
.2

Electromed, Inc. Investor Presentation August 26, 2025 NYSE American: ELMD Innovation Leader in Airway Clearance Technologies

Forward Looking Statements Certain statements in this presentation constitute forward-looking statements as defined in the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “anticipate,” “believe,” “committed,” “continue,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “should,” “will,” and similar expressions, including the negative of these terms, but they are not the exclusive means of identifying such statements. Forward-looking statements cannot be guaranteed, and actual results may vary materially due to the uncertainties and risks, known or unknown associated with such statements. Examples of risks and uncertainties for Electromed include, but are not limited to, competitive nature of our market; changes to Medicare, Medicaid, or private insurance reimbursement policies; changes to state and federal health care laws; changes affecting the medical device industry; our ability to develop new sales channels for our products such as the homecare distributor channel; our need to maintain regulatory compliance and to gain future regulatory approvals and clearances; new drug or pharmaceutical discoveries; general economic and business conditions; component or raw material shortages; changes to lead times or changes to trade regulations; wage and component price inflation; the risks associated with cyberattacks, data breaches, computer viruses and other similar security threats; technical problems with our research and products; our ability to renew our line of credit or obtain additional credit as necessary; our ability to protect and expand our intellectual property portfolio, as well as other factors we may describe from time to time in Electromed’s reports filed with the Securities and Exchange Commission (including Electromed’s most recent Annual Report on Form 10-K, as amended from time to time, and subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K). Investors should