

Corporate Presentation Transformative Targeted Therapeutics December 2025 .2

Disclosures For purposes of this notice, the “presentation” that follows shall mean and include the slides that follow, any oral presentation of the slides by members of management of Immunome, Inc. (“Immunome”) or any person on its behalf, any question-and-answer session that follows that oral presentation, hard copies of this document and any materials distributed at, or in connection with, that presentation. References to “we,” “our,” and “us” refers to Immunome and its subsidiaries. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy or sell Immunome securities. Forward-Looking Statements Statements in this presentation that are not purely historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We use words such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “vision,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “promising,” “projected,” “first step,” “ongoing,” or the negative of these terms, and similar words or expressions to identify these forward-looking statements. These forward-looking statements include, but are not limited to, statements about: the expansion and advancement of Immunome’s platform and pipeline and Immunome’s approach; the expected benefits of Immunome’s proprietary payload, HC74, and strategy related to Immunome’s platform and pipeline; assessments of the clinical efficacy, best-in-class potential, and potential commercial success of Immunome’s product candidates, including IM-1021 and varegacestat; the potential of Immunome’s current and future pipeline to produce first-in-class and/or best-in-class drugs; Immunome’s expectations with respect to future performance, anticipated financial impacts, ability to complete and success of Immunome’s strategic transactions; Immunome’s timeline for filing an NDA, INDs and other regulatory filings, commencing clinical trials, receiving and reporting data from such clinical trials, and seeking regulatory approval, for Immunome’s current and future programs and product candidates and other anticipated milestones; the best-in-class potential of varegacestat and its ability to become the new standard of care for treating desmoid tumors; Immunome’s launch strategy and the expected results therefrom; Immunome’s ability to expand its platform, establish a broad pipeline and advance it through efficient clinical development decisions; and other statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future. These forward-looking statements are based on Immunome’s current expectations and involve assumptions that may never materialize or may prove to be incorrect; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors: the RINGSIDE topline results are based on a preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data and such topline