

JANUARY 2026 We are redefining pregnancy care to reduce the burden of preterm birth

This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The company has no obligation to provide any updates to these forward-looking statements, even if its expectations change, whether as a result of new information, future events or otherwise, except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. Further information on potential factors, risks and uncertainties that could affect operating and financial results is included in the company’s Registration Statement on Form S-1, most recent Annual Report on Form 10-K, and/or subsequent Forms 10-Q, including in each case under the heading RISK FACTORS, and in the company’s other filings with the SEC. The information in this presentation should be considered in conjunction with a review of the company’s filings with the SEC including the information in the company’s Registration Statement on Form S-1, most recent Annual Report on Form 10-K, and/or subsequent Forms 10-Q, under the heading MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIALCONDITION AND RESULTS OF OPERATIONS. Safe Harbor Statement PRESENT-105 rev 6.3

Our vision is to become a global leader in high-value women’s health diagnostics. We’ll do this by taking a holistic approach to providing pivotal information to providers and patients—simultaneously enabling better health for moms and babies while improving the economics of healthcare delivery. Our Bold Ambition We can do better for women’s health. TOGETHER PRESENT-105 rev 6.3
Reference: 1. Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: Final Data for 2022. Natl Vital Stat Rep. 2024 Apr;73(2):1-56. PMID: 38625869 2 Martin JA, Osterman MJK. Increases in neonatal intensive care admissions in the United States, 2016–2023. NCHS Data Brief. 2025 Mar;525 3.marchofdimes.org/peristats/data?reg=99&top=3&stop=362&slev=1&obj=1 Usher in a new era of personalized pregnancy care through predictive diagnostics Use proteomics-based testing for women's health, with the PreTRM® test as our flagship blood-based predictor of spontaneous preterm birth risk PreTRM is the only clinically validated test available for early prediction of premature birth and is verified by 8 independent clinical studies CORPORATE OVERVIEW THE NEED THE SOLUTION POSITIONED FOR COMMERCIAL INFLECTION $102.4M Cash, cash equivalents, and marketable securities as of September 30, 2025. Cash position extends through 2028 to support reaching seminal revenue inflection point Full PRIME data published in Society of Maternal Fetal Medicine journal PREGNANCY; publication of subgroup analyses coming soon Progress in engaging Medicaid plans across wave one and two target states, including those with prior PreTRM study experience; one state Medicaid and one commercial pilot program already launched. Opportunity to address $25.2 billion economic cost of preterm birth in the US3 Turning Data Into Better Maternal & Neonatal Outcomes 1 in 10 babies are born too soon1 Infants were more likely to be admitted to a NICU in 2023 than in 2016, the percentage of infants admitted rose from 8.7% to 9.8%2 The March of Dimes report card continues to show a D-level rating for preterm birth3 PRESENT-105 rev 6.3

THE Need

THE US HAS A PREMATURE BIRTH PROBLEM And identification isn’t getting better National Vital Statistics Data: Infants were more likely to be admitted to a NICU in 2023 than in 2016, according to the Centers for Disease Control. The percentage of infants admitted rose from 8.7% to 9.8% FROM 2013-20243 PRETERM BIRTH RATES IN THE US ROSE FROM 9.4% to 10.4% MARCH OF DIMES References: Martin JA, Osterman MJK. Increases in neonatal intensive care admissions in the United States, 2016–2023. NCHS Data Brief. 2025 Mar;525. DOI: https://dx.doi.org/10.15620/cdc/174581. March of Dimes. The 2025 March of Dimes Report Card: The State of Maternal and Infant Health for American Families. 2025. Available at: https://www.marchofdimes.org/sites/default/files/2025-11/MarchofDimesReportCard-UnitedStates-2025.pdf. PRESENT-105 rev 6.3

HEALTH CARE COSTS OVER 7 YEARS Health Cost & Implications PREMATURE BIRTH LEADS TO 18X+ HIGHER BIRTH COST OF NEWBORNS Extremely Premature < 28 weeks Risk of death, long NICU stay, respiratory issues, decreased organ development, infections Premature < 32 weeks Respiratory issues, significant NICU stay, feeding challenges, temperature regulation, under development Full Term 37-40 weeks Lower healthcare condition burden References: 1.Phibbs CS, et al. Birth Hospitalization Costs and Days of Care for Mothers and Neonates in California, 2009-2011. J Pediatr. 2019 Jan; 204:118-125.e14 2.Beam et al, Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008-2016, Journal of Perinatology (2020) 40:1091–1099. - Preterm births account for 61% of neonatal costs for in-hospital deliveries 3. Full term cost source: https://www.healthsystemtracker.org/brief/health-costs-associated-with-pregnancy-childbirth-and-postpartum-care/ 4. Inflation calculator: https://www.bls.gov/data/inflation_calculator.htm Up to $344,355 $64,815 $18,865 AVERAGE DELIVERY CARE COST $30,889 | ~7X Higher Costs $11,799 | ~3X Higher Costs $4,165 PRESENT-105 rev 6.3
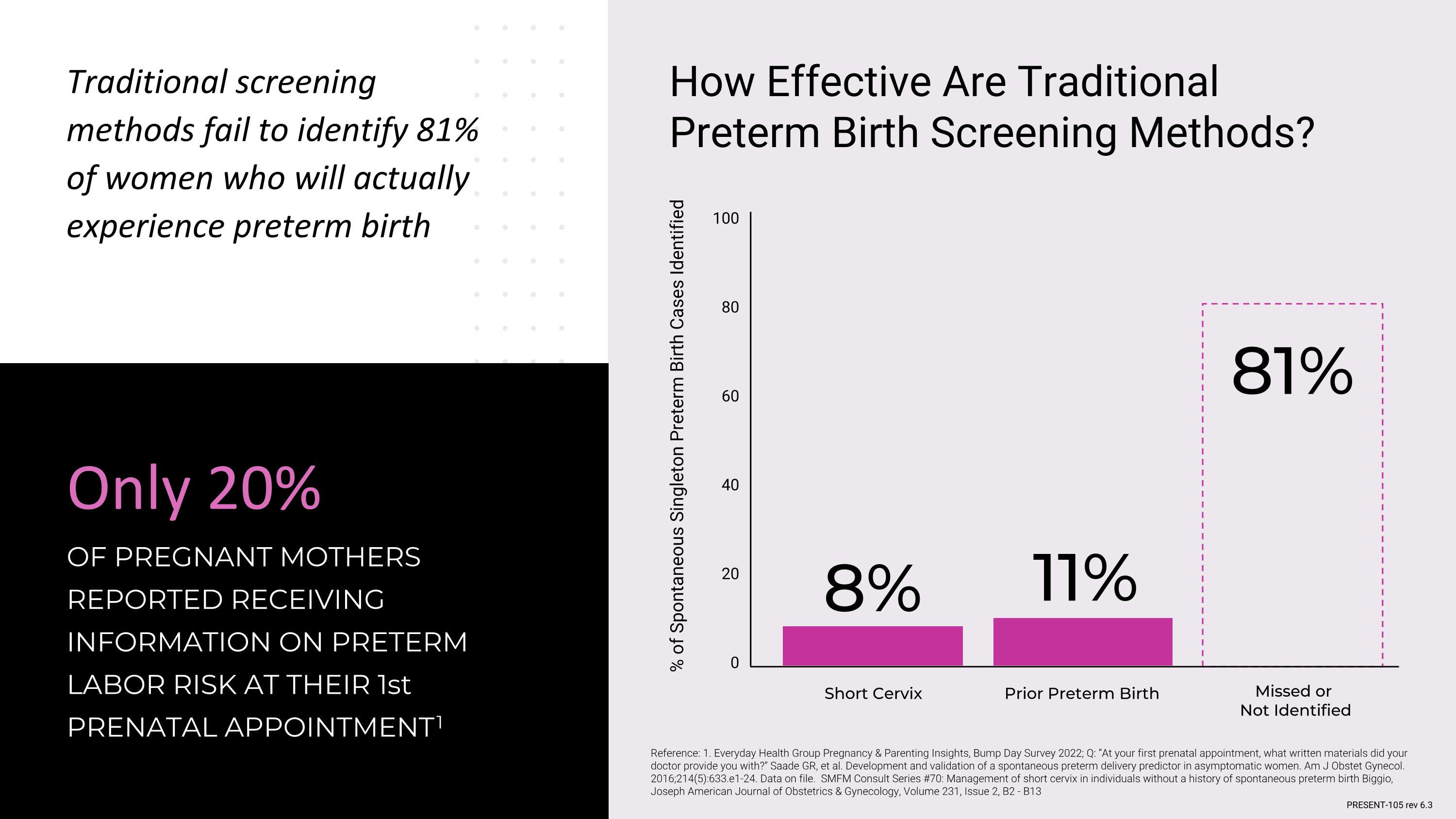
Reference: 1. Everyday Health Group Pregnancy & Parenting Insights, Bump Day Survey 2022; Q: “At your first prenatal appointment, what written materials did your doctor provide you with?” Saade GR, et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol. 2016;214(5):633.e1-24. Data on file. SMFM Consult Series #70: Management of short cervix in individuals without a history of spontaneous preterm birth Biggio, Joseph American Journal of Obstetrics & Gynecology, Volume 231, Issue 2, B2 - B13 Only 20% OF PREGNANT MOTHERS REPORTED RECEIVING INFORMATION ON PRETERM LABOR RISK AT THEIR 1st PRENATAL APPOINTMENT 1 How Effective Are Traditional Preterm Birth Screening Methods? Traditional screening methods fail to identify 81% of women who will actually experience preterm birth 100 80 60 40 20 0 % of Spontaneous Singleton Preterm Birth Cases Identified Prior Preterm Birth Short Cervix 11% 8% 81% Missed or Not Identified PRESENT-105 rev 6.3

