any shares of Myrexis common stock issued after the record date will be issued together with the rights. The rights are not currently exercisable and initially will trade only with shares of Myrexis common stock. However, effective upon the initial public announcement of the Rights Agreement, if any person or group acquires 4.99% or more of the outstanding shares of Myrexis common stock, or if a person or group that already owned 4.99% or more of Myrexis common stock at such time acquires additional shares representing 0.1% or more of the outstanding shares of Myrexis common stock, then, subject to certain exceptions, there would be a triggering event under the plan and the rights would separate from the common stock and become exercisable for shares of Myrexis common stock having a market value equal to twice the exercise price, resulting in significant dilution in the ownership interest of the acquiring person or group. Myrexis’ Board of Directors has the discretion to exempt in advance any acquisition of common stock from the provisions of the plan if it determines that doing so would not limit or impair the availability of the NOLs. Myrexis’ Board of Directors also has the ability to terminate the plan at any time, including but not limited to in connection with a transaction, if it determines that doing so would be in the best interests of the shareholders.
The rights issued under the plan will expire on March 29, 2015. The rights may also expire on an earlier date upon the occurrence of certain events, including a determination by Myrexis’ Board that the Tax Benefits Preservation Rights Plan is no longer necessary for the preservation of tax attributes, or the beginning of a taxable year of Myrexis to which the Board determines that no tax attributes may be carried forward. The rights may also be redeemed, exchanged or terminated prior to their expiration.
| (10) | Commitments and Contingencies |
MGI had entered into a license agreement for exclusive rights to utilize certain intellectual property rights related to the drug candidate Azixa with Maxim Pharmaceuticals, Inc. and Cytovia, Inc. All licensed rights of Maxim and Cytovia were subsequently acquired by EpiCept Corporation, and Maxim, Cytovia and
B-57
EpiCept are collectively referred to herein as EpiCept. Pursuant to the separation agreement with MGI, Myrexis assumed all rights and obligations under this license agreement, including an obligation to pay EpiCept milestone payments upon the occurrence of potential future events.
In September 2011, Myrexis announced that it had suspended any further development of Azixa. On August 28, 2012, Myrexis provided EpiCept notice of termination of the license agreement following its election to terminate all of its efforts to develop and commercialize Azixa in any major market as such products and markets are defined in the agreement. As a result of the termination of the agreement, all rights and licenses granted under the agreement by EpiCept have terminated and reverted to EpiCept. Myrexis has no further obligation for royalty or milestone payments to EpiCept as a result of this notice to terminate.
Various legal claims have been filed against Myrexis that relate to the ordinary course of business and are currently pending resolution. In the opinion of management and upon consultation with legal counsel, the ultimate resolution of these matters is not expected to have a material adverse effect on the financial position or future results of operations of Myrexis.
| (11) | Other Income |
Other income was $0.6 million, $1.7 million and $1.2 million for the years ended June 30, 2012, 2011 and 2010, respectively. Other income in the year ended June 30, 2012 includes interest income, realized gains on Myrexis’ marketable securities and $0.3 million in gains on the disposal of equipment. Other income in the year ended June 30, 2011 includes a one-time $1.2 million grant received in November 2010 as a part of the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code, interest income and realized gains on Myrexis’s marketable securities. Other income for the same period in 2010 reflects interest income and realized gains on Myrexis’s marketable securities, offset by a loss on disposal of assets of $0.2 million.
| (12) | Reorganization |
On November 18, 2011, Myrexis announced a corporate reorganization reducing the Company’s workforce by 20%. In connection with this announcement, the Company recorded severance costs of approximately $0.6 million. These expenses are reflected in the statement of operations, including $50,000 in general and administrative and $550,000 in research and development for the year ended June 30, 2012.
On March 1, 2012, Myrexis announced an alignment of the Company’s resources following the February 2012 announcement to suspend development activities of all its preclinical and clinical programs. The alignment included a phased reduction in the Company’s workforce. The Company currently has 10 employees. In connection with the resource alignment, the Company recorded severance costs of approximately $3.6 million in the year ended June 30, 2012. Of this amount, $2.5 million was paid during the year ended June 30, 2012, and $1.1 million was accrued and is expected to be paid during the first fiscal quarter of 2013. These expenses are reflected in the statement of operations, including $1.0 million in general and administrative and $2.6 million in research and development for the year ended June 30, 2012.
In addition, Myrexis recorded severance expenses of $0.7 million related to the departure of Adrian Hobden, former President and CEO and Wayne Laslie, former COO. These expenses are reflected in the statement of operations in general and administrative for the year ended June 30, 2012.
On May 11, 2012, Myrexis announced its Board of Directors had appointed Richard B. Brewer as President and Chief Executive Officer, and David W. Gryska as Chief Operating Officer. In addition, both Mr. Brewer and Mr. Gryska were appointed to Myrexis’ Board of Directors. In connection with these management changes, Robert J. Lollini stepped down as President and Chief Executive Officer and will continue on Myrexis’ Board for a transition period through November 15, 2012. The Company recorded severance expense of $0.8 million for Mr. Lollini in addition to severance costs previously mentioned during the year ended June 30, 2012. This severance was paid during the year ended June 30, 2012.
On August 15, 2012, Myrexis announced the death of its President and Chief Executive Officer, Richard B. Brewer. David W. Gryska is currently serving as the acting President and Chief Executive Officer while the Board of Directors considers succession plans.
B-58
Also, in conjunction with the March 2012 reorganization, the Company determined that there were indicators of impairment of certain fixed assets, based on quoted market prices, and evaluated whether the carrying value of assets with impairment indicators is recoverable. Management concluded that $281,000 of impairment loss should be recognized during the year. This expense is reflected in the statement of operations in general and administrative for the year ended June 30, 2012.
The Company has evaluated its equipment and management has committed to a plan to sell the Company’s laboratory equipment. Equipment categorized as equipment held for sale on the balance sheet at June 30, 2012 totaled $1.0 million. Equipment held for sale is no longer subject to depreciation, and is recorded at the lower of depreciated carrying value or fair market value less costs to sell. The Company expects to sell these assets by the end of calendar year 2012.
B-59
EXHIBIT INDEX
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
|||||||||||
| 2.1 | Separation and Distribution Agreement, dated June 30, 2009, by and between the Registrant and Myriad Genetics, Inc. | 8-K (Exhibit 2.1) |
7/7/09 | 001-34275 | ||||||||||||
| 3.1 | Amended and Restated Certificate of Incorporation of the Registrant | 10-K (Exhibit 3.1) |
9/13/10 | 001-34275 | ||||||||||||
| 3.1.1 | Certificate of Designation, Preferences and Rights of Series A Junior Participating Preferred Stock | 10-K (Exhibit 3.1.1) |
9/13/10 | 001-34275 | ||||||||||||
| 3.1.2 | Certificate of Amendment to Restated Certificate of Incorporation of the Registrant | 10-K (Exhibit 3.1.2) |
9/13/10 | 001-34275 | ||||||||||||
| 3.1.3 | Amended Certificate of Designation, Rights and Preferences of Series A Junior Participating Preferred Stock | 8-K (Exhibit 3.1) |
3/30/12 | 001-34275 | ||||||||||||
| 3.2 | Amended and Restated Bylaws of the Registrant | 10-K (Exhibit 3.2) |
9/13/10 | 001-34275 | ||||||||||||
| 4.1 | Form of Common Stock Certificate of the Registrant | X | ||||||||||||||
| 4.2 | Shareholder Rights Agreement between the Registrant and American Stock Transfer & Trust Company, LLC, dated June 30, 2009, which includes as Exhibit B the form of Right Certificate | 8-A (Exhibit 4.1) |
6/30/09 | 001-34275 | ||||||||||||
| 4.2.1 | First Amendment, dated March 29, 2012, to Shareholder Rights Agreement by and between the Registrant and American Stock Transfer & Trust Company, LLC, dated June 30, 2009 | 8-K (Exhibit 4.1) |
3/30/12 | 001-34275 | ||||||||||||
| 4.3 | Tax Benefits Preservation Rights Agreement by and between the Registrant and American Stock Transfer & Trust Company, LLC, dated March 29, 2012, which includes as Exhibit B the Form of Right Certificate | 8-K (Exhibit 4.2) |
3/30/12 | 001-34275 | ||||||||||||
| Lease Agreements |
||||||||||||||||
| 10.1 | Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 8-K (Exhibit 10.2) |
7/7/09 | 001-34275 | ||||||||||||
| 10.1.1 | Amendment No. 1, effective November 11, 2009, to Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 10-Q (Exhibit 10.1) |
11/12/09 | 001-34275 | ||||||||||||
| 10.1.2 | Amendment No. 2, dated February 19, 2010, to Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 10-Q (Exhibit 10.2) |
5/17/10 | 001-34275 | ||||||||||||
| Equity Compensation Plans |
||||||||||||||||
| *10.2 | 2009 Employee, Director and Consultant Stock Plan, as amended (the “2009 Plan”) | X | ||||||||||||||
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
|||||||||
| *10.2.1 | Form of Stock Option Agreement under the 2009 Plan | 10/A (Exhibit 10.6.1) |
6/8/09 | 001-34275 | ||||||||||
| *10.2.2 | Form of Restricted Stock Unit Agreement under the 2009 Plan | 10/A (Exhibit 10.6.2) |
6/8/09 | 001-34275 | ||||||||||
| *10.2.3 | Form of Incentive Stock Option Agreement under the 2009 Plan for Rollover Options issued under the Myriad Genetics, Inc. 2003 Employee, Director and Consultant Stock Option Plan, as amended (the “MGI 2003 Plan”) | 10/A (Exhibit 10.6.3) |
6/8/09 | 001-34275 | ||||||||||
| *10.2.4 | Form of Non-Qualified Stock Option Agreement under the 2009 Plan for Rollover Options issued under the MGI 2003 Plan | 10/A (Exhibit 10.6.4) |
6/8/09 | 001-34275 | ||||||||||
| *10.2.5 | Form of Incentive Stock Option Agreement under the 2009 Plan for Rollover Options issued under the Myriad Genetics, Inc. 2002 Employee, Director and Consultant Stock Option Plan, as amended (the “MGI 2002 Plan”) | 10/A (Exhibit 10.6.5) |
6/8/09 | 001-34275 | ||||||||||
| *10.2.6 | Form of Non-Qualified Stock Option Agreement under the 2009 Plan for Rollover Options issued under the MGI 2002 Plan | 10/A (Exhibit 10.6.6) |
6/8/09 | 001-34275 | ||||||||||
| *10.2.7 | Form of Restricted Stock Unit Award Agreement under the 2009 Plan entered into between the Registrant and each of Richard B. Brewer and David W. Gryska on May 11, 2012 | 8-K (Exhibit 10.4) |
5/11/12 | 001-34275 | ||||||||||
| *10.3 | 2009 Employee Stock Purchase Plan | 10/A (Exhibit 10.7) |
6/8/09 | 001-34275 | ||||||||||
| Agreements with Executive Officers and Directors |
||||||||||||||
| 10.4 | Form of Indemnification Agreement between the Registrant and its directors and officers | 10/A (Exhibit 10.8) |
5/29/09 | 001-34275 | ||||||||||
| *10.5 | Non-Employee Director Compensation Policy, as amended November 11, 2010 | 10-Q (Exhibit 10.1) |
2/9/11 | 001-34275 | ||||||||||
| *10.6 | Form of Employment Agreement between the Registrant and its officers | 10-K (Exhibit 10.10) |
9/28/09 | 001-34275 | ||||||||||
| *10.7 | Executive Severance and Change in Control Agreement by and between the Registrant and Adrian N. Hobden, dated February 1, 2010 | 8-K (Exhibit 10.1) |
2/4/10 | 001-34275 | ||||||||||
| *10.8 | Form of Executive Severance and Change in Control Agreement entered into between the Registrant and each of Wayne Laslie and Robert Lollini on February 1, 2010 | 8-K (Exhibit 10.2) |
2/4/10 | 001-34275 | ||||||||||
| *10.9 | Separation Agreement by and between the Registrant and Adrian N. Hobden, dated July 21, 2011 | 8-K (Exhibit 10.1) |
7/22/11 | 001-34275 | ||||||||||
| *10.10 | Offer Letter by and between the Registrant and Robert J. Lollini, dated September 9, 2011 | 8-K (Exhibit 10.1) |
9/12/11 | 001-34275 | ||||||||||
| Exhibit |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
|||||||||||
| *10.11 | First Amendment, dated September 9, 2011, to Executive Severance and Change in Control Agreement by and between the Registrant and Robert J. Lollini, dated February 1, 2010 | 8-K (Exhibit 10.2) |
9/12/11 | 001-34275 | ||||||||||||
| *10.12 | Offer Letter by and between the Registrant and Andrea Kendell, dated September 22, 2011 | 8-K/A (Exhibit 10.1) |
9/28/11 | 001-34275 | ||||||||||||
| *10.13 | Executive Severance and Change in Control Agreement by and between the Registrant and Andrea Kendell, dated September 22, 2011 | 8-K/A (Exhibit 10.2) |
9/28/11 | 001-34275 | ||||||||||||
| 10.14 | Agreement by and between the Registrant and Jason M. Aryeh, dated October 18, 2011 | 8-K (Exhibit 10.2) |
10/21/11 | 001-34275 | ||||||||||||
| *10.15 | Separation Agreement by and between the Registrant and Wayne Laslie, dated December 13, 2011 | 8-K (Exhibit 10.1) |
12/14/11 | 001-34275 | ||||||||||||
| *10.16 | Separation and Consulting Agreement by and between the Registrant and Robert J. Lollini, dated May 11, 2012 | 8-K (Exhibit 10.1) |
5/11/12 | 001-34275 | ||||||||||||
| *10.17 | Employment Agreement by and between the Registrant and Richard B. Brewer, dated May 9, 2012 | 8-K (Exhibit 10.2) |
5/11/12 | 001-34275 | ||||||||||||
| *10.18 | Employment Agreement by and between the Registrant and David W. Gryska, dated May 9, 2012 | 8-K (Exhibit 10.3) |
5/11/12 | 001-34275 | ||||||||||||
| Other Material Agreements |
||||||||||||||||
| 10.19 | Agreement by and among the Registrant and MSMB Healthcare LP, MSMB Healthcare Investors LLC, MSMB Healthcare Management LLC, and MSMB Capital Management LLC, dated October 18, 2011 | 8-K (Exhibit 10.1) |
10/21/11 | 001-34275 | ||||||||||||
| 10.20 | Letter Agreement by and between the Registrant and Martin Shkreli, dated October 18, 2011 | 8-K (Exhibit 10.3) |
10/21/11 | 001-34275 | ||||||||||||
| 10.21 | Letter Agreement by and among the Registrant, Bulldog Investors, and Brooklyn Capital Management LLC, dated August 6, 2012 | 8-K (Exhibit 10.1) |
8/10/12 | 001-34275 | ||||||||||||
| 10.22 | Letter Agreement by and among the Registrant, MSMB Healthcare LP, MSMB Healthcare Investors LLC, MSMB Healthcare Management LLC and MSMB Capital Management LLC, dated August 8, 2012 | 8-K (Exhibit 10.2) |
8/10/12 | 001-34275 | ||||||||||||
| 10.23 | Letter Agreement by and between the Registrant and Martin Shkreli, dated August 8, 2012 | 8-K (Exhibit 10.3) |
8/10/12 | 001-34275 | ||||||||||||
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | X | ||||||||||||||
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number | |||||||
| 31.1 | Certification of the Chief Executive Officer under Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 31.2 | Certification of the Chief Financial Officer under Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 32.1 | Certification of the Chief Executive Officer and the Chief Financial Officer under Section 906 of the Sarbanes- Oxley Act of 2002 | X | ||||||||||
| 101** | The following materials from Myrexis, Inc.’s Annual Report on Form 10-K for the fiscal year ended June 30, 2012, formatted in XBRL (extensible Business Reporting Language): (i) Balance Sheets, (ii) Statements of Operations, (iii) Statements of Stockholders’ Equity and Comprehensive Loss, (iv) Statements of Cash Flows, and (v) Notes to Financial Statements | X | ||||||||||
| * | Management contract, compensatory plan or arrangement. |
| † | Confidential portions of these documents have been filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment. |
| ** | Users of the XBRL data are advised pursuant to Rule 406T of Regulation S-T that this interactive data file is deemed not filed or part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, and otherwise is not subject to liability under these sections. |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K/A
(Amendment No. 1)
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2012
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-34275
MYREXIS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 26-3996918 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 305 Chipeta Way Salt Lake City, Utah |
84108 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code (801) 214-7800
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of each class |
Name of each exchange on which registered | |
| Common Stock, $0.01 Par Value Per Share Preferred Share Purchase Rights |
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | x | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common stock was last sold on December 31, 2011, the last business day of the registrant’s most recently completed second fiscal quarter, was $69,028,808. As of October 25, 2012 the registrant had 26,817,294 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
| Page | ||||||
| Explanatory Note |
||||||
| PART III | ||||||
| Item 10. |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | B-67 | ||||
| Item 11. |
EXECUTIVE COMPENSATION | B-75 | ||||
| Item 12. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
B-96 | ||||
| Item 13. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
B-99 | ||||
| Item 14. |
PRINCIPAL ACCOUNTANT FEES AND SERVICES | B-100 | ||||
| PART IV | ||||||
| Item 15. |
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | B-103 | ||||
| B-104 | ||||||
B-65
Explanatory Note
The purpose of this Amendment No. 1 (the “Amendment”) to the Annual Report on Form 10-K of Myrexis, Inc. (the “Registrant”) for the year ended June 30, 2012 as filed on September 13, 2012 (the “Original Form 10-K”) is to include the disclosure required in Part III, Items 10, 11, 12, 13 and 14. Except for Items 10, 11, 12, 13 and 14 of Part III and Item 15(a)(3) of Part IV, no other information included in the Original Form 10-K is amended or changed by this Amendment.
B-66
PART III
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
THE BOARD OF DIRECTORS
Our Restated Certificate of Incorporation, as amended, and Restated By-Laws provide that our business is to be managed by or under the direction of our Board of Directors. Our Board of Directors is divided into three classes for purposes of election. One class is elected at each annual meeting of stockholders to serve for a three-year term. Our Board of Directors currently consists of eight members, classified into three classes as follows: (1) Jason M. Aryeh, Timothy R. Franson, David W. Gryska and Robert J. Lollini constitute the Class I directors with a term ending at the 2013 annual meeting, however Mr. Lollini will be resigning from the Board of Directors on November 15, 2012; (2) John T. Henderson and Robert Forrester constitute the Class II directors with a term ending at the 2014 annual meeting; and (3) Gerald P. Belle and Dennis H. Langer constitute the Class III directors with a term ending at the 2012 annual meeting.
Effective on October 18, 2011, we entered into agreements with MSMB Healthcare LP and certain of its affiliated funds and entities, referred to hereinafter collectively as MSMB Healthcare, and with Jason M. Aryeh, relating to a notice we received from MSMB Healthcare stating MSMB Healthcare’s intention to nominate two individuals, including Mr. Aryeh, for election as Class II directors of the Board of Directors at the 2011 annual meeting, and its intended proxy solicitation in connection therewith. Pursuant to these agreements, on October 19, 2011, the Board of Directors, accepting the recommendation of the Nominating and Governance Committee of the Board, increased the size of the Board of Directors from six to seven members, and appointed Mr. Aryeh to the Board of Directors as a Class I director to serve in accordance with our Bylaws until the 2013 annual meeting and thereafter until his successor is duly elected and qualified. Mr. Aryeh was also appointed as a member of the Strategy Review Committee of the Board. Pursuant to these agreements, MSMB Healthcare agreed to withdraw its nominations for the upcoming annual meeting and terminate any solicitations in connection therewith, and MSMB Healthcare and Mr. Aryeh have agreed to certain standstill and voting covenants until the completion of, and except in connection with, our 2013 annual meeting. MSMB Healthcare and Mr. Aryeh have each agreed during the standstill period not to, among other things, engage in or otherwise facilitate any proxy solicitation with respect to the securities of Myrexis, acquire or announce an intention to acquire any Myrexis voting securities which would result in such person (together with its or his respective affiliates) owning 5% or more of Myrexis’ voting securities, seek to place any individual on the Board of Directors other than as recommended by the Board of Directors, or become a participant in any election contest involving Myrexis. Martin Shkreli, the Managing Member of the General Partner of MSMB Healthcare LP, has also entered into a letter agreement with us, effective October 18, 2011, pursuant to which he has agreed to be bound by the terms and conditions of the agreement by and among us and MSMB Healthcare.
On August 9, 2012, we entered into a second letter agreement, dated August 8, 2012, with MSMB Healthcare, referred to hereinafter as the 2012 MSMB Letter Agreement. The 2012 MSMB Letter Agreement requires that, through the completion of the 2013 annual meeting, the shareholdings of MSMB Healthcare, Mr. Shkreli and their affiliates must at all times represent less than 4.99% ownership in Myrexis. Also through the completion of the 2013 annual meeting, MSMB Healthcare, Mr. Shkreli and their affiliates must vote the shares of common stock they own on any matter presented to our stockholders for their vote as the Board recommends. The voting requirement is subject to the condition that Mr. Aryeh is included in the Board majority approving the recommendation or, if he abstains or otherwise does not vote as a member of the Board on the matter, that he concurs with the Board’s recommendation. To the extent that the 2012 MSMB Letter Agreement contains provisions which are in addition to or inconsistent with provisions of the October 18, 2011 letter agreement among the Company and MSMB Healthcare described above, referred to hereinafter as the 2011 MSMB Letter Agreement, the 2012 MSMB Letter Agreement provides that such additional and inconsistent provisions in the 2012 MSMB Letter Agreement are incorporated by reference into the 2011 MSMB Letter Agreement, superseding provisions in the 2011 MSMB Letter Agreement which are inconsistent with the
B-67
provisions of the 2012 MSMB Letter Agreement, thereby amending the 2011 MSMB Letter Agreement. Also on August 9, 2012, we entered into a second letter agreement with Mr. Shkreli, which made reference to the 2012 MSMB Letter Agreement. Under this agreement, Mr. Shkreli agrees that he will not, and will not cause or permit any of his affiliates to, take any action or refrain from taking any action which, if done or refrained from being done by MSMB Healthcare, would constitute a breach of the 2011 MSMB Letter Agreement as amended by the 2012 MSMB Letter Agreement.
Set forth below are the names of our directors, their ages, their offices in the Company, if any, their principal occupations or employment for the past five years, the length of their tenure as directors and the names of other public companies in which such persons hold or have held directorships during the past five years.
| Name |
Age | Position with the Company | ||||
| Gerald P. Belle (2) |
66 | Chairman of the Board of Directors | ||||
| Jason M. Aryeh |
44 | Director | ||||
| Robert Forrester, LL.B. (1)(2) |
49 | Director | ||||
| Timothy R. Franson, M.D. (3) |
61 | Director | ||||
| David W. Gryska |
56 | Acting President and Chief Executive Officer, Chief Operating Officer and Director | ||||
| John T. Henderson, M.D. (1)(3) |
68 | Director | ||||
| Dennis H. Langer, M.D., J.D. (1)(2)(3) |
61 | Director | ||||
| Robert J. Lollini |
59 | Director | ||||
| (1) | Member of the Audit Committee. |
| (2) | Member of the Compensation Committee. |
| (3) | Member of the Nominating and Governance Committee. |
Gerald P. Belle was appointed Chairman of the Myrexis Board of Directors on February 19, 2009. He was previously President and Chief Executive Officer, North American Pharmaceuticals, Aventis, Inc. from 2000 to 2004. Over his 35-year career with Aventis and its predecessor companies, Mr. Belle’s responsibilities included executive commercial and general management positions in the U.S., Asia, Europe/Middle East/Africa and Canada. Following his retirement from Aventis in November 2004, he was appointed Executive Chairman of Merial, Ltd., a global leader in animal health and a joint venture between Merck and sanofi-aventis. He retired from Merial, Ltd. in November 2007. Mr. Belle currently serves as the Chairman of the Board of Directors of PDI, Inc. and previously served as a director of Myriad Genetics, Inc. from November 2007 until November 2009. Mr. Belle received his B.S. in Business from Xavier University, and his M.B.A. from Northwestern University.
Our Board of Directors has concluded that Mr. Belle should serve as a director of Myrexis, Inc. due to his knowledge and experience with respect to the biotechnology and pharmaceutical industries, including his background as a senior level executive of a large, global, publicly held pharmaceutical company, service on various boards and his management experience in global operations, international business, strategic planning and finance.
Jason M. Aryeh was appointed a member of the Myrexis Board of Directors on October 19, 2011. Mr. Aryeh is the founder and managing general partner of JALAA Equities, LP, a private hedge fund focused on the biotechnology and specialty pharmaceutical sector, and has served in such capacity since 1997. On June 4, 2012, Mr. Aryeh was elected to the Board of Directors and appointed to serve as Chairman of the Board of QLT, Inc., a biotechnology company dedicated to the development and commercialization of innovative ocular products. Mr. Aryeh also serves on the Board of Directors of Nabi Biopharmaceuticals and Ligand Pharmaceuticals Incorporated, both of which are public biotechnology companies, as well as CorMatrix Cardiovascular, a private medical device company, and the Cystic Fibrosis Foundation’s Therapeutics Board. Mr. Aryeh earned an A.B. in economics, with honors, from Colgate University, and is a member of the Omnicron Delta Epsilon Honor Society in economics.
B-68
Our Board of Directors has concluded that Mr. Aryeh should serve as a director of Myrexis, Inc. due to his capital markets experience, including his service as managing general partner of a hedge fund focused on the biotechnology and specialty pharmaceutical sector, his experience in the biotechnology industry, including his investor-side knowledge of the industry, as well as his experience serving on the boards of directors of publicly traded biotechnology companies.
Robert Forrester, LL.B., joined the Myrexis Board of Directors on June 1, 2009. Mr. Forrester has served as Chief Operating Officer of Verastem, Inc. since March 2011. Prior to joining Verastem, Mr. Forrester served as Chief Operating Officer for Forma Therapeutics, Inc. from April 2010 to January 2011. From February 2004 to January 2010, Mr. Forrester served as Interim President and Chief Executive Officer, and Executive Vice President and Chief Financial Officer of CombinatoRx, Incorporated (now called Zalicus Inc.). Prior to joining CombinatoRx, Mr. Forrester served as Senior Vice President, Finance and Corporate Development at Coley Pharmaceutical Group from 2000 to September 2003. Mr. Forrester was a Managing Director of the proprietary investment group at MeesPierson, part of the Fortis Group, from 1994 to 2000. Prior to MeesPierson, Mr. Forrester worked for BZW, UBS and Clifford Chance LLP. Mr. Forrester holds a LL.B. from Bristol University. Mr. Forrester served as a director of CPEX Pharmaceuticals, Inc. from April 2010 until April 2011.
Our Board of Directors has concluded that Mr. Forrester should serve as a director of Myrexis, Inc. due to his executive level management experience resulting from service on various boards of private and public biotechnology companies and as a senior executive officer at private and publicly held companies in the pharmaceutical and biotechnology industries, including experience in the development of corporate strategy, as well as his significant financial and investment banking expertise.
