
Vibrance-2 Phase 2 Study of Alixorexton in Patients with Narcolepsy Type 2: Positive Topline Results November 12, 2025 .2

Forward Looking Statements Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the potential therapeutic and commercial value of alixorexton (formerly referred to as ALKS 2680) and the company’s expectations related to the alixorexton development program. The company cautions that forward-looking statements are inherently uncertain. Although the company believes that such statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, the forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks, assumptions and uncertainties. These risks, assumptions and uncertainties include, among others: whether initial clinical results for alixorexton will be predictive of results of future stages of ongoing clinical studies, future clinical studies or real-world results; whether ongoing or future clinical studies for alixorexton will be initiated or completed on expected timelines or at all; whether alixorexton could be shown to be ineffective or unsafe; potential changes in the cost, scope and duration of the alixorexton development program; and those risks, assumptions and uncertainties described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K for the year ended Dec. 31, 2024 and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), which are available on the SEC’s website at www.sec.gov, and on the company’s website at www.alkermes.com in the ‘Investors – SEC filings’ section. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation.
Vibrance-2: Successful Phase 2 Study of Alixorexton in Patients With Narcolepsy Type 2 Topline results address the study’s key objectives: Efficacy Safety and Tolerability Phase 3 Readiness
Vibrance-2: Positive Outcome Supports Advancing to Phase 3 Alixorexton is the first orexin 2 receptor agonist to demonstrate an efficacy signal with statistically significant and clinically meaningful improvements in MWT and ESS in a large, multi-dose phase 2 study in narcolepsy type 2 (NT2) Alixorexton was generally well tolerated, with most TEAEs mild to moderate in severity and no serious TEAEs reported Safety and tolerability profile confirmed anticipated dose/response shift in NT2 patients Important new findings from Vibrance-2 inform phase 3 program MWT = Maintenance of Wakefulness Test; ESS = Epworth Sleepiness Scale; NT2 = narcolepsy type 2; TEAE: treatment-emergent adverse events
Narcolepsy Type 2: Heterogeneous Patient Population with Significant Unmet Need Chronic neurological sleep disorder characterized by excessive daytime sleepiness (EDS), without the presence of cataplexy Underlying disease pathology is less clear than NT1; NT2 patients have detectable and variable levels of orexin in CSF Variable disease severity and response to treatment Significant unmet need and limited number