

NYSE: ANVS Annovis Webinar January 28, 2026 Presenter: Maria Maccecchini, Ph.D., President and CEO

2 FORWARD - LOOKING STATEMENTS Forward Looking Statements and Other Important Cautions -- This presentation contains "forward - looking" statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements include, but are not limited to, the Company's plans related to clinical trials and financial condition. Forward - looking statements are based on current expectations and assumptions and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Such risks and uncertainties include, but are not limited to, those related to patient enrollment, the effectiveness of buntanetap, and the timing, effectiveness, and anticipated results of the Company's clinical trials evaluating the efficacy, safety, and tolerabi lit y of buntanetap. Additional risk factors are detailed in the Company's periodic filings with the SEC, including those listed in the "Risk Factors" section of the Company's Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q. All forward - looking statements in this presentation are based on information available to the Company as of the date of this presentation. The Company expressly disclaims any obligation to update or revise its forward - looking statements, whether as a result of new information, future events, or otherwise, except as required by law.
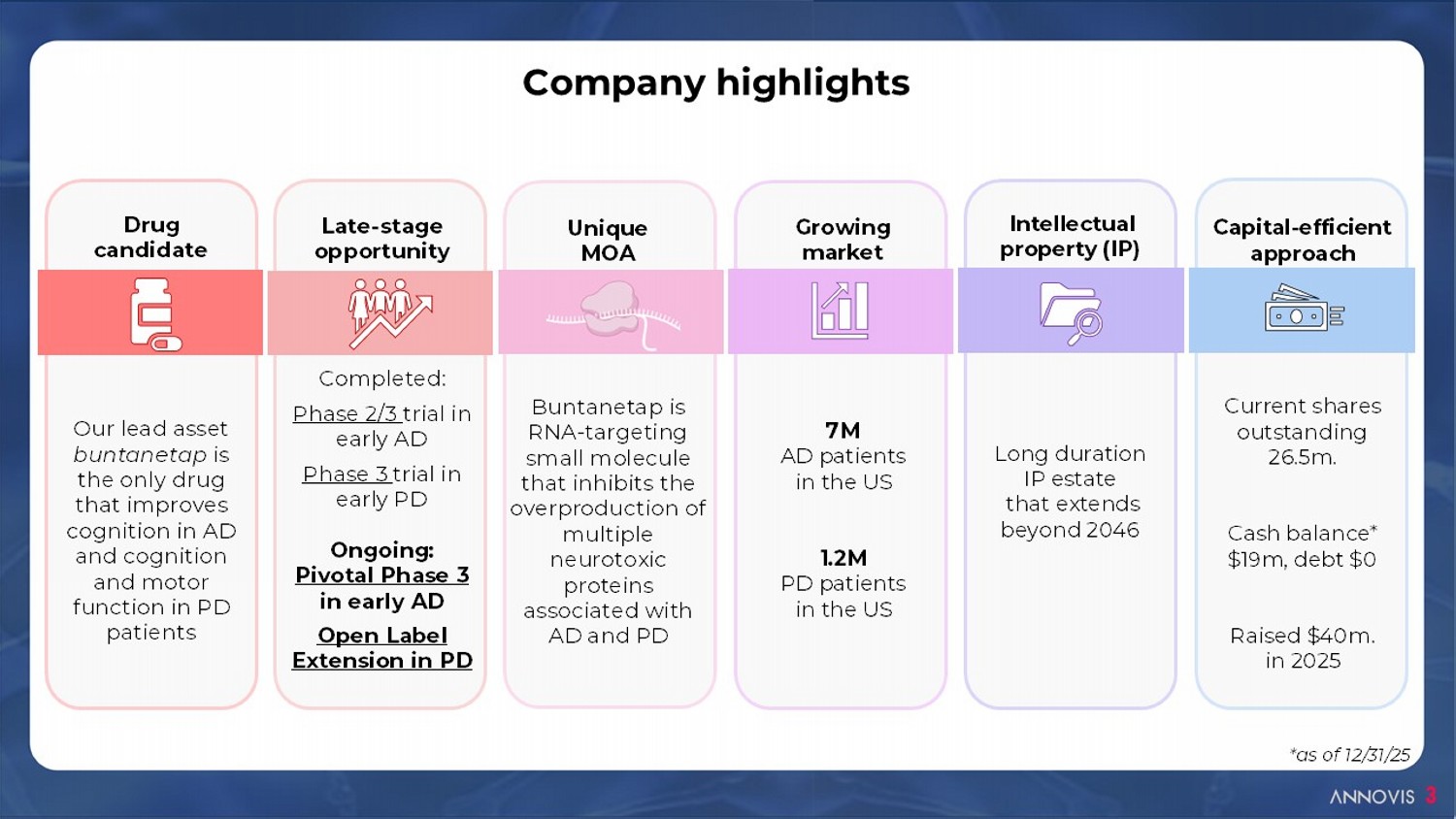