Timothy R. Franson, M.D., joined the Myrexis Board of Directors on September 10, 2009. Dr. Franson has served as Senior Vice President with B&D Consulting since December 2009, and now as Principal at Faegre BD Consulting (a merged firm from Faegre Benson and B&D Consulting) and served as Senior Advisor from August 2008 until December 2009. He also serves as President of the United States Pharmacopeial Convention (2010-2015) and previously served as a Director for Quadraspec, Inc., a small technology firm in West Lafayette, Indiana. Until his retirement in June 2008, Dr. Franson was with Eli Lilly and Company for over 20 years, most recently as Vice President of Global Regulatory Affairs and Drug Safety. Previous positions held at Lilly included Group Medical Director for Europe, Executive Director for North American Regulatory, Chemistry Manufacturing Control, Planning & Global Operations and Vice President of Clinical Research and Regulatory Affairs-US. Dr. Franson has served as chair of the Clinical Steering Committee and as a member of the Regulatory Affairs Coordinating Committee of the Pharmaceutical Research and Manufacturers’ Association (PhRMA) and until recently, chaired PhRMAs FDA Committee Staff Work Group (2000-2008). He was co-chair of the joint FDA-industry working group addressing clinical aspects of the FDA Modernization Act of 1997, including the Prescription Drug User Fee Act (PDUFA) renewal; and from 2000-2003 he co-chaired the overall industry-FDA committees for PDUFA-3 renewal. Dr. Franson received his undergraduate degree in Pharmacy at Drake University and his M.D. degree at the University of Illinois. He is Board Certified in Internal Medicine and Infectious Diseases and prior to joining Lilly was Assistant Professor of Medicine at the Medical College of Wisconsin where he was a member of the Governor’s Task Force on AIDS. He was also an Assistant Professor of Medicine at Indiana University School of Medicine (1987-2008) and on the Board of Directors of the National Patient Safety Foundation (2001-2006).
Our Board of Directors has concluded that Dr. Franson should serve as a director of Myrexis, Inc. due particularly to his knowledge and experience in policymaking and regulatory and compliance issues in the pharmaceutical industry in both the United States and internationally, as well as his extensive clinical and senior management experience at a large, global, publicly held pharmaceutical company.
David W. Gryska was appointed Chief Operating Officer of Myrexis and a member of our Board of Directors on May 11, 2012. On August 15, 2012, Mr. Gryska was appointed Acting President and Chief Executive Officer upon the death of our Chief Executive Officer, Richard B. Brewer. From December 2006 to
B-69
October 2010, Mr. Gryska was Senior Vice President and Chief Financial Officer of Celgene Corporation. Previously, from October 2004 to December 2006, he was a principal at Strategic Consulting Group, where he provided strategic consulting to early-stage biotechnology companies. From 1998 to 2004, Mr. Gryska was Senior Vice President and Chief Financial Officer at Scios, Inc., a biopharmaceutical company, where he helped lead the transaction effort for the successful sale of the company to Johnson & Johnson for $2.5 billion in February 2003. Previously, Mr. Gryska served as a partner at Ernst & Young. During his eleven years at Ernst & Young, he focused on technology industries, with an emphasis on biotechnology and healthcare companies. Mr. Gryska also serves on the Board of Directors of Seattle Genetics, Inc. and Hyperion Therapeutics. He holds a B.A. in accounting and finance from Loyola University and an M.B.A. from Golden Gate University.
Our Board of Directors has concluded that Mr. Gryska should serve as a director of Myrexis, Inc. based on his valuable and relevant experience as a senior financial executive at life science and biotechnology companies dealing with financings, mergers, acquisitions and global expansion and other strategic transactions, his extensive knowledge of accounting principles and financial reporting rules and regulations, tax compliance and oversight of the financial reporting processes of several large, publicly traded corporations.
John T. Henderson, M.D., was appointed a member of the Myrexis Board of Directors on February 19, 2009. Since December 2000, Dr. Henderson has served as a consultant to the pharmaceutical industry as President of FuturePharm LLC. Until his retirement in December 2000, Dr. Henderson was with Pfizer for over 25 years, most recently as a Vice President in the Pfizer Pharmaceuticals Group. Dr. Henderson previously held Vice President level positions with Pfizer in Research and Development in Europe and later in Japan. He was also Vice President, Medical for the Europe, U.S. and International Pharmaceuticals groups at Pfizer. Dr. Henderson earned his bachelor’s and medical degree from the University of Edinburgh and is a Fellow of the Royal College of Physicians (Ed.) and a Fellow of the Faculty of Pharmaceutical Medicine. Dr. Henderson has served as a director of Myriad Genetics, Inc. since May 2004 and Chairman of the Board of Directors since April 2005, and also serves on the Board of Directors of Cytokinetics, Inc.
Our Board of Directors has concluded that Dr. Henderson should serve as a director of Myrexis, Inc. due to his experience and understanding of a broad range of global drug development and pharmaceutical industry issues, his senior management experience at a large, global, publicly held pharmaceutical company, his general knowledge and experience with respect to the biotechnology and pharmaceutical industries as well as his understanding of corporate governance as a Chairman and his service on various boards.
Dennis H. Langer, M.D., J.D., was appointed a member of the Myrexis Board of Directors on February 19, 2009. From August 2005 to May 2010, Dr. Langer served as Managing Partner of Phoenix IP Ventures, LLC. From January 2004 to July 2005, Dr. Langer served as President, North America for Dr. Reddy’s Laboratories, Inc. From September 1994 until January 2004, Dr. Langer held several high-level positions at GlaxoSmithKline, and its predecessor, SmithKline Beecham, including most recently as Senior Vice President, Project, Portfolio and Alliance Management, Senior Vice President, Product Development Strategy, and Senior Vice President, Healthcare Services R&D. From 1991 to 1994, Dr. Langer was President and CEO of Neose Pharmaceuticals, Inc. From 1983 to 1991, Dr. Langer held positions in clinical research and marketing at Eli Lilly, Abbott and Searle. He is also a Clinical Professor at the Department of Psychiatry, Georgetown University School of Medicine. Dr. Langer received a J.D. (cum laude) from Harvard Law School, an M.D. from Georgetown University School of Medicine, and a B.A. in Biology from Columbia University. Dr. Langer has served as a director of Myriad Genetics, Inc. since 2004, and served on the Board of Directors of Auxilium Pharmaceuticals, Inc. from 2007 until 2010, Pharmacopeia, Inc. from 2006 until 2008, and Cytogen Corporation from 2005 until 2008.
Our Board of Directors has concluded that Dr. Langer should serve as a director of Myrexis, Inc. due particularly to his broad leadership experience resulting from service on various boards and as a Chief Executive Officer, his extensive business and scientific expertise due to his background in the development and commercialization of pharmaceutical products in the United States and internationally, and his entrepreneurial experience in the creation and oversight of new life-sciences companies.
B-70
Robert J. Lollini was appointed a member of the Myrexis Board of Directors on September 6, 2011, and previously served as the Company’s President and Chief Executive Officer from September 6, 2011 to May 11, 2012 and as the Company’s Interim President and Chief Executive Officer from July 21, 2011 to September 6, 2011. Mr. Lollini joined Myrexis, Inc. in February 2009 and served as Chief Financial Officer from February 17, 2009 to July 21, 2011. In addition, Mr. Lollini served as Treasurer from February 17, 2009 until September 6, 2011, and as Secretary from May 25, 2011 until September 6, 2011. Prior to joining the Company, Mr. Lollini held several executive management positions with Iomed, Inc., an international drug delivery company, serving as President and Chief Executive Officer and a director from November 2002 to August 2007, Chief Operating Officer from October 2001 to November 2002 and as Executive Vice President, Finance, Chief Financial Officer and Secretary from January 1993 to October 2001. Between 1989 and 1992, Mr. Lollini worked for R.P. Scherer Corporation, also an international drug delivery company, as Vice President, Finance, Chief Financial Officer and Secretary, and between 1981 and 1988, as its Corporate Controller and Chief Accounting Officer and in various other management capacities. Between 1978 and 1981, Mr. Lollini was with the accounting firm of Arthur Andersen & Co. Mr. Lollini is a Certified Public Accountant and received a Bachelor of Arts degree in Accounting from Michigan State University and an MBA in Finance/Economics from the University of Detroit.
Our Board of Directors has concluded that Mr. Lollini should serve as a director of Myrexis, Inc. because, having been with Myrexis since its transition to an independent public company, as well as in his previous professional experience, he has demonstrated outstanding management and leadership skills as well as the ability to make decisions effectively and to execute through appropriate action. In addition, Mr. Lollini has an extensive understanding and command of Myrexis’s business, a combination of strategic thinking and operational effectiveness, leadership skills, and a commitment to pursue the best interests of Myrexis’s shareholders as his highest priority.
Pursuant to the terms of our Separation and Consulting Agreement with Mr. Lollini, dated May 11, 2012, Mr. Lollini will resign from the Board of Directors effective on November 15, 2012.
Committees of the Board of Directors
Our Board of Directors has established the following committees:
Audit Committee
Our Audit Committee currently has three members, Robert Forrester (Chairman), John Henderson and Dennis Langer. Our Audit Committee’s role and responsibilities are set forth in the Audit Committee’s written charter and include the authority to retain and terminate the services of our independent registered public accounting firm. In addition, the Audit Committee reviews annual financial statements, considers matters relating to accounting policy and internal controls and reviews the scope of annual audits. Our Board of Directors has determined that all members of the Audit Committee satisfy the current independence standards promulgated by the SEC and by The NASDAQ Stock Market LLC, as such standards apply specifically to members of audit committees. The Board has determined that Mr. Forrester is an “audit committee financial expert,” as the Securities and Exchange Commission has defined that term in Item 407 of Regulation S-K.
A copy of the Audit Committee’s written charter is publicly available on the “Investors—Corporate Governance” section of our website at www.myrexis.com.
Compensation Committee
Our Compensation Committee currently has three members, Dennis Langer (Chairman), Gerald Belle and Robert Forrester. Our Compensation Committee’s role and responsibilities are set forth in the Compensation Committee’s written charter and include reviewing, approving and making recommendations regarding our compensation policies, practices and procedures to ensure that legal and fiduciary responsibilities of the Board of
B-71
Directors are carried out and that such policies, practices and procedures contribute to our success. The Compensation Committee is responsible for the determination of the compensation of our executive officers, and conducts its decision making process with respect to that issue without such officers present. Our Board of Directors has determined that all members of the Compensation Committee qualify as independent under the definition promulgated by The NASDAQ Stock Market LLC.
A copy of the Compensation Committee’s written charter is publicly available on the “Investors—Corporate Governance” section of our website at www.myrexis.com.
Nominating and Governance Committee
Our Nominating and Governance Committee currently has three members, John Henderson (Chairman), Dennis Langer and Timothy Franson. Our Nominating and Governance Committee’s role and responsibilities are set forth in the Nominating and Governance Committee’s written charter and include identifying and nominating members of our Board of Directors, developing and recommending to our Board of Directors a set of corporate governance principles applicable to our company and overseeing the evaluation of the performance of our Board of Directors. The committee also oversees our policy on plurality voting for director elections, under which, in non-contested elections, if a director receives a greater number of WITHHOLD votes than FOR votes, the Board will decide, through a process managed by the Nominating and Governance Committee and excluding the nominee in question, whether it should request that the director submit his or her resignation, maintain the director but address what the Nominating and Governance Committee believes is the underlying cause of the WITHHOLD votes, or resolve not to re-nominate the director in the future for election. A copy of this policy is publicly available on the “Investors—Corporate Governance” section of our website at www.myrexis.com. The Board of Directors has determined that all members of the Nominating and Governance Committee qualify as independent under the definition promulgated by The NASDAQ Stock Market LLC.
Under our current corporate governance policies, the Nominating and Governance Committee may consider candidates recommended by stockholders as well as from other sources such as other directors or officers, third party search firms or other appropriate sources. For all potential candidates, the Nominating and Governance Committee may consider all factors it deems relevant, such as a candidate’s personal integrity and sound judgment, business and professional skills and experience, independence, knowledge of the industry in which we operate, possible conflicts of interest, diversity, the extent to which the candidate would fill a present need on the Board, and the long-term interests of the stockholders. In general, persons recommended by stockholders will be considered on the same basis as candidates from other sources. If a stockholder wishes to propose a candidate for consideration as a nominee by the Nominating and Governance Committee under our corporate governance policies, for future annual meetings, the Nominating and Governance Committee will consider only one recommended nominee from any stockholder or group of affiliated stockholders for each annual meeting, and such recommending stockholder or group must have held at least 5% of our common stock for at least one year. All stockholder recommendations for proposed director nominees must be in writing to the Nominating and Governance Committee, care of Myrexis’s Secretary at 305 Chipeta Way, Salt Lake City, Utah 84108, and must be received no later than 120 days prior to the first anniversary of the date of the proxy statement for the previous year’s annual meeting or, in certain circumstances, such as there was no previous annual meeting, a reasonable time in advance of the mailing of our proxy statement for such annual meeting. The recommendation must be accompanied by the following information concerning the recommending stockholder:
| • | the name, address and telephone number of the recommending stockholder; |
| • | the number of shares of our common stock owned by the recommending stockholder and the time period for which such shares have been held; |
| • | if the recommending stockholder is not a stockholder of record, a statement from the record holder verifying the holdings of the recommending stockholder and a statement from the recommending stockholder of the length of time such shares have been held (alternatively the recommending stockholder may furnish a current Schedule 13D, Schedule 13G, Form 3, Form 4 or Form 5 filed with the SEC, together with a statement of the length of time that the shares have been held); and |
B-72
| • | a statement from the recommending stockholder as to a good faith intention to continue to hold such shares through the date of the next annual meeting. |
The recommendation must also be accompanied by the following information concerning the proposed nominee:
| • | the information required by Items 401, 403 and 404 of Regulation S-K under the Securities Act; |
| • | a description of all relationships between the proposed nominee and any stockholder of the Company, including the recommending stockholder, including any agreements or understandings regarding the nomination; |
| • | a description of all relationships between the proposed nominee and any of our competitors, customers, suppliers, labor unions or other persons with special interests regarding the Company; and |
| • | the contact information of the proposed nominee. |
The recommending stockholder must also furnish a statement supporting a view that the proposed nominee possesses the minimum qualifications as set forth below for director nominees and describing the contributions that the proposed nominee would be expected to make to the Board and to the governance of Myrexis and must state whether, in its view, the proposed nominee, if elected, would represent all stockholders and not serve for the purpose of advancing or favoring any particular stockholder or other constituency of Myrexis. The recommendation must also be accompanied by the written consent of the proposed nominee (i) to be considered by the Nominating and Governance Committee and interviewed if the committee chooses to do so in its discretion, and (ii) if nominated and elected, to serve as a director.
For all potential candidates, the Nominating and Governance Committee may consider all factors it deems relevant, including the following threshold criteria:
| • | candidates should possess the highest personal and professional standards of integrity and ethical values; |
| • | candidates must be committed to promoting and enhancing the long-term value of Myrexis for its stockholders; |
| • | candidates must be able to represent fairly and equally all stockholders without favoring or advancing any particular stockholder or other constituency of Myrexis; |
| • | candidates must have demonstrated achievement in one or more fields of business, professional, governmental, community, scientific or educational endeavor, and possess mature and objective business judgment and expertise; |
| • | candidates are expected to have sound judgment, derived from management or policy making experience that demonstrates an ability to function effectively in an oversight role; |
| • | candidates must have a general appreciation regarding major issues facing public companies of a size and operational scope similar to Myrexis, including, governance concerns, regulatory obligations, strategic business planning, competition and basic concepts of accounting and finance; and |
| • | candidates must have, and be prepared to devote, adequate time to the Board of Directors and its committees. |
In addition, the Nominating and Governance Committee will also take into account the extent to which the candidate would fill a present need on the Board, including the extent to which a candidate meets the independence and experience standards promulgated by the SEC and by The NASDAQ Stock Market LLC.
The Board and Nominating and Governance Committee do not have a formal policy with respect to the consideration of diversity in identifying nominees for a director position. However, the Board and Nominating
B-73
and Governance Committee strive to nominate individuals with a variety of diverse backgrounds, skills, qualifications, attributes and experience such that the Board, as a group, will possess the appropriate expertise, talent and skills to fulfill its oversight responsibilities with respect to the long-term interests of the stockholders.
A copy of the Nominating and Governance Committee’s written charter is publicly available on the “Investors—Corporate Governance” section of our website at www.myrexis.com.
EXECUTIVE OFFICERS
The following table sets forth certain information regarding our executive officers.
| Name |
Age | Position | ||||
| David W. Gryska |
56 | Acting President and Chief Executive Officer, Chief Operating Officer and Director | ||||
| Andrea Kendell |
40 | Chief Financial Officer, Treasurer and Secretary | ||||
David W. Gryska—Please see Mr. Gryska’s biography above under “The Board of Directors.”
Andrea Kendell joined Myrexis, Inc. in May 2009 as Corporate Controller and became Vice President, Finance and Human Resources in June 2010. On September 6, 2011, the Board appointed Ms. Kendell as Chief Financial Officer, Treasurer and Secretary. Prior to joining Myrexis, Ms. Kendell held senior management positions with Moog Medical Devices Group, formerly ZEVEX, Inc., a publicly traded international medical device manufacturing company serving as Corporate Controller from January 1997 to March 2007, and Group Financial Manager from March 2007 to May 2009. Ms. Kendell received a B.A. in Accounting and a M.A. in Accounting from Westminster College, Salt Lake City, Utah.
Section 16(a) Beneficial Ownership Reporting Compliance
Our records reflect that all reports which were required to be filed pursuant to Section 16(a) of the Exchange Act were filed on a timely basis.
An Annual Statement of Beneficial Ownership on Form 5 is not required to be filed if there are no previously unreported transactions or holdings to report. Nevertheless, we are required to disclose the names of directors, officers and 10% stockholders who did not file a Form 5 unless we have obtained a written statement that no filing is required. We received either a written statement from our directors, officers and 10% stockholders or know from other means that no Forms 5 were required to be filed.
Corporate Code of Conduct and Ethics
We have adopted a Corporate Code of Conduct and Ethics that applies to all of our directors and employees, including our chief executive officer and chief financial and accounting officer. A copy of the Corporate Code of Conduct and Ethics is publicly available on the “Investors—Corporate Governance” section of our website at www.myrexis.com. To the extent permissible under applicable law, the rules of the SEC or The Nasdaq Stock Market, we also intend to post on our website any amendment to the Corporate Code of Conduct and Ethics, or any grant of a waiver from a provision of the Corporate Code of Conduct and Ethics, that requires disclosure under applicable law, SEC rules or Nasdaq listing standards.
B-74
| Item 11. | EXECUTIVE COMPENSATION |
COMPENSATION DISCUSSION AND ANALYSIS
We became an independent, publicly traded biopharmaceutical company on June 30, 2009, the last day of our 2009 fiscal year, as a result of our separation and spin-off from our former parent company Myriad Genetics, Inc. This Compensation Discussion and Analysis discusses the compensation of our named executive officers for fiscal year 2012, our most recently completed fiscal year.
At our 2011 annual meeting, our stockholders cast their votes in support of the Board of Directors’ recommendations on the advisory vote regarding the compensation of our named executive officers. Based on the favorable response we received from our stockholders on this advisory vote, there were no changes made to our compensation policies and decisions as result of this vote.
Fiscal Year 2012 Named Executive Officers
Our named executive officers for the fiscal year ended June 30, 2012 were:
| • | Richard B. Brewer, our former President and Chief Executive Officer (May 11, 2012 – August 15, 2012). |
| • | Robert J. Lollini, our former President and Chief Executive Officer (July 21, 2011 – May 11, 2012) and former Chief Financial Officer (February 17, 2009 – September 6, 2011). |
| • | Adrian N. Hobden, Ph.D., our former President and Chief Executive Officer (February 19, 2009 – July 21, 2011). |
| • | Andrea Kendell, our Chief Financial Officer (September 6, 2011 – present). |
| • | Wayne Laslie, our former Chief Operating Officer (February 19, 2009 – February 29, 2012). |
Although David W. Gryska was appointed our Chief Operating Officer on May 11, 2012, under SEC rules he was not deemed a named executive officer for fiscal year 2012 and as a result does not appear in this Compensation Discussion and Analysis and the executive compensation tables that follow. As he was appointed our acting President and Chief Executive Officer on August 15, 2012, following the death of Richard B. Brewer, he will be a named executive officer for fiscal year 2013.
Executive Summary of Fiscal Year 2012 Company Events and Management Changes
Effective July 21, 2011, Adrian N. Hobden, Ph.D., resigned as our President and Chief Executive Officer and as a member of our Board of Directors, and Robert J. Lollini, our then serving Chief Financial Officer, was appointed our interim President and Chief Executive Officer. On September 6, 2011, the Board of Directors appointed Mr. Lollini permanent President and Chief Executive Officer, and as a Class I member of the Board of Directors, and Andrea Kendell, our then serving Vice President, Finance and Human Resources, as Chief Financial Officer. In connection with these management changes, we entered into a Separation Agreement with Dr. Hobden on July 21, 2011, modified our employment and compensation arrangements with Mr. Lollini, including entering into a new offer letter of employment and an amendment to his Executive Severance and Change in Control Agreement on September 9, 2011, and modified our employment and compensation arrangements with Ms. Kendell, including entering into an offer letter of employment and an Executive Severance and Change in Control Agreement on September 22, 2011, each of which is further described below under the heading “Fiscal Year 2012 Compensation of Named Executive Officers.”
On September 8, 2011, we announced that we had completed an in-depth review of our drug development pipeline, incorporating extensive inputs from both internal and independent external analyses. As a result, we
B-75
made a strategic business decision to suspend any further development of our lead drug candidate Azixa, which was in Phase 2 development for the treatment of advanced primary and metastatic tumors with brain involvement. Following this decision, in November 2011, we announced a corporate reorganization to realign our resources with our development strategy and clinical initiatives following the suspension of our lead drug candidate. The reorganization included an immediate reduction in our workforce by 15 employees or approximately 20%.
On November 16, 2011, Wayne Laslie, our Chief Operating Officer, resigned, which resignation became effective on February 29, 2012. In connection with Mr. Laslie’s resignation, we entered into a Separation Agreement with Mr. Laslie on December 13, 2011, which is further described below under the heading “Fiscal Year 2012 Compensation of Named Executive Officers.”
In February 2012, we announced that we had suspended development activity on all of our preclinical and clinical programs and retained Stifel Nicolaus Weisel, an investment banking firm, to assist in reviewing and evaluating a full range of strategic alternatives to enhance shareholder value. Thereafter, in March 2012, we initiated an alignment of our resources involving a phased reduction in our workforce from approximately 59 employees to 10 current employees.
Based on our evaluation of strategic alternatives, we determined to pursue the acquisition of one or more commercial-stage biopharmaceutical assets, with the goal of building a commercial-stage biopharmaceutical company by optimizing their performance and profitability. Integral to these efforts, on May 11, 2012, we appointed Richard B. Brewer as President and Chief Executive Officer and David W. Gryska as Chief Operating Officer and entered into employment agreements and restricted stock unit agreements with them. The terms of Mr. Brewer’s agreements are described below under the heading “Fiscal Year 2012 Compensation of Named Executive Officers.” In addition, both Mr. Brewer and Mr. Gryska were appointed as members of our Board of Directors.
In connection with the appointment of Mr. Brewer, Robert J. Lollini resigned as President and Chief Executive Officer effective May 11, 2012. In connection with Mr. Lollini’s resignation, we entered into a Separation and Consulting Agreement with Mr. Lollini on May 11, 2012, which is further described below under the heading “Fiscal Year 2012 Compensation of Named Executive Officers.”
After the death of Richard B. Brewer, our President and Chief Executive Officer, on August 15, 2012, the Board of Directors appointed David W. Gryska as our acting President and Chief Executive Officer while considering succession plans.
Detailed Discussion and Analysis
Objectives and Elements of Our Compensation Program
Following our separation and spin-off from Myriad Genetics, our Compensation Committee established the objectives of our compensation programs and implemented plans, policies, and practices to achieve these objectives, and since that time we have been continuously engaged in reviewing and revising our executive compensation policies and practices in light of the changing economic environment, our evolution as an independent company, and, in particular with respect to fiscal year 2012, corporate developments, while striving to achieve the following objectives:
| • | attract and retain the best possible executive talent, |
| • | motivate our executive officers, |
| • | reward executive officers for their contribution to achieving our objectives through the recognition of individual leadership, initiatives, achievements and other contributions, and |
| • | increase long-term shareholder value. |
B-76
The compensation program for our executive officers has historically consisted principally of a combination of base salary, a cash bonus payable under an annual management performance program, and long-term compensation in the form of stock options and restricted stock unit awards designed to be competitive with those of comparable companies and to align executive performance with the long-term interests of our stockholders. An annual base salary provides the foundation of our compensation program and ensures that the executive officer is being paid ongoing compensation which allows us to attract and retain high-quality talent. An annual incentive bonus forms an important part of our compensation strategy by providing an incentive to reward short-term performance as measured by our performance and accomplishment of individual management business objectives, or MBOs. Stock option awards and restricted stock unit awards reward our executive officers for our long-term performance, and help to ensure that our executive officers have a stake in our long-term success by providing an incentive to improve our overall growth and value as measured by our stock price. This aligns the executive officer’s interests with stockholders’ long-term interests. In addition, to motivate our executive officers to stay with us during periods of uncertainty and to keep them focused on the Company’s interests, in February 2010, we entered into Executive Severance and Change in Control Agreements with our then serving executive officers, and on September 22, 2011, with Andrea Kendell in connection with her appointment as Chief Financial Officer, to provide certain severance benefits upon termination. As further discussed herein, our only Executive Severance and Change in Control Agreement currently in effect with any of our executive officers is our agreement with Ms. Kendell. We also have an Employee Stock Purchase Plan that provides all of our eligible employees, including our executive officers, with an opportunity to purchase our common stock semi-annually at a purchase price equal to 85% of the reported last sale price of our common stock on either the first or last day of each offering period, whichever is less, and we provide various benefit programs to all of our employees, including health and dental insurance, life and disability insurance, and a 401(k) plan where the Company makes matching contributions of 50% of each employee’s contribution with the employer’s contribution not to exceed 4% of the employee’s compensation.
While certain of these elements, including base salary and equity awards, were used in fiscal year 2012 to compensate certain of our named executive officers, due to the several management changes that we experienced during the fiscal year resulting in termination-based compensation arrangements for some named executive officers and modified compensation arrangements for other named executive officers, the following discussion analyzes the compensation of each named executive officer individually.
Formulating and Setting Executive Compensation and Role of Management
In accordance with the specific directives of our Compensation Committee as set forth in its charter, the Compensation Committee is responsible for formulating, evaluating, determining, and, at times, recommending that the Board of Directors approve, appropriate short- and long-term compensation and incentives, in the form of cash and equity, that are intended to motivate and reward the accomplishment of individual and corporate objectives and align executive officer compensation with creation of long-term shareholder value. The Compensation Committee also assists the full Board of Directors in establishing and administering appropriate incentive compensation and equity-based plans. Members of management support the Compensation Committee, attend portions of its meetings upon request, and perform various administrative functions at its request. No executive officer is present during Compensation Committee or Board discussions regarding his or her own compensation.
