
1 Bettering the Lives of People Impacted by Kidney DiseaseCorporate Presentation January 2026 NASDAQ: AKBA JOHN BUTLER Chief Executive Officer

Cautionary note on forward-looking statements Statements in this presentation regarding Akebia Therapeutics, Inc.’s (“Akebia’s”) strategy, plans, prospects, expectations, beliefs, intentions and goals are forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, and include, but are not limited to, statements regarding: Akebia's plans, strategies and prospects for its business; Akebia’s plans with respect to its U.S. commercial launch of Vafseo®, including the potential U.S. market opportunity; Akebia’s plans for Vafseo to become standard of care for treatment of anemia due to CKD in dialysis, including its ability to build on the body of evidence demonstrating Vafseo’s value potential, and progress towards that goal; Akebia’s expectations and beliefs about demand for Vafseo, including the number of patients with access to Vafseo and the focus of dialysis organizations; Akebia’s beliefs with respect to patient dosing demand for Vafseo in 2026; Akebia’s plans and expectations with respect to publication of additional analyses of INNO2VATE data; Akebia’s plans and expectations with respect to the VOICE trial, including the timing of top-line data and potential to demonstrate favorable outcomes in the composite of all-cause mortality and hospitalization in patients treated with vadadustat compared to ESA; Akebia’s plans and expectations with respect to the VOCAL trial, including timing of top-line data; Akebia’s plans and expectations with respect to the praliciguat trial, including to assess the use of praliciguat in other rare podocytopathies, the number of patients be enrolled in the trial and its potential for successful development and regulatory path; Akebia’s plans and expectations with respect AKB-097, including the timing of initiation of, and initial data from, an open label Phase 2 basket study and the indications to be evaluated, other potential indications for consideration and its potential for pipeline in a product and to achieve opportunities to address unmet need; and Akebia’s plans and expectations with respect to AKB-9090, including the timing of initiation of, and top-line data from, a Phase 1 trial and the indication to be evaluated. The terms "intend," "believe," "plan," "goal," "potential," "anticipate, "estimate," "expect," "future," "will," "continue," derivatives of these words, and similar references are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Actual results, performance or experience may differ materially from those expressed or implied by any forward-looking statement as a result of various risks, uncertainties and other factors, including, but not limited to, risks associated with: the potential therapeutic benefits, safety profile, and effectiveness of Vafseo and Akebia’s development candidates; the results of preclinical and clinical research; Akebia’s ability to initiate and enroll patients in its clinical trials; decisions made by health authorities, such as the FDA, with respect to