
Summit Therapeutics WCLC Update Call September 8, 2025 8:00am ET

Forward Looking Statement Any statements in this press release about the Company’s future expectations, plans and prospects, including but not limited to, statements about the clinical and preclinical development of the Company’s product candidates, entry into and actions related to the Company’s partnership with Akeso Inc., the Company's anticipated spending and cash runway, the therapeutic potential of the Company’s product candidates, the potential commercialization of the Company’s product candidates, the timing of initiation, completion and availability of data from clinical trials, the potential submission of applications for marketing approvals, potential acquisitions, statements about the previously disclosed At-The-Market equity offering program (“ATM Program”), the expected proceeds and uses thereof, the Company’s estimates regarding stock-based compensation, and other statements containing the words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "would," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the Company’s ability to sell shares of our common stock under the ATM Program, the conditions affecting the capital markets, general economic, industry, or political conditions, the results of our evaluation of the underlying data in connection with the development and commercialization activities for ivonescimab, the outcome of discussions with regulatory authorities, including the Food and Drug Administration, the uncertainties inherent in the initiation of future clinical trials, availability and timing of data from ongoing and future clinical trials, the results of such trials, and their success, global public health crises, that may affect timing and status of our clinical trials and operations, whether preliminary results from a clinical trial will be predictive of the final results of that trial or whether results of early clinical trials or preclinical studies will be indicative of the results of later clinical trials, whether business development opportunities to expand the Company’s pipeline of drug candidates, including without limitation, through potential acquisitions of, and/or collaborations with, other entities occur, expectations for regulatory approvals, laws and regulations affecting government contracts and funding awards, availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements and other factors discussed in the "Risk Factors" and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of filings that the Company makes with the Securities and Exchange Commission. Any change to our ongoing trials could cause delays, affect our future