3 Company highlights Capital - efficient approach Current shares outstanding 26.5m. Cash balance* $19m, d ebt $0 Raised $40m. in 2025 Drug candidate Our lead asset buntanetap is the only drug that improves cognition in AD and cognition and motor function in PD patients Late - stage opportunity Completed: Phase 2/3 trial in early AD Phase 3 trial in early PD Ongoing: Pivotal Phase 3 in early AD Open Label Extension in PD Unique MOA Buntanetap is RNA - targeting small molecule that inhibits the overproduction of multiple neurotoxic proteins associated with AD and PD Growing market 7M AD patients in the US 1.2M PD patients in the US Intellectual property (IP) Long duration IP estate that extends beyond 2046 *as of 12/31/25
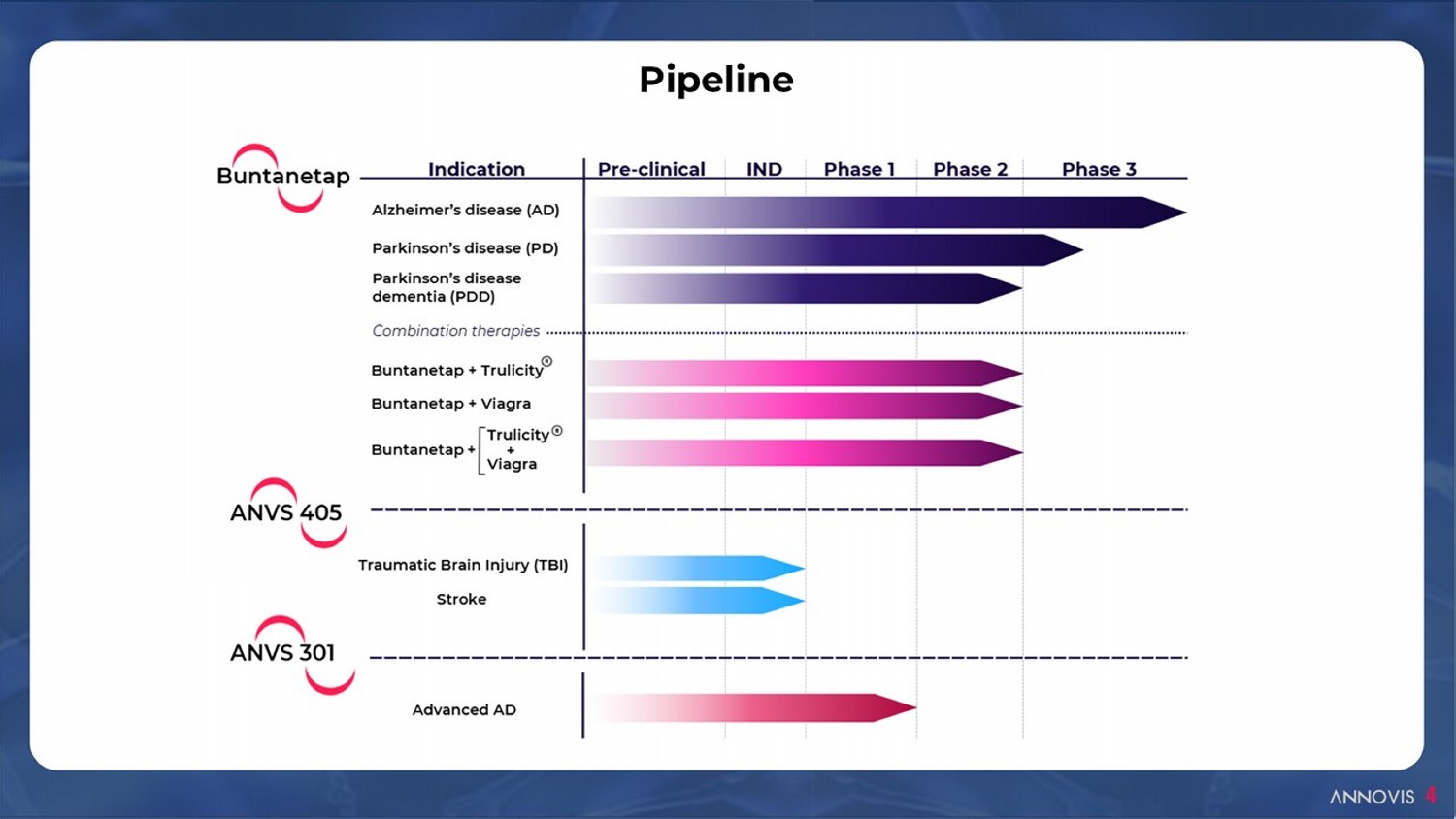
Pipeline 4
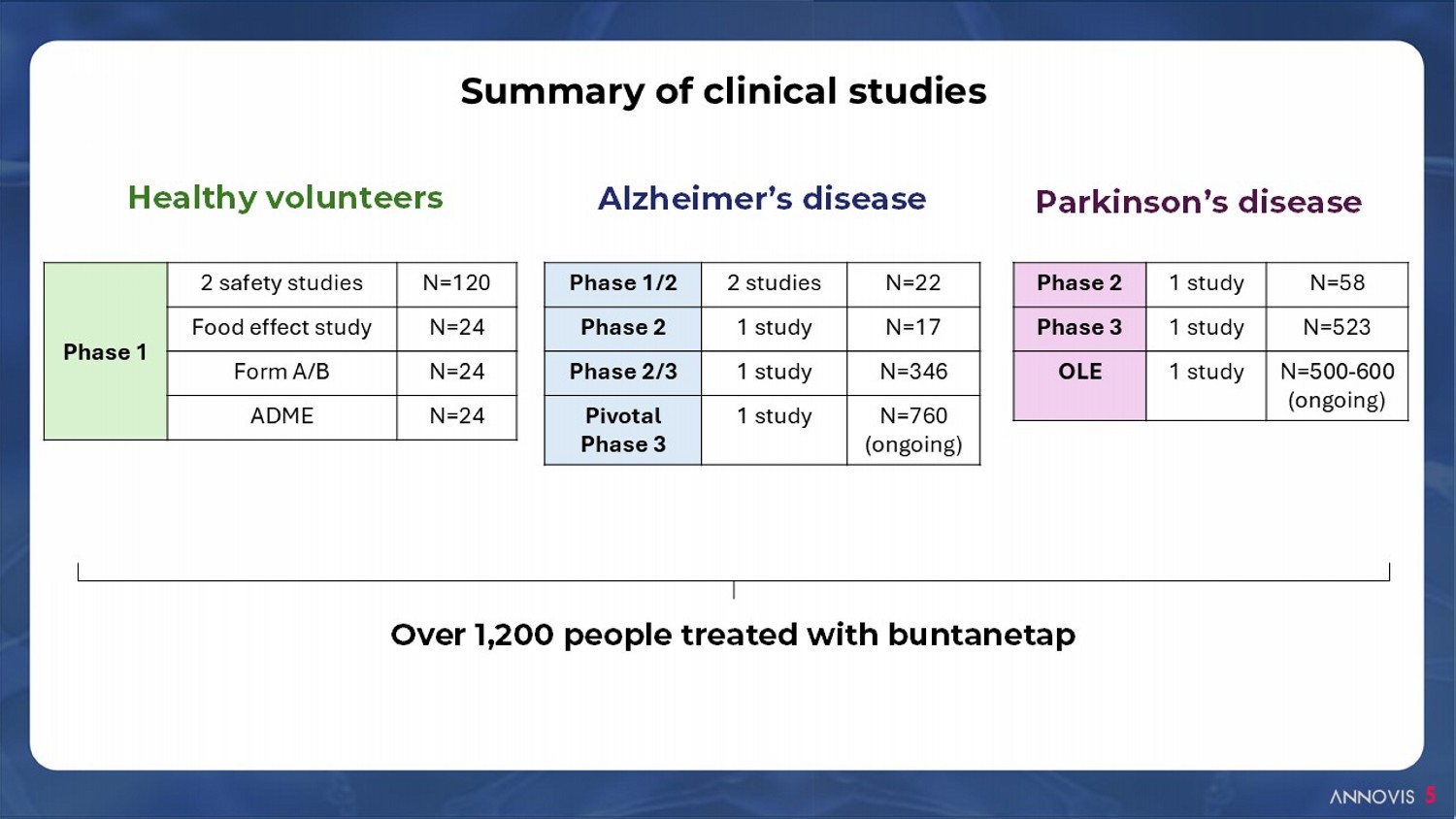
5 Over 1, 2 00 people treated with buntanetap Healthy volunteers Alzheimer’s disease Parkinson’s disease Summary of clinical studies N=22 2 studies Phase 1/2 N=17 1 study Phase 2 N=346 1 study Phase 2/3 N=760 (ongoing) 1 study Pivotal Phase 3 N=58 1 study Phase 2 N=523 1 study Phase 3 N=500 - 600 (ongoing) 1 study OLE N=120 2 safety studies Phase 1 N=24 Food effect study N=24 Form A/B N=24 ADME
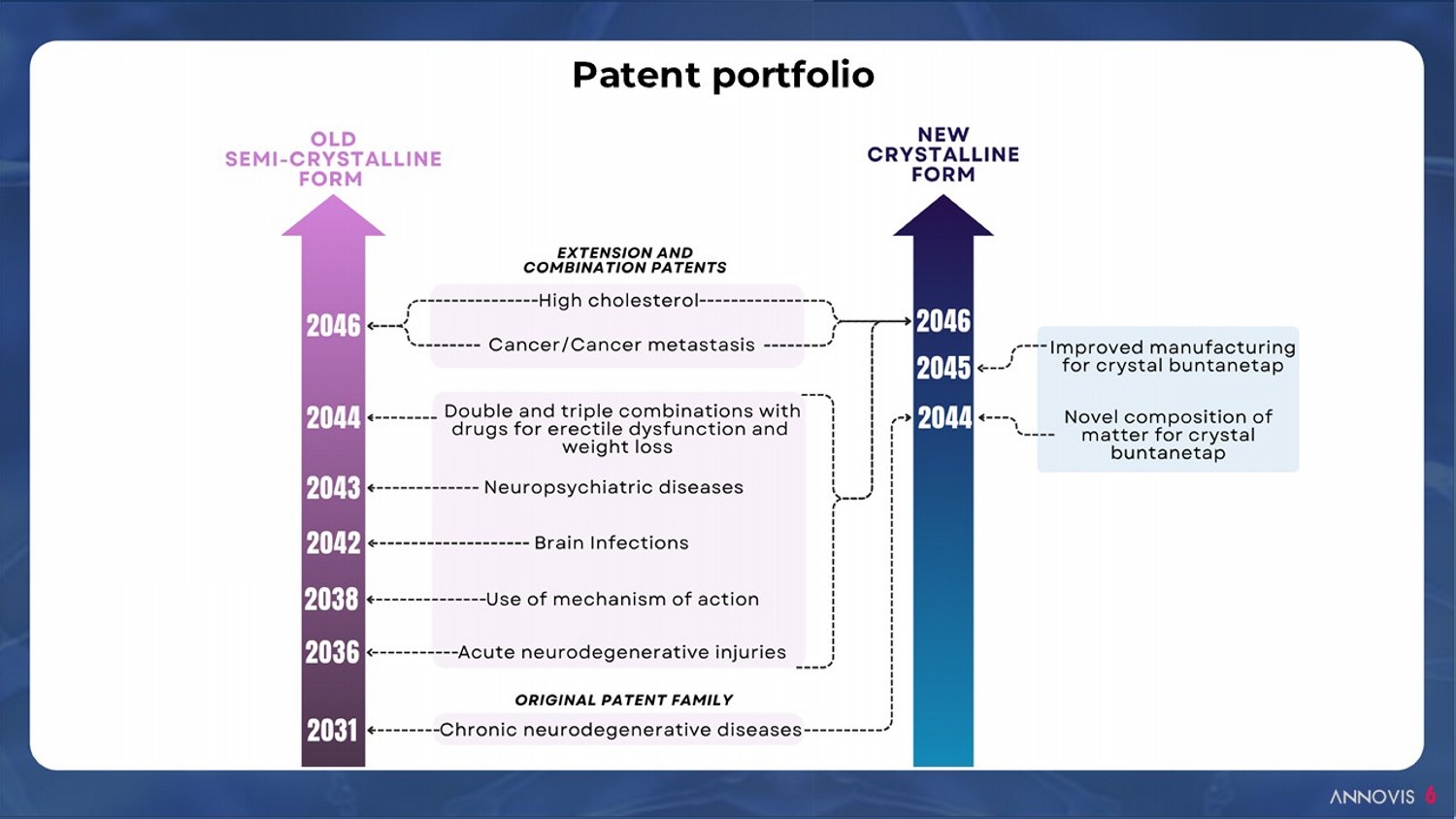
6 Patent portfolio

Alzheimer’s disease
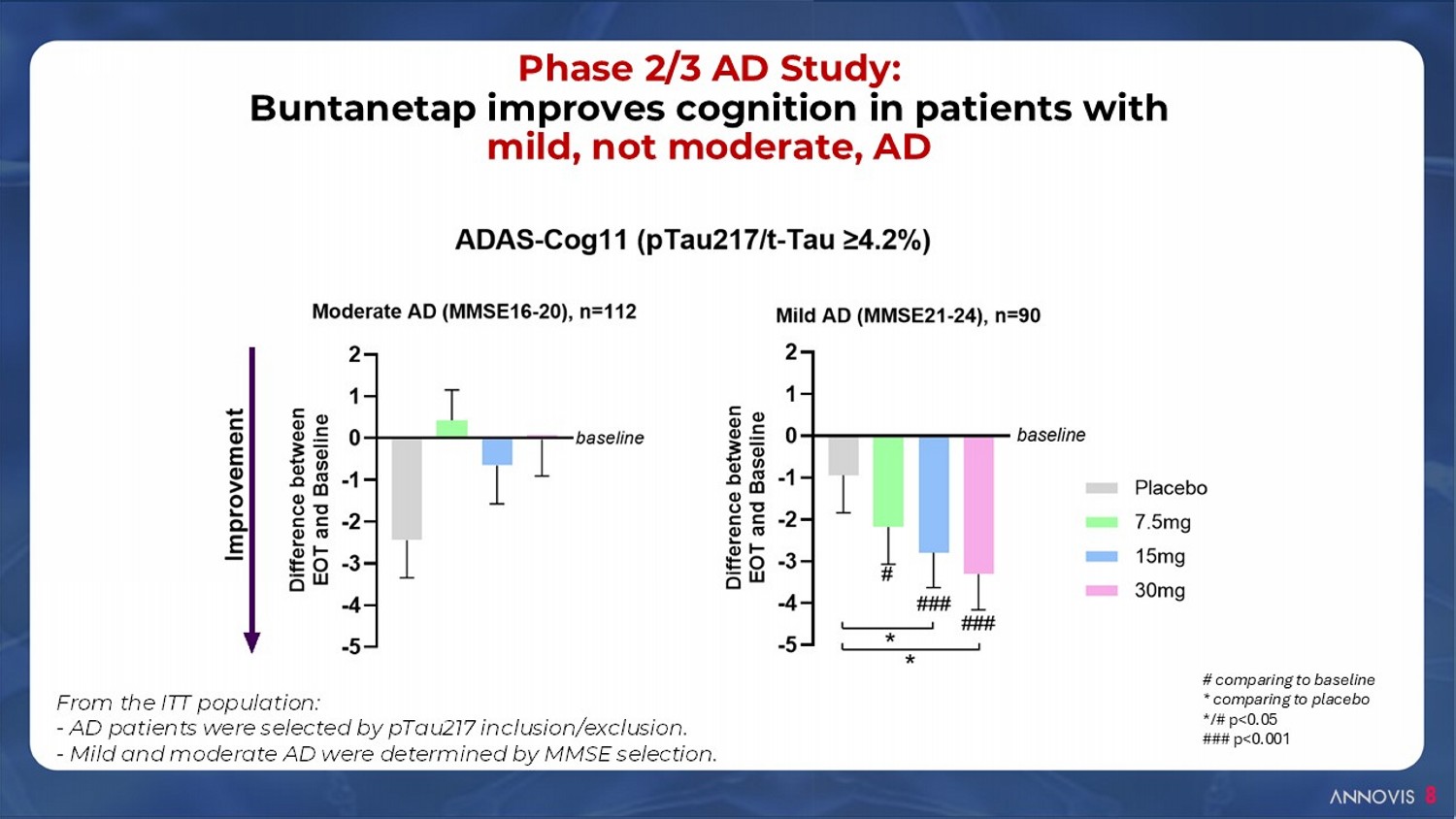
8 Phase 2/3 AD Study: Buntanetap improves cognition in patients with mild, not moderate, AD # comparing to baseline * comparing to placebo */# p<0.05 ### p<0.001 From the ITT population: - AD patients were selected by pTau217 inclusion/exclusion. - Mild and moderate AD were determined by MMSE selection.
