

NASDAQ: ATOS www.atossatherapeutics.com Corporate Presentation January 8, 2026 COVER

Disclaimer This presentation may contain certain forward-looking statements related to Atossa Therapeutics, Inc. (the “Company”) that involve risks and uncertainties. 01 Actual results, outcomes, or the timing of actual results or outcomes may differ significantly from those discussed in forward-looking statements. 02 Factors that might cause or contribute to such differences include, but are not limited to, those discussed in “Risk Factors” in the Company’s Annual Reports on Form 10-K and subsequent Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission. 03 The Company undertakes no obligation to update publicly any forward-looking statements to reflect new information, events, or circumstances. 04 This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdictions in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. 05 The Company may not yet have received clearance from the FDA or any other regulatory agency for some of the products described in this presentation. 06
Corporate Highlights Multi-billion market opportunity across the rapidly growing estrogen receptor positive breast cancer market Demonstrated broad utility across the breast cancer continuum… Recurrence following surgery Following DCIS High-risk dense breast tissue Neoadjuvant Metastatic Robust and Growing IP Portfolio with broad protections in the U.S. and globally Strong financial position with over a year of runway, and zero debt Experienced Leadership Team and world-renowned advisors Lead candidate oral (Z)-endoxifen is a potent selective estrogen receptor modulator/degrader (SERM/SERD) currently in Phase 2 trials
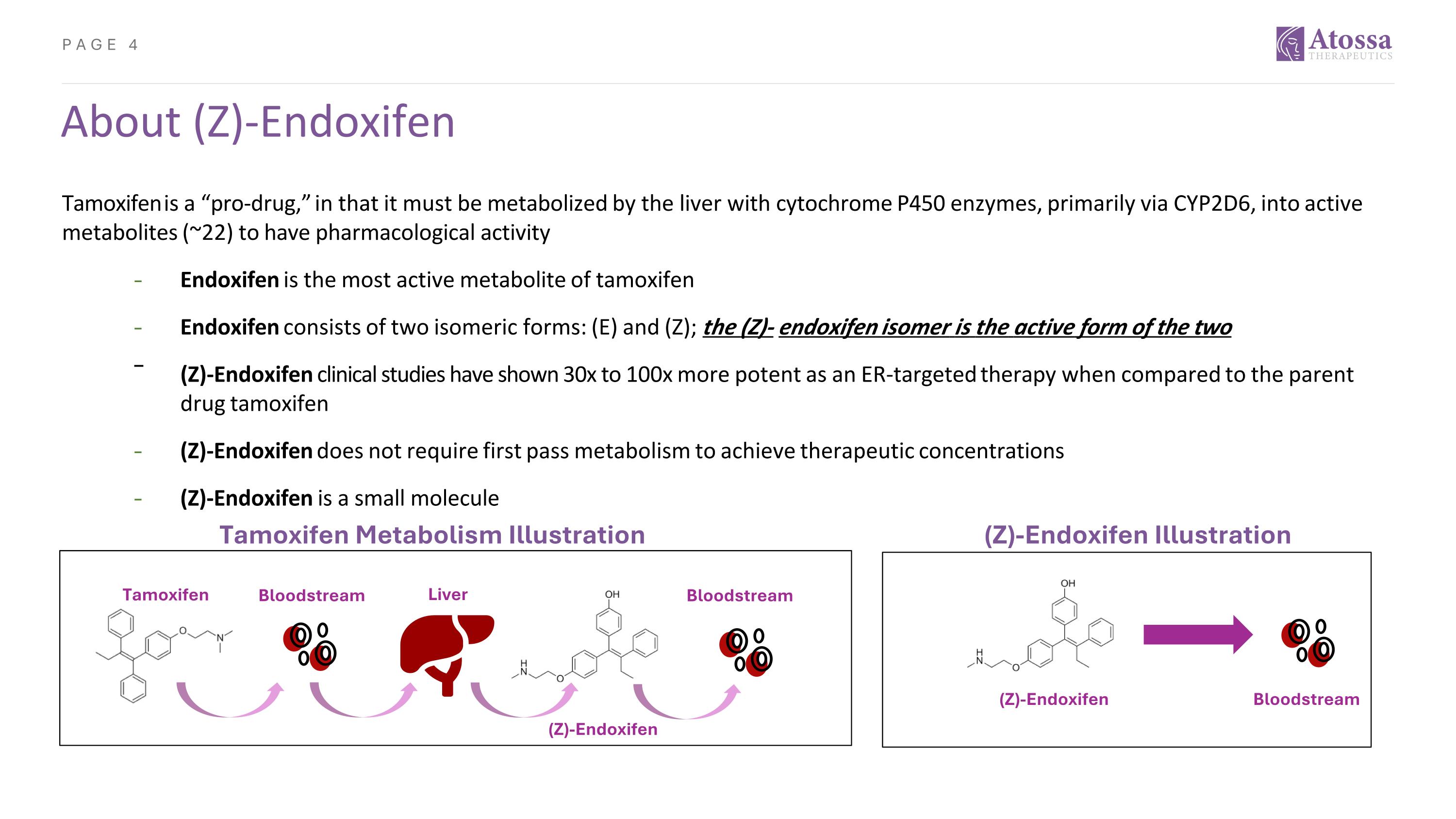
About (Z)-Endoxifen Tamoxifen is a “pro-drug,” in that it must be metabolized by the liver with cytochrome P450 enzymes, primarily via CYP2D6, into active metabolites (~22) to have pharmacological activity ⎼ Endoxifen is the most active metabolite of tamoxifen ⎼ Endoxifen consists of two isomeric forms: (E) and (Z); the (Z)- endoxifen isomer is the active form of the two (Z)-Endoxifen clinical studies have shown 30x to 100x more potent as an ER-targeted therapy when compared to the parent drug tamoxifen ⎼ (Z)-Endoxifen does not require first pass metabolism to achieve therapeutic concentrations ⎼ (Z)-Endoxifen is a small molecule Tamoxifen Bloodstream Bloodstream (Z)-Endoxifen Liver (Z)-Endoxifen Bloodstream Tamoxifen Metabolism Illustration (Z)-Endoxifen Illustration
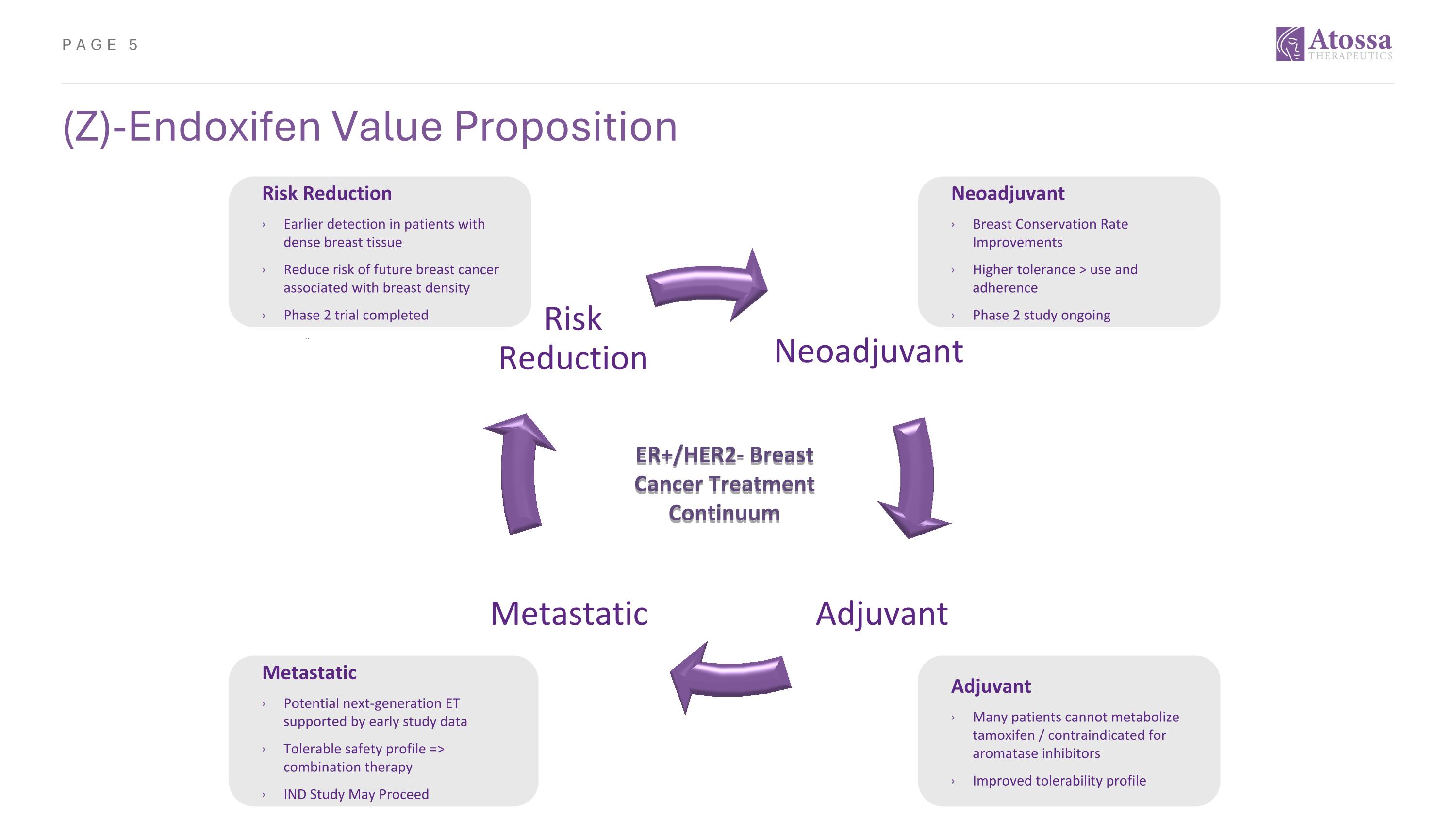
(Z)-Endoxifen Value Proposition Neoadjuvant Adjuvant Metastatic Risk Reduction Risk Reduction Earlier detection in patients with dense breast tissue Reduce risk of future breast cancer associated with breast density Phase 2 trial completed Neoadjuvant