of available treatment options 1 Ruoff C, Rye D. The ICSD-3 and DSM-5 guidelines for diagnosing narcolepsy: clinical relevance and practicality. Curr Med Res Opin. 2016;32(10):1611-1622. doi:10.1080/03007995.2016.1208643 2 Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy – clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519-539. NT1 = narcolepsy type 1; NT2 = narcolepsy type 2; EDS = excessive daytime sleepiness; CSF = cerebral spinal fluid.
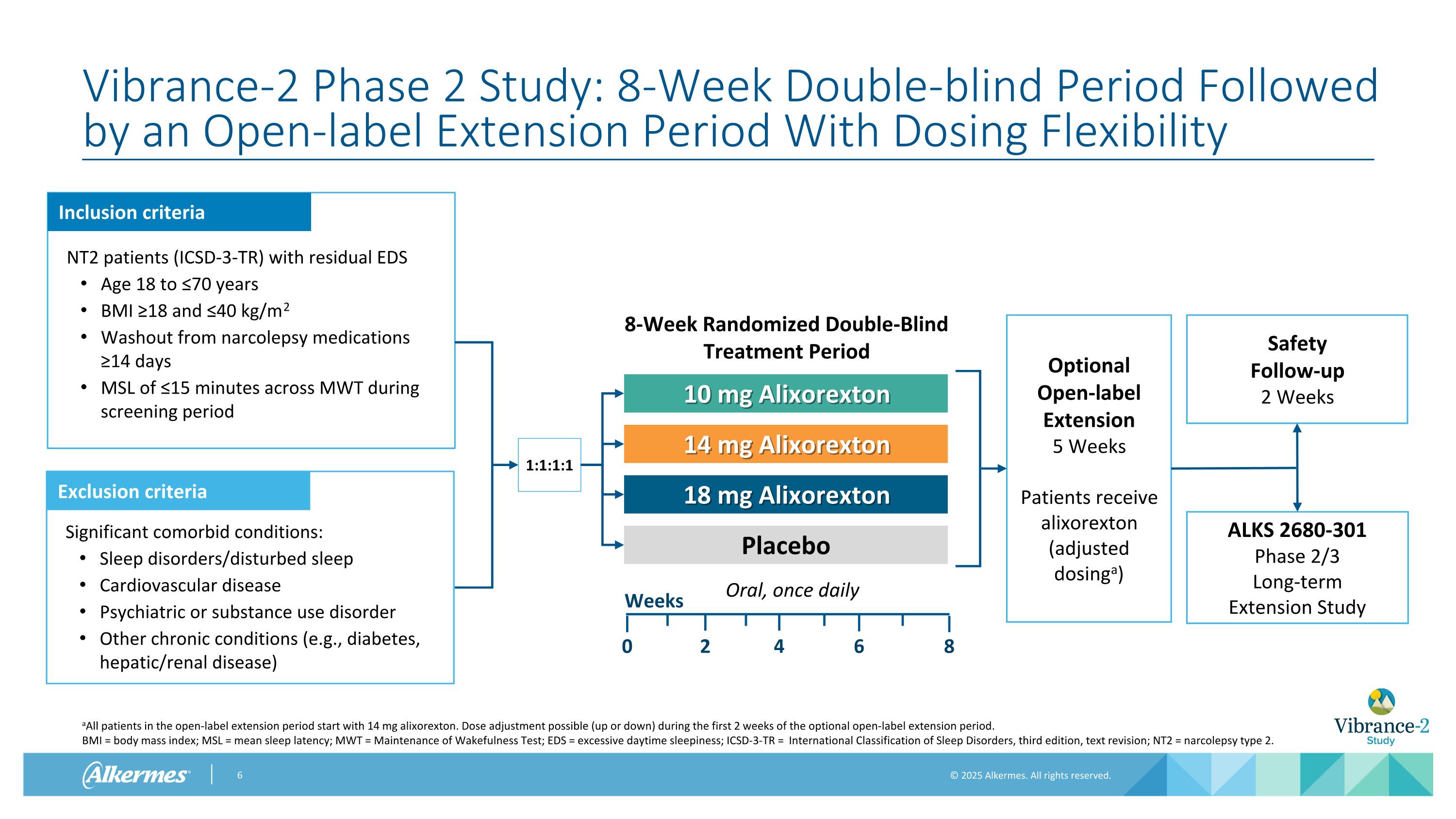
Vibrance-2 Phase 2 Study: 8-Week Double-blind Period Followed by an Open-label Extension Period With Dosing Flexibility aAll patients in the open-label extension period start with 14 mg alixorexton. Dose adjustment possible (up or down) during the first 2 weeks of the optional open-label extension period. BMI = body mass index; MSL = mean sleep latency; MWT = Maintenance of Wakefulness Test; EDS = excessive daytime sleepiness; ICSD-3-TR = International Classification of Sleep Disorders, third edition, text revision; NT2 = narcolepsy type 2. Optional Open-label Extension 5 Weeks Patients receive alixorexton (adjusted dosinga) Safety Follow-up 2 Weeks ALKS 2680-301 Phase 2/3 Long-term Extension Study Inclusion criteria Exclusion criteria NT2 patients (ICSD-3-TR) with residual EDS Age 18 to ≤70 years BMI ≥18 and ≤40 kg/m2 Washout from narcolepsy medications ≥14 days MSL of ≤15 minutes across MWT during screening period Significant