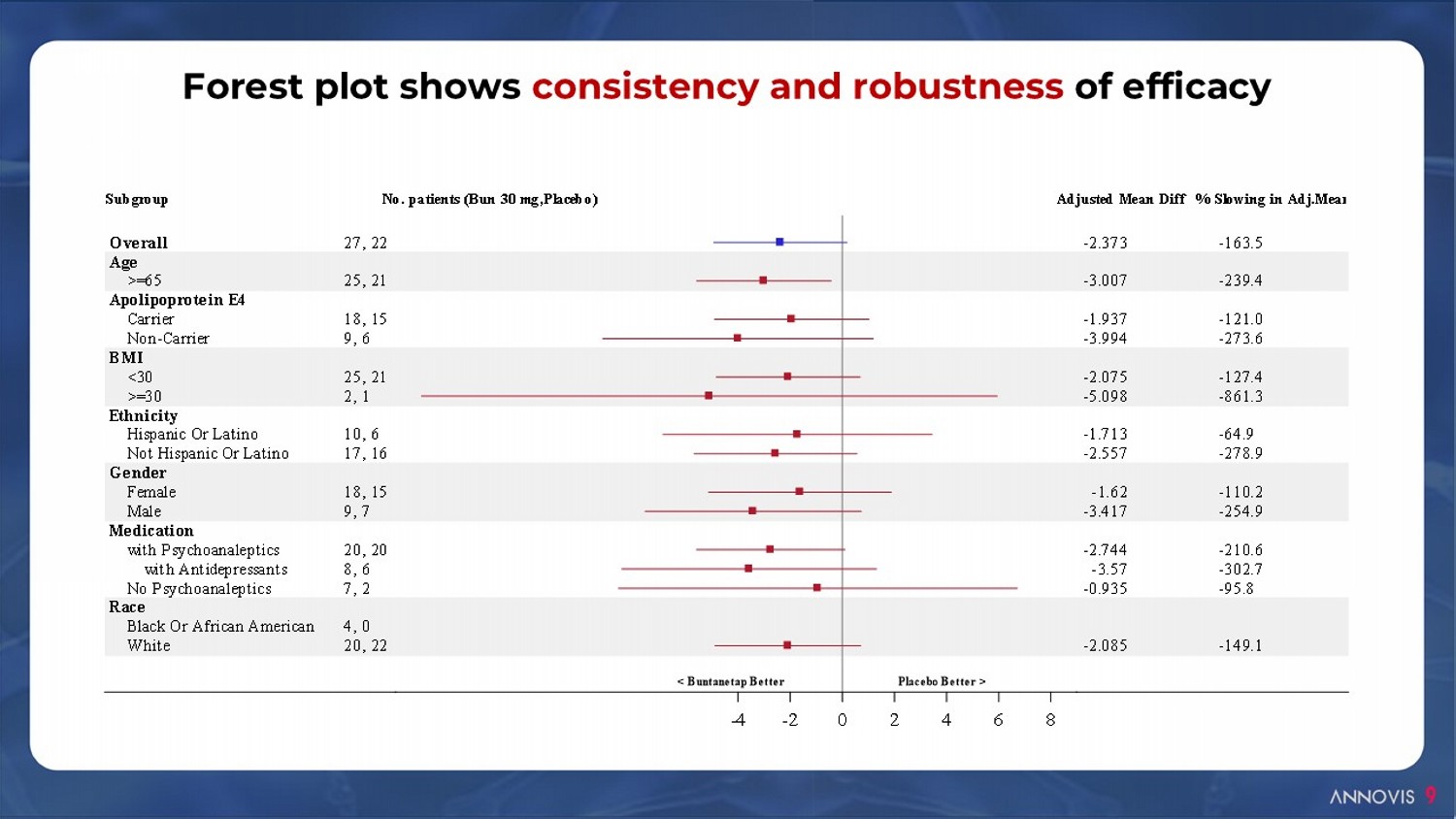
% Slowing in Adj.MeanAdjusted Mean DiffNo. patients (Bun 30 mg,Placebo)Subgroup Overall Age >=65 Apolipoprotein E4 Carrier Non-Carrier BMI <30 >=30 Ethnicity Hispanic Or Latino Not Hispanic Or Latino Gender Female Male Medication with Psychoanaleptics with Antidepressants No Psychoanaleptics Race Black Or African American White 27, 22 25, 21 18, 15 9, 6 25, 21 2, 1 10, 6 17, 16 18, 15 9, 7 20, 20 8, 6 7, 2 4, 0 20, 22 -2.373 -3.007 -1.937 -3.994 -2.075 -5.098 -1.713 -2.557 -1.62 -3.417 -2.744 -3.57 -0.935 -2.085 -163.5 -239.4 -121.0 -273.6 -127.4 -861.3 -64.9 -278.9 -110.2 -254.9 -210.6 -302.7 -95.8 -149.1 -4 -2 0 2 4 6 8 < Buntanetap Better Placebo Better > Forest plot shows consistency and robustness of efficacy 9
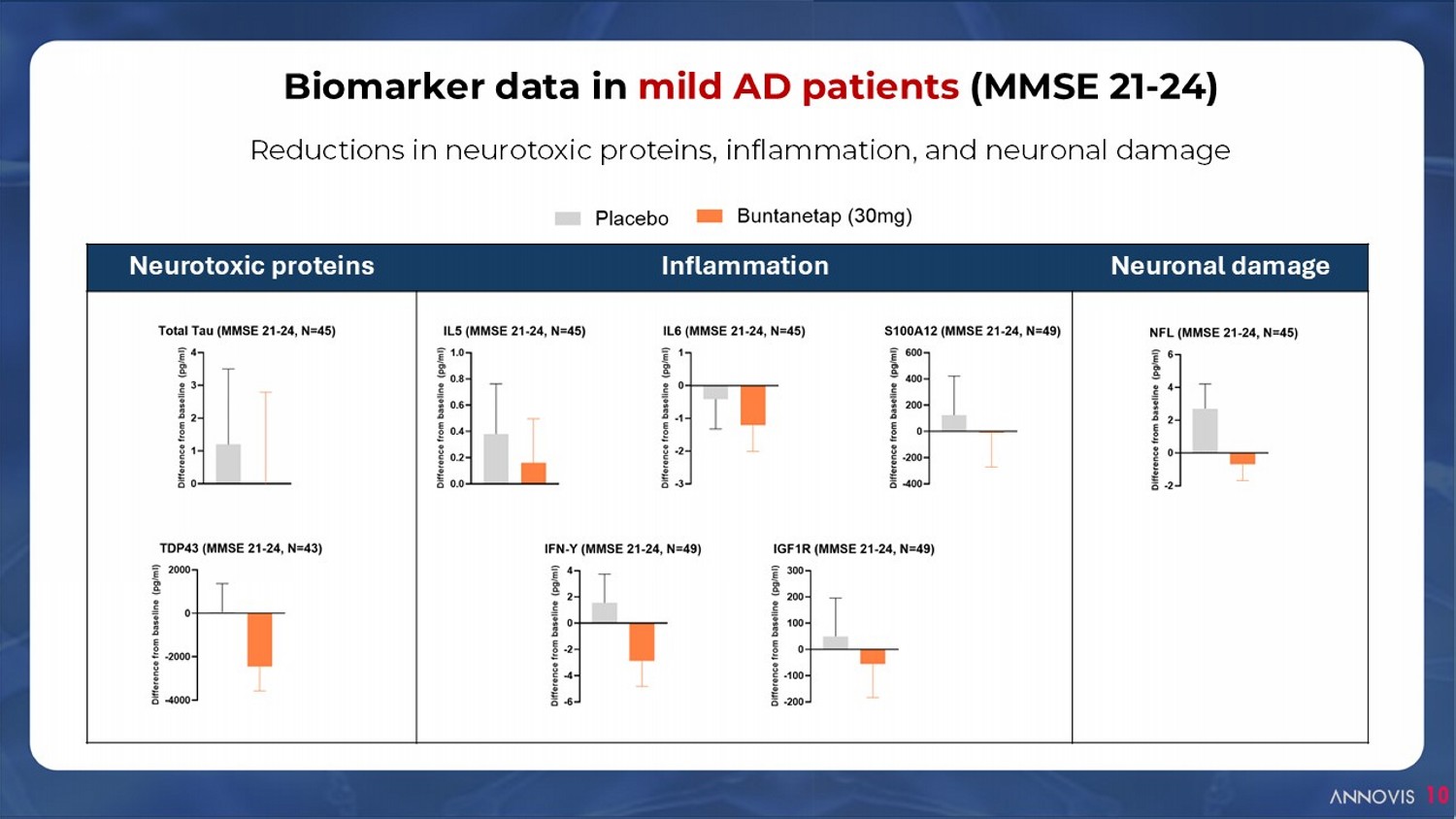