Breast Conservation Rate Improvements Higher tolerance > use and adherence Phase 2 study ongoing Adjuvant Many patients cannot metabolize tamoxifen / contraindicated for aromatase inhibitors Improved tolerability profile Metastatic Potential next-generation ET supported by early study data Tolerable safety profile => combination therapy IND Study May Proceed ER+/HER2- Breast Cancer Treatment Continuum
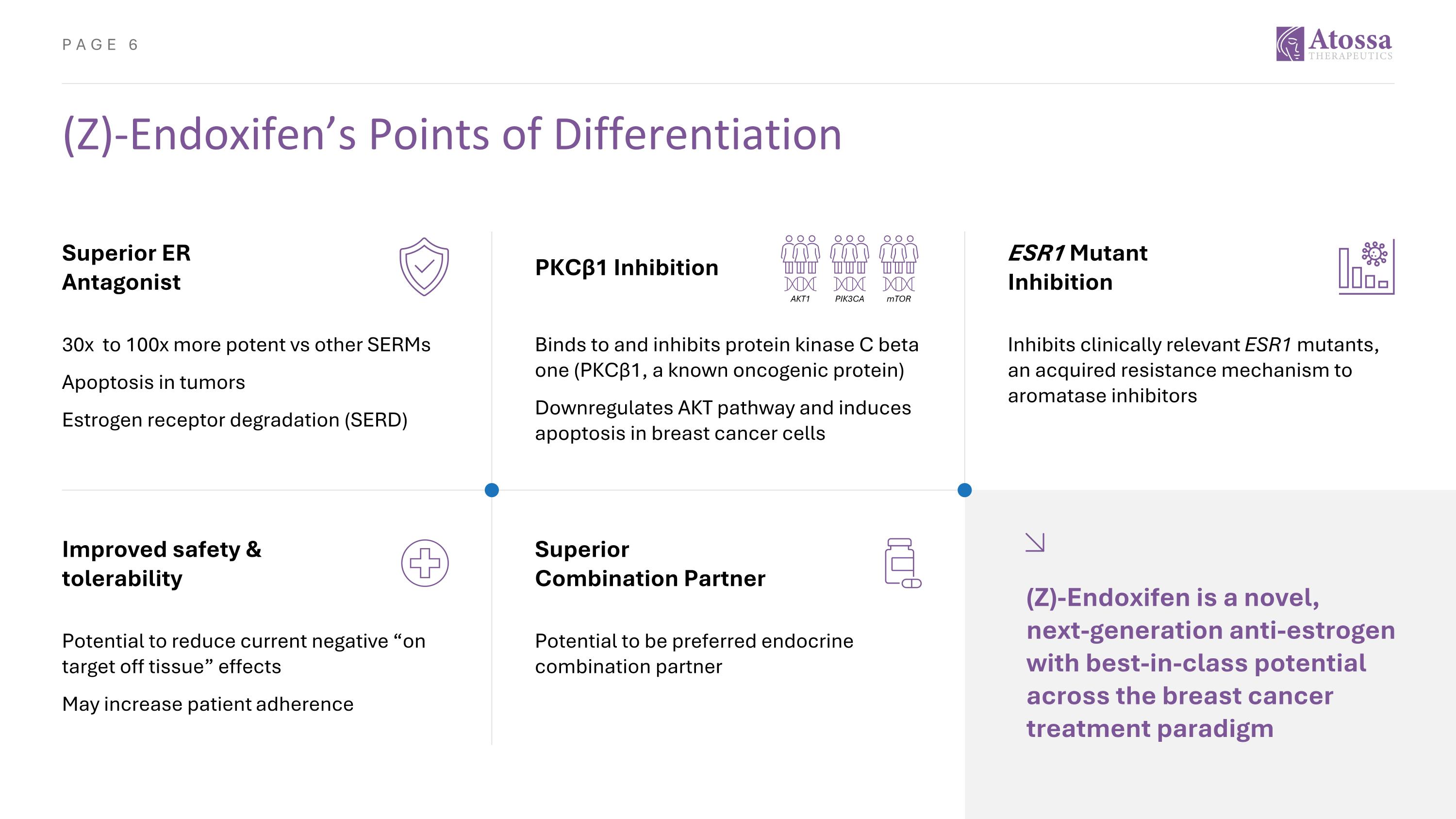
(Z)-Endoxifen’s Points of Differentiation Improved safety & tolerability Potential to reduce current negative “on target off tissue” effects May increase patient adherence Superior Combination Partner Potential to be preferred endocrine combination partner (Z)-Endoxifen is a novel, next-generation anti-estrogen with best-in-class potential across the breast cancer treatment paradigm Superior ER Antagonist 30x to 100x more potent vs other SERMs Apoptosis in tumors Estrogen receptor degradation (SERD) ESR1 Mutant Inhibition Inhibits clinically relevant ESR1 mutants, an acquired resistance mechanism to aromatase inhibitors PKCβ1 Inhibition Binds to and inhibits protein kinase C beta one (PKCβ1, a known oncogenic protein) Downregulates AKT pathway and induces apoptosis in breast cancer cells PIK3CA AKT1 mTOR
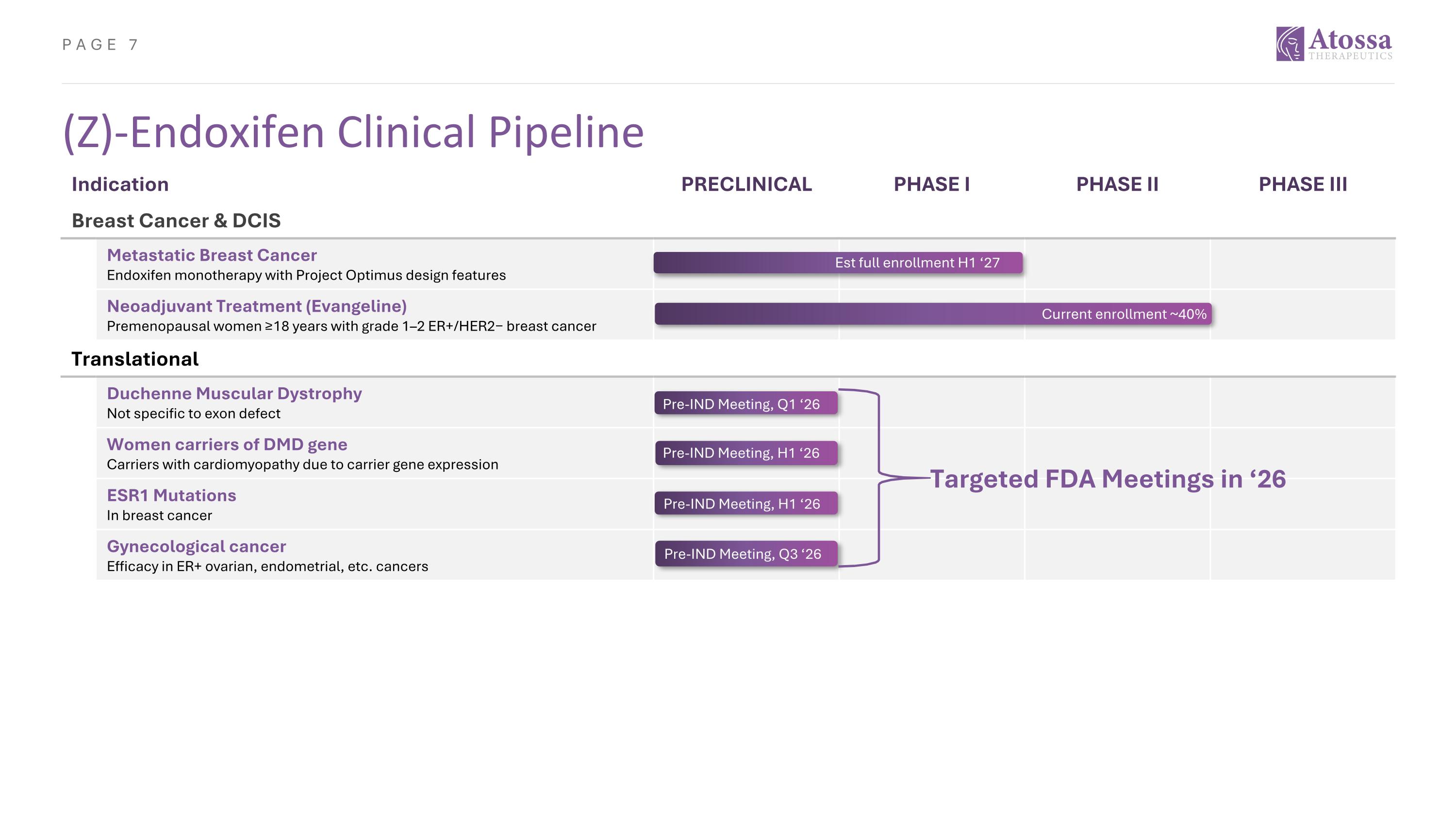