comorbid conditions: Sleep disorders/disturbed sleep Cardiovascular disease Psychiatric or substance use disorder Other chronic conditions (e.g., diabetes, hepatic/renal disease) 1:1:1:1 Oral, once daily 10 mg Alixorexton 14 mg Alixorexton 18 mg Alixorexton Placebo Weeks 2 4 0 8-Week Randomized Double-Blind Treatment Period 8 6
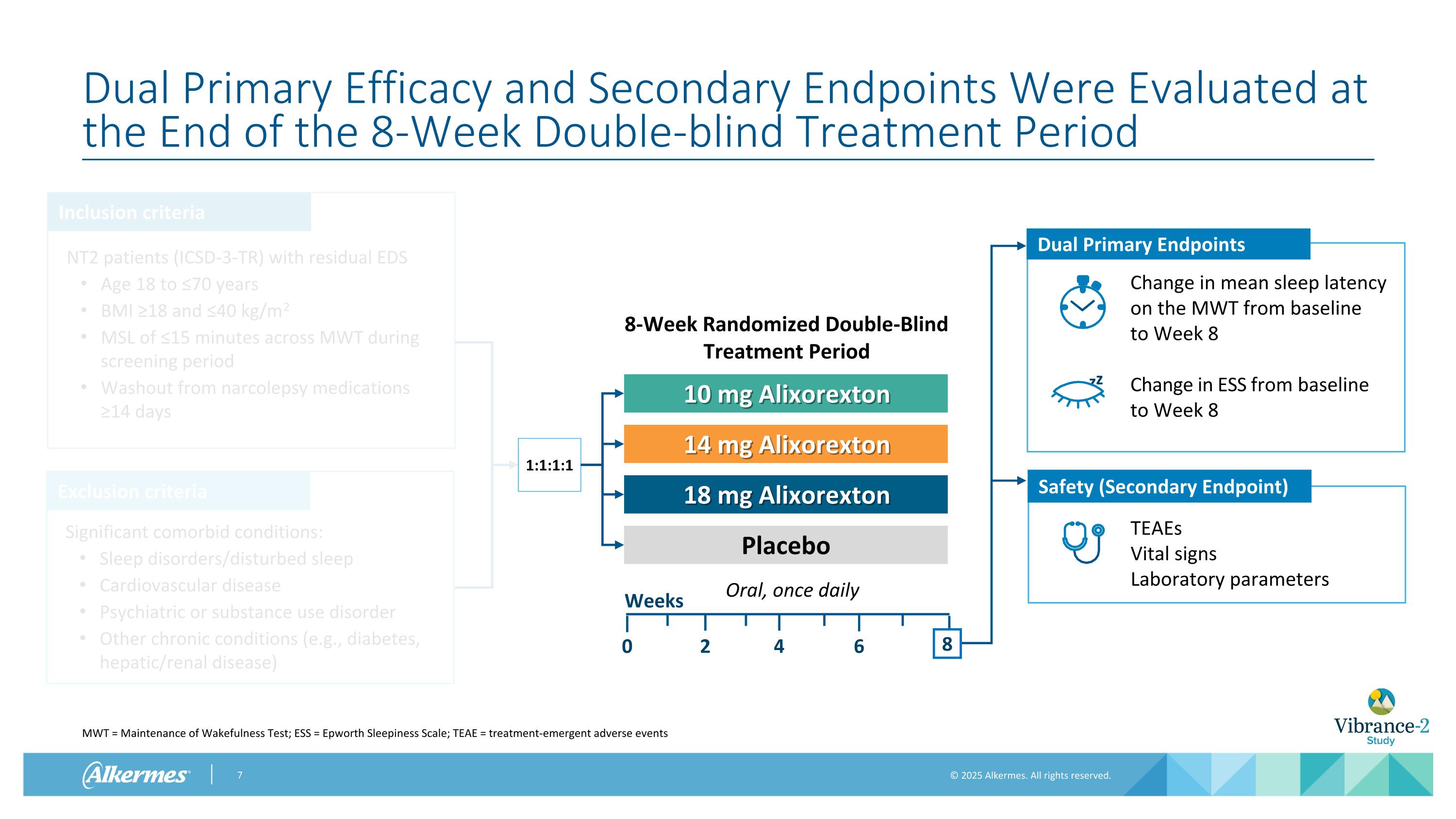
Dual Primary Efficacy and Secondary Endpoints Were Evaluated at the End of the 8-Week Double-blind Treatment Period MWT = Maintenance of Wakefulness Test; ESS = Epworth Sleepiness Scale; TEAE = treatment-emergent adverse events Inclusion criteria Exclusion criteria NT2 patients (ICSD-3-TR) with residual EDS Age 18 to ≤70 years BMI ≥18 and ≤40 kg/m2 MSL of ≤15 minutes across MWT during screening period Washout from narcolepsy medications ≥14 days Significant comorbid conditions: Sleep disorders/disturbed sleep Cardiovascular disease Psychiatric or substance use disorder Other chronic conditions (e.g., diabetes, hepatic/renal disease) 1:1:1:1 Oral, once daily 10 mg Alixorexton 14 mg Alixorexton 18 mg Alixorexton Placebo Weeks 2 4 0 8-Week Randomized Double-Blind Treatment Period 8 6 Change in ESS from baseline to Week 8 Dual Primary Endpoints Change in mean sleep latency on the MWT from baseline to Week 8 z z TEAEs Vital signs Laboratory parameters Safety (Secondary Endpoint) 8
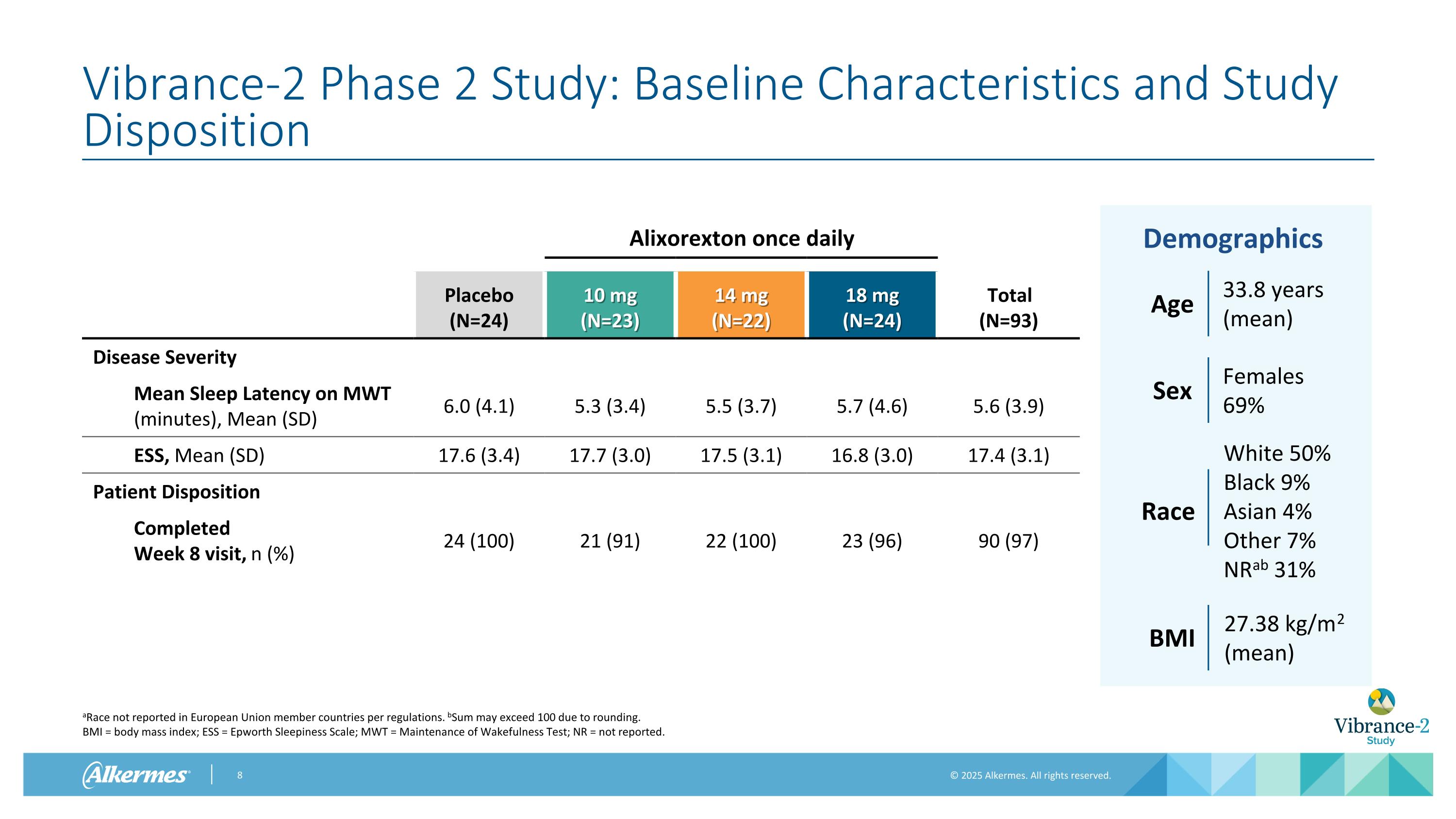