Neuronal damage Inflammation Neurotoxic proteins Biomarker data in mild AD patients (MMSE 21 - 24) 10 Reductions in neurotoxic proteins, inflammation, and neuronal damage
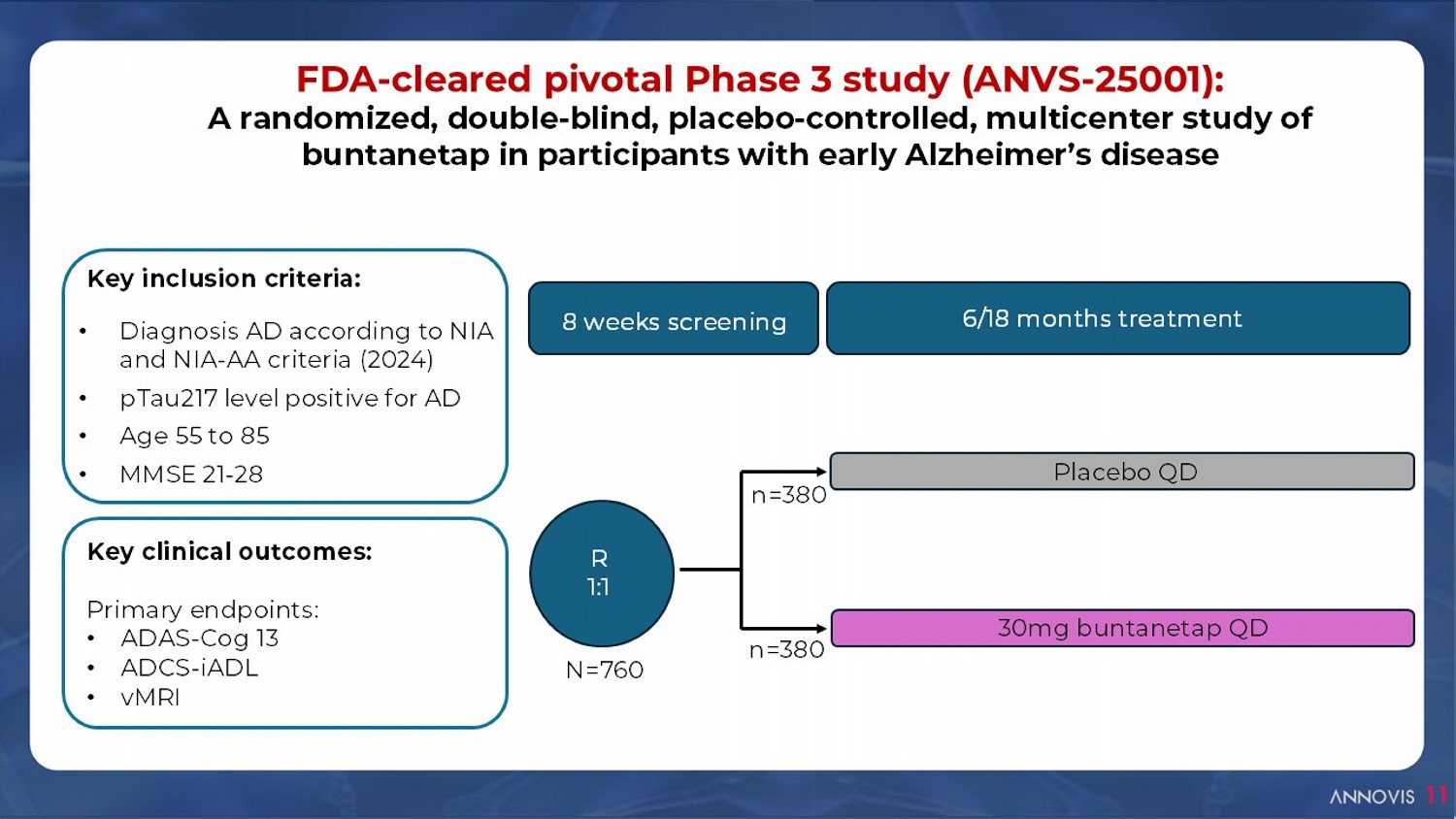
11 FDA - cleared pivotal Phase 3 study (ANVS - 25001): A randomized, double - blind, placebo - controlled, multicenter study of buntanetap in participants with early Alzheimer’s disease R 1:1 Placebo QD 30mg buntanetap QD 8 weeks screening 6/18 months treatment n=380 n=380 N=760 Key inclusion c riteria: • Diagnosis AD according to NIA and NIA - AA criteria (2024) • pTau217 level positive for AD • Age 55 to 85 • MMSE 21 - 28 Key clinical o utcomes : Primary endpoints: • ADAS - Cog 13 • ADCS - iADL • vMRI
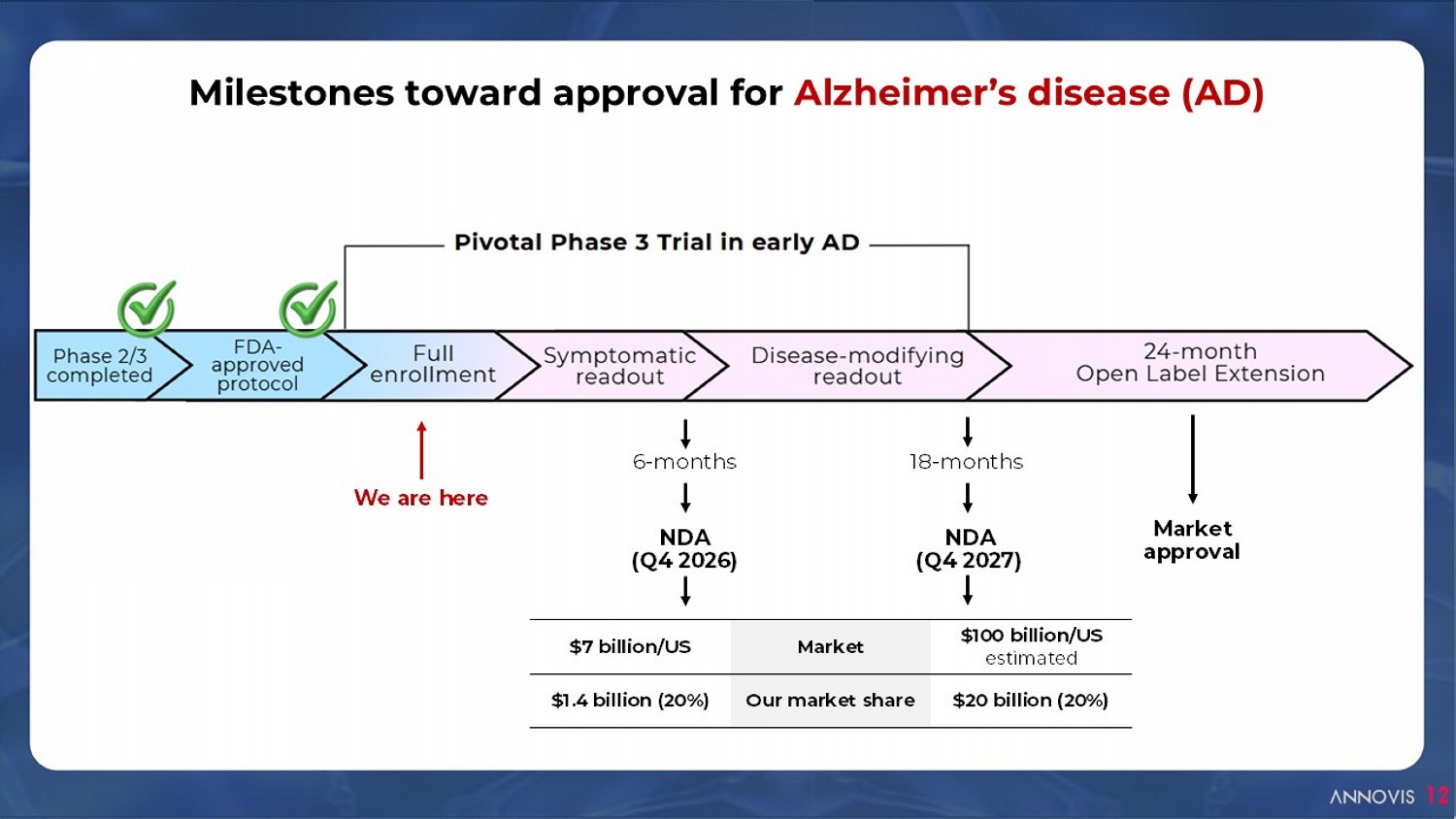
6 - months 18 - months NDA (Q4 2026) NDA (Q4 2027) Milestones toward approval for Alzheimer’s disease (AD) 12 $100 billion/US estimated Market $7 billion/US $20 billion (20%) Our market share $1.4 billion (20%) We are here Market approval
