

January 29, 2026 Positive 6-Month Randomized Data from REMAIN-1 Midpoint Cohort - Accelerating Momentum Towards Revita’s Potential Approval and Launch Revita is for investigational use only in the United States. Revita has a CE mark in the EU/UK. .2

Legal disclaimer The study database has not been locked as this is an ongoing study, and the data are subject to further cleaning and validation. Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact are forward-looking statements. These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will” and variations of these words or similar expressions that are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements in this presentation include, without limitation, statements regarding the promise and potential impact of our product candidates, including Revita’s potential for preserving weight loss after GLP-1 drug discontinuation; the design, initiation, timing, primary and secondary endpoints, and results of clinical enrollment and any clinical studies or readouts, including readouts from the REVEAL-1 and REMAIN-1 Cohorts; the content, information used for, timing or results of any IND-enabling studies, IND applications or Clinical Trial Applications, the potential launch or commercialization of any of our product candidates or products, our regulatory strategy, including potential benefits of the De Novo pathway, the potential treatment population or benefits for any of our product candidates, our expected cash runway and financial conditions, and our strategic and product development objectives and goals, including with respect to enabling long-term control over obesity and type 2 diabetes without the burden of chronic therapies, redefining the future of metabolic disease treatment, and positioning our Company at the forefront at the global opportunity for metabolic care; and the timing of any of the foregoing. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to factors discussed under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2024 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2025 filed with the Securities and Exchange Commission (the “SEC”) on November 12, 2025 and in our other filings with the SEC. These forward-looking statements are based on management’s current estimates and expectations. While the Company may elect to update such forward-looking statements at some point in the future, the Company disclaims any obligation to do so, even if subsequent events cause its views to change. Industry data This presentation also contains estimates, projections and other information concerning our industry, our business and the markets in which we operate. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market, and other data from our own internal estimates and research as well as from reports, research, surveys, studies, and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. While we are not aware of any misstatements regarding any third-party information presented in this presentation, their estimates, in particular, as they relate to projections, involve numerous assumptions, are subject to risks and uncertainties and are subject to change based on various factors. Trademarks This presentation may contain trademarks, service marks, trade names and copyrights of the Company and other companies, which are the property of their respective owners. The use herein does not imply an affiliation with, or endorsement by, the owners of these trademarks, service marks, trade names and copyrights. Third-party logos herein may represent be provided simply for illustrative purposes only. Inclusion of such logos does not necessarily imply affiliation with or endorsement by such firms or businesses. There is no guarantee that the Company will work, or continue to work, with any of the firms or businesses whose logos are included herein in the future. future. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. ©2026 Fractyl Health, Inc. All Rights Reserved.
Accelerating Momentum Towards Revita’s FDA Submission and Potential Commercial Launch Positive 6-Month Data from REMAIN-1 Midpoint Cohort Strong evidence of sustained weight maintenance vs. sham with excellent safety and tolerability profile Patients with greater1 GLP-1-associated weight loss experienced ~ 70% less post-GLP-1 weight regain with Revita vs sham at 6 months (p=0.004, one-sided) Reinforced confidence in ongoing pivotal study Progress in US Regulatory Strategy Requested to reclassify Revita under the De Novo pathway (vs. PMA); FDA feedback expected Q2 2026 Potential to enable a more efficient, risk-based regulatory review process and reduce cost Advancing Commercial Readiness Leveraging well-understood FDA Breakthrough and CMS TPT timelines Focused, capital-efficient go-to-market strategy aligned with existing endoscopy care pathway 1. Above median. Abbreviations: CMS, Centers for Medicare and Medicaid Services; FDA, Food and Drug Administration; GLP-1, glucagon-like peptide-1; PMA, premarket approval; TPT, Transitional Pass-Through.
Revita®: Potential new backbone therapy in obesity A one-time, < 1 hr endoscopic procedure designed to address gut-level metabolic dysfunction Abbreviations: FDA, Food and Drug Administration; GLP-1, glucagon-like peptide-1. Single outpatient endoscopic procedure Consistent clinical results across studies FDA Breakthrough Device designation Performed by endoscopists; leverages familiar skills Up to 2 years of durable activity after a single treatment across multiple clinical studies 2026 is a pivotal and registrational year in post-GLP-1 weight maintenance Positive 6-month Midpoint Cohort data Provides increased confidence in the ongoing pivotal study
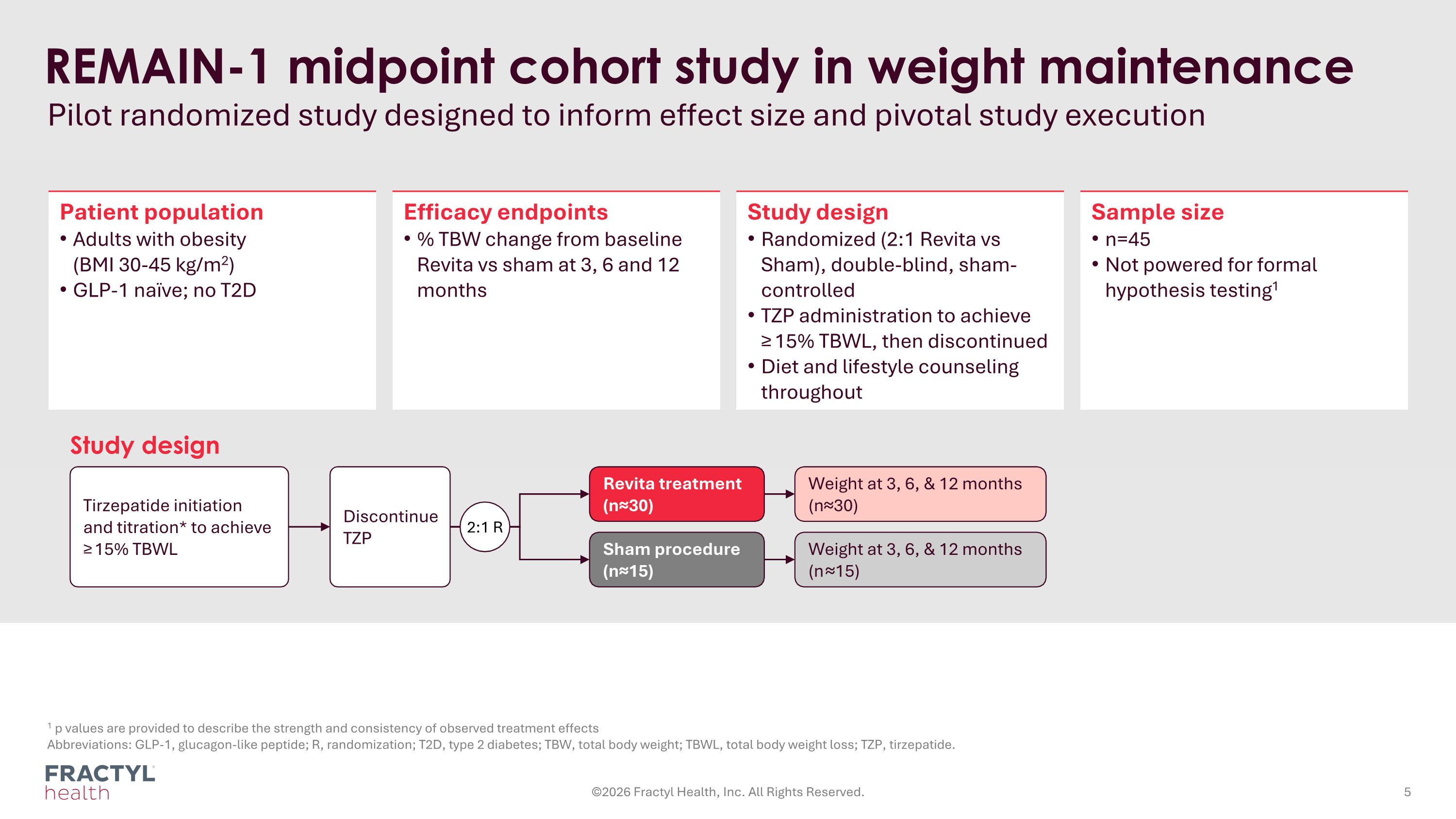
REMAIN-1 midpoint cohort study in weight maintenance Pilot randomized study designed to inform effect size and pivotal study execution 1 p values are provided to describe the strength and consistency of observed treatment effects Abbreviations: GLP-1, glucagon-like peptide; R, randomization; T2D, type 2 diabetes; TBW, total body weight; TBWL, total body weight loss; TZP, tirzepatide. Patient population Adults with obesity (BMI 30-45 kg/m2) GLP-1 naïve; no T2D Efficacy endpoints % TBW change from baseline Revita vs sham at 3, 6 and 12 months Study design Randomized (2:1 Revita vs Sham), double-blind, sham-controlled TZP administration to achieve ≥ 15% TBWL, then discontinued Diet and lifestyle counseling throughout Sample size n=45 Not powered for formal hypothesis testing1 Tirzepatide initiation and titration* to achieve ≥ 15% TBWL Revita treatment (n≈30) Weight at 3, 6, & 12 months (n≈30) Sham procedure (n≈15) Weight at 3, 6, & 12 months (n ≈15) Study design Discontinue TZP 2:1 R ©2026 Fractyl Health, Inc. All Rights Reserved.
