
Protein Degraders to Outmatch Cancer and Autoimmune Disease J.P. Morgan Healthcare Conference January 12, 2026

Important Notice and Disclaimers This presentation contains statements that relate to future events and expectations and as such constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. When or if used in this presentation, the words “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “outlook,” “plan,” “predict,” “should,” “will,” and similar expressions and their variants, as they relate to Nurix Therapeutics, Inc. (“Nurix”, the “Company,” “we,” “us” or “our”), may identify forward-looking statements. All statements that reflect Nurix’s expectations, assumptions or projections about the future, other than statements of historical fact, are forward-looking statements, including, without limitation, statements regarding the therapeutic potential of Nurix's drug candidates; Nurix's plans for the clinical development of its drug candidates; the planned timing for the initiation and enrollment of patients in current and future clinical trials of Nurix's drug candidates; the planned timing for the provision of updates and findings from Nurix's clinical trials; our future financial or business plans; our future performance, prospects and strategies; future conditions, trends, and other financial and business matters; our current and prospective drug candidates; the planned timing and conduct of the clinical trial programs for our drug candidates; the potential benefits of our collaborations, including potential milestone and sales- related payments; the potential advantages of DEL-AI and our drug candidates; the extent to which our scientific approach, our drug discovery engine, targeted protein degradation and degrader antibody conjugates may potentially address a broad range of diseases; the extent animal model data, in vitro potency data, and proteomics data predicts human efficacy; the timing and success of the development and commercialization of our current and anticipated drug candidates; and our ability to fund our operations into 2028. Forward-looking statements reflect Nurix’s current beliefs, expectations, and assumptions. Although Nurix believes the expectations and assumptions reflected in such forward-looking statements are reasonable, Nurix can give no assurance that they will prove to be correct. Forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and changes in circumstances that are difficult to predict, which could cause Nurix’s actual activities and results to differ materially from those expressed in any forward-looking statement. Such risks and uncertainties include, but are not limited to: (i) whether Nurix will be able to advance, obtain regulatory approval of and ultimately commercialize its current and prospective drug candidates, including bexobrutideg, zelebrudomide or NX-1607; (ii) risks and uncertainties inherent in the drug discovery and drug development process; (iii) the