
ABBEY Living with Chronic Neutropenia Driving progress for patients with chronic neutropenic disorders Investor Deck – May 2025 .2

This presentation including any printed or electronic copy of these slides, the talks given by the presenters, the information communicated during any delivery of the presentation and any question and answer sessions and any documents or materials distributed at or in connection with the presentation, contains forward-looking statements within the meaning of applicable securities laws, including the Private Securities Litigation Reform Act of 1995, as amended. These statements may be identified by the words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target,” or other similar terms or expressions that concern X4's expectations, strategy, business, plans, or intentions. Forward-looking statements include, without limitation, implied or express statements regarding X4’s expectations as to X4’s ability to advance or commercialize mavorixafor to treat chronic neutropenia or WHIM syndrome, including ongoing engagement and feedback from regulatory authorities, and to increase sales of or to optimize the U.S. promotion of XOLREMDI® (mavorixafor); the potential number of patients in the United States with WHIM syndrome or chronic neutropenia and the potential market for mavorixafor due to unmet potential patient needs, as well as related market opportunities and potential benefits from partnerships; the initiation, timing, progress, and results of current and future preclinical studies and clinical trials; and the sufficiency of X4’s cash resources and pro-forma cash resources, including expectations regarding X4’s cash runway. Any forward-looking statements in this presentation are based on management's current expectations and beliefs. These forward-looking statements are neither promises nor guarantees of future performance, and are subject to a variety of risks and uncertainties, many of which are beyond X4’s control, which could cause actual results to differ materially from those contemplated in these forward- looking statements, including the risks that: X4 may be unable to advance and commercialize mavorixafor to treat chronic neutropenia, to gain ex-U.S. approval for the treatment of WHIM, or to optimize the U.S. promotion of XOLREMDI® (mavorixafor); the expected sufficiency of X4’s existing cash resources and runway may not be accurate, resulting in the need for additional financing sooner than anticipated or unexpected liquidity constraints; the internal and external costs required for X4’s ongoing and planned activities, and the resulting impact on expense and use of cash, may be higher than expected and may cause the company to use cash more quickly than expected or to change or curtail some of X4’s plans or both; the expected availability, content, and timing of clinical data from X4’s ongoing clinical trials of mavorixafor may be delayed or unavailable, including the ongoing Phase 3 clinical trial in chronic neutropenia; trials, studies and research programs may not have satisfactory outcomes; earlier trials and studies may not be predictive of later trials and studies; the design and rate of enrollment for clinical trials, including the ongoing Phase 3 clinical trial evaluating mavorixafor in certain chronic neutropenic disorders may not enable successful completion of the trial(s); the commercial opportunity for mavorixafor in chronic neutropenic disorders may be smaller than anticipated; X4 may be unable to obtain and maintain regulatory approvals; X4 may not obtain regulatory approval for X4’s product candidates, including additional indications for mavorixafor, such as chronic neutropenia; X4 may face uncertainties inherent in the initiation and completion of preclinical studies and clinical trials and clinical development; adverse safety effects may arise from the testing or use of the company’s product and product candidates; the need to align with partners may hamper or delay development and commercialization efforts or increase costs; business may be adversely affected and costs may increase if any key collaborators fail to perform obligations or terminate the collaboration; and other risks and