data may not accurately reflect the complete results of the trial; the risk that Immunome’s NDA submission for varegacestat is delayed based on regulatory feedback or otherwise, and that regulatory approvals for Immunome’s programs and product candidates are not obtained, are delayed or are subject to unanticipated conditions, including the risk that the results of Immunome’s trials for varegacestat may not be deemed sufficient by the FDA to serve as the basis for an NDA submission or regulatory approval of varegacestat; the risks associated with the potential safety and other complications from varegacestat; the labelling for varegacestat, if approved; the scope, progress and expansion of developing and commercializing varegacestat, if approved; the size and growth of the market for varegacestat and the rate and degree of market acceptance thereof; the risk that Immunome will not be able to realize the benefits of its strategic transactions; the risk that regulatory approvals for Immunome’s programs and product candidates are not obtained, are delayed or are subject to unanticipated conditions; the risk that preclinical or early clinical data may not be predictive of future results; the risk that Immunome’s product candidates and development candidates fail to achieve their intended endpoints; the reliance on Immunome’s management; the prior experience and successes of Immunome’s management team not being indicative of any future success; the risk of reliance on vendors; uncertainties related to Immunome’s capital requirements and Immunome’s expected cash runway; Immunome’s ability to grow and successfully execute on Immunome’s business plan, including the development and commercialization of its pipeline and integration of newly acquired assets; and other risks and uncertainties indicated from time to time described in Immunome’s Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 19, 2025, and in Immunome’s other filings with the SEC. Except as required by law, Immunome assumes no obligation and does not intend to update any forward-looking statements included in this presentation. Product Candidates In this presentation, we may discuss current and potential future product candidates that have not yet undergone clinical trials or been approved for marketing by the U.S. Food and Drug Administration or other governmental authority. No representation is made as to the safety or effectiveness of these current or potential future product candidates for the use for which such product candidates are being studied. Industry and Market Data In this presentation, we rely on and refer to publicly available information and statistics regarding market participants in the sectors in which we compete and other industry data. Any comparison of us to the industry or to any of our competitors is based on this