timing and results of clinical trials; (iv) Nurix’s ability to fund development activities and achieve development goals; (v) risks and uncertainties relating to Nurix's collaboration partners, including the speed of development of partnered programs and the timing and receipt of payments from Nurix's collaboration partners, including milestone payments and royalties on future potential product sales; (vi) the impact of macroeconomic events and conditions, including increasing financial market volatility and uncertainty, inflation, interest rate fluctuations, instability in the global banking system, uncertainty with respect to the federal budget and debt ceiling, war, military or regional conflicts, and global health pandemics, on Nurix’s clinical trials and operations; (vii) Nurix’s ability to protect intellectual property and (viii) other risks and uncertainties described under the heading “Risk Factors” in Nurix’s Quarterly Report on Form 10-Q for the fiscal quarter ended August 31, 2025, and other SEC filings. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. The statements in this presentation speak only as of the date of this presentation, even if subsequently made available by Nurix on its website or otherwise. Nurix disclaims any intention or obligation to update publicly any forward-looking statements, whether in response to new information, future events, or otherwise, except as required by applicable law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Furthermore, while we believe our own internal estimates and research are reliable, such estimates and research have not been verified by any independent source. 2

3 OUR MISSION To establish degrader-based medicines at the forefront of patient care
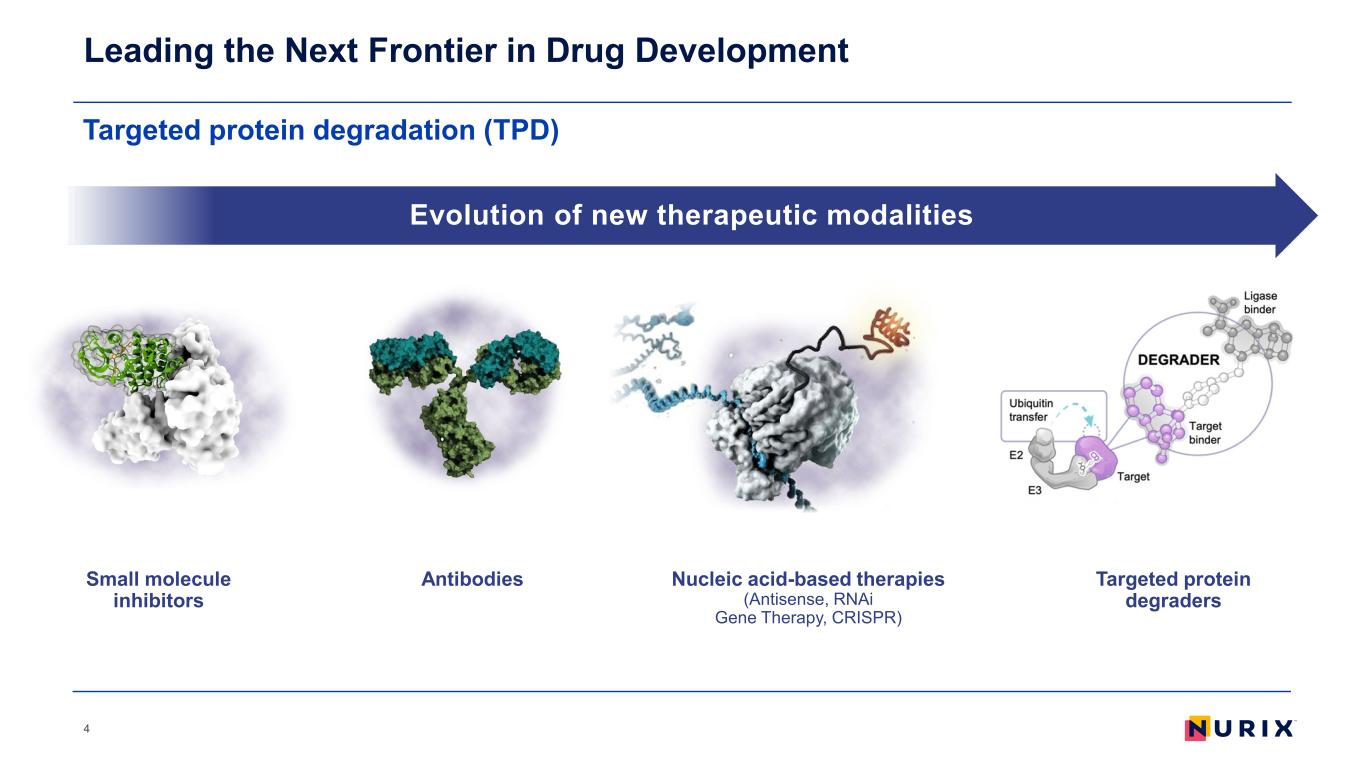
Targeted protein degradation (TPD) Leading the Next Frontier in Drug Development 4 Evolution of new therapeutic modalities AntibodiesSmall molecule inhibitors Nucleic acid-based therapies (Antisense, RNAi Gene Therapy, CRISPR) Targeted protein degraders
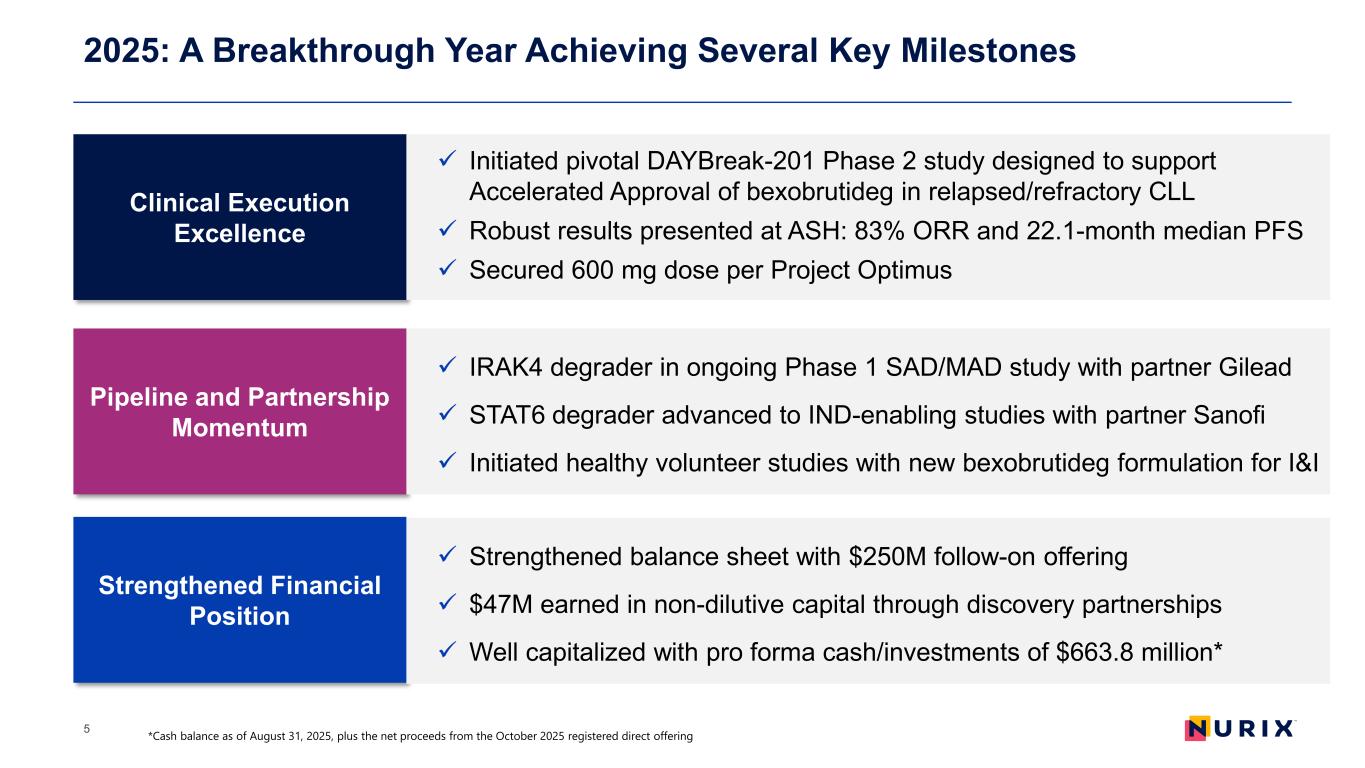
2025: A Breakthrough Year Achieving Several Key Milestones 5 Clinical Execution Excellence Initiated pivotal DAYBreak-201 Phase 2 study designed to support Accelerated Approval of bexobrutideg in relapsed/refractory CLL Robust results presented at ASH: 83% ORR and 22.1-month median PFS Secured 600 mg dose per Project Optimus Pipeline and Partnership Momentum Strengthened Financial Position IRAK4 degrader in ongoing Phase 1 SAD/MAD study with partner Gilead STAT6 degrader advanced to IND-enabling studies with partner Sanofi Initiated healthy volunteer studies with new bexobrutideg formulation for I&I Strengthened balance sheet with $250M follow-on offering $47M earned in non-dilutive capital through discovery partnerships Well capitalized with pro forma cash/investments of $663.8 million* *Cash balance as of August 31, 2025, plus the net proceeds from the October 2025 registered direct offering