uncertainties, including those described in the section entitled “Risk Factors” in X4’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) and in other filings X4 makes with the SEC from time to time. X4 undertakes no obligation to update the information contained in this presentation to reflect new events or circumstances, except as required by law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and X4’s own internal estimates and research. While X4 believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information obtained from third-party sources. Finally, while X4 believes its own internal research is reliable, such research has not been verified or validated by any independent source. X4 is the owner of various trademarks, trade names and service marks. Certain other trademarks, trade names and service marks appearing in this presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this presentation are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. Forward-Looking Statements 2
Addressing unmet needs in rare immune disorders Fully Integrated Company Delivering on the Promise of Mavorixafor 3 1. WHIM (Warts, Hypogammaglobulinemia, Infections, Myelokathexis), a rare primary immunodeficiency and chronic neutropenic disorder. 2. Funds include restricted cash. 3. Assuming continued compliance with debt covenants as described in the company’s Form 10-Q filed on May 1, 2025. • XOLREMDI® (mavorixafor) approved and launched in the U.S. in 2024 - first therapy indicated for patients with WHIM syndrome • MAA submission for WHIM accepted by EMA in January 2025 • EXPANDING the global reach of mavorixafor via partnerships in EU, ANZ, MENA BALANCE SHEET SUPPORTS 4WARD TRIAL • Funds of $87.7 million as of 03/31/20252 • Runway expected into the first half of 20263 MAXIMIZING THE POTENTIAL OF MAVORIXAFOR IN WHIM SYNDROME1 • 4WARD full enrollment expected in 3Q or 4Q 2025 • MAA Approval and EU WHIM launch (via Norgine partnership) expected in 1H 2026 • Top-line 4WARD data expected in 2H 2026 NEXT VALUE DRIVERS: LARGER INDICATION OF CHRONIC NEUTROPENIA & TARGETED EUROPEAN WHIM APPROVAL
4 1. U.S. Prevalence Based on ICD-10 Code Research, Average Across 3 Years (2018, 2019, & 2021); >90% greater than 18 years of age, ~2/3 female, mixed G-CSF use. 2. . https://www.cancernetwork.com/view/fda-approves-new-indication-neupogen-chronic-neutropenia No Innovation for People with Chronic Neutropenia in More Than 30 years Low levels of neutrophils High risk of infections 1 Therapy Approved for Severe Chronic Neutropenia Only One ~50,0001 Estimated Chronic Neutropenia Patients in the U.S. Injectable Granulocyte Colony-Stimulating Factor (G-CSF) • Approved to treat severe chronic neutropenia in 19952 • Used as a chronic daily injection or as rescue during serious infection episodes • Frequent treatment-related / treatment-limiting bone pain other adverse events, and long-term risk of myelodysplastic syndrome and/or leukemia Innovation needed to address unmet patient needs
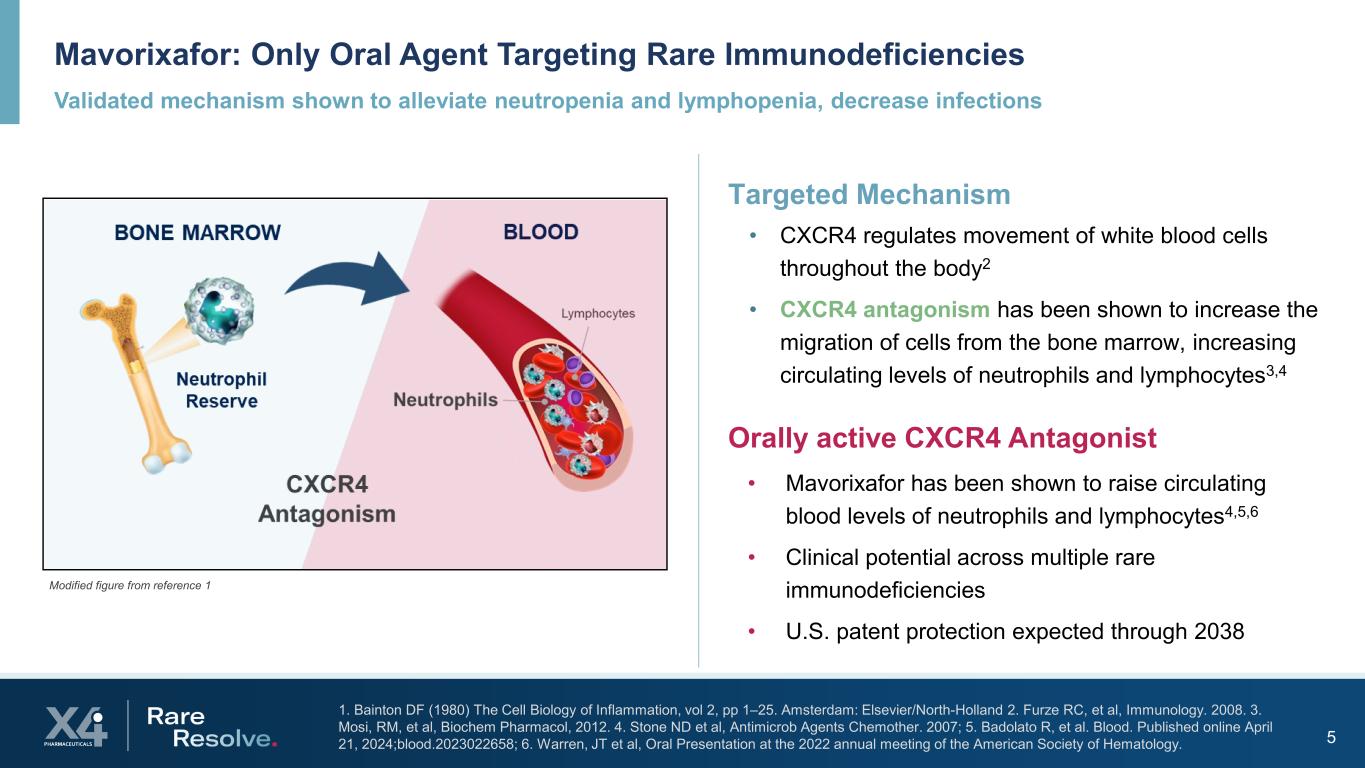
Validated mechanism shown to alleviate neutropenia and lymphopenia, decrease infections Mavorixafor: Only Oral Agent Targeting Rare Immunodeficiencies 5 1. Bainton DF (1980) The Cell Biology of Inflammation, vol 2, pp 1–25. Amsterdam: Elsevier/North-Holland 2. Furze RC, et al, Immunology. 2008. 3. Mosi, RM, et al, Biochem Pharmacol, 2012. 4. Stone ND et al, Antimicrob Agents Chemother. 2007; 5. Badolato R, et al. Blood. Published online April 21, 2024;blood.2023022658; 6. Warren, JT et al, Oral Presentation at the 2022 annual meeting of the American Society of Hematology. Targeted Mechanism • CXCR4 regulates movement of white blood cells throughout the body2 • CXCR4 antagonism has been shown to increase the migration of cells from the bone marrow, increasing circulating levels of neutrophils and lymphocytes3,4 Modified figure from reference 1 Orally active CXCR4 Antagonist • Mavorixafor has been shown to raise circulating blood levels of neutrophils and lymphocytes4,5,6 • Clinical potential across multiple rare immunodeficiencies • U.S. patent protection expected through 2038