To assist in carrying out its responsibilities, the Compensation Committee utilizes publicly available compensation data and subscription compensation survey data for national and regional companies in the biotechnology and life science industry. Since December 2008, the Compensation Committee has retained Radford, An Aon Hewitt Consulting Company, for the purpose of reviewing the compensation of our executive officers. Radford has provided us with competitive market data on the compensation of executive officers at comparable companies within our industry and has provided the Compensation Committee analyses of, and recommendations for, cash and equity compensation for our executive officers. We believe that the information provided by Radford aids us in determining the compensation of our executive officers.
B-77
In June 2011, Radford reviewed our existing fiscal year 2011 peer group to determine its comparability to Myrexis based on our stage of development, employee headcount, market value, financial profile and business focus. This evaluation resulted in the removal of 11 companies (Alexza Pharmaceuticals, Arena Pharmaceuticals, ARIAD Pharmaceuticals, Inc., Array BioPharma Inc., Dyax Corp., ImmunoGen, Inc., Incyte Corporation, Inspire Pharmaceuticals, Inc., Pain Therapeutics, Inc., VIVUS, Inc. and Vical Incorporated) and the addition of 11 companies (Aastrom Biosciences, Athersys, AVI BioPharma, Celldex Therapeutics, Curis, Endocyte, Idera Pharma, Inhibitex, Novavax, Peregrine Pharma and Threshold Pharma) based on employee headcount, development stage and market cap. The peer group approved by the Compensation Committee is comprised of the below listed companies. We refer to this peer group herein as the “2012 peer group” and the data derived from the 2012 peer group as the “Radford Report.”
| Aastrom Biosciences |
Idera Pharma | |
| Affymax, Inc. |
Immunomedics, Inc. | |
| Amicus Therapeutics, Inc. |
Infinity Pharmaceuticals, Inc. | |
| ArQule, Inc. |
Inhibitex | |
| Athersys |
Maxygen, Inc. | |
| AVI BioPharma |
Neurocrine Biosciences, Inc. | |
| Celldex Therapeutics |
Novavax | |
| Curis |
Peregrine Pharma | |
| Cytokinetics, Incorporated |
Rigel Pharmaceuticals, Inc. | |
| Endocyte |
Synta Pharmaceuticals Corp. | |
| Geron Corporation |
Threshold Pharma |
Fiscal Year 2012 Compensation of Named Executive Officers
Richard B. Brewer, former President and Chief Executive Officer (May 11, 2012 – August 15, 2012)
Description of Compensation Arrangements
In connection with Mr. Brewer’s appointment as President and Chief Executive Officer, we entered into an employment agreement with Mr. Brewer, effective May 11, 2012, pursuant to which Mr. Brewer was employed on an at-will basis with no specified term of employment.
Pursuant to Mr. Brewer’s employment agreement, his initial base salary was $575,000 per year. As incentive compensation to align his interests with those of our stockholders, on May 11, 2012, Mr. Brewer was granted restricted stock units (“RSUs”) under our 2009 Employee, Director and Consultant Equity Incentive Plan (the “Equity Incentive Plan”), representing a contingent entitlement to receive 1,069,615 shares of our common stock, representing 4% of our outstanding shares of common stock as of May 11, 2012, pursuant to a Restricted Stock Unit Award Agreement (the “RSU Agreement”). Pursuant to the terms of the RSU Agreement, provided Myrexis had completed the acquisition of another company or business whether by merger, reverse merger, combination, acquisition of all or substantially all of a company’s assets, purchase of securities or similar transaction (an “Acquisition”) on or before May 11, 2013, which date could have been extended for up to two 90-day extensions in the sole discretion of the Board of Directors if it believed significant progress had been made toward achieving an Acquisition, the shares underlying the RSUs would commence vesting as described below after an Acquisition and upon the achievement of the following performance-based criteria:
| • | If the fair market value of Myrexis common stock during any five trading days within a 30-trading day period equaled or exceeded twice the average closing price of the common stock on The NASDAQ Global Market over the 10 trading day period ending on May 10, 2012 (the “First Price Increase”), then the performance milestone with respect to 75% of the shares underlying the RSUs would have been achieved and vesting would have commenced as hereinafter described. On the day following the First Price Increase, half of the shares underlying the RSUs that had been earned upon achievement of the First Price Increase would have immediately vested and the remaining half would have vested quarterly over a 24-month period thereafter, provided Mr. Brewer was still employed by Myrexis or an affiliate on each applicable vesting date. |
B-78
| • | If the fair market value of Myrexis common stock during any five trading days within a 30-trading day period equaled or exceeded three times the average closing price of the common stock on The NASDAQ Global Market over the 10 trading day period ending on May 10, 2012 (the “Second Price Increase”), then the performance milestone with respect to 25% of the shares underlying the RSUs would have been achieved and vesting would have commenced as hereinafter described. On the day following the Second Price Increase, half of the shares underlying the RSUs that had been earned upon achievement of the Second Price Increase would have immediately vested and the remaining half would have vested quarterly over a 24-month period thereafter, provided Mr. Brewer was still employed by Myrexis or an affiliate on each applicable vesting date. In addition, in the event of a sale of Myrexis prior to the Second Price Increase, the Board had the sole discretion to waive the requirement to achieve the Second Price Increase and deem the shares underlying the RSUs to be earned and commence time-based vesting as of the date of the closing of a sale of Myrexis. |
As no Acquisition occurred prior to Mr. Brewer’s death on August 15, 2012, the RSU Agreement was terminated with no shares of common stock issued.
In addition, the Board agreed to increase Mr. Brewer’s salary, subject to and following achievement of the First Price Increase, if necessary, to such amount that equaled the 75th percentile of the base salaries paid to the highest level executive officer in Myrexis’ then applicable peer group, and to establish an annual performance-based bonus plan that would have allowed for Mr. Brewer to earn a bonus with a target payment to be calculated based on a percentage of salary that was considered to be appropriate for his position in relation to companies in Myrexis’ then applicable peer group. Following the establishment of such bonus plan, payments pursuant thereto would have been made based on achievement of performance objectives as determined by the Board of Directors. As no Acquisition, and therefore no First Price Increase, occurred prior to Mr. Brewer’s death, there was no salary adjustment or bonus plan established during fiscal year 2012 or thereafter for Mr. Brewer.
Mr. Brewer’s employment agreement also contained confidentiality, non-competition, and non-solicitation provisions effective during the term of employment and for certain specified periods thereafter.
Upon Mr. Brewer’s death on August 15, 2012, his employment agreement was terminated with no amounts payable thereunder.
Determination of Mr. Brewer’s Compensation Arrangements
The terms of Mr. Brewer’s compensation were negotiated between Mr. Brewer and the Compensation Committee. To assist it in the negotiations, the Compensation Committee obtained an analysis of, and a recommendation for, cash and equity compensation for Mr. Brewer from Radford. In addition, the Compensation Committee used data from Radford’s 2011 Global Life Sciences Survey focusing on public biotechnology and pharmaceutical companies with employee size between 75 and 300 employees and revenues between $50 and $150 million for purposes of determining Mr. Brewer’s compensation. Mr. Brewer’s compensation package was a combination of base salary and equity incentives that were dependent upon both a strategic acquisition and share price performance designed to tie a substantial portion of his compensation to successful execution of the Company’s business strategy and the creation of shareholder value. Specifically, Mr. Brewer’s RSUs would vest only upon the consummation of a strategic acquisition and achievement of established share price performance thresholds within a certain period of time.
Robert J. Lollini, former President and Chief Executive Officer (July 21, 2011 – May 11, 2012) and former Chief Financial Officer (February 17, 2009 – September 6, 2011)
Fiscal Year 2012 Compensation of Mr. Lollini as Chief Financial Officer and Interim President and Chief Executive Officer
In June 2011, the Compensation Committee reevaluated the base salaries of our then serving executive officers and recommended, and the Board of Directors approved, that there be no increases to the base salaries of our executive officers for fiscal year 2012, with the exception of a 5% increase of the base salary of Mr. Lollini,
B-79
who was serving as our Chief Financial Officer at that time, from $285,000 to $300,000. In addition, the Compensation Committee also recommended, and the Board of Directors approved, increasing Mr. Lollini’s target bonus award opportunity for fiscal year 2012 performance from 35% to 40% of his base salary. The Compensation Committee determined to increase Mr. Lollini’s base salary and his target bonus award opportunity based on the Radford Report, targeting a range between the 50th and 75th percentile for base salary and target bonus.
Following the resignation of Adrian N. Hobden, Ph.D., as our President and Chief Executive Officer in July 2011, Mr. Lollini was appointed interim President and Chief Executive Officer while retaining his position as Chief Financial Officer. At the time of this appointment, there were no changes made to Mr. Lollini’s compensation.
Fiscal Year 2012 Compensation of Mr. Lollini as President and Chief Executive Officer
In September 2011, Mr. Lollini was appointed permanent President and Chief Executive Officer and resigned as Chief Financial Officer. In connection with his appointment, we entered into a new offer letter with Mr. Lollini that provided for the following compensation arrangements for Mr. Lollini’s service as President and Chief Executive Officer and replaced the offer letter that we had entered into with Mr. Lollini upon his appointment as Chief Financial Officer on February 4, 2009:
| • | Base Salary: Effective September 6, 2011, Mr. Lollini’s annual base salary was increased from $300,000 to $395,000. |
| • | Payment for Service as Interim Chief Executive Officer: For his services as interim President and Chief Executive Officer from July 21, 2011 until September 6, 2011, while also serving as Chief Financial Officer, Mr. Lollini received a $25,000 cash payment. |
| • | Annual Target Bonus: For fiscal year 2012 performance, Mr. Lollini was eligible to receive an annual target bonus of 50% of his base salary (increased from 40%). |
| • | Stock Options: On September 22, 2011, Mr. Lollini was granted a stock option to purchase up to 300,000 shares of common stock under our Equity Incentive Plan at an exercise price of $2.75 per share, which was equal to the closing price of the common stock on the date of grant, and which was scheduled to vest over four years as to 25% of the shares on each anniversary of the date of grant. |
The Compensation Committee determined Mr. Lollini’s base salary and target bonus amount based on the Radford Report, targeting a range between the 25th and 50th percentile for base salary and target bonus.
Mr. Lollini’s equity compensation was determined with reference to the Radford Report, targeting a range between the 50th and 75th percentile, as well as Radford’s 2011 Global Life Sciences Survey focusing on public biotechnology and pharmaceutical companies with employee size between 50 and 200 employees. Radford determined a “Market Composite” of equity compensation at the 25th, 50th and 75th percentiles for Mr. Lollini. The Market Composite was determined by weighting the compensation data from the 2012 peer group proxy statements by 50%, to the extent proxy data was available, and Radford’s 2011 Global Life Sciences Survey by 50%. Utilizing the data provided to us in the Radford Report, we analyzed, amongst other criteria, the Market Composite equity compensation (using the Black Scholes value of options, the number of option equivalents, and grant as a percent of company), for Mr. Lollini at the 25th, 50th and 75th percentile range. We also analyzed our expected gross equity burn rate, issued equity overhang and total equity overhang at the 50th and 75th percentile range as compared to the 22 companies reported in our peer group of companies from equity compensation information for this peer group from publicly available regulatory filings, including proxy statements. Mr. Lollini’s stock option award was set at approximately the 50th percentile range of aggregate value of awards for chief executive officers represented in the Radford Report.
B-80
In addition, Mr. Lollini’s Executive Severance and Change in Control Agreement, dated February 1, 2010, was amended on September 9, 2011, pursuant to which the benefits Mr. Lollini was eligible to receive upon a termination by us that was not in connection with a Change in Control (other than for Cause, Disability or death) or by Mr. Lollini for Good Reason (as such capitalized terms are defined in the agreement), were amended, as follows:
| • | Payment in a lump sum amount equal to one times his then current annual base salary (increased from six months of base salary); |
| • | payment in a lump sum amount equal to one times his then current fiscal year target bonus amount (increased from 50%); and |
| • | continuation of health benefits for up to 12 months (increased from six months). |
In the event of the above-described termination, Mr. Lollini was also entitled to payment in a lump sum amount of his base salary through the date of termination, a pro rata portion of his then current fiscal year target bonus amount, and any accrued vacation pay to the extent not previously paid, in accordance with the original terms of his agreement. The amendment to Mr. Lollini’s Executive Severance and Change in Control Agreement was recommended by the Compensation Committee, and approved by the Board of Directors, in order to provide Mr. Lollini with the same severance and change in control benefits that Dr. Hobden, our previous President and Chief Executive Officer, had been eligible to receive under his Executive Severance and Change in Control Agreement.
May 11, 2012 Separation and Consulting Agreement with Mr. Lollini
Mr. Lollini resigned as President and Chief Executive Officer effective May 11, 2012. In connection with Mr. Lollini’s resignation, we entered into a Separation and Consulting Agreement with Mr. Lollini, dated May 11, 2012 (the “Separation Agreement”). Mr. Lollini received the payments and benefits to which he was entitled under his Executive Severance and Change in Control Agreement, dated February 1, 2010, as amended on September 9, 2011, in connection with a termination without Cause (as defined in the agreement). In addition, under the Separation Agreement, Mr. Lollini agreed to resign as a director of Myrexis as of November 15, 2012, and to provide consulting services to Myrexis through November 15, 2012 in exchange for (i) continued vesting of his outstanding stock options and restricted stock units until November 15, 2012, (ii) the vesting on November 15, 2012 of options to purchase 75,000 shares of Myrexis common stock that would not otherwise have vested by their terms as of that date, and (iii) the extension of the expiration date of all his vested stock options until November 15, 2013. Further details regarding Mr. Lollini’s Separation Agreement are discussed below under the heading “Payments Upon Termination or Change in Control.”
Adrian N. Hobden, Ph.D., former President and Chief Executive Officer (February 19, 2009 – July 21, 2011)
Effective July 21, 2011, Adrian N. Hobden, Ph.D., resigned as our President and Chief Executive Officer and as a member of our Board of Directors. Prior to his resignation, there were no adjustments made during the 2012 fiscal year to Dr. Hobden’s compensation nor was he awarded any equity grants in the 2012 fiscal year. In connection with Dr. Hobden’s resignation, we negotiated and entered into a Separation Agreement with Dr. Hobden, dated July 21, 2011. In consideration for the payments and benefits under the Separation Agreement, Dr. Hobden agreed to assist us with an orderly transition for a three month period following July 21, 2011, during which he agreed to be available to provide consulting services to us for up to 10 hours per week. Pursuant to the terms and conditions of the Separation Agreement, Dr. Hobden received (i) a lump sum payment equal to six months of his gross monthly base salary, (ii) a lump sum payment equal to 50% of his target bonus for the 2012 fiscal year, (iii) COBRA benefits, and (iv) accelerated vesting of stock options and restricted stock units that would have vested through July 21, 2012. Further details regarding Dr. Hobden’s Separation Agreement are discussed below under the heading “Payments Upon Termination or Change in Control.”
B-81
Andrea Kendell, Chief Financial Officer (September 6, 2011 – present)
Fiscal Year 2012 Compensation of Ms. Kendell as Chief Financial Officer
Prior to Ms. Kendell’s appointment as our Chief Financial Officer on September 6, 2011, Ms. Kendell served as our Vice President, Finance and Human Resources. In connection with Ms. Kendell’s appointment as Chief Financial Officer, we entered into an offer letter with Ms. Kendell, dated September 22, 2011, which provided for the following compensation arrangements for Ms. Kendell’s service as Chief Financial Officer:
| • | Base Salary: Effective September 22, 2011, Ms. Kendell’s annual base salary was $255,000. |
| • | Annual Target Bonus: For fiscal year 2012 performance, Ms. Kendell was eligible to receive an annual target bonus of up to 35% of her base salary. |
| • | Stock Options: On September 22, 2011, Ms. Kendell was granted a stock option to purchase up to 160,000 shares of common stock under our Equity Incentive Plan at an exercise price of $2.75 per share, which was equal to the closing price of the common stock on the date of grant, which vests over four years as to 25% of the shares on each anniversary of the date of grant. |
In addition, on September 22, 2011, we entered into an Executive Severance and Change in Control Agreement with Ms. Kendell, the terms of which are described below under the heading “Payments Upon Termination or Change in Control.”
We also have a standard form of employment agreement with Ms. Kendell, dated June 29, 2009. Pursuant to our employment agreement with Ms. Kendell, either party may terminate employment at any time for any reason, with or without notice or cause. The employment agreement also provides that Ms. Kendell will not disclose confidential information of Myrexis during and after employment and will not compete with Myrexis nor solicit customers or employees during the term of employment and for one year thereafter.
The Compensation Committee determined Ms. Kendell’s base salary and target bonus amount based on the Radford Report, targeting a range between the 25th and 50th percentile for base salary and target bonus. Ms. Kendell’s equity compensation was determined with reference to the Radford Report, as well as Radford’s 2011 Global Life Sciences Survey, in the same manner that Mr. Lollini’s equity compensation was determined as described above. Ms. Kendell’s stock option award was set at approximately the 75th percentile range of aggregate value of awards for chief financial officers represented in the Radford Report.
Ms. Kendell’s Fiscal Year 2012 Bonus and Post-2012 Fiscal Year End Compensation Adjustments
At the end of our 2012 fiscal year, the Compensation Committee determined, in an exercise of its sole discretion, to award Ms. Kendell a $75,000 bonus for fiscal year 2012 performance, representing approximately 84% of her target bonus for the year. The Compensation Committee determined to award this bonus in consideration of Ms. Kendell’s management and oversight of several reductions in force and corporate reorganizations throughout the fiscal year, implementation of strong documentation processes in connection therewith, supporting the Board of Directors through multiple management changes, ensuring internal control over financial reporting activities and supporting the Company and the Board of Directors in its pursuit of other strategic alternatives.
In addition, effective July 2, 2012, the Compensation Committee approved the following adjustments to Ms. Kendell’s compensation:
| • | Base Salary: Effective July 2, 2012, Ms. Kendell’s annual base salary was increased from $255,000 to $280,000. |
| • | Annual Target Bonus: For fiscal year 2013 performance, Ms. Kendell’s annual target bonus percentage was increased from 35% to 40% of her base salary. |
B-82
| • | Equity Grants: On July 2, 2012, Ms. Kendell was granted (i) a stock option to purchase up to 60,000 shares of common stock under our Equity Incentive Plan at an exercise price of $2.65 per share, which was equal to the closing price of the common stock on the date of grant, which vests with respect to 10,000 of the shares on the last day of each calendar quarter beginning on September 30, 2012 through September 30, 2013, and with respect to the final 10,000 shares on November 1, 2013, and (ii) 20,000 restricted stock units representing contingent rights to receive shares of our common stock, which vests in full on November 1, 2013, provided Ms. Kendell is employed by the Company on such date. |
| • | Retention Bonus: A $100,000 retention bonus payable in a lump sum on the earlier of (i) November 10, 2013, provided Ms. Kendell is employed by the Company on such date, and (ii) the date Ms. Kendell’s employment is terminated by the Company without Cause or Ms. Kendell resigns for Good Reason (as such capitalized terms are defined in Ms. Kendell’s Executive Severance and Change in Control Agreement). |
The equity grants and the retention bonus described above were approved by the Compensation Committee in order to provide an incentive for Ms. Kendell to continue her employment with the Company in its pursuit of strategic alternatives.
Wayne Laslie, former Chief Operating Officer (February 19, 2009 – February 29, 2012)
December 13, 2011 Separation Agreement with Wayne Laslie
On November 16, 2011, Wayne Laslie, our then serving Chief Operating Officer, notified the Company of his intention to resign effective on February 29, 2012. Prior to his resignation, there were no adjustments made during the 2012 fiscal year to Mr. Laslie’s compensation nor was he awarded any equity grants in the 2012 fiscal year. In connection with Mr. Laslie’s resignation, we entered into a Separation Agreement with Mr. Laslie on December 13, 2011. Pursuant to the terms and conditions of the Separation Agreement, Mr. Laslie received the payments and benefits to which he was entitled under his Executive Severance and Change in Control Agreement, dated February 1, 2010, in connection with a termination without Cause (as defined in the agreement). In addition, Mr. Laslie received accelerated vesting of stock options and restricted stock units through February 28, 2013. Further details regarding Mr. Laslie’s Separation Agreement are discussed below under the heading “Payments Upon Termination or Change in Control.”
COMPENSATION COMMITTEE REPORT
The Compensation Committee of our Board of Directors has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K, which appears above in this Form 10-K, with our management. Based on this review and discussion, the Compensation Committee has recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Form 10-K.
| MEMBERS OF THE MYREXIS, INC. COMPENSATION COMMITTEE: | ||
| Dennis H. Langer, M.D., J.D., Chairman | ||
| Gerald P. Belle | ||
| Robert Forrester | ||
B-83
EXECUTIVE OFFICER AND DIRECTOR COMPENSATION
Summary Compensation Table
The following table shows the total compensation paid or accrued during the fiscal years ended June 30, 2012, 2011 and 2010 to (1) our former President and Chief Executive Officer, Richard B. Brewer, who died on August 15, 2012, (2) our former President and Chief Executive Officer and former Chief Financial Officer, Robert J. Lollini, who resigned on May 11, 2012, (3) our former President and Chief Executive Officer, Adrian N. Hobden, Ph.D., who resigned on July 21, 2011, (4) our Chief Financial Officer, Andrea Kendell, and (5) our former Chief Operating Officer, Wayne Laslie, who resigned effective February 29, 2012.
| Name and Principal Position |
Fiscal Year |
Salary ($) |
Bonus ($)(1) |
Stock Awards ($)(2) |
Option Awards ($)(2) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||||||||
| Richard B. Brewer (3) |
2012 | 54,597 | 0 | (4) | 0 | 0 | 54,597 | |||||||||||||||||||||
| Former President and Chief Executive Officer |
||||||||||||||||||||||||||||
| Robert J. Lollini (5) |
2012 | 339,387 | (6) | 25,507 | 0 | 550,650 | 810,579 | (7) | 1,726,123 | |||||||||||||||||||
| Former President and Chief Executive Officer and Former Chief Financial Officer |
|
2011 2010 |
|
|
285,552 285,552 |
|
|
50,382 507 |
|
|
85,565 84,630 |
|
|
169,096 411,119 |
|
|
9,875 9,018 |
|
|
600,470 790,820 |
| |||||||
| Adrian N. Hobden, Ph.D. (8) |
2012 | 44,629 | 0 | 0 | 0 | 489,050 | (9) | 533,679 | ||||||||||||||||||||
| Former President and Chief Executive Officer |
|
2011 2010 |
|
|
535,552 535,552 |
|
|
507 507 |
|
|
160,835 201,500 |
|
|
317,850 433,816 |
|
|
9,875 9,875 |
|
|
1,024,619 1,181,250 |
| |||||||
| Andrea Kendell (10) |
2012 | 244,999 | (11) | 75,540 | 0 | 293,680 | 9,623 | (12) | 623,842 | |||||||||||||||||||
| Chief Financial Officer |
||||||||||||||||||||||||||||
| Wayne Laslie (13) |
2012 | 269,535 | 547 | 0 | 0 | 424,087 | (14) | 694,169 | ||||||||||||||||||||
| Former Chief Operating Officer |
|
2011 2010 |
|
|
380,552 380,552 |
|
|
524 524 |
|
|
96,500 68,510 |
|
|
190,710 146,413 |
|
|
9,875 9,875 |
|
|
678,161 605,874 |
| |||||||
| (1) | The amounts cited for fiscal year 2012 represent for Mr. Lollini a $25,000 cash payment for services as interim President and Chief Executive Officer from July 21, 2011 – September 6, 2011, which was paid during the fiscal year, as well as a holiday cash bonus of $507; for Ms. Kendell a $75,000 cash bonus for performance in fiscal year 2012, which was paid in the following fiscal year, as well as a holiday cash bonus of $540; and for Mr. Laslie a holiday cash bonus. |
| (2) | Represents the aggregate grant date fair value of stock awards and option awards, respectively, granted in each year presented calculated in accordance with FASB ASC Topic 718. Information regarding the assumptions used in the valuation of these awards can be found in the footnote to our financial statements entitled “Share-Based Compensation” in this Form 10-K. See also our discussion of stock-based compensation under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Use of Estimates” in this Form 10-K. |
| (3) | Mr. Brewer served as our President and Chief Executive Officer from May 11, 2012 to August 15, 2012. |
| (4) | As discussed in the Compensation Discussion and Analysis, Mr. Brewer was awarded a grant of restricted stock units on May 11, 2012 that would vest only upon the achievement of certain performance criteria, as described in the Compensation Discussion and Analysis. On the date of grant, the performance criteria were not probable of being achieved; therefore, no stock-based compensation expense has been recorded for this grant. Assuming achievement of all of the performance criteria, the grant date fair value of the award is $2,941,441. Upon Mr. Brewer’s death on August 15, 2012, the restricted stock units were terminated and no shares were issued. |
B-84
| (5) | During fiscal year 2012, Mr. Lollini served as our Chief Financial Officer from July 1, 2011 – September 6, 2011, as interim President and Chief Executive Officer from July 21, 2011 – September 6, 2011, and as President and Chief Executive Officer from September 6, 2011 until his resignation effective May 11, 2012. |
| (6) | Represents salary earned at an annual base rate of $300,000 from July 1, 2011 – September 5, 2011, and at an annual base rate of $395,000 from September 6, 2011 – May 11, 2012. |
| (7) | Represents (i) $6.28 per month for July 2011 through December 2011 and $5.53 per month for January 2012 through May 2012 of premiums paid by Myrexis with respect to term life insurance for the benefit of Mr. Lollini, (ii) $10,022 for matching contributions made under the Myrexis 401(k) plan on behalf of Mr. Lollini, and (iii) the following payments made to Mr. Lollini in connection with his resignation effective May 11, 2012, and pursuant to the terms of his Separation and Consulting Agreement, dated May 11, 2012: (a) $395,000 representing twelve months of Mr. Lollini’s annual base salary on the date of his termination, (b) $197,500 representing 100% of Mr. Lollini’s 2012 fiscal year target bonus amount, (c) $181,042 representing a pro-rated portion of Mr. Lollini’s 2012 fiscal year target bonus amount, (d) $25,919 representing accrued vacation as of the date of his termination, and (e) $1,031, representing COBRA benefits. |
| (8) | Dr. Hobden resigned as our President and Chief Executive Officer effective July 21, 2011. |
| (9) | Represents (i) $6.28 per month during fiscal year 2012 of premiums paid by Myrexis with respect to term life insurance for the benefit of Dr. Hobden, (ii) $3,096 for matching contributions made under the Myrexis 401(k) plan on behalf of Dr. Hobden, and (iii) the following payments made to Dr. Hobden in connection with his resignation on July 21, 2011, and pursuant to the terms of his Separation Agreement, dated July 21, 2011: (a) $267,500 representing six months of Dr. Hobdens’ annual base salary on the date of his termination, (b) $133,750 representing 50% of Dr. Hobdens’ 2012 fiscal year target bonus amount, (c) $79,218 representing accrued vacation as of the date of his termination, and (d) $5,480 representing COBRA benefits. |
| (10) | Ms. Kendell was appointed Chief Financial Officer on September 6, 2011. Compensation data is disclosed for Ms. Kendell only for those years for which she was a named executive officer. |
| (11) | Represents salary earned at an annual base rate of $220,000 from July 1, 2011 – September 21, 2011, and at an annual base rate of $255,000 from September 22, 2011 – June 30, 2012. |
| (12) | Represents (i) $6.28 per month for July 2011 through December 2011 and $5.53 per month for January 2012 through June 2012 of premiums paid by Myrexis with respect to term life insurance for the benefit of Ms. Kendell and (ii) $9,552 for matching contributions made under the Myrexis 401(k) plan on behalf of Ms. Kendell. |
| (13) | Mr. Laslie resigned as our Chief Operating Officer effective February 29, 2012. |
| (14) | Represents (i) $6.28 per month for July 2011 through December 2011 and $5.53 per month for January 2012 through February 2012 of premiums paid by Myrexis with respect to term life insurance for the benefit of Mr. Laslie, (ii) $7,098 for matching contributions made under the Myrexis 401(k) plan on behalf of Mr. Laslie, and (iii) the following payments made to Mr. Laslie in connection with his resignation effective February 29, 2012, and pursuant to the terms of his Separation Agreement, dated December 13, 2011: (a) $190,000 representing six months of Mr. Laslie’s annual base salary on the date of his termination, (b) $101,333 representing a pro-rated portion of Mr. Laslie’s 2012 fiscal year target bonus amount, (c) $76,000 representing 50% of Mr. Laslie’s 2012 fiscal year target bonus amount offset by the amount of base salary paid for December 2011 through February 2012, (d) $43,474 representing accrued vacation as of the date of his termination, and (e) $6,133 representing COBRA benefits. |
B-85
2012 Fiscal Year Grants of Plan-Based Awards
The following table shows information regarding grants of equity awards that we made during the fiscal year ended June 30, 2012 to the executive officers named in the Summary Compensation Table.
| Name |
Grant Date |
Approval Date |
Estimated Future Payouts Under Equity Incentive Plan Awards |
All Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Share) |
Grant Date Fair Value of Stock and Option Awards ($)(1) |
||||||||||||||||||||||||||
| Threshold (#) |
Target (#) |
Maximum (#) |
||||||||||||||||||||||||||||||
| Richard B. Brewer Former President |
5/11/12 | 5/9/12 | 802,211 | (2) | 1,069,615 | (2) | 1,069,615 | (2) | — | — | 0 | (2) | ||||||||||||||||||||
| Robert J. Lollini Former President |
9/22/11 | 9/22/11 | — | — | — | 300,000 | 2.75 | 550,650 | ||||||||||||||||||||||||
| Adrian N. Hobden, Ph.D. Former President |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Andrea Kendell Chief Financial |
9/22/11 | 9/22/11 | — | — | — | 160,000 | 2.75 | 293,680 | ||||||||||||||||||||||||
| Wayne Laslie Former Chief |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| (1) | See our discussion in the footnote to our financial statements entitled “Share-Based Compensation” in this Form 10-K for details as to the assumptions used to determine the grant date fair values of these awards. See also our discussion of stock-based compensation under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Use of Estimates” in this Form 10-K. |
| (2) | As discussed in the Compensation Discussion and Analysis, Mr. Brewer was awarded a grant of restricted stock units on May 11, 2012 that would vest with respect to 75% and 25% of the shares, respectively, only upon the achievement of certain performance criteria, as described in the Compensation Discussion and Analysis. On the date of grant, the performance criteria were not probable of being achieved; therefore, no stock-based compensation expense has been recorded for this grant. Assuming achievement of all of the performance criteria, the grant date fair value of the award is $2,941,441. Upon Mr. Brewer’s death on August 15, 2012, the restricted stock units were terminated and no shares were issued. |
Narrative Disclosure to Summary Compensation Table and 2012 Fiscal Year Grants of Plan-Based Awards Table
Employment Agreements
The terms of our employment agreement, offer letter, and/or separation agreement, as applicable, with each of our named executive officers are described in the Compensation Discussion and Analysis and below under the heading “Payments Upon Termination or Change in Control.”
