

NASDAQ: CTXR Citius Pharmaceuticals, Inc. (NASDAQ: CTXR) Corporate Overview DECEMBER 2025

FORWARD - LOOKING STATEMENTS NASDAQ: CTXR 2 This presentation has been prepared by Citius Pharmaceuticals, Inc . (the “Company”) for informational purposes only and not for any other purpose . Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the Company or any director, employee, agent, or adviser of the Company . This presentation does not purport to be all - inclusive or to contain all of the information you may desire . The information contained in this presentation and the comments and remarks of the representatives of the Company made during any presentation to which this presentation relates are integrally related and, as such, are intended to be delivered and understood together . Information provided in this presentation speaks only as of the date hereof . The Company assumes no obligation to update any statement after the date of this presentation as a result of new information, subsequent events or any other circumstances . This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with the Company or its affiliates . The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation . This presentation contains forward - looking statements that involve substantial risks and uncertainties . In some cases, you can identify forward - looking statements by the words “may”, “might”, “will”, “could”, “would”, “should”, “expect”, “intend”, “plan”, “goal”, “objective”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “continue” and “ongoing”, or the negative of these terms, or other comparable terminology intended to identify statements about the future . These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward - looking statements . The forward - looking statements and opinions contained in this presentation are based upon estimates and information available to us as of the date of this presentation . While we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information . Factors that could cause actual results to differ from those discussed in the forward - looking statements include, but are not limited to : our need for substantial additional funds and our ability to raise additional money to fund our operations for at least the next 12 months as a going concern ; our ability to successfully commercialize LYMPHIR, including covering the costs of licensing payments, product manufacturing and other third - party goods and services, through our majority - owned subsidiary and any of our other product candidates that may be approved by the FDA ; our ability to obtain, perform under and maintain financing and strategic agreements and relationships ; our ability to maintain