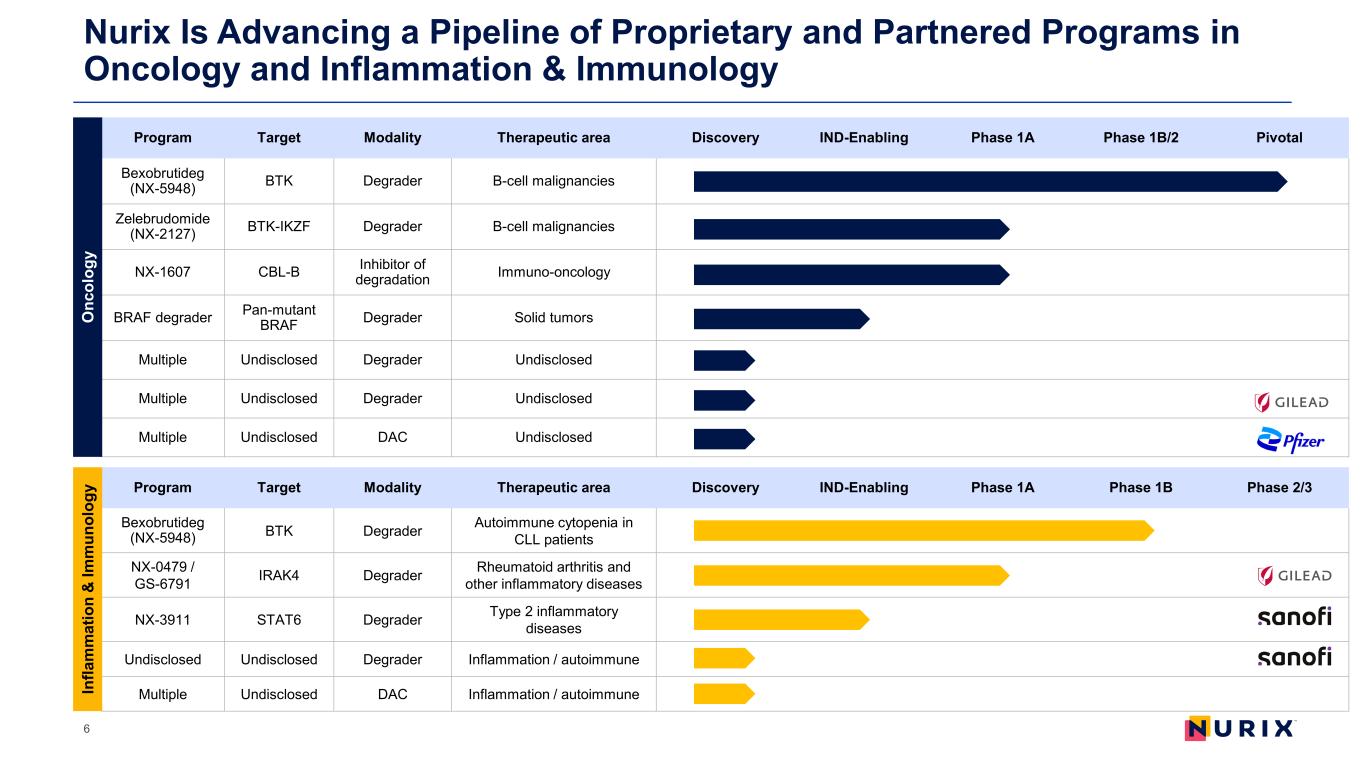
Nurix Is Advancing a Pipeline of Proprietary and Partnered Programs in Oncology and Inflammation & Immunology 6 Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B/2 Pivotal Bexobrutideg (NX-5948) BTK Degrader B-cell malignancies Zelebrudomide (NX-2127) BTK-IKZF Degrader B-cell malignancies NX-1607 CBL-B Inhibitor of degradation Immuno-oncology BRAF degrader Pan-mutant BRAF Degrader Solid tumors Multiple Undisclosed Degrader Undisclosed Multiple Undisclosed Degrader Undisclosed Multiple Undisclosed DAC Undisclosed Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B Phase 2/3 Bexobrutideg (NX-5948) BTK Degrader Autoimmune cytopenia in CLL patients NX-0479 / GS-6791 IRAK4 Degrader Rheumatoid arthritis and other inflammatory diseases NX-3911 STAT6 Degrader Type 2 inflammatory diseases Undisclosed Undisclosed Degrader Inflammation / autoimmune Multiple Undisclosed DAC Inflammation / autoimmune O nc ol og y In fla m m at io n & Im m un ol og y
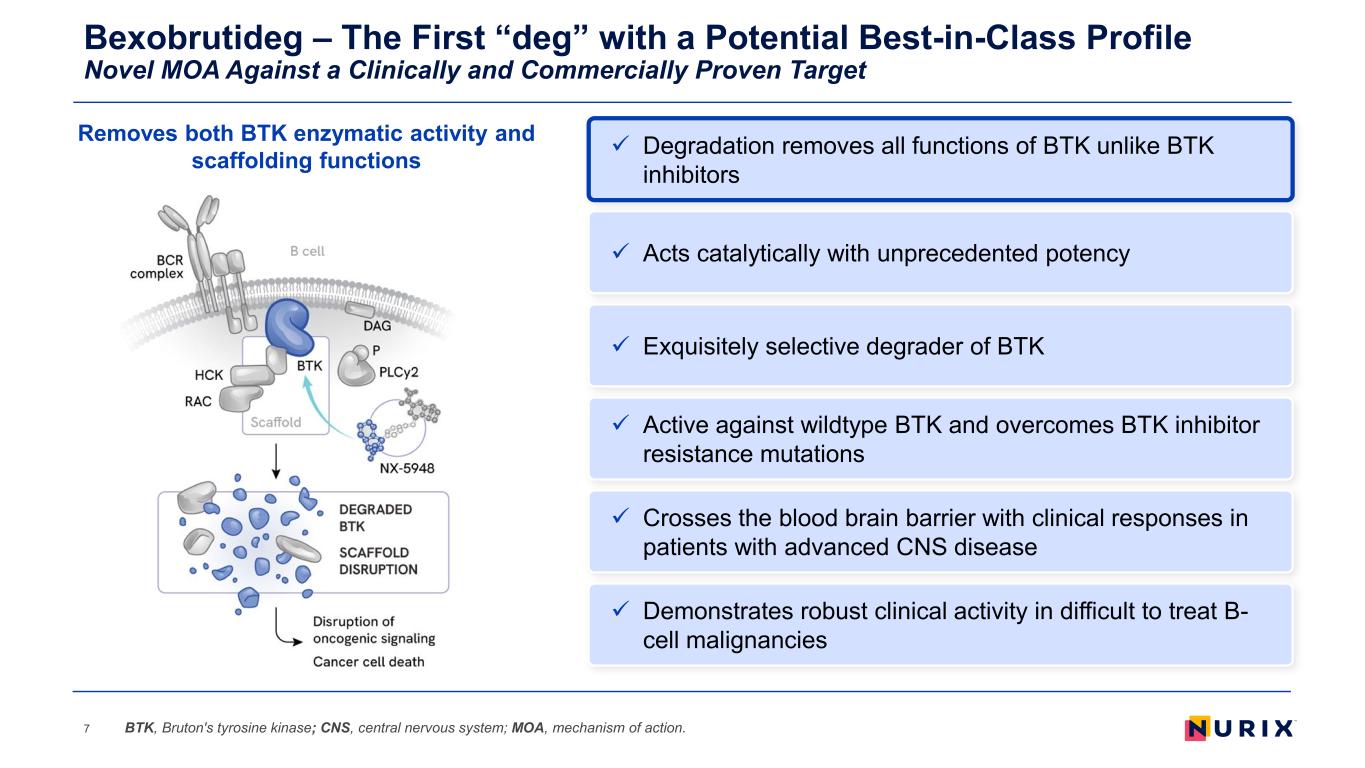
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 7 Exquisitely selective degrader of BTK Active against wildtype BTK and overcomes BTK inhibitor resistance mutations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Acts catalytically with unprecedented potency Crosses the blood brain barrier with clinical responses in patients with advanced CNS disease BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Degradation removes all functions of BTK unlike BTK inhibitors Removes both BTK enzymatic activity and scaffolding functions
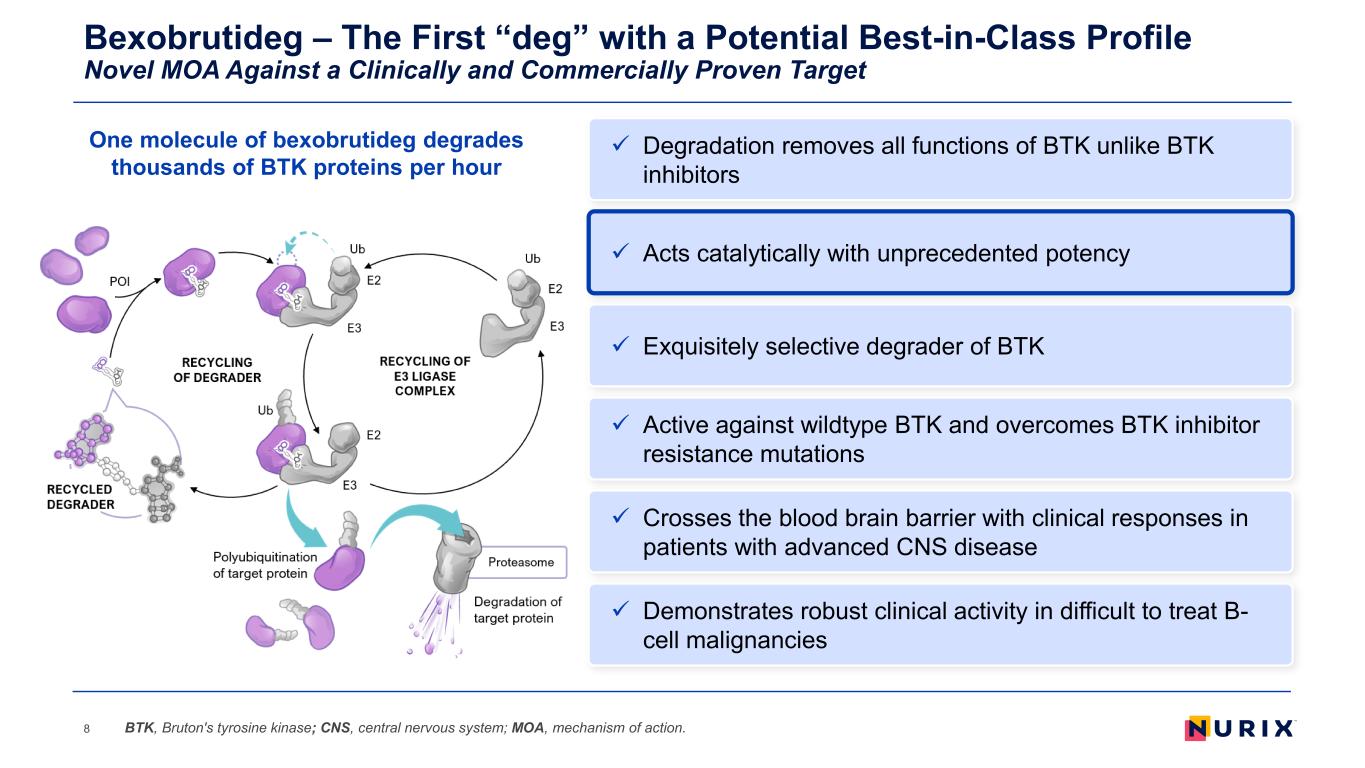
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 8 Exquisitely selective degrader of BTK Active against wildtype BTK and overcomes BTK inhibitor resistance mutations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Acts catalytically with unprecedented potency Crosses the blood brain barrier with clinical responses in patients with advanced CNS disease BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Degradation removes all functions of BTK unlike BTK inhibitors One molecule of bexobrutideg degrades thousands of BTK proteins per hour
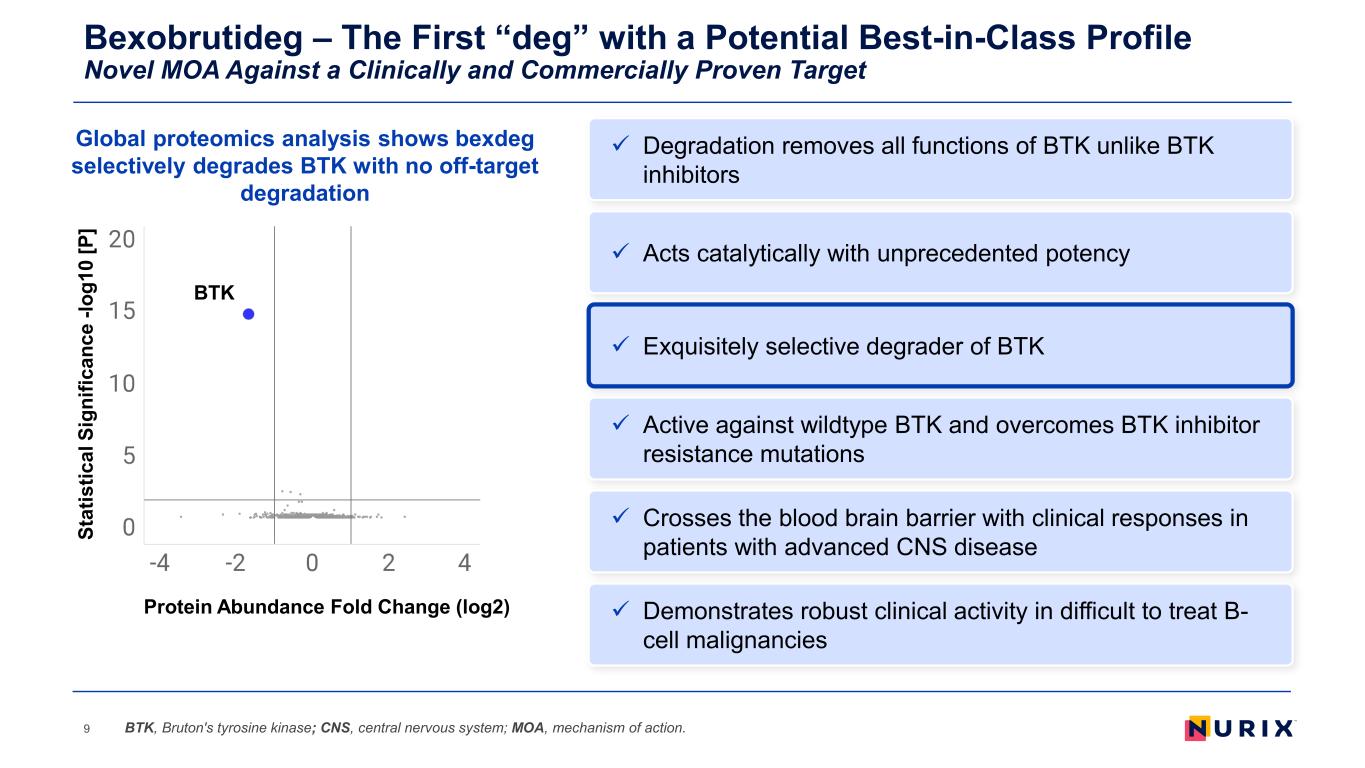
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 9 Exquisitely selective degrader of BTK Active against wildtype BTK and overcomes BTK inhibitor resistance mutations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Acts catalytically with unprecedented potency Crosses the blood brain barrier with clinical responses in patients with advanced CNS disease BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Degradation removes all functions of BTK unlike BTK inhibitors BTK St at is tic al S ig ni fic an ce -l og 10 [P ] Protein Abundance Fold Change (log2) Global proteomics analysis shows bexdeg selectively degrades BTK with no off-target degradation
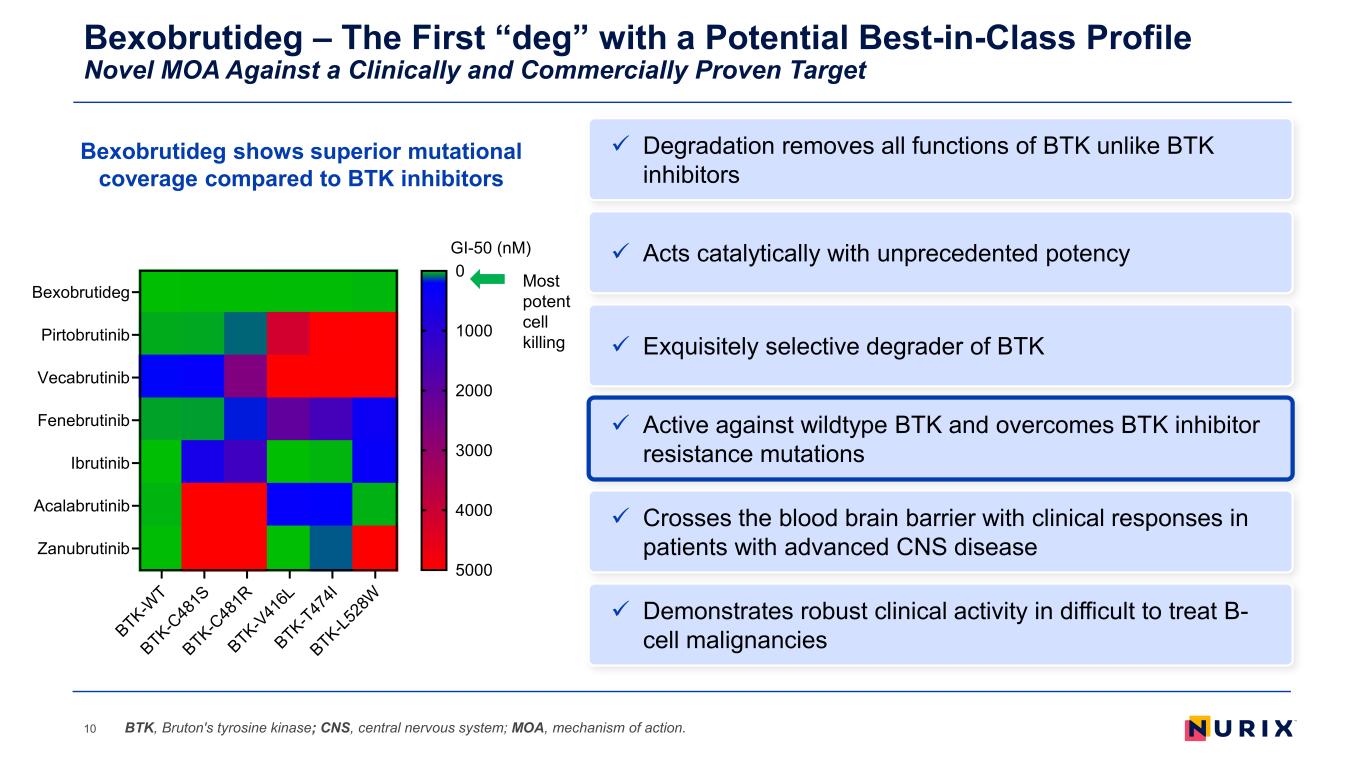