regulatory filings and other interactions; the potential demand and market potential and acceptance of, as well as coverage and reimbursement related to, Vafseo®, including estimates regarding the potential market opportunity; the competitive landscape for Auryxia® and Vafseo, including generic entrants and the timing thereof; the ability of Akebia to attract and retain qualified personnel; Akebia's ability to achieve and maintain profitability and to maintain operating expenses consistent with its operating plan; manufacturing, supply chain and quality matters and any recalls, write-downs, impairments or other related consequences or potential consequences; early termination of any of Akebia's collaborations; and changes in the geopolitical environment and uncertainty surrounding U.S. trade policy on tariffs. Other risks and uncertainties include those identified under the heading "Risk Factors" in Akebia's Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, and other filings that Akebia may make with the U.S. Securities and Exchange Commission in the future. These forward-looking statements (except as otherwise noted) speak only as of the date of this presentation, and, except as required by law, Akebia does not undertake, and specifically disclaims, any obligation to update any forward-looking statements contained in this presentation. Akebia Therapeutics®, Auryxia® and Vafseo® are registered trademarks of Akebia Therapeutics, Inc. and its affiliates. 2
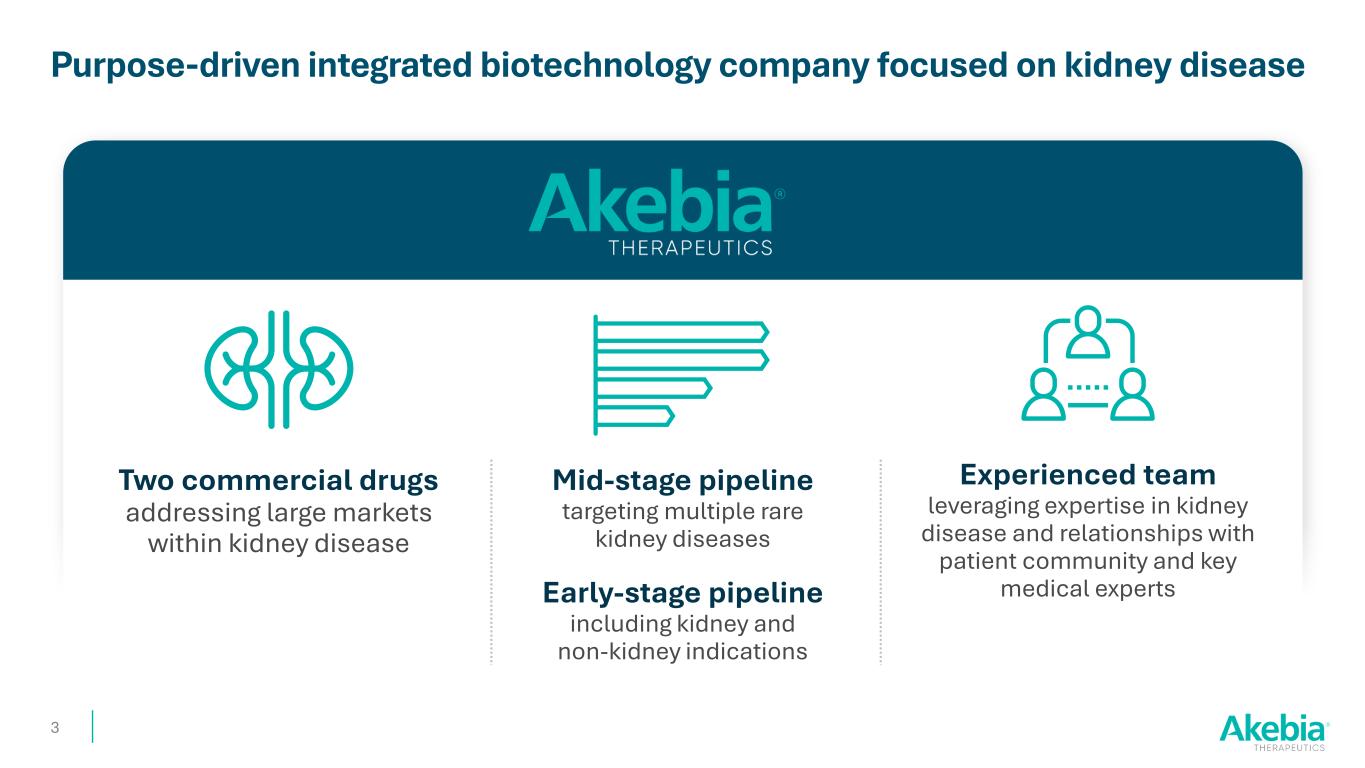
Purpose-driven integrated biotechnology company focused on kidney disease 3 Experienced team leveraging expertise in kidney disease and relationships with patient community and key medical experts Two commercial drugs addressing large markets within kidney disease Mid-stage pipeline targeting multiple rare kidney diseases Early-stage pipeline including kidney and non-kidney indications
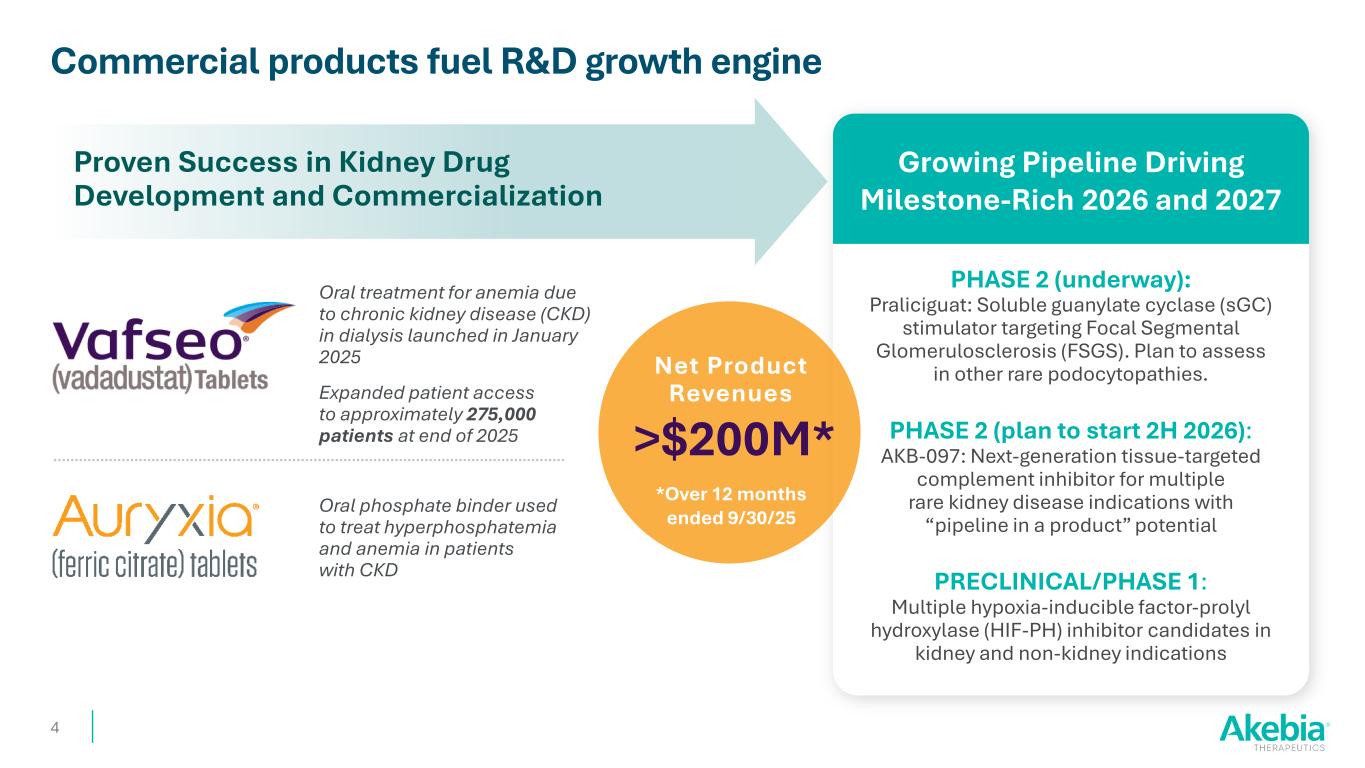
Commercial products fuel R&D growth engine Proven Success in Kidney Drug Development and Commercialization Growing Pipeline Driving Milestone-Rich 2026 and 2027 Oral phosphate binder used to treat hyperphosphatemia and anemia in patients with CKD Oral treatment for anemia due to chronic kidney disease (CKD) in dialysis launched in January 2025 Expanded patient access to approximately 275,000 patients at end of 2025 PHASE 2 (underway): Praliciguat: Soluble guanylate cyclase (sGC) stimulator targeting Focal Segmental Glomerulosclerosis (FSGS). Plan to assess in other rare podocytopathies. PHASE 2 (plan to start 2H 2026): AKB-097: Next-generation tissue-targeted complement inhibitor for multiple rare kidney disease indications with “pipeline in a product” potential PRECLINICAL/PHASE 1: Multiple hypoxia-inducible factor-prolyl hydroxylase (HIF-PH) inhibitor candidates in kidney and non-kidney indications 4 Net Product Revenues >$200M* *Over 12 months ended 9/30/25