PRODUCT AND Science

THE PURPOSE Predictive test to detect biomarkers in the blood before symptoms arise WHAT IT MEASURES Risk of developing a condition, and biological effect HOW IT WORKS Uses advanced technologies (mass spectrometry) to analyze proteins and reveals patterns of protein expression and modification THE SCIENCE What is a proteomic test? Measures types & quantities of proteins present in a sample Reference: https://www.healthcaredive.com/spons/proteomic-tests-empower-precision-medicine/635232/ PRESENT-105 rev 6.3
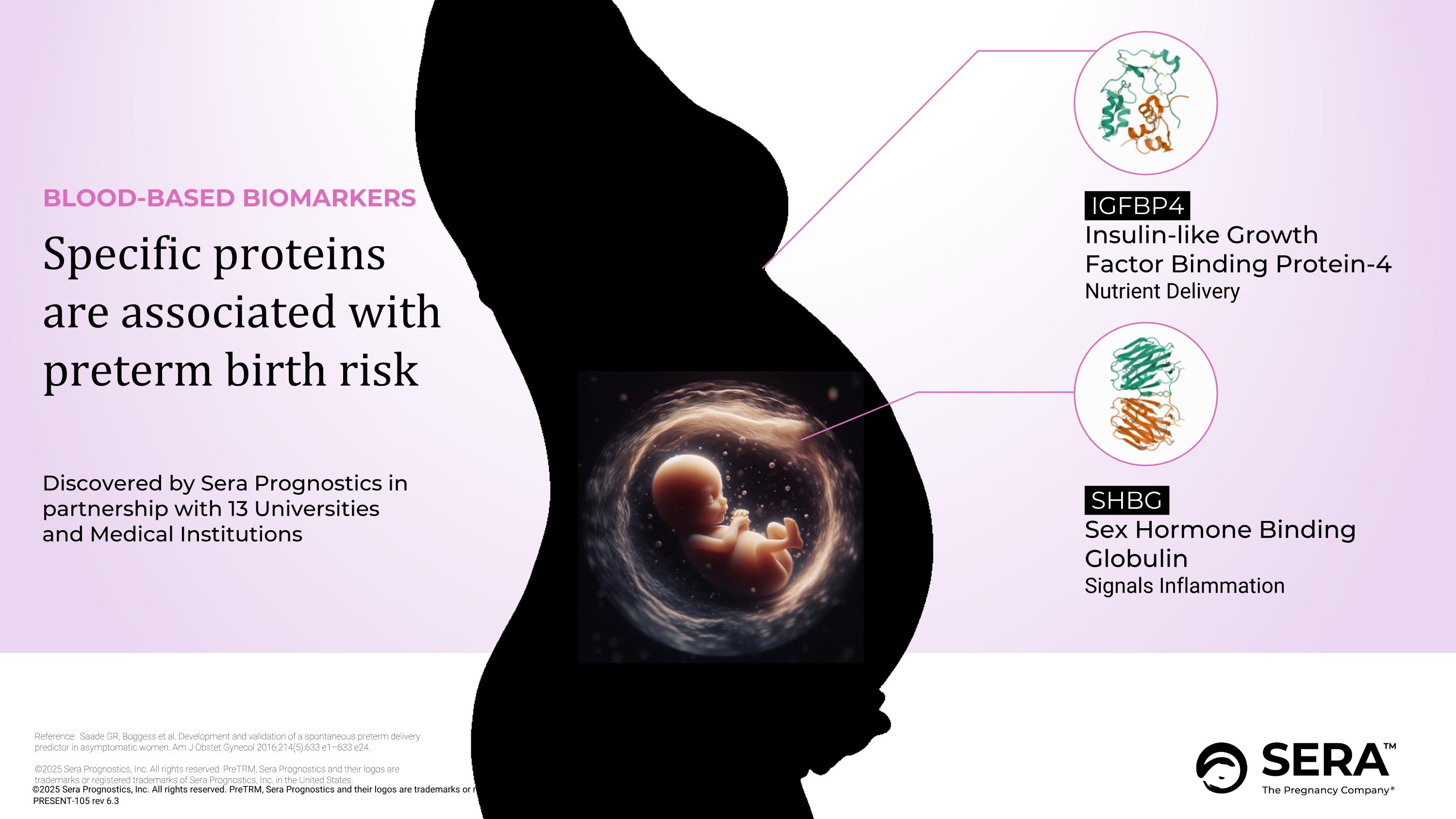
BLOOD-BASED BIOMARKERS Specific proteins are associated with preterm birth risk IGFBP4 Insulin-like Growth Factor Binding Protein-4 Nutrient Delivery Reference: Saade GR, Boggess et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol 2016;214(5):633 e1–633 e24. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. SHBG Sex Hormone Binding Globulin Signals Inflammation Discovered by Sera Prognostics in partnership with 13 Universities and Medical Institutions PRESENT-105 rev 6.3

Results delivered in an average of five days from a CLIA-certified, CAP-accredited lab Blood sample kits are provided for testing during 180/7-206/7 weeks with prepaid shipment to Sera’s lab Sample collection can be performed by staff and aligns with preexisting appointments ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PreTRM SEAMLESSLY INTEGRATES INTO AND COMPLEMENTS PRENATAL CARE WORKFLOWS PRESENT-105 rev 6.3

Pregnancies identified as higher risk by the PreTRM Test are at increased risk for*: Communicates a patient’s risk of spontaneous preterm birth & helps physicians engage with patients TEST REPORT Spontaneous preterm birth Severe adverse neonatal outcomes Longer neonatal hospital length of stay Reference: Burchard J, et al. Clinical Validation of a Proteomic Biomarker Threshold for Increased Risk of Spontaneous Preterm Birth and Associated Clinical Outcomes: A Replication Study. J. Clin. Med. 2021, 10, 5088. doi: 10.3390/jcm10215088. Available at https://www.mdpi.com/2077-0383/10/21/5088 Final Pregnancies identified as not higher risk have peace of mind due to a negative predictive value of 97% PRESENT-105 rev 6.3

Evidence ROBUST CLINICAL
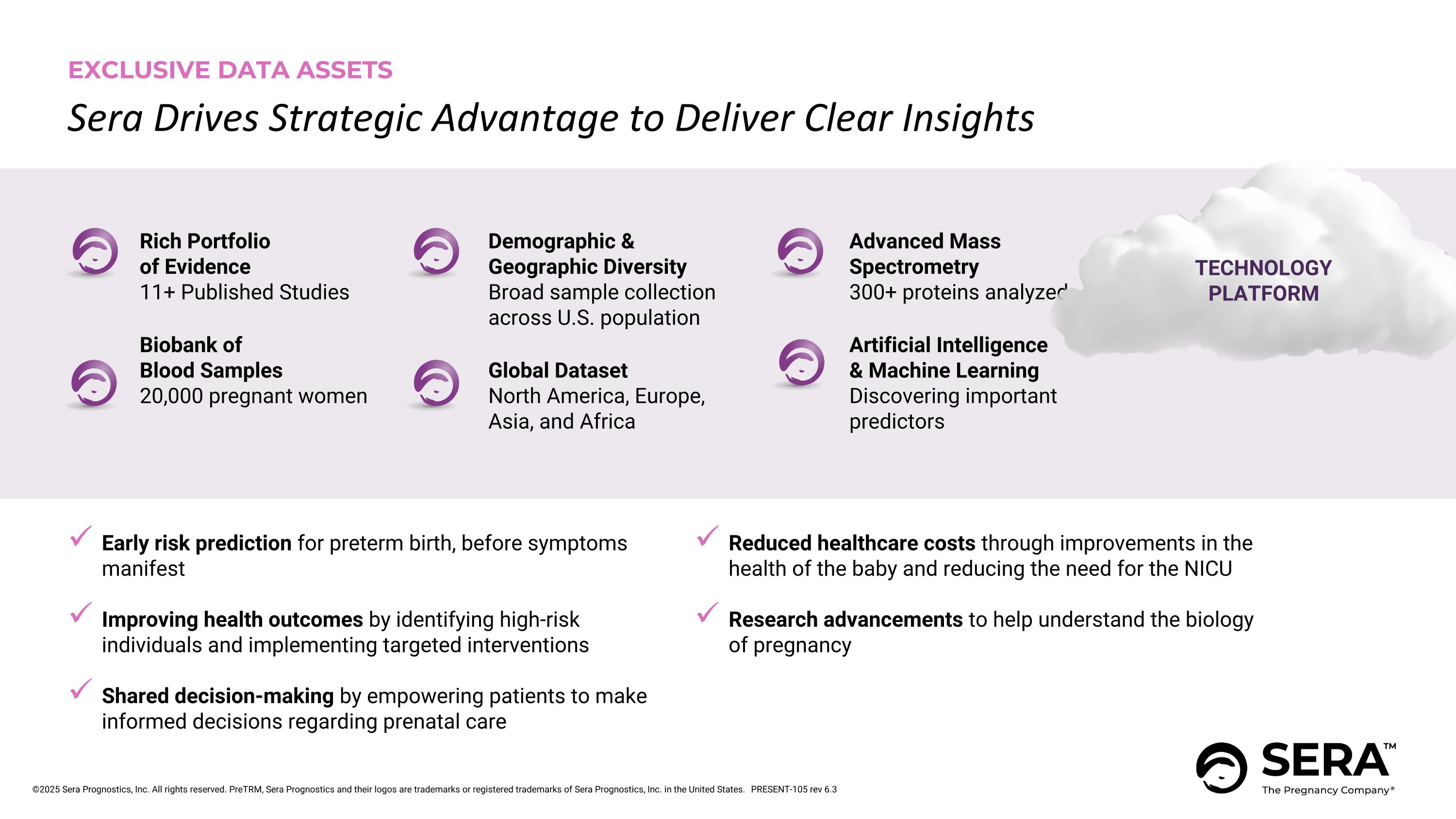
Sera Drives Strategic Advantage to Deliver Clear Insights Rich Portfolio of Evidence 11+ Published Studies Biobank of Blood Samples 20,000 pregnant women EXCLUSIVE DATA ASSETS Advanced Mass Spectrometry 300+ proteins analyzed Artificial Intelligence & Machine Learning Discovering important predictors Demographic & Geographic Diversity Broad sample collection across U.S. population Global Dataset North America, Europe, Asia, and Africa Early risk prediction for preterm birth, before symptoms manifest Improving health outcomes by identifying high-risk individuals and implementing targeted interventions Shared decision-making by empowering patients to make informed decisions regarding prenatal care Reduced healthcare costs through improvements in the health of the baby and reducing the need for the NICU Research advancements to help understand the biology of pregnancy TECHNOLOGY PLATFORM PRESENT-105 rev 6.3

