
.2

Electromed, Inc. Investor Presentation February 10, 2026 NYSE American: ELMD Innovation Leader in Airway Clearance Technologies

Forward looking statements - Certain statements in this presentation constitute forward-looking statements as defined in the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “anticipate,” “believe,” “committed,” “continue,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “should,” “will,” and similar expressions, including the negative of these terms, but they are not the exclusive means of identifying such statements. Forward-looking statements cannot be guaranteed, and actual results may vary materially due to the uncertainties and risks, known or unknown associated with such statements. Examples of risks and uncertainties for Electromed include, but are not limited to, competitive nature of our market; changes to Medicare, Medicaid, or private insurance reimbursement policies; changes to state and federal health care laws; changes affecting the medical device industry; our ability to develop new sales channels for our products such as the homecare distributor channel; our need to maintain regulatory compliance and to gain future regulatory approvals and clearances; new drug or pharmaceutical discoveries; general economic and business conditions; component or raw material shortages; changes to lead times or changes to trade regulations; wage and component price inflation; the risks associated with cyberattacks, data breaches, computer viruses and other similar security threats; technical problems with our research and products; our ability to renew our line of credit or obtain additional credit as necessary; our ability to protect and expand our intellectual property portfolio, as well as other factors we may describe from time to time in Electromed’s reports filed with the Securities and Exchange Commission (including Electromed’s most recent Annual Report on Form 10-K, as amended from time to time, and subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K). Investors should not consider any list of such factors to be an exhaustive statement of all the risks, uncertainties or potentially inaccurate assumptions investors should take into account when making investment decisions. Shareholders and other readers should not place undue reliance on “forward-looking statements,” as such statements speak only as of the date of this press release. We undertake no obligation to update them in light of new information or future events.

Electromed – Who We Are - Electromed, Inc. is a growing medical device company focused on airway management to help people around the world breathe better, stay healthier, and lead active and fulfilling lives. Key Stats: Headquarters:
New Prague, MN Ticker: ELMD Manufacturing: Minnesota, USA Established:
1992 Treatment Focus: HFCWO Employees: 186* LTM Net Revenues:
$68.9M* Market Cap: $241.1M* Cash / Debt: $13.8M / $0.0M* *As of and for the 12 months ended 12/31/2025
Electromed Highlights Growing and profitable medical technology company
» A leader in the large and expanding airway clearance market
» The SmartVest® Airway Clearance System’s
High Frequency Chest Wall Oscillation (“HFCWO”) technology supported by clinical outcomes data with strong reimbursement.
» Attractive direct-to-patient and provider model
» Strong financial profile with attractive gross margins and well-capitalized balance sheet
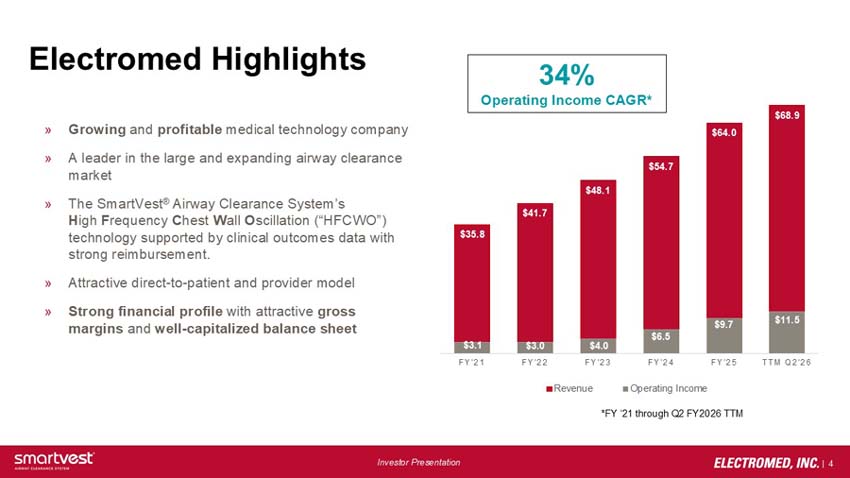
34% Operating Income CAGR*
*FY ‘21 through Q2 FY2026 TTM
FY21: Operating Income: $3.1M Revenue: $35.8M
FY22: Operating Income: $3.0M Revenue: $41.7M
FY23: Operating Income: $4.0M Revenue: $48.1M
FY24: Operating Income: $6.5M Revenue: $54.7M
FY25: Operating Income: $9.7M Revenue: $64.0M
TTM Q2’26: Operating Income: $11.5M Revenue: $68.9M
What is Bronchiectasis (BE)? BE is a condition that is prevalent, but underdiagnosed and often misdiagnosed
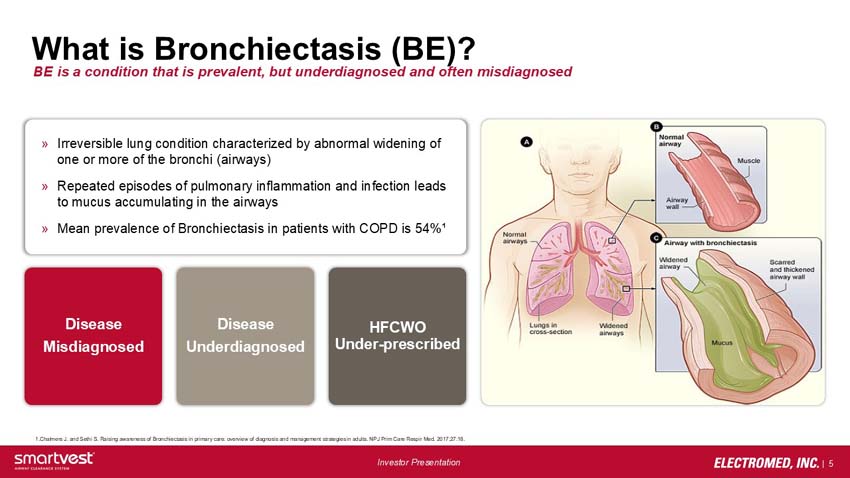
Irreversible lung condition characterized by abnormal widening of one or more of the bronchi (airways)
Repeated episodes of pulmonary inflammation and infection leads to mucus accumulating in the airways
Mean prevalence of Bronchiectasis in patients with COPD is 54%¹
1.Chalmers J. and Sethi S. Raising awareness of Bronchiectasis in primary care: overview of diagnosis and management strategies in adults. NPJ Prim Care Respir Med. 2017;27:18.
Disease Misdiagnosed – Disease Underdiagnosed – HFCWO Under-prescribed
Normal airways, lungs in cross-section, widened airways – normal airway – airway wall – muscle
Airway with bronchiectasis – widened airway, mucus, scarred and thickened airway wall
U.S. Market: Large, Growing, Underpenetrated The Company is expanding awareness within the large BE patient population
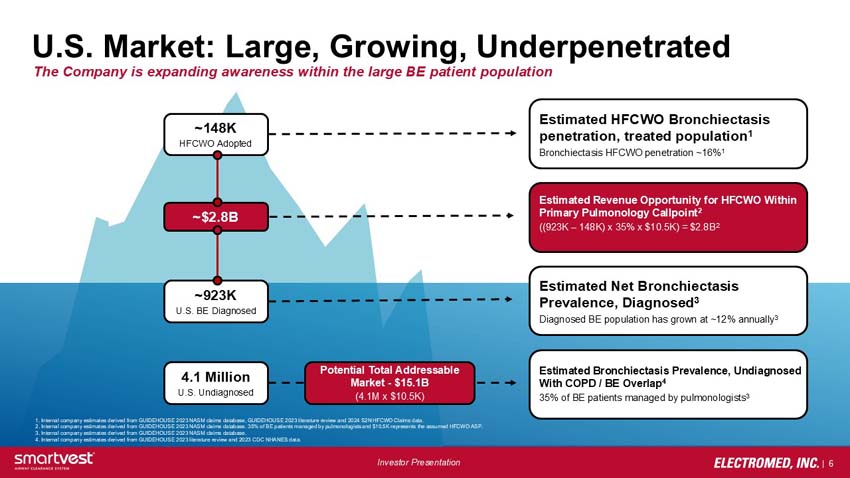
~148K HFCWO Adopted - Estimated HFCWO Bronchiectasis penetration, treated population1 Bronchiectasis HFCWO penetration ~16%1
~$2.8B - Estimated Revenue Opportunity for HFCWO Within Primary Pulmonology Callpoint2 ((923K – 148K) x 35% x $10.5K) = $2.8B2
~923K U.S. BE Diagnosed - Estimated Net Bronchiectasis Prevalence, Diagnosed3
Diagnosed BE population has grown at ~12% annually3
4.1 Million U.S. Undiagnosed - Potential Total Addressable Market - $15.1B
(4.1M x $10.5K) - Estimated Bronchiectasis Prevalence, Undiagnosed With COPD / BE Overlap4 35% of BE patients managed by pulmonologists3
1. Internal company estimates derived from GUIDEHOUSE 2023 NASM claims database, GUIDEHOUSE 2023 literature review and 2024 S2N HFCWO Claims data.