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 10 Exquisitely selective degrader of BTK Active against wildtype BTK and overcomes BTK inhibitor resistance mutations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Acts catalytically with unprecedented potency Crosses the blood brain barrier with clinical responses in patients with advanced CNS disease BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Degradation removes all functions of BTK unlike BTK inhibitors Bexobrutideg shows superior mutational coverage compared to BTK inhibitors Most potent cell killing BTK-W T BTK-C 48 1S BTK-C 48 1R BTK-V 41 6L BTK-T47 4I BTK-L5 28 W Bexobrutideg Pirtobrutinib Vecabrutinib Fenebrutinib Ibrutinib Acalabrutinib Zanubrutinib GI-50 (nM) 0 1000 2000 3000 4000 5000
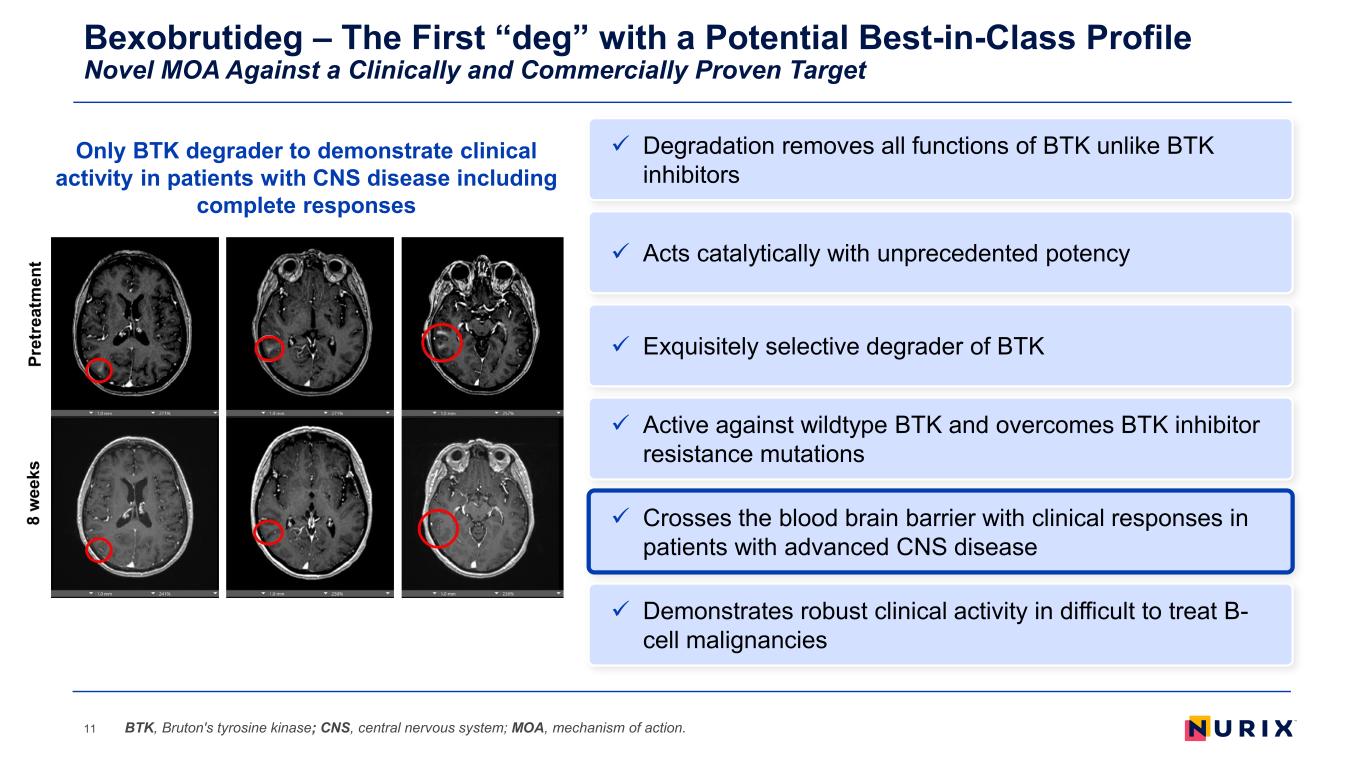
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 11 Exquisitely selective degrader of BTK Active against wildtype BTK and overcomes BTK inhibitor resistance mutations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Acts catalytically with unprecedented potency Crosses the blood brain barrier with clinical responses in patients with advanced CNS disease BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action. Degradation removes all functions of BTK unlike BTK inhibitors Only BTK degrader to demonstrate clinical activity in patients with CNS disease including complete responses
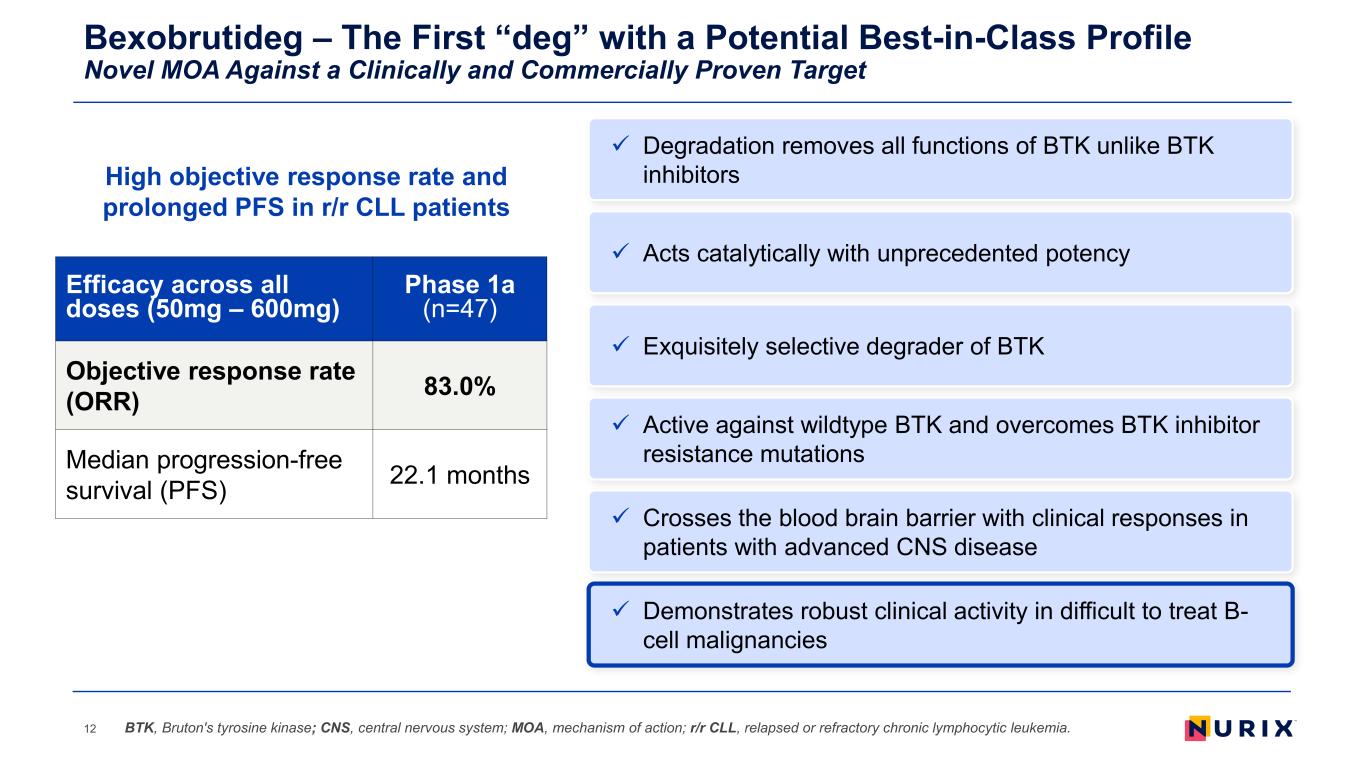
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 12 Exquisitely selective degrader of BTK Active against wildtype BTK and overcomes BTK inhibitor resistance mutations Demonstrates robust clinical activity in difficult to treat B- cell malignancies Acts catalytically with unprecedented potency Crosses the blood brain barrier with clinical responses in patients with advanced CNS disease BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action; r/r CLL, relapsed or refractory chronic lymphocytic leukemia. Degradation removes all functions of BTK unlike BTK inhibitorsHigh objective response rate and prolonged PFS in r/r CLL patients Efficacy across all doses (50mg – 600mg) Phase 1a (n=47) Objective response rate (ORR) 83.0% Median progression-free survival (PFS) 22.1 months
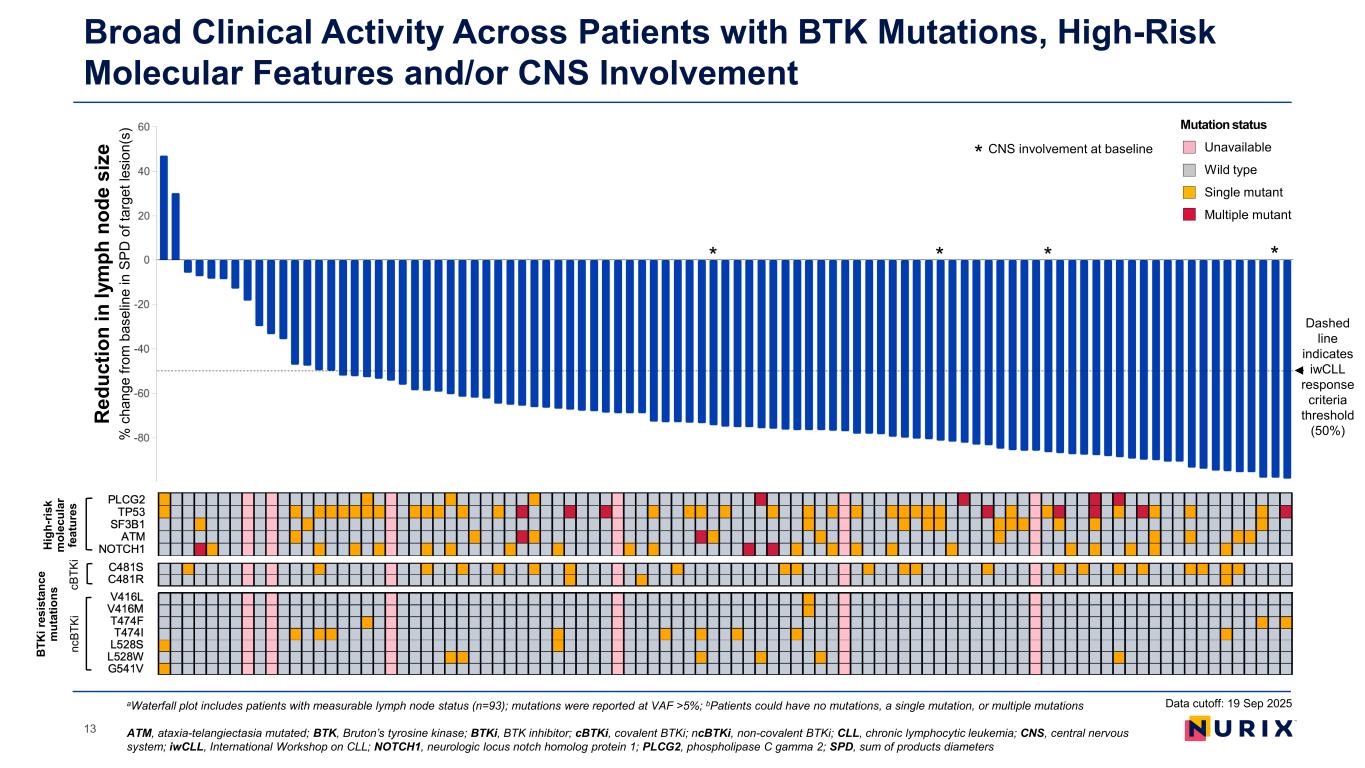
Broad Clinical Activity Across Patients with BTK Mutations, High-Risk Molecular Features and/or CNS Involvement aWaterfall plot includes patients with measurable lymph node status (n=93); mutations were reported at VAF >5%; bPatients could have no mutations, a single mutation, or multiple mutations ATM, ataxia-telangiectasia mutated; BTK, Bruton’s tyrosine kinase; BTKi, BTK inhibitor; cBTKi, covalent BTKi; ncBTKi, non-covalent BTKi; CLL, chronic lymphocytic leukemia; CNS, central nervous system; iwCLL, International Workshop on CLL; NOTCH1, neurologic locus notch homolog protein 1; PLCG2, phospholipase C gamma 2; SPD, sum of products diameters Dashed line indicates iwCLL response criteria threshold (50%) Data cutoff: 19 Sep 2025 CNS involvement at baseline * * *** R ed uc tio n in ly m ph n od e si ze % c ha ng e fro m b as el in e in S PD o f t ar ge t l es io n( s) Mutation status Unavailable Wild type Single mutant Multiple mutant Hi gh -r is k m ol ec ul ar fe at ur es B TK i r es is ta nc e m ut at io ns nc BT Ki cB TK i 13