The PreTRM® Test is highly predictive of spontaneous preterm birth with a single blood draw during 180/7 through 206/7 weeks of gestation2 Three Independent Studies (discovery, verification, validation) reported in a large, multi-center trial Published as Editor’s Choice article in American Journal of Obstetrics & Gynecology (May 2016) References: 1. Saade GR, et al. development and validation of spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol. 2016;214:633e1-24. 2. Burchard, J., et al. Better Estimation of Spontaneous Preterm Birth Prediction Performance through Gestational Age Dating. J. Clin. Med. 2022, 11, 2885. doi.org/10.3390/jcm11102885 PAPR Validation Study1 (n=5501, 11 centers) THE PRETRM® TEST The Only Clinically Validated Test Available for Early Prediction of Premature Birth PRESENT-105 rev 6.3

PRIME & AVERT STUDIES Multiple studies demonstrate an effective approach to managing patients identified as higher risk with the PreTRM® Test PRIME STUDY The Randomized Controlled Trial Involved: 19 U.S. sites, including community practices and university-based or -associated medical centers Enrolled 5018 patients From November 2020 – December 2023 Published in PREGNANCY (2025) AVERT STUDY The Historically Controlled Trial Involved: ChristianaCare Hospital (Newark, DE) Enrolled 1873 patients in the prospective arm compared to 10,000 historical controls From June 2018 – September 2020 Published in Diagnostics (2024) PRESENT-105 rev 6.3 References: 1 Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 2 Iriye BK, O'Brien JM, Ennen CS, et al. Neonatal impact of maternal biomarker screening for risk of preterm birth with targeted interventions (PRIME): A multicenter, randomized, controlled trial. Pregnancy 2026;2(1):e70202. DOI: https://doi.org/10.1002/pmf2.70202.

Finds PreTRM® Test Reduces Earliest Preterm Births & Newborn Complications A pioneering clinical trial assessing the efficacy of the PreTRM Test and preventive interventions in lowering the occurrence of adverse pregnancy outcomes. THE PRIME STUDY HIGHER RISK NOT HIGHER RISK NOT HIGHER RISK PRESENT-105 rev 6.3
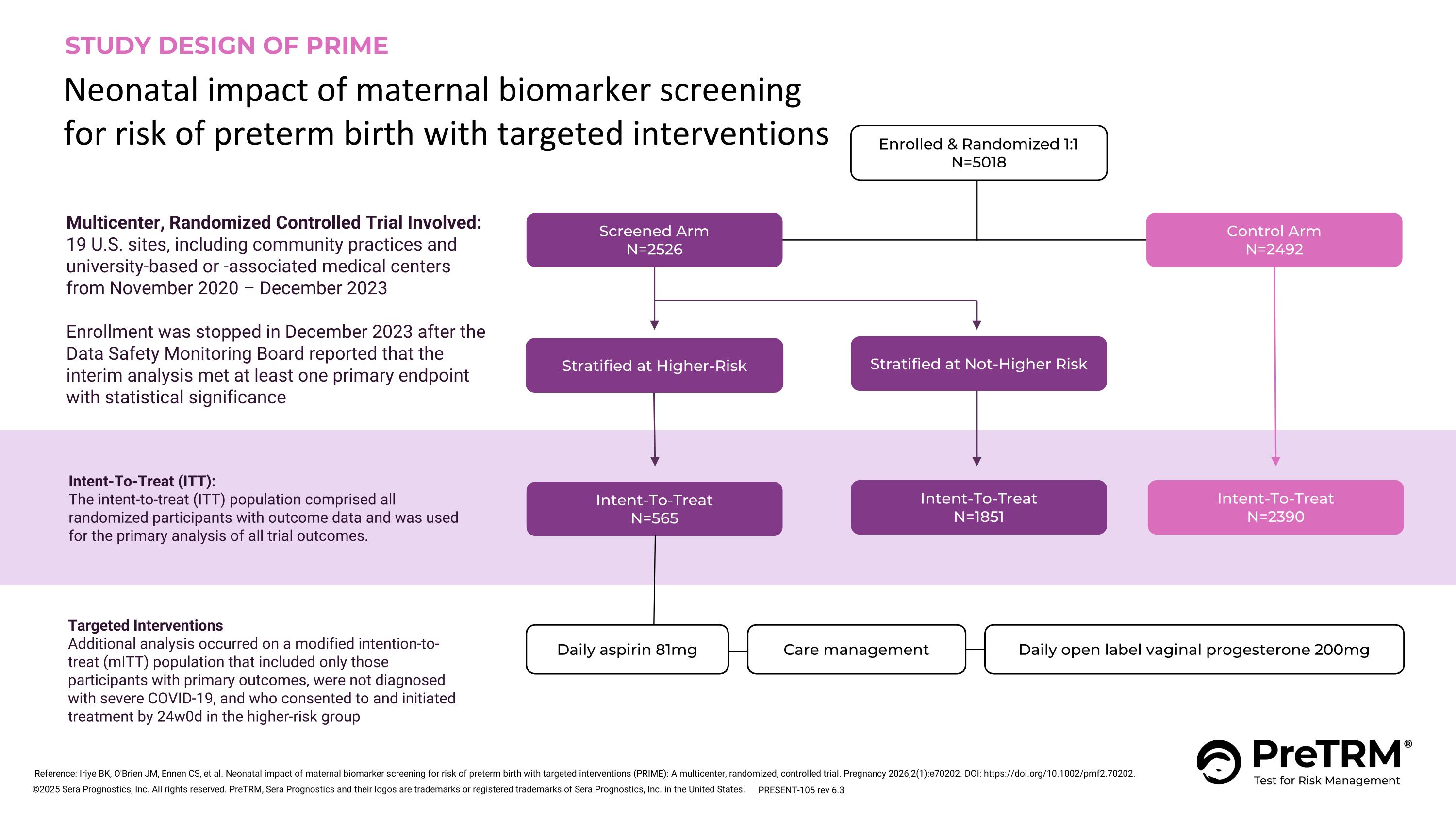
Control Arm N=2492 Intent-To-Treat N=2390 Screened Arm N=2526 Enrolled & Randomized 1:1 N=5018 Intent-To-Treat N=1851 Intent-To-Treat (ITT): The intent-to-treat (ITT) population comprised all randomized participants with outcome data and was used for the primary analysis of all trial outcomes. STUDY DESIGN OF PRIME Neonatal impact of maternal biomarker screening for risk of preterm birth with targeted interventions Stratified at Higher-Risk Stratified at Not-Higher Risk Intent-To-Treat N=565 Targeted Interventions Additional analysis occurred on a modified intention-to-treat (mITT) population that included only those participants with primary outcomes, were not diagnosed with severe COVID-19, and who consented to and initiated treatment by 24w0d in the higher-risk group Multicenter, Randomized Controlled Trial Involved: 19 U.S. sites, including community practices and university-based or -associated medical centers from November 2020 – December 2023 Enrollment was stopped in December 2023 after the Data Safety Monitoring Board reported that the interim analysis met at least one primary endpoint with statistical significance Daily aspirin 81mg Daily open label vaginal progesterone 200mg Care management Reference: Iriye BK, O'Brien JM, Ennen CS, et al. Neonatal impact of maternal biomarker screening for risk of preterm birth with targeted interventions (PRIME): A multicenter, randomized, controlled trial. Pregnancy 2026;2(1):e70202. DOI: https://doi.org/10.1002/pmf2.70202. PRESENT-105 rev 6.3