not consider any list of such factors to be an exhaustive statement of all the risks, uncertainties or potentially inaccurate assumptions investors should take into account when making investment decisions. Shareholders and other readers should not place undue reliance on “forward-looking statements,” as such statements speak only as of the date of this press release. We undertake no obligation to update them in light of new information or future events.

Electromed – Who We Are Electromed, Inc. is a growing medical device company focused on airway management to help people around the world breathe better, stay healthier, and lead active and fulfilling lives. Headquarters:New Prague, MN Ticker:ELMD Established:1992 Annual Revenue:$64.0M Market Cap:$187M Share Count:8.5M 179 Employees Manufacturing in Minnesota HFCWO Market Focus
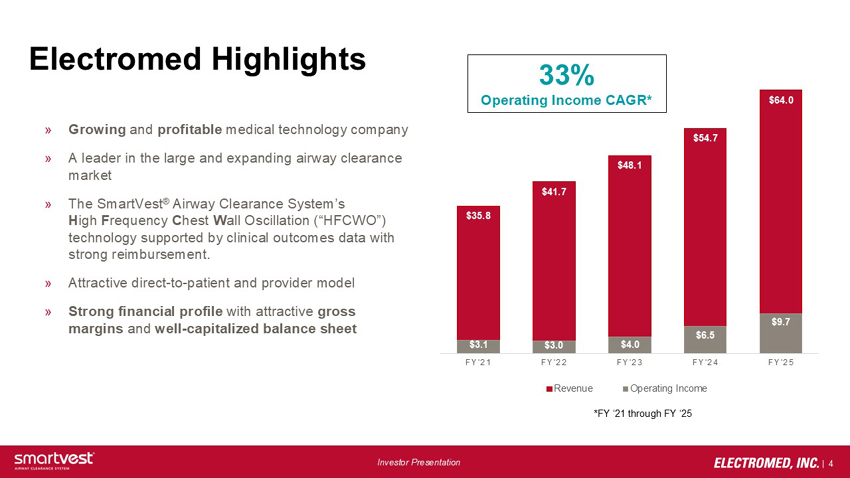
a. Electromed Highlights - Growing and profitable medical technology company - A leader in the large and expanding airway clearance market - The SmartVest® Airway Clearance System’s High Frequency Chest Wall Oscillation (“HFCWO”) technology supported by clinical outcomes data with strong reimbursement -Attractive direct-to-patient and provider model -Strong financial profile with attractive gross margins and well-capitalized balance sheet - 33% Operating Income CAGR* FY’21 Revenue = $35.8, FY’21 Operating Income = $3.1, FY’22 Revenue = $41.7, FY’22 Operating Income = $3.0, FY’23 Revenue = $48.1, FY’23 Operating Income = $4.0, FY’24 Revenue = $54.7, FY’24 Operating Income = $6.5, FY'25 Revenue = $64.0, FY’25 Operating Income = $9.7 (Amounts in millions)
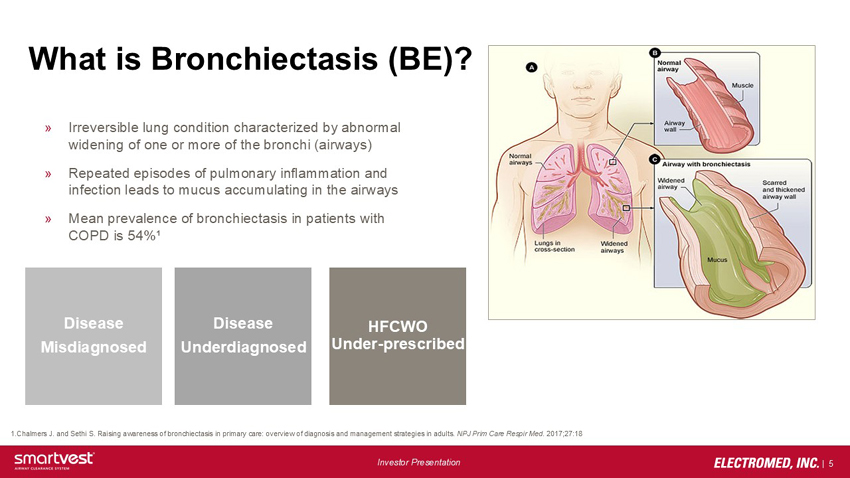
What is Bronchiectasis (BE)? Irreversible lung condition characterized by abnormal widening of one or more of the bronchi (airways) Repeated episodes of pulmonary inflammation and infection leads to mucus accumulating in the airways Mean prevalence of bronchiectasis in patients with COPD is 54%¹ Disease Misdiagnosed Disease Underdiagnosed HFCWO Under-prescribed 1.Chalmers J. and Sethi S. Raising awareness of bronchiectasis in primary care: overview of diagnosis and management strategies in adults. NPJ Prim Care Respir Med. 2017;27:18