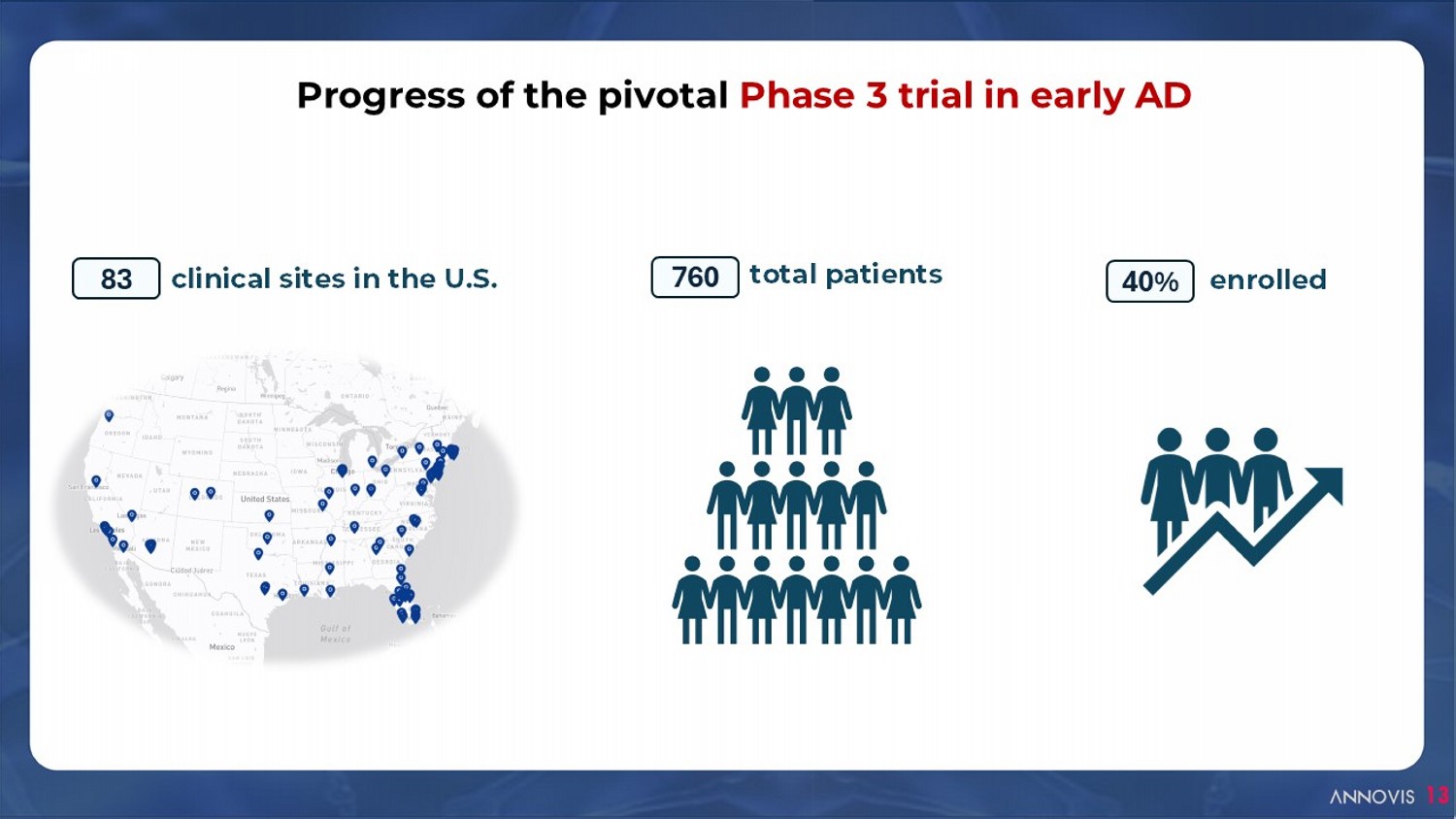
clinical sites in the U.S. 83 total patients 760 Progress of the pivotal Phase 3 trial in early AD enrolled 40% 13

Parkinson’s disease
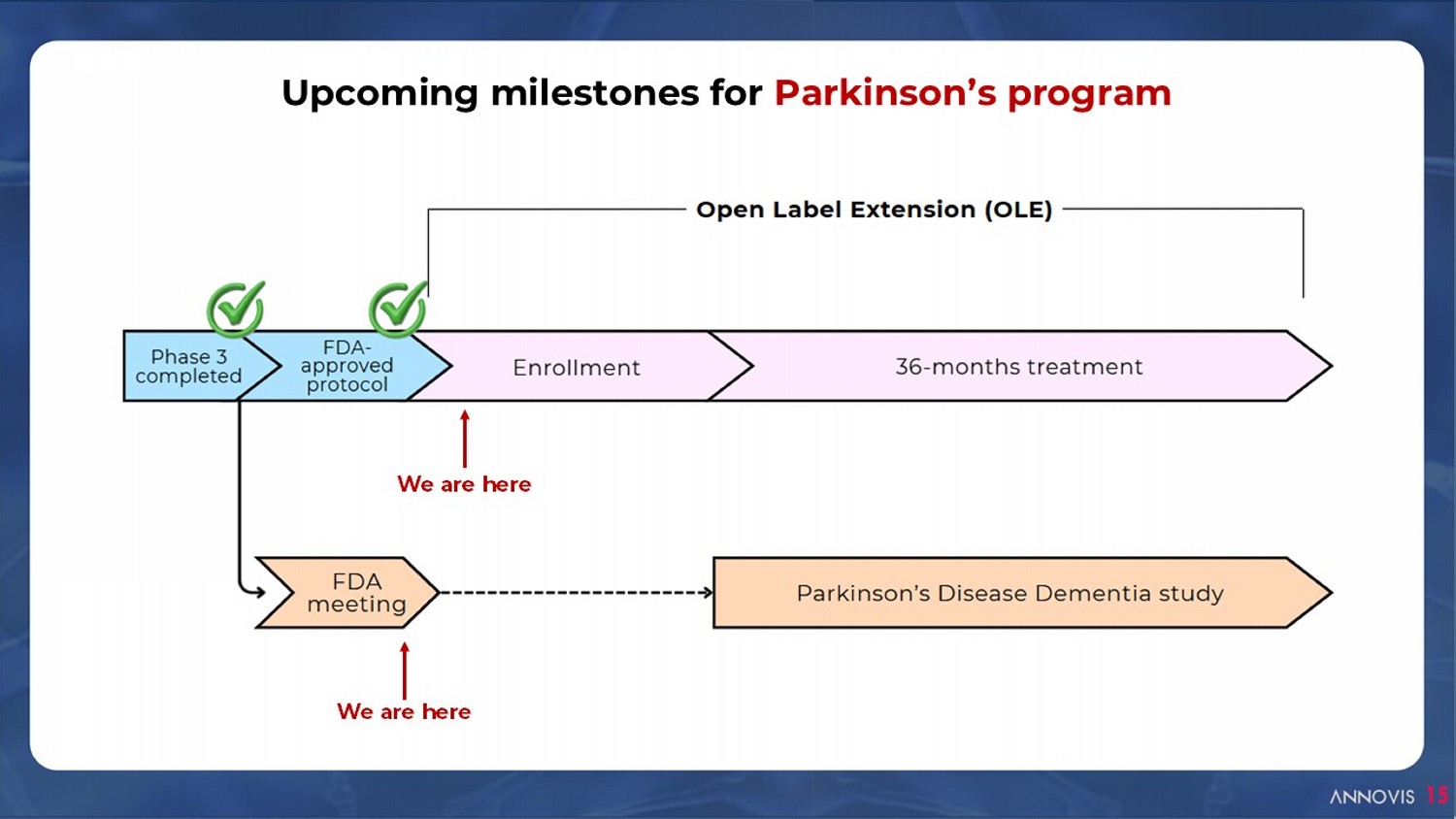
Upcoming milestones for Parkinson’s program 15 We are here We are here
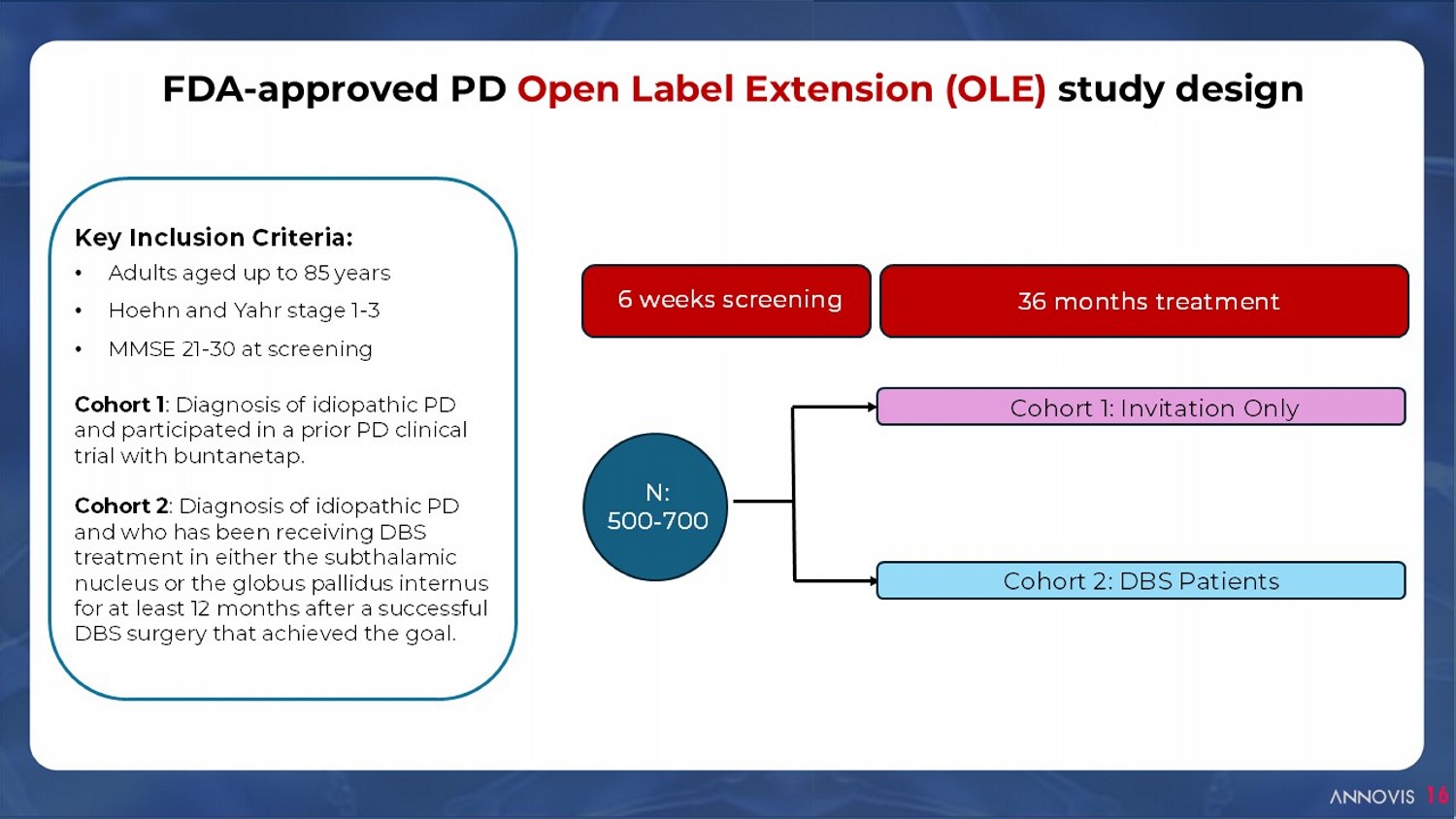
16 FDA - approved PD Open Label Extension (OLE) study design Early AD Cohort 1: Invitation Only Cohort 2: DBS Patients 6 weeks screening 36 months treatment N: 500 - 700 Key Inclusion Criteria: • Adults aged up to 85 years • Hoehn and Yahr stage 1 - 3 • MMSE 21 - 30 at screening Cohort 1 : Diagnosis of idiopathic PD and participated in a prior PD clinical trial with buntanetap. Cohort 2 : Diagnosis of idiopathic PD and