Recruitment November 2020-December 2023 The study was presented as an oral abstract at the 2025 SMFM Annual Pregnancy Meeting Included a diverse population with no significant differences in patient demographics in the control vs treatment arm. Demonstrates Significant Improvements in Neonatal Outcomes With Early Risk Screening With PreTRM® and Targeted Interventions PRIME: A PROSPECTIVE, RANDOMIZED CONTROLLED TRIAL Published in the Society of Maternal Fetal Medicine Journal, PREGNANCY PRIME Study1 (n=5,018, 19 centers) Key findings include: 20% fewer babies admitted to the NICU 56% and 32% fewer babies were born before 32 and 35 weeks, respectively Fewer health complications for newborns (20% reduction in odds of neonatal morbidity) A NICU day was saved for every 4.2 patients screened Reference: 1Iriye BK, O'Brien JM, Ennen CS, et al. Neonatal impact of maternal biomarker screening for risk of preterm birth with targeted interventions (PRIME): A multicenter, randomized, controlled trial. Pregnancy 2026;2(1):e70202. DOI: https://doi.org/10.1002/pmf2.70202. PRESENT-105 rev 6.3

EACH DAY MATTERS Maternal Focused Strategies Help Increase Gestational Age Reducing Deliveries Before 32 Weeks 56% reduction in deliveries before 32 weeks gestation1 Reducing Deliveries Before 35 Weeks 32% reduction in deliveries before 35 weeks gestation1 Increase in Gestational Age Gestational age amongst the earliest ~10% of births was prolonged by 4.5 days, on average1 Reference: 1 Iriye BK, O'Brien JM, Ennen CS, et al. Neonatal impact of maternal biomarker screening for risk of preterm birth with targeted interventions (PRIME): A multicenter, randomized, controlled trial. Pregnancy 2026;2(1):e70202. DOI: https://doi.org/10.1002/pmf2.70202. 2Neonatal morbidities measured using a composite scoring index included: intraventricular hemorrhage; periventricular leukomalacia; necrotizing enterocolitis; respiratory distress syndrome/hyaline membrane disease; bronchopulmonary dysplasia; and sepsis. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PRESENT-105 rev 6.3
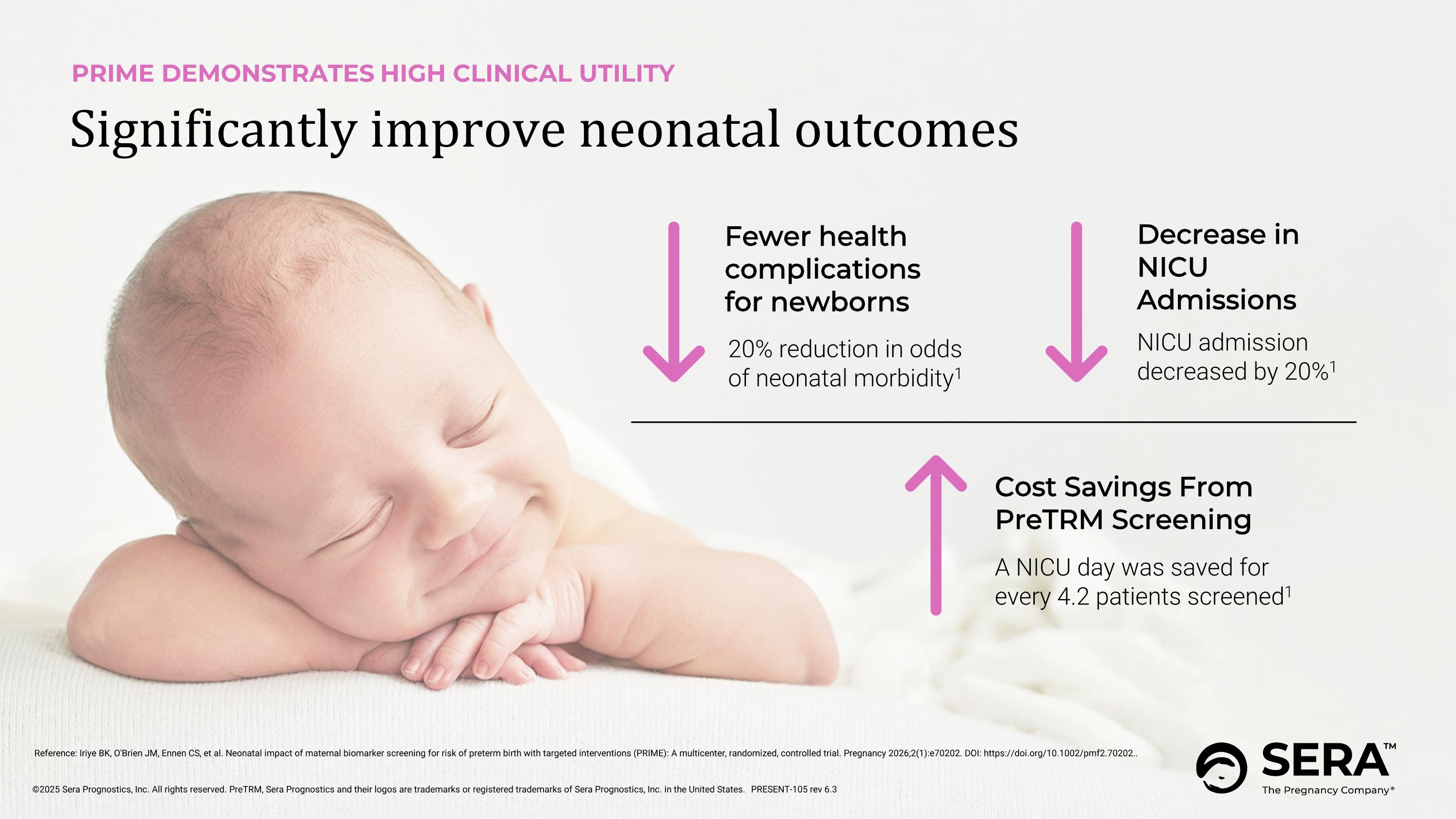
PRIME DEMONSTRATES HIGH CLINICAL UTILITY Significantly improve neonatal outcomes Fewer health complications for newborns 20% reduction in odds of neonatal morbidity1 Decrease in NICU Admissions NICU admission decreased by 20%1 Cost Savings From PreTRM Screening A NICU day was saved for every 4.2 patients screened1 Reference: Iriye BK, O'Brien JM, Ennen CS, et al. Neonatal impact of maternal biomarker screening for risk of preterm birth with targeted interventions (PRIME): A multicenter, randomized, controlled trial. Pregnancy 2026;2(1):e70202. DOI: https://doi.org/10.1002/pmf2.70202.. PRESENT-105 rev 6.3
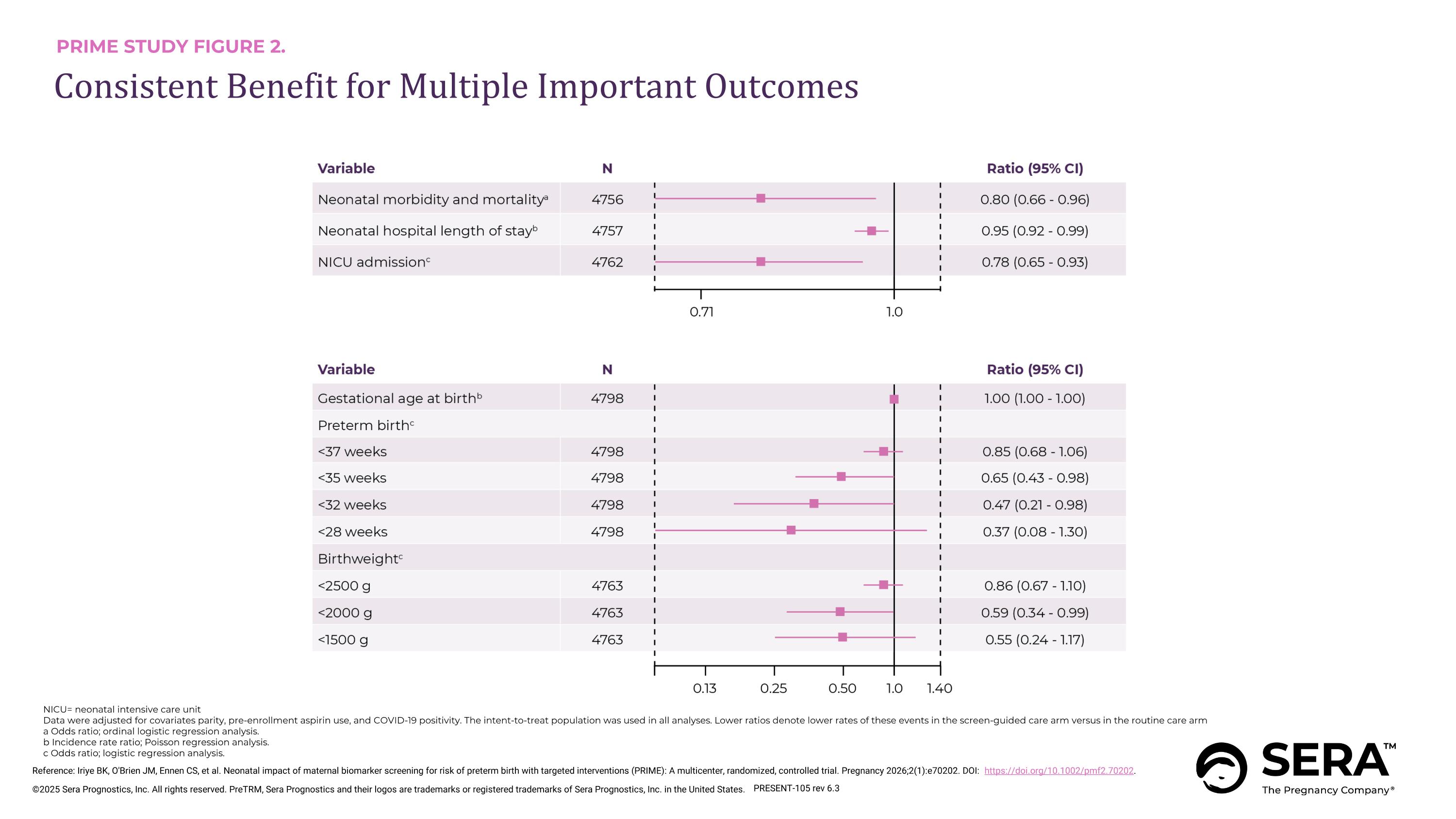
PRIME STUDY FIGURE 2. Consistent Benefit for Multiple Important Outcomes Reference: Iriye BK, O'Brien JM, Ennen CS, et al. Neonatal impact of maternal biomarker screening for risk of preterm birth with targeted interventions (PRIME): A multicenter, randomized, controlled trial. Pregnancy 2026;2(1):e70202. DOI: https://doi.org/10.1002/pmf2.70202. PRESENT-105 rev 6.3 NICU= neonatal intensive care unit Data were adjusted for covariates parity, pre-enrollment aspirin use, and COVID-19 positivity. The intent-to-treat population was used in all analyses. Lower ratios denote lower rates of these events in the screen-guided care arm versus in the routine care arm a Odds ratio; ordinal logistic regression analysis. b Incidence rate ratio; Poisson regression analysis. c Odds ratio; logistic regression analysis.