6 Mavorixafor: Pipeline and Partners Indication Pre-clinical Phase 1 Phase 2 Phase 3 Approved Partners Expected Milestones M av or ix af or WHIM Syndrome (Warts, Hypogammaglobulinemia, Infections, Myelokathexis) Launched as XOLREMDI® in U.S. in May 2024 EU approval/ launch possible in 1H 2026 Chronic Neutropenia (Congenital, Autoimmune, Idiopathic) Full enrollment in 3Q/4Q 2025 Data in 2H 2026 U.S. FDA Approved Global Phase 3 Trial Ongoing MAA Under Review in EU Seeking Approvals in MENA Region

WHIM Syndrome
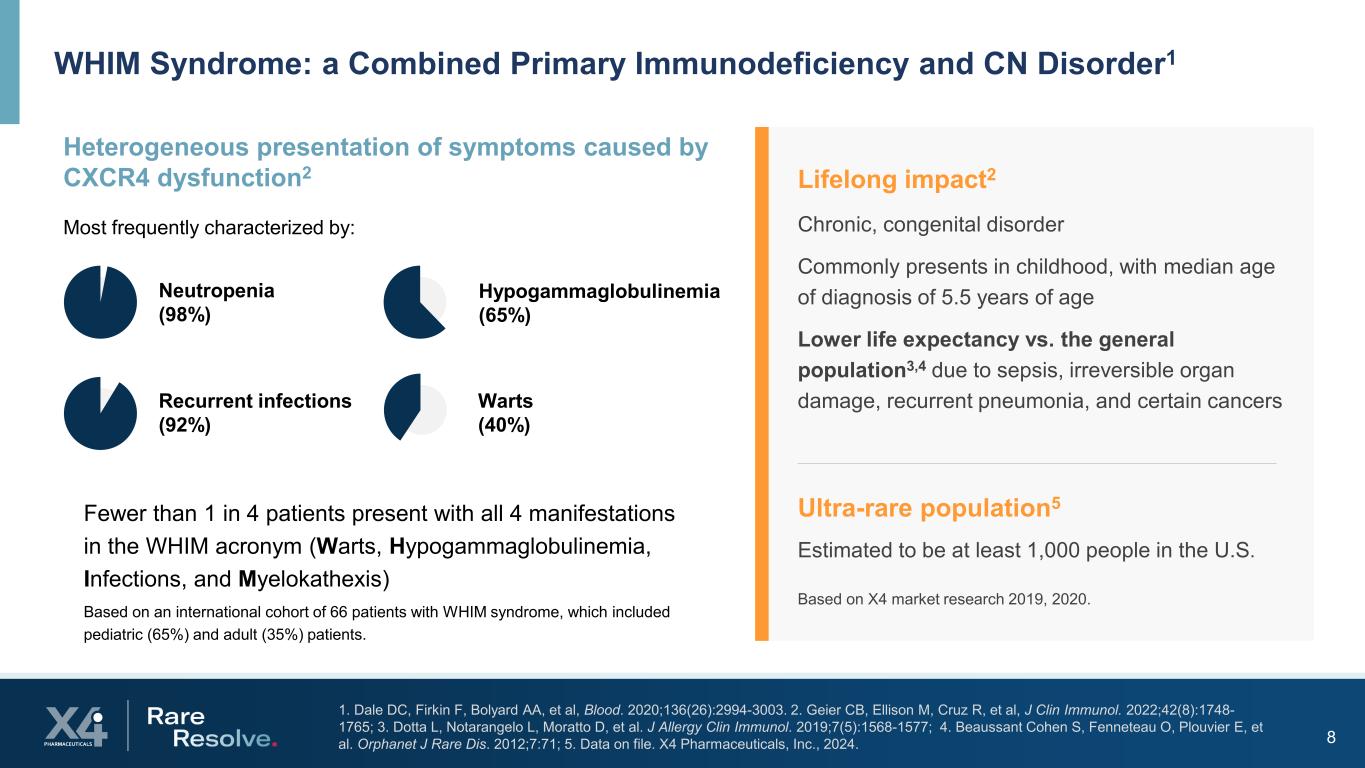
8 1. Dale DC, Firkin F, Bolyard AA, et al, Blood. 2020;136(26):2994-3003. 2. Geier CB, Ellison M, Cruz R, et al, J Clin Immunol. 2022;42(8):1748- 1765; 3. Dotta L, Notarangelo L, Moratto D, et al. J Allergy Clin Immunol. 2019;7(5):1568-1577; 4. Beaussant Cohen S, Fenneteau O, Plouvier E, et al. Orphanet J Rare Dis. 2012;7:71; 5. Data on file. X4 Pharmaceuticals, Inc., 2024. WHIM Syndrome: a Combined Primary Immunodeficiency and CN Disorder1 Lifelong impact2 Chronic, congenital disorder Commonly presents in childhood, with median age of diagnosis of 5.5 years of age Lower life expectancy vs. the general population3,4 due to sepsis, irreversible organ damage, recurrent pneumonia, and certain cancers Ultra-rare population5 Estimated to be at least 1,000 people in the U.S. Based on X4 market research 2019, 2020. Heterogeneous presentation of symptoms caused by CXCR4 dysfunction2 Most frequently characterized by: Fewer than 1 in 4 patients present with all 4 manifestations in the WHIM acronym (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) Based on an international cohort of 66 patients with WHIM syndrome, which included pediatric (65%) and adult (35%) patients. Neutropenia (98%) Recurrent infections (92%) Hypogammaglobulinemia (65%) Warts (40%)
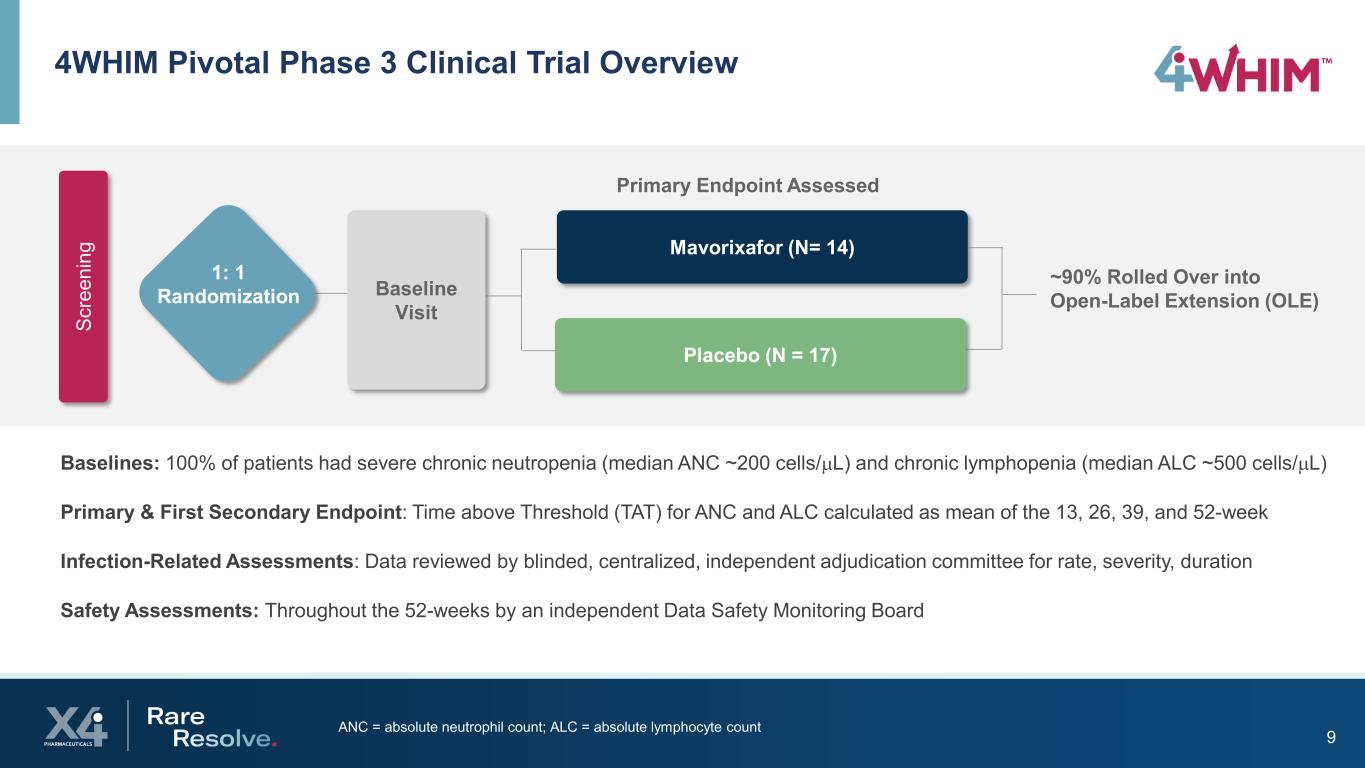
9ANC = absolute neutrophil count; ALC = absolute lymphocyte count 4WHIM Pivotal Phase 3 Clinical Trial Overview Baselines: 100% of patients had severe chronic neutropenia (median ANC ~200 cells/µL) and chronic lymphopenia (median ALC ~500 cells/µL) Primary & First Secondary Endpoint: Time above Threshold (TAT) for ANC and ALC calculated as mean of the 13, 26, 39, and 52-week Infection-Related Assessments: Data reviewed by blinded, centralized, independent adjudication committee for rate, severity, duration Safety Assessments: Throughout the 52-weeks by an independent Data Safety Monitoring Board Baseline Visit Mavorixafor (N= 14) Placebo (N = 17) Primary Endpoint Assessed ~90% Rolled Over into Open-Label Extension (OLE) Sc re en in g 1: 1 Randomization
10 1. Published Trial Results: Badolato R, et al., A phase 3 randomized trial of mavorixafor, a CXCR4 antagonist, for WHIM syndrome, Blood (2024) 144 (1): 35–45 (https://doi.org/10.1182/blood.2023022658) Reduced RATE of infections 1
111. Footnote Placeholder U.S. Launch in May 2024 (zōl-RĔM-dee) For use in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes. See full prescribing information at xolremdi.com Breakthrough Therapy Designation
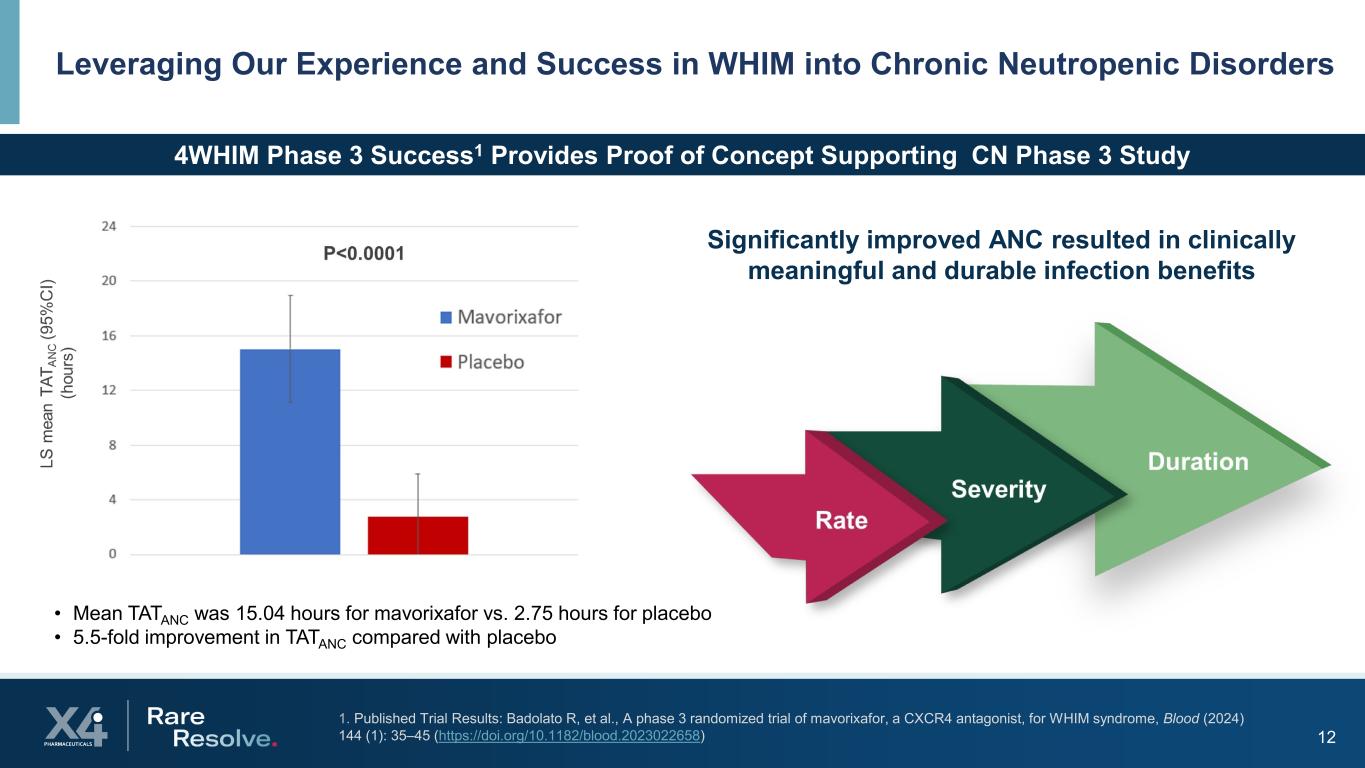
12 Leveraging Our Experience and Success in WHIM into Chronic Neutropenic Disorders 4WHIM Phase 3 Success1 Provides Proof of Concept Supporting CN Phase 3 Study • Mean TATANC was 15.04 hours for mavorixafor vs. 2.75 hours for placebo • 5.5-fold improvement in TATANC compared with placebo Significantly improved ANC resulted in clinically meaningful and durable infection benefits 1. Published Trial Results: Badolato R, et al., A phase 3 randomized trial of mavorixafor, a CXCR4 antagonist, for WHIM syndrome, Blood (2024) 144 (1): 35–45 (https://doi.org/10.1182/blood.2023022658)
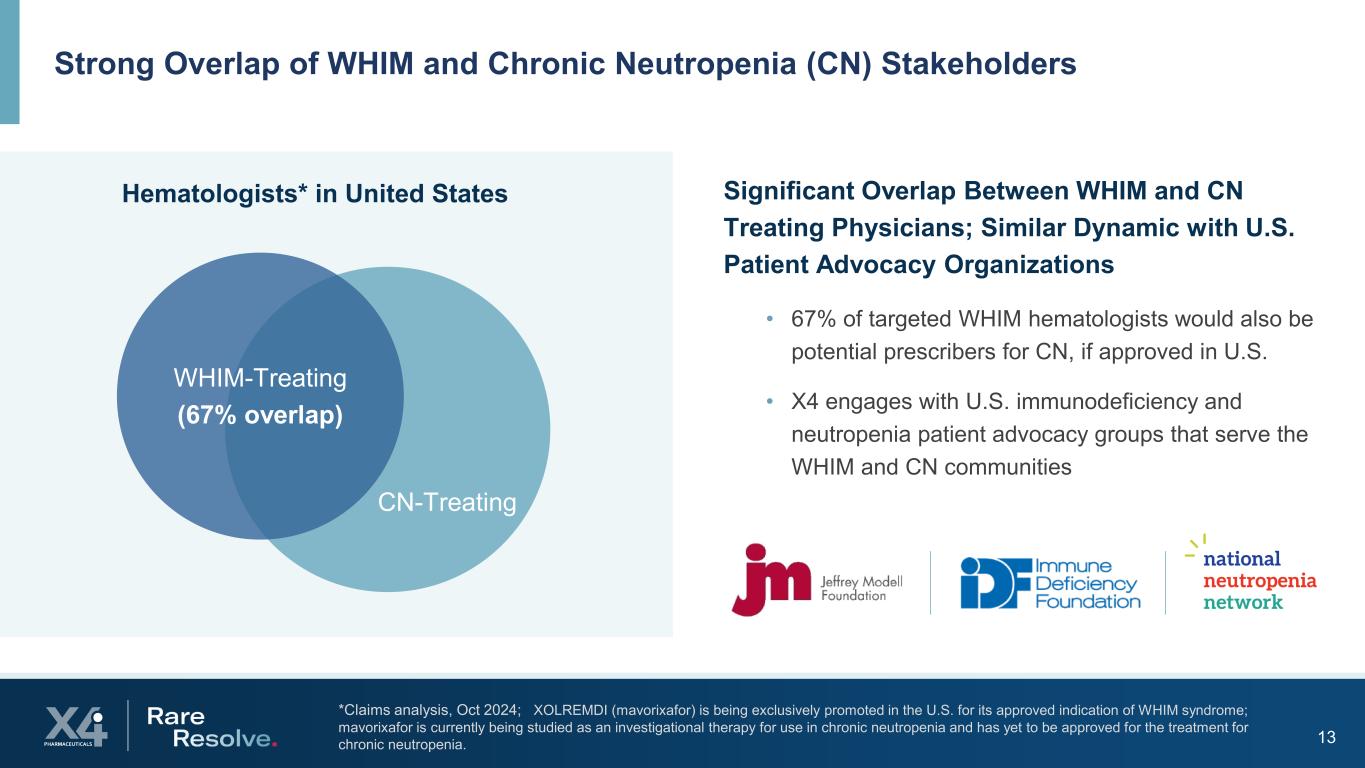
13 *Claims analysis, Oct 2024; XOLREMDI (mavorixafor) is being exclusively promoted in the U.S. for its approved indication of WHIM syndrome; mavorixafor is currently being studied as an investigational therapy for use in chronic neutropenia and has yet to be approved for the treatment for chronic neutropenia. Strong Overlap of WHIM and Chronic Neutropenia (CN) Stakeholders • 67% of targeted WHIM hematologists would also be potential prescribers for CN, if approved in U.S. • X4 engages with U.S. immunodeficiency and neutropenia patient advocacy groups that serve the WHIM and CN communities Hematologists* in United States Significant Overlap Between WHIM and CN Treating Physicians; Similar Dynamic with U.S. Patient Advocacy Organizations WHIM-Treating (67% overlap) CN-Treating