who has been receiving DBS treatment in either the subthalamic nucleus or the globus pallidus internus for at least 12 months after a successful DBS surgery that achieved the goal.
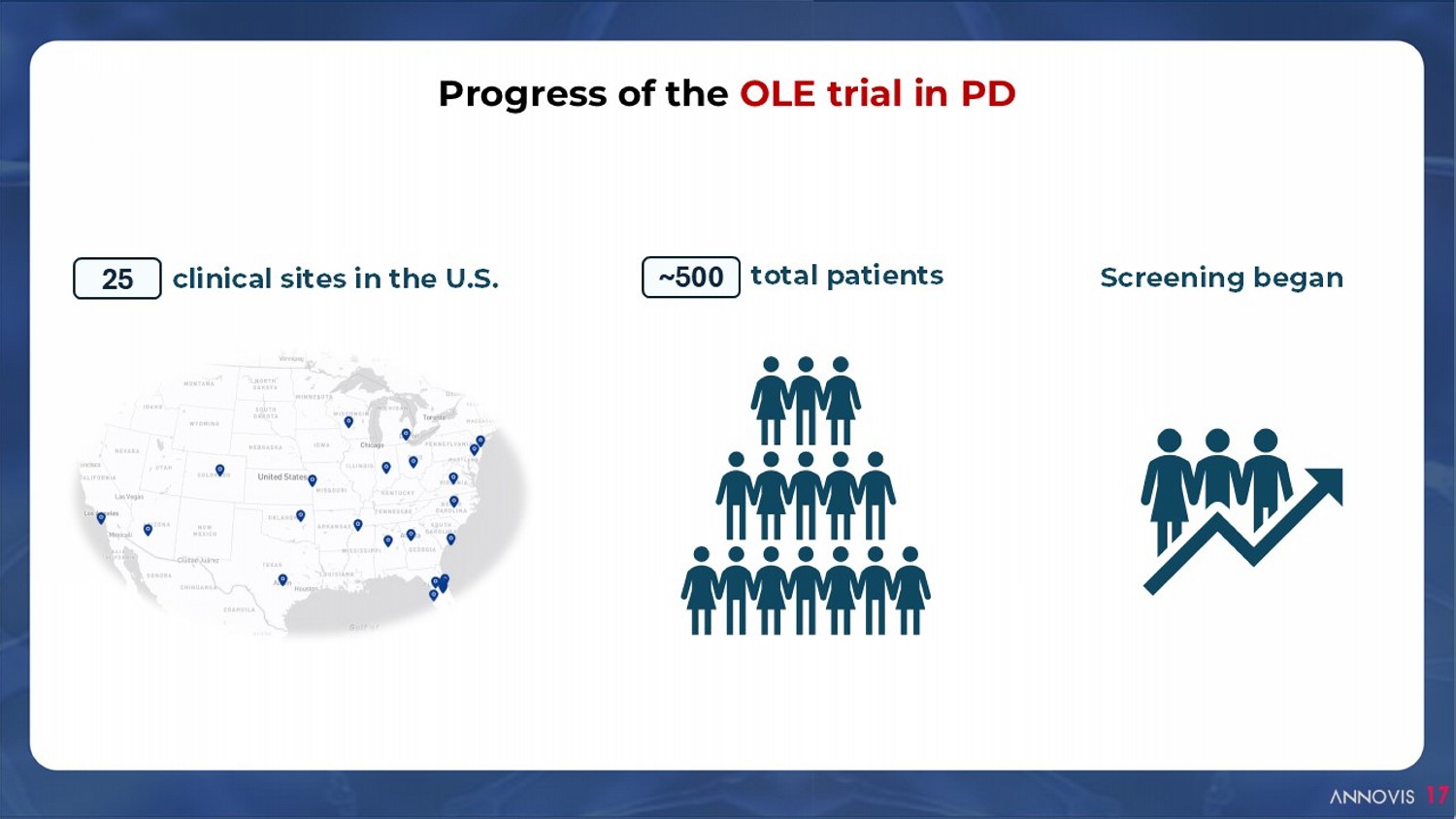
clinical sites in the U.S. 25 total patients ~500 Progress of the OLE trial in PD Screening began 17
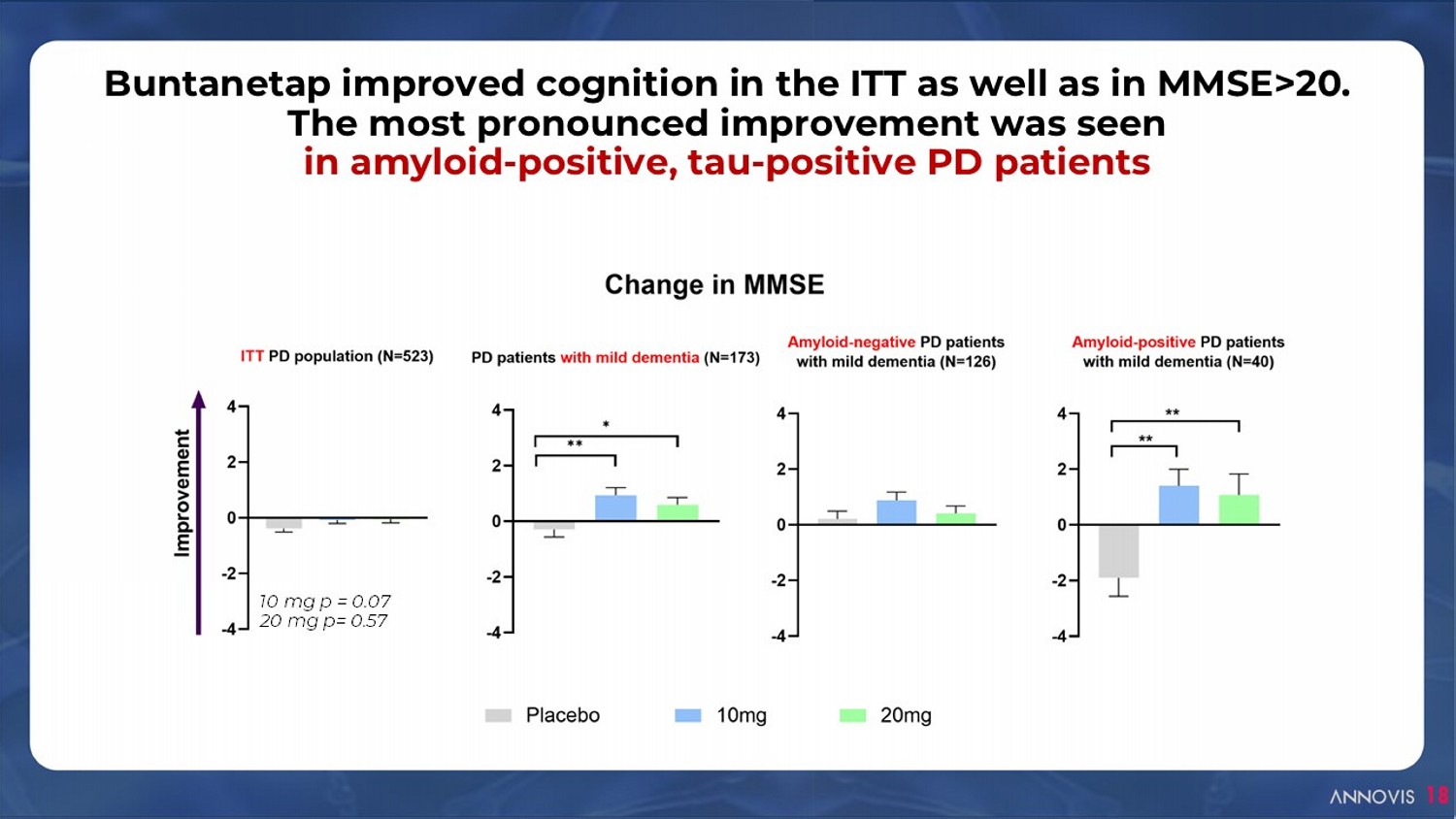
Buntanetap improved cognition in the ITT as well as in MMSE>20. The most pronounced improvement was seen in amyloid - positive, tau - positive PD patients 18 10 mg p = 0.07 20 mg p= 0.57
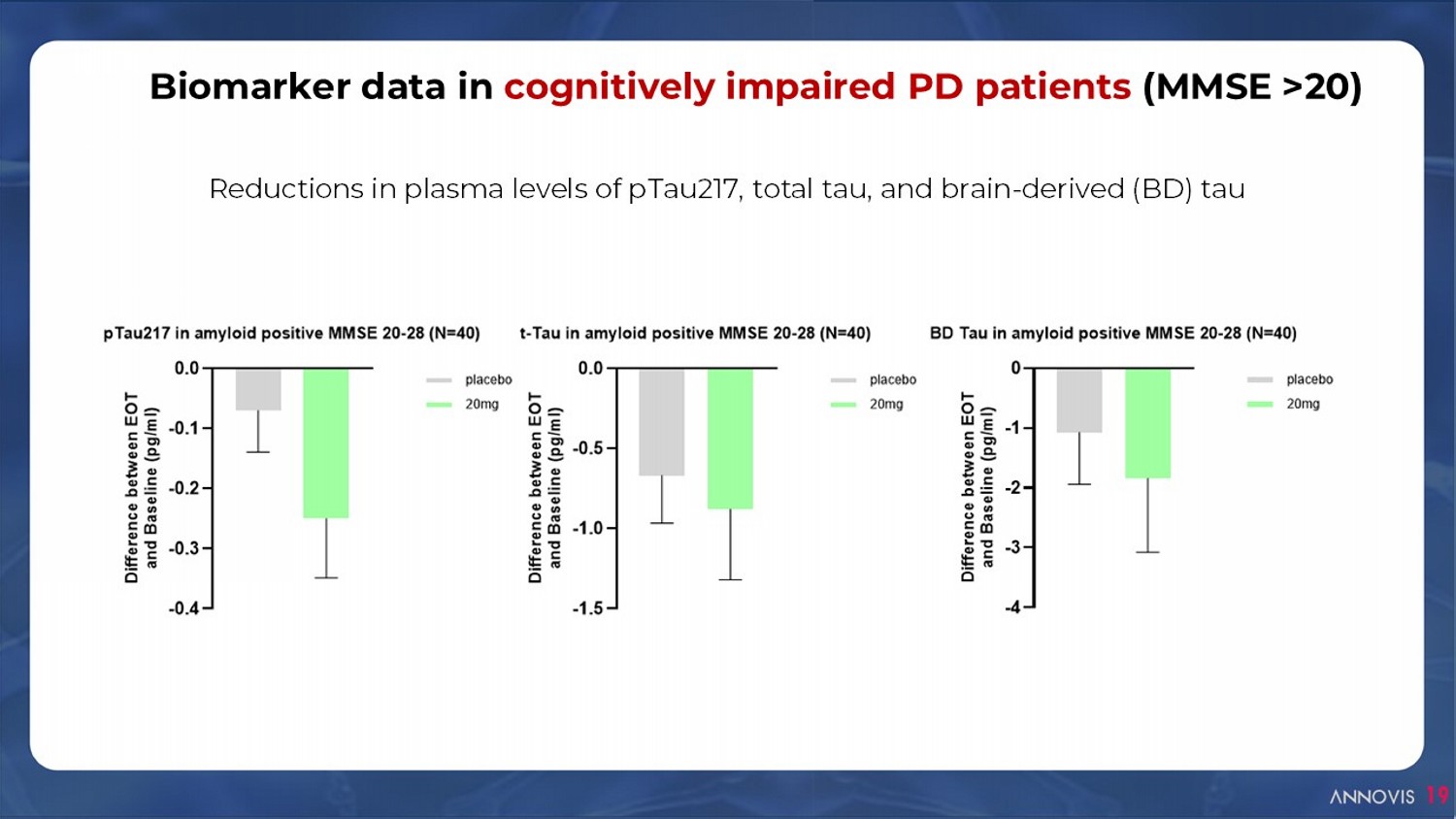
Biomarker data in cognitively impaired PD patients (MMSE >20) 19 Reductions in plasma levels of pTau217, total tau, and brain - derived (BD) tau
