| ATI-052: Anti-TSLP x IL-4Rα Bispecific Antibody Program Highly Potent and Bioactive Investigational Product Candidate January 6, 2026 |
| 2 Any statements contained in this presentation that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “anticipate,” “believe,” “expect,” “intend,” “may,” “plan,” “potential,” “will,” and similar expressions, and are based on Aclaris’ current beliefs and expectations. These forward-looking statements include expectations regarding its development plans for ATI-052, including the timing to initiate and report results from its Phase 1b trials of ATI-052 in asthma and atopic dermatitis (AD), the timing to initiate a Phase 2b trial of ATI-052 in AD, potential indications for future development, and the therapeutic potential for ATI-052, including the potential to be best-in-class, the potential to show superior activity compared to other therapies, and the potential for dosing of up to three months. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the conduct of clinical trials, potential changes to interim, top line, and preliminary data as more subject data become available, Aclaris’ reliance on third parties over which it may not always have full control, Aclaris’ ability to enter into strategic partnerships on commercially reasonable terms, the uncertainty regarding the macroeconomic environment and other risks and uncertainties that are described in the “Risk Factors” section of Aclaris’ Annual Report on Form 10-K for the year ended December 31, 2024, and other filings Aclaris makes with the U.S. Securities and Exchange Commission from time to time. These documents are available under the “SEC Filings” page of the “Investors” section of Aclaris’ website at www.aclaristx.com. Any forward-looking statements speak only as of the date of this presentation and are based on information available to Aclaris as of the date of this presentation, and Aclaris assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise. Tradenames, trademarks and service marks of other companies appearing in this presentation are the property of their respective owners. All future development, clinical, and regulatory timelines are expectations, are based on current beliefs and assumptions, and are subject to change based on a variety of factors. Disclaimer and Cautionary Note Regarding Forward-Looking Statements |