PROACTIVE CARE STRATEGIES USED FOR HIGHER RISK MOMS Clear direction on managing higher-risk pregnancies Low-Dose Aspirin (81 mg) Taken orally daily until 366/7 weeks gestation Vaginal Progesterone (200 mg) Administered daily as a 200 mg micronized progesterone suppository until 366/7 weeks’ gestation Care Management Weekly check-ins with the patient, either virtual or by phone PRESENT-105 rev 6.3

PATHWAY TO Commercialization PRESENT-105 rev 6.3
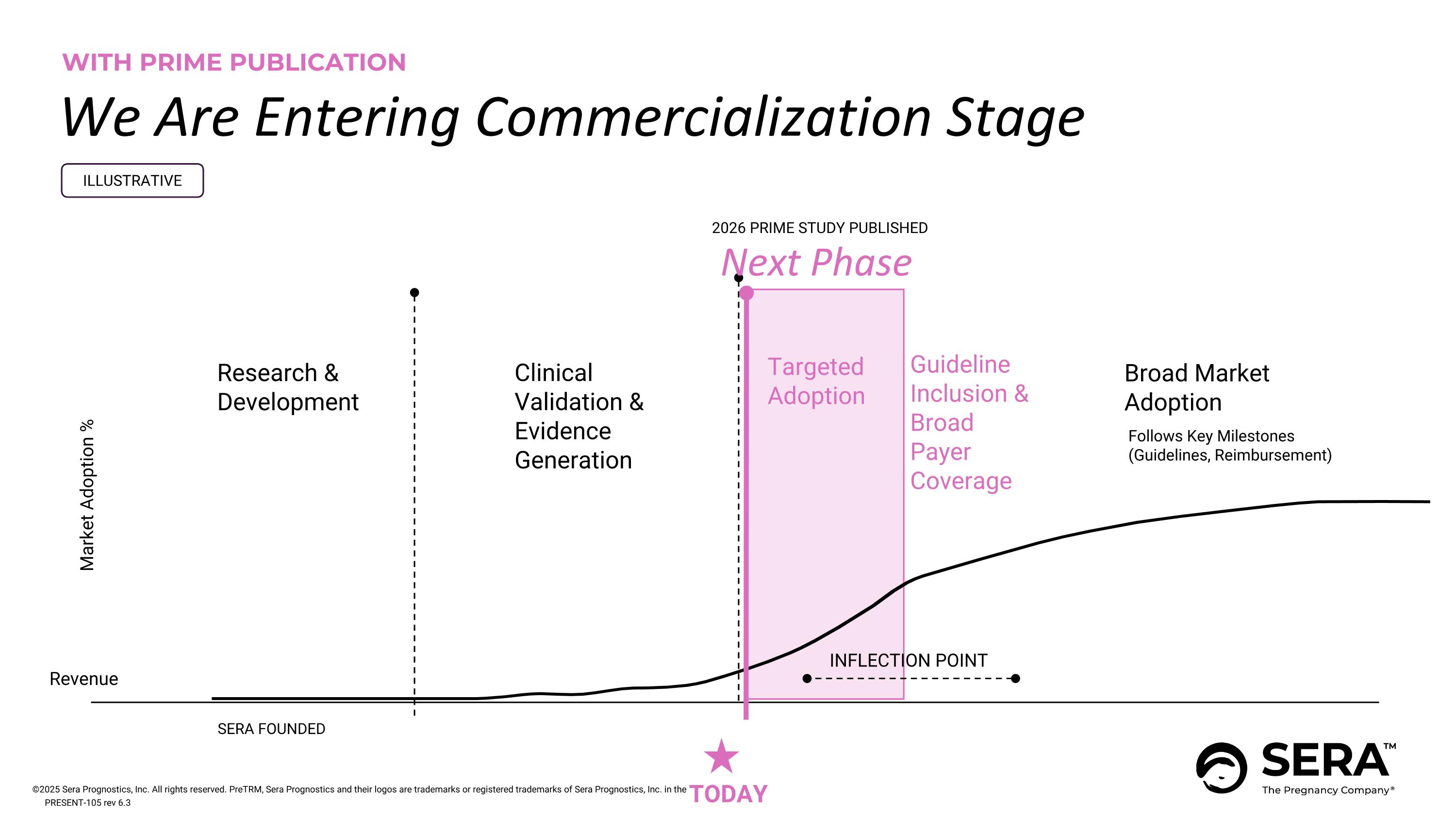
WITH PRIME PUBLICATION We Are Entering Commercialization Stage Follows Key Milestones (Guidelines, Reimbursement) Market Adoption % Research & Development SERA FOUNDED 2026 PRIME STUDY PUBLISHED Broad Market Adoption Clinical Validation & Evidence Generation Targeted Adoption INFLECTION POINT ILLUSTRATIVE Revenue TODAY Next Phase Guideline Inclusion & Broad Payer Coverage PRESENT-105 rev 6.3
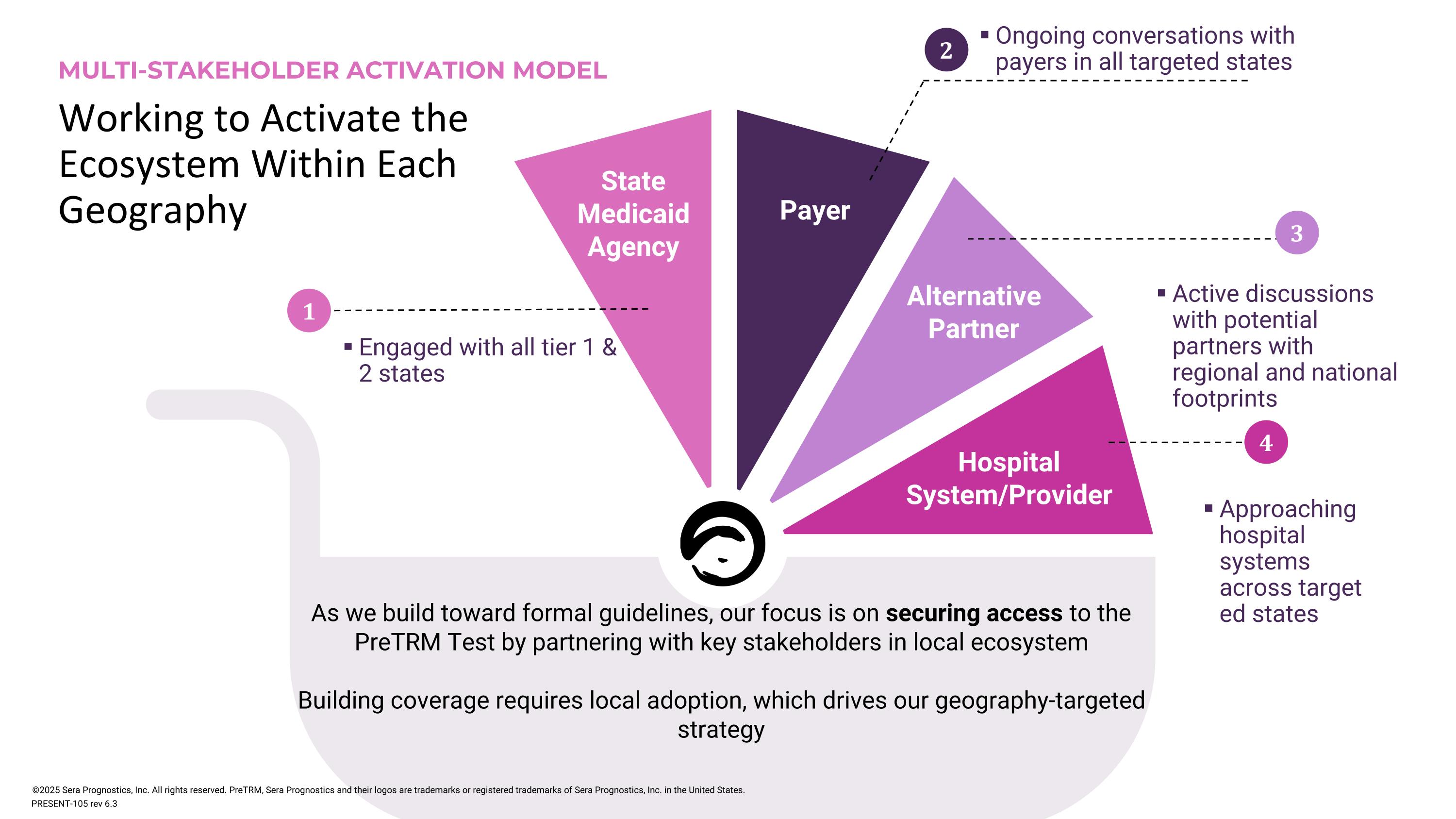
Working to Activate the Ecosystem Within Each Geography MULTI-STAKEHOLDER ACTIVATION MODEL State Medicaid Agency Hospital System/Provider Payer Alternative Partner Engaged with all tier 1 & 2 states 1 2 Ongoing conversations with payers in all targeted states As we build toward formal guidelines, our focus is on securing access to the PreTRM Test by partnering with key stakeholders in local ecosystem Building coverage requires local adoption, which drives our geography-targeted strategy ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. 3 Active discussions with potential partners with regional and national footprints Approaching hospital systems across targeted states 4 PRESENT-105 rev 6.3
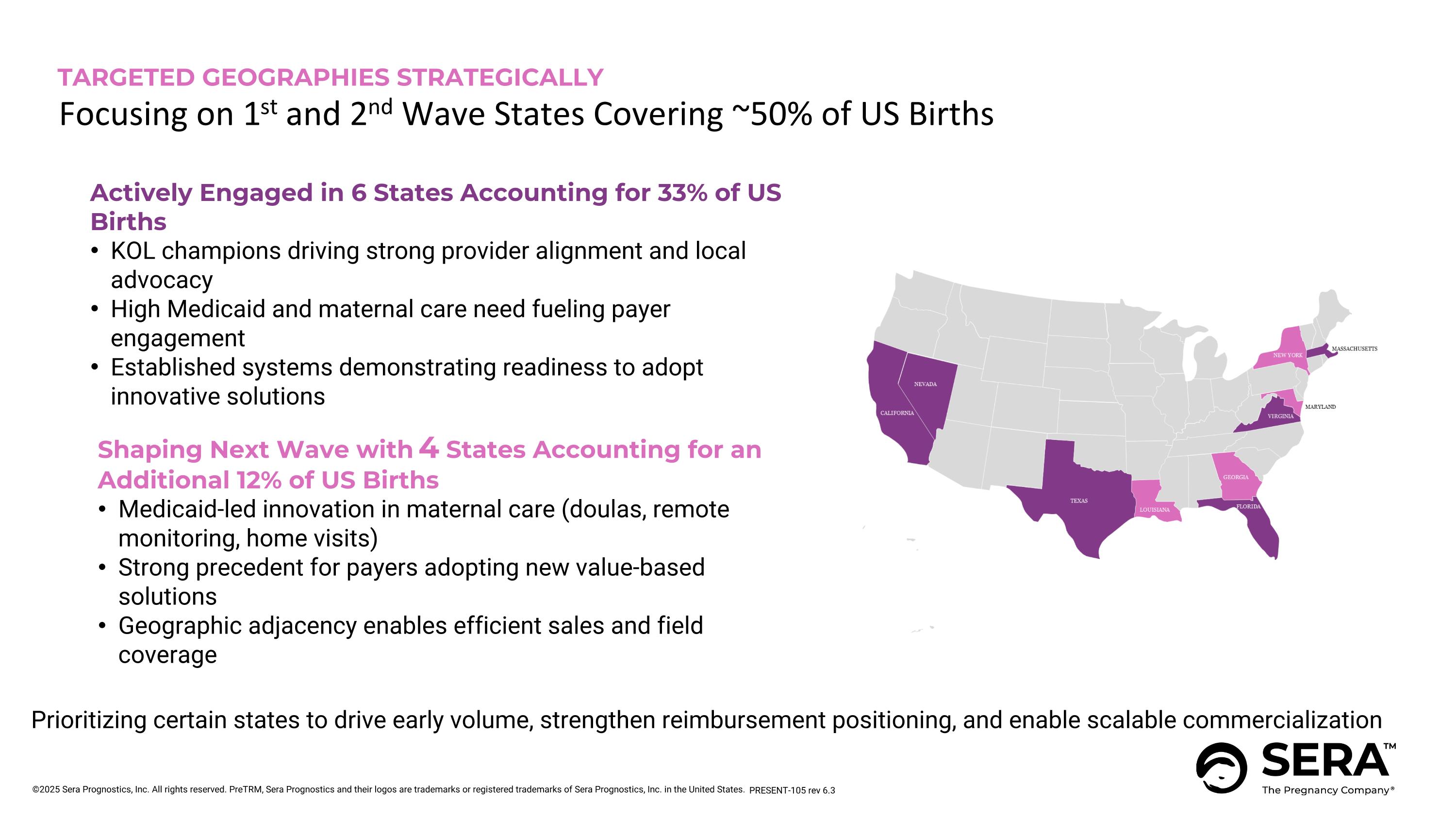
Shaping Next Wave with 4 States Accounting for an Additional 12% of US Births Medicaid-led innovation in maternal care (doulas, remote monitoring, home visits) Strong precedent for payers adopting new value-based solutions Geographic adjacency enables efficient sales and field coverage TARGETED GEOGRAPHIES STRATEGICALLY Focusing on 1st and 2nd Wave States Covering ~50% of US Births Prioritizing certain states to drive early volume, strengthen reimbursement positioning, and enable scalable commercialization Actively Engaged in 6 States Accounting for 33% of US Births KOL champions driving strong provider alignment and local advocacy High Medicaid and maternal care need fueling payer engagement Established systems demonstrating readiness to adopt innovative solutions PRESENT-105 rev 6.3

