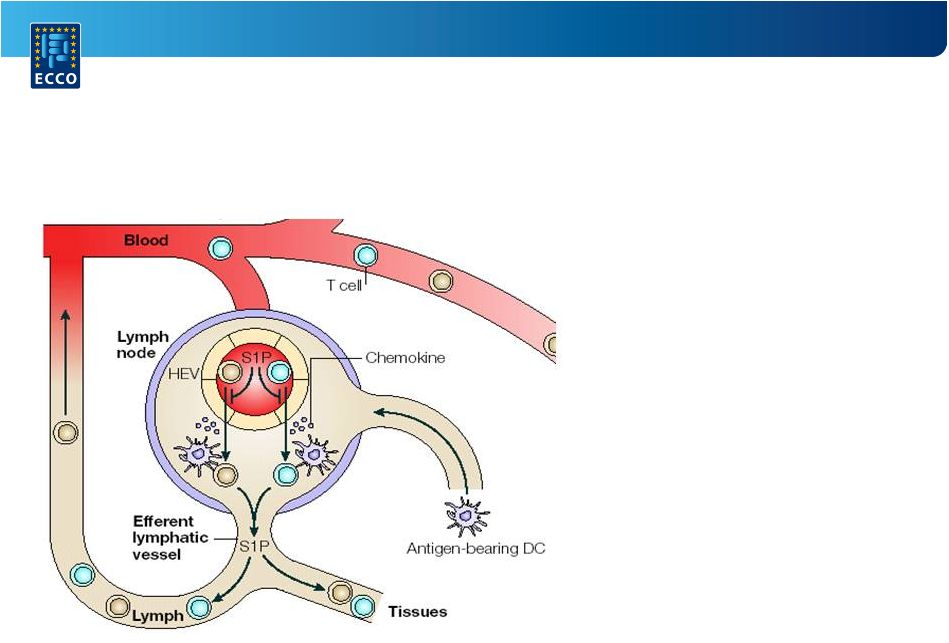
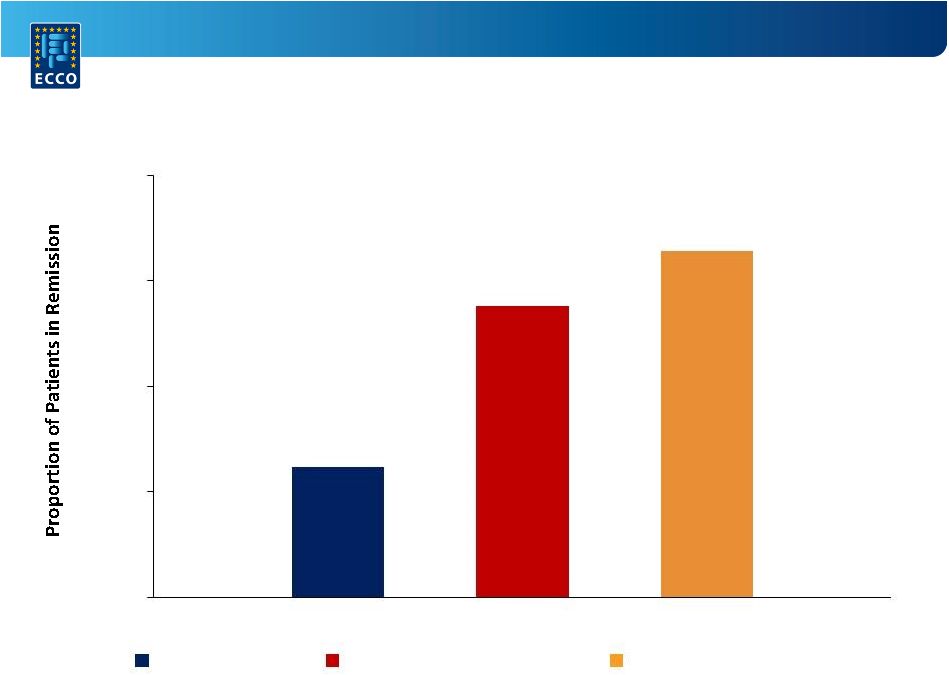
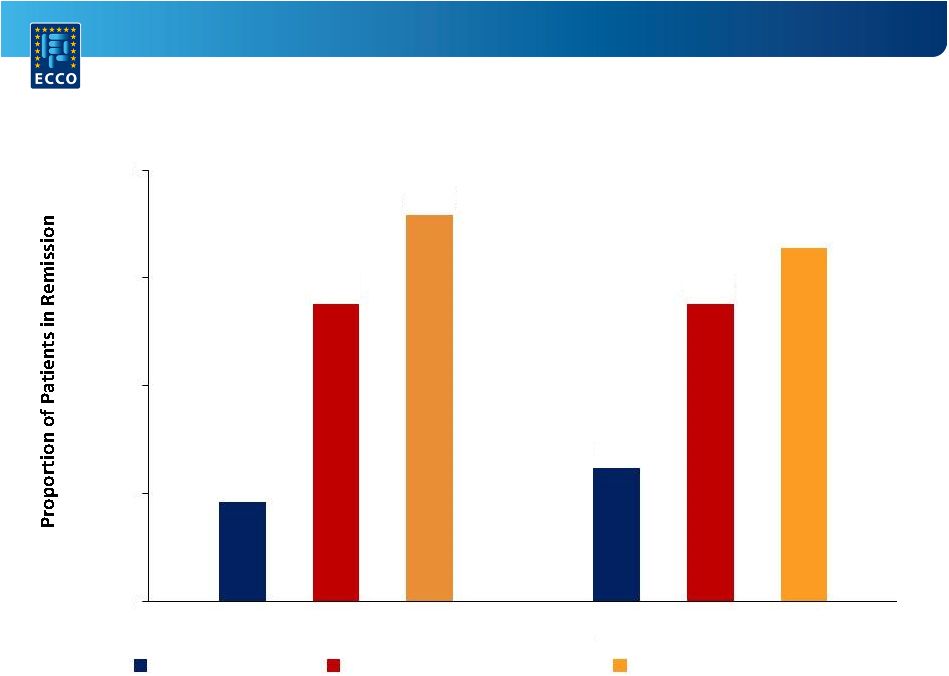
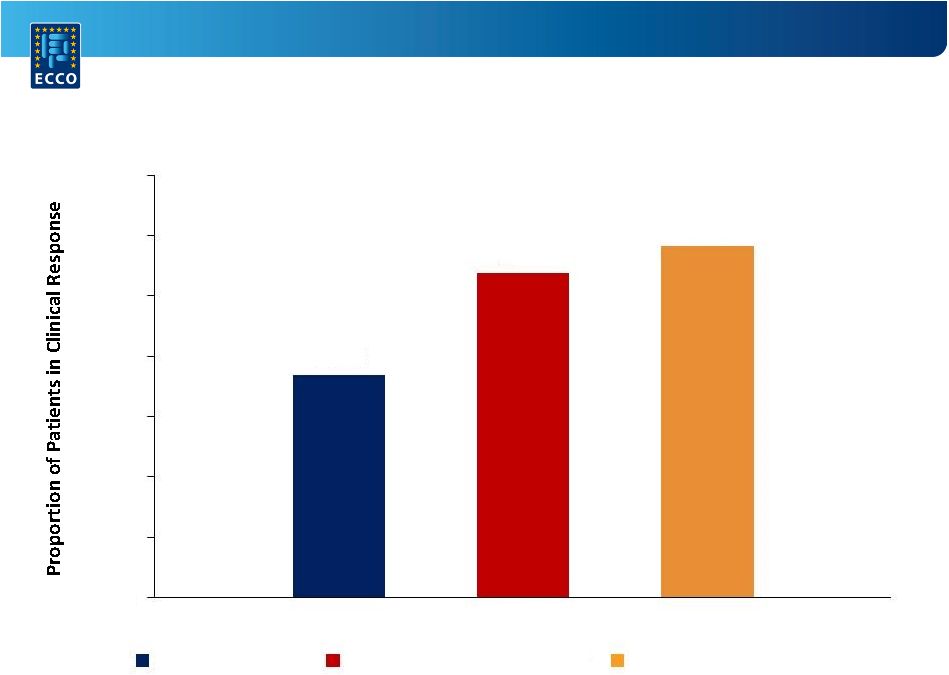
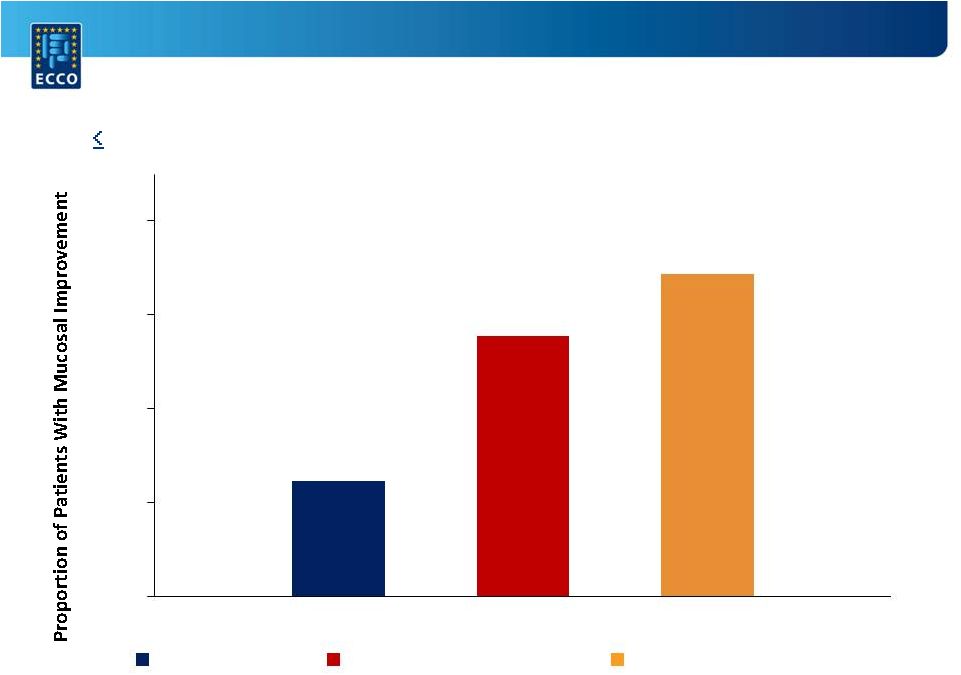
A Randomized,
Double-Blind, Placebo-Controlled Induction Trial of an Oral S1P Receptor
Modulator Ozanimod (RPC1063) in Moderate to Severe Ulcerative Colitis:
Results of the TOUCHSTONE Study
William Sandborn, MD
21 February 2015
Barcelona, Spain
W.
Sandborn¹,
B.
Feagan²,
D.
Wolf³,
G.
D'Haens
4
,
S.
Vermeire
5
,
S.
Hanauer
6
,
S. Ghosh
7
, H. Smith
8
, M. Cravets
8
, P. Frohna
8
, S. Gujrathi
8
, A. Olson
8
1
University of California San Diego, Division of Gastroenterology, La Jolla, United
States 2
University of Western Ontario, Department of Gastroenterology, London, Canada
3
Atlanta Gastroenterology Associates, Emory Saint Joseph's, Atlanta, United States
4
Academic Medical Centre, Inflammatory Bowel Disease Centre, Amsterdam, Netherlands
5
University Hospital Leuven, Department of Gastroenterology, Leuven, Belgium
6
Northwestern University Feinberg School of Medicine, Digestive Health Center, Chicago, United
States 7
University of Calgary, Department of Gastroenterology, Alberta, Canada
8
Receptos, Inc., Clinical Development, San Diego, United States
|