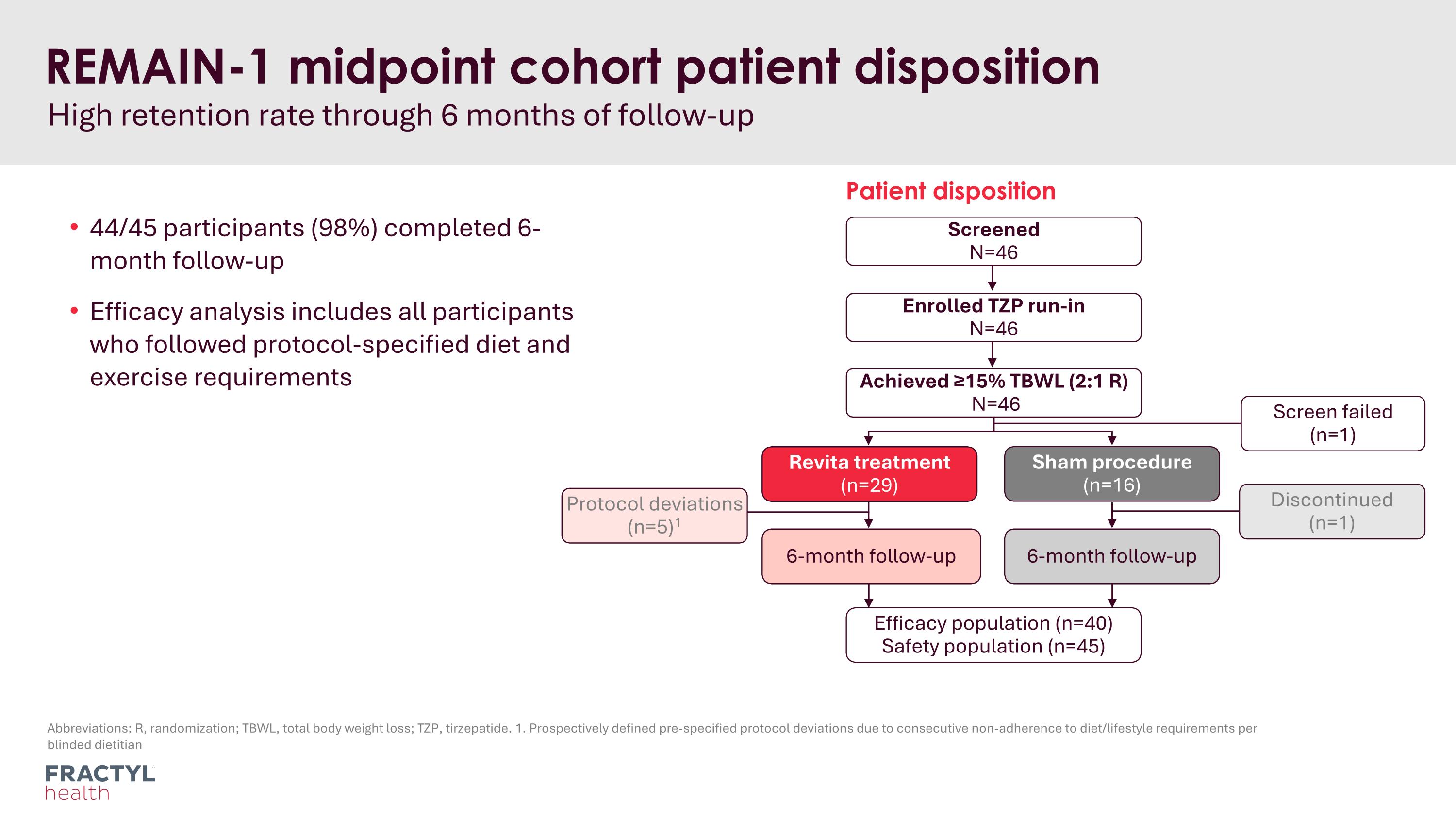
Abbreviations: R, randomization; TBWL, total body weight loss; TZP, tirzepatide. 1. Prospectively defined pre-specified protocol deviations due to consecutive non-adherence to diet/lifestyle requirements per blinded dietitian REMAIN-1 midpoint cohort patient disposition High retention rate through 6 months of follow-up 44/45 participants (98%) completed 6-month follow-up Efficacy analysis includes all participants who followed protocol-specified diet and exercise requirements Screened N=46 Enrolled TZP run-in N=46 Achieved ≥15% TBWL (2:1 R) N=46 Patient disposition Sham procedure (n=16) 6-month follow-up 6-month follow-up Discontinued (n=1) Efficacy population (n=40) Safety population (n=45) Protocol deviations (n=5)1 Revita treatment (n=29) Screen failed (n=1)
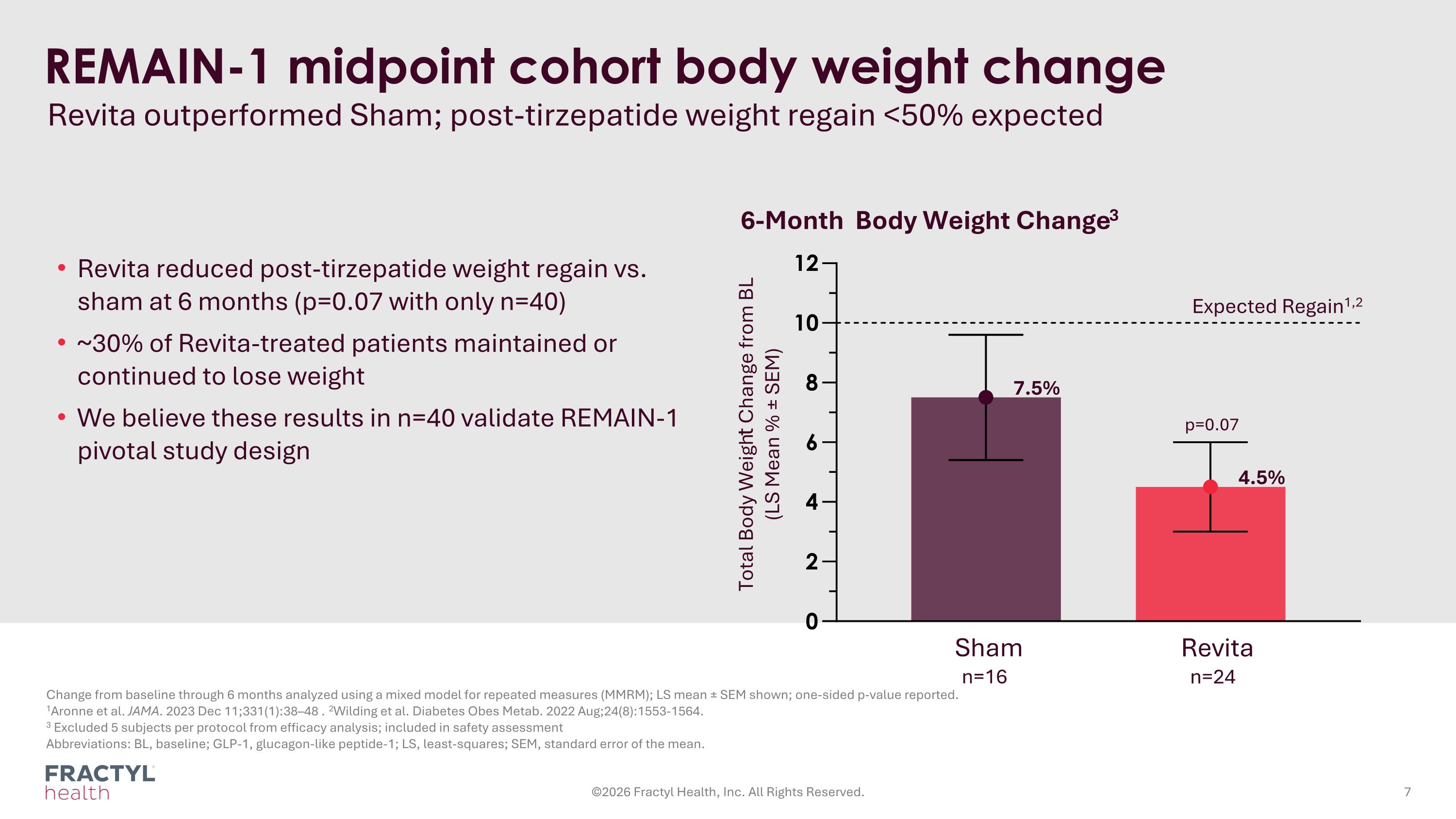
REMAIN-1 midpoint cohort body weight change Revita outperformed Sham; post-tirzepatide weight regain <50% expected Change from baseline through 6 months analyzed using a mixed model for repeated measures (MMRM); LS mean ± SEM shown; one-sided p-value reported. 1Aronne et al. JAMA. 2023 Dec 11;331(1):38–48 . 2Wilding et al. Diabetes Obes Metab. 2022 Aug;24(8):1553-1564. 3 Excluded 5 subjects per protocol from efficacy analysis; included in safety assessment Abbreviations: BL, baseline; GLP-1, glucagon-like peptide-1; LS, least-squares; SEM, standard error of the mean. ©2026 Fractyl Health, Inc. All Rights Reserved. Total Body Weight Change from BL (LS Mean % ± SEM) 6-Month Body Weight Change3 Revita n=24 Sham n=16 Revita reduced post-tirzepatide weight regain vs. sham at 6 months (p=0.07 with only n=40) ~30% of Revita-treated patients maintained or continued to lose weight We believe these results in n=40 validate REMAIN-1 pivotal study design Expected Regain1,2 4.5% 7.5% p=0.07
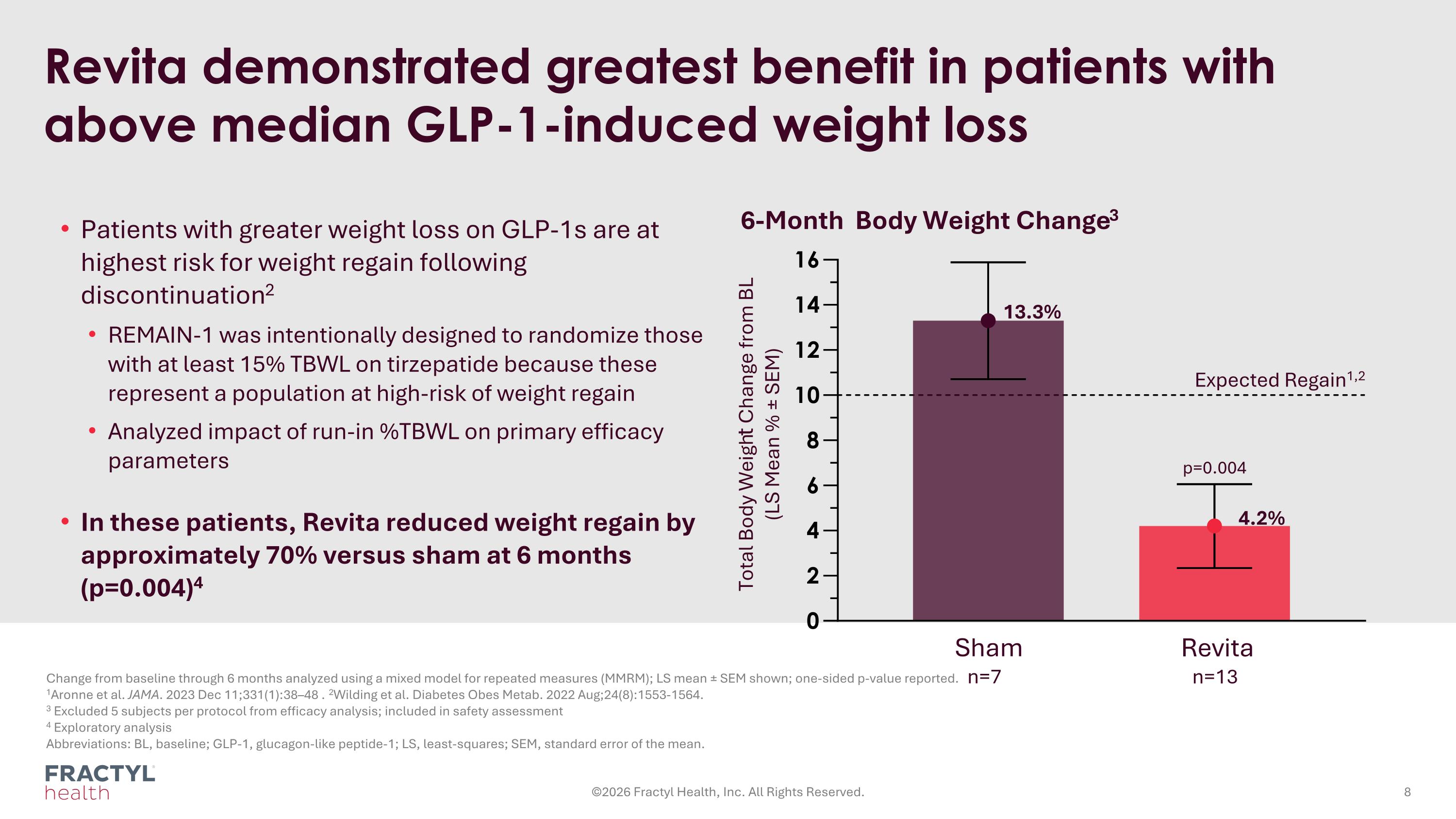