expenses, and add uncertainty to our commercialization efforts, as well as to affect the likelihood of the successful completion of clinical development of ivonescimab. Accordingly, readers should not place undue reliance on forward-looking statements or information. In addition, any forward-looking statements included in this press release represent the Company’s views only as of the date of this release and should not be relied upon as representing the Company’s views as of any subsequent date. The Company specifically disclaims any obligation to update any forward- looking statements included in this press release. Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics WCLC Update Call - September 2025 2 Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.

Ivonescimab vs Placebo Plus Chemo, Phase 3 in Patients with EGFR+ NSCLC Progressed with 3rd gen EGFR-TKI Treatment: HARMONi Jonathan W. Goldman1, Antonio Passaro2, Janessa Laskin3, Delvys Rodrigues-Abreu4, Antonio Calles5, Lyudmila Bazhenova6, Giuseppe Lo Russo7, Natasha Leighl8, Frederico Cappuzzo9, Nicolas Girard10, Sanjay Popat11, Wenfeng Fang12, Yongzhong Luo13, Runxiang Yang14, Wenting Li15, Jianling Li16, Lori Styles16, Benjamin Thompson16, Li Zhang17, Xiuning Le18. 1UCLA Health, Santa Monica, CA, USA; 2European Institute of Oncology, Milan, Italy; 3British Columbia Cancer Research Institute, Vancouver, Canada; 4Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain; 5Hospital General Universitario Gregorio Marañón, Madrid, Spain; 6UC San Diego Moores Cancer Center, San Diego, CA, USA; 7Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy; 8Princess Margaret Cancer Centre University of Toronto, Toronto, Ontario, Canada; 9Regina Elena National Cancer Institute, Rome, Italy; 10Institut Curie, Paris, France; 11Lung Unit, Royal Marsden Hospital, London, UK; 12Sun Yat-sen University Cancer Center, Guangzhou, China; 13Hunan Cancer Hospital, Changsha, China; 14Yunnan Cancer Hospital, Kunming, China; 15Akeso Biopharma, Inc., Zhongshan, China; 16Summit Therapeutics, Menlo Park, CA, USA; 17Sun Yat-sen University Cancer Center, Guangzhou, China; 18The University of Texas MD Anderson Cancer Center, Houston, TX, USA. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
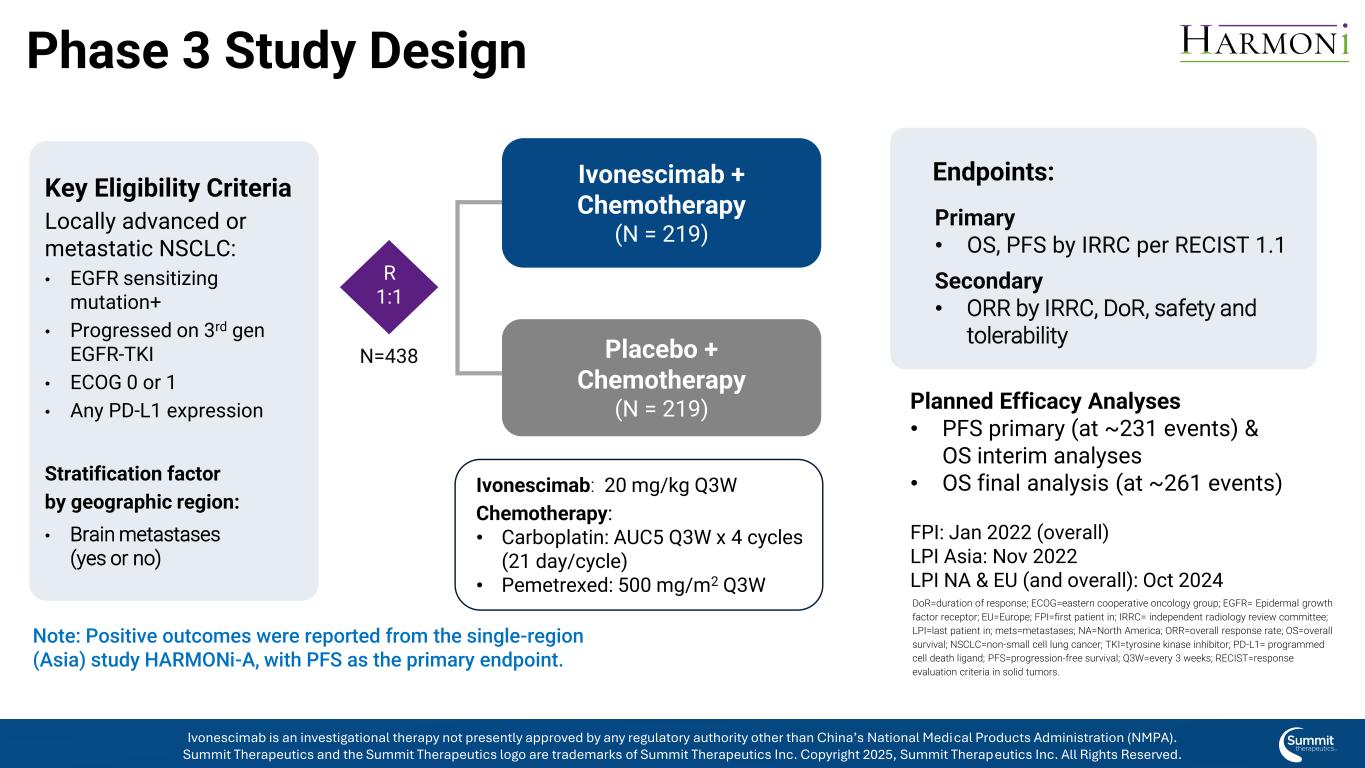