2012 Equity Awards
As discussed in the Compensation Discussion and Analysis, on May 11, 2012, Richard B. Brewer was awarded a grant of 1,069,615 restricted stock units under our Equity Incentive Plan, representing 4% of our outstanding shares of common stock as of May 11, 2012, in connection with his appointment as our President and Chief Executive Officer, which were subject to the vesting and other conditions set forth in the restricted stock unit agreement that we entered into with Mr. Brewer on May 11, 2012 and described in the Compensation Discussion and Analysis. Upon Mr. Brewer’s death on August 15, 2012, the restricted stock unit agreement with Mr. Brewer terminated and no shares of common stock were issued thereunder.
B-86
As discussed in the Compensation Discussion and Analysis, in connection with their respective appointments as President and Chief Executive Officer and Chief Financial Officer effective September 6, 2011 and as set forth in their respective offer letters, Robert J. Lollini and Andrea Kendell were granted options to purchase 300,000 and 160,000 shares of common stock, respectively, on September 22, 2011, under our Equity Incentive Plan. These options were granted with an exercise price equal to the fair market value of our common stock on the date of grant as reported by The NASDAQ Global Market, and a four-year vesting schedule with 25% of the shares vesting on each anniversary of the date of grant. In accordance with the terms of our Separation Agreement with Mr. Lollini, 75,000 shares underlying his option will vest on November 15, 2012, the date of his resignation from the Board of Directors, and the option will cease vesting on such date.
Fiscal Year 2012 Performance Bonus Awards
In the first quarter of fiscal year 2012, the Compensation Committee established target bonus award opportunities for each then serving executive officer and certain employees, based on a percentage of base salary. These percentages were set at the market 50th percentile. In connection with Mr. Lollini’s appointment as President and Chief Executive Officer on September 6, 2011, his target bonus percentage was increased from 40% to 50%. In connection with Ms. Kendell’s appointment as Chief Financial Officer on September 6, 2011, her target bonus percentage was increased from 30% to 35%. Dr. Hobden’s and Mr. Laslie’s target percentages remained at 50% and 40%, respectively, for fiscal year 2012. Mr. Brewer was not entitled to any bonus compensation under his compensation arrangements with us. Although target bonus award opportunities were established for fiscal year 2012 performance, as a result of the changing strategy and direction of the Company during the year, formal objectives were ultimately never established and approved by the Compensation Committee for purposes of evaluating performance and awarding bonuses following the end of the fiscal year, as had been done in prior years.
Our only executive officer eligible to receive a bonus for fiscal year 2012 performance and employed with us on the last day of the 2012 fiscal year was Andrea Kendell, our Chief Financial Officer. Although no formal objectives were established during fiscal year 2012 for purposes of evaluating Ms. Kendell’s performance, the Compensation Committee exercised its discretion and awarded Ms. Kendell a $75,000 bonus, representing 84% of her target bonus. This bonus was awarded by the Compensation Committee in recognition of Ms. Kendell’s management and oversight of several reductions in force and corporate reorganizations, implementation of strong documentation processes in connection therewith, supporting the Board through multiple management changes, ensuring internal control over financial reporting activities and supporting the Company and the Board in its pursuit of other strategic alternatives.
Outstanding Equity Awards at 2012 Fiscal Year-End
Our separation and spin-off from Myriad Genetics on June 30, 2009 was effectuated by way of a pro rata dividend to Myriad Genetics stockholders of one share of our common stock for every four shares of Myriad Genetics common stock. In connection with the spin-off and pursuant to the terms of Myriad Genetics’ stock option plans, each outstanding Myriad Genetics stock option on the date of the separation was adjusted. Each adjusted Myriad Genetics stock option remains exercisable for the same number of shares of Myriad Genetics common stock as the original Myriad Genetics option, and for each Myriad Genetics option outstanding, a new Myrexis stock option, exercisable for one-fourth of the number of shares of our common stock as the original Myriad Genetics option was issued. The exercise price of each adjusted Myriad Genetics option and each new Myrexis stock option was determined in accordance with Section 409A and Section 422 of the Internal Revenue Code and preserved the intrinsic value of the pre-separation Myriad Genetics option.
B-87
The following table shows grants of stock options and grants of unvested stock awards outstanding on June 30, 2012, the last day of our fiscal year, held by each of the executive officers named in the Summary Compensation Table. All options with grant dates noted in the “Date of Grant” column prior to July 1, 2009 represent the Myrexis stock options that were granted on June 30, 2009 in connection with the separation as described above and the “Date of Grant” cited in the below table represents the date that the original Myriad Genetics options were granted as that is the date based on which the associated Myrexis stock options vest and terminate. All of these options were issued under our 2009 Equity Plan with the same terms as the original Myriad Genetics option, except that the vesting, if any, and expiration of both the Myriad Genetics and the new Myrexis options are based on the optionholder’s continuing employment with us following the separation.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||
| Name |
Date of Grant |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($)(1) |
|||||||||||||||||||||
| Richard B. Brewer Former President and |
05/11/2012 | — | — | — | — | 1,069,615 | (2) | 2,791,695 | ||||||||||||||||||||
| Robert J. Lollini (3) |
07/01/2009 | 50,000 | 50,000 | (4) | 4.03 | 11/15/2013 | — | — | ||||||||||||||||||||
| Former President and Chief Executive Officer and Former Chief Financial Officer |
09/10/2009 | 16,500 | 16,500 | (4) | 4.63 | 11/15/2013 | — | — | ||||||||||||||||||||
| 02/18/2010 | 16,500 | 16,500 | (4) | 4.83 | 11/15/2013 | — | — | |||||||||||||||||||||
| 09/24/2010 | — | — | — | — | 16,625 | (5) | 43,391 | |||||||||||||||||||||
| 09/24/2010 | 16,625 | 49,875 | (4) | 3.86 | 11/15/2013 | — | — | |||||||||||||||||||||
| 9/22/2011 | 0 | 150,000 | (6) | 2.75 | 11/15/2013 | — | — | |||||||||||||||||||||
| Adrian N. Hobden, Ph.D. |
02/19/2004 | 2,946 | 0 | 0.93 | 02/19/2014 | — | — | |||||||||||||||||||||
| Former President and Chief Executive Officer (7) |
02/17/2005 | 2,260 | 0 | 1.22 | 02/17/2015 | — | — | |||||||||||||||||||||
| 02/16/2006 | 13,951 | 0 | 1.34 | 02/16/2016 | — | — | ||||||||||||||||||||||
| 09/06/2006 | 16,000 | 0 | 1.40 | 09/06/2016 | — | — | ||||||||||||||||||||||
| 02/21/2007 | 15,548 | 0 | 1.89 | 02/21/2017 | — | — | ||||||||||||||||||||||
| 09/26/2007 | 22,500 | 0 | 2.80 | 09/26/2017 | — | — | ||||||||||||||||||||||
| 02/28/2008 | 26,169 | 0 | 2.06 | 02/28/2018 | — | — | ||||||||||||||||||||||
| 09/10/2008 | 16,875 | 0 | 3.56 | 09/10/2018 | — | — | ||||||||||||||||||||||
| Andrea Kendell |
07/01/2009 | 2,500 | 2,500 | (8) | 4.03 | 07/01/2019 | — | — | ||||||||||||||||||||
| Chief Financial Officer |
09/10/2009 | 5,310 | 5,310 | (8) | 4.63 | 09/10/2019 | — | — | ||||||||||||||||||||
| 02/18/2010 | 5,538 | 5,536 | (8) | 4.83 | 02/18/2020 | — | — | |||||||||||||||||||||
| 09/24/2010 | — | — | — | — | 6,465 | (9) | 16,874 | |||||||||||||||||||||
| 09/24/2010 | 4,315 | 12,945 | (8) | 3.86 | 09/24/2020 | — | — | |||||||||||||||||||||
| 09/22/2011 | 0 | 160,000 | (8) | 2.75 | 09/22/2021 | — | — | |||||||||||||||||||||
| Wayne Laslie |
11/11/2004 | 4,244 | 0 | 1.07 | 11/11/2014 | — | — | |||||||||||||||||||||
| Former Chief Operating Officer (10) |
09/14/2005 | 2,774 | 0 | 1.13 | 09/14/2015 | — | — | |||||||||||||||||||||
| 02/16/2006 | 7,951 | 0 | 1.34 | 02/16/2016 | — | — | ||||||||||||||||||||||
| 09/06/2006 | 10,000 | 0 | 1.40 | 09/06/2016 | — | — | ||||||||||||||||||||||
| 02/21/2007 | 9,548 | 0 | 1.89 | 02/21/2017 | — | — | ||||||||||||||||||||||
| 09/26/2007 | 6,500 | 0 | 2.80 | 09/26/2017 | — | — | ||||||||||||||||||||||
| 02/28/2008 | 16,169 | 0 | 2.06 | 02/28/2018 | — | — | ||||||||||||||||||||||
| 09/10/2008 | 14,000 | 0 | 3.56 | 09/10/2018 | — | — | ||||||||||||||||||||||
| (1) | The market value of the unvested restricted stock units was determined by multiplying the number of unvested units by $2.61, the closing price of our common stock on June 29, 2012, the last business day of our 2012 fiscal year. |
| (2) | Represents a grant of restricted stock units to Mr. Brewer that would vest only upon the achievement of certain performance criteria, as described in the Compensation Discussion and Analysis. Upon Mr. Brewer’s death on August 15, 2012, the restricted stock units were terminated and no shares were issued. |
| (3) | Mr. Lollini resigned as President and Chief Executive Officer effective May 11, 2012 and will resign from the Board of Directors effective November 15, 2012. Pursuant to the Separation and Consulting Agreement entered into with Mr. Lollini on May 11, 2012, in consideration for Mr. Lollini’s agreement to provide consulting services to Myrexis through November 15, 2012, Mr. Lollini received (i) continued vesting of his outstanding stock options and restricted stock units until November 15, 2012, (ii) the vesting on November 15, 2012 of options to purchase 75,000 shares of Myrexis common stock that were granted on September 22, 2011 that would not otherwise have vested by their terms, and (iii) the extension of the expiration date of all of his vested stock options until November 15, 2013. |
| (4) | The options vested or vest as to 25% of the shares on each anniversary of the date of grant but, as described in footnote (3), will cease vesting on November 15, 2012. |
B-88
| (5) | Represents restricted stock units that vested or vest as to one-quarter of the units on each anniversary of the date of grant but, as described in footnote (3), will cease vesting and terminate to the extent unvested on November 15, 2012. |
| (6) | The options vested or vest as to 25% of the shares on each anniversary of the date of grant but, as described in footnote (3), will vest as to 75,000 shares on November 15, 2012 and cease vesting thereafter. |
| (7) | Dr. Hobden resigned as President and Chief Executive Officer and as a member of the Board of Directors effective July 21, 2011. In connection with Dr. Hobden’s resignation, we entered into a Separation Agreement with Dr. Hobden pursuant to which, among other things, the vesting of Dr. Hobden’s stock options and restricted stock units that would have vested through July 21, 2012 was accelerated. All unvested options and unvested restricted stock units held by Dr. Hobden and not subject to accelerated vesting were terminated on July 21, 2011. All of Dr. Hobden’s vested stock options are exercisable following his resignation in accordance with their original terms. |
| (8) | The options vested or vest as to 25% of the shares on each anniversary of the date of grant. |
| (9) | Represents restricted stock units that vested or vest as to one-quarter of the units on each anniversary of the date of grant. |
| (10) | Mr. Laslie resigned as Chief Operating Officer effective February 29, 2012. In connection with Mr. Laslie’s resignation, we entered into a Separation Agreement with Mr. Laslie pursuant to which, among other things, Mr. Laslie received twelve months accelerated vesting of equity awards from February 29, 2012. All unvested options and unvested restricted stock units held by Mr. Laslie and not subject to accelerated vesting terminated on February 29, 2012. All of Mr. Laslie’s vested stock options are exercisable following his resignation in accordance with their original terms. |
2012 Fiscal Year Option Exercises and Stock Vested
The following table shows information regarding the exercise of stock options and the vesting of stock awards held by each executive officer named in the Summary Compensation Table during the fiscal year ended June 30, 2012.
| Option Awards | Stock Awards | |||||||||||||||
| Name |
Number of Shares Acquired on Exercise (#) |
Value Realized on Exercise ($)(1) |
Number of Shares Acquired on Vesting (#) |
Value Realized on Vesting ($)(2) |
||||||||||||
| Richard B. Brewer |
— | — | — | — | ||||||||||||
| Robert J. Lollini |
— | — | 12,542 | 31,621 | ||||||||||||
| Adrian N. Hobden, Ph.D. |
120,914 | 243,576 | 27,083 | 94,249 | ||||||||||||
| Andrea Kendell |
— | — | 3,655 | 9,436 | ||||||||||||
| Wayne Laslie |
14,955 | 5,420 | 18,166 | 55,062 | ||||||||||||
| (1) | Amounts shown in this column do not necessarily represent actual value realized from the sale of the shares acquired upon exercise of options because in many cases the shares are not sold on exercise but continue to be held by the executive officer exercising the option. The amounts shown represent the difference between the option exercise price and the market price on the date of exercise, which is the amount that would have been realized if the shares had been sold immediately upon exercise. All of the options exercised by the executive officers in this table were exercised following such executive officer’s date of termination with the Company. |
| (2) | Represents the vesting of restricted stock units. The value realized is calculated by multiplying the number of units that vested by the closing price of our common stock on the applicable date of vesting. |
B-89
Pension Benefits
We do not have any qualified or non-qualified defined benefit plans.
Nonqualified Deferred Compensation
We do not have any nonqualified defined contribution plans or other deferred compensation plan.
Payments Upon Termination or Change in Control
Richard B. Brewer, former President and Chief Executive Officer
As of June 30, 2012, there were no severance or change in control arrangements in place with Richard B. Brewer, our former President and Chief Executive Officer, other than acceleration of his RSUs had milestones been achieved under the RSU Agreement.
May 11, 2012 Separation and Consulting Agreement with Robert J. Lollini, former President and Chief Executive Officer
Robert J. Lollini resigned as President and Chief Executive Officer effective May 11, 2012. In connection with Mr. Lollini’s resignation, we entered into a Separation and Consulting Agreement with Mr. Lollini, dated May 11, 2012 (the “Separation Agreement”). Pursuant to the terms of the Separation Agreement, Mr. Lollini received the following amounts and benefits to which he was entitled pursuant to the terms of his Executive Severance and Change in Control Agreement, dated February 1, 2010, as amended September 9, 2011, in connection with a termination without Cause (as defined in the agreement):
| (i) | $395,000 representing payment in a lump sum amount equal to one times his then current annual base salary; |
| (ii) | $197,500 representing payment in a lump sum amount equal to one times his fiscal year 2012 target bonus amount; |
| (iii) | $181,042 representing 11 months accrual of his fiscal year 2012 target bonus amount; |
| (iv) | $25,919 representing accrued vacation as of the date of termination; and |
| (v) | 1,031 representing COBRA benefits. |
In addition, Mr. Lollini agreed to resign as a director of Myrexis as of November 15, 2012, and to provide consulting services to Myrexis through November 15, 2012. In consideration of Mr. Lollini’s agreement to provide consulting services to Myrexis, Mr. Lollini received:
| • | continued vesting of his outstanding stock options and restricted stock units until November 15, 2012; |
| • | the vesting on November 15, 2012 of options to purchase 75,000 shares of Myrexis common stock that would not otherwise have vested by their terms, which options have an exercise price of $2.75 per share, which exceeds the closing price of our common stock on June 29, 2012, the last business day of our fiscal year; and |
| • | the extension of the expiration date of all his vested stock options until November 15, 2013. |
In addition, the Separation Agreement contains Mr. Lollini’s general release of any claims against us, and Mr. Lollini’s agreement that the non-disclosure, intellectual property assignment, non-competition (as modified by the Separation Agreement) and non-solicitation provisions set forth in his employment agreement with us, dated July 1, 2009, will continue to apply in accordance with their terms.
B-90
July 21, 2011 Separation Agreement with Adrian N. Hobden, Ph.D., former President and Chief Executive Officer
Effective July 21, 2011, Adrian N. Hobden, Ph.D., resigned as our President and Chief Executive Officer and as a member of our Board of Directors. In connection with Dr. Hobden’s resignation, we negotiated a Separation Agreement with Dr. Hobden. Pursuant to the terms and conditions of the Separation Agreement, Dr. Hobden received:
| (i) | $267,500 representing payment in a lump sum amount equal to six months of his then current annual base salary; |
| (ii) | $133,750 representing payment in a lump sum amount equal to 50% of his fiscal year 2012 target bonus amount; |
| (iii) | $79,218 representing accrued vacation as of the date of termination; |
| (iv) | $5,480 representing COBRA benefits; |
| (v) | accelerated vesting of 89,375 stock options that would have vested through July 21, 2012, valued at $13,558 determined by multiplying the number of vesting in-the-money options by the spread between the closing price of our common stock on July 21, 2011, which was $3.4776 per share, and the exercise price of such accelerated in-the-money options; and |
| (vi) | accelerated vesting of 10,417 restricted stock units that would have vested through July 21, 2012, valued at $36,226 determined by multiplying the number of vesting restricted stock units by $3.4776, the closing price of our common stock on July 21, 2011. |
In exchange for the foregoing, Dr. Hobden agreed to assist us with an orderly transition for a three month period following July 21, 2011, during which he agreed to be available to provide consulting services to us for up to 10 hours per week. In addition, the Separation Agreement contains Dr. Hobden’s release of claims against us, and Dr. Hobden’s agreement that the non-disclosure, intellectual property assignment, non-competition and non-solicitation provisions set forth in his employment agreement with us, dated July 1, 2009, will continue to apply in accordance with their terms.
September 22, 2011 Executive Severance and Change in Control Agreement with Andrea Kendell, Chief Financial Officer
As of June 30, 2012, our only severance and change in control arrangement in effect with a named executive officer is our Executive Severance and Change in Control Agreement (the “Severance and Change in Control Agreement”) with Andrea Kendell, our Chief Financial Officer, dated September 22, 2011.
Termination Payments and Benefits
Under the terms of the Severance and Change in Control Agreement with Ms. Kendell, if (1) a Change in Control (as defined in the agreement) occurs and within 12 months of the Change in Control the employment of Ms. Kendell is terminated by the Company (other than for Cause, Disability or death) or by Ms. Kendell for Good Reason (as such capitalized terms are defined in the agreement), or (2) Ms. Kendell’s employment is terminated by the Company (other than for Cause, Disability or death) or by Ms. Kendell for Good Reason not in connection with a Change in Control, then Ms. Kendell shall be entitled to the following:
| • | payment in a lump sum amount of her base salary through the date of termination, and any accrued vacation pay to the extent not previously paid; |
| • | payment in a lump sum amount equal to one times her then current annual base salary; |
| • | payment in a lump sum amount equal to one times her then current fiscal year target bonus amount; and |
| • | continuation of health benefits for up to 12 months. |
B-91
Receipt of severance payments under the agreement is conditioned on Ms. Kendell executing and delivering a written general release of claims against the Company and its affiliates within 30 days of termination, and which includes Ms. Kendell’s reaffirmation of her continuing obligations under the assignment of inventions, non-disclosure, non-competition and non-solicitation provisions contained in her employment agreement, dated June 29, 2009, with the Company.
Term
The Severance and Change in Control Agreement with Ms. Kendell has a term (the “Term”) that continues in effect until December 31, 2015 and thereafter for one year terms unless the Company provides notice of non-renewal at least 90 days prior to the end of the expiration of the term then in effect. The rights and obligations under the Severance and Change in Control Agreement will expire on the earlier of (i) the expiration of the Term, (ii) the date that is 12 months after a Change in Control, if Ms. Kendell is still employed by the Company as of such later date, or (iii) the fulfillment by the Company of all of its obligations under the Severance and Change in Control Agreement.
Defined Terms
As defined in the Severance and Change in Control Agreement:
“Cause” means the executive’s willful and continued failure to substantially perform her reasonable assigned duties (other than any such failure resulting from incapacity due to physical or mental illness or any failure after the executive gives notice of termination for Good Reason), which failure is not cured within 30 days after a written demand for substantial performance is received by the executive from the Board of Directors of the Company which specifically identifies the manner in which the Board of Directors believes the executive has not substantially performed the executive’s duties; or the executive’s willful engagement in illegal conduct or gross misconduct which is materially and demonstrably injurious to the Company.
A “Change in Control” means the occurrence of any of the following events: (1) Ownership. Any “Person” (as such term is used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended) becomes the “Beneficial Owner” (as defined in Rule 13d-3 under said Act), directly or indirectly, of securities of the Company representing 50% or more of the total voting power represented by the Company’s then outstanding voting securities (excluding for this purpose any such voting securities held by the Company or its affiliates or by any employee benefit plan of the Company) pursuant to a transaction or a series of related transactions which the Board of Directors does not approve; or (2) Merger/Sale of Assets. (A) A merger or consolidation of the Company whether or not approved by the Board of Directors, other than a merger or consolidation which would result in the voting securities of the Company outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or the parent of such corporation) more than 50% of the total voting power represented by the voting securities of the Company or such surviving entity or parent of such corporation, as the case may be, outstanding immediately after such merger or consolidation; or (B) the sale or disposition by the Company of all or substantially all of the Company’s assets in a transaction requiring stockholder approval.
“Disability” means the executive’s absence from the full-time performance of the executive’s duties with the Company for 180 consecutive calendar days as a result of incapacity due to mental or physical illness which is determined to be total and permanent by a physician selected by the Company or its insurers and acceptable to the executive or the executive’s legal representative.
“Good Reason” means the occurrence, without the executive’s written consent, of any of the following events or circumstances: (a) a material and continuing diminution of the executive’s position, duties, authority or responsibilities in the operation and management of the Company as compared to such position, duties, authority or responsibilities on the effective date of the Severance and Change in Control Agreement; (b) a material
B-92
reduction in the executive’s then current annual base salary; (c) a change by the Company in the location at which the executive performs her principal duties for the Company to a new location that is more than 50 miles from the location at which the executive performs her principal duties for the Company on the effective date of the Severance and Change in Control Agreement; (d) the failure of the Company to obtain the agreement from any successor to the Company to assume and agree to perform the Severance and Change in Control Agreement; or (e) any failure of the Company to pay or provide to the executive any portion of the executive’s compensation or any Company-paid health, disability, accident and/or life insurance plans or programs due within seven days of the date such compensation or benefits are due, or any material breach by the Company of the Severance and Change in Control Agreement or any employment agreement with the executive.
The defined terms set forth above are the same defined terms that were contained in our now terminated Executive Severance and Change in Control Agreements with each of Dr. Hobden, Mr. Lollini, and Mr. Laslie.
