
Meeting the Needs of Patients with CLL and WM – Bexobrutideg Clinical Update from ASH 2025 American Society of Hematology December 8, 2025 .3

This presentation contains statements that relate to future events and expectations and as such constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. When or if used in this presentation, the words “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “outlook,” “plan,” “predict,” “should,” “will,” and similar expressions and their variants, as they relate to Nurix Therapeutics, Inc. (“Nurix”, the “Company,” “we,” “us” or “our”), may identify forward-looking statements. All statements that reflect Nurix’s expectations, assumptions or projections about the future, other than statements of historical fact, are forward- looking statements, including, without limitation, statements regarding our future financial or business plans; our future performance, prospects and strategies; future conditions, trends, and other financial and business matters; our current and prospective drug candidates; the planned timing and conduct of the clinical trial programs for our drug candidates; the planned timing for the provision of updates and findings from our clinical studies; the potential benefits of our collaborations, including potential milestone and sales-related payments; the potential advantages of DEL-AI and our drug candidates; the extent to which our scientific approach, our drug discovery engine, targeted protein degradation, and degrader antibody conjugates may potentially address a broad range of diseases; the extent animal model data predicts human efficacy; the timing and success of the development and commercialization of our current and anticipated drug candidates; and our ability to fund our operations into 2028. Forward- looking statements reflect Nurix’s current beliefs, expectations, and assumptions. Although Nurix believes the expectations and assumptions reflected in such forward-looking statements are reasonable, Nurix can give no assurance that they will prove to be correct. Forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and changes in circumstances that are difficult to predict, which could cause Nurix’s actual activities and results to differ materially from those expressed in any forward-looking statement. Such risks and uncertainties include, but are not limited to: (i) risks and uncertainties related to Nurix’s ability to advance its drug candidates, obtain regulatory approval of and ultimately commercialize its drug candidates; (ii) the timing and results of clinical trials; (iii) Nurix’s ability to fund development activities and achieve development goals; (iv) risks and uncertainties relating to the timing and receipt of payments from Nurix's collaboration partners, including milestone payments and royalties on future potential product sales; (v) the impact of macroeconomic events and conditions, including increasing financial market volatility and uncertainty, inflation, interest rate fluctuations, instability in the global banking system, uncertainty with respect to the federal budget and debt ceiling, the impact of war, military or regional conflicts, and global health pandemics, on Nurix’s clinical trials and operations; (vi) Nurix’s ability to protect intellectual property and (vii) other risks and uncertainties described under the heading “Risk Factors” in Nurix’s Quarterly Report on Form 10-Q for the fiscal quarter ended August 31, 2025, and other SEC filings. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. The statements in this presentation speak only as of the date of this presentation, even if subsequently made available by Nurix on its website or otherwise. Nurix disclaims any intention or obligation to update publicly any forward-looking statements, whether in response to new information, future events, or otherwise, except as required by applicable law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Furthermore, while we believe our own internal estimates and research are reliable, such estimates and research have not been verified by any independent source. 2 Important Notice and Disclaimers
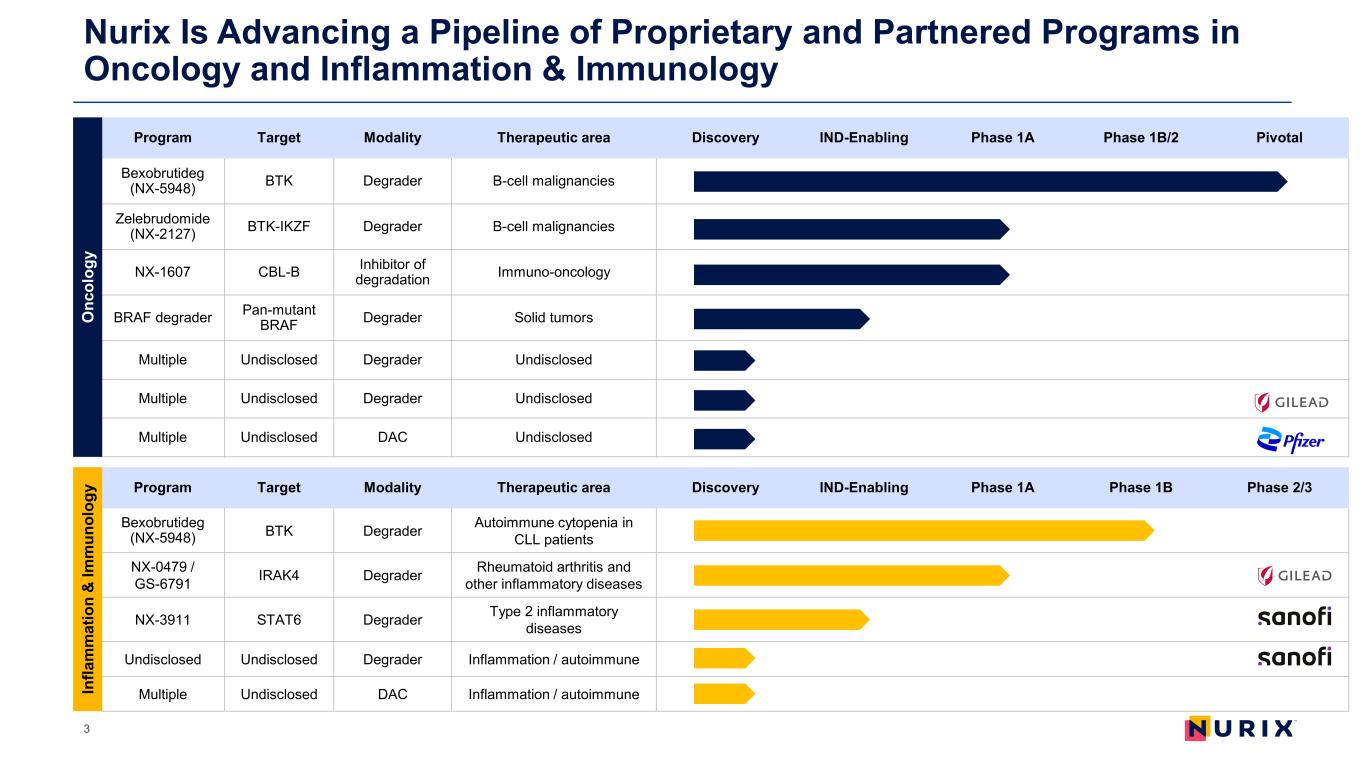
Nurix Is Advancing a Pipeline of Proprietary and Partnered Programs in Oncology and Inflammation & Immunology 3 Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B/2 Pivotal Bexobrutideg (NX-5948) BTK Degrader B-cell malignancies Zelebrudomide (NX-2127) BTK-IKZF Degrader B-cell malignancies NX-1607 CBL-B Inhibitor of degradation Immuno-oncology BRAF degrader Pan-mutant BRAF Degrader Solid tumors Multiple Undisclosed Degrader Undisclosed Multiple Undisclosed Degrader Undisclosed Multiple Undisclosed DAC Undisclosed Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B Phase 2/3 Bexobrutideg (NX-5948) BTK Degrader Autoimmune cytopenia in CLL patients NX-0479 / GS-6791 IRAK4 Degrader Rheumatoid arthritis and other inflammatory diseases NX-3911 STAT6 Degrader Type 2 inflammatory diseases Undisclosed Undisclosed Degrader Inflammation / autoimmune Multiple Undisclosed DAC Inflammation / autoimmune O nc ol og y In fla m m at io n & Im m un ol og y
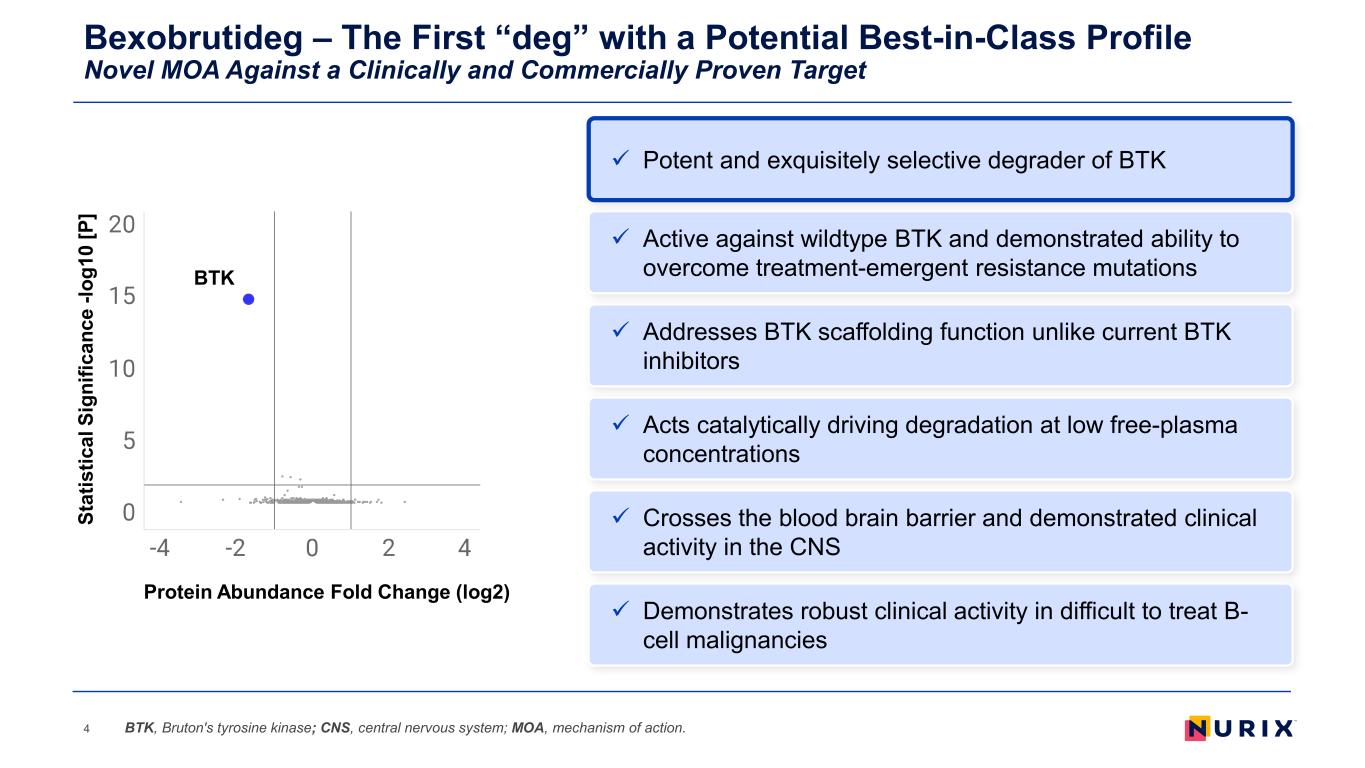
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 4 Addresses BTK scaffolding function unlike current BTK inhibitors Acts catalytically driving degradation at low free-plasma concentrations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Active against wildtype BTK and demonstrated ability to overcome treatment-emergent resistance mutations Crosses the blood brain barrier and demonstrated clinical activity in the CNS BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Potent and exquisitely selective degrader of BTK BTK St at is tic al S ig ni fic an ce -l og 10 [P ] Protein Abundance Fold Change (log2)
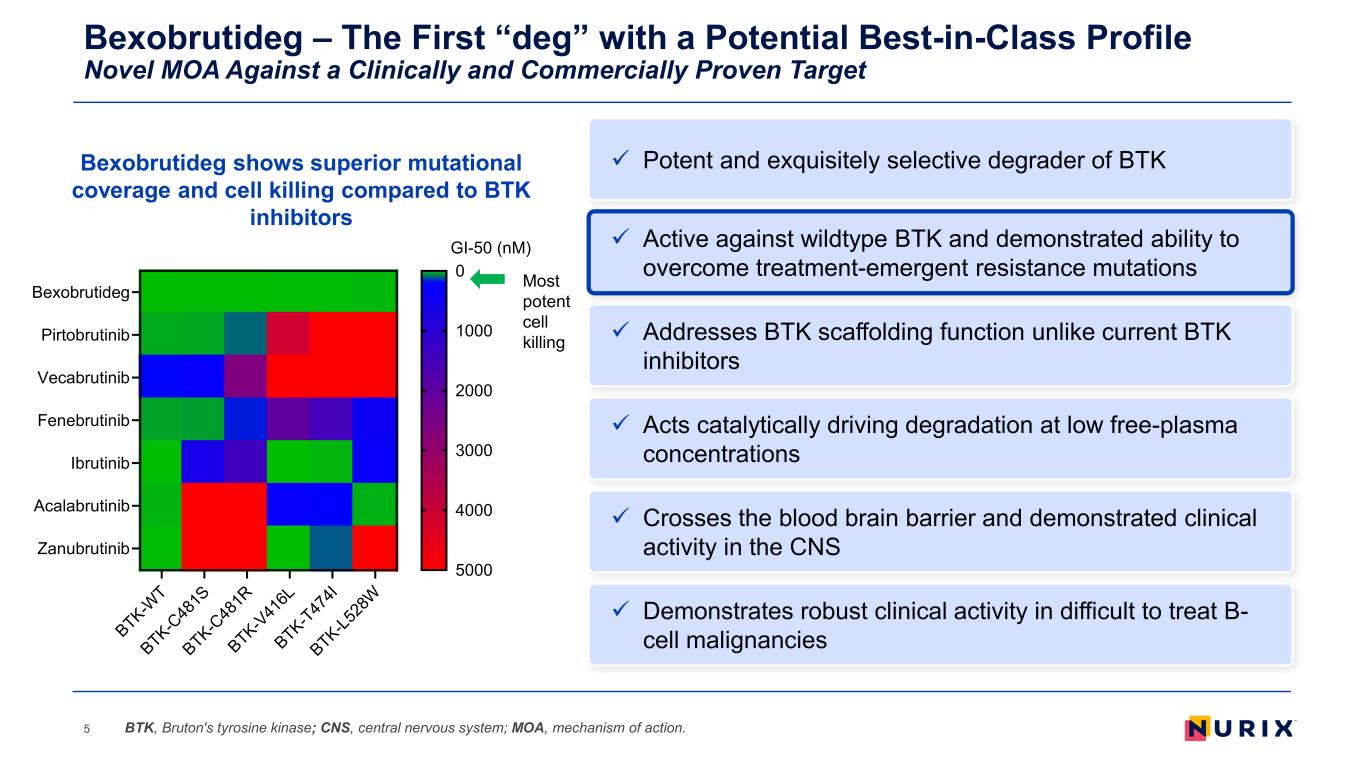