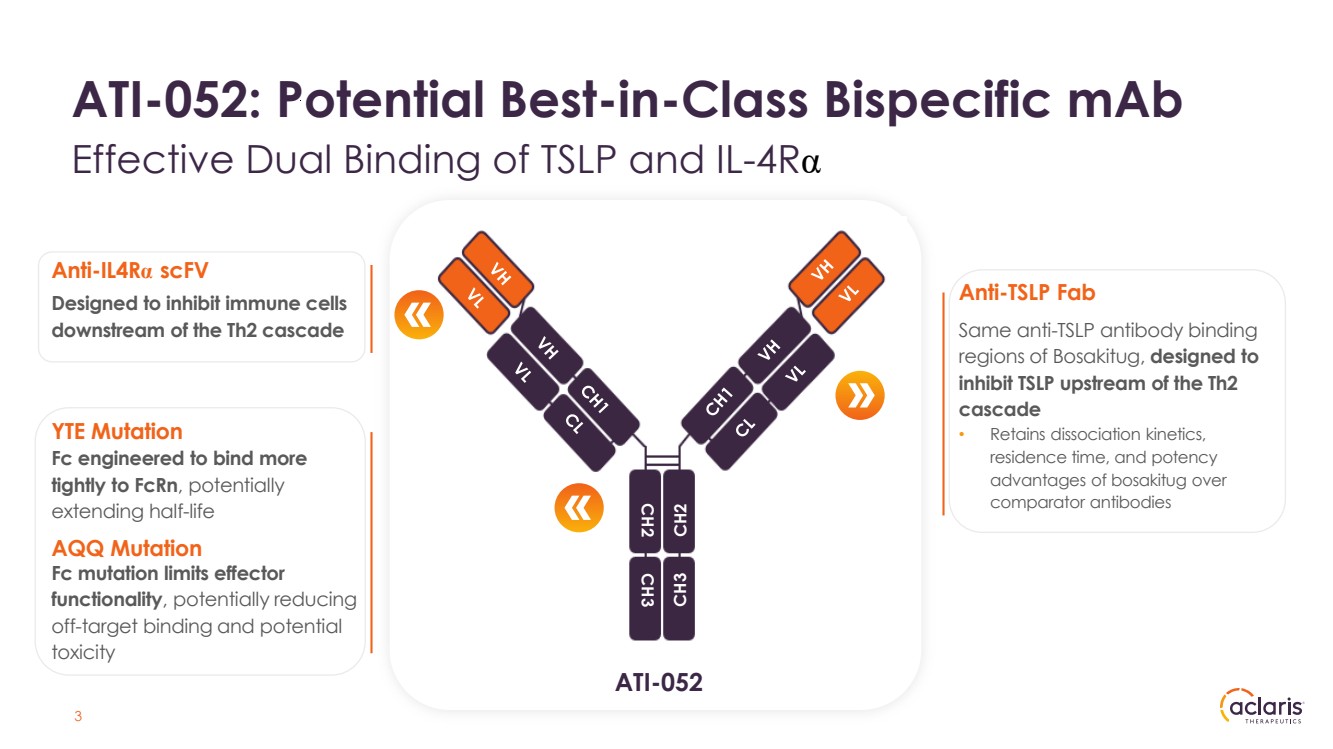
| ATI-052: Potential Best-in-Class Bispecific mAb Effective Dual Binding of TSLP and IL-4Rα ATI-052 CH2 CH3 CH2 CH3 3 Same anti-TSLP antibody binding regions of Bosakitug, designed to inhibit TSLP upstream of the Th2 cascade • Retains dissociation kinetics, residence time, and potency advantages of bosakitug over comparator antibodies Designed to inhibit immune cells downstream of the Th2 cascade Anti-TSLP Fab Anti-IL4Rα scFV Fc engineered to bind more tightly to FcRn, potentially extending half-life YTE Mutation Fc mutation limits effector functionality, potentially reducing off-target binding and potential toxicity AQQ Mutation |
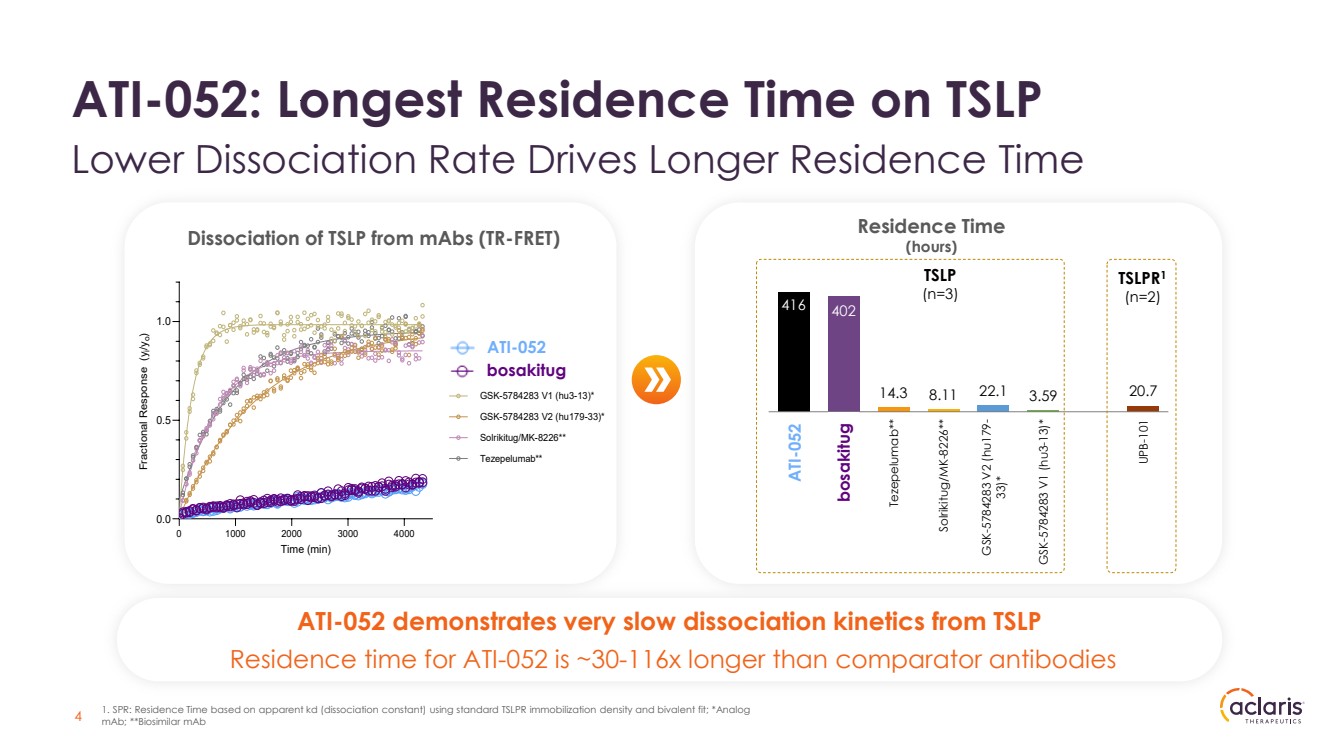
| Dissociation of TSLP from mAbs (TR-FRET) 0 1000 2000 3000 4000 0.0 0.5 1.0 Time (min) Fractional Response (yi/yo) ATI-045 ATI-052 GSK-5784283 V1 (hu3-13)* GSK-5784283 V2 (hu179-33)* Solrikitug/MK-8226** Tezepelumab** Residence Time (hours) 416 402 14.3 8.11 22.1 3.59 20.7 ATI-052 ATI-045 Tezepelumab** Solrikitug/MK-8226** GSK-5784283 V2 (hu179- 33)* GSK-5784283 V1 (hu3-13)* UPB-101 TSLPR1 (n=2) TSLP (n=3) bosakitug ATI-052 demonstrates very slow dissociation kinetics from TSLP Residence time for ATI-052 is ~30-116x longer than comparator antibodies 4 ATI-052: Longest Residence Time on TSLP Lower Dissociation Rate Drives Longer Residence Time 1. SPR: Residence Time based on apparent kd (dissociation constant) using standard TSLPR immobilization density and bivalent fit; *Analog mAb; **Biosimilar mAb bosakitug ATI-052 ATI-052 |
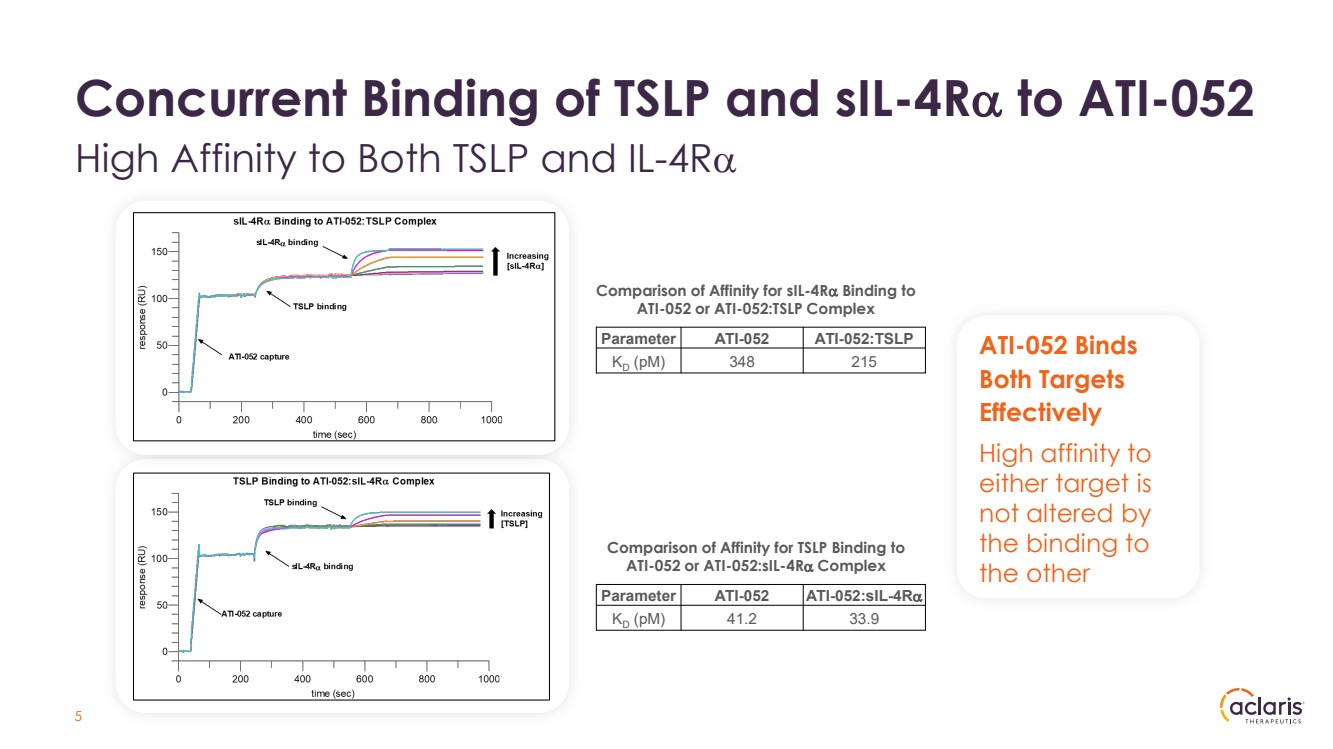
| Concurrent Binding of TSLP and sIL-4Rα to ATI-052 High Affinity to Both TSLP and IL-4Rα 5 a ATI-052 Binds Both Targets Effectively High affinity to either target is not altered by the binding to the other Comparison of Affinity for sIL-4Rα Binding to ATI-052 or ATI-052:TSLP Complex Comparison of Affinity for TSLP Binding to ATI-052 or ATI-052:sIL-4Rα Complex Parameter ATI-052 ATI-052:TSLP KD (pM) 348 215 Parameter ATI-052 ATI-052:sIL-4Rα KD (pM) 41.2 33.9 |