Phase 3 Study Design Key Eligibility Criteria Locally advanced or metastatic NSCLC: • EGFR sensitizing mutation+ • Progressed on 3rd gen EGFR-TKI • ECOG 0 or 1 • Any PD-L1 expression Stratification factor by geographic region: • Brain metastases (yes or no) Ivonescimab + Chemotherapy (N = 219) Placebo + Chemotherapy (N = 219) Primary • OS, PFS by IRRC per RECIST 1.1 Secondary • ORR by IRRC, DoR, safety and tolerability Endpoints: Planned Efficacy Analyses • PFS primary (at ~231 events) & OS interim analyses • OS final analysis (at ~261 events) FPI: Jan 2022 (overall) LPI Asia: Nov 2022 LPI NA & EU (and overall): Oct 2024 Ivonescimab: 20 mg/kg Q3W Chemotherapy: • Carboplatin: AUC5 Q3W x 4 cycles (21 day/cycle) • Pemetrexed: 500 mg/m2 Q3W Note: Positive outcomes were reported from the single-region (Asia) study HARMONi-A, with PFS as the primary endpoint. R 1:1 N=438 DoR=duration of response; ECOG=eastern cooperative oncology group; EGFR= Epidermal growth factor receptor; EU=Europe; FPI=first patient in; IRRC= independent radiology review committee; LPI=last patient in; mets=metastases; NA=North America; ORR=overall response rate; OS=overall survival; NSCLC=non-small cell lung cancer; TKI=tyrosine kinase inhibitor; PD-L1= programmed cell death ligand; PFS=progression-free survival; Q3W=every 3 weeks; RECIST=response evaluation criteria in solid tumors. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
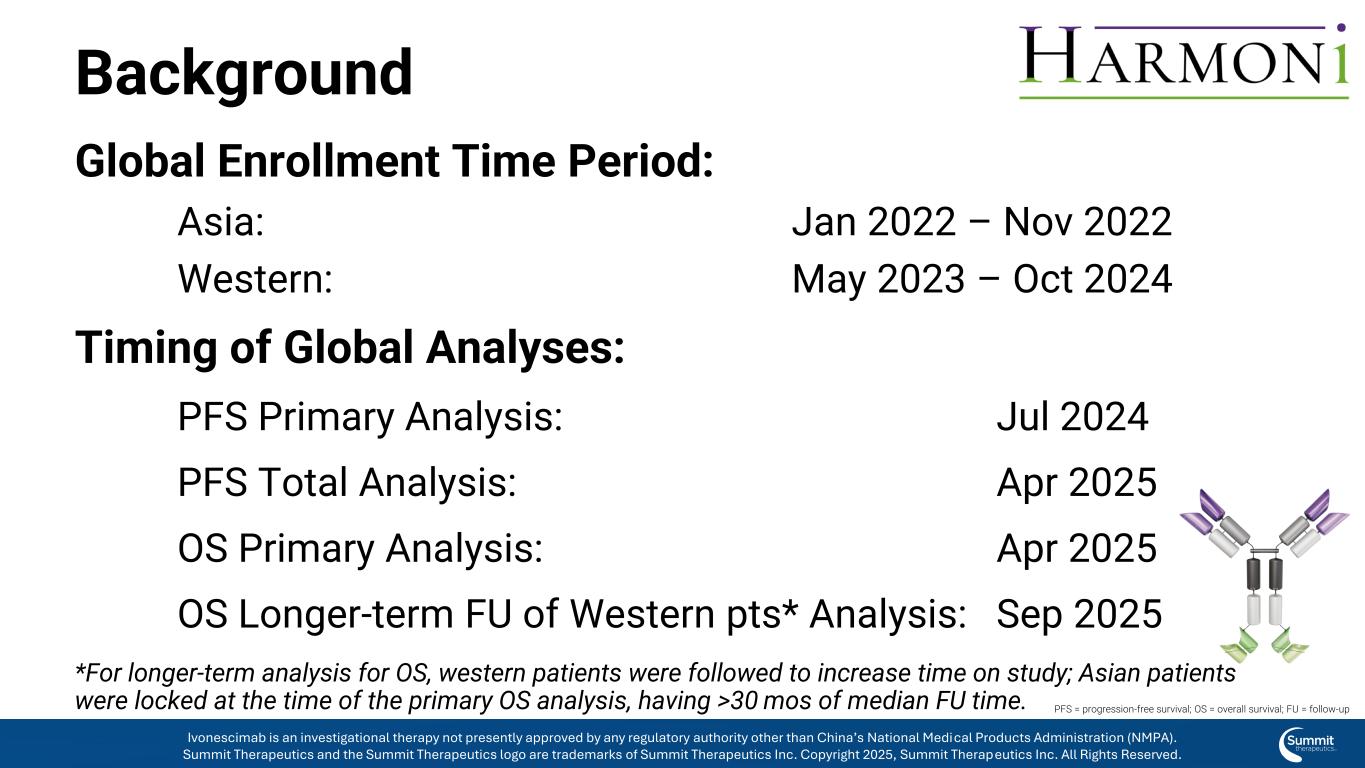
Background Global Enrollment Time Period: Asia: Jan 2022 – Nov 2022 Western: May 2023 – Oct 2024 Timing of Global Analyses: PFS Primary Analysis: Jul 2024 PFS Total Analysis: Apr 2025 OS Primary Analysis: Apr 2025 OS Longer-term FU of Western pts* Analysis: Sep 2025 *For longer-term analysis for OS, western patients were followed to increase time on study; Asian patients were locked at the time of the primary OS analysis, having >30 mos of median FU time. PFS = progression-free survival; OS = overall survival; FU = follow-up Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
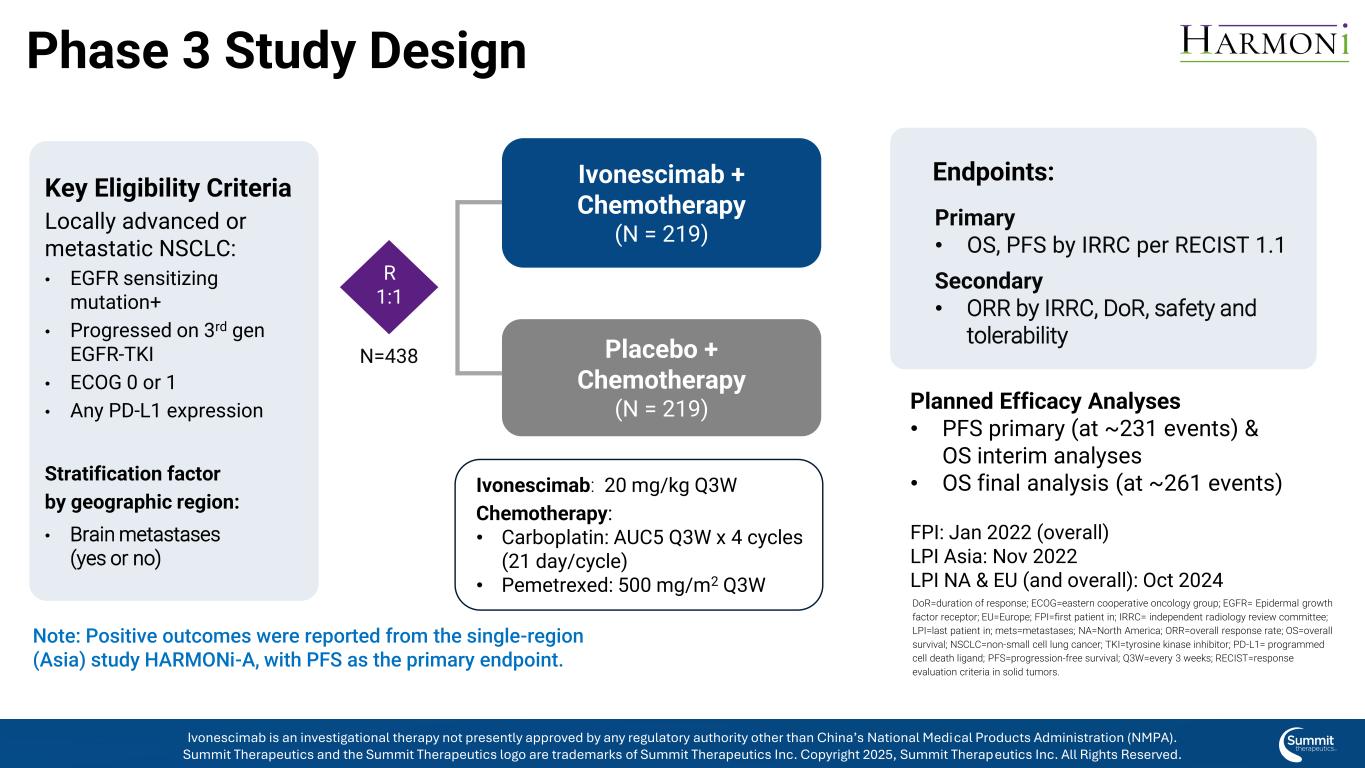
Phase 3 Study Design Key Eligibility Criteria Locally advanced or metastatic NSCLC: • EGFR sensitizing mutation+ • Progressed on 3rd gen EGFR-TKI • ECOG 0 or 1 • Any PD-L1 expression Stratification factor by geographic region: • Brain metastases (yes or no) Ivonescimab + Chemotherapy (N = 219) Placebo + Chemotherapy (N = 219) Primary • OS, PFS by IRRC per RECIST 1.1 Secondary • ORR by IRRC, DoR, safety and tolerability Endpoints: Planned Efficacy Analyses • PFS primary (at ~231 events) & OS interim analyses • OS final analysis (at ~261 events) FPI: Jan 2022 (overall) LPI Asia: Nov 2022 LPI NA & EU (and overall): Oct 2024 Ivonescimab: 20 mg/kg Q3W Chemotherapy: • Carboplatin: AUC5 Q3W x 4 cycles (21 day/cycle) • Pemetrexed: 500 mg/m2 Q3W Note: Positive outcomes were reported from the single-region (Asia) study HARMONi-A, with PFS as the primary endpoint. R 1:1 N=438 DoR=duration of response; ECOG=eastern cooperative oncology group; EGFR= Epidermal growth factor receptor; EU=Europe; FPI=first patient in; IRRC= independent radiology review committee; LPI=last patient in; mets=metastases; NA=North America; ORR=overall response rate; OS=overall survival; NSCLC=non-small cell lung cancer; TKI=tyrosine kinase inhibitor; PD-L1= programmed cell death ligand; PFS=progression-free survival; Q3W=every 3 weeks; RECIST=response evaluation criteria in solid tumors. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
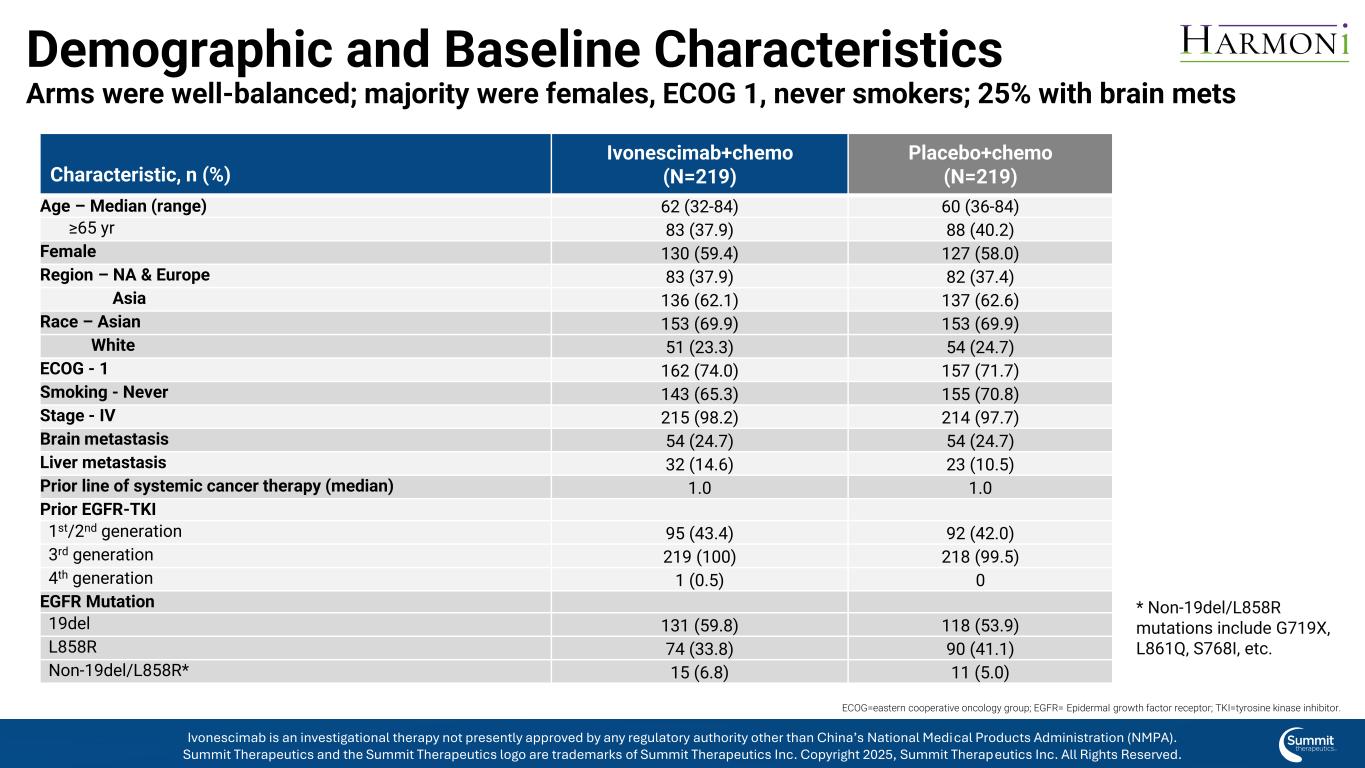
Demographic and Baseline Characteristics Arms were well-balanced; majority were females, ECOG 1, never smokers; 25% with brain mets * Non-19del/L858R mutations include G719X, L861Q, S768I, etc. ECOG=eastern cooperative oncology group; EGFR= Epidermal growth factor receptor; TKI=tyrosine kinase inhibitor. Characteristic, n (%) Ivonescimab+chemo (N=219) Placebo+chemo (N=219) Age – Median (range) 62 (32-84) 60 (36-84) ≥65 yr 83 (37.9) 88 (40.2) Female 130 (59.4) 127 (58.0) Region – NA & Europe 83 (37.9) 82 (37.4) Asia 136 (62.1) 137 (62.6) Race – Asian 153 (69.9) 153 (69.9) White 51 (23.3) 54 (24.7) ECOG - 1 162 (74.0) 157 (71.7) Smoking - Never 143 (65.3) 155 (70.8) Stage - IV 215 (98.2) 214 (97.7) Brain metastasis 54 (24.7) 54 (24.7) Liver metastasis 32 (14.6) 23 (10.5) Prior line of systemic cancer therapy (median) 1.0 1.0 Prior EGFR-TKI 1st/2nd generation 95 (43.4) 92 (42.0) 3rd generation 219 (100) 218 (99.5) 4th generation 1 (0.5) 0 EGFR Mutation 19del 131 (59.8) 118 (53.9) L858R 74 (33.8) 90 (41.1) Non-19del/L858R* 15 (6.8) 11 (5.0) Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
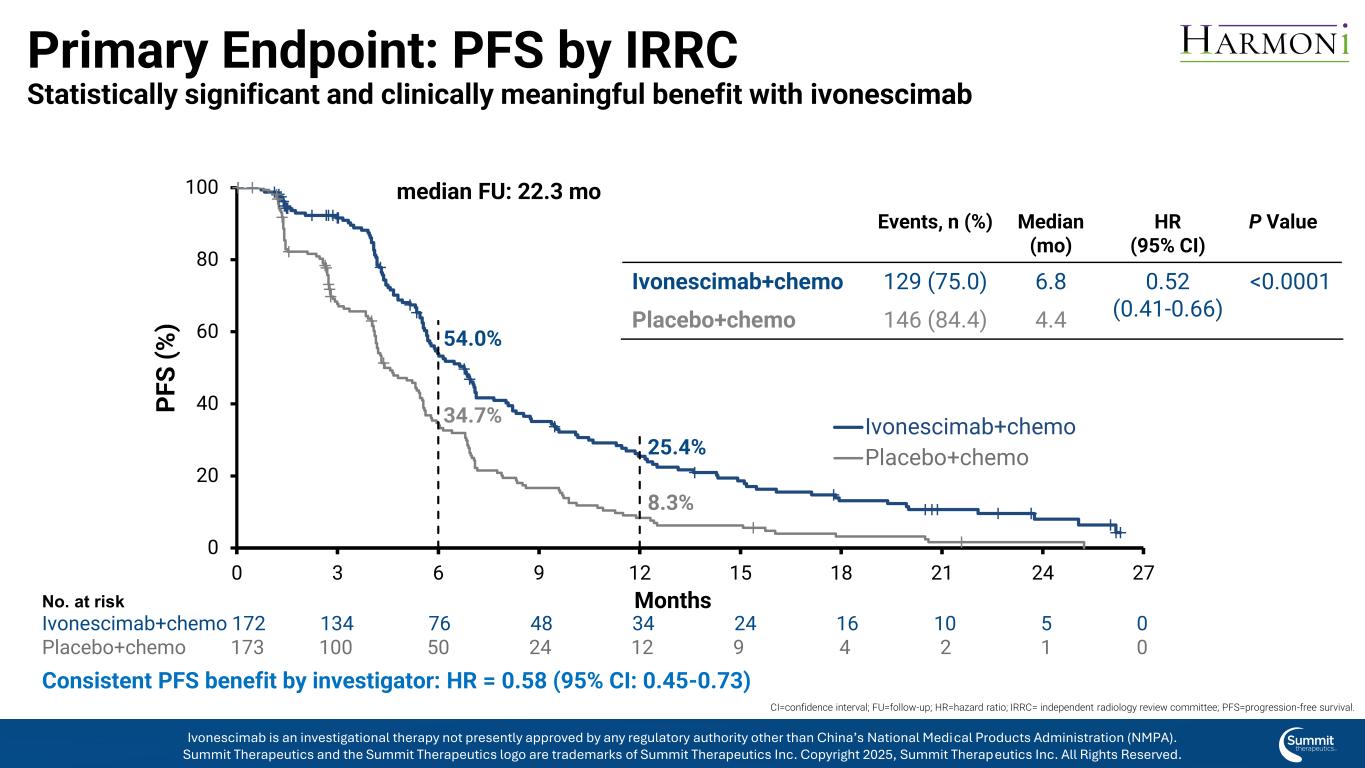
Primary Endpoint: PFS by IRRC Statistically significant and clinically meaningful benefit with ivonescimab Consistent PFS benefit by investigator: HR = 0.58 (95% CI: 0.45-0.73) 0 20 40 60 80 100 0 3 6 9 12 15 18 21 24 27 P F S ( % ) Ivonescimab+chemo Placebo+chemo 54.0% 34.7% 25.4% 8.3% median FU: 22.3 mo No. at risk Ivonescimab+chemo 172 134 76 48 34 24 16 10 5 0 Placebo+chemo 173 100 50 24 12 9 4 2 1 0 Months Events, n (%) Median (mo) HR (95% CI) P Value Ivonescimab+chemo 129 (75.0) 6.8 0.52 (0.41-0.66) <0.0001 Placebo+chemo 146 (84.4) 4.4 CI=confidence interval; FU=follow-up; HR=hazard ratio; IRRC= independent radiology review committee; PFS=progression-free survival. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
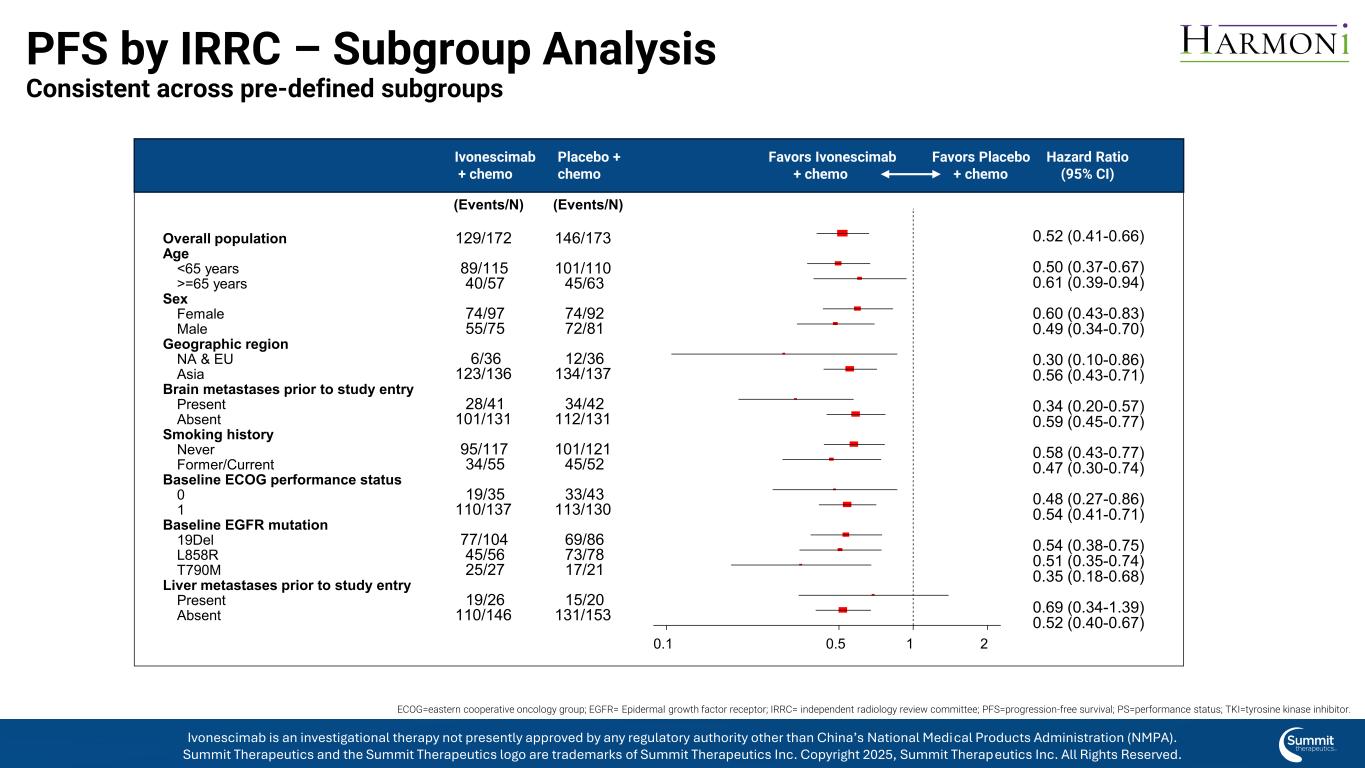