Indication PRECLINICAL PHASE I PHASE II PHASE III Breast Cancer & DCIS Metastatic Breast Cancer Endoxifen monotherapy with Project Optimus design features Neoadjuvant Treatment (Evangeline) Premenopausal women ≥18 years with grade 1–2 ER+/HER2− breast cancer Translational Duchenne Muscular Dystrophy Not specific to exon defect Women carriers of DMD gene Carriers with cardiomyopathy due to carrier gene expression ESR1 Mutations In breast cancer Gynecological cancer Efficacy in ER+ ovarian, endometrial, etc. cancers Pre-IND Meeting, H1 ‘26 (Z)-Endoxifen Clinical Pipeline Est full enrollment H1 ‘27 Current enrollment ~40% Targeted FDA Meetings in ‘26 Pre-IND Meeting, H1 ‘26 Pre-IND Meeting, Q3 ‘26 Pre-IND Meeting, Q1 ‘26 Pre-IND Meeting, H1 ‘26
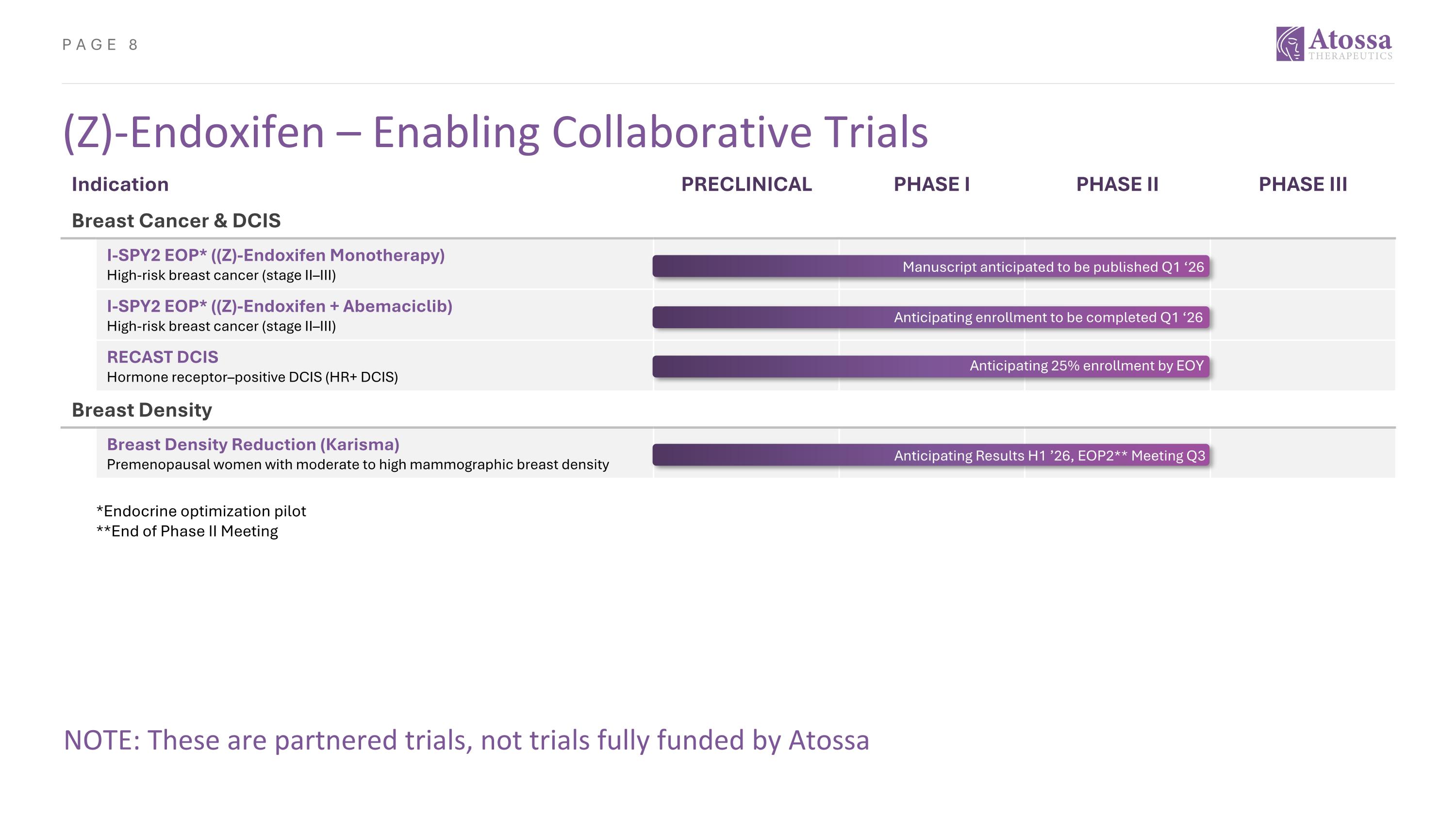
(Z)-Endoxifen – Enabling Collaborative Trials Indication PRECLINICAL PHASE I PHASE II PHASE III Breast Cancer & DCIS I-SPY2 EOP* ((Z)-Endoxifen Monotherapy) High-risk breast cancer (stage II–III) I-SPY2 EOP* ((Z)-Endoxifen + Abemaciclib) High-risk breast cancer (stage II–III) RECAST DCIS Hormone receptor–positive DCIS (HR+ DCIS) Breast Density Breast Density Reduction (Karisma) Premenopausal women with moderate to high mammographic breast density ??? Enrollment ~40% Manuscript anticipated to be published Q1 ‘26 Anticipating enrollment to be completed Q1 ‘26 Anticipating 25% enrollment by EOY NOTE: These are partnered trials, not trials fully funded by Atossa Anticipating Results H1 ’26, EOP2** Meeting Q3 *Endocrine optimization pilot **End of Phase II Meeting