Vibrance-2 Phase 2 Study: Baseline Characteristics and Study Disposition Alixorexton once daily Placebo (N=24) 10 mg (N=23) 14 mg (N=22) 18 mg (N=24) Total (N=93) Disease Severity Mean Sleep Latency on MWT (minutes), Mean (SD) 6.0 (4.1) 5.3 (3.4) 5.5 (3.7) 5.7 (4.6) 5.6 (3.9) ESS, Mean (SD) 17.6 (3.4) 17.7 (3.0) 17.5 (3.1) 16.8 (3.0) 17.4 (3.1) Patient Disposition Completed Week 8 visit, n (%) 24 (100) 21 (91) 22 (100) 23 (96) 90 (97) 33.8 years (mean) Age Females 69% Sex Race White 50% Black 9% Asian 4% Other 7% NRab 31% 27.38 kg/m2 (mean) BMI Demographics aRace not reported in European Union member countries per regulations. bSum may exceed 100 due to rounding. BMI = body mass index; ESS = Epworth Sleepiness Scale; MWT = Maintenance of Wakefulness Test; NR = not reported.
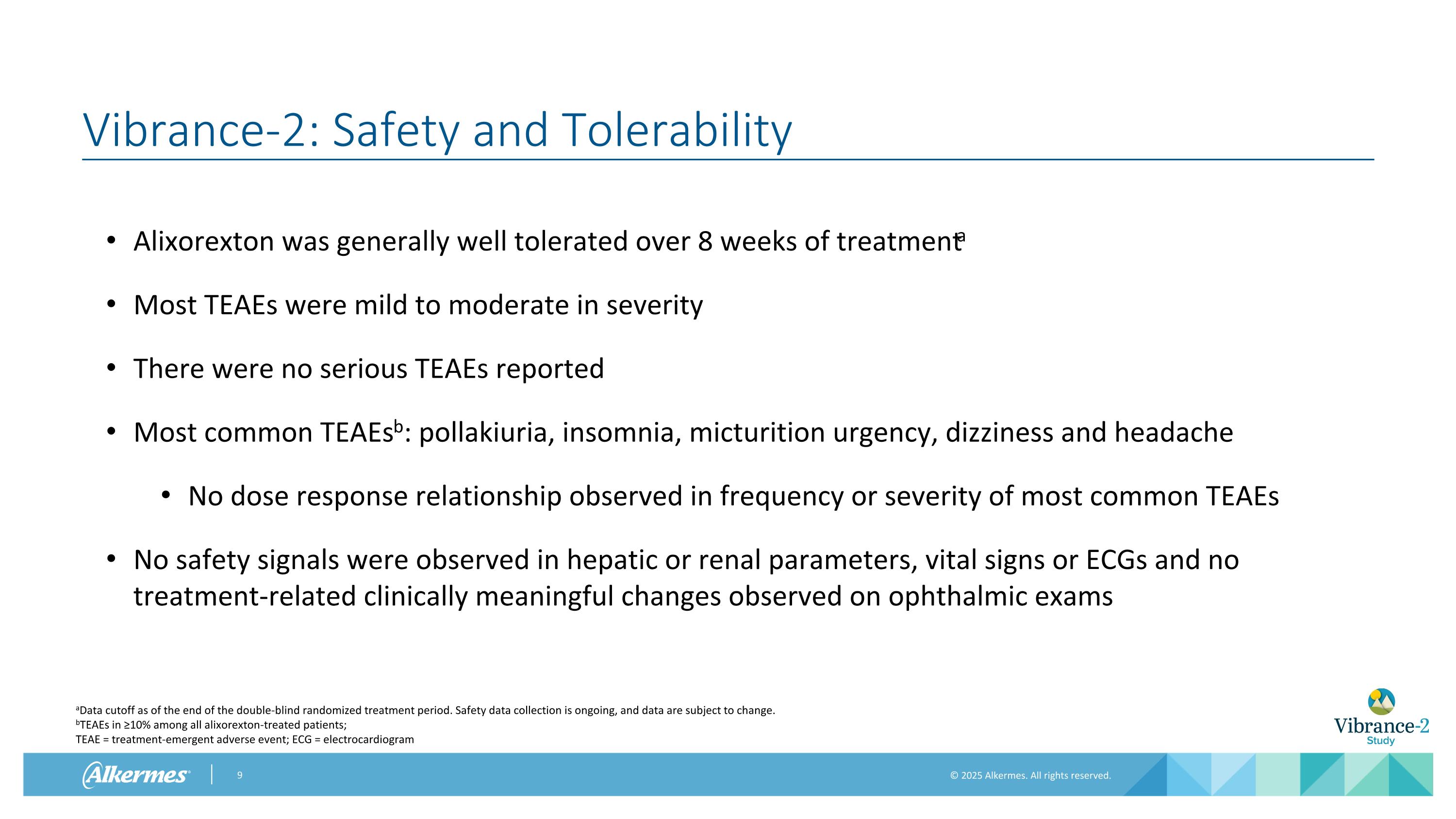
Vibrance-2: Safety and Tolerability Alixorexton was generally well tolerated over 8 weeks of treatmenta Most TEAEs were mild to moderate in severity There were no serious TEAEs reported Most common TEAEsb: pollakiuria, insomnia, micturition urgency, dizziness and headache No dose response relationship observed in frequency or severity of most common TEAEs No safety signals were observed in hepatic or renal parameters, vital signs or ECGs and no treatment-related clinically meaningful changes observed on ophthalmic exams aData cutoff as of the end of the double-blind randomized treatment period. Safety data collection is ongoing, and data are subject to change. bTEAEs in ≥10% among all alixorexton-treated patients; TEAE = treatment-emergent adverse event; ECG = electrocardiogram