Potential Payments to Ms. Kendell Upon a June 30, 2012 Termination
If Ms. Kendell had been terminated as of June 29, 2012, the last business day of our most recently completed fiscal year, under one of the circumstances described in her Severance and Change in Control Agreement, she would have received the following:
| (i) | $255,000 representing payment in a lump sum amount equal to one times her then current annual base salary; |
| (ii) | $89,250 representing payment in a lump sum amount equal to one times her then current fiscal year target bonus amount; |
| (iii) | $14,779 representing continuation of health benefits for up to 12 months; and |
| (iv) | $46,260 representing payment in a lump sum amount of Ms. Kendell’s base salary through the date of termination, and accrued vacation pay. |
December 13, 2011 Separation Agreement with Wayne Laslie, former Chief Operating Officer
On November 16, 2011, Wayne Laslie, our then serving Chief Operating Officer, notified the Company of his intention to resign effective on February 29, 2012. In connection with Mr. Laslie’s resignation, we entered into a Separation Agreement with Mr. Laslie on December 13, 2011. Pursuant to the terms and conditions of the Separation Agreement, Mr. Laslie received the following:
| (i) | $190,000 representing payment in a lump sum amount equal to six months of his then current annual base salary; |
| (ii) | $101,333 representing payment in a lump sum amount equal to 8 months accrual of his fiscal year 2012 target bonus amount; |
| (iii) | $76,000 representing payment in a lump sum amount equal to 50% of his fiscal year target bonus amount offset by the amount of base salary paid for December 2011 through February 2012; |
| (iv) | $43,474 representing accrued vacation as of the date of termination; |
| (v) | $6,133 representing COBRA benefits; |
| (vi) | accelerated vesting of 35,750 stock options that would have vested through February 28, 2013, none of which had an exercise price lower than $3.20 per share, the closing price of our common stock on February 29, 2012; and |
| (vii) | accelerated vesting of 11,916 restricted stock units that would have vested through February 28, 2013, valued at $38,131 determined by multiplying the number of vesting restricted stock units by $3.20, the closing price of our common stock on February 29, 2012. |
B-93
Mr. Laslie’s right to receive the foregoing was subject to his execution of a release of claims against the Company, and his agreement that the non-disclosure, intellectual property assignment, non-competition and non-solicitation provisions set forth in his employment agreement with Myrexis, dated July 1, 2009, will continue to apply in accordance with their terms.
Director Compensation
The following table shows the total compensation paid or accrued during the fiscal year ended June 30, 2012 to each of our non-employee directors. In accordance with the terms of his Separation and Consulting Agreement with us, Mr. Lollini does not receive any compensation for his service as a director.
| Name |
Fees Earned or Paid in Cash ($) |
Option Awards ($)(1) |
Total ($) | |||||||||
| Gerald P. Belle |
157,000 | (2) | 29,949 | 186,949 | ||||||||
| Jason M. Aryeh |
39,250 | 44,898 | 84,148 | |||||||||
| Robert Forrester |
100,000 | 29,949 | 129,949 | |||||||||
| John T. Henderson, M.D. |
71,000 | 29,949 | 100,949 | |||||||||
| Dennis H. Langer, M.D., J.D. |
103,000 | 29,949 | 132,949 | |||||||||
| Timothy R. Franson, M.D. |
59,000 | 29,949 | 88,949 | |||||||||
| (1) | Represents the aggregate grant date fair value of option awards granted in fiscal year 2012 calculated in accordance with FASB ASC Topic 718. Information regarding the assumptions used in the valuation of these awards can be found in the footnote to our financial statements entitled “Share-Based Compensation” in this Form 10-K. See also our discussion of stock-based compensation under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Use of Estimates” in this Form 10-K. In accordance with our Director Compensation Policy described below, on December 8, 2011, the date of our 2011 annual meeting of stockholders, each of our non-employee directors, with the exception of Mr. Aryeh, was granted a non-qualified option to purchase 16,250 shares of common stock, the grant date fair value of which was $29,949. Mr. Aryeh was appointed to our Board of Directors on October 19, 2011. Pursuant to the terms of our Director Compensation Policy, upon his appointment to the Board, Mr. Aryeh was granted a non-qualified option to purchase 25,000 shares of common stock at an exercise price of $2.74 per share, the closing price of our common stock on the date of grant, with a grant date fair value of $44,898. This option vested in full on October 19, 2012. The following table shows the total number of outstanding and vested stock options held by our non-employee directors (excluding Mr. Lollini) as of June 30, 2012: |
| Name |
Options Outstanding (#) |
Vested Options (#) |
||||||
| Gerald P. Belle |
72,500 | 56,250 | ||||||
| Jason M. Aryeh |
25,000 | — | ||||||
| Robert Forrester |
57,500 | 41,250 | ||||||
| John T. Henderson, M.D. |
97,500 | 81,250 | ||||||
| Dennis H. Langer, M.D., J.D. |
75,000 | 58,750 | ||||||
| Timothy R. Franson, M.D. |
57,500 | 41,250 | ||||||
| (2) | In addition to the cash fees paid to Mr. Belle pursuant to our Director Compensation Policy described below, this amount also includes $25,000 paid to Mr. Belle in September 2011 for his services and assistance in connection with Dr. Hobden’s departure as President and Chief Executive Officer and the subsequent management transition. |
B-94
Director Compensation Policy
Our non-employee directors are compensated as follows.
Annual Retainer
Our non-employee directors are compensated on a role-based model and are paid cash fees based on the following annual retainers (25% paid following each quarter of service):
| All members |
$35,000 base retainer | |||
| Chairman of the Board |
$50,000 additional retainer | |||
| Chairman of the Audit Committee |
$18,000 additional retainer | |||
| Chairman of the Compensation Committee |
$14,000 additional retainer | |||
| Chairman of the Nominating and Governance Committee |
$10,000 additional retainer | |||
| Members of the Audit Committee |
$9,000 additional retainer | |||
| Members of the Compensation Committee |
$7,000 additional retainer | |||
| Members of the Nominating and Governance Committee |
$5,000 additional retainer |
Attendance
In addition to the annual retainer amounts, we pay each non-employee director a per meeting cash fee of $2,000 for in-person attendance and $1,000 for telephonic attendance at any Board meetings in excess of five meetings per fiscal year. We also pay each non-employee director a per meeting cash fee of $2,000 for in-person attendance and $1,000 for telephonic attendance at committee meetings in excess of five Audit Committee meetings, four Compensation Committee meetings, and three Nominating and Governance Committee meetings, per fiscal year. All directors are also reimbursed for their out-of pocket expenses incurred in attending meetings.
In addition to the compensation described above, members of the Board’s Strategy Review Committee, comprised of Gerald P. Belle, Dennis Langer, Robert Forrester, and Jason M. Aryeh, are paid a per meeting cash fee of $1,000 for in-person or telephonic attendance or participation in committee meetings.
Stock Option Awards
Our non-employee directors are entitled to receive options to purchase our common stock under Equity Incentive Plan. Each year on the date of our annual meeting of stockholders, each non-employee director, other than new non-employee directors appointed within six months of the annual meeting, will automatically be granted a non-qualified option to purchase 16,250 shares of common stock at an exercise price equal to the closing price of our common stock on the date of grant. In addition, upon initial election to the Board each new non-employee director is granted a non-qualified option to purchase 25,000 shares of common stock at an exercise price equal to the closing price of our common stock on the date of grant. Options granted to our non-employee directors will vest in full on the first anniversary of the date of grant, assuming continued membership on the Board. Options granted to our non-employee directors will be exercisable after the termination of the director’s service on the Board to the extent exercisable on the date of such termination for the remainder of the life of the option. All options granted to our non-employee directors will become fully exercisable upon a change in control or upon the death of the director.
Risks Related to Compensation Practices and Policies
The Compensation Committee maintains a pay-for-performance compensation philosophy, but also recognizes that providing certain types of compensation incentives may inadvertently motivate individuals to act in ways that could be detrimental to the organization as a whole in order to maximize personal compensation. To minimize such risk, the Compensation Committee reviews at least annually the overall structure and individual
B-95
components of our compensation program. The Compensation Committee also performs an annual evaluation to ensure that salary levels, equity awards and other elements of compensation are benchmarked against appropriate standards and that incentives provided for achievement of target goals are balanced between short-term rewards and longer-term enhancement of shareholder value. Based on its review, the Compensation Committee has concluded that any risks created by our compensation policies and procedures are not reasonably likely to have a material adverse effect on our Company or business.
Compensation Committee Interlocks and Insider Participation
Our Compensation Committee has three members, Dennis Langer (Chairman), Gerald Belle and Robert Forrester. No member of our Compensation Committee has at any time been an employee of ours. None of our executive officers is a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of September 30, 2012 (except as otherwise indicated) for (a) each stockholder known by us to own beneficially more than 5% of our common stock, (b) the executive officers named in the Summary Compensation Table of this Form 10-K, (c) each of our directors, and (d) all of our current directors and executive officers as a group. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. We deem shares of common stock that may be acquired by an individual or group within 60 days of September 30, 2012 pursuant to the exercise of options or the vesting of restricted stock units to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Percentage of ownership is based on 26,811,906 shares of common stock outstanding on September 30, 2012. Attached to each share of common stock is a Preferred Share Purchase Right to acquire one one-thousandth of a share of our Series A Junior Participating Preferred Stock, par value $.01 per share, which Preferred Share Purchase Rights are not presently exercisable.
B-96
Except as indicated in the footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them. Unless otherwise indicated, the address for each director and executive officer listed is c/o Myrexis, Inc., 305 Chipeta Way, Salt Lake City, UT 84108.
| Shares of Common Stock Beneficially Owned |
||||||||
| Beneficial Owner |
Number | Percentage | ||||||
| Principal Stockholders: |
||||||||
| Biotechnology Value Fund, L.P. (1) 900 North Michigan Avenue, Suite 1100 Chicago, IL 60611 |
1,344,900 | 5.02 | % | |||||
| Bulldog Investors (2) Park 80 West, 250 Pehle Avenue, Suite 708 Saddle Brook, NJ 07663 |
1,936,564 | 7.22 | % | |||||
| First Eagle Investment Management, LLC (3) 1345 Avenue of the Americas New York, New York 10105 |
2,741,604 | 10.23 | % | |||||
| ICS Opportunities, Ltd. (4) c/o Millennium International Management LP 666 Fifth Avenue New York, New York 10103 |
1,413,300 | 5.27 | % | |||||
| Executive Officers and Directors: |
||||||||
| Andrea Kendell (5) |
75,360 | * | ||||||
| Robert J. Lollini (6) |
268,683 | 1.00 | % | |||||
| Adrian N. Hobden, Ph.D. (7) |
208,014 | * | ||||||
| Wayne Laslie (8) |
120,722 | * | ||||||
| Gerald P. Belle (9) |
133,480 | * | ||||||
| Jason M. Aryeh (10) |
580,145 | 2.16 | % | |||||
| Robert Forrester (11) |
41,250 | * | ||||||
| Timothy R. Franson, M.D. (11) |
41,250 | * | ||||||
| David W. Gryska |
0 | 0 | ||||||
| John T. Henderson, M.D. (12) |
82,325 | * | ||||||
| Dennis H. Langer, M.D., J.D. (11) |
58,750 | * | ||||||
| All current executive officers and directors as a group (9 persons) (13) |
1,281,243 | 4.68 | % | |||||
| * | Represents beneficial ownership of less than 1% of the shares of common stock. |
| (1) | This information is based on a Schedule 13G filed with the SEC on October 28, 2011. As of the close of business on October 27, 2011, (i) Biotechnology Value Fund, L.P. (“BVF”) beneficially owned 280,500 shares of common stock, (ii) Biotechnology Value Fund II, L.P. (“BVF2”) beneficially owned 171,900 shares of common stock, (iii) BVF Investments, L.L.C. (“BVLLC”) beneficially owned 800,200 shares of common stock, and (iv) Investment 10, L.L.C. (“ILL10”) beneficially owned 92,300 shares of common stock. BVF Partners L.P. (“Partners”), as the general partner of BVF and BVF2, the manager of BVLLC and the investment adviser of ILL10, may be deemed to beneficially own the 1,344,900 shares of common stock beneficially owned in the aggregate by BVF, BVF2, BVLLC and ILL10. BVF Inc., as the general partner of Partners, may be deemed to beneficially own the 1,344,900 shares of common stock beneficially owned by Partners. Mark N. Lampert (“Mr. Lampert”), as a director and officer of BVF Inc., may be deemed to beneficially own the 1,344,900 shares of common stock beneficially owned by BVF Inc. Partners, BVF Inc. and Mr. Lampert share voting and dispositive power over the shares of common stock beneficially owned by BVF, BVF2, BVLLC and ILL10. |
B-97
| (2) | This information is based on a Schedule 13G filed with the SEC on February 27, 2012 by Bulldog Investors, Brooklyn Capital Management, Phillip Goldstein and Andrew Dakos, reporting sole power to dispose of all 1,936,564 shares of common stock beneficially owned and shared power to vote certain of the shares of common stock beneficially owned. Phillip Goldstein and Andrew Dakos are principals of Bulldog Investors. On August 7, 2012, we entered into a letter agreement dated August 6, 2012 (the “Bulldog Shareholders Letter Agreement”) with Bulldog Investors and Brooklyn Capital Management LLC (collectively, the “Bulldog Shareholders”). The Bulldog Shareholders Letter Agreement granted to the Bulldog Shareholders an exemption under Section 29 of our Tax Benefits Shareholder Rights Agreement, embodying a shareholder rights plan adopted on March 29, 2012 to protect the use of our net operating losses and certain other tax attributes (the “Plan”). Under the exemption, through the completion of our 2013 annual meeting of shareholders (the “2013 Annual Meeting”), the Bulldog Shareholders’ shareholdings must not at any time represent more than 9.9% ownership in Myrexis. Also through the completion of the 2013 annual meeting, the Bulldog Shareholders must cause the shares of common stock they own to be voted on any matter presented to our stockholders for their vote as our Board of Directors recommends. The voting requirement is subject to the condition that Jason Aryeh is included in the Board of Directors majority approving the recommendation or, if he abstains or otherwise does not vote as a member of the Board of Directors on the matter, that he otherwise concurs with the Board’s recommendation. |
| (3) | This information is based on a Schedule 13D/A filed with the SEC on December 2, 2010. First Eagle Management LLC (“FEM”) is deemed to be the beneficial owner of 2,741,604 shares (which includes 1,729,434 shares for which First Eagle Value in Biotechnology Master Fund Ltd may be deemed to be the beneficial owner). All such shares are held by various clients in accounts that are under management by FEM. |
| (4) | This information is based on a Schedule 13D/A filed with the SEC on February 1, 2012 filed by ICS Opportunities, Ltd. (“ICS Opportunities”). ICS Opportunities is the beneficial owner of 1,413,300 shares of our common stock. Millennium International Management LP, a Delaware limited partnership (“Millennium International Management”), is the investment manager to ICS Opportunities and may be deemed to have shared voting control and investment discretion over securities owned by ICS Opportunities. Millennium International Management GP LLC, a Delaware limited liability company (“Millennium International Management GP”), is the general partner of Millennium International Management and may also be deemed to have shared voting control and investment discretion over securities owned by ICS Opportunities. Millennium Management LLC, a Delaware limited liability company (“Millennium Management”), is the general partner of the 100% shareholder of ICS Opportunities and may be deemed to have shared voting control and investment discretion over securities owned by ICS Opportunities. Israel A. Englander, a United States citizen (“Mr. Englander”), is the managing member of Millennium International Management GP and Millennium Management and consequently may also be deemed to have shared voting control and investment discretion over securities owned by ICS Opportunities. |
| (5) | Includes 65,883 shares of common stock issuable upon the exercise of options currently exercisable or exercisable within 60 days of September 30, 2012. |
| (6) | Includes 224,500 shares of common stock issuable upon the exercise of options currently exercisable or exercisable within 60 days of September 30, 2012. |
| (7) | Includes 116,249 shares of common stock issuable upon the exercise of currently exercisable options. |
| (8) | Includes 71,186 shares of common stock issuable upon the exercise of currently exercisable options. |
| (9) | Includes 56,250 shares of common stock issuable upon the exercise of currently exercisable options. |
| (10) | Represents 545,245 shares of common stock held by JALAA Equities, LP, of which Mr. Aryeh is the founder and general partner, 9,900 shares of common stock held in trust, and 25,000 shares of common stock issuable upon the exercise of currently exercisable options. |
| (11) | Represents shares of common stock issuable upon the exercise of currently exercisable options. |
| (12) | Represents 1,000 shares of common stock beneficially owned directly by Dr. Henderson, 75 shares of common stock owned by Dr. Henderson’s spouse, and 81,250 shares of common stock issuable upon the exercise of currently exercisable options. |
| (13) | Consists of the shares of common stock and shares of common stock issuable upon the exercise options held by Ms. Kendell and Messrs. Lollini, Belle, Aryeh, Forrester, Franson, Henderson, and Langer. |
B-98
Equity Compensation Plan Information
The following table provides certain aggregate information with respect to all of our equity compensation plans in effect as of June 30, 2012.
| (a) | (b) | (c) | ||||||||||
| Plan category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column(a)) |
|||||||||
| Equity compensation plans approved by security holders (1) |
4,806,563 | (2) | $ | 3.30 | (3) | 2,437,149 | (4) | |||||
| Equity compensation plans not approved by security holders |
— | — | — | |||||||||
| Total |
4,806,563 | (2) | $ | 3.30 | (3) | 2,437,149 | (4) | |||||
| (1) | These plans consist of our 2009 Employee, Director and Consultant Equity Incentive Plan (the “2009 Equity Incentive Plan”) and our 2009 Employee Stock Purchase Plan (the “2009 ESPP”). |
| (2) | Includes 2,624,911 shares of common stock to be issued upon the exercise of outstanding stock options under the 2009 Equity Incentive Plan, and 2,181,652 shares of common stock to be issued upon the vesting of restricted stock units granted under the 2009 Equity Incentive Plan as of June 30, 2012. |
| (3) | Weighted-average exercise price relates to outstanding stock options. Restricted stock units are deemed to have an exercise price of zero. |
| (4) | Represents shares of common stock available for future issuance under the 2009 Equity Incentive Plan and the 2009 ESPP as of June 30, 2012. The 2009 Equity Incentive Plan contains an “evergreen provision” which allows for an annual increase in the number of shares available for issuance under the plan on the first day of each of our fiscal years during the period beginning in fiscal year 2011 and ending on the second day of fiscal year 2019. The annual increase in the number of shares shall be equal to the lesser of (i) 2,400,000 shares; (ii) 5% of our outstanding shares on the first day of the fiscal year; and (iii) an amount determined by our Board of Directors. The Board of Directors determined not to increase the number of shares reserved under the Equity Incentive Plan as of July 1, 2012. The 2009 ESPP also contains an evergreen provision which allows for an increase in the number of shares available for issuance under the plan on the first day of each fiscal year beginning with fiscal year 2011. The increase in the number of shares shall be equal to the lesser of (i) 500,000 shares; (ii) 2% of the shares of our common stock outstanding on the last day of the immediately preceding fiscal year; and (iii) an amount determined by our Board of Directors. The Board of Directors determined not to increase the number of shares reserved under the 2009 ESPP as of July 1, 2012. |
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Certain Relationships and Related Transactions
We were not a party to any transactions with related persons since July 1, 2011 that would be required to be disclosed pursuant to Item 404(a) of Regulation S-K.
Policy for Approval of Related Person Transactions
We have adopted a Policy on Related Person Transactions (the “Policy”) under which the Audit Committee reviews, approves or ratifies all related person transactions. Under our Policy, a related person transaction is one in which Myrexis is a participant, and the amount involved exceeds $120,000, and in which any of the following persons has or will have a direct or indirect material interest:
| • | executive officers of the Company; |
| • | members of our Board of Directors; |
B-99
| • | beneficial holders of more than 5% of our securities; |
| • | immediate family members, as defined by Item 404 of Regulation S-K promulgated under the Securities Act, of any of the foregoing persons; |
| • | any firm, corporation or other entity in which any of the foregoing persons is employed or is a partner or principal or in a similar position or in which such person has a 5% or greater beneficial ownership interest; and |
| • | any other persons whom the Board determines may be considered to be related persons as defined by Item 404 of Regulation S-K promulgated under the Securities Act. |
Under the Policy, the Audit Committee shall approve only those related person transactions that are determined to be in, or not inconsistent with, the best interests of Myrexis and its stockholders, taking into account all available facts and circumstances as the Audit Committee determines in good faith to be necessary. These facts and circumstances will typically include, but not be limited to, the benefits of the transaction to Myrexis; the impact on a director’s independence in the event the related person is a director, an immediate family member of a director or an entity in which a director is a partner, shareholder or executive officer; the availability of other sources for comparable products or services; the terms of the transaction; and the terms of comparable transactions that would be available to unrelated third parties or to employees generally. No member of the Audit Committee shall participate in any review, consideration or approval of any related person transaction with respect to which the member or any of his or her immediate family members is the related person.
In reviewing and approving such transactions, the Audit Committee shall obtain, or shall direct management to obtain on its behalf, all information that the Audit Committee believes to be relevant and important to a review of the transaction prior to its approval. Following receipt of the necessary information, a discussion shall be held of the relevant factors if deemed to be necessary by the Audit Committee prior to approval. If a discussion is not deemed to be necessary, approval may be given by written consent of the Audit Committee. This approval authority may also be delegated to the Chairperson of the Audit Committee in some circumstances. It is contemplated that no related person transaction shall be entered into prior to the completion of these procedures; however, where permitted, a related person transaction may be ratified upon completion of these procedures.
The Audit Committee may adopt any further policies and procedures relating to the approval of related person transactions that it deems necessary or advisable from time to time. A copy of our Policy on Related Person Transactions is publicly available on the “Investors—Corporate Governance” section of our website at www.myrexis.com.
Director Independence
Our Board of Directors has reviewed the materiality of any relationship that each of our directors has with Myrexis, either directly or indirectly. Based upon this review, our Board has determined that the following members of the Board are “independent directors” as defined by The NASDAQ Stock Market: Mr. Belle, Mr. Aryeh, Mr. Forrester, Dr. Franson, Dr. Henderson and Dr. Langer.
| Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The Audit Committee has appointed Ernst & Young LLP, independent registered public accounting firm, to audit our financial statements for the fiscal year ending June 30, 2013. Ernst & Young LLP audited our financial statements for the fiscal year ended June 30, 2012.
B-100
Accounting Fees and Services
The following table presents fees for professional audit services rendered by Ernst & Young LLP for the audit of the Company’s annual financial statements for the years ended June 30, 2012, and June 30, 2011, and fees billed for other services rendered by Ernst & Young LLP during those periods.
| 2012 | 2011 | |||||||
| Audit fees: (1) |
$ | 232,096 | $ | 249,949 | ||||
| Audit related fees: (2) |
— | — | ||||||
| Tax fees: (3) |
22,900 | 85,829 | ||||||
| All other fees: (4) |
1,995 | 1,995 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 256,991 | $ | 337,773 | ||||
| (1) | Audit fees consisted of audit work performed in the preparation of financial statements, as well as work generally only the independent registered public accounting firm can reasonably be expected to provide, such as statutory audits. |
| (2) | We did not engage Ernst & Young LLP to perform audit related services during fiscal year 2012 or 2011. |
| (3) | Tax fees in fiscal 2012 consisted principally of assistance with matters related to a Section 382 study. Tax fees in fiscal year 2011 consisted principally of assistance with matters related to an R&D tax credit study and the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code. |
| (4) | All other fees in fiscal year 2012 and 2011 consisted principally of access fees to the Ernst & Young LLP on-line Global Accounting & Auditing Information Tool. |
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services
Consistent with SEC policies regarding auditor independence, the Audit Committee has responsibility for appointing, setting compensation and overseeing the work of our independent registered public accounting firm. In recognition of this responsibility, the Audit Committee has established a policy to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm.
Prior to engagement of an independent registered public accounting firm for the next year’s audit, management will submit an aggregate of services expected to be rendered during that year for each of four categories of services to the Audit Committee for approval.
| 1. | Audit services include audit work performed in the preparation of financial statements, as well as work that generally only an independent registered public accounting firm can reasonably be expected to provide, including comfort letters, statutory audits, and attest services and consultation regarding financial accounting and/or reporting standards. |
| 2. | Audit-Related services are for assurance and related services that are traditionally performed by an independent registered public accounting firm, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements. |
| 3. | Tax services include all services performed by an independent registered public accounting firm’s tax personnel except those services specifically related to the audit of the financial statements, and includes fees in the areas of tax compliance, tax planning, and tax advice. |
| 4. | Other Fees are those associated with services not captured in the other categories. |
Prior to engagement, the Audit Committee pre-approves these services by category of service. The fees are budgeted and the Audit Committee requires our independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage our independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the Audit Committee requires specific pre-approval before engaging our independent registered public accounting firm.
B-101
The Audit Committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the Audit Committee at its next scheduled meeting.
B-102
PART IV
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are filed as part of this Annual Report on Form 10-K:
1. Financial Statements
See “Index to Financial Statements” at Item 8 of this Annual Report on Form 10-K.
2. Financial Statement Schedules
The financial statements beginning on page F-1 are filed as part of this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
3. Exhibits
The exhibits which are filed with or incorporated by reference into this Annual Report on Form 10-K are set forth in the Exhibit Index to this Annual Report on Form 10-K, which is incorporated herein by reference.