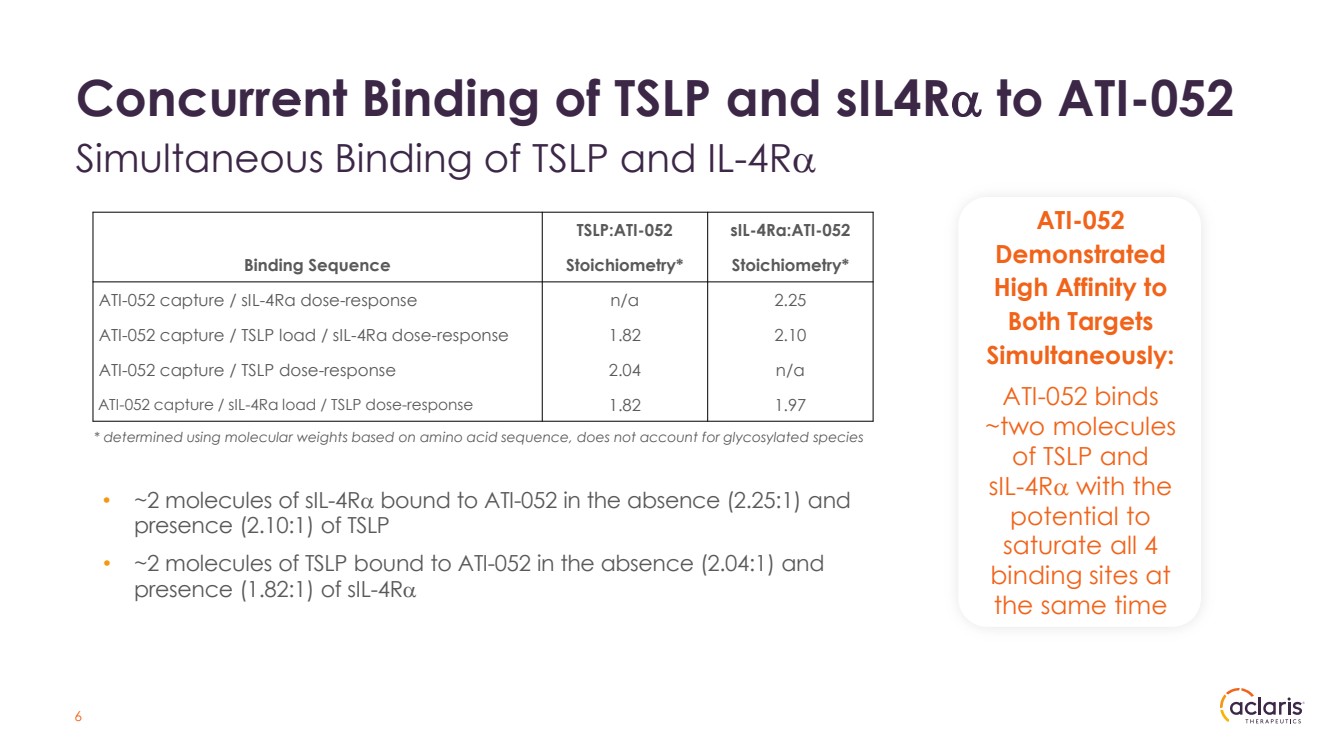
| Concurrent Binding of TSLP and sIL4Rα to ATI-052 Simultaneous Binding of TSLP and IL-4Rα 6 a ATI-052 Demonstrated High Affinity to Both Targets Simultaneously: ATI-052 binds ~two molecules of TSLP and sIL-4Rα with the potential to saturate all 4 binding sites at the same time TSLP:ATI-052 sIL-4Ra:ATI-052 Binding Sequence Stoichiometry* Stoichiometry* ATI-052 capture / sIL-4Ra dose-response n/a 2.25 ATI-052 capture / TSLP load / sIL-4Ra dose-response 1.82 2.10 ATI-052 capture / TSLP dose-response 2.04 n/a ATI-052 capture / sIL-4Ra load / TSLP dose-response 1.82 1.97 * determined using molecular weights based on amino acid sequence, does not account for glycosylated species • ~2 molecules of sIL-4Rα bound to ATI-052 in the absence (2.25:1) and presence (2.10:1) of TSLP • ~2 molecules of TSLP bound to ATI-052 in the absence (2.04:1) and presence (1.82:1) of sIL-4Rα |
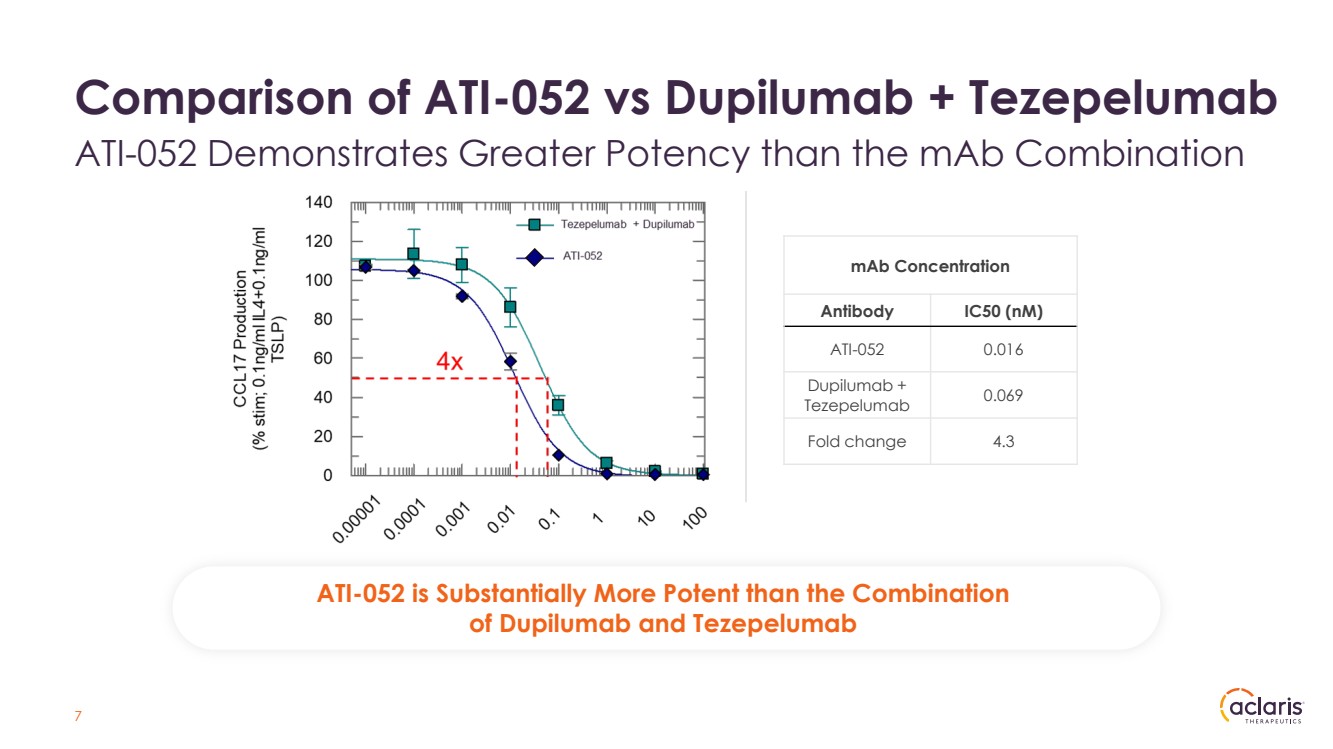
| Comparison of ATI-052 vs Dupilumab + Tezepelumab ATI-052 Demonstrates Greater Potency than the mAb Combination ATI-052 is Substantially More Potent than the Combination of Dupilumab and Tezepelumab mAb Concentration Antibody IC50 (nM) ATI-052 0.016 Dupilumab + Tezepelumab 0.069 Fold change 4.3 7 |
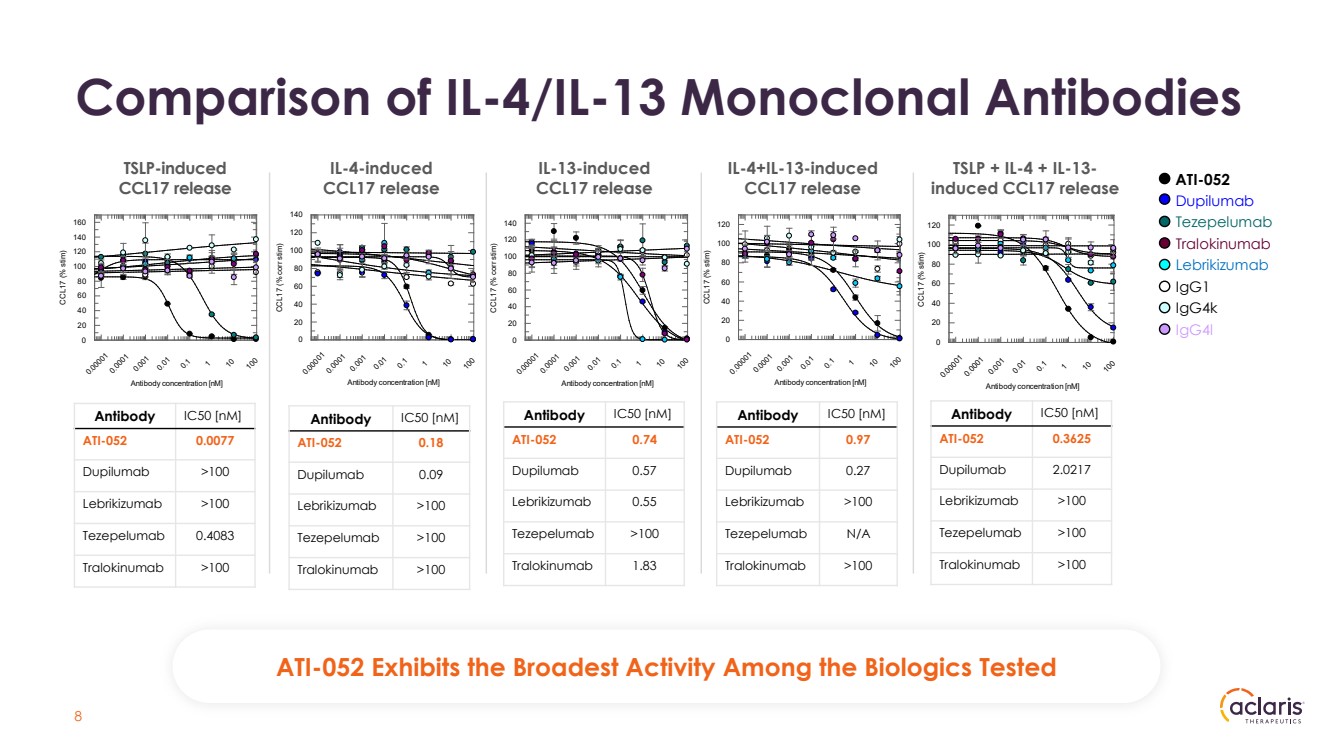
| Comparison of IL-4/IL-13 Monoclonal Antibodies IL-4-induced CCL17 release IL-13-induced CCL17 release TSLP-induced CCL17 release IL-4+IL-13-induced CCL17 release Antibody concentration [nM] 0.00001 0.0001 0.001 0.01 0.1 1 10 100 CCL17 (% stim) 0 20 40 60 80 100 120 140 160 Antibody concentration [nM] 0.00001 0.0001 0.001 0.01 0.1 1 10 100 CCL17 (% corr stim) 0 20 40 60 80 100 120 140 Antibody concentration [nM] 0.00001 0.0001 0.001 0.01 0.1 1 10 100 CCL17 (% corr stim) 0 20 40 60 80 100 120 140 Antibody concentration [nM] 0.00001 0.0001 0.001 0.01 0.1 1 10 100 CCL17 (% stim) 0 20 40 60 80 100 120 TSLP + IL-4 + IL-13- induced CCL17 release Antibody concentration [nM] 0.00001 0.0001 0.001 0.01 0.1 1 10 100 CCL17 (% stim) 0 20 40 60 80 100 120 ATI-052 Dupilumab Tezepelumab Tralokinumab Lebrikizumab IgG1 IgG4k IgG4l 8 Antibody IC50 [nM] ATI-052 0.0077 Dupilumab >100 