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 5 Addresses BTK scaffolding function unlike current BTK inhibitors Acts catalytically driving degradation at low free-plasma concentrations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Active against wildtype BTK and demonstrated ability to overcome treatment-emergent resistance mutations Crosses the blood brain barrier and demonstrated clinical activity in the CNS BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Potent and exquisitely selective degrader of BTKBexobrutideg shows superior mutational coverage and cell killing compared to BTK inhibitors Most potent cell killing BTK-W T BTK-C 48 1S BTK-C 48 1R BTK-V 41 6L BTK-T47 4I BTK-L5 28 W Bexobrutideg Pirtobrutinib Vecabrutinib Fenebrutinib Ibrutinib Acalabrutinib Zanubrutinib GI-50 (nM) 0 1000 2000 3000 4000 5000
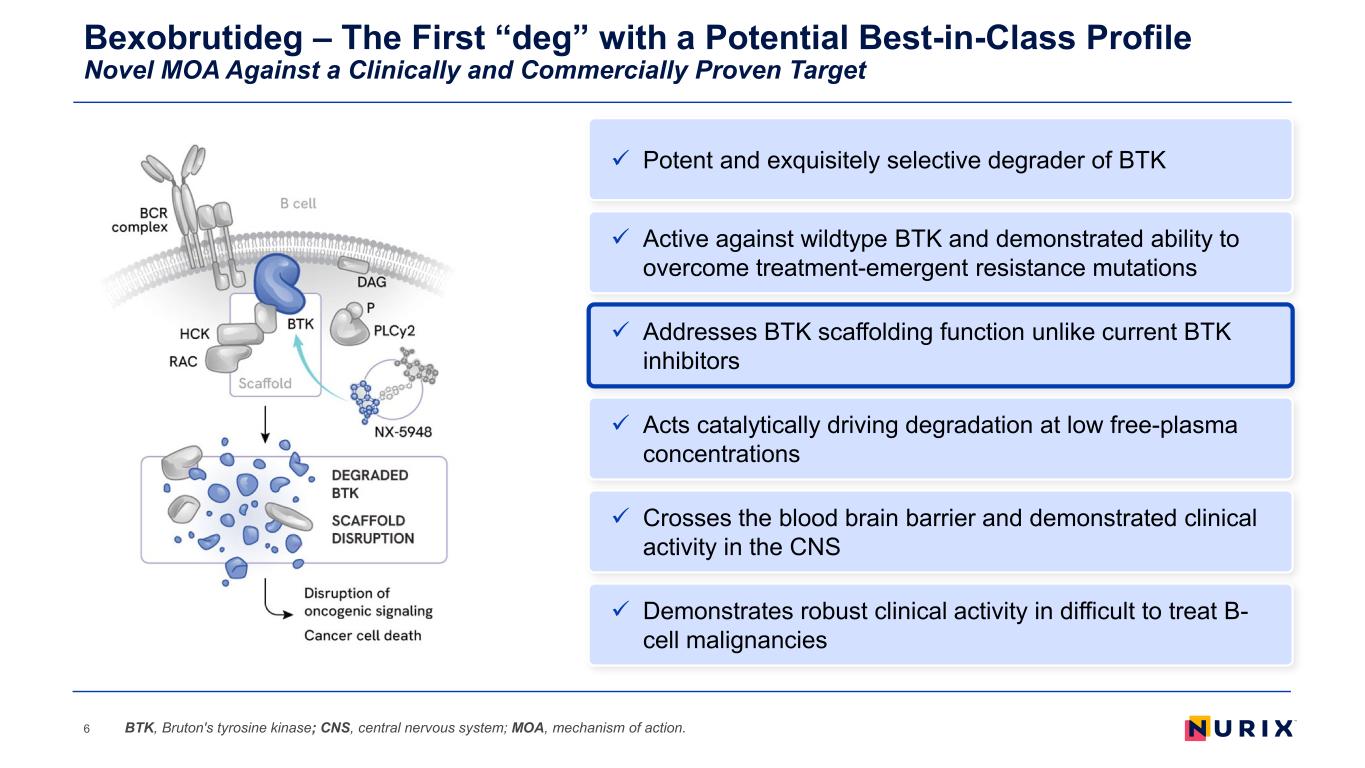
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 6 Addresses BTK scaffolding function unlike current BTK inhibitors Acts catalytically driving degradation at low free-plasma concentrations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Active against wildtype BTK and demonstrated ability to overcome treatment-emergent resistance mutations Crosses the blood brain barrier and demonstrated clinical activity in the CNS BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Potent and exquisitely selective degrader of BTK
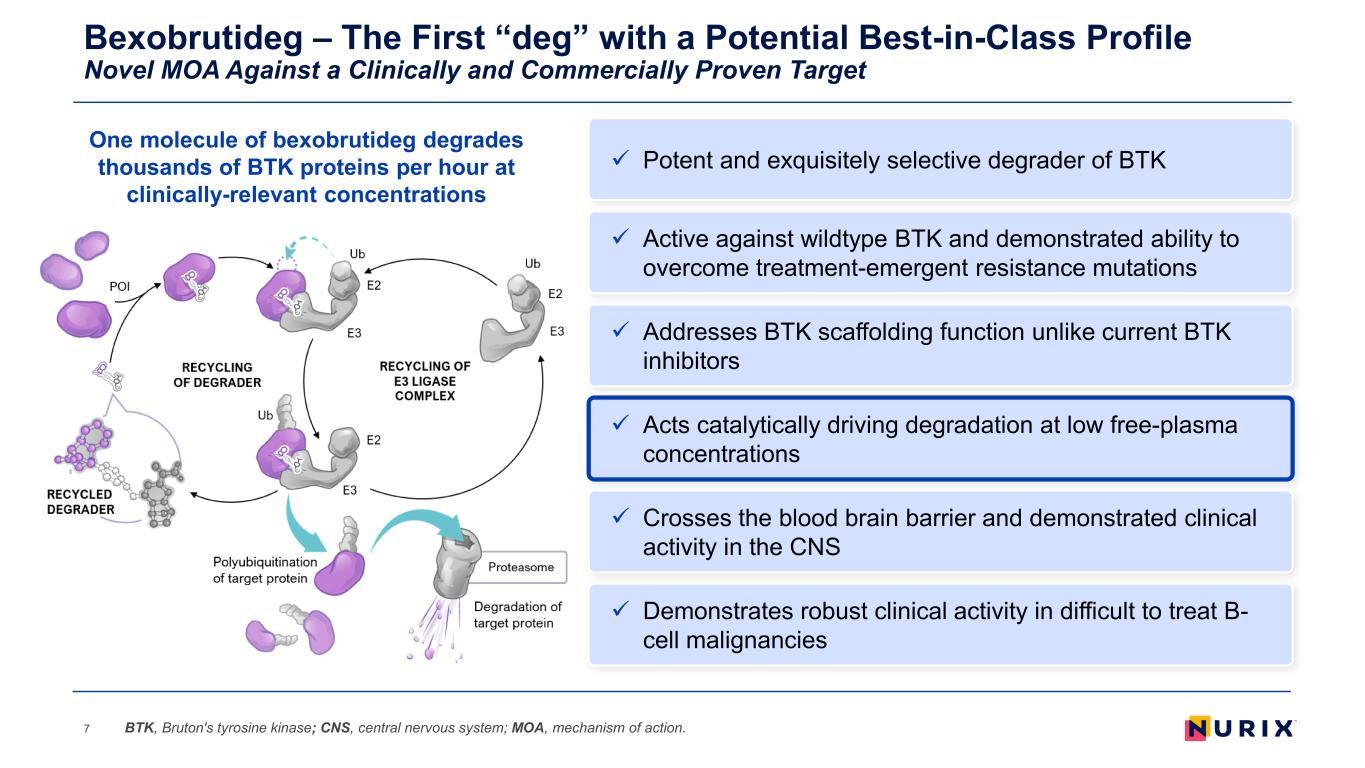
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 7 Addresses BTK scaffolding function unlike current BTK inhibitors Acts catalytically driving degradation at low free-plasma concentrations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Active against wildtype BTK and demonstrated ability to overcome treatment-emergent resistance mutations Crosses the blood brain barrier and demonstrated clinical activity in the CNS BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Potent and exquisitely selective degrader of BTK One molecule of bexobrutideg degrades thousands of BTK proteins per hour at clinically-relevant concentrations
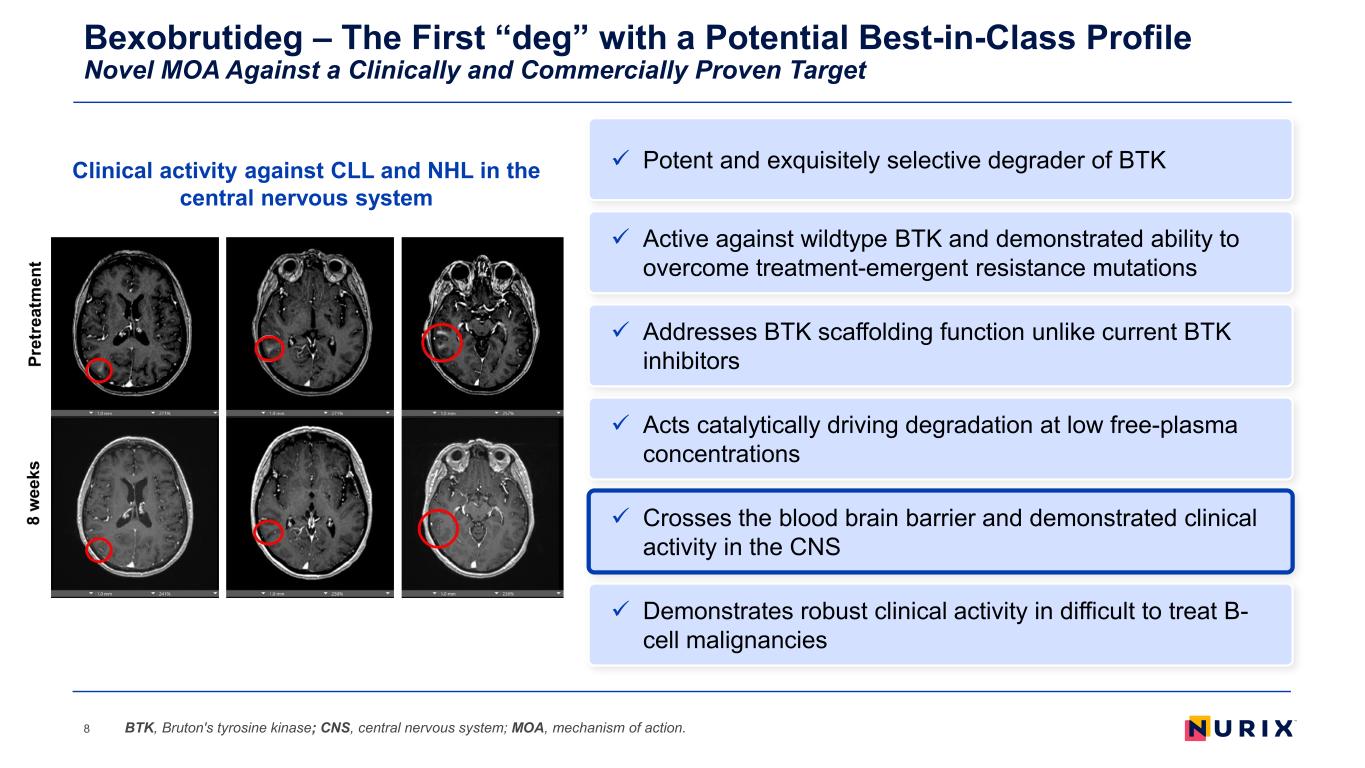
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 8 Addresses BTK scaffolding function unlike current BTK inhibitors Acts catalytically driving degradation at low free-plasma concentrations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Active against wildtype BTK and demonstrated ability to overcome treatment-emergent resistance mutations Crosses the blood brain barrier and demonstrated clinical activity in the CNS BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Potent and exquisitely selective degrader of BTKClinical activity against CLL and NHL in the central nervous system
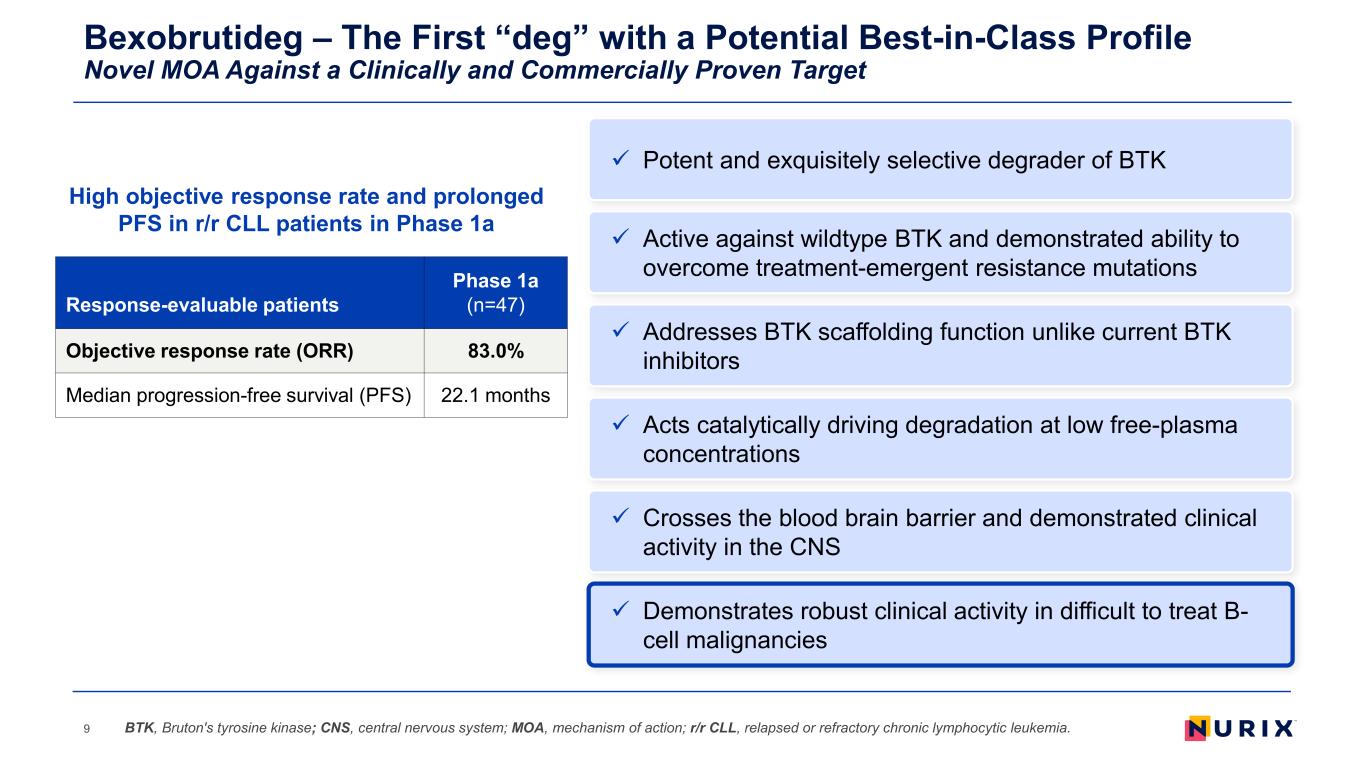
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 9 Addresses BTK scaffolding function unlike current BTK inhibitors Acts catalytically driving degradation at low free-plasma concentrations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Active against wildtype BTK and demonstrated ability to overcome treatment-emergent resistance mutations Crosses the blood brain barrier and demonstrated clinical activity in the CNS BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action; r/r CLL, relapsed or refractory chronic lymphocytic leukemia. Potent and exquisitely selective degrader of BTK High objective response rate and prolonged PFS in r/r CLL patients in Phase 1a Response-evaluable patients Phase 1a (n=47) Objective response rate (ORR) 83.0% Median progression-free survival (PFS) 22.1 months
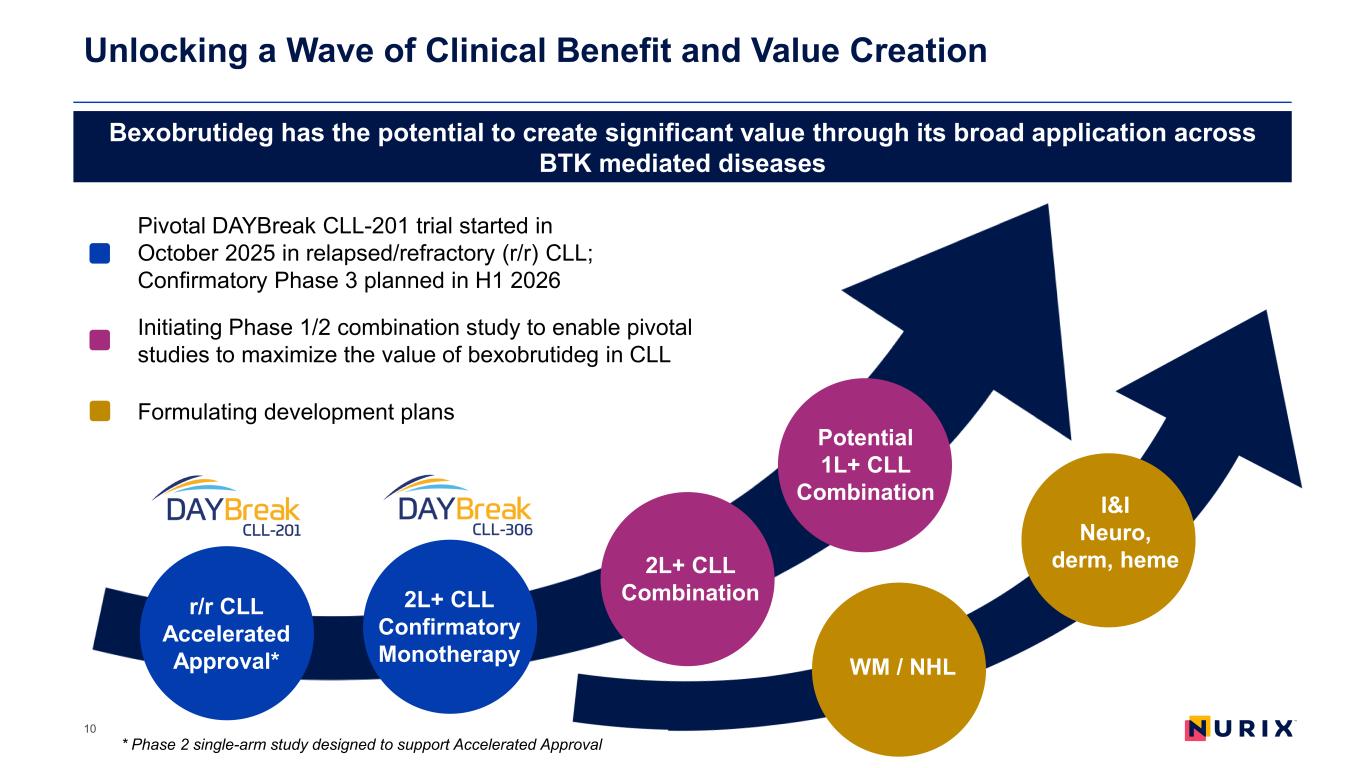
Unlocking a Wave of Clinical Benefit and Value Creation 10 r/r CLL Accelerated Approval* 2L+ CLL Confirmatory Monotherapy 2L+ CLL Combination Potential 1L+ CLL Combination Bexobrutideg has the potential to create significant value through its broad application across BTK mediated diseases Pivotal DAYBreak CLL-201 trial started in October 2025 in relapsed/refractory (r/r) CLL; Confirmatory Phase 3 planned in H1 2026 Initiating Phase 1/2 combination study to enable pivotal studies to maximize the value of bexobrutideg in CLL Formulating development plans I&I Neuro, derm, heme WM / NHL * Phase 2 single-arm study designed to support Accelerated Approval
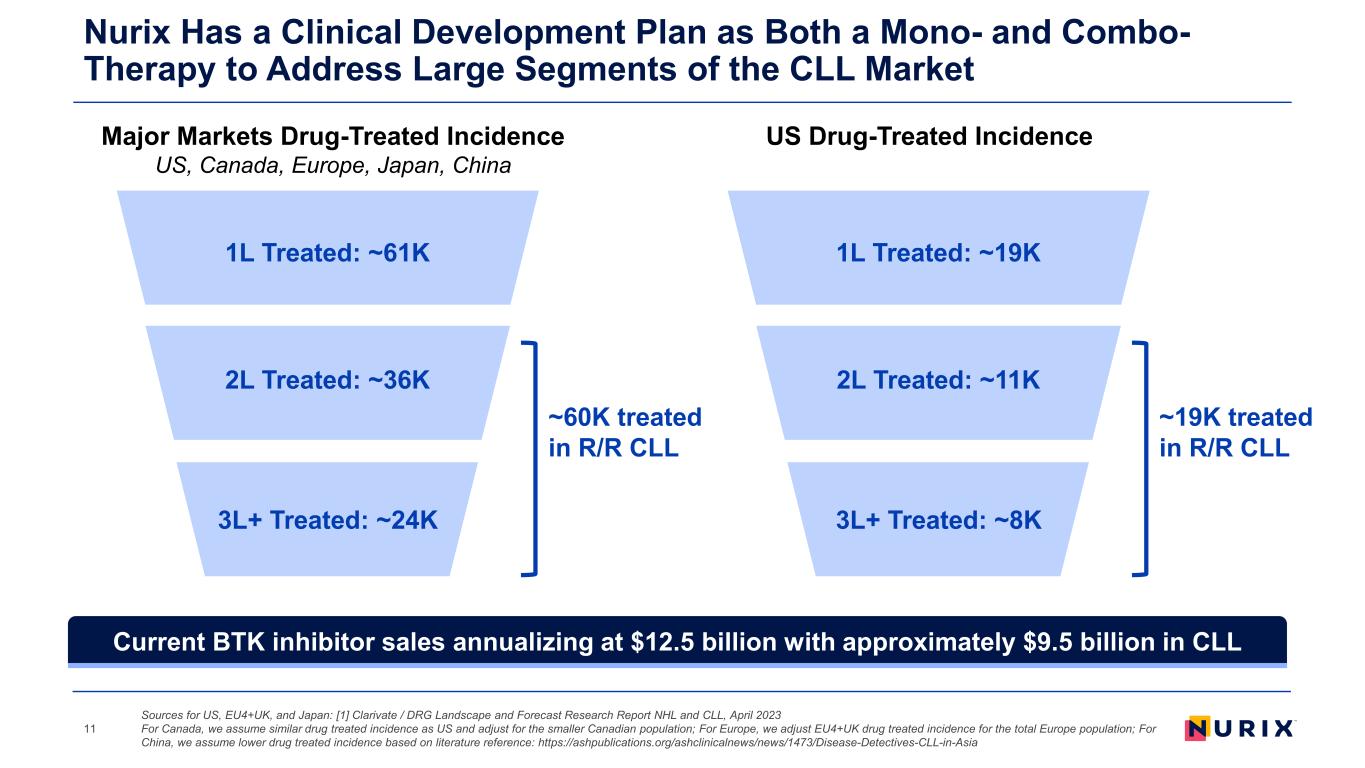
1L Treated: ~61K 2L Treated: ~36K 3L+ Treated: ~24K ~60K treated in R/R CLL Nurix Has a Clinical Development Plan as Both a Mono- and Combo- Therapy to Address Large Segments of the CLL Market 11 1L Treated: ~19K 2L Treated: ~11K 3L+ Treated: ~8K ~19K treated in R/R CLL Major Markets Drug-Treated Incidence US, Canada, Europe, Japan, China US Drug-Treated Incidence Sources for US, EU4+UK, and Japan: [1] Clarivate / DRG Landscape and Forecast Research Report NHL and CLL, April 2023 For Canada, we assume similar drug treated incidence as US and adjust for the smaller Canadian population; For Europe, we adjust EU4+UK drug treated incidence for the total Europe population; For China, we assume lower drug treated incidence based on literature reference: https://ashpublications.org/ashclinicalnews/news/1473/Disease-Detectives-CLL-in-Asia Current BTK inhibitor sales annualizing at $12.5 billion with approximately $9.5 billion in CLL

ASH 2025: Two Clinical Updates for Bexobrutideg 12 Saturday, December 6, 9:45 a.m. – 10:00 a.m. ET Bexobrutideg (NX-5948), a Novel Bruton’s Tyrosine Kinase (BTK) Degrader, Demonstrates Rapid and Durable Clinical Responses in Relapsed / Refractory Chronic Lymphocytic Leukemia (CLL): New and Updated Findings from an Ongoing Phase 1a/b Trial Presenting author: Zulfa Omer, M.D. Abstract # 86 ORAL SESSION I Session title: Chronic Lymphocytic Leukemia: Clinical and Epidemiological: Treatment of CLL in Relapse and in Richter Transformation Monday, December 8, 2025, 6:00 p.m. – 8:00 p.m. ET Bexobrutideg (NX-5948), a Novel Bruton’s Tyrosine Kinase (BTK) Degrader, Shows High Clinical Activity and Tolerable Safety in Patients with Waldenström Macroglobulinemia: Updated Results from an Ongoing Phase 1a/b Study Presenting author: Scott Huntington M.D., MPH Abstract # 5359 POSTER SESSION III Session title: 623. Mantle Cell, Follicular, Waldenstrom's, and Other Indolent B Cell Lymphomas: Clinical and Epidemiological:
Investor Call Agenda 13 Bexobrutideg (NX-5948), a Novel Bruton’s Tyrosine Kinase (BTK) Degrader, Demonstrates Rapid and Durable Clinical Responses in Relapsed / Refractory Chronic Lymphocytic Leukemia (CLL): New and Updated Findings from an Ongoing Phase 1a/b Trial Bexobrutideg (NX-5948), a Novel Bruton’s Tyrosine Kinase (BTK) Degrader, Shows High Clinical Activity and Tolerable Safety in Patients with Waldenström Macroglobulinemia: Updated Results from an Ongoing Phase 1a/b Study Q&A to follow Paula G. O’Connor, M.D. Chief Medical Officer, Nurix Therapeutics Bexobrutideg Program Updates and Next Steps Arthur T. Sands, M.D., Ph.D. Chief Executive Officer, Nurix Therapeutics 2025 Highlights and 2026 Preview Q&A 01 02 03 Alvaro Alencar, M.D. University of Miami Sylvester Cancer Center

Bexobrutideg (NX-5948), a novel Bruton’s tyrosine kinase (BTK) degrader, demonstrates rapid and durable clinical responses in relapsed/refractory chronic lymphocytic leukemia (CLL): New and updated findings from an ongoing Phase 1a/b trial 1Zulfa Omer, 2Alexey Danilov, 3Francesco Forconi, 4Talha Munir, 5,6Mary Gleeson, 7Nirav N. Shah, 8Graham P. Collins, 9Alvaro Alencar, 10Jane Robertson, 11Jonathon B. Cohen, 12Karan Dixit, 13Danielle Brander, 1John C. Byrd, 14Allison Winter, 15Jeffery Smith, 16Dima El-Sharkawi, 17Michal Kwiatek, 18Iwona Hus, 19Prioty Islam, 20Sebastian Grosicki, 21Michael Tees, 22Thorsten Zenz, 23Joanna Romejko-Jarosinska, 24Sarah Injac, 25Wojciech Jurczak 1University of Cincinnati, Cincinnati, OH, USA; 2City of Hope National Medical Center, Duarte, CA, USA; 3University Hospital Southampton NHS Trust, Southampton, UK; 4St James’s Hospital, Leeds, UK; 5Guy’s and St Thomas’ NHS Foundation Trust, London, UK; 6Sarah Cannon Research Institute, London, UK; 7Medical College of Wisconsin, Milwaukee, WI, USA; 8Oxford Cancer and Haematology Centre, Churchill Hospital, Oxford, UK; 9Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, FL, USA; 10The Christie Hospital NHS Foundation Trust, Manchester, UK; 11Emory University Winship Cancer Institute, Atlanta, GA, USA; 12Feinberg School of Medicine, Northwestern University, Chicago, IL, USA; 13Duke Cancer Institute, Durham, NC, USA; 14Cleveland Clinic Foundation, Cleveland, OH, USA; 15The Clatterbridge Cancer Centre, Liverpool, UK; 16Royal Marsden NHS Foundation Trust, Sutton, UK; 17AidPort Hospital, Skórzewo (Poznań), Poland; 18Medical University of Lublin, Lublin, Poland; 19Memorial Sloan Kettering Cancer Center, New York, NY, USA; 20Medical University of Silesia, Katowice, Poland; 21Colorado Blood Cancer Institute/Sarah Cannon Research Institute, Denver, CO, USA; 22Department of Medical Oncology and Hematology, University of Zurich & University Hospital Zurich, Zurich, Switzerland; 23Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland; 24Nurix Therapeutics, Inc., San Francisco, CA, USA; 25Maria Sklodowska-Curie National Research Institute of Oncology, Kraków, Poland ASH 2025 Annual Meeting, Orlando, FL, USA, 6–9 December 2025
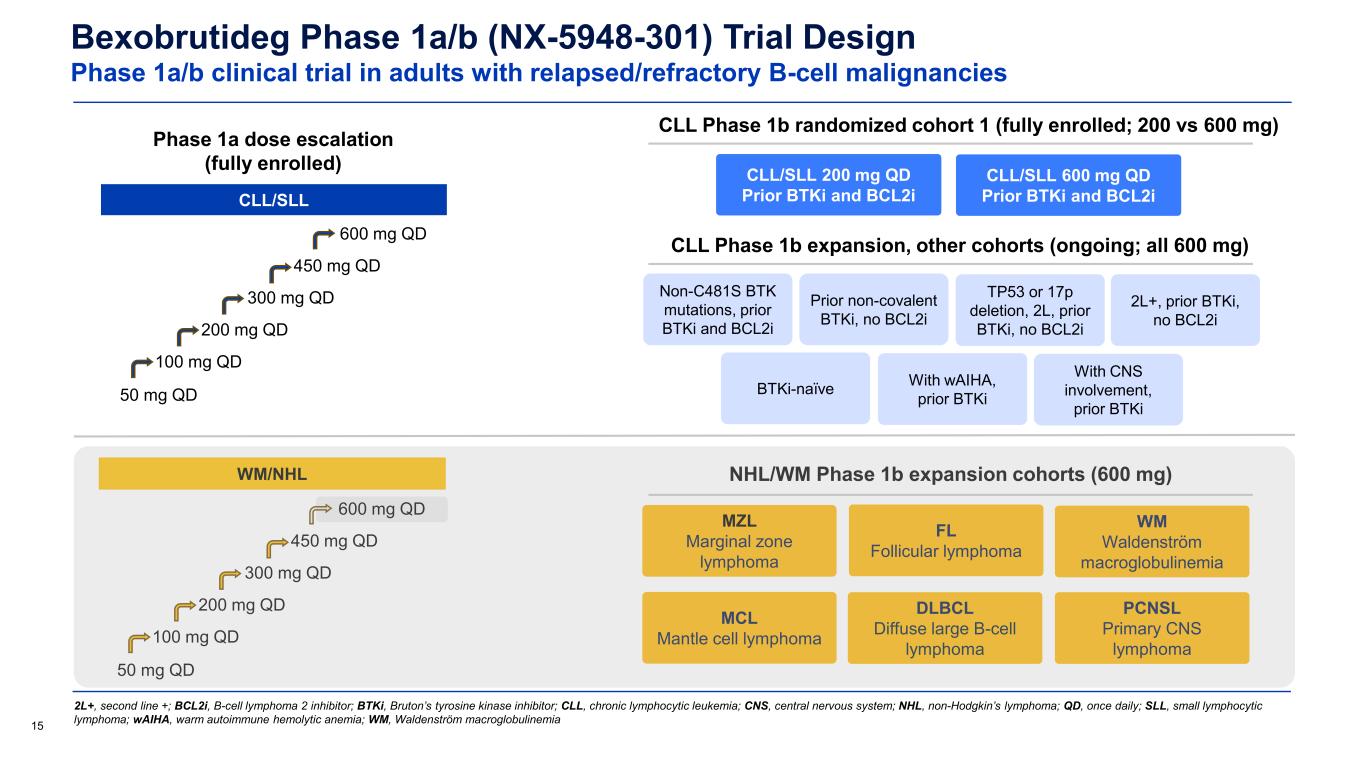
Bexobrutideg Phase 1a/b (NX-5948-301) Trial Design Phase 1a/b clinical trial in adults with relapsed/refractory B-cell malignancies 15 2L+, second line +; BCL2i, B-cell lymphoma 2 inhibitor; BTKi, Bruton’s tyrosine kinase inhibitor; CLL, chronic lymphocytic leukemia; CNS, central nervous system; NHL, non-Hodgkin’s lymphoma; QD, once daily; SLL, small lymphocytic lymphoma; wAIHA, warm autoimmune hemolytic anemia; WM, Waldenström macroglobulinemia Phase 1a dose escalation (fully enrolled) CLL/SLL CLL Phase 1b randomized cohort 1 (fully enrolled; 200 vs 600 mg) CLL Phase 1b expansion, other cohorts (ongoing; all 600 mg) Non-C481S BTK mutations, prior BTKi and BCL2i Prior non-covalent BTKi, no BCL2i TP53 or 17p deletion, 2L, prior BTKi, no BCL2i 2L+, prior BTKi, no BCL2i BTKi-naïve With wAIHA, prior BTKi With CNS involvement, prior BTKi WM/NHL 50 mg QD 100 mg QD 200 mg QD 300 mg QD 450 mg QD WM Waldenström macroglobulinemia MZL Marginal zone lymphoma FL Follicular lymphoma DLBCL Diffuse large B-cell lymphoma MCL Mantle cell lymphoma CLL/SLL 200 mg QD Prior BTKi and BCL2i CLL/SLL 600 mg QD Prior BTKi and BCL2i 50 mg QD 100 mg QD 200 mg QD 300 mg QD 450 mg QD 600 mg QD NHL/WM Phase 1b expansion cohorts (600 mg) PCNSL Primary CNS lymphoma 600 mg QD
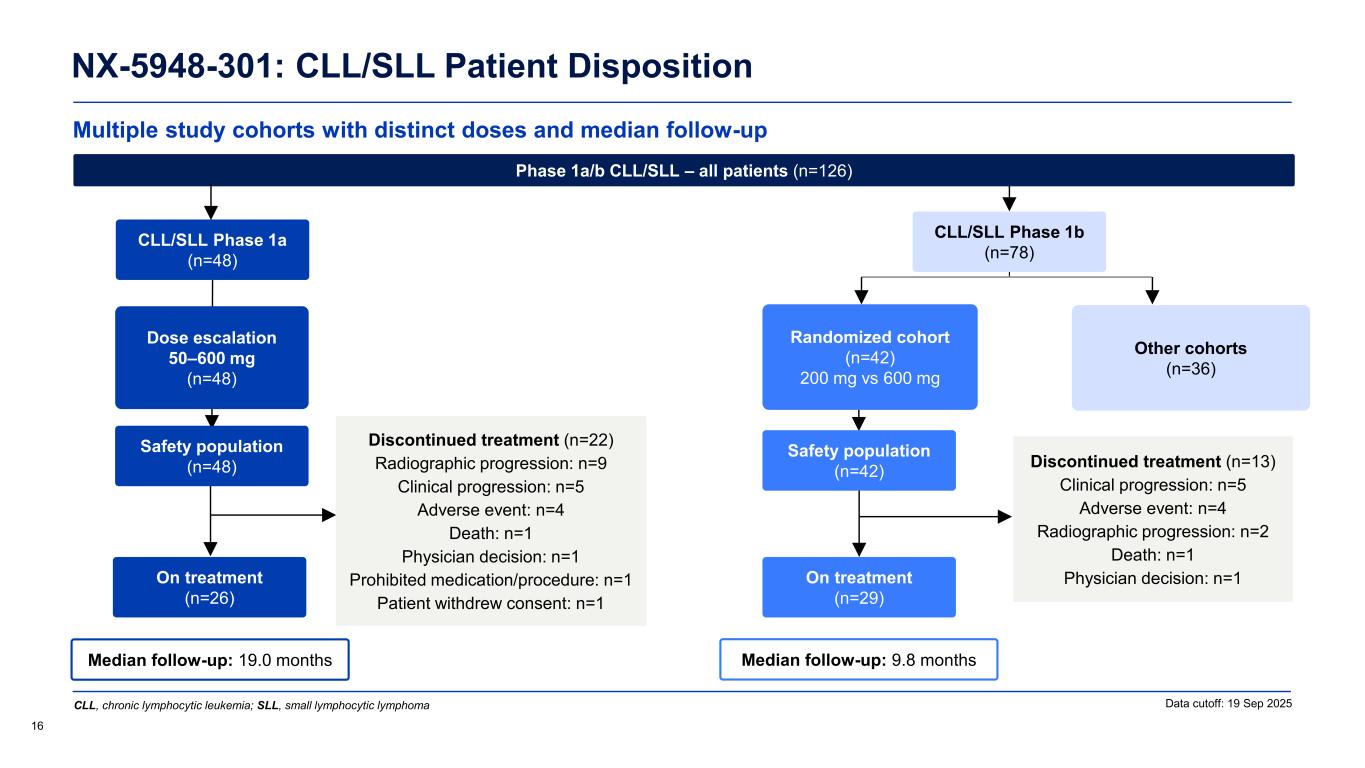
Multiple study cohorts with distinct doses and median follow-up NX-5948-301: CLL/SLL Patient Disposition 16 Data cutoff: 19 Sep 2025 Median follow-up: 9.8 months Safety population (n=42) On treatment (n=29) Randomized cohort (n=42) 200 mg vs 600 mg Other cohorts (n=36) Median follow-up: 19.0 months CLL/SLL Phase 1a (n=48) Safety population (n=48) On treatment (n=26) Dose escalation 50–600 mg (n=48) Discontinued treatment (n=22) Radiographic progression: n=9 Clinical progression: n=5 Adverse event: n=4 Death: n=1 Physician decision: n=1 Prohibited medication/procedure: n=1 Patient withdrew consent: n=1 Phase 1a/b CLL/SLL – all patients (n=126) Discontinued treatment (n=13) Clinical progression: n=5 Adverse event: n=4 Radiographic progression: n=2 Death: n=1 Physician decision: n=1 CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma CLL/SLL Phase 1b (n=78)
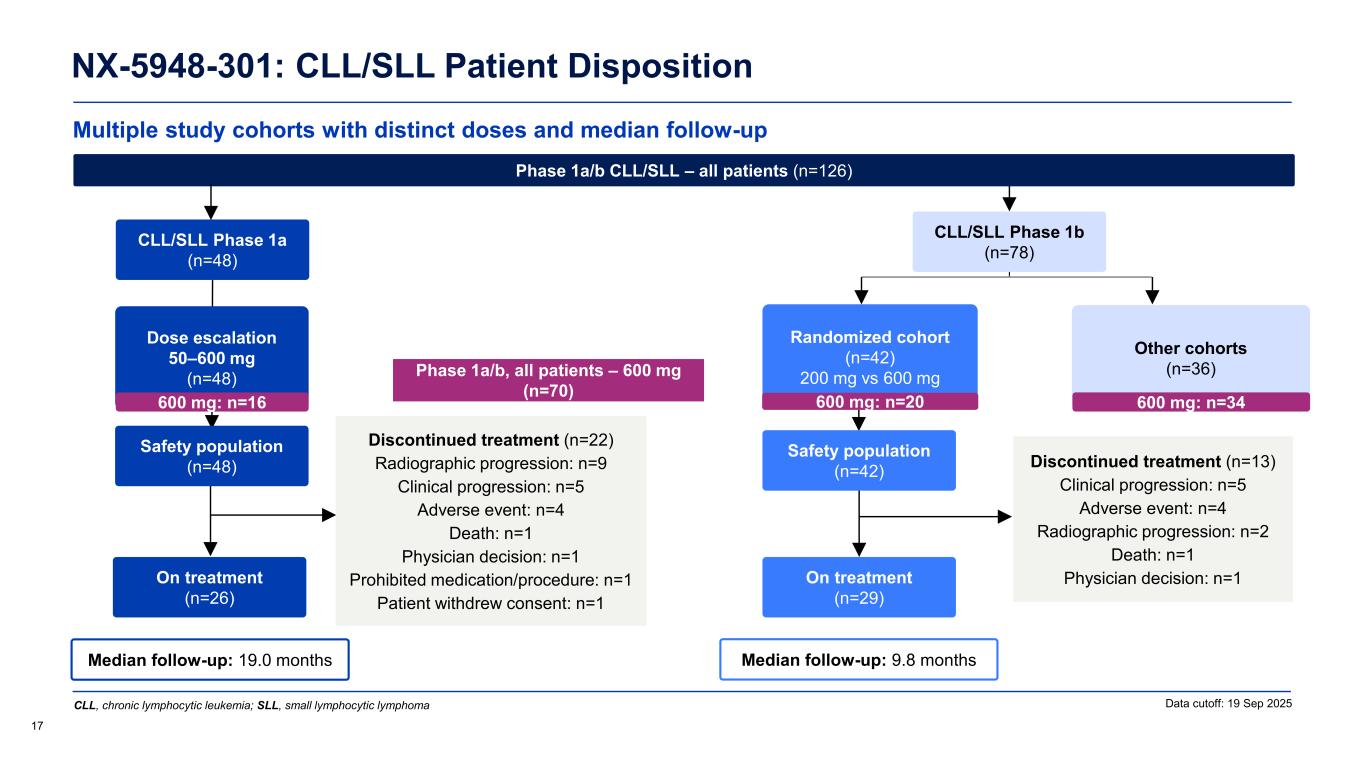