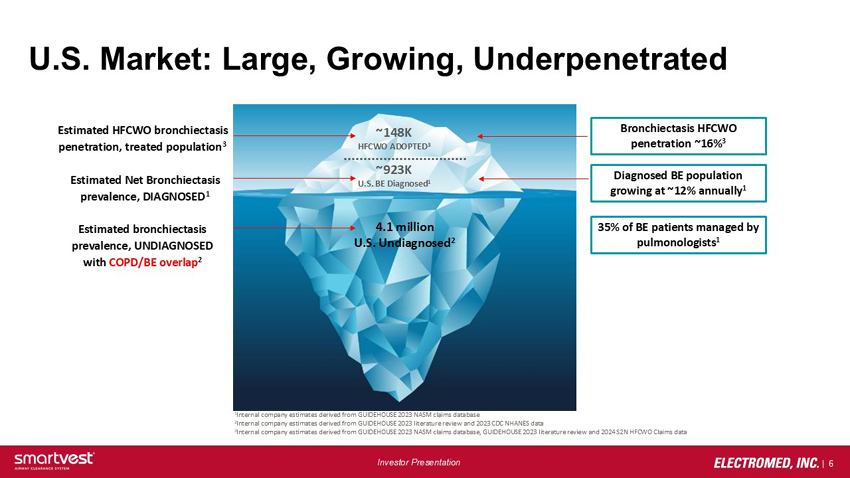
U.S. Market: Large, Growing, Underpenetrated Estimated HFCWO bronchiectasis penetration, treated population3 ~148k HFCWO ADOPTED3 Bronchiectasis HFCWO penetration ~16%3 Estimated Net Bronchiectasis prevalence, DIAGNOSED1 ~923k U.S. BE Diagnosed1 Diagnosed BE population growing at ~12% annually1 Estimated bronchiectasis prevalence, UNDIAGNOSED with COPD/BE overlap2 4.1 million U.S. Undiagnosed2 35% of BE patients managed by pulmonologists1 1Internal company estimates derived from GUIDEHOUSE 2023 NASM claims database 2Internal company estimates derived from GUIDEHOUSE 2023 literature review and 2023 CDC NHANES data 3Internal company estimates derived from GUIDEHOUSE 2023 NASM claims database, GUIDEHOUSE 2023 literature review and 2024 S2N HFCWO Claims data
How is Bronchiectasis Treated? Antibiotics, Anti-Inflammatories, Airway Clearance
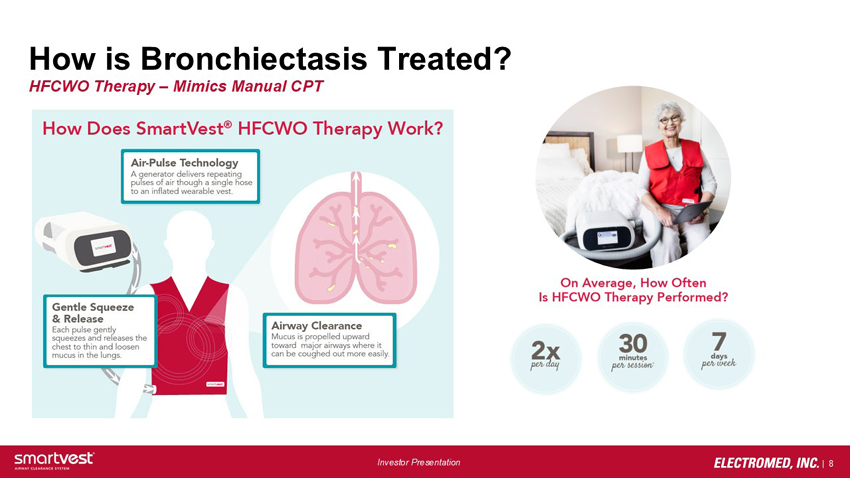
How is Bronchiectasis Treated? HFCWO Therapy – Mimics Manual CPT

SmartVest Clearway® HFCWO Designed with the Patient in Mind An Enhanced Patient Experience Sleek and light weight generator Intuitive user interface for better patient adherence More portable and easier for travel SmartVest® has a well-established reimbursement code from CMS – E0483; We estimate we have over 250 million contracted lives in the US
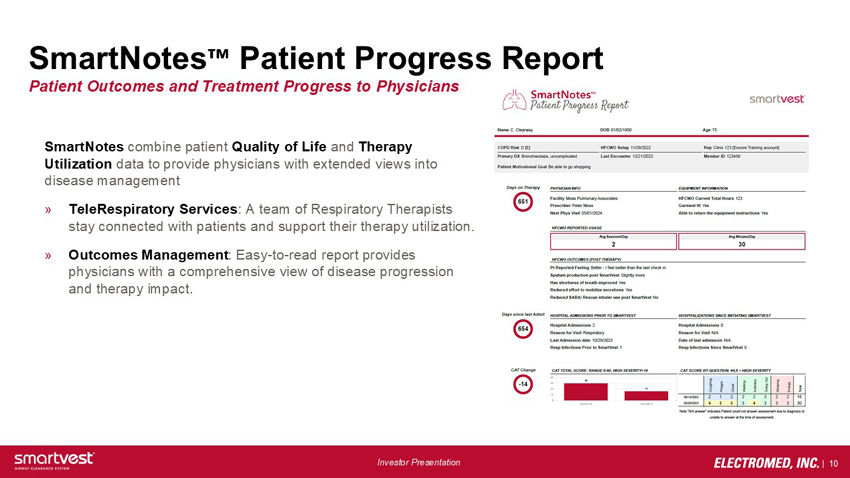
SmartNotes™ Patient Progress Report Patient Outcomes and Treatment Progress to Physicians SmartNotes combine patient Quality of Life and Therapy Utilization data to provide physicians with extended views into disease management TeleRespiratory Services: A team of Respiratory Therapists stay connected with patients and support their therapy utilization. Outcomes Management: Easy-to-read report provides physicians with a comprehensive view of disease progression and therapy impact.