Lebrikizumab >100 Tezepelumab 0.4083 Tralokinumab >100 Antibody IC50 [nM] ATI-052 0.18 Dupilumab 0.09 Lebrikizumab >100 Tezepelumab >100 Tralokinumab >100 Antibody IC50 [nM] ATI-052 0.74 Dupilumab 0.57 Lebrikizumab 0.55 Tezepelumab >100 Tralokinumab 1.83 Antibody IC50 [nM] ATI-052 0.97 Dupilumab 0.27 Lebrikizumab >100 Tezepelumab N/A Tralokinumab >100 Antibody IC50 [nM] ATI-052 0.3625 Dupilumab 2.0217 Lebrikizumab >100 Tezepelumab >100 Tralokinumab >100 ATI-052 Exhibits the Broadest Activity Among the Biologics Tested |
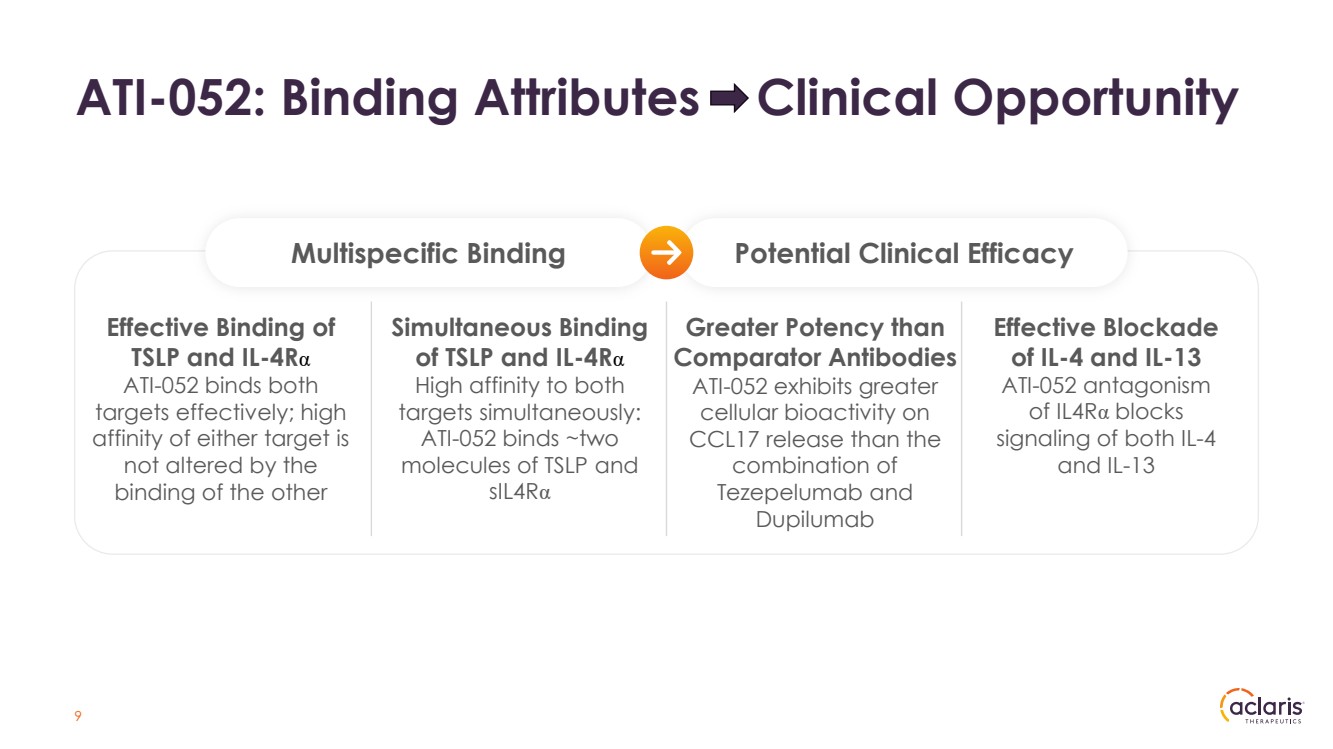
| 9 ATI-052: Binding Attributes Clinical Opportunity Multispecific Binding Potential Clinical Efficacy Simultaneous Binding of TSLP and IL-4Rα High affinity to both targets simultaneously: ATI-052 binds ~two molecules of TSLP and sIL4Rα Effective Binding of TSLP and IL-4Rα ATI-052 binds both targets effectively; high affinity of either target is not altered by the binding of the other Greater Potency than Comparator Antibodies ATI-052 exhibits greater cellular bioactivity on CCL17 release than the combination of Tezepelumab and Dupilumab Effective Blockade of IL-4 and IL-13 ATI-052 antagonism of IL4Rα blocks signaling of both IL-4 and IL-13 |
| Positive Interim Data: ATI-052 Healthy Volunteer Phase 1a SAD and MAD Trial Patient Focused Innovation Interim results as of December 31, 2025 |
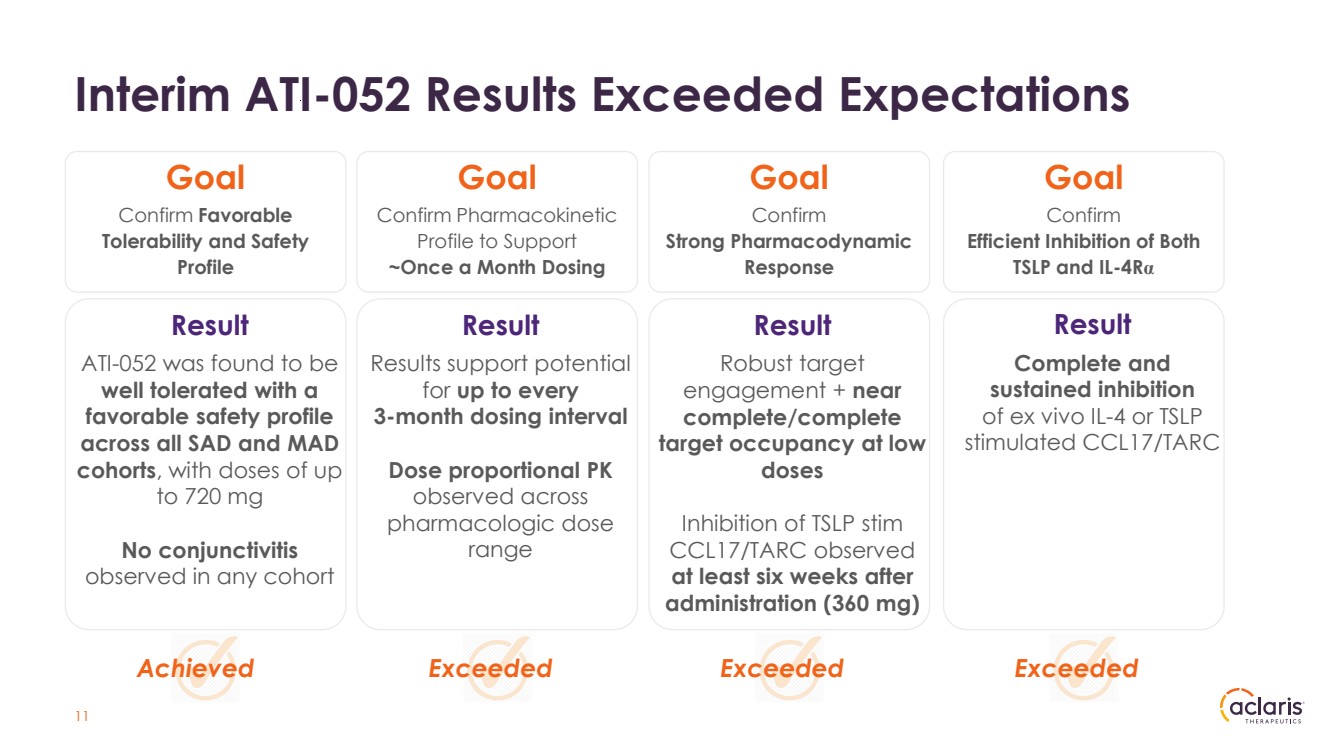
| Interim ATI-052 Results Exceeded Expectations 11 Result ATI-052 was found to be well tolerated with a favorable safety profile across all SAD and MAD cohorts, with doses of up to 720 mg No conjunctivitis observed in any cohort Confirm Favorable Tolerability and Safety Profile Confirm Pharmacokinetic Profile to Support ~Once a Month Dosing Confirm Strong Pharmacodynamic Response Confirm Efficient Inhibition of Both TSLP and IL-4Rα Result Results support potential for up to every 3-month dosing interval Dose proportional PK observed across pharmacologic dose range Result Robust target engagement + near complete/complete target occupancy at low doses Inhibition of TSLP stim CCL17/TARC observed at least six weeks after administration (360 mg) Result Complete and sustained inhibition of ex vivo IL-4 or TSLP stimulated CCL17/TARC Goal Goal Goal Goal Achieved Exceeded Exceeded Exceeded |