compliance with Nasdaq's continued listing requirements ; risks relating to the results of research and development activities, including those from our existing and any new pipeline assets ; risks related to research using our assets but conducted by third parties ; the estimated markets for our product candidates and the acceptance thereof by any market ; the ability of our product candidates to impact the quality of life of our target patient populations ; our dependence on third - party suppliers ; our ability to procure cGMP commercial - scale supply ; uncertainties relating to preclinical and clinical testing ; the early stage of products under development ; market and other conditions ; risks related to our growth strategy ; patent and intellectual property matters ; our ability to identify, acquire, close and integrate product candidates and companies successfully and on a timely basis ; government regulation ; competition ; and other assumptions described in this presentation underlying or relating to any forward - looking statements Investors are strongly encouraged to carefully review the Company’s SEC filings for a listing of the risks that could cause actual results to differ from these forward - looking statements . These forward - looking statements speak only as of the date of this presentation and should not be construed as statements of facts . As a matter of course, we do not make public projections as to our expected sales or profitability due to, among other reasons, the inherent uncertainty of the underlying assumptions and estimates . Similarly, as a matter of course, we do not comment on ongoing or potential partnership discussions, the expected timing of future financial raises or potential long - term strategic plans .
INVESTMENT HIGHLIGHTS Biopharmaceutical company with multiple advanced development programs • LYMPHIR (denileukin diftitox - cxdl) • LAUNCHED December 2025 for the treatment of adult patients with relapsed or refractory Stage I - III cutaneous T - cell lymphoma (CTCL) after at least one prior systemic therapy • Spun - off into Citius Oncology, Inc. (NASDAQ: CTOR) in August 2024 • Mino - Lok ® • Only treatment designed to salvage infected catheters causing CLABSI • Phase 3 trial completed in 2024 • Positive Topline data – met primary and secondary endpoints • Halo - Lido • Only Rx therapy under development for hemorrhoids • Phase 2b trial completed NASDAQ: CTXR 3