Revita demonstrated greatest benefit in patients with above median GLP-1-induced weight loss Change from baseline through 6 months analyzed using a mixed model for repeated measures (MMRM); LS mean ± SEM shown; one-sided p-value reported. 1Aronne et al. JAMA. 2023 Dec 11;331(1):38–48 . 2Wilding et al. Diabetes Obes Metab. 2022 Aug;24(8):1553-1564. 3 Excluded 5 subjects per protocol from efficacy analysis; included in safety assessment 4 Exploratory analysis Abbreviations: BL, baseline; GLP-1, glucagon-like peptide-1; LS, least-squares; SEM, standard error of the mean. ©2026 Fractyl Health, Inc. All Rights Reserved. Total Body Weight Change from BL (LS Mean % ± SEM) 6-Month Body Weight Change3 Revita n=13 Sham n=7 Patients with greater weight loss on GLP-1s are at highest risk for weight regain following discontinuation2 REMAIN-1 was intentionally designed to randomize those with at least 15% TBWL on tirzepatide because these represent a population at high-risk of weight regain Analyzed impact of run-in %TBWL on primary efficacy parameters In these patients, Revita reduced weight regain by approximately 70% versus sham at 6 months (p=0.004)4 Expected Regain1,2 4.2% 13.3% p=0.004
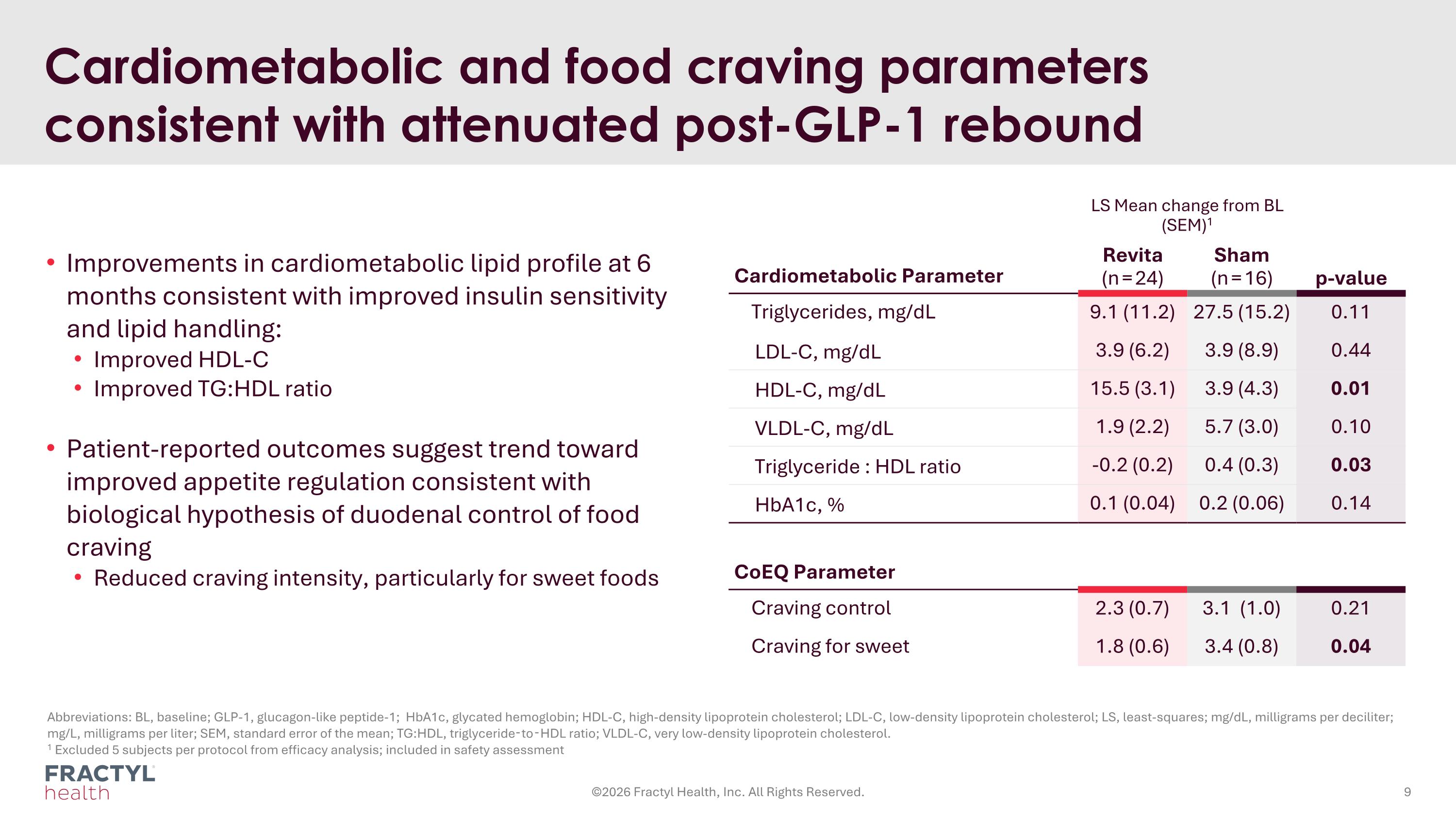
Improvements in cardiometabolic lipid profile at 6 months consistent with improved insulin sensitivity and lipid handling: Improved HDL-C Improved TG:HDL ratio Patient-reported outcomes suggest trend toward improved appetite regulation consistent with biological hypothesis of duodenal control of food craving Reduced craving intensity, particularly for sweet foods CoEQ Parameter Craving control 2.3 (0.7) 3.1 (1.0) 0.21 Craving for sweet 1.8 (0.6) 3.4 (0.8) 0.04 Cardiometabolic and food craving parameters consistent with attenuated post-GLP-1 rebound LS Mean change from BL (SEM)1 Cardiometabolic Parameter Revita (n = 24) Sham (n = 16) p-value Triglycerides, mg/dL 9.1 (11.2) 27.5 (15.2) 0.11 LDL-C, mg/dL 3.9 (6.2) 3.9 (8.9) 0.44 HDL-C, mg/dL 15.5 (3.1) 3.9 (4.3) 0.01 VLDL-C, mg/dL 1.9 (2.2) 5.7 (3.0) 