Update on the FDA meeting for PDD study 20 • Cognitive outcome (MMSE) and plasma biomarkers (pTau217) from previous studies opened the door for a new trial – to test buntanetap in amyloid positive PD patients with Parkinson’s disease dementia (PDD). • We received guidance from the FDA in January about this new PDD study. • The FDA expressed no objections, however there is currently no consensus on the endpoints in PDD. • We are currently in discussion with KOLs to identify appropriate endpoints that can best capture the treatment effect and lead to regulatory approval.

Summary: buntanetap and cognition in Alzheimer’s and Parkinson’s disease
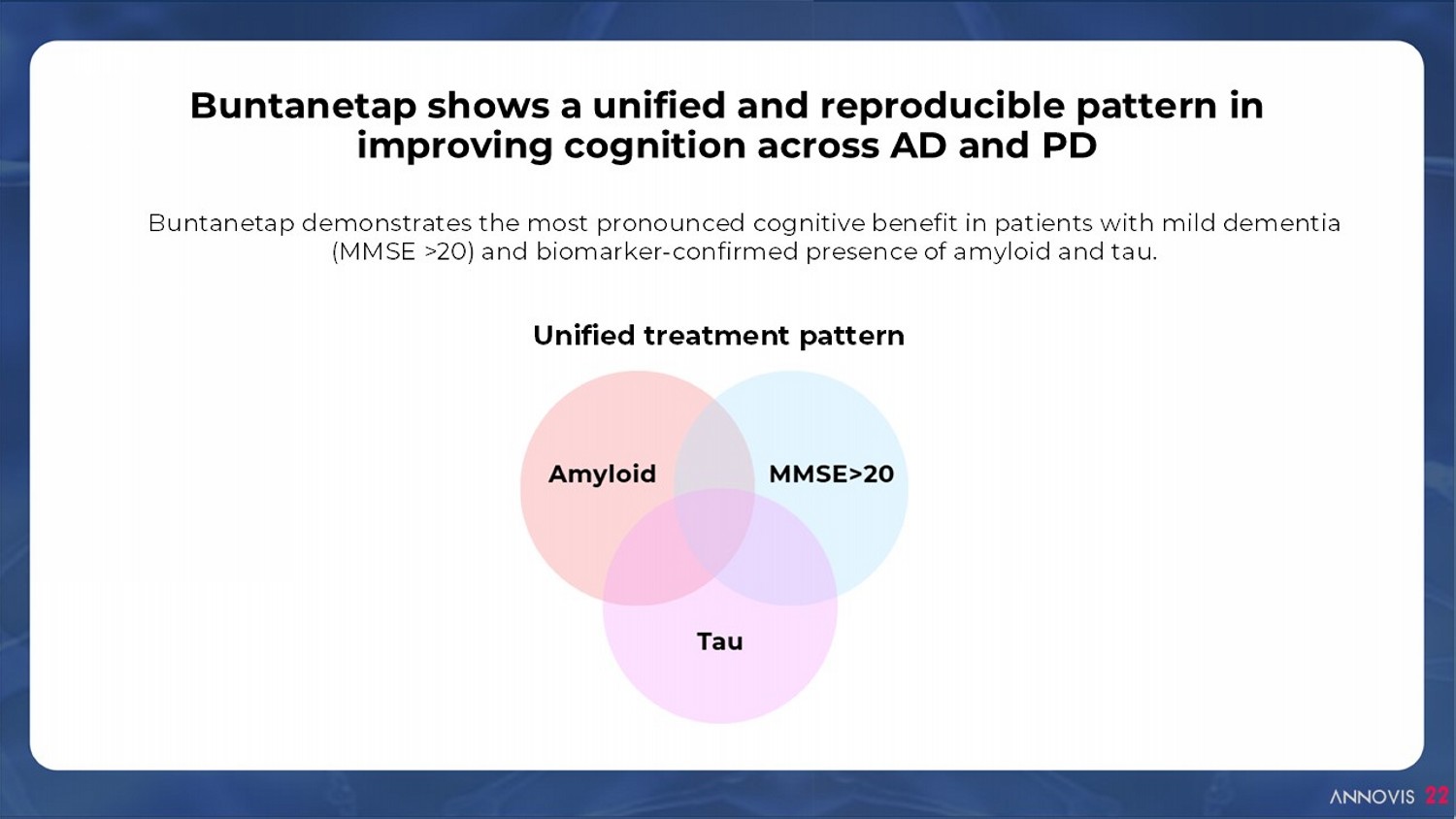
Buntanetap shows a unified and reproducible pattern in improving cognition across AD and PD 22 Unified treatment pattern Buntanetap demonstrates the most pronounced cognitive benefit in patients with mild dementia (MMSE >20) and biomarker - confirmed presence of amyloid and tau.