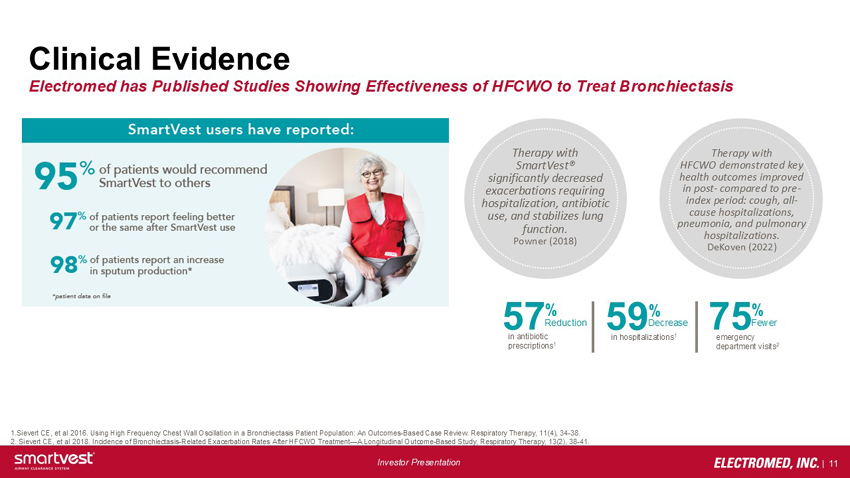
Clinical Evidence Electromed has Published Studies Showing Effectiveness of HFCWO to Treat Bronchiectasis Therapy with SmartVest® significantly decreased exacerbations requiring hospitalization, antibiotic use, and stabilizes lung function. Powner (2018) Therapy with HFCWO demonstrated key health outcomes improved in post- compared to pre-index period: cough, all-cause hospitalizations, pneumonia, and pulmonary hospitalizations. DeKoven (2022) 57% Reduction in antibiotic prescriptions1 59% Decrease in hospitalizations1 75% Fewer emergency department visits2 1.Sievert CE, et al 2016. Using High Frequency Chest Wall Oscillation in a Bronchiectasis Patient Population: An Outcomes-Based Case Review. Respiratory Therapy, 11(4), 34-38. 2. Sievert CE, et al 2018. Incidence of Bronchiectasis-Related Exacerbation Rates After HFCWO Treatment—A Longitudinal Outcome-Based Study, Respiratory Therapy, 13(2), 38-41.