Vafseo® (vadadustat) Tablets indicated for the treatment of anemia due to CKD in adults who have been receiving dialysis for at least three months Click here for the Full Prescribing Information, including BOXED WARNING and Medication Guide. Foundational Launch Year Sets the Stage for Goal to Become Standard of Care 5 This presentation is intended for investor purposes only and is not intended for promotional purposes

An oral HIF-PH inhibitor 6 Unique mechanism of action built on Nobel Prize-winning science Stimulates body’s natural response to hypoxia Enhances body’s natural production of EPO Activates iron mobilization Simple titration and fewer dose modifications Convenient oral dosing Controls hemoglobin (Hb) levels over time
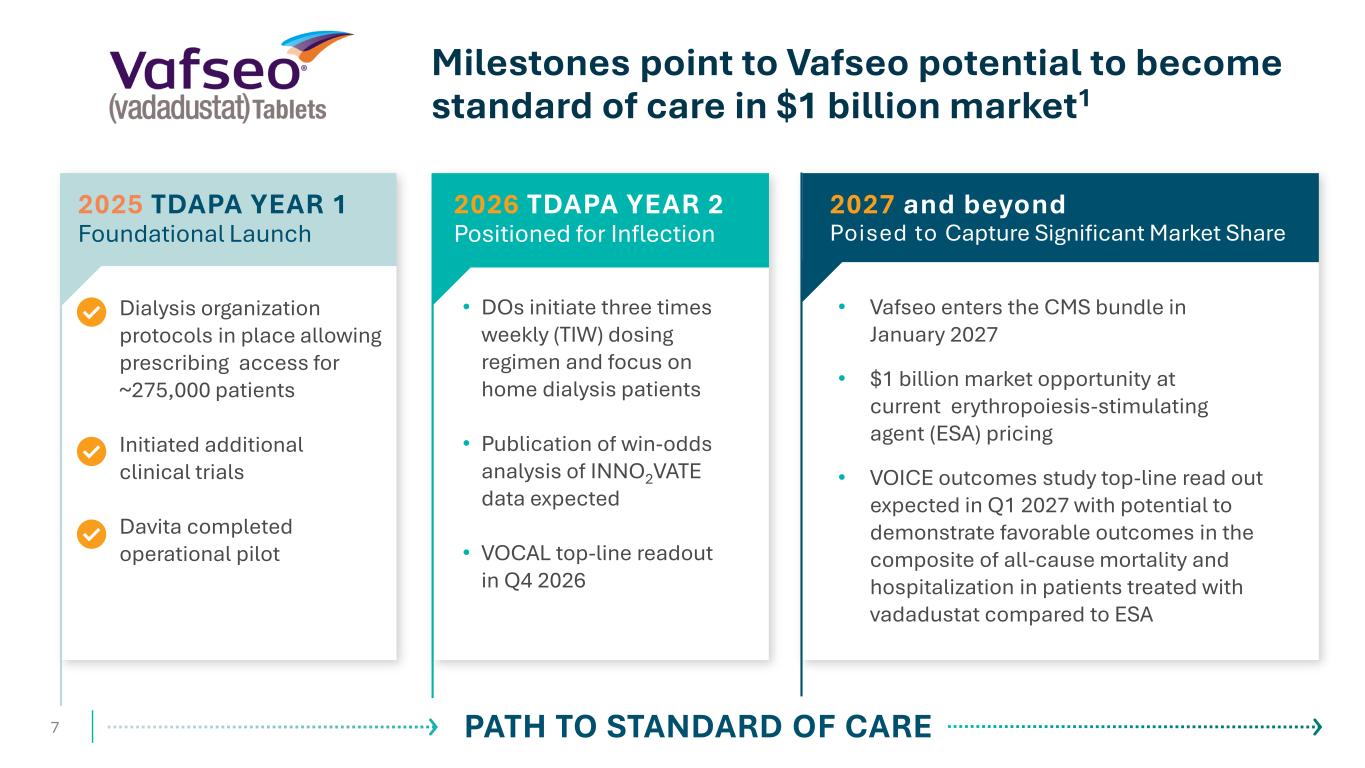
Dialysis organization protocols in place allowing prescribing access for ~275,000 patients Initiated additional clinical trials Davita completed operational pilot 7 Milestones point to Vafseo potential to become standard of care in $1 billion market1 • DOs initiate three times weekly (TIW) dosing regimen and focus on home dialysis patients • Publication of win-odds analysis of INNO2VATE data expected • VOCAL top-line readout in Q4 2026 2026 TDAPA YEAR 2 Positioned for Inflection PATH TO STANDARD OF CARE 2025 TDAPA YEAR 1 Foundational Launch • Vafseo enters the CMS bundle in January 2027 • $1 billion market opportunity at current erythropoiesis-stimulating agent (ESA) pricing • VOICE outcomes study top-line read out expected in Q1 2027 with potential to demonstrate favorable outcomes in the composite of all-cause mortality and hospitalization in patients treated with vadadustat compared to ESA 2027 and beyond Poised to Capture Significant Market Share
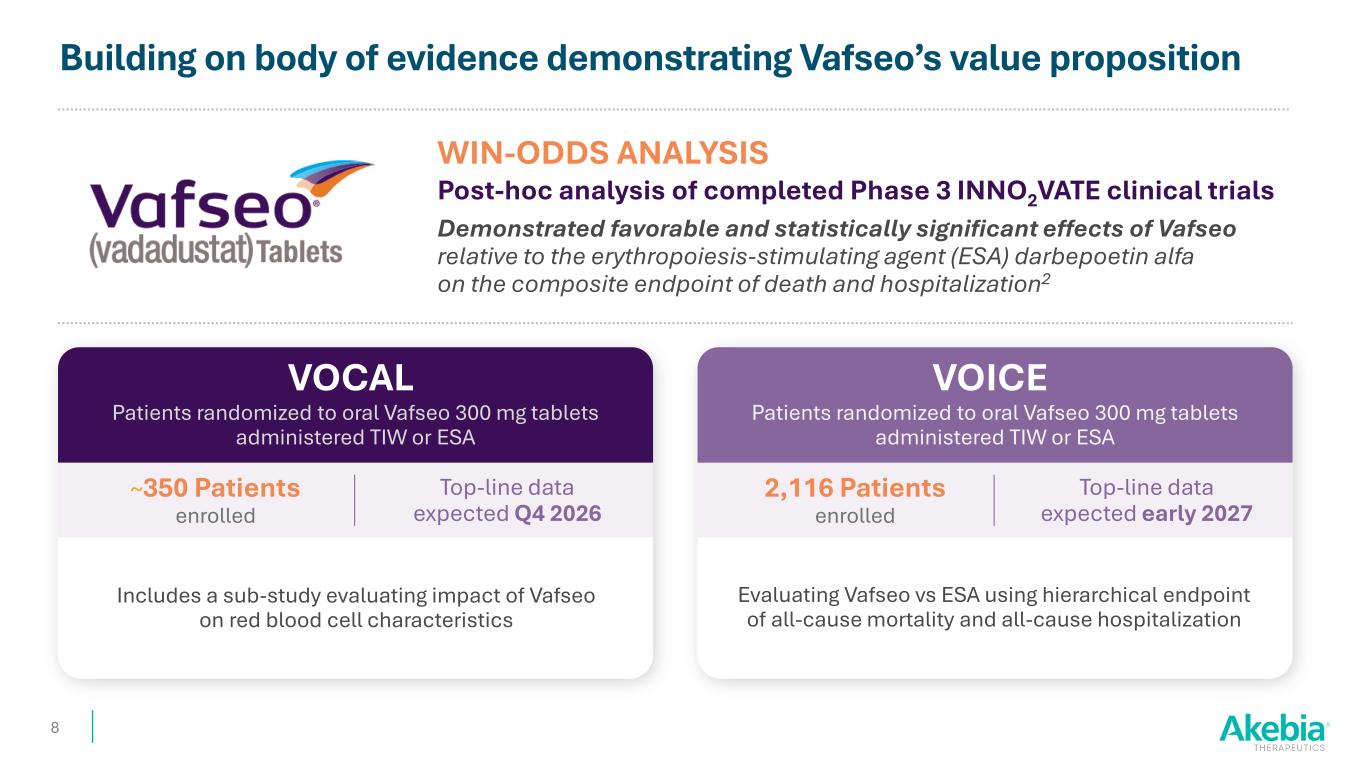
Building on body of evidence demonstrating Vafseo’s value proposition VOCAL 8 Patients randomized to oral Vafseo 300 mg tablets administered TIW or ESA WIN-ODDS ANALYSIS Post-hoc analysis of completed Phase 3 INNO2VATE clinical trials Demonstrated favorable and statistically significant effects of Vafseo relative to the erythropoiesis-stimulating agent (ESA) darbepoetin alfa on the composite endpoint of death and hospitalization2 Includes a sub-study evaluating impact of Vafseo on red blood cell characteristics ~350 Patients enrolled Top-line data expected Q4 2026 VOICE Patients randomized to oral Vafseo 300 mg tablets administered TIW or ESA Evaluating Vafseo vs ESA using hierarchical endpoint of all-cause mortality and all-cause hospitalization 2,116 Patients enrolled Top-line data expected early 2027
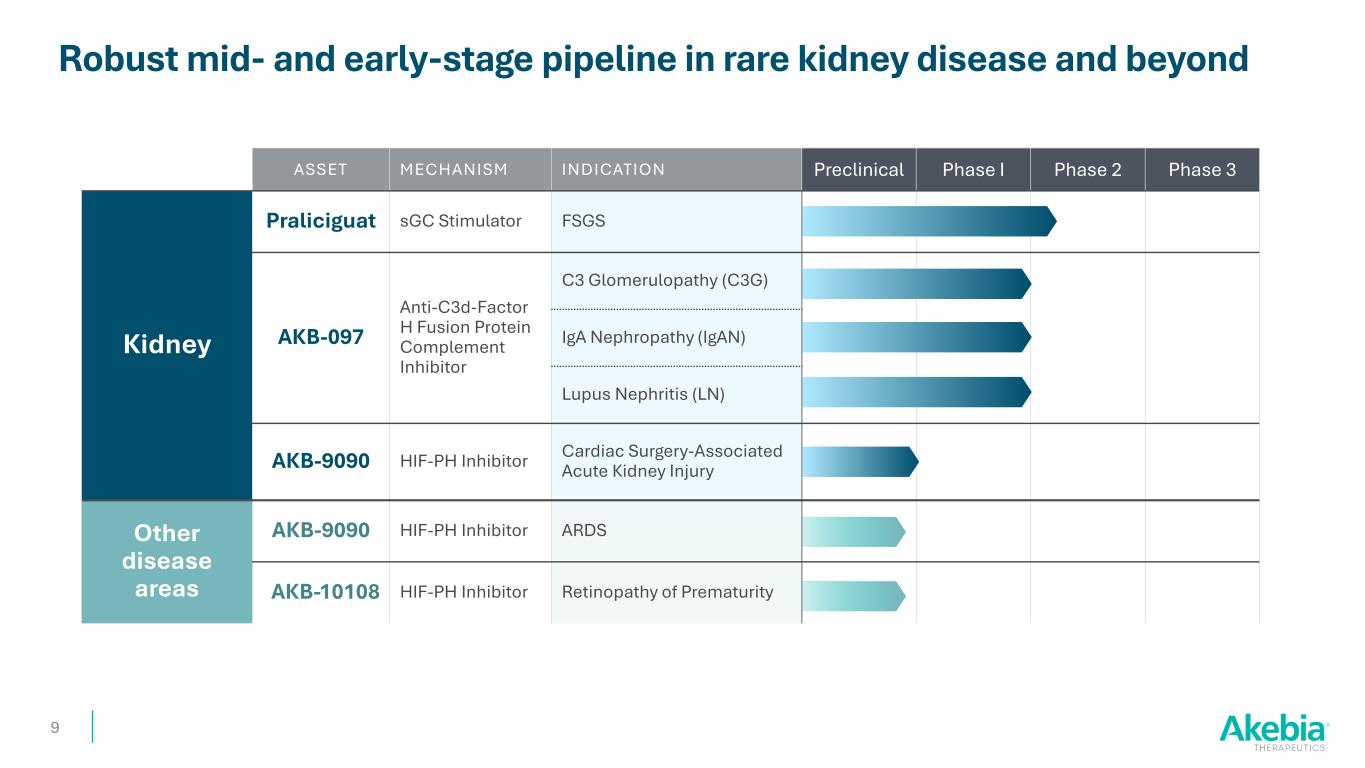
ASSET MECHANISM INDICATION Preclinical Phase I Phase 2 Phase 3 Kidney Praliciguat sGC Stimulator FSGS AKB-097 Anti-C3d-Factor H Fusion Protein Complement Inhibitor C3 Glomerulopathy (C3G) IgA Nephropathy (IgAN) Lupus Nephritis (LN) AKB-9090 HIF-PH Inhibitor Cardiac Surgery-Associated Acute Kidney Injury Other disease areas AKB-9090 HIF-PH Inhibitor ARDS AKB-10108 HIF-PH Inhibitor Retinopathy of Prematurity Robust mid- and early-stage pipeline in rare kidney disease and beyond 9
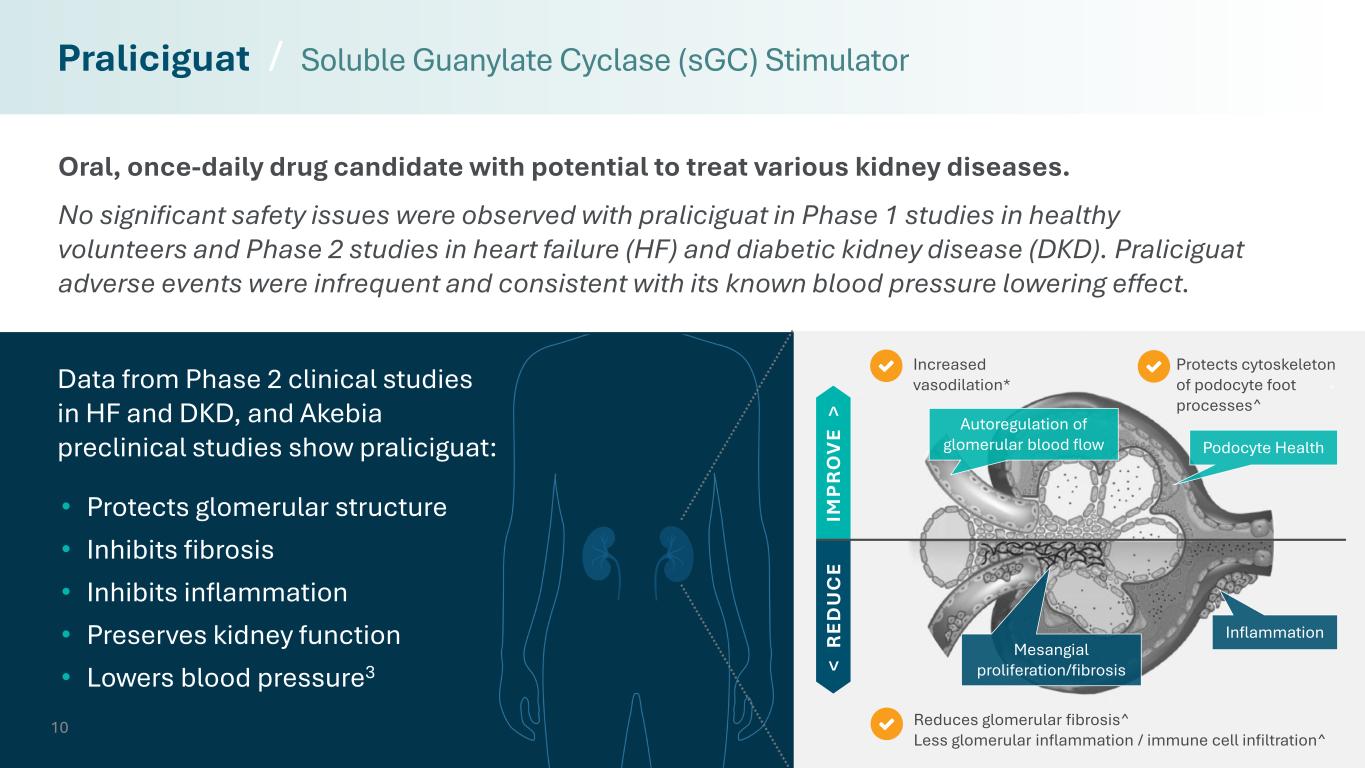