Multiple study cohorts with distinct doses and median follow-up NX-5948-301: CLL/SLL Patient Disposition 17 Data cutoff: 19 Sep 2025 Phase 1a/b, all patients – 600 mg (n=70) Median follow-up: 9.8 months Safety population (n=42) On treatment (n=29) Randomized cohort (n=42) 200 mg vs 600 mg Other cohorts (n=36) Median follow-up: 19.0 months CLL/SLL Phase 1a (n=48) Safety population (n=48) On treatment (n=26) Dose escalation 50–600 mg (n=48) Discontinued treatment (n=22) Radiographic progression: n=9 Clinical progression: n=5 Adverse event: n=4 Death: n=1 Physician decision: n=1 Prohibited medication/procedure: n=1 Patient withdrew consent: n=1 Phase 1a/b CLL/SLL – all patients (n=126) Discontinued treatment (n=13) Clinical progression: n=5 Adverse event: n=4 Radiographic progression: n=2 Death: n=1 Physician decision: n=1 CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma 600 mg: n=16 600 mg: n=20 600 mg: n=34 CLL/SLL Phase 1b (n=78)
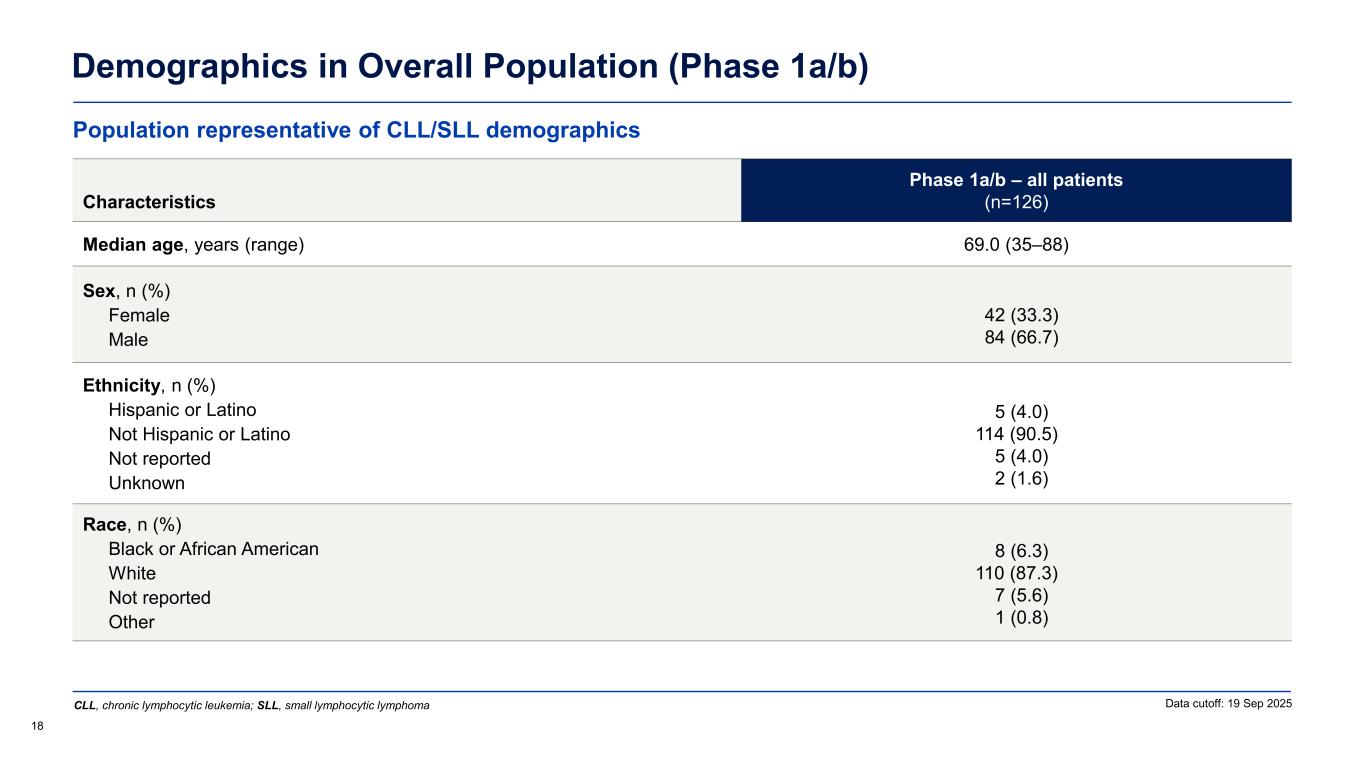
Demographics in Overall Population (Phase 1a/b) 18 Data cutoff: 19 Sep 2025 Characteristics Phase 1a/b – all patients (n=126) Median age, years (range) 69.0 (35–88) Sex, n (%) Female Male 42 (33.3) 84 (66.7) Ethnicity, n (%) Hispanic or Latino Not Hispanic or Latino Not reported Unknown 5 (4.0) 114 (90.5) 5 (4.0) 2 (1.6) Race, n (%) Black or African American White Not reported Other 8 (6.3) 110 (87.3) 7 (5.6) 1 (0.8) Population representative of CLL/SLL demographics CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma
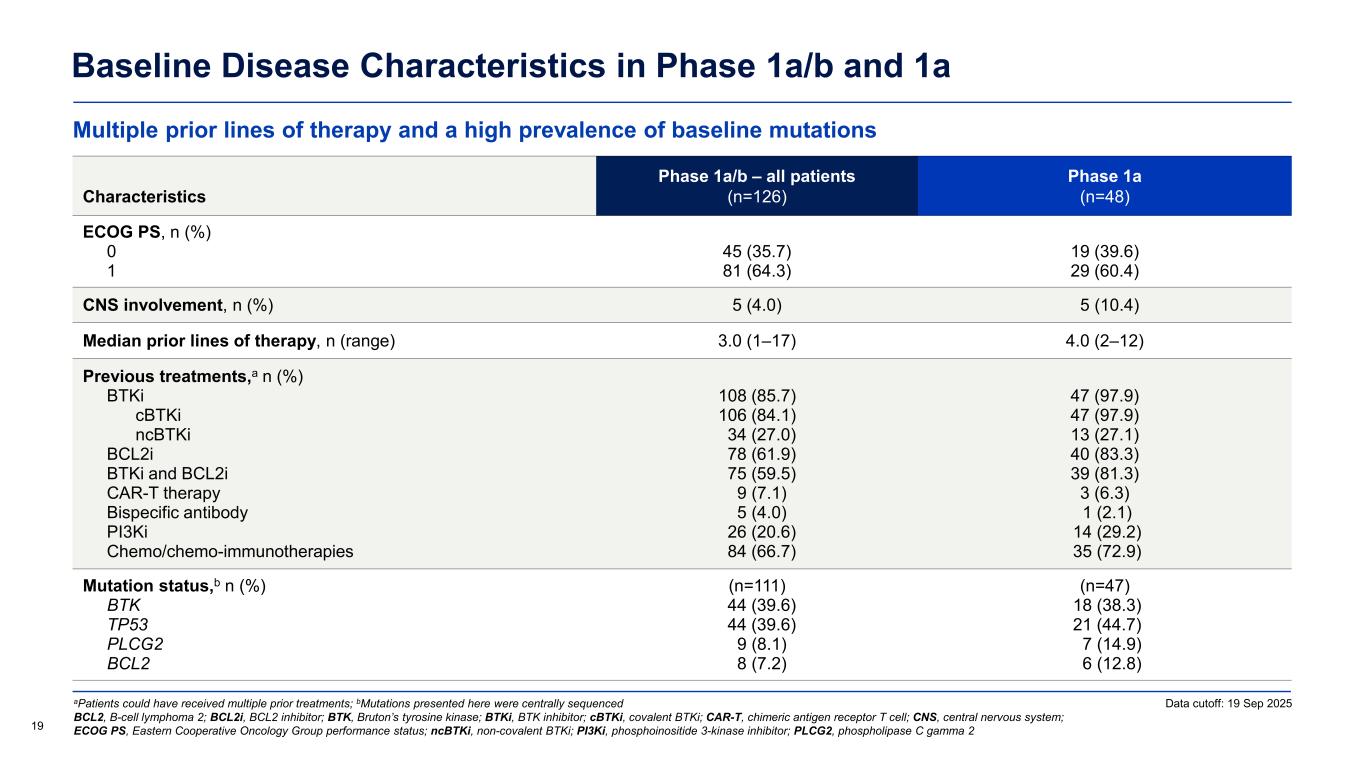
Multiple prior lines of therapy and a high prevalence of baseline mutations Baseline Disease Characteristics in Phase 1a/b and 1a aPatients could have received multiple prior treatments; bMutations presented here were centrally sequenced BCL2, B-cell lymphoma 2; BCL2i, BCL2 inhibitor; BTK, Bruton’s tyrosine kinase; BTKi, BTK inhibitor; cBTKi, covalent BTKi; CAR-T, chimeric antigen receptor T cell; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; ncBTKi, non-covalent BTKi; PI3Ki, phosphoinositide 3-kinase inhibitor; PLCG2, phospholipase C gamma 2 Characteristics Phase 1a/b – all patients (n=126) Phase 1a (n=48) ECOG PS, n (%) 0 1 45 (35.7) 81 (64.3) 19 (39.6) 29 (60.4) CNS involvement, n (%) 5 (4.0) 5 (10.4) Median prior lines of therapy, n (range) 3.0 (1–17) 4.0 (2–12) Previous treatments,a n (%) BTKi cBTKi ncBTKi BCL2i BTKi and BCL2i CAR-T therapy Bispecific antibody PI3Ki Chemo/chemo-immunotherapies 108 (85.7) 106 (84.1) 34 (27.0) 78 (61.9) 75 (59.5) 9 (7.1) 5 (4.0) 26 (20.6) 84 (66.7) 47 (97.9) 47 (97.9) 13 (27.1) 40 (83.3) 39 (81.3) 3 (6.3) 1 (2.1) 14 (29.2) 35 (72.9) Mutation status,b n (%) BTK TP53 PLCG2 BCL2 (n=111) 44 (39.6) 44 (39.6) 9 (8.1) 8 (7.2) (n=47) 18 (38.3) 21 (44.7) 7 (14.9) 6 (12.8) 19 Data cutoff: 19 Sep 2025
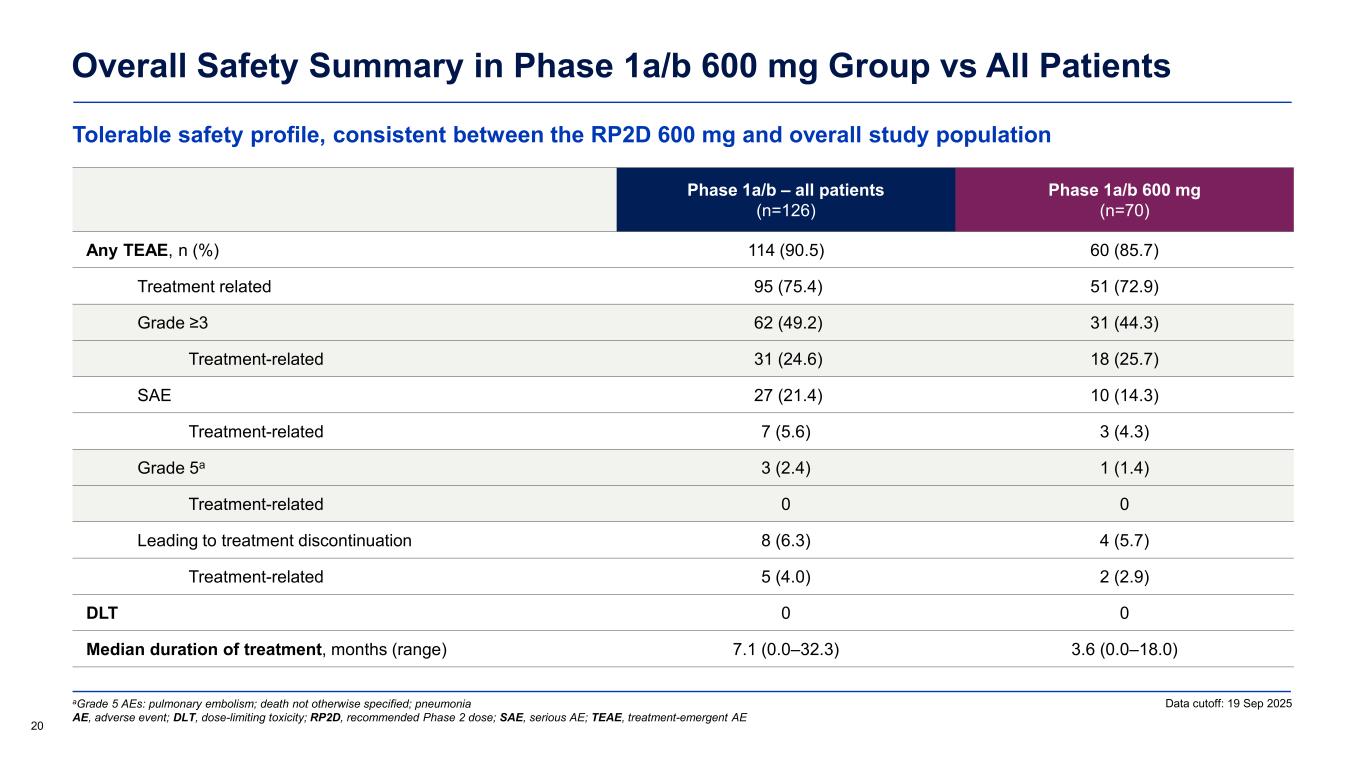
Tolerable safety profile, consistent between the RP2D 600 mg and overall study population Overall Safety Summary in Phase 1a/b 600 mg Group vs All Patients 20 Phase 1a/b – all patients (n=126) Phase 1a/b 600 mg (n=70) Any TEAE, n (%) 114 (90.5) 60 (85.7) Treatment related 95 (75.4) 51 (72.9) Grade ≥3 62 (49.2) 31 (44.3) Treatment-related 31 (24.6) 18 (25.7) SAE 27 (21.4) 10 (14.3) Treatment-related 7 (5.6) 3 (4.3) Grade 5a 3 (2.4) 1 (1.4) Treatment-related 0 0 Leading to treatment discontinuation 8 (6.3) 4 (5.7) Treatment-related 5 (4.0) 2 (2.9) DLT 0 0 Median duration of treatment, months (range) 7.1 (0.0–32.3) 3.6 (0.0–18.0) aGrade 5 AEs: pulmonary embolism; death not otherwise specified; pneumonia AE, adverse event; DLT, dose-limiting toxicity; RP2D, recommended Phase 2 dose; SAE, serious AE; TEAE, treatment-emergent AE Data cutoff: 19 Sep 2025
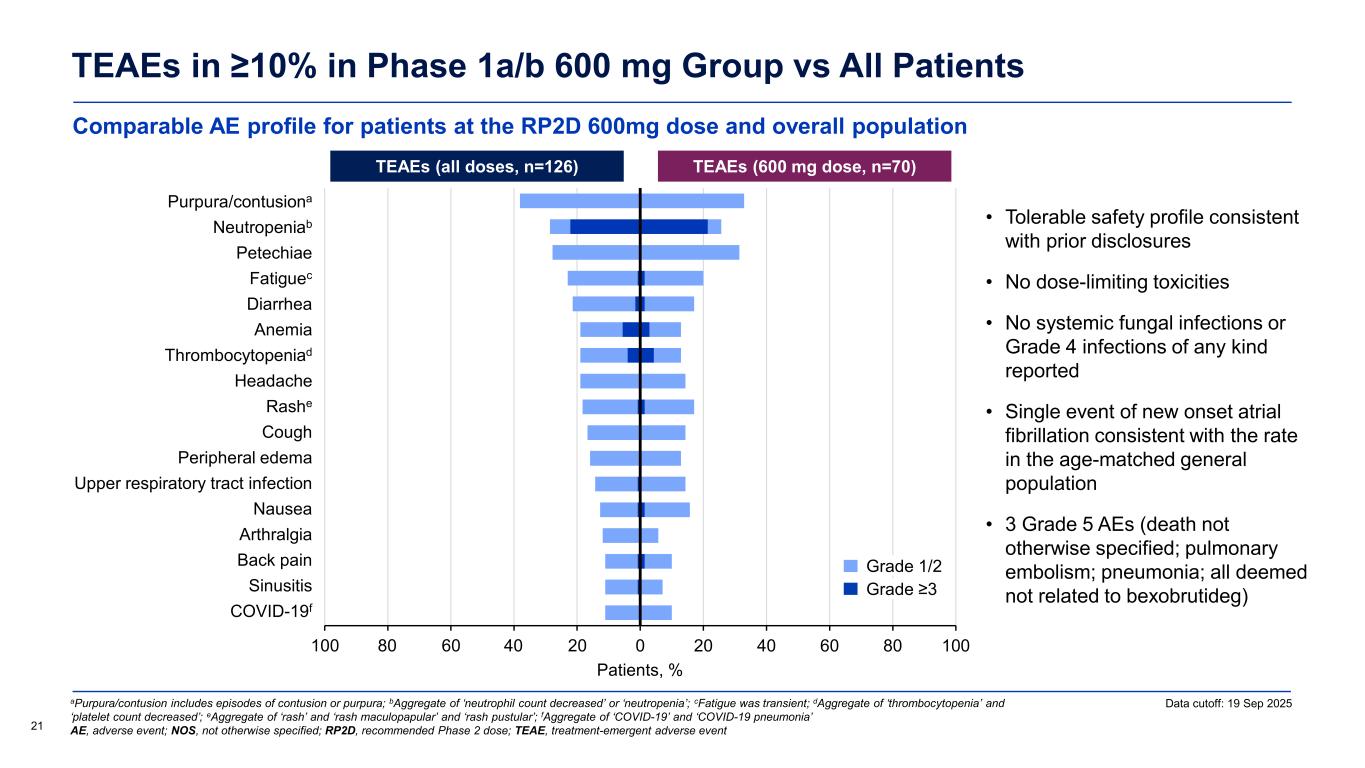
Comparable AE profile for patients at the RP2D 600mg dose and overall population TEAEs in ≥10% in Phase 1a/b 600 mg Group vs All Patients 21 Data cutoff: 19 Sep 2025aPurpura/contusion includes episodes of contusion or purpura; bAggregate of ‘neutrophil count decreased’ or ‘neutropenia’; cFatigue was transient; dAggregate of ‘thrombocytopenia’ and ‘platelet count decreased’; eAggregate of ‘rash’ and ‘rash maculopapular’ and ‘rash pustular’; fAggregate of ‘COVID-19’ and ‘COVID-19 pneumonia’ AE, adverse event; NOS, not otherwise specified; RP2D, recommended Phase 2 dose; TEAE, treatment-emergent adverse event • Tolerable safety profile consistent with prior disclosures • No dose-limiting toxicities • No systemic fungal infections or Grade 4 infections of any kind reported • Single event of new onset atrial fibrillation consistent with the rate in the age-matched general population • 3 Grade 5 AEs (death not otherwise specified; pulmonary embolism; pneumonia; all deemed not related to bexobrutideg) TEAEs (all doses, n=126) TEAEs (600 mg dose, n=70) Patients, % -100 -80 -60 -40 -20 0 20 40 60 80 100 COVID-19 Sinusitis Back pain Arthralgia Nausea Upper respiratory tract infection Peripheral edema Cough Rash Headache Thrombocytopenia Anemia Diarrhea Fatigue Petechiae Neutropenia Purpura/contusion 4080 Grade 1/2 Grade ≥3 Purpura/contusiona eutropeniab t i Fatiguec i rr i Thro bocytopeniad ashe ri r l r r ir t r tr t i f ti rt r l i i i iti f
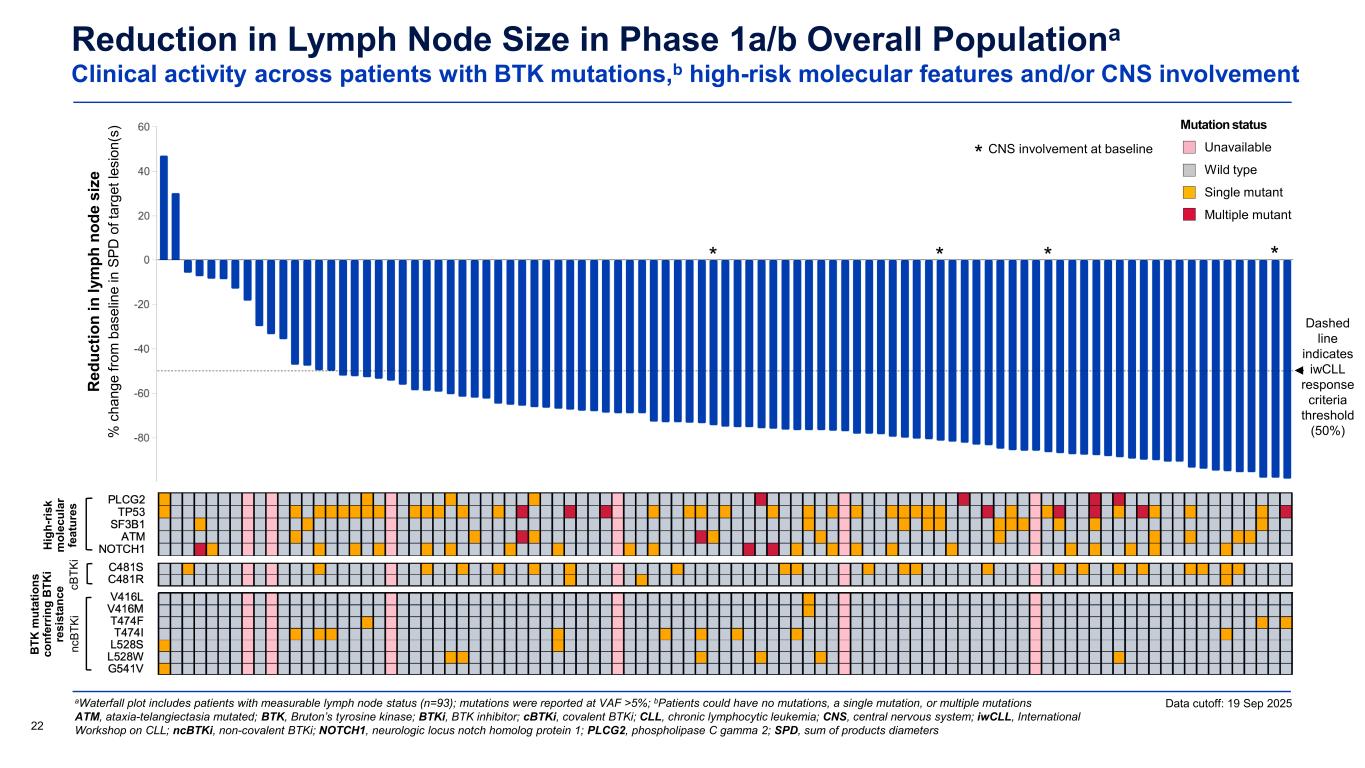
Reduction in Lymph Node Size in Phase 1a/b Overall Populationa Clinical activity across patients with BTK mutations,b high-risk molecular features and/or CNS involvement aWaterfall plot includes patients with measurable lymph node status (n=93); mutations were reported at VAF >5%; bPatients could have no mutations, a single mutation, or multiple mutations ATM, ataxia-telangiectasia mutated; BTK, Bruton’s tyrosine kinase; BTKi, BTK inhibitor; cBTKi, covalent BTKi; CLL, chronic lymphocytic leukemia; CNS, central nervous system; iwCLL, International Workshop on CLL; ncBTKi, non-covalent BTKi; NOTCH1, neurologic locus notch homolog protein 1; PLCG2, phospholipase C gamma 2; SPD, sum of products diameters22 Dashed line indicates iwCLL response criteria threshold (50%) Data cutoff: 19 Sep 2025 CNS involvement at baseline * * *** R ed uc tio n in ly m ph n od e si ze % c ha ng e fro m b as el in e in S PD o f t ar ge t l es io n( s) Mutation status Unavailable Wild type Single mutant Multiple mutant Hi gh -r is k m ol ec ul ar fe at ur es B TK m ut at io ns co nf er rin g B TK i re si st an ce nc BT Ki cB TK i