2. Internal company estimates derived from GUIDEHOUSE 2023 NASM claims database. 35% of BE patients managed by pulmonologists and $10.5K represents the assumed HFCWO ASP.
3. Internal company estimates derived from GUIDEHOUSE 2023 NASM claims database.
4. Internal company estimates derived from GUIDEHOUSE 2023 literature review and 2023 CDC NHANES data.
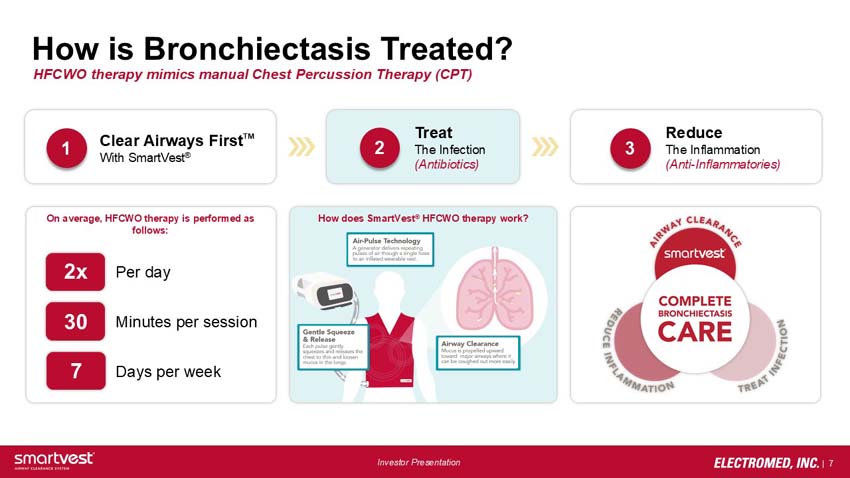
How is Bronchiectasis Treated? HFCWO therapy mimics manual Chest Percussion Therapy (CPT)
1. Clear Airways FirstTM With SmartVest®
2. Treat The Infection (Antibiotics)
3. Reduce The Inflammation (Anti-Inflammatories)
On average, HFCWO therapy is performed as follows: 2x per day, 30 minutes per session, 7 days a week
How does SmartVest® HFCWO therapy work? Air-pulse technology – a generator delivers repeating pulses of air through a single hose to an inflated wearable vest.
Gentle squeeze and release – each pulse gently squeezes and releases the chest to thin and loosen mucus in the lungs.
Airway clearance – mucus is propelled upward toward major airways where it can be coughed and more easily.
Airway clearance – smartvest – reduce inflammation – treat infection – complete bronchiectasis care

SmartVest ® Clearway HFCWO designed with the patient in mind
SmartVest® has a well-established reimbursement code from CMS – E0483; the Company estimates it has over 270 million contracted lives in the U.S.