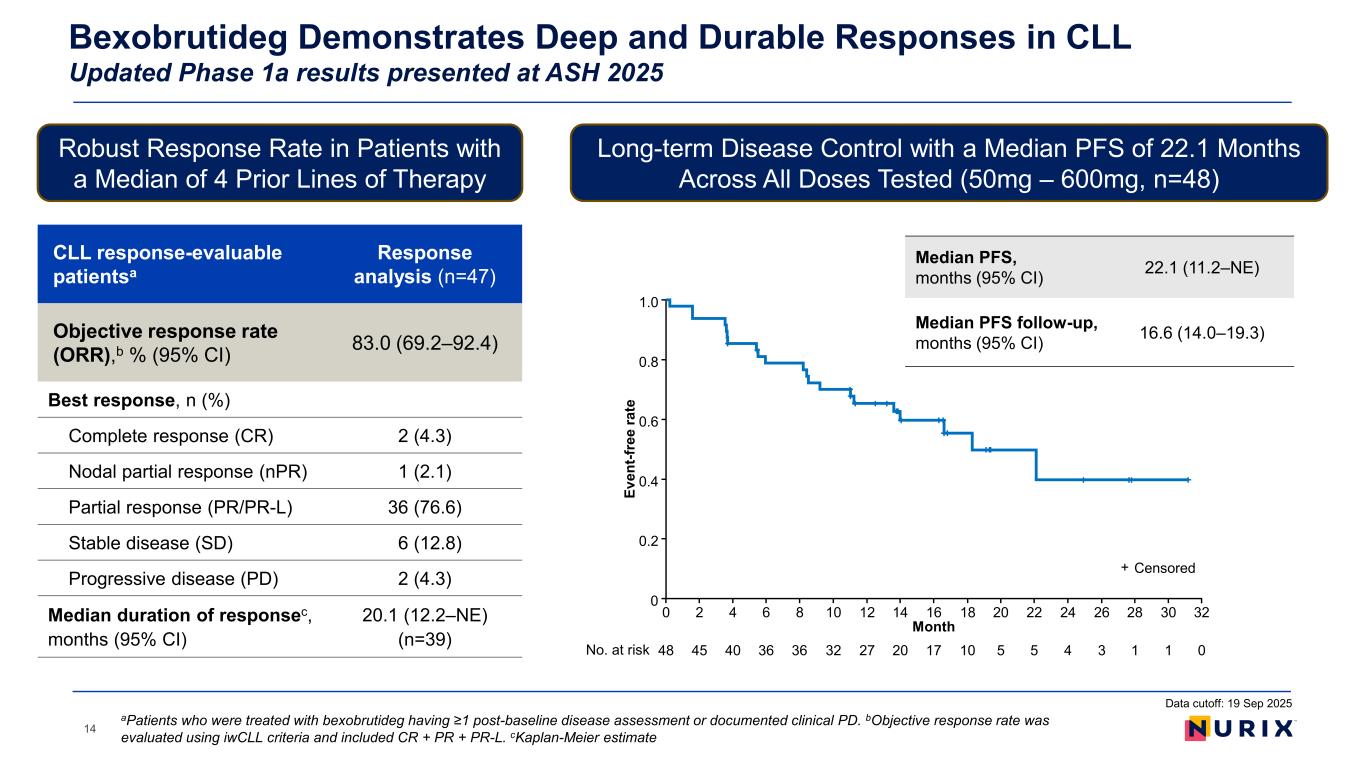
Bexobrutideg Demonstrates Deep and Durable Responses in CLL Updated Phase 1a results presented at ASH 2025 14 Robust Response Rate in Patients with a Median of 4 Prior Lines of Therapy Long-term Disease Control with a Median PFS of 22.1 Months Across All Doses Tested (50mg – 600mg, n=48) CLL response-evaluable patientsa Response analysis (n=47) Objective response rate (ORR),b % (95% CI) 83.0 (69.2–92.4) Best response, n (%) Complete response (CR) 2 (4.3) Nodal partial response (nPR) 1 (2.1) Partial response (PR/PR-L) 36 (76.6) Stable disease (SD) 6 (12.8) Progressive disease (PD) 2 (4.3) Median duration of responsec, months (95% CI) 20.1 (12.2–NE) (n=39) aPatients who were treated with bexobrutideg having ≥1 post-baseline disease assessment or documented clinical PD. bObjective response rate was evaluated using iwCLL criteria and included CR + PR + PR-L. cKaplan-Meier estimate Ev en t-f re e ra te Month 0 No. at risk 48 40 36 27 17 10 3 0 16 1.0 0.2 0.4 0.6 0.8 1412108642 30282624222018 45 36 32 20 5 5 4 1 1 0 32 Censored+ Median PFS, months (95% CI) 22.1 (11.2–NE) Median PFS follow-up, months (95% CI) 16.6 (14.0–19.3) Data cutoff: 19 Sep 2025
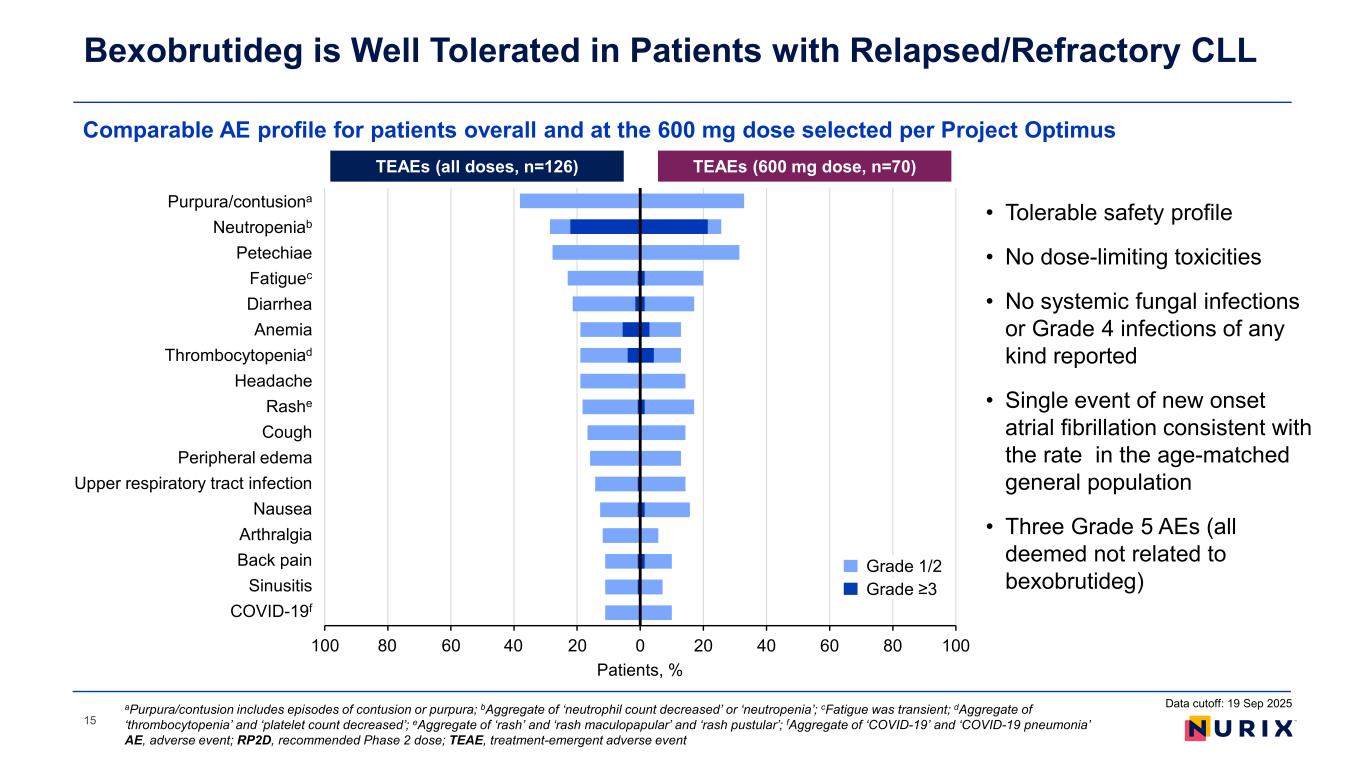
Comparable AE profile for patients overall and at the 600 mg dose selected per Project Optimus Bexobrutideg is Well Tolerated in Patients with Relapsed/Refractory CLL Data cutoff: 19 Sep 2025aPurpura/contusion includes episodes of contusion or purpura; bAggregate of ‘neutrophil count decreased’ or ‘neutropenia’; cFatigue was transient; dAggregate of ‘thrombocytopenia’ and ‘platelet count decreased’; eAggregate of ‘rash’ and ‘rash maculopapular’ and ‘rash pustular’; fAggregate of ‘COVID-19’ and ‘COVID-19 pneumonia’ AE, adverse event; RP2D, recommended Phase 2 dose; TEAE, treatment-emergent adverse event • Tolerable safety profile • No dose-limiting toxicities • No systemic fungal infections or Grade 4 infections of any kind reported • Single event of new onset atrial fibrillation consistent with the rate in the age-matched general population • Three Grade 5 AEs (all deemed not related to bexobrutideg) TEAEs (all doses, n=126) TEAEs (600 mg dose, n=70) Patients, % -100 -80 -60 -40 -20 0 20 40 60 80 100 COVID-19 Sinusitis Back pain Arthralgia Nausea Upper respiratory tract infection Peripheral edema Cough Rash Headache Thrombocytopenia Anemia Diarrhea Fatigue Petechiae Neutropenia Purpura/contusion 4080 Grade 1/2 Grade ≥3 Purpura/contusiona eutropeniab t i Fatiguec i rr i Thro bocytopeniad ashe ri r l r r ir t r tr t i f ti rt r l i i i iti f 15