Targeted Adoption 2026 2027 2028 PRE-GUIDELINES Achieving coverage and reimbursement in target geographies building on provider momentum Partner pilots across first wave of target states, a key unlock SUCCESS FACTORS National Adoption POST-GUIDELINES National field sales presence Achieving broad coverage Conferences Develop local ecosystem expansion approach with roles of field sales, advocacy, and marketing Traditional marketing education, speaker programs, CME, etc. Potential co-marketing with large strategic players 2027 2028 2029 COMMERCIALIZATION STRATEGY IN TWO PARTS Engagement with key opinion leaders around data and interventions Partner to incorporate PreTRM Test into screening pathways to better risk tier moms missed by current screening tools to deliver higher risk care needs with accessible PRIME interventions. Target population-based prioritization: all pregnant moms and first-time moms with no prior history Guidelines BECOMING PART OF SUCCESS FACTORS PRIORITIES ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PRESENT-105 rev 6.3
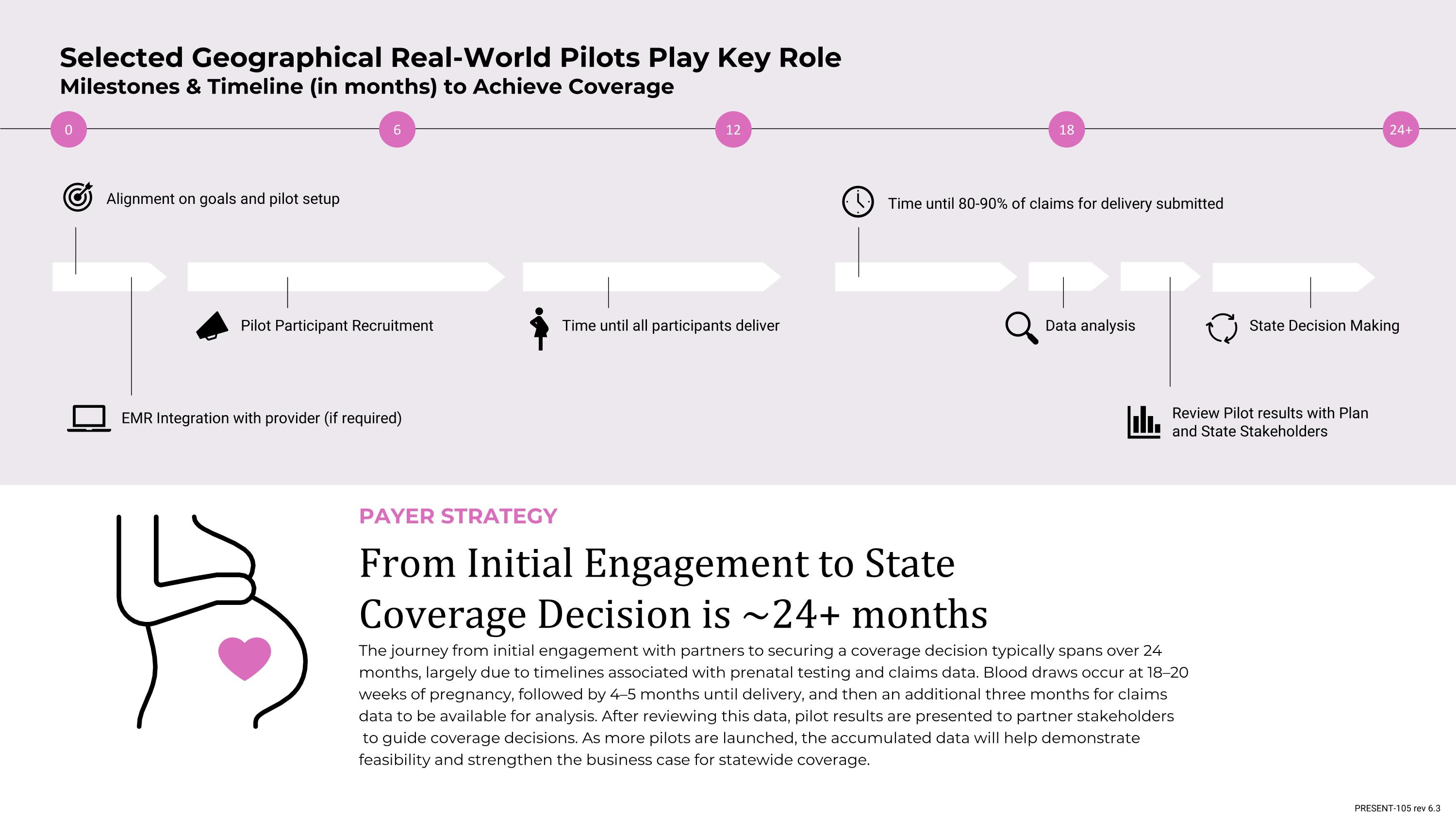
12 6 0 18 24+ Selected Geographical Real-World Pilots Play Key Role Milestones & Timeline (in months) to Achieve Coverage EMR Integration with provider (if required) Review Pilot results with Plan and State Stakeholders Alignment on goals and pilot setup Time until 80-90% of claims for delivery submitted Data analysis State Decision Making Pilot Participant Recruitment Time until all participants deliver From Initial Engagement to State Coverage Decision is ~24+ months The journey from initial engagement with partners to securing a coverage decision typically spans over 24 months, largely due to timelines associated with prenatal testing and claims data. Blood draws occur at 18–20 weeks of pregnancy, followed