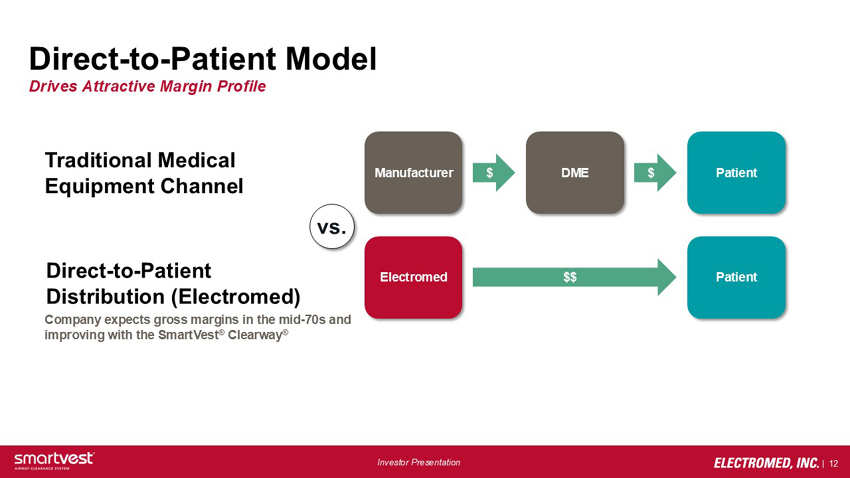
Direct-to-Patient Model Drives Attractive Margin Profile Traditional Medical Equipment Channel Direct-to-Patient Distribution (Electromed) Company expects gross margins in the mid-70s and improving with the SmartVest® Clearway® Manufacturer $ DME $ Patient vs. Electromed $$ Patient
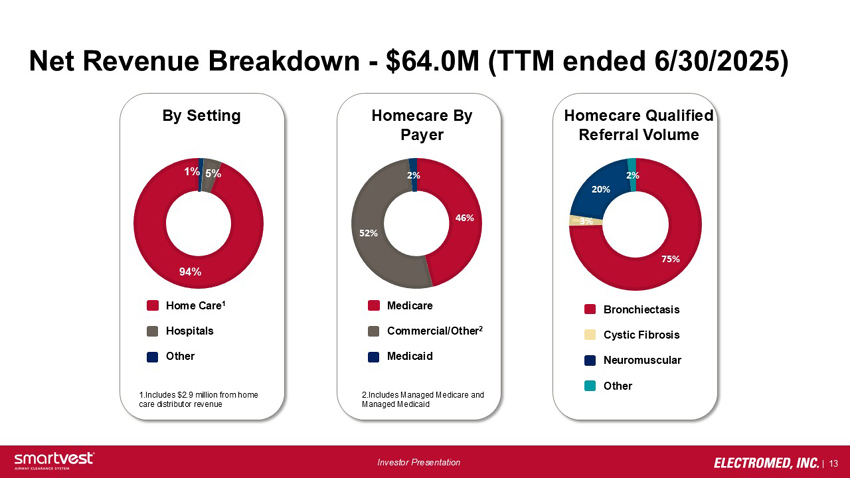
Net Revenue Breakdown - $64.0M (TTM ended 6/30/2025) - By Setting: 1% (Other), 5% (Hospitals), 94% (Home Care, includes $2.9M from home care distributor revenue). Homecare By Payer: 2% (Medicaid), 46% (Medicare), 52% (Commercial/Other, Includes Managed Medicare and Managed Medicaid). Homecare Qualified Referral Volume: 2% (Other), 20% (Neuromuscular), 3% (Cystic Fibrosis), 75% (Bronchiectasis).
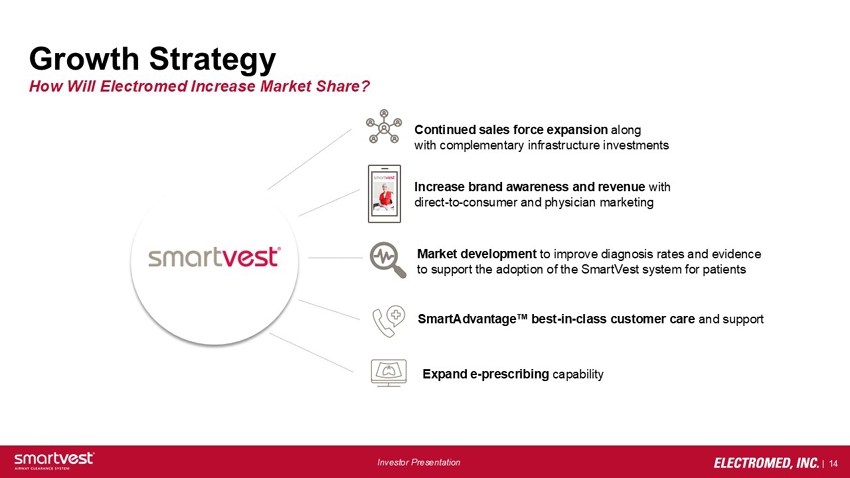
Growth Strategy How Will Electromed Increase Market Share? Continued sales force expansion along with complementary infrastructure investments Increase brand awareness and revenue with direct-to-consumer and physician marketing Market development