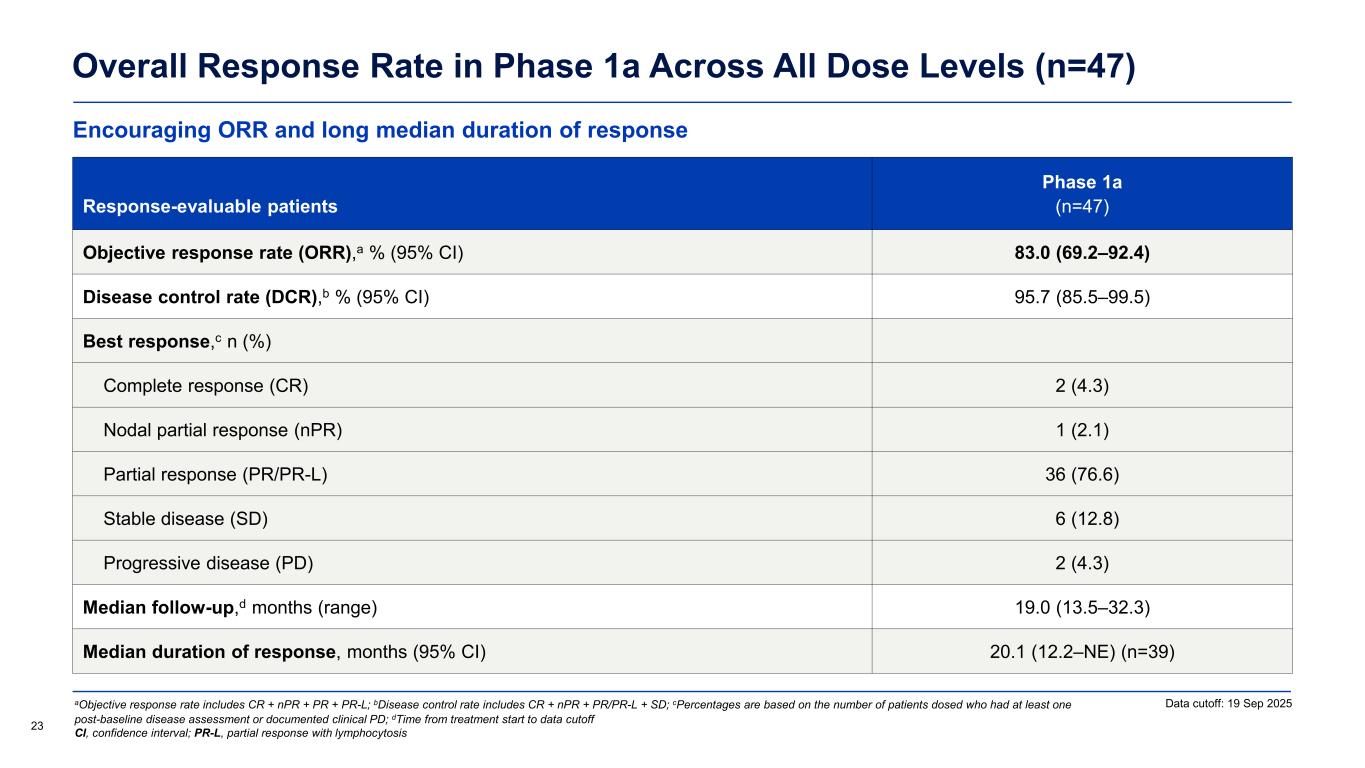
Encouraging ORR and long median duration of response Overall Response Rate in Phase 1a Across All Dose Levels (n=47) Response-evaluable patients Phase 1a (n=47) Objective response rate (ORR),a % (95% CI) 83.0 (69.2–92.4) Disease control rate (DCR),b % (95% CI) 95.7 (85.5–99.5) Best response,c n (%) Complete response (CR) 2 (4.3) Nodal partial response (nPR) 1 (2.1) Partial response (PR/PR-L) 36 (76.6) Stable disease (SD) 6 (12.8) Progressive disease (PD) 2 (4.3) Median follow-up,d months (range) 19.0 (13.5–32.3) Median duration of response, months (95% CI) 20.1 (12.2–NE) (n=39) Data cutoff: 19 Sep 2025 23 aObjective response rate includes CR + nPR + PR + PR-L; bDisease control rate includes CR + nPR + PR/PR-L + SD; cPercentages are based on the number of patients dosed who had at least one post-baseline disease assessment or documented clinical PD; dTime from treatment start to data cutoff CI, confidence interval; PR-L, partial response with lymphocytosis
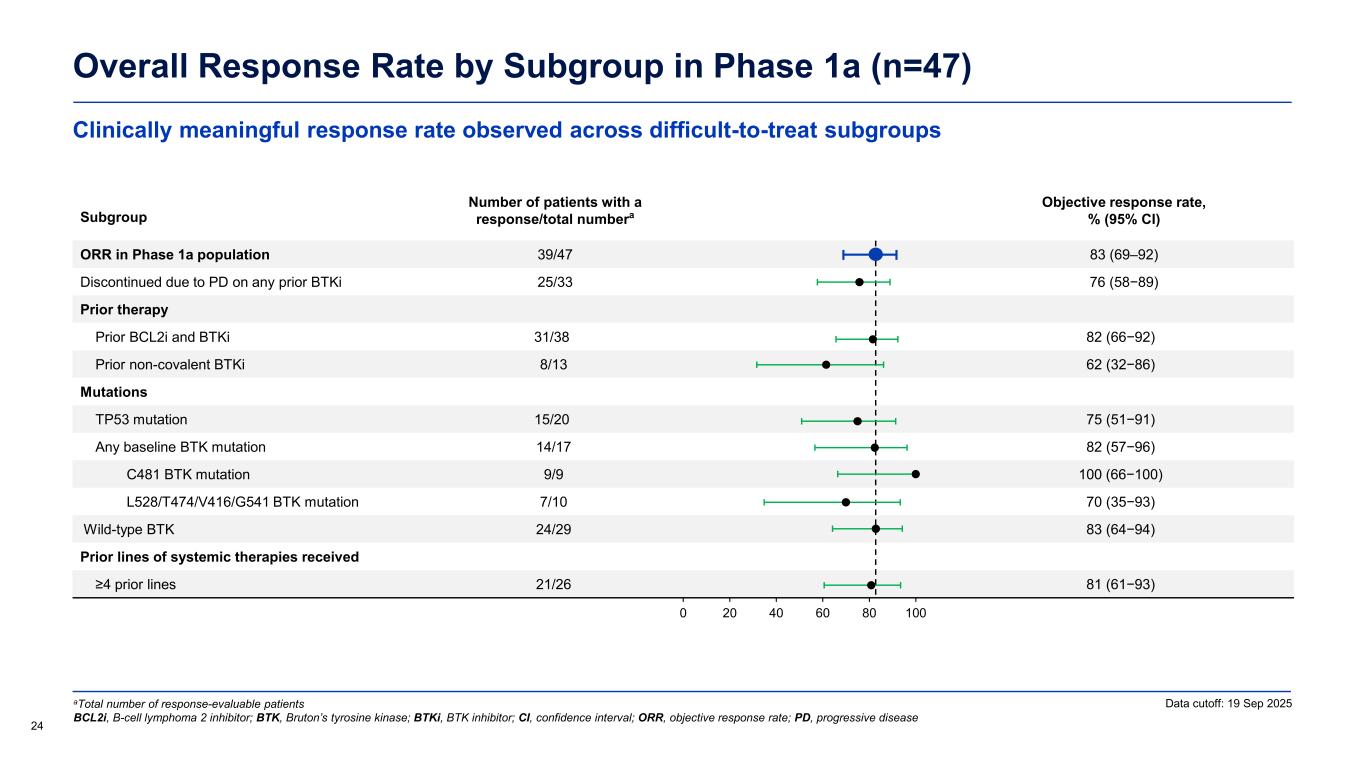
Clinically meaningful response rate observed across difficult-to-treat subgroups Overall Response Rate by Subgroup in Phase 1a (n=47) 24 Data cutoff: 19 Sep 2025aTotal number of response-evaluable patients BCL2i, B-cell lymphoma 2 inhibitor; BTK, Bruton’s tyrosine kinase; BTKi, BTK inhibitor; CI, confidence interval; ORR, objective response rate; PD, progressive disease Subgroup Number of patients with a response/total numbera Objective response rate, % (95% CI) ORR in Phase 1a population 39/47 83 (69–92) Discontinued due to PD on any prior BTKi 25/33 76 (58−89) Prior therapy Prior BCL2i and BTKi 31/38 82 (66−92) Prior non-covalent BTKi 8/13 62 (32−86) Mutations TP53 mutation 15/20 75 (51−91) Any baseline BTK mutation 14/17 82 (57−96) C481 BTK mutation 9/9 100 (66−100) L528/T474/V416/G541 BTK mutation 7/10 70 (35−93) Wild-type BTK 24/29 83 (64−94) Prior lines of systemic therapies received ≥4 prior lines 21/26 81 (61−93) 0 20 40 60 80 100
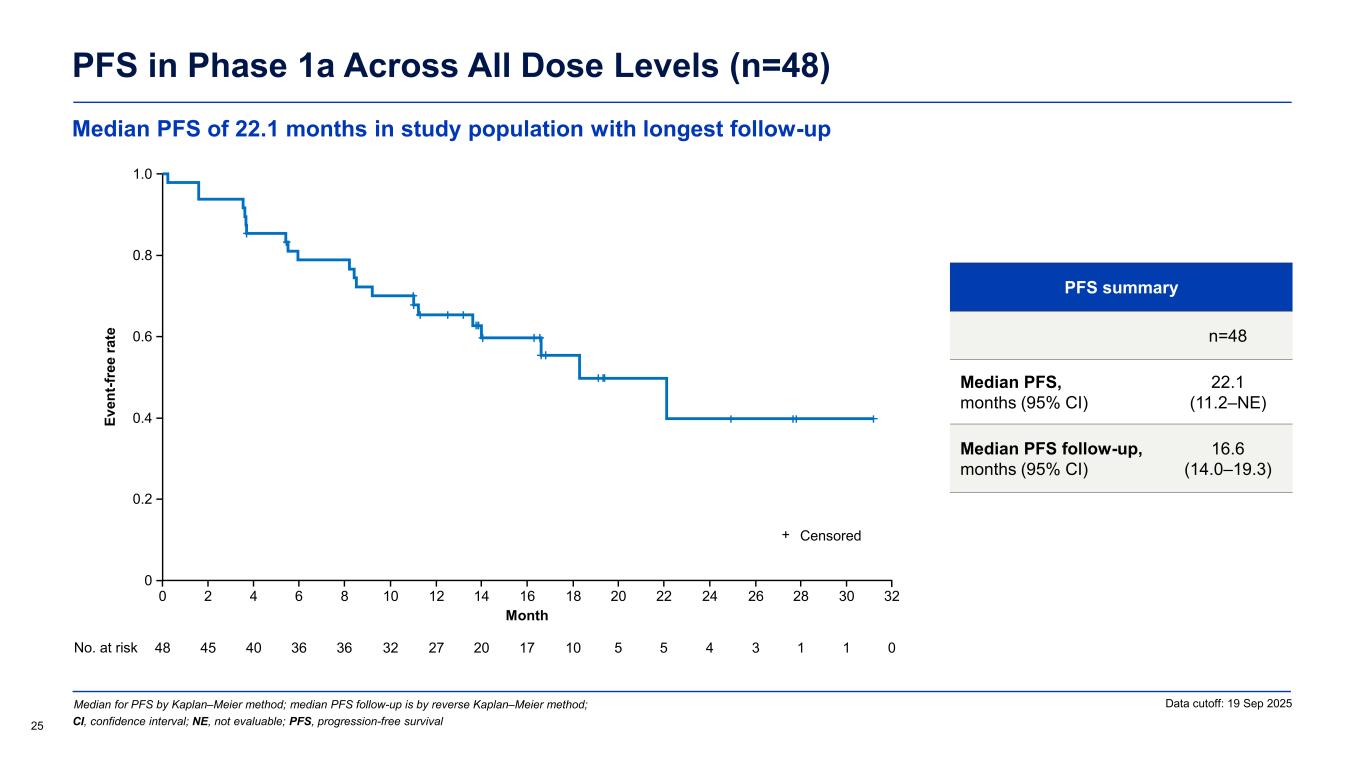
PFS in Phase 1a Across All Dose Levels (n=48) PFS summary n=48 Median PFS, months (95% CI) 22.1 (11.2–NE) Median PFS follow-up, months (95% CI) 16.6 (14.0–19.3) Median for PFS by Kaplan–Meier method; median PFS follow-up is by reverse Kaplan–Meier method; 25 Data cutoff: 19 Sep 2025 CI, confidence interval; NE, not evaluable; PFS, progression-free survival Ev en t-f re e ra te Month 0 No. at risk 48 40 36 27 17 10 3 0 16 1.0 0.2 0.4 0.6 0.8 1412108642 30282624222018 45 36 32 20 5 5 4 1 1 0 32 Censored+ Median PFS of 22.1 months in study population with longest follow-up
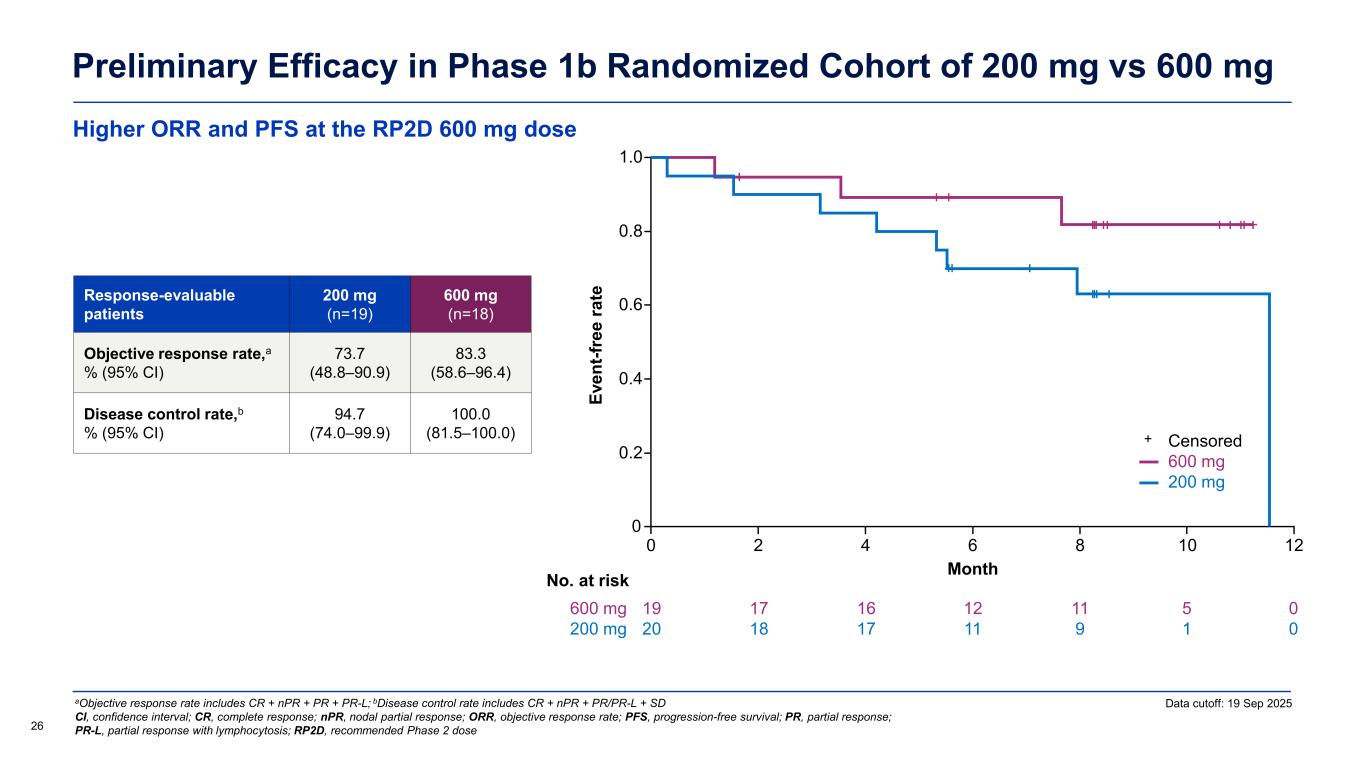
Higher ORR and PFS at the RP2D 600 mg dose Preliminary Efficacy in Phase 1b Randomized Cohort of 200 mg vs 600 mg Data cutoff: 19 Sep 2025 26 aObjective response rate includes CR + nPR + PR + PR-L; bDisease control rate includes CR + nPR + PR/PR-L + SD CI, confidence interval; CR, complete response; nPR, nodal partial response; ORR, objective response rate; PFS, progression-free survival; PR, partial response; PR-L, partial response with lymphocytosis; RP2D, recommended Phase 2 dose Response-evaluable patients 200 mg (n=19) 600 mg (n=18) Objective response rate,a % (95% CI) 73.7 (48.8–90.9) 83.3 (58.6–96.4) Disease control rate,b % (95% CI) 94.7 (74.0–99.9) 100.0 (81.5–100.0) Ev en t-f re e ra te Month 0 0 No. at risk 0.2 0.4 0.6 0.8 1.0 2 4 6 8 10 19 20 17 18 16 17 12 11 11 9 5 1 600 mg 200 mg 12 0 0 Censored 600 mg 200 mg +
Conclusions • In this Phase 1a/b trial, bexobrutideg (NX-5948), a novel BTK degrader with high selectivity for BTK, was well tolerated in a heavily pretreated population of patients with relapsed/refractory CLL/SLL: – Tolerable safety profile consistent with prior disclosures, and consistent between the RP2D 600 mg and overall trial population • In the Phase 1a portion of the trial with a median follow-up of 19 months: – Bexobrutideg demonstrated an ORR of 83% with a CR rate of 4.3% – Median DOR was 20.1 months – Median PFS was 22.1 months across all doses (50–600 mg) with data continuing to mature – High response rates were observed in the overall population including those in difficult-to-treat subgroups with baseline BTK mutations, high-risk molecular features and CNS involvement • In the Phase 1b portion of the trial: – A randomized cohort, conducted in accordance with Project Optimus, was fully enrolled: higher ORR and superior PFS were observed at the 600 mg dose, underpinning its selection as the RP2D – Non-randomized cohorts in CLL subsets of interest, treated at the RP2D dose, are ongoing • Based on the totality and consistency of safety and efficacy findings, including the Phase 1b randomized controlled cohort, the RP2D of 600 mg has been selected. Bexobrutideg will be evaluated in the ongoing pivotal Phase 2 DAYBreak CLL-201 and planned Phase 3 DAYBreak CLL-306 trials 27 BTK, Bruton's tyrosine kinase; CLL, chronic lymphocytic leukemia; CNS, central nervous system; CR, complete response; DOR, duration of response; ORR, objective response rate; RP2D, recommended Phase 2 dose; PFS, progression-free survival; SLL, small lymphocytic lymphoma
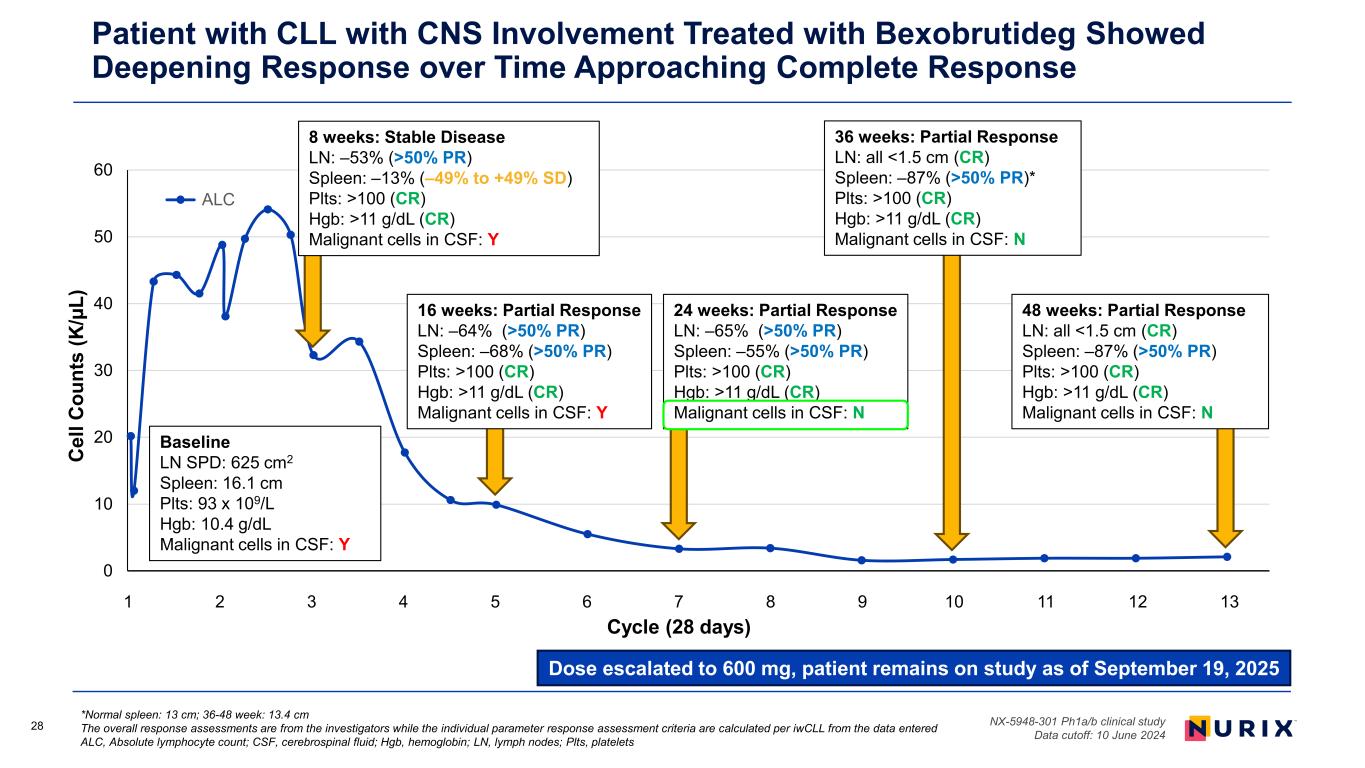