publicly available information and statistics and such comparisons assume the reliability of the information available to us. We obtained this information and statistics from third-party sources, including reports by market research firms and company filings. While we believe such third-party information is reliable, there can be no assurance as to the accuracy or completeness of the indicated information. We have not independently verified the information provided by the third-party sources. Trademarks This presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this presentation may be listed without the TM, SM © or ® symbols, but we will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade names and copyrights. Disclaimer and Forward-Looking Statements

Establishing a Premier Targeted Oncology Company i Varegacestat, an oral, once-daily gamma secretase inhibitor for the treatment of desmoid tumors, with positive Phase 3 topline data NDA submission planned for 2Q 2026 Positioned to be a best-in-class option Deep expertise in ADCs driving differentiated early-stage pipeline leveraging proprietary HC74 TOP1i payload ROR1 ADC in dose escalation with activity observed at multiple dose levels Pairing first-in-class targets with exceptional technology Positioned to deliver multiple INDs per year driven by a team that combines deep expertise and decisive execution Complementary radioligand therapy entering Phase 1 Experienced, successful leadership team Delivering Breakthroughs In Oncology
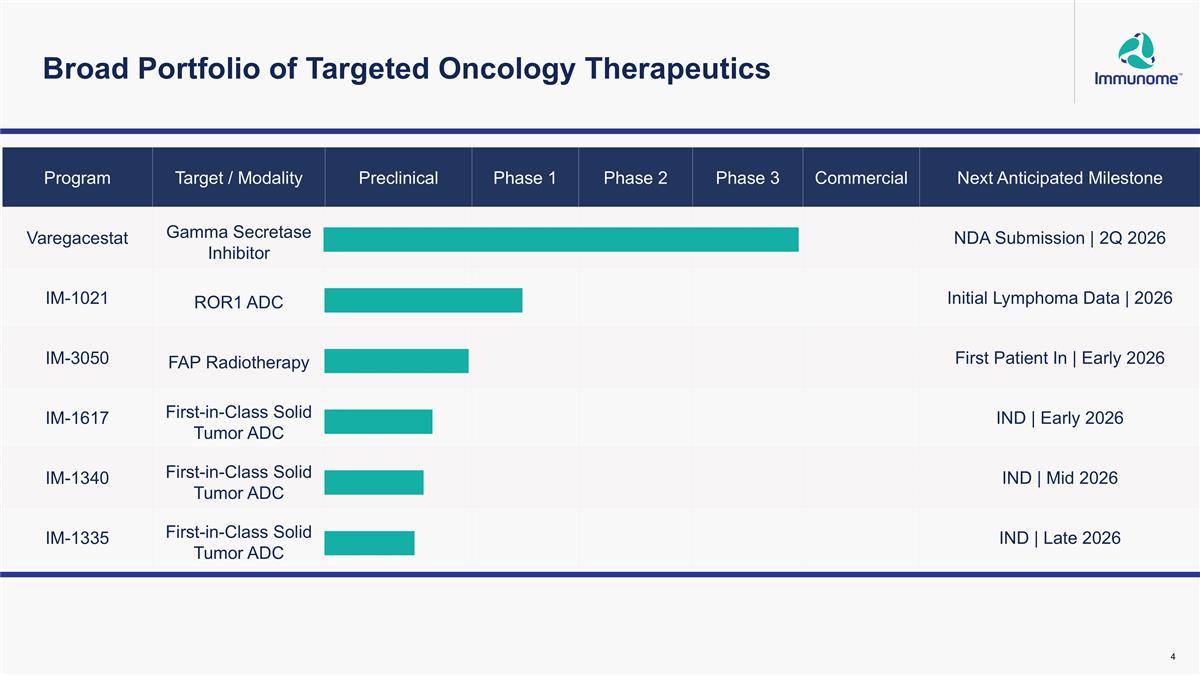
Broad Portfolio of Targeted Oncology Therapeutics Program Target / Modality Preclinical Phase 1 Phase 2 Phase 3 Commercial Next Anticipated Milestone Varegacestat Gamma Secretase Inhibitor NDA Submission | 2Q 2026 IM-1021 ROR1 ADC Initial Lymphoma Data | 2026 IM-3050 FAP Radiotherapy First Patient In | Early 2026 IM-1617 First-in-Class Solid Tumor ADC IND | Early 2026 IM-1340 First-in-Class Solid Tumor ADC IND | Mid 2026 IM-1335 First-in-Class Solid Tumor ADC IND | Late 2026

Varegacestat Phase 3 Oral, Once-Daily Gamma Secretase Inhibitor
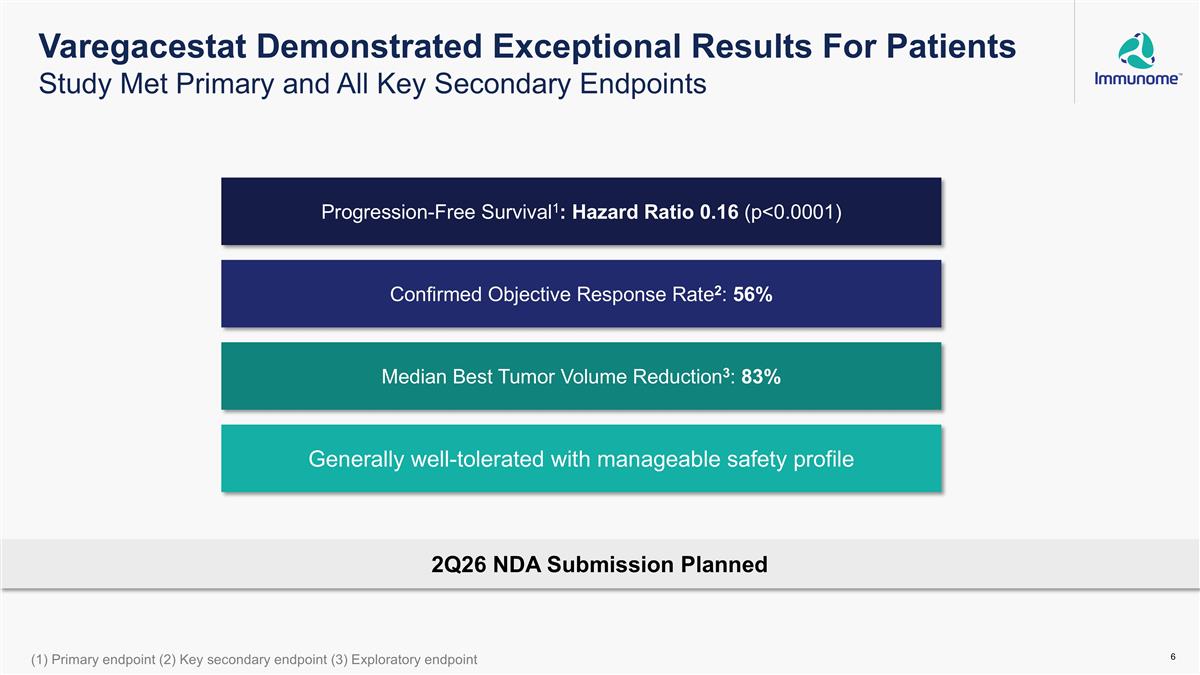
Generally well-tolerated with manageable safety profile Confirmed Objective Response Rate2: 56% Progression-Free Survival1: Hazard Ratio 0.16 (p<0.0001) Median Best Tumor Volume Reduction3: 83% (1) Primary endpoint (2) Key secondary endpoint (3) Exploratory endpoint 2Q26 NDA Submission Planned Varegacestat Demonstrated Exceptional Results For Patients Study Met Primary and All Key Secondary Endpoints