An Enhanced Patient Experience - Sleek and lightweight generator - Intuitive user interface for better patient adherence - More portable and easier for travel
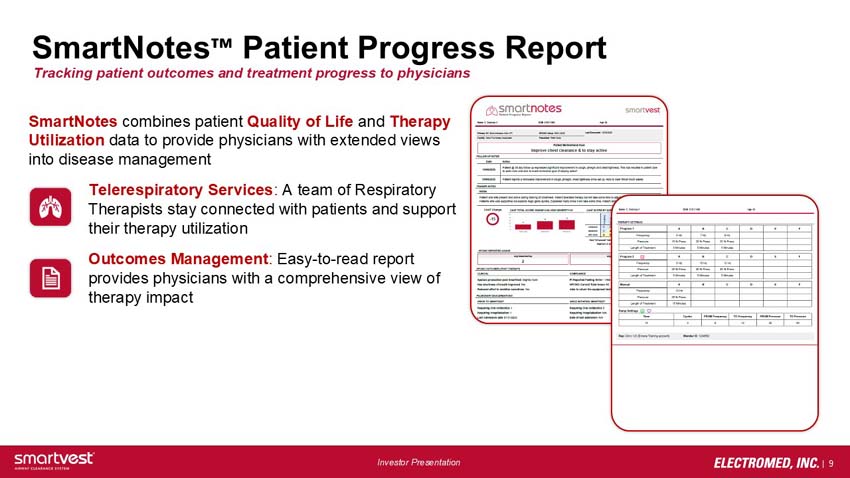
SmartNotes™ Patient Progress Report - Tracking patient outcomes and treatment progress to physicians
SmartNotes combines patient Quality of Life and Therapy Utilization data to provide physicians with extended views into disease management
» Telerespiratory Services: A team of Respiratory Therapists stay connected with patients and support their therapy utilization
» Outcomes Management: Easy-to-read report provides physicians with a comprehensive view of therapy impact
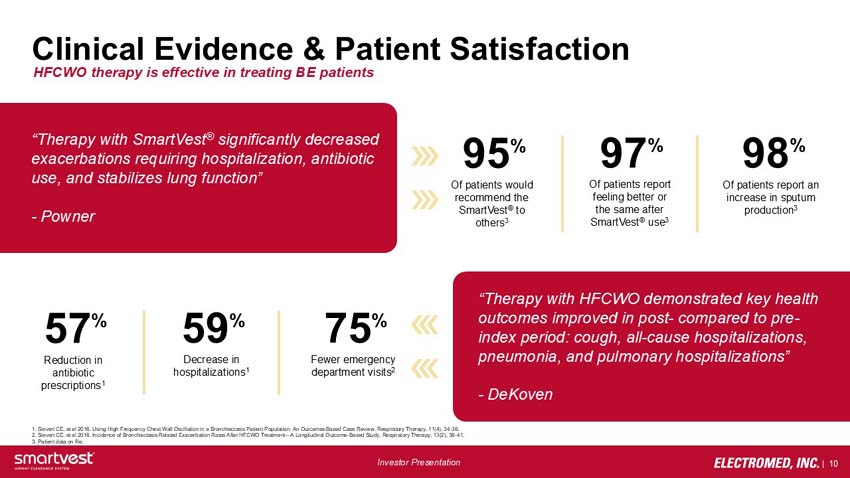
Clinical Evidence & Patient Satisfaction - HFCWO therapy is effective in treating BE patients
“Therapy with SmartVest® significantly decreased exacerbations requiring hospitalization, antibiotic use, and stabilizes lung function”
- Powner
95% Of patients would recommend the SmartVest® to others3
97% Of patients report feeling better or the same after SmartVest® use3
98% Of patients report an increase in sputum production3
57% Reduction in antibiotic prescriptions1
59% Decrease in hospitalizations1
75% Fewer emergency department visits2
1. Sievert CE, et al 2016. Using High Frequency Chest Wall Oscillation in a Bronchiectasis Patient Population: An Outcomes-Based Case Review. Respiratory Therapy, 11(4), 34-38.
2. Sievert CE, et al 2018. Incidence of Bronchiectasis-Related Exacerbation Rates After HFCWO Treatment—A Longitudinal Outcome-Based Study, Respiratory Therapy, 13(2), 38-41.