PFS by IRRC – Subgroup Analysis Consistent across pre-defined subgroups (Events/N)(Events/N) HRPbo + chemoIvo + chemo 0.52 (0.41-0.66) 0.50 (0.37-0.67) 0.61 (0.39-0.94) 0.60 (0.43-0.83) 0.49 (0.34-0.70) 0.30 (0.10-0.86) 0.56 (0.43-0.71) 0.34 (0.20-0.57) 0.59 (0.45-0.77) 0.58 (0.43-0.77) 0.47 (0.30-0.74) 0.48 (0.27-0.86) 0.54 (0.41-0.71) 0.54 (0.38-0.75) 0.51 (0.35-0.74) 0.35 (0.18-0.68) 0.69 (0.34-1.39) 0.52 (0.40-0.67) 0.1 0.5 1 2 146/173 101/110 45/63 74/92 72/81 12/36 134/137 34/42 112/131 101/121 45/52 33/43 113/130 69/86 73/78 17/21 15/20 131/153 129/172 89/115 40/57 74/97 55/75 6/36 123/136 28/41 101/131 95/117 34/55 19/35 110/137 77/104 45/56 25/27 19/26 110/146 Overall population Age Sex Geographic region Brain metastases prior to study entry Smoking history Baseline ECOG performance status Baseline EGFR mutation Liver metastases prior to study entry <65 years >=65 years Female Male NA & EU Asia Present Absent Never Former/Current 0 1 19Del L858R T790M Present Absent Ivonescimab + chemo Placebo + chemo Hazard Ratio (95% CI) Favors Ivonescimab + chemo Favors Placebo + chemo ECOG=eastern cooperative oncology group; EGFR= Epidermal growth factor receptor; IRRC= independent radiology review committee; PFS=progression-free survival; PS=performance status; TKI=tyrosine kinase inhibitor. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
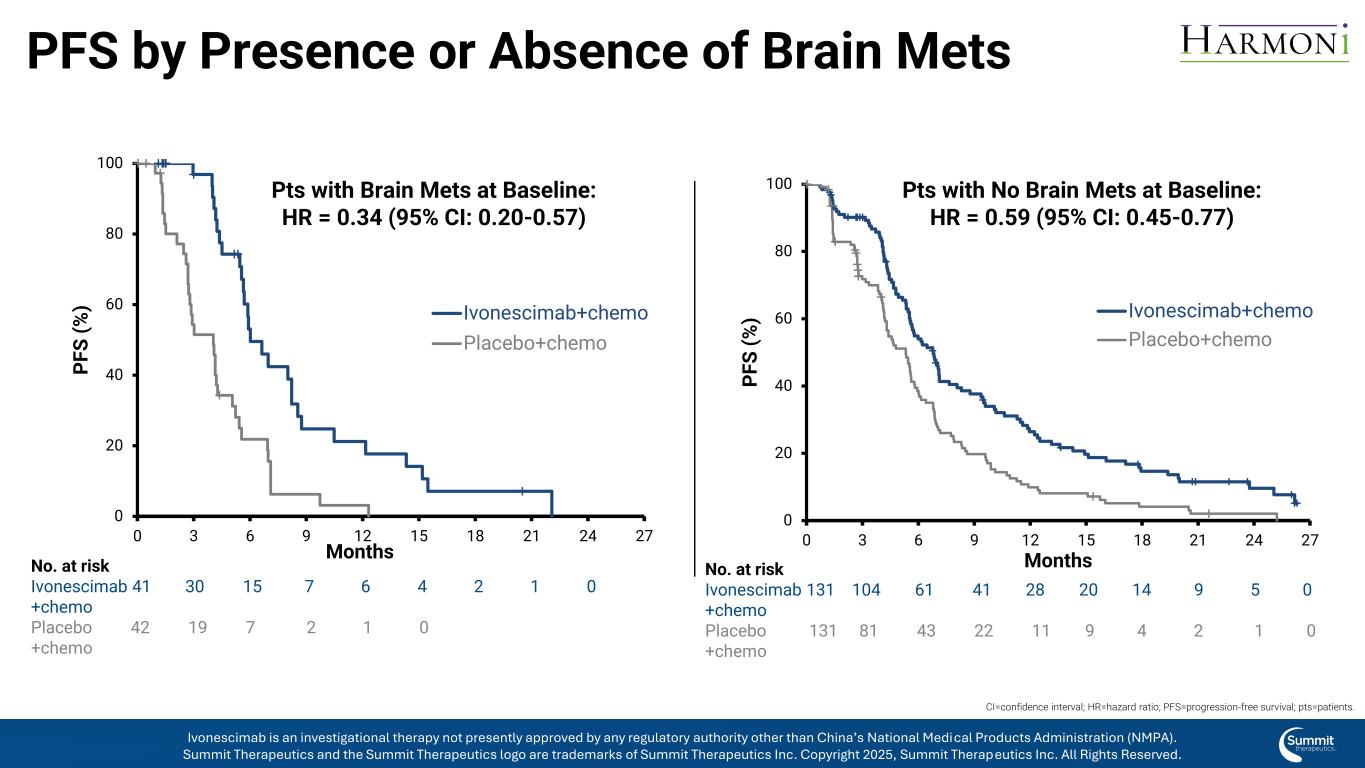
PFS by Presence or Absence of Brain Mets 0 20 40 60 80 100 0 3 6 9 12 15 18 21 24 27 P F S ( % ) Ivonescimab+chemo Placebo+chemo Pts with Brain Mets at Baseline: HR = 0.34 (95% CI: 0.20-0.57) 0 20 40 60 80 100 0 3 6 9 12 15 18 21 24 27 P F S ( % ) Months Ivonescimab+chemo Placebo+chemo Pts with No Brain Mets at Baseline: HR = 0.59 (95% CI: 0.45-0.77) Months No. at risk Ivonescimab 41 30 15 7 6 4 2 1 0 +chemo Placebo 42 19 7 2 1 0 +chemo No. at risk Ivonescimab 131 104 61 41 28 20 14 9 5 0 +chemo Placebo 131 81 43 22 11 9 4 2 1 0 +chemo CI=confidence interval; HR=hazard ratio; PFS=progression-free survival; pts=patients. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
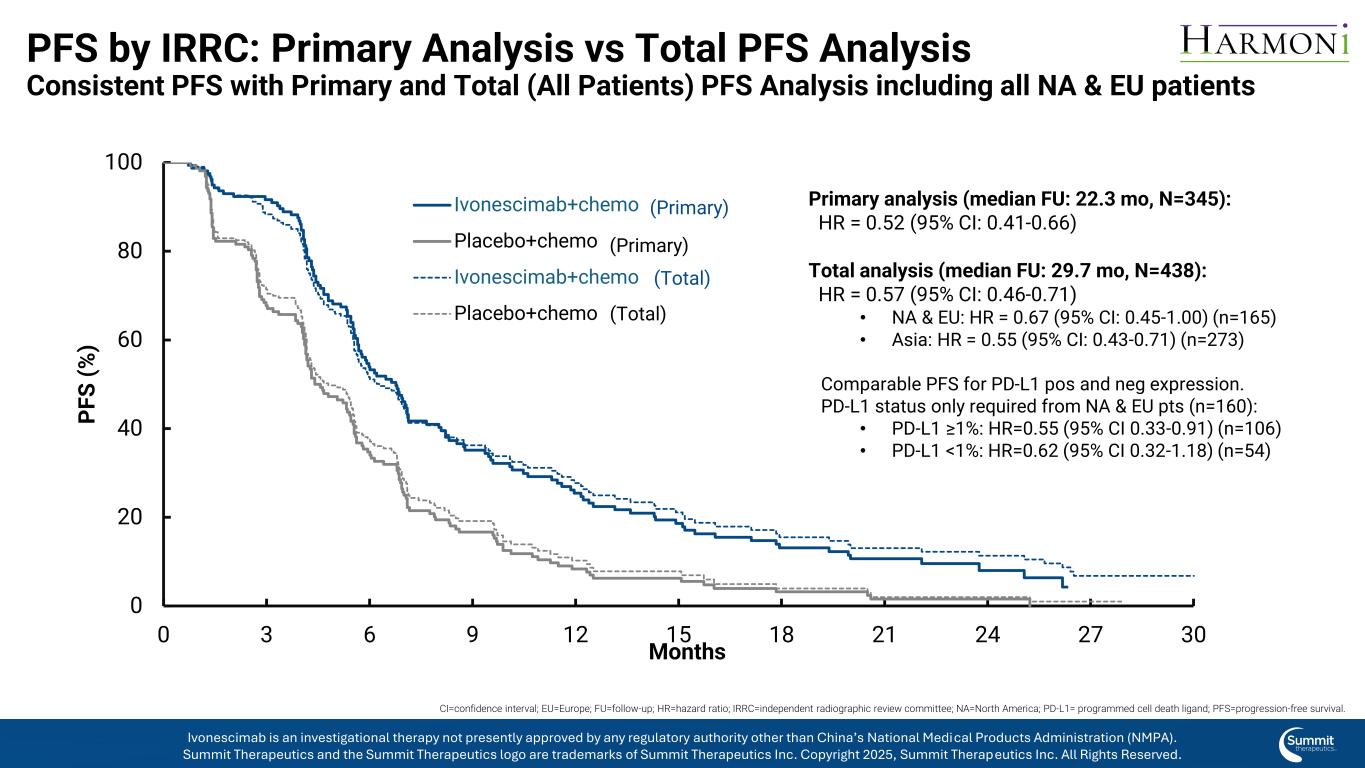
PFS by IRRC: Primary Analysis vs Total PFS Analysis Consistent PFS with Primary and Total (All Patients) PFS Analysis including all NA & EU patients 0 20 40 60 80 100 0 3 6 9 12 15 18 21 24 27 30 P F S ( % ) Months Ivonescimab+chemo Placebo+chemo Ivonescimab+chemo (LTF) Placebo+chemo (LTF) Primary analysis (median FU: 22.3 mo, N=345): HR = 0.52 (95% CI: 0.41-0.66) Total analysis (median FU: 29.7 mo, N=438): HR = 0.57 (95% CI: 0.46-0.71) • NA & EU: HR = 0.67 (95% CI: 0.45-1.00) (n=165) • Asia: HR = 0.55 (95% CI: 0.43-0.71) (n=273) Comparable PFS for PD-L1 pos and neg expression. PD-L1 status only required from NA & EU pts (n=160): • PD-L1 ≥1%: HR=0.55 (95% CI 0.33-0.91) (n=106) • PD-L1 <1%: HR=0.62 (95% CI 0.32-1.18) (n=54) (Primary) (Primary) CI=confidence interval; EU=Europe; FU=follow-up; HR=hazard ratio; IRRC=independent radiographic review committee; NA=North America; PD-L1= programmed cell death ligand; PFS=progression-free survival. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved. ( otal) (Total)