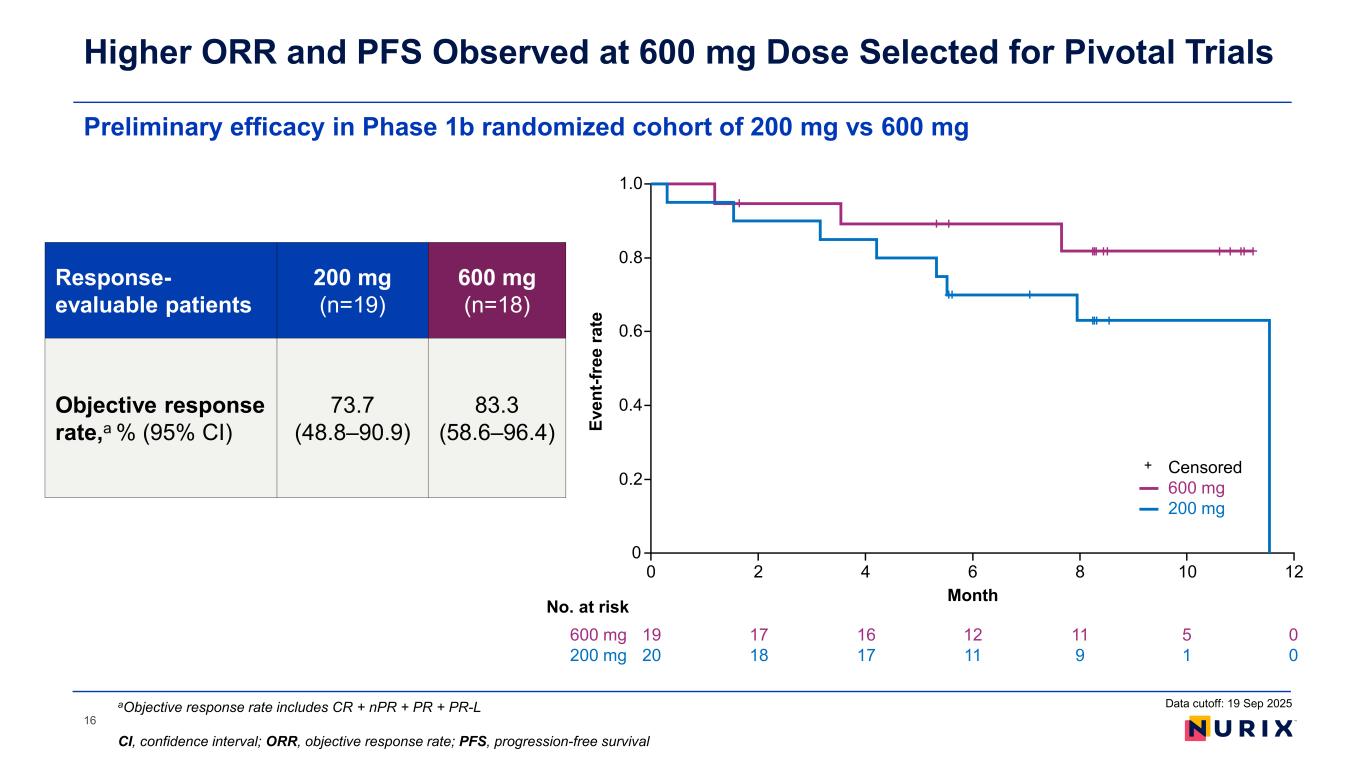
Preliminary efficacy in Phase 1b randomized cohort of 200 mg vs 600 mg Higher ORR and PFS Observed at 600 mg Dose Selected for Pivotal Trials Data cutoff: 19 Sep 2025aObjective response rate includes CR + nPR + PR + PR-L CI, confidence interval; ORR, objective response rate; PFS, progression-free survival Response- evaluable patients 200 mg (n=19) 600 mg (n=18) Objective response rate,a % (95% CI) 73.7 (48.8–90.9) 83.3 (58.6–96.4) Ev en t-f re e ra te Month 0 0 No. at risk 0.2 0.4 0.6 0.8 1.0 2 4 6 8 10 19 20 17 18 16 17 12 11 11 9 5 1 600 mg 200 mg 12 0 0 Censored 600 mg 200 mg + 16
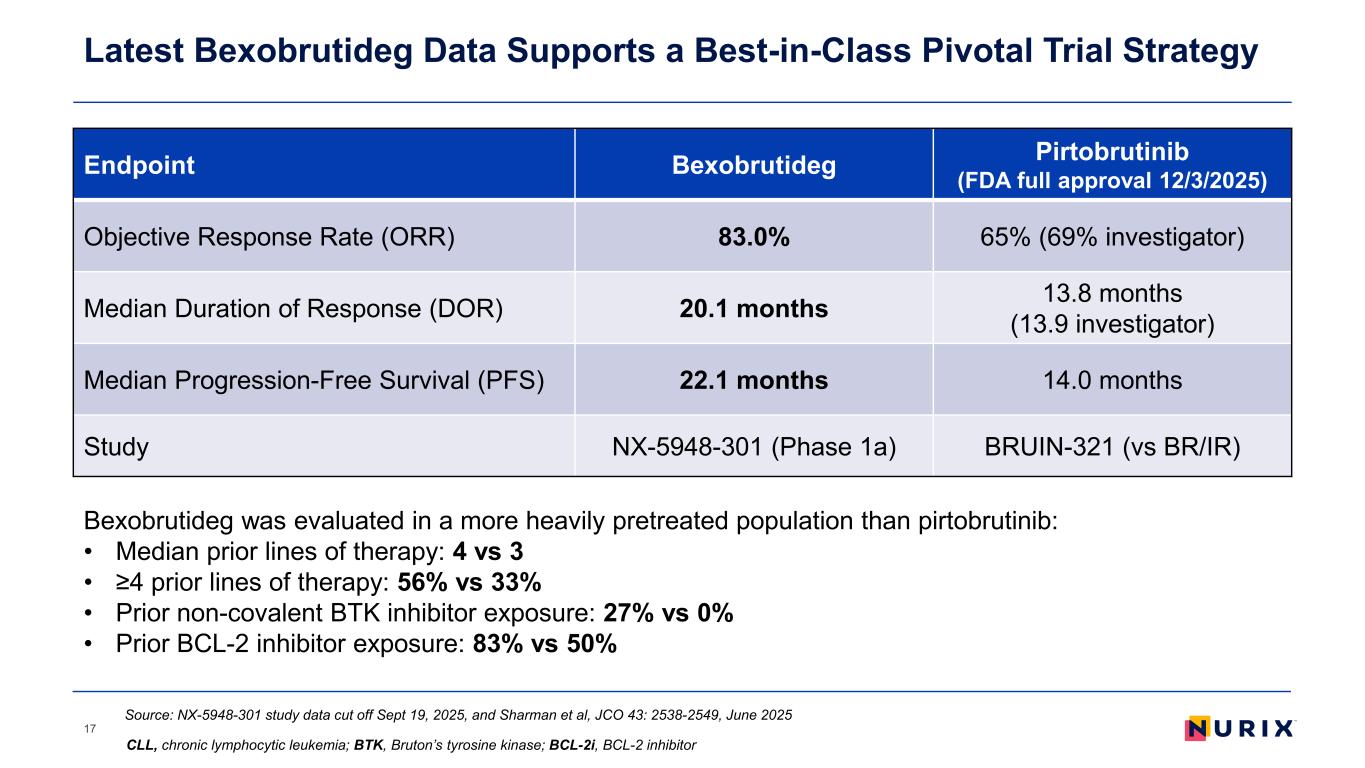
Latest Bexobrutideg Data Supports a Best-in-Class Pivotal Trial Strategy 17 Endpoint Bexobrutideg Pirtobrutinib (FDA full approval 12/3/2025) Objective Response Rate (ORR) 83.0% 65% (69% investigator) Median Duration of Response (DOR) 20.1 months 13.8 months (13.9 investigator) Median Progression-Free Survival (PFS) 22.1 months 14.0 months Study NX-5948-301 (Phase 1a) BRUIN-321 (vs BR/IR) Bexobrutideg was evaluated in a more heavily pretreated population than pirtobrutinib: • Median prior lines of therapy: 4 vs 3 • ≥4 prior lines of therapy: 56% vs 33% • Prior non-covalent BTK inhibitor exposure: 27% vs 0% • Prior BCL-2 inhibitor exposure: 83% vs 50% Source: NX-5948-301 study data cut off Sept 19, 2025, and Sharman et al, JCO 43: 2538-2549, June 2025 CLL, chronic lymphocytic leukemia; BTK, Bruton’s tyrosine kinase; BCL-2i, BCL-2 inhibitor
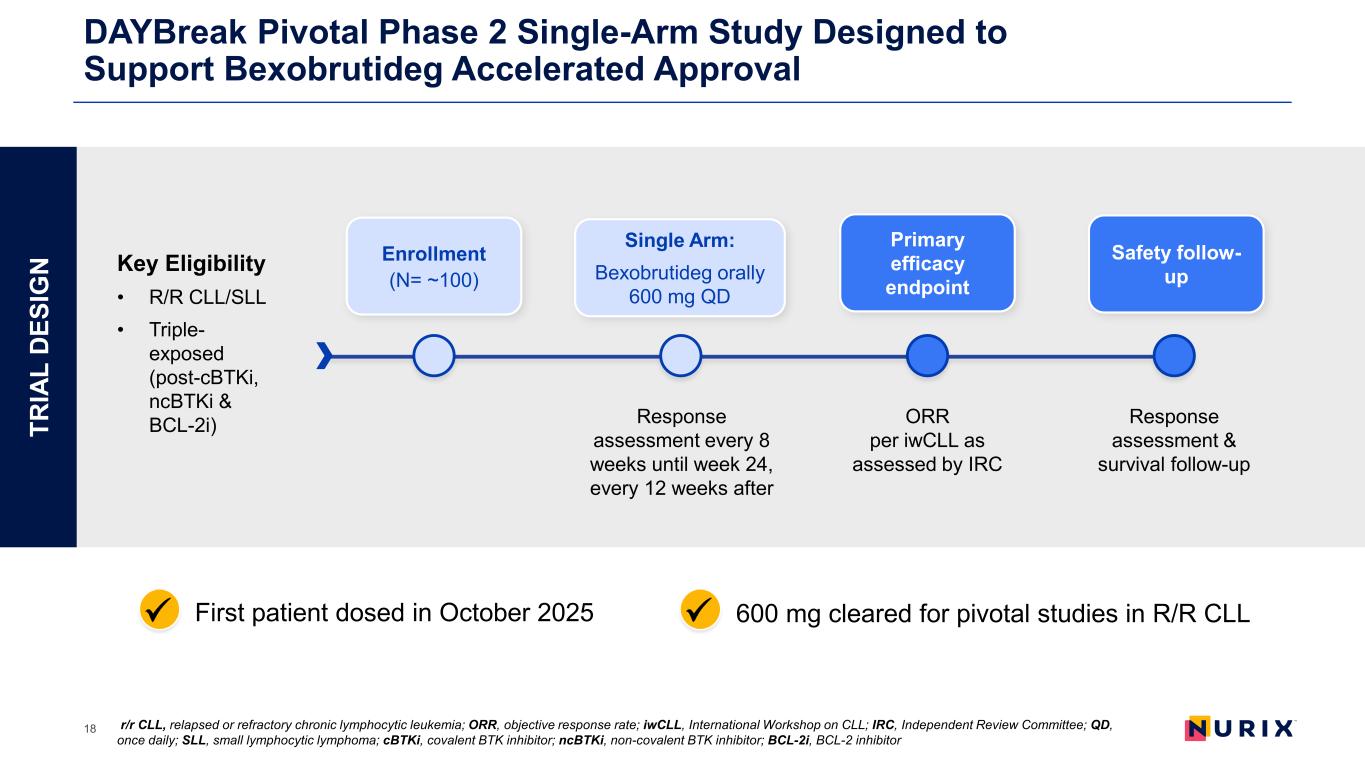
DAYBreak Pivotal Phase 2 Single-Arm Study Designed to Support Bexobrutideg Accelerated Approval 18 600 mg cleared for pivotal studies in R/R CLLFirst patient dosed in October 2025 Key Eligibility • R/R CLL/SLL • Triple- exposed (post-cBTKi, ncBTKi & BCL-2i) Response assessment every 8 weeks until week 24, every 12 weeks after ORR per iwCLL as assessed by IRC Response assessment & survival follow-up Enrollment (N= ~100) TR IA L D ES IG N Single Arm: Bexobrutideg orally 600 mg QD Primary efficacy endpoint Safety follow- up r/r CLL, relapsed or refractory chronic lymphocytic leukemia; ORR, objective response rate; iwCLL, International Workshop on CLL; IRC, Independent Review Committee; QD, once daily; SLL, small lymphocytic lymphoma; cBTKi, covalent BTK inhibitor; ncBTKi, non-covalent BTK inhibitor; BCL-2i, BCL-2 inhibitor
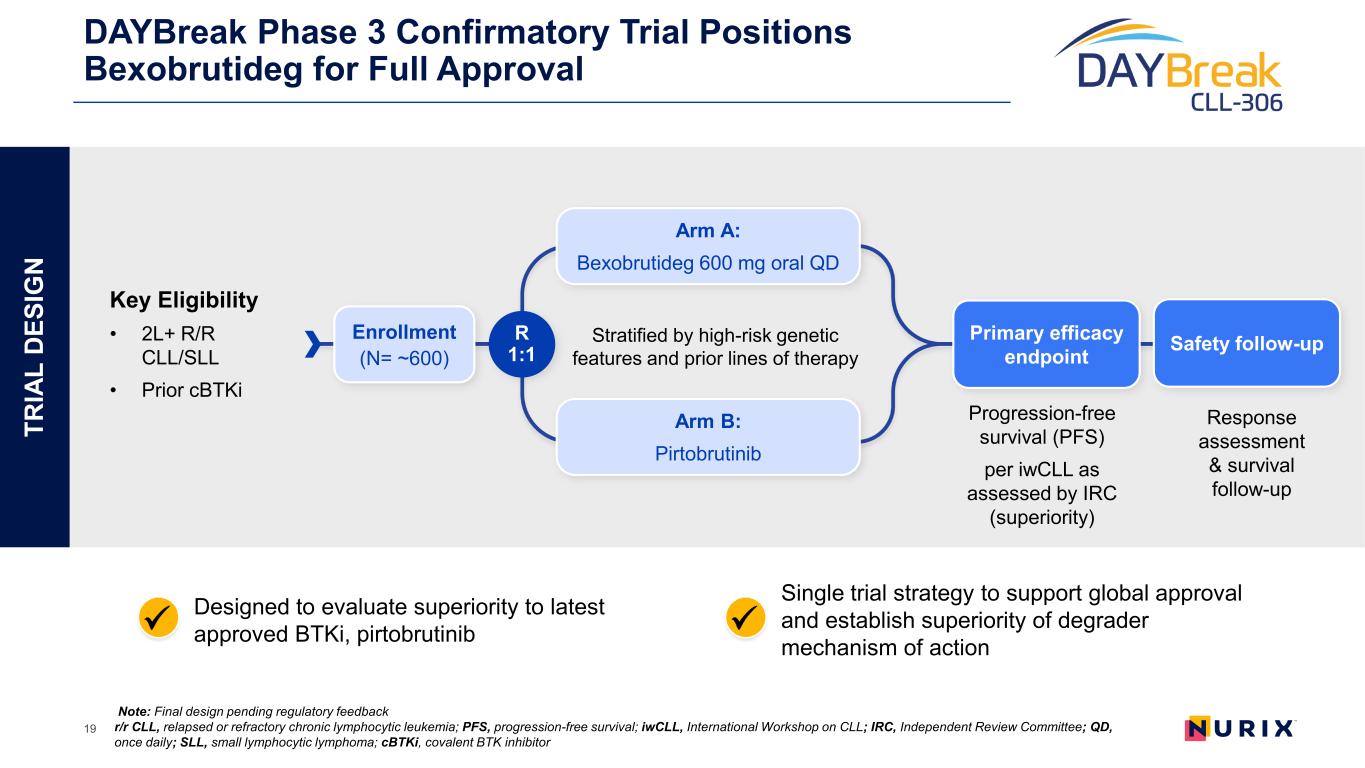
TR IA L D ES IG N DAYBreak Phase 3 Confirmatory Trial Positions Bexobrutideg for Full Approval 19 Stratified by high-risk genetic features and prior lines of therapy Note: Final design pending regulatory feedback r/r CLL, relapsed or refractory chronic lymphocytic leukemia; PFS, progression-free survival; iwCLL, International Workshop on CLL; IRC, Independent Review Committee; QD, once daily; SLL, small lymphocytic lymphoma; cBTKi, covalent BTK inhibitor R 1:1 Primary efficacy endpoint Enrollment (N= ~600) Progression-free survival (PFS) per iwCLL as assessed by IRC (superiority) Response assessment & survival follow-up Arm A: Bexobrutideg 600 mg oral QD Key Eligibility • 2L+ R/R CLL/SLL • Prior cBTKi Arm B: Pirtobrutinib Safety follow-up Single trial strategy to support global approval and establish superiority of degrader mechanism of action Designed to evaluate superiority to latest approved BTKi, pirtobrutinib