0.10 Triglyceride : HDL ratio -0.2 (0.2) 0.4 (0.3) 0.03 HbA1c, % 0.1 (0.04) 0.2 (0.06) 0.14 ©2026 Fractyl Health, Inc. All Rights Reserved. Abbreviations: BL, baseline; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LS, least-squares; mg/dL, milligrams per deciliter; mg/L, milligrams per liter; SEM, standard error of the mean; TG:HDL, triglyceride‑to‑HDL ratio; VLDL-C, very low-density lipoprotein cholesterol. 1 Excluded 5 subjects per protocol from efficacy analysis; included in safety assessment
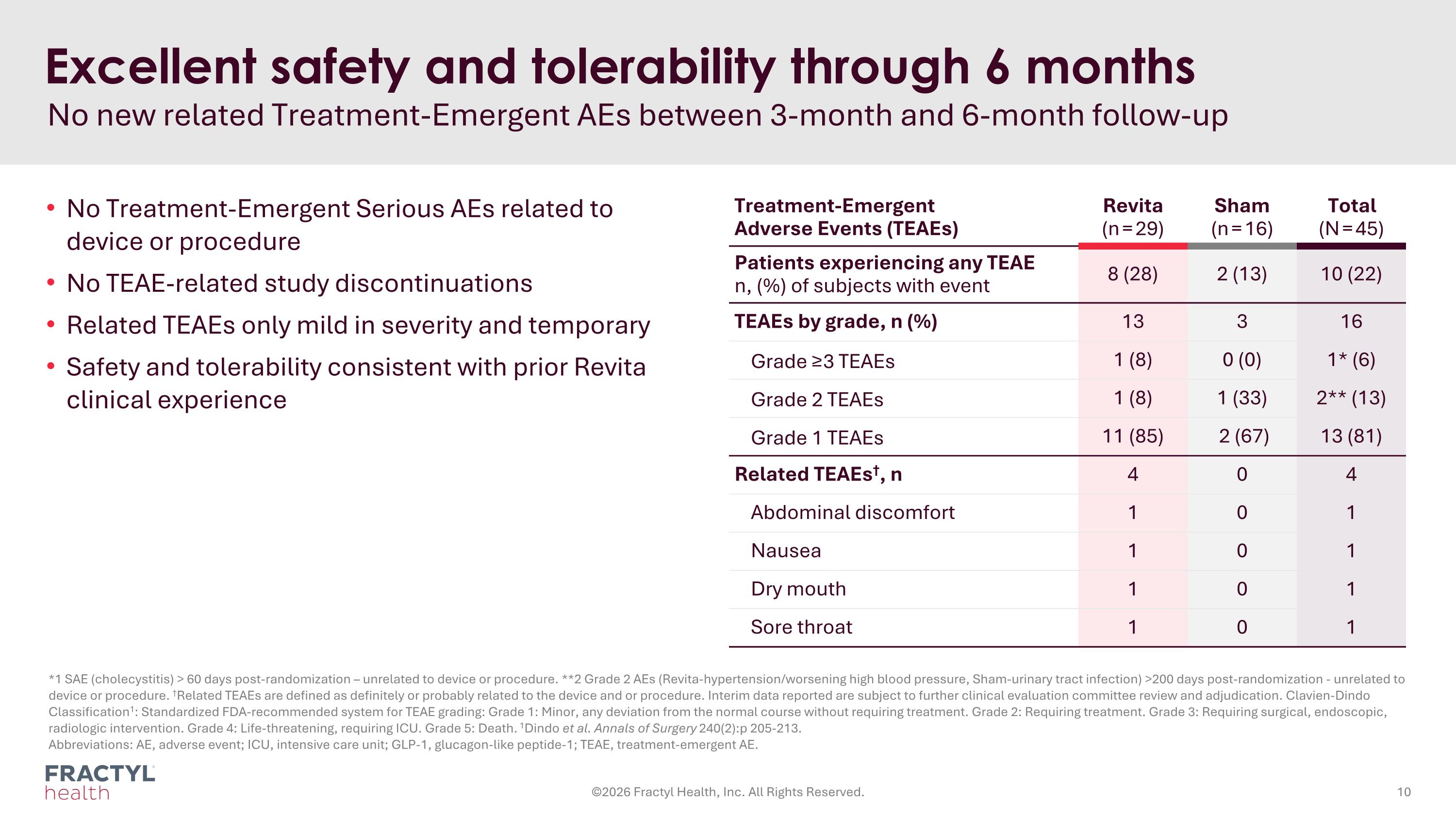
Treatment-Emergent Adverse Events (TEAEs) Revita (n = 29) Sham (n = 16) Total (N = 45) Patients experiencing any TEAE n, (%) of subjects with event 8 (28) 2 (13) 10 (22) TEAEs by grade, n (%) 13 3 16 Grade ≥3 TEAEs 1 (8) 0 (0) 1* (6) Grade 2 TEAEs 1 (8) 1 (33) 2** (13) Grade 1 TEAEs 11 (85) 2 (67) 13 (81) Related TEAEs†, n 4 0 4 Abdominal discomfort 1 0 1 Nausea 1 0 1 Dry mouth 1 0 1 Sore throat 1 0 1 *1 SAE (cholecystitis) > 60 days post-randomization – unrelated to device or procedure. **2 Grade 2 AEs (Revita-hypertension/worsening high blood pressure, Sham-urinary tract infection) >200 days post-randomization - unrelated to device or procedure. †Related TEAEs are defined as definitely or probably related to the device and or procedure. Interim data reported are subject to further clinical evaluation committee review and adjudication. Clavien-Dindo Classification1: Standardized FDA-recommended system for TEAE grading: Grade 1: Minor, any deviation from the normal course without requiring treatment. Grade 2: Requiring treatment. Grade 3: Requiring surgical, endoscopic, radiologic intervention. Grade 4: Life-threatening, requiring ICU. Grade 5: Death. 