by 4–5 months until delivery, and then an additional three months for claims data to be available for analysis. After reviewing this data, pilot results are presented to partner stakeholders to guide coverage decisions. As more pilots are launched, the accumulated data will help demonstrate feasibility and strengthen the business case for statewide coverage. PAYER STRATEGY PRESENT-105 rev 6.3
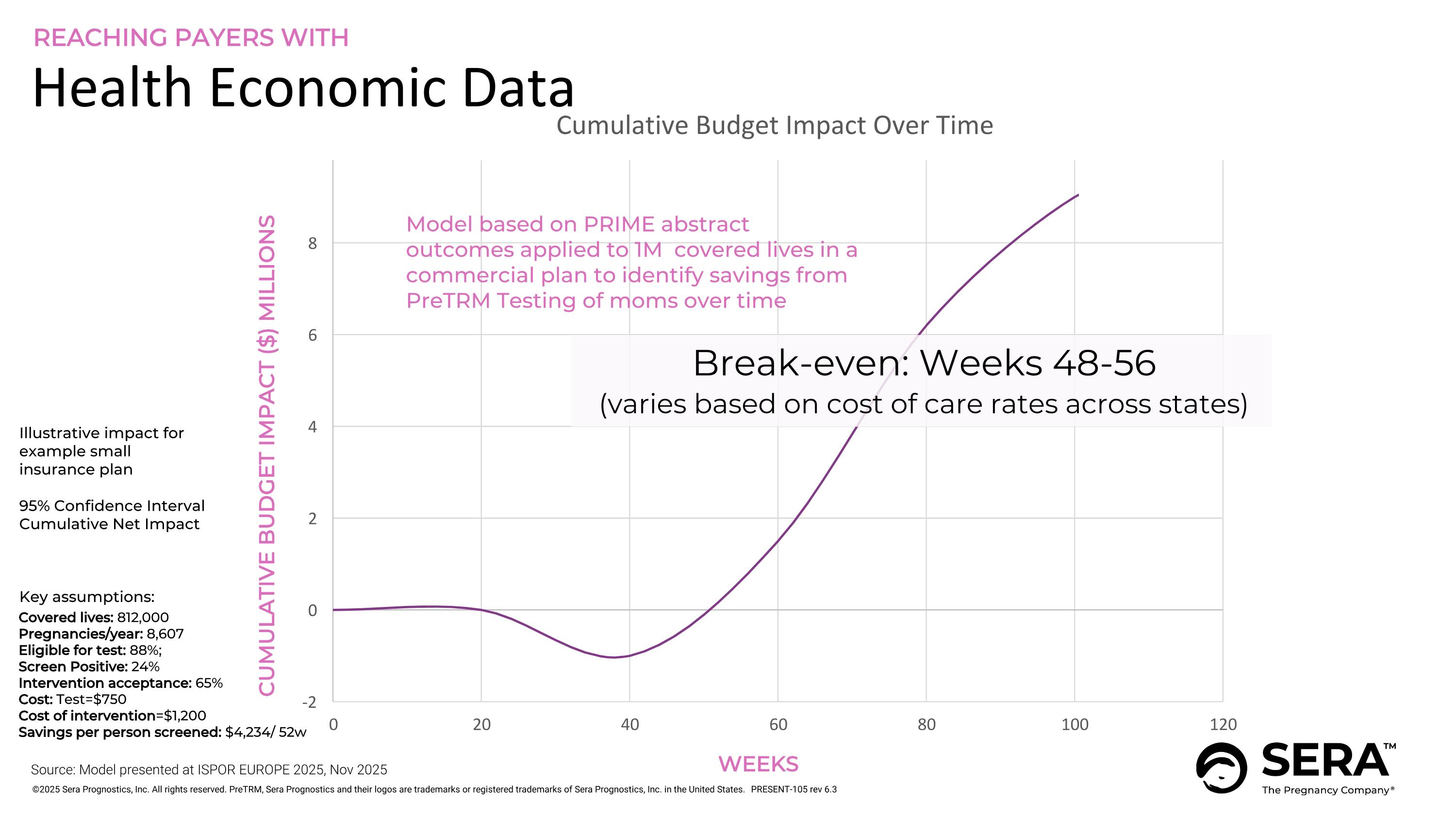
Illustrative impact for example small insurance plan 95% Confidence Interval Cumulative Net Impact Key assumptions: Covered lives: 812,000 Pregnancies/year: 8,607 Eligible for test: 88%; Screen Positive: 24% Intervention acceptance: 65% Cost: Test=$750 Cost of intervention=$1,200 Savings per person screened: $4,234/ 52w Break-even: Weeks 48-56 (varies based on cost of care rates across states) Health Economic Data REACHING PAYERS WITH WEEKS CUMULATIVE BUDGET IMPACT ($) MILLIONS Model based on PRIME abstract outcomes applied to 1M covered lives in a commercial plan to identify savings from PreTRM Testing of moms over time Source: Model presented at ISPOR EUROPE 2025, Nov 2025 PRESENT-105 rev 6.3
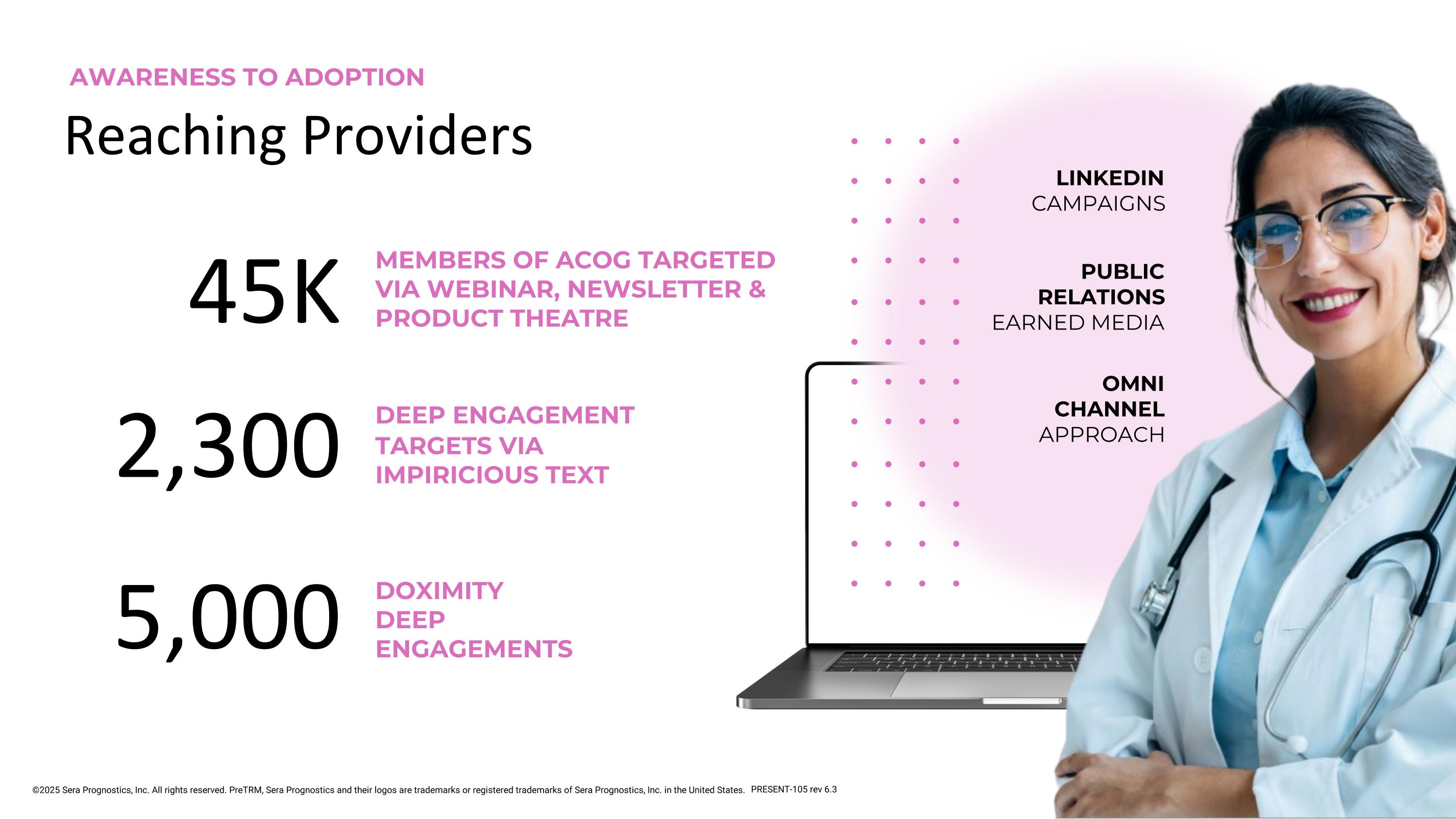
LINKEDIN CAMPAIGNS PUBLIC RELATIONS EARNED MEDIA OMNI CHANNEL APPROACH Reaching Providers MEMBERS OF ACOG TARGETED VIA WEBINAR, NEWSLETTER & PRODUCT THEATRE DOXIMITY DEEP ENGAGEMENTS 5,000 DEEP ENGAGEMENT TARGETS VIA IMPIRICIOUS TEXT AWARENESS TO ADOPTION 2,300 45K ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PRESENT-105 rev 6.3
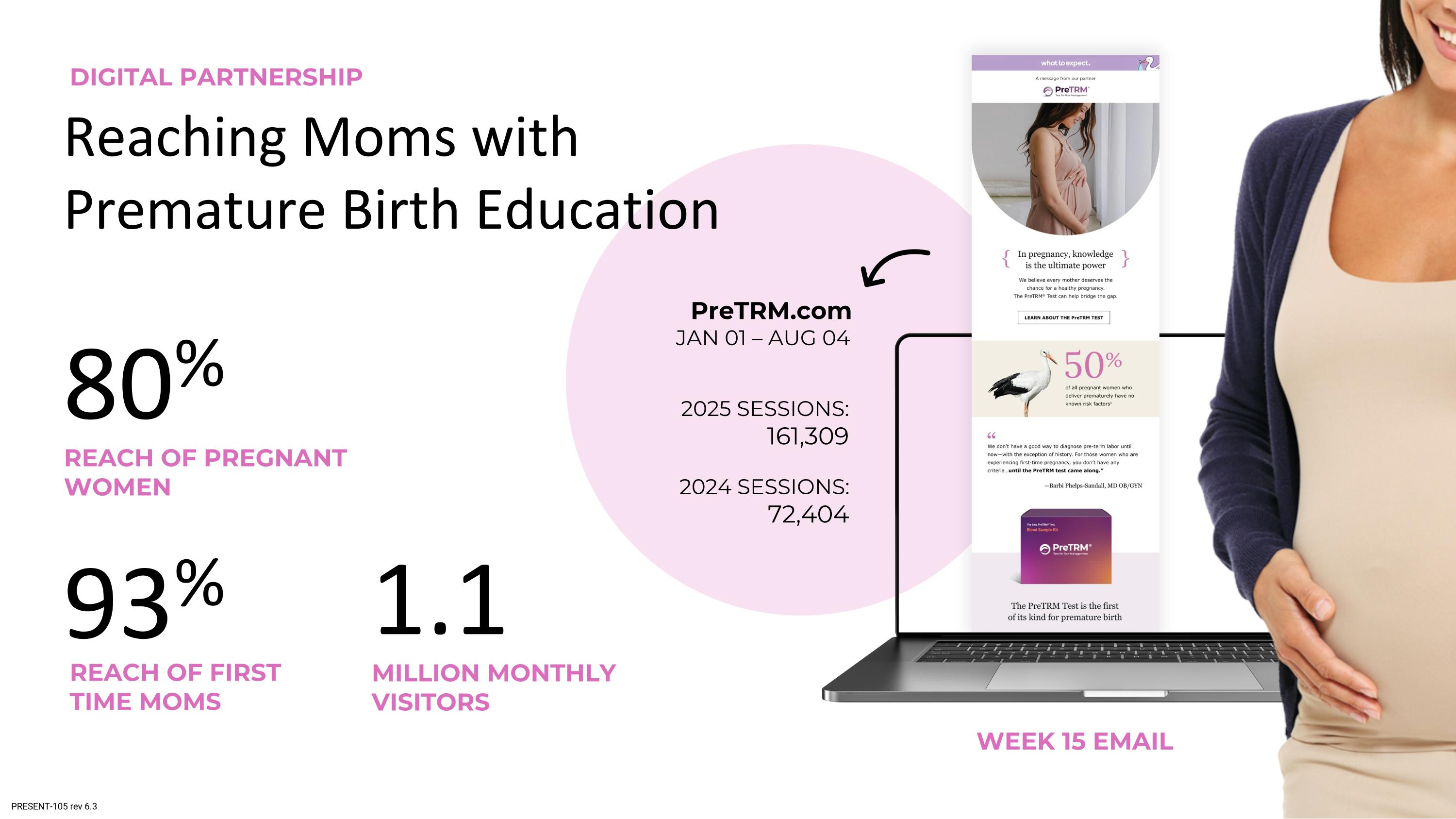
Reaching Moms with Premature Birth Education WEEK 15 EMAIL PreTRM.com JAN 01 – AUG 04 REACH OF PREGNANT WOMEN 80% MILLION MONTHLY VISITORS 1.1 93% REACH OF FIRST TIME MOMS DIGITAL PARTNERSHIP 2025 SESSIONS: 161,309 2024 SESSIONS: 72,404 PRESENT-105 rev 6.3