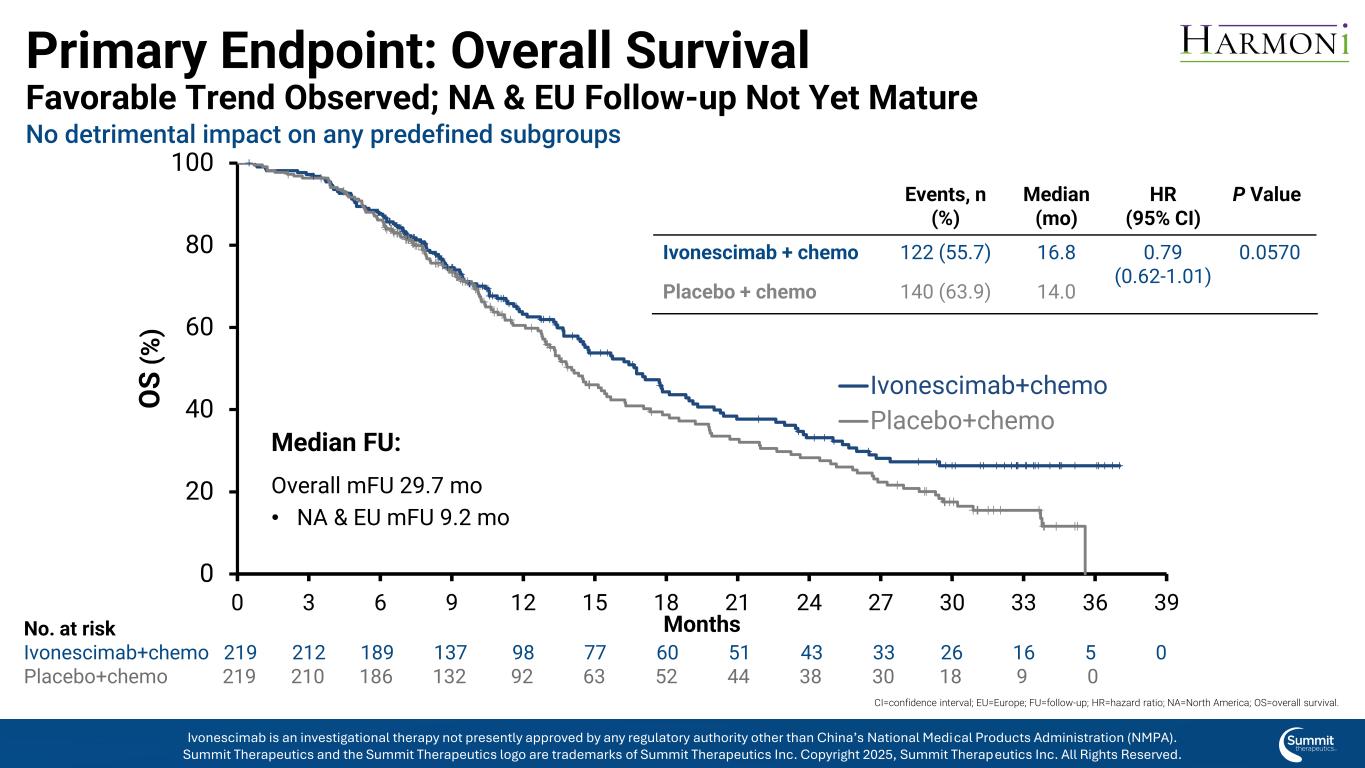
Primary Endpoint: Overall Survival Favorable Trend Observed; NA & EU Follow-up Not Yet Mature 0 20 40 60 80 100 0 3 6 9 12 15 18 21 24 27 30 33 36 39 O S ( % ) Months Ivonescimab+chemo Placebo+chemo Median FU: Overall mFU 29.7 mo • NA & EU mFU 9.2 mo No. at risk Ivonescimab+chemo 219 212 189 137 98 77 60 51 43 33 26 16 5 0 Placebo+chemo 219 210 186 132 92 63 52 44 38 30 18 9 0 CI=confidence interval; EU=Europe; FU=follow-up; HR=hazard ratio; NA=North America; OS=overall survival. Events, n (%) Median (mo) HR (95% CI) P Value Ivonescimab + chemo 122 (55.7) 16.8 0.79 (0.62-1.01) 0.0570 Placebo + chemo 140 (63.9) 14.0 Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved. No detrimental impact on any predefined subgroups
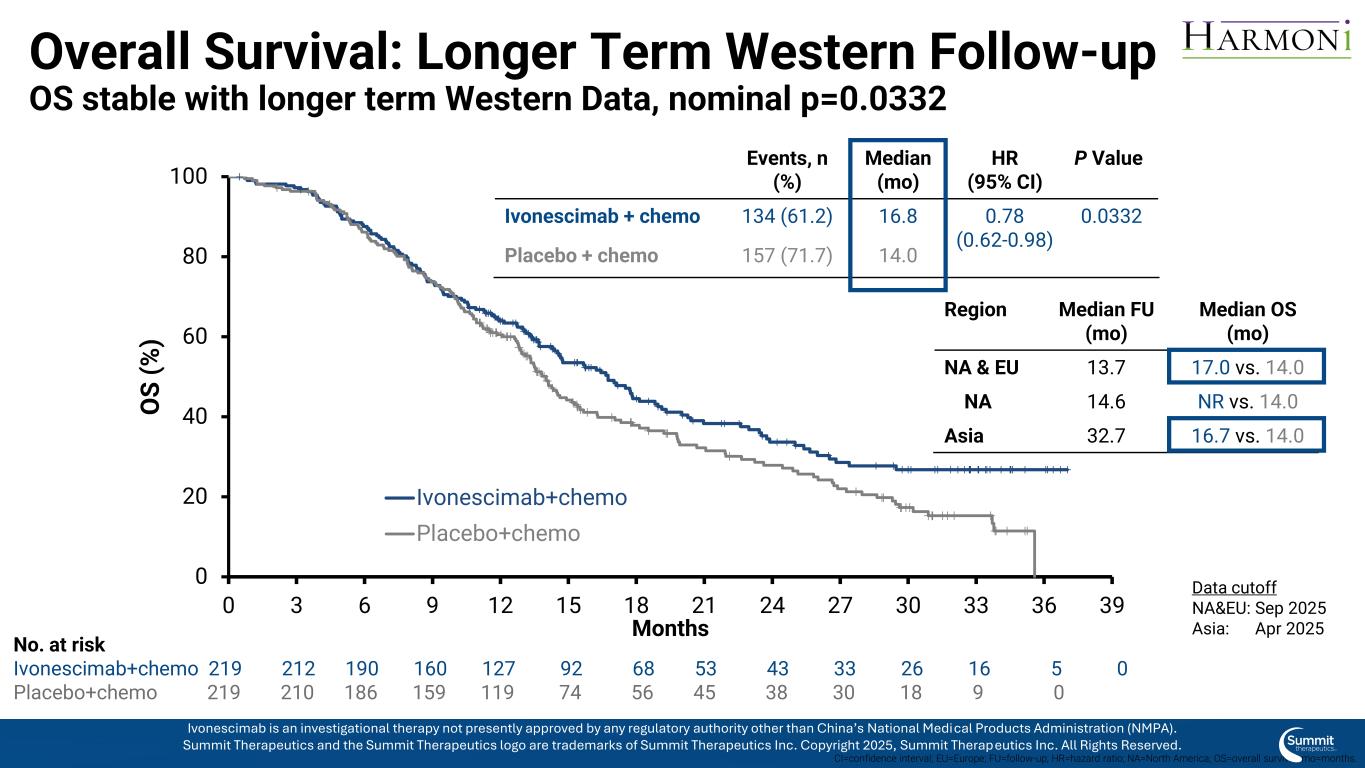
Overall Survival: Longer Term Western Follow-up OS stable with longer term Western Data, nominal p=0.0332 0 20 40 60 80 100 0 3 6 9 12 15 18 21 24 27 30 33 36 39 O S ( % ) Months Ivonescimab+chemo Placebo+chemo No. at risk Ivonescimab+chemo 219 212 190 160 127 92 68 53 43 33 26 16 5 0 Placebo+chemo 219 210 186 159 119 74 56 45 38 30 18 9 0 CI=confidence interval; EU=Europe; FU=follow-up; HR=hazard ratio; NA=North America; OS=overall survival; mo=months. Events, n (%) Median (mo) HR (95% CI) P Value Ivonescimab + chemo 134 (61.2) 16.8 0.78 (0.62-0.98) 0.0332 Placebo + chemo 157 (71.7) 14.0 Region Median FU (mo) Median OS (mo) NA & EU 13.7 17.0 vs. 14.0 NA 14.6 NR vs. 14.0 Asia 32.7 16.7 vs. 14.0 Data cutoff NA&EU: Sep 2025 Asia: Apr 2025 Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
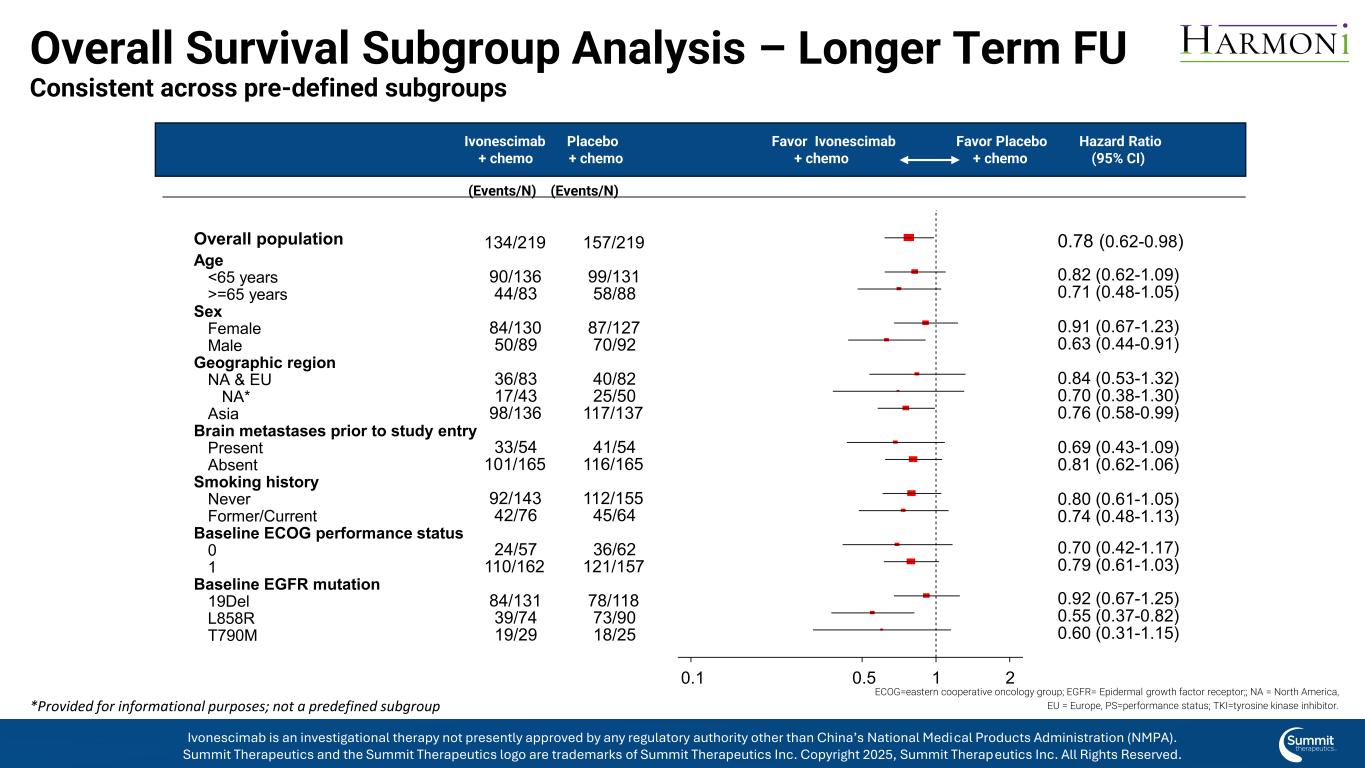
Overall Survival Subgroup Analysis – Longer Term FU Consistent across pre-defined subgroups ECOG=eastern cooperative oncology group; EGFR= Epidermal growth factor receptor;; NA = North America, EU = Europe, PS=performance status; TKI=tyrosine kinase inhibitor. Ivonescimab Placebo Favor Ivonescimab Favor Placebo Hazard Ratio + chemo + chemo + chemo + chemo (95% CI) (Events/N) (Events/N) 0.78 (0.62-0.98) 0.82 (0.62-1.09) 0.71 (0.48-1.05) 0.91 (0.67-1.23) 0.63 (0.44-0.91) 0.84 (0.53-1.32) 0.70 (0.38-1.30) 0.76 (0.58-0.99) 0.69 (0.43-1.09) 0.81 (0.62-1.06) 0.80 (0.61-1.05) 0.74 (0.48-1.13) 0.70 (0.42-1.17) 0.79 (0.61-1.03) 0.92 (0.67-1.25) 0.55 (0.37-0.82) 0.60 (0.31-1.15) 0.1 0.5 1 2 157/219 99/131 58/88 87/127 70/92 40/82 25/50 117/137 41/54 116/165 112/155 45/64 36/62 121/157 78/118 73/90 18/25 134/219 90/136 44/83 84/130 50/89 36/83 17/43 98/136 33/54 