Chronic Neutropenia
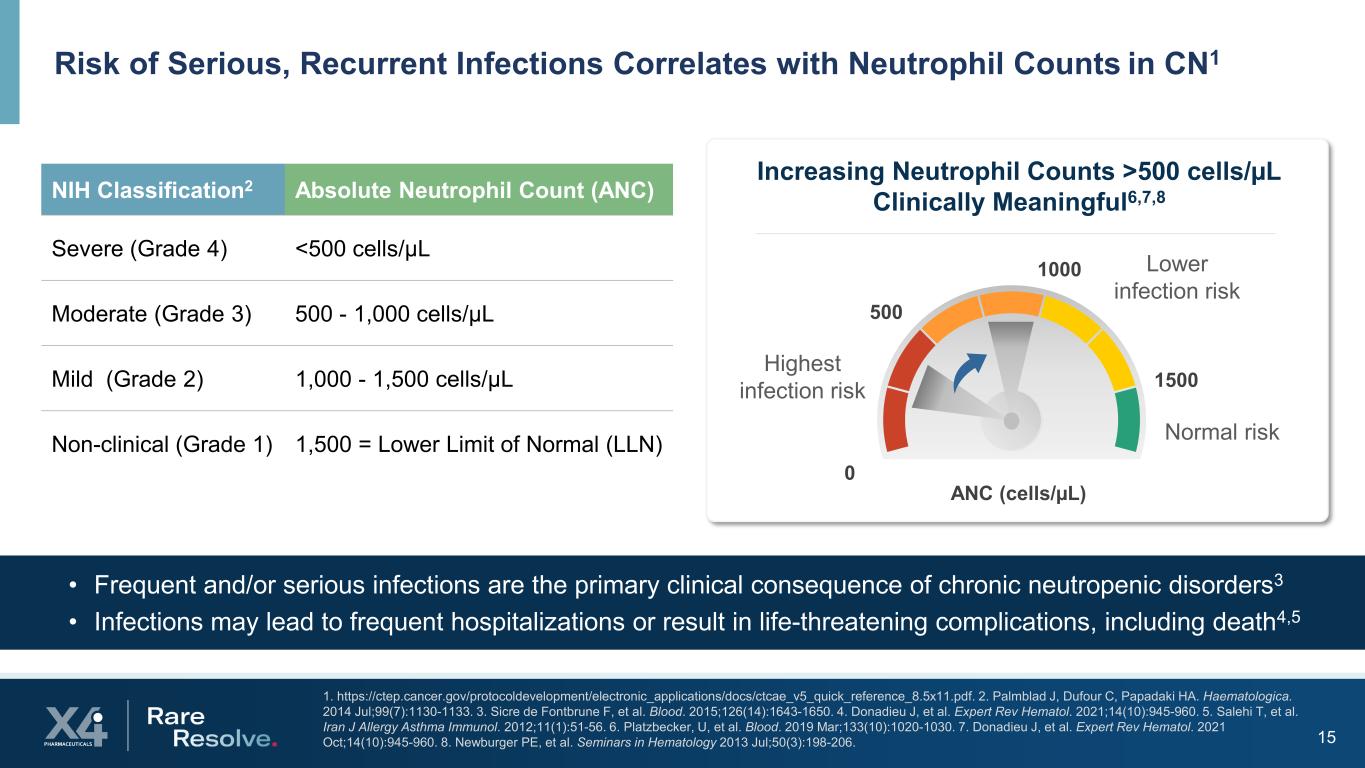
15 1. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf. 2. Palmblad J, Dufour C, Papadaki HA. Haematologica. 2014 Jul;99(7):1130-1133. 3. Sicre de Fontbrune F, et al. Blood. 2015;126(14):1643-1650. 4. Donadieu J, et al. Expert Rev Hematol. 2021;14(10):945-960. 5. Salehi T, et al. Iran J Allergy Asthma Immunol. 2012;11(1):51-56. 6. Platzbecker, U, et al. Blood. 2019 Mar;133(10):1020-1030. 7. Donadieu J, et al. Expert Rev Hematol. 2021 Oct;14(10):945-960. 8. Newburger PE, et al. Seminars in Hematology 2013 Jul;50(3):198-206. Risk of Serious, Recurrent Infections Correlates with Neutrophil Counts in CN1 • Frequent and/or serious infections are the primary clinical consequence of chronic neutropenic disorders3 • Infections may lead to frequent hospitalizations or result in life-threatening complications, including death4,5 NIH Classification2 Absolute Neutrophil Count (ANC) Severe (Grade 4) <500 cells/µL Moderate (Grade 3) 500 - 1,000 cells/µL Mild (Grade 2) 1,000 - 1,500 cells/µL Non-clinical (Grade 1) 1,500 = Lower Limit of Normal (LLN) 1500 500 0 ANC (cells/µL) Increasing Neutrophil Counts >500 cells/µL Clinically Meaningful6,7,8 Highest infection risk Lower infection risk 1000 Normal risk
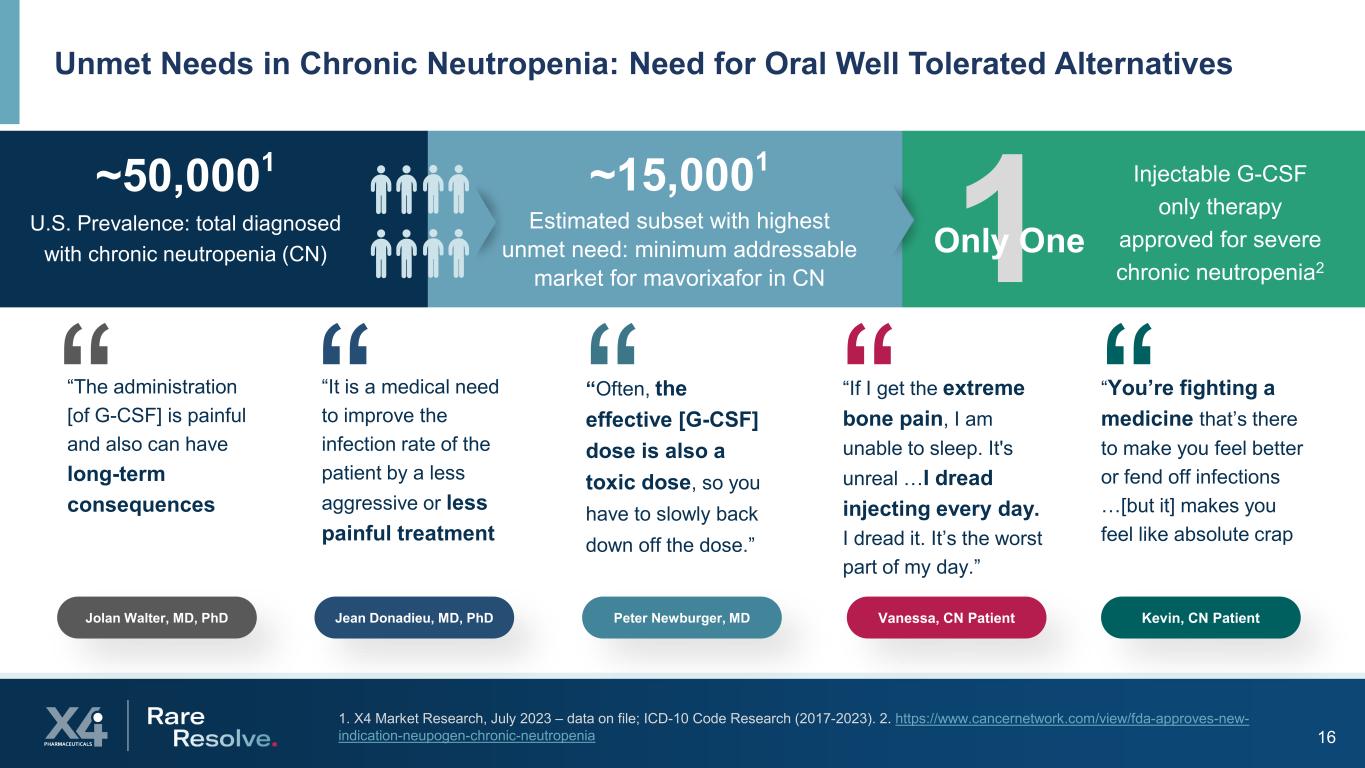
16 1. X4 Market Research, July 2023 – data on file; ICD-10 Code Research (2017-2023). 2. https://www.cancernetwork.com/view/fda-approves-new- indication-neupogen-chronic-neutropenia Unmet Needs in Chronic Neutropenia: Need for Oral Well Tolerated Alternatives 1Only One ~50,0001 U.S. Prevalence: total diagnosed with chronic neutropenia (CN) ~15,0001 Estimated subset with highest unmet need: minimum addressable market for mavorixafor in CN Injectable G-CSF only therapy approved for severe chronic neutropenia2 Jolan Walter, MD, PhD “The administration [of G-CSF] is painful and also can have long-term consequences.” Jean Donadieu, MD, PhD “It is a medical need to improve the infection rate of the patient by a less aggressive or less painful treatment.” “Often, the effective [G-CSF] dose is also a toxic dose, so you have to slowly back down off the dose.” Peter Newburger, MD “If I get the extreme bone pain, I am unable to sleep. It's unreal …I dread injecting every day. I dread it. It’s the worst part of my day.” Vanessa, CN Patient “You’re fighting a medicine that’s there to make you feel better or fend off infections …[but it] makes you feel like absolute crap.” Kevin, CN Patient “ “ “ “ “
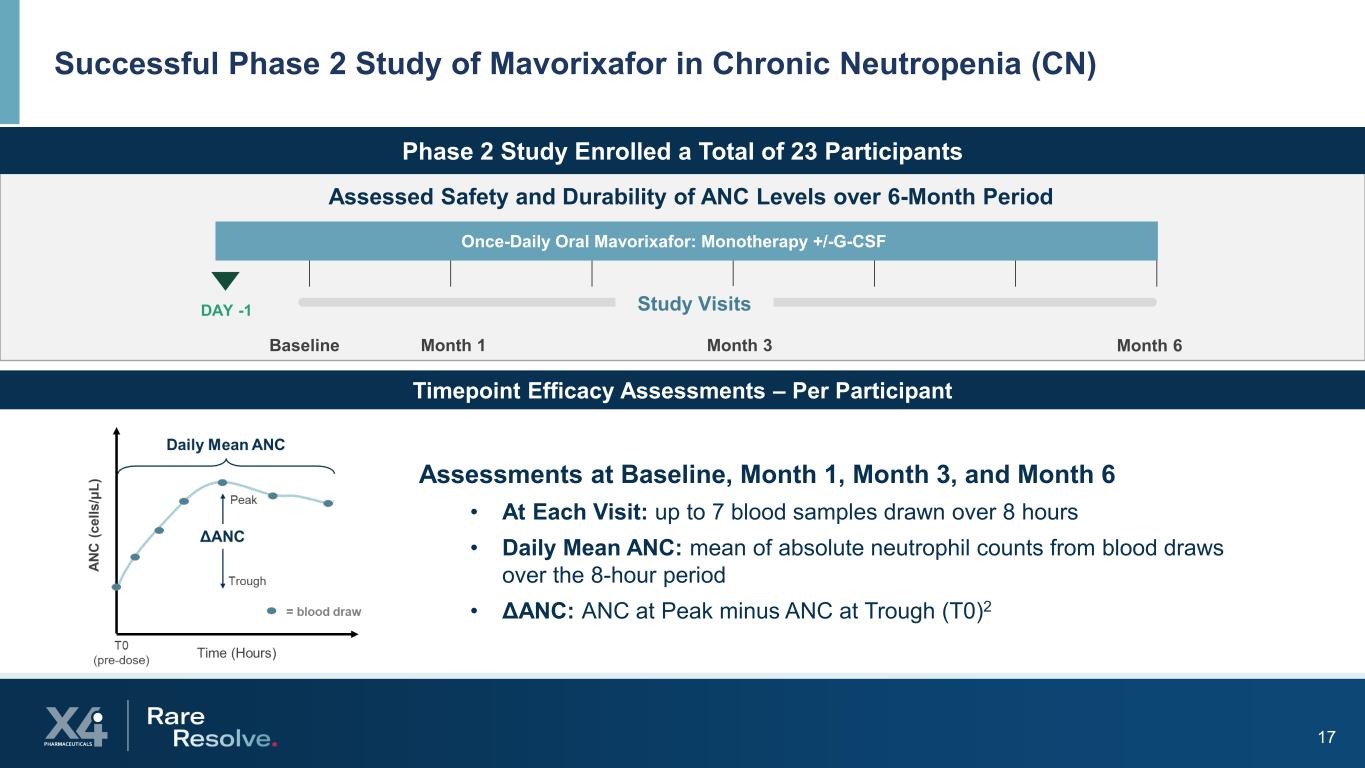
17 Successful Phase 2 Study of Mavorixafor in Chronic Neutropenia (CN) Phase 2 Study Enrolled a Total of 23 Participants Baseline Assessed Safety and Durability of ANC Levels over 6-Month Period Once-Daily Oral Mavorixafor: Monotherapy +/-G-CSF DAY -1 Month 1 Month 3 Month 6 Study Visits Assessments at Baseline, Month 1, Month 3, and Month 6 • At Each Visit: up to 7 blood samples drawn over 8 hours • Daily Mean ANC: mean of absolute neutrophil counts from blood draws over the 8-hour period • ΔANC: ANC at Peak minus ANC at Trough (T0)2 Timepoint Efficacy Assessments – Per Participant
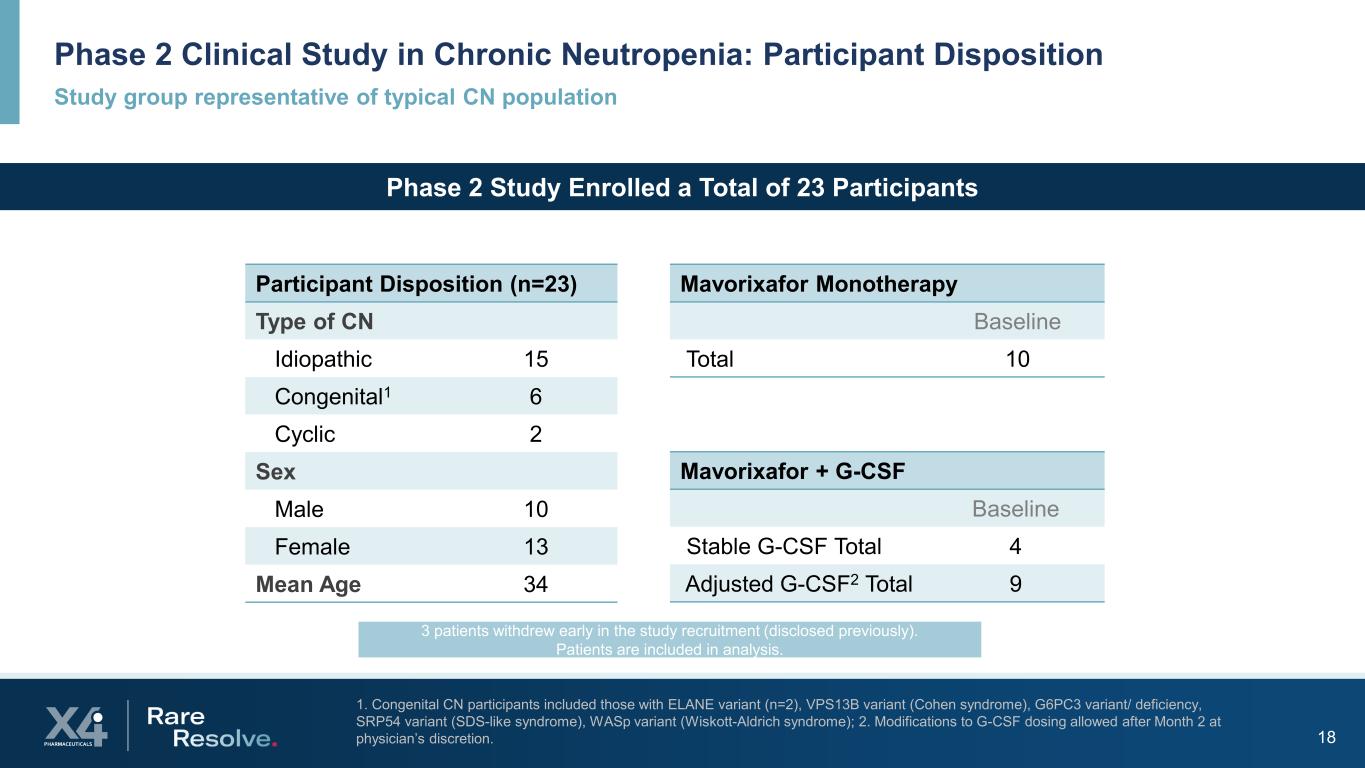
Study group representative of typical CN population Phase 2 Clinical Study in Chronic Neutropenia: Participant Disposition 18 1. Congenital CN participants included those with ELANE variant (n=2), VPS13B variant (Cohen syndrome), G6PC3 variant/ deficiency, SRP54 variant (SDS-like syndrome), WASp variant (Wiskott-Aldrich syndrome); 2. Modifications to G-CSF dosing allowed after Month 2 at physician’s discretion. Participant Disposition (n=23) Type of CN Idiopathic 15 Congenital1 6 Cyclic 2 Sex Male 10 Female 13 Mean Age 34 Mavorixafor + G-CSF Baseline Stable G-CSF Total 4 Adjusted G-CSF2 Total 9 Mavorixafor Monotherapy Baseline Total 10 Phase 2 Study Enrolled a Total of 23 Participants 3 patients withdrew early in the study recruitment (disclosed previously). Patients are included in analysis.