to improve diagnosis rates and evidence to support the adoption of the SmartVest system for patients SmartAdvantage™ best-in-class customer care and support Expand e-prescribing capability
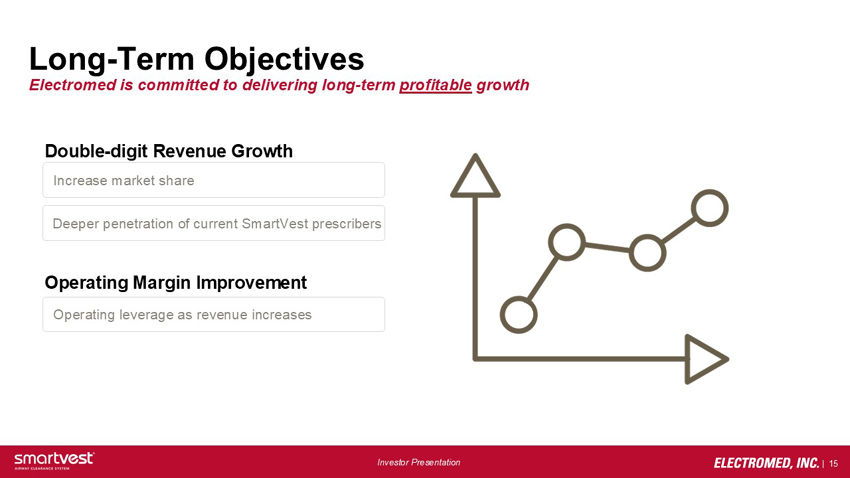
Long-Term Objectives Electromed is committed to delivering long-term profitable growth Double-digit Revenue Growth Increase market share Deeper penetration of current SmartVest prescribers Operating Margin Improvement Operating leverage as revenue increases
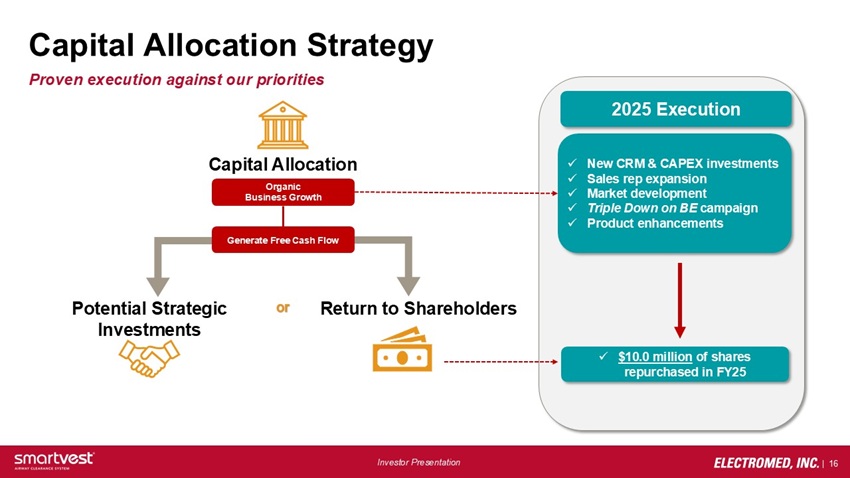
Capital Allocation Strategy Proven execution against our priorities Capital Allocation Support organic business growth Generate Free Cash Flow Potential Strategic Investments Strategic M&A Opportunities or Return to Shareholders Share Buybacks 2025 Execution New CRM & CAPEX investments Sales rep expansion Market development Triple Down on BE campaign Product enhancements $10.0 million of shares repurchased in FY25