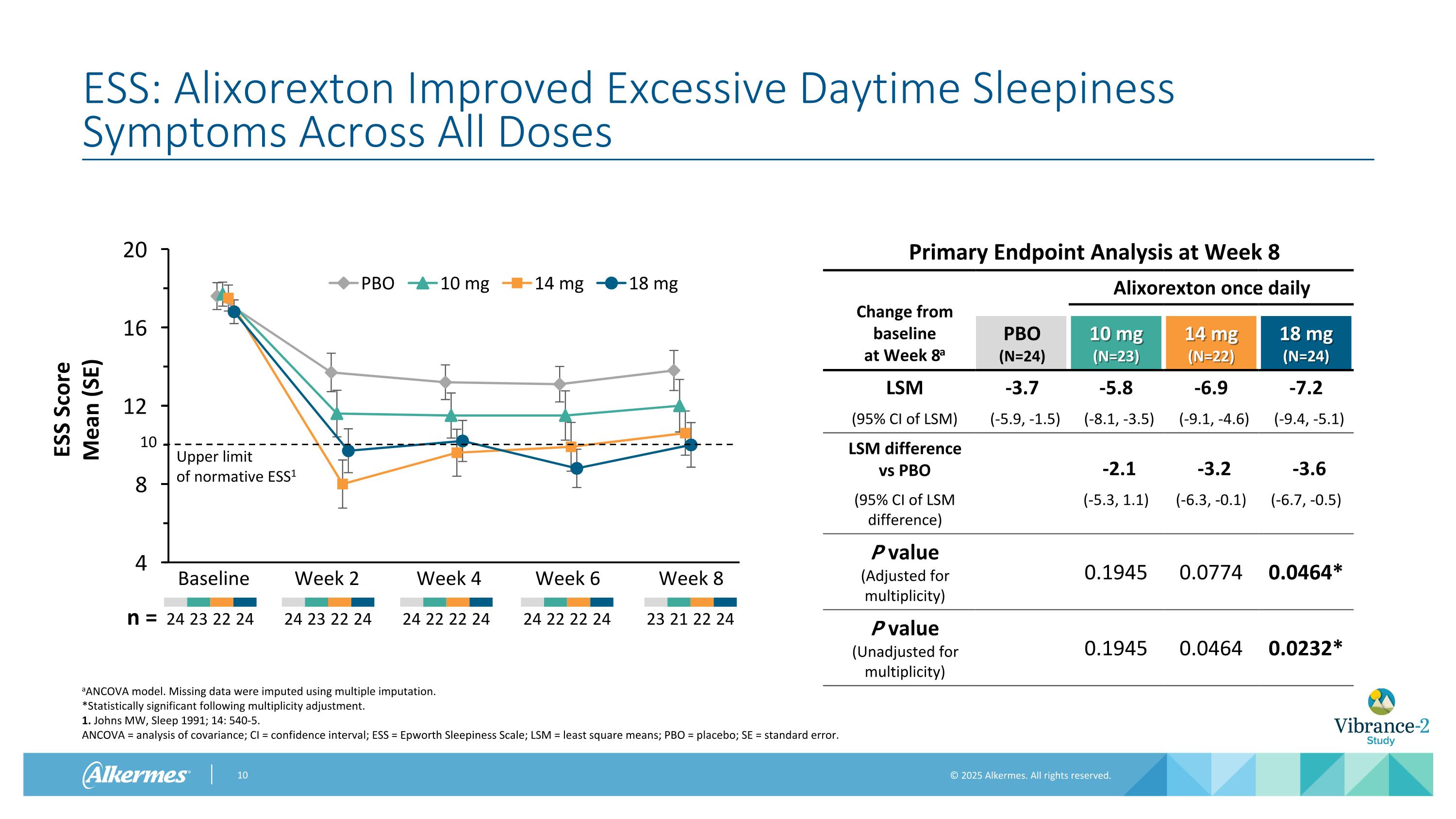
ESS: Alixorexton Improved Excessive Daytime Sleepiness Symptoms Across All Doses aANCOVA model. Missing data were imputed using multiple imputation. *Statistically significant following multiplicity adjustment. 1. Johns MW, Sleep 1991; 14: 540-5. ANCOVA = analysis of covariance; CI = confidence interval; ESS = Epworth Sleepiness Scale; LSM = least square means; PBO = placebo; SE = standard error. Baseline Week 2 Week 4 Week 6 24 23 22 24 24 22 22 24 24 23 22 24 n = 24 22 22 24 Upper limit of normative ESS1 10 Week 8 23 21 22 24 Primary Endpoint Analysis at Week 8 Change from baseline at Week 8a Alixorexton once daily PBO (N=24) 10 mg (N=23) 14 mg (N=22) 18 mg (N=24) LSM -3.7 -5.8 -6.9 -7.2 (95% CI of LSM) (-5.9, -1.5) (-8.1, -3.5) (-9.1, -4.6) (-9.4, -5.1) LSM difference vs PBO -2.1 -3.2 -3.6 (95% CI of LSM difference) (-5.3, 1.1) (-6.3, -0.1) (-6.7, -0.5) P value (Adjusted for multiplicity) 0.1945 0.0774 0.0464* P value (Unadjusted for multiplicity) 0.1945 0.0464 0.0232*
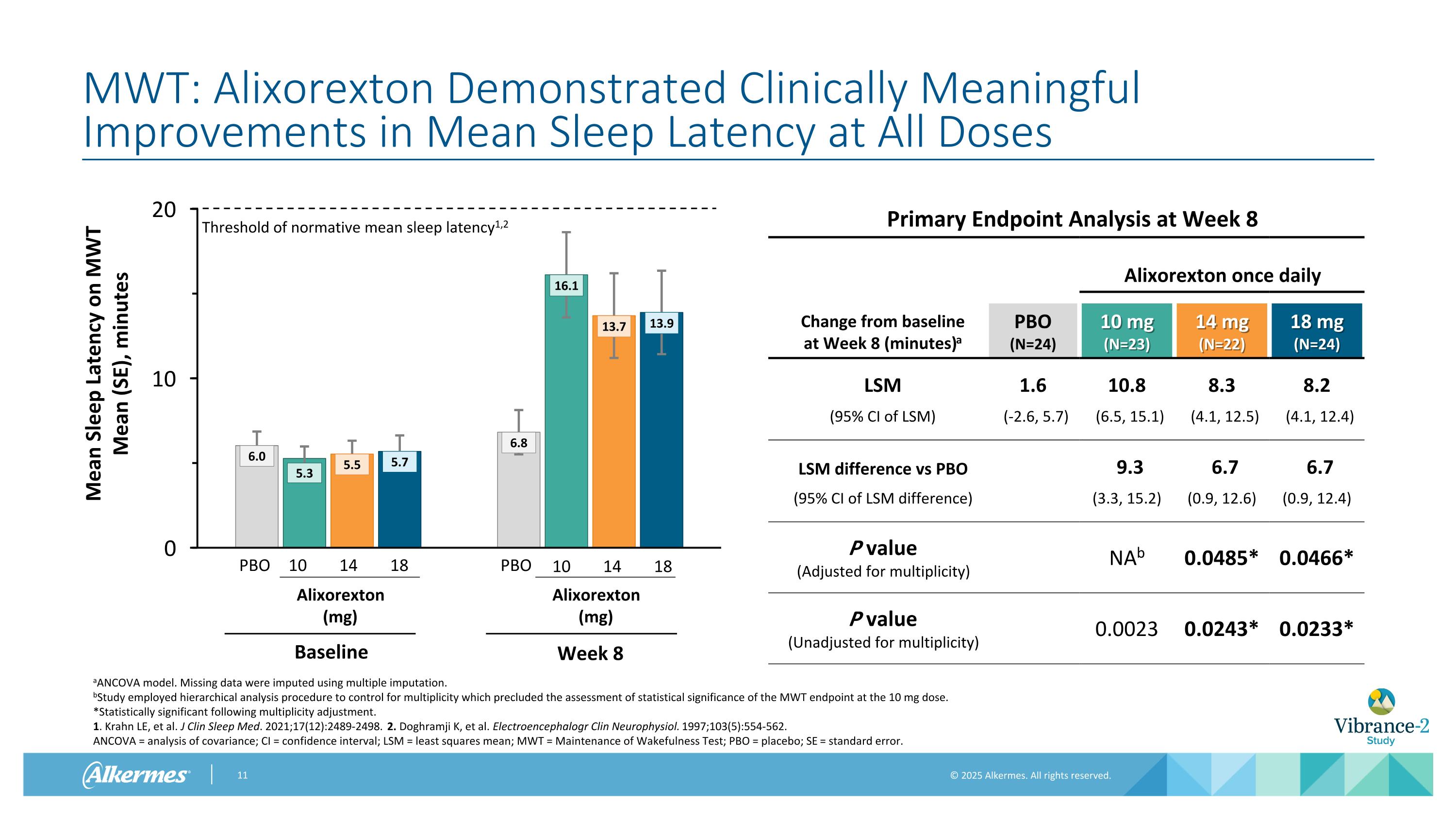
MWT: Alixorexton Demonstrated Clinically Meaningful Improvements in Mean Sleep Latency at All Doses Primary Endpoint Analysis at Week 8 Alixorexton once daily Change from baseline at Week 8 (minutes)a PBO (N=24) 10 mg (N=23) 14 mg (N=22) 18 mg (N=24) LSM 1.6 10.8 8.3 8.2 (95% CI of LSM) (-2.6, 5.7) (6.5, 15.1) (4.1, 12.5) (4.1, 12.4) LSM difference vs PBO 9.3 6.7 6.7 (95% CI of LSM difference) (3.3, 15.2) (0.9, 12.6) (0.9, 12.4) P value (Adjusted for multiplicity) NAb 0.0485* 0.0466* P value (Unadjusted for multiplicity) 0.0023 0.0243* 0.0233* aANCOVA model. Missing data were imputed using multiple imputation. bStudy employed hierarchical analysis procedure to control for multiplicity which precluded the assessment of statistical significance of the MWT endpoint at the 10 mg dose. *Statistically significant following multiplicity adjustment. 1. Krahn LE, et al. J Clin Sleep Med. 2021;17(12):2489-2498. 2. Doghramji K, et al. Electroencephalogr Clin Neurophysiol. 1997;103(5):554-562. ANCOVA = analysis of covariance; CI = confidence interval; LSM = least squares mean; MWT = Maintenance of Wakefulness Test; PBO = placebo; SE = standard error. Threshold of normative mean sleep latency1,2 10 14 18 PBO Alixorexton (mg) Baseline PBO Alixorexton (mg) Week 8 10 14 18
MWT Efficacy Insights in Vibrance-2 Intra-day time course of MWT response Strong, consistent response observed primarily at the 2-hour and 4-hour post-dose assessments Mean wakefulness more variable at the 6-hour and 8-hour post-dose assessments Pattern not previously observed with shorter duration exposures Pharmacodynamic response was inconsistent with plasma PK Phase 3 program expected to advance a range of doses and incorporate split dosing regimens MWT = Maintenance of Wakefulness Test; PK = pharmacokinetic