10 Reduces glomerular fibrosis^ Less glomerular inflammation / immune cell infiltration^ Protects cytoskeleton of podocyte foot processes^ Increased vasodilation* Oral, once-daily drug candidate with potential to treat various kidney diseases. No significant safety issues were observed with praliciguat in Phase 1 studies in healthy volunteers and Phase 2 studies in heart failure (HF) and diabetic kidney disease (DKD). Praliciguat adverse events were infrequent and consistent with its known blood pressure lowering effect. Praliciguat / Soluble Guanylate Cyclase (sGC) Stimulator < R ED U C E IM P R O V E > Inflammation Mesangial proliferation/fibrosis • Protects glomerular structure • Inhibits fibrosis • Inhibits inflammation • Preserves kidney function • Lowers blood pressure3 Autoregulation of glomerular blood flow Podocyte Health Data from Phase 2 clinical studies in HF and DKD, and Akebia preclinical studies show praliciguat:
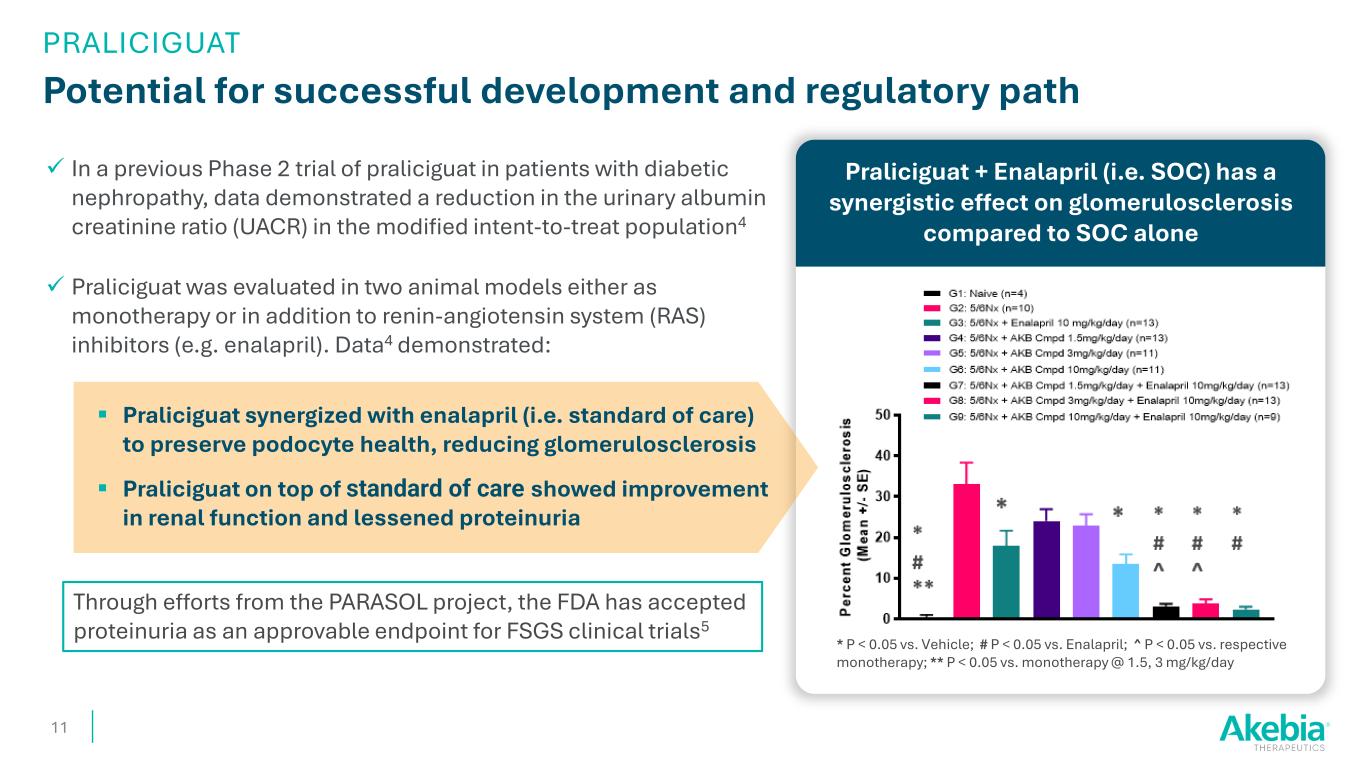
In a previous Phase 2 trial of praliciguat in patients with diabetic nephropathy, data demonstrated a reduction in the urinary albumin creatinine ratio (UACR) in the modified intent-to-treat population4 Praliciguat was evaluated in two animal models either as monotherapy or in addition to renin-angiotensin system (RAS) inhibitors (e.g. enalapril). Data4 demonstrated: Praliciguat synergized with enalapril (i.e. standard of care) to preserve podocyte health, reducing glomerulosclerosis Praliciguat on top of standard of care showed improvement in renal function and lessened proteinuria Praliciguat + Enalapril (i.e. SOC) has a synergistic effect on glomerulosclerosis compared to SOC alone Potential for successful development and regulatory path 11 * P < 0.05 vs. Vehicle; # P < 0.05 vs. Enalapril; ^ P < 0.05 vs. respective monotherapy; ** P < 0.05 vs. monotherapy @ 1.5, 3 mg/kg/day Through efforts from the PARASOL project, the FDA has accepted proteinuria as an approvable endpoint for FSGS clinical trials5 PRALICIGUAT
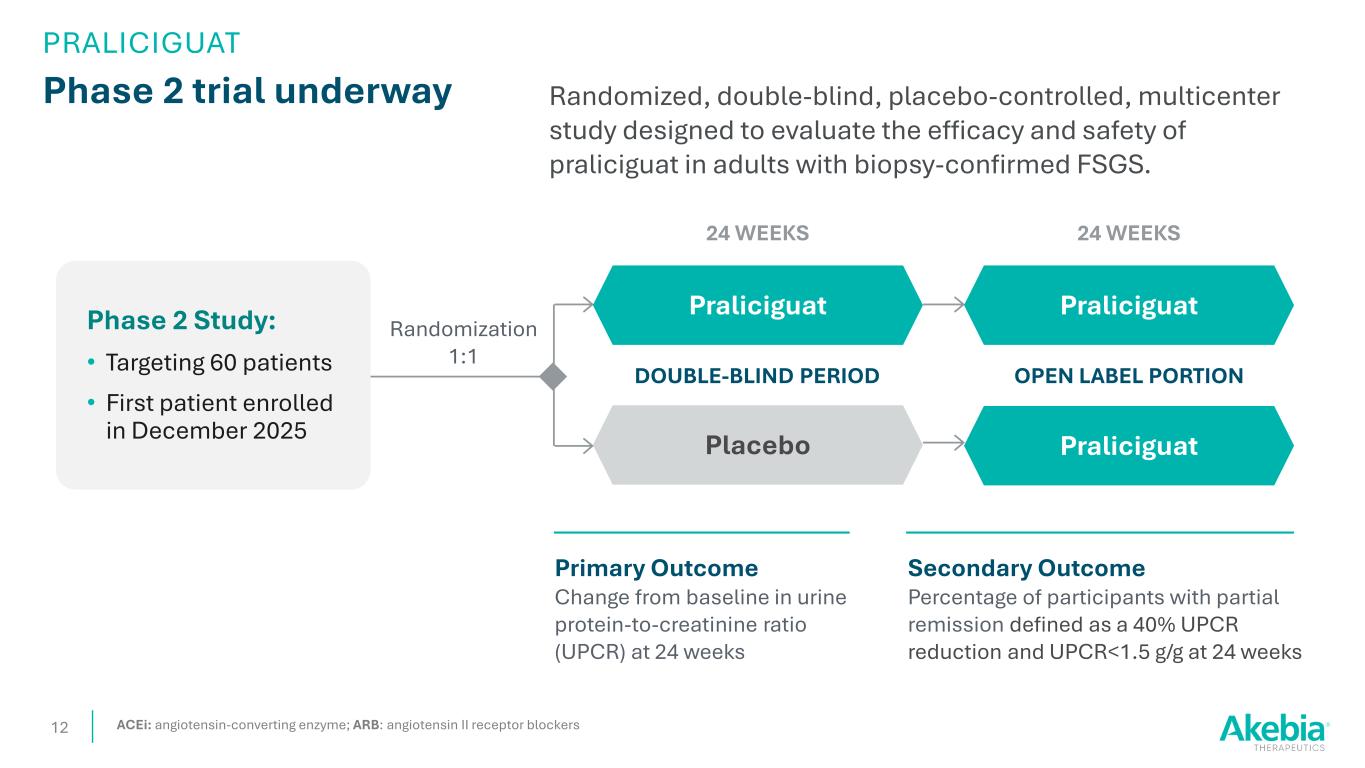
Phase 2 trial underway 12 Randomized, double-blind, placebo-controlled, multicenter study designed to evaluate the efficacy and safety of praliciguat in adults with biopsy-confirmed FSGS. Randomization 1:1 Placebo Praliciguat Secondary Outcome Percentage of participants with partial remission defined as a 40% UPCR reduction and UPCR<1.5 g/g at 24 weeks DOUBLE-BLIND PERIOD Primary Outcome Change from baseline in urine protein-to-creatinine ratio (UPCR) at 24 weeks 24 WEEKS OPEN LABEL PORTION Praliciguat Praliciguat 24 WEEKS ACEi: angiotensin-converting enzyme; ARB: angiotensin II receptor blockers PRALICIGUAT Phase 2 Study: • Targeting 60 patients • First patient enrolled in December 2025
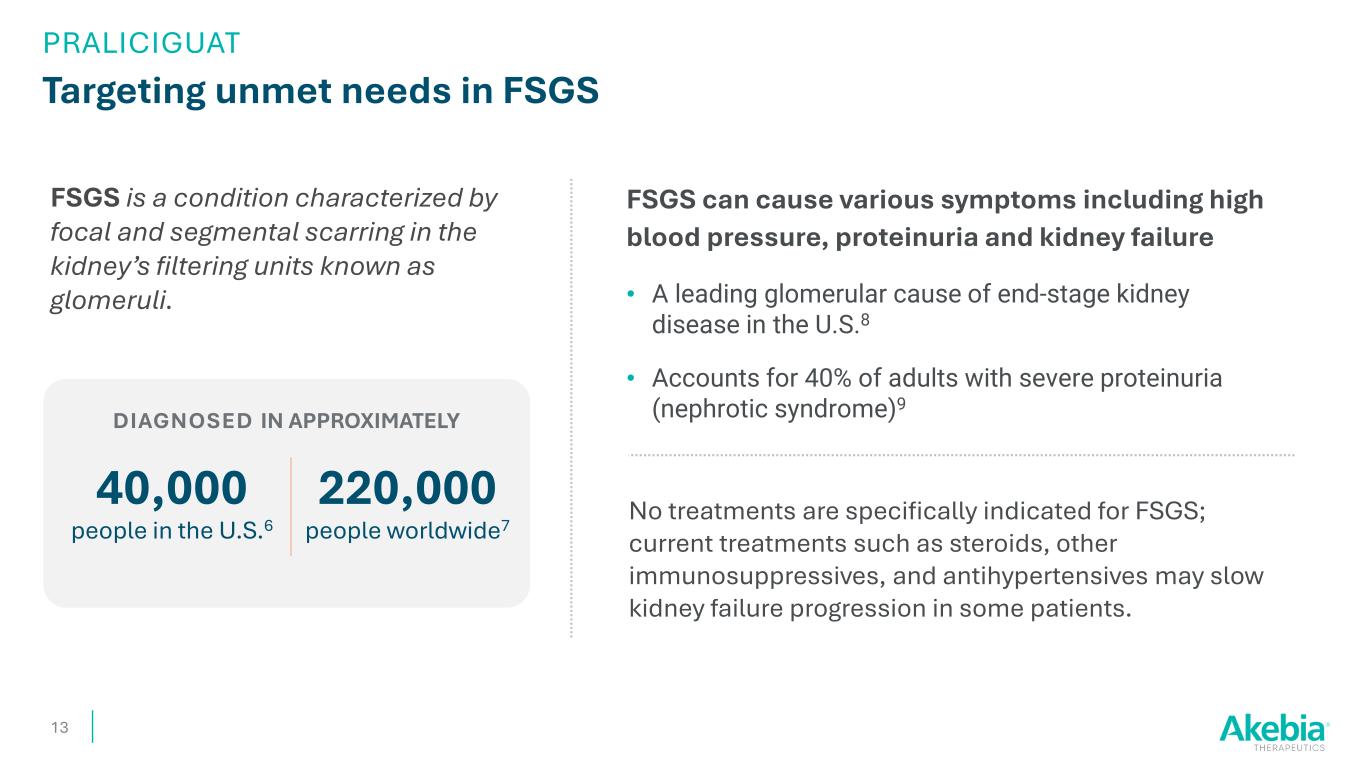
Targeting unmet needs in FSGS FSGS can cause various symptoms including high blood pressure, proteinuria and kidney failure • A leading glomerular cause of end-stage kidney disease in the U.S.8 • Accounts for 40% of adults with severe proteinuria (nephrotic syndrome)9 13 FSGS is a condition characterized by focal and segmental scarring in the kidney’s filtering units known as glomeruli. 40,000 people in the U.S.6 No treatments are specifically indicated for FSGS; current treatments such as steroids, other immunosuppressives, and antihypertensives may slow kidney failure progression in some patients. 220,000 people worldwide7 DIAGNOSED IN APPROXIMATELY PRALICIGUAT