Desmoid tumors often strike in young adulthood, with 1,000-1,650 patients diagnosed each year and 10-11,000 actively managed patients in the US1 Can lead to debilitating pain, deformity, and life-threatening organ damage depending on location1 Quality of life is a major challenge with a majority of patients experiencing chronic pain that can significantly limit physical functioning1 Up to ~60-80% of patients experience recurrence, which can be exacerbated by surgery1 Following progression during initial active surveillance, systemic therapy is recommended for ~75% of tumors based on location2,3 Approved systemic therapy options remain limited with only one approved therapy Desmoid Tumors are Locally Aggressive & Debilitating Sources: (1) Bektas et al., Advances in Therapy, 2023; (2) The Desmoid Tumor Working Group, European Journal of Cancer, 2020; (3) The Desmoid Tumor Working Group, JAMA Oncology, 2024
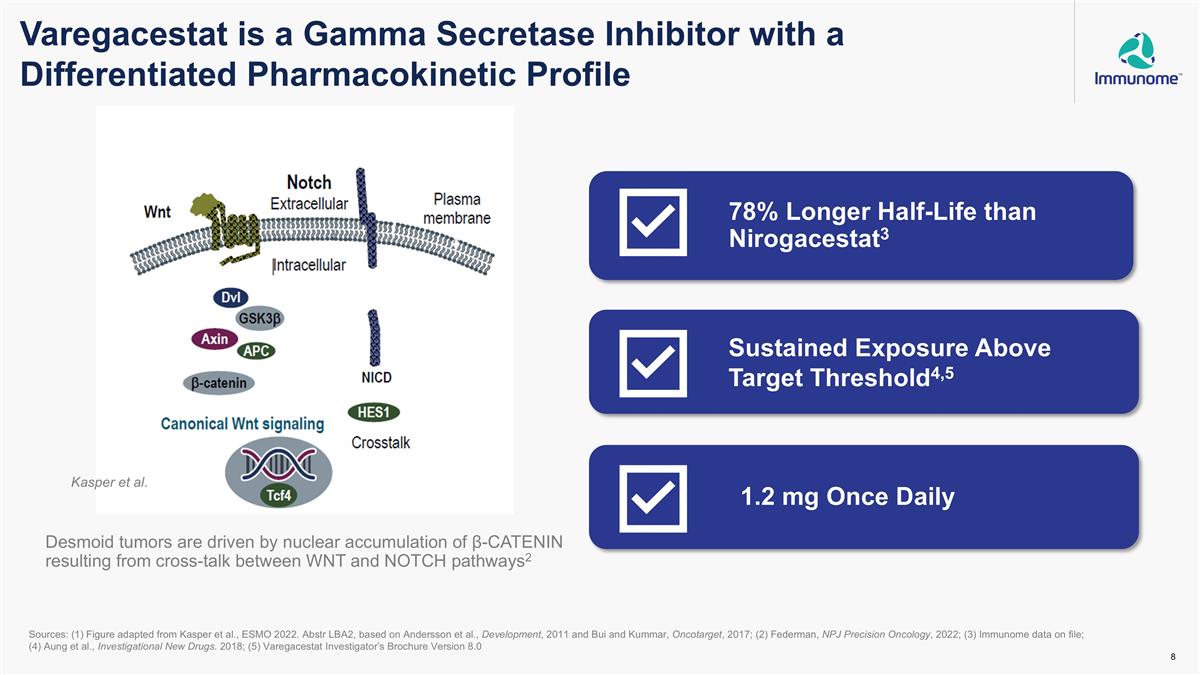
Varegacestat is a Gamma Secretase Inhibitor with a Differentiated Pharmacokinetic Profile Desmoid tumors are driven by nuclear accumulation of β-CATENIN resulting from cross-talk between WNT and NOTCH pathways2 Kasper et al. Sources: (1) Figure adapted from Kasper et al., ESMO 2022. Abstr LBA2, based on Andersson et al., Development, 2011 and Bui and Kummar, Oncotarget, 2017; (2) Federman, NPJ Precision Oncology, 2022; (3) Immunome data on file; (4) Aung et al., Investigational New Drugs. 2018; (5) Varegacestat Investigator’s Brochure Version 8.0 78% Longer Half-Life than Nirogacestat3 Sustained Exposure Above Target Threshold4,5 1.2 mg Once Daily
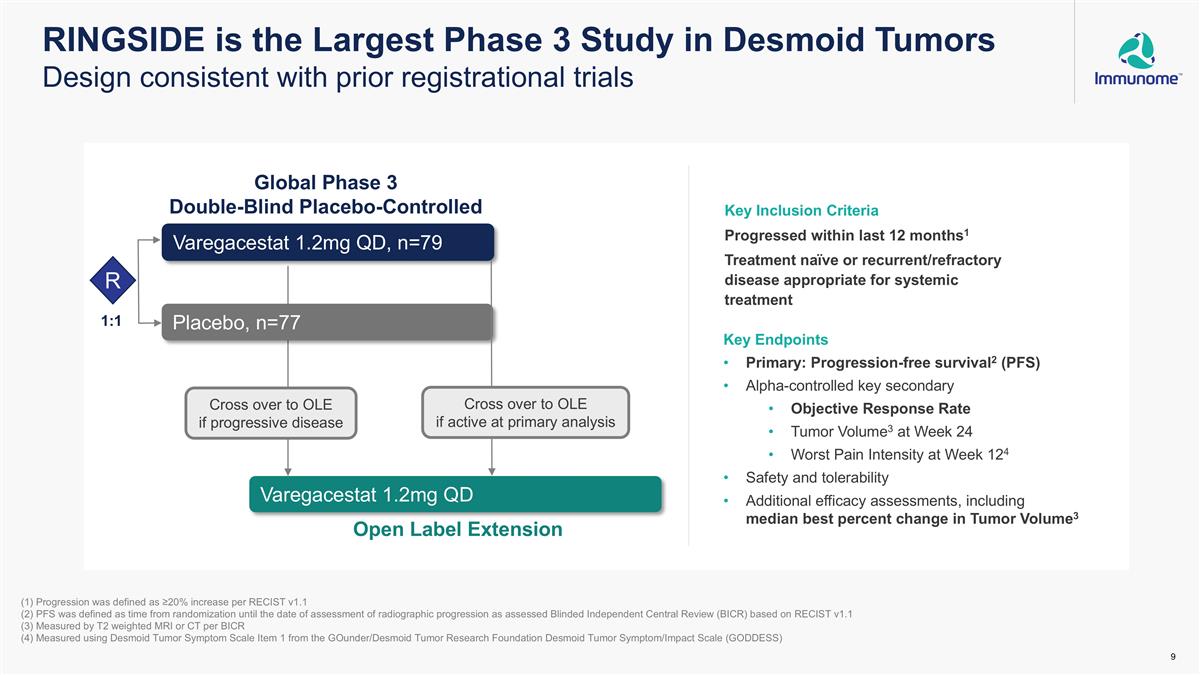
Key Inclusion Criteria Progressed within last 12 months1 Treatment naïve or recurrent/refractory disease appropriate for systemic treatment RINGSIDE is the Largest Phase 3 Study in Desmoid Tumors Design consistent with prior registrational trials Key Endpoints Primary: Progression-free survival2 (PFS) Alpha-controlled key secondary Objective Response Rate Tumor Volume3 at Week 24 Worst Pain Intensity at Week 124 Safety and tolerability Additional efficacy assessments, including median best percent change in Tumor Volume3 (1) Progression was defined as ≥20% increase per RECIST v1.1 (2) PFS was defined as time from randomization until the date of assessment of radiographic progression as assessed Blinded Independent Central Review (BICR) based on RECIST v1.1 (3) Measured by T2 weighted MRI or CT per BICR (4) Measured using Desmoid Tumor Symptom Scale Item 1 from the GOunder/Desmoid Tumor Research Foundation Desmoid Tumor Symptom/Impact Scale (GODDESS) Global Phase 3 Double-Blind Placebo-Controlled R Varegacestat 1.2mg QD, n=79 Placebo, n=77 Varegacestat 1.2mg QD Open Label Extension Cross over to OLE if progressive disease Cross over to OLE if active at primary analysis 1:1
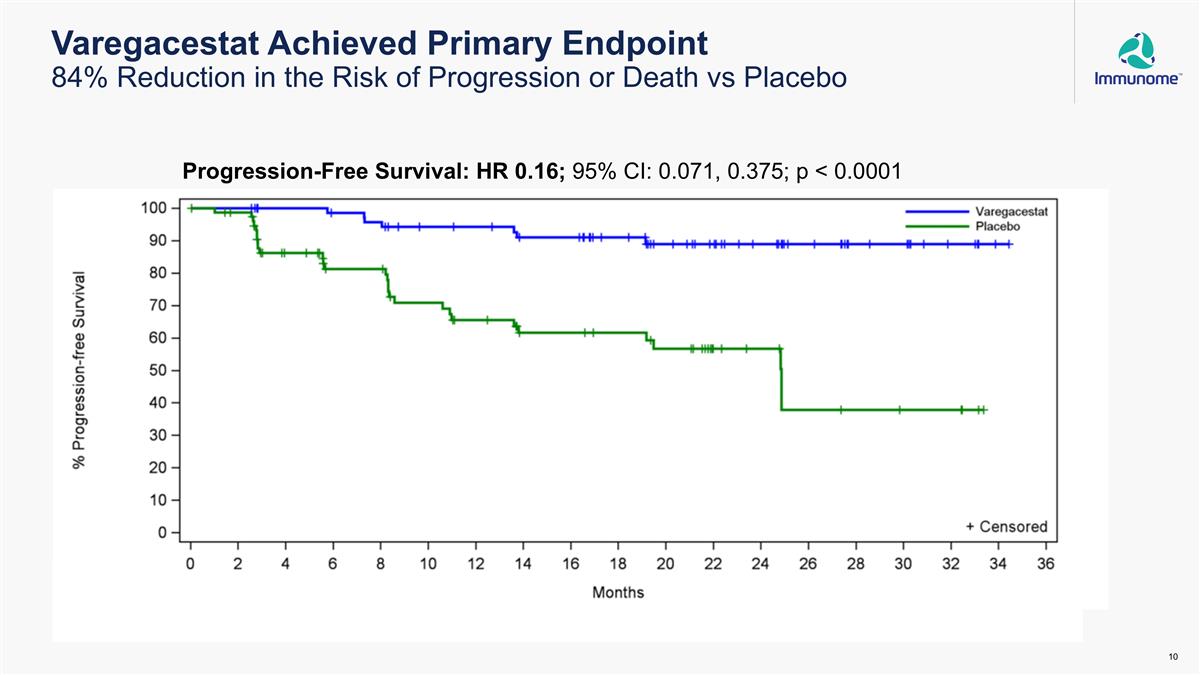
Varegacestat Achieved Primary Endpoint 84% Reduction in the Risk of Progression or Death vs Placebo Progression-Free Survival: HR 0.16; 95% CI: 0.071, 0.375; p < 0.0001
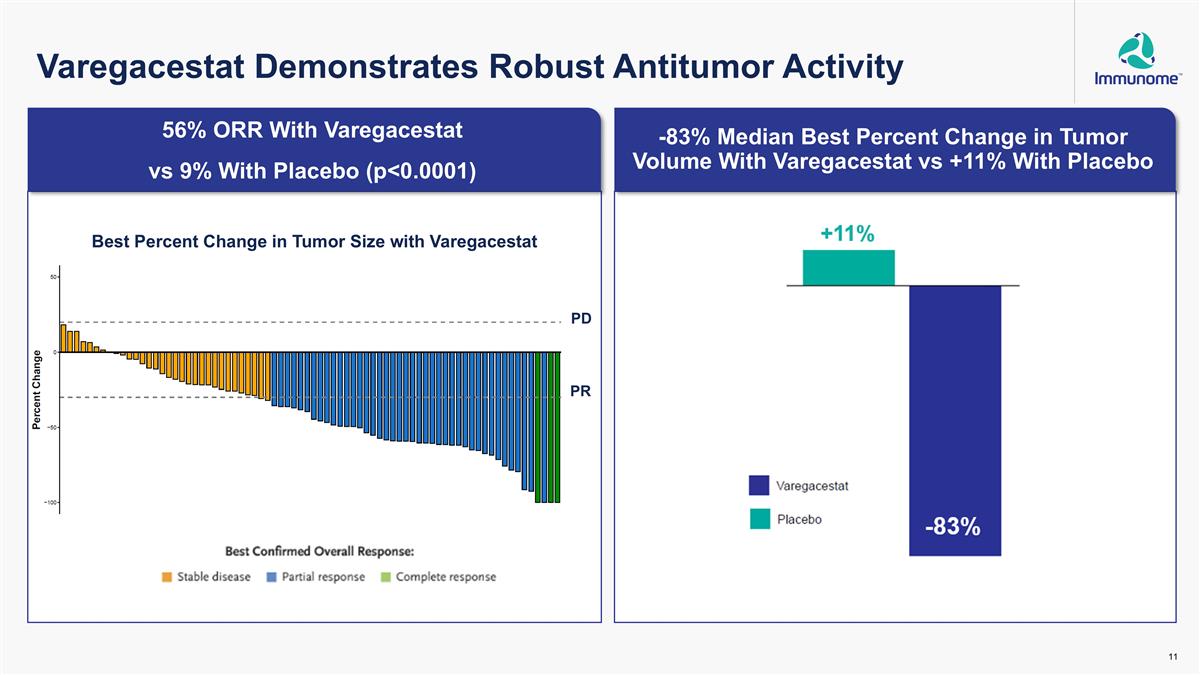