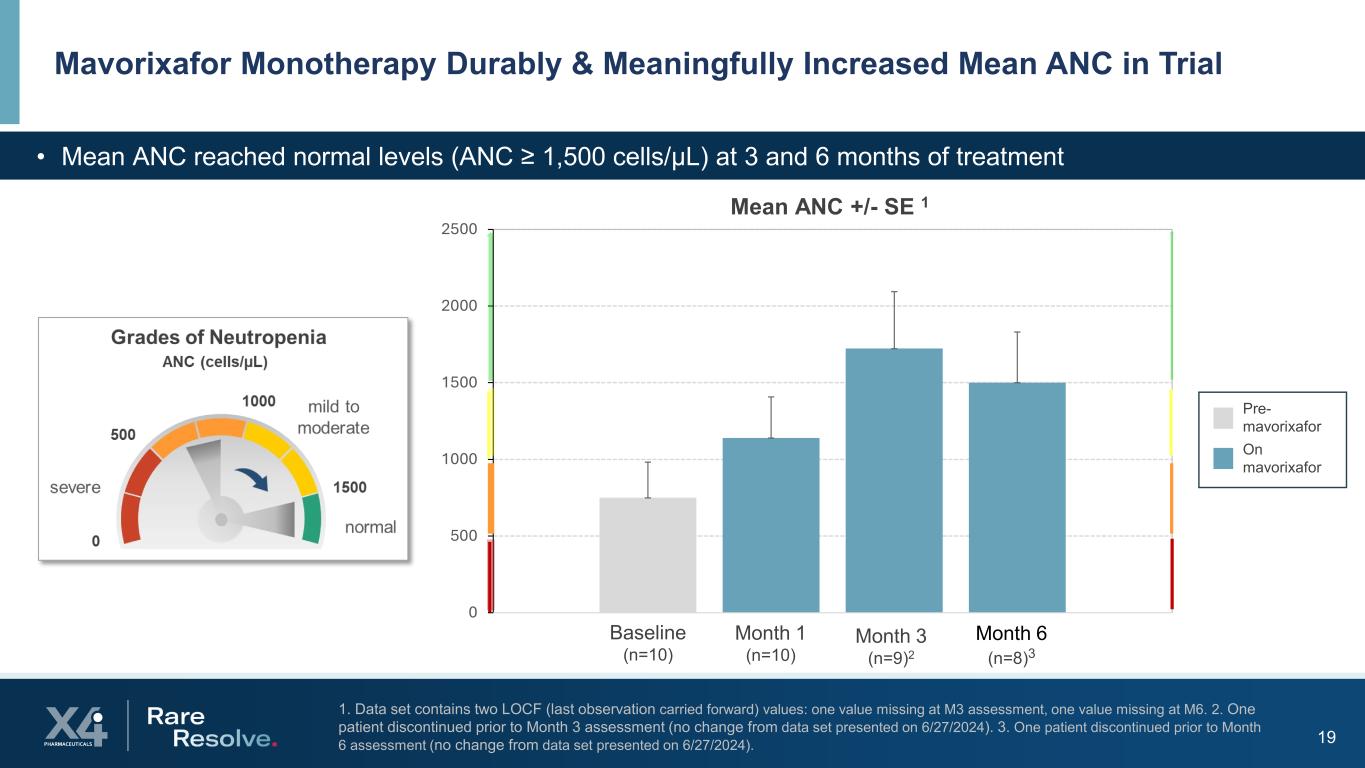
19 1. Data set contains two LOCF (last observation carried forward) values: one value missing at M3 assessment, one value missing at M6. 2. One patient discontinued prior to Month 3 assessment (no change from data set presented on 6/27/2024). 3. One patient discontinued prior to Month 6 assessment (no change from data set presented on 6/27/2024). Mavorixafor Monotherapy Durably & Meaningfully Increased Mean ANC in Trial • Mean ANC reached normal levels (ANC ≥ 1,500 cells/µL) at 3 and 6 months of treatment Pre- mavorixafor On mavorixafor 0 500 1000 1500 2000 2500 Mean Mean ANC +/- SE 1 Month 3 (n=9)2 Month 6 (n=8)3 Month 1 (n=10) Baseline (n=10)
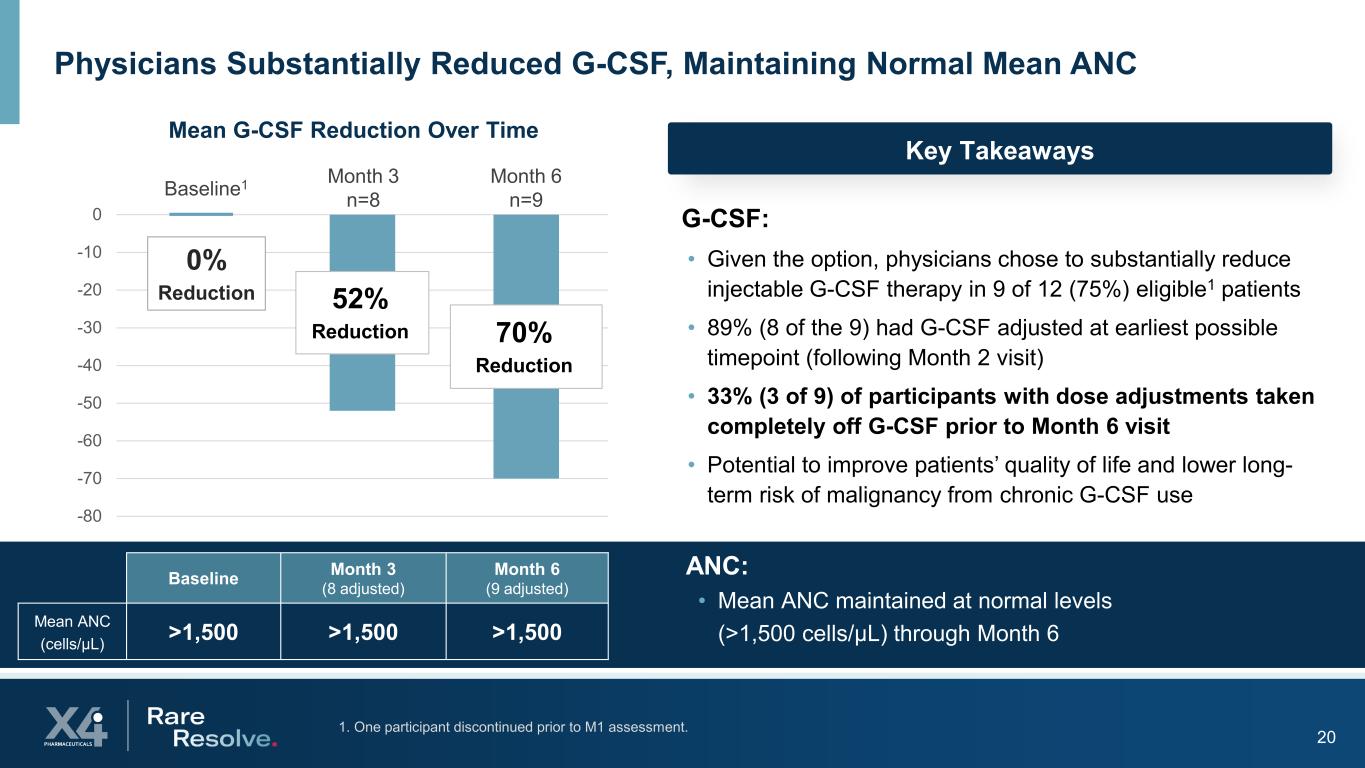
201. One participant discontinued prior to M1 assessment. Physicians Substantially Reduced G-CSF, Maintaining Normal Mean ANC G-CSF: • Given the option, physicians chose to substantially reduce injectable G-CSF therapy in 9 of 12 (75%) eligible1 patients • 89% (8 of the 9) had G-CSF adjusted at earliest possible timepoint (following Month 2 visit) • 33% (3 of 9) of participants with dose adjustments taken completely off G-CSF prior to Month 6 visit • Potential to improve patients’ quality of life and lower long- term risk of malignancy from chronic G-CSF use Baseline Month 3 (8 adjusted) Month 6 (9 adjusted) Mean ANC (cells/µL) >1,500 >1,500 >1,500 52% Reduction 70% Reduction -80 -70 -60 -50 -40 -30 -20 -10 0 Mean G-CSF Reduction Over Time Month 3 n=8 Month 6 n=9Baseline1 ANC: • Mean ANC maintained at normal levels (>1,500 cells/µL) through Month 6 0% Reduction Key Takeaways
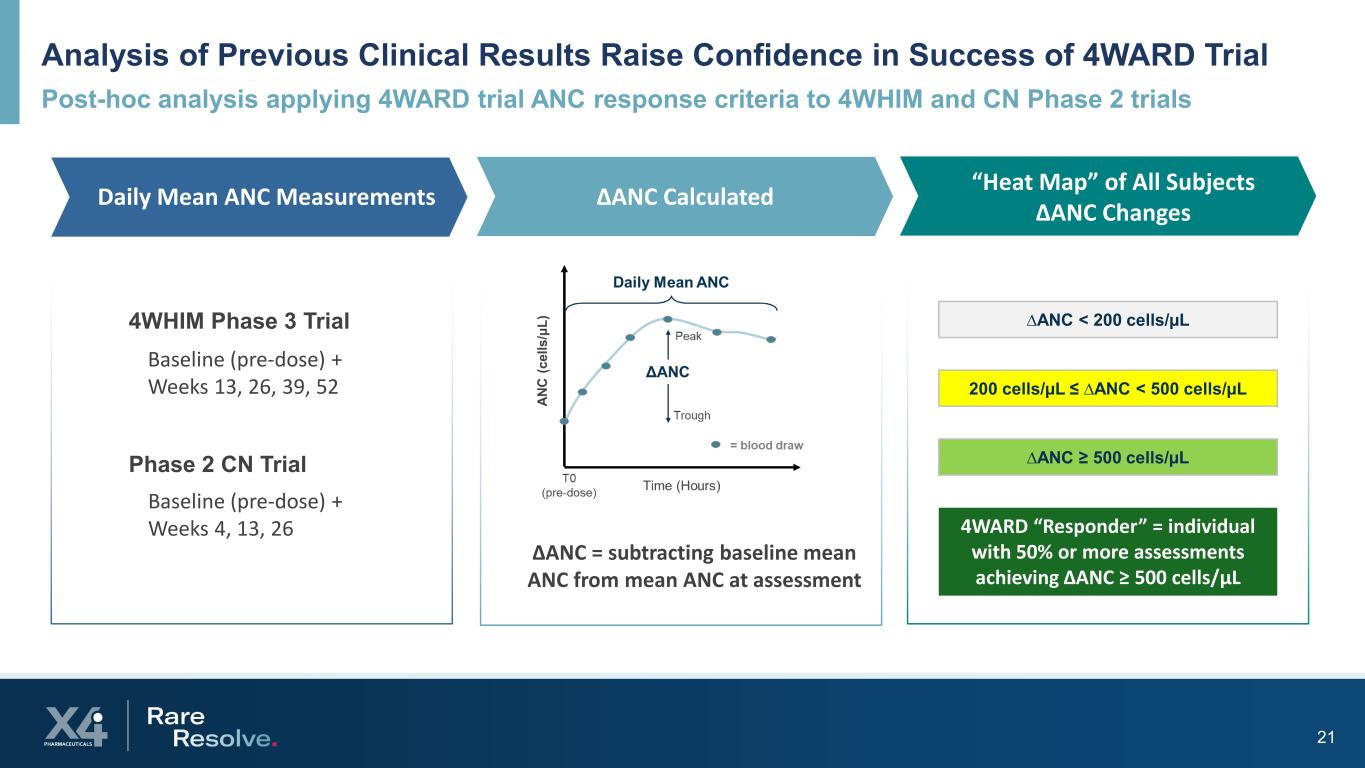
Analysis of Previous Clinical Results Raise Confidence in Success of 4WARD Trial Post-hoc analysis applying 4WARD trial ANC response criteria to 4WHIM and CN Phase 2 trials 21 Baseline (pre-dose) + Weeks 13, 26, 39, 52 ∆ANC = subtracting baseline mean ANC from mean ANC at assessment ∆ANC ≥ 500 cells/µL 200 cells/µL ≤ ∆ANC < 500 cells/µL ∆ANC < 200 cells/µL Daily Mean ANC Measurements ∆ANC Calculated “Heat Map” of All Subjects ∆ANC Changes Baseline (pre-dose) + Weeks 4, 13, 26 4WHIM Phase 3 Trial Phase 2 CN Trial 4WARD “Responder” = individual with 50% or more assessments achieving ∆ANC ≥ 500 cells/µL