3. Patient data on file.
“Therapy with HFCWO demonstrated key health outcomes improved in post- compared to pre-index period: cough, all-cause hospitalizations, pneumonia, and pulmonary hospitalizations”
- DeKoven
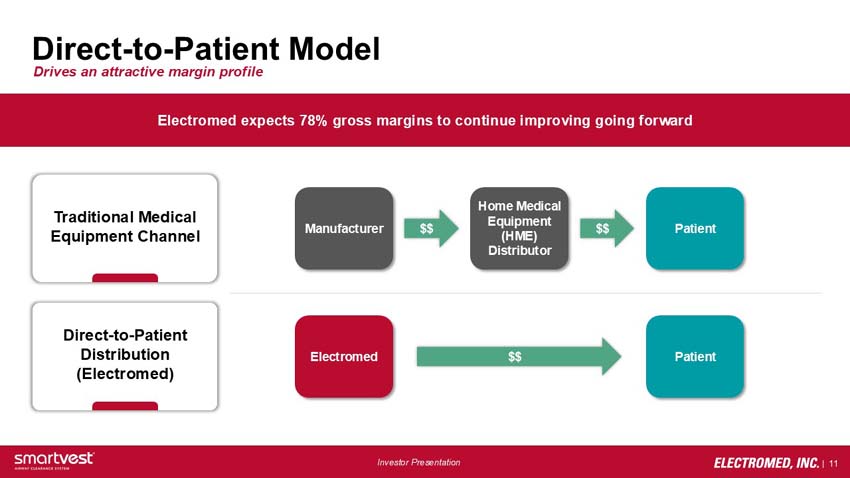
Direct-to-Patient Model - Drives an attractive margin profile
Electromed expects 78% gross margins to continue improving going forward
Traditional Medical Equipment Channel: Manufacturer > Home Medical Equipment (HME) Distributor > Patient
Direct-to-Patient Distribution (Electromed): Electromed > Patient
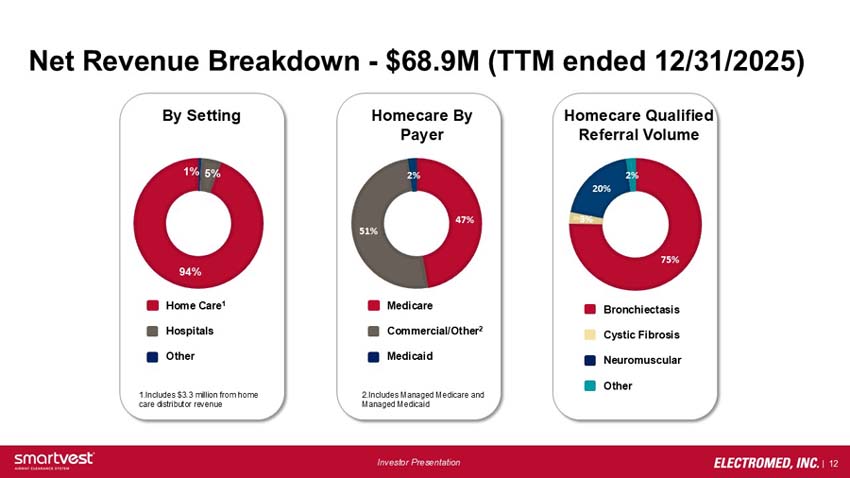
Net Revenue Breakdown - $68.9M (TTM ended 12/31/2025)
By Setting: 94% Home Care (Includes $3.3 million from home care distributor revenue), 5% Hospitals, 1% Other
Homecare by Payer: 51% Commercial/Other (Includes Managed Medicare and Managed Medicaid), 47% Medicare, 2% Medicaid
Homecare Qualified Referral Volume: 75% Bronchiectasis, 20% Neuromuscular, 3% Cystic Fibrosis, 2% Other
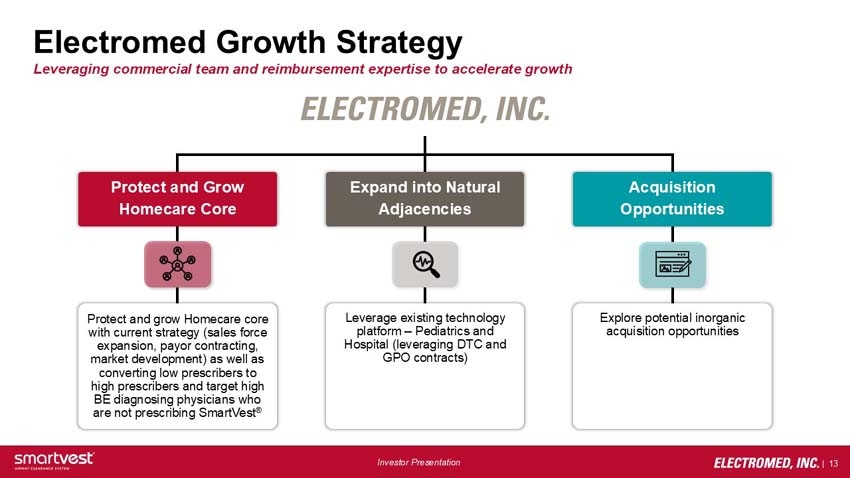
Electromed Growth Strategy - Leveraging commercial team and reimbursement expertise to accelerate growth
Electromed, Inc:
- Protect and grow homecare core
o Protect and grow Homecare core with current strategy (sales force expansion, payor contracting, market development) as well as converting low prescribers to high prescribers and target high BE diagnosing physicians who are not prescribing SmartVest®