Par 56% ORR With Varegacestat vs 9% With Placebo (p<0.0001) -83% Median Best Percent Change in Tumor Volume With Varegacestat vs +11% With Placebo -83% Best Percent Change in Tumor Size with Varegacestat PR Varegacestat Demonstrates Robust Antitumor Activity PD

Varegacestat was generally well-tolerated, with a manageable safety profile consistent with the GSI class of medicines The most common adverse events for participants in the treatment arm were diarrhea (82%), fatigue (44%), rash (43%), nausea (35%) and cough (34%) Most events were grade 1 or 2 55.6% of premenopausal women experienced ovarian toxicity There were no deaths on study Varegacestat Was Generally Well-tolerated With a Manageable Safety Profile
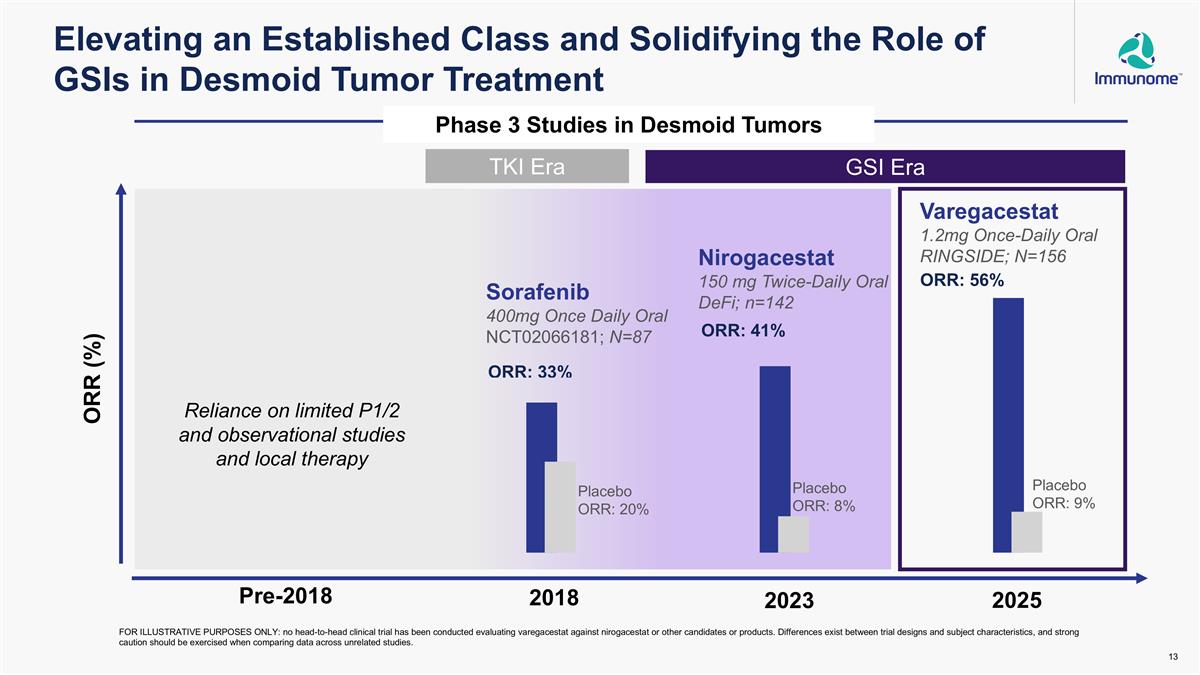
Elevating an Established Class and Solidifying the Role of GSIs in Desmoid Tumor Treatment ORR (%) 2018 2023 2025 Sorafenib 400mg Once Daily Oral NCT02066181; N=87 Nirogacestat 150 mg Twice-Daily Oral DeFi; n=142 Varegacestat 1.2mg Once-Daily Oral RINGSIDE; N=156 TKI Era GSI Era Phase 3 Studies in Desmoid Tumors Reliance on limited P1/2 and observational studies and local therapy FOR ILLUSTRATIVE PURPOSES ONLY: no head-to-head clinical trial has been conducted evaluating varegacestat against nirogacestat or other candidates or products. Differences exist between trial designs and subject characteristics, and strong caution should be exercised when comparing data across unrelated studies. Placebo ORR: 9% Pre-2018
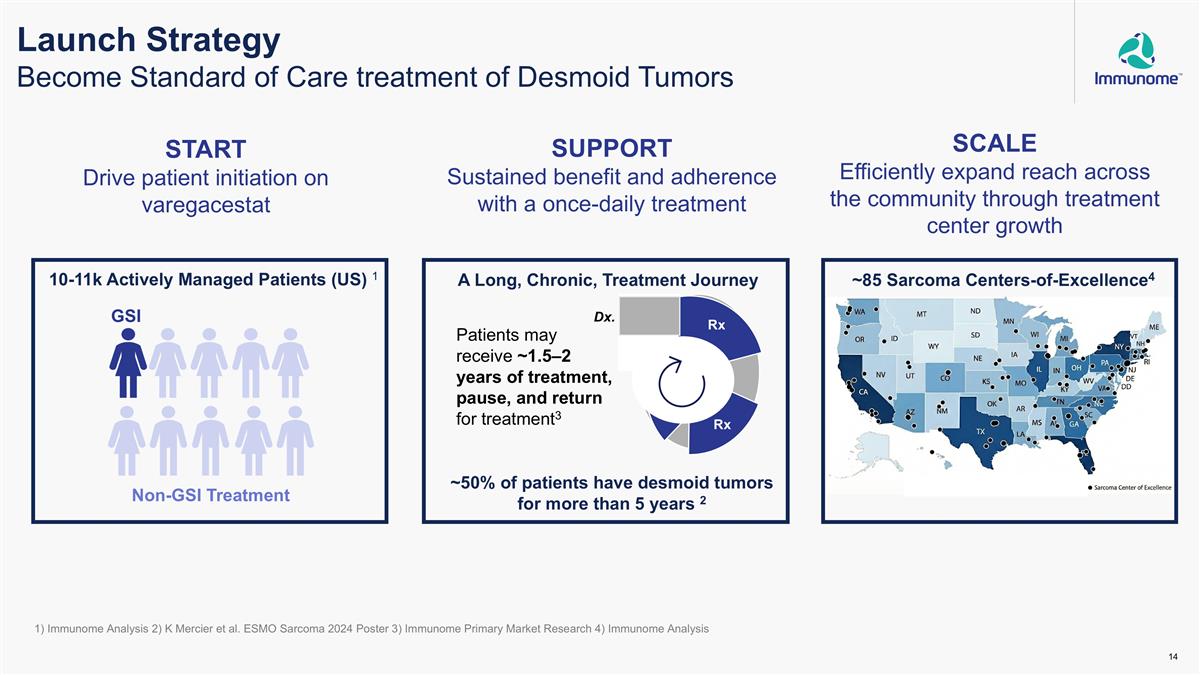
Launch Strategy Become Standard of Care treatment of Desmoid Tumors START Drive patient initiation on varegacestat SUPPORT Sustained benefit and adherence with a once-daily treatment SCALE Efficiently expand reach across the community through treatment center growth GSI Non-GSI Treatment 10-11k Actively Managed Patients (US) 1 ~85 Sarcoma Centers-of-Excellence4 ~50% of patients have desmoid tumors for more than 5 years 2 A Long, Chronic, Treatment Journey 1) Immunome Analysis 2) K Mercier et al. ESMO Sarcoma 2024 Poster 3) Immunome Primary Market Research 4) Immunome Analysis Dx. Rx Rx Patients may receive ~1.5–2 years of treatment, pause, and return for treatment3

IM 1021 ROR1 ADC
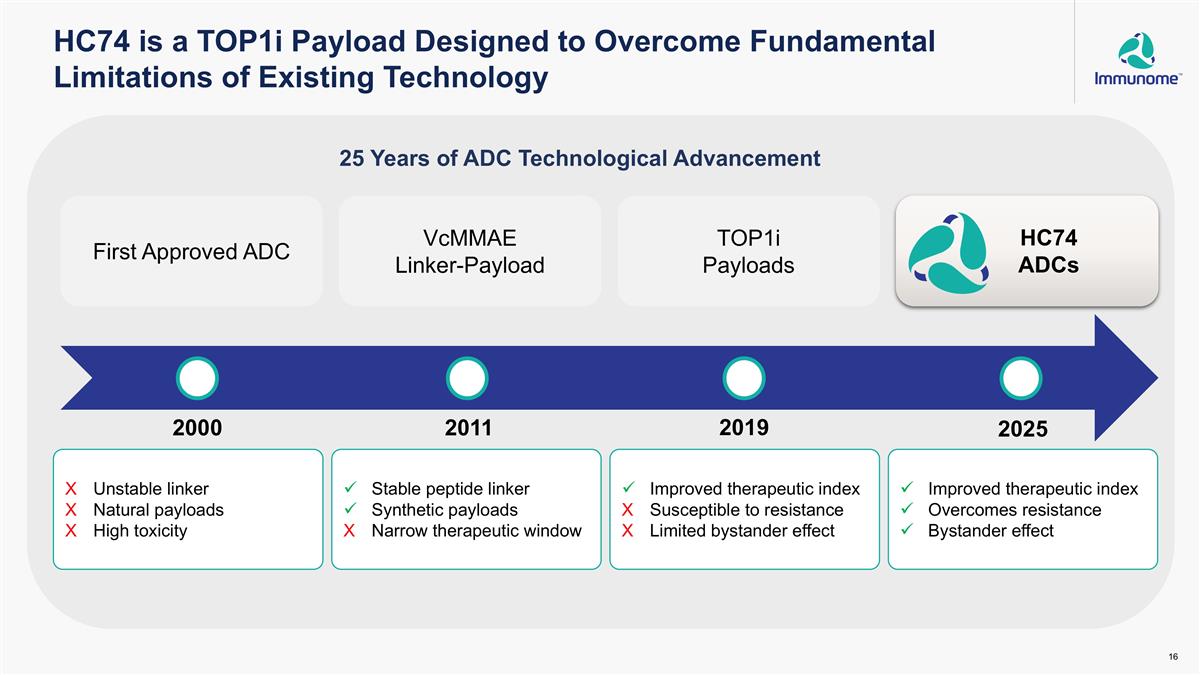
HC74 is a TOP1i Payload Designed to Overcome Fundamental Limitations of Existing Technology Unstable linker Natural payloads High toxicity Stable peptide linker Synthetic payloads Narrow therapeutic window Improved therapeutic index Susceptible to resistance Limited bystander effect Improved therapeutic index Overcomes resistance Bystander effect 25 Years of ADC Technological Advancement HC74 ADCs 2000 2011 2019 2025 First Approved ADC VcMMAE Linker-Payload TOP1i Payloads
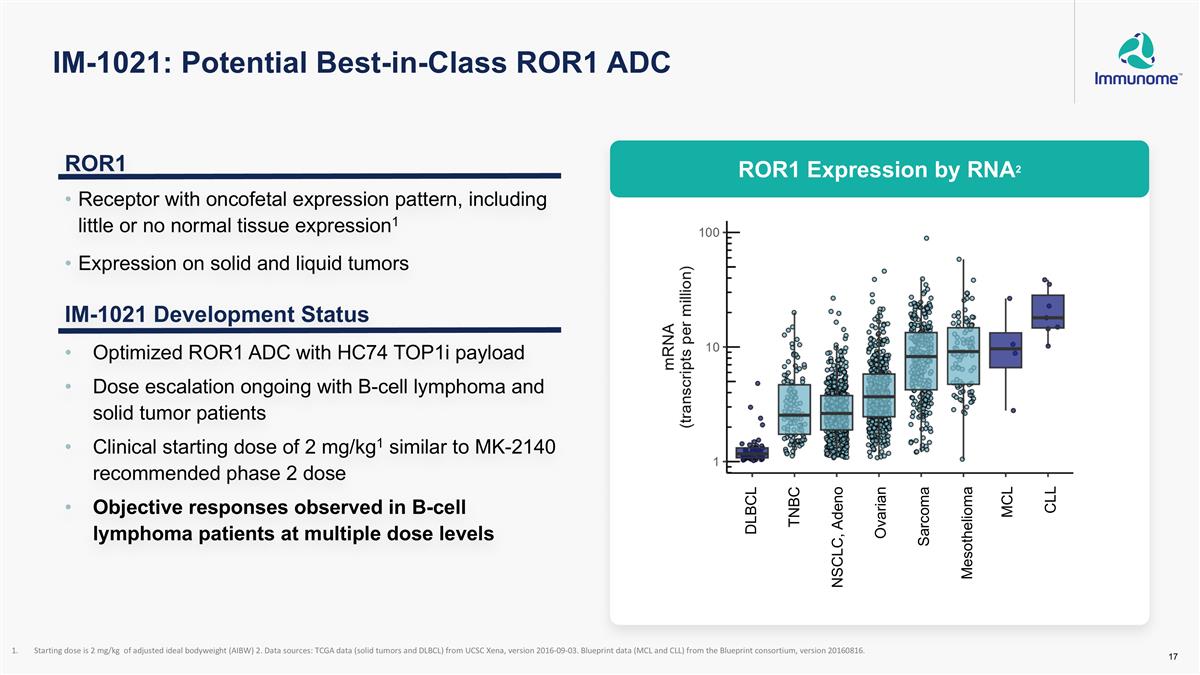