NASDAQ: CTXR ABOUT CITIUS ONCOLOGY, INC. 4 1. Internal estimates based on IQVIA market research. Biopharmaceutical company focused on developing and commercializing innovative targeted oncology therapies • NASDAQ: CTOR | Stand - alone public company since August 2024 • Majority - owned (~79%) subsidiary of Citius Pharmaceuticals (NASDAQ: CTXR) • Shared management services agreement with CTXR for operational efficiency • LYMPHIR : First commercial product launched December 2025 • $400M+ est. addressable U.S. market with strong growth opportunities 1 • Rights to all markets except India, Japan and certain parts of Asia • Mission: Deliver innovative, targeted oncology therapies that transform patient outcomes

LYMPHIR NOW COMMERCIALLY AVAILABLE IN THE U.S. INDICATION: LYMPHIR is an IL2 - receptor - directed cytotoxin indicated for the treatment of adult patients with relapsed or refractory Stage I - III cutaneous T - cell lymphoma (CTCL) after at least one prior systemic therapy • First marketed product by Citius Oncology • Commercial supply shipped to national wholesalers • Accessible to providers and patients across the U.S. NASDAQ: CTXR 5

EXPERIENCED MANAGEMENT TEAM LEONARD MAZUR CHAIRMAN & CEO JAIME BARTUSHAK EVP, CFO & CBO DR. MYRON CZUCZMAN EVP, CHIEF MEDICAL OFFICER OMAR LANSARI DIR, MARKETING MICHAEL MCGUIRE VP, COMMERCIAL MYRON HOLUBIAK EXECUTIVE VICE CHAIRMAN Shared management services agreement with Citius Pharmaceuticals mitigates execution risk, maximizes capital efficiency and leverages industry expertise NASDAQ: CTXR 6
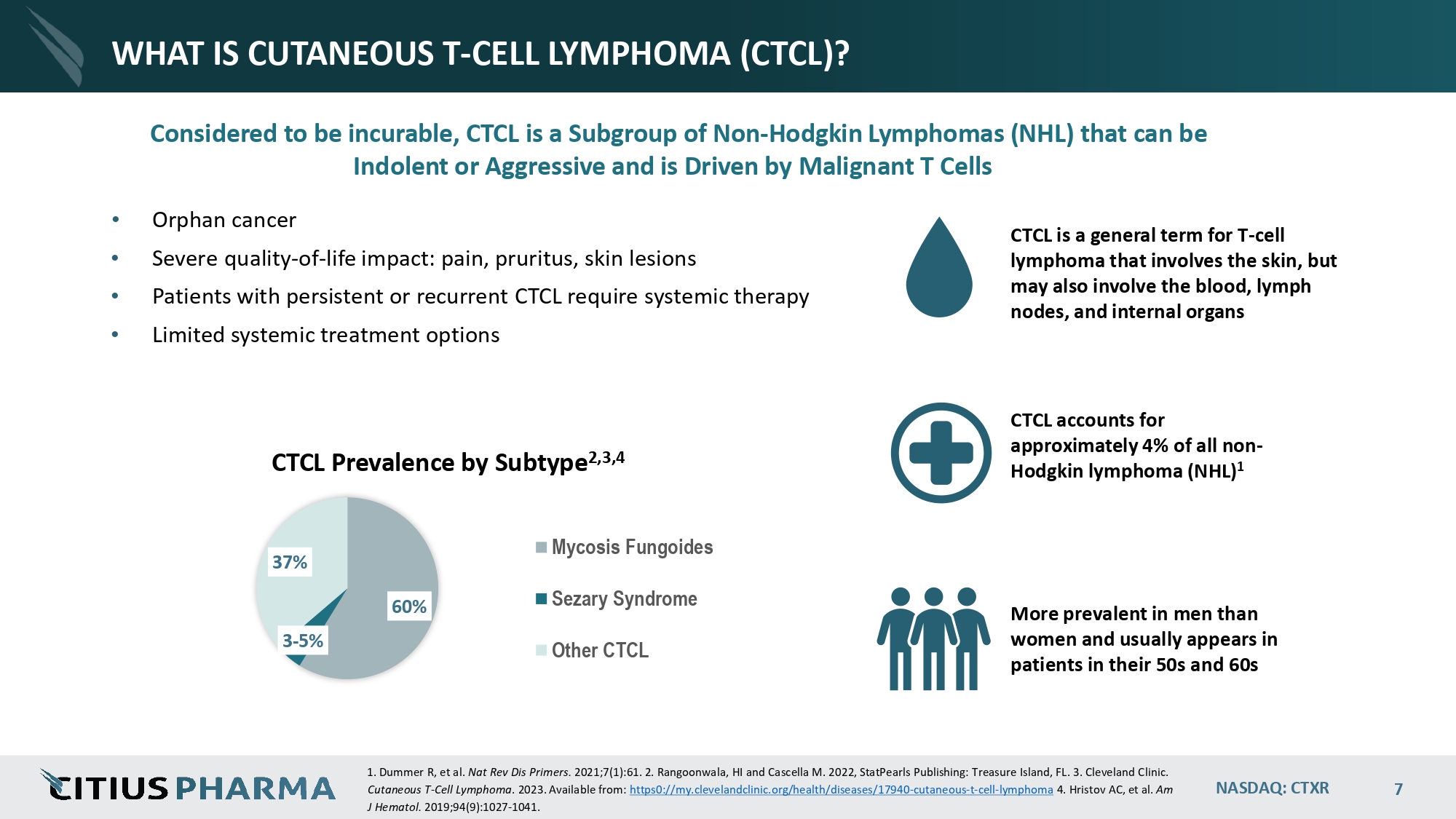
NASDAQ: CTXR 7 WHAT IS CUTANEOUS T - CELL LYMPHOMA (CTCL)? • Orphan cancer • Severe quality - of - life impact: pain, pruritus, skin lesions • Patients with persistent or recurrent CTCL require systemic therapy • Limited systemic treatment options CTCL is a general term for T - cell lymphoma that involves the skin, but may also involve the blood, lymph nodes, and internal organs CTCL accounts for approximately 4% of all non - Hodgkin lymphoma (NHL) 1 More prevalent in men than women and usually appears in patients in their 50s and 60s 60% 3 - 5% 37% CTCL Prevalence by Subtype 2,3,4 Mycosis Fungoides Sezary Syndrome Other CTCL J Hematol. 2019;94(9):1027 - 1041. 1. Dummer R, et al. Nat Rev Dis Primers. 2021;7(1):61. 2. Rangoonwala, HI and Cascella M. 2022, StatPearls Publishing: Treasure Island, FL. 3. Cleveland Clinic. Cutaneous T - Cell Lymphoma . 2023. Available from: https0://my.clevelandclinic.org/health/diseases/17940 - cutaneous - t - cel l - l ymphoma 4. Hristov AC, et al. Am Considered to be incurable, CTCL is a Subgroup of Non - Hodgkin Lymphomas (NHL) that can be Indolent or Aggressive and is Driven by Malignant T Cells
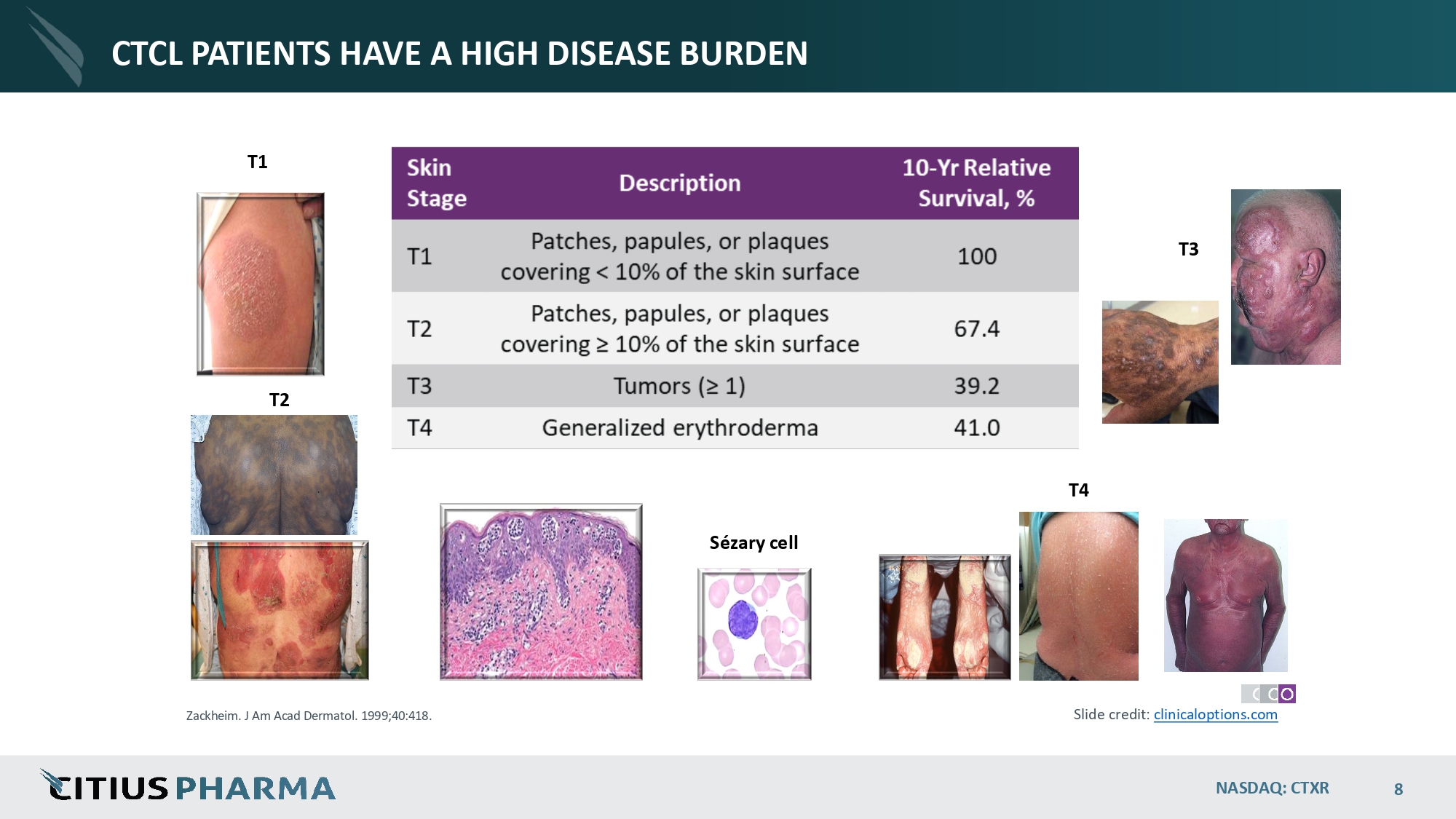
CTCL PATIENTS HAVE A HIGH DISEASE BURDEN T1 T4 Sézary cell T2 T3 Zackheim. J Am Acad Dermatol. 1999;40:418. Slide credit: clinicaloptions.com NASDAQ: CTXR 8
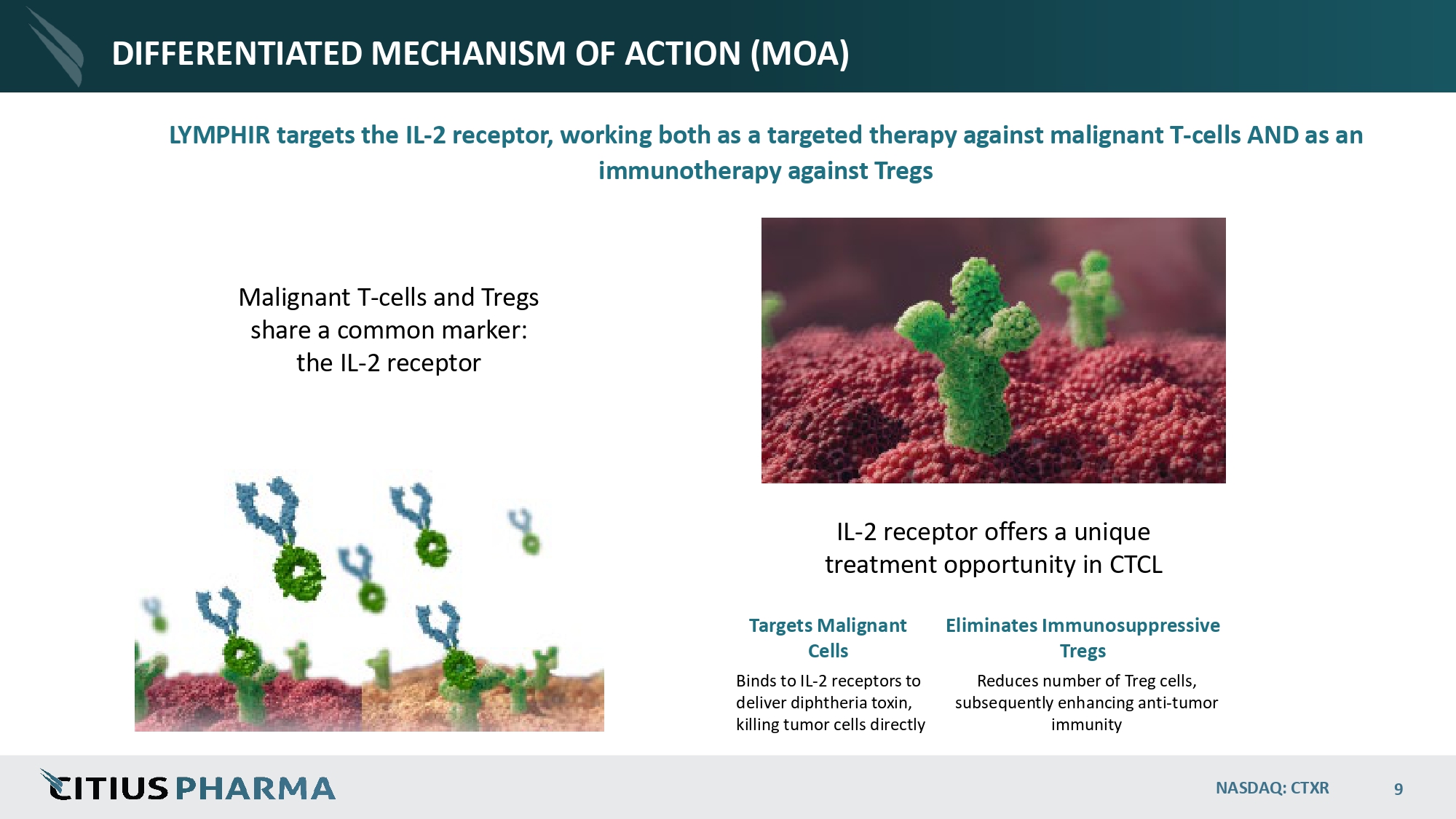