28 *Normal spleen: 13 cm; 36-48 week: 13.4 cm The overall response assessments are from the investigators while the individual parameter response assessment criteria are calculated per iwCLL from the data entered ALC, Absolute lymphocyte count; CSF, cerebrospinal fluid; Hgb, hemoglobin; LN, lymph nodes; Plts, platelets NX-5948-301 Ph1a/b clinical study Data cutoff: 10 June 2024 Patient with CLL with CNS Involvement Treated with Bexobrutideg Showed Deepening Response over Time Approaching Complete Response Dose escalated to 600 mg, patient remains on study as of September 19, 2025 0 10 20 30 40 50 60 0 50 100 150 200 250 300 350 C el l C ou nt s (K /µ L) Study day ALC 16 weeks: Partial Response LN: –64% (>50% PR) Spleen: –68% (>50% PR) Plts: >100 (CR) Hgb: >11 g/dL (CR) Malignant cells in CSF: Y Baseline LN SPD: 625 cm2 Spleen: 16.1 cm Plts: 93 x 109/L Hgb: 10.4 g/dL Malignant cells in CSF: Y 24 weeks: Partial Response LN: –65% (>50% PR) Spleen: –55% (>50% PR) Plts: >100 (CR) Hgb: >11 g/dL (CR) Malignant cells in CSF: N 36 weeks: Partial Response LN: all <1.5 cm (CR) Spleen: –87% (>50% PR)* Plts: >100 (CR) Hgb: >11 g/dL (CR) Malignant cells in CSF: N 8 weeks: Stable Disease LN: –53% (>50% PR) Spleen: –13% (–49% to +49% SD) Plts: >100 (CR) Hgb: >11 g/dL (CR) Malignant cells in CSF: Y 48 weeks: Partial Response LN: all <1.5 cm (CR) Spleen: –87% (>50% PR) Plts: >100 (CR) Hgb: >11 g/dL (CR) Malignant cells in CSF: N 1 2 3 4 5 6 7 8 9 10 11 12 13 Cycle (28 days)

Bexobrutideg (NX-5948), a Novel Bruton’s Tyrosine Kinase (BTK) Degrader, Shows High Clinical Activity and Tolerable Safety in Patients with Waldenström Macroglobulinemia: Updated Results from an Ongoing Phase 1a/b Study 1Nirav N. Shah, 2Scott Huntington, 3David Lewis, 4Tahla Munir, 5Graham P. Collins, 6Alvaro Alencar, 7Kim Linton, 8Zulfa Omer, 9Dima El- Sharkawi, 10,11Mary Gleeson, 12Pam McKay, 13Jeanette K. Doorduijn, 14Jeffery Smith, 15Daniel Morillo, 16Pau Abrisqueta, 17Sarah Injac, 18Astrid Pulles 1Medical College of Wisconsin, Milwaukee, WI, USA; 2Yale School of Medicine, New Haven, CT, USA; 3Derriford Hospital, Plymouth, UK; 4St. James’s Hospital, Leeds, UK; 5Oxford Cancer and Haematology Centre, Churchill Hospital, Oxford, UK; 6Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, FL, USA; 7Division of Cancer Sciences, The University of Manchester, Manchester, UK; 8University of Cincinnati, Cincinnati, OH, USA; 9Royal Marsden NHS Foundation Trust, Sutton, UK; 10Guy’s and St Thomas’ NHS Foundation Trust, London, UK; 11Sarah Cannon Research Institute, London, UK; 12Beatson West of Scotland Cancer Centre, Glasgow, Scotland; 13Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Hematology, The Netherlands, on behalf of the Lunenburg Lymphoma Phase I/II Consortium – HOVON/LLPC; 14The Clatterbridge Cancer Centre, Liverpool, UK; 15Fundación Jiménez Díaz University Hospital, START Madrid-FJD Early Phase Unit, Madrid, Spain; 16Hospital Universitari Vall d’Hebron, Barcelona, Spain; 17Nurix Therapeutics, Inc., San Francisco, CA, USA; 18UMC Utrecht Cancer Center, University Medical Center Utrecht, The Netherlands, on behalf of the Lunenburg Lymphoma Phase I/II Consortium – HOVON/LLPC ASH 2025 Annual Meeting, Orlando, 6–9 December 2025 B4PYY3 B4PYY3
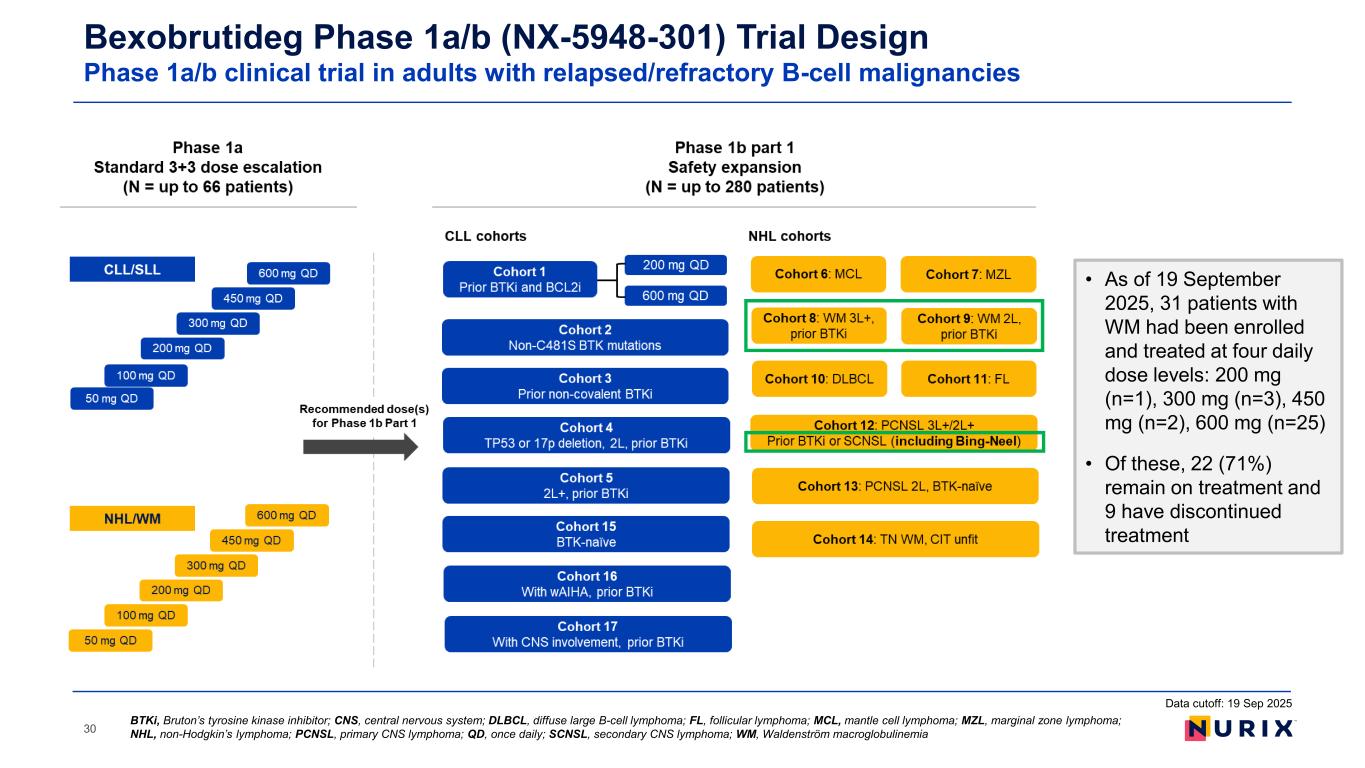
30 BTKi, Bruton’s tyrosine kinase inhibitor; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NHL, non-Hodgkin’s lymphoma; PCNSL, primary CNS lymphoma; QD, once daily; SCNSL, secondary CNS lymphoma; WM, Waldenström macroglobulinemia Bexobrutideg Phase 1a/b (NX-5948-301) Trial Design Phase 1a/b clinical trial in adults with relapsed/refractory B-cell malignancies Data cutoff: 19 Sep 2025 • As of 19 September 2025, 31 patients with WM had been enrolled and treated at four daily dose levels: 200 mg (n=1), 300 mg (n=3), 450 mg (n=2), 600 mg (n=25) • Of these, 22 (71%) remain on treatment and 9 have discontinued treatment
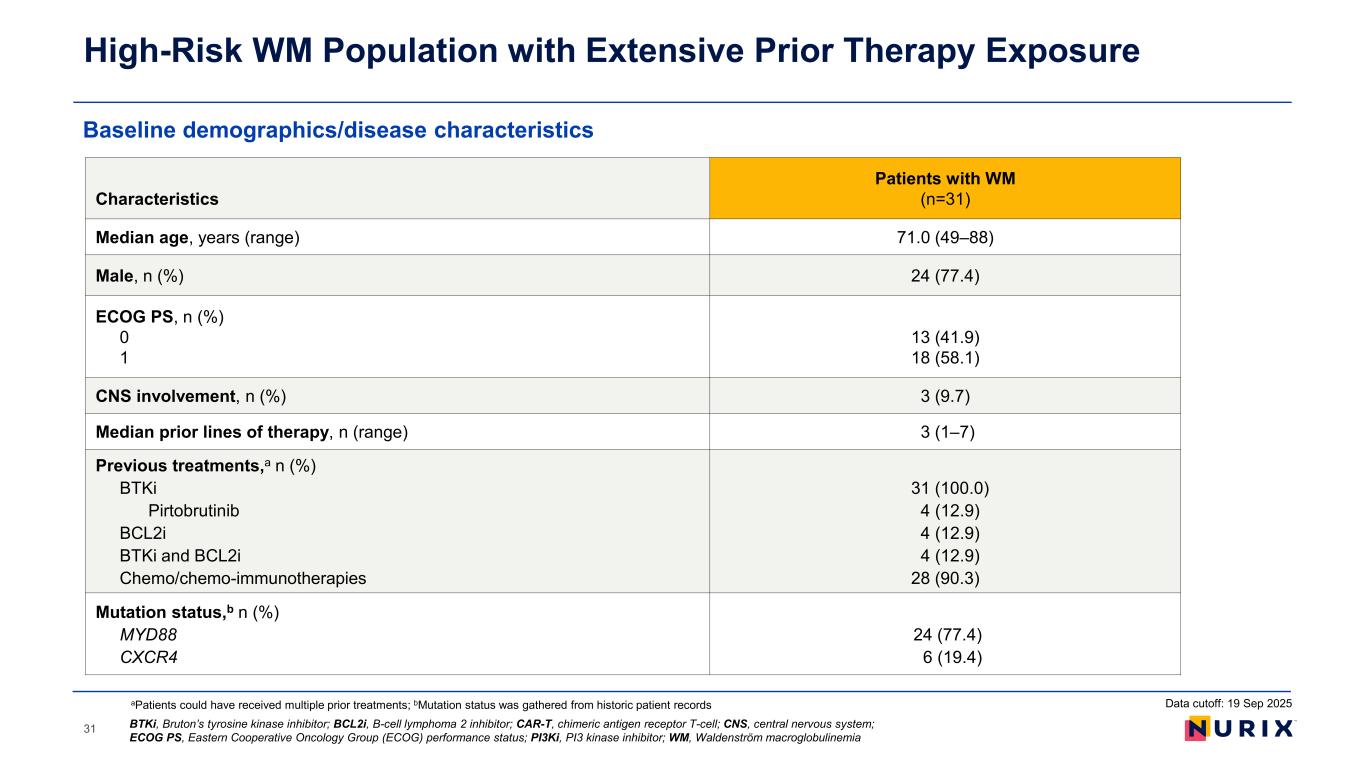
Baseline demographics/disease characteristics High-Risk WM Population with Extensive Prior Therapy Exposure 31 Characteristics Patients with WM (n=31) Median age, years (range) 71.0 (49–88) Male, n (%) 24 (77.4) ECOG PS, n (%) 0 1 13 (41.9) 18 (58.1) CNS involvement, n (%) 3 (9.7) Median prior lines of therapy, n (range) 3 (1–7) Previous treatments,a n (%) BTKi Pirtobrutinib BCL2i BTKi and BCL2i Chemo/chemo-immunotherapies 31 (100.0) 4 (12.9) 4 (12.9) 4 (12.9) 28 (90.3) Mutation status,b n (%) MYD88 CXCR4 24 (77.4) 6 (19.4) aPatients could have received multiple prior treatments; bMutation status was gathered from historic patient records BTKi, Bruton’s tyrosine kinase inhibitor; BCL2i, B-cell lymphoma 2 inhibitor; CAR-T, chimeric antigen receptor T-cell; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group (ECOG) performance status; PI3Ki, PI3 kinase inhibitor; WM, Waldenström macroglobulinemia Data cutoff: 19 Sep 2025
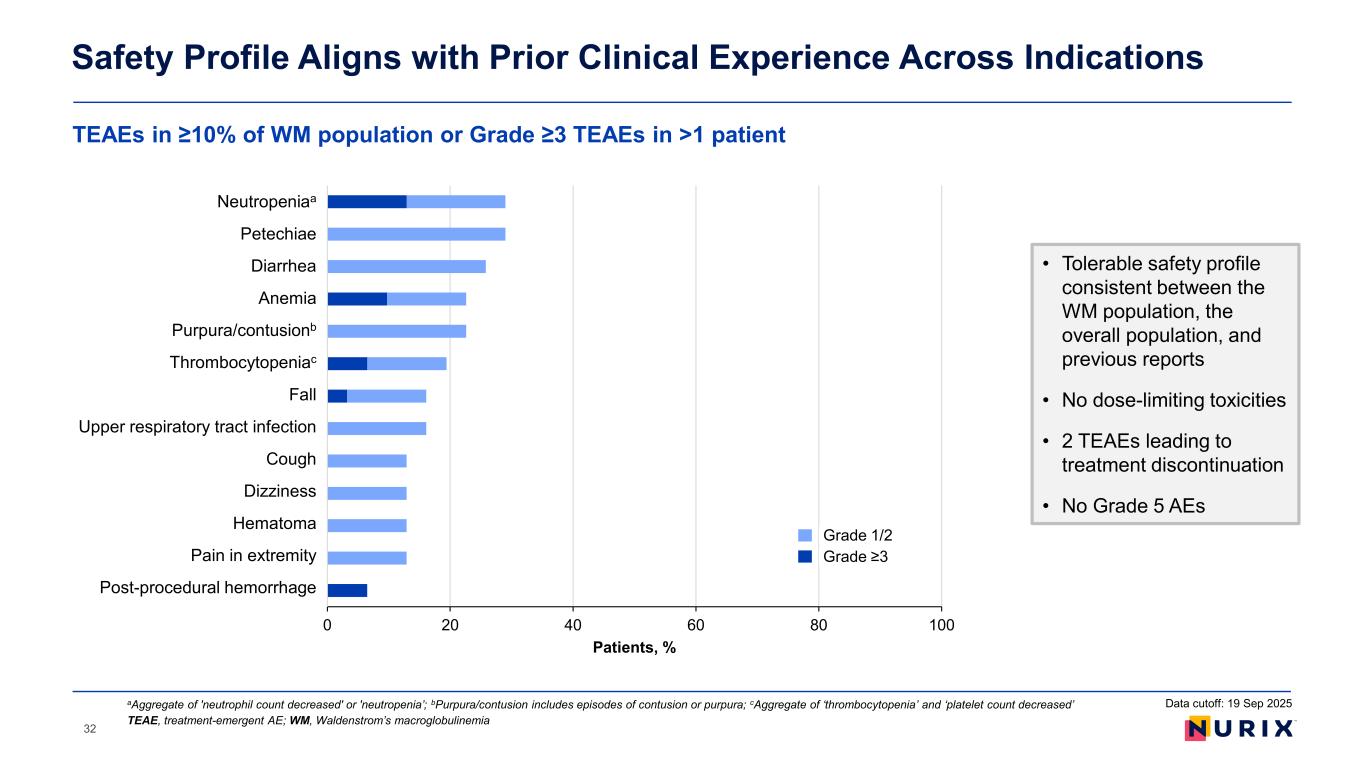
TEAEs in ≥10% of WM population or Grade ≥3 TEAEs in >1 patient Safety Profile Aligns with Prior Clinical Experience Across Indications TEAE, treatment-emergent AE; WM, Waldenstrom’s macroglobulinemia aAggregate of 'neutrophil count decreased' or 'neutropenia’; bPurpura/contusion includes episodes of contusion or purpura; cAggregate of ‘thrombocytopenia’ and ‘platelet count decreased’ Data cutoff: 19 Sep 2025 • Tolerable safety profile consistent between the WM population, the overall population, and previous reports • No dose-limiting toxicities • 2 TEAEs leading to treatment discontinuation • No Grade 5 AEs 0 20 40 60 80 100 Post-procedural hemorrhage Pain in extremity Hematoma Dizziness Cough Upper respiratory tract infection Fall Thrombocytopenia Purpura/contusion Anemia Diarrhea Petechiae Neutropenia Patients, % Grade 1/2 Grade ≥3 Neutropeniaa t chiae i i Purpura/contusionb Thrombocytopeniac ll Upper respiratory tract i f i i He t Pain in tr it Post-procedural he rr 32
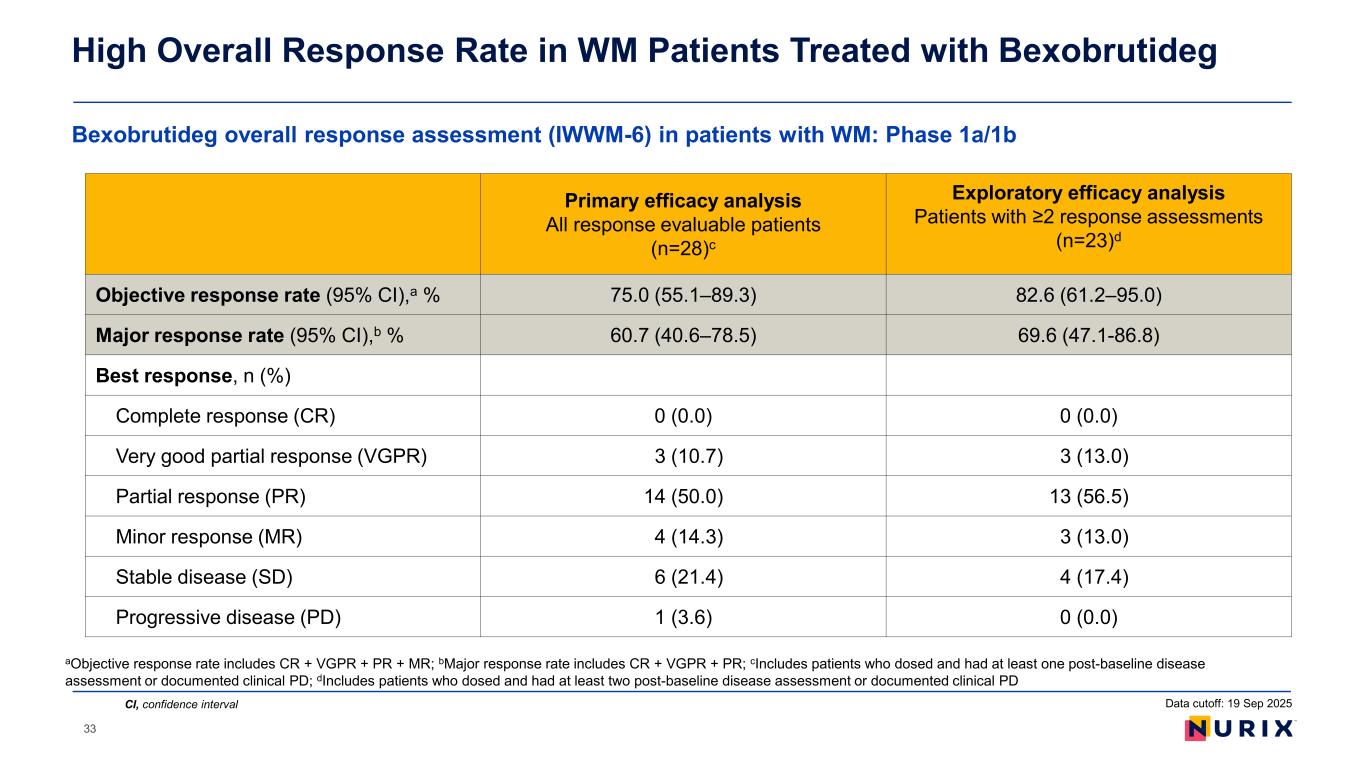