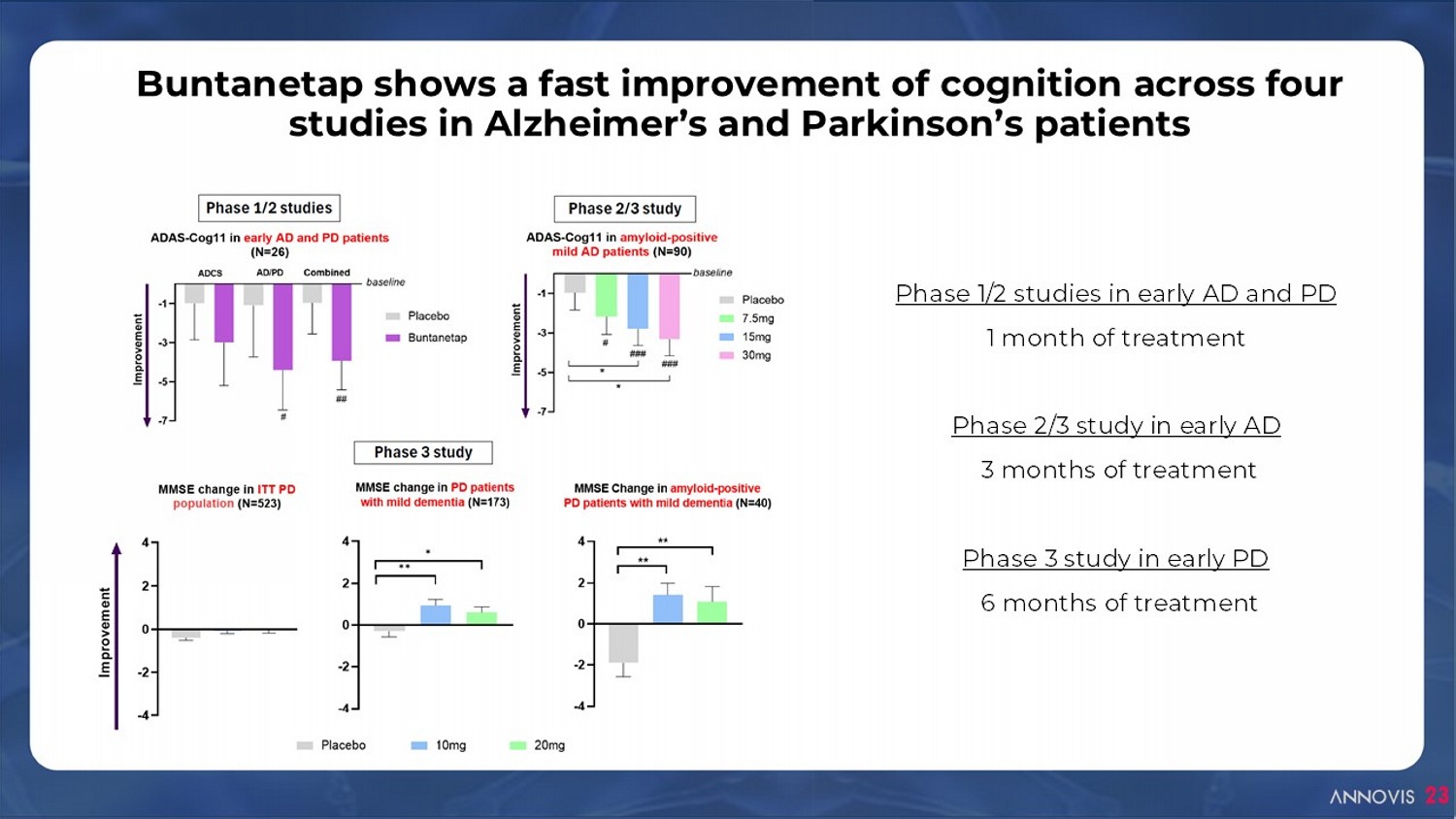
Buntanetap shows a fast improvement of cognition across four studies in Alzheimer’s and Parkinson’s patients Phase 1/2 studies in early AD and PD 1 month of treatment Phase 2/3 study in early AD 3 months of treatment Phase 3 study in early PD 6 months of treatment 23
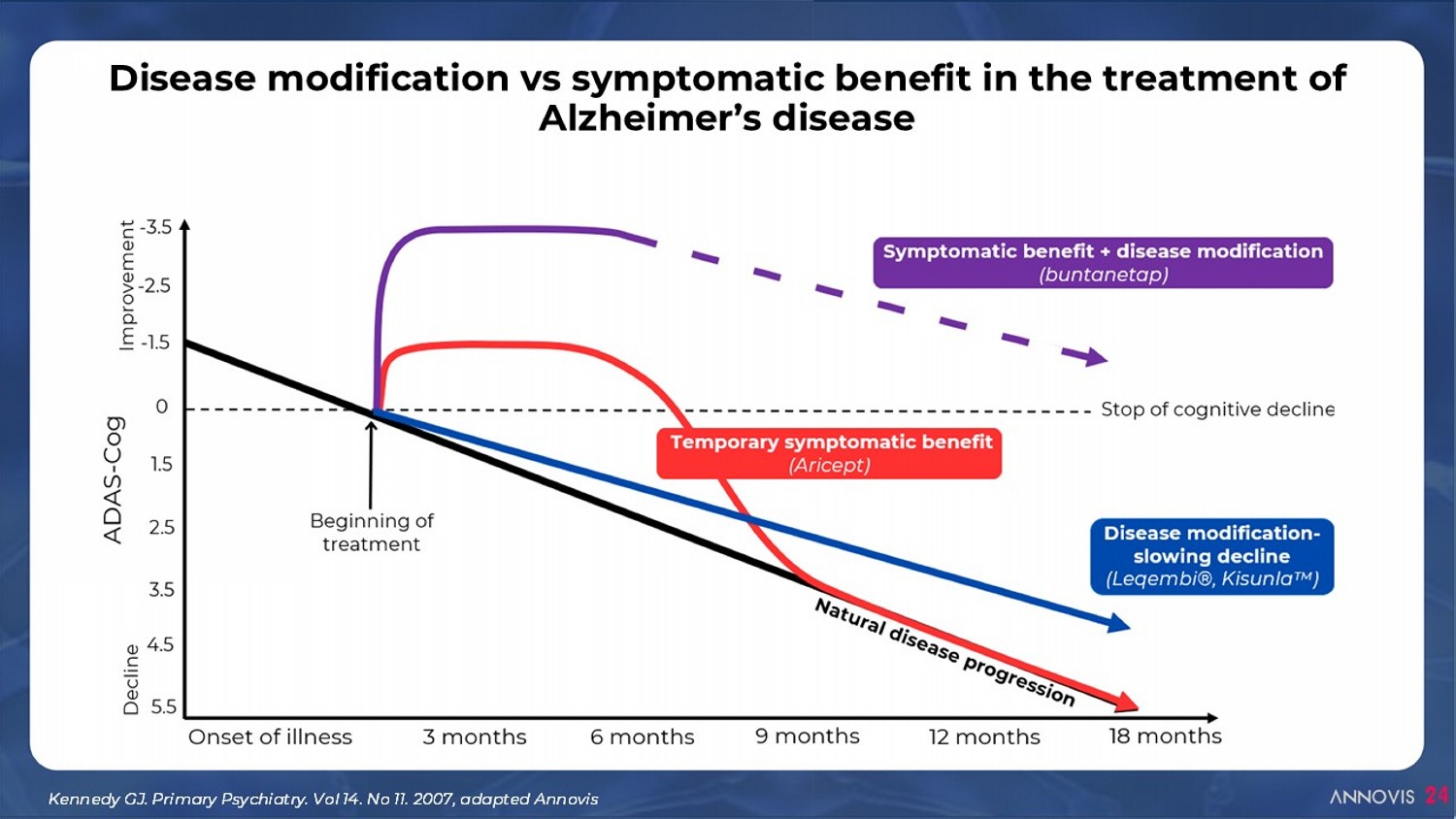
24 Disease modification vs symptomatic benefit in the treatment of Alzheimer’s disease Kennedy GJ. Primary Psychiatry. Vol 14. No 11. 2007, adapted Annovis
Summary 25 1. The enrollment for the pivotal Phase 3 study in early AD is actively progressing, with the full recruitment completion estimated in Spring 2026. 2. The OLE PD study has begun screening patients. 3. The FDA agrees with us conducting a PDD study and we are discussing the details with KOLs in Parkison’s dementia. 4. So far, buntanetap has shown significant cognitive improvement across AD and PD as well as a potential disease - modifying activity as measured by profound reductions in biomarkers linked to neurodegeneration.

Thank you