DIFFERENTIATED MECHANISM OF ACTION (MOA) LYMPHIR targets the IL - 2 receptor, working both as a targeted therapy against malignant T - cells AND as an immunotherapy against Tregs IL - 2 receptor offers a unique treatment opportunity in CTCL Malignant T - cells and Tregs share a common marker: the IL - 2 receptor Targets Malignant Cells Binds to IL - 2 receptors to deliver diphtheria toxin, killing tumor cells directly NASDAQ: CTXR 9 Eliminates Immunosuppressive Tregs Reduces number of Treg cells, subsequently enhancing anti - tumor immunity
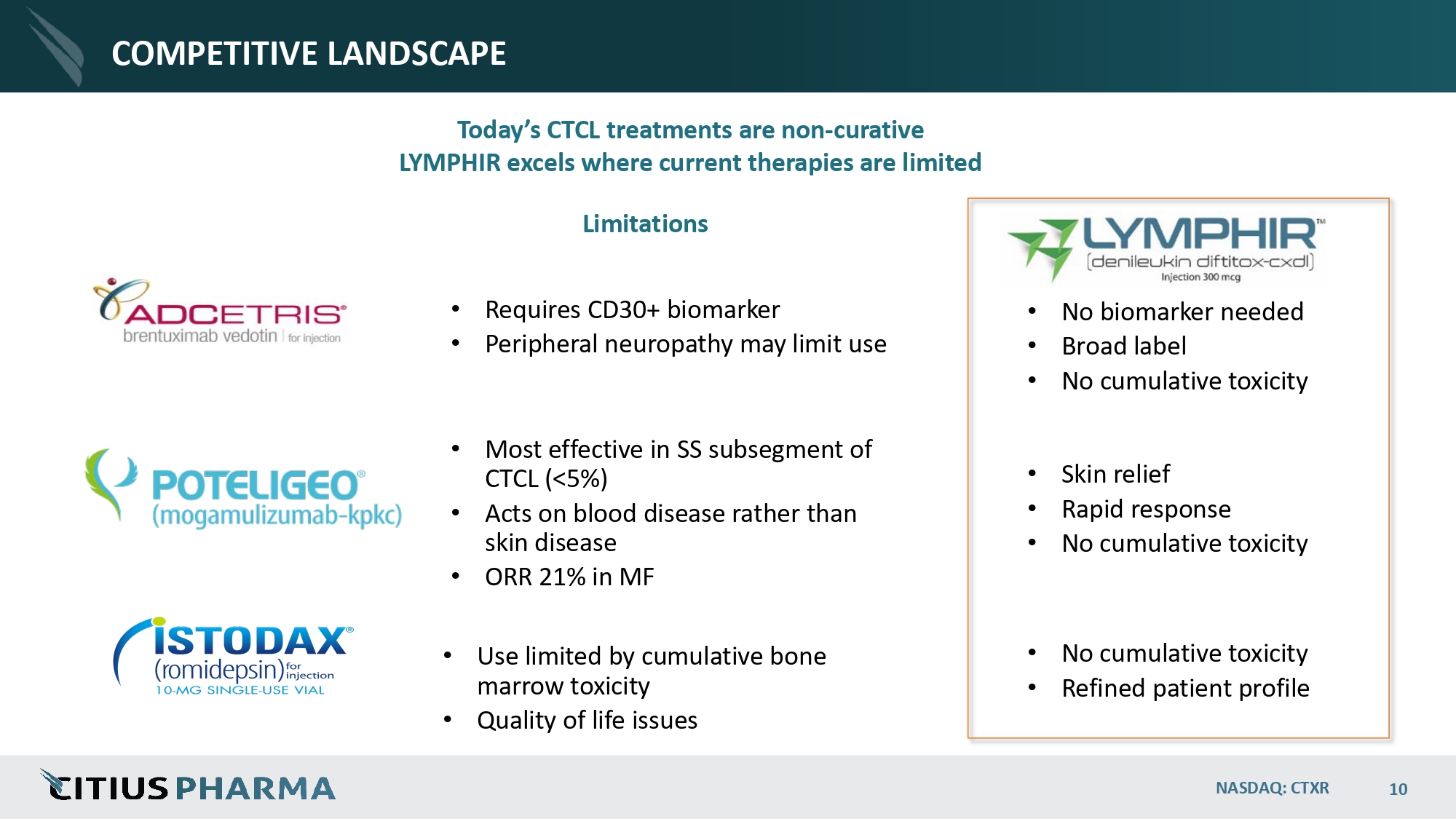
COMPETITIVE LANDSCAPE Today’s CTCL treatments are non - curative LYMPHIR excels where current therapies are limited • Requires CD30+ biomarker • Peripheral neuropathy may limit use • Most effective in SS subsegment of CTCL (<5%) • Acts on blood disease rather than skin disease • ORR 21% in MF • Use limited by cumulative bone marrow toxicity • Quality of life issues • No biomarker needed • Broad label • No cumulative toxicity • Skin relief • Rapid response • No cumulative toxicity • No cumulative toxicity • Refined patient profile Limitations NASDAQ: CTXR 10
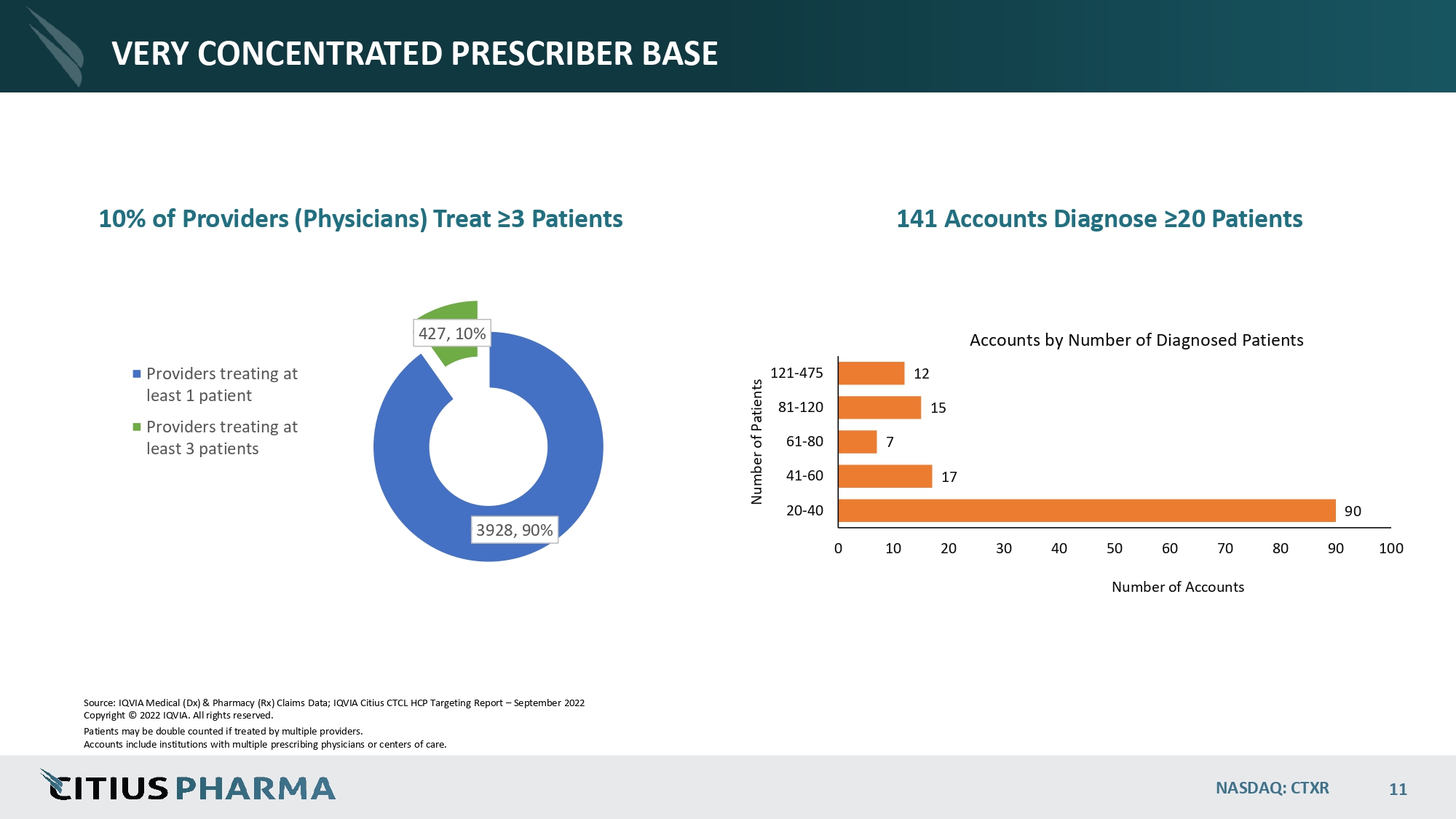
VERY CONCENTRATED PRESCRIBER BASE 10% of Providers (Physicians) Treat ≥3 Patients 90 17 7 15 12 0 10 20 30 40 50 60 70 Number of Accounts 80 90 100 20 - 40 41 - 60 61 - 80 81 - 120 121 - 475 Number of Patients Accounts by Number of Diagnosed Patients 3928, 90% 427, 10% Providers treating at least 1 patient Providers treating at least 3 patients Source: IQVIA Medical (Dx) & Pharmacy (Rx) Claims Data; IQVIA Citius CTCL HCP Targeting Report – September 2022 Copyright © 2022 IQVIA. All rights reserved. Patients may be double counted if treated by multiple providers. Accounts include institutions with multiple prescribing physicians or centers of care. NASDAQ: CTXR 11 141 Accounts Diagnose ≥20 Patients
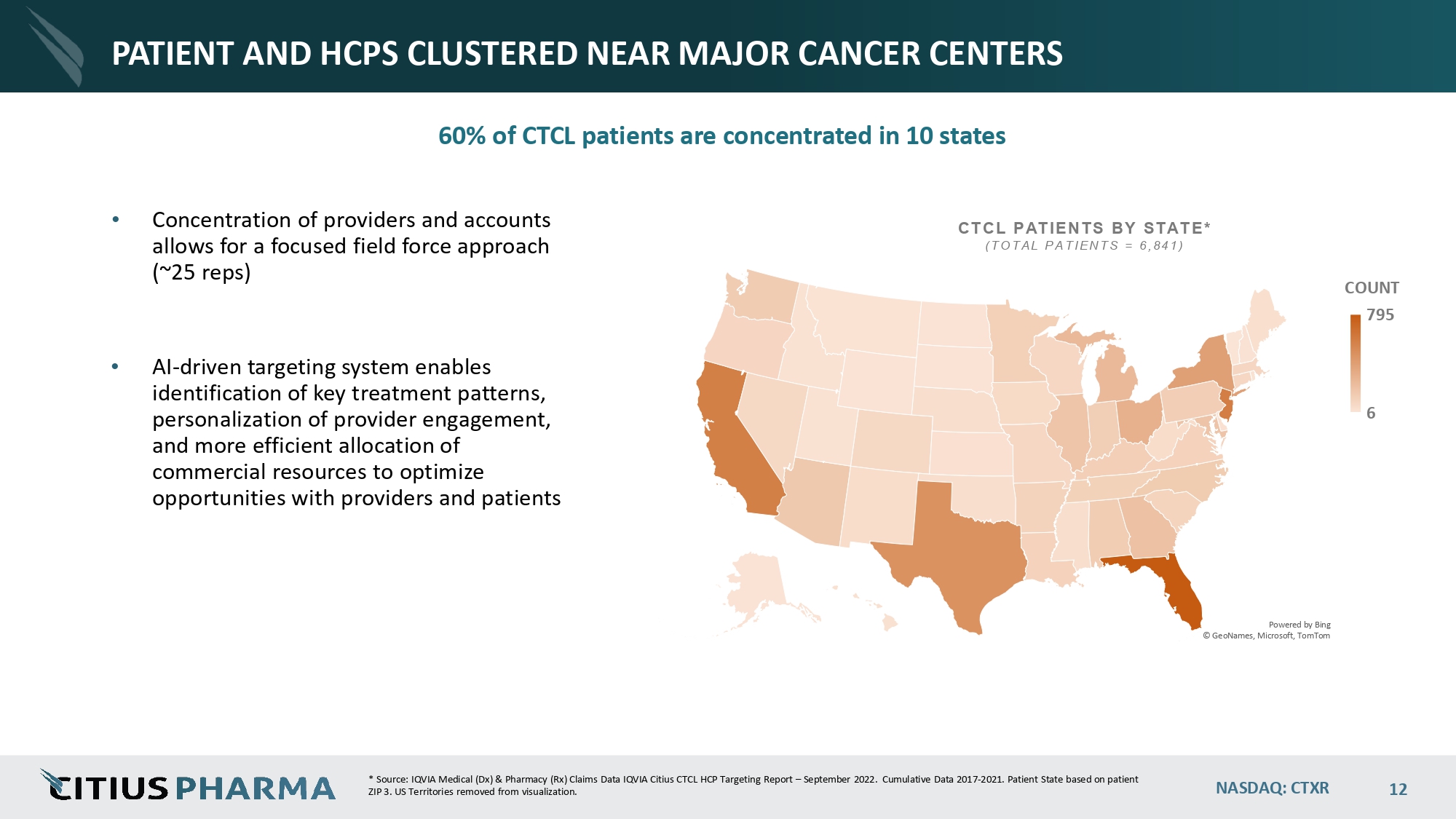
NASDAQ: CTXR 12 PATIENT AND HCPS CLUSTERED NEAR MAJOR CANCER CENTERS 60% of CTCL patients are concentrated in 10 states • Concentration of providers and accounts allows for a focused field force approach (~ 25 reps) • AI - driven targeting system enables identification of key treatment patterns, personalization of provider engagement, and more efficient allocation of commercial resources to optimize opportunities with providers and patients Powered by Bing © GeoNames, Microsoft, TomTom 6 COUNT 795 CTCL PATIENTS BY STATE* ( TOTAL PATIENTS = 6 ,841 ) * Source: IQVIA Medical (Dx) & Pharmacy (Rx) Claims Data IQVIA Citius CTCL HCP Targeting Report – September 2022. Cumulative Data 2017 - 2021. Patient State based on patient ZIP 3. US Territories removed from visualization.