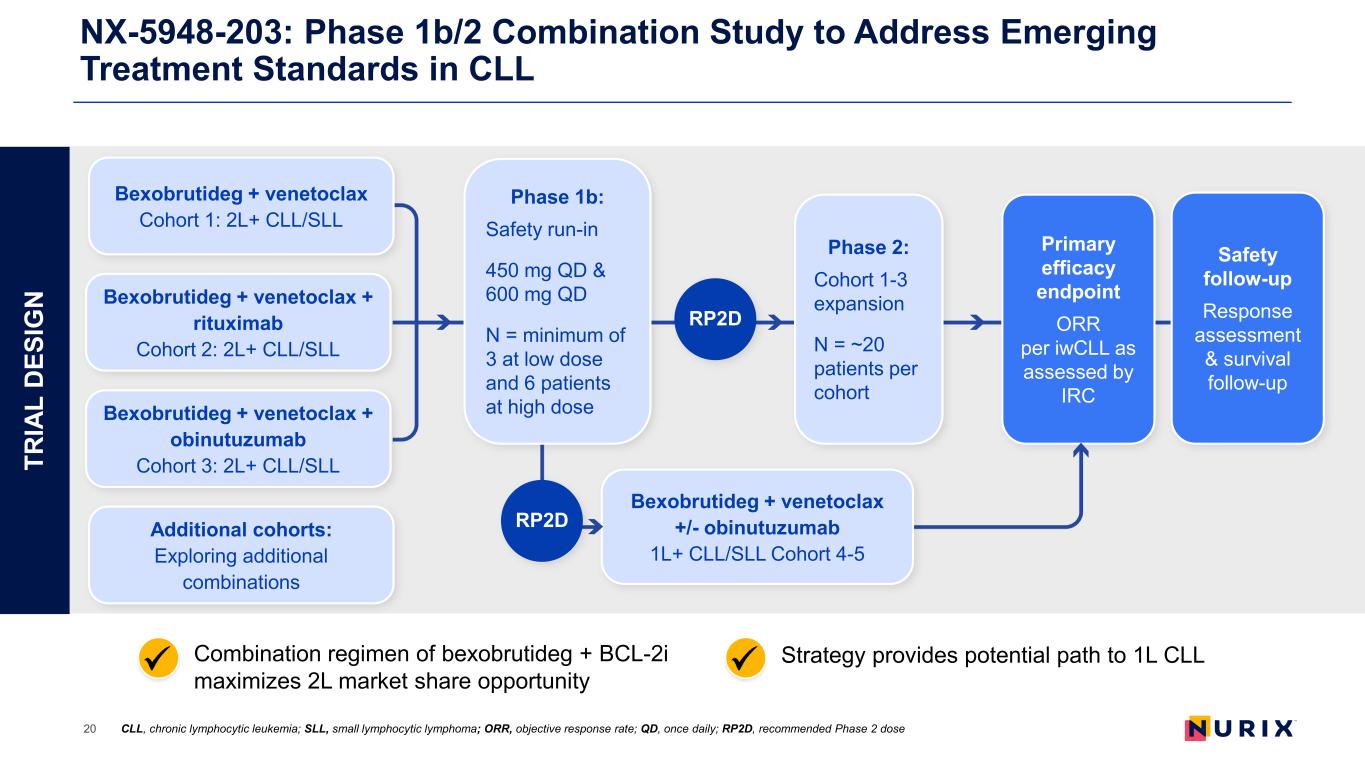
NX-5948-203: Phase 1b/2 Combination Study to Address Emerging Treatment Standards in CLL 20 CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; ORR, objective response rate; QD, once daily; RP2D, recommended Phase 2 dose Strategy provides potential path to 1L CLLCombination regimen of bexobrutideg + BCL-2i maximizes 2L market share opportunity TR IA L D ES IG N Bexobrutideg + venetoclax Cohort 1: 2L+ CLL/SLL Bexobrutideg + venetoclax + rituximab Cohort 2: 2L+ CLL/SLL Bexobrutideg + venetoclax + obinutuzumab Cohort 3: 2L+ CLL/SLL Additional cohorts: Exploring additional combinations RP2D Primary efficacy endpoint ORR per iwCLL as assessed by IRC Safety follow-up Response assessment & survival follow-up RP2D Phase 1b: Safety run-in 450 mg QD & 600 mg QD N = minimum of 3 at low dose and 6 patients at high dose Phase 2: Cohort 1-3 expansion N = ~20 patients per cohort Bexobrutideg + venetoclax +/- obinutuzumab 1L+ CLL/SLL Cohort 4-5
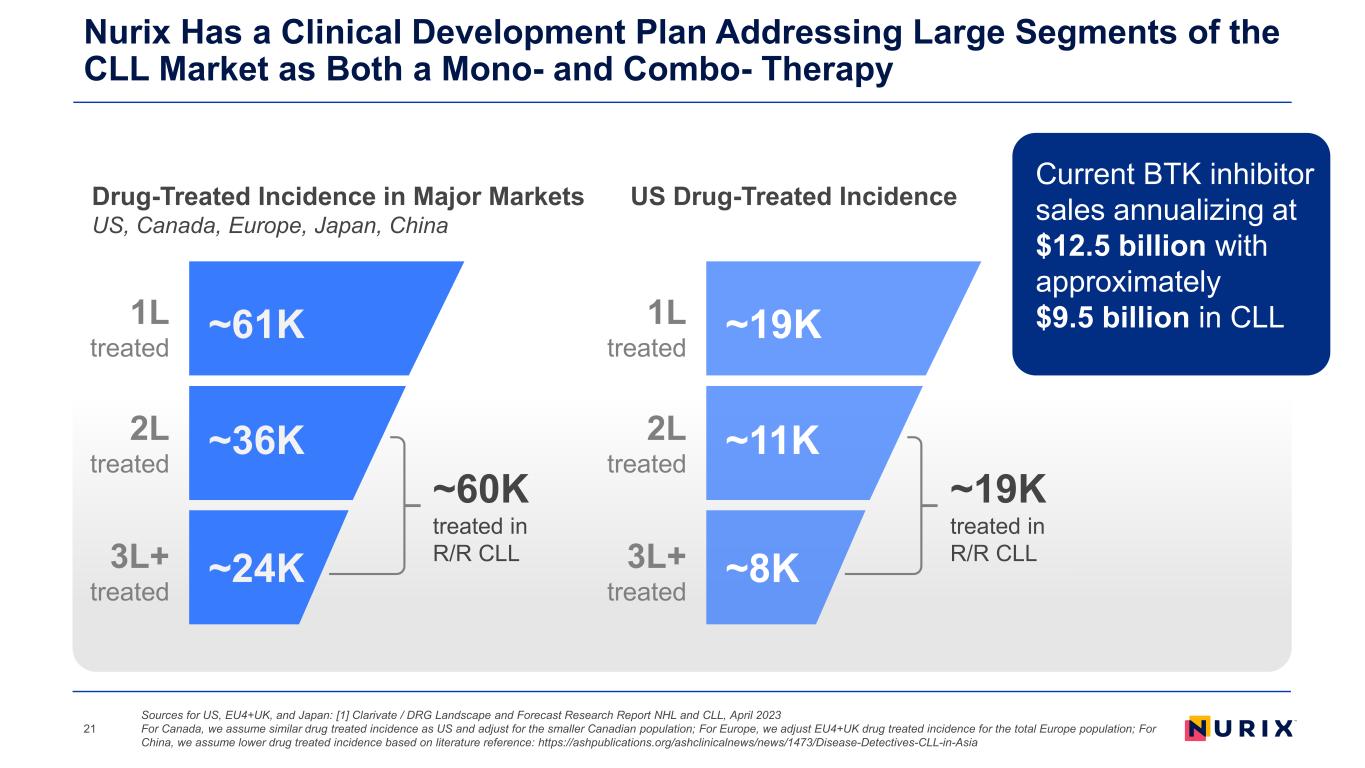
Nurix Has a Clinical Development Plan Addressing Large Segments of the CLL Market as Both a Mono- and Combo- Therapy 21 ~19K treated in R/R CLL ~19K1L treated ~11K2L treated ~8K3L+ treated US Drug-Treated IncidenceDrug-Treated Incidence in Major Markets US, Canada, Europe, Japan, China ~60K treated in R/R CLL ~61K1L treated ~36K2L treated ~24K3L+ treated Current BTK inhibitor sales annualizing at $12.5 billion with approximately $9.5 billion in CLL Sources for US, EU4+UK, and Japan: [1] Clarivate / DRG Landscape and Forecast Research Report NHL and CLL, April 2023 For Canada, we assume similar drug treated incidence as US and adjust for the smaller Canadian population; For Europe, we adjust EU4+UK drug treated incidence for the total Europe population; For China, we assume lower drug treated incidence based on literature reference: https://ashpublications.org/ashclinicalnews/news/1473/Disease-Detectives-CLL-in-Asia
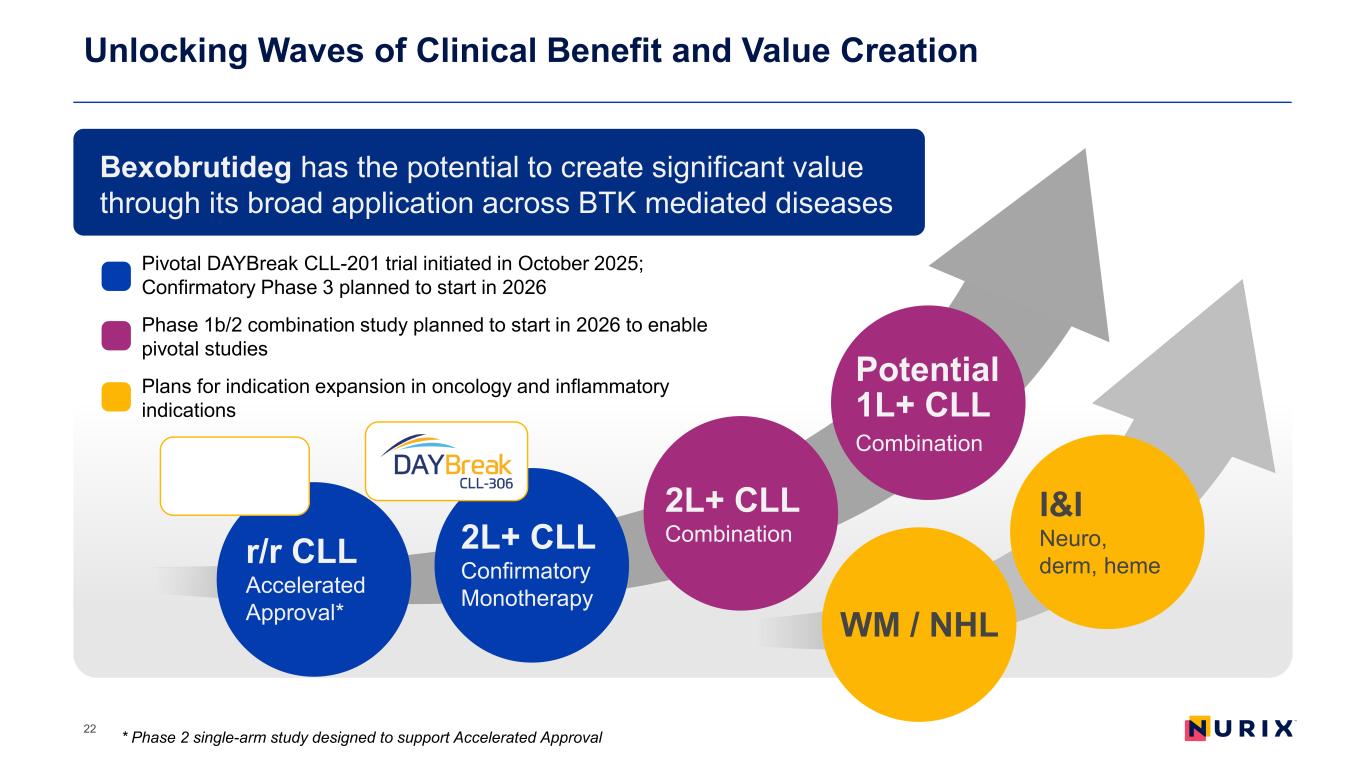
Unlocking Waves of Clinical Benefit and Value Creation 22 * Phase 2 single-arm study designed to support Accelerated Approval Pivotal DAYBreak CLL-201 trial initiated in October 2025; Confirmatory Phase 3 planned to start in 2026 Phase 1b/2 combination study planned to start in 2026 to enable pivotal studies Plans for indication expansion in oncology and inflammatory indications Bexobrutideg has the potential to create significant value through its broad application across BTK mediated diseases r/r CLL Accelerated Approval* 2L+ CLL Confirmatory Monotherapy 2L+ CLL Combination Potential 1L+ CLL Combination I&I Neuro, derm, heme WM / NHL