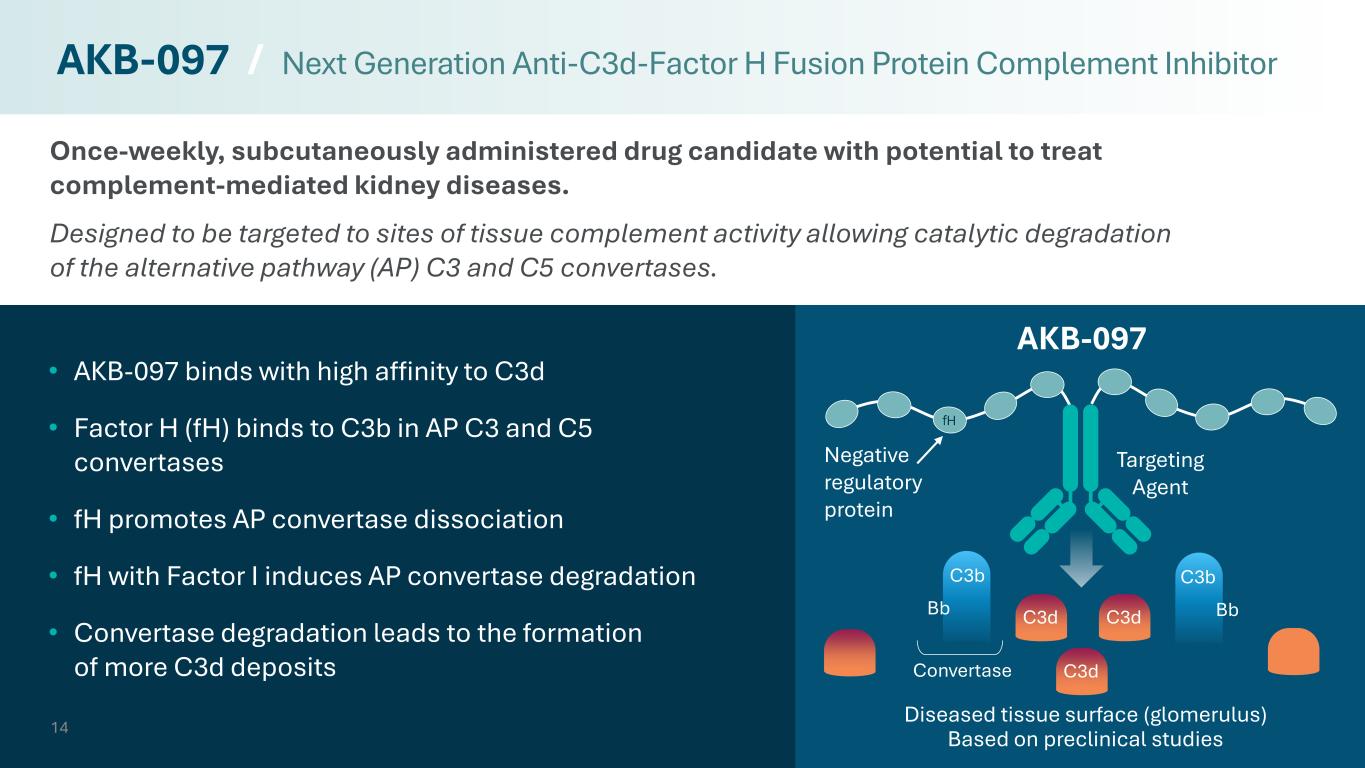
Once-weekly, subcutaneously administered drug candidate with potential to treat complement-mediated kidney diseases. Designed to be targeted to sites of tissue complement activity allowing catalytic degradation of the alternative pathway (AP) C3 and C5 convertases. 14 • AKB-097 binds with high affinity to C3d • Factor H (fH) binds to C3b in AP C3 and C5 convertases • fH promotes AP convertase dissociation • fH with Factor I induces AP convertase degradation • Convertase degradation leads to the formation of more C3d deposits AKB-097 / Next Generation Anti-C3d-Factor H Fusion Protein Complement Inhibitor C3b Bb C3b Bb Convertase C3d C3d C3d Targeting Agent Negative regulatory protein AKB-097 Diseased tissue surface (glomerulus) Based on preclinical studies fH
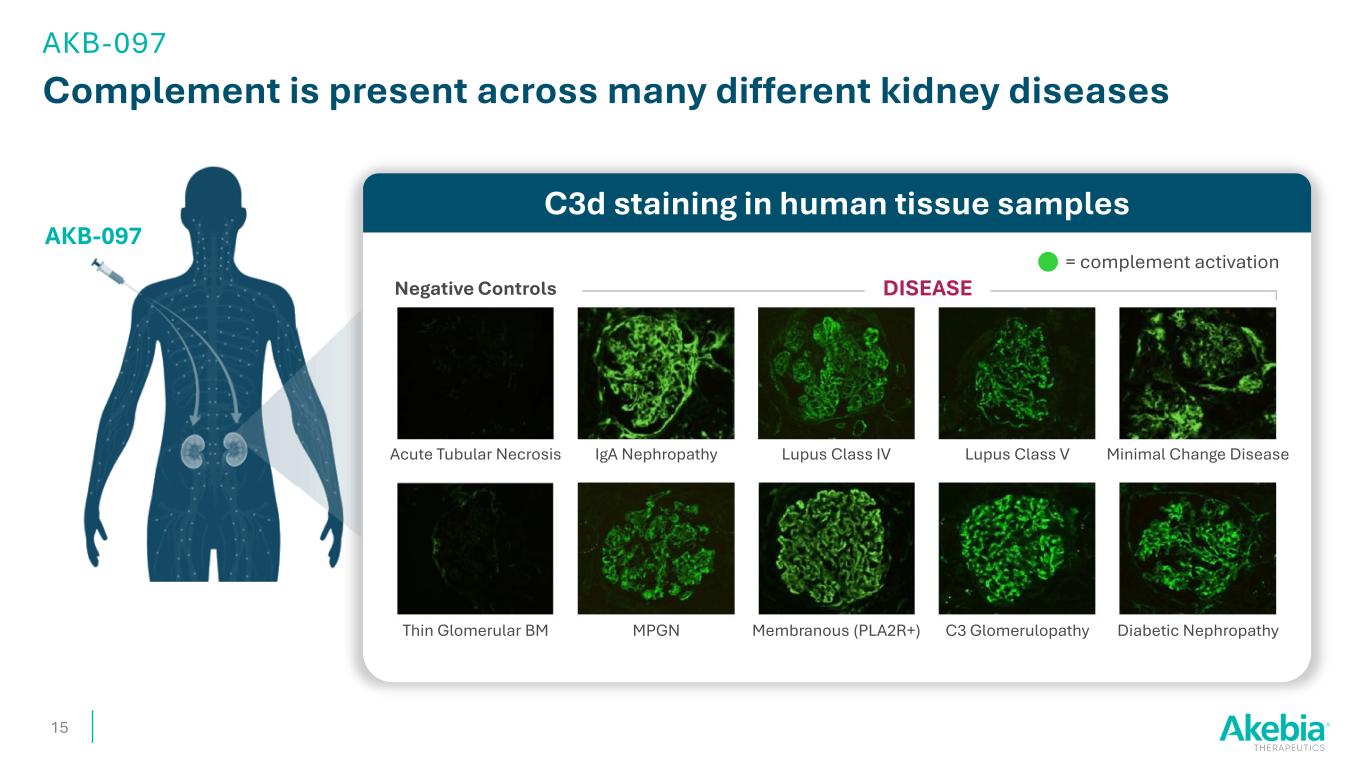
Complement is present across many different kidney diseases 15 AKB-097 C3d staining in human tissue samples DISEASENegative Controls Acute Tubular Necrosis Thin Glomerular BM MPGN Membranous (PLA2R+) C3 Glomerulopathy Diabetic Nephropathy Minimal Change DiseaseLupus Class VLupus Class IVIgA Nephropathy = complement activation AKB-097
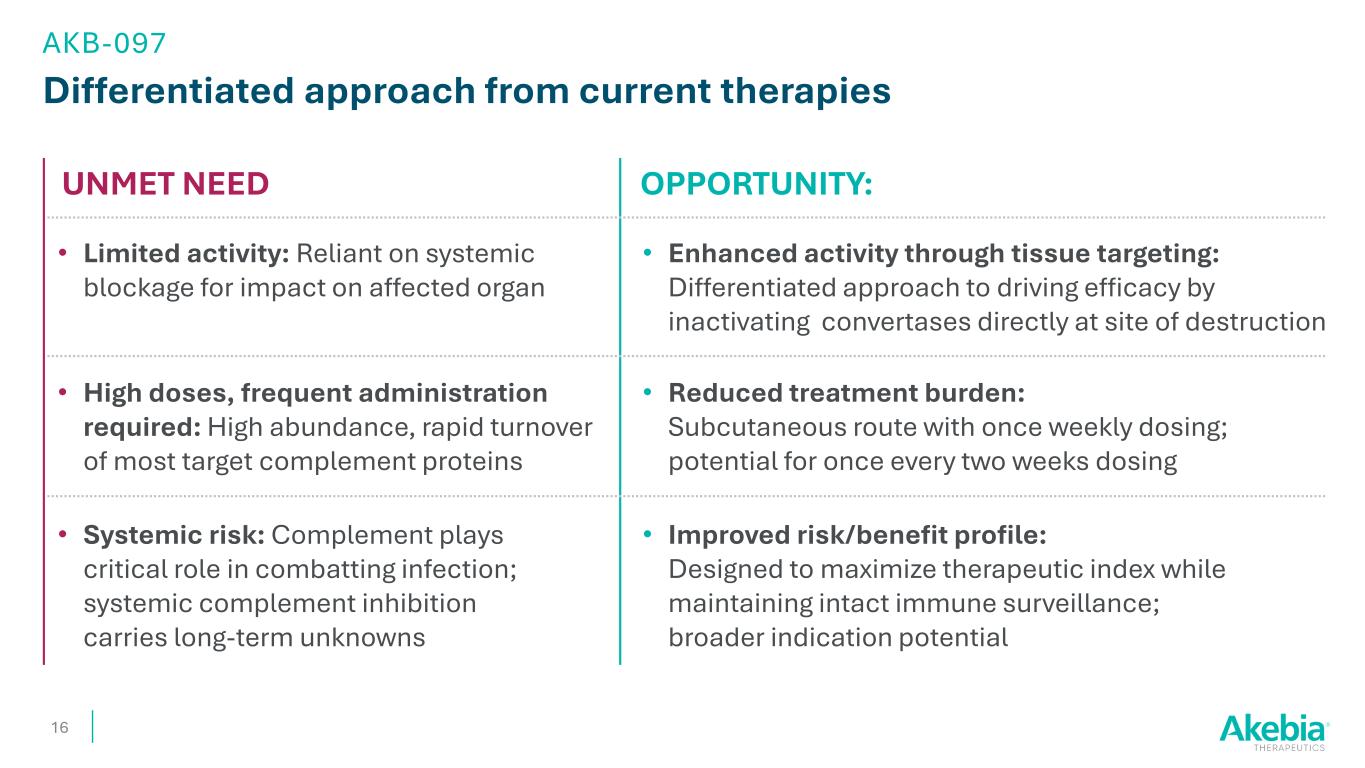
Differentiated approach from current therapies OPPORTUNITY: 16 • Enhanced activity through tissue targeting: Differentiated approach to driving efficacy by inactivating convertases directly at site of destruction • Limited activity: Reliant on systemic blockage for impact on affected organ UNMET NEED • Improved risk/benefit profile: Designed to maximize therapeutic index while maintaining intact immune surveillance; broader indication potential • Systemic risk: Complement plays critical role in combatting infection; systemic complement inhibition carries long-term unknowns • Reduced treatment burden: Subcutaneous route with once weekly dosing; potential for once every two weeks dosing • High doses, frequent administration required: High abundance, rapid turnover of most target complement proteins AKB-097
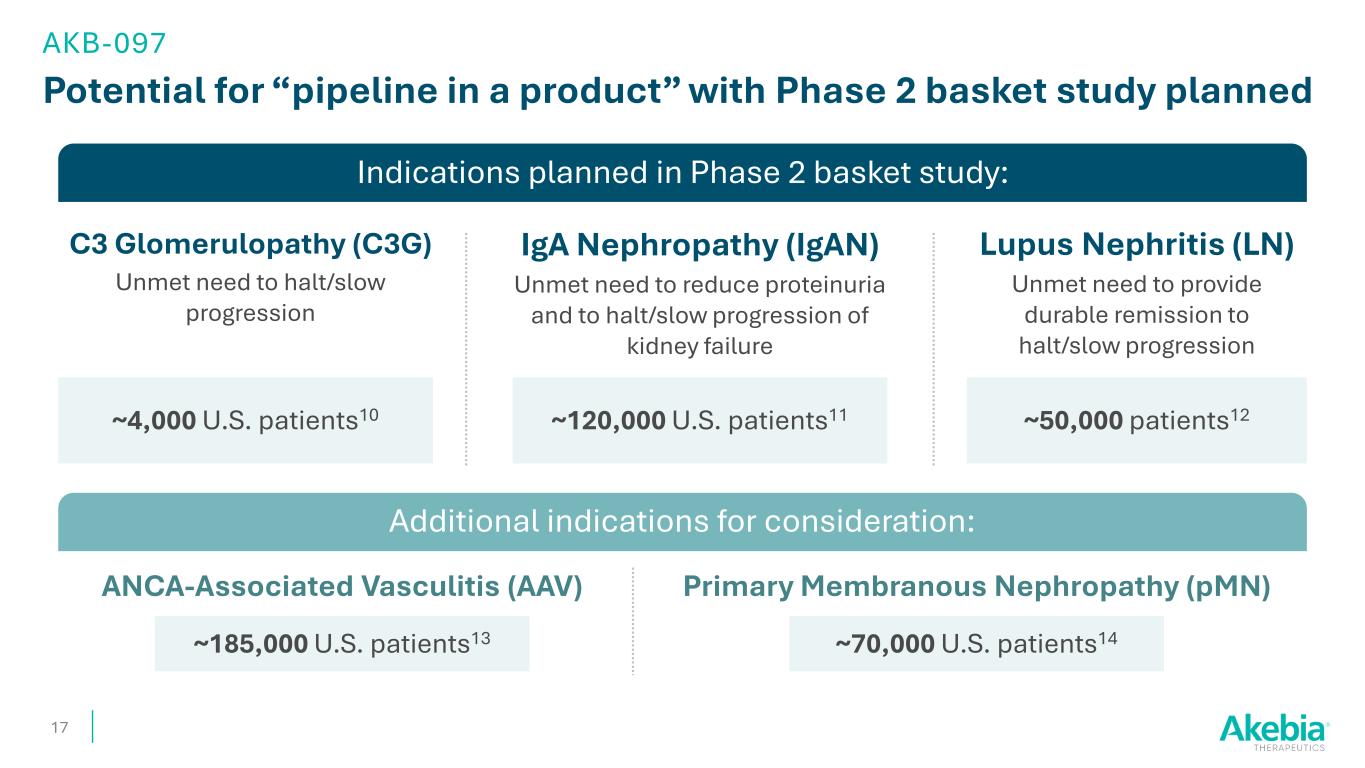
Indications planned in Phase 2 basket study: Potential for “pipeline in a product” with Phase 2 basket study planned 17 AKB-097 C3 Glomerulopathy (C3G) Unmet need to halt/slow progression IgA Nephropathy (IgAN) Unmet need to reduce proteinuria and to halt/slow progression of kidney failure Lupus Nephritis (LN) Unmet need to provide durable remission to halt/slow progression Additional indications for consideration: ~4,000 U.S. patients10 ~50,000 patients12 ANCA-Associated Vasculitis (AAV) Primary Membranous Nephropathy (pMN) ~185,000 U.S. patients13 ~70,000 U.S. patients14 ~120,000 U.S. patients11
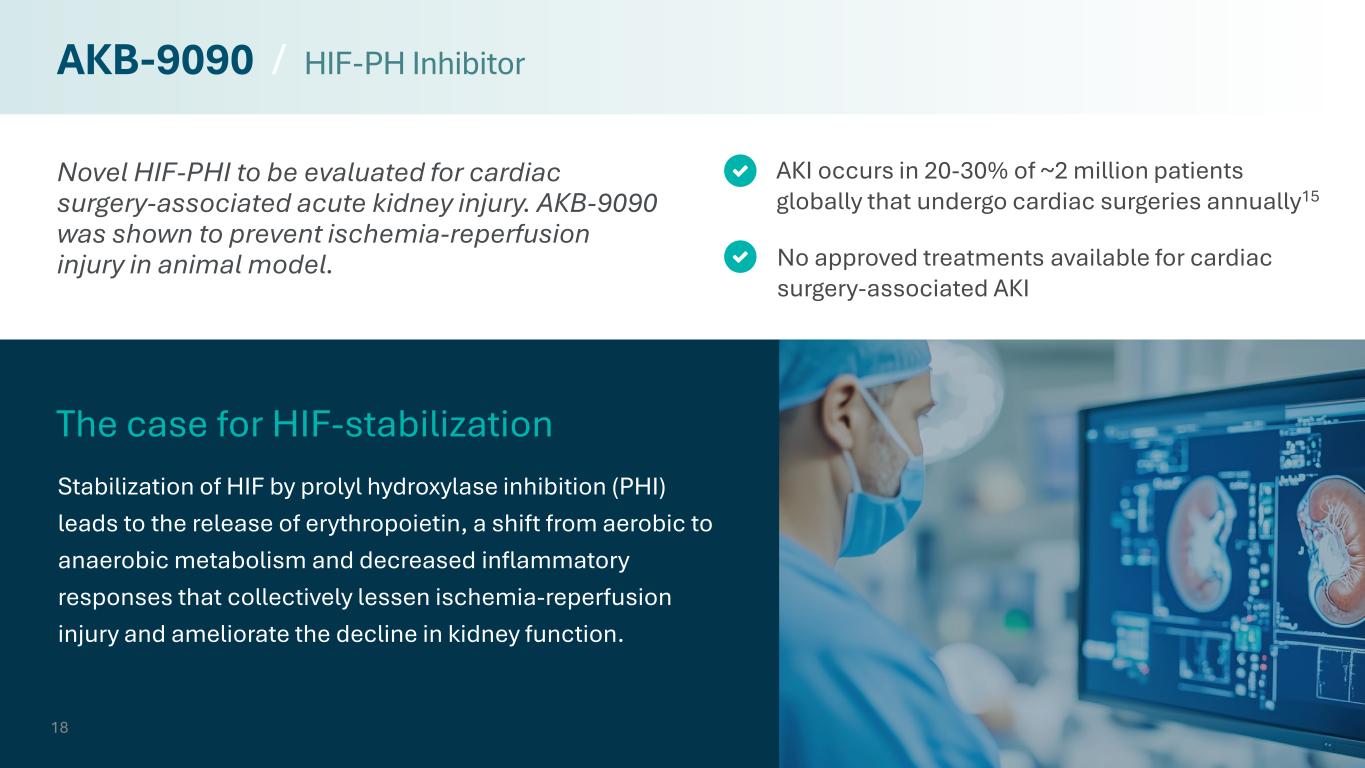