NASDAQ: CTXR DISTRIBUTION READINESS AND CHANNEL ACCESS Patient - Centric Distribution Strategy aims to provide timely product availability for eligible CTCL patients across all care settings, reinforcing Citius’ commitment to access • Nationwide U.S. distribution network established • All major distribution agreements operational • Citius Oncology has executed agreements with Cencora, Cardinal Health, and McKesson to support U.S. distribution of LYMPHIR • These partnerships ensure nationwide coverage across academic centers and community clinics • Commercial - ready inventory with a 60 - month shelf life is in place to meet projected demand 12 – 18 months post - launch • Ex - U.S. strategy to leverage country - specific Named Patient Programs • Exclusive distribution agreement with Integris Pharma S.A. (Oct 2025) • Establishes coverage for 12 markets: Greece, Cyprus, Malta, Bulgaria, Romania, Croatia, Serbia, Albania, Bosnia Herzegovina, Kosovo, Montenegro and North Macedonia • Citius Pharma is in active discussions with multiple additional prospective distribution partners across several European Union member states, in South America, and in select Middle Eastern territories 13
NASDAQ: CTXR PROVIDER & PATIENT SUPPORT Citius Oncology Access & Education Tools • HCP portal: www.lymphirhcp.com • Reimbursement and prior authorization support • Patient assistance via Citius Advantage program • Comprehensive education & prescribing tools 14 NASDAQ: CTOR |
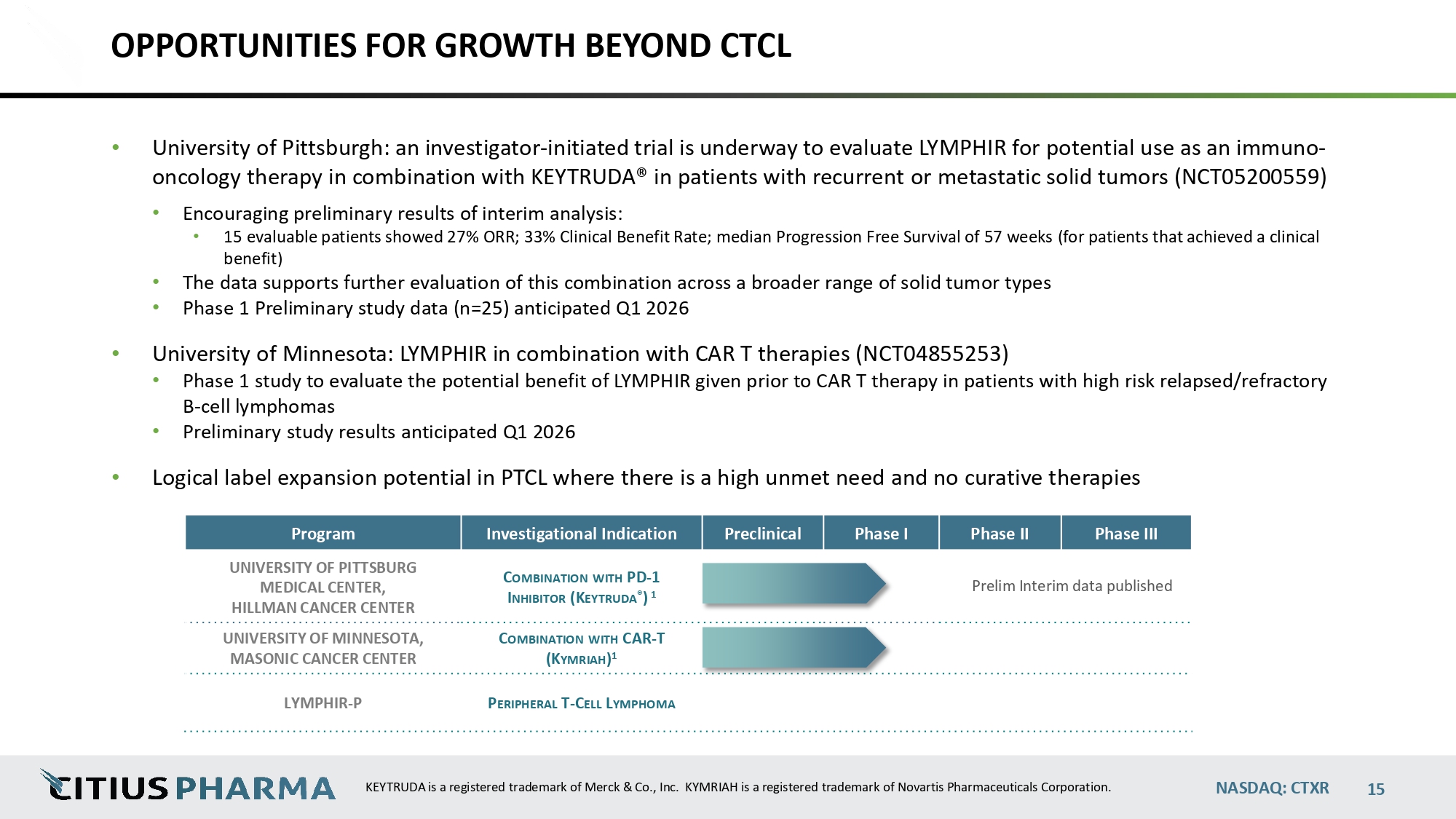
OPPORTUNITIES FOR GROWTH BEYOND CTCL • University of Pittsburgh: an investigator - initiated trial is underway to evaluate LYMPHIR for potential use as an immuno - oncology therapy in combination with KEYTRUDA® in patients with recurrent or metastatic solid tumors (NCT05200559) • Encouraging preliminary results of interim analysis: • 15 evaluable patients showed 27% ORR; 33% Clinical Benefit Rate; median Progression Free Survival of 57 weeks (for patients that achieved a clinical benefit) • The data supports further evaluation of this combination across a broader range of solid tumor types • Phase 1 Preliminary study data (n=25) anticipated Q1 2026 • University of Minnesota: LYMPHIR in combination with CAR T therapies (NCT04855253) • Phase 1 study to evaluate the potential benefit of LYMPHIR given prior to CAR T therapy in patients with high risk relapsed/refractory B - cell lymphomas • Preliminary study results anticipated Q1 2026 • Logical label expansion potential in PTCL where there is a high unmet need and no curative therapies Phase III Phase II Phase I Preclinical Investigational Indication Program data published Prelim Interim p C OMBINATION WITH PD - 1 I NHIBITOR (K EYTRUDA ® ) 1 UNIVERSITY OF PITTSBURG MEDICAL CENTER, HILLMAN CANCER CENTER C OMBINATION WITH CAR - T (K YMRIAH ) 1 UNIVERSITY OF MINNESOTA, MASONIC CANCER CENTER P ERIPHERAL T - C ELL L YMPHOMA LYMPHIR - P NASDAQ: CTXR KEYTRUDA is a registered trademark of Merck & Co., Inc. KYMRIAH is a registered trademark of Novartis Pharmaceuticals Corporation. 15
LYMPHIR IS COMPETITIVELY POSITIONED • Differentiated MOA targeting the IL - 2 receptor reinforces rationale for inclusion among the current core therapeutic options in the U.S. market • CTCL treatments are non - curative , often have a limited duration of response and/or are discontinued early • Patients are put on multiple alternate therapies and cycle to 2nd line treatments within 5 months , on average • Key growth drivers expected to increase overall market size and facilitate market penetration • Evolving treatment paradigm; incremental therapeutic option for pre - treated patients • Historically, market growth has followed introduction of new therapeutics • Competitively priced • No new therapy approved since 2018 Clinical profile and market dynamics supports market entry NASDAQ: CTXR 16

MINO - LOK NASDAQ: CTXR 17 Phase 3 Trial Completed: Positive Topline Data
NASDAQ: CTXR MINO - LOK OVERVIEW Achieved primary and secondary endpoints of Phase 3 Trial x Time to catheter failure exceeded expectations x Majority of patients in the Mino - Lok group achieved overall treatment success x Well tolerated with no drug - related serious adverse events A novel antibiotic lock solution designed to salvage catheters in patients with catheter - related bloodstream infections Mino - Lok addresses the complications, discomfort and cost of catheter removal and replacement No drugs currently approved to salvage catheters in patients with central - line associated bloodstream infections (CLABSI) or catheter - related bloodstream infections (CRBSI) Phase 3 Trial completed: multi - center, randomized, open label, blinded assessor, active control superiority study Estimated global market expected to exceed $2 billion 1 18 1. Market.us. Catheter - Related Bloodstream Infection Market Report. Retrieved from https://market.us/report/catheter - related - bloodstream - infection - market/