1Dindo et al. Annals of Surgery 240(2):p 205-213. Abbreviations: AE, adverse event; ICU, intensive care unit; GLP-1, glucagon-like peptide-1; TEAE, treatment-emergent AE. Excellent safety and tolerability through 6 months No new related Treatment-Emergent AEs between 3-month and 6-month follow-up ©2026 Fractyl Health, Inc. All Rights Reserved. No Treatment-Emergent Serious AEs related to device or procedure No TEAE-related study discontinuations Related TEAEs only mild in severity and temporary Safety and tolerability consistent with prior Revita clinical experience
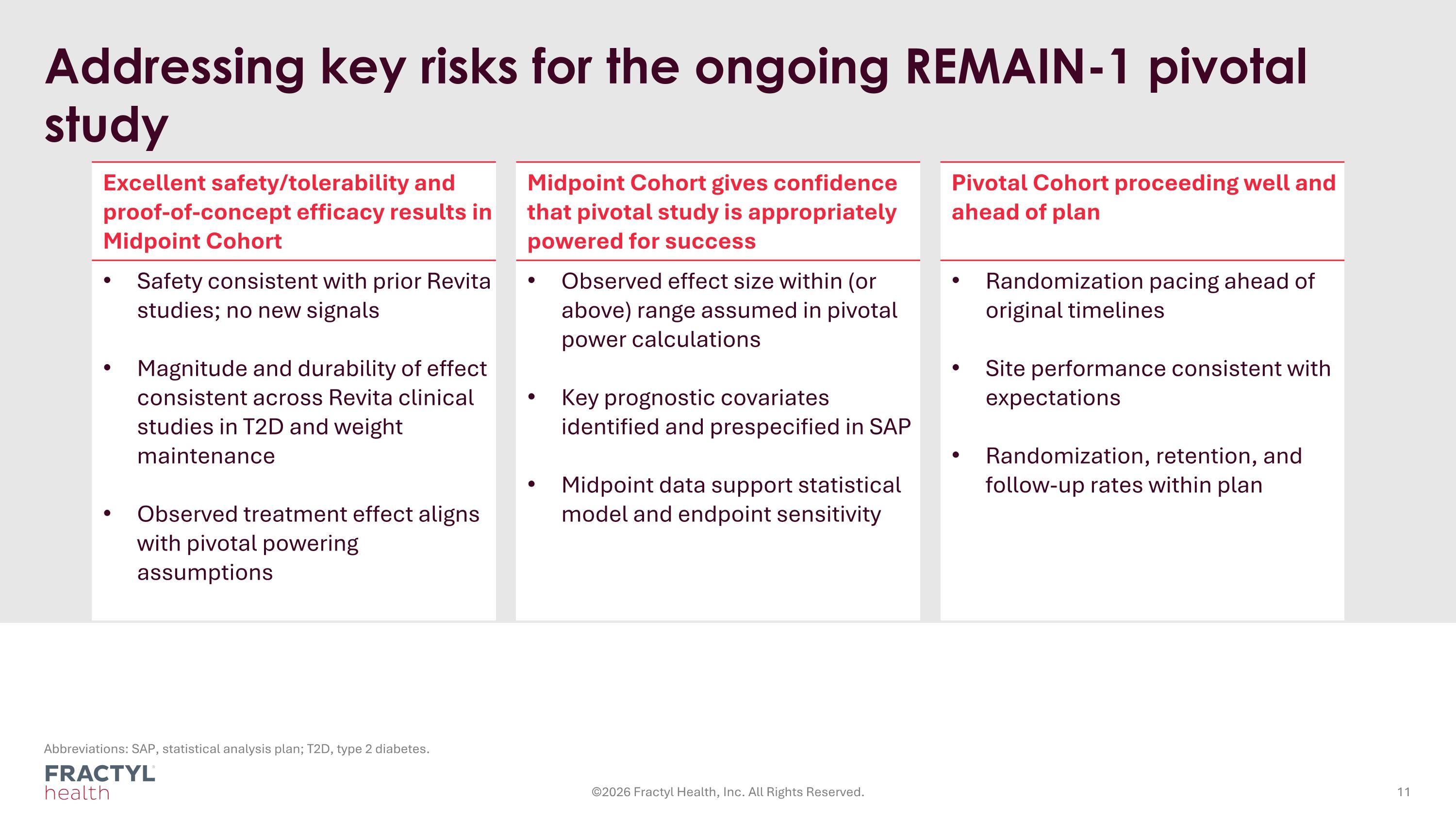
Addressing key risks for the ongoing REMAIN-1 pivotal study Abbreviations: SAP, statistical analysis plan; T2D, type 2 diabetes. ©2026 Fractyl Health, Inc. All Rights Reserved. Excellent safety/tolerability and proof-of-concept efficacy results in Midpoint Cohort Midpoint Cohort gives confidence that pivotal study is appropriately powered for success Pivotal Cohort proceeding well and ahead of plan Safety consistent with prior Revita studies; no new signals Magnitude and durability of effect consistent across Revita clinical studies in T2D and weight maintenance Observed treatment effect aligns with pivotal powering assumptions Observed effect size within (or above) range assumed in pivotal power calculations Key prognostic covariates identified and prespecified in SAP Midpoint data support statistical model and endpoint sensitivity Randomization pacing ahead of original timelines Site performance consistent with expectations Randomization, retention, and follow-up rates within plan
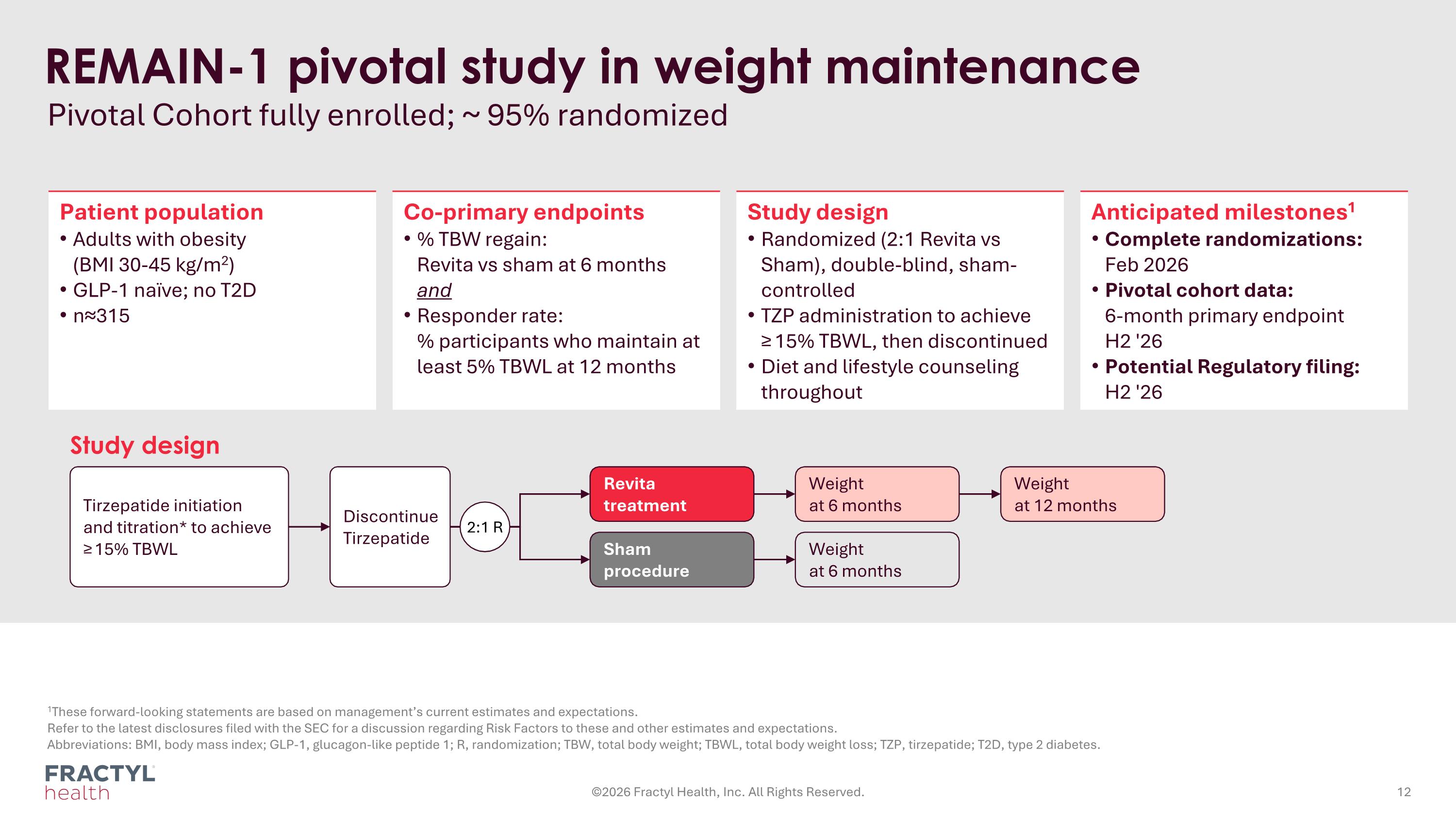
REMAIN-1 pivotal study in weight maintenance Pivotal Cohort fully enrolled; ~ 95% randomized 1These forward-looking statements are based on management’s current estimates and expectations. Refer to the latest disclosures filed with the SEC for a discussion regarding Risk Factors to these and other estimates and expectations. Abbreviations: BMI, body mass index; GLP-1, glucagon-like peptide 1; R, randomization; TBW, total body weight; TBWL, total body weight loss; TZP, tirzepatide; T2D, type 2 diabetes. Patient population Adults with obesity (BMI 30-45 