Novel HIF-PHI to be evaluated for cardiac surgery-associated acute kidney injury. AKB-9090 was shown to prevent ischemia-reperfusion injury in animal model. Stabilization of HIF by prolyl hydroxylase inhibition (PHI) leads to the release of erythropoietin, a shift from aerobic to anaerobic metabolism and decreased inflammatory responses that collectively lessen ischemia-reperfusion injury and ameliorate the decline in kidney function. No approved treatments available for cardiac surgery-associated AKI AKI occurs in 20-30% of ~2 million patients globally that undergo cardiac surgeries annually15 The case for HIF-stabilization 18 AKB-9090 / HIF-PH Inhibitor
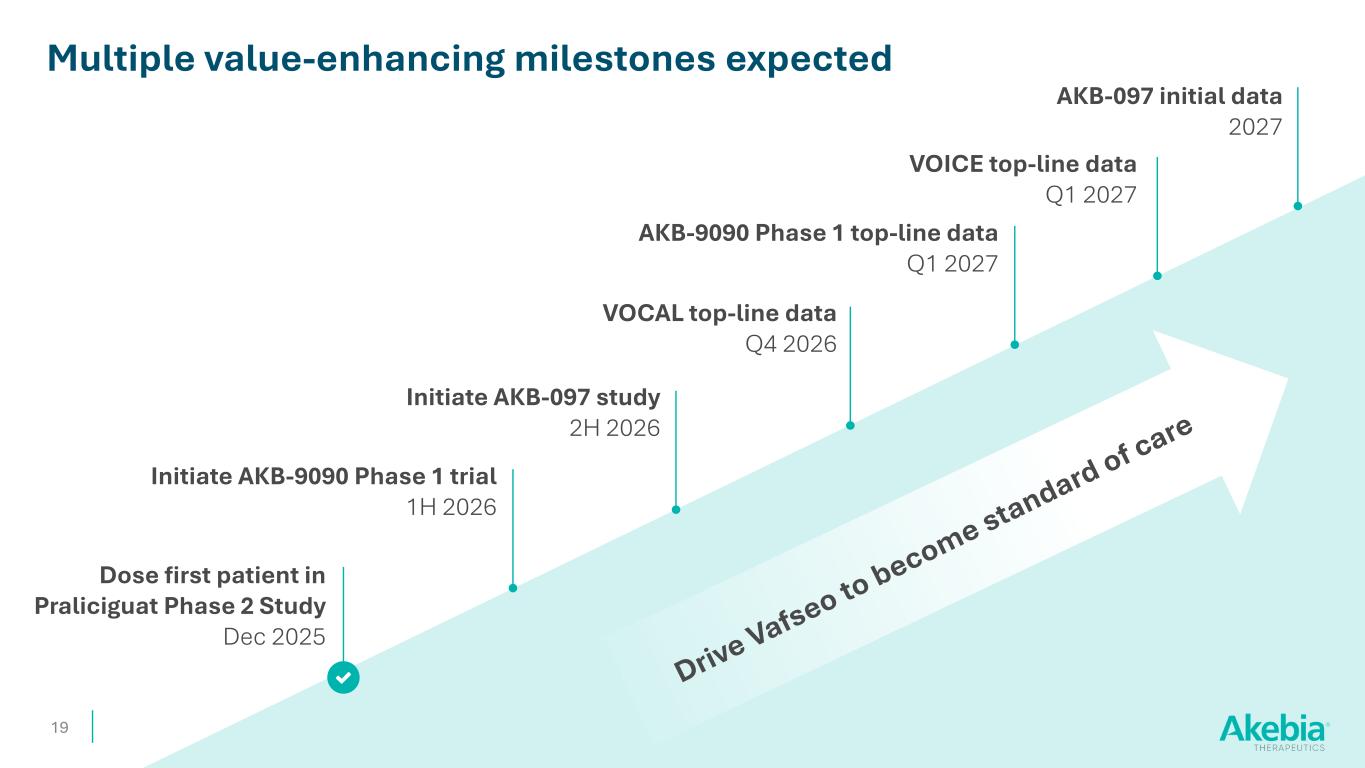
19 Dose first patient in Praliciguat Phase 2 Study Dec 2025 Multiple value-enhancing milestones expected Initiate AKB-9090 Phase 1 trial 1H 2026 VOCAL top-line data Q4 2026 Initiate AKB-097 study 2H 2026 AKB-097 initial data 2027 VOICE top-line data Q1 2027 AKB-9090 Phase 1 top-line data Q1 2027

Akebia: A Compelling Investment Opportunity in the Kidney Space 20 • Two FDA-approved, revenue-generating products; supported by an experienced commercial organization • Potential for Vafseo to be standard of care for treatment of anemia due to CKD in dialysis; $1 billion U.S. market opportunity1 • Advancing differentiated mid-stage pipeline in rare kidney disease • Strong balance sheet; $166 million in cash & cash equivalents as of 9/30/2025 • Multiple value-enhancing milestones expected in 2026 & 2027

21 Appendix

WARNING: INCREASED RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, and THROMBOSIS OF VASCULAR ACCESS. See full prescribing information for complete boxed warning. VAFSEO increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE). Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial and venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels. No trial has identified a hemoglobin target level, dose of VAFSEO, or dosing strategy that does not increase these risks. Use the lowest dose of VAFSEO sufficient to reduce the need for red blood cell transfusions. IMPORTANT SAFETY INFORMATION about VAFSEO (vadadustat) tablets 22

CONTRAINDICATIONS • Known hypersensitivity to VAFSEO or any of its components • Uncontrolled hypertension WARNINGS AND PRECAUTIONS Increased Risk of Death, Myocardial Infarction, Stroke, Venous Thromboembolism, and Thrombosis of Vascular Access A rise in hemoglobin (Hb) levels greater than 1 g/dL over 2 weeks can increase these risks. Avoid use in patients with a history of myocardial infarction, cerebrovascular event, or acute coronary syndrome within the 3 months prior to starting VAFSEO. Targeting a Hb level of greater than 11g/dL is expected to further increase the risk of death and arterial and venous thrombotic events, as occurs with ESAs, which also increase erythropoietin levels. No specific Hb target level, dose of VAFSEO, or dosing strategy has been identified to avoid these risks. Use the lowest effective dose and adhere to dosing and Hb monitoring recommendations to avoid excessive erythropoiesis. Advise patients to seek immediate medical attention if they develop signs or symptoms of myocardial infarction, stroke, venous thromboembolism, or thrombosis of vascular access. Evaluate and manage promptly if these occur. Hepatotoxicity Hepatocellular