IM-1021: Potential Best-in-Class ROR1 ADC Starting dose is 2 mg/kg of adjusted ideal bodyweight (AIBW) 2. Data sources: TCGA data (solid tumors and DLBCL) from UCSC Xena, version 2016-09-03. Blueprint data (MCL and CLL) from the Blueprint consortium, version 20160816. ROR1 Receptor with oncofetal expression pattern, including little or no normal tissue expression1 Expression on solid and liquid tumors IM-1021 Development Status Optimized ROR1 ADC with HC74 TOP1i payload Dose escalation ongoing with B-cell lymphoma and solid tumor patients Clinical starting dose of 2 mg/kg1 similar to MK-2140 recommended phase 2 dose Objective responses observed in B-cell lymphoma patients at multiple dose levels ROR1 Expression by RNA2 DLBCL TNBC NSCLC, Adeno Ovarian Sarcoma Mesothelioma MCL CLL ROR1 Expression by RNA2
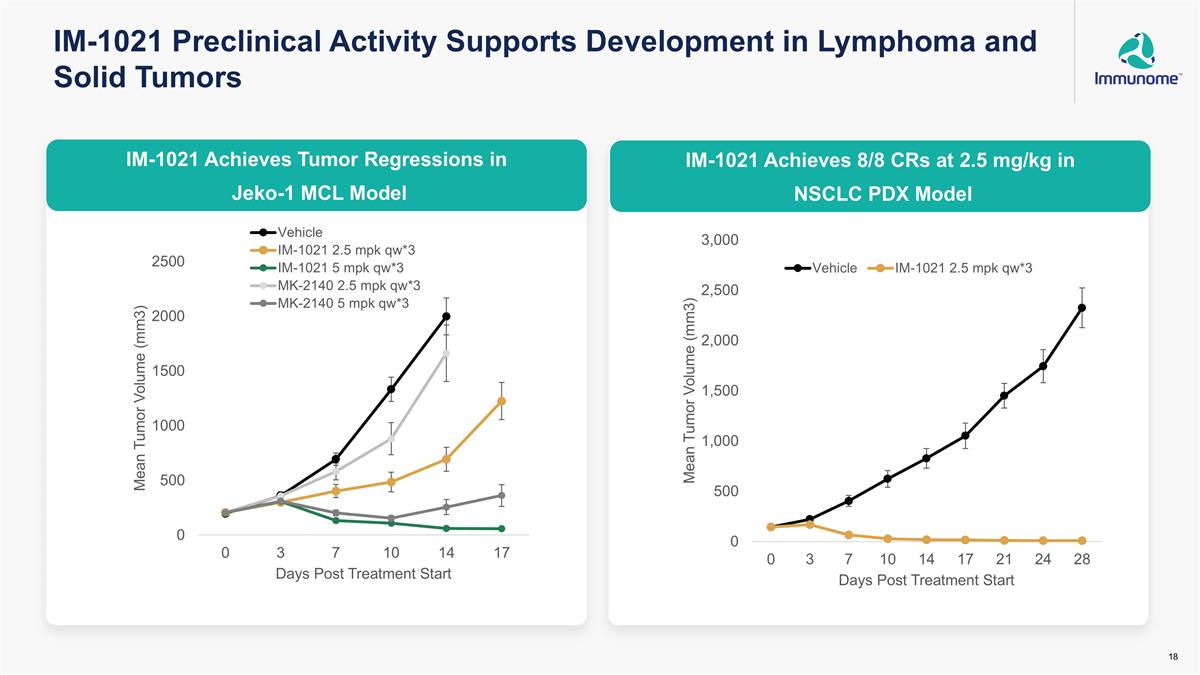
IM-1021 Preclinical Activity Supports Development in Lymphoma and Solid Tumors IM-1021 Achieves Tumor Regressions in Jeko-1 MCL Model IM-1021 Achieves 8/8 CRs at 2.5 mg/kg in NSCLC PDX Model
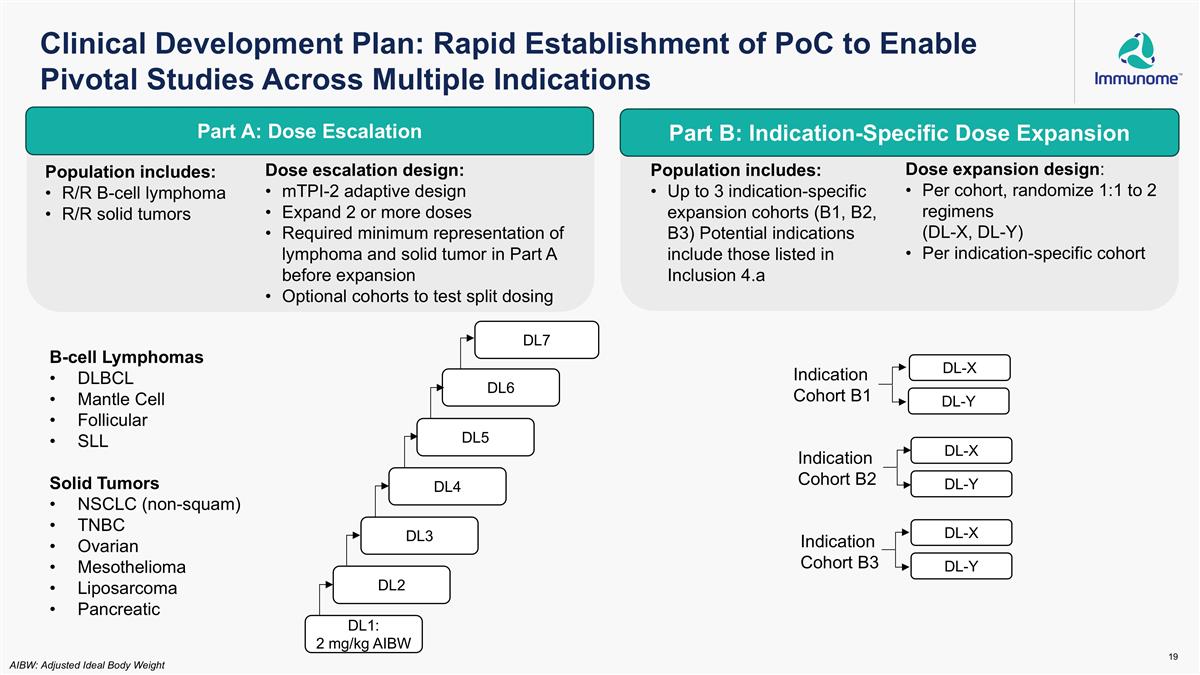
Clinical Development Plan: Rapid Establishment of PoC to Enable Pivotal Studies Across Multiple Indications DL1: 2 mg/kg AIBW DL2 DL3 DL4 DL5 Part A: Dose Escalation Part B: Indication-Specific Dose Expansion DL-Y DL-X Indication Cohort B1 DL-Y DL-X DL-Y DL-X Indication Cohort B2 Indication Cohort B3 AIBW: Adjusted Ideal Body Weight DL6 DL7 B-cell Lymphomas DLBCL Mantle Cell Follicular SLL Solid Tumors NSCLC (non-squam) TNBC Ovarian Mesothelioma Liposarcoma Pancreatic Dose escalation design: mTPI-2 adaptive design Expand 2 or more doses Required minimum representation of lymphoma and solid tumor in Part A before expansion Optional cohorts to test split dosing Population includes: R/R B-cell lymphoma R/R solid tumors Population includes: Up to 3 indication-specific expansion cohorts (B1, B2, B3) Potential indications include those listed in Inclusion 4.a Dose expansion design: Per cohort, randomize 1:1 to 2 regimens (DL-X, DL-Y) Per indication-specific cohort

HC74 Platform
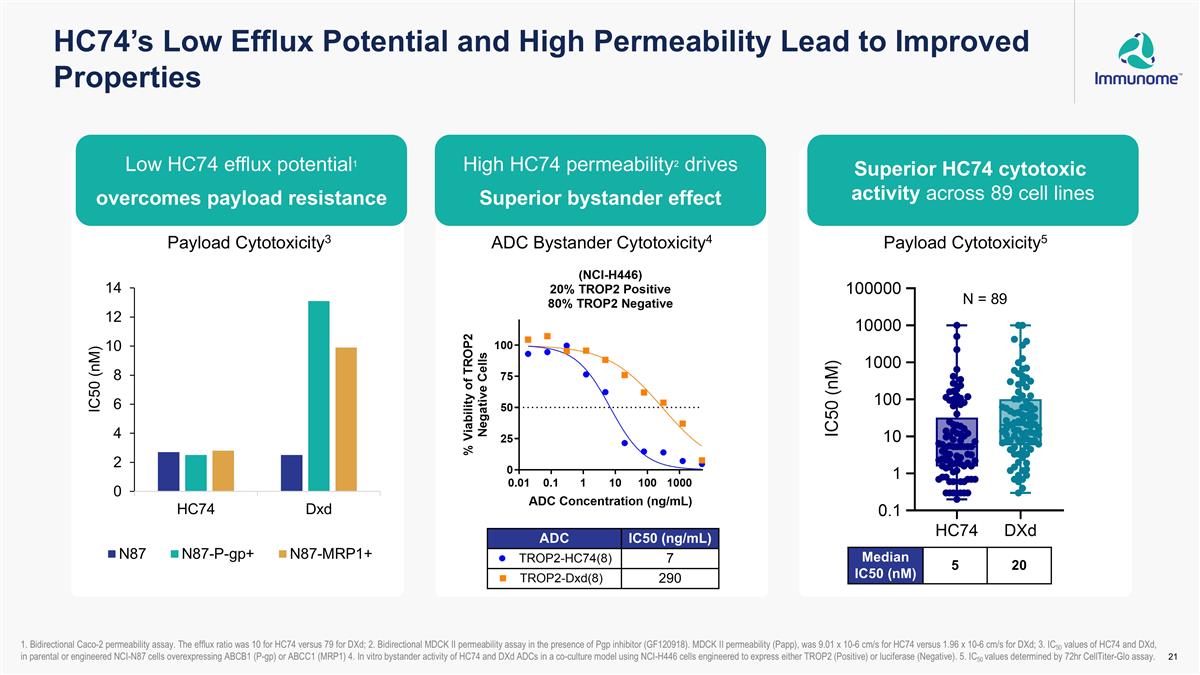
HC74’s Low Efflux Potential and High Permeability Lead to Improved Properties Superior HC74 cytotoxic activity across 89 cell lines High HC74 permeability2 drives superior bystander effect Low HC74 efflux potential1 overcomes payload resistance ADC Bystander Cytotoxicity4 Payload Cytotoxicity3 N = 89 Payload Cytotoxicity5 ADC IC50 (ng/mL) 7 290 1. Bidirectional Caco-2 permeability assay. The efflux ratio was 10 for HC74 versus 79 for DXd; 2. Bidirectional MDCK II permeability assay in the presence of Pgp inhibitor (GF120918). MDCK II permeability (Papp), was 9.01 x 10-6 cm/s for HC74 versus 1.96 x 10-6 cm/s for DXd; 3. IC50 values of HC74 and DXd, in parental or engineered NCI-N87 cells overexpressing ABCB1 (P-gp) or ABCC1 (MRP1) 4. In vitro bystander activity of HC74 and DXd ADCs in a co-culture model using NCI-H446 cells engineered to express either TROP2 (Positive) or luciferase (Negative). 5. IC50 values determined by 72hr CellTiter-Glo assay. Median IC50 (nM) 5 20 Low HC74 efflux potential1 overcomes payload resistance High HC74 permeability2 drives Superior bystander effect Superior HC74 cytotoxic activity across 89 cell lines