Bexobrutideg overall response assessment (IWWM-6) in patients with WM: Phase 1a/1b High Overall Response Rate in WM Patients Treated with Bexobrutideg CI, confidence interval Primary efficacy analysis All response evaluable patients (n=28)c Exploratory efficacy analysis Patients with ≥2 response assessments (n=23)d Objective response rate (95% CI),a % 75.0 (55.1–89.3) 82.6 (61.2–95.0) Major response rate (95% CI),b % 60.7 (40.6–78.5) 69.6 (47.1-86.8) Best response, n (%) Complete response (CR) 0 (0.0) 0 (0.0) Very good partial response (VGPR) 3 (10.7) 3 (13.0) Partial response (PR) 14 (50.0) 13 (56.5) Minor response (MR) 4 (14.3) 3 (13.0) Stable disease (SD) 6 (21.4) 4 (17.4) Progressive disease (PD) 1 (3.6) 0 (0.0) aObjective response rate includes CR + VGPR + PR + MR; bMajor response rate includes CR + VGPR + PR; cIncludes patients who dosed and had at least one post-baseline disease assessment or documented clinical PD; dIncludes patients who dosed and had at least two post-baseline disease assessment or documented clinical PD Data cutoff: 19 Sep 2025 33
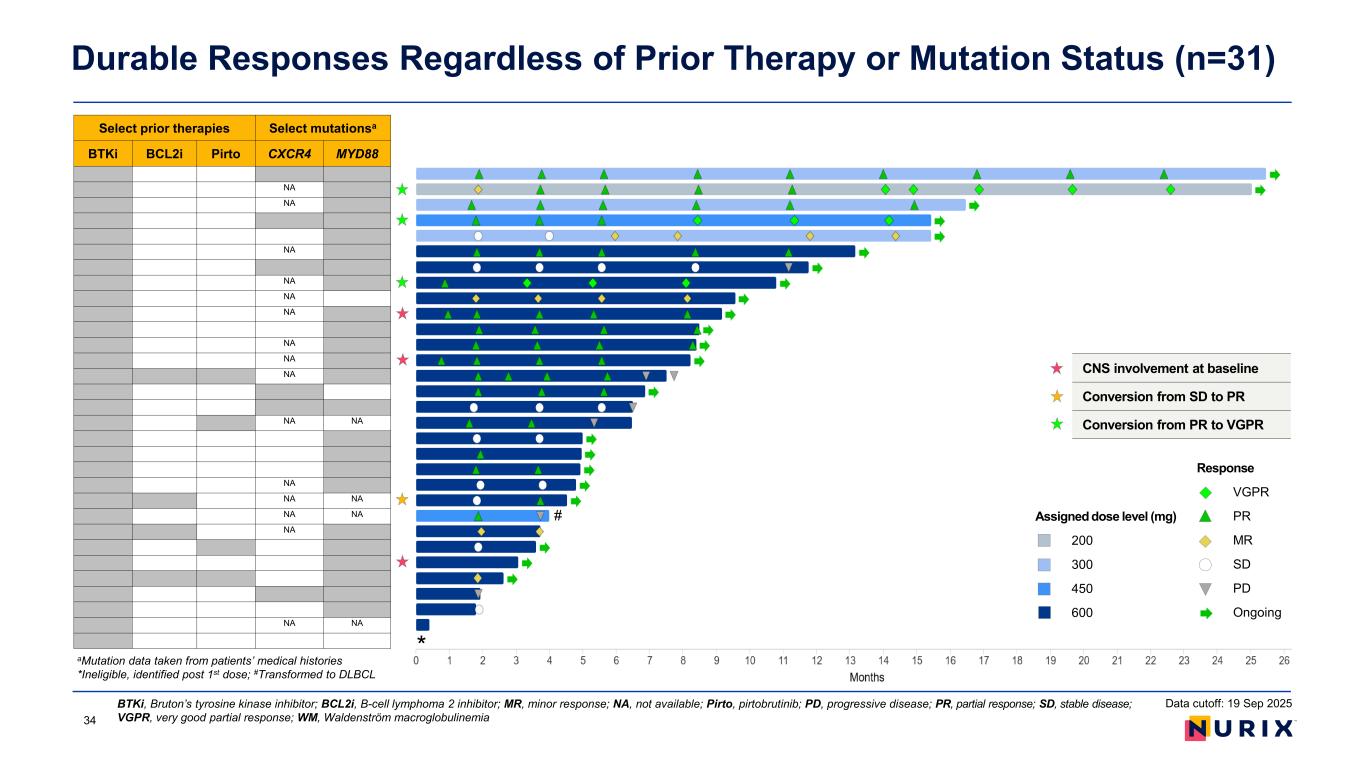
Durable Responses Regardless of Prior Therapy or Mutation Status (n=31) BTKi, Bruton’s tyrosine kinase inhibitor; BCL2i, B-cell lymphoma 2 inhibitor; MR, minor response; NA, not available; Pirto, pirtobrutinib; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response; WM, Waldenström macroglobulinemia Select prior therapies Select mutationsa BTKi BCL2i Pirto CXCR4 MYD88 NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA * # Data cutoff: 19 Sep 2025 aMutation data taken from patients’ medical histories *Ineligible, identified post 1st dose; #Transformed to DLBCL CNS involvement at baseline Conversion from SD to PR Conversion from PR to VGPR Response VGPR PR MR SD PD Ongoing Assigned dose level (mg) 200 300 450 600 34
• Bexobrutideg is a novel small molecule BTK degrader that can overcome treatment-emergent BTKi resistance mutations and disrupt BTK scaffolding. • In the ongoing WM portion of the phase 1 NX-5948-301 study as of the 19 September 2025 data cut: – Median follow-up was 8.1 months, and most patients were still on treatment. – In the WM safety population (31 patients), bexobrutideg was well tolerated, which is consistent with the overall study population and previous disclosures: • AEs were predominantly low grade; the most common AEs were neutropenia, petechiae, diarrhea, anemia, purpura/contusion, and thrombocytopenia. No atrial fibrillation was observed. • No DLTs were noted; two TEAEs led to drug discontinuation. There were no Grade 5 AEs. – In 28 response-evaluable patients, durable and deepening responses were observed in a heavily pre-treated (3 median lines of treatment) population of patients with WM, irrespective of CNS involvement, MYD88 or CXCR4 mutations: • A MRR of 60.7% and ORR of 75.0% was observed (including 3 VGPR and 14 PR), with 3 responses deepening from PR to VGPR with longer duration on treatment, and only one PD (3.6%) as best overall response. • Out of 3 patients with CNS involvement (2 with systemic disease), 2 have responded and none progressed. • A steady reduction in IgM levels occurred in most patients starting from the first IgM assessment (4 weeks), which continued to deepen at 8 weeks and beyond (data shown on the poster). • The median duration of response was not reached. 14 patients continued on treatment for more than 6 months. Conclusions AEs, adverse events; BTKi, Bruton’s tyrosine kinase inhibitor; CNS, central nervous system; DLTs, dose-limiting toxicities; IgM, immunoglobulin M; MRR, major response rate; ORR, objective response rate; PD, progressive disease; PR, partial response; VGPR, very good partial response; TEAE, treatment-emergent AE; WM, Waldenström macroglobulinemia35 Data cutoff: 19 Sep 2025

Paula G. O’Connor, M.D. Chief Medical Officer, Nurix Therapeutics Bexobrutideg: Driving Clinical Momentum and Competitive Leadership
600 mg dose selected per Project Optimus Cleared to move ahead globally (FDA, MHRA, EMA) Pivotal Phase 2 trial initiated – DAYBreak CLL-201 Confirmatory Phase 3 trial initiation planned for H1 2026 New best-in-class in vitro potency and selectivity data Bexobrutideg clinical update at ASH 2025 NEW information: • Bexobrutideg data from Phase 1a in CLL patients demonstrate ORR of 83.0%, median DOR of 20.1 months, and median PFS of 22.1 months Advancing bexobrutideg as a potential best-in-class BTK degrader Positioned for Success – Recent Key Program Updates 37
Current CLL Results for Bexobrutideg Are Highly Differentiated From Pirtobrutinib • Bexobrutideg maturing data from Phase 1a in CLL patients demonstrates ORR of 83.0%, median DOR of 20.1 months, and median PFS of 22.1 months • ORR, DOR, and PFS data for bexobrutideg are highly differentiated from pirtobrutinib based on results from the BRUIN-321 study of pirtobrutinib vs. BR/IR • Pirtobrutinib ORR was 65% (69% by investigator) with a DOR of 13.8 months (13.9 by investigator) • Pirtobrutinib mPFS was 14.0 months overall and 11.4 months in patients with prior cBTKi & BCL-2i (double exposed) • Superior ORR, DOR, and PFS for bexobrutideg compared to pirtobrutinib despite less favorable baseline characteristics: • More prior lines of therapy (median of 4 vs. 3) • More patients with 4+ lines of prior therapy (56.3% vs. 33%) • Prior exposure to ncBTKi (27% vs. 0%) • More patients exposed to prior BCL-2i (83.3% vs. 50%) 38 DOR, duration of response; ORR, objective response rate; PFS, progression-free survival Bexobrutideg: NX-5948-301 study data cut off Sept 19, 2025 Pirtobrutinib: Sharman et al, JCO 43: 2538-2549, June 2025 Data cutoff: 19 Sep 2025
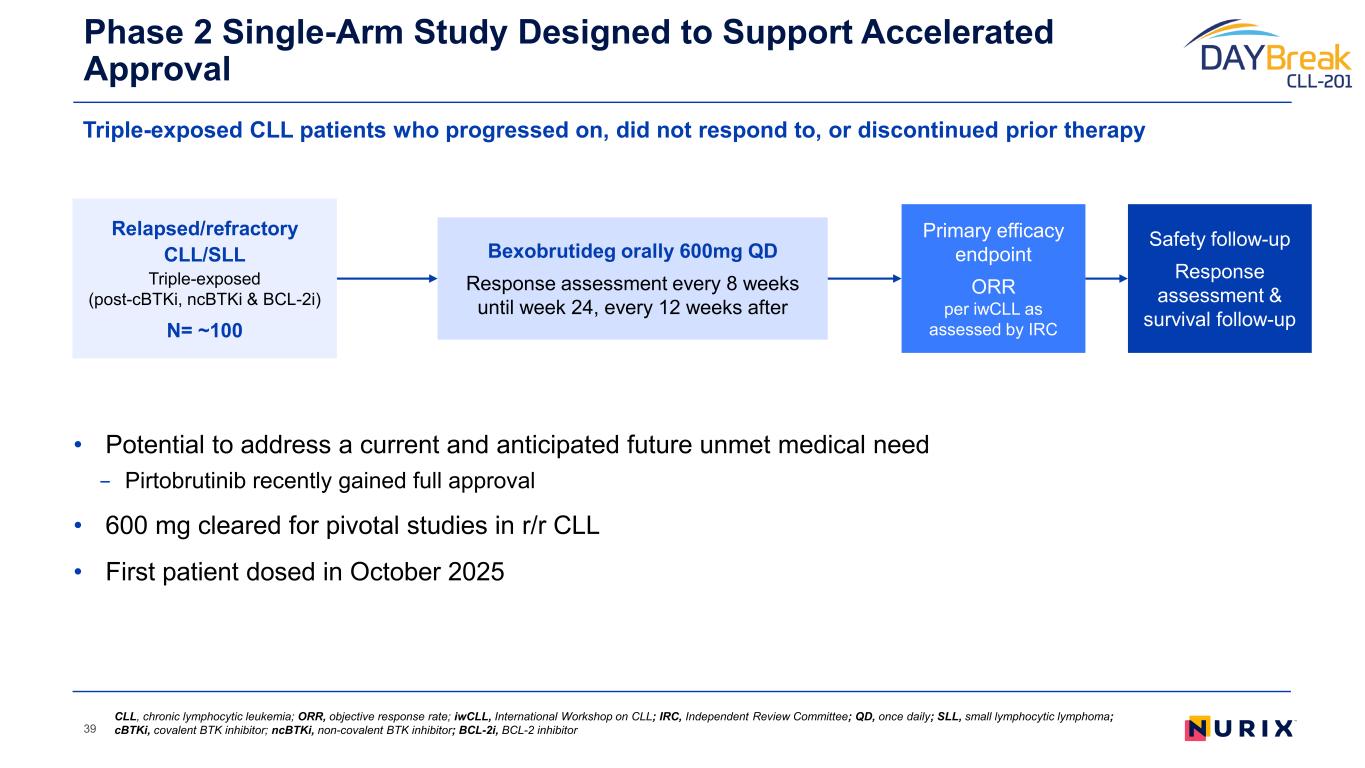