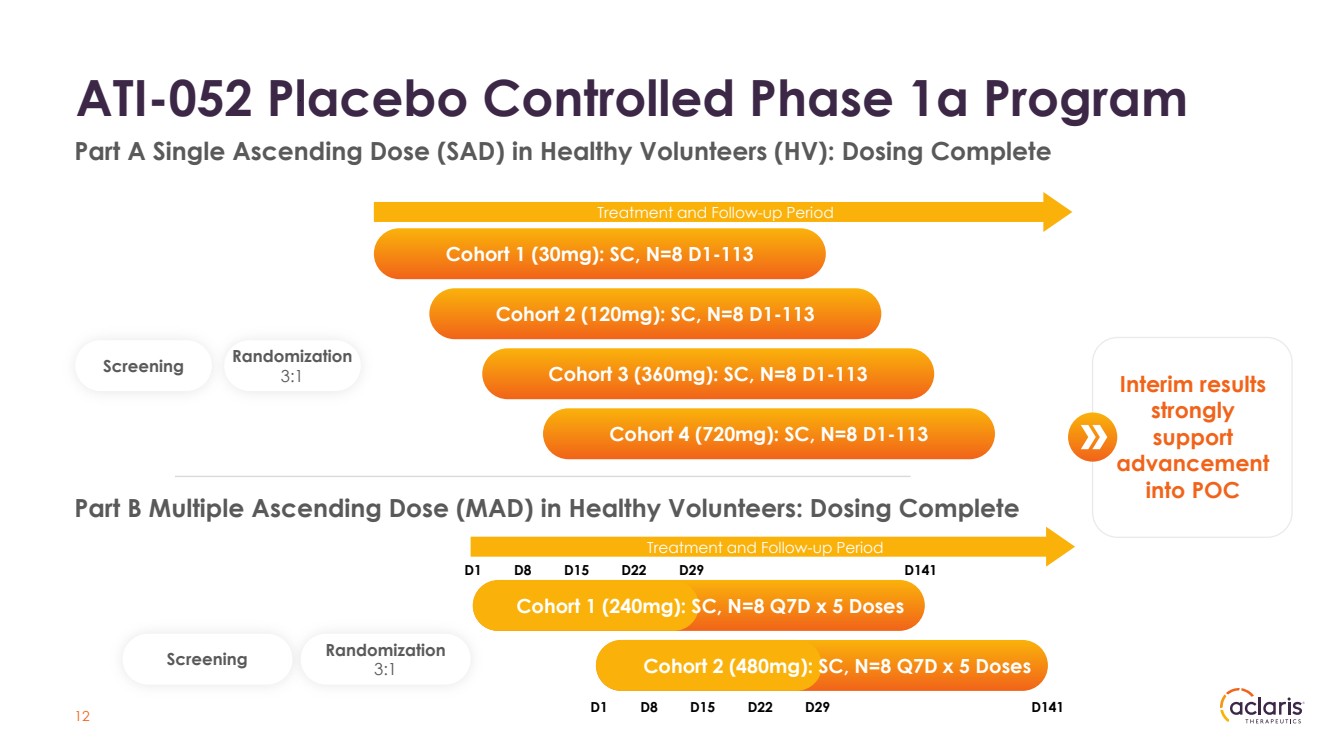
| ATI-052 Placebo Controlled Phase 1a Program 12 Cohort 1 (30mg): SC, N=8 D1-113 Screening Randomization 3:1 Cohort 3 (360mg): SC, N=8 D1-113 Cohort 4 (720mg): SC, N=8 D1-113 Cohort 2 (120mg): SC, N=8 D1-113 Part A Single Ascending Dose (SAD) in Healthy Volunteers (HV): Dosing Complete Part B Multiple Ascending Dose (MAD) in Healthy Volunteers: Dosing Complete Screening Randomization 3:1 D1 D8 D15 D22 D29 D1 D8 D15 D22 D29 D141 D141 Cohort 1 (240mg): SC, N=8 Q7D x 5 Doses Cohort 2 (480mg): SC, N=8 Q7D x 5 Doses Treatment and Follow-up Period Treatment and Follow-up Period Interim results strongly support advancement into POC |
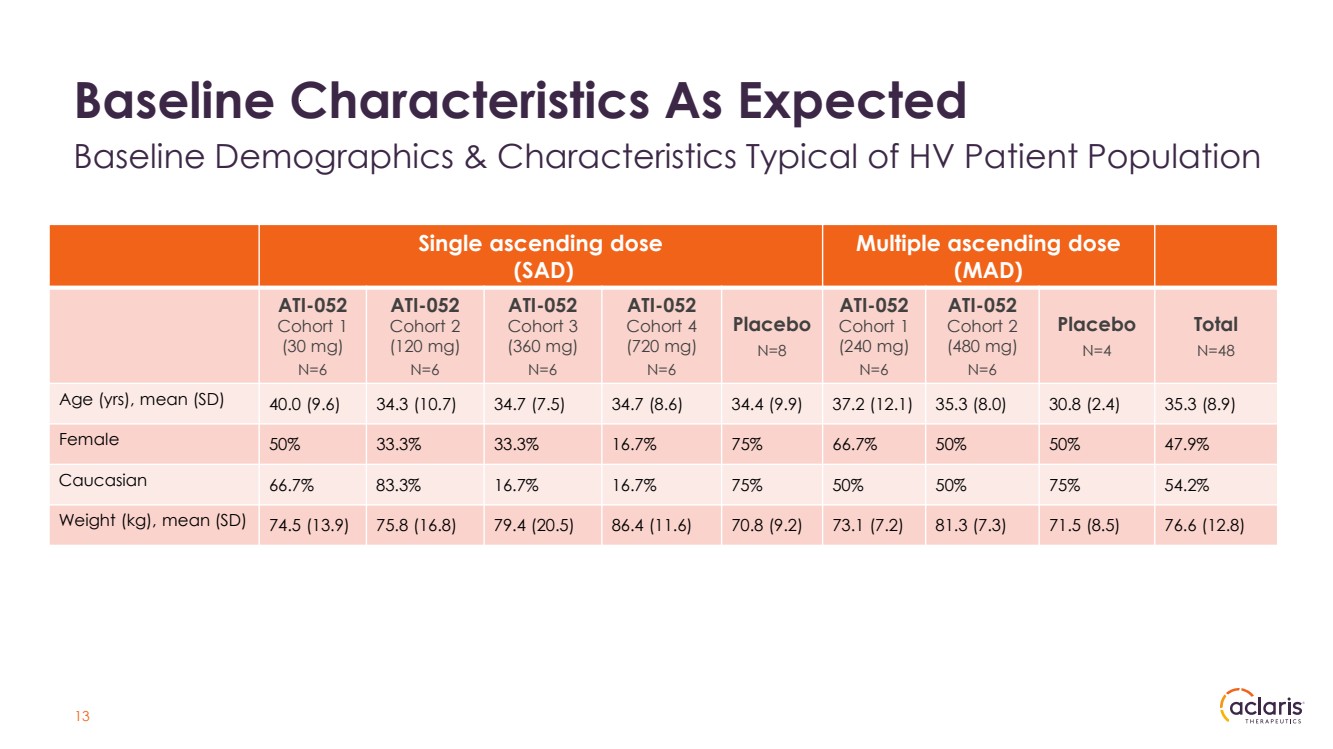
| Single ascending dose (SAD) Multiple ascending dose (MAD) ATI-052 Cohort 1 (30 mg) N=6 ATI-052 Cohort 2 (120 mg) N=6 ATI-052 Cohort 3 (360 mg) N=6 ATI-052 Cohort 4 (720 mg) N=6 Placebo N=8 ATI-052 Cohort 1 (240 mg) N=6 ATI-052 Cohort 2 (480 mg) N=6 Placebo N=4 Total N=48 Age (yrs), mean (SD) 40.0 (9.6) 34.3 (10.7) 34.7 (7.5) 34.7 (8.6) 34.4 (9.9) 37.2 (12.1) 35.3 (8.0) 30.8 (2.4) 35.3 (8.9) Female 50% 33.3% 33.3% 16.7% 75% 66.7% 50% 50% 47.9% Caucasian 66.7% 83.3% 16.7% 16.7% 75% 50% 50% 75% 54.2% Weight (kg), mean (SD) 74.5 (13.9) 75.8 (16.8) 79.4 (20.5) 86.4 (11.6) 70.8 (9.2) 73.1 (7.2) 81.3 (7.3) 71.5 (8.5) 76.6 (12.8) 13 Baseline Characteristics As Expected Baseline Demographics & Characteristics Typical of HV Patient Population |
| ATI-052-PKPD-001 FIH, Phase 1a Healthy Volunteer, SAD and MAD Cohorts Safety and AE Summary |
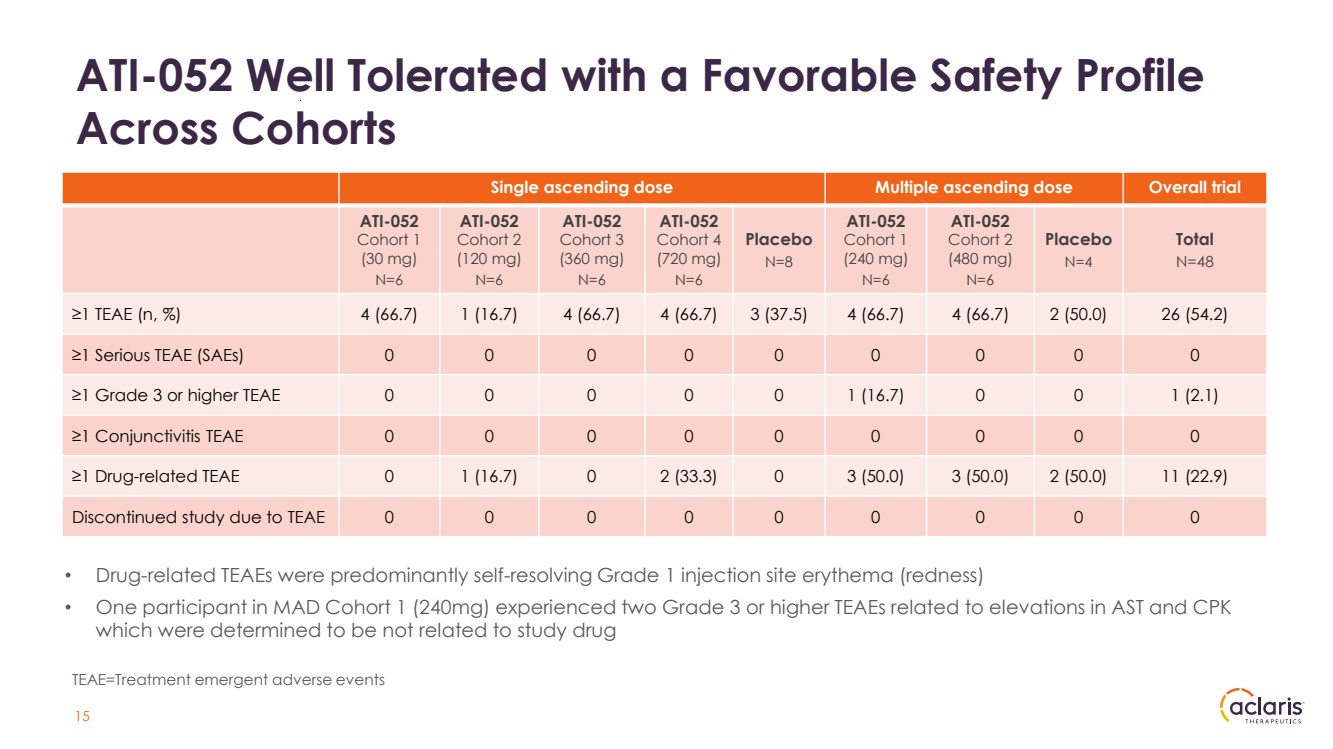
| Single ascending dose Multiple ascending dose Overall trial ATI-052 Cohort 1 (30 mg) N=6 ATI-052 Cohort 2 (120 mg) N=6 ATI-052 Cohort 3 (360 mg) N=6 ATI-052 Cohort 4 (720 mg) N=6 Placebo N=8 ATI-052 Cohort 1 (240 mg) N=6 ATI-052 Cohort 2 (480 mg) N=6 Placebo N=4 Total N=48 ≥1 TEAE (n, %) 4 (66.7) 1 (16.7) 4 (66.7) 4 (66.7) 3 (37.5) 4 (66.7) 4 (66.7) 2 (50.0) 26 (54.2) ≥1 Serious TEAE (SAEs) 0 0 0 0 0 0 0 0 0 ≥1 Grade 3 or higher TEAE 0 0 0 0 0 1 (16.7) 0 0 1 (2.1) ≥1 Conjunctivitis TEAE 0 0 0 0 0 0 0 