B-103
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on October 29, 2012.
| MYREXIS, INC. | ||
| By: |
/S/ DAVID W. GRYSKA | |
| David W. Gryska Acting President and Chief Executive Officer and Chief Operating Officer | ||
B-104
EXHIBIT INDEX
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by |
Filing Date |
SEC File / Registration Number | |||||
| 2.1 | Separation and Distribution Agreement, dated June 30, 2009, by and between the Registrant and Myriad Genetics, Inc. | 8-K (Exhibit 2.1) |
7/7/09 | 001-34275 | ||||||
| 3.1 | Amended and Restated Certificate of Incorporation of the Registrant | 10-K (Exhibit 3.1) |
9/13/10 | 001-34275 | ||||||
| 3.1.1 | Certificate of Designation, Preferences and Rights of Series A Junior Participating Preferred Stock | 10-K (Exhibit 3.1.1) |
9/13/10 | 001-34275 | ||||||
| 3.1.2 | Certificate of Amendment to Restated Certificate of Incorporation of the Registrant | 10-K (Exhibit 3.1.2) |
9/13/10 | 001-34275 | ||||||
| 3.1.3 | Amended Certificate of Designation, Rights and Preferences of Series A Junior Participating Preferred Stock | 8-K (Exhibit 3.1) |
3/30/12 | 001-34275 | ||||||
| 3.2 | Amended and Restated Bylaws of the Registrant | 10-K (Exhibit 3.2) |
9/13/10 | 001-34275 | ||||||
| 4.1 | Form of Common Stock Certificate of the Registrant | 10-K (Exhibit 4.1) |
9/13/12 | 001-34275 | ||||||
| 4.2 | Shareholder Rights Agreement between the Registrant and American Stock Transfer & Trust Company, LLC, dated June 30, 2009, which includes as Exhibit B the form of Right Certificate | 8-A (Exhibit 4.1) |
6/30/09 | 001-34275 | ||||||
| 4.2.1 | First Amendment, dated March 29, 2012, to Shareholder Rights Agreement by and between the Registrant and American Stock Transfer & Trust Company, LLC, dated June 30, 2009 | 8-K (Exhibit 4.1) |
3/30/12 | 001-34275 | ||||||
| 4.3 | Tax Benefits Preservation Rights Agreement by and between the Registrant and American Stock Transfer & Trust Company, LLC, dated March 29, 2012, which includes as Exhibit B the Form of Right Certificate | 8-K (Exhibit 4.2) |
3/30/12 | 001-34275 | ||||||
| Lease Agreements | ||||||||||
| 10.1 | Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 8-K (Exhibit 10.2) |
7/7/09 | 001-34275 | ||||||
| 10.1.1 | Amendment No. 1, effective November 11, 2009, to Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 10-Q (Exhibit 10.1) |
11/12/09 | 001-34275 | ||||||
| 10.1.2 | Amendment No. 2, dated February 19, 2010, to Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 10-Q (Exhibit 10.2) |
5/17/10 | 001-34275 | ||||||
B-105
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by |
Filing Date |
SEC File / Registration Number | |||||||
| Equity Compensation Plans | ||||||||||||
| *10.2 | 2009 Employee, Director and Consultant Stock Plan, as amended (the “2009 Plan”) | 10-K (Exhibit 10.2) |
9/13/12 | 001-34275 | ||||||||
| *10.2.1 | Form of Stock Option Agreement under the 2009 Plan | 10/A (Exhibit 10.6.1) |
6/8/09 | 001-34275 | ||||||||
| *10.2.2 | Form of Restricted Stock Unit Agreement under the 2009 Plan | 10/A (Exhibit 10.6.2) |
6/8/09 | 001-34275 | ||||||||
| *10.2.3 | Form of Incentive Stock Option Agreement under the 2009 Plan for Rollover Options issued under the Myriad Genetics, Inc. 2003 Employee, Director and Consultant Stock Option Plan, as amended (the “MGI 2003 Plan”) | 10/A (Exhibit 10.6.3) |
6/8/09 | 001-34275 | ||||||||
| *10.2.4 | Form of Non-Qualified Stock Option Agreement under the 2009 Plan for Rollover Options issued under the MGI 2003 Plan | 10/A (Exhibit 10.6.4) |
6/8/09 | 001-34275 | ||||||||
| *10.2.5 | Form of Incentive Stock Option Agreement under the 2009 Plan for Rollover Options issued under the Myriad Genetics, Inc. 2002 Employee, Director and Consultant Stock Option Plan, as amended (the “MGI 2002 Plan”) | 10/A (Exhibit 10.6.5) |
6/8/09 | 001-34275 | ||||||||
| *10.2.6 | Form of Non-Qualified Stock Option Agreement under the 2009 Plan for Rollover Options issued under the MGI 2002 Plan | 10/A (Exhibit 10.6.6) |
6/8/09 | 001-34275 | ||||||||
| *10.2.7 | Form of Restricted Stock Unit Award Agreement under the 2009 Plan entered into between the Registrant and each of Richard B. Brewer and David W. Gryska on May 11, 2012 | 8-K (Exhibit 10.4) |
5/11/12 | 001-34275 | ||||||||
| *10.3 | 2009 Employee Stock Purchase Plan | 10/A (Exhibit 10.7) |
6/8/09 | 001-34275 | ||||||||
| Agreements with Executive Officers and Directors | ||||||||||||
| 10.4 | Form of Indemnification Agreement between the Registrant and its directors and officers | 10/A (Exhibit 10.8) |
5/29/09 | 001-34275 | ||||||||
| *10.5 | Non-Employee Director Compensation Policy, as amended November 11, 2010 | 10-Q (Exhibit 10.1) |
2/9/11 | 001-34275 | ||||||||
| *10.6 | Form of Employment Agreement between the Registrant and its officers | 10-K (Exhibit 10.10) |
9/28/09 | 001-34275 | ||||||||
| *10.7 | Executive Severance and Change in Control Agreement by and between the Registrant and Adrian N. Hobden, dated February 1, 2010 | 8-K (Exhibit 10.1) |
2/4/10 | 001-34275 | ||||||||
| *10.8 | Form of Executive Severance and Change in Control Agreement entered into between the Registrant and each of Wayne Laslie and Robert Lollini on February 1, 2010 | 8-K (Exhibit 10.2) |
2/4/10 | 001-34275 | ||||||||
B-106
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by |
Filing Date |
SEC File / Registration Number | |||||||
| *10.9 | Separation Agreement by and between the Registrant and Adrian N. Hobden, dated July 21, 2011 | 8-K (Exhibit 10.1) |
7/22/11 | 001-34275 | ||||||||
| *10.10 | Offer Letter by and between the Registrant and Robert J. Lollini, dated September 9, 2011 | 8-K (Exhibit 10.1) |
9/12/11 | 001-34275 | ||||||||
| *10.11 | First Amendment, dated September 9, 2011, to Executive Severance and Change in Control Agreement by and between the Registrant and Robert J. Lollini, dated February 1, 2010 | 8-K (Exhibit 10.2) |
9/12/11 | 001-34275 | ||||||||
| *10.12 | Offer Letter by and between the Registrant and Andrea Kendell, dated September 22, 2011 | 8-K/A (Exhibit 10.1) |
9/28/11 | 001-34275 | ||||||||
| *10.13 | Executive Severance and Change in Control Agreement by and between the Registrant and Andrea Kendell, dated September 22, 2011 | 8-K/A (Exhibit 10.2) |
9/28/11 | 001-34275 | ||||||||
| 10.14 | Agreement by and between the Registrant and Jason M. Aryeh, dated October 18, 2011 | 8-K (Exhibit 10.2) |
10/21/11 | 001-34275 | ||||||||
| *10.15 | Separation Agreement by and between the Registrant and Wayne Laslie, dated December 13, 2011 | 8-K (Exhibit 10.1) |
12/14/11 | 001-34275 | ||||||||
| *10.16 | Separation and Consulting Agreement by and between the Registrant and Robert J. Lollini, dated May 11, 2012 | 8-K (Exhibit 10.1) |
5/11/12 | 001-34275 | ||||||||
| *10.17 | Employment Agreement by and between the Registrant and Richard B. Brewer, dated May 9, 2012 | 8-K (Exhibit 10.2) |
5/11/12 | 001-34275 | ||||||||
| *10.18 | Employment Agreement by and between the Registrant and David W. Gryska, dated May 9, 2012 | 8-K (Exhibit 10.3) |
5/11/12 | 001-34275 | ||||||||
| Other Material Agreements | ||||||||||||
| 10.19 | Agreement by and among the Registrant and MSMB Healthcare LP, MSMB Healthcare Investors LLC, MSMB Healthcare Management LLC, and MSMB Capital Management LLC, dated October 18, 2011 | 8-K (Exhibit 10.1) |
10/21/11 | 001-34275 | ||||||||
| 10.20 | Letter Agreement by and between the Registrant and Martin Shkreli, dated October 18, 2011 | 8-K (Exhibit 10.3) |
10/21/11 | 001-34275 | ||||||||
| 10.21 | Letter Agreement by and among the Registrant, Bulldog Investors, and Brooklyn Capital Management LLC, dated August 6, 2012 | 8-K (Exhibit 10.1) |
8/10/12 | 001-34275 | ||||||||
B-107
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by |
Filing Date |
SEC File / Registration Number | |||||||
| 10.22 | Letter Agreement by and among the Registrant, MSMB Healthcare LP, MSMB Healthcare Investors LLC, MSMB Healthcare Management LLC and MSMB Capital Management LLC, dated August 8, 2012 | 8-K (Exhibit 10.2) |
8/10/12 | 001-34275 | ||||||||
| 10.23 | Letter Agreement by and between the Registrant and Martin Shkreli, dated August 8, 2012 | 8-K (Exhibit 10.3) |
8/10/12 | 001-34275 | ||||||||
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | 10-K (Exhibit 23.1) |
9/13/12 | 001-34275 | ||||||||
| 31.1 | Certification of the Chief Executive Officer under Section 302 of the Sarbanes-Oxley Act of 2002 | 10-K (Exhibit 31.1) |
9/13/12 | 001-34275 | ||||||||
| 31.1.1 | Certification of the Chief Executive Officer under Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 31.2 | Certification of the Chief Financial Officer under Section 302 of the Sarbanes-Oxley Act of 2002 | 10-K (Exhibit 31.2) |
9/13/12 | 001-34275 | ||||||||
| 31.2.1 | Certification of the Chief Financial Officer under Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 32.1 | Certification of the Chief Executive Officer and the Chief Financial Officer under Section 906 of the Sarbanes- Oxley Act of 2002 | 10-K (Exhibit 32.1) |
9/13/12 | 001-34275 | ||||||||
| 101** | The following materials from Myrexis, Inc.’s Annual Report on Form 10-K for the fiscal year ended June 30, 2012, formatted in XBRL (extensible Business Reporting Language): (i) Balance Sheets, (ii) Statements of Operations, (iii) Statements of Stockholders’ Equity and Comprehensive Loss, (iv) Statements of Cash Flows, and (v) Notes to Financial Statements | 10-K (Exhibit 101) |
9/13/12 | 001-34275 | ||||||||
| * | Management contract, compensatory plan or arrangement. |
| ** | Users of the XBRL data are advised pursuant to Rule 406T of Regulation S-T that this interactive data file is deemed not filed or part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, and otherwise is not subject to liability under these sections. |
B-108
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2012
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-34275
MYREXIS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 26-3996918 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 305 Chipeta Way Salt Lake City, Utah |
84108 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (801) 214-7800
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | x | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of November 2, 2012, the registrant had 26,817,294 shares of common stock outstanding.
MYREXIS, INC.
| Page | ||||
| PART I FINANCIAL INFORMATION | ||||
| Balance Sheets as of September 30, 2012 and June 30, 2012 (unaudited) |
C-3 | |||
| C-4 | ||||
| Statements of Cash Flows for the three months ended September 30, 2012 and 2011 (unaudited) |
C-5 | |||
| C-6 | ||||
| Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations |
C-13 | |||
| Item 3. Quantitative and Qualitative Disclosures About Market Risk |
C-21 | |||
| C-22 | ||||
| PART II OTHER INFORMATION | ||||
| C-23 | ||||
| C-23 | ||||
| Item 2. Unregistered Sales of Equity Securities and Use of Proceeds |
C-26 | |||
| C-26 | ||||
| C-26 | ||||
| C-27 | ||||
| C-27 | ||||
| C-28 | ||||
C-ii
PART I, Item 1—FINANCIAL INFORMATION
MYREXIS, INC.
(In thousands, except per share amounts)
| September 30, 2012 | June 30, 2012 | |||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 36,580 | $ | 19,707 | ||||
| Marketable investment securities |
47,756 | 68,671 | ||||||
| Equipment held for sale |
700 | 974 | ||||||
| Prepaid expenses and other assets |
1,055 | 192 | ||||||
|
|
|
|
|
|||||
| Total current assets |
86,091 | 89,544 | ||||||
|
|
|
|
|
|||||
| Equipment and leasehold improvements: |
||||||||
| Equipment |
836 | 1,298 | ||||||
| Leasehold improvements |
1,197 | 1,197 | ||||||
|
|
|
|
|
|||||
| 2,033 | 2,495 | |||||||
| Less accumulated depreciation |
1,787 | 1,846 | ||||||
|
|
|
|
|
|||||
| Net equipment and leasehold improvements |
246 | 649 | ||||||
|
|
|
|
|
|||||
| Long-term marketable investment securities |
1,123 | 1,248 | ||||||
| Other assets |
210 | 210 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 87,670 | $ | 91,651 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 457 | $ | 197 | ||||
| Accrued liabilities |
1,064 | 2,082 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
1,521 | 2,279 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.01 par value, authorized 5,000 shares; no shares issued and outstanding |
— | — | ||||||
| Common stock, $0.01 par value, authorized 60,000 shares; 26,817 shares issued and outstanding at September 30, 2012; 26,794 shares issued and outstanding at June 30, 2012 |
268 | 268 | ||||||
| Additional paid-in capital |
206,166 | 205,968 | ||||||
| Accumulated other comprehensive income |
4 | 4 | ||||||
| Accumulated deficit |
(120,289 | ) | (116,868 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
86,149 | 89,372 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 87,670 | $ | 91,651 | ||||
|
|
|
|
|
|||||
See accompanying notes to financial statements (unaudited).
C-3
MYREXIS, INC.
Statements of Operations and Comprehensive Loss (Unaudited)
(In thousands, except per share amounts)
| Three Months Ended September 30, | ||||||||
| 2012 | 2011 | |||||||
| Research revenue |
$ | — | $ | — | ||||
| Costs and expenses: |
||||||||
| Research and development expense |
291 | 4,300 | ||||||
| General and administrative expense |
3,485 | 4,385 | ||||||
|
|
|
|
|
|||||
| Total costs and expenses |
3,776 | 8,685 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(3,776 | ) | (8,685 | ) | ||||
|
|
|
|
|
|||||
| Other income, net |
355 | 99 | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (3,421 | ) | $ | (8,586 | ) | ||
|
|
|
|
|
|||||
| Loss per basic and diluted share |
$ | (0.13 | ) | $ | (0.33 | ) | ||
| Weighted-average shares used to compute net loss per basic and diluted share |
26,798 | 26,077 | ||||||
| Comprehensive loss |
$ | (3,421 | ) | $ | (8,560 | ) | ||
|
|
|
|
|
|||||
See accompanying notes to financial statements (unaudited).
C-4
MYREXIS, INC.
Statements of Cash Flows (Unaudited)
(In thousands)
| Three Months Ended September 30, |
||||||||
| 2012 | 2011 | |||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (3,421 | ) | $ | (8,586 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
221 | 336 | ||||||
| Loss on impairment of assets |
20 | — | ||||||
| Share-based compensation expense |
175 | 485 | ||||||
| Gain on sale of assets |
(318 | ) | — | |||||
| Gain on sale of marketable investment securities |
— | (1 | ) | |||||
| Changes in operating assets and liabilities: |
||||||||
| Prepaid expenses |
(257 | ) | 849 | |||||
| Other assets |
(606 | ) | 302 | |||||
| Accounts payable |
260 | (189 | ) | |||||
| Accrued liabilities |
(1,018 | ) | 36 | |||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(4,944 | ) | (6,768 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Capital expenditures for equipment and leasehold improvements |
— | (8 | ) | |||||
| Proceeds from sale of assets |
754 | — | ||||||
| Purchase of marketable investment securities |
(68,238 | ) | (22,787 | ) | ||||
| Proceeds from maturity of marketable investment securities |
89,278 | 31,600 | ||||||
|
|
|
|
|
|||||
| Net cash provided by investing activities |
21,794 | 8,805 | ||||||
|
|
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Net proceeds from common stock issued under share-based compensation plans |
23 | 12 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
23 | 12 | ||||||
|
|
|
|
|
|||||
| Net increase in cash and cash equivalents |
16,873 | 2,049 | ||||||
| Cash and cash equivalents at beginning of period |
19,707 | 19,189 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents at end of period |
$ | 36,580 | $ | 21,238 | ||||
|
|
|
|
|
|||||
See accompanying notes to financial statements (unaudited).
C-5
Notes to Financial Statements (Unaudited)
| (1) | Organization and Basis of Presentation |
| (a) | Organization and Business Description |
Myrexis, Inc. (“Myrexis” or the “Company”) is a biopharmaceutical company that has generated a pipeline of differentiated drug candidates in oncology and autoimmune diseases. The Company currently retains all rights to all of its drug candidates and programs across all geographic markets and therapeutic indications.
In February 2012, the Company announced that it had suspended development activity on all of its preclinical and clinical programs and retained Stifel Nicolaus Weisel, an investment banking firm, to assist in reviewing and evaluating a full range of strategic alternatives to enhance shareholder value. Thereafter, in March 2012, the Company initiated an alignment of its resources involving a phased reduction in its workforce from approximately 59 employees to 10 current employees.
Based on the Company’s evaluation of strategic alternatives, it determined to pursue the acquisition of one or more commercial-stage biopharmaceutical assets, with the goal of building a commercial-stage biopharmaceutical company by optimizing their performance and profitability. Integral to these efforts, on May 11, 2012, the Company announced a change in management, including the appointment of Richard B. Brewer as President and Chief Executive Officer and David W. Gryska as Chief Operating Officer, collectively bringing an extensive track record of commercializing, acquiring and marketing pharmaceutical products throughout their careers. In addition, both Mr. Brewer and Mr. Gryska were appointed as members of the Board of Directors.
On August 15, 2012, the Company announced the death of Richard B. Brewer, its President and Chief Executive Officer. The Board of Directors appointed David W. Gryska as the acting President and Chief Executive Officer while considering succession plans and proceeded to further evaluate the Company’s strategic direction in light of this development and the Company’s progress to date in identifying attractive biopharmaceutical assets.
On November 9, 2012, the Board of Directors concluded that it appeared unlikely that a strategic transaction at a valuation materially in excess of the Company’s estimated liquidation value would be achieved in the near term. Based on these and other factors, the Board of Directors concluded that a statutory dissolution and liquidation was in the best interests of the Company and its stockholders and therefore unanimously adopted a Plan of Complete Liquidation and Dissolution (the “Plan of Dissolution”), subject to stockholder approval. The Company intends to file proxy materials with the Securities and Exchange Commission expeditiously and to hold a special meeting of stockholders as soon as practicable for the purpose of obtaining stockholder approval of the Plan of Dissolution.
The Plan of Dissolution contemplates an orderly wind down of the Company’s business and operations in accordance with the provisions of Delaware law. If the Company’s stockholders approve the Plan of Dissolution, the Company intends to file a Certificate of Dissolution with the Delaware Secretary of State dissolving the Company, delist the Company’s shares of common stock from the NASDAQ Global Market, satisfy or resolve the Company’s remaining liabilities and obligations, including but not limited to contingent liabilities and claims and costs associated with the dissolution and liquidation, make reasonable provisions for unknown claims and liabilities and attempt to convert all of our remaining assets into cash or cash equivalents, and make distributions to the Company’s stockholders of cash available for distribution based upon their proportionate ownership at the time of the dissolution, subject to applicable legal requirements.
Pursuant to Delaware law, the Company will continue to exist for three years after the Certificate of Dissolution is filed or for such longer period as the Delaware Court of Chancery shall direct, solely
C-6
for the purpose of prosecuting and defending suits against it and enabling it to close the Company’s business, to dispose of its property, to discharge its liabilities and to distribute to its stockholders any remaining assets. The Plan of Dissolution contemplates the sale of all of the Company’s remaining non-cash assets, including its intellectual property.
If the Company’s stockholders do not approve the Plan of Dissolution, the Board of Directors and management will continue to explore other strategic alternatives. Even if the Company’s stockholders approve the Plan of Dissolution, the Board of Directors has reserved the right, in its discretion, to the extent permitted by Delaware law, to abandon or delay implementation of the Plan of Dissolution, in order, for example, to permit the Company to pursue any new business opportunities or strategic transactions that may arise.
| (b) | Basis of Accounting and Combination |
The accompanying financial statements have been prepared by Myrexis in accordance with U.S. generally accepted accounting principles (“GAAP”) for interim financial information and pursuant to the applicable rules and regulations of the Securities and Exchange Commission (the “SEC”). In the opinion of management, the accompanying financial statements contain all adjustments necessary to present fairly all financial statements in accordance with GAAP, which consists of only normal recurring adjustments. The financial statements herein should be read in conjunction with the Company’s audited financial statements and notes thereto for the fiscal year ended June 30, 2012, included in the Company’s Annual Report on Form 10-K for the year ended June 30, 2012. Operating results for the three months ended September 30, 2012 may not necessarily be indicative of results to be expected for any other interim period or for the full year.
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
| (2) | Marketable Investment Securities |
The amortized cost, gross unrealized holding gains and losses, and fair value for available-for-sale securities by major security type and class of security at September 30, 2012 and June 30, 2012 were as follows:
| Amortized cost |
Gross unrealized holding gains |
Gross unrealized holding losses |
Estimated fair value |
|||||||||||||
| (In thousands) | ||||||||||||||||
| September 30, 2012: |
||||||||||||||||
| Available-for-sale: |
||||||||||||||||
| Money market funds |
$ | 36,370 | $ | — | $ | — | $ | 36,370 | ||||||||
| Corporate bonds and notes |
33,097 | — | — | 33,097 | ||||||||||||
| Federal agency issues |
15,656 | 3 | — | 15,659 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 85,123 | $ | 3 | $ | — | $ | 85,126 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
C-7
| Amortized cost |
Gross unrealized holding gains |
Gross unrealized holding losses |
Estimated fair value |
|||||||||||||
| (In thousands) | ||||||||||||||||
| June 30, 2012: |
||||||||||||||||
| Available-for-sale: |
||||||||||||||||
| Money market funds |
$ | 19,707 | $ | — | $ | — | $ | 19,707 | ||||||||
| Corporate bonds and notes |
53,989 | 2 | — | 53,991 | ||||||||||||
| Federal agency issues |
15,679 | 2 | — | 15,681 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 89,375 | $ | 4 | $ | — | $ | 89,379 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
In addition, the Company holds $75,000 restricted cash in a 12-month certificate of deposit as collateral for a corporate purchasing card program and $48,000 in a restricted cash account as collateral for office equipment. On June 30, 2012, the Company held $200,000 restricted cash in an 18-month certificate of deposit as collateral for a corporate purchasing card program and $48,000 in a restricted cash account as collateral for office equipment. These amounts are included in long-term marketable securities on the balance sheet as of September 30, 2012 and June 30, 2012.
Maturities of debt securities classified as available-for-sale are as follows at September 30, 2012:
| Amortized cost |
Estimated fair value |
|||||||
| (In thousands) | ||||||||
| September 30, 2012: |
||||||||
| Available-for-sale: |
||||||||
| Due within one year |
$ | 47,753 | $ | 47,756 | ||||
| Due after one year through three years |
1,000 | 1,000 | ||||||
|
|
|
|
|
|||||
| $ | 48,753 | $ | 48,756 | |||||
|
|
|
|
|
|||||
| (3) | Fair Value Measurements |
The fair value of the Company’s financial instruments reflects the amounts that the Company estimates to receive in connection with the sale of an asset or be paid in connection with the transfer of a liability in an orderly transaction between market participants at the measurement date (exit price). The fair value hierarchy prioritizes the use of inputs used in valuation techniques into the following three levels:
Level 1—quoted prices in active markets for identical assets and liabilities.
Level 2—observable inputs other than quoted prices in active markets for identical assets and liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Some of the Company’s marketable securities utilize a the third party pricing service which provides documentation on an ongoing basis that includes, among other things, pricing information with respect to reference data, methodology, inputs summarized by asset class, pricing application, corroborative information, etc. The documentation includes consensus price or weighted average based on reported trades, broker/dealer quotes, benchmark securities, bids, offers, and reference data including market research publications. Also included are data from the vendor trading platform. We review, test and validate this information as appropriate.
Level 3—unobservable inputs.
C-8
The substantial majority of the Company’s financial instruments are valued using quoted prices in active markets or based on other observable inputs. The following table sets forth the fair value of the Company’s financial assets that the Company re-measured at September 30, 2012 and June 30, 2012:
| (In thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| September 30, 2012 |
||||||||||||||||
| Money market funds |
$ | 36,370 | $ | — | $ | — | $ | 36,370 | ||||||||
| Corporate bonds and notes |
— | 33,097 | — | 33,097 | ||||||||||||
| Federal agency issues |
— | 15,659 | — | 15,659 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 36,370 | $ | 48,756 | $ | — | $ | 85,126 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (In thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| June 30, 2012 |
||||||||||||||||
| Money market funds |
$ | 19,707 | $ | — | $ | — | $ | 19,707 | ||||||||
| Corporate bonds and notes |
— | 53,991 | — | 53,991 | ||||||||||||
| Federal agency issues |
— | 15,681 | — | 15,681 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 19,707 | $ | 69,671 | $ | — | $ | 89,379 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
In conjunction with the suspension of all development activities, the Company has evaluated its equipment and management has committed to a plan to sell the Company’s laboratory equipment. Equipment categorized as equipment held for sale on the balance sheet at September 30, 2012 totaled $0.7 million. Equipment held for sale is no longer subject to depreciation, and is recorded at the lower of depreciated carrying value or fair market value less costs to sell. The fair value of the equipment was determined by using broker quotes for similar assets. The Company has classified the inputs used for determining the fair value of these assets as Level II in the fair value hierarchy.
| (4) | Comprehensive Loss |
Comprehensive loss is comprised of net loss and other comprehensive income. Specifically, the Company includes in other comprehensive income the changes in unrealized gains and losses on its holdings of available-for-sale securities, which are excluded from its net loss. The following table sets forth the calculation of the Company’s comprehensive net loss for the three months ended September 30:
| (In thousands) | 2012 | 2011 | ||||||
| Net loss |
$ | (3,421 | ) | $ | (8,586 | ) | ||
| Other comprehensive loss: |
||||||||
| Change in unrealized gain and on marketable securities |
— | 26 | ||||||
|
|
|
|
|
|||||
| Total comprehensive net loss |
$ | (3,421 | ) | $ | (8,560 | ) | ||
|
|
|
|
|
|||||
| (5) | Earnings Per Share |
The loss per basic and diluted share is calculated by dividing net loss by the weighted-average number of shares outstanding during the reported period. For the three months ended September 30, 2012, there were outstanding potential common equivalent shares of 2,102,644, compared to 2,392,617, in the same period in 2011 which were excluded from the computation of diluted earnings per share because the effect would have been anti-dilutive. These potential dilutive common equivalent shares may be dilutive to basic earnings per share in future periods.
The calculation of diluted loss per share is the same as the basic loss per share since the inclusion of any potentially dilutive securities would be anti-dilutive.
C-9
| (6) | Share-Based Compensation |
The Company recognizes compensation expense using a fair-value based method for costs related to stock options and other equity-based compensation. The expense is measured based on the grant date fair value of the awards that are expected to vest, and the expense is recorded over the applicable requisite service period. In the absence of an observable market price for a share-based award, the fair value is based upon a valuation methodology that takes into consideration various factors, including the exercise price of the award, the expected term of the award, the current price of the underlying shares, the expected volatility of the underlying share price based on peer companies, the expected dividends on the underlying shares and the risk-free interest rate.
The Company has adopted two equity incentive plans, the Myrexis, Inc. 2009 Employee, Director and Consultant Equity Incentive Plan (the “Equity Incentive Plan”) and the Myrexis, Inc. 2009 Employee Stock Purchase Plan (the “ESPP”). The Company is authorized to issue a total of 10,063,259 shares under the plans.
The Company’s Equity Incentive Plan provides for the issuance of common stock based awards, including restricted stock, restricted stock units, stock options, stock appreciation rights and other equity based awards to its directors, officers, employees and consultants.
The Company’s ESPP is intended to qualify as an “employee stock purchase plan” under Section 423 of the Internal Revenue Code of 1986, as amended. Full-time employees of Myrexis who will own less than five percent of Myrexis’s outstanding shares of common stock are eligible to contribute a percentage of their base salary, subject to certain limitations, over the course of six-month offering periods for the purchase of shares of common stock. The purchase price for shares of common stock purchased under the ESPP will equal 85 percent of the fair market value of a share of common stock at the beginning or end of the relevant six-month offering period, whichever is lower.