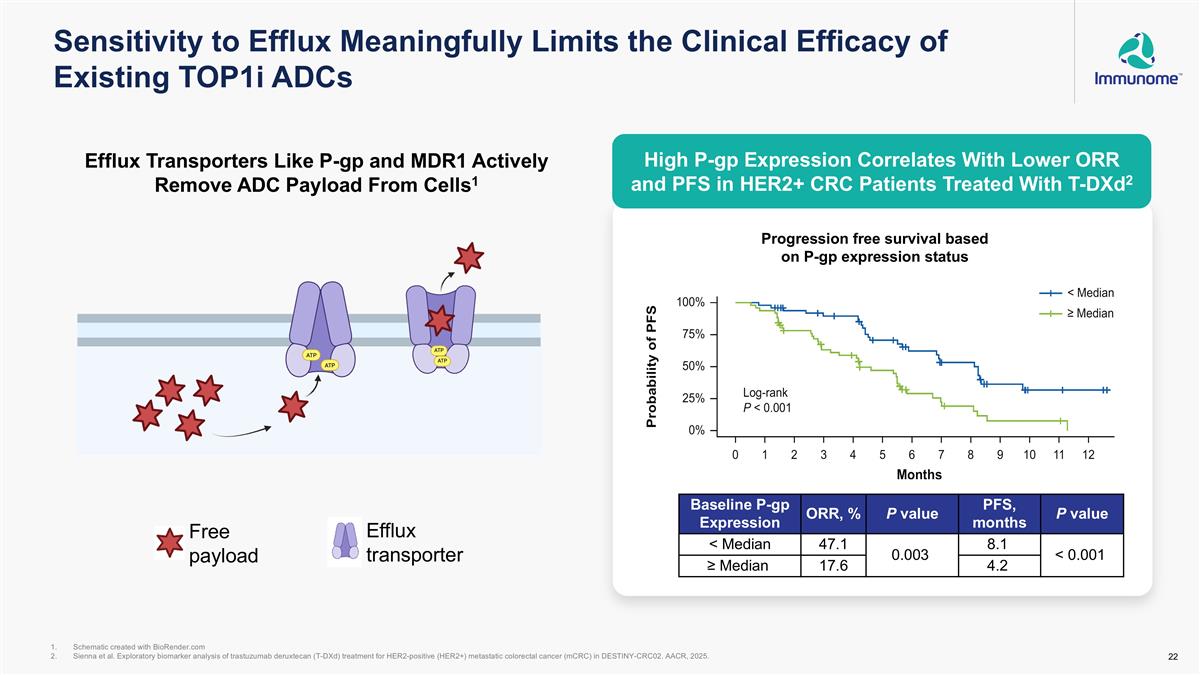
Sensitivity to Efflux Meaningfully Limits the Clinical Efficacy of Existing TOP1i ADCs High P-gp Expression Correlates With Lower ORR and PFS in HER2+ CRC Patients Treated With T-DXd2 Schematic created with BioRender.com Sienna et al. Exploratory biomarker analysis of trastuzumab deruxtecan (T-DXd) treatment for HER2-positive (HER2+) metastatic colorectal cancer (mCRC) in DESTINY-CRC02. AACR, 2025. Baseline P-gp Expression ORR, % P value PFS, months P value < Median 47.1 0.003 8.1 < 0.001 ≥ Median 17.6 4.2 Progression free survival based on P-gp expression status Efflux Transporters Like P-gp and MDR1 Actively Remove ADC Payload From Cells1 Free payload Efflux transporter High P-gp Expression Correlates With Lower ORR and PFS in HER2+ CRC Patients Treated With T-DXd2
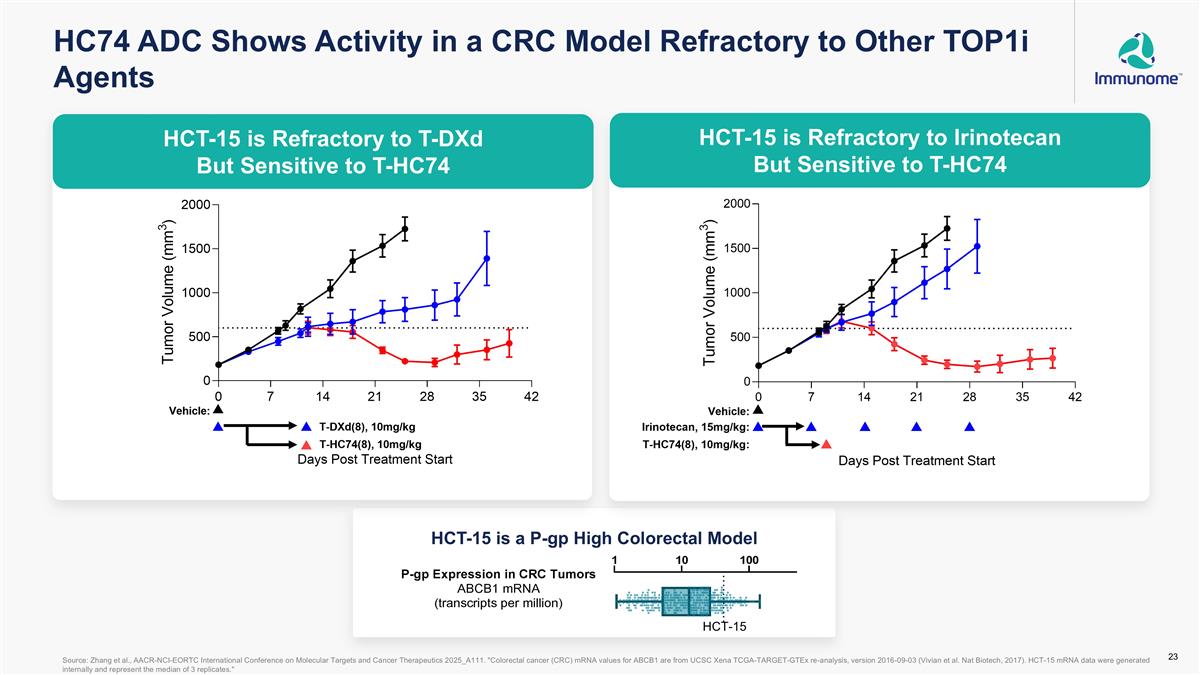
HCT-15 is a P-gp High Colorectal Model HCT-15 is Refractory to T-DXd but Sensitive to T-HC74 HCT-15 is Refractory to Irinotecan but Sensitive to T-HC74 HC74 ADC Shows Activity in a CRC Model Refractory to Other TOP1i Agents Source: Zhang et al., AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2025_A111. "Colorectal cancer (CRC) mRNA values for ABCB1 are from UCSC Xena TCGA-TARGET-GTEx re-analysis, version 2016-09-03 (Vivian et al. Nat Biotech, 2017). HCT-15 mRNA data were generated internally and represent the median of 3 replicates." HCT-15 HCT-15 is Refractory to T-DXd But Sensitive to T-HC74 HCT-15 is Refractory to Irinotecan But Sensitive to T-HC74
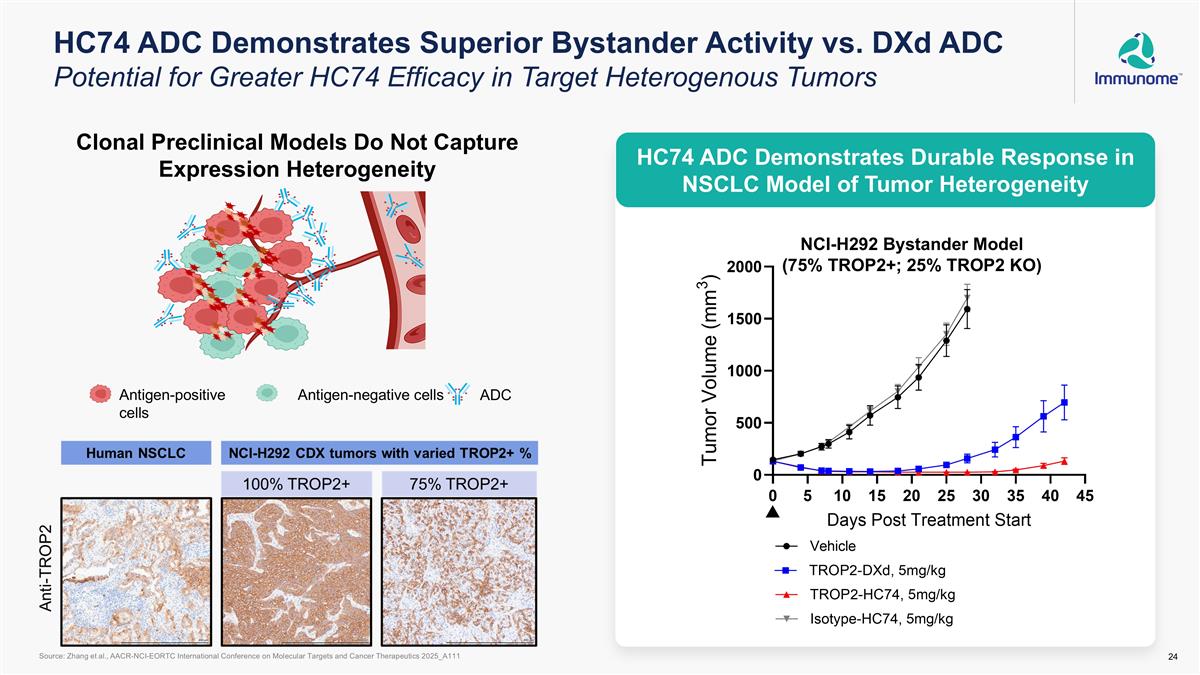
HC74 ADC Demonstrates Superior Bystander Activity vs. DXd ADC Potential for Greater HC74 Efficacy in Target Heterogenous Tumors NCI-H292 Bystander Model (75% TROP2+; 25% TROP2 KO) Clonal Preclinical Models Do Not Capture Expression Heterogeneity Source: Zhang et al., AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2025_A111 Antigen-positive cells Antigen-negative cells ADC HC74 ADC Demonstrates Durable Response in NSCLC Model of Tumor Heterogeneity
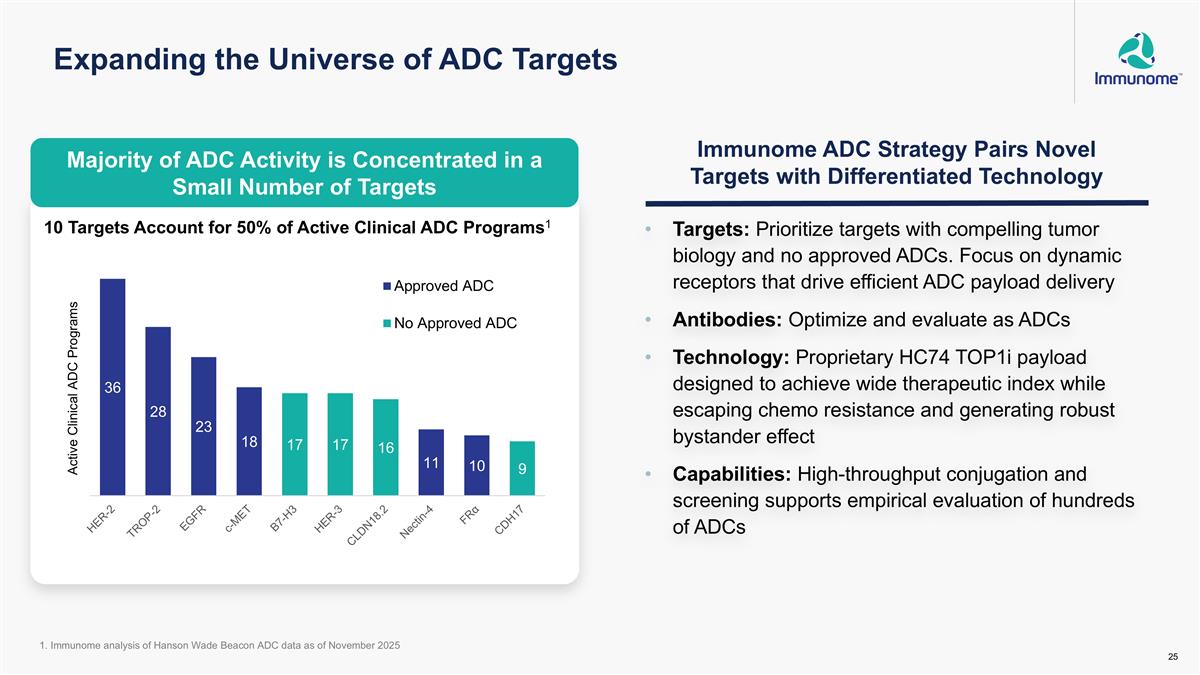
Expanding the Universe of ADC Targets Targets: Prioritize targets with compelling tumor biology and no approved ADCs. Focus on dynamic receptors that drive efficient ADC payload delivery Antibodies: Optimize and evaluate as ADCs Technology: Proprietary HC74 TOP1i payload designed to achieve wide therapeutic index while escaping chemo resistance and generating robust bystander effect Capabilities: High-throughput conjugation and screening supports empirical evaluation of hundreds of ADCs 10 Targets Account for 50% of Active Clinical ADC Programs1 Immunome ADC Strategy Pairs Novel Targets with Differentiated Technology 1. Immunome analysis of Hanson Wade Beacon ADC data as of November 2025 Majority of ADC Activity is Concentrated in a Small Number of Targets