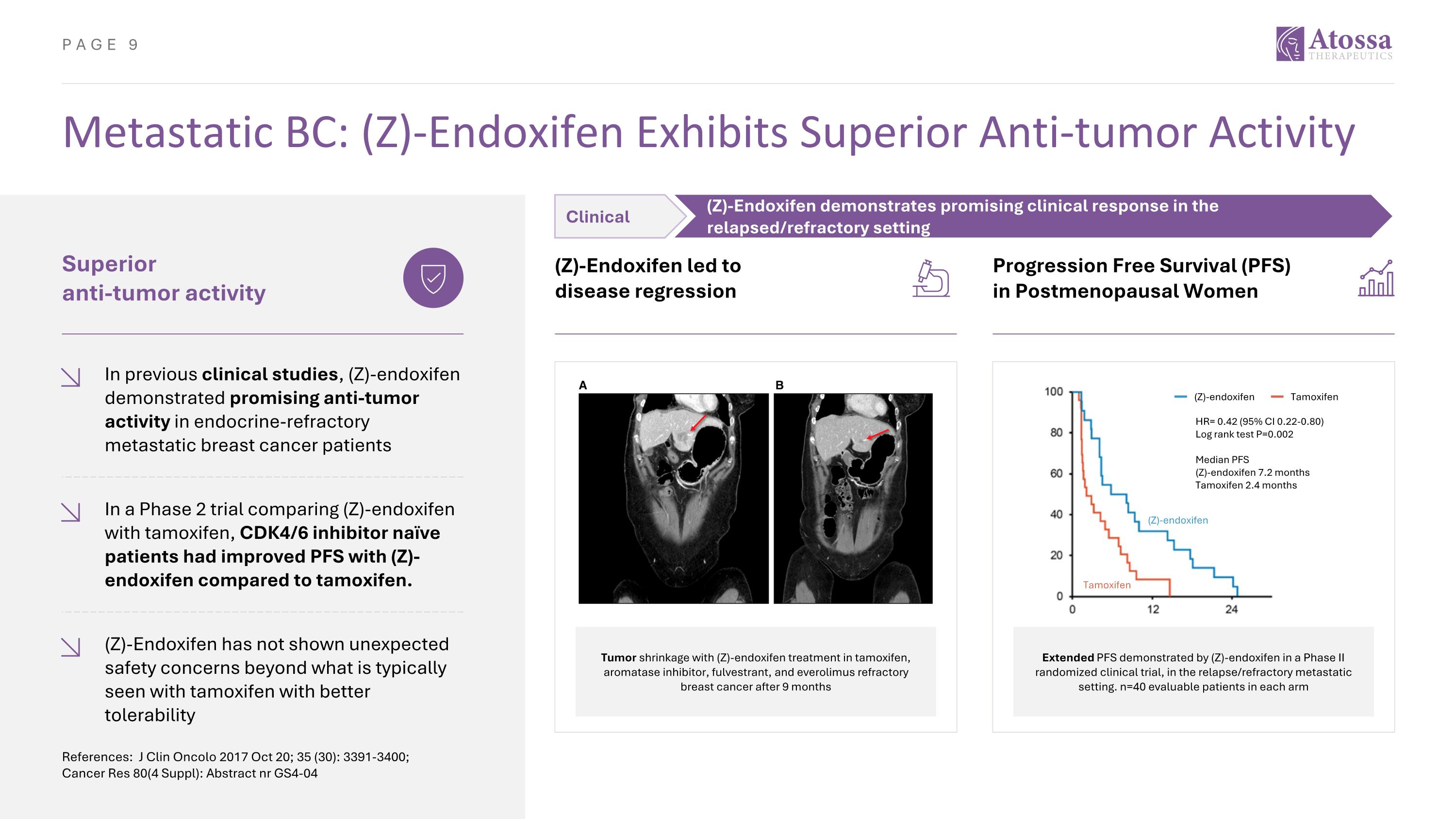
Metastatic BC: (Z)-Endoxifen Exhibits Superior Anti-tumor Activity References: J Clin Oncolo 2017 Oct 20; 35 (30): 3391-3400; Cancer Res 80(4 Suppl): Abstract nr GS4-04 Superior anti-tumor activity In previous clinical studies, (Z)-endoxifen demonstrated promising anti-tumor activity in endocrine-refractory metastatic breast cancer patients (Z)-Endoxifen led to disease regression Tumor shrinkage with (Z)-endoxifen treatment in tamoxifen, aromatase inhibitor, fulvestrant, and everolimus refractory breast cancer after 9 months In a Phase 2 trial comparing (Z)-endoxifen with tamoxifen, CDK4/6 inhibitor naïve patients had improved PFS with (Z)-endoxifen compared to tamoxifen. (Z)-Endoxifen has not shown unexpected safety concerns beyond what is typically seen with tamoxifen with better tolerability Progression Free Survival (PFS) in Postmenopausal Women Extended PFS demonstrated by (Z)-endoxifen in a Phase II randomized clinical trial, in the relapse/refractory metastatic setting. n=40 evaluable patients in each arm (Z)-endoxifen Tamoxifen HR= 0.42 (95% CI 0.22-0.80) Log rank test P=0.002 Median PFS (Z)-endoxifen 7.2 months Tamoxifen 2.4 months (Z)-endoxifen Tamoxifen Clinical (Z)-Endoxifen demonstrates promising clinical response in the relapsed/refractory setting
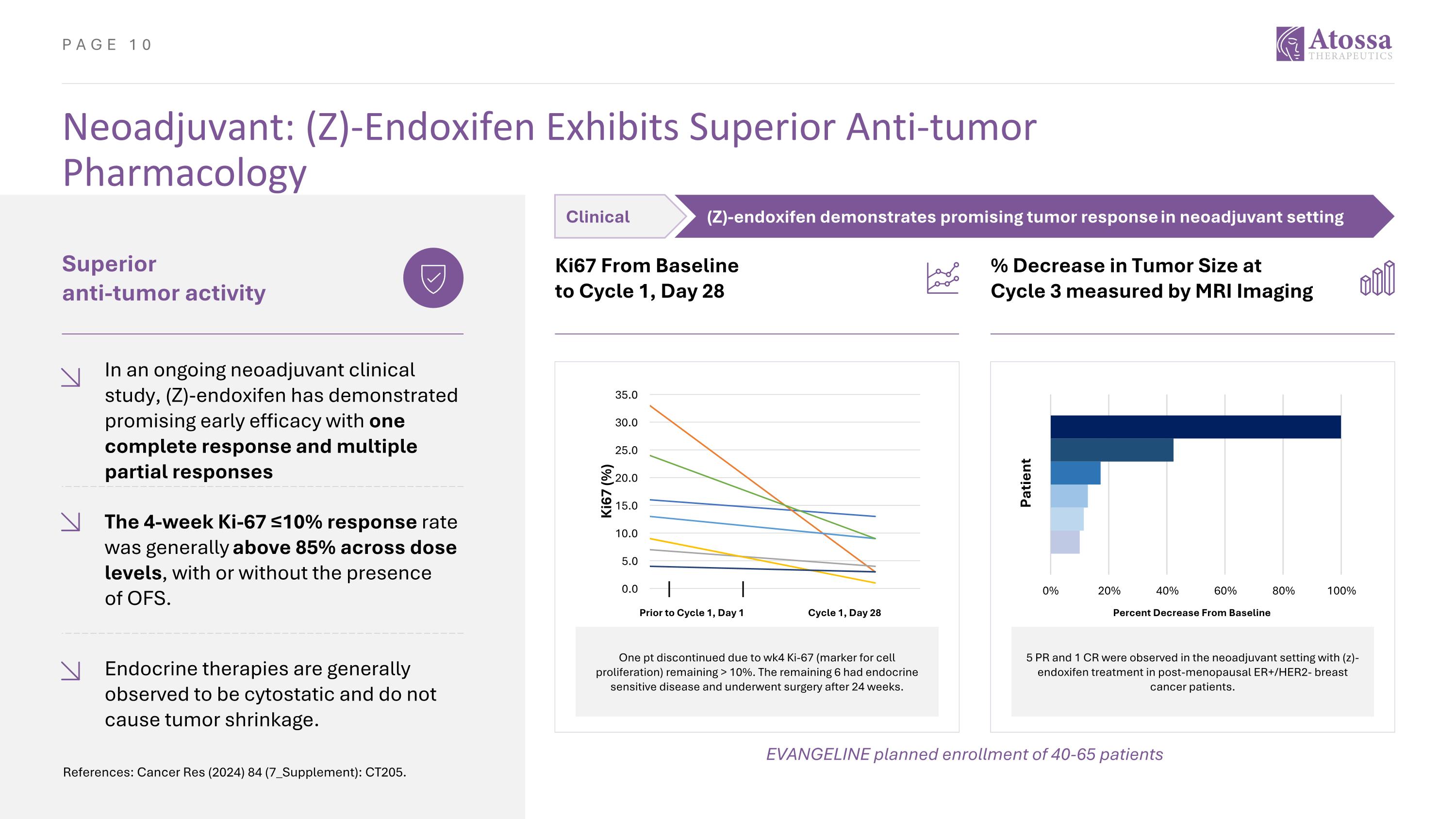