kg/m2) GLP-1 naïve; no T2D n≈315 Co-primary endpoints % TBW regain: Revita vs sham at 6 months and Responder rate: % participants who maintain at least 5% TBWL at 12 months Study design Randomized (2:1 Revita vs Sham), double-blind, sham-controlled TZP administration to achieve ≥ 15% TBWL, then discontinued Diet and lifestyle counseling throughout Anticipated milestones1 Complete randomizations: Feb 2026 Pivotal cohort data: 6-month primary endpoint H2 '26 Potential Regulatory filing: H2 '26 Study design Tirzepatide initiation and titration* to achieve ≥ 15% TBWL Revita treatment Weight at 6 months Weight at 12 months Sham procedure Weight at 6 months Discontinue Tirzepatide 2:1 R ©2026 Fractyl Health, Inc. All Rights Reserved.
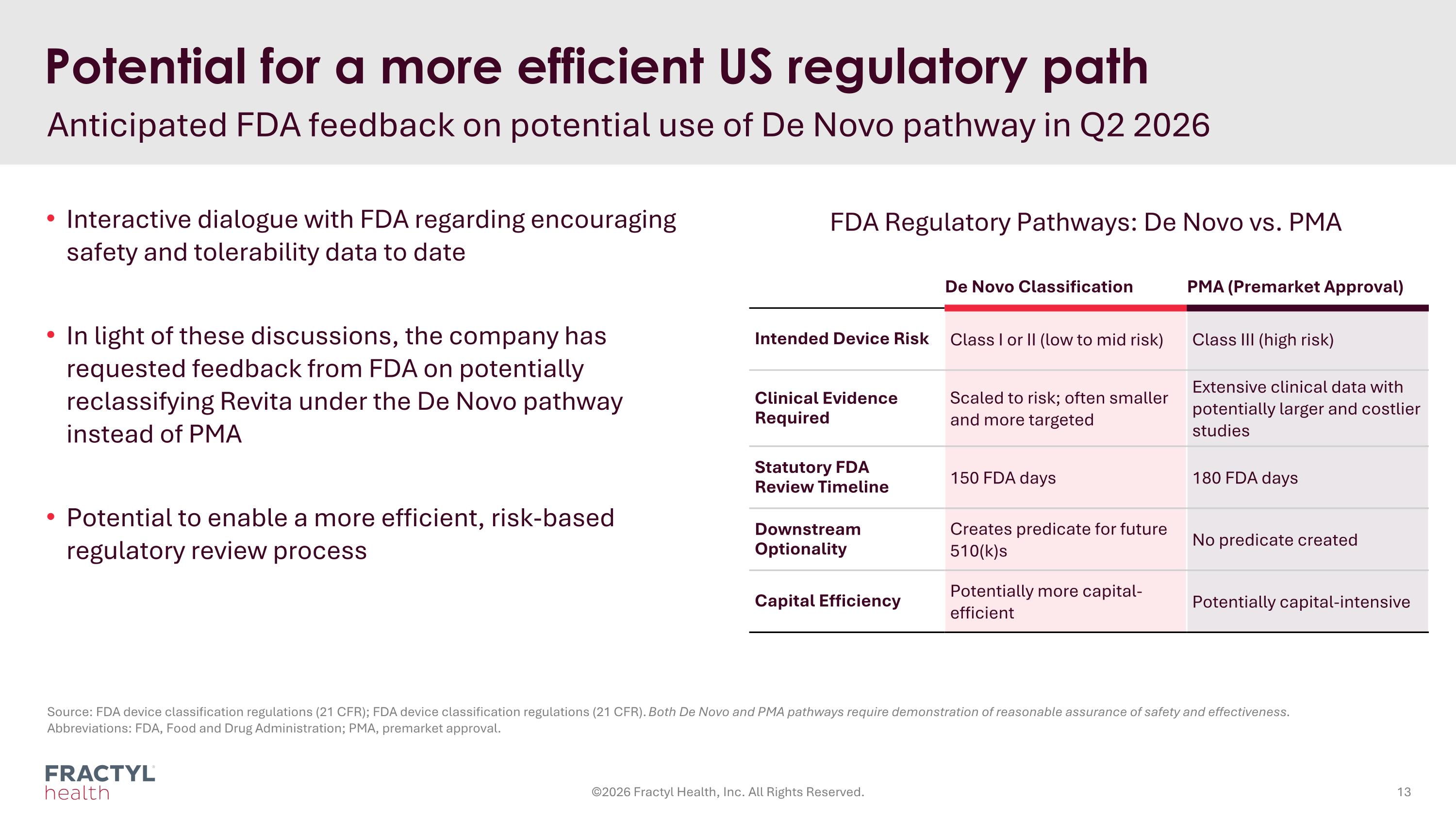
Potential for a more efficient US regulatory path Anticipated FDA feedback on potential use of De Novo pathway in Q2 2026 Interactive dialogue with FDA regarding encouraging safety and tolerability data to date In light of these discussions, the company has requested feedback from FDA on potentially reclassifying Revita under the De Novo pathway instead of PMA Potential to enable a more efficient, risk-based regulatory review process ©2026 Fractyl Health, Inc. All Rights Reserved. Source: FDA device classification regulations (21 CFR); FDA device classification regulations (21 CFR). Both De Novo and PMA pathways require demonstration of reasonable assurance of safety and effectiveness. Abbreviations: FDA, Food and Drug Administration; PMA, premarket approval. De Novo Classification PMA (Premarket Approval) Intended Device Risk Class I or II (low to mid risk) Class III (high risk) Clinical Evidence Required Scaled to risk; often smaller and more targeted Extensive clinical data with potentially larger and costlier studies Statutory FDA Review Timeline 150 FDA days 180 FDA days Downstream Optionality Creates predicate for future 510(k)s No predicate created Capital Efficiency Potentially more capital-efficient Potentially capital-intensive FDA Regulatory Pathways: De Novo vs. PMA