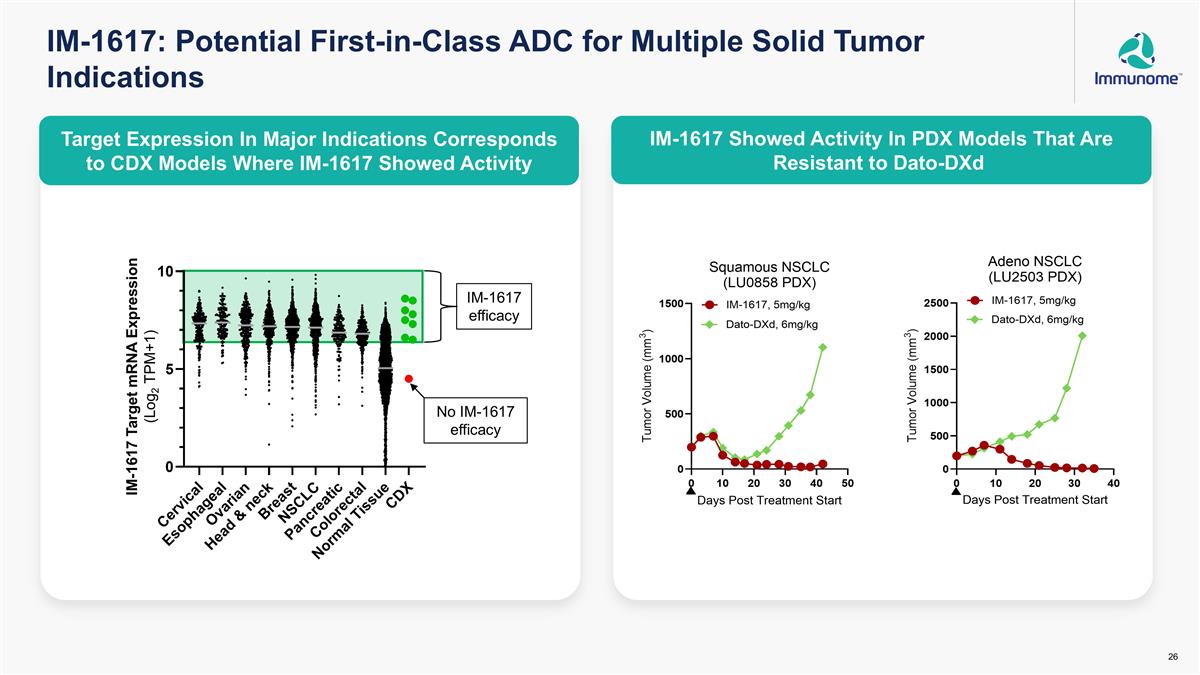
IM-1617: Potential First-in-Class ADC for Multiple Solid Tumor Indications IM-1617 efficacy IM-1617 Target mRNA Expression (Log2 TPM+1) No IM-1617 efficacy Target Expression In Major Indications Corresponds to CDX Models Where IM-1617 Showed Activity IM-1617 Showed Activity In PDX Models That Are Resistant to Dato-DXd
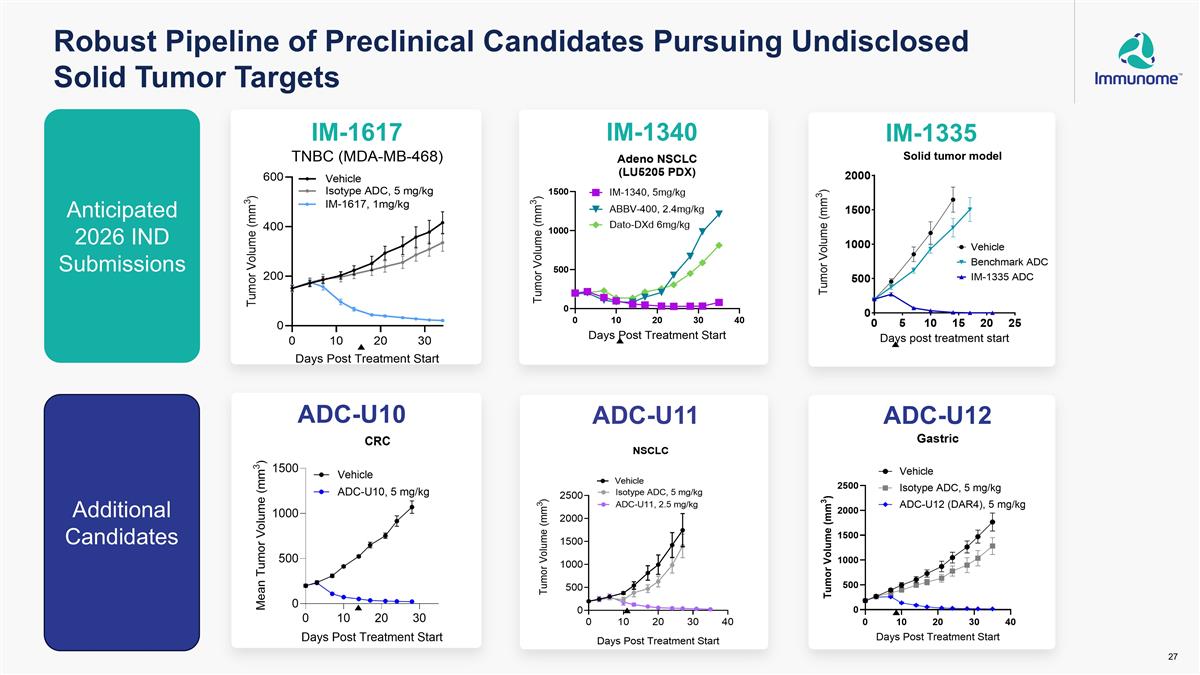
Robust Pipeline of Preclinical Candidates Pursuing Undisclosed Solid Tumor Targets Solid tumor model Anticipated 2026 IND Submissions Additional Candidates IM-1617 ADC-U10 IM-1340 IM-1335 ADC-U11 ADC-U12

IM-3050 FAP-Targeted Radioligand Therapy
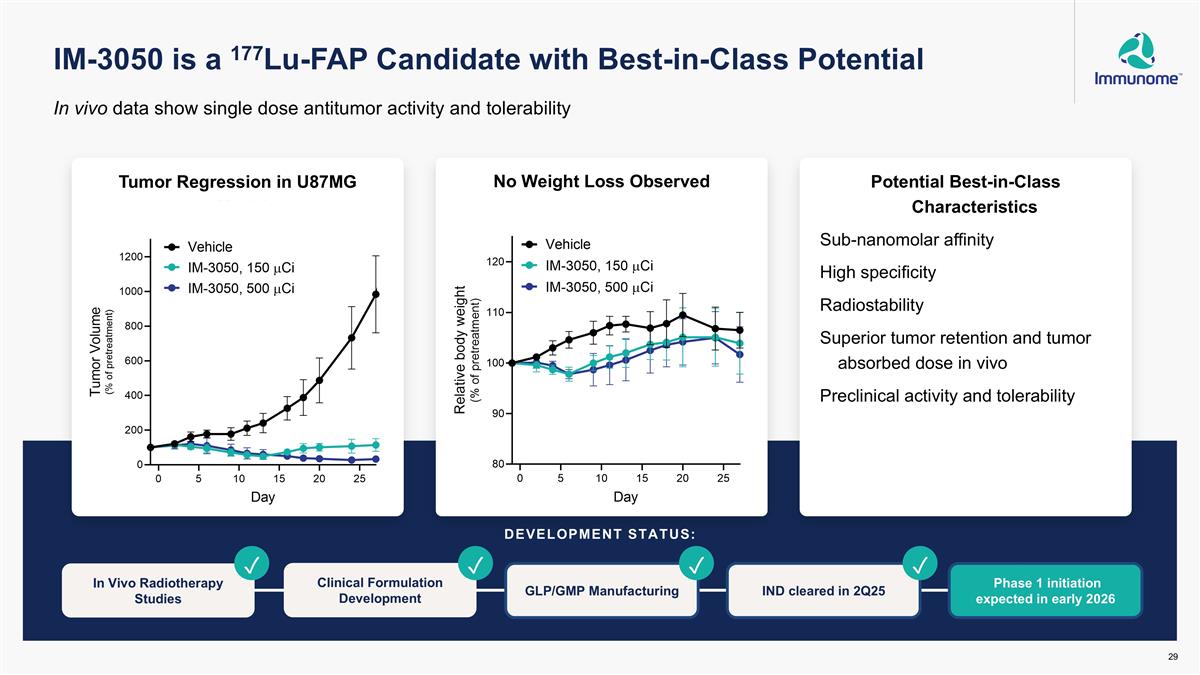
IM-3050 is a 177Lu-FAP Candidate with Best-in-Class Potential Tumor Regression in U87MG Model No Weight Loss Observed Potential Best-in-Class Characteristics Sub-nanomolar affinity High specificity Radiostability Superior tumor retention and tumor absorbed dose in vivo Preclinical activity and tolerability DEVELOPMENT STATUS: Phase 1 initiation expected in early 2026 In Vivo Radiotherapy Studies GLP/GMP Manufacturing IND cleared in 2Q25 ✓ Clinical Formulation Development ✓ In vivo data show single dose antitumor activity and tolerability ✓ ✓

Company Overview

Management Team with a Demonstrated Track Record of Success Chief Executive Officer and Founder, Seagen (1998-2022) Grew company to $2B+ revenue (2022) leading to $43B acquisition Led development of 4 FDA-approved therapeutics Raised over $1B in public and private capital Oversaw acquisition and integration of Cascadian Therapeutics Generated >$3B in partnership and licensing revenue Clay Siegall, Ph.D. PRESIDENT & CHIEF EXECUTIVE OFFICER Sandra Stoneman CHIEF LEGAL OFFICER Jack Higgins, Ph.D. CHIEF SCIENTIFIC OFFICER Max Rosett CHIEF FINANCIAL OFFICER Bob Lechleider, M.D. CHIEF MEDICAL OFFICER Kinney Horn CHIEF BUSINESS OFFICER Phil Tsai CHIEF TECHNICAL OFFICER Roee Shahar EVP, COMMERCIAL
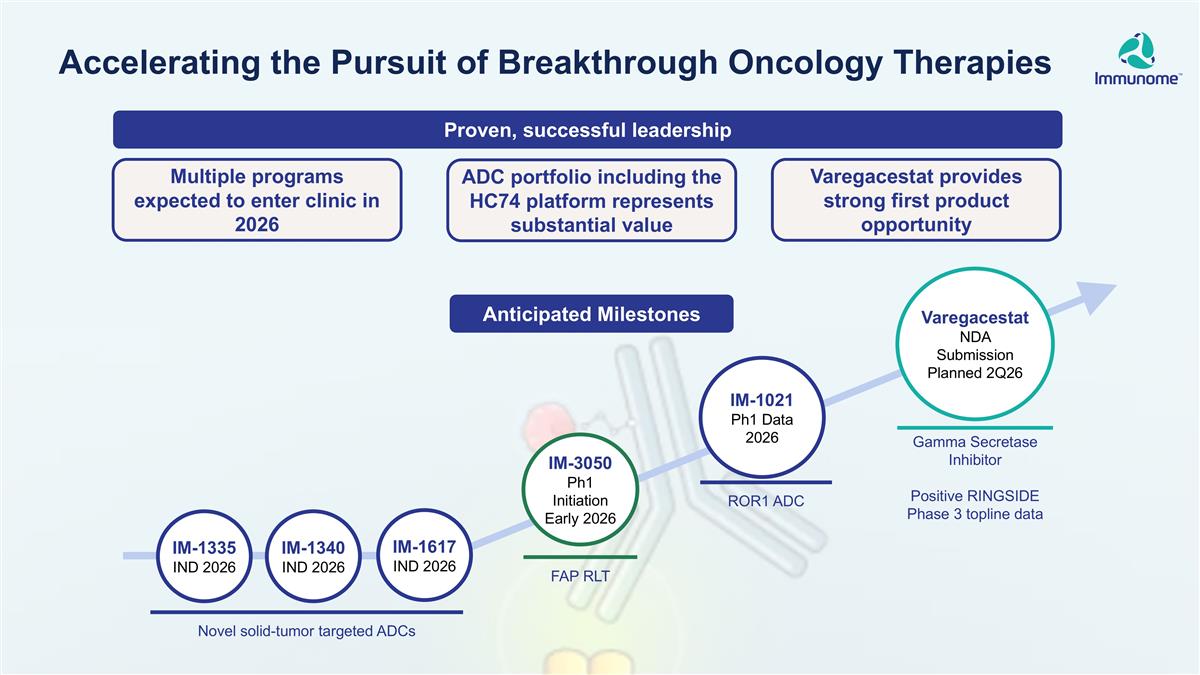
Varegacestat NDA Submission Planned 2Q26 IM-1021 Ph1 Data 2026 Novel solid-tumor targeted ADCs ROR1 ADC Gamma Secretase Inhibitor Positive RINGSIDE Phase 3 topline data IM-1617 IND 2026 IM-1340 IND 2026 IM-1335 IND 2026 ADC portfolio including the HC74 platform represents substantial value Varegacestat provides strong first product opportunity Proven, successful leadership Multiple programs expected to enter clinic in 2026 IM-3050 Ph1 Initiation Early 2026 FAP RLT Accelerating the Pursuit of Breakthrough Oncology Therapies Anticipated Milestones