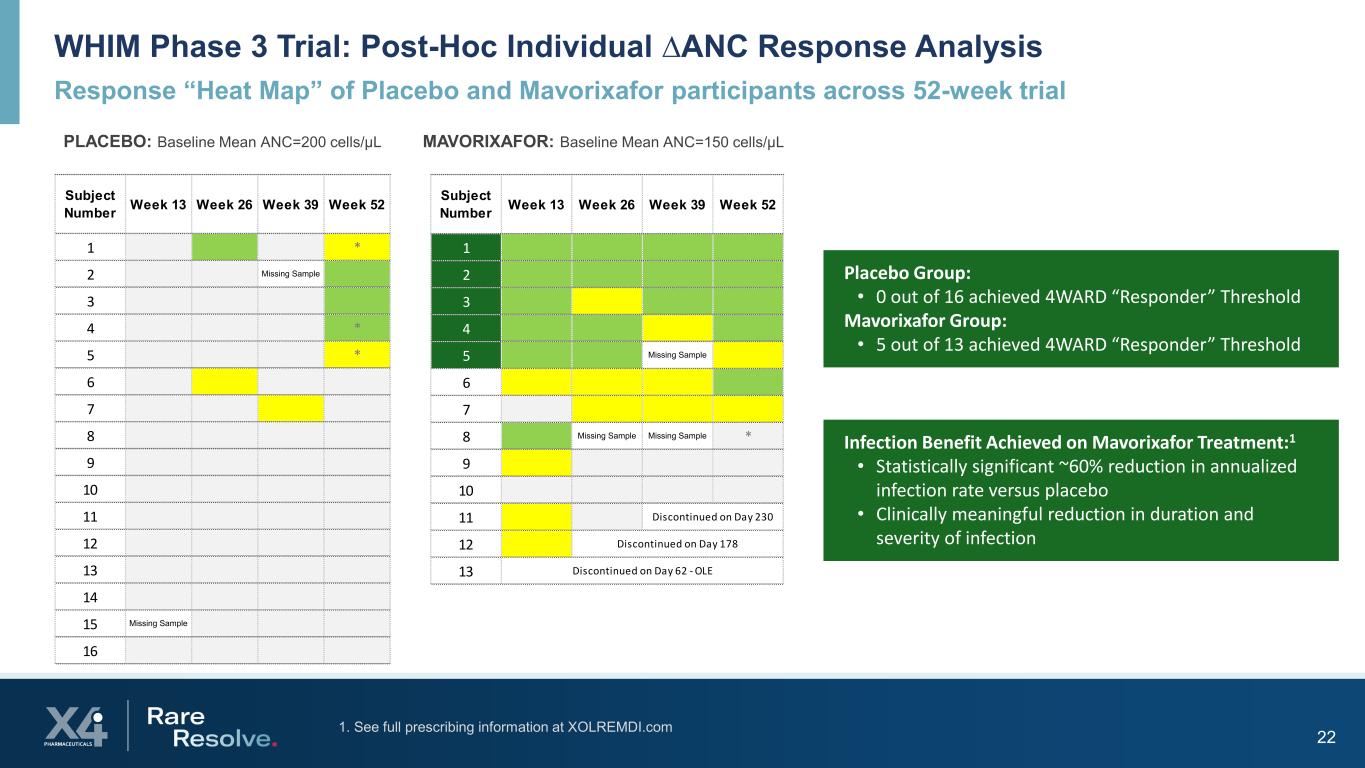
22 Subject Number Week 13 Week 26 Week 39 Week 52 1 * 2 Missing Sample 3 4 * 5 * 6 7 8 9 10 11 12 13 14 15 Missing Sample 16 WHIM Phase 3 Trial: Post-Hoc Individual ∆ANC Response Analysis Response “Heat Map” of Placebo and Mavorixafor participants across 52-week trial PLACEBO: Baseline Mean ANC=200 cells/µL MAVORIXAFOR: Baseline Mean ANC=150 cells/µL Placebo Group: • 0 out of 16 achieved 4WARD “Responder” Threshold Mavorixafor Group: • 5 out of 13 achieved 4WARD “Responder” Threshold Subject Number Week 13 Week 26 Week 39 Week 52 1 2 3 4 5 Missing Sample 6 7 8 Missing Sample Missing Sample * 9 10 11 12 13 Discontinued on Day 230 Discontinued on Day 178 Discontinued on Day 62 - OLE Infection Benefit Achieved on Mavorixafor Treatment:1 • Statistically significant ~60% reduction in annualized infection rate versus placebo • Clinically meaningful reduction in duration and severity of infection 1. See full prescribing information at XOLREMDI.com
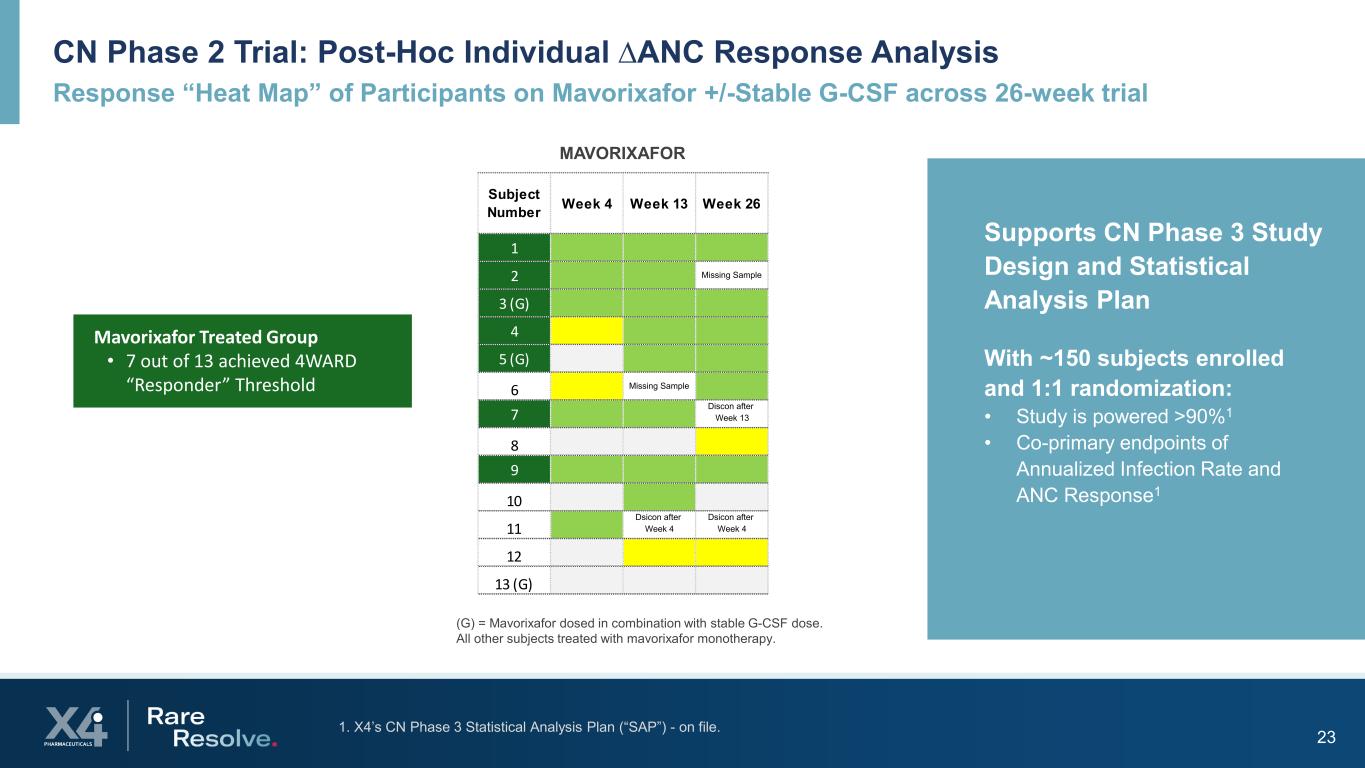
23 MAVORIXAFOR CN Phase 2 Trial: Post-Hoc Individual ∆ANC Response Analysis Response “Heat Map” of Participants on Mavorixafor +/-Stable G-CSF across 26-week trial (G) = Mavorixafor dosed in combination with stable G-CSF dose. All other subjects treated with mavorixafor monotherapy. Supports CN Phase 3 Study Design and Statistical Analysis Plan With ~150 subjects enrolled and 1:1 randomization: • Study is powered >90%1 • Co-primary endpoints of Annualized Infection Rate and ANC Response1 Subject Number Week 4 Week 13 Week 26 1 2 Missing Sample 3 (G) 4 5 (G) 6 Missing Sample 7 Discon after Week 13 8 9 10 11 Dsicon after Week 4 Dsicon after Week 4 12 13 (G) Mavorixafor Treated Group • 7 out of 13 achieved 4WARD “Responder” Threshold 1. X4’s CN Phase 3 Statistical Analysis Plan (“SAP”) - on file.