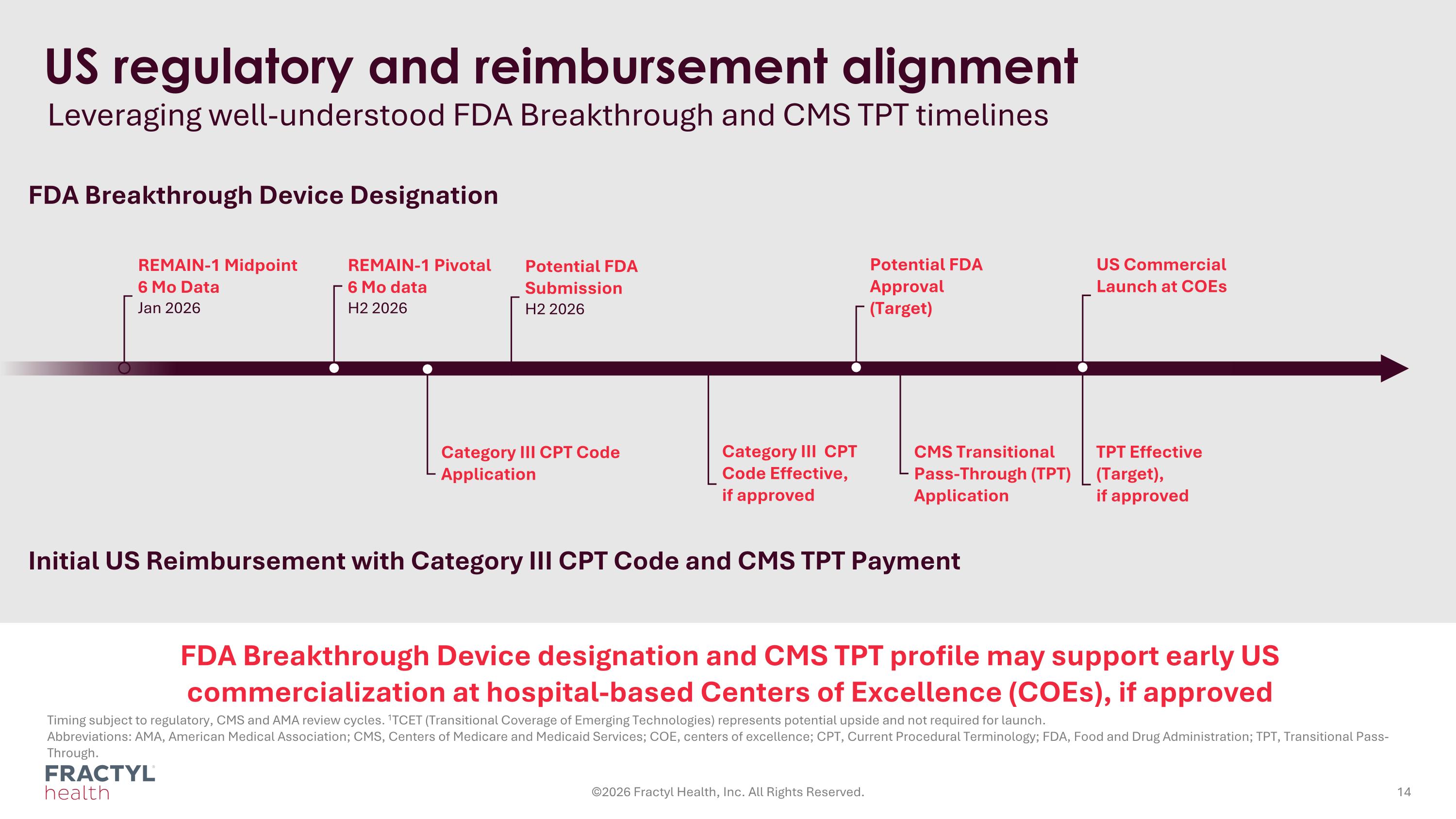
REMAIN-1 Midpoint 6 Mo Data Jan 2026 Leveraging well-understood FDA Breakthrough and CMS TPT timelines Timing subject to regulatory, CMS and AMA review cycles. 1TCET (Transitional Coverage of Emerging Technologies) represents potential upside and not required for launch. Abbreviations: AMA, American Medical Association; CMS, Centers of Medicare and Medicaid Services; COE, centers of excellence; CPT, Current Procedural Terminology; FDA, Food and Drug Administration; TPT, Transitional Pass-Through. ©2026 Fractyl Health, Inc. All Rights Reserved. US regulatory and reimbursement alignment Potential FDA Submission H2 2026 Category III CPT Code Effective, if approved CMS Transitional Pass-Through (TPT) Application TPT Effective (Target), if approved Potential FDA Approval (Target) REMAIN-1 Pivotal 6 Mo data H2 2026 Category III CPT Code Application FDA Breakthrough Device Designation Initial US Reimbursement with Category III CPT Code and CMS TPT Payment US Commercial Launch at COEs FDA Breakthrough Device designation and CMS TPT profile may support early US commercialization at hospital-based Centers of Excellence (COEs), if approved
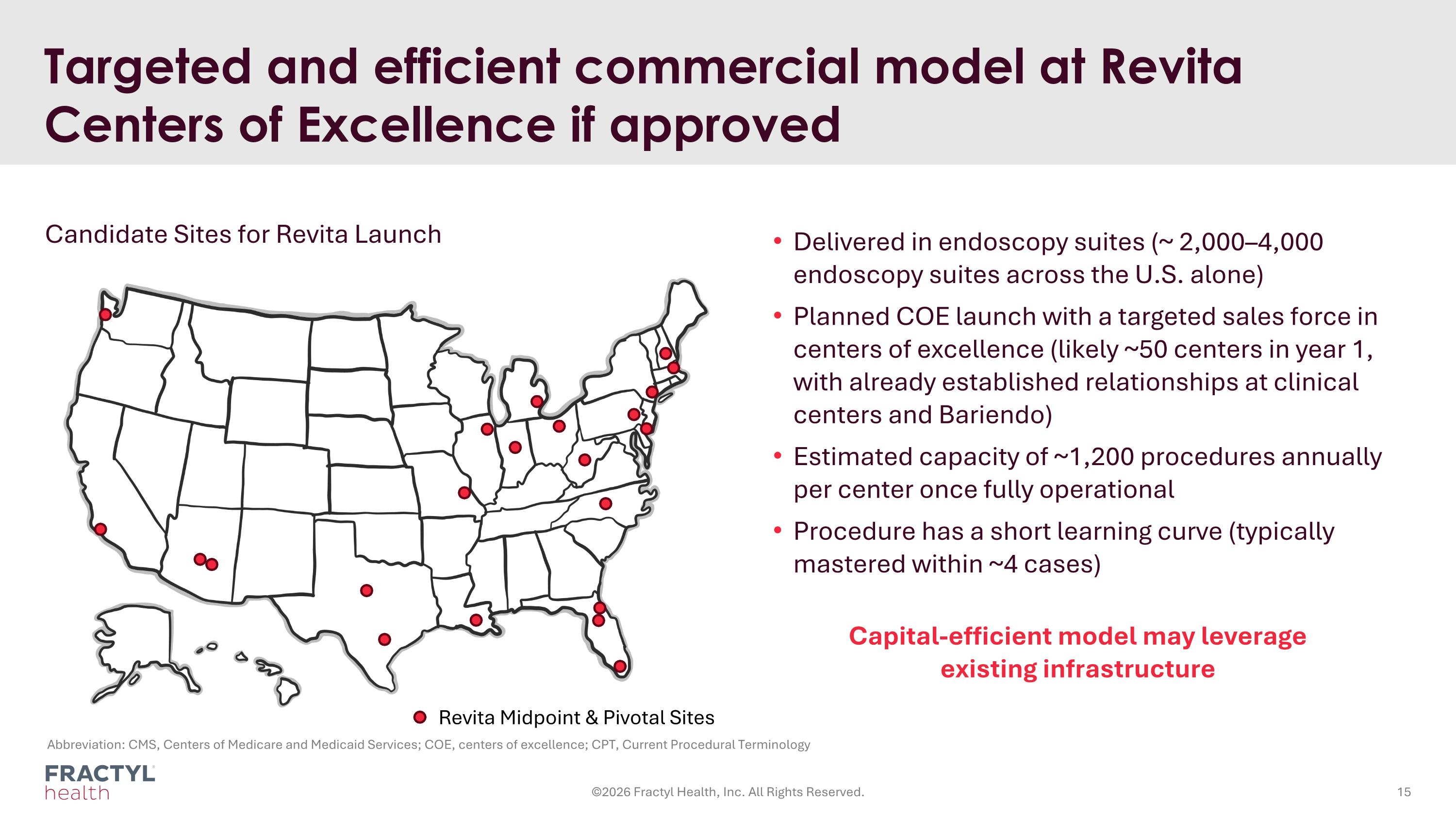
Targeted and efficient commercial model at Revita Centers of Excellence if approved Abbreviation: CMS, Centers of Medicare and Medicaid Services; COE, centers of excellence; CPT, Current Procedural Terminology ©2026 Fractyl Health, Inc. All Rights Reserved. Delivered in endoscopy suites (~ 2,000–4,000 endoscopy suites across the U.S. alone) Planned COE launch with a targeted sales force in centers of excellence (likely ~50 centers in year 1, with already established relationships at clinical centers and Bariendo) Estimated capacity of ~1,200 procedures annually per center once fully operational Procedure has a short learning curve (typically mastered within ~4 cases) Candidate Sites for Revita Launch Revita Midpoint & Pivotal Sites Capital-efficient model may leverage existing infrastructure