101/165 92/143 42/76 24/57 110/162 84/131 39/74 19/29 Overall population Age Sex Geographic region Brain metastases prior to study entry Smoking history Baseline ECOG performance status Baseline EGFR mutation <65 years >=65 years Female Male NA & EU NA* Asia Present Absent Never Former/Current 0 1 19Del L858R T790M Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved. *Provided for informational purposes; not a predefined subgroup
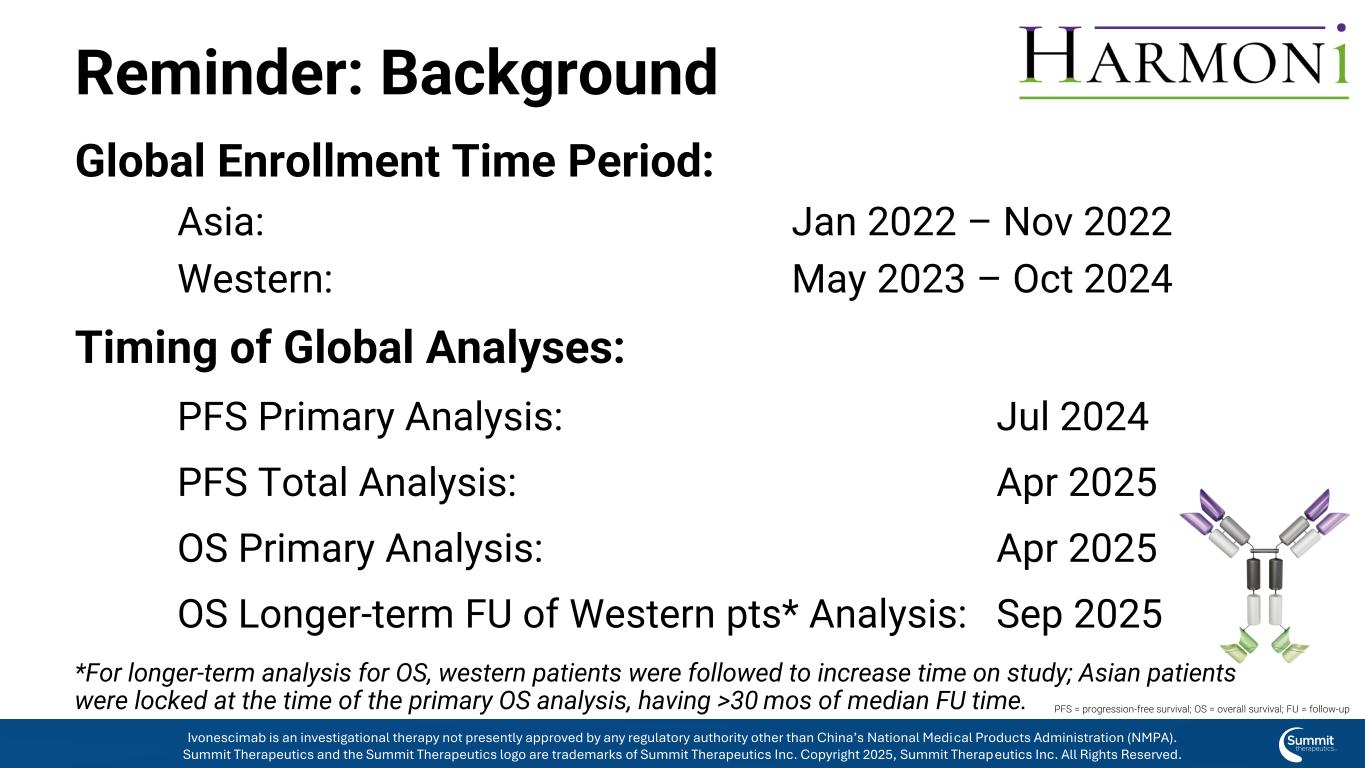
Reminder: Background Global Enrollment Time Period: Asia: Jan 2022 – Nov 2022 Western: May 2023 – Oct 2024 Timing of Global Analyses: PFS Primary Analysis: Jul 2024 PFS Total Analysis: Apr 2025 OS Primary Analysis: Apr 2025 OS Longer-term FU of Western pts* Analysis: Sep 2025 *For longer-term analysis for OS, western patients were followed to increase time on study; Asian patients were locked at the time of the primary OS analysis, having >30 mos of median FU time. PFS = progression-free survival; OS = overall survival; FU = follow-up Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
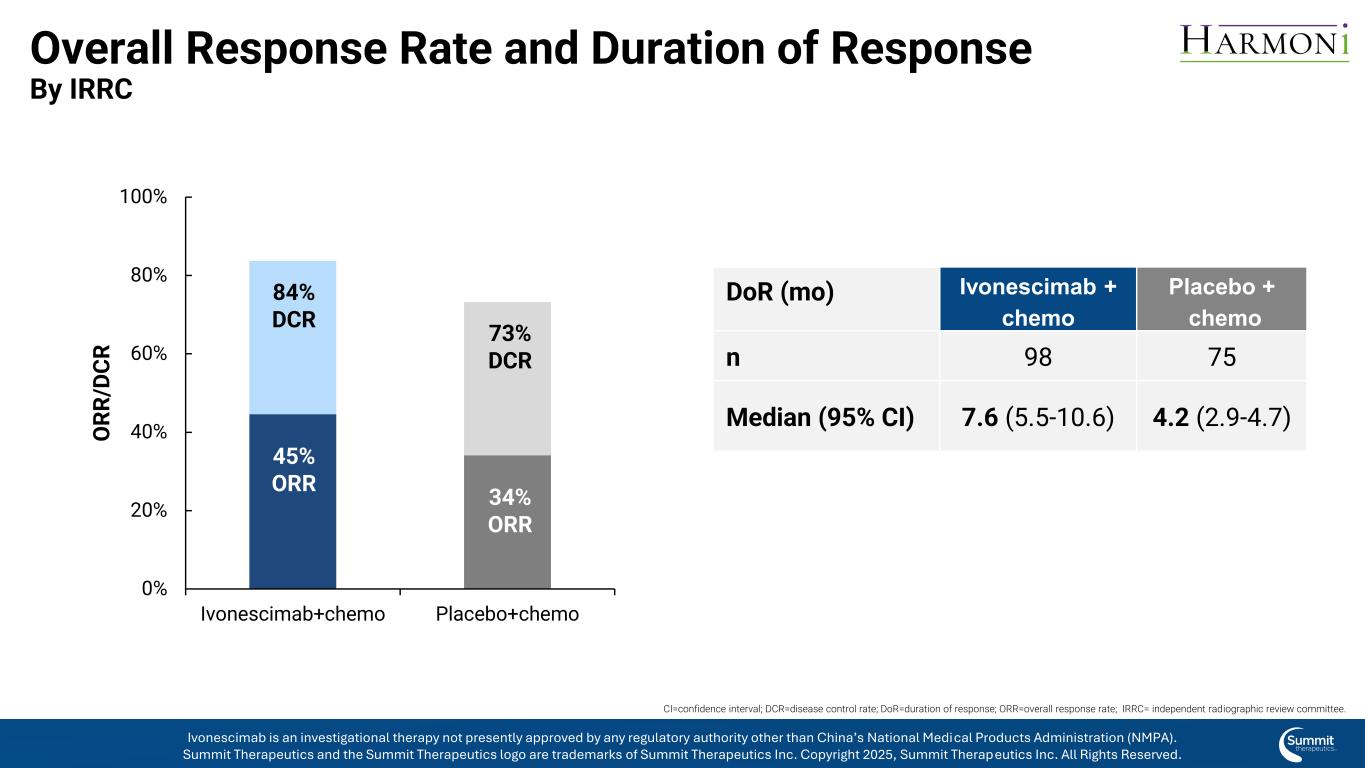
Overall Response Rate and Duration of Response By IRRC 0% 20% 40% 60% 80% 100% Ivonescimab+chemo Placebo+chemo O R R /D C R 84% DCR 45% ORR 73% DCR 34% ORR CI=confidence interval; DCR=disease control rate; DoR=duration of response; ORR=overall response rate; IRRC= independent radiographic review committee. DoR (mo) Ivonescimab + chemo Placebo + chemo n 98 75 Median (95% CI) 7.6 (5.5-10.6) 4.2 (2.9-4.7) Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
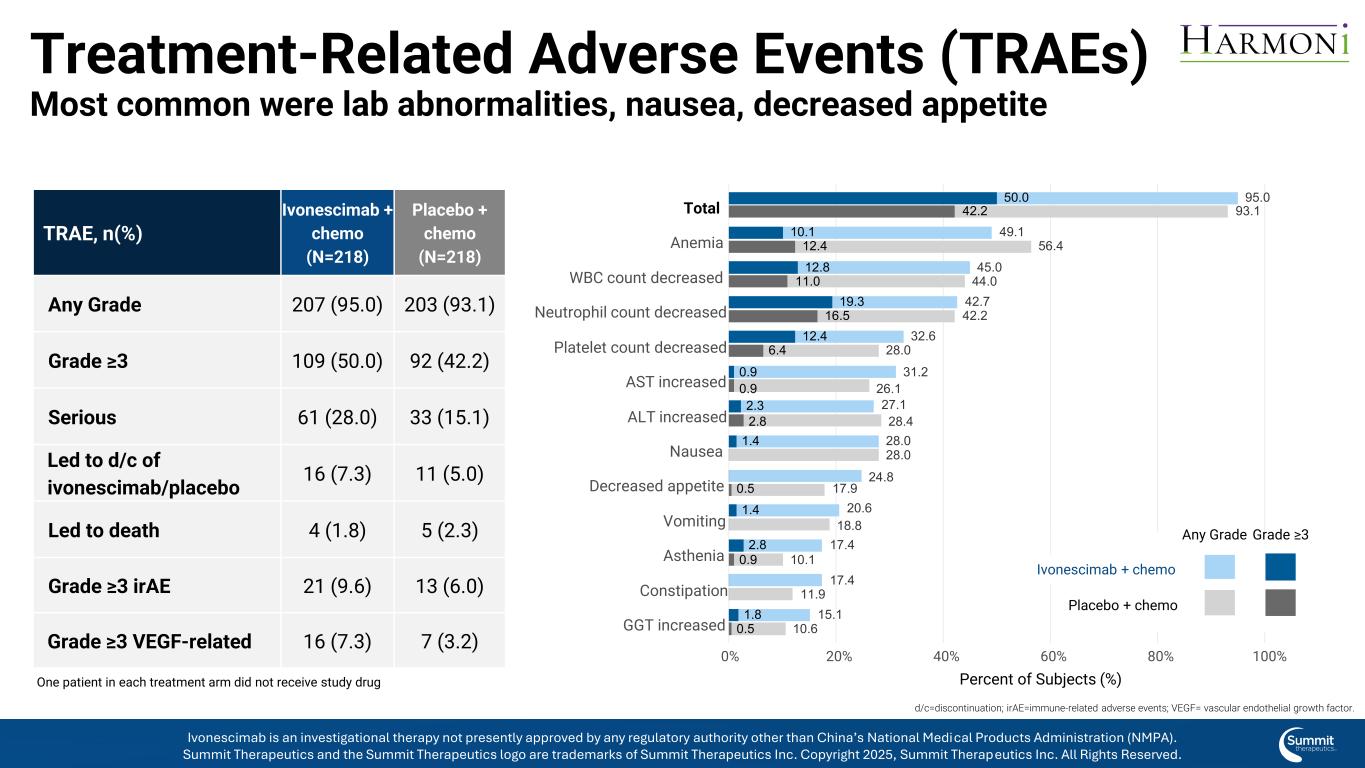
2.3 0.9 10.1 2.8 1.8 1.4 19.3 12.4 1.4 12.8 2.8 0.9 12.4 0.9 0.5 0.5 16.5 6.4 11.0 50.0 42.2 27.1 31.2 49.1 17.4 17.4 24.8 15.1 28.0 42.7 32.6 20.6 45.0 28.4 26.1 56.4 10.1 11.9 17.9 10.6 28.0 42.2 28.0 18.8 44.0 95.0 93.1 GGT increased Constipation Asthenia Vomiting Decreased appetite Nausea ALT increased AST increased Platelet count decreased Neutrophil count decreased WBC count decreased Anemia Total 0% 20% 40% 60% 80% 100% Percent of Subjects (%) Treatment-Related Adverse Events (TRAEs) Most common were lab abnormalities, nausea, decreased appetite Grade ≥3 Ivonescimab + chemo Placebo + chemo Any Grade One patient in each treatment arm did not receive study drug TRAE, n(%) Ivonescimab + chemo (N=218) Placebo + chemo (N=218) Any Grade 207 (95.0) 203 (93.1) Grade ≥3 109 (50.0) 92 (42.2) Serious 61 (28.0) 33 (15.1) Led to d/c of ivonescimab/placebo 16 (7.3) 11 (5.0) Led to death 4 (1.8) 5 (2.3) Grade ≥3 irAE 21 (9.6) 13 (6.0) Grade ≥3 VEGF-related 16 (7.3) 7 (3.2) d/c=discontinuation; irAE=immune-related adverse events; VEGF= vascular endothelial growth factor. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