Share-based compensation expense recognized for Myrexis employees included in the statements of operations for the three months ended September 30, 2012 and 2011 was as follows:
| Three Months Ended September 30, | ||||||||
| 2012 | 2011 | |||||||
| (in thousands) | ||||||||
| Research and development |
$ | (53 | ) | $ | 335 | |||
| General and administrative |
228 | 150 | ||||||
|
|
|
|
|
|||||
| Total employee stock-based compensation expense |
$ | 175 | $ | 485 | ||||
|
|
|
|
|
|||||
During the three months ended September 30, 2012, the Company granted 60,000 options and 56,800 restricted stock units under the Equity Incentive Plan. The weighted-average option exercise price was $2.65 per share for options and the weighted-average grant price was $2.65 per share for restricted stock units.
During the three months ended September 30, 2012, 10,768 stock options were exercised at a weighted average price of $1.69 per share. As of September 30, 2012, unrecognized compensation expense related to the unvested portion of stock options granted to Myrexis employees was approximately $0.9 million, which will be recognized over a weighted-average period of 1.96 years.
The fair value of each option grant is estimated on the grant date using the Black-Scholes option pricing model. Expected option lives were based on historical option lives under the Myrexis equity compensation plan and volatilities used in fair value calculations are based on a benchmark of peer companies with similar expected option lives. The related expense is recognized on a straight-line basis over the vesting period.
Currently eligible Myrexis employees are participating in the ESPP offering period that began June 1, 2012 and will close November 30, 2012. Expense associated with Myrexis employees participating in the ESPP was approximately $6,000 for the period ended September 30, 2012.
C-10
| (7) | Income Taxes |
In accordance with the interim reporting requirements, the Company uses an estimated annual effective rate for computing its provision for income taxes. The effective rate was zero for each of the three month periods ended September 30, 2012 and 2011.
The Company reduces deferred tax assets by a valuation allowance if, based on the weight of evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. At September 30, 2012 the Company has certain deferred tax assets, primarily from NOL’s and research and development tax credits generated since June 30, 2009, which have been offset in total by a valuation allowance.
The Company has adopted Accounting for Uncertainty in Income Taxes. For the three months ended September 30, 2012 and 2011, the Company recorded approximately $0 and $46,000, respectively, of additional liability for unrecognized tax benefits related to research tax credits. The Company includes any interest and penalties associated with any unrecognized tax benefits within the provision for income taxes on the statement of operations and comprehensive loss. The Company does not anticipate any material changes in the liability for unrecognized benefits in the next 12 months.
At September 30, 2012, the Company had Federal and State net operating loss carryforwards of approximately $126,257,000, of which $15,800,000 is attributable to excess tax benefits for which no deferred tax asset has been established. In addition, the Company had Federal research credit carryforwards of $2,650,000 and Utah research credit carryforwards of $1,082,000. These carryforward tax benefits can be used in certain circumstances to offset future tax liabilities. Pursuant to Sections 382 and 383 of the Internal Revenue Code, with which Utah complies, the Company’s use of the carryforward tax benefits may be limited in any given year as a result of certain changes in the Company’s ownership, including significant increases in ownership by the Company’s 5-percent shareholders. While the Company believes that its carryforward tax benefits as of September 30, 2012 are not limited under Sections 382 and 383, significant changes in ownership in the future may limit such usage. In March, 2012, in an effort to protect the use of its carryforward tax benefits, the Company adopted a Tax Benefits Preservation Rights Plan that discourages significant changes in ownership of the Company’s stock that might limit the use of our carryforward tax benefits.
| (8) | Commitments and Contingencies |
Our former parent Myriad Genetics, Inc. (“MGI”) had entered into a license agreement for exclusive rights to utilize certain intellectual property rights related to the drug candidate Azixa with Maxim Pharmaceuticals, Inc. and Cytovia, Inc. All licensed rights of Maxim and Cytovia were subsequently acquired by EpiCept Corporation, and Maxim, Cytovia and EpiCept are collectively referred to herein as EpiCept. Pursuant to the separation agreement with MGI, Myrexis assumed all rights and obligations under this license agreement, including an obligation to pay EpiCept milestone payments upon the occurrence of potential future events.
In September 2011, Myrexis announced that it had suspended any further development of Azixa. On August 28, 2012, Myrexis provided EpiCept notice of termination of the license agreement following its election to terminate all of its efforts to develop and commercialize Azixa in any major market as such products and markets are defined in the agreement. As a result of the termination of the agreement, all rights and licenses granted under the agreement by EpiCept have terminated and reverted to EpiCept. Myrexis has no further obligation for royalty or milestone payments to EpiCept as a result of this notice to terminate.
Various legal claims have been filed against Myrexis that relate to the ordinary course of business and are currently pending resolution. In the opinion of management and upon consultation with legal counsel, the ultimate resolution of these matters is not expected to have a material adverse effect on the financial position or future results of operations of Myrexis.
C-11
| (9) | Reorganization |
In conjunction with the March 2012 reorganization, the Company determined that there were indicators of impairment of certain fixed assets, based on quoted market prices, and evaluated whether the carrying value of assets with impairment indicators is recoverable. Management concluded that $20,000 of additional impairment loss should be recognized during the period ended September 30, 2012. This expense is reflected in the statement of operations and comprehensive income in general and administrative for the period ended September 30, 2012. Impairment charges of $281,000 were recorded in the year ended June 30, 2012 in conjunction with the March 2012 reorganization.
The Company has evaluated its equipment and management has committed to a plan to sell the Company’s laboratory equipment. Equipment categorized as equipment held for sale on the balance sheet at September 30, 2012 totaled $0.7 million. Equipment held for sale is no longer subject to depreciation, and is recorded at the lower of depreciated carrying value or fair market value less costs to sell. For the three months ended September 30, 2012, the Company sold assets with a net book value of $457,000 recognizing a net gain of $318,000. The gain is reflected in other income in the statement of operations and comprehensive loss. The Company expects to sell these assets by the end of calendar year 2012.
| (10) | Subsequent Event |
On November 9, 2012, the Board of Directors of the Company unanimously approved the dissolution of the Company pursuant to a Plan of Complete Liquidation and Dissolution (“Plan of Dissolution”), subject to stockholder approval. The Company intends to file proxy materials with the Securities and Exchange Commission expeditiously and to hold a special meeting of stockholders as soon as practicable for the purpose of obtaining stockholder approval of the Plan of Dissolution.
Upon stockholder approval of the Plan of Dissolution, the Company will adopt and present, as required, future financial statements on a liquidation basis of accounting.
C-12
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
You should read this discussion together with the financial statements, related notes and other financial information included elsewhere in this Quarterly Report on Form 10-Q. The following discussion may contain predictions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under “Risk Factors” in our Annual Report on Form 10-K for the year ended June 30, 2012 filed with the Securities and Exchange Commission, as supplemented under the heading “Risk Factors” in Part II, Item 1A of this Quarterly Report on Form 10-Q. These risks could cause our actual results to differ materially from any future performance suggested below.
Overview
We are a biopharmaceutical company that has generated a pipeline of differentiated drug candidates in oncology and autoimmune diseases. We currently retain all rights to all of our drug candidates and programs across all geographic markets and therapeutic indications.
In February 2012, we announced that we had suspended development activity on all of our preclinical and clinical programs and retained Stifel Nicolaus Weisel, an investment banking firm, to assist in reviewing and evaluating a full range of strategic alternatives to enhance shareholder value. Thereafter, in March 2012, we initiated an alignment of our resources involving a phased reduction in our workforce from approximately 59 employees to 10 current employees.
Based on our evaluation of strategic alternatives, we determined to pursue the acquisition of one or more commercial-stage biopharmaceutical assets, with the goal of building a commercial-stage biopharmaceutical company by optimizing their performance and profitability. Integral to these efforts, on May 11, 2012, we announced a change in management, including the appointment of Richard B. Brewer as President and Chief Executive Officer and David W. Gryska as Chief Operating Officer, collectively bringing an extensive track record of commercializing, acquiring and marketing pharmaceutical products throughout their careers. In addition, both Mr. Brewer and Mr. Gryska were appointed as members of the Board of Directors.
On August 15, 2012, we announced the death of Richard B. Brewer, our President and Chief Executive Officer. The Board of Directors appointed David W. Gryska as the acting President and Chief Executive Officer while considering succession plans and proceeded to further evaluate our strategic direction in light of this development and our progress to date in identifying attractive biopharmaceutical assets.
On November 9, 2012, the Board of Directors concluded that it appeared unlikely that a strategic transaction at a valuation materially in excess of the Company’s estimated liquidation value would be achieved in the near term. Based on these and other factors, the Board of Directors concluded that a statutory dissolution and liquidation was in the best interests of the Company and its stockholders and therefore unanimously adopted a Plan of Complete Liquidation and Dissolution (the “Plan of Dissolution”), subject to stockholder approval. We intend to file proxy materials with the Securities and Exchange Commission expeditiously and to hold a special meeting of stockholders as soon as practicable for the purpose of obtaining stockholder approval of the Plan of Dissolution.
The Plan of Dissolution contemplates an orderly wind down of the Company’s business and operations in accordance with the provisions of Delaware law. If our stockholders approve the Plan of Dissolution, we intend to file a Certificate of Dissolution with the Delaware Secretary of State dissolving the Company, delist the Company’s shares of common stock from the NASDAQ Global Market, satisfy or resolve the Company’s remaining liabilities and obligations, including but not limited to contingent liabilities and claims and costs associated with the dissolution and liquidation, make reasonable provisions for unknown claims and liabilities and attempt to convert all of our remaining assets into cash or cash equivalents, and make distributions to the Company’s stockholders of cash available for distribution based upon their proportionate ownership at the time of the dissolution, subject to applicable legal requirements.
C-13
The Company currently estimates that it will establish a reserve of between $7 million and $12 million, which will be used to pay all expenses (including operating expenses up until the dissolution) and other known, non-contingent liabilities, and includes reasonable provision for expenses of liquidation and contingent and unknown liabilities as required by Delaware law. Based on this estimated reserve, the Company currently estimates that the aggregate amount of an initial liquidating distribution to stockholders will be between $72.9 million and $77.9 million, or between $2.72 to $2.91 per share, based on 26,817,294 shares of common stock outstanding as of November 2, 2012. The Company expects to make an initial liquidating distribution as soon as practicable following the dissolution.
The amount distributable to stockholders, however, may vary substantially from this estimate based on a number of factors, including the resolution of outstanding known and contingent liabilities, the possible assertion of claims that are currently unknown to the Company and costs incurred to wind down the Company’s business. In particular, pursuant to the Company’s Separation and Distribution Agreement with its former parent, Myriad Genetics, Inc., at the time of the Company’s separation from Myriad Genetics in 2009, the Company assumed liability for certain pending or threatened legal matters related to the Company’s business, and is obligated to indemnify Myriad Genetics for any liability arising out of such matters, including any costs of litigating such matters. Although the Company does not believe that any obligation it assumed under the Separation and Distribution Agreement will result in a material liability, it cannot predict with certainty the amount or timing of such liability, if any. The Board of Directors, in consultation with its advisors, has evaluated this contingent liability, as well as other matters, in order to make a determination about reasonable amounts to reserve, which is reflected in the estimated reserve described above. Although the Board of Directors believes there is a reasonable possibility that a substantial amount of the contingency portion of the reserve will ultimately be distributed to the stockholders, Delaware law requires that the Company’s Board of Directors make reasonable provision for contingent and unknown obligations in connection with a dissolution and liquidation of the Company, which requires that a portion of the Company’s assets be reserved until the resolution of such matters. Further, if additional amounts are ultimately determined to be necessary to satisfy any of these obligations, stockholders may receive substantially less than the current estimates.
Pursuant to Delaware law, the Company will continue to exist for three years after the Certificate of Dissolution is filed or for such longer period as the Delaware Court of Chancery shall direct, solely for the purpose of prosecuting and defending suits against it and enabling us to close the Company’s business, to dispose of its property, to discharge its liabilities and to distribute to its stockholders any remaining assets. The Plan of Dissolution contemplates the sale of all of the Company’s remaining non-cash assets, consisting of its Hsp90 Inhibitor program, Cancer Metabolism Inhibitor program, and Oral Anti-interferon program for the treatment of autoimmune diseases (each of which is described below), including all related intellectual property, preclinical and clinical trial data and related regulatory filings and other documentation and supplies. The Plan of Dissolution does not specify the manner in which we may sell the Company’s assets. Such sales could take the form of individual sales of assets, sales of groups of assets organized by type of asset or otherwise, a single sale of all or substantially all of our assets, or some other form of sale. Sales of the Company’s assets will be made on such terms as are approved by the Board of Directors in its sole discretion. The assets may be sold to one or more purchasers in one or more transactions over a period of time. If our stockholders approve the Plan of Dissolution, the Company will be authorized to cease operations, sell or otherwise dispose of its non-cash assets and dissolve the Company and its subsidiaries without further approval of the stockholders, unless required to obtain such approval under Delaware law.
If our stockholders do not approve the Plan of Dissolution, the Board of Directors and management will continue to explore other strategic alternatives. Because the Board of Directors and management believe that they have exhausted all reasonable and viable strategic alternatives, it is possible that the Company would seek voluntary dissolution at a later time and potentially with diminished assets. In addition, the Company could cease operations, make an assignment for the benefit of creditors, turn the Company over to a third-party management company or liquidator or file for bankruptcy protection. Even if our stockholders approve the Plan of Dissolution, the Board of Directors has reserved the right, in its discretion, to the extent permitted by Delaware law, to abandon or delay implementation of the Plan of Dissolution, in order, for example, to permit the Company to pursue any new business opportunities or strategic transactions that may arise.
C-14
Our Oncology Programs
We have two programs in oncology. As indicated in our February 2012 announcement, we have suspended development activities in these programs.
| • | Hsp90 Inhibitor Program. Our compound MPC-3100 is an Hsp90 inhibitor for the treatment of cancer. In November 2011, we presented the results of an open-label, dose-escalating, multiple-dose, Phase 1 study of MPC-3100 in 26 patients with recurrent cancer or cancer refractory to available systemic therapy. Our compound MPC-0767 is a novel L-alanine ester pro-drug of MPC-3100 that was designed to have improved aqueous solubility compared to MPC-3100. We have completed all preclinical activities required for submission of an investigational new drug application, or IND, for MPC-0767. |
| • | Cancer Metabolism Inhibitor Program. MPC-8640 is our lead preclinical compound for the Cancer Metabolism Inhibitor, or CMI, program. As of June 2012, we have completed certain IND enabling studies. |
Our Hsp90 Inhibitor Program for the Treatment of Cancer
Background
Heat shock protein 90, or Hsp90, is involved in the folding and stabilization of many proteins, including mutant oncogenes that become reliant on Hsp90 to maintain their activity, making them particularly sensitive to Hsp90 inhibition. Targeted therapies against such mutant oncogenes, such as ALK, HER2, FLT3 and B-RAF, have proven to be efficacious in the clinic and we believe that by inhibiting these targets through the different mechanism of Hsp90 inhibition, either as monotherapy or in combination with these targeted therapies, clinical efficacy and the duration of response can be improved.
We believe that the potential for Hsp90 inhibitors to improve therapeutic outcomes across a number of oncogene “addicted” cancers, coupled with the oral bioavailability, long half-life and the relative safety profile of our compounds makes our Hsp90 inhibitor program a potentially attractive program.
MPC-3100 and MPC-0767: Preclinical Development
MPC-3100 and MPC-0767, a pro-drug of MPC-3100, are fully synthetic, orally bio-available, non-geldanamycin Hsp90 inhibitors that have shown significant and broad preclinical anti-tumor activity in mouse models of human cancers. These unique molecules are structurally distinct from the geldanamycin family of early Hsp90 inhibitors, which are associated with certain toxicities. MPC-3100 inhibits Hsp90 by binding to the same site as geldanamycin and has displayed potent anti-cancer activity in multiple in vitro and in vivo models. MPC-3100 significantly and dose-dependently reduced tumor growth in studies conducted in mice implanted with a variety of human cancer cell lines, including colon, prostate, myeloid leukemia, small-cell lung, gastric, breast, and ovarian cancers. In April 2011, we reported additional preclinical data on our Hsp90 inhibitor program at the annual meeting of the American Association for Cancer Research in Orlando, Florida. The data presented included a demonstration that the combination of MPC-3100 with other targeted therapies, including erlotinib and sorafenib, showed greater in vivo anti-tumor activity compared to either agent alone, suggesting the potential of combining MPC-3100 with these targeted cancer therapies in the clinic. We also presented a preliminary assessment of MPC-0767, a novel L-alanine ester pro-drug of MPC-3100, which is designed to have improved aqueous solubility. Animal studies showed that MPC-0767 displayed pharmacokinetics comparable to MPC-3100 and equivalent efficacy, inducing significant tumor regressions.
MPC-3100: Clinical Development
We submitted an IND application for MPC-3100 in the first quarter of 2009 and initiated patient enrollment of a Phase 1 clinical trial in the second quarter of 2009 to investigate the safety and tolerability of MPC-3100, pharmacokinetics, and the potential for anti-tumor activity. The Phase 1 study was an open-label, dose-escalating,
C-15
multiple-dose study in which 26 patients aged 45-85 years with recurrent cancer or cancer refractory to available systemic therapy were treated with MPC-3100. Patients received oral MPC-3100 either once daily for 21 days followed by seven days off (cohorts 1-5, at doses of 50, 100, 165, 245, and 340mg/m2, respectively) or continuously for a 28-day cycle at doses spaced 12 hours apart (cohorts 6-7, at total daily doses of 480mg/kg and 640mg/kg, respectively). The primary objective of the Phase 1 study was to determine the safety and tolerability of MPC-3100 in cancer patients. The study also included secondary objectives such as characterization of the pharmacokinetic parameters, determining anti-tumor activity of MPC-3100, and evaluating certain pharmacodynamic biomarkers. In November 2011, we presented the results of this study at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics meeting in San Francisco. The study demonstrated that MPC-3100 was generally safe and well tolerated at doses below 600mg/kg per day. The most common adverse events were gastrointestinal, including diarrhea, nausea, and vomiting. Pharmacokinetic analysis indicated that the maximum plasma concentration, or Cmax, and the area under the curve, or AUC (0-12h), increased proportional to the dose of MPC-3100. The terminal plasma half-life of MPC-3100 ranged from 4.8 to 21.4 hours with a mean half-life of 11.2 hours. The best clinical response was stable disease (12/26; 46%), with a median duration of 11.1 weeks (range 3.0-52.3 weeks). On target activity of MPC-3100 was confirmed by biomarker analysis, which suggested effective and persistent in vivo inhibition of Hsp90.
MPC-3100 and MPC-0767: Future Clinical Development
We have conducted non-clinical studies as well as other technical, regulatory and market assessments with the objective of identifying optimal cancer indications and drug combination regimens to potentially advance one or both of our Hsp90 inhibitor compounds into Phase 2 clinical development. We believe we have completed all preclinical activities required for submission of an IND for MPC-0767.
Our Cancer Metabolism Inhibitor Program
Our CMI program is focused on the inhibition of Nicotinamide phosphoribosyltransferase, or Nampt, an enzyme involved in the production of Nicotinamide Adenine Dinucleotide, or NAD, which is an essential cofactor for the production of cellular energy that is critical for cell survival, growth, and DNA repair.
Cancer cells, in addition to spending energy on rapid, unregulated growth, must also invest significant energy on DNA synthesis and repair mechanisms to cope with the DNA damage. As a result, cancer cells are more susceptible to metabolic downshifts than healthy cells, and the NAD depletion caused by Nampt inhibitors has a greater effect on tumors versus normal tissue.
MPC-8640 is our lead preclinical compound for our CMI program. MPC-8640 is an orally bio-available pro-drug of a follow-on molecule to our prior CMI drug candidate, MPC-9528, that has enhanced solubility and distinct pharmacokinetic advantages and is being developed for the treatment of cancer. Both the active moiety of MPC-8640 (MPI-0487316) as well as MPC-9528 inhibit Nampt in vitro and in cells at picomolar drug concentrations and are tumoricidal in every cancer line tested to date representing 17 different tumor tissue types. Both compounds display on-target activity by potently reducing NAD levels, which leads to inhibition of glycolysis, energy deprivation and cell death in tumor cells, while NAD levels in normal tissues are less affected. In preclinical efficacy studies, MPC-8640 and MPC-9528 causes dramatic tumor regressions in multiple tumor types when administered orally with either a low daily dosing or a higher intermittent dose regimen and are well tolerated. This anti-tumor activity is dose-dependent and tightly correlated to the level of NAD depletion, confirming the on-target mechanism of action. The sensitivity of tumor cells to our Nampt inhibitors in vitro appears to parallel their anti-tumor potency in xenograft models and is linked to basal Nampt expression levels. Nampt expression levels may therefore have utility for predicting tumor response to Nampt inhibitors. Nicotinic acid is converted to NAD through an alternative pathway that is dependent upon the enzyme Nicotinic acid phosphoribosyltransferase (Naprt1) which does not involve Nampt. In tumor cell types with sufficient Naprt expression to support this NAD biosynthetic pathway, nicotinic acid (niacin, Vitamin B3) can completely block the NAD-reducing and tumoricidal activity of MPC-9528. Our studies have found that approximately 40% of
C-16
tumor cell lines are deficient in Naprt1 and in these cells, nicotinic acid has little to no effect on MPC-9528 tumoricidal activity. Furthermore, in animal model studies, a combination of nicotinic acid with MPC-9528 increases the tolerated dose of MPC-9528, while still causing growth inhibition of tumors deficient in Naprt1. This demonstrates the potential to increase the therapeutic index and efficacy of a Nampt inhibitor by combining it with nicotinic acid to treat patients with tumors that are deficient in Naprt1. A diagnostic method designed to measure Naprt1 expression could be used to identify those patients with Naprt1 deficient tumors that are most likely to benefit from this combination therapy.
Additional preclinical studies of MPC-9528 support the potential of Nampt inhibitors for broad spectrum tumoricidal activity as monotherapy and in a variety of combinations with other agents. Inhibition of Nampt by MPC-9528 was shown to exhibit synergistic anti-tumor activity when coupled with DNA damaging agents, such as alkylating agents and thymidylate synthase inhibitors. These common classes of chemotherapy drugs also reduce NAD cellular levels as a result of their mechanism of action, specifically by activating the NAD-consuming enzyme poly (ADP-ribose) polymerase (PARP). The mechanism of action of our Nampt inhibitors is distinct from these other agents, leading to a combined effect on cellular NAD levels and synergistic anti-tumor activity.
In June 2011, preclinical studies on MPC-9528 and MPI-0487316, a structurally distinct Nampt inhibitor and the active moiety of MPC-8640, were presented at the annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. Oral administration of either compound resulted in tumor regressions in animal model studies across multiple dosing schedules. MPC-8640 is a pro-drug of MPI-0487316 with enhanced solubility and distinct pharmacokinetic advantages. In November 2011, we presented data from preclinical studies of MPC-8640 at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics meeting in San Francisco. In these studies, mice with HT1080 human fibrosarcoma xenograft tumors were treated orally with MPC-8640 on either a once-daily or twice-daily dosing schedule. After one week of treatment, the mice demonstrated complete tumor growth inhibition at lower doses and substantial tumor regression at higher doses. Significantly, tumor regression could be achieved well below the maximum tolerated dose of MPC-8640, and the anti-tumor response observed after one week of dosing was maintained for at least one week without further treatment. The results also demonstrated that MPC-8640 is effectively converted into active Nampt inhibitor, either in the gut or immediately upon absorption, as evidenced by the lack of significant plasma concentrations of intact MPC-8640. Taken together, these results demonstrate that oral treatment with MPC-8640 is an effective mode of delivery of active Nampt inhibitor and that administration of this drug results in significant anti-tumor activity in animal models of cancer. We have completed certain IND enabling studies on MPC-8640 and have suspended any new development activities on this program.
Our Small-Molecule Autoimmune Disease Program
Oral Anti-interferon Program for the Treatment of Autoimmune Diseases
MPI-0485520 is our lead preclinical compound in our small-molecule anti-interferon program for autoimmune diseases. It has demonstrated proof of concept activity in an animal model of the autoimmune disease rheumatoid arthritis, or RA. As of June 2012, we have concluded all lead optimization activities and have suspended any new development activities on this program.
IKKe and TBK1 are kinases that serve as key regulators of the pathway that activates alpha and beta interferon expression. Inhibition of these kinases thereby inhibits a major pro-inflammatory pathway involved in a number of autoimmune diseases, including RA, Lupus and psoriasis. We have demonstrated in preclinical studies that treatment with our oral anti-interferons, or OAI’s, inhibits the interferon response in several animal models, including significant inhibition of this response and reduction in the severity of clinical symptoms in a mouse model of RA.
MPI-0485520 is an orally-available small molecule that potently and selectively inhibits IKKe and TBK1 and is our lead preclinical compound in our small molecule anti-interferon program for autoimmune diseases. MPI-0485520 exhibits high oral bio-availability, favorable absorption, distribution, metabolism, and excretion
C-17
pharmacokinetic properties and efficacy in an in vivo mouse model of RA. In cellular models of type I interferon production, MPI-0485520 potently inhibits induction of type I interferons (IFNa/b) following stimulation of a variety of receptors that mediate the type-I interferon to pathogens, such as TLR3, TLR4, RIG-I, and MDA-5. Inhibition of type I interferon production by IKKe/TBK1 inhibitors may benefit patients with autoimmune disorders such as RA, systemic lupus erythematosus (SLE), scleroderma, Sjögren’s syndrome, and polymyositis. In April 2011, results from preclinical studies of MPI-0485520 were presented at the European League Against Rheumatism (EULAR) Annual European Congress of Rheumatology in London. In a proof of concept study, in the well characterized collagen-induced mouse model of arthritis, mice treated with MPI-0485520 show a dose-dependent and statistically significant reduction in the severity of clinical symptoms and paw and joint histopathology, as well as lower weight loss compared to control mice. MPI-0485520 is one compound out of an extensive portfolio of potent and selective IKK epsilon/TBK1 inhibitors identified by our oral anti-interferon program.
Critical Accounting Policies and Use of Estimates
Critical accounting policies are those policies which are both important to the portrayal of a company’s financial condition and results and require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies are as follows:
| • | impairment of long-lived assets. |
Impairment of Long-Lived Assets
We assess the impairment of long-lived assets when events or changes in circumstances indicate that the carrying value of the assets or the asset grouping may not be recoverable. Factors that we consider in deciding when to perform an impairment review include significant negative industry or economic trends, and significant changes or planned changes in our use of the assets. We measure the recoverability of assets that will continue to be used in our operations by comparing the carrying value of the asset grouping to our estimate of the related total future undiscounted net cash flows. If an asset grouping’s carrying value is not recoverable through the related undiscounted cash flows, the asset grouping is considered to be impaired. The impairment is measured by comparing the difference between the asset grouping’s carrying value and its fair value. Fair value is the price that would be received from selling an asset in an orderly transaction between market participants at the measurement date. Long-lived assets such as intangible assets and property, plant and equipment are considered non-financial assets, and are recorded at fair value only when an impairment charge is recognized. We recorded impairment charges for the period ended September 30, 2012 and 2011 of $20,000 and $0, respectively. These charges are reflected in the statement of operations and comprehensive loss in general and administrative expenses.