injury attributed to VAFSEO was reported in less than 1% of patients, including one severe case with jaundice. All events were asymptomatic and resolved after discontinuation of VAFSEO. The time to onset was generally within the first 3 months of treatment. Elevated serum ALT, AST, and bilirubin levels were observed in 1.8%, 1.8%, and 0.3% of CKD patients treated with VAFSEO, respectively. Measure ALT, AST, and bilirubin before treatment and monthly for the first 6 months, then as clinically indicated. Discontinue VAFSEO if ALT or AST is persistently elevated or accompanied by elevated bilirubin. Not recommended in patients with cirrhosis or active, acute liver disease. Hypertension Worsening of hypertension was reported in 14% (9.4 per 100 person-years [PY]) of patients receiving VAFSEO and 17% (11.8 per 100 PY) of patients receiving darbepoetin alfa. Serious worsening of hypertension was reported in 2.7% (1.7 per 100 PY) of patients receiving VAFSEO and 3% (1.8 per 100 PY) of patients receiving darbepoetin alfa. Cases of hypertensive crisis including hypertensive encephalopathy and seizures have also been reported in patients receiving VAFSEO. Monitor blood pressure. Adjust anti-hypertensive therapy as needed. Seizures Seizures occurred in 1.6% (1.0 per 100 PY) of patients who received VAFSEO and 1.6% (1.0 per 100 PY) of patients who received darbepoetin alfa. Following initiation of VAFSEO, monitor patients closely for premonitory neurologic symptoms. Monitor for new-onset seizures, premonitory symptoms, or change in seizure frequency. IMPORTANT SAFETY INFORMATION about VAFSEO (vadadustat) tablets (continued) 23

IMPORTANT SAFETY INFORMATION about VAFSEO (vadadustat) tablets (continued) 24 Gastrointestinal Erosion Gastric or esophageal erosions occurred in 6.4% (4.0 per 100 PY) of patients receiving VAFSEO and 5.3% (3.3 per 100 PY) of darbepoetin alfa-treated patients. Serious gastrointestinal (GI) erosions, including GI bleeding and the need for red blood cell transfusions were reported in 3.4% (2.1 per 100 PY) and 3.3% (2.0 per 100 PY) of those receiving VAFSEO and darbepoetin alfa, respectively. Consider the risk of GI erosion in high-risk patients, including those with a history of GI erosion, peptic ulcer disease, and tobacco or alcohol use. Advise patients of the signs and symptoms of erosions and GI bleeding and urge them to seek prompt medical care if present. Serious Adverse Reactions in Patients with Anemia Due to Chronic Kidney Disease and Not on Dialysis The safety of VAFSEO has not been established for the treatment of anemia due to CKD in adults not on dialysis and its use is not recommended in this setting. In large clinical trials in adults with anemia of CKD who were not on dialysis, an increased risk of mortality, stroke, myocardial infarction, serious acute kidney injury, serious hepatic injury, and serious GI erosions was observed in patients treated with VAFSEO compared to darbepoetin alfa. Malignancy VAFSEO has not been studied and is not recommended in patients with active malignancies. Malignancies were observed in 2.2% (1.3 per 100 PY) of