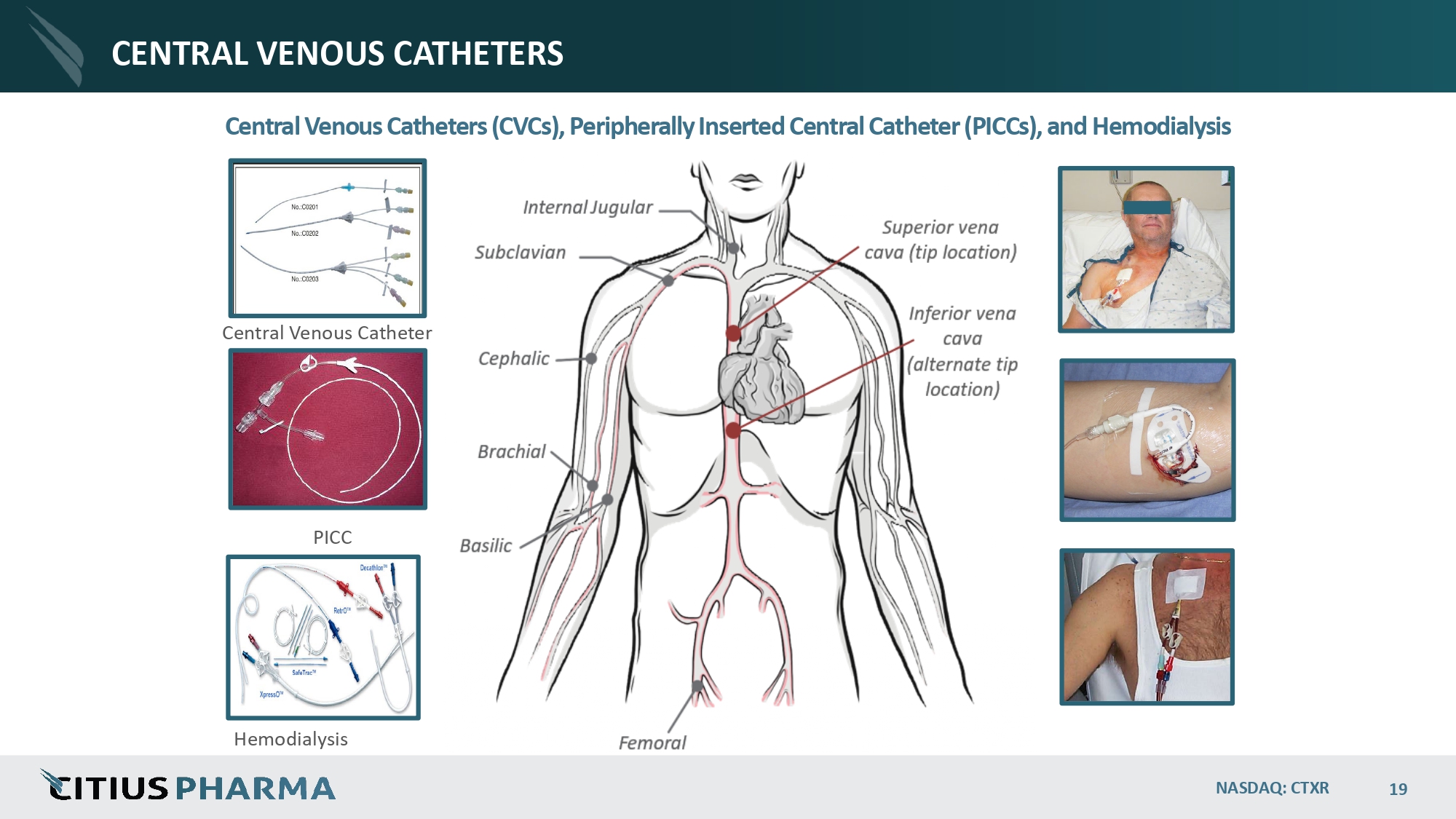
CENTRAL VENOUS CATHETERS NASDAQ: CTXR 19 Central Venous Catheters (CVCs), Peripherally Inserted Central Catheter (PICCs), and Hemodialysis Central Venous Catheter PICC Hemodialysis
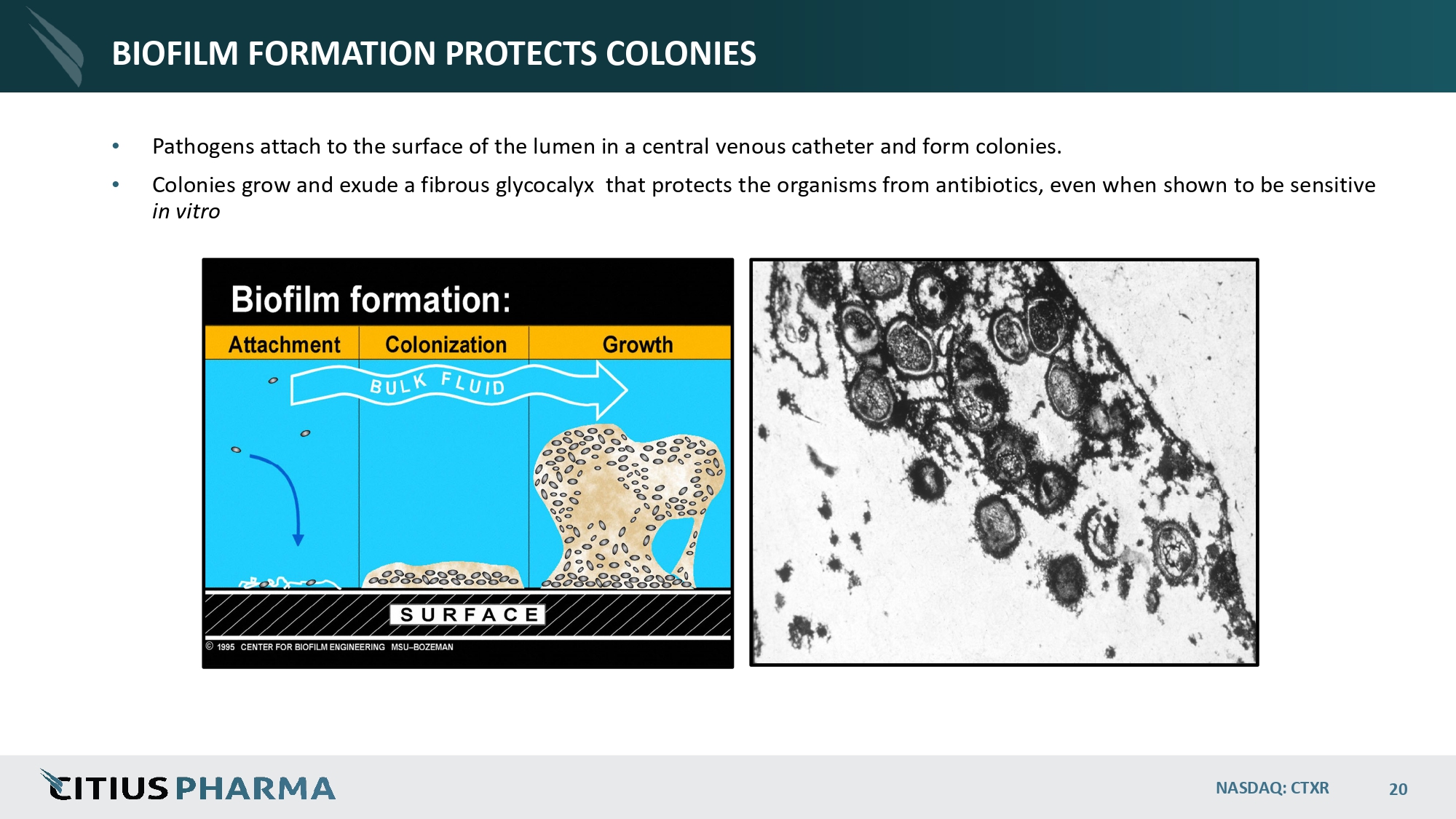
BIOFILM FORMATION PROTECTS COLONIES • Pathogens attach to the surface of the lumen in a central venous catheter and form colonies. • Colonies grow and exude a fibrous glycocalyx that protects the organisms from antibiotics, even when shown to be sensitive in vitro NASDAQ: CTXR 20
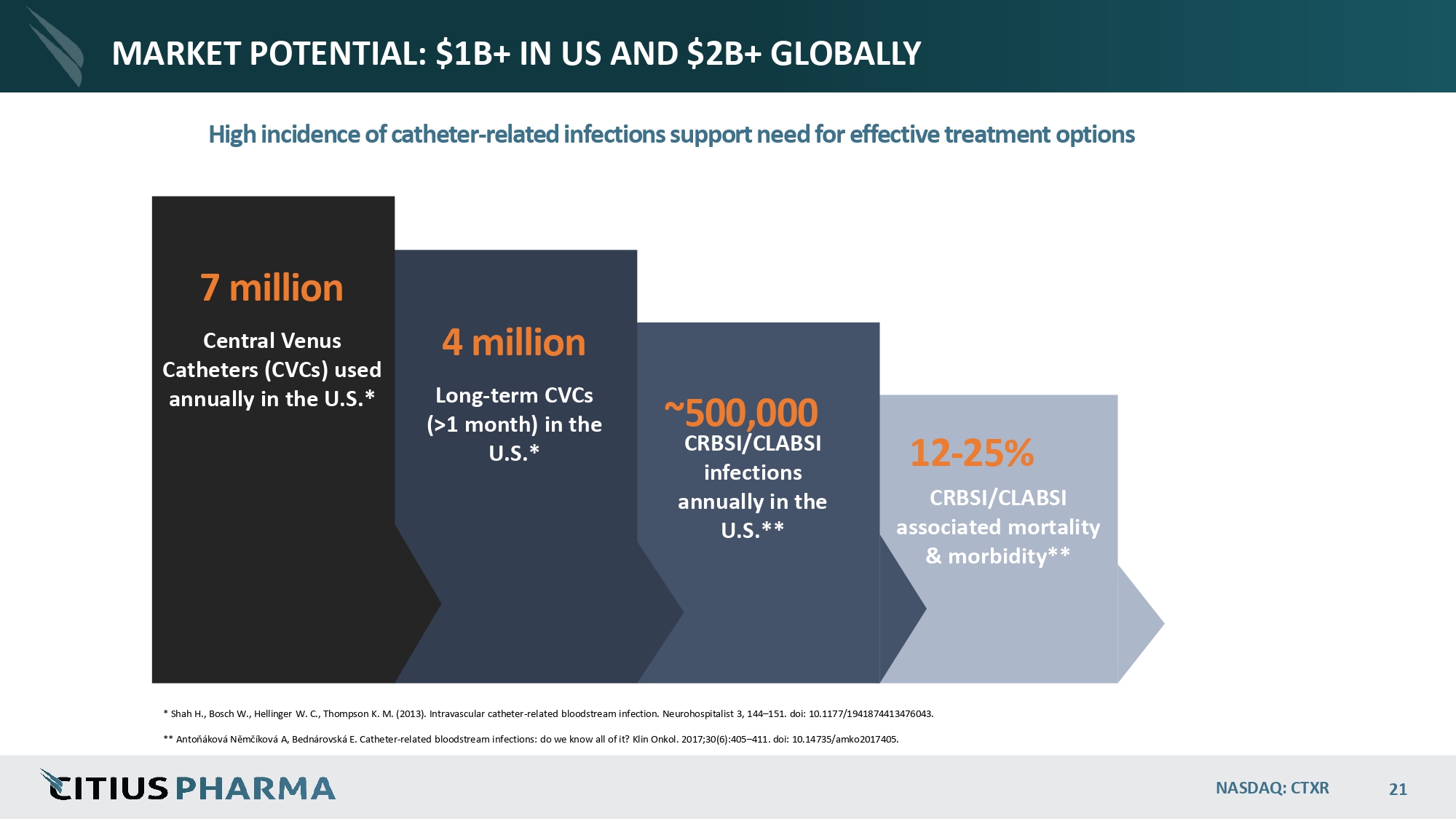
MARKET POTENTIAL: $1B+ IN US AND $2B+ GLOBALLY High incidence of catheter - related infections support need for effective treatment options ~500,000 CRBSI/CLABSI infections annually in the U.S.** 12 - 25% CRBSI/CLABSI associated mortality & morbidity** * Shah H., Bosch W., Hellinger W. C., Thompson K. M. (2013). Intravascular catheter - related bloodstream infection. Neurohospitalist 3, 144 – 151. doi: 10.1177/1941874413476043. ** Antoňáková Němčíková A, Bednárovská E. Catheter - related bloodstream infections: do we know all of it? Klin Onkol. 2017;30(6):405 – 411. doi: 10.14735/amko2017405. NASDAQ: CTXR 21 4 million Long - term CVCs (>1 month) in the U.S.* 7 million Central Venus Catheters (CVCs) used annually in the U.S.*
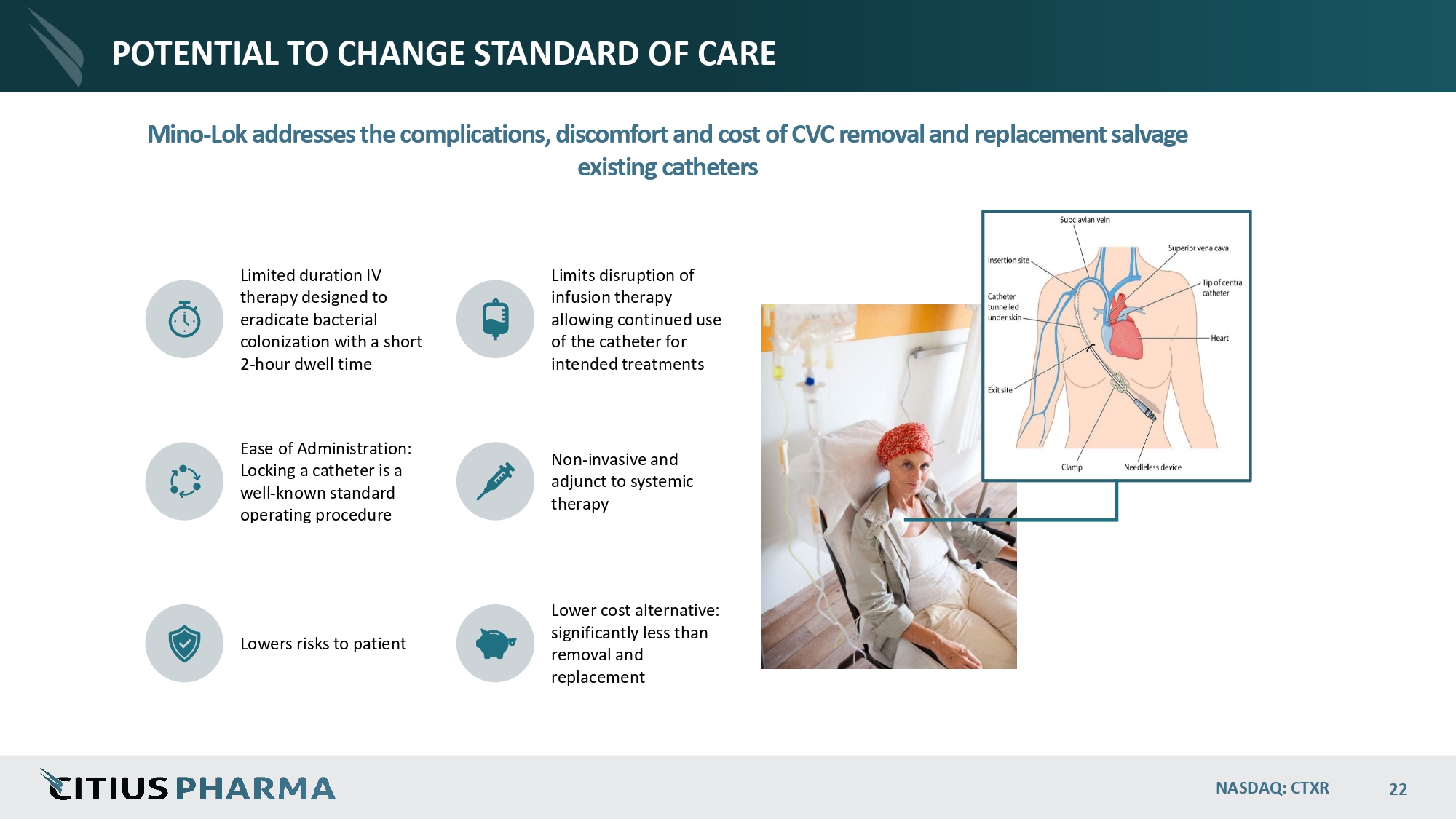
POTENTIAL TO CHANGE STANDARD OF CARE Mino - Lok addresses the complications, discomfort and cost of CVC removal and replacement salvage existing catheters Limited duration IV therapy designed to eradicate bacterial colonization with a short 2 - hour dwell time Limits disruption of infusion therapy allowing continued use of the catheter for intended treatments Ease of Administration: Locking a catheter is a well - known standard operating procedure Non - invasive and adjunct to systemic therapy Lowers risks to patient Lower cost alternative: significantly less than removal and replacement NASDAQ: CTXR 22