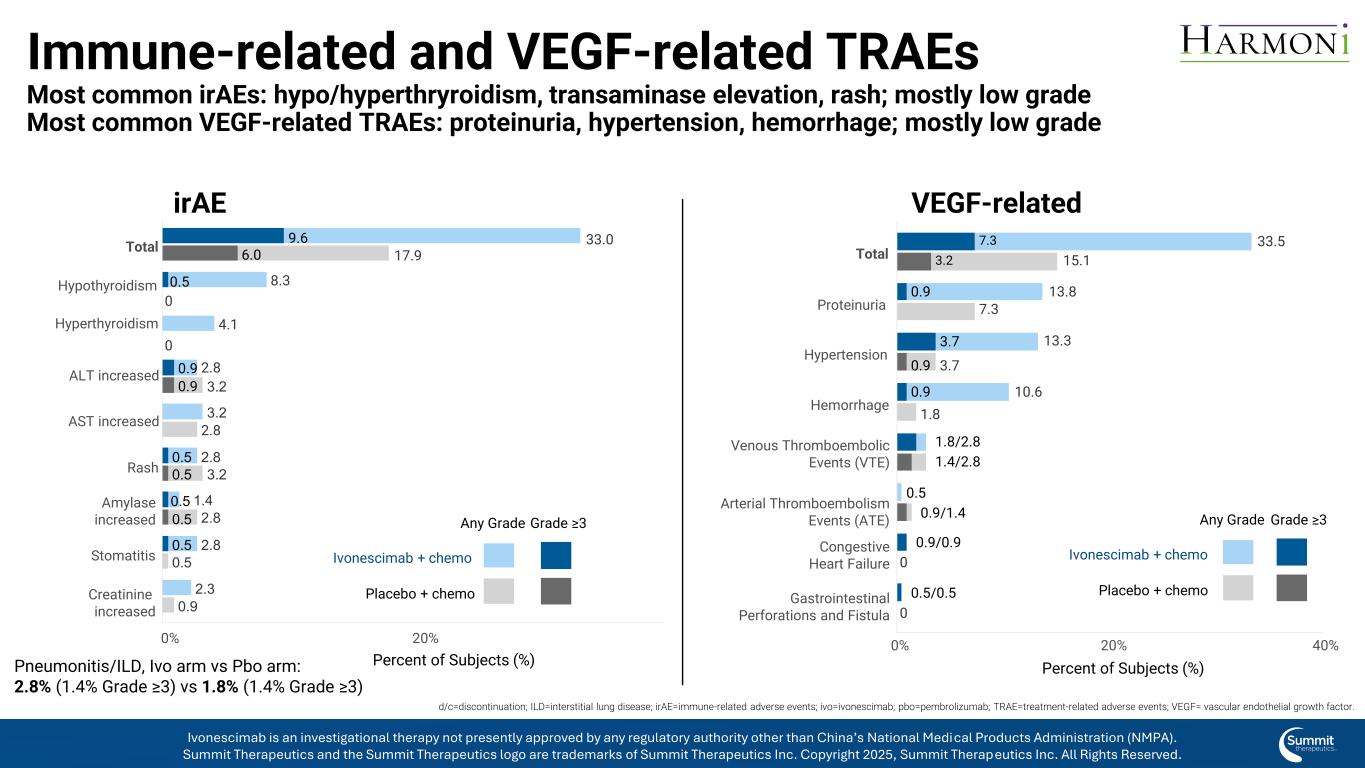
0.9 0.9 0.5 0.5 0.5 0.5 0.5 0.5 9.6 6.0 2.8 3.2 1.4 2.8 3.2 2.8 2.3 0.9 4.1 8.3 2.8 3.2 2.8 0.5 33.0 17.9 0 0 Creatinine increased Stomatitis Amylase increased Rash AST increased ALT increased Hyperthyroidism Hypothyroidism Total 0.5 0.9/1.4 0.9/0.9 0.5/0.5 1.8/2.8 1.4/2.8 0.9 3.7 0.9 0.9 7.3 3.2 10.6 1.8 13.3 3.7 13.8 7.3 33.5 15.1 0 0 Gastrointestinal Perforations and Fistula Congestive Heart Failure Arterial Thromboembolism Events (ATE) Venous Thromboembolic Events (VTE) Hemorrhage Hypertension Proteinuria Total 0% 20% 40% Percent of Subjects (%) Immune-related and VEGF-related TRAEs Most common irAEs: hypo/hyperthryroidism, transaminase elevation, rash; mostly low grade Most common VEGF-related TRAEs: proteinuria, hypertension, hemorrhage; mostly low grade VEGF-related irAE Pneumonitis/ILD, Ivo arm vs Pbo arm: 2.8% (1.4% Grade ≥3) vs 1.8% (1.4% Grade ≥3) Grade ≥3 Ivonescimab + chemo Placebo + chemo Any Grade 0% 20% Percent of Subjects (%) Grade ≥3 Ivonescimab + chemo Placebo + chemo Any Grade d/c=discontinuation; ILD=interstitial lung disease; irAE=immune-related adverse events; ivo=ivonescimab; pbo=pembrolizumab; TRAE=treatment-related adverse events; VEGF= vascular endothelial growth factor. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.
Summary • Ivonescimab had a significant and clinically meaningful PFS benefit in EGFRm+ NSCLC patients post-3rd gen TKI • Reduced risk of progression or death by 48% vs chemotherapy, HR=0.52 • Consistent efficacy across pre-defined subgroups • Increased ORR and DoR • OS final analysis showed favorable trend; HR=0.79 with p=0.0570 • Longer-term follow-up of Western patients showed stable OS; HR=0.78 with nominal p=0.0332 • Western patients’ median OS was numerically higher by 3 months • Ivonescimab well tolerated, with no new safety findings • <1% Grade 3+ bleeding and comparable rates of discontinuation and death between arms DoR=duration of response; EGFRm+=epidermal growth factor receptor mutation positive; EU=Europe; gen=generation; NA=North America; ORR=overall response rate; OS=overall survival; NSCLC=non-small cell lung cancer; TKI=tyrosine kinase inhibitor; PFS=progression-free survival. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.

Acknowledgements Thank you to everyone who made this trial possible: • The patients and their families • The personnel at all the clinical study sites • The scientists, regulatory, operations, and other teams within Summit and Akeso who helped develop ivonescimab and supported the study Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved.

Questions & Answers