Neoadjuvant: (Z)-Endoxifen Exhibits Superior Anti-tumor Pharmacology Superior anti-tumor activity In an ongoing neoadjuvant clinical study, (Z)-endoxifen has demonstrated promising early efficacy with one complete response and multiple partial responses The 4-week Ki-67 ≤10% response rate was generally above 85% across dose levels, with or without the presence of OFS. Ki67 From Baseline to Cycle 1, Day 28 Endocrine therapies are generally observed to be cytostatic and do not cause tumor shrinkage. % Decrease in Tumor Size at Cycle 3 measured by MRI Imaging One pt discontinued due to wk4 Ki-67 (marker for cell proliferation) remaining > 10%. The remaining 6 had endocrine sensitive disease and underwent surgery after 24 weeks. Prior to Cycle 1, Day 1 Cycle 1, Day 28 5 PR and 1 CR were observed in the neoadjuvant setting with (z)-endoxifen treatment in post-menopausal ER+/HER2- breast cancer patients. Percent Decrease From Baseline EVANGELINE planned enrollment of 40-65 patients References: Cancer Res (2024) 84 (7_Supplement): CT205. Clinical (Z)-endoxifen demonstrates promising tumor response in neoadjuvant setting

Chemistry, Manufacturing and Controls (CMC) Active Pharmaceutical Ingredient Defined process Stable, small molecule Reliable high-quality suppliers Enabling redundancies Complex manufacturing process, in development and robust (since 2017) Drug Product Strengths (various up to 40mg) Low dose High dose– proprietary process Stable (Z)-Endoxifen Manufacturing