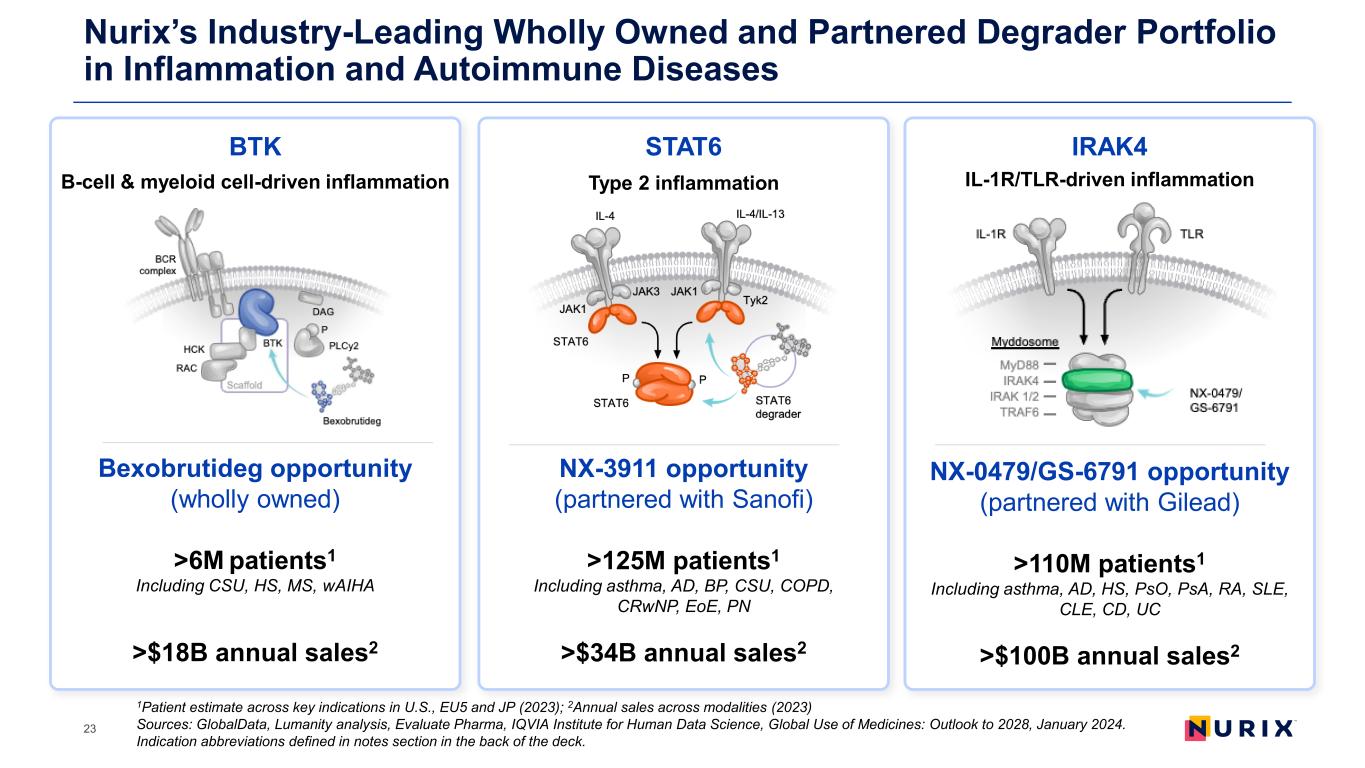
Nurix’s Industry-Leading Wholly Owned and Partnered Degrader Portfolio in Inflammation and Autoimmune Diseases 1Patient estimate across key indications in U.S., EU5 and JP (2023); 2Annual sales across modalities (2023) Sources: GlobalData, Lumanity analysis, Evaluate Pharma, IQVIA Institute for Human Data Science, Global Use of Medicines: Outlook to 2028, January 2024. Indication abbreviations defined in notes section in the back of the deck. 23 BTK Bexobrutideg opportunity (wholly owned) >6M patients1 Including CSU, HS, MS, wAIHA >$18B annual sales2 B-cell & myeloid cell-driven inflammation STAT6 Type 2 inflammation NX-3911 opportunity (partnered with Sanofi) >125M patients1 Including asthma, AD, BP, CSU, COPD, CRwNP, EoE, PN >$34B annual sales2 IRAK4 IL-1R/TLR-driven inflammation NX-0479/GS-6791 opportunity (partnered with Gilead) >110M patients1 Including asthma, AD, HS, PsO, PsA, RA, SLE, CLE, CD, UC >$100B annual sales2
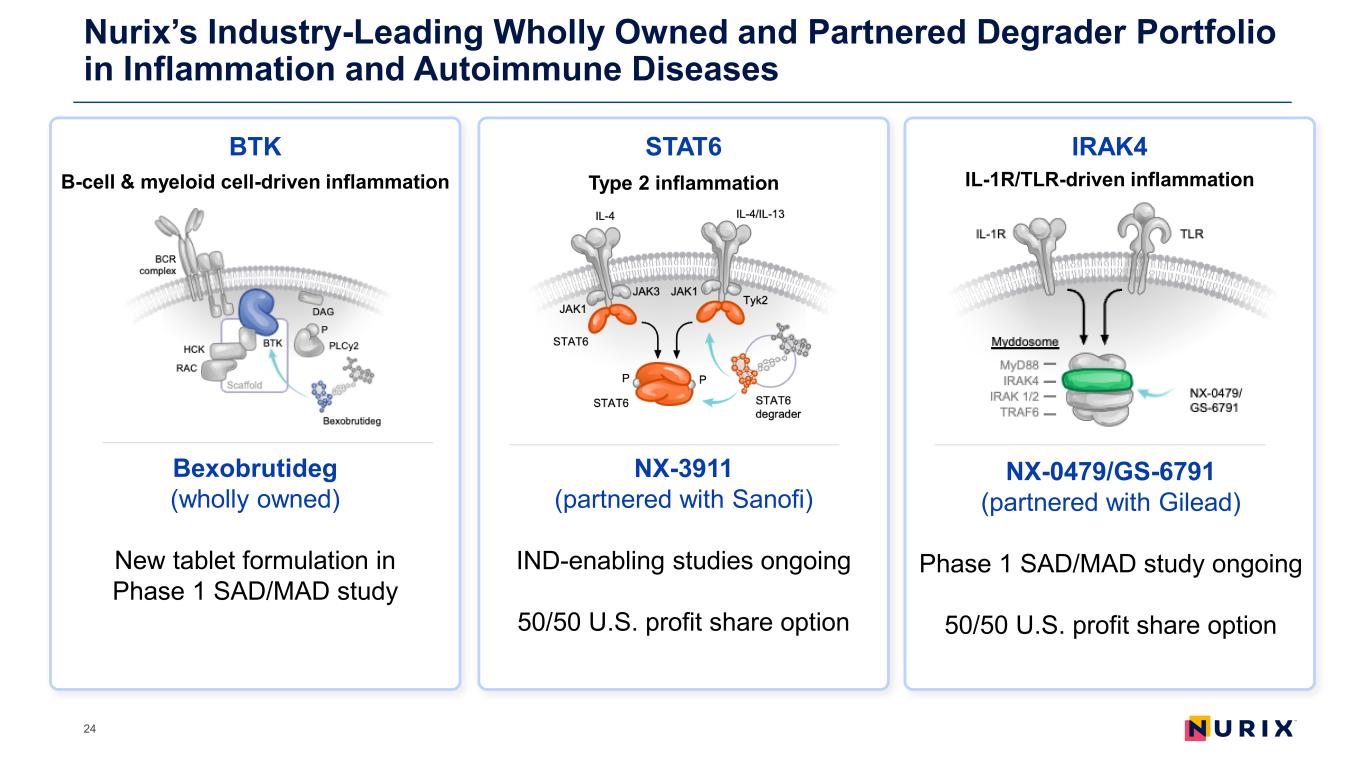
Nurix’s Industry-Leading Wholly Owned and Partnered Degrader Portfolio in Inflammation and Autoimmune Diseases 24 BTK Bexobrutideg (wholly owned) New tablet formulation in Phase 1 SAD/MAD study B-cell & myeloid cell-driven inflammation STAT6 Type 2 inflammation NX-3911 (partnered with Sanofi) IND-enabling studies ongoing 50/50 U.S. profit share option IRAK4 IL-1R/TLR-driven inflammation NX-0479/GS-6791 (partnered with Gilead) Phase 1 SAD/MAD study ongoing 50/50 U.S. profit share option
2026: Building the Future of Protein Degradation 25 Enrollment of Pivotal Phase 2 trial – DAYBreak CLL-201 Initiation of bexobrutideg confirmatory Phase 3 study in r/r CLL – DAYBreak CLL-306 Initiation of bexobrutideg combination study in CLL Potential GS-6791 IRAK4 degrader Phase 1 results from Gilead* Potential NX-3911 STAT6 degrader IND filing by Sanofi* Bexobrutideg new tablet formulation SAD/MAD study supporting IND in I&I Bexobrutideg Phase 1a/b CLL cohorts Bexobrutideg Phase 1a/b NHL cohorts Zelebrudomide Phase 1a cohorts Execute Pivotal Development Pathway in CLL Advance Degrader Programs in I&I Clinical Data Updates * Nurix estimate for partnered programs using industry standard timelines based on current stage of development (not official guidance of partners).

26 Q&A
Abbreviations • AD = Atopic dermatitis • BP = Bullous pemphigoid • COPD = Chronic obstructive pulmonary disease • CD = Crohn’s disease • CRwNP = Chronic rhinosinusitis with nasal polyps • CSU = Chronic spontaneous urticaria • CLE = Cutaneous lupus erythematosus • EoE = Eosinophilic esophagitis • wAIHA = Warm autoimmune hemolytic anemia • HS = Hidradenitis suppurativa • MS = Multiple sclerosis • PsA = Psoriatic Arthritis • PN = Prurigo nodularis • RA = Rheumatoid arthritis • SLE = Systemic lupus erythematosus without Lupus Nephritis • UC = Ulcerative Colitis 27