0 0 ≥1 Drug-related TEAE 0 1 (16.7) 0 2 (33.3) 0 3 (50.0) 3 (50.0) 2 (50.0) 11 (22.9) Discontinued study due to TEAE 0 0 0 0 0 0 0 0 0 ATI-052 Well Tolerated with a Favorable Safety Profile Across Cohorts • Drug-related TEAEs were predominantly self-resolving Grade 1 injection site erythema (redness) • One participant in MAD Cohort 1 (240mg) experienced two Grade 3 or higher TEAEs related to elevations in AST and CPK which were determined to be not related to study drug TEAE=Treatment emergent adverse events 15 |
| 16 Favorable Tolerability and Safety Profile of ATI-052 Provides Confidence in Continued Development • No SAEs; no adverse events led to study discontinuation • Low rate of adverse events (AEs); Predominantly Grade 1 • The most common AE: Injection site redness; self-resolving and generally mild (Grade 1) • No Grade 3 drug-related TEAEs • No conjunctivitis observed in any cohort Favorable tolerability and safety profile demonstrated across all ATI-052 SAD and MAD cohorts, with doses of up to 720 mg/kg |
| ATI-052-PKPD-001 FIH, Phase 1a Healthy Volunteer, SAD and MAD Cohorts Pharmacokinetics Summary |
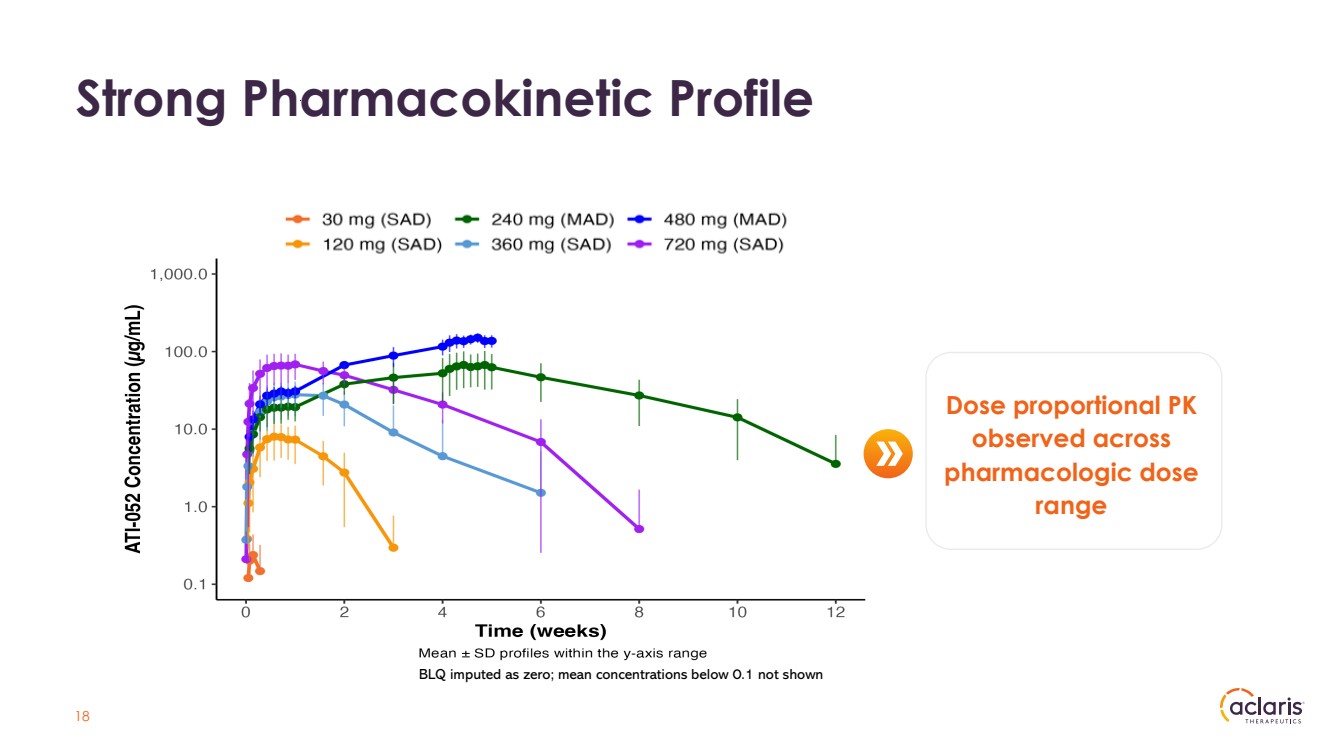
| 18 Strong Pharmacokinetic Profile Dose proportional PK observed across pharmacologic dose range BLQ imputed as zero; mean concentrations below 0.1 not shown |
| 19 Strong PK Profile Support Potential Extended Dosing Dose Proportional PK Observed Across Pharmacologic Dose Range • Dose proportional PK was observed; dose proportional increases in Cmax and AUC observed • PK results provide an effective half-life of at least 26 days Potential best-in-class PK profile |
| ATI-052-PKPD-001 FIH, Phase 1a Healthy Volunteer, SAD and MAD Cohorts Pharmacodynamics Summary |
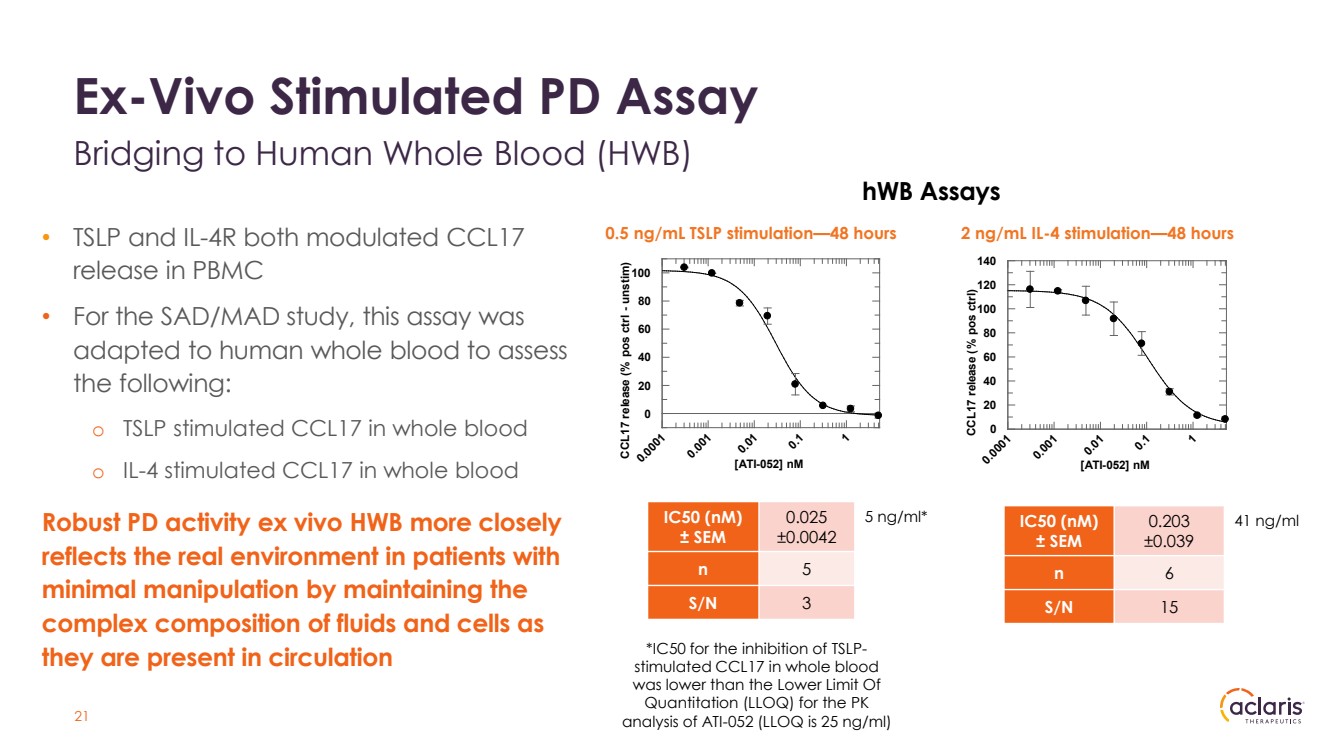
| Ex-Vivo Stimulated PD Assay Bridging to Human Whole Blood (HWB) • TSLP and IL-4R both modulated CCL17 release in PBMC • For the SAD/MAD study, this assay was adapted to human whole blood to assess the following: o TSLP stimulated CCL17 in whole blood o IL-4 stimulated CCL17 in whole blood Robust PD activity ex vivo HWB more closely reflects the real environment in patients with minimal manipulation by maintaining the