We have evaluated our equipment and management has committed to a plan to sell our laboratory equipment. Equipment categorized as equipment held for sale on the balance sheet at September 30, 2012 totaled $0.7 million. Equipment held for sale is no longer subject to depreciation, and is recorded at the lower of depreciated carrying value or fair market value less costs to sell. We expect to sell these assets by the end of calendar year 2012.
Results of Operations for the Three Months Ended September 30, 2012 and 2011
We operate in one reportable operating segment, drug development.
Our drug research and development expenses included costs incurred for our drug candidates. The only costs we tracked for each drug candidate were external costs such as services provided to us by clinical research organizations, manufacturing of drug supply, and other outsourced research. We did not assign or allocate internal costs such as salaries and benefits, facilities costs, lab supplies and the costs of preclinical research and
C-18
studies to individual development programs. All development costs for our drug candidates were expensed as incurred. Our research and development expenses recorded for the period ended September 30, 2012, were expenses associated with research and development activities completed during the quarter that were initiated prior to the announcement of the suspension of all our preclinical and clinical development activities in March 2012.
Research and Development
Research and development expenses are comprised primarily of salaries and related personnel costs, laboratory supplies, equipments costs, facilities expense, and costs associated with our clinical trials. Research and development expenses for the three months ended September 30, 2012 were $0.3 million compared to $4.3 million in the same quarter last year. This 93% decrease was primarily due to:
| • | decreased internal costs of approximately $1.1 million resulting from a reduction in headcount; |
| • | decreased preclinical development costs of $1.4 million resulting from the Company’s decision to suspend development activity on all clinical and preclinical programs; and |
| • | decreased external drug candidate costs of approximately $1.5 million. |
Research and development costs for the three months ended September 30, 2012 and 2011 were as follows:
| Three Months Ended September 30, | ||||||||
| (In thousands) | 2012 | 2011 | ||||||
| External costs, drug candidates: |
||||||||
| Azixa |
$ | 12 | $ | 1,140 | ||||
| MPC-4326 |
3 | 13 | ||||||
| MPC-3100 |
7 | 116 | ||||||
| MPC-0767 |
3 | 318 | ||||||
| MPC-8640 |
145 | 149 | ||||||
| MPI-0485520 |
68 | 3 | ||||||
|
|
|
|
|
|||||
| Sub-total direct costs |
238 | 1,739 | ||||||
| Internal costs: |
||||||||
| Internal costs, drug candidates |
53 | 1,198 | ||||||
| Preclinical development costs |
— | 1,363 | ||||||
|
|
|
|
|
|||||
| Total research and development |
$ | 291 | $ | 4,300 | ||||
|
|
|
|
|
|||||
We expect to see reduced research and development costs as a result of the decision to suspend further development activities for all preclinical and clinical programs and the Board of Directors’ approval of the dissolution and liquidation of the Company.
General and Administrative
General and administrative expenses consist primarily of salaries and related personnel costs for business development, executive, legal, finance and accounting, information technology, human resources, and facilities expenses. General and administrative expenses for the three months ended September 30, 2012 were $3.5 million compared to $4.4 million for the three months ended September 30, 2011. This 20% decrease in general and administrative expenses during the three months ended September 30, 2012, was due primarily to a reduction in headcount as a result of the Company’s decision to suspend development activities for all clinical and preclinical programs. As a result of the Board of Directors’ approval of the dissolution and liquidation of the Company, we expect to see reduced general and administrative expenses.
C-19
Other Income
Other income of $355,000 and $99,000 for the three months ended September 30, 2012 and 2011, respectively, reflects interest income earned on our marketable investment securities of $36,000 for the period ended September 30, 2012 and $88,000 for the same period in 2011, respectively. The decrease in interest income of 59% is a result of the reduction in our invested balance in marketable securities for the three months ended September 30, 2012 as compared to 2011. In addition, other income includes a net gain on the sale of assets of $318,000 for the period ended September 30, 2012 and $0 for the same period in 2011. The increase in gain on disposal of assets is a result of our decision to sell our laboratory equipment after our decision to suspend development activity on all our clinical and preclinical activities. The majority of the gain recorded results from the sale of assets that were fully depreciated or written off as a result of previous reorganizations in the Company.
Liquidity and Capital Resources
Net cash used in operating activities was $4.9 million during the three months ended September 30, 2012 compared to $6.8 million used in operating activities for the same three months in 2011. The change in cash flow from operating activity can be attributed primarily to the timing and payment of liabilities which were offset, in part, by a lower net loss in 2012.
Our investing activities provided $21.8 million in cash during the three months ended September 30, 2012 compared to $8.8 million used for the same three months in 2011. The change is primarily due to a reduction in our overall cash position and timing of new purchases and maturity of our marketable securities.
Approximately $23,000 in cash was provided by financing activities during the three months ended September 30, 2012 as a result of proceeds from stock options exercised during the period compared to $12,000 for the same three months in 2011. The change is primarily due to terminated employees exercising in-the-money stock options during the period ended September 30, 2012.
As of September 30, 2012, we had $85.5 million in cash, cash equivalents and marketable securities. If we do not dissolve and liquidate our assets, and notwithstanding the factors listed below, we believe our cash, cash equivalents and marketable securities are sufficient for at least the next 12 months. If we do not dissolve and liquidate our assets, our future capital requirements, cash flows, and results of operations could be affected by and will depend on many factors that are currently unknown to us, including:
| • | the outcome of our review and evaluation of any additional strategic alternatives; |
| • | changes in our business strategy; |
| • | regulatory developments or enforcement in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | changes in estimates or recommendations by securities analysts, if any cover our common stock; |
| • | the ability to partner, sell or out-license rights to our programs on favorable terms; |
| • | failure to secure adequate capital to fund our operations if and when needed, or the issuance of equity securities at prices below the current market price; |
| • | litigation; |
| • | future sales of our common stock; |
| • | general market conditions; |
| • | economic and other external factors or other disasters or crises; |
C-20
| • | period-to-period fluctuations in our financial results; and |
| • | overall fluctuations in U.S. equity markets. |
If we do not dissolve and liquidate our assets, to the extent our capital resources are insufficient to meet our future capital requirements, we will need to finance our future cash needs through public or private equity offerings, collaboration agreements, debt financings or licensing arrangements. Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. For example, if we raise additional funds by issuing equity securities or by selling convertible debt securities, further dilution to our existing stockholders may result. If we raise funds through licensing arrangements, we may be required to relinquish rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us.
We may elect to raise additional funds even before we need them if the conditions for raising capital are favorable. We have an effective universal shelf registration statement on Form S-3 pursuant to which we have up to $80 million of securities available for issuance.
Certain Factors That May Affect Future Results of Operations
The Securities and Exchange Commission, or SEC, encourages companies to disclose forward-looking information so that investors can better understand a company’s future prospects and make informed investment decisions. This Quarterly Report on Form 10-Q contains such “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995.
Words such as “may,” “anticipate,” “estimate,” “expects,” “projects,” “intends,” “plans,” “believes” and words and terms of similar substance used in connection with any discussion of future operating or financial performance, identify forward-looking statements. All forward-looking statements are management’s present expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to those set forth under the heading “Risk Factors” contained in Item 1A of our Annual Report on Form 10-K for the year ended June 30, 2012 that we have filed with the SEC, as supplemented under the heading “Risk Factors” in Part II, Item 1A of this Quarterly Report on Form 10-Q.
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Quarterly Report on Form 10-Q might not occur. Stockholders are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this Quarterly Report on Form 10-Q. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to Myrexis, Inc. or to any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
| Item 3. | Quantitative and Qualitative Disclosures About Market Risk. |
We maintain a portfolio of cash, cash equivalents and short term and long term marketable securities which are subject to interest rate risk. Our investments consist primarily of highly liquid securities of various types and maturities of two years or less, with a maximum average maturity of one year. Changes in interest rates affect the fair market value of these marketable investment securities. There have been no material changes in our exposure to market risk as compared to our disclosures under Item 7A in our Annual Report on Form 10-K for the year ended June 30, 2012.
C-21
| Item 4. | Controls and Procedures. |
(a) Evaluation of Disclosure Controls and Procedures. Our acting principal executive officer and principal financial officer evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act) as of the end of the period covered by this Quarterly Report on Form 10-Q. Based on this evaluation, our acting principal executive officer and principal financial officer have concluded that our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
(b) Changes in Internal Controls. There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting.
C-22
PART II—OTHER INFORMATION
| Item 1. | Legal Proceedings. |
In the ordinary course of business, various legal claims have been asserted, and in the future may be asserted, against Myrexis. In addition, as previously disclosed, under the terms of our Separation and Distribution Agreement with our former parent Myriad Genetics, Inc. we have the obligation to indemnify Myriad Genetics with respect to certain legal claims against Myriad Genetics which we assumed in connection with our spin-out from Myriad Genetics.
| Item 1A. | Risk Factors. |
In addition to the risk factors described in the “Risk Factors” section in our Annual Report on Form 10-K for the year ended June 30, 2012 filed with the Securities and Exchange Commission on September 13, 2012, as amended on October 29, 2012, the following risk factors should be considered in connection with the proposed liquidation and dissolution of the Company.
The amount we distribute to our stockholders in the initial liquidating distribution may be substantially less than the amount we currently estimate if the amounts of our liabilities, other obligations and expenses or claims against us are higher than we currently anticipate.
We currently estimate that we will establish a reserve of between $7 million and $12 million, which will be used to pay all expenses (including operating expenses up until the dissolution) and other known, non-contingent liabilities, and includes reasonable provision for expenses of liquidation and contingent and unknown liabilities as required by Delaware law. Based on this estimated reserve, we currently estimate that the aggregate amount of an initial liquidating distribution to stockholders will be between $72.9 million and $77.9 million, or between $2.72 to $2.91 per share, based on 26,817,294 shares of common stock outstanding as of November 2, 2012. We expect to make an initial liquidating distribution as soon as practicable following the dissolution. The amount of cash ultimately distributed to our stockholders in liquidating distributions depends on the amount of our liabilities, obligations and expenses and claims against us, and contingency reserves that we establish during the liquidation process. We have attempted to estimate reasonable reserves for such liabilities, obligations, expenses and claims against us. However, those estimates may be inaccurate. Factors that could impact our estimates include the following:
| • | if any of the estimates regarding the Plan of Dissolution, including the expenses to satisfy outstanding obligations, liabilities and claims during the liquidation process, are inaccurate; |
| • | if unforeseen claims are asserted against us, we will have to defend or resolve such claims or establish a reasonable reserve before making distributions to our stockholders; and |
| • | if the estimates regarding the expenses to be incurred in the liquidation process, including expenses of personnel required and other operating expenses (including legal, accounting and other professional fees) necessary to dissolve and liquidate the Company, are inaccurate. |
If any of the foregoing occurs, the amount we initially distribute to our stockholders may be substantially less than the amount we currently estimate.
We cannot assure you of the exact amount or timing of any additional liquidating distributions to our stockholders under the Plan of Dissolution.
We currently estimate that we will establish a reserve of between $7 million and $12 million, which will be used to pay all expenses (including operating expenses up until the dissolution) and other known, non-contingent liabilities, and includes reasonable provision for expenses of liquidation and contingent and unknown liabilities as required by Delaware law. The liquidation and dissolution process is subject to numerous uncertainties, and
C-23
may not result in any remaining capital for additional liquidating distributions to our stockholders following the initial liquidating distribution. The precise nature, amount and timing of any additional liquidating distribution to our stockholders will depend on and could be delayed by, among other things, sales of our non-cash assets, claim settlements with creditors, and unexpected or greater than expected expenses.
In particular, as previously disclosed, pursuant to our Separation and Distribution Agreement with our former parent, Myriad Genetics, Inc., at the time of our separation from Myriad Genetics in 2009, we assumed liability for certain pending or threatened legal matters related to the Company’s business, and we are obligated to indemnify Myriad Genetics for any liability arising out of such matters, including any costs of litigating such matters. Although we do not believe that any obligation we assumed under the Separation and Distribution Agreement will result in a material liability, we cannot predict with certainty the amount or timing of such liability, if any. The Board of Directors, in consultation with its advisors, has evaluated this contingent liability, as well as other matters, in order to make a determination about reasonable amounts to reserve, which is reflected in the estimated reserve described above. Although the Board of Directors believes there is a reasonable possibility that a substantial amount of the contingency portion of the reserve will ultimately be distributed to the stockholders, Delaware law requires that the Company’s Board of Directors make reasonable provision for contingent and unknown obligations in connection with a dissolution and liquidation of the Company, which requires that a portion of the Company’s assets be reserved until the resolution of such matters.
If our stockholders vote against the Plan of Dissolution, it would be very difficult for us to continue our business operations.
If our stockholders do not approve the Plan of Dissolution, we would have to continue our business operations from a difficult position, in light of our announced intent to liquidate and dissolve. We are not actively conducting any clinical development programs and have generally ceased normal business operations and terminated all but ten of our employees. Prospective employees, vendors and other third parties may refuse to form relationships or conduct business with us if they do not believe we will continue to operate as a going concern.
Our Board of Directors may abandon or delay implementation of the Plan of Dissolution even if approved by our stockholders.
Even if our stockholders approve the Plan of Dissolution, our Board of Directors has reserved the right, in its discretion, to the extent permitted by Delaware law, to abandon or delay implementation of the Plan of Dissolution, in order, for example, to permit us to pursue new business opportunities or strategic transactions.
The payment of liquidating distributions, if any, to our stockholders could be delayed.
Although our Board of Directors has not established a firm timetable for liquidating distributions to our stockholders, the Board of Directors intends, subject to contingencies inherent in winding down our business, to make such liquidating distributions, if any, as promptly as practicable as creditor claims and contingent liabilities are paid or settled. However, we are currently unable to predict the precise timing of any such liquidating distributions or whether any liquidating distributions will occur at all. The timing of any such liquidating distributions will depend on and could be delayed by, among other things, the timing of sales of our non-cash assets and claim settlements with creditors. Additionally, a creditor could seek an injunction against the making of such distributions to our stockholders on the ground that the amounts to be distributed were needed to provide for the payment of our liabilities and expenses. Any action of this type could delay or substantially diminish the amount available for such distribution to our stockholders.
We will continue to incur claims, liabilities and expenses that will reduce the amount available for distribution out of the liquidation to stockholders.
Claims, liabilities and expenses from operations, such as operating costs, salaries, directors’ and officers’ insurance payroll and local taxes, legal, accounting and consulting fees and miscellaneous office expenses, will
C-24
continue to be incurred as we wind down. These expenses will reduce the amount of assets available for ultimate distribution to stockholders.
If we fail to create an adequate contingency reserve for payment of our expenses and liabilities, each stockholder receiving liquidating distributions could be held liable for payment to our creditors of his, her or its pro rata share of amounts owed to creditors in excess of the contingency reserve, up to the amount actually distributed to such stockholder in dissolution.
If the Plan of Dissolution is approved by our stockholders, we will file a Certificate of Dissolution with the Delaware Secretary of State dissolving Myrexis, Inc. Pursuant to the Delaware General Corporation Law (the “DGCL”), we will continue to exist for three years after the Certificate of Dissolution is filed or for such longer period as the Delaware Court of Chancery shall direct, for the purpose of prosecuting and defending suits against us and enabling us gradually to close our business, to dispose of our property, to discharge our liabilities and to distribute to our stockholders any remaining assets. Under the DGCL, in the event we fail to create during this three-year period an adequate contingency reserve for payment of our expenses and liabilities, each stockholder of record as of the date of the filing of the Certificate of Dissolution, which is referred to hereinafter as the Final Record Date, could be held liable for payment to our creditors of such stockholder’s pro rata share of amounts owed to creditors in excess of the contingency reserve, up to the amount actually distributed to such stockholder in dissolution.
Although the liability of any stockholder is limited to the amounts previously received by such stockholder from us (and from any liquidating trust or trusts) pursuant to the Plan of Dissolution, this means that a stockholder could be required to return all liquidating distributions previously made to such stockholder and receive nothing from us under the Plan of Dissolution. Moreover, in the event a stockholder has paid taxes on amounts previously received, a repayment of all or a portion of such amount could result in a stockholder incurring a net tax cost if the stockholder’s repayment of an amount previously distributed does not cause a commensurate reduction in taxes payable. While we will endeavor to make adequate reserves for all known, contingent, and unknown liabilities, there is no guarantee that the reserves established by us will be adequate to cover all such expenses and liabilities.
No further stockholder approval will be required.
Approval of the Plan of Dissolution and the actions contemplated thereby requires the affirmative vote of a majority of the votes cast at a meeting of stockholders duly called at which a quorum is present. If our stockholders approve the Plan of Dissolution, we will be authorized to cease operations, sell, license or otherwise dispose of our non-cash assets and dissolve the Company and its subsidiaries without further approval of our stockholders, unless required to do so by Delaware law.
We intend to have our common stock delisted from the NASDAQ Global Market and our stock transfer books closed at the close of business on the date we file the Certificate of Dissolution with the Delaware Secretary of State, after which it will not be possible for stockholders to publicly trade our stock.
We will request that our common stock be delisted from the NASDAQ Global Market at the close of business on the date we file the Certificate of Dissolution with the Delaware Secretary of State and intend to close our stock transfer books and discontinue recording transfers of our common stock at that time. Thereafter, certificates representing our common stock will not be assignable or transferable on our books except by will, intestate succession or operation of law. The proportionate interests of all of our stockholders will be fixed on the basis of their respective stock holdings at the close of business on the Final Record Date, and, after the Final Record Date, any distributions made by us will be made solely to the stockholders of record at the close of business on the Final Record Date, except as may be necessary to reflect subsequent transfers recorded on our books as a result of any assignments by will, intestate succession or operation of law. It is possible that the trading of our common stock on the NASDAQ Global Market will effectively terminate before the Final Record Date if we are unable to meet NASDAQ’s requirements for continued listing.
C-25
We will continue to incur the expenses of complying with public company reporting requirements.
We have an obligation to continue to comply with the applicable reporting requirements of the Exchange Act even though compliance with such reporting requirements is economically burdensome. In order to curtail expenses, we intend, after filing our Certificate of Dissolution, to seek relief from the SEC from the reporting requirements under the Exchange Act. However, the SEC may not grant any such relief, in which case we would be required to continue to bear the expense of being a public reporting company until at least the filing of our Annual Report on Form 10-K for the year ending June 30, 2013.
Our Board of Directors may at any time turn management of the liquidation over to a third party, and some or all of our directors may resign from our Board of Directors at any time.
Our Board of Directors may at any time turn our management over to a third party to complete the liquidation of our remaining assets and distribute the available proceeds to our stockholders, and some or all of our directors may resign from our Board of Directors at any time. If management is turned over to a third party and all of our directors resign from our Board of Directors, the third party would have sole control over the liquidation process, including the sale or distribution of any remaining assets.
If we decide to use a liquidating trust, interests of our stockholders in such a trust may not be transferable.
The interests of our stockholders in a liquidating trust set up by us may not be transferable, which could adversely affect your ability to realize the value of such interests. Even if transferable, the interests are not expected to be listed on a national securities exchange, and the extent of any trading market therein cannot be predicted. Moreover, the interests may not be accepted by commercial lenders as security for loans as readily as more conventional securities with established trading markets. In addition, as stockholders will be deemed to have received a liquidating distribution equal to their pro rata share of the value of the net assets distributed to an entity which is treated as a liquidating trust for tax purposes, the distribution of non-transferable interests would result in tax liability to the interest holders without their being readily able to realize the value of such interest to pay such taxes or otherwise.
Stockholders may not be able to recognize a loss for U.S. federal income tax purposes until they receive a final distribution from us.
As a result of our dissolution and liquidation, for U.S. federal income tax purposes, our stockholders generally will recognize gain or loss equal to the difference between (1) the sum of the amount of cash and the fair market value (at the time of distribution) of property, if any, distributed to them, and (2) their tax basis for their shares of our common stock. Liquidating distributions pursuant to the Plan of Dissolution may occur at various times and in more than one tax year. Any loss generally will be recognized by a stockholder only when the stockholder receives our final liquidating distribution to stockholders, and then only if the aggregate value of all liquidating distributions with respect to a share is less than the stockholder's tax basis for that share. Stockholders are urged to consult their own tax advisors as to the specific tax consequences to them of our dissolution and liquidation pursuant to the Plan of Dissolution.
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds. |
None.
| Item 3. | Defaults Upon Senior Securities. |
None.
| Item 4. | Mine Safety Disclosures. |
Not applicable.
C-26
| Item 5. | Other Information. |
None.
| Item 6. | Exhibits. |
| (a) | Exhibits |
| 2.1 | Plan of Complete Liquidation and Dissolution of Myrexis, Inc. Incorporated by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K filed November 9, 2012 (File No. 001-34275). | |
| 10.1 | Retention Bonus Agreement by and between Myrexis, Inc. and Andrea Kendell, entered into effective July 2, 2012. | |
| 31.1 | Certification of principal executive officer under Section 302(a) of the Sarbanes-Oxley Act of 2002. | |
| 31.2 | Certification of principal financial officer under Section 302(a) of the Sarbanes-Oxley Act of 2002. | |
| 32.1 | Certifications of the principal executive officer and the principal financial officer under Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 101* | The following materials from Myrexis, Inc.’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2012, formatted in XBRL (eXtensible Business Reporting Language): (i) the Unaudited Balance Sheets as of September 30, 2012 and June 30, 2012, (ii) the Unaudited Statements of Operations and Comprehensive Loss for the three months ended September 30, 2012 and 2011, (iii) the Unaudited Statements of Cash Flows for the three months ended September 30, 2012 and 2011, and (iv) Notes to Unaudited Financial Statements. | |
| * | Users of the XBRL data are advised pursuant to Rule 406T of Regulation S-T that this interactive data file is deemed not filed or part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, and otherwise is not subject to liability under these sections. |
C-27
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| MYREXIS, INC. | ||||||
| Date: November 9, 2012 | By: | /s/ DAVID W. GRYSKA | ||||
| David W. Gryska | ||||||
| Acting President and Chief Executive Officer, Chief Operating Officer | ||||||
| (principal executive officer) | ||||||
| Date: November 9, 2012 | By: | /s/ ANDREA KENDELL | ||||
| Andrea Kendell | ||||||
| Chief Financial Officer (principal accounting and financial officer) | ||||||
C-28
SPECIAL MEETING OF STOCKHOLDERS OF
MYREXIS, INC.
January 23, 2013
| PROXY VOTING INSTRUCTIONS |
INTERNET - Access “www.voteproxy.com” and follow the on-screen instructions. Have your proxy card available when you access the web page, and use the Company Number and Account Number shown on your proxy card.
TELEPHONE - Call toll-free 1-800-PROXIES (1-800-776-9437) in the United States or 1-718-921-8500 from foreign countries from any touch-tone telephone and follow the instructions. Have your proxy card available when you call and use the Company Number and Account Number shown on your proxy card.
Vote online/phone until 11:59 PM ET the day before the meeting.
MAIL - Sign, date and mail your proxy card in the envelope provided as soon as possible.
IN PERSON - You may vote your shares in person by attending the Special Meeting.
| COMPANY NUMBER | ||
| ACCOUNT NUMBER | ||
Important Notice Regarding the Availability of Proxy Materials for the Special Meeting:
The Notice and Proxy Statement are available at http://www.amstock.com/ProxyServices/ViewMaterial.asp?CoNumber=16182
i Please detach along perforated line and mail in the envelope provided IF you are not voting via telephone or the
Internet. i
| THE BOARD OF DIRECTORS RECOMMENDS A VOTE FOR PROPOSALS 1, 2 AND 3. PLEASE SIGN, DATE AND RETURN PROMPTLY IN THE ENCLOSED ENVELOPE. PLEASE MARK YOUR VOTE IN BLUE OR BLACK INK AS SHOWN HERE x | ||||||||||||
|
FOR |
AGAINST |
ABSTAIN | ||||||||||
| THE SHARES REPRESENTED BY THIS PROXY WILL BE VOTED IN THE MANNER DIRECTED AND, IF NO INSTRUCTIONS TO THE CONTRARY ARE INDICATED, WILL BE VOTED “FOR” PROPOSALS 1, 2 AND 3. IN THEIR DISCRETION, THE PROXIES ARE AUTHORIZED TO VOTE UPON SUCH OTHER MATTERS AS MAY PROPERLY COME BEFORE THE MEETING OR ANY ADJOURNMENTS OR POSTPONEMENTS OF THE MEETING. | 1. | To approve the dissolution and liquidation of Myrexis, Inc. pursuant to the Plan of Complete Liquidation and Dissolution, as described in the proxy statement. | ¨ | ¨ | ¨ | |||||||
| THE UNDERSIGNED HEREBY ACKNOWLEDGES RECEIPT OF THE | 2. | To grant discretionary authority to the Board of Directors to adjourn the Special | ¨ | ¨ | ¨ | |||||||
| NOTICE OF SPECIAL MEETING OF STOCKHOLDERS AND THE PROXY STATEMENT FURNISHED HEREWITH. | Meeting, even if a quorum is present, to solicit additional proxies in the event that there are insufficient shares present in person or by proxy voting in favor of Proposal 1. | |||||||||||
| PLEASE MARK, SIGN, DATE AND PROMPTLY RETURN THIS PROXY CARD USING THE ENCLOSED ENVELOPE. YOU MAY REVOKE THIS PROXY AT ANY TIME PRIOR TO THE TAKING OF A VOTE ON THE MATTERS HEREIN.
|
3. | To approve, by non-binding, advisory vote, certain compensation arrangements for certain executive officers in connection with the dissolution and liquidation of Myrexis, Inc., as described in the proxy statement. | ¨ | ¨ | ¨ | |||||||
|
|
||||||||||||
| To change the address on your account, please check the box at right and indicate your new address in the address space above. Please note that changes to the registered name(s) on the account may not be submitted via this method. | ¨
|
|||||||||||
| Signature of Stockholder |
Date: | Signature of Stockholder |
Date: | |||||||||||||||
| Note:
|
Please sign exactly as your name or names appear on this Proxy. When shares are held jointly, each holder should sign. When signing as executor, administrator, attorney, trustee or guardian, please give full title as such. If the signer is a corporation, please sign full corporate name by duly authorized officer, giving full title as such. If signer is a partnership, please sign in partnership name by authorized person.
|
|||||||||||||||
MYREXIS, INC.
THIS PROXY IS SOLICITED BY THE BOARD OF DIRECTORS FOR THE SPECIAL MEETING
OF STOCKHOLDERS TO BE HELD ON WEDNESDAY, JANUARY 23, 2013
As an alternative to completing this form, you may enter your vote instruction by telephone at 1-800-PROXIES, or via the Internet at WWW.VOTEPROXY.COM and follow the simple instructions. Use the Company Number and Account Number shown on your proxy card.
The undersigned, revoking any previous proxies relating to these shares, hereby appoints Gerald P. Belle and Andrea Kendell, and each of them (with full power to act alone), proxies, with full power of substitution, to vote all shares of common stock of Myrexis, Inc., a Delaware corporation (the “Company”), registered in the name provided herein which the undersigned is entitled to vote at the Special Meeting of Stockholders of the Company to be held at 305 Chipeta Way, Salt Lake City, Utah, on Wednesday, January 23, 2013, at 9:00 a.m., local time, and at any and all adjournments or postponements thereof.
(Continued and to be signed on the reverse side)