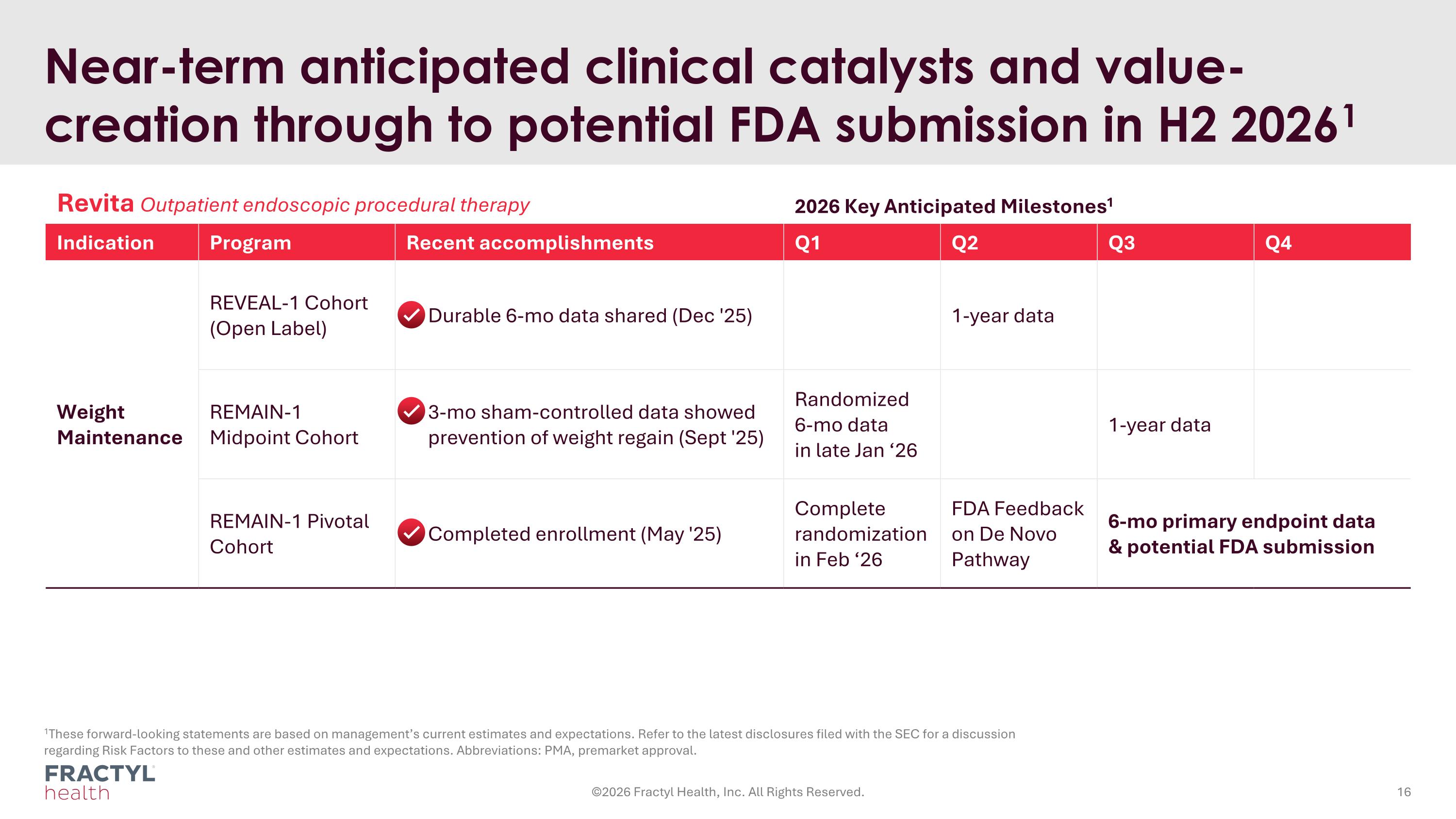
Near-term anticipated clinical catalysts and value-creation through to potential FDA submission in H2 20261 1These forward-looking statements are based on management’s current estimates and expectations. Refer to the latest disclosures filed with the SEC for a discussion regarding Risk Factors to these and other estimates and expectations. Abbreviations: PMA, premarket approval. Revita Outpatient endoscopic procedural therapy 2026 Key Anticipated Milestones1 Indication Program Recent accomplishments Q1 Q2 Q3 Q4 Weight Maintenance REVEAL-1 Cohort (Open Label) Durable 6-mo data shared (Dec '25) 1-year data REMAIN-1 Midpoint Cohort 3-mo sham-controlled data showed prevention of weight regain (Sept '25) Randomized 6-mo data in late Jan ‘26 1-year data REMAIN-1 Pivotal Cohort Completed enrollment (May '25) Complete randomization in Feb ‘26 FDA Feedback on De Novo Pathway 6-mo primary endpoint data & potential FDA submission ©2026 Fractyl Health, Inc. All Rights Reserved.
Key takeaways: Path to pivotal data, FDA submission, reimbursement and commercial readiness ©2026 Fractyl Health, Inc. All Rights Reserved. Clinical evidence: Weight maintenance through 6 months with excellent safety and supportive cardiometabolic and food-craving signals Pivotal study on-track: Midpoint Cohort gives confidence that pivotal study is appropriately powered Regulatory progress: Potential for a more efficient U.S. regulatory path via De Novo; FDA feedback expected Q2 2026 Commercial readiness: Clear reimbursement pathway and focused Centers of Excellence launch creating a predictable path to value creation, if approved

© 2026 Fractyl Health, Inc. All Rights Reserved. Investor Relations Lara Smith Weber CFO 617-835-5912 IR@fractyl.com Thank you