complex composition of fluids and cells as they are present in circulation hWB Assays 0.0001 0.001 0.01 0.1 1 CCL17 release (% pos ctrl) 0 20 40 60 80 100 120 140 [ATI-052] nM IC50 (nM) ± SEM 0.025 ±0.0042 n 5 S/N 3 IC50 (nM) ± SEM 0.203 ±0.039 n 6 S/N 15 0.5 ng/mL TSLP stimulation—48 hours 2 ng/mL IL-4 stimulation—48 hours 5 ng/ml* 41 ng/ml 0.0001 0.001 0.01 0.1 1 CCL17 release (% pos ctrl - unstim) 0 20 40 60 80 100 [ATI-052] nM *IC50 for the inhibition of TSLP-stimulated CCL17 in whole blood was lower than the Lower Limit Of Quantitation (LLOQ) for the PK analysis of ATI-052 (LLOQ is 25 ng/ml) 21 |
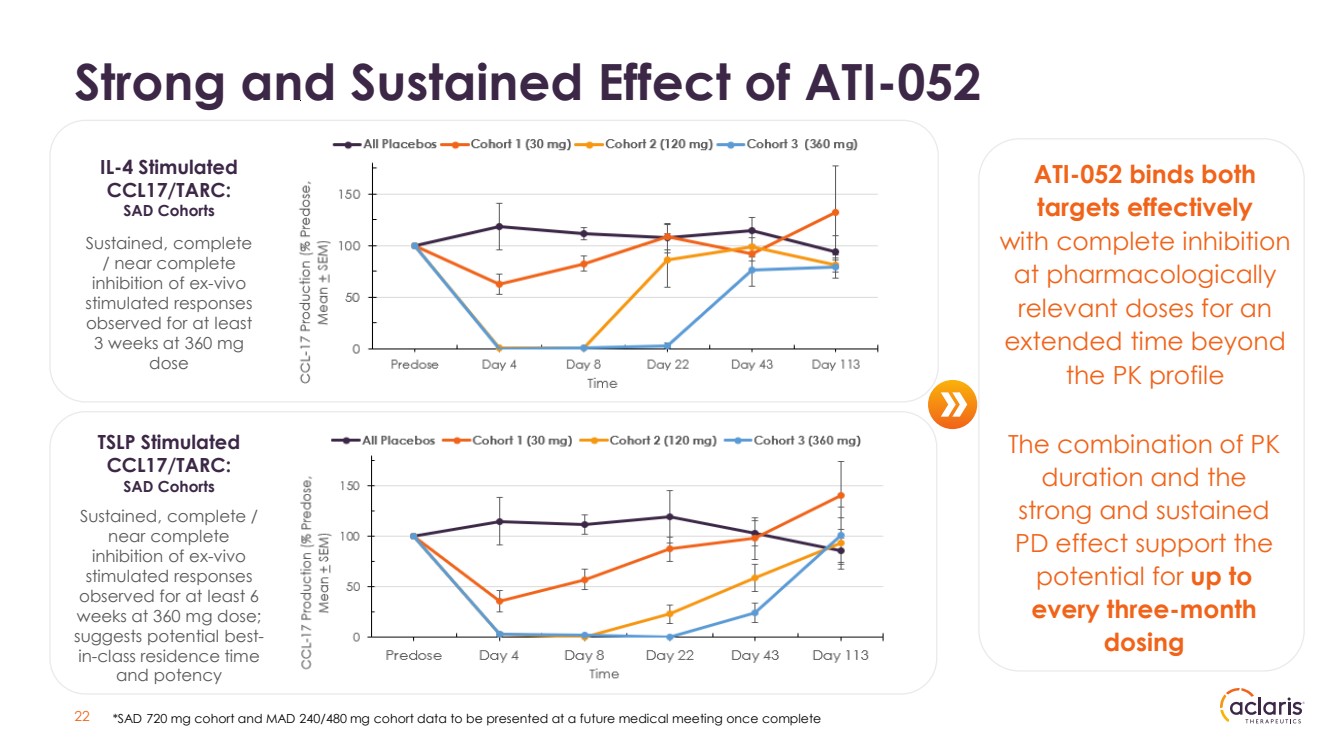
| Strong and Sustained Effect of ATI-052 ATI-052 binds both targets effectively with complete inhibition at pharmacologically relevant doses for an extended time beyond the PK profile Sustained, complete / near complete inhibition of ex-vivo stimulated responses observed for at least 3 weeks at 360 mg dose Sustained, complete / near complete inhibition of ex-vivo stimulated responses observed for at least 6 weeks at 360 mg dose; suggests potential best-in-class residence time and potency IL-4 Stimulated CCL17/TARC: SAD Cohorts TSLP Stimulated CCL17/TARC: SAD Cohorts 22 *SAD 720 mg cohort and MAD 240/480 mg cohort data to be presented at a future medical meeting once complete The combination of PK duration and the strong and sustained PD effect support the potential for up to every three-month dosing |
| 23 Strong Pharmacodynamic Response Robust Target Engagement + Near Complete Occupancy at Low Doses ATI-052 exhibited a potential best-in-class PD profile: • Dose and concentration dependent inhibition of IL-4 and TSLP-stimulated CCL17 release observed • Cohort 1 (30 mg), 2 (120 mg) and 3 (360 mg) data indicate that the time points at which there are detectable levels of ATI-052 present in blood, ATI-052 is active and engaging its target when stimulated ex-vivo • Cohort 3 results demonstrated complete and sustained inhibition of ex vivo IL-4 or TSLP stimulated CCL17/TARC through week three • Near complete inhibition of TSLP stimulated CCL17/TARC was observed at least six weeks after administration for Cohort 3 Observed results further validate the potency of ATI-052 |