Emerging (Z)-Endoxifen Treatment Opportunities DMD Upregulation of utrophin Utrophin is not mutated in DMD (like dystrophin) Mutation agnostic approach with naturally expressed, functionally competent protein Not limited to specific exon mutations or nonsense errors (Z)-Endoxifen’s mechanism of action goes beyond BC New Opportunities Gynecological Cancers Modulates ERα transcriptional activity at low concentrations, inhibiting proliferation in ER+ positive endometrial cancer models. Inhibits PKCβ, suppresses AKT signaling and induces apoptosis, with enhanced anti-tumor activity when combined with CDK4/6 inhibition (abemaciclib) ESR1 Receptor Mutation Resistance Maintains antagonistic activity in Y537S and ESR1 mutants by stabilizing the inactive conformation. Suppresses ER signaling in luciferase assays across Wild-type and ESR1-mutant receptors Reversal of key ESR1-mutant–associated programs; ER, E2F, and Myc targets, and restores phos and p53 signaling pathways Symptomatic Women Carriers of DMD Female DMD carriers have mosaic dystrophin loss, driving subclinical muscle and cardiac fibrosis. Endoxifen modulates estrogen signaling to reduce fibrosis and inflammation, independent of dystrophin restoration. Lowering the dystrophin functional threshold may preserve cardiac and muscle function in carriers.