2026 Milestones Publications Guideline Conversations Commercialization Driving Next Inflection Points Payer Discussions Medicaid Pilot Expansions { ON THE HORIZON } PRESENT-105 rev 6.3

Ushers in a new era of personalized pregnancy care THE PRETRM® TEST

Leadership Team THE PRESENT-105 rev 6.3

Sera Leadership Zhenya Lindgardt President & CEO Former VP of Platform & Customer Engagement, Uber Technologies Former Senior Partner & Managing Director, The Boston Consulting Group Former CEO, The Commons Project Foundation MBA, Harvard University Former VP, Finance & Corporate Controller, Sera Former finance team member, Myriad Former auditor, Ernst & Young LLP Master of Accounting University of Utah; CPA Austin Aerts Chief Financial Officer Former National Precision Oncology General Manager, Commercial team Former VP, US Oncology & Customer Service Lee Anderson Chief Commercial Officer Jay Boniface, Ph.D. Chief Scientific Officer Robert G. Harrison Chief Information Officer Benjamin Jackson General Counsel Michael Foley, M.D. Medical Advisor Paul Kearney, Ph.D Chief Data Officer Angie Fox VP of Clinical Operations Nikki Martin VP of Quality & Regulatory Jennifer Cohrs Head of Marketing Jennifer Zibuda Head of Investor Relations Tiffany Inglis, M.D. Chief Medical Officer

Educate Providers. Empower Moms. HELP Babies. Advance Science. Elevate Care. Reach Maternity Deserts. Inform Payers. Evoke Change. TOGETHER, WE CAN DO BETTER PRESENT-105 rev 6.3