patients treated with VAFSEO and 3.0% (1.8 per 100 PY) of patients treated with darbepoetin alfa. No evidence of increased carcinogenicity was observed in animal studies. ADVERSE REACTIONS The most common adverse reactions (occurring at ≥ 10%) were hypertension and diarrhea. DRUG INTERACTIONS Iron supplements and iron-containing phosphate binders: Administer VAFSEO at least 1 hour before products containing iron. Non-iron-containing phosphate binders: Administer VAFSEO at least 1 hour before or 2 hours after non-iron-containing phosphate binders. BCRP substrates: Monitor for signs of substrate adverse reactions and consider dose reduction. Statins: Monitor for statin-related adverse reactions. Limit the daily dose of simvastatin (20 mg) and rosuvastatin (5 mg). USE IN SPECIFIC POPULATIONS Pregnancy: May cause fetal harm. Lactation: Breastfeeding not recommended until two days after the final dose. Hepatic Impairment: Not recommended for use in patients with cirrhosis or active, acute liver disease. Please note that this information is not comprehensive. Please click here for the Full Prescribing Information, including BOXED WARNING and Medication Guide.

SOURCES 25 1 USRDS (https://usrds-adr.niddk.nih.gov/2022/end-stage-renal-disease/1-incidence-prevalence-patient-characteristics-and-treatment-modalities); DOPPS (https://www.dopps.org/DPM/DPMSlideBrowser.aspx); Based on internal estimates and industry reports estimating ESA pricing 2 https://www.asn-online.org/education/kidneyweek/2025/program-abstract.aspx?controlId=4352988 3 Phase 2 studies and Akebia preclinical studies (data on file with Akebia) 4 Preclinical studies and models (data on file with Akebia) 5 Nephcure/ The PARASOL Project: https://nephcure.org/the-parasol-project/ 6 Nephcure Kidney International: https://nephcure.org/wp-content/uploads/2021/02/nc.factSheet.FSGS_210106.pdf 7 Wedbush, Industry Note: “The IgAN Era Continues, Will FSGS Frenzy Follow?,” May 7, 2025 8 LifeSci Capital Alpha Series, Eledon Pharmaceuticals, Initiating Coverage, March 18, 2021 9 National Organization for Rare Diseases, Focal Segmental Glomerulosclerosis, November 21, 2018: https://rarediseases.org/rare-diseases/focal-segmental-glomerulosclerosis/ 10 C3 glomerulopathy - understanding a rare complement-driven renal disease. Nat Rev Nephrol. 2019 Mar;15(3):129-143. doi: 10.1038/s41581-018-0107-2. PMID: 30692664; PMCID: PMC6876298. https://pmc.ncbi.nlm.nih.gov/articles/PMC6876298/ 11 Evercore ISI, Travere Therapeutics, Inc. October 31, 2024 12 Jefferies Equity Research, Novartis AG, October 26, 2025 13 Systematic Review and Metaanalysis of Worldwide Incidence and Prevalence of Antineutrophil Cytoplasmic Antibody (ANCA) Associated Vasculitis. J Clin Med. 2022 May 4;11(9):2573. doi: 10.3390/jcm11092573. PMID: 35566698; PMCID: PMC9106044 14 Oppenheimer, Equity Research, Climb Bio, Inc. June 6, 2025 15 Cheruku SR, Raphael J, Neyra JA, Fox AA. Acute Kidney Injury after Cardiac Surgery: Prediction, Prevention, and Management. Anesthesiology. 2023 Dec 1;139(6):880-898. doi: 10.1097/ALN.0000000000004734. PMID: 37812758; PMCID: PMC10841304