• Potential to address a current and anticipated future unmet medical need − Pirtobrutinib recently gained full approval • 600 mg cleared for pivotal studies in r/r CLL • First patient dosed in October 2025 Phase 2 Single-Arm Study Designed to Support Accelerated Approval 39 Primary efficacy endpoint ORR per iwCLL as assessed by IRC Relapsed/refractory CLL/SLL Triple-exposed (post-cBTKi, ncBTKi & BCL-2i) N= ~100 Bexobrutideg orally 600mg QD Response assessment every 8 weeks until week 24, every 12 weeks after Safety follow-up Response assessment & survival follow-up CLL, chronic lymphocytic leukemia; ORR, objective response rate; iwCLL, International Workshop on CLL; IRC, Independent Review Committee; QD, once daily; SLL, small lymphocytic lymphoma; cBTKi, covalent BTK inhibitor; ncBTKi, non-covalent BTK inhibitor; BCL-2i, BCL-2 inhibitor Triple-exposed CLL patients who progressed on, did not respond to, or discontinued prior therapy
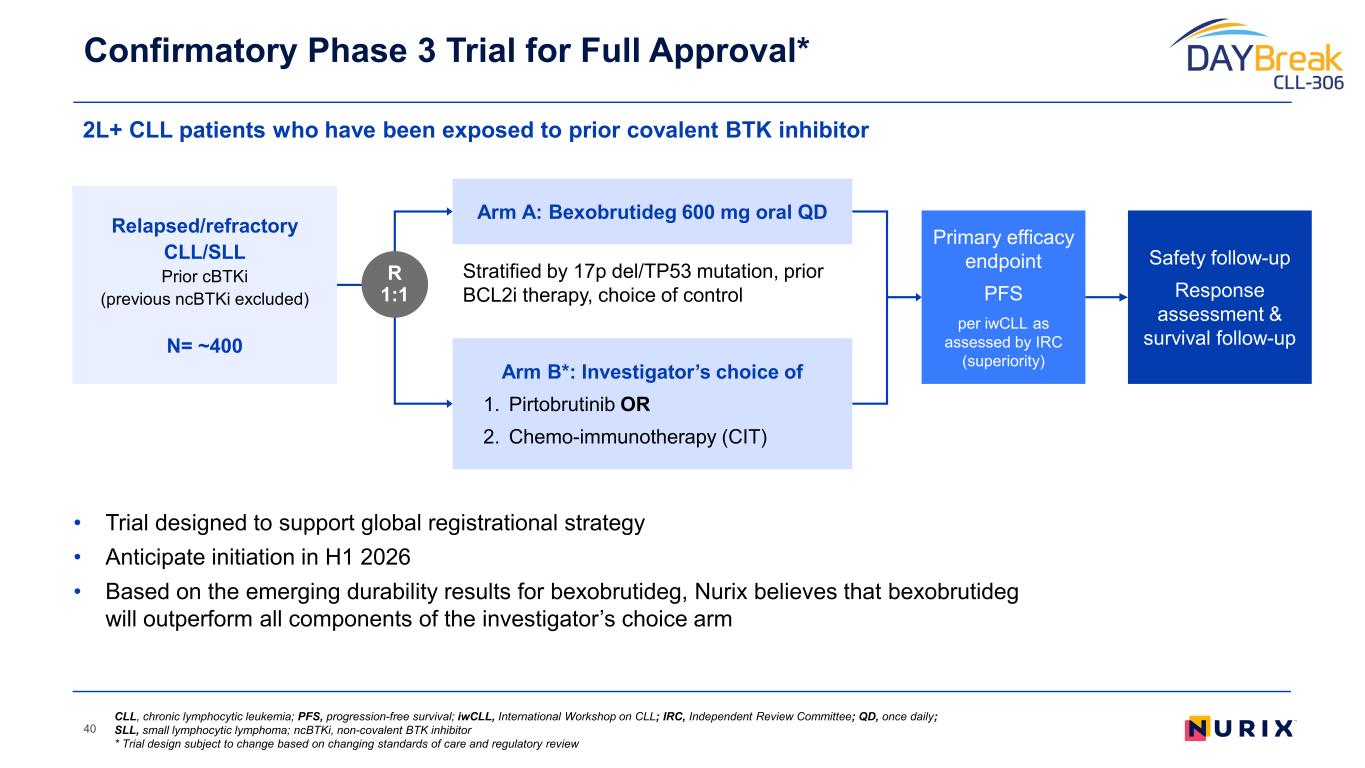
• Trial designed to support global registrational strategy • Anticipate initiation in H1 2026 • Based on the emerging durability results for bexobrutideg, Nurix believes that bexobrutideg will outperform all components of the investigator’s choice arm 2L+ CLL patients who have been exposed to prior covalent BTK inhibitor Confirmatory Phase 3 Trial for Full Approval* 40 Stratified by 17p del/TP53 mutation, prior BCL2i therapy, choice of control Primary efficacy endpoint PFS per iwCLL as assessed by IRC (superiority) Relapsed/refractory CLL/SLL Prior cBTKi (previous ncBTKi excluded) N= ~400 R 1:1 Arm A: Bexobrutideg 600 mg oral QD Arm B*: Investigator’s choice of 1. Pirtobrutinib OR 2. Chemo-immunotherapy (CIT) Safety follow-up Response assessment & survival follow-up CLL, chronic lymphocytic leukemia; PFS, progression-free survival; iwCLL, International Workshop on CLL; IRC, Independent Review Committee; QD, once daily; SLL, small lymphocytic lymphoma; ncBTKi, non-covalent BTK inhibitor * Trial design subject to change based on changing standards of care and regulatory review
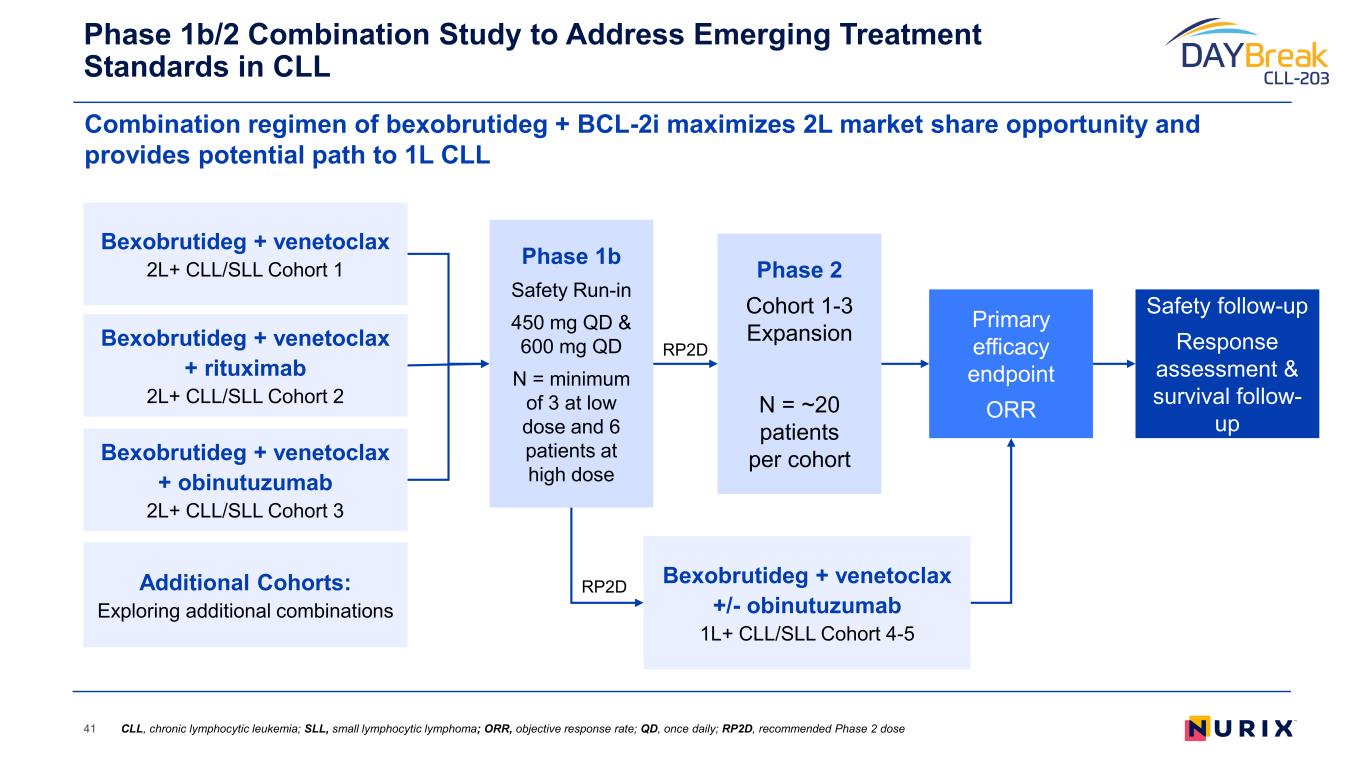
Combination regimen of bexobrutideg + BCL-2i maximizes 2L market share opportunity and provides potential path to 1L CLL Phase 1b/2 Combination Study to Address Emerging Treatment Standards in CLL 41 Bexobrutideg + venetoclax 2L+ CLL/SLL Cohort 1 Bexobrutideg + venetoclax + rituximab 2L+ CLL/SLL Cohort 2 Bexobrutideg + venetoclax + obinutuzumab 2L+ CLL/SLL Cohort 3 Phase 1b Safety Run-in 450 mg QD & 600 mg QD N = minimum of 3 at low dose and 6 patients at high dose Primary efficacy endpoint ORR Safety follow-up Response assessment & survival follow- up Phase 2 Cohort 1-3 Expansion N = ~20 patients per cohort Additional Cohorts: Exploring additional combinations Bexobrutideg + venetoclax +/- obinutuzumab 1L+ CLL/SLL Cohort 4-5 RP2D RP2D CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; ORR, objective response rate; QD, once daily; RP2D, recommended Phase 2 dose
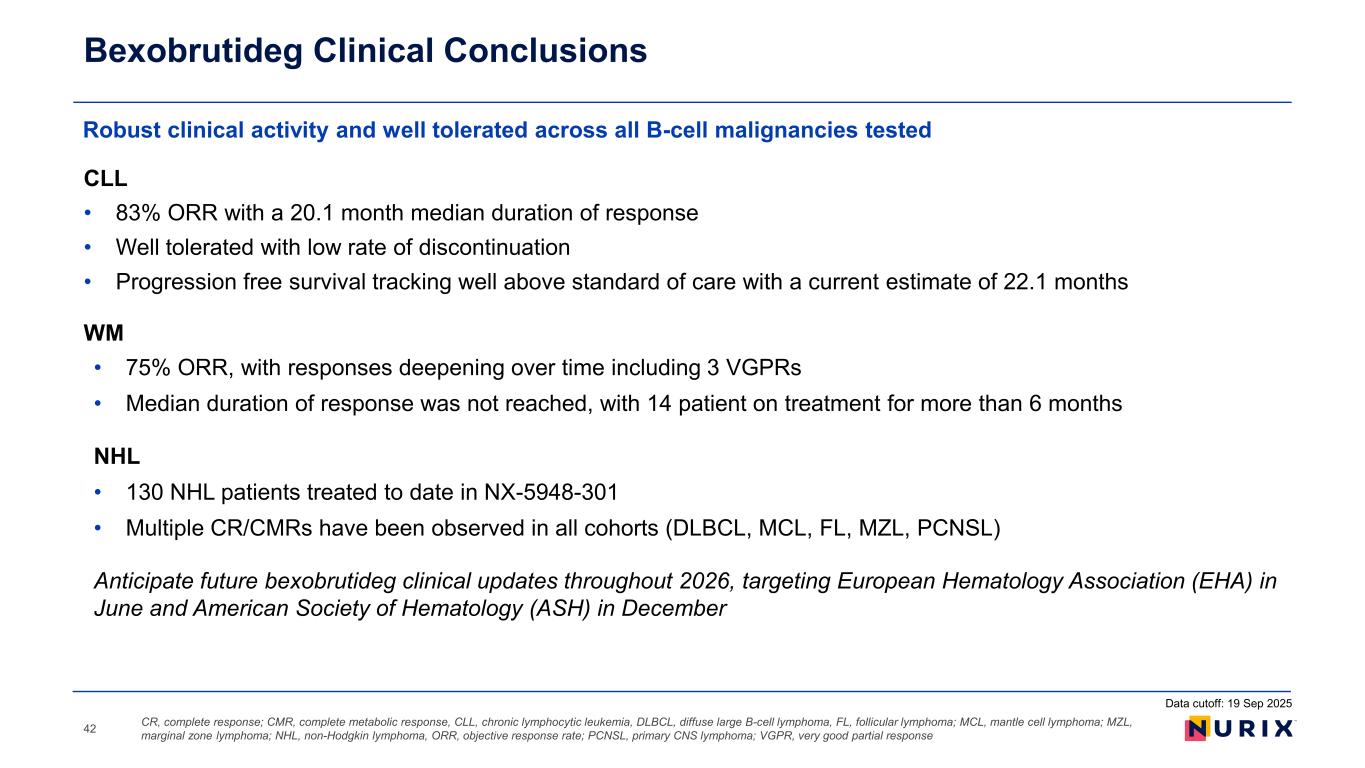
CLL • 83% ORR with a 20.1 month median duration of response • Well tolerated with low rate of discontinuation • Progression free survival tracking well above standard of care with a current estimate of 22.1 months WM • 75% ORR, with responses deepening over time including 3 VGPRs • Median duration of response was not reached, with 14 patient on treatment for more than 6 months NHL • 130 NHL patients treated to date in NX-5948-301 • Multiple CR/CMRs have been observed in all cohorts (DLBCL, MCL, FL, MZL, PCNSL) Anticipate future bexobrutideg clinical updates throughout 2026, targeting European Hematology Association (EHA) in June and American Society of Hematology (ASH) in December Robust clinical activity and well tolerated across all B-cell malignancies tested Bexobrutideg Clinical Conclusions CR, complete response; CMR, complete metabolic response, CLL, chronic lymphocytic leukemia, DLBCL, diffuse large B-cell lymphoma, FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma, ORR, objective response rate; PCNSL, primary CNS lymphoma; VGPR, very good partial response42 Data cutoff: 19 Sep 2025

Arthur T. Sands, M.D., Ph.D. Chief Executive Officer, Nurix Therapeutics Bexobrutideg and Beyond: Building the Next Generation of TPD Therapies
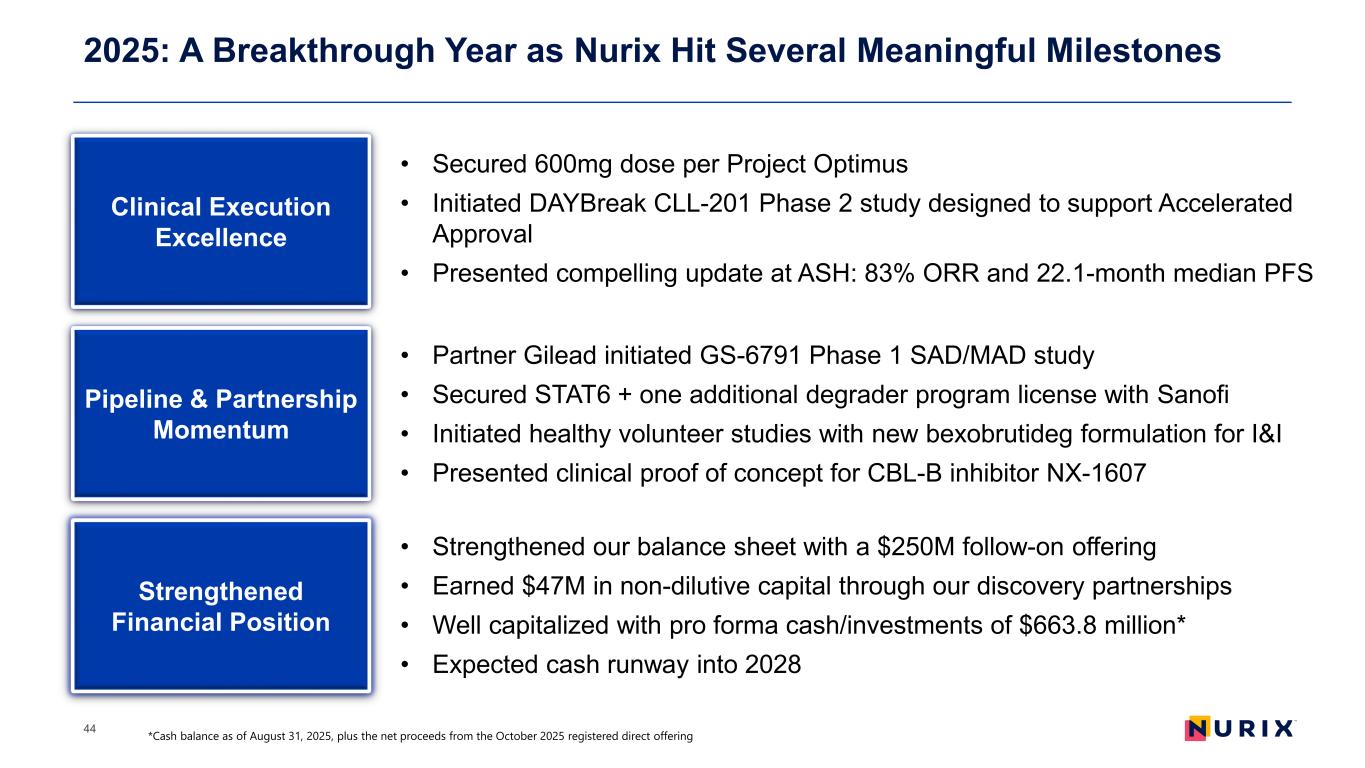
2025: A Breakthrough Year as Nurix Hit Several Meaningful Milestones 44 Clinical Execution Excellence • Secured 600mg dose per Project Optimus • Initiated DAYBreak CLL-201 Phase 2 study designed to support Accelerated Approval • Presented compelling update at ASH: 83% ORR and 22.1-month median PFS Pipeline & Partnership Momentum Strengthened Financial Position • Partner Gilead initiated GS-6791 Phase 1 SAD/MAD study • Secured STAT6 + one additional degrader program license with Sanofi • Initiated healthy volunteer studies with new bexobrutideg formulation for I&I • Presented clinical proof of concept for CBL-B inhibitor NX-1607 • Strengthened our balance sheet with a $250M follow-on offering • Earned $47M in non-dilutive capital through our discovery partnerships • Well capitalized with pro forma cash/investments of $663.8 million* • Expected cash runway into 2028 *Cash balance as of August 31, 2025, plus the net proceeds from the October 2025 registered direct offering
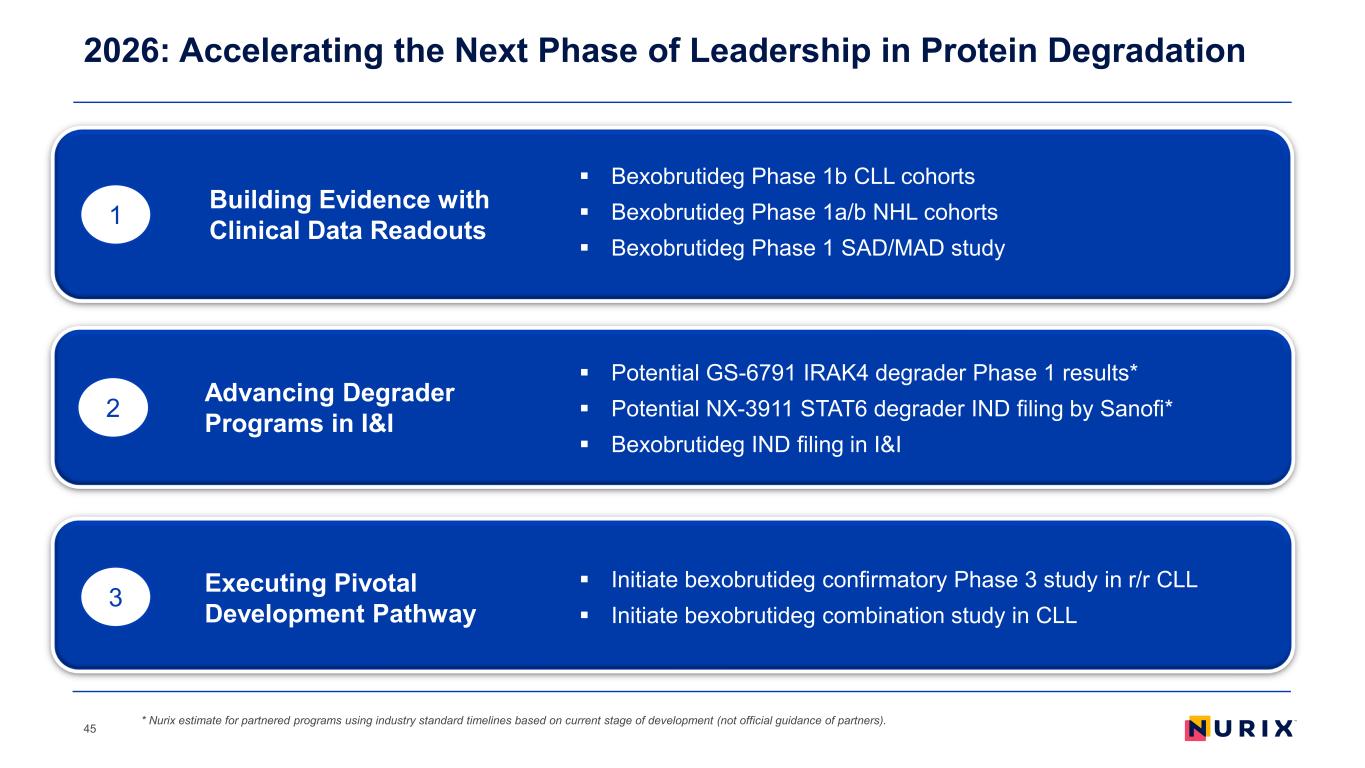
2026: Accelerating the Next Phase of Leadership in Protein Degradation 45 1 2 3 Bexobrutideg Phase 1b CLL cohorts Bexobrutideg Phase 1a/b NHL cohorts Bexobrutideg Phase 1 SAD/MAD study Building Evidence with Clinical Data Readouts Advancing Degrader Programs in I&I Executing Pivotal Development Pathway Potential GS-6791 IRAK4 degrader Phase 1 results* Potential NX-3911 STAT6 degrader IND filing by Sanofi* Bexobrutideg IND filing in I&I Initiate bexobrutideg confirmatory Phase 3 study in r/r CLL Initiate bexobrutideg combination study in CLL * Nurix estimate for partnered programs using industry standard timelines based on current stage of development (not official guidance of partners).

46 Q&A