Vibrance-2: Positive Outcome Supports Advancing to Phase 3 Alixorexton is the first orexin 2 receptor agonist to demonstrate an efficacy signal with statistically significant and clinically meaningful improvements in MWT and ESS in a large, multi-dose phase 2 study in narcolepsy type 2 (NT2) Alixorexton was generally well tolerated, with most TEAEs mild to moderate in severity and no serious TEAEs reported Safety and tolerability profile confirmed anticipated dose/response shift in NT2 patients Important new findings from Vibrance-2 inform phase 3 program MWT = Maintenance of Wakefulness Test; ESS = Epworth Sleepiness Scale; NT2 = narcolepsy type 2; TEAE: Treatment-emergent adverse events
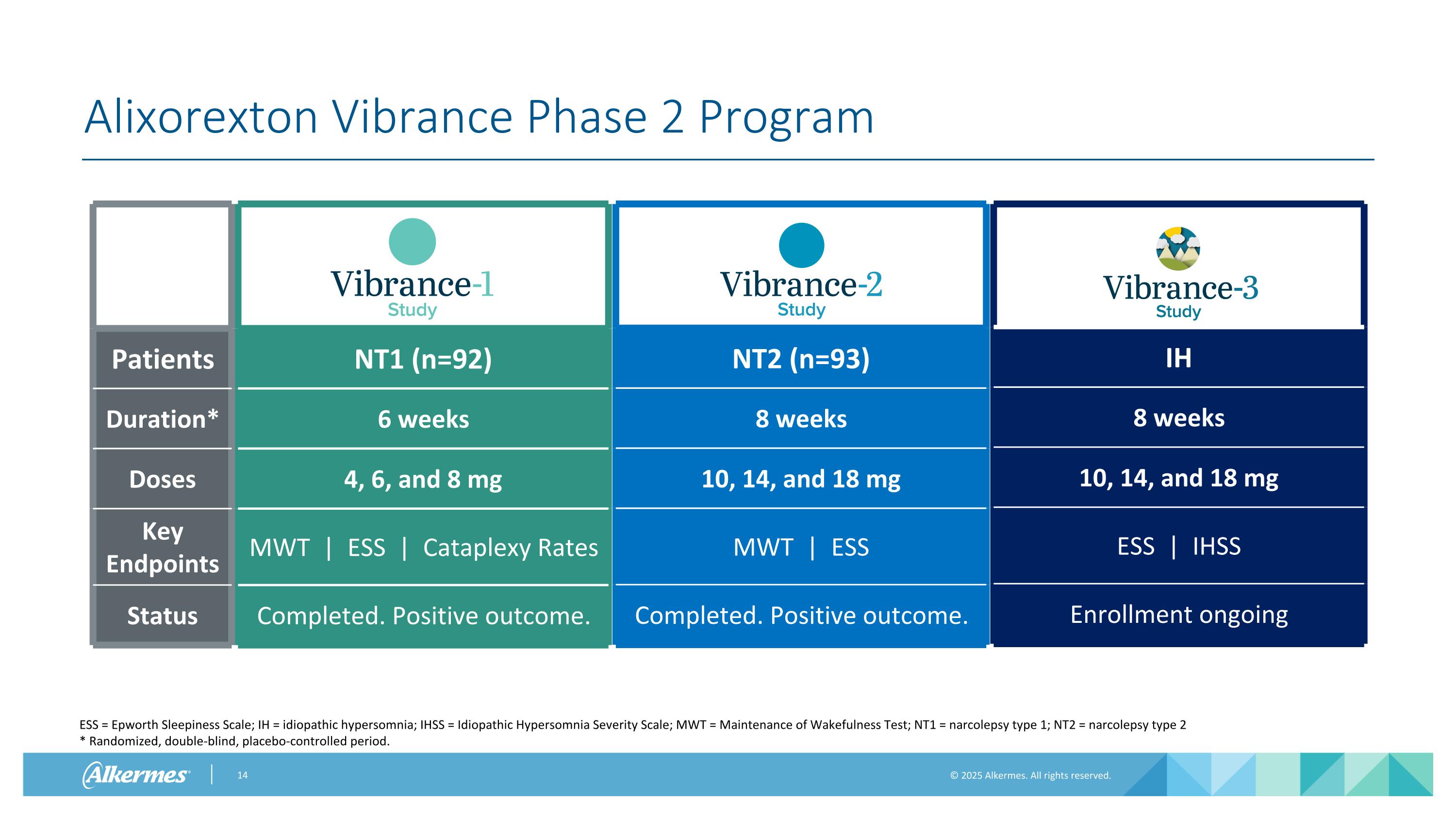
NT1 (n=92) 6 weeks 4, 6, and 8 mg MWT | ESS | Cataplexy Rates Completed. Positive outcome. IH 8 weeks 10, 14, and 18 mg ESS | IHSS Enrollment ongoing NT2 (n=93) 8 weeks 10, 14, and 18 mg MWT | ESS Completed. Positive outcome. Alixorexton Vibrance Phase 2 Program Patients Duration* Doses Key Endpoints Status ESS = Epworth Sleepiness Scale; IH = idiopathic hypersomnia; IHSS = Idiopathic Hypersomnia Severity Scale; MWT = Maintenance of Wakefulness Test; NT1 = narcolepsy type 1; NT2 = narcolepsy type 2 * Randomized, double-blind, placebo-controlled period.

www.alkermes.com