- Expand into natural adjacencies
o Leverage existing technology platform – Pediatrics and Hospital (leveraging DTC and GPO contracts)
- Acquisition opportunities
o Explore potential inorganic acquisition opportunities
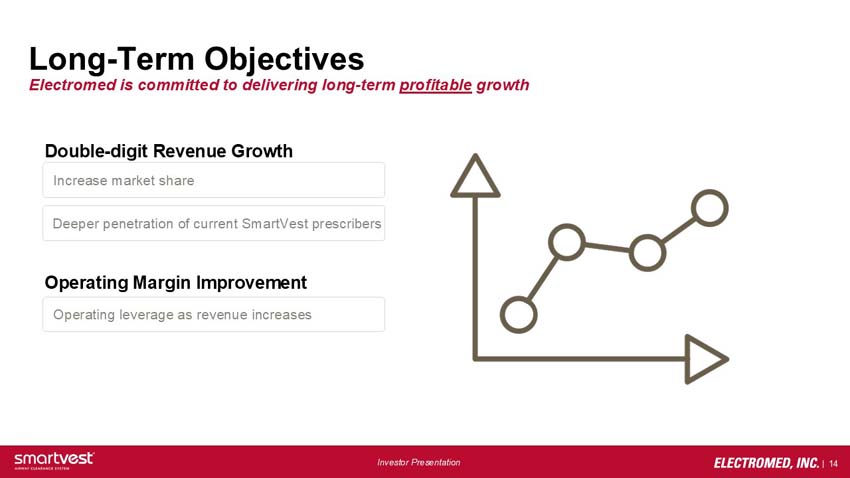
Long-Term Objectives, Electromed is committed to delivering long-term profitable growth
Double-digit revenue growth: increase market share, deeper penetration of current SmartVest prescribers
Operating margin improvement: operating leverage as revenue increases
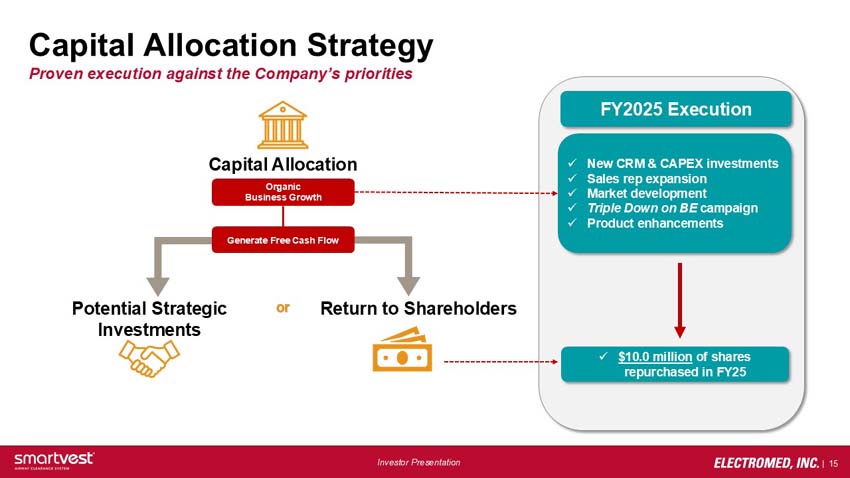
Capital Allocation Strategy: Proven execution against the Company’s priorities
Capital Allocation: Organic Business Growth > Generate Free Cash Flow > Potential Strategic Investments or Return to Shareholders
FY2025 Execution:
- New CRM & CAPEX investments
- Sales rep expansion
- Market development
- Triple Down on BE campaign
- Product enhancements
- $10.0 million of shares repurchased in FY25
Why invest? Large, expanding chronic lung diseases market, clinically proven technology, broad payor coverage, consistent double-digit organic revenue growth, high gross margins, robust cash flow and expanding operating leverage
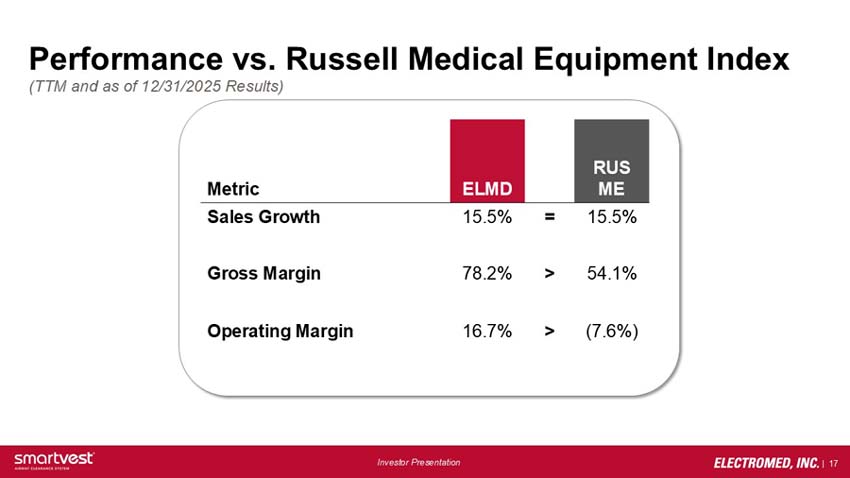
Performance vs. Russell Medical Equipment Index (TTM and as of 12/31/2025 Results):
Sales Growth: ELMD 15.5% = RUS ME 15.5%
Gross Margin: ELMD 78.2% > RUS ME 54.1%
Operating Margin: ELMD 16.7% > RUS ME (7.6)%