MINO - LOK PHASE 3 TRIAL TOPLINE RESULTS NASDAQ: CTXR 23 Mino - Lok significantly outperforms hospital - specific anti - infective lock solutions • Kaplan Meier Analysis demonstrated clear separation between Mino - Lok and control arms, illustrating Mino - Lok’s superiority in extending time to catheter failure Control arm: 33 days Mino - Lok arm: exceeded the trial period (6 weeks) (p - value = 0.0006) Primary Endpoint: Median Time to Failure A greater percentage of patients in the Mino - Lok arm achieved overall treatment success compared to the control arm (p - value = 0.0025) Key Secondary Endpoint: Overall Treatment Success Mino - Lok was well - tolerated with no drug - related serious adverse events Comparable adverse events between Mino - Lok (45.1%) and control (46.1%) arms, as expected in very ill patients Mino - Lok is instilled into the catheter and never enters the patient Safety Profile

HALO - LIDO NASDAQ: CTXR 24 Halobetasol/Lidocaine
NASDAQ: CTXR • 10+ Million patients report symptoms of hemorrhoidal disease; 1/3 seek physician treatment 1 • A cream formulation containing halobetasol propionate (highly potent steroid) and Lidocaine HCl • Phase 2b enrollment completed • 5 cohorts of 60 subjects each • Primary endpoint: reduction in hemorrhoidal symptoms • Subject self - reported using proprietary mobile app (PRO) • Positive Phase 2b results • Meaningful reduction in symptom severity when compared to individual components alone • Dose for Phase 3 trial selected • Trial validates Patient Reported Outcome (PRO) instrument developed to support a pivotal Phase 3 study • Ongoing FDA engagement regarding next steps over the coming months • Citius anticipates monetizing the value of this asset with a strategic or financial partner HALO - LIDO OVERVIEW Potentially the first FDA - approved prescription product to treat hemorrhoids 1. Source: https: //www.mayoclinic.org/medical - professionals/digestive - diseases/news/hemorrhoidal - disease - diagnosis - and - management/mac - 20430067 25

SUMMARY NASDAQ: CTXR 26

WHY INVEST? WHY NOW? NASDAQ: CTXR 27 Diversified late - stage biopharmaceutical company with first commercialized product launched Q4 2025 • LYMPHIR launched December 2025 • $400+M est. addressable market with multiple opportunities for growth • First new systemic CTCL therapy since 2018 • 12 - year BLA exclusivity • Pipeline of additional late - stage assets • Management and shareholder alignment • $26.5 M invested by founders • Successful pharma/biotech track record • Attractive investor entry points

NASDAQ: CTXR Citius Pharmaceuticals, Inc. (NASDAQ: CTXR) Investor Inquiries ir@citiuspharma.com