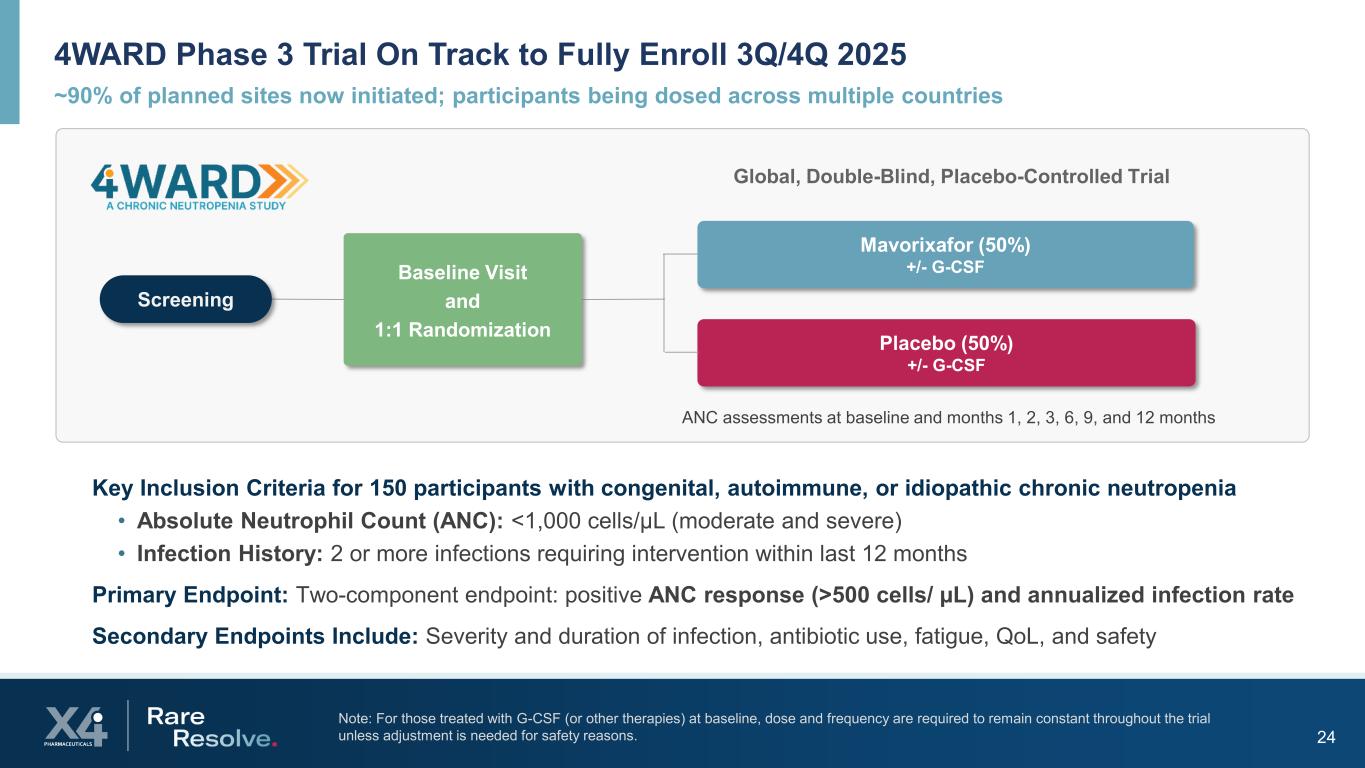
~90% of planned sites now initiated; participants being dosed across multiple countries 4WARD Phase 3 Trial On Track to Fully Enroll 3Q/4Q 2025 24 Note: For those treated with G-CSF (or other therapies) at baseline, dose and frequency are required to remain constant throughout the trial unless adjustment is needed for safety reasons. Mavorixafor (50%) +/- G-CSF Placebo (50%) +/- G-CSF Global, Double-Blind, Placebo-Controlled Trial Screening Baseline Visit and 1:1 Randomization ANC assessments at baseline and months 1, 2, 3, 6, 9, and 12 months Key Inclusion Criteria for 150 participants with congenital, autoimmune, or idiopathic chronic neutropenia • Absolute Neutrophil Count (ANC): <1,000 cells/µL (moderate and severe) • Infection History: 2 or more infections requiring intervention within last 12 months Primary Endpoint: Two-component endpoint: positive ANC response (>500 cells/ µL) and annualized infection rate Secondary Endpoints Include: Severity and duration of infection, antibiotic use, fatigue, QoL, and safety
4WARD on Track to Deliver Potentially Transformative Results 25 12-month 4WARD trial is highly powered (>90%) to meet study’s co-primary endpoints Annualized infection rate and ANC response: co-primary endpoints Clinical operations in full swing and delivering Trial sites are nearly fully activated Screening increasing – full enrollment guidance maintained for 3Q or 4Q 2025 Demographics and quality of subjects demonstrate severity of disease Balanced demographics representative of target populations (congenital, idiopathic CN) Severity of CN disease (both ANC and infection history) is consistent with high unmet need target commercial population Topline Data Still Anticipated in 2H 2026
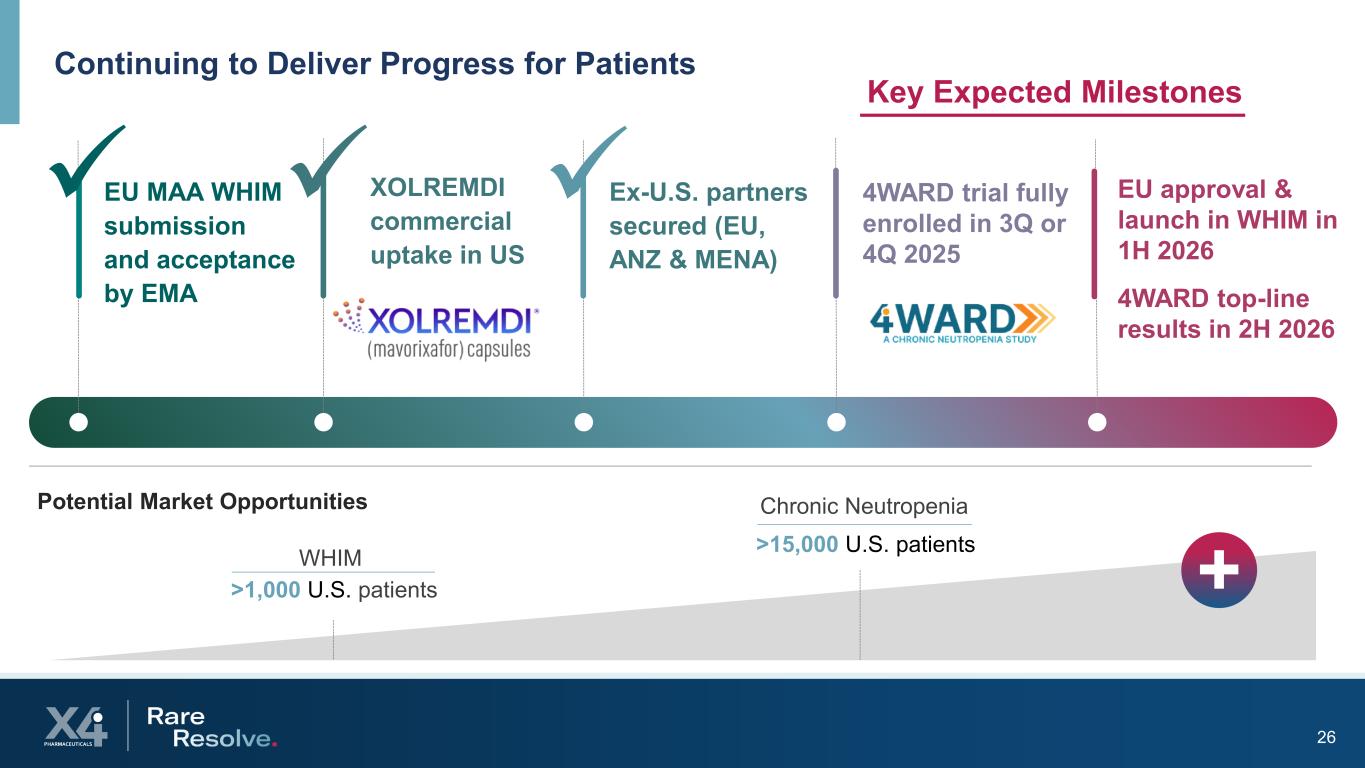
26 Continuing to Deliver Progress for Patients >1,000 U.S. patients >15,000 U.S. patients Potential Market Opportunities +WHIM Chronic Neutropenia EU MAA WHIM submission and acceptance by EMA XOLREMDI commercial uptake in US Ex-U.S. partners secured (EU, ANZ & MENA) 4WARD trial fully enrolled in 3Q or 4Q 2025 EU approval & launch in WHIM in 1H 2026 4WARD top-line results in 2H 2026 Key Expected Milestones

www.x4pharma.com Corporate Headquarters 61 North Beacon Street, 4th Floor Boston, MA 02134 Nasdaq: XFOR

Appendix
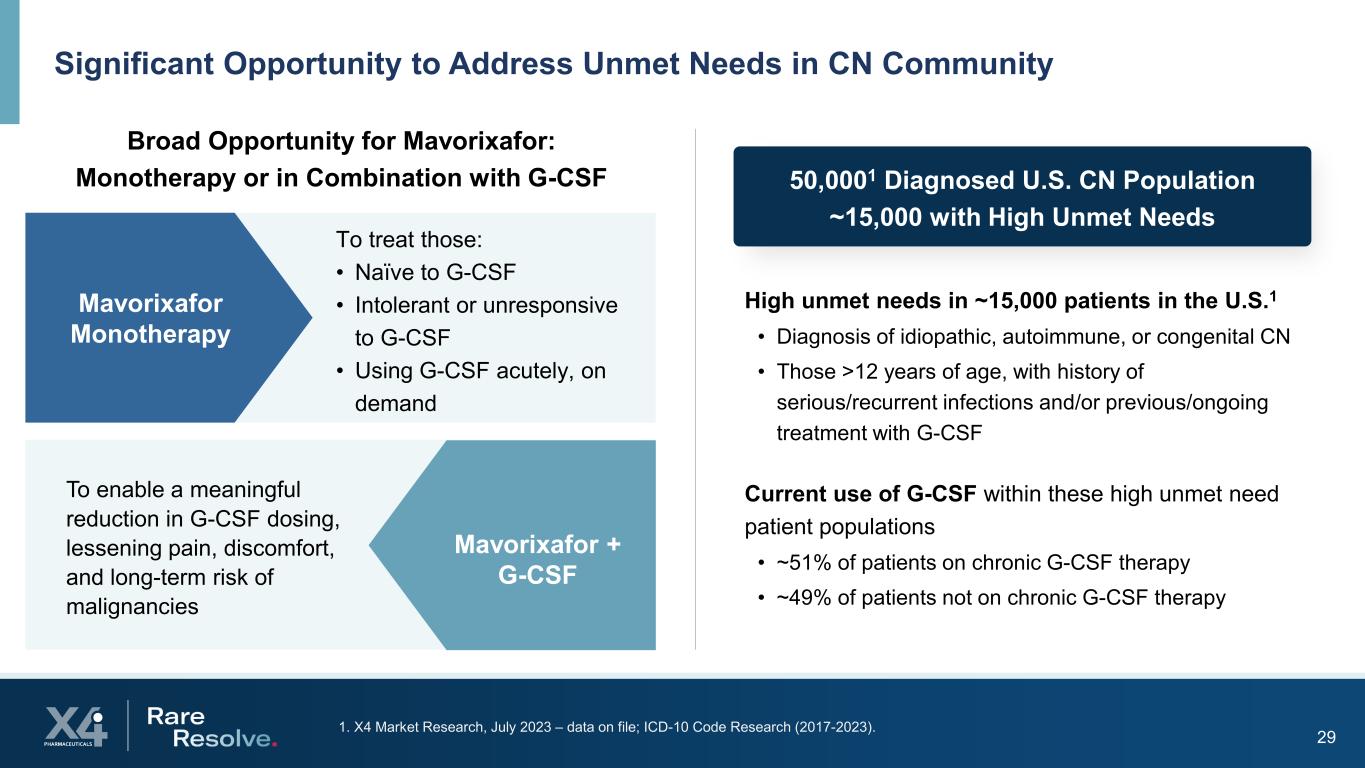