Potent SERM with muscle‑protective, anti‑inflammatory, and anti‑fibrotic effects MDX Mouse Model of DMD Improves muscle strength, motor performance, and phasic force in dystrophic models Reduces plasma CK as well as muscle necrosis toward wild‑type profiles Provides cardiac functional benefits Builds on and shown to exceed clinical findings observed with tamoxifen Strong mechanistic and translational rationale for clinical development Source: CureDuchenne Upcoming milestone: Pre-IND meeting Q1 2026 (Z)-Endoxifen for Duchenne: The Next Big Leap

Finance $ 40M+ Disciplined capital investment following defined regulatory pathway(s) Clinical trial activity represents more than 50% of total spend Annual clinical investment not expected to increase significantly New trials will ramp up as existing trials wind down Estimated Cash and Cash Equivalents (as of 12/31/25) Expected to cover more than one year of working capital and NO debt. No warrants outstanding. Use of Proceeds

Experienced Leadership Chairman, CEO and President Chief Financial Officer SVP, Business Operations Steven Quay, MD, PhD Mark Daniel, CPA Delly Behen, PHR, SHRM-CP SVP, Research & Development Janet Rea, MSPH

World-renowned Thought Leadership Karisma Principal Investigator Per Hall, M.D., PhD i-Spy Principal Investigator Laura Esserman, M.D., MBA EVANGELINE Principal Investigator Matthew Goetz, M.D.

Cautionary Note Regarding Forward Looking Statements This presentation contains certain “forward-looking statements” within the meaning of applicable securities laws, including but not limited to, our expectations regarding the Company’s development and regulatory strategy and related milestones, the potential indications that the Company may pursue for (Z)-Endoxifen, the potential for (Z)-Endoxifen to receive regulatory approval and the timing thereof, the potential market and growth opportunities for the Company and the Company’s estimated cash and cash equivalents and its expectations related thereto. Words such as “expect,” “potential,” “continue,” “may,” “will,” “should,” “could,” “would,” “seek,” “intend,” “plan,” “estimate,” “anticipate,” “believe,” “design,” “predict,” “future,” or other similar expressions or statements regarding intent, belief or current expectations, are forward-looking statements. Forward-looking statements in this presentation are subject to risks and uncertainties that may cause actual results, outcomes, or the timing of actual results or outcomes to differ materially from those projected or anticipated, including, without limitation, risks and uncertainties associated with: our ability to successfully execute our strategy to shorten our clinical development timelines and pursue a metastatic breast cancer indication or other indications for our lead program, (Z)-Endoxifen; expected timing, completion and results of our preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; the timing or likelihood of regulatory filings and approvals; the outcome or timing of necessary regulatory approvals; our ability to regain and maintain compliance with Nasdaq listing requirements; our ability to establish and maintain intellectual property rights covering our products; the impact of general macroeconomic conditions on our business; our ability to raise capital; and other risks and uncertainties detailed from time to time in the Company’s filings with the SEC, including, without limitation, its Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q. Forward-looking statements are presented as of the date of this presentation. Except as required by law, we do not intend to update any forward-looking statements.

NASDAQ: ATOS www.atossatherapeutics.com Thank You Investor and Media Contact: CORE IR ir@atossatherapeutics.com
Appendix

Appendix: Key Scientific References Tamoxifen, Endoxifen & SERM Biology Johnson MD et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004. Goetz MP et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005. Wu X et al. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009. MacGregor & Jordan. Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev. 1998. Endocrine Resistance & ESR1 Mutations Jeselsohn R et al. ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015. Toy W et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013. CDK4/6 Combinations & Endocrine Therapy Limitations Finn RS et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016. Hortobagyi GN et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer). N Engl J Med. 2016. Mammographic Breast Density (MBD) & Prevention Cuzick J et al. Tamoxifen-Induced Reduction in Mammographic Density and Breast Cancer Risk Reduction: A Nested Case–Control Study. JNCI. 2011. Hammarström M et al. Influence of endoxifen on mammographic density: KARISMA-Tam trial. J Natl Cancer Inst. 2025;117(4):629-636. doi:10.1093/jnci/djae280. Clinical Trials of (Z)-Endoxifen Goetz MP et al. First-in-human phase I Study of the tamoxifen metabolite Z-endoxifen in women with endocrine-refractory metastatic breast cancer. J Clin Oncol. 2017. Goetz MP et al. J Clin Oncol. 2023;41(suppl 16):TPS633. DMD Biological Rationale Remmel HL, Hammer SS, Neff LA, et al. A hypothesized therapeutic role of (Z)-endoxifen in Duchenne muscular dystrophy. Degener Neurol Neuromuscul Dis. 2025;15:1–15. doi:10.2147/DNND.S496904. Adherence, Unmet Need & Real-World Burden Hershman DL et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. J Clin Oncol. 2010.

Appendix: NCT References NCT05607004: (Z)-Endoxifen for the Treatment of Premenopausal Women With ER+/HER2- Breast Cancer (EVANGELINE) NCT05068388: Effect of Oral (Z)-Endoxifen in Premenopausal Women With Measurable Breast Density (Sweden) NCT01042379: I-SPY TRIAL: Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer (Quantum Leap Healthcare Collaborative) NCT06075953: DCIS: RECAST Trial Ductal Carcinoma In Situ: Re-Evaluating Conditions for Active Surveillance Suitability as Treatment (Quantum Leap Healthcare Collaborative)