| 24 Unique Binding Attributes Clinical Potential Simultaneous Binding of TSLP and IL-4Rα High affinity to both targets simultaneously: ATI-052 binds two molecules of TSLP and sIL-4Rα Effective Binding of TSLP and IL-4Rα ATI-052 binds both targets effectively; high affinity of either targets is not altered by the binding of the other Higher Potency vs Comparator Antibodies ATI-052 exhibits greater cellular bioactivity on CCL17 release than the combination of Tezepelumab and Dupilumab Effective Blockade of IL-4 and IL-13 ATI-052 antagonism of IL-4Rα blocks signaling of both IL-4 and IL-13 Binding Attributes Phase 1a Interim Clinical Results Favorable Tolerability and Safety Profile Favorable tolerability and safety profile observed across all SAD and MAD cohorts, with doses of up to 720 mg Robust PK Package Dose proportional PK observed across pharmacologic dose range Support potential for up to 3-month dosing Strong Pharmacodynamic Response Robust target engagement + near complete target occupancy, even at very low doses Efficient Inhibition of Both TSLP and IL-4R Dose and concentration dependent inhibition of IL-4 and TSLP-stimulated CCL17 release |
| 25 ATI-052: Next Steps Positive Interim Results Validate ATI-052; Clinical Program Rapidly Advancing Ongoing / Next Steps • Phase 1b AD POC trial imminent • Initiate Phase 1b asthma POC trial: 1Q 2026 • Phase 1b top line POC results: 2H 2026 • Initiate Phase 2b AD trial: 2H 2026 • Complete assessments of additional Phase 2 indications Potential next targets (other non-dermatology) COPD Asthma EOE Phase 1 HV: SAD/MAD Phase 1b POC Atopic dermatitis Phase 1b POC Asthma Initiation Imminent Dosing Complete Initiation 1Q 2026 Initiation 2H 2026 Potential next targets (dermatology) Prurigo Nodularis (PN) Chronic Spontaneous Urticaria (CSU) Phase 2b Atopic dermatitis (in planning) All future development, clinical, and regulatory timelines are expectations, are based on current beliefs and assumptions, and are subject to change based on a variety of factors |
| ATI-052: Anti-TSLP x IL-4Rα Bispecific Antibody Program Highly Potent and Bioactive Investigational Product Candidate January 6, 2026 |