Electromed, Inc.: Jim Cunniff, President & CEO, (952) 758-9299, jcunniff@electromed.com – Brad Nagel, CFO, (952) 758-9299, bnagel@electromed.com
ICR Healthcare: Mike Cavanaugh, (617) 877-8641, mike.cavanaugh@icrhealthcare.com

Appendix
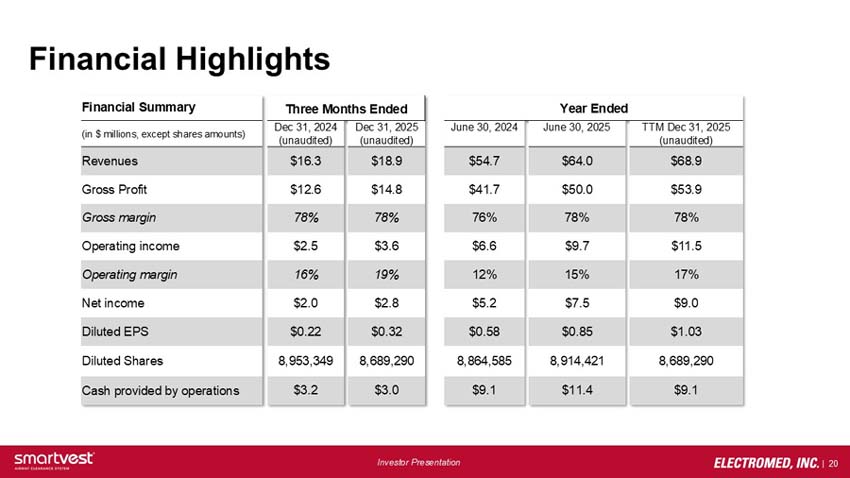
Financial Highlights (in $ millions, except share amounts)
Three Months Ended December 31, 2024: Revenues $16.3 Gross Profit $12.6 Gross Margin 78% Operating Income $2.5 Operating Margin 16% Net Income $2.0 Diluted EPS $0.22 Diluted Shares 8,953,349 Cash Provided by Operations $3.2
Three Months Ended December 31, 2025: Revenues $18.9 Gross Profit $14.8 Gross Margin 78% Operating Income $3.6 Operating Margin 19% Net Income $2.8 Diluted EPS $0.32 Diluted Shares 8,689,290 Cash Provided by Operations $3.0
Year Ended June 30, 2024: Revenues $54.7 Gross Profit $41.7 Gross Margin 76% Operating Income $6.6 Operating Margin 12% Net Income $5.2 Diluted EPS $0.58 Diluted Shares 8,864,585 Cash Provided by Operations $9.1
Year Ended June 30, 2025: Revenues $64.0 Gross Profit $50.0 Gross Margin 78% Operating Income $9.7 Operating Margin 15% Net Income $7.5 Diluted EPS $0.85 Diluted Shares 8,914,421 Cash Provided by Operations $11.4
TTM December 31, 2025 (unaudited): Revenues $68.9 Gross Profit $53.9 Gross Margin 78% Operating Income $11.5 Operating Margin 17% Net Income $9.0 Diluted EPS $1.03 Diluted Shares 8,689,290 Cash Provided by Operations $9.1