291. X4 Market Research, July 2023 – data on file; ICD-10 Code Research (2017-2023). Significant Opportunity to Address Unmet Needs in CN Community Broad Opportunity for Mavorixafor: Monotherapy or in Combination with G-CSF High unmet needs in ~15,000 patients in the U.S.1 • Diagnosis of idiopathic, autoimmune, or congenital CN • Those >12 years of age, with history of serious/recurrent infections and/or previous/ongoing treatment with G-CSF Current use of G-CSF within these high unmet need patient populations • ~51% of patients on chronic G-CSF therapy • ~49% of patients not on chronic G-CSF therapy 50,0001 Diagnosed U.S. CN Population ~15,000 with High Unmet Needs Mavorixafor Monotherapy To treat those: • Naïve to G-CSF • Intolerant or unresponsive to G-CSF • Using G-CSF acutely, on demand Mavorixafor + G-CSF To enable a meaningful reduction in G-CSF dosing, lessening pain, discomfort, and long-term risk of malignancies
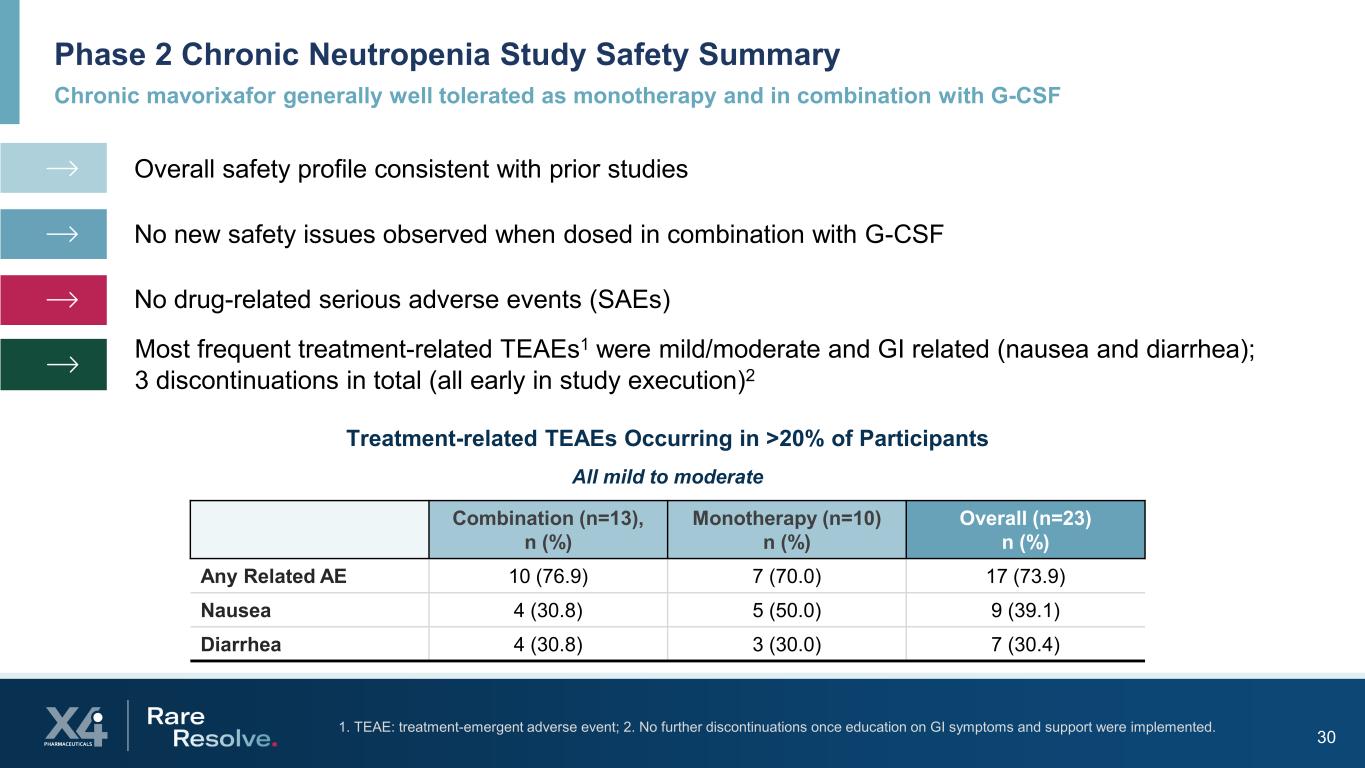
Chronic mavorixafor generally well tolerated as monotherapy and in combination with G-CSF Phase 2 Chronic Neutropenia Study Safety Summary 301. TEAE: treatment-emergent adverse event; 2. No further discontinuations once education on GI symptoms and support were implemented. Combination (n=13), n (%) Monotherapy (n=10) n (%) Overall (n=23) n (%) Any Related AE 10 (76.9) 7 (70.0) 17 (73.9) Nausea 4 (30.8) 5 (50.0) 9 (39.1) Diarrhea 4 (30.8) 3 (30.0) 7 (30.4) Treatment-related TEAEs Occurring in >20% of Participants Overall safety profile consistent with prior studies No new safety issues observed when dosed in combination with G-CSF No drug-related serious adverse events (SAEs) Most frequent treatment-related TEAEs1 were mild/moderate and GI related (nausea and diarrhea); 3 discontinuations in total (all early in study execution)2 All mild to moderate

Experience spans research, development, & commercialization of first-in-class, innovative therapies X4’s Leadership Team – Strength Delivering For Patients 31 c ADAM MOSTAFA Chief Financial Officer c PAULA RAGAN, Ph.D. President & CEO c MARY DIBIASE, Ph.D. Chief Operating Officer c MARK BALDRY Chief Commercial Officer cCHRISTOPHE ARBET- ENGELS, M.D., Ph.D. Chief Medical Officer
32 1. Funds as of March 31, 2025, including restricted cash; 2. Assuming continued compliance with debt covenants as described in the company’s Form 10-Q filed on May 1, 2025. Balance Sheet Supports Expected Upcoming Milestones Funds expected to support operations into 1H 20262 $87.7 million1 Analyst Coverage Top-tier Life Science-Focused Institutional Shareholder Base