Why Invest? Large, expanding chronic lung diseases market Clinically proven technology Broad payor coverage Consistent double-digit organic revenue growth High gross margins, robust cash flow and expanding operating leverage
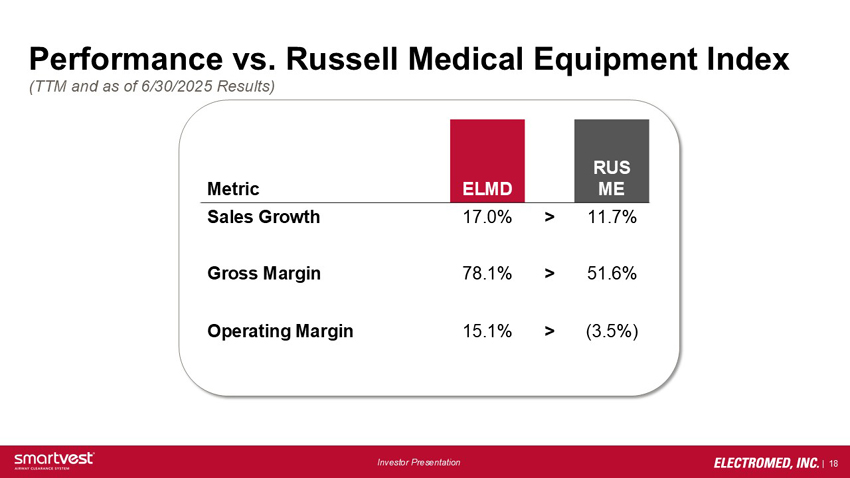
Performance vs. Russell Medical Equipment Index (TTM and as of 6/30/2025 Results) Metric ELMD vs. RUS ME: Sales Growth: 17.0% > 11.7%, Gross Margin: 78.1% > 51.6%, Operating Margin: 15.1% > (3.5%)

Jim Cunniff, President & CEO (952) 758-9299 jcunniff@Electromed.com Brad Nagel, CFO (952) 758-9299 bnagel@Electromed.com Mike Cavanaugh (617) 877-8641 mike.cavanaugh@icrhealthcare.com

APPENDIX
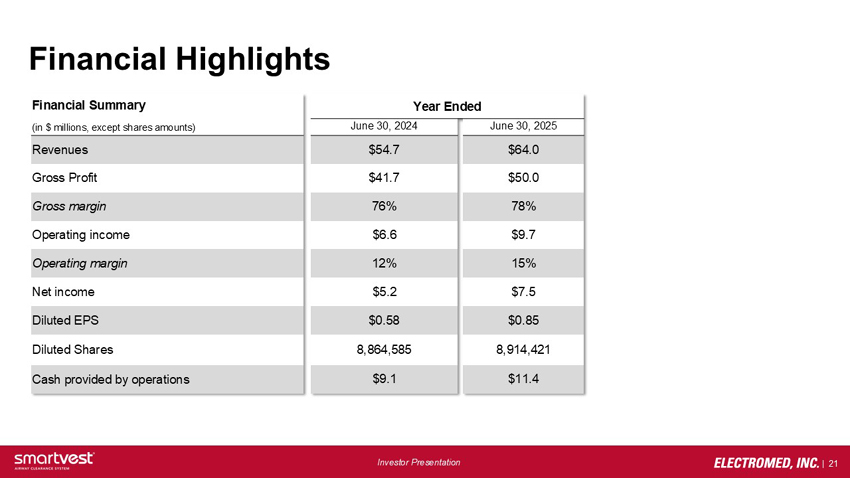
Financial Summary Year Ended (in $ millions, except shares amounts) June 30, 2024 June 30, 2025: Revenues: FY24: $54.7 FY25: $64.0, Gross Profit: FY24 $41.7 FY25: $50.0, Gross margin FY24: 76% FY25: 78%, Operating income: FY24: $6.6 FY25: $9.7, Operating margin: FY24: 12% FY25: 15%, Net income: FY24: $5.2 FY25: $7.5, Diluted EPS: FY24: $0.58 FY25: $0.85, Diluted Shares: FY24: 8,864,585 FY25: 8,914,421, Cash provided by operations: FY24: $9.1 FY25: $11.4